TRANSLATIONAL RESEARCH SECTION
|
|
|
- Zoe Payne
- 5 years ago
- Views:
Transcription
1 Pain Medicine 2011; 12: Wiley Periodicals, Inc. TRANSLATIONAL RESEARCH SECTION Original Research Article Intravenous Injection of Leconotide, an Omega Conotoxin: Synergistic Antihyperalgesic Effects with Morphine in a Rat Model of Bone Cancer Painpme_ Anton Kolosov, PhD,* Lucia Aurini, MD,* Elizabeth D. Williams, PhD, Ian Cooke, PhD, and Colin S. Goodchild, MA, MB, Bchir, PhD, FRCA, FANZCA, FFPMANZCA* *Laboratory for Pain Medicine and Palliative Care, Monash Institute of Medical Research; Centre for Cancer Research, Monash Institute of Medical Research; Office of the Deputy Vice-Chancellor (Research), Administration, Monash University, Clayton, Victoria, Australia Reprint requests to: Anton Kolosov, PhD, Laboratory for Pain Medicine & Palliative Care, Monash Institute of Medical Research, Wright Street, Clayton, Victoria 3168, Australia. Tel: ; Fax: ; anton.kolosov@monash.edu. Abstract Objective. Leconotide (CVID, AM336, CNSB004) is an omega conopeptide similar to ziconotide, which blocks voltage sensitive calcium channels. However, unlike ziconotide, which must be administered intrathecally, leconotide can be given intravenously because it is less toxic. This study investigated the antihyperalgesic potency of leconotide given intravenously alone and in combinations with morphineadministered intraperitoneally, in a rat model of bone cancer pain. Design. Syngeneic rat prostate cancer cells AT3B-1 were injected into one tibia of male Wistar rats. The tumor expanded within the bone causing hyperalgesia to heat applied to the ipsilateral hind paw. Measurements were made of the maximum dose (MD) of morphine and leconotide given alone and in combinations that caused no effect in an open-field activity monitor, rotarod, and blood pressure and heart rate measurements. Paw withdrawal thresholds from noxious heat were measured. Dose response curves for morphine ( mg/kg intraperitoneal) and leconotide ( mg/kg intravenous) given alone were plotted and responses compared with those caused by morphine and leconotide in combinations. Results. Leconotide caused minimal antihyperalgesic effects when administered alone. Morphine given alone intraperitoneally caused dose-related antihyperalgesic effects (ED 50 = mg/kg), which were increased by coadministration of leconotide 20 mg/kg (morphine ED 50 = mg/kg); 0.2 mg/kg (morphine ED 50 = mg/kg); and 0.02 mg/kg (morphine ED 50 = mg/kg). Conclusions. Leconotide caused a significant increase in reversal by morphine of the bone cancerinduced hyperalgesia without increasing the side effect profile of either drug. Clinical Implication. Translation into clinical practice of the method of analgesia described here will improve the quantity and quality of analgesia in patients with bone metastases. The use of an ordinary parenteral route for administration of the calcium channel blocker (leconotide) at low dose opens up the technique to large numbers of patients who could not have an intrathecal catheter for drug administration. Furthermore, the potentiating synergistic effect with morphine on hyperalgesia without increased side effects will lead to greater analgesia with improved quality of life. Key Words. w-conopeptide; Bone Cancer Pain; Hyperalgesia; Ca 2+ -Channels; Synergy; Morphine; Intravenous Injection Introduction As the world population ages, an increasing number of diagnosed malignancies is reported every year [1 3]. The most common tumors have a strong predilection for 923
2 Kolosov et al. bone metastasis and tumor metastases to the skeleton are major contributors to morbidity and mortality in metastatic cancer [4,5]. Cancer-induced bone pain is the major manifestation of the bone metastasis. Unfortunately, current treatment options are severely limited, as most commonly utilized drugs induce inadequate pain relief limited by dose-related side effects. Opioids, the most routinely used drugs in cancer pain management, include morphine, fentanyl, and oxycodone. They can be useful in controlling breakthrough (episodic) pain, but with continuous use, the ability of opioid drugs to relieve pain is markedly reduced, while their side effects are significantly increased [6,7]. Recently, a number of elements in peripheral and central nervous systems (CNSs) that play important roles in induction and maintenance of cancer-related bone pain and subsequent CNS sensitization have been identified. One such target, voltage-gated calcium channels (VGCCs) are well established as modulators of pain and nociception [8,9]. These channels are selectively targeted by w-conotoxins. Over the last 20 years, w-conotoxins MVIIA (ziconotide), GVIA, and leconotide (CVID) have been studied for therapeutic potential in neuropathic pain states. MVIIA and GVIA administered intrathecally cause antinociception in acute, chronic, and neuropathic pain animal models [10]. This line of research has led to the introduction into clinical practice of ziconotide as an intrathecally administered analgesic for severe pain [11 13]. This drug is administered intrathecally in an attempt to target selectively Cav2.2 channels in the spinal cord associated with pain and nociception because some serious side effects have been found to be associated with peripheral autonomic dysfunction after administration of drugs of this type [14 17]. Leconotide is a similar molecule with equal potency to MVIIA but with much higher specificity for sensory compared with sympathetic neuronal calcium channels, and therefore it is less toxic than ziconotide [18,19]. It has been shown that leconotide can penetrate the CNS even when given by non-spinal, parenteral routes of administration (e.g., intravenously [IV]) [20,21]. All this evidence suggests not only that leconotide is a suitable candidate for treatment of persistent pain including cancer-induced bone pain, either as a single drug or as an adjunct analgesic to an existing therapeutic option, but also that it might be administered IV. The intravenous route is clearly far more amenable to widespread clinical use in the management of cancer pain. However, it remains to be seen if there is a significant therapeutic window between side effects caused by the drug when given IV and the desired effect on pain and nociception. The study described in this article utilized the model of direct intratibial rat prostate cancer inoculation with a view of establishing: 1) whether intravenous injection of the novel selective N-type calcium channel (Cav 2.2) blocker toxin leconotide could diminish cancer-induced bone pain; 2) whether coadministration of the leconotide could improve morphine analgesia and also to test if the combination of the two compounds could produce a superior antinociceptive effect compared with either drug used alone with the added benefit of decreased toxicity and sedation; and 3) whether intravenous administration of leconotide in this way caused adverse cardiovascular or CNS side effects at doses used to affect nociceptive behavior. Methods All surgical procedures for intratibial rat prostate cancer cell inoculations were performed on male Wistar rats weighed g. Experiments investigating sedation, cardiovascular effects of the drugs, and antinociception using the heat plantar test paradigm were performed 2 weeks later on the same tumor-bearing male Wistar rats weighed g. This work was carried out with permission of the Monash Medical Centre Committee B on Ethics in Animal Experimentation (Project MMCB 2006/37). In all experiments, attention was paid to ethical guidelines for the investigation of experimental pain in conscious animals and recommendations from the Code of practice for the use of animals from municipal pounds in scientific procedures from the Bureau of Animal Welfare of Victoria, Australia [22,23]. The experiments were performed in an observerblinded fashion with parallel negative placebo (saline vehicle) treatment controls. All drug solutions and vehicle were given either IV in a volume of 100 ml or intraperitoneally (ip) in a volume of 1.0 ml. The maximum doses (MDs) for drugs administered alone and in combinations that caused no effects on blood pressure, heart rate, exploratory activity, or ability to successfully negotiate the rotarod test were defined, and then ranges of doses up to and including those were tested for antihyperalgesia. Materials The AT3B-1 prostate cancer cell line (catalog number CRL-2375) was obtained from American Type Culture Collection (Manassas, VA, USA). The cell line was stored in liquid nitrogen and propagated from frozen stock. DBL Morphine sulfate for injections (Hospira Australia Pty Ltd, Melbourne, Victoria, Australia) in ampoules, saline, and sterile water for injection (Pfizer Australia Pty Ltd, NSW, Australia) were obtained from the pharmacy at Monash Medical Centre. Leconotide (CNSB004, formerly AM336) was obtained by courtesy of CNSBio Pty (Abbotsford, Victoria, Australia) from AMRAD Ltd, later known as Zenyth Therapeutics Ltd (Richmond, Victoria, Australia) as a di-l- tartrate salt. Intravenous catheters were made from lengths of nylon catheter (inner diamater [I.D.] 0.28 mm, outer diameter [O.D.] 0.61 mm). Male Wistar rats were supplied as specified pathogen-free HsdBrlHan:WIST strain by Monash University Animal Services, Clayton. All rats were housed in high-topped cages in groups of four per cage in a temperature controlled room with a 12h-12h light-dark cycle. The rats were allowed food and water ad libitum. Of these, 278 rats had their right tibia inoculated with rat prostate cancer cells, and 46 rats had their right 924
3 Intravenous Leconotide Potentiates Intraperitoneal Morphine tibia injected with phosphate buffered saline (PBS; cell injection vehicle) as age and weight-matched controls. Cell Culture The AT3B-1 rat prostate cancer cells were propagated in T-75 plastic flasks (Greiner Bio-One, Interpath Services Pty. Ltd, Melbourne, Australia), grown in customized RPMI-1640 medium (Invitrogen) with 2 mm L-glutamine supplemented with 10% fetal bovine serum (Invitrogen, Melbourne, Australia) and cultured in a water-saturated incubator in an atmosphere of 5% CO2:95% air. For propagation, cells were detached by rinsing gently with calcium-free and magnesium-free PBS solution, followed by a solution containing 0.25% trypsin and 0.03% EDTA. For injection, the detached cells were first collected by centrifuging 12 ml of medium for 4 minutes. After careful aspiration and discarding the supernatant, the resulting pellet of cells was resuspended in 4 ml of PBS solution for injections (with calcium and magnesium). The cells were counted using a hemocytometer. After the final pellet was recentrifuged for 4 minutes, cells were diluted at final concentration of cells/10 ml PBS solution for injection and kept on ice until injected into rats on that day. Bone Cancer-Induced Nociception The methods used in this model were described previously by Medhurst et al. [24] and Zhang et al. [25]. The experimental paradigm used in this study is shown in Figure 1. Surgical Procedures Male Wistar rats, weighing g, were anesthetized with halothane (0 5%) in oxygen. Following loss of consciousness and righting reflex, each rat was placed supine on a heated animal operating table. The administration of gaseous anesthetic continued via facemask (2 2.5%) with suitable scavenging to minimize pollution and staff exposure to the anesthetic agent. The right hind leg of the rat was shaved and the skin was disinfected with 70% ethanol. A 1-cm-long rostro-caudal incision was made in the skin over the upper medial half of the tibia. The tibia was carefully exposed with minimal damage to the muscle. Using a 23-gauge needle, the bone was pierced 5 mm below the knee joint medial to the tibial tuberosity. The needle was then replaced with a 50-mL Hamilton syringe (SDR Clinical Technology, Sydney, Australia) containing the cells to be injected. A 10-mL volume of prostate cancer cells ( cells) or vehicle (PBS solution only) Figure 1 Protocol used in the rat model of prostate cancer induced bone pain (please see explanations in the text). IV = intravenously; ip = intraperitoneally; PBS = phosphate buffered saline. 925
4 Kolosov et al. was injected into the bone cavity. Immediately following the injection, the syringe was removed, and the injection site was closed using bone wax (Ethicon; Reckitt Benckiser Pty Ltd, Melbourne, Australia). The wound was then irrigated with sterile water to prevent contamination of the surrounding tissues with live cancer cells. The muscle was sutured, the skin wound closed using 2 0 silk suture, and double antibiotic mixture (benzylpenicillin-gentamicin) was injected subcutaneously in a site opposite the surgical incision. The surgical wound was infiltrated with 2% lidocaine, and the general anaesthetic was discontinued. Upon recovery, the animal was returned to its cage. Each rat was monitored for general condition and changes in body weight during the 20-day experiment. Pilot Experiments A pilot series of experiments were performed to assess which stimulus and test of hyperalgesia (von Frey filament, paw pressure, or plantar heat) was most suitable in this model. Nociceptive thresholds were measured in three groups of rats that had been treated with a sham operation on the leg (skin incision only; N = 9), intratibial injection of buffer only (PBS controls; N = 9), or intratibial injection of prostate cancer cells (cancer; N = 8). Three methods were used to assess nociceptive thresholds: 1) paw withdrawal from noxious heat (Hargreaves method as described below) [26]; 2) paw withdrawal from a von Frey filament as described previously in this model [25]; and 3) paw withdrawal from plantar pressure using an Apelex probe (Randall- Selitto method [27]). Nociceptive thresholds were measured in each rat in each treatment group using all three tests, at 6, 10, 13, 17, 20, and 23 days after the treatment. The results for each test were combined for each day of testing and plotted as histograms (means, standard error of the mean [SEM]). Differences between treatments were tested statistically using Kruskall Wallis nonparametric test with Dunn post hoc correction (Figure 2). Thermal Hyperalgesia The pilot experiments showed that the paw withdrawal from the noxious heat nociception paradigm revealed the most consistent and significant hyperalgesic effects in cancer treatment compared with sham and PBS controls. Therefore, in the experiments reported in this article, hyperalgesia was assessed by measurement of ipsilateral paw flick latency (PFL) using the radiant heat plantar test described previously by Hargreaves et al. [26]. Tests of antihyperalgesia took place 14 days after the prostate cancer cells inoculation when the rats had grown to weigh g (Figure 1). They were placed into clear plastic chambers on the glass surface of the Ugo Basile Plantar Test Apparatus (IR 50; Ugo Basile, Comerio VA, Italy) to acclimatize for 20 minutes before the test. A radiant heat stimulus was applied to the plantar surface of hind paw ipsilateral to cancer treatment with a projector lamp bulb (IR 50) beneath the glass floor. PFL (time between start of heat application and paw withdrawal) was automatically recorded when the rat withdrew its paw from the stimulus. A 20-second cutoff was used to prevent tissue damage. Animals that had a PFL equal to or below 8.5 seconds (at least 25% less than the value in PBS-injected agematched and weight-matched rats) were deemed to have developed hyperalgesia/neuropathic pain, and thus used in further experiments to assess drug effects; this was >85% of cancer-treated rats. Up to five experiments involving drug or vehicle placebo injection were performed on each tumor cell inoculated rat, one per day on successive days. The investigator who performed the behavioral tests was unaware of the nature of the treatment, drug, or vehicle. Intravenous Cannulation Two weeks post-inoculation of tumor cells, all rats were anesthetized using inhalation of halothane (0 5% in oxygen). Following loss of consciousness and righting reflex, each rat was placed supine on a heated animal operating table. The administration of gaseous anesthetic continued via facemask with suitable scavenging to minimize pollution and staff exposure to the anesthetic agent. The hair from the skin of the neck (both dorsum and ventrum) was removed, and a small incision was made over the right ventral side of the neck to expose the jugular vein under aseptic conditions. Ligatures were placed around the vein, and an intravenous catheter (I.D mm, O.D mm) was inserted into the vein centrally for 1.5 cm. This was secured in the vein, and then tunnelled subcutaneously to an exit wound in the dorsum of the neck where the catheter was secured with sutures. Wounds were infiltrated with local anesthetic (2% lignocaine) and sutured prior to recovery from anesthesia. Upon recovery, 0.4-mL subcutaneous injection of antibiotic mix (600 mg/kg benzylpenicillin and 80 mg/kg gentamicin diluted in sterile water) was given to each rat. The rats were allowed 24-hour recovery prior to nociception testing. Determination of MDs That Caused No Effects in Cardiovascular, Open Field, and Rotarod Tests Prior to investigation of the antihyperalgesic effects of leconotide administered IV alone and in combinations with intraperitoneal injections of morphine, experiments were performed on tumor cell inoculated rats to define the doses of each drug given alone and in combinations that did not cause nonspecific depressant effects on cardiovascular or CNS (MD). This was achieved in three ways. First, all rats were observed for general behavior by an experimentor who is unaware of the nature of the drug treatment. Second, signs of sedation or CNS depression were assessed by measurement of exploratory activity of rats allowed to move freely in an open field activity monitor. Each rat was subjected to this test on only one occasion to avoid habituation to the test. The rotarod test was also utilized to test for CNS depression, loss of coordination, and stamina for the selected doses of drugs alone and in combination if observed animal behavior fell in the category of suspected sedation, but the sedation was not detected by the open field activity monitor (as in the case with one dose of intraperitoneal morphine). Third, groups of rats treated with each drug and drug combination had 926
5 Intravenous Leconotide Potentiates Intraperitoneal Morphine Figure 2 Results from pilot experiments. Nociceptive thresholds were measured in three groups of rats that had been treated with a sham operation on the leg (skin incision only; N = 9), intratibial injection of buffer only (PBS controls; N = 9), or intratibial injection of prostate cancer cells (cancer; N = 8). Three methods were used to assess nociceptive thresholds: (A) paw withdrawal from noxious heat; (B) paw withdrawal from a von Frey filament; and (C) paw withdrawal from plantar pressure using an Apelex probe (Randall Selitto method). Nociceptive thresholds were measured in each rat in each treatment group using all three tests, on day 6, 10, 13, 17, 20, and 23 days after the treatment. The results for each test were combined for each day of testing and plotted as histograms (means) with SEM (bars). Differences between treatments were tested statistically using the Kruskall Wallis test with Dunn post hoc correction. These revealed that there were no statistically significant differences between sham and PBS treatments at any time and with any of the three nociceptive tests. At one testing time only was there a statistically significant difference between cancer and PBS treatment groups when the von Frey test for allodynia was used, at 6 days posttreatment and at no testing time thereafter. The most consistent and sustained differences between cancer treatment compared with PBS controls was when the nociceptive test used was paw flick from noxious heat (P < 0.05 at 13, 17, and 20 days; P < 0.01 at 23 days). The Randall Selitto method did not show any significant hyperalgesia differences between treatments at any testing time. PBS = phosphate buffered saline; SEM = standard error of the mean. blood pressure and heart rate measurements to find out whether any of the doses or dose combinations caused adverse cardiovascular effects. General Observations General behavioural observations were made. In particular, the rats were observed for startle responses, lethargy, head shakes, and writhing or serpentine movements. Rats were also assessed for general exploratory activity and muscle tone/evading behavior prior to testing in the apparatus. The rats were also inspected daily for signs of spread of the tumor outside the tibia causing fractures or induration and swelling in the skin indicative of skin infiltration. Rats with these signs were killed with an overdose of anesthetic. 927
6 Kolosov et al. Open Field Activity The open field activity monitor (MedAssociates Inc., St. Albans, VT, USA) is an enclosure with a quiet and darkened environment. It contains 16 intersecting beams of infrared light. Interruptions of beams by individual rats moving around the enclosure were recorded by a computer. The time when no beams were interrupted (measured in seconds) during a 20-minute period in the apparatus was called the time of low locomotor activity (LMA). This test has been used in the past to reveal differences in CNS depression caused by neuroactive steroid anesthetics and sedatives as well as differences in sedating properties of omega-conotoxins leconotide and ziconotide [28,29]. Animals naïve to drug treatment were acclimatized to the laboratory environment, and individual cancer-treated rats were selected randomly from a group one at a time. They were injected (ip or IV; see below) with saline, a test drug dose, or a combination of drugs in an operator-blinded fashion. Five minutes after the drug injection, each rat was placed in the open field activity monitor for a 20-minute period. This timing was chosen because preliminary experiments revealed that exploratory activity in the apparatus occurs during the initial 20 minutes only. After 20 minutes in the activity box, the amount of exploratory activity even by untreated normal rats falls to low levels; the time of LMA increases. Thus, the rats were placed in the activity box 5 minutes after drug dosing to allow for drug absorption and stabilization. LMAs were combined for each group of rats that were treated similarly with drug or drug combinations to calculate means (SEM). These were compared with saline-treated controls using one-way analysis of variance (ANOVA) with Dunnett s post hoc test. Groups of rats were tested with the open field activity monitor as above with the following treatments: saline controls (IV + ip), N = 58; leconotide 200 mg/kg IV, N = 10; leconotide 20 mg/kg IV, N = 10; morphine 20 mg/kg ip, N = 12; morphine 10 mg/kg ip, N = 10; morphine 2.5 mg/kg ip + leconotide 200 mg/kg IV, N = 10; morphine 1.25 mg/kg ip + leconotide 200 mg/kg IV, N = 10; and morphine 2.5 mg/kg ip + leconotide 20 mg/kg IV, N = 10. Rotarod Test The rotarod accelerator treadmill (7,650 accelerator rotarod, Ugo Basile) is a rotating drum with gripping surfaces for rats to run on. It consists of four compartments separated by non-transparent walls, which prevent animals from turning on the apparatus excessively. This test has been used in the past to reveal differences in CNS depression caused by neuroactive steroid anesthetics and sedatives as well as differences in sedating properties of omega-conotoxins leconotide and ziconotide [28]. On day 1, rats naïve to the apparatus and the drug treatments were placed on the rotarod set at the minimum rotating speed for two training sessions of 15 minutes separated by an interval of 120 minutes. After the conditioning period, on day 2, an injection (ip or IV) of vehicle or drug was given. Five minutes later, the animals were placed onto the rotarod at a constant speed of 4 rpm. As the animal took grip of the drum, the accelerator mode was switched on, i.e., the rotation rate of the drum was increased linearly at the rate of 20 rpm every minute thereafter. The time was measured from the start of the acceleration period until the rat fell off the apparatus. The cutoff or maximum run time for the test was 2 minutes. Non-sedated rats can all run for 2 minutes at the accelerated speed. This test was performed on each rat for 60 minutes after drug or vehicle injection with intervals between tests of 10 minutes. Run times were combined for each group of rats that were drug-treated to calculate means (SEM). These were compared with saline-treated controls using ANOVA with Dunnett s post hoc test. These comparisons allowed definition of drug doses that caused loss of coordination and stamina in rats, the maximum dose that could be used without causing sedation, as defined by a run time equal to vehicle controls. Groups of rats were tested with the rotarod with the following treatments: saline ip, N = 10; morphine 10 mg/kg ip, N = 10; and morphine 5 mg/kg ip, N = 10. This was done because rats that received morphine 10 mg/kg were sedated on general behavioral observation by the blinded observer but scored normally with the open field activity test. Other drugs and doses were not tested in that way because there was no inconsistency between open field activity test and general behavioral observations. This was to ensure that only non-sedating doses were tested subsequently for antihyperalgesia. Cardiovascular Measurements The indirect tail cuff method of blood pressure measurement is commonly used to investigate the cardiovascular side effect profile of compounds used in CNS modulation (e.g., various analgesics, anesthetics, sedatives, antidepressants) [30]. A good correlation between values obtained with the indirect tail-cuff method and values measured directly with indwelling carotid arterial cannulae has been documented [31,32]. Systolic and diastolic blood pressure and heart rate were measured using a noninvasive tail cuff blood pressure recorder with piezo-ceramic pulse detection (BP Recorders series 58000; Ugo Basile, Via G. Borghi 43, Comerio VA Italy). These parameters were measured every 5 minutes for 10 minutes before and 60 minutes after the following drug treatments: 928
7 Intravenous Leconotide Potentiates Intraperitoneal Morphine saline controls (IV + ip), N = 7; leconotide 200 mg/kg IV, N = 9; morphine 10 mg/kg ip, N = 5; morphine 5 mg/kg ip + leconotide 20 mg/kg IV, N = 6; and morphine 2.5 mg/kg ip + leconotide 20 mg/kg IV, N = 5. Values for each parameter and testing time were combined for each treatment and expressed as means (SEM). Statistical comparisons with saline controls were made using repeated measures ANOVA with Dunnett correction. Nociception Testing After the development of hyperalgesia in cancer-treated animals was confirmed by the radiant heat plantar test, more detailed nociception testing paradigms were carried out in hyperalgesic animals and PBS controls (weight g). The age-matched and weight- matched PBS control animals were used for the same plantar heat tests in parallel with cancer-treated rats. They were subjected to handling and restraint, but they received no intravenous or ip injections. PFL was measured by the method described by Hargreaves et al. [26] using the Ugo Basile Plantar Test Apparatus that allowed assessment of hyperalgesia in freely moving rats. An intense thermal stimulus was applied to the right hind paw until paw withdrawal was elicited or a maximum cutoff time of 20 seconds was reached. Paw withdrawal thresholds were measured as shown in Figure 3 in groups of rats 20 minutes and 10 minutes before, immediately before (time 0) and also at 10-minute intervals for a further 60 minutes after the following treatments: PBS treated, age-matched and weight-matched controls, N = 46; saline controls, N = 41; morphine mg/kg ip, N = 7 9 each; leconotide mg/kg IV, N = 7 17 each; two drug injections combination of leconotide at fixed dose of 20 mg/kg IV combined with morphine doses 0.04, 0.16, 0.31, 0.63, 1.25, and 2.5 mg/kg ip, N = 5 10 each; two drug injections combination of leconotide at fixed dose of 0.2 mg/kg IV combined with morphine doses 0.04, 0.16, 0.31, 0.63, 1.25, and 2.5 mg/kg ip, N = 7 16 each; and two drug injections combination of leconotide at fixed dose of 0.02 mg/kg IV combined with morphine doses 0.04, 0.16, 0.63, 1.25, and 5 mg/kg ip, N = 7 11 each. Each rat had PFL measurements performed every 10 minutes as shown in Figures 1 and 3. Except for the PBS controls, each rat received two drug or saline injections at points shown by the arrows in Figure 3: a 100 ml IV and also a 1.0 ml ip injections were given while the rat was confined closely in a conical plexiglass restrainer. Control rats received saline by both routes, and rats treated with ip morphine alone had intravenous saline as well. Rats that received intravenous conopeptide injections as the sole drug also had ip saline. All of these treatments were performed in an observer-blinded fashion. The PFL values measured in seconds were combined for each time point (-20, -10, 0, 10, 20, 30, 40, 50, and 60 minutes) in all individuals in a treatment group. These were expressed as means (SEM) and plotted on graphs against time of measurement (time response curves). This was done for the PBS-treated rats, saline controls, and for the rats that received injections of the highest MD or dose combinations of drugs. This procedure revealed the timing of the onset and course of responses that would allow valid calculations of drug responses for building dose response curves. The percentage reversal of bone cancer-induced hyperalgesia (calculated as shown in Equation 1) in each experiment was combined with other replicate values from rats treated similarly (saline controls, morphine alone, leconotide alone, and drug combinations) to calculate means (SEM). These were plotted as dose response curves for drugs administered alone and in combinations in GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA, Drug and saline treatments were compared statistically using ANOVA with the Tukey Kramer post hoc test. % reversal of hyperalgesia { mean of 40, 50, and 60 min readings} { mean of hyperalgesic baseline, 20, 10, and 0 min readings} = 100 {( mean of basline readings from PBS controls) ( mean of hyperalgesic baseline, 20, 10, and 0 min readings)} The values that were averaged to calculate the mean hyperalgesic baseline in Equation 1 were the values measured at 20, 10, and 0 minutes before the intravenous and ip injections as shown in Figure 3. The mean of test drug effect in Equation 1 was calculated from averaging the 40-, 50-, and 60-minute values (see Figure 3). The mean paw withdrawal threshold for the age-matched and weight-matched PBS treated rats used in Equation 1 was seconds (Figure 3). Dose Response Curves The measurement values for percentage reversal of hyperalgesia (mean, SEM for each treatment group) were plotted on graphs of dose vs response alongside curves generated from regression analysis. The results for morphine alone and the three combinations of morphine with leconotide 20, 0.2 and 0.02 mg/kg were subjected to regression analysis using GraphPad Prism (version 5.00 for Windows). The data were fitted to a sigmoidal dose response model with parameters as shown in Equation 2 with the top of the curve set at 100% reversal of hyperalgesia and the bottom set at the response in the saline treated group (9.3% reversal of hyperalgesia). The adequacy of curve fit was tested statistically using the (1) 929
8 Kolosov et al. Figure 3 Time response curves for the paw withdrawal latencies of the highest doses (MDs) of each drug and drug combination compared with PBS age-matched and saline controls: (a) leconotide 20 mg/kg IV; (b) morphine 5 mg/kg ip; (c) leconotide 20 mg/kg IV in combination with morphine 2.5 mg/kg ip. These graphs also illustrate the timing of nociception testing every 10 minutes with paw withdrawal from noxious heat, and also the drug and saline injections given at the arrows between time points 0 and 10 minutes. The PBS-injected controls were handled and restrained in exactly the same manner as the drug and saline-treated canceraffected rats but they did not receive any injections. All other rats received an intravenous bolus injection of either saline or conopeptide given at the open arrow. This was performed under close restraint in a plexiglass conical rat restrainer. The rats were then released and given an intraperitoneal injection of morphine or saline shown by the solid arrow. All of this was done in an observer-blinded fashion. Thereafter, the rats were replaced in the paw withdrawal apparatus in which paw withdrawal from noxious heat was measured every 10 minutes. Values shown here are means (SEM). It can be seen that all rats in drug and saline groups had stable baseline readings at -20, -10, and 0 minutes prior to drug or saline injections and that these values were very similar between treatment groups and very much lower than PBS controls; the AT3B-1-induced bone cancer had caused hyperalgesia to paw stimulation by heat. Furthermore, it can be seen that the injection protocol in the saline-treated controls and even the handling and restraint procedures in the PBS controls caused some increase in paw withdrawal thresholds at time markers 10, 20, and even 30 minutes (morphine ip time response curve). However, these effects had subsided, and the responses to drugs were evident and at a plateau at the 40-, 50-, and 60-minute time markers. Leconotide caused no significant antihyperalgesic effect. In Figure 2B, the plot for 5 mg/kg morphine does return to the baseline of the PBS age-matched controls after 30 minutes, but it does not decrease to the hyperalgesic values of the cancer-treated rats. Therefore, the antihyperalgesic actions of morphine appear to be maintained throughout the 60-minute time course, and they are stable at minutes. Leconotide coadministration with morphine did cause an antihyperalgesic effect, which was not only superior to the effect of leconotide alone but also to the effect of morphine. ip = intraperitoneally; IV = intravenously; MDs = maximum doses; SEM = standard error of the mean. 930
9 Intravenous Leconotide Potentiates Intraperitoneal Morphine Plate 1 Photomicrographs of tibias taken from a rat treated by intratibial injection of PBS vehicle (A) and syngeneic rat prostate cancer cells (B). The bone marrow has been filled with cancer cells, which have destroyed the normal cytoarchitecture. PBS = phosphate buffered saline. runs test and drug and saline treatments were compared statistically using ANOVA with Tukey Kramer post hoc test. Results top bottom y = bottom + EC50 x 1 10 Side Effects: Determination of MD General Observations (2) log + Cancer-treated rats gained weight after intratibial inoculations but were slightly smaller when compared with PBStreated control rats. The difference in body weight between two groups did not reach significance until day 15 of the experiment. No excessive weight loss (10% or more compared with the rest of the treatment group) was observed in tumor-bearing animals. Some rats (4%) suffered fractures of the tibia or showed signs of swelling and induration indicating that the cancer cells had eroded the tibia to infiltrate subcutaneous tissues. These rats were killed by administration of an overdose of anesthetic. In the pilot series of experiments, each hind limb was examined postmortem by X-ray and histological examination (demineralization, paraffin embedding, and staining with hematoxylin and eosin). Plates 1 and 2 show histological sections and radiographs of a normal tibia (PBS injection control) and a tibia from a rat that had received intratibial prostate cancer cells. It can be seen that the cancer cell injection led to infiltration of the bone marrow by the cancer cells with subsequent osteolytic changes with bone remodelling as commonly seen with this cancer in human bone metastases. Plate 2 X-ray photographs of tibias taken from a rat treated with intratibial injection of PBS vehicle (A) and syngeneic rat prostate cancer cells (B). PBS = phosphate buffered saline. 931
10 Kolosov et al. Table 1 Open field activity monitor test Treatment Mean SEM N Saline controls (IV + ip) Morphine 20 mg/kg ip 937.4** Morphine 10 mg/kg ip Leconotide 200 mg/kg IV 886.1** Leconotide 20 mg/kg IV Leconotide 200 mg/kg IV + morphine 2.5 mg/kg ip 963.5** Leconotide 200 mg/kg IV + morphine 1.25 mg/kg ip 943.8** Leconotide 20 mg/kg IV + morphine 2.5 mg/kg ip One-way ANOVA with Dunnett post hoc test: leconotide 200 mg/kg, morphine 20 mg/kg and two combinations of leconotide 200 mg/kg plus morphine 2.5 and 1.25 mg/kg were sedating (** P < 0.01). Leconotide at 20 mg/kg and morphine at 10 mg/kg did not cause sedation when administered alone (P > 0.05). The combination of leconotide 20 mg/kg with morphine 2.5 mg/kg was also not sedating (P > 0.05). ANOVA = analysis of variance; ip = intraperitoneally; IV = intravenously; SEM = standard error of the mean. There were no abnormal behaviors noted in any rats in this series, except for the animals that received an ip injection of morphine 10 mg/kg. Despite not being detected as a sedating dose in the open field activity monitor (see below), morphine 10 mg/kg produced mania-like activity noted by the blinded investigators. In particular, rats displayed higher than normal exploratory activity, freezing and unusual ocular stare. They also had intense startle responses to cage tapping and handling. These observations prompted the investigators to test this dose of ip morphine vs saline controls in another test of sedation the rotarod test. All other doses and dose combinations used here, including those subsequently shown to cause sedation, produced no notable adverse responses, other than sedation detected during the open field activity monitor test. No abnormal movements or signs of sedation were detected in animals during nociception testing. Open Field Activity The results from the open field activity monitor experiments are shown in Table 1. The highest doses of drugs administered individually were compared with saline controls statistically. Doses of morphine up to 10 mg/kg and leconotide 20 mg/kg were non-sedating; these doses caused no increase in LMA measurements compared with saline controls (one-way ANOVA Dunnett s post hoc test). The highest dose combination of morphine with leconotide that caused no significant sedation was morphine at 2.5 mg/kg with leconotide 20 mg/kg. Rotarod Table 2 shows the results of the rotarod test for cancertreated animals injected with saline and morphine. These results were analyzed using one-way ANOVA with the Dunnett post-hoc correction comparing drug treatments with saline controls. The rats suspected of having abnormal behavior during open field activity monitor experiments (morphine 10 mg/kg) fell off the accelerated rotarod drum significantly earlier than the controls. The rats treated with morphine 5 mg/kg ip, all remained walking on the rotating drum for periods that were not significantly different compared with the saline controls and the maximum run time of 120 seconds. It can be concluded from the experiments with the open field monitor and rotarod that sedation is caused by doses of morphine greater than 5 mg/kg and of leconotide greater than 20 mg/kg. In addition, the combination of 2.5 mg/kg morphine with 20 mg/kg leconotide was also non-sedating. Cardiovascular Measurements Table 3 shows the results of the measurements of blood pressure and heart rate in groups of rats that were treated with intravenous and ip injections of the highest drug doses and drug combinations used in this study. None of the treatments caused a significant change in systolic or diastolic pressures or heart rate, including the doses of leconotide and morphine that caused sedation in the activity box monitor and rotarod tests. These data are important because it has been shown previously that significant cardiovascular depression may occur Table 2 Rotarod test Treatment Mean SEM N Saline controls (IV + ip) Morphine 10 mg/kg ip 108.9** Morphine 5 mg/kg ip Morphine 10 mg/kg caused loss of stamina and suppressed evoked activity (running) in trained rats when compared with saline controls (** P < 0.01; one way ANOVA with Dunnett post hoc test). Morphine 5 mg/kg ip did not produce such an effect (P > 0.05). ANOVA = analysis of variance; ip = intraperitoneally; IV = intravenously; SEM = standard error of the mean. 932
11 Intravenous Leconotide Potentiates Intraperitoneal Morphine Table 3 Cardiovascular measurements Time (minutes): Drug or Saline Injections Given Immediately after Cardiovascular Measurements Mean SD Mean SD Mean SD Mean SD Mean SD Mean SD Mean SD Mean SD Mean SD N Saline controls (N = 7) Leconotide 200 mg/kg (N = 9) Morphine 10 mg/kg (N = 5) Leconotide 20 mg/kg + morphine 5 mg/kg in combination (N = 6) Leconotide 20 mg/kg + morphine 2.5 mg/kg in combination (N = 5) Systolic BP (mm Hg) Diastolic BP (mm Hg) Heart rate (bpm) Systolic BP (mm Hg) Diastolic BP (mm Hg) Heart rate (bpm) Systolic BP (mm Hg) Diastolic BP (mm Hg) Heart rate (bpm) Systolic BP (mm Hg) Diastolic BP (mm Hg) Heart rate (bpm) Systolic BP (mm Hg) Diastolic BP (mm Hg) Heart rate (bpm) Leconotide (200 mg/kg) and morphine (10 mg/kg) did not cause significant cardiovascular changes in cancer-affected rats. Combinations of leconotide intravenously plus morphine ip also did not have any sizable effect on blood pressure and heart rate in animals (P > 0.05; one-way ANOVA with Dunnett post hoc test). ANOVA = analysis of variance; BP = blood pressure; bpm = beats per minute; ip = intraperitoneally; SD = standard deviation. 933
12 Kolosov et al. following administration of drugs of this type, e.g., ziconotide [29]. The doses used in this study clearly do not cause peripheral autonomic dysfunction, which has been described previously with conopeptide administration [14 17]. Therefore, we conclude that the MDs in rats with cancerinduced hyperalgesia that should be the upper limit of doses for testing for antihyperalgesic effects are: morphine alone 5 mg/kg ip; leconotide alone 20 mg/kg IV; The MD for combination was: morphine 2.5 mg/kg ip in combination with leconotide 20 mg/kg IV. Pilot Experiments Figure 2 shows the results from the pilot experiments. The results for each nociceptive test (paw withdrawal from noxious heat, von Frey filament, and paw pressure probe) were combined for each day of testing and plotted as histograms (means) with SEM (bars). Differences between treatments were tested statistically using the Kruskall Wallis method with Dunn post hoc correction. These revealed that there were no statistically significant differences between sham and PBS treatments at any time and with any of the three nociceptive tests. At one testing time only was there a statistically significant difference between cancer and PBS treatment groups when the von Frey test for allodynia was used; at 6 days posttreatment and at no testing time thereafter. The most consistent and sustained differences between cancer treatment compared with PBS controls was when the nociceptive test used was paw flick from noxious heat (P < 0.05 at 13, 17, and 20 days; P < 0.01 at 23 days). The Randall Selitto method did not show any significant hyperalgesia differences between treatments at any testing time. This test (Randall Selitto [27]) requires manual handling and restraint of the rats and this increased interindividual variability of measurements. The other alternative test for mechanical hyperalgesia, the use of high force Von Frey hairs, proved difficult in distinguishing responses of allodynia and hyperalgesia. The results from the plantar heat test confirmed that hyperalgesia had occurred by 13 days after cancer cells injections, and this was stable and maintained for the subsequent 2 weeks, the time period chosen for experiments with morphine and leconotide. Nociception Testing Hyperalgesia had occurred by 14 days after the intratibial inoculation of the AT3B-1 rat prostate cancer cells, and this was stable for a period of 2 weeks. Hyperalgesia was also stable for all three readings at the bone cancerinduced hyperalgesia baseline (-20, -10, and 0 minutes). The first paw withdrawal latency reading after drug or saline administrations showed that drug effect onset was not fully developed, and there was also a rise in baseline for control saline treatment, indicating some stressinduced analgesia following the brief restraint and subsequent intravenous and ip injections. However, this effect subsided back to baseline levels, and drug effects reached a plateau at the 40, 50, and 60 minutes readings (Figure 3). Therefore, for plotting dose response curves, the values for percent reversal of hyperalgesia were calculated using the 40, 50, and 60 minutes readings as shown in Equation 1 for each rat. The mean paw withdrawal latency for PBS inoculated rats was seconds. The mean paw withdrawal latency in the cancer-treated rats 14 days after inoculation in this study was 8.1 seconds indicating that intratibial injection of prostate cancer cells and subsequent intratibial tumor growth induced changes that caused hyperalgesia in the ipsilateral paw. This was also confirmed by histology and X-ray examinations (Plates 1 and 2). It can be seen in Figure 3 that all rats in drug and saline groups had stable baseline readings at -20, -10, and 0 minutes prior to drug or saline injections and that these values were very similar between treatment groups and very much lower than weight-matched PBS controls revealing the extent of the hyperalgesia to plantar heat stimulation. The results from the bone cancer-induced hyperalgesia experiments are shown in Figures 3 and 4 as well as in Table 4. Time Response Curves The time response curves for the bone cancer-induced hyperalgesia experiments are shown in Figure 3; a) leconotide alone; b) morphine alone; and c) morphine combined with leconotide (20 mg/kg). Paw withdrawal thresholds are shown as means for each treatment group taken at each time of testing. Mean values (SEM) are shown for each group joined by a line. Saline-treated controls and PBS inoculated weight-matched rats are shown on each graph for comparison with drug-treated cancer-treated rats. It can be seen that hyperalgesia occurred by comparison of the PBS treated rats (12.75 seconds) with the values at the hyperalgesia baseline taken at -20, -10, and 0 minutes readings. The hyperalgesia baseline was stable for all three readings at -20, -10, and 0 minutes. The first paw withdrawal latency reading after drug or saline injection (10 minutes) showed that the drug effect was not fully developed. There was also an increase in baseline for control saline treatment at 10 minutes, indicating some stress-induced analgesia following the restraint and intravenous plus ip injections. This had returned to baseline, and drug effects reached a plateau or a peak at the 40, 50, and 60 minutes readings. On looking at Figure 3B, the paw withdrawal latencies after 5 mg/kg morphine do return to the baseline of the PBS age-matched controls by 40 minutes after the drug injections but not to the hyperalgesic values of the cancertreated rats. Therefore, the antihyperalgesic actions of that dose of morphine were stable and maintained throughout the 60 minutes time course. Thus, for plotting dose response curves, the values for percent reversal of hyperalgesia were calculated as shown in Equation 1 for each rat, using the 40-, 50-, and 60-minute readings. An 934
13 Intravenous Leconotide Potentiates Intraperitoneal Morphine Figure 4 Dose response curves for the antihyperalgesic effect of morphine in cancer-induced bone pain effect of coadministration of leconotide (20, 0.2, and 0.02 mg/kg, respectively). Points shown are means (SEM; number of rats in each group as listed in the text). Regression lines for morphine alone (heavy dashed line) and in combination with the different fixed doses of leconotide (solid line) are each bound by 95% CI shown as light dotted lines. CI = confidence interval; ip = intraperitoneally. inspection of the graphs shown in Figure 3 indicates that morphine alone caused measurable antihyperalgesic effect that was dose related. Leconotide was not found to have any significant antihyperalgesic effect in this model of bone cancer pain. However, it is known from previous work that IV leconotide does persist in the nervous system of rats to cause antihyperalgesic effects in a diabetic model lasting at least 60 minutes after the IV injection [29]. We can therefore expect that leconotide is present in the CNS in these rats with cancer-induced bone pain, also for that length of time after IV injection, and therefore available to interact with the ip morphine. The antihyperalgesic effect of the combinations of morphine with leconotide (20 mg/kg) caused greater antihyperalgesic effects than caused by either drug given alone. Combination treatment completely reversed cancer-induced bone pain in this study back to normal levels of the PBS treated controls. Dose Response Curves Dose response curves for reversal of hyperalgesia caused by bone cancer following leconotide and morphine given alone and in combinations are shown in Figure 4; three series of morphine dose response curves in combination with 1) leconotide 20 mg/kg; 2) leconotide 0.2 mg/kg; and 3) leconotide 0.02 mg/kg are shown. Points shown are means and bars SEM for each dose combination and treatment group as well as saline-treated controls. Lines show the mean and 95% confidence intervals of the regression of each data set using Equation 2. Morphine, when administered alone, caused a dose-related antihyperalgesic effect (Figure 4). However, when morphine was given in combination with the fixed doses of leconotide 20, 0.2, or 0.02 mg/kg, there was a larger, highly significant dose-related antihyperalgesic effect and a significant parallel leftward shift of the morphine dose response curve (Figure 4A C, respectively). Table 4 shows aggregate data from the bone cancerinduced hyperalgesia experiments. The runs test assessing goodness of fit for the sigmoid curve regression analysis returned P values greater than 0.05 indicating that the data did not deviate significantly from the regression equation (Equation 2 above). The dose required to cause 50% reversal of bone cancer-induced hyperalgesia (ED 50) was 2.40 mg/kg for morphine given alone. However, coadministration of morphine with leconotide 20 mg/kg led to an ED 50 for morphine that was significantly reduced to 0.16 mg/kg, a 15-fold reduction to a dose of morphine well below the maximum non-sedating dose. This reduction was accompanied by a significant antihyperalgesic effect, causing total reversal of the bone cancer-induced hyperalgesia. Similarly, coadministration of morphine with leconotide 0.2 and 0.02 mg/kg also led to an ED 50 for morphine that was markedly reduced to 0.39 mg/kg (a sixfold reduction) and 1.24 mg/kg (a twofold reduction), respectively. All combinations of morphine with leconotide at non-sedating doses caused significant reduction of pain-related behavior in rats and 935
MODULE 2 THE LABORATORY RAT
 University Animal Care Committee LABORATORY ANIMAL BIOMETHODOLOGY WORKSHOP MODULE 2 THE LABORATORY RAT SUBSTANCE ADMINISTRATION AND BLOOD COLLECTION Substance Administration: Subcutaneous injection Intramuscular
University Animal Care Committee LABORATORY ANIMAL BIOMETHODOLOGY WORKSHOP MODULE 2 THE LABORATORY RAT SUBSTANCE ADMINISTRATION AND BLOOD COLLECTION Substance Administration: Subcutaneous injection Intramuscular
Standard Operating Procedure
 1.0 Purpose: 1.1 Relaxation, dissection, weighing and fixation of heart for histological analysis. Changes in heart weight and wall thickness are linked to cardiovascular phenotypes. This protocol describes
1.0 Purpose: 1.1 Relaxation, dissection, weighing and fixation of heart for histological analysis. Changes in heart weight and wall thickness are linked to cardiovascular phenotypes. This protocol describes
Epidural analgesia technique
 Epidural analgesia technique Martin Pearson School of Veterinary Science University of Queensland, Gatton 0254601834 Peter Best Greencross South Tamworth Animal Hospital 88Duri Road, Tamworth 02 6765 4244
Epidural analgesia technique Martin Pearson School of Veterinary Science University of Queensland, Gatton 0254601834 Peter Best Greencross South Tamworth Animal Hospital 88Duri Road, Tamworth 02 6765 4244
Supporting Information
 Supporting Information Schlosburg et al. 10.1073/pnas.1219159110 SI Materials and Methods: Quantification of Heroin Metabolites Sample Collection. Trunk blood was collected in a 1:1 ratio with acetate
Supporting Information Schlosburg et al. 10.1073/pnas.1219159110 SI Materials and Methods: Quantification of Heroin Metabolites Sample Collection. Trunk blood was collected in a 1:1 ratio with acetate
Physiological normal values (Depends on age, strain, health status, type of anesthesia, etc.)
 UBC Animal Care Guidelines This SOP will be used for: Inhalational anesthesia SOP: ACC-01-2017 Rodent Anesthesia Injectable anesthesia Submitted by: Shelly McErlane both inhalational and injectable anesthesia
UBC Animal Care Guidelines This SOP will be used for: Inhalational anesthesia SOP: ACC-01-2017 Rodent Anesthesia Injectable anesthesia Submitted by: Shelly McErlane both inhalational and injectable anesthesia
MODULE 2 Substance Administration and Blood Collection in the Laboratory Rat
 LABORATORY ANIMAL BIOMETHODOLOGY WORKSHOP MODULE 2 Substance Administration and Blood Collection in the Laboratory Rat Table of Contents 1. GENERAL INFORMATION... 2 2. SUBSTANCE ADMINISTRATION... 3 3.
LABORATORY ANIMAL BIOMETHODOLOGY WORKSHOP MODULE 2 Substance Administration and Blood Collection in the Laboratory Rat Table of Contents 1. GENERAL INFORMATION... 2 2. SUBSTANCE ADMINISTRATION... 3 3.
The Egyptian Journal of Hospital Medicine (January 2018) Vol. 70 (12), Page
 The Egyptian Journal of Hospital Medicine (January 2018) Vol. 70 (12), Page 2172-2177 Blockage of HCN Channels with ZD7288 Attenuates Mechanical Hypersensitivity in Rats Model of Diabetic Neuropathy Hussain
The Egyptian Journal of Hospital Medicine (January 2018) Vol. 70 (12), Page 2172-2177 Blockage of HCN Channels with ZD7288 Attenuates Mechanical Hypersensitivity in Rats Model of Diabetic Neuropathy Hussain
How and why to do an epidural in dogs and cats? Which Indications and which drugs?
 AMVAC/RoSAVA 2014 How and why to do an epidural in dogs and cats? Which Indications and which drugs? Prof. Yves Moens Dipl ECVAA Why do epidurals? A part of a balanced anesthesia A means to provide analgesia
AMVAC/RoSAVA 2014 How and why to do an epidural in dogs and cats? Which Indications and which drugs? Prof. Yves Moens Dipl ECVAA Why do epidurals? A part of a balanced anesthesia A means to provide analgesia
Centrally Mediated Anti-Hyperalgesic and Anti-Allodynic Effect of Tolperisone in Spared Nerve Injury Model of Neuropathic Pain
 Centrally Mediated Anti-Hyperalgesic and Anti-Allodynic Effect of Tolperisone in Spared Nerve Injury Model of Neuropathic Pain Keyur S Patel *, Punam D Sachdeva Department of Pharmacology, A.R.College
Centrally Mediated Anti-Hyperalgesic and Anti-Allodynic Effect of Tolperisone in Spared Nerve Injury Model of Neuropathic Pain Keyur S Patel *, Punam D Sachdeva Department of Pharmacology, A.R.College
Human Mammary Luminal Epithelial Cells. Manual
 Human Mammary Luminal Epithelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0071.00 Human Mammary Luminal Epithelial Cells Orders are delivered via Federal Express courier. All US and Canada
Human Mammary Luminal Epithelial Cell Manual INSTRUCTION MANUAL SHIPPING CONDITIONS ZBM0071.00 Human Mammary Luminal Epithelial Cells Orders are delivered via Federal Express courier. All US and Canada
Paclitaxel, a taxane-derived chemotherapeutic, is a
 The Journal of Pain, Vol 16, No 10 (October), 2015: pp 981-994 Available online at www.jpain.org and www.sciencedirect.com Pharmacological Modulation of the Mitochondrial Electron Transport Chain in Paclitaxel-Induced
The Journal of Pain, Vol 16, No 10 (October), 2015: pp 981-994 Available online at www.jpain.org and www.sciencedirect.com Pharmacological Modulation of the Mitochondrial Electron Transport Chain in Paclitaxel-Induced
IK01400: non-opioid preclinical drug candidate for pain treatment
 IK01400: non-opioid preclinical drug candidate for pain treatment Introduction Bio-Link presents a valuable licensing opportunity for the development of novel nonopioid drug candidates to manage acute
IK01400: non-opioid preclinical drug candidate for pain treatment Introduction Bio-Link presents a valuable licensing opportunity for the development of novel nonopioid drug candidates to manage acute
Evaluation of Gabapentin and S-( )-3-Isobutylgaba in a Rat Model of Postoperative Pain
 0022-3565/97/2823-1242$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 282, No. 3 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in
0022-3565/97/2823-1242$03.00/0 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 282, No. 3 Copyright 1997 by The American Society for Pharmacology and Experimental Therapeutics Printed in
SUPPLEMENTARY INFORMATION. The Calcium-activated Chloride Channel Anoctamin 1 acts as a Heat. Sensor in Nociceptive Neurons
 SUPPLEMENTARY INFORMATION The Calcium-activated Chloride Channel Anoctamin 1 acts as a Heat Sensor in Nociceptive Neurons Hawon Cho, Young Duk Yang, Jesun Lee, Byeongjoon Lee, Tahnbee Kim Yongwoo Jang,
SUPPLEMENTARY INFORMATION The Calcium-activated Chloride Channel Anoctamin 1 acts as a Heat Sensor in Nociceptive Neurons Hawon Cho, Young Duk Yang, Jesun Lee, Byeongjoon Lee, Tahnbee Kim Yongwoo Jang,
ACTIVITY USING RATS A METHOD FOR THE EVALUATION OF ANALGESIC. subject and a variety of stimuli employed. In the examination of new compounds
 Brit. J. Pharmacol. (1946), 1, 255. A METHOD FOR THE EVALUATION OF ANALGESIC ACTIVITY USING RATS BY 0. L. DAVIES, J. RAVENT6S, AND A. L. WALPOLE From Imperial Chemical Industries, Ltd., Biological Laboratories,
Brit. J. Pharmacol. (1946), 1, 255. A METHOD FOR THE EVALUATION OF ANALGESIC ACTIVITY USING RATS BY 0. L. DAVIES, J. RAVENT6S, AND A. L. WALPOLE From Imperial Chemical Industries, Ltd., Biological Laboratories,
Human ipsc-derived Ventricular Cardiomyocytes. Protocol version 3.1
 Human ipsc-derived Ventricular Cardiomyocytes Protocol version 3.1 Protocol version 3.1 Table of Contents Product Information 2 Recommendations 2 Preparing Cardiomyocyte Maintenance Medium 3 Cardiomyocyte
Human ipsc-derived Ventricular Cardiomyocytes Protocol version 3.1 Protocol version 3.1 Table of Contents Product Information 2 Recommendations 2 Preparing Cardiomyocyte Maintenance Medium 3 Cardiomyocyte
Chapter 25. General Anesthetics
 Chapter 25 1. Introduction General anesthetics: 1. Analgesia 2. Amnesia 3. Loss of consciousness 4. Inhibition of sensory and autonomic reflexes 5. Skeletal muscle relaxation An ideal anesthetic: 1. A
Chapter 25 1. Introduction General anesthetics: 1. Analgesia 2. Amnesia 3. Loss of consciousness 4. Inhibition of sensory and autonomic reflexes 5. Skeletal muscle relaxation An ideal anesthetic: 1. A
Controlled Trial of Wound Infiltration with Bupivacaine for Post Operative Pain Relief after Caesarean Section
 Bahrain Medical Bulletin, Vol.23, No.2, June 2001 Controlled Trial of Wound Infiltration with Bupivacaine for Post Operative Pain Relief after Caesarean Section Omar Momani, MD, MBBS, JBA* Objective: The
Bahrain Medical Bulletin, Vol.23, No.2, June 2001 Controlled Trial of Wound Infiltration with Bupivacaine for Post Operative Pain Relief after Caesarean Section Omar Momani, MD, MBBS, JBA* Objective: The
Acute Pain NETP: SEPTEMBER 2013 COHORT
 Acute Pain NETP: SEPTEMBER 2013 COHORT Pain & Suffering an unpleasant sensory & emotional experience associated with actual or potential tissue damage, or described in terms of such damage International
Acute Pain NETP: SEPTEMBER 2013 COHORT Pain & Suffering an unpleasant sensory & emotional experience associated with actual or potential tissue damage, or described in terms of such damage International
Social transfer of pain in mice
 NEUROPSYCHOLOGY Social transfer of pain in mice Monique L. Smith, 1 Caroline M. Hostetler, 1 Mary M. Heinricher, 1,2 Andrey E. Ryabinin 1 * A complex relationship exists between the psychosocial environment
NEUROPSYCHOLOGY Social transfer of pain in mice Monique L. Smith, 1 Caroline M. Hostetler, 1 Mary M. Heinricher, 1,2 Andrey E. Ryabinin 1 * A complex relationship exists between the psychosocial environment
Rapamycin Suppresses Astrocytic and Microglial Activation and Reduced Development of Neuropathic Pain after Spinal Cord Injury in Mice.
 Rapamycin Suppresses Astrocytic and Microglial Activation and Reduced Development of Neuropathic Pain after Spinal Cord Injury in Mice. Satoshi Tateda, M.D., Haruo Kanno, M.D., Ph.D., Hiroshi Ozawa, M.D.,
Rapamycin Suppresses Astrocytic and Microglial Activation and Reduced Development of Neuropathic Pain after Spinal Cord Injury in Mice. Satoshi Tateda, M.D., Haruo Kanno, M.D., Ph.D., Hiroshi Ozawa, M.D.,
Product Use HPSC-CC are for research use only. It is not approved for human or animal use, or for application in in vitro diagnostic procedures.
 HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
HPSC-derived Cardiomyocyte Cells (HPSC-CC) Catalog #6240 Cell Specification Human primary cardiomyocytes and cardiac tissue are superior modeling systems for heart disease studies, drug discovery and toxicity
RODENT Hepatocytes Care Manual
 RODENT Hepatocytes Care Manual INSTRUCTION MANUAL ZBM0054.02 SHIPPING CONDITIONS Rodent Hepatocytes cryopreserved Orders are delivered via Federal Express courier. All US and Canada orders are shipped
RODENT Hepatocytes Care Manual INSTRUCTION MANUAL ZBM0054.02 SHIPPING CONDITIONS Rodent Hepatocytes cryopreserved Orders are delivered via Federal Express courier. All US and Canada orders are shipped
Thawing MEFs (Mouse Embryonic Fibroblasts (MEFs)
 1 FEEDER CULTURES The function of feeder cultures is to support the undifferentiated growth of hpsc. Typically primary fibroblasts are used for this purpose. We prepare our mouse feeder cells from ICR
1 FEEDER CULTURES The function of feeder cultures is to support the undifferentiated growth of hpsc. Typically primary fibroblasts are used for this purpose. We prepare our mouse feeder cells from ICR
Human Umbilical Vein Endothelial Cell Manual
 Human Umbilical Vein Endothelial Cell Manual INSTRUCTION MANUAL ZBM0079.03 SHIPPING CONDITIONS Human Umbilical Vein Endothelial Cells, cryopreserved Cryopreserved cells are shipped on dry ice and should
Human Umbilical Vein Endothelial Cell Manual INSTRUCTION MANUAL ZBM0079.03 SHIPPING CONDITIONS Human Umbilical Vein Endothelial Cells, cryopreserved Cryopreserved cells are shipped on dry ice and should
Implanting an Adult Rat with the Single-Channel Epoch Transmitter for Recording Electrocardiogram in the Type II electrode configuration.
 Implanting an Adult Rat with the Single-Channel Epoch Transmitter for Recording Electrocardiogram in the Type II electrode configuration. Recommended Surgical Tools A. Scalpel handle B. Scalpel blade (#15)
Implanting an Adult Rat with the Single-Channel Epoch Transmitter for Recording Electrocardiogram in the Type II electrode configuration. Recommended Surgical Tools A. Scalpel handle B. Scalpel blade (#15)
Corporate Medical Policy
 Corporate Medical Policy Intravenous Anesthetics for the Treatment of Chronic Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intravenous_anesthetics_for_the_treatment_of_chronic_pain
Corporate Medical Policy Intravenous Anesthetics for the Treatment of Chronic Pain File Name: Origination: Last CAP Review: Next CAP Review: Last Review: intravenous_anesthetics_for_the_treatment_of_chronic_pain
Surgical Care at the District Hospital. EMERGENCY & ESSENTIAL SURGICAL CARE
 Surgical Care at the District Hospital 1 14 Practical Anesthesia Key Points 2 14.1 General Anesthesia Have a clear plan before starting anesthesia Never use an unfamiliar anesthetic technique in an emergency
Surgical Care at the District Hospital 1 14 Practical Anesthesia Key Points 2 14.1 General Anesthesia Have a clear plan before starting anesthesia Never use an unfamiliar anesthetic technique in an emergency
NEONATAL RODENTS SURVIVAL SURGERY
 MCGILL UNIVERSITY UNIVERSITY ANIMAL CARE COMMITTEE UACC Standard Operating Procedure # 11 October 2005 version NEONATAL RODENTS SURVIVAL SURGERY 1. INTRODUCTION Standard Operating Procedures (SOPs) provide
MCGILL UNIVERSITY UNIVERSITY ANIMAL CARE COMMITTEE UACC Standard Operating Procedure # 11 October 2005 version NEONATAL RODENTS SURVIVAL SURGERY 1. INTRODUCTION Standard Operating Procedures (SOPs) provide
General Anesthesia. My goal in general anesthesia is to stop all of these in the picture above (motor reflexes, pain and autonomic reflexes).
 General Anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
General Anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
Supplementary information to. Mesenchymal Stem Cells Reversed Morphine Tolerance and Opioid-induced Hyperalgesia
 Supplementary information to Mesenchymal Stem Cells Reversed Morphine Tolerance and Opioid-induced Hyperalgesia Zhen Hua 1,2 *, LiPing Liu 1 *, Jun Shen 1, Katherine Cheng 1, Aijun Liu 1, Jing Yang 1,
Supplementary information to Mesenchymal Stem Cells Reversed Morphine Tolerance and Opioid-induced Hyperalgesia Zhen Hua 1,2 *, LiPing Liu 1 *, Jun Shen 1, Katherine Cheng 1, Aijun Liu 1, Jing Yang 1,
General anesthesia. No single drug capable of achieving these effects both safely and effectively.
 General anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia, and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
General anesthesia General anesthesia is essential to surgical practice, because it renders patients analgesic, amnesia, and unconscious reflexes, while causing muscle relaxation and suppression of undesirable
Heart Rate and Blood Pressure as Vital Signs
 Heart Rate and Blood Pressure as Vital Signs Computer 10 Since the earliest days of medicine heart rate has been recognized as a vital sign an indicator of health, disease, excitement, and stress. Medical
Heart Rate and Blood Pressure as Vital Signs Computer 10 Since the earliest days of medicine heart rate has been recognized as a vital sign an indicator of health, disease, excitement, and stress. Medical
EVALUATION OF ANTI-NOCICEPTIVE EFFECT OF TOLPERISONE BY COMPARING ITS EFFECT WITH TRAMADOL IN NEUROPATHIC PAIN
 Journal of Pharmaceutical Biology www.jpbjournal.com e-issn - 2249-7560 Print ISSN - 2249-7579 EVALUATION OF ANTI-NOCICEPTIVE EFFECT OF TOLPERISONE BY COMPARING ITS EFFECT WITH TRAMADOL IN NEUROPATHIC
Journal of Pharmaceutical Biology www.jpbjournal.com e-issn - 2249-7560 Print ISSN - 2249-7579 EVALUATION OF ANTI-NOCICEPTIVE EFFECT OF TOLPERISONE BY COMPARING ITS EFFECT WITH TRAMADOL IN NEUROPATHIC
CRITICALLY APPRAISED PAPER (CAP) Evidence / Title of article
 CRITICALLY APPRAISED PAPER (CAP) Evidence / Title of article Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain Schilder A et
CRITICALLY APPRAISED PAPER (CAP) Evidence / Title of article Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain Schilder A et
Protocol for A-549 VIM RFP (ATCC CCL-185EMT) TGFβ1 EMT Induction and Drug Screening
 Protocol for A-549 VIM RFP (ATCC CCL-185EMT) TGFβ1 EMT Induction and Drug Screening Introduction: Vimentin (VIM) intermediate filament (IF) proteins are associated with EMT in lung cancer and its metastatic
Protocol for A-549 VIM RFP (ATCC CCL-185EMT) TGFβ1 EMT Induction and Drug Screening Introduction: Vimentin (VIM) intermediate filament (IF) proteins are associated with EMT in lung cancer and its metastatic
Cryopreserved HepaRG Cells and Media Supplements
 Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
Cryopreserved HepaRG Cells and Media Supplements Catalog No. MMHPR116 Catalog No. MMADD621 Catalog No. MMADD631 Catalog No. MMADD641 Catalog No. MMADD651 Catalog No. MMADD671 FOR RESEARCH USE ONLY Not
IV Catheter Placement
 Year Group: BVSc3 + Document number: CSL_A06 Equipment for this station: Equipment list: IV catheter model, with giving set and red fluid bag IV catheter Bung or T-port Tape two strips cut to size before
Year Group: BVSc3 + Document number: CSL_A06 Equipment for this station: Equipment list: IV catheter model, with giving set and red fluid bag IV catheter Bung or T-port Tape two strips cut to size before
Human TRPC6 Ion Channel Cell Line
 TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
TECHNICAL DATA SHEET ValiScreen Ion Channel Cell Line Caution: For Laboratory Use. A research product for research purposes only Human TRPC6 Ion Channel Cell Line Product No.: AX-012-C Lot No.: 512-548-A
Supporting Information
 Supporting Information Synthesis and Evaluation of Antiallodynic and Anticonvulsant Activity of Novel Amide and Urea Derivatives of Valproic Acid Analogues Dan Kaufmann, Meir Bialer, $, Jakob Avi Shimshoni,
Supporting Information Synthesis and Evaluation of Antiallodynic and Anticonvulsant Activity of Novel Amide and Urea Derivatives of Valproic Acid Analogues Dan Kaufmann, Meir Bialer, $, Jakob Avi Shimshoni,
Effects of meperidine or saline on thermal, mechanical and electrical nociceptive thresholds in cats
 Veterinary Anaesthesia and Analgesia, 2008, 35, 543 547 doi:10.1111/j.1467-2995.2008.00419.x SHORT COMMUNICATION Effects of meperidine or saline on thermal, mechanical and electrical nociceptive thresholds
Veterinary Anaesthesia and Analgesia, 2008, 35, 543 547 doi:10.1111/j.1467-2995.2008.00419.x SHORT COMMUNICATION Effects of meperidine or saline on thermal, mechanical and electrical nociceptive thresholds
Procedures/Risks:central venous catheter
 Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
Procedures/Risks:central venous catheter Central Venous Catheter Placement Procedure: Placement of the central venous catheter will take place in the Interventional Radiology Department (IRD) at The Ohio
PARTIAL KNEE REPLACEMENT
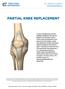 PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
PARTIAL KNEE REPLACEMENT A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
To place an order, please visit lifelinecelltech.com or call customer service at
 Human Melanocyte Cells Instruction Manual HEMn Human Epidermal Melanocytes, Neonatal HEMn-HP Human Epidermal Melanocytes, Neonatal-Highly Pigmented HEMa Human Epidermal Melanocytes, Adult HEMa-HP Human
Human Melanocyte Cells Instruction Manual HEMn Human Epidermal Melanocytes, Neonatal HEMn-HP Human Epidermal Melanocytes, Neonatal-Highly Pigmented HEMa Human Epidermal Melanocytes, Adult HEMa-HP Human
Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male but not female offspring
 1 2 3 4 5 6 7 8 9 1 11 12 13 14 Supplementary information for: Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male but not female offspring Stephane L Bourque,
1 2 3 4 5 6 7 8 9 1 11 12 13 14 Supplementary information for: Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male but not female offspring Stephane L Bourque,
Partial Knee Replacement
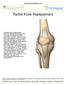 Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Partial Knee Replacement A partial knee replacement removes damaged cartilage from the knee and replaces it with prosthetic implants. Unlike a total knee replacement, which removes all of the cartilage,
Human Apolipoprotein A1 EIA Kit
 A helping hand for your research Product Manual Human Apolipoprotein A1 EIA Kit Catalog Number: 83901 96 assays 1 Table of Content Product Description 3 Assay Principle 3 Kit Components 3 Storage 4 Reagent
A helping hand for your research Product Manual Human Apolipoprotein A1 EIA Kit Catalog Number: 83901 96 assays 1 Table of Content Product Description 3 Assay Principle 3 Kit Components 3 Storage 4 Reagent
Assay Sensitivity.
 Assay Sensitivity Michael C. Rowbotham, MD Professor of Neurology UCSF-Mount Zion Pain Management Center Senior Scientist and IRB Chair, CPMC Research Institute Michael.Rowbotham@ucsf.edu Outline What
Assay Sensitivity Michael C. Rowbotham, MD Professor of Neurology UCSF-Mount Zion Pain Management Center Senior Scientist and IRB Chair, CPMC Research Institute Michael.Rowbotham@ucsf.edu Outline What
LONG-TERM postoperative pain follows many common
 Prolonged Suppression of Postincisional Pain by a Slow-release Formulation of Lidocaine Chi-Fei Wang, M.D.,* Carlo Pancaro, M.D., Peter Gerner, M.D., Gary Strichartz, Ph.D. ABSTRACT Background: Postoperative
Prolonged Suppression of Postincisional Pain by a Slow-release Formulation of Lidocaine Chi-Fei Wang, M.D.,* Carlo Pancaro, M.D., Peter Gerner, M.D., Gary Strichartz, Ph.D. ABSTRACT Background: Postoperative
SURGICAL PROCEDURE DESCRIPTIONS
 SURGICAL PROCEDURE DESCRIPTIONS GONADECTOMY: CASTRATION USING SCROTAL METHOD 1. The animal is anesthetized and placed in dorsal recumbency with the tail toward the surgeon. 2. The abdominal and scrotal
SURGICAL PROCEDURE DESCRIPTIONS GONADECTOMY: CASTRATION USING SCROTAL METHOD 1. The animal is anesthetized and placed in dorsal recumbency with the tail toward the surgeon. 2. The abdominal and scrotal
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress REDUCING THE PAIN FACTOR AN UPDATE ON PERI-OPERATIVE ANALGESIA Sandra Forysth, BVSc DipACVA Institute of Veterinary,
ONLINE. F.M. Teixeira*, L.L. Castro*, R.T. Ferreira, P.A. Pires, F.A. Vanderlinde and M.A. Medeiros
 Brazilian Journal of Medical and Biological Research Online Provisional Version ISSN 0100-879X Short Communication This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully
Brazilian Journal of Medical and Biological Research Online Provisional Version ISSN 0100-879X Short Communication This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully
CONTRACTING ORGANIZATION: Johns Hopkins University Baltimore MD
 AD Award Number: W81XWH-06-1-0176 TITLE: Neurofibromatosis and the Painful Neuroma PRINCIPAL INVESTIGATOR: Allan Joel Belzberg, M.D. CONTRACTING ORGANIZATION: Johns Hopkins University Baltimore MD 21218-2686
AD Award Number: W81XWH-06-1-0176 TITLE: Neurofibromatosis and the Painful Neuroma PRINCIPAL INVESTIGATOR: Allan Joel Belzberg, M.D. CONTRACTING ORGANIZATION: Johns Hopkins University Baltimore MD 21218-2686
Pre-operative Care For Surgery of Forearm Fracture. WONG Mei Chee OT (CMC)
 Pre-operative Care For Surgery of Forearm Fracture WONG Mei Chee OT (CMC) Pre-operative Nursing Considerations for Surgery of Forearm Fracture 1. Patient s problems - Diagnosis: Clinical features, x-ray
Pre-operative Care For Surgery of Forearm Fracture WONG Mei Chee OT (CMC) Pre-operative Nursing Considerations for Surgery of Forearm Fracture 1. Patient s problems - Diagnosis: Clinical features, x-ray
Human Keratinocyte Manual
 INSTRUCTION MANUAL SHIPPING CONDITIONS Human Keratinocyte Cells Human Keratinocyte Manual ZBM0032.09 Orders are delivered via Federal Express courier. All US and Canada orders are shipped via Federal Express
INSTRUCTION MANUAL SHIPPING CONDITIONS Human Keratinocyte Cells Human Keratinocyte Manual ZBM0032.09 Orders are delivered via Federal Express courier. All US and Canada orders are shipped via Federal Express
ASSAY OF SPHINGOMYELINASE ACTIVITY
 ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
ASSAY OF SPHINGOMYELINASE ACTIVITY Protocol for Protein Extraction Stock Solution 1. Leupeptin/hydrochloride (FW 463.0,
SUPPLEMENTARY INFORMATION
 METHODS Mice and rats. Behavioural experiments were performed in male and female 7 12 week old mice or in male 7 10 week old Wistar rats. Permissions for the animal experiments have been obtained from
METHODS Mice and rats. Behavioural experiments were performed in male and female 7 12 week old mice or in male 7 10 week old Wistar rats. Permissions for the animal experiments have been obtained from
Integra B: Camino OLM Intracranial Pressure Monitoring Kit SURGICAL TECHNIQUE
 Integra 110-4B: Camino OLM Intracranial Pressure Monitoring Kit SURGICAL TECHNIQUE Surgical Technique Th OLM ICP Kit was developed in cooperation with Richard C. Ostrup, M.D., Thomas G. Luerssen, M.D.
Integra 110-4B: Camino OLM Intracranial Pressure Monitoring Kit SURGICAL TECHNIQUE Surgical Technique Th OLM ICP Kit was developed in cooperation with Richard C. Ostrup, M.D., Thomas G. Luerssen, M.D.
Data Sheet IL-2-Luciferase Reporter (Luc) - Jurkat Cell Line Catalog # 60481
 642 Cornerstone Court W, Ste B Tel: 1.858.829.382 Data Sheet IL-2-Luciferase Reporter (Luc) - Jurkat Cell Line Catalog # 6481 Description Human IL-2 reporter construct is stably integrated into the genome
642 Cornerstone Court W, Ste B Tel: 1.858.829.382 Data Sheet IL-2-Luciferase Reporter (Luc) - Jurkat Cell Line Catalog # 6481 Description Human IL-2 reporter construct is stably integrated into the genome
Anti-nerve growth factor antibody attenuates chronic morphine treatmentinduced tolerance in the rat
 Cheppudira et al. BMC Anesthesiology (2016) 16:73 DOI 10.1186/s12871-016-0242-x RESEARCH ARTICLE Open Access Anti-nerve growth factor antibody attenuates chronic morphine treatmentinduced tolerance in
Cheppudira et al. BMC Anesthesiology (2016) 16:73 DOI 10.1186/s12871-016-0242-x RESEARCH ARTICLE Open Access Anti-nerve growth factor antibody attenuates chronic morphine treatmentinduced tolerance in
Sensory Assessment of Regional Analgesia in Humans
 REVIEW ARTICLE Dennis M. Fisher, M.D., Editor-in-Chief Anesthesiology 2000; 93:1517 30 2000 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Sensory Assessment of Regional
REVIEW ARTICLE Dennis M. Fisher, M.D., Editor-in-Chief Anesthesiology 2000; 93:1517 30 2000 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Sensory Assessment of Regional
Human CD4+T Cell Care Manual
 Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Human CD4+T Cell Care Manual INSTRUCTION MANUAL ZBM0067.04 SHIPPING CONDITIONS Human CD4+T Cells, cryopreserved Cryopreserved human CD4+T cells are shipped on dry ice and should be stored in liquid nitrogen
Acute painful crisis in patients with sickle cell disease: Clinical Guidelines (HN-506a)
 Acute painful crisis in patients with sickle cell disease: Clinical Guidelines (HN-506a) Introduction The majority of acute painful crises in patients with sickle cell disease will be managed independently
Acute painful crisis in patients with sickle cell disease: Clinical Guidelines (HN-506a) Introduction The majority of acute painful crises in patients with sickle cell disease will be managed independently
Induction of Solid Mammary, Subcutaneous and Lung Cancers in Rats and Mice. To investigate rodent models of cancer
 THE UNIVERSITY OF QUEENSLAND Page 1 of 6 SOP No: AHP 30 DATE ISSUED: 29/09/2017 SUBJECT: REASON FOR USE: Induction of Solid Mammary, Subcutaneous and Lung Cancers in Rats and Mice To investigate rodent
THE UNIVERSITY OF QUEENSLAND Page 1 of 6 SOP No: AHP 30 DATE ISSUED: 29/09/2017 SUBJECT: REASON FOR USE: Induction of Solid Mammary, Subcutaneous and Lung Cancers in Rats and Mice To investigate rodent
For research or further manufacturing use only. Not for injection or diagnostic procedures.
 PRIME-XV T cell Expansion XSFM PRIME-XV T Cell Expansion XSFM is a xeno-free, serum-free medium optimized for the activation and expansion of human T lymphocytes. This medium contains gentamicin and requires
PRIME-XV T cell Expansion XSFM PRIME-XV T Cell Expansion XSFM is a xeno-free, serum-free medium optimized for the activation and expansion of human T lymphocytes. This medium contains gentamicin and requires
EQUIPMENT: Nitrous Oxygen Delivery System:
 Policy: Nitrous Oxide Use in the Intrapartum and Immediate Postpartum Period for Obstetrical Patients in the Family Birth Place Approvers: CEO. CNO, Medical Staff President, Anesthesia Chair, OB Medical
Policy: Nitrous Oxide Use in the Intrapartum and Immediate Postpartum Period for Obstetrical Patients in the Family Birth Place Approvers: CEO. CNO, Medical Staff President, Anesthesia Chair, OB Medical
Cardiac Output Technique For Small Animals
 Cardiac Output Technique For Small Introduction Cardiac output (CO) is a measure of the quantity of blood pumped by the heart each minute and is the product of stroke volume (ie. volume of blood ejected
Cardiac Output Technique For Small Introduction Cardiac output (CO) is a measure of the quantity of blood pumped by the heart each minute and is the product of stroke volume (ie. volume of blood ejected
Specimen Paper. Biology. - Specialists in Science and Maths Education. Paper 2
 www.londonsciencetutors.com - Specialists in Science and Maths Education Specimen Paper Centre Number Candidate Number!"#$%&'()*+#,-$.-+ Surname Other Names %&'()*+#,-$/*)0)'1- Candidate Signature Question
www.londonsciencetutors.com - Specialists in Science and Maths Education Specimen Paper Centre Number Candidate Number!"#$%&'()*+#,-$.-+ Surname Other Names %&'()*+#,-$/*)0)'1- Candidate Signature Question
Department of Orthopaedic Surgery, Tohoku University Graduate School of Medicine, Sendai, Japan, 2
 Low-energy Extracorporeal Shock Wave Therapy Promotes VEGF Expression and Angiogenesis and Improve Locomotor and Sensory Functions after spinal cord injury Kenichiro Yahata 1, Hiroshi Ozawa, M.D., Ph.D.
Low-energy Extracorporeal Shock Wave Therapy Promotes VEGF Expression and Angiogenesis and Improve Locomotor and Sensory Functions after spinal cord injury Kenichiro Yahata 1, Hiroshi Ozawa, M.D., Ph.D.
Potential for delta opioid receptor agonists as analgesics in chronic pain therapy
 Potential for delta opioid receptor agonists as analgesics in chronic pain therapy David Kendall & Bengt von Mentzer; PharmNovo AB/UK Alex Conibear & Eamonn Kelly, University of Bristol Junaid Asghar,
Potential for delta opioid receptor agonists as analgesics in chronic pain therapy David Kendall & Bengt von Mentzer; PharmNovo AB/UK Alex Conibear & Eamonn Kelly, University of Bristol Junaid Asghar,
MTS assay in A549 cells
 Project: VIGO MTS assay in A549 cells Detection of cell viability/activity AUTHORED BY: DATE: Cordula Hirsch 20.01.2014 REVIEWED BY: DATE: Harald Krug 10.04.2014 APPROVED BY: DATE: DOCUMENT HISTORY Effective
Project: VIGO MTS assay in A549 cells Detection of cell viability/activity AUTHORED BY: DATE: Cordula Hirsch 20.01.2014 REVIEWED BY: DATE: Harald Krug 10.04.2014 APPROVED BY: DATE: DOCUMENT HISTORY Effective
International Conference on Biological Sciences and Technology (BST 2016)
 International Conference on Biological Sciences and Technology (BST 2016) Analgesic Contrast of Diverse Administrations of Botulinum Neurotoxin A (BoTN/A) Using Rat Neuropathic Pain Model Qiong-Fang YANG1,3,
International Conference on Biological Sciences and Technology (BST 2016) Analgesic Contrast of Diverse Administrations of Botulinum Neurotoxin A (BoTN/A) Using Rat Neuropathic Pain Model Qiong-Fang YANG1,3,
Intraspinal (Neuraxial) Analgesia Community Nurses Competency Test
 Intraspinal (Neuraxial) Analgesia Community Nurses Competency Test 1 Intraspinal (Neuraxial) Analgesia for Community Nurses Competency Test 1) Name the two major classifications of pain. i. ii. 2) Neuropathic
Intraspinal (Neuraxial) Analgesia Community Nurses Competency Test 1 Intraspinal (Neuraxial) Analgesia for Community Nurses Competency Test 1) Name the two major classifications of pain. i. ii. 2) Neuropathic
Extended therapeutic validation of an anti-mc4 receptor antibody in an animal model of anorexia nervosa
 Extended therapeutic validation of an anti-mc4 receptor antibody in an animal model of anorexia nervosa (project no. 04-12B) Authors Guus Akkermans, Martien Kas Preliminary data report Introduction In
Extended therapeutic validation of an anti-mc4 receptor antibody in an animal model of anorexia nervosa (project no. 04-12B) Authors Guus Akkermans, Martien Kas Preliminary data report Introduction In
GTB 214/3 BASIC PHARMACOLOGY
 GTB 214/3 BASIC PHARMACOLOGY This course exposes students to the basic pharmacology with drawing of knowledge, concepts & techniques derived from biochemistry, physiology, biophysics and other divisions
GTB 214/3 BASIC PHARMACOLOGY This course exposes students to the basic pharmacology with drawing of knowledge, concepts & techniques derived from biochemistry, physiology, biophysics and other divisions
Japan. J. Pharmacol. 50, (1989) 327. Effects of Central Nervous System-Acting Drugs on Urinary Bladder Contraction in Unanesthetized Rats
 Japan. J. Pharmacol. 50, 327-332 (1989) 327 Effects of Central Nervous System-Acting Drugs on Urinary Bladder Contraction in Unanesthetized Rats Hitoshi KONTANI, Mikiko NAKAGAWA and Takeshi SAKAI Department
Japan. J. Pharmacol. 50, 327-332 (1989) 327 Effects of Central Nervous System-Acting Drugs on Urinary Bladder Contraction in Unanesthetized Rats Hitoshi KONTANI, Mikiko NAKAGAWA and Takeshi SAKAI Department
Research and Reviews: Journal of Medical and Health Sciences
 Research and Reviews: Journal of Medical and Health Sciences Evaluation of Epidural Clonidine for Postoperative Pain Relief. Mukesh I Shukla, Ajay Rathod, Swathi N*, Jayesh Kamat, Pramod Sarwa, and Vishal
Research and Reviews: Journal of Medical and Health Sciences Evaluation of Epidural Clonidine for Postoperative Pain Relief. Mukesh I Shukla, Ajay Rathod, Swathi N*, Jayesh Kamat, Pramod Sarwa, and Vishal
Supporting Information File S2
 Pulli et al. Measuring Myeloperoxidase Activity in Biological Samples Page 1 of 6 Supporting Information File S2 Step-by-Step Protocol Reagents: Sucrose (Sigma, S3089) CaCl 2 (Sigma, C5770) Heparin Sodium
Pulli et al. Measuring Myeloperoxidase Activity in Biological Samples Page 1 of 6 Supporting Information File S2 Step-by-Step Protocol Reagents: Sucrose (Sigma, S3089) CaCl 2 (Sigma, C5770) Heparin Sodium
Repeated intra-articular injections of acidic saline produce long-lasting joint pain and. widespread hyperalgesia
 Repeated intra-articular injections of acidic saline produce long-lasting joint pain and widespread hyperalgesia N. Sugimura 1, M. Ikeuchi 1 *, M. Izumi 1, T. Kawano 2, K. Aso 1, T. Kato 1, T. Ushida 3,
Repeated intra-articular injections of acidic saline produce long-lasting joint pain and widespread hyperalgesia N. Sugimura 1, M. Ikeuchi 1 *, M. Izumi 1, T. Kawano 2, K. Aso 1, T. Kato 1, T. Ushida 3,
PATIENT CARE MANUAL POLICY
 PATIENT CARE MANUAL POLICY NUMBER VII-A-15 PAGE 1 OF 3 APPROVED BY: CATEGORY: Vice President and Senior Operating Officer; Rural Health Services & Professional Practice Lead Body Systems; Central Nervous
PATIENT CARE MANUAL POLICY NUMBER VII-A-15 PAGE 1 OF 3 APPROVED BY: CATEGORY: Vice President and Senior Operating Officer; Rural Health Services & Professional Practice Lead Body Systems; Central Nervous
A Patient s Guide to Pain Management: Complex Regional Pain Syndrome
 A Patient s Guide to Pain Management: Complex Regional Pain Syndrome Suite 11-13/14/15 Mount Elizabeth Medical Center 3 Mount Elizabeth Singapore, 228510 Phone: (65) 6738 2628 Fax: (65) 6738 2629 DISCLAIMER:
A Patient s Guide to Pain Management: Complex Regional Pain Syndrome Suite 11-13/14/15 Mount Elizabeth Medical Center 3 Mount Elizabeth Singapore, 228510 Phone: (65) 6738 2628 Fax: (65) 6738 2629 DISCLAIMER:
Novel ω-conotoxins from C. catus reverse signs of mouse inflammatory pain after systemic administration
 Sadeghi et al. Molecular Pain 2013, 9:51 MOLECULAR PAIN RESEARCH Open Access Novel ω-conotoxins from C. catus reverse signs of mouse inflammatory pain after systemic administration Mahsa Sadeghi 1, Swetha
Sadeghi et al. Molecular Pain 2013, 9:51 MOLECULAR PAIN RESEARCH Open Access Novel ω-conotoxins from C. catus reverse signs of mouse inflammatory pain after systemic administration Mahsa Sadeghi 1, Swetha
Follicle Dermal Papilla Cells
 Follicle Dermal Papilla Cells Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product
Follicle Dermal Papilla Cells Instruction Manual Product Size Catalog Number Human Follicle Dermal Papilla Cells (HFDPC) 500,000 cryopreserved cells 500,000 proliferating cells C-12071 C-12072 Product
The Mechanics of the Dental Anesthetic Cartridge
 The Mechanics of the Dental Anesthetic Cartridge Sponsored by Pierrel, makers of Orabloc (Articaine hydrochloridre 40mg/ml and epinephrine Injection) Abstract Local anesthesia is the fundamental part of
The Mechanics of the Dental Anesthetic Cartridge Sponsored by Pierrel, makers of Orabloc (Articaine hydrochloridre 40mg/ml and epinephrine Injection) Abstract Local anesthesia is the fundamental part of
Biological Consulting Services
 Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
Biological Consulting Services of North Florida/ Inc. May 13, 2009 Aphex BioCleanse Systems, Inc. Dear Sirs, We have completed antimicrobial efficacy study on the supplied Multi-Purpose Solution. The testing
1/21/14. Cancer Related Pain: Case-Based Pharmacology. Conflicts of Interest. Learning Objective
 Cancer Related Pain: Case-Based Pharmacology Jeannine M. Brant, PhD, APRN, AOCN Oncology Clinical Nurse Specialist Nurse Scientist Billings Clinic Conflicts of Interest Jeannine Brant has served on the
Cancer Related Pain: Case-Based Pharmacology Jeannine M. Brant, PhD, APRN, AOCN Oncology Clinical Nurse Specialist Nurse Scientist Billings Clinic Conflicts of Interest Jeannine Brant has served on the
ORS 2015 Annual Meeting (Orthopedic Research Society)
 ORS 2015 Annual Meeting (Orthopedic Research Society) Poster No: 1045 CERAMENT Bone Void Filler Impregnated with Gentamicin Increases Bone Formation and Decreases the Rate of Detectable Infection after
ORS 2015 Annual Meeting (Orthopedic Research Society) Poster No: 1045 CERAMENT Bone Void Filler Impregnated with Gentamicin Increases Bone Formation and Decreases the Rate of Detectable Infection after
ClinialTrials.gov Identifier: Sponsor/company: sanofi-aventis
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
NMDA-Receptor Antagonists and Opioid Receptor Interactions as Related to Analgesia and Tolerance
 Vol. 19 No. 1(Suppl.) January 2000 Journal of Pain and Symptom Management S7 Proceedings Supplement NDMA-Receptor Antagonists: Evolving Role in Analgesia NMDA-Receptor Antagonists and Opioid Receptor Interactions
Vol. 19 No. 1(Suppl.) January 2000 Journal of Pain and Symptom Management S7 Proceedings Supplement NDMA-Receptor Antagonists: Evolving Role in Analgesia NMDA-Receptor Antagonists and Opioid Receptor Interactions
Utilizing AlphaLISA Technology to Screen for Inhibitors of the CTLA-4 Immune Checkpoint
 APPLICATION NOTE AlphaLISA Technology Authors: Matthew Marunde Stephen Hurt PerkinElmer, Inc. Hopkinton, MA Utilizing AlphaLISA Technology to Screen for Inhibitors of the CTLA-4 Immune Checkpoint Introduction
APPLICATION NOTE AlphaLISA Technology Authors: Matthew Marunde Stephen Hurt PerkinElmer, Inc. Hopkinton, MA Utilizing AlphaLISA Technology to Screen for Inhibitors of the CTLA-4 Immune Checkpoint Introduction
GUIDELINES AND AUDIT IMPLEMENTATION NETWORK
 GUIDELINES AND AUDIT IMPLEMENTATION NETWORK General Palliative Care Guidelines The Management of Pain at the End Of Life November 2010 Aim To provide a user friendly, evidence based guide for the management
GUIDELINES AND AUDIT IMPLEMENTATION NETWORK General Palliative Care Guidelines The Management of Pain at the End Of Life November 2010 Aim To provide a user friendly, evidence based guide for the management
USA Product Label SYNOVEX C. Fort Dodge CALF IMPLANTS. For Increased Rate of Weight Gain. NADA 9-576, Approved by FDA
 FORT DODGE ANIMAL HEALTH Division of Wyeth, a subsidiary of Pfizer, Inc. USA Product Label http://www.vetdepot.com 235 E. 42ND ST., NEW YORK, NY, 10017 Telephone: 269-833-4000 Customer Service: 800-733-5500
FORT DODGE ANIMAL HEALTH Division of Wyeth, a subsidiary of Pfizer, Inc. USA Product Label http://www.vetdepot.com 235 E. 42ND ST., NEW YORK, NY, 10017 Telephone: 269-833-4000 Customer Service: 800-733-5500
4766 Research Dr. San Antonio, TX insightdentalsystems.com
 OVERVIEW OF THE INSIGHT DENTAL IMPLANT DELIVERY SYSTEM The IDS system comes in a unit dose implant system where its advantage provides sterile instrumentation in one single-use kit. It is organized to
OVERVIEW OF THE INSIGHT DENTAL IMPLANT DELIVERY SYSTEM The IDS system comes in a unit dose implant system where its advantage provides sterile instrumentation in one single-use kit. It is organized to
Human Umbilical Cord Blood CD34 + /133+ Progenitor Cell Care Manual
 Human Umbilical Cord Blood CD34 + /133+ Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.04 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+/133+ Progenitor Cells, cryopreserved Cryopreserved
Human Umbilical Cord Blood CD34 + /133+ Progenitor Cell Care Manual INSTRUCTION MANUAL ZBM0065.04 SHIPPING CONDITIONS Human Umbilical Cord Blood CD34+/133+ Progenitor Cells, cryopreserved Cryopreserved
Role of CB1 and CB2 cannabinoid receptors in the development of joint pain. induced by monosodium iodoacetate
 *Manuscript 1 1 1 1 1 1 1 Role of CB1 and CB cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate Carmen La Porta 1*, Simona Andreea Bura 1*, Auxiliadora Aracil-Fernández,
*Manuscript 1 1 1 1 1 1 1 Role of CB1 and CB cannabinoid receptors in the development of joint pain induced by monosodium iodoacetate Carmen La Porta 1*, Simona Andreea Bura 1*, Auxiliadora Aracil-Fernández,
FOCUS SubCell. For the Enrichment of Subcellular Fractions. (Cat. # ) think proteins! think G-Biosciences
 169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
169PR 01 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name FOCUS SubCell For the Enrichment of Subcellular Fractions (Cat. # 786 260) think
OPIOID ANTINOCICEPTION AND ANTIHYPERALGESIA IN COMBINATION WITH METABOTROPIC GLUTAMATE RECEPTOR ANTAGONISM. Dana Elaine Daugherty
 OPIOID ANTINOCICEPTION AND ANTIHYPERALGESIA IN COMBINATION WITH METABOTROPIC GLUTAMATE RECEPTOR ANTAGONISM Dana Elaine Daugherty A thesis submitted to the faculty of the University of North Carolina at
OPIOID ANTINOCICEPTION AND ANTIHYPERALGESIA IN COMBINATION WITH METABOTROPIC GLUTAMATE RECEPTOR ANTAGONISM Dana Elaine Daugherty A thesis submitted to the faculty of the University of North Carolina at
Type of intervention Anaesthesia. Economic study type Cost-effectiveness analysis.
 Comparison of the costs and recovery profiles of three anesthetic techniques for ambulatory anorectal surgery Li S T, Coloma M, White P F, Watcha M F, Chiu J W, Li H, Huber P J Record Status This is a
Comparison of the costs and recovery profiles of three anesthetic techniques for ambulatory anorectal surgery Li S T, Coloma M, White P F, Watcha M F, Chiu J W, Li H, Huber P J Record Status This is a
