.Duke. SEnergyW. Duke Energy Oconee Nuclear Station 7800 Rochester Highway Seneca, SC (864) OFFICE (864) FAX
|
|
|
- Magnus Lloyd
- 5 years ago
- Views:
Transcription
1 .Duke SEnergyW W R. McCollum, Jr. Vice President Duke Energy Oconee Nuclear Station 7800 Rochester Highway Seneca, SC (864) OFFICE (864) FAX February 6, 2001 U. S. Nuclear Regulatory Commission Document Control Desk Washington, D. C Subject: Oconee Nuclear Station Docket Nos , -270, -287 Emergency Plan Implementing Procedures Manual Volume B, Revision Please find attached for your use and review copies of the revision to the Oconee Nuclear Station Emergency Plan: Volume B Revision February 2001 This revision is being submitted in accordance with 10 CFR 50-54(q) and does not decrease the effectiveness of the Emergency Plan or the Emergency Plan Implementing Procedures. Any questions or concerns pertaining to this revision please call Mike Thorne, Emergency Planning Manager at By copy of this letter, two copies of this revision are being provided to the NRC, Region II, Atlanta, Georgia. Very truly yours, W. R. McCollum, Jr. VP, Oconee Nuclear jgte xc: (w/2 copies of attachments) Mr. Luis Reyes, Regional Administrator, Region II U. S. Nuclear Regulatory Commission 61 Forsyth St., SW, Suite 24T23 Atlanta, Georgia w/copy of attachments Mr. Steven Baggett Rockville, Maryland (w/o Attachments, Oconee Nuclear Station) NRC Resident Inspector M. D. Thorne, Manager, Emergency Planning
2 February 6, 2001 OCONEE NUCLEAR SITE SUBJECT: Emergency Plan Implementing Procedures Volume B, Revision Please make the following changes to the Emergency Plan, Volume B by following these instructions. REMOVE Cover Sheet Rev Table of Contents page 1 HP/0/B/1009/012-06/15/99 ADD Cover Sheet Rev Table of Contents page 1 HP/0/B/1009/012-01/09/01
3 DUKE POWER EMERGENCY PLAN IMPLEMENTING PROCEDURES VOLUME B APPROVED: W.. Fstr, Maage W. W. Foster, Manager Safety Assurance 02/06/2001 Date Approved 02/06/2001 Effective Date VOLUME B REVISION February, 2001
4 VOLUME B Page 1 TABLE OF CONTENTS Chemistry Lab LM-O-PO03C Chemistry Lab LM-O-P919 CP/I/A/2002/004C CP/1 &2/A/2002/005 CP/2/A/2002/004C CP/3/A/2002/004C CP/3/A/2002/005 HP/O/B/1009/009 HP/O/B/1009/012 HP/0/B/1009/015 HP/0/B/1009/016 HP/I/A/1009/017 HP/2/A/1009/017 HP/3/A/1009/017 RP/O/B/1000/011 RP/0/B/1000/025 RP/0/B/1000/027 Determination Of Boron By Manual Colorimetric Titration - (11/18/96) Boron Analysis by Mettler DL 58 Boron Titration - (10/26/99) Operating Procedure for the Post Accident Liquid Sampling System (PALSS) (12/16/99) Post Accident Caustic Injection into the Low Pressure Injection System (05/23/00) Operating Procedure for the Post Accident Liquid Sampling System (PALSS) (12/16/99) Operation Procedure for Operation of the Post-Accident Liquid Sampling System (PALSS) - (12/16/99) Post Accident Caustic Injection into the Low Pressure Injection System (05/23/00) Procedure for Determining The Inplant Airborne Radioiodine Concentration During Accident Conditions - (12/03/97) Distribution of Potassium Iodide Tablets In The Event Of A Radioiodine Release - (01/09/01) Procedure for Sampling and Quantifying High Level Gaseous Radioiodine And Particulate Radioactivity - (06/16/99) Procedure for Emergency Decontamination of Personnel and Vehicles On-Site And From Off-Site Remote Assembly Area - (12/29/97) Operating Procedure For Post-Accident Containment Air Sampling System - (09/13/00) Operating Procedure For Post-Accident Containment Air Sampling System - (09/13/00) Operating Procedure For Post-Accident Containment Air Sampling System - (09/13/00) Planned Emergency Exposure - (02/01/94) Operational Support Center Manager Procedure - (06/26/00) Re-Entry Recovery Procedure - (05/30/00) Revision February, 2001
5 Duke Power Company (I) ID No HP/O/B/1009/012 INFORMATION PROCEDURE PROCESS RECORD Revision No 012 ONLY P,,.-ARATION (2) Station OCONEE NUCLEAR STATION (3) Procedure Title Distribution Of Potassium Iodide Tablets In The Event Of A Radioiodine Release (4) Prepared By I r 7 /{ O Date 1/4/01 (5) Requires 10CFR50.59 evaluation? O Yes (New procedure or revision with major changes) 01 No (Revision with minor changes) O1 No (To incorporate previou approved changes) (6) Reviewed By (QR) Date I 'ko I Cross-Disciplinary Review By (QR)NA_6±t_ Date ± f-01 Reactivity Mgmt. Review By (QR)NA S'- Date LLEL (7) Additional Reviews Reviewed By Reviewed By (8) Temporary Approval (if necessary) By (SRO/QR) Date By (QR) Date F (9) Approved By Date /i b PERFORMANCE ýmpare with control c Date Date ry 14 calendar days while work is being performed.) (10) Compared with Control Copy Date Compared with Control Copy Compared with Control Copy_ (11) Date(s) Performed Work Order Number (WO#) COMPLETION (12) Procedure Completion Verification 0 Yes D NA Checklists and/or blanks initialed, signed, dated, or filled in NA, as appropriate? 0l Yes I1 NA Listed enclosures attached? o Yes 0 NA Data sheets attached, completed, dated, and signed? El Yes Dl NA Charts, graphs, etc. attached, dated, identified, and marked? o Yes 11 NA Procedure requirements met? Verified By Date (12" Procedure Completion Approved Date (., Remarks (Attach additional pages, if necessary) Date Date
6 Duke Power Company Oconee Nuclear Station Distribution Of Potassium Iodide Tablets In The Event Of A Radioiodine Release I Procedure No. HP/O/B/1009/012 Revision No. 012 Information Use Electronic Reference No. OX002SD1
7 Distribution Of Potassium Iodide Tablets In The Event Of A Radioiodine Release HP/O/B/1009/012 Page 2 of 5 1. Purpose 1.1 This procedure provides information necessary to distribute Potassium Iodide (KI) tablets to in-plant personnel in the event of a release of radioiodine. 1.2 This procedure is an Emergency Plan Implementing Procedure (EPIP). It must be forwarded to the Emergency Planning Group within three working days of approval by the responsible group. {PIP ) 2. References 2.1 NCRP Report No. 55; Protection of the Thyroid Gland in the Event of Releases of Radioiodine NCRP Report No. 65; Management of Persons Accidentally Contaminated with Radioiodine SH/0/B/2001/001, Internal Dose Assessment 2.4 NUREG RP/O/B/1000/01 1, Planned Emergency Exposure 2.6 PIP , Distribution Of Emergency Plan Procedures 3. Limits And Precautions 3.1 Persons who are allergic to KI should NOT receive these tablets. 3.2 Nursing mothers who receive KI tablets must be advised to use nutrient substitutes (ex: milk or a formula) for children for the duration of the ten-day tablet use period. 3.3 Personnel shall be advised NOT to deviate from prescribed dosages and dosage rates. 3.4 Best results will be achieved when KI tablets are administered prior to the exposure but at least within 4 hours after an exposure.
8 HPIO/B/1009/012 Page 3 of Do NOT distribute discolored or disfigured tablets, or tablets that have exceeded their expiration date. Such tablets shall be discarded: The expiration date listed on the bottle may have been extended by the manufacturer. Documentation of extension shall be kept with the supply of KI tablets. 3.6 Hands of personnel shall be free of contamination prior to taking KI tablets. 3.7 The RP Manager may approve the use of KI to block thyroid uptake of radioiodines during emergency situations. If the thyroid CDE is anticipated to exceed 25 REM (which is 1000 iodine DAC hours as calculated by Enclosure 5.1), the radiation risk is considered to be greater than the risk of side effects from the KI in most cases. Personnel to be considered for KI include: Personnel suspected of having been in the affected area prior to detection and during a release; Persons present in the affected area; and Persons who will enter the area while a significant amount of radioiodine is present, as defined in Step Each new bottle of KI contains 14 tablets and will require removal of sufficient tablets to ensure only 10 tablets are issued to each affected person. 3.9 Replacement tablets should be ordered at least three months prior to the KI expiration date, to ensure an adequate supply of viable tablets KI tablets shall be stored under the following conditions: temperature range of 68 degrees F to 77 degrees F; low humidity; and away from direct exposure to light.
9 HP/O/B/1009/012 Page 4 of 5 4. Procedure 4.1 Calculate total iodine DAC-hours expected from task using Enclosure 5.1. NOTE: If total iodine DAC-hours expected from task is less than O.RR equal to 1000, administration of KI is NOT required. 4.2 IF total iodine DAC-hours expected from task > 1000 (which is 25 REM), administer KI to affected personnel. 4.3 Secure tablets from emergency inventory: Inspect bottles for evidence of tampering (example: seal broken etc.) Ensure issue date or extension letter date has NOT expired. 4.4 Record data required on Enclosure 5.2 for personnel arriving at distribution site. 4.5 Issue one (1) tablet to each affected person for immediate consumption: Remove sufficient tablets, if necessary, to ensure only nine (9) tablets remain in the bottle/vial Store tablets removed from new bottles of KI in a plastic vial or other similar container Record the expiration date of the bottle or extension letter and the name of the RP representative issuing the tablets on the vial. 4.6 Issue each affected person one (1) bottle/vial containing nine (9) KI tablets: 0 Issue the accumulated vials of KI to avoid opening new bottles until necessary. 4.7 Issue each affected person the package insert for use of the tablets (refer to Enclosure 5.3 for example of the package insert). 4.8 Instruct each person receiving KI to take one (1) tablet per day for 10 days, as near as 24 hour schedule as possible, unless otherwise directed by the Radiation Protection Manager or designee. NOTE: If the number of persons affected render it impractical to give all a BBA, the RP Shift General Supervisor or designee shall draw a sample of persons to receive a BBA. 4.9 Schedule personnel ingesting KI for a BBA.
10 HP/O/B/1009/012 Page 5 of Ensure proper disposition of Enclosures 5.1 and 5.2: "* Original to Master File, under appropriate (S)RWP. "* Copy to the Radiation Protection Manager. "* Copy to the RP Shift General Supervisor. 5. Enclosures 5.1 Example Calculation Of Iodine DAC-hours 5.2 Example Potassium Iodide Tablet Distribution Data Sheet 5.3 Example Package Insert For Thyro-BlockTM Tablets And solution
11 Enc.& -are 5.1 Example Calculation Of Iodine DAC-Hours HP/0/B/l1009/Oiz Page 1 of 1 (S)RWP [(1-131 uci/ml =.) + (1-133 uci/ml =.) + (1-135 uci/ml = 2E-8uCi/ml 1E-7uCi/ml per DAC per DAC 7E-7uCi/ml per DAC )] X time in hrs = # of iodine DAC-hours expected from task Where: 1-131, 1-133, and are the respective iodine isotope concentrations either actually in or potentially in the workplace during task. NOTE: If another iodine isotope(s) is in or expected to be in the workplace, divide its concentration (in uci/ml) by its DAC, as shown above and add to the other radioiodine DACs and multiply the sum by the time spent in the radioiodine atmosphere. 2E-8uCi/ml per DAC = the concentration of equivalent to one DAC 1E-7uCi/ml per DAC = the concentration of equivalent to one DAC 7E-7uCi/ml per DAC = the concentration of equivalent to one DAC Time = the total time in hours that personnel are expected to be in the radioiodine atmosphere
12 RP Badge Number Name Enclosure 5.2 Example Potassium Iodide Tablet Distribution Data Sheet Department Date and Time Of Suspected Exposure HP/O/B/1009/012 Page 1 of 1 Date and Time Of Initial Issue Disposition: "* Original to Master File "* Copy to: RP Manager RP Shift General Supervisor
13 Enclosure 5.3 Example Package Insert For Thyro-BlockTm Tablets And Solution HP/O/B/1009/012 Page 1 of 2 Patient Package Inse4 For THYRO-BLOCK TABLETS (POTASSIUM IODIDE TABLETS, USP) (pro e poe-tass-e-i EYE-Oh-dyed) (abbreviated: 10) IF YOU ARE TOLD TO. TAKE THIS MEDICINE, TAKE IT ONE TIME EVERY 24 HOURS. DO NOT TAKE IT MORE OFTEN. MORE WILL NOT HELP YOU AND MAY INCREASE THE RISK OF SIDE EFFECTS. DO NOT TAKE THIS DRUG IF YOU KNOW YOU ARE ALLERGIC TO IODIDE. (SEE SIDE EFFECTS BELOW.) INDICATIONS THYROID BLOCKING IN A RADIATION EMERGENCY ONLY. DIRECTIONS FOR USE Use only as directed by State or local public health authorities in the event of a radiation emergency. DOSE Tablets: ADULTS AND CHILDREN 1 YEAR OF AGE OR OLDER: One (1) tablet once a day. Crush for small children. BABIES UNDER 1 YEAR OF AGE: One-half (12) tablet once a day. Crush first. Take for 10 days unless directed otherwise by State or local public health authorities. Store at controlled room temperature between 150 and 30'C (590 to 86WF). Keep container tightly closed and protect from light. WARNING Potassium iodide should not be used by people allergic to iodid Keep out of the reach of children. In case of overdose or allergic reaction, contact a physician or the public health authority. DESCRIPTION Each white, round, scored, monogrammed THYRO-BLOCK TABLET contains 130 mg of potassium iodide. Other ingredients: magnesium stearate, microcrystalline cellulose, silica gel, and sodium thiosulfate.
14 Enclosure 5.3 HP/O/B/1009/012 Example Page 2 of 2 Package Insert For Thyro-BlockTm Tablets And Solution HOW POTASSIUM IODIDE WORKS Certain forms of iodine help your thyroid gland work right. Most people get the iodine they need from foods, like iodized salt or fish. The thyroid can "store or hold only a certain amount of iodine. In a radiation emergency, radioactive Iodine miay be released in the air. This material may be breathed or sw lowed It may enter the thyroid gland and damage it. The damage. would probably not show itself for years. Children are most likely to have thyroid damage. If you take potassium iodide, it will l up 7 our thyroid _gand. This reduces the chance that harmfl radoactve iodne wi enter the thyroid gland. WHO SHOULD NOT TAKE POTASSIUM IODIDE The only people who should not take potassium iodide are. people who know they are allergic to iodide. You may take potassium iodide even if you are taking medicines for a thyroid problem (for example, a thyroid hormone or antithyroid drug). Pregnant and nursing women and babies and children may also take this drug. AND WHEN TO TAKE POTASSIUM IODIDE Potassium iodide should be taken as soon as possible after public health officials tell you. You should take one dose every 24 hours. More will not help you because the thyroid can "hold" only limited amounts of iodine. Larger doses win increase the risk of side effects. You will probably be told not to take the drug for more than 10 days. SIDE EFFECTS Usually, side effects of potassium iodide happen when people take higher doses for a long time. You should be careful not to take more than the recommended dose or take it for longer than you are told. Side effects are unlikely because of the low dose and the short time you will be taking the drug. Possible side effects include skin rashes, swelling of the salivary glands, and "iodism" (metallic taste, burning mouth and throat, sore teeth and gums, symptoms of a head cold, and sometimes stomach upset and diarrhea). A few people have an allergic reaction with more serious symptoms. These could be fever and joint pains, or swelling of parts of the face and body and at times severe shortness of breath requiring immediate medical attention. Taking iodide may rarely cause overactivity of the thyroid gland, underactivity of the thyroid gland, or enlargement of the thyroid gland (goiter). WHAT TO DO IF SIDE EFFECTS OCCUR If the side effects are severe or if you have an allergic reaction, stop taking potassium iodide. Then, if possible, call a doctor or public health authorit for instructions. HOW SUPPUED THYRO-BLOCKM TABLETS (Potassium Iodide Tablets USP) are white, round tablets, one side scored, other side debo asd 472 WALLACE each containing 130 mg potassium iodide. Available in bottles of 14 tablets (NDC ). SHOW IN WALLACE LABORATORIES Division of CARTER-WALLACE, INC. Cranbury, New Jersey Rev. 5/94
Compounding KI for Radiation Exposure
 PL Detail-Document #270323 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2011 Compounding KI for
PL Detail-Document #270323 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2011 Compounding KI for
Prophylactic Use of Potassium Iodide (KI) in Radiological Emergencies*: Information for Physicians
 Prophylactic Use of Potassium Iodide (KI) in Radiological Emergencies*: Information for Physicians * Based on FDA's guidance document on use of KI as a thyroid blocking agent in radiation emergencies 1.
Prophylactic Use of Potassium Iodide (KI) in Radiological Emergencies*: Information for Physicians * Based on FDA's guidance document on use of KI as a thyroid blocking agent in radiation emergencies 1.
in88015.txt at Page 1 of 5 2/25/00
 in88015.txt at www.nrc.gov Page 1 of 5 UNITED STATES NUCLEAR REGULATORY COMMISSION OFFICE OF NUCLEAR MATERIAL SAFETY AND SAFEGUARDS WASHINGTON, D.C. 20555 NRC INFORMATION NOTICE NO. 88-15: AVAILABILITY
in88015.txt at www.nrc.gov Page 1 of 5 UNITED STATES NUCLEAR REGULATORY COMMISSION OFFICE OF NUCLEAR MATERIAL SAFETY AND SAFEGUARDS WASHINGTON, D.C. 20555 NRC INFORMATION NOTICE NO. 88-15: AVAILABILITY
Potassium Iodide (KI) Questions & Answers for Schools (Revised January 2007)
 General Information on KI Potassium Iodide (KI) Questions & Answers for Schools (Revised ) 1) What is potassium iodide (KI)? Potassium iodide is a U.S. Food and Drug Administration (FDA) approved over-thecounter
General Information on KI Potassium Iodide (KI) Questions & Answers for Schools (Revised ) 1) What is potassium iodide (KI)? Potassium iodide is a U.S. Food and Drug Administration (FDA) approved over-thecounter
PACKAGE LEAFLET: INFORMATION FOR THE USER. Kaliumjodide G.L. 65 mg tabletten Potassium iodide
 PACKAGE LEAFLET: INFORMATION FOR THE USER Kaliumjodide G.L. 65 mg tabletten Potassium iodide Read all of this leaflet carefully because it contains important information for you. This medicine is available
PACKAGE LEAFLET: INFORMATION FOR THE USER Kaliumjodide G.L. 65 mg tabletten Potassium iodide Read all of this leaflet carefully because it contains important information for you. This medicine is available
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. ERLEADA apalutamide tablets
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION ERLEADA apalutamide tablets Read this carefully before you start taking ERLEADA and each time you get a refill. This
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION ERLEADA apalutamide tablets Read this carefully before you start taking ERLEADA and each time you get a refill. This
Potassium Iodide tablets
 Potassium Iodide tablets In an emergency at Hunterston B Power Station Information for people receiving Potassium Iodide tablets This booklet gives you advice about taking Potassium Iodide tablets after
Potassium Iodide tablets In an emergency at Hunterston B Power Station Information for people receiving Potassium Iodide tablets This booklet gives you advice about taking Potassium Iodide tablets after
Procedure: I-131 Tx for Hyperthyroidism
 PURPOSE: To provide a pathway to successfully and safely deliver a prescribed dose of Iodine - 131 to a patient diagnosed with Hyperthyroidism. SCOPE: The stipulation of this procedure shall apply to all
PURPOSE: To provide a pathway to successfully and safely deliver a prescribed dose of Iodine - 131 to a patient diagnosed with Hyperthyroidism. SCOPE: The stipulation of this procedure shall apply to all
'03 MAR 24 $1 :52
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administratin College Pa&, MD 20740 w lza@ 1 38 7 '03 MAR 24 $1 :52 Mr. Scott D. Kumpf General Counsel and Corporate Secretary
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administratin College Pa&, MD 20740 w lza@ 1 38 7 '03 MAR 24 $1 :52 Mr. Scott D. Kumpf General Counsel and Corporate Secretary
Package leaflet for emergency supplies
 Version: August 2014 No.: 57204.00.00 Package leaflet for emergency supplies This is a special medicine to be taken in the event of radiation accidents involving the release of radioactive iodine. It must
Version: August 2014 No.: 57204.00.00 Package leaflet for emergency supplies This is a special medicine to be taken in the event of radiation accidents involving the release of radioactive iodine. It must
RE: CARBIMAZOLE 5MG TABLETS
 RE: CARBIMAZOLE 5MG TABLETS This notification is sent by LINK to inform your organisation that CARBIMAZOLE LINK, carbimazole 5 mg tablets, AUST R 254132 is currently unavailable due to manufacturing delays.
RE: CARBIMAZOLE 5MG TABLETS This notification is sent by LINK to inform your organisation that CARBIMAZOLE LINK, carbimazole 5 mg tablets, AUST R 254132 is currently unavailable due to manufacturing delays.
a condition called secondary hyperparathyroidism (high-per-pear-a-thigh-royd-izm) in adults with kidney disease who require dialysis treatment.
 Page 1 of 6 Sensipar Cinacalcet hydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Sensipar. It does not contain all the available information.
Page 1 of 6 Sensipar Cinacalcet hydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Sensipar. It does not contain all the available information.
Sodium Iodide I 131 Solution. Click Here to Continue. Click Here to Return to Table of Contents
 Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
PATIENT INFORMATION Metformin Hydrochloride Extended-Release Tablets (met-for-min HYE-droe-KLOR-ide) Rx only
 PATIENT INFORMATION Metformin Hydrochloride Extended-Release Tablets (met-for-min HYE-droe-KLOR-ide) Rx only What is the most important information I should know about metformin hydrochloride extended-release
PATIENT INFORMATION Metformin Hydrochloride Extended-Release Tablets (met-for-min HYE-droe-KLOR-ide) Rx only What is the most important information I should know about metformin hydrochloride extended-release
HEALTH INFORMATION FORM
 St. Michael Albertville STUDENT INFORMATION Name: School: HEALTH INFORMATION FORM Grade: DOB: HEALTH INFORMATION Does your child have any health problems (i.e. Asthma, Diabetes, ADHD, Heart Condition,
St. Michael Albertville STUDENT INFORMATION Name: School: HEALTH INFORMATION FORM Grade: DOB: HEALTH INFORMATION Does your child have any health problems (i.e. Asthma, Diabetes, ADHD, Heart Condition,
PRODUCT MONOGRAPH. Pr KYTRIL. granisetron hydrochloride tablets. 1 mg granisetron as hydrochloride. Manufacture Standard
 PRODUCT MONOGRAPH Pr KYTRIL granisetron hydrochloride tablets 1 mg granisetron as hydrochloride Manufacture Standard Antiemetic (5-HT 3 receptor antagonist) Hoffmann-La Roche Limited Date of Revision:
PRODUCT MONOGRAPH Pr KYTRIL granisetron hydrochloride tablets 1 mg granisetron as hydrochloride Manufacture Standard Antiemetic (5-HT 3 receptor antagonist) Hoffmann-La Roche Limited Date of Revision:
Package leaflet: Information for the user. Ebateva 20 mg Orodispersible Tablets. Ebastine
 Package leaflet: Information for the user Ebateva 10 mg Orodispersible Tablets Ebateva 20 mg Orodispersible Tablets Ebastine Read all of this leaflet carefully before you start taking this medicine because
Package leaflet: Information for the user Ebateva 10 mg Orodispersible Tablets Ebateva 20 mg Orodispersible Tablets Ebastine Read all of this leaflet carefully before you start taking this medicine because
Florida A & M University Office of Human Resources INTERNAL OPERATING PROCEDURE. Procedure No. HR-7000
 Subject: Alcohol and Drug Testing Policy Florida A & M University Office of Human Resources INTERNAL OPERATING PROCEDURE Procedure No. HR-7000 Authority: Florida Statutes 1001.74; Chapter 112.0455, Florida
Subject: Alcohol and Drug Testing Policy Florida A & M University Office of Human Resources INTERNAL OPERATING PROCEDURE Procedure No. HR-7000 Authority: Florida Statutes 1001.74; Chapter 112.0455, Florida
RULES AND REGULATIONS
 RI-12 RI-12 BIOASSAYS FOR INTERNAL RADIOACTIVITY PURPOSE This procedure specifies the requirements, responsibilities and methods for performing and reporting measurements for detecting and verifying the
RI-12 RI-12 BIOASSAYS FOR INTERNAL RADIOACTIVITY PURPOSE This procedure specifies the requirements, responsibilities and methods for performing and reporting measurements for detecting and verifying the
FAVISTAN 20 mg, tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER FAVISTAN 20 mg, tablets TIAMAZOL This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER FAVISTAN 20 mg, tablets TIAMAZOL This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
Treatment for Internal Contamination
 Handbook Treatment for Internal Contamination KIRAMS National Radiation Emergency Medical Center Preface With the increased use of radionuclides in all fields of science and technology ; research, medical
Handbook Treatment for Internal Contamination KIRAMS National Radiation Emergency Medical Center Preface With the increased use of radionuclides in all fields of science and technology ; research, medical
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. PIFELTRO doravirine tablets
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION PIFELTRO doravirine tablets Read this carefully before you start taking PIFELTRO (doravirine) and each time you get
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION PIFELTRO doravirine tablets Read this carefully before you start taking PIFELTRO (doravirine) and each time you get
Package leaflet: Information for the patient. Acetylsalicylic acid Bluefish 75 mg tablets Acetylsalicylic acid Bluefish 160 mg tablets
 Package leaflet: Information for the patient Acetylsalicylic acid Bluefish 75 mg tablets Acetylsalicylic acid Bluefish 160 mg tablets Acetylsalicylic acid Read all of this leaflet carefully before you
Package leaflet: Information for the patient Acetylsalicylic acid Bluefish 75 mg tablets Acetylsalicylic acid Bluefish 160 mg tablets Acetylsalicylic acid Read all of this leaflet carefully before you
Nuclear Plant Emergency Response
 Nuclear Plant Emergency Response Acute and Chronic Clinical Health Effects after a NPP Accident Module 5 At the end of this presentation you will be able to: Discuss the initial medical evaluation of a
Nuclear Plant Emergency Response Acute and Chronic Clinical Health Effects after a NPP Accident Module 5 At the end of this presentation you will be able to: Discuss the initial medical evaluation of a
Nuclear Plant Emergency Response
 Nuclear Plant Emergency Response Acute and Chronic Clinical Health Effects after a NPP Accident Module 5 At the end of this presentation you will be able to: Discuss the initial medical evaluation of a
Nuclear Plant Emergency Response Acute and Chronic Clinical Health Effects after a NPP Accident Module 5 At the end of this presentation you will be able to: Discuss the initial medical evaluation of a
Sensipar Cinacalcet hydrochloride
 Sensipar Cinacalcet hydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Sensipar. It does not contain all the available information. It
Sensipar Cinacalcet hydrochloride Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Sensipar. It does not contain all the available information. It
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Doxycycline for Oral Suspension USP, 25 mg/5 ml Manufacturer Distributor Lupin Limited Goa 403722 INDIA. Lupin Pharmaceuticals,
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Doxycycline for Oral Suspension USP, 25 mg/5 ml Manufacturer Distributor Lupin Limited Goa 403722 INDIA. Lupin Pharmaceuticals,
RULES OF TENNESSEE DEPARTMENT OF AGRICULTURE DIVISION OF MARKETING CHAPTER COMMERCIAL FERTILIZER TABLE OF CONTENTS
 RULES OF TENNESSEE DEPARTMENT OF AGRICULTURE DIVISION OF MARKETING CHAPTER 0080-05-10 COMMERCIAL FERTILIZER TABLE OF CONTENTS 0080-05-10-.01 Definitions and Terms 0080-05-10-.07 Labeling of Soil Conditioners
RULES OF TENNESSEE DEPARTMENT OF AGRICULTURE DIVISION OF MARKETING CHAPTER 0080-05-10 COMMERCIAL FERTILIZER TABLE OF CONTENTS 0080-05-10-.01 Definitions and Terms 0080-05-10-.07 Labeling of Soil Conditioners
8.05 days 138 days 7.60 days 0.22 mr/h at 1.0 meter per millicurie 124,068 curies/gram 2 mm = 0.20 cm = 0.08 in 165 cm = 65.0 in = 5.
 Iodine-131 Radiological Safety Guidance Physical Data GAMMA ENERGIES 364 kev (82% abundance) 637 kev (7% abundance) 284 kev (6% abundance) 80 kev (3% abundance) 723 kev (2% abundance) 29-34 kev (4.5%/x-rays)
Iodine-131 Radiological Safety Guidance Physical Data GAMMA ENERGIES 364 kev (82% abundance) 637 kev (7% abundance) 284 kev (6% abundance) 80 kev (3% abundance) 723 kev (2% abundance) 29-34 kev (4.5%/x-rays)
PATIENT INFORMATION. Metformin Hydrochloride Extended-Release Tablets USP (met-for-min HYE-droe-KLOR-ide)
 PATIENT INFORMATION Metformin Hydrochloride Extended-Release Tablets USP (met-for-min HYE-droe-KLOR-ide) What is the most important information I should know about metformin hydrochloride extended-release
PATIENT INFORMATION Metformin Hydrochloride Extended-Release Tablets USP (met-for-min HYE-droe-KLOR-ide) What is the most important information I should know about metformin hydrochloride extended-release
NEO-MERCAZOLE Carbimazole 5 mg tablets
 NEO-MERCAZOLE Carbimazole 5 mg tablets What is in this leaflet This leaflet answers some common questions about NEO-MERCAZOLE tablets. It does not contain all the available information. It does not take
NEO-MERCAZOLE Carbimazole 5 mg tablets What is in this leaflet This leaflet answers some common questions about NEO-MERCAZOLE tablets. It does not contain all the available information. It does not take
Patient Information Losartan Potassium and Hydrochlorothiazide Tablets, USP 50 mg/12.5 mg, 100 mg/12.5 mg and 100 mg/25 mg Rx only
 Patient Information Losartan Potassium and Hydrochlorothiazide Tablets, USP 50 mg/12.5 mg, 100 mg/12.5 mg and 100 mg/25 mg Rx only Read the Patient Information that comes with Losartan Potassium and Hydrochlorothiazide
Patient Information Losartan Potassium and Hydrochlorothiazide Tablets, USP 50 mg/12.5 mg, 100 mg/12.5 mg and 100 mg/25 mg Rx only Read the Patient Information that comes with Losartan Potassium and Hydrochlorothiazide
What is the current risk of radiation-related health problems in Japan to those near the reactor at the time, and those in other parts of Japan?
 What is the current risk of radiation-related health problems in Japan to those near the reactor at the time, and those in other parts of Japan? The actions proposed by the Government of Japan are in line
What is the current risk of radiation-related health problems in Japan to those near the reactor at the time, and those in other parts of Japan? The actions proposed by the Government of Japan are in line
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
ELTROXIN thyroxine (as anhydrous levothyroxine sodium) 50 micrograms, 100 micrograms tablets
 ELTROXIN thyroxine (as anhydrous levothyroxine sodium) 50 micrograms, 100 micrograms tablets Consumer Medicine Information (CMI) What is in this leaflet This leaflet answers some common questions about
ELTROXIN thyroxine (as anhydrous levothyroxine sodium) 50 micrograms, 100 micrograms tablets Consumer Medicine Information (CMI) What is in this leaflet This leaflet answers some common questions about
AUGMENTIN is also used to prevent infection from major surgery.
 Injection Potassium clavulanate/amoxicillin sodium equivalent to 100 mg clavulanic acid/500 mg amoxicillin Potassium clavulanate/amoxicillin sodium equivalent to 200 mg clavulanic acid/1 g amoxicillin
Injection Potassium clavulanate/amoxicillin sodium equivalent to 100 mg clavulanic acid/500 mg amoxicillin Potassium clavulanate/amoxicillin sodium equivalent to 200 mg clavulanic acid/1 g amoxicillin
Medication Summary: Children s Aspirin (Brand Names: Bayer, Bufferin, Ecotrin, others)
 Medication Summary: Children s Aspirin (Brand Names: Bayer, Bufferin, Ecotrin, others) What is this medicine for? - To decrease swelling and inflammation - Used in the treatment of Kawasaki syndrome -
Medication Summary: Children s Aspirin (Brand Names: Bayer, Bufferin, Ecotrin, others) What is this medicine for? - To decrease swelling and inflammation - Used in the treatment of Kawasaki syndrome -
Medical errors: definition, documentation, and reporting. Outline. Do your homework in advance! 8/1/2017
 Medical errors: definition, documentation, and reporting Zoubir Ouhib MS FACR Lynn Cancer Institute AAPM 2017 Outline Be familiar with current and future definition of medical event Understand the importance
Medical errors: definition, documentation, and reporting Zoubir Ouhib MS FACR Lynn Cancer Institute AAPM 2017 Outline Be familiar with current and future definition of medical event Understand the importance
MEDICATION GUIDE. Rabeprazole Sodium Delayed-Release Tablets Rx Only
 MEDICATION GUIDE Rabeprazole Sodium Delayed-Release Tablets Rx Only Read the Medication Guide that comes with rabeprazole sodium delayed-release tablets before you start taking it and each time you get
MEDICATION GUIDE Rabeprazole Sodium Delayed-Release Tablets Rx Only Read the Medication Guide that comes with rabeprazole sodium delayed-release tablets before you start taking it and each time you get
AUSTRAPEN ampicillin (as sodium)
 AUSTRAPEN ampicillin (as sodium) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about AUSTRAPEN. It does not contain all the available information. It
AUSTRAPEN ampicillin (as sodium) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about AUSTRAPEN. It does not contain all the available information. It
PATIENT INFORMATION LEAFLET
 PATIENT INFORMATION LEAFLET Page 1 of 6 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER 1 ethambutol hydrochloride Read all of this leaflet carefully before your child starts taking this medicine
PATIENT INFORMATION LEAFLET Page 1 of 6 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER 1 ethambutol hydrochloride Read all of this leaflet carefully before your child starts taking this medicine
Medication Guide SEGLUROMET (seg-lur-oh-met) (ertugliflozin and metformin hydrochloride) tablets, for oral use
 Medication Guide SEGLUROMET (seg-lur-oh-met) (ertugliflozin and metformin hydrochloride) tablets, for oral use Read this Medication Guide carefully before you start taking SEGLUROMET and each time you
Medication Guide SEGLUROMET (seg-lur-oh-met) (ertugliflozin and metformin hydrochloride) tablets, for oral use Read this Medication Guide carefully before you start taking SEGLUROMET and each time you
How Entecavir GH Works
 entecavir monohydrate tablets ENTECAVIR GH Tablets Consumer Medicine Information What is in this leaflet Read this leaflet carefully before taking. This leaflet answers some common questions about. It
entecavir monohydrate tablets ENTECAVIR GH Tablets Consumer Medicine Information What is in this leaflet Read this leaflet carefully before taking. This leaflet answers some common questions about. It
MATERIAL SAFETY DATA SHEET. Capture Pro Steam Drop 'N Go
 Page 1 of 5 MATERIAL SAFETY DATA SHEET Capture Pro Steam Drop 'N Go 1. PRODUCT AND COMPANY IDENTIFICATION Product Identification Product Name: Capture Pro Steam Drop 'N Go CAS Number: Blend Company Identification
Page 1 of 5 MATERIAL SAFETY DATA SHEET Capture Pro Steam Drop 'N Go 1. PRODUCT AND COMPANY IDENTIFICATION Product Identification Product Name: Capture Pro Steam Drop 'N Go CAS Number: Blend Company Identification
PATIENT INFORMATION LEAFLET
 PATIENT INFORMATION LEAFLET Page 1 of 7 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER Cycloserine Capsules USP 250 mg 1 Cycloserine Read all of this leaflet carefully before you start taking this
PATIENT INFORMATION LEAFLET Page 1 of 7 PATIENT INFORMATION LEAFLET: INFORMATION FOR THE USER Cycloserine Capsules USP 250 mg 1 Cycloserine Read all of this leaflet carefully before you start taking this
Medication Information for Parents and Teachers
 Medication Information for Parents and Teachers General Information About Medication Melatonin Each child and adolescent is different. No one has exactly the same combination of medical and psychological
Medication Information for Parents and Teachers General Information About Medication Melatonin Each child and adolescent is different. No one has exactly the same combination of medical and psychological
Simplotan* tablets Tinidazole (tin-id-azole)
 Simplotan* tablets Tinidazole (tin-id-azole) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Simplotan. It does not contain all the available information.
Simplotan* tablets Tinidazole (tin-id-azole) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Simplotan. It does not contain all the available information.
AUTHORIZATION FOR STUDENTS TO CARRY A PRESCRIPTION INHALER, EPINEPHRINE AUTO INJECTOR, INSULIN, AND DIABETIC SUPPLIES, OR OTHER APPROVED MEDICATION
 AUTHORIZATION FOR STUDENTS TO CARRY A PRESCRIPTION INHALER, EPINEPHRINE AUTO INJECTOR, INSULIN, AND DIABETIC SUPPLIES, OR OTHER APPROVED MEDICATION needs to carry the following prescription labeled inhaler,
AUTHORIZATION FOR STUDENTS TO CARRY A PRESCRIPTION INHALER, EPINEPHRINE AUTO INJECTOR, INSULIN, AND DIABETIC SUPPLIES, OR OTHER APPROVED MEDICATION needs to carry the following prescription labeled inhaler,
PART I. RADIONUCLIDE THERAPY WITH I-131 SODIUM IODIDE: PATIENT & DOSE ADMINISTRATOR PRECAUTIONS; ADMINISTRATION METHODS
 OVERVIEW AND TOPICS Patient and dose administrator precautions and dose administration methods of therapeutic procedures Administration methods for performing therapy with I-131 sodium iodide in either
OVERVIEW AND TOPICS Patient and dose administrator precautions and dose administration methods of therapeutic procedures Administration methods for performing therapy with I-131 sodium iodide in either
TASK 2: ESTABLISH EXERCISE OBJECTIVES AND EXTENT OF PLAY
 Description The RAC Chair establishes a set of objectives for each exercise by negotiations with OROs, RAC members, and the licensee. Responsibility for the demonstration of selected objectives is assigned
Description The RAC Chair establishes a set of objectives for each exercise by negotiations with OROs, RAC members, and the licensee. Responsibility for the demonstration of selected objectives is assigned
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr BACLOFEN Baclofen Tablets 10 mg and 20 mg This leaflet is part III of a three-part "Product Monograph" published when BACLOFEN was approved for sale in Canada and is designed
PART III: CONSUMER INFORMATION Pr BACLOFEN Baclofen Tablets 10 mg and 20 mg This leaflet is part III of a three-part "Product Monograph" published when BACLOFEN was approved for sale in Canada and is designed
Irbenida H 150mg/12,5mg film-coated tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER Irbenida H 150mg/12,5mg film-coated tablets IRBESARTAN/HYDROCHLOROTHIAZIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information
PACKAGE LEAFLET: INFORMATION FOR THE USER Irbenida H 150mg/12,5mg film-coated tablets IRBESARTAN/HYDROCHLOROTHIAZIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
RULES OF TENNESSEE DEPARTMENT OF AGRICULTURE DIVISION OF MARKETING CHAPTER COMMERCIAL FERTILIZERS REGULATIONS TABLE OF CONTENTS
 RULES OF TENNESSEE DEPARTMENT OF AGRICULTURE DIVISION OF MARKETING CHAPTER 0080-5-10 COMMERCIAL FERTILIZERS REGULATIONS TABLE OF CONTENTS 0080-5-10-.01 Definitions and Terms 0080-5-10-.07 Labeling of Soil
RULES OF TENNESSEE DEPARTMENT OF AGRICULTURE DIVISION OF MARKETING CHAPTER 0080-5-10 COMMERCIAL FERTILIZERS REGULATIONS TABLE OF CONTENTS 0080-5-10-.01 Definitions and Terms 0080-5-10-.07 Labeling of Soil
All medicines have risks and benefits. Your doctor has weighed the risks of you taking Triprim against the benefits he or she expects it will have.
 Triprim Tablets Trimethoprim Tablets Consumer Medicine Information What is in this leaflet? The leaflet answers some common questions about Triprim tablets. It does not contain all the available information
Triprim Tablets Trimethoprim Tablets Consumer Medicine Information What is in this leaflet? The leaflet answers some common questions about Triprim tablets. It does not contain all the available information
Package leaflet: Information for the patient. [To be completed nationally] 10 mg film-coated tablets Ebastine
![Package leaflet: Information for the patient. [To be completed nationally] 10 mg film-coated tablets Ebastine Package leaflet: Information for the patient. [To be completed nationally] 10 mg film-coated tablets Ebastine](/thumbs/91/106770890.jpg) Package leaflet: Information for the patient [To be completed nationally] 10 mg film-coated tablets Ebastine Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the patient [To be completed nationally] 10 mg film-coated tablets Ebastine Read all of this leaflet carefully before you start taking this medicine because it contains
PACKAGE LEAFLET. Page 1 of 6
 PACKAGE LEAFLET Page 1 of 6 Package leaflet: Information for the patient Procyclidine Hydrochloride 5 mg Tablets (procyclidine hydrochloride) Read all of this leaflet carefully before you start taking
PACKAGE LEAFLET Page 1 of 6 Package leaflet: Information for the patient Procyclidine Hydrochloride 5 mg Tablets (procyclidine hydrochloride) Read all of this leaflet carefully before you start taking
MEDICATION GUIDE WELLBUTRIN SR (WELL byu-trin) (bupropion hydrochloride) Sustained-Release Tablets
 Page 56 (see CLINICAL TRIALS under CLINICAL PHARMACOLOGY). Based on these limited data, it is unknown whether or not the dose of WELLBUTRIN SR needed for maintenance treatment is identical to the dose
Page 56 (see CLINICAL TRIALS under CLINICAL PHARMACOLOGY). Based on these limited data, it is unknown whether or not the dose of WELLBUTRIN SR needed for maintenance treatment is identical to the dose
PART III: PATIENT MEDICATION INFORMATION. CLINDAMYCINE-150 Pr. CLINDAMYCINE-300 (Clindamycin Hydrochloride Capsules USP) Clindamycin 150 mg and 300 mg
 PART III: PATIENT MEDICATION INFORMATION Pr CLINDAMYCINE-150 Pr CLINDAMYCINE-300 (Clindamycin Hydrochloride Capsules USP) Clindamycin 150 mg and 300 mg Read this carefully before you start taking CLINDAMYCINE
PART III: PATIENT MEDICATION INFORMATION Pr CLINDAMYCINE-150 Pr CLINDAMYCINE-300 (Clindamycin Hydrochloride Capsules USP) Clindamycin 150 mg and 300 mg Read this carefully before you start taking CLINDAMYCINE
PROTECTION IN A NUCLEAR EMERGENCY
 PROTECTION IN A NUCLEAR EMERGENCY There are 99 nuclear power reactors in the United States producing about one-quarter of the country s electricity. But around each plant is a 50-mile ring that could be
PROTECTION IN A NUCLEAR EMERGENCY There are 99 nuclear power reactors in the United States producing about one-quarter of the country s electricity. But around each plant is a 50-mile ring that could be
Package leaflet: Information for the user. Finasterid Actavis 5 mg film coated tablets. Finasteride
 PACKAGE LEAFLET 1 Package leaflet: Information for the user 5 mg film coated tablets Finasteride Read all of this leaflet carefully before you start taking this medicine because it contains important information
PACKAGE LEAFLET 1 Package leaflet: Information for the user 5 mg film coated tablets Finasteride Read all of this leaflet carefully before you start taking this medicine because it contains important information
PHARMACY ACTION COLD & FLU DAY & NIGHT paracetamol, pseudoephedrine hydrochloride, chlorphenamine maleate
 CONSUMER MEDICINE INFORMATION PHARMACY ACTION COLD & FLU DAY & NIGHT paracetamol, pseudoephedrine hydrochloride, chlorphenamine maleate What is in this leaflet This leaflet answers some common questions
CONSUMER MEDICINE INFORMATION PHARMACY ACTION COLD & FLU DAY & NIGHT paracetamol, pseudoephedrine hydrochloride, chlorphenamine maleate What is in this leaflet This leaflet answers some common questions
Working With MDI and Polymeric MDI: What You Should Know
 Working With MDI and Polymeric MDI: What You Should Know Here are six important points you should know about working with MDI or polymeric MDI The Alliance for the Polyurethanes Industry, prepared this
Working With MDI and Polymeric MDI: What You Should Know Here are six important points you should know about working with MDI or polymeric MDI The Alliance for the Polyurethanes Industry, prepared this
PROSCAR 5mg tablets PACKAGE LEAFLET: INFORMATION FOR THE USER. What is in this leaflet? 1. WHAT PROSCAR IS AND WHAT IS IT USED FOR
 PACKAGE LEAFLET: INFORMATION FOR THE USER PROSCAR 5mg tablets FINASTERIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER PROSCAR 5mg tablets FINASTERIDE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
Employee Handout - Alcohol & drugs - driver
 Employee Handout - Alcohol & drugs - driver 1. Materials explaining how you (the employer) implement the requirements of Part 382, and your company's policies, must be provided to each driver. These materials
Employee Handout - Alcohol & drugs - driver 1. Materials explaining how you (the employer) implement the requirements of Part 382, and your company's policies, must be provided to each driver. These materials
JHM-IRB Guidelines for Radiation Statements
 JHM-IRB Guidelines for Radiation Statements When a participant in a research study is subjected to ionizing radiation exposure (other than that which is incidental for the standard medical management of
JHM-IRB Guidelines for Radiation Statements When a participant in a research study is subjected to ionizing radiation exposure (other than that which is incidental for the standard medical management of
Package leaflet: Information for the user. PRED MILD STERILE OPHTHALMIC SUSPENSION 0.12% w/v Eye Drops, Suspension Prednisolone Acetate
 Package leaflet: Information for the user PRED MILD STERILE OPHTHALMIC SUSPENSION 0.12% w/v Eye Drops, Suspension Prednisolone Acetate Read all of this leaflet carefully before you start using this medicine
Package leaflet: Information for the user PRED MILD STERILE OPHTHALMIC SUSPENSION 0.12% w/v Eye Drops, Suspension Prednisolone Acetate Read all of this leaflet carefully before you start using this medicine
PRONUNCIATION: (met-for-min) COMMON BRAND NAME(S): Riomet
 PRONUNCIATION: (met-for-min) COMMON BRAND NAME(S): Riomet HOW TO USE: Read the Patient Information Leaflet if available from your pharmacist before you start taking metformin and each time you get a refill.
PRONUNCIATION: (met-for-min) COMMON BRAND NAME(S): Riomet HOW TO USE: Read the Patient Information Leaflet if available from your pharmacist before you start taking metformin and each time you get a refill.
MEDICATION GUIDE XIGDUO XR (ZIG- DO- OH X- R) (dapagliflozin and metformin HCL extended-release) Tablets
 XIGDUO XR (dapagliflozin and metformin HCl extended-release) tablets 5 MEDICATION GUIDE XIGDUO XR (ZIG- DO- OH X- R) (dapagliflozin and metformin HCL extended-release) Tablets What is the most important
XIGDUO XR (dapagliflozin and metformin HCl extended-release) tablets 5 MEDICATION GUIDE XIGDUO XR (ZIG- DO- OH X- R) (dapagliflozin and metformin HCL extended-release) Tablets What is the most important
What are some things I need to know or do while I take
 PATIENT & CAREGIVER EDUCATION Cyclophosphamide Brand Names: Canada Procytox What is this drug used for? It is used to treat cancer. It is used to treat nephrotic syndrome. It may be g iven to you for other
PATIENT & CAREGIVER EDUCATION Cyclophosphamide Brand Names: Canada Procytox What is this drug used for? It is used to treat cancer. It is used to treat nephrotic syndrome. It may be g iven to you for other
Fukushima Emergency Planning Zones, Use of Potassium Iodide, Health Effects
 Fukushima Emergency Planning Zones, Use of Potassium Iodide, Health Effects Patricia A. Milligan, CHP Senior Technical Advisor Division of Preparedness & Response Office of Nuclear Security and Incident
Fukushima Emergency Planning Zones, Use of Potassium Iodide, Health Effects Patricia A. Milligan, CHP Senior Technical Advisor Division of Preparedness & Response Office of Nuclear Security and Incident
Package leaflet: Information for the user
 Package leaflet: Information for the user Irbesartan 75mg Film-coated Tablets Irbesartan 150mg Film-coated Tablets Irbesartan 300mg Film-coated Tablets irbesartan Read all of this leaflet carefully before
Package leaflet: Information for the user Irbesartan 75mg Film-coated Tablets Irbesartan 150mg Film-coated Tablets Irbesartan 300mg Film-coated Tablets irbesartan Read all of this leaflet carefully before
Package leaflet: Information for the user. Irprestan 150 mg film-coated tablets. Irprestan 300 mg film-coated tablets irbesartan
 Package leaflet: Information for the user 75 mg film-coated tablets 150 mg film-coated tablets 300 mg film-coated tablets irbesartan Read all of this leaflet carefully before you start taking this medicine
Package leaflet: Information for the user 75 mg film-coated tablets 150 mg film-coated tablets 300 mg film-coated tablets irbesartan Read all of this leaflet carefully before you start taking this medicine
Darren Lackan, MD Chris Bajaj, DO Anjanette Tan, MD Christopher Hudak, MD Stefanie Addington, MD
 Darren Lackan, MD Chris Bajaj, DO Anjanette Tan, MD Christopher Hudak, MD Stefanie Addington, MD I-131 (Radioactive Iodine) Treatment Patient Name: : Out of courtesy, our office expects 24 hour notice
Darren Lackan, MD Chris Bajaj, DO Anjanette Tan, MD Christopher Hudak, MD Stefanie Addington, MD I-131 (Radioactive Iodine) Treatment Patient Name: : Out of courtesy, our office expects 24 hour notice
Dorzolamide 20 mg/ml Eye Drops, Solution
 PACKAGE LEAFLET: INFORMATION FOR THE USER Dorzolamide 20 mg/ml Eye Drops, Solution (Dorzolamide hydrochloride) Read all of this leaflet carefully before you start using this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE USER Dorzolamide 20 mg/ml Eye Drops, Solution (Dorzolamide hydrochloride) Read all of this leaflet carefully before you start using this medicine because it contains
Midostaurin (Rydapt )
 Midostaurin (Rydapt ) ( mye doe STAW rin ) How drug is given: By mouth Purpose: To stop the growth of cancer cells in acute myeloid leukemia (AML) and other cancers. How to take this drug 1. Take this
Midostaurin (Rydapt ) ( mye doe STAW rin ) How drug is given: By mouth Purpose: To stop the growth of cancer cells in acute myeloid leukemia (AML) and other cancers. How to take this drug 1. Take this
Acetylcysteine Injection; Acetylcysteine Solution; Mucomyst; Parvolex. It is used to thin mucus so it can be taken from the body by coughing.
 PATIENT & CAREGIVER EDUCATION Acetylcysteine Brand Names: US Acetadote; Cetylev Brand Names: Canada Acetylcysteine Injection; Acetylcysteine Solution; Mucomyst; Parvolex What is this drug used for? It
PATIENT & CAREGIVER EDUCATION Acetylcysteine Brand Names: US Acetadote; Cetylev Brand Names: Canada Acetylcysteine Injection; Acetylcysteine Solution; Mucomyst; Parvolex What is this drug used for? It
Section 7 ALARA Program
 Page 7-1 Section 7 ALARA Program Contents A. ALARA Principle... 7-2 1. Biological Basis... 7-2 2. Applied Practices... 7-3 3. Operational Dose Limits... 7-3 4. Collective Dose... 7-3 B. Radiation Safety
Page 7-1 Section 7 ALARA Program Contents A. ALARA Principle... 7-2 1. Biological Basis... 7-2 2. Applied Practices... 7-3 3. Operational Dose Limits... 7-3 4. Collective Dose... 7-3 B. Radiation Safety
Goiter. This reference summary explains goiters. It covers symptoms and causes of the condition, as well as treatment options.
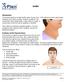 Goiter Introduction The thyroid gland is located at the base of your neck. If the gland becomes abnormally enlarged, it is called a goiter. Goiters usually do not cause pain. But a large goiter could cause
Goiter Introduction The thyroid gland is located at the base of your neck. If the gland becomes abnormally enlarged, it is called a goiter. Goiters usually do not cause pain. But a large goiter could cause
PACKAGE LEAFLET: INFORMATION FOR THE USER Irbea 75 mg film-coated tablets Irbesartan
 PACKAGE LEAFLET: INFORMATION FOR THE USER Irbea 75 mg film-coated tablets Irbesartan Read all of this leaflet carefully before you start taking this medicine because it contains important information for
PACKAGE LEAFLET: INFORMATION FOR THE USER Irbea 75 mg film-coated tablets Irbesartan Read all of this leaflet carefully before you start taking this medicine because it contains important information for
Click Here to Continue. Click Here to Return to Table of Contents
 TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
Protecting Yourself in a Nuclear Emergency with Potassium Iodide
 Protecting Yourself in a Nuclear Emergency with Potassium Iodide By Mark A. Mitchell, MD The possibility of radioactive contamination from a device detonated by terrorists within the country's borders
Protecting Yourself in a Nuclear Emergency with Potassium Iodide By Mark A. Mitchell, MD The possibility of radioactive contamination from a device detonated by terrorists within the country's borders
Patient Guide to Radioiodine Treatment For Thyrotoxicosis (Overactive Thyroid Gland or Hyperthyroidism)
 Patient Guide to Radioiodine Treatment For Thyrotoxicosis (Overactive Thyroid Gland or Hyperthyroidism) Your doctor has referred you to Nuclear Medicine for treatment of your overactive thyroid gland.
Patient Guide to Radioiodine Treatment For Thyrotoxicosis (Overactive Thyroid Gland or Hyperthyroidism) Your doctor has referred you to Nuclear Medicine for treatment of your overactive thyroid gland.
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr BENAZEPRIL Benazepril Hydrochloride tablets Read this carefully before you start taking BENAZEPRIL and each time you get a refill. This leaflet is a summary and will not
PART III: CONSUMER INFORMATION Pr BENAZEPRIL Benazepril Hydrochloride tablets Read this carefully before you start taking BENAZEPRIL and each time you get a refill. This leaflet is a summary and will not
PATIENT MEDICATION INFORMATION
 page 24 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr ATENOLOL Atenolol Tablets, BP Read this carefully before you start taking ATENOLOL and each time you get
page 24 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr ATENOLOL Atenolol Tablets, BP Read this carefully before you start taking ATENOLOL and each time you get
PRED FORTE 1% w/v Eye Drops, Suspension Prednisolone acetate
 Package leaflet: Information for the user PRED FORTE 1% w/v Eye Drops, Suspension Prednisolone acetate Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the user PRED FORTE 1% w/v Eye Drops, Suspension Prednisolone acetate Read all of this leaflet carefully before you start using this medicine because it contains important
What is the most important information I should know about tenofovir? What should I discuss with my healthcare provider before taking tenofovir?
 1 of 6 6/10/2016 4:33 PM Generic Name: tenofovir (ten OF oh vir) Brand Name: Viread What is tenofovir? Tenofovir is an antiviral medicine that prevents human immunodeficiency virus (HIV) or hepatitis B
1 of 6 6/10/2016 4:33 PM Generic Name: tenofovir (ten OF oh vir) Brand Name: Viread What is tenofovir? Tenofovir is an antiviral medicine that prevents human immunodeficiency virus (HIV) or hepatitis B
FAUQUIER COUNTY PUBLIC SCHOOLS Policy: Adopted: 04/10/2012 Revised: 07/23/12, 7/08/13, 08/11/14, 08/14/17 ADMINISTERING MEDICINES TO STUDENTS
 ACCOMPANYING REGULATION REGULATION 7-5.3(B): ADMINISTRATION OF EPINEPHRINE (Severe Allergic Reaction) 1. Generally 1.1. Fauquier County Public Schools Public Schools ( FCPS) anaphylaxis regulation is developed
ACCOMPANYING REGULATION REGULATION 7-5.3(B): ADMINISTRATION OF EPINEPHRINE (Severe Allergic Reaction) 1. Generally 1.1. Fauquier County Public Schools Public Schools ( FCPS) anaphylaxis regulation is developed
247 CMR BOARD OF REGISTRATION IN PHARMACY
 247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
Package leaflet: Information for the patient. Bumetanide 1 mg and 5 mg Tablets (bumetanide)
 Package leaflet: Information for the patient Bumetanide 1 mg and 5 mg Tablets (bumetanide) Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient Bumetanide 1 mg and 5 mg Tablets (bumetanide) Read all of this leaflet carefully before you start taking this medicine because it contains important information
MEDICATION GUIDE. BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use
 MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
MEDICATION GUIDE BENLYSTA (ben-list-ah) (belimumab) Injection for intravenous use Read this Medication Guide before you start receiving BENLYSTA and before each treatment. There may be new information.
Package leaflet: Information for the patient. Torarese 2.5 mg tablets Torarese 5 mg tablets Torarese 10 mg tablets Torarese 20 mg tablets.
 Package leaflet: Information for the patient Torarese 2.5 mg tablets Torarese 5 mg tablets Torarese 10 mg tablets Torarese 20 mg tablets Torasemide Read all of this leaflet carefully before you start taking
Package leaflet: Information for the patient Torarese 2.5 mg tablets Torarese 5 mg tablets Torarese 10 mg tablets Torarese 20 mg tablets Torasemide Read all of this leaflet carefully before you start taking
Curing as a Single Special Process Regulatory Agency Jurisdiction NAME (fill in form)
 Single Hazard Special Process HACCP Template for Curing as a Single Special Process Regulatory Agency Jurisdiction NAME (fill in form) Date Submitted Date Approved Valid until A. General Information This
Single Hazard Special Process HACCP Template for Curing as a Single Special Process Regulatory Agency Jurisdiction NAME (fill in form) Date Submitted Date Approved Valid until A. General Information This
THE THYROID BOOK. Medical and Surgical Treatment of Thyroid Problems
 THE THYROID BOOK Medical and Surgical Treatment of Thyroid Problems Trouble with Your Thyroid Gland The thyroid is a small gland in your neck that plays a big role in how your body functions. It impacts
THE THYROID BOOK Medical and Surgical Treatment of Thyroid Problems Trouble with Your Thyroid Gland The thyroid is a small gland in your neck that plays a big role in how your body functions. It impacts
Medication Guide Fluoxetine Tablets, USP
 Medication Guide Fluoxetine Tablets, USP Read the Medication Guide that comes with fluoxetine before you start taking it and each time you get a refill. There may be new information. This Medication Guide
Medication Guide Fluoxetine Tablets, USP Read the Medication Guide that comes with fluoxetine before you start taking it and each time you get a refill. There may be new information. This Medication Guide
PATIENT INFORMATION LEAFLET
 PATIENT INFORMATION LEAFLET CRESTOVAS (ROSUVASTATIN 5MG, 10MG, 20MG & 40MG, TABLETS USP) Please read this leaflet carefully before you start to take your medicine. It contains important information. Keep
PATIENT INFORMATION LEAFLET CRESTOVAS (ROSUVASTATIN 5MG, 10MG, 20MG & 40MG, TABLETS USP) Please read this leaflet carefully before you start to take your medicine. It contains important information. Keep
Chapter 18 Advanced Life Support (ALS) Controlled Substances March 2009
 Division 05 Emergency Medical March 2009 POLICY This General Order establishes procedures, as mandated by the Comprehensive Drug Abuse Prevention and Control Act of 1970 (Controlled Substances Act), to
Division 05 Emergency Medical March 2009 POLICY This General Order establishes procedures, as mandated by the Comprehensive Drug Abuse Prevention and Control Act of 1970 (Controlled Substances Act), to
Package leaflet: Information for the user Easyhaler Salbutamol Sulfate 100 and 200 micrograms/dose inhalation powder Salbutamol sulfate (salbutamol)
 Package leaflet: Information for the user Easyhaler Salbutamol Sulfate 100 and 200 micrograms/dose inhalation powder Salbutamol sulfate (salbutamol) Read all of this leaflet carefully before you start
Package leaflet: Information for the user Easyhaler Salbutamol Sulfate 100 and 200 micrograms/dose inhalation powder Salbutamol sulfate (salbutamol) Read all of this leaflet carefully before you start
