Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone. the hospital/facility:
|
|
|
- Edgar Carson
- 5 years ago
- Views:
Transcription
1 PATIENT INFORMATION (COMPLETE ONE FORM FOR EACH PERSON TESTED) Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone Patient discharged from Biological Sex: Accession # Hospital / Medical Record # the hospital/facility: Female Male Unknown Yes No Gender identity (if different from above): REPORTING RECIPIENTS Ordering Physician Institution Name (Required for International Clients) Phone Fax ADDITIONAL RECIPIENTS Name Fax Name Fax PAYMENT (FILL OUT ONE OF THE OPTIONS BELOW) SELF PAYMENT Pay With Sample Bill To Patient INSTITUTIONAL BILLING Institution Name Institution Code Institution Contact Name Institution Phone Institution Contact INSURANCE Do Not Perform Test Until Patient is Aware of Out-Of-Pocket Costs (excludes prenatal testing) REQUIRED ITEMS 1. Copy of the Front/Back of Insurance Card(s) 2. ICD10 Diagnosis Code(s) 3. Name of Ordering Physician 4. Insured Signature of Authorization Name of Insured Insured Date of Birth (MM / DD / YYYY) Name of Insured Insured Date of Birth (MM / DD / YYYY) Patient's Relationship to Insured Phone of Insured Patient's Relationship to Insured Phone of Insured Address of Insured Address of Insured City State Zip City State Zip Primary Insurance Co. Name Primary Insurance Co. Phone Secondary Insurance Co. Name Secondary Insurance Co. Phone Primary Member Policy # Primary Member Group # Secondary Member Policy # Secondary Member Group # By signing below, I hereby authorize Baylor Genetics to provide my insurance carrier any information necessary, including test results, for processing my insurance claim. I understand that I am responsible for any co-pay, co-insurance, and unmet deductible that the insurance policy dictates, as well as any amounts not paid by my insurance carrier for reasons including, but not limited to, non-covered and non-authorized services. I understand that I am responsible for sending Baylor Genetics any and all payments that I receive directly from my insurance company in payment for this test. Please note that Medicare does not cover routine screening tests. Patient's Printed Name Patient's Signature Date (MM / DD / YYYY) STATEMENT OF MEDICAL NECESSITY (REQUIRED) This test is medically necessary for the risk assessment, diagnosis, or detection of a disease, illness, impairment, symptom, syndrome, or disorder. The results will determine my patient s medical management and treatment decisions. The person listed as the Ordering Physician is authorized by law to order the test(s) requested herein. I confirm that I have provided genetic testing information to the patient and they have consented to genetic testing. Physician's Printed Name Physician's Signature Date (MM / DD / YYYY) 1 // 8 BAYLORGENETICS.COM
2 ETHNICITY African American Hispanic American Pacific Islander (Philippines, Micronesia, Malaysia, Indonesia) Ashkenazi Jewish Mennonite South Asian (India, Pakistan) East Asian (China, Japan, Korea) Middle Eastern (Saudi Arabia, Qatar, Iraq, Turkey) Southeast Asian (Vietnam, Cambodia, Thailand) Finnish Native American Southern European Caucasian (Spain, Italy, Greece) French Canadian Northern European Caucasian (Scandinavian, UK, Germany) Other (Specify): SAMPLE INDICATION FOR TESTING (REQUIRED) SAMPLE TYPE DATE OF COLLECTION (MM/DD/YYYY) Symptomatic with Positive Family History Blood in EDTA (Purple-top) Cord Blood DNA, Extracted from: Liver Saliva Skin Fibroblast Culture Skeletal Muscle Tissue Symptomatic (Summarize below): Asymptomatic Population Screening Positive Family History Disease Gene Variant NOTE: Extracted DNA/RNA will only be accepted if the isolation of nucleic acids for clinical testing occurs in a CLIA-certified laboratory or a laboratory meeting equivalent requirements as determined by the CAP and/or the CMS. ICD10 Diagnosis Code(s): TESTING OPTIONS Targeted Sequencing for Known Familial Mutation (If selected, specify test code and gene and complete section below) MITOCHONDRIAL PANELS Test Code Gene 2085 Dual Genome Panel by Massively Parallel Sequencing (BCM-MitomeNGS SM ) BE, DNA, SFC, SM Proband Last Name Relationship to Proband Proband testing location (Select one) Proband First Name Date of Birth (MM/DD/YYYY) Dual Genome Leigh Disease Panel by Massively Parallel Sequencing (BCM-MitomeNGS SM ) Comprehensive mtdna by Massively Parallel Sequencing (BCM-MitomeNGS SM ) BE, DNA BE, DNA, L, SM, T Baylor Genetics Lab # Family # Another Laboratory 1. Attach a copy of the Proband test results. 2. A positive control sample of the Proband is requested. Please provide, if available. MASSIVELY PARALLEL SEQUENCING (BCM-MITOMENGS SM ) PANELS Albinism Panel (13 genes) BE, DNA Bardet-Biedl Syndrome Panel (18 genes) BE, DNA 2105 Cholestasis Panel (7 genes) BE, DNA Full Gene Sequencing 2120 Cobalamin Metabolism Panel + Severe MTHFR Deficiency (20 genes) BE, DNA Deletion/ Duplication Analysis 2625 COL1A1 and COL1A2 Panel BE, DNA * Refer to Sample Specifications Table (Page 8) Test list continued on next page 2 // 8 BAYLORGENETICS.COM
3 MASSIVELY PARALLEL SEQUENCING (BCM-MITOMENGS SM ) PANELS 5095 Congenital Disorders of Glycosylation Panel (36 genes) BE, DNA 2100 CoQ10 Deficiency Panel (PDSS1, PDSS2, COQ2, COQ9, and ADCK3(COQ8/CABC1)) BE, DNA 5260 Developmental Glaucoma Panel (8 genes) BE, DNA 5250 Familial Exudative Vitreoretinopathy Panel (FZD4, LRP5, NDP, and TSPAN12) BE, DNA 2095 Fatty Acid Oxidation Panel (20 genes) BE, DNA 2125 Glycogen Storage Disease (GSD) Panel (23 genes) BE, DNA 2126 Glycogen Storage Disease (GSD) Muscle Panel (13 genes) BE, DNA 2127 Glycogen Storage Disease (GSD) Liver Panel (13 genes) BE, DNA 2200 High Bone Mass Panel (14 genes) BE, DNA Hyperinsulinism Panel (8 genes) BE, DNA Hypoglycemia Panel (85 genes) BE, DNA 5090 Leber Congenital Amaurosis Panel (19 genes) BE, DNA Leigh Disease Panel (82 genes) BE, DNA 2090 Low Bone Mass Panel (23 genes) BE, DNA Maple Syrup Urine Disease (MSUD) Panel (BCKHDA, BCKHDB, DBT and DLD) BE, DNA Maturity-Onset Diabetes of the Young (MODY) Panel (25 genes) BE, DNA 2130 mtdna Depletion/Integrity Panel (19 genes) BE, DNA 2155 Mitochondrial Respiratory Chain Complex I Deficiency Panel (21 genes) BE, DNA 2160 Mitochondrial Respiratory Chain Complex II Deficiency Panel (SDHA, SDHB, SDHC, SDHD, and SDHAF1) BE, DNA 2165 Mitochondrial Respiratory Chain Complex III Deficiency Panel (BCS1L, TTC19, UQCRB, and UQCRQ) BE, DNA 2170 Mitochondrial Respiratory Chain Complex IV Deficiency Panel (10 genes) BE, DNA 2175 Mitochondrial Respiratory Chain Complex V Deficiency Panel (ATPAF2, ATP5E, and TMEM70) BE, DNA 2086 Nuclear Panel (162 genes) BE, SFC, DNA, SM 2180 Mitochondrial Respiratory Chain Complex I-V Panel (50 genes) BE, DNA 2300 Myopathy/Rhabdomyolysis Panel (25 genes) BE, DNA Nephronophthisis Panel (NPHP1, INVS, NPHP3, NPHP4) BE, DNA Noonan Spectrum Disorders Panel (12 genes) BE, DNA 2185 PDH & Mitochondrial RC Complex V Panel (9 genes) BE, DNA Peroxisomal Disorders Panel (22 genes) BE, DNA 5255 Primary Open Angle Glaucoma Panel (MYOC, OPTN) BE, DNA 5274 Proximal Urea Cycle Disorders Comprehensive (Seq. & Del/Dup) (CPS1, NAGS, OTC) BE, DNA 2140 Progressive External Ophthalmoplegia Panel (10 genes) BE, DNA 2190 Retinitis Pigmentosa + RPGR orf15 by NGS (66 genes) BE, DNA 2110 Urea Cycle Disorders and Hyperammonemia (8 genes) BE, DNA 2195 Usher Syndrome Panel (9 genes) BE, DNA DNA COPY NUMBER ANALYSIS SPECIFY GENE OF INTEREST 3700 mtdna Content (qpcr) Analysis - Skeletal Muscle SM 3720 mtdna Content (qpcr) Analysis - Liver L 2000 MitoMet Plus acgh Analysis BE, DNA 2001 Oligonucleotide Targeted Array Analysis (Single Target Gene) BE 2003 Oligonucleotide Targeted Array Analysis (Up to 5 Target Genes) BE * Refer to Sample Specifications Table (Page 8) Test list continued on next page 3 // 8 BAYLORGENETICS.COM
4 MITOCHONDRIAL DNA (mtdna) RESPIRATORY CHAIN ENZYME TESTS 3200 Mitochondrial Respiratory Chain Enzyme Analysis (ETC) - Skeletal Muscle SM 3210 Mitochondrial Respiratory Chain Enzyme Analysis (ETC) - Skin Fibroblasts SFC MITOCHONDRIAL DNA (mtdna) MUTATION SCREENS 2010 Advanced mtdna Point Mutations and Deletions by Massively Parallel Sequencing (BCM-MitomeNGS SM ) BE, SM, T MITOCHONDRIAL DNA (mtdna) MUTATION SCREENS 3030 mtdna Nonsyndromic Hearing Loss and Deafness Mutation Panel BE, SA, SM, T SINGLE GENE ANALYSIS If a test is not found on this form, please obtain the test code from our website ( and write in the below space(s). Test Code Gene Test Code Gene Test Code Gene Test Name Test Name Test Name TEST CODE TEST NAME DISORDER SAMPLE TYPE * 3904 ACAD9 Comprehensive (Seq & Del/Dup Analysis) ACAD9 Deficiency BE 2889 ACACA Comprehensive (Seq & Del/Dup Analysis) Acetyl-CoA Carboxylase Deficiency (ACACA-Related Disorders) BE AIFM1 Sequence Analysis AIFM1 Related Disorders BE, DNA APTX Sequence Analysis by NGS Ataxia, early-onset, with oculomotor apraxia and hypoalbuminemia BE, DNA 2219 ATP5A1 Comprehensive (Seq & Del/Dup Analysis) ATP5A1-Related Disorders BE 3614 TAZ Comprehensive (Seq & Del/Dup Analysis) Barth Syndrome (TAZ-Related Disorders) BE 3179 C10orf2 (TWINKLE) Comprehensive (Seq & Del/Dup Analysis) C10orf2 (TWINKLE)-Related Disorders BE 3854 CABC1(ADCK3) Comprehensive (Seq & Del/Dup Analysis) Coenzyme Q10 Deficiency BE 3419 COQ2 Comprehensive (Seq & Del/Dup Analysis) Coenzyme Q10 Deficiency BE 3414 PDSS2 Comprehensive (Seq & Del/Dup Analysis) Coenzyme Q10 Deficiency BE 4800 Coenzyme Q10 Analyte Analysis - Skeletal Muscle Coenzyme Q10 Deficiency SM 2264 GFM1 Comprehensive (Seq & Del/Dup Analysis) Combined Oxidative Phosphorylation Deficiency BE 3649 TSFM Comprehensive (Seq & Del/Dup Analysis) Combined Oxidative Phosphorylation Deficiency BE 2289 MRPS22 Comprehensive (Seq & Del/Dup Analysis) Combined Oxidative Phosphorylation Deficiency BE 2224 C12orf65 Comprehensive (Seq & Del/Dup Analysis) Combined Oxidative Phosphorylation Deficiency BE 2324 AARS2 Comprehensive (Seq & Del/Dup Analysis) Combined Oxidative Phosphorylation Deficiency BE MRPL3 Sequence Analysis Combined Oxidative Phosphorylation Deficiency 9 BE, DNA MTO1 Sequence Analysis Combined Oxidative Phosphorylation Deficiency 10 BE, DNA EARS2 Sequence Analysis Combined Oxidative Phosphorylation Deficiency 12 BE, DNA 2664 FOXRED1 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 3489 NDUFA1 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 2684 NDUFA11 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 3944 NDUFAF1 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 3539 NDUFAF2 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 2694 NDUFAF3 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 2704 NDUFS1 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 3574 NDUFS3 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE * Refer to Sample Specifications Table (Page 8) Test list continued on next page 4 // 8 BAYLORGENETICS.COM
5 SINGLE GENE ANALYSIS TEST CODE TEST NAME DISORDER SAMPLE TYPE * 3564 NDUFS4 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 3569 NDUFS6 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 3849 NDUFS8 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 3594 NDUFV1 Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 2714 NUBPL Comprehensive (Seq & Del/Dup Analysis) Complex I Deficiency BE 3180 SDHA Sequence Analysis Complex II Deficiency BE, SA 3185 SDHB Sequence Analysis Complex II Deficiency BE, SA 3190 SDHC Sequence Analysis Complex II Deficiency BE, SA 3195 SDHD Sequence Analysis Complex II Deficiency BE, SA 3679 SDHAF1 Comprehensive (Seq & Del/Dup Analysis) Complex II Deficiency BE 3114 BCS1L Comprehensive (Seq & Del/Dup Analysis) Complex III Deficiency BE 2719 TTC19 Comprehensive (Seq & Del/Dup Analysis) Complex III Deficiency BE 2734 COX4I1 Comprehensive (Seq & Del/Dup Analysis) Complex IV Deficiency BE 3104 COX10 Comprehensive (Seq & Del/Dup Analysis) Complex IV Deficiency BE 3549 COX15 Comprehensive (Seq & Del/Dup Analysis) Complex IV Deficiency BE 3244 LRPPRC Comprehensive (Seq & Del/Dup Analysis) Complex IV Deficiency BE 3099 SCO1 Comprehensive (Seq & Del/Dup Analysis) Complex IV Deficiency BE 3094 SCO2 Comprehensive (Seq & Del/Dup Analysis) Complex IV Deficiency BE 3089 SURF1 Comprehensive (Seq & Del/Dup Analysis) Complex IV Deficiency BE 2749 TACO1 Comprehensive (Seq & Del/Dup Analysis) Complex IV Deficiency BE 3294 ATP5E Comprehensive (Seq & Del/Dup Analysis) Complex V Deficiency BE 3739 TMEM70 Comprehensive (Seq & Del/Dup Analysis) Complex V Deficiency BE 3344 TIMM8A Comprehensive (Seq & Del/Dup Analysis) Deafness-Dystonia-Optic Neuropathy BE 3079 DGUOK Comprehensive (Seq & Del/Dup Analysis) DGUOK-Related Disorders BE 3749 ETHE1 Comprehensive (Seq & Del/Dup Analysis) Ethylmalonic Encephalopathy BE 2249 FARS2 Comprehensive (Seq & Del/Dup Analysis) FARS2-Related Disorders BE 3559 FASTKD2 Comprehensive (Seq & Del/Dup Analysis) FASTKD2-Related Disorders BE 2314 HARS2 Comprehensive (Seq & Del/Dup Analysis) HARS2-Related Disorders BE 2329 KARS Comprehensive (Seq & Del/Dup Analysis) Intermediate Charcot-Marie-Tooth Neuropathy, KARS-Related BE 2269 ACAT1 Comprehensive (Seq & Del/Dup Analysis) Ketothiolase Deficiency BE SIRT3 Sequence Analysis Li-Fraumeni Syndrome with Brain Tumor BE, DNA 3464 DLD Comprehensive (Seq & Del/Dup Analysis) Maple Syrup Urine Disease Type 3 BE 2229 MARS2 Comprehensive (Seq & Del/Dup Analysis) MARS2 Related Disorders BE MFN2 Sequence Analysis MFN2 Related Disorders BE, DNA NDUFV2 Sequence Analysis Mitochondrial Complex I Deficiency BE, DNA ATP5O Sequence Analysis Mitochondrial Complex V Deficiency - ATP5O Related BE, DNA UQCRC2 Sequence Analysis Mitochondrial Complex III Deficiency Nuclear Type 5 BE, DNA UQCR10 Sequence Analysis Mitochondrial Complex III Deficiency - UQCR10 Related BE, DNA MTHFD1L Sequence Analysis Mitochondrial Disorders - MTHFD1L Related BE, DNA * Refer to Sample Specifications Table (Page 8) Test list continued on next page 5 // 8 BAYLORGENETICS.COM
6 INDIVIDUAL (LISTED BY DISORDER) TEST CODE TEST NAME DISORDER SAMPLE TYPE * POLRMT Sequence Analysis Mitochondrial Disorders - POLRMT Related BE, DNA SIRT5 Sequence Analysis Mitochondrial Disorders - SIRT5 Related BE, DNA TOP1MT Sequence Analysis Mitochondrial Disorders - TOPMT Related BE, DNA TRIT1 Sequence Analysis Mitochondrial Disorders - TRIT1 Related BE, DNA 3964 SUCLG2 Comprehensive (Seq & Del/Dup Analysis) mtdna Depletion Syndrome, SUCLG2-Related BE 3074 TK2 Comprehensive (Seq & Del/Dup Analysis) mtdna Depletion Syndrome, Myopathic Form (TK2-Related Disorders) BE FBXL4 Sequence Analysis by NGS mtdna Depletion Syndrome I3, Encephalomyopathic type BE, DNA 3064 TYMP Comprehensive (Seq & Del/Dup Analysis) MNGIE/MNGIE like Syndrome BE 3324 MPV17 Comprehensive (Seq & Del/Dup Analysis) MPV17-Related Disorders BE 2294 MRPL44 Comprehensive (Seq & Del/Dup Analysis) MRPL44-Related Disorders BE 2235 MTFMT Sequence Analysis MTFMT-Related Disorders BE, SA 3659 ISCU Comprehensive (Seq & Del/Dup Analysis) Myopathy with Deficiency of ISCU BE 3654 PUS1 Comprehensive (Seq & Del/Dup Analysis) Myopathy, Mitochondrial, and Sideroblastic Anemia BE 3959 YARS2 Comprehensive (Seq & Del/Dup Analysis) Myopathy, Mitochondrial, and Sideroblastic Anemia BE GFER Sequence Analysis by NGS Myopathy, mitochondrial progressive, with congenital cataract, hearing loss, and developmental delay 2309 NARS2 Comprehensive (Seq & Del/Dup Analysis) NARS2-Related Disorders BE OPA1 Sequence Analysis by NGS Optic Atrophy Type 1 BE, SA 3529 OPA3 Comprehensive (Seq & Del/Dup Analysis) Optic Atrophy Type 3 BE 3169 PDHA1 Comprehensive (Seq & Del/Dup Analysis) PDH Complex Deficiency BE 3899 PDHB Comprehensive (Seq & Del/Dup Analysis) PDH Complex Deficiency BE 3894 PDP1 Comprehensive (Seq & Del/Dup Analysis) PDH Complex Deficiency BE 3924 PDHX Comprehensive (Seq & Del/Dup Analysis) PDH Complex Deficiency BE 3919 DLAT Comprehensive (Seq & Del/Dup Analysis) PDH Complex Deficiency BE 3069 POLG Comprehensive (Seq & Del/Dup Analysis) POLG-Related Disorders BE 3384 POLG2 Comprehensive (Seq & Del/Dup Analysis) POLG2-Related Disorders BE 3754 PC Comprehensive (Seq & Del/Dup Analysis) Pyruvate Carboxylase Deficiency BE COX6A1 Sequence Analysis Recessive Intermediate D Charcot-Marie-Tooth Disease BE, DNA 3424 RRM2B Comprehensive (Seq & Del/Dup Analysis) RRM2B-Related Disorders BE SIRT1 Sequence Analysis SIRT1 Related Disorders BE, DNA 3174 SLC25A4 (ANT1) Comprehensive (Seq & Del/Dup Analysis) SLC25A4-Related Disorders BE 5335 SPG7 Sequence Analysis Spastic Paraplegia 7, Autosomal Recessive BE, SA 3379 SUCLA2 Comprehensive (Seq & Del/Dup Analysis) SUCLA2-Related Disorders BE 3394 SUCLG1 Comprehensive (Seq & Del/Dup Analysis) SUCLG1-Related Disorders BE GFM2 Sequence Analysis Wolcott-Rallison Syndrome BE, DNA DNAJC19 Sequence Analysis 3-Methyglutaconic Aciduria Type V BE, DNA BE, DNA * Refer to Sample Specifications Table (Page 8) Indications on next page 6 // 8 BAYLORGENETICS.COM
7 INDICATION FOR TESTING (REQUIRED) Clinical management of known diagnosis - Please specify: Diagnostic Testing - Please complete checklist below. CENTRAL NERVOUS SYSTEM VISCERAL SENSORY 101 dd Developmental Delay/ ID 102 ht Hypotonia 103 au Autistic Features 104 enc Dementia/ Encephalopathy 105 ha Headaches/ Migraines 106 stk Stroke, Ischemic Episodes 107 atx Ataxia 108 sz 109 pi Perinatal Insult Intractable/ Refractory/ Myoclonus/Myoclonic Seizures 110 ps Pyramidal Signs 111 hp Hemiparesis 112 spas Spasticity 113 dyst Dystonia 114 cho Chorea 115 sib Self-Injury 116 pan Pancreatitis 117 dia Diarrhea 118 cst Constipation 119 cv Cyclic Vomiting 120 pob Pseudoobstruction 301 gir Gastrointestinal Reflux 302 dge Delayed Gastric Emptying 303 pan Pancreatitis 304 dia Diarrhea 305 cst Constipation 306 cv Cyclic Vomiting 307 pob Pseudoobstruction 308 hpf Hepatic Failure 309 eta Elevated Transaminases 310 rtd Renal Tubular Disease 311 ap Apnea/ Hypoventilation 312 rsf Respiratory Deficiency/Failure 313 ren Renal Dysfunction 314 lc Liver Carcinoma 315 jau Jaundice 316 spm Splenomegaly/Enlarged Spleen 317 hpm Hepatomegaly/Enlarged Liver 318 hd Hepatic Dysfunction 501 rp Retinitis Pigmentosa 502 opa Optic Atrophy 503 cat Cataract 504 hl Sensorineural Hearing Loss 505 trv Tortuous Retinal Vessels 506 crs Cherry Red Spot/Eye 507 co Corneal Opacity 508 el Ectopia Lentis 509 pp Photophobia ENDOCRINE 601 db Diabetes 602 pd Exocrine/Pancreatic Deficiency 603 gf Gonadal Failure 604 hth Hypothyroidism 605 hpt Hypoparathyroidism 606 adr Hypo/Hyper-adrenal Function 607 ss Short Stature 608 adc Adrenal Calcification 609 hf Hydrops Fetalis 610 pg Pregnant NEUROMUSCULAR 201 pn Peripheral Neuropathy 202 exi Exercise Intolerance 203 pmw Progressive Muscle Weakness 204 smw Static Muscle Weakness 205 cr Muscle Cramps after Exercise 206 fat Easy Fatigability 207 dcmyo Dilated Cardiomyopathy 208 hcmyo Hypertrophic Cardiomyopathy 209 hb Heart Block 210 ar Arrhythmia 211 op Ophthalmoparesis, CPEO 212 emg Abnormal EMG/NCV 213 pto Ptosis 214 eh Cardiomegaly/Enlarged Heart METABOLITES / METABOLIC 400 nbs Abnormal Newborn Screen 401 kto Ketosis 402 dca Dicarboxylic Aciduria 403 la Lactic Acidosis 404 csfl High CSF Lactate 405 oa Organic Aciduria 406 lpc Low Plasma Carnitine 407 cpk CPK Abnormalities 408 pyr Elevated Pyruvate 409 ala Elevated Alanine 410 3mg 3-Methylglutaconic Aciduria 411 acid Acidosis 412 NH3 Hypoammonemia 413 hypo Hypoglycemia 414 hyper Hyperglycemia 415 uco Unusual Color/Odor OTHER CLINICAL 701 ftt Failure to Thrive 702 mce Microencephaly 703 sids SIDS/Unexplained Death 704 ca Congenital Anomalies 705 dys Dysmorphic Features 706 id Immunodeficiency 707 ma Macrocytic Anemia 708 pcbm Pancytopenia/Bone Marrow Failure 709 np Neutropenia 710 mc Macrocephaly 711 cf Course Features 712 sa Skeletal Anomalies 713 art Arthritis 7 // 8 BAYLORGENETICS.COM
8 INDICATION FOR TESTING - CONTINUED (REQUIRED) FAMILY HISTORY 001 mut Mutation (Attach details) 002 mi Evidence of Maternal Inheritance ELECTROPHYSIOLOGY 801 baers Abnormal BAERS 802 vers Abnormal VERS 803 eeg Abnormal EEG HAIR/SKIN FINDINGS 714 rash Rashes with Hypopigmentation 715 htii Hyper Trichosis 716 alp Alopecia 717 ac Acrocyanosis 718 ak Angiokeratoma 719 ic Ichthyosis IMAGING/OTHER STUDIES 804 bg Increased Signal Basal Ganglia 805 dmy Delayed Myelination 806 cea Cerebellar Atrophy 807 pstk Posterior Stroke 808 leuk Leukodystrophy 809 mrsl MRS/Lactate Peak 810 mri Abnormal MRI MUSCLE BIOPSY 901 his Abnormal Histology 902 em Abnormal Ultrastructure 903 enz Abnormal Respiratory Enzymes 904 prol Large Mitochondria/Proliferation 905 cox COX Deficiency 906 rrf Ragged Red Fibers SAMPLE SPECIFICATIONS TABLE RECOMMENDED AMOUNT ABBREVIATION SAMPLE NAME (2 YRS - ADULT) (NEWBORN - 2YRS) SHIPPING INSTRUCTIONS SPECIAL NOTES BE Blood in EDTA (purple-top) 3-5 cc 3-5 cc Ship at room temperature in an insulated container by overnight courier. Do not heat or freeze. CB Cord Blood N/A 1-2 cc DNA DNA, Extracted μ μ L Liver 50 mg 50 mg Ship at room temperature in an insulated container by overnight courier. Do not heat or freeze. Ship at room temperature in an insulated container by overnight courier. Do not heat or freeze. Ship frozen sample in insulated container, with 3-5 lbs dry ice, by overnight courier. Ensure properly labeled. Also send 3 cc of maternal blood in properly labeled EDTA tube for MCC studies at no charge as needed. Minimal concentration of 50ng/μ; A260/A280 of ~1.7 Liver should be flash frozen in liquid nitrogen at collection with no media added and stored at -80 C. SA Saliva See Special Notes See Special Notes Ship at room temperature in an insulated container by overnight courier. Do not heat or freeze. Collected with Oragene DNA Self-Collection Kit. SFC Skin Fibroblast Culture (3) T25 flasks (3) T25 flasks Ship at ambient temperature in an insulated container by overnight courier. Send three (3) T25 flasks at approximately 60-80% confluence. SM Skeletal Muscle 150 mg 150 mg T Tissue 50 mg 50 mg Ship frozen sample in insulated container, with 3-5 lbs dry ice, by overnight courier. Ship frozen sample in insulated container, with 3-5 lbs dry ice, by overnight courier. Skeletal Muscle should be flash frozen in liquid nitrogen at collection with no media added, and stored at -80 C. Surgical pathology report required. If a pathology report is not available at this time, please send a clinical summary and the results of any pertinent ancillary testing. Tissue should be flash frozen in liquid nitrogen at collection with no media added, and stored at -80 C. 8 // 8 BAYLORGENETICS.COM
Address City State Zip Phone. the hospital/facility:
 PATIENT INFORMATION (COMPLETE ONE FORM FOR EACH PERSON TESTED) Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone Patient discharged from Biological Sex:
PATIENT INFORMATION (COMPLETE ONE FORM FOR EACH PERSON TESTED) Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone Patient discharged from Biological Sex:
Referring Physician Information Name: (Last, First, Middle):
 Page 1 of 5 Patient Information Clinical Indication: Patient Name: (Last, First, Middle): DOB (M/D/Y): Sex: M F Guardian Name (for minor patients only): Address: City: State: ZIP: Phone: Ethnic Background
Page 1 of 5 Patient Information Clinical Indication: Patient Name: (Last, First, Middle): DOB (M/D/Y): Sex: M F Guardian Name (for minor patients only): Address: City: State: ZIP: Phone: Ethnic Background
COMPREHENSIVE DIAGNOSIS FOR MITOCHONDRIAL DISORDERS
 COMPREHENSIVE DIAGNOSIS FOR MITOCHONDRIAL DISORDERS MITOCHONDRIAL DISORDERS NGS PANELS MITOCHONDRIAL DISORDERS NGS PANELS Name Test code Gene Name Mitome200-Nuclear 2086 (164 nuclear genes) AARS2, ACACA,
COMPREHENSIVE DIAGNOSIS FOR MITOCHONDRIAL DISORDERS MITOCHONDRIAL DISORDERS NGS PANELS MITOCHONDRIAL DISORDERS NGS PANELS Name Test code Gene Name Mitome200-Nuclear 2086 (164 nuclear genes) AARS2, ACACA,
REQUISITION FORM NOTE: ALL FORMS MUST BE FILLED OUT COMPLETELY FOR SAMPLE TO BE PROCESSED. Last First Last First
 #: DEPARTMENT OF NEUROLOGY COLUMBIA COLLEGE OF PHYSICIANS & SURGEONS Room 4-420 630 West 168th Street, New York, NY 10032 Telephone #: 212-305-3947 Fax#: 212-305-3986 REQUISITION FORM NOTE: ALL FORMS MUST
#: DEPARTMENT OF NEUROLOGY COLUMBIA COLLEGE OF PHYSICIANS & SURGEONS Room 4-420 630 West 168th Street, New York, NY 10032 Telephone #: 212-305-3947 Fax#: 212-305-3986 REQUISITION FORM NOTE: ALL FORMS MUST
MNGenome Sequencing Test Request Form
 MNGenome Sequencing Test Request Form Whole Whole Genome Exome Sequencing Note: Clinical Information and Consent Form are required for MNGenome Orders. *Please note if samples are shipping separately as
MNGenome Sequencing Test Request Form Whole Whole Genome Exome Sequencing Note: Clinical Information and Consent Form are required for MNGenome Orders. *Please note if samples are shipping separately as
Movement Disorders Requisition Form
 Movement Disorders Requisition Form The University of Chicago Genetic Services Laboratories 5841 South Maryland Avenue, Room G701/MC0077, Chicago, IL 60637 Toll Free: 888.824.3637 Local: 773.834.0555 Fax:
Movement Disorders Requisition Form The University of Chicago Genetic Services Laboratories 5841 South Maryland Avenue, Room G701/MC0077, Chicago, IL 60637 Toll Free: 888.824.3637 Local: 773.834.0555 Fax:
MNG Exome Sequencing Test Request Form
 Whole Exome Sequencing MNG Exome Sequencing Test Request Form Note: Clinical Information and Consent Form are required for MNG Exome Orders. IMPORTANT: Please note if any additional samples will be shipped
Whole Exome Sequencing MNG Exome Sequencing Test Request Form Note: Clinical Information and Consent Form are required for MNG Exome Orders. IMPORTANT: Please note if any additional samples will be shipped
Presentation and investigation of mitochondrial disease in children
 Presentation and investigation of mitochondrial disease in children Andrew Morris Willink Unit, Manchester Mitochondrial function Carbohydrate Fat Respiratory chain Energy Mitochondria are the product
Presentation and investigation of mitochondrial disease in children Andrew Morris Willink Unit, Manchester Mitochondrial function Carbohydrate Fat Respiratory chain Energy Mitochondria are the product
AnyPanel Requisition Form
 AnyPanel Requisition Form Please complete every field and tick box clearly. PATIENT INFORMATION Patient s First Name Patient s Last Name Biological Sex: Male Female Unknown Gender Identity (if different
AnyPanel Requisition Form Please complete every field and tick box clearly. PATIENT INFORMATION Patient s First Name Patient s Last Name Biological Sex: Male Female Unknown Gender Identity (if different
Ordering Physician Information Additional Report Recipient 1
 Test Selection Targeted Whole Exome Sequencing - Proband Only Targeted Whole Exome Sequencing - Trio Confirmatory Sanger sequencing validation Re-analysis (at least one year after initial analysis) Specimen
Test Selection Targeted Whole Exome Sequencing - Proband Only Targeted Whole Exome Sequencing - Trio Confirmatory Sanger sequencing validation Re-analysis (at least one year after initial analysis) Specimen
panel tests assessing multiple genes at the same time for the diagnosis of one or more related disorders
 NGS tests panel tests assessing multiple genes at the same time for the diagnosis of one or more related disorders UKGTN website lists 13 laboratories offering a total of 56 panel test UKGTN listed panel
NGS tests panel tests assessing multiple genes at the same time for the diagnosis of one or more related disorders UKGTN website lists 13 laboratories offering a total of 56 panel test UKGTN listed panel
A Lawyer s Perspective on Genetic Screening Performed by Cryobanks
 A Lawyer s Perspective on Genetic Screening Performed by Cryobanks As a lawyer practicing in the area of sperm bank litigation, I have, unfortunately, represented too many couples that conceived a child
A Lawyer s Perspective on Genetic Screening Performed by Cryobanks As a lawyer practicing in the area of sperm bank litigation, I have, unfortunately, represented too many couples that conceived a child
MITOCHONDRIAL DISEASE. Amel Karaa, MD Mitochondrial Disease Program Massachusetts General Hospital
 MITOCHONDRIAL DISEASE Amel Karaa, MD Mitochondrial Disease Program Massachusetts General Hospital Disclosures & Disclaimers United Mitochondrial Disease Foundation Research Grant North American Mitochondrial
MITOCHONDRIAL DISEASE Amel Karaa, MD Mitochondrial Disease Program Massachusetts General Hospital Disclosures & Disclaimers United Mitochondrial Disease Foundation Research Grant North American Mitochondrial
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis)
 Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
Newborn Screen & Development Facts about the genetic diseases new since March 2006 (Excluding Cystic Fibrosis) 1) Argininosuccinic acidemia (ASA) a) Incidence: ~1 in 70,000 b) Deficiency in an enzyme of
Intellectual Disability Exome Panel Requisition Form
 Intellectual Disability Exome Panel Requisition Form The University of Chicago Genetic Services Laboratories 5841 South Maryland Avenue, Room G701/MC0077, Chicago, IL 60637 Toll Free: 888.824.3637 Local:
Intellectual Disability Exome Panel Requisition Form The University of Chicago Genetic Services Laboratories 5841 South Maryland Avenue, Room G701/MC0077, Chicago, IL 60637 Toll Free: 888.824.3637 Local:
An Introduction to mitochondrial disease.
 9 th September 2017 An Introduction to mitochondrial disease. Dr Andy Schaefer Consultant Neurologist and Clinical Lead NHS Highly Specialised Rare Mitochondrial Disease Service and Wellcome Trust Centre
9 th September 2017 An Introduction to mitochondrial disease. Dr Andy Schaefer Consultant Neurologist and Clinical Lead NHS Highly Specialised Rare Mitochondrial Disease Service and Wellcome Trust Centre
INBORN ERRORS OF METABOLISM (IEM) IAP UG Teaching slides
 INBORN ERRORS OF METABOLISM (IEM) 1 OBJECTIVES What are IEMs? Categories When to suspect? History and clinical pointers Metabolic presentation Differential diagnosis Emergency and long term management
INBORN ERRORS OF METABOLISM (IEM) 1 OBJECTIVES What are IEMs? Categories When to suspect? History and clinical pointers Metabolic presentation Differential diagnosis Emergency and long term management
Mitochondrial disorders are among the most severe
 NEUROLOGY GRAND ROUNDS Leigh Map: A Novel Computational Diagnostic Resource for Mitochondrial Disease Joyeeta Rahman, BSc, 1 Alberto Noronha, MSc, 2 Ines Thiele, PhD, 2 and Shamima Rahman, FRCP, PhD 1,3
NEUROLOGY GRAND ROUNDS Leigh Map: A Novel Computational Diagnostic Resource for Mitochondrial Disease Joyeeta Rahman, BSc, 1 Alberto Noronha, MSc, 2 Ines Thiele, PhD, 2 and Shamima Rahman, FRCP, PhD 1,3
Clinical Genomics Requisition Form
 Clinical Genomics Requisition Form Please complete every field and tick box clearly. PATIENT INFORMATION Patient s First Name Patient s Last Name Biological Sex: Male Female Unknown Gender Identity (if
Clinical Genomics Requisition Form Please complete every field and tick box clearly. PATIENT INFORMATION Patient s First Name Patient s Last Name Biological Sex: Male Female Unknown Gender Identity (if
Knight Diagnostic Laboratories. Exome Requisition for Proband. Complete Exome Orders Must Include All of the Following:
 Knight Diagnostic Laboratories Fax: (855) 535-1329 Email: KDLClientServices@ohsu.edu Shipping: 2525 SW 3rd Ave, Ste 350, Portland, OR 97201 Questions? (855) 535-1522 Exome Requisition for Proband Complete
Knight Diagnostic Laboratories Fax: (855) 535-1329 Email: KDLClientServices@ohsu.edu Shipping: 2525 SW 3rd Ave, Ste 350, Portland, OR 97201 Questions? (855) 535-1522 Exome Requisition for Proband Complete
Evolution of Genetic Testing. Joan Pellegrino MD Associate Professor of Pediatrics SUNY Upstate Medical University
 Evolution of Genetic Testing Joan Pellegrino MD Associate Professor of Pediatrics SUNY Upstate Medical University Genetic Testing Chromosomal analysis Flourescent in situ hybridization (FISH) Chromosome
Evolution of Genetic Testing Joan Pellegrino MD Associate Professor of Pediatrics SUNY Upstate Medical University Genetic Testing Chromosomal analysis Flourescent in situ hybridization (FISH) Chromosome
PATIENT EDUCATION. carrier screening INFORMATION
 PATIENT EDUCATION carrier screening INFORMATION carrier screening AT A GLANCE Why is carrier screening recommended? Carrier screening is one of many tests that can help provide information to you and your
PATIENT EDUCATION carrier screening INFORMATION carrier screening AT A GLANCE Why is carrier screening recommended? Carrier screening is one of many tests that can help provide information to you and your
Mitochondrial Disorders Genetic Testing
 Mitochondrial Disorders Genetic Testing A Comprehensive Guide 1 Introduction Mitochondrial disorders are a group of related, clinically diverse, genetic diseases with a prevalence of 1/5,000 to 1/8,500
Mitochondrial Disorders Genetic Testing A Comprehensive Guide 1 Introduction Mitochondrial disorders are a group of related, clinically diverse, genetic diseases with a prevalence of 1/5,000 to 1/8,500
Clinical Genomics Requisition Form
 Clinical Genomics Requisition Form Please complete every field and tick box clearly. PATIENT INFORMATION Patient s First Name Patient s Last Name Biological Sex: Male Female Unknown Gender Identity (if
Clinical Genomics Requisition Form Please complete every field and tick box clearly. PATIENT INFORMATION Patient s First Name Patient s Last Name Biological Sex: Male Female Unknown Gender Identity (if
PRICE AND TEST LIST January 4, 2018 (by Test Code)
 Test Code Test Description TAT Price 2018 CPT Codes WHOLE EXOME SEQUENCING WES001 MNG Exome TM Trio Sequencing and Copy Number Analysis + mtdna (Proband + 2 Family Members) 2-4 Weeks $6,900.00 81415, 81416,
Test Code Test Description TAT Price 2018 CPT Codes WHOLE EXOME SEQUENCING WES001 MNG Exome TM Trio Sequencing and Copy Number Analysis + mtdna (Proband + 2 Family Members) 2-4 Weeks $6,900.00 81415, 81416,
7 Medical Genetics. Hemoglobinopathies. Hemoglobinopathies. Protein and Gene Structure. and Biochemical Genetics
 SESSION 7 Medical Genetics Hemoglobinopathies and Biochemical Genetics J a v a d F a s a J a m s h i d i U n i v e r s i t y o f M e d i c a l S c i e n c e s, N o v e m b e r 2 0 1 7 Hemoglobinopathies
SESSION 7 Medical Genetics Hemoglobinopathies and Biochemical Genetics J a v a d F a s a J a m s h i d i U n i v e r s i t y o f M e d i c a l S c i e n c e s, N o v e m b e r 2 0 1 7 Hemoglobinopathies
PRICE AND TEST LIST August 24, 2018 (by Test Code)
 Test Code Test Description TAT Price 2018 CPT Codes WHOLE GENOME SEQUENCING WGS001 MNGenome TRIO Sequencing 2-6 Weeks Call 81425, 81426 x2 WGS003 MNGenome Proband Only Sequencing 2-6 Weeks Call 81425 WHOLE
Test Code Test Description TAT Price 2018 CPT Codes WHOLE GENOME SEQUENCING WGS001 MNGenome TRIO Sequencing 2-6 Weeks Call 81425, 81426 x2 WGS003 MNGenome Proband Only Sequencing 2-6 Weeks Call 81425 WHOLE
Mitochondrial Diseases
 Mitochondrial Diseases Simon Heales SWIM Conference Taunton, November 29 th 2018 Respiratory Failure Cardiomyopathy Optic Atrophy / Retinitis Pigmentosa Seizures / Developmental delay Liver Failure Deafness
Mitochondrial Diseases Simon Heales SWIM Conference Taunton, November 29 th 2018 Respiratory Failure Cardiomyopathy Optic Atrophy / Retinitis Pigmentosa Seizures / Developmental delay Liver Failure Deafness
Talking Genomes with Your Patients. Meagan Cochran, MS, CGC Certified Genetic Counselor HudsonAlpha Institute for Biotechnology
 Talking Genomes with Your Patients Meagan Cochran, MS, CGC Certified Genetic Counselor HudsonAlpha Institute for Biotechnology Objectives Review the importance of physician familiarity with genomic testing
Talking Genomes with Your Patients Meagan Cochran, MS, CGC Certified Genetic Counselor HudsonAlpha Institute for Biotechnology Objectives Review the importance of physician familiarity with genomic testing
Shallotte Vision Care J. Mark Saunders, OD PA 4637 Main Street Shallotte NC Patient Demographic Information
 Shallotte Vision Care J. Mark Saunders, OD PA 4637 Main Street Shallotte NC 28470 Patient Demographic Information Account # Last Name: SSN: / / First: Middle: Marital Status: Single Married Separated Nickname:
Shallotte Vision Care J. Mark Saunders, OD PA 4637 Main Street Shallotte NC 28470 Patient Demographic Information Account # Last Name: SSN: / / First: Middle: Marital Status: Single Married Separated Nickname:
Nutritional Interventions in Primary Mitochondrial Disorders
 Nutritional Interventions in Primary Mitochondrial Disorders Carolyn J Ellaway MBBS PhD FRACP CGHGSA Genetic Metabolic Disorders Service Sydney Children s Hospital Network Disciplines of Child and Adolescent
Nutritional Interventions in Primary Mitochondrial Disorders Carolyn J Ellaway MBBS PhD FRACP CGHGSA Genetic Metabolic Disorders Service Sydney Children s Hospital Network Disciplines of Child and Adolescent
The Premier Vein Center Evan Oblonsky MD 1051 W. Rand Road, Suite 104 Arlington Heights, IL Tel: Fax:
 PATIENT INFORMATION (PLEASE PRINT) Patient Name: Nickname: Guardian: Date of Birth: Sex: Address: 2nd Address: Home Phone: Work Phone: Cell Phone: Best Number: License / ID# Contact Email: Emergency Contact:
PATIENT INFORMATION (PLEASE PRINT) Patient Name: Nickname: Guardian: Date of Birth: Sex: Address: 2nd Address: Home Phone: Work Phone: Cell Phone: Best Number: License / ID# Contact Email: Emergency Contact:
Account # Notes. Physician Name Physician Phone Fax. Diagnosis. CLIA #38D Q11 REQ page 1 of 5
 Knight Diagnostic Laboratories Fax: (855) 535-1329 Email: KDLClientServices@ohsu.edu Shipping: 2525 SW 3rd Ave, Ste 350, Portland, OR 97201 Questions? (855) 535-1522 Molecular Genetics Test Requisition
Knight Diagnostic Laboratories Fax: (855) 535-1329 Email: KDLClientServices@ohsu.edu Shipping: 2525 SW 3rd Ave, Ste 350, Portland, OR 97201 Questions? (855) 535-1522 Molecular Genetics Test Requisition
Patient Information. Account #: Date: Person Responsible For Payment (Other than patient):
 Patient Information Account #: Date: Race: Ethnicity: Tobacco Use: White or Hispanic Asian Black or African American Native Hawaiian or Pacific Islander American Indian or Alaskan Native Hispanic or Latino
Patient Information Account #: Date: Race: Ethnicity: Tobacco Use: White or Hispanic Asian Black or African American Native Hawaiian or Pacific Islander American Indian or Alaskan Native Hispanic or Latino
GASTROCARE, P.C. Contact Preference: HOME: Cell #: Office #: REASON FOR VISIT: Allergies: Current Medications (Name/Dose/How taken):
 GASTROCARE, P.C. DR. A.B. REDDY, M.D., F.A.C.G. DR. REKHA KHURANA, M.D. Referring Physician: First Name: Date of Birth: Last name: Age: Pharmacy (include location): Fax Number: Email Address: Gender: Male
GASTROCARE, P.C. DR. A.B. REDDY, M.D., F.A.C.G. DR. REKHA KHURANA, M.D. Referring Physician: First Name: Date of Birth: Last name: Age: Pharmacy (include location): Fax Number: Email Address: Gender: Male
TEST INFORMATION Test: CarrierMap GEN (Genotyping) Panel: CarrierMap Expanded Diseases Tested: 311 Genes Tested: 299 Mutations Tested: 2647
 Ordering Practice Jane Smith John Smith Practice Code: 675 Miller MD 374 Broadway New York, NY 10000 Physician: Dr. Frank Miller Report Generated: 2016-02-03 DOB: 1973-02-19 Gender: Female Ethnicity: European
Ordering Practice Jane Smith John Smith Practice Code: 675 Miller MD 374 Broadway New York, NY 10000 Physician: Dr. Frank Miller Report Generated: 2016-02-03 DOB: 1973-02-19 Gender: Female Ethnicity: European
The mitochondrion and its disorders
 100 PRACTICAL NEUROLOGY H O W T O U N D E R S T A N D I T The mitochondrion and its disorders Patrick F. Chinnery Department of Neurology, Regional Neurosciences Centre, Newcastle General Hospital and
100 PRACTICAL NEUROLOGY H O W T O U N D E R S T A N D I T The mitochondrion and its disorders Patrick F. Chinnery Department of Neurology, Regional Neurosciences Centre, Newcastle General Hospital and
FIRST NAME MI LAST NAME BIRTH DATE (MM/DD/YYYY) GENDER. Name of person previously tested and relationship:
 REQUEST FOR GERMLINE BAP1TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
REQUEST FOR GERMLINE BAP1TESTING Please provide the following information. We cannot perform your test without ALL of this information. PLEASE PRINT ALL ANSWERS PATIENT INFORMATION* FIRST NAME MI LAST
Metabolic Disorders. Chapter Thomson - Wadsworth
 Metabolic Disorders Chapter 28 1 Metabolic Disorders Inborn errors of metabolism group of diseases that affect a wide variety of metabolic processes; defective processing or transport of amino acids, fatty
Metabolic Disorders Chapter 28 1 Metabolic Disorders Inborn errors of metabolism group of diseases that affect a wide variety of metabolic processes; defective processing or transport of amino acids, fatty
Newborn Screening: Blood Spot Disorders
 Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
Newborn Screening: Blood Spot Disorders Arizona s Newborn Screening Program Program Overview Panel of Disorders Disorder Descriptions Program Components Hospitals ADHS Lab ADHS Follow-up ADHS Billing Medical
THIAMINE TRANSPORTER TYPE 2 DEFICIENCY
 THIAMINE TRANSPORTER TYPE 2 DEFICIENCY WHAT IS THE THIAMINE TRANSPORTER TYPE 2 DEFICIENCY (hthtr2)? The thiamine transporter type 2 deficiency (hthtr2) is a inborn error of thiamine metabolism caused by
THIAMINE TRANSPORTER TYPE 2 DEFICIENCY WHAT IS THE THIAMINE TRANSPORTER TYPE 2 DEFICIENCY (hthtr2)? The thiamine transporter type 2 deficiency (hthtr2) is a inborn error of thiamine metabolism caused by
Secondary Energy Deficiencies in Organic Acidemias. Kimberly A Chapman MD PhD Children s National July 26, 2014
 Secondary Energy Deficiencies in Organic Acidemias Kimberly A Chapman MD PhD Children s National July 26, 2014 2 Goals of this talk Describe the secondary energy deficiencies seen in organic acidemias
Secondary Energy Deficiencies in Organic Acidemias Kimberly A Chapman MD PhD Children s National July 26, 2014 2 Goals of this talk Describe the secondary energy deficiencies seen in organic acidemias
Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone. the hospital/facility:
 PATIENT INFORMATION (COMPLETE ONE FORM FOR EACH PERSON TESTED) Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone Patient discharged from Biological Sex:
PATIENT INFORMATION (COMPLETE ONE FORM FOR EACH PERSON TESTED) Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone Patient discharged from Biological Sex:
CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE. Dr. Bahar Naghavi
 2 CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE Dr. Bahar Naghavi Assistant professor of Basic Science Department, Shahid Beheshti University of Medical Sciences, Tehran,Iran 3 Introduction Over 4000
2 CURRENT GENETIC TESTING TOOLS IN NEONATAL MEDICINE Dr. Bahar Naghavi Assistant professor of Basic Science Department, Shahid Beheshti University of Medical Sciences, Tehran,Iran 3 Introduction Over 4000
Related Policies None
 Medical policy MP 2.04.117 BCBSA Ref. Policy: 2.04.117 Last Review: 06/22/2017 Effective Date: 06/22/2017 Section: Medicine End Date: 06/26/2018 Related Policies None DISCLAIMER Our medical policies are
Medical policy MP 2.04.117 BCBSA Ref. Policy: 2.04.117 Last Review: 06/22/2017 Effective Date: 06/22/2017 Section: Medicine End Date: 06/26/2018 Related Policies None DISCLAIMER Our medical policies are
The University of Arizona Pediatric Residency Program. Primary Goals for Rotation. Genetics
 The University of Arizona Pediatric Residency Program Primary Goals for Rotation Genetics 1. GOAL: Understand the role of the pediatrician in preventing genetic disease, and in counseling and screening
The University of Arizona Pediatric Residency Program Primary Goals for Rotation Genetics 1. GOAL: Understand the role of the pediatrician in preventing genetic disease, and in counseling and screening
SELECTIVE VULNERABILITY (HYPOXIA AND HYPOGLYCEMIA)
 DEFICIENCY OF METABOLITE -HYPOXIA AND HYPOGLYCEMIA -HYPOVITAMINOSIS SELECTIVE VULNERABILITY (HYPOXIA AND HYPOGLYCEMIA) -SPECIFIC CELL TYPE NEURONS>OLIGODENDROCYTES>ASTROCYTES -SPECIFIC BRAIN REGION PYRAMIDAL
DEFICIENCY OF METABOLITE -HYPOXIA AND HYPOGLYCEMIA -HYPOVITAMINOSIS SELECTIVE VULNERABILITY (HYPOXIA AND HYPOGLYCEMIA) -SPECIFIC CELL TYPE NEURONS>OLIGODENDROCYTES>ASTROCYTES -SPECIFIC BRAIN REGION PYRAMIDAL
PATIENT REGISTRATION PATIENT NAME: DOB: SS#: CITY: STATE: ZIP: CELL PHONE: EMPLOYER: EMPLOYER PHONE: ( ) EMERGENCY CONTACT PH# ( ) RELATIONSHIP:
 PATIENT NAME: DOB: SS#: NAME OF PARENTS (if patient is a minor) PATIENT REGISTRATION HOME ADDRESS HOME PHONE: CITY: STATE: ZIP: CELL PHONE: MAILING ADDRESS (if different) CITY: STATE: ZIP: EMPLOYER: EMPLOYER
PATIENT NAME: DOB: SS#: NAME OF PARENTS (if patient is a minor) PATIENT REGISTRATION HOME ADDRESS HOME PHONE: CITY: STATE: ZIP: CELL PHONE: MAILING ADDRESS (if different) CITY: STATE: ZIP: EMPLOYER: EMPLOYER
What s New in Newborn Screening?
 What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is administered by the Illinois Department of Public Health.
What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is administered by the Illinois Department of Public Health.
Illumina Clinical Services Laboratory
 Illumina Clinical Services Laboratory Illumina, Inc. 5200 Illumina Way San Diego, CA 92122, USA Phone: 858.736.8080 Fax: 858.255.5285 everygenome@illumina.com CLIA Certificate No.: 05D1092911 Illumina
Illumina Clinical Services Laboratory Illumina, Inc. 5200 Illumina Way San Diego, CA 92122, USA Phone: 858.736.8080 Fax: 858.255.5285 everygenome@illumina.com CLIA Certificate No.: 05D1092911 Illumina
New Patient Documentation. Name: (Last) (First) (Middle) Address: (Street) (Apt#) (City) (State) (Zip) Home Phone: ( ) Cell: ( ) Work: ( )
 New Patient Documentation Name: (Last) (First) (Middle) Address: (Street) (Apt#) (City) (State) (Zip) Home Phone: ( ) Cell: ( ) Work: ( ) Age: Birthdate: E Email: Social: Sex: Male Female Height: Weight:
New Patient Documentation Name: (Last) (First) (Middle) Address: (Street) (Apt#) (City) (State) (Zip) Home Phone: ( ) Cell: ( ) Work: ( ) Age: Birthdate: E Email: Social: Sex: Male Female Height: Weight:
variant led to a premature stop codon p.k316* which resulted in nonsense-mediated mrna decay. Although the exact function of the C19L1 is still
 157 Neurological disorders primarily affect and impair the functioning of the brain and/or neurological system. Structural, electrical or metabolic abnormalities in the brain or neurological system can
157 Neurological disorders primarily affect and impair the functioning of the brain and/or neurological system. Structural, electrical or metabolic abnormalities in the brain or neurological system can
Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone. the hospital/facility:
 PATIENT INFORMATION (COMPLETE ONE FORM FOR EACH PERSON TESTED) Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone Patient discharged from Biological Sex:
PATIENT INFORMATION (COMPLETE ONE FORM FOR EACH PERSON TESTED) Patient Last Name Patient First Name MI Date of Birth (MM / DD / YYYY) Address City State Zip Phone Patient discharged from Biological Sex:
Subject ID: Date Test Started: Specimen Type: Peripheral Blood Date of Report: Date Specimen Obtained:
 TEST PERFORMED Whole exome sequencing (WES) with Focused Diagnostic Panel(s): Neuropathy, Leukodystrophy, Myopathy See below for a complete list of genes analyzed. RESULTS 1 sequence variant with potential
TEST PERFORMED Whole exome sequencing (WES) with Focused Diagnostic Panel(s): Neuropathy, Leukodystrophy, Myopathy See below for a complete list of genes analyzed. RESULTS 1 sequence variant with potential
When to think about metabolic disorders in adulthood? Wouter Meersseman
 When to think about metabolic disorders in adulthood? Wouter Meersseman General Internal Medicine Adult Metabolic Clinic Wouter Meersseman, Leuven, Belgium Man, 25 year-old Normal development From 15 months
When to think about metabolic disorders in adulthood? Wouter Meersseman General Internal Medicine Adult Metabolic Clinic Wouter Meersseman, Leuven, Belgium Man, 25 year-old Normal development From 15 months
Welcome to Medina Family Chiropractic and Acupuncture!
 Welcome to Medina Family Chiropractic and Acupuncture! Please fill out this form and return it to the front desk. Let us know if you have any questions! Personal information Date: First name: Middle name:
Welcome to Medina Family Chiropractic and Acupuncture! Please fill out this form and return it to the front desk. Let us know if you have any questions! Personal information Date: First name: Middle name:
AUTISM SCIENCE DIGEST: THE JOURNAL OF AUTISMONE ISSUE 01 APRIL 2011 REPRINTED WITH PERMISSION
 FRAN KENDALL, MD, has extensive experience in the diagnosis and management of children and adults with a wide array of inborn errors of metabolism, specifically mitochondrial and metabolic disorders. She
FRAN KENDALL, MD, has extensive experience in the diagnosis and management of children and adults with a wide array of inborn errors of metabolism, specifically mitochondrial and metabolic disorders. She
The spectrum and outcome of the. neonates with inborn errors of. metabolism at a tertiary care hospital
 The spectrum and outcome of the neonates with inborn errors of metabolism at a tertiary care hospital Dr. Sevim Ünal Neonatology Division, Ankara Children s Hematology Oncology Research Hospital, Ankara,
The spectrum and outcome of the neonates with inborn errors of metabolism at a tertiary care hospital Dr. Sevim Ünal Neonatology Division, Ankara Children s Hematology Oncology Research Hospital, Ankara,
Exome Requisition Form
 Patient Information Exome Requisition Form The University of Chicago Genetic Services Laboratories 5841 South Maryland Avenue, Room G701/MC0077, Chicago, IL 60637 Toll Free: 888.824.3637 Local: 773.834.0555
Patient Information Exome Requisition Form The University of Chicago Genetic Services Laboratories 5841 South Maryland Avenue, Room G701/MC0077, Chicago, IL 60637 Toll Free: 888.824.3637 Local: 773.834.0555
Fran D. Kendall, M.D.
 Fran D. Kendall, M.D. VMP, LLC Biochemical Genetics Metabolic, Mitochondrial & Inherited Disorders To provide basic background information on Mitochondrial Disease, its clinical features, diagnosis, treatment,
Fran D. Kendall, M.D. VMP, LLC Biochemical Genetics Metabolic, Mitochondrial & Inherited Disorders To provide basic background information on Mitochondrial Disease, its clinical features, diagnosis, treatment,
Muscle Metabolism. Dr. Nabil Bashir
 Muscle Metabolism Dr. Nabil Bashir Learning objectives Understand how skeletal muscles derive energy at rest, moderate exercise, and strong exercise. Recognize the difference between aerobic and anaerobic
Muscle Metabolism Dr. Nabil Bashir Learning objectives Understand how skeletal muscles derive energy at rest, moderate exercise, and strong exercise. Recognize the difference between aerobic and anaerobic
Patient Medical Information. Last. Sex: M / F Age: Date of Birth: Home Address: City: State: Zip Code: Business Address: City: State: Zip Code:
 Patient Medical Information Name: First Middle Last Sex: M / F Age: Date of Birth: Social Security # Driver s License # Home Address: City: State: Zip Code: Home Phone: Occupation: Cell: Employer: Business
Patient Medical Information Name: First Middle Last Sex: M / F Age: Date of Birth: Social Security # Driver s License # Home Address: City: State: Zip Code: Home Phone: Occupation: Cell: Employer: Business
SCAD and GA-II: Truths and Confusions
 SCAD and GA-II: Truths and Confusions Bill Rhead* Medical College of Wisconsin *MD, PhD GA-II Severe GA-II is as bad as: SCAD + MCAD + COMBINED! VLCAD + IVA + GA-I Severe GA-II is always fatal Mild
SCAD and GA-II: Truths and Confusions Bill Rhead* Medical College of Wisconsin *MD, PhD GA-II Severe GA-II is as bad as: SCAD + MCAD + COMBINED! VLCAD + IVA + GA-I Severe GA-II is always fatal Mild
Hospital he hospital is located near the interchange of highway 217 and (US 26).
 Welcome to our Clinic! Our goal is to provide you with the highest quality medical care available. Please bring the completed enclosed paperwork along with your insurance card and legal picture ID to your
Welcome to our Clinic! Our goal is to provide you with the highest quality medical care available. Please bring the completed enclosed paperwork along with your insurance card and legal picture ID to your
DNA CENTER New Patient Information
 DNA CENTER New Patient Information Name Email: Address City State Zip Home Phone Work Cell Phone Social Security Number Date of birth Gender ( Male/Female) Age Please Circle: Hispanic/Latin or Non Hispanic/Latin
DNA CENTER New Patient Information Name Email: Address City State Zip Home Phone Work Cell Phone Social Security Number Date of birth Gender ( Male/Female) Age Please Circle: Hispanic/Latin or Non Hispanic/Latin
The laboratory investigation of lactic acidaemia. J Bonham/T Laing
 The laboratory investigation of lactic acidaemia J Bonham/T Laing Reference range Typical ranges for blood lactate are: Newborn 0.3-2.2 mmol/l Nielsen J et al1 1994 1-12mo 0.9-1.8 mmol/l Bonnefont et al
The laboratory investigation of lactic acidaemia J Bonham/T Laing Reference range Typical ranges for blood lactate are: Newborn 0.3-2.2 mmol/l Nielsen J et al1 1994 1-12mo 0.9-1.8 mmol/l Bonnefont et al
Fatty Acid Oxidation Disorders- an update. Fiona Carragher Biochemical Sciences, GSTS Pathology St Thomas Hospital, London
 Fatty Acid Oxidation Disorders- an update Fiona Carragher Biochemical Sciences, GSTS Pathology St Thomas Hospital, London An update. Overview of metabolism Clinical presentation and outcome Diagnostic
Fatty Acid Oxidation Disorders- an update Fiona Carragher Biochemical Sciences, GSTS Pathology St Thomas Hospital, London An update. Overview of metabolism Clinical presentation and outcome Diagnostic
Address City State Zip. Home Phone Cell Work. (For SHPT use only) Emergency Contact Phone
 Somerset Hills Physical Therapy, PC 180 Mount Airy Road, Suite 103 Basking Ridge, NJ 07920 Phone (908) 766-1407 Fax (908) 953-8454 wwwsomersethillsptcom Patient Information: Name Sex M F Date of Birth
Somerset Hills Physical Therapy, PC 180 Mount Airy Road, Suite 103 Basking Ridge, NJ 07920 Phone (908) 766-1407 Fax (908) 953-8454 wwwsomersethillsptcom Patient Information: Name Sex M F Date of Birth
Inborn Errors of Metabolism (IEM)
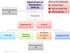 Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
Clinical Presentation Inborn Errors of Metabolism (IEM) Click on the following: - Clinical Pearl - link to movie clip - link to picture Investigations Blood Work Urine No Acidosis NH 4 + Metabolic Acidosis
Acknowledgement of receipt of notice of privacy practices
 Acknowledgement of receipt of notice of privacy practices NOTICE OF PRIVACY PRACTICES I acknowledge that I have received a Notice of Privacy Practices from Kettering Physician Network (dba Kettering Cancer
Acknowledgement of receipt of notice of privacy practices NOTICE OF PRIVACY PRACTICES I acknowledge that I have received a Notice of Privacy Practices from Kettering Physician Network (dba Kettering Cancer
3855 Burton Street SE Suite A, Grand Rapids, MI Phone Fax Patient Information. Address: City: State: Zip:
 3855 Burton Street SE Suite A, Grand Rapids, MI 49546 Phone 616.323.3102 Fax 616.323.3061 Patient Information Patient Name: Preferred Language: Address: City: State: Zip: Home Phone: Cell Phone: Cell Carrier:
3855 Burton Street SE Suite A, Grand Rapids, MI 49546 Phone 616.323.3102 Fax 616.323.3061 Patient Information Patient Name: Preferred Language: Address: City: State: Zip: Home Phone: Cell Phone: Cell Carrier:
Metabolic Changes in ASD. Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius
 Metabolic Changes in ASD Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius 12 patients 3 Autism: Ages 3/3/3.7 3 PDD: Ages 3/3/6 3 Asperger: Ages 6/7/15.1 3 Speech delay and Sensory Problems (SHL):
Metabolic Changes in ASD Norma J. Arciniegas, MD Simón E. Carlo, MD Instituto Filius 12 patients 3 Autism: Ages 3/3/3.7 3 PDD: Ages 3/3/6 3 Asperger: Ages 6/7/15.1 3 Speech delay and Sensory Problems (SHL):
Patient Name: First MI Last Preferred Name. DOB: Sex: MALE FEMALE SSN: Address: City: State: Zip Code:
 PATIENT DEMOGRAPHICS: Patient Name: First MI Last Preferred Name DOB: Sex: MALE FEMALE SSN: Address: City: State: Zip Code: Home Phone: _( ) Marital Status: Married Single Divorced Widowed Cell Phone:
PATIENT DEMOGRAPHICS: Patient Name: First MI Last Preferred Name DOB: Sex: MALE FEMALE SSN: Address: City: State: Zip Code: Home Phone: _( ) Marital Status: Married Single Divorced Widowed Cell Phone:
Inborn Errors of Metabolism in the Emergency Department. Will Davies June 2014
 Inborn Errors of Metabolism in the Emergency Department Will Davies June 2014 Inborn Errors of Metabolism in the Emergency Department Overview Although individually rare, altogether they are 1:1000-2500
Inborn Errors of Metabolism in the Emergency Department Will Davies June 2014 Inborn Errors of Metabolism in the Emergency Department Overview Although individually rare, altogether they are 1:1000-2500
New Patient Information & Consents
 New Patient Information & Consents Name: DOB: SSN: Gender: Address: City: State: Zip: Home #: Cell #: Other#: Employment Status: Occupation: Email Address: Marital Status: S M D W How did you hear about
New Patient Information & Consents Name: DOB: SSN: Gender: Address: City: State: Zip: Home #: Cell #: Other#: Employment Status: Occupation: Email Address: Marital Status: S M D W How did you hear about
Fatty Acids Synthesis L3
 Fatty Acids Synthesis L3 The pathway for fatty acid synthesis occurs in the cytoplasm, whereas, oxidation occurs in the mitochondria. The other major difference is the use of nucleotide co-factors. Oxidation
Fatty Acids Synthesis L3 The pathway for fatty acid synthesis occurs in the cytoplasm, whereas, oxidation occurs in the mitochondria. The other major difference is the use of nucleotide co-factors. Oxidation
Brain MRI in Mitochondrial Disorders: Correlating the Phenotype with the Genotype. Bindu Parayil Sankaran
 Brain MRI in Mitochondrial Disorders: Correlating the Phenotype with the Genotype Bindu Parayil Sankaran copyright Bindu Parayil Sankaran, Maastricht 2018 Printing: Datawyse Universitaire Pers Maastricht
Brain MRI in Mitochondrial Disorders: Correlating the Phenotype with the Genotype Bindu Parayil Sankaran copyright Bindu Parayil Sankaran, Maastricht 2018 Printing: Datawyse Universitaire Pers Maastricht
METABOLITE TEST REQUISITION FORM SPECIMEN INFORMATION. Specimen Date: Time: ADDITIONAL INFORMATION
 METABOLITE TEST REQUISITION FORM Baylor Institute of Metabolic Disease Last Name: First Name: DOB/Age: Gender: Male Female Medical Record #/Patient ID #: PATIENT INFORMATION - OR Place Patient Sticker
METABOLITE TEST REQUISITION FORM Baylor Institute of Metabolic Disease Last Name: First Name: DOB/Age: Gender: Male Female Medical Record #/Patient ID #: PATIENT INFORMATION - OR Place Patient Sticker
Immediate Family History Please list Father, Mother, Brother, Sister or Children
 : Social Security # Name: of Birth: Age: Address: City: State: Zip: Home#: Cell#: Work#: E-mail: Status: Married Single Divorced Widowed Work Place/School: Occupation/Grade: Emergency Contact (Name/Phone):
: Social Security # Name: of Birth: Age: Address: City: State: Zip: Home#: Cell#: Work#: E-mail: Status: Married Single Divorced Widowed Work Place/School: Occupation/Grade: Emergency Contact (Name/Phone):
SERVICE SCHEDULE, FEES & GUIDELINES FOR SAMPLE SHIPMENT
 SERVICE SCHEDULE, FEES & GUIDELINES FOR SAMPLE SHIPMENT ACYLCARNITINE PROFILE: PLASMA/SERUM, PKU CARD, WHOLE BLOOD Amount of sample: Plasma (heparinized is preferred, EDTA acceptable) or serum: 0.5 ml
SERVICE SCHEDULE, FEES & GUIDELINES FOR SAMPLE SHIPMENT ACYLCARNITINE PROFILE: PLASMA/SERUM, PKU CARD, WHOLE BLOOD Amount of sample: Plasma (heparinized is preferred, EDTA acceptable) or serum: 0.5 ml
What s New in Newborn Screening?
 What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is mandated and administered by the Illinois Department of
What s New in Newborn Screening? Funded by: Illinois Department of Public Health Information on Newborn Screening Newborn screening in Illinois is mandated and administered by the Illinois Department of
Should Universal Carrier Screening be Universal?
 Should Universal Carrier Screening be Universal? Disclosures Research funding from Natera Mary E Norton MD University of California, San Francisco Antepartum and Intrapartum Management June 15, 2017 Burden
Should Universal Carrier Screening be Universal? Disclosures Research funding from Natera Mary E Norton MD University of California, San Francisco Antepartum and Intrapartum Management June 15, 2017 Burden
CLINICAL SIGNS SUGGESTIVE OF A NEUROMETABOLIC DISEASE. Bwee Tien Poll-The Amsterdam UMC The Netherlands
 CLINICAL SIGNS SUGGESTIVE OF A NEUROMETABOLIC DISEASE Bwee Tien Poll-The Amsterdam UMC The Netherlands FRAMEWORK OF PRINCIPALS 1. Problem-oriented clinical approach 2. Biomarkers in plasma, urine, CSF
CLINICAL SIGNS SUGGESTIVE OF A NEUROMETABOLIC DISEASE Bwee Tien Poll-The Amsterdam UMC The Netherlands FRAMEWORK OF PRINCIPALS 1. Problem-oriented clinical approach 2. Biomarkers in plasma, urine, CSF
METABOLITE TEST REQUISITION FORM
 METABOLITE TEST REQUISITION FORM Baylor Scott and White Research Institute Last Name: First Name: DOB/Age: Gender: Male Female Medical Record #/Patient ID #: PATIENT INFORMATION OR Place Patient Sticker
METABOLITE TEST REQUISITION FORM Baylor Scott and White Research Institute Last Name: First Name: DOB/Age: Gender: Male Female Medical Record #/Patient ID #: PATIENT INFORMATION OR Place Patient Sticker
BROADENING YOUR PATIENT S OPTIONS FOR GENETIC CARRIER SCREENING.
 BROADENING YOUR PATIENT S OPTIONS FOR GENETIC CARRIER SCREENING. The Inheritest SM Carrier Screen provides relevant genetic screening for many inherited diseases found throughout the pan-ethnic US population.
BROADENING YOUR PATIENT S OPTIONS FOR GENETIC CARRIER SCREENING. The Inheritest SM Carrier Screen provides relevant genetic screening for many inherited diseases found throughout the pan-ethnic US population.
PEDIATRIC REGISTRATION FORM Please Print MALE FEMALE
 PEDIATRIC REGISTRATION FORM Please Print MALE FEMALE Name Birth / / LAST FIRST MI Address City State Zip Home Phone ( ) Parent s Work ( ) Social Security # Parent s Cell ( ) Email Address Parent s Marital
PEDIATRIC REGISTRATION FORM Please Print MALE FEMALE Name Birth / / LAST FIRST MI Address City State Zip Home Phone ( ) Parent s Work ( ) Social Security # Parent s Cell ( ) Email Address Parent s Marital
Patient Information. Address: Street Apt. # City State Zip. Seasonal Address: (If different than above address) Address: Street Apt.
 Page 1 of 6 Patient Information Name: Date of Birth: Age: Address: Seasonal Address: (If different than above address) Address: S.S. #: - - Sex: M F Marital Status: M S D Sep W Partnered Phone: Home (
Page 1 of 6 Patient Information Name: Date of Birth: Age: Address: Seasonal Address: (If different than above address) Address: S.S. #: - - Sex: M F Marital Status: M S D Sep W Partnered Phone: Home (
HEADACHE HISTORY FORM
 HEADACHE HISTORY FORM IF THIS IS YOUR FIRST VISIT, PLEASE TAKE THE TIME TO FILL THIS FORM OUT COMPLETELY. Patient Name: Age: Date of Birth: Weight: Height: Address: City: State: Zip: Home Phone: Cell Phone:
HEADACHE HISTORY FORM IF THIS IS YOUR FIRST VISIT, PLEASE TAKE THE TIME TO FILL THIS FORM OUT COMPLETELY. Patient Name: Age: Date of Birth: Weight: Height: Address: City: State: Zip: Home Phone: Cell Phone:
Abnormalities of Intermediary Metabolism in Barth Syndrome
 Abnormalities of Intermediary Metabolism in Barth Syndrome Richard I. Kelley, M.D., Ph.D. Kennedy Krieger Institute Department of Pediatrics Johns Hopkins University Is Barth Syndrome a Mitochondrial Disease?
Abnormalities of Intermediary Metabolism in Barth Syndrome Richard I. Kelley, M.D., Ph.D. Kennedy Krieger Institute Department of Pediatrics Johns Hopkins University Is Barth Syndrome a Mitochondrial Disease?
PATIENT REGISTRATION
 P Account# PATIENT REGISTRATION Please answer all questions completely. PAYMENT IS EXPECTED WHEN SERVICES ARE RENDERED Date New Update Name Date of Birth Male Last First Middle Female Home Address City/State/Zip
P Account# PATIENT REGISTRATION Please answer all questions completely. PAYMENT IS EXPECTED WHEN SERVICES ARE RENDERED Date New Update Name Date of Birth Male Last First Middle Female Home Address City/State/Zip
SOC SEC #: - - Date of Birth: - - Age: yrs. State: Zip Code: Employer:
 PATIENT INFORMATION (PLEASE PRINT) SOC SEC #: - - MRN#: Home Phone: Work Phone: Ext: Address: City: Cell Phone: Date of Birth: - - Age: yrs State: Zip Code: Employer: SEX: Male Female Work Address: City:
PATIENT INFORMATION (PLEASE PRINT) SOC SEC #: - - MRN#: Home Phone: Work Phone: Ext: Address: City: Cell Phone: Date of Birth: - - Age: yrs State: Zip Code: Employer: SEX: Male Female Work Address: City:
INFORMED CONSENT FOR ANORECTAL PROCEDURES
 516-248-2422 www.crssny.com Locations in Nassau, Suffolk and Queens INFORMED CONSENT FOR ANORECTAL PROCEDURES You may undergo anoscopy or proctosigmoidoscopy as part of your rectal examination. These tests
516-248-2422 www.crssny.com Locations in Nassau, Suffolk and Queens INFORMED CONSENT FOR ANORECTAL PROCEDURES You may undergo anoscopy or proctosigmoidoscopy as part of your rectal examination. These tests
Robert Barski. Biochemical Genetics St James s University Hospital, Leeds. MetBioNet IEM Introductory Training
 Robert Barski Biochemical Genetics St James s University Hospital, Leeds Lactate is produced as the fate of anaerobic metabolism of pyruvate. It is an important intermediary metabolite especially with
Robert Barski Biochemical Genetics St James s University Hospital, Leeds Lactate is produced as the fate of anaerobic metabolism of pyruvate. It is an important intermediary metabolite especially with
Patient Information. Legal Name: First Middle Last. Street City State Zip
 Patient Information Legal Name: Home Address: First Middle Last Street City State Zip Gender: (circle one) Male Female Date of Birth: Social Security #: - - mm / dd / yyyy Email: Marital Status: Primary
Patient Information Legal Name: Home Address: First Middle Last Street City State Zip Gender: (circle one) Male Female Date of Birth: Social Security #: - - mm / dd / yyyy Email: Marital Status: Primary
Testing for Fabry Disease. What You Need to Know
 Testing for Fabry Disease What You Need to Know Identification of One Family Member Can Enable Earlier Screening for Others A woman with Fabry disease may have inherited it from either her mother or her
Testing for Fabry Disease What You Need to Know Identification of One Family Member Can Enable Earlier Screening for Others A woman with Fabry disease may have inherited it from either her mother or her
Vision/Lifestyle Questionnaire
 Roger J. Meyer, M.D. Retina Fellowship Trained Macular Degeneration Diabetic Eye Care Glaucoma Robert M. Reinauer, M.D. Retina Fellowship Trained Surgical/Medical Treatment of the Retina & Vitreous Macular
Roger J. Meyer, M.D. Retina Fellowship Trained Macular Degeneration Diabetic Eye Care Glaucoma Robert M. Reinauer, M.D. Retina Fellowship Trained Surgical/Medical Treatment of the Retina & Vitreous Macular
Applies to: All Aetna plans, except Traditional Choice plans. All Innovation Health plans, except indemnity plans
 BRCA Precertification Information Request Form Applies to: All Aetna plans, except Traditional Choice plans All Innovation Health plans, except indemnity plans All Health benefits and health insurance
BRCA Precertification Information Request Form Applies to: All Aetna plans, except Traditional Choice plans All Innovation Health plans, except indemnity plans All Health benefits and health insurance
Preferred Name: First Name: Last Name: Middle Initial: Mailing Address: City: State: Zip: Alternate number: address:
 Welcome to our office! We want to provide you with the very best in vision care. In order for us to serve you better, we need certain biographical information from you. Please complete the following data
Welcome to our office! We want to provide you with the very best in vision care. In order for us to serve you better, we need certain biographical information from you. Please complete the following data
Exploding Genetic Knowledge in Developmental Disabilities. Disclosures. The Genetic Principle
 Exploding Genetic Knowledge in Developmental Disabilities How to acquire the data and how to make use of it Elliott H. Sherr MD PhD Professor of Neurology & Pediatrics UCSF Disclosures InVitae: clinical
Exploding Genetic Knowledge in Developmental Disabilities How to acquire the data and how to make use of it Elliott H. Sherr MD PhD Professor of Neurology & Pediatrics UCSF Disclosures InVitae: clinical
Training Syllabus CLINICAL SYLLABUS
 Training Syllabus CLINICAL SYLLABUS SYLLABUS FOR TRAINING IN CLINICAL PAEDIATRIC METABOLIC MEDICINE Updated July 2006 This syllabus is intended as a guide. Whilst the training should be comprehensive,
Training Syllabus CLINICAL SYLLABUS SYLLABUS FOR TRAINING IN CLINICAL PAEDIATRIC METABOLIC MEDICINE Updated July 2006 This syllabus is intended as a guide. Whilst the training should be comprehensive,
