Innovations and Combinations for Novel Anticancer Strategies
|
|
|
- Alfred Rose
- 5 years ago
- Views:
Transcription
1 Innovations and Combinations for Novel Anticancer Strategies Axel-R. Hanauske, MD, Ph.D., MBA Senior Medical Fellow Oncology Early Phase Eli Lilly and Company 2017 Eli Lilly and Company
2 Disclosure Information I am an employee of Eli Lilly and Company. I will not discuss off-label or investigational treatments in my presentation. 6/19/ Eli Lilly and Company 2
3 Oncology Drug Development: The Traditional Rocket Model Lead Optimization Pre- Clinical Develop -ment Phase I Phase II Phase III Registration Global Launch Global Optimization Candidate Development Commercialization 2017 Eli Lilly and Company
4 Cost of Cost Development of (Millions (Million $) $) Cost of Developing a New Drug Over Time $ $ $ 54 $ Adapted from: DiMasi et al. J Health Econ. 10: 107, 1991; Tufts Center for the Study of Drug Development; data for 2000 and 2015 are estimates
5 R&D Efficiency by Year Jack W. Scannell et al; Diagnosing the decline in pharmaceutical R&D efficiency: Nature Reviews/Drug Discovery, Volume 11, March Eli Lilly and Company
6 How Does Anticancer Drug Late Development Compare? Probability of Success in Phase III studies by therapeutic area: Autoimmune: 63% Respiratory: 61% Endocrinology: 60% Infectious Disease: 55% Neurology: 55% Cardio-Vascular: 46% Oncology: 34% M.Silverman; BioStrategics Consulting GmbH, 2012; retrieved 11-Nov-2012; 2017 Eli Lilly and Company
7 Estimated New Cases in USA in 2010 Cancer type Estimated New Cases 2010 # Pipeline Products Phase II + III Cancer type Lung (123) 1,2 Non- Hodgkin s Lymphoma Estimated New Cases 2010 # Pipeline Products Phase II + III (92) Prostate Kidney Breast (82) Thyroid Colon + Rectal and # of Pipeline Compounds US 2015: 836 drugs+vaccines in clinical development 3137 clinical trials active Endometrial (Ovarian) (48) Bladder Pancreatic Melanoma (skin: 53) Leukemia (106) informedrx Drug Information Services. RxOutlook; American Cancer Society. Cancer Facts and Figures, 2010; 1 Buffery Am Health & Drug Benefits 8: 216, Accessed: June Eli Lilly and Company
8 How to Develop the Right Drug for the Right Patient in a Disease with Heterogeneity at Multiple Different Levels Define diversity in disease Genetic somatic & germline Functional Tumor components Segregate by defined criteria Target/pathways Biologic processes chromosomes mutations copy changes Predict clinical benefit of a drug Signatures of target dependence Host genetic makeup overexpression protein IHC on tissue arrays
9 Types of Innovative Trial Designs Master Protocol Basket Trials Umbrella Trials Platform Trials Either Or or Neither Strategy trials Bayesian Adaptive Platform Designs etc.
10 Master Protocol Design
11 Hallmarks of Master Protocols One scoping study protocol Beneath: Separate parallel drug trials with Different biomarkers Different treatment designs Require collaboration of many stakeholders: Academic Industry Diagnostic Pharmaceutical Regulatory
12 Why Master Protocols and not Separate Studies? Enhanced genomic screening efficiency Inclusion of wide array of molecular subtypes willingness of patients and HCPs to participate Deletion/insertion of new subprotocol by amendment instead of completely new protocol development and faster accrual c/w separate studies More rapid clinical development Better streamlined clinical development
13 Master Protocols: Basic Design Renfro Ann Oncol October 11, 2016 Substudies often with low sample size: Single arm trials > focus on large efficacy signal Randomized trial > focus on large treatment effect (vs. marker-negative or SOC)
14 Master Protocols: Higher Efficiency than separate studies if Use of common genomic platform or diagnostic tests Screening for variants of multiple genomic targets in each tumor sample (requires sufficient tumor material) Rapid inclusion of patients based on screening results Organizational setting that allows for addition/deletion of subprotocols
15 Master Protocols: Faster Clinical Development than separate studies if Upfront Regulatory/Institutional review of Master Protocol: Less administrative delay: Subsequent addition of subprotocols are considered amendments and may be reviewed accordingling Simplified process of deletion/addition of new substudies or changes in substudies No need to put complete Master protocol or unaffected substudies on hold Potential for faster approval by Regulatory Agencies in case of convincing treatment effects
16 Master Protocols: Stakeholder Enthusiasm if Availability of a large number of different substudies Inclusion of all marker negative substudy All patients signing the ICF know there will be a treatment option for them Simplified local processes: IRB approval Contracting
17 Basket Design
18 Hallmarks of Basket Protocols Terminology used differently in literature Pragmatic definition: TUMOR TYPE-AGNOSTIC APPROACH = HISTOLOGY-REPLACING APPROACH Hypothesis: The response to targeted therapy is determined by the underlying molecular variant and (largely) independent of histology Renfro Ann Oncol October 11, 2016
19 Basket Trial: Basic Design Enrollment based on molecular variant Subtrials may consist of different histologies or tumor types Randomization within molecular cohorts is uncommon and may be challenging (different SOCs across tumor types) Usually single-arm substudies, hypothesis-generating objectives Renfro Ann Oncol 11-Oct_ 2016; Billingham Lancet Oncol 17-Feb-2016
20 Drug-Development-Oriented Basket Trial: Potential Design Renfro Ann Oncol October 11, 2016
21 Basket Trials: Pros and Cons Prerequisites: 1. Drug must sufficiently inhibit target 2. Tumor must depend on target Benefits: Access to trial for patients with rare tumors (but must have respective molecular marker) Testing may be done locally Small cohorts (usually single arm) may suffice to detect activity Quick results Challenges: Molecular variant(s) may not be the only driver of tumor Contextual complexities in various histologies Single biomarkers may be inferior to multi-gene signature Structural variants may need to be complemented with functional studies Different tumor types have different prognoses: single primary endpoint (eg ORR) may skew results
22 Basket trial: NCI MATCH ( Molecular Analysis for Therapy CHoice ) Phase II study, Target: patients in 25 substudies (N=30), screen 6000 pts US only, > 1071 sites participating in the NCI s National Cancer Trials Network TTYPES: Advanced, recurrent, refractory solid tumors Lymphoma Multiple Myeloma 24 drugs, including: Afatinib AZD5363 (AKT inh.) Binimetinib Crizotinib Dabrafenib Dasatinib Defactinib AZD4547 (FGFR Inh.) Nivolumab Osimertinib Palbociclib GSK (PI3K-ß inh.) Sunitinib Taselisib Tametinib Trastuzumab emtansine Vismodegib accessed 23-Jan-2017 Co-PEPs: ORR, 6-mo-PFS Goal: increase ORR from 5% to >16% Re-bx allowed to understand temporal molecular development and re-assignment of pts
23 Umbrella Design
24 Hallmarks of Umbrella Protocols Terminology used differently in literature Pragmatic definition: TUMOR TYPE-BASED APPROACH = HISTOLOGY-REFINING APPROACH Hypothesis: The response to targeted therapy is primarily determined by histologic context Renfro Ann Oncol October 11, 2016
25 Umbrella Trials: Characteristics Screening and enrollment before identification of a targetable molecular variant Use of central laboratory for standardized screening Subprotocols may be single-arm or randomized Renfro Ann Oncol October 11, 2016
26 Umbrella Trials: Pros and Cons Benefits: Conclusions are specific for a given tumor type Tumor heterogeneity limited to one tumor type For randomized substudies: Potential to better understand the difference of targeted therapy vs SOC Potential to differentiate between prognostic and predictive markers Easier path to negotiate approval with Regulatory Agencies Challenges: Requires Strong collaboration between academia and industry Defined marker profile, comparability of cohorts (bx, assay, Tx) Feasibility Subclassification into rare populations (particularly with rare cancers to start out with) speed of accrual Randomization requiring a larger sample size may be challenging Appearance of new SOCs during trial conduct changes the environment
27 Umbrella Trial: FOCUS 4 Design Kaplan J Clin Oncol 36, 4562, 2013
28 Platform Design ( Standing Trial )
29 Hallmarks of Platform Protocols Randomized One control arm, several experimental arms Allow for exit and entry of experimental arms Use of Bayesian hierarchical models for decision making Biomarker-positive subpopulations not necessarily separated Adaptive randomization with mid-trial randomization ratio shifts Renfro Ann Oncol October 11, 2016
30 Platform Protocols: Design Randomize Control Exp 1 Exp 2 Exp 3 Exp 4 Potential Advantages: Reduced overall sample size Exp 5 Improved efficiency compared to sets of indivdual two-arm trials 30
31 Platform Design ( Standing Trial ) - Adapted for Early Phase Drug Development -
32 Platform Protocols for Phase I Combination Trials One Master Protocol Includes design for dose-escalation AND expansion cohorts Identical Primary Endpoint(s) for all cohorts One backbone agent Tumor-type defined or open Exit and entry of combination cohorts allowed through amendment Use of Bayesian models for decision making allowed Biomarker-positive subpopulations may be used in combination cohorts
33 Platform Protocols: Design Platform Phase 1 Protocol with Expansion Cohorts Backbone compound + Combo agent 1 Backbone compound + Combo agent 2 Backbone compound + Combo agent 3 Backbone compound + Combo agent 4 Backbone compound + Combo agent 5 Potential Advantages: Increased flexibility to include new scientific evidence into ongoing trial Backbone Increased speed to identify active combination compound regimens + Improved efficiency to identify active combination regimens Combo agent 6 33
34 Strategy Trials
35 Hallmarks of Strategy Protocols 2-arm randomized trials: non-targeted SOC vs. targeted tx Cross-over in case of PD SHIVA trial: SOC vs. targeted therapy Refractory solid tumor screened: 741 pts Randomized: 195 pts R R Targeted therapy Physician s choice - if PD on targeted tx cross-over to Physician s choice - 10 regimens with 11 targeted agents were studied PEP: PFS RES: mpfs 2.3 vs. 2.0 months (HR 0.88, p=0.41) NCI MPACT: non-targeted therapy > targeted therapy Renfro Ann Oncol October 11, 2016, LeTourneau Lancet Oncol 16: 1324, 2015; Catenacci Lancet Oncol September 2, 2015
36 Strategy Trial: NCI MPACT Design ( Molecular Profiling-based Assignment of Cancer Therapy ) DNA repair, PI3K RAS/RAF/MEK 2:1 Do Chin Clin Oncol 4: 31, 2015
37 NCI MPACT Total of 4 experimental arms Anticipated sample size: 700 pts screened > 180 pts randomized to the initial 4 arms Possibility to add additional cohorts PEPs: ORR, 4-month PFS Trial is ongoing
38 Bayesian Adaptive Trials
39 Hallmarks of Bayesian Adaptive Design Randomized trial with Master Protocol Subprotocols may leave or enter during trial Pts assigned to specific targeted therapy arms based on molecular signature Adaptive change of randomization based on treatment s observed activity randomization probability if activity is observed randomization probability if unpromising activity Requirements to perform well: swift marker assessment quick endpoint to inform adaptive randomization slow accrual rate to ensure subsequent pts benefit from randomization adaptation Renfro Ann Oncol October 11, 2016
40 Bayesian Adaptive Design Notable Trials: BATTLE-1 BATTLE-2 I-SPY2 Renfro Ann Oncol October 11, 2016
41 Bayesian Adaptive Design: BATTLE-1 ( Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE)-1 ) First completed, biomarker-based, adaptive randomized clinical trial (N = 255) Mandatory bx 11 pre-specified biomarkers (After 1 pt in each subgroup) 20 different treatment-by-biomarker subgroups, evaluation of performance of each treatment arm in each marker group Liu Chin Clin Oncol 4: 33, 2015
42 Bayesian Adoptive Design: Pros and Cons Benefits: Identification of potential new predictive biomarkers Enhanced individual ethics through adaptive randomization Greater efficiency compared to stand-alone randomized Phase II trials Real-time biomarker analysis (if possible) Challenges: Prespecification of biomarkers without information whether they are prognostic or predictive Combination of markers may dilute predictive effects of individual markers Rare biomarkers may lead to late start of adaptive randomization Need of short-term outcome measure to guide adaptive randomization Patients with advanced cancer may not be eligible for treatment with all compounds due to medical condition
43 Novel Study Designs: Summary Potential Benefits: Greater efficiency in drug development Faster detection of innovative cancer agents More accurate selection of patients Exposure of less patients to potentially inactive agents Less resource consumption Potential Challenges: Retention of integrity of trial designs Susceptibility for bias Complexity of safety monitoring Robustness of results observed Overestimation of beneficial effect Assessment by Competent Authorities unclear how central Ct approval ( 2018) will handle new designs
44 THANK YOU
45 The Pharmaceutical Industry has Increased its Oncology Efforts EFPIA: A Vision Towards a Life Science Strategy in Europe, Brussels 2014; May 4, 2015
46 # of Pipeline Compounds: USA drugs+vaccines in clinical development 3137 clinical trials active logy-report-2015.pdf Accessed: June
Basket Trials: Features, Examples, and Challenges
 : Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
: Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
Basket and Umbrella Trial Designs in Oncology
 Basket and Umbrella Trial Designs in Oncology Eric Polley Biomedical Statistics and Informatics Mayo Clinic Polley.Eric@mayo.edu Dose Selection for Cancer Treatment Drugs Stanford Medicine May 2017 1 /
Basket and Umbrella Trial Designs in Oncology Eric Polley Biomedical Statistics and Informatics Mayo Clinic Polley.Eric@mayo.edu Dose Selection for Cancer Treatment Drugs Stanford Medicine May 2017 1 /
Master Protocols FDA Oncology Experience
 Master Protocols FDA Oncology Experience Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Center for Drug Evaluation and Research, USFDA Outline Regulations FDA Experience with Basket, Umbrella
Master Protocols FDA Oncology Experience Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Center for Drug Evaluation and Research, USFDA Outline Regulations FDA Experience with Basket, Umbrella
Design considerations for Phase II trials incorporating biomarkers
 Design considerations for Phase II trials incorporating biomarkers Sumithra J. Mandrekar Professor of Biostatistics, Mayo Clinic Pre-Meeting Workshop Enhancing the Design and Conduct of Phase II Studies
Design considerations for Phase II trials incorporating biomarkers Sumithra J. Mandrekar Professor of Biostatistics, Mayo Clinic Pre-Meeting Workshop Enhancing the Design and Conduct of Phase II Studies
Statistical Considerations for Novel Trial Designs: Biomarkers, Umbrellas and Baskets
 Statistical Considerations for Novel Trial Designs: Biomarkers, Umbrellas and Baskets Bibhas Chakraborty, PhD Centre for Quantitative Medicine, Duke-NUS March 29, 2015 Personalized or Precision Medicine
Statistical Considerations for Novel Trial Designs: Biomarkers, Umbrellas and Baskets Bibhas Chakraborty, PhD Centre for Quantitative Medicine, Duke-NUS March 29, 2015 Personalized or Precision Medicine
Is there a Cookbook for Oncology Clinical Trials?
 Masterclass for Masters See beyond : An Oncology Brainstorm Ghent, 16th of September 2016 Is there a Cookbook for Oncology Clinical Trials? Dimitrios Zardavas MD Associate Scientific Director, Breast International
Masterclass for Masters See beyond : An Oncology Brainstorm Ghent, 16th of September 2016 Is there a Cookbook for Oncology Clinical Trials? Dimitrios Zardavas MD Associate Scientific Director, Breast International
33 rd Annual J.P. Morgan Healthcare Conference. January 2015
 33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective
 PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
Accelerating Innovation in Statistical Design
 Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
IRESSA (Gefitinib) The Journey. Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca
 IRESSA (Gefitinib) The Journey Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca Overview The Drug The Biomarker and Clinical Trials Sampling Lessons Learned The
IRESSA (Gefitinib) The Journey Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca Overview The Drug The Biomarker and Clinical Trials Sampling Lessons Learned The
Adaptive Design of Affordable Clinical Trials Using Master Protocols in the Era of Precision Medicine
 Adaptive Design of Affordable Clinical Trials Using Master Protocols in the Era of Precision Medicine Tze Leung Lai Dept. of Statistics, Biomedical Data Science, Computational & Mathematical Engineering;
Adaptive Design of Affordable Clinical Trials Using Master Protocols in the Era of Precision Medicine Tze Leung Lai Dept. of Statistics, Biomedical Data Science, Computational & Mathematical Engineering;
Precision Genetic Testing in Cancer Treatment and Prognosis
 Precision Genetic Testing in Cancer Treatment and Prognosis Deborah Cragun, PhD, MS, CGC Genetic Counseling Graduate Program Director University of South Florida Case #1 Diana is a 47 year old cancer patient
Precision Genetic Testing in Cancer Treatment and Prognosis Deborah Cragun, PhD, MS, CGC Genetic Counseling Graduate Program Director University of South Florida Case #1 Diana is a 47 year old cancer patient
Oncology Drug Development
 Oncology Drug Development Advantages and challenges of using stratified clinical studies Simon J Hollingsworth The 18th Anti-Tumour Drug Development Forum 21 February Japan Stratified Medicine In The Clinic
Oncology Drug Development Advantages and challenges of using stratified clinical studies Simon J Hollingsworth The 18th Anti-Tumour Drug Development Forum 21 February Japan Stratified Medicine In The Clinic
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era
 Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018
 Celebrating 4 Years of Lung MAP Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018 Slide 1 Lung MAP study leadership Vali Papadimitrakopoulou, MD study chair Roy Herbst, MD PhD study
Celebrating 4 Years of Lung MAP Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018 Slide 1 Lung MAP study leadership Vali Papadimitrakopoulou, MD study chair Roy Herbst, MD PhD study
How to faster integrate new technologies into clinical practice
 How to faster integrate new technologies into clinical practice 31JAN2017 Jens Bjørheim, CMO Ultimovacs Nordic Trial Alliance Stakeholders meeting 2 Oncology New drugs Landscape The current buzz Basket
How to faster integrate new technologies into clinical practice 31JAN2017 Jens Bjørheim, CMO Ultimovacs Nordic Trial Alliance Stakeholders meeting 2 Oncology New drugs Landscape The current buzz Basket
Clinical Trial Design to Expedite Drug Development Mary W. Redman, Ph.D.
 Clinical Trial Design to Expedite Drug Development Mary W. Redman, Ph.D. What do we mean by expediting drug development? Phase I Single Arm Phase II (expansion cohort) Randomized Phase II Phase III Necessary?
Clinical Trial Design to Expedite Drug Development Mary W. Redman, Ph.D. What do we mean by expediting drug development? Phase I Single Arm Phase II (expansion cohort) Randomized Phase II Phase III Necessary?
Master Protocols for Immunotherapy Combinations
 Master Protocols for Immunotherapy Combinations Ahmad Tarhini, MD, PhD Professor of Medicine, CCLCM, CWRU Director, Melanoma and Skin Cancer Program Director, Center for Immuno-Oncology Research Cleveland
Master Protocols for Immunotherapy Combinations Ahmad Tarhini, MD, PhD Professor of Medicine, CCLCM, CWRU Director, Melanoma and Skin Cancer Program Director, Center for Immuno-Oncology Research Cleveland
CUP: Treatment by molecular profiling
 CUP: Treatment by molecular profiling George Pentheroudakis Professor of Oncology Medical School, University of Ioannina Greece Chair, ESMO Guidelines September 2018 Enterprise Interest No disclosures.
CUP: Treatment by molecular profiling George Pentheroudakis Professor of Oncology Medical School, University of Ioannina Greece Chair, ESMO Guidelines September 2018 Enterprise Interest No disclosures.
ArQule Jefferies Global Healthcare Conference June 2015
 ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
Fifteenth International Kidney Cancer Symposium
 The following presentation should not be regarded as an endorsement of a particular product/drug/technique by the speaker. The presentation topics were assigned to the speakers by the scientific committee
The following presentation should not be regarded as an endorsement of a particular product/drug/technique by the speaker. The presentation topics were assigned to the speakers by the scientific committee
Combining HS-110 and anti-pd-1 in NSCLC. September 1, 2015
 Combining HS-110 and anti-pd-1 in NSCLC September 1, 2015 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
Combining HS-110 and anti-pd-1 in NSCLC September 1, 2015 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
Regulatory Considerations in Oncology Trials in China. Jiang, Frank, MD, PhD VP, Asia Pacific R&D, Sanofi
 Regulatory Considerations in Oncology Trials in China Jiang, Frank, MD, PhD VP, Asia Pacific R&D, Sanofi 1 Disclaimer The views and opinions provided are those of the speaker and do not reflect those of
Regulatory Considerations in Oncology Trials in China Jiang, Frank, MD, PhD VP, Asia Pacific R&D, Sanofi 1 Disclaimer The views and opinions provided are those of the speaker and do not reflect those of
Determined to realize a future in which people with cancer live longer and better than ever before
 Determined to realize a future in which people with cancer live longer and better than ever before 3Q 2018 EARNINGS PRESENTATION NOVEMBER 2018 1 Forward-looking statements disclosure This presentation
Determined to realize a future in which people with cancer live longer and better than ever before 3Q 2018 EARNINGS PRESENTATION NOVEMBER 2018 1 Forward-looking statements disclosure This presentation
I. Diagnosis of the cancer type in CUP
 Latest Research: USA I. Diagnosis of the cancer type in CUP II. Outcomes of site-specific therapy of the cancer type in CUP a. Prospective clinical trial b. Retrospective clinical trials 1 Latest Research:
Latest Research: USA I. Diagnosis of the cancer type in CUP II. Outcomes of site-specific therapy of the cancer type in CUP a. Prospective clinical trial b. Retrospective clinical trials 1 Latest Research:
Delivering Value Through Personalized Medicine: An Industry Perspective
 Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Liver and Biliary Tract Cancers Critical Review
 Liver and Biliary Tract Cancers Critical Review Lorenza Rimassa Oncologia Medica e Ematologia Humanitas Cancer Center Humanitas Research Hospital Rozzano (Milano) Critical review Oral presentations Melero
Liver and Biliary Tract Cancers Critical Review Lorenza Rimassa Oncologia Medica e Ematologia Humanitas Cancer Center Humanitas Research Hospital Rozzano (Milano) Critical review Oral presentations Melero
MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS
 MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS DR. RACHEL E. LAING*, OLIVIER LESUEUR*, STAN HOPKINS AND DR. SEAN X. HU MAY 2012 Recent advances in oncology, such
MATCHMAKING IN ONCOLOGY CHALLENGES AND COMBINATION STRATEGY FOR NOVEL TARGETED AGENTS DR. RACHEL E. LAING*, OLIVIER LESUEUR*, STAN HOPKINS AND DR. SEAN X. HU MAY 2012 Recent advances in oncology, such
Looking Beyond the Standard-of- Care : The Clinical Trial Option
 1 Looking Beyond the Standard-of- Care : The Clinical Trial Option Terry Mamounas, M.D., M.P.H., F.A.C.S. Medical Director, Comprehensive Breast Program UF Health Cancer Center at Orlando Health Professor
1 Looking Beyond the Standard-of- Care : The Clinical Trial Option Terry Mamounas, M.D., M.P.H., F.A.C.S. Medical Director, Comprehensive Breast Program UF Health Cancer Center at Orlando Health Professor
TARGET A BETTER NOW FORWARD-LOOKING STATEMENTS NASDAQ: IMGN. Current as of January 2018
 NASDAQ: IMGN TARGET A BETTER NOW Current as of January 2018 FORWARD-LOOKING STATEMENTS This presentation includes forward looking statements based on management's current expectations. These statements
NASDAQ: IMGN TARGET A BETTER NOW Current as of January 2018 FORWARD-LOOKING STATEMENTS This presentation includes forward looking statements based on management's current expectations. These statements
09,00 13,40. I Sessione. Moderatori: Giordano Beretta, Roberto Labianca
 09,00 13,40 I Sessione Moderatori: Giordano Beretta, Roberto Labianca 11,20-11,40 Gli aspetti metodologici in ambito di ricerca Valter Torri Gli aspetti metodologici in ambito di ricerca Valter Torri valter
09,00 13,40 I Sessione Moderatori: Giordano Beretta, Roberto Labianca 11,20-11,40 Gli aspetti metodologici in ambito di ricerca Valter Torri Gli aspetti metodologici in ambito di ricerca Valter Torri valter
Oncology Drug Development Using Molecular Pathology
 Oncology Drug Development Using Molecular Pathology Introduction PRESENTERS Lee Schacter, PhD, MD, FACP Executive Medical Director, Oncology Clinipace Worldwide Martha Bonino Director, Strategic Accounts
Oncology Drug Development Using Molecular Pathology Introduction PRESENTERS Lee Schacter, PhD, MD, FACP Executive Medical Director, Oncology Clinipace Worldwide Martha Bonino Director, Strategic Accounts
Relevance of Innovative Trial Design Today. Basket of Baskets and other Innovative Clinical Trials
 Relevance of Innovative Trial Design Today Basket of Baskets and other Innovative Clinical Trials Outline Precision Medicine Clinical Trials Evolving Field Classic industry-sponsored design: single drug,
Relevance of Innovative Trial Design Today Basket of Baskets and other Innovative Clinical Trials Outline Precision Medicine Clinical Trials Evolving Field Classic industry-sponsored design: single drug,
The Current Status of Immune Checkpoint Inhibitors: Arvin Yang, MD PhD Oncology Global Clinical Research Bristol-Myers Squibb
 The Current Status of Immune Checkpoint Inhibitors: A Global Overview of the Field Arvin Yang, MD PhD Oncology Global Clinical Research Bristol-Myers Squibb Immune Checkpoint Inhibitors Conference, March
The Current Status of Immune Checkpoint Inhibitors: A Global Overview of the Field Arvin Yang, MD PhD Oncology Global Clinical Research Bristol-Myers Squibb Immune Checkpoint Inhibitors Conference, March
6/22/2017 TARGETING THE TARGETS IN 2017 TARGETING THE TARGETS IN 2017
 TARGETING THE TARGETS IN 2017 Primary Care Focus Symposium July 1, 2017 Grace Wang MD I do not have any relevant financial relationships to disclose at this time TARGETING THE TARGETS IN 2017 What are
TARGETING THE TARGETS IN 2017 Primary Care Focus Symposium July 1, 2017 Grace Wang MD I do not have any relevant financial relationships to disclose at this time TARGETING THE TARGETS IN 2017 What are
Jan Bogaerts Scientific Director EORTC Headquarters Belgium
 What defines the disease and populations? How to deal with large subgroups within an indication? Heterogeneity, lack of historical real-life data, rarest subtypes Jan Bogaerts Scientific Director EORTC
What defines the disease and populations? How to deal with large subgroups within an indication? Heterogeneity, lack of historical real-life data, rarest subtypes Jan Bogaerts Scientific Director EORTC
Novel RCC Targets from Immuno-Oncology and Antibody-Drug Conjugates
 Novel RCC Targets from Immuno-Oncology and Antibody-Drug Conjugates Christopher Turner, MD Vice President, Clinical Science 04 November 2016 Uveal Melanoma Celldex Pipeline CANDIDATE INDICATION Preclinical
Novel RCC Targets from Immuno-Oncology and Antibody-Drug Conjugates Christopher Turner, MD Vice President, Clinical Science 04 November 2016 Uveal Melanoma Celldex Pipeline CANDIDATE INDICATION Preclinical
Patient Leader Education Summit. Precision Medicine: Today and Tomorrow March 31, 2017
 Patient Leader Education Summit Precision Medicine: Today and Tomorrow March 31, 2017 Precision Medicine: Presentation Outline Agenda What is a Precision Medicine What is its clinical value Overview of
Patient Leader Education Summit Precision Medicine: Today and Tomorrow March 31, 2017 Precision Medicine: Presentation Outline Agenda What is a Precision Medicine What is its clinical value Overview of
Life Sciences Consortium Task Force Report to The National Cancer Policy Forum Workshop
 Life Sciences Consortium Task Force Report to The National Cancer Policy Forum Workshop Gregory A. Curt, MD, Chair U.S. Medical Science Lead, Emerging Products AstraZeneca -Oncology CEO Roundtable-I May
Life Sciences Consortium Task Force Report to The National Cancer Policy Forum Workshop Gregory A. Curt, MD, Chair U.S. Medical Science Lead, Emerging Products AstraZeneca -Oncology CEO Roundtable-I May
Genomic tests to personalize therapy of metastatic breast cancers. Fabrice ANDRE Gustave Roussy Villejuif, France
 Genomic tests to personalize therapy of metastatic breast cancers Fabrice ANDRE Gustave Roussy Villejuif, France Future application of genomics: Understand the biology at the individual scale Patients
Genomic tests to personalize therapy of metastatic breast cancers Fabrice ANDRE Gustave Roussy Villejuif, France Future application of genomics: Understand the biology at the individual scale Patients
Oncolytic Viruses: Reovirus
 T S X : O N C N A S D A Q : O N C Y International Society for Biological Therapy of Cancer 2008 Oncology Biologics Development Primer Oncolytic Viruses: Reovirus REOLYSIN - mode of action REOLYSIN contains
T S X : O N C N A S D A Q : O N C Y International Society for Biological Therapy of Cancer 2008 Oncology Biologics Development Primer Oncolytic Viruses: Reovirus REOLYSIN - mode of action REOLYSIN contains
Accelerate Your Research with Conversant Bio
 Imagination has given us the steam engine, the telephone, the talkingmachine and the automobile, for these things had to be dreamed of before they became realities. So I believe that dreams... are likely
Imagination has given us the steam engine, the telephone, the talkingmachine and the automobile, for these things had to be dreamed of before they became realities. So I believe that dreams... are likely
Idera Pharmaceuticals
 Idera Pharmaceuticals ILLUMINATE-204 Clinical Data Update December 2018 Forward Looking Statements and Other Important Cautions This presentation contains forward-looking statements within the meaning
Idera Pharmaceuticals ILLUMINATE-204 Clinical Data Update December 2018 Forward Looking Statements and Other Important Cautions This presentation contains forward-looking statements within the meaning
Bayesian hierarchical models for adaptive randomization in biomarker-driven studies: Umbrella and platform trials
 Bayesian hierarchical models for adaptive randomization in biomarker-driven studies: Umbrella and platform trials William T. Barry, PhD Nancy and Morris John Lurie Investigator Biostatistics and Computational
Bayesian hierarchical models for adaptive randomization in biomarker-driven studies: Umbrella and platform trials William T. Barry, PhD Nancy and Morris John Lurie Investigator Biostatistics and Computational
PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM
 HEMATOLOGY ONCOLOGY PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM COMMITTED TO ADVANCING DRUG DEVELOPMENT IN ONCOLOGY $ MARKETPLACE COMPLEXITIES increasingly competitive marketplace and rising cost pressures
HEMATOLOGY ONCOLOGY PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM COMMITTED TO ADVANCING DRUG DEVELOPMENT IN ONCOLOGY $ MARKETPLACE COMPLEXITIES increasingly competitive marketplace and rising cost pressures
LION. Corporate Presentation June 2016 BIOTECHNOLOGIES. Leadership & Innovation in Oncology
 LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
LION BIOTECHNOLOGIES Leadership & Innovation in Oncology Corporate Presentation June 2016 Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private
Improving outcomes as rapidly as possible for patients. Multi-arm, multi stage platform, umbrella and basket protocols
 Improving outcomes as rapidly as possible for patients Multi-arm, multi stage platform, umbrella and basket protocols Mahesh Parmar MRC Clinical Trials Unit at UCL Institute of Clinical Trials and Methdology
Improving outcomes as rapidly as possible for patients Multi-arm, multi stage platform, umbrella and basket protocols Mahesh Parmar MRC Clinical Trials Unit at UCL Institute of Clinical Trials and Methdology
CONSIDERATIONS IN DEVELOPMENT OF PEMBROLIZUMAB IN MSI-H CANCERS
 CONSIDERATIONS IN DEVELOPMENT OF PEMBROLIZUMAB IN MSI-H CANCERS December 2017 Christine K. Gause, Ph.D Executive Director, Biostatistics. 2 Microsatellite Instability-High Cancer - USPI KEYTRUDA is indicated
CONSIDERATIONS IN DEVELOPMENT OF PEMBROLIZUMAB IN MSI-H CANCERS December 2017 Christine K. Gause, Ph.D Executive Director, Biostatistics. 2 Microsatellite Instability-High Cancer - USPI KEYTRUDA is indicated
How to address tumour heterogeneity in next generation oncology trials
 How to address tumour heterogeneity in next generation oncology trials Cihangir YANDIM, PhD Research Associate in Cancer Therapeutics and Clinical Sciences Dr. Cihangir Yandim - CTIP 2016, Hamburg 1 Founded
How to address tumour heterogeneity in next generation oncology trials Cihangir YANDIM, PhD Research Associate in Cancer Therapeutics and Clinical Sciences Dr. Cihangir Yandim - CTIP 2016, Hamburg 1 Founded
GSK Oncology. Axel Hoos, MD, PhD Senior Vice President, Oncology R&D. March 8, 2017
 GSK Oncology Axel Hoos, MD, PhD Senior Vice President, Oncology R&D March 8, 217 GSK pipeline Oncology R&D Strategy Maximizing survival through transformational medicines and combinations Cancer Epigenetics
GSK Oncology Axel Hoos, MD, PhD Senior Vice President, Oncology R&D March 8, 217 GSK pipeline Oncology R&D Strategy Maximizing survival through transformational medicines and combinations Cancer Epigenetics
New Avenues for the development and evaluation of therapy: Complex, multi-pronged, not one size fitting all
 New Avenues for the development and evaluation of therapy: Complex, multi-pronged, not one size fitting all Antoine Yver MD MSC Senior VP & Head Oncology Global Medicines Development AstraZeneca, Gaithersburg
New Avenues for the development and evaluation of therapy: Complex, multi-pronged, not one size fitting all Antoine Yver MD MSC Senior VP & Head Oncology Global Medicines Development AstraZeneca, Gaithersburg
Pfizer Presents Final Phase 2 Data on Investigational PARP Inhibitor Talazoparib in Patients with Germline BRCA-Positive Advanced Breast Cancer
 For immediate release June 3, 2017 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Presents Final Phase 2 Data on Investigational PARP Inhibitor Talazoparib
For immediate release June 3, 2017 Media Contact: Sally Beatty (212) 733-6566 Investor Contact: Ryan Crowe (212) 733-8160 Pfizer Presents Final Phase 2 Data on Investigational PARP Inhibitor Talazoparib
Investor Call. May 19, Nasdaq: IMGN
 Investor Call May 19, 2017 Nasdaq: IMGN Forward-Looking Statements This presentation includes forward-looking statements based on management's current expectations. These statements include, but are not
Investor Call May 19, 2017 Nasdaq: IMGN Forward-Looking Statements This presentation includes forward-looking statements based on management's current expectations. These statements include, but are not
Inibitori delle chinasi ciclino dipendenti nel trattamento della malattia metastatica HR-positiva Gli studi clinici
 Inibitori delle chinasi ciclino dipendenti nel trattamento della malattia metastatica HR-positiva Gli studi clinici Laura Orlando UOC Oncologia & Breast Unit Brindisi Verona 22/04/2016 Summary Studi con
Inibitori delle chinasi ciclino dipendenti nel trattamento della malattia metastatica HR-positiva Gli studi clinici Laura Orlando UOC Oncologia & Breast Unit Brindisi Verona 22/04/2016 Summary Studi con
Immuno-Oncology Clinical Trials Update: Checkpoint Inhibitors Others (not Anti-PD-L1/PD-1) Issue 4 January 2017
 Delivering a Competitive Intelligence Advantage Immuno-Oncology Clinical Trials Update: Checkpoint Inhibitors Others (not Anti-PD-L1/PD-1) Issue 4 January 2017 Immuno-Oncology CLINICAL TRIALS UPDATE The
Delivering a Competitive Intelligence Advantage Immuno-Oncology Clinical Trials Update: Checkpoint Inhibitors Others (not Anti-PD-L1/PD-1) Issue 4 January 2017 Immuno-Oncology CLINICAL TRIALS UPDATE The
Immune Therapy in Clear Cell Ovarian Cancer (ITICC) Hal Hirte Canadian Cancer Clinical Trials Group
 Immune Therapy in Clear Cell Ovarian Cancer (ITICC) Hal Hirte Canadian Cancer Clinical Trials Group Results of Phase II Study of Durvalumab and Tremelimumab in recurrent clear cell ovarian cancer Trial
Immune Therapy in Clear Cell Ovarian Cancer (ITICC) Hal Hirte Canadian Cancer Clinical Trials Group Results of Phase II Study of Durvalumab and Tremelimumab in recurrent clear cell ovarian cancer Trial
National Surgical Adjuvant Breast and Bowel Project (NSABP) Foundation Annual Progress Report: 2009 Formula Grant
 National Surgical Adjuvant Breast and Bowel Project (NSABP) Foundation Annual Progress Report: 2009 Formula Grant Reporting Period July 1, 2011 June 30, 2012 Formula Grant Overview The National Surgical
National Surgical Adjuvant Breast and Bowel Project (NSABP) Foundation Annual Progress Report: 2009 Formula Grant Reporting Period July 1, 2011 June 30, 2012 Formula Grant Overview The National Surgical
Q Financial Update November 6, 2018 NASDAQ:FPRX
 Q3 2018 Financial Update November 6, 2018 NASDAQ:FPRX 2 Forward-Looking Statements Disclaimer Forward-looking statements contained in this presentation include statements regarding (i) the timing of initiation,
Q3 2018 Financial Update November 6, 2018 NASDAQ:FPRX 2 Forward-Looking Statements Disclaimer Forward-looking statements contained in this presentation include statements regarding (i) the timing of initiation,
GSK Oncology R&D Update
 GSK Oncology R&D Update Axel Hoos, MD Senior Vice President, Oncology R&D February 2019 Cautionary statement regarding forward-looking statements This presentation may contain forward-looking statements.
GSK Oncology R&D Update Axel Hoos, MD Senior Vice President, Oncology R&D February 2019 Cautionary statement regarding forward-looking statements This presentation may contain forward-looking statements.
Personalized Medicine in Oncology and the Implication for Clinical Development
 THE POWER OFx Experts. Experienc e. Execution. Personalized Medicine in Oncology and the Implication for Clinical Development Jamal Gasmi MD, PhD, Medical Director, Medpace Introduction The notable success
THE POWER OFx Experts. Experienc e. Execution. Personalized Medicine in Oncology and the Implication for Clinical Development Jamal Gasmi MD, PhD, Medical Director, Medpace Introduction The notable success
SKCC Protocol Review Committee New Study Application
 Instructions: Submit the following documents to prc@jefferson.edu - Completed New Study Application (aka MCSF) - Protocol - Protocol Facilitation Committee Approval (if applicable) - MDG Priority Score
Instructions: Submit the following documents to prc@jefferson.edu - Completed New Study Application (aka MCSF) - Protocol - Protocol Facilitation Committee Approval (if applicable) - MDG Priority Score
Patient Reported Outcomes in an Era of Immunotherapy Drug Development. David Cella, PhD Northwestern University Chicago, IL, USA
 Patient Reported Outcomes in an Era of Immunotherapy Drug Development David Cella, PhD Northwestern University Chicago, IL, USA Disclosures Research Grants: National Institutes of Health, Patient Centered
Patient Reported Outcomes in an Era of Immunotherapy Drug Development David Cella, PhD Northwestern University Chicago, IL, USA Disclosures Research Grants: National Institutes of Health, Patient Centered
Merck ASCO 2015 Investor Briefing
 Merck ASCO 2015 Investor Briefing Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation
Merck ASCO 2015 Investor Briefing Forward-Looking Statement This presentation includes forward-looking statements within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation
Clinical: Ipilimumab (MDX-010) Update and Next Steps
 Clinical: Ipilimumab (MDX-010) Update and Next Steps Geoffrey M. Nichol, M.D., M.B.A. Senior Vice President, Product Development Medarex, Inc. R&D Day December 9, 2005 Ipilimumab: New Class of Cancer Therapy
Clinical: Ipilimumab (MDX-010) Update and Next Steps Geoffrey M. Nichol, M.D., M.B.A. Senior Vice President, Product Development Medarex, Inc. R&D Day December 9, 2005 Ipilimumab: New Class of Cancer Therapy
Translational Platform for the Development of Targeted Therapeutics
 Translational Platform for the Development of Targeted Therapeutics Ondřej Kalous, MD Associate Project Scientist UCLA Translational Oncology Research Laboratories (TORL) Jonsson Comprehensive Cancer Center
Translational Platform for the Development of Targeted Therapeutics Ondřej Kalous, MD Associate Project Scientist UCLA Translational Oncology Research Laboratories (TORL) Jonsson Comprehensive Cancer Center
ESMO 2017, Madrid, Spain Dr. Loredana Vecchione Charite Comprehensive Cancer Center, Berlin HIGHLIGHTS ON CANCERS OF THE UPPER GI TRACT
 ESMO 2017, Madrid, Spain Dr. Loredana Vecchione Charite Comprehensive Cancer Center, Berlin HIGHLIGHTS ON CANCERS OF THE UPPER GI TRACT DOCETAXEL, OXALIPLATIN AND FLUOROURACIL/LEUCOVORIN (FLOT) FOR RESECTABLE
ESMO 2017, Madrid, Spain Dr. Loredana Vecchione Charite Comprehensive Cancer Center, Berlin HIGHLIGHTS ON CANCERS OF THE UPPER GI TRACT DOCETAXEL, OXALIPLATIN AND FLUOROURACIL/LEUCOVORIN (FLOT) FOR RESECTABLE
National Bank 8th Annual Quebec Conference TSX: IMV. May 30, IMV Inc. All rights reserved.
 National Bank 8th Annual Quebec Conference TSX: IMV May 30, 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV Inc.
National Bank 8th Annual Quebec Conference TSX: IMV May 30, 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV Inc.
Opportunities and Challenges in the Development of Companion Diagnostics
 Opportunities and Challenges in the Development of Companion Diagnostics E. Patrick Groody, Ph.D. Divisional Vice President Research and Development Abbott Molecular Agenda Value of Personalized Medicine
Opportunities and Challenges in the Development of Companion Diagnostics E. Patrick Groody, Ph.D. Divisional Vice President Research and Development Abbott Molecular Agenda Value of Personalized Medicine
REWRITING CANCER TREATMENT THROUGH EPIGENETIC MEDICINES
 REWRITING CANCER TREATMENT THROUGH EPIGENETIC MEDICINES May 18, 2017 Molecularly Defined Solid Tumor Program Update FORWARD-LOOKING STATEMENTS Any statements in this press release about future expectations,
REWRITING CANCER TREATMENT THROUGH EPIGENETIC MEDICINES May 18, 2017 Molecularly Defined Solid Tumor Program Update FORWARD-LOOKING STATEMENTS Any statements in this press release about future expectations,
Leerink Immuno-Oncology Roundtable Conference
 Leerink Immuno-Oncology Roundtable Conference September 28, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private
Leerink Immuno-Oncology Roundtable Conference September 28, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private
Highlights from AACR 2015: The Emerging Potential of Immunotherapeutic Approaches in Non-Small Cell Lung Cancer
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Cancer Immunotherapy Survey
 CHAPTER 8: Cancer Immunotherapy Survey All (N=100) Please classify your organization. Academic lab or center Small biopharmaceutical company Top 20 Pharma Mid-size pharma Diagnostics company Other (please
CHAPTER 8: Cancer Immunotherapy Survey All (N=100) Please classify your organization. Academic lab or center Small biopharmaceutical company Top 20 Pharma Mid-size pharma Diagnostics company Other (please
Immune Checkpoint Inhibitors: The New Breakout Stars in Cancer Treatment
 Immune Checkpoint Inhibitors: The New Breakout Stars in Cancer Treatment 1 Introductions Peter Langecker, MD, PhD Executive Medical Director, Global Oncology Clinipace Worldwide Mark Shapiro Vice President
Immune Checkpoint Inhibitors: The New Breakout Stars in Cancer Treatment 1 Introductions Peter Langecker, MD, PhD Executive Medical Director, Global Oncology Clinipace Worldwide Mark Shapiro Vice President
Big data vs. the individual liver from a regulatory perspective
 Big data vs. the individual liver from a regulatory perspective Robert Schuck, Pharm.D., Ph.D. Genomics and Targeted Therapy Office of Clinical Pharmacology Center for Drug Evaluation and Research Food
Big data vs. the individual liver from a regulatory perspective Robert Schuck, Pharm.D., Ph.D. Genomics and Targeted Therapy Office of Clinical Pharmacology Center for Drug Evaluation and Research Food
Panel One: Alternative Trial Designs Based on Tumor Genetics/Pathway Characteristics
 Panel One: Alternative Trial Designs Based on Tumor Genetics/Pathway Characteristics Alternative Trial Designs Based on Tumor Genetics and/or Pathway Characteristics Instead of Histology George D. Demetri,
Panel One: Alternative Trial Designs Based on Tumor Genetics/Pathway Characteristics Alternative Trial Designs Based on Tumor Genetics and/or Pathway Characteristics Instead of Histology George D. Demetri,
Jules Bordet Institute, Brussels, Belgium Université Libre de Bruxelles Breast International Group (BIG aisbl), Chair ESMO President
 Symposium «Evaluation of the Belgian Cancer Plan» Brussels, November 26th, 2012 Personalized oncology in Europe: only a dream if national health systems do not get involved in diagnostics and pivotal cancer
Symposium «Evaluation of the Belgian Cancer Plan» Brussels, November 26th, 2012 Personalized oncology in Europe: only a dream if national health systems do not get involved in diagnostics and pivotal cancer
Lights and sheds of early approval of new drugs in clinical routine. Carmen Criscitiello, MD, PhD European Institute of Oncology Milan, Italy
 Lights and sheds of early approval of new drugs in clinical routine Carmen Criscitiello, MD, PhD European Institute of Oncology Milan, Italy RCT 5-10% pts «real» patients are here Clin. Pharm. Ther. 2012
Lights and sheds of early approval of new drugs in clinical routine Carmen Criscitiello, MD, PhD European Institute of Oncology Milan, Italy RCT 5-10% pts «real» patients are here Clin. Pharm. Ther. 2012
Patients Driving Progress
 Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
My name is Dr. David Ilson, Professor of Medicine at Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center in New York, New York.
 Welcome to this CME/CE-certified activity entitled, Integrating the Latest Advances Into Clinical Experience: Data and Expert Insights From the 2016 Meeting on Gastrointestinal Cancers in San Francisco.
Welcome to this CME/CE-certified activity entitled, Integrating the Latest Advances Into Clinical Experience: Data and Expert Insights From the 2016 Meeting on Gastrointestinal Cancers in San Francisco.
Regulatory Landscape for Precision Medicine
 Regulatory Landscape for Precision Medicine Adam C. Berger, Ph.D. Office of In Vitro Diagnostics and Radiological Health, FDA FOCR-Alexandria A Blueprint for Breakthrough Meeting September 13, 2017 1 What
Regulatory Landscape for Precision Medicine Adam C. Berger, Ph.D. Office of In Vitro Diagnostics and Radiological Health, FDA FOCR-Alexandria A Blueprint for Breakthrough Meeting September 13, 2017 1 What
SU2C TOP SCIENCE ACCOMPLISHMENTS
 SU2C TOP SCIENCE ACCOMPLISHMENTS INTRODUCTION Since our founding in 2008, Stand Up To Cancer has funded 87 team science projects, with budgets ranging from $250,000 for a short research project to over
SU2C TOP SCIENCE ACCOMPLISHMENTS INTRODUCTION Since our founding in 2008, Stand Up To Cancer has funded 87 team science projects, with budgets ranging from $250,000 for a short research project to over
Predicting outcome in metastatic breast cancer
 Predicting outcome in metastatic breast cancer Aleix Prat, MD, PhD Medical Oncology Department Translational Genomics and Targeted Therapeutics in Solid Tumors Monday, 15 th January, Manchester, UK Disclosures
Predicting outcome in metastatic breast cancer Aleix Prat, MD, PhD Medical Oncology Department Translational Genomics and Targeted Therapeutics in Solid Tumors Monday, 15 th January, Manchester, UK Disclosures
AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC
 FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
FOR IMMEDIATE RELEASE AVEO and Astellas Announce Positive Findings from TIVO-1 Superiority Study of Tivozanib in First-Line Advanced RCC - Tivozanib is the First Agent to Demonstrate Greater than One Year
Converting Novel Therapeutic Models into Early Phase Clinical Trials
 Converting Novel Therapeutic Models into Early Phase Clinical Trials James H. Doroshow, M.D. Deputy Director for Clinical and Translational Research National Cancer Institute, NIH IOM National Cancer Policy
Converting Novel Therapeutic Models into Early Phase Clinical Trials James H. Doroshow, M.D. Deputy Director for Clinical and Translational Research National Cancer Institute, NIH IOM National Cancer Policy
Peripheral T-cell lymphoma. Matt Ahearne Clinical Lecturer, Leicester
 Peripheral T-cell lymphoma Matt Ahearne Clinical Lecturer, Leicester PTCL Objectives To understand the natural history of PTCL To appreciate the importance of accurate diagnosis of PTCL including recent
Peripheral T-cell lymphoma Matt Ahearne Clinical Lecturer, Leicester PTCL Objectives To understand the natural history of PTCL To appreciate the importance of accurate diagnosis of PTCL including recent
Personalized medecine Biomarker
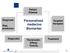 Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Dawson James Conference
 Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Dawson James Conference October 2018 Forward-looking Statements Except for historical information, this presentation contains forward-looking statements, which reflect IMV s current expectations regarding
Durable Response Rate in High Grade Glioma: an Emerging Endpoint for Immunotherapeutics. Timothy Cloughesy, MD University of California, Los Angeles
 Durable Response Rate in High Grade Glioma: an Emerging Endpoint for Immunotherapeutics Timothy Cloughesy, MD University of California, Los Angeles Disclosure 2 FDA Endpoints for the Approval of Cancer
Durable Response Rate in High Grade Glioma: an Emerging Endpoint for Immunotherapeutics Timothy Cloughesy, MD University of California, Los Angeles Disclosure 2 FDA Endpoints for the Approval of Cancer
Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples
 Annals of Oncology 28: 34 43, 2017 doi:10.1093/annonc/mdw413 Published online 11 October 2016 SPECIAL ARTICLE Statistical controversies in clinical research: basket trials, umbrella trials, and other master
Annals of Oncology 28: 34 43, 2017 doi:10.1093/annonc/mdw413 Published online 11 October 2016 SPECIAL ARTICLE Statistical controversies in clinical research: basket trials, umbrella trials, and other master
BIOMARKER DRIVEN EARLY PHASE ONCOLOGY CLINICAL TRIALS; CHALLENGES IN THE REAL WORLD SID KATUGAMPOLA CENTRE FOR DRUG DEVELOPMENT CANCER RESEARCH UK
 BIOMARKER DRIVEN EARLY PHASE ONCOLOGY CLINICAL TRIALS; CHALLENGES IN THE REAL WORLD SID KATUGAMPOLA CENTRE FOR DRUG DEVELOPMENT CANCER RESEARCH UK Cancer; Some Sobering Thoughts Getting cancer is one of
BIOMARKER DRIVEN EARLY PHASE ONCOLOGY CLINICAL TRIALS; CHALLENGES IN THE REAL WORLD SID KATUGAMPOLA CENTRE FOR DRUG DEVELOPMENT CANCER RESEARCH UK Cancer; Some Sobering Thoughts Getting cancer is one of
Triple Negative Breast Cancer. Eric P. Winer, MD Dana-Farber Cancer Institute Harvard Medical School Boston, MA October, 2008
 Triple Negative Breast Cancer Eric P. Winer, MD Dana-Farber Cancer Institute Harvard Medical School Boston, MA October, 2008 Triple Negative Breast Cancer 15% 25% Triple Negative 20% HER2+ ER+ Low Grade
Triple Negative Breast Cancer Eric P. Winer, MD Dana-Farber Cancer Institute Harvard Medical School Boston, MA October, 2008 Triple Negative Breast Cancer 15% 25% Triple Negative 20% HER2+ ER+ Low Grade
Phase II trial designs and endpoints
 Eti Estimating anti-tumour tit activity it Phase II trial designs and endpoints Margaret Hutka MD PhD The Royal Marsden Hospital GI & Lymphoma Unit London, UK margaret.hutka@rmh.nhs.uk www.royalmarsden.nhs.uk
Eti Estimating anti-tumour tit activity it Phase II trial designs and endpoints Margaret Hutka MD PhD The Royal Marsden Hospital GI & Lymphoma Unit London, UK margaret.hutka@rmh.nhs.uk www.royalmarsden.nhs.uk
Carcinosarcoma Trial rial in s a in rare malign rare mali ancy
 Carcinosarcoma Trials in a rare malignancy BACKGROUND Rare and highly aggressive epithelial malignancies Biphasic tumors with epithelial and mesenchymal components Uterine carcinomas (UCS) uncommon with
Carcinosarcoma Trials in a rare malignancy BACKGROUND Rare and highly aggressive epithelial malignancies Biphasic tumors with epithelial and mesenchymal components Uterine carcinomas (UCS) uncommon with
TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive metastatic breast cancer
 TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive metastatic breast cancer Marta Bonotto Department of Oncology University Hospital of Udine TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive
TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive metastatic breast cancer Marta Bonotto Department of Oncology University Hospital of Udine TRIALs of CDK4/6 inhibitor in women with hormone-receptor-positive
Myriad Genetics Corporate Presentation 6/4/13
 Myriad Genetics Corporate Presentation 6/4/13 Forward Looking Statement Some of the information presented here today may contain projections or other forward-looking statements regarding future events
Myriad Genetics Corporate Presentation 6/4/13 Forward Looking Statement Some of the information presented here today may contain projections or other forward-looking statements regarding future events
Phase II Cancer Trials: When and How
 Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
ArQule CorporateUpdate
 ArQule April 2015 1 ArQule CorporateUpdate Safe Harbor This presentation and other statements by ArQule may contain forwardlooking statements within the meaning of the Private Securities Litigation Reform
ArQule April 2015 1 ArQule CorporateUpdate Safe Harbor This presentation and other statements by ArQule may contain forwardlooking statements within the meaning of the Private Securities Litigation Reform
July, ArQule, Inc.
 July, 2012 Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act with respect to clinical
July, 2012 Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act with respect to clinical
2014 San Antonio Breast Cancer Symposium Review
 2014 San Antonio Breast Cancer Symposium Review HER2 Positive Disease 01-10-2015 Elisavet Paplomata, MD Assistant Professor Hematology & Medical Oncology Emory University Winship Cancer Institute S6-01
2014 San Antonio Breast Cancer Symposium Review HER2 Positive Disease 01-10-2015 Elisavet Paplomata, MD Assistant Professor Hematology & Medical Oncology Emory University Winship Cancer Institute S6-01
Targeted Agent and Profiling Utilization Registry
 Targeted Agent and Profiling Utilization Registry Richard L. Schilsky, MD, FACP, FASCO Senior Vice President and Chief Medical Officer American Society of Clinical Oncology Disclosure Information Richard
Targeted Agent and Profiling Utilization Registry Richard L. Schilsky, MD, FACP, FASCO Senior Vice President and Chief Medical Officer American Society of Clinical Oncology Disclosure Information Richard
