ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 02/15/2010
|
|
|
- Bertha Hawkins
- 5 years ago
- Views:
Transcription
1 ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 02/15/2010 Grantor: CDER IND/IDE Number: 49,484 Serial Number: 0278 Efficacy & Safety of Dronedarone Versus Amiodarone for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation (DIONYSOS) This study has been completed. Sponsor: Collaborators: Information provided by: ClinicalTrials.gov Identifier: Sanofi Sanofi NCT Purpose The objective of this study is to compare the efficacy and safety of dronedarone to that of amiodarone for the treatment of patients with atrial fibrillation. Condition Intervention Phase Atrial Fibrillation Drug: dronedarone (SR33589) Drug: amiodarone Phase 3 Study Type: Interventional Study Design: Treatment, Parallel Assignment, Double Blind (Subject, Caregiver, Investigator), Randomized, Efficacy Study Official Title: Randomized Double Blind Trial to Evaluate the Efficacy and Safety of Dronedarone (400mg BID) Versus Amiodarone (600mg Daily for 28 Days, Then 200mg Daily Thereafter) for at Least 6 Months for the Maintenance of Sinus Rhythm in Patients With Atrial Fibrillation (AF) Further study details as provided by Sanofi: Primary Outcome Measure: Treatment Failure [Time Frame: minimum study duration is 6 months (+10 days); maximum is 15 months] [Designated as safety issue: No] The primary event is the treatment failure defined as the first recurrence of atrial fibrillation or premature study drug discontinuation for intolerance or lack of efficacy according to the investigator judgement. The primary efficacy analysis is performed on the time from first study drug intake to this primary event. The "Measured Values" table below presents the numbers of patients with the event at the end of the study period. - Page 1 of 18 -
2 Secondary Outcome Measures: Occurrence of the Main Safety Endpoint (MSE) Defined as Thyroid, Hepatic, Pulmonary, Neurological, Skin, Eye, or Gastrointestinal Specific Treatment Emergent Events or Premature Study Drug Discontinuation Following Any Adverse Event [Time Frame: minimum study duration is 6 months (+10 days); maximum is 15 months] [Designated as safety issue: Yes] The considered event is the occurrence of the MSE defined as thyroid, hepatic, pulmonary, neurological, skin, eye, or gastrointestinal specific treatment emergent events or premature study drug discontinuation following any adverse event (AE), whichever comes first. The analysis is performed on the time from first study drug intake to this event. The "Measured Values" table below presents the numbers of patients with the event at the end of the study period. Other Pre-specified Outcome Measures: Occurrence of the MSE Excluding Gastrointestinal Specific Treatment Emergent Events Defined as Diarrhoea, Nausea, Vomiting [Time Frame: minimum study duration is 6 months (+10 days); maximum is 15 months] [Designated as safety issue: Yes] The considered event is the occurrence of the MSE excluding gastrointestinal specific treatment emergent events defined as diarrhoea, nausea, vomiting. The analysis is performed on the time from first study drug intake to this event. The "Measured Values" table below presents the numbers of patients with the event at the end of the study period. Enrollment: 504 Study Start Date: June 2007 Primary Completion Date: October 2008 Study Completion Date: October 2008 Arms Experimental: Dronedarone 400mg bid dronedarone 400mg tablets administered twice a day (bid) and matching overencapsulated tablets of placebo of amiodarone 200mg Active Comparator: Amiodarone 600mg/200mg od over-encapsulated tablets of amiodarone 200mg (600mg daily for 28 days then 200mg daily) administered once daily (od) and matching placebo of dronedarone 400mg tablets Assigned Interventions Drug: dronedarone (SR33589) oral administration Other Names: Multaq Drug: amiodarone oral administration Eligibility Ages Eligible for Study: 21 Years and older Genders Eligible for Study: Both Accepts Healthy Volunteers: No Criteria Inclusion Criteria: - Page 2 of 18 -
3 Patients with documented atrial fibrillation for more than 72 hours for whom cardioversion and antiarrhythmic treatment is indicated in the opinion of the investigator and under oral anticoagulation Exclusion Criteria: Contraindication to oral anticoagulation Patient having received amiodarone in the past whatever the date (more than a total of twenty 200 mg tablets or more than 5 days intravenous) Patients known to have chronic AF, patients with atrial flutter or paroxysmal atrial fibrillation Severe congestive heart failure with New-York Heart Association (NYHA) class III or IV, severe bradycardia, high degree atrio-ventricular block, ongoing potentially dangerous symptoms when in AF such as angina pectoris, transient ischemic attacks, stroke, syncope, as judged by the investigator, first degree family history of sudden cardiac death below age 50 years in the absence of coronary heart disease, significant sinus node disease without a permanent pacemaker implanted History of torsades de pointes or long QT syndrome or QT- or QTc-interval 500 msecs before randomization Treatment with other class I or III antiarrhythmic drugs which cannot be discontinued Dysthyroidism or other contraindication to amiodarone The above information are not intended to contain all the considerations relevant to a patient's potential participation in a clinical trial. Contacts and Locations Locations United States, New Jersey Bridgewater, New Jersey, United States, Argentina Buenos Aires, Argentina Australia Cove, Australia Austria Vienna, Austria Belgium Diegem, Belgium Canada Laval, Canada Chile Santiago, Chile China Shangaï, China - Page 3 of 18 -
4 Czech Republic Praha, Czech Republic Estonia Tallinn, Estonia Finland Helsinki, Finland France Paris, France Germany Berlin, Germany Italy Milan, Italy Korea, Republic of Seoul, Korea, Republic of Mexico Mexico, Mexico Morocco Casablanca, Morocco Netherlands Gouda, Netherlands Poland Warszawa, Poland Russian Federation Moscow, Russian Federation Sweden Bromma, Sweden Tunisia Megrine, Tunisia Turkey Istanbul, Turkey - Page 4 of 18 -
5 Investigators Study Director: International Clinical Development sanofi-aventis More Information Responsible Party: sanofi-aventis (International Clinical Development, Clinical Study Director) Study ID Numbers: EFC4968 Health Authority: United States: Food and Drug Administration Canada: Health Canada Netherlands: Medicines Evaluation Board (MEB) Participant Flow Study Results Recruitment Details Pre-Assignment Details Reporting Groups Enrollment of patients started on June 12, 2007 and was completed on October 3, The study was conducted in 112 centers in 23 countries. Minimum duration of treatment was 6 months. Minimum duration of observation was last patient's randomization plus 190 days. Planned sample size was 472. Six hundred and eighteen patients (618) were screened of which 113 did not verify one or more selection criteria. One eligible patient received a placebo capsule (morning intake in the amiodarone group) but was not randomized in the trial. This patient did not report any adverse event and was excluded from all analyses. dronedarone tablets 400mg twice daily (bid) Description amiodarone 600mg once daily (od) for 28 days, then amiodarone 200mg once daily (od) Overall Study Started 249 [1] 255 [1] Completed 153 [2] 186 [2] Not Completed Lack of Efficacy Page 5 of 18 -
6 Adverse Event Poor compliance 6 2 Not coded/ Not pre-specified 5 8 [1] Randomized and treated patients [2] completed study drug period Baseline Characteristics Reporting Groups dronedarone tablets 400mg twice daily (bid) Description amiodarone 600mg once daily (od) for 28 days, then amiodarone 200mg once daily (od) Baseline Measures Total Number of Participants Age, Customized [units: participants] 18 to < 65 years to < 75 years >= 75 years Age, Continuous [units: years] Mean (Standard Deviation) Gender, Male/Female [units: participants] 64.4 (10.8) 63.7 (10.6) 64.0 (10.7) Female Male Page 6 of 18 -
7 Outcome Measures 1. Primary Outcome Measure: Measure Title Measure Description Time Frame Safety Issue? Treatment Failure The primary event is the treatment failure defined as the first recurrence of atrial fibrillation or premature study drug discontinuation for intolerance or lack of efficacy according to the investigator judgement. The primary efficacy analysis is performed on the time from first study drug intake to this primary event. The "Measured Values" table below presents the numbers of patients with the event at the end of the study period. minimum study duration is 6 months (+10 days); maximum is 15 months No Analysis Population Description All randomized and treated patients (receiving at least one dose of study drug) were included in the efficacy analysis according to the treatment received. Reporting Groups dronedarone tablets 400mg twice daily (bid) Description amiodarone 600mg once daily (od) for 28 days, then amiodarone 200mg once daily (od) Measured Values Number of Participants Analyzed Treatment Failure [units: participants] Statistical Analysis 1 for Treatment Failure Statistical Analysis Overview Comparison Groups Non-Inferiority or Equivalence Analysis?, No Statistical Test of Hypothesis P-Value < Page 7 of 18 -
8 Method Cumulative incidence functions in each treatment group were calculated using time-toevent non-parametric Kaplan-Meier estimate. The primary comparison was performed at the 5% level using a 2-sided Log rank test. Log Rank Method of Estimation Estimation Parameter Estimated Value 1.59 Hazard Ratio (HR) Confidence Interval (2-Sided) 95% 1.28 to 1.98 Estimation The hazard ratio was estimated by a Cox's proportional hazard model with treatment arm factor. It provides the relative hazard of treatment failure for the dronedarone group compared with the amiodarone group. 2. Secondary Outcome Measure: Measure Title Occurrence of the Main Safety Endpoint (MSE) Defined as Thyroid, Hepatic, Pulmonary, Neurological, Skin, Eye, or Gastrointestinal Specific Treatment Emergent Events or Premature Study Drug Discontinuation Following Any Adverse Event Measure Description Time Frame Safety Issue? The considered event is the occurrence of the MSE defined as thyroid, hepatic, pulmonary, neurological, skin, eye, or gastrointestinal specific treatment emergent events or premature study drug discontinuation following any adverse event (AE), whichever comes first. The analysis is performed on the time from first study drug intake to this event. The "Measured Values" table below presents the numbers of patients with the event at the end of the study period. minimum study duration is 6 months (+10 days); maximum is 15 months Yes Analysis Population Description All randomized and treated patients (receiving at least one dose of study drug) were included in the safety analysis according to the treatment received. Reporting Groups Description dronedarone tablets 400mg twice daily (bid) amiodarone 600mg once daily (od) for 28 days, then amiodarone 200mg once daily (od) Measured Values Number of Participants Analyzed Page 8 of 18 -
9 Occurrence of the Main Safety Endpoint (MSE) Defined as Thyroid, Hepatic, Pulmonary, Neurological, Skin, Eye, or Gastrointestinal Specific Treatment Emergent Events or Premature Study Drug Discontinuation Following Any Adverse Event [units: participants] Statistical Analysis 1 for Occurrence of the Main Safety Endpoint (MSE) Defined as Thyroid, Hepatic, Pulmonary, Neurological, Skin, Eye, or Gastrointestinal Specific Treatment Emergent Events or Premature Study Drug Discontinuation Following Any Adverse Event Statistical Analysis Overview Comparison Groups Non-Inferiority or Equivalence Analysis?, No Statistical Test of Hypothesis Method of Estimation P-Value 0.13 Method Estimation Parameter Estimated Value 0.80 Cumulative incidence functions in each treatment group were calculated using time-toevent non-parametric Kaplan-Meier estimate. The comparison was performed at the 5% level using a 2-sided Log rank test. Log Rank Hazard Ratio (HR) Confidence Interval (2-Sided) 95% 0.60 to 1.07 Estimation The hazard ratio was estimated by a Cox's proportional hazard model with treatment arm factor. It provides the relative hazard of main safety event occurrence for the dronedarone group compared with the amiodarone group. 3. Other Pre-specified Outcome Measure: Measure Title Occurrence of the MSE Excluding Gastrointestinal Specific Treatment Emergent Events Defined as Diarrhoea, Nausea, Vomiting - Page 9 of 18 -
10 Measure Description Time Frame Safety Issue? The considered event is the occurrence of the MSE excluding gastrointestinal specific treatment emergent events defined as diarrhoea, nausea, vomiting. The analysis is performed on the time from first study drug intake to this event. The "Measured Values" table below presents the numbers of patients with the event at the end of the study period. minimum study duration is 6 months (+10 days); maximum is 15 months Yes Analysis Population Description All randomized and treated patients (receiving at least one dose of study drug) were included in the safety analysis according to the treatment received. Reporting Groups Description dronedarone tablets 400mg twice daily (bid) amiodarone 600mg once daily (od) for 28 days, then amiodarone 200mg once daily (od) Measured Values Number of Participants Analyzed Occurrence of the MSE Excluding Gastrointestinal Specific Treatment Emergent Events Defined as Diarrhoea, Nausea, Vomiting [units: participants] Statistical Analysis 1 for Occurrence of the MSE Excluding Gastrointestinal Specific Treatment Emergent Events Defined as Diarrhoea, Nausea, Vomiting Statistical Analysis Overview Comparison Groups Non-Inferiority or Equivalence Analysis?, No Statistical Test of Hypothesis P-Value Cumulative incidence functions in each treatment group were calculated using time-toevent non-parametric Kaplan-Meier estimate. The comparison was performed at the 5% level using a 2-sided Log rank test. Method Log Rank - Page 10 of 18 -
11 Method of Estimation Estimation Parameter Estimated Value 0.61 Hazard Ratio (HR) Confidence Interval (2-Sided) 95% 0.44 to 0.84 Estimation The hazard ratio was estimated by a Cox's proportional hazard model with treatment arm factor. It provides the relative hazard of MSE occurrence excluding gastrointestinal events, for the dronedarone group compared with the amiodarone group. Reported Adverse Events Time Frame Additional Description Reporting Groups From first to last study drug intake +10 days i.e. end of the study. The safety population was the "All randomized and treated patients" population. Description dronedarone tablets 400mg twice daily (bid) amiodarone 600mg once daily (od) for 28 days, then amiodarone 200mg once daily (od) Serious Adverse Events Affected/At Risk (%) Affected/At Risk (%) Total 34/249 (13.65%) 37/255 (14.51%) Blood and lymphatic system disorders Anaemia A * 1/249 (0.4%) 0/255 (0%) Hypoprothrombinaemia A * 0/249 (0%) 1/255 (0.39%) Cardiac disorders Acute coronary syndrome A * 0/249 (0%) 1/255 (0.39%) Acute myocardial infarction A * 0/249 (0%) 1/255 (0.39%) - Page 11 of 18 -
12 Affected/At Risk (%) Affected/At Risk (%) Angina pectoris A * 1/249 (0.4%) 1/255 (0.39%) Arteriosclerosis coronary artery A * 1/249 (0.4%) 0/255 (0%) Atrioventricular block second degree A * 0/249 (0%) 1/255 (0.39%) Bradycardia A * 1/249 (0.4%) 2/255 (0.78%) Cardiac failure A * 6/249 (2.41%) 1/255 (0.39%) Cardiac failure congestive A * 1/249 (0.4%) 6/255 (2.35%) Coronary artery stenosis A * 0/249 (0%) 1/255 (0.39%) Diastolic dysfunction A * 1/249 (0.4%) 0/255 (0%) Left ventricular dysfunction A * 1/249 (0.4%) 0/255 (0%) Pericarditis A * 1/249 (0.4%) 0/255 (0%) Sick sinus syndrome A * 0/249 (0%) 1/255 (0.39%) Sinus bradycardia A * 0/249 (0%) 1/255 (0.39%) Gastrointestinal disorders Duodenal ulcer A * 1/249 (0.4%) 0/255 (0%) Gastric haemorrhage A * 1/249 (0.4%) 0/255 (0%) Gastrointestinal haemorrhage A * 0/249 (0%) 1/255 (0.39%) Gastrointestinal necrosis A * 0/249 (0%) 1/255 (0.39%) Inguinal hernia A * 1/249 (0.4%) 0/255 (0%) General disorders Chest pain A * 0/249 (0%) 1/255 (0.39%) Death A * 1/249 (0.4%) 0/255 (0%) Oedema peripheral A * 1/249 (0.4%) 0/255 (0%) - Page 12 of 18 -
13 Affected/At Risk (%) Affected/At Risk (%) Sudden death A * 0/249 (0%) 1/255 (0.39%) Hepatobiliary disorders Cholangitis A * 0/249 (0%) 1/255 (0.39%) Cholecystitis acute A * 0/249 (0%) 1/255 (0.39%) Hepatocellular injury A * 1/249 (0.4%) 0/255 (0%) Mixed liver injury A * 1/249 (0.4%) 0/255 (0%) Infections and infestations Bronchitis A * 0/249 (0%) 2/255 (0.78%) Cellulitis A * 0/249 (0%) 1/255 (0.39%) Diverticulitis A * 1/249 (0.4%) 1/255 (0.39%) Ear infection A * 0/249 (0%) 1/255 (0.39%) Escherichia sepsis A * 0/249 (0%) 1/255 (0.39%) Gastroenteritis A * 0/249 (0%) 1/255 (0.39%) Oesophageal candidiasis A * 0/249 (0%) 1/255 (0.39%) Pneumonia A * 2/249 (0.8%) 0/255 (0%) Septic shock A * 0/249 (0%) 1/255 (0.39%) Injury, poisoning and procedural complications Accidental overdose A * 0/249 (0%) 2/255 (0.78%) Post procedural haemorrhage A * 1/249 (0.4%) 0/255 (0%) Therapeutic agent toxicity A * 1/249 (0.4%) 3/255 (1.18%) Thoracic vertebral fracture A * 0/249 (0%) 1/255 (0.39%) Investigations Blood creatinine increased A * 1/249 (0.4%) 1/255 (0.39%) - Page 13 of 18 -
14 Affected/At Risk (%) Affected/At Risk (%) Electrocardiogram PR prolongation A * 1/249 (0.4%) 0/255 (0%) International normalised ratio increased A * 0/249 (0%) 2/255 (0.78%) Metabolism and nutrition disorders Diabetic foot A * 1/249 (0.4%) 0/255 (0%) Hyperkalaemia A * 0/249 (0%) 1/255 (0.39%) Hypoglycaemia A * 1/249 (0.4%) 0/255 (0%) Hyponatraemia A * 0/249 (0%) 1/255 (0.39%) Musculoskeletal and connective tissue disorders Muscle haemorrhage A * 0/249 (0%) 1/255 (0.39%) Symphysiolysis A * 1/249 (0.4%) 0/255 (0%) Neoplasms benign, malignant and unspecified (incl cysts and polyps) Adrenal adenoma A * 0/249 (0%) 1/255 (0.39%) Anaplastic thyroid cancer A * 1/249 (0.4%) 0/255 (0%) Bladder cancer A * 1/249 (0.4%) 0/255 (0%) Carcinoma in situ of bladder A * 1/249 (0.4%) 0/255 (0%) Gastrointestinal stromal tumour A * 0/249 (0%) 1/255 (0.39%) Lung cancer metastatic A * 0/249 (0%) 1/255 (0.39%) Lung squamous cell carcinoma stage 1/249 (0.4%) 1/255 (0.39%) unspecified A * Small cell lung cancer stage unspecified A * 0/249 (0%) 1/255 (0.39%) Nervous system disorders Cerebral ischaemia A * 1/249 (0.4%) 0/255 (0%) Cerebrovascular accident A * 1/249 (0.4%) 0/255 (0%) - Page 14 of 18 -
15 Renal and urinary disorders Affected/At Risk (%) Affected/At Risk (%) Haemorrhage intracranial A * 0/249 (0%) 1/255 (0.39%) Respiratory, thoracic and mediastinal disorders Ischaemic stroke A * 1/249 (0.4%) 0/255 (0%) Syncope A * 0/249 (0%) 2/255 (0.78%) Haematuria A * 1/249 (0.4%) 0/255 (0%) Urinary retention A * 0/249 (0%) 1/255 (0.39%) Acute pulmonary oedema A * 1/249 (0.4%) 0/255 (0%) Skin and subcutaneous tissue disorders Vascular disorders Bronchitis chronic A * 1/249 (0.4%) 0/255 (0%) Dyspnoea A * 1/249 (0.4%) 0/255 (0%) Hypoxia A * 0/249 (0%) 1/255 (0.39%) Pneumonitis A * 1/249 (0.4%) 0/255 (0%) Pulmonary embolism A * 1/249 (0.4%) 0/255 (0%) Dermatitis allergic A * 0/249 (0%) 1/255 (0.39%) Pelvic venous thrombosis A * 0/249 (0%) 1/255 (0.39%) * Indicates events were collected by non-systematic methods. A Term from vocabulary, MedDRA 11.0 Other Adverse Events Frequency Threshold Above Which Other Adverse Events are Reported: 5% Affected/At Risk (%) Affected/At Risk (%) Total 146/249 (58.63%) 165/255 (64.71%) - Page 15 of 18 -
16 Affected/At Risk (%) Affected/At Risk (%) Cardiac disorders Any Cardiac disorders A * 21/249 (8.43%) 36/255 (14.12%) Bradycardia A * 4/249 (1.61%) 14/255 (5.49%) Endocrine disorders Any Endocrine disorders A * 3/249 (1.2%) 14/255 (5.49%) Eye disorders Any Eye disorders A * 6/249 (2.41%) 13/255 (5.1%) Gastrointestinal disorders Any Gastrointestinal disorders A * 54/249 (21.69%) 44/255 (17.25%) Diarrhoea A * 23/249 (9.24%) 8/255 (3.14%) Nausea A * 13/249 (5.22%) 9/255 (3.53%) General disorders Any General disorders and administration 14/249 (5.62%) 26/255 (10.2%) site conditions A * Oedema peripheral A * 6/249 (2.41%) 13/255 (5.1%) Infections and infestations Any Infections and infestations A * 35/249 (14.06%) 35/255 (13.73%) Injury, poisoning and procedural complications Any Injury, poisoning and procedural 8/249 (3.21%) 17/255 (6.67%) complications A * Investigations Any Investigations A * 33/249 (13.25%) 35/255 (13.73%) Metabolism and nutrition disorders Any Metabolism and nutrition disorders A * 8/249 (3.21%) 15/255 (5.88%) - Page 16 of 18 -
17 Affected/At Risk (%) Affected/At Risk (%) Musculoskeletal and connective tissue disorders Any Musculoskeletal and connective tissue disorders A * 21/249 (8.43%) 22/255 (8.63%) Nervous system disorders Any Nervous system disorders A * 17/249 (6.83%) 39/255 (15.29%) Dizziness A * 8/249 (3.21%) 16/255 (6.27%) Psychiatric disorders Any Psychiatric disorders A * 6/249 (2.41%) 23/255 (9.02%) Sleep disorder A * 3/249 (1.2%) 19/255 (7.45%) Respiratory, thoracic and mediastinal disorders Any Respiratory, thoracic and mediastinal disorders A * 21/249 (8.43%) 21/255 (8.24%) Skin and subcutaneous tissue disorders Any Skin and subcutaneous tissue disorders 18/249 (7.23%) 23/255 (9.02%) A * Vascular disorders Any Vascular disorders A * 9/249 (3.61%) 23/255 (9.02%) Hypertension A * 7/249 (2.81%) 16/255 (6.27%) * Indicates events were collected by non-systematic methods. A Term from vocabulary, MedDRA 11.0 Limitations and Caveats - Page 17 of 18 -
18 More Information Certain Agreements: Principal Investigators are NOT employed by the organization sponsoring the study. There IS an agreement between the Principal Investigator and the Sponsor (or its agents) that restricts the PI's rights to discuss or publish trial results after the trial is completed. If no publication has occurred within 12 months of the completion of the study, the Investigator shall have the right to publish/present independently the results of the study. The Investigator shall provide the Sponsor with a copy of any such publication for comment at least 45 days before any submission for publication. If requested by the Sponsor, any submission shall be delayed up to 90 days, to allow the Sponsor to preserve its proprietary rights. Results Point of Contact: Name/Official Title: International Clinical Development, Clinical Study Director Organization: sanofi-aventis Phone: GV-Contact-us@sanofi-aventis.com U.S. National Library of Medicine U.S. National Institutes of Health U.S. Department of Health & Human Services - Page 18 of 18 -
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 10/11/2013. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 10/11/2013 ClinicalTrials.gov ID: NCT00168454 Study Identification Unique Protocol ID: 191622-077 Brief Title: A
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 10/11/2013 ClinicalTrials.gov ID: NCT00168454 Study Identification Unique Protocol ID: 191622-077 Brief Title: A
Efficacy Study of Zoledronic Acid and Teriparatide Combination Therapy in Women With Osteoporosis
 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: CZOL446H2409 Previous Study Return to List Next Study Efficacy Study of Zoledronic Acid and Combination Therapy in Women With
A service of the U.S. National Institutes of Health Trial record 1 of 1 for: CZOL446H2409 Previous Study Return to List Next Study Efficacy Study of Zoledronic Acid and Combination Therapy in Women With
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 01/19/2016. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 01/19/2016 ClinicalTrials.gov ID: NCT00595413 Study Identification Unique Protocol ID: 27905 Brief Title: Atacicept
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: 01/19/2016 ClinicalTrials.gov ID: NCT00595413 Study Identification Unique Protocol ID: 27905 Brief Title: Atacicept
Search for studies: ClinicalTrials.gov Identifier: NCT
 ClinicalTrials.gov A service of the U.S. National Institutes of Health Search for studies: Example. "Heart attack" AND "Los Angeles" Advanced Search Help Studies by Topic Glossary Find Studies About Clinical
ClinicalTrials.gov A service of the U.S. National Institutes of Health Search for studies: Example. "Heart attack" AND "Los Angeles" Advanced Search Help Studies by Topic Glossary Find Studies About Clinical
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Full Novartis CTRD Results Template
 Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23138E1 Title A
Dronedarone for the treatment of non-permanent atrial fibrillation
 Dronedarone for the treatment of non-permanent atrial Issued: August 2010 last modified: December 2012 guidance.nice.org.uk/ta197 NICE has accredited the process used by the Centre for Health Technology
Dronedarone for the treatment of non-permanent atrial Issued: August 2010 last modified: December 2012 guidance.nice.org.uk/ta197 NICE has accredited the process used by the Centre for Health Technology
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Synopsis Style Clinical Study Report SR EFC10139 Version number: 1 (electronic 2.0)
 SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, multicenter, multinational study to assess the long-term effect, over 1 year, of rimonabant 10 mg in comparison with rimonabant
SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, multicenter, multinational study to assess the long-term effect, over 1 year, of rimonabant 10 mg in comparison with rimonabant
Full Novartis CTRD Results Template
 Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23137E1 Title A
Full Novartis CTRD Results Template Sponsor Novartis Generic Drug Name vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Protocol Number CLAF237A23137E1 Title A
Study No.: LOV Title: Rationale: Phase: IIB Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary-
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Inspra / Eplerenone
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Inspra / Eplerenone
ClinialTrials.gov Identifier: sanofi-aventis. Sponsor/company: 07/November/2008
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: sanofi-aventis ClinialTrials.gov
dronedarone, 400mg, film-coated tablets (Multaq ) SMC No. (636/10) Sanofi-aventis Ltd
 dronedarone, 400mg, film-coated tablets (Multaq ) SMC No. (636/10) Sanofi-aventis Ltd 6 August 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
dronedarone, 400mg, film-coated tablets (Multaq ) SMC No. (636/10) Sanofi-aventis Ltd 6 August 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT)
 ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
ClinicalTrials.gov "Basic Results" Data Element Definitions (DRAFT) January 9, 2009 * Required by ClinicalTrials.gov [*] Conditionally required by ClinicalTrials.gov (FDAAA) May be required to comply with
National Horizon Scanning Centre. Dronedarone (Multaq) for atrial fibrillation and atrial flutter. December 2007
 Dronedarone (Multaq) for atrial fibrillation and atrial flutter December 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not
Dronedarone (Multaq) for atrial fibrillation and atrial flutter December 2007 This technology summary is based on information available at the time of research and a limited literature search. It is not
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
Sponsor. Novartis. Generic Drug Name. Vildagliptin. Therapeutic Area of Trial. Type 2 diabetes. Approved Indication. Type 2 diabetes.
 Sponsor Page 1 Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLAF237A2308 Title A multicenter, randomized, double-blind,
Sponsor Page 1 Novartis Generic Drug Name Vildagliptin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLAF237A2308 Title A multicenter, randomized, double-blind,
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable: Secondary Outcome/Efficacy Variable(s): Statistical Methods:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Clinical Trial Results Summary Study EN3409-BUP-305
 Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Title of Study: A 52-Week, Open-Label, Long-Term Treatment Evaluation of the Safety and Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Pain Coordinating Investigator: Martin
Previous Study Return to List Next Study
 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: 42603ATT3013 Previous Study Return to List Next Study A Study to Evaluate Effectiveness and Safety of Prolonged Release OROS
A service of the U.S. National Institutes of Health Trial record 1 of 1 for: 42603ATT3013 Previous Study Return to List Next Study A Study to Evaluate Effectiveness and Safety of Prolonged Release OROS
» A new drug s trial
 » A new drug s trial A placebo-controlled, double-blind, parallel arm Trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular Hospitalization or death from any cause
» A new drug s trial A placebo-controlled, double-blind, parallel arm Trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular Hospitalization or death from any cause
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: June 25, ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: June 25, 2014 ClinicalTrials.gov ID: NCT00372385 Study Identification Unique Protocol ID: VX05-950-104EU Brief Title:
ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Release Date: June 25, 2014 ClinicalTrials.gov ID: NCT00372385 Study Identification Unique Protocol ID: VX05-950-104EU Brief Title:
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
A service of the U.S. National Institutes of Health Now Available: Final Rule for FDAAA 801 and NIH Policy on Clinical Trial Reporting
 A service of the U.S. National Institutes of Health w Available: Final Rule for FDAAA 81 and NIH Policy on Clinical Trial Reporting Trial record 1 of 1 for: FENHYDPAI414 Previous Study Return to List Next
A service of the U.S. National Institutes of Health w Available: Final Rule for FDAAA 81 and NIH Policy on Clinical Trial Reporting Trial record 1 of 1 for: FENHYDPAI414 Previous Study Return to List Next
Sponsor Novartis. Generic Drug Name Vildagliptin/Metformin. Therapeutic Area of Trial Type 2 diabetes. Approved Indication Type 2 diabetes
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
EFFECTIVE SHARED CARE AGREEMENT. Dronedarone - For the treatment and management of atrial fibrillation
 EFFECTIVE SHARED CARE AGREEMENT Dronedarone - For the treatment and management of atrial fibrillation Specialist details Patient identifier Name INTRODUCTION This shared care agreement outlines how the
EFFECTIVE SHARED CARE AGREEMENT Dronedarone - For the treatment and management of atrial fibrillation Specialist details Patient identifier Name INTRODUCTION This shared care agreement outlines how the
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Therapeutic Area of Trial L-dopa induced dyskinesias in Parkinson s disease (PD-LID) Approved Indication Not approved yet for any
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Therapeutic Area of Trial L-dopa induced dyskinesias in Parkinson s disease (PD-LID) Approved Indication Not approved yet for any
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Comorbidity or medical history Existing diagnoses between 1 January 2007 and 31 December 2011 AF management care AF symptoms Tachycardia
 Supplementary Table S1 International Classification of Disease 10 (ICD-10) codes Comorbidity or medical history Existing diagnoses between 1 January 2007 and 31 December 2011 AF management care I48 AF
Supplementary Table S1 International Classification of Disease 10 (ICD-10) codes Comorbidity or medical history Existing diagnoses between 1 January 2007 and 31 December 2011 AF management care I48 AF
SYNOPSIS. Clinical Study Report IM Double-blind Period
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Abatacept () Name of Active Ingredient: Abatacept () Individual Study Table Referring to the Dossier SYNOPSIS (For National Authority
Treatment of Atrial Fibrillation in Heart Failure
 Stockholm, September 1st 2010 Treatment of Atrial Fibrillation in Heart Failure Rhythm control: Which drugs? Stefan H. Hohnloser J.W. Goethe University Frankfurt, Germany Presenter disclosure information:
Stockholm, September 1st 2010 Treatment of Atrial Fibrillation in Heart Failure Rhythm control: Which drugs? Stefan H. Hohnloser J.W. Goethe University Frankfurt, Germany Presenter disclosure information:
A Study for Patients With Head and Neck Cancer
 Page 1 of 52 A service of the U.S. National Institutes of Health Now Available: Final Rule for FDAAA 801 and NIH Policy on Clinical Trial Reporting A Study for Patients With Head and Neck Cancer Trial
Page 1 of 52 A service of the U.S. National Institutes of Health Now Available: Final Rule for FDAAA 801 and NIH Policy on Clinical Trial Reporting A Study for Patients With Head and Neck Cancer Trial
Sponsor Novartis Pharmaceuticals
 Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol maleate-glycopyrronium bromide Trial Indication(s) Chronic obstructive pulmonary disease Protocol Number CQVA149A2339 Protocol Title A placebo
Sponsor Novartis Pharmaceuticals Generic Drug Name Indacaterol maleate-glycopyrronium bromide Trial Indication(s) Chronic obstructive pulmonary disease Protocol Number CQVA149A2339 Protocol Title A placebo
PankoMab-GEX Versus Placebo as Maintenance Therapy in Advanced Ovarian Cancer
 1 von 7 13.01.2014 12:26 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: GEXMab25201 Previous Study Return to List Next Study PankoMab-GEX Versus Placebo as Maintenance Therapy
1 von 7 13.01.2014 12:26 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: GEXMab25201 Previous Study Return to List Next Study PankoMab-GEX Versus Placebo as Maintenance Therapy
PRESCRIBING ALERT
 www.empr.com PRESCRIBING ALERT Dear Healthcare Professional, At MPR we strive to bring you important drug information in a concise and timely fashion. In keeping with this goal, we are pleased to bring
www.empr.com PRESCRIBING ALERT Dear Healthcare Professional, At MPR we strive to bring you important drug information in a concise and timely fashion. In keeping with this goal, we are pleased to bring
Supplementary Online Content
 Supplementary Online Content Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line Treatment of Paroxysmal Atrial Fibrillation (RAAFT-2): a randomzied clinical
Supplementary Online Content Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line Treatment of Paroxysmal Atrial Fibrillation (RAAFT-2): a randomzied clinical
Dronedarone: Need to Perform a CV Outcome Safety Study
 Dronedarone: Need to Perform a CV Outcome Safety Study Gerald V. Naccarelli M.D. Consultant: Glaxo-Smith-Kline, Pfizer, Sanofi, Boehringer-Ingelheim, Daiichi-Sankyo, Bristol Myers Squibb, Otsuka, Janssen
Dronedarone: Need to Perform a CV Outcome Safety Study Gerald V. Naccarelli M.D. Consultant: Glaxo-Smith-Kline, Pfizer, Sanofi, Boehringer-Ingelheim, Daiichi-Sankyo, Bristol Myers Squibb, Otsuka, Janssen
Sponsor / Company: Sanofi Drug substance(s): Insulin Glargine. According to template: QSD VERSION N 4.0 (07-JUN-2012) Page 1
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription. Sponsor / Company: Sanofi Drug substance(s):
Kenneth W. Mahaffey, MD and Keith AA Fox, MB ChB
 Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation Kenneth W. Mahaffey, MD and Keith AA Fox, MB ChB on behalf
Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation Kenneth W. Mahaffey, MD and Keith AA Fox, MB ChB on behalf
Polypharmacy - arrhythmic risks in patients with heart failure
 Influencing sudden cardiac death by pharmacotherapy Polypharmacy - arrhythmic risks in patients with heart failure Professor Dan Atar Head, Dept. of Cardiology Oslo University Hospital Ullevål Norway 27.8.2012
Influencing sudden cardiac death by pharmacotherapy Polypharmacy - arrhythmic risks in patients with heart failure Professor Dan Atar Head, Dept. of Cardiology Oslo University Hospital Ullevål Norway 27.8.2012
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objective:
 GSK Medicine: abacavir (ABC)/dolutegravir (DTG)/lamivudine (3TC) Study Number: 201147 Title: A IIIb, randomized, open-label study of the safety, efficacy, and tolerability of switching to a fixed-dose
GSK Medicine: abacavir (ABC)/dolutegravir (DTG)/lamivudine (3TC) Study Number: 201147 Title: A IIIb, randomized, open-label study of the safety, efficacy, and tolerability of switching to a fixed-dose
NHS Kent and Medway Medicines Management. Dronedarone (Multaq ) Shared Care Guideline For Prescribing
 NHS Kent and Medway Medicines Management Dronedarone (Multaq ) Shared Care Guideline For Prescribing Issue No: 2 Review Date (If Applicable): Accountable Officer: Heather Lucas Contact Details: 01233 618158
NHS Kent and Medway Medicines Management Dronedarone (Multaq ) Shared Care Guideline For Prescribing Issue No: 2 Review Date (If Applicable): Accountable Officer: Heather Lucas Contact Details: 01233 618158
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Supplementary Online Content
 Supplementary Online Content Chawla SP, Papai Z, Mukhametshina G, et al. First-line aldoxorubicin vs doxorubicin in metastatic or locally advanced unresectable soft-tissue sarcoma: a phase 2b randomized
Supplementary Online Content Chawla SP, Papai Z, Mukhametshina G, et al. First-line aldoxorubicin vs doxorubicin in metastatic or locally advanced unresectable soft-tissue sarcoma: a phase 2b randomized
Dronedarone For Atrial Fibrillation: Unbridled Enthusiasm Or Just Another Small Step Forward?
 Dronedarone For Atrial Fibrillation: Unbridled Enthusiasm Or Just Another Small Step Forward? James A. Reiffel, M.D. Introduction In July 2009, the federal Food and Drug Administration (FDA) approved the
Dronedarone For Atrial Fibrillation: Unbridled Enthusiasm Or Just Another Small Step Forward? James A. Reiffel, M.D. Introduction In July 2009, the federal Food and Drug Administration (FDA) approved the
The RealiseAF registry:
 The RealiseAF registry: An International, observational, cross-sectional survey evaluating atrial fibrillation management and the cardiovascular risk profile of AF patients initial results PG.Steg on behalf
The RealiseAF registry: An International, observational, cross-sectional survey evaluating atrial fibrillation management and the cardiovascular risk profile of AF patients initial results PG.Steg on behalf
Efficacy and Safety of Diclofenac Potassium 25 mg Tablet Taken Three Times Daily in Subjects With Acute Joint Pain
 Page 1 of 8 A service of the U.S. National Institutes of Health Try our beta test site Trial record 1 of 1 for: 853-P-401 Previous Study Return to List Next Study Efficacy and Safety of Taken Three Times
Page 1 of 8 A service of the U.S. National Institutes of Health Try our beta test site Trial record 1 of 1 for: 853-P-401 Previous Study Return to List Next Study Efficacy and Safety of Taken Three Times
Online Supplementary Data. Country Number of centers Number of patients randomized
 A Randomized, Double-Blind, -Controlled, Phase-2B Study to Evaluate the Safety and Efficacy of Recombinant Human Soluble Thrombomodulin, ART-123, in Patients with Sepsis and Suspected Disseminated Intravascular
A Randomized, Double-Blind, -Controlled, Phase-2B Study to Evaluate the Safety and Efficacy of Recombinant Human Soluble Thrombomodulin, ART-123, in Patients with Sepsis and Suspected Disseminated Intravascular
Candesartan Antihypertensive Survival Evaluation in Japan (CASE-J) Trial of Cardiovascular Events in High-Risk Hypertensive Patients
 1/5 This site became the new ClinicalTrials.gov on June 19th. Learn more. We will be updating this site in phases. This allows us to move faster and to deliver better services. Show less IMPORTANT: Listing
1/5 This site became the new ClinicalTrials.gov on June 19th. Learn more. We will be updating this site in phases. This allows us to move faster and to deliver better services. Show less IMPORTANT: Listing
Etienne Aliot. University of Nancy - France
 Etienne Aliot University of Nancy - France Disclosures Consulting fees : - Bayer, Boehringer Ingelheim,GSK, MedaPharma, Pfizer/BMS,Sanofi Aventis. - Biotronik,Medtronic,St Jude Medical. Electrical vs Pharmacological
Etienne Aliot University of Nancy - France Disclosures Consulting fees : - Bayer, Boehringer Ingelheim,GSK, MedaPharma, Pfizer/BMS,Sanofi Aventis. - Biotronik,Medtronic,St Jude Medical. Electrical vs Pharmacological
Trial record 1 of 1 for: Previous Study Return to List Next Study
 1 von 6 14.01.2014 09:11 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: 2012-001834-33 Previous Study Return to List Next Study Study of Cabozantinib (XL184) Versus Prednisone
1 von 6 14.01.2014 09:11 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: 2012-001834-33 Previous Study Return to List Next Study Study of Cabozantinib (XL184) Versus Prednisone
Sponsor Novartis. Generic Drug Name Pasireotide. Therapeutic Area of Trial Cushing s disease. Protocol Number CSOM230B2208E1
 Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Supplementary Online Content
 Supplementary Online Content Block GA, Bushinsky DA, Cunningham J, et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism:
Supplementary Online Content Block GA, Bushinsky DA, Cunningham J, et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism:
Synopsis Style Clinical Study Report SR EFC5826 Version number: 1 (electronic 2.0)
 SYNOPSIS Title of the study: Randomized, multinational, multicenter, double-blind, placebo-controlled, two-arm parallel group trial of rimonabant 20 mg OD for reducing the risk of major cardiovascular
SYNOPSIS Title of the study: Randomized, multinational, multicenter, double-blind, placebo-controlled, two-arm parallel group trial of rimonabant 20 mg OD for reducing the risk of major cardiovascular
Now Available: Final Rule for FDAAA 801 and NIH Policy on Clinical Trial Reporting
 A service of the U.S. National Institutes of Health Now Available: Final Rule for FDAAA 801 and NIH Policy on Clinical Trial Reporting Trial record 1 of 1 for: Keynote 355 Previous Study Return to List
A service of the U.S. National Institutes of Health Now Available: Final Rule for FDAAA 801 and NIH Policy on Clinical Trial Reporting Trial record 1 of 1 for: Keynote 355 Previous Study Return to List
Trial record 1 of 1 for: Previous Study Return to List Next Study
 A service of the U.S. National Institutes of Health Trial record 1 of 1 for: CR013783 Previous Study Return to List Next Study A Study to Compare Effectiveness and Safety of Darunavir/Ritonavir (DRV/Rtv)
A service of the U.S. National Institutes of Health Trial record 1 of 1 for: CR013783 Previous Study Return to List Next Study A Study to Compare Effectiveness and Safety of Darunavir/Ritonavir (DRV/Rtv)
Update on Dronedarone and Cardiovascular Outcomes
 Update on and Cardiovascular Outcomes Dr. Stuart Connolly MD McMaster University Hamilton Ontario Disclosure: Research grants, speaker fees and consulting honoraria from sanofi aventis has key structural
Update on and Cardiovascular Outcomes Dr. Stuart Connolly MD McMaster University Hamilton Ontario Disclosure: Research grants, speaker fees and consulting honoraria from sanofi aventis has key structural
Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer
 Find Studies About Studies Submit Studies Resources About Site Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer The safety and scientific validity of this study is the responsibility
Find Studies About Studies Submit Studies Resources About Site Trial of Letrozole + Palbociclib/Placebo in Metastatic Endometrial Cancer The safety and scientific validity of this study is the responsibility
Clinical Trial Synopsis TL-OPI-516, NCT#
 Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
Clinical Trial Synopsis, NCT#00225277 Title of Study: A Double-Blind, Randomized, Comparator-Controlled Study in Subjects With Type 2 Diabetes Mellitus Comparing the Effects of Pioglitazone HCl Versus
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only. Please note that the results reported in any single trial may not reflect the overall
APR-DRG Description Ave Charge
 Abdominal Pain 16,500.25 2.8 6,000.09 Acute & Subacute Endocarditis 15,339.30 3.0 5,113.10 Acute Myocardial Infarction 17,687.46 2.6 6,802.87 Alcohol Abuse & Dependence 19,126.64 4.2 4,553.96 Alcoholic
Abdominal Pain 16,500.25 2.8 6,000.09 Acute & Subacute Endocarditis 15,339.30 3.0 5,113.10 Acute Myocardial Infarction 17,687.46 2.6 6,802.87 Alcohol Abuse & Dependence 19,126.64 4.2 4,553.96 Alcoholic
ARTEMIS. ARixtra (fondaparinux) for ThromboEmbolism prevention in. a Medical Indications Study. NV Organon Protocol 63129
 ARTEMIS ARixtra (fondaparinux) for ThromboEmbolism prevention in NV Organon Protocol 63129 a Medical Indications Study Objective To demonstrate efficacy and to assess safety of oncedaily subcutaneous (SC)
ARTEMIS ARixtra (fondaparinux) for ThromboEmbolism prevention in NV Organon Protocol 63129 a Medical Indications Study Objective To demonstrate efficacy and to assess safety of oncedaily subcutaneous (SC)
Asthma J45.20 Mild, uncomplicated J45.21 Mild, with (acute) exacerbation J45.22 Mild, with status asthmaticus
 A Fib & Flutter I48.0 Paroxysmal atrial fibrillation I48.1 Persistent atrial fibrillation I48.2 Chronic atrial fibrillation I48.3 Typical atrial flutter Asthma J45.20 Mild, uncomplicated J45.21 Mild, with
A Fib & Flutter I48.0 Paroxysmal atrial fibrillation I48.1 Persistent atrial fibrillation I48.2 Chronic atrial fibrillation I48.3 Typical atrial flutter Asthma J45.20 Mild, uncomplicated J45.21 Mild, with
The population of subjects which was statistically analyzed was the Intent-to-Treat population
 Study No.: ARIB3003 (Year 1) Title: A Randomized, Double-Blind, Placebo-Controlled, Two-Year Parallel-Group Study of the Efficacy and Safety of GI198745 in the Treatment and Modification of Progression
Study No.: ARIB3003 (Year 1) Title: A Randomized, Double-Blind, Placebo-Controlled, Two-Year Parallel-Group Study of the Efficacy and Safety of GI198745 in the Treatment and Modification of Progression
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
MedDRA Basic Concept
 MedDRA Basic Concept Pansie Zhang MSD R&D (China) Co., Ltd 23Apr2015 Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter and should not
MedDRA Basic Concept Pansie Zhang MSD R&D (China) Co., Ltd 23Apr2015 Disclaimer The views and opinions expressed in the following PowerPoint slides are those of the individual presenter and should not
Ticagrelor compared with clopidogrel in patients with acute coronary syndromes the PLATO trial
 compared with clopidogrel in patients with acute coronary syndromes the PLATO trial August 30, 2009 at 08.00 CET PLATO background In NSTE-ACS and STEMI, current guidelines recommend 12 months aspirin and
compared with clopidogrel in patients with acute coronary syndromes the PLATO trial August 30, 2009 at 08.00 CET PLATO background In NSTE-ACS and STEMI, current guidelines recommend 12 months aspirin and
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See United States Package Insert (USPI)
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Study No.: Title: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Core Safety Profile. Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg. Date of FAR:
 Core Safety Profile Active substance: Bisoprolol Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg P - RMS: FI/H/PSUR/0002/002 Date of FAR: 13.12.2011
Core Safety Profile Active substance: Bisoprolol Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg P - RMS: FI/H/PSUR/0002/002 Date of FAR: 13.12.2011
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Fragmin / sodium
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Fragmin / sodium
Nilotinib AEs (adverse events) in CML population:
 Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
PDF of Trial CTRI Website URL -
 Clinical Trial Details (PDF Generation Date :- Fri, 14 Dec 2018 00:34:23 GMT) CTRI Number Last Modified On 07/02/2014 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Clinical Trial Details (PDF Generation Date :- Fri, 14 Dec 2018 00:34:23 GMT) CTRI Number Last Modified On 07/02/2014 Post Graduate Thesis Type of Trial Type of Study Study Design Public Title of Study
Trial record 1 of 1 for: 075-A-301 Previous Study Return to List Next Study
 Efficacy and Safety of a Sore Throat Lozenge Containing Lidocaine and Cetylpyridinium Chloride in Patients With Sore Throat Due to a Comm... Page 1 of 10 A service of the U.S. National Institutes of Health
Efficacy and Safety of a Sore Throat Lozenge Containing Lidocaine and Cetylpyridinium Chloride in Patients With Sore Throat Due to a Comm... Page 1 of 10 A service of the U.S. National Institutes of Health
Long-term safety, tolerability and efficacy of alirocumab in high cardiovascular risk patients: ODYSSEY LONG TERM
 Long-term safety, tolerability and efficacy of alirocumab in high cardiovascular risk patients: ODYSSEY LONG TERM Efficacy by subgroup, and safety when LDL-C
Long-term safety, tolerability and efficacy of alirocumab in high cardiovascular risk patients: ODYSSEY LONG TERM Efficacy by subgroup, and safety when LDL-C
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
On behalf of the RE-CIRCUIT Investigators. March 19, :45 am 10:55 am. Johns Hopkins Medical Institutions, Baltimore, MD, USA.
 Safety and Efficacy of Uninterrupted Anticoagulation with Dabigatran Etexilate versus in Patients Undergoing Catheter Ablation of Atrial Fibrillation: The RE-CIRCUIT Study Hugh Calkins, M.D., 1 Stephan
Safety and Efficacy of Uninterrupted Anticoagulation with Dabigatran Etexilate versus in Patients Undergoing Catheter Ablation of Atrial Fibrillation: The RE-CIRCUIT Study Hugh Calkins, M.D., 1 Stephan
Common Codes for ICD-10
 Common Codes for ICD-10 Specialty: Cardiology *Always utilize more specific codes first. ABNORMALITIES OF HEART RHYTHM ICD-9-CM Codes: 427.81, 427.89, 785.0, 785.1, 785.3 R00.0 Tachycardia, unspecified
Common Codes for ICD-10 Specialty: Cardiology *Always utilize more specific codes first. ABNORMALITIES OF HEART RHYTHM ICD-9-CM Codes: 427.81, 427.89, 785.0, 785.1, 785.3 R00.0 Tachycardia, unspecified
Transient Atrial Fibrillation and Risk of Stroke after Acute Myocardial Infarction
 Transient Atrial Fibrillation and Risk of Stroke after Acute Myocardial Infarction Doron Aronson MD, Gregory Telman MD, Fadel BahouthMD, Jonathan Lessick MD, DSc and Rema Bishara MD Department of Cardiology
Transient Atrial Fibrillation and Risk of Stroke after Acute Myocardial Infarction Doron Aronson MD, Gregory Telman MD, Fadel BahouthMD, Jonathan Lessick MD, DSc and Rema Bishara MD Department of Cardiology
GSK Medicine: Study Number: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Title A Phase II study of oral LBH589 in adult patients with refractory cutaneous T-Cell lymphoma
 Sponsor Novartis Generic Drug Name Panobinostat Therapeutic Area of Trial Refractory cutaneous T-Cell lymphoma Approved Indication Investigational drug Protocol Number CLBH589B2201 Title A Phase II study
Sponsor Novartis Generic Drug Name Panobinostat Therapeutic Area of Trial Refractory cutaneous T-Cell lymphoma Approved Indication Investigational drug Protocol Number CLBH589B2201 Title A Phase II study
Saudi Arabia February Pr Michel KOMAJDA. Université Pierre et Marie Curie Hospital Pitié Salpétrière
 Prevention of Cardiovascular events with Ivabradine: The SHIFT Study Saudi Arabia February 2011 Pr Michel KOMAJDA Université Pierre et Marie Curie Hospital Pitié Salpétrière Paris FRANCE Declaration Of
Prevention of Cardiovascular events with Ivabradine: The SHIFT Study Saudi Arabia February 2011 Pr Michel KOMAJDA Université Pierre et Marie Curie Hospital Pitié Salpétrière Paris FRANCE Declaration Of
Appendix Criteria used for the automated chart review
 Appendix Criteria used for the automated chart review A. Heart attack i. 410.01 (Acute myocardial infarction of anterolateral wall initial episode of ii. 410.11 (Acute myocardial infarction of other anterior
Appendix Criteria used for the automated chart review A. Heart attack i. 410.01 (Acute myocardial infarction of anterolateral wall initial episode of ii. 410.11 (Acute myocardial infarction of other anterior
Figure 1: Consort diagram; the status of participants in the study
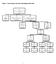 Figure : Consort diagram; the status of participants in the study Figure a. Time to first colorectal cancer in those randomized to aspirin compared with those randomized to aspirin placebo (AP). Kaplan-Meier
Figure : Consort diagram; the status of participants in the study Figure a. Time to first colorectal cancer in those randomized to aspirin compared with those randomized to aspirin placebo (AP). Kaplan-Meier
Sponsor. Generic Drug Name. Trial Indications. Protocol Number. Protocol Title. Clinical Trial Phase. Study Start/End Dates. Reason for Termination
 Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
Sponsor Alcon Research, Ltd. Generic Drug Name Travoprost/timolol maleate Trial Indications Open-angle glaucoma or ocular hypertension Protocol Number C-09-007 Protocol Title An Evaluation of Patient Reported
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert.
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Caduet / Amlodipine
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. PROPRIETARY DRUG NAME / GENERIC DRUG NAME: Caduet / Amlodipine
PALLAS. Dronedarone on Top of Standard Therapy. Stuart J. Connolly MD. on behalf of the PALLAS investigators
 PALLAS Permanent Atrial FibriLLAtion Outcome Study using on Top of Standard Therapy Stuart J. Connolly MD on behalf of the PALLAS investigators http://clinicaltrials.gov Number: NCT01151137 1 Disclosure
PALLAS Permanent Atrial FibriLLAtion Outcome Study using on Top of Standard Therapy Stuart J. Connolly MD on behalf of the PALLAS investigators http://clinicaltrials.gov Number: NCT01151137 1 Disclosure
Supplemental Appendix. 1. Protocol Definition of Sustained Virologic Response. A patient has a sustained virologic response if:
 Supplemental Appendix 1. Protocol Definition of Sustained Virologic Response A patient has a sustained virologic response if: 1. The patient is a responder at the end of treatment and all subsequent planned
Supplemental Appendix 1. Protocol Definition of Sustained Virologic Response A patient has a sustained virologic response if: 1. The patient is a responder at the end of treatment and all subsequent planned
Sponsor Novartis. Generic Drug Name. Valsartan and amlodipine Trial Indication(s) Hypertension Protocol Number CVAA489A2306 Protocol Title
 Sponsor Novartis Generic Drug Name Valsartan and amlodipine Trial Indication(s) Hypertension Protocol Number CVAA489A2306 Protocol Title A randomized, double-blind, multi-center, active-controlled, parallel
Sponsor Novartis Generic Drug Name Valsartan and amlodipine Trial Indication(s) Hypertension Protocol Number CVAA489A2306 Protocol Title A randomized, double-blind, multi-center, active-controlled, parallel
SYNOPSIS. Study centre(s) The study was performed in Denmark, Norway and Sweden at 20 sites.
 Drug substance(s): AZD0837 Edition No.: 1 Study code: D1250C00007 Date: 22 May, 2006 SYNOPSIS (For national authority use only) A Controlled, Randomised, Parallel, Multicentre Study to Assess Safety and
Drug substance(s): AZD0837 Edition No.: 1 Study code: D1250C00007 Date: 22 May, 2006 SYNOPSIS (For national authority use only) A Controlled, Randomised, Parallel, Multicentre Study to Assess Safety and
