Introduction to EU 'SoHO' Sector Screening Meeting with the Republic of Serbia December 5, 2014 Directorate General for Health and Consumers Unit D4
|
|
|
- Cecil Doyle
- 6 years ago
- Views:
Transcription
1 Introduction to EU 'SoHO' Sector Screening Meeting with the Republic of Serbia December 5, 2014 Directorate General for Unit D4
2 1. Introduction: SoHO & the Legal Basis 2. The Legislative Framework 3. Blood / Tissue & Cells / 4. The SoHO Sector and Enlargement 2
3 1. Introduction 1.1 Substances of Human Origin Blood and blood components Tissues and Cells 3
4 1. Introduction 1.2 SoHO Landscape Flow Donor SoHO Recipient Process Donation/ Collection Test Process Store Distribute Human application Actors Procurement site (PS) Blood/Tissue Establishment(s) (TE) Site of Human Application (SiHA) / Transplant Center Oversight National Competent Authority (NCA) 4
5 1. Introduction DONOR (female) LIVING DEAD 1 Donor Donation Episode 1 CORD BLOOD Donation Episode 2 AMNION Donation Episode 3 BRAIN DEATH Recipient 1 Recipient 2 ORGANS TISSUES KIDNEY LIVER PANCREATIC ISLETS CORNEA CV MUSCULO SKELETAL Recipient 3 Right Lobule Left Lobule TE 1 Hepatocites isolation and preservation Recipient 6 TE 2 TE 3 Recipient 7 Recipient 9 Recipient 8 Recipient 10 Recipient 4 Recipient 5 TE: Tissue Establishment R Femur L Femur R Tibia L Tibia R Achiles R Patellar R Humer TE 4 Cleaned Decontaminated Frozen 80ºC Pharma Comp 2 Advanced therapy TE 5 New process T. Plateau Diaphisis PharmaComp 1 Pooled Recipient 11 Recipient 12 Recipient 13 Recipient 14 Recipient 15 Recipients? >100 Recipient 16 Recipient 50 recipients 5 5
6 1. Introduction: SoHO & the Legal Basis 2. The Legislative Framework 3. Blood / Tissue & Cells / 4. The SoHO Sector and Enlargement 6
7 2. The Legislative Framework 2.1 The legal basis - EU action in health The Treaty of the Functioning of the European Union Article 168 (former article 152 TEC) A high level of human health protection shall be ensured in the definition and implementation of all Union policies and activities Health largely falls under the subsidiarity principle Union action shall complement national policies Foster cooperation between Member States (MS) and third countries Exception to the subsidiarity principle: some common safety concerns, including substances of human origin 7
8 2. The Legislative Framework 2.1 The legal basis - SoHO TFEU - Article 168 4(a) "Measures setting high standards of quality and safety of organs and substances of human origin, blood and blood derivatives; these measures shall not prevent any Member State from maintaining or introducing more stringent protective measures." -- Clear Mandate for EU-level action concerning quality & safety of SoHO -- Parallel approach across 3 SOHO segments Blood, T&Cs and 8
9 2. The Legislative Framework EU 'SoHO' Legislation DIRECTIVE 2002/98/EC setting standards of Q&S for the collection, testing, processing, storage and distribution of human blood and blood components DIRECTIVE 2004/23/EC setting standards of Q&S for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells DIRECTIVE 2010/53/EU on standards of Q&S of human organs intended for transplantation 9
10 2. The Legislative Framework EU legislation on SoHO Donation Minimum standards Q&S Distribution Obligations of MS Competent Authorities Donor selection and evaluation Provisions on Quality & Safety Exchange of information, reports, penalties Traceability and SARE reporting Data protection 10
11 2. The Legislative Framework EU 'SoHO' Legislation - Implementing Directives 2004, 2005 COMMISSION DIRECTIVE 2005/61/EC traceability requirements and notification of SARE haemovigilance 2006 COMMISSION DIRECTIVE 2006/86/EC traceability requirements, biovigilance & certain technical requirements [ ] 2012 Implementing Directive 2012/25/EU information procedures for the exchange, between MS, of human organs (transposition deadline April 2014) 11 traceability, biovigilance
12 2. The Legislative Framework 2.3 Framework in Practice: 3 levels of requirements Donation /Collection n Test Process Store Distribute Human application 1 Actors Selection/deferral, consent HIV, Hepatitis B, Hepatitis C.. Quality requirements 2 National Competent Authorities Oversight: vigilance, traceability, accreditation, inspection 3 European Commission EU-level support: rapid alerts, traceability system 12
13 2. The Legislative Framework 2.4 Limits to SoHO Legislation Donation Collect / Procure Testing Preserve Store Process Human application ( > 95%) Transfusion Transplantation Blood, T/Cs Legislation Pharmaceutical Legislation Research Transport Manufacturing Medicinal Products (<5%) 13
14 1. Introduction: SoHO & the Legal Basis 2. The Legislative Framework 3. Blood / Tissue & Cells / 4. The SoHO Sector and Enlargement 14
15 3733 establishments and hospital blood banks (29 countries reporting) units issued (29 countries reporting) units transfused (17 countries reporting) recipients of blood components (16 countries reporting) Data from the 2011 serious adverse reactions and events reports - EU MS, Liechtenstein and Norway 15
16 3.1 Blood and blood components 'Mother' Directive 2002/98/EC - Sets standards for the quality and safety of the; collection, testing, processing, storage and distribution of human blood and blood components Implementing Directives: -2004/33/EC technical requirements for blood -2005/61/EC traceability / notification of SAREs -2005/62/EC quality system for blood establishments 16
17 3.1 Blood and blood components Mother Directive Chapter II OBLIGATIONS ON MEMBER STATES AUTHORITIES - Licensing of establishments - Inspections & Control measures Chapter III PROVISIONS FOR BLOOD ESTABLISHMENTS - Responsible Person - Personnel / Training Chapter IV QUALITY MANAGEMENT -Quality System for BEs -Documentation -Record Keeping Chapter V HAEMOVIGILANCE -Traceability - Notification of SAREs Chapter VI PROVISIONS FOR THE QUALITY AND SAFETY OF BLOOD AND BLOOD COMPONENTS - Info to/from Donors -Donor Eligibility / Examination -VUD Chapter VII DATA PROTECTION Chapter VIII EXCHANGE OF INFORMATION, REPORTS AND PENALTIES 17
18 3.1 Blood and blood components Mother Directive CHAPTER II - OBLIGATIONS ON MEMBER STATES AUTHORITIES Article 5 Designation, authorisation, accreditation or licensing of blood establishments 1.Member States shall ensure that activities relating to the collection and testing of human blood and blood components, whatever their intended purpose, and to their preparation, storage, and distribution when intended for transfusion, are undertaken only by the blood establishments which have been designated, authorised, accredited or licensed by the competent authority for that purpose. 3. The competent authority, having verified whether the blood establishment complies with the requirements set out in this Directive, shall indicate to the blood establishment which activities it may undertake and which conditions apply. Article 8 Inspection and control measures 1.Member States shall ensure that the competent authority organise inspections and appropriate control measures in Blood establishments to ensure that the requirements of this Directive are complied with. 2. Inspection and control measures shall be organised by the competent authority on a regular basis. The interval between two inspections and control measures shall not exceed two years. [..] 18
19 1. Introduction: SoHO & the Legal Basis 2. The Legislative Framework 3. Blood / Tissue & Cells / 4. The SoHO Sector and Enlargement 19
20 TISSUES Bone, tendons, cartilages Are procured from deceased donors. As bone tissue has the ability to completely regenerate bone grafts, it is used to repair bone fractures. Tendons are used mostly for reconstruction in the knee. Skin Skin can be either procured from deceased or from living donors. Skin grafts are mainly used for the treatment of burns. Cardiovascular tissues Are procured from deceased donors, they are mainly used to replace blood vessels or aortic and pulmonary valves for patients with congenital heart defects. Corneas Corneas procured from deceased donors are used in ocular surgery. 20
21 CELLS Haematopoietic stem cells (HSC) HSC are procured from living donors (bone marrow, peripheral blood stem cells and cord blood donation) and mainly used to treat diseases of the blood, bone marrow, or certain types of cancer. Pancreatic islets Pancreatic islets are isolated from the pancreas of a deceased organ donor and can be used to treat type 1 diabetes. Hepatocytes Hepatocytes are cells that can be used for treatment of liver disease. Reproductive cells Eggs and sperm, are used in the field of medically assisted reproductive technology for treating infertility. 21
22 Procurement organisations Reporting countries Number of POs - traditional tissues Number of POs - HPC Number of POs - ART Number of POs- TC for ATMP Number of qualified laboratories for donor testing 27 MS + 2 EEA Procurement Organisations (POs)
23 Tissue establishments Total number of authorised TEs: 2047 (27 MS + 1 EEA countries; 2011 data)
24 Tissue establishments Private and public TEs (24 MS + 1 EEA country; 2011 data) 24
25 3.2 Tissues and Cells Legislation 'Mother' Directive 2004/23/EC - Sets standards for the quality and safety of the; collection, testing, processing, preservation, storage and distribution of human tissues and cells Implementing Directives: -2006/17/EC technical requirements for donation, procurement and testing -2006/86/EC traceability, notification of SAREs and technical requirements for coding, processing, preservation, storage and distribution 25
26 3.2 Tissues and Cells Legislation Mother directive Chapter II - Obligations on NCA s Supervision of procurement Accreditation TE s and preparation processes Inspection and control Traceability and SAR/E Import/export Register of TEs Chapter III - Donor selection & evaluation V.U.D., compensation and promotion Consent Data protection and confidentiality Selection, evaluation and procurement Chapter V, VI coding, reports, penalties consultation of committees Chapter IV - Quality and safety provisions Quality management Responsible person/personnel Reception, processing storage, labelling, packaging, distribution 3 rd parties 26
27 3.2 T&Cs Legislation Obligations on establishments Process Donation Collection Testing Preserve Store Human application Transport Directive 2004/23/EC, Chapter IV (Quality and Safety T&C) Quality Management Responsible person and Personnel T&C reception, processing, storage, distribution Labelling 3rd parties Commission Directive 2006/17/EC technical requirements for donation, procurement and testing 27
28 3.2 T&Cs Legislation Obligations on Member States Process T.E. 1 Donation Collection Testing T.E. 2 Preserve Donation Collection Testing T.E. Store Transport Preserve Donation Collection Testing Human application Process Store Transport Preserve Human application Process Store Transport Human application Directive 2004/23/EC, Chapter II (Obligations CA s) Directive 2004/23/EC, Chapter III (Donation and selection) Supervision of procurement V.U.D., compensation and promotion Accreditation TE s and preparation processes Consent Inspection and control Data protection and confidentiality Traceability and SAR/E (+ Comm Dir 2006/86/EC) Selection, evaluation and procurement Import/export Directive 2004/23/EC, Chapter V: coding, reports, penalties Register of TE s 28
29 T.E. 1 T.E. 2 Do na tio n Colle ction T.E. Do na tio n Te sti ng Colle ction Do na tio n Pr es erv e Te sti ng Colle ction Pr oc es s St or e Tr an sp ort Pr es erv e Te sti ng Human applicati on Pr oc es s St or e Tr an sp ort Pr es erv e Human applicati on Pr oc es s St or e Tr an sp ort Human applicati on T.E. 1 T.E. 2 Do na tio n Colle ction Do na tio n T.E. Te sti ng Colle ction Do na tio n Pr es erv e Te sti ng Colle ction Pr oc es s St or e Tr an sp ort Pr es erv e Te sti ng Human applicati on Pr oc es s St or e Tr an sp ort Pr es erv e Human applicati on Pr oc es s St or e Tr an sp ort Human applicati on T.E. 1 T.E. 2 Do na tio n Colle ction Do na tio n T.E. Te sti ng Colle ction Do na tio n Pr es erv e Te sti ng Colle ction Pr oc es s St or e Tr an sp ort Pr es erv e Te sti ng Human applicati on Pr oc es s St or e Tr an sp ort Pr es erv e Human applicati on Pr oc es s St or e Tr an sp ort Human applicati on 3. Blood / T&Cs / 3.2 T&Cs Legislation Obligations on the Commission M.S.1 M.S.2 M.S.27 Harmonised technical requirements (with Consultative Committee) Donation, procurement and testing (CD 2006/17/EC) Autorisation of TE s and processes (CD 2006/83/EC) Notification of SAR/E (CD 2006/83/EC) Guidelines for inspections Communication of SAR/E between CA s and EC Traceability: EU coding system Monitor and where needed enforce national implementation 29
30 Biovigilance - RATC Launched on 1 February 2013 Web based informatics tool developed in-house Used only for alerts related to human tissues or cells circulated between Member States To be used in parallel with existing national vigilance systems Alerts issued for Q&S defects and / or IFA 30
31 Biovigilance - RATC 31
32 1. Introduction: SoHO & the Legal Basis 2. The Legislative Framework 3. Blood / Tissue & Cells / 4. The SoHO Sector and Enlargement 32
33 Deceased-donors Heart Heart transplantation is performed on patients with endstage heart failure or severe coronary artery disease. Lung Extends life expectancy and enhances the quality of life for end-stage pulmonary patients. Pancreas A pancreas transplant involves implanting a healthy pancreas 33 (one that can produce insulin) into a person who has diabetes.
34 Deceased & living donors Kidney Are the most frequently transplanted organs. Extends life expectancy and enhances the quality of life of a patient with endstage renal disease. Liver Liver transplantation is the replacement of a diseased liver in case of end-stage liver disease and acute liver failure. 34
35 ORGAN TRANSPLANTS IN THE EU Source: Council of Europe / ONT 2014 Newsletter 35
36 DECEASED ORGAN DONORS Source: 2014 Transplant Newsletter (Council of Europe / ONT) 36
37 3.2 Legislation Mother Directive 2010/53/EC - Sets standards for quality and safety of human organs intended for transplantation I. Scope, objectives and definitions II. Quality and safety of organs National Quality Programmes Organ procurement, procurement organisations, transplantation centres Transport of organs Organ and donor characterisation Traceability Reporting of serious adverse events and reactions III. Donor and recipient protection and donor selection and evaluation Unpaid and Voluntary Donation Quality and safety aspects of living donation Data protection IV. National oversight authority or authorities V. Organ Exchange with third countries VI. General provisions 37
38 3.2 Legislation EU Action Plan 3 2 Increasing organ availability Make transplantation systems more efficient and accessible 1 Improve quality and safety EU LEGAL FRAMEWORK 10 PRIORITY ACTIONS ( ) 38
39 EU ACTION PLAN OBJECTIVES Increase Organ Availability Enhance Efficiency and Accessibility of Transplantation Systems Quality and Safety 10 PRIORITY ACTIONS 1 transplant coordinators 2 quality improvement programmes 3 living donation programmes 4 communication skills of professionals 5 information on citizens rights 6 7 EU-wide agreements 8 interchange of organs 6 enhance organisational models 9 evaluation of post-transplant results 10 common accreditation system Working Groups Tx coordinators Living donation Projects Training Tx coordinators Intensive Care Awareness Follow-up registers Interchange of organs Twinning Involves National Authorities, as well as health professionals 39
40 1. Introduction: SoHO & the Legal Basis 2. The Legislative Framework 3. Blood / Tissue & Cells / 4. The SoHO Sector and Enlargement 40
41 4. Commission Support Further use of funding mechanisms such as TAIEX for; Workshops Expert missions Study visits The aim of TAIEX (Technical Assistance and Information Exchange) is to fund short-term peer-to-peer technical assistance, advice and training. Use of IPA projects in Candidate Countries Non-financial support knowledge-sharing etc Participation of Candidate Countries in SoHO CA Expert Group meetings 41
42 Thank you Questions? 42
European actions in the field of transplantation & blood transfusion
 European actions in the field of transplantation & blood transfusion Andrzej Rys Director Directorate Health Systems and Products Madrid, 27 June 2013 1 Blood Tissues and cells Organs 2 Blood transfusion
European actions in the field of transplantation & blood transfusion Andrzej Rys Director Directorate Health Systems and Products Madrid, 27 June 2013 1 Blood Tissues and cells Organs 2 Blood transfusion
European activities in the field of Human Substances - Organ Donation and Transplantation
 European activities in the field of Human Substances - Organ Donation and Transplantation Journalist Workshop 9 October 2012 Hélène Le Borgne, Policy Officer for Organ donation & transplantation, Team
European activities in the field of Human Substances - Organ Donation and Transplantation Journalist Workshop 9 October 2012 Hélène Le Borgne, Policy Officer for Organ donation & transplantation, Team
REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
 EUROPEAN COMMISSION Brussels, 17.6.011 COM(011) 35 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
EUROPEAN COMMISSION Brussels, 17.6.011 COM(011) 35 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
REPUBLIC OF LITHUANIA LAW ON DONATION AND TRANSPLANTATION OF HUMAN TISSUES, CELLS AND ORGANS. 19 November 1996 No I-1626 Vilnius
 REPUBLIC OF LITHUANIA LAW ON DONATION AND TRANSPLANTATION OF HUMAN TISSUES, CELLS AND ORGANS 19 November 1996 No I-1626 Vilnius (As last amended on 12 May 2016 No XII-2344) CHAPTER I GENERAL PROVISIONS
REPUBLIC OF LITHUANIA LAW ON DONATION AND TRANSPLANTATION OF HUMAN TISSUES, CELLS AND ORGANS 19 November 1996 No I-1626 Vilnius (As last amended on 12 May 2016 No XII-2344) CHAPTER I GENERAL PROVISIONS
Transparency, Equity, Ethics: access to care. quality of life. Improving. and patients. An Agency of the French Ministry of Health
 An Agency of the French Ministry of Health Transparency, Equity, Ethics: Improving access to care and patients quality of life AgenceBiomedecine_Plaquette_GB.indd 1 25/08/14 12:15 Contents P.1 Agence de
An Agency of the French Ministry of Health Transparency, Equity, Ethics: Improving access to care and patients quality of life AgenceBiomedecine_Plaquette_GB.indd 1 25/08/14 12:15 Contents P.1 Agence de
EUROPEAN COMMISSION DIRECTORATE GENERAL FOR HEALTH AND FOOD SAFETY
 Ref. Ares(2016)5909621-13/10/2016 EUROPEAN COMMISSION DIRECTORATE GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical products: quality, safety
Ref. Ares(2016)5909621-13/10/2016 EUROPEAN COMMISSION DIRECTORATE GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical products: quality, safety
Accreditation of BMT units & EU Regulations
 Accreditation of BMT units & EU Regulations April 1, 2008 EBMT Annual Meeting Workshop: EU regulation/standardisation of cellular therapy Presentation: JACIE: aims and current activities EU regulation
Accreditation of BMT units & EU Regulations April 1, 2008 EBMT Annual Meeting Workshop: EU regulation/standardisation of cellular therapy Presentation: JACIE: aims and current activities EU regulation
Haemovigilance in Europe: What do health authorities expect from haemovigilance?
 Haemovigilance in Europe: What do health authorities expect from haemovigilance? Deirdre Fehily Substances of Human Origin Team Directorate General for and Food Safety Unit B4 Products: quality, safety
Haemovigilance in Europe: What do health authorities expect from haemovigilance? Deirdre Fehily Substances of Human Origin Team Directorate General for and Food Safety Unit B4 Products: quality, safety
COMMISSION OF THE EUROPEAN COMMUNITIES. Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 8.12.2008 COM(2008) 818 final 2008/0238 (COD) Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on standards of quality and safety
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 8.12.2008 COM(2008) 818 final 2008/0238 (COD) Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL on standards of quality and safety
EU BLOOD AND TISSUES DIRECTIVES
 10th European Haemovigilance Seminar (EHS) February 28 Frankfurt/Main, Germany EU BLOOD AND TISSUES DIRECTIVES European Commission Health and Consumer Protection Directorate General Public Health and Risk
10th European Haemovigilance Seminar (EHS) February 28 Frankfurt/Main, Germany EU BLOOD AND TISSUES DIRECTIVES European Commission Health and Consumer Protection Directorate General Public Health and Risk
EUROPEAN UNION. Brussels, 29 June 2010 (OR. en) 2008/0238 (COD) PE-CONS 19/1/10 REV 1 SAN 114 CODEC 431
 EUROPEAN UNION THE EUROPEAN PARLIAMT THE COUNCIL Brussels, 29 June 2010 (OR. en) 2008/0238 (COD) PE-CONS 19/1/10 REV 1 SAN 114 CODEC 431 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: DIRECTIVE OF THE EUROPEAN
EUROPEAN UNION THE EUROPEAN PARLIAMT THE COUNCIL Brussels, 29 June 2010 (OR. en) 2008/0238 (COD) PE-CONS 19/1/10 REV 1 SAN 114 CODEC 431 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: DIRECTIVE OF THE EUROPEAN
Human tissues and cells annual report 2011
 Human tissues and cells annual report 2011 2012 Human tissues and cells Annual report Danish Health and Medicines Authority, 2012. This publication can be freely cited with a clear indication of source.
Human tissues and cells annual report 2011 2012 Human tissues and cells Annual report Danish Health and Medicines Authority, 2012. This publication can be freely cited with a clear indication of source.
EUROPEAN UNION. Brussels, 15 October 2007 (OR. en) 2005/0227 (COD) PE-CONS 3627/07 MI 149 ECO 83 SAN 123 CODEC 621
 EUROPEAN UNION THE EUROPEAN PARLIAMT Brussels, 15 October 2007 (OR. en) THE COUNCIL 2005/0227 (COD) PE-CONS 3627/07 MI 149 ECO 83 SAN 123 CODEC 621 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: Regulation
EUROPEAN UNION THE EUROPEAN PARLIAMT Brussels, 15 October 2007 (OR. en) THE COUNCIL 2005/0227 (COD) PE-CONS 3627/07 MI 149 ECO 83 SAN 123 CODEC 621 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: Regulation
Competent Authorities on Substances of Human Origin Expert Group (CASoHO E01718) Meeting of the Competent Authorities on Blood and Blood Components
 Ref. Ares(2015)3569906-31/08/2015 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Directorate D - Health systems and products D4 Substances of Human Origin and Tobacco Control Competent
Ref. Ares(2015)3569906-31/08/2015 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Directorate D - Health systems and products D4 Substances of Human Origin and Tobacco Control Competent
Organ Donation. United States and European Union Perspective
 Organ Donation United States and European Union Perspective Carina Sokalski - Gonzalo Perez SANIT- December 14, 2004 Two Types of Organ Donation Deceased Organ Donation The majority of organ donors are
Organ Donation United States and European Union Perspective Carina Sokalski - Gonzalo Perez SANIT- December 14, 2004 Two Types of Organ Donation Deceased Organ Donation The majority of organ donors are
15. Procuring, processing and transporting gametes and
 15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
15. Procuring, processing and transporting gametes and embryos Version 6.0 On this page: : Extracts from the HFE Act Directions HFEA guidance: Documented procedures: general Patient selection and procurement
This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents
 2007R1394 EN 02.07.2012 001.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 1394/2007 OF THE EUROPEAN
2007R1394 EN 02.07.2012 001.001 1 This document is meant purely as a documentation tool and the institutions do not assume any liability for its contents B REGULATION (EC) No 1394/2007 OF THE EUROPEAN
Integrating and strengthening the European research area *
 P5_TA(2003)0506 Integrating and strengthening the European research area * European Parliament legislative resolution on the proposal for a Council decision amending Decision 2002/834/EC on the specific
P5_TA(2003)0506 Integrating and strengthening the European research area * European Parliament legislative resolution on the proposal for a Council decision amending Decision 2002/834/EC on the specific
Single European Code for Tissues and Cells
 Single European Code for Tissues and Cells Mr Richard Forde, Blood Tissues and Organs Inspector Health Products Regulatory Authority 19 th Overview: The Single European Code (SEC): Legislative Basis; Eurocet
Single European Code for Tissues and Cells Mr Richard Forde, Blood Tissues and Organs Inspector Health Products Regulatory Authority 19 th Overview: The Single European Code (SEC): Legislative Basis; Eurocet
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL
 Ref. Ares(2015)2319265-03/06/2015 EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Directorate D - Health systems and products D4 Substances of Human Origin and Tobacco Control Brussels,
Ref. Ares(2015)2319265-03/06/2015 EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Directorate D - Health systems and products D4 Substances of Human Origin and Tobacco Control Brussels,
Paola Di Ciaccio Vanja Nikolac
 Paola Di Ciaccio Vanja Nikolac ITALYintheHSCfield:HOWISTHISRULED? Directive 2004/23/EC of the European Parliament and of the Council of 31March2004 on setting standards of quality and safety for the donation,
Paola Di Ciaccio Vanja Nikolac ITALYintheHSCfield:HOWISTHISRULED? Directive 2004/23/EC of the European Parliament and of the Council of 31March2004 on setting standards of quality and safety for the donation,
Legislation concerning food donations in EU Member States and Croatia. Jasenka Begić, mag. ling., mag. litt. comp.
 Legislation concerning food donations in EU Member States and Croatia Jasenka Begić, mag. ling., mag. litt. comp. Content 1. The scope of the problem 2. Overview of the current legislation and practices
Legislation concerning food donations in EU Member States and Croatia Jasenka Begić, mag. ling., mag. litt. comp. Content 1. The scope of the problem 2. Overview of the current legislation and practices
DIRECTIVES. (Text with EEA relevance)
 L 238/44 DIRECTIVES COMMISSION DIRECTIVE (EU) 2017/1572 of 15 September 2017 supplementing Directive 2001/83/EC of the European Parliament and of the Council as regards the principles and guidelines of
L 238/44 DIRECTIVES COMMISSION DIRECTIVE (EU) 2017/1572 of 15 September 2017 supplementing Directive 2001/83/EC of the European Parliament and of the Council as regards the principles and guidelines of
L 256/32 Official Journal of the European Union
 L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
L 256/32 Official Journal of the European Union 1.10.2005 COMMISSION DIRECTIVE 2005/61/EC of 30 September 2005 implementing Directive 2002/98/EC of the European Parliament and of the Council as regards
Memorandum of Understanding on the working relations between the European Commission and the European Stability Mechanism
 Memorandum of Understanding on the working relations between the European Commission and the European Stability Mechanism 1- General objectives The European Commission (the Commission ) and the European
Memorandum of Understanding on the working relations between the European Commission and the European Stability Mechanism 1- General objectives The European Commission (the Commission ) and the European
ORGAN DONATION AND TRANSPLANTATION POLICY OPTIONS AT EU LEVEL CONSULTATION DOCUMENT* 27 June 2006
 ORGAN DONATION AND TRANSPLANTATION POLICY OPTIONS AT EU LEVEL CONSULTATION DOCUMENT* 27 June 2006 * This document does not represent an official position of the European Commission or its services. It
ORGAN DONATION AND TRANSPLANTATION POLICY OPTIONS AT EU LEVEL CONSULTATION DOCUMENT* 27 June 2006 * This document does not represent an official position of the European Commission or its services. It
Study on the uptake and impact of the EU Action Plan on Organ Donation and Transplantation ( ) in the EU Member States
 Study on the uptake and impact of the EU Action Plan on Organ Donation and Transplantation (2009-2015) in the EU Member States FACTOR Study Written by Renée Bouwman (Ph.D), Trees Wiegers (Ph.D), Steffie
Study on the uptake and impact of the EU Action Plan on Organ Donation and Transplantation (2009-2015) in the EU Member States FACTOR Study Written by Renée Bouwman (Ph.D), Trees Wiegers (Ph.D), Steffie
HUNGARIAN NATIONAL BLOOD TRANSFUSION SERVICE. Dr. Klara Baroti-Tóth, PhD IPFA/PEI 19 th International Workshop Budapest, May, 2012.
 HUNGARIAN NATIONAL BLOOD TRANSFUSION SERVICE Dr. Klara Baroti-Tóth, PhD IPFA/PEI 19 th International Workshop Budapest, 23-24 May, 212. BLOOD DONATION G I V I N G I S G O O D ORGAN COORDINATION OFFICE
HUNGARIAN NATIONAL BLOOD TRANSFUSION SERVICE Dr. Klara Baroti-Tóth, PhD IPFA/PEI 19 th International Workshop Budapest, 23-24 May, 212. BLOOD DONATION G I V I N G I S G O O D ORGAN COORDINATION OFFICE
COMMISSION OF THE EUROPEAN COMMUNITIES. COMMISSION STAFF WORKING DOCUMENT on Joint procurement of vaccine against influenza A(H1N1)
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 15.9.2009 SEC(2009) 1188 final COMMISSION STAFF WORKING DOCUMENT on Joint procurement of vaccine against influenza A(H1N1) Accompanying document to the
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 15.9.2009 SEC(2009) 1188 final COMMISSION STAFF WORKING DOCUMENT on Joint procurement of vaccine against influenza A(H1N1) Accompanying document to the
The TTSN - A Collaborative Biovigilance System
 The TTSN - A Collaborative Biovigilance System 5th World Congress on Tissue Banking 12th International Conference of the APASTB Kuala Lumpur, Malaysia June 4, 2008 Scott A. Brubaker, CTBS American Association
The TTSN - A Collaborative Biovigilance System 5th World Congress on Tissue Banking 12th International Conference of the APASTB Kuala Lumpur, Malaysia June 4, 2008 Scott A. Brubaker, CTBS American Association
European Union legislation on Food additives, Food enzymes, Extractions solvents and Food flavourings
 European Union legislation on Food additives, Food enzymes, Extractions solvents and Food flavourings European Commission, DG, Unit E3 Chemicals, contaminants and pesticides Serbia-Screening meeting on
European Union legislation on Food additives, Food enzymes, Extractions solvents and Food flavourings European Commission, DG, Unit E3 Chemicals, contaminants and pesticides Serbia-Screening meeting on
CTO Establishment Inspections. an Atlantic update - Tissues
 Transplant Atlantic CTO Establishment Inspections an Atlantic update - Tissues October 14, 2010 Compliance & Enforcement Deliver a national compliance and enforcement program, pursuant of the Food & Drugs
Transplant Atlantic CTO Establishment Inspections an Atlantic update - Tissues October 14, 2010 Compliance & Enforcement Deliver a national compliance and enforcement program, pursuant of the Food & Drugs
Vaccine shortages: Improving cooperation, communication and management Michael Sulzner
 Vaccine shortages: Improving cooperation, communication and management Michael Sulzner Public Health Directorate DG Health & Food Safety 13 April 2016, Geneva Decision 1082/2013/EU on serious cross-border
Vaccine shortages: Improving cooperation, communication and management Michael Sulzner Public Health Directorate DG Health & Food Safety 13 April 2016, Geneva Decision 1082/2013/EU on serious cross-border
Jose R. Nuñez MD, PhD Medical Officer Transplantation
 Jose R. Nuñez MD, PhD Medical Officer Transplantation 3 Global Observatory on Donation and Transplantation Organs Transplanted Globally in 2011 112,600 organs transplanted ( 10% of estimated global needs)
Jose R. Nuñez MD, PhD Medical Officer Transplantation 3 Global Observatory on Donation and Transplantation Organs Transplanted Globally in 2011 112,600 organs transplanted ( 10% of estimated global needs)
DIRECTIVE 2011/62/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
 L 174/74 Official Journal of the European Union 1.7.2011 DIRECTIVE 2011/62/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 8 June 2011 amending Directive 2001/83/EC on the Community code relating to
L 174/74 Official Journal of the European Union 1.7.2011 DIRECTIVE 2011/62/EU OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 8 June 2011 amending Directive 2001/83/EC on the Community code relating to
Good Laboratory Practice. EU-Serbia screening meeting Brussels, 19 June 2014
 Good Laboratory Practice EU-Serbia screening meeting Brussels, 19 June 2014 Table of contents 1. Background information on the principles of GLP 2. EU legal basis for GLP 3. Role of Member States 4. Role
Good Laboratory Practice EU-Serbia screening meeting Brussels, 19 June 2014 Table of contents 1. Background information on the principles of GLP 2. EU legal basis for GLP 3. Role of Member States 4. Role
Consultation document on Organ Donation and Transplantation Response Form
 Consultation document on Organ Donation and Transplantation Response Form Contact details of person and/or institution submitting comments Name of person Address e-mail address Knud Erben erben@ceapir.org
Consultation document on Organ Donation and Transplantation Response Form Contact details of person and/or institution submitting comments Name of person Address e-mail address Knud Erben erben@ceapir.org
EUROPEAN COMMISSION. Modus Operandi for the management of new food safety incidents with a potential for extension involving a chemical substance
 EUROPEAN COMMISSION Modus Operandi for the management of new food safety incidents with a potential for extension involving a chemical substance The Health and Consumer Protection Directorate-General of
EUROPEAN COMMISSION Modus Operandi for the management of new food safety incidents with a potential for extension involving a chemical substance The Health and Consumer Protection Directorate-General of
COMMISSION OF THE EUROPEAN COMMUNITIES. Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
 EN EN EN COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 10.12.2008 COM(2008) 663 final 2008/0256 (COD) Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending, as regards information
EN EN EN COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 10.12.2008 COM(2008) 663 final 2008/0256 (COD) Proposal for a DIRECTIVE OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending, as regards information
WHAT DO MANUFACTURERS AND IMPORTERS HAVE TO DO TO PREPARE FOR EU MEMBERSHIP?
 WHAT DO MANUFACTURERS AND IMPORTERS HAVE TO DO TO PREPARE FOR EU MEMBERSHIP? pharmaceutical regulatory affairs consulting and education www.rapharm.eu Vesna Koblar, MD. PhD. EU28: Science, Medicines, Health
WHAT DO MANUFACTURERS AND IMPORTERS HAVE TO DO TO PREPARE FOR EU MEMBERSHIP? pharmaceutical regulatory affairs consulting and education www.rapharm.eu Vesna Koblar, MD. PhD. EU28: Science, Medicines, Health
Meeting of the Competent Authorities on Blood and Blood Components
 Ref. Ares(2013)2586372-08/07/2013 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate D - Health systems and products D4 Substances of Human Origin and Tobacco Control Brussels, SANCO
Ref. Ares(2013)2586372-08/07/2013 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate D - Health systems and products D4 Substances of Human Origin and Tobacco Control Brussels, SANCO
Health & Consumer Protection. EC legislation on food. Olga Solomon Unit E3
 Health & Consumer Protection Directorate-General EC legislation on food additives Olga Solomon Unit E3 1 What is a food additive? Food additives are substances: not normally consumed as food or a characteristic
Health & Consumer Protection Directorate-General EC legislation on food additives Olga Solomon Unit E3 1 What is a food additive? Food additives are substances: not normally consumed as food or a characteristic
Transplantable Organs
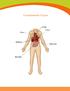 Transplantable Organs Liver Kidneys Pancreas Intestine Organ Information Heart The body s hardest working muscle, the heart beats 70 times each minute as it pumps blood throughout the body. Some conditions
Transplantable Organs Liver Kidneys Pancreas Intestine Organ Information Heart The body s hardest working muscle, the heart beats 70 times each minute as it pumps blood throughout the body. Some conditions
COMMISSION OF THE EUROPEAN COMMUNITIES. Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL
 EN EN EN COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 10.12.2008 COM(2008) 664 final 2008/0257 (COD) Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending, as regards pharmacovigilance
EN EN EN COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 10.12.2008 COM(2008) 664 final 2008/0257 (COD) Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL amending, as regards pharmacovigilance
(Legislative acts) REGULATIONS
 31.12.2010 Official Journal of the European Union L 348/1 I (Legislative acts) REGULATIONS REGULATION (EU) No 1235/2010 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 15 December 2010 amending, as regards
31.12.2010 Official Journal of the European Union L 348/1 I (Legislative acts) REGULATIONS REGULATION (EU) No 1235/2010 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 15 December 2010 amending, as regards
17. Storage of gametes and embryos
 17. Storage of gametes and embryos This guidance note contains: Mandatory requirements Extracts from the HFE Act 1990 (as amended) Extracts from licence conditions Reference to relevant HFEA Directions
17. Storage of gametes and embryos This guidance note contains: Mandatory requirements Extracts from the HFE Act 1990 (as amended) Extracts from licence conditions Reference to relevant HFEA Directions
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) PROGRAMME RESULTS
 COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) PROGRAMME RESULTS 2016-2017 Introduction This summary provides an overview of the activities carried out by
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) PROGRAMME RESULTS 2016-2017 Introduction This summary provides an overview of the activities carried out by
European Stability Mechanism Guideline on Precautionary Financial Assistance
 European Stability Mechanism Guideline on Precautionary Financial Assistance Article 1 Aim and scope The objective of precautionary financial assistance is to support sound policies and prevent crisis
European Stability Mechanism Guideline on Precautionary Financial Assistance Article 1 Aim and scope The objective of precautionary financial assistance is to support sound policies and prevent crisis
BILATERAL SCREENING MEETING
 Negotiating Group for the Accession of the Republic of Serbia to the European Union - Chapter 28, Consumer and Health Protection BILATERAL SCREENING MEETING Chapter 28 Consumer and Health Protection Brussels,
Negotiating Group for the Accession of the Republic of Serbia to the European Union - Chapter 28, Consumer and Health Protection BILATERAL SCREENING MEETING Chapter 28 Consumer and Health Protection Brussels,
EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL
 Ref. Ares(2014)4023682-02/12/2014 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate D - Health systems and products D4 Substances of Human Origin and Tobacco Control Brussels, 2014
Ref. Ares(2014)4023682-02/12/2014 EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL Directorate D - Health systems and products D4 Substances of Human Origin and Tobacco Control Brussels, 2014
COMMISSION REGULATION (EU)
 11.3.2011 Official Journal of the European Union L 64/15 COMMISSION REGULATION (EU) No 234/2011 of 10 March 2011 implementing Regulation (EC) No 1331/2008 of the European Parliament and of the Council
11.3.2011 Official Journal of the European Union L 64/15 COMMISSION REGULATION (EU) No 234/2011 of 10 March 2011 implementing Regulation (EC) No 1331/2008 of the European Parliament and of the Council
AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION. (Moscow, 23 December 2014)
 AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION (Moscow, 23 December 2014) Member States of the Eurasian Economic Union, hereinafter referred
AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION (Moscow, 23 December 2014) Member States of the Eurasian Economic Union, hereinafter referred
Other EU Activities Contributing to Harmonization of Labeling
 Other EU Activities Contributing to Harmonization of Labeling Dr Laurent Brassart European Medicines Agency Medical Information Sector DIA Labeling Harmonisation 2011 Workshop October 13-14. 2011 Disclaimer
Other EU Activities Contributing to Harmonization of Labeling Dr Laurent Brassart European Medicines Agency Medical Information Sector DIA Labeling Harmonisation 2011 Workshop October 13-14. 2011 Disclaimer
REGULATION (EC) No.141/2000
 REGULATION (EC) No.141/2000 Community legislation in force Document 300R0141 Directory chapters where this document can be found: [15.30 Health promotion] Regulation (EC) No.141/2000 of the European Parliament
REGULATION (EC) No.141/2000 Community legislation in force Document 300R0141 Directory chapters where this document can be found: [15.30 Health promotion] Regulation (EC) No.141/2000 of the European Parliament
Wyss Zürich Regulatory Affairs Seminar
 Wyss Zürich Regulatory Affairs Seminar Hospital Exemption & Compassionate Use EU Framework Catherine Longeval Koen T Syen Zürich, 5 July 2017 1 Table of contents Introduction Hospital Exemption Implementation
Wyss Zürich Regulatory Affairs Seminar Hospital Exemption & Compassionate Use EU Framework Catherine Longeval Koen T Syen Zürich, 5 July 2017 1 Table of contents Introduction Hospital Exemption Implementation
PROVISIONAL INSTITUTIONS OF SELF GOVERNMENT FOR BLOOD TRANSFUSION, BLOOD CONTROL AND ITS PRODUCTS
 UNITED NATIONS United Nations Interim Administration Mission in Kosovo UNMIK NATIONS UNIES Mission d Administration Intérimaire des Nations Unies au Kosovo PROVISIONAL INSTITUTIONS OF SELF GOVERNMENT LAW
UNITED NATIONS United Nations Interim Administration Mission in Kosovo UNMIK NATIONS UNIES Mission d Administration Intérimaire des Nations Unies au Kosovo PROVISIONAL INSTITUTIONS OF SELF GOVERNMENT LAW
October 2003 Revision 1, February INTRODUCTION AND SCOPE
 BEST PRACTICE GUIDE FOR THE EXCHANGE OF REGULATORY AND ADMINISTRATIVE INFORMATION REGARDING ORPHAN MEDICINAL PRODUCTS BETWEEN THE EMEA AND THE NATIONAL COMPETENT AUTHORITIES October 2003 Revision 1, February
BEST PRACTICE GUIDE FOR THE EXCHANGE OF REGULATORY AND ADMINISTRATIVE INFORMATION REGARDING ORPHAN MEDICINAL PRODUCTS BETWEEN THE EMEA AND THE NATIONAL COMPETENT AUTHORITIES October 2003 Revision 1, February
ACTOR study. NIVEL - Netherlands institute for health services research
 Study on the set-up of organ donation and transplantation in the EU Member States, uptake and impact of the EU Action Plan on Organ Donation and Transplantation (2009-2015) ACTOR study This study has been
Study on the set-up of organ donation and transplantation in the EU Member States, uptake and impact of the EU Action Plan on Organ Donation and Transplantation (2009-2015) ACTOR study This study has been
ATMPs & EU GMP Update. Bryan J Wright July 2017
 ATMPs & EU GMP Update Bryan J Wright July 2017 Slide 1 PharmOut 2017 Outline ATMPs What they are? Why are we looking at this subject? The Licensing position of ATMPs and use of PRIME ATMPs and GMP Inspections
ATMPs & EU GMP Update Bryan J Wright July 2017 Slide 1 PharmOut 2017 Outline ATMPs What they are? Why are we looking at this subject? The Licensing position of ATMPs and use of PRIME ATMPs and GMP Inspections
NATIONAL BLOOD TRANSFUSION SERVICES STRATEGY
 FEDERAL DEMOCRATIC REPUBLIC OF ETHIOPIA MINISTRY OF HEALTH NATIONAL BLOOD TRANSFUSION SERVICES STRATEGY February 2005 Addis Ababa Ethiopia Acknowledgement The Ministry of Health of the Federal Democratic
FEDERAL DEMOCRATIC REPUBLIC OF ETHIOPIA MINISTRY OF HEALTH NATIONAL BLOOD TRANSFUSION SERVICES STRATEGY February 2005 Addis Ababa Ethiopia Acknowledgement The Ministry of Health of the Federal Democratic
COMPETENT AUTHORITY (UK) MEDICAL DEVICES DIRECTIVES GUIDANCE NOTES FOR MANUFACTURERS OF DENTAL APPLIANCES
 COMPETENT AUTHORITY (UK) 10 EC MEDICAL DEVICES DIRECTIVES GUIDANCE NOTES FOR MANUFACTURERS OF DENTAL APPLIANCES (CUSTOM MADE DEVICES) Updated March 2008 CONTENTS PAGE Introduction 3 Definition of dental
COMPETENT AUTHORITY (UK) 10 EC MEDICAL DEVICES DIRECTIVES GUIDANCE NOTES FOR MANUFACTURERS OF DENTAL APPLIANCES (CUSTOM MADE DEVICES) Updated March 2008 CONTENTS PAGE Introduction 3 Definition of dental
15050/15 JS/pm 1 DGB 3B
 Council of the European Union Brussels, 7 December 2015 (OR. en) 15050/15 SAN 427 OUTCOME OF PROCEEDINGS From: General Secretariat of the Council On: 7 December 2015 To: Delegations No. prev. doc.: 14391/1/15
Council of the European Union Brussels, 7 December 2015 (OR. en) 15050/15 SAN 427 OUTCOME OF PROCEEDINGS From: General Secretariat of the Council On: 7 December 2015 To: Delegations No. prev. doc.: 14391/1/15
THE WORLD MEDICAL ASSOCIATION, INC. WMA STATEMENT ON ORGAN AND TISSUE DONATION
 THE WORLD MEDICAL ASSOCIATION, INC. WMA STATEMENT ON ORGAN AND TISSUE DONATION Adopted by the 63rd WMA General Assembly, Bangkok, Thailand, October 2012 PREAMBLE Advances in medical sciences, especially
THE WORLD MEDICAL ASSOCIATION, INC. WMA STATEMENT ON ORGAN AND TISSUE DONATION Adopted by the 63rd WMA General Assembly, Bangkok, Thailand, October 2012 PREAMBLE Advances in medical sciences, especially
Coding and Labelling of
 Coding and Labelling of Tissues in Europe Deirdre Fehily Why are Tissue Coding and Labelling Important? Anonimity Traceability global uniqueness Standardised product information Potential for electronic
Coding and Labelling of Tissues in Europe Deirdre Fehily Why are Tissue Coding and Labelling Important? Anonimity Traceability global uniqueness Standardised product information Potential for electronic
MEDICAL MANAGEMENT POLICY
 PAGE: 1 of 5 MEDICAL MANAGEMENT POLICY This medical policy is not a guarantee of benefits or coverage, nor should it be deemed as medical advice. In the event of any conflict concerning benefit coverage,
PAGE: 1 of 5 MEDICAL MANAGEMENT POLICY This medical policy is not a guarantee of benefits or coverage, nor should it be deemed as medical advice. In the event of any conflict concerning benefit coverage,
Strategic partner for the Québec health system
 BLOOD PRODUCTS STABLE PRODUCTS STEM CELLS Strategic partner for the Québec health system HUMAN TISSUES MOTHER S MILK CORPORATE PRESENTATION MISSION To efficiently meet the needs of the Québec population
BLOOD PRODUCTS STABLE PRODUCTS STEM CELLS Strategic partner for the Québec health system HUMAN TISSUES MOTHER S MILK CORPORATE PRESENTATION MISSION To efficiently meet the needs of the Québec population
Report on the Transplantation Transmission Sentinel Network (TTSN)
 Report on the Transplantation Transmission Sentinel Network (TTSN) CATB Session - AATB s 31st Annual Meeting, Boston September 15, 2007 Scott Brubaker, CTBS AATB Representative, TTSN Advisory Group TTSN
Report on the Transplantation Transmission Sentinel Network (TTSN) CATB Session - AATB s 31st Annual Meeting, Boston September 15, 2007 Scott Brubaker, CTBS AATB Representative, TTSN Advisory Group TTSN
The European Neighbourhood Policy (ENP) ENP Coordination External Relations Directorate General European Commission
 The European Neighbourhood Policy (ENP) ENP Coordination External Relations Directorate General European Commission Different neighbours, different relations EFTA / EEA Candidate Countries (Croatia, former
The European Neighbourhood Policy (ENP) ENP Coordination External Relations Directorate General European Commission Different neighbours, different relations EFTA / EEA Candidate Countries (Croatia, former
COMMISSION OF THE EUROPEAN COMMUNITIES COMMISSION DECISION. of
 COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 5.10.2009 C(2009)7726 COMMISSION DECISION of 5.10.2009 granting marketing authorisation under Regulation (EC) No 726/2004 of the European Parliament and
COMMISSION OF THE EUROPEAN COMMUNITIES Brussels, 5.10.2009 C(2009)7726 COMMISSION DECISION of 5.10.2009 granting marketing authorisation under Regulation (EC) No 726/2004 of the European Parliament and
 Fact Sheet Blood Collection and Use Hong Kong Red Cross Blood Transfusion Service The Hong Kong Red Cross began its voluntary, nonremunerated blood donation programme to supply blood to all hospitals in
Fact Sheet Blood Collection and Use Hong Kong Red Cross Blood Transfusion Service The Hong Kong Red Cross began its voluntary, nonremunerated blood donation programme to supply blood to all hospitals in
2008 Public Status Report on the Implementation of the European Risk Management Strategy. Executive Summary
 European Medicines Agency London, 17 March 2009 Doc. Ref. EMEA/43556/2009 2008 Status Report on the Implementation of the European Risk Management Strategy Executive Summary The European Risk Management
European Medicines Agency London, 17 March 2009 Doc. Ref. EMEA/43556/2009 2008 Status Report on the Implementation of the European Risk Management Strategy Executive Summary The European Risk Management
Fondazione Banco Alimentare Onlus Via Legnone 4 _ Milano _ T _ F _ E _ CF
 June 2014 Position statement of the Fondazione Banco Alimentare Onlus The Fondazione Banco Alimentare Onlus (hereinafter, FBAO ), through its network of 21 Food Banks in Italy, is committed to fight against
June 2014 Position statement of the Fondazione Banco Alimentare Onlus The Fondazione Banco Alimentare Onlus (hereinafter, FBAO ), through its network of 21 Food Banks in Italy, is committed to fight against
STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH SECTION ON GENERAL FOOD LAW. Summary Record of Meeting of 30 April 2012
 EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH SECTION ON GENERAL FOOD LAW Summary Record of Meeting of 30 April 2012 Chairmen: Mr Basil
EUROPEAN COMMISSION HEALTH & CONSUMERS DIRECTORATE-GENERAL STANDING COMMITTEE ON THE FOOD CHAIN AND ANIMAL HEALTH SECTION ON GENERAL FOOD LAW Summary Record of Meeting of 30 April 2012 Chairmen: Mr Basil
GLP in the European Union Ecolabel detergents, GLP and accreditation
 GLP in the European Union Ecolabel detergents, GLP and accreditation Maik Schmahl Brussels, 25/03/2010 Chemicals Unit Outline What is GLP? How has it developed? The role of the Member States, the European
GLP in the European Union Ecolabel detergents, GLP and accreditation Maik Schmahl Brussels, 25/03/2010 Chemicals Unit Outline What is GLP? How has it developed? The role of the Member States, the European
Medical Devices Act 1
 Issuer: Riigikogu Type: act In force from: 01.01.2016 In force until: 31.05.2016 Translation published: 01.02.2016 Medical Devices Act 1 Amended by the following acts Passed 13.10.2004 RT I 2004, 75, 520
Issuer: Riigikogu Type: act In force from: 01.01.2016 In force until: 31.05.2016 Translation published: 01.02.2016 Medical Devices Act 1 Amended by the following acts Passed 13.10.2004 RT I 2004, 75, 520
Counterfeit Medicinal Products. FINLAND Roschier, Attorneys Ltd.
 Counterfeit Medicinal Products FINLAND Roschier, Attorneys Ltd. CONTACT INFORMATION Mikael Segercrantz Robert Hagelstam Roschier, Attorneys Ltd. Keskuskatu 7 A 00100 Helsinki, Finland 358.20.506.6000 mikael.segercrantz@roschier.com
Counterfeit Medicinal Products FINLAND Roschier, Attorneys Ltd. CONTACT INFORMATION Mikael Segercrantz Robert Hagelstam Roschier, Attorneys Ltd. Keskuskatu 7 A 00100 Helsinki, Finland 358.20.506.6000 mikael.segercrantz@roschier.com
Workshop for OIE national Focal Points for Veterinary Products November 2010, Johannesburg, South Africa. Presentation
 Dr. Anja Holm Chair of CVMP, EMA Danish Medicines Agency Copenhagen, DK - Denmark anh@dkma.dk Overview of existing structures relevant for Veterinary Products Regional Structures: Europe European Medicine
Dr. Anja Holm Chair of CVMP, EMA Danish Medicines Agency Copenhagen, DK - Denmark anh@dkma.dk Overview of existing structures relevant for Veterinary Products Regional Structures: Europe European Medicine
Competent Authorities on Substances of Human Origin Expert Group (CASoHO E01718) Meeting of the Competent Authorities on Blood and Blood Components
 Ref. Ares(2016)1437332-23/03/2016 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical Products: quality, safety,
Ref. Ares(2016)1437332-23/03/2016 EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Directorate B - Health systems, medical products and innovation B4 Medical Products: quality, safety,
COUNCIL OF THE EUROPEAN UNION. Brussels, 7 September 2009 (OR. en) 11261/09 Interinstitutional File: 2008/0002 (COD) DENLEG 51 CODEC 893
 COUNCIL OF THE EUROPEAN UNION Brussels, 7 September 2009 (OR. en) 11261/09 Interinstitutional File: 2008/0002 (COD) DLEG 51 CODEC 893 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: Common Position with
COUNCIL OF THE EUROPEAN UNION Brussels, 7 September 2009 (OR. en) 11261/09 Interinstitutional File: 2008/0002 (COD) DLEG 51 CODEC 893 LEGISLATIVE ACTS AND OTHER INSTRUMTS Subject: Common Position with
An example of intersectoral collaboration: the EU model. European Commission Health and Consumers Directorate-General (DG SANCO)
 An example of intersectoral collaboration: the EU model European Commission Health and Consumers Directorate-General (DG SANCO) The European Union: 27 Member States 3 Candidate Countries European Institutions
An example of intersectoral collaboration: the EU model European Commission Health and Consumers Directorate-General (DG SANCO) The European Union: 27 Member States 3 Candidate Countries European Institutions
COMMISSION DELEGATED REGULATION (EU).../... of XXX
 EUROPEAN COMMISSION Brussels, XXX SANTE/10355/2015 (POOL/E4/2015/10355/10355-EN. doc) [...1(2015) XXX draft COMMISSION DELEGATED REGULATION (EU).../... of XXX supplementmg Regulation (EU) No 609/2013 of
EUROPEAN COMMISSION Brussels, XXX SANTE/10355/2015 (POOL/E4/2015/10355/10355-EN. doc) [...1(2015) XXX draft COMMISSION DELEGATED REGULATION (EU).../... of XXX supplementmg Regulation (EU) No 609/2013 of
A guide for families deciding on organ, eye and tissue donation. When your loved one dies
 A guide for families deciding on organ, eye and tissue donation When your loved one dies Benefits of Donation Donation may help you with your loss. Organ and tissue donation is a unique opportunity to
A guide for families deciding on organ, eye and tissue donation When your loved one dies Benefits of Donation Donation may help you with your loss. Organ and tissue donation is a unique opportunity to
Annex II - List of enforceable provisions of REACH and CLP
 Annex II - List of enforceable provisions of REACH and CLP The provisions below are listed according to the sections of the main report, together with the Titles of the Regulation in the case of REACH.
Annex II - List of enforceable provisions of REACH and CLP The provisions below are listed according to the sections of the main report, together with the Titles of the Regulation in the case of REACH.
IMPORTANT DISCLAIMER. Note
 yn EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL June 2012 DRAFT GUIDANCE DOCUMENT FOR COMPETENT AUTHORITIES FOR THE CONTROL OF COMPLIANCE WITH EU LEGISLATION ON: Regulation (EU) No 1169/2011
yn EUROPEAN COMMISSION HEALTH AND CONSUMERS DIRECTORATE-GENERAL June 2012 DRAFT GUIDANCE DOCUMENT FOR COMPETENT AUTHORITIES FOR THE CONTROL OF COMPLIANCE WITH EU LEGISLATION ON: Regulation (EU) No 1169/2011
NDRI Private Donor Program: Accelerating Biomedical Research via Private Donation
 1 NDRI Private Donor Program: Accelerating Biomedical Research via Private Donation Jeffrey Thomas Vice President, Strategic Initiatives Honesto Nunez III Private Donor/Rare Disease Manager Webinar Objectives
1 NDRI Private Donor Program: Accelerating Biomedical Research via Private Donation Jeffrey Thomas Vice President, Strategic Initiatives Honesto Nunez III Private Donor/Rare Disease Manager Webinar Objectives
COMMISSION IMPLEMENTING DECISION. of
 EUROPEAN COMMISSION Brussels, 14-04-2011 C(2011)2799 COMMISSION IMPLEMENTING DECISION of 14-04-2011 granting marketing authorisation under Regulation (EC) No 726/2004 of the European Parliament and of
EUROPEAN COMMISSION Brussels, 14-04-2011 C(2011)2799 COMMISSION IMPLEMENTING DECISION of 14-04-2011 granting marketing authorisation under Regulation (EC) No 726/2004 of the European Parliament and of
IPEC Europe Suggested Alternative (if none then original text is clear and needs no alteration) Purpose and Scope
 IPEC Europe Observations and Recommendations on Guidelines On The Formalised Risk Assessment For Ascertaining The Appropriate Good Manufacturing Practice For Excipients Of Medicinal Products For Human
IPEC Europe Observations and Recommendations on Guidelines On The Formalised Risk Assessment For Ascertaining The Appropriate Good Manufacturing Practice For Excipients Of Medicinal Products For Human
Human Fertilisation and Embryology Authority 10 Spring Gardens London SW1A 2BU t e w
 Edition 8.0 First published October 2009 Revised April 2010, April 2011, October 2011, April 2012, October 2013, October 2014, April 2015, October 2015, July 2016, May 2017, October 2017 Human Fertilisation
Edition 8.0 First published October 2009 Revised April 2010, April 2011, October 2011, April 2012, October 2013, October 2014, April 2015, October 2015, July 2016, May 2017, October 2017 Human Fertilisation
Structure of the EU Regulation for Organic Farming
 Axmann 2009 SCA in OA - Introduction Structure of the EU Regulation for Organic Farming Council Regulation (EC) No 834/2007 on organic production and labelling of organic products. This is the basic regulation
Axmann 2009 SCA in OA - Introduction Structure of the EU Regulation for Organic Farming Council Regulation (EC) No 834/2007 on organic production and labelling of organic products. This is the basic regulation
COMMISSION REGULATION (EU) / of XXX
 Ref. Ares(2016)5616438-28/09/2016 EUROPEAN COMMISSION Brussels, XXX [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annex III to Directive 2008/98/EC of the European Parliament and of
Ref. Ares(2016)5616438-28/09/2016 EUROPEAN COMMISSION Brussels, XXX [ ](2016) XXX draft COMMISSION REGULATION (EU) / of XXX amending Annex III to Directive 2008/98/EC of the European Parliament and of
Table Of Content. European Organisation for Rare Diseases... 2 Summary... 3 Coordinator, Leader contact and partners... 6 Outputs...
 Table Of Content European Organisation for Rare Diseases... 2 Summary... 3 Coordinator, Leader contact and partners... 6 Outputs... 7 D04 - Electronic Newsletter (EN)... 7 D01 - Activity Report EURORDIS
Table Of Content European Organisation for Rare Diseases... 2 Summary... 3 Coordinator, Leader contact and partners... 6 Outputs... 7 D04 - Electronic Newsletter (EN)... 7 D01 - Activity Report EURORDIS
KIDNEY EXCHANGE PROGRAMMES IN EUROPE. Position Paper of the. European Committee on Organ Transplantation (CD-P-TO) of the Council of Europe
 KIDNEY EXCHANGE PROGRAMMES IN EUROPE Position Paper of the European Committee on Organ Transplantation (CD-P-TO) of the Council of Europe 1. Introduction Approximately one in a thousand European citizens
KIDNEY EXCHANGE PROGRAMMES IN EUROPE Position Paper of the European Committee on Organ Transplantation (CD-P-TO) of the Council of Europe 1. Introduction Approximately one in a thousand European citizens
CONSENT FOR CRYOPRESERVATION OF EMBRYOS
 CONSENT FOR CRYOPRESERVATION OF EMBRYOS We, (Female Partner) and (Partner, Spouse), as participants in the in vitro fertilization (IVF) program at the Reproductive fertility center (REPRODUCTIVE FERTILITY
CONSENT FOR CRYOPRESERVATION OF EMBRYOS We, (Female Partner) and (Partner, Spouse), as participants in the in vitro fertilization (IVF) program at the Reproductive fertility center (REPRODUCTIVE FERTILITY
Organ and Tissue Donation
 Patient & Family Guide 2017 Organ and Tissue Donation www.nshealth.ca Organ and Tissue Donation Health care providers want to be sure that families of all patients who may be eligible are offered the option
Patient & Family Guide 2017 Organ and Tissue Donation www.nshealth.ca Organ and Tissue Donation Health care providers want to be sure that families of all patients who may be eligible are offered the option
Organ and Tissue Donation. Tennessee Donor Registry. Frequently Asked Questions About Donation
 Organ and Tissue Donation 1. What is organ and tissue donation? 2. How many people need donated organs and tissue? 3. What organs and tissues are most commonly donated? 4. How can organs and tissues be
Organ and Tissue Donation 1. What is organ and tissue donation? 2. How many people need donated organs and tissue? 3. What organs and tissues are most commonly donated? 4. How can organs and tissues be
Organ Procurement and Transplantation Network
 OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
OPTN Organ Procurement and Transplantation Network POLICIES This document provides the policy language approved by the OPTN/UNOS Board at its meeting in June 2015 as part of the Operations and Safety Committee
Act on Narcotic Drugs and Psychotropic Substances and Precursors thereof
 Issuer: Riigikogu Type: act In force from: 13.05.2016 In force until: 22.11.2018 Translation published: 06.05.2016 Act on Narcotic Drugs and Psychotropic Substances and Precursors thereof Amended by the
Issuer: Riigikogu Type: act In force from: 13.05.2016 In force until: 22.11.2018 Translation published: 06.05.2016 Act on Narcotic Drugs and Psychotropic Substances and Precursors thereof Amended by the
The Maltese Presidency of the Council of the EU Veterinary Sector
 The Maltese Presidency of the Council of the EU Veterinary Sector 1 January 30 June 2017 Presidency Priority Antimicrobial resistance Global Action Plan on AMR One Health approach addressing the threat
The Maltese Presidency of the Council of the EU Veterinary Sector 1 January 30 June 2017 Presidency Priority Antimicrobial resistance Global Action Plan on AMR One Health approach addressing the threat
The Organ and Tissue Donor Program
 The Organ and Tissue Donor Program If I needed a kidney or some other vital organ to live Would I be able to get one? Maybe. Many people who need organ transplants cannot get them because of a shortage
The Organ and Tissue Donor Program If I needed a kidney or some other vital organ to live Would I be able to get one? Maybe. Many people who need organ transplants cannot get them because of a shortage
LAW ON MINERAL FERTILIZERS. Official Gazette of Bosnia and Herzegovina, 46/04
 LAW ON MINERAL FERTILIZERS Official Gazette of Bosnia and Herzegovina, 46/04 Pursuant to Article IV.4.a) of the Constitution of Bosnia and Herzegovina, the Parliamentary Assembly of Bosnia and Herzegovina,
LAW ON MINERAL FERTILIZERS Official Gazette of Bosnia and Herzegovina, 46/04 Pursuant to Article IV.4.a) of the Constitution of Bosnia and Herzegovina, the Parliamentary Assembly of Bosnia and Herzegovina,
