Articles. Funding US National Institutes of Health grant R44-HL , Advanced Circulatory Systems.
|
|
|
- Amelia Washington
- 5 years ago
- Views:
Transcription
1 Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial Tom P Aufderheide, Ralph J Frascone, Marvin A Wayne, Brian D Mahoney, Robert A Swor, Robert M Domeier, Michael L Olinger, Richard G Holcomb, David E Tupper, Demetris Yannopoulos, Keith G Lurie Summary Background Active compression-decompression cardiopulmonary resuscitation (CPR) with decreased intrathoracic pressure in the decompression phase can lead to improved haemodynamics compared with standard CPR. We aimed to assess effectiveness and safety of this intervention on survival with favourable neurological function after out-ofhospital cardiac arrest. Methods In our randomised trial of 46 emergency medical service agencies (serving 2 3 million people) in urban, suburban, and rural areas of the USA, we assessed outcomes for patients with out-of-hospital cardiac arrest according to Utstein guidelines. We provisionally enrolled patients to receive standard CPR or active compression-decompression CPR with augmented negative intrathoracic pressure (via an impedance-threshold device) with a computer-generated block randomisation weekly schedule in a one-to-one ratio. Adults (presumed age or age 18 years) who had a nontraumatic arrest of presumed cardiac cause and met initial and final selection criteria received designated CPR and were included in the final analyses. The primary endpoint was survival to hospital discharge with favourable neurological function (modified Rankin scale score of 3). All investigators apart from initial rescuers were masked to treatment group assignment. This trial is registered with ClinicalTrials.gov, number NCT Findings 2470 provisionally enrolled patients were randomly allocated to treatment groups. 813 (68%) of 1201 patients assigned to the standard CPR group (controls) and 840 (66%) of 1269 assigned to intervention CPR received designated CPR and were included in the final analyses. 47 (6%) of 813 controls survived to hospital discharge with favourable neurological function compared with 75 (9%) of 840 patients in the intervention group (odds ratio 1 58, 95% CI ; p=0 019]. 74 (9%) of 840 patients survived to 1 year in the intervention group compared with 48 (6%) of 813 controls (p=0 03), with equivalent cognitive skills, disability ratings, and emotional-psychological statuses in both groups. The overall major adverse event rate did not differ between groups, but more patients had pulmonary oedema in the intervention group (94 [11%] of 840) than did controls (62 [7%] of 813; p=0 015). Interpretation On the basis of our findings showing increased effectiveness and generalisability of the study intervention, active compression-decompression CPR with augmentation of negative intrathoracic pressure should be considered as an alternative to standard CPR to increase long-term survival after cardiac arrest. Funding US National Institutes of Health grant R44-HL , Advanced Circulatory Systems. Introduction More than Europeans and North Americans have an out-of-hospital cardiac arrest every year, with overall survival averaging 5%. 1,2 Poor survival rates persist, in part, because manual chest compressions and ventilation, termed standard cardiopulmonary resuscitation (CPR), is inherently inefficient, providing less than 25% of healthy blood flow to the heart and brain. 3 Haemodynamics are often compromised further by poor standard CPR technique, especially inadequate chest compression and incomplete chest recoil. 4 6 Augmentation of negative intrathoracic pressure during the decompression phase can increase cardiac and cerebral perfusion in animals and people during CPR Investigators have shown that a decrease in intrathoracic pressure is linked to a simultaneous decrease in intracranial pressure; these mechanisms underlie the increase in blood flow to the heart and brain Clinical studies 15,16 have also shown substantial improvement in 24-h survival with this approach. Active compression-decompression CPR increases ventilation to 13 5 L per min (SD 5 5) compared with 7 8 L per min (5 3) with standard CPR. 9,12 One study 17 on the mechanism of the combination of active compression-decompression CPR and an impedance-threshold device showed that active compression-decompression CPR alone did not substantially reduce airway pressures during the decompression phase of CPR, because respiratory gases entered the lungs with every chest decompression. However, the study 17 also showed that when used in Published Online January 19, 2011 DOI: /S (10) See Online/Comment DOI: /S (10) Department of Emergency Medicine, Medical College of Wisconsin, Milwaukee, WI, USA (Prof T P Aufderheide MD); Department of Emergency Medicine, Regions Hospital, St Paul, MN, USA (R J Frascone MD); Whatcom County Emergency Medical Services, Department of Emergency Medicine, Peace Health, St Joseph Medical Center, Bellingham, WA, USA (M A Wayne MD); Department of Emergency Medicine, Hennepin County Medical Center, Minneapolis, MN, USA (B D Mahoney MD, Prof K G Lurie MD); Department of Emergency Medicine, William Beaumont Hospital, Royal Oak, MI, USA (Prof R A Swor DO); Department of Emergency Medicine, St Joseph Mercy Hospital, Ann Arbor, MI, USA (R M Domeier MD); Department of Emergency Medicine, Indiana University School of Medicine, Indianapolis, IN, USA (Prof M L Olinger MD); Quintiles Consulting, Rockville, MD, USA (R G Holcomb PhD); Departments of Neurology and Medicine Cardiovascular Division, University of Minnesota Medical Center, Minneapolis, MN, USA (Prof D E Tupper PhD, D Yannopoulos MD, K G Lurie) Correspondence to: Prof Tom P Aufderheide, Department of Emergency Medicine, 9200 West Wisconsin Avenue, Pavilion 1P, Milwaukee, WI 53226, USA taufderh@mcw.edu Published online January 19, 2011 DOI: /S (10)
2 For the study protocol see com/resqtrial-ip.pdf combination with an impedance-threshold device to impede inspiratory gases selectively during the recoil phase, active compression-decompression CPR substantially lowered intrathoracic pressures during chest decompression. The potential effect of augmentation of negative intrathoracic pressure during CPR on long-term survival with good neurological function has not previously been assessed in a clinical trial. We aimed to establish whether active compression-decompression CPR plus a decrease in intrathoracic pressure during the chest recoil phase achieved with an impedance-threshold device would result in improved survival to hospital discharge with favourable neurological function, 18 compared with standard CPR. Methods Study design We undertook our randomised, multicentre trial in seven geographical sites in the USA: Minneapolis and St Paul (MN), Whatcom County (WA), Oshkosh (WI), Oakland and Macomb Counties and Washtenaw and Livingston Counties (MI), and Indianapolis (IN). These sites had 46 emergency medical service agencies in urban, suburban, and rural areas, and served 2 3 million people. Adults (presumed or known to be 18 years of age) with out-of-hospital cardiac arrest were eligible for the study, on the basis of local institutional review board requirements. Patients were initially excluded if the patient was presumed or known to be younger than 18 years of age; had obvious or likely traumatic injuries causing cardiac arrest, a pre-existing do-not-resucitate order, signs of obvious clinical death, conditions that precluded the use of CPR, an in-hospital cardiac arrest, recent sternotomy with wound not appearing completely healed (if unknown) or less than 6 months (if known); or if the family or legal guardians requested that they not be entered in the study at the time of arrest. The final exclusion criteria included all initial criteria and if the patient received less than 1 min of CPR by emergency medical service personnel; had a complete airway obstruction that could not be cleared or if attempts at advanced airway management were unsuccessful; intubation with a leaky or uncuffed advanced airway device; or a stoma, tracheotomy, or tracheostomy. We excluded patients with non-cardiac causes of arrest, including respiratory arrest (eg, pulmonary embolism), arrest due to haemorrhage causes or stroke, metabolic abnormalities (eg, hyperkalaemia), drug overdose, and electrocution, to meet Utstein 19 cardiac arrest criteria for our prespecified primary endpoint analyses. The study was approved by the US Food and Drug Administration (FDA) under the US Code of Federal Regulations (21 CFR 50.24) exemption from informed consent under emergency circumstances, which included community consultation and public notification. Patient or family members provided written informed consent for continued participation in the trial before neurological. The protocol was undertaken with an investigational device exemption (IDE #G050062) and approved by the FDA and 25 institutional review boards at hospitals to which study patients were likely to have been transported. Randomisation and masking We used a prospective computer-generated block randomisation schedule every week, in blocks of 4 weeks, which was prepared by an independent biostatistician (RGH), to assign patients to receive standard CPR or study intervention in a one to one ratio. Apart from rescuer CPR, all aspects of the study, including obtaining of patient consent, medical record review, and neurological s, were done masked to treatment assignment, including during systematic review of hospital charts for adverse events. Study coordinators provided patient information and follow-up documents that did not show treatment assignment to study nurses (who obtained patient consent and did the neurological s) at every site. Hospital personnel who were responsible for postresuscitation care were masked to the CPR method to the extent possible. The study sponsor was masked to all aggregate patient outcomes throughout the study, apart from device failures. An independent data and safety monitoring board monitored safety, ethical, and scientific aspects of the study. An independent clinical events committee was responsible for adjudication of individual patient s adverse events and for exclusion of all those screened who did not meet criteria for enrolment, or did not meet the final selection criteria. With the unavoidable exception of device failures, patient information provided in summary form to the clinical events committee did not show randomised assignment. The data and safety monitoring board was masked to study group assignment when rendering recommendations to the investigators. Procedures Rescuers did active compression-decompression CPR with a hand-held device consisting of a suction cup that was attached to the chest, a handle, an audible metronome set to 80 beats per minute, and a force gauge to guide compression depth and chest wall recoil. 15,16 This CPR technique requires the operator to compress to the same depth as standard CPR and then lift upward to fully decompress the chest. 15,16 An impedance-threshold device, with an inspiratory resistance of 16 cm H 2 O and less than 5 cm H 2 O expiratory impedance, was connected to a facemask or advanced airway. The impedance threshold device lowered intrathoracic pressure during the decompression phase by impeding passive inspiratory gas exchange during the chest recoil phase, yet allowing periodic positive pressure ventilation. 10,17 The CPR device (ResQPump, also called CardioPump) and 2 Published online January 19, 2011 DOI: /S (10)
3 2327 resuscitation not attempted 388 did not meet final selection criteria 256 presumed or known non-cardiac cause 68 pre-existing DNR order discovered or efforts terminated prematurely 23 obvious clinical death or disorder that precluded CPR 17 complete airway obstruction (non-clearable) or unable to place advanced airway 12 leaky or uncuffed advanced airway or stoma, tracheotomy, or tracheostomy 8 received <1 min CPR 3 recent sternotomy 1 prisoner 597 died 335 before hospital admission 262 in emergency department 130 died 6 unknown 1201 enrolled to standard CPR group 2 3 million people served by study sites 5265 confirmed cardiac arrests 2938 resuscitation attempted 80 survived to hospital discharge 73 received neurological 7 declined to participate 1269 enrolled to intervention CPR group 813 provided standard CPR 840 provided intervention CPR 216 survived to hospital admission 2470 provisionally enrolled to receive CPR according to weekly randomisation schedule 237 survived to hospital admission 104 survived to hospital discharge 102 received neurological 2 declined to participate 468 did not meet initial selection criteria 270 trauma 129 aged or presumed aged <18 years 23 resuscitated without EMS CPR 22 prisoners 13 unable to randomly assign 7 return of spontaneous circulation 2 not treated by study participants 4 mass casualty incident and unable to randomise 6 recent sternotomy 3 cardiac arrests in hospital 2 requested exclusion from study at time of arrest 429 did not meet final selection criteria 296 presumed or known non-cardiac cause 54 pre-existing DNR order discovered or efforts terminated prematurely 22 obvious clinical death or disorder that precluded CPR 18 complete airway obstruction (non-clearable) or unable to place advanced airway 21 leaky or uncuffed advanced airway or stoma, tracheotomy, or tracheostomy 13 received <1 min CPR 3 recent sternotomy 1 aged or presumed aged <18 years 1 prisoner 602 died 357 before hospital admission 245 in emergency department 1 lost to follow-up 132 died in hospital 1 unknown* 11 died 4 withdrew from study 2 died 2 withdrew from study 3 lost to follow-up 6 died 3 withdrew from study 1 lost to follow-up 65 survived to 30 days 58 received neurological 7 declined to participate 58 survived to 90 days 50 received neurological 8 declined to participate 48 survived to 1 year 45 received neurological 3 declined to participate 95 survived to 30 days 85 received neurological 10 declined to participate 87 survived to 90 days 77 received neurological 10 declined to participate 74 survived to 1 year 65 received neurological 9 declined to participate 7 died 3 withdrew from study 4 died 2 withdrew from study 1 lost to follow-up 2 died 4 withdrew from study 7 lost to follow-up Figure 1: Study profile Our study was carried out in line with the Utstein guidelines. 19 DNR=do not resuscitate. EMS=emergency medical services. CPR=cardiopulmonary resuscitation. Traditional resuscitation was provided in the standard CPR group. *Patient was confirmed dead by public records. The date of death was before 30 days after cardiac arrest, but whether the death occurred during the index hospital stay is unknown. One patient survived to day 30 and underwent neurological, but had not been discharged from hospital. We searched public death records at 1 year for all patients who withdrew or were lost to follow-up at any stage. Published online January 19, 2011 DOI: /S (10)
4 Standard CPR group (n=813) Intervention* group (n=840) Mean age (years) 66 8 (14 5) 67 0 (15 2) (2%) 11 (1%) (4%) 47 (6%) (14%) 133 (16%) (26%) 179 (21%) (21%) 169 (20%) (20%) 192 (23%) (13%) 109 (13%) Sex (male) 539 (66%) 558 (66%) Arrest surroundings Witnessed before arrival of first responder 383 (47%) 398 (47%) Witnessed after arrival of first responder 76 (9%) 80 (10%) Unwitnessed 353 (43%) 361 (43%) Data not available 1 (<1%) 1 (<1%) Bystander CPR Provided 350 (43%) 357 (43%) Data not available 1 (<1%) Initial recorded cardiac arrest rhythm Ventricular fibrillation and pulseless ventricular tachycardia 247 (30%) 292 (35%) Asystole 379 (47%) 375 (45%) Pulseless electrical activity 180 (22%) 170 (20%) Data not available 7 (<1%) 3 (<1%) Emergency call to first response time (min) 6 5 (3 3) 6 4 (3 1) Emergency call to EMS CPR start time (min) 6 6 (3 4) 6 7 (3 2) Emergency call to placement of study devices (min) 7 1 (3 5) Impedance-threshold device airway attachment site Facemask 717 (85%) Endotracheal tube 586 (70%) Supraglottic airway (eg, laryngeal mask airway, Combitube, King) 169 (20%) Epinephrine dose (mg) 3 3 (2 1) 3 3 (2 1) Patients without ROSC 3 8 (1 9) 3 8 (1 9) Duration of CPR (min) 27 6 (12 2) 28 1 (11 4) Patients without ROSC 32 3 (9 5) 32 3 (8 1) ROSC during CPR before hospital admission 324 (40%) 343 (41%) Enrolment site (15%) 121 (14%) (19%) 169 (20%) (14%) 92 (11%) (23%) 208 (25%) 5 46 (6%) 40 (5%) (18%) 169 (20%) 7 39 (5%) 41 (5%) Admitted to hospital 216 (27%) 237 (28%) In-hospital hypothermia (% admitted) 85 (39%) 92 (39%) Cardiac catheterisation (% admitted) 72 (33%) 100 (42%) Coronary stenting (% admitted) 28 (13%) 38 (16%) Coronary bypass surgery (% admitted) 6 (3%) 15 (6%) Implanted cardiodefibrillator (% admitted) 30 (14%) 41 (17%) Data are number (%) or mean (SD) unless otherwise stated, and include all patients who met final study enrolment endpoint criteria. CPR=cardiopulmonary resuscitation. EMS=emergency medical service. ROSC=return of spontaneous circulation. *Patients received active compression-decompression cardiopulmonary resuscitation plus an impedance-threshold device. Data do not include arrests witnessed by EMS personnel. Table 1: Baseline patient characteristics the impedance-threshold device (ResQPOD) were manufactured by Advanced Circulatory Systems (Roseville, MN, USA). The first basic or advanced life support emergency medical service provider to arrive started chest compressions as soon as possible for both study groups. Standard CPR, defibrillation, and advanced life support treatment were done in accordance with local policy and the American Heart Association guidelines. 20 The compression to ventilation ratio was 30 to 2 during basic life support for both CPR techniques. For the intervention protocol, rescuers provided CPR at 80 compressions per min as soon as possible, with the active compression-decompression CPR device force gauge used to help achieve the recommended compression depth and complete chest recoil. For this group, rescuers initially attached the impedancethreshold device between the ventilation bag and facemask (King Systems, Indianapolis, IN, USA) and the device was subsequently relocated to the advanced airway. The impedance-threshold device was removed if the patient had return of spontaneous circulation and reapplied if rearrest occurred. The devices and facemask for the study intervention group (or a facemask alone for the standard CPR group) were packaged together in a study bag and carried by rescue personnel as per the randomisation calendar. CPR efforts in both groups were encouraged for at least 30 min on scene before the resuscitation attempt was stopped. Study intervention, if in progress, was stopped on arrival to hospital and replaced with traditional CPR, if warranted. Inhospital therapeutic hypothermia and cardiac revascularisa tion for all patients were encouraged by all site investigators emergency medical service personnel underwent didactic and hands-on training before the study started and every 6 months thereafter. Comprehensive training in standard CPR and the study intervention was provided by the principal investigators and research team at each study site, and emphasised the need to start compressions immediately; provide adequate compression depth, rate, and full chest recoil; maximise handson time, appropriate ventilation rate, and duration; ensure appropriate facemask seal for impedancethreshold device use with a facemask (two-handed technique); 20 do active compression-decompression CPR; 21,22 and rotate personnel undertaking CPR every 2 min to avoid fatigue Endpoints The prespecified primary study endpoint was survival to hospital discharge with favourable neurological function, defined as a modified Rankin scale score of 3 or less. 18 This neurological took into account previous neurological deficits and was undertaken at the time of hospital discharge. A secondary safety endpoint assessed the rate of major adverse events until hospital discharge. 4 Published online January 19, 2011 DOI: /S (10)
5 Major adverse events that occurred during the resuscitative effort or subsequent hospital stay that we included in the of secondary safety endpoint were death, cerebral bleeding, bleeding requiring transfusion or requiring surgical intervention, seizures, rearrest, pulmonary oedema, chest fractures (rib or sternum), internal thoracic and abdominal injuries, and device malfunction or defects. To discern if the study intervention resulted in more patients with neurological impairment (ie, modified Rankin scale 4), we assessed additional secondary effectiveness endpoints at 90 days and 365 days after out-of-hospital cardiac arrest. We assessed attention, short-term and longterm memory, judgment, and spatial ability with the cognitive abilities screening instrument. 23 We established amount of functional disability with the disability rating index 24 and depression and emotional stability with the Beck depression inventory. 25 Adverse events We regarded all adverse events reported as major because of their nature and the working understanding that only serious events associated with CPR interventions would be recorded. If a patient died and was not transported to hospital, the only major adverse event assigned to that patient was death. Post-mortems were not routinely reported and a uniform of other adverse events before hospital admission was not possible. However, all patients who were transported to hospital had all reported adverse events before hospital discharge included in calculation of the event rates, including those that occurred before transport to hospital. Events of an equivalent nature were combined for reporting reasons by use of protocol-defined major adverse events. For example, rib, sternal, and spinal fractures were all coded as chest fractures. Chest organ injury and abdominal organ injury were coded as internal organ injury. Adverse events identified as other were individually examined and included in protocol-defined adverse-event groups, if possible. For example, pneumomediastinum was coded into pneumothorax. Adverse events described as fluid in the endotracheal tube or airway were coded as evidence of pulmonary oedema. With only a few exceptions, patients were reported to have only one incident of a particular adverse-event type. In exceptional cases (eg, more than one chest fracture or rearrests) only one event of that type was assigned to an individual patient. Reported rates for individual major adverse event types were based on the percentage of all patients at risk who reported a specific event type. Data collection We obtained out-of-hospital data according to Utstein guidelines 19 from the emergency medical service out-ofhospital medical record. We obtained data for in-hospital treatment, outcomes, and follow-up from hospital records and neurological surveys for all patients who Primary composite study endpoints Standard CPR group (n=813) Intervention* group (n=840) p value Modified Rankin scale score at hospital discharge (<1%) 11 (1%) (1%) 11 (1%) (3%) 30 (4%) (1%) 23 (3%) (1%) 9 (1%) (2%) 18 (2%) (89%) 734 (87%).. Survival data for hospital discharge not available 6 (<1%) 2 (<1%).. Survived, but data for MRS not available 7 (<1%) 2 (<1%).. MRS 3 (primary study endpoint) 47 (6%) 75 (9%) Secondary survival endpoints Survived to 24 h after arrest 176 (22%) 197 (24%) 0 41 Data not available 9 (1%) 6 (<1%).. Survived to hospital discharge 80 (10%) 104 (12%) 0 12 Data not available 6 (<1%) 2 (<1%).. Discharge location (% discharged) Home 47 (59%) 67 (64%) 0 75 Other 28 (35%) 35 (34%).. Data not available 5 (6%) 2 (2%).. Survived to 90 days 58 (7%) 87 (10%) Data not available 15 (2%) 8 (1%).. Survived to 1 year 48 (6%) 74 (9%) Data not available 19 (2%) 19 (2%).. Initial recorded arrest rhythm in patients with MRS 3 Ventricular fibrillation and pulseless ventricular tachycardia 40 (17%) 66 (23%) Asystole 3 (<1%) 6 (2%).. Pulseless electrical activity 3 (2%) 2 (1%).. Unknown 1 (<1%) 1 (<1%).. Neurological CASI (patients with complete score, validity=1) 90 days 93 2 (7 4) 90 4 (13 4) Data not available 19 (33%) 35 (40%) days 92 9 (12 0) 94 5 (4 5) Data not available 16 (33%) 32 (43%).. Beck depression inventory score 90 days 4 8 (3 9) 6 5 (6 8) Data not available 14 (24%) 22 (25%) days 5 2 (6 3) 5 5 (5 9) Data not available 13 (27%) 17 (23%).. Data are number (%) or mean (SD) unless otherwise stated, and include all patients who met final study enrolment endpoint criteria. *Patients received active compression-decompression cardiopulmonary resuscitation plus an impedance-threshold device. For MRS scores, 0 is asymptomatic, 1 is no significant disability, 2 is slight disability, 3 is moderate disability, 4 is moderately severe disability, 5 is severe disability, and 6 is dead. Mantel-Haenszel test statistic across three initial recorded arrest rhythm groups. Assessment of attention, short-term memory, long-term memory, judgment, spatial ability, concentration, based on a scale of 0 100, in which 100 is a perfect score. Assessment of depression with a scale of 0 63, in which 0 13 suggests minimum signs of depression, suggests mild signs of depression, suggests moderate signs of depression, and suggests severe signs of depression. MRS=modified Rankin scale. CASI=cognitive abilities screening instrument. Table 2: Primary and secondary study endpoints Published online January 19, 2011 DOI: /S (10)
6 Patients (n) 80 Intervention (90 days) 70 Intervention (365 days) Standard CPR (90 days) 60 Standard CPR (365 days) Mild (category 1 3) Moderate (category 4 6) Disability rating Extreme (category 7 9) Figure 2: Disability rating scale scores 90 days and 365 days after out-of-hospital cardiac arrest Scores did not differ between study groups at 90 days and 365 days after cardiac arrest. The disabilities rating scale 23 is based on a scale of 0 29 where disability is absent (score 1, category 1), mild (1, 2), partial (2 3, 3), moderate (4 6, 4), moderately severe (7 11, 5), severe (12 16, 6), extremely severe (17 21, 7), vegetative state (22 24, 8), or extreme vegetative state (25 29, 9). CPR=cardiopulmonary resuscitation. consented to be included or until such time as the patient or family refused consent for continued participation in the trial. All neurological s were done by trained and certified nurses who were members of the research team at every study site. We undertook clinical monitoring throughout the study to maximise protocol adherence and quality of rescuer performance of standard CPR and the study intervention. Study sites were required to complete a run-in phase and certification process before beginning enrolment in the study. Statistical analysis On the basis of historical controls in equivalent populations at participating study sites, we estimated that survival to hospital discharge in the control group would be about 6%. Estimates of survival to hospital discharge with a good neurological outcome based on the modified Rankin scale score had not previously been assessed in this population. On the basis of an expected 6% rate in the standard CPR control group for the study endpoint and 10 2% in the intervention group, a sample size of 700 patients per group would have been needed to detect a significant improvement with a final significance level of with 80% statistical power. An interim analysis at the 50% information point of the original study sample was prospectively planned with the Lan-DeMets alpha spending method with O Brien-Fleming boundaries. 26 Following this planned midpoint analysis in September, 2008, the data and safety monitoring board recommended a sample size adjustment to 2696 assessable patients (1348 per group) to maintain the original design objective of 80% power to detect a group difference, without knowledge of the direction of the recorded difference. In July, 2009, the study was stopped early because of a shortage of funding, at which time 1653 patients who met final criteria had been enrolled. At study end, we applied the original prespecified criteria for of statistical significance of study results, which was identical to requirements that would have been applicable to full enrolment. Our original study design initially included a third group of patients who were randomly assigned to standard CPR and an impedance-threshold device. The trial was originally designed to primarily compare standard CPR with active compression-decompression CPR and an impedance-threshold device. The third group was added (with half the proportional enrolment of the other two groups assigned initially to the third arm) to assess the relative contribution of the impedancethreshold device alone to anticipated treatment effect observed for the combination of devices. Although masked to outcome by study group, this group was discontinued in November, 2007, after enrolment of 150 patients because of slower than expected overall enrolment and our intention to funnel remaining funding resources to of the primary outcome. Patients from the third group are not included in analyses presented here. We used Fisher s exact test for analysis of the primary endpoint and p<0 049 was regarded as statistically significant. We did all analyses on the intention-to-treat population of all patients meeting enrolment criteria. We assessed the secondary safety endpoint with an exact binomial test for non-inferiority with the same p-value requirement. We compared proportions of patients who had one or more major adverse events (of any kind) between study groups, and tested whether the patientscale major adverse event rate in the intervention group was inferior or non-inferior to that of the control group with a non-inferiority margin of 5%. We assessed additional prespecified secondary endpoints, including subgroup analyses based upon age, sex, initial rhythm, time to CPR, and whether the arrests were witnessed, with Fisher s exact tests and Student s t tests, but associated p-values were regarded as nominal and unadjusted without associated statistical significance values. We did all analyses with StatXact version 8 and SPSS version Continuous data are expressed as mean (SD). This trial is registered with ClinicalTrials.gov, number NCT Role of the funding source The protocol was approved by the US National Institutes of Health (funding source) and a representative of this institute was on the data and safety monitoring board. The sponsor (Advanced Circulatory Systems) helped investigators to obtain government funding, design the study, interpret the data, write the report, and decide to 6 Published online January 19, 2011 DOI: /S (10)
7 submit the report for publication. The sponsor was not involved in any patient care or of patient neurological status during the 1-year follow-up. The decision to submit the paper was made by all co-authors with no input from the National Institutes of Health. The corresponding author had full access to all the data in the study; all other authors could request examination of any of the data elements. All authors reviewed and approved the final version of the manuscript and had final responsibility for the decision to submit for publication. Results We enrolled a total of 197 patients in a run-in phase across all sites, starting in October, 2005, at the first site, and ending in April, 2009, at the last participating site. Data from the run-in phase were not included in the final analyses. The mean run-in period across the seven sites was 107 days (range 21 to 173 days). Between March, 2006, and July, 2009, we randomly assigned 2470 patients to treatment groups, 1653 of whom met prespecified enrolment criteria (figure 1). We completed the final 1-year follow-up in July, Enrolment was balanced between study groups across all sites (table 1). The site with the lowest enrolment rate enrolled 80 patients (site 7; 4 8% of patients in the study population) and highest enrolled 397 (site 4; 24 0%). For 672 (80%) patients, the impedance-threshold device was used first with a facemask and then switched to an advanced airway. The impedance-threshold device was used only on a facemask in 45 (5 4%) cases and an advanced airway was only used in 73 (8 5%). The impedance-threshold device was not used in 50 cases (5 9%). For the primary endpoint, treatment with study intervention led to a 53% relative increase in survival to hospital discharge with a modified Rankin scale score of 3 or less compared with standard CPR (odds ratio 1 58, 95% CI , p=0 019; table 2). Additionally, there was a shift in the distribution of modified Rankin scale scores in favour of improved outcomes in the intervention group (Kruskal-Wallis test for ordinal responses p=0 039; table 2). Our subgroup analysis of survival based on the first recorded rhythm to hospital discharge with a modified Rankin scale score of 3 or less suggested survival did not differ between patients with ventricular fibrillation and pulseless ventricular tachycardia in the intervention group and those in the standard CPR group (p=0 0645, table 2). 74 of 218 (34%) patients with ventricular fibrillation and pulseless ventricular tachycardia who had a witnessed out-of-hospital cardiac arrest in the intervention group survived to hospital discharge, compared with 50 of 181 (27%) such patients in the standard CPR group (p=0 19). Nearly all survivors had no or mild long-term neurological deficits, which did not differ between groups (table 2). Disabilities rating scale scores, which are a key Standard CPR group (n=813) functional of patients with severe disability, did not differ between groups (figure 2). Overall major adverse event rates did not differ between groups (table 3). Intervention* group (n=840) p value Patients with reported adverse events (94%) 787 (94%) 0 47 (6%) 53 (6%) Adverse events Death 729 (90%) 734 (87%) Rearrest 161 (20%) 184 (22%) Pulmonary oedema 62 (8%) 94 (11%) Seizure after index arrest 13 (2%) 11 (1%) Bleeding requiring transfusion or surgery 3 (<1%) 7 (<1%) Chest fractures 15 (2%) 12 (1%) Pneumothorax 7 (<1%) 10 (1%) Haemothorax 1 (<1%) 2 (<1%) Cardiac tamponade 3 (<1%) 2 (<1%) Cerebral bleeding 3 (<1%) 2 (<1%) Aspiration 7 (<1%) 8 (1%) Internal organ injury 2 (<1%) 4 (<1%) Other 3 (<1%) 1 (<1%) Study device functionality Impedance-threshold device Timing light failure NA 59 (7%) ACD-CPR device Inadequate attachment of suction cup to the chest NA 81 (9%) Data are number (%) or mean (SD), and include all patients who met final study enrolment endpoint criteria. CPR=cardiopulmonary resuscitation. ACD=active compression-decompression. NA=not applicable. *Patients received ACD cardiopulmonary resuscitation plus an impedance-threshold device. p< for non-inferiority within a margin of 5%. Pulmonary oedema included prehospital reports of any fluid or secretions noted in the airway (eg, oedema fluid, blood, or mucus) and pulmonary oedema or pulmonary/pleural effusion that was reported on radiography or CT imaging. The impedance-threshold device timing light is an accessory feature to guide ventilation rate. Primary function of the impedance-threshold device was not affected by timing light failures in any cases as the device provided appropriate inspiratory impedance. Manufacturing changes have been implemented to remedy timing light failures. For 73 of 81 (90%) reported cases of difficulty maintaining suction, use of the ACD-CPR device was continued despite suction cup attachment difficulty. For eight (10%) of these 81 patients, use of the ACD-CPR device was discontinued and replaced with manual standard CPR. Table 3: Major adverse events and device functionality Survivors to hospital discharge with MRS 3 (%) 40 Intervention (n=75) 35 Standard CPR (n=47) Age at time of arrest (years) Figure 3: Age of patients surviving to hospital discharge with favourable neurological function (MRS score 3) CPR=cardiopulmonary resuscitation. MRS=modified Rankin scale Published online January 19, 2011 DOI: /S (10)
8 See Online for webappendix OR (95% CI) All patients 1 58 ( ) Age below median* 1 54 ( ) Age above median* 1 82 ( ) Women 1 65 ( ) Men 1 55 ( ) Witness: no 1 69 ( ) Witness: yes 1 56 ( ) Rhythm: VF/VT 1 48 ( ) Rhythm: other 1 37 ( ) Time to CPR <6 min 1 55 ( ) Time to CPR 6 min 1 73 ( ) Site ( ) Site ( ) Site ( ) Site ( ) Site ( ) Site ( ) Site ( ) Favours standard CPR Favours intervention CPR Figure 4: Effects of age, study site, sex, and treatment intervention on primary study endpoint Estimated odds ratios exceeded 1 00 for subgroups based on age, sex, witnessed status, time to start of CPR, and all study sites apart from Site 1. VF/VT=ventricular fibrillation and pulseless ventricular tachycardia. CPR=cardiopulmonary resuscitation. *Median age was 67 years (IQR 56 79). However, pulmonary oedema was more common in the intervention group (p=0 015). We assessed the effects of age, study site, sex, and date of treatment on the primary endpoint. The average age of survivors at this endpoint was 56 0 years (SD 15 0) in the standard CPR group and 56 4 years (15 4) in the study intervention group (p=0 87). Figure 3 shows age distributions of patients surviving to the primary endpoint. Consistent survival differences between study groups were noted throughout the study, independent of age, study site, sex, and date of treatment (figures 4 and 5). In the standard CPR group, 12 (4%) of 274 women and 35 (6%) of 539 men survived to the primary endpoint compared with 20 (7%) of 282 women and 55 (10%) of 558 men in the intervention group. Furthermore, the therapeutic benefit of CPR in both groups was highly dependent on time to the start of CPR (figure 6). There were no survivors with favourable neurological function in either group when professional rescuer CPR was started more than 10 min after the emergency call. We excluded 817 patients from the primary analysis who did not meet prespecified enrolment criteria (eg, noncardiac causes or inability to ventilate). When data from these patients were combined with those meeting prespecified enrolment criteria, 71 (6%) of 1201 treated with standard CPR survived to the primary endpoint compared with 101 (8%) of 1269 in the intervention group (odds ratio 1 37, 95% CI ; p=0 057). Primary endpoint data were missing for 17 patients who survived to hospital admission, and informed consent for study participation was refused by 14 patients or family members. We also did a sensitivity analysis to establish the effect of missing cases, assuming patients who were known to be dead within 1 year from public death records or those known to be discharged to a nursing home probably had a modified Rankin scale score of more than 3 at hospital discharge. In our sensitivity analysis, assessing the effect of missing data, the difference between the standard CPR and intervention groups remained significant (p=0 018). Webappendix pp 1 4 contains supplementary tables with data for patients in whom resuscitation was not attempted due to clinical death or do-not-resuscitate orders; initial selection criteria were not met; exclusions were made for non-cardiac causes; modified Rankin scale score information was missing; and who were alive at hospital discharge and subsequently died, withdrew from the study, or were lost to follow up. Survivors with MRS 3 (%) Intervention enrolment (cumulative) Standard CPR enrolment (cumulative) Total enrolment (cumulative) Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q Intervention Total Standard CPR Figure 5: Cumulative rates of achievement of primary endpoint (MRS score 3 at discharge from hospital) Results are shown for pivotal phase enrolment (n=1653) by year quarter (Q). Consistent results in both groups were shown throughout the whole study. Enrolment was initiated in the fourth quarter of 2007 in Site 6 and the first quarter of 2009 in Site 7. MRS=modified Rankin scale. CPR=cardiopulmonary resuscitation. 8 Published online January 19, 2011 DOI: /S (10)
9 Survivors to hospital discharge with MRS 3 (%) 16 Intervention (n=75) n=52 Standard CPR (n=47) n=31 n=23 n= >5 10 Time from emergency call to randomised CPR treatment (min) Figure 6: Survival at hospital discharge with favourable neurological function (MRS score 3 at discharge from hospital) by time to CPR treatment Survival at hospital discharge with MRS 3 was significantly higher in the intervention group than in the standard CPR group (odds ratio 1 58, 95% CI , p=0 019). There were no survivors with favourable neurological function in either group if CPR was initiated more than 10 min after the emergency call. MRS=modified Rankin scale. CPR=cardiopulmonary resuscitation. Discussion Our results show that treatment with active compressiondecompression CPR with enhancement of negative intrathoracic pressure during the decompression phase significantly increases survival to hospital discharge with favourable neurological function compared with standard CPR after an out-of-hospital cardiac arrest of presumed cardiac cause (panel). Furthermore, overall survival increased by nearly 50% by 1 year in the intervention group compared with controls. Consistency of benefit was independent of sex, age, date of enrolment, and study site. Neurological function was much the same between groups at 90 days and 365 days after the out-ofhospital cardiac arrest. There was no increase in the number of patients with severe neurological impairment in either group. There were no differences in overall major adverse event rates between groups, although occurrence of pulmonary oedema was increased by 50% in the device group, which was coexistent with the increase in survival with favourable neurological function. The clinical relevance of this finding is unclear: the percentage increase in pulmonary oedema (46%) was proportional to the increase in survival in the intervention group (53%). Our findings strongly support the need for rapid deployment of all CPR interventions to maximise possible benefits. Our investigation builds on previous studies 7 12,15,16,27 31 showing that active compression-decompression CPR and a means to lower intrathoracic pressure during the chest recoil phase transforms the chest into an active bellows to more effectively circulate blood during CPR to the heart and the brain and increase short-term survival. Our study shows that teaching and implementation of active compression-decompression CPR and impedancethreshold device skills in urban, suburban, and rural Panel: Research in context Systematic review Investigators have shown the physiological changes underlying the synergistic effects of a combination of active compression-decompression cardiopulmonary resuscitation (CPR) with an impedance threshold device. Active compression-decompression CPR by itself transforms the human chest into an active bellows ventilation was increased to 13 5 L per min (SD 5 5) compared with 7 8 L per min (5 3) for standard CPR. 9 In that investigation, 9 intrathoracic pressures remained much the same with standard CPR and active compression-decompression CPR unless the endotracheal tube was blocked, thereby preventing respiratory gases from entering the lungs during the chest decompression phase. The mechanical and physiological advantages associated with a lowering of intrathoracic pressure by impedance of inspiration (apart from when active positive pressure ventilation was provided) have been confirmed in various studies of animals and people. Impedance of inspiration lowered intrathoracic pressure and resulted in increased cardiac output, vital organ blood flow, and survival in animals and human patients. 7,10 12,14 17,27 30 Four clinical trials, 15 17,29 including 644 patients, and a meta-analysis 30 done before our clinical investigation provided strong support for the notion that active compression-decompression CPR with augmentation of negative intrathoracic pressure improves haemodynamics, short-term survival, and the potential for longer-term survival with favourable neurological function. Interpretation On the basis of the strong clinical foundation we noted, we provide evidence from a study of 1653 patients that active compression-decompression CPR with augmentation of negative intrathoracic pressure improves survival to hospital discharge with favourable neurological function compared with standard CPR. For the first time, we have shown that a new method of CPR increases hospital-discharge rates and 1-year survival, which are both associated with good neurological outcomes, by nearly 50%, compared with the current standard of care, closed-chest manual CPR. emergency medical services is practicable. Because the US study sites we investigated have much the same practices as do most emergency medical service systems in the USA and because study devices have been successfully integrated into emergency services at locations in Germany and France, this approach should be expandable to any emergency medical service system that follows present European Resuscitation Council or American Heart Association guidelines. We first noted the significant difference in clinical outcomes between intervention groups at the time of hospital discharge; return of spontaneous circulation and hospital admission rates did not differ between groups. On the basis of preclinical and clinical studies showing Published online January 19, 2011 DOI: /S (10)
10 greater blood flow to the heart and brain with active compression-decompression CPR and augmentation of lower intrathoracic pressure, 7,10 12,14,27 29 we suggest that improved cerebral perfusion during CPR in the intervention group resulted in reduced cerebral ischaemia but that recovery and restoration of brain function might take more time than does the recovery of cardiac function. These findings also support the idea that improved perfusion outside the hospital in the intervention group could result in more stable candidates for cardiac catheterisation than were found in the standard CPR group, resulting in more patients in the intervention group being provided cardiac catheterisation. Our study has several limitations. First, emergency medical service rescuers were not blinded to the CPR method; however, assessors of the primary outcome and neurological tests were masked to intervention status, which restricted potential bias. Second, we could not establish the relative contribution of active compressiondecompression CPR alone, the impedance-threshold device alone, or the rescuer feedback elements including the timing lights, metronome, and force gauge to the positive study outcome. Data from studies in animals and human beings suggest that every component is necessary to record benefits with this combined approach. 5,6,11,14,16,30 The study initially included a third arm standard CPR plus an impedance-threshold device study arm to assess the relative contribution of the impedance threshold device only to the anticipated benefit of the combination of active compression-decompression CPR plus an impedance-threshold device versus standard CPR. However, due to slower than anticipated enrolment rates for the entire study and limited funding for the study, enrolment in this third study arm was discontinued early in the course of the study. Another potential limitation of our study was that study enrolment was stopped early because of low funding and extra data could have changed the primary findings. Nevertheless, differences between treatment groups were consistent throughout the study (figure 5). Finally, some surviving patients refused to provide consent for further participation or release of data. However, because of the unique circumstances and limitations associated with obtaining of informed consent under emergency circumstances, collection of follow-up data for all patients is a challenge for all studies such as ours. Thus, compared with standard CPR, active compression-decompression CPR with augmentation of negative intrathoracic pressure results in significantly increased survival to hospital discharge with favourable neurological function, which was observed to 1 year after out-of-hospital cardiac arrest. Contributors TPA, RGH, DET, DY, and KGL designed the study. TPA, RJF, MAW, BDM, RAS, RMD, and MLO obtained the data. RGH did the statistical analysis. All authors participated in the data analysis and interpretation processes and in the review of the final report. Study personnel Data and Safety Monitoring Board L Brent Mitchell (Chairman; Libin Cardiovascular Institute of Alberta, University of Calgary, Calgary, AB, Canada), Dianne M Bartels and Patricia Grambsch (University of Minnesota, Minneapolis, MN, USA), Daniel Hankins (Mayo Clinic Medical Transport, Rochester, MN, USA), James Malec (Rehabilitation Hospital of Indiana/Indiana University School of Medicine, Indianapolis, IN, USA), Jay W Mason (University of Utah, Salt Lake City, UT, USA), and Paul Molinari (Twin Cities Anesthesia Associates, Minneapolis, MN, USA). Clinical Events Committee Carson Harris (Chairman), Avi Nahum, and Dennis Zhu (Regions Hospital, St Paul, MN, USA). Data Co-ordination Terry Arend Provo, Cindy Setum, Lucinda Klann, Nathan Burkhart, and Caleb Sorenson (Roseville, MN, USA). Acknowledgments We thank the many first responders, firefighters, emergency medical technicians, and paramedics at all sites who took care of the patients, and without whom survival from out-of-hospital cardiac arrest would not be possible (see webappendix pp 5 9). The study was funded by the US National Institutes of Health and the federal grant (R44-HL ) was used by Advanced Circulatory Systems to fund the study. Conflicts of interest TPA, RJF, MAW, BDM, RAS, RMD, and MLO all received grant funds to their respective institutions for services related to patient enrolment, follow-up and data management for this clinical study; RGH received consulting fees for statistical analyses (US National Institutes of Health [NIH] R44-HL ). KGL was co-inventor of the impedancethreshold device and active compression-decompression cardiopulmonary resuscitation device, and founded Advanced Circulatory Systems in 1997 after the University of Minnesota (MN, USA) declined the opportunity to apply for patent protection on the impedance threshold device. All investigators feel KGL contributed substantially to this study and therefore should be listed as an author rather than incorporating his role within the activities of the sponsor, Advanced Circulatory Systems. In his role as the Chief Medical Officer of Advanced Circulatory Systems, KGL participated with the other investigators in obtaining the NIH grant funding (KGL was the principal investigator on the Small Business Innovation Research grant), the study design, data interpretation, writing and the decision to submit the paper for publication. KGL was not involved in any patient care or of patient neurological status during the 1-year follow-up. Outside the present study, TPA has board membership for Take Heart America and Citizen CPR Foundation, has consulted for JoLife Medical and Medtronic Foundation, and has received grants/ grants pending from the NIH Immediate Trial, NIH Resuscitation Outcomes Consortium, NIH Neurological Emergency Treatment Trials Network, and NIH Medical College of Wisconsin K12 Research Career Development Program. RJF has received payment for one lecture from Advanced Circulatory Systems. MAW has consulted for Baxter and Vitacare, has received grants/grants pending from the NIH Immediate Trial, payment for lectures or speakers bureau membership from Vitacare and Sub Zero, and royalties from Cook Critical Care. BDM has board membership for Take Heart Minnesota and has received grants/ grants pending from the NIH Neurological Emergency Treatment Trials Network. DET has received grants/grants pending from the Predict HD study, NINDS-6375, NINDS and NIMH-01579, and royalties as a textbook editor. References 1 Nichol G, Thomas E, Callaway CW, et al. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA 2008; 300: Hollenberg J, Herlitz J, Lindqvist J, et al. Improved survival after out-of-hospital cardiac arrest is associated with an increase in proportion of emergency crew-witnessed cases and bystander cardiopulmonary resuscitation. Circulation 2008; 118: Andreka P, Frenneaux MP. Haemodynamics of cardiac arrest and resuscitation. Curr Opin Crit Care 2006; 12: Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA 2005; 293: Published online January 19, 2011 DOI: /S (10)
Introduction. Introduction. Introduction. Results. Method
 Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomized
Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomized
RES TRIAL SUMMARY MARCH 2012 BACKGROUND
 BACKGROUND Even when performed correctly, conventional, manual, standard cardiopulmonary resuscitation (S-CPR) provides only 10 20% of normal blood flow to the heart, and 20 30% of normal blood flow to
BACKGROUND Even when performed correctly, conventional, manual, standard cardiopulmonary resuscitation (S-CPR) provides only 10 20% of normal blood flow to the heart, and 20 30% of normal blood flow to
SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED)
 SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED) I. GENERAL INFORMATION Device Generic Name Device Trade Name Device Procode: Applicant s Name & Address Combination compression/decompression manual chest
SUMMARY OF SAFETY AND EFFECTIVENESS DATA (SSED) I. GENERAL INFORMATION Device Generic Name Device Trade Name Device Procode: Applicant s Name & Address Combination compression/decompression manual chest
Science and Technology of Head Up CPR
 Science and Technology of Head Up CPR Keith Lurie MD Professor of Emergency Medicine and Internal Medicine University of Minnesota Minneapolis, Minnesota USA April 4, 2018 Minneapolis, Minnesota 2 Disclosures
Science and Technology of Head Up CPR Keith Lurie MD Professor of Emergency Medicine and Internal Medicine University of Minnesota Minneapolis, Minnesota USA April 4, 2018 Minneapolis, Minnesota 2 Disclosures
Clinical Investigation and Reports
 Clinical Investigation and Reports Comparison of Standard Cardiopulmonary Resuscitation Versus the Combination of Active Compression-Decompression Cardiopulmonary Resuscitation and an Inspiratory Impedance
Clinical Investigation and Reports Comparison of Standard Cardiopulmonary Resuscitation Versus the Combination of Active Compression-Decompression Cardiopulmonary Resuscitation and an Inspiratory Impedance
MORE HEARTBEAT THAN A. Enhanced CPR. Better neurologic outcomes. Steve Dunn, Ph.D., Professor, University of Wisconsin Oshkosh
 MORE THAN A HEARTBEAT Enhanced CPR. Better neurologic outcomes. Steve Dunn, Ph.D., Professor, University of Wisconsin Oshkosh Thanks to the ResQPOD and a dedicated EMS team, Steve survived sudden cardiac
MORE THAN A HEARTBEAT Enhanced CPR. Better neurologic outcomes. Steve Dunn, Ph.D., Professor, University of Wisconsin Oshkosh Thanks to the ResQPOD and a dedicated EMS team, Steve survived sudden cardiac
Implementing the 2005 American Heart Association Guidelines improves outcomes after out-of-hospital cardiac arrest
 Implementing the 2005 American Heart Association Guidelines improves outcomes after out-of-hospital cardiac arrest Tom P. Aufderheide, MD,* Demetris Yannopoulos, MD, Charles J. Lick, MD, Brent Myers, MD,
Implementing the 2005 American Heart Association Guidelines improves outcomes after out-of-hospital cardiac arrest Tom P. Aufderheide, MD,* Demetris Yannopoulos, MD, Charles J. Lick, MD, Brent Myers, MD,
ROC PRIMED Questions and Answers
 ROC PRIMED Questions and Answers 1) What is the ROC PRIMED study? ROC PRIMED stands for the Resuscitation Outcomes Consortium Prehospital Resuscitation using an IMpedance valve and Early versus Delayed
ROC PRIMED Questions and Answers 1) What is the ROC PRIMED study? ROC PRIMED stands for the Resuscitation Outcomes Consortium Prehospital Resuscitation using an IMpedance valve and Early versus Delayed
Frequently Asked Questions
 Frequently Asked Questions What is this new technique and how does it work? Researchers are testing two new investigational devices - the ResQPump and ResQPOD. The ResQPump assists EMS providers in performing
Frequently Asked Questions What is this new technique and how does it work? Researchers are testing two new investigational devices - the ResQPump and ResQPOD. The ResQPump assists EMS providers in performing
Supplementary Online Content
 Supplementary Online Content Hasegawa K, Hiraide A, Chang Y, Brown DFM. Association of prehospital advancied airway management with neurologic outcome and survival in patients with out-of-hospital cardiac
Supplementary Online Content Hasegawa K, Hiraide A, Chang Y, Brown DFM. Association of prehospital advancied airway management with neurologic outcome and survival in patients with out-of-hospital cardiac
More Than A Heartbeat
 More Than A Heartbeat Improve Perfusion During CPR Today, only a small number of out of hospital cardiac arrest victims survive. A focus on high-quality CPR and adoption of new techniques and technologies
More Than A Heartbeat Improve Perfusion During CPR Today, only a small number of out of hospital cardiac arrest victims survive. A focus on high-quality CPR and adoption of new techniques and technologies
2015 Interim Training Materials
 2015 Interim Training Materials ACLS Manual and ACLS EP Manual Comparison Chart Assessment sequence Manual, Part 2: The Systematic Approach, and Part BLS Changes The HCP should check for response while
2015 Interim Training Materials ACLS Manual and ACLS EP Manual Comparison Chart Assessment sequence Manual, Part 2: The Systematic Approach, and Part BLS Changes The HCP should check for response while
A dose-response curve for the negative bias pressure of an intrathoracic pressure regulator during CPR
 Purdue University Purdue e-pubs Weldon School of Biomedical Engineering Faculty Publications Weldon School of Biomedical Engineering 10-2005 A dose-response curve for the negative bias pressure of an intrathoracic
Purdue University Purdue e-pubs Weldon School of Biomedical Engineering Faculty Publications Weldon School of Biomedical Engineering 10-2005 A dose-response curve for the negative bias pressure of an intrathoracic
Pediatric Cardiac Arrest General
 Date: November 15, 2012 Page 1 of 5 Pediatric Cardiac Arrest General This protocol should be followed for all pediatric cardiac arrests. If an arrest is of a known traumatic origin refer to the Dead on
Date: November 15, 2012 Page 1 of 5 Pediatric Cardiac Arrest General This protocol should be followed for all pediatric cardiac arrests. If an arrest is of a known traumatic origin refer to the Dead on
Michigan Pediatric Cardiac Protocols. Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS
 Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
Michigan Pediatric Cardiac Protocols. Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS
 Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
Date: November 15, 2012 Page 1 of 1 TABLE OF CONTENTS Pediatric Asystole Section 4-1 Pediatric Bradycardia Section 4-2 Pediatric Cardiac Arrest General Section 4-3 Pediatric Narrow Complex Tachycardia
R.J. Frascone, M.D. FACEP FAEMS Medical Director, Regions Hospital EMS Professor, Emergency Medicine University of Minnesota
 R.J. Frascone, M.D. FACEP FAEMS Medical Director, Regions Hospital EMS Professor, Emergency Medicine University of Minnesota Conflicts of Interest I have no conflicts of interest. State of the Future
R.J. Frascone, M.D. FACEP FAEMS Medical Director, Regions Hospital EMS Professor, Emergency Medicine University of Minnesota Conflicts of Interest I have no conflicts of interest. State of the Future
DEVICE DURING STANDARD ACTIVE DECOMPRESSION
 USE OF AN IMPEDANCE THRESHOLD DEVICE DURING STANDARD AND/OR ACTIVE COMPRESSION DECOMPRESSION ITD Bibliography 1. Aufderheide TP, Pirrallo RG, et al. Clinical evaluation of an inspiratory impedance threshold
USE OF AN IMPEDANCE THRESHOLD DEVICE DURING STANDARD AND/OR ACTIVE COMPRESSION DECOMPRESSION ITD Bibliography 1. Aufderheide TP, Pirrallo RG, et al. Clinical evaluation of an inspiratory impedance threshold
² C Y E N G R E M E ssignac Cardiac Arrest Resuscitation Device uob
 E M E R G E N C Y Boussignac Cardiac Arrest Resuscitation Device ² What is b-card? b-card Boussignac Cardiac Arrest Resuscitation Device has been designed specifically for the treatment of cardiac arrest.
E M E R G E N C Y Boussignac Cardiac Arrest Resuscitation Device ² What is b-card? b-card Boussignac Cardiac Arrest Resuscitation Device has been designed specifically for the treatment of cardiac arrest.
Science Behind Resuscitation. Vic Parwani, MD ED Medical Director CarolinaEast Health System August 6 th, 2013
 Science Behind Resuscitation Vic Parwani, MD ED Medical Director CarolinaEast Health System August 6 th, 2013 Conflict of Interest No Financial or Industrial Conflicts Slides: Drs. Nelson, Cole and Larabee
Science Behind Resuscitation Vic Parwani, MD ED Medical Director CarolinaEast Health System August 6 th, 2013 Conflict of Interest No Financial or Industrial Conflicts Slides: Drs. Nelson, Cole and Larabee
2015 AHA Guidelines: Pediatric Updates
 2015 AHA Guidelines: Pediatric Updates Advances in Pediatric Emergency Medicine December 9, 2016 Karen O Connell, MD, MEd Associate Professor of Pediatrics and Emergency Medicine Emergency Medicine and
2015 AHA Guidelines: Pediatric Updates Advances in Pediatric Emergency Medicine December 9, 2016 Karen O Connell, MD, MEd Associate Professor of Pediatrics and Emergency Medicine Emergency Medicine and
Rowan County EMS. I m p r o v i n g C a r d i a c A r r e s t S u r v i v a l. Christopher Warr NREMT-P Lieutenant.
 Rowan County EMS I m p r o v i n g C a r d i a c A r r e s t S u r v i v a l Christopher Warr NREMT-P Lieutenant Rowan County EMS christopher.warr@rowancountync.gov September 9, 2012 2:44 11:44:00 Mr.
Rowan County EMS I m p r o v i n g C a r d i a c A r r e s t S u r v i v a l Christopher Warr NREMT-P Lieutenant Rowan County EMS christopher.warr@rowancountync.gov September 9, 2012 2:44 11:44:00 Mr.
But unfortunately, the first sign of cardiovascular disease is often the last. Chest-Compression-Only Resuscitation Gordon A.
 THE UNIVERSITY OF ARIZONA Sarver Heart Center 1 THE UNIVERSITY OF ARIZONA Sarver Heart Center 2 But unfortunately, the first sign of cardiovascular disease is often the last 3 4 1 5 6 7 8 2 Risk of Cardiac
THE UNIVERSITY OF ARIZONA Sarver Heart Center 1 THE UNIVERSITY OF ARIZONA Sarver Heart Center 2 But unfortunately, the first sign of cardiovascular disease is often the last 3 4 1 5 6 7 8 2 Risk of Cardiac
Strengthening the Chain of Survival
 Impedance Threshold Device Strengthening the Chain of Survival 2007 Advanced Circulatory Systems, Inc. 1 49-0363-000, 01 Katie Talk NREMT-P AHA Faculty (NC) Clinical Educator for Advanced Circulatory Systems,
Impedance Threshold Device Strengthening the Chain of Survival 2007 Advanced Circulatory Systems, Inc. 1 49-0363-000, 01 Katie Talk NREMT-P AHA Faculty (NC) Clinical Educator for Advanced Circulatory Systems,
All under the division of cardiovascular medicine University of Minnesota
 The Team 1) Demetris Yannopoulos M.D. Medical Director, 2) Kim Harkins, Program Manager 3) Lucinda Klann, CARES Data Manager 4) Esther Almeida, Administrative Assistant All under the division of cardiovascular
The Team 1) Demetris Yannopoulos M.D. Medical Director, 2) Kim Harkins, Program Manager 3) Lucinda Klann, CARES Data Manager 4) Esther Almeida, Administrative Assistant All under the division of cardiovascular
Cardiac arrest Cardiac arrest (CA) occurs when the heart ceases to produce an effective pulse and circulate blood It includes four conditions:
 Basic Life Support: Cardiopulmonary Resuscitation (CPR). 2017 Lecture prepared by, Amer A. Hasanien RN, CNS, PhD Cardiac arrest Cardiac arrest (CA) occurs when the heart ceases to produce an effective
Basic Life Support: Cardiopulmonary Resuscitation (CPR). 2017 Lecture prepared by, Amer A. Hasanien RN, CNS, PhD Cardiac arrest Cardiac arrest (CA) occurs when the heart ceases to produce an effective
THE FOLLOWING QUESTIONS RELATE TO THE RESUSCITATION COUNCIL (UK) RESUSCITATION GUIDELINES 2005
 THE FOLLOWING QUESTIONS RELATE TO THE RESUSCITATION COUNCIL (UK) RESUSCITATION GUIDELINES 2005 1. The guidelines suggest that in out-of-hospital cardiac arrests, attended but unwitnessed by health care
THE FOLLOWING QUESTIONS RELATE TO THE RESUSCITATION COUNCIL (UK) RESUSCITATION GUIDELINES 2005 1. The guidelines suggest that in out-of-hospital cardiac arrests, attended but unwitnessed by health care
Cardiopulmonary Resuscitation in Adults
 Cardiopulmonary Resuscitation in Adults Fatma Özdemir, MD Emergency Deparment of Uludag University Faculty of Medicine OVERVIEW Introduction Pathophysiology BLS algorithm ALS algorithm Post resuscitation
Cardiopulmonary Resuscitation in Adults Fatma Özdemir, MD Emergency Deparment of Uludag University Faculty of Medicine OVERVIEW Introduction Pathophysiology BLS algorithm ALS algorithm Post resuscitation
Strengthening the Chain of Survival in Sudden Cardiac Arrest
 Strengthening the Chain of Survival in Sudden Cardiac Arrest Katie Talk CCEMTP AHA Faculty/ Instructor for ACLS, BLS & PALS Clinical Educator for ACSI Employed by Advanced Circulatory Systems, Inc. (manufacture
Strengthening the Chain of Survival in Sudden Cardiac Arrest Katie Talk CCEMTP AHA Faculty/ Instructor for ACLS, BLS & PALS Clinical Educator for ACSI Employed by Advanced Circulatory Systems, Inc. (manufacture
GETTING TO THE HEART OF THE MATTER. Ritu Sahni, MD, MPH Lake Oswego Fire Department Washington County EMS Clackamas County EMS
 GETTING TO THE HEART OF THE MATTER Ritu Sahni, MD, MPH Lake Oswego Fire Department Washington County EMS Clackamas County EMS TAKE HOME POINTS CPR is the most important thing Train like we fight Measure
GETTING TO THE HEART OF THE MATTER Ritu Sahni, MD, MPH Lake Oswego Fire Department Washington County EMS Clackamas County EMS TAKE HOME POINTS CPR is the most important thing Train like we fight Measure
Advanced Resuscitation - Adult
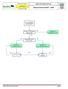 C02A Resuscitation 2017-03-23 17 years & older Office of the Medical Director Advanced Resuscitation - Adult Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia Algorithm
C02A Resuscitation 2017-03-23 17 years & older Office of the Medical Director Advanced Resuscitation - Adult Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia Algorithm
Johnson County Emergency Medical Services Page 23
 Non-resuscitation Situations: Resuscitation should not be initiated in the following situations: Prolonged arrest as evidenced by lividity in dependent parts, rigor mortis, tissue decomposition, or generalized
Non-resuscitation Situations: Resuscitation should not be initiated in the following situations: Prolonged arrest as evidenced by lividity in dependent parts, rigor mortis, tissue decomposition, or generalized
Keywords active compression decompression, cardiopulmonary resuscitation, inspiratory impedance threshold device
 Combination of active compression decompression cardiopulmonary resuscitation and the inspiratory impedance threshold device: state of the art Ralph J. Frascone, a,c Dawn Bitz c and Keith Lurie b,d Purpose
Combination of active compression decompression cardiopulmonary resuscitation and the inspiratory impedance threshold device: state of the art Ralph J. Frascone, a,c Dawn Bitz c and Keith Lurie b,d Purpose
ADVANCED LIFE SUPPORT
 ANSWERS IN ITALICS WITH REFERENCES 1. The guidelines suggest that in out-of-hospital cardiac arrests, attended but unwitnessed by health care professionals equipped with a manual defibrillator, the providers
ANSWERS IN ITALICS WITH REFERENCES 1. The guidelines suggest that in out-of-hospital cardiac arrests, attended but unwitnessed by health care professionals equipped with a manual defibrillator, the providers
Advanced Resuscitation - Child
 C02C Resuscitation 2017-03-23 1 up to 10 years Office of the Medical Director Advanced Resuscitation - Child Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia Algorithm
C02C Resuscitation 2017-03-23 1 up to 10 years Office of the Medical Director Advanced Resuscitation - Child Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia Algorithm
pat hways Medtech innovation briefing Published: 12 February 2015 nice.org.uk/guidance/mib18
 pat hways The AutoPulse non-invasive cardiac support pump for cardiopulmonary resuscitation Medtech innovation briefing Published: 12 February 2015 nice.org.uk/guidance/mib18 Summary The AutoPulse is a
pat hways The AutoPulse non-invasive cardiac support pump for cardiopulmonary resuscitation Medtech innovation briefing Published: 12 February 2015 nice.org.uk/guidance/mib18 Summary The AutoPulse is a
SYSTEMS BASED APPROACH TO OUT-OF-HOSPITAL CARDIAC ARREST
 SYSTEMS BASED APPROACH TO OUT-OF-HOSPITAL CARDIAC ARREST Kenneth A Scheppke, MD Chief Medical Officer Palm Beach County Fire Rescue State EMS Medical Director Florida Department of Health CASE REPORT 42
SYSTEMS BASED APPROACH TO OUT-OF-HOSPITAL CARDIAC ARREST Kenneth A Scheppke, MD Chief Medical Officer Palm Beach County Fire Rescue State EMS Medical Director Florida Department of Health CASE REPORT 42
Advanced Cardiac Life Support (ACLS) Science Update 2015
 1 2 3 4 5 6 7 8 9 Advanced Cardiac Life Support (ACLS) Science Update 2015 What s New in ACLS for 2015? Adult CPR CPR remains (Compressions, Airway, Breathing Chest compressions has priority over all other
1 2 3 4 5 6 7 8 9 Advanced Cardiac Life Support (ACLS) Science Update 2015 What s New in ACLS for 2015? Adult CPR CPR remains (Compressions, Airway, Breathing Chest compressions has priority over all other
Hanna K. Al-Makhamreh, M.D., FACC Interventional Cardiologist
 Hanna K. Al-Makhamreh, M.D., FACC Interventional Cardiologist Introduction. Basic Life Support (BLS). Advanced Cardiac Life Support (ACLS). Cardiovascular diseases (CVDs) are the number one cause of death
Hanna K. Al-Makhamreh, M.D., FACC Interventional Cardiologist Introduction. Basic Life Support (BLS). Advanced Cardiac Life Support (ACLS). Cardiovascular diseases (CVDs) are the number one cause of death
available at journal homepage:
 Resuscitation (2008) 78, 179 185 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/resuscitation CLINICAL PAPER Resuscitation Outcomes Consortium (ROC) PRIMED cardiac arrest
Resuscitation (2008) 78, 179 185 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/resuscitation CLINICAL PAPER Resuscitation Outcomes Consortium (ROC) PRIMED cardiac arrest
The ARREST Trial: Amiodarone for Resuscitation After Out-of-Hospital Cardiac Arrest Due to Ventricular Fibrillation
 The ARREST Trial: Amiodarone for Resuscitation After Out-of-Hospital Cardiac Arrest Due to Ventricular Fibrillation Introduction The ARREST (Amiodarone in out-of-hospital Resuscitation of REfractory Sustained
The ARREST Trial: Amiodarone for Resuscitation After Out-of-Hospital Cardiac Arrest Due to Ventricular Fibrillation Introduction The ARREST (Amiodarone in out-of-hospital Resuscitation of REfractory Sustained
Key statistics from the National Cardiac Arrest Audit: Paediatric arrests April 2012 to March 2017
 Key statistics from the National Cardiac Arrest Audit: Paediatric arrests April 12 to March 17 Supported by Resuscitation Council (UK) and Intensive Care National Audit & Research Centre (ICNARC) Data
Key statistics from the National Cardiac Arrest Audit: Paediatric arrests April 12 to March 17 Supported by Resuscitation Council (UK) and Intensive Care National Audit & Research Centre (ICNARC) Data
Portage County EMS Annual Skills Labs
 Portage County EMS Annual Skills Labs Scope: Provide skills labs for all Emergency Medical Responders and First Response EMTs to assure proficiency of skills and satisfy the Wisconsin State approved Operational
Portage County EMS Annual Skills Labs Scope: Provide skills labs for all Emergency Medical Responders and First Response EMTs to assure proficiency of skills and satisfy the Wisconsin State approved Operational
Traumatic Cardiac Arrest Protocol
 Traumatic Cardiac Arrest Protocol Background: Major Trauma continues to be the leading worldwide cause of death in young adults. Mortality remains high but there are reports of good neurological outcomes
Traumatic Cardiac Arrest Protocol Background: Major Trauma continues to be the leading worldwide cause of death in young adults. Mortality remains high but there are reports of good neurological outcomes
CENTRAL CALIFORNIA EMERGENCY MEDICAL SERVICES A Division of the Fresno County Department of Public Health
 CENTRAL CALIFORNIA EMERGENCY MEDICAL SERVICES A Division of the Fresno County Department of Public Health Manual Subject Emergency Medical Services Administrative Policies and Procedures Table of Contents
CENTRAL CALIFORNIA EMERGENCY MEDICAL SERVICES A Division of the Fresno County Department of Public Health Manual Subject Emergency Medical Services Administrative Policies and Procedures Table of Contents
Advanced Resuscitation - Adolescent
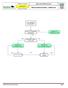 C02B Resuscitation 2017-03-23 10 up to 17 years Office of the Medical Director Advanced Resuscitation - Adolescent Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia
C02B Resuscitation 2017-03-23 10 up to 17 years Office of the Medical Director Advanced Resuscitation - Adolescent Intermediate Advanced Critical From PRIMARY ASSESSMENT Known or suspected hypothermia
Overview and Latest Research on Out of Hospital Cardiac Arrest
 L MODULE 1 Overview and Latest Research on Out of Hospital Cardiac Arrest Jamie Jollis, MD Co PI RACE CARS 2 Out of Hospital Cardiac Arrest in U.S. 236 000 to 325 000 people in the United States each year
L MODULE 1 Overview and Latest Research on Out of Hospital Cardiac Arrest Jamie Jollis, MD Co PI RACE CARS 2 Out of Hospital Cardiac Arrest in U.S. 236 000 to 325 000 people in the United States each year
In-hospital Resuscitation
 In-hospital Resuscitation Introduction This new section in the guidelines describes the sequence of actions for starting in-hospital resuscitation. Hospital staff are often trained in basic life support
In-hospital Resuscitation Introduction This new section in the guidelines describes the sequence of actions for starting in-hospital resuscitation. Hospital staff are often trained in basic life support
Emergency Cardiac Care Guidelines 2015
 Emergency Cardiac Care Guidelines 2015 VACEP 2016 William Brady, MD University of Virginia Guidelines 2015 Basic Life Support & Advanced Cardiac Life Support Acute Coronary Syndrome Pediatric Advanced
Emergency Cardiac Care Guidelines 2015 VACEP 2016 William Brady, MD University of Virginia Guidelines 2015 Basic Life Support & Advanced Cardiac Life Support Acute Coronary Syndrome Pediatric Advanced
Manual Defibrillation. CPR AGE: 18 years LOA: Altered HR: N/A RR: N/A SBP: N/A Other: N/A
 ROC AMIODARONE, LIDOCAINE OR PLACEBO FOR OUT OF HOSPITAL CARDIAC ARREST DUE TO VENTRICULAR FIBRILLATION OR TACHYCARDIA (ALPS) STUDY: MEDICAL CARDIAC ARREST MEDICAL DIRECTIVE An Advanced Care Paramedic
ROC AMIODARONE, LIDOCAINE OR PLACEBO FOR OUT OF HOSPITAL CARDIAC ARREST DUE TO VENTRICULAR FIBRILLATION OR TACHYCARDIA (ALPS) STUDY: MEDICAL CARDIAC ARREST MEDICAL DIRECTIVE An Advanced Care Paramedic
Capnography Connections Guide
 Capnography Connections Guide Patient Monitoring Contents I Section 1: Capnography Introduction...1 I Section 2: Capnography & PCA...3 I Section 3: Capnography & Critical Care...7 I Section 4: Capnography
Capnography Connections Guide Patient Monitoring Contents I Section 1: Capnography Introduction...1 I Section 2: Capnography & PCA...3 I Section 3: Capnography & Critical Care...7 I Section 4: Capnography
Developments in Cardiopulmonary Resuscitation Guidelines
 Developments in Cardiopulmonary Resuscitation Guidelines Bernd W. Böttiger Seite 1 To preserve human life by making high quality resuscitation available to all Outcome after CPR in Germany ROSC ( Return
Developments in Cardiopulmonary Resuscitation Guidelines Bernd W. Böttiger Seite 1 To preserve human life by making high quality resuscitation available to all Outcome after CPR in Germany ROSC ( Return
2016 Top Papers in Critical Care
 2016 Top Papers in Critical Care Briana Witherspoon DNP, APRN, ACNP-BC Assistant Director of Advanced Practice, Neuroscience Assistant in Division of Critical Care, Department of Anesthesiology Neuroscience
2016 Top Papers in Critical Care Briana Witherspoon DNP, APRN, ACNP-BC Assistant Director of Advanced Practice, Neuroscience Assistant in Division of Critical Care, Department of Anesthesiology Neuroscience
Cardiac Arrest Registry Database Office of the Medical Director
 Cardiac Arrest Registry Database 2010 Office of the Medical Director 1 Monthly Statistical Summary Cardiac Arrest, September 2010 Western Western Description Division Division % Totals Eastern Division
Cardiac Arrest Registry Database 2010 Office of the Medical Director 1 Monthly Statistical Summary Cardiac Arrest, September 2010 Western Western Description Division Division % Totals Eastern Division
Cardiac Arrest Registry Database Office of the Medical Director
 Cardiac Arrest Registry Database 2010 Office of the Medical Director 1 Monthly Statistical Summary Cardiac Arrest, December 2010 Western Western Description Division Division % Totals Eastern Division
Cardiac Arrest Registry Database 2010 Office of the Medical Director 1 Monthly Statistical Summary Cardiac Arrest, December 2010 Western Western Description Division Division % Totals Eastern Division
Science Behind CPR Update from Darrell Nelson, MD, FACEP Emergency Medicine Wake Forest University Health Sciences
 Science Behind CPR Update from 2010 Darrell Nelson, MD, FACEP Emergency Medicine Wake Forest University Health Sciences FRAMING THE DISCUSSION NO ONE SURVIVES CARDIAC ARREST, EXCEPT ON TV Conflicts of
Science Behind CPR Update from 2010 Darrell Nelson, MD, FACEP Emergency Medicine Wake Forest University Health Sciences FRAMING THE DISCUSSION NO ONE SURVIVES CARDIAC ARREST, EXCEPT ON TV Conflicts of
Capnography (ILS/ALS)
 Capnography (ILS/ALS) Clinical Indications: 1. Capnography shall be used as soon as possible in conjunction with any airway management adjunct, including endotracheal, Blind Insertion Airway Devices (BIAD)
Capnography (ILS/ALS) Clinical Indications: 1. Capnography shall be used as soon as possible in conjunction with any airway management adjunct, including endotracheal, Blind Insertion Airway Devices (BIAD)
Mechanical devices for cardiopulmonary resuscitation Jane G. Wigginton, Adam H. Miller, Fernando L. Benitez and Paul E. Pepe
 Mechanical devices for cardiopulmonary resuscitation Jane G. Wigginton, Adam H. Miller, Fernando L. Benitez and Paul E. Pepe Purpose of the review For over 40 years, manual chest compressions have been
Mechanical devices for cardiopulmonary resuscitation Jane G. Wigginton, Adam H. Miller, Fernando L. Benitez and Paul E. Pepe Purpose of the review For over 40 years, manual chest compressions have been
1 Chapter 40 Advanced Airway Management 2 Advanced Airway Management The advanced airway management techniques discussed in this chapter are to
 1 Chapter 40 Advanced Airway Management 2 Advanced Airway Management The advanced airway management techniques discussed in this chapter are to introduce the EMT-B student to these procedures only. In
1 Chapter 40 Advanced Airway Management 2 Advanced Airway Management The advanced airway management techniques discussed in this chapter are to introduce the EMT-B student to these procedures only. In
A SYNOPSIS BY ILCOR PEDIATRIC TASK FORCE. Pediatric Basic Life Support, Pediatric Advanced Life Support and Neonatal Resuscitation 2015
 Vol. 2 - No.4 October - December 2015 83 Vol. 2 - No.4 October - December 2015 84 There is new evidence that when treating pediatric septic shock in specific settings, the use of restricted volume of isotonic
Vol. 2 - No.4 October - December 2015 83 Vol. 2 - No.4 October - December 2015 84 There is new evidence that when treating pediatric septic shock in specific settings, the use of restricted volume of isotonic
MICHIGAN. State Protocols. Pediatric Cardiac Table of Contents 6.1 General Pediatric Cardiac Arrest 6.2 Bradycardia 6.
 MICHIGAN State Protocols Protocol Number Protocol Name Pediatric Cardiac Table of Contents 6.1 General Pediatric Cardiac Arrest 6.2 Bradycardia 6.3 Tachycardia PEDIATRIC CARDIAC PEDIATRIC CARDIAC ARREST
MICHIGAN State Protocols Protocol Number Protocol Name Pediatric Cardiac Table of Contents 6.1 General Pediatric Cardiac Arrest 6.2 Bradycardia 6.3 Tachycardia PEDIATRIC CARDIAC PEDIATRIC CARDIAC ARREST
Chapter 40 Advanced Airway Management
 1 2 3 4 5 Chapter 40 Advanced Airway Management Advanced Airway Management The advanced airway management techniques discussed in this chapter are to introduce the EMT-B student to these procedures only.
1 2 3 4 5 Chapter 40 Advanced Airway Management Advanced Airway Management The advanced airway management techniques discussed in this chapter are to introduce the EMT-B student to these procedures only.
The 2015 BLS & ACLS Guideline Updates What Does the Future Hold?
 The 2015 BLS & ACLS Guideline Updates What Does the Future Hold? Greater Kansas City Chapter Of AACN 2016 Visions Critical Care Conference Nicole Kupchik RN, MN, CCNS, CCRN, PCCN, CMC Independent CNS/Staff
The 2015 BLS & ACLS Guideline Updates What Does the Future Hold? Greater Kansas City Chapter Of AACN 2016 Visions Critical Care Conference Nicole Kupchik RN, MN, CCNS, CCRN, PCCN, CMC Independent CNS/Staff
Don t let your patients turn blue! Isn t it about time you used etco 2?
 Don t let your patients turn blue! Isn t it about time you used etco 2? American Association of Critical Care Nurses National Teaching Institute Expo Ed 2013 Susan Thibeault MS, CRNA, APRN, CCRN, EMT-P
Don t let your patients turn blue! Isn t it about time you used etco 2? American Association of Critical Care Nurses National Teaching Institute Expo Ed 2013 Susan Thibeault MS, CRNA, APRN, CCRN, EMT-P
Department of Paediatrics Clinical Guideline. Advanced Paediatric Life Support. Sequence of actions. 1. Establish basic life support
 Advanced Paediatric Life Support Sequence of actions 1. Establish basic life support 2. Oxygenate, ventilate, and start chest compression: - Provide positive-pressure ventilation with high-concentration
Advanced Paediatric Life Support Sequence of actions 1. Establish basic life support 2. Oxygenate, ventilate, and start chest compression: - Provide positive-pressure ventilation with high-concentration
Out-of-hospital Cardiac Arrest. Franz R. Eberli MD, FESC, FAHA Cardiology Triemli Hospital Zurich, Switzerland
 Out-of-hospital Cardiac Arrest Franz R. Eberli MD, FESC, FAHA Cardiology Triemli Hospital Zurich, Switzerland Conflict of Interest I have no conflict of interest to disclose regarding this presentation.
Out-of-hospital Cardiac Arrest Franz R. Eberli MD, FESC, FAHA Cardiology Triemli Hospital Zurich, Switzerland Conflict of Interest I have no conflict of interest to disclose regarding this presentation.
Consider Treatable Underlying Causes Early
 Page 1 of 8 Cardiac Arrest Timeout Checklist Assign roles for Pit Crew CPR o Compressors x 2 o Airway o Lead responsible for coordinating team, making decisions o Medications Continuous compressions at
Page 1 of 8 Cardiac Arrest Timeout Checklist Assign roles for Pit Crew CPR o Compressors x 2 o Airway o Lead responsible for coordinating team, making decisions o Medications Continuous compressions at
CPR What Works, What Doesn t
 Resuscitation 2017 ECMO and ECLS April 1, 2017 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN Circulation 2013;128:417-35
Resuscitation 2017 ECMO and ECLS April 1, 2017 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN Circulation 2013;128:417-35
Out-of-hospital cardiac arrest: incidence, process of care, and outcomes in an urban city, Korea
 Clin Exp Emerg Med 2014;1(2):94-100 http://dx.doi.org/10.15441/ceem.14.021 Out-of-hospital cardiac arrest: incidence, process of care, and outcomes in an urban city, Korea Hanjin Cho 1, Sungwoo Moon 1,
Clin Exp Emerg Med 2014;1(2):94-100 http://dx.doi.org/10.15441/ceem.14.021 Out-of-hospital cardiac arrest: incidence, process of care, and outcomes in an urban city, Korea Hanjin Cho 1, Sungwoo Moon 1,
Adult Advanced Cardiovascular Life Support. Emergency Procedures in PT
 Adult Advanced Cardiovascular Life Support Emergency Procedures in PT BLS Can be learned & practiced by the general public Includes: CPR First Aid (e.g. choking relief) Use of AED ACLS Used by healthcare
Adult Advanced Cardiovascular Life Support Emergency Procedures in PT BLS Can be learned & practiced by the general public Includes: CPR First Aid (e.g. choking relief) Use of AED ACLS Used by healthcare
Outcomes of Therapeutic Hypothermia in Cardiac Arrest. Saad Mohammed Shariff, MBBS Aravind Herle, MD, FACC
 Outcomes of Therapeutic Hypothermia in Cardiac Arrest Saad Mohammed Shariff, MBBS Aravind Herle, MD, FACC https://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_427331.pdf
Outcomes of Therapeutic Hypothermia in Cardiac Arrest Saad Mohammed Shariff, MBBS Aravind Herle, MD, FACC https://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_427331.pdf
San Benito County EMS Agency Section 700: Patient Care Procedures
 Purpose: To outline the steps EMTs & paramedics will take to manage possible life threats in any child or adult patient they encounter. This policy is in effect for all treatment protocols & is to be referred
Purpose: To outline the steps EMTs & paramedics will take to manage possible life threats in any child or adult patient they encounter. This policy is in effect for all treatment protocols & is to be referred
Over the last 3 decades, advances in the understanding of
 Temporal Trends in Sudden Cardiac Arrest A 25-Year Emergency Medical Services Perspective Thomas D. Rea, MD, MPH; Mickey S. Eisenberg, MD, PhD; Linda J. Becker, MA; John A. Murray, MD; Thomas Hearne, PhD
Temporal Trends in Sudden Cardiac Arrest A 25-Year Emergency Medical Services Perspective Thomas D. Rea, MD, MPH; Mickey S. Eisenberg, MD, PhD; Linda J. Becker, MA; John A. Murray, MD; Thomas Hearne, PhD
Sudden cardiac arrest remains a leading cause of prehospital
 The Physiology of Cardiopulmonary Resuscitation Keith G. Lurie, MD,* Edward C. Nemergut, MD, Demetris Yannopoulos, MD, and Michael Sweeney, MD E Review Article Outcomes after cardiac arrest remain poor
The Physiology of Cardiopulmonary Resuscitation Keith G. Lurie, MD,* Edward C. Nemergut, MD, Demetris Yannopoulos, MD, and Michael Sweeney, MD E Review Article Outcomes after cardiac arrest remain poor
Neurologic Recovery Following Prolonged Out-of-Hospital Cardiac Arrest With Resuscitation Guided by Continuous Capnography
 CASE REPORT FULL RECOVERY AFTER PROLONGED CARDIAC ARREST AND RESUSCITATION WITH CAPNOGRAPHY GUIDANCE Neurologic Recovery Following Prolonged Out-of-Hospital Cardiac Arrest With Resuscitation Guided by
CASE REPORT FULL RECOVERY AFTER PROLONGED CARDIAC ARREST AND RESUSCITATION WITH CAPNOGRAPHY GUIDANCE Neurologic Recovery Following Prolonged Out-of-Hospital Cardiac Arrest With Resuscitation Guided by
Sooner to the Ballooner: Going Straight to the Cath Lab with Refractory VF/VT
 Sooner to the Ballooner: Going Straight to the Cath Lab with Refractory VF/VT Marc Conterato, MD, FACEP Office of the Medical Director NMAS and the HC EMS Council/Minnesota Resuscitation Consortium DISCLOSURE
Sooner to the Ballooner: Going Straight to the Cath Lab with Refractory VF/VT Marc Conterato, MD, FACEP Office of the Medical Director NMAS and the HC EMS Council/Minnesota Resuscitation Consortium DISCLOSURE
ECLS: A new frontier for refractory V.Fib and pulseless VT
 ECLS: A new frontier for refractory V.Fib and pulseless VT Ernest L. Mazzaferri, Jr. MD, FACC September 15, 2017 Cardiovascular Emergencies: An exploration into the expansion of time-critical diagnosis
ECLS: A new frontier for refractory V.Fib and pulseless VT Ernest L. Mazzaferri, Jr. MD, FACC September 15, 2017 Cardiovascular Emergencies: An exploration into the expansion of time-critical diagnosis
Management of Cardiac Arrest Based on : 2010 American Heart Association Guidelines
 Management of Cardiac Arrest Based on : 2010 American Heart Association Guidelines www.circ.ahajournals.org Elham Pishbin. M.D Assistant Professor of Emergency Medicine MUMS C H E S Advanced Life Support
Management of Cardiac Arrest Based on : 2010 American Heart Association Guidelines www.circ.ahajournals.org Elham Pishbin. M.D Assistant Professor of Emergency Medicine MUMS C H E S Advanced Life Support
The evidence behind ACLS: the importance of good BLS
 The evidence behind ACLS: the importance of good BLS Benjamin S. Abella, MD, MPhil, FACEP CRS Center for Resuscitation Science Clinical Research Director Center for Resuscitation Science Vice Chair of
The evidence behind ACLS: the importance of good BLS Benjamin S. Abella, MD, MPhil, FACEP CRS Center for Resuscitation Science Clinical Research Director Center for Resuscitation Science Vice Chair of
The ALS Algorithm and Post Resuscitation Care
 The ALS Algorithm and Post Resuscitation Care CET - Ballarat Health Services Valid from 1 st July 2018 to 30 th June 2020 2 Defibrillation Produces simultaneous mass depolarisation of myocardial cells
The ALS Algorithm and Post Resuscitation Care CET - Ballarat Health Services Valid from 1 st July 2018 to 30 th June 2020 2 Defibrillation Produces simultaneous mass depolarisation of myocardial cells
5 Key EMS Articles for 2012
 5 Key EMS Articles for 2012 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN 5 Key Topics Cardiac Arrest Trauma
5 Key EMS Articles for 2012 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN 5 Key Topics Cardiac Arrest Trauma
Resuscitation in infants and children
 Resuscitation in infants and children The importance of respiratory support Dr. Simon Erickson Paediatric Intensive Care Princess Margaret Hospital for Children Paediatric cardiac arrests uncommon (~20/100,000)
Resuscitation in infants and children The importance of respiratory support Dr. Simon Erickson Paediatric Intensive Care Princess Margaret Hospital for Children Paediatric cardiac arrests uncommon (~20/100,000)
PROBLEM: Shock refractory VF/pVT BACKGROUND: Both in 2015 CoSTR. Amiodarone favoured.
 Question Should AMIODARONE vs LIDOCAINE be used for adults with shock refractory VF/pVT PROBLEM: Shock refractory VF/pVT BACKGROUND: Both in 2015 CoSTR. Amiodarone favoured. OPTION: AMIODARONE plus standard
Question Should AMIODARONE vs LIDOCAINE be used for adults with shock refractory VF/pVT PROBLEM: Shock refractory VF/pVT BACKGROUND: Both in 2015 CoSTR. Amiodarone favoured. OPTION: AMIODARONE plus standard
18% Survival from In-Hospital Cardiac Arrest Ways we can do better! National Teaching Institute Denver, CO Class Code: 149 A
 18% Survival from In-Hospital Cardiac Arrest Ways we can do better! National Teaching Institute Denver, CO Class Code: 149 A Nicole Kupchik RN, MN, CCNS, CCRN, PCCN Independent CNS/Staff Nurse Objectives
18% Survival from In-Hospital Cardiac Arrest Ways we can do better! National Teaching Institute Denver, CO Class Code: 149 A Nicole Kupchik RN, MN, CCNS, CCRN, PCCN Independent CNS/Staff Nurse Objectives
CARDIAC ARREST RESEARCH STUDIES. Frequently Asked Questions. Why are these studies being conducted in Milwaukee County?
 CARDIAC ARREST RESEARCH STUDIES Frequently Asked Questions Why are these studies being conducted in Milwaukee County? For the past 20-30 years, Milwaukee County Emergency Medical Services (EMS) System
CARDIAC ARREST RESEARCH STUDIES Frequently Asked Questions Why are these studies being conducted in Milwaukee County? For the past 20-30 years, Milwaukee County Emergency Medical Services (EMS) System
Update on Sudden Cardiac Death and Resuscitation
 Update on Sudden Cardiac Death and Resuscitation Ashish R. Panchal, MD, PhD Medical Director Center for Emergency Medical Services Assistant Professor Clinical Department of Emergency Medicine The Ohio
Update on Sudden Cardiac Death and Resuscitation Ashish R. Panchal, MD, PhD Medical Director Center for Emergency Medical Services Assistant Professor Clinical Department of Emergency Medicine The Ohio
New and Future Trends in EMS. Ron Brown, MD, FACEP Paramedic Lecture Series 2018
 New and Future Trends in EMS Ron Brown, MD, FACEP Paramedic Lecture Series 2018 New technologies and protocols DSD Mechanical Compression ITD BiPAP Ultrasound Double Sequential Defibrillation Two defibrillators
New and Future Trends in EMS Ron Brown, MD, FACEP Paramedic Lecture Series 2018 New technologies and protocols DSD Mechanical Compression ITD BiPAP Ultrasound Double Sequential Defibrillation Two defibrillators
Wake County EMS System Peer Review/Clinical Data/System Performance
 P a g e 1 Wake County EMS System Peer Review/Clinical Data/System Performance Explanations and Definitions for Reports Wake County EMS engages in regular external review. The System makes quarterly reports
P a g e 1 Wake County EMS System Peer Review/Clinical Data/System Performance Explanations and Definitions for Reports Wake County EMS engages in regular external review. The System makes quarterly reports
Tissue Plasminogen Activator in In-Hospital Cardiac Arrest with Pulseless Electrical Activity
 Tissue Plasminogen Activator in In-Hospital Cardiac Arrest with Pulseless Electrical Activity Hannah Jordan A. Study Purpose and Rationale Pulseless electrical activity during cardiac arrest carries a
Tissue Plasminogen Activator in In-Hospital Cardiac Arrest with Pulseless Electrical Activity Hannah Jordan A. Study Purpose and Rationale Pulseless electrical activity during cardiac arrest carries a
Australian Resuscitation Outcomes Consortium (Aus-ROC)
 Australian Resuscitation Outcomes Consortium (Aus-ROC) A NHMRC Centre of Research Excellence (CRE) in Clinical Research, #1029983 Out-of-hospital cardiac arrest registry ( Epistry ) Presented by Prof Judith
Australian Resuscitation Outcomes Consortium (Aus-ROC) A NHMRC Centre of Research Excellence (CRE) in Clinical Research, #1029983 Out-of-hospital cardiac arrest registry ( Epistry ) Presented by Prof Judith
WORKSHEET for Evidence-Based Review of Science for Veterinary CPCR
 RECOVER 2011 1 of 7 WORKSHEET for Evidence-Based Review of Science for Veterinary CPCR 1. Basic Demographics Worksheet author(s) Kate Hopper Mailing address: Dept Vet Surgical & Radiological Sciences Room
RECOVER 2011 1 of 7 WORKSHEET for Evidence-Based Review of Science for Veterinary CPCR 1. Basic Demographics Worksheet author(s) Kate Hopper Mailing address: Dept Vet Surgical & Radiological Sciences Room
What works? What doesn t? What s new? Terry M. Foster, RN
 What works? What doesn t? What s new? Terry M. Foster, RN 2016 Changes Updated every 5 years Last update was 2010 All recommendations have been heavily researched with studies involving large number of
What works? What doesn t? What s new? Terry M. Foster, RN 2016 Changes Updated every 5 years Last update was 2010 All recommendations have been heavily researched with studies involving large number of
HeartCode PALS. PALS Actions Overview > Legend. Contents
 HeartCode PALS PALS Actions Overview > Legend Action buttons (round buttons) Clicking a round button initiates an action. Clicking this button, for example, checks the child s carotid pulse. Menu buttons
HeartCode PALS PALS Actions Overview > Legend Action buttons (round buttons) Clicking a round button initiates an action. Clicking this button, for example, checks the child s carotid pulse. Menu buttons
System Utilization 2013
 System Utilization 2013 8000 7000 Contra Costa County 911 Ambulance Response and Transport By Month 2012 Through 2013 (2013 Data excludes SRVFD) Response 6000 5000 Transport 4000 3000 2000 1000 0 1/1/2012
System Utilization 2013 8000 7000 Contra Costa County 911 Ambulance Response and Transport By Month 2012 Through 2013 (2013 Data excludes SRVFD) Response 6000 5000 Transport 4000 3000 2000 1000 0 1/1/2012
ICEMA Protocol Updates. October 15, 2016
 ICEMA Protocol Updates October 15, 2016 ICEMA disclosure The following protocol updates include changes in ICEMA s paramedic (EMT P) optional scope of practice. Medical Advisory Committee (MAC) representatives,
ICEMA Protocol Updates October 15, 2016 ICEMA disclosure The following protocol updates include changes in ICEMA s paramedic (EMT P) optional scope of practice. Medical Advisory Committee (MAC) representatives,
Epinephrine Cardiovascular Emergencies Symposium 2018
 Epinephrine Cardiovascular Emergencies Symposium 218 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN High Quality
Epinephrine Cardiovascular Emergencies Symposium 218 Corey M. Slovis, M.D. Vanderbilt University Medical Center Metro Nashville Fire Department Nashville International Airport Nashville, TN High Quality
Advanced Life Support
 Standard Operating Procedure 2.1 Advanced Life Support Position Responsible: Head of Operations CGC Approved: October 2017 Related Documents Further Information 1.0 Background Magpas Resuscitation Policy
Standard Operating Procedure 2.1 Advanced Life Support Position Responsible: Head of Operations CGC Approved: October 2017 Related Documents Further Information 1.0 Background Magpas Resuscitation Policy
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes
A mong patients who have an out-of-hospital cardiac
 1114 CARDIOVASCULAR MEDICINE Can we define patients with no chance of survival after outof-hospital cardiac arrest? J Herlitz, J Engdahl, L Svensson, M Young, K-A Ängquist, S Holmberg... See end of article
1114 CARDIOVASCULAR MEDICINE Can we define patients with no chance of survival after outof-hospital cardiac arrest? J Herlitz, J Engdahl, L Svensson, M Young, K-A Ängquist, S Holmberg... See end of article
Remote management of heart failure using implanted devices and formalized follow-up procedures (REM-HF)
 Remote management of heart failure using implanted devices and formalized follow-up procedures (REM-HF) Martin R Cowie Professor of Cardiology, Imperial College London (Royal Brompton Hospital) London,
Remote management of heart failure using implanted devices and formalized follow-up procedures (REM-HF) Martin R Cowie Professor of Cardiology, Imperial College London (Royal Brompton Hospital) London,
