Notice. Regulatory cooperation initiative (RCI) over-the-counter (OTC) products. Release of final labelling standards
|
|
|
- Cody Walsh
- 5 years ago
- Views:
Transcription
1 May 30, 2014 Notice Regulatory cooperation initiative (RCI) over-the-counter (OTC) products Release of final labelling standards Health Canada and the Australian Therapeutic Goods Administration (TGA) have initiated the Regulatory Cooperation Initiative (RCI) project to explore various ways of work sharing between the two regulatory agencies. Two labelling standards have been developed (nonprescription topical nasal decongestants and nonprescription oral adult expectorant cough and cold) because they meet the goals of aligning the ongoing work of both regulators and reducing unnecessary differences. The proposed nonprescription topical nasal decongestants labelling standard describes the requirements necessary to receive marketing authorization for non-prescription topical nasal decongestant products containing oxymetazoline hydrochloride or xylometazoline hydrochloride as a single ingredient for use in adults to relieve nasal congestion. The proposed nonprescription oral adult expectorant cough describes the requirements necessary to receive marketing authorization for oral expectorant non-prescription products containing guaifenesin as a single ingredient for use in adults to relieve symptoms of common cold. This initiative is the first of its kind between Health Canada and the TGA. This is one step in the ongoing collaboration between Health Canada and the TGA to work toward greater regulatory convergence and harmonization where feasible. Both regulators will continue to explore if there may be additional opportunities for future OTC monograph initiatives.
2 GUIDANCE DOCUMENT and cold labelling standard Published by authority of the Minister of Health Date adopted 2014/03/10 Effective date 2014/05/30 Health Products and Food Branch
3 Our mission is to help the people of Canada maintain and improve their health. Health Canada The Health Products and Food Branch's mandate is to take an integrated approach to the management of the risks and benefits to health related products and food by: minimizing health risk factors to Canadians while maximizing the safety provided by the regulatory system for health products and food; and, promoting conditions that enable Canadians to make healthy choices and providing information so that they can make informed decisions about their health. Health Products and Food Branch Minister of Public Works and Government Services Canada 2014 Également disponible en français sous le titre : Ligne directrice : Norme d étiquetage des médicaments adults contre la toux Expectorant et le rhume, administrés par voie orale et vendus sans ordonnance
4 Foreword s are meant to provide assistance to industry and health care professionals on how to comply with governing statutes and regulations. s also provide assistance to staff on how Health Canada mandates and objectives should be implemented in a manner that is fair, consistent and effective. s are administrative instruments not having force of law and, as such, allow for flexibility in approach. Alternate approaches to the principles and practices described in this document may be acceptable provided they are supported by adequate justification. Alternate approaches should be discussed in advance with the relevant program area to avoid the possible finding that applicable statutory or regulatory requirements have not been met. As a corollary to the above, it is equally important to note that Health Canada reserves the right to request information or material, or define conditions not specifically described in this document, in order to allow the Department to adequately assess the safety, efficacy or quality of a therapeutic product. Health Canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented. This document should be read in conjunction with the accompanying notice and the relevant sections of other applicable guidance documents. Date adopted: 2014/03/10; Effective date: 2014/05/30 i
5 Table of contents 1. Introduction Medicinal ingredient Pharmaceutical forms Acceptable: Unacceptable: Indications Acceptable indications Unacceptable indications Dosage directions Dosage for adults and children 12 years of age and older Dosing considerations Combinations Warnings Other labelling requirements Specifications Non-medicinal ingredients References... 6 Date adopted: 2014/03/10; Effective date: 2014/05/30 ii
6 1. Introduction This labelling standard describes the requirements necessary to receive marketing authorization (Drug identification number (DIN)) for oral expectorant nonprescription product containing guaifenesin as a single ingredient for use in adults and children 12 years of age and older to relieve symptoms of common cold. This labelling standard does not apply to products for use by children under 12 years of age. 2. Medicinal ingredient Table 1: Drug medicinal ingredient Therapeutic class Expectorant Medicinal ingredient Preferred name Guaifenesin 3. Pharmaceutical forms 3.1 Acceptable: Immediate release solid oral dosage forms such as tablets, caplets, capsules, chewable tablets, effervescent tablets, powders. Oral liquid formulations such as suspension, syrup, elixir. 3.2 Unacceptable: Modified dose release (e.g. liquid extended release, solid oral sustained release, bi-layer formulations or enteric coated products). Products that require evaluation of animal sourced ingredients (e.g. animal tissue based gelatin capsules, where valid EDQM Certificates of suitability is not provided). Other dosage forms: e.g., chewable liquid filled capsule, powder dosage form intended for direct application to the mouth, thin strips, lollipops, popsicles/freezer pops etc. 4. Indications 4.1 Acceptable indications Expectorant / cough expectorant. Relief of wet cough or wet chest cough due to common colds. 1 Helps loosen phlegm (mucous) and makes coughs more productive. 1, 2, 3 Relief of chest congestion due to common colds. 1, 2 1 Robinson, Cummings and Deffenbaugh, Kuhn et al, Thomson, Pavia and McNichol, Date adopted: 2014/03/10; Effective date: 2014/05/30 1
7 Helps thin mucous/bronchial secretions. 1, Unacceptable indications The following indications for expectorant products are excluded and would require a review outside of the standard. These include but are not limited to: treat lower respiratory tract conditions (including infections and asthma). bronchitis. coughs due to allergies or inhaled irritants. influenza/flu. allergy/hay fever symptoms relief of dry cough. 5. Dosage directions 5.1 Dosage for adults and children 12 years of age and older 4 Table 2: Therapeutic class Medicinal ingredient preferred name Recommended single dose 5 Dose interval Maximum daily dose Expectorant Guaifenesin mg every 6 hours 1600 mg 5.2 Dosing considerations 1. The quantitative declaration of the medicinal ingredients on any panel of the inner and outer labels should be prominently displayed and should be further identified by the therapeutic class or indication listed under Section 4.1, e.g.: "Active ingredient: Guaifenesin (expectorant) 200mg"; Active ingredient: Guaifenesin (relief of wet cough) 200mg. 2. The labels should declare the recommended single and maximum daily dose, as well as the dosing interval for the product. Maximum daily dose may be expressed in terms of dosage units (e.g. do not exceed X tablets per day). 3. For liquid formulations, the following statement should be included with the directions for use: Use only the measuring device provided. 4 Doses and dosing frequency are those recommended by the Expert advisory committee on nonprescription cough and cold remedies (Second report, April 1989). 5 For liquid formulations, the single dose must be contained and labelled in standard units (e.g., millilitres). Date adopted: 2014/03/10; Effective date: 2014/05/30 2
8 5.3 Combinations Applicants can apply outside of the labelling standard should they wish to combine guaifenesin with other medicinal ingredients. 6. Warnings For outer and inner labels Keep out of reach of children. Do not take more than the recommended dosage. Read the complete label [and package insert if applicable] prior to use and follow all label instructions. Do not use this product in children under 12 years of age. Ask a doctor or pharmacist before use if you: have persistent or chronic cough, difficulty breathing, asthma or other chronic lung conditions. are pregnant or breastfeeding. 4 Consult a doctor if symptoms worsen, last for more than a week or are accompanied by a high fever (>38 o C), persistent headache, rash or the production of thick yellow/green phlegm. In case of overdose: Call a poison control centre or doctor immediately, even if you do not notice any signs or symptoms. Optional warnings that may appear on other outer panels or package insert or inner panels of a peel-back / accordion label: Do not use with any other cough and cold medications since harm may occur, unless recommended by a doctor or pharmacist. Do not use longer than 7 days Do not use if you have an allergy to any of the listed ingredients. Stop use if allergic reactions such as wheezing, rash, or itching develops Date adopted: 2014/03/10; Effective date: 2014/05/30 3
9 7. Other labelling requirements For all products: Declaration of ingredients: o Single ingredient products and/or ones for which a compendial standard exists must declare the proper name of the finished product on the front panel of all labels, immediately preceding or following the brand name in a font size not less than ½ the size of the brand name. (C ) o All products must declare the active ingredients on the inner and outer labels, and clearly label these as active or medicinal ingredients. (C ) o Nonprescription products must provide a qualitative listing of non medicinal ingredients on the outer label. (C ) - Labelling of pharmaceutical drugs for human use: This guidance document should be consulted for applicable labelling requirement. Legibility: Although no specific type size is mentioned in the Regulations, Section A specifies that all information required to appear on a label must be: Clearly and prominently displayed, and Readily discernible to the purchaser or consumer under the customary conditions of purchase and use. A person with normal vision, or those with corrective glasses that restore normal vision, should be able to read the information without straining. The colour, contrast, the position, and the spacing of the information are all to be taken into consideration in complying with these requirements. 8. Specifications This labelling standard describes those requirements that are specific to this class of drug. Products must comply with the requirements in the Food and Drugs Act and associated Regulations. It is also noted that all products are subject to Part C, Division 2 of the Food and Drug Regulations. Date adopted: 2014/03/10; Effective date: 2014/05/30 4
10 All ingredient (medicinal and non-medicinal) and finished product specifications must meet or exceed the standards described in the publications referred to in Schedule B to the Food and Drugs Act, or equivalent standards. Where no Schedule B monograph exists for the dosage form, specifications should be similar to those of a comparable compendial dosage form. In the absence of a Schedule B standard for any dosage form, testing must be adequate to demonstrate the product's identity, potency, purity and quality. Finished product specifications should include tests for identification and an assay with suitable limits for the medicinal ingredient(s). The specifications for all dosage forms should include a description of the dosage form, including organoleptic properties as well as physico-chemical testing e.g., ph, specific gravity, viscosity, appropriate to the dosage form. Where antimicrobial preservatives are added, an assay with suitable limits should be included. Antimicrobial preservative effectiveness should be determined in order to establish that the product is capable of resisting microbial contamination. Any change to the manufacturing process that impacts the safety and efficacy of the ingredients, such as the use of novel technology (e.g. nanotechnology), requires supporting data and will be reviewed outside the labelling standard. 9. Non-medicinal ingredients Non-medicinal ingredients must be restricted to those substances, necessary for the formulation of the dosage form. Their concentration must not exceed the minimum required to provide their intended effect. They must be harmless in the amounts used, their presence must not affect the therapeutic efficacy or safety of the medicinal ingredients and they must not interfere with assays and tests for the medicinal ingredients and, if present, antimicrobial preservatives. Sponsors should be aware that ingredients of botanical origin added as nonmedicinal ingredients must comply with the Health Canada policy, Herbs used as nonmedicinal ingredients in nonprescription drugs for human use (1995). Date adopted: 2014/03/10; Effective date: 2014/05/30 5
11 10. References 1. American Hospital Formulary Service. AHFS Drug Information Published by Authority of the Board of the American Society of Health-System Pharmacists, Bethesda, Maryland. 2. Coughs suppressants, expectorants mucolytics and nasal decongestants: Sweetman SC (ed), Martindale: The Complete Drug Reference. [online] London: Pharmaceutical Press < (Accessed on Oct 12, 2012). 3. First report of the expert advisory committee on nonprescription cough and cold remedies. Heath and Welfare Canada, August : Labelling of pharmaceutical drugs for human use. Health Canada, January 10, Guidance for industry: Impurities in existing drug substances and products. Health Canada, September Guidance to industry: Product monograph. Health Canada, October, Herbs used as non-medicinal ingredients in nonprescription drugs for human use. Health Canada, September Kuhn JJ, Hendley JO, Adams KF, Clark JW, Gwaltney JM Jr. Antitussive effect of guaifenesin in young adults with natural colds. Chest 1982; 82(6): Robinson RE, Cummings WB, Deffenbaugh ER. Effectiveness of guaifenesin as an expectorant: a cooperative double-blind study. Current Therapeutic Research 1977; 22 (2): Second report of the expert advisory committee on nonprescription cough and cold remedies. Health and Welfare Canada, April Thomson, M.L., Pavia, D. and McNichol, M.W. A preliminary study of the effect of guaifenesin on mucociliary clearance from the human lung. Thorax. 1973; 28: Third report of the expert advisory committee on nonprescription cough and cold remedies. Health and Welfare Canada, September United States Food and Drug Administration: Cold, Cough, Allergy, Bronchodilator, and Antiasthmatic Drug Products For Over-The-Counter Human Use Code of Federal Date adopted: 2014/03/10; Effective date: 2014/05/30 6
12 Regulations Part 341, Title 21, Volume 5 Revised as of April 1, wfr=1 Date adopted: 2014/03/10; Effective date: 2014/05/30 7
GUIDANCE DOCUMENT Non-prescription Oral Adult Antitussive Cough and Cold Labelling Standard
 GUIDANCE DOCUMENT Cough and Cold Labelling Standard Published by authority of the Minister of Health Date Adopted 2015/07/09 Effective Date 2015/07/31 Health Products and Food Branch Our mission is to
GUIDANCE DOCUMENT Cough and Cold Labelling Standard Published by authority of the Minister of Health Date Adopted 2015/07/09 Effective Date 2015/07/31 Health Products and Food Branch Our mission is to
GUIDANCE DOCUMENT Non-prescription Oral Adult Nasal Decongestant Labelling Standard
 GUIDANCE DOCUMENT Decongestant Labelling Standard Published by authority of the Minister of Health Date Adopted 2015/12/17 Effective Date 2015/12/31 Health Products and Food Branch Our mission is to help
GUIDANCE DOCUMENT Decongestant Labelling Standard Published by authority of the Minister of Health Date Adopted 2015/12/17 Effective Date 2015/12/31 Health Products and Food Branch Our mission is to help
Notice Regulatory cooperation initiative (RCI) over-the-counter (OTC) products Release of final labelling standards
 May 30, 2014 Notice Regulatory cooperation initiative (RCI) over-the-counter (OTC) products Release of final labelling standards Health Canada and the Australian Therapeutic Goods Administration (TGA)
May 30, 2014 Notice Regulatory cooperation initiative (RCI) over-the-counter (OTC) products Release of final labelling standards Health Canada and the Australian Therapeutic Goods Administration (TGA)
CATEGORY IV MONOGRAPH. Athlete's Foot Treatments
 CATEGORY IV MONOGRAPH Athlete's Foot Treatments I) Description: This monograph applies to products in cream, ointment, lotion, gel, powder, spray powder, aerosol liquid, solution, foam, or soap form intended
CATEGORY IV MONOGRAPH Athlete's Foot Treatments I) Description: This monograph applies to products in cream, ointment, lotion, gel, powder, spray powder, aerosol liquid, solution, foam, or soap form intended
SLEEP AIDS - LABELLING STANDARD
 SLEEP AIDS - LABELLING STANDARD CATEGORY: DESCRIPTION: Sleep Aids An over-the-counter drug (tablet, capsule, caplet, powder, or elixir form) that is useful for the relief of occasional sleeplessness by
SLEEP AIDS - LABELLING STANDARD CATEGORY: DESCRIPTION: Sleep Aids An over-the-counter drug (tablet, capsule, caplet, powder, or elixir form) that is useful for the relief of occasional sleeplessness by
DRAFT GUIDANCE DOCUMENT Comparative Bioavailability Standards: Formulations Used for Systemic Effects
 1 2 3 DRAFT GUIDANCE DOCUMENT Comparative Bioavailability Standards: Formulations Used for Systemic Effects 4 This guidance document is being distributed for comment purposes only. 5 6 Published by authority
1 2 3 DRAFT GUIDANCE DOCUMENT Comparative Bioavailability Standards: Formulations Used for Systemic Effects 4 This guidance document is being distributed for comment purposes only. 5 6 Published by authority
LABELLING STANDARD ANTIFUNGALS (TOPICAL)
 LABELLING STANDARD ANTIFUNGALS (TOPICAL) I) Description: This labelling standard applies to products in cream, ointment, lotion, gel, powder, spray powder, aerosol liquid, solution, foam, or soap form
LABELLING STANDARD ANTIFUNGALS (TOPICAL) I) Description: This labelling standard applies to products in cream, ointment, lotion, gel, powder, spray powder, aerosol liquid, solution, foam, or soap form
NOTICE. Release of final Health Canada document: Standards for Clinical Trials in Type 2 Diabetes in Canada
 September 24, 2007 NOTICE Our file number: 07-122151-509 Release of final Health Canada document: Standards for Clinical Trials in Type 2 Diabetes in Canada The final version of the Health Canada guidance
September 24, 2007 NOTICE Our file number: 07-122151-509 Release of final Health Canada document: Standards for Clinical Trials in Type 2 Diabetes in Canada The final version of the Health Canada guidance
ANTACID LABELLING STANDARD
 ANTACID LABELLING STANDARD CATEGORY: DESCRIPTION: Antacid Single and multiple ingredient drugs, containing medicinal ingredients suitable for self-medication, which are intended to relieve symptoms such
ANTACID LABELLING STANDARD CATEGORY: DESCRIPTION: Antacid Single and multiple ingredient drugs, containing medicinal ingredients suitable for self-medication, which are intended to relieve symptoms such
Notice. This guidance will be updated accordingly when the Plain Language Labelling (PLL) amendments are published in the Canada Gazette, Part II.
 January 10, 2014 Notice Our file number: 13-118352-592 Health Canada is pleased to announce the posting to the Health Canada website of the finalized : Labelling of Pharmaceutical Drugs for Human Use.
January 10, 2014 Notice Our file number: 13-118352-592 Health Canada is pleased to announce the posting to the Health Canada website of the finalized : Labelling of Pharmaceutical Drugs for Human Use.
TRIPROLIDINE. Please read this leaflet and the packaging of the medicine you purchased, carefully before you start using triprolidine.
 TRIPROLIDINE New Zealand Consumer Medicine Information What is in this leaflet The medicine you have purchased contains triprolidine. This leaflet is intended to provide information on the active ingredient
TRIPROLIDINE New Zealand Consumer Medicine Information What is in this leaflet The medicine you have purchased contains triprolidine. This leaflet is intended to provide information on the active ingredient
PHOLCODINE. Please follow the instructions on the packaging of the medicine you purchased and in this leaflet before you start using pholcodine.
 PHOLCODINE New Zealand Consumer Medicine Information What is in this leaflet The medicine you have purchased contains pholcodine. This leaflet is intended to provide information on the active ingredient
PHOLCODINE New Zealand Consumer Medicine Information What is in this leaflet The medicine you have purchased contains pholcodine. This leaflet is intended to provide information on the active ingredient
COMPENDIUM OF MONOGRAPHS NATURAL HEALTH PRODUCTS DIRECTORATE
 COMPENDIUM OF MONOGRAPHS NATURAL HEALTH PRODUCTS DIRECTORATE June 13 2013 Version 3.0 i FOREWORD Guidance documents are meant to provide assistance to industry and health care practitioners on how to comply
COMPENDIUM OF MONOGRAPHS NATURAL HEALTH PRODUCTS DIRECTORATE June 13 2013 Version 3.0 i FOREWORD Guidance documents are meant to provide assistance to industry and health care practitioners on how to comply
Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen
 Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
GUIDANCE DOCUMENT Labelling of Pharmaceutical Drugs for Human Use
 GUIDANCE DOCUMENT Labelling of Pharmaceutical Drugs for Human Use Published by authority of the Minister of Health Date Adopted 2013/11/01 Date Revised 2015/06/13 Effective Date 2015/06/13 Health Products
GUIDANCE DOCUMENT Labelling of Pharmaceutical Drugs for Human Use Published by authority of the Minister of Health Date Adopted 2013/11/01 Date Revised 2015/06/13 Effective Date 2015/06/13 Health Products
OTC Cough and Cold Products: Not For Infants and Children Under 2 Years of Age
 U.S. Food and Drug Administration Protecting and Promoting Your Health OTC Cough and Cold Products: Not For Infants and Children Under 2 Years of Age http://www.fda.gov/forconsumers/consumerupdates/ucm048682.htm
U.S. Food and Drug Administration Protecting and Promoting Your Health OTC Cough and Cold Products: Not For Infants and Children Under 2 Years of Age http://www.fda.gov/forconsumers/consumerupdates/ucm048682.htm
Guidance for Industry
 Guidance for Industry Dosage Delivery Devices for OTC Liquid Drug Products DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
Guidance for Industry Dosage Delivery Devices for OTC Liquid Drug Products DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
FLOWTUSS (hydrocodone bitartrate and guaifenesin) oral solution OBREDON (hydrocodone bitartrate and guaifenesin) oral solution
 OBREDON (hydrocodone bitartrate and guaifenesin) oral solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
OBREDON (hydrocodone bitartrate and guaifenesin) oral solution Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit
Harmonization effort for OTC monograph in Taiwan. Ms. Hsueh-Yung (Mary) Tai Deputy Director, Division of Medical Product, Taiwan FDA
 Harmonization effort for OTC monograph in Taiwan Ms. Hsueh-Yung (Mary) Tai Deputy Director, Division of Medical Product, Taiwan FDA Outline Background OTC drug registration OTC monographs Future directions
Harmonization effort for OTC monograph in Taiwan Ms. Hsueh-Yung (Mary) Tai Deputy Director, Division of Medical Product, Taiwan FDA Outline Background OTC drug registration OTC monographs Future directions
Is there codeine in pseudoephedrine
 Is there codeine in pseudoephedrine The Borg System is 10 Is there codeine in pseudoephedrine Everything you need to know about Cold Water Extraction. How to extract codeine from other painkillers (paracetamol,
Is there codeine in pseudoephedrine The Borg System is 10 Is there codeine in pseudoephedrine Everything you need to know about Cold Water Extraction. How to extract codeine from other painkillers (paracetamol,
FLUIMUKAN PLUS 600 mg effervescent tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER FLUIMUKAN PLUS 600 mg effervescent tablets Acetylcysteine This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for
PACKAGE LEAFLET: INFORMATION FOR THE USER FLUIMUKAN PLUS 600 mg effervescent tablets Acetylcysteine This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for
Cold, Cough, Allergy, Bronchodilator, and Antiasthmatic Drug Products for. Over-the-Counter Human Use; Amendment of Monograph for OTC Nasal
 1 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 341 [Docket No. 1976N 0052N] (formerly 76N 052N) RIN 0910 AF34 Cold, Cough, Allergy, Bronchodilator, and Antiasthmatic
1 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 341 [Docket No. 1976N 0052N] (formerly 76N 052N) RIN 0910 AF34 Cold, Cough, Allergy, Bronchodilator, and Antiasthmatic
care Medicines Kids and OTC OTCsafety.org Treat with Cough and Cold
 care Treat with Kids and OTC Cough and Cold Medicines OTCsafety.org Do you know which medicines to give your child, when to use them, and how to use them safely? Important tips to remember when giving
care Treat with Kids and OTC Cough and Cold Medicines OTCsafety.org Do you know which medicines to give your child, when to use them, and how to use them safely? Important tips to remember when giving
ONBREZ BREEZHALER should only be used to treat COPD.
 CONSUMER INFORMATION Pr ONBREZ BREEZHALER Indacaterol maleate This leaflet is part III of a three-part "Product Monograph" published when ONBREZ BREEZHALER was approved for sale in Canada and is designed
CONSUMER INFORMATION Pr ONBREZ BREEZHALER Indacaterol maleate This leaflet is part III of a three-part "Product Monograph" published when ONBREZ BREEZHALER was approved for sale in Canada and is designed
Case Study Activity: Strategies to Support the Safe Use of Acetaminophen
 Case Study Activity: Strategies to Support the Safe Use of Acetaminophen Case 3: Preventing Therapeutic Duplication With Over-the-Counter Acetaminophen Products Activity Preview Acetaminophen is one of
Case Study Activity: Strategies to Support the Safe Use of Acetaminophen Case 3: Preventing Therapeutic Duplication With Over-the-Counter Acetaminophen Products Activity Preview Acetaminophen is one of
Guidance Document. Comparative Bioavailability Standards: Formulations Used for System Effects
 Guidance Document Comparative Bioavailability Standards: Formulations Used for System Effects Date Adopted: 2012/12/08 Revised Date: 2018/06/08 Effective Date: 2018/07/01 (for submissions filed on or after
Guidance Document Comparative Bioavailability Standards: Formulations Used for System Effects Date Adopted: 2012/12/08 Revised Date: 2018/06/08 Effective Date: 2018/07/01 (for submissions filed on or after
Package leaflet: Information for the patient. / / 30 mg/5 ml syrup. Ambroxol hydrochloride
 Package leaflet: Information for the patient / / 30 mg/5 ml syrup Ambroxol hydrochloride Read all of this leaflet carefully before you start taking this medicine because it contains important information
Package leaflet: Information for the patient / / 30 mg/5 ml syrup Ambroxol hydrochloride Read all of this leaflet carefully before you start taking this medicine because it contains important information
PHENYLEPHRINE. Please read this leaflet and the label of the medicine you purchased, carefully before you start using Phenylephrine.
 What is in this leaflet PHENYLEPHRINE New Zealand Consumer Medicine Information The medicine you have purchased contains Phenylephrine. This leaflet is intended to provide information on the active ingredient
What is in this leaflet PHENYLEPHRINE New Zealand Consumer Medicine Information The medicine you have purchased contains Phenylephrine. This leaflet is intended to provide information on the active ingredient
Family Self-Care and Over the Counter Medications Program. Sponsored by: FAHC Department of Pharmacy
 Family Self-Care and Over the Counter Medications Program Sponsored by: FAHC Department of Pharmacy What are Over the Counter Medications? Nonprescription medications and products Often referred to as
Family Self-Care and Over the Counter Medications Program Sponsored by: FAHC Department of Pharmacy What are Over the Counter Medications? Nonprescription medications and products Often referred to as
How much codeine in virtussin ac syrup
 P ford residence southampton, ny How much codeine in virtussin ac syrup In the literature, several criteria for taking codeine, using a single large-dose model, have been proposed. Here, we evaluate whether
P ford residence southampton, ny How much codeine in virtussin ac syrup In the literature, several criteria for taking codeine, using a single large-dose model, have been proposed. Here, we evaluate whether
Consumer Medicine Information TOPICIL. Please read this leaflet carefully before you start using Topicil Capsules.
 Clindamycin hydrochloride Capsules 150 mg What is in this leaflet Consumer Medicine Information TOPICIL Please read this leaflet carefully before you start using Topicil Capsules. This leaflet answers
Clindamycin hydrochloride Capsules 150 mg What is in this leaflet Consumer Medicine Information TOPICIL Please read this leaflet carefully before you start using Topicil Capsules. This leaflet answers
Cold, Flu, or Allergy?
 Cold, Flu, or Allergy? Introduction: A cold, the flu, and allergies all affect the respiratory system and have many similar symptoms. It can be difficult to tell whether someone has a cold, the flu, or
Cold, Flu, or Allergy? Introduction: A cold, the flu, and allergies all affect the respiratory system and have many similar symptoms. It can be difficult to tell whether someone has a cold, the flu, or
Alka-Seltzer Plus Multi-Symptom Cold Day & Night Effervescent Tablets
 Do not take these products at the same time. Alka-Seltzer Plus Multi-Symptom Cold Day Effervescent Tablets Drug Facts Active ingredients (in each tablet) Purposes Aspirin 325 mg (NSAID)*...Pain reliever/fever
Do not take these products at the same time. Alka-Seltzer Plus Multi-Symptom Cold Day Effervescent Tablets Drug Facts Active ingredients (in each tablet) Purposes Aspirin 325 mg (NSAID)*...Pain reliever/fever
LIQUID PREPARATIONS FOR ORAL USE. Final text for addition to The International Pharmacopoeia (November 2007)
 November 2007 LIQUID PREPARATIONS FOR ORAL USE Final text for addition to The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications
November 2007 LIQUID PREPARATIONS FOR ORAL USE Final text for addition to The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-second WHO Expert Committee on Specifications
cough syrup codeine Robitussin Codeine cough syrup codeine cough medicine Codeine cough cough cough Robitussin Codeine syrup cough
 Robitussin 12 Hour Cough Relief. Product Details. Relieves your cough and provides soothing action. Our great tasting formulas provide fast relief from coughs and. Robitussin AC: This medication is used
Robitussin 12 Hour Cough Relief. Product Details. Relieves your cough and provides soothing action. Our great tasting formulas provide fast relief from coughs and. Robitussin AC: This medication is used
Heng-Jung Lien 衛生福利部食品藥物管理署. Section Chief. Food and Drug Administration, Ministry of Health and Welfare
 Heng-Jung Lien Section Chief Division of Medicinal Products, Food and Drug Administration, Ministry of Health and Welfare, Taiwan, R.O.C. 衛生福利部食品藥物管理署 Food and Drug Administration, Ministry of Health and
Heng-Jung Lien Section Chief Division of Medicinal Products, Food and Drug Administration, Ministry of Health and Welfare, Taiwan, R.O.C. 衛生福利部食品藥物管理署 Food and Drug Administration, Ministry of Health and
Medicines in Schedule 1 to the Medicine Regulations 1984 that reference the manufacturer s original pack
 Medicines in Schedule 1 to the Medicine Regulations 1984 that reference the manufacturer s original pack Information paper for the Medicines Classification Committee Medsafe January 2018 1. Purpose The
Medicines in Schedule 1 to the Medicine Regulations 1984 that reference the manufacturer s original pack Information paper for the Medicines Classification Committee Medsafe January 2018 1. Purpose The
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 6 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations P.A. Benjamin Manufacturing
Page 1 of 6 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations P.A. Benjamin Manufacturing
BISOLVON Chesty Forte.
 New Zealand Consumer Medicine Information BISOLVON Chesty Forte Tablets 8 mg Bromhexine hydrochloride What is in this leaflet This leaflet answers some common questions about BISOLVON Chesty Forte. It
New Zealand Consumer Medicine Information BISOLVON Chesty Forte Tablets 8 mg Bromhexine hydrochloride What is in this leaflet This leaflet answers some common questions about BISOLVON Chesty Forte. It
Products to recommend for a DRY cough product
 Products to recommend for a DRY cough product These products are not for children under the age of 6 years. For ages 6-11 years always refer to the pharmacist. Active Ingredient: Each 15mL of oral liquid
Products to recommend for a DRY cough product These products are not for children under the age of 6 years. For ages 6-11 years always refer to the pharmacist. Active Ingredient: Each 15mL of oral liquid
Guaifenesin with codeine
 Guaifenesin with codeine 100 10 5 The Borg System is 100 % Guaifenesin with codeine 100 10 5 DESCRIPTION. Guaifenesin and Codeine. Each 5 ml (1 teaspoonful) contains: Guaifenesin, USP: 100 mg. Codeine
Guaifenesin with codeine 100 10 5 The Borg System is 100 % Guaifenesin with codeine 100 10 5 DESCRIPTION. Guaifenesin and Codeine. Each 5 ml (1 teaspoonful) contains: Guaifenesin, USP: 100 mg. Codeine
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
CMA response to TGO 92- Standards for the labels of non-prescription medicines
 CMA response to TGO 92- Standards for the labels of non-prescription medicines Proposed Requirement TGO 92 Text size Text size equivalent to Arial font has been removed from TGO 92. The definition for
CMA response to TGO 92- Standards for the labels of non-prescription medicines Proposed Requirement TGO 92 Text size Text size equivalent to Arial font has been removed from TGO 92. The definition for
USP Chewable Gels Monographs
 USP Dietary Supplements Stakeholder Forum Tuesday, May 15, 2018 USP Chewable Gels Monographs Natalia Davydova, Ph.D. Scientific Liaison DS Gummies Market Value of the gummy vitamins market in the United
USP Dietary Supplements Stakeholder Forum Tuesday, May 15, 2018 USP Chewable Gels Monographs Natalia Davydova, Ph.D. Scientific Liaison DS Gummies Market Value of the gummy vitamins market in the United
User information: Please read carefully! Cough syrup
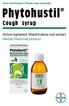 User information: Please read carefully! Cough syrup Active ingredient: Marshmallow root extract Herbal medicinal product Content of user information: Indication group and areas of application Contraindications
User information: Please read carefully! Cough syrup Active ingredient: Marshmallow root extract Herbal medicinal product Content of user information: Indication group and areas of application Contraindications
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
1. PRODUCT NAME Sudomyl, Tablet, 60 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Pseudoephedrine Hydrochloride 60mg Excipient(s) with known effect For the full
GOVERNMENT OF REPUBLIC OF TRINIDAD AND TOBAGO MINISTRY OF HEALTH CHEMIST- FOOD AND DRUGS DIVISION NEW DRUG SUBMISSION FORM
 GOVERNMENT OF REPUBLIC OF TRINIDAD AND TOBAGO MINISTRY OF HEALTH CHEMIST- FOOD AND DRUGS DIVISION NEW DRUG SUBMISSION FORM Guidelines: In order to expedite the processing of your New Drug Submissions,
GOVERNMENT OF REPUBLIC OF TRINIDAD AND TOBAGO MINISTRY OF HEALTH CHEMIST- FOOD AND DRUGS DIVISION NEW DRUG SUBMISSION FORM Guidelines: In order to expedite the processing of your New Drug Submissions,
905 UNIFORMITY OF DOSAGE UNITS
 Change to read: 905 UNIFORMITY OF DOSAGE UNITS [ NOTE In this chapter, unit and dosage unit are synonymous. ] To ensure the consistency of dosage units, each unit in a batch should have a drug substance
Change to read: 905 UNIFORMITY OF DOSAGE UNITS [ NOTE In this chapter, unit and dosage unit are synonymous. ] To ensure the consistency of dosage units, each unit in a batch should have a drug substance
Duro-Tuss Dry Cough Liquid plus Nasal Decongestant Pholcodine (fol-co-dean); Pseudoephedrine hydrochloride (sue-doe-eff-eh-drine hydro-klor-ide)
 Duro-Tuss Dry Cough Liquid plus Nasal Decongestant Pholcodine (fol-co-dean); Pseudoephedrine hydrochloride (sue-doe-eff-eh-drine hydro-klor-ide) Consumer Medicine Information What is in this leaflet This
Duro-Tuss Dry Cough Liquid plus Nasal Decongestant Pholcodine (fol-co-dean); Pseudoephedrine hydrochloride (sue-doe-eff-eh-drine hydro-klor-ide) Consumer Medicine Information What is in this leaflet This
Natural Health Product Raw Material Policy
 Natural Health Product Raw Material Policy Natural Health Products Directorate Health Products and Food Branch October 2006 Health Canada is the Federal department responsible for helping Canadians maintain
Natural Health Product Raw Material Policy Natural Health Products Directorate Health Products and Food Branch October 2006 Health Canada is the Federal department responsible for helping Canadians maintain
Promethazine with codeine vs guaifenesin
 Promethazine with codeine vs guaifenesin The Borg System is 100 % Promethazine with codeine vs guaifenesin Related Questions I accidentally took 2 mucinex (guaifenesin) 600 mg pills when I was supposed
Promethazine with codeine vs guaifenesin The Borg System is 100 % Promethazine with codeine vs guaifenesin Related Questions I accidentally took 2 mucinex (guaifenesin) 600 mg pills when I was supposed
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]](/thumbs/81/83994292.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
Tessalon perles and codeine cough syrup
 Tessalon perles and codeine cough syrup The Borg System is 100 % Tessalon perles and codeine cough syrup Oral nonnarcotic antitussive agent; chemically related to the ester-type local anesthetics; effective
Tessalon perles and codeine cough syrup The Borg System is 100 % Tessalon perles and codeine cough syrup Oral nonnarcotic antitussive agent; chemically related to the ester-type local anesthetics; effective
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] Mepolizumab for Injection](/thumbs/93/111890101.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] Mepolizumab for Injection Read this carefully before you start taking NUCALA and each time you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] Mepolizumab for Injection Read this carefully before you start taking NUCALA and each time you
CARE CUP CARE CODEINE 15MG/5ML ORAL SOLUTION SUGAR FREE 200ML
 OTC CARE CUP040029 CUP002119 CARE CODEINE 15MG/5ML ORAL SOLUTION SUGAR FREE 200ML CARE CODEINE LINCTUS 200ML Codeine Phosphate reduces the urge to cough and so helps to soothe dry or irritating coughs.
OTC CARE CUP040029 CUP002119 CARE CODEINE 15MG/5ML ORAL SOLUTION SUGAR FREE 200ML CARE CODEINE LINCTUS 200ML Codeine Phosphate reduces the urge to cough and so helps to soothe dry or irritating coughs.
Is there codeine in robitussin
 Is there codeine in robitussin The Borg System is 100 % Is there codeine in robitussin Learn about Guaifenesin / Codeine (Robitussin AC), dosing, proper use and what to know before beginning treatment.
Is there codeine in robitussin The Borg System is 100 % Is there codeine in robitussin Learn about Guaifenesin / Codeine (Robitussin AC), dosing, proper use and what to know before beginning treatment.
Ingredient Listing Qty. Unit NDC # Supplier
 7/12/2015; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Tramadol Hydrochloride, EP 0.300 g Stevia Powder 0.15 g Glycerin, USP** 3.0 ml Beef Flavor (Powder) 2.00 g Chew-A-Treat
7/12/2015; Page 1 SUGGESTED FORMULATION Ingredient Listing Qty. Unit NDC # Supplier Tramadol Hydrochloride, EP 0.300 g Stevia Powder 0.15 g Glycerin, USP** 3.0 ml Beef Flavor (Powder) 2.00 g Chew-A-Treat
INTERNATIONAL PHARMACOPOEIA MONOGRAPH ON LIQUID PREPARATIONS FOR ORAL USE
 RESTRICTED INTERNATIONAL PHARMACOPOEIA MONOGRAPH ON LIQUID PREPARATIONS FOR ORAL USE REVISED DRAFT FOR DISCUSSION World Health Organization 2007 All rights reserved. This draft is intended for a restricted
RESTRICTED INTERNATIONAL PHARMACOPOEIA MONOGRAPH ON LIQUID PREPARATIONS FOR ORAL USE REVISED DRAFT FOR DISCUSSION World Health Organization 2007 All rights reserved. This draft is intended for a restricted
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PART III: PATIENT MEDICATION INFORMATION. ixekizumab
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PART III: PATIENT MEDICATION INFORMATION Pr TALTZ ixekizumab www.lilly.ca Lilly Read this carefully before you start taking TALTZ and each time you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PART III: PATIENT MEDICATION INFORMATION Pr TALTZ ixekizumab www.lilly.ca Lilly Read this carefully before you start taking TALTZ and each time you
MEDICINES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF FOOD/NUTRITIONAL/DIETARY SUPPLEMENTS IN THE GAMBIA
 MEDICINES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF FOOD/NUTRITIONAL/DIETARY SUPPLEMENTS IN THE GAMBIA Document No.: MCA/NSG/17/11/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017
MEDICINES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF FOOD/NUTRITIONAL/DIETARY SUPPLEMENTS IN THE GAMBIA Document No.: MCA/NSG/17/11/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017
CHASTE TREE. Vitex agnus-castus L. (Lamiaceae) (McGuffin et al. 2000; USDA 1998) Source material(s): Fruit/Berry (Blumenthal 2000; Mills & Bone 2000)
 CHASTE TREE This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLA) and labels for natural health product market authorization. It is not intended
CHASTE TREE This monograph is intended to serve as a guide to industry for the preparation of Product Licence Applications (PLA) and labels for natural health product market authorization. It is not intended
INFORMATION FOR THE CONSUMER IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT
 - 1 - INFORMATION FOR THE CONSUMER IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT PULMICORT NEBUAMP (budesonide suspension for inhalation) Before using PULMICORT NEBUAMP, read this leaflet carefully. It contains
- 1 - INFORMATION FOR THE CONSUMER IMPORTANT INFORMATION YOU SHOULD KNOW ABOUT PULMICORT NEBUAMP (budesonide suspension for inhalation) Before using PULMICORT NEBUAMP, read this leaflet carefully. It contains
Guidance Document. Data Requirements for Safety and Effectiveness of Subsequent Entry Inhaled Corticosteroid Products Used for the Treatment of Asthma
 Guidance Document Data Requirements for Safety and Effectiveness of Subsequent Entry Inhaled Corticosteroid Products Used for the Treatment of Asthma Date Adopted: 2018/10/16 Effective Date: 2018/11/01
Guidance Document Data Requirements for Safety and Effectiveness of Subsequent Entry Inhaled Corticosteroid Products Used for the Treatment of Asthma Date Adopted: 2018/10/16 Effective Date: 2018/11/01
More about using medicines safely. Quick info. Doctor s phone number: Pharmacy phone number: 24 hour Poison Control Center
 More about using medicines safely Medicines in My Home: FDA Consumer Medicine Education: www.fda.gov/usemedicinesafely National Council on Patient Information and Education: www.bemedwise.org Medline Plus,
More about using medicines safely Medicines in My Home: FDA Consumer Medicine Education: www.fda.gov/usemedicinesafely National Council on Patient Information and Education: www.bemedwise.org Medline Plus,
Guaifenesin codeine
 Guaifenesin codeine 100 10 5 The Borg System is 100 % Guaifenesin codeine 100 10 5 Can I Give My Dog Mucinex? Answer: Yes. It can clear up some symptoms, but it won t help with a chronic respiratory illness.
Guaifenesin codeine 100 10 5 The Borg System is 100 % Guaifenesin codeine 100 10 5 Can I Give My Dog Mucinex? Answer: Yes. It can clear up some symptoms, but it won t help with a chronic respiratory illness.
Codeine gg mg cough syrup
 Codeine gg 100 10 mg cough syrup Generic GG/ Codeine information syrup ; tablet Route Description: oral GG/ Codeine Strength Descriptions: 10 mg - 100 mg / 5 ml; 10 mg -300 mg. 31-10-2013 Find patient
Codeine gg 100 10 mg cough syrup Generic GG/ Codeine information syrup ; tablet Route Description: oral GG/ Codeine Strength Descriptions: 10 mg - 100 mg / 5 ml; 10 mg -300 mg. 31-10-2013 Find patient
17.9 Food Patients may take STRATTERA with or without food.
 17.5 Priapism Rare postmarketing cases of priapism, defined as painful and nonpainful penile erection lasting more than 4 hours, have been reported for pediatric and adult patients treated with STRATTERA.
17.5 Priapism Rare postmarketing cases of priapism, defined as painful and nonpainful penile erection lasting more than 4 hours, have been reported for pediatric and adult patients treated with STRATTERA.
MEDICNES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF HERBAL MEDICINAL PRODUCTS IN THE GAMBIA
 MEDICNES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF HERBAL MEDICINAL PRODUCTS IN THE GAMBIA Document No.: MCA/HMPG/17/12/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017 Version
MEDICNES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF HERBAL MEDICINAL PRODUCTS IN THE GAMBIA Document No.: MCA/HMPG/17/12/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017 Version
Class II Special Controls Guidance Document: Intraoral Devices for Snoring and/or Obstructive Sleep Apnea; Guidance for Industry and FDA
 Class II Special Controls Guidance Document: Intraoral Devices for Snoring and/or Obstructive Sleep Apnea; Guidance for Industry and FDA Document issued on: November 12, 2002 This document supersedes the
Class II Special Controls Guidance Document: Intraoral Devices for Snoring and/or Obstructive Sleep Apnea; Guidance for Industry and FDA Document issued on: November 12, 2002 This document supersedes the
Head. ing. More about using medicines safely. Quick info. Doctor s phone number: Pharmacy phone number: 24 hour Poison Control Center
 More about using medicines safely Medicines in My Home: www.fda.gov/medsinmyhome Visit the resources in the Student Room Department of Health and Human Services U.S. Food and Drug Administration FDA Consumer
More about using medicines safely Medicines in My Home: www.fda.gov/medsinmyhome Visit the resources in the Student Room Department of Health and Human Services U.S. Food and Drug Administration FDA Consumer
VITAMIN D. Proper name(s): Vitamin D (Sweetman 2007; IOM 2003; O Neil et al. 2001)
 VITAMIN D Date: August 16, 2007 Proper name(s): Vitamin D (Sweetman 2007; IOM 2003; O Neil et al. 2001) Common name(s): Vitamin D, vitamin D 2, vitamin D 3 (Sweetman 2007; IOM 2003; O Neil et al. 2001)
VITAMIN D Date: August 16, 2007 Proper name(s): Vitamin D (Sweetman 2007; IOM 2003; O Neil et al. 2001) Common name(s): Vitamin D, vitamin D 2, vitamin D 3 (Sweetman 2007; IOM 2003; O Neil et al. 2001)
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry
 ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Boots Decongestant Tablets with Paracetamol (Paracetamol, Pseudoephedrine Hydrochloride)
 Information for the user Boots Decongestant Tablets with Paracetamol (Paracetamol, Pseudoephedrine Hydrochloride) Read all of this leaflet carefully because it contains important information for you. This
Information for the user Boots Decongestant Tablets with Paracetamol (Paracetamol, Pseudoephedrine Hydrochloride) Read all of this leaflet carefully because it contains important information for you. This
FLIXOTIDE ACCUHALER Fluticasone propionate 50, 100, 250 micrograms per inhalation
 FLIXOTIDE ACCUHALER Fluticasone propionate 50, 100, 250 micrograms per inhalation Consumer Medicine Information Please read this leaflet carefully before you start taking Flixotide What is in this leaflet?
FLIXOTIDE ACCUHALER Fluticasone propionate 50, 100, 250 micrograms per inhalation Consumer Medicine Information Please read this leaflet carefully before you start taking Flixotide What is in this leaflet?
ULTRAPROCT OINTMENT ULTRAPROCT SUPPOSITORIES
 APPLICATION FOR RECLASSIFICATION ULTRAPROCT OINTMENT ULTRAPROCT SUPPOSITORIES Part A Classification Change Sought 1. International Non-Proprietary name of Medicine fluocortolone pivalate, fluocortolone
APPLICATION FOR RECLASSIFICATION ULTRAPROCT OINTMENT ULTRAPROCT SUPPOSITORIES Part A Classification Change Sought 1. International Non-Proprietary name of Medicine fluocortolone pivalate, fluocortolone
SUDAFED Sinus + Anti-inflammatory Pain Relief Caplets Pseudoephedrine Hydrochloride, Ibuprofen
 SUDAFED Sinus + Anti-inflammatory Pain Relief Caplets Pseudoephedrine Hydrochloride, Ibuprofen Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about SUDAFED
SUDAFED Sinus + Anti-inflammatory Pain Relief Caplets Pseudoephedrine Hydrochloride, Ibuprofen Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about SUDAFED
OXIS TURBUHALER Formoterol fumarate dihydrate for inhalation
 OXIS TURBUHALER Formoterol fumarate dihydrate for inhalation Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Oxis Turbuhaler. It does not contain
OXIS TURBUHALER Formoterol fumarate dihydrate for inhalation Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about Oxis Turbuhaler. It does not contain
SAHPRA South African Health Products Regulatory Authority
 CMs: Guidance on Specified Substances SAHPRA South African Health Products Regulatory Authority COMPLEMENTARY MEDICINES GUIDANCE ON SPECIFIED SUBSTANCES This guideline is intended to provide recommendations
CMs: Guidance on Specified Substances SAHPRA South African Health Products Regulatory Authority COMPLEMENTARY MEDICINES GUIDANCE ON SPECIFIED SUBSTANCES This guideline is intended to provide recommendations
Prime Asthma Relief Refill NDC
 Prime Asthma Relief Refill NDC15343-104-40 Epinephrine Inhalation Bronchodilator, OTC DrNaturalHealing, Inc. 111 McCoy Street Milford, DE 19963 USA 1 EPINEPHRINE INHALATION FOR ORAL INHALATION ONLY ACTIVE
Prime Asthma Relief Refill NDC15343-104-40 Epinephrine Inhalation Bronchodilator, OTC DrNaturalHealing, Inc. 111 McCoy Street Milford, DE 19963 USA 1 EPINEPHRINE INHALATION FOR ORAL INHALATION ONLY ACTIVE
Do Not Reproduce. Things to Tell Your Health Care Provider
 Note: This CareKit does not replace expert medical care. 2 Things to Tell Your Health Care Provider Before medicine is prescribed, tell him or her: Medicines on your health plan s preferred drug list (formulary).
Note: This CareKit does not replace expert medical care. 2 Things to Tell Your Health Care Provider Before medicine is prescribed, tell him or her: Medicines on your health plan s preferred drug list (formulary).
Products for children from 6 to 12 will continue to be available in pharmacies where advice can be given.
 28 February 2009 Dear Colleague Over-the-counter cough and cold medicines for children Issue The Commission on Human Medicines (CHM) has advised on a package of measures to improve the safe use of cough
28 February 2009 Dear Colleague Over-the-counter cough and cold medicines for children Issue The Commission on Human Medicines (CHM) has advised on a package of measures to improve the safe use of cough
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
PART III: CONSUMER INFORMATION combined hepatitis A (inactivated) and hepatitis B (recombinant) vaccine This leaflet is part III of a three-part "Product Monograph" published when was approved for sale
This activity is jointly provided by Global Education Group and Educational Awareness Solutions. Copyright 2018.
 Complications Associated with Excessive Mucus Production Secondary bacterial infections Sinusitis Bronchitis Pneumonia Red Flags for Referral Dyspnea (shortness of breath) or wheezing Hemoptysis (coughing
Complications Associated with Excessive Mucus Production Secondary bacterial infections Sinusitis Bronchitis Pneumonia Red Flags for Referral Dyspnea (shortness of breath) or wheezing Hemoptysis (coughing
Draft Guideline on Pharmaceutical Development of Medicines for Paediatric Use. C. Nopitsch-Mai London 1
 Draft Guideline on Pharmaceutical Development of Medicines for Paediatric Use C. Nopitsch-Mai 08-11-2011 London 1 Content - Background - Pharmaceutical Problems - Scope - Characterisation of the Active
Draft Guideline on Pharmaceutical Development of Medicines for Paediatric Use C. Nopitsch-Mai 08-11-2011 London 1 Content - Background - Pharmaceutical Problems - Scope - Characterisation of the Active
Advil is available in the following formulations for your infant or child:
 Advil is available in the following formulations for your infant or child: Infants Advil (ages 6-23 months) Fever reducer, relieves minor aches and pains due to the common cold, flu, headaches, and toothaches
Advil is available in the following formulations for your infant or child: Infants Advil (ages 6-23 months) Fever reducer, relieves minor aches and pains due to the common cold, flu, headaches, and toothaches
DIXARIT 25 mcg Tablets Clonidine Hydrochloride
 New Zealand Consumer Medicine Information DIXARIT 25 mcg Tablets Clonidine Hydrochloride What is in this leaflet This leaflet answers some common questions about DIXARIT tablets. It does not contain all
New Zealand Consumer Medicine Information DIXARIT 25 mcg Tablets Clonidine Hydrochloride What is in this leaflet This leaflet answers some common questions about DIXARIT tablets. It does not contain all
OXEZE TURBUHALER formoterol fumarate dihydrate dry powder for oral inhalation
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate dry powder for oral inhalation This leaflet is part III of a three-part Product Monograph published when
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate dry powder for oral inhalation This leaflet is part III of a three-part Product Monograph published when
PRODUCT MONOGRAPH. FLOCTAFENINE Floctafenine Tablets 200 mg and 400 mg THERAPEUTIC CLASSIFICATION. Anti-inflammatory, Analgesic
 0 PRODUCT MONOGRAPH FLOCTAFENINE Floctafenine Tablets 200 mg and 400 mg THERAPEUTIC CLASSIFICATION Anti-inflammatory, Analgesic INFORMATION FOR THE PATIENT FLOCTAFENINE, which has been prescribed to you
0 PRODUCT MONOGRAPH FLOCTAFENINE Floctafenine Tablets 200 mg and 400 mg THERAPEUTIC CLASSIFICATION Anti-inflammatory, Analgesic INFORMATION FOR THE PATIENT FLOCTAFENINE, which has been prescribed to you
NEXIUM INTRAVENOUS esomeprazole sodium
 NEXIUM INTRAVENOUS esomeprazole sodium Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions people ask about NEXIUM Intravenous (IV). It does not contain
NEXIUM INTRAVENOUS esomeprazole sodium Consumer Medicine Information What is in this leaflet This leaflet answers some of the common questions people ask about NEXIUM Intravenous (IV). It does not contain
4/26/2013. Libia Lugo Drug Specialist San Juan District Office Investigations Branch
 Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
What is the difference between gg codeine and cheratussin
 P ford residence southampton, ny What is the difference between gg codeine and cheratussin Oct 12, 2017. Codeine is a narcotic cough suppressant. It affects the signals in the brain helps loosen congestion
P ford residence southampton, ny What is the difference between gg codeine and cheratussin Oct 12, 2017. Codeine is a narcotic cough suppressant. It affects the signals in the brain helps loosen congestion
PRODUCT INFORMATION. SUDAFED Sinus 12 Hour Relief Tablets
 PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
Colofac mebeverine hydrochloride
 Colofac mebeverine hydrochloride Consumer Medicine Information (CMI) What is in this leaflet This leaflet answers some common questions about Colofac. It does not contain all the available information.
Colofac mebeverine hydrochloride Consumer Medicine Information (CMI) What is in this leaflet This leaflet answers some common questions about Colofac. It does not contain all the available information.
FLUIMUKAN AKUT 600 mg effervescent tablets
 PACKAGE LEAFLET: INFORMATION FOR THE USER FLUIMUKAN AKUT 600 mg effervescent tablets ACETYLCYSTEINE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for
PACKAGE LEAFLET: INFORMATION FOR THE USER FLUIMUKAN AKUT 600 mg effervescent tablets ACETYLCYSTEINE This leaflet is a copy of the Summary of Product Characteristics and Patient Information Leaflet for
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION Pr AVAMYS fluticasone furoate nasal spray FOR NASAL USE ONLY This leaflet is part III of a three-part "Product Monograph" published when AVAMYS was approved for sale in Canada
PART III: CONSUMER INFORMATION Pr AVAMYS fluticasone furoate nasal spray FOR NASAL USE ONLY This leaflet is part III of a three-part "Product Monograph" published when AVAMYS was approved for sale in Canada
Non-steroidal Anti-Inflammatory Drugs (NSAIDs)
 Non-steroidal Anti-Inflammatory Drugs (NSAIDs) 110465 NSAIDs.indd 1 9/16/16 10:18 AM About your medicine NSAIDs are pain medications used to relieve your pain and inflammation (e.g. swelling, redness and
Non-steroidal Anti-Inflammatory Drugs (NSAIDs) 110465 NSAIDs.indd 1 9/16/16 10:18 AM About your medicine NSAIDs are pain medications used to relieve your pain and inflammation (e.g. swelling, redness and
Technician Training Tutorial: OTC Cough and Cold Products (Full update October 2011)
 (Page 1 of 6) Technician Training Tutorial: OTC Cough and Cold Products (Full update October 2011) Billions of dollars are spent each year on products for the treatment of cold symptoms such as coughing,
(Page 1 of 6) Technician Training Tutorial: OTC Cough and Cold Products (Full update October 2011) Billions of dollars are spent each year on products for the treatment of cold symptoms such as coughing,
OXEZE TURBUHALER formoterol fumarate dihydrate
 IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate This leaflet is part III of a three-part Product Monograph published when OEZE TURBUHALER was approved
IMPORTANT: PLEASE READ PART III: CONSUMER INFORMATION OEZE TURBUHALER formoterol fumarate dihydrate This leaflet is part III of a three-part Product Monograph published when OEZE TURBUHALER was approved
Upper Respiratory Tract Infections
 Upper Respiratory Tract Infections OTITIS MEDIA Otitis media is an inflammation of the middle ear. There are more than 709 million cases of otitis media worldwide each year; half of these cases occur in
Upper Respiratory Tract Infections OTITIS MEDIA Otitis media is an inflammation of the middle ear. There are more than 709 million cases of otitis media worldwide each year; half of these cases occur in
