Elements for a Public Summary
|
|
|
- Elfreda Gilbert
- 5 years ago
- Views:
Transcription
1 Ibandronat Stada 50 mg film-coated tablets Ibandronat Stada 150 mg film-coated tablets , Version V2.1 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN VI.2 Elements for a Public Summary <[Ibandronic acid 50 mg film-coated tablets]> VI.2.1a Overview of disease epidemiology Breast cancer is the most common cancer in women. Whereas 1.15 million patients were newly diagnosed in 2002, it is estimated that 2.7 million will be affected in Rates for Asian and Black females (appr per 100,000) are lower than for White females (122 to 125 per 100,000). Among men, less than 1% of cancer cases account for breast cancer. The risk for developing breast cancer increases with age in both males and females. Bone metastases are a significant complication in patients with breast cancer, and occur in % of the patients. Bone metastases lead to pain and more complicated events such as fractures, bone marrow infiltrations or hypercalcaemia. VI.2.2a Summary of treatment benefits <Ibandronic acid 50 mg> contains the active substance ibandronic acid. This belongs to a group of medicines called bisphosphonates. <Ibandronic acid 50 mg> is used in adults and is prescribed to you if you have breast cancer that has spread to your bones (called bone metastases ). It helps to prevent your bones from breaking (fractures). It also helps to prevent other bone problems that may need surgery or radiotherapy. <Ibandronic acid 50 mg> works by reducing the amount of calcium that is lost from your bones. This helps to stop your bones from getting weaker. <[Ibandronic acid 150 mg film-coated tablets]> VI.2.1b Overview of disease epidemiology In the EU, 22 million women and 5.5 million men were estimated to suffer from osteoporosis in Bone is a living tissue. Old bone is constantly removed from your skeleton and replaced with new bone. Osteoporosis is a disease of the bone commonly occuring with age. Bone density reduces, the bones become weaker, more fragile and more likely to break after a fall or strain. In 80% of the cases, osteoporosis occurs in postmenopausal women (women after the menopause). Many patients with osteoporosis have no symptoms and not even have known that they had it. In 30 % of the cases, the osteoporosis is clinically relevant and requires treatment. Osteoporosis is more likely to occur in women who have reached the menopause early and also in patients treated long-term with steroids. At the menopause, a woman s STADA Arzneimittel AG C O N F I D E N T I A L Page 1 of 5
2 ovaries stop producing the female hormone, oestrogen, which helps to keep her skeleton healthy. Other things that can increase the risk of fractures include: not enough calcium and vitamin D in the diet smoking, or drinking too much alcohol not enough walking or other weight-bearing exercise a family history of osteoporosis A healthy lifestyle will also help you to get the most benefit from your treatment. This includes: eating a balanced diet rich in calcium and vitamin D walking or any other weight-bearing exercise not smoking; and not drinking too much alcohol. VI.2.2b Summary of treatment benefits <Ibandronic acid 150 mg> is prescribed to treat postmenopausal osteoporosis. <Ibandronic acid 150 mg> belongs to a group of medicines called bisphosphonates. It contains the active substance ibandronic acid. <Ibandronic acid 150 mg> may reverse bone loss by stopping more loss of bone and increasing bone mass in most women who take it, even though they won t be able to see or feel a difference. <Ibandronic acid 150 mg> may help lower the chances of breaking bones (fractures). This reduction in fractures was shown for the spine but not for the hip. <[Ibandronic acid 50 mg film-coated tablets]> <[Ibandronic acid 150 mg film-coated tablets]> VI.2.3 Unknowns relating to treatment benefits The treatment benefit in children and adolescents below 18 years of age has not been established. Efficacy of <[ibandronic acid 150mg]> in femoral neck fractures has not been established yet. VI.2.4 Summary of safety concerns Important identified risks Risk What is known Preventability Dead bone tissue in the jaw bone (Osteonecrosis of the jaw) This side effect my affect up to 1 in 10,000 people. Pain or sore in your mouth or jaw are early signs of severe jaw problems. A dental examination with appropriate preventive dentistry should be considered prior to treatment, especially if you have the following risk factors: e.g. cancer, chemotherapy, radiotherapy, corticosteroids, poor oral hygiene. If you have one of these risk factors, you should avoid invasive dental STADA Arzneimittel AG C O N F I D E N T I A L Page 2 of 5
3 procedures if possible while on treatment. If you are having dental treatment or surgery or know that you need some in the future, tell your dentist that you are being treated with <Ibandronic acid >. Low calcium levels in your blood (Hypocalcaemia) Up to 1 in 10 people may experience low calcium levels in your blood DO NOT take <Ibandronic acid> if you have or have ever had low calcium in your blood. In particular, tell your doctor or pharmacist if you are taking any supplements containing calcium. Inflammatory immune response in the whole body (Acute phase reaction) Severe irrittation of the tube that connects your mouth and your stomach (esophagus) (severe esophageal irritation) This side effect may affect up to 1 in 100 people. The symptomy are similar to flu-like symtoms and you could feel generally unwell or in pain. This side effect may affect up to 1 in 100 people. The risk of severe irrittation of the tube that connects your mouth and your stomach (esophagus) appears to be greater in patients who do not comply with the dosing instruction and/or who continue to take <Ibandronic acid> after developing symptoms suggestive of irritation (such as heartburn, acid reflux,hoarse voice, chest pain when eating, difficulty or pain when swallowing). Always take this medicine as prescribed by your doctor and as indicated in the Package Leaflet. This will minimise the risk of developing adverse drug reactions. If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in the package leaflet. Always take this medicine as prescribed by your doctor and as indicated in the Package Leaflet. This will minimise the risk of developing adverse drug reactions. Tell your doctor if you have active upper gastrointestinal problems. Stop taking <Ibandronic acid> and contact your doctor immediately if you have difficulty swallowing, painful swallowing, pain behind the breastbone, or new or worsening heartburn. STADA Arzneimittel AG C O N F I D E N T I A L Page 3 of 5
4 Important potential risks Risk Atypical fractures of the femur What is known (Including reason why it is considered a potential risk) The long-term use of bisphosphonates is thought to be the main risk factor for atypical femoral fractures. Some patients experience thigh or groin pain, often associated with imaging features of stress fractures, weeks to months before presenting with a completed femoral fracture. During bisphosphonate treatment, you should report any thigh, hip or groin pain. Atrial fibrillation Patients treated with the medicinal product may be at an increased risk of developing atrial fibrillation. Cases of atrial fibrillation have been reported with the use of pamidronic acid, another bisphosphonate. However, a causal relationship to ibandronic acid has not been established. Kidney dysfunction (renal dysfunction) Patients treated with the medicinal product may be at an increased risk of developing kidney problems. There are concerns about using bisphosphonates in patients with existing kidney problems and also in older people. Tell your doctor if you experience urination changes, ankle swelling, weakness, dizziness, skin rash or itching, or a metallic taste in your mouth. Missing information Risk None What is known NA VI.2.5 Summary of risk minimisation measures by safety concern All medicines have a Summary of Product Characteristics (SPC) which provides physicians, pharmacists and other health care professionals with details on how to use the medicine, the risks and recommendations for minimising them. An abbreviated version of this in lay language is provided in the form of the package leaflet (PL). The measures in these documents are known as routine risk minimisation measures. This medicine has no additional risk minimisation measures. VI.2.6 Planned post authorisation development plan No post-authorisation studies have been imposed or are planned. STADA Arzneimittel AG C O N F I D E N T I A L Page 4 of 5
5 VI.2.7 Summary of changes to the Risk Management Plan over time Version Date Safety Concerns Comment V Sep-21 Atypical fractures of the femur V Jun-16 Important identified risks: Osteonecrosis of the jaw Hypocalcaemia Acute phase reaction Important potential risk: Atrial fibrillation Missing information: None V Nov-03 Important identififed risks: Osteonecrosis of the jaw Hypocalcaemia Acute phase reaction Severe oesophageal irritation Important potential risk: Atrial fibrillation Renal dysfunction Missing information: None STADA Arzneimittel AG C O N F I D E N T I A L Page 5 of 5
Summary of the risk management plan by product
 Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Summary of the risk management plan by product 1 Elements for summary tables in the EPAR 1.1 Summary table of Safety concerns Summary of safety concerns Important identified risks Important potential risks
Zerlinda (MRP DK/H/2265/001)
 Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer
 Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer Category: Summary: Guideline Adjuvant Bisphosphonate treatment for Post-Menopausal
Oxford University Hospitals Guidelines for Adjuvant Bisphosphonate treatment for Post-Menopausal Women with Early Breast Cancer Category: Summary: Guideline Adjuvant Bisphosphonate treatment for Post-Menopausal
MEDICATION GUIDE. Boniva (bon-ee-va) (ibandronate sodium) Tablets
 MEDICATION GUIDE Boniva (bon-ee-va) (ibandronate sodium) Tablets Read the Medication Guide that comes with BONIVA before you start taking it and each time you get a refill. There may be new information.
MEDICATION GUIDE Boniva (bon-ee-va) (ibandronate sodium) Tablets Read the Medication Guide that comes with BONIVA before you start taking it and each time you get a refill. There may be new information.
Package leaflet: Information for the user. Alendronat 1A Farma Veckotablett 70 mg Film-coated tablets Alendronic acid
 Package leaflet: Information for the user Alendronat 1A Farma Veckotablett 70 mg Film-coated tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it
Package leaflet: Information for the user Alendronat 1A Farma Veckotablett 70 mg Film-coated tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it
Package leaflet: Information for the user. Ibandroninezuur STADA 3 mg/3 ml, oplossing voor injectie. Ibandronic acid
 Package leaflet: Information for the user Ibandroninezuur STADA 3 mg/3 ml, oplossing voor injectie Ibandronic acid Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user Ibandroninezuur STADA 3 mg/3 ml, oplossing voor injectie Ibandronic acid Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the patient. Risedronate sodium 35 mg film-coated tablets. Risedronate sodium
 Package leaflet: Information for the patient Risedronate sodium 35 mg film-coated tablets Risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the patient Risedronate sodium 35 mg film-coated tablets Risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE USER. Risedronat Jubilant 5 mg film-coated tablets risedronate sodium
 PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 5 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 5 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER. Alendronic Acid 10 mg Tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER Alendronic Acid 10 mg Tablets (Alendronic Acid) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
PACKAGE LEAFLET: INFORMATION FOR THE USER Alendronic Acid 10 mg Tablets (Alendronic Acid) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet. You may need to read
Etoricoxib STADA 30 mg, 60 mg, 90 mg and 120 mg film-coated tablets , Version V1.2 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN
 Etoricoxib STADA 30 mg, 60 mg, 90 mg and 120 mg film-coated tablets 23.5.2016, Version V1.2 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN VI.2 Elements for a Public Summary Etoricoxib STADA 30 mg film-coated
Etoricoxib STADA 30 mg, 60 mg, 90 mg and 120 mg film-coated tablets 23.5.2016, Version V1.2 PUBLIC SUMMARY OF THE RISK MANAGEMENT PLAN VI.2 Elements for a Public Summary Etoricoxib STADA 30 mg film-coated
2 What you need to know before you take Osbonelle 150 mg Do not take Osbonelle 150 mg:
 Package leaflet: Information for the patient Osbonelle 150 mg film-coated tablets ibandronic acid Read all of this leaflet carefully before you start taking this medicine because it contains important
Package leaflet: Information for the patient Osbonelle 150 mg film-coated tablets ibandronic acid Read all of this leaflet carefully before you start taking this medicine because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER. Risedronat Jubilant 35 mg film-coated tablets risedronate sodium
 PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 35 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 35 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER. Alendroninezuur Mylan 70 mg, tabletten. (sodium alendronate trihydrate)
 PACKAGE LEAFLET: INFORMATION FOR THE USER Alendroninezuur Mylan 70 mg, tabletten (sodium alendronate trihydrate) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet.
PACKAGE LEAFLET: INFORMATION FOR THE USER Alendroninezuur Mylan 70 mg, tabletten (sodium alendronate trihydrate) Read all of this leaflet carefully before you start taking this medicine. Keep this leaflet.
Package leaflet: Information for the user. Zoledronic Acid Accord 4 mg/5 ml concentrate for solution for infusion Zoledronic acid
 Package leaflet: Information for the user Zoledronic Acid Accord 4 mg/5 ml concentrate for solution for infusion Zoledronic acid Read all of this leaflet carefully before you start taking this medicine
Package leaflet: Information for the user Zoledronic Acid Accord 4 mg/5 ml concentrate for solution for infusion Zoledronic acid Read all of this leaflet carefully before you start taking this medicine
Dorset Pathway for the use of bisphosphonates for post-menopausal women with breast cancer. Summary
 Summary Appendix 1: Selection of patients suitable for adjuvant bisphosphonates* Appendix 2 Adjuvant Bisphosphonates for Early Breast Cancer Information for patients This leaflet is intended to support
Summary Appendix 1: Selection of patients suitable for adjuvant bisphosphonates* Appendix 2 Adjuvant Bisphosphonates for Early Breast Cancer Information for patients This leaflet is intended to support
ACLASTA Zoledronic acid
 ACLASTA Zoledronic acid Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Aclasta. It does not contain all the available information. It does not take
ACLASTA Zoledronic acid Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Aclasta. It does not contain all the available information. It does not take
Bonefos 400 mg capsules sodium clodronate
 Due to regulatory changes, the content of the following Patient Information Leaflet may vary from the one found in your medicine pack. Please compare the 'Leaflet prepared/revised date' towards the end
Due to regulatory changes, the content of the following Patient Information Leaflet may vary from the one found in your medicine pack. Please compare the 'Leaflet prepared/revised date' towards the end
Package Leaflet: Information for the user. FOSAVANCE 70 mg/2,800 IU tablets FOSAVANCE 70 mg/5,600 IU tablets Alendronic acid /colecalciferol
 Package Leaflet: Information for the user FOSAVANCE 70 mg/2,800 IU tablets FOSAVANCE 70 mg/5,600 IU tablets Alendronic acid /colecalciferol Read all of this leaflet carefully before you start taking this
Package Leaflet: Information for the user FOSAVANCE 70 mg/2,800 IU tablets FOSAVANCE 70 mg/5,600 IU tablets Alendronic acid /colecalciferol Read all of this leaflet carefully before you start taking this
PACKAGE LEAFLET: INFORMATION FOR THE USER. Zoledronic Acid 5mg/100 ml solution for infusion Zoledronic acid
 PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid 5mg/100 ml solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given this medicine, because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid 5mg/100 ml solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given this medicine, because it contains important
Package leaflet: Information for the patient. Alendronic Acid 70 mg Tablets. sodium alendronate
 Package leaflet: Information for the patient Alendronic Acid 70 mg Tablets sodium alendronate Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient Alendronic Acid 70 mg Tablets sodium alendronate Read all of this leaflet carefully before you start taking this medicine because it contains important information
PACKAGE LEAFLET: INFORMATION FOR THE USER. Risedronat Jubilant 30 mg film-coated tablets risedronate sodium
 PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 30 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
PACKAGE LEAFLET: INFORMATION FOR THE USER Risedronat Jubilant 30 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
Medication Guide ACTONEL (AK-toh-nel) (risedronate sodium) Tablets
 Medication Guide ACTONEL (AK-toh-nel) (risedronate sodium) Tablets Read the Medication Guide that comes with ACTONEL before you start taking it and each time you get a refill. There may be new information.
Medication Guide ACTONEL (AK-toh-nel) (risedronate sodium) Tablets Read the Medication Guide that comes with ACTONEL before you start taking it and each time you get a refill. There may be new information.
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT. Alendronat ratiopharm Veckotablett 70 mg tablets. Alendronic acid
 PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Alendronat ratiopharm Veckotablett 70 mg tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT Alendronat ratiopharm Veckotablett 70 mg tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the patient. Optinate 5 mg film-coated tablets risedronate sodium
 Package leaflet: Information for the patient Optinate 5 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
Package leaflet: Information for the patient Optinate 5 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
FOSAMAX Once Weekly Alendronate sodium
 Alendronate sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It is particularly important that you read the sections "When to take it" and
Alendronate sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It is particularly important that you read the sections "When to take it" and
PACKAGE LEAFLET: INFORMATION FOR THE USER. Zoledronic Acid IBIGEN 4 mg powder and solvent for solution for infusion Zoledronic acid
 PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid IBIGEN 4 mg powder and solvent for solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given Zoledronic Acid
PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid IBIGEN 4 mg powder and solvent for solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given Zoledronic Acid
1231 Zoledronic acid in early breast cancer Page 1 of 5
 Zoledronic acid in early breast cancer Zoledronic acid in early breast cancer Zoledronic acid is a bisphosphonate. This is treatment which works by slowing down the rate of bone change. In the bone there
Zoledronic acid in early breast cancer Zoledronic acid in early breast cancer Zoledronic acid is a bisphosphonate. This is treatment which works by slowing down the rate of bone change. In the bone there
Package leaflet: information for the user. Zoledronic Acid 5 mg / 100 ml solution for infusion. Zoledronic acid
 Package leaflet: information for the user Zoledronic Acid 5 mg / 100 ml solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet: information for the user Zoledronic Acid 5 mg / 100 ml solution for infusion Zoledronic acid Read all of this leaflet carefully before you are given this medicine because it contains important
Elements for a Public Summary
 VI.2 Elements for a Public Summary 25 microgram tablets 50 microgram tablets 75 microgram tablets 100 microgram tablets 125 microgram
VI.2 Elements for a Public Summary 25 microgram tablets 50 microgram tablets 75 microgram tablets 100 microgram tablets 125 microgram
It is used to prevent or treat soft, brittle bones (osteoporosis). It may be g iven to you for other reasons. Talk with the doctor.
 PATIENT & CAREGIVER EDUCATION Alendronate Brand Names: US Binosto; Fosamax Brand Names: Canada Fosamax What is this drug used for? It is used to prevent or treat soft, brittle bones (osteoporosis). It
PATIENT & CAREGIVER EDUCATION Alendronate Brand Names: US Binosto; Fosamax Brand Names: Canada Fosamax What is this drug used for? It is used to prevent or treat soft, brittle bones (osteoporosis). It
Package leaflet: Information for the user. Risedronate Bluefish 35 mg film-coated tablets Risedronate sodium
 Package leaflet: Information for the user Risedronate Bluefish 35 mg film-coated tablets Risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the user Risedronate Bluefish 35 mg film-coated tablets Risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the patient. FOSAMAX Once Weekly 70 mg Tablets Alendronic acid
 Package leaflet: Information for the patient FOSAMAX Once Weekly 70 mg Tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient FOSAMAX Once Weekly 70 mg Tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains important information
Understanding Bone. Osteoporosis
 Apo-Alendronate Once Tablets contains the active ingredient, Alendronate sodium For a copy of a large print leaflet, ph: 1800 195 055 Consumer Medicine Information What is in this leaflet This leaflet
Apo-Alendronate Once Tablets contains the active ingredient, Alendronate sodium For a copy of a large print leaflet, ph: 1800 195 055 Consumer Medicine Information What is in this leaflet This leaflet
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
PACKAGE LEAFLET: INFORMATION FOR THE USER. Raloxifen STADA 60 mg film-coated tablets (raloxifene hydrochloride)
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Raloxifen STADA 60 mg film-coated tablets (raloxifene hydrochloride) Please read this information carefully before you start to take your medicine.
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Raloxifen STADA 60 mg film-coated tablets (raloxifene hydrochloride) Please read this information carefully before you start to take your medicine.
FOSAMAX(R) Once Weekly
 FOSAMAX(R) Once Alendronate sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about FOSAMAX Once. It is particularly important that you read the sections
FOSAMAX(R) Once Alendronate sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about FOSAMAX Once. It is particularly important that you read the sections
FOSAMAX Alendronate sodium
 FOSAMAX Alendronate sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about FOSAMAX. It is particularly important that you read the sections "When
FOSAMAX Alendronate sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about FOSAMAX. It is particularly important that you read the sections "When
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis. Date: Date:
 Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
PART III: CONSUMER INFORMATION Pr Zoledronic Acid A (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when Zoledronic Acid A was
Package leaflet: Information for the patient. Optinate 30 mg film-coated tablets risedronate sodium
 Package leaflet: Information for the patient Optinate 30 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
Package leaflet: Information for the patient Optinate 30 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains important
PART III: CONSUMER INFORMATION
 PART III: CONSUMER INFORMATION Pr ACLASTA (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when ACLASTA was approved for sale
PART III: CONSUMER INFORMATION Pr ACLASTA (zoledronic acid injection) for intravenous infusion This leaflet is part III of a three-part "Product Monograph" published when ACLASTA was approved for sale
Osteoporosis Agents Drug Class Prior Authorization Protocol
 Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
Osteoporosis Agents Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review of
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
MAN SHORTER CAN MAKE A HOW OSTEOPOROSIS. Ask your doctor if you have osteoporosis and if Prolia may be right for you. AND WHAT YOU CAN DO NEXT
 Ask your doctor if you have osteoporosis and if Prolia may be right for you. Prolia is a prescription medicine used to increase bone mass in men with osteoporosis who: are at high risk for fracture, meaning
Ask your doctor if you have osteoporosis and if Prolia may be right for you. Prolia is a prescription medicine used to increase bone mass in men with osteoporosis who: are at high risk for fracture, meaning
Actonel risedronate sodium
 risedronate sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain all the available information. It does not take the place
risedronate sodium Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain all the available information. It does not take the place
What is in this leaflet
 Contains the active ingredient risedronate sodium (as hemipentahydrate) Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet Read this leaflet carefully
Contains the active ingredient risedronate sodium (as hemipentahydrate) Consumer Medicine Information For a copy of a large print leaflet, Ph: 1800 195 055 What is in this leaflet Read this leaflet carefully
Package leaflet: Information for the patient. Optinate Septimum 35 mg film-coated tablets risedronate sodium
 Package leaflet: Information for the patient Optinate Septimum 35 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the patient Optinate Septimum 35 mg film-coated tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION FOSAVANCE 70/2800 (alendronate sodium/cholecalciferol) Once Weekly Tablets 70 mg alendronate + 70 mcg cholecalciferol (2800 IU vitamin D 3 ) FOSAVANCE 70/5600 (alendronate
PART III: CONSUMER INFORMATION FOSAVANCE 70/2800 (alendronate sodium/cholecalciferol) Once Weekly Tablets 70 mg alendronate + 70 mcg cholecalciferol (2800 IU vitamin D 3 ) FOSAVANCE 70/5600 (alendronate
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Lung cancer is one of the most common types of cancer in European men and women. There are two main types of lung cancer: small
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Lung cancer is one of the most common types of cancer in European men and women. There are two main types of lung cancer: small
AACE/ACE Osteoporosis Treatment Decision Tool
 AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
AACE/ACE Osteoporosis Treatment Decision Tool What is Osteoporosis? OSTEOPOROSIS is defined as reduced bone strength leading to an increased risk of fracture. Osteoporosis, or porous bones, occurs when
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Actavis is a cancer medicine used to treat two types of lung cancer, malignant pleural mesothelioma and advanced non-small-cell
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Actavis is a cancer medicine used to treat two types of lung cancer, malignant pleural mesothelioma and advanced non-small-cell
Package leaflet: Information for the patient. Optinate Septimum 35 mg gastro-resistant tablets. risedronate sodium
 Package leaflet: Information for the patient Optinate Septimum 35 mg gastro-resistant tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
Package leaflet: Information for the patient Optinate Septimum 35 mg gastro-resistant tablets risedronate sodium Read all of this leaflet carefully before you start taking this medicine because it contains
PACKAGE LEAFLET: INFORMATION FOR THE USER. Zoledronic Acid 4 mg/5 ml concentrate for solution for infusion. Zoledronic acid
 PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid 4 mg/5 ml concentrate for solution for infusion Zoledronic acid Read all of this leaflet carefully before you start using this medicine because
PACKAGE LEAFLET: INFORMATION FOR THE USER Zoledronic Acid 4 mg/5 ml concentrate for solution for infusion Zoledronic acid Read all of this leaflet carefully before you start using this medicine because
Management of complications and side-effects of myeloma. Jackie Quinn Myeloma CNS Belfast Trust
 Management of complications and side-effects of myeloma Jackie Quinn Myeloma CNS Belfast Trust Common problems in myeloma Myeloma-related complications/symptoms Treatment-related side-effects Myeloma bone
Management of complications and side-effects of myeloma Jackie Quinn Myeloma CNS Belfast Trust Common problems in myeloma Myeloma-related complications/symptoms Treatment-related side-effects Myeloma bone
ALENDRONATE PLUS D3 SANDOZ Contains the active ingredient alendronate sodium and colecalciferol
 Contains the active ingredient alendronate sodium and colecalciferol Consumer Medicine Information What is in this leaflet Read this leaflet carefully before taking your medicine. This leaflet answers
Contains the active ingredient alendronate sodium and colecalciferol Consumer Medicine Information What is in this leaflet Read this leaflet carefully before taking your medicine. This leaflet answers
Rheumatology Department Patient Information Leaflet. Introduction Prolia (denosumab) is a treatment for post-menopausal osteoporosis.
 Prolia (denosumab) Rheumatology Department Patient Information Leaflet Introduction Prolia (denosumab) is a treatment for post-menopausal osteoporosis. What is osteoporosis? Osteoporosis is a condition
Prolia (denosumab) Rheumatology Department Patient Information Leaflet Introduction Prolia (denosumab) is a treatment for post-menopausal osteoporosis. What is osteoporosis? Osteoporosis is a condition
Osteoporosis. Definition
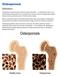 Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Osteoporosis Definition Osteoporosis causes bones to become weak and brittle so brittle that a fall or even mild stresses like bending over or coughing can cause a fracture. Osteoporosis-related fractures
Chemmart Alendronate Plus D3 Tablets Contains the active ingredient alendronate acid (as alendronate sodium) and colecalciferol (vitamin D3)
 Chemmart Alendronate Plus D3 Tablets Contains the active ingredient alendronate acid (as alendronate sodium) and colecalciferol (vitamin D3) Consumer Medicine Information For a copy of a large print leaflet,
Chemmart Alendronate Plus D3 Tablets Contains the active ingredient alendronate acid (as alendronate sodium) and colecalciferol (vitamin D3) Consumer Medicine Information For a copy of a large print leaflet,
Prolia 2 shots a year proven to help strengthen bones.
 Ask your doctor if Prolia (denosumab) is right for you and visit us at www.prolia.com For women with postmenopausal osteoporosis at high risk for fracture: there s Prolia. Prolia 2 shots a year proven
Ask your doctor if Prolia (denosumab) is right for you and visit us at www.prolia.com For women with postmenopausal osteoporosis at high risk for fracture: there s Prolia. Prolia 2 shots a year proven
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss. A Publication of The Bone and Cancer Foundation
 Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
Questions and Answers About Breast Cancer, Bone Metastases, & Treatment-Related Bone Loss A Publication of The Bone and Cancer Foundation Contents This publication includes important information about
Package leaflet: Information for the patient. FOSAMAX Once Weekly 70 mg Tablets Alendronic acid
 Package leaflet: Information for the patient FOSAMAX Once Weekly 70 mg Tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient FOSAMAX Once Weekly 70 mg Tablets Alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains important information
Prolia 2 shots a year proven to help strengthen bones. next shot appointment
 Ask your doctor if Prolia (denosumab) is right for you and visit us at www.prolia.com next shot appointment For women with postmenopausal osteoporosis at high risk for fracture: there s Prolia. Prolia
Ask your doctor if Prolia (denosumab) is right for you and visit us at www.prolia.com next shot appointment For women with postmenopausal osteoporosis at high risk for fracture: there s Prolia. Prolia
Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures
 APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
Package leaflet: Information for the user. Alendroarrow Veckotablett 70mg tablets. alendronic acid
 Package leaflet: Information for the user Alendroarrow Veckotablett 70mg tablets alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the user Alendroarrow Veckotablett 70mg tablets alendronic acid Read all of this leaflet carefully before you start taking this medicine because it contains important information
Osteoporosis. Skeletal System
 Osteoporosis Introduction Osteoporosis is a very common bone disease that causes bone to become weak. Bone weakness can lead to fractures of the spine, hip, and wrist from simple falls or even a sneeze
Osteoporosis Introduction Osteoporosis is a very common bone disease that causes bone to become weak. Bone weakness can lead to fractures of the spine, hip, and wrist from simple falls or even a sneeze
(sunitinib malate) for Kidney Cancer
 Sutent (sunitinib malate) for Kidney Cancer Sutent is a medication used to treat adult patients with kidney cancer that has been surgically removed and at high risk of recurrence, or advanced kidney cancer
Sutent (sunitinib malate) for Kidney Cancer Sutent is a medication used to treat adult patients with kidney cancer that has been surgically removed and at high risk of recurrence, or advanced kidney cancer
It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
 Actonel Combi D risedronate sodium, calcium carbonate, cholecalciferol (Vitamin D3) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Actonel Combi
Actonel Combi D risedronate sodium, calcium carbonate, cholecalciferol (Vitamin D3) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Actonel Combi
Prolia 2 shots a year proven to help strengthen bones.
 For women with postmenopausal osteoporosis at high risk for fracture: there s Prolia. Prolia 2 shots a year proven to help strengthen bones. Blythe Danner, Award winning actress taking Prolia. Prolia is
For women with postmenopausal osteoporosis at high risk for fracture: there s Prolia. Prolia 2 shots a year proven to help strengthen bones. Blythe Danner, Award winning actress taking Prolia. Prolia is
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP
 Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Clodronate BE/H/PSUR/001/001 October 2011 Agreed CSP 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Intravenous use Treatment of hypercalcemia due to malignancy. Oral use Treatment of hypercalcemia
Osteoporosis challenges
 Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
RABEPRAZOL 10mg and 20mg Gastro-resistant Tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER RABEPRAZOL 10mg and 20mg Gastro-resistant Tablets RABEPRAZOLE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet
PACKAGE LEAFLET: INFORMATION FOR THE USER RABEPRAZOL 10mg and 20mg Gastro-resistant Tablets RABEPRAZOLE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet
Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene)
 EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
EMA/679870/2014 Summary of the risk management plan (RMP) for Duavive (conjugated oestrogens / bazedoxifene) This is a summary of the risk management plan (RMP) for Duavive, which details the measures
Package leaflet: information for the user. Tevanate Once Weekly 70 mg Tablets Alendronic acid
 Package leaflet: information for the user Tevanate Once Weekly 70 mg Tablets Alendronic acid Read all of this leaflet carefully before you start using this medicine because it contains important information
Package leaflet: information for the user Tevanate Once Weekly 70 mg Tablets Alendronic acid Read all of this leaflet carefully before you start using this medicine because it contains important information
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: , VERSION 1.
 RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
RISK MANAGEMENT PLAN (RMP) PUBLIC SUMMARY ETORICOXIB ORION (ETORICOXIB) 30 MG, 60 MG, 90 MG & 120 MG FILM-COATED TABLET DATE: 07-10-2016, VERSION 1.2 VI.2 Elements for a Public Summary Etoricoxib Orion
Common Technical Document Ibandronate 150 mg (as sodium monohydrate) Film-coated tablets. Package leaflet: Information for the user.
 1.3.1 Core Patient Information Leaflet Labelling and PIL page 1/10 Package leaflet: Information for the user Ibandroninezuur Polpharma 150 mg, filmomhulde tabletten Ibandronic acid Read all of this leaflet
1.3.1 Core Patient Information Leaflet Labelling and PIL page 1/10 Package leaflet: Information for the user Ibandroninezuur Polpharma 150 mg, filmomhulde tabletten Ibandronic acid Read all of this leaflet
Package leaflet: Information for the patient
 Package leaflet: Information for the patient Norsed Combi D 35 mg + 1000 mg / 880 IU film-coated tablets + effervescent granules Risedronate sodium + calcium / colecalciferol Read all of this leaflet carefully
Package leaflet: Information for the patient Norsed Combi D 35 mg + 1000 mg / 880 IU film-coated tablets + effervescent granules Risedronate sodium + calcium / colecalciferol Read all of this leaflet carefully
The Osteoporosis Center at St. Luke s Hospital
 The Osteoporosis Center at St. Luke s Hospital Desloge Outpatient Center (on the west side of 141) 121 St. Luke s Center Drive, Suite 504 Chesterfield, MO 63017 Phone 314 205-6633 Fax 314 590-5909 NEW
The Osteoporosis Center at St. Luke s Hospital Desloge Outpatient Center (on the west side of 141) 121 St. Luke s Center Drive, Suite 504 Chesterfield, MO 63017 Phone 314 205-6633 Fax 314 590-5909 NEW
FOSAMAX (Alendronic acid as alendronate sodium trihydrate)
 CSP - UK/H/PSUR/0070 - March 2012 EUROPEAN UNION CORE SAFETY PROFILE FOSAMAX (Alendronic acid as alendronate sodium trihydrate) 4.2 Posology and method of administration The recommended dosage is one 70
CSP - UK/H/PSUR/0070 - March 2012 EUROPEAN UNION CORE SAFETY PROFILE FOSAMAX (Alendronic acid as alendronate sodium trihydrate) 4.2 Posology and method of administration The recommended dosage is one 70
FOSAMAX PLUS(TM) Once Weekly
 (TM) Once Weekly Alendronate sodium/colecalciferol Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It is particularly important that you read the
(TM) Once Weekly Alendronate sodium/colecalciferol Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It is particularly important that you read the
VI.2 Elements for a Public Summary. VI.2.1 Overview of disease epidemiology
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Febuxostat is a medicine used in adults with gout to reduce high levels of uric acid in the blood. Gout results from a build up
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Febuxostat is a medicine used in adults with gout to reduce high levels of uric acid in the blood. Gout results from a build up
Understanding NICE guidance. NICE technology appraisal guidance advises on when and how drugs and other treatments should be used in the NHS.
 Understanding NICE guidance Information for people who use NHS services Alendronate, etidronate, risedronate, strontium ranelate and raloxifene for preventing bone fractures in postmenopausal women with
Understanding NICE guidance Information for people who use NHS services Alendronate, etidronate, risedronate, strontium ranelate and raloxifene for preventing bone fractures in postmenopausal women with
EU RISK MANAGEMENT PLAN (EU RMP)
 EU RISK MANAGEMENT PLAN (EU RMP) Active substance(s) (INN or common name): Esomeprazole Pharmaco-therapeutic group (ATC Code): A02B C05 Name of Marketing Authorisation Holder or Applicant: Strength and
EU RISK MANAGEMENT PLAN (EU RMP) Active substance(s) (INN or common name): Esomeprazole Pharmaco-therapeutic group (ATC Code): A02B C05 Name of Marketing Authorisation Holder or Applicant: Strength and
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 21 July 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 21 July 2010 Review of the dossier of the medicinal product included on the list of reimbursable medicines for a period
Elements for a Public Summary
 VI.2 Elements for a Public Summary / / 10 mg/10 mg tablets / / 10 mg/20 mg tablets / / 10 mg/40 mg tablets / / 10 mg/80 mg tablets VI.2.1 Overview of disease epidemiology Prevention of cardiovascular events
VI.2 Elements for a Public Summary / / 10 mg/10 mg tablets / / 10 mg/20 mg tablets / / 10 mg/40 mg tablets / / 10 mg/80 mg tablets VI.2.1 Overview of disease epidemiology Prevention of cardiovascular events
Aredia. For more info on Aredia, please see this link from MedlinePlus:
 Aredia Mr. Reeve, in his appearance on the Larry King Show on September 23, 2002 (rebroadcast 2/23/03 and several more times since) said the following: The way you prevent osteoporosis is by bearing weight,
Aredia Mr. Reeve, in his appearance on the Larry King Show on September 23, 2002 (rebroadcast 2/23/03 and several more times since) said the following: The way you prevent osteoporosis is by bearing weight,
Adjuvant bisphosphonate
 Adjuvant bisphosphonate Information for patients from the Kent Oncology Centre This leaflet is intended to support you in making decisions about the role of bisphosphonates in your breast cancer care.
Adjuvant bisphosphonate Information for patients from the Kent Oncology Centre This leaflet is intended to support you in making decisions about the role of bisphosphonates in your breast cancer care.
Management of the complications of myeloma and side-effects of treatment Christine Morris Clinical Nurse Specialist in Myeloma Royal Derby Hospital
 Management of the complications of myeloma and side-effects of treatment Christine Morris Clinical Nurse Specialist in Myeloma Royal Derby Hospital Common problems in myeloma Myeloma-related complications/symptoms
Management of the complications of myeloma and side-effects of treatment Christine Morris Clinical Nurse Specialist in Myeloma Royal Derby Hospital Common problems in myeloma Myeloma-related complications/symptoms
Risedronate Winthrop EC Combi D risedronate sodium (enteric-coated), calcium carbonate, cholecalciferol (Vitamin D3)
 risedronate sodium (enteric-coated), calcium carbonate, cholecalciferol (Vitamin D3) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Risedronate Winthrop
risedronate sodium (enteric-coated), calcium carbonate, cholecalciferol (Vitamin D3) Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Risedronate Winthrop
Teriparatide (also known as Forsteo) Rheumatology Department Patient Information Leaflet
 Teriparatide (also known as Forsteo) Rheumatology Department Patient Information Leaflet Introduction This information leaflet is about a medicine called teriparatide (also known as Forsteo) which is used
Teriparatide (also known as Forsteo) Rheumatology Department Patient Information Leaflet Introduction This information leaflet is about a medicine called teriparatide (also known as Forsteo) which is used
Understanding bone metastases and XGEVA
 Understanding bone metastases and XGEVA Contents About bone metastases 3 About XGEVA 5 Dealing with common side effects of XGEVA 8 FAs about living with bone metastases 9 Notes 10 About bone metastases
Understanding bone metastases and XGEVA Contents About bone metastases 3 About XGEVA 5 Dealing with common side effects of XGEVA 8 FAs about living with bone metastases 9 Notes 10 About bone metastases
Osteoporosis. Overview
 v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
v2 Osteoporosis Overview Osteoporosis is defined as compromised bone strength that increases risk of fracture (NIH Consensus Conference, 2000). Bone strength is characterized by bone mineral density (BMD)
DENOSUMAB SHARED CARE GUIDLINES
 DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
Summary of the risk management plan (RMP) for Pemetrexed Actavis (pemetrexed)
 EMA/58503/2016 Summary of the risk management plan (RMP) for Pemetrexed Actavis (pemetrexed) This is a summary of the risk management plan (RMP) for Pemetrexed Actavis, which details the measures to be
EMA/58503/2016 Summary of the risk management plan (RMP) for Pemetrexed Actavis (pemetrexed) This is a summary of the risk management plan (RMP) for Pemetrexed Actavis, which details the measures to be
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures
 Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
Administration of Denosumab (PROLIA ) for the treatment of osteoporosis in postmenopausal women and in men at increased risk of fractures 2017 18 1. Purpose of Agreement This agreement outlines the expectations
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Alendronat Orifarm Veckotablett 70 mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains alendronate sodium trihydrate
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Alendronat Orifarm Veckotablett 70 mg tablets 2 QUALITATIVE AND QUANTITATIVE COMPOSITION One tablet contains alendronate sodium trihydrate
BONIVA (ibandronate sodium)
 BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
BONIVA (ibandronate sodium) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices
Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION should be recommended
 Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION Denosumab is suitable for patients with established osteoporosis for both primary and secondary fracture prevention
Guidance for the administration of Denosumab (Prolia ) in Primary Care GENERAL INFORMATION Denosumab is suitable for patients with established osteoporosis for both primary and secondary fracture prevention
