On the trail of milk bioactive peptides in human and animal intestinal tracts during digestion: A review
|
|
|
- Russell Jordan
- 6 years ago
- Views:
Transcription
1 Dairy Sci. & Technol. (2015) 95: DOI /s REVIEW PAPER On the trail of milk bioactive peptides in human and animal intestinal tracts during digestion: A review Rachel Boutrou & Gwénaële Henry & Laura Sanchez-Rivera Received: 3 June 2014 / Revised: 14 November 2014 / Accepted: 16 December 2014 / Published online: 28 January 2015 # INRA and Springer-Verlag France 2015 Abstract Digestion of proteins leads to the release of numerous peptides in the gastrointestinal tract, among them several bioactive peptides. Thisreviewcompiles, organises and critically analyses the information available on bioactive peptides present in vivo in the digestive tract of adult humans and animals. It focuses on milk proteins, which are recognised as one of the main sources of bioactive peptides with different biological functions. To date, few studies have been performed on the presence of milk-derived peptides in the intestinal tract in vivo. The diversity of these studies is remarkable in terms of the milk products that have been tested, the animal models selected and the segment of the digestive tract considered. Altogether, 69 bioactive peptides identified in vivo are reviewed. Most of them are released from casein, and especially from β-casein, to which 38 bioactive peptides are attributed. These sequences can be classified mainly as angiotensin-converting enzyme (ACE) inhibitors, opioids and antimicrobial peptides. Particular attention is paid to β- casomorphins and caseinomacropeptide-derived peptides. The function of caseinophosphopeptides as a mineral carrier is also discussed. Few bioactive peptides derive from whey proteins. Keywords Milk. Bioactive peptides. Intestinal tract. In vivo R. Boutrou (*): G. Henry INRA, UMR1253, Science et Technologie du Lait et de l Œuf, Rennes, France rachel.boutrou@rennes.inra.fr R. Boutrou: G. Henry AGROCAMPUS OUEST, UMR1253, Science et Technologie du Lait et de l Œuf, Rennes, France L. Sanchez-Rivera Instituto de Investigación en Ciencias de la Alimentación, CIAL (CSIC-UAM), C/ Nicolas Cabrera 9, Campus de Cantoblanco de la Universidad Autónoma de Madrid, Madrid, Spain
2 816 R. Boutrou et al. 1 Introduction Numerous peptides are usually found in the gastrointestinal tract (GIT). Some derive from food-protein digestion while others are released from intestinal cells. Among the food-derived peptides are biologically active peptides, commonly called bioactive peptides. These peptides have been defined as specific protein fragments that have a positive impact on body functions and may ultimately influence health (Kitts and Weiler 2003). Upon oral administration, bioactive peptides may exert an effect on the major body systems, namely the cardiovascular, digestive, immune and nervous systems. Much effort has been devoted to evaluating in vivo effects produced by milk proteins and/or peptides (Fitzgerald et al. 2004; Jäkälä and Vapaatalo 2010; Phelan and Kerins 2011; Ramabadran and Bansinath 1989; Ricci-Cabello et al. 2012; Rutherfurd-Markwick 2012). These studies considered the ingestion of proteins and peptides and either subsequent health effects or the presence of the bioactive peptide in the blood. Few studies have focused on the presence of bioactive peptides in the digestive tract. However, to understand how food provides health benefits, it is necessary to know how food is processed throughout digestion and/or how the peptides consumed are preserved from the action of digestive enzymes. This review focuses on bioactive peptides that have been identified in the digestive tract of adult humans and animals, since digestion, the first process that ingested food undergoes, determines their formation from food proteins and their fate. A wide range of food-derived peptides with a variety of physiological functions has been discovered over recent decades. In particular, milk represents one of the main sources of bioactive peptides. Their beneficial health effects may be classified as antimicrobial, antioxidative, antithrombotic, antihypertensive or immunomodulatory (Choi et al. 2012; Korhonen and Pihlanto 2006). In this review, milk-derived bioactive peptides present in vivo in the GIT were considered in order to provide information about their release and stability throughout digestion. The main difficulty in exhaustively collecting these data lies in compiling two kinds of information: the peptides identified in vivo in the GIT and their reported activity. We gathered peptides that are identified as bioactive in the literature and present in the GIT, even if they need to be subsequently absorbed to reach their target. We also discuss the challenge of identifying bioactive peptides within digestive effluents, which are complex mixtures of peptides and many other components. 2 Diversity of the in vivo studies Few studies have been performed on the presence of milk-derived peptides in the GIT in vivo (Table 1). Their diversity is remarkable in terms of the milk products consumed and the segment of the GIT studied, whether in humans or in animals. The milk products consumed ranged from single molecules to complex dairy matrices. Some examples include purified products, such as a mix of peptides of interest, i.e. phosphopeptides (Brommage et al. 1991; Hirayama et al. 1992; Kasai et al. 1995), caseinomacropeptide (Fosset et al. 2002; Ledoux et al. 1999), a single protein such as lactoferrin (Troost et al. 2001), or milk-protein fractions, i.e. casein and
3 Milk bioactive peptides in intestinal tract 817 Table 1 Studies of milk products present in vivo in the gastrointestinal tract Diet Human Mini-pig Calf Rat Site Products Reference Skim milk x Duodenum Peptides Barbé et al Casein, whey proteins x Jejunum Peptides Boutrou et al Meal with 48% Phe-CMP x Stomach, small intestine CMP Fosset et al Lactoferrin x Stomach Lactoferrin Troost et al α-lactalbumin, whey protein x Stomach, small intestine α-lactalbumin, peptides Pantako et al Whey protein, casein, yogurt x Jejunum, ileum CMP Ledoux et al Milk, yogurt x Stomach, duodenum Peptides Chabance et al CPPs, casein x Jejunum, ileum, cecum CPPs Kasai et al Casein phosphopeptides x Duodenum, jejunum, ileum CPPs Brommage et al Casein phosphopeptides x Duodenum, jejunum, ileum CPPs Hirayama et al Skim milk x Stomach CMP, peptides Scanff et al Casein, skim milk x Stomach Peptides Yvon and Pelissier 1987 Casein x Duodenum, distal jejunum CPPs, β-casomorphin Meisel and Frister 1989 Milk x Small intestine β-casomorphins Svedberg et al CMP caseinomacropeptide, CPPs casein phosphopeptides
4 818 R. Boutrou et al. whey proteins (Boutrou et al. 2013; Meisel and Frister 1989). Some information has been gathered on skim milk, which is more complex and consists of several proteins, salts, minerals and vitamins that interact with each other (Chabance et al. 1998; Scanff et al. 1992; Yvon and Pelissier 1987). Raw whole milk, which contains lipids in addition to these components, is an emulsion of fat globules formed within a waterbased fluid (Svedberg et al. 1985). Some reports also exist on in vivo digestion of yogurt, which is fermented milk whose proteins have been degraded by starter bacteria (Chabance et al. 1998; Ledoux et al. 1999). Furthermore, skim milk can be heated and processed to form a gel to evaluate the influence of matrix structure on protein digestion (Barbé et al. 2014). One must consider the impacts of the food matrix (milk versus casein meal, for example) in terms of human nutrition and biological effects (Ross et al. 2013), not just the peptides released. Barbé et al. (2014) aimed to determine the effect of dairy matrix structure on the milk peptidome generated in the gut lumen of mini-pigs after ingestion of raw and heated milk and their corresponding gels. They concluded that matrix structure strongly affects the kinetics of milk-protein digestion, with raw and heated liquid milk having approximately two and three times as many peptides identified as rennet gels, respectively. The number of bioactive peptides identified was a function of the total number of peptides. The number of biopeptides derived from β- casein in gels decreased most compared to the number in the liquid milks. The authors suggested a higher resistance of rennet gels to hydrolysis; this resistance was not observed in acid gels. All the animal species used in digestion studies are mammals. Calves act as monogastric animals for the first 2 weeks of life, using only the abomasum to digest milk (Scanff et al. 1992; Yvon and Pelissier 1987). Rodents such as rats can be used in physiological studies of the GIT, but the results are less relevant for digestion studies because the digestive enzymes of rodents are more adapted to a cellulose-based diet (e.g. seeds and grains). In contrast, the pig represents the most relevant model to assess the human digestion process because its enzymes and the physiology of its digestive tract are the closest to those of humans (Deglaire et al. 2009).Thediettobetestedcan be a determining factor in the choice of the animal model. A calf needs a 5-kg test meal, i.e. milk (Yvon and Pelissier 1987), while mini-pigs need from 0.1 to 1.0 kg of liquid milk or dairy gels (Barbé et al. 2014) or 100 g of casein (Meisel and Frister 1989). However, a few g of purified protein were adequate for a rat meal (Brommage et al. 1991; Kasai et al. 1995). Around 30 g of casein (Boutrou et al. 2013) or5gof lactoferrin (Troost et al. 2001) was suitable to study their digestion in human. As for the segment of the GIT studied, the literature describes studies conducted from the stomach to the cecum, involving various digestive enzymes. Pepsin is the main digestive enzyme acting on proteins in the stomach. Proteolytic pancreatic enzymes, i.e. trypsin, chymotrypsin and carboxypeptidases, are delivered to the duodenum and thus are present in the small intestine. Enzymes from gut microbiota can also hydrolyse proteins and peptides, especially in the ileum and colon. Other factors play a key role in the digestion of milk proteins, such as the hydrolysis rate of proteins through digestion time. Boirie et al. (1997) defined casein and whey protein as slow and fast proteins, respectively. In addition to the factors cited above, the analysis strategy and the techniques used to detect bioactive peptides within digestive effluents are important issues. The method of analysis applied in these studies has changed in recent decades. Until the 2000s, studies
5 Milk bioactive peptides in intestinal tract 819 mainly dealt with (i) a given bioactive peptide that was detected, and even quantified, using immunological techniques such as ELISA or (ii) a small number of peptides that were identified using liquid chromatography (LC) and Edman degradation to determine the N-terminal amino acid sequence. Since 2010, the use of LC coupled with mass spectrometry (MS) represented a breakthrough and made possible the extensive characterisation of the peptides in digestive effluents. For instance, Boutrou et al. (2013) identified more than 400 peptides from casein and whey proteins in the jejunal content of humans after ingestion of these proteins. Moreover, the use of nanolc-ms/ MS allowed more concentrated samples to be analysed in an extended separation time than conventional LC-MS/MS. Barbé et al. (2014) detected 2975 sequences in effluents from mini-pigs fed raw milk. Both studies give an overall view of the peptidic profile generated in vivo, in which bioactive peptides were particularly tracked. The application of MS-based techniques on the formation of milk peptides, especially bioactive ones, and their changes throughout digestion has been recently reviewed (Picariello et al. 2013; Sanchez-Rivera et al. 2014). 3 Bioactive peptides generated in the digestive tract through in vivo digestion of milk proteins 3.1 Bioactive peptides from β-casein Some bioactive peptides from β-casein (38) have been observed within the GIT in adult animals and humans fed milk products (Table 2). Diverse biological activities have been attributed to these β-casein sequences. The most frequent are angiotensin-converting enzyme (ACE)-inhibitory peptides (22), which originate from different regions of the entire sequence of the protein. ACE is one of the key enzymes implicated in the regulation of blood pressure, and its inhibition represents one well-known mechanism of action for milk-derived peptides (Phelan and Kerins 2011). In addition, several peptides with other reported activities have been found in digestive effluents in vivo. For instance, ten opioid peptides originate mainly from the region 59 80, and four immunomodulatory peptides originate from the C-terminal end. Noteworthy is the similarity displayed by two of the three antioxidant peptides: β-casein( ) has only one more amino acid residue than β-casein( ). Considering the three inhibitors of proteolytic enzymes, one of the sequences, β-casein(59 67) is included in the other, β-casein(58 72). These observations question the minimal active sequence required for a peptide to exert a physiological function in vivo. Particular attention has focused on β-casomorphins (β-cm), opioid-like peptides that behave like a μ-type opioid agonist. They consist of fragments from the β-casein sequence (YPFPGPIPNSL), such as YPFPGPI (β-cm 7) or even shorter fragments from the C-terminal portion, i.e. β-cm 6, β-cm 5 and β-cm 4. The first in vivo demonstration of the presence of β-cm immunoreactive material in intestinal effluents of humans fed raw milk was reported by Svedberg et al. (1985). Although they detected β-cm 7 in duodenal effluents, chromatographical characterisation showed that most of the immunoreactive material was larger than β-cm 7, meaning that it might have been a precursor. This hypothesis was verified in the duodenal chyme
6 820 R. Boutrou et al. Table 2 Sequences of bioactive peptides derived from β-casein and that have been identified in vivo in the gastrointestinal tract of humans or animals Sequence Protein fragment Activity In vivo in GI tract LNVPGEIVE β-casein (6 14) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) NVPGEIVE β-casein (7 14) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) DKIHPF β-casein (47 52) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) SLVYPFPGPI β-casein (57 66) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) LVYPFPGPIPNSLPQ β-casein (58 72) Protease/peptidase inhibitor, antihypertensive (ACE inhibitor) VYPFPGPI β-casein (59 66) Opioid agonist, antihypertensive (ACE inhibitor) Mini-pig (Barbé et al. 2014) Human (Boutrou et al. 2013) VYPFPGPIP β-casein (59 67) Protease/peptidase inhibitor Mini-pig (Barbé et al. 2014); human (Boutrou et al. 2013) VYPFPGPIPN β-casein (59 68) Opioid agonist, antihypertensive Human (Boutrou et al. 2013); mini-pig (ACE inhibitor) (Barbé et al. 2014) YPFP β-casein (60 63) Opioid agonist Human (Svedberg et al. 1985) YPFPGP β-casein (60 65) Opioid agonist Human (Boutrou et al. 2013; Svedberg et al. 1985) YPFPGPI β-casein (60 66) Opioid agonist Human (Boutrou et al. 2013; Svedberg et al. 1985) YPFPGPIP β-casein (60 67) Opioid agonist Human (Boutrou et al. 2013) YPFPGPIPN β-casein (60 68) Opioid agonist, antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013); mini-pig (Barbé et al. 2014) YPFPGPIPNSL β-casein (60 70) Opioid agonist Mini-pig (Meisel and Frister 1989) YPFPGPIPNSLPQNIPPLTQT β-casein (60 80) Opioid agonist Mini-pig (Barbé et al. 2014) PGPIPN β-casein (63 68) Immunomodulator Human (Boutrou et al. 2013) NIPPLTQTPV β-casein (73 82) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) PPLTQTPV β-casein (75 82) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) TPVVVPPFLQP β-casein (80 90) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013)
7 Milk bioactive peptides in intestinal tract 821 Table 2 (continued) Sequence Protein fragment Activity In vivo in GI tract VKEAMAPK β-casein (98 105) Antioxidant Mini-pig (Barbé et al. 2014) EMPFPK β-casein ( ) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) YPVEPF β-casein ( ) Opioid agonist Human (Boutrou et al. 2013); mini-pig (Barbé et al. 2014) VENLHLPLPLL β-casein ( ) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013); mini-pig (Barbé et al. 2014) NLHLPLPLL β-casein ( ) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013); mini-pig (Barbé et al. 2014) LHLPLP β-casein ( ) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) HLPLPLL β-casein ( ) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) SQSKVLPVPQ β-casein ( ) Antihypertensive (ACE inhibitor) Mini-pig (Barbé et al. 2014) SKVLPVPQK β-casein ( ) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) KVLPVPQK β-casein ( ) Antioxidant Mini-pig (Barbé et al. 2014) VLPVPQK β-casein ( ) Antioxidant Human (Boutrou et al. 2013) AVPYPQR β-casein ( ) Antihypertensive (ACE inhibitor) Mini-pig (Barbé et al. 2014) LLYQQPVLGPVRGPFPIIV β-casein ( ) Antihypertensive (ACE inhibitor) Mini-pig (Barbé et al. 2014) LYQEPVLQPVRGPFPIIV β-casein ( ) Immunomodulator Mini-pig (Barbé et al. 2014) YQEPVLQPVR β-casein ( ) Immunomodulator Mini-pigs (Barbé et al. 2014) YQEPVLGPVRGPFPI β-casein ( ) Antimicrobial Mini-pig (Barbé et al. 2014) YQEPVLQPVRGPFPIIV β-casein ( ) Immunomodulator Calf (Yvon and Pelissier 1987); mini-pig (Barbé et al. 2014) QEPVLQPVRGPFPIIV β-casein ( ) Antihypertensive (ACE inhibitor), protease/peptidase inhibitor Mini-pig (Barbé et al. 2014) EPVLQPVRGPFP β-casein ( ) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013)
8 822 R. Boutrou et al. of mini-pigs fed casein, in which β-cm 11 was the main β-cm present (Meisel and Frister 1989). In the human jejunum, β-cms were the most frequently identified bioactive peptides during 6 h of digestion after casein ingestion, while β-cm 11 was not identified using MS (Boutrou et al. 2013). Altogether, these results suggest the presence of β-cm precursors in the duodenum and that of β-cms only in the jejunum, where they can bind opioid receptors. 3.2 Bioactive peptides from α s1 -, α s2 -andκ-caseins Bioactive peptides from α s1 -, α s2 - and κ-caseins (18, seven and six peptides, respectively)havebeenidentifiedinthegitinvivo(table3). Among them, 13 antihypertensive peptides from α s1 -casein (nine peptides), α s2 -casein(twopeptides) and κ-casein (two peptides) have been reviewed. In addition, seven antimicrobial peptides have been reported, including three peptides from the region of α s2 -casein. The other 11 bioactive peptides have diverse activities: antioxidant, opioid, antistress, antithrombotic, protease and peptidase inhibitor, and phosphodiesterase inhibitor. We find only one immunomodulator peptide in vivo in the GIT. 3.3 Caseinomacropeptide within in vivo digests The release of caseinomacropeptide (CMP) during milk-protein digestion in the intestine could be of nutritional and physiological significance since this peptide has been shown to have various biological activities as regulatory compounds, either directly as a neurotransmitter or indirectly by modulating the secretion of intestinal hormones (Thoma-Worringer et al. 2006). CMP in the intestine has an inhibitory effect on gastric acid secretion and stimulates pancreatic secretion through the release of cholecystokinin, although no effect on satiety could be demonstrated. CMP can modulate the cardiovascular system through its antithrombotic and antihypertensive activities. It has also been shown that CMP can exert immunomodulating and antimicrobial effects by inhibiting pathogen adhesion to cells,aswellasinfluenzaviruses. Digestion of CMP was widely studied in the 1990s. It is a 64-amino acid peptide released from κ-casein during the first phase of digestion through the action of gastric enzymes, which rapidly cleaves the peptide bond Phe 105 -Met 106 and produces an N- terminal fragment (para-κ-casein, residue 1 105) and CMP (residue ). CMP is a predominant breakdown product in the stomach of humans (Ledoux et al. 1999) and calves (Yvon and Pelissier 1987) after ingestion of casein, skim milk or yogurt. It is detected in stomach contents during the first few hours of digestion; thus, it resists the action of gastric enzymes. Chabance et al. (1998) also identified the antithrombotic peptide κ-casein( ) in human gastric samples from 20 min to 4 h after milk ingestion. In vivo studies have demonstrated that most intact CMP is rapidly released in the stomach (Fosset et al. 2002; Ledoux et al. 1999). CMP is thus present in the human duodenum and jejunum following casein and yogurt ingestion (Chabance et al. 1998; Ledoux et al. 1999). Thereafter, it undergoes partial hydrolysis by pancreatic enzymes to an extent that depends on its transit rate in the lumen (Ledoux et al. 1999). Some derived fragments are also present in the proximal and median intestine of rats fed Phe-
9 Milk bioactive peptides in intestinal tract 823 Table 3 Sequences of bioactive peptides derived from αs1-, αs2- and κ-casein and that have been identified in vivo in the gastrointestinal tract of humans or animals Sequence Protein fragment Activity In vivo GI tract RPKHPIKHQGLPQEVLNENLLRF αs1-cn (1 23) Antibacterial, immunomodulator Mini-pig (Barbé et al. 2014); calf (Yvon and Pelissier 1987) FFVAPFPEVFGK αs1-cn (23 34) Antihypertensive (ACE inhibitor) Mini-pig (Barbé et al. 2014) FVAPFPEV αs1-cn (24 31) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) FVAPFPEVF α S1 -CN (24 32) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) FVAPFPEVFG αs1-cn (24 33) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) VAPFPEVF αs1-cn (25 32) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) HIQKEDVPSER α S1 -CN (80 90) Antioxidant Human (Boutrou et al. 2013); mini-pig (Barbé et al. 2014) RYLGYL αs1-cn (90 95) Opioid Calf (Yvon and Pelissier 1987) YLGYLEQ αs1-cn (91 97) Antistress Human (Boutrou et al. 2013) YLGYLEQL α s1 -CN (91 98) Antistress Human (Boutrou et al. 2013) YLGYLEQLLR αs1-cn (91 100) Antistress Mini-pig (Barbé et al. 2014) LRLKKYKVPQL αs1-cn (99 109) Antimicrobial Mini-pig (Barbé et al. 2014) KKYKVPQL α S1 -CN ( ) Antihypertensive (ACE inhibitor) Mini-pigs (Barbé et al. 2014) YKVPQL αs1-cn ( ) Antihypertensive (ACE inhibitor) Human (Boutrou et al. 2013) AYFYPEL αs1-cn ( ) Antihypertensive (ACE inhibitor) Human (Chabance et al. 1998); calf (Yvon and Pelissier 1987) YFYPEL αs1-cn ( ) Antioxidant Human (Chabance et al. 1998) DAYPSGAW α s1 -CN ( ) Antihypertensive (ACE inhibitor) Calf (Yvon and Pelissier 1987) SDIPNPIGSENSEK αs1-cn ( ) Antimicrobial Mini-pig (Barbé et al. 2014) ALPQYLKTVYQHQKAMKPWIQPKTKVIPYVRYL αs2-cn ( ) Antimicrobial Mini-pig (Barbé et al. 2014) KTVYQHQKAMKPWIQPKTKVIPYVRYL α s2 -CN ( ) Antimicrobial Mini-pig (Barbé et al. 2014) VYQHQKAMKPWIQPKTKVIPYVRY αs2-cn ( ) Phosphodiesterase inhibitor, protease/ peptidase inhibitor Mini-pig (Barbé et al. 2014)
10 824 R. Boutrou et al. Table 3 (continued) Sequence Protein fragment Activity In vivo GI tract VYQHQKAMKPWIQPKTKVIPYVRYL αs2-cn ( ) Antimicrobial, phosphodiesterase and protease/peptidase inhibitor Mini-pig (Barbé et al. 2014) AMKPWIQPK α s2 -CN ( ) Antihypertensive (ACE inhibitor) Mini-pig (Barbé et al. 2014) AMKPWIQPKTKVIPYVRYL αs2-cn ( ) Phosphodiesterase inhibitor t Mini-pig (Barbé et al. 2014) MKPWIQPK αs2-cn ( ) Antihypertensive (ACE inhibitor) Mini-pig (Barbé et al. 2014) FSDKIAK κ-cn (18 24) Antihypertensive (ACE inhibitor), antimicrobial Mini-pig (Barbé et al. 2014) ARHPHPHLSFM κ-cn (96 106) Antioxidant Mini-pig (Barbé et al. 2014) HPHPHLSF κ-cn (98 105) Antihypertensive (ACE inhibitor) Mini-pig (Barbé et al. 2014) MAIPPK κ-cn ( ) Antithrombotic Human (Boutrou et al. 2013) MAIPPKKNQDK κ-cn ( ) Antithrombotic Human (Chabance et al. 1998; Boutrou et al. 2013); mini-pig (Barbé et al. 2014) KNQDK κ-cn ( ) Antithrombotic Human (Boutrou et al. 2013)
11 Milk bioactive peptides in intestinal tract 825 CMP, but none is bioactive; nonetheless, it is notable that two of them contain the antithrombotic sequence κ-casein( ) (Fosset et al. 2002). Both κ-casein( ) and the aforementioned κ-casein( ) have been identified in the human intestine (Boutrou et al. 2013). 3.4 Caseinophosphopeptides identified in the GIT in vivo Milk s high calcium availability has been attributed to caseinophosphopeptides (CPPs) formed during digestion of casein in the small intestine. CPPs have been described as mineral carriers because of the phosphorylated serine residues in their sequence that bind minerals such as calcium, iron and zinc and thus play an important role in mineral bioavailability by forming soluble complexes that prevent, for example, calcium phosphate from precipitating (Ait-Oukhatar et al. 2002; Sato et al. 1991). Kasai et al. (1995) reported CPPs in the digestive tract of rats fed casein, from the stomach to the colon. They also estimated that the residual CPPs of rats fed casein are not distinguishable from those of rats fed a CPP diet. The large CPP α s1 -casein( ) was found in gastric effluents of calves after ingestion of skim milk (Scanff et al. 1992). In vivo formation of CPP α s1 -casein(66 74) was demonstrated in the distal jejunum of cannulated mini-pigs fed casein (Meisel and Frister 1989). The most abundant CPPs from α s1 -casein present in the ileum of rats when CPPs were ingested were the peptides α s1 - casein(61 74) (Hirayama et al. 1992) andα s1 -casein(59 79) (Brommage et al. 1991). Regarding CPPs from β-casein present in the ileum of rats fed CPPs, the following have been reported: β-casein(1 25), β-casein(1 28), β-casein(33 48) (Brommage et al. 1991) and β-casein(7 24) (Hirayama et al. 1992). Overall, in these studies, CPPs were determined according to the phosphorylation of amino acids in soluble fractions prepared from digestive effluents. In studies whose strategy was to track bioactive peptides within an entire profile of peptides identified by MS, sequences, but not always their degree of phosphorylation, was considered. For CPPs, the presence of the complete phosphorylated seryl-cluster (SpSpSpEE) in the sequence has been described as the main factor influencing their interaction with minerals, although the surrounding residues or the length of the sequence may also have an influence (Cross et al. 2005; Reynolds et al. 1994). Barbé et al. (2014) identifiedalmost150cpps in vivo at different times of digestion in duodenal effluents of mini-pigs fed a variety of dairy products. Most were either mono- or diphosphorylated; Table 4 presents the most frequently identified CPPs. It is important to note that some of the CPPs identified in vivo might be considered mineral carriers due to the presence of phosphoseryl residues in their sequences, whereas their bioactivity has not been experimentally established. Furthermore, some CPPs have been demonstrated to be active when administered individually, while others are active when present in a mixture of CPPs (Ait-Oukhatar et al. 2002). This reinforces the difficulty in identifying active CPPs within digestive effluents. 3.5 Identification of bioactive peptides by MS It is difficult to identify all peptides in a digestive effluent with a single type MS analysis because all ranges of molecular weights (from entire proteins to dipeptides) cannot be screened (Panchaud et al. 2012). C18 reversed-phase LC is the most common
12 826 R. Boutrou et al. Table 4 CPPs derived from caseins that have been identified in vivo in the gastrointestinal tract of mini-pigs (data collected from Barbé et al. 2014) Number of phosphorylated sites Sequence Protein fragment incriminated Numbers of identified CPP Some of the most frequently identified CPP 3 SpSpSpEE β-cn (17 21) 4 β-cn (1 24), β-cn (1 29), α s1 -CN (66 70) 0 β-cn (6 25) α s2 -CN (8 12) 4 α s2 -CN (1 24) α s2 -CN (56 60) 0 2 SpTSpEE α s2 -CN ( ) 7 α s2 -CN ( ), α s2 -CN ( ), α s2 -CN ( ), α s2 -CN ( ) 2 SpESpTE α s1 -CN (46 50) 25 α s1 -CN (40 52), α s1 -CN (40 58), α s1 -CN (40 61), α s1 -CN (40 63), α s1 -CN (43 58) 1 SpEE β-cn (35 37) 51 β-cn (29 44), β-cn (29 45), β-cn (30 52), β-cn (33 48) a, β-cn (33 52) 1 SpTE α s2 -CN ( ) 11 α s2 -CN ( ), α s2 -CN ( ) 1 SpAEE α s1 -CN ( ) 45 α s1 -CN ( ) a, α s1 -CN ( ), α s1 -CN ( ) a, α s1 -CN ( ) Sp=phospho-serine a Also identified in humans (Boutrou et al. 2013) method used to separate peptides before their characterisation by MS. However, small peptides containing some hydrophobic amino acids are not retained in C18 columns (Panchaud et al. 2012). In addition, peptides composed of less than six amino acids are not usually addressed using MS because it is difficult to unambiguously infer them back to one precursor protein. For very small peptides such as di- and tripeptides, separation is required prior to injection into the mass spectrometer (Panchaud et al. 2012). For example, the ACE-inhibitory peptides Ile-Pro-Pro (IPP), Leu-Pro-Pro (LPP), AW, IW, LW, VY, IY and FY were identified in plasma of humans fed a peptide-enriched milk using a LC-multiple reaction ion monitoring-ms (LC-MRM- MS) method(foltz et al. 2007). The authors assessed the bioavailability of IPP and seven other ACE-inhibitory peptides present in a lactotripeptide-enriched yogurt beverage to determine whether meal intake affects IPP bioavailability in humans. They concluded that the tripeptide IPP selectively escapes from intestinal degradation and reaches the blood circulation undegraded. Similarly, IPP, LPP and VPP were intragastrically administered to pigs and reached blood circulation in an intact form (van der Pijl et al. 2008). Meta-analyses on the effect of lactotripeptides on blood pressure in humans also exist (Pripp 2008; Qin et al. 2013). However, to our knowledge, there is no evidence for the identification of di- and tripeptides in vivo in the GIT. Considering the currently identified bioactive peptides from casein (Tables 2 and 3), the smallest is β-cm 4, with four amino acids; it was detected as an immunoreactive material by Svedberg et al. (1985). Sixty-seven bioactive peptides identified in vivo in
13 Milk bioactive peptides in intestinal tract 827 the GIT (Tables 2 and 3) contain more than five amino acids. Focusing on bioactive peptides from casein composed of more than five amino acids, 129 and 126 peptides are referenced in our research unit homemade database and in the BIOPEP database ( respectively. Two assumptions explain why almost half of the reported bioactive peptides are not identified in vivo in the GIT, each with completely different consequences from a nutritional viewpoint: either the region of the protein where the peptide is encrypted was almost completely digested, leading to a negligible concentration of the bioactive peptide, or the bioactive peptides were too large for efficient identification by MS in digestive effluents (Boutrou et al. 2013; Ross et al. 2013). 3.6 Bioactive peptides released from whey proteins in vivo According to databases, a hundred or so bioactive peptides are encrypted in milk whey proteins; however, few have been identified in digestive effluents in vivo. Whey proteins are relatively resistant to peptic digestion, especially β-lactoglobulin (β-lg), due to its particular folding (Dalgalarrondo et al. 1995). Troost et al. (2001) studied human gastric digestion of lactoferrin, an iron-binding protein that favours iron absorption and also displays antioxidative, anti-inflammatory, immunomodulatory and antimicrobial activities (Garcia-Montoya et al. 2012). They concluded that more than 65% of lactoferrin survives gastric transit. Using gel permeation chromatography, they found several lactoferrin fragments of different sizes throughout digestion. The most abundant fragments had molecular weights of 76 and 41 kda and were able to bind the antibodies against intact lactoferrin. Pantako et al. (2001) studied the effect of digestion on dry matter recovery and peptide distribution in the gastrointestinal content of rats fed α-lactalbumin, a metalloprotein that strongly binds calcium and zinc ions and has bactericidal or antitumor activity. Their results indicated that α-lactalbumin rapidly left the stomach and was quickly digested into amino acids and absorbed in the proximal small intestine. It would be interesting to study in vivo the digestion of an α-lactalbumin/oleic acid complex, which has been reported to induce the selective death of tumour cells (Brück et al. 2014). Recently, certain bioactive peptides from whey proteins were identified in digestive effluents using MS. Only three bioactive peptides derived from β-lg were identified in duodenal effluents of mini-pigs fed raw milk (Barbé et al. 2014): two antihypertensive sequences, i.e. β-lg(32 40) and β-lg( ), and the antimicrobial peptide β- Lg(92 100). The latter peptide was not detected in jejunal effluents from humans fed whey proteins. Instead, various related fragments were identified, such as β-lg(92 98), (92 99), (92 103) and (93 100) (Boutrou et al. 2013). Whether these sequences exert any biological activity deserves further investigation. Indeed, some peptides not identified as bioactive can act in situ in the GIT. Further research on the interaction of peptides with intestinal receptors would constitute a prospective strategy on this topic. Among food-derived peptides identified in the GIT, it is also important to note that some may interact in situ with receptors located in the gut. Bioactive peptides that regulate gastrointestinal function and intake have been described (Froetschel 1996; Shimizu and Ok Son 2007), and some of them were detected in digestive effluents, such as CPPs that enhance mineral absorption and opioid peptides that regulate intestinal motility (Shimizu and Ok Son 2007).
14 828 R. Boutrou et al. 4Conclusion This review collected comprehensive data that provides valuable information for (i) understanding physico-chemical parameters of protein digestion (for instance, peptides identified in vivo are important data for correlating the in vitro tools necessary to develop models that mimic protein digestion), (ii) improving protein-design or dietstructure approaches to optimise the release of bioactive peptides and (iii) shedding light on nutrient bioaccessibility (i.e. the percentage of a nutrient digested and released from the food matrix in its absorbable form). Acknowledgments The authors are involved in the Food and Agriculture COST (European Cooperation in Science and Technology) Action FA1005 Improving health properties of food by sharing our knowledge on the digestive process (INFOGEST). Conflict of interest conflicts of interest. Rachel Boutrou, Gwénaële Henry and Laura Sanchez-Rivera declare that they have no References Ait-Oukhatar N, Peres JM, Bouhallab S, Neuville D, Bureau F, Bouvard G, Arhan P, Bouglé D (2002) Bioavailability of caseinophosphopeptide-bound iron. J Lab Clin Med 140: Barbé F, Le Feunteun S, Rémond D, Ménard O, Jardin J, Henry G, Laroche B, Dupont D (2014) Tracking the in vivo release of bioactive peptides in the gut during digestion: mass spectrometry peptidomic characterization of effluents collected in the gut of dairy matrices fed mini-pigs. Food Res Int 63(Part B): Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B (1997) Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci 94: Boutrou R, Gaudichon C, Dupont D, Jardin J, Airinei G, Marsset-Baglieri A, Benamouzig R, Tomé D, Léonil J (2013) Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am J Clin Nutr 19: Brommage R, Juillerat MA, Jost R (1991) Influence of casein phosphopeptides and lactulose on intestinal calcium absorption in adult female rats. Lait 71: Brück WM, Gibson GR, Brück TB (2014) The effect of proteolysis on the induction of cell death by monomeric alpha-lactalbumin. Biochimie 97: Chabance B, Marteau P, Rambaud JC, Migliore-Samour D, Boynard M, Perrotin P, Guillet R, Jolles P, Fiat AM (1998) Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 80: Choi J, Sabikhi L, Hassan A, Anand S (2012) Bioactive peptides in dairy products. Int J Dairy Technol 65:1 12 Cross KJ, Huq NL, Palamara JE, Perich JW, Reynolds EC (2005) Physicochemical characterization of casein phosphopeptide-amorphous calcium phosphate nanocomplexes. J Biol Chem 280: Dalgalarrondo M, Dufour E, Chobert JM, Bertrand-Harb C, Haertlé T (1995) Proteolysis of β-lactoglobulin and β-casein by pepsin in ethanolic media. Int Dairy J 5:1 14 Deglaire A, Bos C, Tome D, Moughan PJ (2009) Ileal digestibility of dietary protein in the growing pig and adult human. Br J Nutr 102: Fitzgerald RJ, Murray BA, Walsh DJ (2004) Hypotensive peptides from milk proteins. J Nutr 134:980S 988S Foltz M, Meynen EE, Bianco V, van Platerink C, Koning TMMG, Kloek J (2007) Angiotensin converting enzyme inhibitory peptides from a lactotripeptide-enriched milk beverage are absorbed intact into the circulation. J Nutr 137: Fosset S, Fromentin G, Gietzen DWU, Dubarry M, Huneau JF, Antoine J, Lang V, Mathieu-Casseron F, Tome D (2002) Peptide fragments released from Phe-caseinomacropeptide in vivo in the rat. Peptides 23: Froetschel MA (1996) Bioactive peptides in digesta that regulate gastrointestinal function and intake. J Anim Sci 74:
15 Milk bioactive peptides in intestinal tract 829 Garcia-Montoya IA, Cendon TS, Arévalo-Gallegos S, Rascon-Cruz Q (2012) Lactoferrin a multiple bioactive protein: an overview. Biochim Biophys Acta Gen Subj 1820: Hirayama M, Toyota K, Hidaka H, Naito H (1992) Phosphopeptides in rat intestinal digests after ingestion casein phosphopeptides. Biosci Biotechnol Biochem 56: Jäkälä P, Vapaatalo H (2010) Antihypertensive peptides from milk proteins. Pharmaceuticals 3: Kasai T, Iwasaki R, Tanaka M, Kiriyama S (1995) Caseinphosphopeptide (CPP) in feces and contents in digestive tract of rats fed casein and CPP preparations. Biosci Biotechnol Biochem 59:26 30 Kitts DD, Weiler K (2003) Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr Pharm Des 9: Korhonen H, Pihlanto A (2006) Bioactive peptides: production and functionality. Int Dairy J 16: Ledoux N, Mahé S, Dubarry M, Bourras M, Benamouzig R, Tomé D (1999) Intraluminal immunoreactive caseinomacropeptide after milk protein ingestion in humans. Nahrung/Food 43: Meisel H, Frister H (1989) Chemical characterization of bioactive peptides from in vivo digests of casein. J Dairy Res 56: Panchaud A, Affolter M, Kussmann M (2012) Mass spectrometry for nutritional peptidomics: how to analyze food bioactives and their health effects. J Proteome 75: Pantako OT, Lemieux L, Amiot J (2001) The effects of alpha-lactabumin and whey protein concentrate on dry matter recovery, TCA soluble protein levels, and peptide distribution in the rat gastrointestinal tract. Can J Physiol Pharmacol 79: Phelan M, Kerins D (2011) The potential role of milk-derived peptides in cardiovascular disease. Food Funct 2: Picariello G, Mamone G, Nitride C, Addeo F, Ferranti P (2013) Protein digestomics: integrated platforms to study food-protein digestion and derived functional and active peptides. Trac-Trends Anal Chem 52: Pripp AH (2008) Effect of peptides derived from food proteins on blood pressure: a meta-analysis of randomised controlled trials. Food Nutr Res 52:8p Qin LQ, Xu JY, Dong JY, Zhao Y, van Bladeren P, Zhang W (2013) Lactotripeptides intake and blood pressure management: a meta-analysis of randomised controlled clinical trials. Nutr Metab Carbiovasc Dis 23: Ramabadran K, Bansinath M (1989) Pharmacology of beta-casomorphins, opioid peptides derived from milk protein. Asia Pac J Pharmacol 4:45 58 Reynolds EC, Riley PF, Adamson NJ (1994) A selective precipitation purification procedure for multiple phosphoseryl-containing peptides and methods for their identification. Anal Biochem 217: Ricci-Cabello I, Olalla Herrera M, Artacho R (2012) Possible role of milk-derived bioactive peptides in the treatment and prevention of metabolic syndrome. Nutr Rev 70: Ross RP, Fitzgerald GF, Stanton C (2013) Unraveling the digestion of milk protein. Am J Clin Nutr 97: Rutherfurd-Markwick KJ (2012) Food proteins as a source of bioactive peptides with diverse functions. Br J Nutr 108:S149 S157 Sanchez-Rivera L, Martinez-Maqueda D, Cruz-Huerta E, Miralles B, Recio I (2014) Peptidomics for discovery, bioavailability and monitoring of dairy bioactive peptides. Food Res Int 63(Part B): Sato R, Shindo M, Gunshin H, Noguchi T, Naito H (1991) Characterization of phosphopeptide derived from bovine beta casein: an inhibitor to intra-intestinal precipitation of calcium phosphate. Biochim Biophys Acta 1077: Scanff P, Yvon M, Thirouin S, Pelissier JP (1992) Characterization and kinetics of gastric emptying of peptides derived from milk proteins in the preruminant calf. J Dairy Res 59: Shimizu M, Ok Son D (2007) Food-derived peptides and intestinal functions. Curr Pharm Des 13: Svedberg J, de Haas J, Leimenstoll G, Paul F, Teschemacher H (1985) Demonstration of [beta]-casomorphin immunoreactive materials in in vitro digests of bovine milk and in small intestine contents after bovine milk ingestion in adult humans. Peptides 6: Thoma-Worringer C, Sorensen J, Lopez-fandino R (2006) Health effects and technological features of caseinomacropeptide. Int Dairy J 16: Troost FJ, Steijns J, Saris WHM, Brummer RJ (2001) Gastric digestion of bovine lactoferrin in vivo in adults. J Nutr 131: van der Pijl PC, Kies AK, Ten Have GAM, Duchateau GSMJ, Deutz NEP (2008) Pharmacokinetics of proline-rich tripeptides in the pig. Peptides 29: Yvon M, Pelissier JP (1987) Characterization and kinetics of evacuation of peptides resulting from casein hydrolysis in the stomach of the calf. J Agric Food Chem 35:
The Science of Proteins in Milk (including A1 vs A2 Milk) Dr. Sabrina Greenwood Department of Animal & Veterinary Sciences
 The Science of Proteins in Milk (including A1 vs A2 Milk) Dr. Sabrina Greenwood Department of Animal & Veterinary Sciences Milk protein 20% 80% Whey proteins Lactalbumin (4%) Lactoglobulin (12%) Other
The Science of Proteins in Milk (including A1 vs A2 Milk) Dr. Sabrina Greenwood Department of Animal & Veterinary Sciences Milk protein 20% 80% Whey proteins Lactalbumin (4%) Lactoglobulin (12%) Other
MILK. Nutritious by nature. The science behind the health and nutritional impact of milk and dairy foods
 MILK Nutritious by nature The science behind the health and nutritional impact of milk and dairy foods Dairy matrix effects It is increasingly recognised that the effects of milk and dairy foods on health
MILK Nutritious by nature The science behind the health and nutritional impact of milk and dairy foods Dairy matrix effects It is increasingly recognised that the effects of milk and dairy foods on health
Biopeptides of milk: caseinophosphopeptides and mineral bioavailability
 Biopeptides of milk: caseinophosphopeptides and mineral bioavailability Saïd Bouhallab, Dominique Bouglé To cite this version: Saïd Bouhallab, Dominique Bouglé. Biopeptides of milk: caseinophosphopeptides
Biopeptides of milk: caseinophosphopeptides and mineral bioavailability Saïd Bouhallab, Dominique Bouglé To cite this version: Saïd Bouhallab, Dominique Bouglé. Biopeptides of milk: caseinophosphopeptides
Caseinophosphopeptides released after tryptic hydrolysis versus simulated. gastrointestinal digestion of a casein-derived by-product
 1 2 Caseinophosphopeptides released after tryptic hydrolysis versus simulated gastrointestinal digestion of a casein-derived by-product 3 4 E. Cruz-Huerta, M. J. García-Nebot, B. Miralles, I. Recio, L.
1 2 Caseinophosphopeptides released after tryptic hydrolysis versus simulated gastrointestinal digestion of a casein-derived by-product 3 4 E. Cruz-Huerta, M. J. García-Nebot, B. Miralles, I. Recio, L.
Chapter 8 The DIDGI System
 Chapter 8 The DIDGI System Olivia Ménard, Daniel Picque, and Didier Dupont Abstract A simple two-compartment in vitro dynamic gastrointestinal digestion system allowing the study of the disintegration
Chapter 8 The DIDGI System Olivia Ménard, Daniel Picque, and Didier Dupont Abstract A simple two-compartment in vitro dynamic gastrointestinal digestion system allowing the study of the disintegration
Dairy matrices: effect of processing and composition on microstructure and nutritional properties Sylvie Turgeon, Laure Rinaldi, Erik Ayala-Bribiesca
 Dairy matrices: effect of processing and composition on microstructure and nutritional properties Sylvie Turgeon, Laure Rinaldi, Erik Ayala-Bribiesca 30 mai 2011 Michel Britten, Sylvie Gauthier, André
Dairy matrices: effect of processing and composition on microstructure and nutritional properties Sylvie Turgeon, Laure Rinaldi, Erik Ayala-Bribiesca 30 mai 2011 Michel Britten, Sylvie Gauthier, André
In vitro digestibility testing
 In vitro digestibility testing Improving health properties of food by sharing our knowledge on the digestive process Prof Alan Mackie, University of Leeds a.r.mackie@leeds.ac.uk Dr. Didier DUPONT, Senior
In vitro digestibility testing Improving health properties of food by sharing our knowledge on the digestive process Prof Alan Mackie, University of Leeds a.r.mackie@leeds.ac.uk Dr. Didier DUPONT, Senior
Development of Nutrient Delivery Systems: Ingredients & Challenges
 Development of Nutrient Delivery Systems David Julian McClements and Hang Xiao Department of Food Science University of Massachusetts Development of Nutrient Delivery Systems: Ingredients & Challenges
Development of Nutrient Delivery Systems David Julian McClements and Hang Xiao Department of Food Science University of Massachusetts Development of Nutrient Delivery Systems: Ingredients & Challenges
Digestive Lecture Test Questions Set 4
 Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
Digestive Lecture Test Questions Set 4 1. Which of the following is not associated directly with the small intestine: a. villi b. circular folds c. microvilli d. haustrae e. secretin 2. The largest (longest)
Selecting Beneficial Protein Components From all Dairy Animals for Manufacturing Next Generation Infant Formulas FOOD AND NUTRITION
 Selecting Beneficial Protein Components From all Dairy Animals for Manufacturing Next Generation Infant Formulas FOOD AND NUTRITION Jared Raynes 14 th September 2017 What is the Problem? Breast milk is
Selecting Beneficial Protein Components From all Dairy Animals for Manufacturing Next Generation Infant Formulas FOOD AND NUTRITION Jared Raynes 14 th September 2017 What is the Problem? Breast milk is
Calf Notes.com. happens to the rest of the protein? It s an interesting observation and may provide some insights into the newborn calf s metabolism.
 Calf Notes.com Calf Note 168 Where does the protein go? Introduction Colostrum is special stuff. The composition of maternal colostrum (MC) is profoundly different from that of milk; it s so different
Calf Notes.com Calf Note 168 Where does the protein go? Introduction Colostrum is special stuff. The composition of maternal colostrum (MC) is profoundly different from that of milk; it s so different
Digestive System Processes *
 OpenStax-CNX module: m44742 1 Digestive System Processes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
OpenStax-CNX module: m44742 1 Digestive System Processes * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you
Physiology 12. Overview. The Gastrointestinal Tract. Germann Ch 19
 Physiology 12 The Gastrointestinal Tract Germann Ch 19 Overview 1 Basic functions of the GI tract Digestion Secretion Absorption Motility Basic functions of the GI tract Digestion: : Dissolving and breaking
Physiology 12 The Gastrointestinal Tract Germann Ch 19 Overview 1 Basic functions of the GI tract Digestion Secretion Absorption Motility Basic functions of the GI tract Digestion: : Dissolving and breaking
Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, ,
 IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
IDP Biological Systems Gastrointestinal System Section Coordinator: Jerome W. Breslin, PhD, Assistant Professor of Physiology, MEB 7208, 504-568-2669, jbresl@lsuhsc.edu Overall Learning Objectives 1. Characterize
DIGESTION SBI 3C: NOVEMBER 2010
 DIGESTION SBI 3C: NOVEMBER 2010 DIAGRAM OF DIGESTIVE SYSTEM: Mouth Esophagus Liver Gallbladder Large Intestine Appendix Stomach Pancreas Small Intestine Rectum Anus STAGES OF DIGESTION: 1. INGESTION Taking
DIGESTION SBI 3C: NOVEMBER 2010 DIAGRAM OF DIGESTIVE SYSTEM: Mouth Esophagus Liver Gallbladder Large Intestine Appendix Stomach Pancreas Small Intestine Rectum Anus STAGES OF DIGESTION: 1. INGESTION Taking
Enhancing Bioavailability of Nutrients
 Round Table Enhancing Bioavailability of Nutrients Yrjö H. Roos 1 April 2013 ESPCA/São Paulo School of Advanced Science Advances in Molecular Structuring of Food Materials Faculty of Animal Science and
Round Table Enhancing Bioavailability of Nutrients Yrjö H. Roos 1 April 2013 ESPCA/São Paulo School of Advanced Science Advances in Molecular Structuring of Food Materials Faculty of Animal Science and
AN ANIMAL S DIET MUST SUPPLY CHEMICAL ENERGY, ORGANIC MOLECULES, AND ESSENTIAL NUTRIENTS
 1 ANIMAL NUTRITION 2 3 4 5 6 7 Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores eat mainly plants and algae Carnivores
1 ANIMAL NUTRITION 2 3 4 5 6 7 Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores eat mainly plants and algae Carnivores
The gallbladder. Bile secretion:
 The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
The gallbladder is a thin walled green muscular sac on the inferior surface of the liver. The gallbladder stores bile that is not immediately needed for digestion and concentrates it. When the muscular
Peptide mapping during dynamic gastric digestion of heated and unheated. skimmed milk powder
 1 2 Peptide mapping during dynamic gastric digestion of heated and unheated skimmed milk powder 3 4 Laura Sánchez-Rivera 1 *, Olivia Ménard 2,3, Isidra Recio 1, Didier Dupont 2,3 5 1 Instituto de Investigación
1 2 Peptide mapping during dynamic gastric digestion of heated and unheated skimmed milk powder 3 4 Laura Sánchez-Rivera 1 *, Olivia Ménard 2,3, Isidra Recio 1, Didier Dupont 2,3 5 1 Instituto de Investigación
NOTES: The Digestive System (Ch 14, part 2)
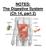 NOTES: The Digestive System (Ch 14, part 2) PANCREAS Structure of the pancreas: The pancreas produces PANCREATIC JUICE that is then secreted into a pancreatic duct. The PANCREATIC DUCT leads to the The
NOTES: The Digestive System (Ch 14, part 2) PANCREAS Structure of the pancreas: The pancreas produces PANCREATIC JUICE that is then secreted into a pancreatic duct. The PANCREATIC DUCT leads to the The
10/23/2013 ANIMAL NUTRITION ANIMAL NUTRITION ESSENTIAL NUTRIENTS AN ANIMAL S DIET MUST STUPPLY: AMINO ACIDS
 ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
Chapter 20 The Digestive System Exam Study Questions
 Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
PROTEIN METABOLISM DEPT OF BIOCHEMISTRY ACS MEDICAL COLLEGE CHENNAI - 77
 PROTEIN METABOLISM DEPT OF BIOCHEMISTRY ACS MEDICAL COLLEGE CHENNAI - 77 DIGESTION & ABSORPTION DIETARY PROTEINS SERVE 3 FUNCTIONS 1. THEIR CONSTITUTENT AMINOACIDS ARE USED FOR SYNTHESIS OF BODY PROTEINS
PROTEIN METABOLISM DEPT OF BIOCHEMISTRY ACS MEDICAL COLLEGE CHENNAI - 77 DIGESTION & ABSORPTION DIETARY PROTEINS SERVE 3 FUNCTIONS 1. THEIR CONSTITUTENT AMINOACIDS ARE USED FOR SYNTHESIS OF BODY PROTEINS
Week 3 The Pancreas: Pancreatic ph buffering:
 Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
Week 3 The Pancreas: A gland with both endocrine (secretion of substances into the bloodstream) & exocrine (secretion of substances to the outside of the body or another surface within the body) functions
Milk protein profile: measure from mid infrared spectra and identification of influence factors
 EAAP 2016, Belfast, 1 Sept. 2016 Milk protein profile: measure from mid infrared spectra and identification of influence factors Session 71 «Milk and meat quality highlighting knowledge gaps in the supply
EAAP 2016, Belfast, 1 Sept. 2016 Milk protein profile: measure from mid infrared spectra and identification of influence factors Session 71 «Milk and meat quality highlighting knowledge gaps in the supply
Overview. Physiology 1. The Gastrointestinal Tract. Guyton section XI
 Overview Physiology 1 The Gastrointestinal Tract Guyton section XI Basic functions of the GI tract Digestion Secretion Absorption Motility Basic functions of the GI tract Digestion: : Dissolving and breaking
Overview Physiology 1 The Gastrointestinal Tract Guyton section XI Basic functions of the GI tract Digestion Secretion Absorption Motility Basic functions of the GI tract Digestion: : Dissolving and breaking
10/18/2017 ANIMAL NUTRITION ANIMAL NUTRITION ESSENTIAL NUTRIENTS AN ANIMAL S DIET MUST STUPPLY: AMINO ACIDS
 ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
ANIMAL NUTRITION Food is taken in, taken apart, and taken up in the process of animal nutrition In general, animals fall into three categories: Herbivores Carnivores Omnivores ANIMAL NUTRITION Chapter
ANTIOXIDATIVE PROPERTY OF COW MILK CASEINATES HYDROLYZED WITH DIFFERENT PROTEASES
 Academic Sciences International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 5, Suppl 2, 2013 Research Article ANTIOXIDATIVE PROPERTY OF COW MILK CASEINATES HYDROLYZED WITH DIFFERENT
Academic Sciences International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 5, Suppl 2, 2013 Research Article ANTIOXIDATIVE PROPERTY OF COW MILK CASEINATES HYDROLYZED WITH DIFFERENT
Topic 6: Human Physiology
 Topic 6: Human Physiology 6.1 Digestion and Absorption D.1 Human Nutrition D.2 Digestion Essential Understandings: The structure of the digestive system allows it to move, digest, and absorb food. A balanced
Topic 6: Human Physiology 6.1 Digestion and Absorption D.1 Human Nutrition D.2 Digestion Essential Understandings: The structure of the digestive system allows it to move, digest, and absorb food. A balanced
Physiology Unit 4 DIGESTIVE PHYSIOLOGY
 Physiology Unit 4 DIGESTIVE PHYSIOLOGY In Physiology Today Functions Motility Ingestion Mastication Deglutition Peristalsis Secretion 7 liters/day! Exocrine/endocrine Digestion Absorption Digestion of
Physiology Unit 4 DIGESTIVE PHYSIOLOGY In Physiology Today Functions Motility Ingestion Mastication Deglutition Peristalsis Secretion 7 liters/day! Exocrine/endocrine Digestion Absorption Digestion of
Includes mouth, pharynx, esophagus, stomach, small intestine, large intestine, rectum, anus. Salivary glands, liver, gallbladder, pancreas
 Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
Chapter 14 The Digestive System and Nutrition Digestive System Brings Nutrients Into the Body The digestive system includes Gastrointestinal (GI) tract (hollow tube) Lumen: space within this tube Includes
Energy, Chemical Reactions and Enzymes
 Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
Phosphorylation Hydrolysis Energy, Chemical Reactions and Enzymes Chapter 2 (selections) What is Energy? Energy is the capacity to do work Potential Energy Kinetic Energy Chemical Bond Energy Like a rechargeable
Dietary Fibres Soluble Fibres: can be.. Insoluble Fibres : can be..
 Dietary Fibres The fraction of edible parts of plants or analogous carbohydrates that are: Resistant to digestion and absorption in the human small intestine with.. Complete or partial fermentation in
Dietary Fibres The fraction of edible parts of plants or analogous carbohydrates that are: Resistant to digestion and absorption in the human small intestine with.. Complete or partial fermentation in
Physicochemical characterisation of dietary fibre and the implication for the gut environment
 1 AARHUS 31 OCTOBER 2013 Physicochemical characterisation of dietary fibre and the implication for the gut environment KNUD ERIK BACH KNUDSEN DEPARTMENT OF ANIMAL SCIENCE 2 AARHUS 31 OCTOBER 2013 POINTS
1 AARHUS 31 OCTOBER 2013 Physicochemical characterisation of dietary fibre and the implication for the gut environment KNUD ERIK BACH KNUDSEN DEPARTMENT OF ANIMAL SCIENCE 2 AARHUS 31 OCTOBER 2013 POINTS
Iron Tissue Storage and Hemoglobin Levels of Deficient Rats Repleted with Iron Bound to the Caseinophosphopeptide
 Iron Tissue Storage and Hemoglobin Levels of Deficient Rats Repleted with Iron Bound to the Caseinophosphopeptide 1 25 of β-casein Nabil Aît-Oukhatar, Said Bouhallab, Pierre Arhan, Jean-Louis Maubois,
Iron Tissue Storage and Hemoglobin Levels of Deficient Rats Repleted with Iron Bound to the Caseinophosphopeptide 1 25 of β-casein Nabil Aît-Oukhatar, Said Bouhallab, Pierre Arhan, Jean-Louis Maubois,
PHYSIOLOGY OF THE DIGESTIVE SYSTEM
 Student Name CHAPTER 26 PHYSIOLOGY OF THE DIGESTIVE SYSTEM D igestion is the process of breaking down complex nutrients into simpler units suitable for absorption. It involves two major processes: mechanical
Student Name CHAPTER 26 PHYSIOLOGY OF THE DIGESTIVE SYSTEM D igestion is the process of breaking down complex nutrients into simpler units suitable for absorption. It involves two major processes: mechanical
100 Points NAME: KEY Lab section:
 ANSC 324 Spring, 2007 EXAM 1 100 Points NAME: KEY Lab section: Instructions: Make sure that you take time to carefully read each question, and then answer the question appropriately. Answers to essay questions
ANSC 324 Spring, 2007 EXAM 1 100 Points NAME: KEY Lab section: Instructions: Make sure that you take time to carefully read each question, and then answer the question appropriately. Answers to essay questions
IMPORTANCE OF ALPHA-LACTALBUMIN IN INFANT NUTRITION
 IMPORTANCE OF ALPHA-LACTALBUMIN IN INFANT NUTRITION By Dr Dan Alaro Learning Objective Describe the roles of α-lactalbumin as an important nutrients for infants. Protein Composition : Human milk The whey-to
IMPORTANCE OF ALPHA-LACTALBUMIN IN INFANT NUTRITION By Dr Dan Alaro Learning Objective Describe the roles of α-lactalbumin as an important nutrients for infants. Protein Composition : Human milk The whey-to
2- Minimum toxic concentration (MTC): The drug concentration needed to just produce a toxic effect.
 BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
BIOPHARMACEUTICS Drug Product Performance Parameters: 1- Minimum effective concentration (MEC): The minimum concentration of drug needed at the receptors to produce the desired pharmacologic effect. 2-
Easy to digest. Kabrita protein digestibility (1)
 Easy to digest Kabrita protein digestibility (1) In vitro analyses of human milk, Kabrita goat milk infant formula (IF) and cow s milk IF in the gastrointestinal model Tiny-TIM agc 90 80 Cum. nitrogen
Easy to digest Kabrita protein digestibility (1) In vitro analyses of human milk, Kabrita goat milk infant formula (IF) and cow s milk IF in the gastrointestinal model Tiny-TIM agc 90 80 Cum. nitrogen
Isolation and Molecular Characterization of Local Goat Milk Casein for Nutraceutical Value
 Isolation and Molecular Characterization of Local Goat Milk Casein for Nutraceutical Value Mohd Akmal Azhar 1,*, and Norshafiqa Salim 2 1 Faculty of Engineering Technology, UMP Gambang, Lebuhraya Tun Razak,
Isolation and Molecular Characterization of Local Goat Milk Casein for Nutraceutical Value Mohd Akmal Azhar 1,*, and Norshafiqa Salim 2 1 Faculty of Engineering Technology, UMP Gambang, Lebuhraya Tun Razak,
Digestive System. Why do we need to eat? Growth Maintenance (repair tissue) Energy
 Digestive System Why do we need to eat? Growth Maintenance (repair tissue) Energy Nutrients Nutrient = chemical that must be obtained by an organism from it s environment in order to survive; nutrients
Digestive System Why do we need to eat? Growth Maintenance (repair tissue) Energy Nutrients Nutrient = chemical that must be obtained by an organism from it s environment in order to survive; nutrients
Global Market for Clinical Nutrition and Dairy Ingredients
 Global Market for Clinical Nutrition and Dairy Ingredients 2015-2020 Published in September 2016 1 Agenda 1. Introduction 2. Global market overview 3. Clinical nutrition ingredients 4. Conclusions 5. Appendix
Global Market for Clinical Nutrition and Dairy Ingredients 2015-2020 Published in September 2016 1 Agenda 1. Introduction 2. Global market overview 3. Clinical nutrition ingredients 4. Conclusions 5. Appendix
REVIEW PeptoPro in Sports Performance
 REVIEW PeptoPro in Sports Performance Tammy Wolhuter, RD (SA) & Anne Till, RD(SA) From: Anne Till & Associates, Registered Dietitians 1. Nutrition and Sporting Performance Optimal and good nutrition is
REVIEW PeptoPro in Sports Performance Tammy Wolhuter, RD (SA) & Anne Till, RD(SA) From: Anne Till & Associates, Registered Dietitians 1. Nutrition and Sporting Performance Optimal and good nutrition is
Digestion and Absorption
 Digestion and Absorption General Considerations - No absorption in esophagus, little in the stomach and vast majority of absorption occurs in small intestine. - The small intestine has specialized structures
Digestion and Absorption General Considerations - No absorption in esophagus, little in the stomach and vast majority of absorption occurs in small intestine. - The small intestine has specialized structures
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 13 THE DIGESTIVE SYSTEM
 ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 13 THE DIGESTIVE SYSTEM The digestive system also known as the alimentary canal or gastrointestinal tract consists of a series of hollow organs joined in a
ANATOMY & PHYSIOLOGY ONLINE COURSE - SESSION 13 THE DIGESTIVE SYSTEM The digestive system also known as the alimentary canal or gastrointestinal tract consists of a series of hollow organs joined in a
Latest developments in policy and research on the DIAASmethod to determine protein quality
 Latest developments in policy and research on the DIAASmethod to determine protein quality Daniel Tomé AgroParisTech, INRA, France and Wageningen University, The Netherlands Event name Introduction The
Latest developments in policy and research on the DIAASmethod to determine protein quality Daniel Tomé AgroParisTech, INRA, France and Wageningen University, The Netherlands Event name Introduction The
c.uma sankar.kanchipuram.
 NAME: GLOBAL COACHING CENTRE XII STANDARD BIO ZOOLOGY DIGESTION ONE MARK PRACTICE PAPER 1. serves to transfer organic molecules, salts and water from the external environment to the body s internal environment.
NAME: GLOBAL COACHING CENTRE XII STANDARD BIO ZOOLOGY DIGESTION ONE MARK PRACTICE PAPER 1. serves to transfer organic molecules, salts and water from the external environment to the body s internal environment.
The Small Intestine. The pyloric sphincter at the bottom of the stomach opens, squirting small amounts of food into your small intestine.
 The Small Intestine The pyloric sphincter at the bottom of the stomach opens, squirting small amounts of food into your small intestine. approximately six metres (the longest section of your digestive
The Small Intestine The pyloric sphincter at the bottom of the stomach opens, squirting small amounts of food into your small intestine. approximately six metres (the longest section of your digestive
Animal Nutrition. Chapter 41. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 41 Animal Nutrition PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Chapter 41 Animal Nutrition PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp
Nutrition and Digestion
 Nutrition and Digestion Classes of Nutrients Carbohydrates Lipids Proteins Minerals Vitamins Water Macronutrients Carbon-containing compounds Energy and raw material Includes carbohydrates, lipids, & proteins
Nutrition and Digestion Classes of Nutrients Carbohydrates Lipids Proteins Minerals Vitamins Water Macronutrients Carbon-containing compounds Energy and raw material Includes carbohydrates, lipids, & proteins
NUTRIENT DIGESTION & ABSORPTION
 NUTRIENT DIGESTION & ABSORPTION NUTR 2050: Nutrition for Nursing Professionals Mrs. Deborah A. Hutcheon, MS, RD, LD Lesson Objectives At the end of the lesson, the student will be able to: 1. Differentiate
NUTRIENT DIGESTION & ABSORPTION NUTR 2050: Nutrition for Nursing Professionals Mrs. Deborah A. Hutcheon, MS, RD, LD Lesson Objectives At the end of the lesson, the student will be able to: 1. Differentiate
Digestive System 7/15/2015. Outline Digestive System. Digestive System
 Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
Digestive System Biology 105 Lecture 18 Chapter 15 Outline Digestive System I. Functions II. Layers of the GI tract III. Major parts: mouth, pharynx, esophagus, stomach, small intestine, large intestine,
BPK 312 Nutrition for Fitness & Sport. Lecture 2. Digestion & Absorption of Food Nutrients
 BPK 312 Nutrition for Fitness & Sport Lecture 2 Digestion & Absorption of Food Nutrients 1. Overview of digestion & absorption of nutrients 2. Functional anatomy of the gastrointestinal (GI) tract 3. Digestion
BPK 312 Nutrition for Fitness & Sport Lecture 2 Digestion & Absorption of Food Nutrients 1. Overview of digestion & absorption of nutrients 2. Functional anatomy of the gastrointestinal (GI) tract 3. Digestion
SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES
 1 SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES Proteins are important in food processing and food product development, as they are
1 SYNOPSIS STUDIES ON THE PREPARATION AND CHARACTERISATION OF PROTEIN HYDROLYSATES FROM GROUNDNUT AND SOYBEAN ISOLATES Proteins are important in food processing and food product development, as they are
New Studies Continue to Reveal the Health Benefits of Colostrum
 New Studies Continue to Reveal the Health Benefits of Colostrum by Barbara L. Minton (see all articles by this author) (NaturalNews) Colostrum is the thin yellowish fluid produced during the first few
New Studies Continue to Reveal the Health Benefits of Colostrum by Barbara L. Minton (see all articles by this author) (NaturalNews) Colostrum is the thin yellowish fluid produced during the first few
Digestive System. Part 3
 Digestive System Part 3 Digestion Ingested materials must be broken down for absorption Majority of absorption in small intestine Water and alcohol in stomach mucosa Some salts and vitamins in large intestine
Digestive System Part 3 Digestion Ingested materials must be broken down for absorption Majority of absorption in small intestine Water and alcohol in stomach mucosa Some salts and vitamins in large intestine
Chapter 6 Reading Guide
 Chapter 6 Reading Guide 1. Describe the structure of an amino acid. 2. What s the difference between an amino acid and a protein? Where do dipeptides, tripeptides and polypeptides fit in? 3. How many amino
Chapter 6 Reading Guide 1. Describe the structure of an amino acid. 2. What s the difference between an amino acid and a protein? Where do dipeptides, tripeptides and polypeptides fit in? 3. How many amino
Evaluation of radical Scavenging of peptides after in vitro digests of chicken protein. Gema Nieto Martínez
 Evaluation of radical Scavenging of peptides after in vitro digests of chicken protein Gema Nieto Martínez Table of contents 1 Introduction 2 Hypothesis 3 Objectives 4 Methodology 5 Results 6 Conclusion
Evaluation of radical Scavenging of peptides after in vitro digests of chicken protein Gema Nieto Martínez Table of contents 1 Introduction 2 Hypothesis 3 Objectives 4 Methodology 5 Results 6 Conclusion
Station 1. Identify (= name) the spaces or structures labeled 1 9.
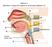 Station 1. Identify (= name) the spaces or structures labeled 1 9. 1 9 8 2 7 3 4 5 6 Station 2. Identify/name the parts or structures labeled 10 20. 10. Is this the right or left lung? 15 13 14 16 11 12
Station 1. Identify (= name) the spaces or structures labeled 1 9. 1 9 8 2 7 3 4 5 6 Station 2. Identify/name the parts or structures labeled 10 20. 10. Is this the right or left lung? 15 13 14 16 11 12
In vivo milk digestion in the calf abomasum. II. Milk and whey proteolysis
 In vivo milk digestion in the calf abomasum. II. Milk and whey proteolysis Mireille YVON Isabelle VAN HILLE, J.-P. PÉLISSIER P. GUILLOTEAU R. TOULLEC Laboratoire de Biochimie et Technologie Laitières,
In vivo milk digestion in the calf abomasum. II. Milk and whey proteolysis Mireille YVON Isabelle VAN HILLE, J.-P. PÉLISSIER P. GUILLOTEAU R. TOULLEC Laboratoire de Biochimie et Technologie Laitières,
Chemical Digestion and Absorption: A Closer Look
 Chemical Digestion and Absorption: A Closer Look Bởi: OpenStaxCollege As you have learned, the process of mechanical digestion is relatively simple. It involves the physical breakdown of food but does
Chemical Digestion and Absorption: A Closer Look Bởi: OpenStaxCollege As you have learned, the process of mechanical digestion is relatively simple. It involves the physical breakdown of food but does
Physiologically relevant in vitro methodology to determine true digestibility of carbohydrates and to predict the glycaemic response
 Physiologically relevant in vitro methodology to determine true digestibility of carbohydrates and to predict the glycaemic response TNO Quality of Life, Zeist, The Netherlands TIM-Carbo Robert Havenaar
Physiologically relevant in vitro methodology to determine true digestibility of carbohydrates and to predict the glycaemic response TNO Quality of Life, Zeist, The Netherlands TIM-Carbo Robert Havenaar
Chapter 3 Reading Guide Be sure to use the many figures and tables provided by the book to help answer these questions.
 Chapter 3 Reading Guide Be sure to use the many figures and tables provided by the book to help answer these questions. 1. What is digestion? What is the difference between mechanical and enzymatic digestion?
Chapter 3 Reading Guide Be sure to use the many figures and tables provided by the book to help answer these questions. 1. What is digestion? What is the difference between mechanical and enzymatic digestion?
Chapter 20 The Digestive System Exam Study Questions
 Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
Chapter 20 The Digestive System Exam Study Questions 20.1 Overview of GI Processes 1. Describe the functions of digestive system. 2. List and define the four GI Processes: 20.2 Functional Anatomy of the
Ingredients for the next generation
 Arla Foods Ingredients Ingredients for the next generation Using infant formulas is second to breastfeeding, a fact supported by WHO. This information is for professional use only. 002 003 Infant nutrition
Arla Foods Ingredients Ingredients for the next generation Using infant formulas is second to breastfeeding, a fact supported by WHO. This information is for professional use only. 002 003 Infant nutrition
Chapter 11: Range Animal Nutrition
 Chapter 11: Range Animal Nutrition 1. Nutritional Components of Forages a. Protein b. Energy c. Phosphorus d. Vitamin A 2. Comparative Nutrition of Forages a. Grasses b. Forbs c. Shrubs 3. Comparative
Chapter 11: Range Animal Nutrition 1. Nutritional Components of Forages a. Protein b. Energy c. Phosphorus d. Vitamin A 2. Comparative Nutrition of Forages a. Grasses b. Forbs c. Shrubs 3. Comparative
Human Complex Milk Lipids: Concentrations, benefits and the implications for Paediatric Nutrition
 Human Complex Milk Lipids: Concentrations, benefits and the implications for Paediatric Nutrition Alastair MacGibbon, Bertram Fong, Angela Rowan and Paul McJarrow Fonterra Research and Development Centre,
Human Complex Milk Lipids: Concentrations, benefits and the implications for Paediatric Nutrition Alastair MacGibbon, Bertram Fong, Angela Rowan and Paul McJarrow Fonterra Research and Development Centre,
Gastrointestinal Anatomy and Physiology. Bio 219 Napa Valley College Dr. Adam Ross
 Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
Gastrointestinal Anatomy and Physiology Bio 219 Napa Valley College Dr. Adam Ross Functions of digestive system Digestion Breakdown of food (chemically) using enzymes, acid, and water Absorption Nutrients,
Digestion and Absorption
 Digestion and Absorption Digestion and Absorption Digestion is a process essential for the conversion of food into a small and simple form. Mechanical digestion by mastication and swallowing Chemical digestion
Digestion and Absorption Digestion and Absorption Digestion is a process essential for the conversion of food into a small and simple form. Mechanical digestion by mastication and swallowing Chemical digestion
Health-promoting properties of bioactive peptides derived from milk proteins in infant food: a review
 Health-promoting properties of bioactive peptides derived from milk proteins in infant food: a review Vassilios Raikos, Theodore Dassios To cite this version: Vassilios Raikos, Theodore Dassios. Health-promoting
Health-promoting properties of bioactive peptides derived from milk proteins in infant food: a review Vassilios Raikos, Theodore Dassios To cite this version: Vassilios Raikos, Theodore Dassios. Health-promoting
- Digestion occurs during periods of low activity - Produces more energy than it uses. - Mucosa
 Introduction Digestive System Chapter 29 Provides processes to break down molecules into a state easily used by cells - A disassembly line: Starts at the mouth and ends at the anus Digestive functions
Introduction Digestive System Chapter 29 Provides processes to break down molecules into a state easily used by cells - A disassembly line: Starts at the mouth and ends at the anus Digestive functions
Modified Monogastric Digestive System
 Modified Monogastric Digestive System Digestive System of the Horse 8/7/2014 1 The Digestive Tract Horses and rabbits are modified monogastric herbivores. Horses are able to utilize large amounts of roughage
Modified Monogastric Digestive System Digestive System of the Horse 8/7/2014 1 The Digestive Tract Horses and rabbits are modified monogastric herbivores. Horses are able to utilize large amounts of roughage
The Detection of Allergens in Food Products with LC-MS
 The Detection of Allergens in Food Products with LC-MS Something for the future? Jacqueline van der Wielen Scope of Organisation Dutch Food and Consumer Product Safety Authority: Law enforcement Control
The Detection of Allergens in Food Products with LC-MS Something for the future? Jacqueline van der Wielen Scope of Organisation Dutch Food and Consumer Product Safety Authority: Law enforcement Control
The digestion system and nutrient requirements
 Principles of nutrition 1 TechNote 1 The digestion system and nutrient requirements IN THIS TECHNOTE 1.1 Functions of the ruminant digestive system 1.2 Requirements of the dairy cow 1.3 Further reading
Principles of nutrition 1 TechNote 1 The digestion system and nutrient requirements IN THIS TECHNOTE 1.1 Functions of the ruminant digestive system 1.2 Requirements of the dairy cow 1.3 Further reading
Digestive System. Digestive System. Digestion is the process of reducing food to small molecules that can be absorbed into the body.
 Digestive System Digestion is the process of reducing food to small molecules that can be absorbed into the body. 2 Types of Digestion Mechanical digestion physical breakdown of food into small particles
Digestive System Digestion is the process of reducing food to small molecules that can be absorbed into the body. 2 Types of Digestion Mechanical digestion physical breakdown of food into small particles
The Digestive System. What is the advantage of a one-way gut? If you swallow something, is it really inside you?
 The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
The Digestive System What is the advantage of a one-way gut?! If you swallow something, is it really inside you? Functions and Processes of the Digestive System: Move nutrients, water, electrolytes from
Nutrients, Enzymes and Digestion Lesson 4: Digestion and Absorption. Digestive Tract and Accessory Organs
 Nutrients, Enzymes and Digestion Lesson 4: Digestion and Absorption Digestive Tract and Accessory Organs http://highered.mheducation.com/sites/0072495855/student_view0/chapter26/animation organs_of_digestion.html
Nutrients, Enzymes and Digestion Lesson 4: Digestion and Absorption Digestive Tract and Accessory Organs http://highered.mheducation.com/sites/0072495855/student_view0/chapter26/animation organs_of_digestion.html
P A T I E N T H A N D B O O K
 PATIENT HANDBOOK Heal Your Gut, Heal Your Body The gastrointestinal (GI) tract is one of the most sophisticated systems of the human body. We often think of the GI tract for its primary role in digesting
PATIENT HANDBOOK Heal Your Gut, Heal Your Body The gastrointestinal (GI) tract is one of the most sophisticated systems of the human body. We often think of the GI tract for its primary role in digesting
MCAT Biology Problem Drill 20: The Digestive System
 MCAT Biology Problem Drill 20: The Digestive System Question No. 1 of 10 Question 1. During the oral phase of swallowing,. Question #01 A. Initially, the food bolus is moved to the back of the tongue and
MCAT Biology Problem Drill 20: The Digestive System Question No. 1 of 10 Question 1. During the oral phase of swallowing,. Question #01 A. Initially, the food bolus is moved to the back of the tongue and
Lab #12: Digestive Physiology
 Background In order for the nutrients in food to be absorbed, they must first be broken down into particles that are small enough to be transported through carrier proteins into the epithelial cells that
Background In order for the nutrients in food to be absorbed, they must first be broken down into particles that are small enough to be transported through carrier proteins into the epithelial cells that
The bovine milk proteome: what s in it and how can it be manipulated?
 Bovine milk protein The bovine milk proteome: what s in it and how can it be manipulated? Sabrina Greenwood Bovine Milk Proteins (30 35g/L) 80% Casein Casein composition αs1 Casein 32% αs2 Casein 8% Κ
Bovine milk protein The bovine milk proteome: what s in it and how can it be manipulated? Sabrina Greenwood Bovine Milk Proteins (30 35g/L) 80% Casein Casein composition αs1 Casein 32% αs2 Casein 8% Κ
1. Which nutrient is so vital to health that you wouldn't live more than a few days without it? A) vitamins B) water C) minerals D) protein
 Nutrition & You, 4e (Blake) Chapter 1 What Is Nutrition? Legend: For sentences / statements that DO NOT HAVE answer choices, Answer A if TRUE Answer B IF False 1. Which nutrient is so vital to health that
Nutrition & You, 4e (Blake) Chapter 1 What Is Nutrition? Legend: For sentences / statements that DO NOT HAVE answer choices, Answer A if TRUE Answer B IF False 1. Which nutrient is so vital to health that
Holistic Healing Professional Practitioner Diploma Course Sample Pages Page 1
 The last phase is called the intestinal phase and takes place about four hours after the gastric phase. The chyme passes through the small intestine, or duodenum, through the pyloric sphincter. This is
The last phase is called the intestinal phase and takes place about four hours after the gastric phase. The chyme passes through the small intestine, or duodenum, through the pyloric sphincter. This is
Not all Proteins are Equal. Maretha Vermaak CEP of Milk SA Dietitian SASDT 2015
 Not all Proteins are Equal Maretha Vermaak CEP of Milk SA Dietitian SASDT 2015 Introduction Dairy products are a feasible means to help meet the demands of a growing world market as well as the nutritional
Not all Proteins are Equal Maretha Vermaak CEP of Milk SA Dietitian SASDT 2015 Introduction Dairy products are a feasible means to help meet the demands of a growing world market as well as the nutritional
Separation of Main Proteins in Plasma and Serum
 BCH 471 Experiment (2) Separation of Main Proteins in Plasma and Serum PLASMA PROTEINS Mw The main plasma proteins are: þ Albumin (36-50 g/l), Mw 66.241kDa. þ Globulins (18-32 g/l), Mw of globulins Cover
BCH 471 Experiment (2) Separation of Main Proteins in Plasma and Serum PLASMA PROTEINS Mw The main plasma proteins are: þ Albumin (36-50 g/l), Mw 66.241kDa. þ Globulins (18-32 g/l), Mw of globulins Cover
- Most nutrients are absorbed before reaching the ileum. - Colon is responsible for final removal of electrolytes and water.
 University of Jordan Department of physiology and Biochemistry Gastro-Intestinal physiology, Medical, Pt III. ---------------------------------------------------------------------------- Academic year:
University of Jordan Department of physiology and Biochemistry Gastro-Intestinal physiology, Medical, Pt III. ---------------------------------------------------------------------------- Academic year:
Calf Notes.com. Calf Note #146 Waste milk vs. milk replacer, revisited
 Calf Notes.com Calf Note #146 Waste milk vs. milk replacer, revisited Introduction A question frequently considered by calf raisers is the use of waste milk for liquid fed calves. Waste milk also called
Calf Notes.com Calf Note #146 Waste milk vs. milk replacer, revisited Introduction A question frequently considered by calf raisers is the use of waste milk for liquid fed calves. Waste milk also called
Enzymes in Human Milk
 Human Milk Oligosaccharides Isolauri E, Sherman PM, Walker WA (eds): Intestinal Microbiome: Functional Aspects in Health and Disease. Nestlé Nutr Inst Workshop Ser, vol 88, pp 129 136, ( DOI: 10.1159/000455250
Human Milk Oligosaccharides Isolauri E, Sherman PM, Walker WA (eds): Intestinal Microbiome: Functional Aspects in Health and Disease. Nestlé Nutr Inst Workshop Ser, vol 88, pp 129 136, ( DOI: 10.1159/000455250
The Effect of Casein Ingestion within a Milk Matrix on Muscle Protein Synthesis
 The Effect of Casein Ingestion within a Milk Matrix on Muscle Protein Synthesis Department of Human Movement Science s.reiners@student.maastrichtuniversity.nl Abstract Isolated micellar casein has been
The Effect of Casein Ingestion within a Milk Matrix on Muscle Protein Synthesis Department of Human Movement Science s.reiners@student.maastrichtuniversity.nl Abstract Isolated micellar casein has been
Lecture 3. Tandem MS & Protein Sequencing
 Lecture 3 Tandem MS & Protein Sequencing Nancy Allbritton, M.D., Ph.D. Department of Physiology & Biophysics 824-9137 (office) nlallbri@uci.edu Office- Rm D349 Medical Science D Bldg. Tandem MS Steps:
Lecture 3 Tandem MS & Protein Sequencing Nancy Allbritton, M.D., Ph.D. Department of Physiology & Biophysics 824-9137 (office) nlallbri@uci.edu Office- Rm D349 Medical Science D Bldg. Tandem MS Steps:
What Are Proteins? Lecture 9: Proteins. Proteins: large complex molecules composed of amino acids. Nutrition 150 Shallin Busch, Ph.D.
 What Are Proteins? Lecture 9: Proteins Nutrition 150 Shallin Busch, Ph.D. Proteins: large complex molecules composed of amino acids. Contain carbon, hydrogen, oxygen, nitrogen Primary source of nitrogen
What Are Proteins? Lecture 9: Proteins Nutrition 150 Shallin Busch, Ph.D. Proteins: large complex molecules composed of amino acids. Contain carbon, hydrogen, oxygen, nitrogen Primary source of nitrogen
SNC4M The Digestive System
 SNC4M The Digestive System What is digestion? Chemical and mechanical breakdown of organic molecules into units small enough for the body to absorb These molecules provide: 1. Energy resources 2. Essential
SNC4M The Digestive System What is digestion? Chemical and mechanical breakdown of organic molecules into units small enough for the body to absorb These molecules provide: 1. Energy resources 2. Essential
Digestive Care Advisor Training #1. Digestion 101 & H.O.P.E.
 Digestive Care Advisor Training #1 & H.O.P.E. The Digestive System in Brief The Process of Digestion The human digestive system is a complex series of organs and glands that process food and excrete waste.
Digestive Care Advisor Training #1 & H.O.P.E. The Digestive System in Brief The Process of Digestion The human digestive system is a complex series of organs and glands that process food and excrete waste.
- Digestion occurs during periods of low activity - Produces more energy than it uses. 3 Copyright 2016 by Elsevier Inc. All rights reserved.
 Introduction Digestive System Chapter 29 Provides processes to break down molecules into a state easily used by cells - A disassembly line: Starts at the mouth and ends at the anus Digestive functions
Introduction Digestive System Chapter 29 Provides processes to break down molecules into a state easily used by cells - A disassembly line: Starts at the mouth and ends at the anus Digestive functions
Digestive System. How your body obtains nutrients. Wednesday, March 2, 16
 Digestive System How your body obtains nutrients Vocabulary Ingestion: food enters the system Physical and enzymatic breakdown begins Digestion: Further breakdown Chemical/enzymatic Vocabulary Absorption:
Digestive System How your body obtains nutrients Vocabulary Ingestion: food enters the system Physical and enzymatic breakdown begins Digestion: Further breakdown Chemical/enzymatic Vocabulary Absorption:
The four stomachs of a dairy cow
 The four stomachs of a dairy cow Left side view 1) Rumen 2) Reticulum 3) Omasum 4) Abomasum Reticulo-omasal orifice (reticulo-rumen exit) (on the right side of the cow) (on the right side of the cow) Esophagus
The four stomachs of a dairy cow Left side view 1) Rumen 2) Reticulum 3) Omasum 4) Abomasum Reticulo-omasal orifice (reticulo-rumen exit) (on the right side of the cow) (on the right side of the cow) Esophagus
Lesson Overview The Digestive System
 30.3 THINK ABOUT IT The only system in the body that food actually enters is the digestive system. So how does food get to the rest of the body after the process of digestion? Functions of the Digestive
30.3 THINK ABOUT IT The only system in the body that food actually enters is the digestive system. So how does food get to the rest of the body after the process of digestion? Functions of the Digestive
Unit 2: Animals on the land
 GCSE Animal Nutrition Unit 2: Animals on the land For first teaching from September 2013 For first award in Summer 2015 Animal Nutrition Learning Outcomes At the end of this unit students should be able
GCSE Animal Nutrition Unit 2: Animals on the land For first teaching from September 2013 For first award in Summer 2015 Animal Nutrition Learning Outcomes At the end of this unit students should be able
