Processed nerve allografts to repair peripheral nerve discontinuities
|
|
|
- Aubrie Cummings
- 5 years ago
- Views:
Transcription
1 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Processed nerve allografts to repair peripheral nerve discontinuities Accidents or major surgery can damage nerves, causing pain, reduced sensation and lack of movement. If the ends of the damaged nerve are too far apart to be stitched together, the gap (discontinuity) needs bridging. In this procedure, a specially treated nerve (an allograft) taken from a human donor after death is used to bridge the gap. The aim is to restore function of the damaged nerve. The National Institute for Health and Care Excellence (NICE) is examining processed nerve allografts to repair peripheral nerve discontinuities and will publish guidance on its safety and efficacy to the NHS. NICE s interventional procedures advisory committee has considered the available evidence and the views of specialist advisers, who are consultants with knowledge of the procedure. The advisory committee has made draft recommendations about processed nerve allografts to repair peripheral nerve discontinuities. This document summarises the procedure and sets out the draft recommendations made by the advisory committee. It has been prepared for public consultation. The advisory committee particularly welcomes: comments on the draft recommendations the identification of factual inaccuracies additional relevant evidence, with bibliographic references where possible. Note that this document is not NICE s formal guidance on this procedure. The recommendations are provisional and may change after consultation. The process that NICE will follow after the consultation period ends is as follows. The advisory committee will meet again to consider the original evidence and its draft recommendations in the light of the comments received during consultation. Page 1 of 10
2 The advisory committee will then prepare draft guidance which will be the basis for NICE s guidance on the use of the procedure in the NHS. For further details, see the Interventional Procedures Programme process guide, which is available from the NICE website. Through its guidance NICE is committed to promoting race and disability equality, equality between men and women, and to eliminating all forms of discrimination. One of the ways we do this is by trying to involve as wide a range of people and interest groups as possible in the development of our interventional procedures guidance. In particular, we aim to encourage people and organisations from groups who might not normally comment on our guidance to do so. In order to help us promote equality through our guidance, we should be grateful if you would consider the following question: Are there any issues that require special attention in light of NICE s duties to have due regard to the need to eliminate unlawful discrimination, advance equality of opportunity, and foster good relations between people with a characteristic protected by the equalities legislation and others? Please note that NICE reserves the right to summarise and edit comments received during consultations or not to publish them at all where in the reasonable opinion of NICE, the comments are voluminous, publication would be unlawful or publication would otherwise be inappropriate. Closing date for comments: 20 July Target date for publication of guidance: 8 November Draft recommendations 1.1 Current evidence on the safety and efficacy of processed nerve allografts to repair peripheral nerve discontinuities is adequate to support the use of this procedure for digital nerves provided that standard arrangements are in place for clinical governance, consent and audit. 1.2 The evidence on the safety of processed nerve allografts to repair peripheral nerve discontinuities in other sites raises no major safety concerns. However, current evidence on its efficacy in these sites Page 2 of 10
3 is limited in quantity. Therefore, for indications other than digital nerve repair, this procedure should only be used with special arrangements for clinical governance, consent and audit or research. 1.3 Clinicians wishing to do processed nerve allografts to repair peripheral nerve discontinuities in sites other than the digital nerves should: Inform the clinical governance leads in their NHS trusts. Ensure that patients understand the uncertainty about the procedure s efficacy on mixed nerve repair and provide them with clear written information. In addition, the use of NICE s information for the public is recommended. Audit and review clinical outcomes of all patients having processed nerve allografts to repair peripheral nerve discontinuities (see section 7.1). 1.4 This procedure should only be done by surgeons with training and experience in peripheral nerve repair. 1.5 Patient selection should take into consideration the site, type of nerve (motor, sensory, mixed) and the size of the defect. 1.6 NICE encourages further research into processed nerve allografts to repair peripheral nerve discontinuities and this should include information on the type of nerve repaired, the anatomical site, the size of the defect, patient reported outcome measures, functional outcomes, time to recovery and long-term outcomes (12 18 months). Page 3 of 10
4 2 Indications and current treatments 2.1 Peripheral nerve damage can be caused by trauma or surgery, and can lead to reduced sensation and mobility of the affected limb or region. If direct repair is not possible because the section of nerve discontinuity is too long, grafts or artificial nerve conduits can be used. 2.2 Autologous nerve grafting (using another nerve from the same patient) is used most frequently (usually using the sural nerve from the leg). However, this can be associated with donor site morbidity. Untreated allografts (using a nerve from a donor) have been used but, after these, immunosuppressive treatment is needed. 3 The procedure 3.1 Acellular processed nerve allografts are nerves from deceased human donors which have had their immunogenic components removed using tissue processing techniques. They are stored frozen until implantation and are available in different sizes. Immunosuppressive treatment is not needed. 3.2 The procedure is done under general anaesthesia. The injured nerve is exposed and the nerve ends are cleared of necrotic tissues and resected to allow for tension-free alignment with the graft. The graft is sutured to the exposed nerve ends. After grafting, limb splinting may be needed for several weeks to allow optimal nerve regeneration. The typical length of an allograft implant is 1 3 cm. Page 4 of 10
5 3.3 The aim of the procedure is to bridge the peripheral nerve discontinuity to allow axonal regeneration and growth through the allograft towards the distal nerve. 4 Efficacy This section describes efficacy outcomes from the published literature that the committee considered as part of the evidence about this procedure. For more detailed information on the evidence, see the interventional procedure overview. 4.1 In a randomised control trial (RCT) of 23 patients needing digital nerves repair comparing processed nerve allograft (PNA) with treated bovine graft), at 12-month follow-up, static 2-point discrimination assessment (s2pd, which tests the ability to discern the difference between 1 and 2 static pressure points) was statistically significantly better in the PNA group (n=5) than the bovine graft group (n=7; 5±1 mm versus 8±5 mm, p<0.05). In the same study, moving 2-point discrimination assessment (m2pd) was not statistically significantly different between the PNA group and the bovine graft group (5±1 mm versus 7±5 mm, p>0.05) at 12-month follow-up. In a non-randomised comparative study of 153 patients needing digital nerve repair comparing PNA repair (n=72) with tension-free suture nerve repair (n=81), s2pd scores (excellent plus good, defined as the ability to distinguish between 2 static pressure points at a maximum distance of 15 mm) were not statistically significantly different between the PNA group (67% [48/72]) and the tension-free suture group (64% [52/81]) at 6-month follow-up (p=0.749). In a case series of 17 patients with digital nerve injuries treated by PNA grafting, s2pd was excellent or good in 78% (14/18) of digits repaired, at a mean follow-up of 15 months. Page 5 of 10
6 In the RCT of 23 patients, Semmes-Weinstein monofilament test (testing of pressure threshold using a monofilament; range: 2.833=normal sensation to 6.650=residual sensation) was statistically significantly better in the PNA group than the treated bovine graft group (3.6±0.7 versus 4.4±1.4, p<0.05) at 12-month follow-up. In the same study, thermal sensation was totally improved from baseline at 12-month follow-up and not statistically significantly different between the treatment (PNA group: from 7% [1/14] to 100% [6/6] and bovine graft group: from 33% [3/9] to 100% [7/7]). 4.2 In a case series of 64 patients needing nerve repair in the upper extremity and treated by grafting using PNA, there was meaningful recovery in 75% (48/64) of all patients. Univariate analysis showed that distal site of injuries have a statistically significantly higher likelihood of recovery than proximal upper limb sites (odds ratio [OR] 5.606, 95% confidence interval [CI] to ; p<0.05). In the same study, discontinuities smaller than 30 mm had a statistically significantly greater likelihood of meaningful repair than those greater than 50 mm (OR , 95% CI to ; p<0.05). In a case series of 26 patients with lingual nerve and inferior alveolar nerve discontinuities treated by PNA grafting, meaningful sensory recovery was assessed using a neurosensory test improvement tool (ranging from normal=best, through mild, moderate and severe to complete=worse). At 12-month follow-up, neurosensory test improvement scores were normal in 52% (12/23), mild in 9% (2/23), moderate in 26% (6/23) and severe in 13% (3/23) of patients. In the same study, neurosensory improvement was reported in 86% (12/14) of patients with Page 6 of 10
7 discontinuities 8 20 mm in length and 89% (8/9) of patients with discontinuities mm in length. 4.3 In the RCT of 23 patients, disability of the arm, shoulder and hand score (DASH: 0= no disability, 100=most severe disability) was not statistically significantly different between the PNA group (5±6.5) and the bovine graft group (8±6.3) at 12-month follow-up (p=0.318). 4.4 In a case series of 108 patients needing nerve repair, there was no sensory recovery because of graft failure in 5% (4/76) of patients at last follow-up and surgical revision was needed. 4.5 In the RCT of 23 patients, at 12-month follow-up, pain measured using a visual analogue scale (VAS, 0=no pain, 10 =extreme pain) had improved from baseline in both groups (PNA group: from 4.7±3.4 to 0.5±0.6; treated bovine graft: from 4.4±2.1 to 0.9±1.0) but there was no statistically significant difference between the groups (p=0.432). In another case series of 26 patients needing PNA after resection of neuromas of the foot and ankle, mean ordinal pain score (0=no pain to 10=worse pain) statistically significantly reduced from 7.5 points at baseline to 4.9 points at a mean 66-week follow-up (difference 2.6, range +2.0 to -8.0; p=0.016). In the same study, patient reported outcomes measurement information system scores were used to assess the impact of pain on patients behaviour and daily function (reported as T-scores with a population mean of 50 and a standard deviation of 10). Pain behaviour T-score decreased by 7.3 (range+2.0 to ) from 63.0 at baseline (percentile decrease of 24%, p<0.003). Pain interference T-score decreased by 11.3 (range +2.0 to -27.0) from 68.0 at baseline (mean percentile change of 31%, p<0.003). In a case series of 17 patients with digital nerve injury treated by Page 7 of 10
8 grafting with PNA, pain (measured using a VAS: 0=no pain, 10 =extreme pain) worsened in 1 patient (VAS score increased from 5 at baseline to 8 at 15-month follow). 4.6 In the non-randomised comparative study of 153 patients, difference in satisfaction rate was not statistically significantly different between the PNA group and the tension-free suture group (2.02%, 95% CI: to 10.87), at 6-month follow-up. 4.7 The specialist advisers listed key efficacy outcomes as reinnervation of target organs, nerve regeneration rate, clinical sensory and motor outcome scales, and patient reported outcomes. 5 Safety This section describes safety outcomes from the published literature that the committee considered as part of the evidence about this procedure. For more detailed information on the evidence, see the interventional procedure overview. 5.1 Tenolysis was needed in 3% (2/78) of patients at 6-month follow-up in a non-randomised comparative study of 153 patients needing digital nerve repair comparing processed nerve allograft (PNA) repair (n=72) with tension-free suture nerve repair (n=81). 5.2 Neuroma was reported after 1 nerve repair of 132 nerves in a case series of 108 patients needing nerve repair. 5.3 Local infection that improved after treatment (not specified) was reported in 1 patient in a case series of 15 patients treated by PNA grafting. Page 8 of 10
9 5.4 In addition to safety outcomes reported in the literature, specialist advisers are asked about anecdotal adverse events (events which they have heard about) and about theoretical adverse events (events which they think might possibly occur, even if they have never done so). For this procedure, specialist advisers listed the following anecdotal adverse events: immunological reaction or rejection, and inflammatory reaction to preservatives. They considered that the following were theoretical adverse events: immunological reaction or rejection, inflammatory reaction to preservatives and sub-optimal results because of preference in using the allograft when patients could be treated by more established interventions. 6 Committee comments 6.1 The grafts used in this procedure are regulated by the Human Tissue Authority. 6.2 The grafts can be used in a variety of anatomical sites but most published evidence reviewed by the committee came from the repair of digital nerves. 6.3 The type of nerve being repaired (motor, sensory, mixed) and the size of the defect potentially affect the outcome. 6.4 The use of this type of graft avoids the need to harvest a donor nerve from the same patient, and avoids the use of non-human derived tissue and of immunosuppression. 7 Further information 7.1 For related NICE guidance, see the NICE website. Page 9 of 10
10 7.2 Patient commentary was not sought because the procedure is only being done in research setting in the UK. 7.3 This guidance requires that clinicians doing the procedure make special arrangements for audit. NICE has identified relevant audit criteria and is developing an audit tool (which is for use at local discretion). This tool will be available when the guidance is published. Tom Clutton-Brock Chairman, interventional procedures advisory committee May 2017 Page 10 of 10
Total distal radioulnar joint replacement for symptomatic joint instability or arthritis
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Total distal radioulnar joint replacement for symptomatic joint instability or arthritis Instability of
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Total distal radioulnar joint replacement for symptomatic joint instability or arthritis Instability of
Inserting a biodegradable subacromial spacer for rotator cuff tears
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Inserting a biodegradable subacromial spacer for rotator cuff tears The rotator cuff is a group of muscles
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Inserting a biodegradable subacromial spacer for rotator cuff tears The rotator cuff is a group of muscles
Liposuction for chronic lymphoedema
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Liposuction for chronic lymphoedema Chronic lymphoedema is the swelling and build-up of body fluid and fat,
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Liposuction for chronic lymphoedema Chronic lymphoedema is the swelling and build-up of body fluid and fat,
Surgical repair of vaginal wall prolapse using mesh
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Surgical repair of vaginal wall prolapse using mesh Vaginal wall prolapse happens when the normal support
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Surgical repair of vaginal wall prolapse using mesh Vaginal wall prolapse happens when the normal support
Extracranial to intracranial bypass for intracranial atherosclerosis
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Extracranial to intracranial bypass for intracranial atherosclerosis In cerebrovascular disease, blood vessels
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Extracranial to intracranial bypass for intracranial atherosclerosis In cerebrovascular disease, blood vessels
Implantation of a sphenopalatine ganglion stimulation device for chronic cluster headache
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a sphenopalatine ganglion stimulation device for chronic cluster headache Cluster headaches
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a sphenopalatine ganglion stimulation device for chronic cluster headache Cluster headaches
Transcutaneous stimulation of the cervical branch of the vagus nerve for cluster headache and migraine
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Transcutaneous stimulation of the cervical branch of the vagus nerve for cluster headache and migraine Cluster
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Transcutaneous stimulation of the cervical branch of the vagus nerve for cluster headache and migraine Cluster
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of processed nerve allografts to repair peripheral nerve discontinuities Accidents
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of processed nerve allografts to repair peripheral nerve discontinuities Accidents
Endobronchial valve insertion to reduce lung volume in emphysema
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
Implanting a baroreceptor stimulation device for resistant hypertension
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implanting a baroreceptor stimulation device for resistant hypertension Hypertension (or high blood pressure)
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implanting a baroreceptor stimulation device for resistant hypertension Hypertension (or high blood pressure)
Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer Radiotherapy
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer Radiotherapy
Repetitive transcranial magnetic stimulation for depression
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Repetitive transcranial magnetic stimulation for depression Depression causes low mood or sadness that can
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Repetitive transcranial magnetic stimulation for depression Depression causes low mood or sadness that can
Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer Radiotherapy
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Biodegradable spacer insertion to reduce rectal toxicity during radiotherapy for prostate cancer Radiotherapy
Endoscopic carbon dioxide laser cricopharyngeal myotomy for relief of oropharyngeal dysphagia
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endoscopic carbon dioxide laser cricopharyngeal myotomy for relief of oropharyngeal dysphagia Difficulty
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endoscopic carbon dioxide laser cricopharyngeal myotomy for relief of oropharyngeal dysphagia Difficulty
Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Ultrasound-enhanced, catheter-directed thrombolysis for pulmonary embolism A pulmonary embolism (PE) is
Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Subcutaneous implantable cardioverter defibrillator insertion for preventing sudden cardiac death A subcutaneous
Insertion of an epiretinal prosthesis for retinitis pigmentosa
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Insertion of an epiretinal prosthesis for retinitis pigmentosa Retinitis pigmentosa is a disease that affects
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Insertion of an epiretinal prosthesis for retinitis pigmentosa Retinitis pigmentosa is a disease that affects
Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes The cornea
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes The cornea
Interventional procedures guidance Published: 28 June 2017 nice.org.uk/guidance/ipg583
 Sacrocolpopexy using mesh to repair vaginal vault prolapse Interventional procedures guidance Published: 28 June 2017 nice.org.uk/guidance/ipg583 Your responsibility This guidance represents the view of
Sacrocolpopexy using mesh to repair vaginal vault prolapse Interventional procedures guidance Published: 28 June 2017 nice.org.uk/guidance/ipg583 Your responsibility This guidance represents the view of
Transcatheter aortic valve implantation for aortic stenosis
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Transcatheter aortic valve implantation for aortic stenosis Aortic stenosis occurs when the aortic valve
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Transcatheter aortic valve implantation for aortic stenosis Aortic stenosis occurs when the aortic valve
Extracorporeal shockwave therapy for refractory Achilles tendinopathy
 Extracorporeal shockwave therapy for refractory Achilles Issued: August 2009 www.nice.org.uk/ipg312 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce
Extracorporeal shockwave therapy for refractory Achilles Issued: August 2009 www.nice.org.uk/ipg312 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce
Infracoccygeal sacropexy using mesh for uterine prolapse repair
 Infracoccygeal sacropexy using mesh for uterine Issued: January 2009 www.nice.org.uk/ipg280 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Infracoccygeal sacropexy using mesh for uterine Issued: January 2009 www.nice.org.uk/ipg280 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600
 Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566
 Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
Single-incision short sling mesh insertion for stress urinary incontinence in women Interventional procedures guidance Published: 12 October 2016 nice.org.uk/guidance/ipg566 Your responsibility This guidance
Peripheral Nerve Reconstruction
 Reconstruction Following Nerve Injury, Nerve Grafts & Nerve Transfers Peripheral Nerve Reconstruction Surgical approach to nerve repair is dependent upon type of injury, gap length, nerve type, and surgeon
Reconstruction Following Nerve Injury, Nerve Grafts & Nerve Transfers Peripheral Nerve Reconstruction Surgical approach to nerve repair is dependent upon type of injury, gap length, nerve type, and surgeon
A70.4 Insertion of neurostimulator electrodes into peripheral nerve Z12.2 Posterior tibial nerve R15.X Faecal incontinence
 The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
The National Institute for Health and Clinical Excellence (NICE) has issued full guidance to the NHS in England, Wales, Scotland and Northern Ireland on Percutaneous tibial nerve stimulation (PTNS) for
Prosthetic intervertebral disc replacement in the cervical spine: Cost-effectiveness compared with cervical discectomy with or without vertebral
 Prosthetic intervertebral disc replacement in the cervical spine: Cost-effectiveness compared with cervical discectomy with or without vertebral fusion Author: William Horsley NHS North East Treatment
Prosthetic intervertebral disc replacement in the cervical spine: Cost-effectiveness compared with cervical discectomy with or without vertebral fusion Author: William Horsley NHS North East Treatment
Translaryngeal tracheostomy
 Translaryngeal tracheostomy Issued: August 2013 NICE interventional procedure guidance 462 guidance.nice.org.uk/ipg462 NICE has accredited the process used by the NICE Interventional Procedures Programme
Translaryngeal tracheostomy Issued: August 2013 NICE interventional procedure guidance 462 guidance.nice.org.uk/ipg462 NICE has accredited the process used by the NICE Interventional Procedures Programme
Nerve Autografts, Allografts, Conduits, Wraps, and Glue. What Should I Do?
 Nerve Autografts, Allografts, Conduits, Wraps, and Glue. What Should I Do? David Kahan, MD Fellow, Hand & Upper Extremity Surgery Rothman Institute at Thomas Jefferson University Outline Wallerian Degeneration
Nerve Autografts, Allografts, Conduits, Wraps, and Glue. What Should I Do? David Kahan, MD Fellow, Hand & Upper Extremity Surgery Rothman Institute at Thomas Jefferson University Outline Wallerian Degeneration
Haemorrhoidal artery ligation
 Haemorrhoidal artery ligation Issued: May 2010 NICE interventional procedure guidance 342 www.nice.org.uk/ipg342 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme
Haemorrhoidal artery ligation Issued: May 2010 NICE interventional procedure guidance 342 www.nice.org.uk/ipg342 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme
Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41
 Senza spinal cord stimulation system for delivering ering HF10 therapy to treat chronic neuropathic pain Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41 NICE 2019. All
Senza spinal cord stimulation system for delivering ering HF10 therapy to treat chronic neuropathic pain Medical technologies guidance Published: 23 January 2019 nice.org.uk/guidance/mtg41 NICE 2019. All
Low back pain and sciatica in over 16s NICE quality standard
 March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Single Technology Appraisal (STA) Ruxolitinib for the treatment of myelofibrosis
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Al Hess MD NERVE REPAIR
 Al Hess MD NERVE REPAIR Historical Aspects 300 BC Hippocrates, description of nervous system 200 AD Galen of Pergamon, nerve injury, questioned possibility of regeneration 600 AD Paul of Arginia, first
Al Hess MD NERVE REPAIR Historical Aspects 300 BC Hippocrates, description of nervous system 200 AD Galen of Pergamon, nerve injury, questioned possibility of regeneration 600 AD Paul of Arginia, first
Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181)
 Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta459
 Collagenase clostridium histolyticum for treating Dupuytren's contracture Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta459 NICE 2017. All rights reserved. Subject to Notice
Collagenase clostridium histolyticum for treating Dupuytren's contracture Technology appraisal guidance Published: 26 July 2017 nice.org.uk/guidance/ta459 NICE 2017. All rights reserved. Subject to Notice
Surveillance report Published: 17 March 2016 nice.org.uk
 Surveillance report 2016 Ovarian Cancer (2011) NICE guideline CG122 Surveillance report Published: 17 March 2016 nice.org.uk NICE 2016. All rights reserved. Contents Surveillance decision... 3 Reason for
Surveillance report 2016 Ovarian Cancer (2011) NICE guideline CG122 Surveillance report Published: 17 March 2016 nice.org.uk NICE 2016. All rights reserved. Contents Surveillance decision... 3 Reason for
Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia
 Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Issued: September 2013 guidance.nice.org.uk/ipg466 NICE has accredited the process used
Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Issued: September 2013 guidance.nice.org.uk/ipg466 NICE has accredited the process used
Medical technologies guidance Published: 2 October 2018 nice.org.uk/guidance/mtg39
 ifuse for treating chronic sacroiliac joint pain Medical technologies guidance Published: 2 October 2018 nice.org.uk/guidance/mtg39 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
ifuse for treating chronic sacroiliac joint pain Medical technologies guidance Published: 2 October 2018 nice.org.uk/guidance/mtg39 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
10 year surveillance (2017) Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy) (2007) NICE guideline CG53
 10 year surveillance (2017) Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy) (2007) NICE guideline CG53 Stakeholder consultation comments form - proposal for no update Consultation
10 year surveillance (2017) Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy) (2007) NICE guideline CG53 Stakeholder consultation comments form - proposal for no update Consultation
National Institute for Health and Care Excellence. NICE Quality Standards Consultation Surgical Site Infection. Closing date: 5pm 17 June 2013
 National Institute for Health and Care Excellence NICE Quality Standards Consultation Surgical Site Infection Closing date: 5pm 17 June 2013 Organisation Title (e.g. Dr, Mr, Ms, Prof) Name Job title or
National Institute for Health and Care Excellence NICE Quality Standards Consultation Surgical Site Infection Closing date: 5pm 17 June 2013 Organisation Title (e.g. Dr, Mr, Ms, Prof) Name Job title or
DR SHRENIK M SHAH SHREY HOSPITAL AHMEDABAD
 DR SHRENIK M SHAH SHREY HOSPITAL AHMEDABAD Surgical anatomy Physiology of healing Classification Pre-operative evaluation OVERVIEW Ultrastructure of the nerve Fragile handle with care Damaged by pressure,
DR SHRENIK M SHAH SHREY HOSPITAL AHMEDABAD Surgical anatomy Physiology of healing Classification Pre-operative evaluation OVERVIEW Ultrastructure of the nerve Fragile handle with care Damaged by pressure,
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to give us a statement on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Interventional procedures guidance Published: 25 September 2013 nice.org.uk/guidance/ipg466
 Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Interventional procedures guidance Published: 25 September 2013 nice.org.uk/guidance/ipg466
Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Interventional procedures guidance Published: 25 September 2013 nice.org.uk/guidance/ipg466
Uterine artery embolisation for treating adenomyosis
 Uterine artery embolisation for treating Issued: December 2013 guidance.nice.org.uk/ipg NICE has accredited the process used by the NICE Interventional Procedures Programme to produce interventional procedures
Uterine artery embolisation for treating Issued: December 2013 guidance.nice.org.uk/ipg NICE has accredited the process used by the NICE Interventional Procedures Programme to produce interventional procedures
Interventional procedures guidance Published: 16 December 2015 nice.org.uk/guidance/ipg540
 Electrical stimulation of the lower oesophageal sphincter for treating gastro-oesophageal reflux disease Interventional procedures guidance Published: 16 December 2015 nice.org.uk/guidance/ipg540 Your
Electrical stimulation of the lower oesophageal sphincter for treating gastro-oesophageal reflux disease Interventional procedures guidance Published: 16 December 2015 nice.org.uk/guidance/ipg540 Your
Technology appraisal guidance Published: 24 January 2018 nice.org.uk/guidance/ta499
 Glecaprevir pibrentasvir for treating chronic hepatitis C Technology appraisal guidance Published: 24 January 2018 nice.org.uk/guidance/ta499 NICE 2018. All rights reserved. Subject to Notice of rights
Glecaprevir pibrentasvir for treating chronic hepatitis C Technology appraisal guidance Published: 24 January 2018 nice.org.uk/guidance/ta499 NICE 2018. All rights reserved. Subject to Notice of rights
Medical technologies guidance Published: 10 September 2018 nice.org.uk/guidance/mtg38
 Neuropad for detecting preclinical diabetic peripheral neuropathy Medical technologies guidance Published: 10 September 2018 nice.org.uk/guidance/mtg38 NICE 2018. All rights reserved. Subject to Notice
Neuropad for detecting preclinical diabetic peripheral neuropathy Medical technologies guidance Published: 10 September 2018 nice.org.uk/guidance/mtg38 NICE 2018. All rights reserved. Subject to Notice
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Single Technology Appraisal (STA)
 Thank you for agreeing to give us your views on the technology and the way it should be used in the NHS. Primary Care Trusts (PCTs) provide a unique perspective on the technology, which is not typically
Thank you for agreeing to give us your views on the technology and the way it should be used in the NHS. Primary Care Trusts (PCTs) provide a unique perspective on the technology, which is not typically
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex
 Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
CUBITAL TUNNEL SYNDROME GUIDANCE
 CUBITAL TUNNEL SYNDROME GUIDANCE Author Louise Ross (Louise.Ross@ggc.scot.nhs.uk) Organisation NHS Greater Glasgow and Clyde Created 15/04/2016 15:32:54 Modified 26/10/2016 16:17:15 Modified By Louise
CUBITAL TUNNEL SYNDROME GUIDANCE Author Louise Ross (Louise.Ross@ggc.scot.nhs.uk) Organisation NHS Greater Glasgow and Clyde Created 15/04/2016 15:32:54 Modified 26/10/2016 16:17:15 Modified By Louise
Interventional procedures guidance Published: 26 September 2014 nice.org.uk/guidance/ipg504
 Transcatheter valve-in-valve e implantation for aortic bioprosthetic valve dysfunction Interventional procedures guidance Published: 26 September 2014 nice.org.uk/guidance/ipg504 Your responsibility This
Transcatheter valve-in-valve e implantation for aortic bioprosthetic valve dysfunction Interventional procedures guidance Published: 26 September 2014 nice.org.uk/guidance/ipg504 Your responsibility This
Stapled transanal rectal resection for obstructed defaecation syndrome
 Stapled transanal rectal resection for obstructed Issued: June 2010 www.nice.org.uk/ipg351 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Stapled transanal rectal resection for obstructed Issued: June 2010 www.nice.org.uk/ipg351 NHS Evidence has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
National Institute for Health and Clinical Excellence. NICE Quality Standards Consultation attention deficit hyperactivity disorder
 National Institute for Health and Clinical Excellence NICE Quality Standards Consultation attention deficit hyperactivity disorder Closing date: 5pm Thursday 21 st March 2013 Organisation Title (e.g. Dr,
National Institute for Health and Clinical Excellence NICE Quality Standards Consultation attention deficit hyperactivity disorder Closing date: 5pm Thursday 21 st March 2013 Organisation Title (e.g. Dr,
Arthroscopic knee washout, with or without debridement, for the treatment of osteoarthritis
 Arthroscopic knee washout, with or without debridement, for the treatment of osteoarthritis Issued: August 2007 www.nice.org.uk/ipg230 Copyright NICE 2007 Contents 1 Guidance... 3 2 The procedure... 4
Arthroscopic knee washout, with or without debridement, for the treatment of osteoarthritis Issued: August 2007 www.nice.org.uk/ipg230 Copyright NICE 2007 Contents 1 Guidance... 3 2 The procedure... 4
Prostate artery embolisation for benign prostatic hyperplasia
 Prostate artery embolisation for benign prostatic Issued: April 2013 guidance.nice.org.uk/ipg NICE has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
Prostate artery embolisation for benign prostatic Issued: April 2013 guidance.nice.org.uk/ipg NICE has accredited the process used by the NICE Interventional Procedures Programme to produce interventional
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC)
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Sept 2015 Review: Sept 2018 Bulletin 227: EXOGEN ultrasound bone healing system used for the management of long bone fractures JPC Recommendations:
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE (JPC) Sept 2015 Review: Sept 2018 Bulletin 227: EXOGEN ultrasound bone healing system used for the management of long bone fractures JPC Recommendations:
Technology appraisal guidance Published: 7 February 2018 nice.org.uk/guidance/ta505
 Ixazomib with lenalidomide and dexamethasone for treating relapsed or refractory multiple myeloma Technology appraisal guidance Published: 7 February 2018 nice.org.uk/guidance/ta505 NICE 2018. All rights
Ixazomib with lenalidomide and dexamethasone for treating relapsed or refractory multiple myeloma Technology appraisal guidance Published: 7 February 2018 nice.org.uk/guidance/ta505 NICE 2018. All rights
Technology appraisal guidance Published: 28 June 2017 nice.org.uk/guidance/ta448
 Etelcalcetide for treating secondary hyperparathyroidism Technology appraisal guidance Published: 28 June 2017 nice.org.uk/guidance/ta448 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Etelcalcetide for treating secondary hyperparathyroidism Technology appraisal guidance Published: 28 June 2017 nice.org.uk/guidance/ta448 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
South East Coast Operational Delivery Network. Critical Care Rehabilitation
 South East Coast Operational Delivery Networks Hosted by Medway Foundation Trust South East Coast Operational Delivery Network Background Critical Care Rehabilitation The optimisation of recovery from
South East Coast Operational Delivery Networks Hosted by Medway Foundation Trust South East Coast Operational Delivery Network Background Critical Care Rehabilitation The optimisation of recovery from
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6
 Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Absorbable Woven Polyglycolic Acid Mesh Tube (Absorbable Nerve Conduit Tube) INSTRUCTIONS FOR USE 2 6 1 0086 SYMBOL DEFINITIONS ENGLISH Do not Reuse Consult Instructions For Use Ethylene Oxide Sterilized
Ixekizumab for treating moderate to severe plaque psoriasis [ID904]
![Ixekizumab for treating moderate to severe plaque psoriasis [ID904] Ixekizumab for treating moderate to severe plaque psoriasis [ID904]](/thumbs/86/94899473.jpg) Thank you for agreeing to make a submission on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to make a submission on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta465
 Olaratumab atumab in combination with doxorubicin orubicin for treating advanced soft tissue sarcoma Technology appraisal guidance Published: 9 August 17 nice.org.uk/guidance/ta465 NICE 17. All rights
Olaratumab atumab in combination with doxorubicin orubicin for treating advanced soft tissue sarcoma Technology appraisal guidance Published: 9 August 17 nice.org.uk/guidance/ta465 NICE 17. All rights
pat hways Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166
 pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
pat hways Galaxy UNYCO for temporary stabilisation of lower limb fractures Medtech innovation briefing Published: 14 December 2018 nice.org.uk/guidance/mib166 Summary The technology described in this briefing
Smoking cessation interventions and services
 National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
Slide 1. Slide 2. Slide 3. The Role Of Plastic Surgery In Reducing A Patient s Disability Score A Reconstructive Approach. Peripheral Nerve Surgery
 Slide 1 The Role Of Plastic Surgery In Reducing A Patient s Disability Score A Reconstructive Approach Andrew I. Elkwood MD FACS Director of the Center for Treatment of Paralysis and Reconstructive Nerve
Slide 1 The Role Of Plastic Surgery In Reducing A Patient s Disability Score A Reconstructive Approach Andrew I. Elkwood MD FACS Director of the Center for Treatment of Paralysis and Reconstructive Nerve
Technology appraisal guidance Published: 16 May 2018 nice.org.uk/guidance/ta520
 Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy Technology appraisal guidance Published: 16 May 2018 nice.org.uk/guidance/ta520 NICE 2018. All rights
Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy Technology appraisal guidance Published: 16 May 2018 nice.org.uk/guidance/ta520 NICE 2018. All rights
Technology appraisal guidance Published: 18 July 2018 nice.org.uk/guidance/ta531
 Pembrolizumab for untreated PD- L1-positive metastatic non-small-cell lung cancer Technology appraisal guidance Published: 18 July 2018 nice.org.uk/guidance/ta531 NICE 2018. All rights reserved. Subject
Pembrolizumab for untreated PD- L1-positive metastatic non-small-cell lung cancer Technology appraisal guidance Published: 18 July 2018 nice.org.uk/guidance/ta531 NICE 2018. All rights reserved. Subject
Technology appraisal guidance Published: 28 March 2018 nice.org.uk/guidance/ta516
 Cabozantinib for treating medullary thyroid cancer Technology appraisal guidance Published: 28 March 2018 nice.org.uk/guidance/ta516 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Cabozantinib for treating medullary thyroid cancer Technology appraisal guidance Published: 28 March 2018 nice.org.uk/guidance/ta516 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
IPAC date: May of 13
 National Institute for Health and Care Excellence IP685/3 Transcatheter aortic valve implantation (transcatheter aortic valve replacement) for aortic stenosis Consultation Comments table IPAC date: May
National Institute for Health and Care Excellence IP685/3 Transcatheter aortic valve implantation (transcatheter aortic valve replacement) for aortic stenosis Consultation Comments table IPAC date: May
Project Initiation Document:
 Project Initiation Document: Lancashire Support Services for Children, Young People, Families and Carers Affected by Autistic Spectrum Disorder (ASD) and Diagnosis 1. Background The Children and Young
Project Initiation Document: Lancashire Support Services for Children, Young People, Families and Carers Affected by Autistic Spectrum Disorder (ASD) and Diagnosis 1. Background The Children and Young
Technology appraisal guidance Published: 26 April 2017 nice.org.uk/guidance/ta442
 Ixekizumab for treating moderate to severe ere plaque psoriasis Technology appraisal guidance Published: 26 April 2017 nice.org.uk/guidance/ta442 NICE 2017. All rights reserved. Subject to Notice of rights
Ixekizumab for treating moderate to severe ere plaque psoriasis Technology appraisal guidance Published: 26 April 2017 nice.org.uk/guidance/ta442 NICE 2017. All rights reserved. Subject to Notice of rights
Technology appraisal guidance Published: 21 November 2018 nice.org.uk/guidance/ta546
 Padeliporfin for untreated localised prostate cancer Technology appraisal guidance Published: 21 November 2018 nice.org.uk/guidance/ta546 NICE 2019. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Padeliporfin for untreated localised prostate cancer Technology appraisal guidance Published: 21 November 2018 nice.org.uk/guidance/ta546 NICE 2019. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Peripheral Nerve Problems
 Patient Education Peripheral Nerve Problems How they develop and ways to treat them This handout provides general information about how nerves work, what happens when they are injured, and how peripheral
Patient Education Peripheral Nerve Problems How they develop and ways to treat them This handout provides general information about how nerves work, what happens when they are injured, and how peripheral
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Peripheral Nerve Problems
 UW MEDICINE PATIENT EDUCATION Peripheral Nerve Problems How they develop and ways to treat them This handout explains how nerves work, what happens when they are injured, and how peripheral nerve problems
UW MEDICINE PATIENT EDUCATION Peripheral Nerve Problems How they develop and ways to treat them This handout explains how nerves work, what happens when they are injured, and how peripheral nerve problems
Audit support for continuous subcutaneous insulin infusion for the treatment of diabetes mellitus (review of technology appraisal guidance 57)
 Audit support for continuous subcutaneous insulin (review of technology appraisal guidance 57) Issue date: 2008 Audit support Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus
Audit support for continuous subcutaneous insulin (review of technology appraisal guidance 57) Issue date: 2008 Audit support Continuous subcutaneous insulin infusion for the treatment of diabetes mellitus
Technology appraisal guidance Published: 6 December 2017 nice.org.uk/guidance/ta493
 Cladribine tablets for treating relapsing remitting multiple sclerosis Technology appraisal guidance Published: 6 December 2017 nice.org.uk/guidance/ta493 NICE 2018. All rights reserved. Subject to Notice
Cladribine tablets for treating relapsing remitting multiple sclerosis Technology appraisal guidance Published: 6 December 2017 nice.org.uk/guidance/ta493 NICE 2018. All rights reserved. Subject to Notice
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal. Serelaxin for treating acute decompensation of heart failure
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Serelaxin for treating acute decompensation of heart Draft scope (pre-referral) Draft remit/appraisal objective To
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Serelaxin for treating acute decompensation of heart Draft scope (pre-referral) Draft remit/appraisal objective To
ifuse implant system for treating chronic sacroiliac joint pain
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance SCOPE ifuse implant system for treating chronic sacroiliac joint pain 1 Technology 1.1 Description of the technology The ifuse
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Medical technology guidance SCOPE ifuse implant system for treating chronic sacroiliac joint pain 1 Technology 1.1 Description of the technology The ifuse
Alcohol interventions in secondary and further education
 National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
National Institute for Health and Care Excellence Guideline version (Draft for Consultation) Alcohol interventions in secondary and further education NICE guideline: methods NICE guideline Methods
NICE guideline Published: 17 February 2016 nice.org.uk/guidance/ng38
 Fractures (non-complex): assessment and management NICE guideline Published: 17 February 2016 nice.org.uk/guidance/ng38 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Fractures (non-complex): assessment and management NICE guideline Published: 17 February 2016 nice.org.uk/guidance/ng38 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Specialised Services Policy:
 Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
Specialised Services Policy: CP35 Cochlear Implants Document Author: Specialised Planner for Women & Children s Services Executive Lead: Director of Planning Approved by: Executive Board Issue Date: 05
GDm-Health is free to download and use. Its use may result in efficiency savings from reducing face-to-face clinic appointments.
 pat hways Health app: GDm-Health for people with gestational diabetes Medtech innovation briefing Published: 13 November 2017 nice.org.uk/guidance/mib131 Summary About this app GDm-Health is a health application
pat hways Health app: GDm-Health for people with gestational diabetes Medtech innovation briefing Published: 13 November 2017 nice.org.uk/guidance/mib131 Summary About this app GDm-Health is a health application
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedures overview of arthroscopic femoro acetabular surgery for hip impingement syndrome Hip impingement
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedures overview of arthroscopic femoro acetabular surgery for hip impingement syndrome Hip impingement
National Institute for Health and Clinical Excellence
 National Institute for Health and Clinical Excellence NICE pilot quality standards for social care: Consultation on the draft quality standard for Dementia: Supporting people to live well with dementia
National Institute for Health and Clinical Excellence NICE pilot quality standards for social care: Consultation on the draft quality standard for Dementia: Supporting people to live well with dementia
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technology guidance SCOPE The UroLift system for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia 1
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technology guidance SCOPE The UroLift system for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia 1
Arthroscopic shoulder stabilisation surgery
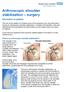 Arthroscopic shoulder stabilisation surgery Information for patients The aim of this leaflet is to answer some of the questions you may have about having an arthroscopic shoulder stabilisation. It explains
Arthroscopic shoulder stabilisation surgery Information for patients The aim of this leaflet is to answer some of the questions you may have about having an arthroscopic shoulder stabilisation. It explains
WORKING TOGETHER FOR THE NHS 20/07/2018
 20/07/2018 NHS Improvement and NHS England Wellington House 133-155 Waterloo Road London SE1 8UG 020 3747 0000 www.england.nhs.uk www.improvement.nhs.uk To: Regional Directors, Trust Medical Directors,
20/07/2018 NHS Improvement and NHS England Wellington House 133-155 Waterloo Road London SE1 8UG 020 3747 0000 www.england.nhs.uk www.improvement.nhs.uk To: Regional Directors, Trust Medical Directors,
Extracorporeal Shockwave Therapy (ESWT) and Radial Pulse Therapy (RPT) August 2016
 Commissioning Policy Extracorporeal Shockwave Therapy (ESWT) and Radial Pulse Therapy (RPT) August 2016 This policy applies to patients for whom the following Clinical Commissioning Groups are responsible:
Commissioning Policy Extracorporeal Shockwave Therapy (ESWT) and Radial Pulse Therapy (RPT) August 2016 This policy applies to patients for whom the following Clinical Commissioning Groups are responsible:
Deep brain stimulation for difficultto-treat
 Issue date January 2012 Understanding NICE guidance Information for people who use NHS services Deep brain stimulation for difficultto-treat epilepsy NICE interventional procedures guidance advises the
Issue date January 2012 Understanding NICE guidance Information for people who use NHS services Deep brain stimulation for difficultto-treat epilepsy NICE interventional procedures guidance advises the
Ankle arthroscopy. If you have any further questions, please speak to a doctor or nurse caring for you
 Ankle arthroscopy This leaflet aims to answer your questions about having an ankle arthroscopy. It explains the benefits, risks and alternatives, as well as what you can expect when you come to hospital.
Ankle arthroscopy This leaflet aims to answer your questions about having an ankle arthroscopy. It explains the benefits, risks and alternatives, as well as what you can expect when you come to hospital.
NeuroMend. Collagen Wrap Conduits. Operative Technique
 NeuroMend Collagen Wrap Conduits Operative Technique Stryker NeuroMend Collagen Wrap Conduits Wrap a protective environment around your patients injured peripheral nerves Common Indications The NeuroMend
NeuroMend Collagen Wrap Conduits Operative Technique Stryker NeuroMend Collagen Wrap Conduits Wrap a protective environment around your patients injured peripheral nerves Common Indications The NeuroMend
National Cancer Peer Review Sarcoma. Julia Hill Acting Deputy National Co-ordinator
 National Cancer Peer Review Sarcoma Julia Hill Acting Deputy National Co-ordinator Improving Outcomes Guidance The Intentions of Improving Outcomes for People with Sarcoma Changes in the provision of care
National Cancer Peer Review Sarcoma Julia Hill Acting Deputy National Co-ordinator Improving Outcomes Guidance The Intentions of Improving Outcomes for People with Sarcoma Changes in the provision of care
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464
 Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Bisphosphonates for treating osteoporosis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta464 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
There are three referral categories used in the dental referral system:
 Restorative Dentistry Referral Criteria Restorative Dentistry referral criteria are outlined to provide General Dental Practitioners (GDPs), Community Dental Service (CDS) Dentists, Primary Care Specialists,
Restorative Dentistry Referral Criteria Restorative Dentistry referral criteria are outlined to provide General Dental Practitioners (GDPs), Community Dental Service (CDS) Dentists, Primary Care Specialists,
Diagnostics consultation document
 National Institute for Health and Care Excellence Diagnostics consultation document Myocardial infarction (acute): Early rule out using high-sensitivity troponin tests (Elecsys Troponin T high-sensitive,
National Institute for Health and Care Excellence Diagnostics consultation document Myocardial infarction (acute): Early rule out using high-sensitivity troponin tests (Elecsys Troponin T high-sensitive,
