TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING October2010. Issue Summary
|
|
|
- Caroline Stafford
- 5 years ago
- Views:
Transcription
1 TRANSMISSIBLE SPONGIFORM ENCEPHALOPATHIES ADVISORY COMMITTEE MEETING 28-29October2010 Issue Summary Informational Topic: FDA s Geographic Donor Deferral Policy to Reduce the Possible Risk of Transmission of Creutzfeldt-Jakob Disease and Variant Creutzfeldt-Jakob Disease by Blood and Blood Products and Human Cells, Tissues and Cellular and Tissuebased Products Issue: FDA wishes to update the Committee on the current regulatory considerations relating to Relevant Communicable Disease Agents and Diseases (RCDADs), of which TSEs are a part, for human cell, tissue, and cellular and tissue-based products (HCT/Ps), and also would like to identify current criteria that would render an HCT/P donor ineligible as a result of TSE risk. There is no decisional issue for the committee at this time. Regulatory Background: The Office of Cellular, Tissue and Gene Therapies (OCTGT) within CBER regulates human cells, tissues, and cellular and tissue-based products (HCT/Ps) in order to prevent the introduction, transmission, or spread of communicable diseases. HCT/Ps cover a broad range of individual products. The regulations define HCT/Ps (21 CFR (d)) as articles containing or consisting of human cells or tissues that are intended for implantation, transplantation, infusion, or transfer into a human recipient. Donors of HCT/Ps may be either living or deceased (i.e., cadaveric). Examples of HCT/Ps include bone, ligament, skin, dura mater, heart valve, and cornea from deceased donors; hematopoietic stem cells derived from peripheral and cord blood, manipulated autologous chondrocytes, cells transduced with gene therapy vectors, epithelial cells on a synthetic matrix, and semen or other reproductive tissue from living donors. The following articles are not considered HCT/Ps: Vascularized human organs for transplantation; Whole blood or blood components or blood derivative products subject to listing under 21 CFR 607 and 207, respectively; Secreted or extracted human products, such as milk, collagen, and cell factors; except that semen is considered an HCT/P; Minimally manipulated bone marrow for homologous use and not combined with a drug or a device (except for a sterilizing, preserving, or storage agent, if the addition of the agent does not raise new clinical safety concerns with respect to the bone marrow); 1
2 Ancillary products used in the manufacture of HCT/P; Cells, tissues, and organs derived from animals other than humans; and In vitro diagnostic products as defined in 21 CFR 809.3(a). Relevant Communicable Disease Agents or Diseases and Donor Screening HCT/Ps carry the risk of communicable disease transmission from the donor to the recipient. To minimize this risk, FDA has established regulatory requirements designed to prevent the introduction, transmission, or spread of communicable disease by screening and testing the donor, and ensuring that the cells or tissues are not contaminated during recovery, processing, storage or distribution. Individuals who have communicable diseases that their donated tissues can transmit to transplant recipients are not eligible to serve as donors. In accordance with the risk-based approach developed for 21 CFR Part 1271 certain exceptions are made for use of HCT/Ps from donors who are determined to be ineligible. These include HCT/Ps from a: First degree or second degree blood relative for hematopoietic stem cell donors, Directed (known) reproductive donors Donors where there is a documented urgent medical need In order for there to be a requirement to screen or test HCT/P donors for an infectious agent, that agent must be considered by FDA to be a relevant communicable disease agent or disease (RCDAD). RCDADs are designated two ways in the regulations; some diseases are specifically listed in the regulation 21 CFR (r)(1), while others must meet certain criteria, listed in 21 CFR (r)(2) to be considered a RCDAD. The provisions in (r)(2) were established to allow FDA to address emerging infectious diseases. Disease agents specifically listed in 21 CFR (r)(1) include the following for all HCT/Ps: Human immunodeficiency virus (HIV), types 1 and 2; Hepatitis B virus (HBV); Hepatitis C virus (HCV); Human transmissible spongiform encephalopathy (TSE), including Creutzfeldt-Jakob disease (CJD); and Treponema pallidum (syphilis). In addition to the above list of RCDADs for all HCT/Ps, a communicable disease agent or disease meeting the following criteria (Sec (r) (2)), but not specifically listed, is relevant if it is one: a. For which there may be a risk of transmission by an HCT/P, either to the recipient of the HCT/P or to those people who may handle or otherwise come in contact with the HCT/P, such as medical personnel, because the disease agent or disease: 2
3 i. is potentially transmissible by an HCT/P; and ii. either (1) has sufficient incidence and/or prevalence to affect the potential donor population (Sec (r)(2)(i)(B)(1)), or (2) may have been released accidentally or intentionally in a manner that could place potential donors at risk of infection (Sec (r)(2)(i)(B)(2)); b. That could be fatal or life-threatening, could result in permanent impairment of a body function or permanent damage to body structure, or could necessitate medical or surgical intervention to preclude permanent impairment of body function or permanent damage to a body structure (Sec (r)(2)(ii)); and c. For which appropriate screening measures have been developed and/or an appropriate screening test for donor specimens has been licensed, approved, or cleared for such use by FDA and is available (Sec (r)(2)(iii)). The following are additional RCDADs for all HCT/Ps that were added according to the (r)(2) definition: West Nile Virus; Sepsis; and Vaccinia (the virus used in smallpox vaccine). 3
4 Current requirements for Donor Screening and Testing Relevant communicable disease agents or diseases for which a donor must be screened or screened and tested include: Agent HCT/Ps for Screening Testing which evaluation is required HIV-1 and -2 All X X Hepatitis B All X X Hepatitis C All X X Syphilis All X X TSE * All X WNV All X Sepsis All X Vaccinia (recent smallpox All X vaccination) HTLV-I and II Viable, X X Leukocyte-Rich CMV ** Viable, X Leukocyte-Rich Chlamydia trachomatis Reproductive X X Neisseria Gonorrhea Reproductive X X * Including CJD and variant CJD ** Although CMV is not a relevant communicable disease agent or disease, donors of viable, leukocyte-rich HCT/Ps must be tested for evidence of infection due to CMV in order to adequately and appropriately reduce the risk of transmission Examples of viable, leukocyte-rich HCT/Ps include hematopoietic stem/progenitor cells and semen. FDA interprets reproductive HCT/Ps to include semen, oocytes, and embryos to which the donor contributed the spermatozoa or oocyte. The term donor screening has a specific meaning in the context of HCT/Ps. In some circumstances, the term donor screening may also encompass donor testing. However, the HCT/P regulations distinguish between donor screening (medical history interview, physical assessment and medical record review) and donor testing. HCT/P donors are tested using donor screening tests. The screening process includes: Physical assessment or examination Review of potential donor s medical records Interviewing donor (if the donor is living) about his or her social behavior 4
5 Interviewing person who is knowledgeable about the donor s social behavior (if the donor is non-living) The key information needed to determine donor eligibility is: Relevant risk factors (e.g., non-medical drug injection during the past five years, geographic regions living in/ visited) Clinical or physical evidence of communicable disease agents that could be transferred to a recipient of donated HCT/Ps Results of testing for relevant communicable disease agents HCT/P Donor Screening for TSE, including vcjd The current TSE risk factors for which HCT/P donors should be excluded from donation are as follows 1 : 19. Persons who have been diagnosed with vcjd or any other form of CJD. Note: Numbers 19 to 26 in this section are designed to screen for TSEs, including CJD and vcjd. If the living donor or the individual knowledgeable about the donor s medical and travel history is not familiar with the term Creutzfeldt-Jakob Disease or variant Creutzfeldt-Jakob Disease, you may try to describe those in layman s terms. If the person being interviewed is still not familiar with those terms, you may consider the lack of familiarity with those terms as a negative response to questions using those terms. 20. Persons who have been diagnosed with dementia or any degenerative or demyelinating disease of the central nervous system or other neurological disease of unknown etiology. Potential donors who have a diagnosis of delirium (e.g., delirium caused by toxic/metabolic diseases or recent head trauma) would not necessarily be considered to have a diagnosis of dementia and should be evaluated by the Medical Director. (HCT/Ps from donors with dementia confirmed by gross and microscopic examination of the brain to be caused by cerebrovascular accident or brain tumor and who are confirmed not to have evidence of TSE on microscopic examination of the brain may be acceptable based on an evaluation by the Medical Director). 21. Persons who are at increased risk for CJD. Donors are considered to have an increased risk for CJD if they have received a non-synthetic dura mater transplant, human pituitary-derived growth hormone, or have one or more blood relatives diagnosed with CJD (see criterion 22 of this section). 1 Guidance for Industry: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products e/ucm htm#donorscreening
6 22. Persons who have a history of CJD in a blood relative unless: The diagnosis of CJD was subsequently found to be an incorrect diagnosis; The CJD was iatrogenic; or Laboratory testing (gene sequencing) shows that the donor does not have a mutation associated with familial CJD. 23. Persons who spent three months or more cumulatively in the United Kingdom (U.K.) (see Appendix 5) from the beginning of 1980 through the end of Persons who are current or former U.S. military members, civilian military employees, or dependents of a military member or civilian employee who resided at U.S. military bases in Northern Europe (Germany, Belgium, and the Netherlands) for 6 months or more cumulatively from 1980 through 1990, or elsewhere in Europe (Greece, Turkey, Spain, Portugal, and Italy) for 6 months or more cumulatively from 1980 through Persons who spent 5 years or more cumulatively in Europe (see Appendix 5) from 1980 until the present (note this criterion includes time spent in the U.K. from 1980 through 1996). 26. Persons who received any transfusion of blood or blood components in the U.K. or France between 1980 and the present. 6
Case 1. FDA, Legal, and EBAA Medical Standards Panel. Case 1. Case 1 6/25/2012. Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies
 Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Case 1 FDA, Legal, and EBAA Medical Standards Panel Samuel B. Barone, M.D. Office of Cellular, Tissue, and Gene Therapies Medical Directors Symposium Eye Bank Association of America Annual Meeting 47 y/o
Directed Semen Donor Eligibility Requirements
 Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Directed Semen Donor Eligibility Requirements Directed (known) reproductive donors using the University Andrology Sperm Banking Program must follow FDA requirements to prevent the transmission of relevant
Question: 1. Are you currently taking an antibiotic?
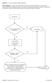 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic?
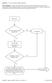 Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
Question: 1. Are you currently taking an antibiotic? Donor Eligibility: A donor with an infection that could cause bacteremia/sepsis or other relevant communicable disease should not donate. The reason
DHQ Flowcharts v2.0 eff. February 2016
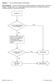 Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
Question: 1. Are you feeling healthy and well today? Donor Eligibility: A person should be free of infectious diseases, including colds, on the day of donation. A person who is not in good health should
FORM TITLE: Risk Behavior Assessment. 1. Are you a male who has had sex with another male in the preceding five years?
 RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
RELESE DTE: 12/07/2012 EFFECTIVE DTE: 12/13/2012 1. re you a male who has had sex with another male in the preceding five years? 2. Have you injected drugs for non-medical reason in the preceding five
DHQ v2.0, February 2016 Table Detailing Changes from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you
 DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
DHQ v2.0, February 2016 Table Detailing s from v1.3 v1.3 Revised language in v2.0 Rationale Question 1: Are you Q 1, No Feeling healthy and well today? Question 2: Are you Q 2, No change Currently taking
Relevant Communicable Diseases in HCT/Ps
 Relevant Communicable Diseases in HCT/Ps Phyllis I. Warkentin, MD Professor of Pathology and Pediatrics University of Nebraska Medical Center FACT Chief Medical Officer Relevant Communicable Diseases in
Relevant Communicable Diseases in HCT/Ps Phyllis I. Warkentin, MD Professor of Pathology and Pediatrics University of Nebraska Medical Center FACT Chief Medical Officer Relevant Communicable Diseases in
The 2008 Guidelines for Gamete and Embryo Donation
 2008 Guidelines for gamete and embryo donation: a Practice Committee report Practice Committee of the American Society for Reproductive Medicine, and the Practice Committee of the Society for Assisted
2008 Guidelines for gamete and embryo donation: a Practice Committee report Practice Committee of the American Society for Reproductive Medicine, and the Practice Committee of the Society for Assisted
MEDICAL ADVISORY. July 18, 2017
 MEDICAL ADVISORY July 18, 2017 The following changes were made at the June 16, 2017 meeting of the Medical Advisory Board, and will become effective immediately. B1.100 Eye Bank Inspection The Accreditation
MEDICAL ADVISORY July 18, 2017 The following changes were made at the June 16, 2017 meeting of the Medical Advisory Board, and will become effective immediately. B1.100 Eye Bank Inspection The Accreditation
[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019
![[Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019 [Type here] Version Approval Dates Announcement Date Effective Standards Committee: Jan 2019](/thumbs/95/123651537.jpg) SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
SECTION D AUTHORIZATION, INFORMED CONSENT, DONOR SCREENING, AND TISSUE RECOVERY, COLLECTION, AND ACQUISITION D1.000 GENERAL POLICIES In addition to the requirements at the series of standards at B1.500,
Joint UKBTS / NIBSC Professional Advisory Committee ( 1 ) Summary Sheet
 JPAC 11-05 Joint UKBTS / NIBSC Professional Advisory Committee ( 1 ) Summary Sheet 1. Paper for the JPAC meeting on: 10/3/11 2. Date submitted: 17 February 2011 3. Title (including version no.): Recommendations
JPAC 11-05 Joint UKBTS / NIBSC Professional Advisory Committee ( 1 ) Summary Sheet 1. Paper for the JPAC meeting on: 10/3/11 2. Date submitted: 17 February 2011 3. Title (including version no.): Recommendations
Cord Blood Medical History/ Health Assessment Questionnaire
 OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
OTT BRM EDM VAN Date: YYYY / MM / DD Phone Interview (if applicable) Informed Consent on file * Mandatory fields GENERAL INFORMATION *Last Name As per government ID *First Name As per government ID *DOB
CREUTZFELDT-JAKOB DISEASE (CJD)
 Cause/Epidemiology CREUTZFELDT-JAKOB DISEASE (CJD) The agent causing CJD and other human transmissible spongiform encephalopathy (TSE) has not yet been definitively identified. It was originally thought
Cause/Epidemiology CREUTZFELDT-JAKOB DISEASE (CJD) The agent causing CJD and other human transmissible spongiform encephalopathy (TSE) has not yet been definitively identified. It was originally thought
The DRAI (Donors >12 yrs old) versus Requirements
 Q# or Pg # Pg 1 Question or section as it appears on the DRAI questionnaire (Donors >12 yrs old) 5-7-12 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
Q# or Pg # Pg 1 Question or section as it appears on the DRAI questionnaire (Donors >12 yrs old) 5-7-12 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
HEALTH SCREENING QUESTIONNAIRE. Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
HEALTH SCREENING QUESTIONNAIRE Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca CT
Hot Topics in Tissue Safety
 Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
Hot Topics in Tissue Safety 37 th Annual AATB Meeting National Harbor, Maryland 03 October 2013 Scott A. Brubaker, CTBS Chief Policy Officer PHS Guideline for Reducing Human Immunodeficiency Virus, Hepatitis
Uniform DRAI - Donor >12 yrs old Requirements Crosswalk
 Q# or Pg # Pg 1 Question or section as it appears on the Uniform DRAI - Donor >12 yrs old 9-10-14 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
Q# or Pg # Pg 1 Question or section as it appears on the Uniform DRAI - Donor >12 yrs old 9-10-14 Donor Name, Person Interviewed (name, relationship), Contact Information (phone, address, city, state,
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Tested pathogen Donor test/ technique
2006 Guidelines for gamete and embryo donation
 2006 Guidelines for gamete and embryo donation The Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology Birmingham,
2006 Guidelines for gamete and embryo donation The Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology Birmingham,
USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire. Table of Contents
 Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Page 1 of 9 USER INSTRUCTIONS HPC, Cord Blood Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials Structure and Content Reformatting
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Appendix A: Disease-Specific Chapters
 Ministry of Health and Long-Term Care Infectious Diseases Protocol Appendix A: Disease-Specific Chapters Chapter: Creutzfeldt-Jakob Disease, all types Revised March 2017 Creutzfeldt-Jakob Disease, all
Ministry of Health and Long-Term Care Infectious Diseases Protocol Appendix A: Disease-Specific Chapters Chapter: Creutzfeldt-Jakob Disease, all types Revised March 2017 Creutzfeldt-Jakob Disease, all
CJD (Creutzfeldt-Jakob disease)
 CJD - lyodura and the risk of exposure during healthcare - frequently asked questions We would like to reassure all our patients that tight regulations govern all infection control processes at Addenbrooke's,
CJD - lyodura and the risk of exposure during healthcare - frequently asked questions We would like to reassure all our patients that tight regulations govern all infection control processes at Addenbrooke's,
The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC
 The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC Ryan A. Maddox, PhD Epidemiologist 2015 CJD Foundation Family Conference July 12, 2015 National Center for Emerging
The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC Ryan A. Maddox, PhD Epidemiologist 2015 CJD Foundation Family Conference July 12, 2015 National Center for Emerging
Pathogen Safety and BSE / variant CJD
 Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
Pathogen Safety and BSE / variant CJD Thomas R. Kreil, Global Pathogen Safety Parenteral Drug Industry Plasma Protein Industry Summit September 7, 2017; Beijing Pathogen Safety Those who cannot remember
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legally binding on national level More stringent testing - recommended on national level Not legally binding
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legally binding More stringent - recommended Not legally binding and not recommended Non-reproductive tissues and cells
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legally binding More stringent - recommended Not legally binding and not recommended Non-reproductive tissues and cells
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Capture Questions Methods of Administration DHQ Materials - Structure and
Non-reproductive tissues and cells
 Colour key Tested pathogen VIRAL Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recommended on national level Not
Colour key Tested pathogen VIRAL Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recommended on national level Not
Guidance for Industry
 Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
Guidance for Industry Informed Consent Recommendations for Source Plasma Donors Participating in Plasmapheresis and Immunization Programs Additional copies of this guidance are available from the Office
HEALTH SCREENING QUESTIONNAIRE CT Work-up. Donor ID EdgeCell #:
 HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
HEALTH SCREENING QUESTIONNAIRE CT Work-up Héma-Québec Registre des Donneurs de Cellules Souches 4045 Côte-vertu, St-Laurent, QC, Canada, H4R 2W7 Tél : + 514-832-1031 Fax : + 514-832-0266 www.hema-quebec.qc.ca
NATIONAL MARROW DONOR PROGRAM. Creating Connections. Saving Lives. Fran Rabe
 Creating Connections. Saving Lives. National Marrow Donor Program (NMDP) Screening of International Donors Fran Rabe FDA Liaison Meeting January 4, 2007 U.S. Importation of Peripheral Blood Stem Cells
Creating Connections. Saving Lives. National Marrow Donor Program (NMDP) Screening of International Donors Fran Rabe FDA Liaison Meeting January 4, 2007 U.S. Importation of Peripheral Blood Stem Cells
Creutzfeldt-Jakob Disease
 Community Infection Prevention and Control Guidance for Health and Social Care Creutzfeldt-Jakob Disease Version 1.01 May 2015 Harrogate and District NHS Foundation Trust Creutzfeldt-Jakob Disease May
Community Infection Prevention and Control Guidance for Health and Social Care Creutzfeldt-Jakob Disease Version 1.01 May 2015 Harrogate and District NHS Foundation Trust Creutzfeldt-Jakob Disease May
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Non-reproductive tissues and cells VIRAL
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More stringent - recommended Not legy binding and not recommended Non-reproductive tissues and cells VIRAL
Chronic Wasting Disease (CWD)
 Blood Safety The American Red Cross (ARC) is denying blood donations from individuals who have spent six months or more in Europe since 1980, as well as that of any blood relative of a CJD victim. Sporadic
Blood Safety The American Red Cross (ARC) is denying blood donations from individuals who have spent six months or more in Europe since 1980, as well as that of any blood relative of a CJD victim. Sporadic
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK
 RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
RECALL / EVENT INVESTIGATION & PATIENT LOOK BACK Federal, State and AABB requirements for nonconforming product investigation and recipient look back for adverse impact Stacy Ralston MPH, CLS (ASCP), RAC
Guidance for Industry
 Guidance for Industry Adequate and Appropriate Donor Screening Tests for Hepatitis B; Hepatitis B Surface Antigen (HBsAg) Assays Used to Test Donors of Whole Blood and Blood Components, Including Source
Guidance for Industry Adequate and Appropriate Donor Screening Tests for Hepatitis B; Hepatitis B Surface Antigen (HBsAg) Assays Used to Test Donors of Whole Blood and Blood Components, Including Source
Non-reproductive tissues and cells
 Ministry of Health: Institute for Transplantation and Biomedicine / Colour key VIRAL HIV 1 and HIV 2 Hepatitis B Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More
Ministry of Health: Institute for Transplantation and Biomedicine / Colour key VIRAL HIV 1 and HIV 2 Hepatitis B Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding More
The Centers for Disease Control and Prevention Report: A CDC CJD Q&A
 The Centers for Disease Control and Prevention Report: A CDC CJD Q&A Ryan A. Maddox, PhD Epidemiologist 2016 CJD Foundation Family Conference July 10, 2016 National Center for Emerging and Zoonotic Infectious
The Centers for Disease Control and Prevention Report: A CDC CJD Q&A Ryan A. Maddox, PhD Epidemiologist 2016 CJD Foundation Family Conference July 10, 2016 National Center for Emerging and Zoonotic Infectious
Non-reproductive tissues and cells Recommending authority/ association
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recomd on national level Not legy binding and not recomd
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent testing - legy binding on national level More stringent testing - recomd on national level Not legy binding and not recomd
Implementation: To be determined by each Service. Change Notification UK National Blood Services No
 Issued by JPAC: 18 July 2017 Implementation: To be determined by each Service Change Notification UK National Blood Services No. 17-2017 Malaria These changes apply to the individual Tissue and Cells Donor
Issued by JPAC: 18 July 2017 Implementation: To be determined by each Service Change Notification UK National Blood Services No. 17-2017 Malaria These changes apply to the individual Tissue and Cells Donor
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding on national level More stringent - recommended on national level Not legy binding and not recommended on
Colour key Minimum requirements as set out in Directive 2004/23/EC More stringent - legy binding on national level More stringent - recommended on national level Not legy binding and not recommended on
Creutzfeldt-Jakob disease (CJD)
 Creutzfeldt-Jakob disease (CJD) A topic in the Alzheimer s Association series on understanding dementia. Dementia is a condition in which a person has significant difficulty with daily functioning because
Creutzfeldt-Jakob disease (CJD) A topic in the Alzheimer s Association series on understanding dementia. Dementia is a condition in which a person has significant difficulty with daily functioning because
Guidance for Industry
 Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Guidance for Industry Use of Nucleic Acid Tests on Pooled and Individual Samples from Donors of Whole Blood and Blood Components (including Source Plasma and Source Leukocytes) to Adequately and Appropriately
Creutzfeldt-Jakob Disease Fact Sheet
 What is Creutzfeldt-Jakob Disease? Cretuzfeldt-Jakob disease (CJD) is a rare, degenerative, invariably fatal brain disorder. It affects about one person in every one million people worldwide and about
What is Creutzfeldt-Jakob Disease? Cretuzfeldt-Jakob disease (CJD) is a rare, degenerative, invariably fatal brain disorder. It affects about one person in every one million people worldwide and about
Not Under Document Control If Printed. Donor Educational Materials. If you are deferred
 Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
Jul 2009 (yellow version) Donor Educational Materials Thank you for coming in today! These educational materials explain how you can help us make the donation process safe for you and patients who might
LEVEL 3 CERTIFICATE / DIPLOMA 4463U10-1A. MEDICAL SCIENCE UNIT 1: Human Health and Disease
 LEVEL 3 CERTIFICATE / DIPLOMA 4463U10-1A S18-4463U10-1A MEDICAL SCIENCE UNIT 1: Human Health and Disease Summer 2018 Pre-Release Article for use in the following examination on 21 May 2018 Level 3 Diploma
LEVEL 3 CERTIFICATE / DIPLOMA 4463U10-1A S18-4463U10-1A MEDICAL SCIENCE UNIT 1: Human Health and Disease Summer 2018 Pre-Release Article for use in the following examination on 21 May 2018 Level 3 Diploma
Current Infectious Disease Screening for the Live Organ Donor
 2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
2013 Public Health and Safety Guidelines for reducing HIV, HBV and HCV Transmission Though Organ Transplantation: Implications for Counseling and Disclosure Dianne LaPointe Rudow DNP, ANP-BC, CCTC Director
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS
 EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
EDUCATIONAL COMMENTARY EMERGING INFECTIOUS DISEASE AGENTS Educational commentary is provided through our affiliation with the American Society for Clinical Pathology (ASCP). To obtain FREE CME/CMLE credits
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire. Table of Contents
 User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Methods of Administration DHQ Materials - Structure and Content Reformatting
User Instructions Allogeneic HPC, Apheresis and HPC, Marrow Donor History Questionnaire Table of Contents Purpose Introduction Methods of Administration DHQ Materials - Structure and Content Reformatting
CREUTZFELDT-JAKOB DISEASE (CJD), CLASSIC
 CREUTZFELDT-JAKOB DISEASE (CJD), CLASSIC SPADIC CREUTZFELDT-JAKOB DISEASE (SCJD) Case definition CONFIRMED CASE Neuropathologically and/or immunocytochemically and/or biochemically confirmed, through observation
CREUTZFELDT-JAKOB DISEASE (CJD), CLASSIC SPADIC CREUTZFELDT-JAKOB DISEASE (SCJD) Case definition CONFIRMED CASE Neuropathologically and/or immunocytochemically and/or biochemically confirmed, through observation
Department of Health and Human Services
 Tuesday, May 25, 2004 Part II Department of Health and Human Services Food and Drug Administration 21 CFR Parts 210, 211, 820, and 1271 Eligibility Determination for Donors of Human Cells, Tissues, and
Tuesday, May 25, 2004 Part II Department of Health and Human Services Food and Drug Administration 21 CFR Parts 210, 211, 820, and 1271 Eligibility Determination for Donors of Human Cells, Tissues, and
Non-reproductive tissues and cells
 Colour key Minimum requirements as set out in Directive 2004/23/EC and its technical Directives (particularly 2006/17/EC) More stringent -legally binding, applies for all donations and all donor profiles
Colour key Minimum requirements as set out in Directive 2004/23/EC and its technical Directives (particularly 2006/17/EC) More stringent -legally binding, applies for all donations and all donor profiles
The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC
 The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC Ryan A. Maddox, PhD Epidemiologist CJD 2013 and the 11 th Annual CJD Foundation Family Conference July 14, 2013 National
The Centers for Disease Control and Prevention Report: Prion Disease Activities at CDC Ryan A. Maddox, PhD Epidemiologist CJD 2013 and the 11 th Annual CJD Foundation Family Conference July 14, 2013 National
Human tissues and cells annual report 2011
 Human tissues and cells annual report 2011 2012 Human tissues and cells Annual report Danish Health and Medicines Authority, 2012. This publication can be freely cited with a clear indication of source.
Human tissues and cells annual report 2011 2012 Human tissues and cells Annual report Danish Health and Medicines Authority, 2012. This publication can be freely cited with a clear indication of source.
DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J
 JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
JON S. CORZINE Governor DEPARTMENT OF HEALTH AND SENIOR SERVICES PO BOX 360 TRENTON, N.J. 08625-0360 www.nj.gov/health HEATHER HOWARD Commissioner TO: FROM: Public Health Council Heather Howard Commissioner
CORD BLOOD TRANSPLANTATION STUDY CBU EXCLUSION AND QUARANTINE RELEASE FORM
 (Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
(Any answer of Yes indicates exclusion) 1. Informed Consent and Maternal/Infant Demographics a. Lack of confirmation of signed and witnessed informed consent 1 Yes 2 No Is the following information missing
Draft Agreed by Biologics Working Party December Draft Agreed by Blood Products Working party December 2010
 15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
15 December 2011 EMA/CHMP/BWP/360642/2010 rev. 1 Committee for Medicinal Products for Human Use (CHMP) Guideline on the warning on transmissible agents in summary of product characteristics (SmPCs) and
Screening donors and donations for transfusion transmissible infectious agents. Alan Kitchen
 Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
Screening donors and donations for transfusion transmissible infectious agents Alan Kitchen Aim Not to teach you microbiology To provide and awareness of the big picture To provide an understanding of
REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
 EUROPEAN COMMISSION Brussels, 17.6.011 COM(011) 35 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
EUROPEAN COMMISSION Brussels, 17.6.011 COM(011) 35 final REPORT FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
Guidance for Industry
 Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
Guidance for Industry Advisement for the Use of Gen-Probe Procleix Ultrio Plus Assay for NAT Testing on Cadaveric Donors Release Date: 08/03/14 American Medical Education and Research Association 4757
The New Regulations - Special IVD Issues
 The New Regulations - Special IVD Issues Dirk Stynen, Ph. D. President - Principal Consultant Qarad Geel, Belgium RMD Brussels October 2018 The IVD Regulation 2017/746 October 29, 2018 www.qarad.com 2
The New Regulations - Special IVD Issues Dirk Stynen, Ph. D. President - Principal Consultant Qarad Geel, Belgium RMD Brussels October 2018 The IVD Regulation 2017/746 October 29, 2018 www.qarad.com 2
Inspections, Compliance, Enforcement, and Criminal Investigations. Central Texas Regional Blood & Tissue Center 07-Nov-03
 1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
1 of 7 6/10/2009 2:20 PM Inspections, Compliance, Enforcement, and Criminal Investigations Department of Health and Human Services Public Health Service Food and Drug Administration Dallas District 4040
Donor Risk Assessment Interview (Donor >12 yrs old)
 Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
Your logo Donor Risk Assessment Interview (Donor >12 yrs old) Your address Donor Name: First Middle Last Person Interviewed: Name Relationship Contact Information: ( ) Phone Address City State Zip The
Creutzfeldt-Jakob Disease
 Community Infection Prevention and Control Guidance for Health and Social Care Creutzfeldt-Jakob Disease Version 1.00 October 2015 Cumbria County Council Creutzfeldt-Jakob Disease October 2015 Version
Community Infection Prevention and Control Guidance for Health and Social Care Creutzfeldt-Jakob Disease Version 1.00 October 2015 Cumbria County Council Creutzfeldt-Jakob Disease October 2015 Version
Breastfeeding Center Milk Connection Questionnaire and Release for Donor
 Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
Breastfeeding Center Milk Connection Questionnaire and Release for Donor All of us at The Breastfeeding Center are deeply grateful to you for your important gift to infants in our community. Your donated
CHAPTER 1: SEXUALLY TRANSMITTED AND BLOODBORNE INFECTIONS
 CHAPTER : SEXUALLY TRANSMITTED AND BLOODBORNE INFECTIONS Highlights In Peel, the incidence of Acquired Immunodeficiency Syndrome (AIDS) has remained low (. to. cases per,) since the introduction of the
CHAPTER : SEXUALLY TRANSMITTED AND BLOODBORNE INFECTIONS Highlights In Peel, the incidence of Acquired Immunodeficiency Syndrome (AIDS) has remained low (. to. cases per,) since the introduction of the
Strategic partner for the Québec health system
 BLOOD PRODUCTS STABLE PRODUCTS STEM CELLS Strategic partner for the Québec health system HUMAN TISSUES MOTHER S MILK CORPORATE PRESENTATION MISSION To efficiently meet the needs of the Québec population
BLOOD PRODUCTS STABLE PRODUCTS STEM CELLS Strategic partner for the Québec health system HUMAN TISSUES MOTHER S MILK CORPORATE PRESENTATION MISSION To efficiently meet the needs of the Québec population
COMMUNICABLE DISEASES EPIDEMIOLOGIC REPORT
 NEW BRUNSWICK COMMUNICABLE DISEASES EPIDEMIOLOGIC REPORT 2004 COMMUNICABLE DISEASES EPIDEMIOLOGIC REPORT 2004 2 The New Brunswick Communicable Diseases Epidemiologic Report is published by Public Health
NEW BRUNSWICK COMMUNICABLE DISEASES EPIDEMIOLOGIC REPORT 2004 COMMUNICABLE DISEASES EPIDEMIOLOGIC REPORT 2004 2 The New Brunswick Communicable Diseases Epidemiologic Report is published by Public Health
INFECTED BLOOD INQUIRY TERMS OF REFERENCE
 INFECTED BLOOD INQUIRY TERMS OF REFERENCE What happened and why? 1. To examine the circumstances in which men, women and children 1 treated by national Health Services in the United Kingdom (collectively,
INFECTED BLOOD INQUIRY TERMS OF REFERENCE What happened and why? 1. To examine the circumstances in which men, women and children 1 treated by national Health Services in the United Kingdom (collectively,
Policy for Infection Prevention and Control of Transmissible Spongiform Encephalopathies (TSE)
 Policy for Infection Prevention and Control of Transmissible Spongiform Encephalopathies (TSE) Page 1 of 9 Document Control Sheet Name of document: Policy for Infection Prevention and Control of Transmissible
Policy for Infection Prevention and Control of Transmissible Spongiform Encephalopathies (TSE) Page 1 of 9 Document Control Sheet Name of document: Policy for Infection Prevention and Control of Transmissible
HIV/AIDS. Kuna High School Mr. Stanley
 HIV/AIDS Kuna High School Mr. Stanley Questions 1. Write an example of how your immune system helps prevent you from getting diseases. Terms to know Epidemic - a widespread occurrence of an infectious
HIV/AIDS Kuna High School Mr. Stanley Questions 1. Write an example of how your immune system helps prevent you from getting diseases. Terms to know Epidemic - a widespread occurrence of an infectious
BLOOD COMPONENTS are regulated as
 Managing Recalls and Withdrawals of Blood Components Glenn Ramsey Donor centers are issuing a growing number of recalls and market withdrawals to hospital transfusion services about blood components. More
Managing Recalls and Withdrawals of Blood Components Glenn Ramsey Donor centers are issuing a growing number of recalls and market withdrawals to hospital transfusion services about blood components. More
FAMILY EDITION IN THIS ISSUE
 IN THIS ISSUE Introduction New Cerebrospinal Fluid Protein Tests Offered Genetics and Creutzfeldt-Jakob Disease Second Case of Variant CJD in Canada You Asked Us Consent Form for Donation of Biological
IN THIS ISSUE Introduction New Cerebrospinal Fluid Protein Tests Offered Genetics and Creutzfeldt-Jakob Disease Second Case of Variant CJD in Canada You Asked Us Consent Form for Donation of Biological
June 8, Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
June 8, 2018 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA 2016 D 0545,
New Zealand Bone Marrow Donor Registry
 Title: SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION Authorised by: Executive Officer Contributing Authors: ABMDR Dr Hilary Blacklock Raewyn Fisher NZBMDR-Guidelines-004-13 Date
Title: SECTION 4.0 NZBMDR STANDARDS DONOR RECRUITMENT, ENROLMENT & INFORMATION Authorised by: Executive Officer Contributing Authors: ABMDR Dr Hilary Blacklock Raewyn Fisher NZBMDR-Guidelines-004-13 Date
MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE BACKGROUND
 MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE Countries around the world are reviewing the policy of deferring from blood donation men who have had sex with men (MSM). Both Canadian Blood Services
MSM DEFERRAL POLICY ISSUE DOCUMENT: MSM DEFERRAL ISSUE Countries around the world are reviewing the policy of deferring from blood donation men who have had sex with men (MSM). Both Canadian Blood Services
BLOOD DONATION Northern Ireland Blood Tranfusion Service
 FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
FREQUENTLY ASKED QUESTIONS BLOOD DONATION Northern Ireland Blood Tranfusion Service Why do we need donated blood? Everyone knows blood is literally a lifesaver for those who have been in an accident or
Patient Name: MRN: DOB: Treatment Location:
 Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Page 1 of 5 I. TO (Required) This Section is required to be completed by all patients who undergo kidney transplant surgery. I hereby consent to and authorize Dr. and his/her assistant(s), including supervised
Creutzfeldt-Jakob Disease
 Creutzfeldt-Jakob Disease Other Dementias Introduction Alzheimer s disease is one type of a large group of disorders known as dementias. It is an irreversible disease of the brain in which the progressive
Creutzfeldt-Jakob Disease Other Dementias Introduction Alzheimer s disease is one type of a large group of disorders known as dementias. It is an irreversible disease of the brain in which the progressive
Guidance for Industry
 Reprinted from FDA s website by EAS Consulting Group, LLC Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening
Reprinted from FDA s website by EAS Consulting Group, LLC Guidance for Industry Recommendations for Screening, Testing, and Management of Blood Donors and Blood and Blood Components Based on Screening
Structure and duties of Blood service
 NATIONAL CENTER FOR TRANSFUSION MEDICINE Prevalence of infectious diseases among Mongolian blood donors Namjil Erdenebayar General director of The National Center for Transfusion Medicine, Mongolia NCTM,
NATIONAL CENTER FOR TRANSFUSION MEDICINE Prevalence of infectious diseases among Mongolian blood donors Namjil Erdenebayar General director of The National Center for Transfusion Medicine, Mongolia NCTM,
UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID
 UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID CONSENT FOR MINOR s SPERM OR TESTIS CRYOPRESERVATION
UNIVERSITY OF WASHINGTON MEDICAL CENTER Men s Health Center and Male Fertility Laboratory Sperm & Testis Cryopreservation Program Patient NAME and ID CONSENT FOR MINOR s SPERM OR TESTIS CRYOPRESERVATION
Egg and Sperm Bank Edinburgh Fertility & Reproductive Endocrine Centre (EFREC) DONOR INFORMATION Screening Tests
 Egg and Sperm Bank Edinburgh Fertility & Reproductive Endocrine Centre (EFREC) DONOR INFORMATION Screening Tests Introduction As a potential donor, it is important you are aware of the screening tests
Egg and Sperm Bank Edinburgh Fertility & Reproductive Endocrine Centre (EFREC) DONOR INFORMATION Screening Tests Introduction As a potential donor, it is important you are aware of the screening tests
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services
 Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
Donation Criteria for Men who have Sex with Men (MSM): Update from Canadian Blood Services Outline 1 Current criteria Proposed criteria Stakeholder outreach Outline 2 Safety Assessment Results since implementation
London, 24 April 2001 EMEA/CPMP/BWP/819/01
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 24 April 2001 EMEA/CPMP/BWP/819/01 QUESTIONS AND ANSWERS ON BOVINE SPONGIFORM ENCEPHALOPATHIES
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 24 April 2001 EMEA/CPMP/BWP/819/01 QUESTIONS AND ANSWERS ON BOVINE SPONGIFORM ENCEPHALOPATHIES
QSEAL Recovered Plasma Specification
 QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
QSEAL Recovered Plasma Specification Background The is part of a series of standards that comprise the Plasma Protein Therapeutics Association (PPTA) QSEAL Standards Program. PPTA's Voluntary Standards
Understanding IVF Processes in Surrogacy
 Melvin H. Thornton II MD Medical Director CT Fertility Understanding IVF Processes in Surrogacy The Basics Surrogacy involves multiple parties IVF CLINIC Egg donors screening and matching* Medical process
Melvin H. Thornton II MD Medical Director CT Fertility Understanding IVF Processes in Surrogacy The Basics Surrogacy involves multiple parties IVF CLINIC Egg donors screening and matching* Medical process
Zika Virus. Report to the Standing Committee on Health. Dr. Graham Sher Chief Executive Officer, Canadian Blood Services
 Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
Dr. Graham Sher Chief Executive Officer, Services Dr. Dana Devine Chief Medical and Scientific Officer, Services Services Services is an arm s-length organization within the larger health-care system of
DONOR ELIGIBILITY EYE & TISSUE DREW TIMMONS, RN, CTBS TRANSPLANT SERVICES CENTER UT SOUTHWESTERN MEDICAL CENTER
 DONOR ELIGIBILITY EYE & TISSUE DREW TIMMONS, RN, CTBS TRANSPLANT SERVICES CENTER UT SOUTHWESTERN MEDICAL CENTER FACTORS IMPACTING EYE & TISSUE DONOR EVALUATION Institution Policy Clinical Experience Training
DONOR ELIGIBILITY EYE & TISSUE DREW TIMMONS, RN, CTBS TRANSPLANT SERVICES CENTER UT SOUTHWESTERN MEDICAL CENTER FACTORS IMPACTING EYE & TISSUE DONOR EVALUATION Institution Policy Clinical Experience Training
More Fun Than Giving Blood CLINIC RECRUITED
 More Fun Than Giving Blood CLINIC RECRUITED Are you thinking of becoming a sperm donor? Choosing to become a sperm donor is a generous act, and by entering our sperm donor program you can help make someone
More Fun Than Giving Blood CLINIC RECRUITED Are you thinking of becoming a sperm donor? Choosing to become a sperm donor is a generous act, and by entering our sperm donor program you can help make someone
For more information about how to cite these materials visit
 Author(s): Robertson Davenport, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Noncommercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Robertson Davenport, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Noncommercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood. Liz Caffrey, Patricia Hewitt and John Barbara
 CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
CHAPTER 1 The Blood Donor: Demographics, Donor Selection and Tests on Donor Blood Liz Caffrey, Patricia Hewitt and John Barbara 0.3 OVERVIEW Total 0.254 0.247 A safe and sufficient blood supply depends
Center for Biologics Evaluation and Research
 Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
Center for Biologics Evaluation and Research Current Activities Future Directions Washington, DC January 25, 2010 Karen Midthun, MD Acting Director, CBER CBER Our Mission To ensure the safety, purity,
COMMUNICABLE DISEASE REPORT
 June 2011 Department of Health and Community Services Government of Newfoundland and Labrador COMMUNICABLE DISEASE REPORT Reporting Sexually Transmitted and Bloodborne Infections All laboratory-confirmed
June 2011 Department of Health and Community Services Government of Newfoundland and Labrador COMMUNICABLE DISEASE REPORT Reporting Sexually Transmitted and Bloodborne Infections All laboratory-confirmed
TRANSFUSION ASSOCIATED DISEASE, RECALL, OR COMPLICATION INVESTIGATION POLICY I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION:
 I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
I. FATALITIES AND COMPLICATIONS ASSOCIATED WITH TRANSFUSION: A. TRANSFUSION RELATED FATALITY: FDA and MEDIC must be notified immediately, and subsequently in writing, when a possible transfusion related
2.0 MINIMUM PROCUREMENT STANDARDS FOR AN ORGAN PROCUREMENT ORGANIZATION (OPO)
 2.0 MINIMUM PROCUREMENT STANDARDS FOR AN ORGAN PROCUREMENT ORGANIZATION (OPO) In order to maximize the gift of donation and optimize recipient outcomes and safety, the Organ Procurement Organization (OPO)
2.0 MINIMUM PROCUREMENT STANDARDS FOR AN ORGAN PROCUREMENT ORGANIZATION (OPO) In order to maximize the gift of donation and optimize recipient outcomes and safety, the Organ Procurement Organization (OPO)
23 November Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm Rockville, MD 20852
 23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
23 November 2016 Division of Dockets Management (HFA 305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Submitted via http://www.regulations.gov Re: Docket No. FDA-2016-N-1502,
Guidance Document for Source Establishments Reporting Adverse Reactions to Human Cells, Tissues and Organs
 Guidance Document for Source Establishments Reporting Adverse Reactions to Human Cells, Tissues and Organs (Effective Date: 2010-05-25) Canada Vigilance Adverse Reaction Monitoring Program and Database,
Guidance Document for Source Establishments Reporting Adverse Reactions to Human Cells, Tissues and Organs (Effective Date: 2010-05-25) Canada Vigilance Adverse Reaction Monitoring Program and Database,
