Urease from a Potentially Pathogenic Coccoid Isolate: Purification, Characterization, and Comparison to Other Microbial Ureases
|
|
|
- Brett Owens
- 5 years ago
- Views:
Transcription
1 INFECTION AND IMMUNITY, Oct. 1997, p Vol. 65, No /97/$ Copyright 1997, American Society for Microbiology Urease from a Potentially Pathogenic Coccoid Isolate: Purification, Characterization, and Comparison to Other Microbial Ureases SUNG G. LEE 1,2 AND DAVID H. CALHOUN 1,3,4 * Center for Applied Biomedicine and Biotechnology, 3 Biochemistry and Biology Doctoral Programs, 4 The Graduate School and University Center, 2 and Department of Chemistry, City College of New York, 1 The City University of New York, New York, New York Received 25 March 1997/Returned for modification 28 May 1997/Accepted 1 July 1997 Strain SL100 is a gram-positive coccoid isolate prototype with an adhesin specific for gastric mucin and is representative of potentially pathogenic organisms obtained at biopsy from patients with gastric disorders. The urease of this isolate constitutes a significant fraction of the total cell protein, and the outcome of the purification strategy described herein suggests that it is associated with a cell wall fraction. The urease was purified 138-fold to apparent homogeneity, as indicated by gel electrophoresis, to a specific activity of 1,120 U/mg. The urease was unstable during purification in the absence of nickel, which is present in a metallocenter in other microbial ureases. When nickel sulfate was present during growth (5 M) and in buffers during sonication and purification (100 M), the urease was completely stable at room temperature during the purification procedure. The native urease was approximately 260 kda and was composed of three subunits of 65 kda and three subunits of 21 kda. The purified urease was relatively stable in acid and retained most of its activity after incubation for 30 min at ph 1.3. The K m s for urease measured from whole cells and for the purified enzyme were 0.56 and 1.7 mm, respectively, indicating that some cell wall component(s) affects the affinity of the enzyme for urea. The V max s for urea hydrolysis measured from whole cells and for the purified enzyme were 8.1 and 1,120 mol/min/mg of protein, respectively. The kinetic parameters, relative abundance, and subunit composition are more similar to those of the ureases of Helicobacter than to those of the ureases of other microbial species. These similarities are consistent with an adaptation of this organism to colonization of the stomach and indicate that the urease may be a virulence factor during colonization. Downloaded from It is known that many cases of gastritis and gastric ulceration in humans are caused by Helicobacter pylori infection (6, 27, 39). Similar gastric disorders are caused by Helicobacter felis infection in cats, dogs, and mice (3, 36) and by Helicobacter mustelae infection in ferrets (13, 14, 35, 36), while Helicobacter heilmannii causes gastric disorders in a wide range of animals (29, 42). One shared feature among the Helicobacter species is high urease activity. Evidence has been presented which indicates that ureases protect these gastric organisms from gastric acid by neutralizing the acid with the ammonia generated through urea hydrolysis (12, 21). In addition, ureases have been shown to promote colonization of the stomachs of gnotobiotic piglets and ferrets by H. pylori (9, 10) and H. mustelae (1), respectively. Various toxic effects of ureases have also been documented (17, 40, 45). There are many bacterial species with strong ureases (30, 31), and several of these species infect the urinary tract. They include Proteus mirabilis (28), Ureaplasma urealyticum (44), Staphylococcus saprophyticus (15, 34), and others (32). Some urease-positive bacteria can infect other human organs (33, 37, 43). The ureases of Helicobacter species are composed of two subunits of approximately 26.5 (UreA) and 60.3 (UreB) kda. The ureases of non-helicobacter bacterial species have three subunits of 6 to 14 kda (UreA), 11 to 20 kda (UreB), and 60 * Corresponding author. Mailing address: Department of Chemistry, City College of New York, Convent Ave. and 138th St., New York, NY Phone: (212) Fax: (212) calhoun@scisun.sci.ccny.cuny.edu. Present address: Laboratory of Bacterial Pathogenesis and Immunology, Rockefeller University, New York, NY to 70 kda (UreC) (2, 4, 5, 7, 15, 20, 23, 25, 33, 41). The amino acid sequence of the large UreC subunit of the non-helicobacter bacterial species is homologous to that of the large UreB subunit of Helicobacter species. The nucleotide and amino acid sequence homologies are consistent with fusion of the genes encoding the UreA and UreB of non-helicobacter bacterial species to generate the Helicobacter urease subunit of 26.5 kda (31). The isolation of a mucinophilic gram-positive staphylococcal species from the stomachs of gastric patients was recently reported, and these isolates were shown to exhibit a strong urease activity (24). We describe here the purification and characterization of the urease of this organism. Using purified urease, we found that the enzyme retained 75% of its original activity after exposure to ph 1.3, indicating that this urease is well-suited to an acidic environment. In addition, the urease was found to be composed of subunits of 21 and 65 kda. As the only other bacterial ureases with two subunits are those of Helicobacter species, this urease may be more closely related to the urease of H. pylori than to the ureases of gram-positive bacteria such as Staphylococcus species. MATERIALS AND METHODS Cell growth and disruption. The SL100 prototype of the coccoid organism isolated from the antra of human gastric patients was grown as described previously (24) except for the inclusion of 5 M nickel sulfate in the medium. Cells scraped from 40 confluent-culture plates were suspended in 240 ml of distilled water and were pelleted by 10 min of centrifugation at 12,000 rpm with an SS34 rotor and a Sorval centrifuge. The cell pellets were then suspended in 100 ml of 20 mm Tris-HCl buffer (ph 7.8) containing 1 mm EDTA and 10 mg of lysozyme, and the suspension was sonicated for 5 min at 50% of the maximum energy output using a sonicator, model W-385 (Heat Systems-Ultrasonics, Inc., Plainview, N.Y.). The suspension was then incubated for 4 h at 37 C, phenylmethyl- on September 30, 2018 by guest 3991
2 3992 LEE AND CALHOUN INFECT. IMMUN. FIG. 1. Molecular size estimate of the microbial urease obtained by Sephadex G-200 chromatography. The column was calibrated with hemoglobin, IgG, and amylase, and the urease activity peak was eluted just ahead of amylase. OD, optical density. sulfonyl fluoride and nickel sulfate were added to it at final concentrations of 0.5 and 0.1 mm respectively, and the suspension was sonicated again for 5 min as described above. The sonicated cell suspension was centrifuged for 10 min at 12,000 rpm with an SS34 rotor and a Sorval centrifuge, and the supernatant was collected. The pellets, consisting mainly of unbroken cells, were suspended in 80 ml of 20 mm Tris-HCl buffer (ph 7.8) and sonicated and centrifuged as described above, and the supernatant was collected. This process of sonication and centrifugation was repeated three or four more times, and the pooled supernatant was used as a starting material for urease purification. Enzyme purification. The pooled supernatant (375 ml) was applied to a DEAE-cellulose column (7 by 2 cm) equilibrated with 30 mm Tris-HCl buffer (ph 7.8), and the turbid eluant containing unadsorbed materials was saved. This turbid eluant contained cell wall fragments and over half of the urease activity of the supernatant. The column was then washed with 0.25 M KCl in 30 mm Tris-HCl buffer (ph 7.8) and the eluant, which did not contain urease activity, was discarded. The column was then washed with 0.4 M KCl in 30 mm Tris-HCl buffer (ph 7.8), and a clear eluant containing urease activity was saved. The turbid eluant containing urease activity (which initially passed through the column) was rechromatographed as described above, and the urease that partitioned into the 0.4 M KCl eluant was saved, while the urease that was eluted with unbound materials was rechromatographed again. The combined eluants in 0.4 M KCl were concentrated to 10 ml by means of ultrafiltration and applied to a Sephadex G-200 column (60 by 3 cm) equilibrated with 0.1 M KCl in 30 mm Tris-HCl buffer, ph 7.8, and the column was eluted with the same buffer. The fractions with urease activity (Fig. 1) were pooled and applied to a DEAEcellulose column (6 by 1.5 cm). This column was washed with 0.25 KCl in 30 mm Tris-HCl buffer (ph 7.8), and the urease was eluted with 300 ml of 0.25 to 0.4 M KCl gradient in the same buffer. The fractions with urease activity that was eluted between 0.29 and 0.34 M KCl were pooled and applied to a hydroxylapatite column (5 by 1.5 cm) equilibrated with 30 mm Tris-HCl buffer (ph 7.2). This column was washed with Tris-HCl buffer, and the urease was eluted with a 200-ml gradient from 30 mm Tris-HCl (ph 7.2) to 0.15 M phosphate buffer (ph 7.2). The fractions with urease activity that was eluted at 0.03 M phosphate were pooled and concentrated to 3 ml by means of ultrafiltration. This urease preparation was loaded onto two preparative 7.5% polyacrylamide slab gels (14 cm by 14 cm by 1 mm; Hoefer Scientific Instruments) and the buffer system of Laemmli (22) was used, with the omission of sodium dodecyl sulfate (SDS). After polyacrylamide gel electrophoresis (PAGE) at 65 V for 22 h at room temperature, a section of the gel with urease activity was removed, and urease was electroeluted from the gel section. Enzyme assays. During enzyme purification urease activity was monitored by observing the color change of a phenol red ph indicator from yellow to red, which results from a ph increase due to urea hydrolysis. Enzyme solution (10 l) was mixed with 100 l of a yellow test solution, ph 3.0, containing a1mm concentration of the free-acid form of phenol red and 100 mm urea, and red color development was observed. To determine the urease activity in a polyacrylamide gel, 5-mm-thick gel sections were introduced into test tubes containing 1 ml of the test solution, and color development was observed. Quantitative measurements of urea hydrolysis were determined spectrophotometrically by assaying glutamate dehydrogenase-mediated NADPH oxidation which was coupled to ammonia utilization (21). This assay was carried out at room temperature in a 1-ml volume in a cuvette containing 240 M NADPH, 810 M -ketoglutarate, 15 U of glutamate dehydrogenase (Sigma type II), 10 mm urea, 30 mm Tris-HCl (ph 8.0), and 10 l of urease solution. Urea hydrolysis was determined by measuring NADPH oxidation at 340 nm. Amylase from sweet potatoes (Sigma) and immunoglobulin G (IgG) were used as molecular weight markers for Sephadex G200 chromatography (Fig. 1). To determine amylase activity, 5gofcorn starch was suspended in 100 ml of 50 mm acetate buffer (ph 5.0) and the suspension was boiled for 5 min. The suspension was centrifuged for 10 min at 3,000 rpm with a Beckman microcentrifuge, and the turbid supernatant was collected. Aliquots (2 ml) of the starch solution were mixed in test tubes with 100- l aliquots of the fractions from the Sephadex G200 column and the mixtures were incubated overnight at 37 C. The amount of free sugar with a reducing end was determined by Fehling s reaction. For this procedure 2 ml of 0.1% CuSO 4 and 0.1% sodium tartrate in 0.1 N NaOH was added to each reaction tube, the tubes were placed in boiling water, and the amount of reddish metallic Cu 2 O deposited on the bottom of the tubes was recorded. To detect IgG, 20- l aliquots of the fractions from the Sephadex G200 column were spotted on nitrocellulose, blocked with 2% skim milk, and then incubated with a solution of covalently protein A-linked horseradish peroxidase. The peroxidase activity bound to the spots was determined with 0.05% diaminobenzidine and 0.03% H 2 O 2. Acid stability of urease. The phosphate concentration of the urease preparation from the hydroxylapatite step was reduced 10-fold to 3 mm by means of ultrafiltration. Aliquots (20 l) of this enzyme solution were mixed in microcentrifuge tubes with 20 l each of buffers at ph 1.3 (100 mm HCl), 3.0 (100 mm citrate), 4.0 (100 mm formate), 5.0 (100 mm acetate), 6.0 (100 mm succinate), and 7.0 (100 mm phosphate). After incubation for 30 min at room temperature, 1 l of phenol red ph indicator solution was added to each tube, and 1 M Tris-HCl (ph 9.0) was added dropwise until the indicator turned red. Volume adjustments were made by adding water to the enzyme solutions at or near neutral ph. The neutralized enzyme solutions were allowed to stand at room temperature for 30 min, after which 10- l aliquots of the enzyme solutions were assayed for urease activity by measuring glutamate dehydrogenase-mediated NADPH oxidation. RESULTS Cell disruption. Initial attempts to break open cells revealed that the external integument of this strain has a structure that makes it resistant to routine cell disruption by sonication, ly-
3 VOL. 65, 1997 UREASE OF POTENTIALLY PATHOGENIC GASTRIC ISOLATE 3993 Purification step TABLE 1. Purification of urease of strain SL100 Amt of total protein (mg) Total activity (10 3 mol/ min) Sp act ( mol/ min/mg) Yield (%) Purification (fold) Crude extract DEAE cellulose (0.4 M eluate) Sephadex G DEAE-cellulose (0.2 to 0.4 M gradient) Hydroxylapatite Nondenaturing acrylamide gel (electroelution) , sozyme, or the use of a French press. However, a combination of sonication at maximum energy output and prolonged incubation with lysozyme was effective in cell disruption. Cells suspended in buffer containing lysozyme were sonicated in an effort to expose peptidoglycan sites to digestion. Five successive sonications of 5 min each followed by 4 h of incubation with lysozyme resulted in the disruption of over two thirds of the cells as judged by the amount of protein in the supernatant after centrifugation. The relative number of cells lysed by this procedure increased when the cells were harvested several days after reaching confluence. Enzyme purification. In a typical purification procedure (Table 1), approximately 375 ml of turbid cell extract was passed through a DEAE-cellulose column. The first 220 ml of eluant was clear in appearance and was devoid of urease activity, but the following 150 ml was turbid and contained 42% of the urease activity. The turbid fraction eluted from the DEAE column was clarified by centrifugation and all of the urease activity was found in the pellet. The remaining portion (58%) of the urease activity was tightly bound to DEAE-cellulose, and it was eluted with 0.4 M KCl. When the 0.4 M KCl eluant from the DEAE-cellulose column was chromatographed on a Sephadex G-200 column, urease activity was eluted at approximately 260 kda (Fig. 1). The peak urease fractions from the G-200 column were applied to a DEAE-cellulose column and then eluted with a 0.25 to 0.4 M KCl gradient, and the urease activity was eluted between 0.29 and 0.34 M KCl. The urease fraction from this step was applied to a hydroxylapatite column and was eluted with a potassium phosphate gradient. The major urease peak was eluted at 0.03 M and a minor peak was eluted at 0.08 M potassium phosphate. It is not clear how the activities in the major and minor peaks differ. The hydroxylapatite step did not increase specific activity appreciably, because the same two major protein species eluted with the urease in both the DEAE-cellulose and the hydroxylapatite steps (Fig. 2). The major urease fraction that was eluted at 0.03 M phosphate was purified by preparative PAGE in the absence of SDS. The urease activity migrated as a broad band to a position between the two major protein species. All enzyme preparations (Fig. 2) yielded an identical broad band when stained with Coomassie blue, and this band corresponded to the urease activity in a parallel gel. The urease preparation from the gel electrophoresis step showed this band only (Fig. 2, lane 6), indicating that these urease fractions were homogeneous by these criteria. Molecular size and subunit composition of urease. Initial experiments revealed that treatment of the urease in 0.2% SDS at room temperature for 30 min resulted in partial denaturation of the enzyme, with some fraction of the enzyme retaining catalytic activity detected in situ after SDS gel electrophoresis. Prior to PAGE, aliquots of the crude extract, hydroxlyapatite eluate, and final purified enzyme preparation were incubated in 0.2% SDS at room temperature for 30 min and duplicate samples were boiled in 0.4% SDS containing 2 mm dithiothreitol to completely denature the enzyme (Fig. 3). One section of the gel was stained with Coomassie blue (Fig. 3, lanes 1 to 8), and another gel section (Fig. 3, lanes 10 to 15) was immersed in a solution containing phenol red and urea to detect urease activity. The activity of the urease was detected in the purified enzyme, hydroxlyapatite eluate, and crude extract incubated at room temperature prior to electrophoresis (Fig. 3, lanes 10 to 12, respectively). The Coomassie bluestained section of the gel revealed that the purified enzyme (Fig. 3, lane 3) contained dissociated subunits of 21 and 65 kda, as well as detectable levels of native urease that migrated above the 200-kDa size marker (Fig. 3, lane 8). This result was consistent with the molecular mass estimate of approximately 260 kda for the native enzyme (Fig. 1) and indicated the presence of a hexameric holoenzyme containing three subunits each of the 65- and 21-kDa monomers. When these samples were boiled prior to being loaded on the gel, all catalytic activity of the urease was lost (Fig. 3, lanes 13 to 15) and the purified urease showed only the subunits of 65 and 21 kda (Fig. 3, lane 6). In order to test for the possible presence of smaller subunits in the purified enzyme preparation that may FIG. 2. Electrophoresis of various urease preparations on a nondenaturing polyacrylamide gel. One gel section (lanes 1 to 7) was stained with Coomassie blue and the other section (lanes 8 to 13) was immersed in a solution containing 100 mm urea and 1 mm phenol red ph indicator to measure urease activity. The urease preparations analyzed were the crude extract (lanes 2 and 9), the fractions from Sephadex G-200 (lanes 3 and 10), the DEAE-cellulose gradient (lanes 4 and 11), the hydroxylapatite (lanes 5 and 12), and the preparation from electroelution from a nondenaturing gel (lanes 6 and 13). Lanes 1, 7, and 8 were not loaded with samples.
4 3994 LEE AND CALHOUN INFECT. IMMUN. FIG. 3. Identification of urease catalytic form and subunits by nondenaturing SDS-PAGE. One set of urease preparations (lanes 1 through 3 and 10 through 12) was incubated with 0.2% SDS at room temperature, and a second set of the same preparations (lanes 4 through 6 and 13 through 15) was boiled with 0.5% SDS and 2 mm dithiothreitol. The urease preparations analyzed were the crude extract (lanes 1, 4, 10, and 13), the hydroxylapatite eluate (lanes 2, 5, 11, and 14), and the electroeluted sample (lanes 3, 6, 12, and 15), with Coomassie blue and urease assays as described in the legend to Fig. 2. FIG. 4. Effect of ph on stability of the microbial urease. The urease preparation from the hydroxylapatite step was incubated for 30 min in buffers at ph 1.3 to 7.0, the solutions were neutralized, and enzyme activities were determined as described in Materials and Methods. not be detectable with the quantities of protein loaded in Fig. 3 (lanes 3 and 6), 10 times more purified enzyme was analyzed by SDS-PAGE using a 16% acrylamide gel. Additional minor contaminants were detected between the 31- and 65-kDa subunits, but no protein bands migrating faster than the 31-kDa subunit were detected. Stability of urease in cell extracts. In the initial attempts at urease purification, nickel was not added at any stage. Under these conditions urease activity decayed rapidly during purification. Subsequently, nickel sulfate (5 M) was added to the culture medium and to the cell suspension (100 M) after incubation with lysozyme and prior to sonication. When these techniques were used, urease activity remained completely stable at room temperature throughout the purification steps. Stability of the purified urease at low ph. Because the coccus isolate from which the urease was purified would be exposed to gastric acid during the course of an infection, the stability of the urease at an acidic ph was examined. The urease was incubated for 30 min at room temperature in buffers with phs ranging from 1.3 to 7. The ph was then adjusted to a neutral level and enzyme activity was determined quantitatively by measuring NADPH oxidation in a glutamate dehydrogenase-coupled reaction. The urease exposed to phs as low as 5.0 retained full activity, and even the urease exposed to the lowest ph tested (1.3) retained most of the activity (Fig. 4). The purified urease was also completely stable when treated with 5% SDS for 30 min at room temperature. Enzyme kinetics. The kinetics of urea hydrolysis was determined from Lineweaver-Burk plots for urease activity detectable in the whole cell and for the purified enzyme (Fig. 5). The calculated K m values for the whole cell and the purified enzyme were 0.56 and 1.7 mm, respectively, and the maximum velocities of urea hydrolysis were 9.3 for the whole cell and 1,350 mol/min/mg of protein for purified urease, respectively. The approximately threefold-higher affinity for urea when the urease is present in intact cells apparently is due to some association of the enzyme with accessory proteins or cellular components. The activity of the urease in intact cells is consistent with a role of this enzyme as a virulence factor during initial colonization of the stomach. DISCUSSION With the exception of those of H. pylori, most microbial ureases appear to be located in the cytoplasmic fraction, although conflicting reports have appeared in this regard (31). The urease of H. pylori was shown to be readily released from the cell upon exposure to water, 0.15 M NaCl, or nondenaturing detergents (8). In contrast, a prolonged exposure of strain SL100 to these agents did not release urease. Ureases of Helicobacter species provide protection from gastric acid, as demonstrated by the observation that H. pylori survived at ph 1.5 only if urea was present in the medium (26). Since the purified urease of strain SL100 retained most of its enzyme activity after exposure to a ph of 1.3 (Fig. 3), it should be active during colonization of stomach mucin. However, strain SL100 survived after overnight exposure to ph 1.3 even in the absence of urea (unpublished data). The ability to withstand an acid ph would be expected to be important for the survival of this organism because it appears to colonize the surface of the mucus layer (24) and would thus be fully exposed to gastric acid. In contrast, Helicobacter species reside within and under the mucus attached to stomach epithelial cells in a location more protected from gastric acid. The urease of strain SL100 is composed of subunits of 21 and 65 kda (Fig. 4). The ureases of Helicobacter species are composed of two subunits, whereas the ureases of all other non-helicobacter bacterial species, including Staphylococcus xylosus, are composed of three distinct subunits (2, 4, 5, 7, 19, 20, 23, 25, 33, 38, 41). Initial taxonomic classification of the coccus based on fatty acid composition and metabolic characterizations showed relationships to Staphylococcus cohnii and S. xylosus, respectively (24). Thus, the coccus resembles S. xylosus
5 VOL. 65, 1997 UREASE OF POTENTIALLY PATHOGENIC GASTRIC ISOLATE 3995 FIG. 5. Lineweaver-Burk plots showing urease activity in whole cells and purified enzyme. Urease activity was determined by measuring glutamate dehydrogenasemediated NADPH oxidation as described in Materials and Methods. and S. cohnii if classified by standard typing approaches, but its urease is more similar to that of the gram-negative gastric pathogen Helicobacter. The urease of H. pylori has one of the lowest K m values (0.17 mm) for urea (18), in contrast to the higher K m values (13 to 130 mm) for enzymes in microorganisms that are found in the urinary tract or soil (31). This has been interpreted to suggest a correlation between the K m and an organism s ecological niche. H. pylori colonizes the gastric mucosal lining and depends upon the low concentrations of urea supplied from the serum. The K m values for strain SL100 (0.56 mm for the whole cell and 1.7 mm for the purified enzyme) are more similar to the K m values of 0.2 to 0.5 mm reported for the ureases of various Helicobacter species (8, 11, 16, 18) than to the values for other Staphylococcus species (e.g., 9.5 mm for S. saprophyticus [38]) or other bacteria that are urinary tract pathogens or soil microorganisms. Thus, the properties of the urease of strain SL100, including its subunit composition, K m for urea, and relative abundance as a percentage of total cell protein, are not typical of ureases of previously described Staphylococcus species. These properties are more similar to those of the urease of the known stomach pathogen H. pylori and provide further support for the pathogenic potential of these coccoid isolates. ACKNOWLEDGMENTS This work was supported by NIH RCMI grant RR03060, NIH MBRS grant RR08168, the Center for Applied Biomedicine and Biotechnology of the Applied Science Coordinating Institute, and the Graduate Center of the City University of New York. We thank Nadia Allen, Enrique Arevalo, Lisa Liu, and Veronica Gibaja for their excellent technical assistance in some phases of this project. REFERENCES 1. Andrutis, K. A., J. G. Fox, D. B. Schauer, R. P. Marini, J. C. Murphy, L. Yan, and J. V. Solnick Inability of an isogenic urease-negative mutant strain of Helicobacter mustelae to colonize the ferret stomach. Infect. Immun. 63: Blanchard, A Ureaplasma urealyticum urease genes: use of UGA tryptophan codon. Mol. Microbiol. 4: Chen, M., A. Lee, and S. L. Hazell Immunization against gastric Helicobacter infection in a mouse/helicobacter felis model. Lancet 339: Christians, S., J. Jose, U. Schfer, and H. Kaltwasser Purification and subunit determination of the nickel-dependent Staphylococcus xylosus urease. FEMS Microbiol. Lett. 80: Clemens, D. L., B.-Y. Lee, and M. A. Horwitz Purification, characterization, and genetic analysis of Mycobacterium tuberculosis urease, a potentially critical determinant of host-pathogen interaction. J. Bacteriol. 177: Cover, T. L., and M. J. Blaser Helicobacter pylori: a bacterial cause of gastritis, peptic ulcer disease, and gastric cancer. ASM News 61: de Koning-Ward, T. F., A. C. Ward, and R. M. Robins-Browne Characterization of the urease-encoding gene complex of Yersinia enterocolitica. Gene 142: Dunn, B. E., G. P. Campbell, G. I. Perez-Perez, and M. J. Blaser Purification and characterization of urease from Helicobacter pylori. J. Biol. Chem. 265: Eaton, K. A., C. L. Brooks, D. R. Morgan, and S. Krakowka Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect. Immun. 59: Eaton, K. A., and S. Krakowka Effect of gastric ph on urease-dependent colonization of gnotobiotic piglets by Helicobacter pylori. Infect. Immun. 62: Evans, D. J., D. G. Evans, S. S. Kirkpatrick, and D. Y. Graham Characterization of Helicobacter pylori urease and purification of its subunits. Microbiol. Pathol. 10: Ferrero, R. L., and A. Lee The importance of urea in acid protection for the gastric-colonizing bacteria H. pylori and felis sp. nov. Microb. Ecol. Health Dis. 4: Fox, J. G., P. Correa, N. S. Taylor, A. Lee, G. Otto, J. C. Murphy, and R. Rose Helicobacter mustelae-associated gastritis in ferrets. An animal model of Helicobacter pylori gastritis in humans. Gastroenterology 99: Fox, J. G., G. Otto, N. S. Taylor, W. Rosenblad, and J. C. Murphy Helicobacter mustelae-induced gastritis and elevated gastric ph in the ferret (Mustela putorius furo). Infect. Immun. 59: Gatermann, S., J. John, and R. Marre Staphylococcus saprophyticus urease: characterization and contribution to uropathogenicity in unobstructed urinary tract infection of rats. Infect. Immun. 57: Gootz, T. D., G. I. Perez-Perez, J. Clancy, B.-A. Martin, A. Tait-Kamradt, and M. J. Blaser Immunological and molecular characterization of Helicobacter felis urease. Infect. Immun. 62: Hazell, S. L Urease and catalase as virulence factors of Helicobacter
6 3996 LEE AND CALHOUN INFECT. IMMUN. pylori, p In H. Menge et al. (ed.), Helicobacter pylori. Springer-Verlag, Berlin, Germany. 18. Hu, L.-T., and H. L. T. Mobley Purification and N-terminal analysis of urease from Helicobacter pylori. Infect. Immun. 58: Johnson, D. E., R. G. Russell, C. V. Lockatell, J. C. Zulty, J. W. Warren, and H. L. T. Mobley Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect. Immun. 61: Jones, B. D., and H. L. T. Mobley Proteus mirabilis urease: nucleotide sequence determination and comparison with jack bean urease. J. Bacteriol. 171: Kaltwasser, H., and H. G. Schlegel NADH-dependent coupled assay for urease and other ammonia producing systems. Anal. Biochem. 16: Laemmli, U. K Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: Lee, M. H., S. B. Mulrooney, and R. P. Hausinger Purification, characterization, and in vivo reconstitution of Klebsiella aerogenes urease apoenzyme. J. Bacteriol. 172: Lee, S. G., C. Kim, and Y. C. Ha Successful cultivation of a potentially pathogenic coccoid organism with trophism for gastric mucin. Infect. Immun. 65: Maeda, M., M. Hidaka, A. Nakamura, H. Masaki, and T. Uozumi Cloning, sequencing, and expression of thermophilic Bacillus sp. strain TB-90 urease gene complex in Escherichia coli. J. Bacteriol. 176: Marshall, B. J., L. J. Barrett, C. Prakash, R. W. McCallun, and R. L. Guerrant Urea protects Helicobacter (Campylobacter) pylori from the bacterial effect of acid. Gastroenterology 99: Marshall, B. J., and J. R. Warren Unidentified curved bacillus in the stomach of patients with gastritis and peptic ulceration. Lancet ii: Mclean, R. J. C., J. C. Nickel, K.-J. Chang, and J. W. Costerton The ecology and pathogenicity of urease-producing bacteria in the urinary tract. Crit. Rev. Microbiol. 16: McNulty, C. A. M., J. C. Dent, A. Curry, J. S. Uff, G. A. Ford, M. W. Gear, and S. P. Wilkinson New spiral bacterium in gastric mucosa. J. Clin. Pathol. 42: Mobley, H. L. T., and R. P. Hausinger Microbial ureases: significance, regulation, and molecular characterization. Microbiol. Rev. 53: Mobley, H. L. T., M. D. Island, and R. P. Hausinger Molecular biology of microbial ureases. Microbiol. Rev. 59: Mobley, H. L. T., and J. W. Warren Urease-positive bacteriuria and obstruction of long-term urinary catheters. J. Clin. Microbiol. 25: Morsdorf, G., and H. Kaltwasser Cloning of the genes encoding Editor: J. G. Cannon urease from Proteus vulgaris and sequencing of the structural genes. FEMS Microbiol. Lett. 66: Osterberg, E., H. O. Hallander, A. Kallner, A. Lundin, S. B. Svensson, and H. Aberg Female urinary tract infection in primary health care: bacteriological and clinical characteristics. Scand. J. Infect. Dis. 22: Otto, G., J. G. Fox, P.-Y. Wu, and N. S. Taylor Eradication of Helicobacter mustelae from the ferret stomach: an animal model of Helicobacter (Campylobacter) pylori chemotherapy. Antimicrob. Agents Chemother. 34: Paster, B. J., A. Lee, J. G. Fox, F. E. Dewhirst, L. A. Tordoff, G. J. Fraser, J. L. O Rourke, N. S. Taylor, and R. Ferrero Phylogeny of Helicobacter felis sp. nov., Helicobacter mustelae, and related bacteria. Int. J. Syst. Bacteriol. 41: Probst, P., E. Hermann, K.-H. Meyer zum Büschenfelde, and B. Fleischer Identification of the Yersinia enterocolitica urease subunit as a target antigen for human synovial T lymphocytes in reactive arthritis. Infect. Immun. 61: Schfer, U. K., and H. Kaltwasser Urease from Staphylococcus saprophyticus: purification, characterization and comparison to Staphylococcus xylosus urease. Arch. Microbiol. 161: Sipponen, P., M. Siurala, and C. S. Goodwin Histology and ultrastructures of Helicobacter pylori infection: gastritis, duodenitis and peptic ulceration, and their relevance as precancerous conditions, p In C. S. Goodwin and B. W. Worsley (ed.), Helicobacter pylori: biology and clinical practice. CRC Press, Inc., Boca Raton, Fla. 40. Smoot, D. T., H. L. T. Mobley, G. R. Chippendale, J. F. Lewison, and J. H. Resau Helicobacter pylori urease activity is toxic to human gastric epithelial cells. Infect. Immun. 58: Solnick, J. V., J. O Rourke, A. Lee, and L. S. Tompkins Molecular analysis of urease genes from a newly identified uncultured species of Helicobacter. Infect. Immun. 62: Solnick, J. V., J. O Rourke, A. Lee, B. J. Paster, F. E. Dewhirst, and L. S. Tompkins An uncultured gastric spiral organism in a newly identified Helicobacter in humans. J. Infect. Dis. 168: Suzuki, K., Y. Benno, T. Mitsuoka, S. Takebe, K. Kobashi, and J. Hase Urease-producing species of intestinal anaerobes and their activities. Appl. Environ. Microbiol. 37: Takebe, S., A. Numata, and K. Kobashi Stone formation by Ureaplasma urealyticum in human urine and its prevention by urease inhibitors. J. Clin. Microbiol. 20: Xu, J.-K., C. S. Goodwin, M. Cooper, and J. Robinson Intercellular vacuolization caused by the urease of Helicobacter pylori. J. Infect. Dis. 161:
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
SUPPLEMENTARY MATERIAL Purification and biochemical properties of SDS-stable low molecular weight alkaline serine protease from Citrullus Colocynthis Muhammad Bashir Khan, 1,3 Hidayatullah khan, 2 Muhammad
H.Pylori IgM Cat # 1504Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Chapter PURIFICATION OF ALKALINE PROTEASES
 Chapter PURIFICATION OF ALKALINE PROTEASES E /xtracellular alkaline proteases produced by Bacillus sp. K 25 and bacillus pumilus K 242, were purified and the homogeneity was examined by electrophoresis.
Chapter PURIFICATION OF ALKALINE PROTEASES E /xtracellular alkaline proteases produced by Bacillus sp. K 25 and bacillus pumilus K 242, were purified and the homogeneity was examined by electrophoresis.
H.Pylori IgG Cat # 1503Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
See external label 2 C-8 C Σ=96 tests Cat # 1505Z. MICROWELL ELISA H.Pylori IgA Cat # 1505Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
H. pylori Antigen ELISA Kit
 H. pylori Antigen ELISA Kit Catalog Number KA3142 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
H. pylori Antigen ELISA Kit Catalog Number KA3142 96 assays Version: 04 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of
H.pylori IgA Cat #
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
H. pylori IgM. Cat # H. pylori IgM ELISA. ELISA: Enzyme Linked Immunosorbent Assay. ELISA - Indirect; Antigen Coated Plate
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com H. pylori
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com H. pylori
H.Pylori IgG
 DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
DIAGNOSTIC AUTOMATION, INC. 21250 Califa Street, Suite 102 and116, Woodland Hills, CA 91367 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com
Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples:
 Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
Dr. Sanjeeva Srivastava IIT Bombay Work-flow: protein sample preparation Precipitation methods Removal of interfering substances Specific examples: Sample preparation for serum proteome analysis Sample
H. pylori IgM CLIA kit
 H. pylori IgM CLIA kit Cat. No.:DEEL0251 Pkg.Size:96 tests Intended use Helicobacter pylori IgM Chemiluminescence ELISA is intended for use in evaluating the serologic status to H. pylori infection in
H. pylori IgM CLIA kit Cat. No.:DEEL0251 Pkg.Size:96 tests Intended use Helicobacter pylori IgM Chemiluminescence ELISA is intended for use in evaluating the serologic status to H. pylori infection in
Supporting Information
 Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Helicobacter and gastritis
 1 Helicobacter and gastritis Dr. Hala Al Daghistani Helicobacter pylori is a spiral-shaped gram-negative rod. H. pylori is associated with antral gastritis, duodenal (peptic) ulcer disease, gastric ulcers,
1 Helicobacter and gastritis Dr. Hala Al Daghistani Helicobacter pylori is a spiral-shaped gram-negative rod. H. pylori is associated with antral gastritis, duodenal (peptic) ulcer disease, gastric ulcers,
Scholars Research Library. Purification and characterization of neutral protease enzyme from Bacillus Subtilis
 Journal of Microbiology and Biotechnology Research Scholars Research Library J. Microbiol. Biotech. Res., 2012, 2 (4):612-618 (http://scholarsresearchlibrary.com/archive.html) Purification and characterization
Journal of Microbiology and Biotechnology Research Scholars Research Library J. Microbiol. Biotech. Res., 2012, 2 (4):612-618 (http://scholarsresearchlibrary.com/archive.html) Purification and characterization
Helicobacter pylori:an Emerging Pathogen
 Bacteriology at UW-Madison Bacteriology 330 Home Page Helicobacter pylori:an Emerging Pathogen by Karrie Holston, Department of Bacteriology University of Wisconsin-Madison Description of Helicobacter
Bacteriology at UW-Madison Bacteriology 330 Home Page Helicobacter pylori:an Emerging Pathogen by Karrie Holston, Department of Bacteriology University of Wisconsin-Madison Description of Helicobacter
Protein MultiColor Stable, Low Range
 Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
Product Name: DynaMarker Protein MultiColor Stable, Low Range Code No: DM670L Lot No: ******* Size: 200 μl x 3 (DM670 x 3) (120 mini-gel lanes) Storage: 4 C Stability: 12 months at 4 C Storage Buffer:
SUPPLEMENTARY INFORMATION. Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was
 SUPPLEMENTARY INFORMATION Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was grown in a casein-based semisynthetic medium (C+Y) supplemented with yeast extract (1 mg/ml of
SUPPLEMENTARY INFORMATION Bacterial strains and growth conditions. Streptococcus pneumoniae strain R36A was grown in a casein-based semisynthetic medium (C+Y) supplemented with yeast extract (1 mg/ml of
10 mm KCl in a Ti-15 zonal rotor at 35,000 rpm for 16 hr at
 Proc. Nat. Acad. SCi. USA Vol. 68, No. 11, pp. 2752-2756, November 1971 Translation of Exogenous Messenger RNA for Hemoglobin on Reticulocyte and Liver Ribosomes (initiation factors/9s RNA/liver factors/reticulocyte
Proc. Nat. Acad. SCi. USA Vol. 68, No. 11, pp. 2752-2756, November 1971 Translation of Exogenous Messenger RNA for Hemoglobin on Reticulocyte and Liver Ribosomes (initiation factors/9s RNA/liver factors/reticulocyte
Adherence of Isogenic Flagellum-Negative Mutants of Helicobacter pylori and Helicobacter mustelae to Human and Ferret Gastric Epithelial Cells
 INFECTION AND IMMUNITY, July 2000, p. 4335 4339 Vol. 68, No. 7 0019-9567/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Adherence of Isogenic Flagellum-Negative Mutants
INFECTION AND IMMUNITY, July 2000, p. 4335 4339 Vol. 68, No. 7 0019-9567/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Adherence of Isogenic Flagellum-Negative Mutants
Supplementary material: Materials and suppliers
 Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Supplementary material: Materials and suppliers Electrophoresis consumables including tris-glycine, acrylamide, SDS buffer and Coomassie Brilliant Blue G-2 dye (CBB) were purchased from Ameresco (Solon,
Amylase: a sample enzyme
 Amylase: a sample enzyme Objectives: After completion of this laboratory exercise you will be able to: 1. Explain the importance of enzymes in biology. 2. Explain the basic properties of an enzyme as a
Amylase: a sample enzyme Objectives: After completion of this laboratory exercise you will be able to: 1. Explain the importance of enzymes in biology. 2. Explain the basic properties of an enzyme as a
Mammalian Membrane Protein Extraction Kit
 Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Mammalian Membrane Protein Extraction Kit Catalog number: AR0155 Boster s Mammalian Membrane Protein Extraction Kit is a simple, rapid and reproducible method to prepare cellular protein fractions highly
Essential Role of Urease in Pathogenesis of Gastritis Induced by Helicobacter pylori in Gnotobiotic Piglets
 INFECTION AND IMMUNITY, JUlY 1991, p. 2470-2475 0019-9567/91/072470-06$02.00/0 Copyright C 1991, American Society for Microbiology Vol. 59, No. 7 Essential Role of Urease in Pathogenesis of Gastritis Induced
INFECTION AND IMMUNITY, JUlY 1991, p. 2470-2475 0019-9567/91/072470-06$02.00/0 Copyright C 1991, American Society for Microbiology Vol. 59, No. 7 Essential Role of Urease in Pathogenesis of Gastritis Induced
Aperto Cell Lysis and Protein Solubilization Users Manual
 Aperto Cell Lysis and Protein Solubilization Users Manual Revision 2 THIS MANUAL APPLIES TO THE FOLLOWING PRODUCTS: 3A8600 Aperto, 5X Cell Lysis Buffer. 20mL 3A8610 Aperto, 5X Cell Lysis Buffer. 100mL
Aperto Cell Lysis and Protein Solubilization Users Manual Revision 2 THIS MANUAL APPLIES TO THE FOLLOWING PRODUCTS: 3A8600 Aperto, 5X Cell Lysis Buffer. 20mL 3A8610 Aperto, 5X Cell Lysis Buffer. 100mL
EFFECT OF SOME CHEMICAL AND PHYSICAL FACTORS ON THE COCCI SHAPE FORMATION OF HELICOBACTER PYLORI ISOLATED FROM PATIENTS WITH DUODENUM ULCER
 . Helicobacter pylori. Helicobacterpylori (Duodenal ulcer) (Ski row medium) H. pylori (Nalidixic Acid).(Cephalothin) H. pylori (%). ( ) ().(86.9) (PH) (%) EFFECT OF SOME CHEMICAL AND PHYSICAL FACTORS ON
. Helicobacter pylori. Helicobacterpylori (Duodenal ulcer) (Ski row medium) H. pylori (Nalidixic Acid).(Cephalothin) H. pylori (%). ( ) ().(86.9) (PH) (%) EFFECT OF SOME CHEMICAL AND PHYSICAL FACTORS ON
Prerequisites Protein purification techniques and protein analytical methods. Basic enzyme kinetics.
 Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Supporting information (protein purification, kinetic characterization, product isolation, and characterization by NMR and mass spectrometry):
 Supporting Information Mechanistic studies of a novel C-S lyase in ergothioneine biosynthesis: the involvement of a sulfenic acid intermediate Heng Song, 1 Wen Hu, 1,2 Nathchar Naowarojna, 1 Ampon Sae
Supporting Information Mechanistic studies of a novel C-S lyase in ergothioneine biosynthesis: the involvement of a sulfenic acid intermediate Heng Song, 1 Wen Hu, 1,2 Nathchar Naowarojna, 1 Ampon Sae
His n. Vivapure Metal Chelate Mega spin columns. Technical data and operating instructions. For in vitro use only.
 His n Vivapure Metal Chelate Mega spin columns Technical data and operating instructions. For in vitro use only. columns - for the purification of proteins with poly-histidine tags Storage conditions columns
His n Vivapure Metal Chelate Mega spin columns Technical data and operating instructions. For in vitro use only. columns - for the purification of proteins with poly-histidine tags Storage conditions columns
Acetyl CoA Carboxylase: The Purified Transcarboxylase Component
 Proc. Nat. Acad. Sci. USA Vol. 68, No. 6, pp. 12591263, June 1971 Acetyl CoA Carboxylase: The Purified Transcarboxylase Component (acyl CoA binding/carboxylation/exchange reactions/biotin) ALFRED W. ALBERTS,
Proc. Nat. Acad. Sci. USA Vol. 68, No. 6, pp. 12591263, June 1971 Acetyl CoA Carboxylase: The Purified Transcarboxylase Component (acyl CoA binding/carboxylation/exchange reactions/biotin) ALFRED W. ALBERTS,
Identification of NADPH-thioredoxin reductase system
 Proc. Nat. Acad. Sci. USA Vol. 72, No. 11, pp. 4233-4237, November 1975 Biochemistry Identification of NADPH-thioredoxin reductase system in Euglena gracilis* (ribonucleotide reduction) S. MUNAVALLIO,
Proc. Nat. Acad. Sci. USA Vol. 72, No. 11, pp. 4233-4237, November 1975 Biochemistry Identification of NADPH-thioredoxin reductase system in Euglena gracilis* (ribonucleotide reduction) S. MUNAVALLIO,
Glutathione Synthesis in Human Erythrocytes
 Glutathione Synthesis in Human Erythrocytes II. PURIFICATION AND PROPERTIES OF THE ENZYMES OF GLUTATHIONE BIOSYNTHESIS PHILI W. MAjEUS, M. J. BRAUNER, M. B. SMITH, and VIRGINIA MINNICH From the Departments
Glutathione Synthesis in Human Erythrocytes II. PURIFICATION AND PROPERTIES OF THE ENZYMES OF GLUTATHIONE BIOSYNTHESIS PHILI W. MAjEUS, M. J. BRAUNER, M. B. SMITH, and VIRGINIA MINNICH From the Departments
Comparative study of invasive methods for diagnosis of Helicobacter pylori in humans
 ISSN: 2319-7706 Volume 2 Number 7 (2013) pp. 63-68 http://www.ijcmas.com Original Research Article Comparative study of invasive methods for diagnosis of Helicobacter pylori in humans V.Subbukesavaraja
ISSN: 2319-7706 Volume 2 Number 7 (2013) pp. 63-68 http://www.ijcmas.com Original Research Article Comparative study of invasive methods for diagnosis of Helicobacter pylori in humans V.Subbukesavaraja
Glutathione Assay Kit
 Glutathione Assay Kit Catalog Number KA1649 250 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Glutathione Assay Kit Catalog Number KA1649 250 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH
 Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
Practical Manual Food Chemistry and Physiology EXPERIMENT 4 DETERMINATION OF REDUCING SUGARS, TOTAL REDUCING SUGARS, SUCROSE AND STARCH Structure 4.1 Introduction Objectives 4.2 Experiment 4a: Reducing
Luminescent platforms for monitoring changes in the solubility of amylin and huntingtin in living cells
 Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is The Royal Society of Chemistry 2016 Contents Supporting Information Luminescent platforms for monitoring changes in the
Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin
 SUPPORTING INFORMATION Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin Anna R. Arnold, Andy Zhou, and Jacqueline K. Barton Division of Chemistry and Chemical Engineering, California
SUPPORTING INFORMATION Characterization of the DNA-mediated Oxidation of Dps, a Bacterial Ferritin Anna R. Arnold, Andy Zhou, and Jacqueline K. Barton Division of Chemistry and Chemical Engineering, California
Title: Column Chromatography of Green Fluorescent Protein
 Title: Column Chromatography of Green Fluorescent Protein Approvals: Preparer Date_07Oct06 Reviewer: Mary Jane Kurtz Date 09Jul13 Part I Crude Isolation of GFP from Lysed Cells q Page 1 of 6 1. Purpose:
Title: Column Chromatography of Green Fluorescent Protein Approvals: Preparer Date_07Oct06 Reviewer: Mary Jane Kurtz Date 09Jul13 Part I Crude Isolation of GFP from Lysed Cells q Page 1 of 6 1. Purpose:
Reconstitution of Neutral Amino Acid Transport From Partially Purified Membrane Components From Ehrlich Ascites Tumor Cells
 Journal of Supramolecular Structure 7:481-487 (1977) Molecular Aspects of Membrane Transport 5 1 1-5 17 Reconstitution of Neutral Amino Acid Transport From Partially Purified Membrane Components From Ehrlich
Journal of Supramolecular Structure 7:481-487 (1977) Molecular Aspects of Membrane Transport 5 1 1-5 17 Reconstitution of Neutral Amino Acid Transport From Partially Purified Membrane Components From Ehrlich
BIL 256 Cell and Molecular Biology Lab Spring, Tissue-Specific Isoenzymes
 BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
BIL 256 Cell and Molecular Biology Lab Spring, 2007 Background Information Tissue-Specific Isoenzymes A. BIOCHEMISTRY The basic pattern of glucose oxidation is outlined in Figure 3-1. Glucose is split
Date... Name... Group... Urine sample (Tube No 2)
 Date... Name... Group... Instructions for the practical lesson on biochemistry Topic: Non-protein nitrogen compounds Task 1: Estimation of creatinine in serum and urine 1. Trichloroacetic acid 1.22 mol/l
Date... Name... Group... Instructions for the practical lesson on biochemistry Topic: Non-protein nitrogen compounds Task 1: Estimation of creatinine in serum and urine 1. Trichloroacetic acid 1.22 mol/l
Phospholipase D Activity of Gram-Negative Bacteria
 JOURNAL OF BACTERIOLOGY, Dec. 1975, p. 1148-1152 Copyright 1975 American Society for Microbiology Vol. 124, No. 3 Printed in U.S.A. Phospholipase D Activity of Gram-Negative Bacteria R. COLE AND P. PROULX*
JOURNAL OF BACTERIOLOGY, Dec. 1975, p. 1148-1152 Copyright 1975 American Society for Microbiology Vol. 124, No. 3 Printed in U.S.A. Phospholipase D Activity of Gram-Negative Bacteria R. COLE AND P. PROULX*
<Supplemental information>
 The Structural Basis of Endosomal Anchoring of KIF16B Kinesin Nichole R. Blatner, Michael I. Wilson, Cai Lei, Wanjin Hong, Diana Murray, Roger L. Williams, and Wonhwa Cho Protein
The Structural Basis of Endosomal Anchoring of KIF16B Kinesin Nichole R. Blatner, Michael I. Wilson, Cai Lei, Wanjin Hong, Diana Murray, Roger L. Williams, and Wonhwa Cho Protein
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Helicobacter Species from Humans and Animals
 INFECrION AND IMMUNITY, Dec. 1992, p. 5259-5266 0019-9567/92/125259-08$02.00/0 Copyright 1992, American Society for Microbiology Vol. 60, No. 12 Purification and Characterization of the Urease Enzymes
INFECrION AND IMMUNITY, Dec. 1992, p. 5259-5266 0019-9567/92/125259-08$02.00/0 Copyright 1992, American Society for Microbiology Vol. 60, No. 12 Purification and Characterization of the Urease Enzymes
HiPer Western Blotting Teaching Kit
 HiPer Western Blotting Teaching Kit Product Code: HTI009 Number of experiments that can be performed: 5/20 Duration of Experiment: ~ 2 days Day 1: 6-8 hours (SDS- PAGE and Electroblotting) Day 2: 3 hours
HiPer Western Blotting Teaching Kit Product Code: HTI009 Number of experiments that can be performed: 5/20 Duration of Experiment: ~ 2 days Day 1: 6-8 hours (SDS- PAGE and Electroblotting) Day 2: 3 hours
Assay Kit for Measurement of Proteoglycan. (Sulfated Glycosaminoglycan Quantification Kit)
 Assay Kit for Measurement of Proteoglycan. (Sulfated Glycosaminoglycan Quantification Kit) Cat. No. 280560-N INTRODUCTION Glycosaminoglycans (GAGs) are a major component of the extracellular matrix (ECM)
Assay Kit for Measurement of Proteoglycan. (Sulfated Glycosaminoglycan Quantification Kit) Cat. No. 280560-N INTRODUCTION Glycosaminoglycans (GAGs) are a major component of the extracellular matrix (ECM)
2010 Annual report. Principle Investigator: Dr. John W. Frost. Draths Corporation. ONR Award Number: N
 2010 Annual report Principle Investigator: Dr. John W. Frost Draths Corporation ONR Award Number: N000140710076 Award Title: Green Synthesis of Phloroglucinol: Exploiting Pseudomonas fluorescens and Scale-
2010 Annual report Principle Investigator: Dr. John W. Frost Draths Corporation ONR Award Number: N000140710076 Award Title: Green Synthesis of Phloroglucinol: Exploiting Pseudomonas fluorescens and Scale-
TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
 Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
Journal of Supramolecular Structure 4:441 (401)-447 (407) (1976) TRANSPORT OF AMINO ACIDS IN INTACT 3T3 AND SV3T3 CELLS. Binding Activity for Leucine in Membrane Preparations of Ehrlich Ascites Tumor Cells
THE UREASES OF PROTEUS STRAINS IN RELATION TO VIRULENCE FOR THE URINARY TRACT
 I. MED. MICP.QBIOL.-VOL. 13 (1980), 507-512 0 1980 The Pathological Society of Great Britain and Ireland 0022-26 15/80/0063 0507 $02.00 THE UREASES OF PROTEUS STRAINS IN RELATION TO VIRULENCE FOR THE URINARY
I. MED. MICP.QBIOL.-VOL. 13 (1980), 507-512 0 1980 The Pathological Society of Great Britain and Ireland 0022-26 15/80/0063 0507 $02.00 THE UREASES OF PROTEUS STRAINS IN RELATION TO VIRULENCE FOR THE URINARY
A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* Previous studies (1, 2) have shown that after the ingestion of caffeine
 A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* BY HERBERT H. CORNISH AND A. A. CHRISTMAN (From the Department of Biological Chemistry, Medical School, University of Michigan,
A STUDY OF THE METABOLISM OF THEOBROMINE, THEOPHYLLINE, AND CAFFEINE IN MAN* BY HERBERT H. CORNISH AND A. A. CHRISTMAN (From the Department of Biological Chemistry, Medical School, University of Michigan,
6. C-type cytochrome, soluble and membrane protein
 185 6. C-type cytochrome, soluble and membrane protein analysis of Rhodobacter sp SW2 and Rhodopseudomonas palustris TIE-1 ABSTRACT The ability to grown on Fe(II) is thought to be a primitive metabolism
185 6. C-type cytochrome, soluble and membrane protein analysis of Rhodobacter sp SW2 and Rhodopseudomonas palustris TIE-1 ABSTRACT The ability to grown on Fe(II) is thought to be a primitive metabolism
Chromatin IP (Isw2) Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles.
 Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
antigen Y. Kajita, D. Morgan, A.B. Parkes and B. Rees Smith
 Volume 87, number 2 FEBS 2756 August 985 Labelling and immunoprecipitation antigen of thyroid microsomal Y. Kajita, D. Morgan, A.B. Parkes and B. Rees Smith Endocrine Immunology Unit, 7th Floor Medicine.
Volume 87, number 2 FEBS 2756 August 985 Labelling and immunoprecipitation antigen of thyroid microsomal Y. Kajita, D. Morgan, A.B. Parkes and B. Rees Smith Endocrine Immunology Unit, 7th Floor Medicine.
LOCALIZATION OF ACID AND ALKALINE PHOSPHATASES IN Myxococcus coralloides D
 LOCALIZATION OF ACID AND ALKALINE PHOSPHATASES IN Myxococcus coralloides D Francisco González, M.Magdalena Martínez-Cañamero, M.Esther Fárez-Vidal & José M.Arias Departamento de Microbiología, Facultad
LOCALIZATION OF ACID AND ALKALINE PHOSPHATASES IN Myxococcus coralloides D Francisco González, M.Magdalena Martínez-Cañamero, M.Esther Fárez-Vidal & José M.Arias Departamento de Microbiología, Facultad
Recombinant Trypsin, Animal Origin Free
 Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Recombinant Trypsin, Animal Origin Free PRODUCT INFORMATION: BioGenomics r-trypsin powder is ready to use, animal origin free optimized for cell culture applications. It is derived by r-dna technology.
Framing Helicobacter pylori: The Etiology of Peptic Ulcers and Gastritis
 Framing Helicobacter pylori: The Etiology of Peptic Ulcers and Gastritis By Aja Dunn Gastritis (inflammation of the stomach); Etiologic agent - Helicobacter pylori (1). Transmission H. pylori infection
Framing Helicobacter pylori: The Etiology of Peptic Ulcers and Gastritis By Aja Dunn Gastritis (inflammation of the stomach); Etiologic agent - Helicobacter pylori (1). Transmission H. pylori infection
Mouse Cathepsin B ELISA Kit
 GenWay Biotech, Inc. 6777 Nancy Ridge Drive San Diego, CA 92121 Phone: 858.458.0866 Fax: 858.458.0833 Email: techline@genwaybio.com http://www.genwaybio.com Mouse Cathepsin B ELISA Kit Catalog No. GWB-ZZD154
GenWay Biotech, Inc. 6777 Nancy Ridge Drive San Diego, CA 92121 Phone: 858.458.0866 Fax: 858.458.0833 Email: techline@genwaybio.com http://www.genwaybio.com Mouse Cathepsin B ELISA Kit Catalog No. GWB-ZZD154
Case 19 Purification of Rat Kidney Sphingosine Kinase
 Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Case 19 Purification of Rat Kidney Sphingosine Kinase Focus concept The purification and kinetic analysis of an enzyme that produces a product important in cell survival is the focus of this study. Prerequisites
Nitrate and Nitrite Key Words: 1. Introduction 1.1. Nature, Mechanism of Action, and Biological Effects (Fig. 1)
 7 Nitrate and Nitrite Key Words: Nitrate; nitrite; methemoglobin; blood pressure; asphyxia; spinach; spongy cadmium column; zinc metal; sodium nitrate; sodium nitrite; ammonia buffer solution; Jones reductor.
7 Nitrate and Nitrite Key Words: Nitrate; nitrite; methemoglobin; blood pressure; asphyxia; spinach; spongy cadmium column; zinc metal; sodium nitrate; sodium nitrite; ammonia buffer solution; Jones reductor.
Studies on Glucose Isomerase from a Streptomyces Species
 APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Oct. 1976, P. 489-493 Copyright ) 1976 American Society for Microbiology Vol. 32, No. 4 Printed in U.S.A. Studies on Glucose Isomerase from a Streptomyces Species
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Oct. 1976, P. 489-493 Copyright ) 1976 American Society for Microbiology Vol. 32, No. 4 Printed in U.S.A. Studies on Glucose Isomerase from a Streptomyces Species
ab Urease Activity Assay Kit (Colorimetric)
 ab204697 Urease Activity Assay Kit (Colorimetric) Instructions for Use For rapid, sensitive and accurate measuring of Urease activity in biological samples and soil. This product is for research use only
ab204697 Urease Activity Assay Kit (Colorimetric) Instructions for Use For rapid, sensitive and accurate measuring of Urease activity in biological samples and soil. This product is for research use only
SCS MOLECULAR WEIGHT MARKERS 2,500-17,000 Caltons
 LECTROPHORES/S Revised November 1992 SCS MOLECULAR WEIGHT MARKERS 2,500-17,000 Caltons I NTRODUCTION Electrophoresis in polyacrylamide gels in the presence of sodium dodecyl sulfate (SDS), an anionic detergent,
LECTROPHORES/S Revised November 1992 SCS MOLECULAR WEIGHT MARKERS 2,500-17,000 Caltons I NTRODUCTION Electrophoresis in polyacrylamide gels in the presence of sodium dodecyl sulfate (SDS), an anionic detergent,
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
BIOC2060: Purication of alkaline phosphatase
 BIOC2060: Purication of alkaline phosphatase Tom Hargreaves December 2008 Contents 1 Introduction 1 2 Procedure 2 2.1 Lysozyme treatment......................... 2 2.2 Partial purication..........................
BIOC2060: Purication of alkaline phosphatase Tom Hargreaves December 2008 Contents 1 Introduction 1 2 Procedure 2 2.1 Lysozyme treatment......................... 2 2.2 Partial purication..........................
Purification and Properties of Nicotinamide Adenine Dinucleotide-Dependent D- and L- Lactate Dehydrogenases in a Group N Streptococcus
 JOURNAL OF BACTERIOLOGY, Aug. 1972, P. 392-396 Copyright 0 1972 American Society for Microbiology Vol. 111, No. 2 Printed in U.S.A. Purification and Properties of Nicotinamide Adenine Dinucleotide-Dependent
JOURNAL OF BACTERIOLOGY, Aug. 1972, P. 392-396 Copyright 0 1972 American Society for Microbiology Vol. 111, No. 2 Printed in U.S.A. Purification and Properties of Nicotinamide Adenine Dinucleotide-Dependent
2-Deoxyglucose (2DG) Uptake Measurement kit
 Document#:K2DG13516E For research use only. Not for clinical diagnosis. Catalog No. CSR-OKP-PMG-K1E 2-Deoxyglucose (2DG) Uptake Measurement kit Introduction Measurement of 2-deoxyglucose (2DG) uptake in
Document#:K2DG13516E For research use only. Not for clinical diagnosis. Catalog No. CSR-OKP-PMG-K1E 2-Deoxyglucose (2DG) Uptake Measurement kit Introduction Measurement of 2-deoxyglucose (2DG) uptake in
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/ MCQs. Location : 102, 105, 106, 301, 302
 FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
FIRST BIOCHEMISTRY EXAM Tuesday 25/10/2016 10-11 40 MCQs. Location : 102, 105, 106, 301, 302 The Behavior of Proteins: Enzymes, Mechanisms, and Control General theory of enzyme action, by Leonor Michaelis
Mouse TrkB ELISA Kit
 Mouse TrkB ELISA Kit CATALOG NO: IRKTAH5472 LOT NO: SAMPLE INTENDED USE For quantitative detection of mouse TrkB in cell culture supernates, cell lysates and tissue homogenates. BACKGROUND TrkB receptor
Mouse TrkB ELISA Kit CATALOG NO: IRKTAH5472 LOT NO: SAMPLE INTENDED USE For quantitative detection of mouse TrkB in cell culture supernates, cell lysates and tissue homogenates. BACKGROUND TrkB receptor
Dental Research Institute, Faculty of Dentistry, University of Toronto, Toronto, Canada *For correspondence:
 Zymogram Assay for the Detection of Peptidoglycan Hydrolases in Streptococcus mutans Delphine Dufour and Céline M. Lévesque * Dental Research Institute, Faculty of Dentistry, University of Toronto, Toronto,
Zymogram Assay for the Detection of Peptidoglycan Hydrolases in Streptococcus mutans Delphine Dufour and Céline M. Lévesque * Dental Research Institute, Faculty of Dentistry, University of Toronto, Toronto,
ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA
 J. Gen. App!. Microbiol., 34, 213-219 (1988) ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA TOSHIRO HAYASHI, RYO IOROI,*
J. Gen. App!. Microbiol., 34, 213-219 (1988) ON THE DIFFERENCE IN ADSORPTION ON SEPHADEX GEL OF THE DEXTRANSUCRASE OF STREPTOCOCCUS BOVIS GROWN ON SUCROSE AND GLUCOSE MEDIA TOSHIRO HAYASHI, RYO IOROI,*
Rapid-VIDITEST. Helicobacter pylori. One step Helicobacter pylori Blister test. Instruction manual
 Rapid-VIDITEST Helicobacter pylori One step Helicobacter pylori Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565,
Rapid-VIDITEST Helicobacter pylori One step Helicobacter pylori Blister test. Instruction manual Producer: VIDIA spol. s r.o., Nad Safinou II 365, 252 50 Vestec, Czech Republic, Tel.: +420 261 090 565,
Received 31 July 1992/Accepted 12 November 1992
 ANTMCROBAL AGENTS AND CHEMOTHERAPY, Feb 1993, p 32-36 66-8/93/232-5$2/ Copyright 1993, American Society for Microbiology Vol 37, No 2 Abnormal Peptidoglycan Produced in a Methicillin-Resistant Strain of
ANTMCROBAL AGENTS AND CHEMOTHERAPY, Feb 1993, p 32-36 66-8/93/232-5$2/ Copyright 1993, American Society for Microbiology Vol 37, No 2 Abnormal Peptidoglycan Produced in a Methicillin-Resistant Strain of
The Nobel Prize in Physiology or Medicine for 2005
 The Nobel Prize in Physiology or Medicine for 2005 jointly to Barry J. Marshall and J. Robin Warren for their discovery of "the bacterium Helicobacter pylori and its role in gastritis and peptic ulcer
The Nobel Prize in Physiology or Medicine for 2005 jointly to Barry J. Marshall and J. Robin Warren for their discovery of "the bacterium Helicobacter pylori and its role in gastritis and peptic ulcer
Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit
 PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
PROTOCOL Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit DESCRIPTION Mitochondrial Trifunctional Protein (TFP) Protein Quantity Microplate Assay Kit Sufficient materials
January 07, ANIMALS Digestive System Stomach.notebook. The Stomach. (cardiac sphincter) bbbbbbbbbbbbbbbbbb
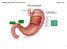 (cardiac sphincter) bbbbbbbbbbbbbbbbbb 1 Location: thoracic cavity Physical description: a "J" shaped organ with muscular walls lined with folds it is the widest part of the digestive tract has 2 muscular
(cardiac sphincter) bbbbbbbbbbbbbbbbbb 1 Location: thoracic cavity Physical description: a "J" shaped organ with muscular walls lined with folds it is the widest part of the digestive tract has 2 muscular
BabyBio IMAC columns DATA SHEET DS
 BabyBio IMAC columns DATA SHEET DS 45 655 010 BabyBio columns for Immobilized Metal Ion Affinity Chromatography (IMAC) are ready-to-use for quick and easy purification of polyhistidine-tagged (His-tagged)
BabyBio IMAC columns DATA SHEET DS 45 655 010 BabyBio columns for Immobilized Metal Ion Affinity Chromatography (IMAC) are ready-to-use for quick and easy purification of polyhistidine-tagged (His-tagged)
The Immunoassay Guide to Successful Mass Spectrometry. Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013
 The Immunoassay Guide to Successful Mass Spectrometry Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013 What is it? Hey! Look at that! Something is reacting in here! I just wish I knew what it
The Immunoassay Guide to Successful Mass Spectrometry Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013 What is it? Hey! Look at that! Something is reacting in here! I just wish I knew what it
Screening of bacteria producing amylase and its immobilization: a selective approach By Debasish Mondal
 Screening of bacteria producing amylase and its immobilization: a selective approach By Debasish Mondal Article Summary (In short - What is your article about Just 2 or 3 lines) Category: Bacillus sp produce
Screening of bacteria producing amylase and its immobilization: a selective approach By Debasish Mondal Article Summary (In short - What is your article about Just 2 or 3 lines) Category: Bacillus sp produce
Isolation, Separation, and Characterization of Organic Acids*
 In Dashek, William V., ed. Methods in plant biochemistry and molecular biology. Boca Raton, FL: CRC Press: pp. 107-113. Chapter 9.1997. Isolation, Separation, and Characterization of Organic Acids* William
In Dashek, William V., ed. Methods in plant biochemistry and molecular biology. Boca Raton, FL: CRC Press: pp. 107-113. Chapter 9.1997. Isolation, Separation, and Characterization of Organic Acids* William
N α -Acetylation of yeast ribosomal proteins and its effect on protein synthesis
 JOURNAL OF PROTEOMICS 74 (2011) 431 441 available at www.sciencedirect.com www.elsevier.com/locate/jprot N α -Acetylation of yeast ribosomal proteins and its effect on protein synthesis Masahiro Kamita
JOURNAL OF PROTEOMICS 74 (2011) 431 441 available at www.sciencedirect.com www.elsevier.com/locate/jprot N α -Acetylation of yeast ribosomal proteins and its effect on protein synthesis Masahiro Kamita
Isolation, Purification, and Characterization of Horseradish Peroxidase (HRP) J. Kane, T. Schweickart
 Isolation, Purification, and Characterization of Horseradish Peroxidase (HRP) J. Kane, T. Schweickart From the Department of Chemistry, Elon University, Elon, North Carolina 27244 Running title: Analysis
Isolation, Purification, and Characterization of Horseradish Peroxidase (HRP) J. Kane, T. Schweickart From the Department of Chemistry, Elon University, Elon, North Carolina 27244 Running title: Analysis
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
Europium Labeling Kit
 Europium Labeling Kit Catalog Number KA2096 100ug *1 Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Europium Labeling Kit Catalog Number KA2096 100ug *1 Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Inositol Phosphate Phosphatases of Microbiological Origin: the Inositol Pentaphosphate Products of Aspergillus ficuum
 JOURNAL OF BACTERIOLOGY, OCt. 1972, p. 434-438 Copyright 1972 American Society for Microbiology Vol. 112, No. 1 Printed in U.S.A. Inositol Phosphate Phosphatases of Microbiological Origin: the Inositol
JOURNAL OF BACTERIOLOGY, OCt. 1972, p. 434-438 Copyright 1972 American Society for Microbiology Vol. 112, No. 1 Printed in U.S.A. Inositol Phosphate Phosphatases of Microbiological Origin: the Inositol
colorimetrically by the methylene blue method according to Fogo and manometrically. In the presence of excess sulfur the amount of oxygen taken up
 GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
GLUTA THIONE AND SULFUR OXIDATION BY THIOBACILLUS THIOOXIDANS* BY ISAMU SUZUKI AND C. H. WERKMAN DEPARTMENT OF BACTERIOLOGY, IOWA STATE COLLEGE Communicated December 15, 1958 The ability of Thiobacillus
Virus Harvest AAV 15 cm 2
 Virus Harvest AAV 15 cm 2 1) Gently scrape cells from plate in 10 ml of media, place remaining 10 ml of media in plate lid. 2) Once cells are removed from plate, wash plate by pipetting 20 ml of media
Virus Harvest AAV 15 cm 2 1) Gently scrape cells from plate in 10 ml of media, place remaining 10 ml of media in plate lid. 2) Once cells are removed from plate, wash plate by pipetting 20 ml of media
Human Cathepsin D ELISA Kit
 GenWay Biotech, Inc. 6777 Nancy Ridge Drive San Diego, CA 92121 Phone: 858.458.0866 Fax: 858.458.0833 Email: techline@genwaybio.com http://www.genwaybio.com Human Cathepsin D ELISA Kit Catalog No. GWB-J4JVV9
GenWay Biotech, Inc. 6777 Nancy Ridge Drive San Diego, CA 92121 Phone: 858.458.0866 Fax: 858.458.0833 Email: techline@genwaybio.com http://www.genwaybio.com Human Cathepsin D ELISA Kit Catalog No. GWB-J4JVV9
320 MBIO Microbial Diagnosis. Aljawharah F. Alabbad Noorah A. Alkubaisi 2017
 320 MBIO Microbial Diagnosis Aljawharah F. Alabbad Noorah A. Alkubaisi 2017 Pathogens of the Urinary tract The urinary system is composed of organs that regulate the chemical composition and volume of
320 MBIO Microbial Diagnosis Aljawharah F. Alabbad Noorah A. Alkubaisi 2017 Pathogens of the Urinary tract The urinary system is composed of organs that regulate the chemical composition and volume of
Student Number: THE UNIVERSITY OF MANITOBA April 11, 2011, 1:00 PM - 4:00 PM Page 1 (of 3)
 Name: Student Number: THE UNIVERSITY OF MANITOBA April 11, 2011, 1:00 PM - 4:00 PM Page 1 (of 3) Biochemistry II Laboratory Section Examiners: Drs. J. Galka 1. Answer ALL questions in the space provided.
Name: Student Number: THE UNIVERSITY OF MANITOBA April 11, 2011, 1:00 PM - 4:00 PM Page 1 (of 3) Biochemistry II Laboratory Section Examiners: Drs. J. Galka 1. Answer ALL questions in the space provided.
Tivadar Orban, Beata Jastrzebska, Sayan Gupta, Benlian Wang, Masaru Miyagi, Mark R. Chance, and Krzysztof Palczewski
 Structure, Volume Supplemental Information Conformational Dynamics of Activation for the Pentameric Complex of Dimeric G Protein-Coupled Receptor and Heterotrimeric G Protein Tivadar Orban, Beata Jastrzebska,
Structure, Volume Supplemental Information Conformational Dynamics of Activation for the Pentameric Complex of Dimeric G Protein-Coupled Receptor and Heterotrimeric G Protein Tivadar Orban, Beata Jastrzebska,
TECHNICAL BULLETIN. Sialic Acid Quantitation Kit. Catalog Number SIALICQ Storage Temperature 2 8 C
 Sialic Acid Quantitation Kit Catalog Number SIALICQ Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description The Sialic Acid Quantitation Kit provides a rapid and accurate determination of total
Sialic Acid Quantitation Kit Catalog Number SIALICQ Storage Temperature 2 8 C TECHNICAL BULLETIN Product Description The Sialic Acid Quantitation Kit provides a rapid and accurate determination of total
SUPPRESSION OF HELICOBACTER PYLOR! UREASE ACTIVITY BY SUCRALFATE AND SULGLYCOTIDE
 Vol. 42, No. 1, June 1997 Pages 155-161 SUPPRESSION OF HELICOBACTER PYLOR! UREASE ACTIVITY BY SUCRALFATE AND SULGLYCOTIDE Rcccivcd March 5, 1997 B.L. Slomiany, J. Piotrowski and A. Slomiany Research Center
Vol. 42, No. 1, June 1997 Pages 155-161 SUPPRESSION OF HELICOBACTER PYLOR! UREASE ACTIVITY BY SUCRALFATE AND SULGLYCOTIDE Rcccivcd March 5, 1997 B.L. Slomiany, J. Piotrowski and A. Slomiany Research Center
Sponsors. Logo Design Ruth Cronje, and Jan Swanson; based on the original design by Dr. Robert Dunlop. Cover Design Sarah Summerbell
 Sponsors University of Minnesota College of Veterinary Medicine College of Agricultural, Food and Environmental Sciences Extension Service Swine Center Editors W. Christopher Scruton Stephen Claas Layout
Sponsors University of Minnesota College of Veterinary Medicine College of Agricultural, Food and Environmental Sciences Extension Service Swine Center Editors W. Christopher Scruton Stephen Claas Layout
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN
 THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
THE ESTIMATION OF TRYPSIN WITH HEMOGLOBIN BY M. L. ANSON Am) A. E. MIRSKY (From the Laboratories of The Rockefeller Institute for Medical Research, Princeton, N. J., and the Hospital of The Rockefeller
Supporting Information for:
 Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Supporting Information for: Methylerythritol Cyclodiphosphate (MEcPP) in Deoxyxylulose Phosphate Pathway: Synthesis from an Epoxide and Mechanisms Youli Xiao, a Rodney L. Nyland II, b Caren L. Freel Meyers
Manja Henze, Dorothee Merker and Lothar Elling. 1. Characteristics of the Recombinant β-glycosidase from Pyrococcus
 S1 of S17 Supplementary Materials: Microwave-Assisted Synthesis of Glycoconjugates by Transgalactosylation with Recombinant Thermostable β-glycosidase from Pyrococcus Manja Henze, Dorothee Merker and Lothar
S1 of S17 Supplementary Materials: Microwave-Assisted Synthesis of Glycoconjugates by Transgalactosylation with Recombinant Thermostable β-glycosidase from Pyrococcus Manja Henze, Dorothee Merker and Lothar
Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard. Product Number: AD0014
 TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
TECHNICAL DATA SHEET Lance Caution: For Laboratory Use. A product for research purposes only. Eu-W1284 Iodoacetamido Chelate & Europium Standard Product Number: AD0014 INTRODUCTION: Iodoacetamido-activated
