C. Bachmann This article was published as. Centre Hospitalier Universitaire Vaudois Number 3 in May 2000 CH 1011 Lausanne, Switzerland
|
|
|
- Tracey Simon
- 5 years ago
- Views:
Transcription
1 The control of analytical accuracy and day to day precision helps for the follow-up of patients and is essential when using biochemical data from the literature. C. Bachmann This article was published as Laboratoire Central de Chimie Clinique ERNDIM Biomed2 Newsletter Centre Hospitalier Universitaire Vaudois Number 3 in May 2000 CH 1011 Lausanne, Switzerland The theoretical background of Quality Control (QC) has been presented in an earlier BIOMEDII ERNDIM Newsletter ( February 1999). For stimulating participants of the ERNDIM QAP we want to remind you that the internal QC results allow you to assess rationally (no guess-work!) whether consecutive changes of laboratory results are most likely reflecting the biochemical course of the disease and of its treatment or if such changes can merely be explained by the existing day to day imprecision of the analytical process. In the appendix we describe our recipe for preparing QC-material for amino acid analysis to allow you to do this yourself. Finally we discuss the need for external quality control and awareness of the accuracy of your results in making recommendations or comparisons with data from the literature for managing your patients. Precision Let us assume that you are monitoring the treatment of a 2 month old patient in the out-patient clinic. PKU has been detected in this boy by newborn screening; you have confirmed the increased plasma phenylalanine in plasma by chromatography and a defect of tetrahydrobiopterin metabolism has been excluded. At the last control, 2 weeks ago, his phenylalanine was 150 µmol/l with a prescribed nutritional intake of 45 mg Phe /kg per day. You assay the phenylalanine now and find 64 µmol/l. Can this decrease between the 2 consultations merely be explained by the expected analytical imprecision or is it due to overanxious parents who restrict the intake of natural protein even more than you prescribed and thereby excessive restriction of phenylalanine? How can you find out? Your analytical imprecision should be known to you from the interseries imprecision results of the internal quality control of your lab. This is the day to day variance of the analytical process tested in a sample material which is identical to the patients analytical samples ( i.e. heparinized plasma in the example) at a concentration which is reasonably close to the critical levels you face ( e.g. intervention or diagnostic limits) and for which you have determined in your setting the acceptable mean (and confidence limits calculated from SEM = SD/ n) and multiples of standard deviation and calculated the coefficient of variation (CV= SD/mean). Population variance (SD 2 ) of a analyte (e.g. in reference values) is the sum of the inter-individual, the intraindividual and the analytical variance. In the situation presented here i.e. in the follow-up with 2 sequential values, we deal with a single subject, so that inter-individual variance does not apply. We are interested in determining which part of the fluctuations of the two results can be attributed to the analytical variance and which is due to intra-individual variance. The latter is dependent on the impact of treatment and evolution of the disorder. Also it should not be forgotten that preanalytical factors also play an important role. These are e.g. the time of sampling (fasting, time after last meal), the delay and ambient temperature during any delay ( cystine drops!) before centrifugation of the blood, the patients muscular activity (increase of glutamine after seizures) or the type of sample (capillary vs. venous) CBachmann preaccur edited.doc
2 If it is ensured that the sampling conditions are well controlled and the analytical imprecision is known, we can assess whether the critical difference for analytical results of two consecutive samples can be attributed, with a 95 probability, to analytical variance alone: To perform the calculation we need: 1. The two results (x 1 et x 2 ) of the patient and compute the actual difference 2. The coefficient of variation of the day to day quality control for which the target value is of the order of magnitude of the higher of the two patient samples. One compares the actual (absolute) difference with the critical difference. For those who like statistics : as in the t-test, p = 2.5% to p=97.5% the integral of which with Gaussian distribution = 1.96 =Z ; Test quotient compared to Z : Difference between the 2 values/ (SD of difference). SD = mean Coefficient of variation (as %)/100. Actual difference = x1 x2 Critical difference = x max CV (%) / 100 = 2.82 x max CV (%) / 100 Critical Difference 3 xmax CV (%) /100 where x max is the higher of the 2 results. If the actual difference is larger than the critical difference, the decrease (or increase) observed cannot be merely explained by the analytical imprecision. Taking the example above : Actual difference of phenylalanine (µmol/l) = = 86 µmol/l If your day to day imprecision at the time is 7% (CV at a level of e.g. 96 µmol/l (target value)) the critical difference will be : 2.82 * 150 µmol/l * 0.07 = 30 µmol/l The actual difference is greater than this critical difference, thus the drop cannot be explained solely by analytical imprecision. If however a method with a higher CV, of let us say 18%, is used e.g. the Guthrie Test, the critical difference will be 76 µmol/l ( 2.82 * 150 µmol/l * 0.18), and the change from one control visit to the next can then be fully explained by the analytical variance of this screening method (which is not designed for the follow-up of patients). With such an imprecision the conclusion that the patient tends to be dangerously deprived of phenylalanine by excessive treatment cannot be made since the drop of phenylalanine can fully be attributed to the analytical imprecision. You will have to use another method and rapidly control the patient again. From the responses to the questionnaire for the ERNDIM Laboratory Directory it is evident that some laboratories do not perform internal quality control routinely; thus a practical procedure for preparing the QCmaterial yourself is presented in the appendix. The results of three critical amino acids in the QC of one batch used during 17 months is shown in Figure 1. It demonstrates that such a material stored at 80 C in aliquots is stable for a year. The cause of the increase during the last few months could be the water loss by lyophilisation when sample tubes are not hermetically closed. Since amino acids such as glutamine are only stable at an acid ph the QC material we use is not untreated plasma but includes the sample treatment before chromatography. Thus in addition to the amino acid concentrations in the QC material we also evaluate the areas of the internal standards in the patients samples and compare them to those of the QC material to make sure that there was no error made in the sample preparation (pipetting imprecision when adding 25 µl of the deproteinisation-internal standard solution to 100 µl patient plasma!). 2
3 Accuracy Due to the rarity of patients with IEMs it is virtually impossible for any single centre to follow enough patients and practice independently of observations or recommendations of other groups. We all use data from the literature, preferably from prospective multicenter studies which pool a significant number of affected patients. Lessons can be learnt from the exemplary work which has been carried out in the domain of assessing cardiovascular risk factors. Method standardisation and external quality control programs (WHO) allow us to minimise the bias of cholesterol determination (below 3%). We propose that in our field of rare inherited errors of metabolism we should also aim at the goal reached with cholesterol determination. We have chosen that example to illustrate the impact of analytical inaccuracy on preventive therapeutic decisions. What would be the consequences if a laboratory had a bias for cholesterol determination of plus / minus 10% at a decision level of 6.5 mmol/l? Figure 2 shows the histogram of serum cholesterol levels in our normal male population of years of age. 30% of the population has a level above 6.5 mmol/l and comes into consideration for chronic preventive treatment. If a laboratory had a bias of cholesterol measurement of +10%, which means that it would give a result of 6.5 mmol/l when the true cholesterol is actually 5.85 mmol/l, an additional 21 % of the patients would be alarmed and possibly subjected to an excessive preventive treatment. Worse, if the bias were 10% the laboratory would miss 17% of the patients who would actually need a treatment. As already mentioned we are often forced to use data from the literature for amino acids, organic acids, carnitine, lactate, pyruvate and other special assays. For example in adapting treatment in PKU we feel secure if the phenylalanine values lie between 40 and 250 µmol/l until puberty. We might decide to increase the supply of essential amino acids in urea cycle disorders or supplement isoleucine in propionic or methylmalonic acidaemia when fasting isoleucine in plasma drops below 25 µmol/l in order to avoid appetite loss and chronic catabolism. Lactate/pyruvate ratios above 30 lead us to focus investigations on respiratory chain disorders or pyruvate carboxylase deficiency and dismiss other genetic causes of lactic acidemia. In those cases where we have not established our own age dependent reference values but use those of the literature are we really sure that our own analytical methods (and sampling conditions) correspond to those of the authors whose magic numbers we use? The results of the QAP programs lead us think otherwise and that major efforts need to be made to correct the analytical bias in order to avoid false decisions. Especially for the follow-up of patients and the optimisation of treatment we have definitely evolved from qualitative or semi-quantitative assay results to quantitative results with their inherent imprecision and inaccuracy which we must take into account. However waiting for clinical signs and symptoms cannot be the criteria for assuring the plausibility of laboratory data since irreversible damage might have already occurred. It is obvious that multicenter studies with several laboratories involved need to have a reliable QAP for avoiding systematic errors. Summary The results of internal quality control are not only helpful for early detection of random or systematic analytical errors by using the Westgard rules ( but also for a rational use of consecutive results in the follow-up of patients. For collaborative studies and the use of published reference values or recommendations based on biochemical data, information about the accuracy of own measurements is a necessity for applying extraneous data correctly. When using data from other sources one should not only compare the methodology but also the performance including accuracy and precision. Such data, even if they are not published, can be requested from the authors of published literature. We encourage all ERNDIM participants to review their latest QAP results in conjunction with their decision algorithms for prevention, diagnosis and adaptation of treatment as illustrated above for phenylalanine and to draw the necessary conclusions. References: Fraser CG, Petersen PH (1991) The importance of imprecision. Ann Clin Biochem 28: Jones RG, Payne RB (Eds) Clinical Investigation and Statistics in Laboratory Medicine, ACB Venture Publications, Association of Clinical Biochemists, London 1997 (ISBN ) 3
4 Appendix Preparation of QC-material for internal quality control. First calculate approximately how much material you will need for a year. We perform an internal QC analysis after every tenth analysis (and at least once per week) and after every calibration (and change of reagents). To this sum you add 30 samples (for establishing the initial mean and standard deviation). Multiply the number of samples by the sample volume and add 10 % for pipetting loss. Multiply the whole by the number of levels at which you will do a control (decision levels), e.g. 2 levels, one rather low or normal and one high. Estimate from this how much fresh blood you need assuming a hematocrit of 50%. Obtain from the transfusion centre enough fresh blood collected from a fasting subject with the identical anticoagulant (e.g. lithium heparin) you use for your patient sampling (not ACD-blood!). Have this tested for HIV and hepatitis. It might be advisable to check also that the subject has no cryoglobulins. Centrifuge rapidly and collect the plasma. Assay it for establishing the amino acid concentration and freeze all the plasma. Remark : Commercial preparations of serum ( e.g. calf serum ) are not suitable because they usually have been heated which leads to losses of amino acids and gross increase of glutamate (with relatively low glutamine). Deproteinize the plasma as you do for patients samples but on a higher scale: Per 100 ml of plasma ( e.g. in 50 ml Falcon tubes) add 25 ml of the deproteinisation solution which also contains the internal standard(s).you might choose to add solid amino acids for spiking. The deproteinisation solution contains 16.0g of sulfosalicylic acid / 100 ml of nanopure water. Vortex for 2 minutes, distribute it into tubes which withstand centrifugation at 15'000 x g Centrifuge : 30 min at 4 C at 15'000 x g Recover supernatant and repeat the centrifugation of the supernatants twice. Pool the final supernatants and dilute with buffer, as you would do for the sample preparation before chromatography mixing carefully in the cold. We use a ph 3.3 chromatography buffer (1:1 v/v) which results in a final ph of 2.2. An acid ph is needed for avoiding losses of glutamine and asparagine even if the samples are deep frozen. Verify the ph, aliquot the whole pool (e.g. 180µl) into Eppendorf tubes which should be tightly closed. The tubes are labelled and stored at -80 C. Assay twenty samples of QC with recalibrations of your method between each of the assays in order to calculate the starting target values of mean and standard deviation of this QC material. 4
5 Stability of QC-material over 17 months Cystine, Glutamate (µmol/l) Cystine Glutamate Glutamine /04/ /05/ /05/ /06/ /06/ /07/ /07/ /07/ /08/ /08/ /09/ /09/ /10/ /10/ /11/ /11/ /12/ /12/ /12/ /01/ /01/ /02/ /02/ /03/ /03/ /04/ /04/ /05/ /05/ /06/ /06/ /07/1998 Glutamine (µmol/l) 15/07/ /07/ /08/ /08/ /09/ /09/ Figure 1 Figure 2 5
PKU (Phenylketonuria) Serum HPLC Analysis Kit User Manual
 Page 1 / 20 PKU (Phenylketonuria) Serum HPLC Analysis Kit User Manual ZV-4003-0200-10 200 2-8 C Page 2 / 20 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST PRINCIPLE... 3
Page 1 / 20 PKU (Phenylketonuria) Serum HPLC Analysis Kit User Manual ZV-4003-0200-10 200 2-8 C Page 2 / 20 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST PRINCIPLE... 3
Annual Report ERNDIM-EQAS Quantitative Amino Acids 2004
 Annual Report ERNDIM-EQAS Quantitative Amino Acids 2004 1. Purpose The purpose of the ERNDIM External Quality Assurance Scheme for Quantitative Organic Acids is the monitoring of the analytical quality
Annual Report ERNDIM-EQAS Quantitative Amino Acids 2004 1. Purpose The purpose of the ERNDIM External Quality Assurance Scheme for Quantitative Organic Acids is the monitoring of the analytical quality
Lab Guide 2019 Metabolic Section Lab Guide
 Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
Lab Guide 2019 Metabolic Section Lab Guide Quantitative Amino acids Plasma Plasma. Container/Tube: Preferred EDTA, Place immediately in ice. Acceptable: lithium heparin, sodium heparin. Patient preparation:
PKU (Phenylketonuria) Serum LC-MS/MS Analysis Kit User Manual
 Page 1 / 24 PKU (Phenylketonuria) Serum LC-MS/MS Analysis Kit User Manual ZV-3003-0200-10 200 2-8 C Page 2 / 24 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST PRINCIPLE...
Page 1 / 24 PKU (Phenylketonuria) Serum LC-MS/MS Analysis Kit User Manual ZV-3003-0200-10 200 2-8 C Page 2 / 24 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST PRINCIPLE...
Forum for Collaborative HIV Research External Validation of CD4 and Viral Load Assays Paris, France June 29, 2007
 Forum for Collaborative HIV Research External Validation of CD4 and Viral Load Assays Paris, France June 29, 2007 Thomas J. Spira, M.D. International Laboratory Branch Global AIDS Program Centers for Disease
Forum for Collaborative HIV Research External Validation of CD4 and Viral Load Assays Paris, France June 29, 2007 Thomas J. Spira, M.D. International Laboratory Branch Global AIDS Program Centers for Disease
Annual Report ERNDIM-EQAS Quantitative Amino Acids 2002
 Annual Report ERNDIM-EQAS Quantitative Amino Acids 2002 1. Purpose The purpose of the ERNDIM External Quality Assurance Scheme for Quantitative Organic Acids is the monitoring of the analytical quality
Annual Report ERNDIM-EQAS Quantitative Amino Acids 2002 1. Purpose The purpose of the ERNDIM External Quality Assurance Scheme for Quantitative Organic Acids is the monitoring of the analytical quality
Week 17 and 21 Comparing two assays and Measurement of Uncertainty Explain tools used to compare the performance of two assays, including
 Week 17 and 21 Comparing two assays and Measurement of Uncertainty 2.4.1.4. Explain tools used to compare the performance of two assays, including 2.4.1.4.1. Linear regression 2.4.1.4.2. Bland-Altman plots
Week 17 and 21 Comparing two assays and Measurement of Uncertainty 2.4.1.4. Explain tools used to compare the performance of two assays, including 2.4.1.4.1. Linear regression 2.4.1.4.2. Bland-Altman plots
MS/MS APPROACHES TO CLINICAL TESTING FOR INBORN ERRORS OF METABOLISM
 MS/MS APPROACHES TO CLINICAL TESTING FOR INBORN ERRORS OF METABOLISM Tina M. Cowan, PhD Director, Clinical Biochemical Genetics Laboratory Stanford University Medical Center Presented at the 2011 Stanford
MS/MS APPROACHES TO CLINICAL TESTING FOR INBORN ERRORS OF METABOLISM Tina M. Cowan, PhD Director, Clinical Biochemical Genetics Laboratory Stanford University Medical Center Presented at the 2011 Stanford
The analytical phase
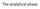 The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
INTERNAL QUALITY CONTROL ( Q I C) QC)
 EXTERNAL QUALITY ASSESSMENT PROGRAM (EQAP) BIOCHEMISTRY DEPARTMENT R. Mohammadi Biochemist (Ph.D.) Faculty member of Medical Faculty TWO COMPLEMENTARY COMPONENETS OF TQM ARE Internal Quality Control (IQC)
EXTERNAL QUALITY ASSESSMENT PROGRAM (EQAP) BIOCHEMISTRY DEPARTMENT R. Mohammadi Biochemist (Ph.D.) Faculty member of Medical Faculty TWO COMPLEMENTARY COMPONENETS OF TQM ARE Internal Quality Control (IQC)
METHOD VALIDATION: WHY, HOW AND WHEN?
 METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
METHOD VALIDATION: WHY, HOW AND WHEN? Linear-Data Plotter a,b s y/x,r Regression Statistics mean SD,CV Distribution Statistics Comparison Plot Histogram Plot SD Calculator Bias SD Diff t Paired t-test
USING THE ACCESS AMH ASSAY IN YOUR LABORATORY
 INFORMATION BULLETIN USING THE ACCESS AMH ASSAY IN YOUR LABORATORY ///////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
INFORMATION BULLETIN USING THE ACCESS AMH ASSAY IN YOUR LABORATORY ///////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
[ APPLICATION NOTE ] UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research INTRODUCTION APPLICATION BENEFITS
![[ APPLICATION NOTE ] UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research INTRODUCTION APPLICATION BENEFITS [ APPLICATION NOTE ] UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research INTRODUCTION APPLICATION BENEFITS](/thumbs/88/117895443.jpg) UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research Padhraic Rossiter, 1 Jaime Salcedo Dominguez, 1 Jennifer Warren, 1 Norma Breen, 1 Lisa Calton 2 1 Waters Corporation,
UPLC-MS/MS Analysis of 45 Amino Acids Using the Kairos Amino Acid Kit for Biomedical Research Padhraic Rossiter, 1 Jaime Salcedo Dominguez, 1 Jennifer Warren, 1 Norma Breen, 1 Lisa Calton 2 1 Waters Corporation,
SEED HAEMATOLOGY. Medical statistics your support when interpreting results SYSMEX EDUCATIONAL ENHANCEMENT AND DEVELOPMENT APRIL 2015
 SYSMEX EDUCATIONAL ENHANCEMENT AND DEVELOPMENT APRIL 2015 SEED HAEMATOLOGY Medical statistics your support when interpreting results The importance of statistical investigations Modern medicine is often
SYSMEX EDUCATIONAL ENHANCEMENT AND DEVELOPMENT APRIL 2015 SEED HAEMATOLOGY Medical statistics your support when interpreting results The importance of statistical investigations Modern medicine is often
Guide to Fulfillment of Measurement Uncertainty
 DIAGNOSTIC ACCREDITATION PROGRAM College of Physicians and Surgeons of British Columbia 300 669 Howe Street Telephone: 604-733-7758 ext. 2635 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca
DIAGNOSTIC ACCREDITATION PROGRAM College of Physicians and Surgeons of British Columbia 300 669 Howe Street Telephone: 604-733-7758 ext. 2635 Vancouver BC V6C 0B4 Toll Free: 1-800-461-3008 (in BC) www.cpsbc.ca
Polymer Technology Systems, Inc. CardioChek PA Comparison Study
 Polymer Technology Systems, Inc. CardioChek PA Comparison Study Evaluation Protocol: Accuracy Precision Clinical Correlation PTS Panels Lipid Panel Test Strips For Use in Comparisons to a Reference Laboratory
Polymer Technology Systems, Inc. CardioChek PA Comparison Study Evaluation Protocol: Accuracy Precision Clinical Correlation PTS Panels Lipid Panel Test Strips For Use in Comparisons to a Reference Laboratory
Human Alpha 1 microglobulin ELISA Kit
 Human Alpha 1 microglobulin ELISA Kit Catalogue No.: EH4144 Size: 48T/96T Reactivity: Human Range:0.625-40ng/ml Sensitivity:
Human Alpha 1 microglobulin ELISA Kit Catalogue No.: EH4144 Size: 48T/96T Reactivity: Human Range:0.625-40ng/ml Sensitivity:
UK NATIONAL METABOLIC BIOCHEMISTRY NETWORK GUIDELINES FOR THE INVESTIGATION OF HYPERAMMONAEMIA
 UK NATIONAL METABOLIC BIOCHEMISTRY NETWORK GUIDELINES FOR THE INVESTIGATION OF HYPERAMMONAEMIA Hyperammonaemia results from defective catabolism of amino acids to urea. Recognition and treatment of hyperammonaemia,
UK NATIONAL METABOLIC BIOCHEMISTRY NETWORK GUIDELINES FOR THE INVESTIGATION OF HYPERAMMONAEMIA Hyperammonaemia results from defective catabolism of amino acids to urea. Recognition and treatment of hyperammonaemia,
Phenylalanine Assay Kit
 ab83376 Phenylalanine Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Phenylalanine levels in various samples This product is for research use only and is not intended
ab83376 Phenylalanine Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Phenylalanine levels in various samples This product is for research use only and is not intended
BIOLOGICAL VARIABILITY: THE CIRME CONTRIBUTION. F. Braga Centre for Metrological Traceability in Laboratory Medicine (CIRME)
 BIOLOGICAL VARIABILITY: THE CIRME CONTRIBUTION F. Braga Centre for Metrological Traceability in Laboratory Medicine (CIRME) TOTAL VARIATION (CV (CV T ) T ) PREANALYTICAL VARIATION ANALYTICAL VARIATION
BIOLOGICAL VARIABILITY: THE CIRME CONTRIBUTION F. Braga Centre for Metrological Traceability in Laboratory Medicine (CIRME) TOTAL VARIATION (CV (CV T ) T ) PREANALYTICAL VARIATION ANALYTICAL VARIATION
Farouk AL Quadan lecture 3 Implentation of
 How to implement a program? Establish written policies and procedures Assign responsibility for monitoring and reviewing Train staff Obtain control materials Collect data Set target values (mean, SD) Establish
How to implement a program? Establish written policies and procedures Assign responsibility for monitoring and reviewing Train staff Obtain control materials Collect data Set target values (mean, SD) Establish
Four rules to remember: 1) Addition, subtraction, multiplication and division of SD and/or CV.
 Analytical Quality Specifications for Thyroid Hormones Bayer Nordic Users Meeting, 17. September 2001. Frode Engbaek, Ph. D., Department of Clinical Biochemistry, Aarhus Kommunehospital, DK-8000 Aarhus
Analytical Quality Specifications for Thyroid Hormones Bayer Nordic Users Meeting, 17. September 2001. Frode Engbaek, Ph. D., Department of Clinical Biochemistry, Aarhus Kommunehospital, DK-8000 Aarhus
CoQ10(Coenzyme Q10) ELISA Kit
 CoQ10(Coenzyme Q10) ELISA Kit Catalogue No.: EU0196 Size: 48T/96T Reactivity: Universal Detection Range: 0.781-50ng/ml Sensitivity:
CoQ10(Coenzyme Q10) ELISA Kit Catalogue No.: EU0196 Size: 48T/96T Reactivity: Universal Detection Range: 0.781-50ng/ml Sensitivity:
EliKine Free Thyroxine (ft4) ELISA Kit
 EliKine Free Thyroxine (ft4) ELISA Kit Booklet Item NO. KET0005 Product Name EliKine Free Thyroxine (ft4) ELISA Kit ATTENTION For laboratory research use only. Not for clinical or diagnostic use TABLE
EliKine Free Thyroxine (ft4) ELISA Kit Booklet Item NO. KET0005 Product Name EliKine Free Thyroxine (ft4) ELISA Kit ATTENTION For laboratory research use only. Not for clinical or diagnostic use TABLE
RayBio Phenylalanine Fluorometric Assay Kit
 RayBio Phenylalanine Fluorometric Assay Kit User Manual Version 1.0 June 25, 2014 RayBio Phenylalanine Fluorometric (Cat#: 68-Phenyl-S100) RayBiotech, Inc. We Provide You With Excellent Support And Service
RayBio Phenylalanine Fluorometric Assay Kit User Manual Version 1.0 June 25, 2014 RayBio Phenylalanine Fluorometric (Cat#: 68-Phenyl-S100) RayBiotech, Inc. We Provide You With Excellent Support And Service
Individual Lab Report Ci-Trol Nov,2016. Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC Your Lab
 Individual Lab Report Ci-Trol Nov,2016 ST VINCENT MEDICAL CENTER LABORATORY(LAB# 7300 ) 2131 WEST THIRD STREET LOS ANGELES CA USA 90057 Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC SYSMEX
Individual Lab Report Ci-Trol Nov,2016 ST VINCENT MEDICAL CENTER LABORATORY(LAB# 7300 ) 2131 WEST THIRD STREET LOS ANGELES CA USA 90057 Abnormal Fibrinogen (mg/dl) Abnormal Fbg Control - Lot# LFC SYSMEX
Human HIV (1+2) antigen&antibody ELISA Kit
 Human HIV (1+2) antigen&antibody ELISA Kit Catalog Number. CSB-E18042h For the qualitative determination of human HIV (1+2) antibody and P24 antigen concentrations in serum, plasma. This package insert
Human HIV (1+2) antigen&antibody ELISA Kit Catalog Number. CSB-E18042h For the qualitative determination of human HIV (1+2) antibody and P24 antigen concentrations in serum, plasma. This package insert
Glutamine Kit. For the determination of glutamine and glutamate in human EDTA plasma and serum. For research use only K 7732.
 Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Manual Kit For the determination of glutamine and glutamate
Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Manual Kit For the determination of glutamine and glutamate
Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on. CUBE analyser (CA0100)
 Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on CUBE analyser (CA0100) Location Location: Eurolyser Diagnostica GmbH Operators: Simone Wieser; Franz Helminger; Michael Gruber Date: July-November
Evaluation Report: Eurolyser CRP test (ST0100 and ST0102) on CUBE analyser (CA0100) Location Location: Eurolyser Diagnostica GmbH Operators: Simone Wieser; Franz Helminger; Michael Gruber Date: July-November
DIHYDROSTREPTOMYCIN and STREPTOMYCIN First draft prepared by Lynn G. Friedlander, Rockville, MD, USA Rainer W. Stephany, Bilthoven, The Netherlands
 DIHYDROSTREPTOMYCIN and STREPTOMYCIN First draft prepared by Lynn G. Friedlander, Rockville, MD, USA Rainer W. Stephany, Bilthoven, The Netherlands ADDENDUM To the monograph and addenda prepared by the
DIHYDROSTREPTOMYCIN and STREPTOMYCIN First draft prepared by Lynn G. Friedlander, Rockville, MD, USA Rainer W. Stephany, Bilthoven, The Netherlands ADDENDUM To the monograph and addenda prepared by the
Mouse C-Peptide ELISA Kit
 Mouse C-Peptide ELISA Kit Cat.No: DEIA4507 Lot. No. (See product label) Size 96T Intended Use The Mouse C-Peptide ELISA kit is for the quantitative determination of c-peptide in mouse serum, plasma, and
Mouse C-Peptide ELISA Kit Cat.No: DEIA4507 Lot. No. (See product label) Size 96T Intended Use The Mouse C-Peptide ELISA kit is for the quantitative determination of c-peptide in mouse serum, plasma, and
Practical relevance of measurement uncertainty in inborn errors of metabolism. Prof Jim Bonham Clinical Director Sheffield Children s NHS FT
 Practical relevance of measurement uncertainty in inborn errors of metabolism Prof Jim Bonham Clinical Director Sheffield Children s NHS FT Outline Is it important for inborn errors? What is measurement
Practical relevance of measurement uncertainty in inborn errors of metabolism Prof Jim Bonham Clinical Director Sheffield Children s NHS FT Outline Is it important for inborn errors? What is measurement
Procine sphingomyelin ELISA Kit
 Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Procine sphingomyelin ELISA Kit For the quantitative in vitro determination of Procine sphingomyelin concentrations in serum - plasma - celiac fluid - tissue homogenate - body fluid FOR LABORATORY RESEARCH
Urea Nitrogen (BUN) detection Kit
 K-ASSAY KAMIYA BIOMEDICAL COMPANY KAMIYA BIOMEDICAL COMPANY Urea Nitrogen (BUN) detection Kit For the quantitative determination of urea nitrogen in saliva and TCM Cat. No. KT-747 For Research Use Only.
K-ASSAY KAMIYA BIOMEDICAL COMPANY KAMIYA BIOMEDICAL COMPANY Urea Nitrogen (BUN) detection Kit For the quantitative determination of urea nitrogen in saliva and TCM Cat. No. KT-747 For Research Use Only.
APOB (Human) ELISA Kit
 APOB (Human) ELISA Kit Catalog Number KA4330 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
APOB (Human) ELISA Kit Catalog Number KA4330 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
GlucoMen LX PLUS blood glucose and blood ketone meter. Accuracy Evaluation to New ISO 15197:2013, with Technical and Specification Data
 GlucoMen LX PLUS: Accuracy Evaluations to ISO 15197:2013 with Technical and Specification Data GlucoMen LX PLUS blood glucose and blood ketone meter Accuracy Evaluation to New ISO 15197:2013, with Technical
GlucoMen LX PLUS: Accuracy Evaluations to ISO 15197:2013 with Technical and Specification Data GlucoMen LX PLUS blood glucose and blood ketone meter Accuracy Evaluation to New ISO 15197:2013, with Technical
Carnitine / Acylcarnitines Dried Blood Spots LC-MS/MS Analysis Kit User Manual
 Page 1 / 14 Carnitine / Acylcarnitines Dried Blood Spots LC-MS/MS Analysis Kit User Manual ZV-3051-0200-15 200 Page 2 / 14 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST
Page 1 / 14 Carnitine / Acylcarnitines Dried Blood Spots LC-MS/MS Analysis Kit User Manual ZV-3051-0200-15 200 Page 2 / 14 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST
Annual Report Zurich 2017 Date of issue: 19 th April 2018 Amended report issued: 1 st May
 ERNDIM Administration Office Manchester Centre for Genomic Medicine 6 th Floor, St Mary's Hospital, Oxford Road, Manchester M13 9WL, United Kingdom. Tel: +44 161 276 6741 Fax: +44 161 850 1145 Email: admin@erndim.org
ERNDIM Administration Office Manchester Centre for Genomic Medicine 6 th Floor, St Mary's Hospital, Oxford Road, Manchester M13 9WL, United Kingdom. Tel: +44 161 276 6741 Fax: +44 161 850 1145 Email: admin@erndim.org
Branched Chain Amino Acid (Leu, Ile, Val) Assay Kit
 Branched Chain Amino Acid (Leu, Ile, Val) Assay Kit Catalog Number KA0824 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
Branched Chain Amino Acid (Leu, Ile, Val) Assay Kit Catalog Number KA0824 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
Myeloperoxidase (MPO) ELISA Stool, Urine KIT
 Myeloperoxidase (MPO) ELISA Stool, Urine KIT Cat. No.:DEIA6211 Pkg.Size:96T Intended use The Myeloperoxidase Assay is intended for the quantitative determination of Myeloperoxidase in urine and stool,
Myeloperoxidase (MPO) ELISA Stool, Urine KIT Cat. No.:DEIA6211 Pkg.Size:96T Intended use The Myeloperoxidase Assay is intended for the quantitative determination of Myeloperoxidase in urine and stool,
(a) y = 1.0x + 0.0; r = ; N = 60 (b) y = 1.0x + 0.0; r = ; N = Lot 1, Li-heparin whole blood, HbA1c (%)
 cobas b system - performance evaluation Study report from a multicenter evaluation of the new cobas b system for the measurement of HbAc and lipid panel Introduction The new cobas b system provides a point-of-care
cobas b system - performance evaluation Study report from a multicenter evaluation of the new cobas b system for the measurement of HbAc and lipid panel Introduction The new cobas b system provides a point-of-care
retardation in infants. A wide variety of analytical methods for the analysis of
 IN THE NAME OF GOD Diagnosis and measurment of Phenylalanine in plasma and dried bilood spot (DBS) by a simple and sensitive HPLC method and compaire with Recipe Reference Health Laboratory Research Center,
IN THE NAME OF GOD Diagnosis and measurment of Phenylalanine in plasma and dried bilood spot (DBS) by a simple and sensitive HPLC method and compaire with Recipe Reference Health Laboratory Research Center,
A validation study of after reconstitution stability of diabetes: level 1 and diabetes level 2 controls
 A validation study of after reconstitution stability of diabetes: level 1 and diabetes level 2 controls Shyamali Pal R B Diagnostic Private Limited, Lake Town, Kolkata, India INFO ABSTRACT Corresponding
A validation study of after reconstitution stability of diabetes: level 1 and diabetes level 2 controls Shyamali Pal R B Diagnostic Private Limited, Lake Town, Kolkata, India INFO ABSTRACT Corresponding
Serodos and Serodos plus
 Design Verification Serodos and Serodos plus Contents 1 Value Adjustment... 2 2 Target Determination... 2 3 Stability... 2 Real-Time Stability... 3 Stability after Reconstitution... 4 Stability after Reconstitution
Design Verification Serodos and Serodos plus Contents 1 Value Adjustment... 2 2 Target Determination... 2 3 Stability... 2 Real-Time Stability... 3 Stability after Reconstitution... 4 Stability after Reconstitution
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme
 Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Acylcarnitine measurement in blood spots: methodological aspects, problems and pitfalls with reference to the ERNDIM QA scheme Charles Turner Laboratory Guy s Hospital (Evelina Childrens Hospital, St Thomas
Instructions for Use. IA-2 Antibody RIA. for the Quantitative Determination of Antibodies against Protein-Tyrosine-Phosphatase (IA2) in Serum
 Instructions for Use IA-2 Antibody RIA 125I-Radioimmunoassay for the Quantitative Determination of Antibodies against Protein-Tyrosine-Phosphatase (IA2) in Serum REF RA101/50 RA104/100 50 100 2 8 C I V
Instructions for Use IA-2 Antibody RIA 125I-Radioimmunoassay for the Quantitative Determination of Antibodies against Protein-Tyrosine-Phosphatase (IA2) in Serum REF RA101/50 RA104/100 50 100 2 8 C I V
Abraxis Progesterone (bovine) ELISA Kit
 Abraxis Progesterone (bovine) ELISA Kit Enzyme immunoassay for the quantitative determination of progesterone in bovine milk/serum/plasma samples PN5081M 96 Tests For Research Use Only. Not for use in
Abraxis Progesterone (bovine) ELISA Kit Enzyme immunoassay for the quantitative determination of progesterone in bovine milk/serum/plasma samples PN5081M 96 Tests For Research Use Only. Not for use in
Acetyl-CoA Assay Kit. Catalog Number KA assays Version: 04. Intended for research use only.
 Acetyl-CoA Assay Kit Catalog Number KA0803 100 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials Supplied...
Acetyl-CoA Assay Kit Catalog Number KA0803 100 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials Supplied...
Chymotrypsin ELISA Kit
 Chymotrypsin ELISA Kit Cat. No.:DEIA10041 Pkg.Size:96T Intended use The Chymotrypsin ELISA Kit is a sandwich Enzyme Immuno Assay intended for the quantitative determination of Chymotrypsin in stool. General
Chymotrypsin ELISA Kit Cat. No.:DEIA10041 Pkg.Size:96T Intended use The Chymotrypsin ELISA Kit is a sandwich Enzyme Immuno Assay intended for the quantitative determination of Chymotrypsin in stool. General
Insulin Rodent (Mouse/Rat) Chemiluminescence ELISA
 Insulin Rodent (Mouse/Rat) Chemiluminescence ELISA For the quantitative determination of insulin in rodent serum, plasma, and tissue culture supernatants. For Research use Only. Not For Use In Diagnostic
Insulin Rodent (Mouse/Rat) Chemiluminescence ELISA For the quantitative determination of insulin in rodent serum, plasma, and tissue culture supernatants. For Research use Only. Not For Use In Diagnostic
FinTest IgG4 Screen 20 ELISA KIT
 FinTest IgG4 Screen 20 ELISA KIT Cat. No.:DEIA6196 Pkg.Size:96T Intended use Enzyme immunoassay (microtiter strips) for the detection and the quantitative determination of IgG4 antibodies against 20 Food
FinTest IgG4 Screen 20 ELISA KIT Cat. No.:DEIA6196 Pkg.Size:96T Intended use Enzyme immunoassay (microtiter strips) for the detection and the quantitative determination of IgG4 antibodies against 20 Food
ab Creatinine Assay Kit (Colorimetric)
 ab204537 Creatinine Assay Kit (Colorimetric) Instructions for Use For the quantitative determination of Creatinine in urine samples. This product is for research use only and is not intended for diagnostic
ab204537 Creatinine Assay Kit (Colorimetric) Instructions for Use For the quantitative determination of Creatinine in urine samples. This product is for research use only and is not intended for diagnostic
FreeStyle Lite A Blood Glucose Meter That Requires No Coding
 Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
Journal of Diabetes Science and Technology Volume 2, Issue 4, July 2008 Diabetes Technology Society SYMPOSIUM Shridhara, Ph.D. Abstract Background: Abbott Diabetes Care introduced the FreeStyle Lite blood
Amal Alamri. Phenylketonuria
 Amal Alamri Phenylketonuria Norwegian doctor Asbjørn Følling (left) discovered phenylpyrouvica (later termed phenylketonuria) in 1934 upon discovering that ten mentally retarded patients had phenylpyruvic
Amal Alamri Phenylketonuria Norwegian doctor Asbjørn Følling (left) discovered phenylpyrouvica (later termed phenylketonuria) in 1934 upon discovering that ten mentally retarded patients had phenylpyruvic
Erythrocyte and Plasma Volume Measurement
 Erythrocyte and Plasma Volume Measurement JP Esser, Meander Medical Centre, Amersfoort NOTE: no changes have been made since the version of 2007 Warning: I human serum albumin and 131 I human serum albumin
Erythrocyte and Plasma Volume Measurement JP Esser, Meander Medical Centre, Amersfoort NOTE: no changes have been made since the version of 2007 Warning: I human serum albumin and 131 I human serum albumin
MyBioSource.com. Instructions for use. Histamine Release
 Instructions for use Histamine Release Supplementary kit for the determination of the release of histamine from heparinized whole blood (this kit has to be used in combination with the Histamine ELISA,
Instructions for use Histamine Release Supplementary kit for the determination of the release of histamine from heparinized whole blood (this kit has to be used in combination with the Histamine ELISA,
DKK-1 ELISA, Cat.No. BI For the quantitative determination of DKK-1 in human serum
 DKK-1 ELISA, Cat.No. BI-20413 For the quantitative determination of DKK-1 in human serum ASSAY CHARACTERISTICS Method Standard range Conversion factor Sample volume / well Incubation time, temp. Sensitivity
DKK-1 ELISA, Cat.No. BI-20413 For the quantitative determination of DKK-1 in human serum ASSAY CHARACTERISTICS Method Standard range Conversion factor Sample volume / well Incubation time, temp. Sensitivity
Proinsulin (Total) Chemiluminescence ELISA
 Proinsulin (Total) Chemiluminescence ELISA For the quantitative determination of proinsulin in human serum, EDTA plasma, heparin plasma, and tissue culture supernatants For In Vitro Diagnostic use within
Proinsulin (Total) Chemiluminescence ELISA For the quantitative determination of proinsulin in human serum, EDTA plasma, heparin plasma, and tissue culture supernatants For In Vitro Diagnostic use within
LIPASE liquicolor. Design Verification. Multipurpose Reagent
 Design Verification LIPASE liquicolor Multipurpose Reagent CONTENTS 1 Introduction... 2 2 Imprecision... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 3 4 Recovery of
Design Verification LIPASE liquicolor Multipurpose Reagent CONTENTS 1 Introduction... 2 2 Imprecision... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 3 4 Recovery of
Glucose Meter. User Guide. Veterinary Monitoring System. For dog and cat use only
 Glucose Meter User Guide Veterinary Monitoring System For dog and cat use only Gpet instruction Manual 31/5/09 18:06 Page 2 Gpet instruction Manual 31/5/09 18:06 Page 3 TABLE OF CONTENTS Your g-pet system
Glucose Meter User Guide Veterinary Monitoring System For dog and cat use only Gpet instruction Manual 31/5/09 18:06 Page 2 Gpet instruction Manual 31/5/09 18:06 Page 3 TABLE OF CONTENTS Your g-pet system
Quantitative Method for Amphetamines, Phentermine, and Designer Stimulants Using an Agilent 6430 LC/MS/MS
 Quantitative Method for Amphetamines, Phentermine, and Designer Stimulants Using an Agilent 6430 LC/MS/MS Application Note Forensic Toxicology Authors Jason Hudson, Ph.D., James Hutchings, Ph.D., and Rebecca
Quantitative Method for Amphetamines, Phentermine, and Designer Stimulants Using an Agilent 6430 LC/MS/MS Application Note Forensic Toxicology Authors Jason Hudson, Ph.D., James Hutchings, Ph.D., and Rebecca
1 PROTOCOL. Comparison Study Summary. Springs Memorial Hospital. Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC (803)
 Comparison Study Summary Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC 29720 (803) 286-1480 February 10, 2016 1 PROTOCOL This evaluation was conducted on February 10, 2016 at Springs Memorial
Comparison Study Summary Springs Memorial Hospital 800 W. Meeting St. Lancaster, SC 29720 (803) 286-1480 February 10, 2016 1 PROTOCOL This evaluation was conducted on February 10, 2016 at Springs Memorial
Design Verification IMTEC-TSH R -A C Intended Use... 2 Diagnostic Sensitivity and Diagnostic Specificity... 2 Interferences... 4 Imprecision...
 Design Verification IMTEC-TSH RECEPTOR-ANTIBODIES CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Diagnostic Specificity... 2 2.1 Determination of Diagnostic Sensitivity and Diagnostic Specificity...
Design Verification IMTEC-TSH RECEPTOR-ANTIBODIES CONTENTS 1 Intended Use... 2 2 Diagnostic Sensitivity and Diagnostic Specificity... 2 2.1 Determination of Diagnostic Sensitivity and Diagnostic Specificity...
Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column
 Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
Application Note Pharmaceutical and Food Testing Analysis of Amino Acids Derived Online Using an Agilent AdvanceBio AAA Column Author Lu Yufei Agilent Technologies, Inc. Abstract A liquid chromatographic
Coenzyme A Assay Kit. Catalog Number KA assays Version: 02. Intended for research use only.
 Coenzyme A Assay Kit Catalog Number KA0809 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials Supplied...
Coenzyme A Assay Kit Catalog Number KA0809 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials Supplied...
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 21 September 2006 EMEA/CHMP/BWP/298390/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 21 September 2006 EMEA/CHMP/BWP/298390/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON
VGKC-Autoantibody Assay RIA
 Instructions for Use VGKC-Autoantibody Assay RIA 125I-Radio Immuno Assay for the Quantitative Determination of Antibodies to the Voltage-Gated Potassium Channel (VGKC) in Serum I V D REF RA115/25 25 2
Instructions for Use VGKC-Autoantibody Assay RIA 125I-Radio Immuno Assay for the Quantitative Determination of Antibodies to the Voltage-Gated Potassium Channel (VGKC) in Serum I V D REF RA115/25 25 2
QUALITROL 2H Insert. 01 Inglês - Ref.: 72. Ref.:72. Precautions and warnings
 QUALITROL 2H Insert Ref.:72 Lot 6002 Expiration 2019/08/23 Precautions and warnings Qualitrol 2H is for in vitro diagnostic use only. Attention. It is suggested to verify carefully if the lot number pressed
QUALITROL 2H Insert Ref.:72 Lot 6002 Expiration 2019/08/23 Precautions and warnings Qualitrol 2H is for in vitro diagnostic use only. Attention. It is suggested to verify carefully if the lot number pressed
02006B 1 vial 02006B 1 vial Store at -20 C. Lyophilized recombinant IL-2
 For detection and measurement of human interleukin 2 Catalog #02006 Catalog #02007 2 Plates 10 Plates Product Description The Human Interleukin 2 (IL-2) ELISA Kit is designed for the quantitative detection
For detection and measurement of human interleukin 2 Catalog #02006 Catalog #02007 2 Plates 10 Plates Product Description The Human Interleukin 2 (IL-2) ELISA Kit is designed for the quantitative detection
Canine Thyroid Stimulating Hormone, TSH ELISA Kit
 Canine Thyroid Stimulating Hormone, TSH ELISA Kit Catalog No: E0463c 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Canine Thyroid Stimulating Hormone, TSH ELISA Kit Catalog No: E0463c 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
High-density Lipoprotein Cholesterol (HDL-C) Assay Kit
 (FOR RESEARCH USE ONLY. DO NOT USE IT IN CLINICAL DIAGNOSIS!) High-density Lipoprotein Cholesterol (HDL-C) Assay Kit (Double reagents) Catalog No: E-BC-K221 Method: Colorimetric method Specification: 96T
(FOR RESEARCH USE ONLY. DO NOT USE IT IN CLINICAL DIAGNOSIS!) High-density Lipoprotein Cholesterol (HDL-C) Assay Kit (Double reagents) Catalog No: E-BC-K221 Method: Colorimetric method Specification: 96T
The laboratory investigation of lactic acidaemia. J Bonham/T Laing
 The laboratory investigation of lactic acidaemia J Bonham/T Laing Reference range Typical ranges for blood lactate are: Newborn 0.3-2.2 mmol/l Nielsen J et al1 1994 1-12mo 0.9-1.8 mmol/l Bonnefont et al
The laboratory investigation of lactic acidaemia J Bonham/T Laing Reference range Typical ranges for blood lactate are: Newborn 0.3-2.2 mmol/l Nielsen J et al1 1994 1-12mo 0.9-1.8 mmol/l Bonnefont et al
Panther has new prey
 Raising the Bar for Performance Testing Panther has new prey The Aptima HIV-1 Quant Dx assay leads the hunt for HIV-1 diagnosis and viral load monitoring. Freedom to work the way you choose Run what assays
Raising the Bar for Performance Testing Panther has new prey The Aptima HIV-1 Quant Dx assay leads the hunt for HIV-1 diagnosis and viral load monitoring. Freedom to work the way you choose Run what assays
Diagnostic reagent for quantitative in vitro determination of Urea / Blood Urea Nitrogen in serum, plasma and urine by colorimetry.
 2013/07/30 A93A01315AUS A11A01641 60 ml 15 ml Pentra C400 Diagnostic reagent for quantitative in vitro determination of Urea / Blood Urea Nitrogen in serum, plasma and urine by colorimetry. QUAL-QA-TEMP-0846
2013/07/30 A93A01315AUS A11A01641 60 ml 15 ml Pentra C400 Diagnostic reagent for quantitative in vitro determination of Urea / Blood Urea Nitrogen in serum, plasma and urine by colorimetry. QUAL-QA-TEMP-0846
Morinaga Mouse C-peptide ELISA Kit
 Morinaga Mouse C-peptide ELISA Kit For the quantitative determination of C-peptide in mouse serum, plasma, and fluid 96wells For Laboratory Use Only, not for use in diagnostic procedure Please read full
Morinaga Mouse C-peptide ELISA Kit For the quantitative determination of C-peptide in mouse serum, plasma, and fluid 96wells For Laboratory Use Only, not for use in diagnostic procedure Please read full
liquicolor Design Verification
 Design Verification liquicolor 1 Introduction... 2 2 Imprecision... 2 2.1 Imprecision for... 2 2.2 Imprecision for... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 4
Design Verification liquicolor 1 Introduction... 2 2 Imprecision... 2 2.1 Imprecision for... 2 2.2 Imprecision for... 2 3 Linearity and Detection Limit... 2 3.1 Linearity... 2 3.2 Detection Limit... 4
What is the normal reference range for TAS? The normal reference range is quoted as mmol/l
 What is the normal reference range for TAS? The normal reference range is quoted as 1.30-1.77mmol/l How sensitive is the TAS assay? The sensitivity of the test is dependent on the actual antioxidants being
What is the normal reference range for TAS? The normal reference range is quoted as 1.30-1.77mmol/l How sensitive is the TAS assay? The sensitivity of the test is dependent on the actual antioxidants being
Thin-Layer Chromatography of Amino Acids HASPI Medical Biology Lab 15b Background Macromolecules
 Thin-Layer Chromatography of s HASPI Medical Biology Lab 15b Background Macromolecules Name: Period: Date: There are four major types of biological macromolecules that make up the human body: nucleic acids
Thin-Layer Chromatography of s HASPI Medical Biology Lab 15b Background Macromolecules Name: Period: Date: There are four major types of biological macromolecules that make up the human body: nucleic acids
Reliability, validity, and all that jazz
 Reliability, validity, and all that jazz Dylan Wiliam King s College London Published in Education 3-13, 29 (3) pp. 17-21 (2001) Introduction No measuring instrument is perfect. If we use a thermometer
Reliability, validity, and all that jazz Dylan Wiliam King s College London Published in Education 3-13, 29 (3) pp. 17-21 (2001) Introduction No measuring instrument is perfect. If we use a thermometer
ANA and Antibody Series Changes in ANA and Antibody Levels in Scleroderma
 ANA and Antibody Series Changes in ANA and Antibody Levels in Scleroderma Background This article was prompted by an excellent question that was recently sent to the Scleroderma Education Project: You
ANA and Antibody Series Changes in ANA and Antibody Levels in Scleroderma Background This article was prompted by an excellent question that was recently sent to the Scleroderma Education Project: You
IV2-113E Use by. Invitron Glargine ELISA Kit REF LOT IVD. Definitions. English. For in-vitro diagnostic use. Instructions for use.
 Definitions Instructions for use REF Catalogue number IV2-113E Use by English Invitron Glargine ELISA Kit For in-vitro diagnostic use Σ 96 LOT IVD Lot/Batch Code Storage temperature limitations In vitro
Definitions Instructions for use REF Catalogue number IV2-113E Use by English Invitron Glargine ELISA Kit For in-vitro diagnostic use Σ 96 LOT IVD Lot/Batch Code Storage temperature limitations In vitro
DETERMINATION OF COMPOSITION OF TRIACYLGLYCEROLS AND COMPOSITION AND CONTENT OF DI-ACYLGLYCEROLS BY CAPILLARY GAS CHROMATOGRAPHY, IN VEGETABLE OILS
 INTERNATIONAL OLIVE COUNCIL COI/T.20/Doc. No 32 November 2013 ENGLISH Original: ENGLISH Príncipe de Vergara, 154 28002 Madrid España Telef.: +34 915 903 638 Fax: +34 915 631 263 - e-mail: iooc@internationaloliveoil.org
INTERNATIONAL OLIVE COUNCIL COI/T.20/Doc. No 32 November 2013 ENGLISH Original: ENGLISH Príncipe de Vergara, 154 28002 Madrid España Telef.: +34 915 903 638 Fax: +34 915 631 263 - e-mail: iooc@internationaloliveoil.org
Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4000
 v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
Accuracy and Precision. Intra- and inter-assay accuracy and precision for both rifapentine
 Supplemental Materials Assay Validation Methods Accuracy and Precision. Intra- and inter-assay accuracy and precision for both rifapentine (RPT) and desacetyl-rifapentine (desrpt) were determined through
Supplemental Materials Assay Validation Methods Accuracy and Precision. Intra- and inter-assay accuracy and precision for both rifapentine (RPT) and desacetyl-rifapentine (desrpt) were determined through
VITAMINES A / E IN PLASMA BY UV - FAST CODE Z18610
 VITAMINES A / E IN PLASMA BY UV - FAST CODE Z18610 BIOCHEMISTRY Vitamins A and E are two fat-soluble vitamins indispensable to animals life for a correct growth and development. Vitamin A exists in three
VITAMINES A / E IN PLASMA BY UV - FAST CODE Z18610 BIOCHEMISTRY Vitamins A and E are two fat-soluble vitamins indispensable to animals life for a correct growth and development. Vitamin A exists in three
510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE
 A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
A. 510(k) Number: k100322 510(k) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY AND INSTRUMENT COMBINATION TEMPLATE B. Purpose for Submission: Clearance of a new device C. Measurand: Whole
Gentian Canine CRP Immunoassay Application Note for scil VitroVet*
 v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
BNP Fragment EIA (Cat.No. BI-20852W) For the Determination of BNP Fragment in Human Samples
 BNP Fragment EIA (Cat.No. BI-20852W) For the Determination of BNP Fragment in Human Samples ASSAY CHARACTERISTICS Method Competitive Enzyme Immunoassay, HRP/TMB. Microtiter plates are coated with a polyclonal
BNP Fragment EIA (Cat.No. BI-20852W) For the Determination of BNP Fragment in Human Samples ASSAY CHARACTERISTICS Method Competitive Enzyme Immunoassay, HRP/TMB. Microtiter plates are coated with a polyclonal
The Virtues and Pitfalls of Implementing a New Test
 The Virtues and Pitfalls of Implementing a New Test James H. Nichols, Ph.D., DABCC, FACB Professor of Clinical Pathology, Microbiology and Immunology Associate Medical Director for Clinical Operations
The Virtues and Pitfalls of Implementing a New Test James H. Nichols, Ph.D., DABCC, FACB Professor of Clinical Pathology, Microbiology and Immunology Associate Medical Director for Clinical Operations
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit
 Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
Mouse HBsAg(Hepatitis B Virus Surface Antigen) ELISA Kit Catalogue No.: EM0002 Size: 96T Reactivity: Mouse Application: This immunoassay kit allows for the qualitative determination of HBsAg in Mouse serum
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up
 HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
HA Convention 2016 Master course How to Handle Abnormal Newborn Metabolic Screening Results Causes, Management and Follow up Dr. Josephine Chong Clinical Professional Consultant Centre of Inborn Errors
Human Obestatin ELISA
 K-ASSAY Human Obestatin ELISA For the quantitative determination of obestatin in human serum and plasma Cat. No. KT-495 For Research Use Only. 1 Rev. 081309 K-ASSAY PRODUCT INFORMATION Human Obestatin
K-ASSAY Human Obestatin ELISA For the quantitative determination of obestatin in human serum and plasma Cat. No. KT-495 For Research Use Only. 1 Rev. 081309 K-ASSAY PRODUCT INFORMATION Human Obestatin
DetectX. Urinary Creatinine Detection Kit. Catalog Number K002-H1. Sample Types Validated: Human, Monkey, Dog, Rat and Mouse Urine
 World s Only One Component Assay Simple & Easy to Use DetectX Urinary Creatinine Detection Kit Catalog Number K002-H1 Sample Types Validated: Human, Monkey, Dog, Rat and Mouse Urine Please read this insert
World s Only One Component Assay Simple & Easy to Use DetectX Urinary Creatinine Detection Kit Catalog Number K002-H1 Sample Types Validated: Human, Monkey, Dog, Rat and Mouse Urine Please read this insert
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism
 Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
Newborn Screening & Methods for Diagnosing Inborn Errors of Metabolism Patricia Jones, PhD DABCC FACB UT Southwestern Medical Center Children s Medical Center Dallas, Texas Learning Objectives Justify
Bias in clinical chemistry. Elvar Theodorsson EFLM and Linköping University
 Bias in clinical chemistry Elvar Theodorsson EFLM and Linköping University Bias a controversial subject Different perspectives Reseachers Users Regulatory bodies Standardisation organisations Metrologists
Bias in clinical chemistry Elvar Theodorsson EFLM and Linköping University Bias a controversial subject Different perspectives Reseachers Users Regulatory bodies Standardisation organisations Metrologists
Rat cholesterol ELISA Kit
 Rat cholesterol ELISA Kit Catalog No. CSB-E11706r (96T) This immunoassay kit allows for the in vitro quantitative determination of rat Cholesterol concentrations in serum, plasma and other biological fluids.
Rat cholesterol ELISA Kit Catalog No. CSB-E11706r (96T) This immunoassay kit allows for the in vitro quantitative determination of rat Cholesterol concentrations in serum, plasma and other biological fluids.
Measuring Lipid Composition LC-MS/MS
 Project: Measuring Lipid Composition LC-MS/MS Verification of expected lipid composition in nanomedical controlled release systems by liquid chromatography tandem mass spectrometry AUTHORED BY: DATE: Sven
Project: Measuring Lipid Composition LC-MS/MS Verification of expected lipid composition in nanomedical controlled release systems by liquid chromatography tandem mass spectrometry AUTHORED BY: DATE: Sven
GlucCell TM SYSTEM USER S GUIDE ver 2.3 CELL CULTURE GLUCOSE METER. Important Information. Intended Use. Caution. About the System
 GlucCell TM SYSTEM USER S GUIDE ver 2.3 Intended Use The GlucCell TM Cell Culture Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during
GlucCell TM SYSTEM USER S GUIDE ver 2.3 Intended Use The GlucCell TM Cell Culture Glucose Monitoring System (The GlucCell TM System) is designed to quantitatively measure the concentration of glucose during
AF HDL and LDL/VLDL Assay Kit
 A HDL and LDL/VLDL Assay Kit Catalog Number KA1656 100 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3
A HDL and LDL/VLDL Assay Kit Catalog Number KA1656 100 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Principle of the Assay... 3
