Germline Replacement by Transfer of Primordial Germ Cells into Partially Sterilized Embryos in the Chicken 1
|
|
|
- Agatha Carter
- 6 years ago
- Views:
Transcription
1 BIOLOGY OF REPRODUCTION 83, (2010) Published online before print 31 March DOI /biolreprod Germline Replacement by Transfer of Primordial Germ Cells into Partially Sterilized Embryos in the Chicken 1 Yoshiaki Nakamura, 3,4 Fumitake Usui, 3 Tamao Ono, 3 Kumiko Takeda, 4 Keijiro Nirasawa, 4 Hiroshi Kagami, 3 and Takahiro Tagami 2,4 Faculty of Agriculture, 3 Shinshu University, Nagano, Japan National Institute of Livestock and Grassland Science, 4 Tsukuba, Japan ABSTRACT We report a novel technique for almost complete replacement of the recipient germline with donor germ cells in the chicken. Busulfan solubilized in a sustained-release emulsion was injected into the yolk of fertile eggs before incubation. A dose of 100 lg was found to provide the best outcome in terms of reducing the number of endogenous primordial germ cells (PGCs) in embryonic gonads (0.6% of control numbers) and hatchability (36.4%). This was applied for preparing partially sterilized embryos to serve as recipients for the transfer of exogenous PGCs. Immunohistochemical analysis showed that the proportion of donor PGCs in busulfan-treated embryos was significantly higher than in controls (98.6% vs. 6.4%). Genetic cross-test analysis revealed that the germline transmission rate in busulfan-treated chickens was significantly higher than in controls (99.5% vs. 6.0%). Of 11 chimeras, 7 produced only donor-derived progenies, suggesting that these produced only donor-derived gametes in the recipient s gonads. This novel germline replacement technique provides a powerful tool for studying germline differentiation, for generating transgenic individuals, and for conserving genetic resources in birds. chicken, germline replacement, primordial germ cells, sterilization INTRODUCTION Primordial germ cells (PGCs) are the progenitor cells of gametes. Recently, Tsunekawa et al. [1] clarified that the chicken PGC precursors comprise just six cells expressing the chicken VASA homolog (CVH) protein among approximately 300 residing in the center of the blastodisc at stage IX (Roman numerals refer to the staging system of Eyal-Giladi and Kochav [2]). Thereafter, they increase to about cells in the central zone of the area pellucida of stage X embryos, which contain about cells [1, 3]. With the formation of the primitive streak, PGCs move anteriorly to the edge of the extraembryonic region, the so-called germinal crescent region [4]. They enter the developing vascular system from stage 11 1 Supported by the Genebank Project research project of the National Institute of Agrobiological Science (NIAS) to T.T. and a Research Fellowship for Young Scientists from the Japan Society for the Promotion of Sciences (JSPS) to Y.N. 2 Correspondence: Takahiro Tagami, National Institute of Livestock and Grassland Science, Tsukuba, Ibaraki , Japan. FAX: ; tagami@affrc.go.jp Received: 4 February First decision: 23 February Accepted: 22 March Ó 2010 by the Society for the Study of Reproduction, Inc. This is an Open Access article, freely available through Biology of Reproduction s Authors Choice option. eissn: ISSN: (Arabic numerals refer to the staging system of Hamburger and Hamilton [5]) and then circulate temporarily throughout the embryo by stage 18. Finally, PGCs leave the blood vessels and migrate to the intermediate mesoderm from stage 15 [3] and then differentiate into gametes. Since the clarification of the timing and migration pathway of avian PGCs, production of germline chimeric birds by the transfer of foreign PGCs has been a topic of interest. The transfer of PGCs provides a means to study avian germ cell development and differentiation. Establishment of embryo manipulation techniques has made it possible to produce somatic as well as germline chimeric chickens by transferring blastodermal cells containing PGCs from stage X embryos into the subgerminal cavity of recipient embryos at stage X [6]. In addition, the unique migration pattern of avian PGCs via the vascular network enables researchers to produce germline chimeric chickens by transferring PGCs retrieved from the germinal crescent region [7], from blood [8], and from gonads [9] into the vascular system of recipient embryos. Avian germline chimeras using blastodermal cells or PGCs have potential application in generating transgenic birds [10, 11] and preserving endangered species [12, 13]. However, there is no definitive method to incorporate donor PGCs into avian host germlines. Therefore, development of a germline replacement technique is needed. To produce only donor-derived sperm or eggs from germline chimeric animals, the use of sterile recipients is essential. Transplantation of donor spermatogonia of rainbow trout into sterile triploid salmon has resulted in the successful production of only donor-derived sperm or eggs [14]. In normal chickens, as in other birds, the male is the homogametic sex (ZZ) and the female is heterogametic (ZW). At present, three genotypes of triploid embryos have been identified: ZWW, ZZW, and ZZZ. Embryos with ZWW sex chromosomes cannot survive to hatching [15]. On the other hand, some ZZW and ZZZ triploid embryos are viable and can survive to maturity, although neither genotype is fertile [16]. In ZZW chicken, the right gonad develops into a testis and the left gonad develops into an ovotestis at hatching. However, leukocytes infiltrate both gonads at approximately 9 mo of age, and the seminiferous epithelium degenerates in most chicken aged over 1 yr [17]. In ZZZ chicken, gonadal and excurrent duct development is normal [17]. ZZZ triploid chicken embryos can be used as recipients for germline chimera production, but this strategy is only available for males. Genetic mutants lacking germ cells are also unavailable in the chicken. Alternatively, several methods to eliminate the endogenous PGCs or spermatogonia of recipients have been applied for germline replacement in zebrafish [18] and mice [19, 20], respectively. Therefore, partial, if not complete, sterilization of recipient embryos prior to the transfer of donor 130
2 GERMLINE REPLACEMENT IN CHICKEN 131 PGCs would be an ideal approach for germline replacement in chicken. We have developed an efficient method for delivering busulfan (1,4-butanediol dimethanesulfonate), an alkylating agent with cytotoxic effect on chicken germ cells [21, 22], to the chicken embryo [23]. This method enables an efficient depletion of the endogenous PGCs and repopulation with exogenous PGCs in early chicken embryos [24]. Here we have exploited these advances to produce chicken chimeras in which recipient germlines were almost totally replaced by donor cells. MATERIALS AND METHODS Fertilized Eggs and Animal Care All animal care and use in this study was conducted in accordance with the animal experimentation guidelines of our institute (NILGS Animal Care Committee). White Leghorn (WL) and Barred Plymouth Rock (BPR) chickens were maintained at the NILGS farm. Administration of Busulfan A sustained-release emulsion [23] was used as the vehicle for busulfan delivery. Busulfan (Sigma-Aldrich, St. Louis, MO) was dissolved in N,Ndimethyl formamide (DMF; Nacalai Tesque, Kyoto, Japan), diluted 10-fold in Ca 2þ - and Mg 2þ -free PBS (PBS[ ]). A volume of 1 ml busulfan solubilized solution and the same volume of sesame oil (Nacalai Tesque) were separately added in each of the two 2.5-ml syringes (Terumo, Tokyo, Japan). These syringes were connected with a Shirasu porous glass (SPG) membrane filter (SPG pumping connector, pore diameter 10 lm; SPG Technology, Miyazaki, Japan) and the busulfan solubilized solution and sesame oil were emulsified by passing through the membrane 100 times. The sustained-release emulsion was stored for a few minutes for cooling to room temperature and then used for injection. In fresh egg, the position of blastodisc is unsettled because a high amount of thick albumen covering with the yolk blocks the yolk rotation. To position the blastodisc at the top of the yolk, the thick albumen was detached from the yolk. It is well known that the proportional amount of thick albumen decreases gradually, whereas that of thin albumen increases gradually during the storage of chicken eggs [25]. Fertilized WL eggs were obtained freshly from our farm and then stored for 7 days at 148C in an incubator to reduce the proportional amount of thick albumen. Subsequently, detachment of thick albumen from the yolk was carried out by vortexing the egg for 10 sec at the lowest speed using a mixer with a pop-off cup (Vortex-Genie 2; Scientific Industries, Bohemia, NY). A small hole was made at the blunt end of each egg without damaging the inner shell membrane. Doses of 0, 25, 50, 75, 100, or 125 lg of busulfan in 50 ll sustained-release emulsion were injected horizontally into the yolk through a small hole using a sharp needle (32 gauge cm; Hamilton, Reno, NV) attached to a 50-ll syringe (Hamilton) and then the hole was sealed with wax. Embryo Culture Treated or untreated control eggs were incubated for h until they reached stage 14 at 39.08C and relative humidity of 50% 60%, with tilting at a 908 angle once an hour, in a forced-air incubator (P-008B Biotype; Showa Furankli, Saitama, Japan). A window with diameter 20 mm was opened in the narrow end and a portion (4 8 ml) of albumen was discarded. After the window was sealed with clingfilm wrap, the embryos were incubated for 6 days until they reached stage 29 or until hatching in the same forced-air incubator conditions with tilting at a 308 angle once every 2 h. Immunohistochemistry and Counting PGCs Whole gonads attached with the adjacent mesonephros were collected from stage 29 embryos, fixed overnight in 4% paraformaldehyde (PFA) in PBS( ) and washed three times in PBS( ) containing 0.1% Tween-20 (PBS-T) for 15 min each. Fixed gonads were treated with methanol containing 2% H 2 O 2 for 30 min to inactivate endogenous peroxidase activity and rinsed twice with PBS-T for 20 min each. Immunohistochemical staining was performed using the biotin/avidin-conjugated horseradish peroxidase system (Vectorstain Elite ABC Rabbit IgG Kit; Vector Laboratories, Burlingame, CA) and NovaRed substrate (NovaRed substrate kit for peroxidase; Vector Laboratories). After blocking in 4.5% normal goat serum in PBS-T for 6 h, gonads were incubated overnight in a 1: dilution of rabbit anti-cvh antibody [3] and then washed three times in PBS-T for 25 min each. Gonads were incubated with biotinylated secondary antibody (1:200) overnight and then rinsed three times with PBS-T for 25 min each. After incubation with Vectorstain Elite ABC Reagent for 30 min, gonads were washed four times in PBS( ) for 5 min each. Finally, gonads were stained using NovaRed solution according to the manufacturer s instructions. All steps were carried out at 48C. Both left and right gonads were detached from mesonephros and then the labeled cells in the stained gonads were counted using a microscope (DFC480-Note OY; Leica Microsystems, Tokyo, Japan). Preparation of Donor PGCs Fertilized BPR eggs were incubated in the same conditions as described above for 55 h to obtain embryo stages Blood was collected from the dorsal aorta of the embryos with a fine glass micropipette under a microscope (MS5; Leica Microsystems). Each collected blood sample was added to 100 ll of PBS( ). After sexing of each sample, male and female blood samples were pooled separately in PBS( ). PGCs were concentrated from embryonic blood using Nycodenz density gradient centrifugation [26] with some modification. Briefly, PBS( ) containing 10% fetal bovine serum (FBS) was used as a buffer instead of KAv-1 medium [27]. Some concentrated PGCs were labeled with the fluorescent lipophilic carbocyanine dye PKH-26 (Zynaxis, Malvern, PA) according to the protocol of Yamamoto et al. [28]. Chicken PGCs are easily distinguished from erythrocytes by their morphological characteristics, such as large size and the presence of many refractive granules in the cytoplasm observed using phase-contrast microscopy [29]. Collected PGCs were stored in 1 ml of PBS( ) containing 10% FBS for 4 h until transfer at room temperature. Repopulation of Recipient Germline with Donor PGCs WL embryos treated with 100 lg busulfan or untreated controls were prepared as recipients and then incubated for h until they reached stage 14 in the same conditions as described above. Approximately 0.5 ll of blood was collected from the dorsal aorta of recipient embryos through a window (diameter 20 mm) in the narrow end of the egg. Blood collection from recipient embryos was finished within 60 min. Each collected blood sample was diluted to 100 ll with PBS( ) and used for sex determination. Sets of 200 PKH-26-labeled PGCs from the BPR strain were microinjected into the dorsal aorta of recipient embryos at stages with the same sexes of donors and recipients. Manipulated embryos were incubated further and whole gonads attached with the adjacent mesonephros were collected at stage 29. After fixing in 4% PFA in PBS( ), gonads were rinsed three times with PBS-T for 15 min each. Fluorescent immunostaining was done as described elsewhere [23]. After blocking in 4.5% normal goat serum in PBS-T for 6 h, gonads were incubated overnight with rabbit anti-cvh antibody (1:10 000) and were then rinsed three times with PBS-T for 25 min each. Gonads were incubated overnight in a 1:400 dilution of Alexa 488-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) and washed three times in PBS-T for 25 min each. After detachment of both left and right gonads from mesonephros, fluorescent PKH-26-labeled cells (donor PGCs) and CVH-positive cells (donor and recipient PGCs) in the immunostained gonads were monitored by fluorescence microscopy (DFC480- Note OY, Leica Microsystems). When the immunostained samples were obtained from male embryos, the numbers of PKH-26-labeled cells and CVHpositive cells in both right and left gonads were counted. The numbers of PKH- 26-labeled cells and CVH-positive cells were counted in the left gonad in female embryos because only this one develops into a functional ovary in birds. Production of Chimeric Chickens Recipient WL embryos treated with 100 lg busulfan or untreated controls were incubated for h until they reached stage 14 in the same conditions as described above. About 0.5 ll of blood was drawn from the dorsal aorta of recipient embryos through a window (diameter 20 mm) in the narrow end of the egg. Blood collection from recipient embryos was completed within 60 min. Each collected blood sample was added to 100 ll of PBS( ) and used for sexing. Sets of 200 PGCs from BPR embryos were transferred to the dorsal aorta of recipient embryos at stages with the same sex combinations of donors and recipients. Manipulated embryos were incubated until hatching. Sexing The tissues obtained from donor BPR embryos were digested in 70 ll of buffer composed of 10 mm Tris-HCl (ph 7.5), 5 mm EDTA, 0.5% Tween 20, and 50 lg/ml Proteinase K (ph 7.5) using vigorous vortexing for 20 min at room temperature. Blood samples from recipient WL embryos were digested in 10 ll of the same buffer using vigorous vortexing for 20 min at room temperature. Molecular sexing was conducted by detecting conserved regions of the CHD-W and CHD-Z genes using primers 2550 F and 2718 R and
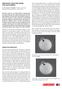
3 132 NAKAMURA ET AL. regenerating BPR chickens as a result of fertilizing eggs with spermatozoa, both derived from donor PGCs. The hatchlings were classified as black (BPR 3 BPR), spotted white (BPR 3 WL), or pure white (WL 3 WL). Statistical Analysis All data are expressed as the mean 6 SD. The survival rate of the embryos in each group was analyzed using the chi-square test. The numbers of PGCs in the gonads in each of the seven groups was analyzed by one-way ANOVA. If model effect was significant, differences between mean values for each treatment were then evaluated using the Bonferroni test. Differences in the numbers of PKH-26-labeled PGCs and CVH-positive cells, and in the proportions of donor PGCs and donor-derived offspring, were compared between busulfan-treated and untreated control groups using unpaired Student t-tests. Differences in survivability and the other factors were considered significant at P, 0.05 and P, 0.01, respectively. FIG. 1. The number of endogenous PGCs in embryonic gonads after 6 days incubation. Endogenous PGCs were detected by immunohistochemical staining using a specific antibody raised against chicken VASA homolog protein. A) Untreated control embryo. Embryos treated with doses of 0 (B), 25 (C), 50 (D), 75 (E), 100 (F), and 125 lg (G) busulfan solubilized in 50 ll sustained-release emulsion. Bar ¼ 100 lm. H) The numbers of endogenous PGCs in whole gonads at embryo stage 29 were reduced by busulfan treatment dose-dependently. Means with different superscripts are significantly different (P, 0.01). The number of embryos evaluated is shown below each treatment. RESULTS PGC Depletion in Embryonic Gonads Embryos treated with busulfan did not show developmental delay after 6 days of incubation regardless of the dosage when compared to the untreated control embryos. Whole gonads were collected from morphologically normal embryos at stage 29. Immunohistochemical analysis confirmed that the five busulfan-treated groups (25, 50, 75, 100, or 125 lg) resulted in fewer PGCs compared with the sham-operated control group (receiving sustained-release emulsion without busulfan) and the untreated control group (Fig. 1, A G). The numbers of PGCs in whole gonads of embryos at stage 29 in each group are shown in Figure 1H. The mean number (range) of PGCs in sham-operated controls ( ; ) was almost the same as in untreated controls ( ; ). The mean number of PGCs in whole mounts of gonads at stage 29 was reduced by administration of busulfan in a dose-dependent manner up to 100 lg (P, 0.01), with the values for 25-, 50-, 75-, and 100-lg groups being ( ), ( ), (0 142), and (0 34), respectively. No statistically significant difference in the numbers of PGCs was observed between the 100- and 125-lg treatment groups ( ; 0 35). Embryonic Development and Hatchability Teratogenic effects increased with the dose of busulfan (Table 1). The most common abnormalities observed in the treated embryos were anangioplasia, abnormal limb buds, and small eyes. These embryos showing developmental abnormalities did not survive to hatching. The effects of busulfan administration on the survivability and hatchability of chicken polymerase chain reaction amplification, following the protocol of Fridolfsson and Ellegren [30]. Sex determination of donors and recipients was completed within 3 h. Progeny Testing Putative germline chimeric chickens derived from PGC transfer that survived to sexual maturity were crossed with BPR (i/i) chickens by artificial insemination and the feather color of their offspring was examined. Black offspring (i/i) indicated that the offspring was derived from BPR donor PGCs, whereas white offspring with small patches of black pigmentation (I/i) indicated that the offspring was derived from recipient WL PGCs. Regeneration of BPR Individuals from Germline Chimeras When complete germline replacement has been identified by test mating, the chimeric chickens were crossed using artificial insemination for TABLE 1. Teratogenic effects of busulfan on chicken embryos and their hatching rate. Dose (lg) No. of embryos Percentage of embryos showing abnormalities Hatching rate of embryos showing abnormalities a Untreated controls 74 0 NA 0 (Sham-operated 85 0 NA controls) a NA, not available.
4 GERMLINE REPLACEMENT IN CHICKEN 133 TABLE 2. Survival and hatching rates of chicken embryos in windowed eggs with and without busulfan. Survivability of embryos on incubation day (%) Dose (lg) No. of embryos Hatched (%) Untreated controls a 93.2 a 93.2 a 89.1 a 87.8 a 83.8 a 74.3 a 0 (Sham-operated controls) ab 87.1 ab 83.5 ab 80.0 ab 80.0 ab 77.6 ab 68.2 ab ab 79.3 abc 77.0 abc 75.9 ab 73.6 abc 67.8 abc 54.0 abc ab 75.6 abc 74.4 abc 73.2 ab 70.7 abc 63.4 abc 46.3 bcd ab 74.4 abc 70.0 bc 65.6 b 62.2 bc 60.0 bc 41.1 cd b 68.2 bc 67.0 bc 63.6 bc 61.3 bc 58.0 bc 36.4 cd b 63.8 c 61.9 c 59.0 b 56.2 c 52.4 c 29.5 d a d Means with different superscript in the same column are significantly different (chi-square test, P, 0.05). embryos are shown in Table 2. The survival and hatching rates of embryos in the sham control group was slightly lower than in the untreated control group, but the difference was not statistically significant. Survival and hatching rates were both decreased dose-dependently by the application of busulfan (P, 0.05). Germline Chimerism in Embryonic Gonads To monitor whether donor PGCs could repopulate PGCdepleted gonads after busulfan treatment, sets of 200 fluorescently labeled PGCs from BPR embryos were transferred at stages Gonadal migration of donor PGCs was observed in all manipulated embryos, as shown in Figure 2. Embryonic germline chimerism, estimated by the number of PKH-26-labeled cells (donor PGCs) and CVH-positive cells (donor and recipient PGCs) in busulfan-treated (100 lg) and untreated control groups in males and females, is listed in Tables 3 and 4, respectively. The number of PKH-26-labeled PGCs was more than fivefold higher (P, 0.01) in busulfantreated than in untreated control groups in both male (embryo FIG. 2. Migration of donor PGCs to recipient gonads at embryo stage 29. Male gonads of busulfan-treated (A A ) and untreated control embryos (B B ). A and B) Bright-field images of whole mounts of gonads attached with the adjacent mesonephros. Gonadal migration of donor PGCs was tracked by staining with the fluorescent lipophilic carbocyanine dye PKH- 26 (A, B ). Chimeric gonads were immunostained with an antibody raised against CVH protein (A, B ). Bar ¼ 100 lm. numbers BME-1 13 vs. UME-1 9) and female embryos (BFE vs. UFE-1 8). On the other hand, the number of CVHpositive cells in the busulfan-treated group was significantly lower than the number in the untreated control group (P, 0.01). The proportion of donor PGCs in the busulfan-treated group was 98.5% 6 2.1%, whereas in the untreated control group it was 6.4% 6 2.0% (P, 0.01). Germline Chimerism in Sexually Mature Birds For chimera production, WL embryos were treated with 100 lg of busulfan, and these embryos and untreated controls received sets of 200 PGCs from BPR embryos at stages with the same sexes of donors and recipients. In the busulfantreated group, 50 embryos were manipulated (25 male and 25 female), 17 (8 male and 9 female) hatched, and 11 (5 male and 6 female) reached sexual maturity. In the untreated control group, 20 embryos were manipulated (9 male and 11 female), 15 (7 male and 8 female) hatched, and 12 (5 male and 7 female) survived to sexual maturity. Genetic crossing analysis with BPR chickens showed that all sexually matured chickens both in the busulfan-treated group (BMC-2 8 and BFC-1 9) and in the untreated control group (UMC-1 6 and UFC-1 8) TABLE 3. Proportion of donor primordial germ cells (PGCs) in male gonads of 6-day-old embryos with and without busulfan treatment. Recipient treatment/ embryo ID No. of PKH-26- labeled cells (donor PGCs) No. of CVHpositive cells (donor and recipient PGCs) Proportion of donor PGCs (%) Busulfan (100 lg) BME BME BME BME BME BME BME BME BME BME Mean 6 SD a a a Untreated controls UME UME UME UME UME UME UME UME Mean 6 SD b b b a,b Different superscripts in the proportion of donor PGCs were significantly different, P, 0.01.
5 134 NAKAMURA ET AL. TABLE 4. Proportion of donor primordial germ cells (PGCs) in female gonads of 6-day-old embryos with and without busulfan treatment.* Recipient treatment/ embryo ID were germline chimeras (Table 5). The frequency of obtaining donor-derived offspring from germline chimeric chickens in the busulfan-treated group was 99.5% 6 0.9%, whereas in the untreated control group it was 6.0% 6 2.9% (P, 0.01). Moreover, seven chimeras (three male and four female) produced only donor-derived black progenies, suggesting that these birds produced only donor-derived gametes. Regeneration of BPR Chickens by Crossing Germline Replacement Chimeras Four germline-replaced hens (BFC-1, BFC-2, BFC-5, and BFC-9) were artificially inseminated with semen from each of three germline-replaced cocks (BMC-2, BMC-4, and BMC-8). Donor-derived black embryos in recipient-derived whiteshelled eggs were observed by mating male and female germline chimeric birds (Fig. 3A). All of the progenies obtained from each combination of germline chimeric birds were donor-derived black chickens (Fig. 3B; Table 6). DISCUSSION This is the first report of a technique that allows for the almost complete replacement of a recipient germline following transplantation of donor PGCs in birds. Stable production of recipient embryos lacking endogenous PGCs was achieved following the injection of busulfan solubilized in a sustainedrelease emulsion into the yolk of fertile eggs before incubation. Donor PGCs transferred to partially sterilized recipient embryos were successfully incorporated into the recipient germline and gave rise to functional gametes. Successful replacement of a recipient s germline with donor germ cells requires prior sterilization of the recipients. Up to the present, three different approaches have been applied to sterilize recipients in vertebrates. Administration of busulfan has been used for eliminating testicular cells in mice [19]. Repeated exposure to gamma radiation has also been used for sterilizing male mice [20] and roosters [31]. In these studies, subsequent transplantation of testicular cells containing spermatogonial stem cells to the sterilized testes showed TABLE 5. Progeny test of same-sex germline chimeras produced after transfer of primordial germ cells from Barred Plymouth Rock into White Leghorn embryos with and without busulfan treatment. Recipient treatment/ chimera ID No. of PKH-26- labeled cells (donor PGCs) Sexuality of chimeras No. of CVHpositive cells (donor and recipient PGCs) Proportion of donor PGCs (%) Busulfan (100 lg) BFE BFE BFE BFE BFE BFE BFE BFE BFE BFE Mean 6 SD a a a Untreated controls UFE UFE UFE UFE UFE UFE UFE UFE Mean 6 SD b b b * Data were obtained from only left gonads. a,b Different superscripts in the proportion of donor PGCs were significantly different, P, No. of black chicks (donor-derived progenies) No. of white chicks (recipient-derived progenies) Proportion of donor-derived progenies (%) Busulfan (100 lg) BMC-2 Male BMC-4 Male BMC-6 Male BMC-7 Male BMC-8 Male BFC-1 Female BFC-2 Female BFC-4 Female BFC-5 Female BFC-7 Female BFC-9 Female Mean 6 SD a Untreated controls UMC-1 Male UMC-2 Male UMC-3 Male UMC-4 Male UMC-6 Male UFC-1 Female UFC-2 Female UFC-3 Female UFC-4 Female UFC-5 Female UFC-7 Female UFC-8 Female Mean 6 SD b a,b Different superscripts in the proportion of donor-derived progenies were significantly different, P, 0.01.
6 GERMLINE REPLACEMENT IN CHICKEN 135 TABLE 6. Regeneration of Barred Plymouth Rock (BPR) progenies by crossing White Leghorn (WL) chickens that have germlines replaced by BPR. Male chimera ID Female chimera ID Phenotypes of offspring* Black Spotted white Pure white BMC-2 BFC BMC-2 BFC BMC-2 BFC BMC-2 BFC BMC-4 BFC BMC-4 BFC BMC-4 BFC BMC-4 BFC BMC-8 BFC BMC-8 BFC * Black progenies are typical BPR 3 BPR; spotted white progenies are BPR 3 WL; white progenies are WL. FIG. 3. Regeneration of donor-derived individuals by crossing germlinereplaced chimeras. A) A black embryo in a white-shelled egg (left) was obtained by mating germline-replaced chimeras with transplanted PGCs from BPR into WL embryos. A colored egg (center) and a white egg (right) were laid by BPR and WL hens, respectively. B) Donor-derived BPR hatchlings and a male (left, BMC-1) and female (right, BFC-2) germline replacement chimera. successful colonization of recipient testes by the exogenous germlines. In zebrafish, microinjection of a morpholino antisense oligonucleotide directed against the dead end mrna in 1- to 2-cell stage embryos effectively ablated the recipient germline, and germline replacement was achieved successfully by transferring donor PGCs [18]. In practice, the use of gamma irradiation is limited because of the high cost and special equipment needed. A morpholino oligonucleotide approach is also restricted in avian species because of the technical difficulties in microinjecting the ovum immediately after fertilization and subsequent culture of the manipulated embryo. Therefore, busulfan treatment seems to be a better option for germline replacement in birds. However, the efficacy of this approach has been highly variable because of the difficulty in delivering a constant amount of busulfan to the target embryo [21]. Furthermore, busulfan treatment is feasible only when the residual busulfan does not interfere with the transferred PGCs [22]. To overcome these problems, we developed an efficient technique to deliver consistent amounts of busulfan using a sustained-release emulsion as a vehicle [23]. To maintain a consistent level of busulfan in a vehicle, the sustained-release emulsion is made by emulsifying busulfan solubilized in PBS( ) containing 10% DMF and an equal amount of sesame oil. Because the sustained-release emulsion had a lower density than the yolk, this vehicle rapidly rose and contacted the developing embryo that was lying at the top of the yolk when it was injected into the yolk. According to these advantages, the injection of busulfan solubilized in the sustained-release emulsion into the yolk resulted in a consistent and extensive depletion of endogenous PGCs. Subsequently, we showed that early application of busulfan to the recipient embryos allowed reduction of the sterilizing effect of the residual busulfan on donor PGCs [24]. In the present study, germline chimera production and subsequent genetic cross testing were performed to validate our germline replacement technique. The number of endogenous PGCs in the whole gonads of chicken embryos incubated for 6 days was reduced following busulfan administration in a dose-dependent manner. This suggests that a constant amount of drug was delivered successfully to the blastodisc when using the sustained-release emulsion as a vehicle. Doses of 100 or 125 lg resulted in nearly total depletion of the endogenous PGCs. The hatchability of the embryos was also decreased in a dose-dependent manner. A dose of 100 lg was found to be optimal for preparing partially sterilized recipient embryos for the germline replacement study, considering both the number of endogenous PGCs remaining (0.6% of control value) and hatchability (36.4%). The average proportion of chicken PGCs that migrated into the gonadal area of quail embryos within 24 h after PGC transfer was 50.7% regardless of the number of PGCs transferred [32]. In the present study, the mean number of donor PGCs observed in the gonads of untreated control embryos within 4 days of transfer was appreciably lower than the number transferred. In contrast, the number of donor PGCs in the gonads of busulfan-treated embryos within 4 days of transplantation was far higher than the number transplanted. This indicates that exogenous PGCs proliferated to repopulate the recipient s gonads without any marked sterilizing effect of the residual busulfan. Thus, the proliferation profile of exogenous PGCs after gonadal migration seems to be closely associated with the number of residual PGCs in the recipient s gonads. We were able to produce germline chimeras by transferring sets of 200 isolated PGCs from BPR embryos into the vascular systems of recipient WL embryos treated with 100 lg of busulfan and into untreated controls. It has been reported that female PGCs in male gonads are capable of passing through the first and second meiotic divisions by adapting themselves to a male environment; however, they can barely complete spermatogenesis [33]. Accordingly, in this study sex-matched germline chimeras were produced by prior sex identification of the donor PGCs and recipient embryos to overcome this sexual mismatch problem. Crossbreeding analysis showed that the frequency of germline transmission of produced chimeric chickens in the busulfan-treated group was 99.5% whereas the rate in the untreated control group was only 6.0%. Moreover, 7 of the 11 chimeric chickens (64%; 3 male and 4 female) in the busulfan-treated group produced only donor-derived chickens. This suggests that the recipient s germline had been completely replaced by the donor germ cells and produced only donor-
7 136 NAKAMURA ET AL. derived gametes. This established method has dramatically enhanced the germline transmission rate when compared with the previously reported busulfan approach [34] (97.5% 100.0% vs. 0% 13.9%). This difference of germline transmission rate would be caused by the drug delivery system and the timing of busulfan treatment. Our established method resulted in a stable removal of endogenous PGCs, as shown in Figure 1, because of the steady delivery of consistent amounts of drug to the blastodisc. By contrast, constant PGC depletion could not be achieved using the method of Song et al. [34] because the vehicle, which was made by mixing DMF with an equal amount of sesame oil, quickly separated into DMF and oil immediately just after injection [23]. Additionally, we have elucidated that application of busulfan before incubation leads the reduction of the sterilizing effect of the residual busulfan at the time of donor PGC transfer when compared to application of busulfan after 24 h of incubation. Thus, we treated recipient embryos with busulfan before incubation, although Song et al. [34] treated recipient embryos after 24 h of incubation. Apart from the busulfan approach, several techniques have been attempted to improve the germline transmission rate of donorderived gametes in chicken by reducing the endogenous PGCs of recipient embryos prior to donor PGC transfer. The frequencies of obtaining donor-derived offspring using gamma ray sterilization [35], surgical removal of the central zone of the area pellucida [36], or surgical removal of early embryonic blood [37] were 6.0% (0% 100%), 32.5% (0% 100%) and 33.5% (0% 95.8%), respectively. Our established method seems to be the most practical and efficient method presently available for chimera production because the frequency of germline transmission can be stabilized at a high rate. Cryopreservation of PGCs as genetic material seems to be an effective conservation strategy because techniques for freezing ova and producing cloned individuals from somatic cells are not available in birds. Here we demonstrate the ability to produce both male and female chimeric chickens that have germlines almost completely reconstituted by donor germ cells. Moreover, purely donor-derived individuals were successfully regenerated by mating between germline-replaced chimeras. Thus, this technique will promote not only the banking of PGCs from existing chicken breeds, but also the augmentation of individual genetic stock using germline replacement chimeras. Although studies in zebrafish have demonstrated that a single PGC has the ability to repopulate the germline and give rise to functional gametes [38], little is known about the number of PGCs required for normal germline development in the chicken. We aim to determine this using our established system. Such an approach will create better conditions for enhancing PGC-mediated genetic resource conservation systems. Additionally, combining this germline replacement technique with PGC-mediated transgenesis will allow us to generate homozygous mutants efficiently in the F1 generation. Therefore, our novel technique offers a new means to study germline differentiation, conserve genetic resources, and produce transgenic individuals in birds. REFERENCES 1. Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 2000; 127: Eyal-Giladi H, Kochav S. From cleavage to primitive streak formation: a complementary normal table and a new look at the first stages of the development of the chick. I. General morphology. Dev Biol 1976; 49: Nakamura Y, Yamamoto Y, Usui F, Mushika T, Ono T, Setioko AR, Takeda K, Nirasawa K, Kagami H, Tagami T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult Sci 2007; 86: Ginsburg M, Eyal-Giladi H. Temporal and spatial aspects of the gradual migration of primordial germ cells from the epiblast into the germinal crescent in the avian embryo. J Embryol Exp Morphol 1986; 95: Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 1951; 88: Petitte JN, Clark ME, Liu G, Verrinder Gibbins AM, Etches RJ. Production of somatic and germline chimeras in the chicken by transfer of early blastodermal cells. Development 1990; 108: Vick L, Luke G, Simkiss K. Germ-line chimaeras can produce both strains of fowl with high efficiency after partial sterilization. J Reprod Fertil 1993; 98: Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ line chimera by transfer of primordial germ cells in the domestic chicken (Gallus domesticus). Theriogenology 1993; 40: Tajima A, Naito M, Yasuda Y, Kuwana T. Production of germ-line chimeras by transfer of cryopreserved gonadal primordial germ cells (gpgcs) in chicken. J Exp Zool 1998; 280: van de Lavoir MC, Diamond JH, Leighton PA, Mather-Love C, Heyer BS, Bradshaw R, Kerchner A, Hooi LT, Gessaro TM, Swanberg SE, Delany ME, Etches RJ. Germline transmission of genetically modified primordial germ cells. Nature 2006; 441: Shin SS, Kim TM, Kim SY, Kim TW, Seo HW, Lee SK, Kwon SC, Lee GS, Kim H, Lim JM, Han JY. Generation of transgenic quail through germ cell-mediated germline transmission. FASEB J 2008; 22: Kuwana T, Kawashima T, Naito M, Yamashita H, Matsuzaki M, Takano T. Conservation of a threatened indigenous fowl (Kureko Dori) using the germline chimeras transplanted from primordial germ cells. J Poult Sci 2006; 43: Kang SJ, Choi JW, Kim SY, Park KJ, Kim TM, Lee YM, Kim H, Lim JM, Han JY. Reproduction of wild birds via interspecies germ cell transplantation. Biol Reprod 2008; 79: Okutsu T, Shikina S, Kanno M, Takeuchi Y, Yoshizaki G. Production of trout offspring from triploid salmon parents. Science 2007; 317: Bloom SE. Chromosome abnormalities in chicken (Gallus domesticus) embryos: types, frequencies and phenotypic effects. Chromosoma 1972; 37: Abdel-Hameed F, Shoffner RN. Intersexes and sex determination in chickens. Science 1971; 172: Lin M, Thorne MH, Martin IC, Sheldon BL, Jones RC. Development of the gonads in the triploid (ZZW and ZZZ) fowl, Gallus domesticus, and comparison with normal diploid males (ZZ) and females (ZW). Reprod Fertil Dev 1995; 7: Ciruna B, Weidinger G, Knaut H, Thisse B, Thisse C, Raz E, Schier AF. Production of maternal-zygotic mutant zebrafish by germ-line replacement. Proc Natl Acad Sci U S A 2002; 99: Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A 1994; 91: Creemers LB, Meng X, den Ouden K, van Pelt AM, Izadyar F, Santoro M, Sariola H, de Rooij DG. Transplantation of germ cells from glial cell linederived neurotrophic factor-overexpressing mice to host testes depleted of endogenous spermatogenesis by fractionated irradiation. Biol Reprod 2002; 66: Aige-Gil V, Simkiss K. Sterilisation of avian embryos with busulphan. Res Vet Sci 1991; 50: Hallett JS, Wentworth BC. The effects of busulfan on gonadal differentiation and development in Japanese quail (Coturnix coturnix japonica). Poult Sci 1991; 70: Nakamura Y, Yamamoto Y, Usui F, Atsumi Y, Ito Y, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Increased proportion of donor primordial germ cells in chimeric gonads by sterilisation of recipient embryos using busulfan sustained-release emulsion in chicken. Reprod Fertil Dev 2008; 20: Nakamura Y, Usui F, Atsumi Y, Otomo A, Teshima A, Ono T, Takeda K, Nirasawa K, Kagami H, Tagami T. Effects of busulfan sustained-release emulsion on depletion and repopulation of primordial germ cells in early chicken embryos. J Poult Sci 2009; 46: Romanoff AL, Romanoff AJ. The Avian Egg. New York: John Wiley and Sons Inc.; 1949: Zhao DF, Kuwana T. Purification of avian circulating primordial germ cells by Nycodenz density gradient centrifugation. Br Poult Sci 2003; 44: Kuwana T, Hashimoto K, Nakanishi A, Yasuda Y, Tajima A, Naito M. Long-term culture of avian embryonic cells in vitro. Int J Dev Biol 1996; 40: Yamamoto Y, Usui F, Nakamura Y, Ito Y, Tagami T, Nirasawa K,
8 GERMLINE REPLACEMENT IN CHICKEN 137 Matsubara Y, Ono T, Kagami H. A novel method to isolate primordial germ cells and its use for the generation of germline chimeras in chicken. Biol Reprod 2007; 77: Fujimoto T, Ukeshima A, Kiyofuji R. The origin, migration and morphology of the primordial germ cells in the chick embryo. Anat Rec 1976; 185: Fridolfsson A, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 1999; 30: Trefil P, Micáková A, Mucksová J, Hejnar J, Poplstein M, Bakst MR, Kalina J, Brillard JP. Restoration of spermatogenesis and male fertility by transplantation of dispersed testicular cells in the chicken. Biol Reprod 2006; 75: Ishiguro S, Minematsu T, Naito M, Kanai Y, Tajima A. Migratory ability of chicken primordial germ cells transferred into quail embryos. J Reprod Dev 2009; 55: Tagami T, Kagami H, Matsubara Y, Harumi T, Naito M, Takeda K, Hanada H, Nirasawa K. Differentiation of female primordial germ cells in the male testes of chicken (Gallus gallus domesticus). Mol Reprod Dev 2007; 74: Song Y, D Costa S, Pardue SL, Petitte JN. Production of germline chimeric chickens following the administration of a busulfan emulsion. Mol Reprod Dev 2005; 70: Carsience RS, Clark ME, Verrinder Gibbins AM, Etches RJ. Germline chimeric chickens from dispersed donor blastodermal cells and compromised recipient embryos. Development 1993; 117: Kagami H, Tagami T, Matsubara Y, Harumi T, Hanada H, Maruyama K, Sakurai M, Kuwana T, Naito M. The developmental origin of primordial germ cells and the transmission of the donor-derived gametes in mixed-sex germline chimeras to the offspring in the chicken. Mol Reprod Dev 1997; 48: Naito M, Tajima A, Yasuda Y, Kuwana T. Production of germline chimeric chickens, with high transmission rate of donor-derived gametes, produced by transfer of primordial germ cells. Mol Reprod Dev 1994; 39: Saito T, Goto-Kazeto R, Arai K, Yamaha E. Xenogenesis in teleost fish through generation of germ-line chimeras by single primordial germ cell transplantation. Biol Reprod 2008; 78:
Research Fellow of the Japan Society for the Promotion of Science, Japan
 http://www.jstage.jst.go.jp/browse/jpsa doi: +*.,+.+/jpsa.*+**2/ Copyright,*++, Japan Poultry Science Association. +,-,, + + + + Yoshiaki Nakamura, Fumitake Usui, Daichi Miyahara, Takafumi Mori, Haruhi
http://www.jstage.jst.go.jp/browse/jpsa doi: +*.,+.+/jpsa.*+**2/ Copyright,*++, Japan Poultry Science Association. +,-,, + + + + Yoshiaki Nakamura, Fumitake Usui, Daichi Miyahara, Takafumi Mori, Haruhi
Evidence of in vivo transfection of circulating primordial germ cells in chickens
 Evidence of in vivo transfection of circulating primordial germ cells in chickens M. NAITO 1 *, T. HARUMI 2, Y. MATSUBARA 1 and T. KUWANA 3 1 Transgenic Animal Research Center, 2 Animal Genome Research
Evidence of in vivo transfection of circulating primordial germ cells in chickens M. NAITO 1 *, T. HARUMI 2, Y. MATSUBARA 1 and T. KUWANA 3 1 Transgenic Animal Research Center, 2 Animal Genome Research
Avian Germplasm Preservation: Embryonic Stem Cells or Primordial Germ Cells? 1
 Avian Germplasm Preservation: Embryonic Stem Cells or Primordial Germ Cells? 1 J. N. Petitte 2 Department of Poultry Science, College of Agriculture and Life Sciences North Carolina State University, Raleigh
Avian Germplasm Preservation: Embryonic Stem Cells or Primordial Germ Cells? 1 J. N. Petitte 2 Department of Poultry Science, College of Agriculture and Life Sciences North Carolina State University, Raleigh
, Takahiro Tagami, Hiroshi Kagami and Tamao Ono
 The Journal of Poultry Science,./:,3,,31,,**2 http://www.jstage.jst.go.jp/browse/jpsa Copyright,**2, Japan Poultry Science Association. doi: +*.,+.+/jpsa../.,3, + Yusuke Atsumi, Takahiro Tagami, Hiroshi
The Journal of Poultry Science,./:,3,,31,,**2 http://www.jstage.jst.go.jp/browse/jpsa Copyright,**2, Japan Poultry Science Association. doi: +*.,+.+/jpsa../.,3, + Yusuke Atsumi, Takahiro Tagami, Hiroshi
DIFFERENTIATION OF FEMALE PRIMORDIAL GERM CELLS IN THE MALE TESTES OF CHICKEN
 DIFFERENTIATION OF FEMALE PRIMORDIAL GERM CELLS IN THE MALE TESTES OF CHICKEN (Diferensiasi Primordial Grem Sel dalam Testes Ayam) TAKAHIRO TAGAMI National Institute of Livestock and Grassland Science
DIFFERENTIATION OF FEMALE PRIMORDIAL GERM CELLS IN THE MALE TESTES OF CHICKEN (Diferensiasi Primordial Grem Sel dalam Testes Ayam) TAKAHIRO TAGAMI National Institute of Livestock and Grassland Science
Germ Cell Transplantation in Fish
 Larvi 2009 Germ Cell Transplantation in Fish Goro Yoshizaki (Tokyo University of Marine Science and Technology, SORST/JST) Tuna Mackerel Body weight; 300 kg 300 g Body length; 3 m 30 cm Scombridae family
Larvi 2009 Germ Cell Transplantation in Fish Goro Yoshizaki (Tokyo University of Marine Science and Technology, SORST/JST) Tuna Mackerel Body weight; 300 kg 300 g Body length; 3 m 30 cm Scombridae family
Production of Offspring from Cryopreserved Chicken Testicular Tissue 1
 Production of Offspring from Cryopreserved Chicken Testicular Tissue 1 Y. Song and F. G. Silversides 2 Agassiz Research Centre, British Columbia, Canada, V0M 1A0 ABSTRACT Cryopreservation of avian germplasm
Production of Offspring from Cryopreserved Chicken Testicular Tissue 1 Y. Song and F. G. Silversides 2 Agassiz Research Centre, British Columbia, Canada, V0M 1A0 ABSTRACT Cryopreservation of avian germplasm
Triploidy and other chromosomal
 Triploidy and other chromosomal abnormalities in a selected line of chickens MH Thorne, RK Collins, BL Sheldon CSIRO Division of Animal Production, Poultry Genetics, PO Box 184, North Ryde, NSW 211,!,
Triploidy and other chromosomal abnormalities in a selected line of chickens MH Thorne, RK Collins, BL Sheldon CSIRO Division of Animal Production, Poultry Genetics, PO Box 184, North Ryde, NSW 211,!,
Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish
 Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish Tomoyuki Okutsu*, Kensuke Suzuki*, Yutaka Takeuchi*, Toshio Takeuchi*, and Goro Yoshizaki*
Testicular germ cells can colonize sexually undifferentiated embryonic gonad and produce functional eggs in fish Tomoyuki Okutsu*, Kensuke Suzuki*, Yutaka Takeuchi*, Toshio Takeuchi*, and Goro Yoshizaki*
Sexual plasticity of ovarian germ cells in rainbow trout
 CORRIGENDUM Development 137, 1584 (2010) doi:10.1242/dev.051821 Sexual plasticity of ovarian germ cells in rainbow trout Goro Yoshizaki, Masaki Ichikawa, Makoto Hayashi, Yoshiko Iwasaki, Misako Miwa, Shinya
CORRIGENDUM Development 137, 1584 (2010) doi:10.1242/dev.051821 Sexual plasticity of ovarian germ cells in rainbow trout Goro Yoshizaki, Masaki Ichikawa, Makoto Hayashi, Yoshiko Iwasaki, Misako Miwa, Shinya
Yusaku Kohara, Yukio Kanai and Atsushi Tajima
 The Journal of Poultry Science,./: /1 0+,,**2 http://www.jstage.jst.go.jp/browse/jpsa Copy right,**2, Japan Poultry Science Association. doi: +*.,+.+/jpsa.././1 Yusaku Kohara, Yukio Kanai and Atsushi Tajima
The Journal of Poultry Science,./: /1 0+,,**2 http://www.jstage.jst.go.jp/browse/jpsa Copy right,**2, Japan Poultry Science Association. doi: +*.,+.+/jpsa.././1 Yusaku Kohara, Yukio Kanai and Atsushi Tajima
A Method for Cryopreserving Semen from Yakido Roosters using. N-Methylacetamide as a Cryoprotective Agent
 Research Note A Method for Cryopreserving Semen from Yakido Roosters using N-Methylacetamide as a Cryoprotective Agent Kenji Sasaki 1), Toshiaki Tatsumi 1), Mariko Tsutsui 2), Tatsuya Niinomi 2), Takayuki
Research Note A Method for Cryopreserving Semen from Yakido Roosters using N-Methylacetamide as a Cryoprotective Agent Kenji Sasaki 1), Toshiaki Tatsumi 1), Mariko Tsutsui 2), Tatsuya Niinomi 2), Takayuki
Compensatory proliferation of endogenous chicken primordial germ cells after elimination by busulfan treatment
 Lee et al. Stem Cell Research & Therapy 2013, 4:136 RESEARCH Open Access Compensatory proliferation of endogenous chicken primordial germ cells after elimination by busulfan treatment Hyung Chul Lee 1,
Lee et al. Stem Cell Research & Therapy 2013, 4:136 RESEARCH Open Access Compensatory proliferation of endogenous chicken primordial germ cells after elimination by busulfan treatment Hyung Chul Lee 1,
Spermatogonial Transplantation in Fish: Production of Trout Offspring from Salmon Parents
 K. Tsukamoto, T. Kawamura, T. Takeuchi, T. D. Beard, Jr. and M. J. Kaiser, eds. Fisheries for Global Welfare and Environment, 5th World Fisheries Congress 2008, pp. 209 219. by TERRAPUB 2008. Spermatogonial
K. Tsukamoto, T. Kawamura, T. Takeuchi, T. D. Beard, Jr. and M. J. Kaiser, eds. Fisheries for Global Welfare and Environment, 5th World Fisheries Congress 2008, pp. 209 219. by TERRAPUB 2008. Spermatogonial
University of Science and Technology in Bydgoszcz. Faculty of Animal Breeding and Biology. Animal Biochemistry and Biotechnology Department
 University of Science and Technology in Bydgoszcz Faculty of Animal Breeding and Biology Animal Biochemistry and Biotechnology Department Behavior of chicken primordial germ cells injected into the bloodstream
University of Science and Technology in Bydgoszcz Faculty of Animal Breeding and Biology Animal Biochemistry and Biotechnology Department Behavior of chicken primordial germ cells injected into the bloodstream
Isolation and Characterization of Gonadal Primordial Germ Cells (gpgcs) of Turkey (Meleagris gallopavo) from Days Old Embryos
 International Journal of Agricultural Technology 2017 Vol. 13(7.2): 1579-1589 Available online http://www.ijat-aatsea.com ISSN 1686-9141 Isolation and Characterization of Gonadal Primordial Germ Cells
International Journal of Agricultural Technology 2017 Vol. 13(7.2): 1579-1589 Available online http://www.ijat-aatsea.com ISSN 1686-9141 Isolation and Characterization of Gonadal Primordial Germ Cells
SUMMARY. Keywords: quail, Coturnix japonica, morphology, ovary, oviduct, neurotrophins, immunohistochemistry
 SUMMARY Keywords: quail, Coturnix japonica, morphology, ovary, oviduct, neurotrophins, immunohistochemistry Studies on the development of biological systems have expanded using animal models, always to
SUMMARY Keywords: quail, Coturnix japonica, morphology, ovary, oviduct, neurotrophins, immunohistochemistry Studies on the development of biological systems have expanded using animal models, always to
(FITC) or rhodamine blue isothiocyanate (RBITC) for use in mixed egg-transfer experiments. Both FITC and RBITC bind to the zona pellucida
 THE LABELLING OF LIVING RABBIT OVA WITH FLUORESCENT DYES J. W. OVERSTREET Department of Anatomy and International Institute for the Study of Human Reproduction, Columbia University, College of Physicians
THE LABELLING OF LIVING RABBIT OVA WITH FLUORESCENT DYES J. W. OVERSTREET Department of Anatomy and International Institute for the Study of Human Reproduction, Columbia University, College of Physicians
Terms. Primary vs. secondary sexual differentiation. Development of gonads vs. physical appearances
 SEX DETERMINATION How is the sex of an organism determined? Is this process the same for all organisms? What is the benefit of sexual reproduction anyway? Terms Primary vs. secondary sexual differentiation
SEX DETERMINATION How is the sex of an organism determined? Is this process the same for all organisms? What is the benefit of sexual reproduction anyway? Terms Primary vs. secondary sexual differentiation
REPRODUCTIVE BIOTECHNOLOGY IN SWINE
 REPRODUCTIVE BIOTECHNOLOGY IN SWINE References * Animal breeding and infertility by M. J. Meredith * Controlled reproduction in pigs by I. Gordon * Reproduction in farm animals by E.S.E. Hafez * Progress
REPRODUCTIVE BIOTECHNOLOGY IN SWINE References * Animal breeding and infertility by M. J. Meredith * Controlled reproduction in pigs by I. Gordon * Reproduction in farm animals by E.S.E. Hafez * Progress
II. The effect of strain and IGF-1 gene transfer using SMGT techniques on some semen characteristics:-
 The experimental work of this study was carried out in poultry farm of the Department of Animal Production, Faculty of Agriculture, Benha University, Egypt with cooperation of Genetic Engineering and Biotechnology
The experimental work of this study was carried out in poultry farm of the Department of Animal Production, Faculty of Agriculture, Benha University, Egypt with cooperation of Genetic Engineering and Biotechnology
Sex Determination and Gonadal Sex Differentiation in Fish
 Sex Determination and Gonadal Sex Differentiation in Fish Yoshitaka Nagahama Okazaki National Research Institutes, Japan This first slide shows the processes of gonadal sex differentiation and gametogenesis
Sex Determination and Gonadal Sex Differentiation in Fish Yoshitaka Nagahama Okazaki National Research Institutes, Japan This first slide shows the processes of gonadal sex differentiation and gametogenesis
TRANSPLANTATION OF SPERMATOGONIA ISOLATED FROM GIANT GOURAMI COLD PRESERVED TESTIS INTO NILE TILAPIA LARVAE
 TRANSPLANTATION OF SPERMATOGONIA ISOLATED FROM GIANT GOURAMI COLD PRESERVED TESTIS INTO NILE TILAPIA LARVAE Irma Andriani Dept of Biology, Faculty of Math and Science, Hasanuddin University, Makassar E-mail
TRANSPLANTATION OF SPERMATOGONIA ISOLATED FROM GIANT GOURAMI COLD PRESERVED TESTIS INTO NILE TILAPIA LARVAE Irma Andriani Dept of Biology, Faculty of Math and Science, Hasanuddin University, Makassar E-mail
To General Embryology Dr: Azza Zaki
 Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Introduction To General Embryology The Human Development is a continuous process that begins when an ovum from a female is fertilized by a sperm from a male. Cell division, growth and differentiation transform
Pakistan Veterinary Journal
 RESEARCH ARTICLE Pakistan Veterinary Journal ISSN: 0253-8318 (PRINT), 2074-7764 (ONLINE) Accessible at: www.pvj.com.pk The Development of Primordial Germ Cells (PGCs) and Testis in the Quail Embryo G.
RESEARCH ARTICLE Pakistan Veterinary Journal ISSN: 0253-8318 (PRINT), 2074-7764 (ONLINE) Accessible at: www.pvj.com.pk The Development of Primordial Germ Cells (PGCs) and Testis in the Quail Embryo G.
Effects of 17beta-estradiol on distribution of primordial germ cell migration in male chicks
 Asian J Androl 2008; 10 (2): 243 248 DOI: 10.1111/j.1745-7262.2008.00330.x www.asiaandro.com. Original Article. Effects of 17beta-estradiol on distribution of primordial germ cell migration in male chicks
Asian J Androl 2008; 10 (2): 243 248 DOI: 10.1111/j.1745-7262.2008.00330.x www.asiaandro.com. Original Article. Effects of 17beta-estradiol on distribution of primordial germ cell migration in male chicks
GENETIC EVALUATION OF ROOSTERS FOR FERTILITY AND HATCHABILITY ACCORDING TO SEMEN INDEX AND INDIVIDUAL SEMEN TRAITS
 GENETIC EVALUATION OF ROOSTERS FOR FERTILITY AND HATCHABILITY ACCORDING TO SEMEN INDEX AND INDIVIDUAL SEMEN TRAITS Firas R. Al-Samarai¹, Thamer K. Al-Ganabi², Ahmed M. Al-Nedawi³, Kalid A. Al-Soudi³ ¹Department
GENETIC EVALUATION OF ROOSTERS FOR FERTILITY AND HATCHABILITY ACCORDING TO SEMEN INDEX AND INDIVIDUAL SEMEN TRAITS Firas R. Al-Samarai¹, Thamer K. Al-Ganabi², Ahmed M. Al-Nedawi³, Kalid A. Al-Soudi³ ¹Department
Morphology and Regional Distribution of the Primordial Germ Cells in the Giant Freshwater Prawn, Macrobrachium rosenbergii
 R ESEARCH ARTICLE ScienceAsia 27 (2001) : 15-19 Morphology and Regional Distribution of the Primordial Germ Cells in the Giant Freshwater Prawn, Macrobrachium rosenbergii Praneet Damrongphol* and Pleanphit
R ESEARCH ARTICLE ScienceAsia 27 (2001) : 15-19 Morphology and Regional Distribution of the Primordial Germ Cells in the Giant Freshwater Prawn, Macrobrachium rosenbergii Praneet Damrongphol* and Pleanphit
Title. Author(s)TEKELI, Tevfik; KWEON, Oh Kyeong; KANAGAWA, Hiroshi. CitationJapanese Journal of Veterinary Research, 35(4): 283-
 Title THE VIABILITY OF DEEP-FROZEN AGGREGATED MOUSE EMBRYO Author(s)TEKELI, Tevfik; KWEON, Oh Kyeong; KANAGAWA, Hiroshi CitationJapanese Journal of Veterinary Research, 35(4): 283- Issue Date 1987-10-30
Title THE VIABILITY OF DEEP-FROZEN AGGREGATED MOUSE EMBRYO Author(s)TEKELI, Tevfik; KWEON, Oh Kyeong; KANAGAWA, Hiroshi CitationJapanese Journal of Veterinary Research, 35(4): 283- Issue Date 1987-10-30
SUPPLEMENTARY MATERIAL. Sample preparation for light microscopy
 SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
SUPPLEMENTARY MATERIAL Sample preparation for light microscopy To characterize the granulocytes and melanomacrophage centers, cross sections were prepared for light microscopy, as described in Material
Bi-potent Gonads. Sex Determination
 יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
יצירת הגונדות Primordial Germ Cells (PGCs) Somatic cells Genital ridge Bi-potent Gonads Sex Determination Testis and Sperm Ovary and Oocyte Migration of Primordial Germ Cells in the Chick Embryo The
Biology 4361 Developmental Biology Exam 1 ID#: October 11, 2005
 Biology 4361 Developmental Biology Name: Key Exam 1 ID#: October 11, 2005 Multiple choice (one point each) 1. Primordial germ cells a. are immortal b. produce polar bodies c. are haploid d. are somatic
Biology 4361 Developmental Biology Name: Key Exam 1 ID#: October 11, 2005 Multiple choice (one point each) 1. Primordial germ cells a. are immortal b. produce polar bodies c. are haploid d. are somatic
Sex differentiation of germ cells in the teleost, Oryzias latipes, during normal embryonic development
 J. Embryol. exp. Morph. Vol. 2, 2, pp. -, 2 Printed in Great Britain Sex differentiation of germ cells in the teleost, Oryzias latipes, during normal embryonic development ByNORIYUKI SATOH AND NOBUO EGAMI
J. Embryol. exp. Morph. Vol. 2, 2, pp. -, 2 Printed in Great Britain Sex differentiation of germ cells in the teleost, Oryzias latipes, during normal embryonic development ByNORIYUKI SATOH AND NOBUO EGAMI
Monitoring of the effects of various spiking techniques in broiler breeder flocks by counting of IPVL holes of the eggs
 Monitoring of the effects of various spiking techniques in broiler breeder flocks by counting of IPVL holes of the eggs B.VÉGI*, ZS. SZİKE, J. BARNA Research Institute for Animal Breeding and Nutrition,
Monitoring of the effects of various spiking techniques in broiler breeder flocks by counting of IPVL holes of the eggs B.VÉGI*, ZS. SZİKE, J. BARNA Research Institute for Animal Breeding and Nutrition,
In domestic animals, we have limited period of estrus (sexual receptivity) and the term estrous
 REPRODUCTIVE CYCLES 1. Estrous cycle 2. Menstrual cycle In domestic animals, we have limited period of estrus (sexual receptivity) and the term estrous cycle is used. The onset of proestrus defines the
REPRODUCTIVE CYCLES 1. Estrous cycle 2. Menstrual cycle In domestic animals, we have limited period of estrus (sexual receptivity) and the term estrous cycle is used. The onset of proestrus defines the
Sexual Reproduction. For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species.
 Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
Sex Determination Sexual Reproduction For most diploid eukaryotes, sexual reproduction is the only mechanism resulting in new members of a species. Meiosis in the sexual organs of parents produces haploid
THERE is considerable evidence that
 Effects of High Temperature on Semen Production and Fertility in the Domestic Fowl M. A. BOONE Poultry Department, Clemson College, Clemson, S.C. AND T. M. HUSTON Poultry Department, University of Georgia,
Effects of High Temperature on Semen Production and Fertility in the Domestic Fowl M. A. BOONE Poultry Department, Clemson College, Clemson, S.C. AND T. M. HUSTON Poultry Department, University of Georgia,
SUPPLEMENTAL INFORMATION FOR. PAX7 expression defines germline stem cells in the adult testis
 SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
SUPPLEMENTAL INFORMATION FOR PAX7 expression defines germline stem cells in the adult testis Gina M. Aloisio, Yuji Nakada, Hatice D. Saatcioglu, Christopher G. Peña, Michael D. Baker, Edward D. Tarnawa,
The development of ovary in quail s embryo
 African Journal of Biotechnology Vol. 10 (4), pp. 712-717, 24 January, 2011 Available online at http://www.academicjournals.org/ajb DOI: 10.5897/AJB09.1519 ISSN 1684 5315 2011 Academic Journals Full Length
African Journal of Biotechnology Vol. 10 (4), pp. 712-717, 24 January, 2011 Available online at http://www.academicjournals.org/ajb DOI: 10.5897/AJB09.1519 ISSN 1684 5315 2011 Academic Journals Full Length
ICSI with sperm derived from cultured testes (Nature 2011) Establishment of rabbit ips cells (J Biol Chem 2010)
 Efficient production of offspring in wild-derived strains of mice (Biol Reprod in press) (Presented by Keiji Mochida) Birth of offspring from ectopically transplanted PGCs (Biol Reprod 211) ICSI with sperm
Efficient production of offspring in wild-derived strains of mice (Biol Reprod in press) (Presented by Keiji Mochida) Birth of offspring from ectopically transplanted PGCs (Biol Reprod 211) ICSI with sperm
PHYSIOLOGY AND REPRODUCTION. Fertility Rate of Daily Collected and Cryopreserved Fowl Semen
 PHYSIOLOGY AND REPRODUCTION Fertility Rate of Daily Collected and Cryopreserved Fowl Semen A. VAN VOORST and F. R. LEENSTRA Institute for Animal Science and Health (ID-DLO) "Het Spelderholt", P.O. Box,
PHYSIOLOGY AND REPRODUCTION Fertility Rate of Daily Collected and Cryopreserved Fowl Semen A. VAN VOORST and F. R. LEENSTRA Institute for Animal Science and Health (ID-DLO) "Het Spelderholt", P.O. Box,
Testicular stem cells
 Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Testicular stem cells Dirk G. de Rooij Department of Endocrinology Faculty of Biology, Utrecht University 1. Knowledge on the development of the spermatogenic stem cell lineage 2. Principals of the nature
Microinsemination (Intracytoplasmic Sperm Injection) Microinsemination schedule. 1. Preparation of mediums
 Microinsemination (Intracytoplasmic Sperm Injection) Masumi Hirabayashi Section of Mammalian Transgenesis, Center for Genetic Analysis of Behavior, National Institute for Physiological Sciences, National
Microinsemination (Intracytoplasmic Sperm Injection) Masumi Hirabayashi Section of Mammalian Transgenesis, Center for Genetic Analysis of Behavior, National Institute for Physiological Sciences, National
Rejuvenation of Gamete Cells; Past, Present and Future
 Rejuvenation of Gamete Cells; Past, Present and Future Denny Sakkas PhD Scientific Director, Boston IVF Waltham, MA, USA Conflict of Interest I have no conflict of interest related to this presentation.
Rejuvenation of Gamete Cells; Past, Present and Future Denny Sakkas PhD Scientific Director, Boston IVF Waltham, MA, USA Conflict of Interest I have no conflict of interest related to this presentation.
SEX BEHAVIOUR AND SEX DETERMINATION IN CREPIDVLA FORNICATA L.
 [34] SEX BEHAVIOUR AND SEX DETERMINATION IN CREPIDVLA FORNICATA L. BY JAN Z. WILCZYNSKI Lebanese State University, Beirut, Lebanon (Received 21 November 1957) INTRODUCTION The problem of sex determination
[34] SEX BEHAVIOUR AND SEX DETERMINATION IN CREPIDVLA FORNICATA L. BY JAN Z. WILCZYNSKI Lebanese State University, Beirut, Lebanon (Received 21 November 1957) INTRODUCTION The problem of sex determination
A Diluent for Deep Freezing Preservation of Fowl Spermatozoa
 J. Fac. Fish. Anim. Husb., Hiroshima Univ. (1977), 16: 59-64 A Diluent for Deep Freezing Preservation of Fowl Spermatozoa Moriyuki WATANABE, Takato TERADA and Yoshihisa SHIRAKAWA. Department of Animal
J. Fac. Fish. Anim. Husb., Hiroshima Univ. (1977), 16: 59-64 A Diluent for Deep Freezing Preservation of Fowl Spermatozoa Moriyuki WATANABE, Takato TERADA and Yoshihisa SHIRAKAWA. Department of Animal
Unit B: Anatomy and Physiology of Poultry. Lesson 4: Artificial Poultry Reproduction
 Unit B: Anatomy and Physiology of Poultry Lesson 4: Artificial Poultry Reproduction 1 1 Terms Artificial insemination Sexed semen Standing heat 2 2 I. Artificial insemination is the placing of semen in
Unit B: Anatomy and Physiology of Poultry Lesson 4: Artificial Poultry Reproduction 1 1 Terms Artificial insemination Sexed semen Standing heat 2 2 I. Artificial insemination is the placing of semen in
BREAKOUT ANALYSIS GUIDE FOR HATCHERIES
 BREAKOUT ANALYSIS GUIDE FOR HATCHERIES By Dr. Joseph M. Mauldin, University of Georgia, Cooperative Extension Service, Athens, USA Breakout analyses are useful hatchery management procedures that provide
BREAKOUT ANALYSIS GUIDE FOR HATCHERIES By Dr. Joseph M. Mauldin, University of Georgia, Cooperative Extension Service, Athens, USA Breakout analyses are useful hatchery management procedures that provide
Accelerating Embryonic Growth During Incubation Following Prolonged Egg Storage 2. Embryonic Growth and Metabolism 1
 Accelerating Embryonic Growth During Incubation Following Prolonged Egg Storage 2. Embryonic Growth and Metabolism 1 V. L. Christensen, 2 J. L. Grimes, M. J. Wineland, and G. S. Davis Department of Poultry
Accelerating Embryonic Growth During Incubation Following Prolonged Egg Storage 2. Embryonic Growth and Metabolism 1 V. L. Christensen, 2 J. L. Grimes, M. J. Wineland, and G. S. Davis Department of Poultry
In vitro Culture, Storage and Transfer of Goat Embryos
 Aust. J. Bio!. Sci., 1976,29, 125-9 In vitro Culture, Storage and Transfer of Goat Embryos R. J. Bilton and N. W. Moore Department of Animal Husbandry, University of Sydney, Camden, N.S.W. 2570. Abstract
Aust. J. Bio!. Sci., 1976,29, 125-9 In vitro Culture, Storage and Transfer of Goat Embryos R. J. Bilton and N. W. Moore Department of Animal Husbandry, University of Sydney, Camden, N.S.W. 2570. Abstract
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
11. SEXUAL DIFFERENTIATION. Germinal cells, gonocytes. Indifferent stage INDIFFERENT STAGE
 11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
11. SEXUAL DIFFERENTIATION INDIFFERENT STAGE Early in pregnancy, (within 10-15 % of the pregnancy s expected length) a genital ridge is formed in the sides of the embryonic tissue, ventral to the mesonephros
Cell Divisions. The autosomes represent the whole body. * Male Sex Chromosomes: XY * Female Sex Chromosomes: XX
 Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
Cell Divisions Each Cell (including gonads) has 46 chromosomes (23 pairs of chromosomes: 22 pairs of autosomes, 1 pair of sex chromosomes) which are located in the nucleus). The autosomes represent the
BIOL2005 WORKSHEET 2008
 BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
BIOL2005 WORKSHEET 2008 Answer all 6 questions in the space provided using additional sheets where necessary. Hand your completed answers in to the Biology office by 3 p.m. Friday 8th February. 1. Your
Temperature during the initial phases of incubation
 Temperature during the initial phases of incubation By R. Meijerhof Introduction For commercial and logistical reasons it is often necessary to store hatching eggs for several days or even weeks before
Temperature during the initial phases of incubation By R. Meijerhof Introduction For commercial and logistical reasons it is often necessary to store hatching eggs for several days or even weeks before
List of Equipment, Tools, Supplies, and Facilities:
 Unit B: Understanding Animal Body Systems Lesson 6: Anatomy and Physiology of Animal Reproductive Systems Student Learning Objectives: Instruction in this lesson should result in students achieving the
Unit B: Understanding Animal Body Systems Lesson 6: Anatomy and Physiology of Animal Reproductive Systems Student Learning Objectives: Instruction in this lesson should result in students achieving the
Draft. Draft. 2. The system of breeding which breeds a registered male to a registered female animal of the same breed is:
 Student Name: Draft Teacher: Date: District: Wake County Assessment: 9_12 Agriculture AA21 - Animal Science I Test 4 Description: Test 7: Reproduction & Genetics Form: 501 Draft 1. Superior traits of offspring
Student Name: Draft Teacher: Date: District: Wake County Assessment: 9_12 Agriculture AA21 - Animal Science I Test 4 Description: Test 7: Reproduction & Genetics Form: 501 Draft 1. Superior traits of offspring
Research Note. Kenji Sasaki, Toshiaki Tatsumi, Mariko Tsutsui, Tatsuya Niinomi,, -. + Takayuki Imai, Mitsuru Naito, Atsushi Tajima and Yasuhiro Nishi
 http://www.jstage.jst.go.jp/browse/jpsa doi: +*.,+. + /jpsa.**3+++ Copyright,*+* Poultry Science Association. Research Note, + +,, Kenji Sasaki, Toshiaki Tatsumi, Mariko Tsutsui, Tatsuya Niinomi,, -. +
http://www.jstage.jst.go.jp/browse/jpsa doi: +*.,+. + /jpsa.**3+++ Copyright,*+* Poultry Science Association. Research Note, + +,, Kenji Sasaki, Toshiaki Tatsumi, Mariko Tsutsui, Tatsuya Niinomi,, -. +
A technique for introducing localized long-lasting implants in the chick embryo
 /. Embryol. exp. Morph. Vol. 39, pp. 261-266, 1977 261 Printed in Great Britain SHORT PAPERS A technique for introducing localized long-lasting implants in the chick embryo By MARIETA B. HEATON 1 From
/. Embryol. exp. Morph. Vol. 39, pp. 261-266, 1977 261 Printed in Great Britain SHORT PAPERS A technique for introducing localized long-lasting implants in the chick embryo By MARIETA B. HEATON 1 From
Chromosome Theory & Sex-Linked Transmission
 Chromosome Theory & Sex-Linked Transmission (CHAPTER 3 & 5- Brooker Text) Feb 14, 2006 BIO 184 Dr. Tom Peavy - Maturation of the sperm in the epididymis - Mammalian sperm become capable of fertilizing
Chromosome Theory & Sex-Linked Transmission (CHAPTER 3 & 5- Brooker Text) Feb 14, 2006 BIO 184 Dr. Tom Peavy - Maturation of the sperm in the epididymis - Mammalian sperm become capable of fertilizing
Semen Production by the Emu (Dromaius novaehollandiae). 2. Effect of Collection Frequency on the Production of Semen and Spermatozoa
 Semen Production by the Emu (Dromaius novaehollandiae). 2. Effect of Collection Frequency on the Production of Semen and Spermatozoa I. A. MALECKI,*, G. B. MARTIN,*, and D. R. LINDSAY* *Animal Science,
Semen Production by the Emu (Dromaius novaehollandiae). 2. Effect of Collection Frequency on the Production of Semen and Spermatozoa I. A. MALECKI,*, G. B. MARTIN,*, and D. R. LINDSAY* *Animal Science,
Male Anatomy. testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics
 Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Male Anatomy Male Anatomy Primary Organ testes, genetically determined in mammals - testis releases hormones that then control the development of secondary sex characteristics 1) Secondary Organs internal
Faculty of Agriculture, Department of Animal Science, Ege University, Izmir, Turkey. * Corresponding author:
 The effect of exogenous melatonin administration on sperm quality and some stress related parameters of broiler breeder males under natural summer conditions H. KOZANOĞLU *, E. BABACANOĞLU, S. ÖZKAN Faculty
The effect of exogenous melatonin administration on sperm quality and some stress related parameters of broiler breeder males under natural summer conditions H. KOZANOĞLU *, E. BABACANOĞLU, S. ÖZKAN Faculty
Biology Developmental Biology Spring Quarter Midterm 1 Version A
 Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Biology 411 - Developmental Biology Spring Quarter 2013 Midterm 1 Version A 75 Total Points Open Book Choose 15 out the 20 questions to answer (5 pts each). Only the first 15 questions that are answered
Changes in Quail Blastodermal Cell Status as a Result of Selection*
 PL-ISSN 0015-5497 (print), ISSN 1734-9168 (online) Folia Biologica (Kraków), vol. 63 (2015), No 1 Institute of Systematics and Evolution of Animals, PAS, Kraków, 2015 doi:10.3409/fb63_1.63 Changes in Quail
PL-ISSN 0015-5497 (print), ISSN 1734-9168 (online) Folia Biologica (Kraków), vol. 63 (2015), No 1 Institute of Systematics and Evolution of Animals, PAS, Kraków, 2015 doi:10.3409/fb63_1.63 Changes in Quail
MEASURING FERTILITY & EARLY DEADS LEVELS PART 1
 MEASURING FERTILITY & EARLY DEADS LEVELS PART 1 Why measure fertility and early deads? An unfertilised egg cannot produce a chick. Flock fertility is governed by management of males and females on the
MEASURING FERTILITY & EARLY DEADS LEVELS PART 1 Why measure fertility and early deads? An unfertilised egg cannot produce a chick. Flock fertility is governed by management of males and females on the
Animal Development. Lecture 3. Germ Cells and Sex
 Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Animal Development Lecture 3 Germ Cells and Sex 1 The ovary of sow. The ovary of mare. The ovary of cow. The ovary of ewe. 2 3 The ovary. A generalized vertebrate ovary. (Wilt and Hake, Ch 2, 2004) 4 The
Scanning Electron Microscopic Observations on the Sperm Penetration through the Zona Pellucida of Mouse Oocytes Fertilized in vitro
 Scanning Electron Microscopic Observations on the Sperm Penetration through the Zona Pellucida of Mouse Oocytes Fertilized in vitro Masatsugu MOTOMURA and Yutaka TOYODA School of Veterinary Medicine and
Scanning Electron Microscopic Observations on the Sperm Penetration through the Zona Pellucida of Mouse Oocytes Fertilized in vitro Masatsugu MOTOMURA and Yutaka TOYODA School of Veterinary Medicine and
Chapter 4 The Chromosome Theory of Inheritance
 Chapter 4 The Chromosome Theory of Inheritance 4-1 Sections to study 4.1 Chromosomes: The carriers of genes 4.2 Mitosis: Cell division that preserves chromosome number 4.3 Meiosis: Cell division that halve
Chapter 4 The Chromosome Theory of Inheritance 4-1 Sections to study 4.1 Chromosomes: The carriers of genes 4.2 Mitosis: Cell division that preserves chromosome number 4.3 Meiosis: Cell division that halve
Spermatogenesis. What is it and what does it look like? How do hormones regulate spermatogenesis?
 Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Spermatogenesis What is it and what does it look like? How do hormones regulate spermatogenesis? FSH, androgens, growth factors Animal Physiology (Hill, Wise, Anderson): Ch. 15 435-438 1 Spermatogenesis:
Preimplantation genetic diagnosis: polar body and embryo biopsy
 Human Reproduction, Vol. 15, (Suppl. 4), pp. 69-75, 2000 Preimplantation genetic diagnosis: polar body and embryo biopsy Luca Gianaroli SISMER, Via Mazzini 12, 40138 Bologna, Italy Scientific Director
Human Reproduction, Vol. 15, (Suppl. 4), pp. 69-75, 2000 Preimplantation genetic diagnosis: polar body and embryo biopsy Luca Gianaroli SISMER, Via Mazzini 12, 40138 Bologna, Italy Scientific Director
Interval between PMSG Priming and hcg Injection in Superovulation of the Mongolian Gerbil
 J. Mamm. Ova Res. Vol. 21, 105 109, 2004 105 Original Interval between PMSG Priming and hcg Injection in Superovulation of the Mongolian Gerbil Yuichi Kameyama 1 *, Kaori Arai 1 and Yoshiro Ishijima 1
J. Mamm. Ova Res. Vol. 21, 105 109, 2004 105 Original Interval between PMSG Priming and hcg Injection in Superovulation of the Mongolian Gerbil Yuichi Kameyama 1 *, Kaori Arai 1 and Yoshiro Ishijima 1
SEX DETERMINATION AND SEX CHROMOSOMES
 Klug et al. 2006, 2009 Concepts of Genetics Chapter 7 STUDY UNIT 5 SEX DETERMINATION AND SEX CHROMOSOMES Some species reproduce asexually Most diploid eukaryotes reproduce sexually Parent (2n) Parent (2n)
Klug et al. 2006, 2009 Concepts of Genetics Chapter 7 STUDY UNIT 5 SEX DETERMINATION AND SEX CHROMOSOMES Some species reproduce asexually Most diploid eukaryotes reproduce sexually Parent (2n) Parent (2n)
ASA Presentation Notes Lesson 6.2
 ASA Presentation Notes Lesson 6.2 Unit Name: Unit 6 - Animal Reproduction Lesson Topic: Lesson 6.2 Generating Generations Presentation on: Livestock Breeding Systems Breeding Systems. Straight breeding.
ASA Presentation Notes Lesson 6.2 Unit Name: Unit 6 - Animal Reproduction Lesson Topic: Lesson 6.2 Generating Generations Presentation on: Livestock Breeding Systems Breeding Systems. Straight breeding.
Basic information on the cryopreservation process
 COST Action FA1205 AQUAGAMETE 5 th AQUAGAMETE Training School Valencia, Spain, 7-11 th March, 2016 Basic information on the cryopreservation process Ákos Horváth Department of Aquaculture, Szent István
COST Action FA1205 AQUAGAMETE 5 th AQUAGAMETE Training School Valencia, Spain, 7-11 th March, 2016 Basic information on the cryopreservation process Ákos Horváth Department of Aquaculture, Szent István
DRB666 Applied Developmental and Reproductive Biology (Spring 2013)
 DRB666 Applied Developmental and Reproductive Biology (Spring 2013) Director: 651 Ilalo Street, BSB163-3 e-mail: yyamazak@hawaii.edu Phone: (808) 692-1416 Instructors (e-mail): Steve Ward Yusuke Marikawa
DRB666 Applied Developmental and Reproductive Biology (Spring 2013) Director: 651 Ilalo Street, BSB163-3 e-mail: yyamazak@hawaii.edu Phone: (808) 692-1416 Instructors (e-mail): Steve Ward Yusuke Marikawa
Sexual Reproduction and Meiosis
 Sexual Reproduction and Meiosis Meiosis sexual reproduction! Meiosis makes the cells that are responsible for sexual reproduction Sexual Reproduction Producing a new organism by combining chromosomes from
Sexual Reproduction and Meiosis Meiosis sexual reproduction! Meiosis makes the cells that are responsible for sexual reproduction Sexual Reproduction Producing a new organism by combining chromosomes from
Influence of genetic factors on the fertilization of mouse ova in vitro
 Influence of genetic factors on the fertilization of mouse ova in vitro El\l=z:\b\l=i:\etaKaleta Department of Genetics and Evolution, Institute of Zoology, Jagellonian University, Krupnicza 50, 30-060
Influence of genetic factors on the fertilization of mouse ova in vitro El\l=z:\b\l=i:\etaKaleta Department of Genetics and Evolution, Institute of Zoology, Jagellonian University, Krupnicza 50, 30-060
Unit B Understanding Animal Body Systems. Lesson 7 Understanding Animal Reproduction
 Unit B Understanding Animal Body Systems Lesson 7 Understanding Animal Reproduction 1 Terms Anestrus Artificial insemination Castration Cervix Copulation Diestrus Egg Ejaculation Estrous cycle Estrus Fertilization
Unit B Understanding Animal Body Systems Lesson 7 Understanding Animal Reproduction 1 Terms Anestrus Artificial insemination Castration Cervix Copulation Diestrus Egg Ejaculation Estrous cycle Estrus Fertilization
Selection of Young Broiler Breeders for Semen Quality Improves Hatchability in an Industry Field Trial 1
 2002 Poultry Science Association, Inc. Selection of Young Broiler Breeders for Semen Quality Improves Hatchability in an Industry Field Trial 1 H. M. Parker and C. D. McDaniel 2 Poultry Science Department,
2002 Poultry Science Association, Inc. Selection of Young Broiler Breeders for Semen Quality Improves Hatchability in an Industry Field Trial 1 H. M. Parker and C. D. McDaniel 2 Poultry Science Department,
RECIPROCAL TRANSLOCATIONS AND REPRODUCTIVE CAPACITY IN RABBITS FOLLOWING EXTERNAL GAMMA IRRADIATION
 Bulgarian Journal of Veterinary Medicine (2005), 8, No 4, 227232 RECIPROCAL TRANSLOCATIONS AND REPRODUCTIVE CAPACITY IN RABBITS FOLLOWING EXTERNAL GAMMA IRRADIATION S. GEORGIEVA 1, TS. YABLANSKI 1, P.
Bulgarian Journal of Veterinary Medicine (2005), 8, No 4, 227232 RECIPROCAL TRANSLOCATIONS AND REPRODUCTIVE CAPACITY IN RABBITS FOLLOWING EXTERNAL GAMMA IRRADIATION S. GEORGIEVA 1, TS. YABLANSKI 1, P.
Strategic delivery: Setting standards Increasing and. Details: Output: Demonstrating efficiency. informing choice.
 Strategic delivery: Setting standards Increasing and informing choice Demonstrating efficiency economy and value Details: Meeting Scientific and Clinical Advances Advisory Committee Agenda item 6 Paper
Strategic delivery: Setting standards Increasing and informing choice Demonstrating efficiency economy and value Details: Meeting Scientific and Clinical Advances Advisory Committee Agenda item 6 Paper
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
F ertilizability of Rabbit Ova after Removal of the Corona Radiata
 F ertilizability of Rabbit Ova after Removal of the Corona Radiata M. C. CHANG, Ph.D., and J. M. BEDFORD, M.R.C.V.S." FRESHLY ovulated rabbit ova are surrounded by a mass of follicular cells in a mucous
F ertilizability of Rabbit Ova after Removal of the Corona Radiata M. C. CHANG, Ph.D., and J. M. BEDFORD, M.R.C.V.S." FRESHLY ovulated rabbit ova are surrounded by a mass of follicular cells in a mucous
Chapter 22 The Reproductive System (I)
 Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Chapter 22 The Reproductive System (I) An Overview of Reproductive Physiology o The Male Reproductive System o The Female Reproductive System 22.1 Reproductive System Overview Reproductive system = all
Fresh and Frozen Ovary Tissue Transplants: Mechanism of Adult Primordial Follicle Recruitment And Fetal Oocyte Arrest
 Fresh and Frozen Ovary Tissue Transplants: Mechanism of Adult Primordial Follicle Recruitment And Fetal Oocyte Arrest Locking and Unlocking: Oocyte Meiosis and PGC differentiation Yasui et al 2012 Factors
Fresh and Frozen Ovary Tissue Transplants: Mechanism of Adult Primordial Follicle Recruitment And Fetal Oocyte Arrest Locking and Unlocking: Oocyte Meiosis and PGC differentiation Yasui et al 2012 Factors
Scanning Electron Microscopical Observation on the Penetration Mechanism of Fowl Spermatozoa into the Ovum in the Process of Fertilization
 J. Fac. Fish. Anim. Husb., Hiroshima Univ. (1976), 15: 85-92 Scanning Electron Microscopical Observation on the Penetration Mechanism of Fowl Spermatozoa into the Ovum in the Process of Fertilization Shunsaku
J. Fac. Fish. Anim. Husb., Hiroshima Univ. (1976), 15: 85-92 Scanning Electron Microscopical Observation on the Penetration Mechanism of Fowl Spermatozoa into the Ovum in the Process of Fertilization Shunsaku
EOG Practice:,Evolution & Genetics [126663]
![EOG Practice:,Evolution & Genetics [126663] EOG Practice:,Evolution & Genetics [126663]](/thumbs/87/96059079.jpg) EOG Practice:,Evolution & Genetics [126663] Student Class Date 1. A particular peach tree produces peaches that are more resistant to disease than other peaches. What method would reproduce these EXACT
EOG Practice:,Evolution & Genetics [126663] Student Class Date 1. A particular peach tree produces peaches that are more resistant to disease than other peaches. What method would reproduce these EXACT
A comparison of the effects of estrus cow. nuclear maturation of bovine oocytes
 A comparison of the effects of estrus cow serum and fetal calf serum on in vitro nuclear maturation of bovine oocytes J Spiropoulos, SE Long University of Bristol, School of Veterinary Science, Department
A comparison of the effects of estrus cow serum and fetal calf serum on in vitro nuclear maturation of bovine oocytes J Spiropoulos, SE Long University of Bristol, School of Veterinary Science, Department
Correspondence: mirna regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration
 Correspondence: mirna regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration Alison A. Staton, Holger Knaut, and Antonio J. Giraldez Supplementary Note Materials and
Correspondence: mirna regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration Alison A. Staton, Holger Knaut, and Antonio J. Giraldez Supplementary Note Materials and
DRB666 Applied Developmental and Reproductive Biology Spring Semester, 2011
 DRB666 Applied Developmental and Reproductive Biology Spring Semester, 2011 Director: 651 Ilalo Street, BSB163-3 e-mail: yyamazak@hawaii.edu Phone: (808) 692-1416 Instructors (e-mail): Steve Ward Yusuke
DRB666 Applied Developmental and Reproductive Biology Spring Semester, 2011 Director: 651 Ilalo Street, BSB163-3 e-mail: yyamazak@hawaii.edu Phone: (808) 692-1416 Instructors (e-mail): Steve Ward Yusuke
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
PHYSIOLOGY AND REPRODUCTION. Fertility of Male and Female Broiler Breeders Following Exposure to Elevated Ambient Temperatures 1
 PHYSIOLOGY AND REPRODUCTION Fertility of Male and Female Broiler Breeders Following Exposure to Elevated Ambient Temperatures 1 CHRISTOPHER D. MCDANIEL, 2 R. KEITH BRAMWELL, JEANNA L. WILSON, and BIRKETT
PHYSIOLOGY AND REPRODUCTION Fertility of Male and Female Broiler Breeders Following Exposure to Elevated Ambient Temperatures 1 CHRISTOPHER D. MCDANIEL, 2 R. KEITH BRAMWELL, JEANNA L. WILSON, and BIRKETT
Biology 4361 Developmental Biology. October 11, Multiple choice (one point each)
 Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
Biology 4361 Developmental Biology Exam 1 October 11, 2005 Name: ID#: Multiple choice (one point each) 1. Sertoli cells a. surround spermatocytes b. are the structural components of the seminiferous tubules
FERTIUP PM 1 ml / 0.5 ml - CARD MEDIUM Set
 Product manual FERTIUP PM 1 ml / 0.5 ml - CARD MEDIUM Set Cat. No. KYD-004-EX Size: 1 SET KYD-005-EX 1 SET Department of Reproductive Engineering, Center for Animal Resources and Development, Kumamoto
Product manual FERTIUP PM 1 ml / 0.5 ml - CARD MEDIUM Set Cat. No. KYD-004-EX Size: 1 SET KYD-005-EX 1 SET Department of Reproductive Engineering, Center for Animal Resources and Development, Kumamoto
Oocyte and Sperm from ipscs
 Key Points University of Miyazaki, RIKEN, Kyoto University, Shiga University of Medical Science, Hokkaido University Oocyte and Sperm from ipscs of an Endangered Species -Sexual flexibility of endangered
Key Points University of Miyazaki, RIKEN, Kyoto University, Shiga University of Medical Science, Hokkaido University Oocyte and Sperm from ipscs of an Endangered Species -Sexual flexibility of endangered
3.1 Meiosis
 3.1 Meiosis Chromosome Number Individuals of the same species have the same number of chromosomes. Meiosis is used to half the number of chromosomes passed to the offspring. What is meiosis Meiosis happens
3.1 Meiosis Chromosome Number Individuals of the same species have the same number of chromosomes. Meiosis is used to half the number of chromosomes passed to the offspring. What is meiosis Meiosis happens
TEST NAME:review TEST ID: GRADE:07 Seventh Grade SUBJECT:Life and Physical Sciences TEST CATEGORY: My Classroom
 TEST NAME:review TEST ID:1070005 GRADE:07 Seventh Grade SUBJECT:Life and Physical Sciences TEST CATEGORY: My Classroom review Page 1 of 18 Student: Class: Date: 1. There are four blood types: A, B, AB,
TEST NAME:review TEST ID:1070005 GRADE:07 Seventh Grade SUBJECT:Life and Physical Sciences TEST CATEGORY: My Classroom review Page 1 of 18 Student: Class: Date: 1. There are four blood types: A, B, AB,
EFFECT OF THAWING RATE AND POST-THAW TEMPERATURE ON MOTILITY AND ACROSOMAL MAINTENANCE IN BOVINE SEMEN FROZEN IN PLASTIC STRAWS l,2
 EFFECT OF THAWING RATE AND POST-THAW TEMPERATURE ON MOTILITY AND ACROSOMAL MAINTENANCE IN BOVINE SEMEN FROZEN IN PLASTIC STRAWS l,2 P. L. Senger, W. C. Becker and J. K. Hillers Washington State University
EFFECT OF THAWING RATE AND POST-THAW TEMPERATURE ON MOTILITY AND ACROSOMAL MAINTENANCE IN BOVINE SEMEN FROZEN IN PLASTIC STRAWS l,2 P. L. Senger, W. C. Becker and J. K. Hillers Washington State University
저작권법에따른이용자의권리는위의내용에의하여영향을받지않습니다.
 저작자표시 - 비영리 - 변경금지 2.0 대한민국 이용자는아래의조건을따르는경우에한하여자유롭게 이저작물을복제, 배포, 전송, 전시, 공연및방송할수있습니다. 다음과같은조건을따라야합니다 : 저작자표시. 귀하는원저작자를표시하여야합니다. 비영리. 귀하는이저작물을영리목적으로이용할수없습니다. 변경금지. 귀하는이저작물을개작, 변형또는가공할수없습니다. 귀하는, 이저작물의재이용이나배포의경우,
저작자표시 - 비영리 - 변경금지 2.0 대한민국 이용자는아래의조건을따르는경우에한하여자유롭게 이저작물을복제, 배포, 전송, 전시, 공연및방송할수있습니다. 다음과같은조건을따라야합니다 : 저작자표시. 귀하는원저작자를표시하여야합니다. 비영리. 귀하는이저작물을영리목적으로이용할수없습니다. 변경금지. 귀하는이저작물을개작, 변형또는가공할수없습니다. 귀하는, 이저작물의재이용이나배포의경우,
