Protocol for the Examination of Specimens From Patients With Malignant Germ Cell and Sex Cord-Stromal Tumors of the Testis
|
|
|
- Rodger Stephens
- 6 years ago
- Views:
Transcription
1 Protocol for the Examination of Specimens From Patients With Malignant Germ Cell and Sex Cord-Stromal Tumors of the Testis Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation purposes, this protocol should be used for the following procedures AND tumor types: Procedure Description Radical Orchiectomy Includes specimens designated orchiectomy and orchidectomy Tumor Type Description Germ cell tumors Includes seminoma and variants, all non-seminomatous germ cell tumors, mixed germ cell tumors, Leydig cell tumors, Sertoli cell tumors, granulosa cell tumors, and placental site trophoblastic tumors Sex cord-stromal tumors Includes Leydig cell tumors, Sertoli cell tumors, granulosa cell tumors, and mixed sex cord tumors This protocol is NOT required for accreditation purposes for the following: Procedure Retroperitoneal lymphadenectomy Biopsy (needle, incisional or wedge) Primary resection specimen with no residual cancer (eg, following neoadjuvant therapy) Cytologic specimens The following tumor types should NOT be reported using this protocol: Tumor Type Paratesticular malignancies (consider Soft Tissue protocol) Non-testis germ cell tumors (consider Extragonadal Germ Cell protocol) Lymphoma (consider the Hodgkin or non-hodgkin Lymphoma protocols) Sarcoma (consider the Soft Tissue protocol) Authors Satish K. Tickoo, MD*; Ming Zhou, MD, PhD*; Robert Allan, MD; Mahul B. Amin, MD; Sam S. Chang, MD; Peter A. Humphrey, MD, PhD; James McKiernan, MD; Jason Pettus, MD; Victor E. Reuter, MD; John R. Srigley, MD; Thomas M. Ulbright, MD With guidance from the CAP Cancer and CAP Pathology Electronic Reporting Committees. * Denotes primary authors. All other contributing authors are listed alphabetically College of American Pathologists (CAP). All rights reserved. For Terms of Use please visit
2 Accreditation Requirements This protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Core data elements are required in reports to adequately describe appropriate malignancies. For accreditation purposes, essential data elements must be reported in all instances, even if the response is not applicable or cannot be determined. Conditional data elements are only required to be reported if applicable as delineated in the protocol. For instance, the total number of lymph nodes examined must be reported, but only if nodes are present in the specimen. Optional data elements are identified with + and although not required for CAP accreditation purposes, may be considered for reporting as determined by local practice standards. The use of this protocol is not required for recurrent tumors or for metastatic tumors that are resected at a different time than the primary tumor. Use of this protocol is also not required for pathology reviews performed at a second institution (ie, secondary consultation, second opinion, or review of outside case at second institution). Synoptic Reporting All core and conditionally required data elements outlined on the surgical case summary from this cancer protocol must be displayed in synoptic report format. Synoptic format is defined as: Data element: followed by its answer (response), outline format without the paired "Data element: Response" format is NOT considered synoptic. The data element must be represented in the report as it is listed in the case summary. The response for any data element may be modified from those listed in the case summary, including Cannot be determined if appropriate. Each diagnostic parameter pair (Data element: Response) is listed on a separate line or in a tabular format to achieve visual separation. The following exceptions are allowed to be listed on one line: o Anatomic site or specimen, laterality, and procedure o Pathologic Stage Classification (ptnm) elements o Negative margins, as long as all negative margins are specifically enumerated where applicable The synoptic portion of the report can appear in the diagnosis section of the pathology report, at the end of the report or in a separate section, but all Data element: Responses must be listed together in one location Organizations and pathologists may choose to list the required elements in any order, use additional methods in order to enhance or achieve visual separation, or add optional items within the synoptic report. The report may have required elements in a summary format elsewhere in the report IN ADDITION TO but not as replacement for the synoptic report i.e. all required elements must be in the synoptic portion of the report in the format defined above. CAP Laboratory Accreditation Program Protocol Required Use Date: March 2018* * Beginning January 1, 2018, the 8th edition AJCC Staging Manual should be used for reporting ptnm. CAP Testis Protocol Summary of Changes The following data elements were modified: Pathologic Stage Classification (ptnm, AJCC 8th Edition) 2
3 CAP Approved Surgical Pathology Cancer Case Summary Protocol posting date: June 2017 TESTIS: Radical Orchiectomy Select a single response unless otherwise indicated. Specimen Laterality Right Left Not specified Tumor Focality Unifocal Multifocal Cannot be determined Tumor Size Greatest dimension of main tumor mass (centimeters): cm + Additional dimensions: x cm Greatest dimensions of additional tumor nodules (centimeters) (required only if applicable): cm # Cannot be determined (explain): # Note: Include additional greatest dimensions for additional nodules as necessary Histologic Type (Notes A, B, and C) Intratubular Germ Cell Neoplasia Germ cell neoplasia in situ (GCNIS) Intratubular seminoma Intratubular embryonal carcinoma Other intratubular germ cell tumor (specify): Seminoma Seminoma Seminoma with syncytiotrophoblastic cells Seminoma with associated scar Embryonal carcinoma Yolk sac tumor, postpubertal type Choriocarcinoma Mixed germ cell tumor Seminoma (specify percentage): % Embryonal carcinoma (specify percentage): % Yolk sac tumor, postpubertal type (specify percentage): % Choriocarcinoma (specify percentage): % Teratoma (specify percentage): % Other (specify type and percentage): % Non-Choriocarcinomatous Trophoblastic Tumor Non-choriocarcinomatous trophoblastic tumor, NOS Placental site trophoblastic tumor Epithelioid trophoblastic tumor Cystic trophoblastic tumor + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 3
4 CAP Approved Teratoma, postpubertal type Teratoma with somatic-type malignancy (specify type): Testicular Scar/Regressed Germ Cell Tumor Scar diagnostic of regressed germ cell tumor Scar suspicious for regressed germ cell tumor Spermatocytic tumor Spermatocytic tumor with a sarcomatous component + Prepubertal type teratoma + Dermoid cyst + Epidermoid cyst + Well-differentiated neuroendocrine tumor (monodermal teratoma) + Other (specify): Mixed germ cell-sex cord stromal tumor, gonadoblastoma Sex Cord-Stromal Tumor Leydig cell tumor Malignant Leydig cell tumor Sertoli cell tumor, NOS Sertoli cell tumor, malignant Sertoli cell tumor, large cell calcifying Sertoli cell tumor, intratubular large cell hyalinizing Granulosa cell tumor, adult type Granulosa cell tumor, juvenile type Fibroma-thecoma Sex cord-stromal tumor, mixed type (specify components and approximate percentages): Sex cord-stromal tumor type, unclassified Other histologic type not listed, (specify): Tumor Extension (select all that apply) (Note D) No evidence of primary tumor Germ cell neoplasia in situ only Tumor limited to testis Tumor invades rete testis # Tumor invades hilar soft tissue Tumor invades epididymis Tumor invades tunica albuginea (perforates mesothelium) Tumor invades spermatic cord Tumor invades scrotum Tumor invades other structures (specify): Cannot be assessed # See Note D for definition of rete testis invasion. Margins Spermatic Cord Margin Cannot be assessed Uninvolved by tumor Involved by tumor Other Margin(s) (required only if applicable) Cannot be assessed Uninvolved by tumor (specify margin(s)): + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 4
5 CAP Approved Involved by tumor (specify margin(s)): Lymphovascular Invasion (Note E) Not identified Present Cannot be determined Regional Lymph Nodes No lymph nodes submitted or found Lymph Node Examination (required only if lymph nodes are present in the specimen) Number of Lymph Nodes Involved: Number cannot be determined (explain): Specify Site(s) (if applicable) # : # Sites may include interaortocaval, paraaortic, paracaval, preaortic, precaval, retroaortic, retrocaval, or other lymph nodes. Number of Lymph Nodes Examined: Number cannot be determined (explain): Lymph Node Metastasis (required if any lymph nodes are involved) Size of Largest Lymph Node (or Nodal Mass) Involved (centimeters): cm + Size of Largest Metastatic Deposit (millimeters): mm + Specify Site: Extranodal Extension (required only if lymph nodes involved) Not identified Present Cannot be determined Histologic subtype of germ cell tumor in involved lymph nodes (if applicable, specify): Pathologic Stage Classification (ptnm, AJCC 8 th Edition) (Note F) Note: Reporting of pt, pn, and (when applicable) pm categories is based on information available to the pathologist at the time the report is issued. Only the applicable T, N, or M category is required for reporting; their definitions need not be included in the report. The categories (with modifiers when applicable) can be listed on 1 line or more than 1 line. TNM Descriptors (required only if applicable) (select all that apply) m (multiple) r (recurrent) y (posttreatment) Primary Tumor (pt) ptx: Primary tumor cannot be assessed pt0: No evidence of primary tumor ptis: Germ cell neoplasia in situ pt1: Tumor limited to testis (including rete testis invasion) without lymphovascular invasion pt1a: Tumor smaller than 3 cm in size # pt1b: Tumor 3 cm or larger in size # pt2: Tumor limited to testis (including rete testis invasion) with lymphovascular invasion OR Tumor invading hilar soft tissue or epididymis or penetrating visceral mesothelial layer covering the external surface of tunica albuginea with or without lymphovascular invasion + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 5
6 CAP Approved pt3: pt4: Tumor invades spermatic cord with or without lymphovascular invasion Tumor invades scrotum with or without lymphovascular invasion # Subclassification of pt1 applies only to pure seminoma Regional Lymph Nodes (pn) pnx: Regional lymph node cannot be assessed pn0: No regional lymph node metastasis pn1: Metastasis with a lymph node mass 2 cm or smaller in greatest dimension and less than or equal to five nodes positive, none larger than 2 cm in greatest dimension pn2: Metastasis with a lymph node mass larger than 2 cm but not larger than 5 cm in greatest dimension; or more than five nodes positive, none larger than 5 cm; or evidence of extranodal extension of tumor pn3: Metastasis with a lymph node mass larger than 5 cm in greatest dimension Distant Metastasis (pm) (required only if confirmed pathologically in this case) pm1: Distant metastasis pm1a: Nonretroperitoneal nodal or pulmonary metastases pm1b: Nonpulmonary visceral metastases Specify site(s), if known: + Pre-Orchiectomy Serum Tumor Markers (select all that apply) (Notes F and G) + Unknown + Serum marker studies within normal limits + Alpha-fetoprotein (AFP) elevation + Beta subunit of human chorionic gonadotropin (b-hcg) elevation + Lactate dehydrogenase (LDH) elevation + Post-Orchiectomy Serum Tumor Markers (select all that apply) (Notes F and G) + Unknown + Serum marker studies within normal limits + Alpha-fetoprotein (AFP) elevation + Beta subunit of human chorionic gonadotropin (b-hcg) elevation + Lactate dehydrogenase (LDH) elevation + Serum Tumor Markers (S) (Note G) + SX: Serum marker studies not available or performed + S0: Serum marker study levels within normal limits LDH HCG (miu/ml) AFP (ng/ml) + S1: <1.5 X N # and <5,000 and <1,000 + S2: X N or 5,000-50,000 or 1,000-10,000 + S3: >10 X N or >50,000 or >10,000 # N indicates the upper limit of normal for the LDH assay. + Additional Pathologic Findings (select all that apply) (Note H) + None identified + Germ cell neoplasia in situ (GCNIS) + Microlith + Sertoli cell nodule (Pick s adenoma) + Atrophy + Other (specify): + Comment(s) + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 6
7 CAP Approved Surgical Pathology Cancer Case Summary Protocol posting date: June 2017 TESTIS: Retroperitoneal Lymphadenectomy Note: This case summary is recommended for reporting retroperitoneal lymphadenectomy specimens, but is not required for accreditation purposes. Select a single response unless otherwise indicated. + Specimen Site(s) (Note A) + Specify: + Prelymphadenectomy Treatment + Chemo/radiation therapy + No chemo/radiation therapy + Unknown + Number of Nodal Groups Present + Specify: + Cannot be determined Histologic Viability of Tumor (if applicable) (select all that apply) Viable teratoma present Viable nonteratomatous tumor present No viable tumor present Histologic Type of Metastatic Tumor (Note B) Seminoma Seminoma with syncytiotrophoblastic cells Embryonal carcinoma Yolk sac tumor, postpubertal type Choriocarcinoma Mixed germ cell tumor, specify components and approximate percentages: Non-choriocarcinomatous trophoblastic tumor, NOS Placental site trophoblastic tumor Epithelioid trophoblastic tumor Cystic trophoblastic tumor Teratoma, postpubertal type Teratoma with somatic-type malignancy (specify type): Spermatocytic tumor Spermatocytic tumor with a sarcomatous component Well-differentiated neuroendocrine tumor (monodermal teratoma) Other histologic type not listed(specify): Regional Lymph Nodes Number of Lymph Nodes Involved: Number cannot be determined (explain): Specify Site(s): Note: Sites may include interaortocaval, paraaortic, paracaval, preaortic, precaval, retroaortic, retrocaval, or other lymph nodes. Number of Lymph Nodes Examined: + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 7
8 CAP Approved Number cannot be determined (explain): Size of Largest Lymph Node (or Nodal Mass) Involved (centimeters): cm + Size of Largest Metastatic Deposit (millimeters): mm + Specify Site: Extranodal Extension (ENE) Not identified Present Cannot be determined Histologic subtype of germ cell tumor in involved lymph nodes (if applicable, specify): Nonregional Lymph Node Metastasis (M1a, AJCC 8 th Edition) (Note I) Not applicable Not identified Present + Number of Lymph Nodes Involved: + Number cannot be determined (explain): + Specify site(s): + Number of Lymph Nodes Examined: + Number cannot be determined (explain): Regional Lymph Nodes (pn, AJCC 8 th Edition) (Note I) Note: Reporting of pn category is based on information available to the pathologist at the time the report is issued. pnx: Regional lymph nodes cannot be assessed pn0: No regional lymph node metastasis pn1: Metastasis with a lymph node mass 2 cm or smaller in greatest dimension and less than or equal to five nodes positive, none larger than 2 cm in greatest dimension pn2: Metastasis with a lymph node mass larger than 2 cm but not larger than 5 cm in greatest dimension; or more than five nodes positive, none larger than 5 cm; or evidence of extranodal extension of tumor pn3: Metastasis with a lymph node mass larger than 5 cm in greatest dimension + Comment(s) + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 8
9 Background Documentation Explanatory Notes A. Tissues Submitted for Microscopic Evaluation The entire testicular tumor may be blocked if it requires 10 blocks or less (tissue may be retained for special studies); 10 blocks of larger tumors may be taken, unless the tumor is greater than 10 cm, in which case 1 block may be submitted for every 1 cm of maximum tumor dimension. Blocks must contain the interface with nontumorous testis, as well as the tunica albuginea, even away from the tumor, because lymphatic invasion is best appreciated in the peritumoral tissue, as well as in the vessels within and under/parallel to the tunica. When there are multifocal tumors (with 2 separate tumor nodules), additional tumor nodule(s) should also be sampled. Tissues to be sampled include: Tumor, including interface with surrounding testis, and tunica albuginea All of the grossly different appearing areas in the tumor Testicular hilum/mediastinum testis Uninvolved testis, including tunica albuginea Epididymis Spermatic cord, including cord margin Other lesion(s) All identifiable lymph nodes # Other tissue(s) submitted with specimen # For large masses that have obliterated individual nodes, 1 section for every centimeter of maximum tumor dimension, including grossly different looking areas, is recommended. The margins in a specimen resected for a malignant tumor of the testis, depending on the extent of the surgery, include spermatic cord margin, the parietal layer of tunica vaginalis, and scrotal skin. B. Histologic Type The protocol mainly applies to malignant tumors of the testis, the vast majority of which are of germ cell origin. It may also be applied to other malignant or potentially malignant tumors of the testis included in the classification shown below For hematolymphoid neoplasms involving the testis, refer to the corresponding CAP protocols. World Health Organization (WHO) Histologic Classification of Testicular Tumors (2016) 13 Germ Cell Tumors Derived From Germ Cell Neoplasia In Situ Noninvasive germ cell neoplasia Germ cell neoplasia in situ Specific forms of intratubular germ cell neoplasia Tumors of a single histologic type (pure forms) Seminoma Seminoma with syncytiotrophoblastic cells Nonseminomatous germ cell tumors Embryonal carcinoma Yolk sac tumor, postpubertal type Trophoblastic tumors Choriocarcinoma Nonchoriocarcinomatous trophoblastic tumors Placental site trophoblastic tumor Epidermoid trophoblastic tumor Cystic trophoblastic tumor Teratoma, postpubertal type Teratoma with somatic-type malignancy Nonseminomatous germ cell tumors of more than one histologic type Mixed germ cell tumor Germ cell tumors of unknown type Regressed germ cell tumor 9
10 Background Documentation Germ Cell Tumors Unrelated to Germ Cell Neoplasia In Situ Spermatocytic tumor Teratoma, prepubertal type Dermoid cyst Epidermoid cyst Well-differentiated neuroendocrine tumor (monodermal teratoma) Yolk sac tumor, prepubertal type Mixed teratoma and yolk sac tumor, prepubertal type York sac tumor, prepubertal type Sex Cord-Stromal Tumors Pure tumors Leydig cell tumor Malignant Leydig cell tumor Sertoli cell tumor Malignant Sertoli cell tumor Large cell calcifying Sertoli cell tumor Intratubular large cell hyalinizing Sertoli cell neoplasia Granulosa cell tumor Adult granulosa cell tumor Juvenile granulosa cell tumor Tumors in the fibroma-thecoma group Mixed and unclassified sex cord stromal tumor Mixed sex cord-stromal tumor Unclassified sex cord-stromal tumor Tumor Containing Both Germ Cell and Sex Cord-Stromal Elements Gonadoblastoma Miscellaneous Ovarian epithelial-type tumors Serous cystadenoma Serous tumor of borderline malignancy Serous cystadenocarcinoma Mucinous cystadenoma Mucinous borderline tumor Mucinous cuystadenocarcinoma Endometrioid adenocarcinoma Clear cell adenocarcinoma Brenner tumor Juvenile xanthogranuloma Hemangioma Hematolymphoid Tumors Diffuse large B-cell lymphoma Follicular lymphoma Extranodal NI/T-cell lymphoma, nasal type Plasmacytoma Myeloid sarcoma Rosai-Dorfman disease Tumors of Collecting Duct and Rete Testis Adenoma Adenocarcinoma 10
11 Background Documentation Tumors of Paratesticular Structures Adenomatoid tumor Mesothelioma Well-differentiated papillary mesothelioma Epididymal tumors Cystadenoma of the epididymis Papillary cystadenoma Adenocarcinoma of the epididymis Squamous cell carcinoma Melanotic neuroectodermal tumor Nephroblastoma Paraganglioma Mesenchymal Tumors of the Spermatic Cord and Testicular Adnexa Apipocytic tumors Lipoma Well-differentiated liposarcoma Dedifferentiated liposarcoma Myxoid liposarcoma Pleomorphic liposarcoma C. Scar Testicular scars, particularly in patients presenting with metastatic disease and clinically inapparent testicular primaries, may represent regressed, burnt-out testicular germ cell tumors. There are 2 established criteria to indicate a scar is diagnostic of a regressed germ cell tumor (GCT): a scar with associated germ cell neoplasia in situ (GCNIS) or a scar that contains coarse intratubular calcifications within expanded tubular profiles, which correspond to dystrophic calcifications that occurred in completely necrotic intratubular embryonal carcinoma. Features that are suspicious for, although not diagnostic of, regressed germ cell tumors include testicular atrophy, microlithiasis, and, in the scar, lymphoplasmacytic infiltrates and prominent vascularity. 14 In otherwise pure seminoma, such partial regression may have clinically important implications, since it is possible that some of these scars may represent regression of a nonseminomatous germ cell tumor component of the tumor. D. Invasion of the Rete Testis, Hilar/Mediastinal Soft Tissue, Epididymis or Tunica Vaginalis Tumors invading the tunica vaginalis (perforating the mesothelial lining) (Figure 1, Tumor A) are considered as stage pt2 by the American Joint Committee on Cancer (AJCC) TNM staging system. Invasion of rete testis is not assigned a higher pt stage than that for a tumor limited to the testis. Rete testis invasion has been reported by some to be associated with higher risk of relapse in clinical stage I seminoma. 15 Rete testis invasion is that the invasive tumor involves the rete testis stroma, with or without luminal involvement. Pagetoid extension of GCNIS into the rete testis should not be considered rete testis invasion. Hilar soft tissue invasion (Figure 1, Tumor B) is the predominant pathway of extratesticular extension for testicular tumors. 16,17 There is evidence beginning to accumulate that rete testis and hilar soft tissue invasion have predictive value for metastatic disease in patients with nonseminomatous GCTs. 17 Invasion of epididymis and hilar soft tissue is staged as pt2 by the 8 th edition of AJCC TNM
12 Background Documentation Figure 1. Diagrammatic representation of a tumor (Tumor A) invading tunica vaginalis, perforating through the mesothelium, and another tumor (Tumor B) partly involving the rete testis and invading the hilar soft tissue. Figure courtesy of Satish K. Tickoo, MD. E. Venous/Lymphatic Vessel Invasion In several studies, the presence of vascular space invasion (usually lymphatic but possibly also capillary or venous invasion) has been correlated with a significantly elevated risk for distant metastasis This observation, therefore, is most pertinent for patients who have clinical stage I disease, ie, those who have no evidence of spread beyond the testis by clinical examination (including radiographic and serum marker studies). Some clinicians manage the patients with clinical stage I disease who lack evidence of lymphatic or vascular invasion in their orchiectomy specimens (with possibly other favorable prognostic features, such as relatively small amounts of embryonal carcinoma) by close follow-up examinations rather than intervention. According to the 8 th edition AJCC TNM staging system, discontinuous involvement of the spermatic cord soft tissue via a vascular thrombus is better regarded as a metastatic deposit (pm1). Presence of only an intravascular tumor in the spermatic cord in the absence of parenchymal invasion is considered pt2. 26 F. Staging The protocol recommends staging according to the AJCC TNM staging system. 25 Additional criteria for staging seminomas according to a modification of the Royal Marsden system are also recommended. 27 Some studies suggest that the staging of patients with seminoma by the TNM system is less meaningful therapeutically than staging by a modification of the Royal Marsden method. 25,27 Also, the data from a large Danish study of seminomas clinically limited to the testis do not support the conclusion that local staging of the primary tumor, as performed in the TNM system, provides useful prognostic information; rather, the most valuable prognostic indicator was the size of the seminoma. 28 This protocol therefore encourages the use of the TNM system with optional use of the modified Royal Marsden staging system for patients with seminoma. AJCC/UICC TNM and Stage Groupings By AJCC convention, the designation T refers to a primary tumor that has not been previously treated. The symbol p refers to the pathologic classification of the TNM, as opposed to the clinical classification, and is based on gross and microscopic examination. pt entails a resection of the primary tumor or biopsy adequate to evaluate the highest pt category, pn entails removal of nodes adequate to validate lymph node metastasis, and pm implies 12
13 Background Documentation microscopic examination of distant lesions. Clinical classification (ctnm) is usually carried out by the referring physician before treatment during initial evaluation of the patient or when pathologic classification is not possible. Pathologic staging is usually performed after surgical resection of the primary tumor. Pathologic staging depends on pathologic documentation of the anatomic extent of disease, whether or not the primary tumor has been completely removed. If a biopsied tumor is not resected for any reason (eg, when technically unfeasible) and if the highest T and N categories or the M1 category of the tumor can be confirmed microscopically, the criteria for pathologic classification and staging have been satisfied without total removal of the primary cancer. TNM Descriptors For identification of special cases of TNM or ptnm classifications, the m suffix and y, r, and a prefixes are used. Although they do not affect the stage grouping, they indicate cases needing separate analysis. The m suffix indicates the presence of multiple primary tumors in a single site and is recorded in parentheses: pt(m)nm. The y prefix indicates those cases in which classification is performed during or following initial multimodality therapy (ie, neoadjuvant chemotherapy, radiation therapy, or both chemotherapy and radiation therapy). The ctnm or ptnm category is identified by a y prefix. The yctnm or yptnm categorizes the extent of tumor actually present at the time of that examination. The y categorization is not an estimate of tumor prior to multimodality therapy (ie, before initiation of neoadjuvant therapy). The r prefix indicates a recurrent tumor when staged after a documented disease-free interval, and is identified by the r prefix: rtnm. The a prefix designates the stage determined at autopsy: atnm. Additional Descriptors Residual Tumor (R) Tumor remaining in a patient after therapy with curative intent (eg, surgical resection for cure) is categorized by a system known as R classification, shown below. RX R0 R1 R2 Presence of residual tumor cannot be assessed No residual tumor Microscopic residual tumor Macroscopic residual tumor For the surgeon, the R classification may be useful to indicate the known or assumed status of the completeness of a surgical excision. For the pathologist, the R classification is relevant to the status of the margins of a surgical resection specimen. That is, tumor involving the resection margin on pathologic examination may be assumed to correspond to residual tumor in the patient and may be classified as macroscopic or microscopic according to the findings at the specimen margin(s). Modified Royal Marsden Staging System Stage I Tumor confined to the testis Stage II Infradiaphragmatic nodal involvement IIA greatest dimension of involved nodes less than 2 cm IIB greatest dimension of involved nodes 2 cm or more but less than 5 cm IIC greatest dimension of involved nodes 5 cm or more but less than 10 cm IID greatest dimension of involved nodes 10 cm or more Stage III Supraclavicular or mediastinal involvement Stage IV Extranodal metastases 13
14 Background Documentation G. Serum Markers The protocol emphasizes the importance of relevant clinical information in the pathologic evaluation of specimens. Serum marker studies play a key role in the clinical management of patients with testicular germ cell tumors The occurrence of elevated serum levels of alpha-fetoprotein (AFP) or the beta subunit of human chorionic gonadotropin (b-hcg) may indicate the need for additional sections of certain specimens if the initial findings do not account for such elevations. Information regarding preorchiectomy serum marker status (lactate dehydrogenase [LDH], AFP, and b-hcg) is also important in the S categorization of the tumor for stage groupings. Postorchiectomy serum markers are important for the assignment of stage IS only. Anatomic Stage/Prognostic Groups Group T N M S Stage 0 ptis N0 M0 S0 Stage I pt1-4 N0 M0 SX Stage IA pt1 N0 M0 S0 Stage IB pt2 N0 M0 S0 pt3 N0 M0 S0 pt4 N0 M0 S0 Stage IS Any pt/tx N0 M0 S1-3 (measured post orchiectomy) Stage II Any pt/tx N1,N2,N3 M0 SX Stage IIA Any pt/tx N1 M0 S0 Any pt/tx N1 M0 S1 Stage IIB Any pt/tx N2 M0 S0 Any pt/tx N2 M0 S1 Stage IIC Any pt/tx N3 M0 S0 Any pt/tx N3 M0 S1 Stage III Any pt/tx Any N M1 SX Stage IIIA Any pt/tx Any N M1a S0 Any pt/tx Any N M1a S1 Stage IIIB Any pt/tx N1,N2,N3 M0 S2 Any pt/tx Any N M1a S2 Stage IIIC Any pt/tx N1,N2,N3 M0 S3 Any pt/tx Any N M1a S3 Any T Any N M1b Any S Prognostic Factors Serum Tumor Markers (S) SX Serum marker studies not available or performed S0 Serum marker study levels within normal limits LDH HCG (miu/ml) AFP (ng/ml) S1 <1.5 X N # and <5,000 and <1,000 S X N or 5,000-50,000 or 1,000-10,000 S3 >10 X N or >50,000 or >10,000 # N indicates the upper limit of normal for the LDH assay. The serum tumor markers (S) category comprises the following: Alpha fetoprotein (AFP) half-life 5 to 7 days Human chorionic gonadotropin (hcg) half-life 1 to 3 days Lactate dehydrogenase (LDH) H. Additional Pathologic Findings Important findings include Leydig cell hyperplasia, which may be correlated with b-hcg elevation; scarring, the presence of hemosiderin-laden macrophages, and coarse intratubular calcifications in expanded tubular profiles (distinct from microlithiasis), which may indicate regression of a tumor; testicular atrophy; sertoli cell nodules 14
15 Background Documentation (Pick s adenoma), which most often are associated with undescended testes, and abnormal testicular development (eg, dysgenesis or androgen-insensitivity syndrome). 32,33 I. Metastatic Tumor Often the most important distinction in patients with metastatic testicular germ cell tumor following initial chemotherapy is the differentiation of metastatic residual teratoma from nonteratomatous types of germ cell tumor. Pure teratomatous metastasis is generally treated by surgical excision alone, whereas patients who have other residual germ cell tumor components are usually treated with additional chemotherapy. References 1. Lawrence WD, Young RH, Scully RE. Sex cord-stromal tumors. In: Talerman A, Roth LM, eds. Pathology of the Testis and Its Adnexa. New York, NY: Churchill Livingstone; 1986: Proppe KH, Scully RE. Large-cell calcifying Sertoli cell tumor of the testis. Am J Clin Pathol. 1980;74: Young RH, Talerman A. Testicular tumors other than germ cell tumors. Semin Diagn Pathol. 1987;4: Kim I, Young RH, Scully RE. Leydig cell tumors of the testis: a clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol. 1985;9: Mostofi FK, Price EBJ. Tumors of the Male Genital System: Atlas of Tumor Pathology. 2 nd series. Fascicle 8. Washington DC: Armed Forces Institute of Pathology; Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press; Mostofi FK, Spaander P, Grigor K, Parkinson CM, Skakkebaek NE, Oliver RT. Consensus on pathological classifications of testicular tumours. Prog Clin Biol Res. 1990;357: Young RH, Scully RE. Testicular Tumors. Chicago, IL: ASCP Press; Ulbright TM. Testicular and paratesticular tumors. In: Mills SE, ed. Sternberg s Diagnostic Surgical Pathology. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004: Ulbright TM, Amin MB, Young RH. Tumors of the Testis, Adnexa, Spermatic Cord, and Scrotum. Third Series. Fascicle 25. Washington, DC: Armed Forces Institute of Pathology; Ro JY, Dexeus FH, El-Naggar A, Ayala AG. Testicular germ cell tumors: clinically relevant pathologic findings. Pathol Annu. 1991;26: Ferry JA, Harris NL, Young RH, Coen J, Zietman A, Scully RE. Malignant lymphoma of the testis, epididymis, and spermatic cord: a clinicopathologic study of 69 cases with immunophenotypic analysis. Am J Surg Pathol. 1994;18: Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO Classification of Tumours of the Urinary System and Male Genital Organs. Geneva, Switzerland: WHO Press; Balzer BL, Ulbright TM. Spontaneous regression of testicular germ cell tumors: an analysis of 42 cases. Am J Surg Pathol. 2006;30(7): Warde P, Specht L, Horwich A, Oliver T, Panzarella T, Gospodarowicz M, von der Maase H. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20: Dry SM, Renshaw AA. Extratesticular extension of germ cell tumors preferentially occurs at the hilum. Am J Clin Pathol. 1999;111: Yilmaz A, Cheng T, Zhang J, Trpkov K. Testicular hilum and vascular invasion predict advanced clinical stage in nonseminomatous germ cell tumors. Mod Pathol. 2013;26(4): Jacobsen GK, Rorth M, Osterlind K, et al. Histopathological features in stage I non-seminomatous testicular germ cell tumours correlated to relapse: Danish Testicular Cancer Study Group. APMIS. 1990;98: Marks LB, Rutgers JL, Shipley WU, et al. Testicular seminoma: clinical and pathological features that may predict para-aortic lymph node metastasis. J Urol. 1990;143: Hoeltl W, Pont J, Kosak D, Honetz N, Marberger M. Treatment decision for stage I non-seminomatous germ cell tumours based on the risk factor vascular invasion. Br J Urol. 1992;69: Sesterhenn IA, Weiss RB, Mostofi FK, et al. Prognosis and other clinical correlates of pathologic review in stage I and II testicular carcinoma: a report from the Testicular Cancer Intergroup Study. J Clin Oncol. 1992;10: Horwich A, Alsanjari N, A Hern R, Nicholls J, Dearnaley DP, Fisher C. Surveillance following orchidectomy for stage I testicular seminoma. Br J Cancer. 1992;65:
16 Background Documentation 23. Sturgeon JF, Jewett MA, Alison RE, et al. Surveillance after orchidectomy for patients with clinical stage I nonseminomatous testis tumors. J Clin Oncol. 1992;10: Moul JW, McCarthy WF, Fernandez EB, Sesterhenn IA. Percentage of embryonal carcinoma and of vascular invasion predicts pathological stage in clinical stage I nonseminomatous testicular cancer. Cancer Res. 1994;54: Edge SB, Byrd DR, Carducci MA, Compton CC, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; Thomas G, Jones W, VanOosterom A, Kawai T. Consensus statement on the investigation and management of testicular seminoma Prog Clin Biol Res. 1990;357: von der Maase H, Specht L, Jacobsen GK, et al. Surveillance following orchidectomy for stage I seminoma of the testis. Eur J Cancer. 1993;29A: Chisolm GG. Tumour markers in testicular tumours. Prog Clin Biol Res. 1985;203: Javadpour N. Tumor markers in testicular cancer: an update. Prog Clin Biol Res. 1985;203: Aass N, Klepp O, Cavallin-Stahl E, et al. Prognostic factors in unselected patients with nonseminomatous metastatic testicular cancer: a multicenter experience. J Clin Oncol. 1991;9: Rutgers JL, Scully RE. Pathology of the testis in intersex syndromes. Semin Diagn Pathol. 1987;4: Wallace TM, Levin HS. Mixed gonadal dysgenesis: a review of 15 patients reporting single cases of malignant intratubular germ cell neoplasia of the testis, endometrial adenocarcinoma, and a complex vascular anomaly. Arch Pathol Lab Med. 1990;114:
Protocol for the Examination of Lymphadenectomy Specimens From Patients With Malignant Germ Cell and Sex Cord-Stromal Tumors of the Testis
 Protocol for the Examination of Specimens From Patients With Malignant Germ Cell and Sex Cord-Stromal Tumors of the Testis Version: Testis 4.0.1.1 Protocol Posting Date: February 2019 Accreditation Requirements
Protocol for the Examination of Specimens From Patients With Malignant Germ Cell and Sex Cord-Stromal Tumors of the Testis Version: Testis 4.0.1.1 Protocol Posting Date: February 2019 Accreditation Requirements
Testis. Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies.
 Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
Testis. Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies.
 Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2004 Based on AJCC/UICC
Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2004 Based on AJCC/UICC
Testis. Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies.
 Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
This protocol is intended to assist pathologists in providing
 Protocol for the Examination of Specimens From Patients With Malignant Germ Cell and Sex Cord Stromal Tumors of the Testis, Exclusive of Paratesticular Malignancies A Basis for Checklists Thomas M. Ulbright,
Protocol for the Examination of Specimens From Patients With Malignant Germ Cell and Sex Cord Stromal Tumors of the Testis, Exclusive of Paratesticular Malignancies A Basis for Checklists Thomas M. Ulbright,
Testicular Malignancies /8/15
 Collecting Cancer Data: Testis 2014-2015 NAACCR Webinar Series January 8, 2015 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
Collecting Cancer Data: Testis 2014-2015 NAACCR Webinar Series January 8, 2015 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
*OPERATIVE PROCEDURE. Serum tumour markers within normal limits S1.04 PRINCIPAL CLINICIAN
 Neoplasia of the Testis - Orchidectomy Histopathology Reporting Proforma Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Indigenous
Neoplasia of the Testis - Orchidectomy Histopathology Reporting Proforma Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Indigenous
GUIDELINES ON TESTICULAR CANCER
 38 (Text updated March 2005) P. Albers (chairman), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, A. Horwich, O. Klepp, M.P. Laguna, G. Pizzocaro Introduction Compared with other types of cancer
38 (Text updated March 2005) P. Albers (chairman), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, A. Horwich, O. Klepp, M.P. Laguna, G. Pizzocaro Introduction Compared with other types of cancer
Note: The cause of testicular neoplasms remains unknown
 - In the 15- to 34-year-old age group, they are the most common tumors of men. - Tumors of the testis are a heterogeneous group of neoplasms that include: I. Germ cell tumors : 95%; all are malignant.
- In the 15- to 34-year-old age group, they are the most common tumors of men. - Tumors of the testis are a heterogeneous group of neoplasms that include: I. Germ cell tumors : 95%; all are malignant.
Quiz 1. Assign Race 1, Race 2 and Spanish Hispanic Origin to the following scenarios.
 Quiz 1 Assign Race 1, Race 2 and Spanish Hispanic Origin to the following scenarios. 1. 62 year old Brazilian female Race 1 Race 2 Spanish/Hispanic Origin 2. 43 year old Asian male born in Japan Race 1
Quiz 1 Assign Race 1, Race 2 and Spanish Hispanic Origin to the following scenarios. 1. 62 year old Brazilian female Race 1 Race 2 Spanish/Hispanic Origin 2. 43 year old Asian male born in Japan Race 1
-The cause of testicular neoplasms remains unknown
 - In the 15- to 34-year-old age group, they are the most common tumors of men. - include: I. Germ cell tumors : (95%); all are malignant. II. Sex cord-stromal tumors: from Sertoli or Leydig cells; usually
- In the 15- to 34-year-old age group, they are the most common tumors of men. - include: I. Germ cell tumors : (95%); all are malignant. II. Sex cord-stromal tumors: from Sertoli or Leydig cells; usually
Male Genital Cancers in the US in Frequency of Types
 Germ Cell Tumors of the Testis Pathology, Immunohistochemistry, and the Often Confusing Appearance of Their Metastases Charles Zaloudek, MD Department of Pathology UCSF Male Genital Cancers in the US in
Germ Cell Tumors of the Testis Pathology, Immunohistochemistry, and the Often Confusing Appearance of Their Metastases Charles Zaloudek, MD Department of Pathology UCSF Male Genital Cancers in the US in
Extratesticular Extension of Germ Cell Tumors Preferentially Occurs at the Hilum
 Anatomic Pathology / EXTRATESTICULAR EXTENSION OF GERM CELL TUMORS Extratesticular Extension of Germ Cell Tumors Preferentially Occurs at the Hilum Sarah M. Dry, MD, and Andrew A. Renshaw, MD Key Words:
Anatomic Pathology / EXTRATESTICULAR EXTENSION OF GERM CELL TUMORS Extratesticular Extension of Germ Cell Tumors Preferentially Occurs at the Hilum Sarah M. Dry, MD, and Andrew A. Renshaw, MD Key Words:
Testicular germ cell tumors
 Testicular germ cell tumors Introduction Most common solid tumor in young adult men with 3 6 new cases/100,000 men/year. They acc ount for 1.5% of male malignancies and 5% of urological tumors. Bilateral
Testicular germ cell tumors Introduction Most common solid tumor in young adult men with 3 6 new cases/100,000 men/year. They acc ount for 1.5% of male malignancies and 5% of urological tumors. Bilateral
EAU GUIDELINES ON TESTICULAR CANCER
 EAU GUIDELINES ON TESTICULAR CANCER (Limited text update March 2018) P. Albers (Chair), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi, A. Horwich, M.P. Laguna (Vice-chair), N. Nicolai,
EAU GUIDELINES ON TESTICULAR CANCER (Limited text update March 2018) P. Albers (Chair), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi, A. Horwich, M.P. Laguna (Vice-chair), N. Nicolai,
EAU GUIDELINES ON TESTICULAR CANCER
 EU GUIDELINES ON TESTICULR CNCER (Limited text update March 2017) P. lbers (Chair), W. lbrecht, F. lgaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi,. Horwich, M.P. Laguna, N. Nicolai, J. Oldenburg Introduction
EU GUIDELINES ON TESTICULR CNCER (Limited text update March 2017) P. lbers (Chair), W. lbrecht, F. lgaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi,. Horwich, M.P. Laguna, N. Nicolai, J. Oldenburg Introduction
Case Scenario 1 Discharge Summary Pathology Report Final Diagnosis: Oncology Consult
 Case Scenario 1 Discharge Summary A 31-year-old Brazilian male presented with a 6 month history of right-sided scrotal swelling. Backache was present for 2 months and a history of right epididymitis was
Case Scenario 1 Discharge Summary A 31-year-old Brazilian male presented with a 6 month history of right-sided scrotal swelling. Backache was present for 2 months and a history of right epididymitis was
Case Scenario 1 Discharge Summary Pathology Report Final Diagnosis: Oncology Consult
 Case Scenario 1 Discharge Summary A 31-year-old Brazilian male presented with a 6 month history of right-sided scrotal swelling. Backache was present for 2 months and a history of right epididymitis was
Case Scenario 1 Discharge Summary A 31-year-old Brazilian male presented with a 6 month history of right-sided scrotal swelling. Backache was present for 2 months and a history of right epididymitis was
STAGE CATEGORY DEFINITIONS
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
EAU GUIDELINES ON TESTICULAR CANCER
 EAU GUIDELINES ON TESTICULAR CANCER (Limited text update March 2015) P. Albers (Chair), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi, A. Horwich, M.P. Laguna, N. Nicolai, J. Oldenburg
EAU GUIDELINES ON TESTICULAR CANCER (Limited text update March 2015) P. Albers (Chair), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi, A. Horwich, M.P. Laguna, N. Nicolai, J. Oldenburg
Collecting Cancer Data: Testis 2/3/11. Collecting Cancer Data: NAACCR Webinar Series 1. Agenda. Fabulous Prizes
 Collecting Cancer Data: Testis February 3, 2011 NAACCR 2010-2011 Webinar Series Agenda Coding moment Race/Hispanic origin Overview Collaborative Stage Treatment Exercises Fabulous Prizes NAACCR 2010-2011
Collecting Cancer Data: Testis February 3, 2011 NAACCR 2010-2011 Webinar Series Agenda Coding moment Race/Hispanic origin Overview Collaborative Stage Treatment Exercises Fabulous Prizes NAACCR 2010-2011
Exercise. Discharge Summary
 Exercise Discharge Summary A 32-year-old Brazilian male presented with a 6 month history of right-sided scrotal swelling. Backache was present for 2 months and a history of right epididymitis was present
Exercise Discharge Summary A 32-year-old Brazilian male presented with a 6 month history of right-sided scrotal swelling. Backache was present for 2 months and a history of right epididymitis was present
Descriptor Definition Author s notes TNM descriptors Required only if applicable; select all that apply multiple foci of invasive carcinoma
 S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
Testicular Tumors: What s New, True, Important Cristina Magi-Galluzzi, MD, PhD
 Testicular Tumors: What s New, True, Important Cristina Magi-Galluzzi, MD, PhD Director, Genitourinary Pathology R.J. Tomsich Pathology & Laboratory Medicine Institute Professor of Pathology, Lerner College
Testicular Tumors: What s New, True, Important Cristina Magi-Galluzzi, MD, PhD Director, Genitourinary Pathology R.J. Tomsich Pathology & Laboratory Medicine Institute Professor of Pathology, Lerner College
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
Protocol for the Examination of Specimens From Patients With Carcinoma of the Urethra and Periurethral Glands
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Urethra and Periurethral Glands Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition,
Protocol for the Examination of Specimens From Patients With Carcinoma of the Urethra and Periurethral Glands Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition,
Definition of Synoptic Reporting
 Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
STAGING AND FOLLOW-UP STRATEGIES
 ATHENS 4-6 October 2018 European Society of Urogenital Radiology STAGING AND FOLLOW-UP STRATEGIES Ahmet Tuncay Turgut, MD Professor of Radiology Hacettepe University, Faculty of Medicine Ankara 2nd ESUR
ATHENS 4-6 October 2018 European Society of Urogenital Radiology STAGING AND FOLLOW-UP STRATEGIES Ahmet Tuncay Turgut, MD Professor of Radiology Hacettepe University, Faculty of Medicine Ankara 2nd ESUR
Cardiff MRCS OSCE Courses Testicular Cancer
 Testicular Cancer Scenario: A 40-year-old male presents to the surgical out-patient clinic with a 6-8 week history of a painless lump in his left scrotum. He however complains of a dull ache in the scrotum
Testicular Cancer Scenario: A 40-year-old male presents to the surgical out-patient clinic with a 6-8 week history of a painless lump in his left scrotum. He however complains of a dull ache in the scrotum
Protocol for the Examination of Cystectomy Specimens From Patients With Carcinoma of the Urinary Bladder
 Protocol for the Examination of Cystectomy Specimens From Patients With Carcinoma of the Urinary Bladder Version: UrinaryBladder 4.0.1.1 Protocol Posting Date: February 2019 CAP Laboratory Accreditation
Protocol for the Examination of Cystectomy Specimens From Patients With Carcinoma of the Urinary Bladder Version: UrinaryBladder 4.0.1.1 Protocol Posting Date: February 2019 CAP Laboratory Accreditation
Tinh hoàn
 Tinh hoàn Tinh hoàn Tinh hoàn Tiền liệt tuyến Tiền liệt tuyến Mào tinh hoàn Mào tinh hoàn Túi tinh Túi tinh Túi tinh Túi tinh So-called cystadenoma of seminal vesicle. Gross appearance of granulomatous
Tinh hoàn Tinh hoàn Tinh hoàn Tiền liệt tuyến Tiền liệt tuyến Mào tinh hoàn Mào tinh hoàn Túi tinh Túi tinh Túi tinh Túi tinh So-called cystadenoma of seminal vesicle. Gross appearance of granulomatous
Prognostic factors of genitourinary tumors: Do we have to care?
 Prognostic factors of genitourinary tumors: Do we have to care? Jae Y. Ro, MD, PhD Professor and Director of Surgical Pathology The Methodist Hospital, Weill Medical College of Cornell University, Houston,
Prognostic factors of genitourinary tumors: Do we have to care? Jae Y. Ro, MD, PhD Professor and Director of Surgical Pathology The Methodist Hospital, Weill Medical College of Cornell University, Houston,
Original Articles. The Significance of Lymphovascular Invasion of the Spermatic Cord in the Absence of Cord Soft Tissue Invasion
 Original Articles The Significance of Lymphovascular Invasion of the Spermatic Cord in the Absence of Cord Soft Tissue Invasion Brandi C. McCleskey, MD; Jonathan I. Epstein, MD; Constantine Albany, MD;
Original Articles The Significance of Lymphovascular Invasion of the Spermatic Cord in the Absence of Cord Soft Tissue Invasion Brandi C. McCleskey, MD; Jonathan I. Epstein, MD; Constantine Albany, MD;
47. Melanoma of the Skin
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Uterine Cervix. Protocol applies to all invasive carcinomas of the cervix.
 Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Protocol for the Examination of Specimens From Patients With Thymic Tumors
 Protocol for the Examination of Specimens From Patients With Thymic Tumors Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Protocol for the Examination of Specimens From Patients With Thymic Tumors Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Teratocarcinoma In A Young Boy- An Unusual Presentation
 Human Journals Case Report November 2015 Vol.:2, Issue:1 All rights are reserved by Atia Zaka-ur-Rab et al. Teratocarcinoma In A Young Boy- An Unusual Presentation Keywords: Boy, Testicular Mass, Teratocarcinoma
Human Journals Case Report November 2015 Vol.:2, Issue:1 All rights are reserved by Atia Zaka-ur-Rab et al. Teratocarcinoma In A Young Boy- An Unusual Presentation Keywords: Boy, Testicular Mass, Teratocarcinoma
Procedures Needle Biopsy Transurethral Prostatic Resection Suprapubic or Retropubic Enucleation (Subtotal Prostatectomy) Radical Prostatectomy
 Prostate Gland Protocol applies to invasive carcinomas of the prostate gland. Protocol web posting date: July 2006 Protocol effective date: April 2007 Based on AJCC/UICC TNM, 6 th edition Procedures Needle
Prostate Gland Protocol applies to invasive carcinomas of the prostate gland. Protocol web posting date: July 2006 Protocol effective date: April 2007 Based on AJCC/UICC TNM, 6 th edition Procedures Needle
Testicular Germ Cell Tumors; A Simplistic Approach
 Testicular Germ Cell Tumors; A Simplistic Approach Merce Jorda, MD, PhD, MBA Professor and Vice Chair, Director of Anatomic Pathology Director of Genitourinary Pathology Service Interim Director of Cytopathology
Testicular Germ Cell Tumors; A Simplistic Approach Merce Jorda, MD, PhD, MBA Professor and Vice Chair, Director of Anatomic Pathology Director of Genitourinary Pathology Service Interim Director of Cytopathology
TESTICULAR CANCER Updated March 2016 by Dr. Safiya Karim (PGY-5 Medical Oncology Resident, University of Toronto)
 TESTICULAR CANCER Updated March 2016 by Dr. Safiya Karim (PGY-5 Medical Oncology Resident, University of Toronto) Reviewed by Dr. Aaron Hansen (Staff Medical Oncologist, University of Toronto) DISCLAIMER:
TESTICULAR CANCER Updated March 2016 by Dr. Safiya Karim (PGY-5 Medical Oncology Resident, University of Toronto) Reviewed by Dr. Aaron Hansen (Staff Medical Oncologist, University of Toronto) DISCLAIMER:
Testicular Tumors Including Secondary and Unusual Tumors of the Testis
 Testicular Tumors Including Secondary and Unusual Tumors of the Testis Milton W. Datta Partner, Hospital Pathology Associates University of Minnesota Minneapolis, MN Topics Review Features of Germ Cell
Testicular Tumors Including Secondary and Unusual Tumors of the Testis Milton W. Datta Partner, Hospital Pathology Associates University of Minnesota Minneapolis, MN Topics Review Features of Germ Cell
Patient identifiers Date of request Accession/Laboratory number
 Neoplasia of the Testis - Retroperitoneal Lymphadenectomy Histopathology Reporting Guide Family/Last name Given name(s) Date of birth Patient identifiers Date of request Accession/Laboratory number DD
Neoplasia of the Testis - Retroperitoneal Lymphadenectomy Histopathology Reporting Guide Family/Last name Given name(s) Date of birth Patient identifiers Date of request Accession/Laboratory number DD
Small Intestine. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition
 Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Protocol for the Examination of Specimens From Patients With Carcinoma of the Prostate Gland
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Prostate Gland Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual
Protocol for the Examination of Specimens From Patients With Carcinoma of the Prostate Gland Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual
Kidney. Protocol applies to all invasive carcinomas of renal tubular origin. It excludes Wilms tumors and tumors of urothelial origin.
 Kidney Protocol applies to all invasive carcinomas of renal tubular origin. It excludes Wilms tumors and tumors of urothelial origin. Procedures Incisional Biopsy (Needle or Wedge) Partial Nephrectomy
Kidney Protocol applies to all invasive carcinomas of renal tubular origin. It excludes Wilms tumors and tumors of urothelial origin. Procedures Incisional Biopsy (Needle or Wedge) Partial Nephrectomy
6. Cervical Lymph Nodes and Unknown Primary Tumors of the Head and Neck
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Disclosure of Relevant Financial Relationships
 Evening Specialty Conference - Genitourinary Pathology Case 2 Disclosure of Relevant Financial Relationships Sean R Williamson, MD Henry Ford Health System, Detroit, MI @Williamson_SR USCAP requires that
Evening Specialty Conference - Genitourinary Pathology Case 2 Disclosure of Relevant Financial Relationships Sean R Williamson, MD Henry Ford Health System, Detroit, MI @Williamson_SR USCAP requires that
Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin
 Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual
Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual
Protocol applies to melanoma of cutaneous surfaces only.
 Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
Interactive Staging Bee
 Interactive Staging Bee ROBIN BILLET, MA, CTR GA/SC REGIONAL CONFERENCE NOVEMBER 6, 2018? Clinical Staging includes any information obtained about the extent of cancer obtained before initiation of treatment
Interactive Staging Bee ROBIN BILLET, MA, CTR GA/SC REGIONAL CONFERENCE NOVEMBER 6, 2018? Clinical Staging includes any information obtained about the extent of cancer obtained before initiation of treatment
GUIDELINES ON TESTICULAR CANCER
 European Association of Urology GUIDELINES ON TESTICULAR CANCER P. Albers (chairman), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, A. Horwich, O. Klepp, M.P. Laguna, G. Pizzocaro UPDATE MARCH
European Association of Urology GUIDELINES ON TESTICULAR CANCER P. Albers (chairman), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, A. Horwich, O. Klepp, M.P. Laguna, G. Pizzocaro UPDATE MARCH
Thyroid Gland. Protocol applies to all malignant tumors of the thyroid gland, except lymphomas.
 Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas.
 Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist)
Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist)
Urinary Bladder, Ureter, and Renal Pelvis
 Urinary Bladder, Ureter, and Renal Pelvis Protocol applies to all carcinomas of the urinary bladder, ureter, and renal pelvis. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition Procedures
Urinary Bladder, Ureter, and Renal Pelvis Protocol applies to all carcinomas of the urinary bladder, ureter, and renal pelvis. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition Procedures
Chapter 2 Staging of Breast Cancer
 Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Guidelines on Testicular Cancer
 Guidelines on Testicular Cancer P. Albers (chairman), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi, A. Horwich, M.P. Laguna European Association of Urology 2009 TABLE OF CONTENTS
Guidelines on Testicular Cancer P. Albers (chairman), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi, A. Horwich, M.P. Laguna European Association of Urology 2009 TABLE OF CONTENTS
B REAST STAGING FORM. PATHOLOGIC Extent of disease through completion of definitive surgery. CLINICAL Extent of disease before any treatment
 B REAST STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi c a b c d TUMOR SIZE:
B REAST STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi c a b c d TUMOR SIZE:
B REAST STAGING FORM. PATHOLOGIC Extent of disease through completion of definitive surgery. CLINICAL Extent of disease before any treatment
 B REAST STAGING FORM Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi a b c a b c d TUMOR SIZE: S TAGE
B REAST STAGING FORM Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi a b c a b c d TUMOR SIZE: S TAGE
Staging for Residents, Nurses, and Multidisciplinary Health Care Team
 Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
Neoplasia of the Testis - Orchidectomy Histopathology Reporting Guide
 Neoplasia of the Testis - Orchidectomy Histopathology Reporting Guide Family/Last name Given name(s) Date of birth Patient identifiers Date of request Accession/Laboratory number DD MM YYYY Elements in
Neoplasia of the Testis - Orchidectomy Histopathology Reporting Guide Family/Last name Given name(s) Date of birth Patient identifiers Date of request Accession/Laboratory number DD MM YYYY Elements in
Transitional Cell Papilloma 2-3% Inverted Papilloma Rare
 BLADDER TUMORS Benign Transitional Cell Papilloma 2-3% Inverted Papilloma Rare Malignant Transitional (Urothelial) Carcinoma90% Carcinoma In-Situ (By Itself) 5-10% Squamous Cell Carcinoma 3-7% Adenocarcinoma
BLADDER TUMORS Benign Transitional Cell Papilloma 2-3% Inverted Papilloma Rare Malignant Transitional (Urothelial) Carcinoma90% Carcinoma In-Situ (By Itself) 5-10% Squamous Cell Carcinoma 3-7% Adenocarcinoma
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
Gastric Cancer Histopathology Reporting Proforma
 Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy
 Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC
Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC
Protocol for the Examination of Specimens From Patients With Carcinoma of the Perihilar Bile Ducts
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Perihilar Bile Ducts Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging
Protocol for the Examination of Specimens From Patients With Carcinoma of the Perihilar Bile Ducts Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging
L ARYNX S TAGING F ORM
 CLI N I CA L Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 Tis a b L ARYNX S TAGING F ORM LATERALITY: TUMOR SIZE: left
CLI N I CA L Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 Tis a b L ARYNX S TAGING F ORM LATERALITY: TUMOR SIZE: left
M ALIGNANT MELANOMA OF THE UVEA STAGING FORM
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 a a a d b c d a TUMOR SIZE: S TAGE C ATEGORY D EFINITIONS LATERALITY:
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 a a a d b c d a TUMOR SIZE: S TAGE C ATEGORY D EFINITIONS LATERALITY:
Carcinoma of the Renal Pelvis and Ureter Histopathology
 Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Regressed Testicular Seminoma with Extensive Metastases. S Andhavarapu, B Low, J Raj, S Skinner, J Armenta-Corona
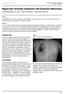 ISPUB.COM The Internet Journal of Oncology Volume 5 Number 1 S Andhavarapu, B Low, J Raj, S Skinner, J Armenta-Corona Citation S Andhavarapu, B Low, J Raj, S Skinner, J Armenta-Corona.. The Internet Journal
ISPUB.COM The Internet Journal of Oncology Volume 5 Number 1 S Andhavarapu, B Low, J Raj, S Skinner, J Armenta-Corona Citation S Andhavarapu, B Low, J Raj, S Skinner, J Armenta-Corona.. The Internet Journal
Testicular tumours: What s new and what s
 USCAP Vancouver 2012 Testicular tumours: What s new and what s relevant? Dan Berney Introduction The rarity and complexity of testicular tumours results in unique challenges for pathologists. The difficulty
USCAP Vancouver 2012 Testicular tumours: What s new and what s relevant? Dan Berney Introduction The rarity and complexity of testicular tumours results in unique challenges for pathologists. The difficulty
Carcinoma of the Urinary Bladder Histopathology
 Carcinoma of the Urinary Bladder Histopathology Reporting Proforma (Radical & Partial Cystectomy, Cystoprostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Carcinoma of the Urinary Bladder Histopathology Reporting Proforma (Radical & Partial Cystectomy, Cystoprostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Protocol for the Examination of Specimens From Patients With Carcinoma of the Urinary Bladder
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Urinary Bladder Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual
Protocol for the Examination of Specimens From Patients With Carcinoma of the Urinary Bladder Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual
EAU Guidelines on Testicular Cancer
 EAU Guidelines on Testicular Cancer P. Albers (Chair), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi, A. Horwich, M.P. Laguna, N. Nicolai, J. Oldenburg Guidelines Associates: J.L.
EAU Guidelines on Testicular Cancer P. Albers (Chair), W. Albrecht, F. Algaba, C. Bokemeyer, G. Cohn-Cedermark, K. Fizazi, A. Horwich, M.P. Laguna, N. Nicolai, J. Oldenburg Guidelines Associates: J.L.
Ultrasound of malignant testicular lesions. Arne Hørlyck Department of Radiology Aarhus University Hospital, Skejby
 Ultrasound of malignant testicular lesions Arne Hørlyck Department of Radiology Aarhus University Hospital, Skejby Testis Ultrasound is fantastic!! Scrotum Extratesticular mass: Benign Intratesticular
Ultrasound of malignant testicular lesions Arne Hørlyck Department of Radiology Aarhus University Hospital, Skejby Testis Ultrasound is fantastic!! Scrotum Extratesticular mass: Benign Intratesticular
THE TESTICULAR Cancer Intergroup Study (TCIS)
 Prognosis and Other Clinical Correlates of Pathologic Review in Stage I and II Testicular Carcinoma: A Report From the Testicular Cancer Intergroup Study By Isabell A. Sesterhenn, Raymond B. Weiss, F.
Prognosis and Other Clinical Correlates of Pathologic Review in Stage I and II Testicular Carcinoma: A Report From the Testicular Cancer Intergroup Study By Isabell A. Sesterhenn, Raymond B. Weiss, F.
FINALIZED SEER SINQ QUESTIONS
 0076 Source 1: WHO Class CNS Tumors pgs: 33 MP/H Rules/Histology--Brain and CNS: What is the histology code for a tumor originating in the cerebellum and extending into the fourth ventricle described as
0076 Source 1: WHO Class CNS Tumors pgs: 33 MP/H Rules/Histology--Brain and CNS: What is the histology code for a tumor originating in the cerebellum and extending into the fourth ventricle described as
This form may provide more data elements than required for collection by standard setters such as NCI SEER, CDC NPCR, and CoC NCDB.
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
H&E, IHC anti- Cytokeratin
 Cat No: OVC2281 - Ovary cancer tissue array Lot# Cores Size Cut Format QA/QC OVC228101 228 1.1mm 4um 12X19 H&E, IHC anti- Cytokeratin Recommended applications: For Research use only. RNA or protein ovary
Cat No: OVC2281 - Ovary cancer tissue array Lot# Cores Size Cut Format QA/QC OVC228101 228 1.1mm 4um 12X19 H&E, IHC anti- Cytokeratin Recommended applications: For Research use only. RNA or protein ovary
Prof. Dr. med. Beata BODE-LESNIEWSKA Institute of Pathology and Molecular Pathology University Hospital; Zurich
 Prof. Dr. med. Beata BODE-LESNIEWSKA Institute of Pathology and Molecular Pathology University Hospital; Zurich 32 year old man 2 months history of growing left supraclavicular lymph nodes Antibiotic treatment
Prof. Dr. med. Beata BODE-LESNIEWSKA Institute of Pathology and Molecular Pathology University Hospital; Zurich 32 year old man 2 months history of growing left supraclavicular lymph nodes Antibiotic treatment
Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013
 bs_bs_banner doi:10.1111/jog.12360 J. Obstet. Gynaecol. Res. Vol. 40, No. 2: 338 348, February 2014 Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013 Daisuke
bs_bs_banner doi:10.1111/jog.12360 J. Obstet. Gynaecol. Res. Vol. 40, No. 2: 338 348, February 2014 Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013 Daisuke
VULVAR CARCINOMA. Page 1 of 5
 VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
10. HPV-Mediated (p16+) Oropharyngeal Cancer
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Analysis of the prognosis of patients with testicular seminoma
 ONCOLOGY LETTERS 11: 1361-1366, 2016 Analysis of the prognosis of patients with testicular seminoma WEI DONG 1, WANG GANG 1, MIAOMIAO LIU 2 and HONGZHEN ZHANG 2 1 Department of Urology; 2 Department of
ONCOLOGY LETTERS 11: 1361-1366, 2016 Analysis of the prognosis of patients with testicular seminoma WEI DONG 1, WANG GANG 1, MIAOMIAO LIU 2 and HONGZHEN ZHANG 2 1 Department of Urology; 2 Department of
Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do?
 Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
46. Merkel Cell Carcinoma
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Bilateral Testicular Germ Cell Tumors
 1228 Bilateral Testicular Germ Cell Tumors Twenty-Year Experience at M. D. Anderson Cancer Center Mingxin Che, M.D., Ph.D. 1 Pheroze Tamboli, M.D. 1 Jae Y. Ro, M.D., Ph.D. 1 Dong Soo Park, M.D. 2 Jung
1228 Bilateral Testicular Germ Cell Tumors Twenty-Year Experience at M. D. Anderson Cancer Center Mingxin Che, M.D., Ph.D. 1 Pheroze Tamboli, M.D. 1 Jae Y. Ro, M.D., Ph.D. 1 Dong Soo Park, M.D. 2 Jung
Take Home Quiz 1 Please complete the quiz below prior to the session. Use the Multiple Primary and Histology Rules
 Take Home Quiz 1 Please complete the quiz below prior to the session. Use the Multiple Primary and Histology Rules Case 1 72 year old white female presents with a nodular thyroid. This was biopsied in
Take Home Quiz 1 Please complete the quiz below prior to the session. Use the Multiple Primary and Histology Rules Case 1 72 year old white female presents with a nodular thyroid. This was biopsied in
Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin
 Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin Protocol applies to Merkel cell carcinoma of cutaneous surfaces only. Based on AJCC/UICC TNM, 7th edition
Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin Protocol applies to Merkel cell carcinoma of cutaneous surfaces only. Based on AJCC/UICC TNM, 7th edition
Colon and Rectum. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition
 Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
The College of American Pathologists offers these
 CAP Laboratory Improvement Programs Protocol for the Examination of Specimens From Patients With Carcinoma of the Intrahepatic Bile Ducts Mary Kay Washington, MD, PhD; Jordan Berlin, MD; Philip A. Branton,
CAP Laboratory Improvement Programs Protocol for the Examination of Specimens From Patients With Carcinoma of the Intrahepatic Bile Ducts Mary Kay Washington, MD, PhD; Jordan Berlin, MD; Philip A. Branton,
Protocol for the Examination of Specimens From Patients With Carcinoma of the Ureter and Renal Pelvis
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Ureter and Renal Pelvis Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging
Protocol for the Examination of Specimens From Patients With Carcinoma of the Ureter and Renal Pelvis Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging
This form may provide more data elements than required for collection by standard setters such as NCI SEER, CDC NPCR, and CoC NCDB.
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology
 bs_bs_banner doi:10.1111/jog.12596 J. Obstet. Gynaecol. Res. Vol. 41, No. 2: 167 177, February 2015 Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology
bs_bs_banner doi:10.1111/jog.12596 J. Obstet. Gynaecol. Res. Vol. 41, No. 2: 167 177, February 2015 Annual report of the Committee on Gynecologic Oncology, the Japan Society of Obstetrics and Gynecology
Protocol for the Examination of Specimens from Patients with Carcinoma of the Urinary Bladder
 Protocol for the Examination of Specimens from Patients with Carcinoma of the Urinary Bladder Protocol applies primarily to invasive and noninvasive carcinomas, including carcinoma in situ. Version: Protocol
Protocol for the Examination of Specimens from Patients with Carcinoma of the Urinary Bladder Protocol applies primarily to invasive and noninvasive carcinomas, including carcinoma in situ. Version: Protocol
A RARE CASE OF FIBROUS PSEUDO TUMOR
 IJCRR Vol 06 issue 14 Section: Healthcare Category: Case Report Received on: 14/05/14 Revised on: 09/06/14 Accepted on: 10/07/14 Sudha V., Ganthimathy Sekhar, Karunakumar, Jayaganesh, Vimal Chander R.
IJCRR Vol 06 issue 14 Section: Healthcare Category: Case Report Received on: 14/05/14 Revised on: 09/06/14 Accepted on: 10/07/14 Sudha V., Ganthimathy Sekhar, Karunakumar, Jayaganesh, Vimal Chander R.
Leydig cell tumour. Testis: non-germ cell tumours. Testis: sex cord-stromal tumours. Differential diagnosis of Leydig cell tumour TTAGS
 Non-germ cell s of the testis Dr Jonathan H Shanks The Christie NHS Foundation Trust, Manchester, UK Testis: non-germ cell s Sex cord-stromal s Haemolymphoid neoplasms Other neoplasms Tumour-like conditions
Non-germ cell s of the testis Dr Jonathan H Shanks The Christie NHS Foundation Trust, Manchester, UK Testis: non-germ cell s Sex cord-stromal s Haemolymphoid neoplasms Other neoplasms Tumour-like conditions
