The Effect of Using Different Aerosol Devices and Masks on Aerosol Deposition during Noninvasive Positive Pressure Ventilation in an Adult Lung Model
|
|
|
- Dinah Fleming
- 5 years ago
- Views:
Transcription
1 Georgia State University Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy Fall The Effect of Using Different Aerosol Devices and Masks on Aerosol Deposition during Noninvasive Positive Pressure Ventilation in an Adult Lung Model Maher M. AlQuaimi Respiratory Therapy at Byrdine F. Lewis School of Nursing and Health Professions Follow this and additional works at: Part of the Medicine and Health Sciences Commons Recommended Citation AlQuaimi, Maher M., "The Effect of Using Different Aerosol Devices and Masks on Aerosol Deposition during Noninvasive Positive Pressure Ventilation in an Adult Lung Model." Thesis, Georgia State University, This Thesis is brought to you for free and open access by the Department of Respiratory Therapy at Georgia State University. It has been accepted for inclusion in Respiratory Therapy Theses by an authorized administrator of Georgia State University. For more information, please contact scholarworks@gsu.edu.
2 ACCEPTANCE This thesis, THE EFFECT OF USING DIFFERENT AEROSOL DEVICES AND MASKS ON AEROSOL DEPOSITION DURING NONINVASIVE POSITIVE PRESSURE VENTILATION IN AN ADULT LUNG MODEL, by Maher M. AlQuaimi was prepared under the direction of the Master s Thesis Advisory Committee of the Respiratory Therapy department at Georgia State University. It is accepted by the committee in partial fulfillment of requirements for the Master s of Science degree in Respiratory Therapy at Byrdine F. Lewis School of Nursing and Health Professions, Georgia State University. The Master s Thesis Advisory Committee, as representatives of the faculty, certifies that this thesis has met all standards of excellence and scholarship as determined by the faculty. Arzu Ari, PhD, RRT, PT, CPFT, FAARC Robert Harwood, MSA, RRT Lawrence Bryant, PhD, RRT Date
3 AUTHOR S STATEMENT In presenting this thesis as partial fulfillment of the requirements for the Master s Degree in Respiratory Therapy from Georgia State University, I agree that the library of Georgia State University shall make it available for inspection and circulation in accordance with its regulation governing materials of this type. I agree that the permission to quote, to copy from or to publish this thesis may be granted by the professors under whose direction this thesis was written or by the Byrdine F. Lewis School of Nursing and Health Professions or by me. Such quoting, copying or publishing must be solely for scholarly purposes and will not involve potential financial gain. It is understood that any copying from, quoting or publication of this thesis which involves potential financial gain will not be allowed without my written permission. Signature of Author
4 NOTICE TO BORROWERS All thesis deposited in Georgia State University library must be used in accordance with stipulations prescribed by the author in the preceding statement. The author of this thesis is: Maher M. AlQuaimi 907 Huntcliff Village Court Sandy Springs, GA The director of this thesis is: Arzu Ari, PhD, RRT, PT, CPFT, FAARC Associate Professor Division of Respiratory Therapy Byrdine F. Lewis School of Nursing and Health Professions Georgia State University P. O. Box 4019 Atlanta, Georgia 30303
5 VITA Maher M. AlQuaimi ADDRESS: 907 Huntcliff Village Court Sandy Springs, GA EDUCATION B.S King Faisal University Respiratory therapy PROFESSIONAL EXPERIENCE July 2007 present Faculty member in King Faisal University Dammam, Saudi Arabia Aug 2006 July 2007 Staff respiratory therapist in King Faisal Specialist Hospital and Research Center Riyadh, Saudi Arabia Aug 2005 July 2006 Internship in King Faisal Specialist Hospital and Research Center Riyadh, Saudi Arabia PROFESSIONAL SOCIETIES AND ORGANIZATIONS 2005 Present American Association for Respiratory Care 2007 Present Saudi Society for Respiratory Care PRESENTATIONS AND PUBLICATIONS 2011 Efficiency of Aerosol Devices During Noninvasive Positive Pressure Ventilation in a Simulated Adult Lung Model: A poster presentation presented at the 57th International Respiratory Convention & Exhibition. Tampa, Florida 2008 Status of Respiratory Therapists in Saudi Arabia: A National Survey. Poster presented at the first international symposium of respiratory care, Dahran, Saudi Arabia.
6 ABSTRACT Introduction: Although patients with an acute increase in airflow resistance may require aerosol therapy and noninvasive positive pressure ventilation (NIPPV), the efficiency of different aerosol devices and masks during NIPPV is not well understood. The purpose of this study was to determine the efficiency of a jet nebulizer (JN), a vibrating mesh nebulizer (VMN) and a pressurized metered-dose inhaler (pmdi) and three different masks during NIPPV. Method: An in vitro lung model consisted of the upper airway of an adult teaching manikin with a collecting filter at the level of the bronchi attached to a passive test lung. NIPPV was administered via full face mask for the first experiment (AF531 oro-nasal) with an IPAP/EPAP of 20/5 cm H 2 O and a respiratory rate of 15 Breath per minute (BPM). Aerosol generators were placed between the leak in the circuit and the mask. Albuterol sulfate (2.5 mg/ 3 ml) was nebulized with the JN (Micromist) and the VMN (Aeroneb Solo). Four puffs (108 µg/puff) were administered with the pmdi (ProAir HFA) with a spacer (Aerovent) that first was placed in the recommended normal position (pmdi-n) with aerosol plume directed towards patient, and then in the reversed position (pmdi-r), with aerosol directed away from patient (n=3). In the second experiment, three masks were used 1) the Performax mask, 2) the AF531 oro-nasal mask, and 3) the Performa track mask. Performa track mask was tested with only Aeroneb solo while other masks were tested with both Aeroneb solo and NIVO VMNs. In both experiments, filters were eluted with 0.1 HCl and analyzed by a spectrophotometer at 276 nm. Residual volumes were determined gravimetrically.
7 Result: Descriptive statistics, one-way analysis of variance (ANOVA), and independent t tests were used. Statistical significance was set at p<0.05. During NIPPV, inhaled mass (IM) and inhaled mass percent (IM %) varied significantly (p=0.042 and p=0.028, respectively). Aerosol delivery with the JN was the lowest during NIPPV. The VMN has a significantly lower residual volume than the JN (p=0.0001). No statistical difference in efficiency was found between the two pmdi orientations (p=0.253). In the second experiment, oro nasal mask with Aeroneb Solo VMN results in the highest IM which was significant when compared with all other masks(p=0.0001). No statistical difference can be found between other masks. Conclusion: The JN was less efficient than the VMN and the pmdi in either orientation. The type of aerosol device used during NIPPV influenced aerosol delivery in this simulated adult lung model. Oro nasal mask with Aeroneb Solo VMN provided the highest IM.
8 THE EFFECT OF USING DIFFERENT AEROSOL DEVICES AND MASKS ON AEROSOL DEPOSITION DURING NONINVASIVE POSITIVE PRESSURE VENTILATION IN AN ADULT LUNG MODEL By Maher M. AlQuaimi A Thesis Presented in Partial Fulfillment of Requirements for the Degree of Masters of Science in Health Sciences in the Division of Respiratory Therapy in Byrdine F. Lewis School of Nursing and Health Professions Georgia State University Atlanta, Georgia 2011
9 Acknowledgments I thank God for his guidance and support throughout my life. God gave me the power and health to function and think. My father, you are the nondepletable motivator in my life. Thanks for all what you have done. Your words are being felt deep inside my heart. I would like to thank the Division of Respiratory Therapy for the use of their laboratory facilities while conducting the research for this thesis. Special thanks to Mr. Robert Harwood. I know I used a lot of your weekends, days offs, and travel time! Sorry for that. Hope it was worth it in the end! Special thanks to the smiley professor in the department. Dr. Bryant, your input and support were amazing. Having you in my committee was a special gift for me. I learned from you a lot, specifically how to take it easy. They say behind every great man is a great woman. Well, that is not exactly the case for me. I have three ladies who pushed me hard and helped me a lot with my thesis. Dr. Arzu Ari, was a teacher, an advisor, and a close friend who helped me wisely, made me think deeply, and taught me how to be a researcher. You are very supportive when needed, very flexible and hard worker with optimum accuracy. I thank my wife Amirah Alrasheed, who gave me love, support, and was patient with me during my extremely busy days. To my mother, the most wonderful and important person in my life, you taught how to overcome challenge, how to smile everyday, how to be passionate about something. My mom I can not than you in words, but I will try to make you proud of me. ii
10 TABLE OF CONTENTS Page No List of Tables... v List of Figures... vi List of Abbreviations... vii Chapter I Introduction... 1 Significance of The Study... 3 Purpose of The Study... 4 Research Questions of The Study... 4 Chapter II Review of Literature... 5 Bench Studies... 6 Clinical Studies... 8 Studies including in-vitro and in-vivo components Noninvasive Positive Pressure Ventilation Aerosol Generators Chapter III Methodology Lung Model First Experiment Aerosol Generators Type, Dose, Location, and Operation Second Experiment Masks Types Measurement of Aerosol deposition Data Analysis iii
11 Chapter IV Results Chapter V Discussion References iv
12 LIST OF TABLES Page No Table 1 Means and SDs of inhaled mass, inhaled mass percent and residual volume of each aerosol device used in this study Table 2 Inhaled Mass Among the Five VMN Mask Combinations v
13 LIST OF FIGURES Page No Figure 1. Lung model with the jet nebulizer Figure 2. Lung model with the pmdi Figure 3. Lung model with the vibrating mesh nebulizer Figure 4. Performax mask, AF531 oro-nasal mask, Performa track mask...23 Figure 5. Inhaled mass percentage differences between devices Figure 6. Residual volume between JN and VMN Figure 7. Inhaled mass percentage differences among different masks 28 vi
14 LIST OF ABBREVIATIONS ANOVA BiPAP BPM COPD CPAP EPAP analysis of variance bilevel positive airway pressure breaths per minute chronic obstructive pulmonary disease continuous positive airway pressure expiratory positive airway pressure FEF 25%-75% forced expiratory flow between 25 and 75% FiO 2 FVC JN HFA ICU IM IM% IPAP IPPB LPM MMAD NIPPV PaO 2 fraction of inspired oxygen forced vital capacity Jet nebulizer hydrofluoroalkane intensive care unit inhaled mass inhaled mass percentage inspiratory positive airway pressure intermittent positive pressure breathing liters per minute mass median aerosol diameter noninvasive positive pressure ventilation arterial oxygen partial pressure vii
15 SVN VMN small volume nebulizer vibrating mesh nebulizer viii
16 CHAPTER I INTRODUCTION Noninvasive positive pressure ventilation (NIPPV) can be delivered to a patient with an intact airway through a face mask or similar interface (Crummy & Naughton, 2007). By leaving the upper airway intact, NIPPV preserves airway defense mechanisms, and patients are able to eat and drink, speak, cough and expectorate secretions, and avoid specific complications associated with an endotracheal tube, such as ventilator-associated pneumonia, sinusitis, and tracheal stenosis (Crummy & Naughton, 2007). In addition, patients may not need to be on sedatives, analgesics, or paralytics to start or maintain NIPPV. In a 2003 meta-analysis involving eight studies with more than 600 patients, Lightowler and his colleagues showed that the use of NIPPV resulted in a highly significant reduction in mortality rate (relative risk 41%) and the need for intubation (relative risk 42%). They concluded that NIPPV is an effective intervention in the management of acute respiratory failure secondary to acute hypercapnic exacerbations of chronic obstructive pulmonary disease (COPD). NIPPV is a very cost-effective intervention, avoiding intubation and intensive care unit (ICU) admission, without prolonging hospital length of stay (Plant, Owen, Parrott, & Elliott, 2003). It also can be used safely on the general ward (Al-Mutairi & Al- Deen, 2004; Crummy & Naughton, 2007), which could avoid the need for ICU admission. Plant, Owen, Parrott, and Elliott (2003) have suggested that treatment with NIPPV may avoid between three and nine ICU admissions per year in a regional UK general hospital, resulting in a potential annual cost saving of up to (US$125,000). 1
17 During NIPPV, alveolar ventilation is improved by the application of positive pressure through a nasal mask, oral mask, face mask, full face mask, or hamlet, thereby avoiding the need for an endotracheal or tracheostomy tube to perform invasive ventilation. Inhaled aerosol therapy has gained widespread acceptance because of its advantages over other alternative routes for drug administration (Ari, Hess, Myers, & Rau, 2009). A medical aerosol is any suspension of liquid (nebulizer or pressurized metered-dose inhaler [pmdi]) or solid drug particles (pmdi or dry powder inhaler) in a carrier gas (Ari et al., 2009). Aerosol delivered through the inhalation route has been used widely to treat different diseases such as COPD, asthma, cystic fibrosis, and pneumonia. It has advantages over the systematic route, such as the ability to use smaller doses, a faster onset of the effects, the topical application of a drug to the lungs, and less systematic side effects. In addition, aerosol therapy is painless, and it often results in better clinical outcomes when compared with a much larger oral dose or even with a subcutaneous injection of the drug (Ari et al., 2009). Aerosolized medications include but are not limited to: long- and short-acting adrenergic bronchodilators, long- and shortacting anticholinergic bronchodilators, inhaled corticosteroid, xanthines, mucuscontrolling drugs, surfactant agents, mast cell stabilizing agents, antileukotriene agents, and anti-infective agents (Gardenhire, 2007). Short-acting beta agonists such as albuterol are the most common inhaled medication that is being used for NIPPV and aerosol research, and it is administered either by a pmdi or by a small volume nebulizer (SVN) (Branconnier & Hess, 2005; Mukhopadhyay, Dela Pena, Wadden, Procyshyn, & Keang Lim, 2009; Nava, Karakurt, Rampulla, Braschi, & Fanfulla, 2001). 2
18 Patients receiving NIPPV may require aerosol therapy for several reasons because NIPPV and aerosol are two modalities that are administered for similar diseases and conditions. COPD and asthma, for example, are two common pulmonary diseases with airflow limitation and may benefit from aerosol therapy, NIPPV, or both. Combining both modalities could result in better deposition and consequently better clinical improvement (Brandao et al., 2009; Nava et al., 2001; Pollack, Fleisch, & Dowsey, 1995) or at least fewer complications from discontinuation of mechanical support. In addition, providing this combination could save therapist time, effort, and cost. Unfortunately, there is a lack of in vivo and in vitro research regarding use of aerosol therapy in patients receiving NIPPV. Significance of The Study More research is needed to understand the efficacy of aerosol therapy in conjunction with NIPPV. To improve aerosol delivery during NIPPV, the physical and physiological aspects of this type of therapy during NIPPV need to be studied. Additionally, research thus far has not definitively determined which device should be used to optimize aerosol deposition. Clearly, more studies are needed to establish which mask achieves the highest possible aerosol deposition. In addition, no published study has compared the JN, the pmdi, and the VMN. Also, there no study in the literature evaluating the efficiency of the HFA format of pmdi during NIPPV. The efficiency of different masks used during NIPPV is also unknown. No study thus far has looked at the efficiency of the new VMN (NIVO) that is designed specifically for NIPPV. 3
19 Purpose of The Study The purpose of this study was first to determine the efficiency of a JN versus a vibrating mesh nebulizer versus a pressurized metered-dose inhaler, and second to compare the efficiency of the Performax mask, the AF531 oro-nasal mask, and the Performa track mask during NIPPV using two different VMNs. Research Questions of The Study The research questions for this study include the following: 1) What is the efficiency of JN, VMN, and pmdi during NIPPV? 2) What is the difference in aerosol deposition among three different masks during NIPPV? 4
20 CHAPTER II REVIEW OF LITERATURE This literature review examines articles published in the area of aerosol delivery during NIPPV. The search terms used to gather studies for this review included the following: nebulizer, pmdi, SVN, mesh, bronchodilator, nebulization, inhalation therapy, metered-dose inhaler, small volume nebulizer, inhalers, aerosol therapy, noninvasive positive pressure ventilation, mask ventilation, positive pressure respiration, continuous positive airway pressure, positive airway pressure, bilevel positive airway pressure, and noninvasive ventilation. These search terms were utilized together to find articles in different databases such as Cochrane Reviews, Sciencedirect, Web of Science, the Cumulative Index to Nursing and Allied Health Literature, and MEDLINE (pubmed). Relevant articles are presented in the following five sections: (1) bench model studies, (2) clinical studies, (3) studies that include both a bench model and a clinical aspect, (4) NIPPV, and (5) aerosol generators. In vitro studies are included in the bench model section only, and in vivo studies are included only in the clinical section. If the same study includes both in vivo and in vitro components, it is presented in the section entitled studies including in-vitro and in-vivo components. 5
21 Bench Studies Chatmongkolchart, Schettino, Dillman, Kacmarek, and Hess s (2002) in vitro study evaluated the effects of ventilator settings and nebulizer position on aerosol bronchodilator delivery during NIPPV. Their model consisted of 1) a double-chamber lung model, 2) a mechanical ventilator, 3) a bilevel positive airway pressure (BiPAP) machine (Respironics, Murrysville, PA), and 4) a small volume nebulizer (SVN) (Micromist, Hudson RCI, Temecula, CA), which was connected either proximally or distally to the BiPAP. Albuterol delivery with BiPAP was evaluated at a respiratory rate of 10 BPM and 20 BPM at inspiratory positive airway pressures and expiratory positive airway pressures (IPAP/EPAP) of 10/5, 15/5, 20/5, 15/10, 20/10, and 25/10 cm H 2 O, respectively. The albuterol delivery from the SVN with the BiPAP ventilator ranged from 5.2 ± 0.4 to 24.5 ±1.3% of the nominal albuterol dose. They reported that albuterol delivery was significantly affected by the BiPAP settings, the nebulizer position, and the respiratory rate. They detected the greatest albuterol delivery when the nebulizer was connected at the distal position with a respiratory rate of 20 BPM. Under these conditions, as the inspiratory pressure levels increased, so did the albuterol delivery. In addition, increasing expiratory pressure levels decreased albuterol delivery. Branconnier and Hess (2005) evaluated the effect of two aerosol delivery devices and the leak port position on albuterol delivery during NIPPV. The authors used a SVN (Micromist, Hudson RCI, Temecula, CA) and a BiPAP machine (BiPAP S/T30, Respironics, Murrysville, PA). They reported that the location of the leak port, the type of aerosol delivery device, and the timing of actuation of the pmdi have an impact on albuterol delivery with NIPPV. They reported three main findings from this study. First, 6
22 higher aerosolized bronchodilator delivery was detected when the leak port was in the circuit rather than the mask. Second, the aerosolized bronchodilator delivery using the pmdi with a spacer and the nebulizer was similar when the leak port was in the circuit, but when the leak port was in the mask, delivery was greater with the pmdi. Finally, the researchers detected a significant reduction in aerosolized bronchodilator delivery when the pmdi was actuated during the expiratory phase. Aerosol deposition percentage with both aerosol devices was about 9% when using a spectrum mask (Respironics, Murrysville, PA). When the pmdi was used with a mirage mask (resmed, Poway, CA) it was also 9%; however, it dropped to approximately 4% when an SVN was used with the mirage mask. Calvert et al. (2006) investigated the differences in inhaled salbutamol during NIPPV at three different aerosol location compared with during spontaneous breathing( without circuit). The authors utilized a Pari COMPAS breath simulator (Pari GmbH, Starnberg, Germany) to model a patient with a sinusoidal breathing pattern. A bilevel ventilator (Knightstar 335, Mallincroft, UK) was connected to the breath simulator via a 187-cm length NIPPV corrugated circuit. The bilevel ventilator was set on spontaneous mode with an IPAP/EPAP of 20/5. Five mg of salbutamol with 2.5 ml normal saline was nebulized for 5 minutes by a JN (Cirrus, Intersurgical Ltd., Wokingham, UK) before the expiration port (position A), after the expiration port (position B) or pre-ventilator ( position C). The authors concluded that IM was the highest at position B (647±76 µg) which was slightly more than position B (544±85µg). IM at position C was (267±26µg) which was less than IM (424±61 µg) during spontaneous. Significance can be seen only between position B and C and between B and spontaneous. 7
23 Abdelrahim, Plant, and Chrystyn (2010) conducted an in vitro study to detect the differences in IM when delivered during NIPPV at two different positions and via two different nebulizers. The authors contrasted an Aeroneb Professional (Aerogen Inc., Ireland) and a sidestream JN. The positions were either before expiratory port (position A) or after expiratory port (position B). The researchers utilized a breathing simulator machine (Compass; Pari GmbH, Germany) that was connected to a bilevel ventilator (Nippy2; B&D Electromedical, UK) via a 180-cm length of corrugated circuit with a fixed leak port. They found that the deposition was highest with the Aeroneb at position A ( ±150.9 µg) and second highest with the sidestream at position A ( ±1613µg). Clinical Studies Pollack et al. (1995) conducted a randomized prospective clinical study on 100 afebrile, wheezing patients between the ages of 18 and 40 to find out whether betaadrenergic agonist aerosol is more effective in improving acute bronchospasm if delivered by nasal BiPAP (BiPAP S/T ventilator, Respironics) with an in-line SVN than by an SVN alone. The patients were randomly assigned to receive two doses of aerosolized albuterol via either an SVN alone or an SVN during BiPAP (either nose mask or face mask). In the SVN BiPAP group, aerosol was delivered through the circuit of the BiPAP with an IPAP of 10 cm H 2 O and an EPAP of 5 cm H 2 O. Arterial blood oxygen saturation (SpO 2 ), peak expiratory flow rate, heart rate, and respiratory rate were measured before and after each treatment. The researchers found no differences in any of the measures between the two groups except for the percentage of the predicted peak 8
24 expiratory flow rate, which was significantly higher in the SVN BiPAP group after each treatment (p=.0011) and from their baseline to their final treatment (p=.0013). Nava et al. (2001) conducted a randomized control study on 18 stable patients with COPD to evaluate the feasibility and efficacy of delivery of inhaled salbutamol solution via an SVN with intermittent positive pressure breathing (IPPB) (PR II Respiratory Unit, Puritan Bennett), a pmdi with a spacer (Volumatic, Allen & Hanburys, Greenford, UK) in a spontaneous breathing situation, or a pmdi during NIPPV (Helia, Saime, Savigny Le Temple, France). Patients were randomly assigned to receive one of four possible modalities of treatment for four consecutive days: 1) placebo via a pmdi with a spacer; 2) 400 µg of salbutamol via a pmdi with a spacer during spontaneous breathing; 3) 400 µg of salbutamol via a spacer fitted into the inspiratory limb of the circuit, directly after the wye of the circuit, with use of a pmdi and NIPPV; 4) 5 mg of salbutamol with 5 ml of saline solution delivered by IPPB. The NIPPV settings were as follows: pressure support of 14.3±1.8 cm H 2 O, a tidal volume guarantee of 10 ml/kg, and inspiratory trigger of 0.5 cm H 2 O, which were delivered by a full face mask. The authors found that salbutamol delivery during NIPPV is feasible and effective, and showed significant bronchodilation, which was confirmed by the measurement of forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) compared with the placebo group; however, the delivery using pmdis with a spacer resulted in the best bronchodilation effect. França et al. (2006) conducted a clinical study on 13 healthy subjects to evaluate pulmonary radioaerosol deposition by a JN during NIPPV (BiPAP synchrony Respironics, Inc.; Murrysville, PA) with an IPAP setting of 12 cm H 2 O and an EPAP 9
25 setting of 5 cm H 2 O compared with spontaneous breathing nebulization. The radioaerosol used with both groups was Tc99m-DTPA (25 mci), which was nebulized by a JN (ST3; NS; Sao Paulo, Brazil). Immediately after nebulization, images were taken of the patients pulmonary fields using a scintigraphy camera. França et al. compared the pulmonary fields of each group. They reported that there was less radioaerosol deposition during NIPPV compared with spontaneous breathing nebulization (p<0.001), and they found a significant correlation between tidal volume and radioaerosol deposition. Reychler et al. (2007) conducted a clinical study on six healthy subjects to compare lung deposition of amikacin measured indirectly by urinary excretion nebulized by a JN (sidestream; Medic-aid; West Sussex) with or without continuous positive airway pressure (CPAP) (Boussignac; Vygon; Belgium) at a pressure of 6 cm H 2 O. Patients underwent two nebulization sessions (one during CPAP and one spontaneous) in a random order with a one-week washout period between the sessions. After one day of nebulization, urine was collected at each spontaneous urination. The authors found that urine excretion of amikacin in the CPAP group was significantly lower (1.97% initial dose versus 4.88 initial dose, p<0.001) compared with those who received nebulization without CPAP. Brandao et al. (2009) conducted a randomized controlled study on 36 patients with severe asthma to compare the effect of jet nebulization (ST3; NS; Sao Paulo, Brazil) during spontaneous breathing with nebulization during NIPPV (BiPAP synchrony Respironics, Inc.; Murrysville, PA). The pressure settings for NIPPV were an IPAP of 15 cm H 2 O and an EPAP of 5 cm H 2 O, or an IPAP of 15 cm H 2 O and an EPAP of 10 cm H 2 O. Respiratory rate, heart rate, S P O 2, peak expiratory flow, FEV 1, FVC, and forced 10
26 expiratory flow between 25 and 75% (FEF 25%-75% ) were measured before and 30 minutes after each treatment. The authors stated that nebulization during NIPPV resulted in significant improvement in peak expiratory flow, FVC, FEV 1, and FEF 25%-75% compared with jet nebulization alone. This improvement was better when the delta pressure was smaller and the EPAP was higher. The researchers suggested that this result could be explained by the higher possibility of laminar flow when delta pressure is smaller. Studies including in-vitro and in-vivo components Parkes and Bersten (1997) conducted in vitro study followed by a clinical study on nine stable asthmatic subjects to investigate the effects of CPAP on aerosol kinetics and bronchodilator efficacy. In their bench model, spontaneous breathing was simulated by using two compartments connected by a bar. The compartments simulated the respiratory muscle and the lung, which was attached to a circuit and a face mask, connected to a ventilator. The ventilator settings were either a fixed tidal volume of 500 ml with a varied inspiratory flow, frequency, and minute volume or a fixed minute volume of 10 liter per minute (LPM) with a varied tidal volume. CPAP was set at a pressure of 10 cm H 2 O with a flow of 50 LPM. Technetium with 3 ml of saline was nebulized with a flow rate of 6 LPM and connected proximally to the mask and the CPAP machine. In this clinical study all subjects underwent nebulization during CPAP and nebulization only. Salbutamol was given at incremental doses of 250 µg, 250 µg, 500 µg, and 1000 µg at 30-minute intervals. For the nebulizer (Bird Micronebuliser, Bird, CA) group, the same doses were given but without the CPAP. FEV1 was measured after 5 and 30 minutes of nebulization. The researchers found that CPAP resulted in a lower IM%, from 6.85± 1.52 % in the control group to 1.3±0.37% in the CPAP group. In the clinical 11
27 study, CPAP did not result in a significant change in the bronchodilation response measured by FEV1 when compared to the control group. Smedsaas-Löfvenberg, Nilsson, Moa, and Axelsson (1999) conducted an in vitro study followed by a clinical study on five infants to develop and evaluate a safe method to provide nebulization during CPAP. The bench model was conducted by modifying a nasal CPAP system (Infant Flow ; EME, Brighton, UK) to add the ability to deliver nebulization (Aiolos AB, Karlstad, Sweden). The particle size was measured using a Malvern Laser 2600 at a pressure of bars (corresponding to a gas flow of LPM-1). The mass median aerosol diameter (MMAD) of the surfactant was µm, which is very similar to the MMAD for ribavirin generated by the Small Particle Aerosol Generator Model-2. The same model was used to deliver ribavirin to treat five infants with a respiratory syncytial virus infection. All patients made a complete recovery with minimal complications. Fauroux et al. (2000) conducted in vitro and in vivo studies to evaluate the efficacy of NIPPV (Onyx; Mallinckrodt, Les Ulis, France) in optimizing IM. In the in vitro study, the researchers compared the performance of two actuated nebulizers, of which one was patient-triggered (Optined; Air Liquid Santé, Paris, France) and the other was positive pressure triggered (Optiplus; Air Liquid Santé). They reported that the MMAD for both nebulizers was very similar (3.21±0.13 and 3.16 ± 0.02, respectively). In the in vivo study, 18 clinically stable cystic fibrosis patients underwent one control session (nebulization alone) and one session of nebulization during NIPPV. Aerosol deposition, which was estimated by radioactive imaging, was significantly higher in the nebulization-during-nippv sessions (15.3 ± 8.3 compared with 11.5 ± 5.7, respectively). 12
28 The researchers concluded that adding NIPPV to nebulization could enhance total IM without impacting the regional deposition. Noninvasive Positive Pressure Ventilation Criner, Travaline, Brennan, and Kreimer (1994) conducted a clinical study on nine patients with chronic respiratory failure to evaluate the efficacy of using a full face mask during NIPPV. The researchers utilized three different types of masks: 1) a full face mask (Total, Respironics, Monroeville, PA), 2) a nasal CPAP mask (Respironics), and 3) a nasal/oral mask (Vitalog, Vital Signs). Patients were assigned to receive NIPPV for 20 to 30 minutes via different masks in a random order. In addition, multiple tests were performed on patients before initiating NIPPV and after each session of NIPPV. All masks resulted in a significant improvement in gas exchange, with the best results attained using the full face mask. However, dyspnea, discomfort, and leakage were significant (p<0.05) when the full face mask was used. Plant, Own, and Elliott (2001) conducted a prospective, multicenter, randomized, controlled study on 236 patients with acute exacerbation of COPD. The researchers had two aims. First, they hoped to determine the variables that could be used to divide patients according to their risk of needing invasive mechanical ventilation. The second aim was to ascertain long-term mortality rates associated with using or not using NIPPV. Patients were randomized to receive either NIPPV or a standard treatment. Arterial blood gases and respiratory rate were recorded at admission, after one hour, and after four hours. In the standard treatment group, 27% of the patients required intubation compared to only 15.3% of the NIPPV group (p<0.02). The in-hospital mortality rate was 20% for the standard group and 10% for the NIPPV group. The authors recommended the use of 13
29 NIPPV as a first line of intervention in addition to standard medical care to manage acute exacerbation of COPD. Miyoshi, Fujino, Uchiyama, Mashimo, and Nishimura (2005) studied the effects of the gas leak that commonly occurs during NIPPV on triggering function, humidification, and fraction of inspired oxygen (FiO 2 ) in bilevel pressure ventilators and ICU ventilators. The authors evaluated two ICU ventilators (Puritan Bennett 7200ae and Puritan Bennett 840; Tyco Healthcare; Mansfield, MA) and two bilevel pressure ventilators (BiPAP S/T-D and BiPAP vision; Respironics; Murrysville, PA). The researchers set the machines to an IPAP of 15, an EPAP of 5 cm H 2 O, and an FIO 2 of A leak was created in the airway opening of the lung model with several holes sizes to allow gas to leak from 1.1 to 44.2 LPM. They reported that bilevel pressure ventilators were able to compensate for all leak sizes adequately, whereas ICU ventilators were not able to do so with large leaks, which resulted in uncontrolled triggering. The authors were able to predict the FiO 2 when the leak was small but were unable to predict it when the leak was large (>15 LPM). Gas leaks caused minimal changes in humidification. Moerer et al. (2006) conducted an in vitro study to evaluate two types of interface for NIPPV, comparing them with invasive ventilation. The following interfaces were used by the authors: 1) a helmet (Starmed Castar R; Mirandola; Modena, Italy) and 2) a face mask (King Systems Corporation; Noblesville, IN). For the invasive ventilation, the model was connected to an endotracheal tube (Portex, 7.5 mm; Portex, Ltd.; Kent, UK). All systems were evaluated in terms of delay times, pressure time products, and wasted effort during inspiration, defined as the failure of the ventilator-driven chamber to activate the passively driven lung chamber. They reported that delay time was 14
30 significantly longer (p<0.001) when the helmet was used (144 to 174 ms) compared to invasive ventilation (66 to 76 ms) or the use of the face mask (70 to 74 ms); however, there were no significant differences in mean pressure time products among all the systems. The frequency of wasted efforts (failure of the ventilator-driven chamber to activate the passively driven lung chamber) increased with higher pressure support, higher respiratory rate, lower sensitivity, higher compliance, and the helmet interface. Conti et al. s (2007) clinical study compared the efficacy of NIPPV delivered by helmet (CaStar, Starmed, Mirandola, Italy) versus face mask (Vital Signs, Totowa, NJ, or Dar-Tyco, Mirandola, Italy) in 50 patients with acute respiratory failure after abdominal surgery. The control group of 25 patients was selected from a historical group based on numerous factors to match with the intervention group. The matching was based on age, simplified acute physiology score II, and the ratio of arterial oxygen partial pressure to the fraction of inspired oxygen (PaO 2 /FiO 2 ). The ventilator (300, Siemens, Uppsala, Sweden) was started at IPAP of 10 cm H 2 O with an incremental increase to achieve patient comfort of less than 25 BPM. EPAP was increased up to 12 cm H 2 O in order to maintain SpO 2 over 90% with the minimal FiO 2 possible. The authors reported that mask intolerance, major leaks, or ventilator-associated pneumonia were significantly higher in the face mask group (p<0.03). Roy, Cordova, Travaline, D Alonzo, and Criner (2007) conducted a clinical study on 10 nonambulatory patients with acute respiratory failure to determine the effectiveness of a total face mask in the application of NIPPV. None of the patients were able to tolerate NIPPV via nasal mask (Respironics, Inc., Monroeville, PA) or oro-nasal mask (Respironics, Inc., Monroeville, PA); however, eight out of the 10 patients were managed 15
31 successfully when NIPPV was delivered via a total face mask (Respironics, Inc., Monroeville, PA), and the other two required endotracheal intubation due to excessive secretions. Patients were ventilated by NIPPV (BiPAP, Respironics, Inc., Monroeville, PA) with an initial IPAP of 21 cm H 2 O and EPAP of 3 cm H 2 O with an average delta pressure of 18±4 cm H 2 O. The authors suggested using a total face mask with patients who could not tolerate NIPPV with a nasal or oro-nasal mask. Aerosol Generators Deerojanawong et al. (2005) conducted a double-blind, randomized controlled trial on 47 young children with wheezing to compare the efficacy of salbutamol aerosol therapy delivered by either a pmdi or a JN. Each patient was given either a placebo pmdi followed by a jet nebulization treatment or a placebo jet nebulization followed by a pmdi. Clinical outcomes were quantified by measuring each patient s heart rate, respiratory rate, clinical scores, SpO 2, and pulmonary function before and 30 minutes after each treatment. The authors concluded that there was no statistical difference between the groups in terms of clinical outcomes; however, heart rate was significantly higher in the jet nebulization group when compared with the pmdi group. Pitance et al. (2010) conducted both in vitro and in vivo studies to compare the IM and urinary drug concentration of amikacin in three aerosol delivery systems: a standard JN (sidestream, Philips-Respironics, Pittsburgh, PA) with and without a 110-ml corrugated piece of tubing and a VMN (e-flow Rapid, PARI, Pharma GmbH, Munich, Germany). All systems were assessed for particle size distribution using a Malvern Mastersizer-X laser particle sizer (Malvern Instruments Ltd., Worcestershire, UK) during the in vitro study; however, during the in vivo study the IM was measured indirectly by 16
32 the urinary excretion of amikacin. The authors reported that during the in vitro portion of the study, the IM% of the nominal dose was approximately 40% for the mesh nebulizer, 25% for the JN with the corrugated tube, and 15% for the JN alone; however, there was no significant difference in MMAD among all three systems. The daily percentage of amikacin from the initial dose was 11.6% with the mesh nebulizer, 8.2% with the JN with the corrugated tube, and 6.15% with the JN alone. Both results suggest that the mesh nebulizer was able to deliver the highest amount of aerosol mass among the three systems. Skaria and Smaldone (2010) conducted an in vitro study comparing IM, residual activity, MMAD, and run time (which is the time required to provide the nebulization by the system) with a radiolabeled albuterol for four aerosol systems. The systems compared in this study included a sidestream JN (Philips/Respironics, Parsippany, NJ), a breathenhanced nebulizer (Pari LC Plus, Pari Respiratory Equipment, Midlothian, VA), and a mesh nebulizer (Omron Healthcare, Inc., Bannockburn, IL), which was tested in both the tilted and the horizontal positions. All systems were ventilated with a Harvard pump and set to simulate a COPD patient with a tidal volume of 450 ml, a respiratory rate of 15 BPM, and a duty cycle of Particle distribution was quantified by using low flow cascade impaction. The authors found that the IM by the Omron and Pari were comparable (20% of the nominal dose) and higher than the sidestream (10%). However, MMADs were comparable for all systems ( )µm, but variability ( ) µm was higher for the Omron in the horizontal position. Residual activates for the Omron in the tilted position were 25% CI (22 32) and 22% CI (18 25) in the horizontal position. Residual activates for the Pari LC were 57% CI(55-60) and 47% CI(45-49) for the 17
33 sidestream. The run time was significantly lower for the Omron in both positions compared with the JNs; however, it was about three times greater in the tilted position. In an in vitro study, Ari, Areabi, and Fink (2010) compared the performance of four aerosol generators at three different locations in humidified and nonhumidified circuits during adult mechanical ventilation. They examined four aerosol generators a JN, a VMN, an ultrasonic nebulizer, and a pmdi with a spacer at three different locations: 1) between the endotracheal tube and the Y-piece, 2) 15 cm from the Y-piece, and 3) 15 cm from the ventilator. The ventilator settings were a tidal volume of 500 ml, a ramp flow pattern, 15 BPM, a peak inspiratory flow of 60 LPM, and a positive end expiratory pressure of 5 cm H 2 O. The drug deposition was collected by an absolute filter distal to an 8.0-mm endotracheal tube and was measured by spectrophotometry (276 mm). The VMN was able to deliver 30.2% ±1.0, which was the highest IM% throughout the study, at location 2 with a nonhumidified circuit; in contrast, the pmdi delivered the least IM% 2.5% ± 0.8 at location 3 with a heated circuit. The authors reported that the humidified circuit had the overall effect of decreasing IM in all positions and with all generators. Ari, Atalay, et al. (2010) conducted an in vitro study to determine the impact of nebulizer type, position on the ventilator circuit, and bias flow on aerosol delivery in simulated and mechanically ventilated pediatric and adult lung models. The study used a JN and a VMN. The JN was set at 15 cm proximal to the wye (position 1) or 15 cm prior to the heated humidifier (position 2). The VMN was attached directly to the wye, at the inlet of the humidifier. Bias flow was either 2 liters per minute (LPM) or 5 LPM. The highest IM%, 23.8% ±1.0, was attained with the adult lung model at position 2 with a 2 18
34 LPM bias flow, delivered by VMN; in contrast, the lowest IM%, 3.8 %± 0.3, was measured using the pediatric lung model at position 1 with a 5 LPM bias flow delivered by JN. In all conditions, the IM was two- to four folds greater when using the VMN. 19
35 CHAPTER III METHODOLOGY Lung Model This model consists of a BiPAP ventilator (BiPAP S/T30, Respironics, Murrysville, PA) and a single-limb NIPPV circuit (Philips, Respironics, PA). In this invitro model, a breathing adult was simulated. The BiPAP machine was set on the spontaneous/time mode with a respiratory rate of 15 BPM and an inspiratory time of 1.0 second, with pressures settings of IPAP/ EPAP 20/5 cm H 2 O. The single-limb NIPPV circuit was connected between the BiPAP machine and the mask, which was tightly strapped to the head of the manikin. Each aerosol generator that was used in this study was attached between the mask and the single-limb circuit. First Experiment Aerosol Generators Type, Dose, Location, and Operation Three different aerosol generators were investigated in this study: 1) a JN (Micro Mist, Hudson RCI, Temecula, CA), 2) a pmdi (ProAir HFA, Teva Specialty Pharmaceuticals, Atlanta, GA) with a spacer, and 3) a VMN (Aeroneb Solo, Aerogen, Mountain View, CA). All generators were placed between the AF531 oro-nasal mask (Respironics, Inc., Murrysville, PA) and the circuit. The JN was pressurized with 8 LPM O 2 and a 2.5 mg/3 ml unit dose of albuterol (Nephron Pharmaceuticals, Orlando, FL) and connected at the vertical position (Figure 1). Nebulization continued until sputter. The pmdi was shaken and primed, then connected at the same location (Figure 2). The pmdi actuation synchronized at the beginning of the inspiratory cycle for a total of four actuations, separated by at least 15 seconds. The pmdi was connected to a spacer either 20
36 at the normal position (recommended by the company) which was abbreviated as pmdi- N or the reversed position (pmdi-r). The VMN was connected at the same location, and a 2.5 mg/3 ml unit dose was placed into the nebulizer (Figure 3). The nebulizer was run continuously until sputter. Figure 1. Lung model with the jet nebulizer. 21
37 Figure 2. Lung model with the pmdi. Figure 3. Lung model with the vibrating mesh nebulizer. 22
38 Second Experiment Masks Types The same lung model was reassembled for the second experiment. Two different VMNs were used: an Aeroneb Solo (Aerogen, Mountain View, CA) and a NIVO (Phillips Respironics, Ireland). Three masks were used: 1) a Performax mask (Respironics, Inc., Murrysville, PA), 2) an AF531 oro-nasal mask (Respironics, Inc., Murrysville, PA), and 3) a Performa track mask (Respironics, Inc., Murrysville, PA). The Performax mask was investigated with both mesh nebulizers (Aeroneb Solo and NIVO). The Performa track mask was only tested with the Aeroneb Solo due to the inability to connect with the NIVO. Figure 4. From left to right, Performax mask, AF531 oro-nasal mask, Performa track mask Measurement of Aerosol deposition An absolute filter (Respirgard II, 303, Vital Signs, Totowa, NY) was attached and placed at the wye connection, where two simulated bronchi from the manikin meet. After each test was completed, the filter was shaken for at least one minute after being washed with 0.1 molar N HCl. The concentration of albuterol from the filter was measured via a spectrophotometer (Beckman Instruments, Fullerton, CA). 23
39 Data Analysis Albuterol deposition was quantified and reported as a percentage of the nominal dose. Data analysis and graphs were created using Statistical Package for the Social Sciences (IBM SPSS, 18.0, Armonk, NY) and Microsoft office Excel (2007,Redmond, WA). Means and standard deviations were calculated for the three generators. A oneway ANOVA test was used not only to compare the means of albuterol deposition of the three generators on the first experiment, but also to compare means of albuterol depositions and residual volume among the three different masks on the second experiment. An independent t-test was used to compare residual volume in the VMN and the JN on the first experiment and to compare the Aeroneb Solo and the NIVO in the second experiment. A significance level of 0.05 was used for all comparisons. 24
40 CHAPTER IV RESULTS In the first experiment, descriptive statistics were computed for IM, IM%, and residual volume (Table 1). In addition, inferential statistics were used to compare the means and to identify the significance levels IM, IM%, and residual volume. The ANOVA comparing the means of IM during NIPPV varied significantly (p<0.05): the JN and the VMN resulted in a significance level of p=0.005; the JN and the pmdi in the reversed position (pmdi-r) had a significance level of p=0.002; the JN and the pmdi in the normal position (pmdi-n) was significant at the p=0.001 level; the VMN and the pmdi-r resulted in a significance level of p=0.002; and the VMN and the pmdi (in both positions) had a significance level of p= The difference between the pmdi-n and the pmdi-r was not significant (p=0.253). Of the delivery devices tested, the VMN produced the highest IM (0.72 mg ± 0.48 mg) with the highest IM% (28.83% ± 1.93%). In addition, the VMN had a significantly lower residual volume than the JN (p=.000). The mean residual volume of the VMN was 0.10 ml ± 0.069, whereas the mean residual volume of the JN was ml 1.65 ±
41 Table 1 Means and SDs of inhaled mass, inhaled mass percent and residual volume of each aerosol device used in this study. JN: jet nebulizer VMN: vibrating mesh nebulizer, pmdi-n= pmdi normal position, pmdi-r: pmdi retrograde position JN VMN pmdi-n pmdi-r Inhaled Mass (mg) 0.33 ± ± ± ± 0.01 Inhaled Mass Percent (%) ± ± ± ± 0.32 Residual volume (g) 1.65 ± ± 0.07 * * * * Figure 5. Inhaled mass percentage differences between devices. * p<
42 * Figure 6. Residual volume between JN and VMN. * p<0.05 In the second experiment, results indicate that aerosol delivery during NIPPV with the three different masks and the two different VMNs varied significantly (p<0.05). However, the only statistically significant relations are between the oro-nasal mask Aeroneb Solo VMN setup and each of the other setups. For example, the oro-nasal mask Aeroneb Solo setup compared with the Performax mask Aeoneb Solo was significant at the p= level. This same setup had the following results when compared with other combinations: the oro-nasal mask NIVO VMN (p=0.023), the Performax mask NIVO VMN (p=0.001), and the Performa track mask Aeroneb Solo VMN (p=.000). All other relations were not significant. In conclusion, the findings of this study showed that VMN was the most efficient aerosol generator with the lowest residual volume. However, pmdi could be an attractive alternative to VMN since it provide a close IM%. Oro-nasal mask with Aeroneb solo 27
43 provided the highest aerosol deposition and may be consider as a first option for aerosol therapy during NIPPV. Other masks did not differ statistically. Therefore, the mask selection should be based on patient s need and comfort level, and mask availability.. Table 2 Inhaled Mass and Inhaled Mass Percent Among the Five VMN Mask Combinations Performax Performax Oro-nasal Oro-nasal Performa mask NIVO mask mask mask track mask VMN Aeroneb Solo NIVO VMN Aeroneb Solo Solo VMN VMN VMN Inhaled mass 0.49 ± ± ± ± ±0.062 Inhaled mass % 20 ± ± ±.70 29± ±2.46 * * * * Figure 7. Inhaled mass percentage differences among different masks. * p<
600 RESPIRATORY CARE MAY 2016 VOL 61 NO 5
 Quantifying Aerosol Delivery in Simulated Spontaneously Breathing Patients With Tracheostomy Using Different Humidification Systems With or Without Exhaled Humidity Arzu Ari PhD RRT PT CPFT FAARC, Robert
Quantifying Aerosol Delivery in Simulated Spontaneously Breathing Patients With Tracheostomy Using Different Humidification Systems With or Without Exhaled Humidity Arzu Ari PhD RRT PT CPFT FAARC, Robert
NIV and Aerosoltherapy
 NIV and Aerosoltherapy Workshop Jean-Bernard Michotte (Lausanne-CH) Simone Gambazza (Milano-IT) Jean-Bernard Michotte Haute Ecole de Santé Vaud, 1011 Lausanne - Suisse Cliniques Universitaires Saint-Luc,
NIV and Aerosoltherapy Workshop Jean-Bernard Michotte (Lausanne-CH) Simone Gambazza (Milano-IT) Jean-Bernard Michotte Haute Ecole de Santé Vaud, 1011 Lausanne - Suisse Cliniques Universitaires Saint-Luc,
Pediatrics in mechanical ventilation
 Pediatrics Optimization Intitulé du cours of aerosol therapy in mechanical ventilation Thèmes donnés Ermindo Di Paolo, PhD Departments of Pharmacy and Pediatrics Lausanne University Hospital Switzerland
Pediatrics Optimization Intitulé du cours of aerosol therapy in mechanical ventilation Thèmes donnés Ermindo Di Paolo, PhD Departments of Pharmacy and Pediatrics Lausanne University Hospital Switzerland
The Impact of Aerosol Devices and Delivery Interfaces on Aerosol Deposition in Children Receiving Noninvasive Ventilation
 Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy Spring 4-4-2018 The Impact of Aerosol Devices and Delivery Interfaces on Aerosol
Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy Spring 4-4-2018 The Impact of Aerosol Devices and Delivery Interfaces on Aerosol
Rush University, College of Health Sciences
 Rush University, College of Health Sciences An Evaluation of the Prototype OxyMulti Mask Prototype Compared to the Oxymask Aerosol, OxyMulti Mask and Airlife Aerosol Mask for Aerosol Delivery in Adults
Rush University, College of Health Sciences An Evaluation of the Prototype OxyMulti Mask Prototype Compared to the Oxymask Aerosol, OxyMulti Mask and Airlife Aerosol Mask for Aerosol Delivery in Adults
What is the next best step?
 Noninvasive Ventilation William Janssen, M.D. Assistant Professor of Medicine National Jewish Health University of Colorado Denver Health Sciences Center What is the next best step? 65 year old female
Noninvasive Ventilation William Janssen, M.D. Assistant Professor of Medicine National Jewish Health University of Colorado Denver Health Sciences Center What is the next best step? 65 year old female
Effects of Heat and Moisture Exchangers and Exhaled Humidity on Aerosol Deposition in a Simulated Ventilator-Dependent Adult Lung Model
 Effects of Heat and Moisture Exchangers and Exhaled Humidity on Aerosol Deposition in a Simulated Ventilator-Dependent Adult Lung Model Arzu Ari PhD RRT PT CPFT FAARC, Khalid S Alwadeai MSc RRT-NPS, and
Effects of Heat and Moisture Exchangers and Exhaled Humidity on Aerosol Deposition in a Simulated Ventilator-Dependent Adult Lung Model Arzu Ari PhD RRT PT CPFT FAARC, Khalid S Alwadeai MSc RRT-NPS, and
Effective Aerosol Delivery in Acute Care
 Effective Aerosol Delivery in Acute Care Jim Fink, RRT, PhD, FAARC, FCCP Chief Science Officer, Aerogen Pharma Corp., San Mateo, CA USA Visiting Professor, Dept of Physical therapy, Universidade Federal
Effective Aerosol Delivery in Acute Care Jim Fink, RRT, PhD, FAARC, FCCP Chief Science Officer, Aerogen Pharma Corp., San Mateo, CA USA Visiting Professor, Dept of Physical therapy, Universidade Federal
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI)
 I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
Factors affecting bronchodilator delivery in mechanically ventilated adults
 LITERATURE REVIEW Factors affecting bronchodilator delivery in mechanically ventilated adults Arzu Ari and James B Fink ABSTRACT Background: Bronchodilators are increasingly being used in patients undergoing
LITERATURE REVIEW Factors affecting bronchodilator delivery in mechanically ventilated adults Arzu Ari and James B Fink ABSTRACT Background: Bronchodilators are increasingly being used in patients undergoing
Misty Max 10 nebulizer
 AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
Dolci U 1, Sidler-Moix AL 1, Di Paolo ER 1,Berger- Gryllaki 1 M, Pannatier A 1, Cotting J 2
 SALBUTAMOL DELIVERY IN AN IN VITRO PAEDIATRIC VENTILATOR-LUNG MODEL: COMPARISON OF JET, ULTRASONIC AND MESH NEBULISERS Dolci U 1, Sidler-Moix AL 1, Di Paolo ER 1,Berger- Gryllaki 1 M, Pannatier A 1, Cotting
SALBUTAMOL DELIVERY IN AN IN VITRO PAEDIATRIC VENTILATOR-LUNG MODEL: COMPARISON OF JET, ULTRASONIC AND MESH NEBULISERS Dolci U 1, Sidler-Moix AL 1, Di Paolo ER 1,Berger- Gryllaki 1 M, Pannatier A 1, Cotting
The Effects of Aerosol Drug Delivery on Airway Resistance through Heat-Moisutre Exchangers
 Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 9-15-2009 The Effects of Aerosol Drug Delivery on Airway Resistance through
Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 9-15-2009 The Effects of Aerosol Drug Delivery on Airway Resistance through
I. Subject: Continuous Aerosolization of Bronchodilators
 I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
Mechanical Ventilation Principles and Practices
 Mechanical Ventilation Principles and Practices Dr LAU Chun Wing Arthur Department of Intensive Care Pamela Youde Nethersole Eastern Hospital 6 October 2009 In this lecture, you will learn Major concepts
Mechanical Ventilation Principles and Practices Dr LAU Chun Wing Arthur Department of Intensive Care Pamela Youde Nethersole Eastern Hospital 6 October 2009 In this lecture, you will learn Major concepts
How to write bipap settings
 How to write bipap settings 6-6-2013 Living On O2 for Life If you use a bipap machine, like I do, this post is for you. I've been using a bipap machine since 1993 which is a pretty long time. BiPAP 's
How to write bipap settings 6-6-2013 Living On O2 for Life If you use a bipap machine, like I do, this post is for you. I've been using a bipap machine since 1993 which is a pretty long time. BiPAP 's
Delivering Aerosol Medication in ICU
 Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
Georgia State University. Georgia State University. Waleed A. Alzahrani KSAU-HS
 Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 12-14-2010 Comparison of Albuterol Delivery between High Frequency Oscillatory
Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 12-14-2010 Comparison of Albuterol Delivery between High Frequency Oscillatory
In-Vitro Comparison of Aerosol Drug Delivery in Pediatrics Using Pressurized Metered Dose Inhaler, Jet Nebulizer, and Vibrating Mesh Nebulizers
 Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 7-31-2012 In-Vitro Comparison of Aerosol Drug Delivery in Pediatrics Using
Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 7-31-2012 In-Vitro Comparison of Aerosol Drug Delivery in Pediatrics Using
The Effect of Different Interfaces on Aerosol Delivery in Simulated Spontaneously Breathing Adult with Tracheostomy
 Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy Fall 12-15-2010 The Effect of Different Interfaces on Aerosol Delivery in Simulated
Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy Fall 12-15-2010 The Effect of Different Interfaces on Aerosol Delivery in Simulated
Ron Hosp, MS-HSA, RRT Regional Respiratory Specialist. This program has been approved for 1 hour of continuing education credit.
 Ron Hosp, MS-HSA, RRT Regional Respiratory Specialist This program has been approved for 1 hour of continuing education credit. Course Objectives Identify at least four goals of home NIV Identify candidates
Ron Hosp, MS-HSA, RRT Regional Respiratory Specialist This program has been approved for 1 hour of continuing education credit. Course Objectives Identify at least four goals of home NIV Identify candidates
Archives of Pulmonology and Respiratory Care
 v Clinical Group Archives of Pulmonology and Respiratory Care DOI CC By Marwa Mohsen 1, Ahmed A Elberry 2, Abeer Salah Eldin 3, Raghda RS Hussein 1 and Mohamed EA Abdelrahim 1,4 * Research Article Effects
v Clinical Group Archives of Pulmonology and Respiratory Care DOI CC By Marwa Mohsen 1, Ahmed A Elberry 2, Abeer Salah Eldin 3, Raghda RS Hussein 1 and Mohamed EA Abdelrahim 1,4 * Research Article Effects
Articles. The Advantages of Nebulization in the Treatment of Mechanically Ventilated Neonates. Kristin Smith, RRT-NPS
 Articles The Advantages of Nebulization in the Treatment of Mechanically Ventilated Neonates Kristin Smith, RRT-NPS A major goal in the care of premature babies is growth, and so all therapies are applied
Articles The Advantages of Nebulization in the Treatment of Mechanically Ventilated Neonates Kristin Smith, RRT-NPS A major goal in the care of premature babies is growth, and so all therapies are applied
POLICY. Number: Title: APPLICATION OF NON INVASIVE VENTILATION FOR ACUTE RESPIRATORY FAILURE. Authorization
 POLICY Number: 7311-60-024 Title: APPLICATION OF NON INVASIVE VENTILATION FOR ACUTE RESPIRATORY FAILURE Authorization [ ] President and CEO [ x ] Vice President, Finance and Corporate Services Source:
POLICY Number: 7311-60-024 Title: APPLICATION OF NON INVASIVE VENTILATION FOR ACUTE RESPIRATORY FAILURE Authorization [ ] President and CEO [ x ] Vice President, Finance and Corporate Services Source:
Airway Clearance Devices
 Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
Print Page 1 of 11 Wisconsin.gov home state agencies subject directory department of health services Search Welcome» August 2, 2018 5:18 PM Program Name: BadgerCare Plus and Medicaid Handbook Area: Durable
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE
 Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Emergency Medicine High Velocity Nasal Insufflation (Hi-VNI) VAPOTHERM POCKET GUIDE Indications for Vapotherm High Velocity Nasal Insufflation (Hi-VNI ) administration, the patient should be: Spontaneously
Supplementary Online Content 2
 Supplementary Online Content 2 van Meenen DMP, van der Hoeven SM, Binnekade JM, et al. Effect of on demand vs routine nebulization of acetylcysteine with salbutamol on ventilator-free days in intensive
Supplementary Online Content 2 van Meenen DMP, van der Hoeven SM, Binnekade JM, et al. Effect of on demand vs routine nebulization of acetylcysteine with salbutamol on ventilator-free days in intensive
Latex Free. An affordable, easy to use, high density, small volume nebulizer with a breath enhanced design! Breath Enhanced High Density Jet Nebulizer
 Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
NON INVASIVE LIFE SAVERS. Non Invasive Ventilation (NIV)
 Table 1. NIV: Mechanisms Of Action Decreases work of breathing Increases functional residual capacity Recruits collapsed alveoli Improves respiratory gas exchange Reverses hypoventilation Maintains upper
Table 1. NIV: Mechanisms Of Action Decreases work of breathing Increases functional residual capacity Recruits collapsed alveoli Improves respiratory gas exchange Reverses hypoventilation Maintains upper
NIV - BI-LEVEL POSITIVE AIRWAY PRESSURE (BIPAP)
 Introduction NIV - BI-LEVEL POSITIVE AIRWAY PRESSURE (BIPAP) Noninvasive ventilation (NIV) is a method of delivering oxygen by positive pressure mask that allows for the prevention or postponement of invasive
Introduction NIV - BI-LEVEL POSITIVE AIRWAY PRESSURE (BIPAP) Noninvasive ventilation (NIV) is a method of delivering oxygen by positive pressure mask that allows for the prevention or postponement of invasive
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device
 I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
Pap Settings. A review of fine tuning settings For patient comfort and compliance Wendy Cook BSRT Judy Salisbury RPGST
 Pap Settings A review of fine tuning settings For patient comfort and compliance Wendy Cook BSRT Judy Salisbury RPGST Conflict of Interest Disclosure x 1. I do not have any relationships with any entities
Pap Settings A review of fine tuning settings For patient comfort and compliance Wendy Cook BSRT Judy Salisbury RPGST Conflict of Interest Disclosure x 1. I do not have any relationships with any entities
JAMES B. FINK, RAJIV DHAND, JERRY GRYCHOWSKI, PATRICK J. FAHEY, and MARTIN J. TOBIN
 Reconciling In Vitro and In Vivo Measurements of Aerosol Delivery from a Metered-Dose Inhaler during Mechanical Ventilation and Defining Efficiency-enhancing Factors JAMES B. FINK, RAJIV DHAND, JERRY GRYCHOWSKI,
Reconciling In Vitro and In Vivo Measurements of Aerosol Delivery from a Metered-Dose Inhaler during Mechanical Ventilation and Defining Efficiency-enhancing Factors JAMES B. FINK, RAJIV DHAND, JERRY GRYCHOWSKI,
Lecture Notes. Chapter 3: Asthma
 Lecture Notes Chapter 3: Asthma Objectives Define asthma and status asthmaticus List the potential causes of asthma attacks Describe the effect of asthma attacks on lung function List the clinical features
Lecture Notes Chapter 3: Asthma Objectives Define asthma and status asthmaticus List the potential causes of asthma attacks Describe the effect of asthma attacks on lung function List the clinical features
Respiratory Care in PICU Aerosol Therapy ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า
 Respiratory Care in PICU Aerosol Therapy น.อ.หญ ง ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า Aerosol liquid droplets or solid particles suspended in a gas(air) visible like fog Aerosol vs Humidity Humidity
Respiratory Care in PICU Aerosol Therapy น.อ.หญ ง ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า Aerosol liquid droplets or solid particles suspended in a gas(air) visible like fog Aerosol vs Humidity Humidity
AFCH NEUROMUSCULAR DISORDERS (NMD) PROTOCOL
 AFCH NEUROMUSCULAR DISORDERS (NMD) PROTOCOL A. Definition of Therapy: 1. Cough machine: 4 sets of 5 breaths with a goal of I:E pressures approximately the same of 30-40. Inhale time = 1 second, exhale
AFCH NEUROMUSCULAR DISORDERS (NMD) PROTOCOL A. Definition of Therapy: 1. Cough machine: 4 sets of 5 breaths with a goal of I:E pressures approximately the same of 30-40. Inhale time = 1 second, exhale
Natural performance. Introducing the BiPAP A30 - because ease of use and therapy efficacy are key to patient well-being
 Natural performance Introducing the BiPAP A30 - because ease of use and therapy efficacy are key to patient well-being Because our innovations are inspired by you and your patients, the bi-level ventilator
Natural performance Introducing the BiPAP A30 - because ease of use and therapy efficacy are key to patient well-being Because our innovations are inspired by you and your patients, the bi-level ventilator
Small Volume Nebulizer Treatment (Hand-Held)
 Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS
 NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
Metered Dose Inhalers with Valved Holding Chamber: A Pediatric Hospital Experience
 Metered Dose Inhalers with Valved Holding Chamber: A Pediatric Hospital Experience 8th Annual North Regional Respiratory Care Conference Minnesota & Wisconsin Societies for Respiratory Care Mayo Civic
Metered Dose Inhalers with Valved Holding Chamber: A Pediatric Hospital Experience 8th Annual North Regional Respiratory Care Conference Minnesota & Wisconsin Societies for Respiratory Care Mayo Civic
(To be filled by the treating physician)
 CERTIFICATE OF MEDICAL NECESSITY TO BE ISSUED TO CGHS BENEFICIAREIS BEING PRESCRIBED BILEVEL CONTINUOUS POSITIVE AIRWAY PRESSURE (BI-LEVEL CPAP) / BI-LEVEL VENTILATORY SUPPORT SYSTEM Certification Type
CERTIFICATE OF MEDICAL NECESSITY TO BE ISSUED TO CGHS BENEFICIAREIS BEING PRESCRIBED BILEVEL CONTINUOUS POSITIVE AIRWAY PRESSURE (BI-LEVEL CPAP) / BI-LEVEL VENTILATORY SUPPORT SYSTEM Certification Type
Intracheal antibiotics administration
 Intracheal antibiotics administration Jean Chastre, M.D. www.reamedpitie.com Disclosure Conflicts of interest: Consulting or Lecture fees: Bayer, Pfizer, Cubist/Merck, Basilea, Kenta/Aridis, Roche, AstraZeneca/Medimmune
Intracheal antibiotics administration Jean Chastre, M.D. www.reamedpitie.com Disclosure Conflicts of interest: Consulting or Lecture fees: Bayer, Pfizer, Cubist/Merck, Basilea, Kenta/Aridis, Roche, AstraZeneca/Medimmune
NI 60. Non-invasive ventilation without compromise. Homecare Pneumology Neonatology Anaesthesia. Sleep Diagnostics Service Patient Support
 NI 60 Non-invasive ventilation without compromise Homecare Pneumology Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support NI 60 Non-invasive ventilation without
NI 60 Non-invasive ventilation without compromise Homecare Pneumology Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support NI 60 Non-invasive ventilation without
STATE OF OKLAHOMA 2014 EMERGENCY MEDICAL SERVICES PROTOCOLS
 3K NON-INVASIVE POSITIVE PRESSURE VENTILATION (NIPPV) ADULT EMT EMT-INTERMEDIATE 85 ADVANCED EMT PARAMEDIC Indications: 1. Dyspnea Uncertain Etiology Adult. 2. Dyspnea Asthma Adult. 3. Dyspnea Chronic
3K NON-INVASIVE POSITIVE PRESSURE VENTILATION (NIPPV) ADULT EMT EMT-INTERMEDIATE 85 ADVANCED EMT PARAMEDIC Indications: 1. Dyspnea Uncertain Etiology Adult. 2. Dyspnea Asthma Adult. 3. Dyspnea Chronic
BiLevel Pressure Device
 PROCEDURE - Page 1 of 7 Purpose Scope Classes/ Goals Define indications and care settings for acute and chronic initiation of Noninvasive Positive Pressure Ventilation. Identify the role of Respiratory
PROCEDURE - Page 1 of 7 Purpose Scope Classes/ Goals Define indications and care settings for acute and chronic initiation of Noninvasive Positive Pressure Ventilation. Identify the role of Respiratory
NIV use in ED. Dr. Khalfan AL Amrani Emergency Resuscitation Symposium 2 nd May 2016 SQUH
 NIV use in ED Dr. Khalfan AL Amrani Emergency Resuscitation Symposium 2 nd May 2016 SQUH Outline History & Introduction Overview of NIV application Review of proven uses of NIV History of Ventilation 1940
NIV use in ED Dr. Khalfan AL Amrani Emergency Resuscitation Symposium 2 nd May 2016 SQUH Outline History & Introduction Overview of NIV application Review of proven uses of NIV History of Ventilation 1940
Basics of NIV. Dr Shrikanth Srinivasan MD,DNB,FNB,EDIC. Consultant, Critical Care Medicine Medanta, The Medicity
 Basics of NIV Dr Shrikanth Srinivasan MD,DNB,FNB,EDIC Consultant, Critical Care Medicine Medanta, The Medicity Objectives: Definitions Advantages and Disadvantages Interfaces Indications Contraindications
Basics of NIV Dr Shrikanth Srinivasan MD,DNB,FNB,EDIC Consultant, Critical Care Medicine Medanta, The Medicity Objectives: Definitions Advantages and Disadvantages Interfaces Indications Contraindications
Titration protocol reference guide
 PN 1079754_Cover:22037_Cov_Canada 11/22/10 Philips Healthcare is part of Royal Philips Electronics How to reach us www.philips.com/healthcare healthcare@philips.com Asia +49 7031 463 2254 Europe, Middle
PN 1079754_Cover:22037_Cov_Canada 11/22/10 Philips Healthcare is part of Royal Philips Electronics How to reach us www.philips.com/healthcare healthcare@philips.com Asia +49 7031 463 2254 Europe, Middle
Using an Inhaler and Nebulizer
 Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
Using an Inhaler and Nebulizer Introduction An inhaler is a handheld device that is used to deliver medication directly to your airways. A nebulizer is an electric or battery powered machine that turns
Pediatric Aerosol Therapy
 Pediatric Aerosol Therapy Ariel Berlinski MD Introduction Delivery Device Selection for the Treatment of Acute Pediatric Asthma Transnasal Aerosol Delivery in Pediatric Patients Delivery Device Selection
Pediatric Aerosol Therapy Ariel Berlinski MD Introduction Delivery Device Selection for the Treatment of Acute Pediatric Asthma Transnasal Aerosol Delivery in Pediatric Patients Delivery Device Selection
Vibrating Mesh Nebulizer Compared With Metered-Dose Inhaler in Mechanically Ventilated Subjects
 Vibrating Mesh Nebulizer Compared With Metered-Dose Inhaler in Mechanically Ventilated Subjects Meagan N Dubosky MSc RRT, Yi-Fan Chen PhD, Mary E Henriksen MSc RRT, and David L Vines MHS RRT FAARC BACKGROUND:
Vibrating Mesh Nebulizer Compared With Metered-Dose Inhaler in Mechanically Ventilated Subjects Meagan N Dubosky MSc RRT, Yi-Fan Chen PhD, Mary E Henriksen MSc RRT, and David L Vines MHS RRT FAARC BACKGROUND:
Noninvasive ventilation: Selection of patient, interfaces, initiation and weaning
 CME article Johnson S, et al: Noninvasive ventilation Noninvasive ventilation: Selection of patient, interfaces, initiation and weaning Saumy Johnson, Ramesh Unnikrishnan * Email: ramesh.unnikrishnan@manipal.edu
CME article Johnson S, et al: Noninvasive ventilation Noninvasive ventilation: Selection of patient, interfaces, initiation and weaning Saumy Johnson, Ramesh Unnikrishnan * Email: ramesh.unnikrishnan@manipal.edu
Respiratory Management- Your Questions Answered! Michelle Chatwin, PhD Consultant Physiotherapist
 Respiratory Management- Your Questions Answered! Michelle Chatwin, PhD Consultant Physiotherapist Why Are People Affected Differently Neuromuscular Disease; A Spectrum Its severity varies widely within
Respiratory Management- Your Questions Answered! Michelle Chatwin, PhD Consultant Physiotherapist Why Are People Affected Differently Neuromuscular Disease; A Spectrum Its severity varies widely within
Paramedic Rounds. Pre-Hospital Continuous Positive Airway Pressure (CPAP)
 Paramedic Rounds Pre-Hospital Continuous Positive Airway Pressure (CPAP) Morgan Hillier MD Class of 2011 Dr. Mike Peddle Assistant Medical Director SWORBHP Objectives Outline evidence for pre-hospital
Paramedic Rounds Pre-Hospital Continuous Positive Airway Pressure (CPAP) Morgan Hillier MD Class of 2011 Dr. Mike Peddle Assistant Medical Director SWORBHP Objectives Outline evidence for pre-hospital
Web Appendix 1: Literature search strategy. BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up. Sources to be searched for the guidelines;
 Web Appendix 1: Literature search strategy BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up Sources to be searched for the guidelines; Cochrane Database of Systematic Reviews (CDSR) Database of
Web Appendix 1: Literature search strategy BTS Acute Hypercapnic Respiratory Failure (AHRF) write-up Sources to be searched for the guidelines; Cochrane Database of Systematic Reviews (CDSR) Database of
Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler
 Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler Ariel Berlinski MD, Dirk von Hollen, John N Pritchard PhD, and Ross HM Hatley PhD BACKGROUND: Inhaled
Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler Ariel Berlinski MD, Dirk von Hollen, John N Pritchard PhD, and Ross HM Hatley PhD BACKGROUND: Inhaled
I. Subject: Pressure Support Ventilation (PSV) with BiPAP Device/Nasal CPAP
 I. Subject: Pressure Support Ventilation (PSV) with BiPAP Device/Nasal CPAP II. Policy: PSV with BiPAP device/nasal CPAP will be initiated upon a physician's order by Respiratory Therapy personnel trained
I. Subject: Pressure Support Ventilation (PSV) with BiPAP Device/Nasal CPAP II. Policy: PSV with BiPAP device/nasal CPAP will be initiated upon a physician's order by Respiratory Therapy personnel trained
In Vitro Comparison of Aerosol Delivery Using Different Face Masks and Flow Rates With a High-Flow Humidity System
 In Vitro Comparison of Aerosol Delivery Using Different Face Masks and Flow Rates With a High-Flow Humidity System Hui-Ling Lin MSc RRT RN FAARC, Robert J Harwood MSA RRT FAARC, James B Fink PhD RRT FAARC,
In Vitro Comparison of Aerosol Delivery Using Different Face Masks and Flow Rates With a High-Flow Humidity System Hui-Ling Lin MSc RRT RN FAARC, Robert J Harwood MSA RRT FAARC, James B Fink PhD RRT FAARC,
Charisma High-flow CPAP solution
 Charisma High-flow CPAP solution Homecare PNEUMOLOGY Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support charisma High-flow CPAP solution Evidence CPAP therapy
Charisma High-flow CPAP solution Homecare PNEUMOLOGY Neonatology Anaesthesia INTENSIVE CARE VENTILATION Sleep Diagnostics Service Patient Support charisma High-flow CPAP solution Evidence CPAP therapy
GE Healthcare. Non Invasive Ventilation (NIV) For the Engström Ventilator. Relief, Relax, Recovery
 GE Healthcare Non Invasive Ventilation (NIV) For the Engström Ventilator Relief, Relax, Recovery COPD is currently the fourth leading cause of death in the world, and further increases in the prevalence
GE Healthcare Non Invasive Ventilation (NIV) For the Engström Ventilator Relief, Relax, Recovery COPD is currently the fourth leading cause of death in the world, and further increases in the prevalence
8/13/11. RSPT 1410 Humidity & Aerosol Therapy Part 3. Humidification Equipment. Aerosol Therapy
 1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
Georgia State University. Georgia State University. Abdullah ALQarni
 Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 11-30-2011 Efficiency of Aerosol Therapy through Jet Nebulizer, Breath-Actuated
Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 11-30-2011 Efficiency of Aerosol Therapy through Jet Nebulizer, Breath-Actuated
1.40 Prevention of Nosocomial Pneumonia
 1.40 Prevention of Nosocomial Pneumonia Purpose Audience Policy Statement: The guideline is designed to reduce the incidence of pneumonia and other acute lower respiratory tract infections. All UTMB healthcare
1.40 Prevention of Nosocomial Pneumonia Purpose Audience Policy Statement: The guideline is designed to reduce the incidence of pneumonia and other acute lower respiratory tract infections. All UTMB healthcare
Simone Gambazza, PT Cystic Fibrosis Centre, Milan Fondazione I.R.C.C.S. Cà Granda Ospedale Maggiore Policlinico
 Simone Gambazza, PT Cystic Fibrosis Centre, Milan Fondazione I.R.C.C.S. Cà Granda Ospedale Maggiore Policlinico Aerosol Deposition and Particles Sizes þ Inertial impaction It occurs with larger (>3µm)
Simone Gambazza, PT Cystic Fibrosis Centre, Milan Fondazione I.R.C.C.S. Cà Granda Ospedale Maggiore Policlinico Aerosol Deposition and Particles Sizes þ Inertial impaction It occurs with larger (>3µm)
BiPAPS/TVAPSCPAPASV???? Lori Davis, B.Sc., R.C.P.T.(P), RPSGT
 BiPAPS/TVAPSCPAPASV???? Lori Davis, B.Sc., R.C.P.T.(P), RPSGT Modes Continuous Positive Airway Pressure (CPAP): One set pressure which is the same on inspiration and expiration Auto-PAP (APAP) - Provides
BiPAPS/TVAPSCPAPASV???? Lori Davis, B.Sc., R.C.P.T.(P), RPSGT Modes Continuous Positive Airway Pressure (CPAP): One set pressure which is the same on inspiration and expiration Auto-PAP (APAP) - Provides
An update on inhalation devices
 OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
PEDIATRIC PAP TITRATION PROTOCOL
 PURPOSE In order to provide the highest quality care for our patients, our sleep disorders facility adheres to the AASM Standards of Accreditation. The accompanying policy and procedure on pediatric titrations
PURPOSE In order to provide the highest quality care for our patients, our sleep disorders facility adheres to the AASM Standards of Accreditation. The accompanying policy and procedure on pediatric titrations
Respiratory Therapy. Medical/Scientific/General Background
 Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
Respiratory Therapy Medical/Scientific/General Background Marketing Europe Dr. Rainer Jakobs PMM Europe 1 Dr. Rainer Jakobs, PMM Europe RT Medical/Scientific/General Background 2 Dr. Rainer Jakobs, PMM
In Vitro Evaluation of Radio-Labeled Aerosol Delivery Via a Variable-Flow Infant CPAP System
 In Vitro Evaluation of Radio-Labeled Aerosol Delivery Via a Variable-Flow Infant CPAP System Kimberly D Farney RRT, Brandon T Kuehne RRT MBA, Laurie A Gibson RT (N), Leif D Nelin MD, and Edward G Shepherd
In Vitro Evaluation of Radio-Labeled Aerosol Delivery Via a Variable-Flow Infant CPAP System Kimberly D Farney RRT, Brandon T Kuehne RRT MBA, Laurie A Gibson RT (N), Leif D Nelin MD, and Edward G Shepherd
Latex Free. An affordable, easy to use, high density, small volume nebulizer with a revolutionary breath enhanced design!
 Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a revolutionary breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients
Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a revolutionary breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients
Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask
 Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask Abstract This study s objective was to model the Amsino OneMask Oxygen Mask using Computational Fluid Dynamics (CFD). A three-dimensional
Computational Fluid Dynamics Modeling of Amsino OneMask Oxygen Mask Abstract This study s objective was to model the Amsino OneMask Oxygen Mask using Computational Fluid Dynamics (CFD). A three-dimensional
Non-invasive Ventilation
 Non-invasive Ventilation 163 29 Non-invasive Ventilation AM BHAGWATI Artificial ventilatory support has became an integral component in the management of critically ill patients in the intensive care units.
Non-invasive Ventilation 163 29 Non-invasive Ventilation AM BHAGWATI Artificial ventilatory support has became an integral component in the management of critically ill patients in the intensive care units.
WHAT DO YOU WANT FROM A HOME VENTILATION SYSTEM? 8322_RS_HomeNIV_brochure_v14.ind1 1 4/7/06 12:57:35
 WHAT DO YOU WANT FROM A HOME VENTILATION SYSTEM? 8322_RS_HomeNIV_brochure_v14.ind1 1 4/7/06 12:57:35 D I L E M M A DIFFERENT VENTILATORS DIFFERENT ALGORITHMS TO KNOW YOU VE CHANGED PATIENT LIVES?PATIENT??
WHAT DO YOU WANT FROM A HOME VENTILATION SYSTEM? 8322_RS_HomeNIV_brochure_v14.ind1 1 4/7/06 12:57:35 D I L E M M A DIFFERENT VENTILATORS DIFFERENT ALGORITHMS TO KNOW YOU VE CHANGED PATIENT LIVES?PATIENT??
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION
 CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) DEFINITION Method of maintaining low pressure distension of lungs during inspiration and expiration when infant breathing spontaneously Benefits Improves oxygenation
11/20/2015. Beyond CPAP. No relevant financial conflicts of interest. Kristie R Ross, M.D. November 12, Describe advanced ventilation options
 Beyond CPAP Kristie R Ross, M.D. November 12, 2015 No relevant financial conflicts of interest Sponsored by The Warren Alpert Medical School of Brown University Describe advanced ventilation options Compare
Beyond CPAP Kristie R Ross, M.D. November 12, 2015 No relevant financial conflicts of interest Sponsored by The Warren Alpert Medical School of Brown University Describe advanced ventilation options Compare
Hypoventilation? Obstructive Sleep Apnea? Different Tests, Different Treatment
 Hypoventilation? Obstructive Sleep Apnea? Different Tests, Different Treatment Judith R. Fischer, MSLS, Editor, Ventilator-Assisted Living (fischer.judith@sbcglobal.net) Thanks to Josh Benditt, MD, University
Hypoventilation? Obstructive Sleep Apnea? Different Tests, Different Treatment Judith R. Fischer, MSLS, Editor, Ventilator-Assisted Living (fischer.judith@sbcglobal.net) Thanks to Josh Benditt, MD, University
Prepared by : Bayan Kaddourah RN,MHM. GICU Clinical Instructor
 Mechanical Ventilation Prepared by : Bayan Kaddourah RN,MHM. GICU Clinical Instructor 1 Definition Is a supportive therapy to facilitate gas exchange. Most ventilatory support requires an artificial airway.
Mechanical Ventilation Prepared by : Bayan Kaddourah RN,MHM. GICU Clinical Instructor 1 Definition Is a supportive therapy to facilitate gas exchange. Most ventilatory support requires an artificial airway.
Continuous Aerosol Therapy
 PROCEDURE - : Page 1 of 5 Purpose Policy Physician's Order To standardize the administration of continuous aerosol therapy. Respiratory Care Services provides equipment and therapy according to physician
PROCEDURE - : Page 1 of 5 Purpose Policy Physician's Order To standardize the administration of continuous aerosol therapy. Respiratory Care Services provides equipment and therapy according to physician
Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients
 Thorax 1987;42:457-461 Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients with chronic asthma D M MITCHELL, M A SOLOMON, S E J TOLFREE, M SHORT,
Thorax 1987;42:457-461 Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients with chronic asthma D M MITCHELL, M A SOLOMON, S E J TOLFREE, M SHORT,
Abstract. , FiO2, volume flow, and airway pressure.
 Abstract A Bench Study to Compare Portable Therapies for Respiratory Insufficiency: Continuous Flow Oxygen (CF), Intermittent Flow Oxygen (IF), and Non-Invasive Open Ventilation (NIOV) Background Respiratory
Abstract A Bench Study to Compare Portable Therapies for Respiratory Insufficiency: Continuous Flow Oxygen (CF), Intermittent Flow Oxygen (IF), and Non-Invasive Open Ventilation (NIOV) Background Respiratory
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Aerosolized Antibiotics in Mechanically Ventilated Patients
 Aerosolized Antibiotics in Mechanically Ventilated Patients Gerald C Smaldone MD PhD Introduction Topical Delivery of Antibiotics to the Lung Tracheobronchitis Aerosolized Antibiotic Delivery in the Medical
Aerosolized Antibiotics in Mechanically Ventilated Patients Gerald C Smaldone MD PhD Introduction Topical Delivery of Antibiotics to the Lung Tracheobronchitis Aerosolized Antibiotic Delivery in the Medical
Clinical White Paper
 Clinical White Paper Table of Contents 1 Abstract 1 2 High Efficiency Aerosol Drug Delivery During Ventilation 2 3 A Lung Recruitment Strategy for Patients with Respiratory Failure utilising Aerogen 5
Clinical White Paper Table of Contents 1 Abstract 1 2 High Efficiency Aerosol Drug Delivery During Ventilation 2 3 A Lung Recruitment Strategy for Patients with Respiratory Failure utilising Aerogen 5
DreamStation. Provider guide
 Provider guide Accessing the Provider mode screens Accessing Provider mode unlocks settings that cannot be modified by the user. To access Provider mode: 1. ce the device is powered, press and hold both
Provider guide Accessing the Provider mode screens Accessing Provider mode unlocks settings that cannot be modified by the user. To access Provider mode: 1. ce the device is powered, press and hold both
Aerosol Delivery and Modern Mechanical Ventilation In Vitro/In Vivo Evaluation
 Aerosol Delivery and Modern Mechanical Ventilation In Vitro/In Vivo Evaluation Dorisanne D. Miller, Mohammad M. Amin, Lucy B. Palmer, Akbar R. Shah, and Gerald C. Smaldone Department of Respiratory Care,
Aerosol Delivery and Modern Mechanical Ventilation In Vitro/In Vivo Evaluation Dorisanne D. Miller, Mohammad M. Amin, Lucy B. Palmer, Akbar R. Shah, and Gerald C. Smaldone Department of Respiratory Care,
Nasal High Flow Humidification with or without Oxygen for COPD Management. Shereen Bailey, RCP, RRT, NPS
 Nasal High Flow Humidification with or without Oxygen for COPD Management Shereen Bailey, RCP, RRT, NPS Objectives How it works COPD Management today The role of NHFC Evidence Research/Case Studies Types
Nasal High Flow Humidification with or without Oxygen for COPD Management Shereen Bailey, RCP, RRT, NPS Objectives How it works COPD Management today The role of NHFC Evidence Research/Case Studies Types
Comparison of patient spirometry and ventilator spirometry
 GE Healthcare Comparison of patient spirometry and ventilator spirometry Test results are based on the Master s thesis, Comparison between patient spirometry and ventilator spirometry by Saana Jenu, 2011
GE Healthcare Comparison of patient spirometry and ventilator spirometry Test results are based on the Master s thesis, Comparison between patient spirometry and ventilator spirometry by Saana Jenu, 2011
Noninvasive Mechanical Ventilation in Children ศ.พญ.อร ณวรรณ พฤทธ พ นธ หน วยโรคระบบหายใจเด ก ภาคว ชาก มารเวชศาสตร คณะแพทยศาสตร โรงพยาบาลรามาธ บด
 Noninvasive Mechanical Ventilation in Children ศ.พญ.อร ณวรรณ พฤทธ พ นธ หน วยโรคระบบหายใจเด ก ภาคว ชาก มารเวชศาสตร คณะแพทยศาสตร โรงพยาบาลรามาธ บด Noninvasive Mechanical Ventilation Provide support without
Noninvasive Mechanical Ventilation in Children ศ.พญ.อร ณวรรณ พฤทธ พ นธ หน วยโรคระบบหายใจเด ก ภาคว ชาก มารเวชศาสตร คณะแพทยศาสตร โรงพยาบาลรามาธ บด Noninvasive Mechanical Ventilation Provide support without
It is recommended that a mask and protective eyewear be worn when providing care to a patient with a cough
 UNIVERSITY HEALTH NETWORK POLICY #: PAGE 1 OF 7 POLICY AND PROCEDURE MANUAL: RESPIRATORY THERAPY DEPT PATIENT CARE SECTION ORIGINAL DATE: 04/03 ISSUED BY: SITE LEADER APPROVED BY: Infection Prevention
UNIVERSITY HEALTH NETWORK POLICY #: PAGE 1 OF 7 POLICY AND PROCEDURE MANUAL: RESPIRATORY THERAPY DEPT PATIENT CARE SECTION ORIGINAL DATE: 04/03 ISSUED BY: SITE LEADER APPROVED BY: Infection Prevention
Nebulizers. Small Volume Nebulizers
 Small Volume MICRO MIST Nebulizer The MICRO MIST small volume nebulizer is designed for performance and economy and includes the design features needed by today s respiratory care practitioners. It can
Small Volume MICRO MIST Nebulizer The MICRO MIST small volume nebulizer is designed for performance and economy and includes the design features needed by today s respiratory care practitioners. It can
The International Palestinian Congress in Sleep Medicine
 The International Palestinian Congress in Sleep Medicine Temporomandibular Disorders and Sleep Apnea 26 and 27 October, 2017 Notre Dame Hotel, Jerusalem Using PAP Downloads to Manage Sleep Apnea Patients
The International Palestinian Congress in Sleep Medicine Temporomandibular Disorders and Sleep Apnea 26 and 27 October, 2017 Notre Dame Hotel, Jerusalem Using PAP Downloads to Manage Sleep Apnea Patients
Redefining Continuous Aerosol Drug Delivery 2015 PM223
 Redefining Continuous Aerosol Drug Delivery 2015 PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize
Redefining Continuous Aerosol Drug Delivery 2015 PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize
Systems differ in their ability to deliver optimal humidification
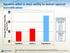 Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Aerosol Delivery Through Adult High Flow Nasal Cannula With Heliox and Oxygen
 Aerosol Delivery Through Adult High Flow Nasal Cannula With Heliox and Oxygen Patricia A Dailey RRT, Robert Harwood MSA RRT-NPS FAARC, Kyle Walsh, James B Fink PhD RRT FAARC, Tina Thayer RRT, Greg Gagnon,
Aerosol Delivery Through Adult High Flow Nasal Cannula With Heliox and Oxygen Patricia A Dailey RRT, Robert Harwood MSA RRT-NPS FAARC, Kyle Walsh, James B Fink PhD RRT FAARC, Tina Thayer RRT, Greg Gagnon,
Frequently Asked Questions.
 Frequently Asked Questions www.aerogen.com 1 FAQs Frequently asked questions are answered below and specifically for each of our products. If you have a question that is not listed, please feel free to
Frequently Asked Questions www.aerogen.com 1 FAQs Frequently asked questions are answered below and specifically for each of our products. If you have a question that is not listed, please feel free to
Redefining Continuous Aerosol Drug Delivery PM223
 Redefining Continuous Aerosol Drug Delivery PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize medication
Redefining Continuous Aerosol Drug Delivery PM223 Explanation of Continuous via Traditional Small Volume (Jet) Nebulizer Traditional small volume (jet) nebulizers require compressed gas to aerosolize medication
Vancouver Coastal Health Guidelines for the use of Respiratory Equipment for Patients on Airborne Precautions in Acute Care Facilities
 Vancouver Coastal Health Guidelines for the use of Respiratory Equipment for Patients on Airborne Precautions in Acute Care Facilities Goals 1. To meet respiratory care needs in patients who are on airborne
Vancouver Coastal Health Guidelines for the use of Respiratory Equipment for Patients on Airborne Precautions in Acute Care Facilities Goals 1. To meet respiratory care needs in patients who are on airborne
AEROSOL THERAPY: THE PRACTICALITIES
 AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
Average volume-assured pressure support
 Focused review Average volume-assured pressure support Abdurahim Aloud MD Abstract Average volume-assured pressure support (AVAPS) is a relatively new mode of noninvasive positive pressure ventilation
Focused review Average volume-assured pressure support Abdurahim Aloud MD Abstract Average volume-assured pressure support (AVAPS) is a relatively new mode of noninvasive positive pressure ventilation
