Ohio 8401 Chagrin Road, Suite 18 Chagrin Falls
|
|
|
- Brandon Dickerson
- 6 years ago
- Views:
Transcription
1 For Year ended June 15, 2009 State Address of principal executive offices City Zip Code Telephone number, including area code Ohio 8401 Chagrin Road, Suite 18 Chagrin Falls
2
3 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT: ORTHOWORLD Inc. All rights reserved. This copyrighted document may not be reproduced in any form by any means in part or in whole without the written permission of ORTHOWORLD Inc Chagrin Road, Suite 18, Chagrin Falls, OH T: F: W: orthoworld.com
4
5 ORTHOWORLD Inc. is the only publisher in the world solely focused on the global orthopaedic market. Its singular mission is helping orthopaedic companies and individuals achieve their growth directives. The company was founded in 1992 by industry executives who realized that the orthopaedic industry had nowhere to turn for objective, accurate, strategically-oriented assistance. Since then it has specialized in products and services that allow companies to more aggressively expand their orthopaedic businesses. ORTHOWORLD s product offerings are coveted for the immediate, usable knowledge their succinct and contemporary industry coverage produces. Professionals and businesses improve their positions by getting really smart, really fast. The orthopaedic portal, houses and delivers many of the company s product offerings in addition to providing an array of helpful information assets in its ongoing mission to help industry participants be better at what they do.
6
7 TABLE OF CONTENTS OVERVIEW OF THE MARKET... 1 JOINT REPLACEMENT... 5 FRACTURE REPAIR Vertebroplasty/Kyphoplasty Bone Growth Stimulation ARTHROSCOPY AND SOFT TISSUE REPAIR SPINAL IMPLANTS AND INSTRUMENTATION Disc Replacement Facet Replacement Nucleus Replacement Dynamic Stabilization COMPUTER ASSISTED SURGERY AND ROBOTICS SYSTEMS ORTHOBIOLOGICS Biologics for Pain Relief and Adhesion Prevention SUPPLIERS SUMMARY THE COMPETITIVE ORTHOPAEDIC ENVIRONMENT APPENDICES INDEX OF COMPANIES/ENTITIES
8 LIST OF EXHIBITS EXHIBIT 1 MUSCULOSKELETAL DIAGNOSES DELINEATED BY AGE WORLDWIDE ORTHOPAEDIC PRODUCT SALES: BY MARKET SEGMENT AND GEOGRAPHIC REGION ($BILLIONS) POPULATION (MM) AND GROWTH BY GEOGRAPHIC REGION: 2005 TO ARTHRITIS AND ITS HEALTH IMPACT GLOBAL JOINT REPLACEMENT SALES IN 2008: REVENUES BY SEGMENT ($BILLIONS) THE WORLD S POPULATION BY AGE GROUP: 2005 AND GLOBAL FRACTURE REPAIR SALES IN 2008: REVENUES BY SEGMENT ($BILLIONS) OSTEOPOROSIS AND ITS HEALTH IMPACT MOTION PRESERVATION LANDSCAPE: ARTIFICIAL DISCS MOTION PRESERVATION LANDSCAPE: FACET ARTHROPLASTY MOTION PRESERVATION LANDSCAPE: NUCLEUS REPLACEMENT MOTION PRESERVATION LANDSCAPE: OTHER TECHNOLOGIES MOTION PRESERVATION LANDSCAPE: DYNAMIC STABILIZATION THE 2008 WORLDWIDE ORTHOPAEDIC MARKET: SALES FOR THE TOP TEN COMPANIES AND ALL OTHERS BY MARKET SEGMENT ($MILLIONS) ORTHOPAEDIC COMPANIES WITH 2008 SALES >$5MM: ESTIMATED SALES AND BREADTH/DEPTH OF PRODUCT LINE...150
9 APPENDICES APPENDIX A: RECONSTRUCTIVE DEVICE COMPANIES APPENDIX B: FRACTURE REPAIR COMPANIES APPENDIX C: ARTHROSCOPY/SOFT TISSUE/SPORTSMED COMPANIES APPENDIX D: SPINAL IMPLANTS/INSTRUMENTATION COMPANIES APPENDIX E: BIOLOGICS & CEMENT COMPANIES
10 This page intentionally blank.
11 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 1 Orthopaedics (variation of orthopedics) ôr-thə-'pē-diks circa 1853 a branch of medicine concerned with the correction or prevention of skeletal deformities, disorders, or injuries French orthopédique, from orthopédie orthopedics, from orth- + Greek paid-, pais child (Source: Merriam-Webster, Incorporated) OVERVIEW OF THE MARKET Orthopaedics relates to the treatment of musculoskeletal disorders, injuries, diseases and deformities arthritis, osteoporosis, fractures, back pain, scoliosis, soft tissue disorders, etc. According to the World Bank Group s 2006 assessment of disease burdens worldwide, the number of people suffering from musculoskeletal conditions increased 25 percent over the past ten years, and musculoskeletal conditions now account for two percent of the global disease burden. Estimates place the prevalence of physical disabilities caused by musculoskeletal conditions at four to five percent of the adult population. Musculoskeletal conditions are the most common cause of chronic disability, the most common medical cause of long-term absence, the second most common reason for consulting a doctor and, in the U.S., the greatest cause of lost work days and medical bed days. Chronic musculoskeletal pain reportedly affects more than 100 million Europeans and one in four people in other parts of the world. In the U.S., for instance, nearly one in two persons aged 18 or older (approximately 108 million people) reported musculoskeletal conditions, with low back pain, chronic joint pain and arthritis being the most common. In Japan, some 41 percent of adults might suffer from musculoskeletal pain, most commonly in the low back. Of those with musculoskeletal pain in Japan, nearly nine percent encounter interference with daily activities due to musculoskeletal pain. The
12 2 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT prevalence of rheumatic complaints in China ranges from 12 to 46 percent of adults, depending on locality. In addition, from five to 20 percent of the Chinese population suffers from OA, primarily in spine and knee. Worldwide, musculoskeletal conditions remain among the most costly illnesses to treat. In Australia, Canada, France, the U.S. and the U.K., the annual cost of treating musculoskeletal conditions is estimated to be between one and three percent of these countries gross national product. According to Bone and Joint Decade, the annual estimated direct and indirect cost associated with persons with a musculoskeletal disease (and treated for it only) is $267.2 billion in the U.S. alone. In Germany, orthopaedic disorders run up costs of 25 billion annually, making them the largest single contributors to lost productivity in that country, while in Sweden, musculoskeletal conditions account for 23 percent of the total cost of all illness. From arthritis to osteoporosis and fractures to dislocations, musculoskeletal conditions and diseases know no age bounds. Those under the age of 18 are most likely to incur fractures and sprains, while the elderly (mostly women over the age of 50) are most likely to suffer from osteoporosis. Furthermore, 65 percent of arthritis sufferers are under the age of 65, and the vast majority of people over the age of 30 will have back problems at some point in their lives. Exhibit 1 illustrates the diversity of the patient population presenting with musculoskeletal problems. EXHIBIT 1 MUSCULOSKELETAL DIAGNOSES DELINEATED BY AGE < Arthropathies and related disorders 9% 29% 62% Dorsopathies 21% 38% 41% Rheumatism, excluding the back 21% 34% 45% Osteopathies, chondropathies and acquired deformities 23% 33% 44% Osteoporosis 6% 18% 77% Fractures 33% 22% 45% Dislocations 45% 29% 27% Sprains/strains 34% 28% 37% (Source: HCUPnet.ahrq.gov, 2006)
13 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 3 A variety of interventions are used to treat the more than 150 diseases and conditions within the musculoskeletal realm. Major product categories within orthopaedics include joint replacement, fracture repair/fixation, arthroscopy and soft tissue repair and spinal fusion and repair. Treatments within these segments may also be supplemented with orthobiologics, computer-assisted surgery, bone growth stimulation, viscoelastic substances and/or bone cements. In 2008, revenues generated by sales of orthopaedic products worldwide neared $36 billion, an increase of just under ten percent over 2007 global revenues. Exhibit 2 summarizes the global orthopaedic market and growth from EXHIBIT WORLDWIDE ORTHOPAEDIC PRODUCT SALES: BY MARKET SEGMENT AND GEOGRAPHIC REGION ($BILLIONS) Product Segment U.S. Ex-U.S. Total vs Reconstructive Devices $6.7 $6.0 $ % Fracture Repair $2.6 $2.3 $ % Arthroscopy/Soft Tissue Repair $1.9 $1.3 $ % Spinal Implants/Instrumentation $4.6 $1.9 $ % Orthobiologics $2.7 $1.0 $ % Other Products $3.1 $1.7 $ % Total Market $21.7 $14.0 $ % Change vs % 10.6% 9.9% (Note: numbers may not add up due to rounding) Products included in each segment: Reconstructive Devices: hip, knee, shoulder, elbow, wrist, ankle and digit implants Fracture Fixation: internal (plates, screws, intramedullary nails, pins, wires) and external fixation products (includes craniomaxillofacial fixation) Spinal Implants/Instrumentation: pedicle screws, plates, rods, hooks, screws, artificial discs, motion preserving devices, and discectomy and vertebroplasty/kyphoplasty products Arthroscopy/Soft Tissue Repair: scopes, cameras, instruments, soft tissue implants and repair kits Orthobiologics: bone graft substitutes, allograft distribution/processing, autogenous bone and soft tissue replacement products, growth factors and viscoelastics Other Products: power equipment (large and small bone and high speed), casting materials, soft goods, bracing systems, bone growth stimulators, cement and cement mixing/delivery systems, infection control equipment, pulsed lavage/irrigation systems, image-guided surgery systems, etc. (Source: ORTHOWORLD Inc.)
14 4 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT In 2008, the U.S., Europe and Japan accounted for more than 80 percent of the global orthopaedic marketplace; however, these geographic regions are peopled with less than 20 percent of the world s population. This disparity illustrates the orthopaedic opportunities that exist outside the three major orthopaedic markets. That is, most of the world s people live in other areas and will need musculoskeletal care throughout their lives. At this time, however, many do not have access to the care they may need, including orthopaedic intervention. As infrastructures improve in developing nations and standards of living rise along with them, the majority of the world s people will gain access to orthopaedic technologies, with the end result of solid, healthy growth in orthopaedic procedures into the future. Exhibit 3 provides estimates of population growth by region from 2005 to EXHIBIT 3 POPULATION (MM) AND GROWTH BY GEOGRAPHIC REGION: 2005 TO 2020 Average Population (MM) Annual Continent Growth Africa 896 1, % North America % South America % Asia 3,911 4, % Europe % Oceania % Total World Population 6,451 7, % (Source: U.S. Bureau of the Census)
15 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 5 JOINT REPLACEMENT Through joint replacement, metal (titanium, cobalt chrome and stainless steel alloys) or ceramic and plastic (ultra high molecular weight polyethylene or UHMWPe) devices replace diseased or damaged bone and cartilage, restoring alignment and function. Fixation of the implants into the bone can be accomplished either with bone cement (typically polymethylmethacrylate or PMMA), which serves as a grout to fix implants into place, or through cementless means, which typically involve a roughened texture or coating. Bone either adheres to the implant coating or incorporates into the surface of the implant. More than 2.7 million joint replacement procedures took place worldwide in 2008 more than 1.3 million hip, 1.1 million knee and nearly 90,000 shoulder replacements. U.S. volumes of joint replacement procedures totaled 48 percent of the number performed, with Western Europe accounting for 30 percent and Japan an additional seven percent. People suffering from arthritis pain remain the primary patient population undergoing joint replacement procedures. Worldwide, the number of arthritis sufferers approximates 355 million people, more than 151 million with osteoarthritis (OA) and 24 million with rheumatoid arthritis (RA). One in five adults in the U.S., nearly one in five Australians, 20 percent of those in the U.K., 16 percent of Canadians over 15, five percent of the Japanese population and an estimated 100 million Chinese suffer with arthritis. OA remains the predominant diagnosis leading to hip, knee and shoulder replacement, with RA more often appearing as an underlying diagnosis for replacement of digits. Most people develop OA after the age of 45, but it can occur at any age, with men and women equally likely to suffer with the disease. OA is the sixth leading cause of years lost to disability worldwide and 80 percent of people with OA report some form of limitation in movement or activities, with 25 percent noting that they cannot perform their major daily activities of life. Knee OA has been found to be as disabling as any cardiovascular disease. For those with RA, more than half find themselves unable to work at all within ten years of onset of the disease. RA affects women three times more often than men. Medical care of arthritis and joint pain in the U.S. is estimated to cost $282 billion annually. It is the leading cause of disability; a more frequent cause of activity limitation than heart disease, cancer or diabetes; the second most frequently reported chronic condition and the third leading cause of work
16 6 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT limitation there. It is also Australia s major cause of disability and pain and accounts for more disability among the elderly in Europe than any other disease. More people in Europe are affected by arthritis than any other chronic medical condition. Exhibit 4 summarizes select arthritis statistics. EXHIBIT 4 ARTHRITIS AND ITS HEALTH IMPACT People with Arthritis Australia 3.85MM (18.5% of population) U.K. 1 in 5 adults; 8.5MM with OA, 387,000 people with RA Canada ~4MM China 100MM Europe >100MM (3MM with RA) Japan 6MM (5% of the population) South Africa 1 in 7 people Shenzhen (South China) 22% of residents over 16 U.S. 46MM (1 in 5 adults); to rise to 67MM by MM with OA and 2MM with RA Costs of Arthritis to the Economy Australia $23.9BB Canada $4.4BB U.K. 5.5BB U.S. $281.5BB In 2008, global sales of joint replacement products (hips, knees, shoulders, elbows, wrists, digits) exceeded $12.7 billion, an increase of just under ten percent over sales generated in Knees comprised the largest subsegment of the joint replacement market, as illustrated in Exhibit 5.
17 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 7 EXHIBIT 5 GLOBAL JOINT REPLACEMENT SALES IN 2008: REVENUES BY SEGMENT ($BILLIONS) Shoulders, $0.5 Other Joints, $0.3 Hips, $5.4 Knees, $6.5 Total Market $12.7BB (Source: ORTHOWORLD Inc.) Geographically, sales in the U.S. accounted for 53 percent of global joint replacement revenues. The world s seven largest joint replacement companies (and the only ones with global joint replacement sales in excess of $200 million) Zimmer, Johnson & Johnson, Stryker, Smith & Nephew, Biomet, Wright Medical and Aesculap generated 91 percent of hip, knee, shoulder and other joint product sales in Other companies that sell joint replacement products number more than 100 worldwide. Many focus predominantly on particular geographic areas, as exemplified by Japan Medical Materials and Japan MDM in Japan; United Orthopedic and Tianjin Taishan Medical in Taiwan and China, respectively; Fournitures Hospitalieres (FH), Intelligent Orthopaedics (IO), Joint Replacement Instrumentation (JRI), Lima-Lto, Mathys and Waldemar Link predominantly in Europe; Baumer, Implantes Fico and Ortosintese in South America; Protetim in Eastern Europe; Roth Medical in South Africa; Sushrut and Uma Surgicals in India; etc. Other companies have built respectable joint replacement franchises by focusing on specific types of products such as Link and Stanmore in salvage/revision implants and Symbios in customs, the latter manufactured for individual patients based on preoperative CT scans and x-rays. A list of companies with reconstructive device franchises can be found in Appendix A at the end of this report.
18 8 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT Within the hip and knee markets, the largest five companies globally have controlled more than 86 percent of total revenues for the past ten years (based on pro forma sales) with shares shifting one to the other, typically with the introduction of novel products that command higher prices and attract competitive surgeons (e.g. metal-on-metal hips in the U.S.) or with the filling of a product matrix to maintain or regain market position (e.g. porous metals from Biomet, Smith & Nephew and Stryker; enhanced poly from Exactech). In 2008, however, additional share shift occurred due to product recalls by Stryker and Zimmer and some surgeon attrition from Zimmer. In late 2007, the top five companies in the joint replacement market avoided prosecution but gained oversight from the Department of Justice (DOJ) relative to the companies financial consulting or professional service agreements with orthopaedic surgeons relating to various joint replacement/ reconstructive devices. Biomet, Johnson & Johnson s DePuy Orthopaedics, Smith & Nephew and Zimmer paid fines and entered into 18-month Deferred Prosecution Agreements (DPA) with the DOJ, and Stryker paid no fine, but entered into an 18-month Non-Prosecution Agreement (NPA) with the DOJ. Under the DPAs, the four companies had to substantially rewrite their compliance programs and retain a federally-selected Monitor to oversee the companies compliance activities related to the DPAs. In addition, companies were required to publish lists of their consultants and the remuneration to the consultants on their corporate websites. Additionally, day-to-day operations related to interactions with orthopaedic surgeons with consulting or professional services agreements were significantly controlled. In late 2007, Exactech and Wright Medical also received subpoenas from the DOJ, requesting similar information related to consulting or professional service agreements with orthopaedic surgeons, although substantially less information related to these two companies activity related to the DOJ requests has been available. In March 2009, the original five companies Deferred Prosecution and Non-Prosecution Agreements expired without prosecution, but the companies were required to put Corporate Integrity Agreements (CIAs) in place governing behavior related to each company s relationships with consulting surgeons going forward. The investigations with Exactech and Wright are still ongoing.
19 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 9 Further, in January 2009, the 111th U.S. Congress reintroduced the Physician Payments Sunshine Act, a bill to require medical device and biologics manufacturers as of 2011 to report online any transfer of value greater than $100 given to doctors in each preceding year, which, if passed, would add significant transparency to these relationships, but also would add significant regulatory reporting to medical device manufacturers. As a result of DOJ scrutiny and calls for increased transparency, the ways that companies and surgeons interact in the U.S. will be significantly more guarded in the future. While changes in primary total hip and knee replacements were primarily incremental technological adjustments, Zimmer has differentiated its line by marketing products designed to address gender differences among arthroplasty patients. For instance, the company s Gender Solutions Knee is specifically designed to address the unique anatomy of a woman s knee. The product continues to help drive growth. In 2008, Zimmer extended the line with the full release of the Gender Solutions Natural Knee Flex and further expanded its Gender Solutions platform to include two hip systems: the Gender Solutions M/L Taper Stem with Kinectiv Technology, a system of modular stem and neck components, and the Gender Solutions VerSys Epoch Hip, a gender-specific stem made from medical grade PEEK. Both products have been released to the market. Furthering patient-specific product development, Zimmer recently announced that it is examining the particular demands of the obese patient, and is working to develop products specifically targeted to this segment of the patient population. The company also expects the revision segment of the market to one day exceed the market for primary joint replacements, and has identified the revision segment as one with growth potential for the company. The success of a gender-specific joint replacement system has not gone unnoticed by other implant makers. Several have developed products or requirements for this and other subsegments of the knee market, or have begun to highlight those (already existing) features of their joint replacement products that they believe are gender beneficial. Others, such as DJO and Consensus Orthopedics (formerly Hayes Medical), claim that gender-related differences are not as crucial as size matching, which is already addressed in their component designs. Smith & Nephew introduced gender-specific product requirements for its major knee product lines claims to have the largest breadth of knee products cleared for gender specific attributes, with an
20 10 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT anatomic tibial baseplate for both right and left knees in an asymmetric design that allows for an accurate fit of the components to the proximal tibia for both male and female patients. In early 2009, Smith & Nephew launched its VISIONAIRE Patient Matched instrumentation system that uses MRI and x-rays to create custom instrumentation for use with the company s knee implants. While not gender- or patient-specific, DePuy s Sigma line was expanded to include the High Performance Partial Knee and the Sigma CR150 High-Flex Knee, as well as TruMatch Personalized Solutions for total knee replacement, joining the chorus of companies offering customized total knee instruments. Also addressing different subsegments of the knee market are Wright Medical s Advance Stature femoral components with reduced width and contoured anterior flange to address the needs of patients with a narrower knee anatomy or smaller skeletal frame. Wright expanded the Stature franchise in early 2009 with a variety of reconstructive hip products featuring the ProFemur modular necks. And, as has been the case with many orthopaedic companies marketing total joints, Wright launched the PROPHECY Pre-Operative Navigation Guides, instrumentation designed to work with MRI or CT scans to provide more accurate implant positioning. Other companies have begun to market customized 3D instrumentation developed from MRIs, sourced by OtisMed. OtisMed has developed the TRIOS, a custom disposable alignment cutting guide tailored to the anatomy of individual knee replacement surgery patients. Taking preoperative CT or MRI scans, OtisMed applies its proprietary preoperative planning software and rapid manufacturing technology to create a template and cutting jig that accurately fit the unique anatomy of each individual patient. The company markets its own total knee under the brand name OtisKnee. In an early report (three month follow up of 48 patients), the OtisMed OtisKnee was deemed to be properly aligned and there were no adverse events. The OtisMed instruments are also currently optimized for use with knee implants from Biomet and Stryker and employ MRI and 3D images to create a ShapeMatched implant image for a custom fit total knee replacement. Although most hip and knee replacement procedures today are performed on people over the age of 65, over the past few years, in the U.S. at least, a trend has emerged with more younger patients undergoing joint replacement procedures. In 1997, people over the age of 65 comprised 66 percent of primary hip and knee replacement patients in the U.S. By 2006, they accounted for 54 percent. A
21 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 11 recent study projected that by 2030, the proportion of patients between the age of 45 and 54 that need knee replacement will increase 17 times from just under 60,000 to nearly one million. In Canada, a shift to a younger population for joint replacement has also occurred. Over the last decade there, the rate of knee replacements in those aged 45 to 54 tripled in males and more than tripled in females. Hip replacement procedures in this age group rose 68 percent in males and 52 percent in females during the same timeframe. Younger patients are undergoing joint replacement more frequently, due in large part to the development of technologies and procedures that are more amenable and applicable to their activity levels and life expectancies. In the earlier days of joint replacement, orthopaedic surgeons delayed the procedure in their younger patients due to concerns with the longevity of certain implant components, in particular, polyethylene. A primary constituent of hip and knee constructs, UHMWPe has been shown to wear. Its wear particles have been implicated in the creation of osteolysis or bone death, which subsequently contributes to implant failure. While surgeons outside the U.S have long had at their disposal alternate bearing materials in hip and knee replacement, only within the last decade have surgeons in the U.S. witnessed the commercialization of non-polyethylene bearing materials with primary application in the treatment of a more active, often younger population. Of note, in Canada, alternate bearing materials account for the vast majority of hip procedures, with metal-onmetal penetration at six percent, CoC at 14 percent and enhanced/crosslinked polyethylene at 68 percent of metal/poly bearing combinations. Biomet, DePuy, DJO, Smith & Nephew, Wright Medical and Zimmer all market MoM systems in the U.S. Most companies market larger diameter head systems to further accommodate a younger patient population through increased range of motion and lower risk of postoperative dislocation. Biomet s M2a-Magnum large metal articulation system continues to help drive that company s hip sales, while Zimmer had expected its Durom acetabular cups with Metasul large diameter heads to contribute to sales in In mid-2008, however, Zimmer voluntarily suspended U.S. marketing and distribution of the Durom Acetabular component following reports of cup loosening and revisions of the acetabular component. Zimmer will update labeling to provide more detailed surgical instruction for U.S. surgeons, but has delayed the U.S. launch of the product until The company continues to
22 12 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT market the device outside the U.S. DePuy launched the Ultamet XL hip bearing to be used with the Pinnacle Acetabular System, while Stryker will continue to monitor the metal-on-metal hip opportunity, but does not have immediate plans to enter the segment. Ceramic-on-ceramic systems hit the U.S. in 2003 when FDA granted clearance for Wright Medical and Stryker to sell their products. Today, Biomet, DePuy, DJO, Exactech, Smith & Nephew, StelKast, Stryker and Zimmer also market CoC systems in the U.S. In mid-2008, Exactech received FDA clearance to market the Novation Element femoral stem, a tapered wedge design that is promoted to facilitate low profile incisions and surgical approaches. The Novation system reportedly offers the use of larger femoral heads in the majority of patients. Smith & Nephew announced the global launch of the R3 Acetabular System, which allows multiple bearing options. Most companies market ceramic femoral heads along with the more traditional metal (cobalt chrome) heads. DePuy and Stryker each introduced new ceramics heads in 2007, the Biolox Delta TX ceramic heads and the Delta Ceramic Anatomic heads, respectively, and Zimmer and Smith & Nephew added Biolox heads in CeramTec supplies the Biolox products, and reports implantation of more than four million Biolox heads and half a million cup inserts implanted since CeramTec claims that 60 percent of hip replacement patients in Europe receive a Biolox component. Due to deviation from internal specifications regarding manufacturing residuals, in early 2008 Stryker voluntarily recalled its Trident PSL and Hemispherical Acetabular Cups manufactured at its Ireland facility. The manufacturing process for these cups in Cork is now validated, product shipments have resumed and the company has increased production at both its U.S. and Ireland facilities. Companies continue to investigate other hard hip bearing materials with various degrees of success. DePuy continues its focus on novel materials, including combinations of metal and ceramic. Dimicron, which had been developing polycrystalline diamond compact hip bearings through an agreement with Exactech, reportedly faces challenges that may adversely impact its ability to produce the product. In addition to the hip joint, ceramics are used as bearing surfaces in knee, shoulder, elbow and ankle systems outside the U.S. Smith & Nephew recently received regulatory clearance in Japan to market
23 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 13 its Oxinium oxidized zirconium material, a proprietary transformed metal alloy with a ceramic bearing surface. Regulatory clearance was followed by C1 Category Reimbursement clearance, allowing Smith & Nephew to obtain a higher reimbursement price, the first C1 Category Reimbursement for an orthopaedic product. The Genesis II knee will be the first implant with Oxinium technology in Japan. Efforts are underway to bring ceramic technology for joints other than the hip to the U.S. Kinamed partnered with Kyocera to develop a zirconia ceramic femoral knee component, which is part of the Gem knee system currently under investigation in the U.S. Kyocera Industrial Ceramics will be the North American representative of JMM for sales of ceramic structural components used in hip and knee implants, as well as other implant devices available from JMM. JMM currently offers ceramic implants for the knee, hip, shoulder, ankle and elbow in Japan. Aesculap introduced its Alternative Surface technology zirconium nitride coating for knee implants (Columbus Knee System). The company reports that the material has been used in more than 1,200 procedures to date, and has been shown to almost eliminate metal ion release while reducing polyethylene wear by up to 60 percent. Metal-on-metal and ceramic-on-ceramic hip bearings each have a place in less than 30 percent of hip replacement procedures. Polyethylene, particularly in its enhanced form, remains the bearing material of choice in joint replacement procedures. Through radiation processes, these enhanced polyethylenes undergo a structural change (a.k.a. crosslinking) that more tightly bonds their molecules. The resultant material reportedly exhibits improved abrasion and wear characteristics over traditional UHMWPe, thereby extending its longevity. Although some concern exists about the longterm wear properties of some of these highly crosslinked polyethylenes, they are used in the majority of hip cases and an increasing number of knee cases. Biomet s E-poly hip cup liners, the first vitamin E stabilized highly crosslinked polyethylene product, represent a different approach to polyethylene material on the market. A strategic alliance with Massachusetts General Hospital for clinical outcomes research will enable the hospital to manage multicenter clinical trials of this technology. In 2009, Mathys Medical intends to introduce vitamys, a vitamin E-enriched cup. Zimmer holds a license for vitamin E stabilized material, as well. Larger femoral head systems are making their way into the traditional metal-on-poly constructs. Similar to their function in MoM systems, larger femoral heads purportedly reduce the risk of postoperative dislocation and can address the needs of younger patient populations. DePuy, DJO,
24 14 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT Smith & Nephew and Wright all launched highly crosslinked polyethylene hip liners which incorporate larger diameter femoral heads within the last year. Smith & Nephew combined Oxinium material with crosslinked poly in its Verilast product, also launched in Active Implants is investigating the use of a pliable polyurethane structure as replacement for polyethylene. Research sponsored by the company suggests a seven times reduction in harmful chemicals formed as a result of the presence of wear particle debris, as compared to polyethylene. The TriboFit Acetabular Buffer is now available in Europe for use in hip arthroplasty and hip resurfacing. Active Implants signed an agreement granting exclusive distribution rights to JRI for the TriboFit Hip System in the U.K. Active Implants second product, the NUsurface implant, with proprietary polyurethane under the tradename Cartelast, a synthetic meniscal replacement, obtained regulatory clearance in the European Union via a CE Mark and is scheduled for a European release in While alternative materials may help mitigate implant wear in younger, more active patient populations, specific implant designs (e.g. mobile bearing knees or MBKs) may also address wear issues. Through their less-constrained design, MBKs reportedly reduce contact stress and subsequent potential wear of the polyethylene. These devices are marketed outside the U.S. through the major joint replacement companies as well as Aesculap, Consensus Orthopedics, Corin, Dedienne Sante (which merged with Serf in 2009), FH, FII, Finsbury, Groupe Lepine, Medacta, Tornier, etc. Exactech expects its patented rotating bearing addition to the Optetrak line will move market share for the company in Europe, and also announced line extensions focused on tibial inserts with varying slopes to achieve better function with less wear. Further, Exactech announced an agreement with the Hospital for Special Surgery to introduce a new knee implant, the Optetrak Logic, planned for the second half of For more than 20 years, DePuy had the only mobile bearing knee systems approved for use in the U.S., including a posterior stabilized version. In 2008, Zimmer obtained clearance via a Premarket Approval (PMA) to market its MBK, which is compatible with minimally invasive surgical (MIS) techniques. Zimmer launched the NexGen LPS-Flex Mobile Knee in 2008 (Zimmer also markets the Innex Knee, a system with both fixed and mobile bearings, in Europe and Asia Pacific). Stryker continues its clinical trial in the U.S. of the Scorpio Plus Mobile Bearing tibial component, which was
25 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 15 launched outside the U.S. in Other companies have reportedly postponed submissions for clinical trials of mobile bearing knees in the U.S., believing that FDA may downclassify the products. Mobile bearing technology is also used in ankle replacement outside the U.S., where the devices are used almost exclusively over fixed-bearing, two-piece devices. Corin, Dedienne Sante, ESKA, Integra, Tornier and Van Straten all market mobile bearing ankles outside the U.S. Endotec continues to pursue FDA clearance of its mobile bearing ankle device by seeking a 510(k) exemption for mobile bearing ankles, and in 2008 announced a U.S. Federal court ruling in favor of the company s position that their ankle implants were custom devices exempt from PMA requirements. However, the company was enjoined by a U.S. District court ruling from manufacturing and distributing its FlexGlide Knee Bearing with Anterior Stop and the Fenning Modular Bearing until it obtains appropriate IDE, PMA or 510(k) clearances. Biomet, DePuy, Stryker and Zimmer all have built fairly strong franchises in shoulders, and several companies (DePuy, Exactech, Tornier and Zimmer) have launched reverse shoulder implant systems for patients with severe rotator cuff pain. These prostheses reverse the anatomy of the shoulder to use the healthy deltoid muscle. Both Encore and Zimmer have noted that their reverse shoulder systems have contributed to growth in their extremities product sales and, for Zimmer, its reverse shoulder is reportedly its most successful shoulder in Europe. Mathys Medical added the Affinis inverse shoulder prosthesis to its line-up of shoulder products as well. In mid-2008, Tornier noted that more than 100,000 Aequalis Shoulders had been implanted since their European introduction in The company released a resurfacing head for the Aequalis line and noted the first human implantation of a shoulder arthroplasty prosthesis featuring the company s pyrocarbon technology. Smith & Nephew plans to release the Promos reverse shoulder, a PLUS product, in Stryker s Solar Total Shoulder system, while not a reverse design, offers both conventional and bipolar heads for use in arthritic shoulders. Finally, Synthes noted the release of its Epoch Shoulder Arthroplasty System, but has positioned it as a fracture repair device, in addition to a total joint. Bone conserving designs are also seen in shoulder devices. Ascension Orthopedics launched the TITAN humeral resurfacing device worldwide early in 2008, after a limited U.S. release. The product offers a four-finned design for greater purchase into bone and enhanced stability.
26 16 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT In small joint initiatives, DePuy, Integra, Tornier and Wright market total ankle systems in the U.S. Outside the U.S., Baumer, Corin, ESKA, Euros, FH, Integra, JMM, Link, Protetim, Sovereign Medical and Tornier sell their ankle systems. Zimmer has reportedly also begun an ankle initiative. Given the difficulties in reclassifying mobile bearing knees, it appears that FDA is unlikely to grant a reclassification for ankles. However, of significant note, Small Bone Innovations (SBi) will enter the mobile bearing ankle market in the U.S. with the Scandinavian Total Ankle Replacement system (the S.T.A.R. ankle ) obtained through its acquisition of Link America, and other assets from Waldemar Link. The S.T.A.R. Ankle was approved for marketing in May 2009 and is both the first mobile bearing ankle approved by FDA and the only one approved for use without cement. The S.T.A.R. Ankle has been used since 1990 in 27 countries and over 10,000 implantations. Ascension markets FDA cleared and CE Mark approved silicone and pyrocarbon digit implants, as well as the first pyrocarbon trapeziometacarpal implant, a hemi-arthroplasty device for treatment of thumb-based arthritis and an implant for treatment of arthritis in the fourth and fifth tarsometatarsal joints of the foot. Arthrosurface, BioPRO, Orthosonics and OsteoMed market hemi and total metatarsophalangeal joints. Other small joint implants can be found in the portfolios of Aptis Medical (CE Mark approved and FDA cleared distal radioulnar joint), ESKA (proximal interphalangeal/pip, MCP, ankle and Great Toe), Merete Medical (FDA-cleared ToeMobile Anatomical Great Toe Resurfacing System) Finsbury (PIP and TMP), Mathys (finger), Metasurg (subtalar implant) and Tecres (MCP implant), etc. Wright Medical offers a broad small joint product range, including shoulder, elbow, radial head, ulnar head, wrist, trapezium, lunate, scaphoid, finger, thumb, ceramic interpositional implants, great toe, hinge toe and subtalar and hammertoe implants, developed internally and acquired through recent acquisitions. Artimplant and Small Bone Innovations reached a licensing agreement for Artimplant s Artelon resurfacing concept for joint applications in the hand and wrist. The two companies revised existing agreements covering the Artelon Spacer, and under these new agreements, Artimplant will develop spacer products for three new joints, but the agreements are no longer exclusive. The company s Artelon STT Spacer and CMC Spacer Arthro have been cleared by FDA for treatment of OA in the
27 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 17 scapho-trapezio-trapezoidial joint and thumb base arthritis, respectively. The Artelon Resurfacing concept involves a minimal resection of the joint afflicted by OA, creating the preconditions for integration of tissue in the implant, thus creating a new joint surface. A variety of less traditional joint replacement products from surface replacement to interpositional devices continue to penetrate the markets and offer options for typically younger patients whose disease process may affect only part of the joint and thus, may not warrant total replacement of hip, knee, shoulder, elbow or other joint. For instance, with a surface replacement hip, the surgeon removes only the surface of the femoral head, replacing it with a hemispherical implant that fits within the acetabular shell. Because a minimal amount of bone is resected, surface replacement surgery is far more conservative and thus, may be more appropriate to the management of the disease state of a younger patient. According to implant registries, resurfacing hip procedures accounted for eight percent of primary hip procedures in Australia, eight percent in the U.K., less than two percent in Sweden and less than one percent in Canada, although resurfacing is employed in approximately four percent of hip revisions in Canada. Interestingly, the use of resurfacing in Australia has declined over the past two years, as was the case in the U.K., as well. Used outside the U.S. for decades, MoM hip resurfacing products only received FDA s blessing in mid-2006, when the agency approved Smith & Nephew s Birmingham resurfacing hip (BHR) (manufactured by Finsbury Orthopaedics). The clearance marked the first time since the 1980s that the agency cleared a device for marketing in the U.S. based solely on ex-u.s. data and that from a single clinician. Smith & Nephew claims to own more than half of the global market for hip resurfacing, and is continuing to expand its products offerings in this area, as evidenced by its R3 Acetabular System, which incorporates a new hip resurfacing option that is awaiting FDA clearance. In 2007, Stryker joined Smith & Nephew in the U.S. hip resurfacing market. Upon completion of an exclusive ten-year marketing and distribution agreement with Corin, Stryker began distributing the Cormet hip resurfacing system in late In early 2008, however, Stryker notified Corin that, based on current inventory, Stryker did not plan to order additional Cormet hip resurfacing devices from
28 18 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT Corin until at least the second quarter of 2009, and no additional instrument sets for the entire 2009 year. Outside the U.S., Stryker distributes the MITCH TRH resurfacing hip system, developed in conjunction with Finsbury. Other resurfacing systems available outside the U.S. include those from ESKA, IO and Van Straten. Global Orthopaedic Technology distributes IO s ICON implants in Australia. IO s implants reportedly derive from the BHR developers but feature titanium nitride coating and are specially designed for MIS approaches. Other companies seek to join Smith & Nephew and Stryker in the hip resurfacing market in the U.S. In 2007, FDA accepted for filing DePuy s Premarket Approval Application for the ASR Hip Surface Replacement System. Wright received regulatory clearance in Japan for the CONSERVE Plus Hip Resurfacing System, which is currently available in Europe, Canada, Australia and other international markets, while Wright s U.S. regulatory submission remains under review at FDA. Zimmer offers the Durom hip resurfacing system in Europe, but expects its lack of availability in the U.S. (due to the voluntary suspension of marketing in the U.S.) to negatively impact sales as adoption rates for this procedure continue to rise. Biomet markets its ReCap hip resurfacing implant internationally while its U.S. IDE clinical trial continues, with enrollment of over 200 patients to date. Conservative joint replacement can be found in the knee joint with unicompartmental devices, which address OA in a single compartment of the knee. These usually take the form of unicondylar implants, which replace either the medial or lateral femorotibial compartment. The patellofemoral compartment can also be replaced independently. In addition to preserving more bone and soft tissue function, unicondylar procedures are conducive to MIS techniques. Unicondylar procedures make up approximately eight percent of all primary knee replacement procedures worldwide seven percent in Sweden, eight percent in Canada and the U.K., nine percent in Australia and the U.S. and 12 percent in Norway. Interestingly, the use of unis in knee replacement procedures has dropped in both Australia and Sweden over the past couple of years and no Unispacer or similar device has been used in Australia since In the U.S. Biomet markets the Oxford Partial Knee, the only free-floating mobile bearing partial knee cleared by FDA. To date, more than 2,000 U.S. surgeons have been trained on the device. Biomet reports a 98 percent success rate at ten years with the product, and 95 percent at 15 years. Smith &
29 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT - 19 Nephew s uni knee features the company s Oxinium material. Stryker Canada distributes Corin s Uniglide mobile bearing unicondylar knee and Corin has a similar long term exclusive distribution agreement with Stryker in the U.S. for Uniglide, subject to FDA approval. In 2008, Corin initiated a randomized, double-blink clinical study for the Uniglide to obtain FDA clearance for the device. The study is still underway. Rather than beginning with a total knee and progressing to unicompartmental devices, Cardo Medical used the uni device to pave the way for the Align 360 Total Knee, which was introduced to the U.S. market in late 2008 after receiving FDA clearance. The company s Align 360 Uni-Compartmental System has been used in over 500 surgeries to date. Most unicompartmental knee implants are fixed bearing devices and represent an important offering in the knee replacement portfolio of the joint replacement companies. In 2008, Stryker launched the Triathlon PKR, a partial knee resurfacing system that incorporates the company s X3 bearing technology, to round out its Triathlon Knee System. DePuy conducted a clinical study in 2008 on its Preservation uni, which encompassed both fixed and mobile bearing designs, although the DePuy device was recalled in the U.K. in 2009 due to an unexpectedly high revision rate. Other technologies that offer conservative alternatives to total joint replacement include ConforMIS CE Mark approved iuni unicompartmental knee resurfacing device, available for medial and lateral use, and its accompanying ijig disposable instrumentation. In 2008, the company also launched the iduo bicompartmental knee resurfacing implant that resurfaces one side of the knee and a portion of the patellofemoral joint. Each iuni, iduo and corresponding ijig cutting and placement guide is designed from a patient s CT scan using the company s proprietary ifit technology to eliminate manual sizing during surgery. The iuni, cleared by FDA, was launched in the U.S. in early ConforMIS also entered into a multi-year partnership with AG Mednet, giving the company access to a global diagnostic imaging network, allowing transmission of DICOM-based diagnostic image studies (CT, MRI, ultrasound, PET and digital X-ray) in support of development of the ConforMIS custom devices. Smith & Nephew s Journey Deuce Bi-Compartment Knee also replaces two of three compartments of the knee. Cleared in Australia, Canada, Europe and the U.S., the device replaces the areas most commonly affected by OA and leaves the lateral femur and tibia intact. Also receiving FDA clearance was the Solo Partial unicompartmental knee system from VOT Arthroscopic Solutions, which reported a successful initial surgery through a 2-inch incision; with the goal of decreasing the incision to one inch for a full resurfacing of the medial condyle. The Solo can be
30 20 THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT implanted using an arthroscopic assisted technique. Advanced Bio-Surfaces offers another conservative option for moderate OA of the medial knee in its OrthoGlide Medial Knee. This minimally invasive device has been implanted in more than 300 patients in the U.S. Arthrosurface s HemiCAP (Contoured Articular Prosthesis) line of products provide for replacement of only damaged, irreparable articular cartilage. The technology incorporates a rounded CoCr cap-like implant that sits flush with the joint surface. Arthrosurface s femoral condyle implant is currently in Phase III clinical trials in the U.S. for the treatment of full thickness femoral condyle cartilage lesions in middle-aged patients. In mid 2008, the company received CE Mark and FDA clearance for its UniCAP unicondylar resurfacing system that is performed as an arthroscopically assisted surgery and features a CoCr femoral implant combined with a small, UHMWPe implant that allows preservation of the meniscus. Aesculap also announced activity on the Univation unicondylar knee, which reportedly offers more than 20 percent more bone preservation than traditional three-plane unis. BioPoly RS, a subsidiary of Schwartz Biomedical, will move forward with development of its BioPoly RS (ReSurfacing) device. Intended to replace only the damaged portion of a joint (hip, knee, shoulder, digits, etc.), the BioPoly RS partial resurfacing implant technology features a hydrophilic polymer (UHMWPe + a naturally occurring polysaccharide molecule) with mechanical and biochemical properties similar to those of articular cartilage. Market launch for the technology could come by the end of Arthritis affecting the patellofemoral compartment of the knee can also be addressed through the use of patellofemoral joint (PFJ) replacement implants. Although not widely used, these implants (like unis and resurfacing hips) do offer a more conservative approach to a particular disease state. In the U.K., patellofemoral knee designs accounted for one percent of knee replacement procedures performed. Outside the U.S., companies as varied as Ceraver Osteal and Wright Medical market PFJ implants, with the clearance for PFJs in the U.S. granted to Biomet, Kinamed, Smith & Nephew, Stryker and Zimmer. Zimmer launched its Gender Solutions Patello-Femoral Joint System in 2008, and Kinamed reports that studies of its KineMatch Patello-Femoral Knee Replacement showed that 100 percent of implants were in place and functioning well at an average follow-up of greater than six years.
Hip and Knee Orthopedic Surgical Implants: Market Shares, Strategies, and Forecasts, Worldwide,
 Hip and Knee Orthopedic Surgical Implants: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Implants: Executive Summary The study is designed
Hip and Knee Orthopedic Surgical Implants: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Implants: Executive Summary The study is designed
Hip and Knee Orthopedic Surgical Implants Market Growth, Research & Trend to Radiant Insights, Inc
 Hip and Knee Orthopedic Surgical Implants Market Growth, Research & Trend to 2016-2022 Radiant Insights, Inc Orthopedic surgical implants help replace deteriorated joints. This deterioration could be due
Hip and Knee Orthopedic Surgical Implants Market Growth, Research & Trend to 2016-2022 Radiant Insights, Inc Orthopedic surgical implants help replace deteriorated joints. This deterioration could be due
Orthopaedics Business. Dave Illingworth, President February
 Orthopaedics Business Dave Illingworth, President February 23 2005 1 Smith & Nephew Orthopaedics Attractive market Strong performance Momentum 2 Attractive market Demographics - 2x Growth by 2020 Active
Orthopaedics Business Dave Illingworth, President February 23 2005 1 Smith & Nephew Orthopaedics Attractive market Strong performance Momentum 2 Attractive market Demographics - 2x Growth by 2020 Active
Hip and Knee Orthopedic Surgical Implants Market Shares, Strategies, and Forecasts, Worldwide, 2016 to 2022
 Published on Market Research Reports Inc. (https://www.marketresearchreports.com) Home > Hip and Knee Orthopedic Surgical Implants Market Shares, Strategies, and Forecasts, Worldwide, 2016 to 2022 Hip
Published on Market Research Reports Inc. (https://www.marketresearchreports.com) Home > Hip and Knee Orthopedic Surgical Implants Market Shares, Strategies, and Forecasts, Worldwide, 2016 to 2022 Hip
HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX
 HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX HIP IMPLANTS TOTAL HIPS Ultra High Demand (metal on metal) Femur, ingrowth Femoral head, metal Acetabular shell, ingrowth Acetabular liner,
HOSPITAL "A" (2007) ORTHOPEDIC JOINT IMPLANT COMPONENT MATRIX HIP IMPLANTS TOTAL HIPS Ultra High Demand (metal on metal) Femur, ingrowth Femoral head, metal Acetabular shell, ingrowth Acetabular liner,
National Joint Replacement Registry. Lay Summary 2015 Annual Report Hip and Knee Replacement
 National Joint Replacement Registry Lay Summary 2015 Annual Report Hip and Knee Replacement SUPPLEMENTARY REPORT 2015 TABLE OF CONTENTS Introduction... 1 A brief history of the Registry origins... 1 The
National Joint Replacement Registry Lay Summary 2015 Annual Report Hip and Knee Replacement SUPPLEMENTARY REPORT 2015 TABLE OF CONTENTS Introduction... 1 A brief history of the Registry origins... 1 The
JOINT RECONSTRUCTION AND REPLACEMENT: MATERIALS, TECHNOLOGIES, AND GLOBAL MARKETS. HLC165A July Dr. Ritu Thakur Dangi Project Analyst
 JOINT RECONSTRUCTION AND REPLACEMENT: MATERIALS, TECHNOLOGIES, AND GLOBAL MARKETS HLC165A July 2014 Dr. Ritu Thakur Dangi Project Analyst ISBN: 1-56965-877-3 BCC Research 49 Walnut Park, Building 2 Wellesley,
JOINT RECONSTRUCTION AND REPLACEMENT: MATERIALS, TECHNOLOGIES, AND GLOBAL MARKETS HLC165A July 2014 Dr. Ritu Thakur Dangi Project Analyst ISBN: 1-56965-877-3 BCC Research 49 Walnut Park, Building 2 Wellesley,
# $ pages In Stock. Report Description
 Global Knee and Hip Replacements Market (By Type, Material,Fixation, Approach): Opportunities and Forecasts (2016-2021) (By Type - Total Knee & Hip Replacement, Partial Knee & Hip Replacement, Revision
Global Knee and Hip Replacements Market (By Type, Material,Fixation, Approach): Opportunities and Forecasts (2016-2021) (By Type - Total Knee & Hip Replacement, Partial Knee & Hip Replacement, Revision
Partial Knee Replacement
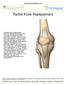 Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Spinal Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide,
 Spinal Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Spinal Surgical Robot: Executive Summary The study is designed to give a comprehensive overview
Spinal Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Spinal Surgical Robot: Executive Summary The study is designed to give a comprehensive overview
TOTAL HIP REPLACEMENT:
 THR Prosthesis Design TOTAL HIP REPLACEMENT: PROSTHESIS DESIGN FEATURES JESS JOHNSTON & MELINDA ZIETH History of Hip Prosthesis Joint Replacement Registry Implant Design Technology & Future History and
THR Prosthesis Design TOTAL HIP REPLACEMENT: PROSTHESIS DESIGN FEATURES JESS JOHNSTON & MELINDA ZIETH History of Hip Prosthesis Joint Replacement Registry Implant Design Technology & Future History and
Hip and Knee Orthopedic Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide,
 Hip and Knee Orthopedic Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Robots: Executive Summary The study is designed
Hip and Knee Orthopedic Surgical Robots: Market Shares, Strategies, and Forecasts, Worldwide, 2016-2022 Table of Contents Hip and Knee Orthopedic Surgical Robots: Executive Summary The study is designed
The Leader in Orthopaedic Innovation
 The Leader in Orthopaedic Innovation Wright is a leading international manufacturer and distributor of superior, easy to use, and innovative orthopaedic implants and instrumentation. For over 50 years,
The Leader in Orthopaedic Innovation Wright is a leading international manufacturer and distributor of superior, easy to use, and innovative orthopaedic implants and instrumentation. For over 50 years,
PARTIAL KNEE REPLACEMENT
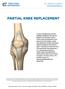 PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
ESC. Enhanced Stability Liners. Design Rationale & Surgical Technique
 ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
ESC Enhanced Stability Liners Design Rationale & Surgical Technique Choice Without Compromise DePuy Synthes PINNACLE Hip Solutions are designed with a wide range of acetabular cup options, biological and
Over 20 Years of Proven Clinical Success. Zimmer Natural-Knee II System
 Over 20 Years of Proven Clinical Success Zimmer Natural-Knee II System CSTi Porous Coating Structurally similar to human bone CSTi porous coating combines the excellent biocompatibility of titanium with
Over 20 Years of Proven Clinical Success Zimmer Natural-Knee II System CSTi Porous Coating Structurally similar to human bone CSTi porous coating combines the excellent biocompatibility of titanium with
Transparency Market Research
 Transparency Market Research IGBT and Super Junction MOSFET Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019 Single User License: USD 4595 Multi User License: USD
Transparency Market Research IGBT and Super Junction MOSFET Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013-2019 Single User License: USD 4595 Multi User License: USD
Mr Aslam Mohammed FRCS, FRCS (Orth) Consultant Orthopaedic Surgeon Specialising in Lower Limb Arthroplasty and Sports Injury
 Mr Aslam Mohammed FRCS, FRCS (Orth) Consultant Orthopaedic Surgeon Specialising in Lower Limb Arthroplasty and Sports Injury I qualified from the Welsh National School of Medicine in Cardiff in 1984. I
Mr Aslam Mohammed FRCS, FRCS (Orth) Consultant Orthopaedic Surgeon Specialising in Lower Limb Arthroplasty and Sports Injury I qualified from the Welsh National School of Medicine in Cardiff in 1984. I
Knee Replacement Implants
 Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Knee Replacement Implants During knee replacement surgery, an orthopaedic surgeon will resurface your damaged knee with artificial components, called implants. There are many different types of implants.
Table of Contents: JULY TH EDITION
 Table of Contents: Table of Contents of the worldwide Orthopaedic Contract manufacturing Market 2016-2021 & Top 100 player profiles 275+ pages 450+ exhibits July 2017 5 th edition I- Worldwide orthopaedic
Table of Contents: Table of Contents of the worldwide Orthopaedic Contract manufacturing Market 2016-2021 & Top 100 player profiles 275+ pages 450+ exhibits July 2017 5 th edition I- Worldwide orthopaedic
Integra. Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION
 Integra Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal
Integra Salto Talaris Total Ankle Prosthesis PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal
Total Knee Replacement
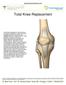 Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
Total Knee Replacement A total knee replacement, also known as total knee arthroplasty, involves removing damaged portions of the knee, and capping the bony surfaces with man-made prosthetic implants.
Integra. Titan Modular Shoulder System, 2.5
 Titan Modular Shoulder System, 2.5 Limit uncertainty with a shoulder implant system that redefines modularity, addresses multiple indications, and allows for reproducible results. Titan Modular Shoulder
Titan Modular Shoulder System, 2.5 Limit uncertainty with a shoulder implant system that redefines modularity, addresses multiple indications, and allows for reproducible results. Titan Modular Shoulder
MAKO Surgical Corp. January MAKO Surgical Corp
 MAKO Surgical Corp. January 2012 MAKO Surgical Corp. 2012 1 Forward Looking Statement This presentation contains forward-looking statements regarding, among other things, statements related to expectations,
MAKO Surgical Corp. January 2012 MAKO Surgical Corp. 2012 1 Forward Looking Statement This presentation contains forward-looking statements regarding, among other things, statements related to expectations,
Optimum implant geometry
 Design Rationale Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Design Rationale Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Comprehensive Solutions
 Comprehensive Solutions Supporting healthcare professionals Flexibility Today s knee implant patients present increasingly diverse scenarios. The versatility of the JOURNEY II Total allows surgeons to
Comprehensive Solutions Supporting healthcare professionals Flexibility Today s knee implant patients present increasingly diverse scenarios. The versatility of the JOURNEY II Total allows surgeons to
Bone Preservation Stem
 TRI-LOCK Bone Preservation Stem Featuring GRIPTION Coating Surgical Technique Implant Geometry Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
TRI-LOCK Bone Preservation Stem Featuring GRIPTION Coating Surgical Technique Implant Geometry Extending the TRI-LOCK Stem heritage The original TRI-LOCK Stem was introduced in 1981. This implant was
Integra. Thumb Joint Replacement PATIENT INFORMATION. with PyroCarbon implants
 Integra Thumb Joint Replacement with PyroCarbon implants PATIENT INFORMATION Anatomy of the Hand The hand is composed of many different bones, muscles and ligaments that allow for a large amount of movement
Integra Thumb Joint Replacement with PyroCarbon implants PATIENT INFORMATION Anatomy of the Hand The hand is composed of many different bones, muscles and ligaments that allow for a large amount of movement
WHAT IS THE BEST BONE FIXATION TYPE? 2/11/2011
 WHAT IS THE BEST BONE FIXATION TYPE? 2/11/2011 A Comparison of cement vs. bone ingrowth. Thomas P. Gross, M.D. At 2 years of follow-up cemented and uncemented femoral resurfacing is equivalent. Femoral
WHAT IS THE BEST BONE FIXATION TYPE? 2/11/2011 A Comparison of cement vs. bone ingrowth. Thomas P. Gross, M.D. At 2 years of follow-up cemented and uncemented femoral resurfacing is equivalent. Femoral
A Patient s Guide to Partial Knee Resurfacing
 A Patient s Guide to Partial Knee Resurfacing Surgical Outcomes System (SOS ) www.orthoillustrated.com OrthoIllustrated is a leading Internet-based resource for patient education. Please visit this website
A Patient s Guide to Partial Knee Resurfacing Surgical Outcomes System (SOS ) www.orthoillustrated.com OrthoIllustrated is a leading Internet-based resource for patient education. Please visit this website
Integra Cadence Total Ankle System PATIENT INFORMATION
 Integra Cadence Total Ankle System PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal Fibular
Integra Cadence Total Ankle System PATIENT INFORMATION Fibula Articular Surface Lateral Malleolus Tibia Medial Malleolus Talus Anterior view of the right ankle region Talo-fibular Ligament Calcaneal Fibular
First Albany Orthopedics Conference Marina Village, San Diego February 13, 2007 Orthofix International
 First Albany Orthopedics Conference Marina Village, San Diego February 13, 2007 Orthofix International Tom Hein, CFO Safe Harbor Statement Except for historical information contained herein, the statements
First Albany Orthopedics Conference Marina Village, San Diego February 13, 2007 Orthofix International Tom Hein, CFO Safe Harbor Statement Except for historical information contained herein, the statements
National Joint Replacement Registry. Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY
 National Joint Replacement Registry Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY Report 2017 AOAnjrr 2016 supplementary report AOAnjrr 2016 supplementary report Contents SUMMARY...
National Joint Replacement Registry Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY Report 2017 AOAnjrr 2016 supplementary report AOAnjrr 2016 supplementary report Contents SUMMARY...
Optimum implant geometry
 Design Rationale Optimum implant geometry Extending the proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Design Rationale Optimum implant geometry Extending the proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
The information contained in this document is intended for healthcare professionals only.
 The information contained in this document is intended for healthcare professionals only. Trident Poly Acetabular System Trident PSL HA Acetabular Shell with X3 Poly insert Trident Hemispherical Acetabular
The information contained in this document is intended for healthcare professionals only. Trident Poly Acetabular System Trident PSL HA Acetabular Shell with X3 Poly insert Trident Hemispherical Acetabular
Enhanced Stability Constrained Liners. Design Rationale Surgical Technique
 Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
Enhanced Stability Constrained Liners Design Rationale Surgical Technique The Pinnacle Acetabular Cup System was designed to maximize the number of options available to the surgeon, and provide those options
I want that next-generation knee
 I want that next-generation knee High flex Potentially longer-lasting More normal feeling Gender-optimized Less-invasive procedure Advanced design technology The last ten years have yielded an explosion
I want that next-generation knee High flex Potentially longer-lasting More normal feeling Gender-optimized Less-invasive procedure Advanced design technology The last ten years have yielded an explosion
Optimum implant geometry
 Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
Surgical Technique Optimum implant geometry Extending proven Tri-Lock heritage The original Tri-Lock was introduced in 1981. This implant was the first proximally coated tapered-wedge hip stem available
SAMPLE. South Korea Orthopedic Procedures Outlook to Reference Code: GDMEMC0008PDB. Publication Date: March 2014
 South Korea Orthopedic Procedures Outlook to 2020 Reference Code: GDMEMC0008PDB Publication Date: March 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2 List of Figures...
South Korea Orthopedic Procedures Outlook to 2020 Reference Code: GDMEMC0008PDB Publication Date: March 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2 List of Figures...
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma >
 Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 5 December 2013 1 Version control Version number Date Amendments
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 5 December 2013 1 Version control Version number Date Amendments
The information contained in this document is intended for healthcare professionals only.
 The information contained in this document is intended for healthcare professionals only. Trident Hemispherical Acetabular Shell Trident PSL HA Acetabular Shell Featuring: The Trident Acetabular System
The information contained in this document is intended for healthcare professionals only. Trident Hemispherical Acetabular Shell Trident PSL HA Acetabular Shell Featuring: The Trident Acetabular System
Specializing in Mechanical Testing for Medical Devices. Office:
 Specializing in Mechanical Testing for Medical Devices Office: 260.489.1444 Email: sales@jtlamerica.com www.jtlamerica.com Spine Spinal implant test standards can t keep up with the rapid pace of device
Specializing in Mechanical Testing for Medical Devices Office: 260.489.1444 Email: sales@jtlamerica.com www.jtlamerica.com Spine Spinal implant test standards can t keep up with the rapid pace of device
Operations included in the National Joint Registry (NJR)
 Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 8 June 2018 1 Version control Version number Date Amendments 1
Operations included in the National Joint Registry (NJR) Quick links, go to: Hips > Knees > Ankles > Elbows > Shoulders > Trauma > Version 8 June 2018 1 Version control Version number Date Amendments 1
CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR
 CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR Evolution of TKR In 1860, Verneuil proposed interposition arthroplasty, involving the insertion of soft tissue
CLINICAL AND OPERATIVE APPROACH FOR TOTAL KNEE REPLACEMENT DR.VINMAIE ORTHOPAEDICS PG 2 ND YEAR Evolution of TKR In 1860, Verneuil proposed interposition arthroplasty, involving the insertion of soft tissue
G7 ACETABULAR SYSTEM. beautifully. efficient.
 G7 ACETABULAR SYSTEM beautifully efficient. HERITAGE MEETS MODERN DESIGN SIMPLICITY, EFFICIENCY AND PERFORMANCE The G7 System is a multi-bearing acetabular platform delivering simplicity, efficiency and
G7 ACETABULAR SYSTEM beautifully efficient. HERITAGE MEETS MODERN DESIGN SIMPLICITY, EFFICIENCY AND PERFORMANCE The G7 System is a multi-bearing acetabular platform delivering simplicity, efficiency and
Institute for Bone, Joint Replacement, Orthopaedics Spine and Sports Medicine
 24-Hour Helpline: 011-30403040 BLK Super Speciality Hospital Pusa Road, New Delhi - 110005 (India). www.blkhospital.com Institute for Bone, Joint Replacement, Orthopaedics Spine and Sports Medicine Introduction
24-Hour Helpline: 011-30403040 BLK Super Speciality Hospital Pusa Road, New Delhi - 110005 (India). www.blkhospital.com Institute for Bone, Joint Replacement, Orthopaedics Spine and Sports Medicine Introduction
China Orthopedics Implants Medical Devices Market Brief
 China Orthopedics Implants Medical Devices Market Brief By: PIM LTD. August 2017 reproduced for distribution outside the client organization without prior written approval from PIM Ltd. 1 Definition and
China Orthopedics Implants Medical Devices Market Brief By: PIM LTD. August 2017 reproduced for distribution outside the client organization without prior written approval from PIM Ltd. 1 Definition and
CONTRIBUTING SURGEON. Barry Waldman, MD Director, Center for Joint Preservation and Replacement Sinai Hospital of Baltimore Baltimore, MD
 CONTRIBUTING SURGEON Barry Waldman, MD Director, Center for Joint Preservation and Replacement Sinai Hospital of Baltimore Baltimore, MD System Overview The EPIK Uni is designed to ease the use of the
CONTRIBUTING SURGEON Barry Waldman, MD Director, Center for Joint Preservation and Replacement Sinai Hospital of Baltimore Baltimore, MD System Overview The EPIK Uni is designed to ease the use of the
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Ardaugh BM, Graves SE, Redberg RF. The 510(k) ancestry of a
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Ardaugh BM, Graves SE, Redberg RF. The 510(k) ancestry of a
Total Ankle Arthroplasty. Joseph P. McCormick, M.D. Affinity Orthopedics & Sports Medicine the original 2014
 Total Ankle Arthroplasty Joseph P. McCormick, M.D. Affinity Orthopedics & Sports Medicine the original 2014 Ankle Anatomy The ankle is a hinge or ginglymus joint Made up of the tibia, fibula, & talus
Total Ankle Arthroplasty Joseph P. McCormick, M.D. Affinity Orthopedics & Sports Medicine the original 2014 Ankle Anatomy The ankle is a hinge or ginglymus joint Made up of the tibia, fibula, & talus
China Cranio Maxillofacial Fixation (CMF) Market Outlook to 2020
 China Cranio Maxillofacial Fixation (CMF) Market Outlook to 2020 Reference Code: GDMECC0079DB Publication Date: March 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2
China Cranio Maxillofacial Fixation (CMF) Market Outlook to 2020 Reference Code: GDMECC0079DB Publication Date: March 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2
The S.T.A.R. Scandinavian Total Ankle Replacement. Patient Information
 The S.T.A.R. Scandinavian Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please
The S.T.A.R. Scandinavian Total Ankle Replacement Patient Information Patient Information This patient education brochure is presented by Small Bone Innovations, Inc. Patient results may vary. Please
2016 CELEBRATING 15 YEARS OF DATA REPORT NATIONAL JOINT REPLACEMENT REGISTRY. Outcomes of Classes No Longer Used Hip and Knee Arthroplasty
 NATIONAL JOINT REPLACEMENT REGISTRY Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY REPORT 2016 CELEBRATING 15 YEARS OF DATA Contents INTRODUCTION...3 HIP REPLACEMENT...4 Partial
NATIONAL JOINT REPLACEMENT REGISTRY Outcomes of Classes No Longer Used Hip and Knee Arthroplasty SUPPLEMENTARY REPORT 2016 CELEBRATING 15 YEARS OF DATA Contents INTRODUCTION...3 HIP REPLACEMENT...4 Partial
CUSTOMIZED KNEE IMPLANTS. One Patient. One Implant.
 CUSTOMIZED KNEE IMPLANTS One Patient. One Implant. UNDERSTANDING YOUR KNEE To understand the benefits of the ConforMIS personalized approach, it s important to understand the anatomy of your knee. Your
CUSTOMIZED KNEE IMPLANTS One Patient. One Implant. UNDERSTANDING YOUR KNEE To understand the benefits of the ConforMIS personalized approach, it s important to understand the anatomy of your knee. Your
U.S. MARKET FOR MINIMALLY INVASIVE SPINAL IMPLANTS
 U.S. MARKET FOR MINIMALLY INVASIVE SPINAL IMPLANTS idata_usmis15_rpt Published in December 2014 By idata Research Inc., 2014 idata Research Inc. Suite 308 4211 Kingsway Burnaby, British Columbia, Canada,
U.S. MARKET FOR MINIMALLY INVASIVE SPINAL IMPLANTS idata_usmis15_rpt Published in December 2014 By idata Research Inc., 2014 idata Research Inc. Suite 308 4211 Kingsway Burnaby, British Columbia, Canada,
Extramedullary Tibial Preparation
 Surgical Technique Extramedullary Tibial Preparation Primary Total Knee Arthroplasty LEGION Total Knee System Extramedullary tibial preparation Contents Introduction...2 EM tibial highlights...3 Preoperative
Surgical Technique Extramedullary Tibial Preparation Primary Total Knee Arthroplasty LEGION Total Knee System Extramedullary tibial preparation Contents Introduction...2 EM tibial highlights...3 Preoperative
Patient-specific bicompart mental knee resurfacing system
 iduog2 Patient-specific bicompart mental knee resurfacing system Superior implant fit and performance require a patient-specific approach. The ConforMIS Partial Knee Resurfacing Systems use proprietary
iduog2 Patient-specific bicompart mental knee resurfacing system Superior implant fit and performance require a patient-specific approach. The ConforMIS Partial Knee Resurfacing Systems use proprietary
REFERENCE CODE GDME1072CFR PUBLICATION DATE NOVEMBER 2013 KNEE REPLACEMENT - US ANALYSIS AND MARKET FORECASTS
 REFERENCE CODE GDME1072CFR PUBLICATION DATE NOVEMBER 2013 KNEE REPLACEMENT - Executive Summary Knee Replacement: Key Metrics in the US Market Prevalence of symptomatic knee osteoarthritis (OA) in 2013
REFERENCE CODE GDME1072CFR PUBLICATION DATE NOVEMBER 2013 KNEE REPLACEMENT - Executive Summary Knee Replacement: Key Metrics in the US Market Prevalence of symptomatic knee osteoarthritis (OA) in 2013
Metha Short Hip Stem System
 Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
Metha Short Hip Stem System Accuracy That Stands Alone Aesculap Orthopaedics Metha Short Hip Stem System Designed For Anatomic Accuracy The Metha Short Hip Stem is designed for anatomic accuracy to restore
Revolution. Unicompartmental Knee System
 Revolution Unicompartmental Knee System While Total Knee Arthroplasty (TKA) is one of the most predictable procedures in orthopedic surgery, many patients undergoing TKA are in fact excellent candidates
Revolution Unicompartmental Knee System While Total Knee Arthroplasty (TKA) is one of the most predictable procedures in orthopedic surgery, many patients undergoing TKA are in fact excellent candidates
INVISION Total Ankle Replacement System with PROPHECY Preoperative Navigation Revision of a Failed Agility Total Ankle Replacement
 016625 REVISION R INVISION Total Ankle Replacement System with PROPHECY Preoperative Navigation Revision of a Failed Agility Total Ankle Replacement CASE STUDY Patient History The patient was a 65-year-old
016625 REVISION R INVISION Total Ankle Replacement System with PROPHECY Preoperative Navigation Revision of a Failed Agility Total Ankle Replacement CASE STUDY Patient History The patient was a 65-year-old
A Summary of Knee Recalls Consumers Union Safe Patient Project September 9, 2013
 A Summary of Knee Recalls Consumers Union Safe Patient Project September 9, 213 An estimated 4.4 million Americans are living with knee implants. In 211, over 711, 1 knee replacements were performed primarily
A Summary of Knee Recalls Consumers Union Safe Patient Project September 9, 213 An estimated 4.4 million Americans are living with knee implants. In 211, over 711, 1 knee replacements were performed primarily
Encina Taper Stem. Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA
 Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Table of Contents Introduction 3 Features 4 Surgical Technique 5 Preoperative
Stinson Orthopedics Inc. 303 Twin Dolphin Drive, Suite 600 Redwood City, CA 94065 info@stinsonortho.com www.stinsonortho.com Table of Contents Introduction 3 Features 4 Surgical Technique 5 Preoperative
Progeny Hip Stem. Surgical Protocol and Product Specifications
 Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Progeny Hip Stem Surgical Protocol and Product Specifications Progeny Hip Stem Introduction With emphasis on maximum stability and ease of use, the StelKast ProgenyTM Hip System provides the surgeon with
Intramedullary Tibial Preparation
 Surgical Technique Intramedullary Tibial Preparation Primary Total Knee Arthroplasty LEGION Total Knee System Intramedullary tibial preparation Contents Introduction...2 IM tibial highlights...3 Preoperative
Surgical Technique Intramedullary Tibial Preparation Primary Total Knee Arthroplasty LEGION Total Knee System Intramedullary tibial preparation Contents Introduction...2 IM tibial highlights...3 Preoperative
Hip & Knee Orthopedic Surgical Implants Market: Country wise Outlook
 Global Hip & Knee Orthopedic Surgical Implants market is projected to reach more than US$ 12 Billion by 2021, at a CAGR of XX% from 2016 to 2021. The growing prevalence of symptomatic osteoarthritis and
Global Hip & Knee Orthopedic Surgical Implants market is projected to reach more than US$ 12 Billion by 2021, at a CAGR of XX% from 2016 to 2021. The growing prevalence of symptomatic osteoarthritis and
Sulzer Orthopedics Joint & Fracture Care
 Sulzer Orthopedics Joint & Fracture Care Natural-Knee Family Anatomic design for superior clinical results. Natural-Knee Natural-Knee Family: A solution for every knee. Asymmetric design increases coverage
Sulzer Orthopedics Joint & Fracture Care Natural-Knee Family Anatomic design for superior clinical results. Natural-Knee Natural-Knee Family: A solution for every knee. Asymmetric design increases coverage
Shoulder Joint Replacement
 Shoulder Joint Replacement Although shoulder joint replacement is less common than knee or hip replacement, it is just as successful in relieving joint pain. Shoulder replacement surgery was first performed
Shoulder Joint Replacement Although shoulder joint replacement is less common than knee or hip replacement, it is just as successful in relieving joint pain. Shoulder replacement surgery was first performed
CAUTION: Ceramic liners are not approved for use in the United States.
 Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
Total Hip Prostheses, Self-Centering Hip Prostheses and Hemi-Hip Prostheses IMPORTANT: This essential product information sheet does not include all of the information necessary for selection and use of
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS
 SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
SURGICAL TECHNIQUE CEMENTED & PRESS-FIT UNIFIED INSTRUMENTATION INTRAOPERATIVE FLEXIBILITY PROVEN BIOMECHANICS INTRODUCTION The Summit Tapered Hip System s comprehensive set of implants and instruments
Reaching new heights. Comprehensive. Efficient. Simple.
 Reaching new heights Comprehensive. Efficient. Simple. Various acetabular cup choices Compatible with the different head and liner options including VERILAST Technology Reach for proven OR efficient Instrumentation
Reaching new heights Comprehensive. Efficient. Simple. Various acetabular cup choices Compatible with the different head and liner options including VERILAST Technology Reach for proven OR efficient Instrumentation
Featuring. Technology. Product Rationale
 Featuring Technology Product Rationale 2 Optimum implant geometry Extending proven TRI-LOCK heritage The original TRI-LOCK Stem was introduced in 1981. This implant was the first proximally coated tapered-wedge
Featuring Technology Product Rationale 2 Optimum implant geometry Extending proven TRI-LOCK heritage The original TRI-LOCK Stem was introduced in 1981. This implant was the first proximally coated tapered-wedge
Luminus Flex Total Knee System (Fixed Type) The Innovative Technology for Knee Replacement
 Luminus Flex Total Knee System (Fixed Type) The Innovative Technology for Knee Replacement Intro The Luminus-Flex Total Knee system takes its name from the English word "Luminous" An adjective meaning
Luminus Flex Total Knee System (Fixed Type) The Innovative Technology for Knee Replacement Intro The Luminus-Flex Total Knee system takes its name from the English word "Luminous" An adjective meaning
Consistency in U2 Knee System over Time
 U2 TM Knee System Consistency in U2 Knee System over Time Share the same femoral profile of all types allow inter-changibility Uniform AP/ML dimensions in all tibial components enhance intraoperative flexibility
U2 TM Knee System Consistency in U2 Knee System over Time Share the same femoral profile of all types allow inter-changibility Uniform AP/ML dimensions in all tibial components enhance intraoperative flexibility
Surgical Technique. Poly UHMWPE. Bio Hyaluronic Acid. Advancing Materials. Advancing Outcomes.
 Surgical Technique Bio Hyaluronic Acid Poly UHMWPE Advancing Materials. Advancing Outcomes. Surgical 2 Assessment 1. Proper implant sizing is determined by placing the appropriate sized trial over the
Surgical Technique Bio Hyaluronic Acid Poly UHMWPE Advancing Materials. Advancing Outcomes. Surgical 2 Assessment 1. Proper implant sizing is determined by placing the appropriate sized trial over the
RECOVERY. P r o t r u s i o
 RECOVERY P r o t r u s i o TM C a g e RECOVERY P r o t r u s i o TM C a g e Design Features Revision acetabular surgery is a major challenge facing today s total joint revision surgeon. Failed endo/bi-polars,
RECOVERY P r o t r u s i o TM C a g e RECOVERY P r o t r u s i o TM C a g e Design Features Revision acetabular surgery is a major challenge facing today s total joint revision surgeon. Failed endo/bi-polars,
Why precision is powerful
 Why precision is powerful A new answer for isolated patellofemoral OA First generation PFJ implants had sharp, constraining trochlear grooves and were prone to complications such as maltracking and catching
Why precision is powerful A new answer for isolated patellofemoral OA First generation PFJ implants had sharp, constraining trochlear grooves and were prone to complications such as maltracking and catching
A Patient s Guide to Hallux Rigidus
 A Patient s Guide to Hallux Rigidus Suite 11-13/14/15 Mount Elizabeth Medical Center 3 Mount Elizabeth Singapore, 228510 Phone: (65) 6738 2628 Fax: (65) 6738 2629 DISCLAIMER: The information in this booklet
A Patient s Guide to Hallux Rigidus Suite 11-13/14/15 Mount Elizabeth Medical Center 3 Mount Elizabeth Singapore, 228510 Phone: (65) 6738 2628 Fax: (65) 6738 2629 DISCLAIMER: The information in this booklet
Regenerex Porous Titanium Construct. Knees Hips Extremities Cement and Accessories PMI Trauma Technology
 Regenerex Porous Titanium Construct Knees Hips Extremities Cement and Accessories PMI Trauma Technology One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized
Regenerex Porous Titanium Construct Knees Hips Extremities Cement and Accessories PMI Trauma Technology One Surgeon. One Patient. Over 1 million times per year, Biomet helps one surgeon provide personalized
WHAT YOU IS BACK WITHIN ARM S REACH
 YOUR TOTAL SHOULDER REPLACEMENT SURGERY STEPS TO RETURNING TO A LIFESTYLE YOU DESERVE WHAT YOU IS BACK WITHIN ARM S REACH Nathan Richardson, MD Orthopedics, Shoulder & Elbow Surgeon Board Certified in
YOUR TOTAL SHOULDER REPLACEMENT SURGERY STEPS TO RETURNING TO A LIFESTYLE YOU DESERVE WHAT YOU IS BACK WITHIN ARM S REACH Nathan Richardson, MD Orthopedics, Shoulder & Elbow Surgeon Board Certified in
What Are Hip and Knee Replacement Implants Made Of?
 What Are Hip and Knee Replacement Implants Made Of? Hip and knee replacement surgery involve replacing the worn-out bone and cartilage lining your hip or knee joint with new implants that are composed
What Are Hip and Knee Replacement Implants Made Of? Hip and knee replacement surgery involve replacing the worn-out bone and cartilage lining your hip or knee joint with new implants that are composed
Furlong H-A.C. THR System
 Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
Important Information Please read prior to use in a clinical setting. The Surgeon should be familiar with the operative technique. Caution Federal (U.S.A) law restricts this device to sale by or on the
Unicondylar Knee Vs Total Knee Replacement: Is Less Better In the Middle Aged Athlete
 Unicondylar Knee Vs Total Knee Replacement: Is Less Better In the Middle Aged Athlete Chair: Maurilio Marcacci, MD Alois Franz "Basic principles and considerations of the Unis" Joao M. Barretto "Sport
Unicondylar Knee Vs Total Knee Replacement: Is Less Better In the Middle Aged Athlete Chair: Maurilio Marcacci, MD Alois Franz "Basic principles and considerations of the Unis" Joao M. Barretto "Sport
The challenge. Knee replacement patients report lower levels of satisfaction versus hip replacement patients. Return to sports activity post surgery
 The challenge Knee replacement patients report lower levels of satisfaction versus hip replacement patients. Return to sports activity post surgery THA 52% Post-op TKA 42% Preop 36% Preop 34% Post-op Total
The challenge Knee replacement patients report lower levels of satisfaction versus hip replacement patients. Return to sports activity post surgery THA 52% Post-op TKA 42% Preop 36% Preop 34% Post-op Total
EC Certification. FULL QUALITY ASSURANCE SYSTEM Directive 93/42/EEC for Medical Devices, Annex II excluding (4)
 EC Certification FULL QUALITY ASSURANCE SYSTEM Directive 93/42/EEC for Medical Devices, Annex II excluding (4) We hereby declare that an examination of the under mentioned full quality assurance system
EC Certification FULL QUALITY ASSURANCE SYSTEM Directive 93/42/EEC for Medical Devices, Annex II excluding (4) We hereby declare that an examination of the under mentioned full quality assurance system
JOINT RULER. Surgical Technique For Knee Joint JRReplacement
 JR JOINT RULER Surgical Technique For Knee Joint JRReplacement INTRODUCTION The Joint Ruler * is designed to help reduce the incidence of flexion, extension, and patellofemoral joint problems by allowing
JR JOINT RULER Surgical Technique For Knee Joint JRReplacement INTRODUCTION The Joint Ruler * is designed to help reduce the incidence of flexion, extension, and patellofemoral joint problems by allowing
ReCap Product Rationale
 ReCap Product Rationale A Complete resurfacing system designe engineering principles, tried and tested m clinically proven fixation methods Biomet UK Ltd Waterton Industrial Estate Bridgend, South Wales
ReCap Product Rationale A Complete resurfacing system designe engineering principles, tried and tested m clinically proven fixation methods Biomet UK Ltd Waterton Industrial Estate Bridgend, South Wales
HIP CONTINUUM OF CARE. Trauma and Joint Reconstruction Hip Portfolio
 HIP CONTINUUM OF CARE Trauma and Joint Reconstruction Hip Portfolio TABLE OF CONTENTS HIP CONTINUUM OF CARE Overview 4 Portfolio Highlight 6 PRODUCT SOLUTIONS Pelvic Fractures 10 Femoral Neck Fractures
HIP CONTINUUM OF CARE Trauma and Joint Reconstruction Hip Portfolio TABLE OF CONTENTS HIP CONTINUUM OF CARE Overview 4 Portfolio Highlight 6 PRODUCT SOLUTIONS Pelvic Fractures 10 Femoral Neck Fractures
FLH183 04/08. Biomet UK Ltd Waterton Industrial Estate Bridgend, South Wales CF31 3XA, United Kingdom. Tel. +44 (0) Fax: +44 (0)
 FLH183 04/08 Biomet UK Ltd Waterton Industrial Estate Bridgend, South Wales CF31 3XA, United Kingdom Tel. +44 (0)1656 655221 Fax: +44 (0)1656 645454 Exceed ABT Operative Technique The Exceed ABT TM acetabular
FLH183 04/08 Biomet UK Ltd Waterton Industrial Estate Bridgend, South Wales CF31 3XA, United Kingdom Tel. +44 (0)1656 655221 Fax: +44 (0)1656 645454 Exceed ABT Operative Technique The Exceed ABT TM acetabular
Hip Resurfacing System
 Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
Hip Resurfacing System The Arthrosurface HemiCAP Hip Hemiarthroplasty System restores the articular surface geometry of the femoral head and preserves functional structures using an innovative 3 dimensional
JOINT RECONSTRUCTION PORTFOLIO OVERVIEW
 JOINT RECONSTRUCTION PORTFOLIO OVERVIEW At DePuy Synthes Companies, we are inspired by you. DEPUY SYNTHES JOINT RECONSTRUCTION Uniquely focused on understanding today s opportunities and tomorrow s DePuy
JOINT RECONSTRUCTION PORTFOLIO OVERVIEW At DePuy Synthes Companies, we are inspired by you. DEPUY SYNTHES JOINT RECONSTRUCTION Uniquely focused on understanding today s opportunities and tomorrow s DePuy
comprehensive Design Rationale shoulder system Knees Hips Extremities Cement and Accessories PMI TEchnology
 comprehensive shoulder system Design Rationale Knees Hips Extremities Cement and Accessories PMI TEchnology . INTRODUCTION The Comprehensive Shoulder System is an evolutionary design based on the successful
comprehensive shoulder system Design Rationale Knees Hips Extremities Cement and Accessories PMI TEchnology . INTRODUCTION The Comprehensive Shoulder System is an evolutionary design based on the successful
ConforMIS, Inc. 28 Crosby Drive Bedford, MA Phone: Fax:
 ConforMIS, Inc. 28 Crosby Drive Bedford, MA 01730 Phone: 781.345.9001 Fax: 781.345.0147 www.conformis.com 0086 Authorized Representative: Medical Device Safety Service, GMBH Schiffgraben 41, 30175 Hannover,
ConforMIS, Inc. 28 Crosby Drive Bedford, MA 01730 Phone: 781.345.9001 Fax: 781.345.0147 www.conformis.com 0086 Authorized Representative: Medical Device Safety Service, GMBH Schiffgraben 41, 30175 Hannover,
Adult Reconstruction Hip Education Tracks
 Adult Reconstruction Hip Education Tracks Adult Reconstruction Hip Track for the Specialist - HIP1 ICL 281 A Case-based Approach to High Risk Total Hip - When Do I Do Something Differently? ICL 241 The
Adult Reconstruction Hip Education Tracks Adult Reconstruction Hip Track for the Specialist - HIP1 ICL 281 A Case-based Approach to High Risk Total Hip - When Do I Do Something Differently? ICL 241 The
Bone Bangalore
 Dr Suresh Annamalai MBBS, MRCS(Edn), FRCS( Tr & Orth)(Edn), FEBOT(European Board), Young Hip and Knee Fellowship(Harrogate, UK) Consultant Arthroplasty and Arthroscopic Surgeon Manipal Hospital, Whitefield,
Dr Suresh Annamalai MBBS, MRCS(Edn), FRCS( Tr & Orth)(Edn), FEBOT(European Board), Young Hip and Knee Fellowship(Harrogate, UK) Consultant Arthroplasty and Arthroscopic Surgeon Manipal Hospital, Whitefield,
A Systematic Review on Performance of the Vanguard Complete Knee System
 A Systematic Review on Performance of the Vanguard Complete Knee System Author: Jing Xie, Ph.D. June 30, 2011 Abstract A systematic review was conducted to evaluate the clinical performance of the Vanguard
A Systematic Review on Performance of the Vanguard Complete Knee System Author: Jing Xie, Ph.D. June 30, 2011 Abstract A systematic review was conducted to evaluate the clinical performance of the Vanguard
TOTAL KNEE ARTHROPLASTY (TKA)
 TOTAL KNEE ARTHROPLASTY (TKA) 1 Anatomy, Biomechanics, and Design 2 Femur Medial and lateral condyles Convex, asymmetric Medial larger than lateral 3 Tibia Tibial plateau Medial tibial condyle: concave
TOTAL KNEE ARTHROPLASTY (TKA) 1 Anatomy, Biomechanics, and Design 2 Femur Medial and lateral condyles Convex, asymmetric Medial larger than lateral 3 Tibia Tibial plateau Medial tibial condyle: concave
The Japanese Society For Replacement Arthroplasty The Japan Arthroplasty Register 2017/3/31
 The Japanese Society For Replacement Arthroplasty The Japan Arthroplasty Register 2017/3/31 90000 Total:88038 cases 80000 70000 60000 50000 40000 30000 20000 10000 0 2006 2007 20082009 20102011 2012 20132014
The Japanese Society For Replacement Arthroplasty The Japan Arthroplasty Register 2017/3/31 90000 Total:88038 cases 80000 70000 60000 50000 40000 30000 20000 10000 0 2006 2007 20082009 20102011 2012 20132014
Section of total knee replacement. Total Knee Replacement System. Knieendoprothesen System. Système de prothèse totale de genou
 Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
Section of total knee replacement Total Knee Replacement System Knieendoprothesen System Système de prothèse totale de genou Introduction: This knee system features great versality with its modular component
