MicroLab Operating Manual
|
|
|
- Antonia Carroll
- 5 years ago
- Views:
Transcription
1 MicroLab Operating Manual Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner. MAN (Part 1) Issue 1.2 June 2016 Micro Direct, Inc. 803 Webster Street Lewiston, ME Mail In Yellow Warranty Card Receive 25 PFT Filters FREE!!
2 Indications for Spirometry Spirometry has been used extensively to measure lung function capability and to recognize and treat many diseases associated with the impairment of healthy lung functions. Spirometry today provides great insight into the status of any person s health. Generally speaking, spirometry is a simple diagnostic tool used to define a subject s lung condition. The major indications for spirometry are: Dyspnea (shortness of breath) Exercise induced coughing Chest tightness Smokers over 45 years of age (NLHEP recommendations) Obesity Pre-operative testing Occupational exposure to dust and/or chemicals Ongoing assessment of patients receiving bronchodilator treatments Determination and/or documentation of pulmonary disability Asthma diagnosis Pre-existing pulmonary disease Frequent colds Assessment of congestive heart failure CPT Codes for Spirometry Spirometry Complete Includes graphic record total and timed vital capacity, expiratory flow rate measurement (s) with or without maximal voluntary ventilation Bronchodilation Responsiveness Spirometry as in 94010, pre and post bronchodilator or exercise Bronchospasm Provocation Evaluation Multiple spirometric determinations after bronchodilator with spirometry as in Vital Capacity Total (separate procedure) Maximal Voluntary Ventilation Maximum breath capacity Flow Volume Loop Respiratory Flow Volume Loop Inhalation Bronchial Challenge Testing (Not including necessary pulmonary function tests), with histamine, methacholine or similar compounds Bronchodilator Administration Demonstration and/or evaluation of patient utilization of an aerosol generator, nebulizer and meter dose inhaler or IPPB device Diagnosis and ICD-9-CM Codes on back cover
3 Contents Introduction... 1 Contraindications... 1 Warning and Cautions... 2 Indication for Use... 3 Overview... 4 Intended Use... 5 Getting Started... 5 Calibration Check (Verification) Customization Administration Mode Paper Loading Switching Off Charging Procedure PC connection using SPCS Looking after your Spirometer Product Lifetime Cleaning Instructions External Surfaces of the Spirometer Cleaning the Accessories Cleaning the Transducer Servicing Troubleshooting Information Safety Designation per IEC Electromagnetic Compatibility (EMC) to EN : Symbols Specifications Spirometry Measurements Consumables / Supporting Products ICD-10 Codes for Spirometry... 36
4 Introduction The MicroLab is a mains/battery operated portable spirometer with the unique combination of ease of use and sophistication. Ease of use is assured through the use of context sensitive help screens, accessed at a touch of a button, that explain every MicroLab feature. The MicroLab uses a Digital Volume Transducer, an extremely stable form of volume transducer, which measures expired air directly at B.T.P.S (Body Temperature and Pressure with Saturated water vapor) thus avoiding the inaccuracies of temperature corrections. The transducer is insensitive to the effects of condensation and temperature and avoids the need for individual calibration prior to performing a test Test results may be uploaded to a PC using the optional Spirometry PC Software and patient details may be downloaded to the MicroLab. Stored data may be printed to the integral thermal. The MicroLab utilizes a single patient use disposable mouthpiece or filter that must be disposed of after use. The MicroLab provides a suggested interpretation that must be supported by clinical judgment. Contraindications Acute disorders affecting test performance (e.g. vomiting, nausea, vertigo) Recent eye surgery (increases in intraocular pressure during spirometry) Oral or facial pain exacerbated by a mouthpiece Recent myocardial infarction Post-operative thoracic surgery Hyperventilation syndrome Note: Extensive exhalation might lead to syncope. 1
5 Warning and Cautions The following terms are used as follows in this manual CAUTION: Possibility of injury or serious damage WARNING: Conditions or practices that could result in personal injury Note: Important information for avoiding damage to the instrument or facilitating operation of the instrument. Note: Patients below the age of four (4) may struggle to perform spirometry correctly and reproducibly. Note: The device should be used by trained and qualified personnel. CAUTION: Read the manual before use. WARNING: The instrument is not suitable for use in the presence of explosive or flammable gases, flammable anesthetic mixtures or in oxygen rich environments. CAUTION: Mouthpieces are single patient use. If used on more than one patient, there is a risk of cross-infection. Repeat use may degrade materials and lead to an incorrect measurement. CAUTION: Pulmonary filters are single patient use. If used on more than one patient, there is a risk of cross-infection. Repeat use may increase air resistance and lead to an incorrect measurement. PLEASE NOTE: The product you have purchased should not be disposed of as unsorted waste. Please utilize your local recycling facility for the disposal of this product. 2
6 PLEASE NOTE: Degree of protection against Ingress of Water is IPX0. WARNING: To avoid risk of electric shock, this equipment must only be connected to a supply mains with protective earth. WARNING: Do not connect devices that are not specified as part of the system. Indication for Use The MicroLab spirometer is intended, for prescription use only, to measure the maximal volume and flow of air that can be moved in and out of a patient's lungs. The system is intended for use with pediatric (4 to 17 years) and adult (18 to 99 years) patients in hospitals, physician offices, laboratories and occupational health testing environments. 3
7 Overview The MicroLab uses a touch screen with icons representing each function available. A stylus, housed in the left hand side of the unit, is provided for icon screen activation. Touch the displayed time to adjust time and date. Touch the toolbox icon to adjust volume and brightness. Unused icons may be disabled by touching the blue background and selecting from the list displayed. Four levels of battery charge are indicated by the segmented battery icon. When this icon turns red the battery is nearly exhausted and the batteries must be charged see Charging Procedure.. The complete functionality is described on the help screen. This is obtained by pressing the help button (?). Help text exists for every screen viewed during the operation of the MicroLab. You are recommended to make full use of the extensive Help screens provided. 4
8 Intended Use The MicroLab spirometer is intended for prescription use only, to measure the maximal volume and flow of air that can be moved in and out of a patient s lungs. The system is intended for use with pediatric (4 to 17 years of age) and adult (18 to 99 years of age) patients in hospitals, physician offices, laboratories and occupational health testing environments. Getting Started When performing a spirometry test the recommended workflow is to enter the patient s details, or retrieve them from memory, perform the required test and then print and save the results. 5
9 Please ensure that the turbine transducer is plugged in to either of the two sockets on the right hand side of the instrument. Select the Patients icon to enter the patient database. The required patient may be selected from the stored patient list. If the patient details have not been previously stored then select Add to enter the new patient s details. The patient details may also be downloaded from the optional Spirometry PC Software. Once selected, the patient s name will appear at the bottom of the screen. Use the help button to obtain further information. 6
10 To add a patient to the database, use the on screen keyboard to type a unique patient ID and then touch the enter key. You will then be prompted for Last Name, First Name, Sex, Ethnic Origin, Height, Weight, date of Birth and Factor. A factor can be applied when testing individuals of other ethnic origins who would not normally be tested against the countries set of predicted values. The factor alters the predicted value set on volume indices by the percentage applied. If NHANES predicted values are selected, then the ethnic origin field should be chosen but a factor correction is not required. Once all the patient details are added, the patient is added to the database and the main menu is displayed with the patient name displayed at the bottom of the screen. From the main menu select the required test, by touching the icon with the stylus. If the displayed patient is not required for testing then touch the patients name and options to change or remove the current patient will become available. 7
11 If Relaxed Spirometry is selected then a volume/time graph will be displayed. Note the unit may be customized to perform a relaxed Vital Capacity with tidal breathing or from a single expiration or single inspiration. When a maneuver has been obtained select Results to view the indices, Again to repeat the maneuver, Reject to delete the maneuver or Done to end the test. All the active indices are displayed for any of the maneuvers selected together with an option to review the volume/ time curves. The active indices listed can be changed by using the customization option. Select Done to proceed to the Spirometry Main Menu. 8
12 From this menu the results of the test may be viewed, saved, or printed and notes may be added. It is also possible to proceed to a forced baseline spirometry test, or a post medication relaxed spirometry test. Select Exit when all the required functions have been used. If forced spirometry is selected the default graph will be displayed. This may be changed by touching the arrows at the top of the screen. Flow/Volume, Volume/time or child incentive default displays may be selected using the customize option from the main menu. When the spirometry maneuver has been completed options to repeat the test, reject the test, and view results will be available. At the end of the test options to view results, save results, print results, and to add notes will be available from the spirometry main menu. 9
13 Select the MVV icon to select this mode of testing and the display will instruct the patient to start breathing hard to commence the test. It is recommended that the patient perform 3 tidal breathing maneuvers prior to performing hard and fast rapid breathing (required for the MVV maneuver). The patient should be instructed to tidal breath. The tidal breaths are automatically detected prior to commencing the MVV maneuver. Once tidal breathing is complete, the display will change and an audible beep heard to instruct the patient to start rapid, fast breathing. The start button should be touched using the stylus to start registering the MVV maneuver. 10
14 The current maneuver will be displayed in black. During the maneuver, the breath rate (BR) will be displayed in green if the breath rate is acceptable (> 65 breaths per minute). If the breath rate falls below this level, it will be displayed in red to show the operator that the patient needs to be instructed to breathe harder and faster during the maneuver. After 12 seconds of hard, fast and rapid breathing, the display will show a green line indicating 12 seconds of the maneuver have elapsed the patient should be encouraged to continue until the display changes to signify the end of the test. The MVV rate, the % variation between maneuvers, the breath rate and an ATS quality warning for the maneuver will be displayed. Note: The patient s effort is acceptable when patient made a maximum effort indicated to the user by the breath rate being displayed in green (> 65 breaths per minute); and the maneuver lasted a full 12 seconds indicated by a green line being displayed. The patient should ideally continue until the test is automatically terminated at 15 seconds with no interruption (i.e. did not cough) 11
15 Once the test has finished, the display will show current test (shown in black if more than one maneuver has been performed, the best maneuver will also be displayed in blue) the MVV rate, the % variation between maneuvers, the breath rate and the ATS quality warning for the test session. Select Again to repeat the maneuver, Reject to reject the current maneuver, Results to display a list of indices, the values obtained, % predicted where applicable and also a quality statement concerning the test session. To meet the ATS quality criteria for a good blow, the maneuver should last 15 seconds with a breath rate greater than 65 breaths per minute. The ATS reproducibility criterion is two maneuvers with a good blow and the MVV variability between maneuvers should not exceed 20%. Note: The MVV test is an exhausting test. It should not be repeated without a rest period. Some elderly or ill people cannot repeat this test even after the rest period. 12
16 Select Back to return to testing and the current maneuver. NOTE: If the breathing rate is insufficient (less than 65 breaths per minute) then the BR value will be displayed in red an MVV value will be calculated and a message displayed that the MVV results was extrapolated from a maneuver with a poor breath rate. Once the number of maneuvers has been completed and the test session has finished, select Done and the results with selected indices will be displayed. Each maneuver will be numbered and the best maneuver highlighted with an asterisk (*). Select Graphs to view the graphs of the currently selected maneuver and best maneuver. Select Set Best to manually select the best maneuver. Select Done to return to the main MVV menu. 13
17 Once testing is complete the MVV main menu will be displayed Select the appropriate icon to allow a Post 1 MVV test to be performed, View Results, Print Results, add notes for the patient s examination, Save the tests or Exit to return to the main spirometer menu. Calibration Check (Verification) The spirometer is calibrated to read in liters at Body Temperature, Barometric Pressure Saturated with water vapor (BTPS). The calibration should remain stable indefinitely, unless the transducer is physically damaged, and the unit should not require re-calibration. However, to ensure the correct functioning of the unit, we do recommend that a calibration check (verification) is performed periodically and after the transducer was removed for cleaning. 14
18 Connect a 3-liter syringe to the transducer with the minimum of adapters and empty by pushing the handle fully in. Note: It is recommended that the transducer is disinfected prior to a calibration check (verification) or a SpiroSafe filter is used during the procedure. Select Calibration Check from the main menu and then select Check Calibration. Fill the syringe by pulling the handle at a constant rate until the end stop is reached and then immediately empty the syringe completely. Try to maintain a flow rate that keeps the trace within the grey bands on the display. Select Reject to retry the calibration check (verification) at the required flow rate. Select Again to repeat the calibration check (verification) at a low flow rate. Select Again to repeat the calibration check (verification) at a high flow rate. When a calibration check (verification) at all three flow rates has been completed select Done to view the calibration check (verification) report screen. 15
19 The calibration error for expiration and inspiration at each flow rate are displayed. The calibration error should be less than 3.5%. If a greater error is shown, repeat the procedure ensuring that the syringe is emptied and filled in a smooth manner without jerking the handle. If an error greater than 3.5% is still shown, inspect the turbine transducer and clean if necessary. Customization The Customize option from the main menu may be used to configure many of the features of your MicroLab and are divided into system, spirometry options and MVV options. System options allow you to configure the following: Language Height and weight units Date format Date separator Color or monochrome printing (on external printer) Personalized printout heading Spirometry options allow you to configure the following: Relaxed spirometry mode (with or without tidal breathing) Predicted value sets Predicted area or line display Display default Incentive display type Printed graphs Best test criteria 16
20 Interpretation and Lung Age indication Dyspnea score and smoking status Daily calibration reminder Manual temperature adjustment Indices selection MVV options allow you to configure the following: Choice of predicted values Display ambient temperature during MVV test Include graph of MVV maneuver in the final printout Note: that when the language is selected, the height and weight units, date format, and date separator will be automatically changed. However this automatic selection may be overridden manually. Administration Mode Administration mode allows the administrator to restrict the availability of functions to the user by disabling icons on the main menu. For example, after the unit has been configured to the administrator s requirements, disabling of the Customize icon will prevent any further adjustment by the user. Similarly, disabling of the Database Management icon will prevent the user from deleting any patient details or test results. 17
21 To enter administration mode, turn the unit on while holding down the help key. The default access code is Type this number in using the on-screen keyboard. A number of functions are now available. Please note: if you change the access code, make sure you document the number in case you forget it. Press the help button to obtain a full description of the functions. Paper Loading To load a new roll of thermal paper lift the paper cover using the side levers, place the paper into the compartment as shown and close the cover firmly. It is recommended that only Micro Direct thermal printer paper (Cat No. 3327) be used with the MicroLab to avoid damage to the thermal printer head. 18
22 To tear off the paper pull the paper towards you and to the right as shown below: Switching Off The unit is switched off by pressing the On/Off button. The unit can be disconnected from the mains by unplugging the charger from the mains socket or unplugging the USB cable if no mains supply is connected. Charging Procedure The MicroLab s internal batteries are discharged when shipped from the factory and should be fully charged on first use. Plug the AC adapter into the mains supply and plug the adapter output plug into the power input socket on the right-hand side of the instrument. The orange charging light next to the power input socket will flash to indicate charging and will turn on constantly to indicate full charge. The batteries will take approximately 4 hours to become fully charged. Note: Use only the AC adapter supplied. Use of any other type may cause permanent damage to the MicroLab and cause a fire or electrical hazard. Do not plug in and remove the power lead from the AC adapter repeatedly. 19
23 Please Note: Dispose of the waste battery in accordance with your local waste management regulations. PC connection using SPCS The optional Spirometry PC Software (SPCS) is an easy to use PC based windows application that interfaces to the MicroLab via the USB port. It incorporates a database into which patient details can be entered and downloaded to the MicroLab or test results may be uploaded from the MicroLab to the PC. Using SPCS and the MicroLab, live blows can be performed with the PC directly controlling the operation of the MicroLab. The results and graphs produced are displayed directly on the PC screen. The spirometer is connected from the USB port on the PC, to the USB port on the right hand side of the instrument using the USB cable provided with the Spirometry PC Software. Note: The MicroLab should only be connected to a computer that is manufactured in accordance with EN / Safety of Information Technology Equipment including Electrical Business Equipment. Note: Keep the PC and monitor out of reach of the patient at all times. It is recommended that while the unit is connected to a computer, the mains adapter is used. Looking after your Spirometer Please observe the following precautions: Do not touch the screen with fingers. Use only the stylus provided. Use only a damp, lint free, cloth to clean the screen. Do not keep the spirometer in a damp place or expose it to extremes of temperature. Do not direct the transducer holder towards a strong light source while operating the spirometer. Check the AC charger for compatibility with local power rating. 20
24 Product Lifetime The MicroLab spirometer is designed for a product lifetime of 7 years. Cleaning Instructions Disinfection of contaminated parts is only effective after having them carefully pre-cleaned. Micro Direct recommends using a cold sterilizing solution that is NOT chlorine or alcohol based for pre-cleaning and disinfection. Please follow the manufacturer s instructions. The device must not be wiped with any aqueous solutions and must not be exposed to solvents i.e. alcohol or chloride solutions as there are electronic components inside that will be permanently damaged. CAUTION: Switch off the device and always unplug the MicroLab before cleaning. External Surfaces of the Spirometer CAUTION: Do not attempt to wash or immerse the MicroLab transducer housing in water or cleaning fluid, as there are electronic components inside that will be permanently damaged. The external housing of the spirometer may be wipe with sterile wipes or a damp cloth that has been immersed in a cold sterilizing solution, when required. CAUTION: Do not wipe the touch screen. Cleaning the Accessories With the use of a SpiroSafe filter (#3385) or a MicroCheck oneway valve safety mouthpieces (#3395) for each patient, cleaning of the transducer is recommended once a month. When using the disposable cardboard mouthpiece (adult: #3314SB or #3314B5, pediatric: #3301) without a filter and under the prerequisite that the patient was instructed only to exhale into 21
25 the transducer, the following parts have to be cleaned once a day: transducer and pediatric adapter (if one was used). With any other use as described above, all contaminated parts must be disinfected between patients. IMPORTANT NOTE: Used single patient nose clips, mouthpieces and SpiroSafe filters must be disposed of immediately after use. If there are changes on the material surfaces (cracks, brittleness) the respective parts must be disposed of. Cleaning the Transducer The transducer requires no routine maintenance or servicing. However, if you wish to disinfecting or clean the transducer, it may be removed by means of the following procedure: 1. Rotating the turbine transducer anti-clockwise until the locating pip lines up with the small rectangular cut-out in the housing as shown below. 2. Gently pull the transducer away from the housing. 3. The transducer may now be immersed in warm soapy water for routine cleaning or immersed in cold disinfecting solutions for a maximum of 10 minutes (Alcohol and chlorine solutions MUST be avoided). 4. After cleaning/disinfecting, the transducer should be immersed briefly in distilled water and left to dry. 5. Re-assemble the mouthpiece holder. 22
26 t uo-t u C ip p g int acol g in suo H r ecudsnart e in br ut CAUTION: Do not attempt to wash or immerse the MicroLab transducer housing in water or cleaning fluid, as there are electronic components inside that will be permanently damaged. Servicing There is no routine maintenance required for the MicroLab and there are no user serviceable parts in this instrument. Please return the unit to Micro Direct or an authorized agent if servicing is required. If your unit requires service or repair, please see page 35 for contact details 23
27 Troubleshooting Information Should you encounter problems operating your MicroLab meter, please consult the table below: Problem Display freezes and the unit does not respond to any key presses No display present Does not register a blow Blows are inverted on the display Blows tracking ends abruptly although patient is still exhaling Battery does not hold a charge Paper not printing Possible Cause Multiple icons have selected or accidently pressed Charger not connected or battery is exhausted Head assembly or cable broken Head assembly or cable broken Turbine sticking Exhausted battery Mains charger fault Check paper is housed in printer compartment correctly Incorrect thermal paper being used Solution Hold the on/off button down (approximately 10 seconds) until the unit switches off and then turn on again Connect charger to the mains and leave unit to fully charge, or return the unit for servicing Replacement of head assembly or return the unit for servicing Replacement of head assembly or return unit for servicing Clean turbine in warm soapy water or sterilizing solution; if problem continues, a replacement turbine may be required Return the unit for servicing Replace the mains charger Refer to section paper loading of this manual and follow the instructions. Ensure you are using Micro Direct recommended thermal paper (See Consumables/Accessories section). 24
28 Stylus does not register icons on the display Icons missing from the display Calibration procedure failed or cannot be completed Touch screen display requires calibration Icon has been de-selected Turbine may be faulty Turbine not fitted tightly to calibration syringe Calibration syringe does not have an inspiratory seal or seal is leaking Shaft of the syringe is being pushed down Select the calibration check icon and choose touch screen and follow the instructions Hold stylus on the blue area of the display, a list will appear, ensure required icon is selected Repeat calibration procedure, if problem persists, replace turbine or return unit for servicing Ensure the syringe is fitted to the turbine using an adapter if necessary Ensure you are using manufacturers recommended syringe The syringe should be emptied and filled with one smooth stroke, avoid pushing down on the shaft or banging at the end of each maneuver 25
29 Safety Designation per IEC Type of protection against electrical shock Degree of protection against electrical shock Power Equipment Degree of Electrical connection between equipment and patient Degree of mobility Mode of operation Classifications according to IEC MicroLab Volume Transducer Internally powered Equipment and Class I Type B applied part Battery type: NiMH battery pack, 8.4V, 1100mAh Equipment designed as non-electrical connection to the patient Transportable Continuous Applied part, type B Applied part, type B WARNING: No modification of this equipment is allowed. Note: When you connect other equipment to the unit, always make sure that the whole combination complies with the international safety standard IEC for medical electrical systems. During measurements, connect the MicroLab only to printers and computers that comply with IEC / ANSI/AAMI ES :2005 /CAN/CSA-C22.2 No :14. WARNING: The user must not touch any voltage carrying parts and the patient at the same time. During database upload, the MicroLab may be connected to a computer that complies with EN Information technology equipment Safety Part 1: General requirements. IMPORTANT: Only use the mains adapter supplied (PSU V DC 2.5A). The adapter contains a transformer. Do not cut off the adapter to replace it with another plug as this causes a hazardous situation. 26
30 The adapter transforms the mains voltage ( Volts) to a safe voltage (12V DC) Make sure the adapter does not get wet Do not use a damaged adapter Always unplug your MicroLab before cleaning WARNING: Do not connect devices that are not specified as part of the system. Note: If an MPSO (Multiple Portable Socket Outlet) is used with the system, the maximum permitted load should not be exceeded. Do not connect electrical equipment that has not been supplied as part of the system. Electromagnetic Compatibility (EMC) to EN :2007 WARNING: use of portable phones or other radio frequency (RF) emitting equipment near the system may cause unexpected or adverse operation. The MicroLab has been tested to EN :2007, regarding the ability to operate in an environment containing other electrical/electronic equipment (including other medical devices). The purpose of this testing is to ensure that the MicroLab is not likely to adversely affect the normal operation of other such equipment and that other such equipment is not likely to adversely affect the normal operation of the MicroLab. Despite the testing of the MicroLab that has been undertaken, normal operation of the MicroLab can be affected by other electrical/electronic equipment and portable and mobile RF communications equipment. As the MicroLab is medical equipment, special precautions are needed regarding EMC (electromagnetic compatibility). It is important that the MicroLab is configured and installed /put into service, in accordance with the instructions/guidance provided herein and is used only in the configuration as supplied. Changes or modifications to the MicroLab may results in increased emissions or decreased immunity of the MicroLab in relation to EMC performance. 27
31 The MicroLab should be used only with the accessories (USB cables, mains adapter and turbine transducer) supplied (which are referenced in the accessories section of this manual). None of the MicroLab cables should be extended in length by the user. If any cables are extended by the user or non approved accessories are used, this may result in an increased level of emissions or decreased level of immunity, in relation to the MicroLab s EMC. None of the MicroLabs accessories should be used with other devices, as this may result in an increased level of emissions or decreased level of immunity, in relation to the other device s EMC. The MicroLab has an essential performance the product should continue to operate correctly. In the unlikely event of a Fast Transient / ESD event occurring, the device should be reset and located away from the source of interference. WARNING: The MicroLab should not be used adjacent to or stacked with other equipment. If adjacent or stacked use with other equipment is necessary, the MicroLab and the other equipment should be observed/monitored, to verify normal operation in the configuration in which it will be used. Guidance and Manufacturer s Declaration Electromagnetic Emissions The MicroLab is intended for use in the electromagnetic environment specified below. The customer or the user of the MicroLab should assure that it is used in such an environment Emission Test Compliance Electromagnetic Environment - Guidance RF emissions CISPR 11 Group 1 The MicroLab uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment The MicroLab is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes RF emissions CISPR Group B 11 Harmonic emissions Class A IEC Voltage fluctuations / Complies flicker emissions IEC Guidance and Manufacturer s Declaration Electromagnetic Immunity The MicroLab is intended for use in the electromagnetic environment specified below. The customer or the user of the MicroLab should assure that it is used in such an environment. 28
32 Immunity Test Electrostatic discharge (ESD) IEC Electrical fast transient / burst IEC Surge IEC Voltage dips, short interruptions and voltage variations on power supply input lines IEC Power frequency (50/60 Hz) Magnetic field IEC IEC Test Level +/- 6 kv contact +/- 8 kv air +/- 2 kv for power supply lines +/- 1 kv for input / output lines +/- 1 kv line(s) to line(s) +/- 2 kv line(s) to earth < 5% U T (> 95% dip in U T) For 0.5 cycle 40% U T (60% dip in U T) for 5 cycles 70% U T (30% dip in U T) for 25 cycles <5% U T (> 95% dip in U T) for 5 s Compliance Level +/- 6 kv contact +/- 8 kv air +/- 2 kv for power supply lines Input/output line tests not applicable (< 3 m) +/- 1 kv line(s) to line(s) +/- 2 kv line(s) to earth < 5% U T (> 95% dip in U T) For 0.5 cycle 40% U T (60% dip in U T) for 5 cycles 70% U T (30% dip in U T) for 25 cycles <5% U T (> 95% dip in U T) for 5 s Electromagnetic Environment - Guidance Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30% Mains power quality should be that of a typical commercial or hospital environment Mains power quality should be that of a typical commercial or hospital environment Mains power quality should be that of a typical commercial or hospital environment. If the user of the MicroLab requires continued operation during power mains interruptions, it is recommended that the MicroLab be powered from an uninterruptible power supply or a battery 3 A / m 3 A / m If incorrect operation occurs, it may be necessary to position the MicroLab further from sources of power frequency magnetic fields or to install magnetic shielding. The power frequency magnetic field should be measured in the intended installation location to assure that it is sufficiently low. NOTE U T is the a.c. mains voltage prior to application of the test level. 29
33 Guidance and Manufacturer s Declaration Electromagnetic Immunity The MicroLab is intended for use in the electromagnetic environment specified below. The customer or the user of the MicroLab should assure that it is used in such an environment. Immunity Test Conducted RF IEC IEC Test Level 3 Vrms 150 khz to 80 MHz Compliance Level 3 Vrms Electromagnetic Environment - Guidance Portable and mobile RF communications equipment should be used no closer to any part of the MicroLab, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance (d) d = 1.2 P Radiated RF IEC V/m 80 Mhz to 2.5 Ghz 3 V/m d = 1.2 P 80 MHz to 800 MHz d = 2.3 P 800 MHz to 2.5 GHz Where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Fields strengths from fixed RF transmitters, as determined by an electromagnetic site survey, a should be less than the compliance level in each frequency range. b Interference may occur in the vicinity of equipment marked with the following symbol: NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular / cordless) telephones and land mobile radios, amateur radios, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the MicroLab is used exceeds the applicable RF compliance level above, the MicroLab should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorientating or relocating the MicroLab 30
34 b Over the frequency range 150 khz to 80 MHz, field strengths should be less than 3 V / m Recommended separation distances between portable and mobile RF communications equipment and the MicroLab The MicroLab is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the MicroLab can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the MicroLab as recommended below, according to the maximum output power of the communications equipment. Rated Maximum Output Power of Transmitter in Watts (W) Separation Distance in Meters (m) according to Frequency of Transmitter 150 KHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz d = 1.2 P d = 1.2 P d = 2.3 P For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equipment applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures objects and people. 31
35 Symbols Type B device In accordance with Directive 93/42/EEC Disposal in compliance with WEEE Consult the instructions for use Manufacturer Date of Manufacture Serial Number Direct Current Single Patient Use Rx only Federal U.S. law restricts this device to sale by or on the order of a physician (Rx only) 32
36 Specifications General Storage : >2000 tests including Flow/Volume loops and Volume/Time curves Display: Color 1/4VGA LCD. Power supply: Input 100 to 240V, 47 to 63Hz. Output 12V 2.5A (Class 1) Type:MENB1030A1200F03 Battery Pack: Rechargeable NiMH 8.4V 1A-hours Battery Life: Approximately 30 hours with a fully charged new battery Dimensions: 1.4" x 10 x Transducer 2 x 2.4 x 3.5 Weight: Unit: 1.4 pounds Operating Temperature: 32 to 104 degrees Fahrenheit Operating Humidity: 30% to 90% RH Transport and Storage Temperature: -4 to 158 degrees Fahrenheit Transport and Storage Humidity: 10% to 90% RH Transport and Storage Pressure: 650 to 1060 hpa Spirometry Measurements Relaxed Expiratory Vital Capacity (VC) Forced Expired Volume in 0.75 seconds (FEV.75) Forced Expired Volume in 1 second (FEV1) Forced Expired Volume in 3 second (FEV3) Forced Expired Volume in 6 seconds (FEV6) Forced Vital Capacity (FVC) Peak Expiratory Flow Rate (PEF) FEV 0.75 as a percentage of VC (FEV.75/VC) FEV 0.75 as a percentage of FVC (FEV.75/FVC) FEV 1 as a percentage of VC (FEV1/VC) FEV 1 as a percentage of FVC (FEV1/FVC) FEV 3 as a percentage of VC (FEV3/VC) FEV 3 as a percentage of FVC (FEV3/FVC) FEV 0.75 as a percentage of FEV6 (FEV.75/FEV6) FEV1 as a percentage of FEV6 (FEV1/FEV6) Maximum Expired Flow at 75% of FVC remaining (MEF75) Maximum Expired Flow at 50% of FVC remaining (MEF50) Maximum Expired Flow at 25% of FVC remaining (MEF25) Mean Mid-Expiratory Flow Rate (MMEF) Forced expiratory flow at 50% of volume as a percentage of VC (FEF50/VC) Forced expiratory flow at 50% of volume as a percentage of FVC (FEF50/FVC) Maximal voluntary ventilation indicated (MVV (ind)) Forced inspired volume in 1 second (FIV1) Forced inspiratory Vital Capacity (FIVC) Peak Inspiratory Flow Rate (PIF) FIV 1 as a percentage of FIVC (FIV1/FIVC) Forced inspiratory flow at 25% of inhaled volume (FIF25) Forced inspiratory flow at 50% of inhaled volume (FIF50) Forced inspiratory flow at 75% of inhaled volume (FIF75) 33
37 Forced expiratory flow at 50% of volume as a percentage of FIF50 (FEF50/FIF50) The time taken between 25% and 75% of the forced expired volume (MET2575) Forced Expiratory Time (FET) Tidal Volume (TV) Expiratory reserve volume (ERV) Inspiratory reserve volume (IRV) Inspiratory capacity (IC) Expiratory Relaxed vital capacity (EVC) Inspiratory vital capacity (IVC) Breathing frequency rate (FR) Inspiratory time (Ti) Expiratory time (Te) Ti as a % of total breath time (Ti/Ttot) Tidal volume as a % of Ti (TV/Ti) Breath Rate BR Breathing Time B.T Volume Tidal VT Expiratory Time average time of expiration per Te breaths in seconds Inspiratory Time average time of inspiration per Ti breath in seconds Total Tidal Breath Time in Seconds TTOT=Ti + Te Ratio of Average Expiratory and Inspiratory Breaths Ti/Te Average Time of Expiration per Breath as a ratio to Ti/TTOT The Total Tidal Breath Time Tests per subject: 5 VC maneuver 8 FVC maneuvers Predicted Values: Various depends upon national preference Transducer: Bi-Directional Digital Volume. Resolution: 10ml volume 0.03l/s flow Accuracy: +/-3%. To ATS recommendations Standardization of spirometry 1994 update for flows and volumes. 34
38 Consumables / Supporting Products Cat. No. Description 3327 Thermal Printer Paper (10 rolls) 3314SB Adult Disposable Mouthpieces (200 per box) 3314B5 Adult Disposable Mouthpieces (500 per box) 3395 MicroCheck One-way Cardboard Mouthpieces (200 per box) 3397 MicroCheck One-way Plastic Mouthpiece (200 per box) 3301 Pediatric Disposable Mouthpieces (100 per bag) PSA1100 Pediatric Adaptor 3385 SpiroSafe Pulmonary Filters (100 per box) 3304 Nose Clips (20 per bag) Liter Calibration Syringe SPC1000 Spirometry PC Software (Optional) CAB7800 USB Lead (PC) PSU1012 Mains Adapter BAT1038 Battery TDX1048 Turbine Transducer ASS1206 Transducer Head Assembly Protex Disinfectant Wipe (100/canister) To place an order for consumables / supporting products, for service/repair or for general questions please contact Micro Direct at: Toll Free: Telephone: Fax: sales@mdspiro.com support@mdspiro.com Website: Or contact your local Micro Direct distributor. 35
39 ICD-10 Codes for Spirometry Acute Bronchitis Diagnosis Allergic Rhinitis, Other J20.0-J20.9 Code J30.81-J30.89 Allergic Rhinitis, Unspecified J30.9 Vasomotor and Allergic Rhinitis Asthma, Mild, Intermittent Asthma, Mild, Persistent Asthma, Moderate, Persistent Asthma, Severe, Persistent Asthma, Unspecified J30.0-J30.5 J45.20-J45.22 J45.30-J45.32 J45.40-J45.42 J45.50-J45.52 J J Cough Variant Asthma J Other Asthma J Cystic Fibrosis with Pulmonary Manifestations Bronchiectasis Encounter for Preprocedural Respiratory Examination Other Interstitial Pulmonary Disease with Fibrosis in diseases classified elsewhere Other Specified Interstitial Pulmonary Disease Interstitial Pulmonary Diseases, Unspecified Pneumoconiosis Due to Asbestos and Other Mineral Fibers Pneumonitis 36 E84.10 J47.0-J47.9 Z J84.17 J84.89 J84.9 J61 J67.0-J67.9
40 Pulmonary, Fibrosis J84.10 Respiratory conditions due to inhalation of chemicals, gases, fumes and vapors Respiratory conditions due to unspecified external agent J68.0-J68.9 J70.9 Sarcoidosis of the Lung D86.0 Sarcoidosis of the Lung with sacoidosis of the lymph nodes Bronchiolitis, Acute Bronchitis, Not Specified as Acute or Chronic D86.2 J21.0-J21.9 J40 Bronchospasm, Acute J98.01 Bronchospasm, Exercised Induced Chronic Bronchitis, Simple J J41.0-J41.8 Chronic Bronchitis, Unspecified COPD Cough J42 J44.0-J44.9 R05 Emphysema Other Long Term (Current) Drug Therapy 37 J43.0-J43.9 Z Shortness of Breath R06.02 Systemic Sclerosis with lung involvement Contact with and (suspected) exposure to environmental tobacco smoke (acute) (chronic)** Nicotine Dependence** M34.81 Z77.22 Tobacco Use (NOS)** Z72.0 F F17.299
41 Occupational exposure to environmental tobacco smoke** Personal history of nicotine dependence** Smoking (tobacco) complicating pregnancy, childbirth, and the puerperium** Z57.31 Z Wheezing R06.2 O O **Use additional code after the primary diagnosis to identify any tobacco use, dependence or exposure to tobacco smoke 38 Rev. 03/17-2
MicroLab Operating Manual
 MicroLab Operating Manual Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner. MAN1300 085-73 (Part 1) Issue 1.0 October 2012 Micro Direct, Inc. 803
MicroLab Operating Manual Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner. MAN1300 085-73 (Part 1) Issue 1.0 October 2012 Micro Direct, Inc. 803
Office Spirometry Guide
 Office Spirometry Guide MD Spiro 803 Webster Street, Lewiston ME 04240 Telephone 207-786-7808 1-800-588-3381 Fax 207-786-7280 www.mdspiro.com e-mail: sales@mdspiro.com Why should you perform spirometry
Office Spirometry Guide MD Spiro 803 Webster Street, Lewiston ME 04240 Telephone 207-786-7808 1-800-588-3381 Fax 207-786-7280 www.mdspiro.com e-mail: sales@mdspiro.com Why should you perform spirometry
Smart Control remote guide
 Smart Control remote guide Thank you Thank you for choosing Unitron Smart Control remote for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing
Smart Control remote guide Thank you Thank you for choosing Unitron Smart Control remote for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing
BiPAP Pro Bi-Flex. Accessing the Provider Mode Screens PROVIDER GUIDE
 BiPAP Pro Bi-Flex PROVIDER GUIDE IMPORTANT! Remove this guide before giving the device to the patient. Only medical professionals should adjust pressure settings. This guide provides you with instructions
BiPAP Pro Bi-Flex PROVIDER GUIDE IMPORTANT! Remove this guide before giving the device to the patient. Only medical professionals should adjust pressure settings. This guide provides you with instructions
Jazz Ultra Instruments
 Jazz Ultra Instruments Instructions for use Next Contents 1. Introduction...p3 2. Symbols...p4 3. Safety 3.1 Device Classification 3.2 Warnings and cautions...p5 4. Cleaning instructions 4.1 Sterilisation...p6
Jazz Ultra Instruments Instructions for use Next Contents 1. Introduction...p3 2. Symbols...p4 3. Safety 3.1 Device Classification 3.2 Warnings and cautions...p5 4. Cleaning instructions 4.1 Sterilisation...p6
This page has been intentionally left blank
 Instruction Manual Table of Contents Introduction... 1 Important Safeguards... 2 Warranty... 5 Classifications and Markings... 6 Aeroneb Go System Parts... 7 Unit Assembly and Usage... 7 Prior to Assembly...
Instruction Manual Table of Contents Introduction... 1 Important Safeguards... 2 Warranty... 5 Classifications and Markings... 6 Aeroneb Go System Parts... 7 Unit Assembly and Usage... 7 Prior to Assembly...
UNIT TWO: OVERVIEW OF SPIROMETRY. A. Definition of Spirometry
 UNIT TWO: OVERVIEW OF SPIROMETRY A. Definition of Spirometry Spirometry is a medical screening test that measures various aspects of breathing and lung function. It is performed by using a spirometer,
UNIT TWO: OVERVIEW OF SPIROMETRY A. Definition of Spirometry Spirometry is a medical screening test that measures various aspects of breathing and lung function. It is performed by using a spirometer,
GENERAL INFORMATION. Page 1 z 11
 GENERAL INFORMATION MySpiroo is a personal, connected and ultraportable spirometer with a dedicated mobile application for your smartphone. The device encompasses all of the most important and widely used
GENERAL INFORMATION MySpiroo is a personal, connected and ultraportable spirometer with a dedicated mobile application for your smartphone. The device encompasses all of the most important and widely used
Compressor Nebulizer Instruction Manual Part No.: 5055
 Compressor Nebulizer Instruction Manual Part No.: 5055 DISTRIBUTED BY: SAVE THESE INSTRUCTIONS. CAUTION - U.S. Federal Law restricts this device to sale by or on the order of a physician. 666002-6310 V1.3
Compressor Nebulizer Instruction Manual Part No.: 5055 DISTRIBUTED BY: SAVE THESE INSTRUCTIONS. CAUTION - U.S. Federal Law restricts this device to sale by or on the order of a physician. 666002-6310 V1.3
INSTALLATION MANUAL. MedRx TINNOMETER. Revolutionary Tinnitus Assessment.
 INSTALLATION MANUAL Revolutionary Tinnitus Assessment MedRx TINNOMETER www.medrx-usa.com Contents Getting to Know Your Tinnometer 3 Computer Requirements 4 Tinnometer 5 Transducers and Accessories 5 Software
INSTALLATION MANUAL Revolutionary Tinnitus Assessment MedRx TINNOMETER www.medrx-usa.com Contents Getting to Know Your Tinnometer 3 Computer Requirements 4 Tinnometer 5 Transducers and Accessories 5 Software
Instructions for Use. Oscilla TSM400 Screening Tympanometer. Specifications are subject to change without notice ID: 6795 / ver.
 Instructions for Use Oscilla TSM400 Screening Tympanometer Specifications are subject to change without notice 2017-02-09 1 Contents General description... 3 Installation... 4 Device overview... 5 Rear
Instructions for Use Oscilla TSM400 Screening Tympanometer Specifications are subject to change without notice 2017-02-09 1 Contents General description... 3 Installation... 4 Device overview... 5 Rear
Provider s Guide. Table of Contents
 Table of Contents 2 3 5 6 7 8 9 10 10 11 13 14 15 17 21 21 21 22 23 How to Use This Guide Warnings, Cautions, and Contraindications Intended Use What is? System Contents Symbols RUSleeping Display Instructions
Table of Contents 2 3 5 6 7 8 9 10 10 11 13 14 15 17 21 21 21 22 23 How to Use This Guide Warnings, Cautions, and Contraindications Intended Use What is? System Contents Symbols RUSleeping Display Instructions
Video Otoscope MS101. User s Manual UM-AP A
 Video Otoscope MS0 User s Manual UM-AP005-08-A Thank you for choosing the MDSCOPE Video Otoscope, the Video Otoscope choice for health care professionals. The operating and maintenance instructions found
Video Otoscope MS0 User s Manual UM-AP005-08-A Thank you for choosing the MDSCOPE Video Otoscope, the Video Otoscope choice for health care professionals. The operating and maintenance instructions found
ER75 Electro-Acoustic Ear Simulator. Operating Manual
 ER75 Electro-Acoustic Ear Simulator Operating Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. Amplivox Ltd. 6 Oasis Park, Eynsham Oxfordshire, OX29 4TP United
ER75 Electro-Acoustic Ear Simulator Operating Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. Amplivox Ltd. 6 Oasis Park, Eynsham Oxfordshire, OX29 4TP United
Portable Equine Nebuliser System. User Manual
 Portable Equine Nebuliser System User Manual Table of Contents INTENDED USE... 3 SAFETY INFORMATION... 3 TECHNICAL SPECIFICATION... 4 INSTRUCTIONS FOR USE... 6 MAINTENANCE... 12 TROUBLESHOOTING... 13 WARRANTY...
Portable Equine Nebuliser System User Manual Table of Contents INTENDED USE... 3 SAFETY INFORMATION... 3 TECHNICAL SPECIFICATION... 4 INSTRUCTIONS FOR USE... 6 MAINTENANCE... 12 TROUBLESHOOTING... 13 WARRANTY...
Aeroneb Solo System Instruction Manual
 www.aerogen.com Aeroneb Solo System Instruction Manual Aeroneb Solo System Instruction Manual Introduction 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 10 Installation
www.aerogen.com Aeroneb Solo System Instruction Manual Aeroneb Solo System Instruction Manual Introduction 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 10 Installation
PLEASE READ THIS USER GUIDE BEFORE OPERATING THE SYSTEM
 USER GUIDE 1 Intended Use and Indications The LungBoost Respiratory Trainer is a device which assists its user in strengthening their respiratory muscles. This device uses dual purpose training Endurance
USER GUIDE 1 Intended Use and Indications The LungBoost Respiratory Trainer is a device which assists its user in strengthening their respiratory muscles. This device uses dual purpose training Endurance
PC Based and Handheld Spirometers
 Innovation in Spirometry Oximetry Telemedicine PC Based and Handheld Spirometers www.spirometry.com www.oximetry.com PC Based and Handheld Spirometers Spirodoc Slim pocket size Mini-Laboratory with touchscreen
Innovation in Spirometry Oximetry Telemedicine PC Based and Handheld Spirometers www.spirometry.com www.oximetry.com PC Based and Handheld Spirometers Spirodoc Slim pocket size Mini-Laboratory with touchscreen
Instruction Manual Aerogen, Inc. Part No. AG-AL1010 Rev. C
 Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
Instruction Manual 2004 Aerogen, Inc. Part No. AG-AL1010 Rev. C Table of Contents Introduction... 3 System description... 4 Warnings... 5 Cautions... 5 Electromagnetic Susceptibility... 5 Symbols... 6
Spirometry and Flow Volume Measurements
 Spirometry and Flow Volume Measurements Standards & Guidelines December 1998 To serve the public and guide the medical profession Revision Dates: December 1998 Approval Date: June 1998 Originating Committee:
Spirometry and Flow Volume Measurements Standards & Guidelines December 1998 To serve the public and guide the medical profession Revision Dates: December 1998 Approval Date: June 1998 Originating Committee:
Spirodoc. 3D Laboratory for respiratory analysis two functional modes: doctor and patient. Four devices in one. Spirometer with Touch Screen display
 Spirodoc 3D Laboratory for respiratory analysis two functional modes: doctor and patient Four devices in one Spirometer with Touch Screen display Pulse Oximeter intelligent with on screen results 3D Accelerometer
Spirodoc 3D Laboratory for respiratory analysis two functional modes: doctor and patient Four devices in one Spirometer with Touch Screen display Pulse Oximeter intelligent with on screen results 3D Accelerometer
Amplivox Ltd Model 116 Screening Audiometer Operating Manual
 Amplivox Ltd Model 116 Screening Audiometer Operating Manual (Applies from serial number 23310 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29 4TP United Kingdom Tel: +44 (0)1865 880846 Fax:
Amplivox Ltd Model 116 Screening Audiometer Operating Manual (Applies from serial number 23310 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29 4TP United Kingdom Tel: +44 (0)1865 880846 Fax:
3 operating modes: Primary Care, Occupational Health or Diagnostic. Transducers: Turbine, Disposable or Fleisch. Spirometry quality control program.
 DATOSPIR SPIROMETER USB Ethernet HL7 3 operating modes: Primary Care, Occupational Health or Diagnostic. Transducers: Turbine, Disposable or Fleisch. Spirometry quality control program. Connectivity to
DATOSPIR SPIROMETER USB Ethernet HL7 3 operating modes: Primary Care, Occupational Health or Diagnostic. Transducers: Turbine, Disposable or Fleisch. Spirometry quality control program. Connectivity to
System Instruction Manual
 System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 11 Optimum Use 14 Installation
System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 11 Optimum Use 14 Installation
2.0 Scope: This document is to be used by the DCS staff when collecting participants spirometry measurements using the TruFlow Easy-On Spirometer.
 Title: Spirometry Version Date: 2017-MAR-21 Document Effective Date: 2017-MAY-15 Number: Data Collection Site (DCS) Version: 2.3 Number of Pages: SOP_DCS_0012 6 1.0 Purpose: The purpose of this document
Title: Spirometry Version Date: 2017-MAR-21 Document Effective Date: 2017-MAY-15 Number: Data Collection Site (DCS) Version: 2.3 Number of Pages: SOP_DCS_0012 6 1.0 Purpose: The purpose of this document
WARNING The user should read and understand all product literature, labeling and warnings prior to operating the Renaissance II Spirometry System
 WARNING The user should read and understand all product literature, labeling and warnings prior to operating the Renaissance II Spirometry System To obtain information about warranty for this product contact
WARNING The user should read and understand all product literature, labeling and warnings prior to operating the Renaissance II Spirometry System To obtain information about warranty for this product contact
SPIROMETRY. Marijke Currie (CRFS) Care Medical Ltd Phone: Copyright CARE Medical ltd
 SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual can
SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual can
Dear HighQ Check System Owner :
 Dear HighQ Check System Owner : Thank you for purchasing the HighQ Check Blood Glucose Monitoring System. This manual provides important information to help you to use the system properly. Before using
Dear HighQ Check System Owner : Thank you for purchasing the HighQ Check Blood Glucose Monitoring System. This manual provides important information to help you to use the system properly. Before using
Compressor Nebulizer. Guidebook
 NSTRU Compressor Nebulizer Guidebook MODEL: MQ6002 READ THIS INSTRUCTION MANUAL CAREFULLY BEFORE USE Compressor Nebulizer MODEL NO: MQ6002 INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
NSTRU Compressor Nebulizer Guidebook MODEL: MQ6002 READ THIS INSTRUCTION MANUAL CAREFULLY BEFORE USE Compressor Nebulizer MODEL NO: MQ6002 INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
Operating Manual. Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner.
 Spiro Home Monitor Operating Manual Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner. Micro Direct, Inc. 803 Webster Street Lewiston, ME 04240
Spiro Home Monitor Operating Manual Federal (USA) law restricts this device to sale by or on the order of a physician or licensed practitioner. Micro Direct, Inc. 803 Webster Street Lewiston, ME 04240
User Training Manual
 Select Language AIM (Aerosol Inhalation Monitor) Model 4500 User Training Manual https://vitalograph.com/downloads/display/41 1/17 UK Sales Vitalograph Ltd. Maids Moreton, Buckingham, MK18 1SW, England
Select Language AIM (Aerosol Inhalation Monitor) Model 4500 User Training Manual https://vitalograph.com/downloads/display/41 1/17 UK Sales Vitalograph Ltd. Maids Moreton, Buckingham, MK18 1SW, England
Model 170. Operation Manual
 Model 170 Operation Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. This manual is valid for the Model 170 (applies from firmware version 2N22 onwards please
Model 170 Operation Manual ABOUT THIS MANUAL READ THIS OPERATING MANUAL BEFORE ATTEMPTING TO USE THE INSTRUMENT. This manual is valid for the Model 170 (applies from firmware version 2N22 onwards please
INSTALLATION MANUAL. AVANT Air, Bone, Speech and Masking Audiometry AUDIOMETERS.
 INSTALLATION MANUAL AVANT Air, Bone, Speech and Masking Audiometry AUDIOMETERS www.medrx-int.com Contents Getting to Know Your Audiometer.. 3 Computer Requirements.. 4 Avant A2D+ 5 Avant Stealth... 6 Transducers
INSTALLATION MANUAL AVANT Air, Bone, Speech and Masking Audiometry AUDIOMETERS www.medrx-int.com Contents Getting to Know Your Audiometer.. 3 Computer Requirements.. 4 Avant A2D+ 5 Avant Stealth... 6 Transducers
System Instruction Manual
 System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 11 Optimum Use 13 Installation
System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 4 Assembly & Installation 6 Installation For Use With A Ventilator 11 Optimum Use 13 Installation
Amplivox Ltd Otosure Automatic Audiometer Operating Manual (Desktop Version) (Audibase Software Version 5.5)
 Amplivox Ltd Otosure Automatic Audiometer Operating Manual (Desktop Version) (Audibase Software Version 5.5) (Applies from serial number 58000 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29
Amplivox Ltd Otosure Automatic Audiometer Operating Manual (Desktop Version) (Audibase Software Version 5.5) (Applies from serial number 58000 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29
System Instruction Manual
 System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 5 Assembly & Installation 9 Installation For Use With A Ventilator 12 Nebulization 16 Installation
System Instruction Manual www.aerogen.com Contents Introduction 2 Indications for Use 2 System Warnings 5 Assembly & Installation 9 Installation For Use With A Ventilator 12 Nebulization 16 Installation
DIAGNOSTIC ACCREDITATION PROGRAM. Spirometry Quality Control Plan
 DIAGNOSTIC ACCREDITATION PROGRAM Spirometry Quality Control Plan Table of Contents Introduction...1 Spirometry Definitions and Requirements...2 Spirometry Requirements... 2...4 Daily Quality Control (see
DIAGNOSTIC ACCREDITATION PROGRAM Spirometry Quality Control Plan Table of Contents Introduction...1 Spirometry Definitions and Requirements...2 Spirometry Requirements... 2...4 Daily Quality Control (see
Vitalograph Alpha Spirometer
 Product Catalogue Vitalograph Alpha Spirometer Dedicated all-in-one desktop unit Simple to use Rapid and low cost testing Clear and bright color screen Accurate Fleisch pneumotachograph Measures VC, FVC,
Product Catalogue Vitalograph Alpha Spirometer Dedicated all-in-one desktop unit Simple to use Rapid and low cost testing Clear and bright color screen Accurate Fleisch pneumotachograph Measures VC, FVC,
Operator s Manual FLASH GLUCOSE MONITORING SYSTEM. CAUTION: Federal law restricts this device to sale by or on the order of a physician.
 Operator s Manual FLASH GLUCOSE MONITORING SYSTEM CAUTION: Federal law restricts this device to sale by or on the order of a physician. Reader Symbols... 1 Contents Important Safety Information... 2 Indications
Operator s Manual FLASH GLUCOSE MONITORING SYSTEM CAUTION: Federal law restricts this device to sale by or on the order of a physician. Reader Symbols... 1 Contents Important Safety Information... 2 Indications
Compressor Nebulizer. Guidebook
 NSTRU Compressor Nebulizer Guidebook MODEL: CN02WS (MQ6003) READ ALL INSTRUCTION BEFORE USE Airial TM Compressor Nebulizer INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
NSTRU Compressor Nebulizer Guidebook MODEL: CN02WS (MQ6003) READ ALL INSTRUCTION BEFORE USE Airial TM Compressor Nebulizer INSTRUCTIONS INDEX 1. Introduction ----------------------------------------------------------------
Medescan Nebuliser Med - S600A Instructions Manual
 Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
Medescan Nebuliser Med - S600A Instructions Manual Please read this guidebook carefully before operating this unit Your Nebuliser is intended for use in the treatment of asthma, COPD and other respiratory
S P I R O M E T R Y. Objectives. Objectives 2/5/2019
 S P I R O M E T R Y Dewey Hahlbohm, PA-C, AE-C Objectives To understand the uses and importance of spirometry testing To perform spirometry testing including reversibility testing To identify normal and
S P I R O M E T R Y Dewey Hahlbohm, PA-C, AE-C Objectives To understand the uses and importance of spirometry testing To perform spirometry testing including reversibility testing To identify normal and
ALPHA 300. Installation and operation manual YL V1.0 04/
 YL071600 V1.0 04/2013 ALPHA 300 Installation and operation manual EN www.airliquide.com www.airliquidemedicalsystems.com I. Introduction 5 I.1 About this manual 5 I.2 Symbols in the manual and on the
YL071600 V1.0 04/2013 ALPHA 300 Installation and operation manual EN www.airliquide.com www.airliquidemedicalsystems.com I. Introduction 5 I.1 About this manual 5 I.2 Symbols in the manual and on the
I N S T R U C T I O N
 I N S T R U C T I O N M ANUAL model V2x Acoustical airway clearance device. 5.04.1 SAFETY SYMBOLS This manual uses the following safety symbols. They denote critical information. Please read them carefully.
I N S T R U C T I O N M ANUAL model V2x Acoustical airway clearance device. 5.04.1 SAFETY SYMBOLS This manual uses the following safety symbols. They denote critical information. Please read them carefully.
Compressor Nebulizer. Model UN-014
 Compressor Nebulizer Model UN-014 Instruction Manual Original Manuel d instructions Traduction Manual de Instrucciones Traducción Manuale di Istruzioni Traduzione ITALIANO ESPAÑOL FRANÇAIS ENGLISH 1WMPD4003398
Compressor Nebulizer Model UN-014 Instruction Manual Original Manuel d instructions Traduction Manual de Instrucciones Traducción Manuale di Istruzioni Traduzione ITALIANO ESPAÑOL FRANÇAIS ENGLISH 1WMPD4003398
Blood Glucose Monitoring System. User Guide
 Blood Glucose Monitoring System User Guide Table of Contents Introduction...2 Important Safety Instructions...2 About ipet PRO Blood Glucose Monitoring System...3 About ipet PRO Meter...4 About the ipet
Blood Glucose Monitoring System User Guide Table of Contents Introduction...2 Important Safety Instructions...2 About ipet PRO Blood Glucose Monitoring System...3 About ipet PRO Meter...4 About the ipet
SuperSpiro. The complete solution to respiratory diagnostics
 SuperSpiro The complete solution to respiratory diagnostics SuperSpiro Complete respiratory diagnostics Developed for the professional, SuperSpiro is a self contained, respiratory diagnostic system. Full
SuperSpiro The complete solution to respiratory diagnostics SuperSpiro Complete respiratory diagnostics Developed for the professional, SuperSpiro is a self contained, respiratory diagnostic system. Full
2.0 Scope: This document is to be used by the DCS staff when collecting participants spirometry measurements using the TruFlow Easy-On Spirometer.
 Title: Spirometry Version Date: 2014-AUG-20 Effective Date: 2014-OCT-15 Data Collection Site (DCS) Version: 2.2 Document Number: Number of Pages: SOP_DCS_0012 7 1.0 Purpose: The purpose of this document
Title: Spirometry Version Date: 2014-AUG-20 Effective Date: 2014-OCT-15 Data Collection Site (DCS) Version: 2.2 Document Number: Number of Pages: SOP_DCS_0012 7 1.0 Purpose: The purpose of this document
Table of Contents. Introduction Indications For Use Contraindications Warnings Precautions...5
 User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
User Manual 3 Table of Contents Introduction....4 1. Indications For Use...4 2. Contraindications...4 3. Warnings...5 4. Precautions...5 5. Adverse Reactions...5 6. Step-By-Step Instructions...6 A. Contents...6
Content Indica c tion Lung v olumes e & Lung Indica c tions i n c paci c ties
 Spirometry Content Indication Indications in occupational medicine Contraindications Confounding factors Complications Type of spirometer Lung volumes & Lung capacities Spirometric values Hygiene &
Spirometry Content Indication Indications in occupational medicine Contraindications Confounding factors Complications Type of spirometer Lung volumes & Lung capacities Spirometric values Hygiene &
#7 - Respiratory System
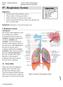 #7 - Respiratory System Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Perform spirometry to measure lung volumes Define and understand the lung volumes and
#7 - Respiratory System Objectives: Study the parts of the respiratory system Observe slides of the lung and trachea Perform spirometry to measure lung volumes Define and understand the lung volumes and
PainShield MD User Manual
 PainShield MD User Manual Cat. # PSUM003 Ver. 07 (KEV) Table of Contents GENERAL INFORMATION 4 IMPORTANT GENERAL SAFETY CAUTIONS AND NOTICES 7 CLINICAL INFORMATION 8 GENERAL DESCRIPTION 10 PAINSHIELD
PainShield MD User Manual Cat. # PSUM003 Ver. 07 (KEV) Table of Contents GENERAL INFORMATION 4 IMPORTANT GENERAL SAFETY CAUTIONS AND NOTICES 7 CLINICAL INFORMATION 8 GENERAL DESCRIPTION 10 PAINSHIELD
Breathing and pulmonary function
 EXPERIMENTAL PHYSIOLOGY EXPERIMENT 5 Breathing and pulmonary function Ying-ying Chen, PhD Dept. of Physiology, Zhejiang University School of Medicine bchenyy@zju.edu.cn Breathing Exercise 1: Tests of pulmonary
EXPERIMENTAL PHYSIOLOGY EXPERIMENT 5 Breathing and pulmonary function Ying-ying Chen, PhD Dept. of Physiology, Zhejiang University School of Medicine bchenyy@zju.edu.cn Breathing Exercise 1: Tests of pulmonary
Sleep Apnea Therapy Software Clinician Manual
 Sleep Apnea Therapy Software Clinician Manual Page ii Sleep Apnea Therapy Software Clinician Manual Notices Revised Notice Trademark Copyright Sleep Apnea Therapy Software Clinician Manual 103391 Rev A
Sleep Apnea Therapy Software Clinician Manual Page ii Sleep Apnea Therapy Software Clinician Manual Notices Revised Notice Trademark Copyright Sleep Apnea Therapy Software Clinician Manual 103391 Rev A
19 LUNG FUNCTION USING NDD EASY ON-PC
 UK Data Archive Study Number 7251 - Understanding Society: Nurse Health Assessment 19 LUNG FUNCTION USING NDD EASY ON-PC 19.1 Introduction Lung function tests objectively assess respiratory function and
UK Data Archive Study Number 7251 - Understanding Society: Nurse Health Assessment 19 LUNG FUNCTION USING NDD EASY ON-PC 19.1 Introduction Lung function tests objectively assess respiratory function and
User Manual RECHARGEABLE KIT. Includes: 1 PLUS+ rechargeable pack 2 PLUS+ batteries 1 wall charger 1 USB cable 3 Classic Tobacco flavor tanks
 User Manual RECHARGEABLE KIT Includes: 1 PLUS+ rechargeable pack 2 PLUS+ batteries 1 wall charger 1 USB cable 3 Classic Tobacco flavor tanks For optimum performance, it is recommended that you charge your
User Manual RECHARGEABLE KIT Includes: 1 PLUS+ rechargeable pack 2 PLUS+ batteries 1 wall charger 1 USB cable 3 Classic Tobacco flavor tanks For optimum performance, it is recommended that you charge your
BETTER SPIROMETRY. Marijke Currie (CRFS) Care Medical Ltd Phone: Copyright CARE Medical ltd
 BETTER SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual
BETTER SPIROMETRY Marijke Currie (CRFS) Care Medical Ltd Phone: 0800 333 808 Email: sales@caremed.co.nz What is spirometry Spirometry is a physiological test that measures the volume of air an individual
Toll-Free Customer Service: Instruction Manual. with SAFE-T-Energetics. Toll-Free Customer Service
 Toll-Free Customer Service: 1-877-970-4970 5301 North Pima Road Scottsdale, AZ 85250 www.regenesisbio.com info@regenesisbio.com K with SAFE-T-Energetics Instruction Manual Toll-Free Customer Service 1-877-970-4970
Toll-Free Customer Service: 1-877-970-4970 5301 North Pima Road Scottsdale, AZ 85250 www.regenesisbio.com info@regenesisbio.com K with SAFE-T-Energetics Instruction Manual Toll-Free Customer Service 1-877-970-4970
The Biomet EBI Bone Healing System. Patient Manual
 The Biomet EBI Bone Healing System Patient Manual Contents Introduction... Page 1 Symbol Description... Page 2 Warnings... Page 3 Battery Warning... Page 5 Indications, Contraindications, Usage and Adverse
The Biomet EBI Bone Healing System Patient Manual Contents Introduction... Page 1 Symbol Description... Page 2 Warnings... Page 3 Battery Warning... Page 5 Indications, Contraindications, Usage and Adverse
COMPREHENSIVE RESPIROMETRY
 INTRODUCTION Respiratory System Structure Complex pathway for respiration 1. Specialized tissues for: a. Conduction b. Gas exchange 2. Position in respiratory pathway determines cell type Two parts Upper
INTRODUCTION Respiratory System Structure Complex pathway for respiration 1. Specialized tissues for: a. Conduction b. Gas exchange 2. Position in respiratory pathway determines cell type Two parts Upper
Compressor Nebulizer System
 MedPro The Professional Choice Le choix des professionnels MedProTM MC Compressor Nebulizer System 705-445 Instruction Manual Index 1. Introduction...2 2. Product Identification...2 3. Important Safeguards...3
MedPro The Professional Choice Le choix des professionnels MedProTM MC Compressor Nebulizer System 705-445 Instruction Manual Index 1. Introduction...2 2. Product Identification...2 3. Important Safeguards...3
User Manual Verizon Wireless. All Rights Reserved. verizonwireless.com OM2260VW
 User Manual 2010 Verizon Wireless. All Rights Reserved. verizonwireless.com OM2260VW Home Phone Connect Welcome to Verizon Wireless Thank you for choosing Verizon Wireless Home Phone Connect. You re now
User Manual 2010 Verizon Wireless. All Rights Reserved. verizonwireless.com OM2260VW Home Phone Connect Welcome to Verizon Wireless Thank you for choosing Verizon Wireless Home Phone Connect. You re now
English. Innovation in Spirometry Oximetry Telemedicine
 English Innovation in Spirometry Oximetry Telemedicine Spirolab 7 inch Touchscreen All in one portable Desktop Spirometer with Oximetry option Wireless Real Time test on PC via Bluetooth Each function
English Innovation in Spirometry Oximetry Telemedicine Spirolab 7 inch Touchscreen All in one portable Desktop Spirometer with Oximetry option Wireless Real Time test on PC via Bluetooth Each function
PilotOne II. User Guide
 PilotOne II User Guide Contents 1. Welcome 4 2. Description 5 3. Using Phonak PilotOne II 6 3.1 Inserting a new battery 6 3.2 Switching On / Off 7 3.3 Holding correctly 7 3.4 Changing hearing aid volume
PilotOne II User Guide Contents 1. Welcome 4 2. Description 5 3. Using Phonak PilotOne II 6 3.1 Inserting a new battery 6 3.2 Switching On / Off 7 3.3 Holding correctly 7 3.4 Changing hearing aid volume
ihealth PO3 Fingertip Pulse Oximeter OPERATION GUIDE INDEX
 ihealth PO3 Fingertip Pulse Oximeter OPERATION GUIDE INDEX INTRODUCTION AND INTENDED USE...2 CONTENTS AND DISPLAY INDICATORS...2 PRODUCT DESCRIPTION...3 SPECIFICATIONS...3 CAUTIONS...3 Cautions...3 USING
ihealth PO3 Fingertip Pulse Oximeter OPERATION GUIDE INDEX INTRODUCTION AND INTENDED USE...2 CONTENTS AND DISPLAY INDICATORS...2 PRODUCT DESCRIPTION...3 SPECIFICATIONS...3 CAUTIONS...3 Cautions...3 USING
Operation Manual MA 27 / MA 27e
 Operation Manual MA 27 / MA 27e Table of Contents Page 1 Introduction 2 2 Intended Use 3 2.1 Description 3 2.2 Extended Function 3 3 Important Safety Note 5 4 Getting started 8 4.1 Unpacking and Checking
Operation Manual MA 27 / MA 27e Table of Contents Page 1 Introduction 2 2 Intended Use 3 2.1 Description 3 2.2 Extended Function 3 3 Important Safety Note 5 4 Getting started 8 4.1 Unpacking and Checking
TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS. Otthon. Ultrasonic handheld spirometer
 TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS Otthon Ultrasonic handheld spirometer OTTHON ULTRASONIC HANDHELD SPIROMETER TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS OTTHON
TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS Otthon Ultrasonic handheld spirometer OTTHON ULTRASONIC HANDHELD SPIROMETER TECHNOLOGY - INNOVATION IN ULTRASONIC RESPIRATORY DIAGNOSTICS OTTHON
Mini Remote Microphone OPERATIONS MANUAL
 Mini Remote Microphone OPERATIONS MANUAL Table of Contents Overview..................................... 4 Basic Use..................................... 7 Daily Use.....................................
Mini Remote Microphone OPERATIONS MANUAL Table of Contents Overview..................................... 4 Basic Use..................................... 7 Daily Use.....................................
MicroLoop. The world s most advanced hand held Spirometer
 MicroLoop The world s most advanced hand held Spirometer MicroLoop A new generation Spirometer designed for the professional Developed for the professional, the new generation MicroLoop employs Micro Medical
MicroLoop The world s most advanced hand held Spirometer MicroLoop A new generation Spirometer designed for the professional Developed for the professional, the new generation MicroLoop employs Micro Medical
CentriVet GK Blood Glucose & Ketone Monitoring System
 CentriVet GK Blood Glucose & Ketone Monitoring System FOR ANIMAL USE. NOT FOR HUMAN USE. Welcome and thank you for choosing the CentriVet GK Blood Glucose & Ketone Monitoring System. The CentriVet GK Blood
CentriVet GK Blood Glucose & Ketone Monitoring System FOR ANIMAL USE. NOT FOR HUMAN USE. Welcome and thank you for choosing the CentriVet GK Blood Glucose & Ketone Monitoring System. The CentriVet GK Blood
Glucose Meter. User Guide. Veterinary Monitoring System. For dog and cat use only
 Glucose Meter User Guide Veterinary Monitoring System For dog and cat use only Gpet instruction Manual 31/5/09 18:06 Page 2 Gpet instruction Manual 31/5/09 18:06 Page 3 TABLE OF CONTENTS Your g-pet system
Glucose Meter User Guide Veterinary Monitoring System For dog and cat use only Gpet instruction Manual 31/5/09 18:06 Page 2 Gpet instruction Manual 31/5/09 18:06 Page 3 TABLE OF CONTENTS Your g-pet system
PULMONARY FUNCTION. VOLUMES AND CAPACITIES
 PULMONARY FUNCTION. VOLUMES AND CAPACITIES The volume of air a person inhales (inspires) and exhales (expires) can be measured with a spirometer (spiro = breath, meter = to measure). A bell spirometer
PULMONARY FUNCTION. VOLUMES AND CAPACITIES The volume of air a person inhales (inspires) and exhales (expires) can be measured with a spirometer (spiro = breath, meter = to measure). A bell spirometer
COMPRESSOR NEBULIZER MODEL: JB USER S MANUAL
 COMPRESSOR NEBULIZER MODEL: JB0112-062 USER S MANUAL Read this manual before operating the nebulizer Save these instructions for future reference Caution: Federal law restricts this device to sale by or
COMPRESSOR NEBULIZER MODEL: JB0112-062 USER S MANUAL Read this manual before operating the nebulizer Save these instructions for future reference Caution: Federal law restricts this device to sale by or
MA 25 Operating Manual
 Table of Contents Page 1. Introduction... 1 2. Description... 2 2.1 Important safety note... 3 2.2 Unpacking and checking the MA 25... 5 2.3 Standard accessories... 5 2.4 Optional accessories... 5 2.5
Table of Contents Page 1. Introduction... 1 2. Description... 2 2.1 Important safety note... 3 2.2 Unpacking and checking the MA 25... 5 2.3 Standard accessories... 5 2.4 Optional accessories... 5 2.5
MESH NEBULIZER INSTRUCTION MANUAL. Model NE-U22
 EN-p2-20-(NE-U22)-3 3/25/02 4:48 PM Page 1 INSTRUCTION MANUAL MESH NEBULIZER Model NE-U22 Thank you very much for purchasing OMRON Mesh Nebulizer. Be sure to read this Instruction Manual before using the
EN-p2-20-(NE-U22)-3 3/25/02 4:48 PM Page 1 INSTRUCTION MANUAL MESH NEBULIZER Model NE-U22 Thank you very much for purchasing OMRON Mesh Nebulizer. Be sure to read this Instruction Manual before using the
Blood Glucose & Ketone Monitoring System
 Blood Glucose & Ketone Monitoring System Self monitoring of blood glucose is an integral part of diabetes care, but the high cost of testing can make this impossible. At ACON, our goal is to provide high
Blood Glucose & Ketone Monitoring System Self monitoring of blood glucose is an integral part of diabetes care, but the high cost of testing can make this impossible. At ACON, our goal is to provide high
INSTRUCTION MANUAL. Compressor Nebulizer NE-C801
 active forever.com INSTRUCTION MANUAL TM Compressor Nebulizer NE-C801 Caution: Federal (USA) law restricts this device to sale by or on the order of a physician or licensed healthcare practitioner ENGLISH
active forever.com INSTRUCTION MANUAL TM Compressor Nebulizer NE-C801 Caution: Federal (USA) law restricts this device to sale by or on the order of a physician or licensed healthcare practitioner ENGLISH
6- Lung Volumes and Pulmonary Function Tests
 6- Lung Volumes and Pulmonary Function Tests s (PFTs) are noninvasive diagnostic tests that provide measurable feedback about the function of the lungs. By assessing lung volumes, capacities, rates of
6- Lung Volumes and Pulmonary Function Tests s (PFTs) are noninvasive diagnostic tests that provide measurable feedback about the function of the lungs. By assessing lung volumes, capacities, rates of
Phonak PilotOne II. User Guide
 Phonak PilotOne II User Guide Contents 1. Welcome 4 2. Description 5 3. Using Phonak PilotOne II 6 3.1 Inserting a new battery 6 3.2 Switching On/Off 7 3.3 Holding PilotOne II 7 3.4 Changing hearing aid
Phonak PilotOne II User Guide Contents 1. Welcome 4 2. Description 5 3. Using Phonak PilotOne II 6 3.1 Inserting a new battery 6 3.2 Switching On/Off 7 3.3 Holding PilotOne II 7 3.4 Changing hearing aid
HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT. Instruction Manual
 HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT Instruction Manual TABLE OF CONTENTS IEC Symbols...2 Important Safeguards...2 Introduction...3 Specifications...4
HCS70004P, HCS70004G HCS70004C, HCS70004BL HCS70004DP, HCS70004 AEROMIST COLORS NEBULIZER COMPRESSOR KIT Instruction Manual TABLE OF CONTENTS IEC Symbols...2 Important Safeguards...2 Introduction...3 Specifications...4
Remote control 2 guide
 Remote control 2 guide Thank you Thank you for choosing remote control 2 for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing healthcare
Remote control 2 guide Thank you Thank you for choosing remote control 2 for your Unitron hearing aids. At Unitron, we care deeply about people with hearing loss. We work closely with hearing healthcare
What do pulmonary function tests tell you?
 Pulmonary Function Testing Michael Wert, MD Assistant Professor Clinical Department of Internal Medicine Division of Pulmonary, Critical Care, and Sleep Medicine The Ohio State University Wexner Medical
Pulmonary Function Testing Michael Wert, MD Assistant Professor Clinical Department of Internal Medicine Division of Pulmonary, Critical Care, and Sleep Medicine The Ohio State University Wexner Medical
PULMONARY FUNCTION TESTS
 Chapter 4 PULMONARY FUNCTION TESTS M.G.Rajanandh, Department of Pharmacy Practice, SRM College of Pharmacy, SRM University. OBJECTIVES Review basic pulmonary anatomy and physiology. Understand the reasons
Chapter 4 PULMONARY FUNCTION TESTS M.G.Rajanandh, Department of Pharmacy Practice, SRM College of Pharmacy, SRM University. OBJECTIVES Review basic pulmonary anatomy and physiology. Understand the reasons
GYMTOP USB PROFESSIONAL 20143
 GYMTOP USB PROFESSIONAL 20143 CONTENTS 1 x Gymtop USB 1 x CD Please note: please see PC requirements below. ABOUT THIS PRODUCT Can help develop users motor skills including planning Gymtop uses proprioceptors
GYMTOP USB PROFESSIONAL 20143 CONTENTS 1 x Gymtop USB 1 x CD Please note: please see PC requirements below. ABOUT THIS PRODUCT Can help develop users motor skills including planning Gymtop uses proprioceptors
S P I R O M E T R Y. Objectives. Objectives 3/12/2018
 S P I R O M E T R Y Dewey Hahlbohm, PA-C, AE-C Objectives To understand the uses and importance of spirometry testing To perform spirometry testing including reversibility testing To identify normal and
S P I R O M E T R Y Dewey Hahlbohm, PA-C, AE-C Objectives To understand the uses and importance of spirometry testing To perform spirometry testing including reversibility testing To identify normal and
SPORTSART C521M BI-DIRECTIONAL BIKE
 2011.12 C521M BIKE SPORTSART C521M BI-DIRECTIONAL BIKE TABLE OF CONTENTS 1. INTRODUCTION... 2. IMPORTANT SAFETY PRECAUTIONS... 3. LIST OF PARTS... 1 2 6 4. ASSEMBLING THE PRODUCT STEP 0 Separate the Product
2011.12 C521M BIKE SPORTSART C521M BI-DIRECTIONAL BIKE TABLE OF CONTENTS 1. INTRODUCTION... 2. IMPORTANT SAFETY PRECAUTIONS... 3. LIST OF PARTS... 1 2 6 4. ASSEMBLING THE PRODUCT STEP 0 Separate the Product
OARTEC TRAINING MONITOR OTM-2
 OARTEC TRAINING MONITOR OTM-2 OPERATION MANUAL Introduction Thankyou for purchasing the Oartec DX with our new training monitor, the OTM-2. The OTM-2 has a number of advanced features including automatic
OARTEC TRAINING MONITOR OTM-2 OPERATION MANUAL Introduction Thankyou for purchasing the Oartec DX with our new training monitor, the OTM-2. The OTM-2 has a number of advanced features including automatic
Fitting System Instructions for Use
 Including 2017 2018.2 Fitting System Instructions for Use Version 1.0 www.sonici.com Table of contents 1. Introduction 4 2. Installation 5 3. System requirements 6 4. Getting started with Expressfit Pro
Including 2017 2018.2 Fitting System Instructions for Use Version 1.0 www.sonici.com Table of contents 1. Introduction 4 2. Installation 5 3. System requirements 6 4. Getting started with Expressfit Pro
PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606
 PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606 Please read this guidebook carefully before operating this unit. Your Nebulizer is intended for use in the treatment of asthma, COPD and
PORTABLE COMPRESSOR NEBULIZER Guidebook & Manual Reorder No. 5606 Please read this guidebook carefully before operating this unit. Your Nebulizer is intended for use in the treatment of asthma, COPD and
DATOSPIR TOUCH USER S MANUAL SPIROMETER 511-B00-MU2 REV
 DATOSPIR TOUCH SPIROMETER USER S MANUAL 511-B00-MU2 REV. 1.07 2015-06 DATOSPIR TOUCH User s Manual 2 1 SIBEL S.A.U., Rosellón 500 bajos, 08026 BARCELONA (Spain) National Sales: Tel. 93 436 00 08 e-mail:
DATOSPIR TOUCH SPIROMETER USER S MANUAL 511-B00-MU2 REV. 1.07 2015-06 DATOSPIR TOUCH User s Manual 2 1 SIBEL S.A.U., Rosellón 500 bajos, 08026 BARCELONA (Spain) National Sales: Tel. 93 436 00 08 e-mail:
Respiratory Physiology In-Lab Guide
 Respiratory Physiology In-Lab Guide Read Me Study Guide Check Your Knowledge, before the Practical: 1. Understand the relationship between volume and pressure. Understand the three respiratory pressures
Respiratory Physiology In-Lab Guide Read Me Study Guide Check Your Knowledge, before the Practical: 1. Understand the relationship between volume and pressure. Understand the three respiratory pressures
For more information visit or contact hearx:
 USER MANUAL hearscope - Ground Floor, Building 2, Ashlea Gardens Office Park, 180 Garsfontein Road, Ashlea Gardens, Pretoria, 0081, South Africa hearscope v2. HSCP-MN-EN hearscope IFU v1.0 For more information
USER MANUAL hearscope - Ground Floor, Building 2, Ashlea Gardens Office Park, 180 Garsfontein Road, Ashlea Gardens, Pretoria, 0081, South Africa hearscope v2. HSCP-MN-EN hearscope IFU v1.0 For more information
Charging base guide. A Sonova brand
 Charging base guide A Sonova brand Thank you Thank you for choosing this rechargeable solution. We care deeply about people with hearing loss. We work closely with hearing healthcare professionals to make
Charging base guide A Sonova brand Thank you Thank you for choosing this rechargeable solution. We care deeply about people with hearing loss. We work closely with hearing healthcare professionals to make
Amplivox Ltd Model 240 Diagnostic Audiometer Operating Manual
 Amplivox Ltd Model 240 Diagnostic Audiometer Operating Manual (Applies from serial number 31657 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29 4TP United Kingdom Tel: +44 (0)1865 880846 Fax:
Amplivox Ltd Model 240 Diagnostic Audiometer Operating Manual (Applies from serial number 31657 onwards) Amplivox Ltd 6 Oasis Park Eynsham Oxfordshire OX29 4TP United Kingdom Tel: +44 (0)1865 880846 Fax:
THE TOTALLY WICKED TORNADO NC CONGRATULATIONS ON PURCHASING YOUR TOTALLY WICKED TORNADO NC ELECTRONIC CIGARETTE KIT
 USER MANUAL THE TOTALLY WICKED TORNADO NC CONGRATULATIONS ON PURCHASING YOUR TOTALLY WICKED TORNADO NC ELECTRONIC CIGARETTE KIT 3 QUICK START GUIDE For those familiar with e-cigarettes, the steps below
USER MANUAL THE TOTALLY WICKED TORNADO NC CONGRATULATIONS ON PURCHASING YOUR TOTALLY WICKED TORNADO NC ELECTRONIC CIGARETTE KIT 3 QUICK START GUIDE For those familiar with e-cigarettes, the steps below
Frequently asked questions to Oticon ConnectClip
 Frequently asked questions to Oticon ConnectClip 1. Pairing Question Answer How many devices can ConnectClip be paired to? ConnectClip can be paired to up to 8 Bluetooth devices. What happens when a 9th
Frequently asked questions to Oticon ConnectClip 1. Pairing Question Answer How many devices can ConnectClip be paired to? ConnectClip can be paired to up to 8 Bluetooth devices. What happens when a 9th
CardioPerfect Workstation SpiroPerfect Module - User Manual
 SpiroPerfect Module - User Manual Welch Allyn, Inc. 4341 State Street Road Skaneateles Falls, NY 13153 USA www.welchallyn.com 901051 SPIROMETER 0297 DIR 80012334 Ver. H Revision date: 2015-05 Copyright
SpiroPerfect Module - User Manual Welch Allyn, Inc. 4341 State Street Road Skaneateles Falls, NY 13153 USA www.welchallyn.com 901051 SPIROMETER 0297 DIR 80012334 Ver. H Revision date: 2015-05 Copyright
PORTO 2 VENT CPAP OS. Operator s Manual. PORTO 2VENT CPAP OS System Operator s Manual Part Number Rev I
 PORTO 2 VENT CPAP OS Operator s Manual 1 2 TABLE of CONTENTS 1. Introduction 3. Operating Instructions 1a. Definitions 3a. Setting the CPAP Level 1b. General Description 3b. Applying the Breathing Circuit
PORTO 2 VENT CPAP OS Operator s Manual 1 2 TABLE of CONTENTS 1. Introduction 3. Operating Instructions 1a. Definitions 3a. Setting the CPAP Level 1b. General Description 3b. Applying the Breathing Circuit
PULMONARY FUNCTION TESTING. By: Gh. Pouryaghoub. MD Center for Research on Occupational Diseases (CROD) Tehran University of Medical Sciences (TUMS)
 PULMONARY FUNCTION TESTING By: Gh. Pouryaghoub. MD Center for Research on Occupational Diseases (CROD) Tehran University of Medical Sciences (TUMS) PULMONARY FUNCTION TESTS CATEGORIES Spirometry Lung volumes
PULMONARY FUNCTION TESTING By: Gh. Pouryaghoub. MD Center for Research on Occupational Diseases (CROD) Tehran University of Medical Sciences (TUMS) PULMONARY FUNCTION TESTS CATEGORIES Spirometry Lung volumes
