Objectives Primary Objective: Secondary Objectives For T4 a, b, c tumors:
|
|
|
- Melinda Griffith
- 5 years ago
- Views:
Transcription
1 Z1031 A randomized phase III trial comparing 16 to 18 weeks of neoadjuvant exemestane (25mg daily), letrozole (2.5mg), or anastrozole (1mg) in postmenopausal women with clinical stage II and III estrogen receptor positive breast cancer Study Chair: Matthew Ellis, MD Activation Date: 1/9/2006 Estimated Closure Date: 07/2009 Accrual Goal: 375 patients Current Accrual: 345 patients Current Amendment: A5 Participating Groups: ACOSOG CALGB CTSU
2 Objectives Primary Objective: To determine whether anastrozole, exemestane or letrozole administered for 16 to 18 weeks as neoadjuvant endocrine treatment for postmenopausal patients with stage II or stage III ER+ breast cancer should be chosen as the aromatase inhibitor arm of a future study that will compare neoadjuvant aromatase inhibitor treatment with neoadjuvant chemotherapy. Secondary Objectives: To compare the neoadjuvant treatment regimens relative to the rates of improvement in surgical outcome defined as follows: For T4 a, b, c tumors: mastectomy with primary skin closure and negative surgical margins For T3 tumors and T2 tumors classified as requiring mastectomy at baseline: breast conserving surgery with negative final margins. For T2 tumors classified as potential candidates for breast conservation: breast conserving surgery at first attempt. To compare and confirm the radiological response rates (mammography and ultrasound by central radiological analysis) between these three neoadjuvant treatment regimens. To compare the relative safety of the neoadjuvant treatment regimens in terms of reported adverse events. To compare the tumor pathologic size between the neoadjuvant treatment regimens and to compare the rates of pathological CR (defined as no invasive cancer in the post-treatment specimen), and to compare down-staging to Stage I, defined and finding 2cm or less invasive cancer in the breast at surgery, with pathologically negative lymph nodes To compare the rate of complete cell cycle response between the three treatment regimens (Ki67 staining of 1% or less in the post treatment sample). To compare the incidence of metastatic lymph node involvement on the three arms of the study in patients who have a lymph node dissection at the end of neoadjuvant treatment. To determine the 5-year incidence of local recurrence after neoadjuvant aromatase inhibitor therapy. To collect tumor tissue, serum specimens, and plasma specimens to develop predictive biomarkers that can be used to select tumors for neoadjuvant aromatase inhibitor therapy. To collect all surgical specimens post-ai neoadjuvant therpy to identify markers of de novo resistance to AI therapy Summary Statement The first patient was registered to this study on April 9, 2006, with 345 patients accrued as of May 4, 2009, 114 on Arm 1, 116 on Arm 2, and 115 on Arm 3. On Arm 1 (exemestane), three patients have reported Grade 4 adverse events: one secondary malignency, unrelated to to study treatment, one blood/bone marrow-other, possibly related to study treatment, and one patient with hyperglycemia, unlikely to be related to study treatment, and glucose intolerance, unrelated to study treatment. On Arm 2 (letrozole), seven patients have reported Grade 4 adverse events: one patient with rectal hemorrhage and GIother, unrelated to study treatment, one patient with anxiety and agitation unlikely related to study treatment, fatigue and depression possibly related to study treatment, and hot flashes definitely related to study treatment, one patient with amylase and lipase unrelated to study treatment, one patient with hot flashes definitely related to study treatment, one patient with hematoma unrelated to study treatment, one patient with joint pain and muscle pain definitely related to study treatment, and another patient with thrombosis unrelated to study treatment. On Arm 3 (anastrozole), two patients have reported Grade 4 adverse events: one hyperglycemia unrelated to study treatment and one dyspnea unrelated to study treatment. The third interim analysis was presented to the Data Monitoring Committee (DMC) at their January 2009 meeting. The study is continuing as planned. Interim analyses are presented to the DMC semi-annually: the next DMC review of interim analyses is in June 2009.
3 Table 1: Accrual By Site Institution Name Frequency Count CTSU Data Operations Center 61 M.D. Anderson Cancer Center (Univ of Texas) 55 Washington University (Barnes Jewish Hospital) 39 Doctors Hospital of Laredo 21 Duke University Medical Center 20 University of Texas Southwestern Medical Center (Dallas) 14 Saint Elizabeth Medical Center South 12 Covenant Medical Center-Lakeside 8 Anne Arundel Medical Center 7 Mayo Clinic (Rochester) 6 Morton Plant Hospital (MPMHC) 6 Baptist Hospital Nashville TN 5 Beaumont Hospital 5 Good Samaritan Hospital (Cincinnati) 5 University of Michigan Univ. Hospital (Compr. Cancer Ctr) 5 University of New Mexico 5 CCOP, Christiana Care Health Services, Incorporated 4 Froedtert Memorial Lutheran Hospital (Medical College of Wisconsin) 4 Johns Hopkins University Hospital 4 Lehigh Valley Hospital 4 Memorial Medical Center Savannah GA 4 Northwestern University 4 Wake Forest University (Baptist Medical Center) 4 Wayne State University 4 Akron General Medical Center 3 Central Baptist Hospital 3 Methodist Hospital 3 University of California at San Francisco Medical Center (Mount Zion) 3 Henry Ford Hospital 2 Oklahoma University Medical Center 2 Saint Joseph Medical Center 2 Surgical Oncology Associates 2
4 Institution Name Frequency Count Swedish Hospital and Medical Center 2 University of South Alabama Mitchell Cancer Institute 2 Hilton Head Regional Medical Center 1 Altru Cancer Center 1 Breastlink Medical Group Inc. 1 Dallas Surgical Group 1 Evanston Northwestern Healthcare 1 Hope, A Women's Cancer Center 1 Presbyterian Kaseman Hospital 1 Roger Williams Medical Center 1 Sacred Heart Hospital 1 Saint Joseph's Hospital (Candler Health System) 1 Sinai Hospital of Baltimore 1 Upstate Carolina CCOP 1 Virtua Memorial Hospital Burlington 1 Western Pennsylvania Hospital 1 York Hospital 1 Total Study Accrual 345
5 Figure 1: Accrual by Month 20 Actual Expected Month Table 2: Demographics Exemestane (N=114) Letrozole (N=116) Anastrozole (N=115) Age Median Range ( ) ( ) ( ) Race White 94 (82.5%) 88 (75.9%) 89 (77.4%) Black 17 (14.9%) 19 (16.4%) 15 (13%) Asian 0 (0%) 2 (1.7%) 1 (0.9%) American Indian 0 (0%) 0 (0%) 1 (0.9%) Unknown 3 (2.6%) 7 (6%) 9 (7.8%) Ethnicity Hispanic or Latino 10 (8.8%) 19 (16.4%) 12 (10.4%) Not Hispanic or Latino 99 (86.8%) 90 (77.6%) 93 (80.9%) Unknown 5 (4.4%) 7 (6%) 10 (8.7%) Gender Female 113 (99.1%) 113 (97.4%) 112 (97.4%) Missing 1 (0.9%) 3 (2.6%) 3 (2.6%)
6 Table 3: Adverse Events, Regardless of Attribution Number of Evaluable Patients: Exemestane=107 Letrozole=108 Anastrozole=105 Patients with at least one: Arm N % Grade 3+ Event Exemestane 26 (24.3%) Grade 4+ Event Exemestane 3 (2.8%) Grade 5 Event Exemestane 0 (0.0%) Grade 3+ Heme Adverse Event Exemestane 1 (0.9%) Grade 4+ Heme Adverse Event Exemestane 1 (0.9%) Grade 3+ Non-Heme Adverse Event Exemestane 25 (23.4%) Grade 4+ Non-Heme Adverse Event Exemestane 2 (1.9%) Grade 3+ Event Letrozole 23 (21.3%) Grade 4+ Event Letrozole 7 (6.5%) Grade 5 Event Letrozole 0 (0.0%) Grade 3+ Heme Adverse Event Letrozole 2 (1.9%) Grade 4+ Heme Adverse Event Letrozole 0 (0.0%) Grade 3+ Non-Heme Adverse Event Letrozole 22 (20.4%) Grade 4+ Non-Heme Adverse Event Letrozole 7 (6.5%) Grade 3+ Event Anastrozole 16 (15.2%) Grade 4+ Event Anastrozole 2 (1.9%) Grade 5 Event Anastrozole 0 (0.0%) Grade 3+ Heme Adverse Event Anastrozole 0 (0.0%) Grade 4+ Heme Adverse Event Anastrozole 0 (0.0%) Grade 3+ Non-Heme Adverse Event Anastrozole 16 (15.2%) Grade 4+ Non-Heme Adverse Event Anastrozole 2 (1.9%)
7 Grade N % N % N % Body System Adverse Event Arm Hematology ANEMIA HEMOLYSIS BLOOD /BONE MARR-OTH Exemestane Hemorrhage HEMATOMA Letrozole RECTAL HEMORR HEMORRHAGE-SURG
8 Infection/Febrile Neutropenia Metabolic/Laboratory PNEUMONIA NOS URNRY TRCT INFECTN WOUND INFECTN INFECTION CELLULITES INFECTN AMYLASE HYPERGLYCEMIA HYPOKALEMIA LIPASE Grade N % N % N % Exemestane Anastrozole
9 Musculoskeletal Neurology FRACTURE MUSCLE WEAKNESS AGITATION ANXIETY ISCHEMIA-CEREBRAL CONFUSION STATE SEIZURE DEPRESSION PSYCHOSIS AGGRAV Grade N % N % N % Letrozole Letrozole
10 Ocular/Visular Pain Pulmonary EYELID DYSFUNCTION GLAUCOMA PAIN-ABDOMINAL ARTHRALGIA PAIN-BACK PAIN-BREAST PAIN-HEADACHE MYALGIA PAIN DYSPNEA Grade N % N % N % Letrozole Letrozole Anastrozole
11 Allergy/Immunology Other Auditory/Hearing Cardiovascular Constitutional Symptoms Dermatology/Skin HYPERSENSITIVITY SECONDARY MALIGNANCY INNER EAR HYPERTENSION THROMBOSIS CARDIOVASCULAR FATIGUE INJECTION SITE RXN SKIN IRRITATION Grade N % N % N % Exemestane Exemestane Letrozole Anastrozole Letrozole Anastrozole
12 Endocrine Gastrointestinal GLUCOSE INTOLERANCE HOT FLASHES ABD DISTENTION DEHYDRATION DIARRHEA-NO COLOSTOM DYSPHAGIA NAUSEA VOMITING GI - Other Grade N % N % N % Exemestane Letrozole Anastrozole Exemestane Letrozole Exemestane
Objectives Primary Objectives:
 Z1031 A randomized phase III trial comparing 16 to 18 weeks of neoadjuvant exemestane (25mg daily), letrozole (2.5mg), or anastrozole (1mg) in postmenopausal women with clinical stage II and III estrogen
Z1031 A randomized phase III trial comparing 16 to 18 weeks of neoadjuvant exemestane (25mg daily), letrozole (2.5mg), or anastrozole (1mg) in postmenopausal women with clinical stage II and III estrogen
January Schema: S T R A T I F Y. Age 1. <50 2. >50
 January 2004 9813-1 RTOG Protocol No: 9813 Protocol Status: ECOG Protocol No: R9813 Phase I: Opened June 16, 2000 NCCTG Protocol No: R9813 Closed January 25, 2002 SWOG Protocol No: R9813 Phase III: Opened
January 2004 9813-1 RTOG Protocol No: 9813 Protocol Status: ECOG Protocol No: R9813 Phase I: Opened June 16, 2000 NCCTG Protocol No: R9813 Closed January 25, 2002 SWOG Protocol No: R9813 Phase III: Opened
NCCTG Status Report for Study N September 2007
 Phase I/II Study of Concurrent Chemotherapy and Escalating Doses 3-D Conformal Radiotherapy (RT) Followed by Three Cycles of Chemotherapy for Unresectable Non-Small Cell Lung Cancer (NSCLC) Using a New
Phase I/II Study of Concurrent Chemotherapy and Escalating Doses 3-D Conformal Radiotherapy (RT) Followed by Three Cycles of Chemotherapy for Unresectable Non-Small Cell Lung Cancer (NSCLC) Using a New
June Schema: S T R A I T I F Y. Age 1. <50 2. >50
 June 2003 9813-1 RTOG Protocol No: 9813 Protocol Status: ECOG Protocol No: R9813 Opened: June 16, 2000 NCCTG Protocol No: R9813 SWOG Protocol No: R9813 Title: A Phase / Randomized Study of Radiation Therapy
June 2003 9813-1 RTOG Protocol No: 9813 Protocol Status: ECOG Protocol No: R9813 Opened: June 16, 2000 NCCTG Protocol No: R9813 SWOG Protocol No: R9813 Title: A Phase / Randomized Study of Radiation Therapy
GOG PROTOCOL #209. David Miller, MD, Gini Fleming, MD, Richard Zaino, MD, and David Cella, PhD
 GOG PROTOCOL #209 PROTOCOL Study Chairs Statistician Data Coordinator Randomized Phase III Trial of Doxorubicin/Cisplatin/Paclitaxel and G-CSF Versus Carboplatin/Paclitaxel in Patients with Stage III and
GOG PROTOCOL #209 PROTOCOL Study Chairs Statistician Data Coordinator Randomized Phase III Trial of Doxorubicin/Cisplatin/Paclitaxel and G-CSF Versus Carboplatin/Paclitaxel in Patients with Stage III and
GOG PROTOCOL #209 GOG 209
 GOG PROTOCOL #209 PROTOCOL Study Chairs Statistician Data Coordinator Randomized Phase III Trial of Doxorubicin/Cisplatin/Paclitaxel and G-CSF Versus Carboplatin/Paclitaxel in Patients with Stage III and
GOG PROTOCOL #209 PROTOCOL Study Chairs Statistician Data Coordinator Randomized Phase III Trial of Doxorubicin/Cisplatin/Paclitaxel and G-CSF Versus Carboplatin/Paclitaxel in Patients with Stage III and
January NCCTG Protocol No: N0628 Opened: November 27, 2007 CALGB Protocol No: ECOG Protocol No: R0617
 January 2010 0617-1 RTOG Protocol No: 0617 Protocol Status: NCCTG Protocol No: N0628 Opened: November 27, 2007 CALGB Protocol No: 30609 ECOG Protocol No: R0617 Title: A Randomized Phase III Comparison
January 2010 0617-1 RTOG Protocol No: 0617 Protocol Status: NCCTG Protocol No: N0628 Opened: November 27, 2007 CALGB Protocol No: 30609 ECOG Protocol No: R0617 Title: A Randomized Phase III Comparison
Welcome to. American College of Surgeons. Clinical Research Program (ACS-CRP) Breast Surgical Trial Webinar
 American College of Surgeons Clinical Research Program Kelly K. Hunt, M.D. Program Director Welcome to American College of Surgeons Clinical Research Program (ACS-CRP) Breast Surgical Trial Webinar Moderator:
American College of Surgeons Clinical Research Program Kelly K. Hunt, M.D. Program Director Welcome to American College of Surgeons Clinical Research Program (ACS-CRP) Breast Surgical Trial Webinar Moderator:
Emerging Approaches for (Neo)Adjuvant Therapy for ER+ Breast Cancer
 Emerging Approaches for (Neo)Adjuvant Therapy for E+ Breast Cancer Cynthia X. Ma, M.D., Ph.D. Associate Professor of Medicine Washington University in St. Louis Outline Current status of adjuvant endocrine
Emerging Approaches for (Neo)Adjuvant Therapy for E+ Breast Cancer Cynthia X. Ma, M.D., Ph.D. Associate Professor of Medicine Washington University in St. Louis Outline Current status of adjuvant endocrine
EASTERN COOPERATIVE ONCOLOGY GROUP
 EASTERN COOPERATIVE ONCOLOGY GROUP E5204 INTERGROUP RANDOMIZED PHASE III STUDY OF POSTOPERATIVE OXALIPLATIN, 5-FLUOROURACIL AND LEUCOVORIN VS OXALIPLATIN, 5-FLUOROURACIL, LEUCOV- ORIN AND BEVACIZUMAB FOR
EASTERN COOPERATIVE ONCOLOGY GROUP E5204 INTERGROUP RANDOMIZED PHASE III STUDY OF POSTOPERATIVE OXALIPLATIN, 5-FLUOROURACIL AND LEUCOVORIN VS OXALIPLATIN, 5-FLUOROURACIL, LEUCOV- ORIN AND BEVACIZUMAB FOR
First-Line Ribociclib + Letrozole for Postmenopausal Women With HR+, HER2-, Advanced Breast Cancer: First Results From the Phase III MONALEESA-2 Study
 First-Line Ribociclib + Letrozole for Postmenopausal Women With HR+, HER2-, Advanced Breast Cancer: First Results From the Phase III MONALEESA-2 Study Abstract LBA1 Hortobagyi GN, Stemmer SM, Burris HA,
First-Line Ribociclib + Letrozole for Postmenopausal Women With HR+, HER2-, Advanced Breast Cancer: First Results From the Phase III MONALEESA-2 Study Abstract LBA1 Hortobagyi GN, Stemmer SM, Burris HA,
June 2009 Breast Committee CALGB 40502
 CALGB 40502/CTSU 40502 A randomized phase III trial of weekly paclitaxel compared to weekly nanoparticle albumin bound nabpaclitaxel or ixabepilone combined with bevacizumab as first-line therapy for locally
CALGB 40502/CTSU 40502 A randomized phase III trial of weekly paclitaxel compared to weekly nanoparticle albumin bound nabpaclitaxel or ixabepilone combined with bevacizumab as first-line therapy for locally
Welcome to Oncology as a Nurse Practitioner!
 Welcome to Oncology as a Nurse Practitioner! As a nurse practitioner in cancer care there are many resources that can help orient you to the care of the patient and family with cancer. The members of the
Welcome to Oncology as a Nurse Practitioner! As a nurse practitioner in cancer care there are many resources that can help orient you to the care of the patient and family with cancer. The members of the
Nilotinib AEs (adverse events) in CML population:
 Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
For more information, call AstraZeneca Access 360 at ASK-A360, Monday through Friday, 8:00 AM to 8:00 PM ET.
 1-844-ASK-A360 1-844-275-2360 1-844-FAX-A360 1-844-329-2360 www.myaccess360.com For more information, call AstraZeneca Access 360 at 1-844-ASK-A360, Monday through Friday, 8:00 AM to 8:00 PM ET. Indications
1-844-ASK-A360 1-844-275-2360 1-844-FAX-A360 1-844-329-2360 www.myaccess360.com For more information, call AstraZeneca Access 360 at 1-844-ASK-A360, Monday through Friday, 8:00 AM to 8:00 PM ET. Indications
June 2009 Respiratory Committee CALGB 30610
 30610 Phase III comparison of thoracic radiotherapy regimens in patients with limited small cell lung cancer also receiving cisplatin and etoposide Activated: March 15, 2008 Study Chairpersons: J. Bogart
30610 Phase III comparison of thoracic radiotherapy regimens in patients with limited small cell lung cancer also receiving cisplatin and etoposide Activated: March 15, 2008 Study Chairpersons: J. Bogart
S0106 Phase III. Coordinating Group: SWOG
 S0106 Phase III Coordinating Group: SWOG A Phase III Study of the Addition of Gemtuzumab Ozogamicin (Mylotarg ) Induction Therapy Versus Standard Induction with Daunomycin and Cytosine Arabinoside Followed
S0106 Phase III Coordinating Group: SWOG A Phase III Study of the Addition of Gemtuzumab Ozogamicin (Mylotarg ) Induction Therapy Versus Standard Induction with Daunomycin and Cytosine Arabinoside Followed
June Patient Population:
 June 2003 9802-1 RTOG Protocol No: 9802 Protocol Status: ECOG Protocol No: R9802 Opened: October 31, 1998 NCCT Protocol No: R9802 Closed: June 27, 2002 SWOG Protocol No: R9802 Title: A Phase II Study of
June 2003 9802-1 RTOG Protocol No: 9802 Protocol Status: ECOG Protocol No: R9802 Opened: October 31, 1998 NCCT Protocol No: R9802 Closed: June 27, 2002 SWOG Protocol No: R9802 Title: A Phase II Study of
Breast Program. Scientific Coordinator: Edith A Perez, MD. Community Co-Chair: Kendrith M Rowland Jr, MD Lead Statistician: Amylou C Dueck, PhD
 Breast Program Scientific Coordinator: Edith A Perez, MD Community Co-Chair: Kendrith M Rowland Jr, MD Lead Statistician: Amylou C Dueck, PhD Studies Open for Patient Enrollment Spring 2011 I. Surgery
Breast Program Scientific Coordinator: Edith A Perez, MD Community Co-Chair: Kendrith M Rowland Jr, MD Lead Statistician: Amylou C Dueck, PhD Studies Open for Patient Enrollment Spring 2011 I. Surgery
Clinical Trial Results Database Page 1
 Page 1 Sponsor Novartis UK Limited Generic Drug Name Letrozole/FEM345 Therapeutic Area of Trial Localized ER and/or PgR receptor positive breast cancer Study Number CFEM345EGB07 Protocol Title This study
Page 1 Sponsor Novartis UK Limited Generic Drug Name Letrozole/FEM345 Therapeutic Area of Trial Localized ER and/or PgR receptor positive breast cancer Study Number CFEM345EGB07 Protocol Title This study
Lessons Learnt from Neoadjuvant Hormone Therapy. 10 Lessons Learnt from Neoadjuvant Endocrine Therapy. Lesson 1
 Lessons Learnt from Neoadjuvant Hormone Therapy Mike Dixon Clinical Director Breakthrough Research Unit Edinburgh 10 Lessons Learnt from Neoadjuvant Endocrine Therapy 10 Lessons Learnt from Neoadjuvant
Lessons Learnt from Neoadjuvant Hormone Therapy Mike Dixon Clinical Director Breakthrough Research Unit Edinburgh 10 Lessons Learnt from Neoadjuvant Endocrine Therapy 10 Lessons Learnt from Neoadjuvant
Lessons Learnt from Neoadjuvant Hormone Therapy. Mike Dixon Clinical Director Breakthrough Research Unit Edinburgh
 Lessons Learnt from Neoadjuvant Hormone Therapy Mike Dixon Clinical Director Breakthrough Research Unit Edinburgh 10 Lessons Learnt from Neoadjuvant Endocrine Therapy 10 Lessons Learnt from Neoadjuvant
Lessons Learnt from Neoadjuvant Hormone Therapy Mike Dixon Clinical Director Breakthrough Research Unit Edinburgh 10 Lessons Learnt from Neoadjuvant Endocrine Therapy 10 Lessons Learnt from Neoadjuvant
Drafting a Coverage Authorization Request Letter
 Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
Drafting a Coverage Authorization Request Letter The following information is presented for informational purposes only and is not intended to provide reimbursement or legal advice. Laws, regulations,
It is a malignancy originating from breast tissue
 59 Breast cancer 1 It is a malignancy originating from breast tissue including both early stages which are potentially curable, and metastatic breast cancer (MBC) which is usually incurable. Most breast
59 Breast cancer 1 It is a malignancy originating from breast tissue including both early stages which are potentially curable, and metastatic breast cancer (MBC) which is usually incurable. Most breast
HORMONAL THERAPY IN ADJUVANT CARE
 ADVANCES IN ENDOCRINE THERAPY FOR BREAST CANCER* Matthew J. Ellis, MD, PhD ABSTRACT Endocrine therapy is used frequently in breast cancer management, particularly in the setting of adjuvant care, but outstanding
ADVANCES IN ENDOCRINE THERAPY FOR BREAST CANCER* Matthew J. Ellis, MD, PhD ABSTRACT Endocrine therapy is used frequently in breast cancer management, particularly in the setting of adjuvant care, but outstanding
Sponsor / Company: Sanofi Drug substance(s): SAR (iniparib)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
REFERENCE NUMBER: NH.PST.05 EFFECTIVE DATE: 10/10
 PAGE: 1 of 5 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
PAGE: 1 of 5 IMPORTANT REMINDER This Clinical Policy has been developed by appropriately experienced and licensed health care professionals based on a thorough review and consideration of generally accepted
BREAST CANCER RISK REDUCTION (PREVENTION)
 BREAST CANCER RISK REDUCTION (PREVENTION) Articles Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled
BREAST CANCER RISK REDUCTION (PREVENTION) Articles Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled
RIBOCICLIB EN PRIMERA LINEA DE TRATAMIENTO. Dra. Elena Aguirre H.U. Miguel Servet
 RIBOCICLIB EN PRIMERA LINEA DE TRATAMIENTO Dra. Elena Aguirre H.U. Miguel Servet INTRODUCTION ADVANCED BREAST CANCER HR+/HER2- YES Consider Chemo VISCERAL CRISIS? NO Endocrine Therapy X3 Toxicity Progresive
RIBOCICLIB EN PRIMERA LINEA DE TRATAMIENTO Dra. Elena Aguirre H.U. Miguel Servet INTRODUCTION ADVANCED BREAST CANCER HR+/HER2- YES Consider Chemo VISCERAL CRISIS? NO Endocrine Therapy X3 Toxicity Progresive
Patient Interview Form
 Page 1 of 5 Orange Coast Memorial Office: 18111 Brookhurst Ave. Suite 5200, Fountain Valley, CA 92708 * Tel: (714) 962-7705 * Fax: (714) 861-4552 www.unitedgi.com Patient Interview Form Patient Information
Page 1 of 5 Orange Coast Memorial Office: 18111 Brookhurst Ave. Suite 5200, Fountain Valley, CA 92708 * Tel: (714) 962-7705 * Fax: (714) 861-4552 www.unitedgi.com Patient Interview Form Patient Information
Objectives Critically review presentations on 1. Local therapy 2. Adjuvant chemotherapy for isolated local regional recurrence 3. The optimal duration
 Objectives Critically review presentations on 1. Local therapy 2. Adjuvant chemotherapy for isolated local regional recurrence 3. The optimal duration of endocrine therapy 4. Advances in HER2 directed
Objectives Critically review presentations on 1. Local therapy 2. Adjuvant chemotherapy for isolated local regional recurrence 3. The optimal duration of endocrine therapy 4. Advances in HER2 directed
Long Term Toxicity of Endocrine Therapy for premenopausal women with ER positive breast cancer
 Global Breast Cancer Conference 2017 21 st Apr, 2017@Chezu Island Long Term Toxicity of Endocrine Therapy for premenopausal women with ER positive breast cancer Shinji Ohno, M.D., Ph.D., F.A.C.S. Breast
Global Breast Cancer Conference 2017 21 st Apr, 2017@Chezu Island Long Term Toxicity of Endocrine Therapy for premenopausal women with ER positive breast cancer Shinji Ohno, M.D., Ph.D., F.A.C.S. Breast
Modesto Gastroenterology Medical Corporation
 Page 1 of 5 Modesto Gastroenterology Medical Corporation Magdy S. Elsakr, M.D. Board Certified Gastroenterologist 2336 Sylvan Avenue, Suite A, Modesto, CA 95355, Phone: 209-338-0292, Fax: 209-338-0298
Page 1 of 5 Modesto Gastroenterology Medical Corporation Magdy S. Elsakr, M.D. Board Certified Gastroenterologist 2336 Sylvan Avenue, Suite A, Modesto, CA 95355, Phone: 209-338-0292, Fax: 209-338-0298
June Arm 1 = Observe R A N D O M I Z E
 June 2004 9802-1 RTOG Protocol No: 98-02 Protocol Status: ECOG Protocol No: R9802 Opened: October 31, 1998 NCCT Protocol No: R9802 Closed: June 27, 2002 SWOG Protocol No: R9802 Title: A Phase II Study
June 2004 9802-1 RTOG Protocol No: 98-02 Protocol Status: ECOG Protocol No: R9802 Opened: October 31, 1998 NCCT Protocol No: R9802 Closed: June 27, 2002 SWOG Protocol No: R9802 Title: A Phase II Study
American Society of Clinical Oncology June , New Orleans
 American Society of Clinical Oncology June 5-8 2004, New Orleans The 2004 annual meeting of the American Society of Clinical Oncology (ASCO) was held June 5 8 in New Orleans, Louisiana. This conference
American Society of Clinical Oncology June 5-8 2004, New Orleans The 2004 annual meeting of the American Society of Clinical Oncology (ASCO) was held June 5 8 in New Orleans, Louisiana. This conference
NSABP: FB-11. Shannon Puhalla, MD
 NSABP: FB-11 Phase II Randomized Study Evaluating the Biological and Clinical Effects of the Combination of Palbociclib with Letrozole as Neoadjuvant Therapy in Post- Menopausal Women with Estrogen Receptor
NSABP: FB-11 Phase II Randomized Study Evaluating the Biological and Clinical Effects of the Combination of Palbociclib with Letrozole as Neoadjuvant Therapy in Post- Menopausal Women with Estrogen Receptor
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD
 Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
Open Clinical Trials: What s Out There Now Paula D. Ryan, MD, PhD Hanahan and Weinberg, 2000 Acquired Capabilities of Cancer Clinical Trials When should I consider a clinical trial? How do I find the right
The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2 invasive breast cancer
 Review Article Page 1 of 7 The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2 invasive breast cancer Vera Jean Suman 1, Matthew J. Ellis
Review Article Page 1 of 7 The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of post-menopausal women with ER+/Her2 invasive breast cancer Vera Jean Suman 1, Matthew J. Ellis
Breast Cancer Treatment
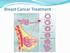 Breast Cancer Treatment Treatment 2 aspects 1. Treatment of the breast itself: Local Treatment 2. Treatment of the whole body = Systemic treatment Local Treatment Surgery +/- Radiation Usually: a. Breast
Breast Cancer Treatment Treatment 2 aspects 1. Treatment of the breast itself: Local Treatment 2. Treatment of the whole body = Systemic treatment Local Treatment Surgery +/- Radiation Usually: a. Breast
Metastatic breast cancer: sequence of therapies
 Metastatic breast cancer: sequence of therapies Clinical Case Discussion Nadia Harbeck, MD PhD Breast Center, Department of Gynecology and Obstetrics University of Munich, Ludwig-Maximilians University
Metastatic breast cancer: sequence of therapies Clinical Case Discussion Nadia Harbeck, MD PhD Breast Center, Department of Gynecology and Obstetrics University of Munich, Ludwig-Maximilians University
Full Novartis CTRD Results Template
 Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Patient Interview Form
 Patient Interview Form Patient Information First Name: Last Name: Date of Birth: Age: Email Personal: Race Select one or more Referring Physician White Black or African Asian American Indian Native Hawaiian
Patient Interview Form Patient Information First Name: Last Name: Date of Birth: Age: Email Personal: Race Select one or more Referring Physician White Black or African Asian American Indian Native Hawaiian
Hospital he hospital is located near the interchange of highway 217 and (US 26).
 Welcome to our Clinic! Our goal is to provide you with the highest quality medical care available. Please bring the completed enclosed paperwork along with your insurance card and legal picture ID to your
Welcome to our Clinic! Our goal is to provide you with the highest quality medical care available. Please bring the completed enclosed paperwork along with your insurance card and legal picture ID to your
Arm A: Induction Gemcitabine 1000 mg/m 2 IV once a week for 6 weeks.
 ECOG-4201 (RTOG Endorsed) ECOG 4201 Pancreas (RTOG Endorsed)-1 Protocol Status: Opened: April 10, 2003 Closed: December 15, 2005 Title: A Randomized Phase III Study of Gemcitabine in Combination with Radiation
ECOG-4201 (RTOG Endorsed) ECOG 4201 Pancreas (RTOG Endorsed)-1 Protocol Status: Opened: April 10, 2003 Closed: December 15, 2005 Title: A Randomized Phase III Study of Gemcitabine in Combination with Radiation
Health Risks and Benefits 3 Years After Stopping Randomized Treatment With Estrogen and Progestin. The WHI Investigators
 Health Risks and Benefits 3 Years After Stopping Randomized Treatment With Estrogen and Progestin The WHI Investigators 1 Background: WHI Hormone Program Design YES N= 10,739 Conjugated equine estrogen
Health Risks and Benefits 3 Years After Stopping Randomized Treatment With Estrogen and Progestin The WHI Investigators 1 Background: WHI Hormone Program Design YES N= 10,739 Conjugated equine estrogen
Supplementary Online Content
 Supplementary Online Content Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. Published
Supplementary Online Content Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. Published
SANTA MONICA BREAST CENTER INTAKE FORM
 SANTA MONICA BREAST CENTER Who referred you to see us today? Who is your primary care physician? Are there any other MDs who you would like to receive today s visit information? No Yes MD contact info
SANTA MONICA BREAST CENTER Who referred you to see us today? Who is your primary care physician? Are there any other MDs who you would like to receive today s visit information? No Yes MD contact info
NSABP PROTOCOL B-34. Date Opened to Randomization: December 1, 2000 Date Closed to Randomization: March 31, 2004 Number of Patients Randomized: 3,323
 NSABP PROTOCOL B-34 A Clinical Trial Comparing Adjuvant Clodronate Therapy versus Placebo in Early-Stage Breast Cancer Patients Receiving Systemic Chemotherapy and/or Tamoxifen or No Therapy Date Opened
NSABP PROTOCOL B-34 A Clinical Trial Comparing Adjuvant Clodronate Therapy versus Placebo in Early-Stage Breast Cancer Patients Receiving Systemic Chemotherapy and/or Tamoxifen or No Therapy Date Opened
Targeting CDK 4/6. Jee Hyun Kim, M.D., Ph.D. Seoul National University College of Medicine
 2016.04.30 GBCC Education Symposium Targeting CDK 4/6 Jee Hyun Kim, M.D., Ph.D. Seoul National University College of Medicine Contents Cyclins -CDKs in cell cycle control CDK 4/6 in breast cancer Preclinical
2016.04.30 GBCC Education Symposium Targeting CDK 4/6 Jee Hyun Kim, M.D., Ph.D. Seoul National University College of Medicine Contents Cyclins -CDKs in cell cycle control CDK 4/6 in breast cancer Preclinical
Novel Strategies in Systemic Therapies: Overcoming Endocrine Therapy Resistance
 Novel Strategies in Systemic Therapies: Overcoming Endocrine Therapy Resistance Richard S. Finn, MD Division of Hematology/ Oncology Director, Translational Oncology Laboratory Geffen School of Medicine
Novel Strategies in Systemic Therapies: Overcoming Endocrine Therapy Resistance Richard S. Finn, MD Division of Hematology/ Oncology Director, Translational Oncology Laboratory Geffen School of Medicine
Johns Hopkins Clinical Update Webinar
 Johns Hopkins Clinical Update Webinar Ben Ho Park, M.D., Ph.D. Department of Oncology Johns Hopkins University February 2015 This presentation is the intellectual property of the author/presenter. Contact
Johns Hopkins Clinical Update Webinar Ben Ho Park, M.D., Ph.D. Department of Oncology Johns Hopkins University February 2015 This presentation is the intellectual property of the author/presenter. Contact
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Adjuvant therapy, for early-stage triple-negative breast cancer, 740 742 in older early-stage breast cancer patients, 790 795 anti-her2-directed
Note: Page numbers of article titles are in boldface type. A Adjuvant therapy, for early-stage triple-negative breast cancer, 740 742 in older early-stage breast cancer patients, 790 795 anti-her2-directed
WELCOME TO OUR OFFICE
 WELCOME TO OUR OFFICE Name: Today s Date: First Middle Last Gender: Male Female Date of birth: Age: Home Address: City: State: Zip: Home Phone:( ) Cell Phone:( ) Occupation: SSN: Employer: Time of employment
WELCOME TO OUR OFFICE Name: Today s Date: First Middle Last Gender: Male Female Date of birth: Age: Home Address: City: State: Zip: Home Phone:( ) Cell Phone:( ) Occupation: SSN: Employer: Time of employment
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Manejo do câncer de mama RH+ na adjuvância: o que há de novo?
 II Simpósio Internacional de Câncer de Mama para o Oncologista Clínico Manejo do câncer de mama RH+ na adjuvância: o que há de novo? INGRID A. MAYER, MD, MSCI Assistant Professor of Medicine Director,
II Simpósio Internacional de Câncer de Mama para o Oncologista Clínico Manejo do câncer de mama RH+ na adjuvância: o que há de novo? INGRID A. MAYER, MD, MSCI Assistant Professor of Medicine Director,
PATIENT INFORMATION SHEET
 ALAMO NEUROSURGICAL INSTITUTE 414 W SUNSET, SUITE 205 SAN ANTONIO, TEXAS 78209 WWW.ANI-ONLINE.COM OFF: 210.564.8300 FAX: 210.564.8399 PATIENT INFORMATION SHEET Patient Name (Last, First, Mi): SSN: Street
ALAMO NEUROSURGICAL INSTITUTE 414 W SUNSET, SUITE 205 SAN ANTONIO, TEXAS 78209 WWW.ANI-ONLINE.COM OFF: 210.564.8300 FAX: 210.564.8399 PATIENT INFORMATION SHEET Patient Name (Last, First, Mi): SSN: Street
Patient Interview Form
 Page 1 of 5 Patient Interview Form Patient Information First Name: MRN: Last Name: Date Of Birth: Contact Preference Email Telephone call- Work Telephone call - Home Email Please check one as your preferred
Page 1 of 5 Patient Interview Form Patient Information First Name: MRN: Last Name: Date Of Birth: Contact Preference Email Telephone call- Work Telephone call - Home Email Please check one as your preferred
Certified Breast Care Nurse (CBCN ) Test Content Outline (Effective 2018)
 Certified Breast Care Nurse (CBCN ) Test Content Outline (Effective 2018) I. Coordination of Care - 26% A. Breast health, screening, early detection, risk assessment and reduction 1. Issues related to
Certified Breast Care Nurse (CBCN ) Test Content Outline (Effective 2018) I. Coordination of Care - 26% A. Breast health, screening, early detection, risk assessment and reduction 1. Issues related to
Figure 1: PALLAS Study Schema. Endocrine adjuvant therapy may have started before randomization and be ongoing at that time.
 Figure 1: PALLAS Study Schema Endocrine adjuvant therapy may have started before randomization and be ongoing at that time. Approximately 4600 patients from approximately 500 global sites will be randomized
Figure 1: PALLAS Study Schema Endocrine adjuvant therapy may have started before randomization and be ongoing at that time. Approximately 4600 patients from approximately 500 global sites will be randomized
J. Van Lier Ribbink, M.D., F.A.C.S. Center for Endocrine and Pancreas Surgery at Honor Health
 J. Van Lier Ribbink, M.D., F.A.C.S. Center for Endocrine and Pancreas Surgery at Honor Health Patient Clinical Information Questionnaire 1.0 Date of Questionnaire Completion; / / 2.0 Patient Data 2.1 Name:
J. Van Lier Ribbink, M.D., F.A.C.S. Center for Endocrine and Pancreas Surgery at Honor Health Patient Clinical Information Questionnaire 1.0 Date of Questionnaire Completion; / / 2.0 Patient Data 2.1 Name:
6 cycles* January Opened: January 17, 2006
 January 2008 0525-1 RTOG Protocol No: 0525 Protocol Status: Opened: January 17, 2006 Title: Phase III Trial Comparing Conventional Adjuvant Temozolomide with Dose-Intensive Temozolomide in Patients with
January 2008 0525-1 RTOG Protocol No: 0525 Protocol Status: Opened: January 17, 2006 Title: Phase III Trial Comparing Conventional Adjuvant Temozolomide with Dose-Intensive Temozolomide in Patients with
FDA APPROVES HERCEPTIN FOR THE ADJUVANT TREATMENT OF HER2-POSITIVE NODE-POSITIVE BREAST CANCER
 NEWS RELEASE Media Contact: Kimberly Ocampo (650) 467-0679 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Ajanta Horan (650) 467-1741 FDA APPROVES HERCEPTIN FOR THE ADJUVANT TREATMENT OF
NEWS RELEASE Media Contact: Kimberly Ocampo (650) 467-0679 Investor Contact: Sue Morris (650) 225-6523 Advocacy Contact: Ajanta Horan (650) 467-1741 FDA APPROVES HERCEPTIN FOR THE ADJUVANT TREATMENT OF
Acupuncture Needles. Overview. Gary Deng, MD,PhD. Traditional Theories. Acupuncture in Cancer Care: the USA Experience. History. Research Activities
 , MD,PhD Acupuncture in Cancer Care: the USA Experience History Overview First ISS-ARTOI Conference on Integrative Oncology Fifth ARTOI International Congress Rome, Italy Nov 7, 2013, MD, PhD Integrative
, MD,PhD Acupuncture in Cancer Care: the USA Experience History Overview First ISS-ARTOI Conference on Integrative Oncology Fifth ARTOI International Congress Rome, Italy Nov 7, 2013, MD, PhD Integrative
TEXAS VASCULAR ASSOCIATES, P.A. PATIENT CLINICAL INTAKE FORM
 TEXAS VASCULAR ASSOCIATES, P.A. PATIENT CLINICAL INTAKE FORM PATIENT NAME: DATE OF BIRTH: TVA Physician being seen: Date of Visit: PAST MEDICAL HISTORY HEART PROBLEMS NEUROLOGICAL Congestive Heart Failure
TEXAS VASCULAR ASSOCIATES, P.A. PATIENT CLINICAL INTAKE FORM PATIENT NAME: DATE OF BIRTH: TVA Physician being seen: Date of Visit: PAST MEDICAL HISTORY HEART PROBLEMS NEUROLOGICAL Congestive Heart Failure
Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers
 日大医誌 75 (1): 10 15 (2016) 10 Original Article Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers Naotaka Uchida 1), Yasuki Matsui 1), Takeshi Notsu 1) and Manabu
日大医誌 75 (1): 10 15 (2016) 10 Original Article Implications of Progesterone Receptor Status for the Biology and Prognosis of Breast Cancers Naotaka Uchida 1), Yasuki Matsui 1), Takeshi Notsu 1) and Manabu
Recent advances in the management of metastatic breast cancer in older adults
 Recent advances in the management of metastatic breast cancer in older adults Laura Biganzoli Medical Oncology Dept New Hospital of Prato Istituto Toscano Tumori Italy Important recent advances in the
Recent advances in the management of metastatic breast cancer in older adults Laura Biganzoli Medical Oncology Dept New Hospital of Prato Istituto Toscano Tumori Italy Important recent advances in the
Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma (BCC): 24-month update of the pivotal ERIVANCE BCC study
 Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma (BCC): 24-month update of the pivotal ERIVANCE BCC study A Sekulic, 1 MR Migden, 2 AE Oro, 3 L Dirix, 4 K Lewis,
Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma (BCC): 24-month update of the pivotal ERIVANCE BCC study A Sekulic, 1 MR Migden, 2 AE Oro, 3 L Dirix, 4 K Lewis,
Supplementary Online Content
 Supplementary Online Content Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. Published online
Supplementary Online Content Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. Published online
The 2010 Gastrointestinal Cancers Symposium Oral Abstract Session: Cancers of the Pancreas, Small Bowel and Hepatobilliary Tract
 The 2010 Gastrointestinal Cancers Symposium : Cancers of the Pancreas, Small Bowel and Hepatobilliary Tract Abstract #131: Phase I study of MK 0646 (dalotuzumab), a humanized monoclonal antibody against
The 2010 Gastrointestinal Cancers Symposium : Cancers of the Pancreas, Small Bowel and Hepatobilliary Tract Abstract #131: Phase I study of MK 0646 (dalotuzumab), a humanized monoclonal antibody against
Supplementary Online Content
 Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Advances in the Diagnosis and Treatment of Breast Cancer. Carol Tweed, M.D. Anne Arundel Medical Center DeCesaris Cancer Institute Annapolis, MD
 Advances in the Diagnosis and Treatment of Breast Cancer Carol Tweed, M.D. Anne Arundel Medical Center DeCesaris Cancer Institute Annapolis, MD Disclosures Genomic Health: Speaker and Consultant AstraZeneca:
Advances in the Diagnosis and Treatment of Breast Cancer Carol Tweed, M.D. Anne Arundel Medical Center DeCesaris Cancer Institute Annapolis, MD Disclosures Genomic Health: Speaker and Consultant AstraZeneca:
*** ADDRESS: (If address is not provided, you MUST write Patient denied.)
 PATIENT INFORMATION NORTHWEST BROWARD ORTHOPAEDICS DATE: ***E-MAIL ADDRESS: (If e-mail address is not provided, you MUST write Patient denied.) Pharmacy Name: Pharmacy Phone Number: Pharmacy Location PATIENT
PATIENT INFORMATION NORTHWEST BROWARD ORTHOPAEDICS DATE: ***E-MAIL ADDRESS: (If e-mail address is not provided, you MUST write Patient denied.) Pharmacy Name: Pharmacy Phone Number: Pharmacy Location PATIENT
Disclosures. Dr. Hall is a paid consultant to the American College of Surgeons (ACS) as Associate Director of ACS-NSQIP
 Does Routine Drainage of the Operative Bed following Elective Distal Pancreatectomy reduce Complications? An Analysis of the ACS-NSQIP Pancreatectomy Demonstration Project Stephen W. Behrman, MD 1, Ben
Does Routine Drainage of the Operative Bed following Elective Distal Pancreatectomy reduce Complications? An Analysis of the ACS-NSQIP Pancreatectomy Demonstration Project Stephen W. Behrman, MD 1, Ben
Breast Cancer? Breast cancer is the most common. What s New in. Janet s Case
 Focus on CME at The University of Calgary What s New in Breast Cancer? Theresa Trotter, MD, FRCPC Breast cancer is the most common malignancy affecting women in Canada, accounting for almost a third of
Focus on CME at The University of Calgary What s New in Breast Cancer? Theresa Trotter, MD, FRCPC Breast cancer is the most common malignancy affecting women in Canada, accounting for almost a third of
Surgical Therapy: Sentinel Node Biopsy and Breast Conservation
 Surgical Therapy: Sentinel Node Biopsy and Breast Conservation Stephen B. Edge, MD Professor of Surgery and Oncology Roswell Park Cancer Institute University at Buffalo Dr. Roswell Park: Tradition in Cancer
Surgical Therapy: Sentinel Node Biopsy and Breast Conservation Stephen B. Edge, MD Professor of Surgery and Oncology Roswell Park Cancer Institute University at Buffalo Dr. Roswell Park: Tradition in Cancer
Distal Pancreatectomy with Celiac Axis Resection: What Are the Added Risks?
 Distal Pancreatectomy with Celiac Axis Resection: What Are the Added Risks? Joal D. Beane, MD a, Michael G. House, MD a, Susan C. Pitt, MD c, E. Molly Kilbane a, Bruce L. Hall c, Abishek Parmar d, Taylor.
Distal Pancreatectomy with Celiac Axis Resection: What Are the Added Risks? Joal D. Beane, MD a, Michael G. House, MD a, Susan C. Pitt, MD c, E. Molly Kilbane a, Bruce L. Hall c, Abishek Parmar d, Taylor.
PATIENT HEALTH QUESTIONNAIRE Radiation Oncology
 REVIEWED DATE / INITIALS Safety: Yes No Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? Allergies: Yes No If YES, please list medication allergies:
REVIEWED DATE / INITIALS Safety: Yes No Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? Allergies: Yes No If YES, please list medication allergies:
PATIENT HEALTH QUESTIONNAIRE Radiation Oncology
 REVIEWED DATE / INITIALS Safety: Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? Allergies: If YES, please list medication allergies: Do you have
REVIEWED DATE / INITIALS Safety: Are you at risk for falls? Do you have a Pacemaker? Females; Is there a possibility you may be pregnant? Allergies: If YES, please list medication allergies: Do you have
Study Period: 27 March 2008 (first subject enrolled) to 05 May 2010 (data cutoff date for primary analysis)
 Date: 20 July 2011 Page 2 of 3375 2. SYNOPSIS Name of Sponsor: mgen Inc., Thousand Oaks, C US Name of Finished Product: Not applicable Name of ctive Ingredient: Ganitumab (MG 479) Title of Study: n International,
Date: 20 July 2011 Page 2 of 3375 2. SYNOPSIS Name of Sponsor: mgen Inc., Thousand Oaks, C US Name of Finished Product: Not applicable Name of ctive Ingredient: Ganitumab (MG 479) Title of Study: n International,
Aromatase Inhibitors & Osteoporosis
 Aromatase Inhibitors & Osteoporosis Miss Sarah Horn Consultant Oncoplastic Breast Surgeon April 2018 Aims Role of Aromatase Inhibitors (AI) in breast cancer treatment AI s effects on bone health Bone health
Aromatase Inhibitors & Osteoporosis Miss Sarah Horn Consultant Oncoplastic Breast Surgeon April 2018 Aims Role of Aromatase Inhibitors (AI) in breast cancer treatment AI s effects on bone health Bone health
Oncotype DX testing in node-positive disease
 Should gene array assays be routinely used in node positive disease? Yes Christy A. Russell, MD University of Southern California Oncotype DX testing in node-positive disease 1 Validity of the Oncotype
Should gene array assays be routinely used in node positive disease? Yes Christy A. Russell, MD University of Southern California Oncotype DX testing in node-positive disease 1 Validity of the Oncotype
New Patient Specialty Intake Form Department of Surgery
 This form contains questions specific to the Department of Surgery. If you are new to Baylor College of Medicine and have not been seen in any of our offices, please be sure to complete our New Patient
This form contains questions specific to the Department of Surgery. If you are new to Baylor College of Medicine and have not been seen in any of our offices, please be sure to complete our New Patient
725 Jesse Jewell Pkwy, Suite 390 Gainesville, GA (770) (770) (facsimile)
 Charles Nash, III, M.D., F.A.C.P. Richard J. LoCicero, M.D. Anup K. Lahiry, M.D. Timothy M. Carey, M.D. Andrew Johnson, M.D. 725 Jesse Jewell Pkwy, Suite 390 Gainesville, GA 30501 (770) 297-5700 (770)
Charles Nash, III, M.D., F.A.C.P. Richard J. LoCicero, M.D. Anup K. Lahiry, M.D. Timothy M. Carey, M.D. Andrew Johnson, M.D. 725 Jesse Jewell Pkwy, Suite 390 Gainesville, GA 30501 (770) 297-5700 (770)
Subject ID: I N D # # U A * Consent Date: Day Month Year
 IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
Extended Adjuvant Endocrine Therapy
 Extended Adjuvant Endocrine Therapy After all, 5 years Tamoxifen works.. For women with ER+ primary breast cancer, previous studies have shown that treatment with tamoxifen for 5 years has a carry-over
Extended Adjuvant Endocrine Therapy After all, 5 years Tamoxifen works.. For women with ER+ primary breast cancer, previous studies have shown that treatment with tamoxifen for 5 years has a carry-over
The Clinical Research E-News
 Volume 3: ISSUE 9: May 18, 2011 The Clinical Research E-News Jefferson Kimmel Cancer Network: For urgent clinical trial questions or assistance please page: 877-656-9004 Now Open: RTOG 0837, A Randomized,
Volume 3: ISSUE 9: May 18, 2011 The Clinical Research E-News Jefferson Kimmel Cancer Network: For urgent clinical trial questions or assistance please page: 877-656-9004 Now Open: RTOG 0837, A Randomized,
Breast cancer treatment
 Report from the San Antonio Breast Cancer Symposium Breast cancer treatment Determining the best options for select patient groups Sara Soldera, MD, Resident; Nathaniel Bouganim, MD, FRCPC, Medical Oncologist;
Report from the San Antonio Breast Cancer Symposium Breast cancer treatment Determining the best options for select patient groups Sara Soldera, MD, Resident; Nathaniel Bouganim, MD, FRCPC, Medical Oncologist;
Drug Comparison: Lartruvo TM (Olaratumab) for Soft Tissue Sarcoma By: Majd Abedrabbo, PharmD Candidate 2018 Fairleigh Dickinson University School of
 Drug Comparison: Lartruvo TM (Olaratumab) for Soft Tissue Sarcoma By: Majd Abedrabbo, PharmD Candidate 2018 Fairleigh Dickinson University School of Pharmacy May 3, 2017 Introduction Soft Tissue Sarcoma
Drug Comparison: Lartruvo TM (Olaratumab) for Soft Tissue Sarcoma By: Majd Abedrabbo, PharmD Candidate 2018 Fairleigh Dickinson University School of Pharmacy May 3, 2017 Introduction Soft Tissue Sarcoma
Breast Cancer Breast Managed Clinical Network
 Initial Evaluation Clinical Stage Pre-Treatment Evaluation Treatment and pathological stage Less than 4 positive lymph nodes Adjuvant Treatment ER Positive HER2 Negative (see page 2 & 3 ) HER2 Positive
Initial Evaluation Clinical Stage Pre-Treatment Evaluation Treatment and pathological stage Less than 4 positive lymph nodes Adjuvant Treatment ER Positive HER2 Negative (see page 2 & 3 ) HER2 Positive
/ / - - / / Age: USF Cutaneous Oncology Program. Skin Cancer Questionnaire. Patient Information: Fax completed forms to:
 Page 1 of 8 Patient Information: Last Name: First Name: Initial: Address: Address (cont.) : City: State: Zip Code: Phone: - - Social Security Number: Date of Birth: - - Age: Sex: Female Male Email Address:
Page 1 of 8 Patient Information: Last Name: First Name: Initial: Address: Address (cont.) : City: State: Zip Code: Phone: - - Social Security Number: Date of Birth: - - Age: Sex: Female Male Email Address:
Breast Cancer. Most common cancer among women in the US. 2nd leading cause of death in women. Mortality rates though have declined
 Breast Cancer Most common cancer among women in the US 2nd leading cause of death in women Mortality rates though have declined 1 in 8 women will develop breast cancer Breast Cancer Breast cancer increases
Breast Cancer Most common cancer among women in the US 2nd leading cause of death in women Mortality rates though have declined 1 in 8 women will develop breast cancer Breast Cancer Breast cancer increases
ARROCase - April 2017
 ARROCase - April 2017 Radiation Indications in the setting of Neoadjuvant chemotherapy for Breast Cancer Lauren Colbert, MD, MSCR Faculty Mentor: Benjamin Smith, MD UT MD Anderson Cancer Center 37 year
ARROCase - April 2017 Radiation Indications in the setting of Neoadjuvant chemotherapy for Breast Cancer Lauren Colbert, MD, MSCR Faculty Mentor: Benjamin Smith, MD UT MD Anderson Cancer Center 37 year
Health Disparities Advances in Breast Cancer Treatment. Jo Anne Zujewski April 27, 2009
 Health Disparities Advances in Breast Cancer Treatment Jo Anne Zujewski April 27, 2009 Disclaimer Breast Cancer Incidence 1994-2003 Breast Cancer Mortality 1994-2003 Access to Care Comorbidity Biology
Health Disparities Advances in Breast Cancer Treatment Jo Anne Zujewski April 27, 2009 Disclaimer Breast Cancer Incidence 1994-2003 Breast Cancer Mortality 1994-2003 Access to Care Comorbidity Biology
Breast cancer remains the most common malignancy and the second leading. cause of cancer mortality in women in the United States.
 Dr. Andrew Seidman s Commentary I. Breast Cancer Overview Breast cancer remains the most common malignancy and the second leading cause of cancer mortality in women in the United States. The following
Dr. Andrew Seidman s Commentary I. Breast Cancer Overview Breast cancer remains the most common malignancy and the second leading cause of cancer mortality in women in the United States. The following
GASTROCARE, P.C. Contact Preference: HOME: Cell #: Office #: REASON FOR VISIT: Allergies: Current Medications (Name/Dose/How taken):
 GASTROCARE, P.C. DR. A.B. REDDY, M.D., F.A.C.G. DR. REKHA KHURANA, M.D. Referring Physician: First Name: Date of Birth: Last name: Age: Pharmacy (include location): Fax Number: Email Address: Gender: Male
GASTROCARE, P.C. DR. A.B. REDDY, M.D., F.A.C.G. DR. REKHA KHURANA, M.D. Referring Physician: First Name: Date of Birth: Last name: Age: Pharmacy (include location): Fax Number: Email Address: Gender: Male
Extended Hormonal Therapy
 Extended Hormonal Therapy Dr. Caroline Lohrisch, Medical Oncologist, BC Cancer Agency Vancouver Centre November 1, 2014 www.fpon.ca Optimal Endocrine Therapy for Women with Hormone Receptor Positive Early
Extended Hormonal Therapy Dr. Caroline Lohrisch, Medical Oncologist, BC Cancer Agency Vancouver Centre November 1, 2014 www.fpon.ca Optimal Endocrine Therapy for Women with Hormone Receptor Positive Early
So, Who are the appropriate individuals that should consider genetic counseling and genetic testing?
 Hello, I m Banu Arun, Professor of Breast Medical Oncology and Co-Director of Clinical Cancer Genetics at the University of Texas MD Anderson Cancer Center. Today I will be discussing with you Hereditary
Hello, I m Banu Arun, Professor of Breast Medical Oncology and Co-Director of Clinical Cancer Genetics at the University of Texas MD Anderson Cancer Center. Today I will be discussing with you Hereditary
A Slow Starvation: Adjuvant Endocrine Therapy of Breast Cancer
 A Slow Starvation: Adjuvant Endocrine Therapy of Breast Cancer Dr. Susan Ellard Surgical Oncology Update October 24, 2009 Disclosure slide Participant in various meetings or advisory boards sponsored by
A Slow Starvation: Adjuvant Endocrine Therapy of Breast Cancer Dr. Susan Ellard Surgical Oncology Update October 24, 2009 Disclosure slide Participant in various meetings or advisory boards sponsored by
Patient Interview Form
 Page 1 of 7 Patient Interview Form UNIVERSITY GASTROENTEROLOGY 33 Staniford Street, Providence, RI 02905 Phone 401-421-8800 Fax 401-421-2492 Patient Information First Name: MRN: Age: Last Name: Date Of
Page 1 of 7 Patient Interview Form UNIVERSITY GASTROENTEROLOGY 33 Staniford Street, Providence, RI 02905 Phone 401-421-8800 Fax 401-421-2492 Patient Information First Name: MRN: Age: Last Name: Date Of
