Treatment for Internal Contamination
|
|
|
- Jesse Hill
- 5 years ago
- Views:
Transcription
1 Handbook Treatment for Internal Contamination KIRAMS National Radiation Emergency Medical Center Preface With the increased use of radionuclides in all fields of science and technology ; research, medical applications, nuclear power, and industrial processes, it suggests that there is also a concomitant increase in the probability of human exposures to internally-deposited radionuclides. It is important that such exposures be minimized to use appropriate medication as soon as possible. This handbook provides information on some medications that are effective in the treatment or the prevention of persons internally contaminated with radionuclides. Even though some
2 problems exist to use those medications as the literature on medications of such cases is scattered and sparse or only experimental studies have been approved, they are very useful under an emergency situation. Therefore, this handbook will be intended as an aid and guide to find quick reference of treatment for medical personnels; physicians, nurses, physics, ambulance attendants, rescue squads, and paramedic personnels and other resources as well; police officers, fire fighters, military officers, authorities and etc... KI(Potassium Iodine) KI? A Thyroid Blocking Agent as preventing uptake of radioiodine It is the ingredient added to table salt to make it iodized salt. Approximately 76.5% iodine. When KI is available in the blood before any radioactive iodine, the thyroid gland becomes saturated with non-radioactive iodine. When saturated, the thyroid can absorb only about l% as much additional iodine, including radioactive forms: then it is said to be blocked. Excess iodine in the blood is rapidly eliminated by the action of the kidneys.
3 How KI Works Radioactive Iodine Released iodine in fission products I years I days, common cause of internal contamination I hours I minutes I-131 in human via inhalation/ingestion Iodide ions form in the blood saturated in the thyroid gland 10-30%(max) within 24hours retained in salivary gland/stomach mucosa/mammary gland eliminated by respiratory tract, urine/feces Indications After exposure to radioiodine in the air; highly recommended KI to administrate ASAP or within 6 hours Effectiveness within 15minutes: 90-95% within 6 hours: 50% after the first 12 hours: little
4 Guidance Doses for Population Protection Dosage and Administration KI 130mg immediately, then 130mg daily for 7-14days WHO Guidelines for Iodine Age Group Adults and Adolescents iodine (mg) KI (mg) KIO3 (mg) 100mg Children(3-12years) /2 Infants (1month to 3years) Neonates (birth to 1month) / /8 Sheltering in place Evacuation Iodine Prophylaxis Temporary Relocation Adverse Reactions Skin Rash 10 msv 50 msv 100 msv 30 msv/the first month, 10 msv/followed by the next month Swelling of the salivary glands Iodism: metallic taste, burning mouth & throat, sore teeth & gum bleeding, symptoms of a head cold, stomach upset & diarrhea Allergic reaction with serious symptoms: fever & joint pains, or swelling of parts of the face and body, at times severe SOB(shortness of breath) requiring immediate medical attention
5 Rare : overactivity of the thyroid gland, underactivity of the thyroid gland, or enlargement of the thyroid gland(goiter) Ca-DTPA(Trisodium calciumdiethylenetriaminepentaacetate) Ca-DTPA? Calcium salt of DTPA (1g/amp) The Powerful chelating agent as forms stable complexes with plutonium or transuranic metals Effectively exchanges calcium for another metal of greater binding power and carries it to the kidneys The plasma half life of DTPA is minutes; almost the entire administered dose is excreted in 12 hours. Ca-DTPA is rapidly distributed throughout the extracellular fluid space.
6 Ca-DTPA is approximately 10 times more effective than Zn-DTPA for initial chelation of transuranics; therefore, it should be used whenever larger body burdens of transuranics are involved. Ca-DTPA can deplete the body of Zn or Mn with repeating dosing. How DTPA Works Indications Treatment of internal contamination with transuranium ions(plutonium, americium, curium, berkelium, californium) Effectiveness Good for internal contamination with the soluble plutonium salts; however, chelation is highly dependent not only on the actual metal, but also on the chemical & physical characteristics of the compound. As the efficiency of chelation decreases with time, DTPA should be given within 6 hours of exposure, if possible. DTPA treatment of 239 Pu Intake Retention (% of Uptake) Control DTPA Treated Liver Skeleton
7 Dosage & Administration IV Injection of DTPA 1g/4ml with 6ml saline over 5-10 minutes IV infusion of DTPA 1g with 100~250ml D5W/NS/Ringers Lactate over 1hour, but less than 2 hours Aerosol: 1:1 dilution with water or saline via hand-held nebulizer; inhalation takes minutes Contraindications Minors(under 18YOA), pregnant women, patients with nephrotic syndrome, in patients with bone marrow depression; use zinc-dtpa Precautions pre-existing serious kidney disease or depressed myelopoietic function (e.g., leukopenia or thrombocytopenia) Kidney function should be normal; Urinalysis should be normal prior to each use. If proteinuria, hematuria or casts developed during use; discontinue drug administrations. Fractionation of the recommend 1g dose is not recommended. Blood pressure should be monitored closely during drug infusion. Discontinue the drug if diarrhea occurs. Adverse Reactions No serious toxicity in human has been reported in recommended doses. When given repeatedly, with short intervals for recovery, Ca-DTPA treatment may cause nausea/vomiting/diarrhea/chills/fever/pruritus/ muscle cramps in the first 24 hours.
8 Insoluble Prussian Blue(ferric hexacyanlferrate, Fe₄[Fe(CN)₆]₃) Prussian Blue? Fe₄[Fe(CN)₆]₃ Radiogardase R -Cs enhances excretion of isotopes of cesium and thallium from the body by means of ion exchange(0.5g/cap) Cs-137 and Thallium-232 are the most important isotopes. Ions of cesium and thallium are ordinarily excreted into the intestine and reabsorbed from the gut into the bile, and then excreted again into the gastrointestinal(gi) tract. Orally administrated PB traps thallium and Cs in the gut, interrupts its re-absorption from the GI tract and thereby increases fecal excretion. The mechanism of Cs and thallium absorption by hexacyanoferrates is not yet known in full details. Dosage and Administration Depending on the severity of thallium and Cs incorporation, PB may given from 3g to 20g, daily in three divided doses.
9 For internally deposited radiocesium, the initial dosage is 1g three times daily and the dosage may be increased for more severe cases. > 10g daily is preferred in acute poisoning with thallium, and thallium still present in the stomach or the upper part of the small intestine, an initial dose of at least 3g should be given at once. Administrated orally with some liquid or dispersed in warm water and drunk as a solution the solution may also be administrated via stomach tube following gastric lavage If oral ingestion is impossible, PB administration is recommended via a duodenal tube. In cases involving decorporation therapy, daily whole body counting and collection of 24-hour urine and fecal bioassay samples will be performed. This will allow proper evaluation of Cs-137 elimination curve. The patient should be warned that he/she may experience blue colored stool. Precautions PB is effective only if gastrointestinal motility is intact. No known absolute contraindication of the usage of PB when medically indicated; however, given with tetracycline, it may retard the absorption of the tetracycline. Blood samples should be collected every 12 hours for the first 96 hours post-accident for serum chemistry, esp., iron, ferritin, and serum electrolytes). CBC should be collected initially and as indicated clinically thereafter.
10 In severe cases of internal contamination, hemodialysis and other extracorporeal clearance technique have been recently been proposed to aid elimination of radiocesium. Therapy for thallium poisoning is similar to that for cesium, except that an initial loading dose of 3g orally should be used. In cases of severe thallium intoxication, additional types of elimination treatment may be necessary. Induced emesis, followed by gastric intubation and lavage Forced diuresis until urinary thallium excretion is less than 1mg/24h charcoal hemoperfusion hemodialysis Radionuclide Iodine Rare earths Plutonium Transplutonics Yttrium Cesium Rubidium Thallium Uranium Tritium (H-3) medication Administration Principle KI Ca-DTPA Zn-DTPA Prussian blue Bicarbonate water 130mg(tabl) stat, followed by 130mg QD 7 if indicated blocks thyroid deposition 1g DTPA in 150~250 ml chelation D5W (N/S) IV over 60 minutes 1g with 100~200mL water p.o. TID for several days 2 ampls sodium bicarbonate(44.3 meq each; 7.5 alternately, oral administration of 2 bicarbonate tabls every 4 hours until the urine reaches a ph of 8~9 force fluids blocks absorption from GI & prevention recycling alkalization of urine; reduces chance of acute tubular necrosis isotopic dilution
11 REFERENCES 1. KIRAMS NREMC; Hands-on Manual of Radiation Emergency Medical Response(2005) 2. IAEA; Generic procedures for medical response during a nuclear or radiological emergency(epr Medical 2005) 3. KIRAMS NREMC; REAC/TS Lecture Schedule for U.S./Korean Joint Agreement on Nuclear Emergency Training Seoul, Korea(2006) 4. MOST(Ministry of Science & Technology); Standard Guidance in Radiation Terror Attacks 5. NCRP REPORT No. 65; Management of Persons Accidently Contaminated with Radionuclides 6. REAC/TS Guidance for Radiation Accident Management 7. ARP, NSA; Medical Management of Individuals Involved in Radiation Accidents Published by Korea Institute of Radiological & Medical Sciences, National Radiation Emergency Medical Center Address: #75, Nowongil, Nowon-Gu, Seoul, Korea. zip TEL: FAX: URL: Issued December 5, 2008
Radiological Preparedness & Emergency Response. Session V. Objectives. Clinical Evaluation and Management of Internal Contamination
 Radiological Preparedness & Emergency Response Session V Clinical Evaluation and Management of Internal Contamination Objectives Discuss the diagnosis of internal contamination. Describe the health effects
Radiological Preparedness & Emergency Response Session V Clinical Evaluation and Management of Internal Contamination Objectives Discuss the diagnosis of internal contamination. Describe the health effects
Topic: What Emergency Management Professionals Should Know about Antidotes for Radiological Agents
 Presenter: Tareg Bey, MD, FACEP, ABMT, DEAA Professor of Emergency Medicine Director, International Emergency Medicine Department of Emergency Medicine UCI Medical Center 101 The City Drive, Rte. 128 Orange,
Presenter: Tareg Bey, MD, FACEP, ABMT, DEAA Professor of Emergency Medicine Director, International Emergency Medicine Department of Emergency Medicine UCI Medical Center 101 The City Drive, Rte. 128 Orange,
Nuclear Plant Emergency Response
 Nuclear Plant Emergency Response Acute and Chronic Clinical Health Effects after a NPP Accident Module 5 At the end of this presentation you will be able to: Discuss the initial medical evaluation of a
Nuclear Plant Emergency Response Acute and Chronic Clinical Health Effects after a NPP Accident Module 5 At the end of this presentation you will be able to: Discuss the initial medical evaluation of a
Nuclear Plant Emergency Response
 Nuclear Plant Emergency Response Acute and Chronic Clinical Health Effects after a NPP Accident Module 5 At the end of this presentation you will be able to: Discuss the initial medical evaluation of a
Nuclear Plant Emergency Response Acute and Chronic Clinical Health Effects after a NPP Accident Module 5 At the end of this presentation you will be able to: Discuss the initial medical evaluation of a
Ditripentat-Heyl (DTPA)
 SUMMARY OF PRODUCT CHARACTERISTICS Ditripentat-Heyl (DTPA) 1. NAME OF THE MEDICINAL PRODUCT Ditripentat-Heyl (DTPA) 1 000 mg solution for injection Active pharmaceutical ingredient: Calcium trisodium pentetate
SUMMARY OF PRODUCT CHARACTERISTICS Ditripentat-Heyl (DTPA) 1. NAME OF THE MEDICINAL PRODUCT Ditripentat-Heyl (DTPA) 1 000 mg solution for injection Active pharmaceutical ingredient: Calcium trisodium pentetate
Special Topic: Radiological Dispersal Device or Dirty Bomb EXPLOSION AND BLAST INJURIES
 Special Topic: Radiological Dispersal Device or Dirty Bomb EXPLOSION AND BLAST INJURIES Scenario Presentation Possible Scenarios Simple radiological device Improvised nuclear device (IND) Nuclear weapon
Special Topic: Radiological Dispersal Device or Dirty Bomb EXPLOSION AND BLAST INJURIES Scenario Presentation Possible Scenarios Simple radiological device Improvised nuclear device (IND) Nuclear weapon
Ditripentat-Heyl (DTPA)
 Package leaflet: Information for the patient Ditripentat-Heyl (DTPA) 1,000 mg solution for injection Active substance: Calcium trisodium pentetate (Ca-DTPA) Read all of this leaflet carefully before this
Package leaflet: Information for the patient Ditripentat-Heyl (DTPA) 1,000 mg solution for injection Active substance: Calcium trisodium pentetate (Ca-DTPA) Read all of this leaflet carefully before this
Prophylactic Use of Potassium Iodide (KI) in Radiological Emergencies*: Information for Physicians
 Prophylactic Use of Potassium Iodide (KI) in Radiological Emergencies*: Information for Physicians * Based on FDA's guidance document on use of KI as a thyroid blocking agent in radiation emergencies 1.
Prophylactic Use of Potassium Iodide (KI) in Radiological Emergencies*: Information for Physicians * Based on FDA's guidance document on use of KI as a thyroid blocking agent in radiation emergencies 1.
Chatsworth High School Medical Careers Academy. By the Waters of Babylon Highlighting Assignment
 Assignment: 1. Read the following medical article about radiation sickness. 2. Highlight the points of interest: statements that could be investigated further; significance of each section. 3. Make notes
Assignment: 1. Read the following medical article about radiation sickness. 2. Highlight the points of interest: statements that could be investigated further; significance of each section. 3. Make notes
For more information about Thomson Reuters Micromedex, visit Information valid as of March 17, 2011.
 pentetate calcium trisodium Micromedex drugpoints Summary DrugPoints Summaries provide information on dosage, pharmacokinetics, cautions, interactions, clinical applications, adverse effects, comparative
pentetate calcium trisodium Micromedex drugpoints Summary DrugPoints Summaries provide information on dosage, pharmacokinetics, cautions, interactions, clinical applications, adverse effects, comparative
Sodium Iodide I 131 Solution. Click Here to Continue. Click Here to Return to Table of Contents
 Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Sodium Iodide I 131 Solution Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions Click Here to Continue Click Here to Return to
Radiobiology Hall 14: Radiologic Terrorism (Completed)
 Radiobiology Hall 14: Radiologic Terrorism (Completed) What are a few of the possible scenarios of radiologic terrorism? 1. Detonation of a nuclear major city 2. An attack on a nuclear power station 3.
Radiobiology Hall 14: Radiologic Terrorism (Completed) What are a few of the possible scenarios of radiologic terrorism? 1. Detonation of a nuclear major city 2. An attack on a nuclear power station 3.
GALLIUM CITRATE Ga 67 INJECTION
 511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
511945-0903 September 2003 USA Bristol-Myers Squibb Medical Imaging 331 Treble Cove Road N. Billerica, MA 01862 USA GALLIUM CITRATE Ga 67 INJECTION FOR DIAGNOSTIC USE DESCRIPTION: Gallium Citrate Ga 67
Compounding KI for Radiation Exposure
 PL Detail-Document #270323 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2011 Compounding KI for
PL Detail-Document #270323 This PL Detail-Document gives subscribers additional insight related to the Recommendations published in PHARMACIST S LETTER / PRESCRIBER S LETTER March 2011 Compounding KI for
Towards a model of DTPA decorporation therapy
 Towards a model of DTPA decorporation therapy B. Breustedt, KIT, Germany E. Blanchardon, IRSN, France P. Berard, CEA, France P. Fritsch, CEA, France O. Gremy, CEA, France A. Giussani, BfS, Germany M. Kastl,
Towards a model of DTPA decorporation therapy B. Breustedt, KIT, Germany E. Blanchardon, IRSN, France P. Berard, CEA, France P. Fritsch, CEA, France O. Gremy, CEA, France A. Giussani, BfS, Germany M. Kastl,
DOSAGE AND DISTRIBUTION OF POTASSIUM IODIDE TABLETS (A THYROID BLOCKING AGENT) IN RADIATION EMERGENCIES REGULATORY GUIDE
 PNRA-RG-914.01 (Rev. 0) September 2008 DOSAGE AND DISTRIBUTION OF POTASSIUM IODIDE TABLETS (A THYROID BLOCKING AGENT) IN RADIATION EMERGENCIES REGULATORY GUIDE PAKISTAN NUCLEAR REGULATORY AUTHORITY For
PNRA-RG-914.01 (Rev. 0) September 2008 DOSAGE AND DISTRIBUTION OF POTASSIUM IODIDE TABLETS (A THYROID BLOCKING AGENT) IN RADIATION EMERGENCIES REGULATORY GUIDE PAKISTAN NUCLEAR REGULATORY AUTHORITY For
NURSING PROCESS FOCUS: Patients Receiving Amphotericin B (Fungizone, Abelcet)
 NURSING PROCESS FOCUS: Patients Receiving Amphotericin B (Fungizone, Abelcet) ASSESSMENT Prior to administration: Obtain complete health history including allergies, drug history, and possible drug interactions.
NURSING PROCESS FOCUS: Patients Receiving Amphotericin B (Fungizone, Abelcet) ASSESSMENT Prior to administration: Obtain complete health history including allergies, drug history, and possible drug interactions.
8.05 days 138 days 7.60 days 0.22 mr/h at 1.0 meter per millicurie 124,068 curies/gram 2 mm = 0.20 cm = 0.08 in 165 cm = 65.0 in = 5.
 Iodine-131 Radiological Safety Guidance Physical Data GAMMA ENERGIES 364 kev (82% abundance) 637 kev (7% abundance) 284 kev (6% abundance) 80 kev (3% abundance) 723 kev (2% abundance) 29-34 kev (4.5%/x-rays)
Iodine-131 Radiological Safety Guidance Physical Data GAMMA ENERGIES 364 kev (82% abundance) 637 kev (7% abundance) 284 kev (6% abundance) 80 kev (3% abundance) 723 kev (2% abundance) 29-34 kev (4.5%/x-rays)
International Food Safety Authorities Network (INFOSAN) INFORMATION on Nuclear accidents and radioactive contamination of foods 30.
 Food and Agriculture Organization of the United Nations International Food Safety Authorities Network (INFOSAN) INFORMATION on Nuclear accidents and radioactive contamination of foods 30. March 2011 This
Food and Agriculture Organization of the United Nations International Food Safety Authorities Network (INFOSAN) INFORMATION on Nuclear accidents and radioactive contamination of foods 30. March 2011 This
ICRP Perspective on Internal Dosimetry OIR and Radiopharmaceuticals
 ICRP Perspective on Internal Dosimetry OIR and Radiopharmaceuticals Dietmar Noßke dnosske@web.de 1 Disclaimer The information and views set out in this presentation are those of the author and do not necessarily
ICRP Perspective on Internal Dosimetry OIR and Radiopharmaceuticals Dietmar Noßke dnosske@web.de 1 Disclaimer The information and views set out in this presentation are those of the author and do not necessarily
General Nuclear Medicine
 General Nuclear Medicine What is General Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
General Nuclear Medicine What is General Nuclear Medicine? What are some common uses of the procedure? How should I prepare? What does the equipment look like? How does the procedure work? How is the procedure
KELFER Deferiprone. COMPOSITION KELFER-250 Capsules Each capsule contains Deferiprone 250 mg
 KELFER Deferiprone COMPOSITION KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg DOSAGE FORM Capsules PHARMACOLOGY Pharmacodynamics
KELFER Deferiprone COMPOSITION KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg DOSAGE FORM Capsules PHARMACOLOGY Pharmacodynamics
MEDICAL MANAGEMENT FOR NUCLEAR/RADIOLOGIC EVENTS. Frank Guyette, MD, MPH Department of Emergency Medicine University of Pittsburgh
 MEDICAL MANAGEMENT FOR NUCLEAR/RADIOLOGIC EVENTS Frank Guyette, MD, MPH Department of Emergency Medicine University of Pittsburgh Overview Nuclear Scenarios Detonation Issues Reactor Issues Radiation Injury
MEDICAL MANAGEMENT FOR NUCLEAR/RADIOLOGIC EVENTS Frank Guyette, MD, MPH Department of Emergency Medicine University of Pittsburgh Overview Nuclear Scenarios Detonation Issues Reactor Issues Radiation Injury
Quick Reference Information Recommendations of the National Council on Radiation Protection and Measurements
 Quick Reference Information Recommendations of the National Council on Radiation Protection and Measurements The information in this document is adapted, with the permission of the National Council on
Quick Reference Information Recommendations of the National Council on Radiation Protection and Measurements The information in this document is adapted, with the permission of the National Council on
IONIZING RADIATION, HEALTH EFFECTS AND PROTECTIVE MEASURES
 May 2011 IONIZING RADIATION, HEALTH EFFECTS AND PROTECTIVE MEASURES KEY FACTS Ionizing radiation is a type of energy released by atoms in the form of electromagnetic waves or particles. People are exposed
May 2011 IONIZING RADIATION, HEALTH EFFECTS AND PROTECTIVE MEASURES KEY FACTS Ionizing radiation is a type of energy released by atoms in the form of electromagnetic waves or particles. People are exposed
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
Contrast Materials Patient Safety: What are contrast materials and how do they work?
 Contrast Materials Patient Safety: What are contrast materials and how do they work? Which imaging exams use contrast materials? How safe are contrast materials? How should I prepare for my imaging procedure
Contrast Materials Patient Safety: What are contrast materials and how do they work? Which imaging exams use contrast materials? How safe are contrast materials? How should I prepare for my imaging procedure
KELFER Capsules (Deferiprone)
 Published on: 22 Sep 2014 KELFER Capsules (Deferiprone) Composition KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg Dosage Form
Published on: 22 Sep 2014 KELFER Capsules (Deferiprone) Composition KELFER-250 Capsules Each capsule contains Deferiprone 250 mg KELFER-500 Capsules Each capsule contains Deferiprone 500 mg Dosage Form
MATERIAL SAFETY DATA SHEET
 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Cephalexin for Oral Suspension USP 125 mg/5 ml and 250 mg/5 ml Lupin Limited Mandideep 462
MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE AND THE COMPANY Material Manufacturer Distributor Cephalexin for Oral Suspension USP 125 mg/5 ml and 250 mg/5 ml Lupin Limited Mandideep 462
Salicylate (Aspirin) Ingestion California Poison Control Background 1. The prevalence of aspirin-containing analgesic products makes
 Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
Salicylate (Aspirin) Ingestion California Poison Control 1-800-876-4766 Background 1. The prevalence of aspirin-containing analgesic products makes these agents, found in virtually every household, common
P A T I E N T H A N D B O O K
 PATIENT HANDBOOK Heal Your Gut, Heal Your Body The gastrointestinal (GI) tract is one of the most sophisticated systems of the human body. We often think of the GI tract for its primary role in digesting
PATIENT HANDBOOK Heal Your Gut, Heal Your Body The gastrointestinal (GI) tract is one of the most sophisticated systems of the human body. We often think of the GI tract for its primary role in digesting
PARACOD Tablets (Paracetamol + Codeine phosphate)
 Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
Published on: 22 Sep 2014 PARACOD Tablets (Paracetamol + Codeine phosphate) Composition PARACOD Tablets Each effervescent tablet contains: Paracetamol IP...650 mg Codeine Phosphate IP... 30 mg Dosage Form/s
Metabolism Paracetamol is metabolised in the liver and excreted in the urine mainly as glucuronide and sulphate conjugates.
 FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
FEBRAMOL Composition Febramol 150 Suppositories Each suppository contains Paracetamol 150 mg. Suppositories, Tablets & Syrup Febramol 300 Suppositories Each suppository contains Paracetamol 300 mg. Each
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
DRAXIMAGE SODIUM IODIDE I 131 CAPSULES, USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Capsules, USP are color-coded capsules containing sodium iodide I 131 for diagnostic use by oral administration.
INCOMING! Is your ED Ready for a Nuclear Bomb?
 INCOMING! Is your ED Ready for a Nuclear Bomb? Katie Tataris MD, MPH Assistant Professor of Medicine, Section of Emergency Medicine EMS Fellowship Director University of Chicago Medical Center EMS Medical
INCOMING! Is your ED Ready for a Nuclear Bomb? Katie Tataris MD, MPH Assistant Professor of Medicine, Section of Emergency Medicine EMS Fellowship Director University of Chicago Medical Center EMS Medical
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcium Sandoz 500 mg, effervescent tablets Calcium Sandoz 1000 mg, effervescent tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Calcium Sandoz 500 mg, effervescent tablets Calcium Sandoz 1000 mg, effervescent tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Hematopoiesis, The hematopoietic machinery requires a constant supply iron, vitamin B 12, and folic acid.
 Hematopoiesis, 200 billion new blood cells per day The hematopoietic machinery requires a constant supply iron, vitamin B 12, and folic acid. hematopoietic growth factors, proteins that regulate the proliferation
Hematopoiesis, 200 billion new blood cells per day The hematopoietic machinery requires a constant supply iron, vitamin B 12, and folic acid. hematopoietic growth factors, proteins that regulate the proliferation
FUNDAMENTAL SAFETY OVERVIEW VOLUME 2: DESIGN AND SAFETY CHAPTER S: RISK REDUCTION CATEGORIES 3. RADIOLOGICAL CONSEQUENCES OF SEVERE ACCIDENTS
 PAGE : 1 / 10 3. RADIOLOGICAL CONSEQUENCES OF SEVERE ACCIDENTS 3.1. SAFETY REQUIREMENTS 3.1.1. Safety objectives The safety approach for EPR reactors is deterministic, complemented by probabilistic analyses,
PAGE : 1 / 10 3. RADIOLOGICAL CONSEQUENCES OF SEVERE ACCIDENTS 3.1. SAFETY REQUIREMENTS 3.1.1. Safety objectives The safety approach for EPR reactors is deterministic, complemented by probabilistic analyses,
Protecting Yourself in a Nuclear Emergency with Potassium Iodide
 Protecting Yourself in a Nuclear Emergency with Potassium Iodide By Mark A. Mitchell, MD The possibility of radioactive contamination from a device detonated by terrorists within the country's borders
Protecting Yourself in a Nuclear Emergency with Potassium Iodide By Mark A. Mitchell, MD The possibility of radioactive contamination from a device detonated by terrorists within the country's borders
1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small
 Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Lecture-5 1. Gastric Emptying Time Anatomically, a swallowed drug rapidly reaches the stomach. Eventually, the stomach empties its content in the small intestine. Because the duodenum has the greatest
Have a healthy discussion. Use this guide to start a. conversation. with your. healthcare provider
 Have a healthy discussion Use this guide to start a conversation with your healthcare provider MAKE THE CONVERSATION COUNT Here are some things you may want to reflect on and discuss with your healthcare
Have a healthy discussion Use this guide to start a conversation with your healthcare provider MAKE THE CONVERSATION COUNT Here are some things you may want to reflect on and discuss with your healthcare
Nuclear Plant Emergency Response. NPP Function and Malfunction: Historical Overview. Why is this training program important to you?
 Nuclear Plant Emergency Response NPP Function and Malfunction: Historical Overview Module 1 Why is this training program important to you? The Fukushima Daiichi nuclear power plant (NPP) crisis impacted
Nuclear Plant Emergency Response NPP Function and Malfunction: Historical Overview Module 1 Why is this training program important to you? The Fukushima Daiichi nuclear power plant (NPP) crisis impacted
Multiphasic Blood Analysis
 Understanding Your Multiphasic Blood Analysis Test Results Mon General thanks you for participating in the multiphasic blood analysis. This test can be an early warning of health problems, including coronary
Understanding Your Multiphasic Blood Analysis Test Results Mon General thanks you for participating in the multiphasic blood analysis. This test can be an early warning of health problems, including coronary
LUPIN LIMITED SAFETY DATA SHEET. Section 1: Identification. Mandideep India
 LUPIN LIMITED SAFETY DATA SHEET Section 1: Identification Section 1, Identification Material Manufacturer Distributor Cephalexin Capsules USP 250 mg and 500 mg Lupin Limited Mandideep 462 046 India Lupin
LUPIN LIMITED SAFETY DATA SHEET Section 1: Identification Section 1, Identification Material Manufacturer Distributor Cephalexin Capsules USP 250 mg and 500 mg Lupin Limited Mandideep 462 046 India Lupin
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]](/thumbs/81/83994292.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
F. Paquet, M.R. Bailey, R.W. Leggett, T. Fell, T. Smith, V. Berkovski and J.D. Harrison ICRP C2
 F. Paquet, M.R. Bailey, R.W. Leggett, T. Fell, T. Smith, V. Berkovski and J.D. Harrison ICRP C2 What are «Internal Dose Coefficients»? 2 What are «Internal Dose Coefficients»? A Coefficient is a value
F. Paquet, M.R. Bailey, R.W. Leggett, T. Fell, T. Smith, V. Berkovski and J.D. Harrison ICRP C2 What are «Internal Dose Coefficients»? 2 What are «Internal Dose Coefficients»? A Coefficient is a value
Long-term Excretion of Thorium with Faeces and Urine from TIG Welders after Continuos Occupational Handling of Thoriated Electrodes
 Long-term Excretion of Thorium with Faeces and Urine from TIG Welders after Continuos Occupational Handling of Thoriated Electrodes P. Ostapczuk 1, R. Hille 1, A Titze 2, N. Witkowski 1 1 Division of Safety
Long-term Excretion of Thorium with Faeces and Urine from TIG Welders after Continuos Occupational Handling of Thoriated Electrodes P. Ostapczuk 1, R. Hille 1, A Titze 2, N. Witkowski 1 1 Division of Safety
Goiter. This reference summary explains goiters. It covers symptoms and causes of the condition, as well as treatment options.
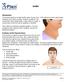 Goiter Introduction The thyroid gland is located at the base of your neck. If the gland becomes abnormally enlarged, it is called a goiter. Goiters usually do not cause pain. But a large goiter could cause
Goiter Introduction The thyroid gland is located at the base of your neck. If the gland becomes abnormally enlarged, it is called a goiter. Goiters usually do not cause pain. But a large goiter could cause
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Treatment of Graves Disease by the Atomic Cocktail by Malcolm R. Powell, M.D., F.A.C.P, F.A.C.N.P
 GRAVES DISEASE FOUNDATION Educate * Encourage * Empower 400 International Drive Williamsville, NY 14221 (877) 643-3123 Treatment of Graves Disease by the Atomic Cocktail by Malcolm R. Powell, M.D., F.A.C.P,
GRAVES DISEASE FOUNDATION Educate * Encourage * Empower 400 International Drive Williamsville, NY 14221 (877) 643-3123 Treatment of Graves Disease by the Atomic Cocktail by Malcolm R. Powell, M.D., F.A.C.P,
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT "Carbellon" Tablets. 2. Qualitative and Quantitative Composition Active Constituents: Activated Charcoal Ph.Eur. Magnesium Hydroxide Ph.Eur.
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT "Carbellon" Tablets. 2. Qualitative and Quantitative Composition Active Constituents: Activated Charcoal Ph.Eur. Magnesium Hydroxide Ph.Eur.
Drugs Used in Anemia
 Drugs Used in Anemia Drugs of Anemia Anemia is defined as a below-normal plasma hemoglobin concentration resulting from: a decreased number of circulating red blood cells or an abnormally low total hemoglobin
Drugs Used in Anemia Drugs of Anemia Anemia is defined as a below-normal plasma hemoglobin concentration resulting from: a decreased number of circulating red blood cells or an abnormally low total hemoglobin
PDP 406 CLINICAL TOXICOLOGY
 PDP 406 CLINICAL TOXICOLOGY Pharm.D Fourth Year Mr.D.Raju.M.Pharm., Lecturer INTRODUCTION It is the process of freeing of a person or object of some contaminating substance from intestine which further
PDP 406 CLINICAL TOXICOLOGY Pharm.D Fourth Year Mr.D.Raju.M.Pharm., Lecturer INTRODUCTION It is the process of freeing of a person or object of some contaminating substance from intestine which further
Radiological Fact Sheet: Controlling contamination
 Radiological Fact Sheet: Controlling contamination Risks Contamination (measured in counts per minute) measures number of bits of radiation that come from a given area, and each of these bits (counts)
Radiological Fact Sheet: Controlling contamination Risks Contamination (measured in counts per minute) measures number of bits of radiation that come from a given area, and each of these bits (counts)
Medical Considerations in a Nuclear Attack. Matt Mihelic, M.D. University of Tennessee Graduate School of Medicine July 13, 2008
 Medical Considerations in a Nuclear Attack Matt Mihelic, M.D. University of Tennessee Graduate School of Medicine July 13, 2008 National Planning Scenarios Scenario 1: Nuclear Detonation 10-Kiloton Improvised
Medical Considerations in a Nuclear Attack Matt Mihelic, M.D. University of Tennessee Graduate School of Medicine July 13, 2008 National Planning Scenarios Scenario 1: Nuclear Detonation 10-Kiloton Improvised
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection](/thumbs/93/111890101.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] Mepolizumab for Injection Read this carefully before you start taking NUCALA and each time you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] Mepolizumab for Injection Read this carefully before you start taking NUCALA and each time you
SODIUM POLYSTYRENE SULFONATE Suspension, USP
 SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance
 Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
Chapter 26 Fluid, Electrolyte, and Acid- Base Balance 1 Body Water Content Infants: 73% or more water (low body fat, low bone mass) Adult males: ~60% water Adult females: ~50% water (higher fat content,
POVIDONE-IODINE BETADINE
 POVIDONE-IODINE BETADINE NAME OF PRODUCT BETADINE 10% OINTMENT Read this leaflet carefully before you start using BETADINE ointment. Keep this leaflet. You may need to read it again. If you have any further
POVIDONE-IODINE BETADINE NAME OF PRODUCT BETADINE 10% OINTMENT Read this leaflet carefully before you start using BETADINE ointment. Keep this leaflet. You may need to read it again. If you have any further
Dimaval (DMPS) 100 mg Hartkapseln
 SUMMARY OF PRODUCT CHARACTERISTICS Dimaval (DMPS) 100 mg Hartkapseln 1. NAME OF THE MEDICINAL PRODUCT Dimaval (DMPS) 100 mg Hartkapseln 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 hard capsule contains
SUMMARY OF PRODUCT CHARACTERISTICS Dimaval (DMPS) 100 mg Hartkapseln 1. NAME OF THE MEDICINAL PRODUCT Dimaval (DMPS) 100 mg Hartkapseln 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 hard capsule contains
HEALTH AND SAFETY DATA SHEET
 HEALTH AND SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND COMPANY/UNDERTAKING PRODUCT NAME: BIOLOGICAL LAUNDRY DETERGENT PART NO: 0980 SUPPLIER: Envomatic Foxholes Road Leicester
HEALTH AND SAFETY DATA SHEET 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND COMPANY/UNDERTAKING PRODUCT NAME: BIOLOGICAL LAUNDRY DETERGENT PART NO: 0980 SUPPLIER: Envomatic Foxholes Road Leicester
PRESCRIBING INFORMATION mg primaquine phosphate equivalent to 15 mg primaquine base. Antimalarial
 PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
PRESCRIBING INFORMATION Pr PRIMAQUINE primaquine phosphate tablets USP 26.3 mg primaquine phosphate equivalent to 15 mg primaquine base Antimalarial sanofi-aventis Canada Inc. 2150 St. Elzear Blvd. West
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION
 Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
Panadeine Tablet, Caplets and Rapid Soluble tablets PRODUCT INFORMATION DESCRIPTION Active Ingredients Paracetamol 500 mg Codeine Phosphate 8 mg Excipients Tablets - Starch - maize, talc - purified, stearic
What are some things I need to know or do while I take
 PATIENT & CAREGIVER EDUCATION Cyclophosphamide Brand Names: Canada Procytox What is this drug used for? It is used to treat cancer. It is used to treat nephrotic syndrome. It may be g iven to you for other
PATIENT & CAREGIVER EDUCATION Cyclophosphamide Brand Names: Canada Procytox What is this drug used for? It is used to treat cancer. It is used to treat nephrotic syndrome. It may be g iven to you for other
FAVISTAN 20 mg, tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER FAVISTAN 20 mg, tablets TIAMAZOL This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
PACKAGE LEAFLET: INFORMATION FOR THE USER FAVISTAN 20 mg, tablets TIAMAZOL This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for a medicine, which outlines
Click Here to Continue. Click Here to Return to Table of Contents
 TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
TechneScan Gluceptate Package inserts are current as of January, 1997. Contact Professional Services, 1-888-744-1414, regarding possible revisions. Click Here to Continue Click Here to Return to Table
What is the current risk of radiation-related health problems in Japan to those near the reactor at the time, and those in other parts of Japan?
 What is the current risk of radiation-related health problems in Japan to those near the reactor at the time, and those in other parts of Japan? The actions proposed by the Government of Japan are in line
What is the current risk of radiation-related health problems in Japan to those near the reactor at the time, and those in other parts of Japan? The actions proposed by the Government of Japan are in line
Potassium Iodide (KI) Questions & Answers for Schools (Revised January 2007)
 General Information on KI Potassium Iodide (KI) Questions & Answers for Schools (Revised ) 1) What is potassium iodide (KI)? Potassium iodide is a U.S. Food and Drug Administration (FDA) approved over-thecounter
General Information on KI Potassium Iodide (KI) Questions & Answers for Schools (Revised ) 1) What is potassium iodide (KI)? Potassium iodide is a U.S. Food and Drug Administration (FDA) approved over-thecounter
STABLE IODINE DISTRIBUTION BRIEFING SESSION
 Prepared by the Medical Services Division, Department of Public Health and Welfare, Ibaraki Prefectural Government (FY2017) STABLE IODINE DISTRIBUTION BRIEFING SESSION Nuclear Disaster Countermeasures
Prepared by the Medical Services Division, Department of Public Health and Welfare, Ibaraki Prefectural Government (FY2017) STABLE IODINE DISTRIBUTION BRIEFING SESSION Nuclear Disaster Countermeasures
Training Course on Medical Preparedness and Response for a Nuclear or Radiological Emergency Pre- Test - BASIC
 Training Course on Medical Preparedness and Response for a Nuclear or Radiological Emergency Pre- Test - BASIC Name Date. (dd/mm/yyyy) Circle the correct answer(s). 1. Delayed effects of radiation exposure
Training Course on Medical Preparedness and Response for a Nuclear or Radiological Emergency Pre- Test - BASIC Name Date. (dd/mm/yyyy) Circle the correct answer(s). 1. Delayed effects of radiation exposure
IAEA Safety Standards for Emergency Preparedness and Response: Focus on criteria for radionuclides in food, milk and drinking water
 TM on the Harmonization Levels for Foodstuff and Drinking Water Contaminated Following a Nuclear Accident 8-12 September 2014, Vienna IAEA Safety Standards for Emergency Preparedness and Response: Focus
TM on the Harmonization Levels for Foodstuff and Drinking Water Contaminated Following a Nuclear Accident 8-12 September 2014, Vienna IAEA Safety Standards for Emergency Preparedness and Response: Focus
Westco Chemicals, Inc Saticoy Street South - North Hollywood, CA TEL: FAX:
 Westco Chemicals, Inc. 12551-61 Saticoy Street South - North Hollywood, CA 91605 TEL:323-877-0077 818-255-3655 FAX: 818-255-3650 Data Sheet MATERIAL SAFETY DATA SHEET SECTION 1: CHEMICAL PRODUCTS & COMPANY
Westco Chemicals, Inc. 12551-61 Saticoy Street South - North Hollywood, CA 91605 TEL:323-877-0077 818-255-3655 FAX: 818-255-3650 Data Sheet MATERIAL SAFETY DATA SHEET SECTION 1: CHEMICAL PRODUCTS & COMPANY
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
III. TOXICOKINETICS. Studies relevant to the toxicokinetics of inorganic chloramines are severely
 III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
III. TOXICOKINETICS Introduction Studies relevant to the toxicokinetics of inorganic chloramines are severely limited. However, studies done with various chlorinated amino compounds (including organic
Modeling of Internal Dose from Insoluble Cesium
 Modeling of Internal Dose from Insoluble Cesium Kentaro Manabe 1 and Masaki Matsumoto 2 1. Japan Atomic Energy Agency 2. National Institutes for Quantum and Radiological Science and Technology ICRP-RERF-JHPS
Modeling of Internal Dose from Insoluble Cesium Kentaro Manabe 1 and Masaki Matsumoto 2 1. Japan Atomic Energy Agency 2. National Institutes for Quantum and Radiological Science and Technology ICRP-RERF-JHPS
Takako Tominaga, M.D.
 ANSN WS 2013 2013/10/03 Takako Tominaga, M.D. Radiation Emergency Medical Assistance Team (REMAT) NIRS About Polonium-210 (1) Almost pure alpha particle emitter A radioactive element that occurs naturally
ANSN WS 2013 2013/10/03 Takako Tominaga, M.D. Radiation Emergency Medical Assistance Team (REMAT) NIRS About Polonium-210 (1) Almost pure alpha particle emitter A radioactive element that occurs naturally
PRODUCT INFORMATION. DESCRIPTION Addaven is a concentrated trace element solution for infusion which is clear and colourless to slightly yellow.
 PRODUCT INFORMATION Addaven NAME OF MEDICINE Addaven is a concentrated trace element solution containing the following simple salts: chromic chloride hexahydrate (CAS No.:10060-12-5), cupric chloride dihydrate
PRODUCT INFORMATION Addaven NAME OF MEDICINE Addaven is a concentrated trace element solution containing the following simple salts: chromic chloride hexahydrate (CAS No.:10060-12-5), cupric chloride dihydrate
Future perspectives in radiation protection
 Future perspectives in radiation protection Sophie Léonard Health & Environment Health Protection 14/10/2016 1 Program of the session 14:00 14:15: 14:15 14:45: Extension of the zones of pre-distribution
Future perspectives in radiation protection Sophie Léonard Health & Environment Health Protection 14/10/2016 1 Program of the session 14:00 14:15: 14:15 14:45: Extension of the zones of pre-distribution
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less. For pack sizes of more than 24 tablets
 SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
SCHEDULING STATUS: S0 For pack sizes of 24 tablets or less S1 For pack sizes of more than 24 tablets PROPRIETARY NAME: AND DOSAGE FORM PANADO MELTABS (Tablets) COMPOSITION: Each tablet contains 500 mg
Radiological Injuries
 Radiological Injuries Chapter 30 Radiological Injuries The reader is strongly advised to supplement material in this chapter with the following two references: 1. Medical Management of Radiological Casualties
Radiological Injuries Chapter 30 Radiological Injuries The reader is strongly advised to supplement material in this chapter with the following two references: 1. Medical Management of Radiological Casualties
Summary of Product Characteristics
 Summary of Product Characteristics Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 1. NAME OF THE MEDICINAL PRODUCT Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 2. QUALITATIVE AND QUANTITATIVE
Summary of Product Characteristics Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 1. NAME OF THE MEDICINAL PRODUCT Sodium Phosphate ( 32 P) 18.5-185 MBq/ml Injection 2. QUALITATIVE AND QUANTITATIVE
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC. For Oral Use DESCRIPTION
 DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
DRAXIMAGE SODIUM IODIDE I 131 SOLUTION USP DIAGNOSTIC For Oral Use DESCRIPTION Sodium Iodide I 131 Solution is an aqueous solution of sodium iodide I-131 for diagnostic use by oral administration. The
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
1. PRODUCT NAME ADDAVEN (infusion, solution concentrate) NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each 10 ml ampoule of Addaven contains: Chromic chloride hexahydrate 53.33 µg
Removal of Strontium by the Chelating Agent Acethylamino Prophylidence Diphosponic Acid in Rats
 Removal of Strontium by the Chelating Agent Acethylamino Prophylidence Diphosponic Acid in Rats S. Fukuda 1, H. Iida 1, Yueming Yan 2, Y. Xie 2 and W. Chen 2. 1 National Institute of Radiological Sciences,
Removal of Strontium by the Chelating Agent Acethylamino Prophylidence Diphosponic Acid in Rats S. Fukuda 1, H. Iida 1, Yueming Yan 2, Y. Xie 2 and W. Chen 2. 1 National Institute of Radiological Sciences,
Frequently Asked Questions & Suggested Answers - Radiation Emergencies
 Frequently Asked Questions & Suggested Answers - Radiation Emergencies CAUTION The answers to some of the questions below are general in nature and may need to be revised based on the details of the actual
Frequently Asked Questions & Suggested Answers - Radiation Emergencies CAUTION The answers to some of the questions below are general in nature and may need to be revised based on the details of the actual
Internal Dosimetry from Radionuclides Intakes
 Internal Dosimetry from Radionuclides Intakes Christian Hurtgen 6 June 2005 Content I Dose (some definitions) Biokinetics (Compartment model) HRTM HAT Wound model Monitoring Internal contamination From
Internal Dosimetry from Radionuclides Intakes Christian Hurtgen 6 June 2005 Content I Dose (some definitions) Biokinetics (Compartment model) HRTM HAT Wound model Monitoring Internal contamination From
SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Charcodote 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Activated charcoal 200mg/ml 3. PHARMACEUTICAL FORM Oral suspension 4. CLINICAL
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Charcodote 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Activated charcoal 200mg/ml 3. PHARMACEUTICAL FORM Oral suspension 4. CLINICAL
Package leaflet: Information for the user. Optiray 320 mg I/ml, solution for injection or infusion. Active substance: Ioversol
 Package leaflet: Information for the user Optiray 320 mg I/ml, solution for injection or infusion Active substance: Ioversol Read all of this leaflet carefully before you start using this medicine because
Package leaflet: Information for the user Optiray 320 mg I/ml, solution for injection or infusion Active substance: Ioversol Read all of this leaflet carefully before you start using this medicine because
Methotrexate. About This Drug. Possible Side Effects. Warnings and Precautions
 Methotrexate About This Drug Methotrexate is used to treat cancer. This drug is given in the vein (IV). Possible Side Effects Soreness of the mouth and throat. You may have red areas, white patches, or
Methotrexate About This Drug Methotrexate is used to treat cancer. This drug is given in the vein (IV). Possible Side Effects Soreness of the mouth and throat. You may have red areas, white patches, or
HEALTH INFORMATION FORM
 St. Michael Albertville STUDENT INFORMATION Name: School: HEALTH INFORMATION FORM Grade: DOB: HEALTH INFORMATION Does your child have any health problems (i.e. Asthma, Diabetes, ADHD, Heart Condition,
St. Michael Albertville STUDENT INFORMATION Name: School: HEALTH INFORMATION FORM Grade: DOB: HEALTH INFORMATION Does your child have any health problems (i.e. Asthma, Diabetes, ADHD, Heart Condition,
1. NAME OF THE MEDICINAL PRODUCT. Irenat Drops 300 mg sodium perchlorate, oral drops Sodium perchlorate monohydrate
 1. NAME OF THE MEDICINAL PRODUCT Irenat Drops 300 mg sodium perchlorate, oral drops Sodium perchlorate monohydrate 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution (approximately 15 drops) contains
1. NAME OF THE MEDICINAL PRODUCT Irenat Drops 300 mg sodium perchlorate, oral drops Sodium perchlorate monohydrate 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution (approximately 15 drops) contains
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES
 PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
PACKAGE INSERT TEMPLATE FOR PARACETAMOL SUPPOSITORIES Brand or Product Name [Product name] Suppositories mg Name and Strength of Active Substance(s) Eg Paracetamol 500mg Paracetamol 250mg Paracetamol 125mg
GLEEVEC PATIENT RESOURCES
 GLEEVEC TALKING WITH YOUR DOCTOR ABOUT KIT+ GIST AND ITS TREATMENT When you have KIT-positive gastrointestinal stromal tumor (KIT+ GIST), you will likely have many questions and may, at times, feel overwhelmed
GLEEVEC TALKING WITH YOUR DOCTOR ABOUT KIT+ GIST AND ITS TREATMENT When you have KIT-positive gastrointestinal stromal tumor (KIT+ GIST), you will likely have many questions and may, at times, feel overwhelmed
Elements for a public summary
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Deferasirox is a medicine used to treat chronic iron overload (an excess of iron in the body). Chronic iron overload is a condition
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Deferasirox is a medicine used to treat chronic iron overload (an excess of iron in the body). Chronic iron overload is a condition
Ca-C 1000 Calvive 1000 mg mg mg effervescent tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER Ca-C 1000 Calvive 1000 mg + 327 mg + 1000 mg effervescent tablets CALCIUM LACTATE GLUCONATE, CALCIUM CARBONATE, ASCORBIC ACID (VITAMIN C) This leaflet is a copy
PACKAGE LEAFLET: INFORMATION FOR THE USER Ca-C 1000 Calvive 1000 mg + 327 mg + 1000 mg effervescent tablets CALCIUM LACTATE GLUCONATE, CALCIUM CARBONATE, ASCORBIC ACID (VITAMIN C) This leaflet is a copy
Major intra and extracellular ions Lec: 1
 Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
Major intra and extracellular ions Lec: 1 The body fluids are solutions of inorganic and organic solutes. The concentration balance of the various components is maintained in order for the cell and tissue
