A Validation Study of Patient Interview Data and Pharmacy Records for Antihypertensive, Statin, and Antidepressant Medication Use among Older Women
|
|
|
- Sophie Davidson
- 5 years ago
- Views:
Transcription
1 American Journal of Epidemiology Copyright 2004 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 159, No. 3 Printed in U.S.A. DOI: /aje/kwh038 A Validation Study of Patient Interview Data and Pharmacy Records for Antihypertensive, Statin, and Antidepressant Medication Use among Older Women Denise M. Boudreau 1,2, Janet R. Daling 3,4, Kathleen E. Malone 3,4, Jacqueline S. Gardner 2, David K. Blough 2, and Susan R. Heckbert 1,2,4 1 Center for Health Studies, Group Health Cooperative, Seattle, WA. 2 Department of Pharmacy, University of Washington, Seattle, WA. 3 Fred Hutchinson Cancer Research Center, Seattle, WA. 4 Department of Epidemiology, University of Washington, Seattle, WA. Received for publication March 24, 2003; accepted for publication July 31, A validation study evaluated the accuracy of self-reported use of commonly used medications among older women. Within a case-control study of breast cancer, drug information was ascertained by interview. Pharmacy records from 1990 to 1999 were obtained from a Washington State health maintenance organization (66% of subjects) and retail pharmacies (34% of subjects) on a sample of subjects (212 cases, 191 controls) and used as the gold standard. Recall accuracy was assessed for 6-month, 2-year, and 8-year time windows. Sensitivity of antihypertensive use was 92% (95% confidence interval (CI): 85, 96) for cases and controls in the 6-month period and slightly lower for the 2-year (90% (95% CI: 82, 94) and 87% (95% CI: 78, 92)) and 8-year (80% (95% CI: 69, 88) and 79% (95% CI: 68, 88)) periods. For statins, sensitivity was 83% (95% CI: 64, 93) for cases and 93% (95% CI: 69, 99) for controls in the 6-month period, 75% (95% CI: 55, 88) and 86% (95% CI: 60, 96) in the 2-year period, and 67% (95% CI: 42, 85) and 75% (95% CI: 41, 93) in the 8-year period. For self-report of antidepressants, sensitivities ranged from 66% (95% CI: 47, 80) in the 6-month period to 44% (95% CI: 30, 60) in the 8-year period. Specificity was high among all drug classes, ranging from 91% to 100%. Recall did not differ by case-control status. Trivial changes in estimates were observed when health maintenance organization records alone were used as the gold standard. Self-reported use of antihypertensives and statins appears to be relatively accurate among older women. antidepressive agents; antihypertensive agents; hydroxymethylglutaryl-coa reductase inhibitors; interviews; validation studies [publication type]; women Abbreviations: CI, confidence interval; GHC, Group Health Cooperative. Observational studies frequently use self-reported medication data. The quality of these data relies on the ability of subjects to recall information accurately. If medication exposures are not adequately recalled or are recalled differently among cases and controls, the misclassified exposure can lead to erroneous risk and prevalence estimates and results in decreased study power (1). The accuracy of self-reported hormone use has been evaluated in previous studies (2 15), but little work has been done to evaluate the accuracy of self-reported nonhormonal medication use (12, 15 19). In general, recall of hormone replacement therapy and oral contraceptives has been shown to be quite accurate and recall of other medications less accurate. To assess recall accuracy for medications, investigators have commonly used medical records or prescription records as the gold standard. Neither medical records nor pharmacy records are subject to recall bias, but pharmacy records are commonly considered more complete for assessment of medication use (20 23). We used pharmacy records from a health maintenance organization and two retail pharmacy chains to evaluate the accuracy of self-reported medication exposures for three (6-month, 2-year, and 8-year) time windows. The following drug classes were evaluated: anti- Correspondence to Dr. Denise M. Boudreau, Group Health Center for Health Studies, 1730 Minor Avenue, Suite 1600, Seattle, WA ( boudreau.d@ghc.org). 308
2 Validation Study of Interview Data and Pharmacy Records 309 hypertensives, statins (class of lipid-lowering drugs), and antidepressants. MATERIALS AND METHODS Study population Study subjects were a sample of women who participated in a population-based, case-control study of various medications and breast cancer risk (D. M. Boudreau, Group Health Cooperative Center for Health Studies, unpublished manuscript). Cases included 975 women identified from the Surveillance, Epidemiology, and End Results Registry of western Washington State who were diagnosed with primary invasive breast cancer in , whose names appeared on a list of Social Security recipients provided by the Centers for Medicare and Medicaid Services, and who were aged years at the time of diagnosis. The comparison group was 1,007 women without breast cancer randomly selected from the same list of Social Security recipients (24). The reference date was the date of breast cancer diagnosis for cases and a comparable date selected for controls. Subjects were identified for the validation study through informed consent forms that were obtained upon entry into the parent study. The names and locations of pharmacies where women had filled their prescriptions were ascertained, and consent was obtained to access pharmacy records for the past 10 years. Subjects without consent were not available for selection into the validation study (cases = 1.6 percent, controls = 7.3 percent). We selected a subset of cases and controls who reported filling prescription drugs exclusively at the retail pharmacy chains Bartell Drugs and Safeway Incorporated or who were enrollees of the health maintenance organization Group Health Cooperative (GHC) and filled any prescriptions at a GHC pharmacy. Reporting exclusive use of the two pharmacy chains was required because there was no means to validate prescriptions filled at other pharmacies. Bartell Drugs and Safeway Incorporated were chosen because they were among the top 10 most frequently used pharmacies by the case-control study participants, had a large number of branches in the study area, maintained computerized pharmacy records, reported keeping prescription records for more than 2 years, and were willing to participate. Of the 462 women in the parent study who reported using the two retail pharmacies, 158 reported using multiple pharmacies, 65 reported not using the pharmacies at the reference date, and 10 provided too little information for data abstraction. The pharmacy records of only 149 of the 229 remaining eligible subjects (65 percent) who reported exclusive use of either of these retail pharmacy chains were located. There were 288 GHC enrollees identified in the parent study, of whom 94 percent (271/288) were eligible and had pharmacy data available. We limited our study sample to include only women with at least 6 months of pharmacy records before the reference date (n = 403). Data collection In a structured in-person interview, participants provided an inventory of lipid-lowering medications, cardiovascular medications, and antidepressant medications used in the past 20 years. For each course of medication use, the brand name, strength, directions for use, indication(s) for use, and start and stop dates were recorded. Information on current medications was transcribed from the prescription labels on the medication containers. A calendar of life events and a photograph book of lipid-lowering drugs and antihypertensive drugs were used to improve subject recall. Information on the drug name, strength, quantity dispensed, fill date, and directions for use was obtained from pharmacy records for each prescription dispensed at GHC and retail pharmacies. Health maintenance organization enrollment dates were also obtained for GHC enrollees. GHC automated pharmacy records contain data on the prescriptions dispensed at GHC outpatient pharmacies since They are considered to be of high quality and complete, and these records have been used extensively for both primary epidemiologic studies of drug-disease hypotheses and methodological research (25). The completeness and quality of the retail pharmacy records are unknown. Therefore, we estimated our reliability measures of selfreported data using 1) combined data (GHC and retail) as the gold standard comparison and 2) only GHC data as the gold standard comparison. Exposure classification For each time window (6 months, 2 years, and 8 years) prior to the reference date, women were considered true users of a medication class if the pharmacy records indicated that they had filled at least two prescriptions for a drug belonging to that class during the time window. Only GHC enrollees had pharmacy records available for the 8-year period; therefore, reliability measures for this time window were calculated using only GHC data. The antihypertensive medication class included calcium channel blockers, betaadrenergic blockers, angiotensin II receptor antagonists, angiotensin-converting enzyme inhibitors, and diuretics (26). Serotonin reuptake inhibitors, tricyclic antidepressants, and miscellaneous antidepressants were included in the antidepressant drug class (26). are a unique class of drugs used to lower cholesterol (26). We determined the number of days a prescription should last (run-out period) on the basis of the pill quantity dispensed and directions for use. In the event of missing directions for use (42 percent of all dispensings), the average daily dose from all prescriptions with complete information filled for the drug of interest was used to estimate the missing daily dose. If another prescription was filled within 60 days after the end of the run-out period, we assumed that use was continuous during the interval between prescriptions. The duration of exposure to a given drug class was estimated by adding together the total number of days of use within the time windows. Using the interview data, we categorized women as users of a particular therapeutic class of medications if they reported using a drug whose name was included in the
3 310 Boudreau et al. class. The duration of exposure for the interview data was calculated by subtracting the start date from the stop date. As with the pharmacy data, all categorizations of use and estimates of duration were done for each of the three time windows. The majority of medication users had complete data on the start and stop year of use: 89 percent and 94 percent for antihypertensives, 97 percent and 98 percent for statins, and 88 percent and 90 percent for antidepressants. July was imputed for missing start months (60 percent of antihypertensives, 52 percent of statins, and 47 percent of antidepressants), and June was imputed for missing stop months (15 percent of antihypertensives, 14 percent of statins, and 16 percent of antidepressants). January was imputed as the start month when subjects knew the duration of use within a given year but not the exact start and stop month (3 percent of antihypertensives, 15 percent of statins, and 11 percent of antidepressants). If the start year or stop year could not be estimated from the interview, subjects were excluded from the analysis (less than five subjects were excluded from any analysis). Statistical analysis We compared self-reported use with that of combined pharmacy records (GHC and retail) and that of GHC pharmacy records. Using pharmacy records as the gold standard, we calculated the sensitivity, specificity, and respective 95 percent confidence intervals of self-reported drug use for each of the three drug classes (27, 28). The chi-square test for between-group differences was used to detect any differences in sensitivity or specificity between cases and controls. To evaluate the agreement between duration of use as reported by subjects and duration of use calculated from pharmacy records, the intraclass correlation coefficient was calculated using the 1,000-replication naïve bootstrap method (27, 29 32). The 2.5th and 97.5th percentiles of the bootstrap distribution of the 1,000 differences in intraclass correlation coefficients of cases and controls were then estimated (30). The intraclass correlation coefficients of cases and controls were not considered different if the intervals included zero. All reliability measures were estimated for the 6-month, 2- year, and 8-year time windows prior to the reference date. Study subjects without pharmacy records available for the entire time window of interest were excluded from those particular analyses. Analyses were stratified by breast cancer case-control status. RESULTS Cases and controls were comparable in all the characteristics shown in table 1, except that cases were more likely than controls (96 percent vs. 90 percent) to be Caucasian. These characteristics are consistent with participants of the casecontrol study from which our subjects were sampled. Of the 403 eligible women for whom pharmacy records were available for at least 6 months prior to the reference date (66 percent with GHC records, 34 percent with retail pharmacy records), 355 had pharmacy records available for at least 2 years (71 percent with GHC records, 29 percent with retail records), and 222 had pharmacy records available for at least 8 years (100 percent with GHC records). Among the 403 subjects included in the 6-month time window, 212 were breast cancer cases and 191 were controls. The prevalence of any medication use, according to pharmacy records, for each time window and by case-control status is summarized in table 1. Antihypertensive use ranged from 48 percent to 62 percent; statins, from 7 percent to 13 percent; and antidepressants, from 15 percent to 39 percent (table 1). Antihypertensives Of all the women classified as antihypertensive users during the 6 months prior to the reference date according to pharmacy records, 92 percent (95 percent confidence interval (CI): 85, 96) of cases and 92 percent (95 percent CI: 85, 96) of controls reported having used antihypertensives (table 2). The sensitivity of self-reported antihypertensive medication use among cases and controls during the 2 years prior to the reference date was 90 percent (95 percent CI: 82, 94) and 87 percent (95 percent CI: 78, 92). Specificity estimates ranged from 91 percent to 97 percent for the same two time periods. There was little change in the estimates when only GHC records were used as the gold standard (table 3). For the 8-year period, the sensitivity was 80 percent (95 percent CI: 69, 88) among cases and 79 percent (95 percent CI: 68, 88) among controls. Specificity was 100 percent for cases and controls in the 8-year period. Sensitivity and specificity estimates were similar between cases and controls. Intraclass correlation coefficient estimates for selfreported duration of antihypertensive medication use among cases and controls were 0.85 and 0.86 for the 6-month period and 0.89 and 0.90 for the 2-year period (table 4). Intraclass correlation coefficients for duration of antihypertensive medication use during the 6-month and 2-year periods were slightly higher when GHC pharmacy records alone were used as the gold standard (table 5). Intraclass correlation coefficient estimates for the 8-year period were 0.88 for cases and controls, but differences in the median duration of use between self-report and pharmacy records were noted in both groups. There was no difference in intraclass correlation coefficients between cases and controls as indicated by the confidence intervals of the distribution of differences that included zero. The sensitivity of self-reported statin use as compared with combined pharmacy records among cases and controls was 83 percent (95 percent CI: 64, 93) and 93 percent (95 percent CI: 69, 99) for the 6-month period and 75 percent (95 percent CI: 55, 88) and 86 percent (95 percent CI: 60, 96) for the 2-year period (table 2). All specificity estimates were close to 100 percent and did not differ between cases and controls. There was little change in the sensitivity or specificity estimates when only GHC records were used as the gold standard (table 3). Sensitivity and specificity estimates for the 8-year period were 67 percent (95 percent CI: 42, 85) and 100 percent (95 percent CI: 96, 100) in cases and 75
4 Validation Study of Interview Data and Pharmacy Records 311 TABLE 1. Characteristics of validation study subjects, Washington State, Cases Controls Total Patient characteristics No. % No. % No. % Age (years) Race* Caucasian African American Asian/Pacific Islander Other Annual income quartile ($) <20, ,000 34, ,000 49, , Missing Education (years) < > Marital status Single Married/living as married Widowed/divorced/separated Prevalence of medication use In 6 months before reference date Antihypertensives Antidepressants In 2 years before reference date Antihypertensives Antidepressants In 8 years before reference date Antihypertensives Antidepressants * Values significantly different between cases and controls. According to combined (Group Health Cooperative and retail) pharmacy records. Denominator: cases = 212, controls = 191, total = 403. Denominator: cases = 190, controls = 165, total = 355. Denominator: cases = 115, controls = 110, total = 225. percent (95 percent CI: 41, 93) and 99 percent (95 percent CI: 95, 100) in controls. Although controls appeared to recall statin use better than cases did in all the time periods, the sensitivity estimates were not statistically different. Intraclass correlation coefficients for the self-reported duration of statin use among cases and controls were 0.86 and 0.85 for the 6-month period and 0.89 and 0.85 for the 2- year period (table 4). Using only GHC records as the
5 312 Boudreau et al. TABLE 2. Sensitivity and specificity of self-reported drug use compared with pharmacy records for all women (Group Health Cooperative and retail users), by case-control status, Washington State, Cases Controls Therapeutic class Sensitivity (%) 95% CI* Specificity (%) 95% CI Sensitivity (%) 95% CI Specificity (%) 95% CI 6-month period Antihypertensives 92 (103), 85, (105) 85, (92) 85, (99) 86, (24) 64, (188) 95, (14) 69, (177) 95, 99 Antidepressants# 64 (36) 48, (175) 95, (29) 47, (162) 95, 99 2-year period Antihypertensives 90 (99) 82, (88) 89, (91) 78, (74) 91, (24) 55, (166) 97, (14) 60, (151) 95, 100 Antidepressants 56 (43) 41, (146) 95, (31) 41, (134) 93, 99 * CI, confidence interval. Antihypertensives include calcium channel blockers, beta-adrenergic blockers, angiotensin II receptor antagonists, angiotensin-converting enzyme inhibitors, and diuretics. Numbers in parentheses, denominator for the measure. Four subjects were excluded from the 6-month antihypertensive analysis, and three subjects were excluded from the 2-year antihypertensive analysis because of missing data on self-reported use. One subject was excluded from the 6-month and 2-year antidepressant analyses because of missing data on self-reported use. # Antidepressants include tricyclics, serotonin reuptake inhibitors, and miscellaneous (trazodone, buproprion, venlafaxine, and nefaxzodone). comparison group led to trivial changes in the intraclass correlation coefficient estimates (table 5). For the 8-year period, the intraclass correlation coefficient estimates were 0.69 in cases and 0.79 in controls, but differences in the median duration of use between self-report and pharmacy records were noted. There was no difference in intraclass correlation coefficients by case-control status. Antidepressants Among those classified by the combined pharmacy records as antidepressant users during the 6 months prior to the reference date, 64 percent (95 percent CI: 48, 78) of cases and 66 percent (95 percent CI: 47, 80) of controls recalled having used antidepressants during this period (table 2). The sensitivity of self-reported antidepressant medication use TABLE 3. Sensitivity and specificity of self-reported drug use compared with pharmacy records for Group Health Cooperative enrollees only, by case-control status, Washington State, Cases Controls Therapeutic class Sensitivity (%) 95% CI* Specificity (%) 95% CI Sensitivity (%) 95% CI Specificity (%) 95% CI 6-month period Antihypertensives 97 (61), 89, (71) 92, (62) 85, (71) 92, (13) 50, (121) 97, (10) 60, (123) 96, 100 Antidepressants 68 (22) 47, (112) 94, (21) 41, (112) 97, year period Antihypertensivesa 90 (61) 80, (63) 92, (66) 76, (60) 94, (13) 42, (113) 97, (11) 52, (115) 95, 100 Antidepressantsb 61 (28) 42, (98) 96, (24) 35, (102) 96, year period Antihypertensivesa 80 (70) 69, (44) 92, (63) 68, (47) 92, (15) 42, (100) 96, (8) 41, (102) 95, 100 Antidepressantsb 49 (45) 35, (70) 95, (36) 30, (74) 95, 100 * CI, confidence interval. Antihypertensives include calcium channel blockers, beta-adrenergic blockers, angiotensin II receptor antagonists, angiotensin-converting enzyme inhibitors, and diuretics. Numbers in parentheses, denominator for the measure. Two subjects were excluded from the 6-month and 2-year antihypertensives analyses, and one subject was excluded from the 8-year antihypertensive analysis because of missing data on self-reported use. Antidepressants include tricyclics, serotonin reuptake inhibitors, and miscellaneous (trazodone, buproprion, venlafaxine, and nefaxzodone).
6 Validation Study of Interview Data and Pharmacy Records 313 TABLE 4. Comparisons between self-report and pharmacy records for duration of use of various drug classes by all women (Group Health Cooperative and retail users), by case-control status, Washington State, Therapeutic class % of users Self-report Median months of use in users * CI, confidence interval. Antihypertensives include calcium channel blockers, beta-adrenergic blockers, angiotensin II receptor antagonists, angiotensin-converting enzyme inhibitors, and diuretics. Antidepressants include tricyclics, serotonin reuptake inhibitors, and miscellaneous (trazodone, buproprion, venlafaxine, and nefaxzodone). Group Health Cooperative and retail pharmacy records % of users Median months of use in users during the 2 years prior to the reference date was 56 percent (95 percent CI: 41, 70) for cases and 58 percent (95 percent CI: 41, 74) for controls. There was no consistent change in the sensitivity estimates when only GHC records were used as the gold standard for the 6-month and 2-year time periods (table 3). For the 8-year period, the sensitivity was 49 percent (95 percent CI: 35, 63) for cases and 44 percent (95 percent CI: 30, 60) for controls. Specificity estimates tended to approach or equal 100 percent for all time periods, regardless of which pharmacy records were used as the gold standard. All sensitivity and specificity estimates were similar between cases and controls. The intraclass correlation coefficients of self-reported duration of antidepressant medication use compared with those of combined pharmacy records for cases and controls were 0.77 and 0.67 for the 6-month period and 0.81 and 0.74 for the 2-year period prior to the reference date (table 4). Using only GHC records as the comparison group led to trivial changes in the intraclass correlation coefficient estimates and respective 95 percent confidence intervals (table 5). For the 8-year period, the intraclass correlation coefficients were 0.77 for cases and 0.79 for controls. There was no difference in the intraclass correlation coefficients of selfreported duration of antidepressant medication use between cases and controls. DISCUSSION Difference in median Intraclass correlation coefficient 6-month period Antihypertensives Cases , 0.91 Controls , 0.92 Cases , 0.93 Controls , 0.95 Antidepressants Cases , 0.87 Controls , year period Antihypertensives Cases , 0.94 Controls , 0.94 Cases , 0.95 Controls , 0.95 Antidepressants Cases , 0.90 Controls , % CI* In general, the recall accuracy of antihypertensives, antidepressants, and statins was best for the 6-month time window prior to the reference date and decreased slightly for the 2-year and 8-year time windows. The antihypertensive medication class had the highest overall sensitivities, followed by statins, and antidepressants. Our study results suggest some underascertainment and little overascertainment of self-reported medication use. In general, the duration of use was similar between self-report and pharmacy records, but both cases and controls tended to report a longer
7 314 Boudreau et al. TABLE 5. Comparisons between self-report and pharmacy records for duration of use of various drug classes by Group Health Cooperative enrollees only, by case-control status, Washington State, Therapeutic class % of users Self-report Median months of use in users Group Health Cooperative pharmacy records % of users Median months of use in users Difference in median Intraclass correlation coefficient 6-month period Antihypertensives Cases , 0.96 Controls , 0.97 Cases , 0.98 Controls , 0.97 Antidepressants Cases , 0.91 Controls , year period Antihypertensives Cases , 0.95 Controls , 0.98 Cases , 0.97 Controls , 0.95 Antidepressants Cases , 0.90 Controls , year period Antihypertensives Cases , 0.93 Controls , 0.93 Cases , 0.86 Controls , 0.95 Antidepressants Cases , 0.90 Controls , 0.92 * CI, confidence interval. Antihypertensives include calcium channel blockers, beta-adrenergic blockers, angiotensin II receptor antagonists, angiotensin-converting enzyme inhibitors, and diuretics. Antidepressants include tricyclics, serotonin reuptake inhibitors, and miscellaneous (trazodone, buproprion, venlafaxine, and nefaxzodone). 95% CI* duration of use than indicated by the pharmacy records. Recall accuracy of drug use and duration of use did not differ markedly between cases and controls. The recall accuracy reported from similar studies of nonhormonal medications is varied. Most published studies assessed agreement using the kappa statistic, while we used the intraclass correlation coefficient since it does not suffer from the many disadvantages of kappa (33, 34). The intraclass correlation coefficient can be a special case of weighted kappa when the classification categories are equally scaled along one dimension (first category is scored 1, second category is scored 2, etc.) (29). Paganini-Hill and Ross (12) validated self-reported medication use in breast cancer cases and controls, and they found no evidence to indicate that cases recall drug use differently from controls. The study was conducted in women aged years, and it used medical records as the gold standard. The level of agreement between interview and medical
8 Validation Study of Interview Data and Pharmacy Records 315 records was kappa = 0.60 for antihypertensives. For antihypertensive use, the percentage of agreement between medical chart and personal interview was 90 percent among cases and 85 percent among controls. The study did not evaluate antidepressants or statins. The Cardiovascular Health Study compared directed recall and medication inventory for current beta-blocker use (antihypertensive medication class) among adults aged 65 years or older (17). The researchers found a moderate level of agreement with kappa = 0.54 (95 percent CI: 0.51, 0.56) for beta blockers. The Rotterdam elderly study evaluated the agreement between patient interview and pharmacy records for cardiovascular drugs used in the 6 months prior to interview among adults aged 55 or more years (18). Agreement between the two measures varied from poor to almost perfect. However, commonly prescribed antihypertensive agents that were included in our study, such as atenolol (kappa = 0.96), furosemide (kappa = 0.90), and enalapril (kappa = 0.93), were found to have excellent agreement. Kehoe et al. (19) found similar results for recall of antihypertensive medications; self-reported antihypertensive medication use of at least 1 month was reported to have a high sensitivity (88 percent) and specificity (89 percent) when compared with physician report. The accuracy of self-reported exposure to cardiovascular drugs appears to be consistently good across the few published studies (12, 17 19). In addition to supporting these findings, our study extends the validity of antihypertensive medication recall to longer time windows and duration of use and across case-control status. No study to date has evaluated the accuracy of self-reported statin exposure, but we hypothesize that the relatively high sensitivity, specificity, and intraclass correlation coefficient estimates for both antihypertensives and statins are due to similarities in the prescribing of these drug classes. Both drug classes are generally used on a long-term basis, taken daily, and commonly indicated for chronic and often overlapping conditions (26). Unless complications or side effects occur, patients typically remain on the same drug. A photograph book of drugs, including statins and certain classes of antihypertensive drugs, was used in the parent case-control study interview to improve recall. Our estimates of reliability for the drug class of statins are somewhat limited by a low prevalence of drug use. were not regularly prescribed for women until the middle of the 1990s, and the drug class was not commonly included on health maintenance organization formularies for women until that time. Studying the reliability of self-reported statin use is becoming more feasible, as both the prevalence of use and the time available on the market increase. Cotterchio et al. (16) compared the accuracy of selfreported antidepressant medication use with that of physician records for adult female cancer cases (non-hodgkin s lymphoma, breast cancer, and kidney cancer) and controls. The study reported substantial agreement (kappa = 0.60, 95 percent CI: 0.47, 0.74; agreement = 80 percent) for overall lifetime antidepressant medication use ( ever or never ). Moderate agreement (kappa = 0.56, 95 percent CI: 0.32, 0.79) was reported for three categories of lifetime duration of antidepressant medication use, but the level of agreement was somewhat greater for cases than for controls. Our study findings combined with those of Cotterchio et al. (16) indicate only moderate recall accuracy of antidepressant medications, especially as compared with the other medications used for chronic conditions. The degree of misclassification appears to be similar for cases and controls. There are several reasons that may explain why antidepressants were recalled less accurately than antihypertensives and statins in this study. It is not uncommon for patients to misunderstand the indication for an antidepressant prescription. Patients commonly report antidepressant use for symptoms related to depression, such as insomnia or anxiety. In addition, antidepressants have broader indications for use as compared with antihypertensives and statins. It has been suggested that the underreporting of antidepressants and other socially undesirable medications is a result of the reluctance of persons to report such medication use (12, 16). Poor recall may also be related to depression, an indication for the prescription. Finally, unlike statins and antihypertensives, photographs of antidepressant medications were not available for the interview. It is reasonable to speculate that photographs of antidepressants might have improved recall of use. Study limitations should be considered when interpreting our study results. Pharmacy records are not subject to recall bias, but they cannot be considered 100 percent complete and do not address compliance. Patients may use medications differently from what is prescribed, which may explain why self-reported duration is longer than the duration calculated by pharmacy records. To minimize misclassifying nonusers who filled a prescription and did not ingest the drug as users in the pharmacy records, we considered subjects to be users of a drug class only if they filled at least two consecutive prescriptions for drugs belonging to that class. Pharmacy records at GHC and the retail pharmacies cover prescriptions filled at only their pharmacies. Subjects may have filled prescriptions at other pharmacies or obtained drug samples from physicians and, as a result, they may have been misclassified as nonusers according to the pharmacy records. We attempted to minimize this limitation by including only women reporting exclusive use of the retail pharmacies or continuous enrollment in the health maintenance organization, which was confirmed by health maintenance organization enrollment records. As a result, our findings may not be generalizable to all women if women who used multiple pharmacies recall medication use less accurately than do women who used only one pharmacy during the study period. Retail pharmacy records were unavailable for 35 percent of the 229 subjects reporting exclusive use of the two retail pharmacies. Routine purging of electronic pharmacy records after required time periods (2 years in Washington State) is the most likely reason for the missing records, however; it is unknown what influence excluding these subjects has on our findings. We attempted to obtain records on all prescriptions from the retail pharmacies and all prescriptions for the drugs of interest from GHC, but it is possible that prescriptions may have been missed by either source. All of the three drug classes studied contain prescription-only medications in the United States, which, unlike over-the-counter medications, are included in pharmacy records. Only commonly used drugs were included in
9 316 Boudreau et al. the antihypertensive and antidepressant drug categories, and caution should be used in generalizing the study findings to drugs or therapeutic classes not included. Retail pharmacy records are rarely used for research purposes. It is fairly well established that the majority of prescriptions from GHC providers are filled at GHC pharmacies (25), but the use of multiple pharmacies may be more likely among retail pharmacy users. However, we observed little change in the sensitivity, specificity, or intraclass correlation coefficient estimates when GHC pharmacy records alone were used as the gold standard. Our study suggests that older women accurately recall their antihypertensive and statin drug therapy. Recall of antidepressant medication use appeared to be less accurate, although this could be due in part to the less detailed approach used in the study to collect data on antidepressants versus the approach used for the other two drug categories. In general, drug recall does not appear to differ by cancer case-control status and, therefore, studies of the association between drug use and outcome will generally be biased toward the null (1). This bias can be substantial, even with high sensitivity and specificity estimates, if the prevalence of exposure in the nondiseased group is small (35). Care should be taken when generalizing our study findings to other populations and to other medications, but our data should prove useful to other epidemiologists concerned with the accuracy of drug information obtained through self-report. Further research in other populations and for other nonhormonal drug classes is needed. REFERENCES 1. Armstrong BK, White E, Saracci R. Principles of exposure measurement in epidemiology. Vol 21. Oxford, United Kingdom: Oxford University Press, Skegg DCG. Potential for bias in case-control studies of oral contraceptives and breast cancer. Am J Epidemiol 1988;127: Norell SE, Boethius G, Persson I. Oral contraceptive use: interview data versus pharmacy records. Int J Epidemiol 1998;27: Nischan P, Ebeling K, Thomas D, et al. Comparison of recalled and validated oral contraceptive histories. Am J Epidemiol 1993;138: Stolley PD, Tonascia JA, Sartwell PE, et al. Agreement rates between oral contraceptive users and prescribers in relation to drug use histories. Am J Epidemiol 1978;107: Coulter A, Vessey M, McPherson K, et al. The ability of women to recall their oral contraceptive histories. Contraception 1986;33: Rosenberg MJ, Layde PM, Ory HW, et al. Agreement between women s histories of oral contraceptive use and physician records. Int J Epidemiol 1983;12: Adam SA, Sheaves J, Wright NH, et al. A case-control study of the possible association between oral contraceptives and malignant melanoma. Br J Cancer 1981;44: Horowitz RI, Feinstein AR, Stremlau JR. Alternative data sources and discrepant results in case-control studies of estrogens and endometrial cancer. Am J Epidemiol 1980;111: Goodman MT, Nomura AM, Wilkens LR, et al. Agreement between interview information and physician records on the history of menopausal estrogen use. Am J Epidemiol 1990;131: Spengler RF, Clark EA, Woolever CA, et al. Exogenous estrogens and endometrial cancer: a case-control study and assessment of potential biases. Am J Epidemiol 1981;114: Paganini-Hill A, Ross R. Reliability of recall of drug usage and other health-related information. Am J Epidemiol 1982;116: Harlow S, Linet M. Agreement between questionnaire data and medical records. Am J Epidemiol 1989;142: Persson I, Bergkvist L, Adami HO. Reliability of women s histories of climacteric oestrogen treatment assessed by prescription forms. Int J Epidemiol 1987;16: West SL, Savitz DA, Koch G, et al. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol 1995;142: Cotterchio M, Kreiger N, Darlington G, et al. Comparison of self-reported and physician-reported antidepressant medication use. Ann Epidemiol 1999;9: Psaty BM, Lee M, Savage PJ, et al. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol 1992;45: Sjahid SI, Van der Linden PD, Stricker BH. Agreement between the pharmacy medication history and patient interview for cardiovascular drugs: the Rotterdam elderly study. Br J Clin Pharmacol 1998;45: Kehoe R, Wu SY, Leske MC, et al. Comparing self-reported and physician-reported medical history. Am J Epidemiol 1994; 139: West SL, Strom BL, Freundlich B, et al. Completeness of prescription recording in outpatient medical records from a health maintenance organization. J Clin Epidemiol 1994;47: West SL, Strom BL, Poole C. Validity of pharmacoepidemiology drug and diagnosis data. In: Strom B, ed. Pharmacoepidemiology. 2nd ed. Chichester, United Kingdom: John Wiley & Sons, Inc, 2000: Christensen DB, Williams B, Goldberg HI, et al. Comparison of prescription and medical records in reflecting patient antihypertensive drug therapy. Ann Pharmacother 1994;28: Heerdink LR, Leufkens HC, Koppedraaijer C, et al. Information on drug use in the elderly: a comparison of pharmacy, general practitioner, and patient data. Pharm World Sci 1995;17: Apodaca R, Judkins D, Lo A, et al. Sampling from CMS lists. In: Proceedings of the section on survey research methods. Alexandria, VA: American Statistical Association, 1992: Saunders KW, Stergachis A, VonKorff M. Group Health Cooperative of Puget Sound. In: Strom B, ed. Pharmacoepidemiology. 2nd ed. Chichester, United Kingdom: John Wiley & Sons, Inc, 1994: Sewester CS, Dombek CE, Olin BR, et al, eds. Drug facts and comparisons St Louis, MO: Facts & Comparisons, Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. Philadelphia, PA: Lippincott-Raven, Altman DG, Bryant TN, Gardner MJ. Statistics with confidence. 2nd ed. Bristol, United Kingdom: British Medical Journal Books, Fleiss J, Cohen J. The equivalence of weighed kappa and the intraclass correlation coefficient as measures of reliability. Educ Psychol Meas 1973;33: Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med 2000;19:
10 Validation Study of Interview Data and Pharmacy Records Morton AP, Dobson AJ. Assessing agreement. Med J Aust 1989;150: Fleiss JL. Statistical methods for rates and proportions. 2nd ed. New York, NY: John Wiley & Sons, Inc, Maclure M, Willett WC. Misinterpretation and misuse of the kappa statistic. Am J Epidemiol 1987;126: Lantz CA, Nebenzahl E. Behavior and interpretation of the kappa statistic: resolution of the two paradoxes. J Clin Epidemiol 1996;49: Copeland KT, Checkoway H, McMichael AJ, et al. Bias due to misclassification in the estimation of relative risk. Am J Epidemiol 1977;105:
Agreement between the pharmacy medication history and patient interview for cardiovascular drugs: the Rotterdam elderly study
 Br J Clin Pharmacol 1998; 45: 591 595 Agreement between the pharmacy and patient interview for cardiovascular drugs: the Rotterdam elderly study Suzy I. Sjahid, 1 Paul D. van der Linden 1 & Bruno H. Ch.
Br J Clin Pharmacol 1998; 45: 591 595 Agreement between the pharmacy and patient interview for cardiovascular drugs: the Rotterdam elderly study Suzy I. Sjahid, 1 Paul D. van der Linden 1 & Bruno H. Ch.
bias (epidemiology); estrogens; pharmacoepidemiology; progestational hormones; questionnaires; women MATERIALS AND METHODS
 American Journal of Epidemiology Copyright 2000 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 152,. 8 Printed in U.S.A. Questionnaire Assessment of Hormone
American Journal of Epidemiology Copyright 2000 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 152,. 8 Printed in U.S.A. Questionnaire Assessment of Hormone
Hypertension and diabetes treatments and risk of adverse outcomes among breast cancer patients. Lu Chen
 Hypertension and diabetes treatments and risk of adverse outcomes among breast cancer patients Lu Chen A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy
Hypertension and diabetes treatments and risk of adverse outcomes among breast cancer patients Lu Chen A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy
Presenter Disclosure Information
 Presenter Disclosure Information Soko Setoguchi, MD DrPH Prescription Drug Data: Advantages, Availability, and Access FINANCIAL DISCLOSURE: Grants/Research Support: NIH, AHRQ UNLABELED/UNAPPROVED USES
Presenter Disclosure Information Soko Setoguchi, MD DrPH Prescription Drug Data: Advantages, Availability, and Access FINANCIAL DISCLOSURE: Grants/Research Support: NIH, AHRQ UNLABELED/UNAPPROVED USES
Comparison of Two Instruments for Quantifying Intake of Vitamin and Mineral Supplements: A Brief Questionnaire versus Three 24-Hour Recalls
 American Journal of Epidemiology Copyright 2002 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 156, No. 7 Printed in U.S.A. DOI: 10.1093/aje/kwf097 Comparison of Two Instruments
American Journal of Epidemiology Copyright 2002 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 156, No. 7 Printed in U.S.A. DOI: 10.1093/aje/kwf097 Comparison of Two Instruments
A Comparison of Self-reported Medication Use to Actual Prescription Records
 Self-reported Medications and Prescription Records 1 A Comparison of Self-reported Medication Use to Actual Prescription Records Grace I. L. Caskie, Faika A. K. Zanjani, K. Warner Schaie, and Sherry L.
Self-reported Medications and Prescription Records 1 A Comparison of Self-reported Medication Use to Actual Prescription Records Grace I. L. Caskie, Faika A. K. Zanjani, K. Warner Schaie, and Sherry L.
Limin X. Clegg, 1 Arnold L. Potosky, 1 Linda C. Harlan, 1 Benjamin F. Hankey, 1 Richard M. Hoffman, 2,3 Janet L. Stanford, 4 and Ann S.
 American Journal of Epidemiology Copyright 001 by the Johns Hopkins University Bloomberg School of Public Health All rights reserved Vol. 154, No. 6 Printed in U.S.A. Self-reported Treatment for Prostate
American Journal of Epidemiology Copyright 001 by the Johns Hopkins University Bloomberg School of Public Health All rights reserved Vol. 154, No. 6 Printed in U.S.A. Self-reported Treatment for Prostate
Observational Study Designs. Review. Today. Measures of disease occurrence. Cohort Studies
 Observational Study Designs Denise Boudreau, PhD Center for Health Studies Group Health Cooperative Today Review cohort studies Case-control studies Design Identifying cases and controls Measuring exposure
Observational Study Designs Denise Boudreau, PhD Center for Health Studies Group Health Cooperative Today Review cohort studies Case-control studies Design Identifying cases and controls Measuring exposure
Reliability of Reported Age at Menopause
 American Journal of Epidemiology Copyright 1997 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 146, No. 9 Printed in U.S.A Reliability of Reported Age at Menopause
American Journal of Epidemiology Copyright 1997 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 146, No. 9 Printed in U.S.A Reliability of Reported Age at Menopause
2. Studies of Cancer in Humans
 346 IARC MONOGRAPHS VOLUME 72 2. Studies of Cancer in Humans 2.1 Breast cancer 2.1.1 Results of published studies Eight studies have been published on the relationship between the incidence of breast cancer
346 IARC MONOGRAPHS VOLUME 72 2. Studies of Cancer in Humans 2.1 Breast cancer 2.1.1 Results of published studies Eight studies have been published on the relationship between the incidence of breast cancer
THERE IS CONSIDERABLE EVIdence
 ORIGINAL CONTRIBUTION Relationship Between Long Durations and Different Regimens of Hormone Therapy and Risk of Breast Cancer Christopher I. Li, MD, PhD Kathleen E. Malone, PhD Peggy L. Porter, MD Noel
ORIGINAL CONTRIBUTION Relationship Between Long Durations and Different Regimens of Hormone Therapy and Risk of Breast Cancer Christopher I. Li, MD, PhD Kathleen E. Malone, PhD Peggy L. Porter, MD Noel
Comparison of Self-reported Fecal Occult Blood Testing with Automated Laboratory Records among Older Women in a Health Maintenance Organization
 American Journal of Epidemiology Copyright 01999 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol.150,. 6 Printed In USA. Comparison of Self-reported Fecal Occult
American Journal of Epidemiology Copyright 01999 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol.150,. 6 Printed In USA. Comparison of Self-reported Fecal Occult
No Association between Calcium Channel Blocker Use and Confirmed Bleeding Peptic Ulcer Disease
 American Journal of Epidemiology Copyright 1998 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 148, No. 4 Printed in U.S.A. A BRIEF ORIGINAL CONTRIBUTION No
American Journal of Epidemiology Copyright 1998 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 148, No. 4 Printed in U.S.A. A BRIEF ORIGINAL CONTRIBUTION No
Pharmacy Technician Course
 Pharmacy Technician Course VERSION HISTORY SECTION 1: History and Scope of the Pharmacy Technician Scope of the Pharmacy Technician Section 1 Quiz Check Your Knowledge Section 1 Quiz Answers SECTION 2:
Pharmacy Technician Course VERSION HISTORY SECTION 1: History and Scope of the Pharmacy Technician Scope of the Pharmacy Technician Section 1 Quiz Check Your Knowledge Section 1 Quiz Answers SECTION 2:
ORIGINAL INVESTIGATION. Hormone Replacement Therapy and Associated Risk of Stroke in Postmenopausal Women
 ORIGINAL INVESTIGATION Hormone Replacement Therapy and Associated Risk of Stroke in Postmenopausal Women Rozenn N. Lemaitre, PhD, MPH; Susan R. Heckbert, MD, PhD; Bruce M. Psaty, MD, PhD; Nicholas L. Smith,
ORIGINAL INVESTIGATION Hormone Replacement Therapy and Associated Risk of Stroke in Postmenopausal Women Rozenn N. Lemaitre, PhD, MPH; Susan R. Heckbert, MD, PhD; Bruce M. Psaty, MD, PhD; Nicholas L. Smith,
Role of Pharmacoepidemiology in Drug Evaluation
 Role of Pharmacoepidemiology in Drug Evaluation Martin Wong MD, MPH School of Public Health and Primary Care Faculty of Medicine Chinese University of Hog Kong Outline of Content Introduction: what is
Role of Pharmacoepidemiology in Drug Evaluation Martin Wong MD, MPH School of Public Health and Primary Care Faculty of Medicine Chinese University of Hog Kong Outline of Content Introduction: what is
Bias and confounding special issues. Outline for evaluation of bias
 EPIDEMIOLOGI BIAS special issues and discussion of paper April 2009 Søren Friis Institut for Epidemiologisk Kræftforskning Kræftens Bekæmpelse AGENDA Bias and confounding special issues Confounding by
EPIDEMIOLOGI BIAS special issues and discussion of paper April 2009 Søren Friis Institut for Epidemiologisk Kræftforskning Kræftens Bekæmpelse AGENDA Bias and confounding special issues Confounding by
Validity of data sources in pharmacoepidemiology
 Validity of data sources in pharmacoepidemiology Jesper Hallas MD DrMedSc Dept of clinical pharmacology University of Southern Denmark, Odense jhallas@health.sdu.dk Disposition 1. Defining validity 2.
Validity of data sources in pharmacoepidemiology Jesper Hallas MD DrMedSc Dept of clinical pharmacology University of Southern Denmark, Odense jhallas@health.sdu.dk Disposition 1. Defining validity 2.
Bias. Zuber D. Mulla
 Bias Zuber D. Mulla Explanations when you Observe or Don t Observe an Association Truth Chance Bias Confounding From Epidemiology in Medicine (Hennekens & Buring) Bias When you detect an association or
Bias Zuber D. Mulla Explanations when you Observe or Don t Observe an Association Truth Chance Bias Confounding From Epidemiology in Medicine (Hennekens & Buring) Bias When you detect an association or
Selection Bias in the Assessment of Gene-Environment Interaction in Case-Control Studies
 American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 158, No. 3 Printed in U.S.A. DOI: 10.1093/aje/kwg147 Selection Bias in the
American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 158, No. 3 Printed in U.S.A. DOI: 10.1093/aje/kwg147 Selection Bias in the
In 1981, we published results from a case-control. study involving 881 cases and 863 controls. not associated with any substantial overall risk,
 Br. J. Cancer (1986) 54, 825-832 Menopausal oestrogens and breast cancer risk: An expanded case-control study L.A. Brinton, R. Hoover & J.F. Fraumeni, Jr Environmental Epidemiology Branch, National Cancer
Br. J. Cancer (1986) 54, 825-832 Menopausal oestrogens and breast cancer risk: An expanded case-control study L.A. Brinton, R. Hoover & J.F. Fraumeni, Jr Environmental Epidemiology Branch, National Cancer
Validity of Methods Used to Assess Vitamin and Mineral Supplement Use
 American Journal of Epidemiology Copyright O 1998 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 148, No. 7 Printed In U.SA. Validity of Methods Used to Assess
American Journal of Epidemiology Copyright O 1998 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 148, No. 7 Printed In U.SA. Validity of Methods Used to Assess
RESEARCH. Katrina Wilcox Hagberg, 1 Hozefa A Divan, 2 Rebecca Persson, 1 J Curtis Nickel, 3 Susan S Jick 1. open access
 open access Risk of erectile dysfunction associated with use of 5-α reductase inhibitors for benign prostatic hyperplasia or alopecia: population based studies using the Clinical Practice Research Datalink
open access Risk of erectile dysfunction associated with use of 5-α reductase inhibitors for benign prostatic hyperplasia or alopecia: population based studies using the Clinical Practice Research Datalink
DECLARATION OF CONFLICT OF INTEREST
 DECLARATION OF CONFLICT OF INTEREST Warfarin and the risk of major bleeding events in patients with atrial fibrillation: a population-based study Laurent Azoulay PhD 1,2, Sophie Dell Aniello MSc 1, Teresa
DECLARATION OF CONFLICT OF INTEREST Warfarin and the risk of major bleeding events in patients with atrial fibrillation: a population-based study Laurent Azoulay PhD 1,2, Sophie Dell Aniello MSc 1, Teresa
NIH Public Access Author Manuscript Cancer Epidemiol Biomarkers Prev. Author manuscript; available in PMC 2011 January 1.
 NIH Public Access Author Manuscript Published in final edited form as: Cancer Epidemiol Biomarkers Prev. 2010 January ; 19(1): 144 147. doi:10.1158/1055-9965.epi-09-0807. Feasibility Study for Collection
NIH Public Access Author Manuscript Published in final edited form as: Cancer Epidemiol Biomarkers Prev. 2010 January ; 19(1): 144 147. doi:10.1158/1055-9965.epi-09-0807. Feasibility Study for Collection
Douglas A. Thoroughman, 1,2 Deborah Frederickson, 3 H. Dan Cameron, 4 Laura K. Shelby, 2,5 and James E. Cheek 2
 American Journal of Epidemiology Copyright 2002 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 155, No. 12 Printed in U.S.A. Racial Misclassification Thoroughman et al.
American Journal of Epidemiology Copyright 2002 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 155, No. 12 Printed in U.S.A. Racial Misclassification Thoroughman et al.
STAT 6395 Special Topics in Statistics: Epidemiology
 STAT 6395 Special Topics in Statistics: Epidemiology Heroy Science Hall, Room 127, Wednesday 2:00 p.m. - 4:50 p.m. Spring 2008 Instructor: Dr. Giovanni Filardo and Dr. H. K. Tony Ng Office: Heroy Science
STAT 6395 Special Topics in Statistics: Epidemiology Heroy Science Hall, Room 127, Wednesday 2:00 p.m. - 4:50 p.m. Spring 2008 Instructor: Dr. Giovanni Filardo and Dr. H. K. Tony Ng Office: Heroy Science
Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis.
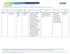 Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. DESCRIPTION NUMERATOR DENOMINATOR DM2012-12 Portion of Days Covered
Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. DESCRIPTION NUMERATOR DENOMINATOR DM2012-12 Portion of Days Covered
Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database
 open access Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database Carol Coupland, 1 Trevor Hill, 1 Richard Morriss,
open access Antidepressant use and risk of suicide and attempted suicide or self harm in people aged 20 to 64: cohort study using a primary care database Carol Coupland, 1 Trevor Hill, 1 Richard Morriss,
Use of Alendronate and Risk of Incident Atrial Fibrillation in Women
 ORIGINAL INVESTIGATION Use of Alendronate and Risk of Incident Atrial Fibrillation in Women Susan R. Heckbert, MD, PhD; Guo Li, MS; Steven R. Cummings, MD; Nicholas L. Smith, PhD; Bruce M. Psaty, MD, PhD
ORIGINAL INVESTIGATION Use of Alendronate and Risk of Incident Atrial Fibrillation in Women Susan R. Heckbert, MD, PhD; Guo Li, MS; Steven R. Cummings, MD; Nicholas L. Smith, PhD; Bruce M. Psaty, MD, PhD
W e have previously described the disease impact
 606 THEORY AND METHODS Impact numbers: measures of risk factor impact on the whole population from case-control and cohort studies R F Heller, A J Dobson, J Attia, J Page... See end of article for authors
606 THEORY AND METHODS Impact numbers: measures of risk factor impact on the whole population from case-control and cohort studies R F Heller, A J Dobson, J Attia, J Page... See end of article for authors
Are hypertensive elderly patients treated differently?
 ORIGINAL RESEARCH Are hypertensive elderly patients treated differently? Amy DG Huebschmann 1 Caroline Bublitz 2 Robert J Anderson 1 1 University of Colorado-Denver Health Sciences Center (UCDHSC), Department
ORIGINAL RESEARCH Are hypertensive elderly patients treated differently? Amy DG Huebschmann 1 Caroline Bublitz 2 Robert J Anderson 1 1 University of Colorado-Denver Health Sciences Center (UCDHSC), Department
Tips for Evolving Medicaid Pharmacy Benefits Management (PBM) Programs. June 5, 2015
 Tips for Evolving Medicaid Pharmacy Benefits Management (PBM) Programs 1 June 5, 2015 Introductions Mark Steck Pharm.D Independent Consultant, MAXIMUS John J.P. Crouse Vice President, MAXIMUS Market Lead
Tips for Evolving Medicaid Pharmacy Benefits Management (PBM) Programs 1 June 5, 2015 Introductions Mark Steck Pharm.D Independent Consultant, MAXIMUS John J.P. Crouse Vice President, MAXIMUS Market Lead
Characteristics of respondents and non-respondents from a case-control study of breast cancer in younger women
 International Epidemiological Association 2000 Printed in Great Britain International Journal of Epidemiology 2000;29:793 798 Characteristics of respondents and non-respondents from a case-control study
International Epidemiological Association 2000 Printed in Great Britain International Journal of Epidemiology 2000;29:793 798 Characteristics of respondents and non-respondents from a case-control study
Increasing Response to Mailed Questionnaires by Including a Pencil/Pen
 American Journal of Epidemiology Copyright ª 2005 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 162, No. 3 Printed in U.S.A. DOI: 10.1093/aje/kwi194 PRACTICE OF EPIDEMIOLOGY
American Journal of Epidemiology Copyright ª 2005 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 162, No. 3 Printed in U.S.A. DOI: 10.1093/aje/kwi194 PRACTICE OF EPIDEMIOLOGY
COMMENTARY: DATA ANALYSIS METHODS AND THE RELIABILITY OF ANALYTIC EPIDEMIOLOGIC RESEARCH. Ross L. Prentice. Fred Hutchinson Cancer Research Center
 COMMENTARY: DATA ANALYSIS METHODS AND THE RELIABILITY OF ANALYTIC EPIDEMIOLOGIC RESEARCH Ross L. Prentice Fred Hutchinson Cancer Research Center 1100 Fairview Avenue North, M3-A410, POB 19024, Seattle,
COMMENTARY: DATA ANALYSIS METHODS AND THE RELIABILITY OF ANALYTIC EPIDEMIOLOGIC RESEARCH Ross L. Prentice Fred Hutchinson Cancer Research Center 1100 Fairview Avenue North, M3-A410, POB 19024, Seattle,
Have you been paying for your prescription drugs? Stop!
 Dear Valued Medtipster Member: Have you been paying for your prescription drugs? Stop! Free prescription drugs are NOW just a Medtipster ID card away. Follow the steps below to obtain thousands of generic
Dear Valued Medtipster Member: Have you been paying for your prescription drugs? Stop! Free prescription drugs are NOW just a Medtipster ID card away. Follow the steps below to obtain thousands of generic
The prevalence and history of knee osteoarthritis in general practice: a case control study
 The Author (2005). Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oupjournals.org doi:10.1093/fampra/cmh700 Family Practice Advance Access
The Author (2005). Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oupjournals.org doi:10.1093/fampra/cmh700 Family Practice Advance Access
COMPARED WITH PLACEBO,
 IGINAL INVESTIGATION Esterified Estrogen and Conjugated Equine Estrogen and the Risk of Incident Myocardial Infarction and Stroke Rozenn N. Lemaitre, PhD, MPH; Noel S. Weiss, MD, DrPH; Nicholas L. Smith,
IGINAL INVESTIGATION Esterified Estrogen and Conjugated Equine Estrogen and the Risk of Incident Myocardial Infarction and Stroke Rozenn N. Lemaitre, PhD, MPH; Noel S. Weiss, MD, DrPH; Nicholas L. Smith,
In Australia, brand substitution of
 Pharmaceutical brand substitution in Australia are there multiple switches per prescription? Abstract Background: In Australia, brand substitution by pharmacists has been possible since 1994. There is
Pharmaceutical brand substitution in Australia are there multiple switches per prescription? Abstract Background: In Australia, brand substitution by pharmacists has been possible since 1994. There is
Supplementary Online Content
 Supplementary Online Content Song Z, Ayanian JZ, Wallace J, He Y, Gibson TB, Chernew ME. Unintended consequences of eliminating Medicare payments for consultations. JAMA Intern Med. Published online November
Supplementary Online Content Song Z, Ayanian JZ, Wallace J, He Y, Gibson TB, Chernew ME. Unintended consequences of eliminating Medicare payments for consultations. JAMA Intern Med. Published online November
JE Ashbury 1,2*, LE Lévesque 2,3, PA Beck 4, KJ Aronson 1,2. Abstract
 RESEARCH ARTICLE Open Access A population-based case-control study of Selective Serotonin Reuptake Inhibitors (SSRIs) and breast cancer: The impact of duration of use, cumulative dose and latency JE Ashbury
RESEARCH ARTICLE Open Access A population-based case-control study of Selective Serotonin Reuptake Inhibitors (SSRIs) and breast cancer: The impact of duration of use, cumulative dose and latency JE Ashbury
THE POSSIBLE ASSOCIATION BEtween
 ORIGINAL CONTRIBUTION Hormone Replacement Therapy in Relation to Breast Cancer Chi-Ling Chen, PhD Noel S. Weiss, MD, DrPH Polly Newcomb, PhD William Barlow, PhD Emily White, PhD THE POSSIBLE ASSOCIATION
ORIGINAL CONTRIBUTION Hormone Replacement Therapy in Relation to Breast Cancer Chi-Ling Chen, PhD Noel S. Weiss, MD, DrPH Polly Newcomb, PhD William Barlow, PhD Emily White, PhD THE POSSIBLE ASSOCIATION
A review of statistical methods in the analysis of data arising from observer reliability studies (Part 11) *
 A review of statistical methods in the analysis of data arising from observer reliability studies (Part 11) * by J. RICHARD LANDIS** and GARY G. KOCH** 4 Methods proposed for nominal and ordinal data Many
A review of statistical methods in the analysis of data arising from observer reliability studies (Part 11) * by J. RICHARD LANDIS** and GARY G. KOCH** 4 Methods proposed for nominal and ordinal data Many
Biases in clinical research. Seungho Ryu, MD, PhD Kanguk Samsung Hospital, Sungkyunkwan University
 Biases in clinical research Seungho Ryu, MD, PhD Kanguk Samsung Hospital, Sungkyunkwan University Learning objectives Describe the threats to causal inferences in clinical studies Understand the role of
Biases in clinical research Seungho Ryu, MD, PhD Kanguk Samsung Hospital, Sungkyunkwan University Learning objectives Describe the threats to causal inferences in clinical studies Understand the role of
Final Report 22 January 2014
 Final Report 22 January 2014 Cohort Study of Pioglitazone and Cancer Incidence in Patients with Diabetes Mellitus, Follow-up 1997-2012 Kaiser Permanente Division of Research Assiamira Ferrara, MD, Ph.D.
Final Report 22 January 2014 Cohort Study of Pioglitazone and Cancer Incidence in Patients with Diabetes Mellitus, Follow-up 1997-2012 Kaiser Permanente Division of Research Assiamira Ferrara, MD, Ph.D.
Timing of Menarche and First Full-Term Birth in Relation to Breast Cancer Risk
 American Journal of Epidemiology ª The Author 2007. Published by the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oxfordjournals.org.
American Journal of Epidemiology ª The Author 2007. Published by the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oxfordjournals.org.
Generalizing the right question, which is?
 Generalizing RCT results to broader populations IOM Workshop Washington, DC, April 25, 2013 Generalizing the right question, which is? Miguel A. Hernán Harvard School of Public Health Observational studies
Generalizing RCT results to broader populations IOM Workshop Washington, DC, April 25, 2013 Generalizing the right question, which is? Miguel A. Hernán Harvard School of Public Health Observational studies
Appendix This appendix was part of the submitted manuscript and has been peer reviewed. It is posted as supplied by the authors.
 Appendix This appendix was part of the submitted manuscript and has been peer reviewed. It is posted as supplied by the authors. Appendix to: Banks E, Crouch SR, Korda RJ, et al. Absolute risk of cardiovascular
Appendix This appendix was part of the submitted manuscript and has been peer reviewed. It is posted as supplied by the authors. Appendix to: Banks E, Crouch SR, Korda RJ, et al. Absolute risk of cardiovascular
Postmenopausal Estrogens and Risk of Myocardial Infarction in Diabetic Women NICHOLAS L. SMITH, PHD KATHERINE M. NEWTON, PHD BRUCE M.
 E p i d e m i o I o g y / H e a 11 h S e r v i c e s / P s y c h o s o c i a I R e s e a r c h N A L A R T I C L E Postmenopausal Estrogens and Risk of Myocardial Infarction in Diabetic Women ROBERT C.
E p i d e m i o I o g y / H e a 11 h S e r v i c e s / P s y c h o s o c i a I R e s e a r c h N A L A R T I C L E Postmenopausal Estrogens and Risk of Myocardial Infarction in Diabetic Women ROBERT C.
Improving Medical Statistics and Interpretation of Clinical Trials
 Improving Medical Statistics and Interpretation of Clinical Trials 1 ALLHAT Trial & ALLHAT Meta-Analysis Critique Table of Contents ALLHAT Trial Critique- Overview p 2-4 Critique Of The Flawed Meta-Analysis
Improving Medical Statistics and Interpretation of Clinical Trials 1 ALLHAT Trial & ALLHAT Meta-Analysis Critique Table of Contents ALLHAT Trial Critique- Overview p 2-4 Critique Of The Flawed Meta-Analysis
Depression and Anxiety Relative Risks in Women Newly Diagnosed with Vulvovaginal Atrophy and Dyspareunia
 Depression and Anxiety Relative Risks in Women Newly Diagnosed with Vulvovaginal Atrophy and Dyspareunia Moyneur, Erick MA 1, Dea, Katherine MSc 1, Derogatis, Len PhD 2, Labrie, Fernand MD, PhD 3 1 StatLog
Depression and Anxiety Relative Risks in Women Newly Diagnosed with Vulvovaginal Atrophy and Dyspareunia Moyneur, Erick MA 1, Dea, Katherine MSc 1, Derogatis, Len PhD 2, Labrie, Fernand MD, PhD 3 1 StatLog
Finland and Sweden and UK GP-HOSP datasets
 Web appendix: Supplementary material Table 1 Specific diagnosis codes used to identify bladder cancer cases in each dataset Finland and Sweden and UK GP-HOSP datasets Netherlands hospital and cancer registry
Web appendix: Supplementary material Table 1 Specific diagnosis codes used to identify bladder cancer cases in each dataset Finland and Sweden and UK GP-HOSP datasets Netherlands hospital and cancer registry
Diabetes and Decline in Heart Disease Mortality in US Adults JAMA. 1999;281:
 ORIGINAL CONTRIBUTION and Decline in Mortality in US Adults Ken Gu, PhD Catherine C. Cowie, PhD, MPH Maureen I. Harris, PhD, MPH MORTALITY FROM HEART disease has declined substantially in the United States
ORIGINAL CONTRIBUTION and Decline in Mortality in US Adults Ken Gu, PhD Catherine C. Cowie, PhD, MPH Maureen I. Harris, PhD, MPH MORTALITY FROM HEART disease has declined substantially in the United States
Appendix 1. Supplementary Methods
 Appendix 1. Supplementary Methods The report on one high-quality study 34 provided only estimates of associations between cervical cancer incidence and IUD use for less than 5 years (versus never) and
Appendix 1. Supplementary Methods The report on one high-quality study 34 provided only estimates of associations between cervical cancer incidence and IUD use for less than 5 years (versus never) and
Mail Service Pharmacy
 Mail Service s At A Glance Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation.
Mail Service s At A Glance Disclaimer: reserves the right to update its measures and measure sets to maintain measure relevancy and to remedy any unintended consequences that may arise during implementation.
Repeatability of a questionnaire to assess respiratory
 Journal of Epidemiology and Community Health, 1988, 42, 54-59 Repeatability of a questionnaire to assess respiratory symptoms in smokers CELIA H WITHEY,' CHARLES E PRICE,' ANTHONY V SWAN,' ANNA 0 PAPACOSTA,'
Journal of Epidemiology and Community Health, 1988, 42, 54-59 Repeatability of a questionnaire to assess respiratory symptoms in smokers CELIA H WITHEY,' CHARLES E PRICE,' ANTHONY V SWAN,' ANNA 0 PAPACOSTA,'
Medicaid Expansion & Adult Dental Benefits: Access to Dental Care among Low-Income Adults
 Medicaid Expansion & Adult Dental Benefits: Access to Dental Care among Low-Income Adults Astha Singhal BDS, MPH, PhD Assistant Professor, Health Policy & Health Services Research Boston University Henry
Medicaid Expansion & Adult Dental Benefits: Access to Dental Care among Low-Income Adults Astha Singhal BDS, MPH, PhD Assistant Professor, Health Policy & Health Services Research Boston University Henry
// Award Number: DAMD TITLE: Markers of Breast Cancer Risk in Women with Benign Breast Disease PRINCIPAL INVESTIGATOR:
 AD Award Number: DAMD17-00-1-0623 TITLE: Markers of Breast Cancer Risk in Women with Benign Breast Disease PRINCIPAL INVESTIGATOR: Margaret Mandelson, Ph.D. CONTRACTING ORGANIZATION: Group Health Cooperative
AD Award Number: DAMD17-00-1-0623 TITLE: Markers of Breast Cancer Risk in Women with Benign Breast Disease PRINCIPAL INVESTIGATOR: Margaret Mandelson, Ph.D. CONTRACTING ORGANIZATION: Group Health Cooperative
Racial Variation In Quality Of Care Among Medicare+Choice Enrollees
 Racial Variation In Quality Of Care Among Medicare+Choice Enrollees Black/white patterns of racial disparities in health care do not necessarily apply to Asians, Hispanics, and Native Americans. by Beth
Racial Variation In Quality Of Care Among Medicare+Choice Enrollees Black/white patterns of racial disparities in health care do not necessarily apply to Asians, Hispanics, and Native Americans. by Beth
In the 1700s patients in charity hospitals sometimes slept two or more to a bed, regardless of diagnosis.
 Control Case In the 1700s patients in charity hospitals sometimes slept two or more to a bed, regardless of diagnosis. This depicts a patient who finds himself lying with a corpse (definitely a case ).
Control Case In the 1700s patients in charity hospitals sometimes slept two or more to a bed, regardless of diagnosis. This depicts a patient who finds himself lying with a corpse (definitely a case ).
Epidemiology: Overview of Key Concepts and Study Design. Polly Marchbanks
 Epidemiology: Overview of Key Concepts and Study Design Polly Marchbanks Lecture Outline (1) Key epidemiologic concepts - Definition - What epi is not - What epi is - Process of epi research Lecture Outline
Epidemiology: Overview of Key Concepts and Study Design Polly Marchbanks Lecture Outline (1) Key epidemiologic concepts - Definition - What epi is not - What epi is - Process of epi research Lecture Outline
Update in Outpatient Medicine JNC 8, Hypertension and More
 Update in Outpatient Medicine JNC 8, Hypertension and More March 6 th 2015 Robert Gluckman, MD, FACP CMO Providence Health Plans Disclosures Stock Holdings Abbott Labs Abbvie Bristol Myers Squibb GE Proctor
Update in Outpatient Medicine JNC 8, Hypertension and More March 6 th 2015 Robert Gluckman, MD, FACP CMO Providence Health Plans Disclosures Stock Holdings Abbott Labs Abbvie Bristol Myers Squibb GE Proctor
Validation of Clinical Outcomes in Electronic Data Sources
 Validation of Clinical Outcomes in Electronic Data Sources Vincent Lo Re, MD, MSCE Assistant Professor of Medicine and Epidemiology Center for Clinical Epidemiology and Biostatistics Center for Pharmacoepidemiology
Validation of Clinical Outcomes in Electronic Data Sources Vincent Lo Re, MD, MSCE Assistant Professor of Medicine and Epidemiology Center for Clinical Epidemiology and Biostatistics Center for Pharmacoepidemiology
Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis.
 COMMUNITY PHARMACY V1.1 MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. # NAME DESCRIPTION NUMERATOR DENOMINATOR
COMMUNITY PHARMACY V1.1 MANDATORY S Note: Mandatory measures are those measures that are a requirement of accreditation and must be reported to URAC on an annual basis. # NAME DESCRIPTION NUMERATOR DENOMINATOR
The Risk of Fracture with Taking Alpha Blockers for Treating Benign Prostatic Hyperplasia
 J Prev Med Public Health 2009;42(3):165-170 DOI: 103961/jpmph2009423165 The Risk of Fracture with Taking Alpha Blockers for Treating Benign Prostatic Hyperplasia Joongyub Lee 1) Nam-Kyoung Choi 13) Sun-Young
J Prev Med Public Health 2009;42(3):165-170 DOI: 103961/jpmph2009423165 The Risk of Fracture with Taking Alpha Blockers for Treating Benign Prostatic Hyperplasia Joongyub Lee 1) Nam-Kyoung Choi 13) Sun-Young
Does Body Mass Index Adequately Capture the Relation of Body Composition and Body Size to Health Outcomes?
 American Journal of Epidemiology Copyright 1998 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 147, No. 2 Printed in U.S.A A BRIEF ORIGINAL CONTRIBUTION Does
American Journal of Epidemiology Copyright 1998 by The Johns Hopkins University School of Hygiene and Public Health All rights reserved Vol. 147, No. 2 Printed in U.S.A A BRIEF ORIGINAL CONTRIBUTION Does
Bias. A systematic error (caused by the investigator or the subjects) that causes an incorrect (overor under-) estimate of an association.
 Bias A systematic error (caused by the investigator or the subjects) that causes an incorrect (overor under-) estimate of an association. Here, random error is small, but systematic errors have led to
Bias A systematic error (caused by the investigator or the subjects) that causes an incorrect (overor under-) estimate of an association. Here, random error is small, but systematic errors have led to
Long-Term Effects on Medical Costs of Improving Depression Outcomes in Patients With Depression and Diabetes
 Epidemiology/Health Services Research O R I G I N A L A R T I C L E Long-Term Effects on Medical Costs of Improving Depression Outcomes in Patients With Depression and Diabetes WAYNE J. KATON, MD 1 JOAN
Epidemiology/Health Services Research O R I G I N A L A R T I C L E Long-Term Effects on Medical Costs of Improving Depression Outcomes in Patients With Depression and Diabetes WAYNE J. KATON, MD 1 JOAN
Title Page. Title Behavioral Influences on Controller Inhaler Use for Persistent Asthma in a Patient-Centered Medical Home
 Title Page Title Behavioral Influences on Controller Inhaler Use for Persistent Asthma in a Patient-Centered Medical Home Authors Sue J. Lee a, Kathleen J. Pincus a, PharmD, BCPS, Adrienne A. Williams,
Title Page Title Behavioral Influences on Controller Inhaler Use for Persistent Asthma in a Patient-Centered Medical Home Authors Sue J. Lee a, Kathleen J. Pincus a, PharmD, BCPS, Adrienne A. Williams,
Supplementary Table 4. Study characteristics and association between OC use and endometrial cancer incidence
 Supplementary Table 4. characteristics and association between OC use and endometrial cancer incidence a Details OR b 95% CI Covariates Region Case-control Parslov, 2000 (1) Danish women aged 25 49 yr
Supplementary Table 4. characteristics and association between OC use and endometrial cancer incidence a Details OR b 95% CI Covariates Region Case-control Parslov, 2000 (1) Danish women aged 25 49 yr
Comparing Vertical and Horizontal Scoring of Open-Ended Questionnaires
 A peer-reviewed electronic journal. Copyright is retained by the first or sole author, who grants right of first publication to the Practical Assessment, Research & Evaluation. Permission is granted to
A peer-reviewed electronic journal. Copyright is retained by the first or sole author, who grants right of first publication to the Practical Assessment, Research & Evaluation. Permission is granted to
Supplementary Online Content
 Supplementary Online Content Rollman BL, Herbeck Belnap B, Abebe KZ, et al. Effectiveness of online collaborative care for treating mood and anxiety disorders in primary care: a randomized clinical trial.
Supplementary Online Content Rollman BL, Herbeck Belnap B, Abebe KZ, et al. Effectiveness of online collaborative care for treating mood and anxiety disorders in primary care: a randomized clinical trial.
THE HYPOTHESIS THAT USE OF ANtibiotics
 ORIGINAL CONTRIBUTION Antibiotic Use in Relation to the Risk of Breast Cancer Christine M. Velicer, PhD Susan R. Heckbert, MD, PhD Johanna W. Lampe, PhD, RD John D. Potter, MD, PhD Carol A. Robertson,
ORIGINAL CONTRIBUTION Antibiotic Use in Relation to the Risk of Breast Cancer Christine M. Velicer, PhD Susan R. Heckbert, MD, PhD Johanna W. Lampe, PhD, RD John D. Potter, MD, PhD Carol A. Robertson,
9/25/15. Pharmacy Quality Measures: Financial Support. Learning Objectives. Speaker Disclosure. Access to Preferred Networks and Clinical Performance
 Pharmacy Quality Measures: Action Steps for Improvement Financial Support Financial support was provided for this activity through an unrestricted grant from Health Mart Systems, Inc. Christine Jacobson
Pharmacy Quality Measures: Action Steps for Improvement Financial Support Financial support was provided for this activity through an unrestricted grant from Health Mart Systems, Inc. Christine Jacobson
Repeat ischaemic heart disease audit of primary care patients ( ): Comparisons by age, sex and ethnic group
 Repeat ischaemic heart disease audit of primary care patients (2002-2003): Comparisons by age, sex and ethnic group Baseline-repeat ischaemic heart disease audit of primary care patients: a comparison
Repeat ischaemic heart disease audit of primary care patients (2002-2003): Comparisons by age, sex and ethnic group Baseline-repeat ischaemic heart disease audit of primary care patients: a comparison
RESEARCH. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database
 Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database Julia Hippisley-Cox, professor of clinical epidemiology and general practice,
Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database Julia Hippisley-Cox, professor of clinical epidemiology and general practice,
Research Article Estimating Measurement Error of the Patient Activation Measure for Respondents with Partially Missing Data
 BioMed Research International Volume 0, Article ID 0, pages http://dx.doi.org/0./0/0 Research Article Estimating Measurement Error of the Patient Activation Measure for Respondents with Partially Missing
BioMed Research International Volume 0, Article ID 0, pages http://dx.doi.org/0./0/0 Research Article Estimating Measurement Error of the Patient Activation Measure for Respondents with Partially Missing
TITLE: Outcomes of Screening Mammography in Elderly Women
 AD Award Number: DAMD17-00-1-0193 TITLE: Outcomes of Screening Mammography in Elderly Women PRINCIPAL INVESTIGATOR: Philip W. Chu Rebecca Smith-Bindman, M.D. CONTRACTING ORGANIZATION: University of California,
AD Award Number: DAMD17-00-1-0193 TITLE: Outcomes of Screening Mammography in Elderly Women PRINCIPAL INVESTIGATOR: Philip W. Chu Rebecca Smith-Bindman, M.D. CONTRACTING ORGANIZATION: University of California,
Supplementary Online Content
 Supplementary Online Content Choudhry NK, Krumme AA, Ercole PM, et al. Effect of reminder devices on medication adherence: the REMIND randomized clinical trial. JAMA Int Med. Published online February
Supplementary Online Content Choudhry NK, Krumme AA, Ercole PM, et al. Effect of reminder devices on medication adherence: the REMIND randomized clinical trial. JAMA Int Med. Published online February
TEACHERS TOPICS. The Role of Matching in Epidemiologic Studies. American Journal of Pharmaceutical Education 2004; 68 (3) Article 83.
 TEACHERS TOPICS American Journal of Pharmaceutical Education 2004; 68 (3) Article 83. The Role of Matching in Epidemiologic Studies Kevin W. Garey, PharmD College of Pharmacy, University of Houston Submitted
TEACHERS TOPICS American Journal of Pharmaceutical Education 2004; 68 (3) Article 83. The Role of Matching in Epidemiologic Studies Kevin W. Garey, PharmD College of Pharmacy, University of Houston Submitted
A Methodological Issue in the Analysis of Second-Primary Cancer Incidence in Long-Term Survivors of Childhood Cancers
 American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 158, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwg278 PRACTICE OF EPIDEMIOLOGY
American Journal of Epidemiology Copyright 2003 by the Johns Hopkins Bloomberg School of Public Health All rights reserved Vol. 158, No. 11 Printed in U.S.A. DOI: 10.1093/aje/kwg278 PRACTICE OF EPIDEMIOLOGY
DRUG UTILIZATION PATTERNS OF ANTIHYPERTENSIVES IN VARIOUS WARDS IN A TERTIARY CARE HOSPITAL IN TAMILNADU
 Original Article DRUG UTILIZATION PATTERNS OF ANTIHYPERTENSIVES IN VARIOUS WARDS IN A TERTIARY CARE HOSPITAL IN TAMILNADU V.Gowri 1, K.Punnagai, K.Vijaybabu 3, Dr.Darling Chellathai 4 1 Assistant Professor
Original Article DRUG UTILIZATION PATTERNS OF ANTIHYPERTENSIVES IN VARIOUS WARDS IN A TERTIARY CARE HOSPITAL IN TAMILNADU V.Gowri 1, K.Punnagai, K.Vijaybabu 3, Dr.Darling Chellathai 4 1 Assistant Professor
Unequal Numbers of Judges per Subject
 The Reliability of Dichotomous Judgments: Unequal Numbers of Judges per Subject Joseph L. Fleiss Columbia University and New York State Psychiatric Institute Jack Cuzick Columbia University Consider a
The Reliability of Dichotomous Judgments: Unequal Numbers of Judges per Subject Joseph L. Fleiss Columbia University and New York State Psychiatric Institute Jack Cuzick Columbia University Consider a
Maltreatment Reliability Statistics last updated 11/22/05
 Maltreatment Reliability Statistics last updated 11/22/05 Historical Information In July 2004, the Coordinating Center (CORE) / Collaborating Studies Coordinating Center (CSCC) identified a protocol to
Maltreatment Reliability Statistics last updated 11/22/05 Historical Information In July 2004, the Coordinating Center (CORE) / Collaborating Studies Coordinating Center (CSCC) identified a protocol to
Biases in clinical research. Seungho Ryu, MD, PhD Kanguk Samsung Hospital, Sungkyunkwan University
 Biases in clinical research Seungho Ryu, MD, PhD Kanguk Samsung Hospital, Sungkyunkwan University Learning objectives Understand goal of measurement and definition of accuracy Describe the threats to causal
Biases in clinical research Seungho Ryu, MD, PhD Kanguk Samsung Hospital, Sungkyunkwan University Learning objectives Understand goal of measurement and definition of accuracy Describe the threats to causal
Impact of Florida s Medicaid Reform on Recipients of Mental Health Services
 Impact of Florida s Medicaid Reform on Recipients of Mental Health Services Jeffrey Harman, PhD John Robst, PhD Lilliana Bell, MHA The Quality of Behavioral Healthcare : A Drive for Change Through Research
Impact of Florida s Medicaid Reform on Recipients of Mental Health Services Jeffrey Harman, PhD John Robst, PhD Lilliana Bell, MHA The Quality of Behavioral Healthcare : A Drive for Change Through Research
Jae Jin An, Ph.D. Michael B. Nichol, Ph.D.
 IMPACT OF MULTIPLE MEDICATION COMPLIANCE ON CARDIOVASCULAR OUTCOMES IN PATIENTS WITH TYPE II DIABETES AND COMORBID HYPERTENSION CONTROLLING FOR ENDOGENEITY BIAS Jae Jin An, Ph.D. Michael B. Nichol, Ph.D.
IMPACT OF MULTIPLE MEDICATION COMPLIANCE ON CARDIOVASCULAR OUTCOMES IN PATIENTS WITH TYPE II DIABETES AND COMORBID HYPERTENSION CONTROLLING FOR ENDOGENEITY BIAS Jae Jin An, Ph.D. Michael B. Nichol, Ph.D.
Rapid Case Ascertainment in Population and Hospital- Based Studies: Notes From the Field
 Rapid Case Ascertainment in Population and Hospital- Based Studies: Notes From the Field James R. Cerhan, M.D., Ph.D. Mayo Clinic College of Medicine University of Iowa College of Public Health Overview
Rapid Case Ascertainment in Population and Hospital- Based Studies: Notes From the Field James R. Cerhan, M.D., Ph.D. Mayo Clinic College of Medicine University of Iowa College of Public Health Overview
UNC Cancer Epidemiology Seminar: Cancer Risk in New Users of Overactive Bladder Drugs
 February 19, 2016 UNC Cancer Epidemiology Seminar: Cancer Risk in New Users of Overactive Bladder Drugs James A. Kaye, MD, DrPH Senior Director, Epidemiology, RTI Health Solutions Collaborators: Andrea
February 19, 2016 UNC Cancer Epidemiology Seminar: Cancer Risk in New Users of Overactive Bladder Drugs James A. Kaye, MD, DrPH Senior Director, Epidemiology, RTI Health Solutions Collaborators: Andrea
Predictors of DEXA Use and Guideline Performance for the Detection of Low Bone Mineral Density in Inflammatory Bowel Disease
 Predictors of DEXA Use and Guideline Performance for the Detection of Low Bone Mineral Density in Inflammatory Bowel Disease Jason Etzel Resident Research Forum Seattle VAMC 6/13/08 Background Increased
Predictors of DEXA Use and Guideline Performance for the Detection of Low Bone Mineral Density in Inflammatory Bowel Disease Jason Etzel Resident Research Forum Seattle VAMC 6/13/08 Background Increased
Alabama Medicaid Pharmacy Override
 Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
Alabama Medicaid Pharmacy Override Therapeutic Duplication, Early Refill, Maximum Unit, Prescription Limit Switchover, Dispense as Written, Accumulation Edit, Maintenance Supply Opt Out, and Maximum Cost
Adjuvant Hormonal Therapy for Breast Cancer and Risk of Hormone Receptor Specific Subtypes of Contralateral Breast Cancer
 Published Online First on August 25, 2009 as 10.1158/0008-5472.CAN-09-1355 Adjuvant Hormonal Therapy for Breast Cancer and Risk of Hormone Receptor Specific Subtypes of Contralateral Breast Cancer Christopher
Published Online First on August 25, 2009 as 10.1158/0008-5472.CAN-09-1355 Adjuvant Hormonal Therapy for Breast Cancer and Risk of Hormone Receptor Specific Subtypes of Contralateral Breast Cancer Christopher
Citation for published version (APA): Faber, A. (2006). Stimulant treatment in children: A Dutch perspective. s.n.
 University of Groningen Stimulant treatment in children Faber, Adrianne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
University of Groningen Stimulant treatment in children Faber, Adrianne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the
Multiple Mediation Analysis For General Models -with Application to Explore Racial Disparity in Breast Cancer Survival Analysis
 Multiple For General Models -with Application to Explore Racial Disparity in Breast Cancer Survival Qingzhao Yu Joint Work with Ms. Ying Fan and Dr. Xiaocheng Wu Louisiana Tumor Registry, LSUHSC June 5th,
Multiple For General Models -with Application to Explore Racial Disparity in Breast Cancer Survival Qingzhao Yu Joint Work with Ms. Ying Fan and Dr. Xiaocheng Wu Louisiana Tumor Registry, LSUHSC June 5th,
Statins and newly diagnosed diabetes
 DOI:10.1111/j.1365-2125.2004.02142.x British Journal of Clinical Pharmacology Statins and newly diagnosed diabetes Susan S. Jick & Brian D. Bradbury Boston Collaborative Drug Surveillance Program, 11 Muzzey
DOI:10.1111/j.1365-2125.2004.02142.x British Journal of Clinical Pharmacology Statins and newly diagnosed diabetes Susan S. Jick & Brian D. Bradbury Boston Collaborative Drug Surveillance Program, 11 Muzzey
The PROMPT criteria: Development and validation of prescribing indicators in middle-aged adults
 The PROMPT criteria: Development and validation of prescribing indicators in middle-aged adults Dr. Janine Cooper, Professor Carmel Hughes, Dr. Cristín Ryan, Professor Susan Smith, Dr. Emma Wallace, Dr.
The PROMPT criteria: Development and validation of prescribing indicators in middle-aged adults Dr. Janine Cooper, Professor Carmel Hughes, Dr. Cristín Ryan, Professor Susan Smith, Dr. Emma Wallace, Dr.
Daily short message service surveys detect greater HIV risk behavior than monthly clinic questionnaires in Kenya
 Daily short message service surveys detect greater HIV risk behavior than monthly clinic questionnaires in Kenya Kathryn Curran, Nelly Mugo, Ann Kurth, Kenneth Ngure, Renee Heffron, Deborah Donnell, Connie
Daily short message service surveys detect greater HIV risk behavior than monthly clinic questionnaires in Kenya Kathryn Curran, Nelly Mugo, Ann Kurth, Kenneth Ngure, Renee Heffron, Deborah Donnell, Connie
Relationship between Menopausal Hormone Therapy and Risk of Ductal, Lobular, and Ductal-Lobular Breast Carcinomas
 43 Relationship between Menopausal Hormone Therapy and Risk of Ductal, Lobular, and Ductal-Lobular Breast Carcinomas Christopher I. Li, 1 Kathleen E. Malone, 1 Peggy L. Porter, 1,2,3 Thomas J. Lawton,
43 Relationship between Menopausal Hormone Therapy and Risk of Ductal, Lobular, and Ductal-Lobular Breast Carcinomas Christopher I. Li, 1 Kathleen E. Malone, 1 Peggy L. Porter, 1,2,3 Thomas J. Lawton,
General practice. Abstract. Subjects and methods. Introduction. examining the effect of use of oral contraceptives on mortality in the long term.
 Mortality associated with oral contraceptive use: 25 year follow up of cohort of 46 000 women from Royal College of General Practitioners oral contraception study Valerie Beral, Carol Hermon, Clifford
Mortality associated with oral contraceptive use: 25 year follow up of cohort of 46 000 women from Royal College of General Practitioners oral contraception study Valerie Beral, Carol Hermon, Clifford
