June Arm 1 = Observe R A N D O M I Z E
|
|
|
- Norman Sanders
- 5 years ago
- Views:
Transcription
1 June RTOG Protocol No: Protocol Status: ECOG Protocol No: R9802 Opened: October 31, 1998 NCCT Protocol No: R9802 Closed: June 27, 2002 SWOG Protocol No: R9802 Title: A Phase II Study of Observation in Favorable Low-Grade Glioma and Phase III Study of Radiation with or without PCV Chemotherapy in Unfavorable Low-Grade Glioma. Patient Population: Histologic proof of a unifocal or multifocal supratentorial WHO grade II astrocytoma (diffuse fibrillary, protoplasmic, or gemistocytic), oligodendroglioma, or oligoastrocytoma. Patients with prior suspected or proven LGG are eligible provided they now have a histologically proven eligible histology. Patients with neurofibromatosis are eligible. Stereotactic biopsies are permitted providing the tissue sample is adequate to make an unequivocal histologic diagnosis. For all patients, tissue must be obtained no more than 12 weeks before the date of registration or randomization. Objectives: Primary: Low Risk Patients To identify the overall (and relapse-free) survival of low-risk adult patients with supratentorial LGG (< 40 years old who undergo gross total resection of a WHO grade II astrocytoma, oligodendroglioma, or mixed oligoastrocytoma) who are observed postoperatively. High Risk Patients To compare the overall (and relapse-free) survival between the two arms of high-risk adult patients with supratentorial LGG ( 40 years old regardless of the degree of surgical resection, or age 18 who undergo subtotal resection or biopsy, of a WHO grade II astrocytoma, oligodendroglioma, or oligoastrocytoma) who receive postoperative external beam radiation therapy with or without PCV chemotherapy. Secondary: To compare the severe or worse toxicities ( grade 3) of unfavorable patients receiving postoperative radiation therapy alone or radiation therapy plus PCV chemotherapy. To compare the neurosurgeon s assessment of gross total resection with that of a postoperative MRI scan interpreted by a neuroradiologist. To collect and store archival, paraffin embedded tissue and peripheral blood samples for concomitant and future correlative studies which will be funded by other mechanisms. Schema: ASSESS RISK LOW RISK Age < 40 AND GROSS TOTAL RESECTION HIGH RISK * Age 40 OR SUBTOTAL RESECTION / BIOPSY Arm 1 = Observe R A N D O M I Z E Arm 2 = Radiation a Alone Arm 3 = Radiation a PCV b x 6 cycles Stratify: Tumor Subtype (astrocytoma [mixed-astro dominant] or equal [astro/oligo mix] vs. oligodendroglioma [mixedoligo dominant]); Age (< 40 vs. > 40); KPS (60-80 vs ); Contrast Enhancement on Pre-Op scan (present vs. absent). Treatment (must begin within 4 weeks after registration) a. Radiation - External Beam Radiation Therapy (EBRT) EBRT: 54 Gy/30 fractions over six weeks, 5 days a week to gross tumor volume defined by a T2 weighted post-op MRI scan (pre-op MRI acceptable if biopsy only) plus a 2 cm margin (tumor edge to block edge. There will be no boost volume. b. PCV Chemotherapy - Procarbazine/CCNU/Vincristine Procarbazine 60mg/m 2 po, Days 8-21 CCNU 110mg/m2 po, Day 1 Vincristine 1.4mg/m 2 iv, Days 8,29 (maximum dose 2 mg) PCV must start within one month following last day of RT. Repeat PCV at 8 week intervals x 6 cycles
2 June Study Chairs: Edward G. Shaw, M.D. (Radiation Oncology) Total patients entered: 370 Jan C. Buckner, M.D. (Medical Oncology) Geoffrey R. Barger, M.D. (Neuro-Oncology) Stephen W. Coons, M.D. (Neuropathology) Peter E. Ricci, M.D. (Neuroradiology) Dennis E. Bullard, M.D. (Neurosurgery) ECOG: Minesh Mehta, M.D. Mark R. Gilbert, M.D. NCCTG: Paul D. Brown, M.D. Jan C. Buckner, M.D. (Radiation Oncology) (Medical Oncology (Radiation Oncology) (Medical Oncology) SWOG: Keith J. Stelzer, M.D., Ph.D. (Radiation Oncology) Geoffrey R. Barger, M.D. (Medical Oncology) Statisticians: Research Associate: Dosimetrist: Protocol Associate: Wendy Seiferheld, M.S. Minhee Won, M.A. Barbara Kaiser, R.N., CCRP Julie McIlvaine, R.T.T. Ellen Aiken, B.A I. Summary: This study opened October 31, 1998, and closed to accrual on June 27, 2002 with 370 patients entered. Two hundred fifty-four high-risk patients were randomized to Arms 2 and 3, while 116 low risk patients were registered to the observation arm. This study used the CTC v. 2.0 and late toxicities were scored using the RTOG/EORTC Late Radiation Morbidity Scoring Schema. Chemotherapy and/or acute radiation toxicities are reported in Tables 4.1 and 4.2. On the RT Alone arm, one patient (1%) had grade 4 toxicity, and eight other patients (6%) had grade 3 toxicity. There were no grade 3 or 4 hematologic toxicities reported. On the RT+PCV arm, 4 patients had non-hematologic grade 4 toxicity (3%), and 29 other patients (23%) had non-hematologic grade 3 toxicity. There were no grade 3 late RT toxicities (Table 4.3). Study chair review of radiation therapy delivery has been completed on 224 patients, 87% of which were determined to be per protocol (Table 5.1). No reviews of chemotherapy delivery have been completed to date. II. Administrative Information: Table 2.1 Patient Accrual Study sample size 370 Total patients entered Arm 1 (Observation) Arm 2 & 3 (Treatment Arms) Average monthly accrual for the study Arm 1 (Observation) Arm 2 & 3 (Treatment Arms)
3 June Table 2.2 Study Accrual by Full Member Institutions RTOG (N=263) Foundation for Cancer Res. and Edu. 49 Cleveland Clinic Foundation 25 Medical College of Wisconsin 23 Notre Dame Hospital/Univ. of Montreal 18 University of Western Ontario 16 Wayne State University 14 Cross Cancer Institute - Univ. of Alberta 12 McGill University 10 Dartmouth Hitchcock Medical Center 8 Univ. of Pennsylvania Medical Center 8 LDS Hospital 7 Univ. of Texas-MD Anderson Cancer Center 7 University of Rochester 6 Wake Forest Univ. Baptist Medical Center 6 Mayo Clinic 5 Metro-MN CCOP 5 Thomas Jefferson Univ. Hospital 5 Radiological Associates of Sacramento 4 Washington University 4 SWOG (N=49) Tumor Institute at Swedish Hospital 11 Univ. of Washington Med. Center 10 St Francis Regional Med Ctr. 9 Grand Rapids CCOP 5 Henry Ford Hospital 4 Louisiana State Univ. Hospital 3 NCCTG (N=37) Rochester Methodist Hospital 11 Des Moines 8 Mayo Clinic - Jacksonville 3 Sioux Falls 3 Cedar Rapids 2 Duluth 2 Peoria CCOP 2 ECOG (N=21) Emory University Affiliated Hospitals 8 West Michigan Cancer Center 4 Vanderbilt University Med. Center 3 St. Vincent Regional Cancer Center CCOP 3 Toledo Comm. Hosp. Oncology Program CCOP 3 Univ. of California San Francisco 3 Univ. of Maryland Med. Systems 3 N. W. Bryant Cancer Center at St. Francis Hosp. 2 SE Cancer Control Consortium, Inc. CCOP 2 Univ. of California Davis Med. Center 2 University of Kentucky Hospital 2 Albert Einstein Medical Center 1 Benefis Healthcare CCOP 1 Colorado Cancer Research Program 1 Fox Chase Cancer Center 1 Greenville S.C. CCOP 1 Gulf Coast MBCCOP 1 Johns Hopkins Hospital 1 Kansas City CCOP 1 Ochsner Clinic CCOP 1 Santa Rosa Memorial Hospital CCOP 1 Univ. of Alabama at Birmingham Med. Ctr. 1 University of Texas Medical Branch 2 City of Hope Medical Center 1 Montana CCOP 1 Multicare - Tacoma General Hospital 1 St. Louis CCOP 1 Wesley Medical Center 1 Bismarck 1 Carle Clinic Association 1 Grand Forks Clinic 1 Rapid City Regional Hospital 1 St Cloud Hospital 1 St Joseph Hospital 1 Yale University-New Haven Hospital 3 West Virginia Univ. Med Center 2 Aultman Hospital 1 Table 2.3a Case Status Observation RT Alone RT+PCV Total Total patients entered Ineligible/canceled* Eligible/pending With on-study information With toxicity information N/A
4 June Table 2.3b *Reasons for Exclusion RX CN Status Reason Observation 337 Canceled Incorrect stratifying registration Observation 369 Ineligible Had hydrea prior to study entry RT Alone 42 Ineligible Central pathology not done prior to randomization RT Alone 59 Ineligible Wrong histology anaplastic astrocytoma RT+PCV 340 Ineligible No pre-operative and post-operative MRI scans Table 2.3c Cases with pending eligibility RX CN Status Reason Observation 368 Pending Missing P4 (central pathology review) RT+PCV 156 Pending No pre-operative and post-operative MRI scans III. Pretreatment Characteristics: Table 3.1 Pretreatment Characteristics Observation (n=114) RT Alone (n=126) RT + PCV (n=125) Age Median Range < (99%) 60 (48%) 56 (45%) 40 1 ( 1%) 66 (52%) 69 (55%) Gender Male 59 (52%) 77 (61%) 65 (52%) Female 55 (48%) 49 (39%) 60 (48%) KPS (12%) 33 (26%) 31 (25%) (88%) 93 (74%) 94 (75%) Neurologic Function 0 69 (61%) 49 (39%) 62 (50%) 1 38 (33%) 67 (53%) 49 (39%) 2 6 ( 5%) 5 ( 4%) 10 ( 8%) 3 1 ( 1%) 5 ( 4%) 4 ( 3%)
5 June Table 3.1 Pretreatment Characteristics Observation (n=114) RT Alone (n=126) RT + PCV (n=125) Surgery Total Resection 111 (97%) 12 (10%) 15 (12%) Subtotal Resection 2 ( 2%) 53 (42%) 48 (38%) Biopsy Only 0 ( 0%) 59 (47%) 59 (47%) Unknown/No Detail/Missing 1 ( 1%) 2 ( 2%) 3 ( 2%) Histology from Central Review Astrocytoma 17 (15%) 29 (23%) 37 (30%) Oligodendroglioma 50 (44%) 57 (45%) 48 (38%) Oligoastrocytoma 46 (40%) 40 (32%) 40 (32%) Pending 1 ( 1%) 0 ( 0%) 0 ( 0%) Contrast Enhancement on Pre-Op Scan Present 54 (47%) 75 (60%) 81 (65%) Absent 60 (53%) 51 (40%) 44 (35%) Pre-Op Non-enhancing Tumor Size (mm) n Mean Median Range Pre-Op Enhancing Tumor Size (mm) n Mean Median Range Risk Low 111 (97%) 1 ( 1%) 1 ( 1%) High 3 ( 3%) 125 (99%) 124 (99%) MMSE n Mean Median Range American Indian or Alaskan Native Table 3.2 Gender/Race Distributions Black, not of Hispanic origin Native Hawaiian or Pacific Islander White, not of Hispanic origin Asian Hispanic Male Female Total Total
6 June IV. Toxicity: Table 4.1 Chemotherapy and Acute Radiotherapy Toxicity RT Alone RT+PCV (n=126) (n=124) Grade Grade Allergy Auditory/hearing Blood/bone marrow Cardiovascular (arrhythmia) Cardiovascular (general) Coagulation Constitutional symptoms Dermatology/skin Endocrine Gastrointestinal Hemorrhage Hepatic Infection/febrile neutropenia Metabolic/laboratory Musculoskeletal Neurology Ocular/visual Pain Pulmonary Renal/genitourinary Sexual/reproductive functions Other tox. From chemotherapy flow sheets Worst non-hematologic (24%) (50%) (6%) (1%) (8%) (46%) (35%) (3%) Worst overall (24%) (50%) (6%) (1%) (4%) (22%) (54%) (12%)
7 June Table 4.2 All Grade 3+ Non-hematologic Toxicities RX CN Group Toxicity Days from RT Start Grade RT Alone 35 Neurology Seizure Gastrointestinal Nausea Vomiting Constitutional Symptoms Weight loss 87 3 Pain Headache 87 3 Chest pain (non-cardiac and non-pleuritic) Neurology Speech impairment Seizure Peripheral motor neuropathy Forgetfulness Constitutional Symptoms Fatigue Neurology Neuropathy cranial Peripheral motor neuropathy Ocular/visual Blurred vision Neurology Ataxia (incoordination) Neurology Seizure Gastrointestinal Nausea Vomiting Cardiovascular (general) Edema RT+PCV 4 Neurology Seizure Hepatic Gama-glutamyltransferase increased Gastrointestinal Nausea 94 3 Metabolic/laboratory Hyponatremia Gastrointestinal Constipation Pain Abdominal Pain Constitutional Symptoms Fatigue Constitutional Symptoms Fatigue 92 4 Dermatology/skin Uriticaria Pain Headache 8 3 Gastrointestinal Nausea 79 3 Vomiting Hepatic SGPT (ALT) Infection/febrile neutropenia Infection with neutropenia Pain Headache Coagulation Thrombotic microangiopathy Gastrointestinal Other-heartburn Vomiting Pain Abdominal Pain or cramping 74 3 Gastrointestinal Diarrhea without colostomy Infection/febrile neutropenia Infection with neutropenia Pulmonary Dyspnea Neurology Seizure 45 3 Constitutional Symptoms Fatigue 98 3
8 June Table 4.2 All Grade 3+ Non-hematologic Toxicities (continued) RX CN Group Toxicity Days from RT Start Grade RT+PCV 125 Hepatic GGT Sexual reproductive function Irregular menses Constitutional Symptoms Weight loss Metabolic/laboratory Hyperglycemia Neurology Speech impairment Gastrointestinal Anal fistula Gastrointestinal Nausea Constitutional Symptoms Fatigue Pain Other-pain in legs Constitutional Symptoms Fatigue Dermatology/skin Alopecia Pulmonary Dyspnea 19 3 Renal/genitourinary Vaginal hemorrhage Dermatology/skin Rash/desquamation Cardiovascular (general) Thrombosis/embolism Metabolic/laboratory Hyperglycemia No RT start date Infection/febrile neutropenia Infection without neutropenia Constitutional Symptoms Weight loss Fever Pain Headache Hepatic SGOT (AST) SGPT (ALT) Alkaline phosphatase Metabolic/laboratory Hyperglycemia/hyponatremia 62 3 Neurology Peripheral motor neuropathy Constitutional Symptoms Fatigue Neurology Insomnia Gastrointestinal Ileus Gastrointestinal Nausea 83 3 Vomiting Gastrointestinal Nausea Vomiting Anorexia Dehydration Constitutional Symptoms Fatigue Weight loss Neurology Peripheral motor neuropathy Hepatic SGPT (ALT) Neurology Speech impairment Neurology Neuralgia 98 3 Pain Neuropathic pain Gastrointestinal Vomiting Constitutional Symptoms Weight loss Allergy/immunology Autoimmune reaction 686 3
9 June Table 4.2 All Grade 3+ Non-hematologic Toxicities (continued) RX CN Group Toxicity Days from RT Start Grade RT+PCV 314 Sexual reproductive function Irregular menses (change from baseline) Dermatology/skin Injection site reaction Endocrine SIADH Hemorrhage Hemorrhage/bleeding Constitutional Symptoms Fatigue Neurology Dizziness/light headedness Hallucinations Memory loss Confusion Mood alternation-anxiety Neuropathy-sensory Constitutional Symptoms Weight loss Gastrointestinal Nausea Table 4.3 Late Radiotherapy Toxicity RT Alone RT+PCV (n=123) (n=118) Grade Grade Brain Skin (within the field) Eye Subcutaneous Tissue Other # Other # Worst overall (28%) (14%) (22%) (16%) V. Modality Reviews: Table 5.1 Radiotherapy Review RT Alone (n=126) RT+PCV (n=125) Not Reviewed 14 (11%) 13 (10%) Reviewed 112 (89%) 112 (90%) Per Protocol 102 (91%) 92 (82%) Variation Acceptable 8 ( 7%) 11 (10%) Deviation unacceptable 1 ( 1%) 5 ( 4%) Incomplete RT/death 0 ( 0%) 2 ( 2%) Not evaluable 1 ( 1%) 2 ( 2%)
June Patient Population:
 June 2003 9802-1 RTOG Protocol No: 9802 Protocol Status: ECOG Protocol No: R9802 Opened: October 31, 1998 NCCT Protocol No: R9802 Closed: June 27, 2002 SWOG Protocol No: R9802 Title: A Phase II Study of
June 2003 9802-1 RTOG Protocol No: 9802 Protocol Status: ECOG Protocol No: R9802 Opened: October 31, 1998 NCCT Protocol No: R9802 Closed: June 27, 2002 SWOG Protocol No: R9802 Title: A Phase II Study of
January Schema: S T R A T I F Y. Age 1. <50 2. >50
 January 2004 9813-1 RTOG Protocol No: 9813 Protocol Status: ECOG Protocol No: R9813 Phase I: Opened June 16, 2000 NCCTG Protocol No: R9813 Closed January 25, 2002 SWOG Protocol No: R9813 Phase III: Opened
January 2004 9813-1 RTOG Protocol No: 9813 Protocol Status: ECOG Protocol No: R9813 Phase I: Opened June 16, 2000 NCCTG Protocol No: R9813 Closed January 25, 2002 SWOG Protocol No: R9813 Phase III: Opened
June Schema: S T R A I T I F Y. Age 1. <50 2. >50
 June 2003 9813-1 RTOG Protocol No: 9813 Protocol Status: ECOG Protocol No: R9813 Opened: June 16, 2000 NCCTG Protocol No: R9813 SWOG Protocol No: R9813 Title: A Phase / Randomized Study of Radiation Therapy
June 2003 9813-1 RTOG Protocol No: 9813 Protocol Status: ECOG Protocol No: R9813 Opened: June 16, 2000 NCCTG Protocol No: R9813 SWOG Protocol No: R9813 Title: A Phase / Randomized Study of Radiation Therapy
January NCCTG Protocol No: N0628 Opened: November 27, 2007 CALGB Protocol No: ECOG Protocol No: R0617
 January 2010 0617-1 RTOG Protocol No: 0617 Protocol Status: NCCTG Protocol No: N0628 Opened: November 27, 2007 CALGB Protocol No: 30609 ECOG Protocol No: R0617 Title: A Randomized Phase III Comparison
January 2010 0617-1 RTOG Protocol No: 0617 Protocol Status: NCCTG Protocol No: N0628 Opened: November 27, 2007 CALGB Protocol No: 30609 ECOG Protocol No: R0617 Title: A Randomized Phase III Comparison
Objectives Primary Objective: Secondary Objectives For T4 a, b, c tumors:
 Z1031 A randomized phase III trial comparing 16 to 18 weeks of neoadjuvant exemestane (25mg daily), letrozole (2.5mg), or anastrozole (1mg) in postmenopausal women with clinical stage II and III estrogen
Z1031 A randomized phase III trial comparing 16 to 18 weeks of neoadjuvant exemestane (25mg daily), letrozole (2.5mg), or anastrozole (1mg) in postmenopausal women with clinical stage II and III estrogen
NCCTG Status Report for Study N September 2007
 Phase I/II Study of Concurrent Chemotherapy and Escalating Doses 3-D Conformal Radiotherapy (RT) Followed by Three Cycles of Chemotherapy for Unresectable Non-Small Cell Lung Cancer (NSCLC) Using a New
Phase I/II Study of Concurrent Chemotherapy and Escalating Doses 3-D Conformal Radiotherapy (RT) Followed by Three Cycles of Chemotherapy for Unresectable Non-Small Cell Lung Cancer (NSCLC) Using a New
Arm A: Induction Gemcitabine 1000 mg/m 2 IV once a week for 6 weeks.
 ECOG-4201 (RTOG Endorsed) ECOG 4201 Pancreas (RTOG Endorsed)-1 Protocol Status: Opened: April 10, 2003 Closed: December 15, 2005 Title: A Randomized Phase III Study of Gemcitabine in Combination with Radiation
ECOG-4201 (RTOG Endorsed) ECOG 4201 Pancreas (RTOG Endorsed)-1 Protocol Status: Opened: April 10, 2003 Closed: December 15, 2005 Title: A Randomized Phase III Study of Gemcitabine in Combination with Radiation
Chemotherapy plus Radiotherapy versus Radiotherapy Alone for Patients with Anaplastic Oligodendroglioma: Long Term Results of RTOG 9402
 Chemotherapy plus Radiotherapy versus Radiotherapy Alone for Patients with Anaplastic Oligodendroglioma: Long Term Results of RTOG 9402 Gregory Cairncross, Meihua Wang, Edward Shaw, Berndt Scheithauer
Chemotherapy plus Radiotherapy versus Radiotherapy Alone for Patients with Anaplastic Oligodendroglioma: Long Term Results of RTOG 9402 Gregory Cairncross, Meihua Wang, Edward Shaw, Berndt Scheithauer
GOG PROTOCOL #209. David Miller, MD, Gini Fleming, MD, Richard Zaino, MD, and David Cella, PhD
 GOG PROTOCOL #209 PROTOCOL Study Chairs Statistician Data Coordinator Randomized Phase III Trial of Doxorubicin/Cisplatin/Paclitaxel and G-CSF Versus Carboplatin/Paclitaxel in Patients with Stage III and
GOG PROTOCOL #209 PROTOCOL Study Chairs Statistician Data Coordinator Randomized Phase III Trial of Doxorubicin/Cisplatin/Paclitaxel and G-CSF Versus Carboplatin/Paclitaxel in Patients with Stage III and
June 2009 Respiratory Committee CALGB 30610
 30610 Phase III comparison of thoracic radiotherapy regimens in patients with limited small cell lung cancer also receiving cisplatin and etoposide Activated: March 15, 2008 Study Chairpersons: J. Bogart
30610 Phase III comparison of thoracic radiotherapy regimens in patients with limited small cell lung cancer also receiving cisplatin and etoposide Activated: March 15, 2008 Study Chairpersons: J. Bogart
EORTC (RTOG 0834 Endorsed) Opened: July 22, 2009
 January 2011 0834-1 EORTC 26053 22054 (RTOG 0834 Endorsed) Protocol Status: Opened: July 22, 2009 Title: Phase III Trial on Concurrent and Adjuvant Temozolomide Chemotherapy in Non-1P/19Q Deleted Anaplastic
January 2011 0834-1 EORTC 26053 22054 (RTOG 0834 Endorsed) Protocol Status: Opened: July 22, 2009 Title: Phase III Trial on Concurrent and Adjuvant Temozolomide Chemotherapy in Non-1P/19Q Deleted Anaplastic
Collection of Recorded Radiotherapy Seminars
 IAEA Human Health Campus Collection of Recorded Radiotherapy Seminars http://humanhealth.iaea.org The Role of Radiosurgery in the Treatment of Gliomas Luis Souhami, MD Professor Department of Radiation
IAEA Human Health Campus Collection of Recorded Radiotherapy Seminars http://humanhealth.iaea.org The Role of Radiosurgery in the Treatment of Gliomas Luis Souhami, MD Professor Department of Radiation
6 cycles* January Opened: January 17, 2006
 January 2008 0525-1 RTOG Protocol No: 0525 Protocol Status: Opened: January 17, 2006 Title: Phase III Trial Comparing Conventional Adjuvant Temozolomide with Dose-Intensive Temozolomide in Patients with
January 2008 0525-1 RTOG Protocol No: 0525 Protocol Status: Opened: January 17, 2006 Title: Phase III Trial Comparing Conventional Adjuvant Temozolomide with Dose-Intensive Temozolomide in Patients with
GOG PROTOCOL #209 GOG 209
 GOG PROTOCOL #209 PROTOCOL Study Chairs Statistician Data Coordinator Randomized Phase III Trial of Doxorubicin/Cisplatin/Paclitaxel and G-CSF Versus Carboplatin/Paclitaxel in Patients with Stage III and
GOG PROTOCOL #209 PROTOCOL Study Chairs Statistician Data Coordinator Randomized Phase III Trial of Doxorubicin/Cisplatin/Paclitaxel and G-CSF Versus Carboplatin/Paclitaxel in Patients with Stage III and
NSABP PROTOCOL B-34. Date Opened to Randomization: December 1, 2000 Date Closed to Randomization: March 31, 2004 Number of Patients Randomized: 3,323
 NSABP PROTOCOL B-34 A Clinical Trial Comparing Adjuvant Clodronate Therapy versus Placebo in Early-Stage Breast Cancer Patients Receiving Systemic Chemotherapy and/or Tamoxifen or No Therapy Date Opened
NSABP PROTOCOL B-34 A Clinical Trial Comparing Adjuvant Clodronate Therapy versus Placebo in Early-Stage Breast Cancer Patients Receiving Systemic Chemotherapy and/or Tamoxifen or No Therapy Date Opened
Prior to 1993, the only data available in the medical
 Neuro-Oncology Prospective clinical trials of intracranial low-grade glioma in adults and children Edward G. Shaw 1 and Jeffrey H. Wisoff Department of Radiation Oncology, Wake Forest University School
Neuro-Oncology Prospective clinical trials of intracranial low-grade glioma in adults and children Edward G. Shaw 1 and Jeffrey H. Wisoff Department of Radiation Oncology, Wake Forest University School
EASTERN COOPERATIVE ONCOLOGY GROUP
 EASTERN COOPERATIVE ONCOLOGY GROUP E5204 INTERGROUP RANDOMIZED PHASE III STUDY OF POSTOPERATIVE OXALIPLATIN, 5-FLUOROURACIL AND LEUCOVORIN VS OXALIPLATIN, 5-FLUOROURACIL, LEUCOV- ORIN AND BEVACIZUMAB FOR
EASTERN COOPERATIVE ONCOLOGY GROUP E5204 INTERGROUP RANDOMIZED PHASE III STUDY OF POSTOPERATIVE OXALIPLATIN, 5-FLUOROURACIL AND LEUCOVORIN VS OXALIPLATIN, 5-FLUOROURACIL, LEUCOV- ORIN AND BEVACIZUMAB FOR
Systemic Treatment. Third International Neuro-Oncology Course. 23 May 2014
 Low-Grade Astrocytoma of the CNS: Systemic Treatment Third International Neuro-Oncology Course São Paulo, Brazil 23 May 2014 John de Groot, MD Associate Professor, Neuro-Oncology UT MD Anderson Cancer
Low-Grade Astrocytoma of the CNS: Systemic Treatment Third International Neuro-Oncology Course São Paulo, Brazil 23 May 2014 John de Groot, MD Associate Professor, Neuro-Oncology UT MD Anderson Cancer
PROCARBAZINE, lomustine, and vincristine (PCV) is
 RAPID PUBLICATION Procarbazine, Lomustine, and Vincristine () Chemotherapy for Anaplastic Astrocytoma: A Retrospective Review of Radiation Therapy Oncology Group Protocols Comparing Survival With Carmustine
RAPID PUBLICATION Procarbazine, Lomustine, and Vincristine () Chemotherapy for Anaplastic Astrocytoma: A Retrospective Review of Radiation Therapy Oncology Group Protocols Comparing Survival With Carmustine
S0106 Phase III. Coordinating Group: SWOG
 S0106 Phase III Coordinating Group: SWOG A Phase III Study of the Addition of Gemtuzumab Ozogamicin (Mylotarg ) Induction Therapy Versus Standard Induction with Daunomycin and Cytosine Arabinoside Followed
S0106 Phase III Coordinating Group: SWOG A Phase III Study of the Addition of Gemtuzumab Ozogamicin (Mylotarg ) Induction Therapy Versus Standard Induction with Daunomycin and Cytosine Arabinoside Followed
Objectives Primary Objectives:
 Z1031 A randomized phase III trial comparing 16 to 18 weeks of neoadjuvant exemestane (25mg daily), letrozole (2.5mg), or anastrozole (1mg) in postmenopausal women with clinical stage II and III estrogen
Z1031 A randomized phase III trial comparing 16 to 18 weeks of neoadjuvant exemestane (25mg daily), letrozole (2.5mg), or anastrozole (1mg) in postmenopausal women with clinical stage II and III estrogen
June Opened: January 17, 2006 Closed: June 13, 2008
 June 2009 0525-1 RTOG Protocol No: 0525 Protocol Status: Opened: January 17, 2006 Closed: June 13, 2008 Title: Phase III Trial Comparing Conventional Adjuvant Temozolomide with Dose-Intensive Temozolomide
June 2009 0525-1 RTOG Protocol No: 0525 Protocol Status: Opened: January 17, 2006 Closed: June 13, 2008 Title: Phase III Trial Comparing Conventional Adjuvant Temozolomide with Dose-Intensive Temozolomide
21/03/2017. Disclosure. Practice Changing Articles in Neuro Oncology for 2016/17. Gliomas. Objectives. Gliomas. No conflicts to declare
 Practice Changing Articles in Neuro Oncology for 2016/17 Disclosure No conflicts to declare Frances Cusano, BScPharm, ACPR April 21, 2017 Objectives Gliomas To describe the patient selection, methodology
Practice Changing Articles in Neuro Oncology for 2016/17 Disclosure No conflicts to declare Frances Cusano, BScPharm, ACPR April 21, 2017 Objectives Gliomas To describe the patient selection, methodology
Supplementary Appendix
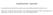 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine,
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus procarbazine,
Phase II Trial of Observation for Low-Risk Meningiomas and of Radiotherapy for Intermediate- and High-Risk Meningiomas
 RTOG 0539 Phase II Trial of Observation for Low-Risk Meningiomas and of Radiotherapy for Intermediate- and High-Risk Meningiomas Volume 4, Issue 2 September 2012 I N T HIS I SSUE Thoughts From the Chair
RTOG 0539 Phase II Trial of Observation for Low-Risk Meningiomas and of Radiotherapy for Intermediate- and High-Risk Meningiomas Volume 4, Issue 2 September 2012 I N T HIS I SSUE Thoughts From the Chair
NCCTG Status Report for Study N May 2010
 MARVEL: Marker Validation of Erlotinib in Lung Cancer - A Phase III Biomarker Validation Study of Second-Line Therapy in Patients With Advanced Non-Small Cell Lung Cancer (NSCLC) Randomized to Pemetrexed
MARVEL: Marker Validation of Erlotinib in Lung Cancer - A Phase III Biomarker Validation Study of Second-Line Therapy in Patients With Advanced Non-Small Cell Lung Cancer (NSCLC) Randomized to Pemetrexed
Radioterapia no Tratamento dos Gliomas de Baixo Grau
 Radioterapia no Tratamento dos Gliomas de Baixo Grau Dr. Luis Souhami University Montreal - Canada Low Grade Gliomas Relatively rare Heterogeneous, slow growing tumors WHO Classification Grade I Pilocytic
Radioterapia no Tratamento dos Gliomas de Baixo Grau Dr. Luis Souhami University Montreal - Canada Low Grade Gliomas Relatively rare Heterogeneous, slow growing tumors WHO Classification Grade I Pilocytic
NCCTG Status Report for Study N April 2008
 Phase II Randomized Study of Pemetrexed With Sorafenib Versus Pemetrexed lone as Second-line Therapy in Patients With dvanced Non-Small Cell Lung Cancer Purpose of - Primary Study: 1) To compare the progression-free
Phase II Randomized Study of Pemetrexed With Sorafenib Versus Pemetrexed lone as Second-line Therapy in Patients With dvanced Non-Small Cell Lung Cancer Purpose of - Primary Study: 1) To compare the progression-free
PRESURGICAL PLANNING. Strongly consider neuropsychological evaluation before functional imaging study Strongly consider functional imaging study
 NOTE: Consider Clinical Trials as treatment options for eligible patients. Page 1 of 6 RADIOLOGICAL PRESENTATION PRESURGICAL PLANNING TREATMENT Imaging study suggestive of glioma 1 Left hemisphere speech/motor
NOTE: Consider Clinical Trials as treatment options for eligible patients. Page 1 of 6 RADIOLOGICAL PRESENTATION PRESURGICAL PLANNING TREATMENT Imaging study suggestive of glioma 1 Left hemisphere speech/motor
Low grade glioma: a journey towards a cure
 Editorial Page 1 of 5 Low grade glioma: a journey towards a cure Ali K. Choucair SIU School of Medicine, Springfield, IL, USA Correspondence to: Ali K. Choucair, MD. Professor of Neurology, Director of
Editorial Page 1 of 5 Low grade glioma: a journey towards a cure Ali K. Choucair SIU School of Medicine, Springfield, IL, USA Correspondence to: Ali K. Choucair, MD. Professor of Neurology, Director of
Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma
 The new england journal of medicine Original Article Radiation plus Procarbazine, CCNU, and Vincristine in Low- Glioma Jan C. Buckner, M.D., Edward G. Shaw, M.D., Stephanie L. Pugh, Ph.D., Arnab Chakravarti,
The new england journal of medicine Original Article Radiation plus Procarbazine, CCNU, and Vincristine in Low- Glioma Jan C. Buckner, M.D., Edward G. Shaw, M.D., Stephanie L. Pugh, Ph.D., Arnab Chakravarti,
CNS Tumors: The Med Onc Perspective. Ronald J. Scheff, MD Associate Clinical Professor Weill Medical College of Cornell U.
 CNS Tumors: The Med Onc Perspective Ronald J. Scheff, MD Associate Clinical Professor Weill Medical College of Cornell U. Disclosure Speakers Bureau, Merck Basic Oncology Concepts Tissue Diagnosis Stage
CNS Tumors: The Med Onc Perspective Ronald J. Scheff, MD Associate Clinical Professor Weill Medical College of Cornell U. Disclosure Speakers Bureau, Merck Basic Oncology Concepts Tissue Diagnosis Stage
Chika Nwachukwu, Ph.D. MS IV Radiation Oncology Rotation
 Chika Nwachukwu, Ph.D. MS IV Radiation Oncology Rotation Background Histology/Tumor Characteristics Presenting Symptoms/diagnosis Treatment/outcome Patient cohort Research on HRQOL Slow growing indolent
Chika Nwachukwu, Ph.D. MS IV Radiation Oncology Rotation Background Histology/Tumor Characteristics Presenting Symptoms/diagnosis Treatment/outcome Patient cohort Research on HRQOL Slow growing indolent
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM ANAPLASTIC GLIOMAS CNS Site Group Anaplastic Gliomas Author: Dr. Norm Laperriere Date: February 20, 2018 1. INTRODUCTION
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM ANAPLASTIC GLIOMAS CNS Site Group Anaplastic Gliomas Author: Dr. Norm Laperriere Date: February 20, 2018 1. INTRODUCTION
January Opened: April 15, 2009
 January 2011 0825-1 RTOG Protocol No: 0825 Protocol Status: Opened: April 15, 2009 Title: Phase III Double-Blind Placebo-Controlled Trial of Conventional Concurrent Chemoradiation and Adjuvant Temozolomide
January 2011 0825-1 RTOG Protocol No: 0825 Protocol Status: Opened: April 15, 2009 Title: Phase III Double-Blind Placebo-Controlled Trial of Conventional Concurrent Chemoradiation and Adjuvant Temozolomide
June 2009 Breast Committee CALGB 40502
 CALGB 40502/CTSU 40502 A randomized phase III trial of weekly paclitaxel compared to weekly nanoparticle albumin bound nabpaclitaxel or ixabepilone combined with bevacizumab as first-line therapy for locally
CALGB 40502/CTSU 40502 A randomized phase III trial of weekly paclitaxel compared to weekly nanoparticle albumin bound nabpaclitaxel or ixabepilone combined with bevacizumab as first-line therapy for locally
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM LOW GRADE GLIOMAS CNS Site Group Low Grade Gliomas Author: Dr. Norm Laperriere 1. INTRODUCTION 3 2. PREVENTION 3 3. SCREENING
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM LOW GRADE GLIOMAS CNS Site Group Low Grade Gliomas Author: Dr. Norm Laperriere 1. INTRODUCTION 3 2. PREVENTION 3 3. SCREENING
Protocol Abstract and Schema
 Protocol Abstract and Schema Phase II study of Bevacizumab plus Irinotecan (Camptosar ) in Children with Recurrent, Progressive, or Refractory Malignant Gliomas, Diffuse/Intrinsic Brain Stem Gliomas, Medulloblastomas,
Protocol Abstract and Schema Phase II study of Bevacizumab plus Irinotecan (Camptosar ) in Children with Recurrent, Progressive, or Refractory Malignant Gliomas, Diffuse/Intrinsic Brain Stem Gliomas, Medulloblastomas,
Disclosures. Dr. Hall is a paid consultant to the American College of Surgeons (ACS) as Associate Director of ACS-NSQIP
 Does Routine Drainage of the Operative Bed following Elective Distal Pancreatectomy reduce Complications? An Analysis of the ACS-NSQIP Pancreatectomy Demonstration Project Stephen W. Behrman, MD 1, Ben
Does Routine Drainage of the Operative Bed following Elective Distal Pancreatectomy reduce Complications? An Analysis of the ACS-NSQIP Pancreatectomy Demonstration Project Stephen W. Behrman, MD 1, Ben
Nilotinib AEs (adverse events) in CML population:
 Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
NCCTG Status Report for Study N May 2010
 Phase I/II Study of Vorinostat (Suberoylanilide Hydroxamic cid SH), Temozolomide, and Radiation Therapy in Patients with Newly Diagnosed Glioblastoma Purpose of - Phase I Study: 1) To determine the maximum
Phase I/II Study of Vorinostat (Suberoylanilide Hydroxamic cid SH), Temozolomide, and Radiation Therapy in Patients with Newly Diagnosed Glioblastoma Purpose of - Phase I Study: 1) To determine the maximum
LOW GRADE ASTROCYTOMAS
 LOW GRADE ASTROCYTOMAS This article was provided to us by David Schiff, MD, Associate Professor of Neurology, Neurosurgery, and Medicine at University of Virginia, Charlottesville. We appreciate his generous
LOW GRADE ASTROCYTOMAS This article was provided to us by David Schiff, MD, Associate Professor of Neurology, Neurosurgery, and Medicine at University of Virginia, Charlottesville. We appreciate his generous
Chemoradiation (CRT) Safety Analysis of ACOSOG Z6041: A Phase II Trial of Neoadjuvant CRT followed by Local Excision in ut2 Rectal Cancer
 Chemoradiation (CRT) Safety Analysis of ACOSOG Z6041: A Phase II Trial of Neoadjuvant CRT followed by Local Excision in ut2 Rectal Cancer Emily Chan, Qian Shi, Julio Garcia-Aguilar, Peter Cataldo, Jorge
Chemoradiation (CRT) Safety Analysis of ACOSOG Z6041: A Phase II Trial of Neoadjuvant CRT followed by Local Excision in ut2 Rectal Cancer Emily Chan, Qian Shi, Julio Garcia-Aguilar, Peter Cataldo, Jorge
Cancer Treatment Trials:
 Neuro-Oncology Program The NCCTG Neuro-Oncology program contains three project areas: (1) cancer treatment trials, (2) neurobehavioral studies, and (3) translational research. Cancer Treatment Trials:
Neuro-Oncology Program The NCCTG Neuro-Oncology program contains three project areas: (1) cancer treatment trials, (2) neurobehavioral studies, and (3) translational research. Cancer Treatment Trials:
Hospital he hospital is located near the interchange of highway 217 and (US 26).
 Welcome to our Clinic! Our goal is to provide you with the highest quality medical care available. Please bring the completed enclosed paperwork along with your insurance card and legal picture ID to your
Welcome to our Clinic! Our goal is to provide you with the highest quality medical care available. Please bring the completed enclosed paperwork along with your insurance card and legal picture ID to your
Update on Pediatric Brain Tumors
 Update on Pediatric Brain Tumors David I. Sandberg, M.D. Director of Pediatric Neurosurgery & Associate Professor Dr. Marnie Rose Professorship in Pediatric Neurosurgery Pre-talk Questions for Audience
Update on Pediatric Brain Tumors David I. Sandberg, M.D. Director of Pediatric Neurosurgery & Associate Professor Dr. Marnie Rose Professorship in Pediatric Neurosurgery Pre-talk Questions for Audience
UPDATES ON CHEMOTHERAPY FOR LOW GRADE GLIOMAS
 UPDATES ON CHEMOTHERAPY FOR LOW GRADE GLIOMAS Antonio M. Omuro Department of Neurology Memorial Sloan-Kettering Cancer Center II International Neuro-Oncology Congress Sao Paulo, 08/17/12 CHALLENGES IN
UPDATES ON CHEMOTHERAPY FOR LOW GRADE GLIOMAS Antonio M. Omuro Department of Neurology Memorial Sloan-Kettering Cancer Center II International Neuro-Oncology Congress Sao Paulo, 08/17/12 CHALLENGES IN
Supplementary Online Content
 Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Supplementary Online Content Powles T, O Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 openlabel
Distal Pancreatectomy with Celiac Axis Resection: What Are the Added Risks?
 Distal Pancreatectomy with Celiac Axis Resection: What Are the Added Risks? Joal D. Beane, MD a, Michael G. House, MD a, Susan C. Pitt, MD c, E. Molly Kilbane a, Bruce L. Hall c, Abishek Parmar d, Taylor.
Distal Pancreatectomy with Celiac Axis Resection: What Are the Added Risks? Joal D. Beane, MD a, Michael G. House, MD a, Susan C. Pitt, MD c, E. Molly Kilbane a, Bruce L. Hall c, Abishek Parmar d, Taylor.
Selecting the Optimal Treatment for Brain Metastases
 Selecting the Optimal Treatment for Brain Metastases Clinical Practice Today CME Co-provided by Learning Objectives Upon completion, participants should be able to: Understand the benefits, limitations,
Selecting the Optimal Treatment for Brain Metastases Clinical Practice Today CME Co-provided by Learning Objectives Upon completion, participants should be able to: Understand the benefits, limitations,
Sponsor / Company: Sanofi Drug substance(s): SAR (iniparib)
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Background: Brain Mets in Breast Cancer
 ANG1005, A Novel Brain-Penetrant Taxane Derivative, for the Treatment of Recurrent Brain Metastases and Leptomeningeal Carcinomatosis from Breast Cancer Priya Kumthekar 1, Shou-Ching Tang 2, Andrew Jacob
ANG1005, A Novel Brain-Penetrant Taxane Derivative, for the Treatment of Recurrent Brain Metastases and Leptomeningeal Carcinomatosis from Breast Cancer Priya Kumthekar 1, Shou-Ching Tang 2, Andrew Jacob
Oncological Management of Brain Tumours. Anna Maria Shiarli SpR in Clinical Oncology 15 th July 2013
 Oncological Management of Brain Tumours Anna Maria Shiarli SpR in Clinical Oncology 15 th July 2013 Outline General considerations of Primary Brain Tumours: epidemiology, pathology, presentation. Diagnosis
Oncological Management of Brain Tumours Anna Maria Shiarli SpR in Clinical Oncology 15 th July 2013 Outline General considerations of Primary Brain Tumours: epidemiology, pathology, presentation. Diagnosis
Imaging for suspected glioma
 Imaging for suspected glioma 1.1.1 Offer standard structural MRI (defined as T2 weighted, FLAIR, DWI series and T1 pre- and post-contrast volume) as the initial diagnostic test for suspected glioma, unless
Imaging for suspected glioma 1.1.1 Offer standard structural MRI (defined as T2 weighted, FLAIR, DWI series and T1 pre- and post-contrast volume) as the initial diagnostic test for suspected glioma, unless
Adjuvant Therapy in Locally Advanced Head and Neck Cancer. Ezra EW Cohen University of Chicago. Financial Support
 Adjuvant Therapy in Locally Advanced Head and Neck Cancer Ezra EW Cohen University of Chicago Financial Support This program is made possible by an educational grant from Eli Lilly Oncology, who had no
Adjuvant Therapy in Locally Advanced Head and Neck Cancer Ezra EW Cohen University of Chicago Financial Support This program is made possible by an educational grant from Eli Lilly Oncology, who had no
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy
PATIENT INFORMATION SHEET
 ALAMO NEUROSURGICAL INSTITUTE 414 W SUNSET, SUITE 205 SAN ANTONIO, TEXAS 78209 WWW.ANI-ONLINE.COM OFF: 210.564.8300 FAX: 210.564.8399 PATIENT INFORMATION SHEET Patient Name (Last, First, Mi): SSN: Street
ALAMO NEUROSURGICAL INSTITUTE 414 W SUNSET, SUITE 205 SAN ANTONIO, TEXAS 78209 WWW.ANI-ONLINE.COM OFF: 210.564.8300 FAX: 210.564.8399 PATIENT INFORMATION SHEET Patient Name (Last, First, Mi): SSN: Street
Contemporary Management of Glioblastoma
 Contemporary Management of Glioblastoma Incidence Rates of Primary Brain Tumors Central Brain Tumor Registry of the United States, 1992-1997 100 Number of Cases per 100,000 Population 10 1 0.1 x I x I
Contemporary Management of Glioblastoma Incidence Rates of Primary Brain Tumors Central Brain Tumor Registry of the United States, 1992-1997 100 Number of Cases per 100,000 Population 10 1 0.1 x I x I
Response to postoperative radiotherapy as a prognostic factor for patients with low-grade gliomas
 ONCOLOGY LETTERS 4: 455-460, 2012 Response to postoperative radiotherapy as a prognostic factor for patients with low-grade gliomas MICHAL SPYCH 1,2, LESZEK GOTTWALD 3, EMILIA JESIEŃ LEWANDOWICZ 1,2, SŁAWOMIR
ONCOLOGY LETTERS 4: 455-460, 2012 Response to postoperative radiotherapy as a prognostic factor for patients with low-grade gliomas MICHAL SPYCH 1,2, LESZEK GOTTWALD 3, EMILIA JESIEŃ LEWANDOWICZ 1,2, SŁAWOMIR
Patient Interview Form
 Page 1 of 6 Patient Interview Form Patient Information First Name: MRN: Age: Last Name: Date Of Birth: Notes: Email Please check one as your preferred email for communications Personal: Work: Race Select
Page 1 of 6 Patient Interview Form Patient Information First Name: MRN: Age: Last Name: Date Of Birth: Notes: Email Please check one as your preferred email for communications Personal: Work: Race Select
Neuro-Oncology. Martin J. van den Bent. Department of Neuro-oncology/Neurology, Erasmus M.C. Cancer Institute, Rotterdam, Netherlands
 Neuro-Oncology Neuro-Oncology 16(12), 1570 1574, 2014 doi:10.1093/neuonc/nou297 Advance Access date 29 October 2014 Practice changing mature results of RTOG study 9802: another positive PCV trial makes
Neuro-Oncology Neuro-Oncology 16(12), 1570 1574, 2014 doi:10.1093/neuonc/nou297 Advance Access date 29 October 2014 Practice changing mature results of RTOG study 9802: another positive PCV trial makes
PATIENT REGISTRATION
 P Account# PATIENT REGISTRATION Please answer all questions completely. PAYMENT IS EXPECTED WHEN SERVICES ARE RENDERED Date New Update Name Date of Birth Male Last First Middle Female Home Address City/State/Zip
P Account# PATIENT REGISTRATION Please answer all questions completely. PAYMENT IS EXPECTED WHEN SERVICES ARE RENDERED Date New Update Name Date of Birth Male Last First Middle Female Home Address City/State/Zip
Supplementary Online Content
 Supplementary Online Content Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes:
Supplementary Online Content Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes:
GASTROCARE, P.C. Contact Preference: HOME: Cell #: Office #: REASON FOR VISIT: Allergies: Current Medications (Name/Dose/How taken):
 GASTROCARE, P.C. DR. A.B. REDDY, M.D., F.A.C.G. DR. REKHA KHURANA, M.D. Referring Physician: First Name: Date of Birth: Last name: Age: Pharmacy (include location): Fax Number: Email Address: Gender: Male
GASTROCARE, P.C. DR. A.B. REDDY, M.D., F.A.C.G. DR. REKHA KHURANA, M.D. Referring Physician: First Name: Date of Birth: Last name: Age: Pharmacy (include location): Fax Number: Email Address: Gender: Male
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Florida Hospital Spine Center Patient Intake Form
 Florida Hospital Spine Center Patient Intake Form Today s Date Last Name First Name Middle Street Address DOB (Address, City, State, Zip Code) First Contact # Please Circle: Home Cell Other Second Contact
Florida Hospital Spine Center Patient Intake Form Today s Date Last Name First Name Middle Street Address DOB (Address, City, State, Zip Code) First Contact # Please Circle: Home Cell Other Second Contact
Patient Interview Form
 Page 1 of 5 Patient Interview Form Patient Information First Name: MRN: Last Name: Date Of Birth: Contact Preference Email Telephone call- Work Telephone call - Home Email Please check one as your preferred
Page 1 of 5 Patient Interview Form Patient Information First Name: MRN: Last Name: Date Of Birth: Contact Preference Email Telephone call- Work Telephone call - Home Email Please check one as your preferred
Irinotecan. Class:Camptothecin. Indications : _Cervical cancer. _CNS tumor. _Esophageal cancer. _Ewing s sarcoma. _Gastric cancer
 Irinotecan Class:Camptothecin Indications : _Cervical cancer _CNS tumor _Esophageal cancer _Ewing s sarcoma _Gastric cancer _Nonsmall cell lung cancer _Pancreatic cancer _Small cell lung cancer _Colorectal
Irinotecan Class:Camptothecin Indications : _Cervical cancer _CNS tumor _Esophageal cancer _Ewing s sarcoma _Gastric cancer _Nonsmall cell lung cancer _Pancreatic cancer _Small cell lung cancer _Colorectal
Supplementary Online Content
 Supplementary Online Content Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. Published online
Supplementary Online Content Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. Published online
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
Special pediatric considerations are noted when applicable, otherwise adult provisions apply.
 DRUG NAME: Lomustine SYNONYM(S): CCNU 1 COMMON TRADE NAME(S): CeeNU CLASSIFICATION: alkylating agent Special pediatric considerations are noted when applicable, otherwise adult provisions apply. MECHANISM
DRUG NAME: Lomustine SYNONYM(S): CCNU 1 COMMON TRADE NAME(S): CeeNU CLASSIFICATION: alkylating agent Special pediatric considerations are noted when applicable, otherwise adult provisions apply. MECHANISM
AMERICAN BRAIN TUMOR ASSOCIATION. Oligodendroglioma and Oligoastrocytoma
 AMERICAN BRAIN TUMOR ASSOCIATION Oligodendroglioma and Oligoastrocytoma ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the
AMERICAN BRAIN TUMOR ASSOCIATION Oligodendroglioma and Oligoastrocytoma ACKNOWLEDGEMENTS ABOUT THE AMERICAN BRAIN TUMOR ASSOCIATION Founded in 1973, the American Brain Tumor Association (ABTA) was the
Modesto Gastroenterology Medical Corporation
 Page 1 of 5 Modesto Gastroenterology Medical Corporation Magdy S. Elsakr, M.D. Board Certified Gastroenterologist 2336 Sylvan Avenue, Suite A, Modesto, CA 95355, Phone: 209-338-0292, Fax: 209-338-0298
Page 1 of 5 Modesto Gastroenterology Medical Corporation Magdy S. Elsakr, M.D. Board Certified Gastroenterologist 2336 Sylvan Avenue, Suite A, Modesto, CA 95355, Phone: 209-338-0292, Fax: 209-338-0298
WARNING, CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS,
 Celgene Corporation 86 Morris Avenue Summit, New Jersey 07901 Tel 908-673-9000 Fax 908-673-9001 October 2012 NEW Indication Announcement for ABRAXANE for Injectable Suspension (paclitaxel protein-bound
Celgene Corporation 86 Morris Avenue Summit, New Jersey 07901 Tel 908-673-9000 Fax 908-673-9001 October 2012 NEW Indication Announcement for ABRAXANE for Injectable Suspension (paclitaxel protein-bound
Allina Health United Lung and Sleep Clinic
 Medical History Form Date Allina Health United Lung and Sleep Clinic Name Last First MI Date of birth What lung problem do you want us to help you with: Who is your primary care provider? Social History
Medical History Form Date Allina Health United Lung and Sleep Clinic Name Last First MI Date of birth What lung problem do you want us to help you with: Who is your primary care provider? Social History
HSV1716 Dose levels and Cohort size Dose level No of Patients HSV1716 Dosage 1* 3 to 6 1 ml of 1 x 10 5 infectious units HSV1716 per ml 2
 Abstract and Schema: Description and Rationale: Pediatric high grade gliomas have a progressive initial course and high risk of relapse/ progression; making the 5-year overall survival rate 15-35% with
Abstract and Schema: Description and Rationale: Pediatric high grade gliomas have a progressive initial course and high risk of relapse/ progression; making the 5-year overall survival rate 15-35% with
Oligodendroglial Tumors: A Review
 Oligodendroglial Tumors: A Review Sajeel Chowdhary, MD H Lee Moffitt Cancer Center and Research Institute Marc C Chamberlain, MD H Lee Moffitt Cancer Center and Research Institute Corresponding author:
Oligodendroglial Tumors: A Review Sajeel Chowdhary, MD H Lee Moffitt Cancer Center and Research Institute Marc C Chamberlain, MD H Lee Moffitt Cancer Center and Research Institute Corresponding author:
Hypofractionated radiation therapy for glioblastoma
 Hypofractionated radiation therapy for glioblastoma Luis Souhami, MD, FASTRO Professor McGill University Department of Oncology, Division of Radiation Oncology Montreal Canada McGill University Health
Hypofractionated radiation therapy for glioblastoma Luis Souhami, MD, FASTRO Professor McGill University Department of Oncology, Division of Radiation Oncology Montreal Canada McGill University Health
NPAC+PERT+TRAS Regimen
 Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM MENINGIOMA CNS Site Group Meningioma Author: Dr. Norm Laperriere Date: February 20, 2018 1. INTRODUCTION 3 2. PREVENTION
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM MENINGIOMA CNS Site Group Meningioma Author: Dr. Norm Laperriere Date: February 20, 2018 1. INTRODUCTION 3 2. PREVENTION
NRG ONCOLOGY NRG-CC003
 NRG ONCOLOGY NRG-CC003 A Randomized Phase II/III Trial of Prophylactic Cranial Irradiation with or without Hippocampal Avoidance for Small Cell Lung Cancer SCHEMA Histologic proof or unequivocal cytologic
NRG ONCOLOGY NRG-CC003 A Randomized Phase II/III Trial of Prophylactic Cranial Irradiation with or without Hippocampal Avoidance for Small Cell Lung Cancer SCHEMA Histologic proof or unequivocal cytologic
SANTA MONICA BREAST CENTER INTAKE FORM
 SANTA MONICA BREAST CENTER Who referred you to see us today? Who is your primary care physician? Are there any other MDs who you would like to receive today s visit information? No Yes MD contact info
SANTA MONICA BREAST CENTER Who referred you to see us today? Who is your primary care physician? Are there any other MDs who you would like to receive today s visit information? No Yes MD contact info
MOLECULAR DIAGNOSTICS OF GLIOMAS
 MOLECULAR DIAGNOSTICS OF GLIOMAS Arie Perry, M.D. Director, Neuropathology Division DIFFUSE GLIOMAS Cell types Astrocytomas (A) Oligodendrogliomas (O) Mixed oligoastrocytoma (MOA) Three WHO grades: II,
MOLECULAR DIAGNOSTICS OF GLIOMAS Arie Perry, M.D. Director, Neuropathology Division DIFFUSE GLIOMAS Cell types Astrocytomas (A) Oligodendrogliomas (O) Mixed oligoastrocytoma (MOA) Three WHO grades: II,
Cilengitide (Impetreve) for glioblastoma multiforme. February 2012
 Cilengitide (Impetreve) for glioblastoma multiforme February 2012 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Cilengitide (Impetreve) for glioblastoma multiforme February 2012 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Pain Management Questionnaire
 In order to make the most of your visit, we require this form to be completed to the best of your ability and sent to the Pain Management Clinic a copy should be shared with your Primary Care Provider
In order to make the most of your visit, we require this form to be completed to the best of your ability and sent to the Pain Management Clinic a copy should be shared with your Primary Care Provider
(7) VITAL SIGNS (8) LEVEL OF CONSCIOUSNESS (9) MENTAL STATUS (10) SPEECH (11) VISION (12) FUNDUS (PAPILLEDEMA)
 Radiation Therapy Oncology Group Phase II CNS Lymphoma Follow-Up Form RTOG Study No. 1114 Case # Amended Data Yes INSTRUCTIONS: Submit this form as indicated in the protocol. All dates need to be recorded
Radiation Therapy Oncology Group Phase II CNS Lymphoma Follow-Up Form RTOG Study No. 1114 Case # Amended Data Yes INSTRUCTIONS: Submit this form as indicated in the protocol. All dates need to be recorded
Subject ID: I N D # # U A * Consent Date: Day Month Year
 IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
Technology appraisal guidance Published: 27 June 2007 nice.org.uk/guidance/ta121
 Carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma Technology appraisal guidance Published: 27 June 2007 nice.org.uk/guidance/ta121 NICE 2018. All rights reserved.
Carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma Technology appraisal guidance Published: 27 June 2007 nice.org.uk/guidance/ta121 NICE 2018. All rights reserved.
NOVEMBER IS NATIONAL PANCREATIC CANCER AWARENESS MONTH NATIONAL BAPTIST CONGRESS/PANCREATIC CANCER ACTION NETWORK
 NOVEMBER IS NATIONAL PANCREATIC CANCER AWARENESS MONTH NATIONAL BAPTIST CONGRESS/PANCREATIC CANCER ACTION NETWORK PURPLELIGHT VIGIL FOR HOPE SUNDAY, NOVEMBER 18, 2012 HOW CAN I ENGAGE MY CONGREGATION?
NOVEMBER IS NATIONAL PANCREATIC CANCER AWARENESS MONTH NATIONAL BAPTIST CONGRESS/PANCREATIC CANCER ACTION NETWORK PURPLELIGHT VIGIL FOR HOPE SUNDAY, NOVEMBER 18, 2012 HOW CAN I ENGAGE MY CONGREGATION?
Patient Interview Form
 Patient Interview Form Patient Information First Name: Last Name: Date of Birth: Age: Email Personal: Race Select one or more Referring Physician White Black or African Asian American Indian Native Hawaiian
Patient Interview Form Patient Information First Name: Last Name: Date of Birth: Age: Email Personal: Race Select one or more Referring Physician White Black or African Asian American Indian Native Hawaiian
Neurosurgical Management of Brain Tumours. Nicholas Little Neurosurgeon RNSH
 Neurosurgical Management of Brain Tumours Nicholas Little Neurosurgeon RNSH General Most common tumours are metastatic 10x more common than primary Incidence of primary neoplasms is 20 per 100000 per year
Neurosurgical Management of Brain Tumours Nicholas Little Neurosurgeon RNSH General Most common tumours are metastatic 10x more common than primary Incidence of primary neoplasms is 20 per 100000 per year
Neuro-Oncology Program
 Neuro-Oncology Program The goals of the Neuro-oncology Committee are: 1) to improve duration and quality of life of brain tumor patients; 2) to assess disease and treatment-related effects on neurocognitive
Neuro-Oncology Program The goals of the Neuro-oncology Committee are: 1) to improve duration and quality of life of brain tumor patients; 2) to assess disease and treatment-related effects on neurocognitive
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine
 BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
Protocol Abstract and Schema
 Protocol Abstract and Schema A Molecular Biology and Phase II Study of Imetelstat (GRN163L) in Children with Recurrent High-Grade Glioma, Ependymoma, Medulloblastoma/Primitive Neuroectodermal Tumor and
Protocol Abstract and Schema A Molecular Biology and Phase II Study of Imetelstat (GRN163L) in Children with Recurrent High-Grade Glioma, Ependymoma, Medulloblastoma/Primitive Neuroectodermal Tumor and
Antiemetic protocol for low-moderately emetogenic chemotherapy (see SCNAUSEA)
 BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
Challenging Paediatric Brain Tumours. ASP Belfast March 2017 Dr Jane Pears Consultant Paediatric Oncologist, Dublin
 Challenging Paediatric Brain Tumours ASP Belfast March 2017 Dr Jane Pears Consultant Paediatric Oncologist, Dublin Overview (i) Paediatric malignancy (ii) Central nervous system tumours (iii) Diffuse Intrinsic
Challenging Paediatric Brain Tumours ASP Belfast March 2017 Dr Jane Pears Consultant Paediatric Oncologist, Dublin Overview (i) Paediatric malignancy (ii) Central nervous system tumours (iii) Diffuse Intrinsic
News Briefing: Treatment Considerations for Focused Populations
 News Briefing: Treatment Considerations for Focused Populations Moderator: Pranshu Mohindra, MD, University of Maryland, Baltimore Reirradiation of Thoracic Cancers with Intensity Modulated Proton Therapy
News Briefing: Treatment Considerations for Focused Populations Moderator: Pranshu Mohindra, MD, University of Maryland, Baltimore Reirradiation of Thoracic Cancers with Intensity Modulated Proton Therapy
Acupuncture Needles. Overview. Gary Deng, MD,PhD. Traditional Theories. Acupuncture in Cancer Care: the USA Experience. History. Research Activities
 , MD,PhD Acupuncture in Cancer Care: the USA Experience History Overview First ISS-ARTOI Conference on Integrative Oncology Fifth ARTOI International Congress Rome, Italy Nov 7, 2013, MD, PhD Integrative
, MD,PhD Acupuncture in Cancer Care: the USA Experience History Overview First ISS-ARTOI Conference on Integrative Oncology Fifth ARTOI International Congress Rome, Italy Nov 7, 2013, MD, PhD Integrative
THE EFFECTIVE OF BRAIN CANCER AND XAY BETWEEN THEORY AND IMPLEMENTATION. Mustafa Rashid Issa
 THE EFFECTIVE OF BRAIN CANCER AND XAY BETWEEN THEORY AND IMPLEMENTATION Mustafa Rashid Issa ABSTRACT: Illustrate malignant tumors that form either in the brain or in the nerves originating in the brain.
THE EFFECTIVE OF BRAIN CANCER AND XAY BETWEEN THEORY AND IMPLEMENTATION Mustafa Rashid Issa ABSTRACT: Illustrate malignant tumors that form either in the brain or in the nerves originating in the brain.
