Postmarketing safety evaluation: depside salt injection made from Danshen (Radix Salviae Miltiorrhizae)
|
|
|
- Naomi Charles
- 5 years ago
- Views:
Transcription
1 Online Submissions: J Tradit Chin Med 2014 December 15; 34(6): info@journaltcm.com ISSN JTCM. All rights reserved. REVIEW TOPIC Postmarketing safety evaluation: depside salt injection made from Danshen (Radix Salviae Miltiorrhizae) Yanpeng Chang, Wen Zhang, Yanming Xie, Xiangyang Xu, Rendi Sun, Zheng Wang, Ruihua Yan Yanpeng Chang, Wen Zhang, Yanming Xie, Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing , China Yanpeng Chang, Intensive Care Unit of Shenyang Orthopedic Hospital, Shenyang , China Xiangyang Xu, Rendi Sun, Zheng Wang, Ruihua Yang, Shanghai Green Valley Pharmaceutical Ltd., Shanghai , China Supported by National Science and Technology Major Projects for "Major New Drugs Innovation and Development": Study on Key Technologies of Postmarketing Evaluation for Chinese Medicine (No. 2009ZX ) Correspondence to: Prof. Yanming Xie, Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing , China. datamining5288@ 163.com Telephone: Accepted: June 19,2014 Abstract OBJECTIVE: To systematically examine the postmarketing safety of depside salt injection made from Danshen (Radix Salviae Miltiorrhizae), identify the potential risk factors, and ensure its clinical safety. METHODS: We examined a comprehensive series of studies on the production process, quality standards, pharmacology, population pharmacokinetics, and safety evaluation of depside salt injection made from Danshen (RadixSalviaeMiltiorrhizae). Data from Ⅰ-Ⅳ clinical drug trials, hospital information systems (HIS), and spontaneous reporting systems (SRS) were also analyzed. RESULTS: The effective components of salvianolic acid salt content reached almost 100%, and the magnesium lithospermate B content reached more than 80%. The median lethal dose (LD50) calculated by the Bliss method was 1.49 g/kg, with 95% confidence intervals of g/kg. Long-term tests on Beagle dogs indicated that doses of less than 80 mg/kg were safe and doses of 320 mg/kg were toxic. Adverse drug reactions (ADRs) included digestive disorders; drug-induced erythrocyte deformation in lung, liver, spleen, kidney, bone marrow, intestinal mucosa, lymph nodes, and other tissues; megakaryocytes in lung, liver, and spleen resulting from mild hemolysis; and mild hyperplasia in bone marrow hematopoietic tissue. Other studies indicated no irritative effect of the injection on local tissues and blood vessels, and no allergic reactions, erythrocyte coagulation, or hemolysis. SRS data showed that the most common ADRs were headache, head distention, dizziness, facial flushing, skin itching, thrombocytopenia, and the reversibility of elevated Aspartate transaminase. HIS data indicated no damage to renal function from using depside salt injection made from Danshen (Radix Salviae Miltiorrhizae) at a dosage higher than the recommended dose. CONCLUSION: This study analyzes the clinical characteristics of ADRs from depside salt injection made from Danshen (Radix Salviae Miltiorrhizae), and discusses the factors influencing such reactions. It provides scientific reference and recommendations for clinically safe medication of the Danshen injection JTCM. All rights reserved. Key words: Product surveillance, postmarketing Chinese medicine; Safety; Danshen (Radix Salviae Miltiorrhizae); Depside salt injection JTCM www. journaltcm. com 749
2 INTRODUCTION The depside salt injection made from Danshen (Radix Salviae Miltiorrhizae) manufactured by Shanghai Green Valley Pharmaceutical Co., Ltd., (Shanghai, China) is prepared from salvianolate compound extracted and refined from Danshen (Radix Salviae Miltiorrhizae), with magnesium lithospermate B (>80%) as the main active ingredient. It activates blood, removes blood stasis, and dredges the channels. It is used to treat patients with stable angina pectoris Classes Ⅰand Ⅱ, associated with coronary heart disease (CHD), whose symptoms are mild or moderate chest pain, chest tightness and palpitations, and who are diagnosed with the Traditional Chinese Medicine (TCM) syndrome of heart blood stasis. MEDICINE QUALITY CONTROL Quality control of raw herbal medicinal material The injection is made from salvianolate compound produced by Shanghai Green Valley Life Pharmaceutical Co., Ltd., and extracted from Danshen (Radix Salviae Miltiorrhizae) grown in Linyi, Shandong Province. This growing area contains the highest salvianolic acid B content (4.5%-9.0%) in the world, and indicators of moisture, total ash, acid-insoluble ash, total metals, harmful elements, extract, and Tanshinone Ⅱ-A satisfy the requirements of the Chinese Pharmacopoeia (2010). 1 Linyi enjoys favorable environmental conditions, such as good soil, water, and air quality to relevant national standards. The development of mountain areas, comprehensive management, and constant adjustment of the growing environment has made this area highly conducive to the cultivation and growth of Danshen (Radix Salviae Miltiorrhizae), producing a medicinal material that has a high concentration of active ingredients in the water-soluble phenolic acids and is especially suitable for the extraction of salvianolate. The chemical compositions of salvianolate include the active ingredient magnesium lithospermate B (80%-90%), as well as purple oxalate and rosemary acid homologues (10%-20% ). These active ingredients can protect the cardiovascular system, 2-4 and together form the effective part of the injection. An effective system has been established for studying and controlling the quality of the injection. Quality control of the production process The injection is manufactured in accordance with process specifications and relevant production quality control requirements, and the balance and deviation in composition are managed to ensure quality, stability, and consistency of products. In addition, the manufacturer has specified the principles and main technical parameters of key production equipment, formulated quality standards for the solvents, adsorbents, decolorizing agents, and clarifying agents to be used in production, and controlled the production process and levels of bacteria and endotoxins generated. Quality control was performed using three levels of fingerprints for raw materials, drug substances (extracts), and final products. Internal control was established over the quality indicators of process water, raw materials, intermediate products, and finished products, such as property, identification, visible foreign objects, variations in amount of packaging, heavy metals, harmful elements, pyrogens, hemolysis and coagulation, abnormal toxicity, antihypertensive substances, allergic reactions, fingerprints, proteins, tannins, resins, oxalates, and potassium ions. The shelf-life stability of the product and its quality within 1 year of the expiration date were examined by accelerated testing, long-term tests, key inspections, and appearance inspections. Product variety was inspected yearly; accelerated tests and long-term stability trials were also conducted when changing drafted quality standards into regular quality standards, commissioning product production, changing raw materials suppliers, and changing packaging materials. All the results showed that the stability of the quality of the product was good. NON-CLINICAL SAFETY AND PHARMACOLOGICAL STUDIES Toxicity tests In September 1997, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, performed acute toxicity tests on mice and long-term toxicity tests on rats and Beagle dogs. Mice received single doses of 1.3, 1.45, 1.61, and 1.79 g/kg of Danshen (Radix Salviae Miltiorrhizae) depside salt injection intravenously. Autopsies revealed no abnormality in the organs of dead mice, and there were no lesions in autopsies of surviving mice conducted 2 weeks after administration. The lethal median dose (LD50) calculated by the Bliss method was 1.49 g/kg, with 95% confidence intervals of g/kg. Sprague Dawley (SD) rats were divided into four groups of 50, 200, and 400 mg/kg dose, and a saline control group. Rats received the injection intraperitoneally for 30 consecutive days. Doses less than 50 mg/kg (about twice as much as the optimal effective dose for rats) were non-toxic; doses of 200 mg/kg or more were toxic. This could cause drug-induced peritonitis to some local tissues, but without obvious damage to the heart, liver, kidneys, and other solid organs. Beagle dogs in the dose groups received 20, 80, and 320 mg/kg injections intravenously, and the animals in the control group received saline intravenous injections, for 30 days. The 20 mg/kg dose (about six times as much as the optimal effective dose for dogs) was non-toxic, doses less than 80 mg/kg (about 24 times as much as the optimal effective dose for dogs) were safe, and doses of 320 mg/kg (about JTCM www. journaltcm. com 750
3 100 times as much as the optimal effective dose for dogs) were toxic. The toxic and side reactions of the 320 mg/kg dose included digestive disorders; drug-induced erythrocyte deformation in the lung, liver, spleen, kidney, bone marrow, intestinal mucosa, lymph nodes, and other tissues; megakaryocytes in the lung, liver, and spleen resulting from mild hemolysis; and mild hyperplasia in bone marrow hematopoietic tissue. Other toxicity studies showed that the injection exerted no irritative effect on local tissues and blood vessels, no allergic reactions, and did not produce any erythrocyte coagulation or hemolysis. In 2013, the detection limit of "abnormal toxicity" as specified in existing product quality standards was revised in accordance with the guideline requirements and approved by the State Pharmacopoeia Commission. The expression was modified to read "Take this product, dissolve and dilute it with sterile water for injection to make a solution of 15 mg/ml, check it by the method (Appendix XIIIE of Volume Ⅱ of Chinese Pharmacopoeia 2010), administer slowly through intravenous injection for at least 30 seconds; all requirements are to be fulfilled." (The National Drug Standards of China Food and Drug Administration, YBZ Z-2013) Pharmacological evaluation of the injection In cardiovascular pharmacodynamic tests, the Pharmacology Lab of Shanghai Institute of Materia Medica, Chinese Academy of Sciences, found that the drug had an anti-myocardial ischemic effect, an effect on cardiac hemodynamics and oxygen consumption of the heart, and inhibited platelet aggregation and thrombosis. Cerebrovascular pharmacodynamic tests indicated a therapeutic effect on the rat model of focal cerebral ischemia arising from cerebral artery occlusion, on the gerbil model of total cerebral ischemia arising from bilateral carotid artery ligation reperfusion, an effect on mice hypobaric hypoxia ability, and on rabbit basilar artery vascular ring tension and PC-12 cell injury induced by hypoxia-reoxygenation. Studies indicated the pharmacological mechanism underlying the injection to be the migration of vascular endothelial cells and tube formation, the regulation of the Ca 2+ channel, regulation of immune/inflammatory physiological processes, anti-platelet aggregation, and the inhibition of Na + / K + ATP enzymes through its anti-oxidative stress effect. As a result, this drug can protect myocardial and endothelial cells and improve blood circulation. PREMARKETING CLINICAL SAFETY STUDIES A Phase Ⅰ human tolerance clinical trial was conducted by Xiyuan Hospital of China Academy of TCM from December 16 to 29, 2002; 36 healthy volunteers (men and women) aged from 18 to 50 participated in the trial. No adverse reactions were found. A Phase Ⅱ clinical safety trial was conducted as a preliminary evaluation of the safety and efficacy of the drug for the treatment of angina pectoris in CHD (heart blood stasis syndrome). After 14 days of treatment, one patient in the experimental group experienced distending pain in full head. In a Phase Ⅲ clinical safety trial on angina pectoris, some subjects experienced mild abnormality in routine blood tests, renal, and hepatic functions, as well as distending pain in full head and dizziness, but no serious ADRs. POSTMARKETING CLINICAL SAFETY STUDIES Postmarketing Phase Ⅳ trials (Randomized Controlled Trials [RCTs]) A multi-center Phase Ⅳ clinical trial evaluated the efficacy and safety of the injection in the treatment of chronic stable angina pectoris in CHD from April 9, 2006 to May 17, Co-led by Huashan Hospital, affiliated with Fudan University, and Ruijin Hospital, affiliated with Shanghai Jiaotong University, the trial incorporated 40 Class Ⅲ hospitals and 10 Class Ⅱ hospitals, with a large sample of ADR incidence rate was 0.56%, mainly involving the digestive, nervous, and cardiovascular systems; ADR symptoms included vomiting, headache, head distention, dizziness, chest distress, palpitations, flushing and thermal discomfort, pruritus, hypotension, insomnia, and thrombocytopenia. 5 Evaluation of clinical applications and therapeutic value A large number of clinical studies indicate that, in addition to cardiovascular [angina pectoris, postoperative coronary stent intervention, postoperative cardiopulmonary bypass (CPB) valve replacement, heart failure, myocardial infarction] and cerebrovascular (ischemic stroke, cerebral infarction) diseases, the injection can treat lung diseases, thoracic obstruction, kidney diseases, lumbar disc herniation, sudden deafness with vertigo, pancreatitis, liver cirrhosis, postoperative anticoagulation, coronary slow flow, tuberculosis, disseminated intravascular coagulation, and other diseases. 6 Because the drug improves microcirculation and resists oxidative stress injury, it was predicted to improve microcirculation of the heart, protect against ischemic-reperfusion injury, and prevent no-reflow restenosis after percutaneous coronary intervention (PCI) and diabetes microvascular pathological changes in clinical applications. Additionally, because it can inhibit thromboxane A2 (TXA 2), and elevate Prostacyclin (PGI 2), it was predicted to treat thrombotic diseases with aspirin and clopidogrel resistance; and because it promotes the proliferation and differentiation of neural stem cells and the formation of neuron axons, it was predicted to treat ischemic cerebrovascular diseases. In Phase Ⅱ JTCM www. journaltcm. com 751
4 and Ⅲ trials, the injection was effective in the treatment of angina pectoris, abnormal electrocardiogram (ECG), TCM symptoms, and exercise tolerance tests (based on pre- and post-treatment comparison). The Phase Ⅳ postmarketing clinical trial showed that the drug was effective in treating patients with angina of varying severity; the ADR incidence rate was low (0.56%), and all ADRs were mild or moderate. Moreover, findings indicated that the drug could be safely applied to elderly CHD patients or those with mild hepatic and renal dysfunction. Pharmacokinetic evaluation On the basis of the Phase Ⅰ clinical pharmacokinetic trial, two other studies were completed after the injection was introduced to the market. A multi-ingredient in vivo and pharmacokinetic study examined the injection's three main ingredients: magnesium lithospermate B, rosemary acid and lithospermic acid. After Beagle dogs received a 6 mg/kg intravenous injection, the average half-lives (T 1/2) of magnesium lithospermate B, rosemary acid, and lithospermic acid were 0.05, 0.04, and 0.07 h respectively; after SD rats received a 60 mg/kg intravenous injection, the average elimination half-lives (T 1/2) of magnesium lithospermate B, rosemary acid, and lithospermic acid were 1.04, 0.75, and 2.00 h, respectively. Another pharmacokinetic study was conducted examining magnesium lithospermate B in selected typical patients with angina pectoris (CHD), who received the injection intravenously. The terminal elimination half-life (T 1/2) was (1.71 ± 0.78) h, much lower than that of healthy people. 7 The role of magnesium lithospermate B in impairing blood circulatory function might be one of the pathological mechanisms reducing drug distribution, thereby shortening the half-life. For CHD patients with blood stasis syndrome, it may be advisable to increase the dose, extend the infusion time, or increase the frequency of administration. Evaluation results showed that the price of the product was reasonable; a single course of treatment costs RMB 2492, which is moderate compared with similar cardiovascular and cerebrovascular therapeutic products. The product showed significant treatment effect, and good safety and reliability. Registry-based postmarketing clinical safety monitoring study As a Chinese medicine parenterally-administered medication under clinical safety monitoring, the injection is covered by the research from a major national science and technology project for "Major New Medicine Development," titled Research on Key Technologies for Evaluation of Postmarketing Traditional Chinese Medicines (No. 2009ZX ), presided over by Xie Yanming, a researcher from the Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences. In December 2012, the project passed a review by the Ethics Committee of the Institute of Basic Research in Clinical Medicine (Approval document No. 16), and completed protocol registration in clinicaltrials.gov (ID: NCT ). The project is currently underway. Spontaneous Reporting System (SRS) data analysis An analysis of the SRS data on ADRs reported for Danshen (Radix Salviae Miltiorrhizae) depside salt injection from the State Adverse Drug Reaction Monitoring Centre revealed 739 ADR case reports involving 1310 adverse reactions of 106 types. Overall, there were 24 cases of serious ADRs, accounting for 3.25% of the total, and 251 new ADR cases, accounting for 33.96% of the total. Among all new ADR cases, there were 238 general cases, accounting for 32.20% of the total and 13 serious cases, accounting for 1.76% of the total; no patients died. In the SRS reports, the proportion of severe ADRs and new severe ADRs was high, and mainly included anaphylactoid reaction, chilling, rash, and abnormal liver function. Among the reported ADRs, abnormal liver function was mentioned in the specification of the solution. In 19 ADR cases, the solution was used to treat the indications, and used for different diseases in the other cases. The severe ADRs could have been caused by super indications medication and clinical use not in accordance with TCM syndrome differentiation and treatment. The 10 most common ADR symptoms were rash, dizziness, itching, headache, chills, difficulty breathing, nausea, palpitations, anaphylactoid reaction, and fever. Proportional reporting ratio (PRR) analysis showed that dizziness, headache, nausea, and itching were early warning signals, while Bayesian confidence propagation neural network method (BCPNN) analysis showed that dizziness and headache were early warning signals. The propensity score analysis indicated that the PRR and BCPNN results were equivalent, and both the headache and dizziness were early warning signals before and after matching. 8 Hospital Information System (HIS) data analysis An analysis was carried out on the clinical safety and effectiveness of the injections using HIS data from 14, 191 patients in 20 Grade Ⅲ, Level A hospitals around China. Most patients were middle-aged or elderly. The injection was mostly used to clinically treat hypertension, atherosclerosis, and ischemic encephalopathy. Most patients received a single dose of mg with a treatment cycle of 1-3 days; the solvent was 0.9% sodium chloride solution. When it was used to treat coronary heart disease, the injection was usually used in combination with clopidogrel hydrogen and isosorbide esters. 9 In patients who stopped administration of the injection within 24 h after commencement of treatment, the potential risk factors were cefazolin, dopamine, mannitol, and epinephrine compared with the control group; however, for patients who stopped administration over 24 h but less than or equal to 48 h after commencement, the potential risk factors were ambroxol, JTCM www. journaltcm. com 752
5 levamlodipine, and papaverine. The propensity score method was used to analyze the confounding factors reflecting the patient's four hepatic and renal function indicators; i.e., alanine aminotransferase, aspartate aminotransferase, serum creatinine, and blood urea nitrogen. The results indicated that the injection might impact alanine aminotransferase and blood urea nitrogen. A dose higher than the recommended one was more likely to lead to abnormal changes in levels of creatinine and blood urea nitrogen than a recommended dose, but the difference was not statistically significant. Use in a treatment cycle longer than that recommended in the medicine specification did not seem to exert a significant effect on hepatic and renal functions. 10 RISK CONTROL A risk management evaluation and necessity of risk minimization action plan has been carried out for the injection, and an action plan has been developed to control the origin of raw medicinal material in accordance with Good Agricultural Practice standards, improve the production process and quality standards, strengthen studies on the material basis of the pharmacological effects and allergic reactions, conduct drug metabolism studies, carry out well-targeted drug safety publicity and education, amend the medicine specification, conduct postmarketing pharmacoepidemiologic studies, and improve corporate internal drug quality and ADR monitoring systems. FUTURE POSTMARKETING EVALUATION PLAN The postmarketing evaluation plan for future use of the depside injection will focus on evaluating the following three areas over the next 3-5 years: pharmacology, indications, and clinical application. The pharmacology evaluation includes more rigorous and advanced evaluation of the production process, quality, and stability. The indications evaluation system will be established by standardizing literal expression, conducting mechanism studies, strengthening pharmacological and toxicological tests and studies, and improving the medicine specification. The clinical evaluation studies, including population and individual evaluation studies, will follow the methodology adopted by the Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences in the "Research on Key Technologies for Evaluation of Postmarketing Traditional Chinese Medicines-Clinical Safety Monitoring of Postmarketing Parenterally Administered Chinese Medicines." Safety monitoring and safety evaluations will be conducted. Additionally, it is imperative to establish a postmarketing safety monitoring network for the injection. In summary, this is the first study to demonstrate that salvianolate and its dominant ingredient magnesium lithospermate B are the most important active ingredients of Danshen (Radix Salviae Miltiorrhizae), and it proposed magnesium lithospermate B as the quality control indicator of Danshen (Radix Salviae Miltiorrhizae) depside salt injection and its powder injection. Our study indicates that the preparation process is original; the fingerprint-based quality standards for the raw medicinal material, drug substances, and finished products have been established; and that the original bottle freeze-drying technology has been adopted in the solution production. Both pharmacological and toxicological studies indicate that Danshen (Radix Salviae Miltiorrhizae) depside salt injection is a high-efficacy and low-toxicity cardiovascular drug. REFERENCES 1 National Pharmacopoeia Committee. Chinese Pharmacopoeia Beijing: China Medical Science Press, 2010: Wu XJ, Wang YP, Wang W, et al. Free radical scavenging and inhibition of lipid peroxidation by magnesium lithospermate B. Acta Pharmacol Sin 2000; 21(9): Wang W, Wang YP, Sun WK, et al. Effects of magnesium lithospermate B on aggregation and 5-HT release in rabbit washed platelets. Acta Pharmacol Sin 2000; 21(9): Luo WB, Wang YP. Effect of magnesium lithospermate B on calcium and nitric oxide in endothelial cells upon hypoxia/reoxygenation. Acta Pharmacologica Sinica 2002; 23 (10): Miao Y, Gao ZY, Xu FQ, et al. Clinical observation on salvianolate for the treatment of angina pectoris in coronary heart disease with heart-blood stagnation syndrome. Zhong Yao Xin Yao Yu Lin Chuang Yao Li 2006; 17(2): Song YQ, Xu XY, Sun RD. A summary of clinical application of salvianolate injection. Chin J Pharmacoepidemiol 2012; 21(8): Zhang Y, Gao R, Liu JX, et al. Pharmacokinetic study of lithospermata B on patients with blood-stasis syndrome of CHD. Zhong Yao Yao Li Yu Lin Chuang 2010; 26(6): Lu PF, Xiang YY, Xie YM, et al. Pharmacovigilance of parenterally administered salvianolate based on analysis of spontaneous reporting system data. Zhong Guo Zhong Yao Za Zhi 2013; 38(18): Chang YP, Zhang H, Xie YM, et al. Analysis of salvianolate injection combined with usual drugs in treatment of coronary heart disease in real world. Zhong Guo Zhong Yao Za Zhi 2013; 38(18): Chang YP, Huo J, Xie YM, et al. Real world study of affect on liver function of overdose of salvianolate extract injection. Zhong Guo Zhong Yao Za Zhi 2013; 38(18): JTCM www. journaltcm. com 753
Postmarketing studies on safety of Dengfeng shenmai injection
 Online Submissions: http://www.journaltcm.com J Tradit Chin Med 2013 December 15; 33(6): 827-831 info@journaltcm.com ISSN 0255-2922 2013 JTCM. All rights reserved. REVIEW TOPIC Postmarketing studies on
Online Submissions: http://www.journaltcm.com J Tradit Chin Med 2013 December 15; 33(6): 827-831 info@journaltcm.com ISSN 0255-2922 2013 JTCM. All rights reserved. REVIEW TOPIC Postmarketing studies on
Post-marketing safety monitoring of Shenqifuzheng injection: a solution
 Online Submissions: http://www.journaltcm.com J Tradit Chin Med 2014 August 15; 34(4): 498-503 info@journaltcm.com ISSN 0255-2922 2014 JTCM. All rights reserved. REVIEW TOPIC Post-marketing safety monitoring
Online Submissions: http://www.journaltcm.com J Tradit Chin Med 2014 August 15; 34(4): 498-503 info@journaltcm.com ISSN 0255-2922 2014 JTCM. All rights reserved. REVIEW TOPIC Post-marketing safety monitoring
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Attia 200 mg Modified-Release Capsules, Hard 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each modified-release capsule contains dipyridamole
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Attia 200 mg Modified-Release Capsules, Hard 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each modified-release capsule contains dipyridamole
Comparative Analgesic and Anti- Inflammatory Effect of Salviae miltiorrhizae Pieces and its Products in Mice
 , 2014, Vol. 1, No. 2,109-114 Available online at http://www.ajethno.com American Journal of Ethnomedicine Comparative Analgesic and Anti- Inflammatory Effect of Salviae miltiorrhizae Pieces and its Products
, 2014, Vol. 1, No. 2,109-114 Available online at http://www.ajethno.com American Journal of Ethnomedicine Comparative Analgesic and Anti- Inflammatory Effect of Salviae miltiorrhizae Pieces and its Products
Zhengao Sun 1*, Fang Lian 1, Jianwei Zhang 2, Jinlong Sun 1, Ying Guo 1, Ying Zhang 2
 A LITERATURE ANALYSIS ON 14 CASES OF ALLERGIC SHOCK CAUSED BY SAFFLOWER INJECTION 563 Zhengao Sun 1*, Fang Lian 1, Jianwei Zhang 2, Jinlong Sun 1, Ying Guo 1, Ying Zhang 2 1 Affiliated Hospital of Shandong
A LITERATURE ANALYSIS ON 14 CASES OF ALLERGIC SHOCK CAUSED BY SAFFLOWER INJECTION 563 Zhengao Sun 1*, Fang Lian 1, Jianwei Zhang 2, Jinlong Sun 1, Ying Guo 1, Ying Zhang 2 1 Affiliated Hospital of Shandong
Diagnosis and Management of Acute Myocardial Infarction
 Diagnosis and Management of Acute Myocardial Infarction Acute Myocardial Infarction (AMI) occurs as a result of prolonged myocardial ischemia Atherosclerosis leads to endothelial rupture or erosion that
Diagnosis and Management of Acute Myocardial Infarction Acute Myocardial Infarction (AMI) occurs as a result of prolonged myocardial ischemia Atherosclerosis leads to endothelial rupture or erosion that
Questions and Answers: The NIH Trial of EDTA Chelation Therapy for Coronary Heart Disease
 Questions and Answers: The NIH Trial of EDTA Chelation Therapy for Coronary Heart Disease Results from the Trial to Assess Chelation Therapy will be published in the Journal of the American Medical Associatio
Questions and Answers: The NIH Trial of EDTA Chelation Therapy for Coronary Heart Disease Results from the Trial to Assess Chelation Therapy will be published in the Journal of the American Medical Associatio
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 5%, 50 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 5% is a solution containing 50 g/l of total
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 5%, 50 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 5% is a solution containing 50 g/l of total
Blood products and plasma substitutes
 Blood products and plasma substitutes Plasma substitutes Dextran 70 and polygeline are macromolecular substances which are metabolized slowly; they may be used to expand and maintain blood volume in shock
Blood products and plasma substitutes Plasma substitutes Dextran 70 and polygeline are macromolecular substances which are metabolized slowly; they may be used to expand and maintain blood volume in shock
Ginkgo biloba extract postconditioning reduces myocardial ischemia reperfusion injury
 Ginkgo biloba extract postconditioning reduces myocardial ischemia reperfusion injury K. Ran 1, D.-L. Yang 1, Y.-T. Chang 1, K.-M. Duan 2, Y.-W. Ou 2, H.-P. Wang 3 and Z.-J. Li 1 1 Department of Anesthesiology,
Ginkgo biloba extract postconditioning reduces myocardial ischemia reperfusion injury K. Ran 1, D.-L. Yang 1, Y.-T. Chang 1, K.-M. Duan 2, Y.-W. Ou 2, H.-P. Wang 3 and Z.-J. Li 1 1 Department of Anesthesiology,
Package Insert. Cognitin
 Package Insert Cognitin Product Summary 1. Name of the medicinal product Cognitin 2. Qualitative and quantitative composition Each film coated tablet contains extract 60 mg 5mg 3. Pharmaceutical form Film
Package Insert Cognitin Product Summary 1. Name of the medicinal product Cognitin 2. Qualitative and quantitative composition Each film coated tablet contains extract 60 mg 5mg 3. Pharmaceutical form Film
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GIAPREZA TM safely and effectively. See full prescribing information for GIAPREZA. GIAPREZA (angiotensin
Elements for a public summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Coronary artery disease and as anticoagulant (inhibiting the clotting of the blood) in patients undergoing surgery to treat blockages
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Coronary artery disease and as anticoagulant (inhibiting the clotting of the blood) in patients undergoing surgery to treat blockages
Design and implementation of fast allergy skin test detector for traditional Chinese medicine injections
 1884 Design and implementation of fast allergy skin test detector for traditional Chinese medicine injections YING XIAO 1*, YUBIN ZHAO 2* and YANMING XIE 3 1 Department of Bioengineering, School of Chemical
1884 Design and implementation of fast allergy skin test detector for traditional Chinese medicine injections YING XIAO 1*, YUBIN ZHAO 2* and YANMING XIE 3 1 Department of Bioengineering, School of Chemical
PACKAGE LEAFLET: INFORMATION FOR THE USER. Glucose Intravenous Infusion BP 10% w/v solution for infusion Glucose (as glucose monohydrate)
 PACKAGE LEAFLET: INFORMATION FOR THE USER Glucose Intravenous Infusion BP 10% w/v solution for infusion Glucose (as glucose monohydrate) Read all of this leaflet carefully before you start using this medicine
PACKAGE LEAFLET: INFORMATION FOR THE USER Glucose Intravenous Infusion BP 10% w/v solution for infusion Glucose (as glucose monohydrate) Read all of this leaflet carefully before you start using this medicine
Cardiovascular Disorders Lecture 3 Coronar Artery Diseases
 Cardiovascular Disorders Lecture 3 Coronar Artery Diseases By Prof. El Sayed Abdel Fattah Eid Lecturer of Internal Medicine Delta University Coronary Heart Diseases It is the leading cause of death in
Cardiovascular Disorders Lecture 3 Coronar Artery Diseases By Prof. El Sayed Abdel Fattah Eid Lecturer of Internal Medicine Delta University Coronary Heart Diseases It is the leading cause of death in
A Chinese Patent Medicine for the Secondary Prevention of Myocardial Infarction
 Section 1 01 Clinical Trials A Chinese Patent Medicine for the Secondary Prevention of Myocardial Infarction China Boli Zhang and Junhua Zhang. Tianjin University of Traditional Chinese Medicine, 312 Anshanxi
Section 1 01 Clinical Trials A Chinese Patent Medicine for the Secondary Prevention of Myocardial Infarction China Boli Zhang and Junhua Zhang. Tianjin University of Traditional Chinese Medicine, 312 Anshanxi
TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain
 TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain Second Low-Dose SoluMatrix NSAID from Iroko Now Available by Prescription PHILADELPHIA, June 29, 2015 Iroko Pharmaceuticals, LLC,
TIVORBEX Now Available in U.S. Pharmacies for the Treatment of Acute Pain Second Low-Dose SoluMatrix NSAID from Iroko Now Available by Prescription PHILADELPHIA, June 29, 2015 Iroko Pharmaceuticals, LLC,
Anticoagulants. Pathological formation of a haemostatic plug Arterial associated with atherosclerosis Venous blood stasis e.g. DVT
 Haemostasis Thrombosis Phases Endogenous anticoagulants Stopping blood loss Pathological formation of a haemostatic plug Arterial associated with atherosclerosis Venous blood stasis e.g. DVT Vascular Platelet
Haemostasis Thrombosis Phases Endogenous anticoagulants Stopping blood loss Pathological formation of a haemostatic plug Arterial associated with atherosclerosis Venous blood stasis e.g. DVT Vascular Platelet
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH)
 COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
COMPANY CORE PACKAGE INSERT CCPI (PI/CORE/ENGLISH) HUMAN ALBUMIN 20 % BEHRING Rev.: 05-MAR-2008 / PEI approval 26.02.08 Supersedes previous versions Rev.: 28-NOV-2007 / Adaptation to Core SPC Rev.: 02-JAN-2007
ACETYLCYSTEINE INJECTION
 ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
ACETYLCYSTEINE INJECTION 1. Name of the medicinal product Acetylcysteine 200 mg/ml Injection 2. Qualitative and quantitative composition Acetylcysteine 200mg per ml (as N-acetylcysteine) Each 10ml ampoule
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid
 Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet: Information for the patient Tranexamic acid 100 mg/ml Solution for Injection tranexamic acid Read all of this leaflet carefully before you are given this medicine because it contains important
International Conference on Biomedical and Biological Engineering (BBE 2016)
 International Conference on Biomedical and Biological Engineering (BBE 2016) The Mechanism of Carthamin Yellow Injection Promote Acute Myocardial Infarction Infarct Border Zone Angiogenesis Xiao-min WANG1,
International Conference on Biomedical and Biological Engineering (BBE 2016) The Mechanism of Carthamin Yellow Injection Promote Acute Myocardial Infarction Infarct Border Zone Angiogenesis Xiao-min WANG1,
M0BCore Safety Profile
 M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
M0BCore Safety Profile Active substance: Aciclovir Pharmaceutical form(s)/strength: Tablets 200, 400 or 800 mg Dispersible tablets 200, 400 or 800 mg Oral suspensions 200 mg or 400 mg per 5 ml. Freeze
DATA SHEET. Product Summary. 1. Trade Name of Medicinal Product. Protamine Sulphate Injection BP. 2. Qualitative and Quantitative Composition
 DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
Angina Pectoris. Edward JN Ishac, Ph.D. Smith Building, Room
 Angina Pectoris Edward JN Ishac, Ph.D. Smith Building, Room 742 eishac@vcu.edu 828-2127 Department of Pharmacology and Toxicology Medical College of Virginia Campus of Virginia Commonwealth University
Angina Pectoris Edward JN Ishac, Ph.D. Smith Building, Room 742 eishac@vcu.edu 828-2127 Department of Pharmacology and Toxicology Medical College of Virginia Campus of Virginia Commonwealth University
Angina Pectoris Dr. Shariq Syed
 Angina Pectoris Dr. Syed 1 What is Angina Pectoris (AP)? Commonly known as angina is chest pain often due to ischemia of the heart muscle, Because of obstruction or spasm of the coronary arteries 2 What
Angina Pectoris Dr. Syed 1 What is Angina Pectoris (AP)? Commonly known as angina is chest pain often due to ischemia of the heart muscle, Because of obstruction or spasm of the coronary arteries 2 What
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) DRAFT COMMUNITY HERBAL MONOGRAPH ON BETULA PENDULA ROTH; BETULA PUBESCENS EHRH.
 European Medicines Agency Evaluation of Medicines for Human Use London, 8 May 2007 Doc. Ref. EMEA/HMPC/260019/2006 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) DRAFT COMMUNITY HERBAL MONOGRAPH ON BETULA
European Medicines Agency Evaluation of Medicines for Human Use London, 8 May 2007 Doc. Ref. EMEA/HMPC/260019/2006 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) DRAFT COMMUNITY HERBAL MONOGRAPH ON BETULA
PACKAGE LEAFLET: INFORMATION FOR THE USER. Active substance: enoximone
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM
 REVIEW DATE REVIEWER'S ID HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM : DISCHARGE DATE: RECORDS FROM: Hospitalization ER Please check all that may apply: Myocardial Infarction Pages 2, 3,
REVIEW DATE REVIEWER'S ID HEART AND SOUL STUDY OUTCOME EVENT - MORBIDITY REVIEW FORM : DISCHARGE DATE: RECORDS FROM: Hospitalization ER Please check all that may apply: Myocardial Infarction Pages 2, 3,
Cardiovascular Diseases and Diabetes
 Cardiovascular Diseases and Diabetes LEARNING OBJECTIVES Ø Identify the components of the cardiovascular system and the various types of cardiovascular disease Ø Discuss ways of promoting cardiovascular
Cardiovascular Diseases and Diabetes LEARNING OBJECTIVES Ø Identify the components of the cardiovascular system and the various types of cardiovascular disease Ø Discuss ways of promoting cardiovascular
Experimental study on anaphylaxis of Qingkailing injection and its components on Beagle dogs
 Online Submissions:http://www.journaltcm.com J Tradit Chin Med December 5; (4): 64-645 info@journaltcm.com ISSN 55-9 JTCM. All rights reserved. BASIC INVESTIGATION TOPIC Experimental study on anaphylaxis
Online Submissions:http://www.journaltcm.com J Tradit Chin Med December 5; (4): 64-645 info@journaltcm.com ISSN 55-9 JTCM. All rights reserved. BASIC INVESTIGATION TOPIC Experimental study on anaphylaxis
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 1 PRODUCT NAME TROPISETRON-AFT tropisetron hydrochloride (equivalent to 2 mg or 5 mg tropisetron) per ampoule. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 1 mg of tropisetron 1 2 ml ampoule
1 PRODUCT NAME TROPISETRON-AFT tropisetron hydrochloride (equivalent to 2 mg or 5 mg tropisetron) per ampoule. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml contains 1 mg of tropisetron 1 2 ml ampoule
Salvianolate injection in the treatment of unstable angina pectoris A systematic review and meta-analysis
 Systematic Review and Meta-Analysis Medicine Salvianolate injection in the treatment of unstable angina pectoris A systematic review and meta-analysis Dan Zhang, MM, Jiarui Wu, MD, PhD, Shi Liu, MM, Xiaomeng
Systematic Review and Meta-Analysis Medicine Salvianolate injection in the treatment of unstable angina pectoris A systematic review and meta-analysis Dan Zhang, MM, Jiarui Wu, MD, PhD, Shi Liu, MM, Xiaomeng
Package leaflet: Information for the user. ReoPro 2 mg/ml solution for injection or infusion. abciximab
 Package leaflet: Information for the user ReoPro 2 mg/ml solution for injection or infusion abciximab Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the user ReoPro 2 mg/ml solution for injection or infusion abciximab Read all of this leaflet carefully before you start using this medicine because it contains important
Public Assessment Report. Scientific discussion. Pentasa Compact 4 g, prolonged-release granules. (mesalazine) NL License RVG:
 Public Assessment Report Scientific discussion Pentasa Compact 4 g, prolonged-release granules (mesalazine) NL License RVG: 114015 Date: 30 March 2015 This module reflects the scientific discussion for
Public Assessment Report Scientific discussion Pentasa Compact 4 g, prolonged-release granules (mesalazine) NL License RVG: 114015 Date: 30 March 2015 This module reflects the scientific discussion for
CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM 10% [100 mg/ml]
![CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM 10% [100 mg/ml] CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM 10% [100 mg/ml]](/thumbs/80/82110087.jpg) CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM 10% [100 mg/ml] Human Normal Immunoglobulin Solution for Intravenous Infusion. OCTAGAM 10% [100 mg/ml] is available in single use bottles of 20 ml, 50 ml, 100
CONSUMER MEDICINE INFORMATION (CMI) OCTAGAM 10% [100 mg/ml] Human Normal Immunoglobulin Solution for Intravenous Infusion. OCTAGAM 10% [100 mg/ml] is available in single use bottles of 20 ml, 50 ml, 100
Research Article Clinical and Epidemiological Investigation of TCM Syndromes of Patients with Coronary Heart Disease in China
 Evidence-Based Complementary and Alternative Medicine Volume 2012, Article ID 714517, 5 pages doi:10.1155/2012/714517 Research Article Clinical and Epidemiological Investigation of TCM Syndromes of Patients
Evidence-Based Complementary and Alternative Medicine Volume 2012, Article ID 714517, 5 pages doi:10.1155/2012/714517 Research Article Clinical and Epidemiological Investigation of TCM Syndromes of Patients
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200 g/l, solution for infusion 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
LACIPIL QUALITATIVE AND QUANTITATIVE COMPOSITION
 LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
LACIPIL lacidipine QUALITATIVE AND QUANTITATIVE COMPOSITION Lacidipine, 2 mg - round shaped white engraved on one face. Lacidipine, 4 mg - oval white with break line on both faces. Lacidipine, 6 mg - oval
ClinicalTrials.gov Identifier: NCT Sponsor/company: Sanofi-Aventis. Date: 08/02/ 2008
 These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Sanofi-Aventis ClinicalTrials.gov
These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert in the country of prescription Sponsor/company: Sanofi-Aventis ClinicalTrials.gov
Online Supplementary Data. Country Number of centers Number of patients randomized
 A Randomized, Double-Blind, -Controlled, Phase-2B Study to Evaluate the Safety and Efficacy of Recombinant Human Soluble Thrombomodulin, ART-123, in Patients with Sepsis and Suspected Disseminated Intravascular
A Randomized, Double-Blind, -Controlled, Phase-2B Study to Evaluate the Safety and Efficacy of Recombinant Human Soluble Thrombomodulin, ART-123, in Patients with Sepsis and Suspected Disseminated Intravascular
Traditional Chinese Medicine syndrome patterns and Qi-regulating, chest-relaxing and blood-activating therapy on cardiac syndrome X
 Online Submissions:http://www.journaltcm.com J Tradit Chin Med 2013 April 15; 33(2): 194-199 info@journaltcm.com ISSN 0255-2922 2013 JTCM. All rights reserved. CLINICAL STUDY TOPIC Traditional Chinese
Online Submissions:http://www.journaltcm.com J Tradit Chin Med 2013 April 15; 33(2): 194-199 info@journaltcm.com ISSN 0255-2922 2013 JTCM. All rights reserved. CLINICAL STUDY TOPIC Traditional Chinese
(angiotensin II) injection for intravenous infusion
 ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
ADMINISTERING GIAPREZA TM (angiotensin II) injection for intravenous infusion Visit www.giapreza.com INITIATE Recommended starting dose of GIAPREZA is 20 ng/kg/min, which is equivalent to 0.02 mcg/kg/min
(human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS
 albunorm TM 5% (human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS UK IRELAND and Ireland Octapharma Limited The Zenith Building, 26 Spring Gardens Manchester M2 1AB United Kingdom 1. Name
albunorm TM 5% (human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS UK IRELAND and Ireland Octapharma Limited The Zenith Building, 26 Spring Gardens Manchester M2 1AB United Kingdom 1. Name
Structure and organization of blood vessels
 The cardiovascular system Structure of the heart The cardiac cycle Structure and organization of blood vessels What is the cardiovascular system? The heart is a double pump heart arteries arterioles veins
The cardiovascular system Structure of the heart The cardiac cycle Structure and organization of blood vessels What is the cardiovascular system? The heart is a double pump heart arteries arterioles veins
Clinical study of Huxinkang tablet in treating unstable angina pectoris
 ISSN : 0974-7435 Volume 10 Issue 17 BTAIJ, 10(17), 2014 [9538-9543] Clinical study of Huxinkang tablet in treating unstable angina pectoris Wang Guoqian 1, Yu Zhengke 2, Cheng Zhihong 2, Xie hongtu 3 1
ISSN : 0974-7435 Volume 10 Issue 17 BTAIJ, 10(17), 2014 [9538-9543] Clinical study of Huxinkang tablet in treating unstable angina pectoris Wang Guoqian 1, Yu Zhengke 2, Cheng Zhihong 2, Xie hongtu 3 1
Public Assessment Report. Scientific discussion MINOXIDIL AT/H/0638/ /DC. Date:
 CMDh/223/2005 February 2014 Public Assessment Report Scientific discussion Alocutan 20 mg/ml Spray zur Anwendung auf der Haut, Lösung, Alocutan 50 mg/ml Spray zur Anwendung auf der Haut, Lösung MINOXIDIL
CMDh/223/2005 February 2014 Public Assessment Report Scientific discussion Alocutan 20 mg/ml Spray zur Anwendung auf der Haut, Lösung, Alocutan 50 mg/ml Spray zur Anwendung auf der Haut, Lösung MINOXIDIL
Iroko Pharmaceuticals Gains Additional Patents for ZORVOLEX and TIVORBEX TM
 Iroko Pharmaceuticals Gains Additional Patents for ZORVOLEX and TIVORBEX TM PHILADELPHIA, April 8, 2015 Iroko Pharmaceuticals, LLC, a global specialty pharmaceutical company dedicated to advancing the
Iroko Pharmaceuticals Gains Additional Patents for ZORVOLEX and TIVORBEX TM PHILADELPHIA, April 8, 2015 Iroko Pharmaceuticals, LLC, a global specialty pharmaceutical company dedicated to advancing the
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fexofenadine hydrochloride 180 mg film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 180mg
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Fexofenadine hydrochloride 180 mg film-coated tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each film coated tablet contains 180mg
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory. Light Liquid Paraffin and White Soft Paraffin Cream
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory OILATUM CREAM / KIDS CREAM Light Liquid Paraffin and White Soft Paraffin Cream QUALITATIVE AND QUANTITATIVE COMPOSITION
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory OILATUM CREAM / KIDS CREAM Light Liquid Paraffin and White Soft Paraffin Cream QUALITATIVE AND QUANTITATIVE COMPOSITION
Antihypertensive drugs SUMMARY Made by: Lama Shatat
 Antihypertensive drugs SUMMARY Made by: Lama Shatat Diuretic Thiazide diuretics The loop diuretics Potassium-sparing Diuretics *Hydrochlorothiazide *Chlorthalidone *Furosemide *Torsemide *Bumetanide Aldosterone
Antihypertensive drugs SUMMARY Made by: Lama Shatat Diuretic Thiazide diuretics The loop diuretics Potassium-sparing Diuretics *Hydrochlorothiazide *Chlorthalidone *Furosemide *Torsemide *Bumetanide Aldosterone
Off-white, round, with faceted edges, scored on one side and bearing the inscription IK10 (10mg) or IK20 (20mg).
 1 TRADE NAME OF THE MEDICINAL PRODUCT Ikorel 10mg and 20mg Tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Nicorandil 10mg or 20mg For a full list of excipients, see section 6.1 3 PHARMACEUTICAL FORM
1 TRADE NAME OF THE MEDICINAL PRODUCT Ikorel 10mg and 20mg Tablet 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Nicorandil 10mg or 20mg For a full list of excipients, see section 6.1 3 PHARMACEUTICAL FORM
Iroko Pharmaceuticals Announces Acceptance for Filing of ZORVOLEX snda for the Treatment of Osteoarthritis Pain in Adults
 Iroko Pharmaceuticals Announces Acceptance for Filing of ZORVOLEX snda for the Treatment of Osteoarthritis Pain in Adults First Lower Dose NSAID Using SoluMatrix Fine Particle Technology to be Reviewed
Iroko Pharmaceuticals Announces Acceptance for Filing of ZORVOLEX snda for the Treatment of Osteoarthritis Pain in Adults First Lower Dose NSAID Using SoluMatrix Fine Particle Technology to be Reviewed
Heart disease remains the leading cause of morbidity and mortality in industrialized nations. It accounts for nearly 40% of all deaths in the United
 Heart disease remains the leading cause of morbidity and mortality in industrialized nations. It accounts for nearly 40% of all deaths in the United States, totaling about 750,000 individuals annually
Heart disease remains the leading cause of morbidity and mortality in industrialized nations. It accounts for nearly 40% of all deaths in the United States, totaling about 750,000 individuals annually
Rasburicase 1.5 mg/ml powder and solvent for concentrate for solution for infusion. FASTURTEC
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
Package leaflet: Information for the user Zinforo 600 mg powder for concentrate for solution for infusion Ceftaroline fosamil
 Package leaflet: Information for the user Zinforo 600 mg powder for concentrate for solution for infusion Ceftaroline fosamil Read all of this leaflet carefully before you start using this medicine because
Package leaflet: Information for the user Zinforo 600 mg powder for concentrate for solution for infusion Ceftaroline fosamil Read all of this leaflet carefully before you start using this medicine because
Management of Stable Ischemic Heart Disease. Vinay Madan MD February 10, 2018
 Management of Stable Ischemic Heart Disease Vinay Madan MD February 10, 2018 1 Disclosure No financial disclosure. 2 Overview of SIHD Diagnosis Outline of talk Functional vs. Anatomic assessment Management
Management of Stable Ischemic Heart Disease Vinay Madan MD February 10, 2018 1 Disclosure No financial disclosure. 2 Overview of SIHD Diagnosis Outline of talk Functional vs. Anatomic assessment Management
Consumer Medicine Information. This leaflet answers some common questions about Calvasc tablets
 Consumer Medicine Information Calvasc Tablets Amlodipine 5 mg tablets and 10 mg tablets What is in this leaflet This leaflet answers some common questions about Calvasc tablets It does not contain all
Consumer Medicine Information Calvasc Tablets Amlodipine 5 mg tablets and 10 mg tablets What is in this leaflet This leaflet answers some common questions about Calvasc tablets It does not contain all
BECLOT 500MG. INJECTION Composition : Each ml. contains : Tranexamic Acid I.P. 100mg.
 BECLOT 500MG. INJECTION Composition : Each ml. contains : Tranexamic Acid I.P. 100mg. DESCRIPTION Tranexamic acid is a synthetic analog of the amino acid lysine. It is used to treat or prevent excessive
BECLOT 500MG. INJECTION Composition : Each ml. contains : Tranexamic Acid I.P. 100mg. DESCRIPTION Tranexamic acid is a synthetic analog of the amino acid lysine. It is used to treat or prevent excessive
Improving the Drug Quality in China Via the Development of the Standard System of Pharmaceutical Exicipent of the 2015 Edition of Chinese Pharmacopeia
 2015 100050 2015 2010 2015 2015 doi 10. 11669 /cpj. 2015. 15. 019 A 1001-2494 2015 15-1353 - 06 Improving the Drug Quality in China Via the Development of the Standard System of Pharmaceutical Exicipent
2015 100050 2015 2010 2015 2015 doi 10. 11669 /cpj. 2015. 15. 019 A 1001-2494 2015 15-1353 - 06 Improving the Drug Quality in China Via the Development of the Standard System of Pharmaceutical Exicipent
Package leaflet: Information for the user. Elaprase 2 mg/ml concentrate for solution for infusion idursulfase
 Package leaflet: Information for the user Elaprase 2 mg/ml concentrate for solution for infusion idursulfase This medicine is subject to additional monitoring. This will allow quick identification of new
Package leaflet: Information for the user Elaprase 2 mg/ml concentrate for solution for infusion idursulfase This medicine is subject to additional monitoring. This will allow quick identification of new
Package leaflet: Information for the patient. Zerbaxa 1 g / 0.5 g powder for concentrate for solution for infusion ceftolozane / tazobactam
 Package leaflet: Information for the patient Zerbaxa 1 g / 0.5 g powder for concentrate for solution for infusion ceftolozane / tazobactam This medicine is subject to additional monitoring. This will allow
Package leaflet: Information for the patient Zerbaxa 1 g / 0.5 g powder for concentrate for solution for infusion ceftolozane / tazobactam This medicine is subject to additional monitoring. This will allow
Hypertension The normal radial artery blood pressures in adults are: Systolic arterial pressure: 100 to 140 mmhg. Diastolic arterial pressure: 60 to
 Hypertension The normal radial artery blood pressures in adults are: Systolic arterial pressure: 100 to 140 mmhg. Diastolic arterial pressure: 60 to 90 mmhg. These pressures are called Normal blood pressure
Hypertension The normal radial artery blood pressures in adults are: Systolic arterial pressure: 100 to 140 mmhg. Diastolic arterial pressure: 60 to 90 mmhg. These pressures are called Normal blood pressure
SUCRALFATE TABLETS, USP
 1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002-01 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
Package leaflet: Information for the patient. Trasylol 10,000 KIU/ml solution for injection or infusion. Aprotinin
 Package leaflet: Information for the patient Trasylol 10,000 KIU/ml solution for injection or infusion Aprotinin Read all of this leaflet carefully before you are given this medicine because it contains
Package leaflet: Information for the patient Trasylol 10,000 KIU/ml solution for injection or infusion Aprotinin Read all of this leaflet carefully before you are given this medicine because it contains
(human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS
 albunorm TM 20% (human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS UK IRELAND and Ireland Octapharma Limited The Zenith Building, 26 Spring Gardens Manchester M2 1AB United Kingdom 1. Name
albunorm TM 20% (human albumin solution) POM SUMMARY OF PRODUCT CHARACTERISTICS UK IRELAND and Ireland Octapharma Limited The Zenith Building, 26 Spring Gardens Manchester M2 1AB United Kingdom 1. Name
PACKAGE LEAFLET: INFORMATION FOR THE USER. SODIPHOS 22mEq / 10ml Concentrate for solution for infusion. Disodium phosphate dihydrate
 PACKAGE LEAFLET: INFORMATION FOR THE USER SODIPHOS 22mEq / 10ml Concentrate for solution for infusion Disodium phosphate dihydrate Read all of this leaflet carefully before you start using this medicine.
PACKAGE LEAFLET: INFORMATION FOR THE USER SODIPHOS 22mEq / 10ml Concentrate for solution for infusion Disodium phosphate dihydrate Read all of this leaflet carefully before you start using this medicine.
24 h. P > h. R doi /j. issn
 38 4 2015 4 Vol. 38 No. 4 Apr. 2015 Journal of Beijing University of Traditional Chinese Medicine 271 2 ~ 3 * 1 2 2 2 2 2 1# 1 201203 2 2 ~ 3 55 2 18 37 24 2 0 8 16 24 24 h 2 P > 0. 05 P < 0. 05 P < 0.
38 4 2015 4 Vol. 38 No. 4 Apr. 2015 Journal of Beijing University of Traditional Chinese Medicine 271 2 ~ 3 * 1 2 2 2 2 2 1# 1 201203 2 2 ~ 3 55 2 18 37 24 2 0 8 16 24 24 h 2 P > 0. 05 P < 0. 05 P < 0.
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Fexofenadine Cipla 120 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each film-coated tablet contains 120 mg fexofenadine
SUMMARY OF PRODUCT CHARACTERISTICS. Medical conditions that require parenteral nutrition for supply of energy and essential fatty acids.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alphalipid 200 mg/ml emulsion for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1000 ml of the emulsion contain: Soya-bean oil,
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Alphalipid 200 mg/ml emulsion for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1000 ml of the emulsion contain: Soya-bean oil,
SUMMARY OF PRODUCT CHARACTERISTICS 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Telfast 120 mg film-coated tablets. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 120 mg of fexofenadine hydrochloride,
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Telfast 120 mg film-coated tablets. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 120 mg of fexofenadine hydrochloride,
PACKAGE LEAFLET TEXT ZOLADEX LA 10.8MG. (goserelin)
 ONC.000-092-861.10.0 PACKAGE LEAFLET TEXT ZOLADEX LA 10.8MG (goserelin) Name of the medicinal product Zoladex LA 10.8mg depot Qualitative and quantitative composition Goserelin acetate (equivalent to 10.8
ONC.000-092-861.10.0 PACKAGE LEAFLET TEXT ZOLADEX LA 10.8MG (goserelin) Name of the medicinal product Zoladex LA 10.8mg depot Qualitative and quantitative composition Goserelin acetate (equivalent to 10.8
Contents of four active components in different commercial crude drugs and preparations of Danshen (Salvia miltiorrhiza)
 Zhang H et al / Acta Pharmacol Sin 2002 Dec; 23 (12): 1163-1168 1163 2002, Acta Pharmacologica Sinica ISSN 1671-4083 Shanghai Institute of Materia Medica Chinese Academy of Sciences http://www.chinaphar.com
Zhang H et al / Acta Pharmacol Sin 2002 Dec; 23 (12): 1163-1168 1163 2002, Acta Pharmacologica Sinica ISSN 1671-4083 Shanghai Institute of Materia Medica Chinese Academy of Sciences http://www.chinaphar.com
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Comfora 595 mg film-coated tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One film-coated tablet contains: glucosamine sulphate
SUCRALFATE TABLETS, USP
 1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
1234567890 10 210002 SUCRALFATE TABLETS, USP DESCRIPTION Sucralfate is an -D-glucopyranoside, -D-fructofuranosyl-, octakis-(hydrogen sulfate), aluminum complex. It has the following structural formula:
CEREZYME Genzyme. 70 mg (52 mg) (18 mg)
 CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
CEREZYME Genzyme Imiglucerase for injection 400 UNITS 200 UNITS DESCRIPTION Cerezyme (imiglucerase for injection) is an analogue of the human enzyme ß-Glucocerebrosidase, produced by recombinant DNA technology.
Cardiovascular Concerns in Intermediate Care
 Cardiovascular Concerns in Intermediate Care GINA ST. JEAN RN, MSN, CCRN-CSC CLINICAL NURSE EDUCATOR HEART AND & CRITICAL AND INTERMEDIATE CARE Objectives: Identify how to do a thorough assessment of the
Cardiovascular Concerns in Intermediate Care GINA ST. JEAN RN, MSN, CCRN-CSC CLINICAL NURSE EDUCATOR HEART AND & CRITICAL AND INTERMEDIATE CARE Objectives: Identify how to do a thorough assessment of the
Pharmacy Instructions for Preparation
 MARQIBO (vincristine sulfate LIPOSOME injection) Pharmacy Instructions for Preparation Important Information for Preparation 1 The instructions for the constitution of MARQIBO are provided in each kit.
MARQIBO (vincristine sulfate LIPOSOME injection) Pharmacy Instructions for Preparation Important Information for Preparation 1 The instructions for the constitution of MARQIBO are provided in each kit.
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) DRAFT COMMUNITY HERBAL MONOGRAPH ON MELILOTUS OFFICINALIS (L.) LAM., HERBA
 European Medicines Agency Evaluation of Medicines for Human Use London, 31 October 2007 Doc. Ref. EMEA/HMPC/354177/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) DRAFT COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 31 October 2007 Doc. Ref. EMEA/HMPC/354177/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) DRAFT COMMUNITY HERBAL MONOGRAPH ON
CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS
 CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS Guideline Title Clinical Investigation of Anti-Anginal Medicinal Products in Stable Angina Pectoris Legislative basis
CLINICAL INVESTIGATION OF ANTI-ANGINAL MEDICINAL PRODUCTS IN STABLE ANGINA PECTORIS Guideline Title Clinical Investigation of Anti-Anginal Medicinal Products in Stable Angina Pectoris Legislative basis
Cardiac Pathophysiology
 Cardiac Pathophysiology Evaluation Components Medical history Physical examination Routine laboratory tests Optional tests Medical History Duration and classification of hypertension. Patient history of
Cardiac Pathophysiology Evaluation Components Medical history Physical examination Routine laboratory tests Optional tests Medical History Duration and classification of hypertension. Patient history of
PATIENT / USER INFORMATION LEAFLET. Cinryze 500 Units powder and solvent for solution for injection C1 inhibitor (human)
 PATIENT / USER INFORMATION LEAFLET Cinryze 500 Units powder and solvent for solution for injection C1 inhibitor (human) Read all of this leaflet carefully before you start taking this medicine. Keep this
PATIENT / USER INFORMATION LEAFLET Cinryze 500 Units powder and solvent for solution for injection C1 inhibitor (human) Read all of this leaflet carefully before you start taking this medicine. Keep this
Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein of which at least 95% is human albumin.
 1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
1. NAME OF THE MEDICINAL PRODUCT Human Albumin 200 g/l Baxter Solution for Infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Human Albumin 200 g/l Baxter is a solution containing 200 g/l of total protein
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion , version 1.1
 EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
EU RISK MANAGEMENT PLAN (EU RMP) Nutriflex Omega peri emulsion for infusion 13.7.2015, version 1.1 III.1. Elements for a Public Summary III.1.1. Overview of disease epidemiology Patients may need parenteral
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use North American Coral Snake Antivenin (Equine) safely and effectively. See full prescribing information
SUMMARY OF PRODUCT CHARACTERISTICS. Flexbumin 200 g/l is a solution containing 200 g/l (20%) of total protein of which at least 95% is human albumin.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Flexbumin 200g/l solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Flexbumin 200 g/l is a solution containing 200 g/l
PARENTERAL PREPARATIONS
 PARENTERAL PREPARATIONS INTRODUCTION Parenteral (Gk, para enteron, beside the intestine) dosage forms differ from all other drug dosage forms, because they are injected directly into body tissue through
PARENTERAL PREPARATIONS INTRODUCTION Parenteral (Gk, para enteron, beside the intestine) dosage forms differ from all other drug dosage forms, because they are injected directly into body tissue through
UW MEDICINE PATIENT EDUCATION. Treatment for blocked heart arteries DRAFT. What are arteries? How do heart arteries become blocked?
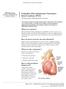 UW MEDICINE PATIENT EDUCATION Complex Percutaneous Coronary Intervention (PCI) Treatment for blocked heart arteries This handout explains complex percutaneous intervention (PCI) treatment of a coronary
UW MEDICINE PATIENT EDUCATION Complex Percutaneous Coronary Intervention (PCI) Treatment for blocked heart arteries This handout explains complex percutaneous intervention (PCI) treatment of a coronary
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: , VERSION 1.
 RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
PHARMACOKINETICS, TISSUE DISTRIBUTION, METABOLISM AND EXCRETION OF DEPSIDE SALTS FROM SALVIA MILTIORRHIZA IN RATS
 DMD Fast This Forward. article has not Published been copyedited on and November formatted. The 28, final 2006 version as may doi:10.1124/dmd.106.013045 differ from this version. PHARMACOKINETICS, TISSUE
DMD Fast This Forward. article has not Published been copyedited on and November formatted. The 28, final 2006 version as may doi:10.1124/dmd.106.013045 differ from this version. PHARMACOKINETICS, TISSUE
ANNEX III LABELLING AND PACKAGE LEAFLET
 ANNEX III LABELLING AND PACKAGE LEAFLET 1 B. PACKAGE LEAFLET 2 PACKAGE LEAFLET Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
ANNEX III LABELLING AND PACKAGE LEAFLET 1 B. PACKAGE LEAFLET 2 PACKAGE LEAFLET Read all of this leaflet carefully before you start taking this medicine. - Keep this leaflet. You may need to read it again.
Pharmacokinetics, Tissue Distribution, Metabolism, and Excretion of Depside Salts from Salvia miltiorrhiza in Rats
 0090-9556/07/3502-234 239$20.00 DRUG METABOLISM AND DISPOSITION Vol. 35, No. 2 Copyright 2007 by The American Society for Pharmacology and Experimental Therapeutics 13045/3173663 DMD 35:234 239, 2007 Printed
0090-9556/07/3502-234 239$20.00 DRUG METABOLISM AND DISPOSITION Vol. 35, No. 2 Copyright 2007 by The American Society for Pharmacology and Experimental Therapeutics 13045/3173663 DMD 35:234 239, 2007 Printed
Construction of the Vesselcollateral. Guidance for Prevention and Treatment of Vasculopathy. Section 1. China. Clinical Trials.
 Section 1 05 Clinical Trials Construction of the Vesselcollateral Theory and its Guidance for Prevention and Treatment of Vasculopathy China Yiling Wu The Integration of Traditional and Western Medical
Section 1 05 Clinical Trials Construction of the Vesselcollateral Theory and its Guidance for Prevention and Treatment of Vasculopathy China Yiling Wu The Integration of Traditional and Western Medical
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200g/l, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Albunorm 20%, 200g/l, solution for infusion 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Albunorm 20% is a solution containing 200 g/l
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
Summary of Product Characteristics 1. NAME OF THE MEDICINAL PRODUCT {To be completed nationally} 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 mg tablets: each tablet contains 1 mg granisetron (as hydrochloride).
1/3/2008. Karen Burke Priscilla LeMone Elaine Mohn-Brown. Medical-Surgical Nursing Care, 2e Karen Burke, Priscilla LeMone, and Elaine Mohn-Brown
 Medical-Surgical Nursing Care Second Edition Karen Burke Priscilla LeMone Elaine Mohn-Brown Chapter 26 Caring for Clients with Coronary Heart Disease and Dysrhythmias Coronary Heart Disease (CHD) Leading
Medical-Surgical Nursing Care Second Edition Karen Burke Priscilla LeMone Elaine Mohn-Brown Chapter 26 Caring for Clients with Coronary Heart Disease and Dysrhythmias Coronary Heart Disease (CHD) Leading
Package leaflet: Information for the user. TRISENOX 1 mg/ml concentrate for solution for infusion arsenic trioxide
 Package leaflet: Information for the user TRISENOX 1 mg/ml concentrate for solution for infusion arsenic trioxide Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user TRISENOX 1 mg/ml concentrate for solution for infusion arsenic trioxide Read all of this leaflet carefully before you start using this medicine because it contains
Acute coronary syndrome. Dr LM Murray Chemical Pathology Block SA
 Acute coronary syndrome Dr LM Murray Chemical Pathology Block SA13-2014 Acute myocardial infarction (MI) MI is still the leading cause of death in many countries It is characterized by severe chest pain,
Acute coronary syndrome Dr LM Murray Chemical Pathology Block SA13-2014 Acute myocardial infarction (MI) MI is still the leading cause of death in many countries It is characterized by severe chest pain,
