Physician Training Manual for Urolastic
|
|
|
- Regina Franklin
- 5 years ago
- Views:
Transcription
1 Physician Training Manual for Urolastic Urogyn BV Jonkerbosplein AB Nijmegen The Netherlands t +31(0) info@urogynbv.com
2 Physician Training Manual for Urolastic In compliance with Directive 93/42/CEE, this product is to be handled and/or implanted solely by qualified physicians who have read these instructions for use. 2 of 36
3 Index Introduction 3 Training requirements 4 Learning objectives 5 History of Urogyn and Urolastic 6 Clinical data 7 Patient selection and screening 8 Patient counseling 9 Benefit and risks 10 Product overview 11 Procedural preparations 14 Pre-operative procedures 15 Peri operative procedure 16 Precautions 24 Case Report Form (CRF) 25 Patient follow-up 26 Adverse events 27 Appendices 28 3 of 36
4 Introduction The purpose of this manual is to provide instructional information about Urolastic, a minimally invasive urethral bulking agent. Unlike most other bulking agents, Urolastic is not based on a resorbable agent. This makes Urolastic a long-lasting solution for stress urinary incontinence (SUI). This manual is to be used in combination with Urogyn-sponsored training. Under no circumstances should the Urolastic procedure be attempted prior to completion of this training. Prior to this training, readers are recommended to refer to the bibliography appended. These references cover clinical studies, animal studies and literature. 4 of 36
5 Training requirements In order to be recognized as a Urolastic-certified physician you must: - be a qualified urologist or gynecologist - have successfully completed the Urolastic training - carry out at least 3 procedures under the supervision of a Urogyn-certified tutor Upon successful completion of the training course, the physician training record will be completed and signed by the certified tutor. You will then be included in the Urogyn register of approved Urolastic specialists and receive a certificate. As the procedure, product and/ or instruments are updated, Urogyn will continue to educate physicians through written communication, seminars and personal visits. In order to remain on the register of approved Urolastic specialists, physicians must be accessible for direct training by Urogyn and attend seminars as required. Training updates are documented in training records. Urogyn will complete and maintain the latest version of this manual and make sure registered physicians have the appropriate version. 5 of 36
6 Learning objectives At the end of this programme you will: - be able to explain the Urolastic procedure. - understand the principle of the Urolastic method. - be able to effectively select and counsel your patients on the benefits and risks of the Urolastic method. - understand the basic procedures for the preparation, placement and follow-up of the Urolastic method. - understand how to manage technical issues and adverse events. 6 of 36
7 History of Urogyn and Urolastic Urogyn was founded in 2009 to develop new products in the field of urology and gynecology, based on a two-component polymer technology. One development was Urolastic, which used the technology to develop a long-lasting bulking agent for the treatment of SUI. Other products based on the same technology include treating incontinence post prostatectomy and anal incontinence. Urolastic is a minimally invasive flexible bulking agent for the treatment of SUI, which is easy to inject and polymerizes in situ into a uniform elastomer within seconds of application. The product remains flexible and adapts to its environment during injection, reducing the chances of migration. It is not biodegradable, resulting in a long-term effect. As the product is clearly visible through imaging techniques, it allows for retrospective investigation or removal in the event of an emergency. Reflux of the material after injection and polymerization as a consequence of interstitial pressure is unlikely. 7 of 36
8 Clinical data Urogyn has conducted several clinical trials to demonstrate the safety and efficacy of the Urolastic procedure. (Intermediate) results of (ongoing) clinical trials are available on our website ( The clinical effectiveness of urethral, nonabsorbable bulking agent in the treatment of recurrent stress urinary incontinence - 6 months of follow-up multicenter study. Dr. T. Rechberger See Appendix A Prospective one-year clinical evaluation of the efficacy and safety of Urolastic, a new bulking agent, for the treatment of stress urinary incontinence. Dr. J. Zajda See Appendix B Safety of novel permanent injectable bulking agent Urolastic for refractory stress urinary incontinence in most demanding patients - results of phase IV one-center study Dr M. Jozwik et al. See Appendix C 8 of 36
9 Patient selection and screening Indications for use Urolastic is intended for use as a urethral bulking agent for the treatment of female stress urinary incontinence (SUI). Urolastic is injected peri-urethrally in the mid urethral submucosal tissue. The injection of Urolastic creates tissue bulking and subsequent coaptation of mid urethral submucosal tissue, to achieve better anatomy, closure of the bladder and prevention of urine leakage. Contra-indications Urolastic is contraindicated in the following conditions: - Acute urogenital tract inflammation - Gross utero-vaginal prolapse - Detrusor overactivity - Neurogenic bladder - Overflow incontinence - Pregnancy, within one year post-partum - Urinary tract infection - Active herpes genitalis - Autoimmune diseases or patients receiving systemic corticosteroid treatment - Patients with MUS procedures less than 6 months before the scheduled procedure. Benefits - The Urolastic method has a long-lasting effect and generally requires no reinjection of material to achieve this effect. - Urolastic does not contain any resorbable materials and therefore maintains its injected volume, resulting in a long-lasting bulking effect. - Urolastic is minimally invasive and may be performed as an outpatient procedure. - The Urolastic procedure does not require any incisions. - The procedure can be performed without general anesthetic. - It is a short and minimally invasive procedure. The treatment takes about 15 minutes and after treatment most women leave the hospital after voiding independently. 9 of 36
10 Patient counseling Physicians should consider the following when counseling patients about the procedure: Patient Information Leaflet Physicians should discuss with the patient the details provided in the Patient Information Leaflet regarding the risks associated with the placement of Urolastic. Effectiveness Like any method for the treatment of stress urinary incontinence, there is no guarantee that the procedure will be 100% effective. However, clinical results showed that 95% of patients treated with Urolastic demonstrated an improvement of at least one Stamey grade, and over 60% of patients were fully cured after an Urolastic treatment. Procedural issues Patients may suffer certain adverse events as result of the procedure (see the chapter on adverse events). Patients should be reminded that a local anesthesia will be used during the procedure, but that they may experience some pain or discomfort during or immediately after the procedure. Patients may experience a form of acute retention during the 48 hours following the procedure, caused by tissue swelling due to the local anesthesia and needle manipulation. During this period, patients may perform self-catheterization. After this 48-hour period, patients should be able to void naturally. Another possible effect is minor discomfort during intercourse following the procedure. This affects a small percentage of patients only and may be resolved by removing the material at the 5 or 7 o clock position by means of a simple procedure. 10 of 36
11 Benefit and risks Acute retention is a side effect seen in all studies. However, it should last no longer than 7 days. The amount of Urolastic to be injected has been carefully checked against the amount of bulking agents used in similar procedures. Also, an application device has been introduced that allows for a reproducible peri-urethral injection of the material. The total amount injected does not exceed 5 ml of Urolastic. If the cough test is not successful, up to 1 ml of extra Urolastic material may be injected at either or both the 3 or 9 o clock position. Peri-urethral application of Urolastic might be considered in any patient with stress urinary incontinence. Its application is simple and may be performed in an accredited day surgery setting or as an outpatient procedure under local anesthesia. This makes it especially suited for patients with an unacceptable risk for major surgery due to co- morbidity. Compared to pubo-vaginal sling procedures, there is little risk of post- operative urinary retention. Patients who find the potential risk of post-operative self- catheterization unacceptable are also deemed good candidates for Urolastic injection therapy. 11 of 36
12 Product overview Product Urolastic is a two-component product supplied in a dual syringe, mixed in a static mixer and subsequently dispensed through an 18G needle. Urolastic is injected peri-urethrally in the mid urethral submucosal tissue using the appropriate Urolastic applicator head and dispenser gun. Injection of Urolastic creates increased tissue bulk and subsequent coaptation of the mid urethral sub mucosal tissue. Over time, collagen is deposited around the elastomeric material. The Urogyn applicator head has been designed with three openings to guide the needle and facilitate injections at different positions. Peri-urethral injection is chosen to prevent back flow of the material. Using the droplet technique, Urolastic is injected at the 2, 5, 7 and 10 o clock positions. Depending on the effect measured after 6 weeks, treatment can be repeated by injecting Urolastic next to the initial deposits. Under cystoscopic vision to prevent overcorrection, total amounts of up to 1.2 ml of Urolastic at both the 5 and 7 o clock positions and up to 1.0 ml at both the 2 and 10 o clock positions may be injected. This procedure can be performed either blind (using the applicator support) or under cystoscopic vision. If there is no, or a moderate, injection result, this may be due to a flawed injection technique or anatomic variations. The optimal position of a bolus of bulking agent is directly under the mucosa. Intra-mucosally injected material will lead to mucosa rupture and loss of the implant. Implants that are injected too deeply will disappear via tissue clefts or will not lead to any visible effect at the location of the urethral lumen. 12 of 36
13 Equipment - A dispenser gun for dosing material from the dual Urolastic syringe. (1) - One dual syringe with 2.5 ml of each component. (2) - Two static mixers with 18G needle attached. (3) - Applicator set, 3 pieces. (4) - Instructions For Use (IFU). (5) - Three patient labels. (6) - Applicator support (if required) 13 of 36
14 Auxiliary Materials - Local anesthetic (lidocaine 1% or similar) and syringe to inject. - A pair of tweezers. - A Foley catheter and inserting gel. - A ml disposable syringe. - Sterile water for irrigation ( ml). - Sterile gauzes. - Standard disinfectant solution. - Sterile cups. - Ruler. 14 of 36
15 Procedural preparations Setting The Urolastic procedure can be performed in an accredited day surgery setting. PRECAUTION: As with all day surgery procedures, appropriate equipment, medications, staff, and training should be present to handle possible emergency situations. Sterility Aseptic and sterile techniques are required during the Urolastic procedure. Considering the time required for preparation, the procedure and recovery, patients should expect to spend several hours at the facility. Staff The physician should be supported by a nurse familiar with the procedure. Pain and/or discomfort Patients should be reminded that a local anesthesia will be used during the procedure but that they may experience some pain or discomfort during or immediately after the procedure. Medication One dose of an oral antibiotic (Ciprofloxacin, 500 mg) and one dose of an oral NSAID should be given at the start of the procedure. For patients allergic to one of these drugs, other drugs may be selected. 15 of 36
16 Pre-operative procedures Prior to their treatment, patients must undergo a physical examination and be thoroughly evaluated to ensure proper patient selection. The patients urine must be checked to exclude urinary tract infection (UTI). Do not proceed if infection is present. Prepare for routine cystoscopy. Place the patient in lithotomy position but more horizontal than vertical to prevent pressure on the urethra. Make sure your own position allows for easy handling. Keep the urethra positioned at chest level. Disinfect according to local routine procedure. Use a Foley catheter to accurately measure the length of the urethra and select the correct applicator (see also 1.0 below). Place anesthetic gel inside the urethra, and/or inject 4 ml lidocaine 1% (or similar) bilaterally to the mucosa along the urethra using the following technique (5 10 minutes prior to the procedure): - Insert the needle at the 9 o clock position, direct it upwards to the 10 o clock position and deposit 1ml of the local anesthetic. - Withdraw the needle slightly and direct it downwards to the 8 o clock position and again deposit 1 ml of the anesthetic. - Repeat the procedure at the 3 o clock position, where the deposit will be close to the 2 and 4 o clock positions, respectively. 16 of 36
17 Peri operative procedure Urolastic is applied peri-urethrally through an 18G needle (under cystoscopic vision if preferred). Considering the initial procedure, the implant is situated at the 2, 5, 7 and 10 o clock positions. The urethra may be flushed during the procedure, e.g. to provide sufficient vision. 1.0 Use a Foley catheter to measure the length of the urethra. After estimating the length of the urethra, unpack the applicator heads Select the correct size for mid-urethral injection and remove the applicators that will not be used immediately. The applicator length of the applicator is marked on the front side: - 20 corresponds with an applicator length of 20mm and an injection depth of 40 mm - 30 corresponds with an applicator length of 30mm and an injection depth of 30 mm - 40 corresponds with an applicator length of 40mm and an injection depth of 20 mm Insert an unused needle in the applicator and hold it next to the Foley catheter to ensure the right depth of injection. NOTE It is important to ensure the right depth of injection to prevent material being injected too deeply or too superficially. 17 of 36
18 1.0.3 Slide the sheath or applicator support into the lumen of the selected applicator until it touches the transparent cap. NOTE It is important to slide the sheath up to the transparent cap to ensure proper needle alignment in relation to the endoscope Pull the lever to clamp the applicator to the sheath or applicator support 18 of 36
19 1.0.5 Remove the transparent cap by the twist-and-pull mechanism Ensure again, by inserting a needle through one of the injection ports, that the needle will end in the mid urethral section. 2.0 Peel open the blister pack and remove the Urolastic syringe from the package. Keep the cap on the tip of the syringe. Using the aseptic technique, remove the static mixer/ needle combination from the package. 3.0 Prepare the syringe according to the following instructions: Make sure the black plunger is fully retracted. 19 of 36
20 3.0.2 Pull out the white plunger for manual ejection from the syringe Peel open the gun blister and remove the gun from the package. Keep the cap on the tip of the syringe while placing the syringe, with the printed side up, into the dispenser gun. 20 of 36
21 3.0.4 Check if the toggle switch of the gun is in the 0.2 ml position (middle) Remove the cap from the Urolastic dual syringe and manipulate the gun to squeeze out a little bit of material (before attaching the static mixer). 21 of 36
22 3.0.6 Make sure both pistons are at the same height. Wipe the syringe off with a sterile cloth Attach the static mixer by firmly tightening the hub of the mixer onto the Urolastic dual syringe s lock tip. Check that the needle is tightly connected to the mixer and remove the needle s protective sleeve. 22 of 36
23 4.0 Prior to inserting the needle into the applicator head, carefully express the Urolastic material until visible at the tip of the needle. Start injecting within 2 minutes, before the increasing viscosity renders it too difficult. 5.0 Before starting the actual procedure the operator of the gun should re-check if the applicator used corresponds with the required depth of injection. 6.0 With the patient under local anesthesia, advance the sheath or applicator support with attached applicator into the urethra. Place the front end of the applicator head against the urethral meatus and rotate to allow injection at 2 o clock position. 7.0 The needle is then passed into the corresponding working channel of the applicator head. The needle is inserted peri-urethrally, on a small angle prefixed by the applicator head. Do not angle the sheath. Angling could lead to either a too deep or too superficial injection. Correct needle placement within the mucosa is checked by injecting a small amount of the implants. If the needle is properly placed, tissue bulking will immediately appear in the urethral mucosa at mid-urethral position. 8.0 Slowly inject 0.8 ml ml of Urolastic at the 2 o clock position. After injection is complete, wait approximately 30 seconds. Reject the needle from the tissue, but leave it inside the applicator. Then turn the applicator clockwise to allow for injection at the 5 o clock position. Eject 1.0 ml - to 1.2 ml and wait 30 seconds before withdrawing the needle. 9.0 Change hands (the gun in your left hand and the sheath or applicator support in your right hand) and rotate the applicator head counter-clockwise. Verify that the unused injection ports are at the 7 and 10 o clock position. Now inject 0.8 ml ml at the 10 o clock position and wait another 30 seconds before retracting the needle. Then inject 1.0 ml - 1.2ml at the remaining 7 o clock position, wait 30 seconds, and retract the needle If the cough test still shows some loss of urine, inject maximally 0.8 ml at either or both the 3 or 9 o clock position (using a new syringe) Depending on the patient s history of a previous surgical incontinence procedure (i.e. bladder neck suspension, sling procedures and morphology of the bladder neck and urethra), the Urolastic implantation sites and volumes may be adjusted to achieve optimal urethral coaption or closure Withdraw the needle prior to removing the sheath or applicator support from the urethra. Specific care must be taken not to introduce the sheath or applicator support again into the bladder, for this will compress the injected bolus. 23 of 36
24 13.0 Ask the patient to cough to verify sufficient effect has been obtained. Then remove any product that remains at the puncture holes, manually or using a pair of tweezers The bladder is emptied with a 12G Nelaton catheter. No indwelling catheter is to be left behind at any stage The patient is asked to stay in the hospital until she is able to void independently At the patient s initiative the physician will verify whether or not to decide for a repeated treatment in case of persistent leakage If retention occurs, the patient should be instructed to use an indwelling Nelaton catheter of max 12G. Retention could normally last up to 48 hours. If after one week catheterization is still required, it may be necessary to remove one (1) deposit on either the 5 or 7 o clock position. NOTE If during the procedure the patient perceives excessive pain, this can be an indication of a too deep injection of material. If this is the case the operator should stop the injection sequence and check that the depth of injection corresponds with the intended depth of injection. If there are any doubts the procedure should be aborted. NOTE If there is an excessive amount of blood loss or the forming of hematoma, this can be an indication of a too deep or wrong injection of material. If this is the case the operator should stop the injection sequence and check that the depth of injection corresponds with the intended depth of injection. If there are any doubts the procedure should be aborted. 24 of 36
25 Precautions Urolastic is to be administered solely by qualified physicians experienced in urological procedures. Improper patient selection, improper surgical implantation and/or overcorrection may result in unsatisfactory performance. Pre-operative microbiological cultures of urine must be performed to ensure the absence of urinary tract infection. Evaluate the condition of the tissue (e.g. hardness, edema, hematoma, atrophy) at the site of injection prior to treatment. Do not inject if the tissue is damaged. Patients receiving treatment interfering with blood coagulation have an increased risk of hematoma or urethral bleeding. Do not inject intravascularly. In the event of accidental contamination of the needle after assembly, discard the device. Do not use needles other than those supplied with the package. The cystoscope used should fit an Olympus A2213, Tian Song A1103.1, Shenda N4021 or equivalent sheath. The performance of a safe procedure relies on the use of the instruments and materials supplied by Urogyn BV. This product is for single use only. In theory, multi-use can increase the risk of cross-infection. In practice multi-use is not possible, since curing of the silicone material during application renders all productcontacted components unusable. Any co-packed instruments are supplied as single use disposables and are not considered to increase the risk of cross-infection through extra-corporal use. - Sterility is guaranteed in undamaged packages. - Do not use the package if it is damaged. - Do not re- sterilize. NOTE that (potentially) contaminated materials may be returned only following Urogyn s instructions, e.g. in a sealed container/ bag. Apply hospital procedures to safely dispose of contaminated material and components, e.g. used needles. 25 of 36
26 Case Report Form (CRF) For each patient, physicians must complete a Case Record Form (see Appendix D). This CRF covers the following: Identification Both the physician and the patient must be identified such that they can be traced. (NOTE on both pages) Screening Several pre-operative procedures are required. Tick all relevant boxes and provide comments before proceeding. Stamey incontinence grade (prior to treatment) Report the Stamey grade of the patient prior to the Urolastic treatment Implantation data Record the following data relevant to the procedure: - Date of procedure - Date of previous procedure (if repeated) - Positions and amount per injection - Additional comments Post-op verification Verification of the success rate immediately after the procedure, until the patient is discharged (generally a couple of hours after the procedure). This may include stress tests and bulk placement verification using ultrasonography. Stamey incontinence grade (after treatment) Report the Stamey grade of the patient after the Urolastic treatment Sign and complete CRF: Sign, complement and complete the CRF by declaring that the treatment was performed in accordance with guidelines. 26 of 36
27 Patient follow-up Patient information The patient should be informed about the intended use, expected results, contraindications, precautions, and potential adverse events. The patient should be advised that Urolastic may not give a permanent therapeutic result and that additional treatment sessions may be required to achieve and maintain the treatment s effect. Re-implantation procedures If a second implantation of additional material is required, re-implantation by injecting next to the initial deposits should not be carried out within six (6) weeks after the procedure. Following these instructions for use, the same procedure may be executed with the amounts of bulk material indicated, depending on the physician s observations. 27 of 36
28 Adverse events The following adverse events are commonly seen with bulking agent injections - urinary tract infection - urinary urgency - dysuria - acute retention ( 7 days) - non-acute urinary retention (> 7 days) - nausea, vomiting, diarrhea - genitourinary problems - dyspareunia, vaginal pain - hematuria - urinary frequency - outlet obstruction (slow, prolonged stream) - excreted bulking material Bladder inflammation may result from procedure handling. For this reason a prophylactic antibiotic (Ciprofloxacin) is recommended. Any known allergy to this drug should be investigated. Some bleeding may occur at the injection site and some difficulty may be experienced with voiding during the first days after the procedure as a consequence of the changed anatomical position of the urethra. A bruised feeling is common during the first days after the procedure Any side-effects or adverse events considered to be related to the product should be reported to the manufacturer or local retailer. 28 of 36
29 Appendix A The clinical effectiveness of urethral, nonabsorbable bulking agent in the treatment of recurrent stress urinary incontinence - 6 months of follow-up multicenter study. Dr. T. Rechberger (ICS 2013) Hypothesis / aims of study Midurethral slings (MUS) are currently the mainstay of surgical anti-incontinence therapy. Patients who experience MUS failures (despite the proper tape position at midurethra found during post-op ultrasound examination) are appropriate candidates for this highly effective and minimally invasive salvage therapy. Urethral bulking agents with specially designed injection devices are one of the minimally invasive options in the treatment of recurrent stress urinary incontinence (RSUI). There are few techniques as well as many different types of materials injected into the tissues surrounding female urethra. The primary aim of our study was to investigate mid-term (6 months) clinical effectiveness of non-absorbable periurethral bulking agent (polydimethylsiloxane (PDMS) polymer, tetrapropoxysilane cross-linking agent and titanium dioxide radio-pacifying agent - Urolastic - in the treatment of RSUI in females. The secondary aim was to investigate the safety as well as early and late complications profile of this procedure. Study design, materials and methods Between February 2012 and October patients with RSUI were treated with Urolastic (Urogyn BV, Nijmegen, Netherlands) in two tertiary gynecological clinics. The demographic patients data are given in Table 1. Urolastic was injected under local anesthesia with 1% Lignocaine according to the instructions given in the device manual at 10, 2, 4 and 8 o clock positions with 0.5 to 0.75 ccm per one spot. If the second injection was needed it was performed 6 weeks after primary procedure and Urolastic was injected only at 4 and 8 o clock with 0.75 ccm per spot. All injections were performed only by one investigator on each center (JD and KF). Immediately after the injection cough test was performed with 200 ccm. Routinely, ciprofloxacin 500 mg bid for 5 days in order to minimize the risk of infection was prescribed. Follow-up visits were scheduled two, six weeks and three and six months after primary procedure. Seventy three patients were available for 6 months follow-up. Efficacy of the procedure was assessed objectively on the follow-up visits. The outcome was considered as cured (no urine leakage), failure (urine leakage during increases of intraabdominal pressure, positive cough tests or pad test weight gain >1g) or improved (pad test weight gain <1g or subjective occasional urinary leakage). Statistical analyses were performed with Statistica package version 8.0 (StatSoft Inc., Tulsa, OK, USA). Results Objective success rate (cured and improved) was found in 52 patients (71,2%) 6 months after primary procedure. In 12 patients in whom primary injection was unsuccessful a second attempt was performed and marked improvement was observed in 6 of them. In 8 patients bladder outlet obstruction (BOO) was observed after injection requiring catheterization for 7 days. Four of them required partial removal of the Urolastic material after that period. In 4 other patients some material had to be removed due to its displacement under the urethra causing pain and dyspareunia. One patient experienced recurrent urinary tract infections and was admitted at urology department one year after procedure in order to remove the material from the bladder. Portion of Urolastic 5 cm in length was found incorporated in the bladder wall surface. No serious complications including hemorrhage, periurethral abscess or vaginal wall erosion were observed. Interpretation of results This multicenter study was designed to assess long term (1 year) efficacy of 29 of 36
30 Appendix A nonabsorbable periurethral bulking agent in the treatment of RSUI. After 6 months the results are very promising as more than 70% of patients are satisfied with the procedure efficacy. One should remember that this group include only patients previously treated with MUS in whom placing another sling in the periurethral area may neither be safe nor effective. This procedure is also safe as no serious complications occurred in the study group. Concluding message Although cure rates after MUS are up to 90% there is still place for less invasive treatment option like periurethral injection of bulking agents, especially in patients with RSUI without urethral hypermobility. Table 1. Patients demographic data and procedure outcome after 6 months. PARAMETER CURED AND IMPROVED FAILURES p value (n=53) (n=20) Age (years) NS Parity (n) NS BMI (kg/m2) NS 30 of 36
31 Appendix B Prospective one-year clinical evaluation of the efficacy and safety of Urolastic, a new bulking agent, for the treatment of stress urinary incontinence. Dr. J. Zajda (ICS 2013) Hypothesis / aims of study Although midurethral slings are currently the mainstay of surgery for SUI, some patients experience MUS failures. Injection of bulking agents as an alternative treatment for SUI has been attempted for years but so far results are disappointing. Every practitioner treating SUI feels that there is a need for satisfying bulking agents.the ideal bulking agent should be easily injectable under local anesthesia, permanent and maintain its shape, volume and therefore its mechanical effect. It should be hypoallergenic and non-immunogenic. We present the data on efficacy and safety of a new injectable, Urolastic in a prospective trial with one-year follow up. Primary objective was to evaluate the improvement of Stamey incontinence grade from baseline to 3 months post treatment. Afterwards the study was extended to one year. Secondary objectives were to evaluate other efficacy endpoints and to evaluate the frequency and severity of complications related to Urolastic for use as an injectable implant. Study design, materials and methods Twenty patients with SUI were enrolled in the study. All patients were determined eligible upon screening following the inclusion and exclusion criteria. Diagnosis was performed through gynecological examination, positive cough test with comfortable full bladder, 1- hour pad test and urodynamics when indicated. All patients signed the informed consent and the study was approved by the Ethics Committee. All patients were treated with Urolastic (Urogyn BV, Nijmegen, The Netherlands) under local anesthesia with 1% lidocaine. Urolastic is a proprietary LSR elastomer composition consisting of vinyldimethyl terminated polydimethylsiloxane (PDMS) polymer. It is presented in a pre-filled, sterile, 5 ml dual syringe with 2 x 2.5 ml, supplied with a static mixer that allows for adequate pre-mixing of the syringe contents. The product will remain flexible and adapt itself to the shape of the environment during injection, thus reducing the chances of migration. Urolastic is biocompatible and not biodegradable, resulting in a long-term effect. It is injected paraurethrally in the submucosal tissue at the midurethra. The bulk product was injected at the 6 o clock position and at the 2 and 10 o clock positions. After the injection a cough test was performed, with the bladder filled with 200 ml of saline solution. In case a repeated treatment was needed, it was performed 6 weeks after the primary procedure. Ciprofloxacin 500 mg was prescribed for 5 days. Study follow-up visits were scheduled at 6 weeks (n=20) 3 months (n=20) and one year (n=18) follow up. During these visits the efficacy of the procedure was assessed by means of a cough test in the supine and standing positions with a comfortably full bladder. The Stamey grade of incontinence was also assessed as well as a standard onehour pad test. A pad count was calculated at each visit as well as the number of incontinence episodes per 24 hours. Quality of life was assessed with the I-QoL. Complications were noted and treated. Results 20 women with a mean age of 56 years (sd 11,8) were treated. Three of them had had other surgical procedures for SUI. 65% Had one treatment session, 35% had two treatment sessions. The average volume of injected Urolastic was 2,1 ml after during the first treatment session and in addition 0,35 ml was extra injected during the second session. The Stamey incontinence grade significantly decreased from 1.9 at baseline to 0.75 (61%) at 6 weeks, to 0.2 (89% improvement) at 3 months and to 0,39 after one year (all 31 of 36
32 Appendix B p<0.001). The one hour pad test decreased for 20,2 g at baseline, to 5,5 g at 6 weeks; 1,6 g at 3 months and 8 g at one year (all p<0.001). The mean number of incontinence episodes per 24 hr went from 6 a baseline to 2,5 at 6 weeks, to 1,7 at 3 months and to 1,8 at one year FU. The development of number of pads used per 72 hours went from 17 to 5 to 7 at baseline, 6 weeks, 3 months and 12 months respectively (all p<0.001). The percentage of patients that were dry were 0% at baseline, 45% at 6 weeks, 80% at 3 months and 72% at one year (fig 1). The I-Qol score increased from 51, to 64, 82 and 76 at baseline, 6 weeks, 3 months and 12 months respectively (all p<0.001). Six patients (30%) had adverse events from the procedure. Side effects were rated moderate by patients. The occurred complications were: small hematoma after first injection in one patient urinary retention due to bladder outlet obstruction and urinary tract infection in three patients dyspareunia and vaginal pain in two patients. In the last two patients, the implant was removed from the 6 o clock position to provide comfort. Interpretation of results Our results after Urolastic injection indicate the product is a safe and effective novel bulking agent. Moderate complications occur but can be treated fairly easily. After one year 72% was still dry whereas the Stamey continence grade, one hour pad test, total pad count, number of incontinence episodes and QoL measures improved significantly. Concluding message Urolastic is a promising, safe and long lasting bulking agent for SUI. 32 of 36
33 Appendix C Safety of novel permanent injectable bulking agent Urolastic for refractory stress urinary incontinence in most demanding patients - results of phase IV one-center study. Dr M. Jozwik et al. (ICS 2013) Aims of study A well-known shortcoming of bulking agents for stress urinary incontinence (SUI) in women is their resorbable character [1]. On the top of that, one randomized controlled trial of autologous fat injection reported a case of fatality from pulmonary embolism [2]. In the present study, our objective was to prospectively evaluate short-term safety of a new non-resorbable injectable for otherwise hardly curable refractory SUI. Study design, materials and methods We studied 21 Caucasian women with urodynamically proven SUI, injected at 4 defined sites of the midurethra with the recently introduced Urolastic (extremely biocompatible rubbery compound) preparation according to the manufacturer s (Urogyn BV, Nijmegen, the Netherlands) instructions under local anesthesia. The application is not transurethral, but done parallelly to the urethal lumen. The exclusion criteria included: severe overactive bladder, urethral hypermobility, cystocele of any type and degree, post-void residual urine volume increased 100 ml, increased total bladder capacity 600 ml, and decreased detrusor contractility. The inclusion criteria were: history of recurrent SUI following at least 1 unsuccessful SUI surgery, and history of serious medical conditions, such as diabetes mellitus, unstable arterial hypertension, myocardial infarction, stroke, and grey matter involution. In other words, the recruited patients were women with medical contraindications for standard surgery, or for whom, in our setting, an artificial sphincter or no other procedure could be offered. Follow-ups were done at 6 weeks with a written questionnaire and pad test, when a possibility for an additional 2 injections for residual SUI was offered, and at 6 months. Results Median age was 64 (range: 36-80) years, mean follow-up: 4.2 ± 0.3 (1 SD) months. One patient had myocardial infarction 8 months before procedure, other two experienced mild stroke in the past, while some others had: morbid obesity (BMI of 47.4), chronic atrial fibrillation, chronic renal insufficiency, Parkinson s disease, severe asthma, emphysema, and active vertebral compression fracture at L2. The mean time required for the 4 primary injections was 22 ± 1.7 minutes. All patients underwent the procedure uneventfully and all were continent in cough test with full bladder immediately post-injection. No local hematomas were observed, but there were displacements of 3 deposits to the bladder or vagina, which needed removal. One submucosal paravaginal deposit was also removed because it was irritating the patient. One patient reported short-term discomfort with sitting. Only 1 woman had one-day acute retention of urine after primary procedure and another had transient urine stream narrowing after repeat procedure. All cases of relapse were early and in relation to excessive physical exertion or strenuous cough. Ten (47.6%) patients required and agreed to repeat injections (10 ± 1.2 minutes) with a low-volume deposit at 6 weeks, whilst 2 refused additional injections. At follow-up, none of the patients reported worsening of her urinary continence, 6 (28.6%) - had no improvement, 1 (4.8%) - a slight improvement, 3 (14.3%) - a moderate improvement, 4 (19.0%) - a significant improvement, and 7 (33.3%) were completely continent. Thus, some form of improvement was observed in over two thirds of the patients, whereas the combined rate for significant improvement or cure was 52.3%. 33 of 36
34 Appendix C Interpretation of results Injection therapy was considered useful as an option for short-term symptomatic relief in selected patients with comorbidities [1], but this review was written before the introduction of permanent injectable Urolastic. The above procedure was well tolerated in these mostly very ill patients, even as early as 8 months after myocardial infarction. It is a truly minimally invasive procedure with no metabolic impact. The newly created mechanism of continence is of delicate nature. Our work concurs with a recent Swiss study which reported marked improvement in incontinence after resorbable bulking therapy according to subjective and objective outcome measures in an elderly population [3]. Concluding message In spite of the limited number of observations, Urolastic injection can be considered as a viable option for medically compromised patients with recurrent SUI. All participants gave informed consent for the study which had been reviewed and approved by the local Ethics Committee. 1. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2012; 2: CD Periurethral autologous fat injection as treatment for female stress urinary incontinence: a randomized double-blind controlled trial. J Urol 2001; 165: Bulking agents: an analysis of 500 cases and review of the literature. Int Urogynecol J Pelvic Floor Dysfunct 2013; 24: of 36
35 Appendix D UROLASTIC CASE REPORT FORM Physician identification: Patient identification: Name: Subject Initials: Hospital: Date of birth: Country: Length (cm): Weight (kg): SCREENING Screening item (tick for checked): Physical examination Comments: X-Thorax Pad weight test 1 Test for urinary tract infection 1 Record amount of urine loss (g), volume (ml) voided after test, and any cystometry results before/after treatment. STAMEY INCONTINENCE GRADE Tick the box applicable to the Stamey Grade observed before treatment: IMPLANTATION DATA Date of procedure: Date of previous procedure (if repeated): Record for each position the amount of material implanted into the patient: Pos. 10 o clock: Pos. 2 o clock: Pos. 5 o clock: Pos. 7 o clock: ml ml ml ml Patient label: Urolastic CRF version date 27MAR2013 Page 1 of 2 35 of 36
36 Appendix D UROLASTIC CASE REPORT FORM Physician identification: Patient identification: Name: Subject Initials: Hospital: Date of birth: Country: Length (cm): Weight (kg): POST-OP VERIFICATION Perform stress test. Stress test: Normal Abnormal* * Description: Perform ultrasonography for bulk placement verification on day of treatment after bulk injections. Is the image: Normal Abnormal* * Description: The patient may be discharged when complete, residue free voiding is possible. Enter date of discharge: DD/MMM/YYYY Record any adverse events (including device deficiencies) and report to distributor/manufacturer. STAMEY INCONTINENCE GRADE Tick the box applicable to the Stamey Grade observed after treatment*: * If the procedure was unsuccessful, indicate follow up (e.g. repeat procedure):.. I am confident that the information supplied in this case report form is complete and accurate data. I confirm that the treatment was conducted in accordance with the Urolastic IFU. Signature of physician Date Urolastic CRF version date 27MAR2013 Page 2 of 2 36 of 36
THE BULKING AGENT WITH LONG-LASTING EFFECT!
 THE BULKING AGENT WITH LONG-LASTING EFFECT! Urogyn BV Transistorweg 5a 6534 AT Nijmegen The Netherlands t +31(0) 24 711 41 30 info@urogynbv.com www.urogynbv.com THE FIRST INJECTABLE IMPLANT THAT CAN COMPETE
THE BULKING AGENT WITH LONG-LASTING EFFECT! Urogyn BV Transistorweg 5a 6534 AT Nijmegen The Netherlands t +31(0) 24 711 41 30 info@urogynbv.com www.urogynbv.com THE FIRST INJECTABLE IMPLANT THAT CAN COMPETE
THE LONG-TERM SOLUTION FOR MALE INCONTINENCE
 THE LONG-TERM SOLUTION FOR MALE INCONTINENCE Urogyn BV Transistorweg 5a 6534 AT Nijmegen The Netherlands t +31(0) 24 711 41 30 info@urogynbv.com www.urogynbv.com THE FIRST INJECTABLE IMPLANT THAT CAN COMPETE
THE LONG-TERM SOLUTION FOR MALE INCONTINENCE Urogyn BV Transistorweg 5a 6534 AT Nijmegen The Netherlands t +31(0) 24 711 41 30 info@urogynbv.com www.urogynbv.com THE FIRST INJECTABLE IMPLANT THAT CAN COMPETE
This information is intended as an overview only
 This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
This information is intended as an overview only Please refer to the INSTRUCTIONS FOR USE included with this device for indications, contraindications, warnings, precautions and other important information
q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE
 493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
493495.q7:480499_P0 6/5/09 10:23 AM Page 1 WHAT YOU SHOULD KNOW ABOUT YOUR DIAGNOSIS OF STRESS URINARY INCONTINENCE 493495.q7:480499_P0 6/5/09 10:23 AM Page 2 What is Stress Urinary Incontinence? Urinary
BULKAMID STANDARD OPERATING PROCEDURE
 BULKAMID STANDARD OPERATING PROCEDURE Contents 1 1 2 3-4 5-6 5-6 7 9-12 9 10 11 12 13 13 13 15 16 Products Bulkamid multiple use instruments Bulkamid single use instruments The Bulkamid system Pre-procedure
BULKAMID STANDARD OPERATING PROCEDURE Contents 1 1 2 3-4 5-6 5-6 7 9-12 9 10 11 12 13 13 13 15 16 Products Bulkamid multiple use instruments Bulkamid single use instruments The Bulkamid system Pre-procedure
THE WOMAN-FRIENDLY STERILIZATION METHOD
 THE WOMAN-FRIENDLY STERILIZATION METHOD Urogyn BV Transistorweg 5a 6534 AT Nijmegen The Netherlands t +31(0) 24 711 41 30 info@urogynbv.com www.urogynbv.com THE MOST WOMAN-FRIENDLY STERILIZATION METHOD
THE WOMAN-FRIENDLY STERILIZATION METHOD Urogyn BV Transistorweg 5a 6534 AT Nijmegen The Netherlands t +31(0) 24 711 41 30 info@urogynbv.com www.urogynbv.com THE MOST WOMAN-FRIENDLY STERILIZATION METHOD
Blue Ridge Urogynecology
 Surgery for Stress Urinary Incontinence Surgery has proved to be a very effective treatment for stress incontinence. The best surgical procedures improve or cure the incontinence in 85 to 90 percent of
Surgery for Stress Urinary Incontinence Surgery has proved to be a very effective treatment for stress incontinence. The best surgical procedures improve or cure the incontinence in 85 to 90 percent of
Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566
 Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
MACROPLASTIQUE IMPLANTS
 UROPLASTY, INC. MACROPLASTIQUE IMPLANTS INSTRUCTIONS FOR USE UROPLASTY, INC. MACROPLASTIQUE IMPLANTS INSTRUCTIONS FOR USE CONTRAINDICATIONS FOR USE Macroplastique must not be used in patients with: Acute
UROPLASTY, INC. MACROPLASTIQUE IMPLANTS INSTRUCTIONS FOR USE UROPLASTY, INC. MACROPLASTIQUE IMPLANTS INSTRUCTIONS FOR USE CONTRAINDICATIONS FOR USE Macroplastique must not be used in patients with: Acute
Desara TV and Desara Blue TV
 Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
Desara TV and Desara Blue TV Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide Available Electronically M Manufactured
A PATIENT GUIDE TO Understanding Stress Urinary Incontinence
 A PATIENT GUIDE TO Understanding Stress Urinary Incontinence Q: What is SUI? A: Stress urinary incontinence is defined as the involuntary leakage of urine. The problem afflicts approximately 18 million
A PATIENT GUIDE TO Understanding Stress Urinary Incontinence Q: What is SUI? A: Stress urinary incontinence is defined as the involuntary leakage of urine. The problem afflicts approximately 18 million
Bard: Continence Therapy. Stress Urinary Incontinence. Regaining Control. Restoring Your Lifestyle.
 Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Urinary incontinence is a common problem and one that can be resolved by working
Bard: Continence Therapy Stress Urinary Incontinence Regaining Control. Restoring Your Lifestyle. Stress Urinary Incontinence Urinary incontinence is a common problem and one that can be resolved by working
Women s & Children s Directorate The TVT Operation - a guide for patients
 Women s & Children s Directorate The TVT Operation - a guide for patients This leaflet was written for women who are considering having a TVT operation. If you have any questions that aren't answered by
Women s & Children s Directorate The TVT Operation - a guide for patients This leaflet was written for women who are considering having a TVT operation. If you have any questions that aren't answered by
What you should know about your diagnosis of incontinence
 What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
What you should know about your diagnosis of incontinence What is Stress Urinary Incontinence? WHAT IS NORMAL URINARY FUNCTION? Urine is a normal waste product of the body that is manufactured by the kidneys
Pelvic Prolapse. A Patient Guide to Pelvic Floor Reconstruction
 Pelvic Prolapse A Patient Guide to Pelvic Floor Reconstruction Pelvic Prolapse When an organ becomes displaced, or slips down in the body, it is referred to as a prolapse. Your physician has diagnosed
Pelvic Prolapse A Patient Guide to Pelvic Floor Reconstruction Pelvic Prolapse When an organ becomes displaced, or slips down in the body, it is referred to as a prolapse. Your physician has diagnosed
Stress Urinary Incontinence in Women. What YOU can do about it...
 Stress Urinary Incontinence in Women What YOU can do about it... www.gynecare.com Stress Urinary Incontinence in Women: It's Common. It's Treatable. Would it surprise you... To learn that more than 13
Stress Urinary Incontinence in Women What YOU can do about it... www.gynecare.com Stress Urinary Incontinence in Women: It's Common. It's Treatable. Would it surprise you... To learn that more than 13
I-STOP TOMS Transobturator Male Sling
 I-STOP TOMS Transobturator Male Sling The CL Medical I-STOP TOMS sling for male stress urinary incontinence was developed in France where it is widely used and is the market leader. It is constructed with
I-STOP TOMS Transobturator Male Sling The CL Medical I-STOP TOMS sling for male stress urinary incontinence was developed in France where it is widely used and is the market leader. It is constructed with
Incontinence. Anatomy The human body has two kidneys. The kidneys continuously filter the blood and make urine.
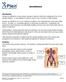 Incontinence Introduction Urinary incontinence occurs when a person cannot control the emptying of his or her urinary bladder. It can happen to anyone, but is very common in older people. Urinary incontinence
Incontinence Introduction Urinary incontinence occurs when a person cannot control the emptying of his or her urinary bladder. It can happen to anyone, but is very common in older people. Urinary incontinence
Urethral Bulking to treat Stress Urinary Incontinence. Patient Information Leaflet
 Urethral Bulking to treat Stress Urinary Incontinence Patient Information Leaflet About this leaflet The information provided in this leaflet should be used as a guide. There may be some variation in how
Urethral Bulking to treat Stress Urinary Incontinence Patient Information Leaflet About this leaflet The information provided in this leaflet should be used as a guide. There may be some variation in how
Surgery for stress incontinence:
 Surgery for stress incontinence: information for you aashara Published February 2005 by the RCOG Contents Key points About this information What is stress incontinence? Do I need an operation? What operation
Surgery for stress incontinence: information for you aashara Published February 2005 by the RCOG Contents Key points About this information What is stress incontinence? Do I need an operation? What operation
Clean Intermittent Self-Catheterisation (CISC)
 Saint Mary s Hospital & Trafford General Hospital Uro-gynaecology Service Information for Patients Clean Intermittent Self-Catheterisation (CISC) What is catheterisation? Catheterisation involves passing
Saint Mary s Hospital & Trafford General Hospital Uro-gynaecology Service Information for Patients Clean Intermittent Self-Catheterisation (CISC) What is catheterisation? Catheterisation involves passing
Management of Female Stress Incontinence
 Management of Female Stress Incontinence Dr. Arvind Goyal Associate Professor (Urology& Renal Transplant) Dayanand Medical College & Hospital, Ludhiana, Punjab, India Stress Incontinence Involuntary loss
Management of Female Stress Incontinence Dr. Arvind Goyal Associate Professor (Urology& Renal Transplant) Dayanand Medical College & Hospital, Ludhiana, Punjab, India Stress Incontinence Involuntary loss
Glossary of terms Urinary Incontinence
 Patient Information English Glossary of terms Urinary Incontinence Anaesthesia (general, spinal, or local) Before a procedure you will get medication to make sure that you don t feel pain. Under general
Patient Information English Glossary of terms Urinary Incontinence Anaesthesia (general, spinal, or local) Before a procedure you will get medication to make sure that you don t feel pain. Under general
If viewing a printed copy of this policy, please note it could be expired. Got to to view current policies.
 If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
If viewing a printed copy of this policy, please note it could be expired. Got to www.fairview.org/fhipolicies to view current policies. Department Policy Code: D: PC-5555 Entity: Fairview Pharmacy Services
3. Urinary Catheters. Indications. Methods of Bladder Catheterization. Hashim Hashim
 3. Urinary Catheters Hashim Hashim Indications Urinary catheters are used to drain urine from the bladder. The main indications are: A. Diagnostic Measure post-void residual in the absence of ultrasound
3. Urinary Catheters Hashim Hashim Indications Urinary catheters are used to drain urine from the bladder. The main indications are: A. Diagnostic Measure post-void residual in the absence of ultrasound
Male and Female Catheterisation
 Male and Female Catheterisation Practical Skills Teaching Year 3 Medical Students MB BCh 2012-2013 Contents Introduction to workshop... 3 Overall Session Aim... 4 Intended Learning Outcomes... 4 Workshop
Male and Female Catheterisation Practical Skills Teaching Year 3 Medical Students MB BCh 2012-2013 Contents Introduction to workshop... 3 Overall Session Aim... 4 Intended Learning Outcomes... 4 Workshop
Aus Artificial Uretheral Sphincter Port System
 NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
NORFOLK VET PRODUCTS the AUS for the long-term relief of incontinence in dogs and cats making life easier for pets Speciality Medical Devices For The Veterinary Community the Aus Artificial Uretheral Sphincter
Bulkamid. Patient Information. Obstetrics & Gynaecology Department
 Bulkamid Patient Information Obstetrics & Gynaecology Department Author ID: JD Leaflet Number: Gyn 050 Version: 5 Name of Leaflet: Bulkamid Date Produced: November 2017 Review Date: November 2019 Bulkamid
Bulkamid Patient Information Obstetrics & Gynaecology Department Author ID: JD Leaflet Number: Gyn 050 Version: 5 Name of Leaflet: Bulkamid Date Produced: November 2017 Review Date: November 2019 Bulkamid
Evidence Summary: The inflow Urinary Prosthesis FDA De Novo Approval DEN130044
 Evidence Summary: The inflow Urinary Prosthesis FDA De Novo Approval DEN130044 Unique alternative to urinary catheters for women with permanent urinary retention Therapeutic benefits o Highly effective
Evidence Summary: The inflow Urinary Prosthesis FDA De Novo Approval DEN130044 Unique alternative to urinary catheters for women with permanent urinary retention Therapeutic benefits o Highly effective
Tools for Evaluation. Urodynamics Case Studies. Case 1. Evaluation. Case 1. Bladder Diary SUI 19/01/2018
 Urodynamics Case Studies Christopher K. Payne, MD Vista Urology & Pelvic Pain Partners Emeritus Professor of Urology, Stanford University Tools for Evaluation Ears, Eyes, and Brain Bladder diary Stress
Urodynamics Case Studies Christopher K. Payne, MD Vista Urology & Pelvic Pain Partners Emeritus Professor of Urology, Stanford University Tools for Evaluation Ears, Eyes, and Brain Bladder diary Stress
Home Health Foundation, Inc. To create more permanent IV access for patients undergoing long term IV therapy.
 PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
PROCEDURE ORIGINAL DATE: 06/99 Revised Date: 09/02 Home Health Foundation, Inc. SUBJECT: PURPOSE: MIDLINE CATHETER INSERTION To create more permanent IV access for patients undergoing long term IV therapy.
Instruction For Use for All Silicon Foley Catheter
 General Description: All Silicone Foley Catheter for single use is a thin, is a flexible tube passed through the urethra and into the bladder to drain urine. It is the most common type of indwelling urinary
General Description: All Silicone Foley Catheter for single use is a thin, is a flexible tube passed through the urethra and into the bladder to drain urine. It is the most common type of indwelling urinary
Loss of Bladder Control
 BLADDER HEALTH Loss of Bladder Control SURGERY TO TREAT URINARY INCONTINENCE AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION What Is Urinary Incontinence? Urinary incontinence
BLADDER HEALTH Loss of Bladder Control SURGERY TO TREAT URINARY INCONTINENCE AUA FOUNDATION OFFICIAL FOUNDATION OF THE AMERICAN UROLOGICAL ASSOCIATION What Is Urinary Incontinence? Urinary incontinence
Female Urinary Incontinence: What It Is and What You Can Do About It
 Female Urinary Incontinence: What It Is and What You Can Do About It Urogynecology Patient Information Sheet What is Urinary Incontinence? Stress Incontinence is a leakage of urine that occurs, for example,
Female Urinary Incontinence: What It Is and What You Can Do About It Urogynecology Patient Information Sheet What is Urinary Incontinence? Stress Incontinence is a leakage of urine that occurs, for example,
A over-the-counter medical device that utilizes muscle stimulation for the treatment of stress, urge and mixed urinary incontinence.
 A over-the-counter medical device that utilizes muscle stimulation for the treatment of stress, urge and mixed urinary incontinence. An over-the-counter medical device that utilizes muscle stimulation
A over-the-counter medical device that utilizes muscle stimulation for the treatment of stress, urge and mixed urinary incontinence. An over-the-counter medical device that utilizes muscle stimulation
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA. A Minimally Invasive Innovative Treatment
 PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
PROSTATIC ARTERY EMBOLISATION (PAE) FOR BENIGN PROSTATIC HYPERPLASIA A Minimally Invasive Innovative Treatment What is the prostate? The prostate is an accessory organ of the male reproductive system.
Procedure for removal and reinsertion of an indwelling urethral catheter (female)
 Procedure for removal and reinsertion of an indwelling urethral catheter (female) Refer to National Infection Prevention and Control Manual for information on aseptic technique/cleaning equipment. Equipment
Procedure for removal and reinsertion of an indwelling urethral catheter (female) Refer to National Infection Prevention and Control Manual for information on aseptic technique/cleaning equipment. Equipment
SOP: Urinary Catheter in Dogs and Cats
 SOP: Urinary Catheter in Dogs and Cats These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
SOP: Urinary Catheter in Dogs and Cats These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during
Intermittent self-catheterisation (ISC) Information for patients Spinal Injuries
 Intermittent self-catheterisation (ISC) Information for patients Spinal Injuries page 2 of 12 What is clean intermittent self-catheterisation (CISC)? Urinary catheterisation is a procedure used to drain
Intermittent self-catheterisation (ISC) Information for patients Spinal Injuries page 2 of 12 What is clean intermittent self-catheterisation (CISC)? Urinary catheterisation is a procedure used to drain
Urogynecology Associates of Philadelphia URODYNAMIC TESTING
 URODYNAMIC TESTING Urogynecology Associates of Philadelphia Most women with urinary incontinence will need to complete a few simple tests, performed in the office, to help your doctor assess your symptoms
URODYNAMIC TESTING Urogynecology Associates of Philadelphia Most women with urinary incontinence will need to complete a few simple tests, performed in the office, to help your doctor assess your symptoms
Stratis INSTRUCTIONS FOR USE. Needle-free Injection System. 0.5mL volume (+/-5%)
 Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stratis Needle-free Injection System INSTRUCTIONS FOR USE 0.5mL volume (+/-5%) Stratis English Symbols Glossary (Note: All symbols are derived from ISO 15223-1, Medical Devices - Symbols to be used with
Stress Incontinence. Susannah Elvy Urogynaecology CNS
 Stress Incontinence Susannah Elvy Urogynaecology CNS Definitions Prevalence Assessment Investigation Treatment Surgery Men International Continence Society define as the complaint of any involuntary leakage
Stress Incontinence Susannah Elvy Urogynaecology CNS Definitions Prevalence Assessment Investigation Treatment Surgery Men International Continence Society define as the complaint of any involuntary leakage
limbsandthings.com Advanced Female Catheterisation Trainer User Guide For more skills training products visit Limbs & Things Ltd.
 Advanced Female Catheterisation Trainer Product No: 60155 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Advanced Female Catheterisation Trainer Product No: 60155 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Suprapubic Catheter Insertion Clinic
 Suprapubic Catheter Insertion Clinic Exceptional healthcare, personally delivered Suprapubic Catheter Why may I need a suprapubic catheter? A suprapubic catheter enters the bladder through an incision
Suprapubic Catheter Insertion Clinic Exceptional healthcare, personally delivered Suprapubic Catheter Why may I need a suprapubic catheter? A suprapubic catheter enters the bladder through an incision
Urogynecology ICD-9 to ICD-10 Crosswalks
 1100 Wayne Ave, Suite 825 Silver Spring, MD 20910 301.273.0570 Fax 301.273.0778 info@augs.org www.augs.org Urogynecology ICD-9 to ICD-10 Crosswalks ICD 9 ICD 9 Description ICD 10 Code ICD 10 Description
1100 Wayne Ave, Suite 825 Silver Spring, MD 20910 301.273.0570 Fax 301.273.0778 info@augs.org www.augs.org Urogynecology ICD-9 to ICD-10 Crosswalks ICD 9 ICD 9 Description ICD 10 Code ICD 10 Description
Procedure for removal and reinsertion of a supra pubic catheter
 Procedure for removal and reinsertion of a supra pubic catheter Equipment required collect prior to procedure Perform this procedure as an aseptic technique to minimise the risk of introducing Clean the
Procedure for removal and reinsertion of a supra pubic catheter Equipment required collect prior to procedure Perform this procedure as an aseptic technique to minimise the risk of introducing Clean the
Advanced Care for Female Overactive Bladder & Urinary Incontinence. Department of Urology Kaiser Permanente Santa Rosa
 Advanced Care for Female Overactive Bladder & Urinary Incontinence Department of Urology Kaiser Permanente Santa Rosa Goals Participants will: Review normal urinary tract anatomy and function Understand
Advanced Care for Female Overactive Bladder & Urinary Incontinence Department of Urology Kaiser Permanente Santa Rosa Goals Participants will: Review normal urinary tract anatomy and function Understand
Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline.
 Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. TARGET POPULATION Eligibility Decidable (Y or N) Inclusion Criterion non-neurogenic OAB Exclusion Criterion
Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. TARGET POPULATION Eligibility Decidable (Y or N) Inclusion Criterion non-neurogenic OAB Exclusion Criterion
Title: EZ-IO. Effective Date: January SOG Number: EMS Rescinds:
 S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
S O G Title: EZ-IO Effective Date: January 2010 SOG Number: EMS - 25 Rescinds: Scope: Providers Authorized are AIC s in the following certifications EMT-I and EMT-P who have been trained and cleared by
Peel-Apart Percutaneous Introducer Kits for
 Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Bard Access Systems Peel-Apart Percutaneous Introducer Kits for Table of Contents Contents Page Bard Implanted Ports Hickman*, Leonard*, Broviac*, Tenckhoff*, and Groshong* Catheters Introduction....................................
Various Types. Ralph Boling, DO, FACOG
 Various Types Ralph Boling, DO, FACOG The goal of this lecture is to increase assessment and treatment abilities for physicians managing urinary incontinence (UI) patients. 1. Effectively communicate with
Various Types Ralph Boling, DO, FACOG The goal of this lecture is to increase assessment and treatment abilities for physicians managing urinary incontinence (UI) patients. 1. Effectively communicate with
Urinary Catheters. Prevalence of Infections
 Urinary Catheterisation Urinary catheterisation is defined as an intervention to enable the emptying of the bladder by insertion of a catheter. Catheters can be short term less than 28 days or long term
Urinary Catheterisation Urinary catheterisation is defined as an intervention to enable the emptying of the bladder by insertion of a catheter. Catheters can be short term less than 28 days or long term
URINARY INCONTINENCE
 Center for Continence Care and Pelvic Medicine What is urinary incontinence? URINARY INCONTINENCE Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only
Center for Continence Care and Pelvic Medicine What is urinary incontinence? URINARY INCONTINENCE Urinary incontinence is the uncontrollable loss of urine. The amount of urine leaked can vary from only
STANDARDIZED PROCEDURE REPROGRAMMING AND REFILLING INTRATHECAL BACLOFEN PUMPS and ACCESSING THE CATHETER ACCESS PORT (Adult,Peds)
 I. Definition The purpose of this procedure is to allow the Advanced Health Practitioner (AHP) to reprogram and refill intrathecal Baclofen pumps, as well as access the catheter access port for those AHPs
I. Definition The purpose of this procedure is to allow the Advanced Health Practitioner (AHP) to reprogram and refill intrathecal Baclofen pumps, as well as access the catheter access port for those AHPs
Desara and Desara Blue
 Desara and Desara Blue Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc.
Desara and Desara Blue Sling for Female Stress Urinary Incontinence Instructions For Use D I Prescription Use only Do not reuse Sterilized using ethylene oxide M Manufactured by: Caldera Medical, Inc.
Surgical treatment of urinary stress incontinence with tension free vaginal tape
 Surgical treatment of urinary stress incontinence with tension free vaginal tape Gynaecology department 01935 384 385 yeovilhospital.nhs.uk Many surgical operations are available for the treatment of
Surgical treatment of urinary stress incontinence with tension free vaginal tape Gynaecology department 01935 384 385 yeovilhospital.nhs.uk Many surgical operations are available for the treatment of
FreshRN Podcast Season 4, Episode 6. All Things Urinary Catheters
 FreshRN Podcast Season 4, Episode 6 All Things Urinary Catheters Key Focus: Catheters can lead to infections, which can be fatal. CAUTI - Catheter Associated Urinary Tract Infection CAUTI is a type of
FreshRN Podcast Season 4, Episode 6 All Things Urinary Catheters Key Focus: Catheters can lead to infections, which can be fatal. CAUTI - Catheter Associated Urinary Tract Infection CAUTI is a type of
Vaginal Tapes to Treat Stress Incontinence. Patient Information
 Vaginal Tapes to Treat Stress Incontinence Patient Information Author ID: JD Leaflet Number: Gyn 044 Version: 6 Name of Leaflet: Vaginal Tapes to Treat Stress Incontinence Date Produced: May 2017 Review
Vaginal Tapes to Treat Stress Incontinence Patient Information Author ID: JD Leaflet Number: Gyn 044 Version: 6 Name of Leaflet: Vaginal Tapes to Treat Stress Incontinence Date Produced: May 2017 Review
Education for Self Administration of Intravenous Therapy HOME IV THERAPY PICC. Portacath
 HOME IV THERAPY PICC Portacath Who To contact Cardio-Respiratory Integrated Specialist Services (CRISS) Office hours 0800 1630 hours Ph: 364 0167 Weekends and after hours, phone Christchurch Hospital operator
HOME IV THERAPY PICC Portacath Who To contact Cardio-Respiratory Integrated Specialist Services (CRISS) Office hours 0800 1630 hours Ph: 364 0167 Weekends and after hours, phone Christchurch Hospital operator
ATI Skills Modules Checklist for Central Venous Access Devices
 For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
For faculty use only Educator s name Score Date ATI Skills Modules Checklist for Central Venous Access Devices Student s name Date Verify order Patient record Assess for procedure need Identify, gather,
Sep \8958 Appell Dmochowski.ppt LMF 1
 Surgical Outcomes (How did we get ourselves into this mess?) Roger R. Dmochowski, MD, FACS Department of Urologic Surgery Vanderbilt University School of Medicine Nashville, Tennessee Considerations Evaluation
Surgical Outcomes (How did we get ourselves into this mess?) Roger R. Dmochowski, MD, FACS Department of Urologic Surgery Vanderbilt University School of Medicine Nashville, Tennessee Considerations Evaluation
Intermittent Self-Catheterization
 Intermittent Self-ization Create Medic Co.,Ltd. Marketing Department Suzuki Masaru If the bodily function emptying bladder, a physiological phenomenon in daily life, is impaired due to some kind of disease,
Intermittent Self-ization Create Medic Co.,Ltd. Marketing Department Suzuki Masaru If the bodily function emptying bladder, a physiological phenomenon in daily life, is impaired due to some kind of disease,
Loss of Bladder Control
 BLADDER HEALTH: Surgery for Urinary Incontinence Loss of Bladder Control Surgery for Urinary Incontinence Don t Let Urinary Incontinence Keep You from Enjoying Life. What is Urinary Incontinence? What
BLADDER HEALTH: Surgery for Urinary Incontinence Loss of Bladder Control Surgery for Urinary Incontinence Don t Let Urinary Incontinence Keep You from Enjoying Life. What is Urinary Incontinence? What
CATHETER ACCESS KIT. For use with Prometra Programmable Infusion Systems
 CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
CATHETER ACCESS KIT Caution: Federal (USA) Law restricts this device to sale by or on the order of a physician. Table of Contents Contents... 3 Description... 3 Indications... 3 Contraindications... 3
Rezūm procedure for the Prostate
 Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Rezūm procedure for the Prostate Mr Jas Kalsi Consultant Urological Surgeon This booklet has been provided to help answer the questions you may have with regards to your enlarged prostate and the Rezūm
Directions For Use. All directions should be read before use
 Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Directions For Use All directions should be read before use DEVICE DESCRIPTION: The CLEANER.XT Rotational Thrombectomy System is a percutaneous, 6Fr catheter based system (single piece construction) that
Prostate surgery. What is the prostate? What is a TURP? Why is a TURP operation necessary? Deciding to have a TURP operation.
 What is the prostate? The prostate is a gland about the size of a walnut that is only present in men. It is located just below the bladder and surrounds the urethra, the tube through which urine flows
What is the prostate? The prostate is a gland about the size of a walnut that is only present in men. It is located just below the bladder and surrounds the urethra, the tube through which urine flows
A Simple Solution. to a Common Problem. Find relief from sudden, unplanned urine leakage.
 A Simple Solution to a Common Problem Find relief from sudden, unplanned urine leakage. A Common Problem Millions of women suffer from stress urinary incontinence (SUI). This condition results in accidental
A Simple Solution to a Common Problem Find relief from sudden, unplanned urine leakage. A Common Problem Millions of women suffer from stress urinary incontinence (SUI). This condition results in accidental
Tension-free Vaginal Tape (TVT)
 Page 1 of 7 Tension-free Vaginal Tape (TVT) Introduction This leaflet will provide you with basic information about the Tension--free Vaginal Tape (TVT) procedure. What is a TVT? TVT is an operation to
Page 1 of 7 Tension-free Vaginal Tape (TVT) Introduction This leaflet will provide you with basic information about the Tension--free Vaginal Tape (TVT) procedure. What is a TVT? TVT is an operation to
In addition to the indications stated above catheterisation may be carried out in female patients for two further reasons:
 Urinary Catheterisation This is the process of inserting a specially designed tube into the urinary bladder using an aseptic technique, for the purpose of draining urine, removing clots and/or debris and
Urinary Catheterisation This is the process of inserting a specially designed tube into the urinary bladder using an aseptic technique, for the purpose of draining urine, removing clots and/or debris and
National Kidney and Urologic Diseases Information Clearinghouse
 Urodynamic Testing National Kidney and Urologic Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is the urinary tract? The urinary tract
Urodynamic Testing National Kidney and Urologic Diseases Information Clearinghouse U.S. Department of Health and Human Services NATIONAL INSTITUTES OF HEALTH What is the urinary tract? The urinary tract
Achieving Independence
 Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization Achieving Independence Introduction This brochure is provided by Bard, a leading provider of urology products since 1907. The best
Bard: Intermittent Self-Catheterization A Guide to Self-Catheterization Achieving Independence Introduction This brochure is provided by Bard, a leading provider of urology products since 1907. The best
You have been booked for a. Flexible Cystoscopy. Under Local Anaesthetic
 You have been booked for a Flexible Cystoscopy Under Local Anaesthetic 1 WHAT IS A FLEXIBLE CYSTOSCOPY A flexible cystoscopy is a test to examine the uretha (waterpipe) and bladder using a thin, lighted
You have been booked for a Flexible Cystoscopy Under Local Anaesthetic 1 WHAT IS A FLEXIBLE CYSTOSCOPY A flexible cystoscopy is a test to examine the uretha (waterpipe) and bladder using a thin, lighted
Suprapubic Catheter Insertion Clinic
 Suprapubic Catheter Insertion Clinic Exceptional healthcare, personally delivered Suprapubic Catheter A urinary catheter is a tube used to drain urine from the bladder. The commonest catheters are ones
Suprapubic Catheter Insertion Clinic Exceptional healthcare, personally delivered Suprapubic Catheter A urinary catheter is a tube used to drain urine from the bladder. The commonest catheters are ones
Voiding Diary. Begin recording upon rising in the morning and continue for a full 24 hours.
 Urodvnamics Your physician has scheduled you for a test called URODYNAMICS. This test is a series of different measurements of bladder function and can be used to determine the cause of a variety of bladder
Urodvnamics Your physician has scheduled you for a test called URODYNAMICS. This test is a series of different measurements of bladder function and can be used to determine the cause of a variety of bladder
limbsandthings.com Advanced Catheterisation Trainer User Guide For more skills training products visit Limbs & Things Ltd.
 Advanced Catheterisation Trainer Product No: 60150 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
Advanced Catheterisation Trainer Product No: 60150 User Guide For more skills training products visit limbsandthings.com Limbs & Things Ltd. Sussex Street, St Philips Bristol, BS2 0RA, UK sales@limbsandthings.com
The Urinary System. 1. Define important words in this chapter. 2. Explain the structure and function of the urinary system
 109 16 The Urinary System 1. Define important words in this chapter 2. Explain the structure and function of the urinary system 3. Discuss changes in the urinary system due to aging 4. List normal qualities
109 16 The Urinary System 1. Define important words in this chapter 2. Explain the structure and function of the urinary system 3. Discuss changes in the urinary system due to aging 4. List normal qualities
Patient Information. Tension Free Vaginal/ Obturator Tape (TVT) Royal Devon and Exeter NHS Foundation Trust
 Tension Free Vaginal/Obturator Tape (TVT) Royal Devon and Exeter NHS Foundation Trust Patient Information Tension Free Vaginal/ Obturator Tape (TVT) Reference Number: CW 08 011 003 (version date: September
Tension Free Vaginal/Obturator Tape (TVT) Royal Devon and Exeter NHS Foundation Trust Patient Information Tension Free Vaginal/ Obturator Tape (TVT) Reference Number: CW 08 011 003 (version date: September
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence in women: the management of urinary incontinence in women 1.1 Short title Urinary incontinence in women
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Urinary incontinence in women: the management of urinary incontinence in women 1.1 Short title Urinary incontinence in women
LESSON ASSIGNMENT. Urinary System Diseases/Disorders. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 4 Urinary System Diseases/Disorders LESSON ASSIGNMENT Paragraphs 4-1 through 4-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Identify the purposes
LESSON ASSIGNMENT LESSON 4 Urinary System Diseases/Disorders LESSON ASSIGNMENT Paragraphs 4-1 through 4-8. LESSON OBJECTIVES After completing this lesson, you should be able to: 4-1. Identify the purposes
AdVance Male Sling System
 AdVance Male Sling System Clinical study summary This document is a compilation and summary of several AdVance Male Sling System peer-reviewed journal articles. The information presented here is taken
AdVance Male Sling System Clinical study summary This document is a compilation and summary of several AdVance Male Sling System peer-reviewed journal articles. The information presented here is taken
A minimally invasive treatment for stress urinary incontinence
 A minimally invasive treatment for stress urinary incontinence This booklet provides information about the Bulkamid hydrogel for the treatment of stress urinary incontinence What is stress urinary incontinence?
A minimally invasive treatment for stress urinary incontinence This booklet provides information about the Bulkamid hydrogel for the treatment of stress urinary incontinence What is stress urinary incontinence?
6 Page Male Incontinence Booklet 10/09/ :44 Page 1. The Natural Non-Surgical Option for Male Urinary Incontinence
 6 Page Male Incontinence Booklet /9/ :44 Page The Natural Non-Surgical Option for Male Urinary Incontinence 6 Page Male Incontinence Booklet /9/ :44 Page Stress Urinary Incontinence - A Stressful Prospect
6 Page Male Incontinence Booklet /9/ :44 Page The Natural Non-Surgical Option for Male Urinary Incontinence 6 Page Male Incontinence Booklet /9/ :44 Page Stress Urinary Incontinence - A Stressful Prospect
Management of Post-Prostatectomy Urinary Incontinence and Sexual Dysfunction
 Management of Post-Prostatectomy Urinary Incontinence and Sexual Dysfunction Robert C. Eyre, MD, FACS Associate Clinical Professor of Surgery (Urology) Harvard Medical School Post-prostatectomy Incontinence
Management of Post-Prostatectomy Urinary Incontinence and Sexual Dysfunction Robert C. Eyre, MD, FACS Associate Clinical Professor of Surgery (Urology) Harvard Medical School Post-prostatectomy Incontinence
Policy #: 213 Latest Review Date: September 2012
 Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Name of Policy: Temporary Prostatic Stent Policy #: 213 Latest Review Date: September 2012 Category: Medicine Policy Grade: Active Policy but no longer scheduled for regular literature reviews and updates.
Medical Review Criteria Invasive Treatment for Urinary Incontinence
 Medical Review Criteria Invasive Treatment for Urinary Incontinence Effective Date: December 21, 2016 Subject: Invasive Treatment for Urinary Incontinence Background: Urinary incontinence (the involuntary
Medical Review Criteria Invasive Treatment for Urinary Incontinence Effective Date: December 21, 2016 Subject: Invasive Treatment for Urinary Incontinence Background: Urinary incontinence (the involuntary
Injection of Urethral Bulking Agents
 Injection of Urethral Bulking Agents Department of Gynaecology Patient Information What are urethral bulking agents? Urethral bulking agents are substances that are injected to support the bladder neck.
Injection of Urethral Bulking Agents Department of Gynaecology Patient Information What are urethral bulking agents? Urethral bulking agents are substances that are injected to support the bladder neck.
Portable Bladder Ultrasound. OHTAC Recommendation. Portable Bladder Ultrasound
 OHTAC Recommendation Portable Bladder Ultrasound April 18, 2006 1 The Ontario Health Technology Advisory Committee (OHTAC) met on April 18, 2006 to review the utility of portable bladder ultrasound for
OHTAC Recommendation Portable Bladder Ultrasound April 18, 2006 1 The Ontario Health Technology Advisory Committee (OHTAC) met on April 18, 2006 to review the utility of portable bladder ultrasound for
INSTRUCTIONS FOR USE
 Comfort Injects 50 Comfort-in INSTRUCTIONS FOR USE English user manual 1 Introduction Thank you for choosing the Comfort in Needle free Injection system, developed with your comfort and convenience in
Comfort Injects 50 Comfort-in INSTRUCTIONS FOR USE English user manual 1 Introduction Thank you for choosing the Comfort in Needle free Injection system, developed with your comfort and convenience in
Suprapubic and Mitrofanoff Catheter Care
 Urinary catheters are tubes that drain urine from your child s bladder. There are many different types of urinary catheters. Your nurse will teach you how to care for these catheters. Here is information
Urinary catheters are tubes that drain urine from your child s bladder. There are many different types of urinary catheters. Your nurse will teach you how to care for these catheters. Here is information
A step-by-step preparation guide
 A step-by-step preparation guide For needle and needle-free systems This guide provides detailed instructions on the reconstitution, dilution, and storage of VELETRI. It is intended to be used after your
A step-by-step preparation guide For needle and needle-free systems This guide provides detailed instructions on the reconstitution, dilution, and storage of VELETRI. It is intended to be used after your
Bill Landry BScPT, BScH, MCPA, CAFCI Family Physiotherapy Centre of London
 Bill Landry BScPT, BScH, MCPA, CAFCI blandry@fpclondon.com Family Physiotherapy Centre of London Objectives To describe the scope of post-prostatectomy incontinence To describe what s been done To provide
Bill Landry BScPT, BScH, MCPA, CAFCI blandry@fpclondon.com Family Physiotherapy Centre of London Objectives To describe the scope of post-prostatectomy incontinence To describe what s been done To provide
Post operative voiding dysfunction and the Value of Urodynamics. Dr Salwan Al-Salihi Urogynaecologist Obstetrician and Gynaecologist
 Post operative voiding dysfunction and the Value of Urodynamics Dr Salwan Al-Salihi Urogynaecologist Obstetrician and Gynaecologist Learning objectives: v Pathophysiology of post op voiding dysfunction.
Post operative voiding dysfunction and the Value of Urodynamics Dr Salwan Al-Salihi Urogynaecologist Obstetrician and Gynaecologist Learning objectives: v Pathophysiology of post op voiding dysfunction.
NHS GREATER GLASGOW & CLYDE CONTROL OF INFECTION COMMITTEE STANDARD OPERATING PROCEDURE (SOP) INSERTION & MAINTENANCE OF INDWELLING
 Page Page 1 of 6 AIM STATEMENT REQUIREMENTS LOCATION TIMING PROCEDURE To minimise the risk of secondary infection as a result of urinary catheterisation. A urinary catheter bypasses the body s normal defence
Page Page 1 of 6 AIM STATEMENT REQUIREMENTS LOCATION TIMING PROCEDURE To minimise the risk of secondary infection as a result of urinary catheterisation. A urinary catheter bypasses the body s normal defence
Urinary Incontinence. Biopolymer GmbH & Co.KG - Germany
 Urinary Incontinence Treatment Options: 1. Physical Therapy: a conservative early stage treatment for UI, including pelvic floor muscles training known as Kegel exercise. 2. Medications: by means of pharmaceutical
Urinary Incontinence Treatment Options: 1. Physical Therapy: a conservative early stage treatment for UI, including pelvic floor muscles training known as Kegel exercise. 2. Medications: by means of pharmaceutical
INCONTINENCE AND OTHER UROLOGICAL DILEMMAS DR. ANNA LAWRENCE UROLOGIST AUCKLAND HOSPITAL 161 UROLOGY
 INCONTINENCE AND OTHER UROLOGICAL DILEMMAS DR. ANNA LAWRENCE UROLOGIST AUCKLAND HOSPITAL 161 UROLOGY COVERING INCONTINENCE BE ON JUST NAPPIES CATHETERS TYPES AVAILABLE AND WHEN TO USE THEM JJ STENTS???
INCONTINENCE AND OTHER UROLOGICAL DILEMMAS DR. ANNA LAWRENCE UROLOGIST AUCKLAND HOSPITAL 161 UROLOGY COVERING INCONTINENCE BE ON JUST NAPPIES CATHETERS TYPES AVAILABLE AND WHEN TO USE THEM JJ STENTS???
MEDICAL POLICY SUBJECT: BULKING AGENTS FOR TREATMENT OF URINARY OR FECAL INCONTINENCE. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: BULKING AGENTS FOR (ARCHIVED DATE: 05/28/09-, EDITED DATE: 05/27/10, 05/19/11, 05/24/12, 05/23/13) PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific
MEDICAL POLICY SUBJECT: BULKING AGENTS FOR (ARCHIVED DATE: 05/28/09-, EDITED DATE: 05/27/10, 05/19/11, 05/24/12, 05/23/13) PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific
P ROLIEVE. Thermodilatation System. The Prolieve System Patient Information is Directed to You, the Patient. A Transurethral Microwave Therapy Device
 P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
P ROLIEVE Thermodilatation System A Transurethral Microwave Therapy Device The Prolieve System Patient Information is Directed to You, the Patient. Contents Why am I being treated with the Prolieve Thermodilatation
Laparoscopic Sacrohysteropexy
 Professor Christian Phillips BSc Hons BM DM FRCOG Consultant Gynaecologist and Urogynaecologist Laparoscopic Sacrohysteropexy What is a prolapse? Uterine prolapse is a bulge or lump in the vagina caused
Professor Christian Phillips BSc Hons BM DM FRCOG Consultant Gynaecologist and Urogynaecologist Laparoscopic Sacrohysteropexy What is a prolapse? Uterine prolapse is a bulge or lump in the vagina caused
Urinary Catheter or Urinary Tract Infection Critical Element Pathway
 Use this pathway for a resident who has a symptomatic urinary tract infection (UTI) and/or an indwelling urinary catheter. Review the Following in Advance to Guide Observations and Interviews: Review the
Use this pathway for a resident who has a symptomatic urinary tract infection (UTI) and/or an indwelling urinary catheter. Review the Following in Advance to Guide Observations and Interviews: Review the
