Pharmacy Logs and Worksheets
|
|
|
- Alexandra Bradford
- 6 years ago
- Views:
Transcription
1 Pharmacy Logs and Worksheets 1
2 Monthly Site Inventory Log Month Year To be filled out by Site Pharmacy monthly and faxed to Clinical Evaluation Research Unit (CERU). Name of Site: Pharmacist: Phone: Product Supplier Minimum Supply needed Actual supplies Amount needed Dipeptiven (100 ml bottles) Fresenius 80 bottles* bottles bottles (10 bottles per carton) Kabi (FK) EN REDOXS formula (500 mls bottles) (12 bottles per carton) AOX + GLN FK 36 bottles bottles bottles AOX FK 36 bottles bottles bottles GLN FK 36 bottles bottles bottles Placebo FK 36 bottles bottles bottles Selenase(10 ml vials) Biosyn 40 vials vials vials * based on 4 patients, each needing 2 bottles per day for 10 days based on 4 patients, each needing 1 bottle per day for 9 days based on 4 patients, each needing approximately 1 vials/day per day for 10 days Checked by CERU Project Assistant Signature of person completing log: Fax completed form to: CERU (613) Date Attention: REDOXS Study (613) ext 6686 or
3 Monthly Site Temperature Log Month Year To be filled out by Site Pharmacy daily and faxed to Clinical Evaluation Research Unit (CERU) monthly. Name of Site: Pharmacist: Phone: Date Temperature Low Température Bas Temperature Current Température Présent Temperature High Température Haut Date Temperature Low Température Bas Temperature Current Température Présent Temperature High Température Haut Signature of person submitting log: Fax completed form to: CERU (613) Attention: REDOXS Study (613) ext 6686 or
4 Enteral Product Label Log Page of Pharmacist to place removable labels here daily (use one page is for 3 days) Patient ID #: Patient Initials: Enrollment#: Treatment Group (circle one): AOX GLN AOX+ GLN Placebo Date dd/mm/yyyy Date dd/mm/yyyy Date dd/mm/yyyy 4
5 Enteral Study Supplement Dispensing Log Page of To be filled out by Pharmacist Patient ID # Patient Initials: Enrollment #: Dose: 480 mls/day Infusion Rate: 20 mls/hour Treatment Group (circle one): AOX GLN AOX+ GLN Placebo Prepared by Checked Checked by Date dd/mm/yyyy Lot # Expiry by study monitor 5
6 Parenteral Study Supplement Dispensing Log Page of To be filled out by Pharmacist Patient ID #: Patient Initials: Height: cms Enrollment #: Treatment Group: (circle one): AOX GLN AOX+ GLN Placebo Infusion Rate of Final Product: 10 ml/hr (or > if tall) Dipeptiven Selenium Saline/D5W Signatures Date dose (mls) Lot # & expiry dose (mls) Lot # & expiry dose (mls) Lot # & expiry Manufacturer Prepared by Checked by Audited by: 6
7 Nutrient Accountability Log Enteral AOX Page of Site #: To be filled out by Pharmacist Date Quantity received or destroyed Lot # Expiry date Quantity dispensed Patient enrollment # Balance of Product Signature 7
8 Nutrient Accountability Log Enteral GLN Page of Site #: To be filled out by Pharmacist Date Quantity received or destroyed Lot # Expiry date Quantity dispensed Patient enrollment # Balance of Product Signature Audited by: 8
9 Nutrient Accountability Log Enteral AOX+GLN Page of Site #: To be filled out by Pharmacist Date Quantity received or destroyed Lot # Expiry date Quantity dispensed Patient enrollment # Balance of Product Signature Audited by: 9
10 Nutrient Accountability Log Enteral Placebo Page of Site #: To be filled out by Pharmacist Date Quantity received or destroyed Lot # Expiry date Quantity dispensed Patient enrollment # Balance of Product Signature Audited by: 10
11 Nutrient Accountability Log Dipeptiven Page of Site #: To be filled out by Pharmacist Date Quantity received or destroyed Lot # Expiry date Quantity dispensed Patient enrollment # Balance of Product Signature Audited by: 11
12 Nutrient Accountability Log Selenase Page of Site #: To be filled out by Pharmacist Date Quantity received or destroyed Lot # Expiry date Quantity dispensed Patient enrollment # Balance of Product Signature Audited by: 12
13 Appendices Appendix A: Site Investigator Delegation of Authority Log Appendix B: Pharmacy Training/Delegation of Authority Log Appendix C: Pharmacy Web Access Signature Log Appendix D: Randomization Process on Web Appendix E: Enteral Study Supplement Label Template Appendix F: Parenteral Study Supplement Worksheets Appendix G: Parenteral Study Supplement Label Template Appendix H: Height and Dose of Dipeptiven 13
14 14
15 15
16 Pharmacy Web access Signature Log INSTITUTION: SITE NUMBER: INVESTIGATOR: Please complete the Electronic Data Capture (EDC) System Access Signature Sheet for each Pharmacist/technician at your site who will be checking the randomization or dispensing/checking study supplements. A signature and address is required to create user accounts for the web based system for the REDOXS Study. NAME TITLE SIGNATURE DATE NOTE: By completing the information in the table above, the individual confirms they have been delegated the responsibility of checking the randomization and dispensing/verifying study supplements for the REDOXS Study. Reference: ICH GCP
17 Appendix D Randomization Process on Web Screen New Subject Only Subjects Who Meet Inclusion Screening Form Inclusion/Exclusion Meets an Exclusion Ends No Exclusion Eligible for Study Pre Randomization Form Eligibility Must Be Confirmed By MD Do you want to obtain consent? Ends Explain why No Pharmacist Receives call from Study Coordinator Pharmacist Logs onto Web Yes Consent Obtained Subject s Height in cm. CLICK here to Randomize Patient button Pharmacist Receives Treatment Assignment Subject s Initials Date of Birth Height Subject is now Randomized Enrollment Number Is Contact your Site Pharmacy and enter date and time of contact. Provide Height/Subject Initials/ DOB/Enrollment Number After Randomization Study Coordinator to Complete Baseline Form & APACHE Score and other Electronic CRFs 17
18 Appendix E Enteral Study Supplement Study: REDOXS Enteral Component For Clinical trial Use Only Enrollment #: Patient ID/CR#: Patient Name: Physician: Directions: Infuse at 20 ml/hr Storage: keep between C Expiry: use within 24 hours Date: 18
19 Appendix F Parenteral Study Supplement Worksheets Use the appropriate worksheet according to the group the patient has been randomized to. These worksheets will assist in calculating the volumes of the parenteral study supplements and normal saline or D5W needed. Worksheet for Antioxidants (AOX) Worksheet for Glutamine (GLN) Worksheet for (Antioxidant + Glutamine) AOX + GLN Worksheet for Placebo 19
20 Parenteral Supplements Worksheet Patient CR# / ID# Patient Initials Enrolment # Antioxidants (AOX) Patient will receive Selenium (Antioxidants) and normal saline (or if hypernatremic D5W) Begin with a bag containing exactly 250 ml of normal saline (i.e. account for overfill): 1. Volume of Dipeptiven to be added = _0_mL 2. Volume of Selenium to be added = _10 ml_ 3. Total Volume to be removed from 250 ml normal saline bag before adding study supplements: (# 1) + (# 2) = _10_ ml volume of dipeptiven volume of selenium 4. Add (#1) + (#2) + normal saline =_250 ml_ 5. Record the volumes of Selenium and Normal Saline on the Parenteral Study Supplement Log for this patient daily. 6. Generate a label and attach to bag. Dipeptiven not required for this treatment arm Note: Height of the patient not required to calculate the AOX dose version: 3-Jul-09 (EU) Replaces version: 9-Sept-07 (EU)
21 Parenteral Supplements Worksheet (Patients > 196 cm tall) Patient CR# / ID# Patient Initials Enrolment # Antioxidants (AOX) Patient will receive Selenium (Antioxidants) and normal saline (or if hypernatremic D5W) Dosing: 1. Dosage of Selenium = 500mcg/day = 10mL/day regardless of height 2. Rate of infusion determined from chart below. 3. Dose will be diluted in a NS bag to a final volume indicated in the chart below. 4. You must account for overfill in the normal saline bags as per table below. Beginning with a bag of exactly 250 ml of normal saline: Go to height chart (see below). Record: 1. Patient height = cm 2. Final total volume of the bag (from chart below) = ml 3. Rate to be infused (from chart below) = ml/hr 4. Volume of Selenium to be added = _10 ml_ Height Final Total Volume of Bag Rate to be Infused Volume of Selenium to be Added Amount of Normal Saline To be removed 196 cm 250 ml 10.4 ml/hr 10 ml 10.0 ml 198 cm 255 ml 10.6 ml/hr 10 ml 15.0 ml To be added 201cm 263 ml 11.0 ml/hr 10 ml 3.0 ml 203 cm 268 ml 11.2 ml/hr 10 ml 8.0 ml 206 cm 275 ml 11.5 ml/hr 10 ml 15.0 ml 208 cm 280 ml 11.7 ml/hr 10 ml 20.0 ml 211 cm 288 ml 12.0 ml/hr 10 ml 28.0 ml 213 cm 293 ml 12.2 ml/hr 10 ml 33.0 ml 5. Volume of NS to be removed or added to bag = ml 6. Add Selenium (#4), as per chart, to normal saline bag. Mix. 7. Record the volumes of Selenium and Normal Saline on the Parenteral Study Supplement Log for this patient daily. 8. Generate a label and attach to bag. Version: 3-Jul-09 (EU) Replaces version: 6-Dec-07 (EU)
22 Parenteral Supplements Worksheet Patient CR# / ID# Patient Initials Enrolment # Glutamine (GLN) Patient will receive Dipeptiven (Glutamine) and normal saline (or if hypernatremic DSW) Begin with a bag containing exactly 250 ml of normal saline (i.e. account for overfill): 1. Patient s height = cm 2. Normal Body Weight = (#1) 100 cm = kg 3. Volume of Dipeptiven to be added = (#2) x 2.5 ml = ml 4. Volume of Selenium to be added = 0 ml_ 5. Total Volume to be removed from 250 ml normal saline bag before adding study supplements: (# 3) + (# 4)_ = ml volume of dipeptiven volume of selenium 7. Add (#3) + (#4) + normal saline =_250 ml_ 8. Record the volumes of Dipeptiven and Normal Saline on the Parenteral Study Supplement Log for this patient daily. 9. Generate a label and attach to bag. Dipeptiven 2.5 ml/kg/day =Glutamine 0.35 g/kg/day = L-alanyl-L-glutamine 0.5 g/kg/day Selenium not required for this treatment arm version: 3-Jul-09 (EU) Replaces version: 9-Sept-07 (EU)
23 Parenteral Supplements Worksheet (Patients > 196 cm tall) Patient CR# / ID# Patient Initials Enrolment # Glutamine (GLN) Patient will receive Dipeptiven (Glutamine) and normal saline (or if hypernatremic D5W) Dosing: 1. Dosage of Glutamine = 0.35g/kg/day = L-alanyl-L-glutamine 0.5g/kg/day= Dipeptivan 2.5mL/kg/day 2. Dosing will be based on patient s Normal Body Weight using Broca Formula as follows: Normal Weight (kg)= Patient s Height (cm) Dose will be diluted in a NS bag to a final volume indicated in the chart below. 4. Rate of infusion determined from chart below. 5. You must account for overfill in the normal saline bags as per table below. Beginning with a bag of exactly 250 ml of normal saline: Go to height chart (see below). Record: 1. Patient height = cm 2. Final total volume of the bag (from chart below) = ml 3. Rate to be infused (from chart below) = ml/hr 4. Volume of Glutamine (Dipeptiven ) to be added = ml Height Final Total Volume of Bag Rate to be Infused Volume of Glutamine to be Added Amount of Normal Saline to be removed 196 cm 253 ml 10.5 ml/hr 240 ml 237 ml 198 cm 258 ml 10.8 ml/hr 245 ml 237 ml 201cm 266 ml 11.1 ml/hr 253 ml 237 ml 203 cm 271 ml 11.3 ml/hr 258 ml 237 ml 206 cm 278 ml 11.6 ml/hr 265 ml 237 ml 208 cm 283 ml 11.8 ml/hr 270 ml 237 ml 211 cm 291 ml 12.1/mL/hr 278 ml 237 ml 213 cm 296 ml 12.3 ml/hr 283 ml 237 ml 5. Volume of NS to be removed from bag = ml 6. Add Dipeptiven (#4), as per chart, to normal saline bag. Mix. 7. Expiry dating = 96 hours at room temperature. 8. Generate a label and attach to bag. Version: 3-Jul-09 (EU) Replaces version: 6-Dec-07 (EU)
24 Parenteral Supplements Worksheet Patient CR# / ID# Patient Initials Enrolment # Antioxidants + Glutamine (AOX+GLN) Patient will receive Selenium (AOX), Dipeptiven (GLN) and normal saline (or if hypernatremic DSW) Begin with a bag containing exactly 250 ml of normal saline (i.e. account for overfill): 1. Patient s height = cm 2. Normal Body Weight = (#1) 100 cm = kg 3. Volume of Dipeptiven to be added = (#2) x 2.5 ml = ml 4. Volume of Selenium to be added = 10 ml_ 5. Total Volume to be removed from 250 ml normal saline bag before adding study supplements: (#3) + (#4) = ml volume of dipeptiven volume of selenium 6. Add (#3) + (#4) + normal saline =_250 ml_ 7. Record the volumes of Dipeptiven, Selenium and Normal Saline on the Parenteral Study Supplement Log for this patient daily. 8. Generate a label and attach to bag. Dipeptiven 2.5 ml/kg/day =Glutamine 0.35 g/kg/day = L-alanyl-L-glutamine 0.5 g/kg/day version: 3-Jul-09 (EU) Replaces version: 9-Sept-07 (EU)
25 Parenteral Supplements Worksheet (Patients > 196 cm tall) Patient CR# / ID# Patient Initials Enrolment # 9. Record the volumes of Dipeptiven and Normal Saline on the Parenteral Study Supplement Log for this patient daily. Antioxidants + Glutamine (AOX+GLN) Patient will receive Selenium (AOX), Dipeptiven (GLN) and normal saline (or if hypernatremic D5W) Dosing: 1. Dosage of Selenium = 500mcg/day = 10mL/day regardless of height; 2. Dosage of Glutamine 0.35g/kg/day = L-alanyl-L-glutamine 0.5g/kg/day= Dipeptivan 2.5mL/kg/day 3. Dosing will be based on patient s Normal Body Weight using Broca Formula as follows: Normal Weight (kg) = Patient s Height(cm) Dose will be diluted in a NS bag to a final volume indicated in the chart below. 5. Rate of infusion determined from chart below. ( rate may be increased up to 2x the amount on day 1 for hours missed to conform to standard dosing times) 6. You must account for overfill in the normal saline bags as per table below. Beginning with a bag of exactly 250 ml of normal saline: Go to height chart (see below). Record: 1. Patient height = cm 2. Final total volume of the bag (from chart below) = ml 3. Rate to be infused (from chart below) = ml/hr 4. Volume of Selenium to be added = _10.0 ml_ 5. Volume of Glutamine (Dipeptiven ) to be added = ml Height Final Total Volume of Bag Rate to be Infused Volume of Selenium to be Added Volume of Glutamine to be Added 196 cm 250 ml 10.4 ml/hr 10 ml 240 ml 198 cm 255 ml 10.6 ml/hr 10 ml 245 ml 201cm 263 ml 11.0 ml/hr 10 ml 253 ml 203 cm 268 ml 11.2 ml/hr 10 ml 258 ml 206 cm 275 ml 11.5 ml/hr 10 ml 265 ml 208 cm 280 ml 11.7 ml/hr 10 ml 270 ml 211 cm 288 ml 12.0 ml/hr 10 ml 278 ml 213 cm 293 ml 12.2 ml/hr 10 ml 283 ml 6. Begin with an empty sterile bag, draw up: Selenium (#4) and Glutamine (#5). Add to the bag and mix. 7. Expiry dating = 96 hours at room temperature. 8. Generate a label and attach to bag. Version: 3-Jul-09 (EU) Replaces version: 6-Dec-07 (EU)
26 Parenteral Supplements Worksheet Patient CR# / ID# Patient Initials Enrolment # Placebo Patient will only receive normal saline (or if hypernatremic D5W) Begin with a bag containing exactly 250 ml of normal saline (i.e. account for overfill): 1. Volume of Dipeptiven to be added = _0_mL 2. Volume of Selenium to be added = 0 ml_ 3. No mixing needed. The patient will receive a 250 ml bag of normal saline. 4. Record the volume of Normal Saline (=250 ml) on the Parenteral Study Supplement Log, for this patient daily. 5. Generate a label and attach to bag. Dipeptiven and Selenium not required for this treatment arm. version: 3-Jul-09 (EU) Replaces version: 9-Sept-07 (EU)
27 Parenteral Supplements Worksheet (Patients > 196 cm tall) Patient CR# / ID# Patient Initials Enrolment # 9. Record the volumes of Selenium and Dipeptiven on the Parenteral Study Supplement Log for this patient daily. * May want to start with empty bag. Placebo Patient will only receive normal saline (or if hypernatremic D5W) Dosing: 1. Dose will consist of a 250mL NS bag with added NS to a final volume as indicated in chart below. 2. Rate of infusion determined from chart below Beginning with a bag of exactly 250 ml of normal saline: Go to height chart (see below). Record: 1. Patient height = cm 2. Final total volume of the bag (from chart below) = ml 3. Rate to be infused (from chart below) = ml/hr 4. Volume of normal saline to be added = ml Height Final total volume of bag Rate to be infused Amount of normal saline to be added 196 cm 250 ml 10.4 ml/hr 198 cm 255 ml 10.6 ml/hr 5 ml 201 cm 263 ml 11.0 ml/hr 13 ml 203 cm 268 ml 11.2 ml/hr 18 ml 206 cm 275 ml 11.5 ml/hr 25 ml 208 cm 280 ml 11.7 ml/hr 30 ml 211 cm 288 ml 12.0 ml/hr 38 ml 213 cm 293 ml 12.2 ml/hr 43 ml 5. Record the volume of Normal Saline (as per final volume on chart above) on the Parenteral Study Supplement Log, for this patient daily. 6. Generate a label and attach to the bag. Version: 3-Jul-09 (EU) Replaces version: 6-Dec-07 (EU)
28 Appendix G Parenteral Study Supplement Study: REDOXS Parenteral Component For Clinical trial Use Only Enrollment #: Directions: Infuse at 10 ml/hr Patient CR#/ID#: Storage: keep between Patient Name: Expiry: Physician: Date: 20
29 Appendix H. Height and Dose of Dipeptiven Ht (ft in) Ht (cms) Dipeptiven Se N/S Total mls mls mls mls
The REDOXS Study. REducing Deaths due to OXidative Stress. A randomized trial of glutamine and antioxidant supplementation in critically ill patients
 The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
The REDOXS Study. REducing Deaths due to OXidative Stress. A randomized trial of glutamine and antioxidant supplementation in critically ill patients
 The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
The REDOXS Study REducing Deaths due to OXidative Stress A randomized trial of glutamine and antioxidant supplementation in critically ill patients Pharmacy Manual This study is registered at Clinicaltrials.gov.
HOW TO MANAGE AND ADMINISTER THE TRIAL TREATMENT
 HOW TO MANAGE AND ADMINISTER THE TRIAL TREATMENT Protocol Code: ISRCTN11225767 How to manage and administer the trial treatment version 2.0 date 23/07/2017 What is the trial treatment? Random allocation
HOW TO MANAGE AND ADMINISTER THE TRIAL TREATMENT Protocol Code: ISRCTN11225767 How to manage and administer the trial treatment version 2.0 date 23/07/2017 What is the trial treatment? Random allocation
REducing Deaths from OXidative Stress
 C a n adian Critic a The REDOXS Study REducing Deaths due to OXidative Stress The REDOXS Study REducing Deaths from OXidative Stress Study Chair Dr. Daren Heyland, MD, FRCPC Project Leaders Rupinder Dhaliwal,
C a n adian Critic a The REDOXS Study REducing Deaths due to OXidative Stress The REDOXS Study REducing Deaths from OXidative Stress Study Chair Dr. Daren Heyland, MD, FRCPC Project Leaders Rupinder Dhaliwal,
Pharmacy Manual. Cambridge University Hospitals NHS Foundation Trust & University of Cambridge
 Page 1 of 11 Pharmacy Manual Trial Title: PRedicting Outcomes For Crohn s disease using a molecular biomarker (PROFILE) trial EudraCT Number: N/A (non-ctimp study) ISRCTN: 11808228 REC Reference: 17/EE/0382
Page 1 of 11 Pharmacy Manual Trial Title: PRedicting Outcomes For Crohn s disease using a molecular biomarker (PROFILE) trial EudraCT Number: N/A (non-ctimp study) ISRCTN: 11808228 REC Reference: 17/EE/0382
Ascribe Rx Online Resource Center User Guide
 Ascribe Rx Online Resource Center User Guide Contents Introduction... 1... 1 Wildcard functionality... 2 Canister Reports... 3 Canister aging... 3 Canister issues... 3 Current inventory... 4 Dispense Reports...
Ascribe Rx Online Resource Center User Guide Contents Introduction... 1... 1 Wildcard functionality... 2 Canister Reports... 3 Canister aging... 3 Canister issues... 3 Current inventory... 4 Dispense Reports...
Ratios and Proportions. Calculations for Pharmacy Technicians 9/21/2017. Presented by: Antonia Kraljevic PGY2 Pharmacy Practice Resident
 Calculations for Pharmacy Technicians Presented by: Antonia Kraljevic PGY2 Pharmacy Practice Resident The speaker has no actual or potential conflict of interest in relation to this presentation. Pharmacy
Calculations for Pharmacy Technicians Presented by: Antonia Kraljevic PGY2 Pharmacy Practice Resident The speaker has no actual or potential conflict of interest in relation to this presentation. Pharmacy
November 9, Clarification Memo 1 Version 3.0 HVTN 704/HPTN 085
 November 9, 2017 Clarification Memo 1 Version 3.0 HVTN 704/HPTN 085 A phase 2b study to evaluate the safety and efficacy of VRC01 broadly neutralizing monoclonal antibody in reducing acquisition of HIV-1
November 9, 2017 Clarification Memo 1 Version 3.0 HVTN 704/HPTN 085 A phase 2b study to evaluate the safety and efficacy of VRC01 broadly neutralizing monoclonal antibody in reducing acquisition of HIV-1
Index No: MMG11/1. Version: 1. Date ratified: 12 th November 2013
 Index No: Intravenous fluid prescription in children For previously well children aged one month to 16 years (excluding renal, cardiac, diabetic ketoacidosis and acute burns patients) Version: 1 Date ratified:
Index No: Intravenous fluid prescription in children For previously well children aged one month to 16 years (excluding renal, cardiac, diabetic ketoacidosis and acute burns patients) Version: 1 Date ratified:
EasyComp. The TPN Compatibility Software. Clinical Nutrition
 EasyComp The TPN Compatibility Software Clinical Nutrition Content 1. General Information 5 1.1 What is EasyComp? 4 1.2 Features and functions of EasyComp 5 1.3 IT requirements 5 t2. Starting EasyComp
EasyComp The TPN Compatibility Software Clinical Nutrition Content 1. General Information 5 1.1 What is EasyComp? 4 1.2 Features and functions of EasyComp 5 1.3 IT requirements 5 t2. Starting EasyComp
CYCLE Vanguard: Flow Sheet for Protocol Schedule of Forms + Procedures. Binder Title and Cover Pages. Required Materials Study Schema RC flowsheet
 Binder Title and Cover Pages Required Materials Study Schema flowsheet 1 1. Screening a. Screen patient for eligibility. ASAP after ICU admission (within 4 days of mechanical ventilation and 7 days of
Binder Title and Cover Pages Required Materials Study Schema flowsheet 1 1. Screening a. Screen patient for eligibility. ASAP after ICU admission (within 4 days of mechanical ventilation and 7 days of
Tech Lectures For the Pharmacy Technician
 1 Tech Lectures For the Pharmacy Technician P.O. Box 19357 Denver, CO 80219-0357 303-984-9877 Lecture 14 - Hospital Calculations It is respectfully requested by the Author that no part of this Tech Lecture
1 Tech Lectures For the Pharmacy Technician P.O. Box 19357 Denver, CO 80219-0357 303-984-9877 Lecture 14 - Hospital Calculations It is respectfully requested by the Author that no part of this Tech Lecture
Guidance on Bulk Prescribing for Care Home Patients
 Guidance on Bulk Prescribing for Care Home Patients Introduction Many patients in care homes taking medicines when required (PRN) can inevitably present problems for the prescriber in determining the quantity
Guidance on Bulk Prescribing for Care Home Patients Introduction Many patients in care homes taking medicines when required (PRN) can inevitably present problems for the prescriber in determining the quantity
Research Compliance and Quality Assurance Program (RCQA): Audit Checklist Subject Specific
 Protocol Title / Code: Sponsor: PI Name: Auditor Name: Audit Date(s): Subject #: Key Dates: Date participant was identified: Date of Initial Consent: Date of Optional Consent: Date of Re-consent(s): Date
Protocol Title / Code: Sponsor: PI Name: Auditor Name: Audit Date(s): Subject #: Key Dates: Date participant was identified: Date of Initial Consent: Date of Optional Consent: Date of Re-consent(s): Date
ARCADIA Study Coordinator Training Investigator Meeting
 ARCADIA Study Coordinator Training Learning Goals 1. The coordinator will be able to identify the 3 screening tests required to meet the atrial cardiopathy criteria for ARCADIA. 2. The coordinator will
ARCADIA Study Coordinator Training Learning Goals 1. The coordinator will be able to identify the 3 screening tests required to meet the atrial cardiopathy criteria for ARCADIA. 2. The coordinator will
Pharmacy Plan Guidance
 Pharmacy Plan Guidance The pharmacy plan is a tool used during the site readiness process to develop and document the site-specific procedures for study drug ordering, labeling and dispensing for the SHINE
Pharmacy Plan Guidance The pharmacy plan is a tool used during the site readiness process to develop and document the site-specific procedures for study drug ordering, labeling and dispensing for the SHINE
Prevention of Transcription Errors with Pediatric TPN Orders Using a Web Interface
 Prevention of Transcription Errors with Pediatric TPN Orders Using a Web Interface Organization Name: University of Maryland Type: Acute Care Hospital Medical Center Contact Person: Belinda Todjo, PharmD
Prevention of Transcription Errors with Pediatric TPN Orders Using a Web Interface Organization Name: University of Maryland Type: Acute Care Hospital Medical Center Contact Person: Belinda Todjo, PharmD
Nonparenteral medications
 Nonparenteral medications Capsules and unscored tablets are rounded to the nearest whole tablet. Scored tablets are rounded to the nearest 1/2 tablet. Liquid medications are rounded to one decimal place
Nonparenteral medications Capsules and unscored tablets are rounded to the nearest whole tablet. Scored tablets are rounded to the nearest 1/2 tablet. Liquid medications are rounded to one decimal place
Review calculation procedures for the following:
 Review calculation procedures for the following: Convert in the Metric System 1000 mg = 1 gram 1000 ml = 1 liter To convert from grams to mg, move the decimal point 3 places to the right (add zeros to
Review calculation procedures for the following: Convert in the Metric System 1000 mg = 1 gram 1000 ml = 1 liter To convert from grams to mg, move the decimal point 3 places to the right (add zeros to
This document is to help guide the use of the provided GRH IV Iron Sucrose package. The documents included in the IV Iron Sucrose Package are:
 This document is to help guide the use of the provided GRH IV Iron Sucrose package. The documents included in the IV Iron Sucrose Package are: 1. Adult Outpatient Iron Sucrose Order set (page 2 and 3)
This document is to help guide the use of the provided GRH IV Iron Sucrose package. The documents included in the IV Iron Sucrose Package are: 1. Adult Outpatient Iron Sucrose Order set (page 2 and 3)
DRUG ORDERING & DISPENSING:
 STUDY DRUG: List the study drugs that will be used at your site based on your hospital formulary 1. Insulin Humulin R 2. Insulin Humalog 3. Insulin Lantus DRUG SUPPLY & STORAGE: Since study drugs are not
STUDY DRUG: List the study drugs that will be used at your site based on your hospital formulary 1. Insulin Humulin R 2. Insulin Humalog 3. Insulin Lantus DRUG SUPPLY & STORAGE: Since study drugs are not
Intravenous Immunoglobulin (IVIg) prescribing guidance
 Intravenous Immunoglobulin (IVIg) prescribing guidance Kejal Mehta (Specialist Pharmacist) [December 2016] Review Date: December 2017 Contents Introduction:... 2 Prior to Intravenous immunoglobulin (IVIg)
Intravenous Immunoglobulin (IVIg) prescribing guidance Kejal Mehta (Specialist Pharmacist) [December 2016] Review Date: December 2017 Contents Introduction:... 2 Prior to Intravenous immunoglobulin (IVIg)
EVERY SHOT COUNTS C O R O N A D O R O O M A U G U S T 1 6,
 EVERY SHOT COUNTS 2016-2 0 1 7 S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S C O R O N A D O R O O M A U G U S T 1 6, 2 0 1 6
EVERY SHOT COUNTS 2016-2 0 1 7 S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S C O R O N A D O R O O M A U G U S T 1 6, 2 0 1 6
TOTAL PARENTERAL NUTRITION
 TOTAL PARENTERAL NUTRITION Indication See algorithm. Timing Start TPN as indicated on algorithm 1. There is no need to build up TPN volume. The volume of TPN (including lipids) should equate to the total
TOTAL PARENTERAL NUTRITION Indication See algorithm. Timing Start TPN as indicated on algorithm 1. There is no need to build up TPN volume. The volume of TPN (including lipids) should equate to the total
MOST Clinical Performing Site Study Drug Procedures
 Abbreviations and Definitions MOST Clinical Performing Site Study Drug Procedures ACS AIS Arm Acute Coronary Syndrome Acute Ischemic Stroke Argatroban (100µg/kg bolus and a 12-hour infusion at 3µg/kg/min)
Abbreviations and Definitions MOST Clinical Performing Site Study Drug Procedures ACS AIS Arm Acute Coronary Syndrome Acute Ischemic Stroke Argatroban (100µg/kg bolus and a 12-hour infusion at 3µg/kg/min)
Chapter 12.0: Test Article Preparation, Accountability, (Pharmacy Manual) and Administration
 Chapter 12.0: Test Article Preparation, Accountability, (Pharmacy Manual) and Administration Table of Contents: 12.1. Randomization...12-2 12.2. Treatment...12-2 12.3. Drug Supply and Storage...12-2 12.4.
Chapter 12.0: Test Article Preparation, Accountability, (Pharmacy Manual) and Administration Table of Contents: 12.1. Randomization...12-2 12.2. Treatment...12-2 12.3. Drug Supply and Storage...12-2 12.4.
Guidance on Bulk Prescribing for Care Home Residents
 Guidance on Bulk Prescribing for Care Home Residents There are many residents in care homes taking medicines when required, which may present problems for the prescriber in determining the quantity to
Guidance on Bulk Prescribing for Care Home Residents There are many residents in care homes taking medicines when required, which may present problems for the prescriber in determining the quantity to
Documenting Patient Immunization. New Brunswick 2018/19
 Documenting Patient Immunization New Brunswick 2018/19 Table of Contents Documenting Patient Immunization New Brunswick...3 Immunization Module Features...4 Configuration...5 Marketing Message Setup...6
Documenting Patient Immunization New Brunswick 2018/19 Table of Contents Documenting Patient Immunization New Brunswick...3 Immunization Module Features...4 Configuration...5 Marketing Message Setup...6
11.1 Supplemental Antioxidant Nutrients: Combined Vitamins and Trace Elements April 2013
 . Supplemental Antioxidant Nutrients: Combined Vitamins and Trace Elements April 23 23 Recommendation: Based on 7 level and 7 level 2 studies, the use of supplemental combined vitamins and trace elements
. Supplemental Antioxidant Nutrients: Combined Vitamins and Trace Elements April 23 23 Recommendation: Based on 7 level and 7 level 2 studies, the use of supplemental combined vitamins and trace elements
Refilling a Prescription with No Refills Remaining
 ................................................................................................... Refilling a Prescription with No Refills Remaining Pharmacy Technology Solutions May, 2013 Refilling
................................................................................................... Refilling a Prescription with No Refills Remaining Pharmacy Technology Solutions May, 2013 Refilling
APIDRA (insulin glulisine) injection vial APIDRA SOLOSTAR (insulin glulisine) subcutaneous solution pen-injector
 APIDRA SOLOSTAR (insulin glulisine) subcutaneous solution pen-injector Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
APIDRA SOLOSTAR (insulin glulisine) subcutaneous solution pen-injector Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Documenting Patient Immunization. Ontario 2018/19
 Documenting Patient Immunization Ontario 2018/19 Table of Contents Documenting Patient Immunization Ontario...3 Immunization Module Features...4 Configuration...5 Marketing Message Setup...6 Paper Mode...9
Documenting Patient Immunization Ontario 2018/19 Table of Contents Documenting Patient Immunization Ontario...3 Immunization Module Features...4 Configuration...5 Marketing Message Setup...6 Paper Mode...9
PAGE 1 of 6. Summative Assessment - Dietary Analysis Report Data Worksheets
 1 PAGE 1 of 6 Name _ S.A. # Hour Summative Assessment - Dietary Analysis Report Data Worksheets READ CAREFULLY!!! Welcome to the PROJECT part of your Dietary Analysis Report. You will be entering information
1 PAGE 1 of 6 Name _ S.A. # Hour Summative Assessment - Dietary Analysis Report Data Worksheets READ CAREFULLY!!! Welcome to the PROJECT part of your Dietary Analysis Report. You will be entering information
Medicines Optimisation Team Standard Operating Procedure Insulin Quantities Audit
 Medicines Optimisation Team Standard Operating Procedure Insulin Quantities Audit Aim Review and rationalise the insulin quantities based on dosage instructions for diabetic patients in order to align
Medicines Optimisation Team Standard Operating Procedure Insulin Quantities Audit Aim Review and rationalise the insulin quantities based on dosage instructions for diabetic patients in order to align
1. Intrathecal must never be abbreviated to IT on a prescription form True / False
 Appendix 1: Intrathecal SACT Assessment Questions Core General Questions must be completed by all staff. 1. Intrathecal must never be abbreviated to IT on a prescription form 2. Intrathecal SACT must be
Appendix 1: Intrathecal SACT Assessment Questions Core General Questions must be completed by all staff. 1. Intrathecal must never be abbreviated to IT on a prescription form 2. Intrathecal SACT must be
Package leaflet: Information for the user. Aminoven 5% Solution for infusion Aminoven 10% Solution for infusion Aminoven 15% Solution for infusion
 Package leaflet: Information for the user Aminoven 5% Solution for infusion Aminoven 10% Solution for infusion Aminoven 15% Solution for infusion Read all of this leaflet carefully before you start using
Package leaflet: Information for the user Aminoven 5% Solution for infusion Aminoven 10% Solution for infusion Aminoven 15% Solution for infusion Read all of this leaflet carefully before you start using
Drug Prior Authorization Form Opdivo (nivolumab)
 This document contains both information and form fields. To read information, use the Down Arrow from a form field. Drug Prior Authorization Form The purpose of this form is to obtain information required
This document contains both information and form fields. To read information, use the Down Arrow from a form field. Drug Prior Authorization Form The purpose of this form is to obtain information required
CHAPTER 6 EXAMINATION SCHEDULE 6.1 OVERVIEW OF SCHEDULE AND DESCRIPTION OF PARTICIPANT VISITS
 CHAPTER 6 EXAMINATION SCHEDULE 6.1 OVERVIEW OF SCHEDULE AND DESCRIPTION OF PARTICIPANT VISITS This chapter presents the examination schedule for AREDS participants during Phase II, highlighting the important
CHAPTER 6 EXAMINATION SCHEDULE 6.1 OVERVIEW OF SCHEDULE AND DESCRIPTION OF PARTICIPANT VISITS This chapter presents the examination schedule for AREDS participants during Phase II, highlighting the important
SECTION 4: RECRUIT PARTICIPANTS
 SECTION 4: RECRUIT PARTICIPANTS Contents Participant Eligibility & Enrollment... 2 Screening... 2 Study ID Numbers... 2 Inclusion Criteria... 2 Exclusion Criteria... 4 Co-Enrollment... 5 Informed Consent
SECTION 4: RECRUIT PARTICIPANTS Contents Participant Eligibility & Enrollment... 2 Screening... 2 Study ID Numbers... 2 Inclusion Criteria... 2 Exclusion Criteria... 4 Co-Enrollment... 5 Informed Consent
Newfoundland and Labrador Pharmacy Board Standards of Practice
 Newfoundland and Labrador Pharmacy Board Standards of Practice Standards for the Safe and Effective Administration of Drug Therapy by Inhalation or Injection June 2015 Table of Contents 1) Introduction...
Newfoundland and Labrador Pharmacy Board Standards of Practice Standards for the Safe and Effective Administration of Drug Therapy by Inhalation or Injection June 2015 Table of Contents 1) Introduction...
Standard Operating Procedures Clinical and Translational Research Center
 Standard Operating Procedures Clinical and Translational Research Center Title: Approved By: Number of Pages: Frequently Sampled Intravenous Glucose Tolerance Test (FSIVGTT) Effective March 19, 2012 Date:
Standard Operating Procedures Clinical and Translational Research Center Title: Approved By: Number of Pages: Frequently Sampled Intravenous Glucose Tolerance Test (FSIVGTT) Effective March 19, 2012 Date:
HIV MANAGEMENT PROGRAMME APPLICATION FORM
 Private Private Bag X82081, Bag X82081, Rustenburg, Rustenburg, 0300 0300 Tel: Tel: (014) 590 5901700 1900 Fax: Fax: 086 (014) 577 0274 591 4570 www.platinumhealth.co.za www.platinumhealth.co.za ZZGPlatinumHealthClinicalMotivation@angloamerican.com
Private Private Bag X82081, Bag X82081, Rustenburg, Rustenburg, 0300 0300 Tel: Tel: (014) 590 5901700 1900 Fax: Fax: 086 (014) 577 0274 591 4570 www.platinumhealth.co.za www.platinumhealth.co.za ZZGPlatinumHealthClinicalMotivation@angloamerican.com
PACKAGE LEAFLET: INFORMATION FOR THE USER
 biosyn Arzneimittel GmbH Schorndorfer Strasse 32, 70734 Fellbach, Germany PACKAGE LEAFLET: INFORMATION FOR THE USER Selenase 100 micrograms, solution for injection (50 micrograms/ml) 100 microgram selenium
biosyn Arzneimittel GmbH Schorndorfer Strasse 32, 70734 Fellbach, Germany PACKAGE LEAFLET: INFORMATION FOR THE USER Selenase 100 micrograms, solution for injection (50 micrograms/ml) 100 microgram selenium
Immunization Toolkit for Immunization Providers
 Immunization Toolkit for Immunization Providers Communicable Disease Prevention and Control Division Public Health Branch Department of Health and Wellness 09/09/2014 1 Contents Introduction... 3 General
Immunization Toolkit for Immunization Providers Communicable Disease Prevention and Control Division Public Health Branch Department of Health and Wellness 09/09/2014 1 Contents Introduction... 3 General
Version: Date. Version 2: 02-September Feb-2016
 A RandomizEd Trial of ENtERal Glutamine to minimize Thermal Injury Pharmacy Manual Intended Audience: Pharmacists, Pharmacy Technicians This study is registered at Clinicaltrials.gov. Identification number
A RandomizEd Trial of ENtERal Glutamine to minimize Thermal Injury Pharmacy Manual Intended Audience: Pharmacists, Pharmacy Technicians This study is registered at Clinicaltrials.gov. Identification number
ProScript User Guide. Pharmacy Access Medicines Manager
 User Guide Pharmacy Access Medicines Manager Version 3.0.0 Release Date 01/03/2014 Last Reviewed 11/04/2014 Author Rx Systems Service Desk (T): 01923 474 600 Service Desk (E): servicedesk@rxsystems.co.uk
User Guide Pharmacy Access Medicines Manager Version 3.0.0 Release Date 01/03/2014 Last Reviewed 11/04/2014 Author Rx Systems Service Desk (T): 01923 474 600 Service Desk (E): servicedesk@rxsystems.co.uk
Investigational Product Procedures Manual
 A Randomized Trial of Supplemental Parenteral Nutrition in Under and Over Weight Critically Ill Patients: The TOP UP Trial Investigational Product Procedures Manual Intended Audience: Research Cooridnators/Pharmacists
A Randomized Trial of Supplemental Parenteral Nutrition in Under and Over Weight Critically Ill Patients: The TOP UP Trial Investigational Product Procedures Manual Intended Audience: Research Cooridnators/Pharmacists
INFORMATION FOR FILLING OUT APPLICATION FORM FOR AN EXEMPTION TO USE A CONTROLLED SUBSTANCE FOR SCIENTIFIC PURPOSES (a Health Canada document)
 INFORMATION FOR FILLING OUT APPLICATION FORM FOR AN EXEMPTION TO USE A CONTROLLED SUBSTANCE FOR SCIENTIFIC PURPOSES (a Health Canada document) Quote from Health Canada website: http://www.hc-sc.gc.ca/hc-ps/substancontrol/exemptions/index-eng.php
INFORMATION FOR FILLING OUT APPLICATION FORM FOR AN EXEMPTION TO USE A CONTROLLED SUBSTANCE FOR SCIENTIFIC PURPOSES (a Health Canada document) Quote from Health Canada website: http://www.hc-sc.gc.ca/hc-ps/substancontrol/exemptions/index-eng.php
Urine Dipstick Medical Mobile Unit: Patient Test Procedure
 Urine Dipstick Medical Mobile Unit: Patient Test Procedure Children s Hospital: Emergency Department Medical Mobile Unit PURPOSE To provide instruction in performing a manual Routine Urine Test using urine
Urine Dipstick Medical Mobile Unit: Patient Test Procedure Children s Hospital: Emergency Department Medical Mobile Unit PURPOSE To provide instruction in performing a manual Routine Urine Test using urine
Health Services. Procedure Manual
 Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Management of Adult Safety Net Vaccine Program All Clinic Sites To ensure uniform management of this resource throughout the agency. REPORT THROUGH AN AGENCY COORDINATOR. 1) Designated staff positions/persons
Common Infusions for Neonatal Use
 Common Infusions for Neonatal Use Document Title and Reference : Common Infusions for Neonatal Use Main Author (s) Dr N B Soni Ratified by: LSC CEG Date Ratified: May 2016 Review Date: May 2018 Version:
Common Infusions for Neonatal Use Document Title and Reference : Common Infusions for Neonatal Use Main Author (s) Dr N B Soni Ratified by: LSC CEG Date Ratified: May 2016 Review Date: May 2018 Version:
Section: Claims Payment - Cognitive Services (PAS Policy Administered by Drug Plan and Extended Benefits Branch)
 Section: Claims Payment - Cognitive Services (PAS Policy Administered by Drug Plan and Extended Benefits Branch) Reference Date of Issue June 3, 2009 Approved by Director of Operations PACT Partnership
Section: Claims Payment - Cognitive Services (PAS Policy Administered by Drug Plan and Extended Benefits Branch) Reference Date of Issue June 3, 2009 Approved by Director of Operations PACT Partnership
Eligibility Form. 1. Patient Profile. (This form must be completed before the first dose is dispensed.) Request prior approval for enrolment
 Bevacizumab in combination with Paclitaxel and Carboplatin - Frontline Treatment (Previously Untreated) Ovarian, Fallopian Tube, and Primary Peritoneal Cancer (This form must be completed before the first
Bevacizumab in combination with Paclitaxel and Carboplatin - Frontline Treatment (Previously Untreated) Ovarian, Fallopian Tube, and Primary Peritoneal Cancer (This form must be completed before the first
Homely Remedies Guidance for Care Homes
 Homely Remedies Guidance for Care Homes What is a homely remedy? Homely or household remedy is another name for a non-prescription medicine which is used in a care home for the short term management of
Homely Remedies Guidance for Care Homes What is a homely remedy? Homely or household remedy is another name for a non-prescription medicine which is used in a care home for the short term management of
ISMP guidelines that support intravenous drug delivery system safety
 ISMP guidelines that support intravenous drug delivery system safety Michael R. Cohen, RPh, MS, ScD (hon), DPS (hon), FASHP Institute for Safe Medication Practices Sterile Compounding Errors and Patient
ISMP guidelines that support intravenous drug delivery system safety Michael R. Cohen, RPh, MS, ScD (hon), DPS (hon), FASHP Institute for Safe Medication Practices Sterile Compounding Errors and Patient
Parenteral Nutrition Recommendations for Pediatric Patients
 Fluid Dextrose Amino acid Lipid Parenteral Nutrition Recommendations for Pediatric Patients (Calculated for normal organ function and normal caloric requirements) PN orders are due by 11 AM daily Newborn
Fluid Dextrose Amino acid Lipid Parenteral Nutrition Recommendations for Pediatric Patients (Calculated for normal organ function and normal caloric requirements) PN orders are due by 11 AM daily Newborn
Controlled Drug Process. Khoa Vo Masters candidate University of Canterbury
 Controlled Drug Process Khoa Vo Masters candidate University of Canterbury Bachelor of Pharmacy (VN) PG Diploma in Palliative Care (NZ) Cancer Patients in Vietnam 250,000 of 89 million Increased by 150,000
Controlled Drug Process Khoa Vo Masters candidate University of Canterbury Bachelor of Pharmacy (VN) PG Diploma in Palliative Care (NZ) Cancer Patients in Vietnam 250,000 of 89 million Increased by 150,000
Cumulative Math Practice Worksheet
 Name: Date: Use the following to answer questions 1-3: Fill in the blank for each pair of ratios to form a proportion: 1. How many capsules are needed to fill a prescription for three days for mefenamic
Name: Date: Use the following to answer questions 1-3: Fill in the blank for each pair of ratios to form a proportion: 1. How many capsules are needed to fill a prescription for three days for mefenamic
PHARMACY DOSING AND ORDERING GUIDE
 PHARMACY DOSING AND ORDERING GUIDE FIRST AND ONLY APPROVED TREATMENT FOR PATIENTS WITH VOD WITH RENAL OR PULMONARY DYSFUNCTION POST HSCT VOD=veno-occlusive disease Indication Defitelio (defibrotide sodium)
PHARMACY DOSING AND ORDERING GUIDE FIRST AND ONLY APPROVED TREATMENT FOR PATIENTS WITH VOD WITH RENAL OR PULMONARY DYSFUNCTION POST HSCT VOD=veno-occlusive disease Indication Defitelio (defibrotide sodium)
Navigating Alliance Protocols
 Navigating Alliance Protocols Morgen Alexander-Young, MPH Alliance Central Protocol Operations Program Alliance Spring 2017 Group Meeting Alliance Protocol History Alliance for Clinical Trials in Oncology
Navigating Alliance Protocols Morgen Alexander-Young, MPH Alliance Central Protocol Operations Program Alliance Spring 2017 Group Meeting Alliance Protocol History Alliance for Clinical Trials in Oncology
IMPAACT MOCHA More Options for Children and Adolescents
 IMPAACT 2017 Phase I/II Study of the Safety, Acceptability, Tolerability, and Pharmacokinetics of Oral and Long-Acting Injectable Cabotegravir and Long-Acting Injectable Rilpivirine in Virologically Suppressed
IMPAACT 2017 Phase I/II Study of the Safety, Acceptability, Tolerability, and Pharmacokinetics of Oral and Long-Acting Injectable Cabotegravir and Long-Acting Injectable Rilpivirine in Virologically Suppressed
EHS Integrator Controlled Substance Waste Pickup Request Help Guide
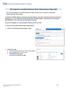 EHS Integrator Controlled Substance Waste Pickup Request Help Guide Use the EHS Integrator Controlled Substance Waste Pickup Form to request a Controlled Substance/Drug waste pickup. To request a controlled
EHS Integrator Controlled Substance Waste Pickup Request Help Guide Use the EHS Integrator Controlled Substance Waste Pickup Form to request a Controlled Substance/Drug waste pickup. To request a controlled
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
Paediatric Prescribing Pocket Guide September 2014 (Version 1)
 PAGE 1 Paediatric Prescribing Pocket Guide September 2014 (Version 1) In children, the risk of medication errors is often exacerbated by the need for calculations to determine the dose. Prescribing errors
PAGE 1 Paediatric Prescribing Pocket Guide September 2014 (Version 1) In children, the risk of medication errors is often exacerbated by the need for calculations to determine the dose. Prescribing errors
Catalogue. Medication Safety Labels CONTENTS 1. For the Safe Management of Medication Version 2. Anaesthetic Syringe Labels
 Medication Safety Labels Catalogue For the Safe Management of Medication Version 2 CONTENTS 1 4 8 11 12 Sterile Labels Injectable Medicines, Fluids & Line Labels Pre-printed Medicine Line Labels Anaesthetic
Medication Safety Labels Catalogue For the Safe Management of Medication Version 2 CONTENTS 1 4 8 11 12 Sterile Labels Injectable Medicines, Fluids & Line Labels Pre-printed Medicine Line Labels Anaesthetic
SAVAYSA (edoxaban tosylate) oral tablet
 SAVAYSA (edoxaban tosylate) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
SAVAYSA (edoxaban tosylate) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Therapy Activation Date: Review Date:
 MEDICAL DIRECTIVE Title: Nicotine Replacement Number: TCFHT-MD10 Therapy Activation Date: 14-06-2011 Review Date: 01-05-2016 Sponsoring/Contact Person(s) (name, position, contact particulars): Sherry Kennedy,
MEDICAL DIRECTIVE Title: Nicotine Replacement Number: TCFHT-MD10 Therapy Activation Date: 14-06-2011 Review Date: 01-05-2016 Sponsoring/Contact Person(s) (name, position, contact particulars): Sherry Kennedy,
Care homes - Homely remedies
 Bulletin 72 August 2014 Care homes - Homely remedies Care home staff have a recognised duty of care to be able to respond to minor symptoms experienced by residents. A homely remedy is a medicinal product
Bulletin 72 August 2014 Care homes - Homely remedies Care home staff have a recognised duty of care to be able to respond to minor symptoms experienced by residents. A homely remedy is a medicinal product
HOSA MEDICAL MATH CONVERSION CHART METRIC SYSTEM
 HOSA MEDICAL MATH CONVERSION CHART METRIC SYSTEM Length 1 meter = 100 centimeters = 1000 millimeters 10 millimeters = 1 centimeter Weight 1 gram = 1000 milligrams 1 milligram = 1000 micrograms 1 kilogram
HOSA MEDICAL MATH CONVERSION CHART METRIC SYSTEM Length 1 meter = 100 centimeters = 1000 millimeters 10 millimeters = 1 centimeter Weight 1 gram = 1000 milligrams 1 milligram = 1000 micrograms 1 kilogram
Education for self administration of intravenous therapy HOME IV THERAPY. 30 minute - Baxter Pump Tobramycin
 HOME IV THERAPY Tobramycin Tobramycin Check the order on the drug chart This can change when the results from your blood test come through. Your doctor will change the order, if required. A copy of the
HOME IV THERAPY Tobramycin Tobramycin Check the order on the drug chart This can change when the results from your blood test come through. Your doctor will change the order, if required. A copy of the
Introduction. Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients)
 Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
Guidelines for patient involvement in the administration of insulin under supervision in hospital (Adult patients) Introduction This guideline is designed to provide a framework for patients to administer
Cox College Springfield, MO. Dosage Calculation Competency Level II Practice Sheet STUDENT NAME: DATE: STUDENT I.D. #: ADVISOR:
 Cox College Springfield, MO Dosage Calculation Competency Level II Practice Sheet Updated 4/2015 STUDENT NAME: DATE: / / STUDENT I.D. #: ADVISOR: A 95% must be achieved on the competency exam to progress
Cox College Springfield, MO Dosage Calculation Competency Level II Practice Sheet Updated 4/2015 STUDENT NAME: DATE: / / STUDENT I.D. #: ADVISOR: A 95% must be achieved on the competency exam to progress
Nancy Knickerbocker State Flu Vaccine Coordinator County of San Diego Immunization Program
 2017-2 0 1 8 S TAT E I N F L U E N Z A VA C C I N E D O C U M E N TAT I O N REQUIREMENTS F O R P U B L I C H E A LT H C E N T E R S C O R O N A D O R O O M Nancy Knickerbocker State Flu Vaccine Coordinator
2017-2 0 1 8 S TAT E I N F L U E N Z A VA C C I N E D O C U M E N TAT I O N REQUIREMENTS F O R P U B L I C H E A LT H C E N T E R S C O R O N A D O R O O M Nancy Knickerbocker State Flu Vaccine Coordinator
Freka Balloon Gastrostomy Feeding. CARE GUIDELINES For Patients & Carers
 Freka Balloon Gastrostomy Feeding CARE GUIDELINES For Patients & Carers Useful Contacts GP Hospital Dietitian Community Dietitian Nutrition Nurse District Nurse Nutrition Feed Company/Nurse Advisor Hospital
Freka Balloon Gastrostomy Feeding CARE GUIDELINES For Patients & Carers Useful Contacts GP Hospital Dietitian Community Dietitian Nutrition Nurse District Nurse Nutrition Feed Company/Nurse Advisor Hospital
LOKELMA (sodium zirconium cyclosilicate) oral suspension
 LOKELMA (sodium zirconium cyclosilicate) oral suspension Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
LOKELMA (sodium zirconium cyclosilicate) oral suspension Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
Diabetes Medical Management Plan/Individualized Healthcare Plan. Part A: Contact Information must be completed by the parent/guardian.
 Middle School 908 689 0750 ext. 2020 WARREN HILLS REGIONAL SCHOOL DISTRICT Washington, NJ 07882 HEALTH OFFICES High School 908 689 3050 ext. 2 MS FAX 908 835 0570 HS FAX 908 835 8511 Diabetes Medical Management
Middle School 908 689 0750 ext. 2020 WARREN HILLS REGIONAL SCHOOL DISTRICT Washington, NJ 07882 HEALTH OFFICES High School 908 689 3050 ext. 2 MS FAX 908 835 0570 HS FAX 908 835 8511 Diabetes Medical Management
1. When are dispensers able to provide publicly funded naloxone emergency kits for Ontarians?
 Ontario Public Drug Programs Division Ontario Naloxone Program for Pharmacies (ONPP) Frequently Asked Questions for Pharmacy Dispensers: Providing Publicly Funded Naloxone Kits and Claims Submission Using
Ontario Public Drug Programs Division Ontario Naloxone Program for Pharmacies (ONPP) Frequently Asked Questions for Pharmacy Dispensers: Providing Publicly Funded Naloxone Kits and Claims Submission Using
*Note - If associate is unsuccessful in their attempt to complete the dosage calculation test
 Competency Statement: Demonstrates ability to use basic mathematic concepts related to the correct administration of medications. Instructional Strategies: Review of basic math concepts, completion of
Competency Statement: Demonstrates ability to use basic mathematic concepts related to the correct administration of medications. Instructional Strategies: Review of basic math concepts, completion of
Good Practice Guidance on Homely Remedy Policy For Adult Service Users in Care Homes
 Good Practice Guidance on Homely Remedy Policy For Adult Service Users in Care Homes This good practice guidance is intended to be used as a framework for care home managers to adapt when writing their
Good Practice Guidance on Homely Remedy Policy For Adult Service Users in Care Homes This good practice guidance is intended to be used as a framework for care home managers to adapt when writing their
Research & Development. J H Pacynko and J Illingworth. Research, pharmacy and R&D staff
 Department Title of SOP Research & Development Safety reporting SOP SOP reference no: R&D GCP SOP 07 Authors: Current version number and date: J H Pacynko and J Illingworth Version 9, 31.01.18 Next review
Department Title of SOP Research & Development Safety reporting SOP SOP reference no: R&D GCP SOP 07 Authors: Current version number and date: J H Pacynko and J Illingworth Version 9, 31.01.18 Next review
3.0 POSITION ON PRE-LOADING SYRINGES WITH VACCINE
 1.0 PURPOSE 1.1 To provide guidance regarding the limited practice of pre-loading syringes with vaccine in controlled mass immunization settings as a part of a strategy to improve clinic efficiency while
1.0 PURPOSE 1.1 To provide guidance regarding the limited practice of pre-loading syringes with vaccine in controlled mass immunization settings as a part of a strategy to improve clinic efficiency while
Before you use NovoThirteen. When NovoThirteen should not be used
 NovoThirteen 2500 U catridecacog (rys) Recombinant coagulation factor X Consumer Medicine nformation What is in this leaflet What NovoThirteen is used for 1 Before you use NovoThirteen 1 Using NovoThirteen...
NovoThirteen 2500 U catridecacog (rys) Recombinant coagulation factor X Consumer Medicine nformation What is in this leaflet What NovoThirteen is used for 1 Before you use NovoThirteen 1 Using NovoThirteen...
TEST ARTICLE TEST PERFORMED PERFORMING LABORATORY SAMPLES SUBMITTED RESULT
 Close To Nature Facility I Facility II 1796-6 Kanaya Kawara. Haibara-Gun. Kanaya, Shizuoka 428-0021 Japan. Phone: +81-547-47-2250 Fax: +81-547-47-2251 4-29-3 Takaoka Higashi 101 Hamamatsu, Shizuoka 433-8117
Close To Nature Facility I Facility II 1796-6 Kanaya Kawara. Haibara-Gun. Kanaya, Shizuoka 428-0021 Japan. Phone: +81-547-47-2250 Fax: +81-547-47-2251 4-29-3 Takaoka Higashi 101 Hamamatsu, Shizuoka 433-8117
C H C S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S
 2017-2 0 1 8 C H C S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S C O R O N A D O R O O M Nancy Knickerbocker State Flu Vaccine
2017-2 0 1 8 C H C S TAT E I N F L U E N Z A VA C C I N E R E Q U I R E M E N T S T R A I N I N G F O R C O M M U N I T Y P R O V I D E R S C O R O N A D O R O O M Nancy Knickerbocker State Flu Vaccine
LEVEMIR (insulin detemir) subcutaneous solution LEVEMIR FLEXTOUCH (insulin detemir) subcutaneous solution pen-injector
 LEVEMIR FLEXTOUCH (insulin detemir) subcutaneous solution pen-injector Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
LEVEMIR FLEXTOUCH (insulin detemir) subcutaneous solution pen-injector Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Creating EVENTS in TPN s Partner Portal Step 1: Scroll down to the footer of the home page and click on PARTNER LOGIN:
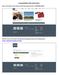 Creating EVENTS in TPN s Partner Portal Step 1: Scroll down to the footer of the home page and click on PARTNER LOGIN: Step 1A: You can also access the login portal by going directly to the following link:
Creating EVENTS in TPN s Partner Portal Step 1: Scroll down to the footer of the home page and click on PARTNER LOGIN: Step 1A: You can also access the login portal by going directly to the following link:
CYSTARAN (cysteamine hydrochloride) ophthalmic solution
 CYSTARAN (cysteamine hydrochloride) ophthalmic solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
CYSTARAN (cysteamine hydrochloride) ophthalmic solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
STATE FUNDED INFLUENZA FINAL OUTCOME REPORT AND SDIR DOCUMENTATION
 2014-2015 STATE FUNDED INFLUENZA FINAL OUTCOME REPORT AND SDIR DOCUMENTATION Nancy Knickerbocker Quality Assurance Specialist County of San Diego Immunization Program 1 DOCUMENTING UNACCOUNTED STATE INFLUENZA
2014-2015 STATE FUNDED INFLUENZA FINAL OUTCOME REPORT AND SDIR DOCUMENTATION Nancy Knickerbocker Quality Assurance Specialist County of San Diego Immunization Program 1 DOCUMENTING UNACCOUNTED STATE INFLUENZA
Vaccine Starter Kit Program FAQs
 Vaccine Starter Kit Program FAQs Program How does this program help Health Mart pharmacies provide vaccines for their customers? McKesson has designed a comprehensive program specifically for Health Mart
Vaccine Starter Kit Program FAQs Program How does this program help Health Mart pharmacies provide vaccines for their customers? McKesson has designed a comprehensive program specifically for Health Mart
Medicare & Dual Options. 1. Every page of the EMR document must include: a. Member Name b. Patient Identifiers (i.e. Date of Birth) c.
 Medicare & SUBMITTING PROGRESS NOTES OR EMR You may use your own progress notes or Electronic Medical Record (EMR) to document the annual comprehensive examination. The EMR must include the elements indicated
Medicare & SUBMITTING PROGRESS NOTES OR EMR You may use your own progress notes or Electronic Medical Record (EMR) to document the annual comprehensive examination. The EMR must include the elements indicated
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Technology Appraisals. Patient Access Scheme Submission Template
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Technology Appraisals Patient Access Scheme Submission Template Bevacizumab in combination with fluoropyrimidine-based chemotherapy for the first-line
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Technology Appraisals Patient Access Scheme Submission Template Bevacizumab in combination with fluoropyrimidine-based chemotherapy for the first-line
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA
 BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
BLOOD GLUCOSE METER TEST STRIP STEP THERAPY CRITERIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
Home Care Services HomeMed MedEQUIP Michigan Visiting Care Michigan Visiting Nurses Wheelchair Seating Service PROCEDURE
 UNIVERSITY OF MICHIGAN HOSPITALS AND HEALTH CENTERS UMHHC-HCS: 253.054 First Approved Date: 3/2010 Home Care Services HomeMed MedEQUIP Michigan Visiting Care Michigan Visiting Nurses Wheelchair Seating
UNIVERSITY OF MICHIGAN HOSPITALS AND HEALTH CENTERS UMHHC-HCS: 253.054 First Approved Date: 3/2010 Home Care Services HomeMed MedEQUIP Michigan Visiting Care Michigan Visiting Nurses Wheelchair Seating
MedsCheck Reviews. Ontario
 MedsCheck Reviews Ontario Contents Configuration... 1 Configuring Electronic Signatures... 1 Configuring Electronic MedsCheck Reviews... 2 Creating an ODB MedsCheck Consent Record... 3 Electronic MedsCheck
MedsCheck Reviews Ontario Contents Configuration... 1 Configuring Electronic Signatures... 1 Configuring Electronic MedsCheck Reviews... 2 Creating an ODB MedsCheck Consent Record... 3 Electronic MedsCheck
Department of Pathology and Laboratory Medicine
 Department of Pathology and Laboratory Medicine Nova Scotia Health Authority Central Zone TITLE: QE BT BloodTrack Enquiry and HemoSafe Doc #: 24764 Section: Management System\PLM\Blood Transfusion Services\HemoSafe
Department of Pathology and Laboratory Medicine Nova Scotia Health Authority Central Zone TITLE: QE BT BloodTrack Enquiry and HemoSafe Doc #: 24764 Section: Management System\PLM\Blood Transfusion Services\HemoSafe
EUROPEAN MEDICINES AGENCY DECISION. of 7 September 2009
 European Medicines Agency Doc. Ref. EMEA/551360/2009 P/175/2009 EUROPEAN MEDICINES AGENCY DECISION of 7 September 2009 on the acceptance of a modification of an agreed Paediatric Investigation Plan for
European Medicines Agency Doc. Ref. EMEA/551360/2009 P/175/2009 EUROPEAN MEDICINES AGENCY DECISION of 7 September 2009 on the acceptance of a modification of an agreed Paediatric Investigation Plan for
MAGDALENE CATHOLIC HIGH SCHOOL Because I have seen the Lord. Anaphylaxis Policy
 MAGDALENE CATHOLIC HIGH SCHOOL Because I have seen the Lord Rationale Anaphylaxis Policy Magdalene Catholic High School endeavours to provide a safe and supportive school environment for all students.
MAGDALENE CATHOLIC HIGH SCHOOL Because I have seen the Lord Rationale Anaphylaxis Policy Magdalene Catholic High School endeavours to provide a safe and supportive school environment for all students.
