REVIEW OF HOMOGENOUS CATEGORIES OF MEDICAL DEVICES. Spinal implants
|
|
|
- Rafe Summers
- 6 years ago
- Views:
Transcription
1 REVIEW OF HOMOGENOUS CATEGORIES OF MEDICAL DEVICES Spinal implants (Interbody cage, interspinous process spacer, spacer, lumbosacral support implant) Texte court Summary March 2013
2 L argumentaire scientifique de cette évaluation The full report supporting this assessment can be downloaded from Haute Autorité de Santé Documentation and Public Information Department 2, avenue du Stade de France - F Saint-Denis La Plaine CEDEX France Tel.: +33 (0) Fax: +33 (0) This document was validated by CNEDiMTS in March Haute Autorité de Santé 2013
3 Table of contents Spinal implants Project Management Team...4 Composition of the working group...5 Summary...7 List of abbreviations
4 Project management Team This report was produced by Albane MAINGUY, project manager, Medical Devices Assessment Department, tel.: , The following also worked on this dossier: - for the analysis and rating of clinical trials and meta-analyses, Estelle PIOTTO-PEYLAN, project manager, Medical Devices Assessment Department, tel.: , e.piotto@has-sante.fr - for the analysis of hospital activity linked to the implantation of spinal implants and for estimating the target population, Emmanuelle SCHAPIRO-DUFOUR, project manager, Medical Devices Assessment Department, tel.: , e.schapiro@hassante.fr The literature research and document management were carried out by Sophie DESPEYROUX (researcher, Documentation and Public Information Department, tel.: , s.despeyroux@has-sante.fr) and Sylvie LASCOLS (assistant researcher, Documentation and Public Information Department, tel: , s.lascols@has-sante.fr). The organisation of meetings and secretarial work were done by Yakaré TOUNKARA (tel.: , y.tounkara@has-sante.fr). Managers : Catherine DENIS (head of the Medical Devices Assessment Department, tel.: , c.denis@has-sante.fr). Corinne COLLIGNON (deputy head of the Medical Devices Assessment Department, tel.: , c.collignon@has-sante.fr). Frédérique PAGÈS (head of the Documentation and Public Information Department, tel. : , f.pages@has-sante.fr). 4
5 Composition of the working group The working group was made up of the following professionals: Dr Mohamed ALLAOUI, neurosurgeon, Roger Salengro Hospital, LILLE (59) Dr Thierry BAZIN, rheumatologist, Louis Dupic Medical Centre, VÉNISSIEUX (69) Dr Luc CHADAN, neurosurgeon in private practice, COMC de Dracy-le-Fort, DRACY-LE-FORT (71) Dr Patrick CHASTANET, radiologist, Roger Salengro Hospital, LILLE (59) Dr Mathieu DE SEZE, physical medicine and rehabilitation, Pellegrin Hospital Group, BORDEAUX (33) Dr Alexis FAURE, neurosurgeon, Parc de Cholet Polyclinic, CHOLET (49) Dr Stéphane GOUTAGNY, neurosurgeon, Beaujon Hospital, CLICHY (92) Prof. Olivier HAUGER, radiologist, Bordeaux University Hospital, BORDEAUX (33) Prof. Bernard IRTHUM, neurosurgeon, Gabriel Montpied Hospital, CLERMONT-FERRAND (63) Dr Mohamed Amine LAHLOU, neurosurgeon, Strasbourg University Hospital, STRASBOURG (67) Prof. Serge NAZARIAN, orthopaedic surgeon, Hospital of the Conception, MARSEILLE (13) Dr Remi PREBET, orthopaedic surgeon, Saint-Léonard Clinic, TRÉLAZÉ (49) Prof. Richard-Alexandre ROCHWERGER, member of CNEDiMTS, orthopaedic surgeon, Hospital of the Conception, MARSEILLE (13) Prof. Nicolas SANS, radiologist, Toulouse University Hospital, TOULOUSE (31) Prof. Jean-Rodolphe VIGNES, neurosurgeon, Bordeaux University Hospital, BORDEAUX (33) The members of the working group were appointed by the CNEDiMTS board from among the experts proposed by the relevant associations or learned societies, experts who responded to an appeal for candidates and experts known to HAS. In accordance with Decree No of 26 October 2004 (Art. R to R of the Social Security Code), all members of the working group completed a declaration of interests, mentioning direct or indirect links with any company or body involved in the field covered by the missions of HAS. These declarations of interests were published on the HAS website. The analysis of the declarations of interests was carried out in accordance with the criteria of the HAS Guide to declarations of interest and the management of conflicts of interests (adopted by 5
6 the HAS Board on 3 March 2010) 1. A table summarising the declared interests was examined by the CNEDiMTS board which decided on the final composition of the working group. The interests declared by the experts selected were all regarded as not major by the CNEDiMTS board. The summary table of declared interests was published and, if necessary, updated on the basis of the experts updated declarations of interests at the start of each meeting of the working group and on presentation of the working group s position at CNEDIMTS. 1 Haute Autorité de Santé. Guide des déclarations d'intérêts et de gestion des conflits d'intérêts. Saint-Denis La Plaine: HAS;
7 Summary Background Spinal implants are used in diseases such as disc hernation, degenerative diseases and post-traumatic instabilities. They are implanted following two surgical procedures which can be combined: arthrodesis (osteosynthesis, whether or not combined with a bone graft) and osteosynthesis alone. Osteosynthesis consists in keeping the fragments of a bone together by means of implants. The equipment used generally consists of internal fixation. The aim of arthrodesis is to permanently lock one or more intervertebral joints in the spinal column by fusing the bone in two or more vertebrae. Fusion is quickest when a graft is used in combination with osteosynthesis. The combination of arthrodesis with osteosynthesis makes it possible to give immediate stability to an unstable spine or one which has been destabilised by an operation, to correct a spinal deformity or intervertebral disc displacement, and to improve the bone fusion rate by reducing the risk of pseudarthrosis. Whatever procedure is used, there are two possible routes of approach (anterior and posterior) and they can either be combined or used alone, depending on the type of deformation, its rigidity and its severity. These two routes use designs of device adapted to the patient s anatomy. This revision on spinal implants was made in the context of an opinion which appeared in the Official Journal on 8 September 2011, and concerns spinal implants belonging to four categories in the nomenclature, defined as follows: - interspinous process spacer; - spacer; - lumbosacral support implant; - interbody cage. Objectives Working method The objectives of this work were to reassess spinal implants so as to: - determine the indications for spinal implants; - assess their actual benefit per indication; - define their place in therapeutic strategy; - characterise the technical specifications which determine the actual benefit, so as to avoid classification mistakes and to clarify which devices are covered by the current generic description; - propose an updated nomenclature based on the form and composition of devices; - estimate their target population; - define the expectations of CNEDiMTS regarding clinical trials needed for inclusion under the brand name on the List of products and services qualifying for reimbursement (LPPR) ; - define the conditions of use and prescription for inclusion of the products on the LPPR. The working method is based on a systematic review of the literature and an analysis of the dossiers submitted by manufacturers, and draws on the expertise of healthcare professionals meeting in the multidisciplinary working group dedicated to the subject. 7
8 Assessment Analysis of the data The assessment covered the following assessment criteria: pain, patient satisfaction, quality of life, the Oswestry Disability Index (ODI score), the fusion rate, the length of the operation, blood loss, complications and the reoperation rate. The literature data show that spinal implants are used in several diseases: - cervical degenerative diseases; - lumbar degenerative diseases, including chronic low back pain. In other indications such as fractures, tumours and specifically in cervical spondylosis, the literature data are limited, or even nonexistent. Studies confirm the benefit of techniques using interbody cages and reconstruction implants after corporectomy, but do not allow an assessment of these implants alone (particularly the cages, which are often combined with osteosynthesis). The data are conflicting as regards the benefit of interspinous process implant. There are no specific data on sacral support implants used in addition to longitudinal osteosynthesis or on spacer. There are no clinical data that can be used to claim the benefit of one implant over another, and there are very few descriptions of the technical specifications of the implants. Working group opinion In the absence of literature with a good level of evidence and or good grades of recommendations, the working group s proposals are based mainly on the members clinical practice. Since no precise definition of spinal implants is available in the literature, the group defined each of these devices by stating their technical specifications, and described the indications in which these implants are used. The group also specified the methods of use, particularly the need for additional osteosynthesis when implanting a cage or a reconstructive vertebral body implant and the limitation to one reconstructive vertebral body implant per level treated if several levels are treated. The working group s main proposals on the nomenclature concerned: - the distinction of interbody cages and reconstruction implants after corporectomy ; - for cages and reconstructive vertebral body implants, the creation of generic descriptions according to their anatomical level (cervical and thoracolumbar), the presence or otherwise of a bone substitute and an integrated fixing system; - the suppression of generic descriptions concerning interspinous process spacer because of the disparity of their designs and their technical specifications and the proposal of registration under the brand name; - the suppression of generic descriptions concerning spacer because of the heterogeneity of these devices, the inability to define and describe the indications and common, minimal technical specifications and the proposal of registration under the brand name; - the inclusion of sacral support implants with longitudinal fusion implants, since they cannot be assessed separately from osteosynthesis implants. General conclusion of the National Committee for the Evaluation of Medical Devices and Health Technologies (CNEDiMTS) The CNEDiMTS accepted most of the working group s proposals, adding a few precisions about the benefit of reconstructive vertebral body implants. In fact, after corporectomy the spine is unstable, and reconstruction implants after corporectomy need an effective anchorage system which is provided by a decompression system in the implant. 8
9 Overall, this assessment has confirmed the benefit of interbody cages, and the distinction of reconstruction implant after corporectomy. In addition, it did not produce any evidence of the benefit of interspinous process spacer or spacer. Two main categories of spinal implants are identified for inclusion under a generic description: 1. interbody cages 2. reconstruction implants after corporectomy The committee recommends, for these two types of implants, the creation of eight generic descriptions according to the level treated (cervical and thoracolumbar) and according to whether or not there is a bone substitute and an integrated fixing system. For a given indication, the CNEDMiTS ruled that there is no added clinical value (ACV V) of one line over another. The committee recommends, for the categories interspinous process spacer and spacer, deletion of these generic descriptions and inclusion by brand name. Finally, for the category lumbosacral support implants, the committee recommends the reinclusion of the generic description for longitudinal reconstructive implants. The CNEDIMTS defined the criteria for assessing devices under their brand name if they do not meet the technical specifications of the proposed generic descriptions. For cages and reconstructive vertebral body implants, manufacturers must prove efficacy by means of a randomised controlled trial versus well-managed medical treatment or versus surgery (conventional arthrodesis with an autologous graft and osteosynthesis) with a follow-up period of not less than 24 months. The recommended outcomes are: - clinical endpoint: the pain assessed on the VAS (visual analogue scale) and radicular pain, quality of life (SF-12 or SF-36 quality of life questionnaire), the neck disability index (NDI) or the lumbar disability index (LDI), functional endpoint: the walking distance (according to the disease treated); the primary endpoint will be selected from among these clinical outcomes; - radiological endpoint (standard X-ray, CT scan or MRI showing the discopathy). For interspinous process spacer and spacer, manufacturers must prove efficacy by means of a randomised controlled trial versus well-managed medical treatment or versus surgery, in the indication claimed (for interspinous process spacer, the identified indication is dynamic stenosis or discopathy). The CNEDiMTS recommends follow-up for 24 months with interim results at 6 months. The recommended outcomes are: - clinical endpoint: lumbar and radicular pain assessed on the VAS (visual analogue scale), quality of life (quality of life questionnaire SF12 or SF36), functional endpoint: the JOA (Japan Orthopaedic Association), the walking distance; - radiological endpoint (standard X-ray, CT scan or MRI, since a dynamic X-ray could be a contraindication to the insertion of an interspinous process implant, bone computed tomography). These outcomes should be adapted according to the disease treated. According to the indication, other outcomes can be considered and the period of follow-up adapted (particularly in oncology). In all cases, the outcomes used must be validated. 9
10 List of abbreviations ACD Anterior cervical discectomy without fusion ACDF Anterior cervical discectomy and fusion AFIDEO Association of European manufacturers, importer and distributors of orthopaedic and traumatologiocal implants AHCPR Agency for Health Care Policy and Research ALIF Anterior lumbar interbody fusion ANSM National Medicines and Health Products Safety Agency (formerly Afssaps) Afssaps French Health Products Safety Agency ACV Added clinical value CCAM Joint classification of medical procedures CEPP Committee for the assessment of products and services CEPS Committee for the Pricing of Healthcare Products CF Circumferential fusion CNAMTS National Salaried Workers Health Insurance Fund CNEDiMTS National Committee for the Evaluation of Medical Devices and Health Technologies DGOS Directorate General for the Provision of Healthcare DGS Directorate-General for Health DSS Social Security Directorate RCT Randomised controlled trial VAS Visual analogue scale HAS Haute Autorité de Santé CI Confidence interval MRI Magnetic resonance imaging JOA Japanese Orthopaedic Association LPPR List of products and services qualifying for reimbursement MCS SF-36 mental composite score MD Median difference NASS North American Spine Society NDI Neck Disability Index NGAP General Nomenclature of Medical Procedures NICE National Institute for Health and Clinical Excellence NR Not known NS Non-significant OCS Oxford Claudication Score ODI Oswestry Disability Index OR Odds ratio PCS SF-36 physical composite score PEEK Polyetheretherketone PEKEKK Polyetherketoneetherketoneketone PLDLLA Poly-L-lactide-co-D,L-lactide PLF Posterolateral fusion 10
11 PLIF PMSI RPC RR RSA RSI SA SD SFNC SFR SIE SMD SNATIH SNITEM SOFCOT SSTF SR SWT TENS TDR TLIF XLIF ZCQ Posterior lumbar interbody fusion Programme for clinical information systems Clinical practice guideline Relative risk Summary of anonymised withdrawals Health and retirement scheme for independent workers Expected clinical value Standard deviation French Society of Neurosurgery French Society of Radiology Interspinous system Standard median difference National System of Information on Hospital Admissions National union of the medical technology industry French Society of Orthopaedic and Trauma Surgery Short segment transpedicular fixation Clinical value Shuttle walk test Transcutaneous electrical nerve stimulation Total disc replacement Transforaminal lumbar interbody fusion extreme lateral interbody fusion Zurich claudication questionnaire 11
Assessment of cardiac defibrillator leads
 REVIEW OF HOMOGENEOUS CATEGORIES OF MEDICAL DEVICES Assessment of cardiac defibrillator leads Summary "Date validated by CNEDiMTS:" 24 January 2017 This dossier can be downloaded at www.has-sante.fr Haute
REVIEW OF HOMOGENEOUS CATEGORIES OF MEDICAL DEVICES Assessment of cardiac defibrillator leads Summary "Date validated by CNEDiMTS:" 24 January 2017 This dossier can be downloaded at www.has-sante.fr Haute
Assessment of an Edge-to-Edge Mitral Valve Repair Clip and its Implantation
 b MEDICAL DEVICES ASSESSMENT DEPARTMENT Assessment of an Edge-to-Edge Mitral Valve Repair Clip and its Implantation Summary of the Technology Assessment report This dossier can be downloaded at www.has-sante.fr
b MEDICAL DEVICES ASSESSMENT DEPARTMENT Assessment of an Edge-to-Edge Mitral Valve Repair Clip and its Implantation Summary of the Technology Assessment report This dossier can be downloaded at www.has-sante.fr
EVALUATION OF NEGATIVE-PRESSURE WOUND THERAPY
 EVALUATION OF NEGATIVE-PRESSURE WOUND THERAPY JANUARY 2010 Medical Devices Assessment Department 1 You can download this document on the following web site www.has-sante.fr Haute Autorité de santé Service
EVALUATION OF NEGATIVE-PRESSURE WOUND THERAPY JANUARY 2010 Medical Devices Assessment Department 1 You can download this document on the following web site www.has-sante.fr Haute Autorité de santé Service
5/19/2017. Interspinous Process Fixation with the Minuteman G3. What is the Minuteman G3. How Does it Work?
 Interspinous Process Fixation with the Minuteman G3 LLOYDINE J. JACOBS, MD CASTELLVI SPINE MEETING MAY 13, 2017 What is the Minuteman G3 The world s first spinous process plating system that is: Minimally
Interspinous Process Fixation with the Minuteman G3 LLOYDINE J. JACOBS, MD CASTELLVI SPINE MEETING MAY 13, 2017 What is the Minuteman G3 The world s first spinous process plating system that is: Minimally
Replacement Code for Interbody Cage for Disc
 +22851 vs. +20931 October 22, 2015 We ve been told we cannot bill +22851 and +20931 with the ACDF code, 22551. Is this true? It is true if you are thinking about reporting +22851 (intervertebral device)
+22851 vs. +20931 October 22, 2015 We ve been told we cannot bill +22851 and +20931 with the ACDF code, 22551. Is this true? It is true if you are thinking about reporting +22851 (intervertebral device)
DEGENERATIVE SPONDYLOLISTHESIS
 AN INTRODUCTION TO DEGENERATIVE SPONDYLOLISTHESIS This booklet is designed to inform you about lumbar degenerative spondylolisthesis. It is not meant to replace any personal conversations that you might
AN INTRODUCTION TO DEGENERATIVE SPONDYLOLISTHESIS This booklet is designed to inform you about lumbar degenerative spondylolisthesis. It is not meant to replace any personal conversations that you might
Original Date: October 2015 LUMBAR SPINAL FUSION FOR
 National Imaging Associates, Inc. Clinical guidelines Original Date: October 2015 LUMBAR SPINAL FUSION FOR Page 1 of 9 INSTABILITY AND DEGENERATIVE DISC CONDITIONS FOR CMS (MEDICARE) MEMBERS ONLY CPT4
National Imaging Associates, Inc. Clinical guidelines Original Date: October 2015 LUMBAR SPINAL FUSION FOR Page 1 of 9 INSTABILITY AND DEGENERATIVE DISC CONDITIONS FOR CMS (MEDICARE) MEMBERS ONLY CPT4
Anterior cervical diskectomy icd 10 procedure code
 Home Anterior cervical diskectomy icd 10 procedure code Access to discounts at hundreds of restaurants, travel destinations, retail stores, and service providers. AAPC members also have opportunities to
Home Anterior cervical diskectomy icd 10 procedure code Access to discounts at hundreds of restaurants, travel destinations, retail stores, and service providers. AAPC members also have opportunities to
TREATMENT WITH IMPLANT-SUPPORTED
 TREATMENT WITH IMPLANT-SUPPORTED DENTURES IN ADULTS WITH MULTIPLE TOOTH AGENESIS RELATED TO A RARE DESEASE - ASSESSMENT OF PROCEDURES ASSOCIATED WITH PRE-IMPLANT SURGERY, IMPLANT AND IMPLANT- SUPPORTED
TREATMENT WITH IMPLANT-SUPPORTED DENTURES IN ADULTS WITH MULTIPLE TOOTH AGENESIS RELATED TO A RARE DESEASE - ASSESSMENT OF PROCEDURES ASSOCIATED WITH PRE-IMPLANT SURGERY, IMPLANT AND IMPLANT- SUPPORTED
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine...2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Innovative Techniques in Minimally Invasive Cervical Spine Surgery. Bruce McCormack, MD San Francisco California
 Innovative Techniques in Minimally Invasive Cervical Spine Surgery Bruce McCormack, MD San Francisco California PCF Posterior Cervical Fusion PCF not currently an ambulatory care procedure Pearl diver
Innovative Techniques in Minimally Invasive Cervical Spine Surgery Bruce McCormack, MD San Francisco California PCF Posterior Cervical Fusion PCF not currently an ambulatory care procedure Pearl diver
EFSPINE CERVICAL COMBINED SET DISC PROTHESIS ORGANIZER BOX
 EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
EFSPINE CERVICAL COMBINED SET INSTRUMENTS CERVICAL CAGE & DISC PROTHESIS ORGANIZER BOX Cervical Thoracic Thoraco - Lumbar Sacral EFSPINE CERVICAL COMBINED SET CERVICAL IMPLANTS INTRODUCTION Cervical Disc
The Fusion of. Strength, Design. Performance. and
 The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
The Fusion of Strength, Design and Performance Interbody Fusion Platform Interbody Fusion Platform The DePuy Spine Interbody Fusion platform is designed to provide an open architecture implant in a load-sharing
Patient Information MIS LLIF. Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
Patient Information MIS LLIF Lateral Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques Table of Contents Anatomy of Spine....2 General Conditions of the Spine....4 What is Spondylolisthesis....5
U.S. MARKET FOR MINIMALLY INVASIVE SPINAL IMPLANTS
 U.S. MARKET FOR MINIMALLY INVASIVE SPINAL IMPLANTS idata_usmis15_rpt Published in December 2014 By idata Research Inc., 2014 idata Research Inc. Suite 308 4211 Kingsway Burnaby, British Columbia, Canada,
U.S. MARKET FOR MINIMALLY INVASIVE SPINAL IMPLANTS idata_usmis15_rpt Published in December 2014 By idata Research Inc., 2014 idata Research Inc. Suite 308 4211 Kingsway Burnaby, British Columbia, Canada,
Uncosectomy Facilitated Cervical Foraminotomy using a new high-speed shielded curved device
 Uncosectomy Facilitated Cervical Foraminotomy using a new high-speed shielded curved device Pierre Bernard, M.D. (1), Michal Tepper, Ph.D. (2), Ely Ashkenazi, M.D. (3) (1) Centre Aquitain du Dos, Hôpital
Uncosectomy Facilitated Cervical Foraminotomy using a new high-speed shielded curved device Pierre Bernard, M.D. (1), Michal Tepper, Ph.D. (2), Ely Ashkenazi, M.D. (3) (1) Centre Aquitain du Dos, Hôpital
2016 OPAM Mid-Year Educational Conference, sponsored by AOCOPM Thursday, March 10, 2016 C-1
 Long-term Outcomes of Lumbar Fusion Among Workers Compensation Subjects : An Historical Cohort Study Trang Nguyen M.D., Ph.D. David C. Randolph M.D, M.P.H. James Talmage MD Paul Succop PhD Russell Travis
Long-term Outcomes of Lumbar Fusion Among Workers Compensation Subjects : An Historical Cohort Study Trang Nguyen M.D., Ph.D. David C. Randolph M.D, M.P.H. James Talmage MD Paul Succop PhD Russell Travis
Prosthetic intervertebral disc replacement in the cervical spine: Cost-effectiveness compared with cervical discectomy with or without vertebral
 Prosthetic intervertebral disc replacement in the cervical spine: Cost-effectiveness compared with cervical discectomy with or without vertebral fusion Author: William Horsley NHS North East Treatment
Prosthetic intervertebral disc replacement in the cervical spine: Cost-effectiveness compared with cervical discectomy with or without vertebral fusion Author: William Horsley NHS North East Treatment
PROCEDURES WE PERFORM
 PROCEDURES WE PERFORM Decompression, Stabilization, and More North American Spine offers a family of advanced, minimally invasive procedures that are highly effective in treating most forms of chronic
PROCEDURES WE PERFORM Decompression, Stabilization, and More North American Spine offers a family of advanced, minimally invasive procedures that are highly effective in treating most forms of chronic
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transaxial interbody lumbosacral fusion for severe chronic low back pain As a
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of transaxial interbody lumbosacral fusion for severe chronic low back pain As a
You Don t Understand This Stuff???
 SPINE SURGERY You Don t Understand This Stuff??? Welcome to the club Most PCP s don t understand what we do Half of other orthopods don t get it Sometimes we can t even explain it to each other Why would
SPINE SURGERY You Don t Understand This Stuff??? Welcome to the club Most PCP s don t understand what we do Half of other orthopods don t get it Sometimes we can t even explain it to each other Why would
2012 CPT Coding Update AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves
 2012 CPT Coding Update AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves Joseph S. Cheng, M.D., M.S. Associate Professor of Neurological Surgery, Orthopedic Surgery, and Rehabilitation
2012 CPT Coding Update AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves Joseph S. Cheng, M.D., M.S. Associate Professor of Neurological Surgery, Orthopedic Surgery, and Rehabilitation
Spine Tango annual report 2012
 DOI 10.1007/s00586-013-2943-x SPINE TANGO REPORT 2012 Spine Tango annual report 2012 M. Neukamp G. Perler T. Pigott E. Munting M. Aebi C. Röder Received: 31 July 2013 / Published online: 30 August 2013
DOI 10.1007/s00586-013-2943-x SPINE TANGO REPORT 2012 Spine Tango annual report 2012 M. Neukamp G. Perler T. Pigott E. Munting M. Aebi C. Röder Received: 31 July 2013 / Published online: 30 August 2013
Systematic review Cervical artificial disc replacement versus fusion in the cervical spine: a systematic review (...)
 Systematic review Cervical artificial disc replacement versus fusion in the cervical spine: a systematic review (...) 59 59 66 Cervical artificial disc replacement versus fusion in the cervical spine:
Systematic review Cervical artificial disc replacement versus fusion in the cervical spine: a systematic review (...) 59 59 66 Cervical artificial disc replacement versus fusion in the cervical spine:
CERVICAL PROCEDURES PHYSICIAN CODING
 CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
CERVICAL PROCEDURES PHYSICIAN CODING Anterior Cervical Discectomy with Interbody Fusion (ACDF) Anterior interbody fusion, with discectomy and decompression; cervical below C2 22551 first interspace 22552
Int J Clin Exp Med 2018;11(2): /ISSN: /IJCEM Yi Yang, Hao Liu, Yueming Song, Tao Li
 Int J Clin Exp Med 2018;11(2):1278-1284 www.ijcem.com /ISSN:1940-5901/IJCEM0063093 Case Report Dislocation and screws pull-out after application of an Isobar TTL dynamic stabilisation system at L2/3 in
Int J Clin Exp Med 2018;11(2):1278-1284 www.ijcem.com /ISSN:1940-5901/IJCEM0063093 Case Report Dislocation and screws pull-out after application of an Isobar TTL dynamic stabilisation system at L2/3 in
Patient Information MIS TLIF. Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine..............................................
Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine..............................................
Surgical Technique INTERSOMATIC CERVICAL CAGE
 R INTERSOMATIC CERVICAL CAGE NEOCIF IMPLANTS NEOCIF is an implant designed to make anterior cervical interbody fusion (ACIF) easier and to remove the need for structural autologous graft. The cage is made
R INTERSOMATIC CERVICAL CAGE NEOCIF IMPLANTS NEOCIF is an implant designed to make anterior cervical interbody fusion (ACIF) easier and to remove the need for structural autologous graft. The cage is made
Departement of Neurosurgery A.O.R.N A. Cardarelli- Naples.
 Percutaneous posterior pedicle screw fixation in the treatment of thoracic, lumbar and thoraco-lumbar junction (T12-L1) traumatic and pathological spine fractures. Report of 45 cases. G. Vitale, A. Punzo,
Percutaneous posterior pedicle screw fixation in the treatment of thoracic, lumbar and thoraco-lumbar junction (T12-L1) traumatic and pathological spine fractures. Report of 45 cases. G. Vitale, A. Punzo,
ProDisc-L Total Disc Replacement. IDE Clinical Study.
 ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
ProDisc-L Total Disc Replacement. IDE Clinical Study. A multi-center, prospective, randomized clinical trial. Instruments and implants approved by the AO Foundation Table of Contents Indications, Contraindications
University of Groningen. Thoracolumbar spinal fractures Leferink, Vincentius Johannes Maria
 University of Groningen Thoracolumbar spinal fractures Leferink, Vincentius Johannes Maria IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
University of Groningen Thoracolumbar spinal fractures Leferink, Vincentius Johannes Maria IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
Patient Information MIS TLIF. Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques
 Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine...2 General Conditions of the Spine...4 6 MIS-TLIF
Patient Information MIS TLIF Transforaminal Lumbar Interbody Fusion Using Minimally Invasive Surgical Techniques MIS TLIF Table of Contents Anatomy of Spine...2 General Conditions of the Spine...4 6 MIS-TLIF
22110 vertebral segment; cervical vertebral segment; thoracic vertebral segment; lumbar
 The following codes are authorized by Palladian Health for applicable product lines. Visit palladianhealth.com to request authorization and to access guidelines. Palladian Musculoskeletal Program Codes
The following codes are authorized by Palladian Health for applicable product lines. Visit palladianhealth.com to request authorization and to access guidelines. Palladian Musculoskeletal Program Codes
PARADIGM SPINE. Anterior Cervical Fusion Cage. Cervical Interbody Fusion
 PARADIGM SPINE Anterior Cervical Fusion Cage Cervical Interbody Fusion DESIGN RATIONALE The OptiStrain TM C* interbody fusion cage follows well established biomechanical principles: The slot design of
PARADIGM SPINE Anterior Cervical Fusion Cage Cervical Interbody Fusion DESIGN RATIONALE The OptiStrain TM C* interbody fusion cage follows well established biomechanical principles: The slot design of
The Role of Surgery in the Treatment of Low Back Pain and Radiculopathy. Christian Etter, MD, Spine Surgeon Zürich, Switzerland
 The Role of Surgery in the Treatment of Low Back Pain and Radiculopathy Christian Etter, MD, Spine Surgeon Zürich, Switzerland WW Fusion Volume by Disorder 2004E % Tumor/Trauma 11% Deformity 15% Degeneration
The Role of Surgery in the Treatment of Low Back Pain and Radiculopathy Christian Etter, MD, Spine Surgeon Zürich, Switzerland WW Fusion Volume by Disorder 2004E % Tumor/Trauma 11% Deformity 15% Degeneration
CPT CODING EXAMPLES FUSION PROCEDURES. Anterior Lumbar Interbody Fusion (ALIF)
 CPT CODING EXAMPLES This list represents coding examples for common spine procedures. The information can also be used in conjunction with the Medicare Fee Calculator on http://www.cms.gov/apps/physician-fee-schedule/
CPT CODING EXAMPLES This list represents coding examples for common spine procedures. The information can also be used in conjunction with the Medicare Fee Calculator on http://www.cms.gov/apps/physician-fee-schedule/
Is unilateral pedicle screw fixation superior than bilateral pedicle screw fixation for lumbar degenerative diseases: a meta-analysis
 Lu et al. Journal of Orthopaedic Surgery and Research (2018) 13:296 https://doi.org/10.1186/s13018-018-1004-x SYSTEMATIC REVIEW Open Access Is unilateral pedicle screw fixation superior than bilateral
Lu et al. Journal of Orthopaedic Surgery and Research (2018) 13:296 https://doi.org/10.1186/s13018-018-1004-x SYSTEMATIC REVIEW Open Access Is unilateral pedicle screw fixation superior than bilateral
Chiropodist-podiatrist consultations for preventing foot lesions in diabetics
 Chiropodist-podiatrist consultations for preventing foot lesions in diabetics Summary document July 2007 Department for the Assessment of Medical and Surgical Procedures 2 avenue du Stade de France 93218
Chiropodist-podiatrist consultations for preventing foot lesions in diabetics Summary document July 2007 Department for the Assessment of Medical and Surgical Procedures 2 avenue du Stade de France 93218
Spine Center. at Stamford Hospital s Orthopedic Institute
 Spine Center at Stamford Hospital s Orthopedic Institute Back pain related to spinal deformity and injury or congenital conditions is a common health complaint, which can be very debilitating. At Stamford
Spine Center at Stamford Hospital s Orthopedic Institute Back pain related to spinal deformity and injury or congenital conditions is a common health complaint, which can be very debilitating. At Stamford
A Structural Service Plan: Towards Better and Safer Spine Surgeries. Department of Orthopaedics & Traumatology Tuen Mun Hospital
 A Structural Service Plan: Towards Better and Safer Spine Surgeries Department of Orthopaedics & Traumatology Tuen Mun Hospital Cheung KK Wong CY Chan Andrew Tse Alfred Chow YY Department of Orthopaedics
A Structural Service Plan: Towards Better and Safer Spine Surgeries Department of Orthopaedics & Traumatology Tuen Mun Hospital Cheung KK Wong CY Chan Andrew Tse Alfred Chow YY Department of Orthopaedics
Original Article Management of Single Level Lumbar Degenerative Spondylolisthesis: Decompression Alone or Decompression and Fusion
 Egyptian Journal of Neurosurgery Volume 9 / No. 4 / October - December 014 51-56 Original Article Management of Single Level Lumbar Degenerative Spondylolisthesis: Decompression Alone or Decompression
Egyptian Journal of Neurosurgery Volume 9 / No. 4 / October - December 014 51-56 Original Article Management of Single Level Lumbar Degenerative Spondylolisthesis: Decompression Alone or Decompression
Interbody fusion cage for the transforaminal approach. Travios. Surgical Technique
 Interbody fusion cage for the transforaminal approach Travios Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes
Interbody fusion cage for the transforaminal approach Travios Surgical Technique Image intensifier control This description alone does not provide sufficient background for direct use of DePuy Synthes
Patient Information ACDF. Anterior Cervical Discectomy and Fusion
 Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Patient Information ACDF Anterior Cervical Discectomy and Fusion Table of Contents Anatomy of the Spine...2-3 General Conditions of the Cervical Spine...4 5 What is an ACDF?...6 How is an ACDF performed?...7
Bare Bones. Use of Systemic Osteoporosis Drugs in Select High Risk Patients Undergoing Spine Surgery
 Use of Systemic Osteoporosis Drugs in Select High Risk Patients Undergoing Spine Surgery 30th Annual Metabolic Bone Disease Society Meeting Denver, CO Oct 11, 2018 Bare Bones 2000 Francesca Pera Kelly
Use of Systemic Osteoporosis Drugs in Select High Risk Patients Undergoing Spine Surgery 30th Annual Metabolic Bone Disease Society Meeting Denver, CO Oct 11, 2018 Bare Bones 2000 Francesca Pera Kelly
Lumbar Spinal Fusion Corporate Medical Policy
 Lumbar Spinal Fusion Corporate Medical Policy File name: Lumbar Spinal Fusion File code: UM.SURG.15 Origination: 09/01/2016 Last Review: 09/29/2016 Next Review: 09/29/2017 Effective Date: 01/01/2017 Populations
Lumbar Spinal Fusion Corporate Medical Policy File name: Lumbar Spinal Fusion File code: UM.SURG.15 Origination: 09/01/2016 Last Review: 09/29/2016 Next Review: 09/29/2017 Effective Date: 01/01/2017 Populations
1105 two (2) vertebrae... 1, add on per additional vertebra
 SPINE STAGE OPERATIONS Staged operations shall be paid at 100% for the first stage and 85% for the second stage. Where the second stage pays a higher fee 100% shall be paid and the first stage shall be
SPINE STAGE OPERATIONS Staged operations shall be paid at 100% for the first stage and 85% for the second stage. Where the second stage pays a higher fee 100% shall be paid and the first stage shall be
NVM5 NERVE MONITORING SYSTEM AN INTRODUCTION TO
 AN INTRODUCTION TO NVM5 NERVE MONITORING SYSTEM This booklet is designed to inform you about the use of NVM5 nerve monitoring in the course of your surgery. It is not meant to replace any personal conversations
AN INTRODUCTION TO NVM5 NERVE MONITORING SYSTEM This booklet is designed to inform you about the use of NVM5 nerve monitoring in the course of your surgery. It is not meant to replace any personal conversations
CPT 2015: Save Your Practice By Shaping Up Your Spinal Procedure Reporting
 2015 Physician Coding Survival Guide CHAPTER 10: NEUROSURGERY CPT 2015: Save Your Practice By Shaping Up Your Spinal Procedure Reporting Sacroplasty codes will now be inclusive of imaging guidance. You
2015 Physician Coding Survival Guide CHAPTER 10: NEUROSURGERY CPT 2015: Save Your Practice By Shaping Up Your Spinal Procedure Reporting Sacroplasty codes will now be inclusive of imaging guidance. You
ACDF. Anterior Cervical Discectomy and Fusion. An introduction to
 An introduction to ACDF Anterior Cervical Discectomy and Fusion This booklet provides general information on ACDF. It is not meant to replace any personal conversations that you might wish to have with
An introduction to ACDF Anterior Cervical Discectomy and Fusion This booklet provides general information on ACDF. It is not meant to replace any personal conversations that you might wish to have with
JCSC INTRODUCTION. Rudolf Morgenstern, MD, PhD
 Diagnosis The lumbar lateral X-ray images in forward flexion and extension showed an anterior displacement of 10 mm in extension and of 6 mm in flexion measured with the methonline ML Comm JCSC DEGENERATIVE
Diagnosis The lumbar lateral X-ray images in forward flexion and extension showed an anterior displacement of 10 mm in extension and of 6 mm in flexion measured with the methonline ML Comm JCSC DEGENERATIVE
PARADIGM SPINE. Brochure. coflex-f Minimally Invasive Lumbar Fusion
 PARADIGM SPINE Brochure coflex-f Minimally Invasive Lumbar Fusion coflex-f THE UNIQUE, MINIMALLY INVASIVE FUSION DEVICE The coflex-f implant is designed to deliver surgeon confidence and patient satisfaction.
PARADIGM SPINE Brochure coflex-f Minimally Invasive Lumbar Fusion coflex-f THE UNIQUE, MINIMALLY INVASIVE FUSION DEVICE The coflex-f implant is designed to deliver surgeon confidence and patient satisfaction.
Medical Policy Original Effective Date: Revised Date: Page 1 of 11
 Page 1 of 11 Content Disclaimer Description Coverage Determination Clinical Indications Lumbar Spine Surgery Lumbar Spine Surgery Description Indication Coding Lumbar Spinal Fusion (single level)surgery
Page 1 of 11 Content Disclaimer Description Coverage Determination Clinical Indications Lumbar Spine Surgery Lumbar Spine Surgery Description Indication Coding Lumbar Spinal Fusion (single level)surgery
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Lumbar Spinal Fusion Page 1 of 28 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Lumbar Spinal Fusion Interspinous Fixation (Fusion) Devices http://www.bcbsks.com/customerservice/providers/medicalpolicies/policies.shtml
Lumbar Spinal Fusion Page 1 of 28 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Lumbar Spinal Fusion Interspinous Fixation (Fusion) Devices http://www.bcbsks.com/customerservice/providers/medicalpolicies/policies.shtml
/ 66 nano-hydroxyapatite/polyamide-66 n-ha/pa66
 1425 / 66 / 66 nano-hydroxyapatite/polyamide-66 n-ha/pa66 2011 1 10 20 n-ha/pa66 8 12 22 80 51 1 24 4 L 4 5 8 L 5 S 1 9 L 4 S 1 3 3 5 9 3 X CT Oswestry ODI SF-36 20 6 9 7 3 d 3 6 P < 0.01P > 0.05 3 9 4
1425 / 66 / 66 nano-hydroxyapatite/polyamide-66 n-ha/pa66 2011 1 10 20 n-ha/pa66 8 12 22 80 51 1 24 4 L 4 5 8 L 5 S 1 9 L 4 S 1 3 3 5 9 3 X CT Oswestry ODI SF-36 20 6 9 7 3 d 3 6 P < 0.01P > 0.05 3 9 4
Interspinous Fusion Devices. Midterm results. ROME SPINE 2012, 7th International Meeting Rome, 6-7 December 2012
 Interspinous Fusion Devices. Midterm results. ROME SPINE 2012, 7th International Meeting Rome, 6-7 December 2012 Posterior distraction and decompression Secure Fixation and Stabilization Integrated Bone
Interspinous Fusion Devices. Midterm results. ROME SPINE 2012, 7th International Meeting Rome, 6-7 December 2012 Posterior distraction and decompression Secure Fixation and Stabilization Integrated Bone
A rare case of spinal injury: bilateral facet dislocation without fracture at the lumbosacral joint
 J Orthop Sci (2012) 17:189 193 DOI 10.1007/s00776-011-0082-y CASE REPORT A rare case of spinal injury: bilateral facet dislocation without fracture at the lumbosacral joint Kei Shinohara Shigeru Soshi
J Orthop Sci (2012) 17:189 193 DOI 10.1007/s00776-011-0082-y CASE REPORT A rare case of spinal injury: bilateral facet dislocation without fracture at the lumbosacral joint Kei Shinohara Shigeru Soshi
A minimally invasive surgical approach reduces cranial adjacent segment degeneration in patients undergoing posterior lumbar interbody fusion
 A minimally invasive surgical approach reduces cranial adjacent segment degeneration in patients undergoing posterior lumbar interbody fusion T. Tsutsumimoto, M. Yui, S. Ikegami, M. Uehara, H. Kosaku,
A minimally invasive surgical approach reduces cranial adjacent segment degeneration in patients undergoing posterior lumbar interbody fusion T. Tsutsumimoto, M. Yui, S. Ikegami, M. Uehara, H. Kosaku,
MAS TLIF MAXIMUM ACCESS SURGERY TRANSFORAMINAL LUMBAR INTERBODY FUSION AN INTRODUCTION TO
 AN INTRODUCTION TO MAS TLIF MAXIMUM ACCESS SURGERY TRANSFORAMINAL LUMBAR INTERBODY FUSION This booklet is designed to inform you about the Maximum Access Surgery (MAS ) Transforaminal Lumbar Interbody
AN INTRODUCTION TO MAS TLIF MAXIMUM ACCESS SURGERY TRANSFORAMINAL LUMBAR INTERBODY FUSION This booklet is designed to inform you about the Maximum Access Surgery (MAS ) Transforaminal Lumbar Interbody
GET BACK TO YOUR FUTURE WITH SPECIALIZED SPINE CARE. A Guide for Patients
 GET BACK TO YOUR FUTURE WITH SPECIALIZED SPINE CARE A Guide for Patients Your Spine Deserves Special Care Your spine is at the center of a delicately balanced system that controls all of your body s movements.
GET BACK TO YOUR FUTURE WITH SPECIALIZED SPINE CARE A Guide for Patients Your Spine Deserves Special Care Your spine is at the center of a delicately balanced system that controls all of your body s movements.
ROME SPINE 2012 VII Annual Congress
 International Conference PRELIMINARY PROGRAMME CALL FOR ABSTRACTS ROME SPINE 2012 VII Annual Congress President F. Postacchini Co President P.P.M. Menchetti Rome 6-7 december 2012 Crowne Plaza St. Pater
International Conference PRELIMINARY PROGRAMME CALL FOR ABSTRACTS ROME SPINE 2012 VII Annual Congress President F. Postacchini Co President P.P.M. Menchetti Rome 6-7 december 2012 Crowne Plaza St. Pater
TABLE OF CONTENTS. ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2. Implant Specifications 3. Instrument Features 4
 Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Surgical Technique TABLE OF CONTENTS ShurFit Anterior Cervical Interbody Fusion (ACIF) System Overview 2 Product Highlights 2 Indications 2 Implant Specifications 3 Instrument Features 4 Surgical Technique
Bioactive glass S53P4 in spine surgery -results from a prospective 11-year-follow-up
 Bioactive glass S53P4 in spine surgery -results from a prospective 11-year-follow-up Janek Frantzén M.D., Neurosurgeon Turku University Hospital, Finland ROME SPINE 2011 7.12.2011 1 Clinical Development
Bioactive glass S53P4 in spine surgery -results from a prospective 11-year-follow-up Janek Frantzén M.D., Neurosurgeon Turku University Hospital, Finland ROME SPINE 2011 7.12.2011 1 Clinical Development
ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc disease: Is there a difference at 12 months?
 Original research ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc ( ) 51 51 56 ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc
Original research ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc ( ) 51 51 56 ProDisc-C versus fusion with Cervios chronos prosthesis in cervical degenerative disc
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Lumbar Spinal Fusion Page 1 of 29 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Lumbar Spinal Fusion Interspinous Fixation (Fusion) Devices http://www.bcbsks.com/customerservice/providers/medicalpolicies/policies.shtml
Lumbar Spinal Fusion Page 1 of 29 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Lumbar Spinal Fusion Interspinous Fixation (Fusion) Devices http://www.bcbsks.com/customerservice/providers/medicalpolicies/policies.shtml
Apache Cervical Interbody Fusion Device. Surgical Technique. Page of 13. LC-005 Rev F
 LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
LC-005 Rev F Apache Cervical Interbody Fusion Device Page of 13 Surgical Technique INDICATIONS: When used as an intervertebral body fusion device, the Genesys Spine Interbody Fusion System is indicated
Management Of Posttraumatic Spinal Instability (Neurosurgical Topics, No 3) READ ONLINE
 Management Of Posttraumatic Spinal Instability (Neurosurgical Topics, No 3) READ ONLINE If you are searching for a ebook Management of Posttraumatic Spinal Instability (Neurosurgical Topics, No 3) in pdf
Management Of Posttraumatic Spinal Instability (Neurosurgical Topics, No 3) READ ONLINE If you are searching for a ebook Management of Posttraumatic Spinal Instability (Neurosurgical Topics, No 3) in pdf
Long term prognosis of young adults after ACDF
 Long term prognosis of young adults after ACDF Tuomas Hirvonen MD 1,2 Johan Marjamaa MD, PhD 1,2 Jari Siironen MD, PhD 1 Anniina Koski-Palkén MD, PhD 1 1 Department of Neurosrugery, Helsinki University
Long term prognosis of young adults after ACDF Tuomas Hirvonen MD 1,2 Johan Marjamaa MD, PhD 1,2 Jari Siironen MD, PhD 1 Anniina Koski-Palkén MD, PhD 1 1 Department of Neurosrugery, Helsinki University
Comprehension of the common spine disorder.
 Objectives Comprehension of the common spine disorder. Disc degeneration/hernia. Spinal stenosis. Common spinal deformity (Spondylolisthesis, Scoliosis). Osteoporotic fracture. Anatomy Anatomy Anatomy
Objectives Comprehension of the common spine disorder. Disc degeneration/hernia. Spinal stenosis. Common spinal deformity (Spondylolisthesis, Scoliosis). Osteoporotic fracture. Anatomy Anatomy Anatomy
L-VARLOCK. Posterior Lumbar Cage with adjustable lordosis. S urgical T echnique
 L-VARLOCK Posterior Lumbar Cage with adjustable lordosis S urgical T echnique Introduction Designed and manufactured by KISCO International, L-VARLOCK cages are made of titanium alloy Ti 6AI 4V (standards
L-VARLOCK Posterior Lumbar Cage with adjustable lordosis S urgical T echnique Introduction Designed and manufactured by KISCO International, L-VARLOCK cages are made of titanium alloy Ti 6AI 4V (standards
Microendoscope-assisted posterior lumbar interbody fusion: a technical note
 Original Study Microendoscope-assisted posterior lumbar interbody fusion: a technical note Hirohiko Inanami 1, Fumiko Saiki 1, Yasushi Oshima 2 1 Department of Orthopaedic Surgery, Inanami Spine and Joint
Original Study Microendoscope-assisted posterior lumbar interbody fusion: a technical note Hirohiko Inanami 1, Fumiko Saiki 1, Yasushi Oshima 2 1 Department of Orthopaedic Surgery, Inanami Spine and Joint
Anthem Blue Cross and Blue Shield Central Region Clinical Claim Edit
 Subject: Laminotomy (Hemilaminectomy) with Decompression of Nerve Root(s), Including Partial Facetectomy, Foraminotomy and/or Excision of Herniated Intervertebral Disc, Reexploration, Single Interspace-Lumbar
Subject: Laminotomy (Hemilaminectomy) with Decompression of Nerve Root(s), Including Partial Facetectomy, Foraminotomy and/or Excision of Herniated Intervertebral Disc, Reexploration, Single Interspace-Lumbar
Biomechanics of Interspinous Process Fixation and Lateral Modular Plate Fixation to Support Lateral Lumbar Interbody Fusion (LLIF)
 Biomechanics of Interspinous Process Fixation and Lateral Modular Plate Fixation to Support Lateral Lumbar Interbody Fusion (LLIF) Calusa Ambulatory Spine Conference 2016 Jason Inzana, PhD 1 ; Anup Gandhi,
Biomechanics of Interspinous Process Fixation and Lateral Modular Plate Fixation to Support Lateral Lumbar Interbody Fusion (LLIF) Calusa Ambulatory Spine Conference 2016 Jason Inzana, PhD 1 ; Anup Gandhi,
Key Primary CPT Codes: Refer to pages: 7-9 Last Review Date: October 2016 Medical Coverage Guideline Number:
 National Imaging Associates, Inc. Clinical guidelines CERVICAL SPINE SURGERY: ANTERI CERVICAL DECOMPRESSION WITH FUSION CERVICAL POSTERI DECOMPRESSION WITH FUSION CERVICAL ARTIFICIAL DISC CERVICAL POSTERI
National Imaging Associates, Inc. Clinical guidelines CERVICAL SPINE SURGERY: ANTERI CERVICAL DECOMPRESSION WITH FUSION CERVICAL POSTERI DECOMPRESSION WITH FUSION CERVICAL ARTIFICIAL DISC CERVICAL POSTERI
ConnectiCare Commercial & Exchange Members Utilization Review Matrix 2018 Spine Surgery, Implantable Infusion Pump Insertion & Other Spine Procedures
 ConnectiCare Commercial & Exchange Members Utilization Review Matrix 2018 Spine Surgery, Implantable Infusion Pump Insertion & Other Spine Procedures The matrix below contains all of the CPT-4 codes for
ConnectiCare Commercial & Exchange Members Utilization Review Matrix 2018 Spine Surgery, Implantable Infusion Pump Insertion & Other Spine Procedures The matrix below contains all of the CPT-4 codes for
Posterior. Lumbar Fusion. Disclaimer. Integrated web marketing. Multimedia Health Education
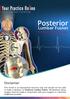 Posterior Lumbar Fusion Disclaimer This movie is an educational resource only and should not be used to make a decision on. All decisions about surgery must be made in conjunction with your surgeon or
Posterior Lumbar Fusion Disclaimer This movie is an educational resource only and should not be used to make a decision on. All decisions about surgery must be made in conjunction with your surgeon or
3D titanium interbody fusion cages sharx. White Paper
 3D titanium interbody fusion cages sharx (SLM selective laser melted) Goal of the study: Does the sharx intervertebral cage due to innovative material, new design, and lordotic shape solve some problems
3D titanium interbody fusion cages sharx (SLM selective laser melted) Goal of the study: Does the sharx intervertebral cage due to innovative material, new design, and lordotic shape solve some problems
Local Organizing Chairman: Bolat Nagymanov APSS President: Dato K.S. Sivananthan
 Local Organizing Chairman: Bolat Nagymanov APSS President: Dato K.S. Sivananthan Dato K.S. Sivananthan APSS President Welcome Message It is our greatest pleasure to welcome you to the APSS Operative Spine
Local Organizing Chairman: Bolat Nagymanov APSS President: Dato K.S. Sivananthan Dato K.S. Sivananthan APSS President Welcome Message It is our greatest pleasure to welcome you to the APSS Operative Spine
When is it appropriate to use codes & in the same setting? the code will describe whether to use interspace or vertebral segment.
 Question What is the difference in the codes 63075 and 22551 Our physician requested that we ask the following coding/billing question: 1) is 62310-50 77003 x 2 appropriate coding, 2) 64483 64484 72275
Question What is the difference in the codes 63075 and 22551 Our physician requested that we ask the following coding/billing question: 1) is 62310-50 77003 x 2 appropriate coding, 2) 64483 64484 72275
EVALUATE, TREAT AND WHEN TO REFER RED FLAGS Mid Atlantic Occupational Regional Conference and Environmental Medicine October 6, 2018
 EVALUATE, TREAT AND WHEN TO REFER RED FLAGS Mid Atlantic Occupational Regional Conference and Environmental Medicine October 6, 2018 Marc J. Levine, MD Rothman Institute Director Spine Surgery Program
EVALUATE, TREAT AND WHEN TO REFER RED FLAGS Mid Atlantic Occupational Regional Conference and Environmental Medicine October 6, 2018 Marc J. Levine, MD Rothman Institute Director Spine Surgery Program
Stand-Alone Technology. Reginald Davis, M.D., FAANS, FACS Director of Clinical Research
 Stand-Alone Technology Reginald Davis, M.D., FAANS, FACS Director of Clinical Research Disclosures Stand-Alone Devices Optio-C Stalif C Coalition Prevail ROI-C Technology Descriptions Optio-C A no profile,
Stand-Alone Technology Reginald Davis, M.D., FAANS, FACS Director of Clinical Research Disclosures Stand-Alone Devices Optio-C Stalif C Coalition Prevail ROI-C Technology Descriptions Optio-C A no profile,
SWESPINE THE SWEDISH SPINE REGISTER 2010 REPORT
 SWESPINE THE SWEDISH SPINE REGISTER 21 REPORT SEPTEMBER 21 SWEDISH SOCIETY OF SPINAL SURGEONS Björn Strömqvist Peter Fritzell Olle Hägg Bo Jönsson ISBN 978-91-978553-8-9 Table of Contents 2 Introduction
SWESPINE THE SWEDISH SPINE REGISTER 21 REPORT SEPTEMBER 21 SWEDISH SOCIETY OF SPINAL SURGEONS Björn Strömqvist Peter Fritzell Olle Hägg Bo Jönsson ISBN 978-91-978553-8-9 Table of Contents 2 Introduction
Free Paper Session IX Spine II
 Free Paper Session IX Spine II 9. Oblique Lateral Interbody Fusion for L5-S Introduction: A minimally invasive modified anterior lumbar interbody fusion technique for the lumbosacral junction is described
Free Paper Session IX Spine II 9. Oblique Lateral Interbody Fusion for L5-S Introduction: A minimally invasive modified anterior lumbar interbody fusion technique for the lumbosacral junction is described
Coflex TM for Lumbar Stenosis with
 Coflex TM for Lumbar Stenosis with Segmental Instability : 1 yr outcomes Eun-Sang Kim, M.D., Ph.D. Clinical Professor Dept of Neurosurgery Samsung Medical Center Seoul, Korea Surgery for Spinal Stenosis
Coflex TM for Lumbar Stenosis with Segmental Instability : 1 yr outcomes Eun-Sang Kim, M.D., Ph.D. Clinical Professor Dept of Neurosurgery Samsung Medical Center Seoul, Korea Surgery for Spinal Stenosis
) ACDF. (Japanese Orthopaedic Association,JOA) ACCF (175.4±12.1ml VS 201.3±80.4ml) ACDF JOA VAS (P=0.000),ACCF :A : X(2015)
 2015 25 5 Chinese Journal of Spine and Spinal Cord,2015,Vol.25,No.5 433 1, 2, 2, 3, 3 (1 ;2 466000 ;3 466000 ) : (anterior cervical discectomy and fusion,acdf) (anterior cervical corpectomy and fusion,accf)
2015 25 5 Chinese Journal of Spine and Spinal Cord,2015,Vol.25,No.5 433 1, 2, 2, 3, 3 (1 ;2 466000 ;3 466000 ) : (anterior cervical discectomy and fusion,acdf) (anterior cervical corpectomy and fusion,accf)
Index. Note: Page numbers of article titles are in boldface type.
 Orthop Clin N Am 38 (2007) 463 468 Index Note: Page numbers of article titles are in boldface type. A Andreas Vesalius, in history of spine pathology, 306 Anesthesia/anesthetics for PECD, 329 in minimally
Orthop Clin N Am 38 (2007) 463 468 Index Note: Page numbers of article titles are in boldface type. A Andreas Vesalius, in history of spine pathology, 306 Anesthesia/anesthetics for PECD, 329 in minimally
Fractures of the thoracic and lumbar spine and thoracolumbar transition
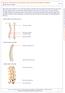 Most spinal column injuries occur in the thoracolumbar transition, the area between the lower thoracic spine and the upper lumbar spine; over half of all vertebral fractures involve the 12 th thoracic
Most spinal column injuries occur in the thoracolumbar transition, the area between the lower thoracic spine and the upper lumbar spine; over half of all vertebral fractures involve the 12 th thoracic
Surgical outcome of posterior lumbar interbody fusion for lumbar lesions in rheumatoid arthritis
 Surgical outcome of posterior lumbar interbody fusion for lumbar lesions in rheumatoid arthritis Fujiwara H*, Kaito T**, Makino T**, Ishii T*, Yonenobu K*** Department of Orthopaedic Surgery, *National
Surgical outcome of posterior lumbar interbody fusion for lumbar lesions in rheumatoid arthritis Fujiwara H*, Kaito T**, Makino T**, Ishii T*, Yonenobu K*** Department of Orthopaedic Surgery, *National
Spinal canal stenosis Degenerative diseases F 06
 What is spinal canal stenosis? The condition known as spinal canal stenosis is a narrowing (stenosis) of the spinal canal that in most cases develops due to the degenerative (wear-induced) deformation
What is spinal canal stenosis? The condition known as spinal canal stenosis is a narrowing (stenosis) of the spinal canal that in most cases develops due to the degenerative (wear-induced) deformation
The Relationship amongst Intervertebral Disc Vertical Diameter, Lateral Foramen Diameter and Nerve Root Impingement in Lumbar Vertebra
 doi: http://dx.doi.org/10.5704/moj.1803.004 The Relationship amongst Intervertebral Disc Vertical Diameter, Lateral Foramen Diameter and Nerve Root Impingement in Lumbar Vertebra Yusof MI, MMed Orth, Hassan
doi: http://dx.doi.org/10.5704/moj.1803.004 The Relationship amongst Intervertebral Disc Vertical Diameter, Lateral Foramen Diameter and Nerve Root Impingement in Lumbar Vertebra Yusof MI, MMed Orth, Hassan
Posterior lumbar interbody fusion using non resorbable poly-ether-ether-ketone versus resorbable poly-l-lactide-co-d,
 Eur Spine J (211) 2:618 622 DOI 1.17/s586-1-1568-6 ORIGINAL ARTICLE Posterior lumbar interbody fusion using non resorbable poly-ether-ether-ketone versus resorbable poly-l-lactide-co-d, L-lactide fusion
Eur Spine J (211) 2:618 622 DOI 1.17/s586-1-1568-6 ORIGINAL ARTICLE Posterior lumbar interbody fusion using non resorbable poly-ether-ether-ketone versus resorbable poly-l-lactide-co-d, L-lactide fusion
porous titanium cervical implants.
 Preliminary results of quantitative functional X-ray analysis of CABO meeting 2017 stand-alone interbody 3D printed porous titanium cervical implants. Mark Arts, MD PhD Jasper Wolfs, MD Neurosurgery Department
Preliminary results of quantitative functional X-ray analysis of CABO meeting 2017 stand-alone interbody 3D printed porous titanium cervical implants. Mark Arts, MD PhD Jasper Wolfs, MD Neurosurgery Department
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2017 5/2018 5/2017 Description of Procedure or Service Low back pain
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: lumbar_spine_fusion_surgery 9/2010 5/2017 5/2018 5/2017 Description of Procedure or Service Low back pain
THRESHOLD POLICY T17 SPINAL SURGERY FOR ACUTE LUMBAR CONDITIONS
 THRESHOLD POLICY T17 SPINAL SURGERY FOR ACUTE LUMBAR CONDITIONS Policy author: Ipswich and East Suffolk and West Suffolk CCGs with support from Public Health Suffolk Policy start date: September 2014 Subsequent
THRESHOLD POLICY T17 SPINAL SURGERY FOR ACUTE LUMBAR CONDITIONS Policy author: Ipswich and East Suffolk and West Suffolk CCGs with support from Public Health Suffolk Policy start date: September 2014 Subsequent
PRELIMINARY PROGRAMME
 MODULE 2 DEGENERATIVE DISEASES OF THE SPINE 4-5 July 2019 PRELIMINARY PROGRAMME QUICK FACTS WHEN: 4-5 July 2019 WHERE: Geneva, Switzerland SWISS Foundation for Innovation and Training in Surgery (SFITS)
MODULE 2 DEGENERATIVE DISEASES OF THE SPINE 4-5 July 2019 PRELIMINARY PROGRAMME QUICK FACTS WHEN: 4-5 July 2019 WHERE: Geneva, Switzerland SWISS Foundation for Innovation and Training in Surgery (SFITS)
ASJ. Outcome of Salvage Lumbar Fusion after Lumbar Arthroplasty. Asian Spine Journal. Introduction. Hussein Alahmadi, Harel Deutsch
 Asian Spine Journal Asian Spine Clinical Journal Study Asian Spine J 2014;8(1):13-18 Lumbar fusion http://dx.doi.org/10.4184/asj.2014.8.1.13 after lumbar arthroplasty 13 Outcome of Salvage Lumbar Fusion
Asian Spine Journal Asian Spine Clinical Journal Study Asian Spine J 2014;8(1):13-18 Lumbar fusion http://dx.doi.org/10.4184/asj.2014.8.1.13 after lumbar arthroplasty 13 Outcome of Salvage Lumbar Fusion
PROGRAMME. IRCAD/EITS 1 Place de l Hôpital Strasbourg, France. Non-member:
 MODULE 2 DEGENERATIVE DISEASES OF THE SPINE 22 & 23 June 2017 PROGRAMME QUICK FACTS WHEN: WHERE: MAXIMUM ATTENDEES: REGISTRATION FEE: CME CREDITS: LANGUAGE: DRESS: IMPORTANT NOTE: 22-23 June 2017 Strasbourg,
MODULE 2 DEGENERATIVE DISEASES OF THE SPINE 22 & 23 June 2017 PROGRAMME QUICK FACTS WHEN: WHERE: MAXIMUM ATTENDEES: REGISTRATION FEE: CME CREDITS: LANGUAGE: DRESS: IMPORTANT NOTE: 22-23 June 2017 Strasbourg,
INTERSPINOUS STABILIZATION-FUSION IN THE UNSTABLE SPINE.
 INTERSPINOUS STABILIZATION-FUSION IN THE UNSTABLE SPINE. A PRELIMINARY REPORT F. POSTACCHINI, A. PALMESI LUMBAR VERTEBRAL INSTABILITY Still the subject of discussion Variously defined and interpreted Postacchini
INTERSPINOUS STABILIZATION-FUSION IN THE UNSTABLE SPINE. A PRELIMINARY REPORT F. POSTACCHINI, A. PALMESI LUMBAR VERTEBRAL INSTABILITY Still the subject of discussion Variously defined and interpreted Postacchini
Spine surgery experience at the Loveland Surgery Center Loveland, Colorado
 Spine surgery experience at the Loveland Surgery Center Loveland, Colorado Kenneth A. Pettine, M.D., M.S. Loveland Surgery Center I.D.E. Site for Twelve F.D.A. Spinal Implant Studies Joint Commission Accredited
Spine surgery experience at the Loveland Surgery Center Loveland, Colorado Kenneth A. Pettine, M.D., M.S. Loveland Surgery Center I.D.E. Site for Twelve F.D.A. Spinal Implant Studies Joint Commission Accredited
Lumbar, cervical and thoracic spine surgery (open, closed or minimally invasive) Adult deformity surgery Implantable infusion pump insertion
 July 30, 2015 Effective Oct. 1, NIA Magellan to Manage Spine Surgery and Interventional Pain Management Program Dear Provider: ConnectiCare is introducing a spine surgery and interventional pain management
July 30, 2015 Effective Oct. 1, NIA Magellan to Manage Spine Surgery and Interventional Pain Management Program Dear Provider: ConnectiCare is introducing a spine surgery and interventional pain management
LIFE MOVES US CORPORATE OVERVIEW
 LIFE MOVES US CORPORATE OVERVIEW 1 Our mission is to deliver cutting-edge technology, research, and innovative solutions, to promote healing in patients with musculoskeletal disorders. Dave Demski, Chief
LIFE MOVES US CORPORATE OVERVIEW 1 Our mission is to deliver cutting-edge technology, research, and innovative solutions, to promote healing in patients with musculoskeletal disorders. Dave Demski, Chief
