FOR PUBLIC CONSULTATION ONLY. Evidence Review: Rituximab for immunoglobulin G4-related disease (IgG4-RD)
|
|
|
- Philip Stephens
- 6 years ago
- Views:
Transcription
1 Evidence Review: Rituximab for immunoglobulin G4-related disease (IgG4-RD)
2 NHS England FOR PUBLIC CONSULTATION ONLY Evidence Review: Rituximab for immunoglobulin G4-related disease (IgG4- RD) First published: January 2016 Updated: Prepared by Not applicable Turnkey Clinical Evidence Review Team on behalf of NHS England Specialised Commissioning 1
3 Contents Introduction 3 Summary of results 3 Research Questions 4 Methodology 5 Results 5 References Literature Search Terms See Appendix 1 See Appendix 2 2
4 1. Introduction Immunoglobulin G4-related disease (IgG4-RD) is an increasingly recognised immune-mediated chronic condition that links several disorders previously seen as unrelated. Recognised as a unified entity only a decade ago, the disease is caused by plasma cells producing the antibody subtype IgG4 which results in mass-forming tissue destructive lesions, with the three key pathologic features of IgG4-RD being lymphoplasmacytic infiltration, storiform fibrosis and obliterative phlebitis. Conditions once regarded as autoimmune/idiopathic disorders but now recognised to be part of IgG4-RD include: autoimmune pancreatitis, cholangitis, periaortitis, retroperitoneal fibrosis with ureteric obstruction, orbital masses, pulmonary nodules / interstitial or airway involvement, thyroiditis, dacryoadenitis, sialadenitis, renal tubulointerstitial nephritis or membranous glomerulonephritis, lymphadenopathy, testicular masses, prostatitis, pericarditis, mastitis and perineural disease. Symptoms, if any, are usually mild and include the presence of painless swellings and mass lesions. Nevertheless, IgG4-RD can cause severe organ damage and even death if left untreated. Rituximab is an anti-cd20 chimeric monoclonal antibody. It depletes circulating B-cells and prevents their maturation into a sub-set of antibody-secreting plasma cells that produce IgG4 autoantibodies. Rituximab has been proposed in IgG4-RD as a third line therapy to control IgG4- RD and prevent further disease progression to fibrosis and organ damage. The eligible patient group is relapsed patients with active disease that is no longer controlled with conventional therapies who, either fail to respond to primary treatment, or with adverse reactions or contraindications to corticosteroids plus azathioprine or methotrexate or mycophenolate mofetil. 2. Summary of results The literature search identified 31 papers, of which 28 were excluded because they did not meet the inclusion criteria. The three papers included in the comparative effectiveness reviews had 44 patients included in them collectively. All three studies were observational with no comparator group. Is Rituximab clinically effective in the treatment of patients with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroid-dependent? The three studies conclude that Rituximab is clinically effective; however caution should be exercised in light of the very small number of patients and study design. Carruthers et al (2015) conclude that their prospective, single-arm safety/efficacy trial of Rituximab (RTX) provides strong evidence that B cell depletion is an effective treatment for IgG4-RD. Thirty patients were recruited into this study: it is not clear whether these were recruited consecutively or the extent to which there may be some selection bias inherent in the study design. The mean age of the study population was 61, with 28 of the 30 being male. 13% of the cohort required retreatment during the 12 months after enrolment. At 12 months only 7% of patients required steroids for their IgG4-RD. Fourteen (47%) and 12 (40%) participants achieved and maintained complete remissions through 6 and 12 months, respectively. Considering the extent of organ involvement, patients with limited organ involvement were more likely to achieve complete remission within 6 months compared with those with multi-organ involvement (12/16 vs 6/14 subjects including serum IgG4 in the assessment; p=0.10; 14/16 vs 7/14 subjects if serum IgG4 excluded; p<0.05). The study concludes that these findings support the observations from smaller retrospective studies, indicating that B cell depletion is an effective and important treatment for IgG4- RD. Gluco-corticoids (GC) should remain the first treatment approach for most patients at the present time, assuming the absence of major contraindications to GC therapy. Khosroshahi A et al (2012) reported in a small uncontrolled observational study with 10 patients that treatment with Rituximab led to prompt clinical and serologic improvement in refractory IgG4-RD in all patients with active inflammation. All patients discontinued steroid and disease-modifying anti-rheumatic drugs (DMARD) following Rituximab treatment; however, four patients were retreated at 6 months. It was reported that repeated courses of Rituximab may lead to progressive declines in serum IgG4 concentrations and better disease control. It was not reported whether the 10 patients were consecutively recruited, nor whether the study was prospective or retrospective. Outcomes were assessed at one month; there is no reporting of longer term outcomes. 3
5 Khosroshahi A et al (2010) performed a small (n=4) efficacy study to assess the clinical and serologic responses to B lymphocyte depletion therapy with Rituximab in patients with systemic IgG4-RD. It was reported that treatment with rituximab led to prompt clinical and serologic improvement in patients with refractory systemic IgG4-RD. The decline in serum IgG4 concentrations was substantially steeper than that of the auto-antibody concentrations in immune-mediated conditions in which Rituximab is effective, such as in Rheumatoid Arthritis. In addition, the reduction in IgG-subclass levels appeared to be specific for IgG4. Given the small number of patients, caution should be warranted in drawing conclusions from this study. Is there any evidence to suggest that either the lymphoma protocol or the rheumatoid arthritis protocol produces better clinical outcomes in patients with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroid-dependent? There was no evidence to answer this question. Is Rituximab more effective than standard treatment in the treatment of patients with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroid-dependent? The three studies were observational in design with no comparator group. It is not possible to give an answer to a question of whether Rituximab is more effective than another treatment. All of the studies were conducted in refractory (to steroid or standard DMARDS) patients. Is Rituximab safe to use in the treatment of patients with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroiddependent? The three studies did not directly address this question, thus it is not possible to provide an evidence based answer. Is Rituximab a cost-effective treatment option for use in patients with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroid-dependent? There were no cost effectiveness studies. It is not possible to answer this question. 3. Research questions Is rituximab clinically effective in the treatment of patients with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroiddependent? Is there any evidence to suggest that either the lymphoma protocol or the rheumatoid arthritis protocol produces better clinical outcomes in patients with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroid-dependent? Is rituximab more effective than standard treatment in the treatment of patients with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroid-dependent? Is rituximab safe to use in the treatment of patients with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroiddependent? Is rituximab a cost-effective treatment option for use in patients with with refractory IgG4-RD which has failed to respond to conventional treatment or with adverse reactions or contraindications to corticosteroids or corticosteroiddependent? 4
6 4. Methodology A review of published, peer reviewed literature has been undertaken based on the research questions set out in Section 3 and a search strategy agreed with the lead clinician and public health lead for this policy area. This has involved a PubMed search and search of the Cochrane database for systematic reviews, in addition to review of any existing NICE or SIGN guidance. The evidence review has been independently quality assured. An audit trail has been maintained of papers excluded from the review on the basis of the inclusion and exclusion criteria agreed within the search strategy. The full list has been made available to the clinicians developing the policy where requested. 5. Results A detailed breakdown of the evidence is included in the Appendix. 5
7 Appendix One Grade Grade of evidence Study design 2- Case series Study design and Study size Intervention Category Primary Outcome mg doses of RTX, administere d approximate ly 15 days apart Clinical effectivenes s of the intervention Disease activity was measured by the IgG4-RD Responder Index (IgG4-RD RI) and physician's global assessment (PGA). Disease response was defined as the improvement of the IgG4-RD RI by two points. The primary outcome, measured at 6 months, was defined as: (1) decline of the IgG4-RD RI 2 points compared with baseline; (2) no disease flares before month 6; and (3) no GC use between months 2 and 6 Outcomes Primary Result The primary outcome was achieved by 23 participants (77%). Fourteen (47%) were in complete remission at 6 months, and 12 (40%) remained in complete remission at 12 months Secondary Outcome Secondary Result Reference Reference none - Carruthers, Mollie N.; Topazian, Mark D.; Khosroshahi, Arezou; Witzig, Thomas E.; Wallace, Zachary S.; Hart, Philip A.; Deshpande, Vikram; Smyrk, Thomas C.; Chari, Suresh; Stone, John H.. Rituximab for IgG4- related disease: a prospective, open-label trial. Ann. Rheum. Dis. 2015;74(6): Complic ations noted Two patients were hospitali sed for infection s during the trial period. Four addition al patients were hospitali sed. Benefits noted Comments Other - The authors conclude that this prospective, singlearm pilot trial of rituximab (RTX) provide strong evidence that B cell depletion is an effective treatment for IgG4-RD. 13% of the cohort required retreatment during the 12m after enrollment. At 12 months only 7% of patients required steroids for their IgG4-RD. Fourteen (47%) and 12 (40%) participants achieved and maintained complete remissions through 6 and 12 months, respectively. Considering the extent of organ involvement, patients with limited organ involvement were more likely to achieve complete remission within 6 months compared with those with multiorgan involvement (12/16 vs 6/14 subjects including serum IgG4 in the assessment; p=0.10; 14/16 vs 7/14 subjects if serum IgG4 excluded; p<0.05). The study concludes that the findings from this prospective pilot trial support the observations from small retrospective studies indicating that B cell depletion is an effective and important treatment for IgG4-RD. Gluco-corticoid (GC) should remain the first treatment approach for most patients at the present time, assuming the absence of major contraindications to GC therapy. However, the incomplete or unsustained responses to GCs observed in many IgG4-RD patients, coupled with the fact that many IgG4-RD patients are middleaged to elderly and have co-comorbidities contraindicating long-term GCs, indicate that B cell depletion may have a substantial role in a large percentage of IgG4-RD patients. This may be particularly true for patients with multiorgan disease. 6
8 2- Case series 10 RTX (2 infusions of 1000 mg, 15 days apart Clinical Clinical effectivenes s of the intervention improvement - assessed by monitoring the patient's ability to taper prednisone to discontinuation and to stop DMARDs; by serial measurements of total IgG and IgG subclasses; and by followup radiologic assessments guided by the patient's particular pattern of organ involvement. Nine of 10 patients demonstrated striking clinical improvement within 1 month of starting RTX. All 10 patients were able to discontinue prednisone and DMARDs following RTX therapy. the IgG4- not RD reported Disease Activity Index and Flare Tool (retrospecti vely applied) Khosroshahi - none Treatment with Rituximab led to prompt clinical and, Arezou; reported serologic improvement in refractory IgG4-RD in all Carruthers, patients with active inflammation. It was reported that Mollie N.; repeated courses of Rituximab may lead to Deshpande, progressive declines in serum IgG4 concentrations Vikram; and better disease control. Unizony, Sebastian; Bloch, Donald B.; Stone, John H.. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore) 2012;91(1):
9 2- Case series 4 RTX (2 infusions of 1000 mg, 15 days apart Clinical Clinical effectivenes s of the intervention improvement was assessed by monitoring the tapering/discont inuation of prednisone and DMARDs, and by measuring the serum concentrations of B lymphocytes, immunoglobulin s, and IgG subclasses before and after therapy Among these patients, the serum IgG4 concentrations declined by a mean of 65% within 2 months of rituximab administration. All 4 patients demonstrated striking clinical improvement within 1 month of the initiation of rituximab therapy, and tapering or discontinuation of their treatment with prednisone and DMARDs was achieved in all 4 patients. A decrease in IgG concentration was observed for the IgG4 subclass only. - - Khosroshahi, Arezou; Bloch, Donald B.; Deshpande, Vikram; Stone, John H.. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4- related systemic disease. Arthritis Rheum. 2010;62(6): none reported This was a small efficacy study to assess the clinical and serologic responses to B lymphocyte depletion therapy with rituximab in patients with IgG4-RSD. It was reported that treatment with rituximab led to prompt clinical and serologic improvement in patients with refractory IgG4-RSD. The decline in serum IgG4 concentrations was substantially steeper than that of the autoantibody concentrations in immune-mediated conditions in which rituximab is effective, such as in rheumatoid arthritis. In addition, the reduction in IgGsubclass levels appeared to be specific for IgG4. 8
10 Appendix Two Literature search terms Assumptions / limits applied to search: n/a Original search terms: Updated search terms - Population igg4-rd OR immunoglobulin g4 OR igg4-related diesease OR igg4 sclerosing disease OR igg4-related systemic disease OR igg4-related sclerosing disease OR igg4-related systemic sclerosing disease OR igg4-related autoimmune disease OR igg4-associated multifocal systemic fibrosis OR igg4-associated disease OR hyper-igg4 disease OR systemic igg4-related plasmacytic syndrome OR igg4-positive multiorgan lymphoproliferative syndrome OR igg4 syndrome Updated search terms - Intervention Updated search terms - Comparator rituximab OR rituxan OR mabthera steroid OR steroids OR azathioprine OR methotrexate OR mycophenolate mofetil OR prednisolone OR prednisone OR methylprednisolone OR corticosteroid OR corticosteroids OR glucocorticoid OR glucocorticoids OR glucocorticosteroid 9
11 OR glucocorticosteroid OR glucocorticosteroids FOR PUBLIC CONSULTATION ONLY Updated search terms - Outcome Inclusion criteria n/a General inclusion criteria In order of decreasing priority, articles will be selected based on the following criteria. 1.All relevant systematic reviews and meta-analysis in the last 5 years and those in 5-10 years period which are still relevant (e.g. no further updated systematic review available) 2.All relevant RCTs and those in the 5-10 years period which are still relevant (e.g. not superseded by a next phase of the trial/ the RCT is one of the few or only high quality clinical trials available) >>>> If studies included reaches 30, inclusion stops here 3.All relevant case control and cohort studies, that qualify after exclusion criteria >>>> If studies included reaches 30, inclusion stops here 4.All relevant non analytical studies (case series/ reports etc.) that qualify after exclusion criteria >>>> If studies included reaches 30, inclusion stops here Specific inclusion criteria The policy working group asked the following paper to be included in the clinical evidence review: Exclusion criteria Treatment approaches to IgG4-related systemic disease, Khosroshahi A, Stone JH. Curr Opin Rheumatol. 2011;23(1):67 General exclusion criteria Studies with the following characteristics will be excluded: 1. Does not answer a PICO research question 2. Comparator differs from the PICO 3. < 50 subjects (where studies with >50 subjects exist) 4. No relevant outcomes 5. Incorrect study type 6. Inclusion of outcomes for only one surgeon/doctor or only one clinical site (where studies with > one surgeon/doctor or one clinical site exist) Specific exclusion criteria n/a 10
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Rituximab for immunoglobulin G4-related disease (IgG4-RD) Reference: NHS England A13X07/01 Information Reader Box (IRB) to be inserted on inside front cover for
Clinical Commissioning Policy Proposition: Rituximab for immunoglobulin G4-related disease (IgG4-RD) Reference: NHS England A13X07/01 Information Reader Box (IRB) to be inserted on inside front cover for
How 5 Diseases Became One. Moez Tajdin R3 McGill University
 How 5 Diseases Became One Moez Tajdin R3 McGill University Conflicts of Interest None! Mr. M. ID: 65 M PMH Benign prostatic hyperplasia Prostate cancer Awaiting biopsy Skin rash Dyslipidemia Hypertension
How 5 Diseases Became One Moez Tajdin R3 McGill University Conflicts of Interest None! Mr. M. ID: 65 M PMH Benign prostatic hyperplasia Prostate cancer Awaiting biopsy Skin rash Dyslipidemia Hypertension
Overview of the Immunoglobulin G4-related Disease Spectrum
 Review Article The Korean Journal of Pancreas and Biliary Tract 2015;20:124-129 http://dx.doi.org/10.15279/kpba.2015.20.3.124 pissn 1976-3573 eissn 2288-0941 면역글로불린 G4 연관질환의개요 1 한림대학교의과대학한림대학교성심병원내과, 2
Review Article The Korean Journal of Pancreas and Biliary Tract 2015;20:124-129 http://dx.doi.org/10.15279/kpba.2015.20.3.124 pissn 1976-3573 eissn 2288-0941 면역글로불린 G4 연관질환의개요 1 한림대학교의과대학한림대학교성심병원내과, 2
Case Report Thoracic Paravertebral Mass as an Infrequent Manifestation of IgG4-Related Disease
 Hindawi Case Reports in Rheumatology Volume 2017, Article ID 4716245, 4 pages https://doi.org/10.1155/2017/4716245 Case Report Thoracic Paravertebral Mass as an Infrequent Manifestation of IgG4-Related
Hindawi Case Reports in Rheumatology Volume 2017, Article ID 4716245, 4 pages https://doi.org/10.1155/2017/4716245 Case Report Thoracic Paravertebral Mass as an Infrequent Manifestation of IgG4-Related
Evidence Review: Title. Month/ Year. Evidence Review:
 Evidence Review: Title Month/ Year Evidence Review: Rituximab for connective tissue disease associated interstitial lung disease October 2014 Standard Operating Procedure: NHS England Evidence Review:
Evidence Review: Title Month/ Year Evidence Review: Rituximab for connective tissue disease associated interstitial lung disease October 2014 Standard Operating Procedure: NHS England Evidence Review:
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Rituximab for the treatment of dermatomyositis and polymyositis (Adults) Reference: NHS England A13X05/01 Information Reader Box (IRB) to be inserted on inside
Clinical Commissioning Policy Proposition: Rituximab for the treatment of dermatomyositis and polymyositis (Adults) Reference: NHS England A13X05/01 Information Reader Box (IRB) to be inserted on inside
IgG4-related disease: features and treatment response in a multi-ethnic cohort in Singapore
 IgG4-related disease: features and treatment response in a multi-ethnic cohort in Singapore W. Fong 1,2,3, I. Liew 1, D. Tan 2,3,4, K.H. Lim 5, A. Low 6, Y.Y. Leung 1,2 1 Department of Rheumatology and
IgG4-related disease: features and treatment response in a multi-ethnic cohort in Singapore W. Fong 1,2,3, I. Liew 1, D. Tan 2,3,4, K.H. Lim 5, A. Low 6, Y.Y. Leung 1,2 1 Department of Rheumatology and
IgG4 Disease. General Principles of IgG4-related disease. EL Cluvar, AC Bateman
 IgG4 Disease General Principles of IgG4-related disease. EL Cluvar, AC Bateman Diagnostic Guidelines for IgG4-related disease with a focus on histopathological criteria. V Deshpande, A Khosroshahi Diagnostic
IgG4 Disease General Principles of IgG4-related disease. EL Cluvar, AC Bateman Diagnostic Guidelines for IgG4-related disease with a focus on histopathological criteria. V Deshpande, A Khosroshahi Diagnostic
Clinical Study Development of an IgG4-RD Responder Index
 International Rheumatology Volume 2012, Article ID 259408, 7 pages doi:10.1155/2012/259408 Clinical Study Development of an IgG4-RD Responder Index Mollie N. Carruthers, 1 John H. Stone, 1 Vikram Deshpande,
International Rheumatology Volume 2012, Article ID 259408, 7 pages doi:10.1155/2012/259408 Clinical Study Development of an IgG4-RD Responder Index Mollie N. Carruthers, 1 John H. Stone, 1 Vikram Deshpande,
Mikulicz s Disease with Progressively Transformed Germinal Centers-type Immunoglobulin G4-related Lymphadenopathy Mimicking Sjögren s Syndrome
 Journal of Rheumatic Diseases Vol. 22, No. 6, December, 2015 http://dx.doi.org/10.4078/jrd.2015.22.6.395 Case Report Mikulicz s Disease with Progressively Transformed Germinal Centers-type Immunoglobulin
Journal of Rheumatic Diseases Vol. 22, No. 6, December, 2015 http://dx.doi.org/10.4078/jrd.2015.22.6.395 Case Report Mikulicz s Disease with Progressively Transformed Germinal Centers-type Immunoglobulin
Clinical Commissioning Policy: Rituximab For ANCA Vasculitis. December Reference : NHSCB/ A3C/1a
 Clinical Commissioning Policy: Rituximab For ANCA Vasculitis December 2012 Reference : NHSCB/ A3C/1a NHS Commissioning Board Clinical Commissioning Policy: Rituximab For The Treatment Of Anti-Neutrophil
Clinical Commissioning Policy: Rituximab For ANCA Vasculitis December 2012 Reference : NHSCB/ A3C/1a NHS Commissioning Board Clinical Commissioning Policy: Rituximab For The Treatment Of Anti-Neutrophil
New Evidence reports on presentations given at EULAR Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis
 New Evidence reports on presentations given at EULAR 2011 Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis Report on EULAR 2011 presentations Anti-TNF failure and response to rituximab
New Evidence reports on presentations given at EULAR 2011 Rituximab for the Treatment of Rheumatoid Arthritis and Vasculitis Report on EULAR 2011 presentations Anti-TNF failure and response to rituximab
TOCILIZUMAB FOR DIFFICULT TO TREAT IDIOPATHIC RETROPERITONEAL FIBROSIS. A PILOT TRIAL
 TOCILIZUMAB FOR DIFFICULT TO TREAT IDIOPATHIC RETROPERITONEAL FIBROSIS. A PILOT TRIAL Dr Augusto Vaglio, Dr Federica Maritati Unit of Nephrology, University Hospital of Parma, Via Gramsci 14, 43126 Parma
TOCILIZUMAB FOR DIFFICULT TO TREAT IDIOPATHIC RETROPERITONEAL FIBROSIS. A PILOT TRIAL Dr Augusto Vaglio, Dr Federica Maritati Unit of Nephrology, University Hospital of Parma, Via Gramsci 14, 43126 Parma
Clinical Study IgG4-Related Disease Is Not Associated with Antibody to the Phospholipase A2 Receptor
 International Rheumatology Volume 2012, Article ID 139409, 6 pages doi:10.1155/2012/139409 Clinical Study IgG4-Related Disease Is Not Associated with Antibody to the Phospholipase A2 Receptor Arezou Khosroshahi,
International Rheumatology Volume 2012, Article ID 139409, 6 pages doi:10.1155/2012/139409 Clinical Study IgG4-Related Disease Is Not Associated with Antibody to the Phospholipase A2 Receptor Arezou Khosroshahi,
ESIM Winter School 2014 Case Presentation
 ESIM Winter School 2014 Case Presentation Hacettepe University School of Medicine Ankara/Turkey Ozant Helvaci, M.D. Patient T.K., 59 years old, male, married with one child, unemployed, place of birth/
ESIM Winter School 2014 Case Presentation Hacettepe University School of Medicine Ankara/Turkey Ozant Helvaci, M.D. Patient T.K., 59 years old, male, married with one child, unemployed, place of birth/
Clinical Commissioning Policy Proposition: Tocilizumab for Takayasu arteritis (adults)
 Clinical Commissioning Policy Proposition: Tocilizumab for Takayasu arteritis (adults) Reference: NHS England A13X06/01 Information Reader Box (IRB) to be inserted on inside front cover for documents of
Clinical Commissioning Policy Proposition: Tocilizumab for Takayasu arteritis (adults) Reference: NHS England A13X06/01 Information Reader Box (IRB) to be inserted on inside front cover for documents of
29/10. Treatment (brand name, manufacturer): For the treatment of: Background: Rituximab (MabThera, Roche) SLE in adults (unlicensed)
 GENERAL POLICY Policy Ref: 29/10 Treatment (brand name, manufacturer): For the treatment of: Background: Commissioning position: Rituximab (MabThera, Roche) SLE in adults (unlicensed) There are frequent
GENERAL POLICY Policy Ref: 29/10 Treatment (brand name, manufacturer): For the treatment of: Background: Commissioning position: Rituximab (MabThera, Roche) SLE in adults (unlicensed) There are frequent
Recovery of renal function after glucocorticoid therapy for IgG4-related kidney disease with renal dysfunction
 Clin Exp Nephrol (2016) 20:87 93 DOI 10.1007/s10157-015-1140-0 ORIGINAL ARTICLE Recovery of renal function after glucocorticoid therapy for IgG4-related kidney disease with renal dysfunction Takako Saeki
Clin Exp Nephrol (2016) 20:87 93 DOI 10.1007/s10157-015-1140-0 ORIGINAL ARTICLE Recovery of renal function after glucocorticoid therapy for IgG4-related kidney disease with renal dysfunction Takako Saeki
Renal manifestations of IgG4-related systemic disease
 Renal manifestations of IgG4-related systemic disease Lynn D. Cornell, M.D. Mayo Clinic Rochester, MN While autoimmune pancreatitis (AIP) has been recognized since the first description by Sarles et al
Renal manifestations of IgG4-related systemic disease Lynn D. Cornell, M.D. Mayo Clinic Rochester, MN While autoimmune pancreatitis (AIP) has been recognized since the first description by Sarles et al
TITLE: Rituximab for Granulomatosis with Polyangiitis or Microscopic Polyangiitis: A Review of the Clinical and Cost-effectiveness
 TITLE: Rituximab for Granulomatosis with Polyangiitis or Microscopic Polyangiitis: A Review of the Clinical and Cost-effectiveness DATE: 28 January 2015 CONTEXT AND POLICY ISSUES Granulomatosis with polyangiitis
TITLE: Rituximab for Granulomatosis with Polyangiitis or Microscopic Polyangiitis: A Review of the Clinical and Cost-effectiveness DATE: 28 January 2015 CONTEXT AND POLICY ISSUES Granulomatosis with polyangiitis
Wang et al. Arthritis Research & Therapy (2018) 20:65
 Wang et al. Arthritis Research & Therapy (2018) 20:65 https://doi.org/10.1186/s13075-018-1567-2 RESEARCH ARTICLE Open Access Failure of remission induction by glucocorticoids alone or in combination with
Wang et al. Arthritis Research & Therapy (2018) 20:65 https://doi.org/10.1186/s13075-018-1567-2 RESEARCH ARTICLE Open Access Failure of remission induction by glucocorticoids alone or in combination with
DRAFT FOR PUBLIC CONSULTATION. Integrated Impact Assessment Report for Clinical Commissioning Policies
 Integrated Impact Assessment Report for Clinical Commissioning Policies Policy Reference Number Policy Title A13X07 Rituximab for immunoglobulin G4-related disease (IgG4-RD) Accountable Commissioner Jon
Integrated Impact Assessment Report for Clinical Commissioning Policies Policy Reference Number Policy Title A13X07 Rituximab for immunoglobulin G4-related disease (IgG4-RD) Accountable Commissioner Jon
Clinical Study Evaluation and Clinical Validity of a New Questionnaire for Mikulicz s Disease
 International Rheumatology Volume 2, Article ID 283459, pages doi:1.1155/2/283459 Clinical Study Evaluation and Clinical Validity of a New Questionnaire for Mikulicz s Disease Motohisa Yamamoto, 1 Hiroki
International Rheumatology Volume 2, Article ID 283459, pages doi:1.1155/2/283459 Clinical Study Evaluation and Clinical Validity of a New Questionnaire for Mikulicz s Disease Motohisa Yamamoto, 1 Hiroki
The CARI Guidelines Caring for Australasians with Renal Impairment. Idiopathic membranous nephropathy: use of other therapies GUIDELINES
 Idiopathic membranous nephropathy: use of other therapies Date written: July 2005 Final submission: September 2005 Author: Merlin Thomas GUIDELINES No recommendations possible based on Level I or II evidence
Idiopathic membranous nephropathy: use of other therapies Date written: July 2005 Final submission: September 2005 Author: Merlin Thomas GUIDELINES No recommendations possible based on Level I or II evidence
Treatment of immunoglobulin G4-related sialadenitis: outcomes of glucocorticoid therapy combined with steroid-sparing agents
 Hong et al. Arthritis Research & Therapy (2018) 20:12 DOI 10.1186/s13075-017-1507-6 RESEARCH ARTICLE Open Access Treatment of immunoglobulin G4-related sialadenitis: outcomes of glucocorticoid therapy
Hong et al. Arthritis Research & Therapy (2018) 20:12 DOI 10.1186/s13075-017-1507-6 RESEARCH ARTICLE Open Access Treatment of immunoglobulin G4-related sialadenitis: outcomes of glucocorticoid therapy
Autoimmune Pancreatitis & Cholangiopathy. Goal and Objectives
 Autoimmune Pancreatitis & Cholangiopathy Kaveh Sharzehi, MD, MS Assistant Professor of Medicine Medical Director of Endoscopy Section of Gastroenterology Lewis Katz School of Medicine at Temple University
Autoimmune Pancreatitis & Cholangiopathy Kaveh Sharzehi, MD, MS Assistant Professor of Medicine Medical Director of Endoscopy Section of Gastroenterology Lewis Katz School of Medicine at Temple University
Immunoglobulin G4-Related Disease with Several Inflammatory Foci
 CASE REPORT Immunoglobulin G4-Related Disease with Several Inflammatory Foci Akira Sakamaki 1, Kenya Kamimura 1, Kazuhiko Shioji 1, Junko Sakurada 2, Takeshi Nakatsue 3, Yoko Wada 3, Michitaka Imai 1,
CASE REPORT Immunoglobulin G4-Related Disease with Several Inflammatory Foci Akira Sakamaki 1, Kenya Kamimura 1, Kazuhiko Shioji 1, Junko Sakurada 2, Takeshi Nakatsue 3, Yoko Wada 3, Michitaka Imai 1,
DRAFT FOR POC BOARD Clinical Commissioning Policy Proposition: Rituximab in Connective Tissue Disease associated Interstitial Lung Disease (adults)
 Clinical Commissioning Policy Proposition: Rituximab in Connective Tissue Disease associated Interstitial Lung Disease (adults) Reference: NHS England A/14/X01 CHECK Information Reader Box (IRB) to be
Clinical Commissioning Policy Proposition: Rituximab in Connective Tissue Disease associated Interstitial Lung Disease (adults) Reference: NHS England A/14/X01 CHECK Information Reader Box (IRB) to be
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December Reference : NHSCB/A3C/1b
 Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
Clinical Commissioning Policy Statement: Rituximab For Systemic Lupus Erythematosus (SLE) December 2012 Reference : NHSCB/A3C/1b NHS Commissioning Board Clinical Commissioning Policy Statement: Rituximab
FOR PUBLIC CONSULTATION ONLY RITUXIMAB FOR CYTOPENIA FROM PRIMARY IMMUNE DEFICIENCY
 RITUXIMAB FOR CYTOPENIA FROM PRIMARY IMMUNE DEFICIENCY QUESTION(S) TO BE ADDRESSED: What is the evidence for the clinical and cost effectiveness for rituximab for the management of auto-immune cytopenia
RITUXIMAB FOR CYTOPENIA FROM PRIMARY IMMUNE DEFICIENCY QUESTION(S) TO BE ADDRESSED: What is the evidence for the clinical and cost effectiveness for rituximab for the management of auto-immune cytopenia
Spuriously Low Serum IgG4 Concentrations Caused by the Prozone Phenomenon in Patients With IgG4-Related Disease
 ARTHRITIS & RHEUMATOLOGY Vol. 66, No. 1, January 2014, pp 213 217 DOI 10.1002/art.38193 2014, American College of Rheumatology BRIEF REPORT Spuriously Low Serum IgG4 Concentrations Caused by the Prozone
ARTHRITIS & RHEUMATOLOGY Vol. 66, No. 1, January 2014, pp 213 217 DOI 10.1002/art.38193 2014, American College of Rheumatology BRIEF REPORT Spuriously Low Serum IgG4 Concentrations Caused by the Prozone
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 08/19/14 SECTION: DRUGS LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 RITUXAN (rituximab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
RITUXAN (rituximab) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Medical Coverage Guideline
Recent advances in management of Pulmonary Vasculitis. Dr Nita MB
 Recent advances in management of Pulmonary Vasculitis Dr Nita MB 23-01-2015 Overview of the seminar Recent classification of Vasculitis What is new in present classification? Trials on remission induction
Recent advances in management of Pulmonary Vasculitis Dr Nita MB 23-01-2015 Overview of the seminar Recent classification of Vasculitis What is new in present classification? Trials on remission induction
A 44-Year-Old Man With Chronic Cough, Weakness, and a Mediastinum Mass
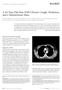 [ Pulmonary, Critical Care, and Sleep Pearls ] A 44-Year-Old Man With Chronic Cough, Weakness, and a Mediastinum Mass Dimitrios Theofilos, MD ; Christina Triantafillidou, MD, PhD ; Athanasios Zetos, MD
[ Pulmonary, Critical Care, and Sleep Pearls ] A 44-Year-Old Man With Chronic Cough, Weakness, and a Mediastinum Mass Dimitrios Theofilos, MD ; Christina Triantafillidou, MD, PhD ; Athanasios Zetos, MD
Infections and Biologics
 Overview Infections and Biologics James Galloway What is the risk of infection with biologics? Are some patients at greater risk? Are some drugs safer? Case scenario You recently commenced Judith, a 54
Overview Infections and Biologics James Galloway What is the risk of infection with biologics? Are some patients at greater risk? Are some drugs safer? Case scenario You recently commenced Judith, a 54
UNFOLDING NATURE S ORIGAMI: MEDICAL TREATMENT OF TAKAYASU ARTERITIS AND GIANT CELL ARTERITIS
 UNFOLDING NATURE S ORIGAMI: MEDICAL TREATMENT OF TAKAYASU ARTERITIS AND GIANT CELL ARTERITIS CanVasc meeting Montreal Nov 22 2012 Patrick Liang Service de rhumatologie Centre Hospitalier Universitaire
UNFOLDING NATURE S ORIGAMI: MEDICAL TREATMENT OF TAKAYASU ARTERITIS AND GIANT CELL ARTERITIS CanVasc meeting Montreal Nov 22 2012 Patrick Liang Service de rhumatologie Centre Hospitalier Universitaire
Year In Review: VasculitisPers. Disclosures. Learning Objectives. none 4/16/2018. Describe new medications for the treatment of vasculitis
 Year In Review: VasculitisPers Cailin Sibley, M.D., M.H.S. Director, Vasculitis Clinic April 27 th, 2018 NTEREST DISCLOSURE Disclosures none Learning Objectives Describe new medications for the treatment
Year In Review: VasculitisPers Cailin Sibley, M.D., M.H.S. Director, Vasculitis Clinic April 27 th, 2018 NTEREST DISCLOSURE Disclosures none Learning Objectives Describe new medications for the treatment
Clinical Commissioning Policy: Anakinra/tocilizumab for the treatment of Adult-Onset Still s Disease refractory to second-line therapy (adults)
 Clinical Commissioning Policy: Anakinra/tocilizumab for the treatment of Adult-Onset Still s Disease refractory to second-line therapy (adults) NHS England Reference: 170056P 1 NHS England INFORMATION
Clinical Commissioning Policy: Anakinra/tocilizumab for the treatment of Adult-Onset Still s Disease refractory to second-line therapy (adults) NHS England Reference: 170056P 1 NHS England INFORMATION
Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency
 Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency Reference: NHS England F06X02/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Rituximab for cytopaenia complicating primary immunodeficiency Reference: NHS England F06X02/01 Information Reader Box (IRB) to be inserted on inside front cover
Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397
 Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
Belimumab for treating active autoantibody-positive systemic lupus erythematosus Technology appraisal guidance Published: 22 June 2016 nice.org.uk/guidance/ta397 NICE 2018. All rights reserved. Subject
Effective Health Care Program
 Comparative Effectiveness Review Number 55 Effective Health Care Program Drug Therapy for Rheumatoid Arthritis in Adults: An Update Executive Summary Background Rheumatoid arthritis (RA), which affects
Comparative Effectiveness Review Number 55 Effective Health Care Program Drug Therapy for Rheumatoid Arthritis in Adults: An Update Executive Summary Background Rheumatoid arthritis (RA), which affects
Clinical Commissioning Policy Proposition: Anakinra/Tocilizumab for the treatment Adult Onset Still s Disease refractory to secondline therapy(adults)
 Clinical Commissioning Policy Proposition: Anakinra/Tocilizumab for the treatment Adult Onset Still s Disease refractory to secondline therapy(adults) Reference: NHS England 1609 First published: TBC Prepared
Clinical Commissioning Policy Proposition: Anakinra/Tocilizumab for the treatment Adult Onset Still s Disease refractory to secondline therapy(adults) Reference: NHS England 1609 First published: TBC Prepared
Autoimmune Pancreatitis: A Great Imitator
 Massachusetts General Hospital Harvard Medical School Autoimmune Pancreatitis: A Great Imitator Dushyant V Sahani MD dsahani@partners.org Autoimmune Pancreatitis: Learning Objectives Clinical manifestations
Massachusetts General Hospital Harvard Medical School Autoimmune Pancreatitis: A Great Imitator Dushyant V Sahani MD dsahani@partners.org Autoimmune Pancreatitis: Learning Objectives Clinical manifestations
Value of Serum IgG4 in the Diagnosis of Autoimmune Pancreatitis and in Distinguishing it from Acute and Chronic Pancreatitis of Other Etiology
 94 Jul 2017 Vol 10 No.3 North American Journal of Medicine and Science Original Research Value of Serum IgG4 in the Diagnosis of Autoimmune Pancreatitis and in Distinguishing it from Acute and Chronic
94 Jul 2017 Vol 10 No.3 North American Journal of Medicine and Science Original Research Value of Serum IgG4 in the Diagnosis of Autoimmune Pancreatitis and in Distinguishing it from Acute and Chronic
Immunoglobulin G4 related Disease in Genitourinary Organs: An Emerging Fibroinflammatory Entity Often Misdiagnosed Preoperatively as Cancer
 EUROPEAN UROLOGY 64 (2013) 865 872 available at www.sciencedirect.com journal homepage: www.europeanurology.com Platinum Opinion Editorial Immunoglobulin G4 related Disease in Genitourinary Organs: An
EUROPEAN UROLOGY 64 (2013) 865 872 available at www.sciencedirect.com journal homepage: www.europeanurology.com Platinum Opinion Editorial Immunoglobulin G4 related Disease in Genitourinary Organs: An
Rituximab for the treatment of adults with idiopathic (immune) thrombocytopenic purpura (ITP)
 Bedfordshire and Luton Joint Prescribing Committee Date: September 2015 Review date: September 2018 Bullletin 221: Rituximab (MabThera ) for the treatment of adults with idiopathic (immune) thrombocytopenic
Bedfordshire and Luton Joint Prescribing Committee Date: September 2015 Review date: September 2018 Bullletin 221: Rituximab (MabThera ) for the treatment of adults with idiopathic (immune) thrombocytopenic
Recommendations for RA management: what has changed?
 The 2016 Update of the EULAR Recommendations for RA management: what has changed? Baltics Rheumatology Conference Vilnius, September 21-22 Prof. Diego Kyburz University Hospital of Basel Switzerland Multiple
The 2016 Update of the EULAR Recommendations for RA management: what has changed? Baltics Rheumatology Conference Vilnius, September 21-22 Prof. Diego Kyburz University Hospital of Basel Switzerland Multiple
Review Article Orbital IgG4-Related Disease: Clinical Features and Diagnosis
 International Scholarly Research Network Volume 2012, Article ID 412896, 5 pages doi:10.5402/2012/412896 Review Article Orbital IgG4-Related Disease: Clinical Features and Diagnosis Toshinobu Kubota 1
International Scholarly Research Network Volume 2012, Article ID 412896, 5 pages doi:10.5402/2012/412896 Review Article Orbital IgG4-Related Disease: Clinical Features and Diagnosis Toshinobu Kubota 1
TREATMENT OF ANCA-ASSOCIATED VASCULITIS
 TREATMENT OF ANCA-ASSOCIATED VASCULITIS Loïc Guillevin Hôpital Cochin, Université Paris Descartes Cours DU, 11 mars 2016 1 Disclosure of interest regarding this presentation Roche has provided, in part,
TREATMENT OF ANCA-ASSOCIATED VASCULITIS Loïc Guillevin Hôpital Cochin, Université Paris Descartes Cours DU, 11 mars 2016 1 Disclosure of interest regarding this presentation Roche has provided, in part,
Understanding Myositis Medications
 Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
Understanding Myositis Medications 2015 TMA Annual Patient Conference Orlando, Florida Chester V. Oddis, MD University of Pittsburgh Director, Myositis Center Disclosures Mallinckrodt: Research Grant Genentech:
Clinical Study Serum Immunoglobulin Free Light Chain Assessment in IgG4-Related Disease
 International Rheumatology Volume 2013, Article ID 426759, 6 pages http://dx.doi.org/10.1155/2013/426759 Clinical Study Serum Immunoglobulin Free Light Chain Assessment in IgG4-Related Disease Aurélie
International Rheumatology Volume 2013, Article ID 426759, 6 pages http://dx.doi.org/10.1155/2013/426759 Clinical Study Serum Immunoglobulin Free Light Chain Assessment in IgG4-Related Disease Aurélie
EudraCT number: Page 8 of 42
 EudraCT number: 2012-001102-14 Page 8 of 42 5 Trial Synopsis Title Sponsor name North American Sponsor Disease Under Investigation An international, open label, randomised, controlled trial comparing rituximab
EudraCT number: 2012-001102-14 Page 8 of 42 5 Trial Synopsis Title Sponsor name North American Sponsor Disease Under Investigation An international, open label, randomised, controlled trial comparing rituximab
Clinical Commissioning Policy Proposition: Tocilizumab for Giant cell arteritis (adults)
 Clinical Commissioning Policy Proposition: Tocilizumab for Giant cell arteritis (adults) Version Number: NHS England A13X12/01 Information Reader Box (IRB) to be inserted on inside front cover for documents
Clinical Commissioning Policy Proposition: Tocilizumab for Giant cell arteritis (adults) Version Number: NHS England A13X12/01 Information Reader Box (IRB) to be inserted on inside front cover for documents
Rheumatoid Arthritis. Module III
 Rheumatoid Arthritis Module III Management: Biological disease modifying anti-rheumatic drugs, glucocorticoids and special situations (pregnancy & lactation) Dr Ved Chaturvedi MD, DM Senior Consultant
Rheumatoid Arthritis Module III Management: Biological disease modifying anti-rheumatic drugs, glucocorticoids and special situations (pregnancy & lactation) Dr Ved Chaturvedi MD, DM Senior Consultant
Effective Health Care Program
 Comparative Effectiveness Review Number 28 Effective Health Care Program Disease-Modifying Antirheumatic Drugs (DMARDs) in Children With Juvenile Idiopathic Arthritis (JIA) Executive Summary Background
Comparative Effectiveness Review Number 28 Effective Health Care Program Disease-Modifying Antirheumatic Drugs (DMARDs) in Children With Juvenile Idiopathic Arthritis (JIA) Executive Summary Background
A case of retroperitoneal fibrosis responding to steroid therapy
 Challenging Clinical Cases Vol. 43 (6): 1185-1189, November - December, 2017 doi: 10.1590/S1677-5538.IBJU.2016.0520 A case of retroperitoneal fibrosis responding to steroid therapy Ryuta Watanabe 1, Akira
Challenging Clinical Cases Vol. 43 (6): 1185-1189, November - December, 2017 doi: 10.1590/S1677-5538.IBJU.2016.0520 A case of retroperitoneal fibrosis responding to steroid therapy Ryuta Watanabe 1, Akira
NEWS RELEASE Genentech Contacts: Media: Joe St. Martin (650) Investor: Karl Mahler Thomas Kudsk Larsen (973)
 NEWS RELEASE Genentech Contacts: Media: Joe St. Martin (650) 467-6800 Investor: Karl Mahler 011 41 61 687 8503 Thomas Kudsk Larsen (973) 235-3655 Biogen Idec Contacts: Media: Christina Chan (781) 464-3260
NEWS RELEASE Genentech Contacts: Media: Joe St. Martin (650) 467-6800 Investor: Karl Mahler 011 41 61 687 8503 Thomas Kudsk Larsen (973) 235-3655 Biogen Idec Contacts: Media: Christina Chan (781) 464-3260
EULAR/ERA-EDTA recommendations for the management of ANCAassociated
 EULAR/ERA-EDTA recommendations for the management of ANCAassociated vasculitis Dr. Meharunnisha Syed III year DNB Resident (General Medicine) Narayana Health-MSH Fifteen recommendations were developed,
EULAR/ERA-EDTA recommendations for the management of ANCAassociated vasculitis Dr. Meharunnisha Syed III year DNB Resident (General Medicine) Narayana Health-MSH Fifteen recommendations were developed,
National Institute for Health and Clinical Excellence SCOPE. Rheumatoid arthritis: the management and treatment of rheumatoid arthritis in adults
 National Institute for Health and Clinical Excellence 1 Guideline title SCOPE Rheumatoid arthritis: the management and treatment of rheumatoid arthritis in adults 1.1 Short title Rheumatoid arthritis 2
National Institute for Health and Clinical Excellence 1 Guideline title SCOPE Rheumatoid arthritis: the management and treatment of rheumatoid arthritis in adults 1.1 Short title Rheumatoid arthritis 2
Case Report IgG4-Related Disease Presenting as Isolated Scleritis
 Hindawi Case Reports in Ophthalmological Medicine Volume 2017, Article ID 4876587, 4 pages https://doi.org/10.1155/2017/4876587 Case Report IgG4-Related Disease Presenting as Isolated Scleritis Eran Berkowitz,
Hindawi Case Reports in Ophthalmological Medicine Volume 2017, Article ID 4876587, 4 pages https://doi.org/10.1155/2017/4876587 Case Report IgG4-Related Disease Presenting as Isolated Scleritis Eran Berkowitz,
National Institute for Health and Clinical Excellence Level 1A, City Tower Piccadilly Plaza Manchester M1 4BD
 xxxxx xxxxxx xxxxxx xxxxxxx - xxxxxxxxx x National Institute for Health and Clinical Excellence Level 1A, City Tower Piccadilly Plaza Manchester M1 4BD Dear xxxxx, 1st September 2011 Comments on the August
xxxxx xxxxxx xxxxxx xxxxxxx - xxxxxxxxx x National Institute for Health and Clinical Excellence Level 1A, City Tower Piccadilly Plaza Manchester M1 4BD Dear xxxxx, 1st September 2011 Comments on the August
Combined Infliximab and Rituximab in Necrotising Scleritis
 This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the article
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/oa-license), applicable to the online version of the article
The newly recognized fibroinflammatory condition. An orphan disease: IgG4-related spinal pachymeningitis: report of 2 cases
 case report J Neurosurg Spine 25:790 794, 2016 An orphan disease: IgG4-related spinal pachymeningitis: report of 2 cases Bishan D. Radotra, MD, 1 Ashish Aggarwal, DNB, MCh, 2 Ankur Kapoor, MS, MCh, 2 Navneet
case report J Neurosurg Spine 25:790 794, 2016 An orphan disease: IgG4-related spinal pachymeningitis: report of 2 cases Bishan D. Radotra, MD, 1 Ashish Aggarwal, DNB, MCh, 2 Ankur Kapoor, MS, MCh, 2 Navneet
IgG4-related sclerosing disease
 IgG4-related sclerosing disease TERUMI KAMISAWA, KENSUKE TAKUMA, NAOTO EGAWA Department of Internal Medicine Tokyo Metropolitan Komagome Hospital 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113-8677, Japan JAPAN
IgG4-related sclerosing disease TERUMI KAMISAWA, KENSUKE TAKUMA, NAOTO EGAWA Department of Internal Medicine Tokyo Metropolitan Komagome Hospital 3-18-22 Honkomagome, Bunkyo-ku, Tokyo 113-8677, Japan JAPAN
Skin cancers in patients treated with immunomodulating drugs. Manuelle Viguier, MD, PhD Dermatology department Saint-Louis Hospital Paris, France
 Skin cancers in patients treated with immunomodulating drugs Manuelle Viguier, MD, PhD Dermatology department Saint-Louis Hospital Paris, France Immunomodulating drugs used in Methotrexate Mycophenolate
Skin cancers in patients treated with immunomodulating drugs Manuelle Viguier, MD, PhD Dermatology department Saint-Louis Hospital Paris, France Immunomodulating drugs used in Methotrexate Mycophenolate
IgG4-Related Sclerosing Cholangitis
 REVIEW IgG4-Related Sclerosing Cholangitis Emma L. Culver, B.Sc., M.B.Ch.B., D.Phil., M.R.C.P.,* and Eleanor Barnes, M.B.B.S., D.Phil., M.R.C.P.*,, BACKGROUND IgG4-related sclerosing cholangitis (IgG4-SC)
REVIEW IgG4-Related Sclerosing Cholangitis Emma L. Culver, B.Sc., M.B.Ch.B., D.Phil., M.R.C.P.,* and Eleanor Barnes, M.B.B.S., D.Phil., M.R.C.P.*,, BACKGROUND IgG4-related sclerosing cholangitis (IgG4-SC)
Risk of Pneumocystosis among Patients Receiving Immunosuppressive Therapies: a population based analysis in the United States
 Risk of Pneumocystosis among Patients Receiving Immunosuppressive Therapies: a population based analysis in the United States Sergey Rekhtman MD, PharmD, MPH Amit Garg, MD Department of Dermatology Zucker
Risk of Pneumocystosis among Patients Receiving Immunosuppressive Therapies: a population based analysis in the United States Sergey Rekhtman MD, PharmD, MPH Amit Garg, MD Department of Dermatology Zucker
Rituximab (MabThera) maintenance therapy for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA)
 NIHR Innovation Observatory Evidence Briefing: August 2017 Rituximab (MabThera) maintenance therapy for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) NIHRIO (HSRIC) ID: 12979
NIHR Innovation Observatory Evidence Briefing: August 2017 Rituximab (MabThera) maintenance therapy for granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) NIHRIO (HSRIC) ID: 12979
Rituximab for the first-line treatment of stage III-IV follicular lymphoma
 Rituximab for the first-line treatment of stage III-IV (review of guidance 110) Issued: January 2012 guidance.nice.org.uk/ta243 NICE has accredited the process used by the Centre for Health Technology
Rituximab for the first-line treatment of stage III-IV (review of guidance 110) Issued: January 2012 guidance.nice.org.uk/ta243 NICE has accredited the process used by the Centre for Health Technology
Successful Treatment of Multi-system IgG4-related Disease with Cyclophosphamide: A Case Report X Zhang, W Qin, J Zhou, L Yang ABSTRACT
 Successful Treatment of Multi-system IgG4-related Disease with Cyclophosphamide: A Case Report X Zhang, W Qin, J Zhou, L Yang ABSTRACT Immunoglobulin G4-related disease (IgG4-RD) is increasingly recognized
Successful Treatment of Multi-system IgG4-related Disease with Cyclophosphamide: A Case Report X Zhang, W Qin, J Zhou, L Yang ABSTRACT Immunoglobulin G4-related disease (IgG4-RD) is increasingly recognized
National Horizon Scanning Centre. Rituximab (MabThera) for chronic lymphocytic leukaemia. September 2007
 Rituximab (MabThera) for chronic lymphocytic leukaemia This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Rituximab (MabThera) for chronic lymphocytic leukaemia This technology summary is based on information available at the time of research and a limited literature search. It is not intended to be a definitive
Vascularites associées aux ANCA Traitement par le RITUXIMAB
 Vascularites associées aux ANCA Traitement par le RITUXIMAB Philippe Vanhille Néphrologie Médecine Interne Hôpital de Valenciennes Aix- en- Provence 2013 Cyclophosphamide therapy of severe systemic necrotizing
Vascularites associées aux ANCA Traitement par le RITUXIMAB Philippe Vanhille Néphrologie Médecine Interne Hôpital de Valenciennes Aix- en- Provence 2013 Cyclophosphamide therapy of severe systemic necrotizing
Lymphoplasmacytic sclerosing pancreatitis without IgG4 tissue infiltration or serum IgG4 elevation: IgG4-related disease without IgG4
 238 & 2015 USCAP, Inc All rights reserved 0893-3952/15 $32.00 Lymphoplasmacytic sclerosing pancreatitis without IgG4 tissue infiltration or serum IgG4 elevation: IgG4-related disease without IgG4 Phil
238 & 2015 USCAP, Inc All rights reserved 0893-3952/15 $32.00 Lymphoplasmacytic sclerosing pancreatitis without IgG4 tissue infiltration or serum IgG4 elevation: IgG4-related disease without IgG4 Phil
Technology appraisal guidance Published: 26 March 2014 nice.org.uk/guidance/ta308
 Rituximab in combination with glucocorticoids for treating antineutrophil cytoplasmic antibody- associated vasculitis Technology appraisal guidance Published: 26 March 2014 nice.org.uk/guidance/ta308 NICE
Rituximab in combination with glucocorticoids for treating antineutrophil cytoplasmic antibody- associated vasculitis Technology appraisal guidance Published: 26 March 2014 nice.org.uk/guidance/ta308 NICE
IgG4-related kidney disease: the effects of a Rituximab-based immunosuppressive therapy
 , 2018, Vol. 9, (No. 30), pp: 21337-21347 IgG4-related kidney disease: the effects of a Rituximab-based immunosuppressive therapy Giacomo Quattrocchio 1, Antonella Barreca 2, Andrea Demarchi 3, Laura Solfietti
, 2018, Vol. 9, (No. 30), pp: 21337-21347 IgG4-related kidney disease: the effects of a Rituximab-based immunosuppressive therapy Giacomo Quattrocchio 1, Antonella Barreca 2, Andrea Demarchi 3, Laura Solfietti
Rituximab treatment for ANCA-associated vasculitis in childhood
 Rituximab treatment for ANCA-associated vasculitis in childhood DISCLOSURE I have no relevant financial relationships to disclose Katharine Moore MD Nov 14, 2012 University of Colorado School of Medicine
Rituximab treatment for ANCA-associated vasculitis in childhood DISCLOSURE I have no relevant financial relationships to disclose Katharine Moore MD Nov 14, 2012 University of Colorado School of Medicine
Tell me more about vasculitis. Lisa Willcocks Consultant in Nephrology and Vasculitis, Addenbrooke s Hospital
 Tell me more about vasculitis Lisa Willcocks Consultant in Nephrology and Vasculitis, Addenbrooke s Hospital Talk overview Case study ANCA-associated vasculitis What is ANCA vasculitis? What causes ANCA
Tell me more about vasculitis Lisa Willcocks Consultant in Nephrology and Vasculitis, Addenbrooke s Hospital Talk overview Case study ANCA-associated vasculitis What is ANCA vasculitis? What causes ANCA
ASSESSMENT OF THE PAEDIATRIC NEEDS IMMUNOLOGY DISCLAIMER
 European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/381922/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS IMMUNOLOGY DISCLAIMER The Paediatric Working Party
European Medicines Agency Evaluation of Medicines for Human Use London, September 2006 Doc. Ref.: EMEA/381922/2006 ASSESSMENT OF THE PAEDIATRIC NEEDS IMMUNOLOGY DISCLAIMER The Paediatric Working Party
New Therapeutic Perspectives in Sjögren's Syndrome
 New Therapeutic Perspectives in Sjögren's Syndrome Claudio Vitali Chairman of the EULAR Task Force for Disease Activity Criteria in Sjögren s Syndrome. Member of the Steering Committee for the ACR- EULAR
New Therapeutic Perspectives in Sjögren's Syndrome Claudio Vitali Chairman of the EULAR Task Force for Disease Activity Criteria in Sjögren s Syndrome. Member of the Steering Committee for the ACR- EULAR
Technology appraisal guidance Published: 25 January 2012 nice.org.uk/guidance/ta243
 Rituximab for the first-line treatment of stage III-IV follicular lymphoma Technology appraisal guidance Published: 25 January 2012 nice.org.uk/guidance/ta243 NICE 2017. All rights reserved. Subject to
Rituximab for the first-line treatment of stage III-IV follicular lymphoma Technology appraisal guidance Published: 25 January 2012 nice.org.uk/guidance/ta243 NICE 2017. All rights reserved. Subject to
Rituximab for the treatment of Immune (Idiopathic) Thrombocytopenic Purpura (ITP)
 Rituximab for the treatment of Immune (Idiopathic) Thrombocytopenic Purpura (ITP) Lead author: Stephen Erhorn Regional Drug & Therapeutics Centre (Newcastle) February 2015 2015 Summary Rituximab (MabThera,
Rituximab for the treatment of Immune (Idiopathic) Thrombocytopenic Purpura (ITP) Lead author: Stephen Erhorn Regional Drug & Therapeutics Centre (Newcastle) February 2015 2015 Summary Rituximab (MabThera,
The Hospital for Sick Children Technology Assessment at SickKids (TASK)
 The Hospital for Sick Children Technology Assessment at SickKids (TASK) THE USE OF BIOLOGIC RESPONSE MODIFIERS IN POLYARTICULAR-COURSE JUVENILE IDIOPATHIC ARTHRITIS Report No. 2010-01 Date: January 11,
The Hospital for Sick Children Technology Assessment at SickKids (TASK) THE USE OF BIOLOGIC RESPONSE MODIFIERS IN POLYARTICULAR-COURSE JUVENILE IDIOPATHIC ARTHRITIS Report No. 2010-01 Date: January 11,
Diagnostic And Therapeutic Challenges in IgG4-Related disease in the Sphenoid Sinus
 Diagnostic And Therapeutic Challenges in IgG4-Related disease in the Sphenoid Sinus Omar Abu Suliman, MBBS, SB-ORL Senior Registrar, otolaryngology Head & neck surgery King Abdullah Medical City, Makkah,
Diagnostic And Therapeutic Challenges in IgG4-Related disease in the Sphenoid Sinus Omar Abu Suliman, MBBS, SB-ORL Senior Registrar, otolaryngology Head & neck surgery King Abdullah Medical City, Makkah,
EudraCT number: Page 9 of 46
 EudraCT number: 2012-001102-14 Page 9 of 46 5 Trial Synopsis Title Sponsor name North American Sponsor Japan Sponsor Ireland Sponsor Disease Under Investigation An international, open label, randomised,
EudraCT number: 2012-001102-14 Page 9 of 46 5 Trial Synopsis Title Sponsor name North American Sponsor Japan Sponsor Ireland Sponsor Disease Under Investigation An international, open label, randomised,
Review Article The Utility of Serum IgG4 Concentrations as a Biomarker
 International Rheumatology Volume 2012, Article ID 198314, 4 pages doi:10.1155/2012/198314 Review Article The Utility of Serum IgG4 Concentrations as a Biomarker Shigeyuki Kawa, 1 Tetsuya Ito, 2 Takayuki
International Rheumatology Volume 2012, Article ID 198314, 4 pages doi:10.1155/2012/198314 Review Article The Utility of Serum IgG4 Concentrations as a Biomarker Shigeyuki Kawa, 1 Tetsuya Ito, 2 Takayuki
Rheumatoid arthritis in adults: diagnosis and management
 National Institute for Health and Care Excellence Consultation Rheumatoid arthritis in adults: diagnosis and management Evidence review F DMARDs NICE guideline CG79 Intervention evidence review January
National Institute for Health and Care Excellence Consultation Rheumatoid arthritis in adults: diagnosis and management Evidence review F DMARDs NICE guideline CG79 Intervention evidence review January
A Case of IgG4-Related Disease Presenting as Massive Pleural Effusion and Thrombophlebitis
 CASE REPORT http://dx.doi.org/10.4046/trd.2014.76.4.179 ISSN: 1738-3536(Print)/2005-6184(Online) Tuberc Respir Dis 2014;76:179-183 A Case of IgG4-Related Disease Presenting as Massive Pleural Effusion
CASE REPORT http://dx.doi.org/10.4046/trd.2014.76.4.179 ISSN: 1738-3536(Print)/2005-6184(Online) Tuberc Respir Dis 2014;76:179-183 A Case of IgG4-Related Disease Presenting as Massive Pleural Effusion
New Evidence reports on presentations given at ACR Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab
 New Evidence reports on presentations given at ACR 2009 Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab From ACR 2009: Rituximab Rituximab in combination with methotrexate
New Evidence reports on presentations given at ACR 2009 Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab From ACR 2009: Rituximab Rituximab in combination with methotrexate
Comments from Wyeth on the Assessment Report for the appraisal of Enbrel in RA General Comments
 General Comments The TAR economic model is a complex model that attempts to reflect the multiple treatment options and pathways an RA patient can follow. This model demonstrates that it would be cost effective
General Comments The TAR economic model is a complex model that attempts to reflect the multiple treatment options and pathways an RA patient can follow. This model demonstrates that it would be cost effective
Efficacy and Safety of Rituximab in the Treatment of Rheumatoid Arthritis and ANCA-associated Vasculitis
 New Evidence reports on presentations given at ACR/ARHP 2010 Efficacy and Safety of Rituximab in the Treatment of Rheumatoid Arthritis and ANCA-associated Vasculitis Report on ACR/ARHP 2010 presentations
New Evidence reports on presentations given at ACR/ARHP 2010 Efficacy and Safety of Rituximab in the Treatment of Rheumatoid Arthritis and ANCA-associated Vasculitis Report on ACR/ARHP 2010 presentations
1 Executive summary. Background
 1 Executive summary Background Rheumatoid Arthritis (RA) is the most common inflammatory polyarthropathy in the UK affecting between.5% and 1% of the population. The mainstay of RA treatment interventions
1 Executive summary Background Rheumatoid Arthritis (RA) is the most common inflammatory polyarthropathy in the UK affecting between.5% and 1% of the population. The mainstay of RA treatment interventions
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults
 Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
REPEATED B CELL DEPLETION IN TREATMENT OF REFRACTORY SYSTEMIC LUPUS ERYTHEMATOSUS
 ARD Online First, published on November 3, 2005 as 10.1136/ard.2005.044487 REPEATED B CELL DEPLETION IN TREATMENT OF REFRACTORY SYSTEMIC LUPUS ERYTHEMATOSUS Concise report Kristine P. Ng 1 Maria J. Leandro
ARD Online First, published on November 3, 2005 as 10.1136/ard.2005.044487 REPEATED B CELL DEPLETION IN TREATMENT OF REFRACTORY SYSTEMIC LUPUS ERYTHEMATOSUS Concise report Kristine P. Ng 1 Maria J. Leandro
Treatment of Rheumatoid Arthritis: The Past, the Present and the Future
 Treatment of Rheumatoid Arthritis: The Past, the Present and the Future Lai-Ling Winchow FCP(SA) Cert Rheum(SA) Chris Hani Baragwanath Academic Hospital University of the Witwatersrand Outline of presentation
Treatment of Rheumatoid Arthritis: The Past, the Present and the Future Lai-Ling Winchow FCP(SA) Cert Rheum(SA) Chris Hani Baragwanath Academic Hospital University of the Witwatersrand Outline of presentation
Coverage Criteria: Express Scripts, Inc. monograph dated 12/15/ months or as otherwise noted by indication
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of document author s Line Manager:
 For Use in: By: For: Guidelines for the Management of Interruption of Biologic Therapies for Division responsible for document: Key words: Name and job title of document author: Name and job title of document
For Use in: By: For: Guidelines for the Management of Interruption of Biologic Therapies for Division responsible for document: Key words: Name and job title of document author: Name and job title of document
Technology appraisal guidance Published: 18 April 2018 nice.org.uk/guidance/ta518
 Tocilizumab for treating giant cell arteritis Technology appraisal guidance Published: 18 April 2018 nice.org.uk/guidance/ta518 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Tocilizumab for treating giant cell arteritis Technology appraisal guidance Published: 18 April 2018 nice.org.uk/guidance/ta518 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
DMARD s in Clinical Practice
 DMARD s in Clinical Practice Professor Md. Mahabubul Islam Majumder Professor & Head, Department of Medicine Comilla Medical College, Comilla Bangladesh Disease-modifying antirheumatic drugs (DMARDs) A
DMARD s in Clinical Practice Professor Md. Mahabubul Islam Majumder Professor & Head, Department of Medicine Comilla Medical College, Comilla Bangladesh Disease-modifying antirheumatic drugs (DMARDs) A
ACTEMRA (tocilizumab)
 Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 1. Active Polyarticular Juvenile Idiopathic Arthritis (PJIA) b. Patient has an intolerance or has experienced
Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 1. Active Polyarticular Juvenile Idiopathic Arthritis (PJIA) b. Patient has an intolerance or has experienced
