AN UPDATE ON FDA S COMPREHENSIVE PLAN ON TOBACCO AND NICOTINE
|
|
|
- Ezra Terry
- 6 years ago
- Views:
Transcription
1 AN UPDATE ON FDA S COMPREHENSIVE PLAN ON TOBACCO AND NICOTINE Mitch Zeller, J.D. Director, FDA May 3, 2018 CENTER FOR TOBACCO PRODUCTS
2 HOW CAN WE MAKE THE GREATEST IMPACT? We truly find ourselves at a crossroads when it comes to efforts to reduce tobacco use. But if we re going to meaningfully improve the public health, we need to be willing to take a hard look at our entire approach. FDA Commissioner Dr. Scott Gottlieb July 28,
3 FDA S NEW COMPREHENSIVE REGULATORY PLAN 2
4 ADDICTIVE NICOTINE IN COMBUSTIBLE CIGARETTES Nicotine, while highly addictive, is delivered through products on a continuum of risk...[and] the combustible cigarette is where nicotine's delivery vehicle leads to incredible amounts of disease and death. FDA Commissioner Dr. Scott Gottlieb October 19,
5 FDA S VISION FOR ADDRESSING NICOTINE FDA envisions a world where cigarettes would no longer create or sustain addiction, and where adults who still seek nicotine could get it from alternative and less harmful sources Decrease the likelihood that future generations will become addicted to cigarettes Allow more addicted smokers to quit Encourage innovation of less harmful products for adults who need them Support innovations to medicinal nicotine and other therapeutic cessation products 4
6 FDA S COMPREHENSIVE REGULATORY PLAN To do this, we ve designed a comprehensive package of actions for FDA to take which places nicotine and the issue of addiction at the center of regulatory efforts All of the programmatic efforts are guided by several principles: Acknowledging that while highly addictive, nicotine is delivered through products on a continuum of risk and cigarettes are the most harmful Striking an appropriate balance between smart regulation and encouraging innovation of satisfying, less harmful products Continuing to base all actions on regulatory and scientific foundation 5
7 FDA S COMPREHENSIVE REGULATORY PLAN These efforts fall under several categories: 1) Regulatory Policies on Addiction, Appeal & Cessation 2) Youth Prevention Plan Access & Marketing Education 3) Science-Based Review of Novel Products 6
8 REGULATORY POLICIES ON ADDICTION, APPEAL & CESSATION 7
9 NICOTINE PRODUCT STANDARD ANPRM On March 15, FDA issued the Tobacco Product Standard for Nicotine Level of Combusted Cigarettes, an Advance Notice of Proposed Rulemaking (ANPRM) Seeks public comment for consideration in developing a potential product standard to lower nicotine to a minimally or non-addictive level in cigarettes What potential maximum nicotine level would be appropriate for the protection of the public health; How a maximum nicotine level should be measured; Whether such a product standard should be implemented all at once or gradually; Whether a nicotine product standard should also cover additional combustible tobacco products; and What unintended consequences might occur as a result of such a standard 8
10 ESTIMATES FROM ONE POSSIBLE NICOTINE PRODUCT STANDARD POLICY Includes newly published estimates of one possible policy scenario to be realized by 2100: 9
11 ENCOURAGING A NATIONAL NICOTINE DIALOGUE Discuss core of the problem but also the solution to addiction Engage public to educate and discuss: Correct common misperceptions: Mistaken beliefs about nicotine and cancer Nicotine s role in continuum of risk: Can be highly addictive; combustible cigarette is the delivery vehicle response for most disease and death; safe and effective in medicinal nicotine Nicotine and youth: Potential for nicotine to rewire a teen s brain and create cravings leading to addiction; potential for future generations to not get addicted Adult smokers and nicotine: How those who still want or need nicotine can get satisfying levels from other and less harmful sources Vulnerable populations: Consider the impact on adult smokers with mental health disorders 10
12 PART 15 HEARING FDA Nicotine Steering Committee formed in September 2017 Includes senior leaders from CDER, CTP & Office of the Commissioner Charged with re-evaluating and modernizing FDA s approach to the development and regulation of nicotine replacement therapy (NRT) products Ensures alignment of FDA s centers and facilitates consensus and development of unified positions on cross-cutting issues Public hearing held in January 2018 to solicit comments on a variety of issues including: New indications such as Reduce to quit for therapeutic product evaluation Investigational New Drug Application vs Investigational Tobacco Product Broadening NRT indications and flexibility on labeling Link to video of January hearing available on our website 11
13 FLAVORS IN TOBACCO PRODUCTS ANPRM On March 20, FDA issued Regulation of Flavors in Tobacco Products, an Advance Notice of Proposed Rulemaking (ANPRM) Seeking comments, research and data on: Role flavors play in initiation & patterns of tobacco use, particularly among youth & young adults; Role flavors may play in helping some adult smokers reduce cigarette use and/or switch to potentially less harmful tobacco products; Consumer perceptions of health risks and addictiveness of flavored products; Whether certain flavors used in tobacco products present potential adverse health effects to users or others 12
14 REGULATION OF PREMIUM CIGARS ANPRM On March 23, FDA issued Regulation of Premium Cigars, an Advance Notice of Proposed Rulemaking (ANPRM) Seeks scientific data on patterns of use and resulting public health impacts of premium cigars The definition of premium cigars; Use patterns of premium cigars generally and among youth and young adults specifically; Public health considerations associated with premium cigars, including the health effects; Studies or information regarding consumer perceptions of the health risks of premium cigars 13
15 YOUTH PREVENTION PLAN 14
16 YOUTH TOBACCO PREVENTION PLAN: ACCESS & MARKETING Last week, Commissioner Gottlieb announced a new focused segment of the Comprehensive Plan to reduce youth use of tobacco products, particularly e-cigarettes But as we work to keep kids from making the deadly progression from experimentation to regular cigarette use, it s imperative that we also make sure children and teenagers aren t getting hooked on more novel nicotine-delivery products. Commissioner Gottlieb, April 24, 2018 The concern is the popularity of products that closely resemble a USB flash drive, have high levels of nicotine, and have emissions that are hard to see These characteristics may facilitate youth use by making products more attractive to youth Several of these products fall under the JUUL brand, but other brands with similar characteristics are emerging (myblu and KandyPens) Kids may be trying these products and liking them without knowing they contain nicotine 15
17 YOUTH TOBACCO PREVENTION PLAN: ACCESS & MARKETING To address these concerns, FDA announced several new enforcement and regulatory steps: In April, conducted a large-scale, undercover nationwide blitz of brick-and-mortar & online retailers Since March, issued 40 Warning Letters to retailers for selling JUUL to minors So that FDA may better understand the reportedly high rates of youth use and appeal, sent official request for information to JUUL Labs requiring them to submit important documents on: Product marketing Research on health, toxicological, behavioral or physiological effects of the product, including: Youth initiation and use Whether certain design features, ingredients, or specifications appeal to different age groups Youth-related adverse events and consumer complaints Worked with ebay to remove listings for JUUL on its website and voluntarily implement new measures to prevent new listings 16
18 YOUTH TOBACCO PREVENTION PLAN: ACCESS & MARKETING On May 1, FDA and the Federal Trade Commission (FTC) issued 13 warning letters to manufacturers, distributors, and retailers for selling e-liquids used in e-cigarettes with labeling and/or advertising that cause them to resemble kid-friendly food products E-liquids resembled juice boxes, candy, cookies, and some included cartoon-like imagery Several of the companies were also cited for illegally selling the products to minors 17
19 YOUTH TOBACCO PREVENTION PLAN: ACCESS & MARKETING Products are considered in violation of the Federal Food, Drug, and Cosmetic Act because their labeling and/or advertising imitating kid-friendly foods is false or misleading. The FTC jointly-issued the letters because Section 5 of the Federal Trade Commission Act prohibits unfair or deceptive advertising Companies are directed to inform each agency of the specific actions taken to address each agency s concerns within 15 working days failure to correct may result in further action(s) 18
20 YOUTH TOBACCO PREVENTION PLAN: EDUCATION This Is Our Watch educates retailers, clerks and the public on how to comply with federal tobacco laws by providing free materials Retailers who comply with laws that prohibit the sale of tobacco products to youth are important intervention points for preventing underage use Even though there are existing industry programs like We Card, retailers want FDA to explain how to comply with federal regulations Printed materials mailed to 350,000+ tobacco retailers and available online for download, sharing and self-printing 19
21 YOUTH TOBACCO PREVENTION PLAN: EDUCATION As part of FDA s regulatory efforts, we have several targeted public education campaigns aimed at educating at-risk teens about the harmful effects of tobacco, including Fresh Empire and This Free Life The Real Cost, FDA s general market campaign, has prevented nearly 350,000 youth aged 11 to 18 nationwide from smoking from 2014 to 2016 For the first time, FDA is expanding The Real Cost to include messaging on electronic nicotine delivery systems (ENDS) Initial messaging launched in Oct. Full-scale effort in Fall
22 YOUTH TOBACCO PREVENTION PLAN: EDUCATION 21
23 SCIENCE-BASED REVIEW OF NOVEL PRODUCTS 22
24 USING SCIENCE TO REVIEW AN EVOLVING MARKETPLACE Since June 2009, FDA has regulated cigarettes, smokeless and rollyour-own tobacco products In August 2016, FDA s regulatory authority over all other tobacco products including electronic nicotine delivery systems (ENDS) became effective (AKA deeming ) Evolving marketplace has seen rise in popularity of certain products like JUUL The challenge for all of us products that are potentially less harmful but enticing to kids 23
25 REVISED APPLICATION DEADLINES FOR DEEMED PRODUCTS Type Example Date Combustible Non-combustible Cigars, pipe tobacco, hookah tobacco E-cigarettes and other ENDS, gels, certain dissolvables Aug. 8, 2021 Aug. 8,
26 REVISED APPLICATION DEADLINES FOR DEEMED PRODUCTS Deadlines were revised to enable FDA to have the latest science and to put regulations, guidances and foundational rules in place before reviewing product applications, e.g.: Product standards (exploding batteries, accidental exposure) Rules for pathway submissions (SE, PMTA, MRTP) Guidance for industry (PMTA for ENDS Final Guidance) Will make the review process more efficient, predictable and transparent while upholding our public health mission All applications will be required to meet regulations issued between now and the deadline 25
27 REVIEWING PRODUCTS IN EVOLVING TOBACCO MARKETPLACE: IQOS MODIFIED RISK APPLICATIONS Pending applications for new and potentially innovative products such as heat-not-burn Most well-known as proprietary technology of Philip Morris International (PMI), sold as iqos Claims to heat tobacco rather than burn to reduce or eliminate formation of many harmful compounds associated with combustion Currently sold in high volumes in Japan, less so in Europe not legally available in U.S. In May 2017, FDA filed three MRTP applications for scientific review from PMI for its iqos system and three HeatStick products The applicant requests modified risk orders to market these products as follows: The IQOS system heats tobacco but does not burn it This significantly reduces the production of harmful and potentially harmful chemicals Scientific studies have shown that switching completely from cigarettes to the IQOS system can reduce the risks of tobacco-related diseases 26
28 REVIEWING PRODUCTS IN EVOLVING TOBACCO MARKETPLACE: IQOS MODIFIED RISK APPLICATIONS TPSAC meeting held Jan for nine members to vote on nine questions The TPSAC votes are non-binding recommendations Comment period on applications is open-ended Our Office of Science will continue its review of these modified risk claims. Any applications for market authorization would be reviewed separately. Our responsibility is to assess the net impact on the population Concerns about teens and nicotine in any form remain 27
29 REVIEWING PRODUCTS IN EVOLVING TOBACCO MARKETPLACE: CAMEL SNUS MODIFIED RISK APPLICATIONS R.J. Reynolds Tobacco Company submitted MRTP applications for smokeless tobacco products: Camel Snus Frost Camel Snus Frost Large Camel Snus Mellow Camel Snus Mint Camel Snus Robust Camel Snus Winterchill Applications are open for public comment by June 18, 2018 TPSAC meeting not yet scheduled 28
30 FDA S STANDARD: ASSESSING OVERALL IMPACT TO PUBLIC HEALTH Smokers Instead unable or of unwilling to quit Patterns quitting, smokers switch of Use become completely dual users to non combustibles Net Impact to the Population Teens At Risk don t tobacco Teens initiate start using Product is is less more Product toxic toxic Toxicity user to user Former Former Former smokers smokers Former Smokers don t Smokers relapse relapse 29
31 QUESTIONS? THANK YOU FOLLOW US ON
FDA S CENTER FOR TOBACCO PRODUCTS: AN UPDATE ON REGULATORY ACTIVITIES AND PRIORITIES
 FDA S CENTER FOR TOBACCO PRODUCTS: AN UPDATE ON REGULATORY ACTIVITIES AND PRIORITIES Mitch Zeller, J.D. (Director, FDA CTP) Matthew R. Holman, Ph.D. (Director, Office of Science, FDA CTP) Kathy Crosby
FDA S CENTER FOR TOBACCO PRODUCTS: AN UPDATE ON REGULATORY ACTIVITIES AND PRIORITIES Mitch Zeller, J.D. (Director, FDA CTP) Matthew R. Holman, Ph.D. (Director, Office of Science, FDA CTP) Kathy Crosby
FDLI s Enforcement, Litigation, and Compliance Conference. Center for Tobacco Products Office of Compliance and Enforcement 2017 Update
 FDLI s Enforcement, Litigation, and Compliance Conference Center for Tobacco Products Office of Compliance and Enforcement 2017 Update Ann Simoneau, Director Office of Compliance and Enforcement Center
FDLI s Enforcement, Litigation, and Compliance Conference Center for Tobacco Products Office of Compliance and Enforcement 2017 Update Ann Simoneau, Director Office of Compliance and Enforcement Center
UNITED STATES REGULATION OF TOBACCO PRODUCTS. Presented by Mitch Zeller Center Director FDA Center for Tobacco Products
 UNITED STATES REGULATION OF TOBACCO PRODUCTS Presented by Mitch Zeller Center Director FDA Center for Tobacco Products May 24, 2016 OVERVIEW OF TODAY S PRESENTATION Highlights of the Deeming Final Rule
UNITED STATES REGULATION OF TOBACCO PRODUCTS Presented by Mitch Zeller Center Director FDA Center for Tobacco Products May 24, 2016 OVERVIEW OF TODAY S PRESENTATION Highlights of the Deeming Final Rule
UPDATES FROM THE FDA CENTER FOR TOBACCO PRODUCTS (CTP)
 UPDATES FROM THE FDA CENTER FOR TOBACCO PRODUCTS (CTP) Mitch Zeller, J.D. Director, FDA May 4, 2017 CENTER FOR TOBACCO PRODUCTS AGENDA Update on Regulations and Guidances: Deeming Update on Science Update
UPDATES FROM THE FDA CENTER FOR TOBACCO PRODUCTS (CTP) Mitch Zeller, J.D. Director, FDA May 4, 2017 CENTER FOR TOBACCO PRODUCTS AGENDA Update on Regulations and Guidances: Deeming Update on Science Update
Mitch Zeller, Director, Center for Tobacco Products, FDA September 19, 2013 Kansas Public Health Association
 Regulatory Public Laws Compliance & Education Policies Science & Enforcement & Communications The FDA Center for Tobacco Products (CTP): Its Role in Reducing Tobacco Use Mitch Zeller, Director, Center
Regulatory Public Laws Compliance & Education Policies Science & Enforcement & Communications The FDA Center for Tobacco Products (CTP): Its Role in Reducing Tobacco Use Mitch Zeller, Director, Center
How to Regulate E-Cigarettes? Are we asking the right questions?
 How to Regulate E-Cigarettes? Are we asking the right questions? Eric N. Lindblom Director, Tobacco Control and Food & Drug Law O Neill Institute for National & Global Health Law Georgetown University
How to Regulate E-Cigarettes? Are we asking the right questions? Eric N. Lindblom Director, Tobacco Control and Food & Drug Law O Neill Institute for National & Global Health Law Georgetown University
The Latest on Vaping Among U.S. Teens
 The Latest on Vaping Among U.S. Teens Jon Macy, PhD, MPH Indiana University School of Public Health Bloomington Presentation Outline Overview of Electronic Nicotine Delivery Systems (ENDS) New Prevalence
The Latest on Vaping Among U.S. Teens Jon Macy, PhD, MPH Indiana University School of Public Health Bloomington Presentation Outline Overview of Electronic Nicotine Delivery Systems (ENDS) New Prevalence
FDA s Action Agenda to Reduce Tobacco Related-Cancer Incidence and Mortality
 FDA s Action Agenda to Reduce Tobacco Related-Cancer Incidence and Mortality Lawrence Deyton, M.S.P.H., M.D. Director, FDA Center for Tobacco Products June 11, 2012 FDA s Vision To make tobaccorelated
FDA s Action Agenda to Reduce Tobacco Related-Cancer Incidence and Mortality Lawrence Deyton, M.S.P.H., M.D. Director, FDA Center for Tobacco Products June 11, 2012 FDA s Vision To make tobaccorelated
Communicating the Risk of Nicotine Delivery Products
 Communicating the Risk of Nicotine Delivery Products August 10, 2014 ACS National Meeting Jim Solyst June 24, 2014 1 Nicotine Background Key Points The popularity of e-cigarettes has increased the need
Communicating the Risk of Nicotine Delivery Products August 10, 2014 ACS National Meeting Jim Solyst June 24, 2014 1 Nicotine Background Key Points The popularity of e-cigarettes has increased the need
Modified Risk Tobacco Product Applications A Succinct ENDS Industry Perspective
 Modified Risk Tobacco Product Applications A Succinct ENDS Industry Perspective Patricia I. Kovacevic General Counsel, Chief Compliance Officer Presentation to the Food and Drug Law Institute Tobacco Conference,
Modified Risk Tobacco Product Applications A Succinct ENDS Industry Perspective Patricia I. Kovacevic General Counsel, Chief Compliance Officer Presentation to the Food and Drug Law Institute Tobacco Conference,
Should FDA try to move smokers to e-cigarettes or other less harmful tobacco-nicotine products and, if so, how?
 Should FDA try to move smokers to e-cigarettes or other less harmful tobacco-nicotine products and, if so, how? Jeff Weiss General Counsel/EVP of Gov. Affairs of NJOY, LLC FDLI - October 20, 2017 FDA s
Should FDA try to move smokers to e-cigarettes or other less harmful tobacco-nicotine products and, if so, how? Jeff Weiss General Counsel/EVP of Gov. Affairs of NJOY, LLC FDLI - October 20, 2017 FDA s
Tobacco-related risk perceptions in the regulation of tobacco products at the FDA Center for Tobacco Products
 Tobacco-related risk perceptions in the regulation of tobacco products at the FDA Center for Tobacco Products David B. Portnoy, PhD, MPH Conrad J. Choiniere, PhD Office of Science FDA, Center for Tobacco
Tobacco-related risk perceptions in the regulation of tobacco products at the FDA Center for Tobacco Products David B. Portnoy, PhD, MPH Conrad J. Choiniere, PhD Office of Science FDA, Center for Tobacco
JUUL and Other Emerging Tobacco Products. Emily McClelland, M.S. December 6, 2018
 JUUL and Other Emerging Tobacco Products Emily McClelland, M.S. December 6, 2018 7.2% for high school students 1.5% for middle school students Preliminary 2018 National Youth Tobacco Survey Findings Surge
JUUL and Other Emerging Tobacco Products Emily McClelland, M.S. December 6, 2018 7.2% for high school students 1.5% for middle school students Preliminary 2018 National Youth Tobacco Survey Findings Surge
TOBACCO PRODUCT OR MEDICAL PRODUCT?
 TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
consistent with the industry documents we note in our September letter.
 February 18, 2010 Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, MD 20852 RE: Docket Number FDA-2010-N-0020
February 18, 2010 Division of Dockets Management (HFA-305) Food and Drug Administration Department of Health and Human Services 5630 Fishers Lane, Room 1061 Rockville, MD 20852 RE: Docket Number FDA-2010-N-0020
MARKETING STANDARDS FOR MEMBERSHIP
 MARKETING STANDARDS FOR MEMBERSHIP The Vapor Technology Association (VTA) is a leading national trade association in the electronic cigarette and vapor product industry. VTA represents the manufacturers,
MARKETING STANDARDS FOR MEMBERSHIP The Vapor Technology Association (VTA) is a leading national trade association in the electronic cigarette and vapor product industry. VTA represents the manufacturers,
FDA Center for Tobacco Products: Tobacco Research and the Population Assessment of Tobacco and Health (PATH) Study
 FDA Center for Tobacco Products: Tobacco Research and the Population Assessment of Tobacco and Health (PATH) Study Regulatory Public Laws Compliance & Education Policies Science & Enforcement & Communications
FDA Center for Tobacco Products: Tobacco Research and the Population Assessment of Tobacco and Health (PATH) Study Regulatory Public Laws Compliance & Education Policies Science & Enforcement & Communications
FDLI Annual Conference
 FDLI Annual Conference Panel Tobacco Harm Reduction: Opportunities & Regulatory Pathways to Achieve May 5, 2017 Joe Murillo Vice President, Regulatory Affairs Altria Client Services Panel Discussion at
FDLI Annual Conference Panel Tobacco Harm Reduction: Opportunities & Regulatory Pathways to Achieve May 5, 2017 Joe Murillo Vice President, Regulatory Affairs Altria Client Services Panel Discussion at
The Legal Resource Center for Public Health Policy provides information and technical assistance on issues related to public health in Maryland.
 October 17, 2016 The Legal Resource Center for Public Health Policy provides information and technical assistance on issues related to public health in Maryland. The legal information and assistance does
October 17, 2016 The Legal Resource Center for Public Health Policy provides information and technical assistance on issues related to public health in Maryland. The legal information and assistance does
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Products
 Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
Effective and Compliance Dates Applicable to Retailers, Manufacturers, Importers, and Distributors of Newly Deemed Tobacco Quick Facts Retailers that mix and prepare e-liquids or create or modify vaporizers
Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products
 Guidance for Industry and FDA Staff Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products June 2010 For questions regarding this guidance, contact the
Guidance for Industry and FDA Staff Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products June 2010 For questions regarding this guidance, contact the
ELECTRONIC CIGARETTES WHAT S THE BOTTOM LINE?
 ELECTRONIC CIGARETTES WHAT S THE BOTTOM LINE? E-cigarettes have the potential to benefit adult smokers who are not pregnant if used as a complete substitute for regular cigarettes and other smoked tobacco
ELECTRONIC CIGARETTES WHAT S THE BOTTOM LINE? E-cigarettes have the potential to benefit adult smokers who are not pregnant if used as a complete substitute for regular cigarettes and other smoked tobacco
SNUS ON THE LOOSE: Big Tobacco s s latest attempt to make nicotine more attractive
 SNUS ON THE LOOSE: Big Tobacco s s latest attempt to make nicotine more attractive U.S. Smokeless Tobacco Market Historically the preserve of smaller, specialized companies: Conwood U.S. U.S. Smokeless
SNUS ON THE LOOSE: Big Tobacco s s latest attempt to make nicotine more attractive U.S. Smokeless Tobacco Market Historically the preserve of smaller, specialized companies: Conwood U.S. U.S. Smokeless
Wednesday, May 30, :00 a.m. 4 th Floor Conference Room E County Office Building AGENDA
 OSWEGO COUNTY LEGISLATURE County Office Building 46 East Bridge Street Oswego, NY 13126 Phone (315)349-8230 Fax (315)349-8237 www.oswegocounty.com TO: FROM: SUBJ: Leg. Morris Sorbello, Vice Chair; Leg.
OSWEGO COUNTY LEGISLATURE County Office Building 46 East Bridge Street Oswego, NY 13126 Phone (315)349-8230 Fax (315)349-8237 www.oswegocounty.com TO: FROM: SUBJ: Leg. Morris Sorbello, Vice Chair; Leg.
RADM Patrick O Carroll, MD, MPH Senior Advisor, Assistant Secretary for Health, US DHSS
 Ending the Tobacco Epidemic RADM Patrick O Carroll, MD, MPH Senior Advisor, Assistant Secretary for Health, US DHSS Tim McAfee, MD, MPH Senior Medical Officer, Office on Smoking and Health, CDC www.nwcphp.org/hot-topics
Ending the Tobacco Epidemic RADM Patrick O Carroll, MD, MPH Senior Advisor, Assistant Secretary for Health, US DHSS Tim McAfee, MD, MPH Senior Medical Officer, Office on Smoking and Health, CDC www.nwcphp.org/hot-topics
Problem Which option Additional option Additional comments definition Yes No change No further observations.
 Department of Health, United Kingdom electronic contribution rec. 317 - by Mr Lee McGill lee.mcgill@dh.gsi.gov.uk Question 1 - scope Problem Which option Recommend option Additional comments Yes No change
Department of Health, United Kingdom electronic contribution rec. 317 - by Mr Lee McGill lee.mcgill@dh.gsi.gov.uk Question 1 - scope Problem Which option Recommend option Additional comments Yes No change
RESPONSE FROM ALTRIA:
 RESPONSE FROM ALTRIA: FDA Regulation of Tobacco http://www.altria.com/en/cms/about_altria/federal_regulation_of_tobacco/default.aspx?src=top_nav http://www.fda.gov/tobaccoproducts/default.htm The Food
RESPONSE FROM ALTRIA: FDA Regulation of Tobacco http://www.altria.com/en/cms/about_altria/federal_regulation_of_tobacco/default.aspx?src=top_nav http://www.fda.gov/tobaccoproducts/default.htm The Food
Jackson Tobacco Reduction Coalition 1715 Lansing Avenue, Jackson, MI Phone (517) FAX (517)
 Jackson Tobacco Reduction Coalition 1715 Lansing Avenue, Jackson, MI 49202 Phone (517) 768-2131 FAX (517) 788-4373 Date: August 16, 2018 Contact: Rhonda Rudolph, Coordinator, Jackson Tobacco Reduction
Jackson Tobacco Reduction Coalition 1715 Lansing Avenue, Jackson, MI 49202 Phone (517) 768-2131 FAX (517) 788-4373 Date: August 16, 2018 Contact: Rhonda Rudolph, Coordinator, Jackson Tobacco Reduction
America s Most Wanted Tobacco Villains The Usual Suspects, New Villains and Emerging Threats
 America s Most Wanted Tobacco Villains The Usual Suspects, New Villains and Emerging Threats The United States has made enormous progress in reducing youth smoking, with the smoking rate among high school
America s Most Wanted Tobacco Villains The Usual Suspects, New Villains and Emerging Threats The United States has made enormous progress in reducing youth smoking, with the smoking rate among high school
FDA Tobacco Retailers Compliance Program Presentation
 Arizona Department of Health Services FDA Tobacco Retailers Compliance Program Presentation June 29, 2011 Revised 06/27/11 FDA Retail Tobacco Compliance Program In contract with Arizona Department of Health
Arizona Department of Health Services FDA Tobacco Retailers Compliance Program Presentation June 29, 2011 Revised 06/27/11 FDA Retail Tobacco Compliance Program In contract with Arizona Department of Health
Flavors Hook Kids. The Tobacco Industry s Kids Menu MARIPOSA COUNTY HEALTH DEPARTMENT
 Flavors Hook Kids The Tobacco Industry s Kids Menu Mariposa County Tobacco Education Program We engage with the community to: Create smoke free environments Counter the aggressive marketing practices of
Flavors Hook Kids The Tobacco Industry s Kids Menu Mariposa County Tobacco Education Program We engage with the community to: Create smoke free environments Counter the aggressive marketing practices of
REPORT OF THE BOARD OF TRUSTEES. Subject: Annual Update on Activities and Progress in Tobacco Control: March 2017 through February 2018
 REPORT OF THE BOARD OF TRUSTEES B of T Report -A- Subject: Annual Update on Activities and Progress in Tobacco Control: March 0 through February 0 Presented by: Gerald E. Harmon, MD, Chair 0 0 0 This report
REPORT OF THE BOARD OF TRUSTEES B of T Report -A- Subject: Annual Update on Activities and Progress in Tobacco Control: March 0 through February 0 Presented by: Gerald E. Harmon, MD, Chair 0 0 0 This report
Three-Month Extension of Certain Tobacco Product Compliance Deadlines Related to the
 This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Challenges and Opportunities: Implementing the Tobacco Control Act
 Challenges and Opportunities: Implementing the Tobacco Control Act Lawrence R. Deyton, M.S.P.H., M.D. Director, Center for Tobacco Products April 5, 2011 1 To make tobaccorelated death and disease part
Challenges and Opportunities: Implementing the Tobacco Control Act Lawrence R. Deyton, M.S.P.H., M.D. Director, Center for Tobacco Products April 5, 2011 1 To make tobaccorelated death and disease part
Tobacco 21 in Oregon 7,000. Leading Causes of Preventable Death in Oregon. Most addiction to tobacco starts in adolescence.
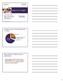 Tobacco 21 in Oregon Luci Longoria, MPH Health Promotion Manager Health Promotion and Chronic Disease Prevention Oregon Health Authority www.nwcphp.org/hot-topics Leading Causes of Preventable Death in
Tobacco 21 in Oregon Luci Longoria, MPH Health Promotion Manager Health Promotion and Chronic Disease Prevention Oregon Health Authority www.nwcphp.org/hot-topics Leading Causes of Preventable Death in
Key findings from a national survey of 1,000 registered voters, conducted February 2-5, Project # 15054
 Key findings from a national survey of 1,000 registered voters, conducted February 2-5, 2015. Project # 15054 Public Opinion Strategies and the Mellman Group are pleased to present the key findings from
Key findings from a national survey of 1,000 registered voters, conducted February 2-5, 2015. Project # 15054 Public Opinion Strategies and the Mellman Group are pleased to present the key findings from
Electronic Cigarettes: What They Are, How They re Marketed, & How We Can Protect Our Youth
 Electronic Cigarettes: What They Are, How They re Marketed, & How We Can Protect Our Youth Amy Barkley Coalition for a Tobacco- Free West Virginia Charleston, WV February 26, 2014 Big Picture Points Tobacco
Electronic Cigarettes: What They Are, How They re Marketed, & How We Can Protect Our Youth Amy Barkley Coalition for a Tobacco- Free West Virginia Charleston, WV February 26, 2014 Big Picture Points Tobacco
RESULTS AT A GLANCE. FDA Oversight of Tobacco Manufacturing Establishments. HHS OIG Data Brief August 2017 OEI
 HHS OIG Data Brief August 2017 OEI-01-15-00300 FDA Oversight of Tobacco Manufacturing Establishments RESULTS AT A GLANCE The Tobacco Control Act authorized FDA to regulate domestic tobacco manufacturers
HHS OIG Data Brief August 2017 OEI-01-15-00300 FDA Oversight of Tobacco Manufacturing Establishments RESULTS AT A GLANCE The Tobacco Control Act authorized FDA to regulate domestic tobacco manufacturers
Smoking, e-cigs and reduced risk products. Dr John Schoonbee, IMS November 2017
 Smoking, e-cigs and reduced risk products Dr John Schoonbee, IMS November 2017 2 The storyline smoking is bad yet many folks still smoke because quitting is very hard is it better, if one cannot quit,
Smoking, e-cigs and reduced risk products Dr John Schoonbee, IMS November 2017 2 The storyline smoking is bad yet many folks still smoke because quitting is very hard is it better, if one cannot quit,
Melodie Tilson, Director of Policy
 Melodie Tilson, Director of Policy Non-Smokers Rights Association/ Smoking and Health Action Foundation PTCC Knowledge Exchange 18 June 2014 Disposable Rechargeable with pre-filled cartridges Rechargeable
Melodie Tilson, Director of Policy Non-Smokers Rights Association/ Smoking and Health Action Foundation PTCC Knowledge Exchange 18 June 2014 Disposable Rechargeable with pre-filled cartridges Rechargeable
The Emergence of JUULs and Heated Tobacco Products
 The Emergence of JUULs and Heated Tobacco Products 2018 TCN Podcast Series Kristy Marynak, CDC/OSH Jeff Willett, Truth Initiative Steven Fiala, Oregon Health Authority Heated tobacco products: What you
The Emergence of JUULs and Heated Tobacco Products 2018 TCN Podcast Series Kristy Marynak, CDC/OSH Jeff Willett, Truth Initiative Steven Fiala, Oregon Health Authority Heated tobacco products: What you
Raze-On. Tobacco 101
 Raze-On Tobacco 101 Key Messages Most people don t use tobacco Tobacco kills Nicotine is addictive The tobacco industry targets youth Tobacco Use in Teens 80% of adult smokers start before the age of 18
Raze-On Tobacco 101 Key Messages Most people don t use tobacco Tobacco kills Nicotine is addictive The tobacco industry targets youth Tobacco Use in Teens 80% of adult smokers start before the age of 18
Understanding the Tobacco Control Act
 Understanding the Tobacco Control Act An Overview of the Tobacco Control Act and Center for Tobacco Products for the National Native Network CAPT Gail Cherry-Peppers, Tribal Liaison Heather Althouse, Senior
Understanding the Tobacco Control Act An Overview of the Tobacco Control Act and Center for Tobacco Products for the National Native Network CAPT Gail Cherry-Peppers, Tribal Liaison Heather Althouse, Senior
REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH
 REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH (A-) Tobacco Harm Reduction: A Comprehensive Nicotine Policy to Reduce Death and Disease Caused by Smoking (Resolution 0-A-) (Reference Committee D) EXECUTIVE
REPORT OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH (A-) Tobacco Harm Reduction: A Comprehensive Nicotine Policy to Reduce Death and Disease Caused by Smoking (Resolution 0-A-) (Reference Committee D) EXECUTIVE
Re: Docket No. FDA-2013-N-0521, Menthol in Cigarettes, Tobacco Products; Request for Comments
 November 22, 2013 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, rm. 1061 Rockville, MD 20852 Re: Docket No. FDA-2013-N-0521, Menthol in Cigarettes, Tobacco Products;
November 22, 2013 Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, rm. 1061 Rockville, MD 20852 Re: Docket No. FDA-2013-N-0521, Menthol in Cigarettes, Tobacco Products;
PMI Representations to TPSAC about Marketing of iqos in the U.S.
 March 23, 2018 Mr. Mitchell Zeller Director, Center for Tobacco Products U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Re: Global Marketing of iqos by PMI Dear Mr.
March 23, 2018 Mr. Mitchell Zeller Director, Center for Tobacco Products U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 Re: Global Marketing of iqos by PMI Dear Mr.
Reduced Risk Review March Keith Lenghaus
 Reduced Risk Review March 2005 Keith Lenghaus Reducing Exposure Add filters (1950s) Rapid gain in market share Reduce tar, nicotine (1960s on) FTC method Competitive advantage Advertising Pollay et al
Reduced Risk Review March 2005 Keith Lenghaus Reducing Exposure Add filters (1950s) Rapid gain in market share Reduce tar, nicotine (1960s on) FTC method Competitive advantage Advertising Pollay et al
Peter G. Shields, MD Tobacco Products Scientific Advisory Committee (TPSAC) August 16, 2013
 Peter G. Shields, MD Tobacco Products Scientific Advisory Committee (TPSAC) August 16, 2013 Conceptual Framework for Evaluating MRTPs: Objectives Provide a framework for evaluating all tobacco products
Peter G. Shields, MD Tobacco Products Scientific Advisory Committee (TPSAC) August 16, 2013 Conceptual Framework for Evaluating MRTPs: Objectives Provide a framework for evaluating all tobacco products
The Tobacco Control Act s Premarket Review Authorities: Reports on Substantial Equivalence and Exemption Requests (905(j))
 The Tobacco Control Act s Premarket Review Authorities: Reports on Substantial Equivalence and Exemption Requests (905(j)) January 12, 2011 Cristi Stark, MS Senior Regulatory Health Project Manager Office
The Tobacco Control Act s Premarket Review Authorities: Reports on Substantial Equivalence and Exemption Requests (905(j)) January 12, 2011 Cristi Stark, MS Senior Regulatory Health Project Manager Office
National Conference of State Legislatures Legislative Summit: Clearing the Air About E-Cigarettes
 National Conference of State Legislatures Legislative Summit: Clearing the Air About E-Cigarettes Jack Henningfield Ph.D. Vice President, Research and Health Policy Pinney Associates And Professor, Adjunct,
National Conference of State Legislatures Legislative Summit: Clearing the Air About E-Cigarettes Jack Henningfield Ph.D. Vice President, Research and Health Policy Pinney Associates And Professor, Adjunct,
The Voice of Local Public Health in New York State. May 12, 2014
 The Voice of Local Public Health in New York State May 12, 2014 Testimony before the Senate Standing Committee on Health To consider including electronic cigarettes in the existing Clean Indoor Air Act
The Voice of Local Public Health in New York State May 12, 2014 Testimony before the Senate Standing Committee on Health To consider including electronic cigarettes in the existing Clean Indoor Air Act
Nebraska Youth Tobacco Survey 2015/2017
 Nebraska Youth Tobacco Survey 2015/2017 TABLE OF CONTENTS Introduction... 1 Background... 1 Method... 1 Sampling Frame and Response Rates... 1 Weighting Data... 2 Terms and Definitions... 3 Executive Summary...
Nebraska Youth Tobacco Survey 2015/2017 TABLE OF CONTENTS Introduction... 1 Background... 1 Method... 1 Sampling Frame and Response Rates... 1 Weighting Data... 2 Terms and Definitions... 3 Executive Summary...
E-Cigs, Etc.: Policy Options for Regulating Nicotine Delivery Devices. Indiana Local Boards of Health Webinar Feb. 12, 2015
 E-Cigs, Etc.: Policy Options for Regulating Nicotine Delivery Devices Indiana Local Boards of Health Webinar Feb. 12, 2015 How to Use Webex If you can hear us through your computer, you do not need to
E-Cigs, Etc.: Policy Options for Regulating Nicotine Delivery Devices Indiana Local Boards of Health Webinar Feb. 12, 2015 How to Use Webex If you can hear us through your computer, you do not need to
Flavored Tobacco and Vaping Products Funding Opportunity New York City Department of Health and Mental Hygiene
 Application DEADLINE: November 7, 2018 at 11:59 pm Summary The New York City (NYC) Department of Health and Mental Hygiene (DOHMH) is pleased to announce the availability of funding for up to five (5)
Application DEADLINE: November 7, 2018 at 11:59 pm Summary The New York City (NYC) Department of Health and Mental Hygiene (DOHMH) is pleased to announce the availability of funding for up to five (5)
Renville County Tobacco Retailers Youth Tobacco Laws A TRAINING BY THE RENVILLE COUNTY PUBLIC HEALTH UPDATED NOVEMBER 2015
 Renville County Tobacco Retailers Youth Tobacco Laws A TRAINING BY THE RENVILLE COUNTY PUBLIC HEALTH UPDATED NOVEMBER 2015 Training Goals Facts about Youth Tobacco Use Proper Identification Federal, State
Renville County Tobacco Retailers Youth Tobacco Laws A TRAINING BY THE RENVILLE COUNTY PUBLIC HEALTH UPDATED NOVEMBER 2015 Training Goals Facts about Youth Tobacco Use Proper Identification Federal, State
Low-Nitrosamine Dissolvable Tobacco Products Star Scientific, Inc. Part III Initiation and Cessation
 Low-Nitrosamine Dissolvable Tobacco Products Star Scientific, Inc. Part III Initiation and Cessation Using science to reduce tobacco-related harm at every level of the population 1 Summary - Parts I &
Low-Nitrosamine Dissolvable Tobacco Products Star Scientific, Inc. Part III Initiation and Cessation Using science to reduce tobacco-related harm at every level of the population 1 Summary - Parts I &
Welcome Superheros! YOUR. Winter Awareness Workshop 2017 BE A HERO IN COMMUNITY!
 Welcome Superheros! BE A HERO IN YOUR COMMUNITY! Winter Awareness Workshop 2017 WHAT ARE E-CIGARETTES /VAPING DEVICES? Devices that allow users to inhale aerosol containing nicotine or other substances.
Welcome Superheros! BE A HERO IN YOUR COMMUNITY! Winter Awareness Workshop 2017 WHAT ARE E-CIGARETTES /VAPING DEVICES? Devices that allow users to inhale aerosol containing nicotine or other substances.
Speakers. Speakers. Tackling Tobacco in California: New Laws Regulating Tobacco Sales 8/18/2016. Derek Carr Legal Fellow ChangeLab Solutions
 Tackling Tobacco in California: New Laws Regulating Tobacco Sales August 17, 2016 Speakers Derek Carr Legal Fellow ChangeLab Solutions Tonia Hagaman Chief Community and Statewide Interventions California
Tackling Tobacco in California: New Laws Regulating Tobacco Sales August 17, 2016 Speakers Derek Carr Legal Fellow ChangeLab Solutions Tonia Hagaman Chief Community and Statewide Interventions California
COMMENT PREPARED AFTER THE TPSAC MEETIING ON CAMEL SNUS. significantly reduce harm to individuals or benefit population health.
 COMMENT PREPARED AFTER THE TPSAC MEETIING ON CAMEL SNUS RJR failed to demonstrate that Camel Snus, as actually used by consumers, will significantly reduce harm to individuals or benefit population health.
COMMENT PREPARED AFTER THE TPSAC MEETIING ON CAMEL SNUS RJR failed to demonstrate that Camel Snus, as actually used by consumers, will significantly reduce harm to individuals or benefit population health.
Local Laws to Raise the Minimum Legal Sale Age for all Tobacco Products 21 Years of Age in the North Country Frequently Asked Questions
 Local Laws to Raise the Minimum Legal Sale Age for all Tobacco Products 21 Years of Age in the North Country Frequently Asked Questions It s Time to Clear the Air in the North Country Tobacco use remains
Local Laws to Raise the Minimum Legal Sale Age for all Tobacco Products 21 Years of Age in the North Country Frequently Asked Questions It s Time to Clear the Air in the North Country Tobacco use remains
COMPREHENSIVE TOBACCO-FREE SCHOOL POLICY
 TOBACCO AND TOBACCO PRODUCTS COMPREHENSIVE TOBACCO-FREE SCHOOL POLICY 1. INTENT All students shall possess the knowledge and skills necessary to avoid all tobacco use, and school leaders shall actively
TOBACCO AND TOBACCO PRODUCTS COMPREHENSIVE TOBACCO-FREE SCHOOL POLICY 1. INTENT All students shall possess the knowledge and skills necessary to avoid all tobacco use, and school leaders shall actively
6/2/2015. Welcome to VAPE U! Module V: Legal Challenges in the E-Liquid Market
 Welcome to VAPE U! Module V: Legal Challenges in the E-Liquid Market 1 Your Instructor: Azim Chowdhury, Esq. Specialist in food, drug, tobacco & e-vapor law. Spearheaded the FDA Tobacco and Electronic
Welcome to VAPE U! Module V: Legal Challenges in the E-Liquid Market 1 Your Instructor: Azim Chowdhury, Esq. Specialist in food, drug, tobacco & e-vapor law. Spearheaded the FDA Tobacco and Electronic
An Overview of the Government of Canada s Approach to Legalize, Regulate and Restrict Access to Cannabis
 An Overview of the Government of Canada s Approach to Legalize, Regulate and Restrict Access to Cannabis I m here today to provide 1 2 3 Context for the Government of Canada s plan to legalize, regulate
An Overview of the Government of Canada s Approach to Legalize, Regulate and Restrict Access to Cannabis I m here today to provide 1 2 3 Context for the Government of Canada s plan to legalize, regulate
UPDATE ON PREMARKET TOBACCO PRODUCT AUTHORIZATION PATHWAY
 UPDATE ON PREMARKET TOBACCO PRODUCT AUTHORIZATION PATHWAY Presented by Ii-Lun Chen, M.D Director Division of Individual Health Science Office of Science, CTP, FDA Disclaimer: This is not a formal dissemination
UPDATE ON PREMARKET TOBACCO PRODUCT AUTHORIZATION PATHWAY Presented by Ii-Lun Chen, M.D Director Division of Individual Health Science Office of Science, CTP, FDA Disclaimer: This is not a formal dissemination
West Milford Township Public Schools. Substance Abuse Policy and Regulation Review. and Vape Education for Parents. April 26, 2018
 West Milford Township Public Schools Substance Abuse Policy and Regulation Review and Vape Education for Parents April 26, 2018 Important to Note Consequences for policy violation include, but are not
West Milford Township Public Schools Substance Abuse Policy and Regulation Review and Vape Education for Parents April 26, 2018 Important to Note Consequences for policy violation include, but are not
Dissolvable Tobacco Products
 Dissolvable Tobacco Products James E. Dillard Senior Vice President, Regulatory Affairs Altria Client Services 1 l Altria Client Services (on behalf of PM USA and USSTC) I Presentation to the Tobacco Products
Dissolvable Tobacco Products James E. Dillard Senior Vice President, Regulatory Affairs Altria Client Services 1 l Altria Client Services (on behalf of PM USA and USSTC) I Presentation to the Tobacco Products
PUBLIC CONSULTATION DOCUMENT
 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate C Public Health and Risk Assessment POSSIBLE REVISION OF THE TOBACCO PRODUCTS DIRECTIVE 2001/37/EC PUBLIC CONSULTATION DOCUMENT
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate C Public Health and Risk Assessment POSSIBLE REVISION OF THE TOBACCO PRODUCTS DIRECTIVE 2001/37/EC PUBLIC CONSULTATION DOCUMENT
Evaluating Interventions to Curb ENDS Use Among Utah Youth
 Evaluating Interventions to Curb ENDS Use Among Utah Youth CDC CC Grant 2016-2017 Braden Ainsworth, MPH, Utah Department of Health Claudia Bohner, MPH, Utah Department of Health Erik Crankshaw, PhD, RTI
Evaluating Interventions to Curb ENDS Use Among Utah Youth CDC CC Grant 2016-2017 Braden Ainsworth, MPH, Utah Department of Health Claudia Bohner, MPH, Utah Department of Health Erik Crankshaw, PhD, RTI
E-Cigarettes and Vapes
 E-Cigarettes and Vapes What You Need to Know Education for Parents/Guardians Electronic Nicotine Delivery Device $3 Billion Global Industry 600 + Brands 8000+ Flavors & Liquids Less than a decade ago,
E-Cigarettes and Vapes What You Need to Know Education for Parents/Guardians Electronic Nicotine Delivery Device $3 Billion Global Industry 600 + Brands 8000+ Flavors & Liquids Less than a decade ago,
SIPP Phase II: Vaping and Vapor Devices. Subcommittee Briefing and Discussion
 SIPP Phase II: Vaping and Vapor Devices Subcommittee Briefing and Discussion August 19, 2015 Welcome and INTRODUCTIONS Subcommittee and Staff Sam Low (Lake Stevens City Council) Linda Grafer (Mukilteo
SIPP Phase II: Vaping and Vapor Devices Subcommittee Briefing and Discussion August 19, 2015 Welcome and INTRODUCTIONS Subcommittee and Staff Sam Low (Lake Stevens City Council) Linda Grafer (Mukilteo
VAPING PREVENTION PUBLIC SERVICE ANNOUNCEMENT CONTEST
 VAPING PREVENTION PUBLIC SERVICE ANNOUNCEMENT CONTEST OVERVIEW What is vaping and why are we doing this contest? Contest requirements Themes to include in your PSA Deadline and submission THANKS! This
VAPING PREVENTION PUBLIC SERVICE ANNOUNCEMENT CONTEST OVERVIEW What is vaping and why are we doing this contest? Contest requirements Themes to include in your PSA Deadline and submission THANKS! This
Tobacco Data, Prevention Spending, and the Toll of Tobacco Use in North Carolina
 Tobacco Data, Prevention Spending, and the Toll of Tobacco Use in North Carolina North Carolina Alliance for Health 2017 0 Table of Contents Highlights from the Surgeon General s Report on E-Cigarette
Tobacco Data, Prevention Spending, and the Toll of Tobacco Use in North Carolina North Carolina Alliance for Health 2017 0 Table of Contents Highlights from the Surgeon General s Report on E-Cigarette
Comprehensive approach to Nicotine: Misperceptions, Regulation and Science
 Comprehensive approach to Nicotine: Misperceptions, Regulation and Science The Public Health View Nicotine Tobacco Products contain many chemicals Hazard Index Analysis Chem Res Toxicol 25, 794, 2012 NICOTINE
Comprehensive approach to Nicotine: Misperceptions, Regulation and Science The Public Health View Nicotine Tobacco Products contain many chemicals Hazard Index Analysis Chem Res Toxicol 25, 794, 2012 NICOTINE
Marlboro Onserts: Another Colorful Addition to the Tobacco Regulatory Landscape
 Marlboro Onserts: Another Colorful Addition to the Tobacco Regulatory Landscape by Stacy Ehrlich and Will Woodlee O n June 17, 2010, the Food and Drug Administration (FDA) sent Philip Morris USA, Inc.,
Marlboro Onserts: Another Colorful Addition to the Tobacco Regulatory Landscape by Stacy Ehrlich and Will Woodlee O n June 17, 2010, the Food and Drug Administration (FDA) sent Philip Morris USA, Inc.,
CHERYL SBARRA, SENIOR STAFF ATTORNEY MASSACHUSETTS ASSOCIATION OF HEALTH BOARDS (MAHB) NATIONAL TOBACCO CONTROL CONFERENCE 2014 DECEMBER 2, 2014
 OUT OF THE BOX LOCAL POLICY CAMPAIGNS IN MASSACHUSETTS CHERYL SBARRA, SENIOR STAFF ATTORNEY MASSACHUSETTS ASSOCIATION OF HEALTH BOARDS (MAHB) NATIONAL TOBACCO CONTROL CONFERENCE 2014 DECEMBER 2, 2014 MODEL
OUT OF THE BOX LOCAL POLICY CAMPAIGNS IN MASSACHUSETTS CHERYL SBARRA, SENIOR STAFF ATTORNEY MASSACHUSETTS ASSOCIATION OF HEALTH BOARDS (MAHB) NATIONAL TOBACCO CONTROL CONFERENCE 2014 DECEMBER 2, 2014 MODEL
Dawn S. Berkowitz, MPH, CHES Director, DHMH Center for Tobacco Prevention and Control 10 th Annual MDQuit Best Practices
 Dawn S. Berkowitz, MPH, CHES Director, DHMH Center for Tobacco Prevention and Control Dawn.Berkowitz@Maryland.gov 10 th Annual MDQuit Best Practices Conference 1.21.16 Under 2% $9.6 Billion/Year Ranks
Dawn S. Berkowitz, MPH, CHES Director, DHMH Center for Tobacco Prevention and Control Dawn.Berkowitz@Maryland.gov 10 th Annual MDQuit Best Practices Conference 1.21.16 Under 2% $9.6 Billion/Year Ranks
Vontobel Viewpoints FDA New Direction on Tobacco Our Take
 Quality Growth Boutique TM Vontobel Viewpoints On July 28, 2017, the new Commissioner of the U.S. Food and Drug Administration (FDA), Scott Gottlieb, surprised the markets by launching a consultation paper
Quality Growth Boutique TM Vontobel Viewpoints On July 28, 2017, the new Commissioner of the U.S. Food and Drug Administration (FDA), Scott Gottlieb, surprised the markets by launching a consultation paper
e-cigarette Regulation
 e-cigarette Regulation The Act prohibits the sale of electronic smoking devices and alternative nicotine products to minors, and requires child-resistant packaging for liquid nicotine containers. The Act
e-cigarette Regulation The Act prohibits the sale of electronic smoking devices and alternative nicotine products to minors, and requires child-resistant packaging for liquid nicotine containers. The Act
THE IMPORTANCE OF POINT OF SALE
 THE IMPORTANCE OF POINT OF SALE Counter Tobacco Allison E. Myers, MPH Kurt M. Ribisl, PhD Adapted from a presentation given January 16, 2013 Office of Smoking and Health Centers for Disease Control and
THE IMPORTANCE OF POINT OF SALE Counter Tobacco Allison E. Myers, MPH Kurt M. Ribisl, PhD Adapted from a presentation given January 16, 2013 Office of Smoking and Health Centers for Disease Control and
Preventing Youth Electronic Cigarette Use: Partnering with Schools
 Preventing Youth Electronic Cigarette Use: Partnering with Schools Kris Minard Montana Office of Public Instruction Tobacco Use Prevention Education Kminard@mt.gov (406) 444-0785 I work for the State of
Preventing Youth Electronic Cigarette Use: Partnering with Schools Kris Minard Montana Office of Public Instruction Tobacco Use Prevention Education Kminard@mt.gov (406) 444-0785 I work for the State of
APPENDIX A: Tobacco Marketing Expenditure Categories
 APPENDIX A: Tobacco Marketing Expenditure Categories The Federal Trade Commission (FTC) issues reports on annual cigarette and smokeless tobacco marketing expenditures, which are based on data from the
APPENDIX A: Tobacco Marketing Expenditure Categories The Federal Trade Commission (FTC) issues reports on annual cigarette and smokeless tobacco marketing expenditures, which are based on data from the
Lindsey White. Tobacco Control Program Coordinator
 Lindsey White Tobacco Control Program Coordinator History Tobacco Targets Tobacco Products Dangers and Tobacco Tolls The Trust America s Brown Gold World War I and II NAACP Nicotine= Insecticide 50-70mg
Lindsey White Tobacco Control Program Coordinator History Tobacco Targets Tobacco Products Dangers and Tobacco Tolls The Trust America s Brown Gold World War I and II NAACP Nicotine= Insecticide 50-70mg
The concept that not all tobacco and nicotine products
 A Model Risk Continuum for Tobacco and Nicotine Products by Chris Proctor, Sudhanshu Patwardhan, and James Murphy The concept that not all tobacco and nicotine products present the same risks to human
A Model Risk Continuum for Tobacco and Nicotine Products by Chris Proctor, Sudhanshu Patwardhan, and James Murphy The concept that not all tobacco and nicotine products present the same risks to human
Global E-Cigarette Market- Industry Analysis & Outlook ( )
 Global E-Cigarette Market- Industry Analysis & Outlook (2016-2020) ----------------------------------------- Executive Summary Electronic cigarettes, also known as E-cigarettes, electronic nicotine delivery
Global E-Cigarette Market- Industry Analysis & Outlook (2016-2020) ----------------------------------------- Executive Summary Electronic cigarettes, also known as E-cigarettes, electronic nicotine delivery
Welcome to the wild, wild west.
 Welcome to the wild, wild west. E-Cigarettes in 2014 Adam Bramwell Utah Department of Health Right now, it s the wild, wild west. -Mitch Zeller Director of the FDA's Center for Tobacco Products The wild
Welcome to the wild, wild west. E-Cigarettes in 2014 Adam Bramwell Utah Department of Health Right now, it s the wild, wild west. -Mitch Zeller Director of the FDA's Center for Tobacco Products The wild
The Tobacco Control Act Four Years Later: Living Up to its Promise? June 24, 2013
 The Tobacco Control Act Four Years Later: Living Up to its Promise? June 24, 2013 Tobacco Control Legal Consortium Webinar Series Providing substantive public health policy knowledge, competencies & research
The Tobacco Control Act Four Years Later: Living Up to its Promise? June 24, 2013 Tobacco Control Legal Consortium Webinar Series Providing substantive public health policy knowledge, competencies & research
Public Health Promise or Peril?
 Public Health Promise or Peril? The Rise of Electronic Tobacco Products and Implications for Tobacco Control Policy and Practice BRIAN A. KING, PHD, MPH DEPUTY DIRECTOR FOR RESEARCH TRANSLATION OFFICE
Public Health Promise or Peril? The Rise of Electronic Tobacco Products and Implications for Tobacco Control Policy and Practice BRIAN A. KING, PHD, MPH DEPUTY DIRECTOR FOR RESEARCH TRANSLATION OFFICE
CBD-BASED PHARMACEUTICAL & CONSUMER PRODUCTS. Corporate Presentation August 23, 2018 Symbol: CVSI
 CBD-BASED PHARMACEUTICAL & CONSUMER PRODUCTS Corporate Presentation August 23, 2018 Symbol: CVSI SAFE HARBOR & DISCLAIMER This presentation may contain certain forward-looking statements and information,
CBD-BASED PHARMACEUTICAL & CONSUMER PRODUCTS Corporate Presentation August 23, 2018 Symbol: CVSI SAFE HARBOR & DISCLAIMER This presentation may contain certain forward-looking statements and information,
Tobacco & Mississippi Youth
 Tobacco & Mississippi Youth Youth Tobacco Use Where we are now: 1998-2012 - Early current tobacco use decreases have leveled off in recent years - Why? Education & community activities Taxes & policies
Tobacco & Mississippi Youth Youth Tobacco Use Where we are now: 1998-2012 - Early current tobacco use decreases have leveled off in recent years - Why? Education & community activities Taxes & policies
Why do Youth Use Tobacco?
 Teens and Tobacco Why do Youth Use Tobacco? Compare your answers how close did you get to the following list? -Social influences Friends Peer pressure / fit in -Parents access to cigarettes attitude toward
Teens and Tobacco Why do Youth Use Tobacco? Compare your answers how close did you get to the following list? -Social influences Friends Peer pressure / fit in -Parents access to cigarettes attitude toward
Public Health Policy Change Series
 Public Health Policy Change Series BEYOND CIGARETTES: FEDERAL REGULATION OF OTHER TOBACCO PRODUCTS Public Health Policy Change Webinar Series Providing substantive public health policy knowledge, competencies
Public Health Policy Change Series BEYOND CIGARETTES: FEDERAL REGULATION OF OTHER TOBACCO PRODUCTS Public Health Policy Change Webinar Series Providing substantive public health policy knowledge, competencies
City of Chicago O Office of the City Clerk. Document Tracking Sheet
 City of Chicago Office of the City Clerk O2018-7371 Document Tracking Sheet Meeting Date: Sponsor(s): Type: Title: Comrnittee(s) Assignment: 9/20/2018 Dept./Agency Ordinance Amendment of Municipal Code
City of Chicago Office of the City Clerk O2018-7371 Document Tracking Sheet Meeting Date: Sponsor(s): Type: Title: Comrnittee(s) Assignment: 9/20/2018 Dept./Agency Ordinance Amendment of Municipal Code
REGULATIONS OF THE PLYMOUTH BOARD OF HEALTH FOR TOBACCO SALES IN CERTAIN PLACES & SALE OF TOBACCO PRODUCTS TO MINORS
 REGULATIONS OF THE PLYMOUTH BOARD OF HEALTH FOR TOBACCO SALES IN CERTAIN PLACES & SALE OF TOBACCO PRODUCTS TO MINORS A. Statement of Purpose: Whereas there exists conclusive evidence that tobacco smoke
REGULATIONS OF THE PLYMOUTH BOARD OF HEALTH FOR TOBACCO SALES IN CERTAIN PLACES & SALE OF TOBACCO PRODUCTS TO MINORS A. Statement of Purpose: Whereas there exists conclusive evidence that tobacco smoke
FDLI Tobacco Products Regulation and Policy Conference Joe Murillo Vice President, Regulatory Affairs October 27, 2017
 FDLI Tobacco Products Regulation and Policy Conference Joe Murillo Vice President, Regulatory Affairs October 27, 2017 Altria Client Services Vice President, Regulatory Affairs FDLI Presentation Oct. 27,
FDLI Tobacco Products Regulation and Policy Conference Joe Murillo Vice President, Regulatory Affairs October 27, 2017 Altria Client Services Vice President, Regulatory Affairs FDLI Presentation Oct. 27,
Electronic Nicotine and Non Nicotine Delivery Systems (ENDS)
 Electronic Nicotine and Non Nicotine Delivery Systems (ENDS) Dr Meena Dawar Medical Health Officer 30 September 2014 1 VCH MHO Recommendations 1. Include e-cigarettes and related ENDS devices in the City
Electronic Nicotine and Non Nicotine Delivery Systems (ENDS) Dr Meena Dawar Medical Health Officer 30 September 2014 1 VCH MHO Recommendations 1. Include e-cigarettes and related ENDS devices in the City
News From the Front: State & Local Regulation of E-Cigarettes
 News From the Front: State & Local Regulation of E-Cigarettes We will begin shortly after 12:00pm Central. Until then you will not hear any audio. Legislation and Advocacy Susan Weisman & Mark Meaney,
News From the Front: State & Local Regulation of E-Cigarettes We will begin shortly after 12:00pm Central. Until then you will not hear any audio. Legislation and Advocacy Susan Weisman & Mark Meaney,
British American Tobacco Snus Marketing Standards
 British American Tobacco Snus Marketing Standards British American Tobacco p.l.c. believes there is sufficient scientific evidence to support a less restrictive regime for the advertising and promotion
British American Tobacco Snus Marketing Standards British American Tobacco p.l.c. believes there is sufficient scientific evidence to support a less restrictive regime for the advertising and promotion
Menthol cigarettes are a public health problem
 Menthol cigarettes are a public health problem ANDREA VILLANTI, PHD MPH JUNE 22, 2016 disclosures Andrea Director The Adjunct Department Johns Villanti, PhD, MPH, CHES for Regulatory Science and Policy
Menthol cigarettes are a public health problem ANDREA VILLANTI, PHD MPH JUNE 22, 2016 disclosures Andrea Director The Adjunct Department Johns Villanti, PhD, MPH, CHES for Regulatory Science and Policy
Welcome to the New England QIN-QIO Webinar: The Truth About E-Cigarettes
 Welcome to the New England QIN-QIO Webinar: The Truth About E-Cigarettes Thank you for joining. Our presentation will begin shortly. If you haven t already, please dial in to the audio line: 888-895-6448
Welcome to the New England QIN-QIO Webinar: The Truth About E-Cigarettes Thank you for joining. Our presentation will begin shortly. If you haven t already, please dial in to the audio line: 888-895-6448
