BIOMARKER DRIVEN EARLY PHASE ONCOLOGY CLINICAL TRIALS; CHALLENGES IN THE REAL WORLD SID KATUGAMPOLA CENTRE FOR DRUG DEVELOPMENT CANCER RESEARCH UK
|
|
|
- Jocelyn Nichols
- 5 years ago
- Views:
Transcription
1 BIOMARKER DRIVEN EARLY PHASE ONCOLOGY CLINICAL TRIALS; CHALLENGES IN THE REAL WORLD SID KATUGAMPOLA CENTRE FOR DRUG DEVELOPMENT CANCER RESEARCH UK
2 Cancer; Some Sobering Thoughts Getting cancer is one of our greatest fears. > 1 in 4 deaths are cancer related Someone in the UK is diagnosed with cancer every 2 minutes. 1 in 2 people in the UK will develop cancer. In 2013, >350,000 new cases of cancer identified and in 2012 >150,000 died of cancer A disease well controlled by surgery and radiotherapy when localised and caught early. A disease that kills when metastasised and where new treatments are desperately needed 2
3 Drug Development the Challenge 5% success rate from Ph 1 to registration - not sustainable 3
4 Shared wish list by patient, physicians, payers and industry More effective Drugs Fewer Failures Faster development cycles CHEAPER BETTER FASTER Right dose/schedule Right patient Monitor impact of therapy Right commercial opportunity IS IT TIME FOR NO BIOMARKER NO EARLY PHASE TRIAL? 4
5 Centre for Drug Development (CDD) Based in London. Manages preclinical development and sponsors phase I and II trials of new anticancer agents (currently~ 30 active projects). agents in multiple tumor types, including on CRUK priority areas (Pancreatic, Lung, Brain, Oesophageal) Small molecule therapeutics, antibodies, cell therapies, gene therapies, imaging agents. Global partnerships with academia and industry Over 100 agents taken into early phase trials and 5 are available to patients (abiraterone, temozolomide, etoposide, pemetrexed, dexrazoxane)
6 So what is the focus of CDD? Develop novel agents where expertise and /or resources to support further development are lacking. - Sourced from academia, biotech & pharma globally. Sweet-spot around translation from preclinical to FIH First in class agents (>80% of portfolio) highly desirable Focus on biomarkers >90% of assays performed in CRUK funded academic labs
7 So what are biomarkers? A characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention (FDA definition) Pharmacodynamic (PD) PoM/PoP/PoC does the compound bind to my target? does the compound alter target-signalling pathway? does the compound modulate tumour response? Predictive Prognostic Safety Early detection/screening biomarkers
8 Ideal PD strategy in early phase trials Exposure in tumour Plasma PK Proof of principle Proof of mechanism Proof of clinical Concept Enrichment/personalised
9 What makes a good biomarker (4S s) 1. Science - is there a scientifically relevant biomarker we can use. Need to understand deep biology and test hypotheses 2. Suitability (fit-for-purpose) - Pre-study assessment of sensitivity, specificity, magnitude of effect and reproducibility are essential to understand the underlying noise of the assay. Include patient material where relevant. Analytical and technical validation 3. Study design - statistics? when do we take samples and from where? Escalation or expansion or both, Use preclinical PKPD as a guide. 4. Sample - how much sample do you need? Stability? How quickly does it need analysing? Freeze thaw? Shipping conditions etc 9
10 Typical early phase oncology study aims & objectives Stopping dose likely to be estimated based on preclinical tox, pre-clinical biomarkers, PK, maximal administered drug Safety, tolerability PK, ADME parameters PoM, PoP biomarkers (primary, secondary or tertiary end points) Starting dose determined by preclinical tox, safety, efficacy, PK, pharmacology..holistic approach (ICH S9) Patients PKPD relationship MTD, BED to determine RP2D early measurement of drug activity/impact on disease on an ongoing basis 10
11 Trial designs in oncology 1-3 subjects 3+3 design 1-3 subjects Intrapatient dose escalation Expansion arm ~10-15 patients at the RD 3 subjects 6 subjects 8 subjects Surrogate PD response detected Surrogate PD response confirmed Surrogate PD response plateau subjects BED reached/expansion in tumour 11
12 Case study 1; Challenges with a PoM PD assay for a FIC agent Compound X is a FIC small molecule transporter inhibitor that prevents excess lactic acid being transported from tumour, that results in acidosis and cell death Pre-clinically lactate accumulation in PBMC (LCMSMS) was shown to be a robust PoM assay Similar responses observed in healthy volunteer blood at therapeutically relevant concentrations in-vitro Assay was selected as a primary end point for the trial Dose escalation underway, 5 th cohort being dosed, drug administered to >25 patients, pharmacological saturation achieved based on PK Result no conclusive PoM response
13 Case study 2; How to select patients most likely to respond to an experimental therapy Compound Y is a FIC antibody targeting a cell surface antigen that is over-expressed in ovarian and other solid tumours. Study will only select patients showing a 3+ staining thus most likely to respond Target antigen has been extensively studies and a range of antibodies exist for IHC evaluation. Literature reports >50% of patients showing positive staining One antibody was chosen during assay development and validation based on ability to demonstrate differential staining intensity in a range of tumour samples/positive/negative controls/independent pathology review etc Key assay for the trial as patients not expressing target unlikely to benefit from drug Currently only 12% success rate implication on timelines, costs, novelty, logistics
14 Case study 3; Requirements for validation, the what, how and why? Compound Z is a cancer vaccine (1 dose, 4 vaccinations) targeting two antigens in solid tumours. The aim of the study is to demonstrate immune responses primary end point assay ELIspot secondary end point ex vivo PBMC assays, genome analysis in PBMC research end points T cell markers, effector mechanisms, cultured ELIspot, MDSCs, tumour IHC Trial complete following 22 patients. ELIspot responses in very few patients and present only at one time point. No clear correlation between ELIspot and genome status or any other research end point. What have we learnt from the trial the vaccine is safe!
15 Challenge 1 How do you go about discovering and validating pharmacodynamic biomarkers for first in class agents with suitable confidence to be tested in the clinic? (case study 1) Novel biology that is evolving with time Biomarker will be tested for the first time in the clinic, so no previous knowledge What degree of validation do you really need to do, can do or pragmatically worth doing?
16 Challenge 2 How do you define patient selection/enrichment criteria for an early phase trial during dose expansion (case study 2) how do you define cut-offs for eligibility what can you do pre-clinically to define acceptance criteria for patient selection assays what other factors would contribute to seeing a difference between validation work and trial samples? What can be done to minimise this effect? how do you validate tumour based PD biomarker assays in the absence of tumour material
17 Challenge 3 Is there a consensus on the extent of validation that would be required for primary vs secondary vs research based assays? (case study 3) Should there be a difference or should all assays be validated to same degree? What does fit-for purpose mean to you? Are there any guidelines or regulatory requirements for biomarker assays validation for primary/secondary or research assays that you can recommend? How do you define acceptance criteria that would give you confidence to detect a signal above noise in a heterogeneous patient group consisting of 3-6 patients per dose?
18 Limitations in the real world Biomarkers are never black and white, mostly shades of grey! Little evidence to correlate PD effect with clinical outcome. How do you correlate expression levels of target with response. What % of cells need to be positive, to what degree of positivity? To what degree, how long, in what tissue do you need to see PD Tumour PD markers not dynamic. Correlation between tumour and blood borne markers not there yet. Resistance and degrees of tumour heterogeneity Challenges at sites with sample handling, processing, shipment and storage Degree of variation in analytical assay validation what does fit for purpose mean to you? 18
19 Summary Biomarkers are key to the success of early phase oncology trials and help minimise phase II attrition Keep a balanced and pragmatic view of the agent/trial. Biomarkers are a key component of future drug development but not the only one! Be clear on which assay you need to address a specific question, the expectation of the assay and how it will help with trial design, success criteria, future investment. Think quality vs quantity, cost to know, value added and accept that their will be limitations with each assay It is challenging to discover, develop and have a robust validated clinical assay, particularly for FIC agents. 19
20 Thank You & Questions? Sidath Katugampola Centre for Drug Development Cancer Research UK
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era
 Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective
 PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
Janet E. Dancey NCIC CTG NEW INVESTIGATOR CLINICAL TRIALS COURSE. August 9-12, 2011 Donald Gordon Centre, Queen s University, Kingston, Ontario
 Janet E. Dancey NCIC CTG NEW INVESTIGATOR CLINICAL TRIALS COURSE August 9-12, 2011 Donald Gordon Centre, Queen s University, Kingston, Ontario Session: Correlative Studies in Phase III Trials Title: Design
Janet E. Dancey NCIC CTG NEW INVESTIGATOR CLINICAL TRIALS COURSE August 9-12, 2011 Donald Gordon Centre, Queen s University, Kingston, Ontario Session: Correlative Studies in Phase III Trials Title: Design
Immuno-Oncology Clinical Trials Update: Checkpoint Inhibitors Others (not Anti-PD-L1/PD-1) Issue 4 January 2017
 Delivering a Competitive Intelligence Advantage Immuno-Oncology Clinical Trials Update: Checkpoint Inhibitors Others (not Anti-PD-L1/PD-1) Issue 4 January 2017 Immuno-Oncology CLINICAL TRIALS UPDATE The
Delivering a Competitive Intelligence Advantage Immuno-Oncology Clinical Trials Update: Checkpoint Inhibitors Others (not Anti-PD-L1/PD-1) Issue 4 January 2017 Immuno-Oncology CLINICAL TRIALS UPDATE The
More cancer patients are being treated with immunotherapy, but
 Bristol-Myers Squibb and Five Prime Present Phase 1a/1b Data Evaluating Cabiralizumab (anti-csf-1 receptor antibody) with Opdivo (nivolumab) in Patients with Advanced Solid Tumors PRINCETON, N.J. & SOUTH
Bristol-Myers Squibb and Five Prime Present Phase 1a/1b Data Evaluating Cabiralizumab (anti-csf-1 receptor antibody) with Opdivo (nivolumab) in Patients with Advanced Solid Tumors PRINCETON, N.J. & SOUTH
NCRI Biomarkers & Imaging CSG Cell-free DNA workshop
 NCRI Biomarkers & Imaging CSG Cell-free DNA workshop Workshop Report Christie Education Centre, Manchester 30th January 2014 Sponsored by Workshop summary 86 delegates from a variety of specialities attended
NCRI Biomarkers & Imaging CSG Cell-free DNA workshop Workshop Report Christie Education Centre, Manchester 30th January 2014 Sponsored by Workshop summary 86 delegates from a variety of specialities attended
PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM
 HEMATOLOGY ONCOLOGY PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM COMMITTED TO ADVANCING DRUG DEVELOPMENT IN ONCOLOGY $ MARKETPLACE COMPLEXITIES increasingly competitive marketplace and rising cost pressures
HEMATOLOGY ONCOLOGY PPD S EXPERT HEMATOLOGY AND ONCOLOGY TEAM COMMITTED TO ADVANCING DRUG DEVELOPMENT IN ONCOLOGY $ MARKETPLACE COMPLEXITIES increasingly competitive marketplace and rising cost pressures
Advanced Epigenetic Technology BIO-Europe Spring 2018 Amsterdam, The Netherlands
 Advanced Epigenetic Technology BIO-Europe Spring 2018 Amsterdam, The Netherlands March 13 th Zenith Profile Founded Company Status Products and Technology Location Financing 2014-2016 Shares Outstanding
Advanced Epigenetic Technology BIO-Europe Spring 2018 Amsterdam, The Netherlands March 13 th Zenith Profile Founded Company Status Products and Technology Location Financing 2014-2016 Shares Outstanding
Accelerate Your Research with Conversant Bio
 Imagination has given us the steam engine, the telephone, the talkingmachine and the automobile, for these things had to be dreamed of before they became realities. So I believe that dreams... are likely
Imagination has given us the steam engine, the telephone, the talkingmachine and the automobile, for these things had to be dreamed of before they became realities. So I believe that dreams... are likely
A Guide for Choosing the Right CRO in Immuno- Oncology Studies. Mitigating Risk in the Clinic.
 A Guide for Choosing the Right CRO in Immuno- Oncology Studies Mitigating Risk in the Clinic Introduction Immunotherapy trials in oncology have shown tremendous promise and may represent the most promising
A Guide for Choosing the Right CRO in Immuno- Oncology Studies Mitigating Risk in the Clinic Introduction Immunotherapy trials in oncology have shown tremendous promise and may represent the most promising
IRESSA (Gefitinib) The Journey. Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca
 IRESSA (Gefitinib) The Journey Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca Overview The Drug The Biomarker and Clinical Trials Sampling Lessons Learned The
IRESSA (Gefitinib) The Journey Anne De Bock Portfolio Leader, Oncology/Infection European Regulatory Affairs AstraZeneca Overview The Drug The Biomarker and Clinical Trials Sampling Lessons Learned The
Spontaneous canine malignancies: Models for precision cancer medicine
 National Cancer Institute Spontaneous canine malignancies: Models for precision cancer medicine Amy K. LeBlanc, DVM DACVIM (Oncology) Director, NCI Comparative Oncology Program NIH/NCI Center for Cancer
National Cancer Institute Spontaneous canine malignancies: Models for precision cancer medicine Amy K. LeBlanc, DVM DACVIM (Oncology) Director, NCI Comparative Oncology Program NIH/NCI Center for Cancer
Oncology Drug Development Using Molecular Pathology
 Oncology Drug Development Using Molecular Pathology Introduction PRESENTERS Lee Schacter, PhD, MD, FACP Executive Medical Director, Oncology Clinipace Worldwide Martha Bonino Director, Strategic Accounts
Oncology Drug Development Using Molecular Pathology Introduction PRESENTERS Lee Schacter, PhD, MD, FACP Executive Medical Director, Oncology Clinipace Worldwide Martha Bonino Director, Strategic Accounts
Patients Driving Progress
 Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Patients Driving Progress Ellen V Sigal, PhD WIN Symposium 2018 June 25, 2018 Paris, France Nothing to disclose The Evolution of Patient Advocacy The Agnew Clinic, Thomas Eakins (1899) Partial mastectomy
Mind the Gap : Challenges in First-in- Man Evaluation of Immuno-Oncology Drugs
 Mind the Gap : Challenges in First-in- Man Evaluation of Immuno-Oncology Drugs Ignacio Garcia-Ribas MD, PhD Senior Medical Director Early Clinical Research & Development, Oncology Therapeutic Area Takeda
Mind the Gap : Challenges in First-in- Man Evaluation of Immuno-Oncology Drugs Ignacio Garcia-Ribas MD, PhD Senior Medical Director Early Clinical Research & Development, Oncology Therapeutic Area Takeda
Cardiovascular Safety Assessments in Early Phase Oncology Clinical Trials
 CSRC Think Tank: Detection, Assessment and Risk Mitigation of Cardiac Safety Signals in Oncology Drug Development Cardiovascular Safety Assessments in Early Phase Oncology Clinical Trials Boaz Mendzelevski,
CSRC Think Tank: Detection, Assessment and Risk Mitigation of Cardiac Safety Signals in Oncology Drug Development Cardiovascular Safety Assessments in Early Phase Oncology Clinical Trials Boaz Mendzelevski,
Liquid Biopsies. Next Generation Cancer Molecular Diagnostics
 Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Liquid Biopsies Next Generation Cancer Molecular Diagnostics Forward Looking Statements 2 Statements pertaining to future financial and/or operating results, future research, diagnostic tests and technology
Spontaneous canine malignancies: Models for precision cancer medicine
 National Cancer Institute Spontaneous canine malignancies: Models for precision cancer medicine Amy K. LeBlanc, DVM DACVIM (Oncology) Director, NCI Comparative Oncology Program NIH/NCI Center for Cancer
National Cancer Institute Spontaneous canine malignancies: Models for precision cancer medicine Amy K. LeBlanc, DVM DACVIM (Oncology) Director, NCI Comparative Oncology Program NIH/NCI Center for Cancer
ArQule Jefferies Global Healthcare Conference June 2015
 ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
Opportunities in Pain Research with the NIH HEAL Initiative
 Opportunities in Pain Research with the NIH HEAL Initiative Clinton Wright, M.D. Director, Division of Clinical Research National Institute of Neurological Disorders and Stroke, NIH September 20, 2018
Opportunities in Pain Research with the NIH HEAL Initiative Clinton Wright, M.D. Director, Division of Clinical Research National Institute of Neurological Disorders and Stroke, NIH September 20, 2018
Survey Results Q1. How would you best describe your organization?
 Survey Results Q1. How would you best describe your organization? Q2. How high would you rate the priority of Circulating Tumor Cells in you organization? Q3. What do you think is the biggest challenge
Survey Results Q1. How would you best describe your organization? Q2. How high would you rate the priority of Circulating Tumor Cells in you organization? Q3. What do you think is the biggest challenge
Is a Maximal Tolerated Dose in Human useful for drug development?
 Is a Maximal Tolerated Dose in Human useful for drug development? How to define an acceptable highest dose to be tested? EuFeMED, London Pre-conference Workshop 17-May-2017 Eric Legangneux Philippe Grosjean
Is a Maximal Tolerated Dose in Human useful for drug development? How to define an acceptable highest dose to be tested? EuFeMED, London Pre-conference Workshop 17-May-2017 Eric Legangneux Philippe Grosjean
Personalised Healthcare. will it deliver? Bob Holland Head of Personalised Healthcare & Biomarkers Innovative Medicines AstraZeneca R&D
 Personalised Healthcare x will it deliver? Bob Holland Head of Personalised Healthcare & Biomarkers Innovative Medicines AstraZeneca R&D Personalised Healthcare It is delivering! Bob Holland Head of Personalised
Personalised Healthcare x will it deliver? Bob Holland Head of Personalised Healthcare & Biomarkers Innovative Medicines AstraZeneca R&D Personalised Healthcare It is delivering! Bob Holland Head of Personalised
Watson Summit Prague 2017
 Watson for Oncology Matěj Adam Watson Health Executive May 2017 Watson Summit Prague 2017 an IBM Company Leadership & 23 Years Patent Leadership Research Spend Global Presence Technology 7300+ 2015 $6B
Watson for Oncology Matěj Adam Watson Health Executive May 2017 Watson Summit Prague 2017 an IBM Company Leadership & 23 Years Patent Leadership Research Spend Global Presence Technology 7300+ 2015 $6B
COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON EVALUATION OF ANTICANCER MEDICINAL PRODUCTS IN MAN
 The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 24 July 2003 EMEA/ COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON EVALUATION
The European Agency for the Evaluation of Medicinal Products Evaluation of Medicines for Human Use London, 24 July 2003 EMEA/ COMMITTEE FOR PROPRIETARY MEDICINAL PRODUCTS (CPMP) NOTE FOR GUIDANCE ON EVALUATION
PK and PKPD considerations for dose selection in the development of pembrolizumab
 PK and PKPD considerations for dose selection in the development of pembrolizumab Dinesh de Alwis, PhD Quantitative Pharmacology and Pharmacometrics. MSD. Disclosure Information Dinesh de Alwis, Ph.D.
PK and PKPD considerations for dose selection in the development of pembrolizumab Dinesh de Alwis, PhD Quantitative Pharmacology and Pharmacometrics. MSD. Disclosure Information Dinesh de Alwis, Ph.D.
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *)
 DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
DOSE SELECTION FOR CARCINOGENICITY STUDIES OF PHARMACEUTICALS *) Guideline Title Dose Selection for Carcinogenicity Studies of Pharmaceuticals *) Legislative basis Directive 75/318/EEC as amended Date
Liver Forum Cirrhosis Working Group Arun J. Sanyal
 Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
Liver Forum Cirrhosis Working Group Arun J. Sanyal Z Reno Vlahcevic Professor of Medicine VCU School of Medicine Richmond, VA 2 Liver Forum 8 DRUG DEVELOPMENT PATHWAYS Regular approval pathway: based on
GSK Oncology. Axel Hoos, MD, PhD Senior Vice President, Oncology R&D. March 8, 2017
 GSK Oncology Axel Hoos, MD, PhD Senior Vice President, Oncology R&D March 8, 217 GSK pipeline Oncology R&D Strategy Maximizing survival through transformational medicines and combinations Cancer Epigenetics
GSK Oncology Axel Hoos, MD, PhD Senior Vice President, Oncology R&D March 8, 217 GSK pipeline Oncology R&D Strategy Maximizing survival through transformational medicines and combinations Cancer Epigenetics
ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals. Step 5
 European Medicines Agency October 2008 EMEA/CHMP/ICH/383/1995 ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON DOSE SELECTION FOR CARCINOGENICITY
European Medicines Agency October 2008 EMEA/CHMP/ICH/383/1995 ICH Topic S1C(R2) Dose Selection for Carcinogenicity Studies of Pharmaceuticals Step 5 NOTE FOR GUIDANCE ON DOSE SELECTION FOR CARCINOGENICITY
The questions are changing in early phase cancer clinical trials: Opportunities for novel statistical designs
 The questions are changing in early phase cancer clinical trials: Opportunities for novel statistical designs Elizabeth Garrett-Mayer, PhD Associate Professor Director of Biostatistics, Hollings Cancer
The questions are changing in early phase cancer clinical trials: Opportunities for novel statistical designs Elizabeth Garrett-Mayer, PhD Associate Professor Director of Biostatistics, Hollings Cancer
Delivering Value Through Personalized Medicine: An Industry Perspective
 Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Shifting the MTD paradigm in Oncology
 Shifting the MTD paradigm in Oncology Kevin Smart and Georgina Meneses-Lorente Clinical Pharmacology, Roche Products Ltd, Roche Innovation Centre, Welwyn, UK. MabCSF-1R Macrophages (Mφ) are Polarized during
Shifting the MTD paradigm in Oncology Kevin Smart and Georgina Meneses-Lorente Clinical Pharmacology, Roche Products Ltd, Roche Innovation Centre, Welwyn, UK. MabCSF-1R Macrophages (Mφ) are Polarized during
PHASE 1 CLINICAL TRIALS: DESIGNS AND CONSIDERATIONS. Sarit Assouline, MD, MSc, FRCPC Associate professor, McGill University Jewish General Hospital
 PHASE 1 CLINICAL TRIALS: DESIGNS AND CONSIDERATIONS Sarit Assouline, MD, MSc, FRCPC Associate professor, McGill University Jewish General Hospital Outline Introduction Goal of Phase I studies in oncology
PHASE 1 CLINICAL TRIALS: DESIGNS AND CONSIDERATIONS Sarit Assouline, MD, MSc, FRCPC Associate professor, McGill University Jewish General Hospital Outline Introduction Goal of Phase I studies in oncology
ArQule CorporateUpdate
 ArQule April 2015 1 ArQule CorporateUpdate Safe Harbor This presentation and other statements by ArQule may contain forwardlooking statements within the meaning of the Private Securities Litigation Reform
ArQule April 2015 1 ArQule CorporateUpdate Safe Harbor This presentation and other statements by ArQule may contain forwardlooking statements within the meaning of the Private Securities Litigation Reform
Combining HS-110 and anti-pd-1 in NSCLC. September 1, 2015
 Combining HS-110 and anti-pd-1 in NSCLC September 1, 2015 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
Combining HS-110 and anti-pd-1 in NSCLC September 1, 2015 Forward Looking Statements This presentation includes statements that are, or may be deemed, forward-looking statements. In some cases, these forward-looking
Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach
 Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach Veterinary Pathology 49(2) 357-361 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
Appropriate Use of Recovery Groups in Nonclinical Toxicity Studies: Value in a Science-Driven Case-by-Case Approach Veterinary Pathology 49(2) 357-361 ª The Author(s) 2012 Reprints and permission: sagepub.com/journalspermissions.nav
Ensuring Success in Early Phase Oncology Clinical Trials
 Ensuring Success in Early Phase Oncology Clinical Trials The numbers of oncology drugs in clinical development is more than twice the number in any other therapeutic area (PharmaLive.com). Yet despite
Ensuring Success in Early Phase Oncology Clinical Trials The numbers of oncology drugs in clinical development is more than twice the number in any other therapeutic area (PharmaLive.com). Yet despite
Cancer Biomarkers: Hope, Hype or Help. Does the past predict the future?
 Cancer Biomarkers: Hope, Hype or Help. Does the past predict the future? ECMC Quality Assurance & Translational Science Network Group 10th Anniversary Symposium of the ECMC QATS Network Group London, May
Cancer Biomarkers: Hope, Hype or Help. Does the past predict the future? ECMC Quality Assurance & Translational Science Network Group 10th Anniversary Symposium of the ECMC QATS Network Group London, May
SURVEY REPORT CLINICAL TRIALS: PAST, PRESENT AND FUTURE
 SURVEY REPORT CLINICAL TRIALS: PAST, PRESENT AND FUTURE + OVERVIEW Clinical trials have changed dramatically in the last 10 years. Sponsors are increasingly pressured to get drugs to market faster and
SURVEY REPORT CLINICAL TRIALS: PAST, PRESENT AND FUTURE + OVERVIEW Clinical trials have changed dramatically in the last 10 years. Sponsors are increasingly pressured to get drugs to market faster and
US Regulatory Considerations for Therapeutic Cancer Vaccines
 US Regulatory Considerations for Therapeutic Cancer Vaccines Peter Bross, M.D., Team Leader, Clinical Oncology, FDA Center for Biologics Evaluation and Research 1 Immune Therapies: The Future Is Now Tuesday,
US Regulatory Considerations for Therapeutic Cancer Vaccines Peter Bross, M.D., Team Leader, Clinical Oncology, FDA Center for Biologics Evaluation and Research 1 Immune Therapies: The Future Is Now Tuesday,
Leerink Immuno-Oncology Roundtable Conference
 Leerink Immuno-Oncology Roundtable Conference September 28, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private
Leerink Immuno-Oncology Roundtable Conference September 28, 2017 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private
NASH Regulatory Landscape. Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH
 NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
NASH Regulatory Landscape Veronica Miller, PhD Forum for Collaborative Research UC Berkeley SPH Disclosures Liver Forum sponsors (last slides) Advisory (Sanofi) Miller_July 7_2017 www.forumresearch.org
Guidance for Industry
 Guidance for Industry Clinical Considerations for Therapeutic Cancer Vaccines DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments on this draft guidance by the date provided
Guidance for Industry Clinical Considerations for Therapeutic Cancer Vaccines DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments on this draft guidance by the date provided
Early drug development and emerging new treatments Unmasking Side Effects from First-in-Man Antineoplastic Medicine
 Early drug development and emerging new treatments Unmasking Side Effects from First-in-Man Antineoplastic Medicine Ulrik Lassen, Professor, MD, PH.D. Phase 1 Unit, www.rigshospitalet.dk/phase1 Department
Early drug development and emerging new treatments Unmasking Side Effects from First-in-Man Antineoplastic Medicine Ulrik Lassen, Professor, MD, PH.D. Phase 1 Unit, www.rigshospitalet.dk/phase1 Department
Oncolytic Viruses: Reovirus
 T S X : O N C N A S D A Q : O N C Y International Society for Biological Therapy of Cancer 2008 Oncology Biologics Development Primer Oncolytic Viruses: Reovirus REOLYSIN - mode of action REOLYSIN contains
T S X : O N C N A S D A Q : O N C Y International Society for Biological Therapy of Cancer 2008 Oncology Biologics Development Primer Oncolytic Viruses: Reovirus REOLYSIN - mode of action REOLYSIN contains
HOW TO MAXIMIZE PATIENT RECRUITMENT IN ONCOLOGY TRIALS A BIOPHARMA DIVE PLAYBOOK
 HOW TO MAXIMIZE PATIENT RECRUITMENT IN ONCOLOGY TRIALS A BIOPHARMA DIVE PLAYBOOK Over the last several decades, patient recruitment for clinical trials has remained a major barrier to rapid execution of
HOW TO MAXIMIZE PATIENT RECRUITMENT IN ONCOLOGY TRIALS A BIOPHARMA DIVE PLAYBOOK Over the last several decades, patient recruitment for clinical trials has remained a major barrier to rapid execution of
Anthony Chalmers University of Glasgow
 Radiotherapy-drug combinations for NSCLC: current and potential UK collaborative initiatives Anthony Chalmers University of Glasgow UK SABR Consortium 25th November 2016 Increase tumour control and cure
Radiotherapy-drug combinations for NSCLC: current and potential UK collaborative initiatives Anthony Chalmers University of Glasgow UK SABR Consortium 25th November 2016 Increase tumour control and cure
R&D UNICANCER Oncology Research
 R&D UNICANCER Oncology Research R&D UNICANCER A national academic sponsor dedicated to clinical research in oncology Our missions: Promote and accelerate Clinical Research in Oncology, especially in areas
R&D UNICANCER Oncology Research R&D UNICANCER A national academic sponsor dedicated to clinical research in oncology Our missions: Promote and accelerate Clinical Research in Oncology, especially in areas
Screening for novel oncology biomarker panels using both DNA and protein microarrays. John Anson, PhD VP Biomarker Discovery
 Screening for novel oncology biomarker panels using both DNA and protein microarrays John Anson, PhD VP Biomarker Discovery Outline of presentation Introduction to OGT and our approach to biomarker studies
Screening for novel oncology biomarker panels using both DNA and protein microarrays John Anson, PhD VP Biomarker Discovery Outline of presentation Introduction to OGT and our approach to biomarker studies
Combination dose finding studies in oncology: an industry perspective
 Combination dose finding studies in oncology: an industry perspective Jian Zhu, Ling Wang Symposium on Dose Selection for Cancer Treatment Drugs Stanford, May 12 th 2017 Outline Overview Practical considerations
Combination dose finding studies in oncology: an industry perspective Jian Zhu, Ling Wang Symposium on Dose Selection for Cancer Treatment Drugs Stanford, May 12 th 2017 Outline Overview Practical considerations
JEFFERIES 2018 LONDON HEALTHCARE CONFERENCE NOVEMBER 15, 2018
 1 JEFFERIES 2018 LONDON HEALTHCARE CONFERENCE NOVEMBER 15, 2018 FORWARD-LOOKING STATEMENTS This presentation includes forward-looking statements that involve risks, uncertainties and other factors, many
1 JEFFERIES 2018 LONDON HEALTHCARE CONFERENCE NOVEMBER 15, 2018 FORWARD-LOOKING STATEMENTS This presentation includes forward-looking statements that involve risks, uncertainties and other factors, many
Basket and Umbrella Trial Designs in Oncology
 Basket and Umbrella Trial Designs in Oncology Eric Polley Biomedical Statistics and Informatics Mayo Clinic Polley.Eric@mayo.edu Dose Selection for Cancer Treatment Drugs Stanford Medicine May 2017 1 /
Basket and Umbrella Trial Designs in Oncology Eric Polley Biomedical Statistics and Informatics Mayo Clinic Polley.Eric@mayo.edu Dose Selection for Cancer Treatment Drugs Stanford Medicine May 2017 1 /
The summit and its purpose
 Sponsors: 1 The summit and its purpose The Translational Hearing Research Summit: Biological and Pharmacological Approaches was an international summit that gathered 159 delegates, from 14 countries to
Sponsors: 1 The summit and its purpose The Translational Hearing Research Summit: Biological and Pharmacological Approaches was an international summit that gathered 159 delegates, from 14 countries to
DOWNLOAD OR READ : UPDATE ON BIOMARKERS IN ASTHMA AN ISSUE OF IMMUNOLOGY AND ALLERGY CLINICS PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : UPDATE ON BIOMARKERS IN ASTHMA AN ISSUE OF IMMUNOLOGY AND ALLERGY CLINICS PDF EBOOK EPUB MOBI Page 1 Page 2 update on biomarkers in asthma an issue of immunology and allergy clinics
DOWNLOAD OR READ : UPDATE ON BIOMARKERS IN ASTHMA AN ISSUE OF IMMUNOLOGY AND ALLERGY CLINICS PDF EBOOK EPUB MOBI Page 1 Page 2 update on biomarkers in asthma an issue of immunology and allergy clinics
Incorporating pharmacodynamic, response and patient selection biomarkers. Paul Elvin PhD Chief Translational Science Officer Aptus Clinical
 Incorporating pharmacodynamic, response and patient selection biomarkers Paul Elvin PhD Chief Translational Science Officer Aptus Clinical 22 Oncology drug development Biomarkers key for: Strong hypothesis
Incorporating pharmacodynamic, response and patient selection biomarkers Paul Elvin PhD Chief Translational Science Officer Aptus Clinical 22 Oncology drug development Biomarkers key for: Strong hypothesis
Phase II Design. Kim N. Chi NCIC/NCIC-CTG NEW INVESTIGATORS CLINICAL TRIALS COURSE
 Phase II Design Kim N. Chi NCIC/NCIC-CTG NEW INVESTIGATORS CLINICAL TRIALS COURSE Phase II Study Results Phase II Study Results Agenda Objectives of phase II trials Endpoints of phase II trials Statistical
Phase II Design Kim N. Chi NCIC/NCIC-CTG NEW INVESTIGATORS CLINICAL TRIALS COURSE Phase II Study Results Phase II Study Results Agenda Objectives of phase II trials Endpoints of phase II trials Statistical
Personalized medecine Biomarker
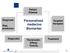 Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Five Prime Therapeutics, Inc. Corporate Overview
 Five Prime Therapeutics, Inc. Corporate Overview June 2015 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities
Five Prime Therapeutics, Inc. Corporate Overview June 2015 NASDAQ:FPRX Forward-Looking Statements Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities
Transforming science into medicine
 Transforming science into medicine 2 Forward-looking statements This presentation contains forward-looking statements. These statements include words like may, expects, believes, plans, scheduled, and
Transforming science into medicine 2 Forward-looking statements This presentation contains forward-looking statements. These statements include words like may, expects, believes, plans, scheduled, and
DMPK. APRIL 27 TH 2017 Jan Neelissen Scientific Adviser Science & Technology
 DMPK APRIL 27 TH 2017 Jan Neelissen Scientific Adviser Science & Technology What I learned is a good DMPK profile have acceptable water solubility for development be completely absorbed, preferably via
DMPK APRIL 27 TH 2017 Jan Neelissen Scientific Adviser Science & Technology What I learned is a good DMPK profile have acceptable water solubility for development be completely absorbed, preferably via
Sviluppo di disegni sperimentali: esperienze e proposte. Gianluca Fincato Medical Director BU Oncology Novartis Farma SpA
 Sviluppo di disegni sperimentali: esperienze e proposte Gianluca Fincato Medical Director BU Oncology Novartis Farma SpA ISS Roma, 17/11/2017 Use of This Slide Deck This material, and all contents cited
Sviluppo di disegni sperimentali: esperienze e proposte Gianluca Fincato Medical Director BU Oncology Novartis Farma SpA ISS Roma, 17/11/2017 Use of This Slide Deck This material, and all contents cited
Novogen Limited. An emerging oncology drug developer with two clinical-stage programs NRT NVGN. April 2017
 Novogen Limited An emerging oncology drug developer with two clinical-stage programs NRT April 2017 NVGN Forward-Looking Statements This presentation contains forward-looking statements within the meaning
Novogen Limited An emerging oncology drug developer with two clinical-stage programs NRT April 2017 NVGN Forward-Looking Statements This presentation contains forward-looking statements within the meaning
CPRIT Overview. Cancer Centers Administrators Forum April 4, 2016
 CPRIT Overview Cancer Centers Administrators Forum April 4, 2016 CPRIT s Unique Role in the Fight Against Cancer Overview: Created by the Texas Legislature and citizens in 2007 $3 billion over 10 years
CPRIT Overview Cancer Centers Administrators Forum April 4, 2016 CPRIT s Unique Role in the Fight Against Cancer Overview: Created by the Texas Legislature and citizens in 2007 $3 billion over 10 years
Corporate Presentation May Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers
 Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Oncology Drug Development
 Oncology Drug Development Advantages and challenges of using stratified clinical studies Simon J Hollingsworth The 18th Anti-Tumour Drug Development Forum 21 February Japan Stratified Medicine In The Clinic
Oncology Drug Development Advantages and challenges of using stratified clinical studies Simon J Hollingsworth The 18th Anti-Tumour Drug Development Forum 21 February Japan Stratified Medicine In The Clinic
ONCOS-102 in mesothelioma. Dr Magnus Jaderberg Chief Medical Officer Targovax
 ONCOS-102 in mesothelioma Dr Magnus Jaderberg Chief Medical Officer Targovax ONCOS CLINICAL DEVELOPMENT STRATEGY 1 2 3 4 Path-to-market Mesothelioma Proof-of-concept CPI refractory Proof-of-concept New
ONCOS-102 in mesothelioma Dr Magnus Jaderberg Chief Medical Officer Targovax ONCOS CLINICAL DEVELOPMENT STRATEGY 1 2 3 4 Path-to-market Mesothelioma Proof-of-concept CPI refractory Proof-of-concept New
33 rd Annual J.P. Morgan Healthcare Conference. January 2015
 33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
33 rd Annual J.P. Morgan Healthcare Conference January 2015 Forward-looking Statements This presentation contains forward-looking statements, which express the current beliefs and expectations of management.
Paediatric Strategy Forum A new concept proposed by ACCELERATE
 A new concept proposed by ACCELERATE 1 2 s Objective - provide a unique opportunity for interaction between all stakeholders (regulators, pharmaceutical companies, clinical and researcher academics and
A new concept proposed by ACCELERATE 1 2 s Objective - provide a unique opportunity for interaction between all stakeholders (regulators, pharmaceutical companies, clinical and researcher academics and
Cancer Immunotherapy Survey
 CHAPTER 8: Cancer Immunotherapy Survey All (N=100) Please classify your organization. Academic lab or center Small biopharmaceutical company Top 20 Pharma Mid-size pharma Diagnostics company Other (please
CHAPTER 8: Cancer Immunotherapy Survey All (N=100) Please classify your organization. Academic lab or center Small biopharmaceutical company Top 20 Pharma Mid-size pharma Diagnostics company Other (please
CTA Strengths. Organisational Structure Board. CTA Board CEO. Background to Cancer Trials Australia What we do well An increasing struggle Conclusions
 Models for Clinical Research: Cancer Trials Australia Professor Mark Rosenthal Chairman; Cancer Trials Australia and Director of Medical Oncology, Royal Melbourne Hospital For discussion: Background to
Models for Clinical Research: Cancer Trials Australia Professor Mark Rosenthal Chairman; Cancer Trials Australia and Director of Medical Oncology, Royal Melbourne Hospital For discussion: Background to
ITCC-P4 International Workshop
 ITCC P4 GA No. 116064 ITCC-P4 International Workshop IMPROVING PEDIATRIC ONCOLOGY DRUG DEVELOPMENT THROUGH PRECLINICAL RESEARCH September 27 th and 28 th, 2018 // Amsterdam, NL By invitation The event
ITCC P4 GA No. 116064 ITCC-P4 International Workshop IMPROVING PEDIATRIC ONCOLOGY DRUG DEVELOPMENT THROUGH PRECLINICAL RESEARCH September 27 th and 28 th, 2018 // Amsterdam, NL By invitation The event
Corporate Presentation September Nasdaq: ADXS
 Corporate Presentation September 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements regarding the ability and
Corporate Presentation September 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements regarding the ability and
PKPD modelling to optimize dose-escalation trials in Oncology
 PKPD modelling to optimize dose-escalation trials in Oncology Marina Savelieva Design of Experiments in Healthcare, Issac Newton Institute for Mathematical Sciences Aug 19th, 2011 Outline Motivation Phase
PKPD modelling to optimize dose-escalation trials in Oncology Marina Savelieva Design of Experiments in Healthcare, Issac Newton Institute for Mathematical Sciences Aug 19th, 2011 Outline Motivation Phase
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Revolutionizing the Treatment of Cancer January 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results
Promising Bifunctional Agents in Immuno- Oncology: A Roundtable Discussion with the Experts
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
How is Merck supporting innovation in oncology?
 How is Merck supporting innovation in oncology? We sponsor research and advanced medical education globally, reflecting our commitment to science, education and patient care We support the development
How is Merck supporting innovation in oncology? We sponsor research and advanced medical education globally, reflecting our commitment to science, education and patient care We support the development
NIH HEAL Initiative: Program Update and Funding Opportunities for Pain Research Available through NIH
 NIH HEAL Initiative: Program Update and Funding Opportunities for Pain Research Available through NIH AAMC Webinar November 8, 2018 Walter J. Koroshetz, MD Director, National Institute of Neurological
NIH HEAL Initiative: Program Update and Funding Opportunities for Pain Research Available through NIH AAMC Webinar November 8, 2018 Walter J. Koroshetz, MD Director, National Institute of Neurological
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer March 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward-looking statements. Rexahn's actual results may
Phase II trial designs and endpoints
 Eti Estimating anti-tumour tit activity it Phase II trial designs and endpoints Margaret Hutka MD PhD The Royal Marsden Hospital GI & Lymphoma Unit London, UK margaret.hutka@rmh.nhs.uk www.royalmarsden.nhs.uk
Eti Estimating anti-tumour tit activity it Phase II trial designs and endpoints Margaret Hutka MD PhD The Royal Marsden Hospital GI & Lymphoma Unit London, UK margaret.hutka@rmh.nhs.uk www.royalmarsden.nhs.uk
Building a Leading Oncology Franchise
 ASDAQ: MEIP Building a Leading Oncology Franchise 17th Annual eedham Healthcare Conference March 27, 2018 Forward-Looking Statements This presentation contains, and our officers and representatives may
ASDAQ: MEIP Building a Leading Oncology Franchise 17th Annual eedham Healthcare Conference March 27, 2018 Forward-Looking Statements This presentation contains, and our officers and representatives may
Innovations and Combinations for Novel Anticancer Strategies
 Innovations and Combinations for Novel Anticancer Strategies Axel-R. Hanauske, MD, Ph.D., MBA Senior Medical Fellow Oncology Early Phase Eli Lilly and Company 2017 Eli Lilly and Company Disclosure Information
Innovations and Combinations for Novel Anticancer Strategies Axel-R. Hanauske, MD, Ph.D., MBA Senior Medical Fellow Oncology Early Phase Eli Lilly and Company 2017 Eli Lilly and Company Disclosure Information
First Quarter 2018 Results
 First Quarter 2018 Results 1 April 27, 2017 Investor call Q1 2017 April 27, 2018 First quarter and after period close highlights and significant events Strong progress in the MIV-818 project Successful
First Quarter 2018 Results 1 April 27, 2017 Investor call Q1 2017 April 27, 2018 First quarter and after period close highlights and significant events Strong progress in the MIV-818 project Successful
Standardization for Preclinical Evaluation of Influenza Vaccines in Animal Models - WHO activities
 Standardization for Preclinical Evaluation of Influenza Vaccines in Animal Models - WHO activities 2 nd WHO Integrated Meeting on development and clinical trials of Influenza vaccines that induce broadly
Standardization for Preclinical Evaluation of Influenza Vaccines in Animal Models - WHO activities 2 nd WHO Integrated Meeting on development and clinical trials of Influenza vaccines that induce broadly
Use of Archived Tissues in the Development and Validation of Prognostic & Predictive Biomarkers
 Use of Archived Tissues in the Development and Validation of Prognostic & Predictive Biomarkers Richard Simon, D.Sc. Chief, Biometric Research Branch National Cancer Institute http://brb.nci.nih.gov Different
Use of Archived Tissues in the Development and Validation of Prognostic & Predictive Biomarkers Richard Simon, D.Sc. Chief, Biometric Research Branch National Cancer Institute http://brb.nci.nih.gov Different
INTERVIEW / GEORGE COUKOS. / κεντρικό πρόσωπο
 10 INTERVIEW / GEORGE COUKOS MD, PhD, Director of the Department of Oncology at the University Hospital of Lausanne (CHUV), Director of the Lausanne Branch of the Ludwig Institute for Cancer Research,
10 INTERVIEW / GEORGE COUKOS MD, PhD, Director of the Department of Oncology at the University Hospital of Lausanne (CHUV), Director of the Lausanne Branch of the Ludwig Institute for Cancer Research,
Preclinical Requirements for Therapeutic Studies in Humans with Advanced Cancer
 Preclinical Requirements for Therapeutic Studies in Humans with Advanced Cancer Scott Laurie MD, FRCPC The Ottawa Hospital Cancer Centre Associate Professor, University of Ottawa Disclosures I have no
Preclinical Requirements for Therapeutic Studies in Humans with Advanced Cancer Scott Laurie MD, FRCPC The Ottawa Hospital Cancer Centre Associate Professor, University of Ottawa Disclosures I have no
How the Changing Landscape of Oncology Drug Development and Approval Will Affect Advanced Practitioners
 How the Changing Landscape of Oncology Drug Development and Approval Will Affect Advanced Practitioners Richard Pazdur, MD Director Oncology Center of Excellence US Food and Drug Administration Learning
How the Changing Landscape of Oncology Drug Development and Approval Will Affect Advanced Practitioners Richard Pazdur, MD Director Oncology Center of Excellence US Food and Drug Administration Learning
Changing the paradigm: Removing barriers, building bridges
 Changing the paradigm: Removing barriers, building bridges Jeffrey S. Hyams, MD Mandell Braunstein Family Endowed Chair in Pediatric IBD Head, Division of Digestive Diseases, Hepatology, and Nutrition
Changing the paradigm: Removing barriers, building bridges Jeffrey S. Hyams, MD Mandell Braunstein Family Endowed Chair in Pediatric IBD Head, Division of Digestive Diseases, Hepatology, and Nutrition
Corporate Presentation October 2018 Nasdaq: ADXS
 Innovations in Immuno-Oncology Corporate Presentation October 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements
Innovations in Immuno-Oncology Corporate Presentation October 2018 Nasdaq: ADXS Forward-Looking Statements This presentation contains forward-looking statements, including, but not limited to, statements
Accelerating Innovation in Statistical Design
 Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
From Patients to Therapies. How could the BADIPS challenge progress towards improved in vitro models and novel patient therapies?
 From Patients to Therapies How could the BADIPS challenge progress towards improved in vitro models and novel patient therapies? 1 Aim of the BADIPS project 2 Overall objective BADIPS project The development
From Patients to Therapies How could the BADIPS challenge progress towards improved in vitro models and novel patient therapies? 1 Aim of the BADIPS project 2 Overall objective BADIPS project The development
Advances in external beam radiotherapy
 International Conference on Modern Radiotherapy: Advances and Challenges in Radiation Protection of Patients Advances in external beam radiotherapy New techniques, new benefits and new risks Michael Brada
International Conference on Modern Radiotherapy: Advances and Challenges in Radiation Protection of Patients Advances in external beam radiotherapy New techniques, new benefits and new risks Michael Brada
Oncology Clinical Research Lifecycle
 Oncology Clinical Research Lifecycle Robert E. Martell, MD, PhD Medical Oncologist Tufts Medical Center Associate Professor Tufts University School of Medicine Oncology Drug Development is Costly and High-Risk
Oncology Clinical Research Lifecycle Robert E. Martell, MD, PhD Medical Oncologist Tufts Medical Center Associate Professor Tufts University School of Medicine Oncology Drug Development is Costly and High-Risk
Corporate Presentation: 2018 Wedbush PacGrow Healthcare Conference August 14, 2018
 Corporate Presentation: 2018 Wedbush PacGrow Healthcare Conference August 14, 2018 2018 CytomX Therapeutics, Inc. 1 Forward Looking Statements Special Note Regarding Forward-Looking Statements This presentation
Corporate Presentation: 2018 Wedbush PacGrow Healthcare Conference August 14, 2018 2018 CytomX Therapeutics, Inc. 1 Forward Looking Statements Special Note Regarding Forward-Looking Statements This presentation
Improving the care of patients suffering from acute or chronic pain
 Improving the care of patients suffering from acute or chronic pain Petra Bloms-Funke, Hiltrud Liedgens, Keith Phillips, Jens Nagel 7 th December 2016 IMI webinar Pain Topics The continued high need for
Improving the care of patients suffering from acute or chronic pain Petra Bloms-Funke, Hiltrud Liedgens, Keith Phillips, Jens Nagel 7 th December 2016 IMI webinar Pain Topics The continued high need for
Asterias Biotherapeutics NYSE American: AST
 Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE American: AST November 2017 Forward-Looking Statements Statements
Clinical-Stage Cell Therapy Programs Addressing Significant Unmet Medical Needs in Neurology and Oncology Asterias Biotherapeutics NYSE American: AST November 2017 Forward-Looking Statements Statements
What are the regulatory issues. prevention trials?
 What are the regulatory issues that impact endpoints for prevention trials? Ann T. Farrell, M.D. Deputy Division Director Division of Drug Oncology Products Office of New Drugs Center for Drug Evaluation
What are the regulatory issues that impact endpoints for prevention trials? Ann T. Farrell, M.D. Deputy Division Director Division of Drug Oncology Products Office of New Drugs Center for Drug Evaluation
Investor Call. May 19, Nasdaq: IMGN
 Investor Call May 19, 2017 Nasdaq: IMGN Forward-Looking Statements This presentation includes forward-looking statements based on management's current expectations. These statements include, but are not
Investor Call May 19, 2017 Nasdaq: IMGN Forward-Looking Statements This presentation includes forward-looking statements based on management's current expectations. These statements include, but are not
Parkinson s Research Program
 Parkinson s Research Program Strategic Plan INTRODUCTION The Congressionally Directed Medical Research Programs (CDMRP) represents a unique partnership among the U.S. Congress, the military, and the public
Parkinson s Research Program Strategic Plan INTRODUCTION The Congressionally Directed Medical Research Programs (CDMRP) represents a unique partnership among the U.S. Congress, the military, and the public
