Received 20 April 2004/Returned for modification 14 June 2004/Accepted 30 June 2004
|
|
|
- Brandon Robertson
- 6 years ago
- Views:
Transcription
1 JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 2004, p Vol. 42, No /04/$ DOI: /JCM Copyright 2004, American Society for Microbiology. All Rights Reserved. Performance Assessment of a Nested-PCR Assay (the RAPID BAP-MTB) and the BD ProbeTec ET System for Detection of Mycobacterium tuberculosis in Clinical Specimens Jann-Yuan Wang, 1 Li-Na Lee, 1,2 Chin-Sheng Chou, 3 Chung-Yi Huang, 3 Shu-Kuan Wang, 2 Hsin-Chih Lai, 2,4 Po-Ren Hsueh, 1,2 * and Kwen-Tay Luh 1,2 Departments of Internal Medicine 1 and Laboratory Medicine, 2 National Taiwan University Hospital, and School of Medical Technology, National Taiwan University College of Medicine, 4 Taipei, and AsiaGen Corporation, Tainan County, 3 Taiwan Received 20 April 2004/Returned for modification 14 June 2004/Accepted 30 June 2004 The performance of a nested PCR-based assay (the RAPID BAP-MTB; AsiaGen, Taichung, Taiwan) and the BD ProbeTec ET (DTB) system (Becton Dickinson, Sparks, Md.) for detection of Mycobacterium tuberculosis was evaluated with 600 consecutive clinical samples. These samples, including 552 respiratory specimens and 48 nonrespiratory specimens, were collected from 333 patients treated at National Taiwan University Hospital from September to October The results of both assays were compared to the gold standard of combined culture results and clinical diagnosis. The overall sensitivity and specificity of the RAPID BAP-MTB assay for respiratory specimens were 66.7% and 97.2%, respectively, and for the DTB assay they were 56.7% and 95.3%, respectively. The positive and negative predictive values for the RAPID BAP-MTB were 74.1% and 96.0%, respectively, and for the DTB assay they were 59.6% and 94.7%, respectively. For smear-negative samples, the sensitivity of the RAPID BAP-MTB and DTB assays was 57.1% and 40.5%, respectively. The RAPID BAP-MTB assay produced 14 false-positive results in 14 samples, including one of the six samples yielding Mycobacterium abscessus, one of the six samples yielding Mycobacterium avium intracellulare, one sample from a patient with a history of pulmonary tuberculosis with complete treatment, and three samples from three patients with a previous diagnosis of tuberculosis who were under treatment at the time of specimen collection. Among the 48 nonrespiratory specimens, the RAPID BAP-MTB assay was positive in one biopsy sample from a patient with lumbar tuberculous spondylitis and one pus sample from a patient with tuberculous cervical lymphadenopathy. Our results showed that the RAPID BAP-MTB assay is better than the DTB assay for both respiratory specimens and nonrespiratory specimens. The overall time for processing this assay is only 5 h. In addition, its diagnostic accuracy in smear-negative samples is as high as in smear-positive samples. Tuberculosis remains one of the deadliest diseases worldwide. The World Health Organization estimated that in this decade, 300 million more people will become infected with tuberculosis, and 30 million people will die from this disease (24). In 2001, the incidence and mortality of tuberculosis in Taiwan were and 5.81 per 100,000, respectively (5). Successful control of tuberculosis depends on effective case finding and rapid detection of Mycobacterium tuberculosis complex. The conventional method for laboratory diagnosis of tuberculosis is based on acid-fast staining and culture. Staining and microscopy are a rapid screening method for detection of acidfast bacilli in clinical specimens but have low sensitivity (3, 14). Moreover, this method does not discriminate M. tuberculosis complex from nontuberculous mycobacteria, which are causing increasing numbers of infections in immunocompromised hosts, particularly in patients with AIDS. Culture has acceptable sensitivity and specificity but may take about 10 days on average to detect positive specimens even with a radiometric procedure (15). Newer diagnostic methods employing nucleic acid amplification and detection may provide very quick and specific tests for identification of M. tuberculosis complex (4, 6, 7, 9-12, 16, 17, 19-21, 25). Among them, the BD ProbeTec ET Direct TB System (DTB), which uses an internal amplification control designed to detect the presence of inhibiting substances, has been reported to have an excellent performance (9, 18, 19). Although these newer amplification methods can theoretically detect a single copy of genomic sequence, their sensitivities are considerably less than that of culture (18). Recently, the Asia- Gen Corporation in Taiwan developed a new assay for detection of M. tuberculosis complex, the RAPID BAP-MTB assay. This technique uses a nested PCR technique, which can improve the sensitivity of detection of M. tuberculosis complex in paucibacillary (smear-negative) specimens (13, 25). In addition, the technique can decrease false-positive events because the probability of a wrong locus being mistakenly amplified twice is very low. The purpose of this study was to compare the value of the DTB assay and the RAPID BAP-MTB assay for the identification of M. tuberculosis complex in clinical samples. * Corresponding author. Mailing address: Department of Laboratory Medicine, National Taiwan University Hospital, No. 7, Chun Shan South Road, Taipei, 100, Taiwan. Phone: , ext Fax: hsporen@ha.mc.ntu.edu.tw. MATERIALS AND METHODS Specimen collection and processing. A total of 600 consecutive clinical specimens were collected, including 552 respiratory specimens (527 sputum and 25 bronchial wash specimens) from 299 patients and 48 nonrespiratory specimens from 34 patients (Table 1). All of these patients were treated at the National 4599
2 4600 WANG ET AL. J. CLIN. MICROBIOL. TABLE 1. Clinical information on the 333 patients and microbiological findings for 600 clinical specimens from these patients Specimen a No. of patients No. of specimens Respiratory Active pulmonary TB, not treated Pulmonary TB, under treatment Old pulmonary TB 5 12 Smear positive M. tuberculosis 9 12 M. abscessus 2 2 Culture negative 5 7 Culture positive M. tuberculosis M. avium intracellulare complex 6 7 M. abscessus 6 6 M. chelonae 3 3 M. fortuitum 2 3 M. kansasii 1 1 Unidentified species 2 2 Nonrespiratory Smear positive/culture positive for 0 0 M. tuberculosis Smear negative/culture positive 1 1 b for M. tuberculosis Smear positive/culture negative for M. tuberculosis 1 1 c a TB, tuberculosis. b This was a pus sample from a patient with tuberculosis-related cervical lymphadenopathy. c This was a lumbar spine biopsy sample from a patient with tuberculosis spondylitis. Taiwan University Hospital from September to October The 48 nonrespiratory specimens included 20 samples from normal sterile body fluids (10 samples from pleural effusion, four from ascites, three from cerebrospinal fluid, two from synovial fluid, and one from pericardial effusion), 15 pus samples (six from tracheostomy, four from soft tissue abscess, three from surgical spinal wound, one from neck lymphadenopathy, and one from liver abscess), 10 urine samples, and three biopsy specimens (one each from neck lymph node, lumbar spine, and pleura). Specimens that could not be processed on receipt were stored at 2 to 8 C for no longer than 48 h. All specimens were processed and treated as previously described (19). Briefly, each specimen was processed by adding an equal volume of NaOH citrate N-acetyl-L-cysteine at room temperature for 15 min. After centrifugation, the precipitate was resuspended in 1 ml of phosphate-buffered saline (ph 7.4). Smear and culture. Smears of the processed specimens for acid-fast bacilli were stained with auramine-rhodamine fluorochrome and examined by standard procedures (15). Fluorochrome stain-positive smears were confirmed by the Kinyoun stain method (15). Cultures were performed by inoculating 0.5 ml of sediment onto Middlebrook 7H11 selective agar with antimicrobials (Remel Inc., Lexena, Kans.) (10) and by the fluorometric BACTEC technique (BACTEC MGIT 960 system; Becton-Dickinson Diagnostic Instrument Systems, Sparks, Md.) as previously described (16). BD ProbeTec ET (DTB) system. The test was done according to the instructions supplied by the manufacturer (package insert; Becton Dickinson). The procedure consisted of two steps, specimen preparation and combined, fully automated amplification and detection. When the amplification signal was greater than 3,400 units of MOTA (metric other than acceleration), the result was considered positive regardless of the values of the internal amplification control. Values lower than 3,400 were considered negative when the internal amplification control value was greater than 5,000 and indeterminate when the internal amplification control value was less than 5,000 (presence of an inhibitor for this assay). In the latter case, samples were retested, and the results of the repeated tests were used in the analysis (19). RAPID BAP-MTB assay. Extract DNA was started by mixing double-distilled H 2 O with resuspended precipitate in a lysis tube. After 20 s of vortexing and 15 min of centrifugation at 3,800 rpm, the supernatant was removed. The pellet was resuspended in 150 l of lysis buffer I (KOH, ph 13.1) at room temperature for 10 min and at 100 C in a water bath for another 20 min. Then, 150 l of lysis buffer II (HCl and acetic acid, ph 1.2) was added. After centrifuging at 10,000 rpm for 2 min, a 3- l volume of supernatant was transferred to an amplification tube containing 50 l of amplification reagent (Tris-HCl, MgCl 2, datp, dgtp, dttp, dctp, external primer, and AmpliTaq DNA polymerase) for nested PCR. The primers for nested PCR were derived from the M. tuberculosis genome, encoding the insertion sequence IS6110 with the sequence of the external primer: 5 -GTGAGGGCATCGAGGTGG-3 and 5 -CGTAGGCGTCGGTCACAA A-3 and internal primers 5 -GATGCACCGTCGAACG-3 and 5 -biotin- CCACGGTAGGCGAACCCT-3. For each assay, one negative control was prepared. The nested PCR was carried out in a thermal reactor. After a 5-min incubation at 94 C, the first amplification was performed for 27 cycles of 94 C for 30 s, 63.7 C for 15 s, and 72 C for 15 s. After the last cycle, the samples were incubated for 10 min at 72 C. controls contained the PCR mixture without the template DNA. The second amplification was performed with the same extension program except the external primer was replaced by the internal primer. In a hybridization tube, 10 l of each amplified DNA sample and 290 l of hybridization reagent, containing 15 l of MagProbe (beads with probe; 5 amine-acctaaccggctgtgggtagcaga) and 150 l of hybridization buffer, was added, vortexed, and incubated at 95 C for 5 min and at 60 C for 20 min in a dry bath. Tubes were then transferred to magnetic wells for 5 min. The hybridization buffer was removed by aspiration without disturbing the Mag- Probe. After adding a 1-ml volume of preheated 60 C wash buffer, the tubes were vortexed and held in a magnetic well for 5 min. The wash buffer was removed by aspiration. The wash steps were then repeated once again. After adding blocking solution and streptavidin-horseradish peroxidase, the hybridization tube was vortexed, kept from light at room temperature for 20 min, and transferred back to the magnetic well for another 5 min. Then the supernatant was removed by careful aspiration. After washing twice and resuspending with phosphate-buffered saline, the tube was placed in a luminometer to determine the number of relative light units (RLU) produced by the reaction. The RAPID BAP-MTB results were interpreted as follows. When the control tube showed an RLU value equal to or greater than 25,000, the test was repeated. When the RLU of the corresponding control was less than 25,000, the sample was considered positive for M. tuberculosis complex if the sample s RLU was equal to or greater than 100,000 and negative if the sample s RLU was less than 25,000. If the sample s RLU was between these values, the sample was retested to verify the results. The sample was considered positive if the retest RLU value was equal to or greater than 25,000 and negative if it was less than 25,000. For the RAPID BAP-MTB assay, there was no means to monitor for the presence of inhibitor. Clinical evaluation of patients. All of the medical records, including history, symptoms, signs, radiology, pathology, microbiology results, and follow-up observations, were carefully reviewed in order to obtain the necessary data from the combination of culture results and observation of clinical condition to perform the assessment which served as the gold standard for diagnosis (resolved results). Clinically, two categories of samples were considered true-positives: (i) samples that were culture positive for M. tuberculosis complex and (ii) samples that were culture negative for M. tuberculosis complex but originated from a patient whose other samples were culture positive or who had a clinical diagnosis of tuberculosis (19, 22). The clinical diagnosis of tuberculosis was established if the biopsy material demonstrated caseating granulomas or the clinical and radiographic presentations were consistent with tuberculosis with marked improvement after antituberculosis therapy. After this analysis, amplification results were reclassified as appropriate. Statistical analysis. Statistical comparisons were calculated by the chi-square test; P 0.05 was considered significant. RESULTS The clinical information on the 333 patients and microbiological findings of 600 clinical specimens from these patients are summarized in Table 1. Altogether, 52 respiratory specimens were culture positive for acid-fast bacilli, 30 (57.7%) isolates were found to be M. tuberculosis complex, whereas the remaining 22 (42.3%) strains were classified as nontuberculous mycobacteria. Based on the clinical and microbiological findings of the 299 patients whose respiratory specimens were collected for this study, 24 patients had active pulmonary tu-
3 VOL. 42, 2004 RAPID DETECTION OF M. TUBERCULOSIS 4601 TABLE 2. Correlation between two amplification assays for detection of M. tuberculosis and culture results among 552 respiratory specimens Assay Acid-fast smear result (no. of samples) Culture positive (n 30) Culture negative (n 522) Predictive values (%) RAPID BAP-MTB (21) (531) Overall (552) DTB (21) (531) Overall (552) berculosis, 20 had a history of pulmonary tuberculosis, and 5 had a previous diagnosis of pulmonary tuberculosis and were currently receiving treatment. Of the 48 nonrespiratory specimens, one lumbar spine biopsy sample from a patient with tuberculosis spondylitis was smear positive and culture negative, and one pus sample from a patient with tuberculosis cervical lymphadenopathy was culture positive and smear negative for M. tuberculosis complex. BD ProbeTec ET (DTB) system and RAPID BAP-MTB assay. Tables 2 and 3 show comparisons of the results of amplification with the two assays in respiratory specimens according to the culture results and gold standard diagnosis made on the basis of culture results and clinical findings. Of the 12 respiratory samples that were smear positive and culture positive for M. tuberculosis complex, 11 were both RAPID BAP-MTB positive and DTB positive. Eighteen samples were smear negative for acid-fast bacilli but culture positive for M. tuberculosis complex; all were RAPID BAP-MTB positive and 11 were DTB positive (P 0.008). There were 24 samples that were both smear and culture negative, collected from 14 patients with a clinical diagnosis of pulmonary tuberculosis. These samples were both RAPID BAP-MTB and DTB positive in three, only RAPID BAP-MTB positive in three, and only DTB positive in the remaining three. The cumulative difference for all M. tuberculosis complex-positive specimens (40 positive by RAPID BAP-MTB and 34 positive by DTB) was not significant (P 0.348). As determined by the findings of culture and clinical diagnosis, a total of 14 respiratory samples had a false-positive result on RAPID BAP-MTB and 23 respiratory samples had a false-positive result on DTB. The possible causes of falsepositive results in both assays are summarized in Table 4. Table 5 compares the results of amplification of nonrespiratory specimens with the culture results and results of gold standard diagnosis based on the result of culture and clinical findings. The cumulative difference between these two methods for all M. tuberculosis complex-positive specimens was not significant. Comparison of the diagnostic value of the two assays. In the 60 respiratory samples considered positive for M. tuberculosis complex based on clinical and microbiologic findings, the results of both amplification assays were concordantly positive in 30 and negative in 16. The results were RAPID BAP-MTB positive and DTB negative in 10 samples, all of which were smear negative. The results were RAPID BAP-MTB negative and DTB positive in four, all except one of which were smear negative. DISCUSSION The major difference between M. tuberculosis complex and nontuberculous mycobacterial infections is that the former can spread via person to person contact. For this reason, it is particularly important to diagnose tuberculosis as early as possible. Because conventional methods, including acid-fast staining and culture, are either insensitive or time-consuming, new technological developments which facilitate rapid diagnosis are of great importance. From a clinical standpoint, the key aspect TABLE 3. Correlation between two amplification assays for detection of M. tuberculosis and resolved results (culture and clinical diagnosis) among 552 respiratory specimens Assay Acid-fast smear result (no. of samples) Resolved result positive (n 60) Resolved result negative (n 492) Predictive value (%) RAPID BAP-MTB (21) (531) Overall (552) DTB (21) (531) Overall (552)
4 4602 WANG ET AL. J. CLIN. MICROBIOL. TABLE 4. Characterizations of specimens with false-positive results in either or the two assays Specimen with false-positive result No. of specimens RAPID BAP-MTB (n 14) DTB (n 23) Pulmonary tuberculosis under treatment 3 6 Old pulmonary tuberculosis 1 5 M. abscessus 1 3 M. avium intracellulare complex 1 0 Unidentified species 0 1 of any new rapid assay for detecting M. tuberculosis complex is its negative predictive value. In the case of respiratory tract disease, it is critical to identify all cases of active tuberculosis and thereby interrupt the dissemination and transmission of the organism. By contrast, in extrapulmonary tuberculosis, it is critical to ensure that a readily treatable infection is not overlooked. The test should be sensitive, specific, and technically simple as well as able to differentiate between live and dead mycobacteria. Although the RAPID BAP-MTB assay is entirely manual, whereas the BD ProbeTec ET system is semiautomated, the present study has demonstrated that both of these amplification assays can detect M. tuberculosis complex in clinical samples within a few hours, fulfilling some if not all of the key requirements for clinical applicability. The negative predictive values of both assays approached 100%. Both kits include all of the reagents needed for sample amplification and detection, and the assays are easy to perform. The differences of the results from cutoff values, values in controls, and values in samples were broad enough to allow easy discrimination by both assays. Although the lack of a means to monitor for inhibition in the RAPID BAP-MTB assay, the two-lysis-buffer system was stringent enough to inactivate the inhibitors, and only one specimen falsely negative for detection of M. tuberculosis complex was found. The specificity and negative predictive value of the DTB assay in this study were in agreement with previous reports, 95.3 and 94.7%, respectively, for respiratory specimens and 95.7 and 97.8%, respectively, for nonrespiratory specimens (2, 12, 19). However, the sensitivity and positive predictive value of the DTB assay were low, 56.7 and 59.6%, respectively, for respiratory specimens and 50.0 and 33.3%, respectively, for nonrespiratory specimens. No significant differences in the diagnostic accuracy of the assay were observed between respiratory and nonrespiratory specimens. The low sensitivity of the DTB assay in this study probably resulted from the generally low bacterial load in the specimens. The percentage (40%) of smear-positive specimens in all culture-positive respiratory specimens was lower than that (64.7 to 83.5%) in previous reports (2, 12, 19). The low positive predictive value of the DTB assay was due to the high false-positive rate. Because the culture yield rate of nontuberculous mycobacteria in this study (4.0%) was lower than in previous reports (8.2 to 9.7%), it did not explain the high false-positive rate (2, 12, 19). In contrast, of the 23 specimens from patients with a positive DTB result but a negative result based on culture and clinical findings, 47.8% were collected either from patients with a history of pulmonary tuberculosis who were not currently being treated or from patients with a previous diagnosis of pulmonary tuberculosis who were currently receiving treatment. These findings reflect the high prevalence of tuberculosis in the general population in Taiwan. It is possible that the dead mycobacteria in the specimens may have caused the DTB test to yield a falsepositive result. By applying the nested PCR technique, the RAPID BAP- MTB assay is able to detect as little as 10 fg, or the equivalent of 1 to 20 copies of M. tuberculosis complex genomic DNA. In addition, false-positive results are decreased because the probability of a wrong locus being mistakenly amplified twice is very low. Thus, the performance of the RAPID BAP-MTB assay in this study was superior to that of the DTB assay in both respiratory and nonrespiratory specimens. For the 18 smear-negative, culture-positive respiratory samples, a small number of mycobacteria, unequally distributed in the test suspension, or a suboptimal target extraction, is perhaps the most likely explanations for the false-negative DTB result in seven (38.9%) samples. However, all 18 samples were RAPID BAP-MTB positive. The problem of smear-negative pulmonary tuberculosis is worth particular attention, because these patients have been reported to be responsible for about 17% of tuberculosis transmission (1, 8). In addition, although many previous studies which used nucleic acid amplification to test extrapulmonary specimens had encouraging results, the sensitivity of this method is still far from ideal when routinely applied to clinical conditions for which proper evaluation may be crucial to outcome (12, 19, 23). Although only 48 nonrespiratory specimens TABLE 5. Correlation between two amplification assays for detection of M. tuberculosis and culture results or resolved results (culture and clinical diagnosis) among 48 nonrespiratory specimens Diagnostic criteria and assay Diagnosis positive Diagnosis negative Predictive value (%) Culture results RAPID BAP-MTB DTB Resolved results RAPID BAP-MTB DTB
5 VOL. 42, 2004 RAPID DETECTION OF M. TUBERCULOSIS 4603 were tested in this study, the results of the RAPID BAP-MTB assay were 100% concordant with the findings of the diagnosis based on the results of culture and clinical findings. Further investigation to assess the performance of this assay in nonrespiratory specimens is needed. In summary, the resurgence of tuberculosis has prompted the need for sensitive, correct, and fast methods for the laboratory detection of M. tuberculosis complex. Our results showed that, although the test is entirely manual, the diagnostic value of the nested PCR-based RAPID BAP-MTB assay is superior to that of the semiautomated DTB assay for both respiratory specimens and nonrespiratory specimens. The overall time for processing this assay is only 5 h. In addition, the diagnostic accuracy of the RAPID BAP-MTB assay in smear-negative samples is as high as that in smear-positive samples. REFERENCES 1. Behr, M. A., S. A. Warren, H. Salamon, P. C. Hopewell, A. Ponce de Leon, C. L. Daley, and P. M. Small Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet 353: Bergmann, J. S., W. E. Keating, and G. L. Woods Clinical evaluation of the BDProbeTec ET system for rapid detection of Mycobacterium tuberculosis. J. Clin. Microbiol. 38: Boyd, J. C., and J. J. Marr Decreasing reliability of acid-fast smear techniques for detection of tuberculosis. Ann. Intern. Med. 82: Brown, T. J., E. G. Power, and G. L. French Evaluation of three commercial detection systems for Mycobacterium tuberculosis where clinical diagnosis is difficult. J. Clin. Pathol. 52: Bureau for Chronic Disease Control Tuberculosis annual report (Taiwan) Bureau for Chronic Disease Control, Taipei, Taiwan. 6. Dalovisio, J. R., S. Montenegro-James, S. A. Kemmerly, C. F. Genre, R. Chambers, D. Greer, G. A. Pankey, D. M. Failla, K. G. Haydel, L. Hutchinson, M. F. Lindley, B. M. Nunez, A. Praba, K. D. Eisenach, and E. S. Cooper Comparison of the amplified Mycobacterium tuberculosis (MTB) direct test, Amplicor MTB PCR, and IS6110-PCR for detection of MTB in respiratory specimens. Clin. Infect. Dis. 23: Eing, B. R., A. Becker, A. Sohns, and R. Ringelmann Comparison of Roche Cobas Amplicor Mycobacterium tuberculosis assay with in-house PCR and culture for detection of M. tuberculosis. J. Clin. Microbiol. 36: Hernandez-Garduno, E., V. Cook, D. Kunimoto, R. K. Elwood, W. A. Black, and J. M. FitzGerald Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax 59: Huang, T. S., W. K. Huang, S. S. Lee, H. Z. Tu, S. H. Chang, and Y. C. Liu Rapid detection of pulmonary tuberculosis using the BDProbeTEC ET Mycobacterium tuberculosis Complex Direct Detection Assay (DTB). Diagn. Microbiol. Infect. Dis. 46: Jan, I. S., P. R. Hsueh, L. J. Teng, L. N. Lee, P. C. Yang, and K. T. Luh Evaluation of an automatic polymerase chain reaction assay for identification of Mycobacterium tuberculosis in respiratory specimens. J. Formos. Med. Assoc. 97: Maugein, J., J. Fourche, S. Vacher, C. Grimond, and C. Bebear Evaluation of the BDProbeTec ET DTB assay(1) for direct detection of Mycobacterium tuberculosis complex from clinical samples. Diagn. Microbiol. Infect. Dis. 44: Mazzarelli, G., L. Rindi, P. Piccoli, C. Scarparo, C. Garzelli, and E. Tortoli Evaluation of the BDProbeTec ET system for direct detection of Mycobacterium tuberculosis in pulmonary and extrapulmonary samples: a multicenter study. J. Clin. Microbiol. 41: Montenegro, S. H., R. H. Gilman, P. Sheen, R. Cama, L. Caviedes, T. Hopper, R. Chambers, and R. A. Oberhelman Improved detection of Mycobacterium tuberculosis in Peruvian children by use of a heminested IS6110 polymerase chain reaction assay. Clin. Infect. Dis. 36: Murray, P. R., C. Elmore, and D. J. Krogstad The acid-fast stain: a specific and predictive test for mycobacterial disease. Ann. Intern. Med. 92: Nolte, F. S., and B. Metchock Mycobacterium, p In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C. 16. O Sullivan, C. E., D. R. Miller, P. S. Schneider, and G. D. Roberts Evaluation of Gen-Probe amplified Mycobacterium tuberculosis direct test by using respiratory and nonrespiratory specimens in a tertiary care center laboratory. J. Clin. Microbiol. 40: Piersimoni, C., A. Callegaro, C. Scarparo, V. Penati, D. Nista, S. Bornigia, C. Lacchini, M. Scagnelli, G. Santini, and G. De Sio Comparative evaluation of the new Gen-Probe Mycobacterium tuberculosis amplified direct test and the semiautomated Abbott LCx Mycobacterium tuberculosis assay for direct detection of Mycobacterium tuberculosis complex in respiratory and extrapulmonary specimens. J. Clin. Microbiol. 36: Piersimoni, C., and C. Scarparo Relevance of commercial amplification methods for direct detection of Mycobacterium tuberculosis complex in clinical samples. J. Clin. Microbiol. 41: Piersimoni, C., C. Scarparo, P. Piccoli, A. Rigon, G. Ruggiero, D. Nista, and S. Bornigia Performance assessment of two commercial amplification assays for direct detection of Mycobacterium tuberculosis complex from respiratory and extrapulmonary specimens. J. Clin. Microbiol. 40: Reischl, U., N. Lehn, H. Wolf, and L. Naumann Clinical evaluation of the automated COBAS AMPLICOR MTB assay for testing respiratory and nonrespiratory specimens. J. Clin. Microbiol. 36: Rimek, D., S. Tyagi, and R. Kappe Performance of an IS6110-based PCR assay and the COBAS AMPLICOR MTB PCR system for detection of Mycobacterium tuberculosis complex DNA in human lymph node samples. J. Clin. Microbiol. 40: Scarparo, C., P. Piccoli, A. Rigon, G. Ruggiero, M. Scagnelli, and C. Piersimoni Comparison of enhanced Mycobacterium tuberculosis amplified direct test with COBAS AMPLICOR Mycobacterium tuberculosis assay for direct detection of Mycobacterium tuberculosis complex in respiratory and extrapulmonary specimens. J. Clin. Microbiol. 38: Woods, G. L., J. S. Bergmann, and N. Williams-Bouyer Clinical Evaluation of the Gen-Probe amplified Mycobacterium tuberculosis direct test for rapid detection of Mycobacterium tuberculosis in select nonrespiratory specimens. J. Clin. Microbiol. 39: World Health Organization Group at risk: W.H.O. report on the tuberculosis epidemic. World Health Organization, Geneva, Switzerland. 25. Yuen, K. Y., W. C. Yam, L. P. Wong, and W. H. Seto Comparison of two automated DNA amplification systems with a manual one-tube nested PCR assay for diagnosis of pulmonary tuberculosis J. Clin. Microbiol. 35:
Microscopic Morphology in Smears Prepared from MGIT Broth Medium for Rapid Presumptive Identification of Mycobacterium tuberculosis
 Annals of Clinical & Laboratory Science, vol. 33, no. 2, 2003 179 Microscopic Morphology in Smears Prepared from MGIT Broth Medium for Rapid Presumptive Identification of Mycobacterium tuberculosis complex,
Annals of Clinical & Laboratory Science, vol. 33, no. 2, 2003 179 Microscopic Morphology in Smears Prepared from MGIT Broth Medium for Rapid Presumptive Identification of Mycobacterium tuberculosis complex,
Use of the BacT/ALERT MB Mycobacteria Blood Culture System for Detecting ACCEPTED
 JCM Accepts, published online ahead of print on December 00 J. Clin. Microbiol. doi:.11/jcm.011-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
JCM Accepts, published online ahead of print on December 00 J. Clin. Microbiol. doi:.11/jcm.011-0 Copyright 00, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved.
Nucleic Acid Amplification Testing for the Diagnosis of TB
 Roche Nucleic Acid Amplification Testing for the Diagnosis of TB David Warshauer, PhD Deputy Director, Communicable Diseases Wisconsin State Laboratory of Hygiene 19 th /20 th Century Traditional Algorithm
Roche Nucleic Acid Amplification Testing for the Diagnosis of TB David Warshauer, PhD Deputy Director, Communicable Diseases Wisconsin State Laboratory of Hygiene 19 th /20 th Century Traditional Algorithm
Performance of Nucleic Acid Amplification Tests for Diagnosis of Tuberculosis in a Large Urban Setting
 MAJOR ARTICLE Performance of Nucleic Acid Amplification Tests for Diagnosis of Tuberculosis in a Large Urban Setting Fabienne Laraque, 1,a Anne Griggs, 2,a Meredith Slopen, 1,a and Sonal S. Munsiff 3,a
MAJOR ARTICLE Performance of Nucleic Acid Amplification Tests for Diagnosis of Tuberculosis in a Large Urban Setting Fabienne Laraque, 1,a Anne Griggs, 2,a Meredith Slopen, 1,a and Sonal S. Munsiff 3,a
ORIGINAL ARTICLE. Department of Microbiology, Military Medical Academy, Belgrade, Yugoslavia. Clin Microbiol Infect 2002; 8:
 ORIGINAL ARTICLE Evaluation of the MB/BacT System for recovery of mycobacteria from clinical specimens in comparison to Lowenstein Jensen medium V. Mirovic and Z. Lepsanovic Department of Microbiology,
ORIGINAL ARTICLE Evaluation of the MB/BacT System for recovery of mycobacteria from clinical specimens in comparison to Lowenstein Jensen medium V. Mirovic and Z. Lepsanovic Department of Microbiology,
Comparison of the automated Cobas Amplicor
 Comparison of the automated Cobas Amplicor Mycobacterium tuberculosis assay with the conventional methods for direct detection of Mycobacterium tuberculosis complex in respiratory and extrapulmonary specimens
Comparison of the automated Cobas Amplicor Mycobacterium tuberculosis assay with the conventional methods for direct detection of Mycobacterium tuberculosis complex in respiratory and extrapulmonary specimens
Diagnosis of Extra Pulmonary Tuberculosis By Using Xpert MTB/RIF Assay (CBNAAT) And MGIT Liquid Culture.
 IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 17, Issue 9 Ver. 10 (September. 2018), PP 65-70 www.iosrjournals.org Diagnosis of Extra Pulmonary Tuberculosis
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-issn: 2279-0853, p-issn: 2279-0861.Volume 17, Issue 9 Ver. 10 (September. 2018), PP 65-70 www.iosrjournals.org Diagnosis of Extra Pulmonary Tuberculosis
The Clinical Utility of Polymerase Chain Reaction for the Diagnosis of Pleural Tuberculosis
 MAJOR ARTICLE The Clinical Utility of Polymerase Chain Reaction for the Diagnosis of Pleural Tuberculosis Jin Wook Moon, 1 Yoon Soo Chang, 1 Se Kyu Kim, 1,2,3 Young Sam Kim, 1,3 Hyuk Min Lee, 4 Sung Kyu
MAJOR ARTICLE The Clinical Utility of Polymerase Chain Reaction for the Diagnosis of Pleural Tuberculosis Jin Wook Moon, 1 Yoon Soo Chang, 1 Se Kyu Kim, 1,2,3 Young Sam Kim, 1,3 Hyuk Min Lee, 4 Sung Kyu
Mycobacterium tuberculosis direct test compared with
 ORIGINAL ARTICLE Clinical utility of the Gen-Probe amplified Mycobacterium tuberculosis direct test compared with smear and culture for the diagnosis of pulmonary tuberculosis Claudio Piersimoni ', Pievgiovgio
ORIGINAL ARTICLE Clinical utility of the Gen-Probe amplified Mycobacterium tuberculosis direct test compared with smear and culture for the diagnosis of pulmonary tuberculosis Claudio Piersimoni ', Pievgiovgio
CDPH - CTCA Joint Guidelines Guideline for Micobacteriology Services In California
 CDPH - CTCA Joint Guidelines Guideline for Micobacteriology Services In California These guidelines are intended to be used as an educational aid to help clinicians make informed decisions about patient
CDPH - CTCA Joint Guidelines Guideline for Micobacteriology Services In California These guidelines are intended to be used as an educational aid to help clinicians make informed decisions about patient
Nucleic Acid Amplification Test for Tuberculosis. Heidi Behm, RN, MPH Acting TB Controller HIV/STD/TB Program Oregon, Department of Health Services
 Nucleic Acid Amplification Test for Tuberculosis Heidi Behm, RN, MPH Acting TB Controller HIV/STD/TB Program Oregon, Department of Health Services What is this test? Nucleic Acid Amplification Test (NAAT)
Nucleic Acid Amplification Test for Tuberculosis Heidi Behm, RN, MPH Acting TB Controller HIV/STD/TB Program Oregon, Department of Health Services What is this test? Nucleic Acid Amplification Test (NAAT)
Laboratory Diagnostic Techniques. Hugo Donaldson Consultant Microbiologist Imperial College Healthcare NHS Trust
 Laboratory Diagnostic Techniques Hugo Donaldson Consultant Microbiologist Imperial College Healthcare NHS Trust Learning Objectives 1) When to consider a diagnosis of TB 2) When to consider a referral
Laboratory Diagnostic Techniques Hugo Donaldson Consultant Microbiologist Imperial College Healthcare NHS Trust Learning Objectives 1) When to consider a diagnosis of TB 2) When to consider a referral
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis
The diagnostic value of gyrb RFLP PCR. Mycobacteria in patients with clinical. in Mazandaran
 Mazandaran University of Medical Sciences The diagnostic value of gyrb RFLP PCR test t in differentiation between pathogenic Mycobacteria in patients with clinical suspicions spicions of tuberculosis in
Mazandaran University of Medical Sciences The diagnostic value of gyrb RFLP PCR test t in differentiation between pathogenic Mycobacteria in patients with clinical suspicions spicions of tuberculosis in
TB Laboratory for Nurses
 TB Laboratory for Nurses Shea Rabley, RN, MN Consultant Mayo Clinic Center for Tuberculosis 2014 MFMER slide-1 Disclosures None 2014 MFMER slide-2 Objectives Participants will be able to: 1. Name 2 safety
TB Laboratory for Nurses Shea Rabley, RN, MN Consultant Mayo Clinic Center for Tuberculosis 2014 MFMER slide-1 Disclosures None 2014 MFMER slide-2 Objectives Participants will be able to: 1. Name 2 safety
of clinical laboratory diagnosis in Extra-pulmonary Tuberculosis
 New approaches and the importance of clinical laboratory diagnosis in Extra-pulmonary Tuberculosis Bahrmand.AR, Hadizadeh Tasbiti.AR, Saifi.M, Yari.SH, Karimi.A, Fateh.A, Tuberculosis Dept. Pasteur Institute
New approaches and the importance of clinical laboratory diagnosis in Extra-pulmonary Tuberculosis Bahrmand.AR, Hadizadeh Tasbiti.AR, Saifi.M, Yari.SH, Karimi.A, Fateh.A, Tuberculosis Dept. Pasteur Institute
International Journal of Health Sciences and Research ISSN:
 International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Laboratory Evaluation of a New Rapid Slide Culture (RSC) Technique for Diagnosis of Extra-
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Laboratory Evaluation of a New Rapid Slide Culture (RSC) Technique for Diagnosis of Extra-
Received 25 March 2011/Returned for modification 9 May 2011/Accepted 25 May 2011
 JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2011, p. 2874 2878 Vol. 49, No. 8 0095-1137/11/$12.00 doi:10.1128/jcm.00612-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Molecular Detection
JOURNAL OF CLINICAL MICROBIOLOGY, Aug. 2011, p. 2874 2878 Vol. 49, No. 8 0095-1137/11/$12.00 doi:10.1128/jcm.00612-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Molecular Detection
Xpert MTB/RIF Training. Indira Soundiram 2012
 Xpert MTB/RIF Training Indira Soundiram 2012 A Better Way to Platform Design GeneXpert Infinity-48 GeneXpert Module GX-I GX-II GX-IV GX-XVI 2 Defining Molecular Diagnostics Any Test Any Time Any Sample
Xpert MTB/RIF Training Indira Soundiram 2012 A Better Way to Platform Design GeneXpert Infinity-48 GeneXpert Module GX-I GX-II GX-IV GX-XVI 2 Defining Molecular Diagnostics Any Test Any Time Any Sample
Chapter 4 Diagnosis of Tuberculosis Disease
 Chapter 4 Diagnosis of Tuberculosis Disease Table of Contents Chapter Objectives.... 75 Introduction.... 77 Medical Evaluation.......................................................... 78 Chapter Summary...
Chapter 4 Diagnosis of Tuberculosis Disease Table of Contents Chapter Objectives.... 75 Introduction.... 77 Medical Evaluation.......................................................... 78 Chapter Summary...
Latent Tuberculosis Infections Controversies in Diagnosis and Management Update 2016
 Latent Tuberculosis Infections Controversies in Diagnosis and Management Update 2016 Randy Culpepper, MD, MPH Deputy Heath Officer/Medical Director Frederick County Health Department March 16, 2016 2 No
Latent Tuberculosis Infections Controversies in Diagnosis and Management Update 2016 Randy Culpepper, MD, MPH Deputy Heath Officer/Medical Director Frederick County Health Department March 16, 2016 2 No
Research Article Factors Associated with Missed Detection of Mycobacterium tuberculosis by Automated BACTEC MGIT 960 System
 BioMed Research International Volume 2016, Article ID 5972021, 4 pages http://dx.doi.org/10.1155/2016/5972021 Research Article Factors Associated with Missed Detection of Mycobacterium tuberculosis by
BioMed Research International Volume 2016, Article ID 5972021, 4 pages http://dx.doi.org/10.1155/2016/5972021 Research Article Factors Associated with Missed Detection of Mycobacterium tuberculosis by
Receipt within 1 day of specimen collection. Report AFB b smear result within 1 day from receipt of specimen
 Recommendation Promote rapid delivery of specimens to the laboratory Use fluorescent acid-fast staining and promptly transmit results by phone, FAX, or electronically Identify growth as acid-fast and use
Recommendation Promote rapid delivery of specimens to the laboratory Use fluorescent acid-fast staining and promptly transmit results by phone, FAX, or electronically Identify growth as acid-fast and use
Evaluation of the Microscopic-Observation. Drug-Susceptibility Assay Drugs Concentration for Detection Of Multidrug-Resistant Tuberculosis
 Evaluation of the Microscopic-Observation Drug-Susceptibility Assay Drugs Concentration for Detection Of Multidrug-Resistant Tuberculosis ABSTRACT New diagnostic tools are urgently needed to interrupt
Evaluation of the Microscopic-Observation Drug-Susceptibility Assay Drugs Concentration for Detection Of Multidrug-Resistant Tuberculosis ABSTRACT New diagnostic tools are urgently needed to interrupt
NAAT in the Clinical Laboratory and Impact on Infection Control 9 th National Conference on Laboratory Aspects of Tuberculosis APHL
 NAAT in the Clinical Laboratory and Impact on Infection Control 9 th National Conference on Laboratory Aspects of Tuberculosis APHL Susan Novak-Weekley, S(M), ASCP, Ph.D., D(ABMM) Director of Microbiology,
NAAT in the Clinical Laboratory and Impact on Infection Control 9 th National Conference on Laboratory Aspects of Tuberculosis APHL Susan Novak-Weekley, S(M), ASCP, Ph.D., D(ABMM) Director of Microbiology,
TB NAAT testing at the Los Angeles County Public Health Laboratory
 TB NAAT testing at the Los Angeles County Public Health Laboratory Hector Rivas Public Health Microbiology Supervisor II Los Angeles County Public Health Laboratory hrivas@ph.lacounty.gov April 2012 1
TB NAAT testing at the Los Angeles County Public Health Laboratory Hector Rivas Public Health Microbiology Supervisor II Los Angeles County Public Health Laboratory hrivas@ph.lacounty.gov April 2012 1
TB/HIV 2 sides of the same coin. Dr. Shamma Shetye, MD Microbiology Metropolis Healthcare, Mumbai
 TB/HIV 2 sides of the same coin Dr. Shamma Shetye, MD Microbiology Metropolis Healthcare, Mumbai Global- Tb new cases Diagnosis-Microscopy ZN,Flourescent microscopy(fm) Rapid, inexpensive test Specificity>95%
TB/HIV 2 sides of the same coin Dr. Shamma Shetye, MD Microbiology Metropolis Healthcare, Mumbai Global- Tb new cases Diagnosis-Microscopy ZN,Flourescent microscopy(fm) Rapid, inexpensive test Specificity>95%
A comparative evaluation of mycobacteria growth indicator tube and Lowenstein-Jensen medium for the isolation of mycobacteria from clinical specimens
 Original Article Vol. 29 No. 2 Comparative evaluation of mycobacteria culture:-kaur KP, et al. 67 A comparative evaluation of mycobacteria growth indicator tube and Lowenstein-Jensen medium for the isolation
Original Article Vol. 29 No. 2 Comparative evaluation of mycobacteria culture:-kaur KP, et al. 67 A comparative evaluation of mycobacteria growth indicator tube and Lowenstein-Jensen medium for the isolation
Received 25 October 2010/Returned for modification 4 February 2011/Accepted 3 March 2011
 JOURNAL OF CLINICAL MICROBIOLOGY, May 2011, p. 1772 1776 Vol. 49, No. 5 0095-1137/11/$12.00 doi:10.1128/jcm.02157-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Comparison of
JOURNAL OF CLINICAL MICROBIOLOGY, May 2011, p. 1772 1776 Vol. 49, No. 5 0095-1137/11/$12.00 doi:10.1128/jcm.02157-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Comparison of
Increasing Trend of Isolation of Non-Tuberculous Mycobacteria in a Tertiary University Hospital in South Korea
 http://dx.doi.org/10.4046/trd.2012.72.5.409 ISSN: 1738-3536(Print)/2005-6184(Online) Tuberc Respir Dis 2012;72:409-415 CopyrightC2012. The Korean Academy of Tuberculosis and Respiratory Diseases. All rights
http://dx.doi.org/10.4046/trd.2012.72.5.409 ISSN: 1738-3536(Print)/2005-6184(Online) Tuberc Respir Dis 2012;72:409-415 CopyrightC2012. The Korean Academy of Tuberculosis and Respiratory Diseases. All rights
Comparison of mechanical disruption techniques for the rapid inactivation of
 JCM Accepted Manuscript Posted Online 10 August 2016 J. Clin. Microbiol. doi:10.1128/jcm.01096-16 Copyright 2016, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6 7 8 9 10 Comparison
JCM Accepted Manuscript Posted Online 10 August 2016 J. Clin. Microbiol. doi:10.1128/jcm.01096-16 Copyright 2016, American Society for Microbiology. All Rights Reserved. 1 2 3 4 5 6 7 8 9 10 Comparison
Isik Somuncu Johansen,* Vibeke Østergaard Thomsen,* Arne Forsgren, Birgit Fischer Hansen, and Bettina Lundgren
 Journal of Molecular Diagnostics, Vol. 6, No. 3, August 2004 Copyright American Society for Investigative Pathology and the Association for Molecular Pathology Detection of Mycobacterium tuberculosis Complex
Journal of Molecular Diagnostics, Vol. 6, No. 3, August 2004 Copyright American Society for Investigative Pathology and the Association for Molecular Pathology Detection of Mycobacterium tuberculosis Complex
Clinical and Public Health Impact of Nucleic Acid Amplification Tests (NAATs) for Tuberculosis
 Clinical and Public Health Impact of Nucleic Acid Amplification Tests (NAATs) for Tuberculosis Amit S. Chitnis, MD, MPH; Pennan M. Barry, MD, MPH; Jennifer M. Flood, MD, MPH. California Tuberculosis Controllers
Clinical and Public Health Impact of Nucleic Acid Amplification Tests (NAATs) for Tuberculosis Amit S. Chitnis, MD, MPH; Pennan M. Barry, MD, MPH; Jennifer M. Flood, MD, MPH. California Tuberculosis Controllers
/01/$ DOI: /JCM Copyright 2001, American Society for Microbiology. All Rights Reserved.
 JOURNAL OF CLINICAL MICROBIOLOGY, Feb. 2001, p. 651 657 Vol. 39, No. 2 0095-1137/01/$04.00 0 DOI: 10.1128/JCM.39.2.651 657.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Comparison
JOURNAL OF CLINICAL MICROBIOLOGY, Feb. 2001, p. 651 657 Vol. 39, No. 2 0095-1137/01/$04.00 0 DOI: 10.1128/JCM.39.2.651 657.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Comparison
Mycobacterial cell wall. Cell Cycle Lengths. Outline of Laboratory Methods. Laboratory Methods
 Laboratory Methods Cell Cycle Lengths Generation time (hrs) Days needed for 26 generations (colony) E. coli 0.33 0.36 Nancy Connell, PhD Professor, nfectious Disease Department of Medicine Center for Emerging
Laboratory Methods Cell Cycle Lengths Generation time (hrs) Days needed for 26 generations (colony) E. coli 0.33 0.36 Nancy Connell, PhD Professor, nfectious Disease Department of Medicine Center for Emerging
TB & HIV CO-INFECTION IN CHILDREN. Reené Naidoo Paediatric Infectious Diseases Broadreach Healthcare 19 April 2012
 TB & HIV CO-INFECTION IN CHILDREN Reené Naidoo Paediatric Infectious Diseases Broadreach Healthcare 19 April 2012 Introduction TB & HIV are two of the leading causes of morbidity & mortality in children
TB & HIV CO-INFECTION IN CHILDREN Reené Naidoo Paediatric Infectious Diseases Broadreach Healthcare 19 April 2012 Introduction TB & HIV are two of the leading causes of morbidity & mortality in children
Evaluation of the Rapid MGIT TBc Identification Test for Culture Confirmation of Mycobacterium tuberculosis Complex Strain Detection
 JOURNAL OF CLINICAL MICROBIOLOGY, Mar. 2011, p. 802 807 Vol. 49, No. 3 0095-1137/11/$12.00 doi:10.1128/jcm.02243-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Evaluation of
JOURNAL OF CLINICAL MICROBIOLOGY, Mar. 2011, p. 802 807 Vol. 49, No. 3 0095-1137/11/$12.00 doi:10.1128/jcm.02243-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. Evaluation of
Overview of Mycobacterial Culture, Identification, and Drug Susceptibility Testing
 Overview of Mycobacterial Culture, Identification, and Drug Susceptibility Testing 1. Essentials for the Mycobacteriology Laboratory: Promoting Quality Practices 1.1 Overview: Mycobacterial Culture, Identification,
Overview of Mycobacterial Culture, Identification, and Drug Susceptibility Testing 1. Essentials for the Mycobacteriology Laboratory: Promoting Quality Practices 1.1 Overview: Mycobacterial Culture, Identification,
Evaluation of Fluorotype MTB for detection of Mycobacterium tuberculosis complex DNA in clinical specimens from a low-incidence country
 Hofmann-Thiel and Hoffmann BMC Infectious Diseases 2014, 14:59 RESEARCH ARTICLE Open Access Evaluation of Fluorotype MTB for detection of Mycobacterium tuberculosis complex DNA in clinical specimens from
Hofmann-Thiel and Hoffmann BMC Infectious Diseases 2014, 14:59 RESEARCH ARTICLE Open Access Evaluation of Fluorotype MTB for detection of Mycobacterium tuberculosis complex DNA in clinical specimens from
Thorax Online First, published on May 31, 2006 as /thx
 Thorax Online First, published on May 31, 2006 as 10.1136/thx.2005.054908 The current evidence on diagnostic accuracy of commercial based nucleic acid amplification tests for the diagnosis of pulmonary
Thorax Online First, published on May 31, 2006 as 10.1136/thx.2005.054908 The current evidence on diagnostic accuracy of commercial based nucleic acid amplification tests for the diagnosis of pulmonary
TB 101 Disease, Clinical Assessment and Lab Testing
 TB 101 Disease, Clinical Assessment and Lab Testing Pacific Islands Tuberculosis Controllers Association Conference (PITCA) Clinical Laboratory Breakout None Disclosure Objectives Be able to list and explain
TB 101 Disease, Clinical Assessment and Lab Testing Pacific Islands Tuberculosis Controllers Association Conference (PITCA) Clinical Laboratory Breakout None Disclosure Objectives Be able to list and explain
Use of BACTEC MGIT 960 for Recovery of Mycobacteria from Clinical Specimens: Multicenter Study
 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 1999, p. 3578 3582 Vol. 37, No. 11 0095-1137/99/$04.00 0 Copyright 1999, American Society for Microbiology. All Rights Reserved. Use of BACTEC MGIT 960 for Recovery
JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 1999, p. 3578 3582 Vol. 37, No. 11 0095-1137/99/$04.00 0 Copyright 1999, American Society for Microbiology. All Rights Reserved. Use of BACTEC MGIT 960 for Recovery
Diagnosis of TB: Laboratory Ken Jost Tuesday April 1, 2014
 TB Nurse Case Management San Antonio, Texas April 1 3, 2014 Diagnosis of TB: Laboratory Ken Jost Tuesday April 1, 2014 Ken Jost, BA has the following disclosures to make: No conflict of interests No relevant
TB Nurse Case Management San Antonio, Texas April 1 3, 2014 Diagnosis of TB: Laboratory Ken Jost Tuesday April 1, 2014 Ken Jost, BA has the following disclosures to make: No conflict of interests No relevant
Diagnosis of Tuberculosis by GeneXpert MTB/RIF Assay Technology: A Short Review
 International Journal of Advanced Microbiology and Health Research www.ijamhr.com Volume 1; Issue 1; September 2017; Page No. 20-24 Review Article Diagnosis of Tuberculosis by GeneXpert MTB/RIF Assay Technology:
International Journal of Advanced Microbiology and Health Research www.ijamhr.com Volume 1; Issue 1; September 2017; Page No. 20-24 Review Article Diagnosis of Tuberculosis by GeneXpert MTB/RIF Assay Technology:
MYCOBACTERIA. Pulmonary T.B. (infect bird)
 MYCOBACTERIA SPP. Reservoir Clinical Manifestation Mycobacterium tuberculosis Human Pulmonary and dissem. T.B. M. lepra Human Leprosy M. bovis Human & cattle T.B. like infection M. avium Soil, water, birds,
MYCOBACTERIA SPP. Reservoir Clinical Manifestation Mycobacterium tuberculosis Human Pulmonary and dissem. T.B. M. lepra Human Leprosy M. bovis Human & cattle T.B. like infection M. avium Soil, water, birds,
Standardized Case Definition for Extrapulmonary Nontuberculous Mycobacteria Infections
 Operational Guidance for Position Statement 17 ID 07: Standardized Case Definition for Extrapulmonary Nontuberculous Mycobacteria Infections Submission Date: April 28, 2017 Committee: Infectious Disease
Operational Guidance for Position Statement 17 ID 07: Standardized Case Definition for Extrapulmonary Nontuberculous Mycobacteria Infections Submission Date: April 28, 2017 Committee: Infectious Disease
Ken Jost, BA, has the following disclosures to make:
 Diagnosis of TB Disease: Laboratory Ken Jost, BA May 10, 2017 TB Intensive May 9-12, 2017 San Antonio, TX EXCELLENCE EXPERTISE INNOVATION Ken Jost, BA, has the following disclosures to make: No conflict
Diagnosis of TB Disease: Laboratory Ken Jost, BA May 10, 2017 TB Intensive May 9-12, 2017 San Antonio, TX EXCELLENCE EXPERTISE INNOVATION Ken Jost, BA, has the following disclosures to make: No conflict
Characteristics of Mycobacterium
 Mycobacterium Characteristics of Mycobacterium Very thin, rod shape. Culture: Aerobic, need high levels of oxygen to grow. Very slow in grow compared to other bacteria (colonies may be visible in up to
Mycobacterium Characteristics of Mycobacterium Very thin, rod shape. Culture: Aerobic, need high levels of oxygen to grow. Very slow in grow compared to other bacteria (colonies may be visible in up to
TB Updates for the Physician Rochester, Minnesota June 19, 2009
 TB Updates for the Physician Rochester, Minnesota June 19, 2009 Mycobacterial Laboratory Science Update Nancy L. Wengenack, Ph.D. Associate Professor of Laboratory Medicine and Pathology Division of Clinical
TB Updates for the Physician Rochester, Minnesota June 19, 2009 Mycobacterial Laboratory Science Update Nancy L. Wengenack, Ph.D. Associate Professor of Laboratory Medicine and Pathology Division of Clinical
Anti-tuberculosis drug resistance in the world and rapid diagnosis of tuberculosis
 Anti-tuberculosis drug resistance in the world and rapid diagnosis of tuberculosis Chiyoji ABE acquired drug resistance primary drug resistance 10 10 HIV 1 3 INHRFP MDR-TB DOTSDirectly Observed Treatment,
Anti-tuberculosis drug resistance in the world and rapid diagnosis of tuberculosis Chiyoji ABE acquired drug resistance primary drug resistance 10 10 HIV 1 3 INHRFP MDR-TB DOTSDirectly Observed Treatment,
Public Health Mycobacteriology (TB) Laboratory Testing Services
 Public Health Mycobacteriology (TB) Laboratory Testing Services Gary Budnick Supervising Microbiologist Connecticut Department of Public Health Laboratory Branch Hartford, Connecticut Specimen Collection
Public Health Mycobacteriology (TB) Laboratory Testing Services Gary Budnick Supervising Microbiologist Connecticut Department of Public Health Laboratory Branch Hartford, Connecticut Specimen Collection
2 ND YEAR RESEARCH ELECTIVE RESIDENT S JOURNAL Volume X, Ellie R. Carmody. A. Background and Study Rationale.
 Operating characteristics and effectiveness of a screening algorithm for the detection of active tuberculosis in adult outpatients with HIV infection in Mozambique Ellie R. Carmody A. Background and Study
Operating characteristics and effectiveness of a screening algorithm for the detection of active tuberculosis in adult outpatients with HIV infection in Mozambique Ellie R. Carmody A. Background and Study
Towards Harmonization of Mycobacteriology in TB Trials. Study 31/ACTG 5349 Key Elements of Mycobacteriology Laboratory Procedures
 Study 31/ACTG 5349 Key Elements of Mycobacteriology Laboratory Procedures: Towards Harmonization of Mycobacteriology in TB Trials Title Author(s) Study 31/ACTG 5349 Key Elements of Mycobacteriology Laboratory
Study 31/ACTG 5349 Key Elements of Mycobacteriology Laboratory Procedures: Towards Harmonization of Mycobacteriology in TB Trials Title Author(s) Study 31/ACTG 5349 Key Elements of Mycobacteriology Laboratory
Polymerase chain reaction (PCR): Its comparison with conventional techniques for diagnosis of extra-pulmonary tubercular diseases
 2003 Indian Journal of Surgery www.indianjsurg.com Original Article Polymerase chain reaction (PCR): Its comparison with conventional techniques for diagnosis of extra-pulmonary tubercular diseases R.
2003 Indian Journal of Surgery www.indianjsurg.com Original Article Polymerase chain reaction (PCR): Its comparison with conventional techniques for diagnosis of extra-pulmonary tubercular diseases R.
Received 25 February 2009/Returned for modification 28 July 2009/Accepted 20 September 2009
 JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2009, p. 3635 3639 Vol. 47, No. 11 0095-1137/09/$12.00 doi:10.1128/jcm.00411-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Direct Detection
JOURNAL OF CLINICAL MICROBIOLOGY, Nov. 2009, p. 3635 3639 Vol. 47, No. 11 0095-1137/09/$12.00 doi:10.1128/jcm.00411-09 Copyright 2009, American Society for Microbiology. All Rights Reserved. Direct Detection
Comparison of the Xpert MTB/RIF Assay and Real-time PCR for the Detection of Mycobacterium tuberculosis
 Available online at www.annclinlabsci.org Annals of Clinical & Laboratory Science, vol. 45, no. 3, 2015 327 Comparison of the Xpert MTB/RIF Assay and Real-time PCR for the Detection of Mycobacterium tuberculosis
Available online at www.annclinlabsci.org Annals of Clinical & Laboratory Science, vol. 45, no. 3, 2015 327 Comparison of the Xpert MTB/RIF Assay and Real-time PCR for the Detection of Mycobacterium tuberculosis
Application of enzyme amplified mycobacterial DNA detection in the diagnosis of pulmonary & extra-pulmonary tuberculosis
 Indian J Med Res 118, December 2003, pp 224-228 Application of enzyme amplified mycobacterial DNA detection in the diagnosis of pulmonary & extra-pulmonary tuberculosis Vandana Tiwari, Amita Jain & R.
Indian J Med Res 118, December 2003, pp 224-228 Application of enzyme amplified mycobacterial DNA detection in the diagnosis of pulmonary & extra-pulmonary tuberculosis Vandana Tiwari, Amita Jain & R.
Int.J.Curr.Microbiol.App.Sci (2018) 7(5):
 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 7 Number 05 (2018) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2018.705.239
International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 7 Number 05 (2018) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2018.705.239
Devasahayam J. Christopher, DTCD DNB FRCP 1, Samuel G. Schumacher, MSc PG
 ERJ Express. Published on July 30, 2013 as doi: 10.1183/09031936.00103213 Performance of Xpert MTB/RIF on pleural tissue for the diagnosis of pleural tuberculosis Devasahayam J. Christopher, DTCD DNB FRCP
ERJ Express. Published on July 30, 2013 as doi: 10.1183/09031936.00103213 Performance of Xpert MTB/RIF on pleural tissue for the diagnosis of pleural tuberculosis Devasahayam J. Christopher, DTCD DNB FRCP
MOLECULAR DETECTION AND IDENTIFICATION OF MYCOBACTERIUM TUBERCULOSIS COMPLEX AND FOUR CLINICALLY IMPORTANT
 JCM Accepts, published online ahead of print on June 0 J. Clin. Microbiol. doi:./jcm.001- Copyright 0, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved. 1 MOLECULAR
JCM Accepts, published online ahead of print on June 0 J. Clin. Microbiol. doi:./jcm.001- Copyright 0, American Society for Microbiology and/or the Listed Authors/Institutions. All Rights Reserved. 1 MOLECULAR
Dr Francis Ogaro MTRH ELDORET
 Dr Francis Ogaro MTRH ELDORET TB in children often severe, disseminated and can progress rapidly and with poor outcome TB diagnosis in children has relied on clinical, imaging, microscopy and TST findings.
Dr Francis Ogaro MTRH ELDORET TB in children often severe, disseminated and can progress rapidly and with poor outcome TB diagnosis in children has relied on clinical, imaging, microscopy and TST findings.
(Mycobacterium abscessus)
 241 (Mycobacterium abscessus) 1 1 1 1,2 1,3 1,4 5 1 2 3 4 5 (Non-tuberculous mycobacterium, NTM) NTM Mycobacterium abscessus PFGE 16 (1) NTM Mycobacterium chelonae 270 CFU/ml (2) 15 NTM 0% 500 CFU/g 200
241 (Mycobacterium abscessus) 1 1 1 1,2 1,3 1,4 5 1 2 3 4 5 (Non-tuberculous mycobacterium, NTM) NTM Mycobacterium abscessus PFGE 16 (1) NTM Mycobacterium chelonae 270 CFU/ml (2) 15 NTM 0% 500 CFU/g 200
Appendix C. Recommendations for Counting Reported Tuberculosis Cases (Revised July 1997)
 Appendix C Recommendations for Counting Reported Tuberculosis Cases (Revised July 1997) Since publication of the Recommendations for Counting Reported Tuberculosis Cases 1 in January 1977, numerous changes
Appendix C Recommendations for Counting Reported Tuberculosis Cases (Revised July 1997) Since publication of the Recommendations for Counting Reported Tuberculosis Cases 1 in January 1977, numerous changes
Rapid PCR TB Testing Results in 68.5% Reduction in Unnecessary Isolation Days in Smear Positive Patients.
 Rapid PCR TB Testing Results in 68.5% Reduction in Unnecessary Isolation Days in Smear Positive Patients. Item Type Thesis Authors Patel, Ravikumar Publisher The University of Arizona. Rights Copyright
Rapid PCR TB Testing Results in 68.5% Reduction in Unnecessary Isolation Days in Smear Positive Patients. Item Type Thesis Authors Patel, Ravikumar Publisher The University of Arizona. Rights Copyright
Introduction. Diagnosis of extrapulmonaryand paediatric tuberculosis. Extrapulmonary tuberculosis EPTB SASCM WORKSHOP 2014/05/24
 Diagnosis of extrapulmonaryand paediatric tuberculosis AW Dreyer Centre for Tuberculosis NICD Introduction Part of the global efforts to control tuberculosis (TB) include improving case detection, especially
Diagnosis of extrapulmonaryand paediatric tuberculosis AW Dreyer Centre for Tuberculosis NICD Introduction Part of the global efforts to control tuberculosis (TB) include improving case detection, especially
Diagnosis of tuberculosis
 Diagnosis of tuberculosis Madhukar Pai, MD, PhD Assistant Professor, Epidemiology McGill University, Montreal, Canada madhukar.pai@mcgill.ca Global TB Case Detection A major concern 2.6 million new smear
Diagnosis of tuberculosis Madhukar Pai, MD, PhD Assistant Professor, Epidemiology McGill University, Montreal, Canada madhukar.pai@mcgill.ca Global TB Case Detection A major concern 2.6 million new smear
Hepatitis B Virus Genemer
 Product Manual Hepatitis B Virus Genemer Primer Pair for amplification of HBV Viral Specific Fragment Catalog No.: 60-2007-10 Store at 20 o C For research use only. Not for use in diagnostic procedures
Product Manual Hepatitis B Virus Genemer Primer Pair for amplification of HBV Viral Specific Fragment Catalog No.: 60-2007-10 Store at 20 o C For research use only. Not for use in diagnostic procedures
MYCOBACTERIOLOGY SERVICE MANUAL
 MYCOBACTERIOLOGY SERVICE MANUAL The Office of Laboratory Services (OLS) provides primary isolation and identification of Mycobacterium species in human diagnostic specimens. Reference specimens of AFB
MYCOBACTERIOLOGY SERVICE MANUAL The Office of Laboratory Services (OLS) provides primary isolation and identification of Mycobacterium species in human diagnostic specimens. Reference specimens of AFB
The clinical utility of PCR using the Cobas Amplicor MTB test for the diagnosis of pleural tuberculosis
 The clinical utility of PCR using the Cobas Amplicor MTB test for the diagnosis of pleural tuberculosis Jin Wook Moon Department of Medicine The Graduate School, Yonsei University The clinical utility
The clinical utility of PCR using the Cobas Amplicor MTB test for the diagnosis of pleural tuberculosis Jin Wook Moon Department of Medicine The Graduate School, Yonsei University The clinical utility
Diagnosis of tuberculosis in children
 Diagnosis of tuberculosis in children H Simon Schaaf Desmond Tutu TB Centre Department of Paediatrics and Child Health, Stellenbosch University, and Tygerberg Children s Hospital (TCH) Estimated TB incidence
Diagnosis of tuberculosis in children H Simon Schaaf Desmond Tutu TB Centre Department of Paediatrics and Child Health, Stellenbosch University, and Tygerberg Children s Hospital (TCH) Estimated TB incidence
Frances Morgan, PhD October 21, Comprehensive Care of Patients with Tuberculosis and Their Contacts October 19 22, 2015 Wichita, KS
 The Laboratory s Role in Caring for Patients Diagnosed with TB Frances Morgan, PhD October 21, 2015 Comprehensive Care of Patients with Tuberculosis and Their Contacts October 19 22, 2015 Wichita, KS EXCELLENCE
The Laboratory s Role in Caring for Patients Diagnosed with TB Frances Morgan, PhD October 21, 2015 Comprehensive Care of Patients with Tuberculosis and Their Contacts October 19 22, 2015 Wichita, KS EXCELLENCE
Incremental cost-effectiveness of the second Xpert MTB/RIF assay to detect Mycobacterium tuberculosis
 Original Article Incremental cost-effectiveness of the second Xpert MTB/RIF assay to detect Mycobacterium tuberculosis Guirong Wang, Shuqi Wang, Guanglu Jiang, Yuhong Fu, Yuanyuan Shang, Hairong Huang
Original Article Incremental cost-effectiveness of the second Xpert MTB/RIF assay to detect Mycobacterium tuberculosis Guirong Wang, Shuqi Wang, Guanglu Jiang, Yuhong Fu, Yuanyuan Shang, Hairong Huang
Original Article Evaluation of Xpert MTB/RIF in detection of pulmonary and extrapulmonary tuberculosis cases in China
 Int J Clin Exp Pathol 2017;10(4):4847-4851 www.ijcep.com /ISSN:1936-2625/IJCEP0045802 Original Article Evaluation of Xpert MTB/RIF in detection of pulmonary and extrapulmonary tuberculosis cases in China
Int J Clin Exp Pathol 2017;10(4):4847-4851 www.ijcep.com /ISSN:1936-2625/IJCEP0045802 Original Article Evaluation of Xpert MTB/RIF in detection of pulmonary and extrapulmonary tuberculosis cases in China
EVALUATION OF THE BACTEC MGIT 960 TB SYSTEM FOR RECOVERY
 Indian Journal of Medical Microbiology, (2009) 27(3): 217-21 Original Article EVALUATION OF THE BACTEC MGIT 960 TB SYSTEM FOR RECOVERY AND IDENTIFICATION OF MYCOBACTERIUM TUBERCULOSIS COMPLEX IN A HIGH
Indian Journal of Medical Microbiology, (2009) 27(3): 217-21 Original Article EVALUATION OF THE BACTEC MGIT 960 TB SYSTEM FOR RECOVERY AND IDENTIFICATION OF MYCOBACTERIUM TUBERCULOSIS COMPLEX IN A HIGH
Biology and Medicine
 eissn: 09748369 Diagnosis of pulmonary tuberculosis by smear microscopy and culture in a tertiary health care facility Biology and Medicine SI Khatib, MT Williamson, R Singh, JM Joshi Accepted: 28 th Feb
eissn: 09748369 Diagnosis of pulmonary tuberculosis by smear microscopy and culture in a tertiary health care facility Biology and Medicine SI Khatib, MT Williamson, R Singh, JM Joshi Accepted: 28 th Feb
Case Report Diagnostic Challenges of Tuberculous Lymphadenitis Using Polymerase Chain Reaction Analysis: A Case Study
 Case Reports in Infectious Diseases Volume 2015, Article ID 723726, 4 pages http://dx.doi.org/10.1155/2015/723726 Case Report Diagnostic Challenges of Tuberculous Lymphadenitis Using Polymerase Chain Reaction
Case Reports in Infectious Diseases Volume 2015, Article ID 723726, 4 pages http://dx.doi.org/10.1155/2015/723726 Case Report Diagnostic Challenges of Tuberculous Lymphadenitis Using Polymerase Chain Reaction
Mycobacterial peritonitis: difference between non-tuberculous mycobacteria and Mycobacterium tuberculosis
 ORIGINAL ARTICLE BACTERIOLOGY Mycobacterial peritonitis: difference between non-tuberculous mycobacteria and Mycobacterium tuberculosis C.-C. Shu 1,2, J.-T. Wang 1, J.-Y. Wang 1, C.-J. Yu 1, L.-N. Lee
ORIGINAL ARTICLE BACTERIOLOGY Mycobacterial peritonitis: difference between non-tuberculous mycobacteria and Mycobacterium tuberculosis C.-C. Shu 1,2, J.-T. Wang 1, J.-Y. Wang 1, C.-J. Yu 1, L.-N. Lee
TB In Detroit 2011* Early TB: Smudge Sign. Who is at risk for exposure to or infection with TB? Who is at risk for TB after exposure or infection?
 Those oral antibiotics are just not working! Inpatient Standards of Care & Discharge Planning S/He s in the Hospital: Now What Do I Do? Dana G. Kissner, MD TB Intensive Workshop, Lansing, MI 2012 Objectives:
Those oral antibiotics are just not working! Inpatient Standards of Care & Discharge Planning S/He s in the Hospital: Now What Do I Do? Dana G. Kissner, MD TB Intensive Workshop, Lansing, MI 2012 Objectives:
Mycobacteriology William H. Benjamin, Jr.
 Mycobacteriology William H. Benjamin, Jr. William H. Benjamin, PhD Department of Pathology UAB 1 Mycobacteria sp. Acid Fast Bacilli (AFB) Mycolic acids (C78-91) Waxes Obligate aerobes Slow growing days
Mycobacteriology William H. Benjamin, Jr. William H. Benjamin, PhD Department of Pathology UAB 1 Mycobacteria sp. Acid Fast Bacilli (AFB) Mycolic acids (C78-91) Waxes Obligate aerobes Slow growing days
The MycoDDR Product Line is Essential for Mycobacteria Survival and Recovery
 The MycoDDR Product Line is Essential for Mycobacteria Survival and Recovery Crider, B.J., Neary, B.P., Bauman, S.K. Abstract Tuberculosis (TB) is a serious disease that affects a significant portion of
The MycoDDR Product Line is Essential for Mycobacteria Survival and Recovery Crider, B.J., Neary, B.P., Bauman, S.K. Abstract Tuberculosis (TB) is a serious disease that affects a significant portion of
MYCOBACTERIOLOGY INTRODUCTION RANGE OF LABORATORY SERVICES
 MYCOBACTERIOLOGY INTRODUCTION The Central Tuberculosis Laboratory (CTBL) of the Department of Pathology provides laboratory diagnosis of tuberculosis (TB) and other mycobacterial diseases for all hospitals,
MYCOBACTERIOLOGY INTRODUCTION The Central Tuberculosis Laboratory (CTBL) of the Department of Pathology provides laboratory diagnosis of tuberculosis (TB) and other mycobacterial diseases for all hospitals,
Pediatric Drug-Resistant TB in China
 Pediatric Drug-Resistant TB in China Shuihua Lu,Tao Li Shanghai Public Health Clinical Center Jan.18,2013 A MDR-TB CASE A four and a half years old boy, spent 4 yeas of his life in hospital. His childhood
Pediatric Drug-Resistant TB in China Shuihua Lu,Tao Li Shanghai Public Health Clinical Center Jan.18,2013 A MDR-TB CASE A four and a half years old boy, spent 4 yeas of his life in hospital. His childhood
Diagnosis of drug resistant TB
 Diagnosis of drug resistant TB Megan Murray, MD, ScD Harvard School of Public Health Brigham and Women s Hospital Harvard Medical School Broad Institute Global burden of TB 9 million new cases year 2 million
Diagnosis of drug resistant TB Megan Murray, MD, ScD Harvard School of Public Health Brigham and Women s Hospital Harvard Medical School Broad Institute Global burden of TB 9 million new cases year 2 million
Tuberculosis Tools: A Clinical Update
 Tuberculosis Tools: A Clinical Update CAPA Conference 2014 JoAnn Deasy, PA-C. MPH, DFAAPA jadeasy@sbcglobal.net Adjunct Faculty Touro PA Program Learning Objectives Outline the pathogenesis of active pulmonary
Tuberculosis Tools: A Clinical Update CAPA Conference 2014 JoAnn Deasy, PA-C. MPH, DFAAPA jadeasy@sbcglobal.net Adjunct Faculty Touro PA Program Learning Objectives Outline the pathogenesis of active pulmonary
Detect TB. Accurately. Easily.
 Detect TB. Accurately. Easily. Fast and robust test results with TB-LAMP Molecular DX Smear microscopy misses nearly every second positive TB case We need new tests to rapidly diagnose people with TB;
Detect TB. Accurately. Easily. Fast and robust test results with TB-LAMP Molecular DX Smear microscopy misses nearly every second positive TB case We need new tests to rapidly diagnose people with TB;
A retrospective evaluation study of diagnostic accuracy of Xpert MTB/RIF assay, used for detection of Mycobacterium tuberculosis in Greece
 Örebro University School of Health and Medical Science Biomedical Laboratory Science Programme 180 credits Degree project in biomedical laboratory science, advanced course, 15 credits May 21, 2015 A retrospective
Örebro University School of Health and Medical Science Biomedical Laboratory Science Programme 180 credits Degree project in biomedical laboratory science, advanced course, 15 credits May 21, 2015 A retrospective
2008/7/21. An Overview. National Taiwan University College of Medicine 西元前 年. 木乃伊 (Nesperhan, priest of Amun)
 Rapid Diagnosis of Tuberculosis An Overview Po-Ren Hsueh National Taiwan University College of Medicine 埃及時代 西元前 3700-1000 年 土偶 木乃伊 (Nesperhan, priest of Amun) 1 Dixon B. Lancet Infect Dis 2007;7:444-5.
Rapid Diagnosis of Tuberculosis An Overview Po-Ren Hsueh National Taiwan University College of Medicine 埃及時代 西元前 3700-1000 年 土偶 木乃伊 (Nesperhan, priest of Amun) 1 Dixon B. Lancet Infect Dis 2007;7:444-5.
Multi-Site Performance Summary of. AEROSPRAY TB SLIDE STAINER/CYTOCENTRIFUGE (Model 7722)
 Multi-Site Performance Summary of AEROSPRAY TB SLIDE STAINER/CYTOCENTRIFUGE (Model 7722) Abstract The new Aerospray TB Slide Stainer/Cytocentrifuge - Model 7722 (ELITechGroup Inc., www.elitechgroup.com)
Multi-Site Performance Summary of AEROSPRAY TB SLIDE STAINER/CYTOCENTRIFUGE (Model 7722) Abstract The new Aerospray TB Slide Stainer/Cytocentrifuge - Model 7722 (ELITechGroup Inc., www.elitechgroup.com)
Nontuberculous Mycobacterial Lung Disease
 Non-TB Mycobacterial Disease Jeffrey P. Kanne, MD Nontuberculous Mycobacterial Lung Disease Jeffrey P. Kanne, M.D. Consultant Disclosures Perceptive Informatics Royalties (book author) Amirsys, Inc. Wolters
Non-TB Mycobacterial Disease Jeffrey P. Kanne, MD Nontuberculous Mycobacterial Lung Disease Jeffrey P. Kanne, M.D. Consultant Disclosures Perceptive Informatics Royalties (book author) Amirsys, Inc. Wolters
Comparison of Four Decontamination Methods for Recovery
 JOURNAL OF CLINICAL MICROBIOLOGY, Feb. 1993, p. 302-306 Vol. 31, No. 2 0095-1137/93/020302-05$02.00/0 Copyright 1993, American Society for Microbiology Comparison of Four Decontamination Methods for Recovery
JOURNAL OF CLINICAL MICROBIOLOGY, Feb. 1993, p. 302-306 Vol. 31, No. 2 0095-1137/93/020302-05$02.00/0 Copyright 1993, American Society for Microbiology Comparison of Four Decontamination Methods for Recovery
Appendix B. Recommendations for Counting Reported Tuberculosis Cases (Revised July 1997)
 Appendix B Recommendations for Counting Reported Tuberculosis Cases (Revised July 1997) Since publication of the Recommendations for Counting Reported Tuberculosis Cases 1 in January 1977, numerous changes
Appendix B Recommendations for Counting Reported Tuberculosis Cases (Revised July 1997) Since publication of the Recommendations for Counting Reported Tuberculosis Cases 1 in January 1977, numerous changes
M. avium M. tuberculosis. number of vials Days
 252 BJID 2001; 5 (August) Detection of Mycobacteria in the Bloodstream of Patients With Acquired Immunodeficiency Syndrome in a University Hospital in Brazil Carmen Paz Oplustil, Olavo H.M. Leite, Marilia
252 BJID 2001; 5 (August) Detection of Mycobacteria in the Bloodstream of Patients With Acquired Immunodeficiency Syndrome in a University Hospital in Brazil Carmen Paz Oplustil, Olavo H.M. Leite, Marilia
Keywords: tuberculosis; laboratory diagnosis; nontuberculous
 J Clin Pathol 2000;53:727 732 727 Leaders Public Health Laboratory Service Mycobacterium Reference Unit, Dulwich Public Health Laboratory and Department of Microbiology, Guy s, King s and St Thomas s School
J Clin Pathol 2000;53:727 732 727 Leaders Public Health Laboratory Service Mycobacterium Reference Unit, Dulwich Public Health Laboratory and Department of Microbiology, Guy s, King s and St Thomas s School
HIDDEN IN PLAIN SITE:
 HIDDEN IN PLAIN SITE: MYCOBACTERIUM ON THE ROUTINE BENCH Christina Partington MT(ASCP) ACL Laboratory 1 Introduction The importance of the possibility of AFB appearing in a routine culture. How to recognize
HIDDEN IN PLAIN SITE: MYCOBACTERIUM ON THE ROUTINE BENCH Christina Partington MT(ASCP) ACL Laboratory 1 Introduction The importance of the possibility of AFB appearing in a routine culture. How to recognize
AFB Identification Texas Approach
 AFB Identification Texas Approach Ken Jost Texas Department of State Health Services 6th National Conference on Laboratory Aspects of TB June 21, 2010 DSHS-Austin TB Lab Customers & Samples Year 2009 175
AFB Identification Texas Approach Ken Jost Texas Department of State Health Services 6th National Conference on Laboratory Aspects of TB June 21, 2010 DSHS-Austin TB Lab Customers & Samples Year 2009 175
Research Methods for TB Diagnostics. Kathy DeRiemer, PhD, MPH University of California, Davis Shanghai, China: May 8, 2012
 Research Methods for TB Diagnostics Kathy DeRiemer, PhD, MPH University of California, Davis Shanghai, China: May 8, 2012 Overview Why do we need good TB diagnostics? What works? What doesn t work? How
Research Methods for TB Diagnostics Kathy DeRiemer, PhD, MPH University of California, Davis Shanghai, China: May 8, 2012 Overview Why do we need good TB diagnostics? What works? What doesn t work? How
Tuberculosis Elimination: The Role of the Infection Preventionist
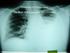 Tuberculosis Elimination: The Role of the Infection Preventionist Preface: What Happens when Health Care Professionals are not familiar with TB? A 15 year old student was diagnosed with highly infectious
Tuberculosis Elimination: The Role of the Infection Preventionist Preface: What Happens when Health Care Professionals are not familiar with TB? A 15 year old student was diagnosed with highly infectious
For Research Use Only Ver
 INSTRUCTION MANUAL Quick-DNA/RNA Viral MagBead Catalog Nos. R2140 & R2141 Highlights High-throughput, magnetic-bead based purification of viral DNA and RNA from plasma, serum, urine, cell culture media,
INSTRUCTION MANUAL Quick-DNA/RNA Viral MagBead Catalog Nos. R2140 & R2141 Highlights High-throughput, magnetic-bead based purification of viral DNA and RNA from plasma, serum, urine, cell culture media,
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
Diagnosis of TB: Laboratory Ken Jost Tuesday April 9, 2013
 TB Nurse Case Management San Antonio, Texas April 9-11, 2013 Diagnosis of TB: Laboratory Ken Jost Tuesday April 9, 2013 Ken Jost has the following disclosures to make: No conflict of interests No relevant
TB Nurse Case Management San Antonio, Texas April 9-11, 2013 Diagnosis of TB: Laboratory Ken Jost Tuesday April 9, 2013 Ken Jost has the following disclosures to make: No conflict of interests No relevant
