COMPENDIUM OF MONOGRAPHS NATURAL HEALTH PRODUCTS DIRECTORATE
|
|
|
- Dortha Holt
- 5 years ago
- Views:
Transcription
1 COMPENDIUM OF MONOGRAPHS NATURAL HEALTH PRODUCTS DIRECTORATE June Version 3.0 i
2 FOREWORD Guidance documents are meant to provide assistance to industry and health care practitioners on how to comply with governing statutes and regulations. Guidance documents also provide assistance to staff on how Health Canada mandates and objectives should be implemented in a manner that is fair, consistent and effective. Guidance documents are administrative instruments and therefore allow for flexibility. Alternate approaches to the principles and practices described in this document may be acceptable; licence applicants are invited to discuss these with the Natural Health Products Directorate prior to submitting an application. As a corollary to the above, it is equally important to note that Health Canada may request information or material, or define conditions not specifically described in this document, in order to enable the Department to adequately assess the safety, efficacy or quality of a health product. Health Canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. This document should be read in conjunction with the Natural Health Products Regulations and relevant sections of other applicable guidance documents. ii
3 TABLE OF CONTENTS INTRODUCTION ABOUT THE COMPENDIUM Compendial Applications Elements of a Monograph References Used in NHPD Monograph Development Revising and Adding Monographs TYPES OF MONOGRAPHS Single Ingredient Monographs Product Monographs Combination of NHPD Pre-cleared Information SPECIFICATIONS... 7
4 INTRODUCTION The Compendium is a compilation of monographs based on natural health product (NHP) ingredients. The Natural Health Products Directorate (NHPD) developed the Compendium of Monographs as a tool for the timely and efficient review of the safety and efficacy of many commonly used NHPs. The NHPD allows applicants to reference NHPD monographs in support of the safety and efficacy of medicinal ingredients as part of their Product Licence Application (PLA). This process is efficient for both applicants and Health Canada, since there is no need to evaluate the safety and efficacy of NHP ingredients that are already known to be safe and efficacious when used under the conditions specified in the NHPD monographs. For more information on the product licence application process and the evidence required to support the safety (risk) and efficacy (benefit) of NHPs, please refer to the Pathway for Licensing Natural Health Products Making Modern Health Claims and the Pathway for Licensing Natural Health Products Used as Traditional Medicines. The Compendium consists of single ingredient monographs, product monographs and criteria for the combination of monographed ingredients. For a list of published NHPD Single Ingredient and Product Monographs, see the Natural Health Product Ingredient Database (NHPID). For acceptable non-medicinal ingredients that can be used in natural health products, see the NHPID. Compendium of Monographs Version 3.0 (May 2013) 1
5 1.0 ABOUT THE COMPENDIUM 1.1 Compendial Applications Natural Health Products Regulations Part 1: PRODUCT LICENCES Sixty-Day Disposition Section 6 6. (1) Subject to subsection (2), the Minister shall dispose of an application submitted under section 5 within 60 days after the day on which it is submitted if, in support of the application, the only information submitted by the applicant under paragraph 5(g) is that which is a. in the case of an application respecting a natural health product that has only one medicinal ingredient, contained in a monograph for that medicinal ingredient in the Compendium; and b. in the case of an application respecting a natural health product that has more than one medicinal ingredient, contained in a monograph for that combination of medicinal ingredients in the Compendium. (2) If the Minister requests that additional information or samples be submitted under section 15, the 60- day period referred to in subsection (1) does not include the number of days beginning on the day on which the request is made and ending on the day on which the additional information or samples are received. (3) For the purposes of this section, the Minister disposes of an application on the earlier of the day on which a. the licence is issued in accordance with section 7; and b. the applicant is sent a notice under subsection 9(1). A compendial application cites NHPD monographs in the Compendium to support the safety and efficacy of medicinal ingredient(s) in a NHP. All other aspects of manufacturing and preparing the product for sale, including good manufacturing practices and labelling, must comply with the Natural Health Product Regulations (NHPR). For more information, see the Quality of Natural Health Products Guide, the Labelling guidance document, the Good Manufacturing Practices guidance document and the relevant sections of the Product Licensing guidance document. 1.2 Elements of a Monograph When submitting a compendial application, several items on the PLA form must match the monograph content exactly or fall within its parameters, including the proper name, common name, source material, route of administration, dosage form and recommended dose. The remaining sections of the monograph - use or purpose, directions of use, duration of use and risk information - may use a statement to the effect of, unless otherwise stated on the monograph. This allows applicants to alter the Compendium of Monographs Version 3.0 (May 2013) 2
6 wording, but not the intent, of the monograph elements. Any statement to the effect of wording may be evaluated to determine whether it has the same intent as the monograph. The following are the parameters for the use of a NHPD monograph. Proper name: The proper name must be chosen from one of the proper name options provided in the monograph. Common name: The common name must be chosen from one of the common name options provided in the monograph. Source material: The source material must be chosen from the options provided in the monograph. More than one source material is acceptable, provided that all source materials listed in the PLA form reflect the same dose and/or use or purpose on the referenced monograph. Route of administration: The route of administration must be chosen from the options provided in the monograph. Please see the Controlled Vocabulary section of the NHPID for a description of the routes of administration. Dosage form: The dosage form must be chosen from the options provided on the monograph and must reflect the route of administration for the product. The dosage form must be chosen from the list of recognized dosage forms, found in the NHPID under the Controlled Vocabulary section. Please note that an NHP in a liposomal formulation is not considered equivalent to an NHP in a non-liposomal formulation. Therefore applicants cannot attest to a monograph for safety and efficacy of an ingredient unless the monograph specifically states that liposomal formulations are acceptable. Products with liposomal formulations must be submitted through the appropriate noncompendial assessment stream with specific evidence to support the liposomal formulation. Recommended use or purpose: Claims have been identified for each monographed ingredient based on NHPD s evaluation of the safety and efficacy data. Applicants may choose one or more claims provided in the monograph or create an alternative using a statement to the effect of, unless otherwise stated. Applicants must ensure that any conditions surrounding the claim (dose, source material, etc.) are met. Dose: The total daily dose must be equal to that noted in the monograph, or, when a range is specified, fall within the range indicated in the monograph. The dose indicated on the monograph may be specific to: Subpopulation: All monographs are intended for adults, unless otherwise specified. Method of preparation: Must be chosen from the list of acceptable methods, if indicated. Furthermore, to make a traditional use claim, the method of preparation must be one that was traditionally used. Please see Compendium of Monographs Version 3.0 (May 2013) 3
7 the Pathway for Licensing Natural Health Products Used as Traditional Medicines guidance document for a list of traditional methods of preparation. Potency: When a monograph includes potency, it must be included in the PLA, unless otherwise specified. Frequency: The frequency must be the same as or fall within the range of the frequency on the monograph, when specified. When the monograph specifies a divided dose the frequency must be more than once daily. If no frequency is specified, the applicant may select an appropriate frequency. Directions of use: Where specified, all directions of use must be included in the PLA. The directions of use may be identical to that on the monograph or may be a statement to the effect of, unless otherwise stated. Duration of use: When the monograph includes a duration of use, it must be included on the PLA. Risk information: All risk information contained in the monograph must be included in the PLA, as applicable. The risk information may be identical to that on the monograph, or may be a statement to the effect of, unless otherwise stated. Non-medicinal ingredients: Only non-medicinal ingredients listed in the NHPID may be used with an appropriate excipient purpose. Any applicable restrictions indicated in the database must be met. The presence of non-medicinal ingredients without conditions on the List of Prohibited and Restricted Cosmetic Ingredients (the Cosmetic Ingredient Hotlist), indicates that there are potentially significant safety issues with these ingredients. If the hotlist indicates that additional evidence is required for an ingredient or if an ingredient is listed with no specified conditions, it is not permitted in a topical product submitted in the compendial stream. If the hotlist specifies certain conditions for an ingredient, or label requirements, it is the responsibility of the license holder to ensure that the ingredient meets the conditions outlined. Requirements for non-medicinal ingredients are outlined in the Quality of Natural Health Products Guide, Pathway for Licensing Natural Health Products Making Modern Health Claims and Pathway for Licensing Natural Health Products Used as Traditional Medicines guidance documents. Storage conditions: When the monograph includes storage conditions, they must appear on the product label as per Section 87 of the NHPR. Specifications: Note that certain monographs include additional specifications relevant to that ingredient or product. This information should be considered when establishing product specifications. Compendium of Monographs Version 3.0 (May 2013) 4
8 1.3 References Used in NHPD Monograph Development The Compendium is comprised of monographs based on information obtained from modern and traditional sources with regard to the safety and/or efficacy associated with the use of NHPs. Developing the monographs involves considering information gathered from sources such as: published compendia such as those of the World Health Organization, European Scientific Cooperative on Phytotherapy, British Herbal Pharmacopoeia, Pharmacopoeia of the People s Republic of China, European Medicines Agency: Committee on Herbal Medicinal Products (HMPC), and the German Commission E; articles published in peer-reviewed journals; information published in national pharmacopoeias such as the United States Pharmacopeia, British Pharmacopoeia, European Pharmacopoeia, Pharmacopée Française; other published expert committee reports such as the Dietary Reference Intakes ( ), and the U.S. Agency for Health Care Research and Quality (ongoing); and Health Canada publications such the Therapeutic Products Directorate s Category IV Monographs and Labelling Standards. 1.4 Revising and Adding Monographs Monographs are revised periodically. If new information is published or if a safety or efficacy issue is identified, a monograph will be updated accordingly. A notice to affected stakeholders requesting information may be issued when a monograph is revised for safety reasons and a risk has been identified. Regarding any other monograph revisions, product licence holders are expected to align products affected by the revision(s) with the current monographs, as applicable. This can be accomplished by submitting a post licensing change. Please see the Post Licensing guidance document for more information on these types of changes and how to submit such changes to the NHPD. Any new PLA s referencing a NHPD monograph will be expected to comply with the current version of the monograph published in the Compendium, found in the NHPID. The NHPD considers the following when determining which monographs may be developed or revised: High proportion of applications in queue with NHPD that contain specific ingredients. Changes to safety and/or efficacy profile of an ingredient. Amount of safety and/or efficacy data available for a particular ingredient. Directorate, Department and/or Government strategic priorities. Compendium of Monographs Version 3.0 (May 2013) 5
9 Signals from international associations, agencies and/or regulatory bodies. Suggestions from stakeholders. Suggestions for revisions to currently published monographs and suggestions for ingredients that should be the subject of a monograph and included in the Compendium can be submitted to NHPD at ingredient_support@hc-sc.gc.ca via the Ingredient Database Issue Form. The form should include the name of the monograph being amended along with the rationale and supporting scientific data for consideration. 2.0 TYPES OF MONOGRAPHS 2.1 Single Ingredient Monographs Applicants may reference a single ingredient monograph that supports the safety and efficacy of a NHP as part of their PLA in the compendial stream. Safety-only monographs address only the safety of the medicinal ingredient. No recommended uses or purposes are associated with these monographs and on their own cannot support the licensing of a product via the compendial stream. The NHPD strives to revise safety-only monographs periodically. When the available body of evidence supports the inclusion of a recommended use or purpose, safety-only monographs will be revised. Applicants cannot reference safety-only monographs in the compendial application stream for single ingredient products; however safety-only monographs, like all monographs, can be used to support the safety of individual ingredients in non-compendial PLA s, provided that the conditions of use on the monographs are met. 2.2 Product Monographs Product monographs are composed of more than one medicinal ingredient and may outline the conditions of use based on a product category (e.g. antiseptic hand cleansers, diaper rash products, Multi-Vitamin/Mineral Supplements, etc.) and not the ingredient. As such, a single ingredient may appear on several product monographs. Therefore, the conditions of use for that ingredient, such as dose, recommended use or purpose and risk information, will differ depending on the product category. Upon the coming into force of the NHPR and the classification of certain ingredients as NHPs, the NHPD adopted relevant Therapeutic Product Directorate (TPD) Category IV Monographs and Labelling Standards (LS) into NHPD product monographs, and has incorporated them in the NHPD Compendium of Monographs. Work is ongoing to convert all relevant Category IV monographs and LS to compendial monographs. Compendium of Monographs Version 3.0 (May 2013) 6
10 2.3 Combination of NHPD Pre-cleared Information Please refer to the Pathway for Licensing Natural Health Products Making Modern Claims for more information regarding combining pre-cleared information. 3.0 SPECIFICATIONS The submission of a signed PLA will be regarded as an attestation acknowledging the licence holder s responsibility to meet the requirements set out in the NHPR and associated guidance documents relating to quality and Good Manufacturing Practices. The Quality of Natural Health Products Guide and the Good Manufacturing Practices guidance document outline expectations and approaches relating to the quality requirements for NHPs. Furthermore, individual monographs and the NHPID may also include ingredient specific considerations pertinent to the quality of the product. By submitting a PLA, the applicant is attesting to meeting the general specifications included in the Quality of Natural Health Products Guide. If the product specifications fall outside the general specifications provided in the Quality of Natural Health Products Guide, the applicant must submit their own product specifications. Any product specifications submitted should be established in accordance with the requirements and principles described in the Quality of Natural Health Products Guide. Applicants are responsible for ensuring that all information is documented, maintained, relevant, accurate, and sufficient to support the quality of their NHPs. This documentation could be requested by the NHPD at any time. Compendium of Monographs Version 3.0 (May 2013) 7
Questions or comments regarding the content of the NHPD Monthly Communiqué may be addressed to
 The NHPD Monthly Communiqué is a publication of Health Canada s Natural Health Products Directorate (NHPD), the federal department responsible for the regulation of natural health products sold in Canada.
The NHPD Monthly Communiqué is a publication of Health Canada s Natural Health Products Directorate (NHPD), the federal department responsible for the regulation of natural health products sold in Canada.
DRAFT GUIDANCE DOCUMENT Comparative Bioavailability Standards: Formulations Used for Systemic Effects
 1 2 3 DRAFT GUIDANCE DOCUMENT Comparative Bioavailability Standards: Formulations Used for Systemic Effects 4 This guidance document is being distributed for comment purposes only. 5 6 Published by authority
1 2 3 DRAFT GUIDANCE DOCUMENT Comparative Bioavailability Standards: Formulations Used for Systemic Effects 4 This guidance document is being distributed for comment purposes only. 5 6 Published by authority
EVIDENCE FOR SAFETY AND EFFICACY OF FINISHED NATURAL HEALTH PRODUCTS NATURAL HEALTH PRODUCTS DIRECTORATE
 EVIDENCE FOR SAFETY AND EFFICACY OF FINISHED NATURAL HEALTH PRODUCTS NATURAL HEALTH PRODUCTS DIRECTORATE December 2006 Version 2.0 Our mission is to help the people of Canada maintain and improve their
EVIDENCE FOR SAFETY AND EFFICACY OF FINISHED NATURAL HEALTH PRODUCTS NATURAL HEALTH PRODUCTS DIRECTORATE December 2006 Version 2.0 Our mission is to help the people of Canada maintain and improve their
GUIDANCE DOCUMENT Non-prescription Oral Adult Antitussive Cough and Cold Labelling Standard
 GUIDANCE DOCUMENT Cough and Cold Labelling Standard Published by authority of the Minister of Health Date Adopted 2015/07/09 Effective Date 2015/07/31 Health Products and Food Branch Our mission is to
GUIDANCE DOCUMENT Cough and Cold Labelling Standard Published by authority of the Minister of Health Date Adopted 2015/07/09 Effective Date 2015/07/31 Health Products and Food Branch Our mission is to
NHPD Monthly Communiqué
 NHPD Monthly Communiqué Vol. 3, Issue 5 February 2008 The NHPD Monthly Communiqué is a publication of Health Canada s Natural Health Products Directorate (NHPD), the federal department responsible for
NHPD Monthly Communiqué Vol. 3, Issue 5 February 2008 The NHPD Monthly Communiqué is a publication of Health Canada s Natural Health Products Directorate (NHPD), the federal department responsible for
M I L L E R T H O M S O N LLP Barristers & Solicitors, Patent & Trade Mark Agents
 M I L L E R T H O M S O N LLP Barristers & Solicitors, Patent & Trade Mark Agents Communiqué for Health Industry Clients on the Legal Retainer Program Canada s New Natural Health Products Regulations On
M I L L E R T H O M S O N LLP Barristers & Solicitors, Patent & Trade Mark Agents Communiqué for Health Industry Clients on the Legal Retainer Program Canada s New Natural Health Products Regulations On
GUIDANCE DOCUMENT Non-prescription Oral Adult Nasal Decongestant Labelling Standard
 GUIDANCE DOCUMENT Decongestant Labelling Standard Published by authority of the Minister of Health Date Adopted 2015/12/17 Effective Date 2015/12/31 Health Products and Food Branch Our mission is to help
GUIDANCE DOCUMENT Decongestant Labelling Standard Published by authority of the Minister of Health Date Adopted 2015/12/17 Effective Date 2015/12/31 Health Products and Food Branch Our mission is to help
in the ICH Regions Table of Content Annexes to Guideline and 3. Why is Q4B necessary? Q4B Annexes? for Human Use
 Frequently Asked Questions Q4B: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions The Q4B Expert Working Group developed a set of frequently asked questions to help users
Frequently Asked Questions Q4B: Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions The Q4B Expert Working Group developed a set of frequently asked questions to help users
CHASTE TREE. Vitex agnus-castus L. (Lamiaceae) (McGuffin et al. 2000; USDA 1998) Source material(s): Fruit/Berry (Blumenthal 2000; Mills & Bone 2000)
 CHASTE TREE This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLA) and labels for natural health product market authorization. It is not intended
CHASTE TREE This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLA) and labels for natural health product market authorization. It is not intended
Interchangeable Drug Products - Additional Criteria
 Interchangeable Drug Products - Additional Criteria Principle: Decisions respecting interchangeability and drug lists remain in the domain of the institution responsible for the costs of the product which
Interchangeable Drug Products - Additional Criteria Principle: Decisions respecting interchangeability and drug lists remain in the domain of the institution responsible for the costs of the product which
Recognized Pharmacopoeia in Registration system
 Recognized Pharmacopoeia in Registration system Mrs. Prapassorn THANAPHOLLERT Acting Director, Bureau of Control Food and, THAILAND What is pharmacopoeia? Legal basis Outline Recognized Official Pharmacopoeia
Recognized Pharmacopoeia in Registration system Mrs. Prapassorn THANAPHOLLERT Acting Director, Bureau of Control Food and, THAILAND What is pharmacopoeia? Legal basis Outline Recognized Official Pharmacopoeia
Guidance Document. Comparative Bioavailability Standards: Formulations Used for System Effects
 Guidance Document Comparative Bioavailability Standards: Formulations Used for System Effects Date Adopted: 2012/12/08 Revised Date: 2018/06/08 Effective Date: 2018/07/01 (for submissions filed on or after
Guidance Document Comparative Bioavailability Standards: Formulations Used for System Effects Date Adopted: 2012/12/08 Revised Date: 2018/06/08 Effective Date: 2018/07/01 (for submissions filed on or after
RE: Permits under the Arizona Pharmacy Act should not be required for dietary supplements
 Board Members Arizona State Board of Pharmacy 1616 W. Adams St., Suite 120 Phoenix, AZ 85007 c/o Kam Gandhi, PharmD Executive Director Arizona State Board of Pharmacy Via email: kgandhi@azpharmacy.gov
Board Members Arizona State Board of Pharmacy 1616 W. Adams St., Suite 120 Phoenix, AZ 85007 c/o Kam Gandhi, PharmD Executive Director Arizona State Board of Pharmacy Via email: kgandhi@azpharmacy.gov
Questions or comments regarding the content of the NHPD Monthly Communiqué may be addressed to
 The NHPD Monthly Communiqué is a publication of Health Canada s Natural Health Products Directorate (NHPD), the federal department responsible for the regulation of natural health products sold in Canada.
The NHPD Monthly Communiqué is a publication of Health Canada s Natural Health Products Directorate (NHPD), the federal department responsible for the regulation of natural health products sold in Canada.
NOTICE. Release of final Health Canada document: Standards for Clinical Trials in Type 2 Diabetes in Canada
 September 24, 2007 NOTICE Our file number: 07-122151-509 Release of final Health Canada document: Standards for Clinical Trials in Type 2 Diabetes in Canada The final version of the Health Canada guidance
September 24, 2007 NOTICE Our file number: 07-122151-509 Release of final Health Canada document: Standards for Clinical Trials in Type 2 Diabetes in Canada The final version of the Health Canada guidance
Natural Health Product Raw Material Policy
 Natural Health Product Raw Material Policy Natural Health Products Directorate Health Products and Food Branch October 2006 Health Canada is the Federal department responsible for helping Canadians maintain
Natural Health Product Raw Material Policy Natural Health Products Directorate Health Products and Food Branch October 2006 Health Canada is the Federal department responsible for helping Canadians maintain
Health Canada s Proposed Approach to Managing Caffeinated Energy Drinks
 Health Canada s Proposed Approach to Managing Caffeinated Energy Drinks October 2011 Food Directorate, Health Products and Food Branch 1 Table of Contents Background... 3 Assessment of the Potential Health
Health Canada s Proposed Approach to Managing Caffeinated Energy Drinks October 2011 Food Directorate, Health Products and Food Branch 1 Table of Contents Background... 3 Assessment of the Potential Health
December 4, 2017 VIA ELECTRONIC SUBMISSION
 VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
Residual Solvents: FDA/ Regulatory Perspective
 Rosa Motta Compliance Officer Residual Solvents: FDA/ Regulatory Perspective PDA/USP Residual Solvents Conference January 18-19, 2007 1 Outline Laws and regulations governing the compliance requirements
Rosa Motta Compliance Officer Residual Solvents: FDA/ Regulatory Perspective PDA/USP Residual Solvents Conference January 18-19, 2007 1 Outline Laws and regulations governing the compliance requirements
NATURAL HEALTH PRODUCT DEVIL S CLAW HARPAGOPHYTUM
 NATURAL HEALTH PRODUCT DEVIL S CLAW HARPAGOPHYTUM This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product
NATURAL HEALTH PRODUCT DEVIL S CLAW HARPAGOPHYTUM This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry
 ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years
 The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years Gary L. Yingling Food Policy Impact December 2011 Copyright 2011 by K&L Gates LLP. All rights reserved. Brief Historical
The Marketing of a Food Ingredient Understanding a System that has Worked for 105 Years Gary L. Yingling Food Policy Impact December 2011 Copyright 2011 by K&L Gates LLP. All rights reserved. Brief Historical
USP Perspective on Atypical Actives November 29, 2017
 USP Perspective on Atypical Actives November 29, 2017 USP Excipients Stakeholder Forum USP Perspective on Atypical Actives Catherine Sheehan, M.S., M.S. Senior Director, Science Excipients Outline Role
USP Perspective on Atypical Actives November 29, 2017 USP Excipients Stakeholder Forum USP Perspective on Atypical Actives Catherine Sheehan, M.S., M.S. Senior Director, Science Excipients Outline Role
Classification of Products at the Cosmetic-Drug Interface
 Health Canada Santé Canada Your health and safety our priority. Votre santé et votre sécurité notre priorité. GUIDANCE DOCUMENT Classification of Products at the Cosmetic-Drug Interface Health Canada is
Health Canada Santé Canada Your health and safety our priority. Votre santé et votre sécurité notre priorité. GUIDANCE DOCUMENT Classification of Products at the Cosmetic-Drug Interface Health Canada is
QUESTIONS AND ANSWERS Access to Drugs in Exceptional Circumstances
 QUESTIONS AND ANSWERS Access to Drugs in Exceptional Circumstances Published by authority of the Minister of Health Date Adopted 2017/06/09 Effective Date 2017/06/28 Health Products and Food Branch Our
QUESTIONS AND ANSWERS Access to Drugs in Exceptional Circumstances Published by authority of the Minister of Health Date Adopted 2017/06/09 Effective Date 2017/06/28 Health Products and Food Branch Our
HOMEOPATHIC MEDICINAL PRODUCT WORKING GROUP (HMPWG) DRAFT (12/08) POINTS TO CONSIDER ON STABILITY TESTING OF HOMEOPATHIC MEDICINAL PRODUCTS
 HOMEOPATHIC MEDICINAL PRODUCT WORKING GROUP (HMPWG) DRAFT (12/08) POINTS TO CONSIDER ON STABILITY TESTING OF HOMEOPATHIC MEDICINAL PRODUCTS DISCUSSION IN THE SUBGROUP STABILITY April / May 2008 DISCUSSION
HOMEOPATHIC MEDICINAL PRODUCT WORKING GROUP (HMPWG) DRAFT (12/08) POINTS TO CONSIDER ON STABILITY TESTING OF HOMEOPATHIC MEDICINAL PRODUCTS DISCUSSION IN THE SUBGROUP STABILITY April / May 2008 DISCUSSION
Notice Regulatory cooperation initiative (RCI) over-the-counter (OTC) products Release of final labelling standards
 May 30, 2014 Notice Regulatory cooperation initiative (RCI) over-the-counter (OTC) products Release of final labelling standards Health Canada and the Australian Therapeutic Goods Administration (TGA)
May 30, 2014 Notice Regulatory cooperation initiative (RCI) over-the-counter (OTC) products Release of final labelling standards Health Canada and the Australian Therapeutic Goods Administration (TGA)
Category Specific Guidance for Temporary Marketing Authorization: Supplemented Food. February 2016
 Category Specific Guidance for Temporary Marketing Authorization: Supplemented Food February 2016 Table of Contents Executive summary... 4 1.0 Introduction... 5 2.0 Background... 6 3.0 Supplemented food
Category Specific Guidance for Temporary Marketing Authorization: Supplemented Food February 2016 Table of Contents Executive summary... 4 1.0 Introduction... 5 2.0 Background... 6 3.0 Supplemented food
WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS
 WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS SEPTEMBER 23, 2009 COUNCIL OF THE CONVENTION SECTION ON THE QUALITY OF FOOD INGREDIENTS AND DIETARY SUPPLEMENTS INTRODUCTION The 1994 Dietary Supplement
WHITE PAPER ACCESS TO GOOD QUALITY DIETARY SUPPLEMENTS SEPTEMBER 23, 2009 COUNCIL OF THE CONVENTION SECTION ON THE QUALITY OF FOOD INGREDIENTS AND DIETARY SUPPLEMENTS INTRODUCTION The 1994 Dietary Supplement
Overview of USP General Chapters <476> and <1086> Prescription/Non-Prescription Stakeholder Forum October 19, 2017
 Overview of USP General Chapters and Prescription/Non-Prescription Stakeholder Forum October 19, 2017 Introduction Periodic review of existing general chapters Typically an approximately 5
Overview of USP General Chapters and Prescription/Non-Prescription Stakeholder Forum October 19, 2017 Introduction Periodic review of existing general chapters Typically an approximately 5
DIRECTIVE 2004/24/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL. of 31 March 2004
 30.4.2004 Official Journal of the European Union L 136/85 DIRECTIVE 2004/24/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 March 2004 amending, as regards traditional herbal medicinal products,
30.4.2004 Official Journal of the European Union L 136/85 DIRECTIVE 2004/24/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 March 2004 amending, as regards traditional herbal medicinal products,
COMPLETION OF THE CTD IN APPLICATION FOR A COMPLEMENTARY MEDICINE
 COMPLETION OF THE CTD IN APPLICATION FOR A COMPLEMENTARY MEDICINE MCC WORKSHOP 31 AUGUST 2015 DR NEIL GOWER MTECH HOM (UJ) CML (UNISA) MEMBER: MEDICINES CONTROL COUNCIL (MCC) CHAIRPERSON: COMPLEMENTARY
COMPLETION OF THE CTD IN APPLICATION FOR A COMPLEMENTARY MEDICINE MCC WORKSHOP 31 AUGUST 2015 DR NEIL GOWER MTECH HOM (UJ) CML (UNISA) MEMBER: MEDICINES CONTROL COUNCIL (MCC) CHAIRPERSON: COMPLEMENTARY
General Chapter/Section: <232> Elemental Impurities - Limits Expert Committee(s): General Chapters Chemical Analysis No.
 General Chapter/Section: Elemental Impurities - Limits Expert Committee(s): General Chapters Chemical Analysis No. of Commenters: 18 Editorial changes suggested by commenters have been reviewed by
General Chapter/Section: Elemental Impurities - Limits Expert Committee(s): General Chapters Chemical Analysis No. of Commenters: 18 Editorial changes suggested by commenters have been reviewed by
OVERVIEW OF COMMENTS RECEIVED ON COMMUNITY HERBAL MONOGRAPH ON HAMAMELIS VIRGINIANA L., FOLIUM (EMEA/HMPC/114586/2008)
 European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/280729/2009 OVERVIEW OF COMMENTS RECEIVED ON COMMUNITY HERBAL MONOGRAPH ON HAMAMELIS VIRGINIANA
European Medicines Agency Evaluation of Medicines for Human Use London, 12 November 2009 Doc. Ref.: EMA/HMPC/280729/2009 OVERVIEW OF COMMENTS RECEIVED ON COMMUNITY HERBAL MONOGRAPH ON HAMAMELIS VIRGINIANA
E11(R1) Addendum to E11: Clinical Investigation of Medicinal Products in the Pediatric Population Step4
 E11(R1) Addendum to E11: Clinical Investigation of Medicinal Products in the Step4 October 2017 International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal
E11(R1) Addendum to E11: Clinical Investigation of Medicinal Products in the Step4 October 2017 International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use 1 Legal
NATURAL HEALTH PRODUCT DEVIL S CLAW HARPAGOPHYTUM
 NATURAL HEALTH PRODUCT DEVIL S CLAW HARPAGOPHYTUM This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product
NATURAL HEALTH PRODUCT DEVIL S CLAW HARPAGOPHYTUM This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product
NATURAL HEALTH PRODUCT FUNGAL PROTEASE
 NATURAL HEALTH PRODUCT FUNGAL PROTEASE This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market
NATURAL HEALTH PRODUCT FUNGAL PROTEASE This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market
Food Labeling: Policy Rationale IFT Food Policy Impact, 2011
 Food Labeling: Policy Rationale IFT Food Policy Impact, 2011 Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements CFSAN-FDA General Labeling Provisions FDA s authority to regulate
Food Labeling: Policy Rationale IFT Food Policy Impact, 2011 Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements CFSAN-FDA General Labeling Provisions FDA s authority to regulate
Proposed Amendments to the Cannabis Regulations: Edible Cannabis, Cannabis Extracts and Cannabis Topicals
 Proposed Amendments to the Cannabis Regulations: Edible Cannabis, Cannabis Extracts and Cannabis Topicals Health Canada s 2019 Regulatory Consultation 1 Purpose The purpose of this presentation is to provide
Proposed Amendments to the Cannabis Regulations: Edible Cannabis, Cannabis Extracts and Cannabis Topicals Health Canada s 2019 Regulatory Consultation 1 Purpose The purpose of this presentation is to provide
EFFECTIVE DATE: 05/02/2017
 Background The purpose of this Guideline is to set forth the criteria that USP uses to determine whether or not a dietary ingredient qualifies for admission to the process for the development of a quality
Background The purpose of this Guideline is to set forth the criteria that USP uses to determine whether or not a dietary ingredient qualifies for admission to the process for the development of a quality
Pathway for Licensing Natural Health Products used as Traditional Medicines
 Pathway for Licensing Natural Health Products used as Traditional Medicines Foreword Guidance documents are meant to provide assistance to industry and health care practitioners on how to comply with governing
Pathway for Licensing Natural Health Products used as Traditional Medicines Foreword Guidance documents are meant to provide assistance to industry and health care practitioners on how to comply with governing
Process Updates and Improvements in the Natural Health Products Regulatory Industry
 Process Updates and Improvements in the Natural Health Products Regulatory Industry Manon Bombardier, Director General Natural and Non-Prescription Health Products Directorate (NNHPD), Health Canada February
Process Updates and Improvements in the Natural Health Products Regulatory Industry Manon Bombardier, Director General Natural and Non-Prescription Health Products Directorate (NNHPD), Health Canada February
MEDICNES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF HERBAL MEDICINAL PRODUCTS IN THE GAMBIA
 MEDICNES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF HERBAL MEDICINAL PRODUCTS IN THE GAMBIA Document No.: MCA/HMPG/17/12/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017 Version
MEDICNES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF HERBAL MEDICINAL PRODUCTS IN THE GAMBIA Document No.: MCA/HMPG/17/12/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017 Version
REGULATORY REQUIREMENTS FOR REGISTRATION OF HEALTH SUPPLEMENTS IN MALAYSIA
 REGULATORY REQUIREMENTS FOR REGISTRATION OF HEALTH SUPPLEMENTS IN MALAYSIA TAN JAS MIN National Pharmaceutical Regulatory Agency Ministry of Health Malaysia Email address: tanjasmin@npra.gov.my ORGANISATION
REGULATORY REQUIREMENTS FOR REGISTRATION OF HEALTH SUPPLEMENTS IN MALAYSIA TAN JAS MIN National Pharmaceutical Regulatory Agency Ministry of Health Malaysia Email address: tanjasmin@npra.gov.my ORGANISATION
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Notice. Regulatory cooperation initiative (RCI) over-the-counter (OTC) products. Release of final labelling standards
 May 30, 2014 Notice Regulatory cooperation initiative (RCI) over-the-counter (OTC) products Release of final labelling standards Health Canada and the Australian Therapeutic Goods Administration (TGA)
May 30, 2014 Notice Regulatory cooperation initiative (RCI) over-the-counter (OTC) products Release of final labelling standards Health Canada and the Australian Therapeutic Goods Administration (TGA)
European Commission request to the European Food Safety Authority for scientific advice on:
 European Commission request to the European Food Safety Authority for scientific advice on: the Community list of permitted health claims pursuant article 13 of Regulation 1924/2006 on nutrition and health
European Commission request to the European Food Safety Authority for scientific advice on: the Community list of permitted health claims pursuant article 13 of Regulation 1924/2006 on nutrition and health
NATURAL HEALTH PRODUCT LINDEN, EUROPEAN TILIA X EUROPAEA
 NATURAL HEALTH PRODUCT LINDEN, EUROPEAN TILIA X EUROPAEA This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health
NATURAL HEALTH PRODUCT LINDEN, EUROPEAN TILIA X EUROPAEA This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health
Draft Agreed by Immunologicals Working Party January Adoption by CVMP for release for consultation 12 March 2009
 15 March 2010 EMA/CVMP/IWP/105506/2007 Committee for medicinal products for veterinary use (CVMP) Guideline on data requirements for multi-strain dossiers for inactivated vaccines against avian influenza
15 March 2010 EMA/CVMP/IWP/105506/2007 Committee for medicinal products for veterinary use (CVMP) Guideline on data requirements for multi-strain dossiers for inactivated vaccines against avian influenza
Bill C-51 and Natural Health Products - The Facts
 Bill C-51 and Natural Health Products - The Facts 1. How will Bill C-51 change the way natural health products are regulated? Bill C-51 will not affect the way that natural health products are regulated
Bill C-51 and Natural Health Products - The Facts 1. How will Bill C-51 change the way natural health products are regulated? Bill C-51 will not affect the way that natural health products are regulated
CMA response to TGO 92- Standards for the labels of non-prescription medicines
 CMA response to TGO 92- Standards for the labels of non-prescription medicines Proposed Requirement TGO 92 Text size Text size equivalent to Arial font has been removed from TGO 92. The definition for
CMA response to TGO 92- Standards for the labels of non-prescription medicines Proposed Requirement TGO 92 Text size Text size equivalent to Arial font has been removed from TGO 92. The definition for
MEDICINES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF FOOD/NUTRITIONAL/DIETARY SUPPLEMENTS IN THE GAMBIA
 MEDICINES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF FOOD/NUTRITIONAL/DIETARY SUPPLEMENTS IN THE GAMBIA Document No.: MCA/NSG/17/11/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017
MEDICINES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF FOOD/NUTRITIONAL/DIETARY SUPPLEMENTS IN THE GAMBIA Document No.: MCA/NSG/17/11/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017
EHR Developer Code of Conduct Frequently Asked Questions
 EHR Developer Code of Conduct Frequently Asked Questions General What is the purpose of the EHR Developer Code of Conduct? EHR Association (the Association) members have a long tradition of working with
EHR Developer Code of Conduct Frequently Asked Questions General What is the purpose of the EHR Developer Code of Conduct? EHR Association (the Association) members have a long tradition of working with
Regulation of FMD vaccines within the European Union
 Introduction Regulation of FMD vaccines within the European Union K De Clercq 1 and D K J Mackay 2 Appendix 36 The EUFMD European Pharmacopoeia Working Group made a proposal for revision of the FMD vaccine
Introduction Regulation of FMD vaccines within the European Union K De Clercq 1 and D K J Mackay 2 Appendix 36 The EUFMD European Pharmacopoeia Working Group made a proposal for revision of the FMD vaccine
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL. PHARMACEUTICAL COMMITTEE 21 October 2015
 EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Health systems and products Medicinal products authorisations, European Medicines Agency PHARM 689 PHARMACEUTICAL COMMITTEE 21 October 2015
EUROPEAN COMMISSION HEALTH AND FOOD SAFETY DIRECTORATE-GENERAL Health systems and products Medicinal products authorisations, European Medicines Agency PHARM 689 PHARMACEUTICAL COMMITTEE 21 October 2015
Classification of Products at the Food Natural Health Product Interface: Products in Food Formats
 Classification of Products at the Food Natural Health Product Interface: Products in Food Formats Natural Health Products Directorate Food Directorate June 2010 Version 2.0 Health Canada is the Federal
Classification of Products at the Food Natural Health Product Interface: Products in Food Formats Natural Health Products Directorate Food Directorate June 2010 Version 2.0 Health Canada is the Federal
Adopted by CVMP 10 March Date for coming into effect 1 July Revised draft guideline agreed by Immunologicals Working Party 22 June 2017
 1 2 3 7 September 2017 EMA/CVMP/IWP/105506/2007-Rev.1 Committee for medicinal products for veterinary use (CVMP) 4 5 6 7 Guideline on data requirements for multi-strain dossiers for inactivated vaccines
1 2 3 7 September 2017 EMA/CVMP/IWP/105506/2007-Rev.1 Committee for medicinal products for veterinary use (CVMP) 4 5 6 7 Guideline on data requirements for multi-strain dossiers for inactivated vaccines
AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION. (Moscow, 23 December 2014)
 AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION (Moscow, 23 December 2014) Member States of the Eurasian Economic Union, hereinafter referred
AGREEMENT ON COMMON PRINCIPLES AND RULES OF CIRCULATION OF MEDICINAL PRODUCTS WITHIN THE EURASIAN ECONOMIC UNION (Moscow, 23 December 2014) Member States of the Eurasian Economic Union, hereinafter referred
Pharmakon drug Poiea to make Government of each country List of Pharmacopeias: British European Indian International United state.
 Pharmacopoeia Derived from Greek word Pharmakon means drug and Poiea means to make. It is a legal and official book issued by recognized authorities usually appointed by Government of each country. It
Pharmacopoeia Derived from Greek word Pharmakon means drug and Poiea means to make. It is a legal and official book issued by recognized authorities usually appointed by Government of each country. It
Research on the Administrative Rules of APIs, Pharmaceutical Excipients and Auxiliary Materials Master File. Translation version
 Research on the Administrative Rules of APIs, Pharmaceutical Excipients and Auxiliary Materials Master File Division of Pharmaceuticals Department of Drug Registration Hou Renping Translation version Main
Research on the Administrative Rules of APIs, Pharmaceutical Excipients and Auxiliary Materials Master File Division of Pharmaceuticals Department of Drug Registration Hou Renping Translation version Main
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency London, 26 July 2006 Doc. Ref. EMEA/186279/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON LEGAL STATUS FOR THE SUPPLY TO THE PATIENT OF CENTRALLY AUTHORISED
European Medicines Agency London, 26 July 2006 Doc. Ref. EMEA/186279/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON LEGAL STATUS FOR THE SUPPLY TO THE PATIENT OF CENTRALLY AUTHORISED
Potential Grounds for Revocation of a Traditional Herbal Registration
 Potential Grounds for Revocation of a Traditional Herbal Registration By Dr Rosanna Cooper In this article we will be exploring the potential grounds for revocation of the Traditional Herbal Registration
Potential Grounds for Revocation of a Traditional Herbal Registration By Dr Rosanna Cooper In this article we will be exploring the potential grounds for revocation of the Traditional Herbal Registration
Advocacy Framework. St. Michael s Hospital Academic Family Health Team
 Advocacy Framework St. Michael s Hospital Academic Family Health Team Purpose To provide a framework by which the St. Michael s Hospital Academic Family Health Team (SMH AFHT) can expand its commitment
Advocacy Framework St. Michael s Hospital Academic Family Health Team Purpose To provide a framework by which the St. Michael s Hospital Academic Family Health Team (SMH AFHT) can expand its commitment
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL PROCEDURE FOR THE PREPARATION OF COMMUNITY MONOGRAPHS FOR TRADITIONAL HERBAL MEDICINAL PRODUCTS
 European Medicines Agency Evaluation of Medicines for Human Use London, 11 January 2007 Ref: EMEA/HMPC/182320/2005 Rev. 2 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL PROCEDURE FOR THE PREPARATION
European Medicines Agency Evaluation of Medicines for Human Use London, 11 January 2007 Ref: EMEA/HMPC/182320/2005 Rev. 2 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL PROCEDURE FOR THE PREPARATION
Requirements to the Registration of Medicinal products in the Republic of Armenia
 Requirements to the Registration of Medicinal products in the Republic of Armenia Yerevan 2010 Requirements to the Registration of Medicinal products in the Republic of Armenia Current requirements to
Requirements to the Registration of Medicinal products in the Republic of Armenia Yerevan 2010 Requirements to the Registration of Medicinal products in the Republic of Armenia Current requirements to
GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS
 1 CAC/GL 23-1997 GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS CAC/GL 23-1997 Nutrition claims should be consistent with national nutrition policy and support that policy. Only nutrition claims that
1 CAC/GL 23-1997 GUIDELINES FOR USE OF NUTRITION AND HEALTH CLAIMS CAC/GL 23-1997 Nutrition claims should be consistent with national nutrition policy and support that policy. Only nutrition claims that
Food and Drug Regulations - Project Number Schedule F
 Graham Spry Building 250 Lanark Avenue Address Locator: 2005D Ottawa, Ontario K1A 0K9 09-128254-482 Provincial and Territorial Deputy Ministers of Health Provincial and Territorial Drug Program Managers
Graham Spry Building 250 Lanark Avenue Address Locator: 2005D Ottawa, Ontario K1A 0K9 09-128254-482 Provincial and Territorial Deputy Ministers of Health Provincial and Territorial Drug Program Managers
Regulations Amending the Food and Drug Regulations (1256
 CANADA GAZETTE, PART II FOOD AND DRUG REGULATIONS - AMENDMENTS WILL BE PUBLISHED IN CANADA GAZETTE, PART II OF OCTOBER 10, 2001 SCHEDULE NO. 1256 (FLUCARBAZONE-SODIUM) P.C. 2001-1648 OF SEPTEMBER 19, 2001
CANADA GAZETTE, PART II FOOD AND DRUG REGULATIONS - AMENDMENTS WILL BE PUBLISHED IN CANADA GAZETTE, PART II OF OCTOBER 10, 2001 SCHEDULE NO. 1256 (FLUCARBAZONE-SODIUM) P.C. 2001-1648 OF SEPTEMBER 19, 2001
Re: National Bioengineered Food Disclosure Standard; Proposed Rule; Request for Comments, 83 Fed. Reg (May 4, 2018), Docket No.
 VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
Interim Policy on the Use of Expired Interim Marketing Authorizations Related to Food Fortification
 Interim Policy on the Use of Expired Interim Marketing Authorizations Related to Food Fortification March 2017 Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch 1 Table
Interim Policy on the Use of Expired Interim Marketing Authorizations Related to Food Fortification March 2017 Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch 1 Table
Regulations Amending the Food and Drug Regulations (1215
 CANADA GAZETTE, PART II FOOD AND DRUG REGULATIONS - AMENDMENTS WILL BE PUBLISHED IN CANADA GAZETTE, PART II OF FEBRUARY 14, 2001 SCHEDULE NO. 1215 (PRIMISULFURON-METHYL MRLs) P.C. 2001-156 OF JANUARY 30,
CANADA GAZETTE, PART II FOOD AND DRUG REGULATIONS - AMENDMENTS WILL BE PUBLISHED IN CANADA GAZETTE, PART II OF FEBRUARY 14, 2001 SCHEDULE NO. 1215 (PRIMISULFURON-METHYL MRLs) P.C. 2001-156 OF JANUARY 30,
THE NEW CANADIAN NATURAL HEALTH PERODUCT REGULATIONS
 THE NEW CANADIAN NATURAL HEALTH PERODUCT REGULATIONS IMPACT AND OPPORTUNITY FOR CHINESE MEDICINE JULY 2006 Yili Bai, PhD Tel: 604-249-2896 Email: ybai@wellgenex.com Michael ZC Li, MSc, MD, MBA Tel: 604-249-2896
THE NEW CANADIAN NATURAL HEALTH PERODUCT REGULATIONS IMPACT AND OPPORTUNITY FOR CHINESE MEDICINE JULY 2006 Yili Bai, PhD Tel: 604-249-2896 Email: ybai@wellgenex.com Michael ZC Li, MSc, MD, MBA Tel: 604-249-2896
DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT
 c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
c t DRUG PRODUCT INTERCHANGEABILITY AND PRICING ACT PLEASE NOTE This document, prepared by the Legislative Counsel Office, is an office consolidation of this Act, current to September 22, 2014. It is intended
Summary of product characteristics (SmPC)
 Summary of product characteristics (SmPC) What is it and what does it contain? An agency of the European Union Table of contents 1.What is the summary of product characteristics (SmPC)? 2.Where SmPC information
Summary of product characteristics (SmPC) What is it and what does it contain? An agency of the European Union Table of contents 1.What is the summary of product characteristics (SmPC)? 2.Where SmPC information
Commentary Pharmacopeial Forum 34(2) March-April 2008 Interim Revision Announcements to USP 30-NF 25 Revised June 30, 2008
 Commentary Pharmacopeial Forum 34(2) March-April 2008 Interim Revision Announcements to USP 30-NF 25 Revised June 30, 2008 Revision proposals published in Pharmacopeial Forum often elicit public comments
Commentary Pharmacopeial Forum 34(2) March-April 2008 Interim Revision Announcements to USP 30-NF 25 Revised June 30, 2008 Revision proposals published in Pharmacopeial Forum often elicit public comments
CARD/MAIL/PRE-APPROVAL/PREFERRED RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date:
![CARD/MAIL/PRE-APPROVAL/PREFERRED RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date: CARD/MAIL/PRE-APPROVAL/PREFERRED RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date:](/thumbs/79/80269988.jpg) RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date: CARD/MAIL/PRE-APPROVAL/PREFERRED The Prescription Drug Coverage under this Rider [replaces] [supplements] the Prescription
RIDER FOR PRESCRIPTION DRUG [INSURANCE] [Policy]holder: Group Policy No: Effective Date: CARD/MAIL/PRE-APPROVAL/PREFERRED The Prescription Drug Coverage under this Rider [replaces] [supplements] the Prescription
Cutanea Life Sciences, Inc. Comprehensive Compliance Program
 Cutanea Life Sciences, Inc. Comprehensive Compliance Program Cutanea engages in research, development, manufacture and marketing of dermatology products, including commercialization of those products.
Cutanea Life Sciences, Inc. Comprehensive Compliance Program Cutanea engages in research, development, manufacture and marketing of dermatology products, including commercialization of those products.
Guidance Document for Source Establishments Reporting Adverse Reactions to Human Cells, Tissues and Organs
 Guidance Document for Source Establishments Reporting Adverse Reactions to Human Cells, Tissues and Organs (Effective Date: 2010-05-25) Canada Vigilance Adverse Reaction Monitoring Program and Database,
Guidance Document for Source Establishments Reporting Adverse Reactions to Human Cells, Tissues and Organs (Effective Date: 2010-05-25) Canada Vigilance Adverse Reaction Monitoring Program and Database,
European Medicines Agency Evaluation of Medicines for Human Use. Superseded COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL
 European Medicines Agency Evaluation of Medicines for Human Use COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL London, 3 July 2008 Doc. Ref. EMEA/HMPC/394894/2007 COMMUNITY HERBAL MONOGRAPH ON EQUISETUM
European Medicines Agency Evaluation of Medicines for Human Use COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL London, 3 July 2008 Doc. Ref. EMEA/HMPC/394894/2007 COMMUNITY HERBAL MONOGRAPH ON EQUISETUM
NOTICE OF INITIATION OF DISQUALIFICATION PROCEEDINGS AND OPPORTUNITY TO EXPLAIN
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 1401 Rockville Pike Rockville, MD 20852-1448 September 26, 2011 By Overnight Delivery Michael Dean Berger, M.D.
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration 1401 Rockville Pike Rockville, MD 20852-1448 September 26, 2011 By Overnight Delivery Michael Dean Berger, M.D.
Dietary Supplement Health and Education Act of 1994 Public Law rd Congress
 Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
NATURAL HEALTH PRODUCT LITHOTHERAPY
 NATURAL HEALTH PRODUCT LITHOTHERAPY This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization.
NATURAL HEALTH PRODUCT LITHOTHERAPY This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health product market authorization.
EVALUATION AND RECOMMENDATION OF PHARMACOPOEIAL TEXTS FOR USE IN THE ICH REGIONS ON DISINTEGRATION TEST GENERAL CHAPTER Q4B ANNEX 5(R1)
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE EVALUATION AND RECOMMENDATION OF PHARMACOPOEIAL
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE EVALUATION AND RECOMMENDATION OF PHARMACOPOEIAL
Module 34: Legal aspects, ADI and GRAS status of food additives
 Paper No.: 13 Paper Title: FOOD ADDITIVES Module 34: Legal aspects, ADI and GRAS status of food additives 34.1 Legal Aspects of Food Additives The data provided by Joint Expert Committee on Food Additives
Paper No.: 13 Paper Title: FOOD ADDITIVES Module 34: Legal aspects, ADI and GRAS status of food additives 34.1 Legal Aspects of Food Additives The data provided by Joint Expert Committee on Food Additives
Regulations Amending the Food and Drug Regulations (1288
 CANADA GAZETTE, PART II FOOD AND DRUG REGULATIONS - AMENDMENTS WILL BE PUBLISHED IN CANADA GAZETTE, PART II OF DECEMBER 5, 2001 SCHEDULE NO. 1288 (FOMESAFEN) P.C. 2001-2143 OF NOVEMBER 21, 2001 SOR/2001-514
CANADA GAZETTE, PART II FOOD AND DRUG REGULATIONS - AMENDMENTS WILL BE PUBLISHED IN CANADA GAZETTE, PART II OF DECEMBER 5, 2001 SCHEDULE NO. 1288 (FOMESAFEN) P.C. 2001-2143 OF NOVEMBER 21, 2001 SOR/2001-514
Ryan Smith, Community Planning Department Manager. Zoning Bylaw Text Amendment for Cannabis Production and Retail Cannabis Sales
 Report to Council Date: August 27, 2018 File: 1250-04 To: From: Subject: Report prepared by: City Manager Ryan Smith, Community Planning Department Manager Zoning Bylaw Text Amendment for Cannabis Production
Report to Council Date: August 27, 2018 File: 1250-04 To: From: Subject: Report prepared by: City Manager Ryan Smith, Community Planning Department Manager Zoning Bylaw Text Amendment for Cannabis Production
Traditional Medicine: Overview on national policies and regulations of traditional medicine
 Traditional Medicine: Overview on national policies and regulations of traditional medicine Kallesh Danappa Jayappa, Ph.D student Infectious minds presentation 22 nd June 2011 WHO initiatives Major challenges:
Traditional Medicine: Overview on national policies and regulations of traditional medicine Kallesh Danappa Jayappa, Ph.D student Infectious minds presentation 22 nd June 2011 WHO initiatives Major challenges:
Update on the Canadian Food Inspection Agency (CFIA) Food Labelling Modernization Initiative
 Update on the Canadian Food Inspection Agency (CFIA) Food Labelling Modernization Initiative Presented to: Food Supply Chain Stakeholder Meeting Date: January 27, 2014 Presented by: Daniel Miller, Food
Update on the Canadian Food Inspection Agency (CFIA) Food Labelling Modernization Initiative Presented to: Food Supply Chain Stakeholder Meeting Date: January 27, 2014 Presented by: Daniel Miller, Food
Regulations Amending the Food and Drug Regulations (1219
 CANADA GAZETTE, PART II FOOD AND DRUG REGULATIONS - AMENDMENTS WILL BE PUBLISHED IN CANADA GAZETTE, PART II OF NOVEMBER 7, 2001 SCHEDULE NO. 1219 (ISOXAFLUTOLE) P.C. 2001-1951 OF OCTOBER 24, 2001 SOR/2001-455
CANADA GAZETTE, PART II FOOD AND DRUG REGULATIONS - AMENDMENTS WILL BE PUBLISHED IN CANADA GAZETTE, PART II OF NOVEMBER 7, 2001 SCHEDULE NO. 1219 (ISOXAFLUTOLE) P.C. 2001-1951 OF OCTOBER 24, 2001 SOR/2001-455
1. NHS (GENERAL DENTAL SERVICES) (SCOTLAND) AMENDMENT REGULATIONS AMENDMENT NO 136 TO THE STATEMENT OF DENTAL REMUNERATION
 MEMORANDUM TO NHS: PCA(D)(2017)6 DENTISTS/DENTAL BODIES CORPORATE NATIONAL HEALTH SERVICE GENERAL DENTAL SERVICES 1. NHS (GENERAL DENTAL SERVICES) (SCOTLAND) AMENDMENT REGULATIONS 2017 2. AMENDMENT NO
MEMORANDUM TO NHS: PCA(D)(2017)6 DENTISTS/DENTAL BODIES CORPORATE NATIONAL HEALTH SERVICE GENERAL DENTAL SERVICES 1. NHS (GENERAL DENTAL SERVICES) (SCOTLAND) AMENDMENT REGULATIONS 2017 2. AMENDMENT NO
NATURAL HEALTH PRODUCT. BILBERRY- VACCINIUM MYRTILLUS Oral
 NATURAL HEALTH PRODUCT BILBERRY- VACCINIUM MYRTILLUS Oral This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health
NATURAL HEALTH PRODUCT BILBERRY- VACCINIUM MYRTILLUS Oral This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLAs) and labels for natural health
Compare Results. 153 Replacements 26 Insertions 344 Deletions. Total Changes. Styling and. Content. 0 Annotations. Old File: New File:
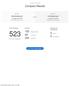 3/1/2019 4:12:31 PM Compare Results Old File: Draft Guidance.pdf 20 pages (438 KB) 3/21/2018 3:55:17 PM versus New File: Final Guidance.pdf 21 pages (323 KB) 2/28/2019 11:42:05 AM Total Changes 523 Text
3/1/2019 4:12:31 PM Compare Results Old File: Draft Guidance.pdf 20 pages (438 KB) 3/21/2018 3:55:17 PM versus New File: Final Guidance.pdf 21 pages (323 KB) 2/28/2019 11:42:05 AM Total Changes 523 Text
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON SOLIDAGO VIRGAUREA L., HERBA
 European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/285758/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
European Medicines Agency Evaluation of Medicines for Human Use London, 4 September 2008 Doc. Ref. EMEA/HMPC/285758/2007 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) FINAL COMMUNITY HERBAL MONOGRAPH ON
COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC)
 European Medicines Agency Post-authorisation Evaluation of Medicines for Human Use London, 7 September 2006 Doc. Ref. EMEA/HMPC/104613/2005 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) GUIDELINE ON THE
European Medicines Agency Post-authorisation Evaluation of Medicines for Human Use London, 7 September 2006 Doc. Ref. EMEA/HMPC/104613/2005 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) GUIDELINE ON THE
Cannabis Retail Store Licensing in Ontario. General Committee December 10, 2018
 1 Cannabis Retail Store Licensing in Ontario General Committee December 10, 2018 Item 1 Item 2 Item 3 Item 4 Item 5 Item 6 Item 7 Item 8 Item 9 Item 10 Item 11 Item 12 Presentation Outline Federal Regulatory
1 Cannabis Retail Store Licensing in Ontario General Committee December 10, 2018 Item 1 Item 2 Item 3 Item 4 Item 5 Item 6 Item 7 Item 8 Item 9 Item 10 Item 11 Item 12 Presentation Outline Federal Regulatory
Technical Monograph n 17, 2nd Edition. Guidelines for Specifying the Shelf Life of Plant Protection Products
 Technical Monograph n 17, 2nd Edition Guidelines for Specifying the Shelf Life of Plant Protection Products June 2009 TABLE OF CONTENTS 1. Introduction 3 2. Background 3 3. Definitions 4 4. Outline of
Technical Monograph n 17, 2nd Edition Guidelines for Specifying the Shelf Life of Plant Protection Products June 2009 TABLE OF CONTENTS 1. Introduction 3 2. Background 3 3. Definitions 4 4. Outline of
HERBAL MEDICINES: Product Licence To Traditional Herbal Registration in the UK. Presented by: Mariam Aslam
 HERBAL MEDICINES: Product Licence To Traditional Herbal Registration in the UK Presented by: Mariam Aslam ESCOP European Scientific Cooperative On Phytotheapy (ESCOP) Founded in June 1989 as an umbrella
HERBAL MEDICINES: Product Licence To Traditional Herbal Registration in the UK Presented by: Mariam Aslam ESCOP European Scientific Cooperative On Phytotheapy (ESCOP) Founded in June 1989 as an umbrella
Developing a Target Product Profile for a Preventive HIV Vaccine
 Developing a Target Product Profile for a Preventive HIV Vaccine Topics to be Covered Overview of TPPs What are they? What is the purpose and benefit of using a TPP? What is usually in a TPP? What are
Developing a Target Product Profile for a Preventive HIV Vaccine Topics to be Covered Overview of TPPs What are they? What is the purpose and benefit of using a TPP? What is usually in a TPP? What are
Business Impact Analysis
 ACTION: Final DATE: 05/23/2018 8:24 AM Business Impact Analysis Agency Name: Ohio Department of Medicaid (ODM) Regulation/Package Title: Dental services Rule Number(s): Rule 5160-5-01 with appendices A
ACTION: Final DATE: 05/23/2018 8:24 AM Business Impact Analysis Agency Name: Ohio Department of Medicaid (ODM) Regulation/Package Title: Dental services Rule Number(s): Rule 5160-5-01 with appendices A
