Project organisation: Flowchart... 5 Background: Design: Methodology: Ethical aspects of the study:... 15
|
|
|
- MargaretMargaret Sheena Jennings
- 5 years ago
- Views:
Transcription
1 Protocol version Protocol version 17 with amendments marked in yellow updated Protocol version 18 further updated before analysis of data, and changes are marked in grey. EASY Early closure of temporary ileostomy - A randomized controlled trial Early versus late closure of temporary ileostomy after rectal resection due to rectal cancer 1
2 Project organisation:... 3 Participating centers:... 4 Flowchart... 5 Background:... 5 The purpose of the study:... 7 Hypotheses:... 8 Endpoints:... 8 Design:... 9 Sample size Methodology: Ethical aspects of the study: Risks and side effects of the study: Scientific approval: Approval of data security: Clinical Trials: Financial support: Remuneration for participating patients: Guidelines for collection of informed consent: Timeline for the project References
3 Project organisation: Steering group: Jacob Rosenberg, Professor, MD, Eva Haglind, Professor, MD, Anne Kjærgaard Danielsen, PhD, Eva Angenete, MD. Project secretariate: Situated at Sahlgrenska Universitety Hospital/Östra, SE Göteborg, Sweden Local investigators: Denmark: Herlev Hospital: Anne Kjærgaard Danielsen, PhD Sweden: Göteborg: Adiela Correra, PhD student A local investigator will be appointed to be responsible at each participating hospital. Publication strategy: Both positive and negative results will be published in internationally recognized journals. If publication in that type of journal is not possible the results will be published on the home page of the research group. Co-authors according to criteria from the Vancouver group ( Data reporting Data will be reported locally in Case Report Forms (CRF), which accurately contain the relevant details necessary to evaluate the questions of the study (CRF attached as appendix 8) The original CRF s will be archived locally in accordance with current regulations. 3
4 Randomisation Patients will be randomized in blocks of 6 from a computer generated list and the necessary number of numbered envelopes will be sent out to participating departments together with CRF for data reporting. Participating centers: The study takes place within the framework of the Scandinavian Surgical Outcomes Research Group (SSORG) and it is expected that the Swedish and Danish surgical departments that are members of the network will participate. There is also the possibility that other surgical departments in Denmark or Sweden may participate. Denmark: Herlev Hospital, University of Copenhagen Nordsjællands Hospital, University of Copenhagen Sverige: Sahlgrenska University Hospital NÄL, Norra Älvsborgs Sjukhus Skaraborgs Sjukhus, Skövde 4
5 Patients with temporary ileostomy after surgery for rectal cancer Flowchart CT-scan 6-8 days after stoma construction Inclusion Randomisation Control group Intervention group Investigation of complications before discharge 3 months (+/- 2 weeks) after stoma construction Investigation of complications Quality of Life assessment (SF-36, OAS + EORTC QLQ- CR30/29 ) Closure of ileostomy 8-13 days after stoma construction Investigation of complications before discharge 3 months (+/- 2 weeks) after stoma construction Investigation of complications Quality of Life assessment (SF-36 + EORTC QLQ- CR30/29) Closure of ileostomy min. 12 weeks after stoma construction 6 months (+/- 2 weeks) after stoma construction Investigation of complications Quality of Life assessnent (SF-36, OAS + EORTC QLQ- CR30/29 ) 6 months (+/- 2 weeks) after stoma construction Investigation of complications Quality of Life assessment (SF-36 + EORTC QLQ- CR30/29) 12 months (+/- 2 weeks) after stoma construction Investigation of complications Quality of Life assessment (SF-36, OAS + EORTC QLQ- CR30/29 ) 12 months (+/- 2 weeks) after stoma construction Investigation of complications Quality of Life assessment (SF-36 + EORTC QLQ- CR30/29 ) Cost calculation Cost calculation 5
6 Background: To have a stoma is not one single condition, but is defined by the fact that there are many types of stoma, many underlying reasons for the stoma and many individual psycho-social factors, which naturally affect people differently (1-4). Patients have to adapt to a different body image (how one views one s body), changed daily routines and for some it will also result in changes to life style, social activity (5) and affect sexuality (6). At the same time the construction of a stoma is also a treatment which eliminates illness, ameliorates pain and prepares for wellness (7), in which case stoma construction can also have a positive meaning (8). The importance of the stoma construction can also be pushed into the background due to other associated processes, e.g. parenteral nutrition (9), complications (10), fecal incontinence (11) and cancer (12). Many factors influence even the simplest adaption to life with a stoma, such as age (13), socio-economic status (14) and gender (15). The stoma construction itself can be associated with complications and can be of a type that in the long term will affects quality of life (10, 16-18). Patients who are admitted for surgical treatment of rectal cancer are in some cases offered a low anastomosis with simultaneous construction of temporary ileostomy for relief of rectal anastomosis. The temporary ileostomy prevents complications in the form of anastomosis leakage requring surgery, but on the other hand, not anastomosis leakage in general (19). Several preliminary studies, however, indicate that the frequency of complications can be reduced for selected patients by closing a temporary stoma within two weeks (20). There is usually low mortality in connection with the closure of temporary ileostomies, regardless of the timing of the closure (20). Though one retrospective study has a noticeably high mortality of 1% and 5.3 % for respectively colostomy and temporary ileostomy (21) where the deaths were connected with anastomosis leakage, sepsis, acute myocardial infarction and one death for unknown reasons. Stoma construction can result in complications which require re-operation and one study concluded that there is a great variation from no cases up to 14%-17%, where only the presence of inflammatory bowel disease is a particular risk factor (20). One prospective study showed that earlier closure of stoma (11 days rather than 2-3 months) was not associated with increased morbidity or mortality (22). A retrospective study including 308 patients who received a temporary loop ileostomy and subsequently underwent 6
7 closure found a positive correlation between creation of a loop ileostomy and a reduced renal function (23). A smaller randomized study examined the importance of early closure (9 days after operation) of ileostomy in 36 patients selected pre-operatively. This shows that hospitalization (overall) was significantly shorter and that bowel function and resumption of oral nutrition was not a problem and concludes that with the study s controlled design in mind there was no increased risk associated with early closure of the ileostomy (24). A randomized and controlled study of 190 patients where the intervention group had their stoma closed after 8 days against the control group, whose stoma was closed after 60 days, showed no significant difference in the number of complications though there was an increase in the time spent in hospital for the group with later closure. There were significant differences in the type of complications in that the incidence of wound complications in the first group was greater, but in the second group there were more patients with obstruction of the small intestine and medical complications (25). Quality of Life assessments are increasingly required in the health sector (26;27), and it is common to measure the effects of interventions on patients quality of life (29). Therefore it seems obvious to examine the impact on patients when a temporary ileostomy is closed and a survey of 76 patients with temporary stoma found that more than half of them felt socially impacted by the stoma, and 12% were completely isolated (5). A prospective study over five years of rectal cancer showed that stoma construction generally decreases quality of life and closure of stoma brings improved quality of life (29). The pressure on the health sector is increasing, resulting in the need to use other tools than the strictly academically orientated, and economic prioritizing of treatment and nursing care for different patient groups (30). Thus a cost analysis can be used to support professional decisions when new treatments and methods are introduced into the health sector (31). The purpose of the study: This study will analyze differences between two different surgical operative regimes in connection with the closure of a protective temporary ileostomy after treatment for rectal cancer. The difference between the two regimes is the interval between constructing the ileostomy (the first operation) and the closure of the stoma (the second operation). The interval until the ileostomy is 7
8 closed will be 8 13 days for the intervention group, and 12 weeks for the control group, which corresponds to normal clinical practice. Differences identified in relation to the frequency and nature of postoperative complications, mortality, the health economic effect and the health related quality of life for the patients. Hypotheses: Primary: Patients who have an ileostomy closed on day 8 13 have fewer complications than patients who have the stoma closed after minimum 12 weeks. Secondary: Cost effectiveness analysis shows that early closure of ileostomy is cost effective in comparison to late closure. Patients with early re-establishment of normal gastrointestinal function (8-13 days postoperatively) will have better health related quality of life in comparison with patients whose gastrointestinal function is re-established after a minimum of 12 weeks. Patients in the control group will have a significant reduction of renal function compared to the intervention group. Endpoints: Primary: Mean number of complications per patient after index-operation and up to 12 months after (covering any complication including stoma related complications). Secondary endpoints: Percentage of patients with at least one complication with Clavien-Dindo Classification of Surgical Complications (34;35) severity grade IIIA, IIIB, IVA, IVB or V after indexoperation and up to 12 months after. Mean number of stoma-related complications after index-operation and up to 12 months after. Supporting endpoint is Comprehensive complication index (CCI) after index-operation and up to 12 months after. 8
9 Analysis and comparison of creatinine levels at both index surgery and prior to loop ileostomy creation. Analysis and comparison of health related quality of life as measured with SF36 3, 6 and 12 months after stoma construction. Analysis and description of quality of life specific to the illness measured with EORTC QLQ-CR29 three, six and twelve months after stoma construction if stoma not closed. Analysis and description of quality of life specific to the illness in the control group measured with Ostomy Adjustment Scale 3, 6 and12 months after stoma construction if stoma not closed. Recording and comparison of the total costs for the control and intervention groups respectively 0-12 months post-operatively. Design: The study will be carried out as a prospective randomized controlled multi-center study of patients, who have temporary ileostomy due to rectal cancer in Denmark and Sweden. The primary and secondary outcomes of the study will be evaluated at all participating centers. Selection: After ileostomy construction patients will be included in the study (see inclusion criteria. Patients will be randomized into the health intervention group where stoma is closed after 8 13 days or into the control group where stoma is closed after minimum 12 weeks. Both groups will be examined for post-operative complications at discharge and at 3, 6 and 12 months after ileostomy construction. Additionally the effect on the patients health-related quality of life will be examined 3, 6 and 12 months after the ileostomy construction. Finally the health economic costs in both groups will be measured and the difference calculated. The final report and analysis of all endpoints will be carried out after 12 months, Inclusion criteria: Adult mentally competent patients who have been operated for rectal cancer with construction of protective temporary ileostomy can be included in the study. The first days after the operation the patient is assessed by a doctor and it is decided if the patient is suitable for inclusion. In order to be included in the study the patient must not show signs of active 9
10 infection or organ failure. Patients must not show signs of anastomosis leakage. This is ensured by testing for radiological signs of anastomosis leakage with a contrast CT scanning or a flexible endoscopy of the anastomosis which will be a standard part of the department during the course of the project. Additionally the patients will have a functioning stoma (both feces and flatus). Patients are included on the basis of professional assessment by experienced colorectal surgeons and the patients post-operative recovery is documented as data describing degrees of post-operative status. This includes: CRP, red blood cells, nutrition, mobilization and stoma function: When the patient is considered suitable for inclusion the patient is informed verbally and in writing and the patient s written consent is documented before the patient is included (see section on consent). Patients will be included 6-11 days after the operation while they are admitted to the ward. Patients are randomized 6 11 days post-operatively to be included in the control or the intervention group. Exclusion criteria: Patients on steroids Patients with diabetes Patients with speech problems Patients with anticipated poor compliance ie for psychiatric reasons. Exclusion: Patients who withdraw their consent to participate Stopping the study: There are no predictable reasons why the study should be terminated. Sample size The calculation of the primary endpoint: Morbidity and mortality Sample size The study protocol specified 72 evaluable patients per group to have 80% power to detect a 66% reduction in the risk of complications 12. Due to slow enrollment fewer evaluable patients were reached and before data analysis we did a recalculation. With the current primary endpoint, 60 evaluable patients per group would have 80% power to detect a 62.5% reduction in the annual mean 10
11 rate of complications using a two-sided test with 5% significance level and assuming a mean rate of 0.45 in the control group. Registration of included patients: We will ensure that all patients who meet the inclusion criteria in practice are included which will be evidenced by the screening log, where non-included patients as well as patients who leave the study will be identified. Patients who after inclusion wish to exit the study will be asked if data already collected can be used in the study or if it should be deleted. Data will be treated according to the patients decision. Methodology: Morbidity: The primary endpoint for the study is the rate of post-operative complications up to 12 months after surgery. The data form is developed based on the Clavien-Dindo Classification of Surgical Complications (C-DCSC)(34;35). C-DCSC is used to ensure that data is collected by objective criteria to minimize the influence of personal and/or cultural bias. The basic concept for the form is to classify complications after treatment and in doing so avoiding individual and ambiguous evaluations such as severe or less severe complications. All complications will be recorded according to type or degree, ie a form for each complication treated will be completed. Stoma related complications will be registered according to symptoms from the stoma and/or the surrounding skin. The classification includes all complications (including stoma related complications) that occur during the six months that the investigation is progressing (34), ie immediately before discharge and 3, 6 and 12 months after construction of ileostomy. Cost calculation: Only the most significant costs will be included in the project, including outpatient contacts for stoma clinics and surgeons as well as individual health benefits associated with municipalities and the private sector. 11
12 Calculation of costs will be made based on recorded admission days, including readmissions, outpatient tests / interviews and reoperations. In addition, questions are asked about demographic data such as level of education and income. The patients' use of services from GPs and community health care and possible return to work will be recorded. It will either record the costs associated with the use of medical services by their GP or nurse, or relate to the reduction of social costs if patients move from being unwell to being part of the working population (36, 37). Social activities and possible return to the workplace will also be recorded, as it indicates that patients have resumed a normal life. The records rely on self-recording in a diary which will be given to patients on discharge, as this kind of information can be difficult to access retrospectively, when patients are likely to forget or overlook visits (38;39). The use of a diary for recording data in combination with cost calculation is a valid method of capturing events that either happen outside the admission period or occur in a patient s daily life. Health related quality of life: The project also intends to assess and compare the health related quality of life with Short Form 36 (SF36) 3, 6 and 12 months post-operatively. In addition we will assess the illness specific quality of life in the form of European Organization for Research and Treatment of Cancer QLQ-CR30+CR29 (EORTC QLQ-CR30+CR29). Further we will assess and describe the stoma specific quality of life in the control group by use of the Ostomy Adjustment Scale (OAS). Short Form 36 (SF36, general quality of life)(40) EORTC QLQ-CR29(41;42) and Ostomy Adjustment Scale (OAS, illness specific quality of life ) (43;44) are all constructed as easily accessible questionnaires designed to be completed in the presence of an interviewer, self-completed or completed by telephone. Additionally patients are asked about demographic data about education, work and marital status. SF-36 was developed in an American study for assessing general health concepts and the form has since been used in many clinical and population studies and has been validated in international and Danish and Swedish studies. SF-36 is a questionnaire which can be used to evaluate people s general health status by completion either by the individual or by an interviewer. It only takes minutes to complete. The questionnaire can be used to assess generic measure of health status which menas that it can be used for all age groups (from 14 years upwards) and for all patient groups. The questionnaire assesses the person s general health status partly via self-evaluated health 12
13 questions and partly by questions that assess physical, social and psychological functions. The questionnaire consists of 36 questions which are divided into 8 scales, where half consolidate into a summary measure of physical health and the other half in a summary measure of mental health to give an assessment of the person's overall health EORTC QLQ-CR 30 + CR29(41;42) was originally developed in 1987 as a cancer specific assessment tool under the leadership of EORTC (CR30), which is an international non-profit organisation. The present version of CR29 has been edited and contains a general cancer specific part (CR30) and a more specific part for gastrointestinal symptoms (CR-29), for practical purposes the two questionnaires are always used together. They contain a total of 59 questions which make up ffunctional scales, symptom-oriented scales and a quality of life scale. OAS is a questionnaire which was developed to evaluate patients subjective adaption to life with stoma focusing on physical, psychological and social changes following stoma construction. There are 34 questions answered on the Likert scale from 1-6. This gives a possible outcome of 34 to 204 points, where the higher score indicates better adaption and consequently a higher health related quality of life. The table is validated and used for patients with different stoma and can be used at various points of time in the context of stoma construction. Both general and illness specific quality of life is assessed at 3 months (+/- 2 weeks), 6 months (+/- 2 weeks) and 12 months (+/- 2 weeks) after construction of a temporary stoma. It is presumed that at the time of the first assessment at 3 months patients in the control group have not undergone stoma closure. The questionnaire is aimed to be used for patients only while they still have a stoma. The assessment will be completed by the patient in the presence of an interviewer to ensure a high response rate and to minimize problems with comprehension. Closure of ileostomy: Carried out after satisfactory examination and evaluation of 1. anastomosis density by contrast CT scan or a flexible endoscopy of the anastomosis 2. whether the patient has a current infection 3. the general health of the patients, as set out in a specific form for post-operative recovery. 13
14 Ad 1. The patient will undergo a localized low radiation CT scan (blank scan) with 5% dilute aqueous contrast in the rectum. Immediately before the examination radiologist, a specially trained registered nurse, or a surgeon (according to local protocol) will insert a Foleycatheter (a short tube made of flexible plastic) into the rectum of the patient and inject ml aqueous contrast in 5% solution. This makes it possible to observe the anastomosis and evaluate the seal. For the duration of the project CT scanning after rectal surgery will be standard and will take place 6-8 days post-operatively and be evaluated by a surgical or radiology specialist. Alternatively, centers may decide to examine the rectum performing a flexible endoscopy of the anastomosis. Ad 2. Post-operatively patients will have daily blood samples taken according to the department s protocol, including creatinine levels. Ad 3. The general condition of patients will be evaluated by a surgeon and documented in the form in CRF: Postoperative recovery CRP (value recorded) Hæmoglobin (value recorded) Nutrition (please tick) Patient eats and drinks sufficiently Patient needs i/v fluids Patient needs total parenteral nutrition Patient needs gavage Mobilising (please tick) Patient gets up >6 times/day Patient gets up <6 times/day Patient gets up <1 time/day Stoma function (please tick) Faeces Flatus Timing of closure operation: Intervention group: Closure of the ileostomy will take place 8 13 days postoperatively and will follow the usual protocol for the department, including in regard to hand stitching or stapling. 14
15 Control group: Closure will take place after minimum 12 weeks and will follow the usual protocol for the department. Stoma training in the control group: Patients will receive the departments usual training to enable them to do their own stoma care until the stoma is closed. This means that patients are assigned to the stoma clinic and will be trained by the department staff and stoma nurses as required. Stoma training in the intervention group: Patients will receive the usual stoma training after inclusion and until randomization. Thereafter it will not be necessary for them to go through the full training schedule. The training will be aimed at completely individual needs such as going home on leave for a couple of days. It may, for example, focus on learning how to empty the bag, switch plate and observing the bag. The patient may want go home on leave a couple of days before the second operation, which will depend on individual conditions. Ethical aspects of the study: Participation in the study is voluntary and happens after verbal and written information to patients in accordance with ethical requirements in Denmark and Sweden. The study will be carried out in accordance with the Helsinki II declaration and law 503 from 1992 covering scientific committee systems and will wait for approval from the local Ethical Committee before starting. The operations will be carried out in surgical departments which specialize in colorectal surgery and where closure of temporary ileostomies and other treatment procedures are part of the standard regimes at the department. Thus it will only be the timing of the closure of the temporary ileostomy that is not part of the routine. Each participant is free to withdraw from the study without affecting the observations, treatment or nursing of the patient. The authorized health personnel involved in the project will demonstrate professional care. Risks and side effects of the study: The actual operation is the same for the intervention and control groups, the control group undergoing surgery according to current and usual local guidelines. The intervention group receives exactly the same treatment and follow-up and nursing care as the control group, other than the 15
16 stoma training, which of course will be individually tailored to the fact that the patient will not need to live with stoma. As we have mentioned, previously published studies do not indicate that there is an increased risk associated with earlier closure of temporary ileostomy provided that the patient has been examined and assessed for this by the surgeon. There are no known risks or side effects connected with completing the questionnaire. It is expected that patients will experience a great improvement of quality of life in connection with earlier return to normal bowel function. It is also expected that patients will be able to return sooner to general activities, such as sport, work and other social and personal activities, including a normal family life. The study will contribute to final recommendations for the timing of closure of temporary ileostomies in relation to morbidity. This will be of importance for future development of clinical guidelines in this area. The study will also contribute new health-economic knowledge used for rankings and comparisons in health care. Additionally the research will provide knowledge of how quality of life is affected during the course of temporary ileostomy, which is of importance for clinical nursing care and treatment. Scientific approval: The project is approved by the Science Ethical Committee for the capital region in Denmark. In Sweden the project will be approved in the same manner by the Ethical Approval committee in Göteborg. Approval of data security: The project is approved by the Data Protection, Denmark and by the Personal Data Representative at the Sahlgrenska Universitety Hospital, Västra Götaland Region in Sverige Clinical Trials: The project is registered at by the principal investigator. Financial support: There is no financial support from commercial sources. If the project achieves financial support the money will be paid via a research account administered by Herlev Hospital. 16
17 If the project does not get financial support, money will be found from the department budgets, as it is mainly about re-scheduling existing treatments. The project was initiated by the principal investigator Jacob Rosenberg, Professor, senior surgeon, MD who is employed and paid by Herlev Hospital. Anne Kjærgaard Danielsen, PhD, RN and also employed and paid by Herlev Hospital. Remuneration for participating patients: Patients will not receive payment for their participation. Guidelines for collection of informed consent: Patients intended for inclusion will be informed verbally and in writing by the local investigator. When patients have agreed to participate and have signed the consent agreement they are included in the study. Patients are randomized into the control or intervention groups after inclusion. Information about the study will happen immediately after the inclusion of the patient ie after CT scan and assessment by a doctor. The written material will be given to the patients to read after the patient has been informed verbally. The verbal information conversation will be held in an undisturbed place or an interview room, or if the patient is confined to bed by making sure that the patient agrees to be informed in the ward. Any other patients in the ward will be asked to leave the ward if this is possible. The patient will be encouraged to have a support person present during the information and the arrival of this person will be awaited. The written material will be delivered by a health care worker who has been appointed by the responsible local investigator. The verbal information will be handled by a health care worker who has been appointed by the local investigator and who is competent to answer the questions that might arise from the patient or the support person. After patients have been informed they have 24 hours to consider giving consent to participate, but they can withdraw their consent at any stage of the study. Inclusion in the project happens after the patient has given written and verbal agreement to participate in the study. Patients will be randomized to the control or intervention groups when they are included in the project. Independently of the randomization the patients will be informed about the operation according to the usual procedure in the department. Timeline for the project Start February 2011 Inclusion of patients February 2011 November
18 Reporting of morbidity until December 2015 Analyses of data and writing up results February
19 References 1. Brown H, Randle J. Living with a stoma: a review of the literature. J Clin Nurs 2005;14: Nugent KP, Daniels P, Stewart B, Patankar R, Johnson CD. Quality of life in stoma patients. Dis Colon Rectum 1999;42: Persson E, Wilde LB. Quality of care after ostomy surgery: a perspective study of patients. Ostomy Wound Manage 2005;51: Persson E, Gustavsson R, Hellstroem A, Lappas G, Hultén L. Ostomy patients perceptions of quality of care. J Adv Nurs 2005;49: Gooszen AW, Geelkerken RH, Hermans J, Lagaay MB, Gooszen HG. Quality of life with a temporary stoma: ileostomy vs. colostomy. Dis Colon Rectum 2000;43: Williams J. Sexual health: case study of a patient who has undergone stoma formation. Br J Nurs 2006;15: Black PK. Psychological, sexual and cultural issues for patients with a stoma. Br J Nurs 2004;13: Rauch P, Miny J, Conroy T, Neyton L, Guillemin F. Quality of life among disease-free survivors of rectal cancer. J Clin Oncol 2004;22: Carlsson E, Bosaeus I, Nordgren S. Quality of life and concerns in patients with short bowel syndrome. Clin Nutr 2003;22: Bloemen JG, Visschers RG, Truin W, Beets GL, Konsten JL. Long-term quality of life in patients with rectal cancer: association with severe postoperative complications and presence of a stoma. Dis Colon Rectum 2009;52: Colquhoun P, Kaiser R, Jr., Efron J et al. Is the quality of life better in patients with colostomy than patients with fecal incontience? World J Surg 2006;30: Krouse R, Grant M, Ferrell B, Nelson R, Chu D. Quality of Life outcomes in 599 cancer and non-cancer patients with colostomies. J Surg Res 2007;138: Ma N, Harvey J, Stewart J, Andrews L, Hill AG. The effect of age on the quality of life of patients living with stomas: a pilot study. ANZ J Surg 2007;77: Holzer B, Matzel K, Schiedeck T et al. Do geographic and educational factors influence the quality of life in rectal cancer patients with a permanent colostomy? Dis Colon Rectum 2005;48: Hamashima C. Long-term quality of life of postoperative rectal cancer patients. J Gastroenterol Hepatol 2002;17: Butler DL. Early postoperative complications following ostomy surgery: a review. J Wound Ostomy Continence Nurs 2009;36: Cottam J, Richards K, Hasted A, Blackman A. Results of a nationwide prospective audit of stoma complications within 3 weeks of surgery. Colorectal Dis 2007;9: Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Dis. In press. 19. Marusch F, Koch A, Schmidt U et al. Value of a protective stoma in low anterior resections for rectal cancer. Dis Colon Rectum 2002;45: Hindenburg T, Rosenberg J. Closing a temporary ileostomy within two weeks. Dan Med Bul 2010;57: Nagell CF, Pedersen CR, Gyrtrup HJ. [Complications after stoma closure. A retrospective study of 11 years' experience]. Ugeskr Laeger 2005;167:
20 22. Bakx R, Busch OR, van GD, Bemelman WA, Slors JF, van Lanschot JJ. Feasibility of early closure of loop ileostomies: a pilot study. Dis Colon Rectum 2003;46: Gessler, B., Haglind, E., & Angenete, E. (2014). A temporary loop ileostomy affects renal function. International Journal of Colorectal Disease, doi: /s Menegaux F, Jordi-Galais P, Turrin N, Chigot JP. Closure of small bowel stomas on postoperative day 10. Eur J Surg 2002;168: Alves A, Panis Y, Lelong B, Dousset B, Benoist S, Vicaut E. Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy. Br J Surg 2008;95: Gronvold M. [Health-related quality of life in cancer patients]. Ugeskr Laeger 2008;170: Jess P. [Quality of life assessments in colorectal surgery]. Ugeskr Laeger 2008;170: Burckhardt CS, Hanestad BR. Nursing strategies and quality of life outcomes: a systematic review. Vard i Norden 2003; Engel J, Kerr J, Schlesinger-Raab A, Eckel R, Sauer H, Holzel D. Quality of life in rectal cancer patients: a four-year prospective study. Ann Surg 2003;238: Wittrup-Jensen KU, Lauridsen J, Pedersen KM. Ønsker danskerne mest mulig sundhed for pengene, når de prioriterer? In: Lauridsen JT, Pedersen KM, eds. Sundhedsøkonomi. Fra Teori til Praksis. Jurist-og Økonomforbundet; 2009; van den Hout WB, van den Brink M, Stiggelbout AM, van de Velde CJ, Kievit J. Costeffectiveness analysis of colorectal cancer treatments. Eur J Cancer 2002;38: Alves A, Panis Y, Lelong B, Dousset B, Benoist S, Vicaut E. Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy. Br J Surg 2008;95: Siassi M, Hohenberger W, Lösel F, Weiss M. Quality of life and patient s expectations after closure of a temporary stoma. Int J Colorectal Dis 2008; Clavien PA, Barkun J, de Oliveira ML et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250: Dindo D, Demartines N, Clavien P-A. Classification of surgical complications. A new proposal with evalutation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240: Drummond MF, Sculpher MJ, Torrance GW, O Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. Third edition ed. Oxford University Press, Kronborg C, Vass M, Lauridsen JAa, Avlund K. Cost effectiveness of preventive home visits to the elderly. Economic evaluation alongside randomized controlled study. Eur J Health Econ 2006;7: van Berge Henegouwen MT, van Driel HF, Kasteleijn-Nolst Trenite DG. A patient diary as a tool to improve medicine compliance. Pharm World Sci 1999;21: Goossens M.E.J.B.:Rutten-van Mölken MPMH, Vlayen JWS, van den Linden SMJP. The cost diary: a method to measure direct and indirect costs in cost-effectiveness resarch. Journal of Clinical Epidemiology 2000; Bue Bjoerner J, Trab Damsgaard M, Watt T et al. Dansk manual til SF-36. Lif, Lægemiddelindustriforeningen,
21 41. Gujral S, Conroy T, Fleissner C et al. Assessing quality of life in patients with colorectal cancer: An update of the EORTC quality of life questionnaire. European Journal of Cancer 2007;43: Whistance RN, Conroy T, Chie W et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer 2009;45: Burckhardt CS. The Ostomy Adjustment Scale: Further evidence of reliability and validity. Rehabil Psychol 1990;35: Olbrisch M. Development and validation of the Ostomy Adjustment Scale. Rehabil Psychol 1983;28:12. 21
Early closure of temporary ileostomy, design of the EASY trial. A randomized controlled trial. For peer review only
 Early closure of temporary ileostomy, design of the EASY trial. A randomized controlled trial. Journal: BMJ Open Manuscript ID: bmjopen-0-000 Article Type: Protocol Date Submitted by the Author: 0-May-0
Early closure of temporary ileostomy, design of the EASY trial. A randomized controlled trial. Journal: BMJ Open Manuscript ID: bmjopen-0-000 Article Type: Protocol Date Submitted by the Author: 0-May-0
Patients with low rectal cancer often receive a temporary ileostomy
 RANDOMIZED CONTROLLED TRIAL Early Closure of a Temporary Ileostomy in Patients With Rectal Cancer A Multicenter Randomized Controlled Trial Anne K. Danielsen, PhD, MA(Ed), MA(ClN), RN, Jennifer Park, MD,y
RANDOMIZED CONTROLLED TRIAL Early Closure of a Temporary Ileostomy in Patients With Rectal Cancer A Multicenter Randomized Controlled Trial Anne K. Danielsen, PhD, MA(Ed), MA(ClN), RN, Jennifer Park, MD,y
Early Vs Delayed Loop Ileostomy Closure: A Comparative Study
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 7 Ver. VI (July. 2017), PP 08-13 www.iosrjournals.org Early Vs Delayed Loop Ileostomy Closure:
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 16, Issue 7 Ver. VI (July. 2017), PP 08-13 www.iosrjournals.org Early Vs Delayed Loop Ileostomy Closure:
WHAT IS LARS? LOW ANTERIOR RESECTIONSYNDROME. Sophie Pilkington. Colorectal Surgeon University Hospital Southampton
 WHAT IS LARS? LOW ANTERIOR RESECTIONSYNDROME Sophie Pilkington Colorectal Surgeon University Hospital Southampton INTRODUCTION UK Bowel cancer 2013 41,100 new cases INTRODUCTION UK Bowel cancer 2013 41,100
WHAT IS LARS? LOW ANTERIOR RESECTIONSYNDROME Sophie Pilkington Colorectal Surgeon University Hospital Southampton INTRODUCTION UK Bowel cancer 2013 41,100 new cases INTRODUCTION UK Bowel cancer 2013 41,100
REVERSAL OF ILEOSTOMY. Patient information Leaflet
 REVERSAL OF ILEOSTOMY Patient information Leaflet April 2017 WHAT IS A REVERSAL OF ILEOSTOMY? A reversal of ileostomy is an operation to close your temporary ileostomy. Your surgeon will make a cut in
REVERSAL OF ILEOSTOMY Patient information Leaflet April 2017 WHAT IS A REVERSAL OF ILEOSTOMY? A reversal of ileostomy is an operation to close your temporary ileostomy. Your surgeon will make a cut in
Establishment of a regional Danish database for patients with a stoma
 Original article doi:10.1111/codi.12848 Establishment of a regional Danish database for patients with a stoma A. K. Danielsen*, B. M. Christensen, J. Mortensen*, L. L. Voergaard, P. Herlufsen and L.Balleby
Original article doi:10.1111/codi.12848 Establishment of a regional Danish database for patients with a stoma A. K. Danielsen*, B. M. Christensen, J. Mortensen*, L. L. Voergaard, P. Herlufsen and L.Balleby
PHYSSURG PHYSICAL ACTIVITY IN RELATION TO SURGICAL A PROSPECTIVE STUDY OF THE LEVEL OF PHYSICAL ACTIVITY OPERATIONS
 PHYSSURG PHYSICAL ACTIVITY IN RELATION TO SURGICAL OPERATIONS A PROSPECTIVE STUDY OF THE LEVEL OF PHYSICAL ACTIVITY AND ITS RELATIONSHIP TO COMPLICATIONS AFTER SURGERY Project plan CONTENT 1.0 Study organisation
PHYSSURG PHYSICAL ACTIVITY IN RELATION TO SURGICAL OPERATIONS A PROSPECTIVE STUDY OF THE LEVEL OF PHYSICAL ACTIVITY AND ITS RELATIONSHIP TO COMPLICATIONS AFTER SURGERY Project plan CONTENT 1.0 Study organisation
Prospective study on the safety and feasibility of early ileostomy closure 2 weeks after lower anterior resection for rectal cancer
 ORIGINAL ARTICLE pissn 2288-6575 eissn 2288-6796 https://doi.org/10.4174/astr.2019.96.1.41 Annals of Surgical Treatment and Research Prospective study on the safety and feasibility of early ileostomy closure
ORIGINAL ARTICLE pissn 2288-6575 eissn 2288-6796 https://doi.org/10.4174/astr.2019.96.1.41 Annals of Surgical Treatment and Research Prospective study on the safety and feasibility of early ileostomy closure
HARTMANNS PROCEDURE. Patient information Leaflet
 HARTMANNS PROCEDURE Patient information Leaflet April 2017 WHAT IS A HARTMANNS PROCEDURE? This operation is necessary to remove the area of bowel that is diseased. The operation removes a piece of your
HARTMANNS PROCEDURE Patient information Leaflet April 2017 WHAT IS A HARTMANNS PROCEDURE? This operation is necessary to remove the area of bowel that is diseased. The operation removes a piece of your
Clavien-Dindo indikator: Et eksempel fra den Danske KoloRektal Cancer database (DCCG)
 Clavien-Dindo indikator: Et eksempel fra den Danske KoloRektal Cancer database (DCCG) Peter-Martin Krarup Overlæge Abdominalcenter K, Bispebjerg Hospital Disclosures AstraZeneca (Research collaboration)
Clavien-Dindo indikator: Et eksempel fra den Danske KoloRektal Cancer database (DCCG) Peter-Martin Krarup Overlæge Abdominalcenter K, Bispebjerg Hospital Disclosures AstraZeneca (Research collaboration)
University College Hospital. Laparoscopic colorectal surgery. Gastrointestinal Services Division
 University College Hospital Laparoscopic colorectal surgery Gastrointestinal Services Division 2 Colon 3 If you would like a large print, audio or translated version of this document contact us on 0845
University College Hospital Laparoscopic colorectal surgery Gastrointestinal Services Division 2 Colon 3 If you would like a large print, audio or translated version of this document contact us on 0845
Colostomy & Ileostomy
 Colostomy & Ileostomy Indications, problems and preference By Waleed Omar Professor of Colorectal surgery, Mansoura University. Disclosure I have no disclosures. Presentation outline Stoma: Definition
Colostomy & Ileostomy Indications, problems and preference By Waleed Omar Professor of Colorectal surgery, Mansoura University. Disclosure I have no disclosures. Presentation outline Stoma: Definition
The challenges faced by people with a stoma and dementia
 The challenges faced by people with a stoma and dementia A holistic approach to person-centred care Rebecca Fossett, Colorectal Nurse Specialist (2018) Safe & Effective Kind & Caring Exceeding Expectation
The challenges faced by people with a stoma and dementia A holistic approach to person-centred care Rebecca Fossett, Colorectal Nurse Specialist (2018) Safe & Effective Kind & Caring Exceeding Expectation
To help you understand your operation, it is helpful to have a basic knowledge of how the body works (see Figure 1).
 Page 1 of 11 Anterior resection Introduction This leaflet tells you about the procedure known as an anterior resection. It explains what the procedure involves and also some of the common complications
Page 1 of 11 Anterior resection Introduction This leaflet tells you about the procedure known as an anterior resection. It explains what the procedure involves and also some of the common complications
Life after stoma creation
 PHD THESIS DANISH MEDICAL JOURNAL Life after stoma creation Anne Kjærgaard Danielsen This review has been accepted as a thesis together with 6 previously published papers by University Copenhagen 7 th
PHD THESIS DANISH MEDICAL JOURNAL Life after stoma creation Anne Kjærgaard Danielsen This review has been accepted as a thesis together with 6 previously published papers by University Copenhagen 7 th
Colorectal Laparoscopic Standards and Coding Protocols July 2015 v2.0
 Laparoscopic Standards and Coding Protocols July 2015 v2.0 COLORECTAL LAPAROSCOPIC STANDARDS AND CODING PROTOCOLS Contents 1 Context... 3 2 Laparoscopic Standards... 3 3 Coding Protocols... 3 Appendix
Laparoscopic Standards and Coding Protocols July 2015 v2.0 COLORECTAL LAPAROSCOPIC STANDARDS AND CODING PROTOCOLS Contents 1 Context... 3 2 Laparoscopic Standards... 3 3 Coding Protocols... 3 Appendix
Chapter I 7. Laparoscopic versus open elective sigmoid resection in diverticular disease: six months follow-up of the randomized control Sigma-trial
 Chapter I 7 Laparoscopic versus open elective sigmoid resection in diverticular disease: six months follow-up of the randomized control Sigma-trial Bastiaan R. Klarenbeek Roberto Bergamaschi Alexander
Chapter I 7 Laparoscopic versus open elective sigmoid resection in diverticular disease: six months follow-up of the randomized control Sigma-trial Bastiaan R. Klarenbeek Roberto Bergamaschi Alexander
Suspected CANcer (SCAN) Pathway Information for patients
 Suspected CANcer (SCAN) Pathway Information for patients page 2 Your GP has advised you may benefit from investigation via the SCAN pathway. The SCAN pathway is part of a national programme called ACE
Suspected CANcer (SCAN) Pathway Information for patients page 2 Your GP has advised you may benefit from investigation via the SCAN pathway. The SCAN pathway is part of a national programme called ACE
YOUR OPERATION EXPLAINED
 RIGHT HEMICOLECTOMY This leaflet is produced by the Department of Colorectal Surgery at Beaumont Hospital supported by an unrestricted grant to better Beaumont from the Beaumont Hospital Cancer Research
RIGHT HEMICOLECTOMY This leaflet is produced by the Department of Colorectal Surgery at Beaumont Hospital supported by an unrestricted grant to better Beaumont from the Beaumont Hospital Cancer Research
University of Groningen. Colorectal Anastomoses Bakker, Ilsalien
 University of Groningen Colorectal Anastomoses Bakker, Ilsalien IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Colorectal Anastomoses Bakker, Ilsalien IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Understanding your bowel surgery
 Understanding your bowel surgery Abdomino Perineal Excision of Rectum (APER) Hartmann s Procedure Pan Proctocolectomy Total Colectomy Subtotal Colectomy Information for patients, relatives and carers This
Understanding your bowel surgery Abdomino Perineal Excision of Rectum (APER) Hartmann s Procedure Pan Proctocolectomy Total Colectomy Subtotal Colectomy Information for patients, relatives and carers This
National Bowel Cancer Audit Supplementary Report 2011
 National Bowel Cancer Audit Supplementary Report 2011 This Supplementary Report contains data from the 2009/2010 reporting period which covers patients in England with a diagnosis date from 1 August 2009
National Bowel Cancer Audit Supplementary Report 2011 This Supplementary Report contains data from the 2009/2010 reporting period which covers patients in England with a diagnosis date from 1 August 2009
Supplementary Online Content
 Supplementary Online Content Schultz JK, Yaqub S, Wallon C, et al. Laparoscopic lavage vs primary resection for acute perforated diverticulitis: the SCANDIV randomized clinical trial. JAMA. doi:10.1001/jama.2015.12076
Supplementary Online Content Schultz JK, Yaqub S, Wallon C, et al. Laparoscopic lavage vs primary resection for acute perforated diverticulitis: the SCANDIV randomized clinical trial. JAMA. doi:10.1001/jama.2015.12076
preparing for surgery
 preparing for surgery Facing the news that you need to have a stoma is very difficult but with thousands of people having stoma surgery each year, it is important to remember that you are not alone and
preparing for surgery Facing the news that you need to have a stoma is very difficult but with thousands of people having stoma surgery each year, it is important to remember that you are not alone and
ABDOMINAL PERINEAL RESECTION. Patient information Leaflet
 ABDOMINAL PERINEAL RESECTION Patient information Leaflet April 2017 WHAT IS AN ABDOMINAL PERINEAL RESECTION? This is an operation which involves removing the lower end of your large bowel along with the
ABDOMINAL PERINEAL RESECTION Patient information Leaflet April 2017 WHAT IS AN ABDOMINAL PERINEAL RESECTION? This is an operation which involves removing the lower end of your large bowel along with the
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician CSCCN PORTSMOUTH HOSPITALS Portsmouth Colorectal MDT (11-2D-1) - 2011/12 Daniel OLeary Compliance Self Assessment COLORECTAL
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician CSCCN PORTSMOUTH HOSPITALS Portsmouth Colorectal MDT (11-2D-1) - 2011/12 Daniel OLeary Compliance Self Assessment COLORECTAL
Laparoscopic radical nephrectomy
 PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label What is a laparoscopic radical nephrectomy? This is a procedure which involves removal
PLEASE PRINT WHOLE FORM DOUBLE SIDED ON YELLOW PAPER Patient Information to be retained by patient affix patient label What is a laparoscopic radical nephrectomy? This is a procedure which involves removal
Ein Leben nach tiefer Rektumresektion: Was erwartet unsere Patienten im Langzeitverlauf?
 Ein Leben nach tiefer Rektumresektion: Was erwartet unsere Patienten im Langzeitverlauf? Dieter Hahnloser Klinik für Viszeral- und Transplantationschirurgie UniverstätsSpital Zürich Low Rectal Resection
Ein Leben nach tiefer Rektumresektion: Was erwartet unsere Patienten im Langzeitverlauf? Dieter Hahnloser Klinik für Viszeral- und Transplantationschirurgie UniverstätsSpital Zürich Low Rectal Resection
Medicines and Technologies Programme Adoption Scoping Report MTG315 Peristeen
 Medicines and Technologies Programme Adoption Scoping Report MTG315 Peristeen SUMMARY for MTAC1 meeting Contributors questioned why NICE are writing guidance on this system alone when it is viewed and
Medicines and Technologies Programme Adoption Scoping Report MTG315 Peristeen SUMMARY for MTAC1 meeting Contributors questioned why NICE are writing guidance on this system alone when it is viewed and
Acute Diverticulitis. Andrew B. Peitzman, MD Mark M. Ravitch Professor of Surgery University of Pittsburgh
 Acute Diverticulitis Andrew B. Peitzman, MD Mark M. Ravitch Professor of Surgery University of Pittsburgh Focus today: when to operate n Recurrent, uncomplicated diverticulitis; after how many episodes?
Acute Diverticulitis Andrew B. Peitzman, MD Mark M. Ravitch Professor of Surgery University of Pittsburgh Focus today: when to operate n Recurrent, uncomplicated diverticulitis; after how many episodes?
Loop ostomy following laparoscopic low anterior resection for rectal cancer after neoadjuvant chemoradiotherapy
 https://doi.org/10.1186/s40001-018-0325-x European Journal of Medical Research RESEARCH Open Access Loop ostomy following laparoscopic low anterior resection for rectal cancer after neoadjuvant chemoradiotherapy
https://doi.org/10.1186/s40001-018-0325-x European Journal of Medical Research RESEARCH Open Access Loop ostomy following laparoscopic low anterior resection for rectal cancer after neoadjuvant chemoradiotherapy
My hip fracture care: 12 questions to ask A guide for patients, their families and carers
 My hip fracture care: 12 questions to ask A guide for patients, their families and carers About this guide This guide is aimed at patients who have a hip fracture, and their families and carers. It explains
My hip fracture care: 12 questions to ask A guide for patients, their families and carers About this guide This guide is aimed at patients who have a hip fracture, and their families and carers. It explains
Incidence of Colorectal Cancers- Australia. Anterior Resection 5/23/2018. What spurs us to investigate?
 Incidence of Colorectal Cancers- Australia 17,000 Colorectal cancers in 2018 20% of Colorectal cancers are in the Rectum 12.3% of all new cancers Anterior Resection Syndrome (ARS) Lisa Wilson. Colorectal
Incidence of Colorectal Cancers- Australia 17,000 Colorectal cancers in 2018 20% of Colorectal cancers are in the Rectum 12.3% of all new cancers Anterior Resection Syndrome (ARS) Lisa Wilson. Colorectal
Appendicitis. Diagnosis and Surgery
 Appendicitis Diagnosis and Surgery What Is Appendicitis? Your side may hurt so much that you called your doctor. Or maybe you went straight to the hospital emergency room. If the symptoms came on quickly,
Appendicitis Diagnosis and Surgery What Is Appendicitis? Your side may hurt so much that you called your doctor. Or maybe you went straight to the hospital emergency room. If the symptoms came on quickly,
Colorectal Surgery. Ostomy
 Colorectal Surgery Ostomy The Department of Surgery sees patients for a wide range of surgical services. These include Colorectal, Endocrine, Breast, Upper GI, Bariatrics, Hepatobiliary, Plastics, Neurosurgery,
Colorectal Surgery Ostomy The Department of Surgery sees patients for a wide range of surgical services. These include Colorectal, Endocrine, Breast, Upper GI, Bariatrics, Hepatobiliary, Plastics, Neurosurgery,
Laparoscopic (keyhole) colorectal (bowel) resection
 Laparoscopic (keyhole) colorectal (bowel) resection Your operation explained Information for patients Colorectal Surgery Introduction This leaflet should be read together with a booklet which explains
Laparoscopic (keyhole) colorectal (bowel) resection Your operation explained Information for patients Colorectal Surgery Introduction This leaflet should be read together with a booklet which explains
Adult organisational audit
 Adult organisational audit Health Information Coding Responses for this section require data for the period between 1/12/13-30/11/14. [Infliximab was introduced for the treatment of ulcerative colitis
Adult organisational audit Health Information Coding Responses for this section require data for the period between 1/12/13-30/11/14. [Infliximab was introduced for the treatment of ulcerative colitis
ILEOSTOMY VERSUS COLOSTOMY searchers conducted the data search independently using the key words ileostomy AND colostomy, and loop ileostomy AND loop
 Original Article Temporary Ileostomy Versus Temporary Colostomy: A Meta-analysis of Complications Panuwat Lertsithichai and Pudsaporn Rattanapichart, Department of Surgery, Ramathibodi Hospital Medical
Original Article Temporary Ileostomy Versus Temporary Colostomy: A Meta-analysis of Complications Panuwat Lertsithichai and Pudsaporn Rattanapichart, Department of Surgery, Ramathibodi Hospital Medical
LAPAROSCOPIC PYELOPLASTY INFORMATION LEAFLET
 LAPAROSCOPIC PYELOPLASTY INFORMATION LEAFLET Laparoscopic Pyeloplasty Page 1 of 8 LAPAROSCOPIC PYELOPLASTY This leaflet has been written to answers questions that you may have about your operation. If
LAPAROSCOPIC PYELOPLASTY INFORMATION LEAFLET Laparoscopic Pyeloplasty Page 1 of 8 LAPAROSCOPIC PYELOPLASTY This leaflet has been written to answers questions that you may have about your operation. If
Quality of Life Questionnaire for a Patient with an. Ostomy (QOL-O) Grant, M., Ferrell, B. R., Dean, G., Uman, G., Chu, D., & Krouse, R.
 Instrument Title: Quality of Life Questionnaire for a Patient with an Ostomy (QOL-O) Instrument Author: Grant, M., Ferrell, B. R., Dean, G., Uman, G., Chu, D., & Krouse, R. Cite instrument as: Grant, M.,
Instrument Title: Quality of Life Questionnaire for a Patient with an Ostomy (QOL-O) Instrument Author: Grant, M., Ferrell, B. R., Dean, G., Uman, G., Chu, D., & Krouse, R. Cite instrument as: Grant, M.,
PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS. A08/S/d Colorectal: Faecal Incontinence (Adult)
 A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
A08/S/d 2013/14 NHS STANDARD CONTRACT FOR COLORECTAL: FAECAL INCONTINENCE (ADULT) PARTICULARS, SCHEDULE 2- THE SERVICES, A- SERVICE SPECIFICATIONS Service Specification No. Service Commissioner Lead Provider
The EORTC QLQ-CR29 quality of life questionnaire for colorectal cancer: validation of the Dutch version
 Qual Life Res (2016) 25:1853 1858 DOI 10.1007/s11136-015-1210-5 BRIEF COMMUNICATION The EORTC QLQ-CR29 quality of life questionnaire for colorectal cancer: validation of the Dutch version A. M. Stiggelbout
Qual Life Res (2016) 25:1853 1858 DOI 10.1007/s11136-015-1210-5 BRIEF COMMUNICATION The EORTC QLQ-CR29 quality of life questionnaire for colorectal cancer: validation of the Dutch version A. M. Stiggelbout
NATIONAL BOWEL CANCER AUDIT The feasibility of reporting Patient Reported Outcome Measures as part of a national colorectal cancer audit
 NATIONAL BOWEL CANCER AUDIT The feasibility of reporting Patient Reported Outcome Measures as part of a national colorectal cancer audit NBOCA: Feasibility Study Date of publication: Thursday 9 th August
NATIONAL BOWEL CANCER AUDIT The feasibility of reporting Patient Reported Outcome Measures as part of a national colorectal cancer audit NBOCA: Feasibility Study Date of publication: Thursday 9 th August
Research Article Temporary Fecal Diversion in the Management of Colorectal and Perianal Crohn s Disease
 Hindawi Publishing Corporation Gastroenterology Research and Practice Volume 2015, Article ID 286315, 5 pages http://dx.doi.org/10.1155/2015/286315 Research Article Temporary Fecal Diversion in the Management
Hindawi Publishing Corporation Gastroenterology Research and Practice Volume 2015, Article ID 286315, 5 pages http://dx.doi.org/10.1155/2015/286315 Research Article Temporary Fecal Diversion in the Management
ABDOMINAL PERINEAL RESECTION
 ABDOMINAL PERINEAL RESECTION This leaflet is produced by the Department of Colorectal Surgery at Beaumont Hospital supported by an unrestricted grant to better Beaumont from the Beaumont Hospital Cancer
ABDOMINAL PERINEAL RESECTION This leaflet is produced by the Department of Colorectal Surgery at Beaumont Hospital supported by an unrestricted grant to better Beaumont from the Beaumont Hospital Cancer
Inflammatory Bowel Disease and Surgery: What You Should Know
 Inflammatory Bowel Disease and Surgery: What You Should Know Ask the Experts March 9, 2019 Kristen Blaker, MD Colon and Rectal Surgery MetroHealth Medical Center Disclosures None Outline Who undergoes
Inflammatory Bowel Disease and Surgery: What You Should Know Ask the Experts March 9, 2019 Kristen Blaker, MD Colon and Rectal Surgery MetroHealth Medical Center Disclosures None Outline Who undergoes
A SAFE AND DIGNIFIED LIFE WITH DEMENTIA
 A SAFE AND DIGNIFIED LIFE WITH DEMENTIA NATIONAL ACTION PLAN ON DEMENTIA 2025 January 2017 A SAFE AN DIGNIFIED LIFE WITH DEMENTIA INTRODUCTION We can do much better In Denmark, we have come a long way
A SAFE AND DIGNIFIED LIFE WITH DEMENTIA NATIONAL ACTION PLAN ON DEMENTIA 2025 January 2017 A SAFE AN DIGNIFIED LIFE WITH DEMENTIA INTRODUCTION We can do much better In Denmark, we have come a long way
Københavns Universitet
 university of copenhagen Københavns Universitet Laparoscopic lavage is superior to colon resection for perforated purulent diverticulitis-a meta-analysis Angenete, Eva; Bock, David; Rosenberg, Jacob; Haglind,
university of copenhagen Københavns Universitet Laparoscopic lavage is superior to colon resection for perforated purulent diverticulitis-a meta-analysis Angenete, Eva; Bock, David; Rosenberg, Jacob; Haglind,
Patient Information Sheet
 Research Trial of Treatments for Patients with Bony Metastatic Cancer of the Prostate. - TRAPEZE Patient Information Sheet Your doctor has explained to you that your prostate cancer is no longer responding
Research Trial of Treatments for Patients with Bony Metastatic Cancer of the Prostate. - TRAPEZE Patient Information Sheet Your doctor has explained to you that your prostate cancer is no longer responding
Complications of laparoscopic protective loop ileostomy in patients with colorectal cancer
 ISPUB.COM The Internet Journal of Surgery Volume 19 Number 2 Complications of laparoscopic protective loop ileostomy in patients with colorectal cancer F Puccio, M Solazzo, G Pandolfo, P Marcianò Citation
ISPUB.COM The Internet Journal of Surgery Volume 19 Number 2 Complications of laparoscopic protective loop ileostomy in patients with colorectal cancer F Puccio, M Solazzo, G Pandolfo, P Marcianò Citation
Transforming Cancer Services for London
 Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Programme Director Paul Roche Status Draft Owner Laura Boyd Version 0.4 Author Jennifer Layburn Date 15/05/13 Transforming Cancer Services for London Best Practice Commissioning Pathway for the early detection
Discharge information for patients Fistula plug for anal fistula
 Discharge information for patients Fistula plug for anal fistula Clinical Sciences Building Colorectal Surgery 0161 206 1249 All Rights Reserved 2017. Document for issue as handout.. What is an anal fistula?
Discharge information for patients Fistula plug for anal fistula Clinical Sciences Building Colorectal Surgery 0161 206 1249 All Rights Reserved 2017. Document for issue as handout.. What is an anal fistula?
Progress in improving cancer services and outcomes in England. Report. Department of Health, NHS England and Public Health England
 Report by the Comptroller and Auditor General Department of Health, NHS England and Public Health England Progress in improving cancer services and outcomes in England HC 949 SESSION 2014-15 15 JANUARY
Report by the Comptroller and Auditor General Department of Health, NHS England and Public Health England Progress in improving cancer services and outcomes in England HC 949 SESSION 2014-15 15 JANUARY
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician ASWCN TAUNTON AND SOMERSET Taunton Lung MDT (11-2C-1) - 2011/12 Dr Sarah Foster Compliance Self Assessment LUNG MDT
DATA COLLECTION SHEET CRF 3M FOLLOW UP AT 3 MONTHS (+/- 2 weeks)
 DATA COLLECTION SHEET CRF 3M FOLLOW UP AT 3 MONTHS (+/- 2 weeks) Center ID: Patient code: Date of evaluation (dd/mm/yyyy): / / 90 day mortality No Yes Date of death (dd/mm/yyyy): / / (If Yes specify cause
DATA COLLECTION SHEET CRF 3M FOLLOW UP AT 3 MONTHS (+/- 2 weeks) Center ID: Patient code: Date of evaluation (dd/mm/yyyy): / / 90 day mortality No Yes Date of death (dd/mm/yyyy): / / (If Yes specify cause
Bowel Cancer in England and Wales A summary report about the management and outcomes of people with bowel cancer
 Bowel Cancer in England and Wales A summary report about the management and outcomes of people with bowel cancer Based on findings from the National Bowel Cancer Audit Background How are patients diagnosed?
Bowel Cancer in England and Wales A summary report about the management and outcomes of people with bowel cancer Based on findings from the National Bowel Cancer Audit Background How are patients diagnosed?
Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy
 Randomized clinical trial Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy A. Alves 1,Y.Panis 1,B.Lelong 2, B. Dousset 3,S.Benoist 4 and E. Vicaut 5 1 Department
Randomized clinical trial Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy A. Alves 1,Y.Panis 1,B.Lelong 2, B. Dousset 3,S.Benoist 4 and E. Vicaut 5 1 Department
National Bowel Cancer Audit. Detection and management of outliers: Clinical Outcomes Publication
 National Bowel Cancer Audit Detection and management of outliers: Clinical Outcomes Publication November 2017 1 National Bowel Cancer Audit (NBOCA) Detection and management of outliers Clinical Outcomes
National Bowel Cancer Audit Detection and management of outliers: Clinical Outcomes Publication November 2017 1 National Bowel Cancer Audit (NBOCA) Detection and management of outliers Clinical Outcomes
Clinical profile and post-operative lifestyle changes in cancer and non-cancer patients with ostomy
 Gastroenterology and Hepatology From Bed to Bench 2012 RIGLD, Research Institute for Gastroenterology and Liver Diseases ORIGINAL ARTICLE Clinical profile and post-operative lifestyle changes in cancer
Gastroenterology and Hepatology From Bed to Bench 2012 RIGLD, Research Institute for Gastroenterology and Liver Diseases ORIGINAL ARTICLE Clinical profile and post-operative lifestyle changes in cancer
COLORECTAL RESECTIONS
 COLORECTAL RESECTIONS What is a colorectal (bowel) resection? Surgery to remove a part of the large bowel is called a resection. Different parts of the colon require different operations and have different
COLORECTAL RESECTIONS What is a colorectal (bowel) resection? Surgery to remove a part of the large bowel is called a resection. Different parts of the colon require different operations and have different
Introduction. Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients)
 Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
Bowel Cancer Information Leaflet THE DIGESTIVE SYSTEM
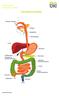 THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
THE DIGESTIVE SYSTEM This factsheet is about bowel cancer Throughout our lives, the lining of the bowel constantly renews itself. This lining contains many millions of tiny cells, which grow, serve their
Hearing aid dispenser approval process review Introduction Hearing aid dispenser data transfer... 6
 Hearing aid dispenser approval process review 2010 11 Content 1.0 Introduction... 4 1.1 About this document... 4 1.2 Overview of the approval process... 4 2.0 Hearing aid dispenser data transfer... 6 2.1
Hearing aid dispenser approval process review 2010 11 Content 1.0 Introduction... 4 1.1 About this document... 4 1.2 Overview of the approval process... 4 2.0 Hearing aid dispenser data transfer... 6 2.1
1st stage neuromodulation test
 1st stage neuromodulation test Information for patients Urology PROUD TO MAKE A DIFFERENCE SHEFFIELD TEACHING HOSPITALS NHS FOUNDATION TRUST page 2 of 12 This leaflet is to give you some background information
1st stage neuromodulation test Information for patients Urology PROUD TO MAKE A DIFFERENCE SHEFFIELD TEACHING HOSPITALS NHS FOUNDATION TRUST page 2 of 12 This leaflet is to give you some background information
PERCUTANEOUS BILIARY DRAINAGE
 PERCUTANEOUS BILIARY DRAINAGE MEDICAL IMAGING INFORMATION FOR PATIENTS Introduction This booklet tells you about the procedure known as percutaneous biliary drainage, explains what is involved and what
PERCUTANEOUS BILIARY DRAINAGE MEDICAL IMAGING INFORMATION FOR PATIENTS Introduction This booklet tells you about the procedure known as percutaneous biliary drainage, explains what is involved and what
Your Bowel Operation Hartmanns Procedure
 Your Bowel Operation Hartmanns Procedure Introduction You are having an operation called Hartmanns Procedure and this booklet aims to help you to understand your condition and this operation. The nurses
Your Bowel Operation Hartmanns Procedure Introduction You are having an operation called Hartmanns Procedure and this booklet aims to help you to understand your condition and this operation. The nurses
Paediatric Organisational Audit
 Paediatric Organisational Audit Health Information Coding Responses for this section require data for the period between 1/12/13-30/11/14. [Infliximab was introduced for the treatment of ulcerative colitis
Paediatric Organisational Audit Health Information Coding Responses for this section require data for the period between 1/12/13-30/11/14. [Infliximab was introduced for the treatment of ulcerative colitis
Can postoperative Nutritional Therapy influence the Convalescent period for patients who have undergone Radical Cystectomy.
 Anja Kort. RN Department of Urology 2114 University Hospital Rigshospitalet Copenhagen Denmark Blegdamsvej 9, 2100 Kbh Ø anja.kort@rh.hosp.dk 0045-35452114 Authors: Anja Kort. RN. Department of Urology
Anja Kort. RN Department of Urology 2114 University Hospital Rigshospitalet Copenhagen Denmark Blegdamsvej 9, 2100 Kbh Ø anja.kort@rh.hosp.dk 0045-35452114 Authors: Anja Kort. RN. Department of Urology
Sphenopalatine ganglion (SPG) stimulation for the treatment of cluster headaches
 Headache Centre Sphenopalatine ganglion (SPG) stimulation for the treatment of cluster headaches This leaflet is for patients, family and carers. It explains about the benefits, risks and any alternatives
Headache Centre Sphenopalatine ganglion (SPG) stimulation for the treatment of cluster headaches This leaflet is for patients, family and carers. It explains about the benefits, risks and any alternatives
Suprapubic catheter insertion in the radiology department. Information for patients Urology
 Suprapubic catheter insertion in the radiology department Information for patients Urology page 2 of 8 What is a suprapubic catheter? A suprapubic catheter is an indwelling tube that drains the bladder
Suprapubic catheter insertion in the radiology department Information for patients Urology page 2 of 8 What is a suprapubic catheter? A suprapubic catheter is an indwelling tube that drains the bladder
Level 2 Leg Ulcer Management Service. Service Level Agreement Background. Contents:
 Level 2 Leg Ulcer Management Service Service Level Agreement 2016-2019 Contents: 1. Background to Leg Ulcer Management Service 2. Service Details 3. Accreditation 4. Service Standards 5. Finance Details
Level 2 Leg Ulcer Management Service Service Level Agreement 2016-2019 Contents: 1. Background to Leg Ulcer Management Service 2. Service Details 3. Accreditation 4. Service Standards 5. Finance Details
Surgery for Inflammatory Bowel Disease
 Surgery for Inflammatory Bowel Disease Emily Steinhagen, MD Assistant Professor Department of Surgery, Division of Colorectal Surgery University Hospitals Cleveland Medical Center Common Questions Why
Surgery for Inflammatory Bowel Disease Emily Steinhagen, MD Assistant Professor Department of Surgery, Division of Colorectal Surgery University Hospitals Cleveland Medical Center Common Questions Why
Transurethral Resection of the Prostate (TURP)
 Transurethral Resection of the Prostate (TURP) UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm Introduction
Transurethral Resection of the Prostate (TURP) UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm Introduction
Geriatric screening in acute care wards a novel method of providing care to elderly patients
 Geriatric screening in acute care wards a novel method of providing care to elderly patients JKH Luk, T Kwok, J Woo Objective. To assess a nurse-implemented geriatric screening system. Design. Descriptive
Geriatric screening in acute care wards a novel method of providing care to elderly patients JKH Luk, T Kwok, J Woo Objective. To assess a nurse-implemented geriatric screening system. Design. Descriptive
Patient information leaflet. Royal Surrey County Hospital. NHS Foundation Trust. Nephrostomy. Radiology
 Patient information leaflet Royal Surrey County Hospital NHS Foundation Trust Nephrostomy Radiology This leaflet informs you about the procedure known as a nephrostomy. It explains what is involved and
Patient information leaflet Royal Surrey County Hospital NHS Foundation Trust Nephrostomy Radiology This leaflet informs you about the procedure known as a nephrostomy. It explains what is involved and
Ileostomy and Colostomy Water Soluble Enema
 Patient information leaflet Royal Surrey County Hospital NHS Foundation Trust Ileostomy and Colostomy Water Soluble Enema Radiology This is an examination to look at the section of bowel leading to or
Patient information leaflet Royal Surrey County Hospital NHS Foundation Trust Ileostomy and Colostomy Water Soluble Enema Radiology This is an examination to look at the section of bowel leading to or
Antigrade Colonic Enema (ACE) Information for patients Spinal Injuries
 Antigrade Colonic Enema (ACE) Information for patients Spinal Injuries page 2 of 8 This leaflet has been produced in support of the explanation and counselling provided by your urologist and nurse specialist.
Antigrade Colonic Enema (ACE) Information for patients Spinal Injuries page 2 of 8 This leaflet has been produced in support of the explanation and counselling provided by your urologist and nurse specialist.
Women s & Children s Directorate The TVT Operation - a guide for patients
 Women s & Children s Directorate The TVT Operation - a guide for patients This leaflet was written for women who are considering having a TVT operation. If you have any questions that aren't answered by
Women s & Children s Directorate The TVT Operation - a guide for patients This leaflet was written for women who are considering having a TVT operation. If you have any questions that aren't answered by
SACRAL NERVE STIMULATION (NEUROMODULATION)
 SACRAL NERVE STIMULATION (NEUROMODULATION) What evidence is this information based on? This leaflet includes advice from consensus panels, ICI and ICS. You should read this leaflet with any advice your
SACRAL NERVE STIMULATION (NEUROMODULATION) What evidence is this information based on? This leaflet includes advice from consensus panels, ICI and ICS. You should read this leaflet with any advice your
Upstate New York Surgical Quality Initiative
 Upstate New York Surgical Quality Initiative 30-Day Readmissions: A Snapshot of Regional Practice Experience in Colorectal Surgery ACS NSQIP National Conference 10 th Annual Meeting, July 27 th, 2015 Bradley
Upstate New York Surgical Quality Initiative 30-Day Readmissions: A Snapshot of Regional Practice Experience in Colorectal Surgery ACS NSQIP National Conference 10 th Annual Meeting, July 27 th, 2015 Bradley
Colectomy. Surgical treatment for Ulcerative Colitis (UC) and Familial Adenomatous Polyposis (FAP) Patient and Family Education
 Patient and Family Education Colectomy Surgical treatment for Ulcerative Colitis (UC) and Familial Adenomatous Polyposis (FAP) A colectomy is a surgery that removes the colon, or large intestine. The colectomy
Patient and Family Education Colectomy Surgical treatment for Ulcerative Colitis (UC) and Familial Adenomatous Polyposis (FAP) A colectomy is a surgery that removes the colon, or large intestine. The colectomy
NATIONAL REHABILITATION HOSPITAL SPINAL CORD SYSTEM OF CARE (SCSC) OUTPATIENT SCOPE OF SERVICE
 NATIONAL REHABILITATION HOSPITAL SPINAL CORD SYSTEM OF CARE (SCSC) OUTPATIENT SCOPE OF SERVICE Introduction: The Spinal Cord System of Care (SCSC) at the National Rehabilitation Hospital (NRH) provides
NATIONAL REHABILITATION HOSPITAL SPINAL CORD SYSTEM OF CARE (SCSC) OUTPATIENT SCOPE OF SERVICE Introduction: The Spinal Cord System of Care (SCSC) at the National Rehabilitation Hospital (NRH) provides
Trial Designs. Professor Peter Cameron
 Trial Designs Professor Peter Cameron OVERVIEW Review of Observational methods Principles of experimental design applied to observational studies Population Selection Looking for bias Inference Analysis
Trial Designs Professor Peter Cameron OVERVIEW Review of Observational methods Principles of experimental design applied to observational studies Population Selection Looking for bias Inference Analysis
Tenant & Service User Involvement Strategy
 Tenant & Service User Involvement Strategy Policy No: HM 07 Page: 1 of 9 Tenant & Service User Involvement Strategy 1. Introduction 1.1 Loreburn's Mission Statement is "Delivering Excellence" and we see
Tenant & Service User Involvement Strategy Policy No: HM 07 Page: 1 of 9 Tenant & Service User Involvement Strategy 1. Introduction 1.1 Loreburn's Mission Statement is "Delivering Excellence" and we see
Adult Patient Information and Consent Form
 The ROAM Trial Radiation versus Observation following surgical resection of Atypical Meningioma: a randomised controlled trial
The ROAM Trial Radiation versus Observation following surgical resection of Atypical Meningioma: a randomised controlled trial
Abdomino Perineal Excision of Rectum (APER)
 PATIENT INFORMATION Abdomino Perineal Excision of Rectum (APER) Introduction/Procedure This leaflet tells you about the procedure known as abdomino perineal excision/resection (APER) of the rectum. What
PATIENT INFORMATION Abdomino Perineal Excision of Rectum (APER) Introduction/Procedure This leaflet tells you about the procedure known as abdomino perineal excision/resection (APER) of the rectum. What
LONG TERM OUTCOME OF ELECTIVE SURGERY
 LONG TERM OUTCOME OF ELECTIVE SURGERY Roberto Persiani Associate Professor Mini-invasive Oncological Surgery Unit Institute of Surgical Pathology (Dir. prof. D. D Ugo) Dis Colon Rectum, March 2000 Dis
LONG TERM OUTCOME OF ELECTIVE SURGERY Roberto Persiani Associate Professor Mini-invasive Oncological Surgery Unit Institute of Surgical Pathology (Dir. prof. D. D Ugo) Dis Colon Rectum, March 2000 Dis
National Emergency Laparotomy Audit. Help Box Text
 National Emergency Laparotomy Audit Help Box Text Version Control Version 1.1 06/12/13 1.2 13/12/13 1.3 20/12/13 1.4 20/01/14 1.5 30/01/14 1.6 13/03/14 1.7 07/04/14 1.8 01/12/14 1.9 05/05/15 1.10 02/07/15
National Emergency Laparotomy Audit Help Box Text Version Control Version 1.1 06/12/13 1.2 13/12/13 1.3 20/12/13 1.4 20/01/14 1.5 30/01/14 1.6 13/03/14 1.7 07/04/14 1.8 01/12/14 1.9 05/05/15 1.10 02/07/15
Attending for your Prostate Scan
 Attending for your Prostate Scan Patient Information The aim of this booklet is to give you enough information: To help you prepare for your hospital visit To give properly informed consent for the procedure
Attending for your Prostate Scan Patient Information The aim of this booklet is to give you enough information: To help you prepare for your hospital visit To give properly informed consent for the procedure
Patient information - General Surgery. What is the Large Bowel (Colon) and Rectum?
 Anterior Resection General Surgery Anterior Resection Patient information - General Surgery Introduction This booklet provides information about your operation. Please do not hesitate to ask any questions
Anterior Resection General Surgery Anterior Resection Patient information - General Surgery Introduction This booklet provides information about your operation. Please do not hesitate to ask any questions
Rectal Cancer. About the Colon and Rectum. Symptoms. Colorectal Cancer Screening
 Patient information regarding care and surgery associated with RECTAL CANCER by Robert K. Cleary, M.D., John C. Eggenberger, M.D., Amalia J. Stefanou., M.D. location: Michigan Heart and Vascular Institute,
Patient information regarding care and surgery associated with RECTAL CANCER by Robert K. Cleary, M.D., John C. Eggenberger, M.D., Amalia J. Stefanou., M.D. location: Michigan Heart and Vascular Institute,
Nephrostomy. Radiology Department. Patient information leaflet
 Nephrostomy Radiology Department Patient information leaflet This leaflet informs you about the procedure known as a nephrostomy. It explains what is involved and the possible risks. The benefits and risks
Nephrostomy Radiology Department Patient information leaflet This leaflet informs you about the procedure known as a nephrostomy. It explains what is involved and the possible risks. The benefits and risks
ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS
 R2 (REVISED MANUSCRIPT BLUE 200208-877OC) ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS Mario Castro, M.D., M.P.H. Nina A. Zimmermann R.N. Sue
R2 (REVISED MANUSCRIPT BLUE 200208-877OC) ONLINE DATA SUPPLEMENT - ASTHMA INTERVENTION PROGRAM PREVENTS READMISSIONS IN HIGH HEALTHCARE UTILIZERS Mario Castro, M.D., M.P.H. Nina A. Zimmermann R.N. Sue
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION
 SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date Gender Female Male Date of surgery INCLUSION & EXCLUSION CRITERIA YES
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date Gender Female Male Date of surgery INCLUSION & EXCLUSION CRITERIA YES
Keywords: Ileostomy; Defunctioning; Anterior resection. Introduction. Methods
 Elmer ress Original Article J Clin Med Res. 2015;7(9):685-689 Defunctioning Ileostomy Reversal Rates and Reasons for Delayed Reversal: Does Delay Impact on Complications of Ileostomy Reversal? A Study
Elmer ress Original Article J Clin Med Res. 2015;7(9):685-689 Defunctioning Ileostomy Reversal Rates and Reasons for Delayed Reversal: Does Delay Impact on Complications of Ileostomy Reversal? A Study
02/GMS/0030 ADULT EPILEPSY SERVICE CCP for General Medical and Surgical POOLE HOSPITAL NHS FOUNDATION TRUST
 Service Specification No. Service Commissioner Leads 02/GMS/0030 ADULT EPILEPSY SERVICE CCP for General Medical and Surgical Provider Lead POOLE HOSPITAL NHS FOUNDATION TRUST Period 1 April 2013 to 31
Service Specification No. Service Commissioner Leads 02/GMS/0030 ADULT EPILEPSY SERVICE CCP for General Medical and Surgical Provider Lead POOLE HOSPITAL NHS FOUNDATION TRUST Period 1 April 2013 to 31
Laser vaporisation of prostate (Green light laser prostate surgery): procedure-specific information
 PATIENT INFORMATION Laser vaporisation of prostate (Green light laser prostate surgery): procedure-specific information What is the evidence base for this information? This leaflet includes advice from
PATIENT INFORMATION Laser vaporisation of prostate (Green light laser prostate surgery): procedure-specific information What is the evidence base for this information? This leaflet includes advice from
