GENERAL INFORMATION/RATIONALE
|
|
|
- Alexina Ward
- 5 years ago
- Views:
Transcription
1 MEASURE DESCRIPTION The percentage of adults age 50 through 75 who had a minimum of one colorectal cancer screening test during the one year measurement period. (Refer to Table CCS-3 for qualifying tests and timeframes) Disclaimer: Measures reported by WCHQ healthcare organizations represent a specific aspect of care in relation to an evidence-based standard, but are not clinical guidelines and do not establish standards of care. All providers should have an individual care plan established with their patient. GENERAL INFORMATION/RATIONALE The United States Preventive Services Task Force (USPSTF) strongly recommends that clinicians screen men and women, at age 50 and older for colorectal cancer. The USPSTF concluded that the benefits from screening for colorectal cancer substantially outweigh potential harms, and that regardless of screening strategy chosen, it is likely to be cost-effective 1. The optimal interval for screening depends on the test. Annual fecal occult blood testing or fecal immunoassay test offers greater reductions in mortality rates than biennial screening. A 10-year interval has been recommended for colonoscopy, but a 5-year interval is recommended for flexible sigmoidoscopies because of their lower sensitivity. Fecal DNA Screening (Cologuard test) has been added as a new option for screening in 2015 (recommended interval every three years) 2. In persons identified as being at high-risk by their health care providers, initiating screening at an earlier age and at more frequent intervals is reasonable. It is recommended that all adults speak with their health care providers to determine, on an individual basis, the age at which to begin and end screenings, the best type of screening for individual circumstances, and the frequency of these screenings. References: 1. The Guide to Clinical Preventive Service 2007-Recommendations and rationale: Screening For Colorectal Cancer U.S. Preventive Services Task Force (USPSTF). Retrieved December 17, 2007 from and Cologuard update from 06/21/2016: 2. CMS Decision Memo for Screening for Colorectal Cancer - Stool DNA Testing, October 9, DEFINITIONS 12 Months: Measurement Period 24 Months: Measurement Period plus Prior Year 36 Months: Measurement Period plus Prior Two Years Primary Care Office Visit: Office visit in an outpatient, non-urgent care setting PCP: For WCHQ measure purposes, a primary care provider is defined as any General Practice, Internal Medicine, Family Practice, Pediatrics provider with the following degree types (MD, DO, PA, NP), and any other practitioners identified by the healthcare system as primary care practitioners. The rationale for the additional practitioner(s) must be documented and must be applied consistently across all measures by the organization. Age Range 50-75: Patients born between 01/01/1943 and 01/01/1968 1
2 DENOMINATOR DESCRIPTION Adults, whose age at the beginning of the one year measurement period is at least 50 and whose age at the end of the one year measurement period is less than 76 and who are alive as of the last day of the Measurement Period. Expired patients for whom a specific date of expiration cannot be found are excluded from the denominator population. The rationale for the denominator population is built from the following criteria: [Question 1] Is this a patient whose care is managed within the physician group? [Question 2] Is this a patient currently managed in our system? [Question 3] Is this a patient that is eligible for colorectal cancer screening? MINIMUM POPULATION SIZE Organization Level Reporting: For every WCHQ Ambulatory Measure, each organization must calculate total denominator population for this measure, not a sample (see Encounter Data section). If the Denominator for any given measure is less than 50 patients, the organization does not have to report the Numerator for the measure to WCHQ. To allow for appropriate comparisons of performance across organizations, a minimum population of 50 patients ensures a maximum of a 2% incremental scale on proportional measures. Publication on the Website: If the Denominator is less than 50, only the Physician Group Name, Population Size (N), and the following statement will display on The patient population is too small (N<50) for purposes of reliably predicting Physician Group performance. Historical Trend for Low Population Sizes: The historical trend display of Physician Group performance will not include measurement periods with population sizes less than 50. For each measurement period with insufficient data, there will be no display for that period. Site Level Reporting: Denominator Minimum: For site level reporting there must be a minimum of 100 patients per clinic in the denominator for each measure. If the clinic denominator for any given measure is less than 100 patients the organization does not publicly report the results for the measure. The results will still be included in the organization level data. Provider Minimum per Clinic: For site level reporting there are two options as follows: o A minimum of 3 providers per clinic who have patients in the measure denominator. There could be a provider or providers in a given clinic who do not get counted because they have no patients in the measure denominator. o If an organization desires, they can report site level data for a clinic with fewer than 3 providers as long as the clinic meets the 100 patient threshold. If your organization is planning to report results publicly for clinics with less than 3 providers, all clinics that meet this criteria will need to be reported. Publication on the Website: Clinics who do not have enough providers or patients to be publicly reported for a given measure or measures will still display on the website by name but without results and with a caveat indicating that data was reported but did not meet the minimum provider or population size. 2
3 Provider and /or Clinic Attrition Recommendation: 1. If a provider or clinic has left the organization prior to the end of the measurement period and if the organization can track the provider termination date, the provider will not be included in the site level reporting results. The provider or clinic is still included in the group level results. 2. If a clinic closes or is no longer affiliated with a health care system after the end of the measurement period and prior to next year s data being published a termination date and verbiage will be added next to that clinic s name on the website. This will require website updating throughout the year. Assignment of Provider to Clinic: Organizations can use their current internal site level reporting methodology to assign a provider to a clinic. A provider must be assigned to a home clinic. Organizations who are not already doing internal site level reporting can work with WCHQ for assistance. Assignment of Patient to Provider: For purposes of WCHQ site level reporting a patient must be attributed to one provider. Organizations can use their own internal algorithm to assign a patient to a provider. Those who are not already doing this can work with WCHQ for assistance. ENCOUNTER DATA Patients eligible for inclusion in the denominator include: (See Figure CCS-1) [Question 1] Is this a patient whose care is managed within the physician group? Patients who had at least two Primary Care office visits (Table CCS-1), regardless of diagnosis code, on different dates of service, to a PCP in the past 36 months (Measurement Period plus Prior Two Years). [Question 2] Is this a patient currently managed in our system? Patients who had at least one Primary Care office visit (Table CCS-1) regardless of diagnosis code, with a PCP in the most recent 24 months (Measurement Period plus Prior Year) [Question 3] Is this a patient that is eligible for Colorectal Cancer Screening? A patient is not eligible for Colorectal Cancer Screening for the following reasons: 1. Exclude those patients who have had a total colectomy (Table CCS-2). The organizations may look for these exclusions as far back as possible in the patient s history, through administrative data and/or medical record review. The exclusion can be identified through an ICD-9 or 10 diagnosis-based problem list. The problem must be ACTIVE. There is no limit on the look back date, but the date of documentation or onset date must occur prior to the end of the measurement period. 2. Patients who are in hospice or using hospice services during the measurement period. Evidence of hospice status can be demonstrated through the following: Administrative Data which can include: I. Billing codes from Table CCS-4 II. status extracted from an EMR 3
4 NUMERATOR DESCRIPTION The number of eligible adults who had one or more screenings for colorectal cancer as defined by the following criteria: Fecal Immunoassay Test (FIT) / Fecal Occult Blood Test (FOBT): One test during the measurement period (12 months) DNA Stool Test (Cologuard): One test during the measurement period or the two years prior to the measurement period Flexible Sigmoidoscopy: During the measurement period or the four years prior to the measurement period. CT Colonography (virtual colonoscopy): During the measurement period or the four years prior to the measurement period. Colonoscopy: During the measurement period or the nine years prior to the measurement period. This may be demonstrated through any of the following: Administrative data, which can include: o Table CCS-3 o Internal, external and/or patient reported screenings extracted electronically from an Electronic Medical Record (EMR), requiring one of the following: Year test was performed Date range test was performed, providing the entire range is within the measurement period. NOTE: This does not include results with a date of documentation only; the actual year or date range of the test must be present and be within the numerator description timeframes for inclusion in the numerator. Medical Record Review (Refer to Medical Record Review for Numerator Inclusion/Denominator Exclusion section) AST he Measurement Period during the Measurement Period 12 months or the four years prior to the Measurement Period) Period 12 months or the four years prior to the Measurement SIGMOIDOSCOPY) 4
5 5
6 SAMPLING METHODOLOGY Organizations unable to collect numerator data by electronic means can do so by using the following criteria for chart review; The Sample size for chart review is determined based in the following criteria: c = 95% Confidence Interval E = 5% Margin of Error N = Total number of patients in the denominator pool Use the Sample Size Generator at the Wisconsin Collaborative for Healthcare Quality website and enter values to generate the sample size appropriate for your organization A 10% over sample is recommended beyond the generated sample size. INTERNALLY DEVELOPED CODES DATA TRANSLATION/MAPPING REQUIREMENTS If a medical group utilizes internally generated codes to identify specific services or events required for a given WCHQ performance measure, the group may translate or map the information to the WCHQ performance measurement specifications. The medical group must assure that the internally generated code matches the clinical specificity of the standard (ICD-9 or 10, CPT) codes included in the WCHQ performance measurement specifications. In order to use internally developed codes for WCHQ performance measure reporting, the medical group needs to document the translation/mapping to the codes in the specifications. This documentation should include the internally generated code, a description of the internally developed code, any additional clinical information for the internally developed code, and the equivalent standard code with description from the WCHQ performance measurement specifications. Once the translation/ mapping documentation is established, the medical group s WCHQ performance measurement team must review the mapping on a yearly basis and document that internally developed codes have not changed and are being used in the manner described in the translation/ mapping document. The medical group must have documented processes in place for adding codes to the medical group s administrative data system and procedures to implement the internally developed codes. MEDICAL RECORD REVIEW FOR NUMERATOR INCLUSION/DENOMINATOR EXCLUSION If appropriate, and/or when necessary, every organization may complement their electronic capture of patient medical history with electronic or manual record review. The following criteria apply only to data captured/reviewed during medical record review. Numerator Inclusion For WCHQ Preventive Screening Measures, which can include, internal, external, and/or patient reported test results, proof of numerator compliance requires one of the following: Year test was performed Date range test was performed, providing the entire range is within the measurement period. 6
7 NOTES: o This does not include results with a date of documentation only; the actual year or date range of the test must be present and be within the numerator description timeframes for inclusion in the numerator. o CPT Code Blood, occult, by perioxidase activity (eg guaiac), qualitative, feces, single specimen is NOT numerator compliant. o A colon polyp pathology report with no colonoscopy procedure report can count towards a colonoscopy or flex sig as one of the two procedures would have to have been done to remove the polyp. This would be found during a chart review from reading a path report, not something that will have a code for electronic data collection. If it is not possible to tell the location of the polyp in the colon then default to flex sig because it is the shorter look back. Denominator Exclusion For all WCHQ Measures, proof of Denominator exclusion requires: Existence of exclusion criteria. These data may be retrieved, in whole or in part, from any of the following: Notation in Progress Note Notation in Medical History or Surgical History Flag/Field in Electronic Medical Record Documentation in patient chart REQUIRED DATA SUBMISSION FIELDS Fields required for data submission for this measure depend upon the methodology used. The fields are as follows: Organization Level Reporting: TOTAL POPULATION METHODOLOGY: Population Denominator (N) (Patients Eligible for Colorectal Cancer Screening) Numerator (Patients who had a minimum of one Colorectal Cancer Screen) Upon entry of these numbers, the rate is automatically calculated RANDOM SAMPLE METHODOLOGY: Population Denominator (N) (Patients Eligible for Colorectal Cancer Screening) Population Sample (n) (r) (Patients in Denominator Population whose records will be reviewed) o (n)=population Sample and (r)=patients Reviewed equal the same number o The Population Sample size must be determined using the WCHQ Sample Calculator Numerator (Patients who had a minimum of one Colorectal Cancer Screen from Population Sample) Upon entry of these numbers, the rate is automatically calculated HYBRID METHODOLOGY: Population Denominator (N) (Patients Eligible for Colorectal Cancer Screening) Administrative Review Denominator (Patients in Total Denominator Population whose numerator information is obtained through administrative data) Administrative Review Numerator (Patients who had a minimum of one Colorectal Cancer Screen found through administrative data) 7
8 Manual Review Denominator (Patients in Total Denominator Population whose numerator information cannot be obtained through administrative data) Manual Review Sample Size (Patients in Manual Review Denominator Population whose records will be reviewed) o The Manual Review Sample size must be determined using the WCHQ Sample Calculator plus a 10% over sample Manual Review Numerator (Patients who had a minimum of one Colorectal Cancer Screen found through Manual Review Sampling) Upon entry of these numbers, the Rates, Weight Factors and Total Reviewed are automatically calculated. Total Reviewed equals Administrative Review Denominator + Manual Review Sample Size. Site Level Reporting: Non-RBS Organizations: 1. Refer to the Non-RBS Site Level Reporting Upload Template.xls for specific instructions. RBS Organizations: 1. Refer to the RBS Site Level Reporting Upload Template.xls for specific instructions. PAYER STRATIFICATION: 1. Denominator data submission is requested by Payer Stratification for Medicare, Medicaid, Commercial and Uninsured. 2. Numerator data submission can be entered by primary payer category only for total population methodology Refer to Appendix A for definitions of the payer stratification categories FIELDS REQUIRED FOR MEASURE VALIDATION Validation of this measure will require patient level data files for Administrative Data and/or for Manual Review. The following indicates fields needed for validation, which may be helpful to consider when querying the measure: Denominator Data File fields: Patient Identifier (can be medical record number or other ID) Primary Care Office Visit Dates Provider Specialty Patient Date of Birth Exclusion codes used for Colectomy Date of Colectomy documentation Status Exclusion and Date Numerator Data File fields: Patient Identifier (can be medical record number or other ID) Colorectal Cancer Screening Code or Colorectal Cancer Screening Test Date Site Level Reporting fields: Clinic Name Period 8
9 Metric ID Clinical Topic Measure Clinic ID Clinic Name Metric Level (for A1C and LDL Testing and Control measures) Payer (optional) Numerator Denominator Percentage Provider Count Provider Minimum Count Flag Patient Minimum Count Flag Appendix A Primary Payer In keeping with the changing atmosphere of quality measurement and reporting, WCHQ would like for participating organizations to include the primary payer source with their data submissions for the ambulatory care measures. The primary payer source should be identified in the denominator upon answering the question, Is this patient current in our system? Once it has been determined that a patient is current because of a visit to their physician within the specified time period (12 months for chronic care measures and 24 months for preventive care measures), the payer should be pulled into the query. The primary payer should be the payer at the most recent office visit within the measurement period. There will be four categories of primary payer that will need to be submitted to WCHQ via the data submission tool: Medicare FFS, Medicaid (all types), Commercial (including Medicare HMO) and Uninsured/Self-Pay. The raw numbers for the denominator and numerator should be included for all three types of data submission, total population, hybrid, and sample. Rationale Opportunities exist for WCHQ to collect and report data on specific populations, like the Medicare population, through grant applications to begin to understand the disparities in quality of care. The purpose of this is to begin to understand the challenges of putting in additional data elements and complexities of data display for public reporting. At this time, the primary payer information will not be publicly reported. Definitions: Commercial: All plans not Medicaid or Medicare FFS (Includes VA, DoD, etc.) FFS Medicare: FFS plans, not Medicare HMO (Medicare Railroad is FFS Medicare) Medicaid: All Medicaid plans including those managed by commercial plans Uninsured: Self-pay individuals 9
10 APPENDIX B Code tables with descriptions. Reference also WCHQ Measure Spec Code List.xls Table CCS-1: CPT Codes to Identify Outpatient Visits CPT Codes Office or OP a visit E&M b, new patient Office or OP visit E&M, established patient Office or other OP consultations Home visit for evaluation and management of an established patient Initial comprehensive preventive medicine E&M, infant age younger than 1 year Initial comprehensive preventive medicine E&M, early childhood, age 1-4 years Initial comprehensive preventive medicine E&M, late childhood, age 5-11 years Initial preventive medicine E&M b Periodic comprehensive preventive medicine, established patient, infant age younger than 1 year Periodic comprehensive preventive medicine E&M, established patient, early childhood, age 1-4 years Periodic comprehensive preventive medicine, established patient, late childhood, age 5-11 years Periodic preventive medicine E&M b Preventive medicine counseling Preventive medicine counseling, group Preventive medicine counseling, group Risk assessment, admin and interpretation Unlisted preventive medicine service Initial care, per day, for evaluation and management of normal newborn infant seen in other than hospital or birthing center (Deleted 01/01/15) Complex chronic care coordination services; first hour of clinical staff time directed by a physician or other qualified health care professional with one face-to-face visit, per calendar month Chronic care management services, at least 20 minutes of clinical staff time directed by a physician or other qualified health care professional, per calendar month, with the following required elements: Multiple (two or more) chronic conditions expected to last at least 12 months or until the death of the patient; Chronic conditions place the patient at significant risk of death, acute exacerbation/decompensation, or functional decline; Comprehensive care plan established, implemented, revised or monitored Transitional Care Management Services (Moderate Complexity) Transitional Care Management Services (High Complexity) HCPCS Code G0402 (Effective 01/01/09) Initial preventive physical examination; face-to-face visit, services limited to new beneficiary during the first 12 months of Medicare enrollment 10
11 G0438 Annual wellness visit; includes a personalized prevention plan of service, initial visit G0439 Annual wellness visit; includes a personalized prevention plan of service, subsequent visit a outpatient b evaluation and management Table CCS-2: Codes to Identify Exclusions for Colorectal Cancer Screening ICD-9-CM Procedure Codes 45.8x Total intra-abdominal colectomy Laparoscopic total intra-abdominal colectomy Open total intra-abdominal colectomy Other and unspecified total intra-abdominal colectomy Effective 10/01/2015 ICD-10-PCS Procedure Codes 0DTE4ZZ Resection of Large Intestine, Percutaneous Endoscopic Approach 0DTE0ZZ Resection of Large Intestine, Open Approach 0DTE7ZZ Resection of Large Intestine, Via Natural or Artificial Opening 0DTE8ZZ Resection of Large Intestine, Via Natural or Artificial Opening Endoscopic ICD-9-CM Diagnosis Codes **V45.72 Effective 10/01/2015 ICD-10-CM Diagnosis Codes **Z90.49 Acquired absence of intestine (large, small) **Code can be included at the organization s discretion. If included, chart review is required for these visits. Acquired absence of other specified parts of digestive tract **Code can be included at the organization s discretion. If included, chart review is required for these visits. CPT Codes Colectomy, total, abdominal, without proctectomy; with ileostomy or ileoproctostomy Colectomy, total, abdominal, without proctectomy; with continent ileostomy (deleted 01/01/07) Colectomy, total, abdominal, without proctectomy; with rectal mucosectomy, ileoanal anastomosis, with or without loop ileostomy (deleted 01/01/07) Colectomy, total, abdominal, without proctectomy; with rectal mucosectomy, ileoanal anastomosis, creation of ileal reservoir (S or J), with or without loop ileostomy 11
12 44155 Colectomy, total, abdominal, with proctectomy; with ileostomy Colectomy, total, abdominal, with proctectomy; with continent ileostomy Colectomy, total abdominal, with proctectomy; with ileoanal anastomosis Colectomy, total abdominal, with proctectomy; with ileoanal anastomosis and creation of ileal reservoir Colectomy, total, abdominal, without proctectomy, with ileostomy or ileoproctostomy Colectomy, total, abdominal, with proctectomy, with ileoanal anastomosis, creationo of ileal reservoir (S or J), with loop ileostomy, includes rectal mucosectomy, when performed Colectomy, total, abdominal, with proctectomy, with ileostomy **44799 Unlisted Procedure, Intestine **Code can be included at the organization s discretion. If included, chart review is required for these visits. **45121 Proctectomy, complete (for congenital megacolon), abdominal and perineal approach; with subtotal or total colectomy, with multiple biopsies **Code can be included at the organization s discretion. If included, chart review is required for these visits. Table CCS-3: Codes to Identify Colorectal Cancer Screening FECAL IMMUNOASSAY TEST (FIT) / FECAL OCCULT BLOOD TEST (FOBT) CODES (This test must have occurred during the Measurement Period 12 months) CPT Codes Blood, occult; feces screening, 1-3 simultaneous determinations Blood, occult, by fecal hemoglobin determination by immunoassay, qualitative, feces, 1-3 simultaneous determinations LOINC Codes Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood Fecal Occult Blood 12
13 HCPCS Codes WCHQ Ambulatory Measure Specification G0328 Colorectal cancer screening, fecal occult blood test, immunoassay, 1-3 simultaneous DNA STOOL TEST (COLOGUARD) (This test must have occurred during the Measurement Period or the two years prior to the Measurement Period) CPT Codes (Effective 01/01/16) HCPCS Code G0464 (Deleted Effective 01/01/16) Oncology (colorectal) screening, quantitative real-time target and signal amplification of 10 DNA markers (KRAS mutations, promoter methylation of NDRG4 and BMP3) and fecal hemoglobin, utilizing stool, algorithm reported as a positive or negative result Colorectal cancer screening; stool-based DNA and fecal occult hemoglobin FLEXIBLE SIGMOIDOSCOPY CODES (This test must have occurred during the Measurement Period 12 months or the four years prior to the Measurement Period) CPT Codes Sigmoidoscopy, flexible; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) Sigmoidoscopy, flexible; with biopsy, single or multiple Sigmoidoscopy, flexible; with removal of foreign body Sigmoidoscopy, flexible; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps or bipolar cautery Sigmoidoscopy, flexible; with removal of foreign body Sigmoidoscopy, flexible; with directed submucosal injection(s), any substance Sigmoidoscopy, flexible; with decompression of volvulus, any method Sigmoidoscopy, flexible; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique Sigmoidoscopy, flexible; with ablation of tumor(s), polyp(s), or other lesion(s) not amenable to removal by hot biopsy forceps, bipolar cautery or snare technique Sigmoidoscopy, flexible; with dilation by balloon, 1 or more strictures Sigmoidoscopy, flexible; with endoscopic ultrasound examination Sigmoidoscopy, flexible; with transendoscopic ultrasound guided intramural or transmural fine needle aspiration/biopsy(s) Sigmoidoscopy, flexible; with transendoscopic stent placement (includes 13
14 predilation) Sigmoidoscopy flexible; with ablation tumor, polyp, other lesion Sigmoidoscopy flexible; placement of endoscopic stent Sigmoidoscopy flexible with endoscopic mucosal resection Sigmoidoscopy flexible with band ligation(s) ICD-9-CM Procedure Codes Flexible sigmoidoscopy Effective 10/01/2015 ICD-10-PCS Procedure Codes 0DJD8ZZ HCPCS Codes G0104 G6022 G6023 Inspection of Lower Intestinal Tract, Via Natural or Artificial Opening Endoscopic (Same as Colonoscopy code) Colorectal cancer screening; flexible sigmoidoscopy Sigmoidoscopy w/ablation of tumor Sigmoidoscopy w/stent CT COLONOGRAPY (Virtual Colonoscopy) CODES (This test must have occurred during the Measurement Period 12 months or the four years prior to the Measurement Period) Category III CPT Codes computerized tomographic colonography (CTC) without IV contrast computerized tomographic colonography (CTC) with IV contrast Screening computerized tomographic colonography (CTC) COLONOSCOPY CODES (This test must have occurred during the Measurement Period 12 months or the nine years prior to the Measurement Period) CPT Codes Colonoscopy through stoma; diagnostic, with or without collection of specimen(s) by brushing or washing (separate procedure) Colonoscopy through stoma; with biopsy, single or multiple Colonoscopy through stoma; with removal of foreign body Colonoscopy through stoma; with control of bleeding, any method Colonoscopy through stoma; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps or bipolar cautery Colonoscopy through stoma; with ablation of tumor(s), polyp(s), or other lesion(s) not amenable to removal by hot biopsy forceps, bipolar cautery or 14
15 snare technique Colonoscopy through stoma; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique Colonoscopy through stoma; with transendoscopic stent placement (includes predilation) Colonoscopy, rigid or flexible, transabdominal via colotomy, single or multiple Colonoscopy, flexible, proximal to splenic flexure; diagnostic, with or without collection of specimen(s) by brushing or washing, with or without colon decompression (separate procedure) Colonoscopy, flexible, proximal to splenic flexure; with removal of foreign body Colonoscopy, flexible, proximal to splenic flexure; with biopsy, single or multiple Colonoscopy, flexible, proximal to splenic flexure; with directed submucosal injection(s), any substance Colonoscopy, flexible, proximal to splenic flexure; with control of bleeding (eg. Injection, bipolar cautery, unipolar cautery, laser, heater probe, stapler, plasma coagulator) Colonoscopy, flexible, proximal to splenic flexure; with ablation of tumor(s), polyp(s), or other lesion(s) not amenable to removal by hot biopsy forceps, bipolar cautery or snare techique Colonoscopy, flexible, proximal to splenic flexure; with removal of tumor(s), polyp(s), or other lesion(s) by hot biopsy forceps or bipolar cautery Colonoscopy, flexible, proximal to splenic flexure; with removal of tumor(s), polyp(s), or other lesion(s) by snare technique Colonoscopy, flexible, proximal to splenic flexure; with dilation by balloon, 1 or more strictures Colonoscopy, flexible, proximal to splenic flexure; with transendoscopic stent placement (includes predilation) Colonoscopy, flexible, proximal to splenic flexure; with endoscopic ultrasound examination Colonoscopy, flexible, proximal to splenic flexure; with transendoscopic ultrasound guided intramural or transmural fine needle aspiration/biopsy(s) Colonoscopy stoma ablation lesion Colonoscopy stoma with endoscopic stent placement Colonoscopy stoma with endoscopic mucosal resection Colonoscopy stoma with submucosal injection Colonoscopy stoma with balloon dilation Colonoscopy stoma with endoscopic ultrasound exam Colonoscopy stoma with ultrasound guided needle aspiration/biopsy Colonoscopy through stoma with decompression Colonoscopy, flexible; with ablation of tumor(s), polyp(s), or other lesion(s) Colonoscopy, flexible; with endoscopic stent placement Colonoscopy, flexible; with endoscopic mucosal resection Colonoscopy flexible; with decompression Colonoscopy flexible; with band ligation(s) 15
16 HCPCS Codes G0105 G0121 G6019 G6020 G6024 G6025 Colorectal cancer screening; colonoscopy on individual at high risk Colorectal cancer screening; colonoscopy on individual not meeting criteria for high risk Colonoscopy lesion removal Colonoscopy w/stent Lesion removal colonoscopy Colonoscopy w/stent ICD-9-CM Procedure Codes Endoscopy of large intestine through artificial stoma Colonoscopy Closed [endoscopic] biopsy of large intestine Endoscopic polypectomy of large intestine Endoscopic destruction of other lesion or tissue of large intestine Effective 10/01/2015 ICD-10-PCS Procedure Codes 0DJD8ZZ 0DBE4ZZ 0DBE8ZZ 0DBF8ZZ 0DBG8ZZ 0DBH8ZZ 0DBK8ZZ 0DBL8ZZ 0DBM8ZZ 0DBN8ZZ 0D5E4ZZ 0D5E8ZZ 0D5F4ZZ 0D5F8ZZ 0D5G4ZZ Inspection of Lower Intestinal Tract, Via Natural or Artificial Opening Endoscopic (Same as Flex Sig code) Excision of Large Intestine, Percutaneous Endoscopic Approach Excision of Large Intestine, Via Natural or Artificial Opening Endoscopic Excision of right large intestine, endoscopic Excision of left large intestine, endoscopic Excision of cecum, endoscopic Excision of ascending colon, endoscopic Excision of transverse colon, endoscopic Excision of descending colon, endoscopic Excision of sigmoid colon, endoscopic Destruction of Large Intestine, Percutaneous Endoscopic Approach Destruction of Large Intestine, Via Natural or Artificial Opening Endoscopic Destruction of Right Large Intestine, Percutaneous Endoscopic Approach Destruction of Right Large Intestine, Via Natural or Artificial Opening Endoscopic Destruction of Left Large Intestine, Percutaneous Endoscopic Approach 16
17 0D5G8ZZ 0D5H4ZZ 0D5H8ZZ 0D5K4ZZ 0D5K8ZZ 0D5L4ZZ 0D5L8ZZ 0D5M4ZZ 0D5M8ZZ 0D5N4ZZ 0D5N8ZZ 0D9E3ZX 0D9E4ZX 0D9E7ZX 0D9E8ZX 0D9F3ZX 0D9F4ZX 0D9F7ZX 0D9F8ZX 0D9G3ZX 0D9G4ZX 0D9G7ZX 0D9G8ZX 0D9H3ZX 0D9H4ZX 0D9H7ZX 0D9H8ZX Destruction of Left Large Intestine, Via Natural or Artificial Opening Endoscopic Destruction of Cecum, Percutaneous Endoscopic Approach Destruction of Cecum, Via Natural or Artificial Opening Endoscopic Destruction of Ascending Colon, Percutaneous Endoscopic Approach Destruction of Ascending Colon, Via Natural or Artificial Opening Endoscopic Destruction of Transverse Colon, Percutaneous Endoscopic Approach Destruction of Transverse Colon, Via Natural or Artificial Opening Endoscopic Destruction of Descending Colon, Percutaneous Endoscopic Approach Destruction of Descending Colon, Via Natural or Artificial Opening Endoscopic Destruction of Sigmoid Colon, Percutaneous Endoscopic Approach Destruction of Sigmoid Colon, Via Natural or Artificial Opening Endoscopic Drainage of Large Intestine, Percutaneous Approach, Drainage of Large Intestine, Percutaneous Endoscopic Approach, Drainage of Large Intestine, Via Natural or Artificial Opening, Drainage of Large Intestine, Via Natural or Artificial Opening Endoscopic, Drainage of Right Large Intestine, Percutaneous Approach, Drainage of Right Large Intestine, Percutaneous Endoscopic Approach, Drainage of Right Large Intestine, Via Natural or Artificial Opening, Drainage of Right Large Intestine, Via Natural or Artificial Opening Endoscopic, Drainage of Left Large Intestine, Percutaneous Approach, Drainage of Left Large Intestine, Percutaneous Endoscopic Approach, Drainage of Left Large Intestine, Via Natural or Artificial Opening, Drainage of Left Large Intestine, Via Natural or Artificial Opening Endoscopic, Drainage of Cecum, Percutaneous Approach, Drainage of Cecum, Percutaneous Endoscopic Approach, Drainage of Cecum, Via Natural or Artificial Opening, Drainage of Cecum, Via Natural or Artificial Opening Endoscopic, 17
18 0D9K3ZX 0D9K4ZX 0D9K7ZX 0D9K8ZX 0D9L3ZX 0D9L4ZX 0D9L7ZX 0D9L8ZX 0D9M3ZX 0D9M4ZX 0D9M7ZX 0D9M8ZX 0D9N3ZX 0D9N4ZX 0D9N7ZX 0D9N8ZX 0DBE3ZX 0DBE4ZX 0DBE7ZX 0DBE8ZX 0DBF3ZX 0DBF4ZX 0DBF7ZX 0DBF8ZX 0DBG3ZX 0DBG4ZX Drainage of Ascending Colon, Percutaneous Approach, Drainage of Ascending Colon, Percutaneous Endoscopic Approach, Drainage of Ascending Colon, Via Natural or Artificial Opening, Drainage of Ascending Colon, Via Natural or Artificial Opening Endoscopic, Drainage of Transverse Colon, Percutaneous Approach, Drainage of Transverse Colon, Percutaneous Endoscopic Approach, Drainage of Transverse Colon, Via Natural or Artificial Opening, Drainage of Transverse Colon, Via Natural or Artificial Opening Endoscopic, Drainage of Descending Colon, Percutaneous Approach, Drainage of Descending Colon, Percutaneous Endoscopic Approach, Drainage of Descending Colon, Via Natural or Artificial Opening, Drainage of Descending Colon, Via Natural or Artificial Opening Endoscopic, Drainage of Sigmoid Colon, Percutaneous Approach, Drainage of Sigmoid Colon, Percutaneous Endoscopic Approach, Drainage of Sigmoid Colon, Via Natural or Artificial Opening, Drainage of Sigmoid Colon, Via Natural or Artificial Opening Endoscopic, Excision of Large Intestine, Percutaneous Approach, Excision of Large Intestine, Percutaneous Endoscopic Approach, Excision of Large Intestine, Via Natural or Artificial Opening, Excision of Large Intestine, Via Natural or Artificial Opening Endoscopic, Excision of Right Large Intestine, Percutaneous Approach, Excision of Right Large Intestine, Percutaneous Endoscopic Approach, Excision of Right Large Intestine, Via Natural or Artificial Opening, Excision of Right Large Intestine, Via Natural or Artificial Opening Endoscopic, Excision of Left Large Intestine, Percutaneous Approach, Excision of Left Large Intestine, Percutaneous Endoscopic Approach, 18
19 0DBG7ZX 0DBG8ZX 0DBH3ZX 0DBH4ZX 0DBH7ZX 0DBH8ZX 0DBK3ZX 0DBK4ZX 0DBK7ZX 0DBK8ZX 0DBL3ZX 0DBL4ZX 0DBL7ZX 0DBL8ZX 0DBM3ZX 0DBM4ZX 0DBM7ZX 0DBM8ZX 0DBN3ZX 0DBN4ZX 0DBN7ZX 0DBN8ZX Excision of Left Large Intestine, Via Natural or Artificial Opening, Excision of Left Large Intestine, Via Natural or Artificial Opening Endoscopic, Excision of Cecum, Percutaneous Approach, Excision of Cecum, Percutaneous Endoscopic Approach, Excision of Cecum, Via Natural or Artificial Opening, Excision of Cecum, Via Natural or Artificial Opening Endoscopic, Excision of Ascending Colon, Percutaneous Approach, Excision of Ascending Colon, Percutaneous Endoscopic Approach, Excision of Ascending Colon, Via Natural or Artificial Opening, Excision of Ascending Colon, Via Natural or Artificial Opening Endoscopic, Excision of Transverse Colon, Percutaneous Approach, Excision of Transverse Colon, Percutaneous Endoscopic Approach, Excision of Transverse Colon, Via Natural or Artificial Opening, Excision of Transverse Colon, Via Natural or Artificial Opening Endoscopic, Excision of Descending Colon, Percutaneous Approach, Excision of Descending Colon, Percutaneous Endoscopic Approach, Excision of Descending Colon, Via Natural or Artificial Opening, Excision of Descending Colon, Via Natural or Artificial Opening Endoscopic, Excision of Sigmoid Colon, Percutaneous Approach, Excision of Sigmoid Colon, Percutaneous Endoscopic Approach, Excision of Sigmoid Colon, Via Natural or Artificial Opening, Excision of Sigmoid Colon, Via Natural or Artificial Opening Endoscopic, TABLE CCS-4: Codes to Identify Status CPT / HCPCS Codes Supervision of a hospice patient minutes Supervision of a hospice patient 30 minutes Physician supervision of a patient under a medicare-approved hospice (patient not present) requiring complex and multidisciplinary care modalities G0182 involving regular physician development and/or revision of care plans, review 19
20 G9473 G9474 G9475 G9476 G9477 G9478 G9479 Q5003 Q5004 Q5005 Q5006 Q5007 Q5008 Q5010 S9126 T2042 T2043 T2044 T2045 T2046 of subsequent reports of patient status, review of laboratory and other studies, communication (including telephone calls) with other health care professionals involved in the patient's care, integration of new information into the medical treatment plan and/or adjustment of medical therapy, within a calendar month, 30 minutes or more (G0182) Services performed by chaplain in the hospice setting, each 15 minutes (G9473) Services performed by dietary counselor in the hospice setting, each 15 minutes (G9474) Services performed by other counselor in the hospice setting, each 15 minutes (G9475) Services performed by volunteer in the hospice setting, each 15 minutes (G9476) Services performed by care coordinator in the hospice setting, each 15 minutes (G9477) Services performed by other qualified therapist in the hospice setting, each 15 minutes (G9478) Services performed by qualified pharmacist in the hospice setting, each 15 minutes (G9479) care provided in nursing long term care facility (ltc) or non-skilled nursing facility (nf) (Q5003) care provided in skilled nursing facility (snf) (Q5004) care provided in inpatient hospital (Q5005) care provided in inpatient hospice facility (Q5006) care provided in long term care facility (Q5007) care provided in inpatient psychiatric facility (Q5008) home care provided in a hospice facility (Q5010) care, in the home, per diem (S9126) routine home care; per diem (T2042) continuous home care; per hour (T2043) inpatient respite care; per diem (T2044) general inpatient care; per diem (T2045) long term care, room and board only; per diem (T2046) UBREV / UBTOB Codes 0115 Room and Board, Private 0125 Room and Board, Semi-private, two beds 0135 Room and Board, Semi-private, three and four beds 0145 Room and Board, Private, Deluxe 0155 Room and Board, Ward 0235 Incremental Nursing Care Charges 0650 Service General 0651 Routine home care 0652 Continuous home care 0655 Inpatient respite care 0656 General inpatient care (nonrespite) 20
21 0657 Physician services 0658 room and board - nursing facility 0659 Other A 081B 081C 081D 081E 081F 081G 081H 081I 081J 081K 081M 081O 081X 081Y 081Z 082A 082B 082C 082D 082E 082F 082G 082H 082I 082J 082K 21
22 082M 082O 082X 082Y 082Z 22
WCHQ Ambulatory Measure Specification Screening For Osteoporosis Measurement Period 07/01/16-06/30/17 Submission Period: 09/05/17-10/20/17
 MEASURE DESCRIPTION The percentage of women age 65 through 85 who had a minimum of one bone densitometry test at age 60 or above or have a diagnosis of osteoporosis or osteopenia. Disclaimer: Measures
MEASURE DESCRIPTION The percentage of women age 65 through 85 who had a minimum of one bone densitometry test at age 60 or above or have a diagnosis of osteoporosis or osteopenia. Disclaimer: Measures
DATA COLLECTION GUIDE Direct Data Submission
 DATA COLLECTION GUIDE Updated 05/08/2014: Colorectal Cancer Screening 2014 (07/01/2013 to 06/30/2014 Dates of Service) Posted 05/08/2014 Final 1. Column V on page 31: Do not enter dates prior to 07/01/2013.
DATA COLLECTION GUIDE Updated 05/08/2014: Colorectal Cancer Screening 2014 (07/01/2013 to 06/30/2014 Dates of Service) Posted 05/08/2014 Final 1. Column V on page 31: Do not enter dates prior to 07/01/2013.
DATA COLLECTION GUIDE Direct Data Submission
 DATA COLLECTION GUIDE Colorectal Cancer Screening 2015 (07/01/2014 to 06/30/2015 Dates of Service) 1 Table of Contents Overview of the Process and Timeline 3 Measure Specifications 4 Summary of Changes
DATA COLLECTION GUIDE Colorectal Cancer Screening 2015 (07/01/2014 to 06/30/2015 Dates of Service) 1 Table of Contents Overview of the Process and Timeline 3 Measure Specifications 4 Summary of Changes
The focus of Chapter 9 is on anoscopy, proctosigmoidoscopy, flexible sigmoidoscopy, and colonoscopy procedures and all
 9 Anoscopy, 45380 45380 45385 Proctosigmoidoscopy, Flexible Sigmoidoscopy, and Colonoscopy 45378 The focus of Chapter 9 is on anoscopy, proctosigmoidoscopy, flexible sigmoidoscopy, and colonoscopy procedures
9 Anoscopy, 45380 45380 45385 Proctosigmoidoscopy, Flexible Sigmoidoscopy, and Colonoscopy 45378 The focus of Chapter 9 is on anoscopy, proctosigmoidoscopy, flexible sigmoidoscopy, and colonoscopy procedures
Colorectal Cancer Screening And Related Ancillary Services
 Manual: Policy Title: Reimbursement Policy Colorectal Cancer Screening And Related Ancillary Services Section: Preventive Services Subsection: None Date of Origin: 11/20/2015 Policy Number: RPM046 Last
Manual: Policy Title: Reimbursement Policy Colorectal Cancer Screening And Related Ancillary Services Section: Preventive Services Subsection: None Date of Origin: 11/20/2015 Policy Number: RPM046 Last
NOTE: Please append modifier 33 when indicated. If modifier 33 is not appended, regular plan benefits will be applied.
 Policy name: Health Guidelines - Men This policy applies only to non-grandfathered plans as defined in the Affordable Care Act section 1251. The following chart contains procedure, diagnosis and modifier
Policy name: Health Guidelines - Men This policy applies only to non-grandfathered plans as defined in the Affordable Care Act section 1251. The following chart contains procedure, diagnosis and modifier
NOTE: Please append modifier 33 when indicated. If modifier 33 is not appended, regular plan benefits will be applied.
 Policy name: Preventive Health Guidelines - Men The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
Policy name: Preventive Health Guidelines - Men The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
Billing Guideline. Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/14 Last revision effective 4/16
 Billing Guideline Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/14 Last revision effective 4/16 Florida Hospital Care Advantage plans include full coverage of in-network
Billing Guideline Subject: Colorectal Cancer Screening Exams (Invasive Procedures) Effective Date: 1/1/14 Last revision effective 4/16 Florida Hospital Care Advantage plans include full coverage of in-network
NOTE: Please append modifier 33 when indicated. If modifier 33 is not appended, regular plan benefits will be applied.
 Policy name: Preventive Health Guidelines - Men The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
Policy name: Preventive Health Guidelines - Men The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
Oncology, Colorectal Surgery, Gastroenterology, Internal Medicine, Family Practice, General Surgery, Geriatric Medicine, Multispecialty
 Measure : Follow-Up After Initial Diagnosis and Treatment Of Colorectal Cancer: Colonoscopy Owner: NQF (#0572) Measure Code: CLF Lab Data: N Steward: Health Benchmarks-IMS Health Rule Description: Percentage
Measure : Follow-Up After Initial Diagnosis and Treatment Of Colorectal Cancer: Colonoscopy Owner: NQF (#0572) Measure Code: CLF Lab Data: N Steward: Health Benchmarks-IMS Health Rule Description: Percentage
FY 2017 MCRCEDP Procedure Code Reference Chart
 45378-53 45380 45381 45382 45384 45385 48388 45390 ; Colonoscopy, flexible, proximal to splenic flexure; diagnostic, with or without collection of specimen(s) by brushing or washing, with or without colon
45378-53 45380 45381 45382 45384 45385 48388 45390 ; Colonoscopy, flexible, proximal to splenic flexure; diagnostic, with or without collection of specimen(s) by brushing or washing, with or without colon
Preventive Services versus Diagnostic and/or Medical Services
 Manual: Policy Title: Reimbursement Policy Preventive Services versus Diagnostic and/or Medical Services Section: Administrative Subsection: None Date of Origin: 1/1/2000 Policy Number: RPM037 Last Updated:
Manual: Policy Title: Reimbursement Policy Preventive Services versus Diagnostic and/or Medical Services Section: Administrative Subsection: None Date of Origin: 1/1/2000 Policy Number: RPM037 Last Updated:
2016 CPT coding changes and their effects
 18 2016 CPT coding changes and their effects by Linda Barney, MD, FACS, and Mark T. Savarise, MD, FACS Significant Current Procedural Terminology (CPT)* coding changes are being implemented in 2016. Notably,
18 2016 CPT coding changes and their effects by Linda Barney, MD, FACS, and Mark T. Savarise, MD, FACS Significant Current Procedural Terminology (CPT)* coding changes are being implemented in 2016. Notably,
COLORECTAL CANCER SCREENING
 COLORECTAL CANCER SCREENING APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE NCQA ACCEPTED CODES DOCUMENTATION REQUIREMENTS What documentation
COLORECTAL CANCER SCREENING APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE NCQA ACCEPTED CODES DOCUMENTATION REQUIREMENTS What documentation
2017 Procedural Reimbursement Guide for Endoscopy
 2017 Procedural Reimbursement Guide for Endoscopy THIS PROCEDURAL REIMBURSEMENT GUIDE, FOR SELECT ENDOSCOPY PROCEDURES, provides coding and reimbursement information for physicians and facilities. The
2017 Procedural Reimbursement Guide for Endoscopy THIS PROCEDURAL REIMBURSEMENT GUIDE, FOR SELECT ENDOSCOPY PROCEDURES, provides coding and reimbursement information for physicians and facilities. The
Information Technology Solutions
 2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
2016 2014 CPT Esophagoscopy Changes - Gastroenterology CPT Changes Information Technology Solutions ASGE LOGO AND INFO Esophagogastroduodenoscopy CPT Codes 43235-43270 The American Society for Gastrointestinal
Quality ID #113 (NQF 0034): Colorectal Cancer Screening National Quality Strategy Domain: Effective Clinical Care
 Quality ID #113 (NQF 0034): Colorectal Cancer Screening National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY MEASURE TYPE: Process DESCRIPTION: Percentage
Quality ID #113 (NQF 0034): Colorectal Cancer Screening National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS ONLY MEASURE TYPE: Process DESCRIPTION: Percentage
Overview 2015 CPT Changes. Kelly A. Kehoe, CPC, CEMC Clinical Documentation Audit Manager
 . Overview 2015 CPT Changes Kelly A. Kehoe, CPC, CEMC Clinical Documentation Audit Manager 1 Resources CPT 2015-American Medical Association CPT Changes, An Insider s View, American Medical Association
. Overview 2015 CPT Changes Kelly A. Kehoe, CPC, CEMC Clinical Documentation Audit Manager 1 Resources CPT 2015-American Medical Association CPT Changes, An Insider s View, American Medical Association
Colonoscopy MM /01/2010. PPO; HMO; QUEST Integration 10/01/2017 Section: Surgery Place(s) of Service: Outpatient
 Colonoscopy Policy Number: Original Effective Date: MM.12.003 12/01/2010 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration 10/01/2017 Section: Surgery Place(s) of Service: Outpatient
Colonoscopy Policy Number: Original Effective Date: MM.12.003 12/01/2010 Line(s) of Business: Current Effective Date: PPO; HMO; QUEST Integration 10/01/2017 Section: Surgery Place(s) of Service: Outpatient
DIGESTIVE SYSTEM SURGICAL PROCEDURES May 1, 2015 INTESTINES (EXCEPT RECTUM) Asst Surg Anae
 ENDOSCOPY Z50 Duodenoscopy (not to be claimed if Z399 and/or Z00 performed on same patient within 3 months)... 92.10 Z9 Subsequent procedure (within three months following previous endoscopic procedure)...
ENDOSCOPY Z50 Duodenoscopy (not to be claimed if Z399 and/or Z00 performed on same patient within 3 months)... 92.10 Z9 Subsequent procedure (within three months following previous endoscopic procedure)...
Quality ID #113 (NQF 0034): Colorectal Cancer Screening National Quality Strategy Domain: Effective Clinical Care
 Quality ID #113 (NQF 0034): Colorectal Cancer Screening National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION:
Quality ID #113 (NQF 0034): Colorectal Cancer Screening National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION:
2011 FITWAY Allowable CPT Codes (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility)
 2011 FITWAY Allowable s (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility) Fecal Immunochemical Test (FIT) G0328/ 82274 Colorectal cancer screening
2011 FITWAY Allowable s (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility) Fecal Immunochemical Test (FIT) G0328/ 82274 Colorectal cancer screening
Clinical Documentation Excellence: CPT Coding Updates for Missy Vance, RHIA, CCS, CPC, AHIMA Approved ICD-10-CM/PCS Trainer & Ambassador
 Clinical Documentation Excellence: CPT Coding Updates for 2015 Missy Vance, RHIA, CCS, CPC, AHIMA Approved ICD-10-CM/PCS Trainer & Ambassador Kelly Spell, CCS, CPC, CPC-H, CAHIMS, AHIMA Approved ICD-10-CM/PCS
Clinical Documentation Excellence: CPT Coding Updates for 2015 Missy Vance, RHIA, CCS, CPC, AHIMA Approved ICD-10-CM/PCS Trainer & Ambassador Kelly Spell, CCS, CPC, CPC-H, CAHIMS, AHIMA Approved ICD-10-CM/PCS
OBSOLETE. NOTE: Please append modifier 33 when indicated. If modifier 33 is not appended, regular plan benefits will be applied.
 This document is obsolete. For current content, please see Preventive Health Guidelines - Women. Policy name: Preventive Health Guidelines - Women The following chart contains procedure and diagnosis code
This document is obsolete. For current content, please see Preventive Health Guidelines - Women. Policy name: Preventive Health Guidelines - Women The following chart contains procedure and diagnosis code
GI Coding Updates. Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC
 GI Coding Updates Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC Copyright/Disclaimer 2014 AAPC text CPT copyright 2016 American Medical Association. All rights reserved.
GI Coding Updates Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC Copyright/Disclaimer 2014 AAPC text CPT copyright 2016 American Medical Association. All rights reserved.
Historical. Note: The parenthetical numbers in the Clinical Indications section refer to the source documents cited in the References Section below.
 Clinical UM Guideline Subject: Colonoscopy Guideline #: CG-SURG-01 Current Effective Date: 01/21/2015 Status: Revised Last Review Date: 05/15/2014 Description Colonoscopy describes the direct visual inspection
Clinical UM Guideline Subject: Colonoscopy Guideline #: CG-SURG-01 Current Effective Date: 01/21/2015 Status: Revised Last Review Date: 05/15/2014 Description Colonoscopy describes the direct visual inspection
The Affordable Care Act (ACA) requires full coverage of the following preventive services for non-grandfathered plans 1 :
 Billing Guideline Subject: Preventive s Effective: 1/1/14 Last revision effective: 9/1/2017 Background We are committed to the wellness of our members and encourage preventive services that can detect
Billing Guideline Subject: Preventive s Effective: 1/1/14 Last revision effective: 9/1/2017 Background We are committed to the wellness of our members and encourage preventive services that can detect
DIGESTIVE SYSTEM SURGICAL PROCEDURES December 22, 2015 (effective March 1, 2016) INTESTINES (EXCEPT RECTUM) Asst Surg Anae
 December 22, 2015 (effective March 1, 201) INTESTINES (EXCEPT RECTUM) Z513 Hydrostatic - Pneumatic dilatation of colon stricture(s) through colonoscope... 10.50 Z50 Fulguration of first polyp through colonoscope...
December 22, 2015 (effective March 1, 201) INTESTINES (EXCEPT RECTUM) Z513 Hydrostatic - Pneumatic dilatation of colon stricture(s) through colonoscope... 10.50 Z50 Fulguration of first polyp through colonoscope...
CPT 2014 Overview of GI Changes
 CPT 2014 Overview of GI Changes The following table is a listing of the new,, and deleted codes in the Esophagus/Endoscopy section effective January 1, 2014. The table lists the CPT code, a brief description
CPT 2014 Overview of GI Changes The following table is a listing of the new,, and deleted codes in the Esophagus/Endoscopy section effective January 1, 2014. The table lists the CPT code, a brief description
2012 FITWAY Allowable CPT Codes (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility)
 2012 FITWAY Allowable s (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility) Fecal Immunochemical Test (FIT) G0328/ 82274 Colorectal cancer screening
2012 FITWAY Allowable s (Modifiers are to be reported with appropriate CPT codes at the discretion of the Provider or Facility) Fecal Immunochemical Test (FIT) G0328/ 82274 Colorectal cancer screening
Clinical Policy: Monitored Anesthesia Care for Gastrointestinal Endoscopy
 Clinical Policy: Monitored Anesthesia Care for Gastrointestinal Endoscopy Reference Number: CP.MP.161 Last Review Date: 05/18 See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Monitored Anesthesia Care for Gastrointestinal Endoscopy Reference Number: CP.MP.161 Last Review Date: 05/18 See Important Reminder at the end of this policy for important regulatory and
Colorectal Cancer Screening and Risk Assessment Workflow. Documentation Guide for Health Center NextGen Users
 Colorectal Cancer Screening and Risk Assessment Workflow Documentation Guide for Health Center NextGen Users Colorectal Cancer Screening and Risk Assessment Workflow and Documentation Guide for Health
Colorectal Cancer Screening and Risk Assessment Workflow Documentation Guide for Health Center NextGen Users Colorectal Cancer Screening and Risk Assessment Workflow and Documentation Guide for Health
The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean?
 The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean? Robert A. Smith, PhD Cancer Control, Department of Prevention and Early Detection American Cancer Society
The New Grade A: USPSTF Updated Colorectal Cancer Screening Guidelines, What does it all mean? Robert A. Smith, PhD Cancer Control, Department of Prevention and Early Detection American Cancer Society
CPT COD1NG UPDATES Gastroenterology CPT Advisors
 2014 CPT COD1NG UPDATES Gastroenterology CPT Advisors Joel V. Brill, MD, AGA CPT Advisor Daniel C. DeMarco, MD, ACG CPT Advisor Glenn D. Littenberg, MD, ASGE CPT Advisor The American College of Gastroenterology
2014 CPT COD1NG UPDATES Gastroenterology CPT Advisors Joel V. Brill, MD, AGA CPT Advisor Daniel C. DeMarco, MD, ACG CPT Advisor Glenn D. Littenberg, MD, ASGE CPT Advisor The American College of Gastroenterology
Sage Program Reimbursement Rates (Effective Jan 1, 2018 through Dec 31, 2018)
 Sage Program Reimbursement Rates Code Description of Service Allowable Rates New Patient 99201 History, exam, straight forward decision-making; 10 $44.47 99202 Expanded history; exam, straightforward decision-making;
Sage Program Reimbursement Rates Code Description of Service Allowable Rates New Patient 99201 History, exam, straight forward decision-making; 10 $44.47 99202 Expanded history; exam, straightforward decision-making;
Medicare Shared Savings Program Accountable Care Organization (ACO) Measures Deep Dive Series
 Medicare Shared Savings Program Accountable Care Organization (ACO) Measures Deep Dive Series Preventive Care and Screening (PREV-6): Measure 19 Colorectal Cancer Screening ACO_QRM19PPTv9_0518_IA Approved
Medicare Shared Savings Program Accountable Care Organization (ACO) Measures Deep Dive Series Preventive Care and Screening (PREV-6): Measure 19 Colorectal Cancer Screening ACO_QRM19PPTv9_0518_IA Approved
Screening & Surveillance Guidelines
 Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Chapter 2 Screening & Surveillance Guidelines I. Eligibility Coloradans ages 50 and older (average risk) or under 50 at elevated risk for colon cancer (personal or family history) that meet the following
Local Coverage Determination for Colorectal Cancer Screening (L29796)
 Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
Page 1 of 15 Home Medicare Medicaid CHIP About CMS Regulations & Guidance Research, Statistics, Data & Systems Outreach & E People with Medicare & Medicaid Questions Careers Newsroom Contact CMS Acronyms
2015 Procedural Reimbursement Guide for Endoscopy
 2015 Procedural Reimbursement Guide for Endoscopy THIS PROCEDURAL REIMBURSEMENT GUIDE, FOR SELECT ENDOSCOPY PROCEDURES, provides coding and reimbursement information for physicians and facilities. The
2015 Procedural Reimbursement Guide for Endoscopy THIS PROCEDURAL REIMBURSEMENT GUIDE, FOR SELECT ENDOSCOPY PROCEDURES, provides coding and reimbursement information for physicians and facilities. The
NOTE: Please append modifier 33 when indicated. If modifier 33 is not appended, regular plan benefits will be applied.
 Policy name: Preventive Health Guidelines - Women The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
Policy name: Preventive Health Guidelines - Women The following chart contains procedure and diagnosis code combinations that identify services covered under HMSA's Preventive Health s policy. * For professional
Measure #425: Photodocumentation of Cecal Intubation National Quality Strategy Domain: Effective Clinical Care
 Measure #425: Photodocumentation of Cecal Intubation National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS, REGISTRY DESCRIPTION: The rate of screening
Measure #425: Photodocumentation of Cecal Intubation National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS, REGISTRY DESCRIPTION: The rate of screening
December 2018 CTC/OHIC Measure Specifications
 Overarching Principles and Definitions Active Patients: Patients seen by a primary care clinician of the PCMH anytime within the last 24 months Definition of primary care clinician includes the following:
Overarching Principles and Definitions Active Patients: Patients seen by a primary care clinician of the PCMH anytime within the last 24 months Definition of primary care clinician includes the following:
Clinical UM Guideline
 Subject: Guideline #: Current Effective Date: 06/28/2016 Status: Revised Last Review Date: 05/05/2016 Description This document addresses colonoscopy, an endoscopic procedure which allows direct visual
Subject: Guideline #: Current Effective Date: 06/28/2016 Status: Revised Last Review Date: 05/05/2016 Description This document addresses colonoscopy, an endoscopic procedure which allows direct visual
Cologuard Screening for Colorectal Cancer
 Pending Policies - Medicine Cologuard Screening for Colorectal Cancer Print Number: MED208.056 Effective Date: 08-15-2016 Coverage: I.Cologuard stool DNA testing may be considered medically necessary for
Pending Policies - Medicine Cologuard Screening for Colorectal Cancer Print Number: MED208.056 Effective Date: 08-15-2016 Coverage: I.Cologuard stool DNA testing may be considered medically necessary for
Appendix 1 (as supplied by the authors): Supplementary tables. Supplementary Table A1. Description of OHIP codes used in the current study.
 Appendix 1 (as supplied by the authors): Supplementary tables Supplementary Table A1. Description of OHIP codes used in the current study. OHIP Billing Code OHIP Billing Code Description Colonoscopy and
Appendix 1 (as supplied by the authors): Supplementary tables Supplementary Table A1. Description of OHIP codes used in the current study. OHIP Billing Code OHIP Billing Code Description Colonoscopy and
2014 CPT Codes: What Your Practice Needs to Know. December 12, 2013
 2014 CPT Codes: What Your Practice Needs to Know December 12, 2013 2014 CPT Changes 335 changes, 175 new codes, 107 revisions, 47 deletions Changes to upper and lower GI codes, breast biopsies, peripheral
2014 CPT Codes: What Your Practice Needs to Know December 12, 2013 2014 CPT Changes 335 changes, 175 new codes, 107 revisions, 47 deletions Changes to upper and lower GI codes, breast biopsies, peripheral
BC ADVANTAGE AUDIO SERIES:
 BC ADVANTAGE AUDIO SERIES: UPDATES FOR 2015 SURGICAL CPT CODES 1 Presented by: Darlene Boschert, RHIA, CPC, CPC-H, CPC-I Providing LOW-COST educational resources for Medical office Professionals OBJECTIVES
BC ADVANTAGE AUDIO SERIES: UPDATES FOR 2015 SURGICAL CPT CODES 1 Presented by: Darlene Boschert, RHIA, CPC, CPC-H, CPC-I Providing LOW-COST educational resources for Medical office Professionals OBJECTIVES
Physician s Compliance Guide
 Physician s Compliance Guide Updates to this guide will be posted on the Optum website and can be found at: http://www.optumcoding.com/product/updates/2013pcg/pcg13 Please use the following password to
Physician s Compliance Guide Updates to this guide will be posted on the Optum website and can be found at: http://www.optumcoding.com/product/updates/2013pcg/pcg13 Please use the following password to
Measure #425: Photodocumentation of Cecal Intubation National Quality Strategy Domain: Effective Clinical Care
 Measure #425: Photodocumentation of Cecal Intubation National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: The
Measure #425: Photodocumentation of Cecal Intubation National Quality Strategy Domain: Effective Clinical Care 2017 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Process DESCRIPTION: The
MEDICAL ASSISTANCE BULLETIN
 ISSUE DATE August 31, 2015 SUBJECT EFFECTIVE DATE September 1, 2015 MEDICAL ASSISTANCE BULLETIN BY NUMBER 99-15-06 2015 HCPCS Updates and Other Procedure Code Changes Leesa M. Allen, Deputy Secretary IMPORTANT
ISSUE DATE August 31, 2015 SUBJECT EFFECTIVE DATE September 1, 2015 MEDICAL ASSISTANCE BULLETIN BY NUMBER 99-15-06 2015 HCPCS Updates and Other Procedure Code Changes Leesa M. Allen, Deputy Secretary IMPORTANT
Supplementary Online Content
 Supplementary Online Content Tran AH, Ngor EWM, Wu BU. Surveillance colonoscopy in elderly patients: a retrospective cohort study. JAMA Intern Med. Published online August 11, 2014. doi:10.1001/jamainternmed.2014.3746
Supplementary Online Content Tran AH, Ngor EWM, Wu BU. Surveillance colonoscopy in elderly patients: a retrospective cohort study. JAMA Intern Med. Published online August 11, 2014. doi:10.1001/jamainternmed.2014.3746
Colorectal Cancer Screening in Ohio CHCs. Ohio Association of Community Health Centers
 Colorectal Cancer Screening in Ohio CHCs Ohio Association of Community Health Centers 2 1/29/2015 Your Speakers Dr. Ted Wymyslo Ashley Ballard Randy Runyon 3 1/29/2015 Facts 3 rd most common cancer in
Colorectal Cancer Screening in Ohio CHCs Ohio Association of Community Health Centers 2 1/29/2015 Your Speakers Dr. Ted Wymyslo Ashley Ballard Randy Runyon 3 1/29/2015 Facts 3 rd most common cancer in
UNHEALTHY ALCOHOL USE SCREENING and FOLLOW-UP (ASF) HEDIS (Administrative)
 UNHEALTHY ALCOHOL USE SCREENING and FOLLOW-UP (ASF) APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE NCQA ACCEPTED CODES HEDIS (Administrative)
UNHEALTHY ALCOHOL USE SCREENING and FOLLOW-UP (ASF) APPLICATIONS OBJECTIVE Purpose of Measure: ELIGIBLE POPULATION Which members are included? STANDARD OF CARE NCQA ACCEPTED CODES HEDIS (Administrative)
HEDIS Guidelines for Health Care Providers
 75 Vanderbilt Ave Staten Island NY 10304 1-844-CPHL-CARES www.centersplan.com HEDIS Guidelines for Health Care Providers Adult BMI Assessment (ABA) Members 18-74 years of age who had an outpatient visit
75 Vanderbilt Ave Staten Island NY 10304 1-844-CPHL-CARES www.centersplan.com HEDIS Guidelines for Health Care Providers Adult BMI Assessment (ABA) Members 18-74 years of age who had an outpatient visit
How to effectively code for Endoscopic procedures in Gastroenterology
 How to effectively code for Endoscopic procedures in Gastroenterology Ariwan Rakvit, MD Associate Professor Division of Gastroenterology Texas Tech University Health Science Center All rights reserved
How to effectively code for Endoscopic procedures in Gastroenterology Ariwan Rakvit, MD Associate Professor Division of Gastroenterology Texas Tech University Health Science Center All rights reserved
Icd 10 code for distal esophageal stricture Address Submit
 Icd 10 code for distal esophageal stricture Email Address Submit If an EGD is performed with a biopsy, and then the physician removes the scope and performs an Esophageal Dilation by unguided sound, it
Icd 10 code for distal esophageal stricture Email Address Submit If an EGD is performed with a biopsy, and then the physician removes the scope and performs an Esophageal Dilation by unguided sound, it
GI Coding Updates. Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC
 GI Coding Updates Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC Copyright/Disclaimer 2014 AAPC text CPT copyright 2016 American Medical Association. All rights reserved.
GI Coding Updates Rhonda Buckholtz, CPC, CPCI, CPMS, CRC, CDEO, CHPSE, CGSC, COBGC, CENTC, CPEDC Copyright/Disclaimer 2014 AAPC text CPT copyright 2016 American Medical Association. All rights reserved.
Clinical Policy: DNA Analysis of Stool to Screen for Colorectal Cancer
 Clinical Policy: to Screen for Colorectal Cancer Reference Number: CP.MP.125 Last Review Date: 07/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding
Clinical Policy: to Screen for Colorectal Cancer Reference Number: CP.MP.125 Last Review Date: 07/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding
2018 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA CMS Web Interface V2.1 Page 1 of 18 06/25/ Contents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA CMS Web Interface V2.1 Page 1 of 18 06/25/ Contents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4 BENEFICIARY
Neoplastic Colon Polyps. Joyce Au SUNY Downstate Grand Rounds, October 18, 2012
 Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
Neoplastic Colon Polyps Joyce Au SUNY Downstate Grand Rounds, October 18, 2012 CASE 55M with Hepatitis C, COPD (FEV1=45%), s/p vasectomy, knee surgery Meds: albuterol, flunisolide, mometasone, tiotropium
Quality ID #439: Age Appropriate Screening Colonoscopy National Quality Strategy Domain: Efficiency and Cost Reduction
 Quality ID #439: Age Appropriate Screening Colonoscopy National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Efficiency DESCRIPTION:
Quality ID #439: Age Appropriate Screening Colonoscopy National Quality Strategy Domain: Efficiency and Cost Reduction 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Efficiency DESCRIPTION:
Colorectal Cancer Screening. Paul Berg MD
 Colorectal Cancer Screening Paul Berg MD What is clinical integration? AMA Definition The means to facilitate the coordination of patient care across conditions, providers, settings, and time in order
Colorectal Cancer Screening Paul Berg MD What is clinical integration? AMA Definition The means to facilitate the coordination of patient care across conditions, providers, settings, and time in order
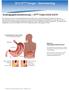
This is the portion of the intestine which lies between the small intestine and the outlet (Anus).
 THE COLON This is the portion of the intestine which lies between the small intestine and the outlet (Anus). 3 4 5 This part is responsible for formation of stool. The large intestine (colon- coloured
THE COLON This is the portion of the intestine which lies between the small intestine and the outlet (Anus). 3 4 5 This part is responsible for formation of stool. The large intestine (colon- coloured
Ontario Association of Gastroenterology
 FEE GUIDE Ontario Association of Gastroenterology OHIP Gastroenterology Fee Guide October 2003* *The fee guide is derived from the Ontario Schedule of Benefits for Physician Services (OHIP fee guide) which
FEE GUIDE Ontario Association of Gastroenterology OHIP Gastroenterology Fee Guide October 2003* *The fee guide is derived from the Ontario Schedule of Benefits for Physician Services (OHIP fee guide) which
2017 CPT coding changes
 16 2017 CPT coding changes by Albert Bothe, MD, FACS; Megan McNally, MD, FACS; and Jan Nagle, MS, RPh V102 No 1 BULLETIN American College of Surgeons Significant changes in Current Procedural Terminology
16 2017 CPT coding changes by Albert Bothe, MD, FACS; Megan McNally, MD, FACS; and Jan Nagle, MS, RPh V102 No 1 BULLETIN American College of Surgeons Significant changes in Current Procedural Terminology
2017 CMS Web Interface Reporting
 2017 CMS Web Interface Reporting Keys to Successful Reporting Part 2 Measures Refresher November 27, 2017 1:30 3:00 p.m. ET Sherry Grund, Telligen Mary Schrader, Telligen Medicare Shared Savings Program
2017 CMS Web Interface Reporting Keys to Successful Reporting Part 2 Measures Refresher November 27, 2017 1:30 3:00 p.m. ET Sherry Grund, Telligen Mary Schrader, Telligen Medicare Shared Savings Program
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT AUGUST 15, 2017
 IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201753 AUGUST 15, 2017 IHCP to unbundle moderate (conscious) sedation from CPT s Prior to January 1, 2017, approximately 400 Current Procedural Terminology
IHCP bulletin INDIANA HEALTH COVERAGE PROGRAMS BT201753 AUGUST 15, 2017 IHCP to unbundle moderate (conscious) sedation from CPT s Prior to January 1, 2017, approximately 400 Current Procedural Terminology
2/14/2018 CODING PITFALLS IN THE ASC AVERAGE RISK VS HIGH RISK SCREENING TOP CODING PITFALLS
 CODING PITFALLS IN THE ASC Presented by: Kristin Vaughn, CPC, CGCS, CPMA, ICDCT-CM Healthcare Consultant and Lead Auditor AskMueller Consulting, LLC Kristin@askmuellerconsulting.com TOP CODING PITFALLS
CODING PITFALLS IN THE ASC Presented by: Kristin Vaughn, CPC, CGCS, CPMA, ICDCT-CM Healthcare Consultant and Lead Auditor AskMueller Consulting, LLC Kristin@askmuellerconsulting.com TOP CODING PITFALLS
CPT CHANGES 2015: What s New, Revised, and Deleted
 CPT CHANGES 2015: What s New, Revised, and Deleted Cristina Bentin, CCS-P, CPC-H, CMA AHIMA Approved ICD-10-CM Trainer Coding Compliance Management, LLC cristina@ccmpro.com * 225.752.8390 Current Procedural
CPT CHANGES 2015: What s New, Revised, and Deleted Cristina Bentin, CCS-P, CPC-H, CMA AHIMA Approved ICD-10-CM Trainer Coding Compliance Management, LLC cristina@ccmpro.com * 225.752.8390 Current Procedural
2019 CMS Web Interface
 CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA CMS Web Interface V3.0 Page 1 of 18 xx/xx/2018 Contents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
CMS Web Interface PREV-5 (NQF 2372): Breast Cancer Screening Measure Steward: NCQA CMS Web Interface V3.0 Page 1 of 18 xx/xx/2018 Contents INTRODUCTION... 3 CMS WEB INTERFACE SAMPLING INFORMATION... 4
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care 2018 OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY ONLY MEASURE TYPE: Outcome DESCRIPTION:
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening
 Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Analysis of Human DNA in Stool Samples as a Technique for Colorectal Cancer Screening Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary,
Preventive Services Based Off 5110
 Preventive Services Based Off 5110 The Greater St. Louis Construction Laborers Welfare Fund will be required to provide all insured members 100% coverage for preventive care services. This means that members
Preventive Services Based Off 5110 The Greater St. Louis Construction Laborers Welfare Fund will be required to provide all insured members 100% coverage for preventive care services. This means that members
7/11/2017 ICD 10 AND MIPS: THE KEY TO ECONOMIC SURVIVAL DIAGNOSIS CODING WITH SPECIFICITY CLINICAL EXAMPLES: SPECIFICITY IS KEY
 ICD 10 AND MIPS: THE KEY TO ECONOMIC SURVIVAL Presented by: Kristin Vaughn, CPC, CGCS, CPMA, ICDCT-CM Healthcare Consultant and Lead Auditor AskMueller Consulting, LLC Kristin@askmuellerconsulting.com
ICD 10 AND MIPS: THE KEY TO ECONOMIC SURVIVAL Presented by: Kristin Vaughn, CPC, CGCS, CPMA, ICDCT-CM Healthcare Consultant and Lead Auditor AskMueller Consulting, LLC Kristin@askmuellerconsulting.com
2019 COLLECTION TYPE: MIPS CLINICAL QUALITY MEASURES (CQMS) MEASURE TYPE: Outcome High Priority
 Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
Quality ID #343: Screening Colonoscopy Adenoma Detection Rate National Quality Strategy Domain: Effective Clinical Care Meaningful Measure Area: Preventive Care 2019 COLLECTION TYPE: MIPS CLINICAL QUALITY
HEDIS. Quick Reference Guide. For more information, visit
 HEDIS Quick Reference Guide For more information, visit www.ncqa.org HEDIS Quick Reference Guide Updated to reflect NCQA HEDIS 2018 Technical Specifications Michigan Complete Health strives to provide
HEDIS Quick Reference Guide For more information, visit www.ncqa.org HEDIS Quick Reference Guide Updated to reflect NCQA HEDIS 2018 Technical Specifications Michigan Complete Health strives to provide
IEHP UM Subcommittee Approved Authorization Guidelines Colorectal Cancer Screening with Cologuard TM for Medicare Beneficiaries
 for Medicare Beneficiaries Policy: Based on our review of the available evidence, the IEHP UM Subcommittee adopts the use of Cologuard TM - a multi-target stool DNA test as a colorectal cancer screening
for Medicare Beneficiaries Policy: Based on our review of the available evidence, the IEHP UM Subcommittee adopts the use of Cologuard TM - a multi-target stool DNA test as a colorectal cancer screening
Improving Outcomes in Colorectal Cancer: The Science of Screening. Colorectal Cancer (CRC)
 Improving Outcomes in Colorectal Cancer: The Science of Screening Tennessee Primary Care Association October 23, 2014 Durado Brooks, MD, MPH Director, Prostate and Colorectal Cancers Colorectal Cancer
Improving Outcomes in Colorectal Cancer: The Science of Screening Tennessee Primary Care Association October 23, 2014 Durado Brooks, MD, MPH Director, Prostate and Colorectal Cancers Colorectal Cancer
Tools of the Gastroenterologist: Introduction to GI Endoscopy
 Tools of the Gastroenterologist: Introduction to GI Endoscopy Objectives Endoscopy Upper endoscopy Colonoscopy Endoscopic retrograde cholangiopancreatography (ERCP) Endoscopic ultrasound (EUS) Endoscopic
Tools of the Gastroenterologist: Introduction to GI Endoscopy Objectives Endoscopy Upper endoscopy Colonoscopy Endoscopic retrograde cholangiopancreatography (ERCP) Endoscopic ultrasound (EUS) Endoscopic
Ultrasound Reimbursement Guide 2015: BioJet Fusion
 Ultrasound Reimbursement Guide 2015: BioJet Fusion Diagnosis codes explain the rationale for a given service and are a key factor in a payer s evaluation of medical necessity and coverage determination
Ultrasound Reimbursement Guide 2015: BioJet Fusion Diagnosis codes explain the rationale for a given service and are a key factor in a payer s evaluation of medical necessity and coverage determination
Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies Modeling Study for the US Preventive Services Task Force
 Clinical Review & Education US Preventive Services Task Force MODELING STUDY Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies Modeling Study for the US Preventive Services
Clinical Review & Education US Preventive Services Task Force MODELING STUDY Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies Modeling Study for the US Preventive Services
Billing Guideline. Subject: Preventive Services. Effective: 1/1/14 Last revision effective: 1/1/15
 Subject: Preventive Services Billing Guideline Effective: 1/1/14 Last revision effective: 1/1/15 Background: We are committed to the wellness of our members, and encourage preventive services that can
Subject: Preventive Services Billing Guideline Effective: 1/1/14 Last revision effective: 1/1/15 Background: We are committed to the wellness of our members, and encourage preventive services that can
Obtaining the diagnosis:
 CANCER SURGERY Obtaining the diagnosis: Most cancers are first biopsied in order to determine tumor type. Incisional biopsies extract small portions of tumor for microscopic examination. Excisional biopsies
CANCER SURGERY Obtaining the diagnosis: Most cancers are first biopsied in order to determine tumor type. Incisional biopsies extract small portions of tumor for microscopic examination. Excisional biopsies
2017 MSSP Clinical Quality Measures
 *The information contained in this document relies heavily on information supplied by CMS. GPRO CARE-1 (NQF 0097): Medication Reconciliation Post-Discharge DESCRIPTION: Percentage of discharges from any
*The information contained in this document relies heavily on information supplied by CMS. GPRO CARE-1 (NQF 0097): Medication Reconciliation Post-Discharge DESCRIPTION: Percentage of discharges from any
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal surgery prior as factor in laparoscopic colorectal surgery, 554 555 Abscess(es) CRC presenting as, 539 540 Adenocarcinoma of
Index Note: Page numbers of article titles are in boldface type. A Abdominal surgery prior as factor in laparoscopic colorectal surgery, 554 555 Abscess(es) CRC presenting as, 539 540 Adenocarcinoma of
2017 Coding & Payment Quick Reference
 2017 Coding & Payment Quick Reference Select Pulmonary Procedures Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements.
2017 Coding & Payment Quick Reference Select Pulmonary Procedures Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding or site of service requirements.
Patient sample criteria for the Preventive Care Measure Group are patients aged 50 years and older with a specific patient encounter:
 2016 Physician Quality Reporting System Data Collection Form: Preventive Care (for patients aged 50 and older) NOTE: Individual measures may have more restrictive age and gender requirements. IMPORTANT:
2016 Physician Quality Reporting System Data Collection Form: Preventive Care (for patients aged 50 and older) NOTE: Individual measures may have more restrictive age and gender requirements. IMPORTANT:
Adult-Peds Quality Measure Information Sheet 2018
 Prevention and Screening Adolescent Preventive Care Measures (ADL) The percentage of adolescents 12-17 years of age who had at least one outpatient visit with a PCP or OB/ GYN practitioner during the measurement
Prevention and Screening Adolescent Preventive Care Measures (ADL) The percentage of adolescents 12-17 years of age who had at least one outpatient visit with a PCP or OB/ GYN practitioner during the measurement
WCHQ MEASURES AT A GLANCE
 WCHQ Ambulatory Measures A1C Blood Sugar Testing A1C Blood Sugar Control Patients with diabetes Patients with diabetes office visit in. Gestational Diabetes (code 648.8) is office visit in. Compliance
WCHQ Ambulatory Measures A1C Blood Sugar Testing A1C Blood Sugar Control Patients with diabetes Patients with diabetes office visit in. Gestational Diabetes (code 648.8) is office visit in. Compliance
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS What is CRC? CRC (CRC) is cancer of the large intestine (colon), the lower part of the digestive system. Rectal cancer is cancer of the last several inches of the colon. Together,
FREQUENTLY ASKED QUESTIONS What is CRC? CRC (CRC) is cancer of the large intestine (colon), the lower part of the digestive system. Rectal cancer is cancer of the last several inches of the colon. Together,
who where symptoms? colon cancer facts affected? what
 who Over 130,000 new cases diagnosed each year is Greater than 50,000 deaths annually attributable to colon cancer Second leading cause of cancer death in the U.S. Equal risk in men and women Women over
who Over 130,000 new cases diagnosed each year is Greater than 50,000 deaths annually attributable to colon cancer Second leading cause of cancer death in the U.S. Equal risk in men and women Women over
Diagnostics for the early detection and prevention of colon cancer
 Diagnostics for the early detection and prevention of colon cancer Cologuard Test Crosswalk Proposal July 14 th, 2014 Colorectal cancer: the second-leading U.S. cancer killer Annual U.S. cancer mortality
Diagnostics for the early detection and prevention of colon cancer Cologuard Test Crosswalk Proposal July 14 th, 2014 Colorectal cancer: the second-leading U.S. cancer killer Annual U.S. cancer mortality
Supplementary Online Content
 Supplementary Online Content Friedberg MW, Rosenthal MB, Werner RM, Volpp KG, Schneider EC. Effects of a medical home and shared savings intervention on quality and utilization of care. Published online
Supplementary Online Content Friedberg MW, Rosenthal MB, Werner RM, Volpp KG, Schneider EC. Effects of a medical home and shared savings intervention on quality and utilization of care. Published online
Diagnostics for the early detection and prevention of colon cancer. Publication of DeeP-C Study Data in New England Journal of Medicine March 2014
 Diagnostics for the early detection and prevention of colon cancer Publication of DeeP-C Study Data in New England Journal of Medicine March 2014 Safe Harbor Statement Certain statements made in this presentation
Diagnostics for the early detection and prevention of colon cancer Publication of DeeP-C Study Data in New England Journal of Medicine March 2014 Safe Harbor Statement Certain statements made in this presentation
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING
 CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
CLINICAL PRACTICE GUIDELINE FOR COLORECTAL CANCER SCREENING This guideline is designed to assist practitioners by providing the framework for colorectal cancer (CRC) screening, and is not intended to replace
Expert Consensus Decision Pathway on Peri- Procedural Management of Anticoagulation
 Expert Consensus Decision Pathway on Peri- Procedural Management of Anticoagulation John U Doherty, MD, FACC Anticoagulation Consortium Roundtable Heart House October 24, 2015 Peri-Procedural Management
Expert Consensus Decision Pathway on Peri- Procedural Management of Anticoagulation John U Doherty, MD, FACC Anticoagulation Consortium Roundtable Heart House October 24, 2015 Peri-Procedural Management
KANSAS MEDICAL ASSISTANCE PROGRAM. Fee-for-Service Provider Manual. Rehabilitative Therapy Services
 Fee-for-Service Provider Manual Rehabilitative Therapy Services Updated 12.2015 PART II (PHYSICAL THERAPY, OCCUPATIONAL THERAPY, SPEECH/LANGUAGE PATHOLOGY) Introduction Section BILLING INSTRUCTIONS Page
Fee-for-Service Provider Manual Rehabilitative Therapy Services Updated 12.2015 PART II (PHYSICAL THERAPY, OCCUPATIONAL THERAPY, SPEECH/LANGUAGE PATHOLOGY) Introduction Section BILLING INSTRUCTIONS Page
OFCCR CLINICAL DIAGNOSIS AND TREATMENT FORM
 OFCCR CLINICAL DIAGNOSIS AND TREATMENT FORM Name: _, OFCCR # _ OCGN # _ OCR Group # _ HIN# Sex: MALE FEMALE UNKNOWN Date of Birth: DD MMM YYYY BASELINE DIAGNOSIS & TREATMENT 1. Place of Diagnosis: Name
OFCCR CLINICAL DIAGNOSIS AND TREATMENT FORM Name: _, OFCCR # _ OCGN # _ OCR Group # _ HIN# Sex: MALE FEMALE UNKNOWN Date of Birth: DD MMM YYYY BASELINE DIAGNOSIS & TREATMENT 1. Place of Diagnosis: Name
A PROVIDER S GUIDE TO PREVENTIVE HEALTH SERVICES FOR YOUR PATIENTS
 ConnectiCare, together with the Centers for Medicare & Medicaid Services, encourages the use of preventive health services. For certain basic preventive health services, ConnectiCare Medicare Plan beneficiaries
ConnectiCare, together with the Centers for Medicare & Medicaid Services, encourages the use of preventive health services. For certain basic preventive health services, ConnectiCare Medicare Plan beneficiaries
The following tables represent services by categories which have been identified as preventive in nature:
 Preventive Services Effective for new groups and existing groups when they renew on or after September 23, 2010, most preventive care services. Groups maintaining "grandfathered" status under the Patient
Preventive Services Effective for new groups and existing groups when they renew on or after September 23, 2010, most preventive care services. Groups maintaining "grandfathered" status under the Patient
