Vitamin K deficiency bleeding (VKDB) was first discovered
|
|
|
- Katrina McGee
- 5 years ago
- Views:
Transcription
1 Preventing Vitamin K Deficiency Bleeding: An Evidence-Based Update for the Midwife BY KELSEY SCHERER Vitamin K deficiency bleeding (VKDB) was first discovered in 1894 when spontaneous bleeding was observed in neonates who did not have any underlying disorders such as hemophilia, and it was named hemorrhagic disease of the newborn (HDN). 1 It was later renamed VKDB for accuracy, as it was discovered that VKDB is not unique to the newborn period, and hemorrhagic disease has multiple causes, though this particular manifestation is specific to vitamin K (VK) deficiency. 1,2 Although VKDB can occur across the lifespan, including in adults, it is most common in infants due to endogenous and exogenous factors that prevent them from developing and maintaining adequate VK levels. VKDB thus describes bleeding secondary to inadequate activity of VK-dependent coagulation factors that can be corrected by VK replacement. 3 In 1961, the American Academy of Pediatrics began to recommend that all newborns be administered a VK injection at birth to help prevent them from developing VKDB. 4 Although another option exists for the prevention of VKDB oral VK supplementation for a variety of reasons this option is not offered as a standard of care in the United States. Midwives practicing in home and birth center settings frequently encounter parents who decline intramuscular VK for their newborns, leaving these infants vulnerable to the rare, though possible, outcome of developing VKDB and its serious sequelae, including brain damage and death. Oral VK is an evidence-based secondary option that can be offered to parents who are unwilling to accept an intramuscular VK injection for their infant. Although offering an oral VK protocol in the United States presents challenges, including lack of supplement regulation and no national standardized oral VK guidelines, it is an option that may be accepted by clients who decline intramuscular prophylaxis for their infant. Because many parents have questions and concerns about whether their infant needs exogenous VK, and if so, which path they should take for administration, care providers need a clear understanding of both the etiology of VKDB and options for prevention. In recent years, concern over administration of the VK injection has led to parental refusal, resulting in severe consequences for some infants, including infant mortality and morbidity. Most modern cases of VKDB occur in infants whose parents decline prophylactic VK, often resulting in life-long implications for the infant. Case reports from hospitals around the country of infants who developed VKDB have led to an awareness that refusal of VK prophylaxis may be increasing. Although the following examples of infant hemorrhage following parental refusal of the VK injection are alarming, it is difficult to say how widespread VKDB is because there is no mandated reporting on or nationwide surveillance of its use in the United States. In 2004, a 5-week-old infant in Michigan developed a thymic hemorrhage and respiratory failure. 5 In 2013, a 3-week-old infant in North Carolina developed an umbilical stump bleed and hemorrhagic shock. 6 In 2006, two 5-week-old infants in Missouri developed intracranial hemorrhages (ICH). 7 In 2009, 2012, and 2015 respectively, a 6-weekold infant in Texas, 8 a 4-week-old infant in Virginia, 9 and a 3-week-old infant in Indiana 10 all also developed ICH. Perhaps most memorably, 7 infants who did not receive VK prophylaxis developed VK deficiency in Tennessee in 2013, drawing major attention and prompting a Centers for Disease Control and Prevention (CDC) investigation. Five of these infants exhibited bleeding, and 4 of those bleeds were intracranial hemorrhages. 11 In all of the cases above, parents declined the VK injection, and it is unknown whether they were offered or aware of oral VK as a prophylactic option because it is not considered the standard of care. While there are no nationwide surveillance data on rates of VK refusal or place of birth for those infants not receiving VK, the CDC investigation in Tennessee revealed that in area hospitals, 96.6% of infants received VK injections, while in area out-of-hospital births, 72% of infants received VK. 11 Larger studies, such as a population-based study of nearly 300,000 births in Alberta, Canada, found that planned home births had SUMMER 2016 / 13
2 a parental VK refusal rate of 14.5%, birth center births a rate of 10.7%, and hospital births a rate of 0.2%. 12 While the midwifery model of care strongly emphasizes informed choice, it is crucial that parents understand the possibility of the rare yet devastating consequences that can occur in infants not receiving VK prophylaxis. In the Tennessee cases, for example, only 1 out of 7 infants parents reported that they were aware that this type of emergency could result when they refused VK. 11 Misinformation on VK abounds both in the midwifery community and more broadly, and many parents may decline prophylaxis without having the necessary information to make a truly informed decision. The full range of options for the prevention of VKDB may not even be available to parents depending on the provider they choose for their pregnancy and infant. To offer evidence-based, client-centered care, the midwifery community must ensure that all providers are well-educated and able to provide clients with comprehensive information on this topic. The following sections will explore the role of VK in the body and the unique physiology that puts infants at particular risk for VKDB; review the benefits and risks of intramuscular and oral VK prophylaxis; and present an evidence-based oral VK prophylactic protocol that care providers can offer parents for the prevention of infant VKDB. Vitamin K in the Human Body Vitamin K is one of the 4 fat-soluble vitamins, though in contrast to the other fat-soluble vitamins, there is little storage of it in the body. Naturally occurring forms of VK include a number of vitamers known as vitamin K1 and vitamin K2. Vitamin K1, or phylloquinone, is the predominant dietary form of VK and is found in green leafy vegetables and some plant oils. Vitamin K2 includes a wide range of VK forms called menaquinones. These forms of VK, known by their length, are designated by MK-n, ranging from MK-2 to MK-14. Menaquinones are primarily of microbial origins and are found in fermented foods, dairy products, and animal liver. K2 is also produced in the human colon by bacteria, but this does not appear to be a major contributor to the body s coagulation status. Finally, there is a synthetic compound of VK known as vitamin K3 or menadione, originally used for newborn prophylaxis but rarely used today in any product for human use, as it can cause hemolytic anemia, hyperbilirubinemia, jaundice, and kernicterus in infants. 3,13,14 Vitamin K performs many functions in the body, including an essential role in blood clotting. Vitamins K1 and K2 combine with enzymes, proteins, and minerals in the 14 / MIDWIFERY MATTERS FIGURE 1. VITAMIN K DEFICIENCY BLEEDING 2,3,4,11,13,16,37,38 body to synthesize clotting factors II (prothrombin), VII, IX, and X in the liver, as well as Protein S, Protein C, and Protein Z. 3,13,15 These clotting factors make up the core of the clotting cascade. Both vitamins K1 and K2 are utilized by the body for clotting purposes, but K1 has been more robustly studied for its role in coagulation. 3 Vitamin K Deficiency Bleeding Infants may experience occasional internal bleeding as a spontaneous event or develop bleeding secondary to a traumatic event. VKDB can occur when the body experiences any type of bleeding, and due to a lack of available VK and VK-dependent coagulation factors, the bleeding persists. VKDB is diagnosed when VK replacement stops a bleeding event. VKDB is divided into three categories: early, classic, and late. Although these categories are based solely on the timing of bleeding, each type is also associated with a different pathogenic mechanism (Figure 1). 3 Early VKDB develops in the first 24 hours of life and occurs secondary to placental transfer of maternal drugs, including anticonvulsants, cephalosporin antibiotics, tuberculostatic agents, and VK antagonists like warfarin, all of which inhibit VK activity in the infant. The incidence of VKDB in newborns of mothers who take any of the above drugs is as high as 6% to 12%. 2 Early VKDB Classic VKDB Late VKDB Age at onset Less than 24 hours Days 1-7 (most likely Weeks 2-12 (possible up to days 2-5) 6 months) Causes and risk factors Drugs taken during Low placental VK transfer; Low VK content of breast pregnancy (anticonvulsants, rapid VK excretion; low milk; rapid VK excretion; anticoagulants, VK content of breast milk; immature VK cycle; liver tuberculostatic agents, inadequate milk intake for stores dominated by cephalosporins). any reason, including but phylloquinone rather not limited to late onset of than menaquinone; gut feeding and low milk supply. bacteria that do not contribute to VK status; physiologic slow rise of clotting factors until ~90 days after birth; VK malabsorptive disorders. Location of bleeding Cephalahematoma, Gastrointestinal tract, Intracranial (30-80%), skin, (in order of frequency) umbilicus, intracranial, umbilicus, nose, nose, gastrointestinal tract, intra-abdominal, venipuncture site, venipuncture site, intrathoracic, circumcision, intracranial umbilicus, urogenital tract, gastrointestinal tract. (rarely). intrathoracic. Avoidance measures Consult or refer prenatally Ensure normal VK VK prophylaxis (single IM to maternal-fetal medicine. introduction to neonate dose on day of birth or Stop or replace problematic by early and adequate oral dosing per Figure drugs if feasible. IM VK breastfeeding. VK 3). Early recognition of prophylaxis immediately prophylaxis day of birth predisposing conditions after birth. (IM and oral deemed (jaundice, failure to thrive) equally effective). and prompt investigation of warning bleeds. Warning signs of VKDB or malabsorptive/ hepatobiliary disorders Management Failure to thrive; vomiting; poor feeding; lethargy; hypothermia; pallor; tense or bulging fontanelle; jaundice beyond 2 weeks of age; warning bleeds: epistaxis, umbilical stump, urethral or gastrointestinal bleeding (blood in diaper, black stool), petechiae, bruising, circumcision site bleeding, mucosal bleeding (mouth, throat, gums), continuous bleeding of a small cut or abrasion. All warning bleeds, jaundice beyond 2 weeks, and any other signs and symptoms should be taken seriously and evaluated. If VKDB is suspected, administer IM VK and send infant to hospital for immediate evaluation. IM VK may be administered to any infant regardless of previous VK status, though if already given IM VK, a bleed is unlikely to be due to VK deficiency. Ensure parents inform the hospital of the infant s VK prophylactic history upon arrival, as previous IM VK administration is likely assumed. 5,6,9,10
3 Classic VKDB develops from 24 hours after birth up to 1 week, most commonly on days 2 to 5. The incidence of classic VKDB is 0.25% to 1.7%, making it the most common manifestation of VKDB. 3,4,16 The etiology of classic VKDB is well understood. Newborns are born with negligible amounts of maternally acquired VK stored in their bodies, and deficiency intensifies in the first few days after birth because VK is a rapidly excreted vitamin. Newborns depend on continual nutritional intake for their VK needs, and colostrum is produced in physiologically small volumes, so in the first few days of life, newborns receive only small amounts of VK. Although delayed initiation of breastfeeding, low milk supply, and birth trauma can play a role in classic VKDB, the disease can occur in any newborn without the presence of these events as well. 2,3,16,17 Late VKDB occurs on or after day 8, most often between 2 and 12 weeks of age. 2,4 The incidence of late VKDB ranges from 4.4 to 7.2 per 100,000 births. 4 While relatively rare, the bleeding is usually severe, with intracranial hemorrhages occurring in 30% to 80% of cases. 11 Bleeding can also occur from the nose, skin (petechiae, bruising, venipuncture sites), umbilicus, urogenital tract, gastrointestinal tract, or intrathoracically. 2,3,11 The etiology of late VKDB is complex. Limited placental VK transfer, low VK content in breast milk, rapid VK excretion, an immature VK cycle, liver stores dominated by K1 rather than K2, gut bacteria that do not contribute to VK status, a slow rise of clotting factors until about 90 days after birth, and the possibility of pathologic contributors such as hepatobiliary or malabsorptive disease all contribute to an infant s risk for late VKDB. Physiologic Causes of VKDB in the Infant Placental Transfer Placental transfer of VK to the fetus is limited, as VK does not easily cross the placenta. 11 There is no evidence that delayed cord clamping increases a newborn s stores of VK, because as measured after birth, cord blood concentrations of VK are low to undetectable. 19 Prenatal maternal supplementation is not a reliable method of transferring VK to the newborn either, 20 as even large pharmacological doses of VK during pregnancy do not have reliable outcomes on cord blood concentrations. 19 Even if there were proven benefit to maternal supplementation during pregnancy or delayed cord clamping at birth, late VKDB occurs until 12 weeks of age, making this an event related to continued intake of VK rather than placental transfer. Dietary Intake of Vitamin K Because diet is the main source of VK for an infant, exclusively breastfed infants have a higher risk of VKDB than formula-fed infants, as formula in the United States is supplemented with VK. 11,15 In fact, late VKDB occurs almost exclusively in breastfed infants. 2 The Adequate Intake for VK is set at 2 mcg/day for infants 0 to 6 months of age. 20 Human breast milk contains relatively low concentrations of vitamin K1 and K2, with average concentrations of 0.25 mcg/dl to 0.5 mcg/dl, 21,22 whereas an average formula may have around 5.5 mcg/dl. 23 Therefore, an infant consuming 750 ml/day would have a total intake of 1.9 mcg to 3.8 mcg of VK via breast milk, or 41.3 mcg of VK via formula. Based on these total daily intakes, many breastfed infants may meet the Adequate Intake guidelines, while formulafed infants exceed them. It is important to consider that the Adequate Intake value is based on the premise that all infants receive an intramuscular VK injection at birth. When breast milk s VK content is combined with other endogenous factors present in the infant, this Adequate Intake value may not be enough to prevent bleeding in the infant who did not receive prophylactic VK. In the first week of life, breastfed infants are at particularly high risk of classic VKDB because of the low volume of colostrum or breast milk they receive. Some studies show colostrum can have a higher (even double) VK content as compared to mature milk, 22,24 while other studies show the content to be about the same or with a difference that is not statistically significant. 25 Regardless, the small volume of colostrum produced makes for a small transfer of VK. Limited studies indicate that maternal supplementation can increase VK content in breast milk, but there are no evidencebased protocols. Two small studies of 20 and 32 breastfeeding women indicate that maternal intakes of 4 mg to 5 mg of K1 each day have been shown to increase breast milk VK content to formula levels. 23,26 Conversely, a study of 77 women showed that maternal intakes as high as 15 mg/day of K2 did not increase VK milk content as significantly as the smaller doses of K1. 27 The bioavailability of different supplements, each individual s ability to absorb supplements, and the reliability of this method of supplementation have not been clearly established. Gut Flora A common refrain among midwives is that gut-produced vitamin K2 is a main contributor to the body s VK needs. It is often repeated that infants are deficient in VK because of their immature, altered, or lack of gut flora, all of which may prevent them from producing K2, thereby putting them at risk for bleeding. Data now demonstrate that the main source of VK in infants and adults alike is dietary, not the gut. K2 production does occur in the adult and formula-fed infant gut because of production by bacteria such as Bacteroides fragilis and Escherichia coli. Meanwhile, because the breastfed infant gut is colonized with bacteria like Bifidobacterium spp., Lactobacillus spp., and Clostridium spp., K2 is not readily produced. 18 The amount of K2 the gut produces, however, is likely of little importance in the prevention of VKDB. Previously, data showed that up to 50% of the human VK requirement might be met by bacterial K2 synthesis, but it is now known that this figure is inaccurately high, with little evidence that gut-produced K2 contributes to coagulation at all. 13 Because VK is fat-soluble, it can only be absorbed from the small intestine in the presence of bile salts, 15 which are absent in the colon (large intestine) where K2 is produced. 33 There is no evidence that gut-produced K2 can be absorbed for use, though it may be possible it is absorbed through a mechanism currently unknown to researchers. The liver stores both vitamin K1 and K2, but just as liver stored K1 is from our diet, so is the majority of K2. 18,28,31 Although liver stores of VK help adults maintain coagulation status, reducing VK intake to zero or negligible levels depletes liver stores, and coagulation becomes abnormal. 28 Even in adults with fully developed gut bacteria, without dietary VK intake, gut-produced K2 is inadequate to maintain normal VK status. Vitamin K Excretion and the Vitamin K Cycle There are relatively low circulating and tissue stores of VK in the human body as compared to the other fat-soluble vitamins, because 60% to 70% of dietary VK intake is rapidly excreted from the body. 28 The adult body recycles VK, allowing it to be reused many times in the body, in part to compensate for the body s tendency to rapidly excrete VK. A single VK molecule is thought to be recycled ~1,000 times. 3,13 The vitamin K cycle may not yet be fully functional in newborns due to enzymes that are absent at birth. 29 Thus, when a breast-fed infant s marginally adequate levels of VK are excreted, the infant may not have sufficient VK to maintain normal coagulation. Formula levels Vitamin K deficiency in infants is part of the process of maturation infants need time to develop their Vitamin K system much the same way they need time to develop their immune system. SUMMER 2016 / 15
4 of VK are higher and therefore a higher circulating level of VK remains in the formula-fed infant s body despite excretion. Liver Storage of Vitamin K Adults and infants have important differences in their liver storage of VK. In adults, liver storage is 10% phylloquinone (K1) and 90% menaquinone (K2). 28 In infants, liver storage is overall more limited, and phylloquinone predominates over menaquinone. 18,30 Menaquinone liver stores increase during the first year of life as complementary foods are added to the infant diet. 18 Because phylloquinone stores are more rapidly excreted from the body than menaquinone, 11,28 infants excrete VK at higher rates than do adults. Slow Rise of Clotting Factors Infant plasma concentrations of all VK-dependent clotting factors are 40% to 60% of adult values and rise slowly during infancy, taking up to 90 days to completely normalize even with adequate VK stores. 11,18,29,31 Data demonstrate that VK prophylaxis does not actually increase clotting factors in infants it just ensures that there is enough VK available to produce clotting factors when the body needs them. As infants mature, they become more efficient producers of clotting factors. Hepatobiliary and Malabsorptive Diseases Hepatobiliary (liver/gallbladder) diseases, including cholestasis and biliary atresia, and malabsorptive diseases, such as cystic fibrosis, are the main pathologic causes of VKDB. Without proper liver, gallbladder, or small intestine function, VK cannot be absorbed or transported to the liver, and clotting factors cannot be produced. 2,11,32 Hepatobiliary and malabsorptive diseases are a main cause of late VKDB and can often result in the failure of oral VK prophylaxis, and though less common, can also result in the failure of IM VK prophylaxis. 33,34,35 Wisdom of the Body Although some may believe that there is a reason infants bodies maintain a low level of VK, data demonstrate that VK deficiency in infants is simply a part of the process of maturation. Just as infants immune systems are immature when they are born, so is their ability to clot. All humans would be deficient in VK without dietary intake even adults develop bleeding problems when VK is removed from their diet. Breast milk, as infants primary food source, happens to have minimally adequate levels of VK. With steady breast milk intake, most infants maintain an adequate though marginal supply of VK; however, when combined with other factors such as limited placental transfer, rapid excretion, an immature VK cycle, liver stores dominated by phylloquinone rather than menaquinone, gut bacteria that do not contribute to VK status, and the slow rise of clotting factors until about 90 days after birth, it seems that infants simply need time to develop their VK system to prevent bleeding, much the same way as they need time to develop their immune system. It doesn t mean they are unwise; it means they are developing. Signs and Symptoms of VKDB All practitioners responsible for infant care must be vigilant for symptoms of VKDB. Although VKDB most often occurs in infants who received no prophylaxis, it can occasionally occur in infants who received prophylaxis. Warning signs of VKDB, such as minimal bleeds and evidence of hepatobiliary disease, have often been present in infants diagnosed with VKDB but were overlooked by care providers. 36 The incidence of intracranial hemorrhage and long-term sequalae to VKDB can be reduced by recognizing the signs of predisposing conditions and promptly investigating warning bleeds. 2 Infants requiring further evaluation for early signs of VKDB or underlying hepatobiliary disease include those experiencing failure to thrive, vomiting, poor feeding, lethargy, hypothermia, pallor, a tense or bulging fontanelle, jaundice beyond 2 weeks of age, or warning bleeds (Figure 1). 3,11,13,16,37 Jaundice should be of particular concern to the midwife, especially in the case of an infant who received no VK or oral VK, as this may be a sign there is an underlying hepatobiliary disorder preventing absorption of VK. Around 15% of breastfed infants have physiologic jaundice at 2 weeks. Referral for jaundice is recommended at 2 weeks if any of the following occur: dark urine; light stools; petechiae, bruising, or other signs of bleeding; poor feeding or weight gain; or if the midwife is unable to monitor the infant in the coming week. 38 Referral is recommended if jaundice persists for 3 weeks, even in the absence of any other symptoms. Prophylactic Options for the Prevention of VKDB The two options for preventing VKDB are intramuscular (IM) and oral VK prophylaxis, with the principal option in the United States being the IM injection. The American Academy of Pediatrics (AAP) recommends an IM injection of 0.5 mg to 1 mg of vitamin K1 after birth for all newborns. 4 Because the incidence of VKDB has gone down since the introduction of the VK injection in the United States, the injection has been deemed highly effective, 4 though there are no surveillance data determining exact past or current rates of effectiveness. Oral prophylaxis is not recommended by the AAP due to a lack of evidence to support it as an alternative to the highly effective IM injection. 4 IM vitamin K remains the standard of care in the US. The effectiveness of VK prophylaxis varies depending on the type of VKDB it is used to prevent. Both oral and IM VK have been demonstrated to reduce the rate of classic VKDB to zero when administered immediately after birth. 15 As for late VKDB, neither oral nor IM prophylaxis provide complete protection, and neither has been tested in randomized controlled trials regarding efficacy in reduction of late VKDB. 3 Instead, population-based surveillance studies are used to determine effectiveness and show that when IM VK is given at birth, the rate of late VKDB is reduced from 4.4 to 7.2 cases per 100,000 births without prophylaxis, 4 to 0.24 to 3.2 cases per 100,000 births with prophylaxis. 11 There is variability in surveillance results due to the rarity of VKDB. For example, the most recent surveillance data from Canada and Australia reveal so few cases of late VKDB after IM prophylaxis that the incidence is 0 cases per 100,000 births. 41 Late VKDB only occasionally occurs after IM VK, usually due to failure of uptake by the body or hepatobiliary disease. 41,42,43,44,45 The IM injection is thus highly effective in preventing late VKDB, though rarely it may fail. As for oral prophylaxis, population-based studies show that a single dose of oral VK prophylaxis at birth lowers the rate of late VKDB to 1.4 to 6.4 per 100,000 births, 4 which is less effective than a single IM VK dose at birth, and demonstrates that oral VK must be repeated to improve effectiveness. International data on the effectiveness of multiple doses of oral VK can be found in Figure 2, with nearly all countries surveillance data demonstrating a comparable or lesser risk of VKDB after multi-dose oral prophylaxis when compared to IM prophylaxis. Parental Refusal of Intramuscular Vitamin K Parental concerns about IM VK do result in decline of this standard of care, as evidenced by multiple case studies and surveillance studies. Common concerns include high dosing, ingredients, and side effects. High Dosing High doses of VK are found in newborn serum after the IM injection. Some studies have shown infant VK serum levels to be 100 to 400 times the concentration of the normal adult range after the IM injection. 19,41,46 There is no evidence, however, that any amount of naturally occurring VK is toxic in the infant or adult body. 20 Ingredients and Side Effects Reports of rare side effects to the injection do occur and are 16 / MIDWIFERY MATTERS
5 FIGURE 2. INTERNATIONAL VITAMIN K PROTOCOLS AND INCIDENCE OF VKDB Country Year Protocol Established Recommended Route of VKDB Prophylaxis Protocol Incidence of VKDB & Product Used Prophylaxis Australia 1999 All newborns should receive VK. IM Dosing Protocol: 36 Classic VKDB: Konakion MM Paediatric For healthy newborns, IM injection Term newborns: 1 mg at birth Eliminated with VK administered via for IM and oral use. 36 recommended, oral VK available. Birth weight <1500 g: 0.5mg any route at birth. 36 For unwell newborns or those at birth Late VKDB: whose mothers took medications Oral Dosing Protocol: 36 Incidence 0.3/100,000 births that interfere with VK metabolism, 2 mg birth following IM injection. 36 IM injection recommended mg at 3-5 days Incidence 1.5/100,000 births 2 mg at exactly 4 weeks following complete oral dosing (no later) protocol (using Konakion cremophor formulation from ). 37 Incidence 2.5/100,000 births following complete or incomplete oral dosing protocol. 37 Canada 1997 IM injection highly recommended, IM Dosing Protocol: 51 Early and Late VKDB: 52 Vitamin K1 IM product; no oral VK available. 51 IM dose within 6 hrs of birth based Incidence 0.45/100,000 births. licensed oral VK product on weight. 6 total cases of VKDB: 1 early and 5 available. 1 mg VK if birth weight >1500 g late. 2 received no VK, 3 received 0.5 mg VK if birth weight <1500 g IM VK, 1 received oral VK. Oral Dosing Protocol: 51 Protocol varies by location. Canadian Paediatric Society and College of Family Physicians of Canada suggest: 2 mg within 6 hours after birth 2 mg at 2-4 weeks 2 mg at 6-8 weeks Denmark 1992 IM injection recommended. Oral IM Dosing Protocol: 44 Late VKDB: Konakion MM from previously recommended for all 2 mg at birth Incidence 0/100,000 births. 44 for IM and oral use; oral infants except those born before Oral Dosing Protocol: 44 preparation currently 33 wks gestation, with a difficult 2 mg after birth unavailable. 44 delivery, having asphyxia requiring 1 mg/week until 3 months resuscitation, and with mothers on (parents administer) as long as antiepileptic drugs. In practice, often baby is 50% or greater breastfed administered to all cesarean babies. 44 Germany 1994 For healthy newborns, IM injection IM Dosing Protocol: 38 Late VKDB: 39 Konakion MM Paediatric since recommended, oral VK available. For Term newborns: 1 mg at birth Incidence following IM injection 1996 for IM and oral use. 39 unwell newborns or preterm infants Preterm or birth weight <1500 g: unavailable. <1500 g, IM injection recommended mg/kg at birth Incidence 0.44/100,000 births following Oral Dosing Protocol: 38 complete oral dosing protocol. 2 mg after birth Incidence 0.56/100,000 births following 2 mg at 3-10 days complete or incomplete oral dosing 2 mg at 4-6 weeks protocol. Majority of cases occurred secondary to cholestasis. Japan 1988 Oral recommended. 41 Oral Dosing Protocol: 41 Late VKDB: Vitamin K2 menaquinone (MK- 2 mg after birth Incidence 1.9/100,000 births 4) oral product. 2 mg at discharge (usually ( ) wk of age) 71 total reported cases: 11 received full 2 mg at 1 month oral prophylaxis. Netherlands 1990 Oral recommended. Oral Dosing Protocol since 2011: 50 Late VKDB: Three K1 products are licensed IM injection recommended for unwell, 1 mg at birth Incidence 1.1/100,000 births ( ). 49 for IM and oral use. 49 preterm, and low birth weight infants mcg/day until 12 weeks Incidence 3.2/100,000 births (2005). 49 Oral Dosing Protocol : 49 Incidence 2.1/100,000 births ( ) mg at birth Majority of cases secondary to 25 mcg/day until 13 weeks cholestasis. The 2011 oral dosing protocol was implemented in light of these data suggesting that 25 mcg/day is insufficient to prevent late VKDB. New Zealand 1995 IM injection recommended, oral VK IM Dosing Protocol: 32 Classic VKDB: 33 Konakion mixed micellar (MM) available. 32 Term newborns: 1 mg at birth Incidence 1.24/100,000 births. Paediatric since 1999 for IM and Birth weight <1000g: 0.5mg 8 total cases: 7 fully breastfed, none oral use. 32 at birth received VK prophylaxis. Oral Dosing Protocol: 32 Late VKDB: 33 2 mg at birth Incidence 1.4/100,000 births. 2 mg at 3-7 days 9 total cases: 8 received no VK, 2 mg at 4-6 weeks 1 received IM VK. Switzerland 2003 Oral recommended. Oral Dosing Protocol: 43 Late VKDB: 43 Unspecified brand of mixed 2 mg by hour 4 after birth Incidence 0.87/100,000 births. micellar (MM) VK since 1995 for 2 mg at 4 days 4 total cases: 3 had cholestasis, IM and oral use mg at 4 weeks 3 refused all VK prophylaxis, 1 forgot Possible benefit to weekly oral third dose of oral VK doses recognized for preventing VKDB in infants with cholestasis, but more complex regimen may reduce compliance. United 2006 IM VK is the most clinically and cost- IM Dosing Protocol: 55 Classic VKDB: 20 Kingdom Konakion MM Pediatric effective method of administration. 1 mg IM at birth No cases reported. for IM and oral use. 20 Parents declining IM VK should be offered Oral Dosing Protocol: Late VKDB: 20 oral VK as a second-line option. 55 Administer multiple oral doses Incidence 0.64/100,000 births. In practice, pediatric units recommend according to manufacturer s 11 total cases: 6 received no VK, preferred routes of administration, with instructions. 55 Maternity units 3 received IM VK (2 had biliary those supporting oral dosing often usually recommend a minimum of atresia, one was born preterm), recommending IM for high-risk three 2 mg doses of oral VK for 2 received incomplete oral VK. newborns. 20 breastfed babies of the 11 infants were jaundiced at presentation to clinic after 21 days. United States 1961 IM injection. 13 IM Dosing Protocol: 13 No data available. Phytonadione for IM use mg at birth SUMMER 2016 / 17
6 generally attributed to other added ingredients in the VK injection. 47 Aluminum, benzyl alcohol and derivatives, polyoxyethlyated castor oil, polysorbate 80, and other ingredients may be among the inactive constituents of VK IM injections. All formulations containing benzyl alcohol or its derivates should be avoided in the neonate due to the possibility of gasping syndrome, which consists of gasping respirations, respiratory distress, metabolic acidosis, hypotension, CNS dysfunction, and cardiovascular collapse. 39 Preservative-free IM formulations are available and may be administered by the midwife after review of the manufacturer s ingredient labeling. With any formulation of IM VK, there is the possibility of rarely reported side effects such as infection at the site of injection, hypersensitivity, shock, cardiac or respiratory arrest, anaphylaxis, or death. Data are scant on how often these side effects occur. 39 Childhood Leukemia A now readily-disproved study from 1992 reported that the IM injection led to a tripling of leukemia risk in children as opposed to the oral or complete refusal route. 48 This study led many countries to develop their oral VK protocols to find a method of administration that was acceptable to parents. While the leukemia connection has been disproven, parents may still be concerned about it. Parental Preference Due to the possible trauma of receiving an injection on the first day of life, a belief in the wisdom of the body not having VK at birth, unknown risks of the injection, and a general preference for avoiding interventions, some parents may not want to administer IM VK to their newborn. Midwives may encounter clients with these concerns and can offer the most current, evidence-based information available, including information on the risks of VKDB, which IM VK is highly effective at preventing. The consequences for the small number of infants who develop uncontrolled bleeding are often catastrophic. Implications of any uncontrolled bleed in the infant include anemia, ischemia, shock, brain injury, disseminated intravascular coagulation, and death. Data from millions of births across the world demonstrate that VK prophylaxis is critical for the infant, and this prompted many countries including Australia, New Zealand, the United Kingdom, the Netherlands, Switzerland, Germany, and Japan to develop an oral VK product and protocol to ensure parents had an option besides the IM injection. 34,49,50,51,52,53,54 When parents decline the IM injection, a full range of options should be available to their infant for the prevention of VKDB, including oral VK prophylaxis. An Optimal Vitamin K Protocol An optimal vitamin K protocol incorporates parental acceptance, ease of use, and effective reduction of VKDB. While IM VK has been verified as simple and effective at preventing VKDB in a majority of cases, IM VK is not always accepted by parents. Because parents continue to refuse the injection, and because the majority of modern United States cases of VKDB occur in babies whose parents declined the injection, 34,42 an alternative option is essential. Most other developed nations with excellent infant health outcomes offer an oral VK product and administration protocol with confidence, and their data demonstrate effective reduction of VKDB with these oral protocols (Figure 2). In the majority of these countries, the IM injection can also be given as an oral dose, and most utilize a mixed micellar (MM) formulation. This product uses a different solubilizing agent than the US IM formulation and was developed because there was some evidence that it may be better absorbed, especially in infants with cholestasis. However, data have not shown a significant improvement in the efficacy of the MM preparation to prevent late VKDB in infants with cholestasis. 34,50 Nonetheless the MM product has stayed on the market and continues to be used for IM and oral administration. In England, 55 Germany, 52 Australia, 51 New Zealand, 50 and Canada, 56 IM injections for the newborn are recommended, but oral administration is available. In Switzerland, 34 Japan, 53 Denmark, 57 and the Netherlands, 58 oral prophylaxis is recommended over IM injections, though IM injections may be recommended if the infant is preterm or unwell. 57,58 Although Denmark historically recommended oral prophylaxis, due to licensing issues, it lost its oral VK product in 2000 and now exclusively offers IM prophylaxis. 57 Vitamin K1 products for oral or IM administration are used in every country except Japan, where vitamin K2 is used. Oral VK has high rates of parental acceptance and effectiveness based on population surveillance when given according to evidence-based dosing and timing protocols. Efficacy may be reduced by forgotten doses or infant sickness. Among infants with an undiagnosed hepatobiliary or malabsorptive disorder who received VK prophylaxis, oral prophylaxis is more likely to fail, but IM prophylaxis may fail as well. 34,42,49,59,60 Both IM and oral VK are effective options for the prevention of VKDB, especially when compared to no prophylaxis. Limitations of Oral Vitamin K Offering oral VK prophylaxis as an alternative to IM VK is a reasonable option when parents decline IM VK prophylaxis; however, both the midwife and client must understand the following limitations of oral prophylaxis. Effectiveness IM and oral VK are equally effective in preventing classic VKDB, 15 but IM VK is more effective than oral VK at preventing late VKDB, due to inconsistent administration and the possibility of hepatobiliary or malabsorptive disorders. Undiagnosed Disorders Not all infants who appear healthy are able to absorb oral VK, particularly infants with hepatobiliary or malabsorptive disorders. Many of these infants appear normal and have no external symptoms. If an infant is diagnosed with one of these diseases, the midwife must remind parents to inform their doctor if the infant did not receive IM VK at birth. These infants cannot absorb oral VK well, including VK from breast milk, and should receive the VK injection. Premature or Low Birth Weight Infants Preterm infants (<37 wks) and low birth weight infants (<2,000 g) should receive IM VK due to issues with limited intestinal absorption of oral VK and elevated risk for bleeds. 60 The IM injection may, however, contain ingredients such as aluminum and polysorbate 80 that are known to have serious side effects in premature neonates, and the manufacturer s ingredient label should be read to avoid these ingredients. 39 The standard IM VK dose of 1 mg has been found to cause excessively high serum levels of VK in premature or low birth weight infants, 61 and midwives may advocate for their clients to receive lower dosing. One study of 0.5 mg of IM VK in preterm infants found high serum VK values, and concluded that preterm infants should at most receive 0.5 mg of IM VK. 62 Another study of preterm infants found that 0.2 mg of IM VK maintained adequate VK status in preterm infants until a median age of 25 days, demonstrating that an even lower dose may be appropriate, with the potential to repeat small VK doses. 63 Limited Product Options The current IM VK product on the market is FDA-approved for injection but not oral use in the neonate. 39 Liquid oral VK products available in the United States include those found online or through compounding pharmacies. These products are not standardized, third-party verified, or FDA-approved, and therefore may not have the high level of effectiveness found in products used in foreign studies. Parental Compliance The midwife and parents must recognize that parental compliance with administration is key. Missed oral VK doses increase the risk for bleeding and related sequelae. Maternal Risk Factors The midwife must consider maternal risk factors. A mother 18 / MIDWIFERY MATTERS
7 who has been taking anti-coagulants, anti-convulsants, cephalosporins, VK antagonists such as warfarin, or tubercolostatic agents during pregnancy will have a newborn requiring immediate IM VK prophylaxis. She will probably not be a candidate for out-of-hospital midwifery care because her infant will need special monitoring due to the high risk of developing VKDB in the first 24 hours of life. 2 Circumcision While there is little current data available, it can be reasonably inferred that an IM VK injection is ideal to mitigate postsurgical, circumcision-related bleeding risk, which is a common manifestation of classic VKDB. 2,3,17 Special Circumstances While there are scant data, in practice some countries recommend IM VK for infants with birth asphyxia requiring resuscitation, those born via cesarean section, and those who experience traumatic birth because trauma can contribute to cases of VKDB. 50,51,52,55,57,59 Unknown Risks Midwives and parents should recognize that while there may be unknown long-term risks to oral VK administration, none have been identified in the literature. The theoretical question remains as to whether oral VK preparations could harm the sensitive intestinal lining of the newborn. An Oral Vitamin K Protocol Based on maternal medical history and a discussion of plans for the infant, the midwife will be able to determine if a client is an appropriate candidate for an oral VK protocol. Eligibility criteria may include the requirements that the mother is not taking any high-risk medications, that the parents do not have plans for circumcision, and that they are committed to regular oral VK administration. If a client understands the main concerns with oral VK in the United States lack of a licensed VK preparation for oral use and the possibility of poor absorption then an oral protocol may be appropriate. Midwives may recommend the evidence-based oral VK protocol detailed in Figure 3 to their clients who decline IM VK prophylaxis. This protocol is based on multiple countries recommendations that 3-part oral VK dosing has demonstrated effectiveness in reducing VKDB according to surveillance studies of the largest populations. Late VKDB is so rare that large populations must be considered to determine the true effectiveness of any protocol, and 3-part dosing appears effective in the largest studied populations. Denmark and the Netherlands recommend daily or weekly dosing, but because the population of these countries is small, the results of their surveillance that show a rate of late VKDB at 0 cases per 100,000 births following the recommended oral prophylaxis protocol may not capture the true likelihood of cases of late VKDB. Combined data from 14 years of surveillance, however, do offer information about the benefit of continued weekly dosing for unwell infants. Denmark and the Netherlands are unique in that their surveillance looks retrospectively at cases of infants who were thought to be healthy but later diagnosed with hepatobiliary or malabsorptive disease. Data from 3.76 million births in Denmark and the Netherlands found that an oral VK dose of 1 mg/wk until week 13 is nearly as effective as IM VK at birth in preventing late VKDB in infants with a hepatobiliary or malabsorptive disease that has gone undiagnosed for a period of time since birth. 60 Conclusion VKDB continues to occur today, most often in infants who did not receive VK prophylaxis at birth. Both case studies and population data across the world demonstrate that VK administration is essential for preventing VKDB, both because there is no way to predict which infants will develop VKDB, and because all infants have physiologic factors that limit their ability to develop and maintain adequate VK stores, putting them at risk. VKDB can be life-threatening when it occurs, and parents FIGURE 3. ORAL VITAMIN K PROPHYLAXIS PROTOCOL AND ADMINISTRATION GUIDELINES Oral Dosing Schedule: 2 mg at birth 2 mg on days mg at 1 month Optional continued dosage of 1 mg/wk from 5 weeks to 13 weeks Determine the dose given in each drop of the oral vitamin K product being used. Dosages vary by drop, and correct dosing is critical. Because vitamin K1 products are the most robustly researched, it is recommended they should be used in this protocol. Parameters for administration include: Midwife ensures infant receives critical doses at birth, on days 4-7, and at 1 month by scheduling an appointment or making a reminder call to the family. Midwife leaves optional continued weekly dosing from week 5 to week 13 up to parents. Midwife administers the at-birth dose, but parents must have their own bottle of Vitamin K to ensure the time-sensitive administration of the remaining doses. Midwife encourages parents to put dosing dates on the calendar. Timing is critical. Midwife instructs parents in proper administration technique. To administer, gently hold the infant s mouth open and place drops on tongue. Close the infant s mouth and observe for any of the dose leaking or being spit out. Re-administer dose if any spitting up, vomiting, or diarrhea occurs within 2 hours of administration. Encourage parents to administer the dose after feeding. Some studies suggest concurrent fat intake makes oral Vitamin K more absorbable. deserve access to the full range of VK prophylactic options for the prevention of this event. Misinformation about VK abounds, including in midwifery circles, and it is the midwife s responsibility to be educated and current on this topic and to ensure that clients are also welleducated and able to make the best decision for themselves and their infants. IM VK remains the standard of care due to its ease of administration and effective reduction of VKDB, but it may not be the option every parent is comfortable to choose. Oral administration of VK is a reasonable and effective option for parents choosing to decline IM VK. Each choice for prophylactic VK for the infant has benefits and risks. Evidence-based education covering the full range of options for the prevention of VKDB is critical in providing client-centered care that ensures access to the standard of care while also leaving space for clients to make the best decisions for themselves and their infants. Kelsey Scherer is a senior student at Birthwise Midwifery School completing preceptorship at the Concord Birth Center in New Hampshire. She can be reached at Kelsey_Scherer@birthwisemidwifery.edu. REFERENCES 1. Jinghe X, Mizuta T, Ozaki I. Vitamin K and hepatocellular carcinoma: The basic and clinic. World J Clin Cases Sept 16;3(9): Sutor AH, von Kries R, Cornelissen EA, McNinch AW, Andrew M. Vitamin K deficiency bleeding (VKDB) in infancy. Thromb Haemost Mar;81(3): van Hasselt PM. Vitamin K prophylaxis revisited: Focus on risk factors [dissertation]. Utrecht, Netherlands: Utrecht University; American Academy of Pediatrics Committee on Fetus and Newborn (USA). Policy statement: Controversies concerning vitamin K and the newborn. Pediatrics Jul;112(1 Pt 1): Thomas MW, Gebara BM. Resident rounds: Difficulty breathing. Clin Pediatr. 2004;43: Woods CW, Woods AG, Cederholm CK. Vitamin K deficiency bleeding: a case study. Adv Neonatal Care Dec;13(6): SUMMER 2016 / 19
8 7. Hubbard D, Tobias JD. Intracerebral hemorrhage due to hemorrhagic disease of the newborn and failure to administer vitamin K at birth. South Med J Nov;99(11): Adame N, Carpenter SL. Closing the loophole: Midwives and the administration of vitamin K in neonates. J Pediatr May;154(5): Burke CW. Vitamin k deficiency bleeding: overview and considerations. J Pedatr Health Care May-June;27(3): Christensen CK, Golomb MR. It s not just a vitamin: comment on Rise in late onset vitamin K deficiency bleeding in young infants because of omission or refusal of prophylaxis at birth by Schulte and colleagues. Pediatr Neurol Mar;52(3):e1-e Schulte R, Jordan LC, Morad A, Naftel RP, Wellons JC, Sidonio R. Rise in late onset vitamin K deficiency bleeding in young infants because of omission or refusal of prophylaxis at birth. Pediatr Neurol. 2014;50: Sahni V, Lai FY, MacDonald SE. Neonatal vitamin K refusal and nonimmunization. Pediatrics. 2014;134(3): Higdon J, Drake V, Delage B, Booth S; Linus Pauling Institute. Micronutrient information center: Vitamin K [Internet]. Corvallis (OR): Oregon State University; 2015 [cited 2015 Nov 29]. Available from: vitamins/vitamin-k#introduction. 14. Post T, editor. Overview of vitamin K. [Internet]. Waltham (MA): UpToDate; 2015 [cited 2016 Jan 11]. Available from: Puckett RM, Offringa M. Prophylactic vitamin K for vitamin K deficiency bleeding in neonates. Cochrane Database of Syst Rev. 2009: (4). Art. No.: CD Mahadevan SB, Beath SV, McKiernan PJ, Kelly DA. Letters to the editor: The vitamin K debacle and infants with cholestatic liver disease. Arch Dis Child. 1999:81; Blackburn ST. Maternal, fetal, & neonatal physiology: A clinical perspective. 4th rev. ed. Maryland Heights (MO): Saunders; Greer FR. Vitamin K the basics what s new? Early Hum Dev. 2010;86(Suppl 1): McNinch AW, Upton C, Samuels M, Shearer MJ, McCarthy P, Tripp JH, L E Orme R. Plasma concentrations after oral or intramuscular vitamin K1 in neonates. Arch Dis Child. 1985;60: Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press;2002: Greer FR. Vitamin K in human milk still not enough. Acta Paediatr. 2004;93: Kojima T, Asoh M, Yamawaki N, Kanno T, Hawegawa H, Yonekubo A. Vitamin K concentrations in the maternal milk of Japanese women. Acta Paediatr. 2004;93: Greer FR, Marshall SP, Foley AL, Suttie JW. Improving the Vitamin K status of breastfeeding infants with maternal vitamin K supplements. Pediatrics. 1997;99(1): von Kries R, Shearer M, McCarthy PT, Haug M, Harzer G, Gobel U. Vitamin K1 content of maternal milk: Influence of the stage of lactation, lipid composition, and vitamin K1 supplements given to the mother. Ped Research. 1987;22(5): Canfield LM, Hopkinson JM, Lima AF, Silva BS, Garza C. Vitamin K in colostrum and mature human milk over the lactation period a cross-sectional study. Am J Clin Nutri. 1991;53: Thijssen HH, Drittij MJ, Vermeer C, Schoffelen E. Menaquinone-4 in breast milk is derived from dietary phylloquinone. Br J Nutr. 2002;87: Nishiguchi T, Yamashita M, Maeda M, Matsuyama K, Kobayashi T, Kanayama N, Terao T. Improvement of vitamin K status of breastfeeding infants with maternal supplement of vitamin K2. Semin Thromb Hemost 2002;28(6): Shearer MJ. Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008; 100: von Kries R, Greer FR, Suttie JW. Assessment of vitamin K status of the newborn infant. J Ped Gastroenter Nutr. 1993;16: Kayata S, Kindberg C, Greer FR, Suttie JW. Vitamin K1 and K2 in infant human liver. J Ped Gastroenter Nutr. 1989;8: Shearer MJ, Rahim S, Barkhan P, Stimmler L. Plasma vitamin K1 in mothers and their newborn babies. Lancet. 1982;2: Beulens JW, Booth SL, van den Heuvel EG, Stocklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K2) in human health. Br J Nutr Oct;110(8): von Kries R, Achmeister A, Gobel U. Can 3 oral 2 mg doses of vitamin K effectively prevent late vitamin K deficiency bleeding? Eur J Pediatr. 1997;158[Suppl 3]:S Laubscher B, Banziger O, Schubiger G; Swiss Paediatric Surveillance Unit (SPSU). Prevention of vitamin K deficiency bleeding with three mixed micellar phylloquinone doses: results of a 6-year ( ) surveillance in Switzerland. Eur J Paediatr Mar;172(3): Visser DY, Jansen NJ, Ijland MM, de Koning TJ, van Hasselt PM. Intracranial bleeding due to Vitamin K deficiency: advantages of using a pediatric intensive care registry. Intensive Care Med. 2011;37(6): Sutor AH. New aspects of Vitamin K prophylaxis. Semin Thromb Hemost Aug;29(4): MacKinlay GA. Jaundice persisting beyond 14 days after birth. Brit Med J May; 306: Bergquist J. Neonatal Cholestasis. [Internet]. Chicago: The University of Chicago Medicine Comer Children s Hospital; 2005 Aug [cited 2015 Dec 7]. Available from: Post T, editor. Vitamin K (phytonadione): Drug information. [Internet]. Waltham (MA): UpToDate; 2015 [cited 2016 January 11]. Available from: www. uptodate.com. 40. Canadian Paediatric Surveillance Program (CPSP). CPSP highlights. Paediatr Child Health Oct;7(8): Schubiger G, Tonz O, Gruter J, Shearer MJ. Vitamin K1 concentration in breast-fed neonates after oral or intramuscular administration of a single dose of a new mixed-micellar preparation of phylloquinone. J Pediatr Gastroentero and Nutr May; 16: Darlow BA, Phillips AA. Dickson NP. New Zealand surveillance of neonatal vitamin K deficiency bleeding (VKDB): J Paediatr Child Health Jul;47(7): McMillan DD, Grenier D, Medaglia A. Canadian paediatric surveillance program confirms low incidence of hemorrhagic disease of the newborn in Canada. Paediatr Child Health. 2004;9(4): Flood VH, Galderisi FC, Lowas SR, Kendrick A, Boshkov LK. Hemorrhagic disease of the newborn despite vitamin K prophylaxis at birth. Pediatr Blood Cancer. 2008;50: Ciantelli M, Bartalena L, Bernardini M, Biver P, Chesi F, Boldrini A, Sigali E. Late vitamin K deficiency bleeding after intramuscular prophylaxis at birth: a case report. J Perinatol. 2009;29(2): Widdershoven J, Lambert W, Motohara K, Monnens L, de Leenher A, Matsuda I, Endo F. Plasma concentrations of vitamin K1 and PIVKA-II in bottle-fed and breast-fed infants with and without vitamin K prophylaxis at birth. Eur J Pediatr. 1988;148: Shearer MJ. Vitamin K in parenteral nutrition. Gastroenterology. 2009;137:S Roman E, Fear NT, Ansell P, Bull D, Draper G, McKinney P, et al. Vitamin K and childhood cancer: analysis of individual patient data from six case-control studies. Br J Cancer. 2002;86(1): Busfield A, Samuel R, McNinch A, Trip JH. Vitamin K deficiency bleeding after NICE guidance and withdrawl of Konakion Neonatal: British paediatric surveillance unit study, Arch Dis Child. 2013;98: Fetus and Newborn Committee of the Paediatric Society of New Zealand. Newborn services clinical guideline: vitamin K prophylaxis and vitamin K deficiency bleeding [Internet]. New Zealand: Paed Soc NZ; 2013 Aug [cited 2016 Jan 23]. Available from: VitaminK.htm. 51. National Health and Medical Research Council. Paediatric Division of the Royal Australian College of Physicians. Royal Australian and New Zealand College of Obstetrics and Gynaecoloy. Royal Australian College of General Practitioners. Australian College of Midwives. Joint statement and recommendations on vitamin K administration to newborn infants to prevent vitamin K deficiency bleeding in infancy [Internet]. Australia: NHMRC; 2010 Oct [cited 2016 Jan 23]. Available from: Buhrer C, Genzel-Boroviczeny O, Jochum F, Kauth T, Kersting M, Koletzko B, et al. Vitamin-K-prophylaxe bei neugeborenen (Vitamin K prophylaxis in newborns). Monatsschrift Kinderheilkinde. 2013;161(4): Takahashi D, Shirahata A, Itoh S, Takahashi Y, Nishiguchi T, Matsuda Y. Vitamin k prophylaxis and late vitamin K deficiency bleeding in infants: fifth nationwide survey in Japan. Pediatr Int Dec;53(6): Ijland MM, Pereira RR, Cornelissen EA. Incidence of late vitamin K deficiency bleeding in newborns in the Netherlands in 2005: evaluation of the current guideline. Eur J Pediatr. 2008;167: Busfield A, Samuel R, McNinch A, Tripp JH. Vitamin K deficiency bleeding after NICE guidance and withdrawal of Konakion Neonatal: British Paediatric Surveillance Unit study, Arch Dis Child. 2013;90: McMillan D; Fetus and Newborn Committee Canadian Paediatric Society; College of Family Physicians of Canda. Position statement: routine administration of vitamin K to newborns. Paediatr Child Health. 1997;2(6): Hansen KN, Minousis M, Ebbesen F. Weekly oral vitamin K prophylaxis in Denmark. Acta Paediatr. 2003;92(7): de Winter JP, Joosten KF, Ijland MM, Verkade HJ, Offringa M, Dorrius MD, van Hasselt PM; Spaarne Ziekenhuis afd. Kindergeneeskunde. New Dutch practice guideline for administration of vitamin K to full-term newborns. Ned Tijdschr Geneeskd. 2011;155(18):A Cornelissen M, von Kries R, Loughnan P, Schubiger G. Prevention of vitamin K deficiency bleeding: efficacy of different multiple oral dose schedules of vitamin K. Eur J Pediatr Feb;156(2): van Hasselt PM, de Koning TJ, Kvist N, de Vries E, Lundin CR, Berger R, et al; Netherlands Study Group for Biliary Atresia Registry. Prevention of vitamin K deficiency bleeding in breastfed infants: lessons from the Dutch and Danish biliary atresia registries. Pediatrics. 2008;121(4):e Costakos DT, Porte M. Did Controversies concerning vitamin K and the newborn cover all controversies? Pediatrics. 2004;113: Costakos DT, Greer FR, Love LA, Dahlen LR, Sutte JW. Vitamin K prophylaxis for premature infants: 1 mg versus 0.5 mg. Am J Perinatol. 2003;20: Clarke P, Mitchell SJ, Wynn R, Sundaram S, Speed V, Gardener E, Roeves D, Shearer MJ. Vitamin K prophylaxis for preterm infants: A randomized, controlled trial of 3 regimens. Pediatrics. 2006;118: National Institute for Clinical Excellence. Postnatal care up to 8 weeks after birth [Internet]. London: NICE; 2006 July [cited 2016 Jan 23]. Available from: Schubiger G, Stocker C, Banzinger O, Laubscher B, Zimmerman H. Oral vitamin K1 prophylaxis for newborns with a new mixed-micellar preparation of phylloquinone: 3 years experience in Switzerland. Eur J Pediatr Jul;158(7): / MIDWIFERY MATTERS
Vitamin K. Emily Rohan Sarah Steinmetz
 Vitamin K Emily Rohan Sarah Steinmetz How much do we need? Adequate Intake (AI) Life Stage Group Adequate Intake Level 0-6 months 2.0 ug/day 7-12 months 2.5 ug/day 1-3 years 30 ug/day 4-8 years 55 ug/day
Vitamin K Emily Rohan Sarah Steinmetz How much do we need? Adequate Intake (AI) Life Stage Group Adequate Intake Level 0-6 months 2.0 ug/day 7-12 months 2.5 ug/day 1-3 years 30 ug/day 4-8 years 55 ug/day
Number of preventable vitamin K deficiency bleeding cases
 preventable vitamin K deficiency bleeding cases No. 2017/04Ae, The Hague, April 11, 2017 Background document to: Vitamin K for infants No. 2017/04e, The Hague, April 11, 2017 preventable vitamin K deficiency
preventable vitamin K deficiency bleeding cases No. 2017/04Ae, The Hague, April 11, 2017 Background document to: Vitamin K for infants No. 2017/04e, The Hague, April 11, 2017 preventable vitamin K deficiency
Vitamin K. Amina Ziyad Elaf Sohaib
 Vitamin K Amina Ziyad Elaf Sohaib What is Vitamin K? Fat soluble compound Necessary for the synthesis of several proteins required for blood clotting 1) Vit K 1 (Phylloquinone) - natural form - found in
Vitamin K Amina Ziyad Elaf Sohaib What is Vitamin K? Fat soluble compound Necessary for the synthesis of several proteins required for blood clotting 1) Vit K 1 (Phylloquinone) - natural form - found in
Vitamin K. Emily Rohan Sarah Steinmetz
 Vitamin K Emily Rohan Sarah Steinmetz How much do we need? Adequate Intake (AI) Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron,
Vitamin K Emily Rohan Sarah Steinmetz How much do we need? Adequate Intake (AI) Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron,
Nottingham Neonatal Service Clinical Guidelines
 Nottingham Neonatal Service Clinical Guidelines Title: VITAMIN K IN THE NEWBORN: Prophylaxis against vitamin K deficiency bleeding in infants Version: 5 (received and circulated July 2015) Date: V4 March
Nottingham Neonatal Service Clinical Guidelines Title: VITAMIN K IN THE NEWBORN: Prophylaxis against vitamin K deficiency bleeding in infants Version: 5 (received and circulated July 2015) Date: V4 March
Personal Practice. Vitamin K During Infancy: Current Status and Recommendations
 Personal Practice Vitamin K During Infancy: Current Status and Recommendations Meharban Singh Vitamin K is usually the first vitamin given at birth. Dietary deficiency of vitamin K in healthy subjects
Personal Practice Vitamin K During Infancy: Current Status and Recommendations Meharban Singh Vitamin K is usually the first vitamin given at birth. Dietary deficiency of vitamin K in healthy subjects
DATE: 28 May 2015 CONTEXT AND POLICY ISSUES
 TITLE: Neonatal Vitamin K Administration for the Prevention of Hemorrhagic Disease: A Review of the Clinical Effectiveness, Comparative Effectiveness, and Guidelines DATE: 28 May 2015 CONTEXT AND POLICY
TITLE: Neonatal Vitamin K Administration for the Prevention of Hemorrhagic Disease: A Review of the Clinical Effectiveness, Comparative Effectiveness, and Guidelines DATE: 28 May 2015 CONTEXT AND POLICY
Implications of Parent Vitamin K & Immunization Refusal
 Implications of Parent & Immunization Refusal Helen Russell, MSN NNP-bc March 2016 OBJECTIVES Understand the physiologic purpose of Identify and known advantages and disadvantage of vitamin K injection
Implications of Parent & Immunization Refusal Helen Russell, MSN NNP-bc March 2016 OBJECTIVES Understand the physiologic purpose of Identify and known advantages and disadvantage of vitamin K injection
Vitamin K in Cystic Fibrosis
 Vitamin K in Cystic Fibrosis Martin J. Shearer PhD, MRCPath Consultant Clinical Scientist Honorary Senior Lecturer Centre for Haemostasis and Thrombosis, Guy s and St. Thomas Foundation Trust London, UK
Vitamin K in Cystic Fibrosis Martin J. Shearer PhD, MRCPath Consultant Clinical Scientist Honorary Senior Lecturer Centre for Haemostasis and Thrombosis, Guy s and St. Thomas Foundation Trust London, UK
Biochemistry of Vitamin K
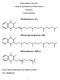
 Lecture 4 Biochemistry of Vitamin K The Objectives Types and chemistry of vitamin K Sources and daily requirements Functions Synthesis of γ-carboxyglutamate in: Prothrombin and blood clotting factors Osteocalcin
Lecture 4 Biochemistry of Vitamin K The Objectives Types and chemistry of vitamin K Sources and daily requirements Functions Synthesis of γ-carboxyglutamate in: Prothrombin and blood clotting factors Osteocalcin
EFFECT OF ORAL WATER SOLUBLE VITAMIN K ON PIVKA-II LEVELS IN NEWBORNS
 EFFECT OF ORAL WATER SOLUBLE VITAMIN K ON PIVKA-II LEVELS IN NEWBORNS R.K. Sharma N. Marwaha P. Kumar A. Narang ABSTRACT Intramuscular administration of vitamin K for prophylaxis against hemorrhagic disease
EFFECT OF ORAL WATER SOLUBLE VITAMIN K ON PIVKA-II LEVELS IN NEWBORNS R.K. Sharma N. Marwaha P. Kumar A. Narang ABSTRACT Intramuscular administration of vitamin K for prophylaxis against hemorrhagic disease
Summary of Product Characteristics
 NAME OF THE MEDICINAL PRODUCT Summary of Product Characteristics Konakion MM Paediatric Ampoules 2 mg/ 0.2 ml oral solution or solution for injection. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ampoule
NAME OF THE MEDICINAL PRODUCT Summary of Product Characteristics Konakion MM Paediatric Ampoules 2 mg/ 0.2 ml oral solution or solution for injection. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ampoule
Vitamin K (Phylloquinone)
 Vitamin K (Phylloquinone) What is Vitamin K? Vitamin K is a fat-soluble vitamin which plays a key role in blood coagulation. Its name comes from the German name, Koagulationsvitamin. There are three forms,
Vitamin K (Phylloquinone) What is Vitamin K? Vitamin K is a fat-soluble vitamin which plays a key role in blood coagulation. Its name comes from the German name, Koagulationsvitamin. There are three forms,
Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review
 OPEN Journal of Perinatology (2016) 36, S29 S34 2016 Nature America, Inc. All rights reserved 0743-8346/16 www.nature.com/jp SYSTEMATIC REVIEWS Vitamin K prophylaxis for prevention of vitamin K deficiency
OPEN Journal of Perinatology (2016) 36, S29 S34 2016 Nature America, Inc. All rights reserved 0743-8346/16 www.nature.com/jp SYSTEMATIC REVIEWS Vitamin K prophylaxis for prevention of vitamin K deficiency
Konakion MM. Phytomenadione Composition. Properties and effects. Pharmacokinetics
 פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר על ידו בנובמבר 2015 Konakion MM Phytomenadione Composition Active ingredient: phytomenadione (synthetic vitamin K 1 ). Ampoules MM 10 mg/ml in a bile
פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר על ידו בנובמבר 2015 Konakion MM Phytomenadione Composition Active ingredient: phytomenadione (synthetic vitamin K 1 ). Ampoules MM 10 mg/ml in a bile
AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1
 9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
9073025 INJECTION AquaMEPHYTON (PHYTONADIONE) Aqueous Colloidal Solution of Vitamin K 1 WARNING - INTRAVENOUS AND INTRAMUSCULAR USE Severe reactions, including fatalities, have occurred during and immediately
PDF hosted at the Radboud Repository of the Radboud University Nijmegen
 PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/24702
PDF hosted at the Radboud Repository of the Radboud University Nijmegen The following full text is a publisher's version. For additional information about this publication click this link. http://hdl.handle.net/2066/24702
ORIGINAL ARTICLES. Frank R Greer, Sharon P Marshall, Rebecca R Severson, David A Smith, Martin J Shearer, Daniel G Pace, Pieter H Joubert
 300 Arch Dis Child 1998;79:300 305 ORIGINAL ARTICLES Department of Pediatrics, University of Wisconsin, Madison, Wisconsin 53715, USA F R Greer S P Marshall Department of Pediatrics, Franciscan-Skemp Medical
300 Arch Dis Child 1998;79:300 305 ORIGINAL ARTICLES Department of Pediatrics, University of Wisconsin, Madison, Wisconsin 53715, USA F R Greer S P Marshall Department of Pediatrics, Franciscan-Skemp Medical
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion , version 1.1
 EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
Minimal Enteral Nutrition
 Abstract Minimal Enteral Nutrition Although parenteral nutrition has been used widely in the management of sick very low birth weight infants, a smooth transition to the enteral route is most desirable.
Abstract Minimal Enteral Nutrition Although parenteral nutrition has been used widely in the management of sick very low birth weight infants, a smooth transition to the enteral route is most desirable.
11/8/12. KERNICTERUS: The reason we have to care about bilirubin. MANAGING JAUNDICE IN THE BREASTFEEDING INFANT AKA: Lack of Breastfeeding Jaundice
 MANAGING JAUNDICE IN THE BREASTFEEDING INFANT AKA: Lack of Breastfeeding Jaundice November 16, 2012 Orange County Lawrence M. Gartner, M.D. University of Chicago and Valley Center, California KERNICTERUS:
MANAGING JAUNDICE IN THE BREASTFEEDING INFANT AKA: Lack of Breastfeeding Jaundice November 16, 2012 Orange County Lawrence M. Gartner, M.D. University of Chicago and Valley Center, California KERNICTERUS:
Maternal and Infant Nutrition Briefs
 Maternal and Infant Nutrition Briefs A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition March/April 2003 New Guidelines on
Maternal and Infant Nutrition Briefs A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition March/April 2003 New Guidelines on
Clinical evaluation Jaundice skin and mucous membranes
 JAUNDICE Framework The definition of Neonatal Jaundice Billirubin Metabolism Special characteristic in neonates Dangerous of the Hyperbillirubinemia The diseases in relation with Neonatal Jaundice Objectives:
JAUNDICE Framework The definition of Neonatal Jaundice Billirubin Metabolism Special characteristic in neonates Dangerous of the Hyperbillirubinemia The diseases in relation with Neonatal Jaundice Objectives:
VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1
 VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1 Ampul Rx only Protect from light. Keep ampuls in tray until time of use. WARNING INTRAVENOUS AND INTRAMUSCULAR
VITAMIN K 1 INJECTION Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K 1 Ampul Rx only Protect from light. Keep ampuls in tray until time of use. WARNING INTRAVENOUS AND INTRAMUSCULAR
Safe and Healthy Beginnings. M. Jeffrey Maisels MD William Beaumont Hospital Royal Oak, MI
 Safe and Healthy Beginnings M. Jeffrey Maisels MD William Beaumont Hospital Royal Oak, MI jmaisels@beaumont.edu Risk Factors There are 2 kinds Those that increase the risk of subsequently developing a
Safe and Healthy Beginnings M. Jeffrey Maisels MD William Beaumont Hospital Royal Oak, MI jmaisels@beaumont.edu Risk Factors There are 2 kinds Those that increase the risk of subsequently developing a
Sultan Qaboos University. College of Agricultural & Marine Sciences. Vitamin K. Lecture Summary
 Sultan Qaboos University College of Agricultural & Marine Sciences Vitamin K Lecture Summary Name: Mohsin Mohammed Taqi Mohsin Al-Saleh ID #: M020944-99 0 Introduction: Vitamin K is a group of structurally
Sultan Qaboos University College of Agricultural & Marine Sciences Vitamin K Lecture Summary Name: Mohsin Mohammed Taqi Mohsin Al-Saleh ID #: M020944-99 0 Introduction: Vitamin K is a group of structurally
Standard of Newborn Care in the Age of Birth Plans. Stephanie Deal, MD Tiffany McKee-Garrett, MD
 Standard of Newborn Care in the Age of Birth Plans Stephanie Deal, MD Tiffany McKee-Garrett, MD Disclosure We have no relevant financial relationships with the manufacturers(s) of any commercial products(s)
Standard of Newborn Care in the Age of Birth Plans Stephanie Deal, MD Tiffany McKee-Garrett, MD Disclosure We have no relevant financial relationships with the manufacturers(s) of any commercial products(s)
ACQUIRED COAGULATION ABNORMALITIES
 ACQUIRED COAGULATION ABNORMALITIES ACQUIRED COAGULATION ABNORMALITIES - causes 1. Liver disease 2. Vitamin K deficiency 3. Increased consumption of the clotting factors (disseminated intravascular coagulation
ACQUIRED COAGULATION ABNORMALITIES ACQUIRED COAGULATION ABNORMALITIES - causes 1. Liver disease 2. Vitamin K deficiency 3. Increased consumption of the clotting factors (disseminated intravascular coagulation
 VITAMIN K1 - phytonadione injection, emulsion Cardinal Health ---------- Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K1 Ampul Rx only Protect from light. Keep ampuls in tray until
VITAMIN K1 - phytonadione injection, emulsion Cardinal Health ---------- Phytonadione Injectable Emulsion, USP Aqueous Dispersion of Vitamin K1 Ampul Rx only Protect from light. Keep ampuls in tray until
Maternal and Infant Nutrition Briefs
 Maternal and Infant Nutrition Briefs November/December 2002 A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition Recent Trends
Maternal and Infant Nutrition Briefs November/December 2002 A research-based newsletter prepared by the University of California for professionals interested in maternal and infant nutrition Recent Trends
The importance of early complementary feeding in the development of oral tolerance: Concerns and controversies
 The importance of early complementary feeding in the development of oral tolerance: Concerns and controversies Prescott SL, Smith P, Tang M, Palmer DJ, Sinn J, Huntley SJ, Cormack B. Heine RG. Gibson RA,
The importance of early complementary feeding in the development of oral tolerance: Concerns and controversies Prescott SL, Smith P, Tang M, Palmer DJ, Sinn J, Huntley SJ, Cormack B. Heine RG. Gibson RA,
Ashley Robson Canyon Creek Dr. Mckinney, TX 75070
 1 Ashley Robson 2212 Canyon Creek Dr. Mckinney, TX 75070 September 2 nd 2014 Debra Brandon PhD, RN, CCNS, FAAN Duke University School of Nursing Durham, NC Dear Mrs. Brandon- I would like the opportunity
1 Ashley Robson 2212 Canyon Creek Dr. Mckinney, TX 75070 September 2 nd 2014 Debra Brandon PhD, RN, CCNS, FAAN Duke University School of Nursing Durham, NC Dear Mrs. Brandon- I would like the opportunity
PACKAGE LEAFLET: Information for the patient VITAMINE E Sugar coated tablets 10 mg (Alpha tocopherol acetate)
 PACKAGE LEAFLET: Information for the patient VITAMINE E Sugar coated tablets 10 mg (Alpha tocopherol acetate) Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet.
PACKAGE LEAFLET: Information for the patient VITAMINE E Sugar coated tablets 10 mg (Alpha tocopherol acetate) Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet.
BREASTFEEDING TO PREVENT DOUBLE BURDEN OF MALNUTRITION
 BREASTFEEDING TO PREVENT DOUBLE BURDEN OF MALNUTRITION Sirinuch Chomtho Department of Pediatrics, Chulalongkorn University, Bangkok, Thailand The double burden of malnutrition means under- and over-nutrition
BREASTFEEDING TO PREVENT DOUBLE BURDEN OF MALNUTRITION Sirinuch Chomtho Department of Pediatrics, Chulalongkorn University, Bangkok, Thailand The double burden of malnutrition means under- and over-nutrition
Intracranial hemorrhages and late hemorrhagic disease associated cholestatic liver disease
 Neurol Sci (2013) 34:51 56 DOI 10.1007/s10072-012-0965-5 ORIGINAL ARTICLE Intracranial s and late hemorrhagic disease associated cholestatic liver disease Hüseyin Per Duran Arslan Hakan Gümüş Abdulhakim
Neurol Sci (2013) 34:51 56 DOI 10.1007/s10072-012-0965-5 ORIGINAL ARTICLE Intracranial s and late hemorrhagic disease associated cholestatic liver disease Hüseyin Per Duran Arslan Hakan Gümüş Abdulhakim
SWISS SOCIETY OF NEONATOLOGY. Spontaneous intestinal perforation or necrotizing enterocolitis?
 SWISS SOCIETY OF NEONATOLOGY Spontaneous intestinal perforation or necrotizing enterocolitis? June 2004 2 Stocker M, Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital of Lucerne,
SWISS SOCIETY OF NEONATOLOGY Spontaneous intestinal perforation or necrotizing enterocolitis? June 2004 2 Stocker M, Berger TM, Neonatal and Pediatric Intensive Care Unit, Children s Hospital of Lucerne,
Vitamin A Facts. for health workers. The USAID Micronutrient Program
 Vitamin A Facts for health workers The USAID Micronutrient Program What is vitamin A? Vitamin A Vitamin A is a nutrient required in small amounts for the body to function properly. It is called a micronutrient
Vitamin A Facts for health workers The USAID Micronutrient Program What is vitamin A? Vitamin A Vitamin A is a nutrient required in small amounts for the body to function properly. It is called a micronutrient
Vancomycin Orion , Version 1.0. Public Summary of the Risk Management Plan
 Vancomycin Orion 18.6.2015, Version 1.0 Public Summary of the Risk Management Plan VI.2 Elements for a Public Summary Vancomycin Orion is intravenously administered glycopeptide antibiotic. It is indicated
Vancomycin Orion 18.6.2015, Version 1.0 Public Summary of the Risk Management Plan VI.2 Elements for a Public Summary Vancomycin Orion is intravenously administered glycopeptide antibiotic. It is indicated
Activity 3-F: Micronutrient Activity Station
 Activity 3-F: Micronutrient Activity Station 1 Vitamin A deficiency 1 Instructions Please read through this Vitamin A information package and discuss amongst your group. You have 15 minutes to review this
Activity 3-F: Micronutrient Activity Station 1 Vitamin A deficiency 1 Instructions Please read through this Vitamin A information package and discuss amongst your group. You have 15 minutes to review this
Contraindications to breast feeding. Dr. Ahmed Isam
 Contraindications to breast feeding Dr. Ahmed Isam When should a mother avoid breastfeeding? Health professionals agree that human milk provides the most complete form of nutrition for infants, including
Contraindications to breast feeding Dr. Ahmed Isam When should a mother avoid breastfeeding? Health professionals agree that human milk provides the most complete form of nutrition for infants, including
Bacteriology. Mycology. Patient: SAMPLE PATIENT DOB: Sex: MRN: Rare. Rare. Positive. Brown. Negative *NG. Negative
 Patient: SAMPLE PATIENT DOB: Sex: MRN: 3.2 0.9-26.8 U/g 1.2 0.2-3.3 mg/g 2.2 1.3-8.6 micromol/g 1.1 1.3-23.7 mg/g 1.1 0.2-3.5 mg/g Rare 1.0 0.2-8.8 mg/g Rare 4.4 2.6-32.4 mg/g 64.6 >= 13.6 micromol/g Bacteriology
Patient: SAMPLE PATIENT DOB: Sex: MRN: 3.2 0.9-26.8 U/g 1.2 0.2-3.3 mg/g 2.2 1.3-8.6 micromol/g 1.1 1.3-23.7 mg/g 1.1 0.2-3.5 mg/g Rare 1.0 0.2-8.8 mg/g Rare 4.4 2.6-32.4 mg/g 64.6 >= 13.6 micromol/g Bacteriology
Optimal Distribution and Utilization of Donated Human Breast Milk: A Novel Approach
 653738JHLXXX10.1177/0890334416653738Journal of Human LactationSimpson et al research-article2016 Original Research: Brief Report Optimal Distribution and Utilization of Donated Human Breast Milk: A Novel
653738JHLXXX10.1177/0890334416653738Journal of Human LactationSimpson et al research-article2016 Original Research: Brief Report Optimal Distribution and Utilization of Donated Human Breast Milk: A Novel
LIFIB. Your Local Infant Feeding Information Board. LIFIB Briefing Paper: Lactose Intolerance in Infants
 LIFIB Your Local Infant Feeding Information Board Briefing Paper 2 January 2015 LIFIB Briefing Paper: in Infants The purpose of this Briefing Paper is to equip Midwives, Health Visitors and partners (including
LIFIB Your Local Infant Feeding Information Board Briefing Paper 2 January 2015 LIFIB Briefing Paper: in Infants The purpose of this Briefing Paper is to equip Midwives, Health Visitors and partners (including
Dr. MUBARAK ABDELRAHMAN MD PEDIATRICS AND CHILD HEALTH Assistant Professor FACULTY OF MEDICINE -JAZAN
 Dr. MUBARAK ABDELRAHMAN MD PEDIATRICS AND CHILD HEALTH Assistant Professor FACULTY OF MEDICINE -JAZAN The student should be able:» To identify the mechanism of homeostasis and the role of vessels, platelets
Dr. MUBARAK ABDELRAHMAN MD PEDIATRICS AND CHILD HEALTH Assistant Professor FACULTY OF MEDICINE -JAZAN The student should be able:» To identify the mechanism of homeostasis and the role of vessels, platelets
Vitamins. Nafith Abu Tarboush, DDS, MSc, PhD
 Vitamins Nafith Abu Tarboush, DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Vitamins Organic compounds required by an organism in tiny amounts as a vital nutrient Cannot be synthesized
Vitamins Nafith Abu Tarboush, DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Vitamins Organic compounds required by an organism in tiny amounts as a vital nutrient Cannot be synthesized
Is It Possible to Prevent Necrotizing Enterocolitis?
 Is It Possible to Prevent Necrotizing Enterocolitis? Ravi Mangal Patel, MD MSc Associate Professor of Pediatrics Emory University School of Medicine, Atlanta, GA, USA @institutopgg @ravimpatelmd Disclosures
Is It Possible to Prevent Necrotizing Enterocolitis? Ravi Mangal Patel, MD MSc Associate Professor of Pediatrics Emory University School of Medicine, Atlanta, GA, USA @institutopgg @ravimpatelmd Disclosures
D. Vitamin K -- Group of naphthoquinones having antihemorrhagic activity.
 D. Vitamin K -- Group of naphthoquinones having antihemorrhagic activity. 1. Structures VITAMIN K1; Phytonadione; 2-Methyl-3-phytyl-1,4-naphthoquinone VITAMIN K2; Menaquinone-6 and 7; N=6,7 n 2. Function
D. Vitamin K -- Group of naphthoquinones having antihemorrhagic activity. 1. Structures VITAMIN K1; Phytonadione; 2-Methyl-3-phytyl-1,4-naphthoquinone VITAMIN K2; Menaquinone-6 and 7; N=6,7 n 2. Function
IHN-CCO DST Final Report and Evaluation
 IHN-CCO DST Final Report and Evaluation Breastfeeding Support Services July 2016 to September 2018 Summary: This pilot reduced the barriers new mothers have in being able to successfully breastfeed their
IHN-CCO DST Final Report and Evaluation Breastfeeding Support Services July 2016 to September 2018 Summary: This pilot reduced the barriers new mothers have in being able to successfully breastfeed their
EDUCATIONAL COMMENTARY ORAL ANTICOAGULANT THERAPEUTIC MONITORING AND POINT-OF-CARE TESTING
 POINT-OF-CARE TESTING Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on
POINT-OF-CARE TESTING Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits click on Continuing Education on
Neonatal Hypoglycemia. Presented By : Kamlah Olaimat 25\7\2010
 Neonatal Hypoglycemia Presented By : Kamlah Olaimat 25\7\2010 Definition The S.T.A.B.L.E. Program defines hypoglycemia as: Glucose delivery or availability is inadequate to meet glucose demand (Karlsen,
Neonatal Hypoglycemia Presented By : Kamlah Olaimat 25\7\2010 Definition The S.T.A.B.L.E. Program defines hypoglycemia as: Glucose delivery or availability is inadequate to meet glucose demand (Karlsen,
Pregnancy and Epilepsy
 Pregnancy and Epilepsy Nowhere is the problem more evident or more complicated than in pregnancy. In the United States, epilepsy affects nearly one million women of childbearing potential. Alarm bells
Pregnancy and Epilepsy Nowhere is the problem more evident or more complicated than in pregnancy. In the United States, epilepsy affects nearly one million women of childbearing potential. Alarm bells
Probiotics. NOW Guide to Probiotics
 Probiotics NOW Guide to Probiotics The Health Benefits of Probiotics Microorganisms for Health Did you know that you have trillions of microorganisms, including bacteria and yeast, living in and on your
Probiotics NOW Guide to Probiotics The Health Benefits of Probiotics Microorganisms for Health Did you know that you have trillions of microorganisms, including bacteria and yeast, living in and on your
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets
 PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
PRODUCT INFORMATION CODAPANE XTRA Paracetamol 500 mg and Codeine Phosphate 15 mg Tablets NAME OF THE MEDICINE Active Ingredients: Paracetamol and Codeine Phosphate Paracetamol: Molecular Formula: C 8 H
The Case Begins. The case continued. Necrotizing Enterocolitis
 Bugs, Drugs and Things that go Bump in the Night From ghoulies to ghosties and long leggety beasties & things that go bump in the night, good lord deliver us Old Cornish Prayer Caring for premature infant
Bugs, Drugs and Things that go Bump in the Night From ghoulies to ghosties and long leggety beasties & things that go bump in the night, good lord deliver us Old Cornish Prayer Caring for premature infant
Nutrition in the premie World
 SURVIVAL AND GROWTH NUTRITION ESSENTIALS Nutrition in the premie World DR VISH SUBRAMANIAN MD MRCP (UK) FAAP NEONATAL CRITICAL CARE MERCY CHILDRENS HOSPITAL., SPRINGFIELD MO Prematurity Nutritional Requirements
SURVIVAL AND GROWTH NUTRITION ESSENTIALS Nutrition in the premie World DR VISH SUBRAMANIAN MD MRCP (UK) FAAP NEONATAL CRITICAL CARE MERCY CHILDRENS HOSPITAL., SPRINGFIELD MO Prematurity Nutritional Requirements
z_4-/01/11 ,c.,u V clf..rm-t PGD for the administration of Phytomenadione (Konakion MM Paediatric 2mg/0.2ml solution for injection) via the oral route
 PGD for the administration of Phytomenadione (Konakion MM Paediatric 2mg/0.2ml solution for injection) via the oral route PGD Ref: p G- D f j Effective From: Feb 2.0 }9 Expiry: Feb 2. 0 2 J by Paediatrics
PGD for the administration of Phytomenadione (Konakion MM Paediatric 2mg/0.2ml solution for injection) via the oral route PGD Ref: p G- D f j Effective From: Feb 2.0 }9 Expiry: Feb 2. 0 2 J by Paediatrics
Product Information: Similac Special Care 24 High Protein
 Product Information: Similac Special Care 24 High Protein 1 of 5 A 24 Cal/fl oz iron-fortified feeding for growing, low-birth-weight infants and premature infants who may need extra protein to help support
Product Information: Similac Special Care 24 High Protein 1 of 5 A 24 Cal/fl oz iron-fortified feeding for growing, low-birth-weight infants and premature infants who may need extra protein to help support
Biliary Atresia. Who is at risk for biliary atresia?
 Biliary Atresia Biliary atresia is a life-threatening condition in infants in which the bile ducts inside or outside the liver do not have normal openings. Bile ducts in the liver, also called hepatic
Biliary Atresia Biliary atresia is a life-threatening condition in infants in which the bile ducts inside or outside the liver do not have normal openings. Bile ducts in the liver, also called hepatic
lactation normally do not cause any adverse long-term consequences to the maternal skeleton.
 MATRIX Matrix Is The First Choice Product Ever Promotes Bone Health Containing A Unique Combination Of 12 Amino Acid Chelated Active Ingredients Formulated In Palatable Toffee Pieces Which Guarantees The
MATRIX Matrix Is The First Choice Product Ever Promotes Bone Health Containing A Unique Combination Of 12 Amino Acid Chelated Active Ingredients Formulated In Palatable Toffee Pieces Which Guarantees The
Classes of Nutrients A Diet
 Ch. 7 Notes Section 1: What is Nutrition? is the science or study of food and the ways the body uses food. are substances in food that provide energy or help form body tissues and are necessary for life
Ch. 7 Notes Section 1: What is Nutrition? is the science or study of food and the ways the body uses food. are substances in food that provide energy or help form body tissues and are necessary for life
Compounds with vitamin K activity all have a common. Vitamin K in Parenteral Nutrition. Metabolic Function
 GASTROENTEROLOGY 2009;137:S105 S118 Vitamin K in Parenteral Nutrition MARTIN J. SHEARER Centre for Haemostasis and Thrombosis, St Thomas Hospital, London, United Kingdom Vitamin K (as phylloquinone and
GASTROENTEROLOGY 2009;137:S105 S118 Vitamin K in Parenteral Nutrition MARTIN J. SHEARER Centre for Haemostasis and Thrombosis, St Thomas Hospital, London, United Kingdom Vitamin K (as phylloquinone and
Drug Shortages with Parenteral Nutrition
 Drug Shortages with Parenteral Nutrition Carol J Rollins, MS, RD, PharmD, BCNSP Coordinator, Nutrition Support Team The University of Arizona Medical Center www.nutritioncare.org Conflict of Interest None
Drug Shortages with Parenteral Nutrition Carol J Rollins, MS, RD, PharmD, BCNSP Coordinator, Nutrition Support Team The University of Arizona Medical Center www.nutritioncare.org Conflict of Interest None
What can we learn from the clinical studies in infants (on thickeners)?
 What can we learn from the clinical studies in infants (on thickeners)? Dominique Turck Member of the FAF WG Re-evaluation of FA in foods for infants below 16 weeks of age FA Stakeholders Workshop 30 November
What can we learn from the clinical studies in infants (on thickeners)? Dominique Turck Member of the FAF WG Re-evaluation of FA in foods for infants below 16 weeks of age FA Stakeholders Workshop 30 November
WHO Growth Grids/ 2012 Risk Changes. Diane Traver Joyce Bryant
 WHO Growth Grids/ 2012 Risk Changes Diane Traver Joyce Bryant Overview CDC vs WHO Growth Charts- Why Change? Transition from
WHO Growth Grids/ 2012 Risk Changes Diane Traver Joyce Bryant Overview CDC vs WHO Growth Charts- Why Change? Transition from
Vancomycin Orion , Version 2 Public Summary of the Risk Management Plan
 Vancomycin Orion 18.1.2016, Version 2 Public Summary of the Risk Management Plan VI.2 Elements for a Public Summary Vancomycin Orion is intravenously administered glycopeptide antibiotic. It is indicated
Vancomycin Orion 18.1.2016, Version 2 Public Summary of the Risk Management Plan VI.2 Elements for a Public Summary Vancomycin Orion is intravenously administered glycopeptide antibiotic. It is indicated
Brief History of Methadone Maintenance Treatment
 METHADONE Brief History of Methadone Maintenance Treatment Methadone maintenance treatment was on the cusp of the social revolution in the sixties. Doctors and public health workers had concluded what
METHADONE Brief History of Methadone Maintenance Treatment Methadone maintenance treatment was on the cusp of the social revolution in the sixties. Doctors and public health workers had concluded what
PART III: PATIENT MEDICATION INFORMATION. CLINDAMYCINE-150 Pr. CLINDAMYCINE-300 (Clindamycin Hydrochloride Capsules USP) Clindamycin 150 mg and 300 mg
 PART III: PATIENT MEDICATION INFORMATION Pr CLINDAMYCINE-150 Pr CLINDAMYCINE-300 (Clindamycin Hydrochloride Capsules USP) Clindamycin 150 mg and 300 mg Read this carefully before you start taking CLINDAMYCINE
PART III: PATIENT MEDICATION INFORMATION Pr CLINDAMYCINE-150 Pr CLINDAMYCINE-300 (Clindamycin Hydrochloride Capsules USP) Clindamycin 150 mg and 300 mg Read this carefully before you start taking CLINDAMYCINE
Neonatal Hypoglycaemia Guidelines
 N.B. Staff should be discouraged from printing this document. This is to avoid the risk of out of date printed versions of the document. The Intranet should be referred to for the current version of the
N.B. Staff should be discouraged from printing this document. This is to avoid the risk of out of date printed versions of the document. The Intranet should be referred to for the current version of the
M3 Pediatric Clerkship
 M3 Pediatric Clerkship The overall goals for the third year Pediatric Clerkship are to educate future physicians to provide competent, effective and compassionate care of patients by developing clinical
M3 Pediatric Clerkship The overall goals for the third year Pediatric Clerkship are to educate future physicians to provide competent, effective and compassionate care of patients by developing clinical
Ca-C 1000 Calvive 1000 mg mg mg effervescent tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER Ca-C 1000 Calvive 1000 mg + 327 mg + 1000 mg effervescent tablets CALCIUM LACTATE GLUCONATE, CALCIUM CARBONATE, ASCORBIC ACID (VITAMIN C) This leaflet is a copy
PACKAGE LEAFLET: INFORMATION FOR THE USER Ca-C 1000 Calvive 1000 mg + 327 mg + 1000 mg effervescent tablets CALCIUM LACTATE GLUCONATE, CALCIUM CARBONATE, ASCORBIC ACID (VITAMIN C) This leaflet is a copy
PRODUCT INFORMATION. DESCRIPTION Addaven is a concentrated trace element solution for infusion which is clear and colourless to slightly yellow.
 PRODUCT INFORMATION Addaven NAME OF MEDICINE Addaven is a concentrated trace element solution containing the following simple salts: chromic chloride hexahydrate (CAS No.:10060-12-5), cupric chloride dihydrate
PRODUCT INFORMATION Addaven NAME OF MEDICINE Addaven is a concentrated trace element solution containing the following simple salts: chromic chloride hexahydrate (CAS No.:10060-12-5), cupric chloride dihydrate
OPO fats By Dr. Annie Salsberg, N.D.
 1 of 5 An inside look at and how y increase absorption of calcium and essential fatty acids while improving digestive health. Close up portrait of eight mounth old baby What are? According to FDA regulations,
1 of 5 An inside look at and how y increase absorption of calcium and essential fatty acids while improving digestive health. Close up portrait of eight mounth old baby What are? According to FDA regulations,
The Importance of Glutamine and Antioxidant Vitamin Supplementation in HIV
 The Importance of Glutamine and Antioxidant Vitamin Supplementation in HIV An Introduction to Glutamine Glutamine is the most abundant amino acid in the human body, and plays extremely important role in
The Importance of Glutamine and Antioxidant Vitamin Supplementation in HIV An Introduction to Glutamine Glutamine is the most abundant amino acid in the human body, and plays extremely important role in
From: Evidence Based Birth-Used with permission. Is Erythromycin Eye Ointment Always Necessary for Newborns?
 From: Evidence Based Birth-Used with permission. Is Erythromycin Eye Ointment Always Necessary for Newborns? November 11, 2012 by Rebecca Dekker, PhD, RN, APRN Copyright Evidence Based Birth. Please see
From: Evidence Based Birth-Used with permission. Is Erythromycin Eye Ointment Always Necessary for Newborns? November 11, 2012 by Rebecca Dekker, PhD, RN, APRN Copyright Evidence Based Birth. Please see
Evidence Based Interventions for Improving Maternal and Child Nutrition: What Can be Done and at What Cost? Lancet, vol 382, , 2013
 Evidence Based Interventions for Improving Maternal and Child Nutrition: What Can be Done and at What Cost? Lancet, vol 382, 452 77, 2013 Dr. S.K Roy Senior Scientist Chairperson, Bangladesh Breastfeeding
Evidence Based Interventions for Improving Maternal and Child Nutrition: What Can be Done and at What Cost? Lancet, vol 382, 452 77, 2013 Dr. S.K Roy Senior Scientist Chairperson, Bangladesh Breastfeeding
 Food, Supplements and Healthy Lifestyles in Slowing and Preventing Macular Degeneration Julie Mares, Professor For the Nutrition and Eye Health Research Team http://nutritionforeyes.ophth.wisc.edu Preserving
Food, Supplements and Healthy Lifestyles in Slowing and Preventing Macular Degeneration Julie Mares, Professor For the Nutrition and Eye Health Research Team http://nutritionforeyes.ophth.wisc.edu Preserving
Background OVER 30 ISSUES IDENTIFIED! Key opportunities. What we ve done. October 31, 2012
 Background Hyperbilirubinemia: Developed by CMNRP s Jaundice Working Group Strategic planning meeting of CMNRP and its committees Multiple tables identified jaundice as a problem/priority Opportunity to
Background Hyperbilirubinemia: Developed by CMNRP s Jaundice Working Group Strategic planning meeting of CMNRP and its committees Multiple tables identified jaundice as a problem/priority Opportunity to
Understanding the potential of cognitive ingredients. Dr Carrie Ruxton Freelance Dietitian
 Understanding the potential of cognitive ingredients Dr Carrie Ruxton Freelance Dietitian Cognitive health important across the lifecycle Higher IQ Diet & Supplements Brain development Slower cognitive
Understanding the potential of cognitive ingredients Dr Carrie Ruxton Freelance Dietitian Cognitive health important across the lifecycle Higher IQ Diet & Supplements Brain development Slower cognitive
NOWS The Time Caring for the Infant with Neonatal Opiate Withdrawal Syndrome
 NOWS The Time Caring for the Infant with Neonatal Opiate Withdrawal Syndrome Meghan Howell, MD FAAP Assistant Professor of Pediatrics Clinical Director, Tulane NICU Graduate Clinic Tulane University School
NOWS The Time Caring for the Infant with Neonatal Opiate Withdrawal Syndrome Meghan Howell, MD FAAP Assistant Professor of Pediatrics Clinical Director, Tulane NICU Graduate Clinic Tulane University School
NEONATOLOGY Healthy newborn. Neonatal sequelaes
 NEONATOLOGY Healthy newborn. Neonatal sequelaes Ágnes Harmath M.D. Ph.D. senior lecturer 11. November 2016. Tasks of the neonatologist Prenatal diagnosed condition Inform parents, preparation of necessary
NEONATOLOGY Healthy newborn. Neonatal sequelaes Ágnes Harmath M.D. Ph.D. senior lecturer 11. November 2016. Tasks of the neonatologist Prenatal diagnosed condition Inform parents, preparation of necessary
Listing of Color Additives Exempt from Certification; Synthetic Iron Oxide
 This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
Introduction to Pharmacokinetics
 - 1 - Introduction to Pharmacokinetics Outline accompanies required webcast for Marie Biancuzzo s Lactation Exam Review and Marie Biancuzzo s Comprehensive Lactation Course Notes We will not cover this
- 1 - Introduction to Pharmacokinetics Outline accompanies required webcast for Marie Biancuzzo s Lactation Exam Review and Marie Biancuzzo s Comprehensive Lactation Course Notes We will not cover this
Prolonged Neonatal Jaundice
 Prolonged Neonatal Jaundice Ahmed Laving KPA Annual Scientific Conference 2018 Prolonged Jaundice? >6 months >3 months >2 weeks >4 weeks Prolonged Jaundice? >6 months >3 months >2 weeks >4 weeks Case Presentation
Prolonged Neonatal Jaundice Ahmed Laving KPA Annual Scientific Conference 2018 Prolonged Jaundice? >6 months >3 months >2 weeks >4 weeks Prolonged Jaundice? >6 months >3 months >2 weeks >4 weeks Case Presentation
Pharmacotherapy Issues in the Pediatric Population
 Pharmacotherapy Issues in the Pediatric Population Continuing Professional Pharmacy Development Dr. Shane Pawluk, PharmD Dr. Andrea Cartwright, PharmD Dr. Maryam Khaja March 26, 2014 Outline Didactic Session
Pharmacotherapy Issues in the Pediatric Population Continuing Professional Pharmacy Development Dr. Shane Pawluk, PharmD Dr. Andrea Cartwright, PharmD Dr. Maryam Khaja March 26, 2014 Outline Didactic Session
Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis
 Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis 1 Journal of Affective Disorders: Volume 69, Issues 1-3, May 2002,
Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis 1 Journal of Affective Disorders: Volume 69, Issues 1-3, May 2002,
Activity 3-F: Micronutrient Activity Station
 Activity 3-F: Micronutrient Activity Station 1 Vitamin A deficiency 1 Instructions Please read through this Vitamin A information package and discuss amongst your group. You have 15 minutes to review this
Activity 3-F: Micronutrient Activity Station 1 Vitamin A deficiency 1 Instructions Please read through this Vitamin A information package and discuss amongst your group. You have 15 minutes to review this
Parenteral Micronutrient Recommendations and Laboratory Monitoring for Infants on Long Term Parenteral Nutrition (PN)
 Parenteral Micronutrient Recommendations and Laboratory Monitoring for nfants on Long Term Parenteral Nutrition (PN) Dosage Micronutrient Preterm Term Trace Elements (mcg/kg/day) (mcg/kg/day) 1 Chromium
Parenteral Micronutrient Recommendations and Laboratory Monitoring for nfants on Long Term Parenteral Nutrition (PN) Dosage Micronutrient Preterm Term Trace Elements (mcg/kg/day) (mcg/kg/day) 1 Chromium
L ate onset vitamin K deficiency bleeding (VKDB) is a
 F546 ORIGINAL ARTICLE Intracranial haemorrhage due to late onset vitamin K deficiency bleeding in Hanoi province, Vietnam N Danielsson, D P Hoa, N V Thang, T Vos, P M Loughnan... See end of article for
F546 ORIGINAL ARTICLE Intracranial haemorrhage due to late onset vitamin K deficiency bleeding in Hanoi province, Vietnam N Danielsson, D P Hoa, N V Thang, T Vos, P M Loughnan... See end of article for
HarvestPlus Statement on the Potential Benefits of Biofortification on the Nutritional Status of Populations
 HarvestPlus Statement on the Potential Benefits of Biofortification on the Nutritional Status of Populations Biofortification is an intervention strategy currently being researched and developed for increasing
HarvestPlus Statement on the Potential Benefits of Biofortification on the Nutritional Status of Populations Biofortification is an intervention strategy currently being researched and developed for increasing
Comparison of cow-milk, breast milk and formula: nutritional, immunologic and developmental considerations
 Comparison of cow-milk, breast milk and formula: nutritional, immunologic and developmental considerations Eugene Dinkevich, MD Downstate Healthy Lifestyles Program Department of Pediatrics SUNY-Downstate
Comparison of cow-milk, breast milk and formula: nutritional, immunologic and developmental considerations Eugene Dinkevich, MD Downstate Healthy Lifestyles Program Department of Pediatrics SUNY-Downstate
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES
 PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
AXITAB-CV TAB. COMPOSITION :
 AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
AXITAB-CV TAB. COMPOSITION : Each film coated tablet contains: Cefuroxime Axetil I.P. Eq. to Anhydrous 500mg. Potassium Clavulanate Diluted I.P. Eq. to Clavulanic Acid 125mg DESCRIPTION : Cefuroxime Axetil
Vitamin K Snapshot Monograph
 vitamins minerals nutrients Vitamin K Snapshot Monograph Vitamin K1 and Vitamin K2 Most Frequent Reported Uses: Blood clotting Anticoagulant reversal; bleeding disorders Osteoporosis prevention Arthritis,
vitamins minerals nutrients Vitamin K Snapshot Monograph Vitamin K1 and Vitamin K2 Most Frequent Reported Uses: Blood clotting Anticoagulant reversal; bleeding disorders Osteoporosis prevention Arthritis,
Evolution and Medicine. Dr. Peter J. Lin Director Primary Care Initiatives Canadian Heart Research Centre
 Evolution and Medicine Dr. Peter J. Lin Director Primary Care Initiatives Canadian Heart Research Centre Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced,
Evolution and Medicine Dr. Peter J. Lin Director Primary Care Initiatives Canadian Heart Research Centre Copyright 2017 by Sea Courses Inc. All rights reserved. No part of this document may be reproduced,
Epatite B: fertilità, gravidanza ed allattamento, aspetti clinici e terapeutici. Ivana Maida
 Epatite B: fertilità, gravidanza ed allattamento, aspetti clinici e terapeutici Ivana Maida Positivity for HBsAg was found in 0.5% of tested women In the 70s and 80s, Italy was one of the European countries
Epatite B: fertilità, gravidanza ed allattamento, aspetti clinici e terapeutici Ivana Maida Positivity for HBsAg was found in 0.5% of tested women In the 70s and 80s, Italy was one of the European countries
Focus Areas for Entry Test (Technical Part) for M. Phil / PhD in Food & Nutrition
 Focus Areas for Entry Test (Technical Part) for M. Phil / PhD in Food & Nutrition Sr. No. Core Areas Percentage 1. 2. Community Nutrition 15% Dietetics and Preventive Nutrition 15% 3. 4. 5. 6. 7. Nutritional
Focus Areas for Entry Test (Technical Part) for M. Phil / PhD in Food & Nutrition Sr. No. Core Areas Percentage 1. 2. Community Nutrition 15% Dietetics and Preventive Nutrition 15% 3. 4. 5. 6. 7. Nutritional
Topic owner: Mollie Grow MD MPH, updated June 2018
 Iron deficiency Anemia UW Pediatrics Outpatient Clinical Guidelines Sources: AAP Clinical Report Diagnosis and Prevention of Iron Deficiency and Iron- Deficiency Anemia in Infants and Young Children (0
Iron deficiency Anemia UW Pediatrics Outpatient Clinical Guidelines Sources: AAP Clinical Report Diagnosis and Prevention of Iron Deficiency and Iron- Deficiency Anemia in Infants and Young Children (0
Dietary Supplements: A Necessity or Folly?
 Dietary Supplements: A Necessity or Folly? Presenter: Dr. Robert Van Saun Professor of Veterinary Science Penn State University September 22, 2015 Host/Moderator: Jay Parsons This webinar is made possible
Dietary Supplements: A Necessity or Folly? Presenter: Dr. Robert Van Saun Professor of Veterinary Science Penn State University September 22, 2015 Host/Moderator: Jay Parsons This webinar is made possible
ANTIRETROVIRAL (ART) DRUG INFORMATION FOR HEALTH CARE PROFESSIONAL
 ZIDOVUDINE (AZT, Retrovir ) Benefits of zidovudine: Zidovudine (ZDV) is an antiretroviral drug that slows the growth of the HIV virus. It has shown in a large randomized, multicentre study to decrease
ZIDOVUDINE (AZT, Retrovir ) Benefits of zidovudine: Zidovudine (ZDV) is an antiretroviral drug that slows the growth of the HIV virus. It has shown in a large randomized, multicentre study to decrease
