Antiviral Therapy 2017; 22:61 70 (doi: /IMP3085)
|
|
|
- Rudolf Watkins
- 6 years ago
- Views:
Transcription
1 Antivirl Therpy 217; 22:61 7 (doi: /IMP385) Originl rticle Comprison of the Abbott RelTime HCV nd Roche COBAS Ampliprep/COBAS TqMn HCV ssys for the monitoring of sofosbuvir-bsed therpy Eiichi Ogw 1 *, Norihiro Furusyo 1, Msyuki Murt 1, Motohiro Shimizu 1, Kzuhiro Toyod 1, Teko Hott 2, Tkeshi Uchiumi 2, Jun Hyshi 3 1 Deprtment of Generl Internl Medicine, Kyushu University Hospitl, Fukuok, Jpn 2 Deprtment of Clinicl Chemistry nd Lbortory of Medicine, Kyushu University Hospitl, Fukuok, Jpn 3 Kyushu Generl Internl Medicine Center, Hrdoi Hospitl, Fukuok, Jpn *Corresponding uthor e-mil: eogw@gim.med.kyushu-u.c.jp Bckground: On-tretment HCV kinetics ply n invluble role in evluting the efficcy of interferon-bsed therpies. However, the importnce of HCV RNA monitoring hs not been well discussed concerning tretment with sofosbuvir (SOF)-bsed regimens, especilly for the utility of the Abbott RelTime HCV (ART) ssy. Methods: This study consisted of 151 ptients infected with HCV genotype-1 or -2, including ptients with prior tretment-experience or cirrhosis. HCV genotype-1 ptients were treted with SOF/ledipsvir nd genotype-2 ptients with SOF/ribvirin, both for 12 weeks. Seril mesurements of HCV RNA were performed with both the ART nd COBAS AmpliPrep/COBAS TqMn v2. (CAP/ CTM) ssys simultneously t weeks, 1, 2, 4, 6, 8, 1 nd 12 of tretment. Results: The rtes of HCV RNA trget not detected (TND) by ART were significntly lower thn those by CAP/CTM between weeks 2 nd 12 (end of tretment [EOT]), irrespective of prior tretment-experience or cirrhosis. 11 (11.6%) genotype-1 nd 8 (14.3%) genotype-2 ptients did not chieve HCV RNA TND by ART t EOT, in contrst to ll hving HCV RNA TND by CAP/CTM; however, ll chieved sustined virologicl response. The time t which HCV RNA becme TND or unquntifible ws not ssocited with tretment outcome by either the ART or CAP/CTM ssy. Conclusions: Over 1% of the ptients continued to hve detectble HCV RNA by ART t EOT, irrespective of HCV genotype, prior tretment-experience nd/or cirrhosis. However, prolonged residul HCV RNA ws not ssocited with tretment filure. Introduction An estimted 17 million people worldwide hve chronic HCV infection [1], which is mjor cuse of liver cirrhosis nd heptocellulr crcinom [2]. Of the rpidly evolving tretments for HCV, vrious interferonfree regimens bsed on direct-cting ntivirl gents (DAAs) hve become the gold stndrd [3,4], lthough mny ptients continue to be treted with pegylted interferon-lph (PEG-IFN-) nd ribvirin (RBV) in Asin nd Africn countries, which hve reltively high rtes of HCV prevlence [5]. On-tretment HCV kinetics ply n invluble role in evluting the efficcy of ntivirl therpies tht include PEG-IFN- nd RBV for chronic HCV infection [6 9]. In prticulr, it contributes to the prediction of tretment outcome nd to decisions on the tretment durtion. Rel-time PCR-bsed ssys with differing sensitivity nd ccurcy re commonly used in clinicl prctice. Among them is the most widely used HCV ssy, the Roche COBAS AmpliPrep/ COBAS TqMn HCV test (CAP/CTM). The utility of more sensitive ssy for very low viremi, the Abbott RelTime HCV (ART) ssy, hs been reported for DAA-bsed triple therpy [1 13]. The mjor difference between these ssys is the discordnce in the detection of HCV RNA t the lower limits of quntifiction, which could influence tretment outcome. Among the IFN-free therpies, regimens contining sofosbuvir (SOF; nucleotide NS5B polymerse inhibitor) re currently used in Europe, North Americ nd Jpn. According to rel-world cohort study, the sustined virologicl response (SVR) rte is over 9% for SOF nd ledipsvir (LDV; NS5A inhibitor) therpy, 217 Interntionl Medicl Press (print) (online) 61
2 E Ogw et l. with or without RBV, due to good tolerbility nd the ntivirl effect [14,15]. The importnce of on-tretment HCV RNA mesurement hs not been well discussed, nd there re questions bout the necessity of predicting tretment outcome becuse lmost ll ptients chieve rpid virologicl response. Recently, comprison of the effect of the CAP/CTM nd ART ssys for SOFbsed regimens ws reported [16,17]; however, there is limited dt on SOF/LDV for ptients with genotype-1, especilly for tretment-experienced nd/or cirrhotic ptients. The purpose of this study is to nlyse HCV kinetics during tretment with SOF/LDV for ptients with HCV genotype-1 nd with SOF/RBV for ptients with genotype-2, using the ART nd CAP/CTM v2. ssys to determine the ssocition of HCV RNA levels during tretment with tretment outcome. Methods Ptients This study consisted of 152 Jpnese ptients with confirmed chronic HCV genotype-1 or -2 infection, including ptients with prior tretment-experience or cirrhosis, who initited SOF-bsed therpy between June 215 nd Jnury 216. Eligible ptients were ged 2 yers nd older who hd HCV monoinfection. Exclusion criteri included lost to follow up (n=1), clinicl or biochemicl evidence of heptic decompenstion (Child Pugh B or C; n=), nd ny non-hcvrelted liver disese, such s utoimmune heptitis or primry biliry cholngitis (n=). After exclusions, the dt of 151 ptients ws vilble for nlysis. The study ws conducted in ccordnce with the ethicl principles of the 28 Declrtion of Helsinki, ws pproved by the Ethics Committee of Kyushu University Hospitl, nd is registered s clinicl study on the University Hospitl Medicl Informtion Network (ID 23115). Clinicl nd lbortory ssessment Clinicl prmeters were mesured by stndrd lbortory techniques t commercil lbortory (SRL Lbortory, Tokyo, Jpn). Blood smples were obtined t weeks, 2, 4, 6, 8, 1 nd 12 (end of tretment [EOT]), then t 4-week intervls until 12 weeks fter the end of tretment. Heptic fibrosis sttus ws ssessed by biopsy or trnsient elstogrphy (FibroScn). Liver biopsy ws performed within 1 month before the initition of tretment. The minimum length of the liver biopsy ws 15 mm, nd t lest 1 complete portl trcts were necessry for inclusion. For ech specimen, the stge of fibrosis ws estblished ccording to the METAVIR score [18]. We defined cirrhosis s METAVIR F4 or FibroScn >14.9 kp [19], which ws used if the META- VIR score ws unvilble. Genotyping of the interleukin-28b (IL28B; rs899917) gene ws performed using the ABI TqMn llelic discrimintion kit (75 RelTime PCR System; Applied Biosystems, Crlsbd, CA, USA). The llele of the IL28B single nucleoside polymorphism ws determined for ll studied ptients. Determintion of the HCV RNA level All serum smples were tested simultneously for HCV RNA level by the CAP/CTM v2. nd ART ssys. Testing ws performed t Kyushu University Hospitl ccording to the mnufcturer s instructions. Performnce chrcteristics of the two ssys hve been described in detil previously [13]. CAP/CTM ssy results re reported s trget not detected (TND), trget detected but lower limittion of quntifiction (TD/<LLOQ; <15 IU/ml) or bsolute number (quntifible results: 15 IU/ml). The rnge for liner quntifiction is between 15 nd IU/ml. ART ssy results re reported s TND, TD/<LLOQ (<12 IU/ml) or bsolute number (quntifible results: 12 IU/ml). The rnge of liner quntifiction is between 12 nd 1 8 IU/ml. Antivirl tretment nd tretment outcome All HCV genotype-1 ptients received combintion of SOF/LDV (Hrvoni; Giled Sciences KK, Tokyo, Jpn) for 12 weeks. All HCV genotype-2 ptients received SOF (Sovldi; Giled Sciences KK) nd RBV (Rebetol; MSD KK, Tokyo, Jpn; bsed on bodyweight, orlly) for 12 weeks. SVR ws defined s HCV RNA <LLOQ 12 weeks fter the end of tretment (SVR12) for both ssys. A virologicl relpse ws defined s recurrence of quntifible HCV RNA fter the end of tretment. Sttisticl nlysis Sttisticl nlyses were conducted using SPSS Sttistics version 22. (IBM SPSS Inc., Chicgo, IL, USA). Bseline continuous dt re expressed s medin (first third qurtiles) nd ctegoricl vribles re reported s frequencies nd percentges. Univrite nlysis ws done using the c 2 or Fisher s exct test, s pproprite. The positive predictive vlue (PPV) of undetectble nd unquntifible HCV RNA is the proportion of ptients with HCV RNA TND nd TND or TD/<LLOQ who chieved SVR12. The negtive predictive vlue (NPV) of detectble nd quntifible HCV RNA is the proportion of ptients with HCV RNA TD/<LLOQ or LLOQ nd LLOQ who filed tretment. These vlues were clculted for both ssys for ech tretment regimen t weeks 2, 4, 6, 8, 1 nd 12. A P-vlue less thn.5 ws regrded s sttisticlly significnt in ll nlyses Interntionl Medicl Press
3 HCV RNA monitoring of sofosbuvir-bsed therpy Results Ptient chrcteristics nd tretment outcome for HCV genotype-1 The bseline demogrphics nd clinicl chrcteristics of the 151 ptients included in this study re shown in Tble 1. Of the 95 (62.9%) infected with HCV genotype-1, lmost ll (n=94, 98.9%) were infected with HCV genotype-1b. Medin ge ws 69, with 87 (91.6%) of the ptients over 6, 52 (54.7%) tretmentexperienced nd 35 (36.8%) with cirrhosis. Of the tretment-experienced ptients, 2 (38.5%) hd been treted with DAA, such s triple therpy with telprevir (n=2) or simeprevir (SMV; n=15) or n IFN-free regimen with dcltsvir (DCV)/sunprevir (n=3). Tble 1. Bseline chrcteristics Genotype-1 Genotype-2 Number Mle 4 (42.1) 29 (51.8) Age, yers 69 (64 76) 64 (5 71) Body mss index, kg/m ( ) 22.4 ( ) Serum lbumin, g/l 4 (38 43) 41 (37 44) Alnine minotrnsferse, U/l 34 (24 5) 32 (18 6) g-glutmyl trnspeptidse, U/l 29 (19 48) 27 (14 5) egfr, ml/min/1.73 m 2 62 (52 72) 76 (66 86) -fetoprotein, ng/ml 5.5 ( ) 3. ( ) Prothrombin time, % 9 (75 98) 91 (78 12) Hemoglobin, g/l 136 ( ) 136 ( ) Pltelet count, 1 9 /l 147 (12 199) 166 (119 26) HCV RNA level, log 1 IU/ml ART, log 1 IU/ml 5.86 ( ) 6.3 ( ) CAP/CTM, v2., log 1 IU/ml 6.1 ( ) 6.3 ( ) HCV genotype 1 1 (1.1) 1b 94 (98.9) 2 27 (48.2) 2b 29 (51.8) Liver cirrhosis 35 (36.8) 15 (26.8) Dibetes mellitus 1 (1.6) 13 (23.2) IL28B SNPs (rs899917) TT 58 (61.1) 39 (69.6) TG/GG 37 (38.9) 17 (3.4) Tretment-nive 43 (45.3) 4 (71.4) Tretment-experienced PEG-IFN/RBV 32 (33.7) 16 (28.6) Telprevir-triple 2 (2.1) Simeprevir-triple 15 (15.8) DCV/ASV 3 (3.2) SVR12 93 (97.9) 5 (89.3) Dt re expressed s n (%) or medin (first third qurtiles). Triple therpy in combintion with PEG-IFN/RBV. ART, Abbott RelTime HCV ssy; CAP/CTM, Roche COBAS AmpliPrep/COBAS TqMn HCV ssy; DCV/ASV, dcltsvir/sunprevir; egfr, estimted glomerulr filtrtion rte; IL28B, interleukin-28b; PEG-IFN, pegylted interferon; RBV, ribvirin; SNP, single nucleoside polymorphism; SVR12, sustined virologicl response 12 weeks fter the end of tretment. All HCV genotype-1 ptients were treted with SOF/ LDV for 12 weeks without temporry interruption or discontinution nd 97.9% (93/95) chieved SVR12. The two non-svr ptients hd evidence of liver cirrhosis nd hd relpse fter the end of tretment. Ptient chrcteristics nd tretment outcome for HCV genotype-2 Of the 56 HCV genotype-2 ptients, 27 (48.2%) were infected with HCV genotype-2 nd 29 (51.8%) with -2b. Medin ge ws 64, with 34 (6.7%) of the ptients over 6, 16 tretment-experienced (28.6%) nd 15 with cirrhosis (26.8%). All tretment-experienced ptients hd been treted with PEG-IFN-/RBV. All HCV genotype-2 ptients were treted with SOF/RBV for 12 weeks without temporry interruption or discontinution nd 89.3% (5/56) chieved SVR12. None experienced virl brekthrough during tretment, in common with SOF/LDV for HCV genotype-1. Of the six non-svr ptients, five were infected with HCV genotype-2, three were tretment-experienced nd two hd evidence of liver cirrhosis. HCV kinetics during SOF/LDV tretment for HCV genotype-1 SOF/LDV tretment brought rpid HCV RNA reduction in the erly stge (medin HCV RNA level t week 1: 2.1 log IU/ml by CAP/CTM nd 1.91 log IU/ml by ART). Overll, the rtes of HCV RNA TND by ART were significntly lower thn those by CAP/CTM between weeks 2 nd 12, s shown in Figure 1A. Of note, there ws n extreme difference between the ssys in the HCV RNA TND rtes t weeks 4 nd 6 (82.1% nd 96.8% by CAP/CTM nd 33.7% nd 57.9% by ART, respectively; both P<.1). Similr results were found for the tretment-experienced nd cirrhotic groups, s shown in Figure 1B nd 1C. Moreover, 11 (11.6%) did not chieve HCV RNA TND by ART t EOT, in contrst to ll hving HCV RNA TND by CAP/CTM. HCV kinetics during SOF/RBV tretment for HCV genotype-2 SOF/RBV tretment lso brought rpid HCV RNA reduction in the erly stge (medin HCV RNA level t week 1: 1.9 log IU/ml by CAP/CTM nd 1.81 log IU/ ml by ART), similr to SOF/LDV tretment. Overll, the HCV RNA TND rtes by ART were significntly lower thn those by CAP/CTM between weeks 2 nd 12, s shown in Figure 2A. Of note, there ws n extreme difference between the ssys in the HCV RNA TND rtes t weeks 2, 4 nd 6 (44.6%, 82.1% nd 96.8% by CAP/CTM nd 12.5%, 39.3% nd 64.3% by ART, respectively; ll P<.1). Similr results were found in the tretment-experienced nd cirrhotic groups, s shown in Figure 2B nd 2C. The sme pttern ws seen Antivirl Therpy
4 E Ogw et l. Figure 1. Rtes of undetectble (trget not detected) HCV RNA during SOF/LDV tretment for HCV genotype-1 ptients A Genotype-1, SOF/LDV, overll B Genotype-1, SOF/LDV, tretment-experienced b c Rtes of undetectble HCV RNA (trget not detected), % w 2w 4w 6w 8w 1w 12w Rtes of undetectble HCV RNA (trget not detected), % b w 2w 4w 6w 8w 1w 12w C Rtes of undetectble HCV RNA (trget not detected), % b Genotype-1, SOF/LDV, cirrhosis w 2w 4w 6w 8w 1w 12w b c CAP/CTM ART (A) Overll (n=95), (B) tretment-experienced (n=52) nd (C) cirrhosis (n=35). P<.1; b P<.1; c P<.5. ART, Abbott RelTime HCV ssy; CAP/CTM, Roche COBAS AmpliPrep/COBAS TqMn HCV ssy; EOT, end of tretment; SOF/LDV, sofosbuvir/ledipsvir. s for SOF/LDV tretment, 8 (14.3%) did not chieve HCV RNA TND by ART t EOT, in contrst to ll hving HCV RNA TND by CAP/CTM. Moreover, residul HCV RNA (TD/<LLOQ) ws detected in two cses t week 4 fter the end of tretment, despite the ptients hving chieved SVR12. Predictive vlue of HCV RNA during SOF/LDV tretment for HCV genotype-1 The predictive vlues of undetectble (TND) nd unquntifible (TND or TD/<LLOQ) HCV RNA by ART during SOF/LDV tretment for HCV genotype-1 re shown in Tbles 2, 3, 4 nd 5. Of the Interntionl Medicl Press
5 HCV RNA monitoring of sofosbuvir-bsed therpy Figure 2. Rtes of undetectble (trget not detected) HCV RNA during SOF/RBV tretment for HCV genotype-2 ptients A Genotype-2, SOF/RBV, overll B Genotype-2, SOF/RBV, tretment-experienced 98.2 b 93.8 Rtes of undetectble HCV RNA (trget not detected), % w 2w 4w 6w 8w 1w 12w 85.7 Rtes of undetectble HCV RNA (trget not detected), % c w 2w 4w 6w 8w 1w 12w C Rtes of undetectble HCV RNA (trget not detected), % c 73.3 Genotype-2, SOF/RBV, cirrhosis w 2w 4w 6w 8w 1w 12w CAP/CTM ART (A) Overll (n=56), (B) tretment-experienced (n=16) nd (C) cirrhosis (n=15). P<.1; b P<.1; c P<.5. ART, Abbott RelTime HCV ssy; CAP/CTM, Roche COBAS AmpliPrep/COBAS TqMn HCV ssy; EOT, end of tretment; SOF/RBV, sofosbuvir/ribvirin. ptients treted with SOF/LDV, only two (2.1%) did not chieve SVR12. Both hd HCV RNA TND by ART t week 8, lthough they hd HCV RNA TND by CAP/CTM t week 2. Therefore, ll ptients with HCV RNA TND by ART t weeks 2, 4 nd 6 chieved SVR12 (PPV %). Otherwise, one (1.1%) hd HCV RNA LLOQ by ART t weeks 8 nd 1, but chieved SVR12. It should be noted tht 11 (11.6%) ptients hd HCV RNA TD/<LLOQ t EOT, but ll of them chieved SVR4 nd SVR12. We lso evluted the predictive vlues of HCV RNA by CAP/CTM, s shown in Additionl file 1. Most of the Antivirl Therpy
6 E Ogw et l. Tble 2. Predictive vlues of undetectble HCV RNA by ART during SOF/LDV therpy for HCV genotype-1 ptients (week 2 to 6) Week 2 Week 4 Week 6 TND TD/<LLOQ, LLOQ TND TD/<LLOQ, LLOQ TND TD/<LLOQ, LLOQ Number Sensitivity, % (n/totl n) 8.6 (8/93) 91.4 (85/93) 34.4 (32/93) 65.6 (61/93) 59.1 (55/93) 4.9 (38/93) Specificity, % (n/totl n) (2/2) (/2) (2/2) (/2) (2/2) (/2) PPV, % (n/totl n) (8/8) 97.7 (85/87) (32/32) 96.8 (61/63) (55/55) 95. (38/4) NPV, % (n/totl n) 2.3 (2/87) (/8) 3.2 (2/63) (/32) 5. (2/4) (/55) ART, Abbott RelTime HCV ssy; LLOQ, lower limit of quntifiction; NPV, negtive predictive vlue; PPV, positive predictive vlue; SOF/LDV, sofosbuvir/ledipsvir; TD, trget detected; TND, trget not detected. Tble 3. Predictive vlues of undetectble HCV RNA by ART during SOF/LDV therpy for HCV genotype-1 ptients (week 8 to 12) Week 8 Week 1 Week 12 TND TD/<LLOQ, LLOQ TND TD/<LLOQ, LLOQ TND TD/<LLOQ, LLOQ Number Sensitivity, % (n/totl n) 73.1 (68/93) 26.9 (25/93) 8.6 (75/93) 19.4 (18/93) 88.2 (82/93) 11.8 (11/93) Specificity, % (n/totl n) (/2) (2/2) (/2) (2/2) (/2) (2/2) PPV, % (n/totl n) 97.1 (68/7) (25/25) 97.4 (75/77) (18/18) 97.6 (82/84) (11/11) NPV, % (n/totl n) (/25) 2.9 (2/7) (/18) 2.6 (2/77) (/11) 2.4 (2/84) ART, Abbott RelTime HCV ssy; EOT, end of tretment; LLOQ, lower limit of quntifiction; NPV, negtive predictive vlue; PPV, positive predictive vlue; SOF/LDV, sofosbuvir/ledipsvir; TD, trget detected; TND, trget not detected. Tble 4. Predictive vlues of unquntifible HCV RNA by ART during SOF/LDV therpy for HCV genotype-1 ptients (week 2 to 6) Week 2 Week 4 Week 6 TND, TD/<LLOQ LLOQ TND, TD/<LLOQ LLOQ TND, TD/<LLOQ LLOQ Number Sensitivity, % (n/totl n) 4.9 (38/93) 59.1 (55/93) 8.6 (75/93) 19.4 (18/93) 95.7 (89/93) 4.3 (4/93) Specificity, % (n/totl n) 5. (1/2) 5. (1/2) (/2) (2/2) (/2) (2/2) PPV, % (n/totl n) 97.4 (38/39) 98.2 (55/56) 97.4 (75/77) (18/18) 97.8 (89/91) (4/4) NPV, % (n/totl n) 1.8 (1/56) 2.6 (1/39) (/18) 2.6 (2/77) (/4) 2.2 (2/91) ART, Abbott RelTime HCV ssy; LLOQ, lower limit of quntifiction; NPV, negtive predictive vlue; PPV, positive predictive vlue; SOF/LDV, sofosbuvir/ledipsvir; TD, trget detected; TND, trget not detected. Tble 5. Predictive vlues of unquntifible HCV RNA by ART during SOF/LDV therpy for HCV genotype-1 ptients (week 8 to 12) Week 8 Week 1 Week 12 TND, TD/<LLOQ LLOQ TND, TD/<LLOQ LLOQ TND, TD/<LLOQ LLOQ Number Sensitivity, % (n/totl n) 98.9 (92/93) 1.1 (1/93) 98.9 (92/93) 1.1 (1/93) (93/93) NA Specificity, % (n/totl n) (/2) (2/2) (/2) (2/2) (/2) NA PPV, % (n/totl n) 97.8 (89/91) (1/1) 97.8 (89/91) (1/1) 97.9 (93/95) NA NPV, % (n/totl n) (/4) 2.1 (2/94) (/4) 2.1 (2/94) NA NA ART, Abbott RelTime HCV ssy; EOT, end of tretment; LLOQ, lower limit of quntifiction; NPV, negtive predictive vlue; PPV, positive predictive vlue; SOF/LDV, sofosbuvir/ledipsvir; TD, trget detected; TND, trget not detected Interntionl Medicl Press
7 HCV RNA monitoring of sofosbuvir-bsed therpy ptients hd undetectble (n=78, 82.1%) nd unquntifible (n=92, 96.8%) HCV RNA t week 4, nd ll hd undetectble HCV RNA t week 8. High PPV nd low NPV vlues t ll points during tretment were seen for both the ART nd CAP/CTM ssys. Predictive vlue of HCV RNA during SOF/RBV tretment for HCV genotype-2 The predictive vlues of undetectble nd unquntifible HCV RNA by ART during SOF/RBV tretment for HCV genotype-2 re shown in Tbles 6, 7, 8 nd 9. Rpid virologicl response (HCV RNA TND) ws found for 7 (12.5%) ptients t week 2 nd for 22 (39.3%) ptients t week 4, but one (PPV 85.7%) nd five (PPV 89.8%) relpsed t the respective time points. Only one ptient (1.8%) hd HCV RNA LLOQ t weeks 8 nd 1, but chieved SVR12. However, s in the cse with SOF/LDV tretment, 8 (14.3%) ptients hd HCV RNA TD/<LLOQ t EOT, two of whom did not chieve SVR4 but did chieve SVR12. The predictive vlues of HCV RNA results by CAP/ CTM for genotype 2 ptients re shown in Additionl file 1. The CAP/CTM ssy ws less sensitive, indicting tht fewer ptients hd quntifible HCV RNA t weeks 2 nd 4 thn were identified by ART. Of the 15 ptients (26.8%) who hd HCV RNA LLOQ t week 2, 12 chieved SVR12 (PPV 8.%). This PPV vlue ws not significnt but ws lower thn tht of ptients who hd unquntifible HCV RNA t week 2 (PPV 92.7%, 38/41). Tken together, the dt indicte tht HCV RNA kinetics were not ssocited with tretment outcome, even with the more sensitive ART ssy, for either SOF/LDV or SOF/RBV tretment. Discussion Mesurement of HCV RNA during ntivirl tretment with PEG-IFN-/RBV hs been shown to be cliniclly useful for predicting tretment outcome nd for mking decisions on tretment durtion [6 9]. Furthermore, monitoring virl kinetics in the erly phse hs been reported to be beneficil for regimens tht include DAA in combintion with PEG-IFN-/ RBV [11 13,2,21]. However, with the pprovl of the IFN-free regimen with SOF, lmost ll ptients chieve SVR, regrdless of erly virl response t week 4. In this study, we followed the virl kinetics in 12-week regimens of SOF/LDV for genotype-1 nd SOF/RBV for genotype-2, using the ART nd CAP/ CTM v2. ssys. More thn 1% of the ptients hd HCV RNA TD/<LLOQ by ART t EOT for both tretments but ll chieved SVR12, indicting tht detectble residul HCV RNA t the end of SOF/LDV or SOF/RBV tretment ws not ssocited with tretment filure. Some studies used the CAP/CTM nd ART ssys to follow virl kinetics during DAA (NS3/4A protese inhibitor)-bsed triple therpy [1 13]. The time of HCV RNA TND ws significntly lter by ART thn by CAP/CTM; however, lte response by ART ws not relted to tretment filure, especilly in fvourble groups with mild fibrosis or prior relpse. ART ws useful for predicting tretment outcome only for difficult-to-tret groups, such s prior null response or dvnced fibrosis/cirrhosis [13]. Another merit of ART is tht HCV RNA TD/<LLOQ during the tretment stge with only PEG-IFN-/RBV (without DAA fter 12 weeks of triple therpy) indicted relpse, irrespective of HCV RNA TND by CAP/CTM [13]. The SOF/LDV regimen hs resulted in remrkbly high SVR rte, over 9% for ptients with genotype-1, even in rel-world clinicl prctice nd subgroups of ptients such s those with prior tretment-experience or who re cirrhotic [14,15]. The vlue of HCV RNA mesurement t specific points during the new tretments is in ensuring ptient dherence: high SVR rtes hve mde it less importnt to predict tretment outcome from on-tretment virl kinetics. However, it continues to be importnt to discuss the utility nd sensitivity of the HCV RNA ssys. In this study, we simultneously determined the HCV RNA level by both CAP/CTM nd ART t 2-week intervls during tretment nd rised three importnt findings in the detiled nlysis of our dt. First, HCV RNA remined detectble by ART for longer durtion during SOF-bsed tretment, irrespective of the HCV genotype. Notbly, the rtes of HCV RNA TND t week 4 were extremely different between the two ssys (genotype-1, CAP/CTM 82.1% nd ART 31.5%: genotype-2, CAP/CTM 85.7% nd ART 35.%; both P<.1). Msoumy et l. [17] provided similr results, but their rtes of HCV RNA TND t week 4 of SOF/DCV ± RBV (CAP/CTM 51% nd ART 1%) nd SOF/SMV ± RBV (CAP/CTM 55% nd ART 14%) therpy were lower thn ours for SOF/LDV. Nevertheless, the HCV RNA TND rtes t weeks 8 nd 12 were similr to our results. Second, detectble residul HCV RNA (TD/<LLOQ) t EOT (week 12) ws found for 11.6% of our genotype-1 ptients nd for 14.3% of those with genotype-2, irrespective of prior tretment experience or cirrhosis; however, ll of them chieved SVR12: this ws not ssocited with relpse by either SOF/LDV or SOF/RBV therpy. In two genotype-2 ptients, residul HCV RNA remined detectble t week 4 fter the end of tretment, but both chieved SVR12. Recently, similr results were reported by Sidhrthn et l. [16] for 6-week SOF-bsed tretment of tretment-nive ptients. This is mjor difference from IFN-bsed regimens in tht detectble residul HCV RNA t EOT ws, Antivirl Therpy
8 E Ogw et l. Tble 6. Predictive vlues of undetectble HCV RNA by ART during SOF/RBV therpy for HCV genotype-2 ptients (week 2 to 6) Week 2 Week 4 Week 6 TND TD/<LLOQ, LLOQ TND TD/<LLOQ, LLOQ TND TD/<LLOQ, LLOQ Number Sensitivity, % (n/totl n) 12. (6/5) 88. (44/5) 36. (18/5) 64. (32/5) 64. (32/5) 36. (18/5) Specificity, % (n/totl n) 83.3 (5/6) 16.7 (1/6) 33.3 (2/6) 66.7 (4/6) 33.3 (2/6) 66.7 (4/6) PPV, % (n/totl n) 85.7 (6/7) 89.8 (44/49) 81.8 (18/22) 94.1 (32/34) 88.9 (32/36) 9. (18/2) NPV, % (n/totl n) 1.2 (5/49) 14.3 (1/7) 5.9 (2/34) 18.2 (4/22) 1. (2/2) 11.1 (4/36) ART, Abbott RelTime HCV ssy; LLOQ, lower limit of quntifiction; NPV, negtive predictive vlue; PPV, positive predictive vlue; SOF/RBV, sofosbuvir/ribvirin; TD, trget detected; TND, trget not detected. Tble 7. Predictive vlues of undetectble HCV RNA by ART during SOF/RBV therpy for HCV genotype-2 ptients (week 8 to 12) Week 8 Week 1 Week 12 TND TD/<LLOQ, LLOQ TND TD/<LLOQ, LLOQ TND TD/<LLOQ, LLOQ Number Sensitivity, % (n/totl n) 72. (36/5) 28. (14/5) 76. (38/5) 2. (1/5) 84. (42/5) 16. (8/5) Specificity, % (n/totl n) 16.7 (1/6) 83.3 (5/6) (/6) (6/6) (/6) (6/6) PPV, % (n/totl n) 87.8 (36/41) 93.3 (14/15) 87. (4/46) (1/1) 87.5 (42/48) (8/8) NPV, % (n/totl n) 6.7 (1/15) 12.2 (5/41) (/1) 13. (6/46) (/8) 12.5 (6/48) ART, Abbott RelTime HCV ssy; EOT, end of tretment; LLOQ, lower limit of quntifiction; NPV, negtive predictive vlue; PPV, positive predictive vlue; SOF/RBV, sofosbuvir/ribvirin; TD, trget detected; TND, trget not detected. Tble 8. Predictive vlues of unquntifible HCV RNA by ART during SOF/RBV therpy for HCV genotype-2 ptients (week 2 to 6) Week 2 Week 4 Week 6 TND, TD/<LLOQ LLOQ TND, TD/<LLOQ LLOQ TND, TD/<LLOQ LLOQ Number Sensitivity, % (n/totl n) 54. (27/5) 46. (23/5) 82. (41/5) 18. (9/5) 94. (47/5) 6. (3/5) Specificity, % (n/totl n) 66.7 (4/6) 33.3 (2/6) 16.7 (1/6) 83.3 (5/6) 16.7 (1/6) 83.3 (5/6) PPV, % (n/totl n) 93.1 (27/29) 85.2 (23/27) 89.1 (41/46) 9. (9/1) 9.4 (47/52) 75. (3/4) NPV, % (n/totl n) 14.8 (4/27) 6.9 (2/29) 1. (1/1) 1.9 (5/46) 25. (1/4) 9.6 (5/52) ART, Abbott RelTime HCV ssy; LLOQ, lower limit of quntifiction; NPV, negtive predictive vlue; PPV, positive predictive vlue; SOF/RBV, sofosbuvir/ribvirin; TD, trget detected; TND, trget not detected. Tble 9. Predictive vlues of unquntifible HCV RNA by ART during SOF/RBV therpy for HCV genotype-2 ptients (week 8 to 12) Week 8 Week 1 Week 12 TND, TD/<LLOQ LLOQ TND, TD/<LLOQ LLOQ TND, TD/<LLOQ LLOQ Number Sensitivity, % (n/totl n) 98. (49/5) 2. (1/5) (5/5) NA (5/5) NA Specificity, % (n/totl n) (/6) (6/6) (/6) NA (/6) NA PPV, % (n/totl n) 89.1 (49/55) (1/1) 89.3 (5/56) NA 89.3 (5/56) NA NPV, % (n/totl n) (/1) 1.9 (6/55) NA NA NA NA ART, Abbott RelTime HCV ssy; EOT, end of tretment; LLOQ, lower limit of quntifiction; NPV, negtive predictive vlue; PPV, positive predictive vlue; SOF/RBV, sofosbuvir/ribvirin; TD, trget detected; TND, trget not detected Interntionl Medicl Press
9 HCV RNA monitoring of sofosbuvir-bsed therpy without exception, relpse sign in DAA-bsed triple therpy. A possible reson for this phenomenon cn be explined by the improvement of the host immune system due to the decline of the HCV RNA level [22,23]. Additionlly, the CAP/CTM ssy hs AmpErse (urcil-n-glycosylse) enzyme, which removes ny potentil contminting DNA from previous mplifictions; ny non-specific product is destroyed by the AmpErse enzyme, thus improving sensitivity nd specificity. Third, the time t which HCV RNA becme undetectble or unquntifible ws not ssocited with tretment outcome by either the ART or CAP/CTM ssy. In fct, PPV ws very high by both ssys t ll points during tretment. There is lck of dt evluting whether or not tretment durtion cn be individulized using on-tretment HCV kinetics, especilly for IFN-free regimens. However, frequent HCV RNA monitoring during SOF-bsed tretment my not be useful in predicting tretment outcome or for the determintion of tretment durtion in response-guided therpy, including for ptients with cirrhosis nd/or those with tretment experience, irrespective of the sensitivity of the HCV ssy. We were ble to provide detiled dt on the kinetics of HCV by the use of two rel-time ssys. Moreover, ll studied ptients were treted with the sme SOF-bsed regimens for fixed tretment durtion, ccording to their HCV genotype. However, the limittions of this study include its reltively smll popultion with tretment filure. We were ble to highlight the importnce of HCV RNA monitoring, but further exmintion in lrger trils will be needed to evlute the clinicl utility. In conclusion, the period of HCV RNA TD/<LLOQ ws significntly longer when mesured by ART thn by CAP/CTM for ptients treted with SOF/LDV or SOF/ RBV, irrespective of tretment-experience or cirrhosis. Furthermore, over 1% of the ptients remined HCV RNA TD/<LLOQ t EOT; however, prolonged HCV RNA TD/<LLOQ ws not ssocited with tretment filure. Contrry to tretment regimens with PEG-IFN/RBV, becuse of the high success rtes frequent monitoring of the HCV RNA level of ptients treted with DAAs my hve limited utility in predicting tretment outcome nd for guiding tretment durtion, irrespective of the sensitivity of the HCV ssy used. Acknowledgements We re grteful to Tkeo Hyshi, Fujiko Mitsumoto- Kseid, Koji Tkym, Kzuy Ur nd Sho Ymski for their ssistnce with dt collection nd to Yoshitk Etoh for his excellent lb work from the Deprtment of Generl Internl Medicine, Kyushu University Hospitl. We re lso grteful to Dongchon Kng from the Deprtment of Clinicl Chemistry nd Lbortory of Medicine, Kyushu University Hospitl. Disclosure sttement NF hs received grnts from Tisho Toym Phrmceuticl Co., Diichi Snkyo Co., Chugi Phrmceuticl Co., Mitsubishi Tnbe Phrm Co., Bristol Myers Squibb nd Jnssen Phrmceuticl KK. The other uthors declre tht they hve no conflicts of interest. This work ws supported by JSPS KAKENHI Grnt Number 15K Additionl file Additionl file 1: Supplementry informtion cn be found t documents/3912_ogw_addfile1.pdf References 1. Perz JF, Armstrong GL, Frrington LA, et l. The contributions of heptitis B virus nd heptitis C virus infections to cirrhosis nd primry liver cncer worldwide. J Heptol 26; 45: Alter HJ, Seeff LB. Recovery, persistence, nd sequele in heptitis C virus infection: perspective on long-term outcome. Semin Liver Dis 2; 2: AASLD/IDSA HCV Guidnce Pnel. Heptitis C guidnce: AASLD-IDSA recommendtions for testing, mnging, nd treting dults infected with heptitis C virus. Heptology 215; 62: Europen Assocition for Study of Liver. EASL Clinicl Prctice Guidelines: mngement of heptitis C virus infection. J Heptol 214; 6: Mohd Hnfih K, Groeger J, Flxmn AD, et l. Globl epidemiology of heptitis C virus infection: new estimtes of ge-specific ntibody to HCV seroprevlence. Heptology 213; 57: Ku A, Vermehren J, Srrzin C. Tretment predictors of sustined virologic response in heptitis B nd C. J Heptol 28; 49: Liu CH, Liu CJ, Lin CL, et l. Pegylted interferonlph-2 plus ribvirin for tretment-nive Asin ptients with heptitis C virus genotype 1 infection: multicenter, rndomized controlled tril. Clin Infect Dis 28; 47: Ferenci P, Lferl H, Scherzer TM, et l. Peginterferon lf-2 nd ribvirin for 24 weeks in heptitis C type 1 nd 4 ptients with rpid virologicl response. Gstroenterology 28; 135: Mngi A, Minerv N, Bcc D, et l. Individulized tretment durtion for heptitis C genotype 1 ptients: rndomized controlled tril. Heptology 28; 47: Ogw E, Furusyo N, Murt M, et l. Erly phse virl kinetics of chronic heptitis C ptients receiving telprevirbsed triple therpy: comprison of two rel-time PCR ssys. Antivirl Res 213; 99: Msoumy B, Hunydy B, Clvruso V, et l. Performnce of two HCV RNA ssys during protese inhibitor-bsed triple therpy in ptients with dvnced liver fibrosis nd cirrhosis. PLoS One 214; 9:e Fevery B, Susser S, Lenz O, et l. Comprison of three quntittive HCV RNA ssys in smples from HCV genotype 1- or 4-infected ptients treted with the NS3/4A protese inhibitor simeprevir. J Clin Virol 215; 72: Antivirl Therpy
10 E Ogw et l. 13. Ogw E, Furusyo N, Murt M, et l. Impct of HCV kinetics on tretment outcome differs by the type of reltime HCV ssy in NS3/4A protese inhibitor-bsed triple therpy. Antivirl Res 216; 126: Bckus LI, Belperio PS, Shhoumin TA, et l. Rel world effectiveness of ledipsvir/sofosbuvir in 4365 tretmentnive genotype 1 heptitis C infected ptients. Heptology 216; 64: Ionnou GN, Beste LA, Chng MF, et l. Effectiveness of sofosbuvir, ledipsvir/sofosbuvir, or pritprevir/ritonvir/ ombitsvir nd dsbuvir regimens for tretment of ptients with heptitis C in the Veterns Affirs Ntionl Helthcre System. Gstroenterology 216; 151: Sidhrthn S, Kohli A, Sims Z, et l. Utility of heptitis C virl lod monitoring on direct-cting ntivirl therpy. Clin Infect Dis 215; 6: Msoumy B, Vermehren J, Welker MW, et l. Clinicl vlue of on-tretment HCV RNA levels during different sofosbuvir-bsed ntivirl regimens. J Heptol 216; 65: The French METAVIR Coopertive Study Group. Introbserver nd interobserver vritions in liver biopsy interprettion in ptients with chronic heptitis C. Heptology 1994; 2: Ogw E, Furusyo N, Toyod K, et l. The longitudinl quntittive ssessment by trnsient elstogrphy of chronic heptitis C ptients treted with pegylted interferon lph-2b nd ribvirin. Antivirl Res 29; 83: Furusyo N, Ogw E, Murt M, et l. Therpeutic drug monitoring of telprevir in chronic heptitis C ptients receiving telprevir-bsed triple therpy is useful for predicting virologicl response. J Antimicrob Chemother 214; 69: Ogw E, Furusyo N, Kjiwr E, et l. Comprtive effectiveness nd sfety study of triple therpy with simeprevir or telprevir for non-cirrhotic ptients with chronic heptitis C virus genotype 1b infection. J Gstroenterol Heptol 215; 3: Meissner EG, Wu D, Osinusi A, et l. Endogenous intrheptic IFNs nd ssocition with IFN-free HCV tretment outcome. J Clin Invest 214; 124: Mrtin B, Hennecke N, Lohmnn V, et l. Restortion of HCV-specific CD8 + T cell function by interferon-free therpy. J Heptol 214; 61: Accepted 7 September 216; published online 15 September Interntionl Medicl Press
Abstract. Background. Aim. Patients and Methods. Patients. Study Design
 Impct of the Use of Drugs nd Substitution Tretments on the Antivirl Tretment of Chronic Heptitis C: Anlysis of Complince, Virologicl Response nd Qulity of Life (CHEOBS). Melin, 1 J.-. Lng, D. Ouzn, 3 M.
Impct of the Use of Drugs nd Substitution Tretments on the Antivirl Tretment of Chronic Heptitis C: Anlysis of Complince, Virologicl Response nd Qulity of Life (CHEOBS). Melin, 1 J.-. Lng, D. Ouzn, 3 M.
Clinical Study Report Synopsis Drug Substance Naloxegol Study Code D3820C00018 Edition Number 1 Date 01 February 2013 EudraCT Number
 EudrCT Number 2012-001531-31 A Phse I, Rndomised, Open-lbel, 3-wy Cross-over Study in Helthy Volunteers to Demonstrte the Bioequivlence of the Nloxegol 25 mg Commercil nd Phse III Formultions nd to Assess
EudrCT Number 2012-001531-31 A Phse I, Rndomised, Open-lbel, 3-wy Cross-over Study in Helthy Volunteers to Demonstrte the Bioequivlence of the Nloxegol 25 mg Commercil nd Phse III Formultions nd to Assess
CheckMate 153: Randomized Results of Continuous vs 1-Year Fixed-Duration Nivolumab in Patients With Advanced Non-Small Cell Lung Cancer
 CheckMte 53: Rndomized Results of Continuous vs -Yer Fixed-Durtion Nivolumb in Ptients With Advnced Non-Smll Cell Lung Cncer Abstrct 297O Spigel DR, McCleod M, Hussein MA, Wterhouse DM, Einhorn L, Horn
CheckMte 53: Rndomized Results of Continuous vs -Yer Fixed-Durtion Nivolumb in Ptients With Advnced Non-Smll Cell Lung Cncer Abstrct 297O Spigel DR, McCleod M, Hussein MA, Wterhouse DM, Einhorn L, Horn
Antiviral Therapy 2015; 20: (doi: /IMP2825)
 Antivirl Therpy 2015; 20:209 216 (doi: 10.3851/IMP2825) Originl rticle Cost-effectiveness of boceprevir co-dministrtion versus pegylted interferon-2b nd ribvirin only for ptients with heptitis C genotype
Antivirl Therpy 2015; 20:209 216 (doi: 10.3851/IMP2825) Originl rticle Cost-effectiveness of boceprevir co-dministrtion versus pegylted interferon-2b nd ribvirin only for ptients with heptitis C genotype
THE CHB TREATMENT GUIDELINE NAVIGATOR REVIEW AN ONLINE INTERACTIVE GUIDE FOR CLINICIANS FEATURING EXPERT AUDIO COMMENTARY
 The Americn Assocition for the Study of Liver Diseses () nd the Europen Assocition for the Study of Liver Disese () provide clinicl prctice guidelines for the mngement nd tretment of chronic heptitis B
The Americn Assocition for the Study of Liver Diseses () nd the Europen Assocition for the Study of Liver Disese () provide clinicl prctice guidelines for the mngement nd tretment of chronic heptitis B
SYNOPSIS Final Abbreviated Clinical Study Report for Study CA ABBREVIATED REPORT
 Finl Arevited Clinicl Study Report Nme of Sponsor/Compny: Bristol-Myers Squi Ipilimum Individul Study Tle Referring to the Dossier (For Ntionl Authority Use Only) Nme of Finished Product: Yervoy Nme of
Finl Arevited Clinicl Study Report Nme of Sponsor/Compny: Bristol-Myers Squi Ipilimum Individul Study Tle Referring to the Dossier (For Ntionl Authority Use Only) Nme of Finished Product: Yervoy Nme of
Supplementary Online Content
 Supplementry Online Content Zulmn DM, Pl Chee C, Ezeji-Okoye SC, et l. Effect of n intensive outptient progrm to ugment primry cre for high-need Veterns Affirs ptients: rndomized clinicl tril. JAMA Intern
Supplementry Online Content Zulmn DM, Pl Chee C, Ezeji-Okoye SC, et l. Effect of n intensive outptient progrm to ugment primry cre for high-need Veterns Affirs ptients: rndomized clinicl tril. JAMA Intern
Efficacy of Pembrolizumab in Patients With Advanced Melanoma With Stable Brain Metastases at Baseline: A Pooled Retrospective Analysis
 Efficcy of Pembrolizumb in Ptients With Advnced Melnom With Stble Brin Metstses t Bseline: A Pooled Retrospective Anlysis Abstrct 1248PD Hmid O, Ribs A, Dud A, Butler MO, Crlino MS, Hwu WJ, Long GV, Ancell
Efficcy of Pembrolizumb in Ptients With Advnced Melnom With Stble Brin Metstses t Bseline: A Pooled Retrospective Anlysis Abstrct 1248PD Hmid O, Ribs A, Dud A, Butler MO, Crlino MS, Hwu WJ, Long GV, Ancell
A review of the patterns of docetaxel use for hormone-resistant prostate cancer at the Princess Margaret Hospital
 MEDICAL ONCOLOGY A review of the ptterns of docetxel use for hormone-resistnt prostte cncer t the Princess Mrgret Hospitl S.N. Chin MD,* L. Wng MSc, M. Moore MD,* nd S.S. Sridhr MD MSc* ABSTRACT Bckground
MEDICAL ONCOLOGY A review of the ptterns of docetxel use for hormone-resistnt prostte cncer t the Princess Mrgret Hospitl S.N. Chin MD,* L. Wng MSc, M. Moore MD,* nd S.S. Sridhr MD MSc* ABSTRACT Bckground
All- Oral 12- Week Combina3on Treatment With Daclatasvir and Sofosbuvir in Pa3ents Infected With HCV Genotype 3: ALLY- 3 Phase 3 Study
 All- Orl 12- Week Combin3on Tretment With Dcltsvir nd Sofosbuvir in P3ents Infected With HCV Genotype 3: ALLY- 3 Phse 3 Study Nelson DR, 1 Cooper JN, 2 Llezri JP, 3 Lwitz E, 4 Pockros P, 5 Freilich BF,
All- Orl 12- Week Combin3on Tretment With Dcltsvir nd Sofosbuvir in P3ents Infected With HCV Genotype 3: ALLY- 3 Phse 3 Study Nelson DR, 1 Cooper JN, 2 Llezri JP, 3 Lwitz E, 4 Pockros P, 5 Freilich BF,
Efficacy of Sonidegib in Patients With Metastatic BCC (mbcc)
 AAD 216 eposter 3368 Efficcy of Sonidegib in Ptients With Metsttic BCC (mbcc) Colin Morton, 1 Michel Migden, 2 Tingting Yi, 3 Mnish Mone, 3 Dlil Sellmi, 3 Reinhrd Dummer 4 1 Stirling Community Hospitl,
AAD 216 eposter 3368 Efficcy of Sonidegib in Ptients With Metsttic BCC (mbcc) Colin Morton, 1 Michel Migden, 2 Tingting Yi, 3 Mnish Mone, 3 Dlil Sellmi, 3 Reinhrd Dummer 4 1 Stirling Community Hospitl,
Body mass index, waist-to-hip ratio, and metabolic syndrome as predictors of middle-aged men's health
 Originl Article - Sexul Dysfunction/Infertility pissn 2005-6737 eissn 2005-6745 Body mss index, wist-to-hip rtio, nd metbolic syndrome s predictors of middle-ged men's helth Jung Hyun Prk *, In-Chng Cho
Originl Article - Sexul Dysfunction/Infertility pissn 2005-6737 eissn 2005-6745 Body mss index, wist-to-hip rtio, nd metbolic syndrome s predictors of middle-ged men's helth Jung Hyun Prk *, In-Chng Cho
Age related differences in prognosis and prognostic factors among patients with epithelial ovarian cancer
 MOLECULAR AND CLINICAL ONCOLOGY 9: 329-334, 2018 Age relted differences in prognosis nd prognostic fctors mong ptients with epithelil ovrin cncer KENJI YOSHIKAWA, TAKESHI FUKUDA, RYO UEMURA, HIROAKI MATSUBARA,
MOLECULAR AND CLINICAL ONCOLOGY 9: 329-334, 2018 Age relted differences in prognosis nd prognostic fctors mong ptients with epithelil ovrin cncer KENJI YOSHIKAWA, TAKESHI FUKUDA, RYO UEMURA, HIROAKI MATSUBARA,
Introduction. These patients benefit less from conventional chemotherapy than patients identified as MMR proficient or microsatellite stable 3-5
 Nivolumb + Ipilimumb Combintion in Ptients With DNA Mismtch Repir-Deficient/Microstellite Instbility-High Metsttic Colorectl Cncer: First Report of the Full Cohort From CheckMte-142 Abstrct 553 André T,
Nivolumb + Ipilimumb Combintion in Ptients With DNA Mismtch Repir-Deficient/Microstellite Instbility-High Metsttic Colorectl Cncer: First Report of the Full Cohort From CheckMte-142 Abstrct 553 André T,
Risks for All-Cause Mortality: Stratified by Age, Estimated Glomerular Filtration Rate and Albuminuria
 Clinicl Prctice: Mini-Review Received: My 20, 2016 Accepted fter revision: December 14, 2016 Published online: Jnury 27, 2017 Risks for All-Cuse Mortlity: Strtified by Age, Estimted Glomerulr Filtrtion
Clinicl Prctice: Mini-Review Received: My 20, 2016 Accepted fter revision: December 14, 2016 Published online: Jnury 27, 2017 Risks for All-Cuse Mortlity: Strtified by Age, Estimted Glomerulr Filtrtion
Diabetes affects 29 million Americans, imposing a substantial
 CLINICAL Comprtive Effectiveness nd Costs of Insulin Pump Therpy for Dibetes Ronld T. Ackermnn, MD, MPH; Amish Wlli, MD, MS; Rymond Kng, MA; Andrew Cooper, MPH; Theodore A. Prospect, FSA, MAAA; Lewis G.
CLINICAL Comprtive Effectiveness nd Costs of Insulin Pump Therpy for Dibetes Ronld T. Ackermnn, MD, MPH; Amish Wlli, MD, MS; Rymond Kng, MA; Andrew Cooper, MPH; Theodore A. Prospect, FSA, MAAA; Lewis G.
PNEUMOVAX 23 is recommended by the CDC for all your appropriate adult patients at increased risk for pneumococcal disease 1,2 :
 PNEUMOVAX 23 is recommended y the CDC for ll your pproprite dult ptients t incresed risk for pneumococcl disese 1,2 : Adults ged
PNEUMOVAX 23 is recommended y the CDC for ll your pproprite dult ptients t incresed risk for pneumococcl disese 1,2 : Adults ged
Trends in antihypertensive and lipidlowering therapy in subjects with type II diabetes: clinical effectiveness or clinical discretion?
 ORIGINAL ARTICLE Trends in ntihypertensive nd lipidlowering therpy in subjects with type II dibetes: clinicl effectiveness or clinicl discretion? MC Gulliford, J Chrlton nd R Ltinovic Deprtment of Public
ORIGINAL ARTICLE Trends in ntihypertensive nd lipidlowering therpy in subjects with type II dibetes: clinicl effectiveness or clinicl discretion? MC Gulliford, J Chrlton nd R Ltinovic Deprtment of Public
Invasive Pneumococcal Disease Quarterly Report. July September 2017
 Invsive Pneumococcl Disese Qurterly Report July September 2017 Prepred s prt of Ministry of Helth contrct for scientific services by Rebekh Roos Helen Heffernn October 2017 Acknowledgements This report
Invsive Pneumococcl Disese Qurterly Report July September 2017 Prepred s prt of Ministry of Helth contrct for scientific services by Rebekh Roos Helen Heffernn October 2017 Acknowledgements This report
The Hepatitis C treatment landscape is changing in Pakistan
 The Heptitis C tretment lndscpe is chnging in Pkistn 01 A new dwn hs emerged in ccess to HCV Cure Ferozsons is herlding the chnge in the Heptitis C lndscpe of Pkistn In prtnership with Giled Sciences,
The Heptitis C tretment lndscpe is chnging in Pkistn 01 A new dwn hs emerged in ccess to HCV Cure Ferozsons is herlding the chnge in the Heptitis C lndscpe of Pkistn In prtnership with Giled Sciences,
Safety and Tolerability of Subcutaneous Sarilumab and Intravenous Tocilizumab in Patients With RA
 Sfety nd Tolerbility of Subcutneous Srilumb nd Intrvenous Tocilizumb in Ptients With RA Pul Emery, 1 Jun Rondon, 2 Anju Grg, 3 Hubert vn Hoogstrten, 3 Neil M.H. Grhm, 4 Ming Liu, 4 Nncy Liu, 3 Jnie Prrino,
Sfety nd Tolerbility of Subcutneous Srilumb nd Intrvenous Tocilizumb in Ptients With RA Pul Emery, 1 Jun Rondon, 2 Anju Grg, 3 Hubert vn Hoogstrten, 3 Neil M.H. Grhm, 4 Ming Liu, 4 Nncy Liu, 3 Jnie Prrino,
Case Report INTRODUCTION CASE REPORT. pissn eissn X
 pissn 2287-2728 eissn 2287-285X Cse Report Clinicl nd Moleculr Heptology 2018;24:424-429 Complete cure of dvnced heptocellulr crcinom with right drenl glnd metstsis nd portl vein thrombosis by multiple
pissn 2287-2728 eissn 2287-285X Cse Report Clinicl nd Moleculr Heptology 2018;24:424-429 Complete cure of dvnced heptocellulr crcinom with right drenl glnd metstsis nd portl vein thrombosis by multiple
Correlations Between Cytogenetic and Molecular Monitoring Among Patients With Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase
 Originl Articles Correltions Between Cytogenetic nd Moleculr Monitoring Among Ptients With Newly Dignosed Chronic Myeloid Leukemi in Chronic Phse Post Hoc Anlyses of the Rtionle nd Insight for Gleevec
Originl Articles Correltions Between Cytogenetic nd Moleculr Monitoring Among Ptients With Newly Dignosed Chronic Myeloid Leukemi in Chronic Phse Post Hoc Anlyses of the Rtionle nd Insight for Gleevec
Factors affecting screening for hepatocellular carcinoma
 204 Al Hsni F, et l., 2014; 13 (2): 204-210 ORIGINAL ARTICLE Mrch-April, Vol. 13 No. 2, 2014: 204-210 Fctors ffecting screening for heptocellulr crcinom Frh Al Hsni,* Mrin Knoepfli, Armin Gemperli, Attil
204 Al Hsni F, et l., 2014; 13 (2): 204-210 ORIGINAL ARTICLE Mrch-April, Vol. 13 No. 2, 2014: 204-210 Fctors ffecting screening for heptocellulr crcinom Frh Al Hsni,* Mrin Knoepfli, Armin Gemperli, Attil
Original Article. T Akter 1, N Islam 2, MA Hoque 3, S Khanam 4, HA khan 5, BK Saha 6. Abstract:
 Fridpur Med. Coll. J. 214;9(2):61-67 Originl Article Nebuliztion by Isotonic Mgnesium Sulphte Solution with Provide Erly nd Better Response s Compred to Conventionl Approch ( Plus Norml Sline) in Acute
Fridpur Med. Coll. J. 214;9(2):61-67 Originl Article Nebuliztion by Isotonic Mgnesium Sulphte Solution with Provide Erly nd Better Response s Compred to Conventionl Approch ( Plus Norml Sline) in Acute
Community. Profile Big Horn County. Public Health and Safety Division
 Community Helth Profile 2015 Big Horn County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community Helth Profile 2015 Big Horn County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
The RUTHERFORD-2 trial in heterozygous FH: Results and implications
 The RUTHERFORD-2 tril in heterozygous FH: Results nd implictions Slide deck kindly supplied s n eductionl resource by Professor Derick Rl MD PhD Crbohydrte & Lipid Metbolism Reserch Unit University of
The RUTHERFORD-2 tril in heterozygous FH: Results nd implictions Slide deck kindly supplied s n eductionl resource by Professor Derick Rl MD PhD Crbohydrte & Lipid Metbolism Reserch Unit University of
Effect on Glycemic, Blood Pressure, and Lipid Control according to Education Types
 Originl Article http://dx.doi.org/10.4093/dmj.2011.35.6.580 pissn 2233-6079 eissn 2233-6087 D I A B E T E S & M E T A B O L I S M J O U R N A L Effect on Glycemic, Blood Pressure, nd Lipid Control ccording
Originl Article http://dx.doi.org/10.4093/dmj.2011.35.6.580 pissn 2233-6079 eissn 2233-6087 D I A B E T E S & M E T A B O L I S M J O U R N A L Effect on Glycemic, Blood Pressure, nd Lipid Control ccording
The Acute Time Course of Concurrent Activation Potentiation
 Mrquette University e-publictions@mrquette Exercise Science Fculty Reserch nd Publictions Exercise Science, Deprtment of 1-1-2010 The Acute Time Course of Concurrent Activtion Potentition Luke Grceu Mrquette
Mrquette University e-publictions@mrquette Exercise Science Fculty Reserch nd Publictions Exercise Science, Deprtment of 1-1-2010 The Acute Time Course of Concurrent Activtion Potentition Luke Grceu Mrquette
Community. Profile Powell County. Public Health and Safety Division
 Community Helth Profile 2015 Powell County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Community Helth Profile 2015 Powell County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Introduction. Open Access
 Clin Chem Lb Med 2017; 55(4): 517 521 Open Access Evelyn Stelzl, Hnnh M. Appel, Rochk Meht, Ed G. Mrins, Jörg Berg, Christin Pr, Hnn Zurl, Brigitte I. Sntner nd Hrld H. Kessler* Evlution of the new cobs
Clin Chem Lb Med 2017; 55(4): 517 521 Open Access Evelyn Stelzl, Hnnh M. Appel, Rochk Meht, Ed G. Mrins, Jörg Berg, Christin Pr, Hnn Zurl, Brigitte I. Sntner nd Hrld H. Kessler* Evlution of the new cobs
Community. Profile Yellowstone County. Public Health and Safety Division
 Community Helth Profile 2015 Yellowstone County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community Helth Profile 2015 Yellowstone County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community. Profile Anaconda- Deer Lodge County. Public Health and Safety Division
 Community Helth Profile 2015 Ancond- Deer Lodge County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12
Community Helth Profile 2015 Ancond- Deer Lodge County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12
Community. Profile Lewis & Clark County. Public Health and Safety Division
 Community Helth Profile 2015 Lewis & Clrk County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community Helth Profile 2015 Lewis & Clrk County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl
Community. Profile Missoula County. Public Health and Safety Division
 Community Helth Profile 2015 Missoul County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Community Helth Profile 2015 Missoul County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
XII. HIV/AIDS. Knowledge about HIV Transmission and Misconceptions about HIV
 XII. HIV/AIDS Knowledge bout HIV Trnsmission nd Misconceptions bout HIV One of the most importnt prerequisites for reducing the rte of HIV infection is ccurte knowledge of how HIV is trnsmitted nd strtegies
XII. HIV/AIDS Knowledge bout HIV Trnsmission nd Misconceptions bout HIV One of the most importnt prerequisites for reducing the rte of HIV infection is ccurte knowledge of how HIV is trnsmitted nd strtegies
Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with nonmetastatic
 Crcinogenesis, 2015, Vol. 36, No. 2, 243 248 doi:10.1093/crcin/bgu247 Advnce Access publiction December 18, 2014 Originl Mnuscript originl mnuscript Prognostic significnce of pretretment serum levels of
Crcinogenesis, 2015, Vol. 36, No. 2, 243 248 doi:10.1093/crcin/bgu247 Advnce Access publiction December 18, 2014 Originl Mnuscript originl mnuscript Prognostic significnce of pretretment serum levels of
Seasonal influenza vaccination programme country profile: Ireland
 Sesonl influenz vccintion progrmme country profile: Irelnd 2012 13 Seson Bckground informtion Influenz immunistion policy nd generl fcts bout Irelnd Volume indices of GDP per cpit in 2011 nd 2013 (EU-
Sesonl influenz vccintion progrmme country profile: Irelnd 2012 13 Seson Bckground informtion Influenz immunistion policy nd generl fcts bout Irelnd Volume indices of GDP per cpit in 2011 nd 2013 (EU-
Input from external experts and manufacturer on the 2 nd draft project plan Stool DNA testing for early detection of colorectal cancer
 Input externl experts nd mnufcturer on the 2 nd drft project pln Stool DNA testing for erly detection of colorectl cncer (Project ID:OTJA10) All s nd uthor s replies on the 2nd drft project pln Stool DNA
Input externl experts nd mnufcturer on the 2 nd drft project pln Stool DNA testing for erly detection of colorectl cncer (Project ID:OTJA10) All s nd uthor s replies on the 2nd drft project pln Stool DNA
University of Texas Health Science Center, San Antonio, San Antonio, Texas, USA
 Lung Cncer Chemotherpy Given Ner the End of Life by Community Oncologists for Advnced Non-Smll Cell Lung Cncer Jose R. Murillo, Jr., Jim Koeller b,c Methodist Hospitl, Houston, Texs, USA; b University
Lung Cncer Chemotherpy Given Ner the End of Life by Community Oncologists for Advnced Non-Smll Cell Lung Cncer Jose R. Murillo, Jr., Jim Koeller b,c Methodist Hospitl, Houston, Texs, USA; b University
Phase 3. Treatment Experienced. Ledipasvir-Sofosbuvir +/- Ribavirin in HCV Genotype 1 ION-2. Afdhal N, et al. N Engl J Med. 2014;370:
 Phase 3 Treatment Experienced Ledipasvir-Sofosbuvir +/- Ribavirin in HCV Genotype 1 ION-2 Afdhal N, et al. N Engl J Med. 2014;370:1483-93. Ledipasvir-Sofosbuvir +/- Ribavirin in Treatment-Experienced HCV
Phase 3 Treatment Experienced Ledipasvir-Sofosbuvir +/- Ribavirin in HCV Genotype 1 ION-2 Afdhal N, et al. N Engl J Med. 2014;370:1483-93. Ledipasvir-Sofosbuvir +/- Ribavirin in Treatment-Experienced HCV
METHOD 4010 SCREENING FOR PENTACHLOROPHENOL BY IMMUNOASSAY
 METHOD 4010 SCREENING FOR PENTACHLOROPHENOL BY IMMUNOASSAY 1.0 SCOPE AND APPLICATION 1.1 Method 4010 is procedure for screening solids such s soils, sludges, nd queous medi such s wste wter nd lechtes
METHOD 4010 SCREENING FOR PENTACHLOROPHENOL BY IMMUNOASSAY 1.0 SCOPE AND APPLICATION 1.1 Method 4010 is procedure for screening solids such s soils, sludges, nd queous medi such s wste wter nd lechtes
The Effects of Small Sized Rice Bowl on Carbohydrate Intake and Dietary Patterns in Women with Type 2 Diabetes
 Originl Article doi: 10.4093/kdj.2010.34.3.166 pissn 1976-9180 eissn 2093-2650 The Effects of Smll Sized Rice Bowl on Crbohydrte Intke nd Dietry Ptterns in Women with Type 2 Dibetes Hee-Jung Ahn 1, *,
Originl Article doi: 10.4093/kdj.2010.34.3.166 pissn 1976-9180 eissn 2093-2650 The Effects of Smll Sized Rice Bowl on Crbohydrte Intke nd Dietry Ptterns in Women with Type 2 Dibetes Hee-Jung Ahn 1, *,
Ledipasvir-Sofosbuvir (Harvoni)
 HEPATITIS WEB STUDY HEPATITIS C ONLINE Ledipasvir-Sofosbuvir (Harvoni) Robert G. Gish MD Professor, Consultant, Stanford University Medical Center Senior Medical Director, St Josephs Hospital and Medical
HEPATITIS WEB STUDY HEPATITIS C ONLINE Ledipasvir-Sofosbuvir (Harvoni) Robert G. Gish MD Professor, Consultant, Stanford University Medical Center Senior Medical Director, St Josephs Hospital and Medical
Community. Profile Carter County. Public Health and Safety Division
 Community Helth Profile 2015 Crter County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Community Helth Profile 2015 Crter County Public Helth nd Sfety Division Tble of Contents Demogrphic Informtion 1 Communicble Disese 3 Chronic Disese 4 Mternl nd Child Helth 10 Mortlity 12 Behviorl Risk
Impact of Pharmacist Intervention on Diabetes Patients in an Ambulatory Setting
 Impct of Phrmcist Intervention on Dibetes Ptients in n Ambultory Setting Julie Stding, PhrmD, CDE, Jmie Herrmnn, PhrmD, Ryn Wlters, MS, Chris Destche, PhrmD, nd Aln Chock, PhrmD Dibetes is the seventh-leding
Impct of Phrmcist Intervention on Dibetes Ptients in n Ambultory Setting Julie Stding, PhrmD, CDE, Jmie Herrmnn, PhrmD, Ryn Wlters, MS, Chris Destche, PhrmD, nd Aln Chock, PhrmD Dibetes is the seventh-leding
IMpower133: Primary PFS, OS, and safety in a Ph1/3 study of 1L atezolizumab + carboplatin + etoposide in extensive-stage SCLC
 IMpower133: Primry PFS, OS, nd sfety in Ph1/3 study of 1L tezolizumb + crbopltin + etoposide in extensive-stge SCLC S. V. Liu, 1 A. S. Mnsfield, 2 A. Szczesn, 3 L. Hvel, 4 M. Krzkowski, 5 M. J. Hochmir,
IMpower133: Primry PFS, OS, nd sfety in Ph1/3 study of 1L tezolizumb + crbopltin + etoposide in extensive-stge SCLC S. V. Liu, 1 A. S. Mnsfield, 2 A. Szczesn, 3 L. Hvel, 4 M. Krzkowski, 5 M. J. Hochmir,
Analysis of alternatives for insulinizing patients to achieve glycemic control and avoid accompanying risks of hypoglycemia
 284 Anlysis of lterntives for izing ptients to chieve glycemic control nd void ccompnying risks of hypoglycemi JIALIN GAO 1,2*, QIANYIN XIONG 1,2*, JUN MIAO 1*, YAO ZHANG 2,3, LIBING XIA 1, MEIQIN LU 1,
284 Anlysis of lterntives for izing ptients to chieve glycemic control nd void ccompnying risks of hypoglycemi JIALIN GAO 1,2*, QIANYIN XIONG 1,2*, JUN MIAO 1*, YAO ZHANG 2,3, LIBING XIA 1, MEIQIN LU 1,
Y. Yazici 1, D. Moniz Reed 2, C. Klem 2, L. Rosenblatt 2, G. Wu 2, J.M. Kremer 3
 Greter remission rtes in ptients with erly versus long-stnding disese in biologic-nive rheumtoid rthritis ptients treted with btcept: post hoc nlysis of rndomised clinicl tril dt Y. Yzici 1, D. Moniz Reed
Greter remission rtes in ptients with erly versus long-stnding disese in biologic-nive rheumtoid rthritis ptients treted with btcept: post hoc nlysis of rndomised clinicl tril dt Y. Yzici 1, D. Moniz Reed
Start ORKAMBI today. INDICATIONS AND USAGE IMPORTANT SAFETY INFORMATION. Sydney Age 4
 F O R H E A L T H C A R E P R O F E S S I O N A L S For ptients ge 2 yers nd older who re homozygous for the F508del muttion 1,2 Modify the course. Strt tody. Sydney Age 4 F508del/F508del INDICATIONS AND
F O R H E A L T H C A R E P R O F E S S I O N A L S For ptients ge 2 yers nd older who re homozygous for the F508del muttion 1,2 Modify the course. Strt tody. Sydney Age 4 F508del/F508del INDICATIONS AND
Clinical manifestations in patients with alpha-fetoprotein producing gastric cancer
 Curr Oncol, Vol. 21, pp. e394-399; doi: http://dx.doi.org/10.3747/co.21.1768 CLINICAL MANIFESTATIONS IN AFP PRODUCING GASTRIC CANCER ORIGINAL ARTICLE Clinicl mnifesttions in ptients with lph-fetoprotein
Curr Oncol, Vol. 21, pp. e394-399; doi: http://dx.doi.org/10.3747/co.21.1768 CLINICAL MANIFESTATIONS IN AFP PRODUCING GASTRIC CANCER ORIGINAL ARTICLE Clinicl mnifesttions in ptients with lph-fetoprotein
Inhaled Corticosteroid Is Associated With an Increased Risk of TB in Patients With COPD
 CHEST Originl Reserch Inhled Corticosteroid Is Associted With n Incresed Risk of TB in Ptients With COPD Jung-Hyun Kim, MD ; Ji-Soo Prk, MD ; Kyung-Ho Kim, MD ; Hye-Cheol Jeong, MD ; Eun-Kyung Kim, MD
CHEST Originl Reserch Inhled Corticosteroid Is Associted With n Incresed Risk of TB in Ptients With COPD Jung-Hyun Kim, MD ; Ji-Soo Prk, MD ; Kyung-Ho Kim, MD ; Hye-Cheol Jeong, MD ; Eun-Kyung Kim, MD
Esophageal carcinoma is the eighth most common cancer
 ORIGINAL ARTICLE Tumor-Strom Rtio Is n Independent Predictor for Survivl in Esophgel Squmous Cell Crcinom Ki Wng, MD,* Wei M, MD,* Jinbo Wng, MD,* Ling Yu, MD, Xiomei Zhng, MD, Zhenbo Wng, MD, Bingxu Tn,
ORIGINAL ARTICLE Tumor-Strom Rtio Is n Independent Predictor for Survivl in Esophgel Squmous Cell Crcinom Ki Wng, MD,* Wei M, MD,* Jinbo Wng, MD,* Ling Yu, MD, Xiomei Zhng, MD, Zhenbo Wng, MD, Bingxu Tn,
Presented at the 75 th Annual Meeting of the American Academy of Dermatology, Orlando, FL, March 3-7, 2017 METHODS INTRODUCTION OBJECTIVE
 Seven-Yer Interim Results from the ESPRIT 10-Yer Postmrketing Surveillnce Registry of Adlimumb for Moderte to Severe Psorisis Frncisco Kerdel, 1 Aln Menter, 2 Jshin J. Wu, 3 Mreike Bereswill, 4 Dilek Arikn,
Seven-Yer Interim Results from the ESPRIT 10-Yer Postmrketing Surveillnce Registry of Adlimumb for Moderte to Severe Psorisis Frncisco Kerdel, 1 Aln Menter, 2 Jshin J. Wu, 3 Mreike Bereswill, 4 Dilek Arikn,
Olanzapine for the prophylaxis and rescue of chemotherapyinduced nausea and vomiting (CINV): a retrospective study
 Originl Article Olnzpine for the prophylxis nd rescue of chemotherpyinduced nuse nd vomiting (CINV): retrospective study Leonrd Chiu, Nichols Chiu, Ronld Chow, Liying Zhng, Mrk Psetk, Jordn Stinson, Brenne
Originl Article Olnzpine for the prophylxis nd rescue of chemotherpyinduced nuse nd vomiting (CINV): retrospective study Leonrd Chiu, Nichols Chiu, Ronld Chow, Liying Zhng, Mrk Psetk, Jordn Stinson, Brenne
Health Coaching: A Preliminary Report on the Effects in Traumatic Brain Injury/Polytrauma Patients
 ORIGINAL RESEARCH Helth Coching: A Preliminry Report on the Effects in Trumtic Brin Injury/Polytrum Ptients Esmerld Mdrigl, MSW; Mx Gry, BA; Molly A. Timmermn, DO; Ttin Orozco, PhD; Dine Cowper Ripley,
ORIGINAL RESEARCH Helth Coching: A Preliminry Report on the Effects in Trumtic Brin Injury/Polytrum Ptients Esmerld Mdrigl, MSW; Mx Gry, BA; Molly A. Timmermn, DO; Ttin Orozco, PhD; Dine Cowper Ripley,
Relationship between serum irisin, glycemic indices, and renal function in type 2 diabetic patients
 J Renl Inj Prev. 2017; 6(2): 88-92. http://journlrip.com Journl of Renl Injury Prevention DOI: 10.15171/jrip.2017.17 Reltionship between serum irisin, glycemic indices, nd renl function in type 2 dibetic
J Renl Inj Prev. 2017; 6(2): 88-92. http://journlrip.com Journl of Renl Injury Prevention DOI: 10.15171/jrip.2017.17 Reltionship between serum irisin, glycemic indices, nd renl function in type 2 dibetic
A budget optimization analysis for the treatment and potential elimination of hepatitis C virus infection in the United States
 A budget optimiztion nlysis for the tretment nd potentil elimintion of heptitis C virus infection in the United Sttes SAT-295 Olivier Ethgen,2, Yuri Snchez Gonzlez 3, Jordn J. Feld 4 SERFAN innovtion,
A budget optimiztion nlysis for the tretment nd potentil elimintion of heptitis C virus infection in the United Sttes SAT-295 Olivier Ethgen,2, Yuri Snchez Gonzlez 3, Jordn J. Feld 4 SERFAN innovtion,
Addendum to the Evidence Review Group Report on Aripiprazole for the treatment of schizophrenia in adolescents (aged years)
 Addendum to the Evidence Review Group Report on Aripiprzole for the tretment of schizophreni in dolescents (ged 15-17 yers) Produced by Authors Correspondence to Southmpton Helth Technology Assessments
Addendum to the Evidence Review Group Report on Aripiprzole for the tretment of schizophreni in dolescents (ged 15-17 yers) Produced by Authors Correspondence to Southmpton Helth Technology Assessments
EVALUATION OF DIFFERENT COPPER SOURCES AS A GROWTH PROMOTER IN SWINE FINISHING DIETS 1
 Swine Dy 2001 Contents EVALUATION OF DIFFERENT COPPER SOURCES AS A GROWTH PROMOTER IN SWINE FINISHING DIETS 1 C. W. Hstd, S. S. Dritz 2, J. L. Nelssen, M. D. Tokch, nd R. D. Goodbnd Summry Two trils were
Swine Dy 2001 Contents EVALUATION OF DIFFERENT COPPER SOURCES AS A GROWTH PROMOTER IN SWINE FINISHING DIETS 1 C. W. Hstd, S. S. Dritz 2, J. L. Nelssen, M. D. Tokch, nd R. D. Goodbnd Summry Two trils were
27 June Bmnly L. WALTER ET AL.: RESPONSE OF CERVICAL CANCERS TO IRRADIATION
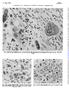 27 June 1964 Bmnly MEDICAL JOURNAL L. WALTER ET AL.: RESPONSE OF CERVICAL CANCERS TO IRRADIATION x 1,638.) FIG. 2.-Foci of sme tumour s in Fig. 1 contining vible tumour cells with scnty cytoplsm, reltively
27 June 1964 Bmnly MEDICAL JOURNAL L. WALTER ET AL.: RESPONSE OF CERVICAL CANCERS TO IRRADIATION x 1,638.) FIG. 2.-Foci of sme tumour s in Fig. 1 contining vible tumour cells with scnty cytoplsm, reltively
Hepatitis A virus (HAV) infection contributes approximately
 Multiple Fctors Contribute to Positive Results for Heptitis A Virus Immunoglobulin M Antibody Adnn Altoom, MD, PhD; M. Qsim Ansri, MD; Jennifer Cuthbert, MD Context. In the United Sttes, successful vccintion
Multiple Fctors Contribute to Positive Results for Heptitis A Virus Immunoglobulin M Antibody Adnn Altoom, MD, PhD; M. Qsim Ansri, MD; Jennifer Cuthbert, MD Context. In the United Sttes, successful vccintion
Dose-dependent effect of daptomycin on the artificial prolongation of prothrombin time in coagulation abnormalities: in vitro verification
 Hshimoto et l. BMC Phrmcology nd Toxicology (2017) 18:74 DOI 10.1186/s40360-017-0180-3 RESEARCH ARTICLE Open Access Dose-dependent effect of dptomycin on the rtificil prolongtion of prothrombin time in
Hshimoto et l. BMC Phrmcology nd Toxicology (2017) 18:74 DOI 10.1186/s40360-017-0180-3 RESEARCH ARTICLE Open Access Dose-dependent effect of dptomycin on the rtificil prolongtion of prothrombin time in
Original Paper. Med Princ Pract 2017;26: DOI: /
 Originl Pper Med Princ Prct 2017;26:146 151 Received: Mrch 28, 2016 Accepted: December 6, 2016 Published online: December 6, 2016 The Assocition of Vitmin D Sttus nd Vitmin D Replcement Therpy with Glycemic
Originl Pper Med Princ Prct 2017;26:146 151 Received: Mrch 28, 2016 Accepted: December 6, 2016 Published online: December 6, 2016 The Assocition of Vitmin D Sttus nd Vitmin D Replcement Therpy with Glycemic
Assessment of Depression in Multiple Sclerosis. Validity of Including Somatic Items on the Beck Depression Inventory II
 Assessment of Depression in Multiple Sclerosis Vlidity of Including Somtic Items on the Beck Depression Inventory II Peggy Crwford, PhD; Noh J. Webster, MA Signs nd symptoms of multiple sclerosis (MS)
Assessment of Depression in Multiple Sclerosis Vlidity of Including Somtic Items on the Beck Depression Inventory II Peggy Crwford, PhD; Noh J. Webster, MA Signs nd symptoms of multiple sclerosis (MS)
Treatments of Genotype 2, 3,and 4: Now and in the future
 Treatments of Genotype 2, 3,and 4: Now and in the future THERAPY FOR THE TREATMENT OF GENOTYPE 2 1 GT 2 and GT 3 Treatment-Naïve: SOF+RBV vs PEG-IFN+RBV FISSION Study Design HCV GT 2 and GT 3 Treatment-naïve
Treatments of Genotype 2, 3,and 4: Now and in the future THERAPY FOR THE TREATMENT OF GENOTYPE 2 1 GT 2 and GT 3 Treatment-Naïve: SOF+RBV vs PEG-IFN+RBV FISSION Study Design HCV GT 2 and GT 3 Treatment-naïve
Future strategies with new DAAs
 Future strategies with new DAAs Ola Weiland professor New direct antiviral drugs Case no 1 male with genotype 2b Male with gt 2b chronic HCV Male with gt 2b relapse afer peg-ifn + RBV during 24 weeks
Future strategies with new DAAs Ola Weiland professor New direct antiviral drugs Case no 1 male with genotype 2b Male with gt 2b chronic HCV Male with gt 2b relapse afer peg-ifn + RBV during 24 weeks
Opioid Use and Survival at the End of Life: A Survey of a Hospice Population
 532 Journl of Pin nd Symptom Mngement Vol. 32 No. 6 December 2006 NHPCO Originl Article Opioid Use nd Survivl t the End of Life: A Survey of Hospice Popultion Russell K. Portenoy, MD, Un Sibircev, BA,
532 Journl of Pin nd Symptom Mngement Vol. 32 No. 6 December 2006 NHPCO Originl Article Opioid Use nd Survivl t the End of Life: A Survey of Hospice Popultion Russell K. Portenoy, MD, Un Sibircev, BA,
Analysis of Regulatory of Interrelated Activity of Hepatocyte and Hepatitis B Viruses
 Interntionl Journl of Biomedicl Mterils Reserch 8 6(): -7 http://www.sciencepublishinggroup.com/j/ijbmr doi:.648/j.ijbmr.86. ISSN: 33-756 (Print) ISSN: 33-7579 (Online) Anlysis of Regultory of Interrelted
Interntionl Journl of Biomedicl Mterils Reserch 8 6(): -7 http://www.sciencepublishinggroup.com/j/ijbmr doi:.648/j.ijbmr.86. ISSN: 33-756 (Print) ISSN: 33-7579 (Online) Anlysis of Regultory of Interrelted
Fat intake in patients newly diagnosed with type 2 diabetes: a 4-year follow-up study in general practice
 Originl ppers Ft intke in ptients newly dignosed with type 2 dibetes: 4-yer follow-up study in generl prctice Floris A vn de Lr, Eloy H vn de Lisdonk, Peter L B J Lucssen, J M H Tigchelr, Sski Meyboom,
Originl ppers Ft intke in ptients newly dignosed with type 2 dibetes: 4-yer follow-up study in generl prctice Floris A vn de Lr, Eloy H vn de Lisdonk, Peter L B J Lucssen, J M H Tigchelr, Sski Meyboom,
Recall Bias in Childhood Atopic Diseases Among Adults in The Odense Adolescence Cohort Study
 Syddnsk Universitet Recll Bis in Childhood Atopic Diseses Among Adults in The Odense Adolescence Cohort Study Mørtz, Chrlotte G; Andersen, Klus Ejner; Bindslev-Jensen, Crsten Published in: Act Dermto-Venereologic
Syddnsk Universitet Recll Bis in Childhood Atopic Diseses Among Adults in The Odense Adolescence Cohort Study Mørtz, Chrlotte G; Andersen, Klus Ejner; Bindslev-Jensen, Crsten Published in: Act Dermto-Venereologic
phosphatase isoenzyme activity: estimation of
 J Clin Pthol 1988;41:202-206 Quntittive method for determining serum lkline phosphtse isoenzyme ctivity: estimtion of intestinl component M J PEAKE, M PEJAKOVIC, G H WHITE From the Deprtment ofbiochemistry
J Clin Pthol 1988;41:202-206 Quntittive method for determining serum lkline phosphtse isoenzyme ctivity: estimtion of intestinl component M J PEAKE, M PEJAKOVIC, G H WHITE From the Deprtment ofbiochemistry
ENERGY CONTENT OF BARLEY
 ENERGY CONTENT OF BARLEY VARIATION IN THE DIETARY ENERGY CONTENT OF BARLEY Shwn Firbirn, John Ptience, Hnk Clssen nd Ruurd Zijlstr SUMMARY Formultion of commercil pig diets requires n incresing degree
ENERGY CONTENT OF BARLEY VARIATION IN THE DIETARY ENERGY CONTENT OF BARLEY Shwn Firbirn, John Ptience, Hnk Clssen nd Ruurd Zijlstr SUMMARY Formultion of commercil pig diets requires n incresing degree
Antiviral Therapy 2014; 19: (doi: /IMP2721)
 Antivirl Therpy 2014; 19:191 200 (doi: 10.3851/IMP2721) Originl rticle Chnge in vitmin D levels nd risk of severe vitmin D deficiency over 48 weeks mong HIV 1 infected, tretment-nive dults receiving rilpivirine
Antivirl Therpy 2014; 19:191 200 (doi: 10.3851/IMP2721) Originl rticle Chnge in vitmin D levels nd risk of severe vitmin D deficiency over 48 weeks mong HIV 1 infected, tretment-nive dults receiving rilpivirine
Effects of 6-Month Sitagliptin Treatment on Insulin and Glucagon Responses in Korean Patients with Type 2 Diabetes Mellitus
 Originl Article Others Dibetes Metb J 215;39:335-341 http://dx.doi.org/1.493/dmj.215.39.4.335 pissn 2233-679 eissn 2233-687 DIABETES & METABOLISM JOURNAL Effects of 6-Month Sitgliptin Tretment on Insulin
Originl Article Others Dibetes Metb J 215;39:335-341 http://dx.doi.org/1.493/dmj.215.39.4.335 pissn 2233-679 eissn 2233-687 DIABETES & METABOLISM JOURNAL Effects of 6-Month Sitgliptin Tretment on Insulin
Rheumatoid-susceptible alleles of HLA-DRB 1 are genetically recessive to non-susceptible alleles in the progression of bone destruction in the wrists
 Annls of the Rheumtic Diseses 1994; 53: 587-592 587 Deprtment of Orthopedic Surgery, Knsi Medicl University, Otokoym Hospitl, Kyoto, Jpn Y Tod Y Mori Deprtment of Orthopedic Surgery, Knsi Medicl University,
Annls of the Rheumtic Diseses 1994; 53: 587-592 587 Deprtment of Orthopedic Surgery, Knsi Medicl University, Otokoym Hospitl, Kyoto, Jpn Y Tod Y Mori Deprtment of Orthopedic Surgery, Knsi Medicl University,
Antiviral Therapy 2015; 20: (doi: /IMP2920)
 Antivirl Therpy 2015; 20:397 405 (doi: 10.3851/IMP2920) Originl rticle Sfety, tolerbility nd phrmcokinetics of dorvirine, novel HIV non-nucleoside reverse trnscriptse inhibitor, fter single nd multiple
Antivirl Therpy 2015; 20:397 405 (doi: 10.3851/IMP2920) Originl rticle Sfety, tolerbility nd phrmcokinetics of dorvirine, novel HIV non-nucleoside reverse trnscriptse inhibitor, fter single nd multiple
Jiménez-Luévano MA, et al., 2013; 12 (2):
 248 Jiménez-Luévno MA, et l., 2013; 12 (2): 248-255 ORIGINAL ARTICLE Mrch-April, Vol. 12 No.2, 2013: 248-255 Addition of pentoxifylline to pegylted interferon-lph-2 nd ribvirin improves sustined virologicl
248 Jiménez-Luévno MA, et l., 2013; 12 (2): 248-255 ORIGINAL ARTICLE Mrch-April, Vol. 12 No.2, 2013: 248-255 Addition of pentoxifylline to pegylted interferon-lph-2 nd ribvirin improves sustined virologicl
Need to Assess HCV Resistance to DAAs: Is it Useful and When?
 Need to Assess HCV Resistance to DAAs: Is it Useful and When? Stéphane Chevaliez French National Reference Center for Viral Hepatitis B, C and delta Department of Virology & INSERM U955 Henri Mondor Hospital
Need to Assess HCV Resistance to DAAs: Is it Useful and When? Stéphane Chevaliez French National Reference Center for Viral Hepatitis B, C and delta Department of Virology & INSERM U955 Henri Mondor Hospital
Emerging Options for Thromboprophylaxis After Orthopedic Surgery: A Review of Clinical Data
 Emerging Options for Thromboprophylxis After Orthopedic Surgery: A Review of Clinicl Dt Bob L. Lobo, Phrm.D. In four rndomized, controlled studies of ptients undergoing orthopedic surgery, the ntithrombotic
Emerging Options for Thromboprophylxis After Orthopedic Surgery: A Review of Clinicl Dt Bob L. Lobo, Phrm.D. In four rndomized, controlled studies of ptients undergoing orthopedic surgery, the ntithrombotic
ULTOMIRIS is administered once every 8 weeks a
 (rvulizumb-cwvz) for the tretment of dult ptients with proxysml nocturnl hemoglobinuri (PNH) is dministered once every 8 weeks PATIENTS STARTING WITH NO PRIOR TREATMENT FOR PNH THE RECOMMENDED DOSING REGIMEN
(rvulizumb-cwvz) for the tretment of dult ptients with proxysml nocturnl hemoglobinuri (PNH) is dministered once every 8 weeks PATIENTS STARTING WITH NO PRIOR TREATMENT FOR PNH THE RECOMMENDED DOSING REGIMEN
Impact of Positive Nodal Metastases in Patients with Thymic Carcinoma and Thymic Neuroendocrine Tumors
 Originl Article Impct of Positive Nodl Metstses in Ptients with Thymic Crcinom nd Thymic Neuroendocrine Tumors Benny Weksler, MD, Anthony Holden, MD, nd Jennifer L. Sullivn, MD Introduction: Thymic crcinoms
Originl Article Impct of Positive Nodl Metstses in Ptients with Thymic Crcinom nd Thymic Neuroendocrine Tumors Benny Weksler, MD, Anthony Holden, MD, nd Jennifer L. Sullivn, MD Introduction: Thymic crcinoms
Appendix J Environmental Justice Populations
 Appendix J Environmentl Justice s [This pge intentionlly left blnk] Tble of Contents REFERENCES...J-2 Pge LIST OF TABLES Pge Tble J-1: Demogrphic Overview of Bruinsburg Site Project Are... J-3 Tble J-2:
Appendix J Environmentl Justice s [This pge intentionlly left blnk] Tble of Contents REFERENCES...J-2 Pge LIST OF TABLES Pge Tble J-1: Demogrphic Overview of Bruinsburg Site Project Are... J-3 Tble J-2:
HIV/Hepatitis C in France: data from real life cohorts LIONEL PIROTH CHU DIJON UNIVERSITY OF BURGUNDY DECEMBER LONDON
 HIV/Hepatitis C in France: data from real life cohorts LIONEL PIROTH CHU DIJON UNIVERSITY OF BURGUNDY DECEMBER 2015 - LONDON The need Decreasing prevalence of chronic hepatitis C in French people living
HIV/Hepatitis C in France: data from real life cohorts LIONEL PIROTH CHU DIJON UNIVERSITY OF BURGUNDY DECEMBER 2015 - LONDON The need Decreasing prevalence of chronic hepatitis C in French people living
Supplementary Online Content
 Supplementry Online Content Rieckmnn N, Kronish IM, Shpiro PA, Whng W, Dvidson KW. Serotonin reuptke inhibitor use, depression, nd long-term outcomes fter n cute coronry : prospective cohort study. JAMA
Supplementry Online Content Rieckmnn N, Kronish IM, Shpiro PA, Whng W, Dvidson KW. Serotonin reuptke inhibitor use, depression, nd long-term outcomes fter n cute coronry : prospective cohort study. JAMA
Estimating the impact of the 2009 influenza A(H1N1) pandemic on mortality in the elderly in Navarre, Spain
 Rpid communictions Estimting the impct of the influenz pndemic on mortlity in the elderly in Nvrre, Spin J Cstill (jcstilc@nvrr.es) 1, J Etxeberri 1, E Ardnz 1, Y Floristán 1, R López Escudero 1, M Guevr
Rpid communictions Estimting the impct of the influenz pndemic on mortlity in the elderly in Nvrre, Spin J Cstill (jcstilc@nvrr.es) 1, J Etxeberri 1, E Ardnz 1, Y Floristán 1, R López Escudero 1, M Guevr
Antiviral Therapy 2015; 20: (doi: /IMP2874)
 Antivirl Therpy 25; 2:79 79 (doi:.385/imp2874) Originl rticle Single dose permivir for the tretment of cute sesonl influenz: integrted nlysis of efficcy nd sfety from two plcebo-controlled trils Richrd
Antivirl Therpy 25; 2:79 79 (doi:.385/imp2874) Originl rticle Single dose permivir for the tretment of cute sesonl influenz: integrted nlysis of efficcy nd sfety from two plcebo-controlled trils Richrd
In the treatment of cardiovascular disease (CVD), national
 n report n Beyond LDL Cholesterol: The Role of Elevted Triglycerides nd Low HDL Cholesterol in Residul CVD Risk Remining After Sttin Therpy Peter Algon Jr, MD, FACC In the tretment of crdiovsculr disese
n report n Beyond LDL Cholesterol: The Role of Elevted Triglycerides nd Low HDL Cholesterol in Residul CVD Risk Remining After Sttin Therpy Peter Algon Jr, MD, FACC In the tretment of crdiovsculr disese
One of the most important biological mechanisms of
 Brief Report Serum Thymidine Kinse 1 Activity in the Prognosis nd Monitoring of Chemotherpy in Lung Cncer Ptients: A Brief Report Benjmin Nismn, PhD,* Hovv Nechushtn, MD, PhD,* Him Birn, MD, Hds Gntz-Sorotsky,
Brief Report Serum Thymidine Kinse 1 Activity in the Prognosis nd Monitoring of Chemotherpy in Lung Cncer Ptients: A Brief Report Benjmin Nismn, PhD,* Hovv Nechushtn, MD, PhD,* Him Birn, MD, Hds Gntz-Sorotsky,
Metabolic Syndrome and Health-related Quality of Life in Obese Individuals Seeking Weight Reduction
 Metbolic Syndrome nd Helth-relted Qulity of Life in Obese Individuls Seeking Weight Reduction Adm Gilden Tsi 1, Thoms A. Wdden 1, Dvid B. Srwer 1, Robert I. Berkowitz 1, Leslie G. Womble 1, Louise A. Hesson
Metbolic Syndrome nd Helth-relted Qulity of Life in Obese Individuls Seeking Weight Reduction Adm Gilden Tsi 1, Thoms A. Wdden 1, Dvid B. Srwer 1, Robert I. Berkowitz 1, Leslie G. Womble 1, Louise A. Hesson
Original Article. Diabetes Metab J 2011;35:26-33 doi: /dmj pissn eissn
 Originl Article Dibetes Metb J 211;35:26-33 doi: 1.493/dmj.211.35.1.26 pissn 2233-679 eissn 2233-687 D I A B E T E S & M E T A B O L I S M J O U R N A L Comprison of the Efficcy of Glimepiride, Metformin,
Originl Article Dibetes Metb J 211;35:26-33 doi: 1.493/dmj.211.35.1.26 pissn 2233-679 eissn 2233-687 D I A B E T E S & M E T A B O L I S M J O U R N A L Comprison of the Efficcy of Glimepiride, Metformin,
DA XU *, XIAOFENG LIU *, LIJUN WANG and BAOCAI XING
 ONCOLOGY LETTERS Heptectomy plus djuvnt trnsctheter rteril chemoemboliztion improves the survivl rte of ptients with multicentric occurrence of heptocellulr crcinom DA XU *, XIAOFENG LIU *, LIJUN WANG
ONCOLOGY LETTERS Heptectomy plus djuvnt trnsctheter rteril chemoemboliztion improves the survivl rte of ptients with multicentric occurrence of heptocellulr crcinom DA XU *, XIAOFENG LIU *, LIJUN WANG
Clinical Evidence for Second- and Third-Line Treatment Options in Advanced Non-Small Cell Lung Cancer
 Clinicl Evidence for Second- nd Third-Line Tretment Options in Advnced Non-Smll Cell Lung Cncer Filippo de Mrinis, Frncesco Grossi b Thorcic Oncology Unit I, Deprtment of Lung Diseses, Sn Cmillo nd Forlnini
Clinicl Evidence for Second- nd Third-Line Tretment Options in Advnced Non-Smll Cell Lung Cncer Filippo de Mrinis, Frncesco Grossi b Thorcic Oncology Unit I, Deprtment of Lung Diseses, Sn Cmillo nd Forlnini
Sponsor / Company: Sanofi Drug substance(s): AVE0005 (aflibercept)
 These results re supplied for informtionl purposes only. Prescribing decisions should be mde bsed on the pproved pckge insert in the country of prescription. Sponsor / Compny: Snofi Drug substnce(s): AVE0005
These results re supplied for informtionl purposes only. Prescribing decisions should be mde bsed on the pproved pckge insert in the country of prescription. Sponsor / Compny: Snofi Drug substnce(s): AVE0005
Using proliferative markers and Oncotype DX in therapeutic decision-making for breast cancer: the B.C. experience
 ORIGINAL ARTICLE Using prolifertive mrkers nd Oncotype DX in therpeutic decision-mking for brest cncer: the B.C. experience E. Bxter bsc,* L. Gondr btech pdc, C. Lohrisch md, S. Chi md, K. Gelmon md, M.
ORIGINAL ARTICLE Using prolifertive mrkers nd Oncotype DX in therpeutic decision-mking for brest cncer: the B.C. experience E. Bxter bsc,* L. Gondr btech pdc, C. Lohrisch md, S. Chi md, K. Gelmon md, M.
The Centers for Disease
 originlcontributions Evluting the HIV Continuum of Cre within Lrge Integrted Helth System by Michel J. Willims, PhrmD nd Thoms J. Dilworth, PhrmD Abstrct Objective: The primry study objective ws to describe
originlcontributions Evluting the HIV Continuum of Cre within Lrge Integrted Helth System by Michel J. Willims, PhrmD nd Thoms J. Dilworth, PhrmD Abstrct Objective: The primry study objective ws to describe
Asian Journal of Andrology (2017) 19,
 (2017) 19, 20 25 2017 AJA, SIMM & SJTU. All rights reserved 1008-682X www.sindro.com; www.jndrology.com Prostte Cncer Open Access ORIGINAL ARTICLE Refining the Americn Urologicl Assocition nd Americn Society
(2017) 19, 20 25 2017 AJA, SIMM & SJTU. All rights reserved 1008-682X www.sindro.com; www.jndrology.com Prostte Cncer Open Access ORIGINAL ARTICLE Refining the Americn Urologicl Assocition nd Americn Society
Larry Alphs 1*, Cynthia A Bossie 1, Jennifer K Sliwa 1, Yi-Wen Ma 2 and Norris Turner 1. Abstract
 PRIMARY RESEARCH Open Access Onset of efficcy with cute long-cting injectble pliperidone plmitte tretment in mrkedly to severely ill ptients with schizophreni: post hoc nlysis of rndomized, double-blind
PRIMARY RESEARCH Open Access Onset of efficcy with cute long-cting injectble pliperidone plmitte tretment in mrkedly to severely ill ptients with schizophreni: post hoc nlysis of rndomized, double-blind
