Effect of Gamma Radiation, XIV)
|
|
|
- Jeffery Summers
- 5 years ago
- Views:
Transcription
1 Radiolytic Effects of Gamma Rays on TitleSolution (Special Issue on Physical Effect of Gamma Radiation, XIV) Author(s) Tanabe, Reiko Citation Bulletin of the Institute for Chemi University (1973), 51(1): Issue Date URL Right Type Departmental Bulletin Paper Textversion publisher Kyoto University
2 Bull. Inst. Chem. Res., Kyoto Univ., Vol. 51, No. 1, 1973 Radiolytic Effects of Gamma Rays on a-amylase in Aqueous Solution Reiko TANABE* Received January 16, 1973 In order to investigate the effects of irradiation on the peptide bonds of this enzyme protein, search for small peptides by gel filtration, measurement of molecular weight by ultracentrifugal analysis and determination of N-terminal amino acid by the DNP method were carried out in the intact enzyme and irradiated one. From the results obtained from these experiments, it was concluded that the peptide bonds did not accept any cleavage by the irradiation. Irradiation of r-rays on a-amylase in aqueous solution was found to reduce the amount of several amino acid residues to some extent, such as lysine, histidine, arginine, tyrosine and so on. Free ammonia were liberated from the enzyme protein by the irradiation of r- rays. The degree of liberation of the free ammonia was proportional to that of inactivation and was independent of the enzyme concentration at the irradiation. It was assumed that the liberation of the ammonia was due to the destruction on the basic amino acid residues in this protein molecule. Inactivation of a-amylase by irradiation may be carried out through the specific damage of amino acid residues which consist in the active center or have great influence upon it. INTRODUCTION In the previous paperl>, it was reported that the inactivation of Bacillus subtilis a-amylase in aqueous solution by r-ray irradiation was caused by the indirect action of radiation, in other words, by the reaction between the solute, a-amylase, and the radiolyzates of the solvent water. It was also found that hydroxyl radicals were more effective to inactivation of the enzyme than other radiolyzates of water. At the begining of investigation of the mechanism of enzyme inactivation by irradiation, experiments concerning the action of radiation on the peptide bonds of enzyme protein were carried out. In order to investigate wheather or not the peptide bonds accept cleavage by irradiation, gel filtration for the search of small peptide, ultracentrifugal analysis for the measurement of molecular weight and the DNP method for the determination of N-terminal amino acid were tried and there results will be described in the present paper. However, a-amylase irradiated in aqueous solution and reduced its activity has not accept any damage in its peptide chain. In order to investigate the inactivation mechanism of a-amylase by r-ray irradiation, secondly was studied the effects of irradiation on the amino acid residues of side chain in this enzyme protein. From this point of view, the experiments treating of the change in the amino acid composition of intact and irradiated a-amylase and the * EQ 2 --f : Department of Chemistry, Faculty of Science, Kyoto University, Kyoto. ( 37 )
3 R. TANABE liberation of free ammonia by irradiation were carried out and the results will be described in this paper. MATERIALS AND METHODS Bacillus subtilis a-amylase used in this work was a crystalline preparation made by Daiwa Kasei Co. Ltd. from the culture of Bacillus subtilis DT-57 strain. To remove the contaminated impurities and stabilizer, the enzyme solution was filtrated through a column of Sephadex G-25. Concentration of the enzyme protein in the solution was estimated from the absorption at 280 mit assuming Ell= For the conversion of gravity to molarity, a molecular weight of this enzyme was assumed to be 48,500, according to the results from the ultracentrifugational analysis described here. Molecular weight of the enzyme protein was determined by the Archibald's method2> with a Hitachi Analytical Ultracentrifuge Apparatus UCA-1 type. An 0.64 per cent a-amylase solution in a 0.1 M sodium acetate-0.08 M NaCI M CaC12 buffer ph 6.9, ionic strength 0.2, was equilibrated by a dialysis versus the same buffer for 24 hrs at 5 C and then centrifuged at a speed setting of 9,690 r.p.m. at 16 C. The exposures were taken with a diagonal slit of 80, 20 min, 40 min, 60 min and 80 min after the rotor had reached full speed. Specific gravity was measured by using a Ostwald picnometer, capacity 1 ml. 1-Ray irradiation was carried out using the 2000 Ci 60Co Irradiation Facility with the dose rate of 1.2 x 105 rad/hr in the Institute for Chemical Research, Kyoto University, and the Toshiba's RE Ci 60Co Irradiation Facility3) with the dose rate of 4.63 x 104 rad/hr. Amino acid composition of the enzyme was determined by the method of Stein and Mooreo with a KLA-2 Hitachi amino acid analyzer. Standard specimens of amino acids used in this determination were of a synthetic pure product of Tanabe Pharmaceutical Chemical Co. Ltd.. In order to discriminate inconsiderable difference of the amino acid composition between non-irradiated and irradiated enzyme, the degree of hydrolysis for various times, 16, 22, 48 and 76 hours, were measured in the non irradiated enzyme and in the irradiated one respectively, according to the method of Stein and Moore. The values obtained from the amino acid analysis were collected by the data from these measurements. Quantitative analysis of tryptophan residue was made by the method of ultra violet light absorptions) separately. The species of N-terminal amino acid was determined by the Sanger's DNP methodo. The free ammonia blended in the enzyme protein was isolated and measured by the Conway's microdiffusion method7) with some improvement as follows : 1 ml of borate- NaOH buffer, ph 11.5, was added into 1-2 ml of protein solution included ammonia in a bottle for diffusion, and immediately a rubber stopper with a glass rod immersed in 5N-H2SO4 was putted on the bottle, which was rotated at 30 C for 1.5 hours. The diffused ammonia was adsorbed in the H2SO4 of the glass rod, washed out into a test tube with 3 ml of distilled water, and then was measured colorimetrically by the method of indophenol8 using ammonium sulfate as standard substance. ( 38 )
4 Radiolytic Effects of Gamma rays on a-amylase in Aqueous Solution Hydrolysis of amides in the enyzme protein was carried out through the two processes, one was an acid hydrolysis by 3N-HC1, and the other was an alkali hydrolysis by saturated NaOH. In the alkali hydrolysis, the mixture of 1 ml of 0.2 percent a- amylase solution and 1 ml of saturated NaOH in the same bottle as described in METHODS, was rotated at 32 C for 2.5 hours. The hydrolyzed and liberated ammonia was adsorbed in 5 N-H2SO4 and measured collorimetrically as described above. In the acid hydrolysis, the mixture of 1 ml of 0.3 percent of a-amylase solution and 1 ml of 6 N-HC1 and several drops of octanol was heated at 110 C for 3 hours in a test tube with a refrigerator. This hydrolyzate was removed into the diffusion bottle and after the addition of 1 ml of saturated NaOH, the ammonia in that was isolated and measured in the same manner. RESULTS Gel filtration Gel filration was applied using a 2 x 30 cm column packed with Sephadex G-75, for non irradiated and irradiated a-amylase solution. The result is shown in Fig. 1. The remaining activity ratio of the enzyme irradiated with 7.8 x 104 rad was about 50 percent. The elution patterns and the retention volumes were almost simillar respectively between non irradiated enzyme and irradiated one. This data indicates that the enzyme did not undergo a change on its molecular size by the irradiation 'S ad2 < E11( uent volume (ml) Fig. 1. Reduction of amino acid residues by irradiation. Moles of amino acid residues reduced per enzyme protein molecule was shown against the inactivation ratio by the irradiation. Dose irradiated in each points was identical with that of Table I. Measurement of molecular weight An alteration of the molecular weight of a-amylase by irradiation was measured according to the Archibald's'method. Each molecular weight shown in Table I is an average of every measurements obtained from the data in the various sedimentation equilibrium runs. It had been reported by Fischer et al.9) or Isemura et al.40,11) that two molecules of a-amylase of Bacillus subtilis with a molecular weight of about 45,000 associate as a dimer bound by a Zn2+ ion usually. According to Fischer et al., the dimer dissociates into two monomers in the presence of metal-binding agents such as calcium salt at ethylenediamine tetra-acetic acid (Ca-EDTA), hydroxyquinoline, citrate and oxalate etc. On the one hand, according to Isemura et al. the monomer- ( 39 )
5 R. TANABE Table I. Molecular weight of non irradiated and irradiated enzyme protein calculated by the Archibald's method. MmMb Survival Enzyme activity rate of none irradiated 97, , % irradiated by 5.9 x 104 rad 96,903 98,21150% Mm : molecular weight calculated at the meniscus Mb : molecular weight calculated at the bottom of cell dimer transformation depends upon the concentration of a-amylase and the separation of the dimer with a sedimentation coefficient of 6 S and the monomer with that of 4.5 S can be carried out through a gel filtration of Sephadex G-100. Under the conditions in the present work, a-amylase should be considered to be exist as a dimer. Therefore, the molecular weight as a dimer was estimated about at 48,500. The centrifuge pattern and the calculated molecular weight of non irradiated enzyme and those of irradiated one were essentially identical. Either any alteration of the molecular weight or dissociation of dimer to monomer was not found from this result. Determination of N-terminal amino acid The determination of N-terminal amino acid of the irradiated and non irradiated a-amylase was carried out by the method of Sanger. As shown in Table II, N- terminal amino acid of both enzymes was found to be valine alone. The results obtained by gel filtration and ultracentrifugation described above, denied the alteration in the molecular weight of this enzyme protein. However, they cannot deny the possiblity of cleavage or isolation of small peptides and amino acids. Table II. N-terminal amino acid of non irradiated and irradiated enzyme protein. (by Sanger's DNP method) irradiation Dose none 4.6 x 104 rad 5.5 x 104 rad 4.6 x 105 rad Survival rate 100%65%50%20% of enz. Activity neutral acidic Valine ValineValineValine basic e-lysine e-lysine e-lysine e-lysine e-lysine e-lysines-lysine e-lysine s-lysine e-lysine di-lysinenonenonenonenone From the fact that any new species of N-terminal amino acid was not found in the irradiated enzyme, it was concluded that the peptide main chain had not accept any cleavage by irradiation. The amino acid composition of intact and irradiated a-amylase was measured by the method of Stein and Moore. Since the degree of hydrolysis of the irradiated enzyme is more or less different from that of non-irradiated enzyme, the values shown in Table III have been collected by the data obtained from the experiments done in the variation of hydrolitic time. For example, in the intact a-amylase, the amounts of aspartic acid, glysine, alanine, threonine, valine, etc. were reached in their maximum value after a hydrolysis as long as 48 hours, but both in the irradiated protein (40)
6 Radiolytic Effects of Gamma rays on a-amylase in Aqueous Solution Table III. Amino acid composition of non-irradiated and irradiated a-amylase. Amino A cid Dose noneradradjunge 5.9 x x 105data by Akabori data by et al.1z) et al.13) Aspartic acid Threonine Serine Glutamic acid Proline Glycine Alanine Cystine----- Valine Methionine Isoleucine Leucine Tyrosine Phenylalanine Tryptophan14.1* 14.3* Lysine Histidine Ammonia Arginine The amounts of each amino acid is described as number of amino acid residues per enzyme molecule. Molecular weight of a-amylase used in this data is 48,000. Inactivation rates of enzyme to irradiation of each dose are, 5.9 x 104 rad : 30 percent. 9.8 x 105 rad : 95 percent. remaining 70 percent of its original activity and the irradiated one remaining only 5 percent, a hydrolysis of 22 hours was found to be satisfactory for the complete hydrolysis in almost all amino acids. The results obstined in these manner, were shown in Table III. Since the result from the analysis of the intact a-amylase were intermediate between the result of Junge et a1.12 and that of Akabori et a1.133, this result was seemed to be reasonable one considering the difference in the strain of Bacillus Subtilis. In the chromatograms of this analysis any unidentified peak was not found even in the irradiated enzymes. The amino acid compositions of both the enzyme irradiated with 5.9 x 104 rad, inactivated 30 percent and that irradiated with 9.8 x 105 rad, inactivated 95 percent, were not so different from that of non irradiated enzyme on the whole. However, the amounts of basic amino acids, such as lysine, histidine and arginine, and tyrosine were found to be reduced a little as the irradiation dose increased. In this irradiated enzyme which are inactivated to 10 percent of its original activity, it has lost 4.5 moles of lysine, 2 moles of histidine and arginine, and 2.7 moles of tyrosine in one enzyme molecule. The amounts of amide in this a-amylase were measured by the method described in METHODS. Intact a-amylase was found to include 36 moles of amide in its mole- ( 41 )
7 R. TANABE cule. Liberation of ammonia by irradiation 0.13 percent, 0.34 percent and 0.69 percent a-amylase solutions were irradiated with various doses of r-rays and the amounts of ammonia liberated from the irradiated protein were measured by the method described in METHODS. Figure 2 shows the number of ammonia liberated, versus the dose irradiated and versus the inactivation ratio of the enzyme. The amount of free ammonia in each solution increased with the irradiation dose and as shown in Fig. 2, the degree of liberation of the free ammonia was found to proceed proportionally to that of inactivation by irradiation. Furthermore this finding was independent of the enzyme concentration at the irradiation. In the irradiated enzyme which was completely inactivated, moles of ammonia were removed per 1 molecule of the enzyme. 5' 1.5 4a;/ n~ x/ a 05- E I o w/-05 O E B I rradiation dose (x 105 rad ) Inactivation rate ('/. Fig. 2. Liberation of ammonia from enzyme protein by irradiation. Moles of removed ammonia per enzyme molecule is shown against irradiated dose and against the inactivation ratio. (0) : The concentration of enzyme solution at the irradiation is 1.3 mg/ml., (x) : 3.4 mg/ml., (0) : 6.9 mg/ml. DISCUSSION Although the amino acid composition of a-amylase was not found to be altered so largely by irradiation, the amounts of tyrosine and several basic amino acids, such as lysine, histidine and arginine, were reduced to some extent as described above. These amino acid residues are well known to be conparatively sensitive to irradiation14), but it is not clear wheather or not they play important parts to reveal the enzyme activity. In the case of this a-amylase, the importance of these residues for this enzyme activity have been suggested from the studies using the methods modifying these amino acid residues by specific chemical reagents. Therefore, the reduction of these amino acid residues by irradiation is likely to be caused by the damage of active sites in this enzyme. On the contrary, considering the high sensitivity of these amino acids to irradiation, it is also assumed that these reduction may be only an accompanying result with the destruction of protein comformation. These problems should be discussed formally standing on the investigations treating with the comformation changes by irradiation elsewhere. As shown in Fig. 2 the free ammonia (42)
8 Radiolytic Effects of Gamma rays on a-amylase in Aqueous Solution was liberated by irradiation. Since it was cleared that the peptide bond of a-amylase was not cleaved by irradiation the free ammonia should originate not in the NH2 of the peptide bonds nor of the N-terminal amino acid, but in the deamination of acid amides or basic amino acid residues in side chains. The results from the amino acid analysis showed a little reduction of basic amino acids and acid amides such as glutamine or asparagine were inclined to be resistant to irradiation in its single solution. These facts seemed to suggest that the ammonia was the reult from the destruction of basic amino acids, but cannot deny the posibility of the destruction in acid amides. ACKNOWLEDGEMENT The author would like to express her sincere thanks to Professor Sakae Shimizu for his kind arrangement to use the Irradiation Facility of the Institute for Chemical Research, Kyoto University, and to Professor Hiroyuki Hatano and Dr. Koichiro Sumizu for their helpfull direction and discussion, and also thanks the Daiwa Kasei Co., Ltd., Osaka, for the kind supply of the crystalline a-amylase. REFERENCES (1) R. Tanabe, This Bulletin, (1973). (2) H. K. Schachman, Methods in Enzymology, Vol. IV, p. 38, (Academic Press, New York 1957). (3) H. Hatano, S. Ganno, K. Sumizu, S. Ohnishi, N. Kimura, T. Imai and T. Tsuzuki, J. Radiat. Res., 7, 67 (1966). (4) D. H. Spackman, W. H. Stein and S. Moore, Anal. Chem., 30, 1190 (1958). (5) T. W. Goodwin and R. A. Morton, Biochem. J., 40, 628 (1946). (6) F. Sanger, Biochem. J., 39, 507 (1945). (7) E. J. Conway, Microdiffusion analysis and volumetric error, p. 87, (Crosby Lockwood and Son Ltd., London 1950). (8) S. Akamatsu, J. Biochem., 39, 203 (1952). (9) E. A. Stein and E. H. Fischer, Biochim. Biophys. Acta, 39, 287 (1960). (10) T. Isemura, K. Kakiuchi and H. Eto, J. Biochem., 47, 548 (1960). (11) K. Kakiuchi, S. Kato, A. Imanishi and T. Isemura, J. Biochem., 55, 102 (1964). (12) J. M. Junge, E. A. Stein, H. Neurath and E. H. Fischer, J. Biol. Chem., 234, 556 (1958). (13) S. Akabori, Y. Okada, S. Fujiwara and K. Sugae, J. Biochem., 43, 741 (1956). (14) G. Barron, J. Ambrose and P. Johonson, Rad. Res., 2, 145 (1955). ( 43 )
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 9. AMINO ACIDS, PEPTIDES AND
Amino acids. (Foundation Block) Dr. Essa Sabi
 Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Amino acids (Foundation Block) Dr. Essa Sabi Learning outcomes What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino acid configuration. Non-standard amino
Author(s) Hatano, Hiroyuki; Ganno, Shigetake;
 Radiation Sensitivity of Amino Acid TitleProtein to Gamma Rays+ (Special Iss Chemical and Biological Effects of Author(s) Hatano, Hiroyuki; Ganno, Shigetake; Citation Bulletin of the Institute for Chemi
Radiation Sensitivity of Amino Acid TitleProtein to Gamma Rays+ (Special Iss Chemical and Biological Effects of Author(s) Hatano, Hiroyuki; Ganno, Shigetake; Citation Bulletin of the Institute for Chemi
Author(s) Hatano, Hiroyuki; Ganno, Shigetake;
 Electron Spin Resonance Studies on Title Inactivation by Direct Action of Ga Issue on Physical, Chemical and Bio Radiation, IV) Author(s) Hatano, Hiroyuki; Ganno, Shigetake; Citation Bulletin of the Institute
Electron Spin Resonance Studies on Title Inactivation by Direct Action of Ga Issue on Physical, Chemical and Bio Radiation, IV) Author(s) Hatano, Hiroyuki; Ganno, Shigetake; Citation Bulletin of the Institute
Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column
 Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS
 AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
AMINO ACIDS STRUCTURE, CLASSIFICATION, PROPERTIES. PRIMARY STRUCTURE OF PROTEINS Elena Rivneac PhD, Associate Professor Department of Biochemistry and Clinical Biochemistry State University of Medicine
Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids.
 Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Today we begin our discussion of the structure and properties of proteins. Proteins consist in whole or large part of amino acids. Simple proteins consist only of amino acids. Conjugated proteins contain
Amino Acid Analyzer AAA400
 Amino Acid Analyzer AAA400 Determination of amino acid of hydrolyzates (food and feed) Column: LG ANB OSTION 3.6x340 12μm Eluents: sodium-citrate buffers, 0.2 M NaOH Aspartic Acid, Threonine, Serine, Glutamic
Amino Acid Analyzer AAA400 Determination of amino acid of hydrolyzates (food and feed) Column: LG ANB OSTION 3.6x340 12μm Eluents: sodium-citrate buffers, 0.2 M NaOH Aspartic Acid, Threonine, Serine, Glutamic
Lecture 11 AMINO ACIDS AND PROTEINS
 Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Lecture 11 AMINO ACIDS AND PROTEINS The word "Protein" was coined by J.J. Berzelius in 1838 and was derived from the Greek word "Proteios" meaning the first rank. Proteins are macromolecular polymers composed
Section 1 Proteins and Proteomics
 Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Section 1 Proteins and Proteomics Learning Objectives At the end of this assignment, you should be able to: 1. Draw the chemical structure of an amino acid and small peptide. 2. Describe the difference
Lecture 10 - Protein Turnover and Amino Acid Catabolism
 Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
Lecture 10 - Protein Turnover and Amino Acid Catabolism Chem 454: Regulatory Mechanisms in Biochemistry University of Wisconsin-Eau Claire 1 Introduction 2 Proteins are degraded into amino acids. Protein
PRO G max Probiotic fermented soybean meal Benefits of PRO G max
 PRO G max Probiotic fermented soybean meal Benefits of PRO G max Probiotic bacteria > 10 10 CFU/kg High protein with low molecular weight protein approaching small peptides enhancing digestion and absorption
PRO G max Probiotic fermented soybean meal Benefits of PRO G max Probiotic bacteria > 10 10 CFU/kg High protein with low molecular weight protein approaching small peptides enhancing digestion and absorption
Lecture 3: 8/24. CHAPTER 3 Amino Acids
 Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
Lecture 3: 8/24 CHAPTER 3 Amino Acids 1 Chapter 3 Outline 2 Amino Acid Are Biomolecules and their Atoms Can Be Visualized by Two Different Ways 1) Fischer projections: Two dimensional representation of
BIOCHEMICAL STUDIES ON PEARL FRACTIONATION AND TERMINAL AMINO ACIDS OF CONCHIOLIN. By SHOZO TANAKA, HIROYUKI HATANO AND GINZABURO SUZUE
 The Journal of Biochemistry, Vol. 47, No. 1, 1960 BIOCHEMICAL STUDIES ON PEARL VII. FRACTIONATION AND TERMINAL AMINO ACIDS OF CONCHIOLIN By SHOZO TANAKA, HIROYUKI HATANO AND GINZABURO SUZUE (From the Department
The Journal of Biochemistry, Vol. 47, No. 1, 1960 BIOCHEMICAL STUDIES ON PEARL VII. FRACTIONATION AND TERMINAL AMINO ACIDS OF CONCHIOLIN By SHOZO TANAKA, HIROYUKI HATANO AND GINZABURO SUZUE (From the Department
I) Choose the best answer: 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine.
 1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
1- All of the following amino acids are neutral except: a) glycine. b) threonine. c) lysine. d) proline. e) leucine. 2- The egg white protein, ovalbumin, is denatured in a hard-boiled egg. Which of the
The Effect of Reducing Agents
 I. Soc. Cosmet. Chem., 22, 571-578 (August 18, 1971) on The Effect of Reducing Agents Fingernail Keratin NANCY F. WOLEJSZA, B.A.,* STANLEY G. ELFBAUM, Ph.D.,* and MARIA A. WOLFRAM, Ph.D.* Synopsis--The
I. Soc. Cosmet. Chem., 22, 571-578 (August 18, 1971) on The Effect of Reducing Agents Fingernail Keratin NANCY F. WOLEJSZA, B.A.,* STANLEY G. ELFBAUM, Ph.D.,* and MARIA A. WOLFRAM, Ph.D.* Synopsis--The
1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids
 Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
Amino acids 1-To know what is protein 2-To identify Types of protein 3- To Know amino acids 4- To be differentiate between essential and nonessential amino acids 5-To understand amino acids synthesis Amino
PHAR3316 Pharmacy biochemistry Exam #2 Fall 2010 KEY
 1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
1. How many protons is(are) lost when the amino acid Asparagine is titrated from its fully protonated state to a fully deprotonated state? A. 0 B. 1 * C. 2 D. 3 E. none Correct Answer: C (this question
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2*
 Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
Analysis of L- and D-Amino Acids Using UPLC Yuta Mutaguchi 1 and Toshihisa Ohshima 2* 1 Department of Biotechnology, Akita Prefectural University, Akita City, Japan; 2 Department of Biomedical Engineering,
Biochemistry - I. Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I
 Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
Biochemistry - I Prof. S. Dasgupta Department of Chemistry Indian Institute of Technology, Kharagpur Lecture 1 Amino Acids I Hello, welcome to the course Biochemistry 1 conducted by me Dr. S Dasgupta,
J. Physiol. (I956) I33,
 626 J. Physiol. (I956) I33, 626-630 ACTIVE TRANSPORT OF AMINO ACIDS BY SACS OF EVERTED SMALL INTESTINE OF THE GOLDEN HAMSTER (MESOCRICETUS AURATUS) BY G. WISEMAN From the Department of Physiology, University
626 J. Physiol. (I956) I33, 626-630 ACTIVE TRANSPORT OF AMINO ACIDS BY SACS OF EVERTED SMALL INTESTINE OF THE GOLDEN HAMSTER (MESOCRICETUS AURATUS) BY G. WISEMAN From the Department of Physiology, University
Midterm 1 Last, First
 Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Midterm 1 BIS 105 Prof. T. Murphy April 23, 2014 There should be 6 pages in this exam. Exam instructions (1) Please write your name on the top of every page of the exam (2) Show all work for full credit
Chemical Nature of the Amino Acids. Table of a-amino Acids Found in Proteins
 Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Chemical Nature of the Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. There are 20 a- amino acids that are relevant to the make-up of mammalian proteins (see below). Several
Amino acids. Ing. Petrová Jaroslava. Workshop on Official Controls of Feed AGR 46230, , Ankara. Turkey ÚKZÚZ - NRL RO Praha 1
 Amino acids Ing. Petrová Jaroslava Workshop on Official Controls of Feed AGR 46230, 6. 7. 12. 2011, Ankara. Turkey 6.12.2011 ÚKZÚZ - NRL RO Praha 1 Content of this presentation 1. Function of amino acids
Amino acids Ing. Petrová Jaroslava Workshop on Official Controls of Feed AGR 46230, 6. 7. 12. 2011, Ankara. Turkey 6.12.2011 ÚKZÚZ - NRL RO Praha 1 Content of this presentation 1. Function of amino acids
Amino acids. You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures.
 Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
Amino acids You are required to know and identify the 20 amino acids : their names, 3 letter abbreviations and their structures. If you wanna make any classification in the world, you have to find what
H 2 C H 2 N C CH O N C CH 3 CH 2 H O. aspartame
 1 The addition of sucrose, table sugar, to food and drink has been linked to the increased risk of obesity and insulin resistance. Aspartame is used as an alternative to sugar. The structure of aspartame
1 The addition of sucrose, table sugar, to food and drink has been linked to the increased risk of obesity and insulin resistance. Aspartame is used as an alternative to sugar. The structure of aspartame
So where were we? But what does the order mean? OK, so what's a protein? 4/1/11
 So where were we? We know that DNA is responsible for heredity Chromosomes are long pieces of DNA DNA turned out to be the transforming principle We know that DNA is shaped like a long double helix, with
So where were we? We know that DNA is responsible for heredity Chromosomes are long pieces of DNA DNA turned out to be the transforming principle We know that DNA is shaped like a long double helix, with
Flagellar Hook Protein from Salmonella SJ25
 JOURNAL OF BACrERIOLOGY, Jan. 1976, p. 68-73 Copyright 1976 American Society for Microbiology Vol. 125, No. 1 Printed in U.S.A. Flagellar Hook Protein from Salmonella SJ25 HIROAKI KAGAWA,* KATSUSHI OWARIBE,
JOURNAL OF BACrERIOLOGY, Jan. 1976, p. 68-73 Copyright 1976 American Society for Microbiology Vol. 125, No. 1 Printed in U.S.A. Flagellar Hook Protein from Salmonella SJ25 HIROAKI KAGAWA,* KATSUSHI OWARIBE,
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay
 Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Introduction to Proteomics Dr. Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology - Bombay Lecture 01 Introduction to Amino Acids Welcome to the proteomic course.
Macromolecules of Life -3 Amino Acids & Proteins
 Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
Macromolecules of Life -3 Amino Acids & Proteins Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: splin@dragon.nchu.edu.tw Website: http://web.nchu.edu.tw/pweb/users/splin/ Amino Acids Proteins
PROTEIN. By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM
 PROTEIN By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM OBJECTIVES OF THE LECTURE By the end of this lecture, student can: Define
PROTEIN By: Shamsul Azahari Zainal Badari Department of Resource Management and Consumer Studies Faculty of Human Ecology UPM OBJECTIVES OF THE LECTURE By the end of this lecture, student can: Define
Biomolecular Mass Spectrometry
 Lipids ot different than other organic small molecules Carbohydrates Polymers of monosaccharides linked via glycosidic bonds (acetals/ ketals) many different combinationsvery interesting no time ucleic
Lipids ot different than other organic small molecules Carbohydrates Polymers of monosaccharides linked via glycosidic bonds (acetals/ ketals) many different combinationsvery interesting no time ucleic
THE AMINO ACID SEQUENCE OF HYPERTENSIN II
 THE AMINO ACID SEQUENCE OF HYPERTENSIN II BY LEONARD T. SKEGGS, JR., PH.D., KENNETH E. LENTZ, PH.D., JOSEPH R. KAHN, M.D., NORMAN P. SHUMWAY, M.D., ~'D KENNETH R. WOODS, I~.D. (From the Department of Medicine
THE AMINO ACID SEQUENCE OF HYPERTENSIN II BY LEONARD T. SKEGGS, JR., PH.D., KENNETH E. LENTZ, PH.D., JOSEPH R. KAHN, M.D., NORMAN P. SHUMWAY, M.D., ~'D KENNETH R. WOODS, I~.D. (From the Department of Medicine
Amino Acids. Amino Acids. Fundamentals. While their name implies that amino acids are compounds that contain an NH. 3 and CO NH 3
 Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
Fundamentals While their name implies that amino acids are compounds that contain an 2 group and a 2 group, these groups are actually present as 3 and 2 respectively. They are classified as α, β, γ, etc..
2. Ionization Sources 3. Mass Analyzers 4. Tandem Mass Spectrometry
 Dr. Sanjeeva Srivastava 1. Fundamental of Mass Spectrometry Role of MS and basic concepts 2. Ionization Sources 3. Mass Analyzers 4. Tandem Mass Spectrometry 2 1 MS basic concepts Mass spectrometry - technique
Dr. Sanjeeva Srivastava 1. Fundamental of Mass Spectrometry Role of MS and basic concepts 2. Ionization Sources 3. Mass Analyzers 4. Tandem Mass Spectrometry 2 1 MS basic concepts Mass spectrometry - technique
QUALITATIVE ANALYSIS OF AMINO ACIDS AND PROTEINS
 QUALITATIVE ANALYSIS OF AMINO ACIDS AND PROTEINS Amino acids are molecules containing an amine group, a carboxylic acid group and a side chain that varies between different amino acids. Amino acids of
QUALITATIVE ANALYSIS OF AMINO ACIDS AND PROTEINS Amino acids are molecules containing an amine group, a carboxylic acid group and a side chain that varies between different amino acids. Amino acids of
Effect of Excess of Individual Essential Amino Acids in Diets on Chicks
 135 Effect of Excess of Individual Essential Amino Acids in Diets on Chicks Jun-ichi OKUMURA and Kiyoto YAMAGUCHI Laboratory of Animal Nutrition, Faculty of Agriculture, Nagoya University, Nagoya-shi 464
135 Effect of Excess of Individual Essential Amino Acids in Diets on Chicks Jun-ichi OKUMURA and Kiyoto YAMAGUCHI Laboratory of Animal Nutrition, Faculty of Agriculture, Nagoya University, Nagoya-shi 464
Reactions and amino acids structure & properties
 Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
Lecture 2: Reactions and amino acids structure & properties Dr. Sameh Sarray Hlaoui Common Functional Groups Common Biochemical Reactions AH + B A + BH Oxidation-Reduction A-H + B-OH + energy ª A-B + H
[NOTE The relative retention times for calcitonin salmon and calcitonin salmon related compound A Change to read:
 . Mode: Revision Bulletin Official April 1, 2012 Calcitonin Salmon 1 LC Calcitonin Salmon Detector: UV 220 nm Column: 4.6-mm 25-cm; packing L1 Column temperature: 65 Flow rate: 1 ml/min Injection volume:
. Mode: Revision Bulletin Official April 1, 2012 Calcitonin Salmon 1 LC Calcitonin Salmon Detector: UV 220 nm Column: 4.6-mm 25-cm; packing L1 Column temperature: 65 Flow rate: 1 ml/min Injection volume:
Controlled biomimetic crystallization of ZIF-8 particles by amino acids
 Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2016 Supporting information for Controlled biomimetic crystallization of ZIF-8 particles by amino
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2016 Supporting information for Controlled biomimetic crystallization of ZIF-8 particles by amino
Gentilucci, Amino Acids, Peptides, and Proteins. Peptides and proteins are polymers of amino acids linked together by amide bonds CH 3
 Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Amino Acids Peptides and proteins are polymers of amino acids linked together by amide bonds Aliphatic Side-Chain Amino Acids - - H CH glycine alanine 3 proline valine CH CH 3 - leucine - isoleucine CH
Reinvestigation on the Amino Acid Composition and C-Terminal Group of Taka-Amylase A. By Kozo NARITA*, HIRONORI MURAKAMI* and TOKUJI IKENAKA**
 The Journal of Biochemistry, Vol. 59, No. 2, 1966 Reinvestigation on the Amino Acid Composition and C-Terminal Group of Taka-Amylase A By Kozo NARITA*, HIRONORI MURAKAMI* and TOKUJI IKENAKA** (From *the
The Journal of Biochemistry, Vol. 59, No. 2, 1966 Reinvestigation on the Amino Acid Composition and C-Terminal Group of Taka-Amylase A By Kozo NARITA*, HIRONORI MURAKAMI* and TOKUJI IKENAKA** (From *the
Protein Folding LARP
 Protein Folding LARP Version: 1.0 Release: April 2018 Amplyus 2018 minipcr TM Protein Folding LARP (Live Action Role Play) Summary Materials In this activity, students will role play to make a folded protein
Protein Folding LARP Version: 1.0 Release: April 2018 Amplyus 2018 minipcr TM Protein Folding LARP (Live Action Role Play) Summary Materials In this activity, students will role play to make a folded protein
Biomolecules: amino acids
 Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Biomolecules: amino acids Amino acids Amino acids are the building blocks of proteins They are also part of hormones, neurotransmitters and metabolic intermediates There are 20 different amino acids in
Amino acids-incorporated nanoflowers with an
 Amino acids-incorporated nanoflowers with an intrinsic peroxidase-like activity Zhuo-Fu Wu 1,2,+, Zhi Wang 1,+, Ye Zhang 3, Ya-Li Ma 3, Cheng-Yan He 4, Heng Li 1, Lei Chen 1, Qi-Sheng Huo 3, Lei Wang 1,*
Amino acids-incorporated nanoflowers with an intrinsic peroxidase-like activity Zhuo-Fu Wu 1,2,+, Zhi Wang 1,+, Ye Zhang 3, Ya-Li Ma 3, Cheng-Yan He 4, Heng Li 1, Lei Chen 1, Qi-Sheng Huo 3, Lei Wang 1,*
Amino acids. Dr. Mamoun Ahram Summer semester,
 Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino acids Dr. Mamoun Ahram Summer semester, 2017-2018 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 3 (pp.66-76) General structure (Chiral carbon) The amino acids that occur in
Amino Acid Composition of Hypertensin II.-- EXPERIMENTAL
 THE AMINO ACID COMPOSITION OF HYPERTENSIN II AND ITS BIOCHEMICAL RELATIONSHIP TO HYPERTENSIN I BY KENNETH E. LENTZ, PH.D., LEONARD T. SKEGGS, JR., PH.D., KENNETH R. WOODS, PH.D., JOSEPH R. KAHN, M.D.,
THE AMINO ACID COMPOSITION OF HYPERTENSIN II AND ITS BIOCHEMICAL RELATIONSHIP TO HYPERTENSIN I BY KENNETH E. LENTZ, PH.D., LEONARD T. SKEGGS, JR., PH.D., KENNETH R. WOODS, PH.D., JOSEPH R. KAHN, M.D.,
Molecular Biology. general transfer: occurs normally in cells. special transfer: occurs only in the laboratory in specific conditions.
 Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Chapter 9: Proteins Molecular Biology replication general transfer: occurs normally in cells transcription special transfer: occurs only in the laboratory in specific conditions translation unknown transfer:
Quantitative Analysis of Underivatized Amino Acids in Plant Matrix by Hydrophilic Interaction Chromatography (HILIC) with LC/MS Detection
 Application Note Food Testing, Metabolomics, Agricultural Chemistry, Environmental Quantitative Analysis of Underivatized Amino Acids in Plant Matrix by Hydrophilic Interaction Chromatography (HILIC) with
Application Note Food Testing, Metabolomics, Agricultural Chemistry, Environmental Quantitative Analysis of Underivatized Amino Acids in Plant Matrix by Hydrophilic Interaction Chromatography (HILIC) with
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
(65 pts.) 27. (10 pts.) 28. (15 pts.) 29. (10 pts.) TOTAL (100 points) Moorpark College Chemistry 11 Spring Instructor: Professor Gopal
 Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2012 Instructor: Professor Gopal Examination # 5: Section Five May 1, 2012 Name: (print) GOOD LUCK! Directions: Make sure your examination contains TWELVE total pages
EFFECT OF SOME AMINO ACIDS ON THE GROWTH AND L-GLUTAMIC ACID FERMENTATION BY AN AUXOTROPHIC MUTANT Micrococcus glutamicus AB 100.
 S. Ganguly et. al. / International Journal on Pharmaceutical and Biomedical Research (IJPBR) Vol. 2(1), 2011, 21-25 EFFECT OF SOME AMINO ACIDS ON THE GROWTH AND L-GLUTAMIC ACID FERMENTATION BY AN AUXOTROPHIC
S. Ganguly et. al. / International Journal on Pharmaceutical and Biomedical Research (IJPBR) Vol. 2(1), 2011, 21-25 EFFECT OF SOME AMINO ACIDS ON THE GROWTH AND L-GLUTAMIC ACID FERMENTATION BY AN AUXOTROPHIC
STUDIES OF STREPTOCOCCAL CELL WALLS
 STUDIES OF STREPTOCOCCAL CELL WALLS V. AMINO ACID COMPOSITION OF CELL WALLS OF VIRULENT AND AVIRULENT GROUP A HEMOLYTIC STREPTOCOCCI' B. S. TEPPER, J. A. HAYASHI, AND S. S. BARKULIS Department of Biological
STUDIES OF STREPTOCOCCAL CELL WALLS V. AMINO ACID COMPOSITION OF CELL WALLS OF VIRULENT AND AVIRULENT GROUP A HEMOLYTIC STREPTOCOCCI' B. S. TEPPER, J. A. HAYASHI, AND S. S. BARKULIS Department of Biological
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 ANSWERS For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the
Annual Report ERNDIM-EQAS Quantitative Amino Acids 2002
 Annual Report ERNDIM-EQAS Quantitative Amino Acids 2002 1. Purpose The purpose of the ERNDIM External Quality Assurance Scheme for Quantitative Organic Acids is the monitoring of the analytical quality
Annual Report ERNDIM-EQAS Quantitative Amino Acids 2002 1. Purpose The purpose of the ERNDIM External Quality Assurance Scheme for Quantitative Organic Acids is the monitoring of the analytical quality
Introduction to Biochemistry Midterm exam )ومن أحياها(
 Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
Introduction to Biochemistry Midterm exam 2016-2017 )ومن أحياها( 1. Which of the following amino (in a peptide chain) would probably be found at a beta bend or turn? a. lysine * b. Gly c. arg d. asn 2.
ADSORPTION AND DESORPTION OF METAL IONS BY SYSTEMS BASED ON CELLULOSE DERIVATIVES THAT CONTAIN AMINO ACID RESIDUES"
 (41) Vol. 41, No.6 (1985) T-235 (Received May 24, 1984) ADSORPTION AND DESORPTION OF METAL IONS BY SYSTEMS BASED ON CELLULOSE DERIVATIVES THAT CONTAIN AMINO ACID RESIDUES" By Toshihiko Sato, Shigenori
(41) Vol. 41, No.6 (1985) T-235 (Received May 24, 1984) ADSORPTION AND DESORPTION OF METAL IONS BY SYSTEMS BASED ON CELLULOSE DERIVATIVES THAT CONTAIN AMINO ACID RESIDUES" By Toshihiko Sato, Shigenori
Hydrolysis of Irradiated Ovalbumin by Pepsin
 Hydrolysis of Irradiated Ovalbumin by Pepsin HECTOR A. DIEU and V. DESREUX From the Department of Physical Chemistry, University of Liege, Liege, Belgium ABSTRACT Solid ovalbumin has been irradiated at
Hydrolysis of Irradiated Ovalbumin by Pepsin HECTOR A. DIEU and V. DESREUX From the Department of Physical Chemistry, University of Liege, Liege, Belgium ABSTRACT Solid ovalbumin has been irradiated at
Chemistry 121 Winter 17
 Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121 Winter 17 Introduction to Organic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. Ohio State) E-mail: upali@latech.edu Office: 311 Carson Taylor Hall ; Phone: 318-257-4941;
[ APPLICATION NOTE ] UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research INTRODUCTION APPLICATION BENEFITS
![[ APPLICATION NOTE ] UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research INTRODUCTION APPLICATION BENEFITS [ APPLICATION NOTE ] UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research INTRODUCTION APPLICATION BENEFITS](/thumbs/88/117895443.jpg) UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research Padhraic Rossiter, 1 Jaime Salcedo Dominguez, 1 Jennifer Warren, 1 Norma Breen, 1 Lisa Calton 2 1 Waters Corporation,
UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research Padhraic Rossiter, 1 Jaime Salcedo Dominguez, 1 Jennifer Warren, 1 Norma Breen, 1 Lisa Calton 2 1 Waters Corporation,
Hydroxyquinoline, 8-Mercaptoquinoli. Eiji Suito on the Occasion of his R.
 Comparison of Properties and Struct Title Hydroxyquinoline, 8-Mercaptoquinoli Selenoquinoline (Commemoration Issu Eiji Suito on the Occasion of his R Author(s) Sekido, Eiichi; Fukui, Nobuo Citation Bulletin
Comparison of Properties and Struct Title Hydroxyquinoline, 8-Mercaptoquinoli Selenoquinoline (Commemoration Issu Eiji Suito on the Occasion of his R Author(s) Sekido, Eiichi; Fukui, Nobuo Citation Bulletin
For questions 1-4, match the carbohydrate with its size/functional group name:
 Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2013 Examination #5 PRACTICE 1 For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
بسم هللا الرحمن الرحيم
 بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
بسم هللا الرحمن الرحيم Biochemistry Lec #1 Dr. Nafith AbuTarboush- (30.6.2014) Amino Acids 1 Campbell and Farrell s Biochemistry, Chapter 3 (pp.66-76) Introduction: Biochemistry is two courses; one is
0010 Amino Acids 40 Profile - Plasma
 Accession #: Order #: G1234567 Date Collected: Date Received: 01/22/2013 Reference #: Patient: Date of Birth: 02/05/1962 Date of Report: Telephone: 7704464583 Ordering Physician: 1234 Main St. Anywhere,
Accession #: Order #: G1234567 Date Collected: Date Received: 01/22/2013 Reference #: Patient: Date of Birth: 02/05/1962 Date of Report: Telephone: 7704464583 Ordering Physician: 1234 Main St. Anywhere,
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PRIMENE 10% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each litre of the infusion solution contains: L-Isoleucine L-Leucine L-Valine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT PRIMENE 10% 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each litre of the infusion solution contains: L-Isoleucine L-Leucine L-Valine
Characterization of Bacteria by Their Degradation of Amino Acids
 APPLIED MICROBIOLOGY, Oct. 1968, P. 1591-1595 Copyright 1968 American Society for Microbiology Vol. 16, No. 10 Printed in U.S.A. Characterization of Bacteria by Their Degradation of Amino Acids M. J. PICKETT
APPLIED MICROBIOLOGY, Oct. 1968, P. 1591-1595 Copyright 1968 American Society for Microbiology Vol. 16, No. 10 Printed in U.S.A. Characterization of Bacteria by Their Degradation of Amino Acids M. J. PICKETT
Product Information: Ketonex -1
 Product Information: 1 of 5 Nutrition support of infants and toddlers with maple syrup urine disease (MSUD). Isoleucine-, leucine- and valine-free. Use under medical supervision. Branched-chain amino acid-free
Product Information: 1 of 5 Nutrition support of infants and toddlers with maple syrup urine disease (MSUD). Isoleucine-, leucine- and valine-free. Use under medical supervision. Branched-chain amino acid-free
Metabolism of Amino Acids in Aquatic Animals II
 Mem. Fac. Fish., Kagoshima Univ. Vol. 26 pp. 45-48 (1977) Metabolism of Amino Acids in Aquatic Animals II The effect of an amino acid supplemented casein diet on the growth rate of carp Yoshito Tanaka,
Mem. Fac. Fish., Kagoshima Univ. Vol. 26 pp. 45-48 (1977) Metabolism of Amino Acids in Aquatic Animals II The effect of an amino acid supplemented casein diet on the growth rate of carp Yoshito Tanaka,
1. Describe the relationship of dietary protein and the health of major body systems.
 Food Explorations Lab I: The Building Blocks STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will be constructing animal and plant proteins using beads to represent the amino acids.
Food Explorations Lab I: The Building Blocks STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will be constructing animal and plant proteins using beads to represent the amino acids.
Date: EXERCISE 4. Figure 1. Amino acid structure.
 Student s name: Date: Points: Assistant s signature: Index numer: /6 EXERISE 4 AMIN AIDS AND PRTEINS. Amino acids are structural units (monomers) of proteins. There are 20 different amino acids coded for
Student s name: Date: Points: Assistant s signature: Index numer: /6 EXERISE 4 AMIN AIDS AND PRTEINS. Amino acids are structural units (monomers) of proteins. There are 20 different amino acids coded for
LAB#23: Biochemical Evidence of Evolution Name: Period Date :
 LAB#23: Biochemical Evidence of Name: Period Date : Laboratory Experience #23 Bridge Worth 80 Lab Minutes If two organisms have similar portions of DNA (genes), these organisms will probably make similar
LAB#23: Biochemical Evidence of Name: Period Date : Laboratory Experience #23 Bridge Worth 80 Lab Minutes If two organisms have similar portions of DNA (genes), these organisms will probably make similar
Thin-Layer Chromatography of Amino Acids HASPI Medical Biology Lab 15b Background Macromolecules
 Thin-Layer Chromatography of s HASPI Medical Biology Lab 15b Background Macromolecules Name: Period: Date: There are four major types of biological macromolecules that make up the human body: nucleic acids
Thin-Layer Chromatography of s HASPI Medical Biology Lab 15b Background Macromolecules Name: Period: Date: There are four major types of biological macromolecules that make up the human body: nucleic acids
0010 Amino Acid Analysis - 40 Plasma
 770.446.5483 770.441.2237 This report contains reference range adjustments from routine revalidation procedures. It also contains the following three upgrades: 1) The amino acids have been reorganized
770.446.5483 770.441.2237 This report contains reference range adjustments from routine revalidation procedures. It also contains the following three upgrades: 1) The amino acids have been reorganized
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy
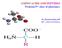 AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
AMINO ACIDS AND PEPTIDES Proteins/3 rd class of pharmacy Dr. Basima Sadiq Jaff PhD. Clinical Biochemistry MEDICAL AND BIOLOGICAL IMPORTANCE 1. building blocks of proteins. Some amino acids are found in
Analysis of Free Amino Acid Pools in Fungal Mycelia
 APPLID MICROBIOLOGY, Feb. 1972, p. 349-353 Copyright 1972 American Society for Microbiology Vol. 23, No. 2 Printed in U.SA Analysis of Free Amino Acid Pools in Fungal Mycelia J. G. HATHCOT, D. M. DAVIS,
APPLID MICROBIOLOGY, Feb. 1972, p. 349-353 Copyright 1972 American Society for Microbiology Vol. 23, No. 2 Printed in U.SA Analysis of Free Amino Acid Pools in Fungal Mycelia J. G. HATHCOT, D. M. DAVIS,
Proteins are sometimes only produced in one cell type or cell compartment (brain has 15,000 expressed proteins, gut has 2,000).
 Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Lecture 2: Principles of Protein Structure: Amino Acids Why study proteins? Proteins underpin every aspect of biological activity and therefore are targets for drug design and medicinal therapy, and in
Cultivation of Yeast Cells and Induction of Autophagy Hayashi Yamamoto, Hitoshi Nakatogawa
 Cultivation of Yeast Cells and Induction of Autophagy Hayashi Yamamoto, Hitoshi Nakatogawa METHOD Preculture 1. Inoculate yeast cells (from a single colony) into 2 ml of liquid medium (YPD, SD/CA, or SD/DO
Cultivation of Yeast Cells and Induction of Autophagy Hayashi Yamamoto, Hitoshi Nakatogawa METHOD Preculture 1. Inoculate yeast cells (from a single colony) into 2 ml of liquid medium (YPD, SD/CA, or SD/DO
Lecture 4. Grouping Amino Acid 7/1/10. Proteins. Amino Acids. Where Are Proteins Located. Nonpolar Amino Acids
 Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Proteins Lecture 4 Proteins - Composition of Proteins (Amino Acids) Chapter 21 ection 1-6! Proteins are compounds of high molar mass consisting almost entirely of amino acid chain(s)! Molar masses range
Product Information: Tyrex -1
 Product Information: Tyrex -1 1 of 5 Nutrition support of infants and toddlers with tyrosinemia types I, II or III. Phenylalanine- and tyrosine-free. Use under medical supervision. Phenylalanine- and tyrosine-free
Product Information: Tyrex -1 1 of 5 Nutrition support of infants and toddlers with tyrosinemia types I, II or III. Phenylalanine- and tyrosine-free. Use under medical supervision. Phenylalanine- and tyrosine-free
The incorporation of labeled amino acids into lens protein. Abraham Speclor and Jin H. Kinoshita
 The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
The incorporation of labeled amino acids into lens protein Abraham Speclor and Jin H. Kinoshita Calf and rabbit lenses cultured in a medium containing a radioactive amino acid incorporate some labeled
and the cells removed by centrifugation. These were resuspended in sterile 1949a), growth was measured in terms of acid production while dextran was
 THE NUTRITIONAL REQUIREMENTS OF LEUCONOSTOC DEXTRANICUM FOR GROWTH AND DEXTRAN SYNTHESIS1 VIRGINIA WHITESIDE-CARLSON AND CARMEN L. ROSANO Biochemistry Department, Medical College of Alabama, Birmingham,
THE NUTRITIONAL REQUIREMENTS OF LEUCONOSTOC DEXTRANICUM FOR GROWTH AND DEXTRAN SYNTHESIS1 VIRGINIA WHITESIDE-CARLSON AND CARMEN L. ROSANO Biochemistry Department, Medical College of Alabama, Birmingham,
Activities for the α-helix / β-sheet Construction Kit
 Activities for the α-helix / β-sheet Construction Kit The primary sequence of a protein, composed of amino acids, determines the organization of the sequence into the secondary structure. There are two
Activities for the α-helix / β-sheet Construction Kit The primary sequence of a protein, composed of amino acids, determines the organization of the sequence into the secondary structure. There are two
Fatty acids and phospholipids
 PYS 4xx Intro 2 1 PYS 4xx Intro 2 - Molecular building blocks We now describe in more detail the nomenclature and composition of several classes of compounds of relevance to the cell, including: membrane
PYS 4xx Intro 2 1 PYS 4xx Intro 2 - Molecular building blocks We now describe in more detail the nomenclature and composition of several classes of compounds of relevance to the cell, including: membrane
AMINO ACIDS NON-ESSENTIAL ESSENTIAL
 Edith Frederika Introduction A major component of food is PROTEIN The protein ingested as part of our diet are not the same protein required by the body Only 40 to 50 gr of protein is required by a normal
Edith Frederika Introduction A major component of food is PROTEIN The protein ingested as part of our diet are not the same protein required by the body Only 40 to 50 gr of protein is required by a normal
CHAPTER 21: Amino Acids, Proteins, & Enzymes. General, Organic, & Biological Chemistry Janice Gorzynski Smith
 CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
CHAPTER 21: Amino Acids, Proteins, & Enzymes General, Organic, & Biological Chemistry Janice Gorzynski Smith CHAPTER 21: Amino Acids, Proteins, Enzymes Learning Objectives: q The 20 common, naturally occurring
2018/9/24 AMINO ACIDS. Important. 436 Notes Original slides. 438 notes Extra information
 2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
2018/9/24 AMINO ACIDS Important. 436 Notes Original slides. 438 notes Extra information Objectives: What are the amino acids? General structure. Classification of amino acids. Optical properties. Amino
Lab Guide 2019 Metabolic Section Lab Guide
 Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
Vamin 18 EF QUALITATIVE AND QUANTITATIVE COMPOSITION
 Title Page Information Dec. 2000 1 (7) Number Vamin 18 EF 121-01 1. NAME OF THE MEDICINAL PRODUCT Vamin 18 g N/l electrolyte-free infusion fluid, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1000
Title Page Information Dec. 2000 1 (7) Number Vamin 18 EF 121-01 1. NAME OF THE MEDICINAL PRODUCT Vamin 18 g N/l electrolyte-free infusion fluid, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1000
Objective: You will be able to explain how the subcomponents of
 Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Objective: You will be able to explain how the subcomponents of nucleic acids determine the properties of that polymer. Do Now: Read the first two paragraphs from enduring understanding 4.A Essential knowledge:
Several Bacteroides Strains
 APPLIED MICROBIOLOGY, Nov., 1966 Vol. 14, No. 6 Copyright @ 1966 American Society for Microbiology Printed in U.S.A. Amino Acid and Vitamin Requirements of Several Bacteroides Strains GRACE QUINTO Cumberland
APPLIED MICROBIOLOGY, Nov., 1966 Vol. 14, No. 6 Copyright @ 1966 American Society for Microbiology Printed in U.S.A. Amino Acid and Vitamin Requirements of Several Bacteroides Strains GRACE QUINTO Cumberland
The Amino Acid Content of Hen's Egg in Relation to Dietary Protein Intake, Breed and Environment 1
 The Amino Acid Content of Hen's Egg in Relation to Dietary Protein Intake, Breed and Environment 1 P. Lunven and C. Le Clément de St. Marcq Protein Food Development Group Nutrition Division In 1963 the
The Amino Acid Content of Hen's Egg in Relation to Dietary Protein Intake, Breed and Environment 1 P. Lunven and C. Le Clément de St. Marcq Protein Food Development Group Nutrition Division In 1963 the
From sugar unit A From sugar unit B From sugar unit C
 ame 215 F12-Exam o. Page 2 I. (26 points) Raffinose is a trisaccharide occurring in cottonseed meal. Answer the following questions as directed in the boxes. (1) ( points) Label each of the glycosidic
ame 215 F12-Exam o. Page 2 I. (26 points) Raffinose is a trisaccharide occurring in cottonseed meal. Answer the following questions as directed in the boxes. (1) ( points) Label each of the glycosidic
(a) (i) Describe how the production and action of interferon differs from the production and action of lysozyme. (3)
 1 Histamine and the proteins interferon and lysozyme are involved in the non-specific responses to infection. (a) (i) escribe how the production and action of interferon differs from the production and
1 Histamine and the proteins interferon and lysozyme are involved in the non-specific responses to infection. (a) (i) escribe how the production and action of interferon differs from the production and
Application Note. Determination of Amino acids by UHPLC with automated OPA- Derivatization by the Autosampler. Summary. Fig. 1.
 Application Note Determination of Amino acids by UHPLC with automated PA- Derivatization by the Autosampler Category Bio Analysis Matrix - Method UHPLC Keywords Proteinogenic Amino acids, Canonical Amino
Application Note Determination of Amino acids by UHPLC with automated PA- Derivatization by the Autosampler Category Bio Analysis Matrix - Method UHPLC Keywords Proteinogenic Amino acids, Canonical Amino
The source of protein structures is the Protein Data Bank. The unit of classification of structure in SCOP is the protein domain.
 UNIT 14 PROTEINS DEFINITION A large molecule composed of one or more chains of amino acids in a specific order; the order is determined by the base sequence of nucleotides in the gene that codes for the
UNIT 14 PROTEINS DEFINITION A large molecule composed of one or more chains of amino acids in a specific order; the order is determined by the base sequence of nucleotides in the gene that codes for the
Product Information: Phenex -1
 Product Information: Phenex -1 1 of 5 For nutrition support of infants and toddlers with phenylketonuria (PKU). Phenylalanine-free Use under medical supervision. Phenylalanine-free to allow greater intake
Product Information: Phenex -1 1 of 5 For nutrition support of infants and toddlers with phenylketonuria (PKU). Phenylalanine-free Use under medical supervision. Phenylalanine-free to allow greater intake
9/6/2011. Amino Acids. C α. Nonpolar, aliphatic R groups
 Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Amino Acids Side chains (R groups) vary in: size shape charge hydrogen-bonding capacity hydrophobic character chemical reactivity C α Nonpolar, aliphatic R groups Glycine (Gly, G) Alanine (Ala, A) Valine
Introduction to Peptide Sequencing
 Introduction to Peptide equencing Quadrupole Ion Traps tructural Biophysics Course December 3, 2014 12/8/14 Introduction to Peptide equencing - athan Yates 1 Why are ion traps used to sequence peptides?
Introduction to Peptide equencing Quadrupole Ion Traps tructural Biophysics Course December 3, 2014 12/8/14 Introduction to Peptide equencing - athan Yates 1 Why are ion traps used to sequence peptides?
1. (38 pts.) 2. (25 pts.) 3. (15 pts.) 4. (12 pts.) 5. (10 pts.) Bonus (12 pts.) TOTAL (100 points)
 Moorpark College Chemistry 11 Spring 2010 Instructor: Professor Torres Examination #5: Section Five May 4, 2010 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total pages
Moorpark College Chemistry 11 Spring 2010 Instructor: Professor Torres Examination #5: Section Five May 4, 2010 ame: (print) ame: (sign) Directions: Make sure your examination contains TWELVE total pages
