Randomized controlled safety and efficacy trial of 2 vitamin A supplementation schedules in Tanzanian infants 1 3
|
|
|
- Howard Newman
- 6 years ago
- Views:
Transcription
1 Randomized controlled safety and efficacy trial of 2 vitamin A supplementation schedules in Tanzanian infants 1 3 Boniphace Idindili, Honarati Masanja, Honorathy Urassa, Wilbert Bunini, Paul van Jaarsveld, John J Aponte, Elizeus Kahigwa, Hassan Mshinda, David Ross, and David M Schellenberg ABSTRACT Background: Vitamin A supplementation reduces morbidity and mortality in children living in areas endemic for vitamin A deficiency. Routine vitamin A supplementation usually starts only at age 9 mo, but high rates of illness and mortality are seen in the first months of life. Objective: The objective of the study was to evaluate the safety and efficacy of vitamin A supplementation at the same time as routine vaccination in infants aged 1 3 mo. Design: We recruited 780 newborn infants and their mothers to a randomized double-blind controlled trial in Ifakara in southern Tanzania. In one group, mothers received g vitamin A palmitate shortly after delivery, and their infants received 7500 g at the same time as vaccinations given at 1, 2, and 3 mo of age. In the other group, mothers received a second g dose when their infant was aged 1 mo, and their infants received g at the same time as the routine vaccinations. VAD was defined as a modified relative dose-response test result of Results: High-dose vitamin A supplementation was well tolerated. The relative risk of VAD at 6 mo in the high-dose group compared with the lower dose group was 0.91 (95% CI: 0.76, 1.09; P 0.32). Serum retinol and incidence of illness did not differ significantly between the 2 groups. Some vitamin A capsules degraded toward the end of the study. Conclusions: Doubling the doses of vitamin A to mothers and their young infants is safe but unlikely to reduce short-term morbidity or to substantially enhance the biochemical vitamin A status of infants at age 6 mo. The stability of vitamin A capsules merits further investigation. Am J Clin Nutr 2007;85: KEY WORDS Tanzania, infants INTRODUCTION Vitamin A, supplementation, safety, efficacy, There is strong evidence that supplementation with vitamin A from the age of 6 mo reduces morbidity and mortality in areas with endemic vitamin A deficiency (VAD) (1, 2). In practice, one of the main opportunities for supplementing this age group is at the time of measles vaccination; this vaccination is usually given as soon as possible after the infant is 9 mo old. However, because a high proportion of all child deaths occur before the age of 9 mo, the effects of earlier vitamin A supplementation were investigated, with conflicting results (3 7). Two trials in Asia gave IU vitamin A to infants within the first few days of life and found 22 64% reductions in infant mortality (3, 4). A more recent trial involving 9208 mother-infant pairs in a less vitamin A deficient population in Zimbabwe showed that giving IU vitamin A to mothers and IU vitamin A to their infants, within 96 h of birth, did not lower infant mortality [hazard ratio: 1.28 (95% CI: 0.83, 1.98)] (5). A further study explored the effect of supplementation in slightly older infants and made use of the Expanded Program on Immunization (EPI) of the World Health Organization (WHO). EPI routinely delivers 3 doses of diphtheria-pertussis-tetanus/ oral polio vaccines (DPT/OPV) (sometimes with hepatitis B or Haemophilus influenzae vaccine or both) to millions of infants aged 6 mo living in VAD-endemic settings. The WHO coordinated a randomized, double-blind, placebo-controlled trial in 9424 infants to investigate the effects of supplementation with IU vitamin A at the time of these routine vaccinations at 6, 10, and 14 wk of age. This study, conducted in Ghana, India, and Peru, showed that the intervention was safe but also that it produced only a modest and short-lived improvement in vitamin A status when infants were aged 6 mo (serum retinol 0.70 mol/l; 29.9% of supplemented infants and 37.1% of unsupplemented infants; risk difference: 7.2% (95% CI: 14.3%, 0.2%). The benefits were no longer apparent on biochemical testing when the infants were 9 mo old (8). Supplementation of mothers has been shown to improve the vitamin A intake of breastfeeding infants. Four studies, all in Asia (9 12), showed that a single vitamin A dose of to 1 From the Ifakara Health Research and Development Centre, Ifakara, Tanzania (BI, HM, HU, EK, HM, and DMS); the St Francis Designated District Hospital, Ministry of Health, Ifakara, Tanzania (WB); the Nutritional Intervention Research Unit, Medical Research Council, Parow, Republic of South Africa (PvJ); the Hospital Clinic Center for International Health, University of Barcelona, Barcelona, Spain (JJA and DMS); the Manhiça Health Research Center, Manhiça, Mozambique (JJA); and the London School of Hygiene & Tropical Medicine, London, United Kingdom (DR and DMS). 2 Supported by Immunization Vaccines & Biologicals (the World Health Organization and the United Nations Foundation), Sight & Life (Hoffman-la Roche Ltd), Micronutrient Initiative, the Canadian International Development Agency, and the United Nations Children s Fund (UNICEF). 3 Reprints not available. Address correspondence to D Schellenberg, Department of Infectious & Tropical Diseases, London School of Hygiene & Tropical Medicine, London WC1E 7HT, United Kingdom. david.schellenberg@lshtm.ac.uk. Received October 20, Accepted for publication January 15, Am J Clin Nutr 2007;85: Printed in USA American Society for Nutrition
2 IFAKARA VITAMIN A INFANT TRIAL IU given to mothers within 3 wk of delivery significantly increased their breast-milk retinol concentration for 3 8 mo. Combined supplementation of mothers shortly after delivery and of their young children may help improve children s vitamin A status. An assessment of vitamin A requirements in infancy suggested that supplementation of women after delivery with 2 doses of IU and of infants with 3 doses of IU in the first 6 mo of life would be sufficient to prevent VAD in most infants (13). Metabolic studies also suggested that this dosing regimen was very unlikely to be toxic (14). We conducted a double-blind, randomized, 2-arm study to compare the safety and efficacy of the previously tested low-dose regimen ( IU at the time of DTP/OPV doses 1, 2, and 3) with those of the suggested high-dose regimen (a second IU dose to mothers when their infants were 1 mo old and IU to infants at the time of DTP/OPV doses 1, 2, and 3). SUBJECTS AND METHODS Setting The study was conducted in Ifakara, in southern Tanzania, where 56% of infants aged 6 mo 6 y had serum retinol concentrations 0.70 mol/l in 1997 (15). The town is served by the St Francis Designated District Hospital (SFDDH) and an adjacent Maternal and Child Health Clinic (MCHC). The MCHC provides routine EPI vaccinations: bacille Calmette-Guerin (BCG) and OPV are given immediately after birth; doses of DPT/OPV are given at age 1, 2, and 3 mo; and measles vaccination is given at age 9 mo. The study area covers a 6-km radius from the SFDDH, which ensures good access to the hospitalbased clinical surveillance systems. Design and intervention This 2-arm, randomized, double-blind trial was designed to compare the safety and efficacy of 2 vitamin A supplementation regimens. In the low-dose group, mothers received g ( IU) vitamin A (as vitamin A palmitate) at the time of their infant s BCG vaccination, and their infants received doses of 7500 g ( IU) at the time of the 3 DPT/OPV vaccinations. In the high-dose group, mothers received an additional dose of g ( IU) when their infant received the first DPT/OPV vaccination, and their infants received 3 doses of g ( IU) at the same time as the 3 DPT/OPV vaccinations. In accordance with national guidelines, all infants received g ( IU) vitamin A at the time of measles vaccination. Vitamin A capsules providing different doses were manufactured by Accucaps Industries (Windsor, Canada) ( and IU) and RpScherer (Aprilia, Italy) ( IU), and were supplied to the project by the WHO. All capsules contained a small amount of vitamin E (10 IU in the IU and IU capsules, 20 IU in the IU capsules, and 40 IU in the IU capsules). Capsules were transported and stored in lightproof boxes and kept in an air-conditioned room at the trial site. Subject recruitment and randomization and administration of vitamin A Recruitment began in April 2002 and lasted until March Mothers who were resident in the study area and brought their infants to the SFDDH and MCHC for BCG vaccination within 7 d of birth were invited to participate in the trial. Details of the trial were explained, and a series of standardized questions were asked to ensure adequate understanding before seeking written informed consent. Demographic information was documented before the lowest available study number was assigned to mother-infant pairs. Individual randomization was achieved by using a list of study numbers that had been randomly assigned to an intervention arm in blocks of 10, generated by the Data and Safety Monitoring Board. Mothers were invited to bring their infant to the MCHC for vaccination and vitamin A dosing on a specific date when the infant would be 1 mo old. Before each supplementation, project clinical officers examined mothers or their infants (or both), and a standardized morbidity questionnaire was completed. The contents of a vitamin A capsule, labeled only with the participant s study number, were squeezed into the recipient s mouth. Mother-infant pairs who had not attended the MCHC within 1 wk of their appointment were visited at home to confirm residence and survival status and, if present, were reminded to attend the study clinic. All mothers provided written informed consent. The trial received ethical approval from the Ifakara Health Research & Development Centre, the National Medical Research Coordinating Committee of Tanzania, the London School of Hygiene and Tropical Medicine, and the WHO; it was registered on the Current Controlled Trials Register (number ISRCTN ). Follow-up The timing of the interventions and follow-up is summarized in Figure 1. Safety was assessed throughout the study by passive case detection with the use of a clinical surveillance system (CSS) and was actively assessed at the participants home on each of the 2 d after each supplementation. The primary assessment of efficacy was based on biochemical indicators of vitamin A status, for whose measurement blood samples were collected during cross-sectional surveys when the infants were aged 6 and 9 mo. Assessment of safety Mothers were encouraged to bring their infant to a CSS location if the infant experienced any illness at any time during follow-up. The CSS was established at SFDDH in 1994 to document all outpatient attendance of study infants and any pediatric admissions, around the clock, every day of the year. Specially trained clinical officers examined the infants and completed a standardized coded morbidity questionnaire including (but not limited to) such factors as the presence or absence of fever, bulging fontanelle, diarrhea, clinical malaria, and anemia. The CSS thus generated detailed clinical data for the assessment of safety and secondary efficacy endpoints. Necessary laboratory tests and appropriate management were provided for free. Active clinical surveillance was by fieldworkers who visited the mothers and their infants at home for 2 consecutive days after each maternal and infant supplementation. A standard form was completed to document any symptoms or clinical signs of illness. Study participants found to have potentially serious clinical symptoms or signs were asked to visit the hospital for reexamination by a study clinician.
3 1314 IDINDILI ET AL Active Clinical Surveillance Demographic Visit Demographic Visit Active Clinical Surveillance Age (mo) Before recruitment Child: BCG/OPV Mother: Low: Vit A IU High: Vit A IU Child: Child: DTP/OPV-2 Low: Vit A IU High: Vit A IU DTP/OPV-3 Low: Vit A IU High: Vit A IU Cross-sectional Survey 1 Measles Child: Low: Vit A IU High: Vit A IU Cross-sectional Survey 2 Mother: Low: Placebo High: Vit A IU Child: DTP/OPV-1 Low: Vit A IU High: Vit A IU FIGURE 1. Individual timeline for passive case detection by outpatient and inpatient surveillance systems. Low, low-dose vitamin A supplementation regimen; High, high-dose vitamin A supplementation regimen; Vit A, vitamin A; BCG, bacille Calmet-Guérin vaccine; OPV, oral polio vaccine; DTP, dipththeria/pertussis/tetanus vaccine. Assessment of efficacy Vitamin A status was assessed by analysis of capillary blood samples collected from infants during cross-sectional surveys at age 6 and 9 mo. A fieldworker invited mother-infant pairs to attend these surveys, having documented that the infant was alive and still resident in the study area. Those who did not attend were reinvited each week for a total of 4 wk. Breast milk was collected into 50-mL screw-top centrifuge tubes (Falcon; Becton Dickinson, Franklin Lakes, NJ) for subsequent analysis of retinol content. A single oral dose of 3,4-didehydroretinyl acetate (DRA) was administered to mothers (8.8 mol) or infants (5.3 mol) (or both) for assessment of the modified relative doseresponse (MRDR test) as described previously (16). Fingerprick capillary blood samples were collected into 500- L microtainer tubes (Becton Dickinson) 3 h after administration of DRA. Height and weight of infants were also recorded by using standard methods (17). Laboratory procedures Blood and milk samples were immediately stored on ice in cool boxes and transported to the laboratory within 2 h. Exposure to light was kept to a minimum. In the base laboratory, serum was immediately separated from whole blood, and aliquots of serum and homogenized milk were stored at 20 C in 2-mL screw-top freezer tubes (Nunc, Roskilde, Denmark). Frozen samples were transported to the Nutritional Intervention Research Unit of the Medical Research Council (Cape Town, South Africa) for analysis by using standard HPLC procedures (18, 19). Data management and statistical analysis Data were double-entered, and daily cross-checking routines were used to detect and correct any discrepancies. A compact disk containing the cleaned and locked database files was exchanged for the treatment randomization code, held by the Data and Safety Monitoring Board. Analyses were conducted, in a masked manner, according to a previously-agreed-upon analytic plan using STATA software (version 7; Stata Corp, College Station, TX). EPINUT software (version 6; EPI Info, Atlanta, GA) was used to generate anthropometric indexes. Before the study, a total of 780 infants (aged 6 mo) were estimated to provide 90% power to detect a relative decrease in the prevalence of VAD from 37% to 25%, at a 5% significance level. The primary definition of VAD was an MRDR value [ie, the ratio of serum 3,4-didehydroretinol to retinol] 3 h after the DRA dose was administered; VAD was evaluated in all randomly assigned infants who had valid laboratory results. As a secondary endpoint, VAD was also defined as a serum retinol concentration 0.70 mol/l; it was considered severe at a concentration 0.35 mol/l. These and other categorical variables were compared by using the chi-square test. Mean breast milk and serum retinol concentrations were compared by using the t test. Separate analyses evaluated endpoints at 6 and 9 mo of age. Safety was analyzed by comparing the incidence rates of clinical signs of illnesses and diagnoses documented by the CSS during the 28 d after each supplementation and over all time at risk. Time at risk began from the time of recruitment and continued until whichever occurred first: a clinical episode or censoring due to withdrawal, death, or end of follow-up. Poisson regression models with random effects to take into account between-infant heterogeneity were used to compare incidence rates. The proportions of participants with clinical signs of illnesses and symptoms detected actively within 48 h after each supplementation were also compared. The effect of the intervention on anemia and clinical malaria was evaluated by comparing incidence rates between the 2 groups. Anemia was defined as either mild [a packed cell volume (PCV) 33%], severe (PCV 25%), or life-threatening (PCV 15%). Anthropometric indexes were assessed on all randomly assigned infants at ages 6 and 9 mo by comparing the proportion of infants in each group with a weightfor-age z score (WAZ) 2, height-for-age z score (HAZ) 2, and weight-for-height z score (WHZ) 2. Quality control Standardized quality-control procedures were used to ensure consistency in data collection and assessment of adverse events. Previously coded forms with labels bearing identification information were used for all routine contacts. Training in the assessment of clinical signs of illness and monitoring of clinical officers and fieldworkers was continued throughout the trial. Field activities were supervised through regular accompanied and repeated interviews. Vitamin A capsules for quality control were transported at regular intervals to the Institute of Nutritional Research (University of Oslo, Oslo, Norway). These analyses confirmed that the and IU capsules contained a
4 IFAKARA VITAMIN A INFANT TRIAL 1315 satisfactory amount of retinyl palmitate throughout the trial period. The IU capsules also had 80% potency up to July 2003, when the last dose of this type of capsule had already been given. The IU capsules that were tested were also fully potent up to and through February However, between February and July 2003, the vitamin A content of the IU capsules declined markedly, so that the 33 capsules that were analyzed between August 2003 and January 2004 contained only IU (mean: IU), or 32% of the expected amount. Taking into consideration the results of similar analyses from a sister trial in Kintampo, Ghana, the investigators, the WHO, and the trial s Data and Safety Monitoring Board reached a consensus, before the code was broken, that the IU capsules in Ifakara degraded rapidly after May Hence, secondary statistical analyses were performed by excluding the 5% of infants who received doses after 31 May 2003, and tertiary analyses were performed after exclusion of the 30% of infants who received doses after 31 January In addition, assessments of both efficacy and safety were evaluated by taking into consideration the time elapsed since the date on which recruitment started, by using the likelihood ratio test comparing a model with the effect of the vitamin A with a model in which the cohort had been split into tertiles by date of recruitment, and by looking for an interaction between the date of recruitment tertile and efficacy. RESULTS Recruitment of 780 mother-infant pairs took from April 2002 to March 2003, and follow-up was completed by the end of Completeness of follow-up (Figure 2) and baseline characteristics (Table 1) did not differ significantly between the 2 groups. Infants who did not complete follow-up were older at the time of their first dose, less likely to be exclusively breastfeed, and more likely to use ITNs, and they had a slightly lower mean serum retinol than did infants who completed follow-up (data not shown). However, none of the baseline comparators differed significantly between the 2 arms for infants who completed follow-up and contributed to the following analyses. The incidence of all clinical signs of illnesses, symptoms, and diagnoses detected by the CSS did not differ significantly between the 2 groups of infants during the 28 d after each supplementation (data not shown) or during all time at risk (Table 2). Active case detection documented a significantly (P 0.01) lower prevalence of fever after the first dose of vitamin A in infants in the high-dose arm [(147 of 308 (48%)] than in the infants in the low-dose arm [183 of 318 (58%); risk ratio: 0.83 (95% CI: 0.71, 0.96)]. No similar pattern was seen after other doses, and the prevalence of other signs and symptoms (eg, fits, jaundice, and chest indrawing) was similar after all doses (data not shown). The proportion of infants with inadequate vitamin A liver stores (MRDR 0.060) at 6 mo was 43% in the high-dose arm and 47% in the low-dose arm (Table 3). VAD of different severities (defined according to serum retinol and mean serum retinol concentrations) also did not differ significantly between the 2 groups. The incidence of malaria and anemia during follow up, the cross-sectional prevalence of anemia and malaria, and the anthropometric indexes did not differ significantly between the 2 groups (Table 4). The secondary and tertiary analyses excluding data from infants who received vitamin A supplements after May 31, 2003 and January 31, 2003, respectively, did not significantly change these results (data not shown). DISCUSSION This double-blind randomized controlled trial has shown that supplementation of mothers with a second dose of IU vitamin A and of their infants with IU at the time of routine vaccinations at 1, 2, and 3 mo of age was safe but not more efficacious than a lower-dose regimen. Neither biochemical markers of vitamin A status nor mean breast-milk retinol concentrations differed significantly between infants in the 2 study arms at 6 and 9 mo of age. Clinical endpoints detected passively and actively did not suggest any consistent clinical benefits of the high-dose regimen. These results are not what had been expected (13), and it is important to consider all possible explanations. The degradation of vitamin A capsules, which was most marked in the IU capsules, is a prime concern. However, the analyses excluding infants who may have received lower amounts of vitamin A than intended reassured us that the deterioration of the capsules was not the reason for the lack of efficacy of the intervention. This finding shows the importance of the quality-control assessments that were included in this clinical trial and emphasizes the importance of such checks both in future clinical trials and in routine supplementation programs. Another possibility is that the measures of VAD used in this study do not accurately reflect the vitamin A status of the very young African infants involved. Young infants are subject to major physiologic changes and a high incidence of infectious disease. The integrity of serum retinol as a measure of VAD may be compromised, for example, by elevated acute phase proteins resulting from infections, which could lead to an underestimate of serum retinol concentration (20) and a reduction of the specificity of serum retinol as a marker of VAD. This reduced specificity would tend to downwardly bias the efficacy estimates based on this measure. However, MRDR tests were used as the primary outcome measure, and they are not affected by subclinical inflammation (21, 22). We collected blood samples for MRDR evaluation 3 h after DRA dosing. It is now clear that samples should be collected 4 h after supplementation because earlier sampling reduces the sensitivity of the test. Nevertheless, we found a high prevalence of VAD and, because the test specificity would be relatively high, we conclude that the timing of MRDR blood sample collection is unlikely to explain the lack of efficacy in this study. The gold standard measure of vitamin A status requires the taking of liver tissue for biopsy, which is too invasive an investigation for a trial such as this. The MRDR test is the most appropriate indicator of vitamin A status, and it has been shown to work well in older populations when the test is tightly controlled and care taken with its interpretation. However, its use in field settings involving young African infants merits further investigation. The study was not placebo controlled because it was not considered ethical to randomly assign some infants to placebo when the low-dose regimen had been shown in a previous trial to have a marginal beneficial effect. Hence, our efficacy estimate may be lower than it would be had this been a placebo-controlled trial. Nevertheless, the prevalence of VAD in the 2 arms was high, and it did not differ significantly between the groups: it seems unlikely that we missed a substantial effect of the high-dose
5 1316 IDINDILI ET AL FIGURE 2. Trial profile. CSV-1, cross-sectional survey conducted at 6 mo of age; CSV-2, cross-sectional survey conducted at 9 mo of age.
6 IFAKARA VITAMIN A INFANT TRIAL 1317 TABLE 1 Baseline characteristics of study subjects 1 Vitamin A supplementation group Variable High-dose (n 390) Low-dose (n 390) P Male [n (%)] 215 (55) 197 (51) Exclusive breastfeeding at age 1 mo [n (%)] 337 (86) 338 (87) VAD (MRDR 0.06) before child s first dose [n (%)] 255 (84) 238 (82) Maternal education [n (%)] None 61 (16) 71 (18) Primary 314 (81) 301 (77) Secondary 15 (4) 18 (5) Distance from child s home to hospital 1st tertile, 0.4 km 112 (34) 124 (37) 2nd tertile, km 112 (34) 107 (32) 3rd tertile, km 108 (33) 100 (30) Insecticide-treated net use [n (%)] 334 (86) 337 (86) Age at dose 1 (mo) Mean birth weight (kg) Serum retinol concentration before child s first dose ( mol/l) Breast milk retinol after delivery ( mol/l) VAD, vitamin A deficiency; MRDR, modified relative dose response. Chi-square test. x SD (all such values). 4 t Test. regimen. It also seems unlikely that losses to follow-up biased our efficacy estimate, because there were no differences in baseline characteristics between the groups of infants completing follow-up. The study benefited from the availability of a CSS, which generated detailed clinical safety and efficacy information. The data generated by this passive case detection system was qualitatively different from that produced by the active case detection used to identify any immediate, short-term side effects of the supplementation. The CSS documented episodes of illness that were severe enough for a child to be brought to the clinic, and it covered the whole 9 mo of follow up for each infant rather TABLE 2 Incidence of signs and symptoms detected by clinical surveillance system from recruitment to end of time at risk 1 High dose Low dose Comparison Outcome Episodes PYAR Rate 2 Episodes PYAR Rate 2 RR 95% CI P Fever , Runny nose , Diarrhea , Vomiting , Coughing , Fast breathing , Convulsion , Pallor , Jaundice Nasal flaring , Chest indrawing , Pus in ears , Bulging fontanelle Wheezing , Stiff neck , Hepatomegaly , Splenomegaly , Oedema , Oral candidiasis , Chest crackles , PYAR, person-years at risk; RR, relative risk. 2 Number of episodes per person-year at risk.
7 1318 IDINDILI ET AL TABLE 3 Prevalence of vitamin A deficiency and mean serum retinol concentration at age 6 and 9 mo 1 Outcome High dose Low dose Relative risk (95% CI) P Age6mo MRDR (125/293) 2 47 (132/282) 0.91 (0.76, 1.09) Serum retinol 0.70 mol/l 36 (111/306) 41 (121/296) 0.89 (0.73, 1.09) Serum retinol 0.35 mol/l 1.0 (3/306) 2.4 (7/296) 0.41 (011, 1.59) Serum retinol ( mol/l) Breast milk retinol ( mol/l) Age9mo MRDR (113/278) 40 (108/269) 1.01 (0.83, 1.24) Serum retinol 0.70 mol/l 29 (85/292) 32 (90/286) 0.93 (0.72, 1.18) Serum retinol 0.35 mol/l 1.7 (5/292) 1.8 (5/286) 0.98 (0.29, 3.35) Serum retinol ( mol/l) Breast milk retinol ( mol/l) MRDR, modified relative dose response. 2 %; n/n in parentheses (all such values). The differences in denominators reflect the number of samples in which no estimate of 3,4-didehydroretinol was possible for technical reasons. 3 Chi-square test. 4 x SD (all such values). 5 t Test. than just the 2 d after supplementation. The fact that there was no increase in either the short-term side effects or passively detected illnesses, including bulging fontanelle, provides reassurance that the high-dose regimen was as safe as the lowdose regimen. However, the lack of any decrease in incidence of illnesses detected through the CSS suggests that the highdose regimen did not confer any clinically important absolute effect on morbidity. The best approach to improving the vitamin A status of young infants remains unclear. It may be that even higher doses of vitamin A given to infants at the time of routine vaccinations, to mothers of young children, or both would have an effect. Alternatively, given the success of immunization campaigns to increase vitamin A supplementation coverage, it may be useful to assess the effects of these on vitamin A status and to consider supplementing infants aged 6 mo in such campaigns. Another TABLE 4 Anthropometric, malaria, and anemia indexes 1 Outcome High dose Low dose Relative risk (95% CI) P Prevalence at age 6 mo [n/n (%)] WAZ 2 12/305 (3.9) 15/289 (5.2) 0.76 (0.36, 1.59) HAZ 2 29/309 (9.4) 18/297 (6.1) 1.55 (0.88, 2.73) WHZ 2 3/304 (1.0) 7/289 (2.4) 0.41 (0.11, 1.56) Moderate anemia, PCV 33% 125/311 (40.2) 121/304 (39.8) 1.01 (0.83, 1.23) Severe anemia, PCV 25% 3/311 (1.0) 3/304 (1.0) 0.98 (0.20, 4.81) Prevalence at age 9 mo WAZ 2 38/287 (13.2) 43/272 (15.8) 0.84 (0.56, 1.25) HAZ 2 39/297 (13.1) 31/279 (11.1) 1.18 (0.76, 1.84) WHZ 2 12/288 (4.2) 12/271 (4.4) 0.94 (0.43, 2.06) Mild anemia, PCV 33% 87/298 (29.2) 89/288 (30.9) 0.94 (0.74, 1.21) Severe anemia, PCV 25% 5/298 (1.7) 5/288 (1.7) 0.97 (0.28, 3.30) Incidence during all time at risk after recruitment First malaria episode Events/PYAR 46/ /242.1 Incidence Incidence rate ratio (95% CI) 1.25 (0.81, 1.93) First mild anemia episode Events/PYAR 50/ /241.3 Incidence Incidence rate ratio (95% CI) 1.11 (0.74, 1.66) WAZ, weight-for-age z score; HAZ, height-for-age z score; WHZ, weight-for-height z score; PCV, packed cell volume; PYAR, person-years at risk. 2 Chi-square test.
8 IFAKARA VITAMIN A INFANT TRIAL 1319 approach would be to further evaluate the effects of early neonatal supplementation. The high-dose supplementation regimen we evaluated has already been recommended by the International Vitamin A Consultative Group [IVACG (23)]. Although not previously tested in a clinical trial, the recommendation was based on theoretical calculations of its likely effect on infants vitamin A status, the potential for reductions in infant morbidity and mortality, and a low probability of any important toxic side effects. Our results do not support this current IVACG recommendation. Instead, our findings highlight the difficulties inherent in balancing the urgency of reducing high infant mortality rates by implementing interventions that seem likely to be beneficial against the time taken to conduct appropriate studies to gain empirical evidence of their efficacy. However, the EPI offers unparalleled opportunities to deliver health interventions to infants the age group of children who suffer the highest mortality rates and it is essential that the safety and efficacy of any potential intervention are tested in carefully conducted clinical trials before implementation. In conclusion, higher-dose vitamin A supplementation was well tolerated by mothers and infants, but it failed to improve the vitamin A status or the incidence of illness episodes in trial participants. The stability of the vitamin A capsules is of great concern and draws attention to the need for quality-assurance checks of such capsules in clinical trials and routine supplementation programs. Specific validation of the biochemical indicators of vitamin A status may be useful in very young infants. This study highlights the potential dangers of basing policy recommendations on theoretical calculations and extrapolation from existing empirical data. We are grateful to the infants and families of study participants, to the staff of the St Francis Designated District Hospital and the Ifakara Maternal and Child Health Clinic, and to Eldrich Harmse (Nutritional Intervention Research Unit, Medical Research Council, South Africa) for performing the modified relative dose-response laboratory analyses. We thank the trial s Data & Safety Monitoring Board for their monitoring of the safety data. The authors responsibilities were as follows DR, EK, and DS: study design; BI and EK: coordinating field work; HM and JJA: statistical analysis; WB: operated the clinical surveillance system used to capture safety data; HU and PvJ: coordinated laboratory activities; HM: assured the availability of institutional resources in Tanzania and assisted with interpretation of data and data dissemination to Tanzanian partners; DR: provided support on technical issues and field activities; DMS: coordinated the research team; and BI and DS: wrote the manuscript, with editing and critiques from all authors. None of the authors had a personal or financial conflict of interest. REFERENCES 1. Beaton GH, Martorell R, L Abbé KA, et al. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. Summary report of research findings presented at the Micronutrient Forum. Internet: archives/scnnews09/ch4.htm (accessed 13 May 2006). 2. Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality. A meta-analysis. JAMA 1993;269: Humphrey JH, Agoestina T, Wu L, et al. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr 1996;128: Rahmathullah L, Tielsch JM, Thulasiraj RD, et al. Impact of supplementing newborn infants with vitamin A on early infant mortality: community-based randomized trial in southern India. BMJ 2003;327: Malaba LC, Iliff PJ, Nathoo KJ, et al. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am J Clin Nutr 2005;81: Daulaire NM, Starbuck ES, Houston RM, Church MS, Stukel TA, Pandey MR. Childhood mortality after a high dose of vitamin A in a high risk population. BMJ 1992;304: West KP Jr, Katz J, Shrestha SR, et al. Mortality of infants 6moofage supplemented with vitamin A: a randomized, double-masked trial in Nepal. Am J Clin Nutr 1995;62: WHO/CHD Immunisation-Linked Vitamin A Supplementation Study Group. Randomised trial to assess benefits and safety of vitamin A supplementation linked to immunisation in early infancy. Lancet 1998; 352: Stoltzfus RJ, Hakimi M, Miller KW, et al. High dose vitamin A supplementation of breast-feeding Indonesian mothers: effects on the vitamin A status of mother and infant. J Nutr 1993;123: Basu S, Sengupta B, Paladhi PK. Single megadose vitamin A supplementation of Indian mothers and morbidity in breastfed young infants. Postgrad Med J 2003;79: Rice AL, Stoltzfus RJ, de Francisco A, Chakraborty J, Kjolhede CL, Wahed MA. Maternal vitamin A or -carotene supplementation in lactating Bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J Nutr 1999;129: Roy SK, Islam A, Molla A, Akramuzzaman SM, Jahan F, Fuchs G. Impact of a single megadose of vitamin A at delivery on breastmilk of mothers and morbidity of their infants. Eur J Clin Nutr 1997;51: Humphrey JH, Rice AL. Vitamin A supplementation of young infants. Lancet 2000;356: Allen LH, Haskell M. Vitamin A requirements of infants under six months of age. Food Nutr Bull 2001;22: Tanzanian Ministry of Health. The national vitamin A deficiency control programme: preliminary report on the national vitamin A survey Dar es Salaam, Tanzania: Tanzanian Food and Nutrition Centre, (Tanzanian Food and Nutrition Centre Report no ) 16. Tanumihardjo SA, Cheng JC, Permaesih D, et al. Refinement of the modified-relative-dose-response test as a method for assessing vitamin A status in a field setting: experience with Indonesian children. Am J Clin Nutr 1996;64: United Nations Department of Technical Co-operation, Development and Statistical Office. How to weigh and measure children in Household Surveys: assessing nutritional status of young children. New York, NY: United Nations, Valentine AR, Tanumihardjo SA. Adjustments to the modified relative dose response (MRDR) test for assessment of vitamin A status minimize the blood volume used in piglets. J Nutr 2004;134: Tanumihardjo SA, Penniston KL. Simplified methodology to determine breast milk retinol concentrations. J Lipid Res 2002;43: Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003;362: Tanumihardjo SA, Permaesih D, Muherdiyantiningsih, et al. Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. J Nutr 1996;126: Wieringa FT, Dijkhuizen MA, West CE, Northrop-Clewes CA, Muhilal. Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J Nutr 2002;132: The International Life Science Institute (ILSI) Research Foundation s Human Nutrition Institute, the International Vitamin A Consultative Group. The Annecy accords to assess and control vitamin A deficiency: summary of recommendations and clarifications. Washington, DC: ILSI, 2002.
Protocol Synopsis. Administrative information
 Protocol Synopsis Item (SPIRIT item no.) Administrative information Title (1) Introduction Description of research question (6a) Description An optimal schedule for the post-polio eradication era: multicentre
Protocol Synopsis Item (SPIRIT item no.) Administrative information Title (1) Introduction Description of research question (6a) Description An optimal schedule for the post-polio eradication era: multicentre
Cite this article as: BMJ, doi: /bmj (published 23 November 2005)
 Randomised study of effect of different doses of vitamin A on childhood morbidity and mortality Christine Stabell Benn, Cesario Martins, Amabelia Rodrigues, Henrik Jensen, Ida Maria Lisse, Peter Aaby Abstract
Randomised study of effect of different doses of vitamin A on childhood morbidity and mortality Christine Stabell Benn, Cesario Martins, Amabelia Rodrigues, Henrik Jensen, Ida Maria Lisse, Peter Aaby Abstract
Prevalence of Vitamin A Deficiency among 6 months to 5 years old Children
 Prevalence of Vitamin A Deficiency among 6 months to 5 years old Children Htin Lin, May Khin Than, Khaing Mar Zaw, Theingi Thwin, Moh Moh Hlaing I. Introduction Myanmar, likewise other South-East Asian
Prevalence of Vitamin A Deficiency among 6 months to 5 years old Children Htin Lin, May Khin Than, Khaing Mar Zaw, Theingi Thwin, Moh Moh Hlaing I. Introduction Myanmar, likewise other South-East Asian
Postpartum Vitamin A Supplementation: Evaluating the Evidence for Action
 TECHNICAL BRIEF Postpartum Vitamin A Supplementation: Evaluating the Evidence for Action Amy L. Rice, PhD BACKGROUND Scientific and policy-making groups have issued somewhat conflicting statements about
TECHNICAL BRIEF Postpartum Vitamin A Supplementation: Evaluating the Evidence for Action Amy L. Rice, PhD BACKGROUND Scientific and policy-making groups have issued somewhat conflicting statements about
Afghanistan: WHO and UNICEF estimates of immunization coverage: 2017 revision
 Afghanistan: WHO and UNICEF estimates of immunization coverage: 2017 revision July 7, 2018; page 1 WHO and UNICEF estimates of national immunization coverage - next revision available July 15, 2019 data
Afghanistan: WHO and UNICEF estimates of immunization coverage: 2017 revision July 7, 2018; page 1 WHO and UNICEF estimates of national immunization coverage - next revision available July 15, 2019 data
Systematic review of the non- specific effects of BCG, DTP and measles containing vaccines
 Systematic review of the non- specific effects of BCG, DTP and measles containing vaccines Higgins JPT, Soares- Weiser K, Reingold A 13 March 2014 Contents 1 Executive Summary... 3 2 Background... 4 3
Systematic review of the non- specific effects of BCG, DTP and measles containing vaccines Higgins JPT, Soares- Weiser K, Reingold A 13 March 2014 Contents 1 Executive Summary... 3 2 Background... 4 3
CHILD HEALTH RECORD BOOK for Girls
 Department of Health CHILD HEALTH RECORD BOOK for Girls EVERY CHILD NEEDS 5 MEALS EVERY DAY Ask your clinic Sister which foods are best to make your child grow well. GROWING STRONG WITH OUR NATION NAME:...
Department of Health CHILD HEALTH RECORD BOOK for Girls EVERY CHILD NEEDS 5 MEALS EVERY DAY Ask your clinic Sister which foods are best to make your child grow well. GROWING STRONG WITH OUR NATION NAME:...
Proceedings of the XX International Vitamin A Consultative Group Meeting
 Proceedings of the XX International Vitamin A Consultative Group Meeting Recommendations for Vitamin A Supplementation 1 David A. Ross 2 Infectious Diseases Epidemiology Unit, London School of Hygiene
Proceedings of the XX International Vitamin A Consultative Group Meeting Recommendations for Vitamin A Supplementation 1 David A. Ross 2 Infectious Diseases Epidemiology Unit, London School of Hygiene
Postpartum Vitamin A Supplementation: Evaluating the Evidence for Action
 TECHNICAL BRIEF Postpartum Vitamin A Supplementation: Evaluating the Evidence for Action Amy L. Rice, PhD BACKGROUND Scientific and policy-making groups have issued somewhat conflicting statements about
TECHNICAL BRIEF Postpartum Vitamin A Supplementation: Evaluating the Evidence for Action Amy L. Rice, PhD BACKGROUND Scientific and policy-making groups have issued somewhat conflicting statements about
Effect of vitamin A supplementation with BCG vaccine at birth on vitamin A status at 6 wk and 4 mo of age 1 3
 Effect of vitamin A supplementation with BCG vaccine at birth on vitamin A status at 6 wk and 4 mo of age 1 3 Ane B Fisker, Ida M Lisse, Peter Aaby, Juergen G Erhardt, Amabelia Rodrigues, Bo M Bibby, and
Effect of vitamin A supplementation with BCG vaccine at birth on vitamin A status at 6 wk and 4 mo of age 1 3 Ane B Fisker, Ida M Lisse, Peter Aaby, Juergen G Erhardt, Amabelia Rodrigues, Bo M Bibby, and
WHITE PAPER. A Summary of Global Immunization Coverage through David W Brown, Anthony H Burton, Marta Gacic-Dobo (alphabetical order)
 WHITE PAPER A Summary of Global Immunization Coverage through 2013 David W Brown, Anthony H Burton, Marta Gacic-Dobo (alphabetical order) for the WHO and UNICEF working group for monitoring national immunization
WHITE PAPER A Summary of Global Immunization Coverage through 2013 David W Brown, Anthony H Burton, Marta Gacic-Dobo (alphabetical order) for the WHO and UNICEF working group for monitoring national immunization
Selected vaccine introduction status into routine immunization
 Selected introduction status into routine infant immunization worldwide, 2003 This report summarizes the current status of national immunization schedules in 2003, as reported by Member States in the /UNICEF
Selected introduction status into routine infant immunization worldwide, 2003 This report summarizes the current status of national immunization schedules in 2003, as reported by Member States in the /UNICEF
h e a l t h l i n e ISSN X Volume 3 Issue 1 January-June 2012
 Original article A study of risk factors of acute respiratory tract infection (ARI) of under five age group in uban and rural communities of Ahmedabad district, Gujarat Bipin Prajapati 1, Niti Talsania
Original article A study of risk factors of acute respiratory tract infection (ARI) of under five age group in uban and rural communities of Ahmedabad district, Gujarat Bipin Prajapati 1, Niti Talsania
2011 UGANDA DHS - ADDENDUM TO CHAPTER 11
 2011 UGANDA DHS - ADDENDUM TO CHAPTER 11 11.9 VITAMIN A 11.9.1 Background and Methodology Vitamin A is an essential micronutrient for vision, for the maintenance of epithelial cells, and for regulation
2011 UGANDA DHS - ADDENDUM TO CHAPTER 11 11.9 VITAMIN A 11.9.1 Background and Methodology Vitamin A is an essential micronutrient for vision, for the maintenance of epithelial cells, and for regulation
Global Update. Reducing Mortality From Major Childhood Killer Diseases. infant feeding, including exclusive breastfeeding.
 INDIAN PEDIATRICS VOLUME 35-FEBRUARY 1998 Global Update Reducing Mortality From Major Childhood Killer Diseases Seven out of 10 childhood deaths in developing countries can be attributed to just five main
INDIAN PEDIATRICS VOLUME 35-FEBRUARY 1998 Global Update Reducing Mortality From Major Childhood Killer Diseases Seven out of 10 childhood deaths in developing countries can be attributed to just five main
Vitamin A Deficiency: Counting the Cost in Women s Lives
 TECHNICAL BRIEF Vitamin A Deficiency: Counting the Cost in Women s Lives Amy L. Rice, PhD INTRODUCTION Over half a million women around the world die each year from conditions related to pregnancy and
TECHNICAL BRIEF Vitamin A Deficiency: Counting the Cost in Women s Lives Amy L. Rice, PhD INTRODUCTION Over half a million women around the world die each year from conditions related to pregnancy and
Pakistan: WHO and UNICEF estimates of immunization coverage: 2017 revision
 Pakistan: WHO and UNICEF estimates of immunization coverage: 2017 revision July 7, 2018; page 1 WHO and UNICEF estimates of national immunization coverage - next revision available July 15, 2019 data received
Pakistan: WHO and UNICEF estimates of immunization coverage: 2017 revision July 7, 2018; page 1 WHO and UNICEF estimates of national immunization coverage - next revision available July 15, 2019 data received
Journal Club 3/4/2011
 Journal Club 3/4/2011 Maternal HIV Infection and Antibody Responses Against Vaccine-Preventable Diseases in Uninfected Infants JAMA. 2011 Feb 9;305(6):576-84. Jones et al Dept of Pediatrics, Imperial College,
Journal Club 3/4/2011 Maternal HIV Infection and Antibody Responses Against Vaccine-Preventable Diseases in Uninfected Infants JAMA. 2011 Feb 9;305(6):576-84. Jones et al Dept of Pediatrics, Imperial College,
PROGRESS REPORT ON CHILD SURVIVAL: A STRATEGY FOR THE AFRICAN REGION. Information Document CONTENTS
 29 June 2009 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-ninth session Kigali, Republic of Rwanda, 31 August 4 September 2009 Provisional agenda item 9.2 PROGRESS REPORT ON CHILD SURVIVAL: A
29 June 2009 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Fifty-ninth session Kigali, Republic of Rwanda, 31 August 4 September 2009 Provisional agenda item 9.2 PROGRESS REPORT ON CHILD SURVIVAL: A
Global landscape analysis and literature review of 2 nd Year of Life immunization platform
 Global landscape analysis and literature review of 2 nd Year of Life immunization platform Global Vaccine and Immunization Research Forum 15-17 March 2016 Johannesburg, South Africa Imran Mirza; Celina
Global landscape analysis and literature review of 2 nd Year of Life immunization platform Global Vaccine and Immunization Research Forum 15-17 March 2016 Johannesburg, South Africa Imran Mirza; Celina
Nigeria: WHO and UNICEF estimates of immunization coverage: 2017 revision
 Nigeria: WHO and UNICEF estimates of immunization coverage: 2017 revision July 7, 2018; page 1 WHO and UNICEF estimates of national immunization coverage - next revision available July 15, 2019 data received
Nigeria: WHO and UNICEF estimates of immunization coverage: 2017 revision July 7, 2018; page 1 WHO and UNICEF estimates of national immunization coverage - next revision available July 15, 2019 data received
How to Use Stable Isotope Techniques for Assessment of Breastfeeding Patterns. Christine Slater Retired IAEA Nutrition Specialist
 How to Use Stable Isotope Techniques for Assessment of Breastfeeding Patterns Christine Slater Retired IAEA Nutrition Specialist Objectively measured breastfeeding Deuterium oxide dose-to-mother technique
How to Use Stable Isotope Techniques for Assessment of Breastfeeding Patterns Christine Slater Retired IAEA Nutrition Specialist Objectively measured breastfeeding Deuterium oxide dose-to-mother technique
Introduction to Global Child Health Elective for Pediatric Residents and Fellows Children s National Medical Center, Washington, DC.
 Introduction to Global Child Health Elective for Pediatric Residents and Fellows Children s National Medical Center, Washington, DC October 11-15, 2010 Pre-Course Test 1. You are preparing for an elective
Introduction to Global Child Health Elective for Pediatric Residents and Fellows Children s National Medical Center, Washington, DC October 11-15, 2010 Pre-Course Test 1. You are preparing for an elective
COUNSELING CARDS FOR IMMUNIZATIONS
 www.calcuttakids.org info@calcuttakids.org COUNSELING CARDS FOR IMMUNIZATIONS For Community Health Workers Created by Calcutta Kids, May 2012 COUNSELING POINTS FOR IMMUNIZATIONS Sheet 1 Tuberculosis Sheet
www.calcuttakids.org info@calcuttakids.org COUNSELING CARDS FOR IMMUNIZATIONS For Community Health Workers Created by Calcutta Kids, May 2012 COUNSELING POINTS FOR IMMUNIZATIONS Sheet 1 Tuberculosis Sheet
Impact of Immunization on Under 5 Mortality
 Annals of Community Health (AoCH) pissn 2347-5455 eissn 2347-5714 An Indexed (Index Medicus, DOAJ and More), Peer Reviewed, Quarterly, International Journal focusing exclusively on Community Medicine and
Annals of Community Health (AoCH) pissn 2347-5455 eissn 2347-5714 An Indexed (Index Medicus, DOAJ and More), Peer Reviewed, Quarterly, International Journal focusing exclusively on Community Medicine and
HOMESTEAD GARDENING FOR COMBATING VITAMIN A DEFICIENCY:THE HELEN KELLER INTERNATIONAL, BANGLADESH, EXPERIENCE
 9 HOMESTEAD GARDENING FOR COMBATING VITAMIN A DEFICIENCY:THE HELEN KELLER INTERNATIONAL, BANGLADESH, EXPERIENCE A.TAHER, A.TALUKDER, N.R. SARKAR,V.N. BUSHAMUKA, A. HALL, S. DE PEE, R. MOENCH-PFANNER, L.
9 HOMESTEAD GARDENING FOR COMBATING VITAMIN A DEFICIENCY:THE HELEN KELLER INTERNATIONAL, BANGLADESH, EXPERIENCE A.TAHER, A.TALUKDER, N.R. SARKAR,V.N. BUSHAMUKA, A. HALL, S. DE PEE, R. MOENCH-PFANNER, L.
Challenges of building a new vaccine delivery platform for LMICs
 Challenges of building a new vaccine delivery platform for LMICs Terri B. Hyde, MD MPH Immunizations Systems Branch, GID, CDC 23 March 2017 Immunization in the Elderly Geneva, Switzerland What has been
Challenges of building a new vaccine delivery platform for LMICs Terri B. Hyde, MD MPH Immunizations Systems Branch, GID, CDC 23 March 2017 Immunization in the Elderly Geneva, Switzerland What has been
Completion rate (upper secondary education, female)
 Annex C. Country profile indicators and data sources Indicator Data source Global database Demographics and contextual factors Demographics Total population Total under-5 population Total adolescent (10
Annex C. Country profile indicators and data sources Indicator Data source Global database Demographics and contextual factors Demographics Total population Total under-5 population Total adolescent (10
THE IMPACT OF PARENTAL EDUCATION AND SOCIOECONOMIC STATUS ON ROUTINE CHILDHOOD VACCINATION: AN OBSEVATIONAL STUDY
 ORIGINAL ARTICLE THE IMPACT OF PARENTAL EDUCATION AND SOCIOECONOMIC STATUS ON ROUTINE CHILDHOOD VACCINATION: AN OBSEVATIONAL STUDY Samreen Ahmad 1, Shahzada Bakhtyar Zahid 2, Anwar Zeb Jan 3 ABSTRACT Objective:
ORIGINAL ARTICLE THE IMPACT OF PARENTAL EDUCATION AND SOCIOECONOMIC STATUS ON ROUTINE CHILDHOOD VACCINATION: AN OBSEVATIONAL STUDY Samreen Ahmad 1, Shahzada Bakhtyar Zahid 2, Anwar Zeb Jan 3 ABSTRACT Objective:
Nutrition Management of HIV-infected Women of Reproductive Age
 Nutrition Management of HIV-infected Women of Reproductive Age Wafaie Fawzi Departments of Global Health and Population, Nutrition and Epidemiology Harvard School of Public Health June 10, 2013 Nutrition
Nutrition Management of HIV-infected Women of Reproductive Age Wafaie Fawzi Departments of Global Health and Population, Nutrition and Epidemiology Harvard School of Public Health June 10, 2013 Nutrition
Your Child s Health Depends on You
 Your Child s Health Depends on You Contents Healthy Living 1 Keeping Infections Away 1 Eating and Drinking 2 Visiting the Clinic 3 Taking Your Well-Child to the Clinic 4 Under 5 Card 4 Free Safe and Available
Your Child s Health Depends on You Contents Healthy Living 1 Keeping Infections Away 1 Eating and Drinking 2 Visiting the Clinic 3 Taking Your Well-Child to the Clinic 4 Under 5 Card 4 Free Safe and Available
ORIGINAL ARTICLES. Missed opportunities for vaccination in health facilities in Swaziland. Methods
 Missed opportunities for vaccination in health facilities in Swaziland AD Daly, M P Nxumalo, R J Biellik Objectives. To determine whether potential exists to increase vaccination coverage in Swaziland
Missed opportunities for vaccination in health facilities in Swaziland AD Daly, M P Nxumalo, R J Biellik Objectives. To determine whether potential exists to increase vaccination coverage in Swaziland
Vitamin A deficiency and child survival in sub-saharan Africa: A reappraisal of challenges and opportunities
 Vitamin A deficiency and child survival in sub-saharan Africa: A reappraisal of challenges and opportunities Victor M. Aguayo and Shawn K. Baker Abstract Background. Children with vitamin A deficiency
Vitamin A deficiency and child survival in sub-saharan Africa: A reappraisal of challenges and opportunities Victor M. Aguayo and Shawn K. Baker Abstract Background. Children with vitamin A deficiency
CHILD HEALTH. There is a list of references at the end where you can find more information. FACT SHEETS
 SOME 18,000 CHILDREN STILL DIE EVERY DAY FROM DISEASES THAT ARE MOSTLY PREVENTABLE. This fact sheet outlines some of the basic information related to the health and wellbeing of children under five years
SOME 18,000 CHILDREN STILL DIE EVERY DAY FROM DISEASES THAT ARE MOSTLY PREVENTABLE. This fact sheet outlines some of the basic information related to the health and wellbeing of children under five years
Pertussis immunisation for pregnant women
 Pertussis immunisation for pregnant women Introduction The routine childhood immunisation programme has been very effective in reducing the overall numbers of cases of pertussis. Before the introduction
Pertussis immunisation for pregnant women Introduction The routine childhood immunisation programme has been very effective in reducing the overall numbers of cases of pertussis. Before the introduction
Requirements for new trials to examine offtarget. vaccination. Workshop on: Off-target (heterologous/non-specific) effects of vaccination.
 Requirements for new trials to examine offtarget effects of vaccination Workshop on: Off-target (heterologous/non-specific) effects of vaccination Les Pensieres Jun 8-10, 2015 PEM Fine London School of
Requirements for new trials to examine offtarget effects of vaccination Workshop on: Off-target (heterologous/non-specific) effects of vaccination Les Pensieres Jun 8-10, 2015 PEM Fine London School of
Ex post evaluation Tanzania
 Ex post evaluation Tanzania Sector: Health, family planning, HIV/AIDS (12250) Project: Promotion of national vaccination programme in cooperation with GAVI Alliance, Phase I and II (BMZ no. 2011 66 586
Ex post evaluation Tanzania Sector: Health, family planning, HIV/AIDS (12250) Project: Promotion of national vaccination programme in cooperation with GAVI Alliance, Phase I and II (BMZ no. 2011 66 586
Pentabio Vaccine (DTP-HB-Hib)
 SUMMARY OF PRODUCT CHARACTERISTICS Product Name Pharmaceutical Form Strength Presentation : Pentabio : Suspension for injection : 1, 5 and 10 doses : Box of 10 vials @ 0.5 ml Box of 10 vials @ 2.5 ml Box
SUMMARY OF PRODUCT CHARACTERISTICS Product Name Pharmaceutical Form Strength Presentation : Pentabio : Suspension for injection : 1, 5 and 10 doses : Box of 10 vials @ 0.5 ml Box of 10 vials @ 2.5 ml Box
Status of RTS,S/AS01 malaria vaccine candidate
 Status of RTS,S/AS01 malaria vaccine candidate Multi-center RTS,S malaria vaccine efficacy trial Phase 3, randomized, controlled, double-blind trial conducted in 11 centers in 7 African countries 15,460
Status of RTS,S/AS01 malaria vaccine candidate Multi-center RTS,S malaria vaccine efficacy trial Phase 3, randomized, controlled, double-blind trial conducted in 11 centers in 7 African countries 15,460
BCG. Program Management. Vaccine Quality
 Program Management 50_16 To change from general to selective BCG vaccination, an efficient notification system must be in place in addition to the following criteria: an average annual notification rate
Program Management 50_16 To change from general to selective BCG vaccination, an efficient notification system must be in place in addition to the following criteria: an average annual notification rate
APPENDIX 4 A4.1 APPENDIX 4
 APPENDIX 4 A4.1 APPENDIX 4 APPENDIX 4 A4.3 DEFINITIONS OF THE INDICATORS 1.1 BCG coverage Number 12 23-month-olds receiving BCG vaccine before 1st birthday 1 1.2 DPT coverage Number 12 23-month-olds receiving
APPENDIX 4 A4.1 APPENDIX 4 APPENDIX 4 A4.3 DEFINITIONS OF THE INDICATORS 1.1 BCG coverage Number 12 23-month-olds receiving BCG vaccine before 1st birthday 1 1.2 DPT coverage Number 12 23-month-olds receiving
Expanded Programme on Immunization (EPI):
 Expanded Programme on Immunization (EPI): Introduction Four to five million annual deaths could be prevented by 2015 through sustained and appropriate immunization efforts, backed by financial support.
Expanded Programme on Immunization (EPI): Introduction Four to five million annual deaths could be prevented by 2015 through sustained and appropriate immunization efforts, backed by financial support.
Nutrition Update Severe acute malnutrition
 Nutrition Update Assessing the nutritional status of children and the presence of anemia is an integral part of the IMCI ask, look and listen strategy. The risk of death from acute respiratory infection,
Nutrition Update Assessing the nutritional status of children and the presence of anemia is an integral part of the IMCI ask, look and listen strategy. The risk of death from acute respiratory infection,
Vitamin A Deficiency and Child Health, Survival and Vision
 This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this
WHO/UNICEF Review of National Immunization Coverage India
 WHO/UNICEF Review of National Immunization Coverage 1980-2002 India October, 2003 India Estimates, 1980-2002 80 66 68 73 73 60 40 30 20 10 0 1980 19 1982 1983 1984 1985 1986 1987 1988 1989 19 1991 1992
WHO/UNICEF Review of National Immunization Coverage 1980-2002 India October, 2003 India Estimates, 1980-2002 80 66 68 73 73 60 40 30 20 10 0 1980 19 1982 1983 1984 1985 1986 1987 1988 1989 19 1991 1992
MOBILISING COMMUNITIES FOR IMMUNIZATION SERVICES
 MOBILISING COMMUNITIES FOR IMMUNIZATION SERVICES Photo of VHT conducting a community meeting What VHTs and community mobilisers need to know and do Printed with support from UNICEF and partners 2 1. Introduction
MOBILISING COMMUNITIES FOR IMMUNIZATION SERVICES Photo of VHT conducting a community meeting What VHTs and community mobilisers need to know and do Printed with support from UNICEF and partners 2 1. Introduction
Children s Health and Nutritional Status. Data from the 2011 Ethiopia Demographic and Health Survey
 Children s Health and Nutritional Status Data from the 2011 Ethiopia Demographic and Health Survey This report summarises the child health and nutrition findings from the 2011 Ethiopia Demographic and
Children s Health and Nutritional Status Data from the 2011 Ethiopia Demographic and Health Survey This report summarises the child health and nutrition findings from the 2011 Ethiopia Demographic and
The National Immunisation Schedule Update and Current issues November Dr Brenda Corcoran National Immunisation Office.
 The National Immunisation Schedule Update and Current issues November 2013 Dr Brenda Corcoran National Immunisation Office Objectives To outline immunisation schedules in Ireland Primary childhood schedule
The National Immunisation Schedule Update and Current issues November 2013 Dr Brenda Corcoran National Immunisation Office Objectives To outline immunisation schedules in Ireland Primary childhood schedule
Hepatitis B vaccine alone or with HBIG in neonates of HBsAg+/HBeAg- mothers: a systematic review and meta-analysis.
 Hepatitis B vaccine alone or with HBIG in neonates of HBsAg+/HBeAg- mothers: a systematic review and meta-analysis. Vana Papaevangelou (Greece) National and Kapodistrian University of Athens Chang SemFNM
Hepatitis B vaccine alone or with HBIG in neonates of HBsAg+/HBeAg- mothers: a systematic review and meta-analysis. Vana Papaevangelou (Greece) National and Kapodistrian University of Athens Chang SemFNM
IMMUNISATION. Diseases that can be prevented by immunisation
 IMMUNISATION THE IDEA Every year, millions of children die, and five million are disabled, from diseases which could have been prevented by immunisation against the germs which cause them. Children can
IMMUNISATION THE IDEA Every year, millions of children die, and five million are disabled, from diseases which could have been prevented by immunisation against the germs which cause them. Children can
The National Immunisation Schedule Update and Current issues. Dr Brenda Corcoran National Immunisation Office.
 The National Immunisation Schedule Update and Current issues Dr Brenda Corcoran National Immunisation Office Objectives To outline immunisation schedules in Ireland Primary childhood schedule Vaccine uptake
The National Immunisation Schedule Update and Current issues Dr Brenda Corcoran National Immunisation Office Objectives To outline immunisation schedules in Ireland Primary childhood schedule Vaccine uptake
How to optimise immunisation: a live vaccine last policy. Frank Shann University of Melbourne
 How to optimise immunisation: a live vaccine last policy Frank Shann University of Melbourne We can no longer assume that a vaccine: 1. Acts independently of other vaccines 2. Influences only infections
How to optimise immunisation: a live vaccine last policy Frank Shann University of Melbourne We can no longer assume that a vaccine: 1. Acts independently of other vaccines 2. Influences only infections
Evaluating Immunisation Dropout Rates in Eight Hard to Reach Unions of Maulvibazar District, Bangladesh
 International Journal of Immunology 2017; 5(1): 5-10 http://www.sciencepublishinggroup.com/j/iji doi: 10.11648/j.iji.20170501.12 ISSN: 2329-177X (Print); ISSN: 2329-1753 (Online) Evaluating Immunisation
International Journal of Immunology 2017; 5(1): 5-10 http://www.sciencepublishinggroup.com/j/iji doi: 10.11648/j.iji.20170501.12 ISSN: 2329-177X (Print); ISSN: 2329-1753 (Online) Evaluating Immunisation
Balance Sheets 1. CHILD HEALTH... PAGE NUTRITION... PAGE WOMEN S HEALTH... PAGE WATER AND ENVIRONMENTAL SANITATION...
 Balance Sheets A summary of the goals, gains and unfinished business of the 1990-2000 decade as included in the Report of the Secretary-General, 'We the Children: End-decade review of the follow-up to
Balance Sheets A summary of the goals, gains and unfinished business of the 1990-2000 decade as included in the Report of the Secretary-General, 'We the Children: End-decade review of the follow-up to
The effect of neonatal vitamin A supplementation on morbidity and mortality at 12 months: a randomized trial
 International Journal of Epidemiology, 2016, 2112 2121 doi: 10.1093/ije/dyw238 Advance Access Publication Date: 27 October 2016 Original article Interventions The effect of neonatal vitamin A supplementation
International Journal of Epidemiology, 2016, 2112 2121 doi: 10.1093/ije/dyw238 Advance Access Publication Date: 27 October 2016 Original article Interventions The effect of neonatal vitamin A supplementation
Undernutrition & risk of infections in preschool children
 Indian J Med Res 130, November 2009, pp 579-583 Undernutrition & risk of infections in preschool children Prema Ramachandran & Hema S. Gopalan Nutrition Foundation of India, New Delhi, India Received April
Indian J Med Res 130, November 2009, pp 579-583 Undernutrition & risk of infections in preschool children Prema Ramachandran & Hema S. Gopalan Nutrition Foundation of India, New Delhi, India Received April
Shabir A. Madhi. Progress and Challenges of Immunization Contributing Toward Attaining the MDG Goal to Reduce under-5 Childhood Mortality.
 Shabir A. Madhi Progress and Challenges of Immunization Contributing Toward Attaining the MDG Goal to Reduce under-5 Childhood Mortality. National Institute for Communicable Diseases & University of Witwatersrand,
Shabir A. Madhi Progress and Challenges of Immunization Contributing Toward Attaining the MDG Goal to Reduce under-5 Childhood Mortality. National Institute for Communicable Diseases & University of Witwatersrand,
Eritrea Health Weekly Update 9 th to 15 th October, 2006
 Eritrea Health Weekly Update 9 th to 15 th October, 26 HIGHLIGHTS Weekly outbreak Monitoring WCO Meets to Develop Strategies HH/C-IMCI Survey Findings Weekly Outbreak Monitoring Week 41 (9 th to 15 th
Eritrea Health Weekly Update 9 th to 15 th October, 26 HIGHLIGHTS Weekly outbreak Monitoring WCO Meets to Develop Strategies HH/C-IMCI Survey Findings Weekly Outbreak Monitoring Week 41 (9 th to 15 th
I mun u i n s i atio i n o n u p u d p a d te
 Immunisation update Brian Eley Paediatric Infectious Diseases Unit Red Cross War Memorial Children s Hospital Department of Paediatrics and Child Health University of Cape Town Immunisation schedules,
Immunisation update Brian Eley Paediatric Infectious Diseases Unit Red Cross War Memorial Children s Hospital Department of Paediatrics and Child Health University of Cape Town Immunisation schedules,
WG: Vaccinations and child survival Focus:
 WG: Vaccinations and child survival Focus: Monitoring childhood interventions including routine services and campaigns => To find possible changes in policy Why is that necessary? Paradox: All interventions
WG: Vaccinations and child survival Focus: Monitoring childhood interventions including routine services and campaigns => To find possible changes in policy Why is that necessary? Paradox: All interventions
The National Immunisation Schedule Update and Current issues. Dr Brenda Corcoran National Immunisation Office.
 The National Immunisation Schedule Update and Current issues Dr Brenda Corcoran National Immunisation Office : Dates vaccines introduced into the Irish immunisation schedule Vaccine 1937-1999 Date introduced
The National Immunisation Schedule Update and Current issues Dr Brenda Corcoran National Immunisation Office : Dates vaccines introduced into the Irish immunisation schedule Vaccine 1937-1999 Date introduced
MCSP Mozambique Program Brief Strengthening Immunization Services
 MCSP Mozambique Program Brief Strengthening Immunization Services November 2018 www.mcsprogram.org Goal The Maternal and Child Survival Program (MCSP) in Mozambique used the Reaching Every District/Reaching
MCSP Mozambique Program Brief Strengthening Immunization Services November 2018 www.mcsprogram.org Goal The Maternal and Child Survival Program (MCSP) in Mozambique used the Reaching Every District/Reaching
VI Child Health. Immunisation
 VI Child Health Immunisation Millennium Development Goal (MDG) 4 is to reduce child mortality by two-thirds between 1990 and 2015. Immunisation plays a key part in this goal. Immunisation has saved the
VI Child Health Immunisation Millennium Development Goal (MDG) 4 is to reduce child mortality by two-thirds between 1990 and 2015. Immunisation plays a key part in this goal. Immunisation has saved the
Immunization and Vaccines
 Immunization and Vaccines A parental choice Dr. Vivien Suttorp BSc, MPH, MD, CCFP, FCFP Lead Medical Officer of Health South Zone, Alberta Health Services November 7, 2013 Overview Facts about vaccines
Immunization and Vaccines A parental choice Dr. Vivien Suttorp BSc, MPH, MD, CCFP, FCFP Lead Medical Officer of Health South Zone, Alberta Health Services November 7, 2013 Overview Facts about vaccines
first three years of life
 Journal of Epidemiology and Community Health, 1981, 35, 18-184 Parental smoking and lower respiratory illness in the first three years of life D. M. FERGUSSON, L. J. HORWOOD, F. T. SHANNON, AND BRENT TAYLOR
Journal of Epidemiology and Community Health, 1981, 35, 18-184 Parental smoking and lower respiratory illness in the first three years of life D. M. FERGUSSON, L. J. HORWOOD, F. T. SHANNON, AND BRENT TAYLOR
Mangement of severe acute malnutrition in Cambodian children 6-59 months
 Mangement of severe acute malnutrition in Cambodian children 6-59 months 14 th LAO PEDIATRIC CONTINUING MEDICAL EDUCATION CONFERENCE March 1-2, 2018 Vientiane, Laos Ms. Sanne Sigh, Cand. scient. Human
Mangement of severe acute malnutrition in Cambodian children 6-59 months 14 th LAO PEDIATRIC CONTINUING MEDICAL EDUCATION CONFERENCE March 1-2, 2018 Vientiane, Laos Ms. Sanne Sigh, Cand. scient. Human
Determinants of Vaccination Status in Rural Guinea-Bissau. Linda Hornshøj
 Determinants of Vaccination Status in Rural Guinea-Bissau Linda Hornshøj Background In1974 World Health organization (WHO) launched the Expanded Program on Immunization (EPI) to provide routine vaccinations
Determinants of Vaccination Status in Rural Guinea-Bissau Linda Hornshøj Background In1974 World Health organization (WHO) launched the Expanded Program on Immunization (EPI) to provide routine vaccinations
Published by HSRC Press Private Bag X9182, Cape Town, 8000, South Africa First published 2013
 Published by HSRC Press Private Bag X9182, Cape Town, 8000, South Africa www.hsrcpress.ac.za First published 2013 ISBN (soft cover) 978-0-7969-2446-9 ISBN (pdf) 978-0-7969-2447-6 ISBN (e-pub) 978-0-7969-2448-3
Published by HSRC Press Private Bag X9182, Cape Town, 8000, South Africa www.hsrcpress.ac.za First published 2013 ISBN (soft cover) 978-0-7969-2446-9 ISBN (pdf) 978-0-7969-2447-6 ISBN (e-pub) 978-0-7969-2448-3
Adherence to case management guidelines of IMCI by health care workers in Tshwane
 Adherence to case management guidelines of IMCI by health care workers in Tshwane S Afr J Child Health 2015;9(3):89-92. DOI:10.7196/SAJCH.7959 Mphele Mulaudzi UPdate 4 March 2016, Menlyn Introduction Integrated
Adherence to case management guidelines of IMCI by health care workers in Tshwane S Afr J Child Health 2015;9(3):89-92. DOI:10.7196/SAJCH.7959 Mphele Mulaudzi UPdate 4 March 2016, Menlyn Introduction Integrated
46825 (260) $UPONT
 Be wise. Immunize. Keeping track of the shots your children receive can be confusing. This is an important responsibility that is shared by you and your immunization providers. This booklet contains the
Be wise. Immunize. Keeping track of the shots your children receive can be confusing. This is an important responsibility that is shared by you and your immunization providers. This booklet contains the
Maternal and Infant Nutrition Briefs
 Maternal and Infant Nutrition Briefs November/December 2002 A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition Recent Trends
Maternal and Infant Nutrition Briefs November/December 2002 A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition Recent Trends
Immunization Graphs: Natural Infectious Disease Declines; Immunization Effectiveness; and Immunization Dangers
 Immunization Graphs: Natural Infectious Disease Declines; Immunization Effectiveness; and Immunization Dangers Prepared by: Raymond Obomsawin Ph.D. December, 2009 FIGURE SET I. Natural Infectious Disease
Immunization Graphs: Natural Infectious Disease Declines; Immunization Effectiveness; and Immunization Dangers Prepared by: Raymond Obomsawin Ph.D. December, 2009 FIGURE SET I. Natural Infectious Disease
The Gates Challenge. Bill Gates Commencement Address Harvard University Class of 2007
 History & Future of the Expanded Programme on Immunization Supplier Meeting, Copenhagen, 3-4 April 2008 Dr Osman David Mansoor Senior Adviser EPI (New Vaccines) UNICEF New York The Gates Challenge If we
History & Future of the Expanded Programme on Immunization Supplier Meeting, Copenhagen, 3-4 April 2008 Dr Osman David Mansoor Senior Adviser EPI (New Vaccines) UNICEF New York The Gates Challenge If we
Care of the HIV-Exposed Infant
 Care of the HIV-Exposed Infant Use of Flipchart To promote quality and consistency of counseling Why use the counseling flipchart? To improve HIV-exposed infant outcomes through high quality counseling.
Care of the HIV-Exposed Infant Use of Flipchart To promote quality and consistency of counseling Why use the counseling flipchart? To improve HIV-exposed infant outcomes through high quality counseling.
Ministry of health. Our Community
 Ministry of health Our Community This flip book is specially developed for Community Health Volunteer CHVs MUST ask 4 questions while using communication materials 1. What is happening in this picture?
Ministry of health Our Community This flip book is specially developed for Community Health Volunteer CHVs MUST ask 4 questions while using communication materials 1. What is happening in this picture?
CHILDHOOD VACCINATION
 EPI (3) Age of Child How and Where is it given? CHILDHOOD VACCINATION Nicolette du Plessis Block 10 28/02/2012 10 weeks DTaP-IPV/Hib (2) Diphtheria, Tetanus, Acellular pertussis, Inactivated polio vaccine,
EPI (3) Age of Child How and Where is it given? CHILDHOOD VACCINATION Nicolette du Plessis Block 10 28/02/2012 10 weeks DTaP-IPV/Hib (2) Diphtheria, Tetanus, Acellular pertussis, Inactivated polio vaccine,
The National Immunisation Schedule. Dr Brenda Corcoran.
 The National Immunisation Schedule Update and Current issues Dr Brenda Corcoran National Immunisation Office Objectives To outline immunisation schedules in Ireland Primary childhood schedule Vaccine uptake
The National Immunisation Schedule Update and Current issues Dr Brenda Corcoran National Immunisation Office Objectives To outline immunisation schedules in Ireland Primary childhood schedule Vaccine uptake
Updated WHO position paper on pertussis vaccines. Geneva, Switzerland October 2010
 Updated WHO position paper on pertussis vaccines Geneva, Switzerland October 2010 Introduction Replaces the position paper on pertussis vaccines published in the Weekly Epidemiological Record in January
Updated WHO position paper on pertussis vaccines Geneva, Switzerland October 2010 Introduction Replaces the position paper on pertussis vaccines published in the Weekly Epidemiological Record in January
ANNEX C: RISK- OF- BIAS ASSESSMENTS
 Systematic review of the non- specific effects of BCG, DTP and measles containing vaccines ANNEX C: RISK- OF- BIAS ASSESSMENTS 1 Risk of bias assessments for randomized trials of BCG... 2 2 Risk of bias
Systematic review of the non- specific effects of BCG, DTP and measles containing vaccines ANNEX C: RISK- OF- BIAS ASSESSMENTS 1 Risk of bias assessments for randomized trials of BCG... 2 2 Risk of bias
Improving Nutrition Through Multisectoral Approaches
 Improving Nutrition Through Multisectoral Approaches Health Undernutrition and health linkages Undernutrition is the single greatest cause of child deaths in most low-income and lower middle-income countries.
Improving Nutrition Through Multisectoral Approaches Health Undernutrition and health linkages Undernutrition is the single greatest cause of child deaths in most low-income and lower middle-income countries.
WHO Updates Essential Nutrition Actions: Improving Women s, Newborn, Infant and Young Child Health and Nutrition
 WHO Updates Essential Nutrition Actions: Improving Women s, Newborn, Infant and Young Child Health and Nutrition Agnes Guyon, MD, MPH Senior Child Health & Nutrition Advisor John Snow, Inc. WCPH-Kolkata
WHO Updates Essential Nutrition Actions: Improving Women s, Newborn, Infant and Young Child Health and Nutrition Agnes Guyon, MD, MPH Senior Child Health & Nutrition Advisor John Snow, Inc. WCPH-Kolkata
Introduction to Measles a Priority Vaccine Preventable Disease (VPD) in Africa
 Introduction to Measles a Priority Vaccine Preventable Disease (VPD) in Africa Nigeria Center for Disease Control Federal Ministry of Health Abuja July 2015 Outline 1. Measles disease 2. Progress towards
Introduction to Measles a Priority Vaccine Preventable Disease (VPD) in Africa Nigeria Center for Disease Control Federal Ministry of Health Abuja July 2015 Outline 1. Measles disease 2. Progress towards
Ma. Erlinda Tarrayo, Imelda Agdeppa, Ph.D., Carmina DD. Cuarteros
 The Interplay of Immunization, Low Birth Weight, Feeding Practices and Food Intake On The Nutritional Status and Anemia Prevalence Among Young Children 6-23 Months old Ma. Erlinda Tarrayo, Imelda Agdeppa,
The Interplay of Immunization, Low Birth Weight, Feeding Practices and Food Intake On The Nutritional Status and Anemia Prevalence Among Young Children 6-23 Months old Ma. Erlinda Tarrayo, Imelda Agdeppa,
Student Guide Module 8: Nutrition and Malnutrition
 Student Guide Module 8: Nutrition and Malnutrition Objectives of the station Plan and develop measures to assess the nutritional status of populations displaced by disasters, and to ensure optimal nutritional
Student Guide Module 8: Nutrition and Malnutrition Objectives of the station Plan and develop measures to assess the nutritional status of populations displaced by disasters, and to ensure optimal nutritional
Guidelines for management of suspected sepsis in young infants where referral is not possible
 Guidelines for management of suspected sepsis in young infants where referral is not possible Kenya Paediatrics Association Conference, 26-29 April 2016, Eldoret Kenya 1 Kenya Paediatrics Association Conference,
Guidelines for management of suspected sepsis in young infants where referral is not possible Kenya Paediatrics Association Conference, 26-29 April 2016, Eldoret Kenya 1 Kenya Paediatrics Association Conference,
THAILAND THAILAND 207
 THAILAND 27 List of Country Indicators Selected Demographic Indicators Selected demographic indicators Child Mortality and Nutritional Status Trends in neonatal, infant and child mortality rates Distribution
THAILAND 27 List of Country Indicators Selected Demographic Indicators Selected demographic indicators Child Mortality and Nutritional Status Trends in neonatal, infant and child mortality rates Distribution
Women by area: Urban F Women by area: Rural F
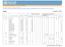 Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Vitamin A Deficiency The Vitamin A Deficiency database includes data by country based on xerophthalmia and/or serum or plasma
Vitamin and Mineral Nutrition Information System (VMNIS) WHO Global Database on Vitamin A Deficiency The Vitamin A Deficiency database includes data by country based on xerophthalmia and/or serum or plasma
Prevalence of tuberculosis (TB) infection and disease among adolescents western Kenya: preparation for future TB vaccine trials
 Prevalence of tuberculosis (TB) infection and disease among adolescents western Kenya: preparation for future TB vaccine trials Videlis Nduba 1,2, Peter Onyango 1, Anja Van t Hoog 1,3, Anthony Hawkridge
Prevalence of tuberculosis (TB) infection and disease among adolescents western Kenya: preparation for future TB vaccine trials Videlis Nduba 1,2, Peter Onyango 1, Anja Van t Hoog 1,3, Anthony Hawkridge
Help protect your child. At-a-glance guide to childhood vaccines.
 Help protect your child. At-a-glance guide to childhood vaccines. Why vaccines matter. Thanks to widespread vaccination programs, several diseases that can infect our children have been eliminated. But
Help protect your child. At-a-glance guide to childhood vaccines. Why vaccines matter. Thanks to widespread vaccination programs, several diseases that can infect our children have been eliminated. But
Help protect your child. At-a-glance guide to childhood vaccines.
 Help protect your child. At-a-glance guide to childhood vaccines. Why vaccines matter. Thanks to widespread vaccination programs, several diseases that can infect our children have been eliminated. But
Help protect your child. At-a-glance guide to childhood vaccines. Why vaccines matter. Thanks to widespread vaccination programs, several diseases that can infect our children have been eliminated. But
Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018
 Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018 1 Objectives Brief review Background EMA positive opinion and WHO recommendations Funding Description
Overview of the Malaria Vaccine Implementation Programme (MVIP) Prof. Fred Were SAGE meeting 17 April, 2018 1 Objectives Brief review Background EMA positive opinion and WHO recommendations Funding Description
Methodological issues in the use of anthropometry for evaluation of nutritional status
 Methodological issues in the use of anthropometry for evaluation of nutritional status Monika Blössner WHO Department of Nutrition for Health and Development Methodological issues in the use of anthropometry?
Methodological issues in the use of anthropometry for evaluation of nutritional status Monika Blössner WHO Department of Nutrition for Health and Development Methodological issues in the use of anthropometry?
Help protect your child. At-a-glance guide to childhood vaccines.
 Help protect your child. At-a-glance guide to childhood vaccines. 40976_CDCupdate.indd 1 Why vaccines matter. Thanks to widespread vaccination programs, several diseases that can infect our children have
Help protect your child. At-a-glance guide to childhood vaccines. 40976_CDCupdate.indd 1 Why vaccines matter. Thanks to widespread vaccination programs, several diseases that can infect our children have
CRED Technical Brief: Outbreaks in Fragile States. Yellow Fever in Darfur September December 2012
 1th December 212 CRED Technical Brief: Outbreaks in Fragile States. Yellow Fever in Darfur September December 212 This technical brief consists of 2 sections: 1. An Overview of Yellow Fever (p 1-3) 2.
1th December 212 CRED Technical Brief: Outbreaks in Fragile States. Yellow Fever in Darfur September December 212 This technical brief consists of 2 sections: 1. An Overview of Yellow Fever (p 1-3) 2.
Routine Immunization Status among Children under 5 Years of Age living in Rural District of Pakistan
 International Journal of Health Research and Innovation, vol. 3, no. 2, 2015, 13-20 ISSN: 2051-5057 (print version), 2051-5065 (online) Scienpress Ltd, 2015 Routine Immunization Status among Children under
International Journal of Health Research and Innovation, vol. 3, no. 2, 2015, 13-20 ISSN: 2051-5057 (print version), 2051-5065 (online) Scienpress Ltd, 2015 Routine Immunization Status among Children under
National immunisation days and vitamin A distribution in Mali: has the vitamin A status of pre-school children improved?
 Public Health Nutrition: 6(3), 233 240 DOI: 10.1079/PHN2002432 National immunisation days and vitamin A distribution in Mali: has the vitamin A status of pre-school children improved? JF Schémann 1, *,
Public Health Nutrition: 6(3), 233 240 DOI: 10.1079/PHN2002432 National immunisation days and vitamin A distribution in Mali: has the vitamin A status of pre-school children improved? JF Schémann 1, *,
Expanded Programme on Immunization (EPI)
 Bhutan 2017 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles containing
Bhutan 2017 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles containing
Baby Friendly Vaccines
 Baby Friendly Vaccines What exactly are Baby Friendly Vaccines? Background on Immunity Immunity is the ability of the body to resist and fight germs (disease causing organisms) that can cause infectious
Baby Friendly Vaccines What exactly are Baby Friendly Vaccines? Background on Immunity Immunity is the ability of the body to resist and fight germs (disease causing organisms) that can cause infectious
Samir K Saha, Ph.D Child Health Research Foundation Dhaka Shishu Hospital Dhaka, Bangladesh
 Barriers Rotavirus to Rotavirus Vaccine Impact Vaccine Beyond Preventing Introduction Diarrhea Samir K Saha, Ph.D Child Health Research Foundation Dhaka Shishu Hospital Dhaka, Bangladesh 13 th International
Barriers Rotavirus to Rotavirus Vaccine Impact Vaccine Beyond Preventing Introduction Diarrhea Samir K Saha, Ph.D Child Health Research Foundation Dhaka Shishu Hospital Dhaka, Bangladesh 13 th International
Expanded Programme on Immunization (EPI)
 Timor-Leste 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
Timor-Leste 217 Expanded Programme on Immunization (EPI) FACT SHEET Acronyms AD Auto disable MCV1 First dose measles containing vaccine AEFI Adverse events following immunization MCV2 Second dose measles
