Gynaecology Forum. Regine Sitruk-Ware. The levonorgestrelreleasing intrauterine. system, Mirena. Vol. 11, No. 2, Guest Editor: In this issue:
|
|
|
- Antony Neal
- 5 years ago
- Views:
Transcription
1 Gynaecology Forum Vol. 11, No. 2, 2006 Guest Editor: Regine Sitruk-Ware In this issue: The levonorgestrelreleasing intrauterine system, Mirena Contraceptive efficacy and safety Use in nulliparous women Alternative to tubal occlusion Menorrhagia Leiomyoma-related bleeding QOL and cost-effectiveness vs. hysterectomy Endometriosis and dysmenorrhoea Peri- and postmenopause Endometrial effects Future options Medical Forum International
2 Vol. 11, No. 2, 2006 Gynaecology Forum is an interactive topical review journal for gynaecologists and other interested physicians Internet address: Guest Editor Regine Sitruk-Ware Rockefeller University and Population Council, New York, NY, USA Editors-in-Chief H. Brölmann, The Netherlands R. Homburg, The Netherlands S.R. Killick, UK A. Kubba, UK R. Massai, Chile J. van der Mooren, The Netherlands Z.M. van der Spuy, South Africa Editorial Board U.J. Gaspard, Belgium P.J. Keller, Switzerland S. Mashiach, Israel D.R. Mishell, USA M.M. Shabaan, Egypt M. Short, Ireland S.O. Skouby, Denmark P. Virutamasen, Thailand A. Webb, UK G. Yeo, Singapore Contents Editorial... 2 Overview article The levonorgestrel-releasing intrauterine system (Mirena ) Regine Sitruk-Ware... 3 Review articles Contraceptive efficacy and safety of the levonorgestrel-releasing intrauterine system (Mirena ) Tapani Luukkainen, Päivi Pakarinen... 4 Can the levonorgestrel-releasing intrauterine system (Mirena ) be used in nulliparous women? Sarah Prager, Philip Darney... 7 The levonorgestrel-releasing intrauterine system (Mirena ) as an alternative to tubal occlusion Ian Milsom The levonorgestrel-releasing intrauterine system (Mirena ) in the treatment of menorrhagia Bilian Xiao The levonorgestrel-releasing intrauterine system (Mirena ) in the treatment of leiomyoma-related bleeding Vera Halpern, Marina Tarasova Quality of life and cost-effectiveness of the levonorgestrel-releasing intrauterine system (Mirena ) and hysterectomy in menorrhagia Ritva Hurskainen The levonorgestrel-releasing intrauterine system (Mirena ) in endometriosis and dysmenorrhoea Luis Bahamondes, Carlos A. Petta The levonorgestrel-releasing intrauterine system (Mirena ) in the peri- and postmenopause David Sturdee Endometrial effects of levonorgestrel intrauterine delivery Takeshi Maruo Future options for the intrauterine delivery of progestogens in the prevention of gynaecological disease Ian S. Fraser ISSN: Medical Forum International All rights reserved. No part of this publication may be translated, reproduced or stored in a retrieval system, in any form, without the written consent of the publisher. Published by: Medical Forum International PO Box AR Zeist The Netherlands correspondence@medforum.nl Subscription rate (Volume 11, 2006, 4 issues): individual 150/year; institutional 175/year (including postage and handling). Subscriptions are accepted with prepayment only. Orders should be addressed to: Medical Forum International, Attn Subscription Dept, PO Box 655, 3700 AR Zeist, The Netherlands, fax: Although great care has been taken to ensure accuracy, Medical Forum International shall not be liable for any errors or inaccuracies in this publication. Opinions expressed are those of the authors. The publishers and editors shall not be liable for the validity of clinical treatments, dosage regimens or other medical statements made. All dosages referred to should be checked against the relevant product data sheets.
3 Editorial EDITORIAL Dear Colleagues Most new medical drugs or developments suffer a pendulum effect with regard to their popularity and use. They are introduced with a fanfare and used widely and enthusiastically, only to find that after a year or two of general use side effects and difficulties emerge which had not been predicted or expected from the phase III studies. Confidence in the method wanes and its use decreases dramatically. There then often follows a realization that the method is useful for a particular population of patients or in certain circumstances and its popularity rises again. The introduction of Mirena has not followed this course; in fact, quite the reverse. Any initial concerns about pelvic inflammatory disease or the use of Mirena in nulliparous women have been dispelled and new indications for its use in conditions such as menorrhagia, postmenopausal hormone replacement therapy, endometriosis, or for contraception in renal disease or after malignancy have emerged. Over 60 million couples currently use female sterilization as their method of contraception. Surely this number needs to be reduced by a cheaper, safer, more efficacious and reversible method. Dr Sitruk-Ware and her team have expertly put together this edition of Gynaecology Forum, which is the third we have produced on the topic of Mirena in the last 10 years. Each new issue illustrates the widening usefulness of the system. We need to use it more often. Stephen Killick Editor-in-Chief 2006 Medical Forum International 2 Gynaecology Forum Vol. 11, No. 2, 2006
4 OVERVIEW ARTICLE The levonorgestrel-releasing intrauterine system (Mirena ) Regine Sitruk-Ware Rockefeller University and Population Council, New York, NY, USA Intrauterine contraceptive devices (IUDs) have been widely used since This method of contraception is extremely effective, with an effectiveness rate that is similar to that of tubal interruption (female sterilization), yet it is rapidly reversible. The types of IUDs have evolved from the original plastic (inert) devices to the current devices that release copper or levonorgestrel (termed intrauterine systems). Unwarranted concerns about IUDs limited their use in the United States to less than 1% of female contraceptive users compared with 30% in Europe and China. The levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) was developed as a long-acting method of contraception. The concept was invented by Tapani Luukkainen and developed by the Population Council in the late 1980s and early 1990s with the objective of improving the contraceptive efficacy and decreasing the bleeding days of the inert IUDs. The development was then completed in collaboration with the pharmaceutical companies Leiras in Finland and then Schering, and the system was first introduced in Finland in This medicated device inserted into the uterus is one of the most effective methods of contraception and is presently approved in over 100 countries for 5 years of use, including in the United States where the system was approved in The LNG-IUS releases daily small amounts of levonorgestrel, a synthetic progestogen derivative, into the uterine cavity during a long wearing period. The system consists of a T-shaped plastic frame, to the vertical arm of which a cylinder containing the active substance mixed with polydimethyl siloxane (PDMS) is attached. The total amount of levonorgestrel in the cylinder is 52 mg. The cylinder is covered by a ratecontrolling PDMS membrane that regulates the daily release rate of the hormone. Initially, the release rate is 20 μg/day, which declines to 11 μg/day at the end of 5 years. The mean release rate is 14 μg/day over the 5-year wearing period. The distal end of the T-frame contains two removal threads. The T-frame also contains barium sulfate, which makes it visible on x-ray examination. The LNG-IUS directly targets the endometrium by releasing the potent progestogen levonorgestrel in low daily doses into the uterine cavity. The result is high local levonorgestrel concentrations that achieve uniform suppression of endometrial proliferation, inactive histology, thin epithelium and decidualization of the stroma. Such dramatic alteration of the endometrium creates an environment within the uterus that is unsuitable for sperm survival and fertilization and is thus a key mechanism of action for contraception. Although the circulating levels of levonorgestrel are very low, side effects related to the androgenic properties of levonorgestrel have been reported. In addition, insertion of the system needs a skilled health provider and may be difficult or painful when the uterus is small or atrophic. The development of smaller devices is warranted in these specific indications. Besides its main indication for contraception, the advantages of direct action of levonorgestrel on the endometrium have been studied in other medical conditions. By inactivating the endometrium, the LNG-IUS is able to provide a range of other health benefits. These include treatment of idiopathic menorrhagia and associated anaemia or menorrhagia due to leiofibromas. The LNG-IUS also provides protection against endometrial proliferation in women receiving estrogen therapy. The guest authors in this issue of Gynaecology Forum have reviewed the major aspects of the efficacy and safety of the LNG-IUS in several populations and provide recommendations about the appropriate indications and safe conditions for use of the system in young women as well as in postmenopausal women in countries where the product is licensed. Unfortunately the system is not yet widely available in developing countries where the dual benefits of a longterm contraceptive that reduces bleeding and hence prevents anaemia are of the utmost interest. The size of the system that contains a reservoir delivering the progestogen may create difficulties for insertion in some women; thus, appropriate training of health care providers is required. New developments of a smaller system are ongoing and will offer an additional option to potential users. Suggested reading Mirena. Physician information. Available at: /products/pi/mirena_ mar_pi.pdf. Accessed 6 April Sitruk-Ware R, Inki P. The levonorgestrel intrauterine system: long-term contraception and therapeutic effects. Women s Health 2005; 1: Gynaecology Forum Vol. 11, No. 2, Medical Forum International
5 Contraceptive efficacy and safety of the levonorgestrelreleasing intrauterine system (Mirena ) Tapani Luukkainen 1 Päivi Pakarinen 2 1 Steroid Research Laboratory, Institute of Biomedicine, University of Helsinki, Helsinki, Finland 2 Department of Obstetrics and Gynecology, Helsinki University Central Hospital, Helsinki, Finland (paivi.pakarinen@hus.fi) Contraceptive efficacy The mode of action of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) is to prevent fertilization not implantation [1]. The best experimental evidence for the mechanism of action was presented in a recent report by Munuce et al. [2]. The authors showed that concentrations of levonorgestrel above 400 ng/ml, which are similar to those found in the uterine cavity, can modify in vitro the expression of zona pellucida receptors on spermatozoa. This could also explain why the LNG-IUS is ineffective if inserted into the myometrium. The local release of levonorgestrel in the LNG-IUS produces concentrations of between 470 and 1500 ng/g wet tissue weight in the endometrium within weeks of insertion [3]. Circulating levels range from 150 to 200 nmol/l after the first few weeks. The contraceptive effectiveness of the LNG-IUS does not depend on the age of the user (Table I). All other contraceptive methods including sterilization have high failure rates in young women. Our large randomized comparative trial in five European countries revealed not a single pregnancy during 5 years of use in women below 25 years of age in any of the participating countries [4]. The higher failure rate in all age groups using a copper-releasing intrauterine device (IUD) shows the superior effectiveness of the LNG-IUS. Faundes et al. [5] reported no pregnancies during 7 years and 1018 woman-years of LNG-IUS use. Cumulative 7-year net rates revealed a low expulsion rate of 4.4 per 100 women and a low discontinuation rate due to bleeding of 1.8 per 100 women. This report is in agreement with other studies reporting a zero pregnancy rate with the LNG-IUS [1]. The report of Faundes et al. has an interesting conclusion for developing countries: Preference for the copper IUD could be a sound public health policy, consistent with scarce resources. It does not take into account, however, the individual needs of women already debilitated by overt or subclinical anaemia, and the long-term costbenefit ratio of saving versus increasing menstrual blood loss in these women. The hypothesized mechanism of action described by Munuce et al. [2] explains the absence of ectopic pregnancies when the LNG-IUS is correctly placed in the uterine cavity. A summary of the above studies covered 12,000 woman-years of LNG-IUS use without the occurrence of a single ectopic pregnancy. In women not using contraception, the expected number of ectopic pregnancies is between 7.5 and 10.6 per 1000 woman-years in those aged years [6]; thus in 12,000 woman-years we would expect between 90 and 127 ectopic pregnancies, which illustrates the effectiveness of the LNG-IUS at preventing ectopic pregnancy. The LNG-IUS has been shown to be effective in a range of situations. Abortion usually results from contraceptive failure or misuse. For example, a woman may have opted for a contraceptive method that was too difficult for her to use consistently or that had side effects leading to discontinuation. Eventually her choice of contraception failed to protect her from unwanted pregnancy. The decision to have an abortion is always a difficult one and afterwards requires an effective and usually long-term contraceptive method. The LNG-IUS can and should be inserted immediately after abortion, since the return of fertility is immediate [7]. The LNG-IUS is highly effective and does not need to be remembered every day. Other alternative reversible contraceptives have a higher Table I: Pregnancy rate according to age during contraceptive use for 5 years (modified from [4]). Age (years) Copper-releasing IUD LNG-IUS (n = 937) (n = 1821) Medical Forum International 4 Gynaecology Forum Vol. 11, No. 2, 2006
6 failure rate and are less cost-effective in the long term [8]. Women at the time of abortion are highly motivated to consider contraception and, because of the surgical procedure, are already in a sterile environment in the operating theatre. Insertion of the LNG- IUS after evacuation of the uterine cavity is easy because the cervical canal is widened and the patient is under anaesthesia. In developed countries a high percentage of women are overweight. The prevalence of obesity is increasing among young, fertile women. Their pregnancies have a high risk of complications. Young, obese women need a highly effective contraceptive method to prevent unplanned pregnancy. Because of the volume and increase in steroid solvent (fat in obese women), oral contraceptives are less effective and the risk of venous thromboembolism is increased [9]. Like oral contraceptives, steroid implants, contraceptive patches and vaginal rings are also less effective in obese women. In these women even a minor surgical procedure is problematic. Abortion is more difficult to carry out and even sterilization has obesity-dependent complications. The World Health Organization gives both the copper-releasing IUD and the LNG-IUS a category 1 rating for obese women, indicating that there are no safety concerns. The interested reader is referred to an excellent editorial in Contraception [10]. The LNG-IUS cannot be used for emergency contraception. If a woman has a cervical or endometrial infection, insertion should be postponed until the infection has been treated. Safety The LNG-IUS has been used by over 8,500,000 women (February 2006) and is marketed as a contraceptive in 107 countries (data from Schering Oy Finland, Information Centre). In the literature there are 1395 scientific reports of the LNG-IUS, none of which mention serious side effects or safety concerns. The LNG-IUS is a well-established contraceptive. However, there are adverse effects related to any contraceptive. During the first months after insertion of the LNG-IUS, the patient may experience transient hormonal side effects including oedema, headache, breast tenderness and acne or other skin problems [11]. Lower abdominal or back pain, vaginal discharge and nausea have also been reported [12]. Functional cysts are typical during the use of progestogen-only contraception, but they vanish spontaneously [13]. Spotting is quite common during the first few months, but by the end of the first year of use 20 35% of women are amenorrhoeic. The reason for the amenorrhoea is the suppression of the endometrium by locally released levonorgestrel. Ovarian function remains unchanged [14]. Before coming to the market, the LNG-IUS was studied in two randomized comparative 5-year trials [12, 15]. Several specific studies were carried out on the effects of use on metabolic events and local microscopic changes in the endometrium during long-term use [16, 17]. All the materials were thoroughly tested and found to be suitable for human use when placed in the uterine cavity. The results of all these studies resulted in approval by all European registration agencies as well as by the United States Food and Drug Administration (FDA). Anaemia and iron stores Compared with the copper-releasing IUD, the LNG- IUS decreases the duration of menstruation and blood loss, thus increasing body iron stores and treating anaemia [18]. Pelvic inflammatory disease The decrease in the duration of menstruation and the associated blood loss, inactivation of the endometrium and possible changes in the cervical mucus help to prevent the progression of cervical infection to endometritis and pelvic inflammatory disease [12, 19]. The rate of infection in young women using the LNG-IUS was significantly lower in comparison with those using the copper-releasing IUD at 3 and 5 years of use. However, insertion-related infections were eliminated by maintaining asepsis during insertion of both devices. This is important to protect future fertility and requires only a minor effort by the attending medical personnel. The recovery of fertility after LNG-IUS removal is rapid and reaches normal values for planned pregnancy [20]. The LNG-IUS is a suitable replacement for irrevocable contraception, sterilization, in women of reproductive age. Lactation Insertion of the LNG-IUS should be performed between 6 and 8 weeks after delivery following these instructions: do not use the sound, release the arms low in the uterine cavity, wait for the arms to open and lift gently to the fundal part of the cavity, then release the device. The levonorgestrel released from the LNG-IUS in the mother s circulation and in her breast milk is very low. Only 0.1% of the maternal daily dose is present in the milk [21 23]. This low amount of levonorgestrel does not harm the development or growth of the newborn [24, 25]. A positive effect of providing safe and effective contraception in breast-feeding mothers was the increase in duration of breast-feeding [24]. Endometrial and cervical safety The LNG-IUS has a strong suppressive effect on the endometrium [16, 17]. The endometrium becomes thin and bleeding decreases. There have been preliminary reports of the LNG-IUS as a therapy for endometrial hyperplasia [26 28]. The suppressive effect on the endometrium is especially beneficial in obese women of reproductive age because of the risk of excessive endometrial proliferation leading to hyperplasia as a result of elevated estradiol production from adipose tissue. Large multicentre studies have detected no differences in cervical cytology between copper-releasing IUD and LNG-IUS users [11]. Gynaecology Forum Vol. 11, No. 2, Medical Forum International
7 Glucose metabolism Contraceptive options for women with insulindependent diabetes mellitus are limited because of concerns about potential vascular and metabolic effects associated with hormonal methods [29]. In a recent study [30], women with insulin-dependent diabetes were randomly assigned to use the LNG-IUS or copper-releasing IUD. It was found that the LNG-IUS had no adverse effect on glucose metabolism. Concern about a potential adverse effect of the LNG-IUS on glucose control is thus unwarranted. Bone mineral density (BMD) Progestogen-only contraception has been found to have adverse effects on bone mineral density (BMD) during long-term use. There is increasing evidence indicating a negative impact of depot medroxyprogesterone acetate on bone health in adolescents [31]. Furthermore, results suggest that low ethinylestradiol-containing (20 μg) oral contraceptive pills are unable to replace normal ovarian estradiol production and have a potentially adverse effect on bone health [31, 32]. On 17 November 2004 the FDA required a warning to be issued with regard to the long-term use of depot medroxyprogesterone acetate. Serial samples of estradiol plasma concentrations have been measured during LNG-IUS use [33, 34]. It was found that even during amenorrhoea ovarian estradiol production was normal as judged by follicular levels in plasma [14]. Ovarian function is equal in both amenorrhoeic and menstruating LNG-IUS users [11]. A decrease in BMD or signs of osteoporosis were not even anticipated because the absence of bleeding is not caused by a lack of estradiol but by the antiproliferative effect of levonorgestrel on the endometrial mucosa. A recent study by Bahamondes et al. [35] reported on the BMD of LNG-IUS users over a 7-year period. No differences in BMD were found between LNG-IUS and copper-releasing IUD users, thus supporting the earlier hypothesis. References 1. Luukkainen T, Pakarinen P, Toivonen J. Progestin-releasing intrauterine systems. Semin Reprod Med 2001; 19: Munuce MJ, Nascimento JA, Rosano G, et al. Doses of levonorgestrel comparable to that delivered by the levonorgestrel-releasing intrauterine system can modify the in vitro expression of zona binding sites of human spermatozoa. Contraception 2006; 73: Nilsson CG, Lähteenmäki PL, Luukkainen T, Robertson DN. Sustained intrauterine release of levonorgestrel over five years. Fertil Steril 1986; 45: Luukkainen T. Levonorgestrel-releasing intrauterine device. Ann N Y Acad Sci 1991; 626: Faundes A, Alvarez F, Díaz J. A Latin American experience with levonorgestrel IUD. Ann Med 1993; 25: Sivin I. Extrauterine pregnancies and intrauterine devices reassessed. In: Bardin CW, Mishell Dr Jr, eds. Proceedings from the Fourth International Conference on IUDs. London: Butterworth-Heinemann, 1994; Lähteenmäki P, Luukkainen T. Return of ovarian function after abortion. Clin Endocrinol (Oxf) 1978; 8: Pakarinen P, Toivonen J, Luukkainen T. Randomized comparison of levonorgestrel- and copper-releasing intrauterine systems immediately after abortion, with 5 years follow-up. Contraception 2003; 68: Abdollahi M, Cushman M, Rosendaal FR. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost 2003; 89: Grimes DA, Shields WC. Family planning for obese women: challenges and opportunities. Contraception 2005; 72: Luukkainen T, Lähteenmäki P, Toivonen J. Levonorgestrel-releasing intrauterine device. Ann Med 1990; 22: Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copperreleasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49: Pakarinen PI, Suvisaari J, Luukkainen T, Lähteenmäki P. Intracervical and fundal administration of levonorgestrel for contraception: endometrial thickness, patterns of bleeding, and persisting ovarian follicles. Fertil Steril 1997; 68: Nilsson CG, Lähteenmäki PL, Luukkainen T. Ovarian function in amenorrheic and menstruating users of a levonorgestrel-releasing intrauterine device. Fertil Steril 1984; 41: Luukkainen T, Allonen H, Haukkamaa M, et al. Five years experience with levonorgestrel-releasing IUDs. Contraception 1986; 33: Nilsson CG, Luukkainen T, Arko H. Endometrial morphology of women using a d-norgestrel-releasing intrauterine device. Fertil Steril 1978; 29: Silverberg SG, Haukkamaa M, Arko H, et al. Endometrial morphology during long-term use of levonorgestrel-releasing intrauterine devices. Int J Gynecol Pathol 1986; 5: Faundes A, Alvarez F, Brache V, Tejada AS. The role of the levonorgestrel intrauterine device in the prevention and treatment of iron deficiency anemia during fertility regulation. Int J Gynecol Obstet 1988; 26: Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol 1991; 77: Belhadj H, Sivin I, Díaz S, et al. Recovery of fertility after use of the levonorgestrel 20 mcg/d or Copper T 380 Ag intrauterine device. Contraception 1986; 34: Heikkilä M. Puerperal insertion of a copper-releasing and a levonorgestrel-releasing intrauterine contraceptive device. Contraception 1982; 25: Heikkilä M, Haukkamaa M, Luukkainen T. Levonorgestrel in milk and plasma of breast-feeding women with a levonorgestrel-releasing IUD. Contraception 1982; 25: Heikkilä M, Nylander P, Luukkainen T. Body iron stores and patterns of bleeding after insertion of a levonorgestrel- or a copper-releasing intrauterine contraceptive device. Contraception 1982; 26: Heikkilä M, Luukkainen T. Duration of breast-feeding and development of children after insertion of a levonorgestrel-releasing intrauterine contraceptive device. Contraception 1982; 25: Shaamash AH, Sayed GH, Hussien MM, Shaaban MM. A comparative study of the levonorgestrel-releasing intrauterine system Mirena versus the Copper T380A intrauterine device during lactation: breast-feeding performance, infant growth and infant development. Contraception 2005; 72: Perino A, Quartararo P, Catinella E, et al. Treatment of endometrial hyperplasia with levonorgestrel releasing intrauterine devices. Acta Eur Fertil 1987; 18: Bahamondes L, Ribeiro-Huguet P, de Andrade KC, et al. Levonorgestrelreleasing intrauterine system (Mirena ) as a therapy for endometrial hyperplasia and carcinoma. Acta Obstet Gynecol Scand 2003; 82: Vereide AB, Kaino T, Sager G, et al. Effect of levonorgestrel IUD and oral medroxyprogesterone acetate on glandular and stromal progesterone receptors (PRA and PRB), and estrogen receptors (ER-alpha and ER-beta) in human endometrial hyperplasia. Gynecol Oncol 2006; 101; World Health Organization. Improving access to quality care in family planning: medical eligibility criteria for contraceptive use. Geneva: WHO, Rogovskaya S, Rivera R, Grimes DA, et al. Effect of a levonorgestrel intrauterine system on women with type 1 diabetes: a randomized trial. Obstet Gynecol 2005; 105: Cromer BA, Stager M, Bonny A, et al. Depot medroxyprogesterone acetate, oral contraceptives and bone mineral density in a cohort of adolescent girls. J Adolesc Health 2004; 35: Rome E, Ziegler J, Secic M, et al. Bone biochemical markers in adolescent girls using either depot medroxyprogesterone acetate or an oral contraceptive. J Pediatr Adolesc Gynecol 2004; 17: Nilsson CG, Lähteenmäki P. Plasma prolactin concentrations in women treated with low dose combination type oral contraceptives and in women using a d-norgestrel-releasing intrauterine device. Ann Clin Res 1978; 10: Nilsson CG, Lähteenmäki P, Luukkainen T. Levonorgestrel plasma concentrations and hormone profiles after insertion and after one year of treatment with a levonorgestrel-iud. Contraception 1980; 21: Bahamondes L, Espejo-Arce X, Hidalgo MM, et al. A cross-sectional study of the forearm bone density of long-term users of levonorgestrel-releasing intrauterine system. Hum Reprod 2006; 21: Medical Forum International 6 Gynaecology Forum Vol. 11, No. 2, 2006
8 Can the levonorgestrelreleasing intrauterine system (Mirena ) be used in nulliparous women? Sarah Prager, Philip Darney Department of Obstetrics, Gynecology and Reproductive Sciences, San Francisco General Hospital, University of California, San Francisco, CA, USA Introduction The levonorgestrel-releasing intrauterine system (LNG-IUS), known as Mirena (or Levonova in Scandinavia), was first introduced in Finland in Since then, it has been approved for use in over 100 countries [1]. In multiple trials it has been shown to be a safe, effective and acceptable method of contraception, with few side effects or negative sequelae [2 4]. Its contraceptive efficacy is comparable to, if not higher than, surgical sterilization, and its use has many non-contraceptive benefits, including improvement in anaemia, menorrhagia and endometriosis, control of uterine fibroids and adenomyosis, and possible protection against and treatment of endometrial hyperplasia and cancer [5, 6]. However, most trials of the LNG-IUS and other forms of intrauterine contraception have limited enrolment to parous women. The limited data in nulliparous women, as well as misunderstandings about the relationship between intrauterine contraception and pelvic inflammatory disease (PID), have caused concerns about its use in nulliparous women. Worries about intrauterine contraception for nulliparous women come largely from misinformation about the risk of infertility associated with intrauterine contraception, but clinicians also worry that it might be less effective and cause more side effects, making it less acceptable to nulliparous women. This article will address the use of the LNG-IUS in nulliparous women with respect to several important outcomes and hopefully dispel these concerns. Mechanical issues Any intrauterine device (IUD) must fit within the contours of the uterine cavity. In general, parous women of reproductive age have higher uterine volume and a greater endometrial surface area than nulliparous and nulligravid women [7]. Clinicians are concerned that a smaller cavity may increase the risk of failure due to expulsion, perforation or early discontinuation in nulliparous compared with parous women. While studies have attempted to estimate the risk of perforation from insertion of intrauterine contraceptives, few have included nulliparous women. The rate of perforation for all women using the LNG-IUS is 0 1.3% [8], and the primary risk factor associated with perforation, lactation [9, 10], is not relevant to nulliparous women. At this time, there are not enough data on nulliparous women specifically to make a statement regarding risk of perforation with the LNG-IUS. The risk of expulsion does not seem to be increased in nulliparous women. A multicentre international trial found that the annual expulsion rate of the LNG- IUS was per hundred (the rates decreasing with increased length of use), but the trial did not include nulliparous women [8]. A Spanish trial of four different copper-releasing IUDs found no difference in expulsion rates by parity [11]. A retrospective cohort study of 129 nulliparous and 332 parous women in the Netherlands found that expulsion rates were 0 1.2% per year for the copper-releasing IUD and 0 0.2% per year for the LNG-IUS [12]. There were no statistically significant differences in expulsion rates between nulliparous and parous women. The LNG- IUS seems unlikely to have a greater risk of perforation or expulsion in nulliparous women, though there are only scant data regarding perforation risks in this population. Efficacy and acceptability The overall efficacy of the LNG-IUS is excellent, with rates ranging from 0 to 1.1 pregnancies per hundred woman-years of use [2, 8, 13]. A recent prospective non-comparative study evaluated efficacy by parity and found no pregnancies at 1 year in either parous or nulliparous women. In this study two different types of levonorgestrel intrauterine systems were used: the Femilis or the Femilis Slim (designed for use in nulliparous women) [13]. However, it is still reasonable to conclude equivalent efficacy, since in other studies of intrauterine contraception, smaller uterine size is not associated with lower efficacy [11]. Gynaecology Forum Vol. 11, No. 2, Medical Forum International
9 Acceptability of the LNG-IUS is evaluated by continuation rate. One study found the 1-year continuation rate in nulliparous women using the LNG-IUS to be 80 per 100, which is comparable to that of multiparous women and better than the continuation rates in nulliparas taking combined oral contraceptive pills [14, 15]. A comparison of side effects experienced by nulliparous women after 1 year of using either the LNG-IUS or the combined oral contraceptive pill revealed significantly less dysmenorrhoea, intermenstrual bleeding and spotting, and significantly fewer total bleeding days in the LNG-IUS group. The LNG- IUS group also had fewer regular cycles when compared with the combined oral contraceptive group, largely due to amenorrhoea [14]. Overall satisfaction at the end of the 1-year study, as indicated by willingness to continue with the same method, was 88% in LNG-IUS users and only 68% in combined oral contraceptive users (p-value 0.003). Data about efficacy of the LNG-IUS in nulliparous women are scant; however they are comparable to data in parous women. Acceptability in nulliparous women appears high when compared with both parous women and nulliparous users of combined oral contraceptives. PID and infertility risks Patients and providers often express concern about the possible relationship between the use of intrauterine contraception and both PID and subsequent infertility. This possible relationship is of particular concern to nulliparous women who do not yet know whether they can become pregnant. Numerous studies in the 1970s and 1980s found an increased risk of PID and infertility in users of intrauterine contraception [16, 17]. Many of these studies were subsequently reanalysed and it was found that most of the increased risk was associated with one type of intrauterine contraception no longer marketed (Dalkon Shield) and with high-risk sexual behaviour [18 20]. In fact, in many studies, the increased risk of PID was only present in the first 20 days after insertion, indicating undiagnosed cervical infection at the time of insertion. Further, many studies had methodological flaws that introduced bias into the results, such as comparing women using intrauterine contraception with those taking combined oral contraceptive pills (who have a decreased risk of PID compared with non-users) [21]. An important error in the early studies was equating nulliparity with high-risk sexual behaviour. Since young women are more likely to acquire sexually transmitted cervical infections, and because young age is associated with nulliparity, many studies erroneously concluded that the increased risk of PID and infertility was attributed to nulliparity. We now realize, based on more recent evidence, that women with high-risk sexual behaviour and sexually transmitted infections at the time of intrauterine contraception placement are at increased risk, and that age and nulliparity were surrogates for cervical infection at the time of insertion of the contraceptive. A case-control study in nulliparous Mexican women who were seeking treatment for primary infertility found no association between tubal infertility and past intrauterine contraceptive use. In this study, 358 women with primary infertility and documented tubal occlusion (cases) were compared with two sets of controls: 953 nulliparous women with primary infertility and no tubal occlusion and 584 primigravid women. Past use of a copper-releasing IUD was not associated with tubal occlusion when compared with either infertile women without tubal occlusion or primigravid controls (p-values 1.0 and 0.9, respectively) [22]. However, tubal infertility was associated with a past Chlamydia infection (as evidenced by Chlamydia antibodies). This study further supports an association between PID and infertility and cervical infection, not IUD use. While this study in nulliparous women evaluated the relationship between tubal infertility and copper-releasing IUD use, it is reasonable to extrapolate the findings of no association between intrauterine contraception and infertility to the LNG-IUS, for which studies have shown the expected protective effect of other hormonal contraceptives. Not only does the LNG-IUS not increase the risk of PID, there is physiological and clinical evidence that the LNG-IUS may be protective against PID. One of the primary physiological effects of progestogen contraception is thickening of the cervical mucus, which protects against ascending genital tract infection and is the cause of decreased PID in women who use combined oral contraceptives, progestogen implants or progestogen injectables [23]. Further, a randomized controlled trial comparing the LNG-IUS with a copper-releasing IUD (Nova-T ) in parous and nulliparous women found that the cumulative 36-month rate of PID was lower in users of the LNG-IUS when compared with users of the Nova-T (0.5 and 2.0, respectively; p < 0.013) [4], especially in women under the age of 25. Though a subanalysis in nulliparous women was not reported, the subjects include nulliparous women, making these data applicable to this population. In spite of limited direct evidence about the relationship between infertility and LNG-IUS use in nulliparous women, we can conclude that nulliparous intrauterine contraceptive users are at no increased risk of infection and infertility over that of multiparous intrauterine contraceptive users. In fact, it is the women who have a sexually transmitted infection present in their cervix at the time of insertion who are at increased risk of PID within the first 20 days after insertion. Thus, it is important to assess each woman s risk of infection based on her sexual behaviour (and not on parity) to screen at the time of, or prior to, insertion, and to treat cervicitis. If amplified DNA screening for Chlamydia or gonococcal infection is positive at the time of insertion, the intrauterine contraceptive can be left in place during treatment as long as upper tract infection does not develop [21] Medical Forum International 8 Gynaecology Forum Vol. 11, No. 2, 2006
10 Postabortal use Between 39% and 52% of all abortions are obtained by nulliparous women [24, 25]. Most women who present for abortion have experienced a contraceptive failure and are at high risk of another, thus making them good candidates for a highly efficacious contraceptive method such as an intrauterine method. Placement of an intrauterine contraceptive immediately following an abortion is ideal, given an already dilated cervix, minimal additional discomfort for the patient, certainty that the patient is not pregnant, and peace of mind for the patient in knowing that her method is in place and requires no further intervention except for string checks. However, there has been concern that postabortal placement of an intrauterine contraceptive might lead to perforation, expulsion and infection. A Cochrane review on this topic indicated that the cumulative net probability of expulsion after second-trimester procedures is approximately 5- to 10-fold higher than the rate after first-trimester procedures for three different copper-releasing IUDs [26]. A comparison of postabortal insertion with interval insertion (3 5 weeks after procedures) found a higher expulsion rate after immediate insertion, but this difference did not reach statistical significance. Studies support our conclusion that it is safe to offer postabortal placement of the LNG-IUS to nulliparous women. A trial that compared postabortal insertion of the LNG-IUS with insertion of various copper-releasing IUDs found significantly fewer pregnancies after placement of the LNG-IUS [26]. When compared with historical controls, overall rates of PID, perforation and other complications after postabortal insertion were similar [26]. Nulliparous women were included in a few of these studies, but analyses of associations between nulliparity and complications or failure of the LNG-IUS were not reported. One observational study of LNG-IUS insertion in 20 nulliparous women following surgical abortion found that all the subjects who completed the study continued with the LNG- IUS, and therefore concluded that it is an acceptable method of contraception and could be offered to young, single, nulliparous women after abortion [27]. In general, these studies support our conclusion that it is safe to offer postabortal placement of the LNG- IUS to nulliparous women. Conclusion Although we need more information about the benefits and risks of LNG-IUS use in nulliparous women, there are extensive data about the efficacy and safety of the device and of other currently available intrauterine contraceptive methods. In order to provide young women, many of whom are nulliparous, with the most effective methods of contraception we feel that the LNG-IUS may be offered to nulliparous women, since it appears to be both safe and extremely efficacious. References 1. INFO Project, Center for Communication Programs, Johns Hopkins Bloomberg School of Public Health. Intrauterine devices. Available at: April 2005, Series M, Number 19. Accessed April 4, Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copperreleasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49: Luukkainen T, Allonen H, Haukkamaa M, et al. Effective contraception with the levonorgestrel-releasing intrauterine device: 12-month report of a European multicenter study. Contraception 1987; 36: Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol 1991; 77: Hubacher D, Grimes DA. Noncontraceptive health benefits of intrauterine devices: a systematic review. Obstet Gynecol Surv 2002; 57: Varma R, Sinha D, Gupta JK. Non-contraceptive uses of levonorgestrelreleasing hormone system (LNG-IUS) a systematic enquiry and overview. Eur J Obstet Gynecol Reprod Biol 2006; 125: Mishell D. Comprehensive gynecology. 3rd ed. St Louis, MO: Mosby, Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the Copper T380 Ag IUDS. Contraception 1991; 44: Andersson K, Ryde-Blomqvist E, Lindell K, et al. Perforations with intrauterine devices. Report from a Swedish survey. Contraception 1998; 57: Caliskan E. Analysis of risk factors associated with uterine perforation by intrauterine devices. Eur J Contracept Reprod Health Care 2003; 8: Duenas J. Intrauterine contraception in nulligravid vs parous women. Contraception 1996; 53: Veldhuis H. Complications of the intrauterine device in nulliparous and parous women. Eur J Gen Pract 2004; 10: Wildemeerch D. Ease of insertion, contraceptive efficacy and safely of new T-shaped levonorgestrel-releasing intrauterine systems. Contraception 2005; 71: Suhonen S. Clinical performance of a levonorgestrel-releasing intrauterine system and oral contraceptives in young nulliparous women: a comparative study. Contraception 2004; 69: Meirik O, Farley TM, Sivin I. Safety and efficacy of levonorgestrel implant, intrauterine device, and sterilization. Obstet Gynecol 2001; 97: Lee NC, Rubin GL, Ory HW, Burkman RT. Type of intrauterine device and the risk of pelvic inflammatory disease. Obstet Gynecol 1983; 62: World Health Organization Task Force on Intrauterine Devices, Special Programme of Research, Development and Research Training in Human Reproduction. PID associated with fertility regulation agents. Contraception 1984; 30: Lee NC, Rubin GL, Borucki R. The intrauterine device and pelvic inflammatory disease revisited: new results from the Women s Health Study. Obstet Gynecol 1988; 72: Gareen IF, Greenland S, Morgenstern H. Intrauterine devices and pelvic inflammatory disease: meta-analyses of published studies, Epidemiology 2000; 11: Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet 1992; 339: Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet 2000; 356: Hubacher D, Lara-Ricalde R, Taylor DJ, et al. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med 2001; 345: Speroff L, Darney PD. A clinical guide for contraception. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins, Jones RK, Darroch JE, Henshaw SK. Contraceptive use among U.S. women having abortions in Perspect Sex Reprod Health 2002; 34: Bartley J, Tongs S, Everington D, Baird DT. Parity is a major determinant of success rate in medical abortion: a retrospective analysis of 3161 consecutive cases of early medical abortion treated with reduced doses of mifepristone and vaginal gemeprost. Contraception 2000; 62: Grimes D, Schulz K, Stanwood N. Immediate postabortal insertion of intrauterine devices. Cochrane Database Syst Rev 2004; 4: CD Li CF, Lee SS, Pun TC. A pilot study on the acceptability of levonorgestrelreleasing intrauterine device by young, single, nulliparous Chinese females following surgical abortion. Contraception 2004; 69: Gynaecology Forum Vol. 11, No. 2, Medical Forum International
11 Tubal occlusion REVIEW ARTICLES The levonorgestrel-releasing intrauterine system (Mirena ) as an alternative to tubal occlusion Ian Milsom Department of Obstetrics and Gynaecology, Sahlgrenska Academy at Göteborg University and Sahlgrenska University Hospital, Göteborg, Sweden Sterilization by tubal occlusion is a common form of contraception in many countries where it accounts for a large proportion of contraceptive use in women over 30 years of age. In the United States male/female sterilization accounts for more than 40% of contraceptive use (Figure 1) [1] and similar sterilization rates have been reported from several European countries [2, 3]. As tubal occlusion is intended to be a permanent form of sterilization, it is considered important that an adequate time interval elapses between counselling and the performance of the surgical procedure in order to ensure a well-considered decision. Thus, tubal occlusion is not a method that can be readily used in an acute situation, as it is important that the woman carefully contemplates her decision before undergoing the surgical procedure [4]. Tubal sterilization is considered to be a highly effective form of contraception, but there is nevertheless a risk of pregnancy, which varies according to the sterilization technique used [5]. The cumulative 10-year probability of pregnancy has been reported to be highest after clip sterilization (36.5/1000 procedures) and lowest after unipolar coagulation (7.5/1000 and postpartum partial salpingectomy (7.5/1000). The cumulative risk of pregnancy has been reported to be highest among women sterilized at a younger age with bipolar coagulation (54.3/1000) and clip application (52.1/1000) [5]. Although a relatively simple procedure, like all surgical procedures it is associated with a risk of complications during surgery. The lowest rate of complications has been reported with laparoscopy ( %); laparotomy has approximately twice the risk of complications [4]. The most common complication arises when there is a need to convert a laparoscopic procedure to a laparotomy because of an inability to complete the sterilization laparoscopically, with most studies describing a 1% incidence. Burns can occur from accidental electrocoagulation resulting in late perforation and peritonitis. Both with laparoscopy Contraceptive use (%) Age Pill IUD Implant Male/female sterilization Injectable None Other Figure 1: Contraceptive use in the United States in 1999, grouped by age (data from [1]). and laparotomy there is a risk of wound infections and/or wound dehiscence (Table I). The mortality rate has been reported to be per 100,000 operations, half of which is due to complications from general anaesthesia and the other half to the operative procedure [4]. Despite counselling, a large proportion of women regret tubal sterilization. The best predictor of regret and a desire for re-anastomosis is age. In addition, it has also been reported that, despite counselling, a large proportion of women regret the 2006 Medical Forum International 10 Gynaecology Forum Vol. 11, No. 2, 2006
12 Table I: Complications of female sterilization [4]. Complication % At laparoscopy Mesosalpingeal tears 0.3 Bleeding 0.3 Bowel burns, which may result in late perforation and peritonitis 0.6 Necessary to convert to laparotomy <1.0 Wound infections or dehiscence 1.0 Total complication rate At laparotomy Total complication rate Late sequelae Regret 2 years post-sterilization 2.7 procedure [4]. It has been shown that the best predictor of regret and a desire for re-anastomosis is age: the younger the woman s age at sterilization the greater the risk of regret. There are also reports that the procedure may cause more long-term somatic symptoms such as pain and bleeding disturbances [4 7]. The occurrence of menstrual disturbances has been shown to vary according to the type of procedure performed. Adverse menstrual changes occurred significantly more often after cauterization and with the Pomeroy technique [6]. The levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) Table III: Cumulative gross termination rates at 60 months reported in a prospective randomized trial comparing the LNG-IUS with a copperreleasing IUD (Nova-T ) [9]. Nova-T LNG-IUS p-value Pregnancy <0.001 Expulsion NS Bleeding problems <0.01 Amenorrhoea <0.001 Pain NS Hormonal side effects <0.001 Pelvic inflammatory disease <0.05 Other medical NS Personal NS Continuation rate NS The levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) has been shown to be a highly effective form of long-term reversible contraception (Table II). In a large European randomized multicentre study [9], the efficacy of the LNG-IUS was compared with that of a copper-releasing intrauterine device (IUD; Nova-T ). The cumulative 5-year gross pregnancy rate was 5.9 for the copper-releasing IUD and 0.5 for the LNG-IUS. Thus, the contraceptive efficacy of the LNG-IUS does not differ greatly from that reported after female sterilization. Women who have an LNG-IUS fitted often experience irregular bleeding after insertion of the device. However, the bleeding is usually scanty and in many cases results in amenorrhoea after a variable period of time. Some women experience hormonal side effects for a short period of time after insertion such as breast tenderness and nausea. The cumulative gross termination rates at 60 months reported in the prospective comparison between the LNG-IUS and a copperreleasing IUD are shown in Table III. There are also several non-contraceptive benefits with the LNG-IUS such as a reduction in menstrual blood loss [12] and dysmenorrhoea [12]. In a study comparing the LNG-IUS with a copper-releasing IUD it was shown that women fitted with an LNG-IUS had an increase in haemoglobin concentration (+2 g/l), whereas women fitted with a copper-releasing IUD had a decrease in haemoglobin ( 3 g/l) following IUD insertion [12]. Return to fertility after removal of the LNG-IUS has also been studied and, although the system induces suppression of the endometrium and in many cases amenorrhoea, the return to fertility was no different from that in women who had been fitted with a copper-releasing IUD [13]. Thus, it is apparent from these data that the LNG- IUS is an equally effective contraceptive method as tubal sterilization but with the advantage of being reversible. In addition, many women also experience the advantage of being amenorrhoeic. In a long-term Table II: Gross cumulative pregnancy rates in four randomized clinical trials comparing the LNG-IUS with different copper-releasing IUDs. Trial Women (n) Follow-up (years) Pregnancy rate (%) Nilsson et al. [8] LNG-IUS Copper IUD Andersson et al. [9] LNG-IUS Copper IUD Sivin et al. [10] LNG-IUS Copper IUD Indian Council of Medical Research [11] LNG-IUS Copper IUD Gynaecology Forum Vol. 11, No. 2, Medical Forum International
13 Effectiveness (%) Cost (US $) LNG-IUS IUD 3-month injectable Tubal ligation Oral contraception Diaphragm Figure 2: Comparison of the cost-effectiveness of various methods of contraception (adapted from [16]). study of the LNG-IUS over 12 years as many as 60% of the women were amenorrhoeic and it was shown to be a safe and effective method of contraception, providing the women at the same time with a prolonged relief of menstrual problems [14]. In the age group where tubal sterilization is most frequently used, a significant proportion of women suffer from menorrhagia, but tubal sterilization does not protect these women from this bleeding disturbance. In contrast, the LNG-IUS offers the advantage of being a simultaneous form of treatment for heavy menstrual bleeding [15] as well as offering effective contraception. In contrast to tubal occlusion the LNG-IUS provides a reversible form of contraception with the added advantage for many women of reduced menstrual blood loss or even amenorrhoea. The cost-effectiveness of various contraceptive techniques has been studied and the LNG-IUS has been shown to be one of the most cost-effective contraceptives available [16]. According to Chiou et al. [16], the effectiveness of tubal ligation and the LNG- IUS was comparable, but the LNG-IUS was less expensive (Figure 2). At present a large percentage of women in the United States and Europe rely on sterilization as their method of contraception. According to a cost-effectiveness analysis performed by Chiou et al. [16], if these women utilized the LNG-IUS instead there would be a considerable economic saving with approximately the same contraceptive efficacy. Conclusion The LNG-IUS is a highly effective form of contraception, with a long-term efficacy comparable to that of tubal occlusion. After insertion of the device many women experience irregular bleeding and some experience hormonal side effects. In contrast to tubal occlusion the LNG-IUS provides a reversible form of contraception with the added advantage for many women of reduced menstrual blood loss or even amenorrhoea. This is particularly important for women in the later stages of the fertile period where menorrhagia is more common. The LNG-IUS is, in addition, a cost-effective form of long-term contraception that has been reported to be superior to that reported after tubal sterilization. References 1. MacKay AP, Kieki BA Jr, Koonin LM, Beattie K. Tubal sterilization in the United States Fam Plann Perspect 2001; 33: Oddens BJ, Vemer HM, Visser AP, Ketting E. Contraception in Germany: a review. Adv Contracept 1993; 9: Oddens BJ, Visser AP, Vemer HM, et al. Contraceptive use and attitudes in Great Britain. Contraception 1994; 49: Kovacs L. Sterilisation. In: Milsom I, ed. Contraception and family planning. European practice in gynaecology and obstetrics, series 8. Edinburgh: Elsevier, 2006; Peterson HB, Xia Z, Hughes JM et al. The risk of pregnancy after tubal sterilization: findings from the US Collaborative Review of Sterilization. Am J Obstet Gynecol 1996; 174: Rosenfeld BL, Taskin O, Kafkashli A et al. Menstrual, psychosexual, psychological and somatic sequelae following postpartum sterilisation. J Obstet Gynaecol 1998; 18: Shain RN, Miller WB, Mitchell GW et al. Menstrual pattern change 1 year after sterilization: results of a controlled, prospective study. Fertil Steril 1989; 52: Nilsson CG, Luukkainen T, Díaz J, Allonen H. Intrauterine contraception with levonorgestrel: a comparative randomised clinical performance study. Lancet 1981; 1: Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copperreleasing (NovaT) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49: Sivin I, Stern J, Coutinho E, et al. Prolonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and Copper T380 Ag IUDS. Contraception 1991; 44: Indian Council of Medical Research. Task Force on IUD. Randomised clinical trial with intrauterine devices (levonorgestrel intrauterine device (LNG), CuT 380Ag, CuT 220C and CuT 200B). A 36-month study. Contraception 1989; 39: Nilsson C-G. Medicated IUDs. In: Milsom I, ed. Contraception and family planning. European practice in gynaecology and obstetrics, series 8. Edinburgh: Elsevier, 2006; Andersson K, Batar I, Rybo G. Return to fertility after removal of a levonorgestrel-releasing intrauterine device and Nova-T. Contraception 1992; 46: Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand 1999; 78: Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol 1991; 164: Chiou CF, Trussell J, Reyes E, et al. Economic analysis of contraceptives for women. Contraception 2003; 68: Medical Forum International 12 Gynaecology Forum Vol. 11, No. 2, 2006
14 The levonorgestrel-releasing intrauterine system (Mirena ) in the treatment of menorrhagia Bilian Xiao National Research Institute for Family Planning, Beijing, People s Republic of China Introduction There are numerous reports in the literature on the use of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) for contraception and the treatment of gynaecological disorders. The first reports of its therapeutic effect on menorrhagia came from Europe. However, use of the LNG-IUS has enormous potential to improve women s health in developing countries, where protection against pregnancies and reproductive health are both important to quality of life. The LNG-IUS is a contraceptive method that can effectively fulfil both functions. Studies in Europe As the LNG-IUS was first developed in Finland for contraceptive purposes, the device has been widely used in European countries such as Finland, Sweden, the Netherlands and the UK. The therapeutic effect of the LNG-IUS in the treatment of menorrhagia was carefully evaluated in a Swedish study [1] which measured menstrual blood loss (MBL) before and after insertion of the device and in comparison with copper-bearing intrauterine devices and other medical treatments. Andersson and Rybo [1] studied 20 healthy parous women with regular periods and MBL >80 ml who were treated with the LNG-IUS. MBL was measured at 3, 6 and 12 months after insertion of the device. A significant reduction in MBL of 86% at 3 months and 97% at 12 months was reported and blood haemoglobin and serum ferritin were significantly increased. In another randomized study comparing the LNG- IUS with crossover oral treatment with flurbiprofen and tranexamic acid in 35 women with idiopathic menorrhagia [2], the LNG-IUS was inserted for 12 months and flurbiprofen and tranexamic acid were used for 2 months each. MBL decreased by 21% with flurbiprofen and by 44% with tranexamic acid, but with the LNG-IUS, MBL was reduced by 82% after 3 months and by 96% after 12 months. This difference was highly significant. A study in the Netherlands by Scholten [3] on 11 women for 12 months showed a similar reduction in MBL and an increase in haemoglobin and serum ferritin. Studies in developing countries The LNG-IUS is not widely used for the treatment of menorrhagia in developing countries except in China and Brazil. China The device was first introduced in China as a contraceptive method in Many studies on clinical performance [4], ovarian function [5, 6] and the endometrium [7] were subsequently carried out. Although all the studies were performed in normal healthy women requesting contraception, the results provided a good insight into the benefits of the LNG- IUS and its mechanism of action, which highlighted its potential in the treatment of menorrhagia. The earliest clinical trial to explore the efficacy and acceptability of the LNG-IUS in the treatment of menorrhagia was carried out in 10 Chinese women in Hong Kong in 1995 [8]. Over a period of 129 patientmonths there was a 54%, 87% and 95% reduction in MBL after 1, 3 and 6 months of treatment, respectively. The reduction was highly significant. There was a median increase in menstrual cycle length of 12 days in 9 months, and 15% of the cycles were longer than 60 days. Most bleeding (76%) was in the form of spotting. These menstrual changes were acceptable to the women, who preferred the device to hysterectomy. Studies of the treatment of idiopathic menorrhagia were begun in Beijing in 1999 [9]. A 3-year protocol was designed with the objective of investigating the effect of the LNG-IUS in the treatment of idiopathic menorrhagia among Chinese women. Thirty-four patients aged years with regular menstrual cycles and heavy bleeding (>80 ml) were recruited. The patients were followed up at 3-monthly intervals for the first year and then annually for 3 years. MBL, blood haemoglobin and serum ferritin were measured before and after insertion at 6, 12, 24 and 36 months. Gynaecology Forum Vol. 11, No. 2, Medical Forum International
15 Table I: Mean (SD) changes in MBL, haemoglobin and serum ferritin before and after LNG-IUS insertion. (Data from [9].) MBL a Haemoglobin Serum ferritin n ml n g/l n ng/ml Before insertion ± ± ± 16.7 After insertion (months) ± ± 14.4 * ± ± ± ± ± ± ± ± ± ± ± ± 50.3 p < throughout, except for * p < a Many cases with amenorrhoea or scanty spotting could not be measured. A total of 1125 post-insertion patient-months were assessed. No intrauterine or extrauterine pregnancies occurred during the 3-year follow-up period. There were two complete expulsions: one during the first month and the other on the second day after insertion due to heavy bleeding. Two partial expulsions were observed on pelvic examination: the lower end of the vertical stem was seen to protrude from the cervical os at 6-month and 15-month check-ups. The devices were removed by the attending doctor. Two patients were lost to follow-up when they moved away from the area. There were two premature removals at 7 months due to prolonged spotting and fatigue, and another at 16 months due to persistent amenorrhoea lasting 7 months. The main outcome results were changes in MBL, which reduced by 78.7%, 83.8%, 97.7% and 85.0% at 6, 12, 24 and 36 months, respectively. Haemoglobin and serum ferritin increased between pre- and post-insertion. The differences were highly significant. The results are shown in Table I. The reduction in MBL in each patient during the course of 3 years is shown in Figure 1. Due to the local effect of progestogens on the endometrium, the prominent feature of the menstrual pattern is amenorrhoea and irregular spotting. Almost one-third of the patients experienced amenorrhoea after 3 months of treatment, which lasted in some cases for more than 6 months. Irregular spotting in the first 3 months was also a frequent occurrence. Measurement of the endocrinological profile during the menstrual cycle showed no or slight suppression of ovarian function, even in patients with amenorrhoea. Slight nausea and breast tenderness were experienced by a few patients. No serious hormonal side effects occurred and no medication was required. Brazil The LNG-IUS was first used for contraception in 256 women in Campinas in A pilot study of the use of the LNG-IUS in 44 women with menorrhagia was published in 2002 [10]. Menstrual patterns were assessed subjectively by the patients and haemoglobin was measured at 3, 6, 9 and 12 months. In the first 3 months spotting was the most common feature, but after 6, 9 and 12 months the majority of women presented with amenorrhoea or oligomenorrhoea. There were six expulsions and one request for removal due to spotting. Haemoglobin levels increased significantly. The overall continuation rate at 12 months was 70.5%. The authors concluded that the LNG-IUS was a good alternative treatment in 75% of Brazilian women with menorrhagia who were either contraindicated for or refused hysterectomy or endometrial ablation. Health benefits and socioeconomic implications The LNG-IUS in the treatment of menorrhagia is a good option amongst the array of available medical and surgical treatments. This is particularly true in developing countries, where women are often in poor health and afraid of operational procedures. Chinese women would first choose traditional medicine to treat the bleeding and turn to surgery only as a last resort. The introduction of the LNG-IUS in recent years has offered these women an alternative method. It not only decreases MBL but also increases haemoglobin and serum ferritin, which is essential to women s health. MBL (ml) Months Figure 1: Changes in MBL in each patient before (two cycles) and 6, 12, 24 and 36 months after insertion of the LNG-IUS. The reduction in MBL was highly significant at 6 months (p < ), except in one case where MBL remained over 100 ml during the first year of treatment. (Reproduced from [9] with permission from the American Society for Reproductive Medicine.) 2006 Medical Forum International 14 Gynaecology Forum Vol. 11, No. 2, 2006
16 Treatment with the LNG-IUS has special implications for women living in rural areas or on a low income: the LNG-IUS costs much less than hysterectomy or even endometrial resection or ablation. In China, the LNG-IUS, including all medical care services before and after insertion, costs about six times less than hysterectomy. As menorrhagia is one of the most common gynaecological disorders, the reduction in surgical procedures and in potential blood transfusions contributes considerably to reducing the medical burden of a family or a community. Unfortunately the benefits of LNG-IUS use in the developing world are not well recognized. Dissemination of information about its health benefits and socioeconomic implications in the treatment of menorrhagia is therefore essential. Unfortunately the benefits of LNG-IUS use in the developing world are not well recognized. Dissemination of information about its health benefits and socioeconomic implications in the treatment of menorrhagia is therefore essential. References 1. Andersson JK, Rybo G. Levonorgestrel-releasing intrauterine device in the treatment of menorrhagia. Br J Obstet Gynaecol 1990; 97: Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol 1991; 164: Scholten PC. The levonorgestrel IUD: clinical performance and impact on menstruation. Utrecht: University of Utrecht, 1989; Thesis. 4. Gao J, Wang SL, Wu SC, et al. Comparison of the clinical performance, contraceptive efficacy and acceptability of levonorgestrel-releasing IUD and Norplant-2 implants in China. Contraception 1990; 41: Xiao BL, Zhou LY, Zhang XL, et al. Pharmacokinetic and pharmacodynamic studies of levonorgestrel-releasing intrauterine device. Contraception 1990; 41: Xiao B, Zeng T, Wu S, et al. Effect of levonorgestrel-releasing intrauterine device on hormonal profile and menstrual pattern after long-term use. Contraception 1995; 51: Zhu P, Liu X, Luo H, et al. The effect of levonorgestrel-releasing intrauterine device (20 μg/day) (LNG-IUD-20) on the morphological structure of human endometrium: a study of the endometrial factor VIII activity in the women before and after insertion of LNG-IUD-20 by the digital image analysis. Contraception 1995; 52: Tang GW, Lo SS. Levonorgestrel intrauterine device in the treatment of menorrhagia in Chinese women: efficacy versus acceptability. Contraception 1995; 51: Xiao BL, Wu SC, Cong J, et al. Therapeutic effects of the levonorgestrelreleasing intrauterine system in the treatment of idiopathic menorrhagia. Fertil Steril 2003; 79: Monteiro I, Bahamondes L, Díaz J, et al. Therapeutic use of levonorgestrel-releasing intrauterine system in women with menorrhagia: a pilot study (1). Contraception 2002; 65: The levonorgestrel-releasing intrauterine system (Mirena ) in the treatment of leiomyoma-related bleeding Vera Halpern 1, Marina Tarasova 2 1 Family Health International, Research Triangle Park, NC, USA; 2 Ott Institute of Obstetrics and Gynaecology, St Petersburg, Russia Introduction Uterine leiomyomas are the most common benign pelvic tumours found in women. Data on the prevalence of uterine leiomyomas vary greatly, with some studies reporting a prevalence as high as 70% among premenopausal women [1]. Peak incidence occurs in women between 35 and 40 years old, when clinical signs are most likely to manifest. Although leiomyomas are often asymptomatic, approximately 20 40% produce symptoms, leading patients to seek therapy [2]. Almost one-third of women with uterine leiomyomas experience excessive or prolonged menstrual bleeding [3]. Menorrhagia associated with uterine leiomyomas can impair social and sexual activities and lead to lost work time, increased cost of sanitary Gynaecology Forum Vol. 11, No. 2, Medical Forum International
17 Table I: Use of the LNG-IUS in women with uterine leiomyomas: summary of the existing evidence. Study Study design and sample size Study Results duration (months) Singer & Ikomi, 1994 [19] Prospective pilot study of 5 women with menorrhagia 6 18 Reduction in MBL treated with intrauterine progesterone device Fong & Singh, 1999 [20] Case report of a renal transplant patient with 12 Reduction in MBL menorrhagia Increase in Hb Starczewski & Iwanicki, 2000 [21] Prospective non-comparative study of 12 women with 6 12 Reduction in MBL severe menstrual bleeding fitted with the LNG-IUS Normalization of Hb Mercorio et al., 2003 [22] Observational study of 19 women with recurrent 12 Reduction in MBL menorrhagia fitted with the LNG-IUS Decrease in Hb 74% reported persistent menorrhagia at the end of the study Grigorieva et al., 2003 [23] Prospective non-comparative study of 67 women with 12 Reduction in MBL uterine leiomyomas fitted with the LNG-IUS Increase in Hb and ferritin 40% reported amenorrhoea at the end of the study Rosa e Silva et al., 2005 [24] Descriptive case series of 10 women with increased 6 Reduction in MBL uterine bleeding fitted with the LNG-IUS Normalization of Hb and haematocrit Soysal & Soysal, 2005 [25] Prospective, historically controlled study of Similar reduction in MBL menorrhagic women fitted with the LNG-IUS Similar increase in Hb and 32 treated with thermal balloon ablation MBL, Menstrual blood loss; Hb, haemoglobin. protection and the need for medical care [4]. Besides being troublesome, bleeding from leiomyomas can result in severe, sometimes life-threatening, anaemia [5]. Approaches to the treatment of uterine leiomyomas Current approaches to dealing with abnormal uterine bleeding associated with leiomyomas fall into two broad categories. The first is surgical therapy, which ranges from traditional hysterectomy to endoscopic uterine-sparing procedures [6]. Hysterectomy for leiomyoma-related menorrhagia is highly successful. However, the existing data demonstrate that in at least a third of all surgical interventions a normal uterus is removed [7]. Furthermore, this option is unacceptable for women who are considering having children. As opposed to the permanent relief from heavy menstrual bleeding provided by hysterectomy, less invasive surgical interventions such as uterine artery embolization or myomectomy may require further invasive treatment [8, 9]. Uterine leiomyomas are the most common benign pelvic tumours found in women. The second strategy for the management of leiomyoma-related bleeding is medically induced suppression of the endometrium. Despite the long-standing appeal of medical therapy, use of agents such as progesterone, gestrinone and danazol has not been successful [2, 10]. Currently, few drugs are known to effectively reduce the heavy menstrual bleeding associated with these tumours. Administration of long-acting gonadotropin-releasing hormone (GnRH) analogues as well as the antiprogestogen mifepristone profoundly reduces menstrual bleeding and thus normalizes haematological indices and improves anaemia [11, 12]. Unfortunately, after the cessation of GnRH agonist therapy, the uterus and leiomyoma rapidly return to their pretreatment volume; menses return 4 10 weeks after cessation of therapy [13, 14]. The long-term administration of GnRH agonists is limited by bone loss [15] and by the high cost of the drug. The major concerns about long-term administration of antiprogestogens are the possible induction of endometrial hyperplasia and transitory increase in liver enzymes, although data on the long-term safety of mifepristone are scant and conflicting [16, 17]. Studies with the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) in leiomyoma-related bleeding The levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) provides a broad spectrum of non-contraceptive health benefits [18], including possible treatment of leiomyoma and related symptoms (Table I). The exact mechanism of the therapeutic effect of the LNG-IUS is unknown. By rendering the endometrium inactive and insensitive to the prolifer Medical Forum International 16 Gynaecology Forum Vol. 11, No. 2, 2006
18 Menstrual blood loss (PBAC score) * * * Baseline 3 months 6 months 12 months Figure 1: Estimated menstrual blood loss (mean ± SD) before and after insertion of the LNG-IUS ( * p < vs. baseline). PBAC, Pictorial Blood Loss Assessment Chart. (Reproduced from [23] with permission from the American Society for Reproductive Medicine.) ative effects of estradiol, intrauterine release of levonorgestrel reduces the amount and duration of menstrual bleeding. Levonorgestrel stimulates the synthesis of insulin-like growth factor (IGF) binding protein (IGFBP)-1. Suppression of endometrial IGF-I, a mediator of estradiol action in the endometrium, by IGFBP-1 may be one of the molecular mechanisms accounting for the antiestrogenic effects of the LNG- IUS in the endometrium [26]. Changes in uterine artery blood flow accompanying the continuous intrauterine release of levonorgestrel may also explain the induced changes in the endometrium [27]. In a large multicentre Population Council study comparing the LNG-IUS with a copper-releasing intrauterine device, LNG-IUS users had less leiomyoma development and a lower rate of uterine surgery after 5 years of use [28]. Several case reports revealed a reduction in leiomyoma size in women using the LNG-IUS as well as significant reduction in menorrhagia [19, 20]. In spite of a large number of studies investigating the effect of the LNG-IUS on idiopathic menorrhagia [29, 30], few have specifically looked into the therapeutic benefits of the LNG-IUS in women with uterine leiomyomas. Although limited, the existing data suggest that the LNG-IUS is a promising treatment for women with heavy bleeding associated with leiomyomas. Researchers from Poland inserted the LNG-IUS in 12 women with uterine leiomyomas and severe menstrual bleeding and followed them for 6 12 months [21]. Amenorrhoea was reported by 50% of women with intramural tumours after 12 weeks, and the remainder had scanty bleeding. Six months after insertion of the device, haemoglobin levels were normal in 11 women. The biggest case series to date found a dramatic reduction in menstrual blood loss accompanied by an improvement in haematological indices in 67 women with uterine leiomyomas treated with the LNG-IUS for 1 year [23]. A profound reduction in menstrual blood loss occurred within 3 months of insertion (Figure 1) and was accompanied by significant increases in blood haemoglobin and serum ferritin levels. Within 3 months of insertion the mean haemoglobin value significantly increased (13.2 ± 1.3 g/dl vs ± 1.2 g/dl; p < 0.001) and remained significantly higher than baseline values at 6 months (13.7 ± 1.0 g/dl; p < 0.001) and 12 months (13.6 ± 0.9 g/dl; p < 0.001). Serum ferritin levels changed little by 3 months (25 ± 22 ng/ml vs. 24 ± 23 ng/ml) but showed a significant increase at 6 and 12 months (32 ± 24 ng/ml; p < 0.001; 41 ± 28 ng/ml; p < 0.001, respectively). Eighteen out of 19 women (95%) who were anaemic at the start of the study were no longer anaemic at 12 months. Moreover, 40% of participants had amenorrhoea at the end of the study. The finding that the LNG-IUS effectively treated iron-deficiency anaemia in women with menorrhagia associated with leiomyomas was in accord with studies comparing the effectiveness of the LNG- IUS with operative treatments of menorrhagia [31, 32]. Only four women with uterine leiomyomas (6%) expelled the device during the study by Grigorieva et al. [23]. This is similar to the 4% expulsion rate reported by Díaz et al. [33] among 1101 women without preexisting gynaecological conditions fitted with the LNG-IUS for contraception only. Soysal & Soysal [25] were the first to evaluate the use of the LNG-IUS in menorrhagic patients with submucous leiomyomas. The authors compared the device with the results of thermal balloon ablation chosen as a historic control. The LNG-IUS was as effective as the surgical procedure in treating the excessive bleeding and related anaemia. Rosa e Silva et al. [24] inserted the LNG-IUS in 10 patients with uterine myomas complaining of increased uterine bleeding. They observed a significant reduction in bleeding and improvement of the haematological indices following 6 months of LNG- IUS use. Most of these studies reported no LNG-IUSinduced side effects other than abnormal bleeding. Only Grigorieva et al. [23] documented breast tenderness as the most frequently encountered side effect other than irregular spotting. According to WHO guidelines for contraceptive use, only pre-existing uterine fibroids that distort the uterine cavity contraindicate the method. A weakness of most of these studies is the lack of a control group. Despite their uncontrolled nature, the observed changes in bleeding patterns and haematological indices are likely to be the result of the LNG- IUS. Given the natural history of untreated leiomyomas in women of reproductive age, the likelihood of such dramatic improvements in bleeding occurring spontaneously is small. Some researchers expressed concerns in the past about the use of the LNG-IUS by women with uterine Gynaecology Forum Vol. 11, No. 2, Medical Forum International
19 leiomyomas reporting higher rates of expulsion in the presence of uterine fibroids, uterine enlargement, heavy bleeding and anaemia [34]. Mercorio et al. [22] found that menorrhagia persisted in 74% of patients after 1 year of LNG-IUS use. These authors concluded that the LNG-IUS should not be considered in the presence of a large myomatous uterus. While these concerns may warrant further research, they should not deter clinicians from using the intrauterine system in women with uterine leiomyomas. Each clinical case, however, requires careful individual assessment. The LNG-IUS should not be a substitute for surgical treatment where surgery is an acceptable option and will clearly provide a better clinical outcome. According to WHO guidelines for contraceptive use [35], only pre-existing uterine fibroids that distort the uterine cavity contraindicate the method. Until more extensive experience with the LNG-IUS in women with leiomyomas is available, compliance with the WHO eligibility criteria for intrauterine contraceptives in women with uterine leiomyomas seems prudent. Conclusion Uterine leiomyomas pose an enormous global health problem. A safe, effective, reversible, long-term, nonsurgical treatment for symptomatic leiomyomas would be of considerable clinical and public health importance. The LNG-IUS has strong potential for providing effective treatment of the excessive uterine bleeding associated with leiomyomas. That the available evidence of LNG-IUS use in women with uterine leiomyomas continues to be based primarily on retrospective or prospective non-comparative research indicates a long-standing need for a well-designed comparative study. References 1. Day Baird D, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003; 188: Chavez NF, Stewart EA. Medical treatment of uterine fibroids. Clin Obstet Gynecol 2001; 44: Schwartz SM. Epidemiology of uterine leiomyomata. Clin Obstet Gynecol 2001; 44: Stovall DW. Clinical symptomatology of uterine leiomyomas. Clin Obstet Gynecol 2001; 44: Wegienka G, Baird DD, Hertz-Picciotto I, et al. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol 2003; 101: Munro MG. Management of leiomyomas: is there a panacea in Pandora s box? Fertil Steril 2006; 85: 40 3; discussion Clarke A, Black N, Rowe P, et al. Indications for and outcome of total abdominal hysterectomy for benign disease: a prospective cohort study. Br J Obstet Gynaecol 1995; 102: Fedele L, Vercellini P, Bianchi S, et al. Treatment with GnRH agonists before myomectomy and the risk of short-term myoma recurrence. Br J Obstet Gynaecol 1990; 97: Broder MS, Goodwin S, Chen G, et al. Comparison of long-term outcomes of myomectomy and uterine artery embolization. Obstet Gynecol 2002; 100 (5 part 1): Coutinho EM, Boulanger GA, Goncalves MT. Regression of uterine leiomyomas after treatment with gestrinone, an antiestrogen, antiprogesterone. Am J Obstet Gynecol 1986; 155: Friedman AJ, Harrison-Atlas D, Barbieri RL, et al. A randomized, placebo-controlled, double-blind study evaluating the efficacy of leuprolide acetate depot in the treatment of uterine leiomyomata. Fertil Steril 1989; 51: Eisinger SH, Meldrum S, Fiscella K, et al. Low-dose mifepristone for uterine leiomyomata. Obstet Gynecol 2003; 101: Matta WH, Shaw RW, Nye M. Long-term follow-up of patients with uterine fibroids after treatment with the LHRH agonist buserelin. Br J Obstet Gynaecol 1989; 96: Friedman AJ, Rein MS, Harrison-Atlas D, et al. A randomized, placebocontrolled, double-blind study evaluating leuprolide acetate depot treatment before myomectomy. Fertil Steril 1989; 52: Tahara M, Matsuoka T, Yokoi T, et al. Treatment of endometriosis with a decreasing dosage of a gonadotropin-releasing hormone agonist (nafarelin): a pilot study with low-dose agonist therapy ( draw-back therapy). Fertil Steril 2000; 73: Kettel LM, Murphy AA, Morales AJ, et al. Treatment of endometriosis with the antiprogesterone mifepristone (RU486). Fertil Steril 1996; 65: Brown A, Cheng L, Lin S, Baird DT. Daily low-dose mifepristone has contraceptive potential by suppressing ovulation and menstruation: a double-blind randomized control trial of 2 and 5 mg per day for 120 days. J Clin Endocrinol Metab 2002; 87: Hubacher D, Grimes DA. Noncontraceptive health benefits of intrauterine devices: a systematic review. Obstet Gynecol Surv 2002; 57: Singer A, Ikomi A. Successful treatment of fibroids using an intrauterine progesterone device. Int J Gynecol Obstet 1994; 46: Fong YF, Singh K. Effect of the levonorgestrel-releasing intrauterine system on uterine myomas in a renal transplant patient. Contraception 1999; 60: Starczewski A, Iwanicki M. [Intrauterine therapy with levonorgestrel releasing IUD of women with hypermenorrhea secondary to uterine fibroids]. Ginekol Pol 2000; 71: Mercorio F, De Simone R, Di Spiezio Sardo A, et al. The effect of a levonorgestrel-releasing intrauterine device in the treatment of myoma-related menorrhagia. Contraception 2003; 67: Grigorieva VA, Chen-Mok M, Tarasova MA, Mikhailov AV. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril 2003; 79: Rosa e Silva JC, de Sá Rosa e Silva AC, dos Reis FJ, et al. Use of a levonorgestrel-releasing intrauterine device for the symptomatic treatment of uterine myomas. J Reprod Med 2005; 50: Soysal S, Soysal ME. The efficacy of levonorgestrel-releasing intrauterine device in selected cases of myoma-related menorrhagia: a prospective controlled trial. Gynecol Obstet Invest 2005; 59: Rutanen EM. Insulin-like growth factors and insulin-like growth factor binding proteins in the endometrium. Effect of intrauterine levonorgestrel delivery. Hum Reprod 2000; 15 (suppl 3): Jarvela I, Tekay A, Jouppila P. The effect of a levonorgestrel-releasing intrauterine system on uterine artery blood flow, hormone concentrations and ovarian cyst formation in fertile women. Hum Reprod 1998; 13: Sivin I, Stern J. Health during prolonged use of levonorgestrel 20 micrograms/d and the copper TCu 380Ag intrauterine contraceptive devices: a multicenter study. International Committee for Contraception Research (ICCR). Fertil Steril 1994; 61: Xiao B, Wu SC, Chong J, et al. Therapeutic effects of the levonorgestrelreleasing intrauterine system in the treatment of idiopathic menorrhagia. Fertil Steril 2003; 79: Monteiro I, Bahamondes L, Díaz J, et al. Therapeutic use of levonorgestrel-releasing intrauterine system in women with menorrhagia: a pilot study (1). Contraception 2002; 65: Istre O, Trolle B. Treatment of menorrhagia with the levonorgestrel intrauterine system versus endometrial resection. Fertil Steril 2001; 76: Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet 2001; 357: Díaz J, Bahamondes L, Monteiro I, et al. Acceptability and performance of the levonorgestrel-releasing intrauterine system (Mirena ) in Campinas, Brazil. Contraception 2000; 62: Ikomi A, Mansell E, Spence-Jones C, Singer A. Treatment of menorrhagia with the levonorgestrel intrauterine system: can we learn from our failures? J Obstet Gynecol 2000; 20: World Health Organization. Medical eligibility criteria for contraceptive use. 3rd edition. Geneva: WHO, Medical Forum International 18 Gynaecology Forum Vol. 11, No. 2, 2006
20 Quality of life and cost-effectiveness of the levonorgestrel-releasing intrauterine system (Mirena ) and hysterectomy in menorrhagia Ritva Hurskainen Department of Obstetrics and Gynaecology, Hyvinkää Hospital, Hyvinkää, Finland (ritva.hurskainen@hus.fi) Introduction Menorrhagia, usually defined as heavy menstrual bleeding over several consecutive cycles, has a significant impact on many women s lives. Rapid developments in medical technology have delivered new management strategies for menorrhagia, prompting clinicians to consider the cost and effectiveness of the treatments available. Accurate information on the costeffectiveness of the different treatment options would ensure the best health outcomes from the available resources. Menorrhagia is usually a benign disorder and treatment is aimed at improving health-related quality of life (HRQOL). Self-reported HRQOL has been regarded as the most reliable key variable of effectiveness. There are many generic and disease-specific tools for assessing HRQOL, but few of them have a single index value that can be used for economic evaluation. Studies of the cost and effectiveness of the different treatment modalities for menorrhagia are rare. Although hysterectomy is by definition more effective than other treatment modalities for menorrhagia, the morbidity and complication rates associated with hysterectomy cannot be ignored [1]. The levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) offers a truly effective and reversible treatment modality for menorrhagia. A number of recent studies have reported improvements in HRQOL after hysterectomy [2 5] and insertion of the LNG-IUS [6], but few have reported on the cost-effectiveness of these two treatments. Effectiveness of the LNG-IUS and hysterectomy LNG-IUS Traditionally, evaluation of the effectiveness of medical treatments has relied upon measures of morbidity (based on clinical, radiological and laboratory tests) and mortality. The most commonly used outcome reflecting the effectiveness of menorrhagia treatments is the reduction in menstrual blood loss (MBL). However, menorrhagia may have a strong effect on the quality of women s lives, including both physical and psychological health. Social and working abilities may be disrupted, and some women report adverse effects in their family life causing considerable strain on family relationships. Women seek professional help for their menstrual problems mostly because of the deleterious effect on their quality of life. To use millilitres of reduced MBL as the only indicator of effectiveness gives a poor estimate of how women feel after treatment. A more appropriate outcome indicator is HRQOL. The tools assessing HRQOL are normally categorized as generic or disease-specific. Generic measures have increased in popularity, because they enable comparison between different health problems. Disease-specific instruments give more accurate information about a specific health problem; thus there are clear reasons for using both types of instrument side by side. In evaluating the success of treatment of menorrhagia the most commonly used validated HRQOL measurement is the Short Form 36 (SF 36). This is a shortened version of a battery of 149 health status questions developed and tested on a large population. It uses eight scales to measure three aspects of health: functional status, well-being and general evaluation of health. The five-dimensional EuroQol (EQ-5D) is an HRQOL instrument which gives a single number score that in a comprehensive and valid way reflects the total change in HRQOL. It can be used to calculate quality-adjusted life years (QALYs). It includes five dimensions: mobility, self-care, usual activities, pain and mood. Gynaecology Forum Vol. 11, No. 2, Medical Forum International
21 To understand the effectiveness of the LNG-IUS and hysterectomy with regard to HRQOL we must be aware of the clinical effects of these treatments. Controlled trials supported by evidence from observational studies show that the LNG-IUS can significantly reduce MBL (up to 94% after 3 months) and is well accepted by most women [7]. The device also provides contraception comparable to that of sterilization, with fertility returning when the system is removed [8]. It also offers other therapeutic effects that can improve HRQOL. Reduction in dysmenorrhoea has been reported in numerous LNG-IUS trials [9, 10] as well as reduction in premenstrual symptoms [11]. There is also preliminary evidence that the LNG- IUS may be therapeutic in women with fibroids [12, 13], endometriosis [14 16] and adenomyosis [17], which are often connected with heavy bleeding. The LNG-IUS releases 20 μg levonorgestrel per day, resulting in low serum concentrations. However, some hormonal side effects are possible especially in the first 3 months. The LNG-IUS frequently causes irregular vaginal bleeding or spotting and some women experience breast tenderness and bloating [18], all of which increase the failure rate. Most side effects, however, resolve within a relatively short time. Hysterectomy Hysterectomy is one of the most common surgeries performed on women and has been the standard treatment for menorrhagia for many decades. Although hysterectomy is a successful operation with high levels of patient satisfaction, there is a risk of complications. Mortality is relatively low (1 2/1000 operations) [19, 20], but the risk of lesser complications is quite high, occurring in one-third of procedures [5, 21, 22]. Hysterectomy may impair ovarian function and cause early menopausal symptoms [23]. Constipation, symptoms arising from adhesions and urinary problems are all connected with the procedure [24, 25]. Effectiveness comparison Treatment of menorrhagia with hysterectomy and the LNG-IUS is the subject of two Cochrane reviews which evaluated among other effects the impact of menorrhagia treatments on HRQOL [4, 6]. In four trials patients in the medical arm received the LNG-IUS and those in the surgery arm underwent transcervical resection of the endometrium (TCRE), thermal balloon ablation or hysterectomy. At 1 year, there was no statistically significant difference between the study arms in satisfaction rates or quality of life (measured by SF 36 or EQ-5D), but endometrial destruction was less likely to cause adverse effects than the LNG-IUS (OR 0.24, 95% CI ). Hysterectomy stopped all bleeding but caused serious complications for some women. The LNG-IUS was superior to oral medication. EQ-5D score Healthy Finnish Menorrhagic Menorrhagic women women at women after (mean age baseline treatment 43 years) (5 years) Figure 1: EQ-5D scores in healthy and in menorrhagic women (data derived from [22]). Economic studies of the LNG-IUS and hysterectomy Randomized health economic studies of menorrhagia are scarce. There are no economic evaluations comparing medical treatments. Five publications, from three randomized trials, compared the overall cost of endometrial resection vs. hysterectomy [2, 26 29]. All these trials reported that endometrial resection cost less than hysterectomy in a 1-year follow-up. The difference in the cost gap narrowed over time because of the re-treatment rate for women who had undergone endometrial resection. Based on 4 months follow-up [29] the cost of resection was 53% of that of hysterectomy (TCRE 560, hysterectomy 1060); after 12 months follow-up the respective value was 76% ( 1001 and 1315) [28]; after 2.8 years of follow-up it was 71% ( 790 and 1110) [2]; and at 4 years it was 93% ( 1231 and 1332) [26]. Only one randomized large-scale study with 236 women compared the cost-effectiveness of the LNG- IUS vs. hysterectomy over 5 years [22]. This study included costs of health care, medication and lost productivity. The total direct health care cost per patient after 1 year was US $1074 in the LNG-IUS group and US $2489 in the hysterectomy group. When lost productivity was added, the total cost per woman in the LNG-IUS group was US $1530 and in the hysterectomy group US $4222 [30]. Although 42% of women assigned to the LNG-IUS eventually underwent hysterectomy, the discounted direct cost after 5 years was US $1892 with the LNG-IUS and US $2787 with hysterectomy; the total costs were US $2817 and US $4660, respectively, at 2001 prices. HRQOL and psychosocial well-being improved significantly in both groups and there was no difference between the groups. The cost per QALY was US $6190 per woman in the LNG-IUS group and US $9017 per woman in the hysterectomy group (Hurskainen R. Unpublished data), which shows that treatment of menorrhagia is significantly more cost-effective with the LNG-IUS than with hysterectomy Medical Forum International 20 Gynaecology Forum Vol. 11, No. 2, 2006
22 Conclusion Heavy menstrual bleeding is a common complaint, which causes much inconvenience as well as physical, mental and emotional problems to many women. It has a prominent effect on HRQOL [30]. Many studies have shown that treatment of menorrhagia significantly improves quality of life (Figure 1) [1, 22, 31 34]. However, increasing health care costs are a worldwide phenomenon and there is a clear need for strategies that produce net cost savings. Data from the 1970s to the 1990s suggest that menstrual complaints are on the increase [22] and call for greater resources. Most of this increase is in menorrhagia. In western countries, about 5% of women of reproductive age seek help annually for menorrhagia [35]. Hysterectomy is one of the most frequently performed major surgical procedures for menorrhagia and therefore the consequences concern a large number of women and a large amount of money. The LNG-IUS seems to offer a good and reversible alternative to hysterectomy. The cost-effectiveness of the LNG-IUS seems to be significantly better than that of hysterectomy. Over 5 years the LNG-IUS offers the same improvement in quality of life as hysterectomy, but the costs are 40% lower [22]. In Finland about 10,000 hysterectomies are performed each year. At least 3000 of these are due to menorrhagia, which could also be treated with the LNG-IUS. If all those women were hysterectomized, the cost of treatment (including sick leave and complications) would be about 14 million. If all those women were first treated with the LNG-IUS and then only those who were not satisfied (42%) were hysterectomized, the treatment cost would be about 8.5 million. By adopting the latter strategy we could save at least 5.5 million without impairing the HRQOL of menorrhagic women. These data are from a study by Hurskainen et al. [22], which included patients who were referred to hospital for hysterectomy. The savings could be even greater if the LNG-IUS were inserted in primary care before referral. It would be a straightforward matter to issue care guidelines recommending LNG-IUS insertion before referral to a specialist if there are no contraindications. Many women prefer less invasive surgical treatment even when they are aware that the success of the treatment is not always assured [36]. The majority of menorrhagic women scheduled for endometrial ablation or LNG-IUS insertion are inclined to risk a 50% likelihood of treatment failure to avoid a hysterectomy [36]. References 1. O Connor H, Broadbent JA, Magos AL, McPherson K. Medical Research Council randomised trial of endometrial resection versus hysterectomy in management of menorrhagia. Lancet 1997; 349: Sculpher MJ, Dwyer N, Byford S, Stirrat GM. Randomised trial comparing hysterectomy and transcervical endometrial resection: effect on health related quality of life and costs two years after surgery. Br J Obstet Gynaecol 1996; 103: Coulter A, Peto V, Jenkinson C. Quality of life and patient satisfaction following treatment for menorrhagia. Fam Pract 1994; 11: Marjoribanks J, Lethaby A, Farquhar C. Surgery versus medical therapy for heavy menstrual bleeding. Cochrane Database Syst Rev 2003; 2: CD Garry R. Health economics of hysterectomy. Best Pract Res Clin Obstet Gynaecol 2005; 19: Lethaby A, Cooke I, Rees M, Lethaby A. Progesterone or progestogenreleasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev 2005; 4: CD Stewart A, Cummins C, Gold L, et al. The effectiveness of the levonorgestrel-releasing intrauterine system in menorrhagia: a systematic review. Br J Obstet Gynaecol 2001; 108: French R, Vliet H, Cowan F, et al. Hormonally impregnated intrauterine systems (IUSs) versus other forms of reversible contraceptives as effective methods of preventing pregnancy. Cochrane Database Syst Rev 2004; 3: CD Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand 1999; 78: Luukkainen T, Allonen H, Haukkamaa M, et al. Five years experience with levonorgestrel-releasing IUDs. Contraception 1986; 33: Barrington JW, Bowen-Simpkins P. The levonorgestrel intrauterine system in the management of menorrhagia. Br J Obstet Gynaecol 1997; 104: Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril 2003; 79: Starczewski A, Iwanicki M. [Intrauterine therapy with levonorgestrel releasing IUD of women with hypermenorrhea secondary to uterine fibroids.] Ginekol Pol 2000; 71: Lockhat FB, Emembolu JO, Konje JC. The evaluation of the effectiveness of an intrauterine-administered progestogen (levonorgestrel) in the symptomatic treatment of endometriosis and in the staging of the disease. Hum Reprod 2004; 19: Vercellini P, Frontino G, De Giorgi O, et al. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril 2003; 80: Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod 2005; 20: Maia H Jr, Maltez A, Coelho G, et al. Insertion of Mirena after endometrial resection in patients with adenomyosis. J Am Assoc Gynecol Laparosc 2003; 10: Lethaby AE, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medication for heavy menstrual bleeding. Cochrane Database Syst Rev 2000; 2: CD Wingo PA, Huezo CM, Rubin GL, et al. The mortality risk associated with hysterectomy. Am J Obstet Gynecol 1985; 152 (7 part 1): Virtanen HS, Makinen JI. Mortality after gynaecologic operations in Finland, Br J Obstet Gynaecol 1995; 102: Dicker RC, Greenspan JR, Strauss LT, et al. Complications of abdominal and vaginal hysterectomy among women of reproductive age in the United States. The Collaborative Review of Sterilization. Am J Obstet Gynecol 1982; 144: Hurskainen R, Teperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. J Am Med Assoc 2004; 291: Halmesmaki K, Hurskainen R, Tiitinen A, et al. A randomized controlled trial of hysterectomy or levonorgestrel-releasing intrauterine system in the treatment of menorrhagia effect on FSH levels and menopausal symptoms. Hum Reprod 2004; 19: McPherson K, Herbert A, Judge A, et al. Self-reported bladder function five years post-hysterectomy. J Obstet Gynaecol 2005; 25: Danforth KN, Townsend MK, Lifford K, et al. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol 2006; 194: Aberdeen Endometrial Ablation Trials Group. A randomised trial of endometrial ablation versus hysterectomy for the treatment of dysfunctional uterine bleeding: outcome at four years. Br J Obstet Gynaecol 1999; 106: Gannon MJ, Holt EM, Fairbank J, et al. A randomised trial comparing endometrial resection and abdominal hysterectomy for the treatment of menorrhagia. Br Med J 1991; 303: Cameron IM, Mollison J, Pinion SB, et al. A cost comparison of hysterectomy and hysteroscopic surgery for the treatment of menorrhagia. Eur J Obstet Gynecol Reprod Biol 1996; 70: Sculpher MJ, Bryan S, Dwyer N, et al. An economic evaluation of transcervical endometrial resection versus abdominal hysterectomy for the treatment of menorrhagia. Br J Obstet Gynaecol 1993; 100: Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet 2001; 357: Gynaecology Forum Vol. 11, No. 2, Medical Forum International
23 31. Cooper KG, Bain C, Parkin DE. Comparison of microwave endometrial ablation and transcervical resection of the endometrium for treatment of heavy menstrual loss: a randomised trial. Lancet 1999; 354: Cooper KG, Jack SA, Parkin DE, Grant AM. Five-year follow up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss: clinical and quality of life outcomes. Br J Obstet Gynaecol 2001; 108: Crosignani PG, Vercellini P, Mosconi P, et al. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstet Gynecol 1997; 90: Alexander DA, Naji AA, Pinion SB, et al. Randomised trial comparing hysterectomy with endometrial ablation for dysfunctional uterine bleeding: psychiatric and psychosocial aspects. Br Med J 1996; 312: Vessey MP, Villard-Mackintosh L, Painter R. Epidemiology of endometriosis in women attending family planning clinics. Br Med J 1993; 306: Bourdrez P, Bongers MY, Mol BW. Treatment of dysfunctional uterine bleeding: patient preferences for endometrial ablation, a levonorgestrelreleasing intrauterine device, or hysterectomy. Fertil Steril 2004; 82: The levonorgestrel-releasing intrauterine system (Mirena ) in endometriosis and dysmenorrhoea Luis Bahamondes, Carlos A. Petta Human Reproduction Unit, Department of Obstetrics and Gynaecology, School of Medicine, Universidade Estadual de Campinas (UNICAMP), Campinas, Brazil Endometriosis affects almost 10% of women of reproductive age. Seventy to ninety percent of women with chronic pelvic pain, dysmenorrhoea, dyspareunia, infertility or menstrual disturbances have endometriosis [1], a disease that impairs their quality of life [2]. Gonadotropin-releasing hormone analogues (GnRHa) Surgical and medical therapies have been used to treat chronic pelvic pain associated with endometriosis, and gonadotropin-releasing hormone analogues (GnRHa) are the gold standard drug treatment. Treatment is based on reducing lesions or suppressing ovarian estrogen production. However, adherence and long-term treatment continue to represent challenges in the management of endometriosis. Because of the profound hypoestrogenism provoked by GnRHa, their use is limited to 6 months, although longer treatment with add-back hormone therapy is possible. Furthermore, GnRHa are expensive and not readily available to women worldwide, especially in developing countries [3]. The levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) Because GnRHa provoke hypoestrogenism and can lead to bone loss, new therapeutic options, including the levonorgestrel-releasing intrauterine system (LNG- IUS; Mirena ), are being investigated [4]. The mechanism of action of the LNG-IUS on endometriosis is unknown but appears to differ from that of GnRHa, since the LNG-IUS neither inhibits ovulation in the majority of women nor provokes hypoestrogenism [5]; moreover, the concentration of levonorgestrel is high only inside the uterus [6]. Levonorgestrel is believed to act on endometriosis lesions by depleting the estrogen and progesterone receptors through inhibition of the synthesis and expression of estrogen receptors, resulting in an antiproliferative effect, glandular atrophy and decidualization. An increase in apoptosis and a reduction in Bcl-2 protein, an inhibitor of apoptosis in the endometrial stroma, have also been described. In addition, the effect of the LNG-IUS on pain control may occur through the reduction of local vascular angiogenesis, reduction in the congestion of pelvic vessels and an increase in apoptosis [7]. Although endometriosis is a frequent cause of infertility, not all patients with endometriosis wish to conceive and some contraceptive methods, including the LNG-IUS, have been used to control pain [4]. Previous studies have suggested that the LNG-IUS offers effective relief from chronic pelvic pain, dyspareunia and dysmenorrhoea associated with endometriosis and adenomyosis, and improves the staging of endometriosis [7 10]. Efficacy studies In two studies, Vercellini et al. [9, 10] evaluated the efficacy of the LNG-IUS in the treatment of recurrent dysmenorrhoea in women following surgery for endometriosis. Both studies reported a drop in visual 2006 Medical Forum International 22 Gynaecology Forum Vol. 11, No. 2, 2006
24 analogue scale (VAS) pain measurements (1 100) from 76 to 34 after 12 months of use. 8 7 LNG-IUS GnRHa The LNG-IUS neither inhibits ovulation in the majority of women nor provokes hypoestrogenism; moreover, the concentration of levonorgestrel is high only inside the uterus. Fedele et al. [8] evaluated the effectiveness of the LNG-IUS in controlling pain and lesion size in women with endometriosis of the rectovaginal septum. Dysmenorrhoea, pelvic pain and deep dyspareunia improved, and the size of the endometriotic lesions reduced significantly on ultrasonographic evaluation. Lockhat et al. [11] evaluated the LNG-IUS over a 6- month period in 34 women with minimal to moderate endometriosis. Among the 29 women who completed the study, significant improvements were observed in the severity and frequency of pain and in the staging of the disease, with 68% of users opting to continue using the LNG-IUS beyond 6 months. Side effects were assessed and pelvic pain was evaluated using a VAS (1 10) in the same women after 3 years of LNG- IUS use [7]. The continuation rate at 36 months was 56%. Most of the discontinuations were due to unacceptable bleeding disturbances and persistent pain. However, these studies were either non-comparative or in comparison with expectant management only. Our group conducted a study in which 82 women with endometriosis were randomly assigned to the LNG-IUS (n = 39) or to GnRHa (n = 43) [12]. All the patients presented with cyclic chronic pelvic pain with or without dysmenorrhoea and all had a VAS (1 10) pain score >3 during the pretreatment cycle. In the pretreatment month, the VAS pain score was 7.3 ± 0.3 (mean ± SEM) in both groups, while 17 women in each treatment group had initial VAS pain scores >3 and <7, and 22 and 26 women in the LNG-IUS and GnRHa groups, respectively, had VAS pain scores >7. A significant reduction in pain score was observed by the end of the first treatment month and this therapeutic effect persisted throughout the 6 months of the study with no statistically significant differences between the two groups. Between baseline and visit 6, there was a six-point decrease in VAS pain score in both patient groups and no significant difference between the groups (Figure 1). No relationship was found between the reduction in pain score at each month of observation and the VAS pain score observed at baseline in either treatment group. Moreover, women with stage III or IV endometriosis experienced a significantly faster improvement in VAS pain score in comparison with women with stage I or II of the disease. Bleeding patterns were evaluated daily in all patients. Amenorrhoea occurred earlier in the group VAS pain score Baseline Months Figure 1: Variation in pain score in each treatment group during 6 months of evaluation (p = NS). of GnRHa users, with 34% and 71% of patients reporting amenorrhoea by the second treatment month in the LNG-IUS and GnRHa groups, respectively. At the end of 6 months of observation, 70% and 98% of users of the LNG-IUS and GnRHa, respectively, were amenorrhoeic (Figure 2). Side effects of abdominal distension or peripheral oedema were similar in both treatments groups; however, more symptoms of breast tenderness were observed in LNG-IUS users. Although the pain score and menstrual bleeding reduced significantly in both treatment groups, there was no significant increase in the Psychological General Well-Being Index in either group at 6 months of follow-up. Discussion Due to the high prevalence of the disease there has been an increased demand for the treatment of endometriosis-related chronic pelvic pain, dysmenorrhoea and dyspareunia. In addition, although endometriosis is linked with infertility, many women wish to postpone pregnancy and others are diagnosed only after their family is already complete. For these women, therapeutic options must be found that offer pain relief, bleeding control and long-term treatment at an affordable cost and with minimal side effects. GnRHa remain the gold standard treatment of endometriosis-associated chronic pelvic pain, mainly because they promote anovulation, hypoestrogenism with amenorrhoea, and a reduction in endometriotic lesions. However, the hypoestrogenism induced by the medication places limitations on its use, principally because of the need to restrict the duration of treatment, the risk of bone loss with consequent osteopenia or osteoporosis, and side effects such as mood changes and vaginal dryness [13]. Although the daily Gynaecology Forum Vol. 11, No. 2, Medical Forum International
25 Amenorrhoea (%) GnRHa LNG-IUS Months Figure 2: Occurrence of amenorrhoea in each treatment group during 6 months of treatment. dose of levonorgestrel in LNG-IUS users is low without peaks in plasma concentration, we cannot ignore the presence of side effects in these users, mainly ascribed to hormonal influence. The more commonly reported were acne, other skin problems, weight changes, nausea, headache, depression, other mood changes and breast tenderness [14]. The three side effects which more frequently accounted for discontinuation were depression, acne and headache; however, the discontinuation rates were low [15]. The LNG-IUS offers several advantages for the treatment of endometriosis and pain control. Although in our study, treatment was limited to 6 months, other studies have evaluated the device over a period of 12 months [11] and 3 years following insertion [7]. Since the LNG-IUS has been approved for 5 years of use, its therapeutic effect is potentially much longer lasting. However, no data on long-term use have been published. The LNG-IUS permits the same system to be used for at least 5 years with no modifications in estrogen levels and consequently few side effects, while at the same time providing highly effective contraception. The rate of release of levonorgestrel from the LNG- IUS is 20 μg/day during the first year, slowly decreasing throughout the 5 years of use [5]. Despite the small amount of levonorgestrel released by the device, amenorrhoea is achieved in approximately 60% of women after 6 months of use and this percentage is maintained throughout the duration of use. Therefore, based on the induced endometrial atrophy and amenorrhoea, it is possible to speculate that the LNG-IUS may be effective in controlling chronic pelvic pain for the same period of time. It is important to note that the longer the effect on pain control, the greater the cost-effectiveness of the LNG-IUS. Moreover, the market cost of the LNG-IUS in countries such as Brazil is only a little higher than the cost of one ampoule of GnRHa. Amenorrhoea was established earlier in GnRHa users than in LNG-IUS users, and the LNG-IUS users experienced light, irregular bleeding during the initial months of use (although with pain control), similar to that observed in users of the device for contraception [16]. However, at 6 months of follow-up, 70% of the women were amenorrhoeic and would probably remain so throughout their use of the method. For women with endometriosis who need long-term treatment, the LNG-IUS may be the treatment of choice, since it permits the same system to be used for at least 5 years with no modifications in estrogen levels and consequently few side effects, while at the same time providing highly effective contraception. Its short-term efficacy was similar to that of GnRHa, but its long-term use requires further evaluation. References 1. Ling F, Pelvic Pain Study Group. Randomized controlled trial of depot leuprolide in patients with chronic pelvic pain and clinically suspected endometriosis. Obstet Gynecol 1999; 93: Marques AA, Bahamondes L, Aldrighi JM, Petta CA. Life quality of women with endometriosis assessed through a medical outcome questionnaire, Short-Form 36 (SF-36), in a cohort study of Brazilian women. J Reprod Med 2004; 49: Gambone JC, Mittman BS, Munro MG, et al., for the Chronic Pelvic Pain/Endometriosis Working Group. Consensus statement for the management of chronic pelvic pain and endometriosis: proceedings of an expert-panel consensus process. Fertil Steril 2002; 78: Fedele L, Berlanda N. Emerging drugs for endometriosis. Exp Opin Emerg Drugs 2004; 9: Luukkainen T, Lähteenmäki P, Toivonen J. Levonorgestrel-releasing intrauterine device. Ann Med 1990; 22: Nilsson CG, Haukkamaa M, Vierola H, Luukkainen T. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol 1982; 17: Lockhat FB, Emembolu JO, Konje JC. The efficacy, side-effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intra-uterine administered progestogen (levonorgestrel): a 3 year follow-up. Hum Reprod 2005; 20: Fedele L, Bianchi S, Zanconato G, et al. Use of a levonorgestrel-releasing intrauterine device in the treatment of rectovaginal endometriosis. Fertil Steril 2001; 75: Vercellini P, De Giorgi O, Oldani S, et al. Depot medroxyprogesterone acetate versus an oral contraceptive combined with very-low-dose danazol for long-term treatment of pelvic pain associated with endometriosis. Am J Obstet Gynecol 1996; 175: Vercellini P, Aimi G, Paonazza S, et al. A levonorgestrel-releasing intrauterine system for the treatment of dysmenorrhea associated with endometriosis: a pilot study. Fertil Steril 1999; 72: Lockhat FB, Emembolu JO, Konje JC. The evaluation of the effectiveness of an intrauterine-administered progestogen (levonorgestrel) in the symptomatic treatment of endometriosis and in the staging of the disease. Hum Reprod 2004; 19: Petta CA, Ferriani RA, Abrao MS, et al. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Hum Reprod 2005; 20: Prentice A, Deary AJ, Goldbeck-Wood S, et al. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst Rev 2000; 2: CD Luukkainen T, Toivonen J. Levonorgestrel-releasing IUD as a method of contraception with therapeutic properties. Contraception 1995; 52: Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copperreleasing (Nova T) IUDs during five years of use: a randomized comparative trial. Contraception 1994; 49: Hidalgo M, Bahamondes L, Perrotti M, et al. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena ) up to two years. Contraception 2002; 65: Medical Forum International 24 Gynaecology Forum Vol. 11, No. 2, 2006
26 The levonorgestrel-releasing intrauterine system (Mirena ) in the peri- and postmenopause David Sturdee Department of Obstetrics and Gynaecology, Heart of England NHS Foundation Trust, Solihull Hospital, Solihull, UK Introduction Women in the perimenopausal years are amongst the most frequent attenders for gynaecological advice, and especially concerning menorrhagia or dysfunctional uterine bleeding. The gradual decline in the control of menstruation prior to the menopause, together with an increasing menstrual loss (Figure 1), can have a major impact on quality of life. Unsatisfactory bleeding is also one of the commonest causes of dissatisfaction with hormone replacement therapy (HRT), which can often be resolved by using a regimen with continuous progestogen in addition to estrogen to keep the endometrium permanently suppressed. The levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) is a most logical and satisfactory measure for resolving both these problems in peri- and postmenopausal women. The perimenopause Irregular dysfunctional uterine bleeding in the perimenopause can often be regulated by oral cyclical progestogen therapy, but regular and heavier periods do not respond well to this regimen and the intrauterine delivery of progestogen is usually more effective. Blood loss (ml) Age (years) Figure 1: Mean menstrual blood loss in women approaching the menopause. In a randomized comparative trial of women with menorrhagia treated with either the LNG-IUS or oral norethisterone 5 mg 8 hourly from days 5 to 26, there was a similar reduction in blood loss after 3 months, but the continuation rates were significantly different. In the LNG-IUS group 17 out of 22 (77%) wished to continue, whereas in the norethisterone group only 4 out of 18 (22%) continued with treatment, indicating a strong preference for the intrauterine treatment [1]. The combination of good control of the menstrual loss and reliable contraception means that the LNG-IUS is becoming increasingly popular for perimenopausal women for whom hysterectomy has for too long provided the only definitive relief from unwanted menstrual problems and pregnancy. The common clinical dilemma of the 45- to 50-year-old woman with distressing and restricting menorrhagia for whom the timing of the menopause is indeterminable, and yet the prospect of possibly an unnecessary hysterectomy is unsatisfactory, can often be resolved by the LNG- IUS. In recent years there has been a welcome gradual reduction in the rates of hysterectomy just for dysfunctional uterine bleeding. This is partly due to wider education on the merits of more conservative managements such as oral tranexamic acid, endometrial ablation techniques and also the LNG-IUS. A long-term follow-up study of 50 women who were on the waiting list for hysterectomy because of menorrhagia reported that 67% managed to avoid hysterectomy by using the LNG-IUS [2]. This could have considerable health cost-savings if such a practice were adopted across large populations. More recent evidence was reported by Hurskainen and colleagues [3] from a randomized controlled trial of 236 women with menorrhagia at a mean age of 43 years. Participants were randomly assigned to treatment with either the LNG-IUS (n = 119) or hysterectomy (n = 117) and were monitored for 5 years. At the end of the study, the two groups did not differ significantly in terms of health-related quality of life (HRQOL) or psychosocial well-being. Although 42% (n = 50) of the LNG-IUS group eventually underwent hysterectomy, Gynaecology Forum Vol. 11, No. 2, Medical Forum International
27 Days without bleeding * the discounted direct and indirect costs in the LNG- IUS group at US $2817 (95% CI ) per participant remained substantially lower than in the hysterectomy group at US $4660 (95% CI ). Satisfaction with treatment was similar in both groups, but the conclusion was that by improving HRQOL at relatively low cost, the LNG-IUS offers a wider availability of choices for the patient and may decrease costs due to interventions involving surgery. Transition to the postmenopause LNG-IUS Oral therapy ** Months of treatment Figure 2: Bleeding profile with the LNG-IUS vs. oral continuous combined therapy with estradiol and norethisterone acetate (*p < 0.05, **p < 0.001). (Reprinted from [5] with permission from Elsevier.) * Many women are using the LNG-IUS for contraception during the years prior to the menopause, but now that the device has been licensed in many countries also for use as the progestogen component of continuous combined HRT regimens, it can provide a convenient transition from contraception to HRT [4]. Traditionally women requiring HRT in the perimenopause are advised to use a sequential estrogen and progestogen regimen, which should give a regular and controlled withdrawal bleed. However, bleeding is one of the commonest reasons for dissatisfaction with HRT, and for longer term use and for better protection of the endometrium the continuous combined estrogen and progestogen therapy (CCEPT) regimens are advocated. Such regimens provide the same benefits of symptom relief as a sequential regimen but avoid cyclical bleeding. As with the use of the LNG-IUS for the control of menorrhagia, after the initial insertion it may take several months for the full effect on bleeding to be realized and women must be encouraged to persevere, but thereafter the bleeding is similar to that of other forms of CCEPT. Figure 2 shows the bleeding profile of women using the LNG- IUS together with a transdermal patch delivering 20 μg estradiol (n = 20) compared with oral estradiol valerate 2 mg combined with norethisterone acetate 1 mg daily (n = 20). For the initial 3 months there was more bleeding in the LNG-IUS group, but thereafter the bleeding was similar in the two groups [5]. In addition, use of sequential HRT regimens for over 5 years has been associated with an increased risk of endometrial carcinoma [6], whereas CCEPT does not increase the risk and may even provide added protection against endometrial carcinoma [7]. Further evidence on the endometrial suppressive effect of intrauterine progestogen comes from a study of 40 postmenopausal women using the LNG-IUS with oral or transdermal estradiol. Endometrial thickness decreased from 5 mm (range 0 14 mm) at baseline to 2.4 mm (range mm) after 5 years. Of the women completing 5 years of therapy, 64% were totally amenorrhoeic [8]. A more recent randomized study of postmenopausal women taking oral estradiol valerate 2 mg daily and using either the LNG-IUS providing continuous progestogen or oral sequential medroxyprogesterone acetate showed a reduction in endometrial proliferation with the LNG-IUS and an increase in progestogen-induced atrophy (Table I). After 6 months, 54 (98%) of the 55 women had no bleeding. Riphagen [10] reviewed the intrauterine application of progestogens in HRT and reported on 16 studies of LNG-IUS use in combination with different types and routes of estrogen in over 800 women. Durations of therapy ranged from 6 months to 5 years and in none of them was there any case of endometrial hyperplasia or carcinoma. He concluded that these data confirm the endometrial safety of this application. The apparent increased risk of breast cancer when progestogen is added to estrogen in HRT regimens as reported by the Women s Health Initiative [11] and Table I: Endometrial histology and thickness at 12 months with the LNG-IUS vs. sequential oral medroxyprogesterone acetate in postmenopausal women taking continuous estradiol valerate [9]. LNG-IUS Medroxyprogesterone acetate (n = 55) (n = 47) No. of women experiencing atrophy 2 (4%) 3 (6%) No. of women experiencing proliferation 0 18 (38%) No. of women experiencing progestogen-induced atrophy 53 (96%) 26 (55%) Endometrial thickness (mm) Medical Forum International 26 Gynaecology Forum Vol. 11, No. 2, 2006
28 the Million Women Study [7] has raised concern about the relative merits of additional progestogen. These studies relate to oral and transdermal administration and with the LNG-IUS the circulating plasma levels of levonorgestrel are much lower than with oral or transdermal HRT regimens. Recent data from the Finnish Cancer Registry provide reassurance that LNG-IUS users have the same incidence of breast cancer as the general population [12]. Conclusions The LNG-IUS is especially suitable for the perimenopausal woman for the control of dysfunctional uterine bleeding. It can be continued through the menopausal transition after which estrogen therapy may be added by any route that is favoured to provide continuous combined HRT for the relief of menopausal symptoms. The LNG-IUS can also provide the most reliable contraception during the menopausal transition at a time when other methods may be less satisfactory. After insertion in the premenopausal woman or as part of an HRT regimen, there may be some initial irregular bleeding, but with perseverance this will usually resolve. References 1. Irvine GA, Campbell-Brown MB, Lumsden MA, et al. Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for the treatment of idiopathic menorrhagia. Br J Obstet Gynaecol 1998; 105: Nagrani R, Bowen-Simpkins P, Barrington JW. Can the levonorgestrel intrauterine system replace surgical treatment for the management of menorrhagia? Br J Obstet Gynaecol 2002; 109: Hurskainen R, Teperi J, Rissanen P, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for the treatment of menorrhagia: randomized trial 5-year follow-up. J Am Med Assoc 2004; 291: Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand 1999; 78: Raudaskoski T, Lahti EI, Kauppila AJ, et al. Transdermal estrogen with a levonorgestrel-releasing intrauterine device for climacteric complaints: clinical and endometrial responses. Am J Obstet Gynecol 1995; 172 (1 part 1): Weiderpass E, Adami HO, Baron JA, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst 1999; 91: Million Women Study Collaborators. Endometrial cancer and hormone replacement therapy in the Million Women Study. Lancet 2005; 365: Varila E, Wahlström T, Rauramo I. A 5-year follow up study on the use of a levonorgestrel intrauterine system in women receiving hormone replacement therapy. Fertil Steril 2001; 76: Raudaskoski T, Tapanainen J, Tomás E, et al. Intrauterine 10 microgram and 20 microgram levonorgestrel systems in postmenopausal women receiving oral oestrogen replacement therapy: clinical, endometrial and metabolic response. Br J Obstet Gynaecol 2002; 109: Riphagen FE. Intrauterine application of progestins in hormone replacement therapy: a review. Climacteric 2000; 3: Writing Group for the Women s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women s Health Initiative randomized controlled trial. J Am Med Assoc 2002; 288: Backman T, Rauramo I, Jaakkola K, et al. Use of the levonorgestrel-releasing intrauterine system and breast cancer. Obstet Gynecol 2005; 106: Endometrial effects of levonorgestrel intrauterine delivery Takeshi Maruo Department of Obstetrics and Gynecology, Kobe University Graduate School of Medicine, Kobe, Japan Introduction The levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena ) was originally developed as a contraceptive but has also been shown to be effective in achieving a significant reduction in menstrual blood loss [1, 2]. This non-contraceptive benefit of the LNG-IUS implies that it may be a safe, non-surgical alternative in the management of menorrhagia. We observed that use of the LNG-IUS resulted in a striking reduction in menorrhagia in all women with adenomyosis and intramural myomas except for women with submucous myomas [3, 4]. Although some women with large intramural myomas had spontaneous expulsion of the device at various intervals, they nevertheless requested reinsertion because of the remarkable reduction in menorrhagia, which resulted in significant increases in haemoglobin levels (Figure 1). By contrast, in menorrhagic women with adenomyosis there were no spontaneous expulsions of the device during 3 years of use and significant increases in haemoglobin levels were recorded 3 months after insertion. Thus, the LNG-IUS has been proven to be an effective modality in the long-term management of menorrhagia due to adenomyosis and uterine myomas except for submucous myomas. These clinical experiences prompted us to characterize the effects of levonorgestrel on endometrial proliferation and apoptosis. Gynaecology Forum Vol. 11, No. 2, Medical Forum International
29 Haemoglobin (g/dl) ± ± ± ± ± 0.91 p < 0.01 p < 0.01 p < 0.01 p < 0.01 Effects of the LNG-IUS on the endometrium The effects of the LNG-IUS on endometrial proliferation and apoptosis were evaluated by proliferating cell nuclear antigen (PCNA) expression, apoptosis, Fas and Bcl-2 protein expression in the endometrium, which were determined at the early proliferative phase of the menstrual cycle before and 3 months after insertion in menorrhagic women with adenomyosis [5]. PCNA, immunolocalized both in the nuclei of the endometrial glands and stroma, became less abundant 3 months after insertion (Figure 2). Determination of the mean percentages of PCNA-positive nuclei in the endometrial glands and endometrial stroma showed that the PCNA-positive rate, both in the endometrial glands and endometrial stroma, was significantly lower after 3 months of LNG-IUS use (p < 0.05). Apoptotic nuclei assessed by the TUNEL method were noted on the endometrial surface and in the endometrial glands and stroma. The apoptotic nuclei in the endometrium seemed more abundant 3 months after insertion (Figure 3). Quantitative analysis of apoptosis on the basis of TUNEL analysis showed that the apoptosis-positive rate in the nuclei of the endometrial glands and endometrial stroma obtained 3 months after insertion was significantly (p < 0.05) higher. Before LNG-IUS insertion, immunohistochemical staining of Fas antigen in the endometrium was scarcely apparent, being only slightly visible in the endometrial glands. The immunostaining intensity of Fas antigen became predominant in both the endometrial glands and stroma 3 months after insertion (Figure 4). On the other hand, Bcl-2 protein, immunolocalized in the cytoplasm of the endometrial glands but not in the stroma, became scanty 3 months after insertion. These results demonstrate that LNG-IUS use resulted in a decrease in endometrial proliferation and in an increase in apoptosis in endometrial glands and stroma. Discussion Before Months after insertion Figure 1: Haemoglobin levels in blood before and after insertion of the LNG-IUS in menorrhagic women with intramural myomas. Levonorgestrel released locally from the LNG-IUS has been shown to inhibit the expression of both estrogen and progesterone receptors in the human endometrium [6]. This decrease in estrogen and progesterone receptors in the endometrium was suggested as a contributory mechanism to the amenorrhoea associated with LNG-IUS use. By contrast, Pakarinen et al. [7] reported that the local release of levonorgestrel abolished cyclical changes in the endometrium in relation to the menstrual cycle and reduced endometrial thickness, as evidenced by ultrasonography. Levonorgestrel caused atrophy of the endometrial glands and decidualization of the stroma [8, 9]. Thinning of the mucosa was evident, and the stroma became swollen during LNG-IUS use. Based on the remarkable reduction in PCNA-positive cells in the endometrium during LNG- IUS use, it is now evident that insertion results in a significant decrease in the proliferative activity of endometrial glands in menorragic women with adenomyosis. The role of apoptosis in the endometrium in menstrual cycle regulation has been reported. Apoptosis was detected in the early proliferative phase, mainly in the glandular epithelium with few positive cells in the stroma, but decreased during the secretory phase of the menstrual cycle [10]. In our study [5], although minimal apoptosis was detected in the endometrial samples taken in the early proliferative phase before LNG-IUS insertion, there was a significant increase in apoptosis-positive nuclei in the endometrium 3 months after LNG-IUS insertion. In accordance with the increased apoptosis in the endometrium, Fas expression in the endometrium became prominent 3 months after insertion, not only in the glandular cells but also in the stromal cells. This finding was of significant interest in understanding the molecular basis of the increased apoptosis that occurs in the endometrium during LNG-IUS use. Consistent with this observation, minimal Bcl-2 protein expression was detected in the endometrial curettage samples obtained during the early proliferative phase before insertion, which became less detectable 3 months after insertion [10]. Decreased Bcl-2 protein expression in the endometrium after 3 months of LNG-IUS use may also be linked to the increased apoptosis that occurs in the endometrium during LNG-IUS use. Although no differences in the levels of expression of Bcl-2, Fas and caspase-3 in the endometrium were noted between levonorgestrel implant (Norplant ) users with and without breakthrough bleeding [11], the lack of effect of levonorgestrel on the endometrium in Norplant users might be due to much lower concentrations of levonorgestrel in the endometrial tissues compared with those in LNG-IUS users. The current study demonstrates a decline in proliferative activity, associated with an increase in apoptosis, in the endometrial glands and stroma after LNG- IUS insertion. The increased apoptosis in the endometrium after 3 months of LNG-IUS use was congruent with a remarkable increase in Fas antigen expression, together with a decline in Bcl-2 protein expression in the endometrium. As Fas antigen is a mediator of apoptosis, and Bcl-2 protein is an apopto Medical Forum International 28 Gynaecology Forum Vol. 11, No. 2, 2006
30 2A 3A 2B 3B Figure 2: Immunohistochemical localization of PCNA before (2A) and 3 months after (2B) LNG-IUS insertion. PCNA expression in the endometrium appeared less abundant 3 months after insertion. E, Endometrial gland epithelium; S, endometrial stroma. Scale bar = 10 μm. Figure 3: Apoptosis as assessed by TUNEL method before (3A) and 3 months after (3B) LNG-IUS insertion. The apoptosis-positive nuclei in the endometrium appeared more abundant 3 months after insertion. E, Endometrial gland epithelium; S, endometrial stroma. Scale bar = 10 μm. sis-inhibiting gene product, the increased apoptosis in the endometrial glands and stroma that occurs during LNG-IUS use may represent an underlying molecular mechanism that causes atrophic changes of the endometrium, leading in turn to the remarkable improvement of menorrhagia. This may explain the molecular basis of the beneficial effect of LNG-IUS on menorrhagia. 4A 4B Figure 4: Immunohistochemical localization of Fas antigen in the endometrium before (4A) and 3 months after (4B) LNG-IUS insertion. Fas antigen expression became more abundant in both the endometrial glands and stroma 3 months after LNG-IUS insertion. E, Endometrial gland epithelium; S, endometrial stroma. Scale bar = 10 μm. Gynaecology Forum Vol. 11, No. 2, Medical Forum International
Product Information. Confidence that lasts
 Confidence that lasts What is Mirena? Inhibition of sperm motility and function inside the uterus and the fallopian tubes, preventing fertilization (Videla-Rivero et al. 1987). Section of system Levonorgestrel
Confidence that lasts What is Mirena? Inhibition of sperm motility and function inside the uterus and the fallopian tubes, preventing fertilization (Videla-Rivero et al. 1987). Section of system Levonorgestrel
What s New in Adolescent Contraception?
 What s New in Adolescent Contraception? Abby Furukawa, MD Legacy Medical Group Portland Obstetrics and Gynecology April 29, 2017 Objectives Provide an update on contraception options for the adolescent
What s New in Adolescent Contraception? Abby Furukawa, MD Legacy Medical Group Portland Obstetrics and Gynecology April 29, 2017 Objectives Provide an update on contraception options for the adolescent
1. Attia AM et al. Role of the levonorgestrel intrauterine system in effective contraception. Patient Prefer Adherence 2013; 7:
 1 2 1. Attia AM et al. Role of the levonorgestrel intrauterine system in effective contraception. Patient Prefer Adherence 2013; 7: 777 85. 3 1. Wu JP et al. Extended use of the intrauterine device: a
1 2 1. Attia AM et al. Role of the levonorgestrel intrauterine system in effective contraception. Patient Prefer Adherence 2013; 7: 777 85. 3 1. Wu JP et al. Extended use of the intrauterine device: a
Application for inclusion of levonorgestrel - releasing IUD for contraception in the WHO Model List of Essential Medicines
 Application for inclusion of levonorgestrel - releasing IUD for contraception in the WHO Model List of Essential Medicines 1. Summary statement of the proposal for inclusion LNG-IUS is an effective contraceptive;
Application for inclusion of levonorgestrel - releasing IUD for contraception in the WHO Model List of Essential Medicines 1. Summary statement of the proposal for inclusion LNG-IUS is an effective contraceptive;
International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage:
 Review Article ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com A REVIEW ON INTRAUTERINE DEVICES Boddu Venkata Komali* 1, M. Kalyani
Review Article ISSN: 2319 9563 International Journal of Research in Pharmaceutical and Nano Sciences Journal homepage: www.ijrpns.com A REVIEW ON INTRAUTERINE DEVICES Boddu Venkata Komali* 1, M. Kalyani
Instruction for the patient
 WS 4 Case 3 STI and IUD Your situation Instruction for the patient You are 32 years old, divorced and have one child; you have just started a new relationship You underwent surgical resection of the left
WS 4 Case 3 STI and IUD Your situation Instruction for the patient You are 32 years old, divorced and have one child; you have just started a new relationship You underwent surgical resection of the left
Levosert levonorgestrel 20mcg/24hour intrauterine device
 Levosert levonorgestrel 20mcg/24hour intrauterine device Verdict: Formulary inclusion: Formulary category: Restrictions: Reason for inclusion: Link to formulary: Link to medicine review summary: Levosert
Levosert levonorgestrel 20mcg/24hour intrauterine device Verdict: Formulary inclusion: Formulary category: Restrictions: Reason for inclusion: Link to formulary: Link to medicine review summary: Levosert
2
 1 2 3 1. Usinger KM et al. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol 2016; 29: 659 67. 2. Kost K et al. Estimates of contraceptive
1 2 3 1. Usinger KM et al. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol 2016; 29: 659 67. 2. Kost K et al. Estimates of contraceptive
1. Ortiz, M. E et al. Mechanisms of action of intrauterine devices. Obstet & Gynl Survey 1996; 51(12), 42S-51S.
 1 2 1. Ortiz, M. E et al. Mechanisms of action of intrauterine devices. Obstet & Gynl Survey 1996; 51(12), 42S-51S. The contraceptive action of all IUDs is mainly in the uterine cavity. The major effect
1 2 1. Ortiz, M. E et al. Mechanisms of action of intrauterine devices. Obstet & Gynl Survey 1996; 51(12), 42S-51S. The contraceptive action of all IUDs is mainly in the uterine cavity. The major effect
Intrauterine delivery of progestogen in the peri- and postmenopausal women. Outline of the presentation. Levonorgestrel releasing IUS - Mirena
 QuickTime ja Valokuva - JPEG pakkauksen purkuohjelma tarvitaan elokuvan katselemiseen. Intrauterine delivery of progestogen in the peri- and postmenopausal women ESHRE Campus meeting 6.-7.10.2008 Oskari
QuickTime ja Valokuva - JPEG pakkauksen purkuohjelma tarvitaan elokuvan katselemiseen. Intrauterine delivery of progestogen in the peri- and postmenopausal women ESHRE Campus meeting 6.-7.10.2008 Oskari
LEARNING OBJECTIVES. Beyond the Pill: Long Acting Contraception. Distribution Of Contraception Use By Women In The Us. Unintended Pregnancy is Common
 4:15 5 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
4:15 5 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system)
 FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system) Mirena does not protect against HIV infection (AIDS) and other sexually transmitted infections
FDA-Approved Patient Labeling Patient Information Mirena (mur-ā-nah) (levonorgestrel-releasing intrauterine system) Mirena does not protect against HIV infection (AIDS) and other sexually transmitted infections
Unintended Pregnancy is Common LEARNING OBJECTIVES. Distribution Of Contraception Use By Women In The Us. Unintended Pregnancy And Contraceptive Use
 3:45 4:30 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
3:45 4:30 pm Beyond the Pill: Long Acting Contraceptives and IUDs Presenter Disclosure Information The following relationships exist related to this presentation: Christine L. Curry, MD, PhD: No financial
Carl Gustaf Nilsson, M.D. t:j: Hannu Allonen, M.D. Juan Diaz, M.D.II Tapani Luukkainen, M.D., Ph.D.t
 FERTILITY AND STERILITY Copyright 0 1983 The American Fertility Society Vol. 39, No.2, February 1983 Printed in U.8A. Two years' experience with two levonorgestrel-releasing intrauterine devices and one
FERTILITY AND STERILITY Copyright 0 1983 The American Fertility Society Vol. 39, No.2, February 1983 Printed in U.8A. Two years' experience with two levonorgestrel-releasing intrauterine devices and one
1. Attia AM et al. Role of the levonorgestrel intrauterine system in effective contraception. Patient Prefer Adherence 2013; 7:
 1 2 1. Attia AM et al. Role of the levonorgestrel intrauterine system in effective contraception. Patient Prefer Adherence 2013; 7: 777 85. 3 1. Wu JP et al. Extended use of the intrauterine device: a
1 2 1. Attia AM et al. Role of the levonorgestrel intrauterine system in effective contraception. Patient Prefer Adherence 2013; 7: 777 85. 3 1. Wu JP et al. Extended use of the intrauterine device: a
levonorgestrel 13.5mg intrauterine delivery system (Jaydess ) SMC No. (1036/15) Bayer
 levonorgestrel 13.5mg intrauterine delivery system (Jaydess ) SMC No. (1036/15) Bayer 6 March 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
levonorgestrel 13.5mg intrauterine delivery system (Jaydess ) SMC No. (1036/15) Bayer 6 March 2015 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and advises
Zurich Open Repository and Archive
 University of Zurich Zurich Open Repository and Archive Winterthurerstr. 190 CH-8057 Zurich http://www.zora.uzh.ch Year: 2008 Partial and complete expulsion of the Multiload 375 IUD and the levonorgestrel-releasing
University of Zurich Zurich Open Repository and Archive Winterthurerstr. 190 CH-8057 Zurich http://www.zora.uzh.ch Year: 2008 Partial and complete expulsion of the Multiload 375 IUD and the levonorgestrel-releasing
Dr. Russo reports no financial relationships relevant to this article. Dr Creinin is a senior clinical advisor for Medicines360.
 CONTRACEPTION Demand for long-acting reversible contraception is growing, including in adolescents and nulliparas. We need to challenge our historical reservations about the IUD and heed the call. Jennefer
CONTRACEPTION Demand for long-acting reversible contraception is growing, including in adolescents and nulliparas. We need to challenge our historical reservations about the IUD and heed the call. Jennefer
LARC: Disclosures. Long Acting Reversible Contraception. Objectives 10/23/2013. I have no relevant financial disclosures
 LARC: Long Acting Reversible Contraception Disclosures I have no relevant financial disclosures Jennifer Kerns, MD, MPH Assistant Professor, UCSF Obstetrics, Gynecology and Reproductive Sciences San Francisco
LARC: Long Acting Reversible Contraception Disclosures I have no relevant financial disclosures Jennifer Kerns, MD, MPH Assistant Professor, UCSF Obstetrics, Gynecology and Reproductive Sciences San Francisco
Key words: Contraception, Copper T380A, Discontinuation.
 Discontinuation Rates among Women Using either the Combined Oral Contraceptive Pills or an Intrauterine Contraceptive Device for Contraception: A Comparative Study Ehab Al-Rayyan MD*, Zakarya Bani Meri
Discontinuation Rates among Women Using either the Combined Oral Contraceptive Pills or an Intrauterine Contraceptive Device for Contraception: A Comparative Study Ehab Al-Rayyan MD*, Zakarya Bani Meri
Contraception and gynecological pathologies
 1 Contraception and gynecological pathologies 18 years old, 2 CMI normal First menstruation at 14 years old Irregular (every 2/3 months), painful + She does not need contraception She is worried about
1 Contraception and gynecological pathologies 18 years old, 2 CMI normal First menstruation at 14 years old Irregular (every 2/3 months), painful + She does not need contraception She is worried about
 Non-contraceptive Uses of the Levonorgestrel Intrauterine Device Elena Gates, MD http://www.mirena-us.com/pvs1/pri/whatisframe.html Progestin levels with LNG- IUS Lower plasma levels Mirena 150-200 pg/ml
Non-contraceptive Uses of the Levonorgestrel Intrauterine Device Elena Gates, MD http://www.mirena-us.com/pvs1/pri/whatisframe.html Progestin levels with LNG- IUS Lower plasma levels Mirena 150-200 pg/ml
THE INTRA--UTERINE DEVICE
 CANADIAN CONSENSUS CONFERENCE THE INTRA--UTERINE DEVICE WHY CONSIDER THIS METHOD? Use of an IUD should be considered for all women who seek a reversible, effective, coitally-independent method of contraception.
CANADIAN CONSENSUS CONFERENCE THE INTRA--UTERINE DEVICE WHY CONSIDER THIS METHOD? Use of an IUD should be considered for all women who seek a reversible, effective, coitally-independent method of contraception.
Ardhanu Kusumanto Oktober Contraception methods for gyne cancer survivors
 Ardhanu Kusumanto Oktober 2017 Contraception methods for gyne cancer survivors Background cancer treatment Care of gyn cancer survivor Promotion of sexual, cardiovascular, bone, and brain health management
Ardhanu Kusumanto Oktober 2017 Contraception methods for gyne cancer survivors Background cancer treatment Care of gyn cancer survivor Promotion of sexual, cardiovascular, bone, and brain health management
REPRODUCTIVE ENDOCRINOLOGY
 FERTILITY AND STERILITY VOL. 79, NO. 4, APRIL 2003 Copyright 2003 American Society for Reproductive Medicine Published by Elsevier Science Inc. Printed on acid-free paper in U.S.A. REPRODUCTIVE ENDOCRINOLOGY
FERTILITY AND STERILITY VOL. 79, NO. 4, APRIL 2003 Copyright 2003 American Society for Reproductive Medicine Published by Elsevier Science Inc. Printed on acid-free paper in U.S.A. REPRODUCTIVE ENDOCRINOLOGY
1. Ng M et a l. Global, regional, and national prevalence of overweight and obesity in children and adults during : A systematic analysis
 1 2 3 1. Ng M et a l. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980 2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet
1 2 3 1. Ng M et a l. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980 2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet
Contraception. Objectives. Unintended Pregnancy. Unintended Pregnancy in the US. What s the Impact? 10/7/2014
 Contraception Tami Allen, RNC OB, MHA Robin Petersen, RN, MSN Perinatal Clinical Nurse Specialist Objectives Discuss the impact of unintended pregnancy in the United States Discuss the risks and benefits
Contraception Tami Allen, RNC OB, MHA Robin Petersen, RN, MSN Perinatal Clinical Nurse Specialist Objectives Discuss the impact of unintended pregnancy in the United States Discuss the risks and benefits
INTRAUTERINE DEVICES AND INFECTIONS. Tips for Evaluation and Management
 INTRAUTERINE DEVICES AND INFECTIONS Tips for Evaluation and Management Objectives At the end of this presentation, the participant should be able to: 1. Diagnose infection after IUD placement 2. Provide
INTRAUTERINE DEVICES AND INFECTIONS Tips for Evaluation and Management Objectives At the end of this presentation, the participant should be able to: 1. Diagnose infection after IUD placement 2. Provide
Treatment of menorrhagia with a novel frameless intrauterine levonorgestrelreleasing drug delivery system: a pilot study
 European Journal of Contraception & Reproductive Health Care, 2001;6:93-101 Treatment of menorrhagia with a novel frameless intrauterine levonorgestrelreleasing drug delivery system: a pilot study D. Wildemeersch*,
European Journal of Contraception & Reproductive Health Care, 2001;6:93-101 Treatment of menorrhagia with a novel frameless intrauterine levonorgestrelreleasing drug delivery system: a pilot study D. Wildemeersch*,
Information for Informed Consent for Insertion of a Mirena IUD
 Information for Informed Consent for Insertion of a Mirena IUD What is an IUD (intrauterine Device)? An intrauterine device (IUD) is a plastic device that is placed into your uterus to prevent pregnancy.
Information for Informed Consent for Insertion of a Mirena IUD What is an IUD (intrauterine Device)? An intrauterine device (IUD) is a plastic device that is placed into your uterus to prevent pregnancy.
Time Topic Speaker Abbreviation
 1. Programme Sunday, 4 th November 2018 Time Topic Speaker Abbreviation 08:00 Welcome, distribution materials 08:30 Overview of the Medical Eligibility Criteria (2015), and the Selected Practices Recommendations
1. Programme Sunday, 4 th November 2018 Time Topic Speaker Abbreviation 08:00 Welcome, distribution materials 08:30 Overview of the Medical Eligibility Criteria (2015), and the Selected Practices Recommendations
Slid e 1. This slide should be shown at each presentation. This slide should be shown at each presentation. Slid e 2. Slid e 3
 e 1 New Developments in Contraception This slide should be shown at each presentation. Counseling and Insertion Training Featuring the Levonorgestrel Intrauterine System Sponsored by Association of Reproductive
e 1 New Developments in Contraception This slide should be shown at each presentation. Counseling and Insertion Training Featuring the Levonorgestrel Intrauterine System Sponsored by Association of Reproductive
Family Planning UNMET NEED. The Nurse Mildred Radio Talk Shows
 Family Planning UNMET NEED The Nurse Mildred Radio Talk Shows TOPIC 9: IUD/COIL Guests FP counsellor from MSU, RHU& UHMG Nurse Mildred Nurse Betty Objectives of the programme: To inform listeners about
Family Planning UNMET NEED The Nurse Mildred Radio Talk Shows TOPIC 9: IUD/COIL Guests FP counsellor from MSU, RHU& UHMG Nurse Mildred Nurse Betty Objectives of the programme: To inform listeners about
LONG-ACTING REVERSIBLE CONTRACEPTION. Summary Tables
 LONG-ACTING REVERSIBLE CONTRACEPTION Summary Tables Bridging the Divide: A Project of the Jacobs Institute of Women s Health June 2016 Table 1. Summary of LARC Methods Available Years Since Effective Copper
LONG-ACTING REVERSIBLE CONTRACEPTION Summary Tables Bridging the Divide: A Project of the Jacobs Institute of Women s Health June 2016 Table 1. Summary of LARC Methods Available Years Since Effective Copper
Wendy Shen, MD, PhD Refresher Course for the Family Physician April 5, 2018 Coralville, Iowa
 Wendy Shen, MD, PhD Refresher Course for the Family Physician April 5, 2018 Coralville, Iowa Objectives Distinguish the different types of IUDs Understand the mechanism of action and selection of candidates
Wendy Shen, MD, PhD Refresher Course for the Family Physician April 5, 2018 Coralville, Iowa Objectives Distinguish the different types of IUDs Understand the mechanism of action and selection of candidates
Review of the safety, effi cacy and patient acceptability of the levonorgestrel-releasing intrauterine system
 REVIEW Review of the safety, effi cacy and patient acceptability of the levonorgestrel-releasing intrauterine system Chandra Kailasam 1 David Cahill 2 1 Bristol Centre for Reproductive Medicine, Southmead
REVIEW Review of the safety, effi cacy and patient acceptability of the levonorgestrel-releasing intrauterine system Chandra Kailasam 1 David Cahill 2 1 Bristol Centre for Reproductive Medicine, Southmead
Intrauterine contraceptive devices (IUDs) are becoming
 Malpositioned Intrauterine Contraceptive Devices Risk Factors, Outcomes, and Future Pregnancies Kari P. Braaten, MD, MPH, Carol B. Benson, MD, Rie Maurer, MA, and Alisa B. Goldberg, MD, MPH OBJECTIVE:
Malpositioned Intrauterine Contraceptive Devices Risk Factors, Outcomes, and Future Pregnancies Kari P. Braaten, MD, MPH, Carol B. Benson, MD, Rie Maurer, MA, and Alisa B. Goldberg, MD, MPH OBJECTIVE:
Reproductive health research at WHO
 Reproductive health research at WHO Paul F.A. Van Look, MD PhD Department of Reproductive Health and Research World Health Organization Geneva, 11 March 2004 PVL_GE_StudCourse_MAR04/1 Health is a state
Reproductive health research at WHO Paul F.A. Van Look, MD PhD Department of Reproductive Health and Research World Health Organization Geneva, 11 March 2004 PVL_GE_StudCourse_MAR04/1 Health is a state
Female Sterilization. Kavita Nanda, MD, MHS FHI 360 Expanding Contraceptive Choice December 6, 2018
 Female Sterilization Kavita Nanda, MD, MHS FHI 360 Expanding Contraceptive Choice December 6, 2018 What is female sterilization? Family planning method that provides permanent contraception to women and
Female Sterilization Kavita Nanda, MD, MHS FHI 360 Expanding Contraceptive Choice December 6, 2018 What is female sterilization? Family planning method that provides permanent contraception to women and
Simplifying Vide Contraception. University of Utah Department of Ob/Gyn Post Grad Course February 13, 2017 David Turok
 Simplifying Vide Contraception University of Utah Department of Ob/Gyn Post Grad Course February 13, 2017 David Turok Background Objectives At the conclusion of this presentation participants will be able
Simplifying Vide Contraception University of Utah Department of Ob/Gyn Post Grad Course February 13, 2017 David Turok Background Objectives At the conclusion of this presentation participants will be able
Picking the Perfect Pill How to Effectively Choose an Oral Contraceptive
 Focus on CME at Queen s University Picking the Perfect Pill How to Effectively Choose an Oral Contraceptive By Susan Chamberlain, MD, FRCSC There are over 20 oral contraceptive (OC) preparations on the
Focus on CME at Queen s University Picking the Perfect Pill How to Effectively Choose an Oral Contraceptive By Susan Chamberlain, MD, FRCSC There are over 20 oral contraceptive (OC) preparations on the
Long Acting Reversible Contraception: First Line Care for Adolescents. David A. Levine, MD, FAAP Melissa Kottke, MD, MPH, FACOG
 Long Acting Reversible Contraception: First Line Care for Adolescents David A. Levine, MD, FAAP Melissa Kottke, MD, MPH, FACOG Disclosures Melissa Kottke is a Nexplanon trainer for Merck Objectives Describe
Long Acting Reversible Contraception: First Line Care for Adolescents David A. Levine, MD, FAAP Melissa Kottke, MD, MPH, FACOG Disclosures Melissa Kottke is a Nexplanon trainer for Merck Objectives Describe
CLEAR COVERAGE HYSTERECTOMY CHECKLISTS
 CLEAR COVERAGE HYSTERECTOMY CHECKLISTS Click on the link below to access the checklist sheet. Abnormal Uterine Bleeding Adenomyosis Chronic Abdominal or Pelvic Pain Endometriosis Fibroids General Guidelines
CLEAR COVERAGE HYSTERECTOMY CHECKLISTS Click on the link below to access the checklist sheet. Abnormal Uterine Bleeding Adenomyosis Chronic Abdominal or Pelvic Pain Endometriosis Fibroids General Guidelines
Case Report Successful Treatment of Early Endometrial Carcinoma by Local Delivery of Levonorgestrel: A Case Report
 Obstetrics and Gynecology International Volume 2010, Article ID 431950, 4 pages doi:10.1155/2010/431950 Case Report Successful Treatment of Early Endometrial Carcinoma by Local Delivery of Levonorgestrel:
Obstetrics and Gynecology International Volume 2010, Article ID 431950, 4 pages doi:10.1155/2010/431950 Case Report Successful Treatment of Early Endometrial Carcinoma by Local Delivery of Levonorgestrel:
REPRODUCTIVE ENDOCRINOLOGY
 REPRODUCTIVE ENDOCRINOLOGY FERTILITY AND STERILITY VOL. 76, NO. 2, AUGUST 2001 Copyright 2001 American Society for Reproductive Medicine Published by Elsevier Science Inc. Printed on acid-free paper in
REPRODUCTIVE ENDOCRINOLOGY FERTILITY AND STERILITY VOL. 76, NO. 2, AUGUST 2001 Copyright 2001 American Society for Reproductive Medicine Published by Elsevier Science Inc. Printed on acid-free paper in
Gayatrri Anipindi *, Vani I. Original Research Article. Abstract
 Original Research Article Role of levonorgestrel releasing intrauterine device in management of heavy menstrual bleeding: A safe and effective option for all PALM COEIN variants Gayatrri Anipindi *, Vani
Original Research Article Role of levonorgestrel releasing intrauterine device in management of heavy menstrual bleeding: A safe and effective option for all PALM COEIN variants Gayatrri Anipindi *, Vani
Heavy Menstrual Bleeding. Mr Nick Nicholas MD FRCOG Grad Dip Law. Consultant Gynaecologist
 Heavy Menstrual Bleeding Mr Nick Nicholas MD FRCOG Grad Dip Law. Consultant Gynaecologist Why is HMB so important? 1:20 women aged 30-49 consult their GP with HMB Once referred to gynaecologist, surgical
Heavy Menstrual Bleeding Mr Nick Nicholas MD FRCOG Grad Dip Law. Consultant Gynaecologist Why is HMB so important? 1:20 women aged 30-49 consult their GP with HMB Once referred to gynaecologist, surgical
BLEEDING PATTERNS AND CONTRACEPTIVE DISCONTINUATION FG MHLANGA MTN ANNUAL MEETING 20 MARCH 2018
 BLEEDING PATTERNS AND CONTRACEPTIVE DISCONTINUATION FG MHLANGA MTN ANNUAL MEETING 20 MARCH 2018 Introduction Bleeding with contraception may lead to discontinuation and possible unintended pregnancy What
BLEEDING PATTERNS AND CONTRACEPTIVE DISCONTINUATION FG MHLANGA MTN ANNUAL MEETING 20 MARCH 2018 Introduction Bleeding with contraception may lead to discontinuation and possible unintended pregnancy What
Topics. Periods Menopause & HRT Contraception Vulva problems
 Girls stuff Topics Periods Menopause & HRT Contraception Vulva problems Menorrhagia Excessive menstrual loss occurring with regular or irregular cycles Ovulatory Anovulatory Usual blood loss 30-40ml per
Girls stuff Topics Periods Menopause & HRT Contraception Vulva problems Menorrhagia Excessive menstrual loss occurring with regular or irregular cycles Ovulatory Anovulatory Usual blood loss 30-40ml per
UKMEC SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION
 SUMMARY TABLE SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION Cu-IUD = Copper-bearing intrauterine device; LNG-IUS = Levonorgestrel-releasing intrauterine system; IMP = Progestogen-only implant;
SUMMARY TABLE SUMMARY TABLE HORMONAL AND INTRAUTERINE CONTRACEPTION Cu-IUD = Copper-bearing intrauterine device; LNG-IUS = Levonorgestrel-releasing intrauterine system; IMP = Progestogen-only implant;
Endometriosis. *Chocolate cyst in the ovary
 Endometriosis What is endometriosis? Endometriosis is a common condition in young women. It's chronic, painful, and it often progressively gets worse over the time. *Chocolate cyst in the ovary Normally,
Endometriosis What is endometriosis? Endometriosis is a common condition in young women. It's chronic, painful, and it often progressively gets worse over the time. *Chocolate cyst in the ovary Normally,
Abnormal Uterine Bleeding Case Studies
 Case Study 1 Abnormal Uterine Bleeding Case Studies Abigail, a 24 year old female, presents to your office complaining that her menstrual cycles have become a problem. They are now lasting 6 7 days instead
Case Study 1 Abnormal Uterine Bleeding Case Studies Abigail, a 24 year old female, presents to your office complaining that her menstrual cycles have become a problem. They are now lasting 6 7 days instead
2
 1 2 3 1. Usinger KM et al. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol 2016; 29: 659 67. 2. Kost K et al. Estimates of contraceptive
1 2 3 1. Usinger KM et al. Intrauterine contraception continuation in adolescents and young women: a systematic review. J Pediatr Adolesc Gynecol 2016; 29: 659 67. 2. Kost K et al. Estimates of contraceptive
Downloaded from:
 French, R; Cowan, F; Mansour, D; Morris, S; Hughes, D; Robinson, A; Proctor, T; Summerbell, C; Logan, S; Guillebaud, J (2001) Hormonally impregnated intrauterine systems (IUSs), versus other forms of reversible
French, R; Cowan, F; Mansour, D; Morris, S; Hughes, D; Robinson, A; Proctor, T; Summerbell, C; Logan, S; Guillebaud, J (2001) Hormonally impregnated intrauterine systems (IUSs), versus other forms of reversible
WHAT ARE CONTRACEPTIVES?
 CONTRACEPTION WHAT ARE CONTRACEPTIVES? Methods used to prevent fertilization *Also referred to as birth control methods With contraceptives, it is important to look at what works for you and your body.
CONTRACEPTION WHAT ARE CONTRACEPTIVES? Methods used to prevent fertilization *Also referred to as birth control methods With contraceptives, it is important to look at what works for you and your body.
the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD the IUD your guide to
 your guide to Helping you choose the method of contraception that s best for you IUD IUD the e IUD IU IUD the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that s put
your guide to Helping you choose the method of contraception that s best for you IUD IUD the e IUD IU IUD the IUD 2 3 The intrauterine device (IUD) An IUD is a small plastic and copper device that s put
Fertility control: what do women want?
 FIAPAC 2018 Fertility control: what do women want? Dr. Raymond H.W. Li MBBS, MMedSC, FRCOG, FHKAM (O&G) Cert RCOG/HKCOG (Reprod Med) Department of O&G, The University of Hong Kong The Family Planning Association
FIAPAC 2018 Fertility control: what do women want? Dr. Raymond H.W. Li MBBS, MMedSC, FRCOG, FHKAM (O&G) Cert RCOG/HKCOG (Reprod Med) Department of O&G, The University of Hong Kong The Family Planning Association
Dr. Nancy Van Eyk Associate Professor, Dalhousie University Chief of Gynaecology, IWK Health Centre
 Dr. Nancy Van Eyk Associate Professor, Dalhousie University Chief of Gynaecology, IWK Health Centre AUB Outline Terminology Classification/Etiology Assessment Treatment Referral to Gynaecology U c pt 4
Dr. Nancy Van Eyk Associate Professor, Dalhousie University Chief of Gynaecology, IWK Health Centre AUB Outline Terminology Classification/Etiology Assessment Treatment Referral to Gynaecology U c pt 4
Excessive menstrual blood loss
 Ian Chilcott Excessive menstrual blood loss >80mls - That interferes with physical, emotional, social and material quality of life 1 in 20 women aged 30 to 49 years consult their GP each year with menorrhagia
Ian Chilcott Excessive menstrual blood loss >80mls - That interferes with physical, emotional, social and material quality of life 1 in 20 women aged 30 to 49 years consult their GP each year with menorrhagia
Breast Cancer Risk in Patients Using Hormonal Contraception
 Breast Cancer Risk in Patients Using Hormonal Contraception Bradley L. Smith, Pharm.D. Smith.bradley1@mayo.edu Pharmacy Ground Rounds Mayo Clinic Rochester April 3 rd, 2018 2017 MFMER slide-1 Presentation
Breast Cancer Risk in Patients Using Hormonal Contraception Bradley L. Smith, Pharm.D. Smith.bradley1@mayo.edu Pharmacy Ground Rounds Mayo Clinic Rochester April 3 rd, 2018 2017 MFMER slide-1 Presentation
Contraception: Common Problems Faced in Office Practice. Jane S. Sillman, MD Brigham and Women s Hospital
 Contraception: Common Problems Faced in Office Practice Jane S. Sillman, MD Brigham and Women s Hospital Disclosures I have no conflicts of interest Contraception: Common Problems How to discuss contraception
Contraception: Common Problems Faced in Office Practice Jane S. Sillman, MD Brigham and Women s Hospital Disclosures I have no conflicts of interest Contraception: Common Problems How to discuss contraception
CURRENT HORMONAL CONTRACEPTION - LIMITATIONS
 CURRENT HORMONAL CONTRACEPTION - LIMITATIONS Oral Contraceptives - Features MERITS Up to 99.9% efficacy if used correctly and consistently Reversible method rapid return of fertility Offer non-contraceptive
CURRENT HORMONAL CONTRACEPTION - LIMITATIONS Oral Contraceptives - Features MERITS Up to 99.9% efficacy if used correctly and consistently Reversible method rapid return of fertility Offer non-contraceptive
A Comparative Study between the Side Effects of Copper Intrauterine Device in Women with Non-scarred and Scarred Uterus
 Iraqi JMS Published by Al-Nahrain College of Medicine ISSN 161-659 Email: Iraqi_jms_alnahrain@yahoo.com http://www. colmed-nahrain.edu.iq/ A Comparative Study between the Side Effects of Copper Intrauterine
Iraqi JMS Published by Al-Nahrain College of Medicine ISSN 161-659 Email: Iraqi_jms_alnahrain@yahoo.com http://www. colmed-nahrain.edu.iq/ A Comparative Study between the Side Effects of Copper Intrauterine
Review of IUCD Complications: Lessons from CAT. Dr FG Mhlanga CAT Meeting 24 September 2016
 Review of IUCD Complications: Lessons from CAT Dr FG Mhlanga CAT Meeting 24 September 2016 INTRODUCTION The intrauterine device (IUD) is a reliable long term reversible, cost-effective,easy to use and
Review of IUCD Complications: Lessons from CAT Dr FG Mhlanga CAT Meeting 24 September 2016 INTRODUCTION The intrauterine device (IUD) is a reliable long term reversible, cost-effective,easy to use and
Instructions how to use the ESC teach the teachers course and self-learning tool
 Instructions how to use the ESC teach the teachers course and self-learning tool Welcome to the ESC advanced learning tool To improve and facilitate knowledge and use of contraception, abortion, sexually
Instructions how to use the ESC teach the teachers course and self-learning tool Welcome to the ESC advanced learning tool To improve and facilitate knowledge and use of contraception, abortion, sexually
38 OBG Management August 2012 Vol. 24 No. 8 obgmanagement.com
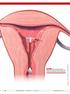 FIGURE 1 Copper intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium. illustrations: craig zuckerman for obg management 38 OBG Management August 2012
FIGURE 1 Copper intrauterine device displaced in the lower uterine segment with the left arm embedded in the myometrium. illustrations: craig zuckerman for obg management 38 OBG Management August 2012
The number of women using long-acting reversible
 Long-acting reversible contraception: Who, what, when, and how This review provides practical tips and dispels some common misconceptions about these devices, which have higher rates of patient satisfaction
Long-acting reversible contraception: Who, what, when, and how This review provides practical tips and dispels some common misconceptions about these devices, which have higher rates of patient satisfaction
Frequency of menses. Duration of menses 3 days to 7 days. Flow/amount of menses Average blood loss with menstruation is 60-80cc.
 Frequency of menses 24 days (0.5%) to 35 days (0.9%) Age 25, 40% are between 25 and 28 days Age 25-35, 60% are between 25 and 28 days Teens and women over 40 s cycles may be longer apart Duration of menses
Frequency of menses 24 days (0.5%) to 35 days (0.9%) Age 25, 40% are between 25 and 28 days Age 25-35, 60% are between 25 and 28 days Teens and women over 40 s cycles may be longer apart Duration of menses
Notes to Teacher continued Contraceptive Considerations
 Abstinence a conscious decision to refrain from sexual intercourse 100% pregnancy will not occur if close contact between the penis and vagina does not take place. The risk of a number of STDs, including
Abstinence a conscious decision to refrain from sexual intercourse 100% pregnancy will not occur if close contact between the penis and vagina does not take place. The risk of a number of STDs, including
A systematic review of some of the side effects of copper T380 intrauterine contraceptive device
 A systematic review of some of the side effects of copper T380 intrauterine contraceptive device Ahmed Nehad Ahmed Hatem Askalani IAMANEH Scholarship Reproductive Health Research Course Geneva, 2006 Introduction
A systematic review of some of the side effects of copper T380 intrauterine contraceptive device Ahmed Nehad Ahmed Hatem Askalani IAMANEH Scholarship Reproductive Health Research Course Geneva, 2006 Introduction
Instructions how to use the ESC teach the teachers course and self-learning tool
 Instructions how to use the ESC teach the teachers course and self-learning tool Welcome to the ESC advanced learning tool To improve and facilitate knowledge and use of contraception, abortion, sexually
Instructions how to use the ESC teach the teachers course and self-learning tool Welcome to the ESC advanced learning tool To improve and facilitate knowledge and use of contraception, abortion, sexually
Menstrual Disorders & Ambulatory Gynaecology
 Menstrual Disorders & Ambulatory Gynaecology Mr. Nagui Lewis Aziz M B, CH B, FRCOG Consultant Gynaecologist The Royal Oldham Hospital 01/09/2018 Heavy menstrual bleeding (HMB ) is a common problem responsible
Menstrual Disorders & Ambulatory Gynaecology Mr. Nagui Lewis Aziz M B, CH B, FRCOG Consultant Gynaecologist The Royal Oldham Hospital 01/09/2018 Heavy menstrual bleeding (HMB ) is a common problem responsible
Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended.
 Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended UK/W/0083/pdWS/001 Rapporteur: UK Finalisation procedure (day 120): 18
Public Assessment Report for paediatric studies submitted in accordance with Article 45 of Regulation (EC) No1901/2006, as amended UK/W/0083/pdWS/001 Rapporteur: UK Finalisation procedure (day 120): 18
Family Planning د. نجمه محمود كلية الطب جامعة بغداد فرع النسائية والتوليد
 Family Planning د. نجمه محمود كلية الطب جامعة بغداد فرع النسائية والتوليد Objectives:- To know the different types of contraception To know the failure rate & mechansim of action of each type To explain
Family Planning د. نجمه محمود كلية الطب جامعة بغداد فرع النسائية والتوليد Objectives:- To know the different types of contraception To know the failure rate & mechansim of action of each type To explain
Welcome to Mirena. The Mirena Handbook: A Personal Guide to Your New Mirena. mirena.com. Mirena is the #1 prescribed IUD * in the U.S.
 Mirena is the #1 prescribed IUD * in the U.S. Welcome to Mirena The Mirena Handbook: A Personal Guide to Your New Mirena *Intrauterine Device Supported by 2015-2016 SHS data INDICATIONS FOR MIRENA Mirena
Mirena is the #1 prescribed IUD * in the U.S. Welcome to Mirena The Mirena Handbook: A Personal Guide to Your New Mirena *Intrauterine Device Supported by 2015-2016 SHS data INDICATIONS FOR MIRENA Mirena
Endometriosis. What you need to know. 139 Dumaresq Street Campbelltown Phone Fax
 Endometriosis What you need to know 139 Dumaresq Street Campbelltown Phone 4628 5292 Fax 4628 0349 www.nureva.com.au September 2015 What is Endometriosis? Endometriosis is a condition whereby the lining
Endometriosis What you need to know 139 Dumaresq Street Campbelltown Phone 4628 5292 Fax 4628 0349 www.nureva.com.au September 2015 What is Endometriosis? Endometriosis is a condition whereby the lining
Disclosures. Learning Objectives 4/18/2017 ADOLESCENT CONTRACEPTION UPDATE APRIL 28, Nexplanon trainer for Merck
 ADOLESCENT CONTRACEPTION UPDATE APRIL 28, 2017 Brandy Mitchell, MN, RN, ANP BC, WHNP BC University of Iowa Hospitals and Clinics Obstetrics and Gynecology Iowa Association of Nurse Practitioners Spring
ADOLESCENT CONTRACEPTION UPDATE APRIL 28, 2017 Brandy Mitchell, MN, RN, ANP BC, WHNP BC University of Iowa Hospitals and Clinics Obstetrics and Gynecology Iowa Association of Nurse Practitioners Spring
INFERTILITY CAUSES. Basic evaluation of the female
 INFERTILITY Infertility is the inability to conceive after 12 months of unprotected intercourse. There are multiple causes of infertility and a systematic way to evaluate the condition. Let s look at some
INFERTILITY Infertility is the inability to conceive after 12 months of unprotected intercourse. There are multiple causes of infertility and a systematic way to evaluate the condition. Let s look at some
Chapter 7 Infertility, Contraception, and Abortion
 Chapter 7 Infertility, Contraception, and Abortion Infertility Incidence Affects about 10% to 15% of reproductive-age population Subfertility: prolonged time to conceive Sterility: inability to conceive
Chapter 7 Infertility, Contraception, and Abortion Infertility Incidence Affects about 10% to 15% of reproductive-age population Subfertility: prolonged time to conceive Sterility: inability to conceive
Endometrial line thickness in different conditions.
 Endometrial line thickness in different conditions 1 Endometrial thickens in response to Rising estrogen levels during the menstrual cycle and then shedding endometrial at the times of menses 2 The thickens
Endometrial line thickness in different conditions 1 Endometrial thickens in response to Rising estrogen levels during the menstrual cycle and then shedding endometrial at the times of menses 2 The thickens
Effective Contraception Utilization. Sarah Laiosa, DO Family Physician Contract Medical Director, EOCCO
 Effective Contraception Utilization Sarah Laiosa, DO Family Physician Contract Medical Director, EOCCO Disclosures Contract Medical Director, EOCCO Objectives Illustrate how to best address contraception
Effective Contraception Utilization Sarah Laiosa, DO Family Physician Contract Medical Director, EOCCO Disclosures Contract Medical Director, EOCCO Objectives Illustrate how to best address contraception
Learning Objectives. Peri menopause. Menopause Overview. Recommendation grading categories
 Learning Objectives Identify common symptoms of the menopause transition Understand the risks and benefits of hormone replacement therapy (HRT) Be able to choose an appropriate hormone replacement regimen
Learning Objectives Identify common symptoms of the menopause transition Understand the risks and benefits of hormone replacement therapy (HRT) Be able to choose an appropriate hormone replacement regimen
Contraception for Adolescents: What s New?
 Contraception for Adolescents: What s New? US Medical Eligibility Criteria for Contraceptive Use Kathryn M. Curtis, PhD Division of Reproductive Health, CDC Expanding Our Experience and Expertise: Implementing
Contraception for Adolescents: What s New? US Medical Eligibility Criteria for Contraceptive Use Kathryn M. Curtis, PhD Division of Reproductive Health, CDC Expanding Our Experience and Expertise: Implementing
Disclosures. Contraceptive Method Use, U.S. Best Practices in Contraception: Advances, Tips, and Tricks
 Best Practices in Contraception: Advances, Tips, and Tricks Disclosures I have no disclosures I may discuss off-label use of some contraceptives Biftu Mengesha MD MAS Department of Obstetrics, Gynecology
Best Practices in Contraception: Advances, Tips, and Tricks Disclosures I have no disclosures I may discuss off-label use of some contraceptives Biftu Mengesha MD MAS Department of Obstetrics, Gynecology
The Balanced Counseling Strategy Plus: A Toolkit for Family Planning Service Providers Working in High STI/HIV Prevalence Settings.
 The Balanced Counseling Strategy Plus: A Toolkit for Family Planning Service Providers Working in High STI/HIV Prevalence Settings Counseling Cards Checklist to be reasonably sure a woman is not pregnant
The Balanced Counseling Strategy Plus: A Toolkit for Family Planning Service Providers Working in High STI/HIV Prevalence Settings Counseling Cards Checklist to be reasonably sure a woman is not pregnant
Primary Care Gynaecology Guidelines: HEAVY REGULAR MENSTRUAL BLEEDING
 Primary Care Guidelines: HEAVY REGULAR MENSTRUAL BLEEDING
Primary Care Guidelines: HEAVY REGULAR MENSTRUAL BLEEDING
Hormonal Contraception: Asian View. Hormonal Contraception: Asian View: focused on Chinese View. Prof.Dr.Xiangyan Ruan, MD.PhD
 Asian View Prof.Dr.Xiangyan Ruan, MD.PhD Beijing Obstetrics & Gynecology Hospital, Capital Medical University (China) WHO Collaborative Centre - Director of Dept. of Gynecological Endocrinology & & - Fertility
Asian View Prof.Dr.Xiangyan Ruan, MD.PhD Beijing Obstetrics & Gynecology Hospital, Capital Medical University (China) WHO Collaborative Centre - Director of Dept. of Gynecological Endocrinology & & - Fertility
Question Bank III - BHMS
 Question Bank III - BHMS Sub:- Ob/Gy -Paper-II 1. Give the definition of Puberty. 2. Enumerate five important physical changes evident during puberty. 3. Write down the vaginal changes during puberty.
Question Bank III - BHMS Sub:- Ob/Gy -Paper-II 1. Give the definition of Puberty. 2. Enumerate five important physical changes evident during puberty. 3. Write down the vaginal changes during puberty.
Management of Gynae Problems in Primary Care David Griffiths FRCOG The Great Western Hospital Swindon. A brief overview
 Management of Gynae Problems in Primary Care David Griffiths FRCOG The Great Western Hospital Swindon A brief overview Pelvic Pain Challenge to the physician In UK 1 Million sufferers 20% of all gynae
Management of Gynae Problems in Primary Care David Griffiths FRCOG The Great Western Hospital Swindon A brief overview Pelvic Pain Challenge to the physician In UK 1 Million sufferers 20% of all gynae
Family Planning and Infertility
 Family Planning and Infertility Chapter 20 Objectives Discuss types of reversible contraception Natural methods Mechanical barrier methods Hormonal contraceptives Discuss types of permanent contraception
Family Planning and Infertility Chapter 20 Objectives Discuss types of reversible contraception Natural methods Mechanical barrier methods Hormonal contraceptives Discuss types of permanent contraception
100% Highly effective No cost No side effects
 effective? Advantages Disadvantages How do I get Cost Abstinence For some it can mean no sexual contact. For others it is no sexual intercourse or vaginal penetration. A permanent surgical procedure available
effective? Advantages Disadvantages How do I get Cost Abstinence For some it can mean no sexual contact. For others it is no sexual intercourse or vaginal penetration. A permanent surgical procedure available
American Journal of Internal Medicine
 American Journal of Internal Medicine 2016; 4(3): 49-59 http://www.sciencepublishinggroup.com/j/ajim doi: 10.11648/j.ajim.20160403.12 ISSN: 2330-4316 (Print); ISSN: 2330-4324 (Online) The Effect of Dose-Reduced
American Journal of Internal Medicine 2016; 4(3): 49-59 http://www.sciencepublishinggroup.com/j/ajim doi: 10.11648/j.ajim.20160403.12 ISSN: 2330-4316 (Print); ISSN: 2330-4324 (Online) The Effect of Dose-Reduced
Chapter 100 Gynecologic Disorders
 Chapter 100 Gynecologic Disorders Episode Overview: 1. Describe the presentation and RF for Adnexal torsion 2. List the imaging findings of adnexal torsion (US vs CT) 3. What is the management of adnexal
Chapter 100 Gynecologic Disorders Episode Overview: 1. Describe the presentation and RF for Adnexal torsion 2. List the imaging findings of adnexal torsion (US vs CT) 3. What is the management of adnexal
Article begins on next page
 Association of cervical microglandular hyperplasia with exogenous progestin exposure Rutgers University has made this article freely available. Please share how this access benefits you. Your story matters.
Association of cervical microglandular hyperplasia with exogenous progestin exposure Rutgers University has made this article freely available. Please share how this access benefits you. Your story matters.
INTERVENTIONAL PROCEDURES PROGRAMME
 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of microwave endometrial ablation Introduction This overview has been prepared to assist
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of microwave endometrial ablation Introduction This overview has been prepared to assist
Emergency Contraception THE FACTS
 Emergency Contraception Quick Facts What is it? Emergency contraception is birth control that you use after you have had unprotected sex--if you didn t use birth control or your regular birth control failed.
Emergency Contraception Quick Facts What is it? Emergency contraception is birth control that you use after you have had unprotected sex--if you didn t use birth control or your regular birth control failed.
Multipurpose Intravaginal Ring: Tenofovir / Levonorgestrel
 MTN Annual Meeting Bethesda, MD March 17, 2015 Multipurpose Intravaginal Ring: Tenofovir / Levonorgestrel Christine Mauck, MD, MPH Why develop a multipurpose ring? Providing drug in a ring is likely to
MTN Annual Meeting Bethesda, MD March 17, 2015 Multipurpose Intravaginal Ring: Tenofovir / Levonorgestrel Christine Mauck, MD, MPH Why develop a multipurpose ring? Providing drug in a ring is likely to
Contraception Choices: An Evidence Based Approach Case Study Approach. Susan Hellier PhD, DNP, FNP-BC, CNE
 Contraception Choices: An Evidence Based Approach Case Study Approach Susan Hellier PhD, DNP, FNP-BC, CNE Objectives Describe the U.S. Medical Eligibility Criteria for Contraceptive Use, 2016 (U.S. MEC)
Contraception Choices: An Evidence Based Approach Case Study Approach Susan Hellier PhD, DNP, FNP-BC, CNE Objectives Describe the U.S. Medical Eligibility Criteria for Contraceptive Use, 2016 (U.S. MEC)
Dysmenorrhoea Gynaecology د.شيماءعبداالميرالجميلي. Aetiology of secondary dysmenorrhea
 30-11-2014 Gynaecology Dysmenorrhoea د.شيماءعبداالميرالجميلي Dysmenorrhoea is defined as painful menstruation. It is experienced by 45 95 per cent of women of reproductive age.primary Spasmodic Dysmenorrhea
30-11-2014 Gynaecology Dysmenorrhoea د.شيماءعبداالميرالجميلي Dysmenorrhoea is defined as painful menstruation. It is experienced by 45 95 per cent of women of reproductive age.primary Spasmodic Dysmenorrhea
2/4/2011. What is your specialty? A. Family practice B. Internal medicine and subs C. OB/GYN D. Peds E. Surgery and subs
 Steve P. Buchanan D.O. FACOOG(Dist.) TOMA Mid Winter February 11, 2011 Dallas,TX Associate Professor OB/GYN UNTHSC/TCOM 1987- present Executive Vice President, American College of Osteopathic Obstetricians
Steve P. Buchanan D.O. FACOOG(Dist.) TOMA Mid Winter February 11, 2011 Dallas,TX Associate Professor OB/GYN UNTHSC/TCOM 1987- present Executive Vice President, American College of Osteopathic Obstetricians
