III. United States Patent (19) Carlsson et al. 11 Patent Number: 5, Date of Patent: Apr. 30, 1996
|
|
|
- Ambrose Kennedy
- 5 years ago
- Views:
Transcription
1 United States Patent (19) Carlsson et al. 54) 75) 73) 21) 22) 63) 51) 52) (58 56 SMOKING SUBSTITUTE Inventors: Thommy Carlsson, Helsingborg; Sven B. Andersson, Odakra, both of Sweden Assignee: Pharmica AB, Sweden Appl. No.: 3,175 Filed: Nov. 7, 1994 Related U.S. Application Data Continuation of Ser. No. 862,533, Jun. 19, Int. Cl.... A23G 3/; A24B 15/00 U.S. Cl /3; 426/650; 131/347; 131/9; 131/369; 424/434; 424/4; 424/465; 424/489; 424/48 Field of Search /3-6, 650: 131/1, 2,9, 369, 366, 367 References Cited U.S. PATENT DOCUMENTS 3,8,217 10/1974 Fernö /3 3,877,468 4/1975 Lichtneckert et al /2 3,901,248 8/1975 Lichtneckert et al III USOO55126A 11 Patent Number: 5,512.6 Date of Patent: Apr., ,267,166 5/1981 Yajima /3 4,284,089 8/1981 Ray ,579,858 4/1986 Fernö et al /9 4,655,231 4/1987 Ray et al /7 4,967,773 1/1990 Shaw / ,055,478 10/1991 Cooper et al /3 5,1,753 8/1992 Baker et al /4 FOREIGN PATENT DOCUMENTS /1985 European Pat. Off France /1988 France /1985 United Kingdom. OTHER PUBLICATIONS Anal. Chem., 1984,56: Chemistry in Britain, 1987, 23:5-8. Primary Examiner-Jeanette Hunter Attorney, Agent, or Firm-Pravel, Hewitt, Kimball & Krieger 57 ABSTRACT The present invention concerns a smoking substitute com position wherein nicotine is the form of an inclusion com plex between nicotine and a cyclodextrincompound. 13 Claims, 1 Drawing Sheet
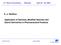
2 U.S. Patent Apr., ,512.6 Egss S. se CO O vs. CN O CONC ng/ml
3 1. SMOKING SUBSTITUTE This is a continuation of application Ser. No. 07/862,533 filed Jun. 19, In the U.S. Surgeon General's 1988 report on The Health Consequences of smoking, it was estimated that in the U.S. alone about 0,000 deaths are caused each yearby diseases related to cigarette smoking. In fact, excessive smoking is now recognized as one of the major health problems throughout the world. The most advantageous thing a heavy smoker can do is, therefore, to reduce or preferably even stop smoking completely. Experience shows, however, that most smokers find this extremely difficult. It is generally accepted that this difficulty results from the fact that heavy smokers are dependent on nicotine, which is considered to be one of the risk factors in tobacco smoke. The most important risk factors, however, are substances which are formed during the combustion of tobacco, such as carbon monoxide, tar products, aldehydes, and hydrocyanic acid. One way to reduce smoking is to provide nicotine in a form or manner other than by smoking and some products have been developed to this end. The most successful product, which is used as a smoking substitute and/or as a smoking cessation aid and which is based on nicotine, is the chewing gum Nicorette(R). To date this product is the only form of nicotine replacement which has been approved by the Food and Drug Administration (FDA). Nicorette(E) has been on the market in about thirty countries for several years. In this chewing gum the nicotine is present in the form of a complex with an insoluble cation-exchanger (polac rilex) which is dispersed in a gum base. A buffering agent is also included in this composition. Patents related to this product are the U.S. Pat. Nos. 3,877,468, 3, and 3,8,217. Another product within this field is Favor(s) which was on the U.S. market for approximately 18 months but which had to be withdrawn as it did not fulfil the requirements set up by the Food and Drug Administration. This product, which is covered by the U.S. Pat. Nos. 4,284,089 and 4,800,903, is a nicotine inhalation device consisting of an elongated tube, in which a porous polymer plug including nicotine free base, is arranged. The main problem with this nicotine delivery system concerned the volatility of the nicotine free base which very rapidly disappeared from the system. It has been estimated that the shelf-life of the unrefrigerated vapor inhalor was approximately 1 month. A product which has not yet been approved by the health authorities but which is now undergoing clinical trials is a nicotine nasal solution of the type disclosed in the U.S. Pat. No. 4,579,858. This product is also based on the use of nicotine free base. Other compositions for nasal use is disclosed in the U.S. Pat. No. 4,655,231. According to this patent the composition is based on water soluble nicotine salts of organic acids, preferably oxalic acid. To the best of our knowledge no clinical studies have been performed with these composi tions. It can be assumed, however, that when the salt dissociates in the nasal cavity, the acid moiety will increase the irritant sensation on the mucous membran originating from the nicotine base formed. The main problems with products based on nicotine free base originate from the volatility of the nicotine, the irritant sensation of the mucous membranes, and the decomposition of nicotine in the presence of oxygen. To some extent these problems are also met with when nicotine is used in the form of a complex with an insoluble cation-exchanger resin as in the Nicorette(E) product, which was developed by our com 5, pany and from which we have by now a broad experience. It should also be born in mind that the nicotine is released from the nicotine resin only after extensive chewing. The present invention concerns compositions to be used as a smoking substitute and/or as a smoking cessation aid comprising nicotine in the form of an inclusion complex formed between nicotine and cyclo compound, preferably a cyclized polysaccaride, and a pharmaceutically acceptable carrier or adjuvant. At present we consider B-cyclodextrin to be the most preferred cyclo compound, but other cyclodextrins such as O- and Y-cyclodextrin can also be used, Also derivatives of cyclodextrines are of interest, e.g. trimethyl-3-cyclodextrin, dimethyl-3-cyclodextrin, hydroxyethyl-3-cyclodextrin and hydroxypropyl-3-cyclodextrin, The compositions of the invention are distinguished by several desirable characteristics making them useful for different types of pharmaceutically acceptable preparations, Such as tablets, capsule, powders, chewing gums, These characteristics include improved stability, improved taste, good bioavailability, ph independent release, reduced irri tant sensation. A preferred embodiment of the composition according to the invention is a tablet for sublingual or buccal adminis tration. In this composition the nicotine in the ram of the inclusion complex may be present in an amount of %, and the rest, apart from a minor amount of conventional lubricant, such as magnesium stearate, is a water-soluble, direct compressible excipient. This excipient should be palatable or tasteless and water soluble and accepted for use in pharmaceutical preparations or as a food additive. With excipients tested so far good results have been obtained with B-cyclodextrin, lactos, sorbitol and mannitol. A special advantage is that this tablet can be very easily prepared with a minimum of additives. Our experiments have shown that the stability of nicotine inclusion complex can be decreased by the addition of common additives and, so far, the best stability results have been obtained with preparations including only the complex, an excipient and a lubricant. Optionally also a flavouring agent could be present. In comparison with the Nicorette() chewing gum it has been found that both the taste and the irritant sensation in the throat are diminished in the sublingual tablet accord ing to the invention. Another interesting finding according to preliminary experiments is that no buffering agent is needed contrary to what could be expected from the experience with the chewing gum, Nicorette(), wherein a buffering agent is necessary. The cyclodextrin inclusion complexes can be prepared according to methods well known to a person skilled in the art. The most common procedures comprise stirring or shaking of an aqueous solution of the particular cyclodextrin with the nicotine. The reaction is preferably carried out in a common solvent like water. After an equilibrium is reached, the solvent can be removed by filtration. The cyclodextrin nicotine complex is then mixed with the selected excipients and compressed into tablets or formulated to a powder for nasal administration. It can also be dispersed in a gum matrix for the preparation of a chewing gum. Of particular value is the improved stability as stability problems with nicotine pharmaceutical preparations are very usual and difficult to solve. The stability can be divided into three categories: a) improved stability as regards evaporation (reduced volatility) b) improved stability as regards decomposition through oxidation (resistance to oxidation) and
4 3 c) improved stability to light which is related to b) as oxidation of nicotine is believed to take place through radical induced reaction. Such reactions are often cata lyzed by light radiation. According to the invention all these three categories of stability are considerably improved if the complexes accord ing to the invention are used instead of nicotine free base. When in proper environment (e.g. saliva in the mouth) the inclusion complex of the invention permits release of nico tine. Furthermore, the complexes are easy to handle and can readily be transferred to different pharmaceutical prepara tions, to which different and desirable release profiles can be imparted. BRIEF DESCRIPTION OF THE FIGURES FIG. 1 shows the results of Experiment 2, the average nicotine plasma level of four persons versus time. The invention is further illustrated by the following examples which should not be construed as limiting the invention: EXAMPLE Preparation of inclusion complex of B-cyclodextrin (B-CD) and nicotine. This complex, which is previously known from Anal. Chem. 1984, , was prepared as follows: 100 g of water was heated to 75 C., g of B-CD were added and dissolved while stirring the solution. 3.5 ml of nicotine (obtained from Eastman Kodak) were added. The mixture was stirred for about 4 h at ambient temperature. The obtained mixture was filtered and dried in a drying oven at C. EXAMPLE tablets having the following composition were prepared. Composition per tablet: Sorbitol powder PEG 6000 Sodium carbonate Ethyl cellulose N-7 Silicone oil 010 cst 34.8 mg (respond to 4 mg nicotine) 500 mg 100 mg 24 mg 12 mg 1,27 mg Sorbitol, PEG-6000, 3-CD nicotine complex and sodium carbonate were sieved and mixed. The silicone oil was added to the ethyl cellulose dissolved in ethanol while stirring. The powder was moistened with the ethanol solution and the obtained product was dried at C. until the ethanol had evaporated and a granulate was obtained. This granulate was sieved and 0.5% Ng stearate was added. The mixture was agitated for 3 minutes, and compressed to tablets. EXAMPLE tablets having the following composition were prepared. Composition per tablet: Sorbitol powder PEG mg (respond to 4 mg nicotine) 500 mg 100 mg 5, Ethyl cellulose N-7 Silicone oil 0,0 cst 4 -continued 12 mg 1,27 mg Sorbitol, PEG-6000, B-CD nicotincomplex were sieved and mixed. The silicone oil was added to the ethyl cellulose dissolved in ethanol while stirring. The powder was moistened with the ethanol solution and the obtained product was dried at C. until the ethanol had evaporated and a granulate was obtained. This granulate was sieved and 0.5% Mg stearate was added. The mixture was agitated and compressed to tablets. EXAMPLE 4 Tablets were prepared from the following ingredients: 3-CD-nicotine 43,5 g (respond to 2 mg nicotine) 3-CD 1792 Mg stearate 2,3 g 00 tablets weigh 2,0 g All the ingredients were placed in an environment with low humidity for more than 24 h. The ingredients were sieved through sieve 1.0 and mixed in a double cone mixer for 5 minutes and compressed to tablets. EXAMPLE 5 described in Example 4 from the following ingredients: 87.0 g (respond to 2 g nicotine) Lactose 8,4 g 5000 tablets weigh 0,0 g EXAMPLE g (respond to 2 g nicotine) Sorbitol 6,0 g Mg stearate 4.5g 5000 tablets weigh 00 g EXAMPLE g (respond to 2 mg nicotine) Starch (Sta. RX 1500) 8,0 g 5000 tablets weigh 0,0g EXAMPLE g (respond to 2 mg nicotine)
5 S -continued Mannit 6,0 g Mg stearate tablets weigh 00 g EXAMPLE 9 3-CD-nicotine 87.0 g (respond to 2 mg nicotine) Avicel ph 101 8,4g 5000 tablets weigh 0,0 g EXPERMENT Tablets prepared according to the above examples were stored for approximately 1 month in an environment having the temperature C. The following observations on the tablets were made: Tablet prepared according to Example No Test time (days) Appearance yellow-brown spots yellow-brown spots without remark 5 38 without remark 7 34 without remark without remark 9 38 without remark Preliminary results also indicated that the loss of nicotine from the tablets was quite small or neglible. Most nicotine had disappeared from the tablets prepared according to the Examples 2 and 3. No increase of oxidation products of nicotine, such as cotinine, myosmine, nicotine-n-oxide, were found in the tablets after storage for one month at C. EXPERIMENT 2. In this experiment nicotine-3-cyclodextrin powder was placed in a porous bag of synthetic material (type tea bag) in an amount corresponding to 6 mg nicotine. A bag was 5,512.6 O 15 6 placed in the mouth of a person and the nicotine plasma level was measured. FIG. 1 discloses the average nicotine plasma level of four persons versus time. We claim: 1. A smoking substitute composition for application to the nose or oral cavity, comprising an inclusion complex of nicotine and a cyclized polysaccharide and an excipient acceptable for pharmaceutical use or as a food additive. 2. The composition of claim 1, wherein said cyclized polysaccharide is a cyclodextrin. 3. The composition of claim 9, wherein said cyclodextrin is selected from the group consisting of alphacyclodextrin, gamma-cyclodextrin, beta-cyclodextrin, and derivatives thereof. 4. The composition of claim3, wherein said cyclodextrine is beta-cyclodextrin. 5. The composition of claim 1 for sublingual or buccal administration, wherein the excipient is a direct compress ible, water soluble substance. 6. The composition of claim 1, wherein said excipient is selected from the group consisting of lactose, Sorbitol, mannitol, and non-complexed cyclodextrin. 7. The composition of claim 1, further comprising a lubricant. 8. The composition of claim 7, wherein said lubricant is magnesium stearate. 9. The composition of claim 1, in the form of a spray for nasal administration. 10. The composition of claim 1, in the form of a powder. 11. The composition of claim 1, in the form of a tablet. 12. The composition of claim 1, wherein the composition is dispersed in a chewing gum matrix. 13. A composition comprising: (1) an inclusion complex of nicotine and cyclodextrin, wherein said inclusion complex comprises from about 0.1% w/w to about 10% w/w of said composition; (2) about 0.5% w/w to about 3% w/w of a lubricant; and (3) an excipient selected from the group consisting of lactose, non-complexed cyclodextrin, sorbitol, and mannitol.
(12) United States Patent (10) Patent No.: US 6,365,596 B1
 USOO63696B1 (12) United States Patent (10) Patent No.: US 6,365,596 B1 Valenti (45) Date of Patent: Apr. 2, 2002 (54) ORAL PHARMACEUTICAL COMPOSITIONS (51) Int. Cl."... A61K 31/44 CONTAINING BUPRENORPHIN
USOO63696B1 (12) United States Patent (10) Patent No.: US 6,365,596 B1 Valenti (45) Date of Patent: Apr. 2, 2002 (54) ORAL PHARMACEUTICAL COMPOSITIONS (51) Int. Cl."... A61K 31/44 CONTAINING BUPRENORPHIN
III. USOO A United States Patent (19) 11. Patent Number: 5,589,191 Ukigaya et al. 45) Date of Patent: Dec. 31, 1996
 III USOO5589191A United States Patent (19) 11. Patent Number: 5,589,191 Ukigaya et al. 45) Date of Patent: Dec. 31, 1996 54) SLOW-RELEASE SODIUM VALPROATE 5,019,398 5/1991 Daste... 424/480 TABLETS 5,055,306
III USOO5589191A United States Patent (19) 11. Patent Number: 5,589,191 Ukigaya et al. 45) Date of Patent: Dec. 31, 1996 54) SLOW-RELEASE SODIUM VALPROATE 5,019,398 5/1991 Daste... 424/480 TABLETS 5,055,306
US A United States Patent (19) 11 Patent Number: 5,635,209 Groenewoud et al. 45 Date of Patent: Jun. 3, 1997
 US005635209A United States Patent (19) 11 Patent Number: Groenewoud et al. 45 Date of Patent: Jun. 3, 1997 (54) STABILIZED COMPOSITION OF LEVOTHYROXNE SODIUM MEDICATION 4,585,652 4,818,531 4/1986 Miller
US005635209A United States Patent (19) 11 Patent Number: Groenewoud et al. 45 Date of Patent: Jun. 3, 1997 (54) STABILIZED COMPOSITION OF LEVOTHYROXNE SODIUM MEDICATION 4,585,652 4,818,531 4/1986 Miller
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 US 2003.01.18647A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0118647 A1 Seth (43) Pub. Date: (54) EXTENDED RELEASE TABLET OF Publication Classification METFORMIN (51)
US 2003.01.18647A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0118647 A1 Seth (43) Pub. Date: (54) EXTENDED RELEASE TABLET OF Publication Classification METFORMIN (51)
(12) Patent Application Publication (10) Pub. No.: US 2001/ A1
 US 2001 0026788A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0026788A1 Piskorz (43) Pub. Date: (54) METHOD OF PRODUCING A NICOTINE (30) Foreign Application Priority Data
US 2001 0026788A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2001/0026788A1 Piskorz (43) Pub. Date: (54) METHOD OF PRODUCING A NICOTINE (30) Foreign Application Priority Data
(51) Int Cl.: A61K 9/20 ( ) A61K 31/41 ( )
 (19) TEPZZ 94677_A_T (11) (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 2.11.1 Bulletin 1/48 (1) Int Cl.: A61K 9/ (06.01) A61K 31/41 (06.01) (21) Application number: 116790.3 (22) Date of
(19) TEPZZ 94677_A_T (11) (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 2.11.1 Bulletin 1/48 (1) Int Cl.: A61K 9/ (06.01) A61K 31/41 (06.01) (21) Application number: 116790.3 (22) Date of
Formulation and Evaluation
 Chapter-5 Formulation and Evaluation 5.1 OBJECTIVE After successful taste masking and solubility enhancement of drugs in preliminary studies, by using Mannitol Solid Dispersion, next step includes the
Chapter-5 Formulation and Evaluation 5.1 OBJECTIVE After successful taste masking and solubility enhancement of drugs in preliminary studies, by using Mannitol Solid Dispersion, next step includes the
(12) United States Patent
 USOO9034382B2 (12) United States Patent Li et al. (54) OSELTAMIVIR PHOSPHATEGRANULE AND PREPARATION METHOD THEREOF (75) Inventors: Song Li, Beijing (CN); Wu Zhong, Beijing (CN); Junhai Xiao, Beijing (CN);
USOO9034382B2 (12) United States Patent Li et al. (54) OSELTAMIVIR PHOSPHATEGRANULE AND PREPARATION METHOD THEREOF (75) Inventors: Song Li, Beijing (CN); Wu Zhong, Beijing (CN); Junhai Xiao, Beijing (CN);
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0193911A1 Ketsela et al. US 2006O193911A1 (43) Pub. Date: (54) (75) (73) (21) (22) (60) CONTROLLED RELEASE VENLAFAXNE FORMULATIONS
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0193911A1 Ketsela et al. US 2006O193911A1 (43) Pub. Date: (54) (75) (73) (21) (22) (60) CONTROLLED RELEASE VENLAFAXNE FORMULATIONS
TEPZZ Z697_5A_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (51) Int Cl.: A61K 9/20 ( ) A61K 31/135 (2006.
 (19) TEPZZ Z697_A_T (11) EP 3 069 7 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 21.09.16 Bulletin 16/38 (1) Int Cl.: A61K 9/ (06.01) A61K 31/13 (06.01) (21) Application number: 160172.1
(19) TEPZZ Z697_A_T (11) EP 3 069 7 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 21.09.16 Bulletin 16/38 (1) Int Cl.: A61K 9/ (06.01) A61K 31/13 (06.01) (21) Application number: 160172.1
A2.2323:23. United States Patent (19) Desai 5,609,884. Mar 11, Patent Number: 45) Date of Patent:
 United States Patent (19) Desai III US005609884A 11 Patent Number: 45) Date of Patent: III Mar 11, 1997 54 CNTRLLED RELEASE NAPRXEN SDIUM PLUS NAPRXEN CMBINATIN TABLET 75 Inventor: Subhash Desai, Grayslake,
United States Patent (19) Desai III US005609884A 11 Patent Number: 45) Date of Patent: III Mar 11, 1997 54 CNTRLLED RELEASE NAPRXEN SDIUM PLUS NAPRXEN CMBINATIN TABLET 75 Inventor: Subhash Desai, Grayslake,
Europaisches Patentamt European Patent Office Office europeen des brevets. Publication number: A2 EUROPEAN PATENT APPLICATION
 Europaisches Patentamt European Patent Office Office europeen des brevets Publication number: 0 345 628 A2 EUROPEAN PATENT APPLICATION Application number: 89109891.5 391.5 Int. CI.4: A61K 9/26 Date of
Europaisches Patentamt European Patent Office Office europeen des brevets Publication number: 0 345 628 A2 EUROPEAN PATENT APPLICATION Application number: 89109891.5 391.5 Int. CI.4: A61K 9/26 Date of
REVISION OF MONOGRAPH ON TABLETS. Tablets
 March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
March 2011 REVISION OF MONOGRAPH ON TABLETS Final text for addition to The International Pharmacopoeia This monograph was adopted by the Forty-fourth WHO Expert Committee on Specifications for Pharmaceutical
(12) Patent Application Publication (10) Pub. No.: US 2011/ A1
 (19) United States US 2011 0112125A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0112125 A1 Liu et al. (43) Pub. Date: May 12, 2011 (54) NOVEL HAIR GROWTH COMPOSITION Publication Classification
(19) United States US 2011 0112125A1 (12) Patent Application Publication (10) Pub. No.: US 2011/0112125 A1 Liu et al. (43) Pub. Date: May 12, 2011 (54) NOVEL HAIR GROWTH COMPOSITION Publication Classification
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION
 PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
PHARMACEUTICS I صيدالنيات 1 UNIT 1 INTRODUCTION 1 PHARMACEUTICS Pharmaceutics is the science of dosage form design. The general area of study concerned with the formulation, manufacture, stability, and
EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2010/39
 (19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 233 131 A1 (43) Date of publication: 29.09. Bulletin /39 (1) Int Cl.: A61K 9/ (06.01) A61K 31/00 (06.01) (21) Application number: 09908.8 (22) Date of filing:
(19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 233 131 A1 (43) Date of publication: 29.09. Bulletin /39 (1) Int Cl.: A61K 9/ (06.01) A61K 31/00 (06.01) (21) Application number: 09908.8 (22) Date of filing:
(12) United States Patent
 USOO7022364B1 (12) United States Patent De Meuter et al. (10) Patent No.: (45) Date of Patent: Apr. 4, 2006 (54) SUGAR-FREE HARD COATINGS PREPARED FROM LIQUID MIXTURES OF ERYTHRTOL AND SORBITOL (75) Inventors:
USOO7022364B1 (12) United States Patent De Meuter et al. (10) Patent No.: (45) Date of Patent: Apr. 4, 2006 (54) SUGAR-FREE HARD COATINGS PREPARED FROM LIQUID MIXTURES OF ERYTHRTOL AND SORBITOL (75) Inventors:
IIII IIHill. United States Patent (19) Shelley et al. 11 Patent Number: 5,505, Date of Patent: Apr. 9, 1996
 United States Patent (19) Shelley et al. IIII IIHill USOO961A 11 Patent Number: Date of Patent: Apr. 9, 1996 (54) 75) 73) 21 22 63 51 (52) 58) 56 GELATIN CAPSULES CONTAINING A HIGHILY CONCENTRATED ACETAMNOPHEN
United States Patent (19) Shelley et al. IIII IIHill USOO961A 11 Patent Number: Date of Patent: Apr. 9, 1996 (54) 75) 73) 21 22 63 51 (52) 58) 56 GELATIN CAPSULES CONTAINING A HIGHILY CONCENTRATED ACETAMNOPHEN
EP B1 (19) (11) EP B1 (12) EUROPEAN PATENT SPECIFICATION
 (19) (11) EP 1 864 677 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 02.01.08 Bulletin 08/01 (1) Int Cl.: A61K 38/ (06.01) A61K 9/ (06.01) A61K 31/39
(19) (11) EP 1 864 677 B1 (12) EUROPEAN PATENT SPECIFICATION (4) Date of publication and mention of the grant of the patent: 02.01.08 Bulletin 08/01 (1) Int Cl.: A61K 38/ (06.01) A61K 9/ (06.01) A61K 31/39
(12) United States Patent
 (12) United States Patent Gabel et al. USOO6294.196B1 (10) Patent No.: () Date of Patent: Sep., 2001 (54) PHARMACEUTICAL COMPOSITION CONTAINING DPHOSPHONCACID OR SALT THEREOF (75) Inventors: Rolf-Dieter
(12) United States Patent Gabel et al. USOO6294.196B1 (10) Patent No.: () Date of Patent: Sep., 2001 (54) PHARMACEUTICAL COMPOSITION CONTAINING DPHOSPHONCACID OR SALT THEREOF (75) Inventors: Rolf-Dieter
SENTRY TM POLYOX Water Soluble Resins
 SENTRY TM POLYOX Water Soluble Resins Technical Information on Stability Introduction Key Points Antioxidants SENTRY POLYOX WSR resins consist of a family of high molecular weight polyethers with nominal
SENTRY TM POLYOX Water Soluble Resins Technical Information on Stability Introduction Key Points Antioxidants SENTRY POLYOX WSR resins consist of a family of high molecular weight polyethers with nominal
PHARMACEUTICAL AID PREPARED BY B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY
 PHARMACEUTICAL AID PREPARED BY B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY Excipients are inactive ingredients used as carriers for the active ingredients in a pharmaceutical product. These may
PHARMACEUTICAL AID PREPARED BY B.KIRUTHIGA LECTURER DEPT OF PHARMACEUTICAL CHEMISTRY Excipients are inactive ingredients used as carriers for the active ingredients in a pharmaceutical product. These may
Formulation and evaluation of sublingual tablets of lisinopril
 Journal of GROVER Scientific & Industrial AGARWAL: Research FORMULATION AND EVALUATION OF SUBLINGUAL TABLETS OF LISINOPRIL Vol. 71, June 2012, pp. 413-417 413 Formulation and evaluation of sublingual tablets
Journal of GROVER Scientific & Industrial AGARWAL: Research FORMULATION AND EVALUATION OF SUBLINGUAL TABLETS OF LISINOPRIL Vol. 71, June 2012, pp. 413-417 413 Formulation and evaluation of sublingual tablets
STABILITY STUDIES OF FORMULATED CONTROLLED RELEASE ACECLOFENAC TABLETS
 Int. J. Chem. Sci.: 8(1), 2010, 405-414 STABILITY STUDIES OF FORMULATED CONTROLLED RELEASE ACECLOFENAC TABLETS V. L. NARASAIAH, T. KARTHIK KUMAR, D. SRINIVAS, K. SOWMYA, P. L. PRAVALLIKA and Sk. Md. MOBEEN
Int. J. Chem. Sci.: 8(1), 2010, 405-414 STABILITY STUDIES OF FORMULATED CONTROLLED RELEASE ACECLOFENAC TABLETS V. L. NARASAIAH, T. KARTHIK KUMAR, D. SRINIVAS, K. SOWMYA, P. L. PRAVALLIKA and Sk. Md. MOBEEN
International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage:
 Research Article CODEN: IJRPJK ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com COMPARARISSION OF SOLUBILITY IMPROVEMENT OF CEFIXIME
Research Article CODEN: IJRPJK ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com COMPARARISSION OF SOLUBILITY IMPROVEMENT OF CEFIXIME
75 Inventors: Ulrik Nosted, Lyngby; Jan Torstensen, 55. o1. Rho 206/364
 US006059107A United States Patent (19) 11 Patent Number: 6,059,107 Nosted et al. (45) Date of Patent: May 9, 2000 54). URINARY CATHETER ASSEMBLY WITH A 3,967,728 7/1976 Gordon et al.. READY-TO-USE CATHETER
US006059107A United States Patent (19) 11 Patent Number: 6,059,107 Nosted et al. (45) Date of Patent: May 9, 2000 54). URINARY CATHETER ASSEMBLY WITH A 3,967,728 7/1976 Gordon et al.. READY-TO-USE CATHETER
(12) United States Patent
 USOO951 0624B2 (12) United States Patent Li et al. (10) Patent No.: (45) Date of Patent: US 9,510,624 B2 Dec. 6, 2016 (54) DISPOSABLE ELECTRONIC CIGARETTE (75) Inventors: Yonghai Li, Shenzhen (CN); Zhongli
USOO951 0624B2 (12) United States Patent Li et al. (10) Patent No.: (45) Date of Patent: US 9,510,624 B2 Dec. 6, 2016 (54) DISPOSABLE ELECTRONIC CIGARETTE (75) Inventors: Yonghai Li, Shenzhen (CN); Zhongli
FLORITER. New Technology for Innovative Formulation Design.
 FLORITER New Technology for Innovative Formulation Design www.tomitaph.co.jp FLORITE Dramatically Change Your Formulation FLORITE is synthetic Calcium Silicate with exceptional liquid absorbency and excellent
FLORITER New Technology for Innovative Formulation Design www.tomitaph.co.jp FLORITE Dramatically Change Your Formulation FLORITE is synthetic Calcium Silicate with exceptional liquid absorbency and excellent
TABLET DESIGN AND FORMULATION
 TABLET DESIGN AND FORMULATION PART 5 Industrial pharmacy 5th class 1st semester TABLET DESIGN AND FORMULATION Conventional oral tablets for ingestion usually contain the same classes of components in addition
TABLET DESIGN AND FORMULATION PART 5 Industrial pharmacy 5th class 1st semester TABLET DESIGN AND FORMULATION Conventional oral tablets for ingestion usually contain the same classes of components in addition
United States Patent (19) James
 United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
United States Patent (19) James 54 DEVICE FOR DISPENSING MEDICAMENTS (75) Inventor: Michael James, Welwyn Garden City, England Allen & Hanburys Limited, London, 73 Assignee: England (21) Appl. No.: 767,518
United States Patent 19
 United States Patent 19 US5866,154A 11 Patent Number: 5,866,154 Bahal et al. (45) Date of Patent: Feb. 2, 1999 54). STABILIZED NALXNE FRMULATINS 56) References Cited 75 Inventors: Surendra Mohan Bahal,
United States Patent 19 US5866,154A 11 Patent Number: 5,866,154 Bahal et al. (45) Date of Patent: Feb. 2, 1999 54). STABILIZED NALXNE FRMULATINS 56) References Cited 75 Inventors: Surendra Mohan Bahal,
LubriTose Mannitol Michael Crowley, Director of R&D, Excipients
 LubriTose Mannitol Michael Crowley, Director of R&D, Excipients Introduction Michael Crowley Director of R&D Excipients 158 St. Highway 320 Norwich, NY 13815 PH 315-802-5970 Michael.Crowley@Kerry.com 2
LubriTose Mannitol Michael Crowley, Director of R&D, Excipients Introduction Michael Crowley Director of R&D Excipients 158 St. Highway 320 Norwich, NY 13815 PH 315-802-5970 Michael.Crowley@Kerry.com 2
EUROPEAN QUALIFYING EXAMINATION 2006
 EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
EUROPEAN QUALIFYING EXAMINATION 2006 PAPER A CHEMISTRY This paper comprises: * Letter from the applicant 2006/A(Ch)/e/1-6 * Document 1 2006/A(Ch)/e/7 * Document 2 2006/A(Ch)/e/8-10 2006/A(Ch)/e - 1 - LETTER
( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / A1
 THE LETTERIN US 20170231969A1 MUTTU 19 United States ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / 0231969 A1 Raneburger et al. ( 43 ) Pub. Date : Aug. 17, 2017 ( 54 ) PHARMACEUTICAL
THE LETTERIN US 20170231969A1 MUTTU 19 United States ( 12 ) Patent Application Publication ( 10 ) Pub. No.: US 2017 / 0231969 A1 Raneburger et al. ( 43 ) Pub. Date : Aug. 17, 2017 ( 54 ) PHARMACEUTICAL
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0118555 A1 Krishnan et al. US 200801185.55A1 (43) Pub. Date: (54) (75) (73) (21) (22) (60) (30) STABLE PHARMACEUTICAL COMPOSITION
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0118555 A1 Krishnan et al. US 200801185.55A1 (43) Pub. Date: (54) (75) (73) (21) (22) (60) (30) STABLE PHARMACEUTICAL COMPOSITION
Easy, fast and reliable!
 Product Overview Easy, fast and reliable! Special easy-to-use preparations for film coating, sugar-coating, colouring and tabletting. Tailormade formulated. s film coating products are one-step coating
Product Overview Easy, fast and reliable! Special easy-to-use preparations for film coating, sugar-coating, colouring and tabletting. Tailormade formulated. s film coating products are one-step coating
Ion Exchange Resins. Unique Solutions to Formulation Problems
 Ion Exchange Resins Unique Solutions to Formulation Problems Lyn Hughes Some of the common problems faced by formulators and how using ion exchange resins may be able to solve them are discussed. PHOTODISC,
Ion Exchange Resins Unique Solutions to Formulation Problems Lyn Hughes Some of the common problems faced by formulators and how using ion exchange resins may be able to solve them are discussed. PHOTODISC,
IIII. United States Patent (19) Nolan et al. 11 Patent Number: 5,776,150 45) Date of Patent: Jul. 7, 1998
 United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
United States Patent (19) Nolan et al. 54) SUTURE ASSIST DEVICE 75) Inventors: Leo J. Nolan; John P. Measamer, both of Cincinnati; James D. Staley, Jr., Loveland; Robert F. Welch, Maineville, all of Ohio
Smoking and Nicotine Replacement Therapy (NRT) Lec:5
 Smoking and Nicotine Replacement Therapy (NRT) Lec:5 Tobacco use remains the single largest preventable cause of mortality. Cigarette smoke is a complex mixture of an estimated 4800 compounds. Approximately
Smoking and Nicotine Replacement Therapy (NRT) Lec:5 Tobacco use remains the single largest preventable cause of mortality. Cigarette smoke is a complex mixture of an estimated 4800 compounds. Approximately
Nov. 20, 1973 G. GERGELY 3,773,922 METHOD FOR THE MANUFACTURE OF EFFERVESCENT TABLETS Filed April 6, 1972
 Nov. 20, 1973 G. GERGELY 3,773,922 METHD FR THE MANUFACTURE F EFFERVESCENT TABLETS Filed April 6, 1972 United States Patent ffice 3,773,922 Patented Nov. 20, 1973 1. 3,773,922 METHD FR THE MANUFACTURE
Nov. 20, 1973 G. GERGELY 3,773,922 METHD FR THE MANUFACTURE F EFFERVESCENT TABLETS Filed April 6, 1972 United States Patent ffice 3,773,922 Patented Nov. 20, 1973 1. 3,773,922 METHD FR THE MANUFACTURE
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States US 20080 107731A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0107731 A1 Kohlrausch et al. (43) Pub. Date: (54) DPP IV INHIBITOR FORMULATIONS (30) Foreign Application
(19) United States US 20080 107731A1 (12) Patent Application Publication (10) Pub. No.: US 2008/0107731 A1 Kohlrausch et al. (43) Pub. Date: (54) DPP IV INHIBITOR FORMULATIONS (30) Foreign Application
(12) United States Patent (10) Patent No.: US 6,692,756 B2
 USOO6692756B2 (12) United States Patent (10) Patent No.: US 6,692,756 B2 Chou (45) Date of Patent: *Feb. 17, 2004 (54) ALOE VERA GLOVE AND 5,679.399 A 10/1997 Shlenker et al. MANUFACTURING METHOD 5,682,617.
USOO6692756B2 (12) United States Patent (10) Patent No.: US 6,692,756 B2 Chou (45) Date of Patent: *Feb. 17, 2004 (54) ALOE VERA GLOVE AND 5,679.399 A 10/1997 Shlenker et al. MANUFACTURING METHOD 5,682,617.
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 US 2008.0092779A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0092779 A1 Wang et al. (43) Pub. Date: Apr. 24, 2008 (54) ULTRAVIOLET ABSORBER FORMULATION (30) Foreign Application
US 2008.0092779A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0092779 A1 Wang et al. (43) Pub. Date: Apr. 24, 2008 (54) ULTRAVIOLET ABSORBER FORMULATION (30) Foreign Application
DRY SYRUPS SWAPNA.M. Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL SEMINAR BY
 DRY SYRUPS SEMINAR BY SWAPNA.M M.PHARMACY Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL CONTENTS DEFINITION CHARACTERISTICS OF SUSPENSIONS
DRY SYRUPS SEMINAR BY SWAPNA.M M.PHARMACY Ist semester DEPARTMENT OF PHARMACEUTICS UNIVERSITY COLLEGE OF PHARMACEUTICAL SCIENCES KAKATIYA UNIVERSITY, WARANGAL CONTENTS DEFINITION CHARACTERISTICS OF SUSPENSIONS
Formulation and evaluation of fast dissolving tablet of aceclofenac
 International Journal of Drug Delivery 2 (2010) 93-97 http://www.arjournals.org/ijdd.html Research article ISSN: 0975-0215 Formulation and evaluation of fast dissolving tablet of aceclofenac Sudhir Bhardwaj
International Journal of Drug Delivery 2 (2010) 93-97 http://www.arjournals.org/ijdd.html Research article ISSN: 0975-0215 Formulation and evaluation of fast dissolving tablet of aceclofenac Sudhir Bhardwaj
(12) Patent Application Publication (10) Pub. No.: US 2006/ A1
 (19) United States US 2006O115435A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0115435 A1 Wilkens (43) Pub. Date: (54) DENTAL TOOTHPASTE, SOLUTION, TABLET AND SYSTEM WITH PLAOUE COLOR INDICATOR
(19) United States US 2006O115435A1 (12) Patent Application Publication (10) Pub. No.: US 2006/0115435 A1 Wilkens (43) Pub. Date: (54) DENTAL TOOTHPASTE, SOLUTION, TABLET AND SYSTEM WITH PLAOUE COLOR INDICATOR
STUDY OF THE DETERIORATION OF ASPIRIN IN THE PRESENCE OF VARIOUS EXCIPIENTS
 STUDY OF THE DETERIORATION OF ASPIRIN IN THE PRESENCE OF VARIOUS EXCIPIENTS D.L.D.A.N DAHANAYAKA, A. MUNISINGHE, D.T.U. ABEYTUNGA DEPARTMENT OF CHEMISTRY, UNIVERSITY OF COLOMBO Abstract Aspirin is a non
STUDY OF THE DETERIORATION OF ASPIRIN IN THE PRESENCE OF VARIOUS EXCIPIENTS D.L.D.A.N DAHANAYAKA, A. MUNISINGHE, D.T.U. ABEYTUNGA DEPARTMENT OF CHEMISTRY, UNIVERSITY OF COLOMBO Abstract Aspirin is a non
Formulation Development of Aceclofenac Tablets Employing Starch Phosphate -A New Modified Starch
 Abstract K.P.R. Chowdary et al. / International Journal of Pharma Sciences and Research (IJPSR) Formulation Development of Aceclofenac Tablets Employing Starch Phosphate -A New Modified Starch K.P.R. Chowdary*,
Abstract K.P.R. Chowdary et al. / International Journal of Pharma Sciences and Research (IJPSR) Formulation Development of Aceclofenac Tablets Employing Starch Phosphate -A New Modified Starch K.P.R. Chowdary*,
LIQUID PREPARATIONS FOR ORAL USE. Final text for addition to The International Pharmacopoeia (November 2007)
 November 2007 LIQUID PREPARATIONS FOR ORAL USE Final text for addition to The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications
November 2007 LIQUID PREPARATIONS FOR ORAL USE Final text for addition to The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications
Application of Starches, Modified Starches and Starch Derivatives in Pharmaceutical Products
 57. Starch Convention, Detmold, April 26-28, 2006 K.-J. Steffens Application of Starches, Modified Starches and Starch Derivatives in Pharmaceutical Products Starches, Pharmaceutical Applications _ Starches
57. Starch Convention, Detmold, April 26-28, 2006 K.-J. Steffens Application of Starches, Modified Starches and Starch Derivatives in Pharmaceutical Products Starches, Pharmaceutical Applications _ Starches
TEPZZ _7584 A_T EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (51) Int Cl.: A61K 9/00 ( ) A61K 31/439 (2006.
 (19) TEPZZ _784 A_T (11) EP 3 17 842 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 07.06.17 Bulletin 17/23 (1) Int Cl.: A61K 9/00 (06.01) A61K 31/439 (06.01) (21) Application number: 1197874.9
(19) TEPZZ _784 A_T (11) EP 3 17 842 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 07.06.17 Bulletin 17/23 (1) Int Cl.: A61K 9/00 (06.01) A61K 31/439 (06.01) (21) Application number: 1197874.9
Industrial Pharmacy (3) Solutions as a dosage form. DR.Saad.M.YACOUB
 Industrial Pharmacy (3) Solutions as a dosage form DR.Saad.M.YACOUB Solutions: definition A solution is a homogenous one-phase system consisting of two or more components. The solvent, or mixture of solvents,
Industrial Pharmacy (3) Solutions as a dosage form DR.Saad.M.YACOUB Solutions: definition A solution is a homogenous one-phase system consisting of two or more components. The solvent, or mixture of solvents,
Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives
 Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives 2.1 Introduction According to the Food Protection Committee of the Food and Nutrition Board, food additives
Paper No.: 13 Paper Title: Food Additives Module 2. Functional Classification of Food Additives 2.1 Introduction According to the Food Protection Committee of the Food and Nutrition Board, food additives
Cessation Medicine Reference Guide Table of Contents
 Cessation Medicine Reference Guide Table of Contents 1. Patch 2. Nicotine Gum 3. Nicotine Lozenge 4. Inhaler 5. Nasal Spray 6. Bupropion SR (Zyban) 7. Chantix (Varenicline) Patch Typical course of therapy
Cessation Medicine Reference Guide Table of Contents 1. Patch 2. Nicotine Gum 3. Nicotine Lozenge 4. Inhaler 5. Nasal Spray 6. Bupropion SR (Zyban) 7. Chantix (Varenicline) Patch Typical course of therapy
(12) United States Patent
 USOO731.4638B2 (12) United States Patent Lee et al. () Patent No.: () Date of Patent: US 7,314.638 B2 Jan. 1, 2008 (54) PREPARING METHOD FOR CONTROLLED RELEASED TYPE TABLET TAMISULOSIN HCL AND THE TABLET
USOO731.4638B2 (12) United States Patent Lee et al. () Patent No.: () Date of Patent: US 7,314.638 B2 Jan. 1, 2008 (54) PREPARING METHOD FOR CONTROLLED RELEASED TYPE TABLET TAMISULOSIN HCL AND THE TABLET
Easy, fast and reliable!
 Product Overview Easy, fast and reliable! Special easy-to-use preparations for film coating, sugar-coating, colouring and tabletting. s film coating products are one-step coating systems for pharmaceutical
Product Overview Easy, fast and reliable! Special easy-to-use preparations for film coating, sugar-coating, colouring and tabletting. s film coating products are one-step coating systems for pharmaceutical
DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN
 Int. J. Chem. Sci.: 10(4), 2012, 2199-2208 ISSN 0972-768X www.sadgurupublications.com DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN K. V. R. N. S. RAMESH *, B. HEMA KIRNAMAYI
Int. J. Chem. Sci.: 10(4), 2012, 2199-2208 ISSN 0972-768X www.sadgurupublications.com DESIGN AND EVALUATION OF CONTROLLED RELEASE MATRIX TABLETS OF FLURBIPROFEN K. V. R. N. S. RAMESH *, B. HEMA KIRNAMAYI
Public Assessment Report Scientific discussion. Nicorette Pepparmint (nicotine) SE/H/904/01/DC
 Public Assessment Report Scientific discussion Nicorette Pepparmint (nicotine) SE/H/904/01/DC This module reflects the scientific discussion for the approval of Nicorette Pepparmint. The procedure was
Public Assessment Report Scientific discussion Nicorette Pepparmint (nicotine) SE/H/904/01/DC This module reflects the scientific discussion for the approval of Nicorette Pepparmint. The procedure was
FORMULATION AND EVALUATION OF VALSARTAN TABLETS EMPLOYING CYCLODEXTRIN-POLOXAMER 407-PVP K30 INCLUSION COMPLEXES
 Int. J. Chem. Sci.: 10(1), 2012, 297-305 ISSN 0972-768X www.sadgurupublications.com FORMULATION AND EVALUATION OF VALSARTAN TABLETS EMPLOYING CYCLODEXTRIN-POLOXAMER 407-PVP K30 INCLUSION COMPLEXES K. P.
Int. J. Chem. Sci.: 10(1), 2012, 297-305 ISSN 0972-768X www.sadgurupublications.com FORMULATION AND EVALUATION OF VALSARTAN TABLETS EMPLOYING CYCLODEXTRIN-POLOXAMER 407-PVP K30 INCLUSION COMPLEXES K. P.
I International Bureau (10) International Publication Number (43) International Publication Date
 (12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International
(12) Patent Application Publication (10) Pub. No.: US 2003/ A1
 US 2003O187062A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0187062 A1 Zenoni et al. (43) Pub. Date: (54) PACLITAXEL-BASEDANTITUMOR (30) Foreign Application Priority
US 2003O187062A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0187062 A1 Zenoni et al. (43) Pub. Date: (54) PACLITAXEL-BASEDANTITUMOR (30) Foreign Application Priority
(12) (10) Patent No.: US 8,512,745 B2. Gainer et al. (45) Date of Patent: Aug. 20, 2013 (54) ULIPRISTALACETATE TABLETS WO 2008/ , 2008
 United States Patent US008512745B2 (12) () Patent No.: US 8,512,745 B2 Gainer et al. (45) Date of Patent: Aug. 20, 2013 (54) ULIPRISTALACETATE TABLETS WO 2008/067086 6, 2008 WO 2008/083.192 T 2008 (75)
United States Patent US008512745B2 (12) () Patent No.: US 8,512,745 B2 Gainer et al. (45) Date of Patent: Aug. 20, 2013 (54) ULIPRISTALACETATE TABLETS WO 2008/067086 6, 2008 WO 2008/083.192 T 2008 (75)
00:08 For decades our scientists have endeavoured to reduce the risks of tobacco use and continue to do so today. 00:15
 00:00 British American Tobacco 00:02 00:03 The Electronic Cigarette 00:07 00:08 For decades our scientists have endeavoured to reduce the risks of tobacco use and continue to do so today. 00:15 00:15 Our
00:00 British American Tobacco 00:02 00:03 The Electronic Cigarette 00:07 00:08 For decades our scientists have endeavoured to reduce the risks of tobacco use and continue to do so today. 00:15 00:15 Our
(12) Patent Application Publication (10) Pub. No.: US 2010/ A1
 (19) United States US 2010O233218A1 (12) Patent Application Publication (10) Pub. No.: US 2010/0233218 A1 Fallon (43) Pub. Date: (54) COMBINATION ENZYME FORCYSTIC Publication Classification FBROSS (51)
(19) United States US 2010O233218A1 (12) Patent Application Publication (10) Pub. No.: US 2010/0233218 A1 Fallon (43) Pub. Date: (54) COMBINATION ENZYME FORCYSTIC Publication Classification FBROSS (51)
SCIENTIFIC DISCUSSION
 This part reflects the scientific knowledge and the information about this product available at the time of prequalification. Thereafter, updates may have become necessary which are included in parts 1
This part reflects the scientific knowledge and the information about this product available at the time of prequalification. Thereafter, updates may have become necessary which are included in parts 1
(12) United States Patent (10) Patent No.: US 6,440,428 B1
 USOO64.40428B1 (12) United States Patent (10) Patent No.: US 6,440,428 B1 Leander et al. (45) Date of Patent: *Aug. 27, 2002 (54) PODOPHYLLOTOXIN PREPARATION 4,235,889 A 11/1980 Evers... 514/863 CONTAINING
USOO64.40428B1 (12) United States Patent (10) Patent No.: US 6,440,428 B1 Leander et al. (45) Date of Patent: *Aug. 27, 2002 (54) PODOPHYLLOTOXIN PREPARATION 4,235,889 A 11/1980 Evers... 514/863 CONTAINING
Preface. Downloaded by on May 1, 2018 https://pubs.acs.org. Publication Date: June 22, 2006 doi: /bk
 Preface Downloaded by 148.251.232.83 on May 1, 2018 https://pubs.acs.org American Viscose Corporation (AVC) was a subsidiary of Courtaulds, when it was established as the first North American producer
Preface Downloaded by 148.251.232.83 on May 1, 2018 https://pubs.acs.org American Viscose Corporation (AVC) was a subsidiary of Courtaulds, when it was established as the first North American producer
Excipient Quality & Trouble Shooting. By Seema Trivedi GM, Technical
 Excipient Quality & Trouble Shooting By Seema Trivedi GM, Technical Back Ground The Society for Pharmaceutical Dissolution Science (SPDS) had held its 6th Annual International Convention Disso India -
Excipient Quality & Trouble Shooting By Seema Trivedi GM, Technical Back Ground The Society for Pharmaceutical Dissolution Science (SPDS) had held its 6th Annual International Convention Disso India -
(12) Patent Application Publication (10) Pub. No.: US 2008/ A1
 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0254.184 A1 Horlacher et al. US 200802541 84A1 (43) Pub. Date: (54) (76) (21) (22) (30) POWDER CONTAINING POLYUNSATURATED FATTY
(19) United States (12) Patent Application Publication (10) Pub. No.: US 2008/0254.184 A1 Horlacher et al. US 200802541 84A1 (43) Pub. Date: (54) (76) (21) (22) (30) POWDER CONTAINING POLYUNSATURATED FATTY
LAB.2. Tablet Production Methods
 LAB.2 Tablet Production Methods Dry methods Direct compression Dry granulation Wet methods Wet granulation Regardless whether tablets are made by direct compression or granulation, the first step, milling
LAB.2 Tablet Production Methods Dry methods Direct compression Dry granulation Wet methods Wet granulation Regardless whether tablets are made by direct compression or granulation, the first step, milling
Student Practical Guide (1) Milk of Magnesia
 School of Pharmacy Student Practical Guide (1b) Milk of Magnesia Facilitators Dr Mark Hewitt M.Hewitt@wlv.ac.uk Required Resources Pre-work: Read this guide Dr Rebecca Butler Rebecca.Butler@wlv.ac.uk Compulsory:
School of Pharmacy Student Practical Guide (1b) Milk of Magnesia Facilitators Dr Mark Hewitt M.Hewitt@wlv.ac.uk Required Resources Pre-work: Read this guide Dr Rebecca Butler Rebecca.Butler@wlv.ac.uk Compulsory:
United States Patent (19) Hensel
 United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
United States Patent (19) Hensel - 54 75 73 1 51 5 58 56) FLUID BEARNG CONSTRUCTION EMPLOYING THRUST PLATE WITH PRESSURE COMPENSATION PORTS Inventor: Robert J. Hensel, Gaston, Oreg. Assignee: Synektron
29 December 2010 ( ) WO 2010/ Al
 (12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date (10) International
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date (10) International
III. United States Patent (19) Hess et al. 11 Patent Number: 5,584,802 (45) Date of Patent: Dec. 17, 1996
 United States Patent (19) Hess et al. (54) ELASTIC KNEE-JOINT BANDAGE (75) Inventors: Heinrich Hess, Saarlouis; Wolfgang Krause, Kassel; Hans B. Bauerfeind, Kempen, all of Germany 73) Assignee: Bauerfeind
United States Patent (19) Hess et al. (54) ELASTIC KNEE-JOINT BANDAGE (75) Inventors: Heinrich Hess, Saarlouis; Wolfgang Krause, Kassel; Hans B. Bauerfeind, Kempen, all of Germany 73) Assignee: Bauerfeind
» Croscarmellose Sodium is a cross linked polymer of carboxymethylcellulose sodium.
 BRIEFING Croscarmellose Sodium, NF 22 page 2856 and page 702 of PF 30(2) [Mar. Apr. 2004]. A modification is made in the test for Degree of substitution to correct the endpoint color to agree with the
BRIEFING Croscarmellose Sodium, NF 22 page 2856 and page 702 of PF 30(2) [Mar. Apr. 2004]. A modification is made in the test for Degree of substitution to correct the endpoint color to agree with the
The Effects of Smoking. Best tip: DONT START
 The Effects of Smoking Best tip: DONT START Why do people start? Feel older Feel cool Feel different Peer pressure Fit in Fun Media: advertising, TV, movies, music Friends / family Relieve stress / relax
The Effects of Smoking Best tip: DONT START Why do people start? Feel older Feel cool Feel different Peer pressure Fit in Fun Media: advertising, TV, movies, music Friends / family Relieve stress / relax
Folic Acid in Human Nutrition
 Folic Acid in Human Nutrition Folic acid is a water soluble B vitamin widely distributed in foods. Deficiencies lead to impaired cell division and altered protein synthesis. Newborn children of women receiving
Folic Acid in Human Nutrition Folic acid is a water soluble B vitamin widely distributed in foods. Deficiencies lead to impaired cell division and altered protein synthesis. Newborn children of women receiving
United States Patent (19)
 United States Patent (19) Gazzaniga et al. 11 Patent Number: () Date of Patent: * May, 1989 54 PHARMACEUTICAL COMPOSITION WITH ANALGESIC ACTIVITY 75 Inventors: Annibale Gazzaniga, Rescaldina, Italy; Walter
United States Patent (19) Gazzaniga et al. 11 Patent Number: () Date of Patent: * May, 1989 54 PHARMACEUTICAL COMPOSITION WITH ANALGESIC ACTIVITY 75 Inventors: Annibale Gazzaniga, Rescaldina, Italy; Walter
H317: May cause allergic skin reaction.
 Safety Data Sheet Date Issued: 2018-02-12 SECTION 1: IDENTIFICATION OF THE PREPARATION AND THE COMPANY PRODUCT NAME: Activa Supersorb ARTICLE NUMBER: 39018, 39021 RECOMMENDED USE: Deodorizer RESTRICTIONS
Safety Data Sheet Date Issued: 2018-02-12 SECTION 1: IDENTIFICATION OF THE PREPARATION AND THE COMPANY PRODUCT NAME: Activa Supersorb ARTICLE NUMBER: 39018, 39021 RECOMMENDED USE: Deodorizer RESTRICTIONS
SAFETY DATA SHEET WF32 OIL
 SAFETY DATA SHEET 1. CHEMICAL PRODUCT AND COMPANY INFORMATION PRODUCT: NRI MINERAL OIL PRODUCT CODE: WF32 CHEMICAL NAME/FAMILY: REFRIGERANT OIL OTHER NAME: Mineral Oil MANUFACTURER / DISTRIBUTOR: NATIONAL
SAFETY DATA SHEET 1. CHEMICAL PRODUCT AND COMPANY INFORMATION PRODUCT: NRI MINERAL OIL PRODUCT CODE: WF32 CHEMICAL NAME/FAMILY: REFRIGERANT OIL OTHER NAME: Mineral Oil MANUFACTURER / DISTRIBUTOR: NATIONAL
Direct Compression. With the right ingredients it s a simple, cost-effective manufacturing process
 Direct With the right ingredients it s a simple, cost-effective manufacturing process TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Speed and savings sounds good to us
Direct With the right ingredients it s a simple, cost-effective manufacturing process TM Trademark of The Dow Chemical Company ( Dow ) or an affiliated company of Dow Speed and savings sounds good to us
United States Patent (19) Blank et al.
 United States Patent (19) Blank et al. 11 Patent Number: 45 Date of Patent: 4,716,042 Dec. 29, 1987 (54. STABILIZED COATED ASPIRIN TABLETS 75 Inventors: Robert G. Blank, Vineland, N.J.; Ronald W. Miller,
United States Patent (19) Blank et al. 11 Patent Number: 45 Date of Patent: 4,716,042 Dec. 29, 1987 (54. STABILIZED COATED ASPIRIN TABLETS 75 Inventors: Robert G. Blank, Vineland, N.J.; Ronald W. Miller,
Effect of magnesium hydroxide as cigarette paper filler to reduce cigarette smoke toxicity
 Journal of Bioresources and Bioproducts. 2016, 1(3):152-158 ORIGINAL PAPER Effect of magnesium hydroxide as cigarette paper filler to reduce cigarette smoke toxicity Xian Lua, Mingyou Liua,*, Zhibin Heb
Journal of Bioresources and Bioproducts. 2016, 1(3):152-158 ORIGINAL PAPER Effect of magnesium hydroxide as cigarette paper filler to reduce cigarette smoke toxicity Xian Lua, Mingyou Liua,*, Zhibin Heb
Published: with international search report (Art. 21(3))
 (12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International
Studies of Rapidly Disintegrating Tablets in the Oral Cavity Using Co-ground Mixtures of Mannitol with Crospovidone
 February 2002 Chem. Pharm. Bull. 50(2) 193 198 (2002) 193 Studies of Rapidly Disintegrating Tablets in the Oral Cavity Using Co-ground Mixtures of Mannitol with Crospovidone Toshifusa SHU,* Hideshi SUZUKI,
February 2002 Chem. Pharm. Bull. 50(2) 193 198 (2002) 193 Studies of Rapidly Disintegrating Tablets in the Oral Cavity Using Co-ground Mixtures of Mannitol with Crospovidone Toshifusa SHU,* Hideshi SUZUKI,
EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2009/36
 (19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 09 81 A1 (43) Date of publication: 02.09.09 Bulletin 09/36 (1) Int Cl.: A61K 9/ (06.01) A61K 31/436 (06.01) (21) Application number: 0838007.3 (22) Date
(19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 09 81 A1 (43) Date of publication: 02.09.09 Bulletin 09/36 (1) Int Cl.: A61K 9/ (06.01) A61K 31/436 (06.01) (21) Application number: 0838007.3 (22) Date
E-Cigarettes: A Game Changer. Dr. Blake Brown Hugh C. Kiger Professor Agriculture and Resource Economics College of Agriculture & Life Sciences
 E-Cigarettes: A Game Changer Dr. Blake Brown Hugh C. Kiger Professor Agriculture and Resource Economics College of Agriculture & Life Sciences What is an e-cigarette? Battery Cartridge Indicator Light
E-Cigarettes: A Game Changer Dr. Blake Brown Hugh C. Kiger Professor Agriculture and Resource Economics College of Agriculture & Life Sciences What is an e-cigarette? Battery Cartridge Indicator Light
METOLOSE: CONTENTS PAGE
 METOLOSE: CONTENTS PAGE 2 Preface What is Metolose Substitution types Specifications 1) Available grades & viscosity 2) Nomenclature 3) Packaging Characteristics of Metolose Properties of Metolose 1) Powder
METOLOSE: CONTENTS PAGE 2 Preface What is Metolose Substitution types Specifications 1) Available grades & viscosity 2) Nomenclature 3) Packaging Characteristics of Metolose Properties of Metolose 1) Powder
III. United States Patent (19) 11 Patent Number: 5,135,768. Campbell et al. (45) Date of Patent: Aug. 4, 1992
 III USOO535768A United States Patent (19) 11 Patent Number: 5,135,768 Campbell et al. (45) Date of Patent: Aug. 4, 1992 (54) NON-DAIRY CREAMS AND PROCESS OF 4,107,343 8/1978 Petricca... 426/570 MAKING
III USOO535768A United States Patent (19) 11 Patent Number: 5,135,768 Campbell et al. (45) Date of Patent: Aug. 4, 1992 (54) NON-DAIRY CREAMS AND PROCESS OF 4,107,343 8/1978 Petricca... 426/570 MAKING
Int. Res J Pharm. App Sci., 2013; 3(6):42-46 ISSN:
 International Research Journal of Pharmaceutical and Applied Sciences (IRJPAS) Available online at www.irjpas.com Int. Res J Pharm. App Sci., 2013; 3(6):42-46 Research Article ENHANCEMENT OF SOLUBILITY
International Research Journal of Pharmaceutical and Applied Sciences (IRJPAS) Available online at www.irjpas.com Int. Res J Pharm. App Sci., 2013; 3(6):42-46 Research Article ENHANCEMENT OF SOLUBILITY
IIIHIIII IIHIII. United States Patent 19. Stroppolo et al. 11 Patent Number: 5,510,385 (45) Date of Patent: Apr. 23, 1996
 United States Patent 19 Stroppolo et al. (54) PHARMACEUTICAL COMPOSITIONS CONTAINING THE SALTS OF S(+)-2-(4-ISOBUTYLPHENYL)PROPIONIC ACID WITH BASIC AMNOACDS 75) Inventors: Federico Stroppolo, Pregassona,
United States Patent 19 Stroppolo et al. (54) PHARMACEUTICAL COMPOSITIONS CONTAINING THE SALTS OF S(+)-2-(4-ISOBUTYLPHENYL)PROPIONIC ACID WITH BASIC AMNOACDS 75) Inventors: Federico Stroppolo, Pregassona,
FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS
 International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
International Journal of PharmTech Research CODEN( USA): IJPRIF ISSN : 0974-4304 Vol.1, No.3, pp 634-638, July-Sept 2009 FORMULATION AND DEVELOPMENT OF ER METOPROLAOL SUCCINATE TABLETS K. Reeta Vijaya
*EP A1* EP A1 (19) (11) EP A1. (12) EUROPEAN PATENT APPLICATION published in accordance with Art.
 (19) Europäisches Patentamt European Patent Office Office européen des brevets *EP00147089A1* (11) EP 1 47 089 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 18(3) EPC (43) Date
(19) Europäisches Patentamt European Patent Office Office européen des brevets *EP00147089A1* (11) EP 1 47 089 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 18(3) EPC (43) Date
CONTENTS PAGE. Please note: Preface Matrix system Selection of METOLOSE grades Specifications
 Hypromellose CONTENTS PAGE 2 Preface Matrix system Selection of METOLOSE grades Specifications Properties Powder Solution Application Related Patents 3 4-5 6 8 10 13 14 17 Please note: The information
Hypromellose CONTENTS PAGE 2 Preface Matrix system Selection of METOLOSE grades Specifications Properties Powder Solution Application Related Patents 3 4-5 6 8 10 13 14 17 Please note: The information
1. PRODUCT NAME 2. SUPPLIER 3. CONTACT FOR PRODUCT INFORMATION OR EMERGENCY. 4. RECOMMENED USE Surfacing Material
 1. PRODUCT NAME 2. SUPPLIER Basix Solid Surface Basix Surfaces 28241 Crown Valley Pkwy. Ste. F #297 Laguna Niguel, CA 92677 Tel: 888-719-1126 3. CONTACT FOR PRODUCT INFORMATION OR EMERGENCY Michelle Kraushaar
1. PRODUCT NAME 2. SUPPLIER Basix Solid Surface Basix Surfaces 28241 Crown Valley Pkwy. Ste. F #297 Laguna Niguel, CA 92677 Tel: 888-719-1126 3. CONTACT FOR PRODUCT INFORMATION OR EMERGENCY Michelle Kraushaar
(12) United States Patent
 USOO879424.4B2 (12) United States Patent Hammel et al. (10) Patent No.: US 8,794,244 B2 (45) Date of Patent: Aug. 5, 2014 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) ELECTRONIC RECHARGEABLESMOKING
USOO879424.4B2 (12) United States Patent Hammel et al. (10) Patent No.: US 8,794,244 B2 (45) Date of Patent: Aug. 5, 2014 (54) (75) (73) (*) (21) (22) (65) (51) (52) (58) ELECTRONIC RECHARGEABLESMOKING
SUSTAINED RELEASE TABLETS USING A PVA DC FORMULATION RESISTANT TO ALCOHOL INDUCED DOSE DUMPING
 SUSTAINED RELEASE TABLETS USING A PVA DC FORMULATION RESISTANT TO ALCOHOL INDUCED DOSE DUMPING Dr. Dieter Lubda MilliporeSigma is a business of Merck KGaA, Darmstadt, Germany Agenda: Sustained release
SUSTAINED RELEASE TABLETS USING A PVA DC FORMULATION RESISTANT TO ALCOHOL INDUCED DOSE DUMPING Dr. Dieter Lubda MilliporeSigma is a business of Merck KGaA, Darmstadt, Germany Agenda: Sustained release
Rationale for dissolution testing. Dissolution testing of nonconventional. Dissolution for IVIVC. Practicality of testing. Apparatus 3 BP 2011
 Rationale for dissolution testing Dissolution testing of nonconventional dosage forms Prof Barbara Conway Annual Symposium for Technical Services 27 th Sept 211 Purpose of dissolution test Product development
Rationale for dissolution testing Dissolution testing of nonconventional dosage forms Prof Barbara Conway Annual Symposium for Technical Services 27 th Sept 211 Purpose of dissolution test Product development
United States Patent Smith, Jr.
 United States Patent Smith, Jr. 4 (72) 73) 22 (21) 2) 1 8 VANADYLOXALATE COMPOUNDS AND PROCESS FOR PRODUCING SAME Inventor: William Novis Smith, Jr., Exton, w Pa. 1934 Assignee: Foote Mineral Company,
United States Patent Smith, Jr. 4 (72) 73) 22 (21) 2) 1 8 VANADYLOXALATE COMPOUNDS AND PROCESS FOR PRODUCING SAME Inventor: William Novis Smith, Jr., Exton, w Pa. 1934 Assignee: Foote Mineral Company,
( 12 ) United States Patent
 ( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
( 12 ) United States Patent Ingwer et al. US009907655B2 ( 10 ) Patent No. : US 9, 907, 655 B2 ( 45 ) Date of Patent : Mar. 6, 2018 ( 54 ) COMPONENTS FOR ARTIFICIAL JOINTS ( 56 ) References Cited U. S.
