Methylnaltrexone Bromide (Relistor)
|
|
|
- Samson Todd Patrick
- 5 years ago
- Views:
Transcription
1 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical edit Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical edit criteria rules Logic diagram: a visual depiction of the clinical edit criteria logic Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes) References: clinical publications and sources relevant to this clinical edit te: Click the hyperlink to navigate directly to that section. Revision tes Initial publication and posting to website August 23, 2013 Copyright 2013 Health Information Designs, LLC 1
2 Drugs Requiring Prior Authorization Drugs Requiring Prior Authorization Label Name GCN RELISTOR 8MG/0.4ML SYRINGE RELISTOR 12MG/0.6ML KIT RELISTOR 12MG/0.6ML SYRINGE RELISTOR 12MG/0.6ML VIAL August 23, 2013 Copyright 2013 Health Information Designs, LLC 2
3 Clinical Edit Criteria Logic 1. Does the client have a diagnosis of opioid induced constipation in the last 730 days? [ ] (Go to #2) [ ] (Deny) 2. Does the client have a history of palliative care in the last 365 days? [ ] (Go to #3) [ ] (Deny) 3. Is the client greater than or equal to ( ) 18 years of age? [ ] (Go to #4) [ ] (Deny) 4. Does the client have a diagnosis of mechanical gastrointestinal obstruction in the last 730 days? [ ] (Deny) [ ] (Go to #5) 5. Does the client have a claim for a laxative in the last 90 days? [ ] (Approve 365 days) [ ] (Deny) August 23, 2013 Copyright 2013 Health Information Designs, LLC 3
4 Clinical Edit Criteria Logic Diagram Step 1 Step 2 Step 3 Does the client have a diagnosis of opioid induced constipation in the past 730 days? Does the client have a history of palliative care in the past 365 days? Is the client 18 years of age? Step 4 Does the client have a diagnosis of mechanical gastrointestinal obstruction in the last 730 days? Step 5 Does the client have a claim for a laxative in the last 90 days? Approve Request (365 days) August 23, 2013 Copyright 2013 Health Information Designs, LLC 4
5 Supporting Tables Step 1 (diagnosis of opioid induced constipation) Required diagnosis: 1 Look back timeframe: 730 days ICD-9 Code Description CONSTIPATION, UNSPECIFIED OTHER CONSTIPATION Step 2 (history of palliative care) Required diagnosis: 1 Look back timeframe: 365 days ICD-9 Code V667 Description ENCOUNTER FOR PALLIATIVE CARE Step 4 (diagnosis of mechanical gastrointestinal obstruction) Required diagnosis: 1 Look back timeframe: 730 days ICD-9 Code Description 552 OTHER HERNIA OF ABDOMINAL CAVITY WITH OBSTRUCTION BUT WITHOUT MENTION OF GANGRENE 5520 FEMORAL HERNIA WITH OBSTRUCTION FEMORAL HERNIA WITH OBSTRUCTION, UNILATERAL OR UNSPECIFIED (NOT SPECIFIED AS RECURRENT) FEMORAL HERNIA WITH OBSTRUCTION, UNILATERAL OR UNSPECIFIED, RECURRENT FEMORAL HERNIA WITH OBSTRUCTION, BILATERAL (NOT SPECIFIED AS RECURRENT) FEMORAL HERNIA WITH OBSTRUCTION, BILATERAL, RECURRENT 5521 UMBILICAL HERNIA WITH OBSTRUCTION 5522 VENTRAL HERNIA WITH OBSTRUCTION VENTRAL, UNSPECIFIED, HERNIA WITH OBSTRUCTION INCISIONAL VENTRAL HERNIA WITH OBSTRUCTION OTHER VENTRAL HERNIA WITH OBSTRUCTION 5523 DIAPHRAGMATIC HERNIA WITH OBSTRUCTION 5528 HERNIA OF OTHER SPECIFIED SITES, WITH OBSTRUCTION August 23, 2013 Copyright 2013 Health Information Designs, LLC 5
6 ICD-9 Code Step 4 (diagnosis of mechanical gastrointestinal obstruction) Description Required diagnosis: 1 Look back timeframe: 730 days 5529 HERNIA OF UNSPECIFIED SITE, WITH OBSTRUCTION 560 INTESTINAL OBSTRUCTION WITHOUT MENTION OF HERNIA 5600 INTUSSUSCEPTION 5601 PARALYTIC ILEUS 5602 VOLVULUS 5603 IMPACTION OF INTESTINE IMPACTION OF INTESTINE, UNSPECIFIED GALLSTONE ILEUS FECAL IMPACTION OTHER IMPACTION OF INTESTINE 5608 OTHER SPECIFIED INTESTINAL OBSTRUCTION INTESTINAL OR PERITONEAL ADHESIONS WITH OBSTRUCTION (POSTOPERATIVE) (POSTINFECTION) OTHER SPECIFIED INTESTINAL OBSTRUCTION 5609 UNSPECIFIED INTESTINAL OBSTRUCTION Step 5 (history of laxative) Number of claims: 1 Look back timeframe: 90 days Label Name GCN AMITIZA 8MCG CAPSULE AMITIZA 24MCG CAPSULE CONSTULOSE 10G/15ML SOLUTION ENULOSE 10 GM/15 ML SOLUTION GENERLAC 10 GM/15 ML SOLUTION KRISTALOSE 10GM PACKET KRISTALOSE 20GM PACKET LACTULOSE 10G/15ML SOLUTION August 23, 2013 Copyright 2013 Health Information Designs, LLC 6
7 Clinical Edit Criteria References 1. Relistor Package Insert, Salix Pharmaceuticals, Inc. Raleigh, NC. Available at Accessed on March 6, Clinical Pharmacology [database online]. Tampa, FL: Gold Standard, Inc.; August Available at Accessed on March 6, ICD-9-CM Diagnosis Codes, Volume 1, Available at Accessed on March 6, August 23, 2013 Copyright 2013 Health Information Designs, LLC 7
8 Publication History The Publication History records the publication iterations and revisions to this document. tes for the most current revision are also provided in the Revision tes on the first page of this document. Publication Date tes 08/23/2013 Initial publication and posting to website August 23, 2013 Copyright 2013 Health Information Designs, LLC 8
Amitiza (Lubiprostone)
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Information Included in this Document Xifaxan 200mg Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Information Included in this Document Xifaxan 200mg Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Xifaxan 200mg Drugs requiring prior authorization: the list of drugs requiring
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Xifaxan 200mg Drugs requiring prior authorization: the list of drugs requiring
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Sitagliptin (Januvia)
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document 25mg Drugs requiring prior authorization: the list of drugs requiring prior
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document 25mg Drugs requiring prior authorization: the list of drugs requiring prior
Diclofenac 3% Gel, Diclofenac 1.5% and 2% Topical Solution
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class, Diclofenac 1.5% and 2% Topical Solution This criteria was recommended for review by an MCO to ensure appropriate and safe utilization
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class, Diclofenac 1.5% and 2% Topical Solution This criteria was recommended for review by an MCO to ensure appropriate and safe utilization
Hypoglycemics, Lantus Insulin
 Texas Prior Authorization Program PDL Edit Criteria Drug/Drug Class Hypoglycemics, Lantus Insulin Information Included in this Document Hypoglycemics, Lantus Insulin Drugs requiring prior authorization:
Texas Prior Authorization Program PDL Edit Criteria Drug/Drug Class Hypoglycemics, Lantus Insulin Information Included in this Document Hypoglycemics, Lantus Insulin Drugs requiring prior authorization:
Victoza (Liraglutide) Solution for Injection
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Lidoderm (Lidocaine) Patch
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Injectable Agents for the Treatment of Pulmonary Arterial Hypertension (PAH)
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Injectable Agents for the Treatment of Pulmonary Arterial Hypertension (PAH) Clinical Edit Information Included in this Document
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Injectable Agents for the Treatment of Pulmonary Arterial Hypertension (PAH) Clinical Edit Information Included in this Document
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Aldara 5% Cream Drugs requiring prior authorization: the list of drugs requiring
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Aldara 5% Cream Drugs requiring prior authorization: the list of drugs requiring
Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document DPP-4 Inhibitor Criteria A: Alogliptin 6.25mg, Januvia 25mg, Nesina 6.25mg, Onglyza
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document DPP-4 Inhibitor Criteria A: Alogliptin 6.25mg, Januvia 25mg, Nesina 6.25mg, Onglyza
Texas Prior Authorization Program Clinical Criteria
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Aldurazyme Adagen Carbaglu Ceprotin Elaprase Fabrazyme Lumizyme Naglazyme Orfandin Ravicti Vimizim Note: Click the hyperlink to navigate
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Aldurazyme Adagen Carbaglu Ceprotin Elaprase Fabrazyme Lumizyme Naglazyme Orfandin Ravicti Vimizim Note: Click the hyperlink to navigate
Agents for the Treatment of Hepatitis C
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria. This criteria was recommended for review by an MCO to ensure appropriate and safe utilization.
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class This criteria was recommended for review by an MCO to ensure appropriate and safe utilization. Clinical Information Included in this
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class This criteria was recommended for review by an MCO to ensure appropriate and safe utilization. Clinical Information Included in this
Agents for Cystic Fibrosis
 Texas Prior Authorization Program Clinical Edit Criteria Clinical Edit Information Included in this Document Kalydeco (Ivacaftor) Drugs requiring prior authorization: the list of drugs requiring prior
Texas Prior Authorization Program Clinical Edit Criteria Clinical Edit Information Included in this Document Kalydeco (Ivacaftor) Drugs requiring prior authorization: the list of drugs requiring prior
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Provigil (Modafinil) Drugs requiring prior authorization: the list of drugs
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Provigil (Modafinil) Drugs requiring prior authorization: the list of drugs
Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Combination Agents
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Combination Agents This criteria was recommended for review by an MCO to ensure appropriate
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Combination Agents This criteria was recommended for review by an MCO to ensure appropriate
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Prior Authorization Neurontin (gabapentin) 2016
 Drugs Requiring Prior Authorization Label Name GCN GABAPENTIN 100 MG CAPSULE 00780 GABAPENTIN 300 MG CAPSULE 00781 GABAPENTIN 400 MG CAPSULE 00782 GABAPENTIN 250 MG/5 ML SOLN 13235 GABAPENTIN 600 MG TABLET
Drugs Requiring Prior Authorization Label Name GCN GABAPENTIN 100 MG CAPSULE 00780 GABAPENTIN 300 MG CAPSULE 00781 GABAPENTIN 400 MG CAPSULE 00782 GABAPENTIN 250 MG/5 ML SOLN 13235 GABAPENTIN 600 MG TABLET
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document - Oral Drugs requiring prior authorization: the list of drugs requiring prior
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document - Oral Drugs requiring prior authorization: the list of drugs requiring prior
Flexeril/Amrix (Cyclobenzaprine)
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria. Allergen Extracts
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Information Included in this Document Oralair (Mixed Grass Pollens Allergen Extract)) Drugs requiring prior authorization: the
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Information Included in this Document Oralair (Mixed Grass Pollens Allergen Extract)) Drugs requiring prior authorization: the
Byetta (Exenatide Injection)
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria. H.P. Acthar
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Movantik (naloxegol), Relistor (methylnaltrexone bromide)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: December 2, 2016 Opioid Antagonist
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: December 2, 2016 Opioid Antagonist
Movantik (naloxegol), Relistor (methylnaltrexone bromide), Symproic (naldemedine)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 7 Last Review Date: November 30, 2018 Opioid Antagonist
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 7 Last Review Date: November 30, 2018 Opioid Antagonist
Amitiza. Amitiza (lubiprostone) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.22 Subject: Amitiza Page: 1 of 5 Last Review Date: March 16, 2018 Amitiza Description Amitiza (lubiprostone)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.22 Subject: Amitiza Page: 1 of 5 Last Review Date: March 16, 2018 Amitiza Description Amitiza (lubiprostone)
Movantik (naloxegol), Relistor (methylnaltrexone bromide), Symproic (naldemedine)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: June 22, 2017 Opioid Antagonist
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.06 Subject: Opioid Antagonist Drug Class Page: 1 of 5 Last Review Date: June 22, 2017 Opioid Antagonist
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Thiazolidinediones Clinical Edit Information Included in this Document Thiazolidinediones Drugs requiring prior authorization: the
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Thiazolidinediones Clinical Edit Information Included in this Document Thiazolidinediones Drugs requiring prior authorization: the
Fentanyl Agents Clinical Edit Criteria
 Fentanyl Agents Clinical Edit Criteria Drug/Drug Class: Fentanyl Agents Superior HealthPlan follows the guidance of the Texas Vendor Drug Program (VDP) for all clinical edit criteria. Superior has adjusted
Fentanyl Agents Clinical Edit Criteria Drug/Drug Class: Fentanyl Agents Superior HealthPlan follows the guidance of the Texas Vendor Drug Program (VDP) for all clinical edit criteria. Superior has adjusted
Specialised Services Policy: CP24 Home Administered Parenteral Nutrition (HPN)
 Specialised Services Policy: CP24 Home Administered Parenteral Nutrition (HPN) Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of
Specialised Services Policy: CP24 Home Administered Parenteral Nutrition (HPN) Document Author: Specialist Services Planning Manager for Neurosciences and Complex Conditions Executive Lead: Director of
Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria
 Drug/Drug Class Antipsychotics Clinical Criteria Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria Prior
Drug/Drug Class Antipsychotics Clinical Criteria Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria Prior
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Relistor) Reference Number: CP.CPA.274 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Relistor) Reference Number: CP.CPA.274 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for
daily; available as 10- mg g PO
 Overview of the PRN: The Pain and Palliative Care PRN of ACCP is an organization of pharmacy practitioners, clinical scientists, pharmacy educators, and others. Its mission is to advance pain and palliative
Overview of the PRN: The Pain and Palliative Care PRN of ACCP is an organization of pharmacy practitioners, clinical scientists, pharmacy educators, and others. Its mission is to advance pain and palliative
ICD-10 Physician Education. General Surgery
 ICD-10 Physician Education General Surgery 1 Training Objectives ICD-9 to ICD-10 Comparison Documentation Tips Additional Educational Opportunities Questions 2 ICD-9 to ICD-10 Comparison Code Structure
ICD-10 Physician Education General Surgery 1 Training Objectives ICD-9 to ICD-10 Comparison Documentation Tips Additional Educational Opportunities Questions 2 ICD-9 to ICD-10 Comparison Code Structure
2015 General Surgery Survival Guide
 2015 General Surgery Survival Guide Chapter 10: Hernia Repair Know What to Look for When Coding Hernia Repair Reporting hernia repair can be tricky. But if you know what to look for then half the work
2015 General Surgery Survival Guide Chapter 10: Hernia Repair Know What to Look for When Coding Hernia Repair Reporting hernia repair can be tricky. But if you know what to look for then half the work
Flexeril/Amrix (Cyclobenzaprine) Clinical Edit Criteria
 Flexeril/Amrix (Cyclobenzaprine) Clinical Edit Criteria Drug/Drug Class: Flexeril/Amrix (Cyclobenzaprine) Superior HealthPlan follows the guidance of the Texas Vendor Drug Program (VDP) for all clinical
Flexeril/Amrix (Cyclobenzaprine) Clinical Edit Criteria Drug/Drug Class: Flexeril/Amrix (Cyclobenzaprine) Superior HealthPlan follows the guidance of the Texas Vendor Drug Program (VDP) for all clinical
Cystic Fibrosis Agents
 Texas Prior Authorization Program Clinical Criteria Clinical Information Included in this Document Kalydeco (Ivacaftor) Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria Clinical Information Included in this Document Kalydeco (Ivacaftor) Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Cystic Fibrosis Agents
 Texas Prior Authorization Program Clinical Criteria Clinical Information Included in this Document Kalydeco (Ivacaftor) Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria Clinical Information Included in this Document Kalydeco (Ivacaftor) Drugs requiring prior authorization: the list of drugs requiring prior authorization
Hernias Umbilical Hernia When to See a Surgeon? What Are Symptoms of an Umbilical Hernia? How is Repair Performed?
 Hernias Umbilical Hernia An umbilical hernia occurs when part of the intestine protrudes through the umbilical opening in the abdominal muscles. Umbilical hernias are common and typically harmless. They
Hernias Umbilical Hernia An umbilical hernia occurs when part of the intestine protrudes through the umbilical opening in the abdominal muscles. Umbilical hernias are common and typically harmless. They
Oral Methylnaltrexone for the. Constipation in Patients with Chronic Non-cancer Pain
 Oral Methylnaltrexone for the Treatment of Opioid-induced Constipation in Patients with Chronic Non-cancer Pain Richard L. Rauck, 1 John F. Peppin, 2 Robert J.Israel, 3 Jennifer Carpenito, 3 Jeffrey Cohn,
Oral Methylnaltrexone for the Treatment of Opioid-induced Constipation in Patients with Chronic Non-cancer Pain Richard L. Rauck, 1 John F. Peppin, 2 Robert J.Israel, 3 Jennifer Carpenito, 3 Jeffrey Cohn,
Drug Regimen Optimization
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Excluding Valsartan / Ramipril Prior authorization criteria logic: a description
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Excluding Valsartan / Ramipril Prior authorization criteria logic: a description
Prior Authorization Flexeril/Amrix (cyclobenzaprine) 2017
 Drugs Requiring Prior Authorization Label Name GCN AMRIX ER 15 MG CAPSULE 97959 AMRIX ER 30 MG CAPSULE 97960 CYCLOBENZAPRINE 10 MG TABLET 18020 CYCLOBENZAPRINE 5 MG TABLET 12805 CYCLOBENZAPRINE 7.5 MG
Drugs Requiring Prior Authorization Label Name GCN AMRIX ER 15 MG CAPSULE 97959 AMRIX ER 30 MG CAPSULE 97960 CYCLOBENZAPRINE 10 MG TABLET 18020 CYCLOBENZAPRINE 5 MG TABLET 12805 CYCLOBENZAPRINE 7.5 MG
3.4 gram/7 gram powder -- $5.63
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Bowel Movements P&T DATE: 9/11/2018 CLASS: Gastrointestinal Disorders REVIEW HISTORY: 12/16, 9/15, 9/12, 5/08 LOB: MCL (MONTH/YEAR)
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Bowel Movements P&T DATE: 9/11/2018 CLASS: Gastrointestinal Disorders REVIEW HISTORY: 12/16, 9/15, 9/12, 5/08 LOB: MCL (MONTH/YEAR)
TRANSPARENCY COMMITTEE OPINION. 10 December 2008
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 RELISTOR 12 mg/0.6 ml solution for injection 1 vial (CIP: 387 365-1) 2 vials + 2 sterile syringes
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 10 December 2008 RELISTOR 12 mg/0.6 ml solution for injection 1 vial (CIP: 387 365-1) 2 vials + 2 sterile syringes
Icd 10 right inguinal pain
 Icd 10 right inguinal pain Search Atherosclerosis of nonbiological bypass graft(s) of the extremities with rest pain, right leg. 2016 2017 2018 Billable/Specific Code Adult Dx (15-124 years) R10.11 is
Icd 10 right inguinal pain Search Atherosclerosis of nonbiological bypass graft(s) of the extremities with rest pain, right leg. 2016 2017 2018 Billable/Specific Code Adult Dx (15-124 years) R10.11 is
University of Bristol - Explore Bristol Research
 Hunt, L., Ben-Shlomo, Y., Whitehouse, M., Porter, M., & Blom, A. (2017). The Main Cause of Death Following Primary Total Hip and Knee Replacement for Osteoarthritis: A Cohort Study of 26,766 Deaths Following
Hunt, L., Ben-Shlomo, Y., Whitehouse, M., Porter, M., & Blom, A. (2017). The Main Cause of Death Following Primary Total Hip and Knee Replacement for Osteoarthritis: A Cohort Study of 26,766 Deaths Following
Surgical Workload, Outcome and Research Database: V1.1
 Technical Guidance for Surgical Workload, Outcome and Research Database: V1.1 Contents 1. Standard Indicators... 5 1.1. Activity Volume... 5 1.2. Average Length of Stay (Days)... 5 1.3. 2/7/30 day Re-admission
Technical Guidance for Surgical Workload, Outcome and Research Database: V1.1 Contents 1. Standard Indicators... 5 1.1. Activity Volume... 5 1.2. Average Length of Stay (Days)... 5 1.3. 2/7/30 day Re-admission
HCPCS Codes (Alphanumeric, CPT AMA) ICD-9-CM Codes Covered by Medicare Program
 HCPCS s (Alphanumeric, CPT AMA) 82272 Blood, occult, by peroxidase activity (e.g., guaiac), qualitative, feces, 1-3 simultaneous determinations, performed for other than colorectal neoplasm screening ICD-9-CM
HCPCS s (Alphanumeric, CPT AMA) 82272 Blood, occult, by peroxidase activity (e.g., guaiac), qualitative, feces, 1-3 simultaneous determinations, performed for other than colorectal neoplasm screening ICD-9-CM
Xyrem (Sodium Oxybate)
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Drugs requiring prior authorization: the list of drugs requiring prior authorization
Glucagon-Like Peptide (GLP-1) Receptor Agonists Clinical Edit Criteria
 Glucagon-Like Peptide (GLP-1) Receptor Agonists Clinical Edit Criteria Drug/Drug Class: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Superior HealthPlan follows the guidance of the Texas Vendor Drug
Glucagon-Like Peptide (GLP-1) Receptor Agonists Clinical Edit Criteria Drug/Drug Class: Glucagon-Like Peptide-1 (GLP-1) Receptor Agonists Superior HealthPlan follows the guidance of the Texas Vendor Drug
Texas Prior Authorization Program Clinical Criteria
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Ketorolac Clinical Criteria Information Included in this Document Ketorolac Oral Drugs requiring prior authorization: the list of drugs
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Ketorolac Clinical Criteria Information Included in this Document Ketorolac Oral Drugs requiring prior authorization: the list of drugs
UNDERSTANDING X-RAYS: ABDOMINAL IMAGING THE ABDOMEN
 UNDERSTANDING X-RAYS: ABDOMINAL IMAGING THE ABDOMEN Radiology Enterprises radiologyenterprises@gmail.com www.radiologyenterprises.com STOMACH AND SMALL BOWEL STOMACH AND SMALL BOWEL Swallowed air is a
UNDERSTANDING X-RAYS: ABDOMINAL IMAGING THE ABDOMEN Radiology Enterprises radiologyenterprises@gmail.com www.radiologyenterprises.com STOMACH AND SMALL BOWEL STOMACH AND SMALL BOWEL Swallowed air is a
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Oral Drugs requiring prior authorization: the list of drugs requiring prior
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class Clinical Edit Information Included in this Document Oral Drugs requiring prior authorization: the list of drugs requiring prior
CENTENE PHARMACY AND THERAPEUTICS DRUG REVIEW 3Q17 July August
 BRAND NAME Symproic GENERIC NAME Naldemedine MANUFACTURER Shionogi Inc. DATE OF APPROVAL March 23, 2017 PRODUCT LAUNCH DATE Anticipated to launch mid-summer 2017 REVIEW TYPE Review type 1 (RT1): New Drug
BRAND NAME Symproic GENERIC NAME Naldemedine MANUFACTURER Shionogi Inc. DATE OF APPROVAL March 23, 2017 PRODUCT LAUNCH DATE Anticipated to launch mid-summer 2017 REVIEW TYPE Review type 1 (RT1): New Drug
Nordic Forum - Trauma & Emergency Radiology. Bowel Obstruction: Imaging Update
 Nordic Forum - Trauma & Emergency Radiology Bowel Obstruction: Imaging Update Borut Marincek Institute of Diagnostic Radiology University Hospital Zurich, Switzerland Acute Abdomen Bowel Obstruction Bowel
Nordic Forum - Trauma & Emergency Radiology Bowel Obstruction: Imaging Update Borut Marincek Institute of Diagnostic Radiology University Hospital Zurich, Switzerland Acute Abdomen Bowel Obstruction Bowel
National Horizon Scanning Centre. Methylnaltrexone (MOA-728) for postoperative ileus. April 2008
 (MOA-728) for postoperative ileus April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
(MOA-728) for postoperative ileus April 2008 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive statement
RELISTOR (methylnaltrexone bromide) INJECTION FOR SUBCUTANEOUS USE
 RELISTOR (methylnaltrexone bromide) INJECTION FOR SUBCUTANEOUS USE Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for
RELISTOR (methylnaltrexone bromide) INJECTION FOR SUBCUTANEOUS USE Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for
OPIOID-INDUCED CONSTIPATION DR ANDREW DAVIES
 OPIOID-INDUCED CONSTIPATION DR ANDREW DAVIES Introduction Introduction Mean faecal weight 128 g / cap / day Mean range 51-796 g Absolute range 15-1505 g Main factors affecting mass are caloric intake,
OPIOID-INDUCED CONSTIPATION DR ANDREW DAVIES Introduction Introduction Mean faecal weight 128 g / cap / day Mean range 51-796 g Absolute range 15-1505 g Main factors affecting mass are caloric intake,
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference
 2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
Dextromethorphan Overutilization
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Drugs Requiring PA: the list of drugs requiring prior authorization for this
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Drugs Requiring PA: the list of drugs requiring prior authorization for this
General Surgery Getting to the Core. Disclaimer
 General Surgery Getting to the Core AAPC Regional Conference Nashville, Tennessee September 2011 1 Disclaimer The information in this presentation was current at the time the presentation was complied
General Surgery Getting to the Core AAPC Regional Conference Nashville, Tennessee September 2011 1 Disclaimer The information in this presentation was current at the time the presentation was complied
Abdominal Assessment
 Abdominal Assessment Mary Marian, MS,RD,CSO University of AZ, Tucson, AZ Neha Parekh, MS,RD,LD,CNSC Cleveland Clinic, Cleveland, OH Objectives: 1. Outline the steps in performing an abdominal examination.
Abdominal Assessment Mary Marian, MS,RD,CSO University of AZ, Tucson, AZ Neha Parekh, MS,RD,LD,CNSC Cleveland Clinic, Cleveland, OH Objectives: 1. Outline the steps in performing an abdominal examination.
Pathology of Intestinal Obstruction. Dr. M. Madhavan, MBBS., MD., MIAC, Professor of Pathology Saveetha Medical College
 Pathology of Intestinal Obstruction Dr. M. Madhavan, MBBS., MD., MIAC, Professor of Pathology Saveetha Medical College Pathology of Intestinal Obstruction Objectives list the causes of intestinal obstruction
Pathology of Intestinal Obstruction Dr. M. Madhavan, MBBS., MD., MIAC, Professor of Pathology Saveetha Medical College Pathology of Intestinal Obstruction Objectives list the causes of intestinal obstruction
Texas Prior Authorization Program Clinical Edit Criteria
 Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class COX-2 Inhibitors Clinical Edit Information Included in this Document COX-2 Inhibitors Celebrex Drugs requiring prior authorization:
Texas Prior Authorization Program Clinical Edit Criteria Drug/Drug Class COX-2 Inhibitors Clinical Edit Information Included in this Document COX-2 Inhibitors Celebrex Drugs requiring prior authorization:
 P R E S E N T S Dr. Mufa T. Ghadiali is skilled in all aspects of General Surgery. His General Surgery Services include: General Surgery Advanced Laparoscopic Surgery Surgical Oncology Gastrointestinal
P R E S E N T S Dr. Mufa T. Ghadiali is skilled in all aspects of General Surgery. His General Surgery Services include: General Surgery Advanced Laparoscopic Surgery Surgical Oncology Gastrointestinal
RELISTOR. Methylnaltrexone bromide, Subcutaneous solution for injection. Consumer Medicine Information
 RELISTOR Methylnaltrexone bromide, Subcutaneous solution for injection Consumer Medicine Information What is in this leaflet? This leaflet answers some common questions about RELISTOR. It does not contain
RELISTOR Methylnaltrexone bromide, Subcutaneous solution for injection Consumer Medicine Information What is in this leaflet? This leaflet answers some common questions about RELISTOR. It does not contain
Clinical Payment and Coding Policy Committee Approval Date: 02/23/2018
 In the event of a conflict between a Clinical Payment and Coding Policy and any plan document under which a member is entitled to Covered Services, the plan document will govern. Plan documents include,
In the event of a conflict between a Clinical Payment and Coding Policy and any plan document under which a member is entitled to Covered Services, the plan document will govern. Plan documents include,
Embeda. Embeda (morphine sulfate and naltrexone hydrochloride) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.39 Subject: Embeda Page: 1 of 6 Last Review Date: March 18, 2016 Embeda Description Embeda (morphine
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.39 Subject: Embeda Page: 1 of 6 Last Review Date: March 18, 2016 Embeda Description Embeda (morphine
Announcing SIBO Testing Updates for February 2018
 Announcing SIBO Testing Updates for February 208 Dear Valued Client, In the spirit of continuous improvement and updating our testing services with the most current science and research, BioHealth is aligning
Announcing SIBO Testing Updates for February 208 Dear Valued Client, In the spirit of continuous improvement and updating our testing services with the most current science and research, BioHealth is aligning
Keyhole Laparoscopic Hernia Repairs: What s the Benefit for Your Patients?
 InTouch ARTICLE Keyhole Laparoscopic Hernia Repairs: What s the Benefit for Your Patients? Author: Mr Steve Warren Date: Mary 2015 17 19 View Road, Highgate, London, N6 4DJ Tel. 020 8341 4182 Email. enquiries@highgatehospital.co.uk
InTouch ARTICLE Keyhole Laparoscopic Hernia Repairs: What s the Benefit for Your Patients? Author: Mr Steve Warren Date: Mary 2015 17 19 View Road, Highgate, London, N6 4DJ Tel. 020 8341 4182 Email. enquiries@highgatehospital.co.uk
Prior Authorization Opioid Overutilization 2017
 Drugs Requiring Prior Authorization Label Name ACETAMINOPHEN/CAFFEINE/DIHYDROCODEINE CAPSULE ACETAMINOPHEN/CODEINE SOLUTION ACETAMINOPHEN/CODEINE TABLET ASCOMP/CODEINE CAPSULE BUTALBITAL/CAFFEINE/ACETAMINOPHEN/CODEINE
Drugs Requiring Prior Authorization Label Name ACETAMINOPHEN/CAFFEINE/DIHYDROCODEINE CAPSULE ACETAMINOPHEN/CODEINE SOLUTION ACETAMINOPHEN/CODEINE TABLET ASCOMP/CODEINE CAPSULE BUTALBITAL/CAFFEINE/ACETAMINOPHEN/CODEINE
Policy No: FCHN.MP Page 1 of 6 Date Originated: Last Review Date Current Revision Date 7/10/07 06/2014 7/2/14
 Page 1 of 6 Date Originated: Last Review Date Current Revision Date 7/10/07 06/2014 7/2/14 SUBJECT: Abdominoplasty, Panniculectomy and Ventral/Incisional Hernia RELATED POLICIES/RELATED DESKTOP PROCEDURES:
Page 1 of 6 Date Originated: Last Review Date Current Revision Date 7/10/07 06/2014 7/2/14 SUBJECT: Abdominoplasty, Panniculectomy and Ventral/Incisional Hernia RELATED POLICIES/RELATED DESKTOP PROCEDURES:
Robotic Ventral Rectopexy
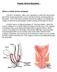 Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
Robotic Ventral Rectopexy What is a robotic ventral rectopexy? The term rectopexy refers to an operation in which the rectum (the part of the bowel nearest the anus) is put back into its normal position
General Surgery Curriculum Royal Australasian College of Surgeons, General Surgeons Australia & New Zealand Association of General Surgeons
 General Surgery Curriculum Royal Australasian College of Surgeons, General Surgeons Australia & New Zealand Association of General Surgeons MODULE TITLE: SMALL BOWEL 7-Nov-2016 DEVELOPED BY: Graham Cullingford,
General Surgery Curriculum Royal Australasian College of Surgeons, General Surgeons Australia & New Zealand Association of General Surgeons MODULE TITLE: SMALL BOWEL 7-Nov-2016 DEVELOPED BY: Graham Cullingford,
Sequelae ICD-10-CM Codes ICD-10 Code Description
 Sequelae These episodes are the avoidable after effects or secondary results of a hysterectomy. Clinicians would be responsible for the cost of the care associated with these conditions. ICD-10-CM Codes
Sequelae These episodes are the avoidable after effects or secondary results of a hysterectomy. Clinicians would be responsible for the cost of the care associated with these conditions. ICD-10-CM Codes
FY 2016 MCRCEDP Approved ICD-10 Code List
 Approved List C18.0 Malignant neoplasm of cecum C18.1 Malignant neoplasm of appendix C18.2 Malignant neoplasm of ascending colon C18.3 Malignant neoplasm of hepatic flexure C18.4 Malignant neoplasm of
Approved List C18.0 Malignant neoplasm of cecum C18.1 Malignant neoplasm of appendix C18.2 Malignant neoplasm of ascending colon C18.3 Malignant neoplasm of hepatic flexure C18.4 Malignant neoplasm of
National Museum of Health and Medicine
 National Museum of Health and Medicine Otis Historical Archives Bower Photograph Collection Date of Records: 1910s-1920s Size: 1 box Finding Aid: by Eric W. Boyle (2012) Biographical Note: Col. Morris
National Museum of Health and Medicine Otis Historical Archives Bower Photograph Collection Date of Records: 1910s-1920s Size: 1 box Finding Aid: by Eric W. Boyle (2012) Biographical Note: Col. Morris
Drug Regimen Optimization
 Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Excluding Valsartan / Ramipril Prior authorization criteria logic: a description
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Clinical Criteria Information Included in this Document Excluding Valsartan / Ramipril Prior authorization criteria logic: a description
DOCTOR DISCUSSION GUIDE
 What your doctor needs to know To make your appointment more productive, share this completed guide with your doctor. That way, he or she can recommend an appropriate treatment plan for you. Check the
What your doctor needs to know To make your appointment more productive, share this completed guide with your doctor. That way, he or she can recommend an appropriate treatment plan for you. Check the
Use of laparoscopy in general surgical operations at academic centers
 Surgery for Obesity and Related Diseases 9 (2013) 15 20 Original article Use of laparoscopy in general surgical operations at academic centers Ninh T. Nguyen, M.D. a, *, Brian Nguyen, B.S. a, Anderson
Surgery for Obesity and Related Diseases 9 (2013) 15 20 Original article Use of laparoscopy in general surgical operations at academic centers Ninh T. Nguyen, M.D. a, *, Brian Nguyen, B.S. a, Anderson
Long-Acting Opioid Analgesics
 Market DC Long-Acting Opioid Analgesics Override(s) Prior Authorization Step Therapy Quantity Limit Approval Duration Initial request: 3 months Maintenance Therapy: Additional prior authorization required
Market DC Long-Acting Opioid Analgesics Override(s) Prior Authorization Step Therapy Quantity Limit Approval Duration Initial request: 3 months Maintenance Therapy: Additional prior authorization required
Long-Acting Opioid Analgesics
 Market DC Long-Acting Opioid Analgesics Override(s) Prior Authorization Step Therapy Quantity Limit Approval Duration Initial request: 3 months Maintenance Therapy: Additional prior authorization required
Market DC Long-Acting Opioid Analgesics Override(s) Prior Authorization Step Therapy Quantity Limit Approval Duration Initial request: 3 months Maintenance Therapy: Additional prior authorization required
Summary of the Home Health Prospective Payment System Final Rule FY 2014
 Summary of the Home Health Prospective Payment System Final Rule FY 2014 Medicare and Medicaid Programs; Home Health Prospective Payment System Rate Update for CY 2014, Home Health Quality Reporting Requirements,
Summary of the Home Health Prospective Payment System Final Rule FY 2014 Medicare and Medicaid Programs; Home Health Prospective Payment System Rate Update for CY 2014, Home Health Quality Reporting Requirements,
Clinical Policy: Budesonide (Uceris) Reference Number: CP.PCH.11 Effective Date: Last Review Date: Line of Business: Commercial, HIM*
 Clinical Policy: (Uceris) Reference Number: CP.PCH.11 Effective Date: 08.14.18 Last Review Date: 11.18 Line of Business: Commercial, HIM* Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Uceris) Reference Number: CP.PCH.11 Effective Date: 08.14.18 Last Review Date: 11.18 Line of Business: Commercial, HIM* Revision Log See Important Reminder at the end of this policy for
PROSPERO International prospective register of systematic reviews
 PROSPERO International prospective register of systematic reviews Evidence in emergency non-trauma gastrointestinal surgery: synthesis of systematic reviews Jelena Savovic, Natalie Blencowe, Sean Strong,
PROSPERO International prospective register of systematic reviews Evidence in emergency non-trauma gastrointestinal surgery: synthesis of systematic reviews Jelena Savovic, Natalie Blencowe, Sean Strong,
Improved Transparency in Key Operational Decisions in Real World Evidence
 PharmaSUG 2018 - Paper RW-06 Improved Transparency in Key Operational Decisions in Real World Evidence Rebecca Levin, Irene Cosmatos, Jamie Reifsnyder United BioSource Corp. ABSTRACT The joint International
PharmaSUG 2018 - Paper RW-06 Improved Transparency in Key Operational Decisions in Real World Evidence Rebecca Levin, Irene Cosmatos, Jamie Reifsnyder United BioSource Corp. ABSTRACT The joint International
Clinical Payment and Coding Policy Committee Approval Date: 02/23/2018
 In the event of conflict between a Clinical Payment and Coding Policy and any plan document under which a member is entitled to Covered Services, the plan document will govern. Plan documents include,
In the event of conflict between a Clinical Payment and Coding Policy and any plan document under which a member is entitled to Covered Services, the plan document will govern. Plan documents include,
Cough/Cold Medications
 Texas Prior Authorization Program Clinical Edit Criteria Cough/Cold Medications NOTE: Claims for cough and cold products containing acetaminophen, ibuprofen, or hydrocodone are not covered by Texas Medicaid
Texas Prior Authorization Program Clinical Edit Criteria Cough/Cold Medications NOTE: Claims for cough and cold products containing acetaminophen, ibuprofen, or hydrocodone are not covered by Texas Medicaid
U Lecture Objectives. U Nordic Forum Trauma & Emergency Radiology. Bowel obstruction. U Bowel Obstruction: Etiologies
 Nordic Forum Trauma & Emergency Radiology Lecture Objectives Bowel Obstruction To illustrate the spectrum of acute obstruction of the small and the large bowel To explain how these bowel obstructions may
Nordic Forum Trauma & Emergency Radiology Lecture Objectives Bowel Obstruction To illustrate the spectrum of acute obstruction of the small and the large bowel To explain how these bowel obstructions may
3/20/2013. "ICD-10 Update Understanding and Analyzing GEMs" March 10, 2013
 "ICD-10 Update Understanding and Analyzing GEMs" March 10, 2013 1 Leola Burke MHSA, CCS AHIMA-approved ICD-10-CM/PCS Trainer Independent Coding Consultant & ICD-10-CM/PCS Expert, Raleigh, NC & Jacksonville,
"ICD-10 Update Understanding and Analyzing GEMs" March 10, 2013 1 Leola Burke MHSA, CCS AHIMA-approved ICD-10-CM/PCS Trainer Independent Coding Consultant & ICD-10-CM/PCS Expert, Raleigh, NC & Jacksonville,
Morphine Sulfate Hydromorphone Oxymorphone
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.02.33 Subject: Morphine Drug Class Page: 1 of 8 Last Review Date: June 19, 2015 Morphine Sulfate Hydromorphone
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.02.33 Subject: Morphine Drug Class Page: 1 of 8 Last Review Date: June 19, 2015 Morphine Sulfate Hydromorphone
Icd 10 pain and swelling of right leg
 Icd 10 pain and swelling of right leg The Borg System is 100 % Icd 10 pain and swelling of right leg Icd 10 code leg pain and swelling. Swelling of toe of right foot; Toe swelling. ICD-10-CM M79.89 is
Icd 10 pain and swelling of right leg The Borg System is 100 % Icd 10 pain and swelling of right leg Icd 10 code leg pain and swelling. Swelling of toe of right foot; Toe swelling. ICD-10-CM M79.89 is
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Lotronex) Reference Number: CP.PMN.153 Effective Date: 11.16.16 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Lotronex) Reference Number: CP.PMN.153 Effective Date: 11.16.16 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
