Citation for published version (APA): Verbeek, H. (2015). Medullary Thyroid Carcinoma: from diagnosis to treatment [S.l.]: [S.n.]
|
|
|
- Kerrie Tiffany French
- 5 years ago
- Views:
Transcription
1 University of Groningen Medullary Thyroid Carcinoma Verbeek, Hans IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2015 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Verbeek, H. (2015). Medullary Thyroid Carcinoma: from diagnosis to treatment [S.l.]: [S.n.] Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date:
2 The effects of four different tyrosine kinase inhibitors on medullary and papillary thyroid cancer cells Hans H. G. Verbeek*, Maria M. Alves*, Jan Willem B. de Groot, Jan Osinga John T. M. Plukker, Thera P. Links, Robert M. W. Hofstra *The first two authors contributed equally to this study J Clin Endocrinol Metab. 2011; 96: E991-5
3 Abstract Context Medullary and papillary thyroid carcinoma (MTC and PTC) are two types of thyroid cancer that can originate from activating mutations or rearrangements in the RET gene. Therapeutic options are limited in recurrent disease, but because RET is a tyrosine kinase (TK) receptor involved in cellular growth and proliferation, treatment with a TK inhibitor might be promising. Several TK inhibitors have been tested in clinical trials, but it is unknown which inhibitor is most effective and whether there is any specificity for particular RET mutations. Objective We aimed to compare the effect of four TK inhibitors (axitinib, sunitinib, vandetanib, and XL184 (cabozantinib)) on cell proliferation, RET expression and autophosphorylation, and ERK activation in cell lines expressing a MEN2A (MTC-TT), a MEN2B (MZ-CRC-1) mutation, and a RET/PTC (TPC-1) rearrangement. Design The three cell lines were cultured and treated with the four TK inhibitors. Effects on cell proliferation and RET and ERK expression and activation were determined. Results XL184 and vandetanib most effectively inhibited cell proliferation, RET autophosphorylation in combination with a reduction of RET expression, and ERK phosphorylation in MTC-TT and MZ-CRC-1, respectively. TPC-1 cells showed a decrease in RET autophosphorylation after treatment with XL184, but no effect was observed on ERK activation. Conclusion There is indeed specificity for different RET mutations, with XL184 being the most potent inhibitor in MEN2A and PTC and vandetanib the most effective in MEN2B in vitro. No TK inhibitor was superior for all the cell lines tested, indicating that mutationspecific therapies could be beneficial in treating MTC and PTC. 110
4 Effects of TK inhibitors on MTC and PTC cells Introduction Medullary thyroid carcinoma (MTC) and papillary thyroid carcinoma (PTC) can be caused by activating mutations or rearrangements of the rearranged during transfection (RET) gene. MTC originates from the calcitonin-producing C cells of the thyroid and can occur sporadically (75%) or as part of a familial cancer syndrome (25%). The latter occurs as multiple endocrine neoplasia (MEN) type 2 syndrome (MEN2A and MEN2B) or as familial MTC. 1-3 PTC is the most common thyroid cancer (80% of all thyroid cancers) and originates from the follicular epithelial cells of the thyroid. In 2.5% 40% of PTC, a RET rearrangement is found, although this percentage is higher in patients exposed to radiation. 1 Total thyroidectomy and extensive lymph node dissection is the curative treatment for MTC and PTC, followed by radioiodine ablation in PTC. However, recurrent disease is often seen in sporadic MTC, and until recently, reoperation was the only therapeutic option. In iodine-refractory PTC, no effective adjuvant therapy is available as well. 4,5 New systemic therapies are therefore needed for both recurrent MTC and PTC. With RET being the gene involved in a subset of MTC and PTC, it is logical to consider the encoded receptor as an important target for systemic therapy. RET is expressed in all tumour cells and continuous autophosphorylation on its tyrosine kinase (TK) residues caused by mutations (MEN2) or rearrangements (PTC) on RET results in a constant activation of downstream signalling pathways that ultimately lead to tumour growth. 1 Therefore, inhibition of RET phosphorylation and its downstream pathways could be of great value. Small-molecule inhibitors that selectively inhibit TK have been proven to be effective in the treatment of several neoplastic diseases. 6-8 A number of these clinically useful inhibitors target TK receptors that belong to the same family group of proteins as RET. 9 Several TK inhibitors have already been tested in vitro and evaluated in phase II clinical trials (Table 1). In a large number of patients (25% 81%), a stable disease state can be established, and some patients even show a partial response (2% 33%) Because most studies have focused on one particular TK inhibitor and have not looked for mutation specificity, it is hard to compare these compounds for the different patient groups. We therefore set out to compare the efficiency of four recently developed TK inhibitors, XL184 (cabozantinib), vandetanib, sunitinib, and axitinib, using three cell lines: MTC-TT reported to be derived from a sporadic MTC expressing a C634W RET mutation, MZ-CRC-1 111
5 derived from a patient with metastatic sporadic MTC expressing an M918T RET mutation, and TPC-1 derived from a patient with PTC expressing a RET/PTC-1 rearrangement. Table 1 Molecular targets and clinical trials performed with tyrosine kinase inhibitors in medullary thyroid carcinoma (MTC) patients. Molecular targets No. of patients Tumor response % (n) Stable disease % (n) Imatinib Bcr-Abl, PDGFR α and β, c-fms, c-kit, RET Sorafenib RAF, VEGFR2 and 3, (2) 86 (18) 12 PDGFR, RET Motesanib VEGFR 1, 2, and 3, 91 2 (2) 81 (74) PDGFR, c-kit, RET 13 Gefitinib* EGFR Vandetanib VEGFR, EGFR, RET Ref (9) 10,11 20 (6) 16 (3) 53 (16) 53 (10) Axitinib VEGFR 1, 2, and 3, (2) 27 (3) 17 Sunitinib* RET, VEGFR, PDGFR (5) 18 XL184 # MET, VEGFR-2, RET 37 (35 with measurable disease) (10) 41 (15) 19 * Clinical trial performed in advanced thyroid cancer patients, not only MTC patients; # Phase 1 trial. Materials and Methods Cell culture MZ-CRC-1, MTC-TT, TPC-1, and HEK293 (human embryonic kidney cells) cell lines were cultured as described in Supplemental Materials and Methods. Tyrosine kinase inhibitors XL184 (Exelixis, San Francisco, CA), vandetanib (AstraZeneca, Zoetermeer, The Netherlands), and sunitinib and axitinib (Pfizer, Capelle a/d IJssel, The Netherlands) were made up as a stock solution of 10 mm in dimethylsulfoxide (DMSO). Cell proliferation assays MTC-TT and MZ-CRC-1cells were plated in 200 µl medium at concentrations of cells per well. TPC-1 and HEK293 cells were plated at a density of cells per well. After overnight incubation, increasing concentrations of TK inhibitor solutions (0, 0.005, 0.05, 0.1, 112
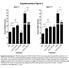
6 Effects of TK inhibitors on MTC and PTC cells 0.5, and 5 µm) were added. A concentration of 0.1% DMSO was used in all experiments. Control cells were grown without DMSO or TK inhibitor. Proliferation was measured at 1, 4, and 7 d using a cell proliferation kit (MTT assay; Roche, Almere, The Netherlands) according to the manufacturer s instructions. The concentration that led to 50% growth inhibition (IC50) was determined using linear interpolation at r=0.5 (Supplemental Table 1). If IC50 concentrations were between 0.5 and 5 µm, additional cell proliferation assays were performed (Supplemental Figure 1, A D). All experiments were performed in triplicate. Cell lysates and Western blot analysis MZ-CRC-1, MTC-TT, and TPC-1 cells were treated with 0, IC50, and maximal concentrations of the different TK inhibitors for 0, 2, and 5 d. Cell lysates were prepared as described in Supplemental Materials and Methods, and supernatants were stored at -80C before they were further processed for SDS-PAGE followed by Western blot analysis. The antibodies used are described in Supplemental Materials and Methods. All experiments were performed in duplicate. RNA extraction and RT-PCR MTC-TT and MZ-CRC-1 cells, treated with 0, IC50, and maximal concentrations (Supplemental Table 1) of XL184 and vandetanib, respectively, were collected after 0, 2, and 5 d. RNA extraction and RT-PCR procedures are described in the Supplemental Materials and Methods. Statistical analysis The statistical analysis was performed using the software program SPSS version Results Effect of different TK inhibitors on cell proliferation A dose-dependent decrease in cell proliferations was seen for all four inhibitors (Figure 1, A C, and Supplemental Figure 1, A D). In contrast, HEK293 cells, who do not endogenously express RET, showed only minor effects on proliferation at concentrations lower than 0.5 µm (Supplemental Figure 2A). Based on the IC50 values (Supplemental Figure 2B and 113
7 Supplemental Table 1), we observed that XL184 was the most effective inhibitor of MTC-TT (IC50=0.04 µm) and TPC-1 (IC50=0.06 µm) proliferation, whereas for MZ-CRC-1, vandetanib inhibited cell proliferation at the lowest concentration (IC50=0.26 µm). DMSO did not seem to have a significant negative effect on cell growth of any of the cell lines tested (Supplemental Table 2). Effect of XL184 and vandetanib on RET autophosphorylation Inhibition of RET autophosphorylation on tyrosine 1062 was observed for the three cell lines tested after 2 d of treatment with XL184 (MTC-TT and TPC-1) and vandetanib (MZ-CRC-1). However, only vandetanib was able to induce this effect with IC50 levels (Figure 1D). For MTC-TT and TPC-1, 5 d exposure to IC50 concentrations of XL184 were necessary to reduce RET autophosphorylation levels (Figure 1, E and F). To investigate whether the decrease in RET autophosphorylation was due to reduced levels of RET expression, the membranes were stripped and probed for RET. MTC-TT and MZ-CRC-1 cells showed a dose-dependent response after 5 d incubation with XL184 and vandetanib, respectively. At IC50 values, a marked decreased in RET expression was observed, and at maximal concentrations, RET was no longer detectable (Figure 1, D and F). To determine whether this effect was due to inhibition of RET transcription, RET expression levels were determined. For MTC-TT, no effect on RET transcription levels was observed. However, for MZ-CRC-1, 5 d exposure to maximal concentrations of vandetanib led to a decrease in RET expression (Supplemental Figure 3, A C). For TPC-1 cells, the total amount of RET remained relatively unchanged even after 5 d exposure to maximal concentrations of XL184 (Figure 1F). Effect of XL184 and vandetanib on RET downstream signalling pathways RET is involved in the activation of several signalling pathways, including the MAPK pathway. 20 Therefore, we evaluated the effect of XL184 and vandetanib in ERK activation. For MTC-TT and MZ-CRC-1, ERK phosphorylation was markedly reduced with IC50 levels of XL184 and vandetanib, respectively, and was totally inhibited when maximal concentrations were used (Figure 1, D and E). However, XL184 was able to exert this effect after only 2 d exposure. Interestingly, this reduction of ERK activation was related to a decrease in ERK expression (Figure 1, D and E). For TPC-1, no effect on ERK expression and activation was observed, even when maximal concentrations of XL184 were used (Figure 1F). 114
8 Effects of TK inhibitors on MTC and PTC cells Figure 1 Effect of XL184, vandetanib, sunitinib, and axitinib on cell proliferation. A C, Dose-response curves of MZ-CRC-1 (A), MTC-TT (B), and TPC-1 (C) cell lines incubated with different concentrations of XL184, vandetanib, sunitinib, and axitinib; D F, effect of XL184 and vandetanib on RET expression, RET autophosphorylation, ERK expression, and ERK phosphorylation: MZ-CRC-1 cells treated with IC50 concentration and 5 μm vandetanib for 2 and 5 d (D), MTC-TT (E), and TPC-1 (F) cells treated with IC50 concentration and 5 μm XL184 for 2 and 5 d. Error bars shown correspond to SD. Discussion We compared the effects of four TK inhibitors, XL184, vandetanib, sunitinib, and axitinib, on cell proliferation and RET inhibition and looked for mutation specificity using cell lines harbouring a MEN2A mutation (MTC-TT), a MEN2B mutation (MZ-CRC-1), and a RET/PTC rearrangement (TPC-1). Our results showed that all four TK inhibitors are capable of reducing cell proliferation to some extent. However, XL184 was found to be the most efficient inhibitor for MEN2A and PTC-derived cell lines, whereas vandetanib proved to be the most potent inhibitor for MEN2B. 115
9 We also showed that XL184 and vandetanib were able to decrease RET autophosphorylation and expression levels in MTC-TT and MZ-CRC-1 cells, respectively. However, only vandetanib exerted this effect by inhibiting RET transcription. It is possible that for XL184, lysosomal or proteasomal degradation is involved, as was described for other inhibitors. 20 For TPC-1 a marked decrease in RET phosphorylation levels was detected, but surprisingly, RET/PTC expression levels increased after exposure to XL184. This dual effect might be the result of a negative feedback mechanism to compensate a reduction in RET activation. Furthermore, it shows that XL184 exerts its effect in PTC by direct inhibition of RET autophosphorylation and that lysosomal or proteasomal degradation may not be effective due to the presence of the fusion protein. Finally, we explored the effect of these drugs in a downstream signalling pathway directly activated by RET, the MAPK pathway. For MTC-TT and MZ-CRC-1, exposure to XL184 and vandetanib, respectively, induced a marked decrease in ERK phosphorylation. Interestingly, a reduction on ERK expression was also observed for these cell lines, suggesting a possible effect of XL184 and vandetanib on ERK transcription. For the TPC-1 PTC model system, no change in ERK phosphorylation was detected after exposure to XL184. ERK phosphorylation through other TK receptors and TK effector molecules, e.g. BRAF, are likely to exert a stronger effect in ERK phosphorylation than RET. It is possible that for PTC, a combination of different inhibitors targeting RET and, e.g. BRAF (serine/threonine-protein kinase B-Raf), could circumvent this problem and thus result in an even more effective treatment for this type of cancer. Because no TK inhibitor was superior for the cell lines tested, our in vitro results suggest that mutation-specific therapies could be beneficial for the treatment of MTC and PTC. However, because only three different mutations were analysed, additional mutational studies are necessary to confirm this specificity. To date, no distinction has been made between the different RET-related mutational subtypes (MEN2A/MEN2B/PTC) in clinical trials, and it is known that aspecific targeting of TK inhibitors can also contribute to antitumor effects. However, the risk of severe side effects for the patients in combination with the development of resistance to the TK inhibitors incorrectly used reinforces the need of mutation specific therapies. Furthermore, the combined use of different TK inhibitors for multiple targeting will also benefit from this knowledge, because only then optimal combinations of inhibitors can be chosen. In conclusion, our results are in general agreement with the outcome of the clinical trials, supporting the idea that XL184 and vandetanib are two potent inhibitors for tumour response 116
10 Effects of TK inhibitors on MTC and PTC cells in MTC. We also showed that there is specificity of these inhibitors for the treatment of different RET mutations, which suggest that mutation-specific therapies will likely improve the outcome of ongoing studies. Thus, reanalysis of already performed trials based on mutation status is more than worthwhile. Acknowledgments We thank Exelixis, AstraZeneca, and Pfizer for providing the TK inhibitors for investigation and Jackie Senior for editing the manuscript. 117
11 References 1. de Groot JW, Links TP, Plukker JT, Lips CJ, Hofstra RM. RET as a diagnostic and therapeutic target in sporadic and hereditary endocrine tumors. Endocr Rev 2006;27: Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994;367: Eng C, Mulligan LM. Mutations of the RET proto-oncogene in the multiple endocrine neoplasia type 2 syndromes, related sporadic tumours, and hirschsprung disease. Hum Mutat 1997;9: Kloos RT, Eng C, Evans DB, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 2009;19: Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2006;16: Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001;344: Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347: McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol 2005;23: Ye L, Santarpia L, Gagel RF. The evolving field of tyrosine kinase inhibitors in the treatment of endocrine tumors. Endocr Rev 2010;31: de Groot JW, Zonnenberg BA, van Ufford-Mannesse PQ, et al. A phase II trial of imatinib therapy for metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab 2007;92: Frank-Raue K, Fabel M, Delorme S, Haberkorn U, Raue F. Efficacy of imatinib mesylate in advanced medullary thyroid carcinoma. Eur J Endocrinol 2007;157: Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 2010;28: Schlumberger MJ, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol 2009;27: Pennell NA, Daniels GH, Haddad RI, et al. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid 2008;18: Wells SA,Jr, Gosnell JE, Gagel RF, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 2010;28: Robinson BG, Paz-Ares L, Krebs A, Vasselli J, Haddad R. Vandetanib (100 mg) in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Endocrinol Metab 2010;95: Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 2008;26: Cohen EE, Needles BM, Cullen KJ, et al. Phase 2 study of sunitinib in refractory thyroid cancer. Journal of Clinical Oncology 2008; Kurzrock R, Sherman S, Hong D, et al. A phase 1 study of XL184, a MET, VEGFR2, and RET kinase inhibitor, administered orally to patients (pts) with advanced malignancies, including a subgroup of pts with medullary thryoid cancer (MTC). EORTC 2008: Plaza-Menacho I, Mologni L, Sala E, et al. Sorafenib functions to potently suppress RET tyrosine kinase activity by direct enzymatic inhibition and promoting RET lysosomal degradation independent of proteasomal targeting. J Biol Chem 2007;282:
12 Effects of TK inhibitors on MTC and PTC cells Supplementary data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum (FBS, BioWhittaker, Lonza,Verviers, Belgium), 1% L-glutamine (Gibco) and 1% penicillin/streptomycin (Gibco). MZ-CRC-1 and HEK293 cell lines were cultured in DMEM high medium containing 4.5 g/l of glucose, L-glutamine and pyruvate (Gibco), supplemented with 15% or 10% (for HEK293) FBS and 1% penicillin/streptomycin. All cells were maintained at 37 C and 5% CO 2 atmosphere. Cell lysates and Western blot analysis Cells were washed with ice-cold PBS and incubated with lysis buffer (m-per, Thermo Fisher Scientific, Rockford, IL, USA) containing protease (Thermo Fisher Scientific) and phosphatase (Thermo Fisher Scientific) inhibitors, for 30 min on ice. Cell lysates were collected by scraping and cleared by centrifugation at 14,000 rpm for 10 min in a pre-cooled (4 C) centrifuge. The following antibodies were used for Western blot: RET (H-300), RET (C-19), p-ret (Y1062) (Santa Cruz Biotechnology, Heidelberg, Germany); ERK, p-erk (Cell Signaling, Danvers, MA, USA) and actin (C4, MP Biomedicals, Illkirch, France). Secondary antibodies used were goat anti-rabbit IgG-HRP (Bio-Rad, Veenendaal, The Netherlands) and goat antimouse IgG-HRP (Bio-Rad). All the experiments were performed in duplicates. RNA extraction and RT-PCR Cells were washed with PBS (Gibco) and RNA extraction was performed using the RNeasy mini kit (Qiagen, Venlo, The Netherlands). First strand cdna was originated using the Ready-To-Go You-Prime First-Strand Beads kit (GE Healthcare, Zeist, The Netherlands) according to the manufacturer s instructions. The following primers were used: RET forward (5 -CCGTGAAGATGCTGAAAGAG-3 ); RET reverse (5 - AGAGGCCGTCGTCATAAATC-3 ); GAPDH forward (5-119
13 TGAAGGTCGGAGTCAACGGATTTGGT-3 ); GAPDH reverse (5 - GCAGAGATGATGACCCTTTTGGCTC -3 ). 120
14 Effects of TK inhibitors on MTC and PTC cells Supplementary Tables Supplementary Table 1 IC50 concentrations of the different TK inhibitors in different cell lines Cell-line Compound MTC-TT MZ-CRC TPC-1 Sunitinib μm μm μm Vandetanib μm μm μm Axitinib μm > 5 μm μm XL μm > 5 μm μm MTC-TT cells express a MEN2A mutation, MZ-CRC-1 cells express a MEN2B mutation, and TPC-1 cells express a RET/PTC rearrangement. Supplementary Table 2 Effect of DMSO on cell proliferation of MZ-CRC-1, MTC-TT and TPC-1 Cell proliferation Control (SD) 0 concentration (SD) Sign. MZ-CRC % (10.2%) 100% (7.0%) 0.35 (NS) MTC-TT 94.2% (18.4%) 100% (14.5%) 0.11 (NS) TPC % (10.4%) 100% (12.1%) 0.93 (NS) Control cells were grown without DMSO or inhibitors, cells in the 0 condition were grown without inhibitors but in the presence of 0.1% DMSO. 121
15 Supplementary Figures Supplementary Figure 1 Additional dose response curves for (A) MTC-TT cells treated with sunitinib, (B) MTC-TT cells treated with axitinib, (C) MZ-CRC-1 cells treated with sunitinib and (D) TPC-1 cells treated with axitinb. 122
16 Effects of TK inhibitors on MTC and PTC cells Supplementary Figure 2 (A) Dose response curves for HEK293 cell line incubated with different concentrations of XL184, vandetanib, sunitinib and axitinib. (B) Overview of the IC50 values (the concentration that lead to 50% cell death) of the four tyrosine kinase inhibitors for MTC-TT, MZ-CRC-1 and TPC-1 cell lines. Error bars shown correspond to standard deviation. * Concentrations are above 5 µm. 123
17 Supplementary Figure 3 Effects of XL184 and vandetanib on RET mrna levels. (A) MTC-TT cells treated with IC50 concentration and 5 µm of XL184 showed no effect on RET transcription levels. (B) MZ-CRC-1 cells treated with IC50 concentration and 5 µm of vandetanib showed a decrease in RET transcription levels after prolonged exposure (5 days) to 5 µm of this inhibitor. (C) Graphical representation of RET bands intensity after correction for GAPDH levels confirmed a reduction of RET mrna levels for MZ-CRC-1 cells after prolonged exposure to 5 µm of vandetanib. MTC-TT cells express a MEN2A mutation (RET C634R) and MZ-CRC-1 cells express a MEN2B mutation (RET M918T). IC50 = concentration that leads to 50% cell death. GAPDH was used as an internal control. 124
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Cabozantinib for medullary thyroid cancer. February 2012
 Cabozantinib for medullary thyroid cancer February 2012 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Cabozantinib for medullary thyroid cancer February 2012 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
An update on systemic treatment of differentiated and medullary thyroid cancers: What to do after RAI
 An update on systemic treatment of differentiated and medullary thyroid cancers: What to do after AI Disclosures: clinical trial support: - Exelixis, BI, Bayer, ECOG, TOG, GSK - Actogenix, Proacta, BMS,
An update on systemic treatment of differentiated and medullary thyroid cancers: What to do after AI Disclosures: clinical trial support: - Exelixis, BI, Bayer, ECOG, TOG, GSK - Actogenix, Proacta, BMS,
Promising New Treatments for Metastatic Differentiated and Medullary Thyroid Cancer. Marcia Brose MD PhD
 Promising New Treatments for Metastatic Differentiated and Medullary Thyroid Cancer Marcia Brose MD PhD Department of Otorhinolaryngology: Head and Neck Surgery Department of Medicine, Division of Hematology/Oncology
Promising New Treatments for Metastatic Differentiated and Medullary Thyroid Cancer Marcia Brose MD PhD Department of Otorhinolaryngology: Head and Neck Surgery Department of Medicine, Division of Hematology/Oncology
National Horizon Scanning Centre. Vandetanib (Zactima) for locally advanced or metastatic medullary thyroid cancer. December 2007
 Vandetanib (Zactima) for locally advanced or metastatic medullary thyroid cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
Vandetanib (Zactima) for locally advanced or metastatic medullary thyroid cancer December 2007 This technology summary is based on information available at the time of research and a limited literature
3/29/2012. Thyroid cancer- what s new. Thyroid Cancer. Thyroid cancer is now the most rapidly increasing cancer in women
 Thyroid cancer- what s new Thyroid Cancer Changing epidemiology Molecular markers Lymph node dissection Technical advances rhtsh Genetic testing and prophylactic surgery Vandetanib What s new? Jessica
Thyroid cancer- what s new Thyroid Cancer Changing epidemiology Molecular markers Lymph node dissection Technical advances rhtsh Genetic testing and prophylactic surgery Vandetanib What s new? Jessica
MINERVA MEDICA COPYRIGHT
 Q J NUCL MED MOL IMAGING 2009;53:520-5 Targeted therapy in radioiodine refractory thyroid cancer The majority of differentiated thyroid carcinomas (DTCs) of follicular cell origin are cured with adequate
Q J NUCL MED MOL IMAGING 2009;53:520-5 Targeted therapy in radioiodine refractory thyroid cancer The majority of differentiated thyroid carcinomas (DTCs) of follicular cell origin are cured with adequate
New Developments in Thyroid Cancer
 New Developments in Thyroid Cancer Eric J. Sherman, MD Professor Vice-Chair for Clinical Operations Chief, Division of Head and Neck Surgery Departments of Otolaryngology, Radiation Oncology, and Immunology
New Developments in Thyroid Cancer Eric J. Sherman, MD Professor Vice-Chair for Clinical Operations Chief, Division of Head and Neck Surgery Departments of Otolaryngology, Radiation Oncology, and Immunology
Medullary Thyroid Carcinoma: New Therapies and Trials
 Medullary Thyroid Carcinoma: New Therapies and Trials Matthew D. Ringel, MD Ralph W. Kurtz Chair and Professor of Medicine Director, Division of Endocrinology, Diabetes, and Metabolism The Ohio State University
Medullary Thyroid Carcinoma: New Therapies and Trials Matthew D. Ringel, MD Ralph W. Kurtz Chair and Professor of Medicine Director, Division of Endocrinology, Diabetes, and Metabolism The Ohio State University
Oncologist. The. The Community Oncologist: Case Report. A Case of Advanced Medullary Thyroid Carcinoma Successfully Treated with Sunitinib
 The Oncologist The Community Oncologist: Case Report A Case of Advanced Medullary Thyroid Carcinoma Successfully Treated with Sunitinib MARIA JOÃO BUGALHO, a c RITA DOMINGUES, b ALEXANDRA BORGES d a Serviço
The Oncologist The Community Oncologist: Case Report A Case of Advanced Medullary Thyroid Carcinoma Successfully Treated with Sunitinib MARIA JOÃO BUGALHO, a c RITA DOMINGUES, b ALEXANDRA BORGES d a Serviço
Medullary Thyroid Cancer: Medullary Thyroid Cancer
 Review & Update Nothing to disclose. Jessica E. Gosnell MD Assistant Professor in Residence Department of Surgery November 9, 2012 Medullary Thyroid Cancer MTC has distinct embryology, genetic association
Review & Update Nothing to disclose. Jessica E. Gosnell MD Assistant Professor in Residence Department of Surgery November 9, 2012 Medullary Thyroid Cancer MTC has distinct embryology, genetic association
Citation for published version (APA): Verbeek, H. (2015). Medullary Thyroid Carcinoma: from diagnosis to treatment [S.l.]: [S.n.]
![Citation for published version (APA): Verbeek, H. (2015). Medullary Thyroid Carcinoma: from diagnosis to treatment [S.l.]: [S.n.] Citation for published version (APA): Verbeek, H. (2015). Medullary Thyroid Carcinoma: from diagnosis to treatment [S.l.]: [S.n.]](/thumbs/75/72603221.jpg) University of Groningen Medullary Thyroid Carcinoma Verbeek, Hans IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Medullary Thyroid Carcinoma Verbeek, Hans IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
2014 Debates and Didactics in Hematology and Oncology New treatments in the management of thyroid cancer
 2014 Debates and Didactics in Hematology and Oncology New treatments in the management of thyroid cancer Taofeek K. Owonikoko, MD/PhD Associate Professor Department of Hematology/Medical Oncology Emory
2014 Debates and Didactics in Hematology and Oncology New treatments in the management of thyroid cancer Taofeek K. Owonikoko, MD/PhD Associate Professor Department of Hematology/Medical Oncology Emory
Vandetanib (ZD6474) in the Treatment of Medullary Thyroid Cancer
 Clinical Medicine Insights: Oncology Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Vandetanib (ZD6474) in the Treatment of Medullary Thyroid Cancer
Clinical Medicine Insights: Oncology Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Vandetanib (ZD6474) in the Treatment of Medullary Thyroid Cancer
Radioiodine-refractory DTC
 Oncology: Radioiodine-refractory DTC New Developments in Giuseppe COSTANTE, MD, Head, Endocrinology Clinic Institut Jules Bordet Université Libre de Bruxelles (U.L.B.) Targeted Therapies Targeted Treatments
Oncology: Radioiodine-refractory DTC New Developments in Giuseppe COSTANTE, MD, Head, Endocrinology Clinic Institut Jules Bordet Université Libre de Bruxelles (U.L.B.) Targeted Therapies Targeted Treatments
Improving quality of care for patients with ovarian and endometrial cancer Eggink, Florine
 University of Groningen Improving quality of care for patients with ovarian and endometrial cancer Eggink, Florine IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if
University of Groningen Improving quality of care for patients with ovarian and endometrial cancer Eggink, Florine IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Rossella Elisei. Department of Endocrinology, University Hospital, Pisa, Italy
 Rossella Elisei Department of Endocrinology, University Hospital, Pisa, Italy THYROID CANCER IS RARE TUMOR AND REPRESENTS ONLY 3.8% OF ALL HUMAN TUMORS All human cancer Thyroid cancer MOST FREQUENT CANCER
Rossella Elisei Department of Endocrinology, University Hospital, Pisa, Italy THYROID CANCER IS RARE TUMOR AND REPRESENTS ONLY 3.8% OF ALL HUMAN TUMORS All human cancer Thyroid cancer MOST FREQUENT CANCER
Thyroid Nodules. Dr. HAKIMI, SpAK Dr. MELDA DELIANA, SpAK Dr. SISKA MAYASARI LUBIS, SpA
 Thyroid Nodules ENDOCRINOLOGY DIVISION ENDOCRINOLOGY DIVISION Dr. HAKIMI, SpAK Dr. MELDA DELIANA, SpAK Dr. SISKA MAYASARI LUBIS, SpA Anatomical Considerations The Thyroid Nodule Congenital anomalies Thyroglossal
Thyroid Nodules ENDOCRINOLOGY DIVISION ENDOCRINOLOGY DIVISION Dr. HAKIMI, SpAK Dr. MELDA DELIANA, SpAK Dr. SISKA MAYASARI LUBIS, SpA Anatomical Considerations The Thyroid Nodule Congenital anomalies Thyroglossal
COME HOME Innovative Oncology Business Solutions, Inc.
 COME HOME Thyroid Cancer pathway development worksheet, v9 April 13, 2015 Required Structured Data: Stage Staging Components Staging Date Histology Quality Measure(s): Staging (clinical or pathologic)
COME HOME Thyroid Cancer pathway development worksheet, v9 April 13, 2015 Required Structured Data: Stage Staging Components Staging Date Histology Quality Measure(s): Staging (clinical or pathologic)
Subject: Vandetanib (Caprelsa ) Tablets
 09-J1000-38 Original Effective Date: 10/15/11 Reviewed: 11/14/18 Revised: 12/15/18 Subject: Vandetanib (Caprelsa ) Tablets THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J1000-38 Original Effective Date: 10/15/11 Reviewed: 11/14/18 Revised: 12/15/18 Subject: Vandetanib (Caprelsa ) Tablets THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Citation for published version (APA): Bleeker, W. A. (2001). Therapeutic considerations in Dukes C colon cancer s.n.
 University of Groningen Therapeutic considerations in Dukes C colon cancer Bleeker, Willem Aldert IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
University of Groningen Therapeutic considerations in Dukes C colon cancer Bleeker, Willem Aldert IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite
Dr Catherine Woolnough, Hospital Scientist, Chemical Pathology, Royal Prince Alfred Hospital. NSW Health Pathology University of Sydney
 Dr Catherine Woolnough, Hospital Scientist, Chemical Pathology, Royal Prince Alfred Hospital NSW Health Pathology University of Sydney Thyroid Cancer TC incidence rates in NSW Several subtypes - Papillary
Dr Catherine Woolnough, Hospital Scientist, Chemical Pathology, Royal Prince Alfred Hospital NSW Health Pathology University of Sydney Thyroid Cancer TC incidence rates in NSW Several subtypes - Papillary
Carcinoma de Tiroide: Teràpies Diana
 Carcinoma de Tiroide: Teràpies Diana Jaume Capdevila, MD GI and Endocrine Tumor Unit Vall d Hebron University Hospital Developmental Therapeutics Unit Vall d Hebron Institute of Oncology THYROID CANCER:
Carcinoma de Tiroide: Teràpies Diana Jaume Capdevila, MD GI and Endocrine Tumor Unit Vall d Hebron University Hospital Developmental Therapeutics Unit Vall d Hebron Institute of Oncology THYROID CANCER:
University of Groningen. Cardiotoxicity after anticancer treatment Perik, Patrick Jozef
 University of Groningen Cardiotoxicity after anticancer treatment Perik, Patrick Jozef IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it.
University of Groningen Cardiotoxicity after anticancer treatment Perik, Patrick Jozef IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it.
2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein
![2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked. amino-modification products by acrolein](/thumbs/86/94743397.jpg) Supplementary Information 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein Ayumi Tsutsui and Katsunori Tanaka* Biofunctional Synthetic Chemistry Laboratory, RIKEN
Supplementary Information 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein Ayumi Tsutsui and Katsunori Tanaka* Biofunctional Synthetic Chemistry Laboratory, RIKEN
Medullary Thyroid Cancer. Caroline S. Kim, MD Perelman School of Medicine at the University of Pennsylvania February 13, 2016
 Medullary Thyroid Cancer Caroline S. Kim, MD Perelman School of Medicine at the University of Pennsylvania February 13, 2016 I have no disclosures 30 minutes on Medullary Thyroid Cancer (MTC) Classification
Medullary Thyroid Cancer Caroline S. Kim, MD Perelman School of Medicine at the University of Pennsylvania February 13, 2016 I have no disclosures 30 minutes on Medullary Thyroid Cancer (MTC) Classification
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
ATA Guidelines for Medullary Thyroid Cancer: approach to initial management of sporadic and inherited disease
 ATA Guidelines for Medullary Thyroid Cancer: approach to initial management of sporadic and inherited disease Richard T. Kloos, M.D. The Ohio State University Divisions of Endocrinology and Nuclear Medicine
ATA Guidelines for Medullary Thyroid Cancer: approach to initial management of sporadic and inherited disease Richard T. Kloos, M.D. The Ohio State University Divisions of Endocrinology and Nuclear Medicine
Persistent & Recurrent Differentiated Thyroid Cancer
 Persistent & Recurrent Differentiated Thyroid Cancer Electron Kebebew University of California, San Francisco Department of Surgery Objectives Risk factors for persistent & recurrent disease Causes of
Persistent & Recurrent Differentiated Thyroid Cancer Electron Kebebew University of California, San Francisco Department of Surgery Objectives Risk factors for persistent & recurrent disease Causes of
BHP 2-7 and Nthy-ori 3-1 cells were grown in RPMI1640 medium (Hyclone) supplemented with 10% fetal bovine serum (Gibco), 2mM L-glutamine, and 100 U/mL
 1 2 3 4 Materials and Methods Cell culture BHP 2-7 and Nthy-ori 3-1 cells were grown in RPMI1640 medium (Hyclone) 5 supplemented with 10% fetal bovine serum (Gibco), 2mM L-glutamine, and 100 U/mL 6 penicillin-streptomycin.
1 2 3 4 Materials and Methods Cell culture BHP 2-7 and Nthy-ori 3-1 cells were grown in RPMI1640 medium (Hyclone) 5 supplemented with 10% fetal bovine serum (Gibco), 2mM L-glutamine, and 100 U/mL 6 penicillin-streptomycin.
The c-ret pathway and. K. Homicsko, Lucerne
 The c-ret pathway and biomarkers K. Homicsko, 2.11.12 Lucerne Origins 1. c-ret is a proto-oncogene on chromosome 10 (10q11.2) 2. «rearranged during transfection» 3. Synonyms: CDHF12, HSCR1, MEN2A, MEN2B,
The c-ret pathway and biomarkers K. Homicsko, 2.11.12 Lucerne Origins 1. c-ret is a proto-oncogene on chromosome 10 (10q11.2) 2. «rearranged during transfection» 3. Synonyms: CDHF12, HSCR1, MEN2A, MEN2B,
Case-Based Discussion of Thyroid Cancer Therapy
 Case-Based Discussion of Thyroid Cancer Therapy Matthew D. Ringel, MD Ralph W. Kurtz Chair and Professor of Medicine Director, Division of Endocrinology The Ohio State University Co-Leader, Molecular Biology
Case-Based Discussion of Thyroid Cancer Therapy Matthew D. Ringel, MD Ralph W. Kurtz Chair and Professor of Medicine Director, Division of Endocrinology The Ohio State University Co-Leader, Molecular Biology
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Section 2 Original Policy Date 2013 Last Review Status/Date September 1, 2014
 Policy Number 2.04.82 Molecular Markers in Fine Needle Aspirates of the Thyroid Medical Policy Section 2 Original Policy Date 2013 Last Review Status/Date September 1, 2014 Disclaimer Our medical policies
Policy Number 2.04.82 Molecular Markers in Fine Needle Aspirates of the Thyroid Medical Policy Section 2 Original Policy Date 2013 Last Review Status/Date September 1, 2014 Disclaimer Our medical policies
University of Groningen
 University of Groningen Cellular effects of imatinib on medullary thyroid cancer cells, harboring multiple endocrine neoplasia Type 2A and 2B associated RET mutations de Groot, J.W.B.; Menacho, I.P.; Schepers,
University of Groningen Cellular effects of imatinib on medullary thyroid cancer cells, harboring multiple endocrine neoplasia Type 2A and 2B associated RET mutations de Groot, J.W.B.; Menacho, I.P.; Schepers,
Supplementary Materials and Methods
 Supplementary Materials and Methods Immunoblotting Immunoblot analysis was performed as described previously (1). Due to high-molecular weight of MUC4 (~ 950 kda) and MUC1 (~ 250 kda) proteins, electrophoresis
Supplementary Materials and Methods Immunoblotting Immunoblot analysis was performed as described previously (1). Due to high-molecular weight of MUC4 (~ 950 kda) and MUC1 (~ 250 kda) proteins, electrophoresis
BLU-667 is a potent and highly selective RET inhibitor in development for RET-driven thyroid cancers
 BLU-667 is a potent and highly selective RET inhibitor in development for RET-driven thyroid cancers Rami Rahal, PhD Blueprint Medicines July 30, 2017 2 Disclosure Employee and shareholder of Blueprint
BLU-667 is a potent and highly selective RET inhibitor in development for RET-driven thyroid cancers Rami Rahal, PhD Blueprint Medicines July 30, 2017 2 Disclosure Employee and shareholder of Blueprint
University of Groningen. Hidradenitis suppurativa Dickinson-Blok, Janine Louise
 University of Groningen Hidradenitis suppurativa Dickinson-Blok, Janine Louise IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen Hidradenitis suppurativa Dickinson-Blok, Janine Louise IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Cell Culture. The human thyroid follicular carcinoma cell lines FTC-238, FTC-236 and FTC-
 Supplemental material and methods Reagents. Hydralazine was purchased from Sigma-Aldrich. Cell Culture. The human thyroid follicular carcinoma cell lines FTC-238, FTC-236 and FTC- 133, human thyroid medullary
Supplemental material and methods Reagents. Hydralazine was purchased from Sigma-Aldrich. Cell Culture. The human thyroid follicular carcinoma cell lines FTC-238, FTC-236 and FTC- 133, human thyroid medullary
Citation for published version (APA): Francken, A. B. (2007). Primary and metastatic melanoma: aspects of follow-up and staging s.n.
 University of Groningen Primary and metastatic melanoma Francken, Anne Brecht IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen Primary and metastatic melanoma Francken, Anne Brecht IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
8/20/2017. Disclosures. Systemic Therapy for Thyroid Cancer: Who, When, and Why? Objectives. Thyroid cancer epidemiology
 Disclosures Systemic Therapy for Thyroid Cancer: Who, When, and Why? Steven P. Weitzman, MD, FACE, ECNU The University of Texas MD Anderson Cancer Center Department of Endocrine Neoplasia and Hormonal
Disclosures Systemic Therapy for Thyroid Cancer: Who, When, and Why? Steven P. Weitzman, MD, FACE, ECNU The University of Texas MD Anderson Cancer Center Department of Endocrine Neoplasia and Hormonal
National Horizon Scanning Centre. Imatinib (Glivec) for adjuvant therapy in gastrointestinal stromal tumours. August 2008
 Imatinib (Glivec) for adjuvant therapy in gastrointestinal stromal tumours August 2008 This technology summary is based on information available at the time of research and a limited literature search.
Imatinib (Glivec) for adjuvant therapy in gastrointestinal stromal tumours August 2008 This technology summary is based on information available at the time of research and a limited literature search.
Votrient. Votrient (pazopanib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.83 Subject: Votrient Page: 1 of 6 Last Review Date: December 2, 2016 Votrient Description Votrient
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.83 Subject: Votrient Page: 1 of 6 Last Review Date: December 2, 2016 Votrient Description Votrient
Votrient. Votrient (pazopanib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.83 Subject: Votrient Page: 1 of 6 Last Review Date: June 22, 2018 Votrient Description Votrient (pazopanib)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.83 Subject: Votrient Page: 1 of 6 Last Review Date: June 22, 2018 Votrient Description Votrient (pazopanib)
Initial Lymph Node Dissection Increases Cure Rates in Patients with Medullary Thyroid Cancer
 Original Article Initial Lymph Node Dissection Increases Cure Rates in Patients with Medullary Thyroid Cancer David Yü Greenblatt, Diane Elson, 1 Eberhard Mack and Herbert Chen, Departments of Surgery
Original Article Initial Lymph Node Dissection Increases Cure Rates in Patients with Medullary Thyroid Cancer David Yü Greenblatt, Diane Elson, 1 Eberhard Mack and Herbert Chen, Departments of Surgery
Transgenic Mice and Genetargeting
 Transgenic Mice and Genetargeting mice In Biomedical Science Techniques of transgenic and gene-targeting mice are indispensable for analyses of in vivo functions of particular genes and roles of their
Transgenic Mice and Genetargeting mice In Biomedical Science Techniques of transgenic and gene-targeting mice are indispensable for analyses of in vivo functions of particular genes and roles of their
Supplementary Figure 1
 Supplementary Figure 1 60 HRAS G12V 40 * 55 35 Percent BRDU Positive 50 45 40 30 20 NS Percent BRDU Positive 30 25 20 15 10 NS 10 5 0 -dox dox doxkt5720 -doxkt5720 Treatment TSHKT5720 0 -dox dox doxkt5720
Supplementary Figure 1 60 HRAS G12V 40 * 55 35 Percent BRDU Positive 50 45 40 30 20 NS Percent BRDU Positive 30 25 20 15 10 NS 10 5 0 -dox dox doxkt5720 -doxkt5720 Treatment TSHKT5720 0 -dox dox doxkt5720
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Review Article Cellular Signaling Pathway Alterations and Potential Targeted Therapies for Medullary Thyroid Carcinoma
 International Journal of Endocrinology Volume 2013, Article ID 803171, 16 pages http://dx.doi.org/10.1155/2013/803171 Review Article Cellular Signaling Pathway Alterations and Potential Targeted Therapies
International Journal of Endocrinology Volume 2013, Article ID 803171, 16 pages http://dx.doi.org/10.1155/2013/803171 Review Article Cellular Signaling Pathway Alterations and Potential Targeted Therapies
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Gerard M. Doherty, MD
 Surgical Management of Differentiated Thyroid Cancer: Update on 2015 ATA Guidelines Gerard M. Doherty, MD Chair of Surgery Utley Professor of Surgery and Medicine Boston University Surgeon-in-Chief Boston
Surgical Management of Differentiated Thyroid Cancer: Update on 2015 ATA Guidelines Gerard M. Doherty, MD Chair of Surgery Utley Professor of Surgery and Medicine Boston University Surgeon-in-Chief Boston
Calcitonin. 1
 Calcitonin Medullary thyroid carcinoma (MTC) is characterized by a high concentration of serum calcitonin. Routine measurement of serum calcitonin concentration has been advocated for detection of MTC
Calcitonin Medullary thyroid carcinoma (MTC) is characterized by a high concentration of serum calcitonin. Routine measurement of serum calcitonin concentration has been advocated for detection of MTC
Review Article Thyroid Cancer: Molecular Aspects and New Therapeutic Strategies
 Journal of Thyroid Research Volume 2012, Article ID 847108, 10 pages doi:10.1155/2012/847108 Review Article Thyroid Cancer: Molecular Aspects and New Therapeutic Strategies Enrique Grande, 1 Juan JoséDíez,
Journal of Thyroid Research Volume 2012, Article ID 847108, 10 pages doi:10.1155/2012/847108 Review Article Thyroid Cancer: Molecular Aspects and New Therapeutic Strategies Enrique Grande, 1 Juan JoséDíez,
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
In vitro studies on the cytoprotective properties of Carbon monoxide releasing molecules and N-acyl dopamine derivatives Stamellou, Eleni
 University of Groningen In vitro studies on the cytoprotective properties of Carbon monoxide releasing molecules and N-acyl dopamine derivatives Stamellou, Eleni IMPORTANT NOTE: You are advised to consult
University of Groningen In vitro studies on the cytoprotective properties of Carbon monoxide releasing molecules and N-acyl dopamine derivatives Stamellou, Eleni IMPORTANT NOTE: You are advised to consult
MANAGEMENT OF REFRACTORY THYROID CANCER RAJKUMAR VENKATRAMANI, MD, MS RARE TUMORS PROGRAM TEXAS CHILDREN S HOSPITAL
 MANAGEMENT OF REFRACTORY THYROID CANCER RAJKUMAR VENKATRAMANI, MD, MS RARE TUMORS PROGRAM TEXAS CHILDREN S HOSPITAL CONFLICTS OF INTEREST Policies and standards of the Texas Medical Association, the Accreditation
MANAGEMENT OF REFRACTORY THYROID CANCER RAJKUMAR VENKATRAMANI, MD, MS RARE TUMORS PROGRAM TEXAS CHILDREN S HOSPITAL CONFLICTS OF INTEREST Policies and standards of the Texas Medical Association, the Accreditation
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 1101-7 Program Prior Authorization/Notification Medication Sutent (sunitinib malate) P&T Approval Date 8/2008, 6/2009, 6/2010,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 1101-7 Program Prior Authorization/Notification Medication Sutent (sunitinib malate) P&T Approval Date 8/2008, 6/2009, 6/2010,
Corporate Medical Policy
 Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Case Report Simultaneous medullary thyroid carcinoma and pheochromocytoma: a case report of MEN2A
 Int J Clin Exp Med 2016;9(6):12269-12274 www.ijcem.com /ISSN:1940-5901/IJCEM0021871 Case Report Simultaneous medullary thyroid carcinoma and pheochromocytoma: a case report of MEN2A Lei Zhao, Cheng Yang,
Int J Clin Exp Med 2016;9(6):12269-12274 www.ijcem.com /ISSN:1940-5901/IJCEM0021871 Case Report Simultaneous medullary thyroid carcinoma and pheochromocytoma: a case report of MEN2A Lei Zhao, Cheng Yang,
OMONDI OGUDE MEDICAL ONCOLOGY
 OMONDI OGUDE MEDICAL ONCOLOGY Personalized medicine (Targeted therapy) In recent years there s been a move from conventional cytotoxic therapy to more targeted therapy Mostly due to the rapid pace of
OMONDI OGUDE MEDICAL ONCOLOGY Personalized medicine (Targeted therapy) In recent years there s been a move from conventional cytotoxic therapy to more targeted therapy Mostly due to the rapid pace of
S4-NEW TREATMENT ALGORITHMS FOR SYSTEMIC THERAPY IN MANAGING AGGRESSIVE THYROID CANCER
 S4-NEW TREATMENT ALGORITHMS FOR SYSTEMIC THERAPY IN MANAGING AGGRESSIVE THYROID CANCER Joshua Klopper and Bryan Haugen University of Colorado School of Medicine An important subset of patients with well
S4-NEW TREATMENT ALGORITHMS FOR SYSTEMIC THERAPY IN MANAGING AGGRESSIVE THYROID CANCER Joshua Klopper and Bryan Haugen University of Colorado School of Medicine An important subset of patients with well
Genetic Testing in Medullary Thyroid Carcinoma
 Genetic Testing in Medullary Thyroid Carcinoma Presenter-Dr Sunil Malla Bujar Barua Moderator- Prof Gaurav Agarwal 1 Genetic testing in MTC 24/4/2012 Background 1959 Hazard et al first described MTC 1961
Genetic Testing in Medullary Thyroid Carcinoma Presenter-Dr Sunil Malla Bujar Barua Moderator- Prof Gaurav Agarwal 1 Genetic testing in MTC 24/4/2012 Background 1959 Hazard et al first described MTC 1961
Clinical Policy: Sorafenib (Nexavar) Reference Number: CP.PHAR.69 Effective Date: Last Review Date: Line of Business: HIM, Medicaid
 Clinical Policy: (Nexavar) Reference Number: CP.PHAR.69 Effective Date: 07.01.11 Last Review Date: 05.18 Line of Business: HIM, Medicaid Revision Log See Important Reminder at the end of this policy for
Clinical Policy: (Nexavar) Reference Number: CP.PHAR.69 Effective Date: 07.01.11 Last Review Date: 05.18 Line of Business: HIM, Medicaid Revision Log See Important Reminder at the end of this policy for
University of Groningen. Metabolic risk in people with psychotic disorders Bruins, Jojanneke
 University of Groningen Metabolic risk in people with psychotic disorders Bruins, Jojanneke IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
University of Groningen Metabolic risk in people with psychotic disorders Bruins, Jojanneke IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from
The significance of Helicobacter pylori in the approach of dyspepsia in primary care Arents, Nicolaas Lodevikus Augustinus
 University of Groningen The significance of Helicobacter pylori in the approach of dyspepsia in primary care Arents, Nicolaas Lodevikus Augustinus IMPORTANT NOTE: You are advised to consult the publisher's
University of Groningen The significance of Helicobacter pylori in the approach of dyspepsia in primary care Arents, Nicolaas Lodevikus Augustinus IMPORTANT NOTE: You are advised to consult the publisher's
Protocol for Gene Transfection & Western Blotting
 The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
The schedule and the manual of basic techniques for cell culture Advanced Protocol for Gene Transfection & Western Blotting Schedule Day 1 26/07/2008 Transfection Day 3 28/07/2008 Cell lysis Immunoprecipitation
Department of Endocrine Neoplasia and Hormonal Disorders. Medical Management of Medullary Thyroid Carcinoma
 ENDOPERSPECTIVES Department of Endocrine Neoplasia and Hormonal Disorders N E W S L E T T E R Volume 1, Issue 2 Medical Management of Medullary Thyroid Carcinoma Mimi I. Hu, M.D., Assistant Professor Department
ENDOPERSPECTIVES Department of Endocrine Neoplasia and Hormonal Disorders N E W S L E T T E R Volume 1, Issue 2 Medical Management of Medullary Thyroid Carcinoma Mimi I. Hu, M.D., Assistant Professor Department
MANAGEMENT OF THYROID MALIGNANCIES
 MANAGEMENT OF THYROID MALIGNANCIES Taofeek K. Owonikoko, MD, PhD Associate Professor Department of Hematology/Medical Oncology Winship Cancer Institute of Emory University Atlanta, GA 1 Disclosures Research
MANAGEMENT OF THYROID MALIGNANCIES Taofeek K. Owonikoko, MD, PhD Associate Professor Department of Hematology/Medical Oncology Winship Cancer Institute of Emory University Atlanta, GA 1 Disclosures Research
Sorting and trafficking of proteins in oligodendrocytes during myelin membrane biogenesis Klunder, Lammert
 University of Groningen Sorting and trafficking of proteins in oligodendrocytes during myelin membrane biogenesis Klunder, Lammert IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
University of Groningen Sorting and trafficking of proteins in oligodendrocytes during myelin membrane biogenesis Klunder, Lammert IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's
Ultrasound-Guided Fine-Needle Aspiration of Thyroid Nodules: New events
 Ultrasound-Guided Fine-Needle Aspiration of Thyroid Nodules: New events Sandrine Rorive, M.D., PhD. Erasme Hospital - Université Libre de Bruxelles (ULB) INTRODUCTION The assessment of thyroid nodules
Ultrasound-Guided Fine-Needle Aspiration of Thyroid Nodules: New events Sandrine Rorive, M.D., PhD. Erasme Hospital - Université Libre de Bruxelles (ULB) INTRODUCTION The assessment of thyroid nodules
Endocrine Oncology Thyroid Carcinoma
 Endocrine Oncology Thyroid Carcinoma Current and Future Perspectives in Thyroid Carcinoma Treatment José Manuel Gómez-Sáez Chief Clinician, Endocrinology and Nutrition Service, Bellvitge University Hospital,
Endocrine Oncology Thyroid Carcinoma Current and Future Perspectives in Thyroid Carcinoma Treatment José Manuel Gómez-Sáez Chief Clinician, Endocrinology and Nutrition Service, Bellvitge University Hospital,
University of Groningen. Non-alcoholic fatty liver disease Sheedfar, Fareeba
 University of Groningen Non-alcoholic fatty liver disease Sheedfar, Fareeba IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen Non-alcoholic fatty liver disease Sheedfar, Fareeba IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
Supplementary Figure 1: Tissue of Origin analysis on 152 cell lines. (a) Heatmap representation of the 30 Tissue scores for the 152 cell lines.
 Supplementary Figure 1: Tissue of Origin analysis on 152 cell lines. (a) Heatmap representation of the 30 Tissue scores for the 152 cell lines. The scores summarize the global expression of the tissue
Supplementary Figure 1: Tissue of Origin analysis on 152 cell lines. (a) Heatmap representation of the 30 Tissue scores for the 152 cell lines. The scores summarize the global expression of the tissue
Citation for published version (APA): Otten, M. P. T. (2011). Oral Biofilm as a Reservoir for Antimicrobials Groningen: University of Groningen
 University of Groningen Oral Biofilm as a Reservoir for Antimicrobials Otten, Marieke Petronella Theodora IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish
University of Groningen Oral Biofilm as a Reservoir for Antimicrobials Otten, Marieke Petronella Theodora IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish
University of Groningen. Real-world influenza vaccine effectiveness Darvishian, Maryam
 University of Groningen Real-world influenza vaccine effectiveness Darvishian, Maryam IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
University of Groningen Real-world influenza vaccine effectiveness Darvishian, Maryam IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please
Supporting Information
 Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
Supporting Information Pang et al. 10.1073/pnas.1322009111 SI Materials and Methods ELISAs. These assays were performed as previously described (1). ELISA plates (MaxiSorp Nunc; Thermo Fisher Scientific)
- RET/PTC rearrangement: 20% papillary thyroid cancer - RET: medullary thyroid cancer
 Thyroid Cancer UpToDate: Introduction: Risk Factors: Biology: Symptoms: Diagnosis: 1. Lenvina is the first line therapy with powerful durable response and superior PFS in pts with RAI-refractory disease.
Thyroid Cancer UpToDate: Introduction: Risk Factors: Biology: Symptoms: Diagnosis: 1. Lenvina is the first line therapy with powerful durable response and superior PFS in pts with RAI-refractory disease.
2. Multiple Endocrine Neoplasia Type 2
 2. Multiple Endocrine Neoplasia Type 2 Mimi I. Hu, MD Robert F. Gagel, MD Introduction Multiple endocrine neoplasia type 2 (MEN-2) is a rare, autosomal dominant inherited syndrome characterized by medullary
2. Multiple Endocrine Neoplasia Type 2 Mimi I. Hu, MD Robert F. Gagel, MD Introduction Multiple endocrine neoplasia type 2 (MEN-2) is a rare, autosomal dominant inherited syndrome characterized by medullary
a b G75 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server.
 a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
a b G75 2 2 G60 Sw-2 Sw-1 Supplementary Figure 1. Structure predictions by I-TASSER Server. a. Overlay of top 10 models generated by I-TASSER illustrates the potential effect of 7 amino acid insertion
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 1113-6 Program Prior Authorization/Notification Medication Votrient TM (pazopanib) P&T Approval Date 1/12/2010, 9/2010, 12/2010,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2017 P 1113-6 Program Prior Authorization/Notification Medication Votrient TM (pazopanib) P&T Approval Date 1/12/2010, 9/2010, 12/2010,
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
Supplementary Figure 1 Supplementary Figure 1. Neither the activation nor suppression of the MAPK pathway affects the ASK1/Vif interaction. (a, b) HEK293 cells were cotransfected with plasmids encoding
THYROID CANCER IN CHILDREN. Humberto Lugo-Vicente MD FACS FAAP Professor Pediatric Surgery UPR School of Medicine
 THYROID CANCER IN CHILDREN Humberto Lugo-Vicente MD FACS FAAP Professor Pediatric Surgery UPR School of Medicine Thyroid nodules Rare Female predominance 4-fold as likely to be malignant Hx Radiation exposure?
THYROID CANCER IN CHILDREN Humberto Lugo-Vicente MD FACS FAAP Professor Pediatric Surgery UPR School of Medicine Thyroid nodules Rare Female predominance 4-fold as likely to be malignant Hx Radiation exposure?
Citation for published version (APA): Ebbes, P. (2004). Latent instrumental variables: a new approach to solve for endogeneity s.n.
 University of Groningen Latent instrumental variables Ebbes, P. IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Latent instrumental variables Ebbes, P. IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
APPROCCIO DIAGNOSTICO-TERAPEUTICO TERAPEUTICO AL CARCINOMA DIFFERENZIATO DELLA TIROIDE Sabato 6 aprile 2013 Aula Magna Nuovo Arcispedale S.
 dal 1846 APPROCCIO DIAGNOSTICO-TERAPEUTICO TERAPEUTICO AL CARCINOMA DIFFERENZIATO DELLA TIROIDE Sabato 6 aprile 2013 Aula Magna Nuovo Arcispedale S. Anna Ruolo dell analisi genetica Maria Chiara Zatelli
dal 1846 APPROCCIO DIAGNOSTICO-TERAPEUTICO TERAPEUTICO AL CARCINOMA DIFFERENZIATO DELLA TIROIDE Sabato 6 aprile 2013 Aula Magna Nuovo Arcispedale S. Anna Ruolo dell analisi genetica Maria Chiara Zatelli
University of Groningen
 University of Groningen Mechanisms of Hemagglutinin Targeted Influenza Virus Neutralization Brandenburg, Boerries; Koudstaal, Wouter; Goudsmit, Jaap; Klaren, Vincent; Tang, Chan; Bujny, Miriam V.; Korse,
University of Groningen Mechanisms of Hemagglutinin Targeted Influenza Virus Neutralization Brandenburg, Boerries; Koudstaal, Wouter; Goudsmit, Jaap; Klaren, Vincent; Tang, Chan; Bujny, Miriam V.; Korse,
Current and Future Perspectives in Thyroid Carcinoma Treatment. José Manuel Gómez-Sáez, MD, PhD
 Current and Future Perspectives in Thyroid Carcinoma Treatment José Manuel Gómez-Sáez, MD, PhD Chief Clinician, Endocrinology and Nutrition Service, Bellvitge University Hospital, Barcelona and Professor,
Current and Future Perspectives in Thyroid Carcinoma Treatment José Manuel Gómez-Sáez, MD, PhD Chief Clinician, Endocrinology and Nutrition Service, Bellvitge University Hospital, Barcelona and Professor,
Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San
 Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San Francisco Lung Cancer Classification Pathological Classification
Osamu Tetsu, MD, PhD Associate Professor Department of Otolaryngology-Head and Neck Surgery School of Medicine, University of California, San Francisco Lung Cancer Classification Pathological Classification
Carcinoma midollare tiroideo familiare
 12 AME Italian Meeting 6 Joint Meeting with AACE Carcinoma midollare tiroideo familiare Profilo genetico e stratificazione del rischio Maria Chiara Zatelli Sezione di Endocrinologia Dipartimento di Scienze
12 AME Italian Meeting 6 Joint Meeting with AACE Carcinoma midollare tiroideo familiare Profilo genetico e stratificazione del rischio Maria Chiara Zatelli Sezione di Endocrinologia Dipartimento di Scienze
Coexistence of parathyroid adenoma and papillary thyroid carcinoma. Yong Sang Lee, Kee-Hyun Nam, Woong Youn Chung, Hang-Seok Chang, Cheong Soo Park
 J Korean Surg Soc 2011;81:316-320 http://dx.doi.org/10.4174/jkss.2011.81.5.316 ORIGINAL ARTICLE JKSS Journal of the Korean Surgical Society pissn 2233-7903 ㆍ eissn 2093-0488 Coexistence of parathyroid
J Korean Surg Soc 2011;81:316-320 http://dx.doi.org/10.4174/jkss.2011.81.5.316 ORIGINAL ARTICLE JKSS Journal of the Korean Surgical Society pissn 2233-7903 ㆍ eissn 2093-0488 Coexistence of parathyroid
University of Groningen. BNP and NT-proBNP in heart failure Hogenhuis, Jochem
 University of Groningen BNP and NT-proBNP in heart failure Hogenhuis, Jochem IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
University of Groningen BNP and NT-proBNP in heart failure Hogenhuis, Jochem IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check
supplementary information
 DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
Citation for published version (APA): Verbeek, H. (2015). Medullary Thyroid Carcinoma: from diagnosis to treatment [S.l.]: [S.n.]
![Citation for published version (APA): Verbeek, H. (2015). Medullary Thyroid Carcinoma: from diagnosis to treatment [S.l.]: [S.n.] Citation for published version (APA): Verbeek, H. (2015). Medullary Thyroid Carcinoma: from diagnosis to treatment [S.l.]: [S.n.]](/thumbs/76/73027555.jpg) University of Groningen Medullary Thyroid Carcinoma Verbeek, Hans IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Medullary Thyroid Carcinoma Verbeek, Hans IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Boucher et al NCOMMS B
 1 Supplementary Figure 1 (linked to Figure 1). mvegfr1 constitutively internalizes in endothelial cells. (a) Immunoblot of mflt1 from undifferentiated mouse embryonic stem (ES) cells with indicated genotypes;
1 Supplementary Figure 1 (linked to Figure 1). mvegfr1 constitutively internalizes in endothelial cells. (a) Immunoblot of mflt1 from undifferentiated mouse embryonic stem (ES) cells with indicated genotypes;
Pharmacoeconomic analysis of proton pump inhibitor therapy and interventions to control Helicobacter pylori infection Klok, Rogier Martijn
 University of Groningen Pharmacoeconomic analysis of proton pump inhibitor therapy and interventions to control Helicobacter pylori infection Klok, Rogier Martijn IMPORTANT NOTE: You are advised to consult
University of Groningen Pharmacoeconomic analysis of proton pump inhibitor therapy and interventions to control Helicobacter pylori infection Klok, Rogier Martijn IMPORTANT NOTE: You are advised to consult
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 1067-7 Program Prior Authorization/Notification Medication Nexavar (sorafenib tosylate) P&T Approval Date 8/2008, 6/2009, 6/2010,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 1067-7 Program Prior Authorization/Notification Medication Nexavar (sorafenib tosylate) P&T Approval Date 8/2008, 6/2009, 6/2010,
The Schedule and the Manual of Basic Techniques for Cell Culture
 The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
The Schedule and the Manual of Basic Techniques for Cell Culture 1 Materials Calcium Phosphate Transfection Kit: Invitrogen Cat.No.K2780-01 Falcon tube (Cat No.35-2054:12 x 75 mm, 5 ml tube) Cell: 293
Circulating Tumor DNA in GIST and its Implications on Treatment
 Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
