Systemic bevacizumab (Avastin) therapy for exudative neovascular age-related macular degeneration. The BEAT-AMD-Study
|
|
|
- Baldwin Stokes
- 5 years ago
- Views:
Transcription
1 1 Department of Ophthalmology, The Ludwig Boltzmann Institute for Retinology and Biomicroscopic Lasersurgery, Rudolf Foundation Clinic, Vienna, Austria; 2 Department of Internal Medicine, Oncology, Rudolf Foundation Clinic, Vienna, Austria Correspondence to: Dr K E Schmid-Kubista, Department of Ophthalmology, Ludwig Boltzmann Institute for Retinology and Biomicroscopic Lasersurgery, Rudolf Foundation Clinic, Juchgasse 25, 1030 Vienna, Austria; katharina. schmid-kubista@wienkav.at Accepted 9 February 2009 Published Online First 29 April 2009 Systemic bevacizumab (Avastin) therapy for exudative neovascular age-related macular degeneration. The BEAT-AMD-Study K E Schmid-Kubista, 1 I Krebs, 1 B Gruenberger, 2 F Zeiler, 1 J Schueller, 2 S Binder 1 ABSTRACT Background: Double-blinded, randomised, prospective, pilot-study to determine the effect of systemic bevacizumab therapy. Methods: Subjects with fibrovascular pigment epithelial detachment, subfoveal choroidal neovascularisation extending under the geometric centre of the foveal avascular zone and/or macular thickness of at least 300 mm in both eyes were included. Sixteen eyes were included and randomised equally to receive either three infusions of 5 mg/kg Avastin or 100 ml of 0.9% sodium chloride every 2 weeks. The main outcome measure was the lesion size. The follow-up time was 24 weeks. Results: Throughout the 24-week follow-up, the lesion size and macular thickness decreased in the Avastin group by 0.5 (SD 0.08) mm and (14.9) ml respectively. In both groups, visual acuity remained stable in seven eyes and decreased in one eye. At the end of follow-up, 50% of the eyes in the Avastin group became fibrotic, 37.5% remained unchanged, and 12.5% developed a subretinal bleeding. There was a treatable rise in blood pressure after Avastin treatment. Conclusion: Systemic Avastin could be offered to patients with exudative age-related macular degeneration in both eyes and/or patients who refuse intravitreal injections if blood pressure is normal and there is no history of thrombosis. Choroidal neovascularisation (CNV) in age-related macular degeneration (AMD) is one of the major causes of severe vision loss in older people in North America and Europe. 1 AMD threatens distance as well as reading acuity, and it is already known that the vascular endothelial growth factor (VEGF) plays a major role in the development of CNV. 2 When we began the present study, in June 2005, promising results were published of phase I/II clinical trials after intravitreal injections of the anti-vegf drugs pegaptanib (Macugen, Eyetech Pharmaceuticals, New York), 3 4 ranibizumab (Lucentis, Genentech, South San Francisco, California), 5 and systemic treatment with bevacizumab (Avastin, Genentech). 6 At that time a systemic treatment, which would need to be administered only a few times within a short time, was very tempting, especially for patients with a low quality of life due to bad visual acuity. Photodynamic therapy (PDT), 7 9 in combination with intravitreal injection of triamcinolone acetonide, and subretinal surgery were the common treatments for AMD. These methods either were very invasive or had to be repeated several times over long periods of time in order to show some effect. PDT was performed only in eyes with predominantly classic lesions and pigment epithelium detachments (PED) with a maximum lesion of 50%, and there was little chance of vision improvement in these eyes. There was no treatment available for larger minimally classic and occult-only lesions or lesions including larger PED. 6 Bevacizumab was a new anti-vegf which showed promising results as a combination therapy with 5-fluorouracil, leucovorin and oxaliplatin in first-line treatment of metastatic colorectal cancer and as systemic therapy against AMD. 6 However, only a case series for AMD treatment existed. The rationale of the present study was to determine the effect of systemic bevacizumab therapy in subjects with fibrovascular PED, involving the geometric centre of the foveal avascular zone, in comparison with placebo treatment with sodium chloride 0.9%. The main outcome measure was the lesion size, while best-corrected visual acuity (BCVA), reading visual acuity (RVA) and macular thickness were additional outcome measures. We believed that a decrease in density of the subfoveal CNV may well lead to a decrease in macular thickness, thereby allowing a better BCVA. Systemic Avastin was a promising treatment option for AMD, although it is mainly administered off-label as an intravitreal injection these days MATERIALS AND METHODS The BEAT-AMD-Study is a prospective, doubleblinded, randomised, monocentric pilot study, and was conducted at our clinic. The study and data accumulation were carried out with approval from the ethics committee of the city of Vienna, and informed consent for research was obtained from all subjects. Eight subjects with fibrovascular PED, subfoveal CNV extending under the geometric centre of the foveal avascular zone and/or a macular thickness of at least 300 mm in both eyes were included. Exclusion criteria were proteinuria or renal impairment, hepatic dysfunction, vision-threatening ophthalmic diseases other than AMD or a history of arterial thromboembolic diseases or cancer. Examinations were performed before the start of infusion therapy, on a weekly basis for the first 6 weeks, every second week for the following 6 weeks, then every 4 weeks for the next 12 weeks. The follow-up time was 24 weeks. Measurement of BCVA, RVA, intraocular pressure (IOP), macular thickness and complete slitlamp examination were performed at every visit, 914 Br J Ophthalmol 2009;93: doi: /bjo
2 Figure 1 Fluorescein angiography and right eye of one patient who received systemic treatment with Avastin. The pictures were taken before treatment (A), after 12 weeks (B) and after 24 weeks (C). The left eye of the same patient is shown in fig 2. while fluorescein angiography (FA) and Indocyanine Green angiography (ICGA) were performed before infusion therapy and after 12 and 24 weeks. BCVA was determined using the ETDRS charts according to the guidelines of the Treatment of Age-related Macular Degeneration with Photodynamic Therapy (TAP) study. 7 It was performed at a test distance of 4 m, with letter-by-letter scoring and a chart luminance of 100 candelas (cd)/m 2. A loss or gain of up to two lines (10 letters) was considered as stable BCVA, and a loss of three lines (15 letters) was considered as a decrease in BCVA. RVA was examined using a standardised, German-language reading test (Radner reading charts). 24 The measurements were performed monocularly with the patient s optimal refractive correction at working distance. The macular thickness was measured using the Stratus OCT 3 (Carl Zeiss Meditec, Dublin, California). Using the macular thickness program, six radial lines through the centre of the foveal avascular zone were made. An internal fixation light was used to maintain the fixation throughout the examination, where possible. FA was performed with the Heidelberg Retina Angiograph (Heidelberg Engineering, Heidelberg, Germany). Six to eight photographs were taken in the early phase after injection of fluorescein, then every 15 s, after 1 min, 2, 5 and 10 min. Distances in the angiograms were measured in millimetres with the help of the Heidelberger software. ICGA was also performed, including late frames after min. Subjects were looked after by an internal medical doctor (oncologist) throughout the entire study. Internal medical examinations including blood pressure, blood sampling, urine sampling, electrocardiogram and weight measurement were performed throughout the first 6 weeks and after 24 weeks, in order to avoid any additional risk related to the administration of Avastin. Subjects were randomised to receive either bevacizumab (Avastin, Genentech) intravenous infusions, three times 5 mg/ kg every 2 weeks (Avastin group) or sodium chloride 0.9% intravenous infusions, three times 100 ml every 2 weeks (NaCl group). Randomisation was performed by the oncologist, by picking an envelope. As the present study is double-blinded, the oncologist and the pharmacist, who prepared the infusion, were the only ones informed about the treatment group in which the subject was randomised. The subject and the examining ophthalmologists were not informed about the randomisation result. Data were collected and calculated using SPSS (Version 11.0). Statistical analysis was not performed due to the small number of subjects included. Data are presented as mean (SD), percentage and difference between measurements at different time points. RESULTS Four subjects received Avastin systemic therapy, and four subjects received NaCl infusions. Four women and four men Br J Ophthalmol 2009;93: doi: /bjo
3 were included. Two women and two men received Avastin, and the others received NaCl. The mean age was 79 (SD 4) (73 82 years) in the Avastin group and 74 (9) (65 83 years) in the NaCl group. All eyes were phakic. Before treatment, the BCVA in the Avastin and in the NaCl group was 0.33 (0.4) and 0.56 (0.33), respectively, while the RVA was 0.6 (0.5)logRAD and 0.5 (0.4)logRAD respectively. At that time the macular thickness and the lesion size were (76.6) mm and 5.42 (2.15) mm respectively in the Avastin group (figs 1, 2) and (81.2) mm and 3.72 (1.88) mm in the NaCl group (figs 3, 4). The mean blood pressure was 140/80 mm Hg in both groups. The IOP was 16 (1) mm Hg in the Avastin group and 16 (4) mm Hg in the NaCl group. At the end of follow-up, after 24 weeks, the BCVA and RVA were 0.33 (0.44) and 0.3 (0.4)logRAD respectively in the Avastin group and 0.53 (0.42) and 0.5 (0.4)logRAD in the NaCl group. The macular thickness and lesion sizes were (91.5) mm and 4.86 (1.92) mm respectively in the Avastin group (figs 1, 2), (78.2) mm and 3.93 (1.93) mm in the NaCl group (figs 3, 4). The mean blood pressure remained 140/80 mm Hg in the NaCl group, while the systolic pressure increased to 150 (12) mm Hg, and the diastolic pressure increased to 90 (12) mm Hg in the Avastin group. The IOP was 15 (1) mm Hg in the Avastin group and 17 (2) mm Hg in the NaCl group. After 10 weeks, the BCVA in the Avastin group dropped to 0.39 (0.46), which was a difference of 0.07 (0.06) compared with BCVA in the same group before the start of therapy. There were Figure 2 Fluorescein angiography and left eye of one patient who received systemic treatment with Avastin. The pictures were taken before treatment (A), after 12 weeks (B) and after 24 weeks (C). The right eye of the same patient is shown in fig 1. no differences in BCVA in the NaCl group and no differences in RVA in either group. Throughout the 24 weeks, the macular thickness decreased in the Avastin group by (14.9) mm, while there was an increase of 22.1 (8.4) mm in the NaCl group (table 1). In the NaCl group, the highest increase in macular thickness was found after 10 weeks, which was an increase of (20.4) mm. In the Avastin group, the thinnest macular thickness was measured at 5 weeks, with a difference of 138 (2.6) mm. The lesion size decreased in the Avastin group from 6.1 (2.2) mm before treatment, to 5.1 (1.9) mm at week 12 and 5.6 (2.1) mm after 24 weeks. In the NaCl group, the lesion size increased from 3.7 (1.9) mm prior to treatment, to 3.8 (2.0) mm after 12 weeks and 3.9 (1.9) at the end of follow-up. In both the Avastin and the NaCl group, five eyes remained stable, with the BCVA being 5 letters throughout the 24 weeks, two remained stable with 10 letters, and one showed a decrease with 215 letters. In the FA, all eight eyes in the NaCl group were shown to remain exudative, while in the Avastin group, four (50%) eyes became dry and fibrotic, three (37.5%) eyes remained exudative, and one (12.5%) eye had subretinal bleeding. The subretinal bleeding began in week 10, while the other eye turned fibrotic. DISCUSSION When Michels and coauthors published the idea of systemic Avastin treatment against AMD, 6 the idea of systemic anti-vegf Br J Ophthalmol: first published as /bjo on 29 April Downloaded from on 15 August 2018 by guest. Protected by copyright. 916 Br J Ophthalmol 2009;93: doi: /bjo
4 Figure 3 Fluorescein angiography and right eye of one patient who received NaCl. The pictures were taken before treatment (A), after 12 weeks (B) and after 24 weeks (C). The left eye of the same patient is shown in fig 4. treatment against AMD was implicated. The idea of treating both eyes at the same time was also very tempting, since it has been proven that patients with occult CNV with serous PED in the first eye have a significantly higher risk of visual loss in the second eye. 25 A systemic treatment with an initial three infusion therapies over 6 weeks seemed very promising. Lesions too large for PDT had no treatment option, since intravitreal anti-vegfs were not yet available, and they were thought to be very invasive bearing the risk of endophthalmitis. Subretinal surgery was too invasive for those eyes, too. The advantage of our BEAT-AMD-Study, compared with the SANA-Study, 6 is that our study is randomised, double-blinded and placebo-controlled. Neither the patient nor the investigating ophthalmologist knew which treatment arm the patient was randomised into. We began our study in August 2005 and recruited until January However, recruitment has been shown to become more difficult after the idea of the off-label use of Avastin intravitreal injections has worked itself out. Those intravitreal injections of anti-vegfs 3 5 have been shown not to be as invasive as assumed, 18 only a few patients develop exudative AMD on both eyes, and of these most have some internal medical problem, which did not allow inclusion into the BEAT-AMD-Study. For those reasons, we decided to discontinue recruitment and publish these data. Our main outcome measure was lesion size, and we were able to show a decrease of 0.5 (0.08) mm in the Avastin group throughout the 24 weeks. Macular thickness and visual acuity were additional outcome measures, whereby we found decreases in macular thickness in the Avastin group already within the first 2 weeks, with a difference of (4.5) mm lasting until week 24. However, neither the BCVA nor the RVA showed any improvement in this group. Unfortunately, there was a decrease in BCVA in week 10 by , which may be due to the development of the subretinal bleeding in one eye of a patient in week 10. Furthermore, there was a slight rise in blood pressure in this group, which began with 140/80 mm Hg and ended with the systolic pressure being 150 (12) mm Hg and the diastolic pressure being 90 (12) mm Hg. The rise in blood pressure was controlled and treated by the specialist for internal medicine and did not exceed 160/100 mm Hg in any patient. In the placebo control group, there was an increase in lesion size throughout the 24 weeks. BCVA and RVA remained stable. Although, after 24 weeks, macular thickness was comparable with the thickness before treatment, a large increase was found at week 10, which showed a difference of (20.4) mm compared with prior treatment. Blood pressure remained stable. When defining the BCVA by loss of lines, both groups had the same outcome. In both groups only one eye showed a loss of three lines (15 letters), while the other eyes remained stable. However, the FA in week 24 showed that 50% of the eyes turned dry and fibrotic, 37.5% remained unchanged and 12.5% developed a subretinal bleeding in the Avastin group, while all eight eyes remained exudative in the NaCl group. IOP remained unchanged in both groups. Even though the macular thickness decreased in the Avastin group, the BCVA did not improve. In the NaCl group, the Br J Ophthalmol 2009;93: doi: /bjo
5 Figure 4 Fluorescein angiography and left eye of one patient who received NaCl. The pictures were taken before treatment (A), after 12 weeks (B) and after 24 weeks (C). The right eye of the same patient is shown in fig 3. BCVA and the macular thickness remained stable. This might be due to the fibrovascular reaction of the macula in the Avastin group, developing a fibrotic scar, with low BCVA, while a PED with a greater macular thickness still seems to have an equivalent BCVA. In conclusion, the lesion size and the macular thickness decreased in the Avastin group. The OCT and FA showed that the macula turns dry and fibrotic under Avastin systemic treatment. Development of BCVA and RVA is comparable in Table 1 Macular thickness in both groups throughout the study Avastin group NaCl group Macular thickness (mm) Macular thickness (mm) Pre (76.6) (81.2) Week (144.8) (99.6) Week (81.1) (119.8) Week (94.7) (96.9) Week (164.0) (39.5) Week (79.2) (39.6) Week (116.2) (95.0) Week (116.7) (75.9) Week (81.9) (44.3) Week (145.9) (104.4) Week (69.0) (89.4) Week (58.9) (46.6) Week (91.5) (78.2) Pre, before infusion therapy. both groups and did not show any variation. Avastin has systemic side effects such as arterial hypertension and thrombosis. Due to its systemic side effects, expensive costs and comparable results with intravitreal injections we suggest offering this systemic therapy to selected patients. After careful selection, according to thrombosis anamnesis, patients with exudative AMD in both eyes, patients who refuse an intravitreal injection and patients who have had previous vitrectomy should be considered for this treatment option. Funding: There was financial support by the Scientific Grant of the Mayor of Vienna, received in June Competing interests: None. Ethics approval: Ethics approval was provided by the Ethics Committee of the City of Vienna. Patient consent: Obtained. REFERENCES 1. Age-Related Eye Disease Study Research Group. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol 2003;121: Kliffen M, Sharma HS, Mooy CM, et al. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol 1997;81: Eyetech Study Group. Preclinical and phase 1A clinical evaluation of an anti-vegf pegylated aptamer (EYE001) for the treatment of exudative age-related macular degeneration. Retina 2002;22: Eyetech Study Group. Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: phase II study results. Ophthalmology 2003;110: Michels S, Rosenfeld PJ. Ranibizumab therapy for neovascular age-related macular degeneration. Retin Physician 2004;1: Br J Ophthalmol 2009;93: doi: /bjo
6 6. Michels S, Rosenfeld PJ, Puliafito CA, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration. Ophthalmology 2005;112: Tretament of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choriodal neovascularisation in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials TAP report. Arch Ophthalmol 1999;117: VIP Study Group. Verteprofin therapy of subfoveal choriodal neovascularisation in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choriodal neovascularisation verteprofin in photodynamic therapy report 2. Am J Ophthalmol 2001;131: Krebs I, Binder S, Stolba U, et al. Reading ability and central visual field after photodynamic therapy. Ophthalmologica 2004;218: Spaide RF, Sorenson J, Maranan L. Combined photodynamic therapy with verteprofin and intravitreal triamcinolone acetonide for choroidal neovascularization. Ophthalmology 2003;110: Rechtman E, Danis RP, Pratt LM, et al. Intravitreal triamcinolone with photodynamic therapy for subfoveal choroidal neovascularisation in age related macular degeneration. Br J Ophthalmol 2004;88: Binder S, Stolba U, Krebs I, et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularisation resulting from age-related macular degeneration: A pilot study. Am J Ophthalmol 2002;133: Binder S, Krebs I, Hilgers RD, et al. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Invest Ophthalmol Vis Sci 2004;45: Thomas MA, Grand MG, Williams DF, et al. Surgical management of subfoveal choriodal neovascularisation. Ophthalmology 1992;99: Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized Phase II trial. J Clin Oncol 2005;23: Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. New Engl J Med 2004;350: Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after intravitreal injection of bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging 2005;36: Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006;113: Jonas JB, Libondi T, Ihloff AK, et al. Visual acuity change after intravitreal bevacizumab for exudative age-related macular degeneration in relation to subfoveal membrane type. Acta Ophthalmol Scand 2007;85: Pedersen R, Soliman W, Lund-Andersen H, et al. Treatment of choroidal neovascularization using intravitreal bevacizumab. Acta Ophthalmol Scand 2007;85: Lux A, Llacer H, Heussen FMA, et al. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophtahlmol 2007;91: Cleary CA, Jungkim S, Ravikumar K, et al. Intravitreal bevacizumab in the treatment of neovascular age-related macular degeneration, 6- and 9-months results. Eye 2008;22: Madhusudhana KC, Hannan SR, Williams CPR, et al. Intravitreal bevacizumab (Avastin) for the treatment of choroidal neovascularization in age-related macular degeneration: results from 118 cases. Br J Ophthalmol 2007;91: Radner W, Obermayer W, Richter-Mueksch S, et al. The validity and reliability of short German sentences for measuring reading speed. Graefes Arch Clin Exp Ophthalmol 2002;240: Pauleikhoff D, Radermacher M, Spital G, et al. Visual prognosis of second eyes in patients with unilateral late exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2002;240: Br J Ophthalmol: first published as /bjo on 29 April Downloaded from on 15 August 2018 by guest. Protected by copyright. Br J Ophthalmol 2009;93: doi: /bjo
Clinical Trials Related to Age Related Macular Degeneration
 Clinical Trials Related to Age Related Macular Degeneration Kirti Singh MD, DNB, FRCS Kirti Singh MD, DNB, FRCS, Pooja Jain MBBS, Nitasha Ahir MBBS, Divya Jain MD, DNB Guru Nanak Eye Centre, Maulana Azad
Clinical Trials Related to Age Related Macular Degeneration Kirti Singh MD, DNB, FRCS Kirti Singh MD, DNB, FRCS, Pooja Jain MBBS, Nitasha Ahir MBBS, Divya Jain MD, DNB Guru Nanak Eye Centre, Maulana Azad
Despite our growing knowledge of the
 OVERVIEW OF AVAILABLE TREATMENTS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Carl D. Regillo, MD ABSTRACT Although potential treatments for neovascular age-related macular degeneration represent
OVERVIEW OF AVAILABLE TREATMENTS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Carl D. Regillo, MD ABSTRACT Although potential treatments for neovascular age-related macular degeneration represent
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article A Multivariate Analysis of Intravitreal Injection of Anti-VEGF Bevacizumab in the Treatment
Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis)
 Aggiornamento biotecnologico: MoAb anti VEGF Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis) Giulia Germena AMD DRY (non-neovascular) Atrophic cell death in the macula No leakage WET
Aggiornamento biotecnologico: MoAb anti VEGF Age-related macular degeneration (AMD) and RANIBIZUMAB (Lucentis) Giulia Germena AMD DRY (non-neovascular) Atrophic cell death in the macula No leakage WET
Efficacy of intravitreal bevacizumab (Avastin TM ) for shortterm treatment of diabetic macular edema
 111 ORIGINAL Efficacy of intravitreal bevacizumab (Avastin TM ) for shortterm treatment of diabetic macular edema Toshihiko Nagasawa, Takeshi Naito, Shingo Matsushita, Hiroyuki Sato, Takashi Katome, and
111 ORIGINAL Efficacy of intravitreal bevacizumab (Avastin TM ) for shortterm treatment of diabetic macular edema Toshihiko Nagasawa, Takeshi Naito, Shingo Matsushita, Hiroyuki Sato, Takashi Katome, and
Efficacy of Anti-VEGF Agents in the Treatment of Age-Related Macular Degeneration
 Efficacy of Anti-VEGF Agents in the Treatment of Age-Related Macular Degeneration Marilita M. Moschos Abstract- Purpose: To evaluate by OCT and mf-erg the macular function in eyes with CNV due to ARMD
Efficacy of Anti-VEGF Agents in the Treatment of Age-Related Macular Degeneration Marilita M. Moschos Abstract- Purpose: To evaluate by OCT and mf-erg the macular function in eyes with CNV due to ARMD
Although photocoagulation and photodynamic PROCEEDINGS PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION *
 PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
PEGAPTANIB SODIUM FOR THE TREATMENT OF AGE-RELATED MACULAR DEGENERATION Evangelos S. Gragoudas, MD ABSTRACT In December 24, the US Food and Drug Administration (FDA) approved pegaptanib sodium. Pegaptanib
Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related macular degeneration.
 Thomas Jefferson University Jefferson Digital Commons Wills Eye Institute Papers Wills Eye Institute 4-1-2008 Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related
Thomas Jefferson University Jefferson Digital Commons Wills Eye Institute Papers Wills Eye Institute 4-1-2008 Retinal pigment epithelial tears after intravitreal bevacizumab injection for exudative age-related
A Treat and Extend Regimen Using Ranibizumab for Neovascular Age-Related Macular Degeneration
 A Treat and Extend Regimen Using Ranibizumab for Neovascular Age-Related Macular Degeneration Clinical and Economic Impact Omesh P. Gupta, MD, MBA, Gary Shienbaum, MD, Avni H. Patel, MD, Christopher Fecarotta,
A Treat and Extend Regimen Using Ranibizumab for Neovascular Age-Related Macular Degeneration Clinical and Economic Impact Omesh P. Gupta, MD, MBA, Gary Shienbaum, MD, Avni H. Patel, MD, Christopher Fecarotta,
Subgroup Analysis of the MARINA Study of Ranibizumab in Neovascular Age-Related Macular Degeneration
 Subgroup Analysis of the MARINA Study of in Neovascular Age-Related Macular Degeneration David S. Boyer, MD, 1 Andrew N. Antoszyk, MD, 2 Carl C. Awh, MD, 3 Robert B. Bhisitkul, MD, PhD, 4 Howard Shapiro,
Subgroup Analysis of the MARINA Study of in Neovascular Age-Related Macular Degeneration David S. Boyer, MD, 1 Andrew N. Antoszyk, MD, 2 Carl C. Awh, MD, 3 Robert B. Bhisitkul, MD, PhD, 4 Howard Shapiro,
Risk factors of a reduced response to ranibizumab treatment for neovascular age-related macular degeneration evaluation in a clinical setting
 Korb et al. BMC Ophthalmology 2013, 13:84 RESEARCH ARTICLE Open Access Risk factors of a reduced response to ranibizumab treatment for neovascular age-related macular degeneration evaluation in a clinical
Korb et al. BMC Ophthalmology 2013, 13:84 RESEARCH ARTICLE Open Access Risk factors of a reduced response to ranibizumab treatment for neovascular age-related macular degeneration evaluation in a clinical
AGE-RELATED MACULAR DEGENERATION (AMD) IS A
 Intravitreal Bevacizumab for the Management of Choroidal Neovascularization in Age-related Macular Degeneration ZIAD F. BASHSHUR, MD, ALI BAZARBACHI, MD, ALEXANDRE SCHAKAL, MD, ZEINA A. HADDAD, MD, CHRISTELLE
Intravitreal Bevacizumab for the Management of Choroidal Neovascularization in Age-related Macular Degeneration ZIAD F. BASHSHUR, MD, ALI BAZARBACHI, MD, ALEXANDRE SCHAKAL, MD, ZEINA A. HADDAD, MD, CHRISTELLE
ANTIVASCULAR ENDOTHELIAL GROWTH FACTOR
 THE ROLE OF RANIBIZUMAB * Michael L. Klein, MD ABSTRACT The positive 1-year results of ranibizumab trials reported at the 23rd Annual Meeting of the American Society of Retina Specialists have generated
THE ROLE OF RANIBIZUMAB * Michael L. Klein, MD ABSTRACT The positive 1-year results of ranibizumab trials reported at the 23rd Annual Meeting of the American Society of Retina Specialists have generated
SUMMARY. Heather Casparis, MD,* and Neil M. Bressler, MD MARINA AND ANCHOR
 The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
The following are summaries of selected presentations and posters from the American Society of Retina Specialists and European VitreoRetinal Society Annual Meeting held September 9 13, 2006, in Cannes,
Evolving European guidance on the medical management of neovascular age related macular degeneration
 Evolving European guidance on the medical management of neovascular age related macular degeneration U Chakravarthy, G Soubrane, F Bandello, V Chong, C Creuzot-Garcher, S A Dimitrakos, II, J-F Korobelnik,
Evolving European guidance on the medical management of neovascular age related macular degeneration U Chakravarthy, G Soubrane, F Bandello, V Chong, C Creuzot-Garcher, S A Dimitrakos, II, J-F Korobelnik,
Digital Journal of Ophthalmology Digital Journal of Ophthalmology. Abstract
 Original Articles Combined intravitreal ranibizumab and verteporfin photodynamic therapy versus ranibizumab alone for the treatment of age-related macular degeneration Rosalia Giustolisi, MD, Nicoletta
Original Articles Combined intravitreal ranibizumab and verteporfin photodynamic therapy versus ranibizumab alone for the treatment of age-related macular degeneration Rosalia Giustolisi, MD, Nicoletta
Christiane I Falkner-Radler, 1 Ilse Krebs, 1 Carl Glittenberg, 1 Boris Povazay, 2 Wolfgang Drexler, 2 Alexandra Graf, 3 Susanne Binder 1
 1 The Ludwig Boltzmann Institute of Retinology and Biomicroscopic Laser surgery, Department of Ophthalmology, Rudolf Foundation Clinic, Vienna, Austria 2 Biomedical Imaging Group, Department of Optometry
1 The Ludwig Boltzmann Institute of Retinology and Biomicroscopic Laser surgery, Department of Ophthalmology, Rudolf Foundation Clinic, Vienna, Austria 2 Biomedical Imaging Group, Department of Optometry
The Natural History of Occult Choroidal Neovascularisation Associated With Age-related Macular Degeneration. A Systematic Review
 Review Article 145 The Natural History of Occult Choroidal Neovascularisation Associated With Age-related Macular Degeneration. A Systematic Review Antonio Polito, 1 MD, Miriam Isola, 2 MHS, Paolo Lanzetta,
Review Article 145 The Natural History of Occult Choroidal Neovascularisation Associated With Age-related Macular Degeneration. A Systematic Review Antonio Polito, 1 MD, Miriam Isola, 2 MHS, Paolo Lanzetta,
Original Article Ranibizumab as needed therapy for wet age-related macular degeneration combined with serous pigment epithelial detachment
 Int J Clin Exp Med 2016;9(7):13650-13656 www.ijcem.com /ISSN:1940-5901/IJCEM0026582 Original Article Ranibizumab as needed therapy for wet age-related macular degeneration combined with serous pigment
Int J Clin Exp Med 2016;9(7):13650-13656 www.ijcem.com /ISSN:1940-5901/IJCEM0026582 Original Article Ranibizumab as needed therapy for wet age-related macular degeneration combined with serous pigment
CENTENE PHARMACY AND THERAPEUTICS NEW DRUG REVIEW 2Q17 April May
 BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
BRAND NAME Lucentis GENERIC NAME ranibizumab MANUFACTURER Genentech, Inc. DATE OF APPROVAL June 30, 2006 PRODUCT LAUNCH DATE July 13, 2006 REVIEW TYPE Review type 1 (RT1): New Drug Review Full review of
A prospective nonrandomized clinical study on exudative age related macular degeneration
 Original Article A prospective nonrandomized clinical study on exudative age related macular degeneration Ayakutty Muni Raja, Siddharam Janti, Charanya Chendilnathan, Adnan Matheen Department of Ophthalmology,
Original Article A prospective nonrandomized clinical study on exudative age related macular degeneration Ayakutty Muni Raja, Siddharam Janti, Charanya Chendilnathan, Adnan Matheen Department of Ophthalmology,
Digital Journal of Ophthalmology, Vol. 21 Digital Journal of Ophthalmology, Vol. 21
 Original Article Feasibility and efficacy of a mass switch from ranibizumab (Lucentis) to bevacizumab (Avastin) for treatment of neovascular age-related macular degeneration Michael T. Andreoli, MD, a,b
Original Article Feasibility and efficacy of a mass switch from ranibizumab (Lucentis) to bevacizumab (Avastin) for treatment of neovascular age-related macular degeneration Michael T. Andreoli, MD, a,b
FEEDER VESSEL TREATMENT
 THERAPEUTIC OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Donald J. D'Amico, MD Dr D Amico: The overview articles in this monograph highlight findings from clinical trials
THERAPEUTIC OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Donald J. D'Amico, MD Dr D Amico: The overview articles in this monograph highlight findings from clinical trials
MEDICAL POLICY SUBJECT: PHOTODYNAMIC THERAPY FOR EFFECTIVE DATE: 07/20/00 SUBFOVEAL CHOROIDAL NEOVASCULARIZATION
 MEDICAL POLICY SUBJECT: PHOTODYNAMIC THERAPY FOR PAGE: 1 OF: 8 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: PHOTODYNAMIC THERAPY FOR PAGE: 1 OF: 8 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Management of Neovascular AMD
 Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
Kapusta AMD Part 1 Management of Neovascular AMD Dr. Michael A. Kapusta, MD, FRCSC Ophthalmologist in Chief Jewish General Hospital Vitreoretinal Surgeon 1 FINANCIAL DISCLOSURES Consulting honoraria Bayer,
Bilateral Elevated Macular Lesions
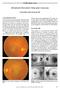 Challenging Case Bilateral Elevated Macular Lesions Section Editor: Alireza Ramezani, MD Case presentation A 65-year-old woman presented with decreased vision in both eyes of 2 months duration. She reported
Challenging Case Bilateral Elevated Macular Lesions Section Editor: Alireza Ramezani, MD Case presentation A 65-year-old woman presented with decreased vision in both eyes of 2 months duration. She reported
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 9.03.20 Intraocular Radiation Therapy for Age-Related Macular Degeneration Photodynamic Therapy for Choroidal
FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 9.03.20 Intraocular Radiation Therapy for Age-Related Macular Degeneration Photodynamic Therapy for Choroidal
TESTING INTRAVITREAL TOXICITY OF BEVACIZUMAB (AVASTIN)
 TESTING INTRAVITREAL TOXICITY OF BEVACIZUMAB (AVASTIN) ROBERTA P. A. MANZANO, MD, GHOLAM A. PEYMAN, MD, PALWASHA KHAN, MD, MUHAMET KIVILCIM, MD Purpose: To evaluate the retinal toxicity of varying doses
TESTING INTRAVITREAL TOXICITY OF BEVACIZUMAB (AVASTIN) ROBERTA P. A. MANZANO, MD, GHOLAM A. PEYMAN, MD, PALWASHA KHAN, MD, MUHAMET KIVILCIM, MD Purpose: To evaluate the retinal toxicity of varying doses
Description. Section: Other Effective Date: July 15, Subsection: Vision Original Policy Date: December 7, 2011 Subject: Page: 1 of 23
 Last Review Status/Date: June 2015 Page: 1 of 23 Description Photodynamic therapy (PDT) is a treatment modality designed to selectively occlude ocular choroidal neovascular tissue. The therapy is a 2-step
Last Review Status/Date: June 2015 Page: 1 of 23 Description Photodynamic therapy (PDT) is a treatment modality designed to selectively occlude ocular choroidal neovascular tissue. The therapy is a 2-step
Photodynamic Therapy for Choroidal Neovascularization
 Photodynamic Therapy for Choroidal Neovascularization Policy Number: 9.03.08 Last Review: 10/2014 Origination: 10/2000 Next Review: 10/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Photodynamic Therapy for Choroidal Neovascularization Policy Number: 9.03.08 Last Review: 10/2014 Origination: 10/2000 Next Review: 10/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
The limited number of currently approved
 CLINICAL TRIALS OF VERTEPORFIN AND PEGAPTANIB: WHAT ARE THE RESULTS? * William F. Mieler, MD ABSTRACT Currently available treatment options for the management of choroidal neovascularization (CNV) in age-related
CLINICAL TRIALS OF VERTEPORFIN AND PEGAPTANIB: WHAT ARE THE RESULTS? * William F. Mieler, MD ABSTRACT Currently available treatment options for the management of choroidal neovascularization (CNV) in age-related
Coding Implications Revision Log. See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
Clinical Policy: Verteporfin (Visudyne) Reference Number: CP.PHAR.187 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important
Angiogenesis and Role of Anti-VEGF Therapy
 Review Article Angiogenesis and Role of Anti-VEGF Therapy P.S. Mahar, Azfar N. Hanfi, Aimal Khan Pak J Ophthalmol 2009, Vol. 25 No. 3.....................................................................................................
Review Article Angiogenesis and Role of Anti-VEGF Therapy P.S. Mahar, Azfar N. Hanfi, Aimal Khan Pak J Ophthalmol 2009, Vol. 25 No. 3.....................................................................................................
CASE STUDIES CURRENT AND FUTURE TREATMENT OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Neil M.
 CURRENT AND FUTURE TREATMENT OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Neil M. Bressler, MD Dr Bressler: The overview articles in this monograph summarize safety, efficacy,
CURRENT AND FUTURE TREATMENT OPTIONS FOR NEOVASCULAR AGE-RELATED MACULAR DEGENERATION * Discussion led by Neil M. Bressler, MD Dr Bressler: The overview articles in this monograph summarize safety, efficacy,
Instudies of vascular endothelial growth factor
 MONITORING THERAPEUTIC EFFICACY IN THE ANTI-VEGF ERA* SriniVas R. Sadda, MD ABSTRACT One of the most important questions with vascular endothelial growth factor (VEGF) inhibitors for age-related macular
MONITORING THERAPEUTIC EFFICACY IN THE ANTI-VEGF ERA* SriniVas R. Sadda, MD ABSTRACT One of the most important questions with vascular endothelial growth factor (VEGF) inhibitors for age-related macular
Comparative Review of Ranibizumab versus Bevacizumab in the Treatment of Neovascular Age-related Macular Degeneration
 Clinical Medicine Insights: Therapeutics Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Comparative Review of Ranibizumab versus Bevacizumab in the
Clinical Medicine Insights: Therapeutics Review Open Access Full open access to this and thousands of other papers at http://www.la-press.com. Comparative Review of Ranibizumab versus Bevacizumab in the
Stabilization of visual acuity with photodynamic therapy in eyes with chorioretinal anastomoses
 Graefe s Arch Clin Exp Ophthalmol (2004) 242:368 376 CLINICAL INVESTIGATION DOI 10.1007/s00417-003-0844-0 Rufino M. Silva José R. Faria de Abreu António Travassos José G. Cunha-Vaz Stabilization of visual
Graefe s Arch Clin Exp Ophthalmol (2004) 242:368 376 CLINICAL INVESTIGATION DOI 10.1007/s00417-003-0844-0 Rufino M. Silva José R. Faria de Abreu António Travassos José G. Cunha-Vaz Stabilization of visual
NEOVASCULAR AGE-RELATED MACULAR DEGENERation
 Randomized, Double-Masked, -Controlled Trial of Ranibizumab for Neovascular Age-Related Macular Degeneration: PIER Study Year 2 PREMA ABRAHAM, HUIBIN YUE, AND LAURA WILSON PURPOSE: To evaluate efficacy
Randomized, Double-Masked, -Controlled Trial of Ranibizumab for Neovascular Age-Related Macular Degeneration: PIER Study Year 2 PREMA ABRAHAM, HUIBIN YUE, AND LAURA WILSON PURPOSE: To evaluate efficacy
MONTHLY ADMINISTERED INTRAVITREOUS PHARmacotherapy
 Polypoidal Choroidal Vasculopathy Masquerading as Neovascular Age-Related Macular Degeneration Refractory to Ranibizumab ALEXANDROS N. STANGOS, JAGDEEP SINGH GANDHI, JAYASHREE NAIR-SAHNI, HEINRICH HEIMANN,
Polypoidal Choroidal Vasculopathy Masquerading as Neovascular Age-Related Macular Degeneration Refractory to Ranibizumab ALEXANDROS N. STANGOS, JAGDEEP SINGH GANDHI, JAYASHREE NAIR-SAHNI, HEINRICH HEIMANN,
EFFICACY OF ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR AGENTS IN RETINAL DISORDER FOR BETTER VISUAL ACUITY
 EFFICACY OF ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR AGENTS IN RETINAL DISORDER FOR BETTER VISUAL ACUITY Diwakar chaudhary *1, 2, Hu shuqiong, Long Yuan and Xiong kun 1 Yangtze University, 1 Nanhuan Road
EFFICACY OF ANTI-VASCULAR ENDOTHELIAL GROWTH FACTOR AGENTS IN RETINAL DISORDER FOR BETTER VISUAL ACUITY Diwakar chaudhary *1, 2, Hu shuqiong, Long Yuan and Xiong kun 1 Yangtze University, 1 Nanhuan Road
Intraocular Radiation Therapy for Age-Related Macular Degeneration
 Intraocular Radiation Therapy for Age-Related Macular Degeneration Policy Number: 9.03.20 Last Review: 9/2014 Origination: 9/2008 Next Review: 9/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Intraocular Radiation Therapy for Age-Related Macular Degeneration Policy Number: 9.03.20 Last Review: 9/2014 Origination: 9/2008 Next Review: 9/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Clinical Study Synopsis
 Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Clinical Study Synopsis This Clinical Study Synopsis is provided for patients and healthcare professionals to increase the transparency of Bayer's clinical research. This document is not intended to replace
Anti VEGF Agents in Retinal Disorders Current Scenario
 Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Retina Anti VEGF Agents in Retinal Disorders Current Scenario Charu Gupta MS Charu Gupta MS, Cyrus M. Shroff MD Shroff Eye Centre, New Delhi T is a group of proteins involved in the regulation of angiogenesis,
Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist
 Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist PRESENT PROFESSIONAL POSITION OPHTALMOLOGIST DOCTOR, Private practice Retinal medical Specialist, DMLA in particular Installation year
Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist PRESENT PROFESSIONAL POSITION OPHTALMOLOGIST DOCTOR, Private practice Retinal medical Specialist, DMLA in particular Installation year
VERTEPORFIN IN PHOTODYNAMIC THERAPY STUDY GROUP
 Verteporfin Therapy of Subfoveal Choroidal Neovascularization in Age-related Macular Degeneration: Two-year Results of a Randomized Clinical Trial Including Lesions With Occult With No Classic Choroidal
Verteporfin Therapy of Subfoveal Choroidal Neovascularization in Age-related Macular Degeneration: Two-year Results of a Randomized Clinical Trial Including Lesions With Occult With No Classic Choroidal
Retinal Pigment Epithelial Tears (Rips) in the ERA of Anti Vegf - When and Why?
 Original Article Retinal Pigment Epithelial Tears (Rips) in the ERA of Anti Vegf - When and Why? Dr Sreelekha S. MS, DNB, DO, FRCS, Dr Manoj Soman DNB, FICO, FRCS, Dr. Unni Nair MS, FRCS, Dr. Ruminder
Original Article Retinal Pigment Epithelial Tears (Rips) in the ERA of Anti Vegf - When and Why? Dr Sreelekha S. MS, DNB, DO, FRCS, Dr Manoj Soman DNB, FICO, FRCS, Dr. Unni Nair MS, FRCS, Dr. Ruminder
Macular Morphology and Visual Acuity in the Comparison of Age-related Macular Degeneration Treatments Trials
 Macular Morphology and Visual Acuity in the Comparison of Age-related Macular Degeneration Treatments Trials Glenn J. Jaffe, MD, 1 Daniel F. Martin, MD, 2 Cynthia A. Toth, MD, 1 Ebenezer Daniel, MPH, PhD,
Macular Morphology and Visual Acuity in the Comparison of Age-related Macular Degeneration Treatments Trials Glenn J. Jaffe, MD, 1 Daniel F. Martin, MD, 2 Cynthia A. Toth, MD, 1 Ebenezer Daniel, MPH, PhD,
Retina Conference. Janelle Fassbender, MD, PhD University of Louisville Department of Ophthalmology and Visual Sciences 09/04/2014
 Retina Conference Janelle Fassbender, MD, PhD University of Louisville Department of Ophthalmology and Visual Sciences 09/04/2014 Subjective CC/HPI: 64 year old Caucasian female referred by outside ophthalmologist
Retina Conference Janelle Fassbender, MD, PhD University of Louisville Department of Ophthalmology and Visual Sciences 09/04/2014 Subjective CC/HPI: 64 year old Caucasian female referred by outside ophthalmologist
Effectiveness of Ranibizumab for Neovascular Age-related Macular Degeneration using Clinician Determined Retreatment Strategy
 Effectiveness of Ranibizumab for Neovascular Age-related Macular Degeneration using Clinician Determined Retreatment Strategy Anupma Kumar, Jayashree N Sahni, Alexandros N Stangos, Claudio Campa, Simon
Effectiveness of Ranibizumab for Neovascular Age-related Macular Degeneration using Clinician Determined Retreatment Strategy Anupma Kumar, Jayashree N Sahni, Alexandros N Stangos, Claudio Campa, Simon
There are no published randomized, double-blind trials comparing aflibercept to other therapies in neovascular AMD.
 Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
Subject: Eylea (aflibercept) Original Effective Date: 7/11/2014 Policy Number: MCP-191 Revision Date(s): 10/11/2016 Review Date(s): 12/16/2015; 10/11/2016, 6/22/2017, 7/10/2018 DISCLAIMER This Medical
P. Stavrakas MD PhD. Consultant Ophthalmologist and vitreoretinal surgeon 2 nd Department of Ophthalmology University of Athens
 P. Stavrakas MD PhD Consultant Ophthalmologist and vitreoretinal surgeon 2 nd Department of Ophthalmology University of Athens Primary diagnose of exudative AMD Anti-VEGF monotherapy treatment Loss of
P. Stavrakas MD PhD Consultant Ophthalmologist and vitreoretinal surgeon 2 nd Department of Ophthalmology University of Athens Primary diagnose of exudative AMD Anti-VEGF monotherapy treatment Loss of
In recent years, the treatment of neovascular age-related
 Clinical Trials Effects of Retinal Morphology on Contrast Sensitivity and Reading Ability in Neovascular Age-Related Macular Degeneration Pearse A. Keane, 1 Praveen J. Patel, 2 Yanling Ouyang, 1 Fred K.
Clinical Trials Effects of Retinal Morphology on Contrast Sensitivity and Reading Ability in Neovascular Age-Related Macular Degeneration Pearse A. Keane, 1 Praveen J. Patel, 2 Yanling Ouyang, 1 Fred K.
Department of Ophthalmology, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea
 Department of Ophthalmology, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea Purpose: To investigate the factors that affect final vision following photodynamic therapy
Department of Ophthalmology, Kangnam Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea Purpose: To investigate the factors that affect final vision following photodynamic therapy
Posterior Vitreomacular Adhesion and Risk of Exudative Age-related Macular Degeneration : Paired Eye Study
 Posterior Vitreomacular Adhesion and Risk of Exudative Age-related Macular Degeneration : Paired Eye Study Sung Jun Lee Department of Medicine The Graduate School, Yonsei University Posterior Vitreomacular
Posterior Vitreomacular Adhesion and Risk of Exudative Age-related Macular Degeneration : Paired Eye Study Sung Jun Lee Department of Medicine The Graduate School, Yonsei University Posterior Vitreomacular
Vascular Endothelial Growth Factor (VEGF) Inhibitors Ocular Use Drug Class Monograph (Medical Benefit)
 Vascular Endothelial Growth Factor (VEGF) Inhibitors Ocular Use Drug Class Monograph (Medical Benefit) Line of Business: Medi-Cal Effective Date: May 17, 2017 Revision Date: May 17, 2017 This policy has
Vascular Endothelial Growth Factor (VEGF) Inhibitors Ocular Use Drug Class Monograph (Medical Benefit) Line of Business: Medi-Cal Effective Date: May 17, 2017 Revision Date: May 17, 2017 This policy has
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 6.01.10 Stereotactic Radiosurgery and Stereotactic Body Radiotherapy 8.01.10 Charged-Particle (Proton
FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 6.01.10 Stereotactic Radiosurgery and Stereotactic Body Radiotherapy 8.01.10 Charged-Particle (Proton
PHOTODYNAMIC THERAPY (PDT) WITH VERTEPORFIN IS
 Verteporfin and Intravitreal Triamcinolone Acetonide Combination Therapy for Occult Choroidal Neovascularization in Age-Related Macular Degeneration ALBERT J. AUGUSTIN, MD, AND URSULA SCHMIDT-ERFURTH,
Verteporfin and Intravitreal Triamcinolone Acetonide Combination Therapy for Occult Choroidal Neovascularization in Age-Related Macular Degeneration ALBERT J. AUGUSTIN, MD, AND URSULA SCHMIDT-ERFURTH,
Intraocular Radiation Therapy for Age-Related Macular Degeneration
 Medical Policy Manual Medicine, Policy No. 134 Intraocular Radiation Therapy for Age-Related Macular Degeneration Next Review: April 2019 Last Review: June 2018 Effective: August 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Medicine, Policy No. 134 Intraocular Radiation Therapy for Age-Related Macular Degeneration Next Review: April 2019 Last Review: June 2018 Effective: August 1, 2018 IMPORTANT REMINDER
Intravitreal Bevacizumab vs Verteporfin Photodynamic Therapy for Neovascular Age-Related Macular Degeneration
 CLINICAL SCIENCES Intravitreal Bevacizumab vs Verteporfin Photodynamic Therapy for Neovascular Age-Related Macular Degeneration Ziad F. Bashshur, MD; Alexandre Schakal, MD; Rola N. Hamam, MD; Christelle
CLINICAL SCIENCES Intravitreal Bevacizumab vs Verteporfin Photodynamic Therapy for Neovascular Age-Related Macular Degeneration Ziad F. Bashshur, MD; Alexandre Schakal, MD; Rola N. Hamam, MD; Christelle
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
Study of clinical significance of optical coherence tomography in diagnosis & management of diabetic macular edema
 Original Research Article Study of clinical significance of optical coherence tomography in diagnosis & management of diabetic macular edema Neha Kantilal Desai 1,*, Somesh Vedprakash Aggarwal 2, Sonali
Original Research Article Study of clinical significance of optical coherence tomography in diagnosis & management of diabetic macular edema Neha Kantilal Desai 1,*, Somesh Vedprakash Aggarwal 2, Sonali
Focal Macular Electroretinograms after Intravitreal Injections of Bevacizumab for Age-Related Macular Degeneration
 Retina Focal Macular Electroretinograms after Intravitreal s of Bevacizumab for Age-Related Macular Degeneration Eiji Iwata, Shinji Ueno, Kohei Ishikawa, Yasuki Ito, Ruka Uetani, Chang-Hua Piao, Mineo
Retina Focal Macular Electroretinograms after Intravitreal s of Bevacizumab for Age-Related Macular Degeneration Eiji Iwata, Shinji Ueno, Kohei Ishikawa, Yasuki Ito, Ruka Uetani, Chang-Hua Piao, Mineo
Anti-VEGF drugs & CATT Results. in AMD. Apollo Hospitals, Hyderabad. Mallika Goyal, MD
 Anti-VEGF drugs & CATT Results in AMD Mallika Goyal, MD Apollo Hospitals, Hyderabad Vascular Endothelial Growth Factor Napoleone Ferrara at Genentech 1989 identified & cloned the gene Protein that causes
Anti-VEGF drugs & CATT Results in AMD Mallika Goyal, MD Apollo Hospitals, Hyderabad Vascular Endothelial Growth Factor Napoleone Ferrara at Genentech 1989 identified & cloned the gene Protein that causes
Age-Related Macular Degeneration (AMD)
 Age-Related Macular Degeneration (AMD) What is the Macula? What is Dry AMD (Age-related Macular Degeneration)? Dry AMD is an aging process that causes accumulation of waste product under the macula leading
Age-Related Macular Degeneration (AMD) What is the Macula? What is Dry AMD (Age-related Macular Degeneration)? Dry AMD is an aging process that causes accumulation of waste product under the macula leading
Ranibizumab and pegaptanib for the treatment of agerelated macular degeneration
 Ranibizumab and pegaptanib for the treatment of agerelated Issued: August 2008 last modified: May 2012 guidance.nice.org.uk/ta155 NHS Evidence has accredited the process used by the Centre for Health Technology
Ranibizumab and pegaptanib for the treatment of agerelated Issued: August 2008 last modified: May 2012 guidance.nice.org.uk/ta155 NHS Evidence has accredited the process used by the Centre for Health Technology
 measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
measure of your overall performance. An isolated glucose test is helpful to let you know what your sugar level is at one moment, but it doesn t tell you whether or not your diabetes is under adequate control
Photodynamic Therapy for Treatment of Subfoveal Choroidal Neovascularization in Exudative Age-Related Macular Degeneration
 Clinical Medicine Journal Vol. 4, No. 4, 2018, pp. 60-66 http://www.aiscience.org/journal/cmj ISSN: 2381-7631 (Print); ISSN: 2381-764X (Online) Photodynamic Therapy for Treatment of Subfoveal Choroidal
Clinical Medicine Journal Vol. 4, No. 4, 2018, pp. 60-66 http://www.aiscience.org/journal/cmj ISSN: 2381-7631 (Print); ISSN: 2381-764X (Online) Photodynamic Therapy for Treatment of Subfoveal Choroidal
CENTRAL SEROUS CHORIORETINOPATHY (CSC) IS
 Association Between the Efficacy of Half-Dose Photodynamic Therapy With Indocyanine Green Angiography and Optical Coherence Tomography Findings in the Treatment of Central Serous Chorioretinopathy MASSIMO
Association Between the Efficacy of Half-Dose Photodynamic Therapy With Indocyanine Green Angiography and Optical Coherence Tomography Findings in the Treatment of Central Serous Chorioretinopathy MASSIMO
Five-year Outcomes of Ranibizumab in Neovascular Age-related Macular Degeneration: Real Life Clinical Experience
 pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2017;31(5):424-430 https://doi.org/10.3341/kjo.2016.0125 Original Article Five-year Outcomes of Ranibizumab in Neovascular Age-related Macular Degeneration:
pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2017;31(5):424-430 https://doi.org/10.3341/kjo.2016.0125 Original Article Five-year Outcomes of Ranibizumab in Neovascular Age-related Macular Degeneration:
HHS Public Access Author manuscript Ophthalmic Surg Lasers Imaging Retina. Author manuscript; available in PMC 2016 January 14.
 High-Speed Ultrahigh-Resolution OCT of Bruch s Membrane in Membranoproliferative Glomerulonephritis Type 2 Mehreen Adhi, MD, Sarah P. Read, MD, PhD, Jonathan J. Liu, PhD, James G. Fujimoto, PhD, and Jay
High-Speed Ultrahigh-Resolution OCT of Bruch s Membrane in Membranoproliferative Glomerulonephritis Type 2 Mehreen Adhi, MD, Sarah P. Read, MD, PhD, Jonathan J. Liu, PhD, James G. Fujimoto, PhD, and Jay
Diabetic retinopathy (DR) progressively
 FOCUS ON DIABETIC MACULAR EDEMA * Frederick L. Ferris III, MD ABSTRACT The current state of treatment for diabetic macular edema (DME) is focused on slowing the rate of vision loss through assessment of
FOCUS ON DIABETIC MACULAR EDEMA * Frederick L. Ferris III, MD ABSTRACT The current state of treatment for diabetic macular edema (DME) is focused on slowing the rate of vision loss through assessment of
Michael P. Blair, MD Retina Consultants, Ltd Libertyville/Des Plaines, Illinois Clinical Associate University of Chicago 17 October 2015
 Michael P. Blair, MD Retina Consultants, Ltd Libertyville/Des Plaines, Illinois Clinical Associate University of Chicago 17 October 2015 So What Parts of the Eye Retina are Affected by VHL Neural tissue
Michael P. Blair, MD Retina Consultants, Ltd Libertyville/Des Plaines, Illinois Clinical Associate University of Chicago 17 October 2015 So What Parts of the Eye Retina are Affected by VHL Neural tissue
Department of Ophthalmology, Kim s Eye Hospital, Konyang University College of Medicine, Seoul, Korea 2
 pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2015;29(5):315-324 http://dx.doi.org/10.3341/kjo.2015.29.5.315 Original Article Clinical Outcomes of Eyes with Submacular Hemorrhage Secondary to Age-related
pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2015;29(5):315-324 http://dx.doi.org/10.3341/kjo.2015.29.5.315 Original Article Clinical Outcomes of Eyes with Submacular Hemorrhage Secondary to Age-related
Clinical Characteristics of Polypoidal Choroidal Vasculopathy Associated with Chronic Central Serous Chorioretionopathy
 pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2012;26(1):15-20 http://dx.doi.org/10.3341/kjo.2012.26.1.15 Original Article Clinical Characteristics of Polypoidal Choroidal Vasculopathy Associated
pissn: 1011-8942 eissn: 2092-9382 Korean J Ophthalmol 2012;26(1):15-20 http://dx.doi.org/10.3341/kjo.2012.26.1.15 Original Article Clinical Characteristics of Polypoidal Choroidal Vasculopathy Associated
Clinical Policy: Neovascular (WET) Macular Degeneration Treatment Reference Number: CP.MP.283
 Clinical Policy: Neovascular (WET) Macular Degeneration Treatment Reference Number: CP.MP.283 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
Clinical Policy: Neovascular (WET) Macular Degeneration Treatment Reference Number: CP.MP.283 Effective Date: 11/16 Last Review Date: 11/17 See Important Reminder at the end of this policy for important
Hyperreflective foci (HFs) have been frequently observed
 Multidisciplinary Ophthalmic Imaging Behavior of SD-OCT Detected Hyperreflective Foci in the Retina of Anti-VEGF Treated Patients with Diabetic Macular Edema Carsten Framme, Paul Schweizer, Manfred Imesch,
Multidisciplinary Ophthalmic Imaging Behavior of SD-OCT Detected Hyperreflective Foci in the Retina of Anti-VEGF Treated Patients with Diabetic Macular Edema Carsten Framme, Paul Schweizer, Manfred Imesch,
London Medicines Evaluation Network Review
 London Medicines Evaluation Network Review Evidence for initiating intravitreal bevacizumab for the management of wet age-related macular degeneration (wet-amd) in eyes with vision better than 6/12 November
London Medicines Evaluation Network Review Evidence for initiating intravitreal bevacizumab for the management of wet age-related macular degeneration (wet-amd) in eyes with vision better than 6/12 November
Computerized Model of Cost-Utility Analysis for Treatment of Age-Related Macular Degeneration
 Computerized Model of Cost-Utility Analysis for Treatment of Age-Related Macular Degeneration E. C. Fletcher, MRCOphth, 1 R. J. Lade, PhD, MBA, 2 T. Adewoyin, MRCOphth, 1 N. V. Chong, FRCOphth, MD 1 Purpose:
Computerized Model of Cost-Utility Analysis for Treatment of Age-Related Macular Degeneration E. C. Fletcher, MRCOphth, 1 R. J. Lade, PhD, MBA, 2 T. Adewoyin, MRCOphth, 1 N. V. Chong, FRCOphth, MD 1 Purpose:
Opinion 4 December 2013
 The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 4 December 2013 LUCENTIS 10 mg/ml, solution for injection Vial of 0.23 ml (CIP: 34009 378 101 5 9) Applicant: Novartis
The legally binding text is the original French version TRANSPARENCY COMMITTEE Opinion 4 December 2013 LUCENTIS 10 mg/ml, solution for injection Vial of 0.23 ml (CIP: 34009 378 101 5 9) Applicant: Novartis
Ranibizumab and Bevacizumab for Treatment of Neovascular Age-related Macular Degeneration
 and for Treatment of Neovascular Age-related Macular Degeneration Two-Year Results Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group* Writing Committee: Daniel F. Martin,
and for Treatment of Neovascular Age-related Macular Degeneration Two-Year Results Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group* Writing Committee: Daniel F. Martin,
Technology appraisal guidance Published: 27 August 2008 nice.org.uk/guidance/ta155
 Ranibizumab and pegaptanib for the treatment of age-related macular degeneration Technology appraisal guidance Published: 27 August 2008 nice.org.uk/guidance/ta155 NICE 2018. All rights reserved. Subject
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration Technology appraisal guidance Published: 27 August 2008 nice.org.uk/guidance/ta155 NICE 2018. All rights reserved. Subject
Department of Ophthalmology, University Hospital of Santiago de Compostela, Ramon Baltar S/N, Santiago de Compostela, Spain 2
 Case Reports in Medicine Volume 2012, Article ID 897097, 10 pages doi:10.1155/2012/897097 Case Report Verteporfin Photodynamic Therapy Combined with Intravitreal Ranibizumab for Polypoidal Choroidal Vasculopathy
Case Reports in Medicine Volume 2012, Article ID 897097, 10 pages doi:10.1155/2012/897097 Case Report Verteporfin Photodynamic Therapy Combined with Intravitreal Ranibizumab for Polypoidal Choroidal Vasculopathy
Ophthalmic VEGF Inhibitors. Eylea (aflibercept), Macugen (pegaptanib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Ophthalmic VEGF Inhibitors Page: 1 of 5 Last Review Date: September 20, 2018 Ophthalmic VEGF Inhibitors
DOME SHAPED MACULOPATHY. Ιωάννης Ν. Βαγγελόπουλος Χειρ. Οφθαλμίατρος - Βόλος
 DOME SHAPED MACULOPATHY Ιωάννης Ν. Βαγγελόπουλος Χειρ. Οφθαλμίατρος - Βόλος DOME SHAPED MACULOPATHY-DEFINITIONS The entity Dome Shaped Macula ( DSM ) was first described by Gaucher and associates in 2008
DOME SHAPED MACULOPATHY Ιωάννης Ν. Βαγγελόπουλος Χειρ. Οφθαλμίατρος - Βόλος DOME SHAPED MACULOPATHY-DEFINITIONS The entity Dome Shaped Macula ( DSM ) was first described by Gaucher and associates in 2008
These issues are covered in more detail below.
 26.3.07 Comments from Novartis on the Assessment Report for the Health Technology Appraisal of Pegaptinib and Ranibizumab for the treatment of age-related macular degeneration In general we feel that the
26.3.07 Comments from Novartis on the Assessment Report for the Health Technology Appraisal of Pegaptinib and Ranibizumab for the treatment of age-related macular degeneration In general we feel that the
Prevention and treatment of agerelated macular degeneration
 THERAPY REVIEW Prevention and treatment of agerelated macular degeneration SARAH HORTON AND CATHERINE GULY Age-related macular degeneration (AMD) is a common cause of visual loss in older people and GPs
THERAPY REVIEW Prevention and treatment of agerelated macular degeneration SARAH HORTON AND CATHERINE GULY Age-related macular degeneration (AMD) is a common cause of visual loss in older people and GPs
Since /01/2014 Private practice Ophthalmology, Retinal Medical Specialty and AMD, private office
 Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist SELARL CABINET DOCTEUR GONZALEZ 27, Boulevard des Minimes 31 200 Toulouse! 05 34 40 77 30 e-mail : cabinet.dr.gonzalez@wanadoo.fr Fax
Corinne GONZALEZ Ophthalmology (MD, Ph.D ) Retinal Medical Specialist SELARL CABINET DOCTEUR GONZALEZ 27, Boulevard des Minimes 31 200 Toulouse! 05 34 40 77 30 e-mail : cabinet.dr.gonzalez@wanadoo.fr Fax
CLINICAL SCIENCES. Verteporfin Therapy for Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration
 CLINICAL SCIENCES Verteporfin Therapy for Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration Three-Year Results of an Open-Label Extension of 2 Randomized Clinical Trials TAP Report
CLINICAL SCIENCES Verteporfin Therapy for Subfoveal Choroidal Neovascularization in Age-Related Macular Degeneration Three-Year Results of an Open-Label Extension of 2 Randomized Clinical Trials TAP Report
Use of Aflibercept in cases of recurrent or refractory neovascular AMD previously treated with other antiangiogenic drug: anatomo-functional outcomes
 Use of Aflibercept in cases of recurrent or refractory neovascular AMD previously treated with other antiangiogenic drug: anatomo-functional outcomes Daniel E. Charles, MD, Martin Charles, MD, Gisela Jelusich,
Use of Aflibercept in cases of recurrent or refractory neovascular AMD previously treated with other antiangiogenic drug: anatomo-functional outcomes Daniel E. Charles, MD, Martin Charles, MD, Gisela Jelusich,
Curriculum Vitae Ryan M. Rich, M.D.
 Retina Consultants of Southern Colorado, P.C. 3030 North Circle Drive, Suite 301 Colorado Springs, CO 80909 (719) 473-9595 DOB: March 20, 1972 Citizenship: USA CURRENT POSITION 2008-present Retina Consultants
Retina Consultants of Southern Colorado, P.C. 3030 North Circle Drive, Suite 301 Colorado Springs, CO 80909 (719) 473-9595 DOB: March 20, 1972 Citizenship: USA CURRENT POSITION 2008-present Retina Consultants
Avastin. Avastin (bevacizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.04 Subject: Avastin Page: 1 of 9 Last Review Date: September 20, 2018 Avastin Description Avastin
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.04 Subject: Avastin Page: 1 of 9 Last Review Date: September 20, 2018 Avastin Description Avastin
NIH Public Access Author Manuscript Ophthalmic Surg Lasers Imaging Retina. Author manuscript; available in PMC 2014 June 24.
 NIH Public Access Author Manuscript Published in final edited form as: Ophthalmic Surg Lasers Imaging Retina. 2014 ; 45(1): 32 37. doi:10.3928/23258160-20131220-04. Analysis of the Short Term Change in
NIH Public Access Author Manuscript Published in final edited form as: Ophthalmic Surg Lasers Imaging Retina. 2014 ; 45(1): 32 37. doi:10.3928/23258160-20131220-04. Analysis of the Short Term Change in
Clinical Study Choroidal Thickness in Eyes with Unilateral Ocular Ischemic Syndrome
 Hindawi Publishing Corporation Journal of Ophthalmology Volume 215, Article ID 62372, 5 pages http://dx.doi.org/1.1155/215/62372 Clinical Study Choroidal Thickness in Eyes with Unilateral Ocular Ischemic
Hindawi Publishing Corporation Journal of Ophthalmology Volume 215, Article ID 62372, 5 pages http://dx.doi.org/1.1155/215/62372 Clinical Study Choroidal Thickness in Eyes with Unilateral Ocular Ischemic
NATURAL COURSE OF PATIENTS DISCONTINUING TREATMENT FOR AGE-RELATED MACULAR DEGENERATION AND FACTORS ASSOCIATED WITH VISUAL PROGNOSIS
 NATURAL COURSE OF PATIENTS DISCONTINUING TREATMENT FOR AGE-RELATED MACULAR DEGENERATION AND FACTORS ASSOCIATED WITH VISUAL PROGNOSIS JAE HUI KIM, MD,* YOUNG SUK CHANG, MD, JONG WOO KIM, MD* Purpose: To
NATURAL COURSE OF PATIENTS DISCONTINUING TREATMENT FOR AGE-RELATED MACULAR DEGENERATION AND FACTORS ASSOCIATED WITH VISUAL PROGNOSIS JAE HUI KIM, MD,* YOUNG SUK CHANG, MD, JONG WOO KIM, MD* Purpose: To
Evaluation of very high- and very low-dose intravitreal aflibercept in patients with neovascular age-related macular degeneration.
 University of Nebraska Medical Center DigitalCommons@UNMC Journal Articles: Ophthalmology Ophthalmology 12-2012 Evaluation of very high- and very low-dose intravitreal aflibercept in patients with neovascular
University of Nebraska Medical Center DigitalCommons@UNMC Journal Articles: Ophthalmology Ophthalmology 12-2012 Evaluation of very high- and very low-dose intravitreal aflibercept in patients with neovascular
Clinically Significant Macular Edema (CSME)
 Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Clinically Significant Macular Edema (CSME) 1 Clinically Significant Macular Edema (CSME) Sadrina T. Shaw OMT I Student July 26, 2014 Advisor: Dr. Uwaydat Clinically Significant Macular Edema (CSME) 2
Fluorescein Angiography Compared to Three-Dimensional Measurements by the Retinal Thickness Analyzer in Classic Choroidal Neovascularization
 Original Paper DOI: 1.1159/99245 Received: June 1, 26 Accepted after revision: November 15, 26 Published online: February 2, 27 Fluorescein Angiography Compared to Three-Dimensional Measurements by the
Original Paper DOI: 1.1159/99245 Received: June 1, 26 Accepted after revision: November 15, 26 Published online: February 2, 27 Fluorescein Angiography Compared to Three-Dimensional Measurements by the
Diabetic Retinopathy A Presentation for the Public
 Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
Diabetic Retinopathy A Presentation for the Public Ray M. Balyeat, MD The Eye Institute Tulsa, Oklahoma The Healthy Eye Light rays enter the eye through the cornea, pupil and lens. These light rays are
Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to retinal vein occlusion
 ORIGINAL RESEARCH Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to retinal vein occlusion Juan Carlos Mesa Gutiérrez Luis Arias Barquet Josep Maria Caminal Mitjana Sergi
ORIGINAL RESEARCH Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to retinal vein occlusion Juan Carlos Mesa Gutiérrez Luis Arias Barquet Josep Maria Caminal Mitjana Sergi
Diagnosis and treatment of diabetic retinopathy. Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City
 Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
Diagnosis and treatment of diabetic retinopathy Blake Cooper MD Ophthalmologist Vitreoretinal Surgeon Retina Associates Kansas City Disclosures Consulted for Novo Nordisk 2017,2018. Will be discussing
