INSTRUCTIONS: 1. Use codetable on page 1 for modifications / termination reasons
|
|
|
- Cameron Bond
- 5 years ago
- Views:
Transcription
1 Radiation Therapy Oncology Group Phase III Head & Neck Cancer Treatment Summary Form AMENDED DATA YES INSTRUCTIONS: 1 Use codetable on page 1 for modifications / termination reasons SUMMARY OF SYSTEMIC TREATMENT 1 ASSIGNED TREATMENT ARM(1) 1 Arm 1 2 Arm 2 2 PATIENT HEIGHT (CM)(2) A REASON FOR DOSE MODIFICATION modification 2 Disease progression, relapse during active treatment 3 Adverse event/side effects/complications 4 Death on study 5 Patient withdrawal / refusal after beginning protocol therapy 6 Patient withdrawal / refusal prior to beginning protocol therapy 7 Alternative therapy 8 Other complicating disease 98 Other B REASON TREATMENT TERMINATED 1 Treatment completed per protocol criteria 2 Disease progression, relapse during active treatment 3 Adverse event / side effects / complications 4 Death on study 5 Patient withdrawal / refusal after beginning protocol therapy 6 Patient withdrawal / refusal prior to beginning protocol therapy 7 Alternative therapy, specify in comments 8 Patient off (protocol) treatment for other complicating disease (followup and data submission will continue) 98 Other, specify in comments 3 ARM 1 ONLY - DAY 1 PATIENT WEIGHT (KG, )(26) BODY SURFACE AREA (M 2, )(27) BODY SURFACE AREA (M 2,VALUE USED TO CALCULATE DOSE IF BSA> 2)(28) Agent Name CISPLATIN Date Agent Administered - - (29) Agent Total Dose (mg) (30) (use code table A) (31) (use code table B) (32) 1016 a of 10
2 3a DAY 22 PATIENT WEIGHT (KG, )(33) BODY SURFACE AREA (M 2, )(34) BODY SURFACE AREA (M 2,VALUE USED TO CALCULATE DOSE IF BSA> 2) (35) Agent Name CISPLATIN Date Agent Administered - - (36) Agent Total Dose (mg) (37) (use code table A) (38) (use code table B) (39) 3b DID PATIENT RECEIVE ANY NON- PROTOCOL CETUXIMAB DURING RADIATION THERAPY?(40) 4 ARM 2 ONLY - WEEK 1 - INITIAL DOSE PATIENT WEIGHT (KG)(41) BODY SURFACE AREA(M 2 )(42) Date Agent Administered - - (43) Agent Total Dose (mg) (44) (use code table A) (45) (use code table B) (46) 4a WEEK 2 PATIENT WEIGHT (KG)(47) BODY SURFACE AREA(M 2 )(48) Date Agent Administered - - (49) Agent Total Dose (mg) (50) (use code table A) (51) (use code table B) (52) 1016 a of 10
3 4b WEEK 3 PATIENT WEIGHT (KG)(53) BODY SURFACE AREA(M 2 )(54) Date Agent Administered - - (55) Agent Total Dose (mg) (56) (use code table A) (57) (use code table B) (58) 4c WEEK 4 PATIENT WEIGHT (KG)(59) BODY SURFACE AREA(M 2 )(60) Date Agent Administered - - (61) Agent Total Dose (mg) (62) (use code table A) (63) (use code table B) (64) 4d WEEK 5 PATIENT WEIGHT (KG)(65) BODY SURFACE AREA(M 2 )(66) Date Agent Administered - - (67) Agent Total Dose (mg) (68) (use code table A) (69) (use code table B) (70) 4e WEEK 6 PATIENT WEIGHT (KG)(71) BODY SURFACE AREA(M 2 )(72) Date Agent Administered - - (73) Agent Total Dose (mg) (74) (use code table A) (75) (use code table B) (76) 1016 a of 10
4 4f WEEK 7 PATIENT WEIGHT (KG)(77) BODY SURFACE AREA(M 2 )(78) Date Agent Administered - - (79) Agent Total Dose (mg) (80) (use code table A) (81) (use code table B) (82) 4g WEEK 8 PATIENT WEIGHT (KG)(83) BODY SURFACE AREA(M 2 )(84) Date Agent Administered - - (85) Agent Total Dose (mg) (86) (use code table A) (87) (use code table B) (88) 4h DID PATIENT RECEIVE ANY NON-PROTOCOL CISPLATIN DURING RADIATION THERAPY?(89) 5 DID THE PATIENT RECEIVE ANY ADDITIONAL SUPPORTIVE CARE DURING THE REPORTING PERIOD? (SEE PROTOCOL 90) (91) 5a IF YES, TYPE OF SUPPORTIVE CARE GIVEN; SPECIFY (92) 6 WAS THE PATIENT HOSPITALIZED FOR ANY TREATMENT RELATED COMPLICATIONS IN THIS REPORT PERIOD?(93) 1016 a of 10
5 7 DOES THE PATIENT CURRENTLY REQUIRE TUBE FEEDING?(94) 7a IF YES, IS PATIENT DEPENDENT ON TUBE FEEDING FOR >50% OF NUTRITIONAL SUPPORT?(95) 8a DENTAL GINGIVAL HEALTH (96) 0 Normal: Edentulous, with no gingival disease; native teeth in place with gingiva in excellent condition 1 Mild changes/good dental health: mild periodontal inflammation-routine cleaning indi cated; < 5 restorations indicated; no extractions indicated 2 Moderate/fair dental health: moderate periodontal inflammation; deep perodontal cleaning indicated; 6 or more restorations indicated; less than full mouth extractions indicated 3 Severe changes in dental health: widespread periodontal disease with extensive procedure/ surgery indicated; full month extractions indicated 4 Life-threatening dental condition: extensive abscess, extensive soft tissue or bone infection, sepsis; urgent intervention indicated 8b NUMBER OF NATIVE TEETH IN PLACE (0-32)(97) 1016 a of 10
6 9 PROTOCOL SPECIFIC ADVERSE EVENT EVALUATION Code each of the following using Version 4 (MedDRA 12) Score most severe grade observed during report period (Grade 1-5) If the AE did not occur, code "0" If unknown code "9" LEAVE NO GRADE BLANK AE's > grade 3 require date of onset Assign attribution to protocol treatment for each AE reported; indicate whether SAE was submitted Document unrelated AE's if > grade 3 and during or within 30 days of completing protocol treatment A ATTRIBUTION CODE See protocol sections 6 & 7 for definitions of "protocol treatment" 1 Unrelated 2 Unlikely 3 Possible 4 Probable 5 Definite B Serious Adverse Event Report Submitted C AE led to discontinuation of treatment TERM GRADE BEGIN DATE A B C Ear and labyrinth disorders Hearing impaired (115) - - (116) (117) (118) (119) Tinnitus (120) - - (121) (122) (123) (124) Gastrointestinal disorders Constipation (125) - - (126) (127) (128) (129) Diarrhea (130) - - (131) (132) (133) (134) Dry mouth (135) - - (136) (137) (138) (139) Dyspepsia (140) - - (141) (142) (143) (144) Dysphagia (145) - - (146) (147) (148) (149) Mucositis oral (150) - - (151) (152) (153) (154) Nausea (155) - - (156) (157) (158) (159) Vomiting (160) - - (161) (162) (163) (164) General disorders & administraton site conditions Fatigue (165) - - (166) (167) (168) (169) Pain (170) - - (171) (172) (173) (174) Injury, poisoning and procedural complications Dermatitis radiation (175) - - (176) (177) (178) (179) Metabolism & nutrition disorders Anorexia (180) - - (181) (182) (183) (184) Musculoskeletal and connective tissue disorders Avascular necrosis (185) - - (186) (187) (188) (189) Head soft tissue necrosis (190) - - (191) (192) (193) (194) Neck soft tissue necrosis (195) - - (196) (197) (198) (199) Trismus (200) - - (201) (202) (203) (204) 1016 a of 10
7 9 PROTOCOL SPECIFIC ADVERSE EVENT EVALUATION Code each of the following using Version 4 (MedDRA 12) Score most severe grade observed during report period (Grade 1-5) If the AE did not occur, code "0" If unknown code "9" LEAVE NO GRADE BLANK AE's > grade 3 require date of onset Assign attribution to protocol treatment for each AE reported; indicate whether SAE was submitted Document unrelated AE's if > grade 3 and during or within 30 days of completing protocol treatment A ATTRIBUTION CODE See protocol sections 6 & 7 for definitions of "protocol treatment" 1 Unrelated 2 Unlikely 3 Possible 4 Probable 5 Definite B Serious Adverse Event Report Submitted C AE led to discontinuation of treatment TERM GRADE BEGIN DATE Nervous system disorders A B C Dysgeusia (205) - - (206) (207) (208) (209) Peripheral sensory neuropathy (210) - - (211) (212) (213) (214) Psychiatric disorders Insomnia (215) - - (216) (217) (218) (219) Respiratory, thoracic and mediastinal disorders Cough (220) - - (221) (222) (223) (224) Pharyngeal mucositis (225) - - (226) (227) (228) (229) Skin and subcutaneous tissue disorders Alopecia (230) - - (231) (232) (233) (234) Dry skin (235) - - (236) (237) (238) (239) Nail loss (240) - - (241) (242) (243) (244) Pruritus (245) - - (246) (247) (248) (249) Rash acneiform (250) - - (251) (252) (253) (254) Rash maculo-papular (255) - - (256) (257) (258) (259) 1016 a of 10
8 10 NEW OR CONTINUING ADVERSE EVENTS? (not reported Q9) (300) (skip to comments) Adverse Events: Use the CTCAE version 4 (MedDRA 12) to code all events Score most severe grade observed during report period (grade 1-5) Adverse Events of grade 3 or higher require start date Assign attribution to protocol treatment for each AE and indicate if an SAE was reported A ATTRIBUTION CODE 1 Unrelated 2 Unlikely 3 Possible 4 Probable 5 Definite B SAE Report Submitted C AE led to discontinuation of treatment MedDRA Code CTCAE Term (specify if "other") CTCAE Grade CTCAE Begin Date A B C (301) (302) (303) - - (304) (305) (306) (307) (308) (309) (310) - - (311) (312) (313) (314) (315) (316) (317) - - (318) (319) (320) (321) (322) (323) (324) - - (325) (326) (327) (328) (329) (330) (331) - - (332) (333) (334) (335) (336) (337) (338) - - (339) (340) (341) (342) (343) (344) (345) - - (346) (347) (348) (349) (350) (351) (352) - - (353) (354) (355) (356) (357) (358) (359) - - (360) (361) (362) (363) (364) (365) (366) - - (367) (368) (369) (370) (371) (372) (373) - - (374) (375) (376) (377) (378) (379) (380) - - (381) (382) (383) (384) (385) (386) (387) - - (388) (389) (390) (391) (392) (393) (394) - - (395) (396) (397) (398) (399) (400) (401) - - (402) (403) (404) (405) (406) (407) (408) - - (409) (410) (411) (412) (413) (414) (415) - - (416) (417) (418) (419) (420) (421) (422) - - (423) (424) (425) (426) (427) (428) (429) - - (430) (431) (432) (433) 1016 a of 10
9 11 INDICATE UNITS TO BE USED FOR LAB VALUES(698) 1 Conventional 2 SI 12 LAB VALUES USING STANDARD US UNITS INSTRUCTIONS: Record laboratory values USING STANDARD US UNITS include normal ranges Normal Range Pre-Rx Date (mm/dd) Year: / / / / / / / / Hgb (g/dl) Hct (%) WBC (x1000) mm 3 Platelets (x1000) mm 3 Neutrophils (%) ANC (mm 3 ) Sodium (meq/l) Potassium (meq/l) Chloride (meq/l) BUN (mg/dl) Creatinine (mg/dl) Calc Creatinine Clear (ml/min) Total Bilirubin (mg/dl) Alk PO4 (ImU/ml) LDH (U/L) SGOT (IU/L) SGPT (IU/L) Total Protein (g/dl) Albumin (g/dl) Uric Acid (mg/dl) Calcium (mg/dl) Glucose(mg/dl) Mg (meq/l) U/A COMMENTS ( ) The reported case report information has been reviewed and confirmed by the principal investigator (1275) Person completing form - - (1276) Date 1016 a of 10
10 13 LAB VALUES USING STANDARD INTERNATIONAL UNITS INSTRUCTIONS: Record laboratory values USING STANDARD INTERNATIONAL UNITS include normal ranges Normal Range Pre-Rx Date (mm/dd) Year: / / / / / / / / Hgb (mmol/l) Hct (volume fraction) WBC (x10 9 liter) Platelets (x10 9 liter) Neutrophils (fraction) ANC (mm 3 ) Sodium (mmol/l) Potassium (mmol/l) Chloride (mmol/l) BUN (mmol/l) Creatinine (umol/l) Calc Creatinine Clear (ml/sec/m 2 ) Total Bilirubin (umol/l) Alk PO4 (U/L) LDH (IU/L) SGOT (U/L) SGPT (U/L) Total Protein (g/l) Albumin (g/l) Uric Acid (mmol/l) Calcium (mmol/l) Glucose (mmol/l) Mg (mmol/l) U/A COMMENTS ( ) The reported case report information has been reviewed and confirmed by the principal investigator (1840) Person completing form - - (1841) Date 1016 a of 10
B. PANITUMUMAB DOSE LEVEL 0 No dose reduction 1 Level -1 2 Level Other, specify in comments for this cycle
 Radiation Therapy Oncology Group Phase II Study Pre-operative Chemo- Radiation + Panitumumab for Potentially Operable Lung Cancer Concurrent Summary Form AMENDED DATA YES INSTRUCTIONS: Submit all pages
Radiation Therapy Oncology Group Phase II Study Pre-operative Chemo- Radiation + Panitumumab for Potentially Operable Lung Cancer Concurrent Summary Form AMENDED DATA YES INSTRUCTIONS: Submit all pages
ASSIGNED TREATMENT ARM
 SF Radiation Therapy Oncology Group Phase III Lung High-dose vs Standard-dose Conformal XRT with Chemotherapy Consolidation Treatment Summary Form RTOG Study No. 0617 Case # AMENDED DATA YES INSTRUCTIONS:
SF Radiation Therapy Oncology Group Phase III Lung High-dose vs Standard-dose Conformal XRT with Chemotherapy Consolidation Treatment Summary Form RTOG Study No. 0617 Case # AMENDED DATA YES INSTRUCTIONS:
(7) VITAL SIGNS (8) LEVEL OF CONSCIOUSNESS (9) MENTAL STATUS (10) SPEECH (11) VISION (12) FUNDUS (PAPILLEDEMA)
 Radiation Therapy Oncology Group Phase II CNS Lymphoma Follow-Up Form RTOG Study No. 1114 Case # Amended Data Yes INSTRUCTIONS: Submit this form as indicated in the protocol. All dates need to be recorded
Radiation Therapy Oncology Group Phase II CNS Lymphoma Follow-Up Form RTOG Study No. 1114 Case # Amended Data Yes INSTRUCTIONS: Submit this form as indicated in the protocol. All dates need to be recorded
PLACE LABEL HERE. Radiation Therapy Oncology Group Phase II Nasopharyngeal Cancer Follow-Up Form
 F1 AMENDED DATA Radiation Therapy Oncology Group Phase II Nasopharyngeal Cancer Follow-Up Form YES No INSTRUCTIONS: Submit this form at the appropriate follow-up interval and at death Dates are recorded
F1 AMENDED DATA Radiation Therapy Oncology Group Phase II Nasopharyngeal Cancer Follow-Up Form YES No INSTRUCTIONS: Submit this form at the appropriate follow-up interval and at death Dates are recorded
MEDICAL HISTORY. 23-Jan-2018 to 23-Jan VCA Miller-Robertson Animal Hospital 8807 Melrose Ave, Los Angeles, CA (310)
 8807 Melrose Ave, Los Angeles, CA 90069 (310) 657-7050 MEDICAL HISTORY 23-Jan-2018 to 23-Jan-2018 Client Linnea Engdahl (1810) C: Linnea: (310) 351-9547 Patient Abby (6487) Canine Mixed Breed 3y (22-Jan-2015)
8807 Melrose Ave, Los Angeles, CA 90069 (310) 657-7050 MEDICAL HISTORY 23-Jan-2018 to 23-Jan-2018 Client Linnea Engdahl (1810) C: Linnea: (310) 351-9547 Patient Abby (6487) Canine Mixed Breed 3y (22-Jan-2015)
Nilotinib AEs (adverse events) in CML population:
 Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Nilotinib AEs (adverse events) in CML population: The percentages below were taken from a randomized trial of nilotinib 300mg BID in newly diagnosed Ph+ CML patients (N=279) taken from the Tasigna 2017
Understanding Blood Tests
 PATIENT EDUCATION patienteducation.osumc.edu Your heart pumps the blood in your body through a system of blood vessels. Blood delivers oxygen and nutrients to all parts of the body. It also carries away
PATIENT EDUCATION patienteducation.osumc.edu Your heart pumps the blood in your body through a system of blood vessels. Blood delivers oxygen and nutrients to all parts of the body. It also carries away
10 Essential Blood Tests PART 1
 Presents 10 Essential Blood Tests PART 1 The Blood Chemistry Webinars With DR. DICKEN WEATHERBY Creator of the Blood Chemistry Software Essential Blood Test #1: Basic Chem Screen and CBC http://bloodchemsoftware.com
Presents 10 Essential Blood Tests PART 1 The Blood Chemistry Webinars With DR. DICKEN WEATHERBY Creator of the Blood Chemistry Software Essential Blood Test #1: Basic Chem Screen and CBC http://bloodchemsoftware.com
CASE-BASED SMALL GROUP DISCUSSION MHD II
 MHD II, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD II Session 11 April 11, 2016 STUDENT COPY MHD II, Session 11, Student Copy Page 2 CASE HISTORY 1 Chief complaint: Our baby
MHD II, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD II Session 11 April 11, 2016 STUDENT COPY MHD II, Session 11, Student Copy Page 2 CASE HISTORY 1 Chief complaint: Our baby
Carboplatin / Liposomal Doxorubicin CARBO/CAELYX Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Supplementary materials
 Supplementary materials Table S Adverse events identified by participants diary logs and blood hematologic and biochemical tests (n=2) group (n=) Placebo group (n=) P value for chi-squared test Asthma
Supplementary materials Table S Adverse events identified by participants diary logs and blood hematologic and biochemical tests (n=2) group (n=) Placebo group (n=) P value for chi-squared test Asthma
Carboplatin / Gemcitabine Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Carboplatin / Gemcitabine Gynaecological Cancer PROCTOCOL REF: MPHAGYNCAG (Version No: 1.0) Approved for use in: Recurrent/metastatic endometrial carcinoma Previously
Systemic Anti Cancer Treatment Protocol Carboplatin / Gemcitabine Gynaecological Cancer PROCTOCOL REF: MPHAGYNCAG (Version No: 1.0) Approved for use in: Recurrent/metastatic endometrial carcinoma Previously
i. Where is the participant seen?
 PFU01 method used: Phone/in-person interview 1 Enter PIP # here: Online survey 2 Enter Web # here: Initials of person completing form: Date Form Completed: / / Form Version: 03 / 01 / 18 Is the participant
PFU01 method used: Phone/in-person interview 1 Enter PIP # here: Online survey 2 Enter Web # here: Initials of person completing form: Date Form Completed: / / Form Version: 03 / 01 / 18 Is the participant
Sponsor Novartis. Generic Drug Name Pasireotide. Therapeutic Area of Trial Cushing s disease. Protocol Number CSOM230B2208E1
 Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
Sponsor Novartis Generic Drug Name Pasireotide Therapeutic Area of Trial Cushing s disease Protocol Number CSOM230B2208E1 Title Extension to a multicenter, open-label study to assess the safety and efficacy
June 2009 Respiratory Committee CALGB 30610
 30610 Phase III comparison of thoracic radiotherapy regimens in patients with limited small cell lung cancer also receiving cisplatin and etoposide Activated: March 15, 2008 Study Chairpersons: J. Bogart
30610 Phase III comparison of thoracic radiotherapy regimens in patients with limited small cell lung cancer also receiving cisplatin and etoposide Activated: March 15, 2008 Study Chairpersons: J. Bogart
Subject ID: I N D # # U A * Consent Date: Day Month Year
 IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
Cisplatin / Paclitaxel Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Cisplatin / Paclitaxel Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIP (Version No: 1.0) Approved for use in: First line treatment for stage Ib-IV with minimal residual
Systemic Anti Cancer Treatment Protocol Cisplatin / Paclitaxel Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIP (Version No: 1.0) Approved for use in: First line treatment for stage Ib-IV with minimal residual
TIP Paclitaxel, Ifosfamide and Cisplatin
 Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Chemistry Reference Ranges and Critical Values
 Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-25 U/L 10-35 U/L 10-30 U/L 10-25 U/L 10-30 U/L 10-35 U/L 10-25 U/L 10-35 U/L 10-25 U/L 10-20 U/L 10-35 U/L Albumin 0-6
Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-25 U/L 10-35 U/L 10-30 U/L 10-25 U/L 10-30 U/L 10-35 U/L 10-25 U/L 10-35 U/L 10-25 U/L 10-20 U/L 10-35 U/L Albumin 0-6
Chemistry Reference Ranges and Critical Values
 Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-30 U/L 10-30 U/L 10-20 U/L Albumin 0-6 days 6 days - 37 months 37 months - 7 years 7-20 years 2.6-3.6 g/dl 3.4-4.2 g/dl
Alanine Aminotransferase (ALT, SGPT) 3-9 years 9-18 years 1-9 years 9-18 years 10-30 U/L 10-30 U/L 10-20 U/L Albumin 0-6 days 6 days - 37 months 37 months - 7 years 7-20 years 2.6-3.6 g/dl 3.4-4.2 g/dl
TABLE FOR GRADING THE SEVERITY OF ADVERSE EVENTS
 TABLE FOR GRADING THE SEVERITY OF ADVERSE EVENTS Green AEs managed by DTUs REMARK Red Patient referral to provincial hospitals for AE management Note: In the case of patients with events at grade of severity
TABLE FOR GRADING THE SEVERITY OF ADVERSE EVENTS Green AEs managed by DTUs REMARK Red Patient referral to provincial hospitals for AE management Note: In the case of patients with events at grade of severity
DEMOGRAPHICS PHYSICAL ATTRIBUTES VITAL SIGNS. Protocol: ABC-123 SCREENING. Subject ID. Subject Initials. Visit Date: / / [ YYYY/MM/DD]
![DEMOGRAPHICS PHYSICAL ATTRIBUTES VITAL SIGNS. Protocol: ABC-123 SCREENING. Subject ID. Subject Initials. Visit Date: / / [ YYYY/MM/DD] DEMOGRAPHICS PHYSICAL ATTRIBUTES VITAL SIGNS. Protocol: ABC-123 SCREENING. Subject ID. Subject Initials. Visit Date: / / [ YYYY/MM/DD]](/thumbs/78/78699510.jpg) SCREENING Visit Date: DEMOGRAPHICS / / [YYYY/MM/DD] Consent Signed Date and time / / [YYYY/MM/DD] : Has written assent been obtained? If no, why not? YES NO Gender: Male Female Birthdate: Permission given
SCREENING Visit Date: DEMOGRAPHICS / / [YYYY/MM/DD] Consent Signed Date and time / / [YYYY/MM/DD] : Has written assent been obtained? If no, why not? YES NO Gender: Male Female Birthdate: Permission given
SMALL ANIMAL SOFT TISSUE CASE- BASED EXAMINATION
 SMALL ANIMAL SOFT TISSUE CASE- BASED EXAMINATION CASE-BASED EXAMINATION INSTRUCTIONS The case-based examination measures surgical principles in case management prior to, during, and after surgery. Information
SMALL ANIMAL SOFT TISSUE CASE- BASED EXAMINATION CASE-BASED EXAMINATION INSTRUCTIONS The case-based examination measures surgical principles in case management prior to, during, and after surgery. Information
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
Ipilimumab in Melanoma
 Ipilimumab in Melanoma Indication: Advanced (unresectable or metastatic) melanoma in adults who have received prior therapy LCNDG criteria to be met: Histologically confirmed unresectable stage III or
Ipilimumab in Melanoma Indication: Advanced (unresectable or metastatic) melanoma in adults who have received prior therapy LCNDG criteria to be met: Histologically confirmed unresectable stage III or
ALIMTA Administration and Education Guide
 ALIMTA Administration and Education Guide A comprehensive guide that includes customizable dosing handouts for patients and caregivers ALIMTA is indicated for the initial treatment of patients with locally
ALIMTA Administration and Education Guide A comprehensive guide that includes customizable dosing handouts for patients and caregivers ALIMTA is indicated for the initial treatment of patients with locally
BIOCHEMICAL REPORT. Parameters Unit Finding Normal Value. Lipase U/L Amylase U/L
 Lipase U/L 88.9 10-195 Amylase U/L 1181.1 371.3-1192.6 West Delhi :- 7/148, Opp. MCD Office, Major Pankaj Batra Marg, Near Ramesh Nagar, New Delhi-15, Ph. : 011-47562566,9999830187 Liver Function Test
Lipase U/L 88.9 10-195 Amylase U/L 1181.1 371.3-1192.6 West Delhi :- 7/148, Opp. MCD Office, Major Pankaj Batra Marg, Near Ramesh Nagar, New Delhi-15, Ph. : 011-47562566,9999830187 Liver Function Test
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS
 Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
CISPLATIN Chemo-radiation regimen Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol CISPLATIN Chemo-radiation regimen Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIX (Version No: 1.0) Approved for use in: Locally advanced cervical cancer (adjuvant/curative)
Systemic Anti Cancer Treatment Protocol CISPLATIN Chemo-radiation regimen Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIX (Version No: 1.0) Approved for use in: Locally advanced cervical cancer (adjuvant/curative)
Cisplatin and Vinorelbine and radiotherapy (NSCLC)
 Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
Weekly Cisplatin + Radiotherapy - Interlace study -
 Weekly Cisplatin + Radiotherapy - Interlace study - A phase III multicentre trial of weekly induction chemotherapy followed by standard chemoradiation versus standard chemoradiation alone in patients with
Weekly Cisplatin + Radiotherapy - Interlace study - A phase III multicentre trial of weekly induction chemotherapy followed by standard chemoradiation versus standard chemoradiation alone in patients with
Cisplatin and Vinorelbine and radiotherapy (NSCLC)
 Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
What is PlaqueOff (PO)? A new study in Beagle dogs. Oral effects of
 Oral effects of What is? PO is a dry food supplement. Sprinkle it onto your pet s food daily. PO is an algae that has been harvested in the Atlantic ocean in northern Norway and contains nothing else such
Oral effects of What is? PO is a dry food supplement. Sprinkle it onto your pet s food daily. PO is an algae that has been harvested in the Atlantic ocean in northern Norway and contains nothing else such
1.) 3 yr old FS Siamese cat: 3 day history of lethargy, anorexia. Dyspneic, thin, febrile.
 1.) 3 yr old FS Siamese cat: 3 day history of lethargy, anorexia. Dyspneic, thin, febrile. NUCLEATED CELLS 19.5 High 4.0-14.0 x 10^3/ul METAMYELOCYTES 9 % 1.8 High 0.0-0.0 x 10^3/ul BAND NEUTROPHILS 61
1.) 3 yr old FS Siamese cat: 3 day history of lethargy, anorexia. Dyspneic, thin, febrile. NUCLEATED CELLS 19.5 High 4.0-14.0 x 10^3/ul METAMYELOCYTES 9 % 1.8 High 0.0-0.0 x 10^3/ul BAND NEUTROPHILS 61
THE CLATTERBRIDGE CANCER CENTRE NHS FOUNDATION TRUST
 Outpatient Anti Cancer Treatment Handbook LCV Lomustine, Cisplatin, Vincristine Packer Regimen Repeated every 6 weeks for a maximum of 6 cycles Approved for use in: Medulloblastoma adjuvant therapy Dosage:
Outpatient Anti Cancer Treatment Handbook LCV Lomustine, Cisplatin, Vincristine Packer Regimen Repeated every 6 weeks for a maximum of 6 cycles Approved for use in: Medulloblastoma adjuvant therapy Dosage:
To report SUSPECTED ADVERSE REACTIONS, contact Millennium Pharmaceuticals at VELCADE or FDA at FDA-1088 or
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VELCADE safely and effectively. See full prescribing information for VELCADE. VELCADE (bortezomib)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VELCADE safely and effectively. See full prescribing information for VELCADE. VELCADE (bortezomib)
Cisplatin Vinorelbine (Oral) therapy +/- radiotherapy
 1 REGIMEN TITLE: Cisplatin Vinorelbine (Oral) therapy +/- radiotherapy Page 1 of 5 Indication: First line in Radical/ Induction, Adjuvant and Advanced & Palliative treatment of Non-small cell lung cancer
1 REGIMEN TITLE: Cisplatin Vinorelbine (Oral) therapy +/- radiotherapy Page 1 of 5 Indication: First line in Radical/ Induction, Adjuvant and Advanced & Palliative treatment of Non-small cell lung cancer
A 73 year old man, presents with anaemia. Describe the CT scan. Should see primary gastric cancer,ascites and liver secondary
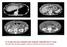 A 73 year old man, presents with anaemia. Describe the CT scan. Should see primary gastric cancer,ascites and liver secondary A 73 year old man PS presents with anaemia. Endoscopy and CT scan thorax/abdomen
A 73 year old man, presents with anaemia. Describe the CT scan. Should see primary gastric cancer,ascites and liver secondary A 73 year old man PS presents with anaemia. Endoscopy and CT scan thorax/abdomen
EASTERN COOPERATIVE ONCOLOGY GROUP
 EASTERN COOPERATIVE ONCOLOGY GROUP E5204 INTERGROUP RANDOMIZED PHASE III STUDY OF POSTOPERATIVE OXALIPLATIN, 5-FLUOROURACIL AND LEUCOVORIN VS OXALIPLATIN, 5-FLUOROURACIL, LEUCOV- ORIN AND BEVACIZUMAB FOR
EASTERN COOPERATIVE ONCOLOGY GROUP E5204 INTERGROUP RANDOMIZED PHASE III STUDY OF POSTOPERATIVE OXALIPLATIN, 5-FLUOROURACIL AND LEUCOVORIN VS OXALIPLATIN, 5-FLUOROURACIL, LEUCOV- ORIN AND BEVACIZUMAB FOR
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck
 Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PERJETA safely and effectively. See full prescribing information for PERJETA. PERJETA (pertuzumab)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use PERJETA safely and effectively. See full prescribing information for PERJETA. PERJETA (pertuzumab)
SMALL ANIMAL SOFT TISSUE CASE-BASED EXAMINATION
 SMALL ANIMAL SOFT TISSUE CASE-BASED EXAMINATION CASE-BASED EXAMINATION INSTRUCTIONS The case-based examination measures surgical principles in case management prior to, during, and after surgery. Information
SMALL ANIMAL SOFT TISSUE CASE-BASED EXAMINATION CASE-BASED EXAMINATION INSTRUCTIONS The case-based examination measures surgical principles in case management prior to, during, and after surgery. Information
Documentation Dissection
 History of Present Illness: Documentation Dissection The patient is a 50-year-old male c/o symptoms for past 4 months 1, severe 2 bloating and stomach cramps, some nausea, vomiting, diarrhea. In last 3
History of Present Illness: Documentation Dissection The patient is a 50-year-old male c/o symptoms for past 4 months 1, severe 2 bloating and stomach cramps, some nausea, vomiting, diarrhea. In last 3
Clinician Blood Panel Results
 Page 1 of 7 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Page 1 of 7 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
VIP (Etoposide, Ifosfamide and Cisplatin)
 VIP (Etoposide, Ifosfamide and Cisplatin) Indication First line treatment for metastatic seminoma, non seminoma or combined tumours where bleomycin is contra-indicated. Usually used for patients with intermediate
VIP (Etoposide, Ifosfamide and Cisplatin) Indication First line treatment for metastatic seminoma, non seminoma or combined tumours where bleomycin is contra-indicated. Usually used for patients with intermediate
Ipilimumab (skin) Indication Advanced (unresectable or metastatic) melanoma in patients who have received prior therapy.
 Ipilimumab (skin) Indication Advanced (unresectable or metastatic) melanoma in patients who have received prior therapy. (NICE TA268) ICD-10 codes Codes prefixed with C43 Regimen details Day Drug Dose
Ipilimumab (skin) Indication Advanced (unresectable or metastatic) melanoma in patients who have received prior therapy. (NICE TA268) ICD-10 codes Codes prefixed with C43 Regimen details Day Drug Dose
* Final Report * ED Triage Entered On: 01/16/2014 8:45 EST Performed On: 01/16/2014 8:42 EST by
 Result date: Result status: 16 January 2014 8:42 EST Auth (Verified) * Final Report * ED Triage Entered On: 01/16/2014 8:45 EST Performed On: 01/16/2014 8:42 EST by Assessment I Chief Complaint : Diarrhea
Result date: Result status: 16 January 2014 8:42 EST Auth (Verified) * Final Report * ED Triage Entered On: 01/16/2014 8:45 EST Performed On: 01/16/2014 8:42 EST by Assessment I Chief Complaint : Diarrhea
H.6.G.2 Non-MAC Studies
 Page 63 H.6.G.2 Non-MAC Studies H.6.G.2.A Non-MAC Studies at the 600 mg dose A 600 mg dose of azithromycin was given in two non-mac studies (354/354A and 167). Please note: Study 354/354A enrolled a mixture
Page 63 H.6.G.2 Non-MAC Studies H.6.G.2.A Non-MAC Studies at the 600 mg dose A 600 mg dose of azithromycin was given in two non-mac studies (354/354A and 167). Please note: Study 354/354A enrolled a mixture
Study No.: Title: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
NORMAL LABORATORY VALUES FOR CHILDREN
 Pediatric Drug Lookup Normal Laboratory Values for NORMAL LABORATORY VALUES FOR CHILDREN CHEMISTRY Normal Values Albumin 0-1 y 2.0-4.0 g/dl 1 y to adult 3.5-5.5 g/dl Ammonia Newborns 90-150 mcg/dl 40-120
Pediatric Drug Lookup Normal Laboratory Values for NORMAL LABORATORY VALUES FOR CHILDREN CHEMISTRY Normal Values Albumin 0-1 y 2.0-4.0 g/dl 1 y to adult 3.5-5.5 g/dl Ammonia Newborns 90-150 mcg/dl 40-120
Sorafenib Side effects and dose adjustment SORAMIC
 Sorafenib Side effects and dose adjustment 1 Adverse events: prevention and management as key Patients should be informed to: Take preventive measures, where possible Be aware and report AEs as soon as
Sorafenib Side effects and dose adjustment 1 Adverse events: prevention and management as key Patients should be informed to: Take preventive measures, where possible Be aware and report AEs as soon as
Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x 4 (3-weekly) in Early Breast Cancer
 Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x Indication: Neoadjuvant or adjuvant therapy for moderate to high risk node positive breast
Breast Pathway Group EC x 4 Paclitaxel x 4 (3-weekly): Epirubicin & Cyclophosphamide x 4 followed by Paclitaxel x Indication: Neoadjuvant or adjuvant therapy for moderate to high risk node positive breast
BCCA Protocol Summary for Treatment of Advanced Squamous Cell Carcinoma of the Head and Neck Cancer Using Fluorouracil and Platinum
 BCCA Protocol Summary for Treatment of Advanced Squamous Cell Carcinoma of the Head and Neck Cancer Using Fluorouracil and Platinum Protocol Code: Tumour Group: Contact Physician: HNAVFUP Head and Neck
BCCA Protocol Summary for Treatment of Advanced Squamous Cell Carcinoma of the Head and Neck Cancer Using Fluorouracil and Platinum Protocol Code: Tumour Group: Contact Physician: HNAVFUP Head and Neck
Synopsis Style Clinical Study Report SR EFC10139 Version number: 1 (electronic 2.0)
 SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, multicenter, multinational study to assess the long-term effect, over 1 year, of rimonabant 10 mg in comparison with rimonabant
SYNOPSIS Title of the study: A randomized, double-blind, parallel-group, multicenter, multinational study to assess the long-term effect, over 1 year, of rimonabant 10 mg in comparison with rimonabant
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix
 Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Study Phase Phase IIIb
 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Methylphenidate hydrochloride Therapeutic Area of Trial Neuroscience/Psychiatry Approved Indication Methylphenidate hydrochloride is currently
Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Methylphenidate hydrochloride Therapeutic Area of Trial Neuroscience/Psychiatry Approved Indication Methylphenidate hydrochloride is currently
PRODUCT MONOGRAPH. Pr Vectibix. (panitumumab) Sterile Solution for Infusion 100, 400 mg (20 mg/ml) Professed Standard.
 PRODUCT MONOGRAPH Pr Vectibix (panitumumab) Sterile Solution for Infusion 100, 400 mg (20 mg/ml) Professed Standard Antineoplastic Amgen Canada Inc. 6775 Financial Drive, Suite 100 Mississauga, Ontario
PRODUCT MONOGRAPH Pr Vectibix (panitumumab) Sterile Solution for Infusion 100, 400 mg (20 mg/ml) Professed Standard Antineoplastic Amgen Canada Inc. 6775 Financial Drive, Suite 100 Mississauga, Ontario
JOB AID CRITICAL VALUES AND TESTS W/O MICRO CRITICAL TESTS. Always call results for the following test(s):
 Facilities: NoCo Laboratories JOB AID CRITICAL VALUES AND TESTS W/O MICRO CRITICAL TESTS Always call results for the following test(s): CONSTITUENT Ethylene Glycol (Performed at CU) Time Interval (from
Facilities: NoCo Laboratories JOB AID CRITICAL VALUES AND TESTS W/O MICRO CRITICAL TESTS Always call results for the following test(s): CONSTITUENT Ethylene Glycol (Performed at CU) Time Interval (from
CASE STUDY. Adverse Events in treatment chronic hepatitis C patients with PegInterferon and Ribavirin What would your management decision be?
 Adverse Events in treatment chronic hepatitis C patients with PegInterferon and Ribavirin What would your management decision be? CASE STUDY Pham Thi Thu Thuy MD, PhD Ho Chi Minh City Vietnam Serious Adverse
Adverse Events in treatment chronic hepatitis C patients with PegInterferon and Ribavirin What would your management decision be? CASE STUDY Pham Thi Thu Thuy MD, PhD Ho Chi Minh City Vietnam Serious Adverse
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine
 BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
NPAC(W)+PERT+TRAS Regimen
 Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
CORE CLINICAL DATASET
 The following pages are the CORE CLINICAL DATASET for Ebolavirus disease. The forms are featured in order of priority. Therefore, when resources are limited, data collection can be reduced as required
The following pages are the CORE CLINICAL DATASET for Ebolavirus disease. The forms are featured in order of priority. Therefore, when resources are limited, data collection can be reduced as required
MANAGEMENT OF LUNG CANCER CYTOTOXIC AND TARGETED THERAPY TOXICITIES
 MANAGEMENT OF LUNG CANCER CYTOTOXIC AND TARGETED THERAPY TOXICITIES CCP Conference Oct 27, 2017 Rick Prayag Pharmacist Lung DSG PRESENTER DISCLOSURE Faculty: Rick Prayag Pharm D Relationships with commercial
MANAGEMENT OF LUNG CANCER CYTOTOXIC AND TARGETED THERAPY TOXICITIES CCP Conference Oct 27, 2017 Rick Prayag Pharmacist Lung DSG PRESENTER DISCLOSURE Faculty: Rick Prayag Pharm D Relationships with commercial
Nivolumab and Ipilimumab
 Nivolumab and Ipilimumab Indication Advanced (unresectable or metastatic) melanoma. (NICE TA400) ICD-10 codes Codes prefixed with C43 Regimen details Cycles 1-4 Nivolumab and Ipilimumab every 3 weeks Day
Nivolumab and Ipilimumab Indication Advanced (unresectable or metastatic) melanoma. (NICE TA400) ICD-10 codes Codes prefixed with C43 Regimen details Cycles 1-4 Nivolumab and Ipilimumab every 3 weeks Day
Case Series Drug Analysis Print Name: Vaxigrip, Fluarix, Inflexal V og Influenzacvaccine 01Sep Oct2014
 - 16Oct2014 Report Run Date: 20-Oct-2014 Data Lock Date: 16-Oct-2014 19:00:06 Earliest Reaction Date: 28-Oct-2009 MedDRA Version: MedDRA 17.0 Vaxigrip, Fluarix, Inflexal V og Influenzacvaccine : Alle cases
- 16Oct2014 Report Run Date: 20-Oct-2014 Data Lock Date: 16-Oct-2014 19:00:06 Earliest Reaction Date: 28-Oct-2009 MedDRA Version: MedDRA 17.0 Vaxigrip, Fluarix, Inflexal V og Influenzacvaccine : Alle cases
Summary ID# Clinical Study Summary: Study B4Z-JE-LYBD
 CT Registry ID#5286 Page 1 Summary ID# 5286 Clinical Study Summary: Study B4Z-JE-LYBD An Open Label, Dose-Titration Safety Study of Hydrochloride in Outpatient Japanese Children with Attention-Deficit/Hyperactivity
CT Registry ID#5286 Page 1 Summary ID# 5286 Clinical Study Summary: Study B4Z-JE-LYBD An Open Label, Dose-Titration Safety Study of Hydrochloride in Outpatient Japanese Children with Attention-Deficit/Hyperactivity
Sponsor Novartis. Generic Drug Name Vildagliptin/Metformin. Therapeutic Area of Trial Type 2 diabetes. Approved Indication Type 2 diabetes
 Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Clinical Trial Results Database Page 1 Sponsor Novartis Generic Drug Name Vildagliptin/Metformin Therapeutic Area of Trial Type 2 diabetes Approved Indication Type 2 diabetes Study Number CLMF237A2309
Cisplatin + Etoposide + Thoracic Radiotherapy (TRT) INDICATIONS FOR USE:
 Cisplatin + Etoposide + Thoracic Radiotherapy (TRT) INDICATIONS FOR USE: Protocol INDICATION ICD10 Code Small cell lung cancer (SCLC) limited disease C34 00279a ELIGIBILTY: Indications as above ECOG 0-2
Cisplatin + Etoposide + Thoracic Radiotherapy (TRT) INDICATIONS FOR USE: Protocol INDICATION ICD10 Code Small cell lung cancer (SCLC) limited disease C34 00279a ELIGIBILTY: Indications as above ECOG 0-2
Clinician Blood Panel Results
 Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
NPAC+PERT+TRAS Regimen
 Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
Regimen Monograph Regimen Name Drug Regimen Cycle Frequency Premedication and Supportive Measures Dose Modifications Adverse Effects Interactions Drug Administration and Special Precautions Recommended
Date Time By Code Description Qty (Variance) Photo
 Adobe Animal Hospital 6331 Haven Ave., Suite 4 Rancho Cucamonga, CA 91737 909-483-3535 Patient Chart Printed: 03-16-17 at 9:44a CLIENT INFORMATION Name Ms. Amanda Barber (1394) Address 10850 Church St.
Adobe Animal Hospital 6331 Haven Ave., Suite 4 Rancho Cucamonga, CA 91737 909-483-3535 Patient Chart Printed: 03-16-17 at 9:44a CLIENT INFORMATION Name Ms. Amanda Barber (1394) Address 10850 Church St.
CABAZITAXEL Prostate Cancer
 Systemic Anti-Cancer Treatment Protocol CABAZITAXEL Prostate Cancer PROCTOCOL REF: MPHACABAZ (Version No: 1.0) Approved for use in: Cabazitaxel in combination with prednisolone is a treatment option for
Systemic Anti-Cancer Treatment Protocol CABAZITAXEL Prostate Cancer PROCTOCOL REF: MPHACABAZ (Version No: 1.0) Approved for use in: Cabazitaxel in combination with prednisolone is a treatment option for
Oxaliplatin and Gemcitabine
 Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
( Thyrotoxicosis ) ( Hyperthyroidism ) ( Coma ) ( Hypercalcemia ) ( thyroid storm )
 2007 18 201-205 ( thyroid storm ) ( 12.4 mg/dl ) ( intact parathyroid hormone ) 32 pg/ml ( 10-60 ) ( 140-150/min ) 36.9 ( 10.5 mg/dl ) ( BUN: 78 mg/dl, creatinine: 1.8 mg/dl ) TSH:
2007 18 201-205 ( thyroid storm ) ( 12.4 mg/dl ) ( intact parathyroid hormone ) 32 pg/ml ( 10-60 ) ( 140-150/min ) 36.9 ( 10.5 mg/dl ) ( BUN: 78 mg/dl, creatinine: 1.8 mg/dl ) TSH:
Sponsor Novartis Pharma GmbH. Generic Drug Name Panobinostat (LBH589) Therapeutic Area of Trial Myelodysplastic Syndrome (MDS)
 Sponsor Novartis Pharma GmbH Generic Drug Name Panobinostat (LBH589) Therapeutic Area of Trial Myelodysplastic Syndrome (MDS) Approved Indication Investigational Protocol Number CLBH589BDE04 Title A one
Sponsor Novartis Pharma GmbH Generic Drug Name Panobinostat (LBH589) Therapeutic Area of Trial Myelodysplastic Syndrome (MDS) Approved Indication Investigational Protocol Number CLBH589BDE04 Title A one
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
MHD I SESSION X. Renal Disease
 MHD I, Session X, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD I SESSION X Renal Disease Monday, November 11, 2013 MHD I, Session X, Student Copy Page 2 Case #1 Cc: I have had weeks of diarrhea
MHD I, Session X, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD I SESSION X Renal Disease Monday, November 11, 2013 MHD I, Session X, Student Copy Page 2 Case #1 Cc: I have had weeks of diarrhea
B3D-MC-GHCY Clinical Study Report Synopsis Page 1Page GHCY Synopsis LY
 B3DMCGHCY Clinical Study Report Page 1Page 1 2. GHCY B3DMCGHCY Clinical ClinicalStudy StudyReport Report Page 2Page 2 Clinical Study Report : Study B3DMCGHCY Title of Study: The Effect of Teriparatide
B3DMCGHCY Clinical Study Report Page 1Page 1 2. GHCY B3DMCGHCY Clinical ClinicalStudy StudyReport Report Page 2Page 2 Clinical Study Report : Study B3DMCGHCY Title of Study: The Effect of Teriparatide
TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour
 TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour Indication: Second line therapy in Relapsed Germ Cell Tumours Regimen details: Paclitaxel 175mg/m 2 IV Cisplatin 20mg/m 2 IV - D5 Ifosfamide
TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour Indication: Second line therapy in Relapsed Germ Cell Tumours Regimen details: Paclitaxel 175mg/m 2 IV Cisplatin 20mg/m 2 IV - D5 Ifosfamide
THE CLATTERBRIDGE CANCER CENTRE NHS FOUNDATION TRUST. Systemic Anti Cancer Treatment Protocol. EDP + mitotane
 Systemic Anti Cancer Treatment Protocol EDP + mitotane PROCEDURE REF: MPHAHANEDP (Version No: 1.0) Approved for use in: Symptomatic treatment for advanced (unresectable, metastatic or relapsed) adrenocortical
Systemic Anti Cancer Treatment Protocol EDP + mitotane PROCEDURE REF: MPHAHANEDP (Version No: 1.0) Approved for use in: Symptomatic treatment for advanced (unresectable, metastatic or relapsed) adrenocortical
Secondary efficacy endpoints for Part 2, the Eltrombopag-Only Period, included the proportion of subjects who
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Age: 14 Houston TX 77007
 Patient Medical History HEIGHTS HOSPITAL FOR ANIMALS Bernie Rogers Patient: JACK DOB: 08/26/1999 720 Courtlandt St. Species: FELINE Age: 14 Houston TX 77007 Breed: Domestic Shorthair Sex: MN Color: Black
Patient Medical History HEIGHTS HOSPITAL FOR ANIMALS Bernie Rogers Patient: JACK DOB: 08/26/1999 720 Courtlandt St. Species: FELINE Age: 14 Houston TX 77007 Breed: Domestic Shorthair Sex: MN Color: Black
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ADCETRIS safely and effectively. See full prescribing information for ADCETRIS. ADCETRIS (brentuximab
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ADCETRIS safely and effectively. See full prescribing information for ADCETRIS. ADCETRIS (brentuximab
Multiphasic Blood Analysis
 Understanding Your Multiphasic Blood Analysis Test Results Mon General thanks you for participating in the multiphasic blood analysis. This test can be an early warning of health problems, including coronary
Understanding Your Multiphasic Blood Analysis Test Results Mon General thanks you for participating in the multiphasic blood analysis. This test can be an early warning of health problems, including coronary
R-GemOx. Lymphoma group INDICATION. Relapsed or Refractory Lymphoma, for patients unsuitable for R-GDP regimen. Omit rituximab if CD20- negative
 R-GemOx INDICATION Relapsed or Refractory Lymphoma, for patients unsuitable for R-GDP regimen. Omit rituximab if CD20- negative TREATMENT INTENT Disease modification PRE-ASSESSMENT 1. Ensure histology
R-GemOx INDICATION Relapsed or Refractory Lymphoma, for patients unsuitable for R-GDP regimen. Omit rituximab if CD20- negative TREATMENT INTENT Disease modification PRE-ASSESSMENT 1. Ensure histology
Paclitaxel/Carboplatin with dose dense EC Neoadjuvant Regimen
 Systemic Anti Cancer Treatment Protocol Paclitaxel/Carboplatin with dose dense EC Neoadjuvant Regimen PROTOCOL REF: MPHAPCECBR (Version No: 1.0) Approved for use in: Neoadjuvant treatment of operable,
Systemic Anti Cancer Treatment Protocol Paclitaxel/Carboplatin with dose dense EC Neoadjuvant Regimen PROTOCOL REF: MPHAPCECBR (Version No: 1.0) Approved for use in: Neoadjuvant treatment of operable,
Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
CHECK LIST FORM-MONTH 27 (Please note Month 27 is from enrolment not randomisation)
 CHECK LIST FORM-MONTH 27 (Please note Month 27 is from enrolment not randomisation) Participant Initials: Date of Birth: Were the following forms completed for this visit? Follow Up Form Done t Done BVASWG
CHECK LIST FORM-MONTH 27 (Please note Month 27 is from enrolment not randomisation) Participant Initials: Date of Birth: Were the following forms completed for this visit? Follow Up Form Done t Done BVASWG
CARDIOVASCULAR CASE-BASED SMALL GROUP DISCUSSION
 MHD I Session VIII Student Copy Page 1 CARDIOVASCULAR CASE-BASED SMALL GROUP DISCUSSION MHD I SESSION VIII OCTOBER 22, 2014 STUDENT COPY MHD I Session VIII Student Copy Page 2 Case 1 Chief Complaint I
MHD I Session VIII Student Copy Page 1 CARDIOVASCULAR CASE-BASED SMALL GROUP DISCUSSION MHD I SESSION VIII OCTOBER 22, 2014 STUDENT COPY MHD I Session VIII Student Copy Page 2 Case 1 Chief Complaint I
Carboplatin + Paclitaxel Cancer of the Cervix
 Carboplatin + Paclitaxel Cancer of the Cervix Background: Topotecan in combination with cisplatin is recommended as a treatment option for women with recurrent or stage IVB cervical cancer only if they
Carboplatin + Paclitaxel Cancer of the Cervix Background: Topotecan in combination with cisplatin is recommended as a treatment option for women with recurrent or stage IVB cervical cancer only if they
Tenapanor, a gastrointestinal NHE3 inhibitor, reduces serum phosphate in patients with chronic kidney disease stage 5D and hyperphosphatemia
 , a gastrointestinal NHE3 inhibitor, reduces serum phosphate in patients with chronic kidney disease stage 5D and hyperphosphatemia Geoffrey A Block, 1 David P Rosenbaum, 2 Maria Leonsson-Zachrisson, 3
, a gastrointestinal NHE3 inhibitor, reduces serum phosphate in patients with chronic kidney disease stage 5D and hyperphosphatemia Geoffrey A Block, 1 David P Rosenbaum, 2 Maria Leonsson-Zachrisson, 3
MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc
 MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc * A hypothetical case study of a patient eligible for first-line mcrc therapy. mcrc = metastatic colorectal cancer. WHAT CLINICAL CHARACTERISTICS AFFECT YOUR
MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc * A hypothetical case study of a patient eligible for first-line mcrc therapy. mcrc = metastatic colorectal cancer. WHAT CLINICAL CHARACTERISTICS AFFECT YOUR
Supplementary Online Content
 Supplementary Online Content Chawla SP, Papai Z, Mukhametshina G, et al. First-line aldoxorubicin vs doxorubicin in metastatic or locally advanced unresectable soft-tissue sarcoma: a phase 2b randomized
Supplementary Online Content Chawla SP, Papai Z, Mukhametshina G, et al. First-line aldoxorubicin vs doxorubicin in metastatic or locally advanced unresectable soft-tissue sarcoma: a phase 2b randomized
This is a controlled document and therefore must not be changed
 AZACITIDINE NICE TA218 Treatment of adults not eligible for haematopoietic stem cell transplantation who have: Intermediate-2 and high-risk MDS according to the International Prognostic Scoring System
AZACITIDINE NICE TA218 Treatment of adults not eligible for haematopoietic stem cell transplantation who have: Intermediate-2 and high-risk MDS according to the International Prognostic Scoring System
Functional Blood Chemistry & CBC Analysis
 Functional Blood Chemistry & CBC Analysis Session 11 Immune Markers Immune Dysfunctions Immune Deficiency, Allergies, Immune Over Activity Causes of Immune Dysregulation External Influences Pharmaceutical
Functional Blood Chemistry & CBC Analysis Session 11 Immune Markers Immune Dysfunctions Immune Deficiency, Allergies, Immune Over Activity Causes of Immune Dysregulation External Influences Pharmaceutical
CASE-BASED SMALL GROUP DISCUSSION
 MHD I, Session XII, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION Session XII MHD I Friday, November 15, 2013 STUDENT COPY MHD I, Session XII, Student Copy Page 2 Case 1 CHIEF COMPLAINT: I am very
MHD I, Session XII, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION Session XII MHD I Friday, November 15, 2013 STUDENT COPY MHD I, Session XII, Student Copy Page 2 Case 1 CHIEF COMPLAINT: I am very
Clinician Blood Panel Results
 Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Page 1 of 8 Blood Panel - Markers Out of Range and Patterns (Pattern: proprietary formula using one or more Blood Markers) Blood Panel: Check for Markers that are out of Lab Range ***NOTE*** Only one supplement
Cetirizine Proposed Core Safety Profile
 Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
Cetirizine Proposed Core Safety Profile Posology and method of administration Elderly subjects: data do not suggest that the dose needs to be reduced in elderly subjects provided that the renal function
Clinical Trial Results Database Page 1
 Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
Clinical Trial Results Database Page 1 Sponsor Novartis Pharmaceuticals Corporation Generic Drug Name Therapeutic Area of Trial Major Depressive Disorder (MDD) Approved Indication Treatment of major depressive
