ANAMORELIN FOR CACHEXIA. Series 20 CASE REPORT FORM
|
|
|
- Buddy Green
- 5 years ago
- Views:
Transcription
1 1 ANAMORELIN FOR CACHEXIA Series 20 CASE REPORT FORM Palliative Care Clinical Studies Collaborative (PaCCSC) RAPID Pharmacovigilance in Palliative Care The case report form (CRF) is to be completed in compliance with PaCCSC Standard Operating Procedures (SOP)
2 2 Staff Participant Id: Date of Baseline: dd/mm/yyyy Demographics Gender Male Female Age years Weight (kg) Height (cm) Primary Cancer Lung Bowel Liver Prostate Head and Neck Gastric Oesophageal Breast Pancreatic Other (please specify) Palliative Care Phase Stable Unstable Deteriorating Terminal Stable: The person's symptoms are adequately controlled by established management. Further interventions to maintain symptom control and quality of life have been planned. Unstable Phase: The person experiences the development of a new problem or a rapid increase in the severity of existing problems either of which requires an urgent change in management or emergency treatment. Deteriorating Phase: The person experiences a gradual worsening of existing symptoms or the development of new but expected problems. These require the application of specific plans of care and regular review but not urgent or emergency treatment. Terminal Care Phase: Death is likely in a matter of days and no acute intervention is planned or required.
3 3 Laboratory tests (Only if available) Serum albumin C-Reactive protein (CRP) Blood sugar level Haemoglobin A1C Creatinine Clearance Result Not available Charlson Comorbidity Index Myocardial infarction Congestive cardiac failure Peripheral vascular disease Cerebrovascular disease Dementia Chronic pulmonary disease Connective tissue disease Ulcer disease Mild liver disease Hemiplegia Moderate or severe renal disease Diabetes with end organ damage Moderate or severe liver disease
4 4 AIDS Diabetes If diabetic please indicate if: Type I Type II Insulin dependent Oral hypoglycaemic agents Controlled by diet alone How often is BSL monitoring being done? At least daily Less than daily No regular monitoring of BSLs. Australian Modified Karnofsky Performance Scale (AKPS) 100 Normal; no complaints; no evidence of disease 90 Able to carry on normal activity; minor signs of symptoms 80 Normal activity with effort; some signs of symptoms or disease 70 Cares for self; unable to carry on normal activity or to do active work 60 Requires occasional assistance but is able to care for most of his needs 50 Requires considerable assistance and frequent medical care 40 In bed more than 50% of the time 30 Almost completely bedfast 20 Totally bedfast and requiring extensive nursing care by professionals and/or family 10 Comatose or barely rousable 0 Dead Not able to determine
5 5 T0 Baseline Medication Commencement Time: Estimated weight loss in the past three months: (kgs; patient s recall) Current weight (kgs) Weight in kgs: % Fat (if available) % water ( if available) % muscle (if available) Anorexia NCI Criteria 1.Loss of appetite without alteration in eating habits 2.Oral intake altered without significant weight loss or malnutrition; oral nutritional supplements indicated 3.Associated with significant weight loss or malnutrition (e.g., inadequate oral caloric and/or fluid intake); tube feeding or TPN indicated 4.Life-threatening consequences; urgent intervention indicated 5.Death Cachexia ungradable no symptom ungradable Fearon and Strasser et al Pre-cachexia-Weight loss </= 5%; Anorexia and metabolic change 2. Cachexia-weight loss >/= 5% or BMI <20% kg/m2 and weight loss > 2%; Often reduced food intake/systemic inflammation 3. Refractory cachexia variable degree of cachexia; Cancer disease both procatabolic and not responsive to anticancer treatment; Low performance score (ECOG/WHO score 3 or 4; less than 3 months survival expected
6 6 Commencement dose Anamorelin Dose (mg) Per 24hrs Toxicity assessment T0 Hyperglycaemia/Hypoglycaemia (since last seen) Symptomatic hypoglycaemia Symptomatic hyperglycaemia Asymptomatic hyperglycaemia Diarrhoea NCI Criteria 1.Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline 2.Increase of 4-6 stools per day over baseline; moderate increase in ostomy output compared to baseline 3.Increase of >=7 stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self-care ADL 4.Life-threatening consequences; urgent intervention indicated 5.Death Other please specify below Other: Additional other please specify below Additional other:
7 7 Which toxicity/symptom is the most troublesome? Select: Anorexia Cachexia Diarrhoea Hypoglycaemia/hyperglycaemia Other Additional other Date of next planned visit: dd/mm/yyyy
8 8 T1-Second visit/assessment Date of visit/assessment dd/mm/yyyy T1: Assessed/Not assessed reason Assessed today (continue) Died (if died please record below) t able to be contacted/located Too unwell Other Date of Death (dd/mm/yyyy) Anorexia NCI Criteria 1.Loss of appetite without alteration in eating habits 2.Oral intake altered without significant weight loss or malnutrition; oral nutritional supplements indicated 3.Associated with significant weight loss or malnutrition (e.g., inadequate oral caloric and/or fluid intake); tube feeding or TPN indicated 4.Life-threatening consequences; urgent intervention indicated 5.Death Cachexia no symptom ungradable Fearon and Strasser et al Pre-cachexia-Weight loss </= 5%; Anorexia and metabolic change 2. Cachexia-weight loss >/= 5% or BMI <20% kg/m2 and weight loss > 2%; Often reduced food intake/systemic inflammation 3. Refractory cachexia variable degree of cachexia; Cancer disease both procatabolic and not responsive to anticancer treatment; Low performance score (ECOG/WHO score 3 or 4; less than 3 months survival expected
9 9 Current weight (kgs) Weight in kgs: % Fat (if available) % water ( if available) % muscle (if available) Australian Modified Karnofsky Performance Scale (AKPS) 100 Normal; no complaints; no evidence of disease 90 Able to carry on normal activity; minor signs of symptoms 80 Normal activity with effort; some signs of symptoms or disease 70 Cares for self; unable to carry on normal activity or to do active work 60 Requires occasional assistance but is able to care for most of his needs 50 Requires considerable assistance and frequent medical care 40 In bed more than 50% of the time 30 Almost completely bedfast 20 Totally bedfast and requiring extensive nursing care by professionals and/or family 10 Comatose or barely rousable 0 Dead Not able to determine
10 10 Toxicity assessment Diarrhoea NCI Criteria 1.Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline 2.Increase of 4-6 stools per day over baseline; moderate increase in ostomy output compared to baseline 3.Increase of >=7 stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self-care ADL 4.Life-threatening consequences; urgent intervention indicated 5.Death Hyperglycaemia/Hypoglycaemia (since last seen) Symptomatic hypoglycaemia Symptomatic hyperglycaemia Asymptomatic hyperglycaemia Other please specify below Other: Additional Other please specify below Additional other: Which toxicity/symptom is the most troublesome? Select: Anorexia Cachexia Diarrhoea Hypoglycaemia/hyperglycaemia Other Additional other
11 11 Symptomatic Benefit Assessment Assessed / Not assessed reason Assessed today (continue) t assessed (go to either date of next planned contact OR completion of data collection form) t able to be contacted / located Too unwell Other Total dose Anamorelin given in last 24 hours (mgs) How long has the patient been on this dose (days) Did the patient perceive any benefit? Medication Changes (pick one of the 4) Anamorelin dose maintained/continue current dose Anamorelin dose increased (please specify below) Anamorelin dose decreased (please specify below) Anamorelin dose ceased If Anamorelin dose increased or decreased above, please specify new dose (mgs) Was a new medication added for side effects? (If yes please specify below) Please specify new medication here
12 12 Key questions derived from the Naranjo modified check list 1. Did the adverse reaction appear after the suspected drug was given? 2. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was given? 3. Are there alternative causes (other than the drug) that could on their own have caused the reaction? 4. Did the patient have a similar reaction to the same or similar drug in any previous exposure? 5. Was the adverse event confirmed by any objective evidence? (if yes please specify below)
13 13 Post toxicity assessment What is the intended treatment based on today s assessment? change to Anamorelin continue current dose Anamorelin ceased Anamorelin reduced Anamorelin increased please specify dose Has a medication been added to treat a specific toxicity? Yes No If yes please specify medication. Based on the assessment today has the toxicity resolved? N/A Date of planned next visit/assessment dd/mm/yyyy
14 14 T2- Assessment/ third visit Date of visit/assessment dd/mm/yyyy T2: Assessed/Not assessed reason Assessed today (continue) Died (if died please record below) t able to be contacted/located Too unwell Other Date of Death (dd/mm/yyyy) Current weight (kgs) Weight in kgs: % Fat (if available) % water ( if available) % muscle (if available) Anorexia NCI Criteria 1.Loss of appetite without alteration in eating habits 2.Oral intake altered without significant weight loss or malnutrition; oral nutritional supplements indicated 3.Associated with significant weight loss or malnutrition (e.g., inadequate oral caloric and/or fluid intake); tube feeding or TPN indicated 4.Life-threatening consequences; urgent intervention indicated 5.Death
15 15 Cachexia Fearon and Strasser et al Criteria 1.Pre-cachexia-Weight loss </= 5%; Anorexia and metabolic change 2. Cachexia-weight loss >/= 5% or BMI <20% kg/m2 and weight loss > 2%; Often reduced food intake/systemic inflammation 3. Refractory cachexia variable degree of cachexia; Cancer disease both procatabolic and not responsive to anticancer treatment; Low performance score (ECOG/WHO score 3 or 4; less than 3 months survival expected Australian Modified Karnofsky Performance Scale (AKPS) 100 Normal; no complaints; no evidence of disease 90 Able to carry on normal activity; minor signs of symptoms 80 Normal activity with effort; some signs of symptoms or disease 70 Cares for self; unable to carry on normal activity or to do active work 60 Requires occasional assistance but is able to care for most of his needs 50 Requires considerable assistance and frequent medical care 40 In bed more than 50% of the time 30 Almost completely bedfast 20 Totally bedfast and requiring extensive nursing care by professionals and/or family 10 Comatose or barely rousable 0 Dead Not able to determine Toxicity assessment Diarrhoea NCI Criteria 1.Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline 2.Increase of 4-6 stools per day over baseline; moderate increase in ostomy output compared to baseline 3.Increase of >=7 stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self-care ADL 4.Life-threatening consequences; urgent intervention indicated 5.Death
16 16 Hyperglycaemia/Hypoglycaemia (since last seen) Symptomatic hypoglycaemia Symptomatic hyperglycaemia Asymptomatic hyperglycaemia Other please specify below Other : Additional other please specify below Additional other: Which toxicity/symptom is the most troublesome? Select: Anorexia Cachexia Diarrhoea Hypoglycaemia/hyperglycaemia Other Additional other Symptomatic Benefit Assessment Assessed/Not assessed reason Assessed today (continue) t assessed (go to either date of next planned contact OR completion of data collection form) t able to be contacted / located Too unwell Other Total dose Anamorelin given in last 24 hours (mgs)
17 17 How long has the patient been on this dose (days) Was there any benefit? Medication Changes (pick one of the 4) Anamorelin dose maintained/continue current dose Anamorelin dose increased (please specify below) Anamorelin dose decreased (please specify below) Anamorelin dose ceased If Anamorelin dose increased or decreased above, please specify new dose (mgs) Was a new medication added for side effects- (if yes please specify below) Key questions derived from the Naranjo modified check list 1. Did the adverse reaction appear after the suspected drug was given? 2. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was given?
18 18 3. Are there alternative causes (other than the drug) that could on their own have caused the reaction? 4. Did the patient have a similar reaction to the same or similar drug in any previous exposure? 5. Was the adverse event confirmed by any objective evidence? (please specify below) Post toxicity assessment What is the intended treatment based on today s assessment? change to Anamorelin/continue current dose Anamorelin ceased Anamorelin dose reduced Anamorelin dose increased please specify dose Has a medication been added to treat a specific toxicity? Yes No If yes please specify medication. Based on the assessment today has the toxicity resolved? Yes N/A Date of planned next visit/assessment dd/mm/yyyy
19 19 Medication Cessation (complete this page at any time the medication of interest is ceased) Date of assessment dd/mm/yyyy Medication was ceased (related to indication of interest): Symptom resolved Symptom continued unchanged Symptom worsened Symptom resolved - date of resolution dd/mm/yyyy Symptom worsened - Grade (NCI) Medication was ceased (related to other reasons): Toxicity Patient unable to take medication Other Please specify the other reason medication was ceased Please specify the patient s inability to take medication
20 20 Unscheduled Adverse Event Assessment (a) Please complete the survey below. Were there any ad hoc toxicities? Yes No Date of assessment dd/mm/yyyy Was there any benefit? Yes No What is the intended treatment based on the assessment today? Medication Changes (pick one of the 4) Anamorelin dose maintained/continue current dose Anamorelin dose increased (please specify below) Anamorelin dose decreased (please specify below) Anamorelin dose ceased If Anamorelin dose increased or decreased above, please specify new dose (mgs) Was a new medication added for side effects (please specify below) Please specify new medication here: Anorexia NCI Criteria 1.Loss of appetite without alteration in eating habits 2.Oral intake altered without significant weight loss or malnutrition; oral nutritional supplements indicated 3.Associated with significant weight loss or malnutrition (e.g., inadequate oral caloric and/or fluid intake); tube feeding or TPN indicated 4.Life-threatening consequences; urgent intervention indicated 5.Death
21 21 Cachexia Fearon and Strasser et al Criteria 1.Pre-cachexia-Weight loss </= 5%; Anorexia and metabolic change 2. Cachexia-weight loss >/= 5% or BMI <20% kg/m2 and weight loss > 2%; Often reduced food intake/systemic inflammation 3. Refractory cachexia variable degree of cachexia; Cancer disease both procatabolic and not responsive to anticancer treatment; Low performance score (ECOG/WHO score 3 or 4; less than 3 months survival expected Diarrhoea NCI Criteria 1.Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline 2.Increase of 4-6 stools per day over baseline; moderate increase in ostomy output compared to baseline 3.Increase of >=7 stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self-care ADL 4.Life-threatening consequences; urgent intervention indicated 5.Death Hyperglycaemia/Hypoglycaemia (since last seen) Symptomatic hypoglycaemia Symptomatic hyperglycaemia Asymptomatic hyperglycaemia Other please specify below Other: Additional other please specify below Additional other:
22 22 Which toxicity/symptom is the most troublesome? Select: Anorexia Cachexia Diarrhoea Hypoglycaemia/hyperglycaemia Other Additional other Key questions derived from the Naranjo modified check list 1. Did the adverse reaction appear after the suspected drug was given? 2. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was given? 3. Are there alternative causes (other than the drug) that could on their own have caused the reaction? 4. Did the patient have a similar reaction to the same or similar drug in any previous exposure? 5. Was the adverse event confirmed by any objective evidence? Please specify objective evidence of adverse event:
23 23 Post toxicity assessment What is the intended treatment based on today s assessment? change to Anamorelin/continue current dose Anamorelin ceased Anamorelin dose reduced Anamorelin dose increased please specify dose Has a medication been added to treat a specific toxicity? Yes No If yes please specify medication. Based on the assessment today has the toxicity resolved? Yes N/A
24 24 Unscheduled Adverse Event Assessment (b) Please complete the survey below. Were there any ad hoc toxicities? Yes No Date of assessment dd/mm/yyyy Was there any benefit? Yes No What is the intended treatment based on the assessment today? Medication Changes (pick one of the 4) Anamorelin dose maintained/continue current dose Anamorelin dose increased (please specify below) Anamorelin dose decreased (please specify below) Anamorelin dose ceased If Anamorelin dose increased or decreased above, please specify new dose (mgs) Was a new medication added for side effects (please specify below) Please specify new medication here: Anorexia NCI Criteria 1.Loss of appetite without alteration in eating habits 2.Oral intake altered without significant weight loss or malnutrition; oral nutritional supplements indicated 3.Associated with significant weight loss or malnutrition (e.g., inadequate oral caloric and/or fluid intake); tube feeding or TPN indicated 4.Life-threatening consequences; urgent intervention indicated 5.Death
25 25 Cachexia Fearon and Strasser et al Criteria 1.Pre-cachexia-Weight loss </= 5%; Anorexia and metabolic change 2. Cachexia-weight loss >/= 5% or BMI <20% kg/m2 and weight loss > 2%; Often reduced food intake/systemic inflammation 3. Refractory cachexia variable degree of cachexia; Cancer disease both procatabolic and not responsive to anticancer treatment; Low performance score (ECOG/WHO score 3 or 4; less than 3 months survival expected Diarrhoea NCI Criteria 1.Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline 2.Increase of 4-6 stools per day over baseline; moderate increase in ostomy output compared to baseline 3.Increase of >=7 stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self-care ADL 4.Life-threatening consequences; urgent intervention indicated 5.Death Hyperglycaemia/Hypoglycaemia (since last seen) Symptomatic hypoglycaemia hyperglycaemia Symptomatic hyperglycaemia Asymptomatic Other please specify below Other: Additional other please specify below Additional other:
26 26 Which toxicity/symptom is the most troublesome? Select: Anorexia Cachexia Diarrhoea Hypoglycaemia/hyperglycaemia Other Additional other Key questions derived from the Naranjo modified check list 1. Did the adverse reaction appear after the suspected drug was given? 2. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was given? 3. Are there alternative causes (other than the drug) that could on their own have caused the reaction? 4. Did the patient have a similar reaction to the same or similar drug in any previous exposure? 5. Was the adverse event confirmed by any objective evidence? Please specify objective evidence of adverse event:
27 27 Post toxicity assessment What is the intended treatment based on today s assessment? change to Anamorelin/continue current dose Anamorelin ceased Anamorelin dose reduced Anamorelin dose increased please specify dose Has a medication been added to treat a specific toxicity? Yes No If yes please specify medication. Based on the assessment today has the toxicity resolved? Yes N/A
28 28 Unscheduled Adverse Event Assessment (c) Please complete the survey below. Were there any ad hoc toxicities? Yes No Date of assessment dd/mm/yyyy Was there any benefit? Yes No What is the intended treatment based on the assessment today? Medication Changes (pick one of the 4) Anamorelin dose maintained/continue current dose Anamorelin dose increased (please specify below) Anamorelin dose decreased (please specify below) Anamorelin dose ceased If Anamorelin dose increased or decreased above, please specify new dose (mgs) Was a new medication added for side effects (please specify below) Please specify new medication here: Anorexia NCI Criteria 1.Loss of appetite without alteration in eating habits 2.Oral intake altered without significant weight loss or malnutrition; oral nutritional supplements indicated 3.Associated with significant weight loss or malnutrition (e.g., inadequate oral caloric and/or fluid intake); tube feeding or TPN indicated 4.Life-threatening consequences; urgent intervention indicated 5.Death
29 29 Cachexia Fearon and Strasser et al Criteria 1.Pre-cachexia-Weight loss </= 5%; Anorexia and metabolic change 2. Cachexia-weight loss >/= 5% or BMI <20% kg/m2 and weight loss > 2%; Often reduced food intake/systemic inflammation 3. Refractory cachexia variable degree of cachexia; Cancer disease both procatabolic and not responsive to anticancer treatment; Low performance score (ECOG/WHO score 3 or 4; less than 3 months survival expected Diarrhoea NCI Criteria 1.Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline 2.Increase of 4-6 stools per day over baseline; moderate increase in ostomy output compared to baseline 3.Increase of >=7 stools per day over baseline; incontinence; hospitalization indicated; severe increase in ostomy output compared to baseline; limiting self-care ADL 4.Life-threatening consequences; urgent intervention indicated 5.Death Hyperglycaemia/Hypoglycaemia (since last seen) Symptomatic hypoglycaemia Symptomatic hyperglycaemia Asymptomatic hyperglycaemia Other please specify below Other: Additional other please specify below Additional other:
30 30 Which toxicity/symptom is the most troublesome? Select: Anorexia Cachexia Diarrhoea Hypoglycaemia/hyperglycaemia Other Additional Other Key questions derived from the Naranjo modified check list 1. Did the adverse reaction appear after the suspected drug was given? 2. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was given? 3. Are there alternative causes (other than the drug) that could on their own have caused the reaction? 4. Did the patient have a similar reaction to the same or similar drug in any previous exposure? 5. Was the adverse event confirmed by any objective evidence? Please specify objective evidence of adverse event:
31 31 Post toxicity assessment What is the intended treatment based on today s assessment? change to Anamorelin/continue current dose Anamorelin ceased Anamorelin dose reduced Anamorelin dose increased please specify dose Has a medication been added to treat a specific toxicity? Yes No If yes please specify medication. Based on the assessment today has the toxicity resolved? Yes N/A
TEMPLATE CASE REPORT FORM. Rapid Response Pharmacovigilance in Palliative Care
 TEMPLATE CASE REPORT FORM Rapid Response Pharmacovigilance in Palliative Care The case report form is to be completed in compliance with PaCCSC Standard Operating Procedures 1 Staff email: Participant
TEMPLATE CASE REPORT FORM Rapid Response Pharmacovigilance in Palliative Care The case report form is to be completed in compliance with PaCCSC Standard Operating Procedures 1 Staff email: Participant
Midazolam for Agitation - Baseline
 Midazolam for Agitation - Baseline Staff email Participant ID Date of Baseline w D-M-Y H:M Initials of person entering data Demographics Gender Male Female Age Weight (kg) Height (cm) Page 1 Primary life
Midazolam for Agitation - Baseline Staff email Participant ID Date of Baseline w D-M-Y H:M Initials of person entering data Demographics Gender Male Female Age Weight (kg) Height (cm) Page 1 Primary life
LABs Albumin. (g/dl) Haemoglobin, (g/l) Creatinin, (mg/dl)
 DATA COLLECTION SHEET CRF B Baseline evaluation Center ID: Patient code: Date of birth (dd/mm/yyyy): / / Sex: Male Female Living situation: home independent home with family/care giver residential care
DATA COLLECTION SHEET CRF B Baseline evaluation Center ID: Patient code: Date of birth (dd/mm/yyyy): / / Sex: Male Female Living situation: home independent home with family/care giver residential care
Diet what helps? Lindsey Allan Macmillan Oncology Dietitian Royal Surrey County Hospital, Guildford
 Diet what helps? Lindsey Allan Macmillan Oncology Dietitian Royal Surrey County Hospital, Guildford Diet and cancer Diet and cancer Nutrition research Lack of funding RCTs Low quality Small sample sizes
Diet what helps? Lindsey Allan Macmillan Oncology Dietitian Royal Surrey County Hospital, Guildford Diet and cancer Diet and cancer Nutrition research Lack of funding RCTs Low quality Small sample sizes
Community and Mental Health Services. Palliative Care. Criteria and
 Community and Mental Health Services Specialist Palliative Care Service Referral Criteria and Guidance November 2018 Specialist Palliative Care Service Referrals These guidelines cover referrals for patients
Community and Mental Health Services Specialist Palliative Care Service Referral Criteria and Guidance November 2018 Specialist Palliative Care Service Referrals These guidelines cover referrals for patients
T: N: M: EORTC SYSTEMIC THERAPY CHECKLIST. Protocol number: SeqID. Copyright EORTC August 2007 COMPARATIVE PERFORMANCE STATUS TABLE
 EORTC SYSTEMIC THERAPY CHECKLIST Copyright EORTC August 2007 (Stick the patient s identification label here) Protocol number: SeqID. T: N: M: WHO / ECOG 0 Normal activity. 1 Symptoms but ambulatory. 2
EORTC SYSTEMIC THERAPY CHECKLIST Copyright EORTC August 2007 (Stick the patient s identification label here) Protocol number: SeqID. T: N: M: WHO / ECOG 0 Normal activity. 1 Symptoms but ambulatory. 2
Specialist Palliative Care Service Referral Criteria and Guidance
 Specialist Palliative Care Service Referral Criteria and Guidance Specialist Palliative Care Service Referrals These guidelines cover referrals for patients with progressive terminal illness, whether
Specialist Palliative Care Service Referral Criteria and Guidance Specialist Palliative Care Service Referrals These guidelines cover referrals for patients with progressive terminal illness, whether
SCALES SCALES SCALES. Performance Scales WHAT SHOULD THE RAINBOW FISH DO WITH ALL OF THESE SCALES?? KPS FAST ECOG PPS NYHA MRI ALSFRS
 SCALES SCALES SCALES WHAT SHOULD THE RAINBOW FISH DO WITH ALL OF THESE SCALES?? Karen L. Cross, MD, FAAHPM Performance Scales KPS FAST ECOG PPS NYHA MRI ALSFRS PPS = 30, 40, or 50 ECOG = 2, 3, or 4 NYHA
SCALES SCALES SCALES WHAT SHOULD THE RAINBOW FISH DO WITH ALL OF THESE SCALES?? Karen L. Cross, MD, FAAHPM Performance Scales KPS FAST ECOG PPS NYHA MRI ALSFRS PPS = 30, 40, or 50 ECOG = 2, 3, or 4 NYHA
Cancer cachexia: assessment and classification. KCH Fearon University of Edinburgh Scotland
 Cancer cachexia: assessment and classification KCH Fearon University of Edinburgh Scotland 1 What is the cancer cachexia phenotype?...the shoulders, clavicles, chest and thighs melt away. This illness
Cancer cachexia: assessment and classification KCH Fearon University of Edinburgh Scotland 1 What is the cancer cachexia phenotype?...the shoulders, clavicles, chest and thighs melt away. This illness
Specialist Palliative Care Referral for Patients
 Specialist Palliative Care Referral for Patients This guideline covers referrals for patients with progressive terminal illness, whether due to cancer or other disease. For many patients in the late stages
Specialist Palliative Care Referral for Patients This guideline covers referrals for patients with progressive terminal illness, whether due to cancer or other disease. For many patients in the late stages
TREATMENTS FOR TYPE 2 DIABETES. Susan Henry Diabetes Specialist Nurse
 TREATMENTS FOR TYPE 2 DIABETES Susan Henry Diabetes Specialist Nurse How can we improve outcomes in Type 2 diabetes? Earlier diagnosis Better patient education Stress central role of lifestyle management
TREATMENTS FOR TYPE 2 DIABETES Susan Henry Diabetes Specialist Nurse How can we improve outcomes in Type 2 diabetes? Earlier diagnosis Better patient education Stress central role of lifestyle management
The structure of PCOC involves three levels. Definitions for each level can be found in this manual. Level 1: Patient level describes demographics
 The PCOC Structure The standardisation of palliative care clinical data items and assessment tools serves the dual purpose of defining a common language to enhance communication between palliative care
The PCOC Structure The standardisation of palliative care clinical data items and assessment tools serves the dual purpose of defining a common language to enhance communication between palliative care
PREMEDICATIONS: Antiemetic protocol for highly emetogenic chemotherapy. May not need any antiemetic with
 BCCA Protocol Summary for Palliative Therapy of Metastatic or Locally Advanced Gastric, Gastroesophageal Junction Adenocarcinoma, Esophageal Squamous Cell Carcinoma, or Anal Squamous Cell Carcinoma using
BCCA Protocol Summary for Palliative Therapy of Metastatic or Locally Advanced Gastric, Gastroesophageal Junction Adenocarcinoma, Esophageal Squamous Cell Carcinoma, or Anal Squamous Cell Carcinoma using
Alzheimer s Disease, Dementia, Related Disorders
 Alzheimer s Disease, Dementia, Related Disorders Stage 7 on the FAST Scale signifies the threshold of activity limitation that would support a six-month prognosis. The FAST Scale does not address the impact
Alzheimer s Disease, Dementia, Related Disorders Stage 7 on the FAST Scale signifies the threshold of activity limitation that would support a six-month prognosis. The FAST Scale does not address the impact
A 73 year old man, presents with anaemia. Describe the CT scan. Should see primary gastric cancer,ascites and liver secondary
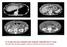 A 73 year old man, presents with anaemia. Describe the CT scan. Should see primary gastric cancer,ascites and liver secondary A 73 year old man PS presents with anaemia. Endoscopy and CT scan thorax/abdomen
A 73 year old man, presents with anaemia. Describe the CT scan. Should see primary gastric cancer,ascites and liver secondary A 73 year old man PS presents with anaemia. Endoscopy and CT scan thorax/abdomen
DATA COLLECTION SHEET CRF 3M FOLLOW UP AT 3 MONTHS (+/- 2 weeks)
 DATA COLLECTION SHEET CRF 3M FOLLOW UP AT 3 MONTHS (+/- 2 weeks) Center ID: Patient code: Date of evaluation (dd/mm/yyyy): / / 90 day mortality No Yes Date of death (dd/mm/yyyy): / / (If Yes specify cause
DATA COLLECTION SHEET CRF 3M FOLLOW UP AT 3 MONTHS (+/- 2 weeks) Center ID: Patient code: Date of evaluation (dd/mm/yyyy): / / 90 day mortality No Yes Date of death (dd/mm/yyyy): / / (If Yes specify cause
Table of Contents: Amyotrophic Lateral Sclerosis (ALS)
 Guidelines for Hospice Admission Amyotrophic Lateral Sclerosis (ALS) Cancer Cerebral Vascular Accident / Stroke or Coma Dementia / Alzheimer s Failure to Thrive Adults Heart Disease / CHF HIV Disease Huntington
Guidelines for Hospice Admission Amyotrophic Lateral Sclerosis (ALS) Cancer Cerebral Vascular Accident / Stroke or Coma Dementia / Alzheimer s Failure to Thrive Adults Heart Disease / CHF HIV Disease Huntington
Determining Eligibility for Hospice Care
 Determining Eligibility for Hospice Care Main Number: 203 739-8300 Toll Free Number: 888 357-3334 www.regionalhospicect.org Many people may not understand all that Regional Hospice can offer or they are
Determining Eligibility for Hospice Care Main Number: 203 739-8300 Toll Free Number: 888 357-3334 www.regionalhospicect.org Many people may not understand all that Regional Hospice can offer or they are
Nutrition. By Dr. Ali Saleh 2/27/2014 1
 Nutrition By Dr. Ali Saleh 2/27/2014 1 Nutrition Functions of nutrients: Providing energy for body processes and movement. Providing structural material for body tissues. Regulating body processes. 2/27/2014
Nutrition By Dr. Ali Saleh 2/27/2014 1 Nutrition Functions of nutrients: Providing energy for body processes and movement. Providing structural material for body tissues. Regulating body processes. 2/27/2014
RECTUM/SIGMOID COLON/BOWEL,
 EMBRACE Follow-up Patient ID: Day Month Year Physician (initials) RECTUM/SIGMOID COLON/BOWEL, morbidity scoring CTC v3.0 1. Diarrhea 1: Increase of
EMBRACE Follow-up Patient ID: Day Month Year Physician (initials) RECTUM/SIGMOID COLON/BOWEL, morbidity scoring CTC v3.0 1. Diarrhea 1: Increase of
Palliative Care Referral/Triage Tool
 Palliative Care Referral/Triage Tool Gippsland Region Palliative Care Consortium Clinical Practice Group Title Keywords Ratified Palliative Care Referral/Triage Tool Palliative Care, triage, symptom assessment,
Palliative Care Referral/Triage Tool Gippsland Region Palliative Care Consortium Clinical Practice Group Title Keywords Ratified Palliative Care Referral/Triage Tool Palliative Care, triage, symptom assessment,
We would like to thank you for completing this audit questionnaire which looks at how you manage nausea and vomiting in palliative care patients.
 We would like to thank you for completing this audit questionnaire which looks at how you manage nausea and vomiting in palliative care patients. The closing date for responses is 19th December The results
We would like to thank you for completing this audit questionnaire which looks at how you manage nausea and vomiting in palliative care patients. The closing date for responses is 19th December The results
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION
 SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date Gender Female Male Date of surgery INCLUSION & EXCLUSION CRITERIA YES
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date Gender Female Male Date of surgery INCLUSION & EXCLUSION CRITERIA YES
Capecitabine + Concurrent Radiotherapy
 Capecitabine + Concurrent Radiotherapy Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient
Capecitabine + Concurrent Radiotherapy Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient
Guidelines to assist General Practitioners in the Management of Type 2 Diabetes. April 2010
 Guidelines to assist General Practitioners in the Management of Type 2 Diabetes April 2010 Foreword The guidelines were devised by the Diabetes Day Centre in Beaumont Hospital in consultation with a number
Guidelines to assist General Practitioners in the Management of Type 2 Diabetes April 2010 Foreword The guidelines were devised by the Diabetes Day Centre in Beaumont Hospital in consultation with a number
Discussing Prognosis. David Ross Russell MD ProHealth Physicians Inc.
 Discussing Prognosis David Ross Russell MD ProHealth Physicians Inc. Prognosis- peeling back the layers Not a new Science Psalm 39 LORD, make me to know mine end, and the measure of my days. Hippocrates
Discussing Prognosis David Ross Russell MD ProHealth Physicians Inc. Prognosis- peeling back the layers Not a new Science Psalm 39 LORD, make me to know mine end, and the measure of my days. Hippocrates
Course Handouts & Post Test
 STROKE/COMA: DISEASE TRAJECTORY AND HOSPICE ELIGIBILITY Terri L. Maxwell PhD, APRN VP, Strategic Initiatives Weatherbee Resources Hospice Education Network Course Handouts & Post Test To download presentation
STROKE/COMA: DISEASE TRAJECTORY AND HOSPICE ELIGIBILITY Terri L. Maxwell PhD, APRN VP, Strategic Initiatives Weatherbee Resources Hospice Education Network Course Handouts & Post Test To download presentation
Glucose Control drug treatments
 Glucose Control drug treatments It should be noted that glitazones are under suspicion of precipitating acute cardiac events and current recommendations contraindicate the use of glitazones in patients
Glucose Control drug treatments It should be noted that glitazones are under suspicion of precipitating acute cardiac events and current recommendations contraindicate the use of glitazones in patients
Appropriate Use of Prescribed Oral Nutritional Supplement (ONS) in the Community
 Appropriate Use of Prescribed Oral Nutritional Supplement (ONS) in the Community Aim This guideline sets out a recommended procedure for the identification and treatment of malnutrition to ensure Oral
Appropriate Use of Prescribed Oral Nutritional Supplement (ONS) in the Community Aim This guideline sets out a recommended procedure for the identification and treatment of malnutrition to ensure Oral
SECTION 4: RECRUIT PARTICIPANTS
 SECTION 4: RECRUIT PARTICIPANTS Contents Participant Eligibility & Enrollment... 2 Screening... 2 Study ID Numbers... 2 Inclusion Criteria... 2 Exclusion Criteria... 4 Co-Enrollment... 5 Informed Consent
SECTION 4: RECRUIT PARTICIPANTS Contents Participant Eligibility & Enrollment... 2 Screening... 2 Study ID Numbers... 2 Inclusion Criteria... 2 Exclusion Criteria... 4 Co-Enrollment... 5 Informed Consent
Palliative Care Clinical Research, Calvary and PaCCSC
 Palliative Care Clinical Research, Calvary and PaCCSC Linda Brown, National Manager Palliative Care Clinical Studies Collaborative (PaCCSC) The mutually beneficial relationship between Calvary and PaCCSC
Palliative Care Clinical Research, Calvary and PaCCSC Linda Brown, National Manager Palliative Care Clinical Studies Collaborative (PaCCSC) The mutually beneficial relationship between Calvary and PaCCSC
PACKAGE LEAFLET: INFORMATION FOR THE USER. Glucose Intravenous Infusion BP 10% w/v solution for infusion Glucose (as glucose monohydrate)
 PACKAGE LEAFLET: INFORMATION FOR THE USER Glucose Intravenous Infusion BP 10% w/v solution for infusion Glucose (as glucose monohydrate) Read all of this leaflet carefully before you start using this medicine
PACKAGE LEAFLET: INFORMATION FOR THE USER Glucose Intravenous Infusion BP 10% w/v solution for infusion Glucose (as glucose monohydrate) Read all of this leaflet carefully before you start using this medicine
Objectives 2/11/2016 HOSPICE 101
 HOSPICE 101 Overview Hospice History and Statistics What is Hospice? Who qualifies for services? Levels of Service The Admission Process Why Not to Wait Objectives Understand how to determine hospice eligibility
HOSPICE 101 Overview Hospice History and Statistics What is Hospice? Who qualifies for services? Levels of Service The Admission Process Why Not to Wait Objectives Understand how to determine hospice eligibility
Inflammatory Bowel Disease
 + Inflammatory Bowel Disease Christina Kalafsky, Dietetic Intern University of Maryland College Park Children s National Medical Center Case Study January 31, 2014 + Outline n Inflammatory Bowel Disease
+ Inflammatory Bowel Disease Christina Kalafsky, Dietetic Intern University of Maryland College Park Children s National Medical Center Case Study January 31, 2014 + Outline n Inflammatory Bowel Disease
Palliative Care Outcomes Collaboration. Clinical Manual
 Palliative Care Outcomes Collaboration Clinical Manual Table of Contents Introduction to this Manual... 5 The Palliative Care Outcomes Collaboration Program... 4 The PCOC Structure... Error! Bookmark not
Palliative Care Outcomes Collaboration Clinical Manual Table of Contents Introduction to this Manual... 5 The Palliative Care Outcomes Collaboration Program... 4 The PCOC Structure... Error! Bookmark not
Ontario s Referral and Listing Criteria for Adult Heart Transplantation
 Ontario s Referral and Listing Criteria for Adult Heart Transplantation Version 3.0 Trillium Gift of Life Network Adult Heart Transplantation Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The
Ontario s Referral and Listing Criteria for Adult Heart Transplantation Version 3.0 Trillium Gift of Life Network Adult Heart Transplantation Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The
Nutritional Assessment of patients in hospital
 Nutritional Assessment of patients in hospital Geoffrey Axiak M.Sc. Nursing (Manchester), B.Sc. Nursing, P.G. Dip. Nutrition & Dietetics Definition of malnutrition Undernutrition can occur as a result
Nutritional Assessment of patients in hospital Geoffrey Axiak M.Sc. Nursing (Manchester), B.Sc. Nursing, P.G. Dip. Nutrition & Dietetics Definition of malnutrition Undernutrition can occur as a result
HOSPICE DIAGNOSIS DETERMINATION ASSESSMENT
 Patient Name: MR #: Date: Objective documentation is required to support hospice admission. This worksheet is intended to gather information on both the severity and trajectory of the patient s condition
Patient Name: MR #: Date: Objective documentation is required to support hospice admission. This worksheet is intended to gather information on both the severity and trajectory of the patient s condition
Intestinal Failure Referral Form
 Intestinal Failure Referral Form This form must be completed in full and emailed to UCLH.IFReferrals@nhs.net or call 07958 263178. Please complete all sections of the form. Please note that incomplete
Intestinal Failure Referral Form This form must be completed in full and emailed to UCLH.IFReferrals@nhs.net or call 07958 263178. Please complete all sections of the form. Please note that incomplete
Legislation POLST. Palliative and Hospice Care: End of Life Decisions. Palliative and Hospice Care End of Life Decisions John F. Bertagnolli, Jr, DO
 Palliative and Hospice Care End of Life Decisions John F. Bertagnolli, Jr, DO Legislation On 12/21/11 Gov. Christie signed legislation that enables patients to indicate their preferences regarding life
Palliative and Hospice Care End of Life Decisions John F. Bertagnolli, Jr, DO Legislation On 12/21/11 Gov. Christie signed legislation that enables patients to indicate their preferences regarding life
Nutrition for Patients with Cancer or HIV/AIDS Chapter 22
 Nutrition for Patients with Cancer or HIV/AIDS Chapter 22 Nutrition for Patients with Cancer or HIV/AIDS Cancer and HIV/AIDS can cause devastating weight loss and malnutrition. Nutrition therapy Cannot
Nutrition for Patients with Cancer or HIV/AIDS Chapter 22 Nutrition for Patients with Cancer or HIV/AIDS Cancer and HIV/AIDS can cause devastating weight loss and malnutrition. Nutrition therapy Cannot
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
Patient Treatment Date/time: Diagnosis: Treatment Regimen:
 Name: Address: DOB: HCRN: Consultant: Ward: Assessment: Pre Systematic Anti Cancer Therapy (SACT) Continuation *This assessment is for patients on multiday regimens Patient Treatment Date/time: Diagnosis:
Name: Address: DOB: HCRN: Consultant: Ward: Assessment: Pre Systematic Anti Cancer Therapy (SACT) Continuation *This assessment is for patients on multiday regimens Patient Treatment Date/time: Diagnosis:
Please inform the Diabetes Nurse Specialist that this patient has been admitted within 24hrs of admission.
 Adult Diabetic Ketoacidosis Care Bundle (V1. Issued October 2014 Review October 2015) Improving patient care This pack includes: DKA Management Guideline Name: (Patient Addressograph) DOB: Hospital No:
Adult Diabetic Ketoacidosis Care Bundle (V1. Issued October 2014 Review October 2015) Improving patient care This pack includes: DKA Management Guideline Name: (Patient Addressograph) DOB: Hospital No:
Case Discussion. Nutrition in IBD. Rémy Meier MD. Ulcerative colitis. Crohn s disease
 26.08.2017 Case Discussion Nutrition in IBD Crohn s disease Ulcerative colitis Rémy Meier MD Case Presentation 30 years old female, with diarrhea for 3 months Shool frequency 3-4 loose stools/day with
26.08.2017 Case Discussion Nutrition in IBD Crohn s disease Ulcerative colitis Rémy Meier MD Case Presentation 30 years old female, with diarrhea for 3 months Shool frequency 3-4 loose stools/day with
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION
 SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date (DD/MMM/YYYY) (DD/MMM/YYYY) Gender Female Male Date of surgery (DD/MMM/YYYY)
SECTION 1: INCLUSION, EXCLUSION & RANDOMISATION INFORMATION DEMOGRAPHIC INFORMATION Given name Family name Date of birth Consent date (DD/MMM/YYYY) (DD/MMM/YYYY) Gender Female Male Date of surgery (DD/MMM/YYYY)
Hospice Eligibility August 2018
 Hospice Eligibility August 2018 Objectives Identify who can make a hospice referral Review hospice eligibility and disease-specific prognostic indicators Review Open Access philosophy Who Can Make A Referral
Hospice Eligibility August 2018 Objectives Identify who can make a hospice referral Review hospice eligibility and disease-specific prognostic indicators Review Open Access philosophy Who Can Make A Referral
Neonatal Parenteral Nutrition Guideline Dr M Hogan, Maire Cullen ANNP, Una Toland Ward Manager, Sandra Kilpatrick Neonatal Pharmacist
 CLINICAL GUIDELINES ID TAG Title: Author: Designation: Speciality / Division: Directorate: Neonatal Parenteral Nutrition Guideline Dr M Hogan, Maire Cullen ANNP, Una Toland Ward Manager, Sandra Kilpatrick
CLINICAL GUIDELINES ID TAG Title: Author: Designation: Speciality / Division: Directorate: Neonatal Parenteral Nutrition Guideline Dr M Hogan, Maire Cullen ANNP, Una Toland Ward Manager, Sandra Kilpatrick
MUST and Malnutrition
 MUST and Malnutrition Presenter Housekeeping Northern Devon Healthcare NHS Trust Confidentiality To respect confidentiality within the group unless it is necessary to address a current concern about the
MUST and Malnutrition Presenter Housekeeping Northern Devon Healthcare NHS Trust Confidentiality To respect confidentiality within the group unless it is necessary to address a current concern about the
Metabolic issues in nutrition: Implications for daily care
 Metabolic issues in nutrition: Implications for daily care Ingvar Bosaeus Dept of Clinical Nutrition Sahlgrenska University Hospital Göteborg, Sweden Nutritional problems in cancer In western countries,
Metabolic issues in nutrition: Implications for daily care Ingvar Bosaeus Dept of Clinical Nutrition Sahlgrenska University Hospital Göteborg, Sweden Nutritional problems in cancer In western countries,
Vildagliptin belongs to a group of medicines called oral antidiabetics.
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vildagliptin belongs to a group of medicines called oral antidiabetics. Vildagliptin is used in a certain form of diabetes (type
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vildagliptin belongs to a group of medicines called oral antidiabetics. Vildagliptin is used in a certain form of diabetes (type
QI Version #: 6.3 MDS 2.0 Form Type: QUARTERLY ASSESSMENT FORM-TWO PAGE DOMAIN: ACCIDENTS
 DOMAIN: ACCIDENTS 1. Incidence of new fractures 1 1.1A0001 Residents with new fractures on most recent Residents who did not have fractures on the previous new hip fracture (J4c is checked on most recent
DOMAIN: ACCIDENTS 1. Incidence of new fractures 1 1.1A0001 Residents with new fractures on most recent Residents who did not have fractures on the previous new hip fracture (J4c is checked on most recent
BCCA Protocol Summary for Second line Treatment of Metastatic or Unresectable Pancreatic Adenocarcinoma Using Capecitabine
 BCCA Protocol Summary for Second line Treatment of Metastatic or Unresectable Pancreatic Adenocarcinoma Using Capecitabine Protocol Code Tumour Group Contact Physician GIPAVCAP Gastrointestinal GI Systemic
BCCA Protocol Summary for Second line Treatment of Metastatic or Unresectable Pancreatic Adenocarcinoma Using Capecitabine Protocol Code Tumour Group Contact Physician GIPAVCAP Gastrointestinal GI Systemic
National Vascular Registry
 National Vascular Registry AAA Repair Patient Details Patient Consent* 0 No 2 Not Required If patient not consented: Date consent recorded / / (DD/MM/YYYY) Do not record NHS number, NHS number* name(s)
National Vascular Registry AAA Repair Patient Details Patient Consent* 0 No 2 Not Required If patient not consented: Date consent recorded / / (DD/MM/YYYY) Do not record NHS number, NHS number* name(s)
Nutritional assessment & support for the upper GI cancer patient
 Nutritional assessment & support for the upper GI cancer patient Catherine Fleuret Specialist Dietitian, The Royal Marsden Outline Nutritional status & implications The role of nutrition & the dietitian
Nutritional assessment & support for the upper GI cancer patient Catherine Fleuret Specialist Dietitian, The Royal Marsden Outline Nutritional status & implications The role of nutrition & the dietitian
Alzheimer s s Disease (AD) Prevalence
 Barriers to Quality End of Life Care for People with Dementia Steve McConnell, PhD Alzheimer s s Association Washington, DC Office Alliance for Health Care Reform Briefing on End of Life Care June 8, 2007
Barriers to Quality End of Life Care for People with Dementia Steve McConnell, PhD Alzheimer s s Association Washington, DC Office Alliance for Health Care Reform Briefing on End of Life Care June 8, 2007
PERIOPERATIVE DIABETES GUIDELINE
 PERIOPERATIVE DIABETES GUIDELINE This Guideline does not replace the need for the application of clinical judgment in respect to each individual patient. Background Diabetes mellitus is estimated to affect
PERIOPERATIVE DIABETES GUIDELINE This Guideline does not replace the need for the application of clinical judgment in respect to each individual patient. Background Diabetes mellitus is estimated to affect
Guideline for Estimating Length of Survival in Palliative Patients
 http://pal 11 ative. into Cornelius Woelk MD, CCFP Medical Director of Palliative Care Regional Health Authority - Central Manitoba 385 Main Street Winkler, Manitoba, Canada R6W 1J2 Ph: 204-325-4312 Fax:
http://pal 11 ative. into Cornelius Woelk MD, CCFP Medical Director of Palliative Care Regional Health Authority - Central Manitoba 385 Main Street Winkler, Manitoba, Canada R6W 1J2 Ph: 204-325-4312 Fax:
Life is pleasant. Death is peaceful. It s the transition that s troublesome. Isaac Asimov ( )
 Life is pleasant. Death is peaceful. It s the transition that s troublesome. Isaac Asimov (1920-1992) Objectives Palliative care versus hospice care. Admission guidelines to hospice services. Having the
Life is pleasant. Death is peaceful. It s the transition that s troublesome. Isaac Asimov (1920-1992) Objectives Palliative care versus hospice care. Admission guidelines to hospice services. Having the
Frailty in Older Adults. Frailty
 Frailty in Older Adults Nancy Stiles, MD Associate Professor Geriatrics i Sanders-Brown Center on Aging University of Kentucky Frailty Global impairment of physiological reserves involving i multiple l
Frailty in Older Adults Nancy Stiles, MD Associate Professor Geriatrics i Sanders-Brown Center on Aging University of Kentucky Frailty Global impairment of physiological reserves involving i multiple l
Diabetes (DIA) Measures Document
 Diabetes (DIA) Measures Document DIA Version: 2.1 - covering patients discharged between 01/07/2016 and present. Programme Lead: Liz Kanwar Clinical Lead: Dr Aftab Ahmad Number of Measures In Clinical
Diabetes (DIA) Measures Document DIA Version: 2.1 - covering patients discharged between 01/07/2016 and present. Programme Lead: Liz Kanwar Clinical Lead: Dr Aftab Ahmad Number of Measures In Clinical
Hospice. Quick Reference Guide for Determining Eligibility for Hospice Care
 Hospice Quick Reference Guide for Determining Eligibility for Hospice Care Hospice is a comprehensive service available to patients and their families who have a life expectancy of six months or less.
Hospice Quick Reference Guide for Determining Eligibility for Hospice Care Hospice is a comprehensive service available to patients and their families who have a life expectancy of six months or less.
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
HOMES AND SENIORS SERVICES. APPROVAL DATE: February 2011 REVISION DATE: July 2018
 Page 1 of 7 POLICY: Each resident s level of nutrition and hydration risk will be identified by the Registered Dietitian during the RAI-MDS Admission Assessment and thereafter during the quarterly, significant
Page 1 of 7 POLICY: Each resident s level of nutrition and hydration risk will be identified by the Registered Dietitian during the RAI-MDS Admission Assessment and thereafter during the quarterly, significant
Dept of Diabetes Main Desk
 Dept of Diabetes Main Desk 01202 448060 Glucose management in Type 2 Diabetes in Adults The natural history of type 2 diabetes is for HbA1c to deteriorate with time. A stepwise approach to treatment is
Dept of Diabetes Main Desk 01202 448060 Glucose management in Type 2 Diabetes in Adults The natural history of type 2 diabetes is for HbA1c to deteriorate with time. A stepwise approach to treatment is
Monash Health Referral Guidelines
 Monash Health Referral Guidelines CLINICAL NUTRITION EXCLUSIONS Services not offered by Monash Health Psychiatric management of eating disorders consider referring to Eating Disorders Unit Full diagnostic
Monash Health Referral Guidelines CLINICAL NUTRITION EXCLUSIONS Services not offered by Monash Health Psychiatric management of eating disorders consider referring to Eating Disorders Unit Full diagnostic
Home Total Parenteral Nutrition for Adults
 Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
Home Total Parenteral Nutrition for Adults Policy Number: Original Effective Date: MM.08.007 05/21/1999 Line(s) of Business: Current Effective Date: PPO, HMO, QUEST Integration 05/27/2016 Section: Home
HPS ALLIANCE MEMBERS ONLY HOSPICE WEBINAR SERIES
 HPS ALLIANCE MEMBERS ONLY HOSPICE WEBINAR SERIES - 2019 PRESENTER(S): LESLIE HEAGY, RN, COS-C & MELINDA A. GABOURY, COS-C Documenting to support the Hospice Terminal Prognosis February 15, 2019 DOCUMENTING
HPS ALLIANCE MEMBERS ONLY HOSPICE WEBINAR SERIES - 2019 PRESENTER(S): LESLIE HEAGY, RN, COS-C & MELINDA A. GABOURY, COS-C Documenting to support the Hospice Terminal Prognosis February 15, 2019 DOCUMENTING
Improving documentation and coding of malnutrition a five year journey
 Improving documentation and coding of malnutrition a five year journey Natalie Simmance, Chief Dietitian Clara Newsome Sally Bell Sonia Grundy St Vincent's Hospital Melbourne Patient Malnutrition common
Improving documentation and coding of malnutrition a five year journey Natalie Simmance, Chief Dietitian Clara Newsome Sally Bell Sonia Grundy St Vincent's Hospital Melbourne Patient Malnutrition common
Core Safety Profile. Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg. Date of FAR:
 Core Safety Profile Active substance: Bisoprolol Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg P - RMS: FI/H/PSUR/0002/002 Date of FAR: 13.12.2011
Core Safety Profile Active substance: Bisoprolol Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg P - RMS: FI/H/PSUR/0002/002 Date of FAR: 13.12.2011
HOSPICE 101. Another choice for patients facing a terminal prognosis. De Anna Looper, RN, CHPN, CHPCA. Carrefour Associates L.L.C.
 HOSPICE 101 Another choice for patients facing a terminal prognosis. De Anna Looper, RN, CHPN, CHPCA Senior Vice President of Clinical Operations Carrefour Associates L.L.C. HOSPICE 101 Patients and their
HOSPICE 101 Another choice for patients facing a terminal prognosis. De Anna Looper, RN, CHPN, CHPCA Senior Vice President of Clinical Operations Carrefour Associates L.L.C. HOSPICE 101 Patients and their
BCCA Protocol Summary for Therapy of Metastatic Breast Cancer using Capecitabine
 BCCA Protocol Summary for Therapy of Metastatic Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAVCAP Breast Dr. Susan Ellard ELIGIBILITY: First line treatment of metastatic
BCCA Protocol Summary for Therapy of Metastatic Breast Cancer using Capecitabine Protocol Code Tumour Group Contact Physician BRAVCAP Breast Dr. Susan Ellard ELIGIBILITY: First line treatment of metastatic
BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using
 BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using Capecitabine Protocol Code Tumour Group Contact Physician GIAJCAP Gastrointestinal GI Systemic Therapy ELIGIBILITY: Resected Stage III or
BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using Capecitabine Protocol Code Tumour Group Contact Physician GIAJCAP Gastrointestinal GI Systemic Therapy ELIGIBILITY: Resected Stage III or
Interventions for ineffective renal perfusion
 Toggle navigation Interventions for ineffective renal perfusion 3-12-2013 This article outlines the possible interventions for the complications associated with lupus. Written for nurses, but suitable
Toggle navigation Interventions for ineffective renal perfusion 3-12-2013 This article outlines the possible interventions for the complications associated with lupus. Written for nurses, but suitable
Measure #403: Adult Kidney Disease: Referral to Hospice National Quality Strategy Domain: Patient and Caregiver-Centered Experience and Outcomes
 Measure #403: Adult Kidney Disease: Referral to Hospice National Quality Strategy Domain: Patient and Caregiver-Centered Experience and Outcomes 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Measure #403: Adult Kidney Disease: Referral to Hospice National Quality Strategy Domain: Patient and Caregiver-Centered Experience and Outcomes 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE
Coding Hints 2 nd Edition
 Coding Hints 2 nd Edition Medicare s guidelines state, Code all documented conditions which co-exist at the time of the visit that require or affect patient care or treatment. Beyond the Basics Incomplete
Coding Hints 2 nd Edition Medicare s guidelines state, Code all documented conditions which co-exist at the time of the visit that require or affect patient care or treatment. Beyond the Basics Incomplete
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens
 EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
EXENATIDE (BYETTA ) PROTOCOL, 5mcg and 10mcg SC injection pre-filled pens This document should be read in conjunction with the current Summary of Product Characteristics http://www.medicines.org.uk 1.
Ceritinib (Zykadia )
 (Ceritinib (Zykadia ) (Xalkori) Ceritinib (Zykadia ) This leaflet is offered as a guide to you and your family. Ceritinib (Zykadia ) is a type of anti-cancer treatment called a targeted therapy. The aim
(Ceritinib (Zykadia ) (Xalkori) Ceritinib (Zykadia ) This leaflet is offered as a guide to you and your family. Ceritinib (Zykadia ) is a type of anti-cancer treatment called a targeted therapy. The aim
PARENTERAL NUTRITION
 PARENTERAL NUTRITION DEFINITION Parenteral nutrition [(PN) or total parenteral nutrition (TPN)] is the intravenous infusion of some or all nutrients for tissue maintenance, metabolic requirements and growth
PARENTERAL NUTRITION DEFINITION Parenteral nutrition [(PN) or total parenteral nutrition (TPN)] is the intravenous infusion of some or all nutrients for tissue maintenance, metabolic requirements and growth
Nutrition Diagnosis: Examples in Two Case Studies
 Nutrition Care Process Model Tutorials Nutrition Care Process and Terminology Committee Academy of Nutrition and Dietetics Nutrition Care Process Terminology 2015 Edition : Examples in Two Case Studies
Nutrition Care Process Model Tutorials Nutrition Care Process and Terminology Committee Academy of Nutrition and Dietetics Nutrition Care Process Terminology 2015 Edition : Examples in Two Case Studies
Primary Endpoint The primary endpoint is overall survival, measured as the time in weeks from randomization to date of death due to any cause.
 CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
CASE STUDY Randomized, Double-Blind, Phase III Trial of NES-822 plus AMO-1002 vs. AMO-1002 alone as first-line therapy in patients with advanced pancreatic cancer This is a multicenter, randomized Phase
Embracing the international agenda The Nutrition Care Process
 Embracing the international agenda The Nutrition Care Process Vera Todorovic Consultant Dietitian Doncaster and Bassetlaw Hospitals NHS Foundation Trust Vera.todorovic@dbh.nhs.uk 11/12 Linking the processes
Embracing the international agenda The Nutrition Care Process Vera Todorovic Consultant Dietitian Doncaster and Bassetlaw Hospitals NHS Foundation Trust Vera.todorovic@dbh.nhs.uk 11/12 Linking the processes
Nutrition Care Process: Case Study B Examples of Charting in Various Formats
 Nutrition Care Process: Case Study B Examples of Charting in Various Formats Case: JG is a 68 year old woman with a history of type 2 diabetes, chronic renal failure which is treated with hemodialysis
Nutrition Care Process: Case Study B Examples of Charting in Various Formats Case: JG is a 68 year old woman with a history of type 2 diabetes, chronic renal failure which is treated with hemodialysis
Results of the ECPC Nutrition and Cancer patient survey
 Results of the ECPC Nutrition and Cancer patient survey Maurizio Muscaritoli filippoossifanelli@uniroma1.it WHY A FOCUS ON NUTRITION IN ONCOLOGY? Nutrition is a fundamental aspect of the therapeutic course
Results of the ECPC Nutrition and Cancer patient survey Maurizio Muscaritoli filippoossifanelli@uniroma1.it WHY A FOCUS ON NUTRITION IN ONCOLOGY? Nutrition is a fundamental aspect of the therapeutic course
National Vascular Registry
 National Vascular Registry AAA Repair Patient Details Patient Consent* 0 No 1 Yes 2 Not Required If patient not consented: Date consent recorded / / (DD/MM/YYYY) Do not record NHS number, NHS number* name(s)
National Vascular Registry AAA Repair Patient Details Patient Consent* 0 No 1 Yes 2 Not Required If patient not consented: Date consent recorded / / (DD/MM/YYYY) Do not record NHS number, NHS number* name(s)
5-FU & Cisplatin + Cetuximab
 5-FU & Cisplatin + Cetuximab Available for Routine Use in Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from either: a) the relevant PCT
5-FU & Cisplatin + Cetuximab Available for Routine Use in Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from either: a) the relevant PCT
Alectinib (Alecensa ) Alectinib (Alecensa )
 Alectinib (Alecensa ) Alectinib (Alecensa ) This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary: for some people, treatment may reduce the risk of the cancer
Alectinib (Alecensa ) Alectinib (Alecensa ) This leaflet is offered as a guide to you and your family. The possible benefits of treatment vary: for some people, treatment may reduce the risk of the cancer
NUTRITION PLANNING FOR PRE AND POST LIVER TRANSPLANT DAPHNEE.D.K HEAD DEPARTMENT OF DIETETICS APOLLO HOSPITALS (MAIN) CHENNAI
 NUTRITION PLANNING FOR PRE AND POST LIVER TRANSPLANT DAPHNEE.D.K HEAD DEPARTMENT OF DIETETICS APOLLO HOSPITALS (MAIN) CHENNAI PRE - OPERATIVE Case Presentation Name: Mr. XXX Age: 51yrs Sex: Male No. of
NUTRITION PLANNING FOR PRE AND POST LIVER TRANSPLANT DAPHNEE.D.K HEAD DEPARTMENT OF DIETETICS APOLLO HOSPITALS (MAIN) CHENNAI PRE - OPERATIVE Case Presentation Name: Mr. XXX Age: 51yrs Sex: Male No. of
Antiemetic protocol for low-moderately emetogenic chemotherapy (see SCNAUSEA)
 BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
Malnutrition in Adults: Guidelines for Identification and Treatment
 Malnutrition in Adults: Guidelines for Identification and Treatment Signatures (e.g. chair of the ratifying committee and lay member) and date Signature...date Designation: Signature...date Designation
Malnutrition in Adults: Guidelines for Identification and Treatment Signatures (e.g. chair of the ratifying committee and lay member) and date Signature...date Designation: Signature...date Designation
A new era of therapeutics for cancer cachexia. Cachexia is a continuum with 3 stages of clinical relevance
 A new era of therapeutics for cancer cachexia I. Depletion of Reserves II. Limitation of food intake III. Catabolic Drivers IV. Impact and outcomes Vickie Baracos PhD Professor and Alberta Cancer Foundation
A new era of therapeutics for cancer cachexia I. Depletion of Reserves II. Limitation of food intake III. Catabolic Drivers IV. Impact and outcomes Vickie Baracos PhD Professor and Alberta Cancer Foundation
DRUG CLASSES BETA-ADRENOCEPTOR ANTAGONISTS (BETA-BLOCKERS)
 DRUG CLASSES BETA-ADRENOCEPTOR ANTAGONISTS (BETA-BLOCKERS) Beta-blockers have been widely used in the management of angina, certain tachyarrhythmias and heart failure, as well as in hypertension. Examples
DRUG CLASSES BETA-ADRENOCEPTOR ANTAGONISTS (BETA-BLOCKERS) Beta-blockers have been widely used in the management of angina, certain tachyarrhythmias and heart failure, as well as in hypertension. Examples
La Nutrizione Artificiale dall ospedale al domicilio
 La Nutrizione Artificiale dall ospedale al domicilio Federico Bozzetti Cagliari 25-26 Marzo 2009 Nutrition of the cancer patient Prevalence of malnutrition Effect of malnutrition on the outcome: - survival
La Nutrizione Artificiale dall ospedale al domicilio Federico Bozzetti Cagliari 25-26 Marzo 2009 Nutrition of the cancer patient Prevalence of malnutrition Effect of malnutrition on the outcome: - survival
Vanderbilt University Medical Center Trauma ICU Nutrition Management Guidelines
 Vanderbilt University Medical Center Trauma ICU Nutrition Management Guidelines Trauma Critical Care Nutrition Guidelines Clinical judgment may supersede guidelines as patient circumstances warrant ASSESSMENT
Vanderbilt University Medical Center Trauma ICU Nutrition Management Guidelines Trauma Critical Care Nutrition Guidelines Clinical judgment may supersede guidelines as patient circumstances warrant ASSESSMENT
All resources are sold in packs of 10, unless otherwise indicated.
 Patient Information Leaflets (No consultation required) 1000 Iron Deficiency Anaemia - Your Diet Can Help Patient Pick Up 6.00 1001 Dietary Advice for Bone Health Patient Pick Up 19.00 1002 Curing Constipation
Patient Information Leaflets (No consultation required) 1000 Iron Deficiency Anaemia - Your Diet Can Help Patient Pick Up 6.00 1001 Dietary Advice for Bone Health Patient Pick Up 19.00 1002 Curing Constipation
Current concepts in Critical Care Nutrition
 Current concepts in Critical Care Nutrition Dr.N.Ramakrishnan AB (Int Med), AB (Crit Care), MMM, FACP, FCCP, FCCM Director, Critical Care Services Apollo Hospitals, Chennai Objectives Why? Enteral or Parenteral
Current concepts in Critical Care Nutrition Dr.N.Ramakrishnan AB (Int Med), AB (Crit Care), MMM, FACP, FCCP, FCCM Director, Critical Care Services Apollo Hospitals, Chennai Objectives Why? Enteral or Parenteral
Patient Group Direction for the Supply of Nitrofurantoin MR 100mg capsules
 October 2016 Patient Group Direction for the Supply of Nitrofurantoin MR 100mg capsules This Patient Group Direction (PGD) is a specific written instruction for the supply and/or administration of nitrofurantoin
October 2016 Patient Group Direction for the Supply of Nitrofurantoin MR 100mg capsules This Patient Group Direction (PGD) is a specific written instruction for the supply and/or administration of nitrofurantoin
