1115 Windfield Way, Suite 100 El Dorado Hills, CA Matthew M. Hull, RAC Phone: (916) / Fax: (916)
|
|
|
- Augustine Atkinson
- 5 years ago
- Views:
Transcription
1 Page 1 of (k) SUMMARY JUN Sponsor Name: 510(k) Contact: Consensus Orthopedics, Inc Windfield Way, Suite 100 El Dorado Hills, CA Matthew M. Hull, RAC Phone: (916) / Fax: (916) mhull@consensusortho.com Date Prepared: 1 April 2011 Trade Name: Common Name: Classification Name: Consensus Knee System, Line Extensions Total knee prosthesis for cemented or uncemented use Knee joint patellofemorotibial metal/polymer/metal semi-constrained cemented prosthesis is a class II device per 21 CFR (Product Code JWH) Knee joint patellofemorotibial metal/polymer porous-coated uncemented prosthesis is a Class 11 device per 21 CFR (Product Code MBH) Review Panel: Orthopedic Devices Device Description: The Consensus Total Knee System (CKS) is a primary fixed-bearing total knee system that has been on the market since the mid-l1990's. The CKS has been designed to replicate the natural anatomy of the knee in order to restore knee function. It has been developed to preserve and utilize healthy ligamentous structures. For cases where the soft tissues are not functional, the PCL substituting tibial inserts or the posterior stabilized system are available for increased stability. The CKS incorporates femoral, tibial, and patellar components and all associated instrumentation needed for implantation. The CKS can be used for total knee replacement with posterior cruciate ligament (PCL) retaining or substituting. The femoral components are provided in left and right side versions and are designed to replicate natural kinematic motion between the femur, tibia and patella. The Consensus femoral component is designed to provide uniform contact zones in the coronal plane throughout the range of motion when the knee is properly aligned. The femoral component is also designed with a large distal radius to optimize contact areas and reduce contact stress. The trochlear groove in
2 the femur is designed to allow the load from the patella to be evenly distributed on the femur with adequate lateral constraint. Page 2 of 4 The CKS metallic components are available in non-porous and porous coated variants for cemented use and in a porous coated (CoCr beads with Titanium) version for uncemented use. Indications for Use: The CONSENSUS KNEE SYSTEM Primary Knee is designed as a system and is not intended for substitution of components from other systems. A. Primary intervention of rheumatoid arthritis, osteoarthritis, post-traumatic arthritis, or degenerative arthritis. B. Failed osteotomy or unicompartmental replacements. C. Replacement of unsatisfactory cemented or press-fit knee components when sufficient bone stock exists. D. The porous coated (CoCr beads with Titanium) components may be used with or without cement. Substantial Equivalence: The CKS components were originally cleared by FDA in K932837* with a posterior stabilizing (PS) version cleared in K954818* & K962215*. The CKS was expanded in K001456** (femoral components) and K983004** (tibial components) and most recently cleared for uncemented use in KlO2927. *These 510(k)'s were cleared prior to the purchase of U.S. Medical Products by Hayes Medical in **The previous 5 10(k)'s for these devices were cleared prior to the change in our company's name from Hayes Medical, inc. to Consensus Orthopedics, Inc. in This submission is to add the following line extensions to the Consensus Knee System: I ) Reduced Lateral Profile (RLP) femoral component in standard and PS versions 2) Porous coated PS femoral component 3) Size 0 tibial baseplate and inserts 4) 20 & 22mmur thick poly inserts 5) Porous coated patella for uncemented use. The CKS implants are also substantially equivalent to the Gender Solutions' m Natural Knee Flex System by Zimmer, Inc. cleared in K & K with the above options.
3 K Io00 Page 3 of 4 Non-Clinical Performance Data: Below is a summary of the testing related to the various aspects of the CKS line extension. Porous Coating: CoCr beads with Ti coating (applicablefo all CoCr implants) Microstructure of the N/A Bead to Bead Neck Diameter 0.33 mm Pore Size modified surface Volume % Porosity 37% mm Coating Thickness mm Corrosion of the modified Equal to or improved Critical Potential Breakdown Potential surface shall be equal of less corrosion resistance when C~ ed 20m 20m than that measured in a compared with CoCr beads Ti Coated legally marketed device, using ASTM F746 & G61 Co Beads_1315_mV _1200_mV Modified surface shall The static tensile strength Static Tensile Strength of exhibit adequate static will exceed 20 MPa MPa tensile strength Modified surface shall The static shear strength Static Shear Strength of exhibit adequate static shear will exceed 20 MPa MPa strength. Modified surface shall The shear fatigue strength 10 million cycles achieved with exhibit adequate shear will exceed 10 million a strength of MPa fatigue strength. cycles. Modified surface shall The rotating beam fatigue 10 million cycles achieved with exhibit adequate rotating strength will exceed 10 a strength of MPa beam fatigue strength. million cycles. Modified surface shall not N/A 200N load: exhibit excessive abrasion. RLP Fernoral Components Avg. mass loss g Avg. thickness loss 6% 1500N load: Avg. Mass loss g Avg. thickness loss 23% The articulating surface of Similar contact area and RLP had the same contact area the RLP components shall surface stress distributions, as the original CKS. The PS match that of their standard RLP matches the articulating and PS counterparts. surface of the RLP. Size 0 Tibial Base Plate and Insert Size 0 tibial baseplate/insert Similar push-ia/push-out Minimum push-out load was assembly shall permit loads when compared with 428 lbs with failure mode being adequate push-ia/push-out existing baseplate/insert deformation of anterior snap force. combinations, recess in poly insert; Similar to other insert/baseplate.
4 K I Oq Page 4 of 4 combinations. Components can be easilv inserted by hand. Thicker Tibial Insert Insert thickness per FDA Insert thickness must be Insert thickness was greater Guidance Jan. 16, >6mm. than 6mm. Po0rous Coated Metal Backed Patella The articulating surface of the Identical articulating surface. Articulating surface of the porous porous coated metal backed coated metal backed patella is patella shall match that of identical to existing patella. current patellas. The mating geometry between Identical mating geometry. The mating geometry of the the UHMWPE and metal back porous coated metal backed of the porous coated metal patella is identical to the existing backed patella shall match that metal backed patella. of current metal backed patella.
5 -4 DEPARTMENT OF HEALTH & HUMAN SERVICES Food Public Health Sulrvice and Dr ug Ad ministration New II ampshlu e Avenute Document Contirol Room -W066-G609 Silver Spring, MID Consensus Orthopedics, Inc. %/ Mr. Matthew Hull JN2 I 1115 Windfield Way, Suite 100 JN271M El Dorado Hills, California Re: K Trade/Device Name: ConsensusD Knee System, Line Extensions Regulation Number: 21 CFR Regulation Namne: Knee joint patellofemiorotibial metal/polymer porous coated uncemnented prosthesis Regulatory Class: Class 11 Product Code: MBHA, JWI-l Dated: April 1, 2011 Received: April 4, 2011 Dear Mr. 1-ull: We have reviewed your Section 5 1 0(k) prernarket notification of intent to market the device referenced above and have determined the device is substantially equivalent (for the indications for use stated in the enclosure) to legally marketed predicate devices marketed in interstate commerce prior to May 28, 1976, the enactment date of the Medical Device Amendments, or to devices that have been reclassified in accordance with the provisions of the Federal Food, Drug, and Cosmetic Act (Act) that do not require approval of a premarket approval application (PMA). You may, therefore, market the device, Subject to the general controls provisions of the Act. The general controls provisions of the Act include requirements for annual registration, listing of devices, good manufacturing practice, labeling, and prohibitions against misbranding and adulteration. If your device is classified (see above) into either class 1I (Special Controls) or class Ill (PMA), it may be subject to additional controls. Existing major regulations affecting your device can be found in the Code of Federal Regulations, Title 2 1, Parts 800 to 898. In addition, FDA may publish further announcements concerning your device in the Federal Register. Please be advised that FDA's issuance of a substantial equivalence determination does not mean that FDA has made a determination that your device complies with other requirements of the Act or any Federal statutes and regulations administered by other Federal agencies. You must comply with all the Act's requirements, including, but not limited to: registration and listing (2 1 CFR Part 807); labeling (21 CFR Part 801); medical device reporting (reporting of medical device-related adverse events) (21 CFR 803); good manufacturing practice requirements as set
6 Page 2 - Mr. Matthew H till forth in the quality systems (QS) regulation (21 CFR Part 820); and if applicable, the electronic product radiation control Provisions (Sections of the Act); 21 CER If you desire specific advice for Your device on our labeling regulation (21 CER Part 801), please go to lhttp://wwvw.f'da.qov/aboutfd)a/ceniter-softices/cdrj-/cdri-otfices/uicii 11I5809. kmi for the Center for Devices and Radiological Health's (CDRH's) Office of Compliance. Also, please note the regulation entitled, "Misbranding by reference to premnarket notification" (21 CER Part ). For questions regarding the reporting of adverse events tinder the MDR regulation (21 CFR Part 803), please go to littp://wvww.i'da.(,ov/medicaldevices/safety/lreportaproblem/dcfault.hitn for the CDRH's Office of Surveillance and Biomietrics/Division of Postmarket Surveillance. You may obtain other general information on your responsibilities under the Act from the Division of Small Manufacturers, International and Consumer Assistance at its toll-free number (800) or (301) or at its Internet address Sincerely yours, Enclosure,f,-Mark N. Melkerson Director Division of Surgical, Orthopedic, and Restorative Devices Office of Device Evaluation Center for Devices and Radiological Health
7 1. INDICATIONS FOR USE STATEME NT 5 10(k) Numrber (if known): 1vieNarne: Consensus Knee System TIdications for Use: The CONSENSUSO KNEE SYSTEM Primary Knee is designed as a system. and is not intended for substitution of components from other systems. A. Primary intervention of rheumatoid arthritis, osteoarthritis, post-traumatic arthritis, or degenerative arthritis. B. Failed osteotomy or unricompartmental replacements. C. Replacement of unsatisfactory cemented *or press-fit knee components when sufficient bone stock exists. D. The porous coated (CoCr beads with. Titanium) components may be used with or without cement. Prescr-iption Use X AND/OR Over the Counter Use (21 CFR Part 80 1 Subpart D) (21 CER Part 80 1 Subpart C) (PLEASE DO NOT WRITE BELOW THIS LINE; CONTINUE ON ANOTHER PAGE IF NEEDED) Concurrence of CDRHI, Office of Device Evaluation (ODE) and Res orative Device 5 10(k) Number
MAY Page 1 of 2
 MAY 2 22012 Page 1 of 2 2. 5 10(k) SUMMARY Sponsor Name: 510(k) Contact: Consensus Orthopedics, Inc. 1115 Windfield Way, Suite 100 El Dorado Hills, CA 95762 Matthew M. Hull, RAC Phone: (916) 355-7156/
MAY 2 22012 Page 1 of 2 2. 5 10(k) SUMMARY Sponsor Name: 510(k) Contact: Consensus Orthopedics, Inc. 1115 Windfield Way, Suite 100 El Dorado Hills, CA 95762 Matthew M. Hull, RAC Phone: (916) 355-7156/
SNuVASivE: 51. 0(k) Premarket Notification Long Lateral Spinal System
 K111410 Page 1 of 2 SNuVASivE: 51 0(k) Premarket Notification Long Lateral Spinal System 510(k) SummaryJU 20201 In accordance with Title 21 of the Code of Federal Regulations, Part 807, and in particular
K111410 Page 1 of 2 SNuVASivE: 51 0(k) Premarket Notification Long Lateral Spinal System 510(k) SummaryJU 20201 In accordance with Title 21 of the Code of Federal Regulations, Part 807, and in particular
U. AUG skeletal dynamics. 510(k) Summary of Safety and Effectiveness Skeletal Dynamics Geminus Volar Distal Radius Plate System
 U. AUG 23 2011 00 skeletal dynamics 510(k) Summary of Safety and Effectiveness Skeletal Dynamics Geminus Volar Distal Radius Plate System Submitter: Skeletal Dynamics, LLC 6905 SW 67 Avenue Suite 201 Miami,
U. AUG 23 2011 00 skeletal dynamics 510(k) Summary of Safety and Effectiveness Skeletal Dynamics Geminus Volar Distal Radius Plate System Submitter: Skeletal Dynamics, LLC 6905 SW 67 Avenue Suite 201 Miami,
Donald W. Guthner Orgenix, LLC Ill Hill Road. Douglassville, PA (888) ORGENIX ( ) (484) (FAX) Intramedullary Nails, Nails
 K070741 ~, SANATMETAL Manufacturer of Orthopaedic and Traumatologic Products H-3301, Eger Faiskola ut 5. Phone: +(36)36-512-900 Fax: +(36)36-512-932 e-mail: metalasanatmetal.hu 510(k) Statement of Summary
K070741 ~, SANATMETAL Manufacturer of Orthopaedic and Traumatologic Products H-3301, Eger Faiskola ut 5. Phone: +(36)36-512-900 Fax: +(36)36-512-932 e-mail: metalasanatmetal.hu 510(k) Statement of Summary
510(k) Summary. Acumed, LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124
 K(102998 Page I of 2 510(k) Summary Device Trade Name: Congruent Bone Plate System J A.N 4 21 Manufacturer: Contact: Prepared by: Acumed, LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124 Mr. Ed Boehmer
K(102998 Page I of 2 510(k) Summary Device Trade Name: Congruent Bone Plate System J A.N 4 21 Manufacturer: Contact: Prepared by: Acumed, LLC 5885 NW Cornelius Pass Road Hillsboro, OR 97124 Mr. Ed Boehmer
MAR (k) SUMMARY. Stanmore Implants Worldwide Ltd JTS Extendible Implant Preparation Date: March 17, A ppl ica nt/spo0nsor:
 510(k) SUMMARY MAR 2 2 201 Stanmore Implants Worldwide Ltd JTS Extendible Implant Preparation Date: March 17, 2011 A ppl ica nt/spo0nsor: Contact Person: Proprietary name: Common or Usual Name: Classification
510(k) SUMMARY MAR 2 2 201 Stanmore Implants Worldwide Ltd JTS Extendible Implant Preparation Date: March 17, 2011 A ppl ica nt/spo0nsor: Contact Person: Proprietary name: Common or Usual Name: Classification
SPINE. 510(k) Summary. Anterior/anterolateral noncervical use (KWQ) (Prodct Cde):Noncervical
 k10 3?I13 SPINE 510(k) Summary FB-22f FB 24f This summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirements of 21 CFR 807.92. Preparation Date: November
k10 3?I13 SPINE 510(k) Summary FB-22f FB 24f This summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirements of 21 CFR 807.92. Preparation Date: November
Ko0 -t. Exhibit 2 MAY 3 0? 510 (K) Summay. Company Name: Columbia Scientific Development, LLC 420 NW I Ith Ave., Suite 617 Portland, Oregon 97209
 Ko0 -t Exhibit 2 MAY 3 0? 510 (K) Summay Company Name: Columbia Scientific Development, LLC 420 NW I Ith Ave., Suite 617 Portland, Oregon 97209 Contact: Stephen Shulman Phone: 734-663-0132 Fax: 734-663-1306
Ko0 -t Exhibit 2 MAY 3 0? 510 (K) Summay Company Name: Columbia Scientific Development, LLC 420 NW I Ith Ave., Suite 617 Portland, Oregon 97209 Contact: Stephen Shulman Phone: 734-663-0132 Fax: 734-663-1306
510(k) SUMMARY STATEMEMT. Mettler Traction Device, MTD 4000 OCT Mettler Electronics Corp South Claudina Street Anaheim, CA 92805
 510(k) SUMMARY STATEMEMT OCT - 8 2009 Mettler Traction Device, MTD 4000 Submitter's Name: Address: Mettler Electronics Corp. 1333 South Claudina Street Anaheim, CA 92805 Telephone: 714-533-2221 x324 Fax:
510(k) SUMMARY STATEMEMT OCT - 8 2009 Mettler Traction Device, MTD 4000 Submitter's Name: Address: Mettler Electronics Corp. 1333 South Claudina Street Anaheim, CA 92805 Telephone: 714-533-2221 x324 Fax:
O(K) SUMMARY. Mega'Gen Co., Ltd , Eupchun-Ri, Jain-Myun. Gyeongsan, Gyeongbuk South Korea Phone: , Fax:
 EZ PLUS IMPLANT SYSTEM 13. 510O(K) SUMMARY Mega'Gen Co., Ltd. 114-8, Eupchun-Ri, Jain-Myun. Gyeongsan, Gyeongbuk South Korea Phone: 82-53-857-5770, Fax: 82-53-857-5432 510(K) Summary 510O(K) SUMMARY AND
EZ PLUS IMPLANT SYSTEM 13. 510O(K) SUMMARY Mega'Gen Co., Ltd. 114-8, Eupchun-Ri, Jain-Myun. Gyeongsan, Gyeongbuk South Korea Phone: 82-53-857-5770, Fax: 82-53-857-5432 510(K) Summary 510O(K) SUMMARY AND
July 10, X-spine Systems, Incorporated David Kirschman, MD Chief Medical Officer 452 Alexandersville Road Miamisburg, Ohio 45342
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service July 10, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 X-spine
5101K] SUMMARY [as required by (c)]
![5101K] SUMMARY [as required by (c)] 5101K] SUMMARY [as required by (c)]](/thumbs/76/73010098.jpg) 735-10, Ancheong-dong, Gwangsan-gu, Gwangju, Korea FAX:+82-62-954-1055 http://www. inarex. co. kr IN AR E)( TEL:+82-62-952-1052 INAREX CORPORATION 5101K] SUMMARY [as required by 807.92(c)] AUG 2 7 2008
735-10, Ancheong-dong, Gwangsan-gu, Gwangju, Korea FAX:+82-62-954-1055 http://www. inarex. co. kr IN AR E)( TEL:+82-62-952-1052 INAREX CORPORATION 5101K] SUMMARY [as required by 807.92(c)] AUG 2 7 2008
K Michael Kolber Regulatory Affairs
 510(k) Number Submitter Name and Address Name: Contact: Address: Consultant, K122105 for the SM I Cardiovascular Patch November 7, 2012 Appendix 4: 510(k) Summary per (21CFRSO7.92) DEC 1 202 K122105 Michael
510(k) Number Submitter Name and Address Name: Contact: Address: Consultant, K122105 for the SM I Cardiovascular Patch November 7, 2012 Appendix 4: 510(k) Summary per (21CFRSO7.92) DEC 1 202 K122105 Michael
March 19, Dear Ms. Strohkirch:
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service March 19, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Treace
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service March 19, 2015 Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Treace
510(k) SUMMARY (Per 21 CFR ) MAY
 K130498 page 1 of 3 General Company Information 510(k) SUMMARY (Per 21 CFR 807.92) MAY 3 12013 Name: Contact: Address: Howard Schrayer Regulatory Affairs Consultant 600 Cruiser Lane Belgrade, MT 59714
K130498 page 1 of 3 General Company Information 510(k) SUMMARY (Per 21 CFR 807.92) MAY 3 12013 Name: Contact: Address: Howard Schrayer Regulatory Affairs Consultant 600 Cruiser Lane Belgrade, MT 59714
January 7, Dear Ms. Chung:
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 January 7, 2015 Jeisys
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 January 7, 2015 Jeisys
ko~ Tk (K) SUMMARY
 ko~3367-3 13. 510(K) SUMMARY 7-Tk 1 92006 Mega'Gen Co., Ltd. 114-8, Eupchun-Ri, Jain-Myun, Gyeongsan, Gyeongbuk South Korea Phone: 82-53-857-5770, Fax: 82-53-857-5432 510(K) Summary 510O(K) SUMMARY AND
ko~3367-3 13. 510(K) SUMMARY 7-Tk 1 92006 Mega'Gen Co., Ltd. 114-8, Eupchun-Ri, Jain-Myun, Gyeongsan, Gyeongbuk South Korea Phone: 82-53-857-5770, Fax: 82-53-857-5432 510(K) Summary 510O(K) SUMMARY AND
NEURONETICS. 510(k) SUMMARY NEUROSTAR TMS THERAPY SYSTEM'
 NEURONETICS 510(k) SUMMARY NEUROSTAR TMS THERAPY SYSTEM' 510(k) Owner: Neuronetics, Inc. DEC 1 6 Z008 1 Great Valley Parkway, Suite 2 Malvern, PA 19355 Phone: 610-640-4202 Fax: 610-640-4206 Company Contact:
NEURONETICS 510(k) SUMMARY NEUROSTAR TMS THERAPY SYSTEM' 510(k) Owner: Neuronetics, Inc. DEC 1 6 Z008 1 Great Valley Parkway, Suite 2 Malvern, PA 19355 Phone: 610-640-4202 Fax: 610-640-4206 Company Contact:
510(k) Summary. BioStructures, LLC. Interface Bone Void Filler
 I UC7 eacv.4df3 5 10(k) Summary DE 3~ 510(k) Summary BioStructures, LLC September 29, 2011 ADMINISTRATIVE INFORMATION Manufacturer Name: Official Contact: Representative/Consultant: BioStructures, LLC
I UC7 eacv.4df3 5 10(k) Summary DE 3~ 510(k) Summary BioStructures, LLC September 29, 2011 ADMINISTRATIVE INFORMATION Manufacturer Name: Official Contact: Representative/Consultant: BioStructures, LLC
Contact [UlS agent: Dong Guk Ha. April Lee MegaGenImplant Co., Ltd.
 5 10kW Submission Xeed PAnyffide Internal Imlant System MAR 2 7 2013 510(k) Summary This summary of 510(k) safety and effectiveness information is being submitted in accordance with requirements of 21
5 10kW Submission Xeed PAnyffide Internal Imlant System MAR 2 7 2013 510(k) Summary This summary of 510(k) safety and effectiveness information is being submitted in accordance with requirements of 21
NOV Jo 6) ' 510(k) Summary
 NOV 2 9 2004Jo 6) ' 510(k) Summary 1. Applicant Amest Corporation 303194 Espetranza Rancho Santa Margarita, CA 92688 Contact person: John Jest Amnest Corporation 30394 Espcranza Rancho Santa Margarita,
NOV 2 9 2004Jo 6) ' 510(k) Summary 1. Applicant Amest Corporation 303194 Espetranza Rancho Santa Margarita, CA 92688 Contact person: John Jest Amnest Corporation 30394 Espcranza Rancho Santa Margarita,
510(K) SUMMARY. Ventus Medical, Inc Shoreway Road, Suite 340 Belmont, CA Menlo Park, CA (650) (phone) (650) (fax)
 510(K) SUMMARY 510(k) Applicant: Contact: Ventus Medical, Inc. 1301 Shoreway Road, Suite 340 Belmont, CA 94002 Menlo Park, CA 94025 (650) 632-4199 (phone) (650) 632-4198 (fax) Cindy Domecus, R.A.C. (US
510(K) SUMMARY 510(k) Applicant: Contact: Ventus Medical, Inc. 1301 Shoreway Road, Suite 340 Belmont, CA 94002 Menlo Park, CA 94025 (650) 632-4199 (phone) (650) 632-4198 (fax) Cindy Domecus, R.A.C. (US
,,~ 30! Premarket Notification - SomnoMed MAS Flex "S"
 510(K) SUMMARY This summary of 510(k) safety and effectiveness infornation is being submitted in accordance with the requirements of 21 CFR 807.92. Submitter Information: SomnoMed Inc Date Summary Prepared:
510(K) SUMMARY This summary of 510(k) safety and effectiveness infornation is being submitted in accordance with the requirements of 21 CFR 807.92. Submitter Information: SomnoMed Inc Date Summary Prepared:
DEC (k) Summary. X-spine Systems, Inc Corporate Way #212 Centerville, OH Telephone (800) FAX (866)
 DEC 1 2 2005 Ksjq 5 1 0(k) Premarket Notification Capless' Pedicle Screw System 510(k) Summary ADMINISTRATIVE INFORMATION NManufacturcr Name: X-spine Systems, Inc. 7026 Corporate Way #212 Centerville,
DEC 1 2 2005 Ksjq 5 1 0(k) Premarket Notification Capless' Pedicle Screw System 510(k) Summary ADMINISTRATIVE INFORMATION NManufacturcr Name: X-spine Systems, Inc. 7026 Corporate Way #212 Centerville,
SECTION 2. SUMMARY AND CERTIFICATION
 SECTION 2. SUMMARY AND CERTIFICATION A. 510(K) SUMMARY JUL 29 Summary of Safety and Effectiveness In accordance with 21 CFR 807.92, the following information constitutes the Oticon Medical summary for
SECTION 2. SUMMARY AND CERTIFICATION A. 510(K) SUMMARY JUL 29 Summary of Safety and Effectiveness In accordance with 21 CFR 807.92, the following information constitutes the Oticon Medical summary for
SECTION 2. SUMMARY AND CERTIFICATIOIov
 SECTION 2. SUMMARY AND CERTIFICATIOIov 1 4 2 00 8 A. 510(K) SUMMARY.Summary of Safetv and Effctiveness In accordance with 21 CFR 807.92, the following information constitutes the Oticon Medical summary
SECTION 2. SUMMARY AND CERTIFICATIOIov 1 4 2 00 8 A. 510(K) SUMMARY.Summary of Safetv and Effctiveness In accordance with 21 CFR 807.92, the following information constitutes the Oticon Medical summary
Bacterin International Incorporated Mr. Howard L. Schrayer 600 Cruiser Lane Belgrade, Montana August 18, 2015
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Bacterin International
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 Bacterin International
510(k) SUMMARY. Bristol, PA President. PhysioFlow Q-Link. Classification Name: Impedance plethysmograph (21 CER 870.
 KC140102 Page 1 of 3 510(k) SUMMARY FB121 This 5 10(k) summary is submitted in accordance with the requirements of SMDA 1990 and 21 CER 807.87 Establishment Relistration Number: 3005467960 Address of Manufacturer:
KC140102 Page 1 of 3 510(k) SUMMARY FB121 This 5 10(k) summary is submitted in accordance with the requirements of SMDA 1990 and 21 CER 807.87 Establishment Relistration Number: 3005467960 Address of Manufacturer:
510(k) 21 Summary of Safety and Effectiveness
 510(k) 21 Summary of Safety and Effectiveness This summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirement of 21 CFR 807.92. Submitter's Identification:
510(k) 21 Summary of Safety and Effectiveness This summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirement of 21 CFR 807.92. Submitter's Identification:
Instrument, Surgical, Powered, Laser 79-GEX 21I CFR878-48
 kor~f ¼ UL 2 62004 This 510(K) Summary of safety and effectiveness for the Millennium Dental Technologies PerioLase Dental Laser System is submitted in accordance with the requirements of the SMDA 1990
kor~f ¼ UL 2 62004 This 510(K) Summary of safety and effectiveness for the Millennium Dental Technologies PerioLase Dental Laser System is submitted in accordance with the requirements of the SMDA 1990
February 15, AtriCure, Inc. Melissa Smallwood Regulatory Affairs Specialist 7555 Innovation Way Mason, Ohio 45040
 February 15, 2018 AtriCure, Inc. Melissa Smallwood Regulatory Affairs Specialist 7555 Innovation Way Mason, Ohio 45040 Re: K180137 Trade/Device Name: AtriCure cryoice cryo-ablation probe (CRYO3), AtriCure
February 15, 2018 AtriCure, Inc. Melissa Smallwood Regulatory Affairs Specialist 7555 Innovation Way Mason, Ohio 45040 Re: K180137 Trade/Device Name: AtriCure cryoice cryo-ablation probe (CRYO3), AtriCure
Matthew M. Hull, RAC Phone: (916) / Fax: (916)
 K130652 Pagel1 of3 2. 510(k) SUMMARY DC 721 Sponsor Name: Consensus Orthopedics, Inc. DC1721 1115 Windfield Way, Suite 100 El Dorado Hills, CA 95762 510(k) Contact: Matthew M. Hull, RAC Phone: (916) 355-7156/
K130652 Pagel1 of3 2. 510(k) SUMMARY DC 721 Sponsor Name: Consensus Orthopedics, Inc. DC1721 1115 Windfield Way, Suite 100 El Dorado Hills, CA 95762 510(k) Contact: Matthew M. Hull, RAC Phone: (916) 355-7156/
March 8, DxNow, Inc. Kevin Sly Senior Advisor to DxNow, Inc. 401 Professional Drive, Suite 130 Gaithersburg, Maryland
 March 8, 2018 Kevin Sly Senior Advisor to 401 Professional Drive, Suite 130 Gaithersburg, Maryland 20879-3429 Re: Trade/Device Name: ZyMot ICSI Sperm Separation Device, ZyMot Multi Sperm Separation Device
March 8, 2018 Kevin Sly Senior Advisor to 401 Professional Drive, Suite 130 Gaithersburg, Maryland 20879-3429 Re: Trade/Device Name: ZyMot ICSI Sperm Separation Device, ZyMot Multi Sperm Separation Device
Section III 510(k) Summary
 510(k) Submission Report for Contec Pocket Fetal Doppler CD Y L- Internal Report SN: A2008-005-031 Section III 510(k) Summary As Required by CFR 807.92 FEB 2 5 2009 Sponsor: Contec Medical Systems Co.,
510(k) Submission Report for Contec Pocket Fetal Doppler CD Y L- Internal Report SN: A2008-005-031 Section III 510(k) Summary As Required by CFR 807.92 FEB 2 5 2009 Sponsor: Contec Medical Systems Co.,
/tj7-0zq0 510 (k) Summary P
 /tj7-0zq0 510 (k) Summary P Date Prepared: November 28, 2012 Sponsor Information: Pulsecor Limited DEC i 2o12 Level 2, 135 Morrin Road Auckland 1072, New Zealand Sponsor Contact: Andrew Lowe Telephone:
/tj7-0zq0 510 (k) Summary P Date Prepared: November 28, 2012 Sponsor Information: Pulsecor Limited DEC i 2o12 Level 2, 135 Morrin Road Auckland 1072, New Zealand Sponsor Contact: Andrew Lowe Telephone:
February 15, AtriCure, Inc. Melissa Smallwood Regulatory Affairs Specialist 7555 Innovation Way Mason, Ohio 45040
 February 15, 2018 AtriCure, Inc. Melissa Smallwood Regulatory Affairs Specialist 7555 Innovation Way Mason, Ohio 45040 Re: K180138 Trade/Device Name: AtriCure cryoice cryo-ablation probe (CRYO2) Regulation
February 15, 2018 AtriCure, Inc. Melissa Smallwood Regulatory Affairs Specialist 7555 Innovation Way Mason, Ohio 45040 Re: K180138 Trade/Device Name: AtriCure cryoice cryo-ablation probe (CRYO2) Regulation
Dear Cecilia Ceng: U.S. Food & Drug Administration New Hampshire Avenue Doc ID# Silver Spring, MD
 November 8, 2017 Cassie Lee Vice President Guangzhou GLOMED Biological Technology Co., Ltd. Suite 306, Kecheng Mansion, No.121 Science Road, Guangzhou Science Park Guangzhou, 510663 CN Re: K170229 Trade/Device
November 8, 2017 Cassie Lee Vice President Guangzhou GLOMED Biological Technology Co., Ltd. Suite 306, Kecheng Mansion, No.121 Science Road, Guangzhou Science Park Guangzhou, 510663 CN Re: K170229 Trade/Device
TISSUELINKO., Simply Better Surgery TM
 Special 510(k): Part A TissueLink Medical, Inc. - Aquamantys Pump Generator System TISSUELINKO., Simply Better Surgery TM OCT 2 5 00 510(k) Summary of Safety and Effectiveness This summary of 510(k)-safety
Special 510(k): Part A TissueLink Medical, Inc. - Aquamantys Pump Generator System TISSUELINKO., Simply Better Surgery TM OCT 2 5 00 510(k) Summary of Safety and Effectiveness This summary of 510(k)-safety
CONSENSUS ORTHOPEDICS INC. CONSENSUS KNEE SYSTEM
 CONSENSUS ORTHOPEDICS INC. CONSENSUS KNEE SYSTEM IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR WITH THE SURGICAL TECHNIQUE.
CONSENSUS ORTHOPEDICS INC. CONSENSUS KNEE SYSTEM IMPORTANT INFORMATION FOR SURGEON: PLEASE READ PRIOR TO IMPLANTING THIS DEVICE IN A CLINICAL SETTING. THE SURGEON SHOULD BE FAMILIAR WITH THE SURGICAL TECHNIQUE.
TELEMED. November 3, Engineering Manager Dariaus ir Gireno str. 42 Vilnius, LT LITHUANIA
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 TELEMED Engineering Manager
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center WO66-G609 Silver Spring, MD 20993-0002 TELEMED Engineering Manager
Over 20 Years of Proven Clinical Success. Zimmer Natural-Knee II System
 Over 20 Years of Proven Clinical Success Zimmer Natural-Knee II System CSTi Porous Coating Structurally similar to human bone CSTi porous coating combines the excellent biocompatibility of titanium with
Over 20 Years of Proven Clinical Success Zimmer Natural-Knee II System CSTi Porous Coating Structurally similar to human bone CSTi porous coating combines the excellent biocompatibility of titanium with
510(k) Premarket Notification Database
 FDA > CDRH > 510(k) Premarket Notification Database Search http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?i... New Search Back To Search Results 510(k) Premarket Notification Database
FDA > CDRH > 510(k) Premarket Notification Database Search http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?i... New Search Back To Search Results 510(k) Premarket Notification Database
510(k) Summary As required by section (c) 89 ITH. James J. Gibson, Jr. Integrity Life Sciences Tel. (702) / Fax (702)
 K (03o1zLAF APR - 520)1 510(k) Summary As required by section 807.92(c) Device Description: Device Trade Name: Integrity Spinal Care System Model Number LCSC 2.0 Common Name: Traction Equipment Classification
K (03o1zLAF APR - 520)1 510(k) Summary As required by section 807.92(c) Device Description: Device Trade Name: Integrity Spinal Care System Model Number LCSC 2.0 Common Name: Traction Equipment Classification
Classification Name, Classification Number, Class, Classification Reference: 74BWS I DSK I DXN I
 510(k) Summary Date of Summary Preparation: February 25,2001 Manufactures Contact Person: Trade Name: Mark Rison Sr. Dir. QARA (2 10) 696-8800 FAX (2 10) 696-8800 Colin Medical Instruments Corp. 5850 Farinon
510(k) Summary Date of Summary Preparation: February 25,2001 Manufactures Contact Person: Trade Name: Mark Rison Sr. Dir. QARA (2 10) 696-8800 FAX (2 10) 696-8800 Colin Medical Instruments Corp. 5850 Farinon
Use of Standards in Substantial Equivalence Determinations
 Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Total Knee Replacement
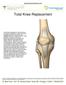 Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
Evolution. Medial-Pivot Knee System The Bi-Cruciate-Substituting Knee. Key Aspects
 Evolution Medial-Pivot Knee System The Bi-Cruciate-Substituting Knee Key Aspects MicroPort s EVOLUTION Medial-Pivot Knee System was designed to recreate the natural anatomy that is lost during a total
Evolution Medial-Pivot Knee System The Bi-Cruciate-Substituting Knee Key Aspects MicroPort s EVOLUTION Medial-Pivot Knee System was designed to recreate the natural anatomy that is lost during a total
Luminus Flex Total Knee System (Fixed Type) The Innovative Technology for Knee Replacement
 Luminus Flex Total Knee System (Fixed Type) The Innovative Technology for Knee Replacement Intro The Luminus-Flex Total Knee system takes its name from the English word "Luminous" An adjective meaning
Luminus Flex Total Knee System (Fixed Type) The Innovative Technology for Knee Replacement Intro The Luminus-Flex Total Knee system takes its name from the English word "Luminous" An adjective meaning
Surgical Technique. VISIONAIRE FastPak Instruments for the LEGION Total Knee System
 Surgical Technique VISIONAIRE FastPak Instruments for the LEGION Total Knee System VISIONAIRE FastPak for LEGION Instrument Technique* Nota Bene The technique description herein is made available to the
Surgical Technique VISIONAIRE FastPak Instruments for the LEGION Total Knee System VISIONAIRE FastPak for LEGION Instrument Technique* Nota Bene The technique description herein is made available to the
FDA 510(k) 101 The Basics
 FDA 510(k) 101 The Basics Floyd G. Larson President, PaxMed International San Diego, CA OMTEC June 17, 2010 Chicago Agenda History of 510(k) process FDA s risk based approach FDA guidance and standards
FDA 510(k) 101 The Basics Floyd G. Larson President, PaxMed International San Diego, CA OMTEC June 17, 2010 Chicago Agenda History of 510(k) process FDA s risk based approach FDA guidance and standards
Constrained Posterior Stabilized (CPS)
 Constrained Posterior Stabilized (CPS) Persona The Personalized Knee Surgical Technique Table of Contents Introduction... 2 Constraint Options Initial Knee Assessment... 3 Femoral Box Cut CPS Tibial Bearing
Constrained Posterior Stabilized (CPS) Persona The Personalized Knee Surgical Technique Table of Contents Introduction... 2 Constraint Options Initial Knee Assessment... 3 Femoral Box Cut CPS Tibial Bearing
Constrained Posterior Stabilized (CPS) Surgical Technique
 Constrained Posterior Stabilized (CPS) Surgical Technique Constrained Posterior Stabilized (CPS) Surgical Technique INTRO Introduction The Constrained Posterior Stabilized (CPS) articular surfaces can
Constrained Posterior Stabilized (CPS) Surgical Technique Constrained Posterior Stabilized (CPS) Surgical Technique INTRO Introduction The Constrained Posterior Stabilized (CPS) articular surfaces can
BRAINSWAY DEEP TMS SYSTEM
 510(K) SUMMARY JAN 0 72013 BRAINSWAY DEEP TMS SYSTEM Applicant Name: Company Name: Address: 510(k) Number K122288 Brainsway Ltd. 19 Hartumn St. (Bynet Bldg) Har Hotzvim, Jerusalem, ISRAEL 91451 Tel: +972-2-5813
510(K) SUMMARY JAN 0 72013 BRAINSWAY DEEP TMS SYSTEM Applicant Name: Company Name: Address: 510(k) Number K122288 Brainsway Ltd. 19 Hartumn St. (Bynet Bldg) Har Hotzvim, Jerusalem, ISRAEL 91451 Tel: +972-2-5813
Knee Replacement Implants
 Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Class II Special Controls Guidance Document: Intraoral Devices for Snoring and/or Obstructive Sleep Apnea; Guidance for Industry and FDA
 Class II Special Controls Guidance Document: Intraoral Devices for Snoring and/or Obstructive Sleep Apnea; Guidance for Industry and FDA Document issued on: November 12, 2002 This document supersedes the
Class II Special Controls Guidance Document: Intraoral Devices for Snoring and/or Obstructive Sleep Apnea; Guidance for Industry and FDA Document issued on: November 12, 2002 This document supersedes the
TOTAL KNEE ARTHROPLASTY (TKA)
 TOTAL KNEE ARTHROPLASTY (TKA) 1 Anatomy, Biomechanics, and Design 2 Femur Medial and lateral condyles Convex, asymmetric Medial larger than lateral 3 Tibia Tibial plateau Medial tibial condyle: concave
TOTAL KNEE ARTHROPLASTY (TKA) 1 Anatomy, Biomechanics, and Design 2 Femur Medial and lateral condyles Convex, asymmetric Medial larger than lateral 3 Tibia Tibial plateau Medial tibial condyle: concave
Encina Taper Stem. Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA
 Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Table of Contents Introduction 3 Features 4 Surgical Technique 5 Preoperative
Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Table of Contents Introduction 3 Features 4 Surgical Technique 5 Preoperative
VOLAR DISTAL RADIUS FRACTURE SOLUTIONS. Value Analysis Committee Resource Guide
 VOLAR DISTAL RADIUS FRACTURE SOLUTIONS Value Analysis Committee Resource Guide Table of Contents About Flower Orthopedics...1 The FlowerCube...2 Flower Locking Mechanism...4 FlowerBasic and FlowerAnatomic...5
VOLAR DISTAL RADIUS FRACTURE SOLUTIONS Value Analysis Committee Resource Guide Table of Contents About Flower Orthopedics...1 The FlowerCube...2 Flower Locking Mechanism...4 FlowerBasic and FlowerAnatomic...5
Patient-specific bicompart mental knee resurfacing system
 iduog2 Patient-specific bicompart mental knee resurfacing system Superior implant fit and performance require a patient-specific approach. The ConforMIS Partial Knee Resurfacing Systems use proprietary
iduog2 Patient-specific bicompart mental knee resurfacing system Superior implant fit and performance require a patient-specific approach. The ConforMIS Partial Knee Resurfacing Systems use proprietary
Consistency in U2 Knee System over Time
 U2 TM Knee System Consistency in U2 Knee System over Time Share the same femoral profile of all types allow inter-changibility Uniform AP/ML dimensions in all tibial components enhance intraoperative flexibility
U2 TM Knee System Consistency in U2 Knee System over Time Share the same femoral profile of all types allow inter-changibility Uniform AP/ML dimensions in all tibial components enhance intraoperative flexibility
Section of total knee replacement. Total Knee Replacement System. Knieendoprothesen System. Système de prothèse totale de genou
 Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Zimmer FuZion Instruments. Surgical Technique (Beta Version)
 Zimmer FuZion Surgical Technique (Beta Version) INTRO Surgical Technique Introduction Surgical goals during total knee arthroplasty (TKA) include establishment of normal leg alignment, secure implant fixation,
Zimmer FuZion Surgical Technique (Beta Version) INTRO Surgical Technique Introduction Surgical goals during total knee arthroplasty (TKA) include establishment of normal leg alignment, secure implant fixation,
Draft Guidance for Industry and FDA Staff
 Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
April 18, Dear Mr. Lei Chen:
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 Beijing Choice Electronic
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 10903 New Hampshire Avenue Document Control Center - WO66-G609 Silver Spring, MD 20993-0002 Beijing Choice Electronic
ANATOMIC Fixed Bearing Total Knee System Posterior Stabilised Version Cemented or Cementless Options
 ANATOMIC Fixed Bearing Total Knee System Posterior Stabilised Version Cemented or Cementless Options ANATOMIC l Anatomic femoral component design based on a study of 420 knees digitised using the AMPLIVISION
ANATOMIC Fixed Bearing Total Knee System Posterior Stabilised Version Cemented or Cementless Options ANATOMIC l Anatomic femoral component design based on a study of 420 knees digitised using the AMPLIVISION
Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs 425 Privet Road Horsham, PA 19044
 DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
Surgical Technique. Poly UHMWPE. Bio Hyaluronic Acid. Advancing Materials. Advancing Outcomes.
 Surgical Technique Bio Hyaluronic Acid Poly UHMWPE Advancing Materials. Advancing Outcomes. Surgical 2 Assessment 1. Proper implant sizing is determined by placing the appropriate sized trial over the
Surgical Technique Bio Hyaluronic Acid Poly UHMWPE Advancing Materials. Advancing Outcomes. Surgical 2 Assessment 1. Proper implant sizing is determined by placing the appropriate sized trial over the
NexGen Legacy LPS-Flex Knee. Brochure
 NexGen Legacy LPS-Flex Knee Brochure What postoperative range of motion can your TKA patients expect? For patients with the ability and desire to perform For patients with the ability high-flexion activities,
NexGen Legacy LPS-Flex Knee Brochure What postoperative range of motion can your TKA patients expect? For patients with the ability and desire to perform For patients with the ability high-flexion activities,
Partial Knee Replacement
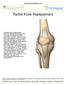 Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
U2 PSA. Revision Knee. Surgical Protocol
 U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
U2 PSA TM Revision Knee Surgical Protocol Table of Contents 1 Component Removal... 1 2 Tibial Preparation... 1 2.1 Tibial Canal Preparation... 1 2.2 Proximal Tibial Resection... 2 2.3 Non Offset Tibial
HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX
 HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX HIP IMPLANTS TOTAL HIPS Ultra High Demand (metal on metal) Femur, ingrowth Femoral head, metal Acetabular shell, ingrowth Acetabular liner,
HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX HIP IMPLANTS TOTAL HIPS Ultra High Demand (metal on metal) Femur, ingrowth Femoral head, metal Acetabular shell, ingrowth Acetabular liner,
JOINT RULER. Surgical Technique For Knee Joint JRReplacement
 JR JOINT RULER Surgical Technique For Knee Joint JRReplacement INTRODUCTION The Joint Ruler * is designed to help reduce the incidence of flexion, extension, and patellofemoral joint problems by allowing
JR JOINT RULER Surgical Technique For Knee Joint JRReplacement INTRODUCTION The Joint Ruler * is designed to help reduce the incidence of flexion, extension, and patellofemoral joint problems by allowing
Surgical Technique. VISIONAIRE Disposable Instruments for the LEGION Total Knee System
 Surgical Technique VISIONAIRE Disposable Instruments for the LEGION Total Knee System VISIONAIRE and LEGION Disposable instrument technique* Note: All disposable instruments are interchangeable with the
Surgical Technique VISIONAIRE Disposable Instruments for the LEGION Total Knee System VISIONAIRE and LEGION Disposable instrument technique* Note: All disposable instruments are interchangeable with the
Biomechanics of. Knee Replacement. Mujda Hakime, Paul Malcolm
 Biomechanics of Knee Replacement Mujda Hakime, Paul Malcolm 1 Table of contents Knee Anatomy Movements of the Knee Knee conditions leading to knee replacement Materials Alignment and Joint Loading Knee
Biomechanics of Knee Replacement Mujda Hakime, Paul Malcolm 1 Table of contents Knee Anatomy Movements of the Knee Knee conditions leading to knee replacement Materials Alignment and Joint Loading Knee
Preparing a US FDA Medical Device 510(K) Submission
 Preparing a US FDA Medical Device 510(K) Submission If you want to introduce your medical device to the US market, you need to obtain clearance from the FDA. This clearance is obtained from the FDA via
Preparing a US FDA Medical Device 510(K) Submission If you want to introduce your medical device to the US market, you need to obtain clearance from the FDA. This clearance is obtained from the FDA via
PARTIAL KNEE REPLACEMENT
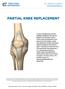 PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Enhanced Stability Constrained Liners. Design Rationale Surgical Technique
 Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
ACETABULAR CUP SURGICAL TECHNIQUE
 ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
ACETABULAR CUP SURGICAL TECHNIQUE ACETABULAR CUP DEVICE INDICATIONS FOR USE The ICONACY I-Hip total hip replacement is indicated for the following conditions: 1. A severely painful and/or disabled hip
Triathlon Tritanium Knee System
 Triathlon Tritanium Knee System Table of Contents Cementless TKA... 4 Why Cementless TKA?... 4 Cementless TKA - Clinical History... 4 Published RSA Results Comparing Fixation Methods... 5 Biologic Fixation...
Triathlon Tritanium Knee System Table of Contents Cementless TKA... 4 Why Cementless TKA?... 4 Cementless TKA - Clinical History... 4 Published RSA Results Comparing Fixation Methods... 5 Biologic Fixation...
MIS Cemented Tibial Component
 MIS Cemented Tibial Component NexGen Complete Knee Solution Surgical Technique Table of Contents Surgical Exposure... 2 Finish the Tibia... 2 Position Based on Anatomic Landmarks... 3 Lateral Posterior
MIS Cemented Tibial Component NexGen Complete Knee Solution Surgical Technique Table of Contents Surgical Exposure... 2 Finish the Tibia... 2 Position Based on Anatomic Landmarks... 3 Lateral Posterior
Sulzer Orthopedics Joint & Fracture Care
 Sulzer Orthopedics Joint & Fracture Care Natural-Knee Family Anatomic design for superior clinical results. Natural-Knee Natural-Knee Family: A solution for every knee. Asymmetric design increases coverage
Sulzer Orthopedics Joint & Fracture Care Natural-Knee Family Anatomic design for superior clinical results. Natural-Knee Natural-Knee Family: A solution for every knee. Asymmetric design increases coverage
Product Portfolio. Consensus Knee Systems. Consensus Hip Systems
 1115 Windfield Way, Suite 100 El Dorado Hills, Ca 95762 916-355-7100, 916-355-7190 info@consensusortho.com consensusortho.com Product Portfolio Consensus Knee Systems Consensus Hip Systems Product Portfolio
1115 Windfield Way, Suite 100 El Dorado Hills, Ca 95762 916-355-7100, 916-355-7190 info@consensusortho.com consensusortho.com Product Portfolio Consensus Knee Systems Consensus Hip Systems Product Portfolio
Vanguard Complete Knee System
 Complete Knee System Design Rationale Knees Hips Extremities Cement and Accessories PMI Technology 1 One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized
Complete Knee System Design Rationale Knees Hips Extremities Cement and Accessories PMI Technology 1 One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized
September 28, Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852
 September 28, 2011 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Docket No. : Center for Devices and Radiological Health 510(k)
September 28, 2011 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Re: Docket No. : Center for Devices and Radiological Health 510(k)
Zimmer NexGen MIS Tibial Component. Cemented Surgical Technique IMAGE TO COME
 Zimmer NexGen MIS Tibial Component Cemented Surgical Technique IMAGE TO COME Zimmer NexGen MIS Tibial Component Cemented Surgical Technique 1 Zimmer NexGen MIS Tibial Component Cemented Surgical Technique
Zimmer NexGen MIS Tibial Component Cemented Surgical Technique IMAGE TO COME Zimmer NexGen MIS Tibial Component Cemented Surgical Technique 1 Zimmer NexGen MIS Tibial Component Cemented Surgical Technique
ClassiQ. Scorpio. Anterior Referencing Surgical Protocol. Anterior Referencing. For use with Scorpio ClassiQ Instrument System
 Scorpio ClassiQ Anterior Referencing Surgical Protocol For use with Scorpio ClassiQ Instrument System For use with Scorpio ClassiQ Single Radius Total Knee System AR Anterior Referencing This document
Scorpio ClassiQ Anterior Referencing Surgical Protocol For use with Scorpio ClassiQ Instrument System For use with Scorpio ClassiQ Single Radius Total Knee System AR Anterior Referencing This document
CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR
 CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR Evolution of TKR In 1860, Verneuil proposed interposition arthroplasty, involving the insertion of soft tissue
CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR Evolution of TKR In 1860, Verneuil proposed interposition arthroplasty, involving the insertion of soft tissue
Zimmer Segmental System
 Zimmer Segmental System Simple solutions for solving complex salvage cases A Step Forward The Zimmer Segmental System is designed to address patients with severe bone loss associated with disease, trauma
Zimmer Segmental System Simple solutions for solving complex salvage cases A Step Forward The Zimmer Segmental System is designed to address patients with severe bone loss associated with disease, trauma
Effects of Posteromedial Vertical Capsulotomy on the Medial Extension Gap in Cruciate-retaining Total Knee Arthroplasty
 Effects of Posteromedial Vertical Capsulotomy on the Medial Extension Gap in Cruciate-retaining Total Knee Arthroplasty Ryutaku Kaneyama, M.D., Hideaki Shiratsuchi, Kazuhiro Oinuma, MD, Yoko Miura, MD,
Effects of Posteromedial Vertical Capsulotomy on the Medial Extension Gap in Cruciate-retaining Total Knee Arthroplasty Ryutaku Kaneyama, M.D., Hideaki Shiratsuchi, Kazuhiro Oinuma, MD, Yoko Miura, MD,
Saiph Knee System. Technical Dossier
 Saiph Knee System Technical Dossier Developed in collaboration with an international surgical team Professor Justin P Cobb Chair in Orthopaedic Surgery Imperial College Hospital, London Professor Fares
Saiph Knee System Technical Dossier Developed in collaboration with an international surgical team Professor Justin P Cobb Chair in Orthopaedic Surgery Imperial College Hospital, London Professor Fares
Overview of the Legal Framework for Medical Device Regulation in the United States
 1 Overview of the Legal Framework for Medical Device Regulation in the United States Ellen J. Flannery This chapter provides an overview of the legal framework for medical device regulation in the United
1 Overview of the Legal Framework for Medical Device Regulation in the United States Ellen J. Flannery This chapter provides an overview of the legal framework for medical device regulation in the United
Responsibilities of Laser Light Show Projector Manufacturers, Dealers, and Distributors; Final Guidance for Industry and FDA.
 Responsibilities of Laser Light Show Projector Manufacturers, Dealers, and Distributors; Final Guidance for Industry and FDA (Laser Notice 51) Document issued on: May 27, 2001 U.S. Department of Health
Responsibilities of Laser Light Show Projector Manufacturers, Dealers, and Distributors; Final Guidance for Industry and FDA (Laser Notice 51) Document issued on: May 27, 2001 U.S. Department of Health
Zimmer NexGen Trabecular Metal Tibial Tray
 Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique Give Bone A Solid Hold Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique
Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique Give Bone A Solid Hold Zimmer NexGen Trabecular Metal Tibial Tray Surgical Technique
ANDA Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY December 3, 2015
 DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
ConforMIS, Inc. 28 Crosby Drive Bedford, MA Phone: Fax:
 ConforMIS, Inc. 28 Crosby Drive Bedford, MA 01730 Phone: 781.345.9001 Fax: 781.345.0147 www.conformis.com 0086 Authorized Representative: Medical Device Safety Service, GMBH Schiffgraben 41, 30175 Hannover,
ConforMIS, Inc. 28 Crosby Drive Bedford, MA 01730 Phone: 781.345.9001 Fax: 781.345.0147 www.conformis.com 0086 Authorized Representative: Medical Device Safety Service, GMBH Schiffgraben 41, 30175 Hannover,
ANATOMIC. Navigated Surgical Technique 4 in 1 TO.G.GB.016/1.0
 ANATOMIC Navigated Surgical Technique 4 in 1 TO.G.GB.016/1.0 SCREEN LAYOUT Take screenshot Surgical step Dynamic navigation zone Information area and buttons 2 SCREEN LAYOUT Indicates action when yellow
ANATOMIC Navigated Surgical Technique 4 in 1 TO.G.GB.016/1.0 SCREEN LAYOUT Take screenshot Surgical step Dynamic navigation zone Information area and buttons 2 SCREEN LAYOUT Indicates action when yellow
Persona. The Personalized Knee. Trabecular Metal Tibia. Surgical Technique
 Persona The Personalized Knee Trabecular Metal Tibia Surgical Technique Table of Contents Resect the Tibia... 4 Size and Finish the Tibia... 4 Trial Fit... 6 Component Implantation... 7 Inserter/Implant
Persona The Personalized Knee Trabecular Metal Tibia Surgical Technique Table of Contents Resect the Tibia... 4 Size and Finish the Tibia... 4 Trial Fit... 6 Component Implantation... 7 Inserter/Implant
Correlation between Knee Kinematics and Patello-femoral Contact Pressure in Total Knee Arthroplasty
 Correlation between Knee Kinematics and Patello-femoral Contact Pressure in Total Knee Arthroplasty Takuya Konno, MD 1, Tomohiro Onodera, MD, PhD 1, Yasuhiko Kasahara, MD, PhD 1, Daisuke Takahashi 1, Norimasa
Correlation between Knee Kinematics and Patello-femoral Contact Pressure in Total Knee Arthroplasty Takuya Konno, MD 1, Tomohiro Onodera, MD, PhD 1, Yasuhiko Kasahara, MD, PhD 1, Daisuke Takahashi 1, Norimasa
Mako Partial Knee Medial bicompartmental
 Mako Partial Knee Medial bicompartmental Surgical reference guide Mako Robotic-Arm Assisted Surgery Table of contents Implant compatibility.... 3 Pre-operative planning.... 4 Intra-operative planning....
Mako Partial Knee Medial bicompartmental Surgical reference guide Mako Robotic-Arm Assisted Surgery Table of contents Implant compatibility.... 3 Pre-operative planning.... 4 Intra-operative planning....
