NEOADJUVANT BEVACIZUMAB, OXALIPLATIN, 5-FLUOROURACIL, AND RADIATION FOR RECTAL CANCER
|
|
|
- Marion Lane
- 6 years ago
- Views:
Transcription
1 doi: /j.ijrobp Int. J. Radiation Oncology Biol. Phys., Vol. 82, No. 1, pp , 2012 Copyright Ó 2012 Elsevier Inc. Printed in the USA. All rights reserved /$ - see front matter CLINICAL INVESTIGATION Gastrointestinal Cancer NEOADJUVANT BEVACIZUMAB, OXALIPLATIN, 5-FLUOROURACIL, AND RADIATION FOR RECTAL CANCER TOM DIPETRILLO, M.D., VICTOR PRICOLO, M.D., JORGE LAGARES-GARCIA, M.D., MATT VREES, M.D., ADAM KLIPFEL, M.D., TOM CATALDO, M.D., WILLIAM SIKOV, M.D., BRENDAN MCNULTY, M.D., JOSHUA SHIPLEY, M.D., ELLIOT ANDERSON, M.D., HUMERA KHURSHID, M.D., BRIGID OCONNOR, M.D., NICKLAS B. E. OLDENBURG, M.D., KATHY RADIE-KEANE, M.D., SYED HUSAIN, M.D., AND HOWARD SAFRAN, M.D. From the Brown University Oncology Group, Providence, RI, USA Purpose: To evaluate the feasibility and pathologic complete response rate of induction bevacizumab + modified infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) 6 regimen followed by concurrent bevacizumab, oxaliplatin, continuous infusion 5-fluorouracil (5-FU), and radiation for patients with rectal cancer. Methods and Materials: Eligible patients received 1 month of induction bevacizumab and mfolfox6. Patients then received 50.4 Gy of radiation and concurrent bevacizumab (5 mg/kg on Days 1, 15, and 29), oxaliplatin (50 mg/ m 2 /week for 6 weeks), and continuous infusion 5-FU (200 mg/m 2 /day). Because of gastrointestinal toxicity, the oxaliplatin dose was reduced to 40 mg/m 2 /week. Resection was performed 4 8 weeks after the completion of chemoradiation. Results: The trial was terminated early because of toxicity after 26 eligible patients were treated. Only 1 patient had significant toxicity (arrhythmia) during induction treatment and was removed from the study. During chemoradiation, Grade 3/4 toxicity was experienced by 19 of 25 patients (76%). The most common Grade 3/4 toxicities were diarrhea, neutropenia, and pain. Five of 25 patients (20%) had a complete pathologic response. Nine of 25 patients (36%) developed postoperative complications including infection (n = 4), delayed healing (n = 3), leak/abscess (n = 2), sterile fluid collection (n = 2), ischemic colonic reservoir (n = 1), and fistula (n = 1). Conclusions: Concurrent oxaliplatin, bevacizumab, continuous infusion 5-FU, and radiation causes significant gastrointestinal toxicity. The pathologic complete response rate of this regimen was similar to other fluorouracil chemoradiation regimens. The high incidence of postoperative wound complications is concerning and consistent with other reports utilizing bevacizumab with chemoradiation before major surgical resections. Ó 2012 Elsevier Inc. Rectal cancer, Neoadjuvant, Bevacizumab, Oxaliplatin. INTRODUCTION There were 40,870 new cases of rectal cancer diagnosed in the United States in 2009 (1). Stage-for-stage, recurrence rates are higher in rectal cancer in comparison to colon cancer and the site of first failure for patients undergoing surgery may be local, within the pelvis, or distant, such as the liver or lungs (2). Preoperative fluorouracil and concurrent radiation is the standard of care for T3-T4 rectal cancer based on the Phase III trial by the German Rectal Cancer Study Group. Preoperative chemoradiation improves local control and is associated with reduced toxicities as compared with postoperative chemoradiation. Pathologic complete response rates for 5-fluorouracil (5-FU) and radiation range from 7% to 29% (2 10). To attempt to increase pathologic complete response rates and reduce local and distant relapses, several strategies have been evaluated. These strategies include the addition of other chemotherapeutic agents and targeted therapies to 5-FU and radiation, as well as the use of induction chemotherapy before concurrent chemoradiation. The addition of oxaliplatin to standard therapy is supported by trials using various infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) regimens that have shown improvements in survival in both the metastatic and adjuvant settings (11 13). The addition of oxaliplatin to preoperative 5-FU/radiation has been reported in rectal cancer (14 16).In Reprint requests to: Howard Safran, M.D., Department of Medicine, The Miriam Hospital, 164 Summit Ave., Providence, RI Tel: (401) ; Fax: (401) ; hsafran@lifespan.org Presented at the Annual Meeting of the American Society of Clinical Oncology, Orlando FL, May 29-June 2, Supported in part by Genentech and Sanofi-Aventis Conflict of interest: none. Received Oct 29, 2009, and in revised form June 10, Accepted for publication Aug 9, 2010.
2 Bevacizumab Rectal Cancer d T. DIPETRILLO et al. 125 randomized trials, the addition of oxaliplatin to capecitabine and concurrent radiation did not statistically increase pathologic complete response but was associated with increased gastrointestinal (GI) toxicities (15, 16). Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, increases survival when combined with chemotherapy for patients with advanced colorectal cancer (17). There is strong rationale to initiate vascular endothelial growth factor inhibition with chemoradiation because these modalities are synergistic in preclinical models (18 20). The addition of bevacizumab to 5-FU/radiation has been described, with complete response rates ranging from 8% to 32%, with an acceptable toxicity profile (21 24). The administration of chemotherapy before neoadjuvant chemoradiation has the theoretical advantages of downstaging a locally advanced tumor, eliminating micrometastatic disease, and improving tolerance to chemotherapy when compared with its administration in the adjuvant setting. A randomized Phase II study of induction chemotherapy administered before chemoradiation and surgery revealed similar pathologic complete response rates and R0 resections, but with improved toxicity and compliance as compared with adjuvant chemotherapy after surgery (25). The purpose of this prospective, Phase II study was to evaluate the pathologic complete response rate, safety, and tolerability of a regimen of induction chemotherapy with two cycles of bevacizumab + modified infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX) 6 regimen, followed by neoadjuvant concurrent radiation/5-fu/oxaliplatin/ bevacizumab. Another six cycles of bevacizumab + mfol- FOX6 were planned to be administered after surgery. PATIENTS AND METHODS Patient eligibility Eligible patients included those with pathologically documented adenocarcinoma of the rectum with no evidence of distant metastasis. Required pretreatment staging studies included a computed tomography (CT) scan or magnetic resonance imaging of the abdomen and pelvis and chest CT or chest x-ray or positron emission tomography/ct scan. Patients were required to have clinical Stage II or III rectal adenocarcinoma, T3-T4, N0, M0 or T1-T4, N1-2, M0. Transrectal ultrasound was performed whenever possible. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 1. Required laboratory parameters included granulocytes $1,500/mL, platelets $100,000/mL, total bilirubin #2.0 mg/dl or direct bilirubin #1.0 mg/dl, alkaline phosphatase #3 Upper Limit of Normal (ULN), alanine transaminase (ALT) #3 ULN, creatinine #1.5 ULN, and urine protein creatinine ratio <1.0. A complete history and physical examination were performed on all patients before treatment. Patients with a history of stroke or myocardial infarction in past 6 months, clinically significant congestive heart failure, peripheral vascular disease, or bleeding diathesis were ineligible for the study. Uncontrolled hypertension $150/100 or >Grade 1 neuropathy were also exclusion criteria. The study was approved by the institutional review boards of all participating hospitals, and all patients gave written informed consent according to federal and institutional guidelines. Chemotherapy Each patient received 1 month of induction treatment with biweekly bevacizumab (5 mg/kg) and mfolfox6 (bevacizumab 5 mg/kg intravenously [IV] over 90 min, followed by oxaliplatin 85 mg/m 2 IVover 2 h with concurrent Leucovorin 400 mg/m 2 IV over 2 h, followed by 5-FU 400 mg/m 2 IV push, followed by 5-FU 2400 mg/m 2 IVover 46 h infusion). Patients then received 50.4 Gy of radiation with concurrent bevacizumab (5 mg/kg on Days 1, 15, and 29), oxaliplatin (50 mg/m 2 /week Days 1, 8, 15, 22, 29, 36), and 5-FU (200 mg/m 2 /day) as a continuous IV infusion throughout radiation. Because of GI toxicity experienced by the first 3 patients in the study, the oxaliplatin dose was reduced to 40 mg/m 2 / week. All patients were premedicated before chemotherapy treatment to minimize nausea and vomiting, usually with 5-HT3 receptor antagonists. Adjuvant chemotherapy was started after 4 but less than 12 weeks after surgical resection and consisted of six biweekly treatments of mfolfox6 and bevacizumab. Radiation therapy All patients received pelvic radiation therapy with concurrent chemotherapy. Treatments were given with a linear accelerator with a minimum energy of 10MeV. The total dose of radiation therapy was 50.4 Gy. Patients underwent CT simulation with oral contrast to visualize the small bowel. All patients underwent three-dimensional conformal radiation treatment planning. The intent of treatment was to include the tumor bed (gross tumor volume) with a margin, the internal iliac nodes, and the presacral nodes (the external iliac nodes were also included if a structure was invaded that drained to the external iliacs) to a total dose of 45 Gy. This was delivered at 1.8 Gy per day, 5 days per week, or 25 fractions over 5 weeks. A minimum tumor boost of 540 cgy, given at 1.8 cgy per fraction, was required for all patients and was given to the gross tumor volume with a 1-cm margin. Normal tissue sparing techniques were employed so that no portion of the small bowel received more than 4500 cgy and less than 10% of the bladder receive greater than 5000 cgy. Surgery Resection was performed 4 8 weeks after the completion of chemoradiotherapy. A total mesorectal excision was the recommended operation for mid and distal rectal tumors. Abdominoperineal resections were done for tumors with sphincter involvement or very distal locations preventing coloanal anastomosis. Complications after surgery were recorded. Assessments Toxicity was graded according to the National Cancer Institute s Common Toxicity Criteria (version 3.0). Patients
3 126 I. J. Radiation Oncology d Biology d Physics Volume 82, Number 1, 2012 were assessed before each treatment during the induction phase. Weekly toxicity assessments were accomplished during the chemoradiation phase. Pathologic response was assessed in all patients undergoing resection of the tumor. A complete pathologic response was defined as no evidence of malignancy on the specimen. Statistical analysis The primary objective was to determine the complete pathologic response rate as determined by surgical resection. Secondary objectives were to determine the safety and tolerability of the treatment regimen. We hypothesized that a complete response rate of 30% would be clinically meaningful. Our accrual goal was 29 evaluable patients. The treatment regimen was to be accepted if at least 6 pathologic complete response rates were observed from the 29 patients. Accrual was stopped after 26 patients when the Brown University Oncology Group data safety monitoring board reviewed the surgical morbidity of this trial. RESULTS Patient characteristics Between April 2005 and July 2008, 26 patients with locally advanced rectal cancer were treated on the study. Table 1 lists the patient characteristics. The median age was 50 years (range, 39 75). Sixteen patients had nodal involvement. Three patients had anal sphincter involvement at presentation. Toxicities: induction chemotherapy One patient experienced a Grade 4 arrhythmia during induction chemotherapy and was withdrawn from the study. Retrospectively, this patient had prolonged QT syndrome at study entry, and the arrhythmia was probably related to the additional effects of diphenhydramine and prochlorperazine. No other Grade 3 or 4 events occurred during induction chemotherapy. Table 1. Patient characteristics (n = 25) Median age 50 (39 75) Stage T2 2 T3 20 T4 3 Node 7 Node + 16 Nx 2 Distance from anal sphincter: Invades sphincter 3 <2 cm cm 7 5cm 13 Surgical procedure Low anterior resection 19 Abdominoperineal resection 6 Toxicities: chemoradiation Acute Grade 2 4 toxicities for all patients are listed in Table 2. Multiple toxicities in the same patient are scored as separate events. Any Grade 3 or 4 toxicities were experienced by 19 of 25 (76%) patients. Grade 3 nonhematologic toxicities included diarrhea (40%), pain (16%), fatigue (8%), nausea (8%), radiation dermatitis (8%), and bleeding with menstruation (4%). Grade 3 hematologic toxicities included neutropenia (16%) and anemia (8%). Grade 4 toxicities included anemia (4%), neutropenia (4%), sepsis (4%), and nausea/diarrhea/weight loss (4%). Because of toxicity, 10 patients (40%) were unable to complete the intended chemotherapy. Hospitalizations occurred in 5 patients (25%) from diarrhea/dehydration (n = 4) and a port infection (n = 1). One patient had a rectovaginal fistula identified incidentally at the time of surgical resection. Postoperative complications The median time from last dose of bevacizumab and surgery was 8.9 weeks. Two operations, performed by non colorectal surgeons, involved a low anterior resection and primary anastomoses with each case was complicated by an anastomic leak. All other operations were performed by colorectal surgeons and involved 6 patients undergoing abdominoperineal resection and 19 patients undergoing a total mesorectal excision with a low anterior resection and a temporary ostomy. Nine of 25 (36%) patients developed postoperative wound complications including infection (n = 4), delayed healing (n = 3), leak/abscess (n = 2), sterile fluid collection (n = 2), ischemic colonic reservoir (n = 1), and fistula (n = 1). Deep venous thrombosis developed postoperatively in 3 patients. One patient expired suddenly at home approximately 10 days after surgical resection. Autopsy revealed the patient had suffered an acute myocardial infarction and had significant three-vessel coronary artery disease. Table 2. Highest toxicity for each patient during chemoradiation (n = 25) Grade 2 Grade 3 Grade 4 Diarrhea 5 (20%) 10 (40%) 1 (4%) Nausea 2 (8%) 2 (8%) 1 (4%) Dehydration 1 (4%) 2 (8%) Pain 7 (28%) 4 (16%) Bleeding 3 (12%) 1 (4%) Neuropathy 1 (4%) 1 (4%) Radiation dermatitis 4 (16%) 2 (8%) Hypokalemia 1 (4%) 2 (8%) Hypocalcemia 3 (12%) Mucositis 2 (8%) 1 (4%) Neutropenia 1 (4%) 4 (16%) 1 (4%) Anemia 2 (8%) 2 (8%) 1 (4%) Fatigue 6 (24%) 2 (8%) Weight loss 4 (16%) 1 (4%) Infection 2 (8%) 1 (4%) 1 (4%)
4 Bevacizumab Rectal Cancer d T. DIPETRILLO et al. 127 Adjuvant treatment Fifteen of 25 patients (60%) received any adjuvant treatment. Only 6 of 25 patients (25%) were able to complete all six cycles of adjuvant treatment. Response and follow-up Five of 25 patients (20%) had a complete pathologic response. With a median follow-up of 36 months, 5 patients have had disease recurrence, 4 with lung metastases and 1 with a pelvic recurrence. None of the patients with recurrence had a pathologic complete response. Follow up ranged from 8 to 54 months. The disease-free and overall survival curves are included in Fig. 1. Discussion In this prospective trial, two cycles of induction bevacizumab + mfolfox6 were safely administered in patients with rectal cancer. However, the addition of oxaliplatin and bevacizumab to 5-FU and radiation had substantial GI toxicity with 44% of patients developing Grade 3/4 diarrhea. Surgical morbidities were concerning with 9 of 25 (36%) patients developing postoperative wound complications. Because of this toxicity, the Brown University Oncology Group Data Safety Monitoring Board recommended closure of this study. Other trials have demonstrated the addition of oxaliplatin to 5-FU and radiation increases GI toxicities in preoperative treatment of rectal cancer. In a Phase III trial, Gerard reported an increase in diarrhea with preoperative capecitabine, oxaliplatin, and radiation compared to capecitabine and radiation alone (16). Furthermore, in the Studio Terapia Adiuvante Retto - 01 (STAR-01) Phase III trial, Aschele et al. reported an increase in GI toxicity with the addition of oxaliplatin to 5-FU infusion and radiation without an increase in pathologic complete response (16). Willett and colleagues reported the addition of neoadjuvant bevacizumab followed by bevacizumab with continuous infusion 5-FU and radiation for patients with rectal cancer. 21 In this study, which did not use induction chemotherapy before chemoradiation, the regimen of bevacizumab, 5-FU, and radiation appeared to be safe. Surgery Fig. Progression-free survival and overall survival. occurred 7 10 weeks after completion of all therapies as compared with 4 8 weeks in our study. In the Willett study, which included 32 patients, postoperative complications included anastomotic leak with presacral abscess requiring drainage (n = 1), vaginal tear (n = 1), pelvic hematoma (n = 1), and delayed healing of perineal incisions. Five of 32 patients (16%) had a pathologic complete response. This treatment resulted in an actuarial 5-year local control and overall survival of 100%. Actuarial 5-year disease-free survival was 75% and 5 patients developed metastases postsurgery. Postoperative complications have been reported in other studies using major resections after bevacizumab, especially when used in the neoadjuvant setting in combination with radiation (22 31). Crane et al. reported a Phase II trial to assess the efficacy and safety of the addition of bevacizumab to concurrent neoadjuvant capecitabinebased chemoradiation in locally advanced rectal cancer (22). Twenty-five patients with clinically staged T3N0-N1 rectal cancer received neoadjuvant therapy with 50.4 Gy radiotherapy with 3 doses of bevacizumab every 2 weeks and capecitabine. Eight (32%) of 25 patients had a pathologic complete response. Three wound complications required surgical intervention (one coloanal anastomotic dehiscence requiring completion abdominal perineal resection and two perineal wound dehiscences after initial abdominal perineal resection APR). Five minor complications occurred that resolved without operative intervention. With a median follow-up of 22.7 months (range, months), all patients were alive; 1 patient has had a recurrence in the pelvis (2-year actuarial rate, 6.2%) and 3 had distant recurrences. The Dutch Colorectal Cancer Group has also evaluated bevacizumab with concurrent capecitabine and radiation in rectal cancer. In a preliminary report of 23 patients, chemoradiation toxicities included 7 patients with Grade 3 toxicities (4 skin, 2 diarrhea, 1 tenesmic) and 1 patient with Grade 4 toxicity (anal mucositis). One patient developed enteritis with diffuse uncontrollable bleeding at the end of the chemoradiation and died before rectal surgery. Two small bowel perforations occurred, with one occurring 70 days after the last administration of bevacizumab. A third patient had an asymptomatic rectal wall perforation at the site of the primary tumor. Surgical complications consisted of one perineal dehiscence, one rectovaginal fistula after a stapling failure, and 1 patient with bleeding (5500 ml). Two of 21 patients had a complete pathologic response. In a trial by Varadhachary evaluating bevacizumab with gemcitabine and radiation as neoadjuvant treatment for pancreatic cancer, major postoperative complications occurred in 5 of the 9 patients (56%) who underwent pancreatic resection including wound dehiscence (n = 3) requiring reoperation, a large ventral hernia related to fascial dehiscence (n = 1), and a biliary anastomotic leak (n =1)(27). After bevacizumab, fewer postoperative complications have been reported in nonirradiated tissues such as resecting liver metastases (31). In our small trial, it is difficult to compare the 20% pathologic complete response rate with other chemoradiation
5 128 I. J. Radiation Oncology d Biology d Physics Volume 82, Number 1, 2012 regimens. Reports of the pathologic complete response rate for 5-FU and radiation have varied widely. For example, the pathologic complete response to fluorouracil and radiation was 5% in the Bosset trial, 8% in the Sauer trial, and 11% in the Gerard trial, respectively (3 5). In Radiation Treatment and Oncology Group 0012, patients with clinical T3/T4 distal rectal cancers were randomly assigned to receive continuous infusion fluorouracil plus pelvic hyperfractionated radiation or continuous infusion fluorouracil and irinotecan. The pathologic complete response rate was 26% in each arm. Furthermore, the impact of bevacizumab and two cycles of induction mfolfox6 and bevacizumab on systemic recurrence cannot be assessed in our trial because the sample size. However, in a large, Phase III randomized trial of 2710 patients with Stage II and Stage III colon cancer, the addition of bevacizumab to mfolfox6 did not significantly prolong disease-free survival compared with mfolfox6 alone (32). It is concerning in our trial that a substantial number of patients did not receive intended postoperative chemotherapy. Fifteen of 25 patients (60%) received any adjuvant treatment and only 6 of 25 patients (25%) were able to complete all six cycles of adjuvant treatment. To improve patient compliance, future Brown University Oncology Group will investigate administering eight cycles of FOLFOX prior to chemoradiation. REFERENCES 1. Jemal A, Siegel R, Ward E, et al. Cancer statistics, CA Cancer J Clin 2009;59: Minsky BD, Mies C, Recht A, et al. Resectable adenocarcinoma of the rectosigmoid and rectum. I. Patterns of failure and survival. Cancer 1988;61: Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006; 355: Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Eng J Med 2004;351: Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD J Clin Oncol 2006;24(28): Roh MS, Colangelo LH, O Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27: Rich TA, Skibber JM, Ajani JA, et al. Preoperative infusional chemoradiation therapy for stage T3 rectal cancer. Int J Radiat Oncol Biol Phys 1995;32: Chari RS, Tyler DS, Anscher MS, et al. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann Surg 1995;221: Mohiuddin M, Winter K, Mitchell E. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial J Clin Oncol 2006;4: Mohiuddin M, Mohiuddin MM, Marks J. Future directions in neoadjuvant therapy of rectal cancer: Maximizing pathological complete response rates. Cancer Treat Rev November 2009; 35(7): Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22: De Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18: Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27: Ryan DP, Niedzwiecki D, Hollis D, et al. Phase I/II Study of preoperative oxaliplatin, fluorouracil, and external-beam radiation therapy in patients with locally advanced rectal cancer: Cancer and Leukemia Group B J Clin Oncol 2006;1: Aschele C, Pinto C, Cordio S, et al., on behalf of STAR Network Investigators. Preoperative fluorouracil (FU)-based chemoradiation with and without weekly oxaliplatin in locally advanced rectal cancer: Pathologic response analysis of the Studio Terapia Adiuvante Retto (STAR)-01 randomized phase III trial [abstract]. J Clin Oncol 2009;27(suppl):18s. abstr CRA Gerard JP, Azria D, Gourgou-Bourgade G, et al. Comparison of two neoadjuvant chemotheradiotherapy regimens for locally advanced rectal cancer: Results of the phase III trial accord 12/0405-Prodige 2. J Clin Oncol 2010;28: Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350: Lee CG, Heijn M, di Tomaso E, et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res 2000;60: Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res 1999;59: Riesterer O, Honer M, Jochum W, et al. Ionizing radiation antagonizes tumor hypoxia induced by antiangiogenic treatment. Clin Cancer Res 2006;12: Willett CG, Duda DG, Tomaso E, et al. Efficacy, Safety, and Biomarkers of Neoadjuvant Bevacizumab, Radiation Therapy, and Fluorouracil in Rectal Cancer: A Multidisciplinary Phase II Study. J Clin Oncol 2009;27: Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2010;76: Czito BG, Bendell JC, Willett CG, et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: Phase I trial results. IntJRadiatOncolBiolPhys2007;68: Marijnen CA, Rutten H, de Wilt H, et al. Preoperative chemoradiotherapy regimen with capecitabine and bevacizumab rectal cancer: A feasibility study of the Dutch Colorectal Cancer Group (DCCG). J Clin Oncol 2008;26. abstract Fernandez-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cancer de Recto 3 Study. J Clin Oncol 2010;28:
6 Bevacizumab Rectal Cancer d T. DIPETRILLO et al Hurwitz H, Fehrenbacher L, Cartwright T, et al. Wound healing/bleeding in metastatic colorectal cancer patients who undergo surgery during treatment with bevacizumab. J Clin Oncol 2004;22(July 15 Supplement) Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine (gem) and bevacizumab (bev)-based chemoradiation for resectable pancreatic adenocarcinoma. J Clin Oncol 2008;26(May 20 Supplement): Nogue M, Salud A, Vicente P, et al. Addition of bevacizumab to induction plus concomitant capecitabine-oxaliplatin (XE- LOX) chemoradiotherapy (CRT) in MRI poor prognosis locally advanced rectal cancer: Avacross study. J Clin Oncol 2009;27(suppl):15s. abstr Crane CH, Eng C, Feig BW, et al. Phase II Trial of Neoadjuvant Bevacizumab, Capecitabine, and Radiotherapy for Locally Advanced Rectal Cancer. Int J Radiat Oncol Biol Phys 2010; 76(3): Bège T, Lelong B, Viret F, et al. Bevacizumab-related surgical site complication despite primary tumor resection in colorectal cancer patients. Ann Surg Oncol 2009;16: Kesmodel SB, Ellis LM, Lin E, et al. Preoperative bevacizumab does not significantly increase postoperative complication rates in patients undergoing hepatic surgery for colorectal cancer liver metastasis. J Clin Oncol 2008;26: Wolmark N, Yothers G, O Connell MJ, et al. A phase III trial comparing mfolfox6 to mfolfox6 plus bevacizumab in stage II or III carcinoma of the colon: Results of NSABP Protocol C-08 [abstract]. J Clin Oncol 2009;27(suppl):18s. abstr LBA4.
Adjuvant Chemotherapy for Rectal Cancer: Are we making progress?
 Adjuvant Chemotherapy for Rectal Cancer: Are we making progress? Hagen Kennecke, MD, MHA, FRCPC Division Of Medical Oncology British Columbia Cancer Agency October 25, 2008 Objectives Review milestones
Adjuvant Chemotherapy for Rectal Cancer: Are we making progress? Hagen Kennecke, MD, MHA, FRCPC Division Of Medical Oncology British Columbia Cancer Agency October 25, 2008 Objectives Review milestones
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
CRT AND SURGICAL resection are important elements
 Current Status and Future Directions in Preoperative Chemoradiotherapy of Rectal Cancer By Claus Rödel, MD Overview: With optimized local treatment for patients with locally advanced rectal cancer, achieved
Current Status and Future Directions in Preoperative Chemoradiotherapy of Rectal Cancer By Claus Rödel, MD Overview: With optimized local treatment for patients with locally advanced rectal cancer, achieved
Case Conference. Craig Morgenthal Department of Surgery Long Island College Hospital
 Case Conference Craig Morgenthal Department of Surgery Long Island College Hospital Neoadjuvant versus Adjuvant Radiation Therapy in Rectal Carcinoma Epidemiology American Cancer Society statistics for
Case Conference Craig Morgenthal Department of Surgery Long Island College Hospital Neoadjuvant versus Adjuvant Radiation Therapy in Rectal Carcinoma Epidemiology American Cancer Society statistics for
Preoperative capecitabine and pelvic radiation in locally advanced rectal cancer: preliminary results (Mansoura experience)
 Original Article Preoperative capecitabine and pelvic radiation in locally advanced rectal cancer: preliminary results (Mansoura experience) Abeer Hussien Anter 1, Ghada Ezzat Eladawei 2, Mahmoud Mosbah
Original Article Preoperative capecitabine and pelvic radiation in locally advanced rectal cancer: preliminary results (Mansoura experience) Abeer Hussien Anter 1, Ghada Ezzat Eladawei 2, Mahmoud Mosbah
Preoperative or Postoperative Therapy for the Management of Patients with Stage II or III Rectal Cancer
 Evidence-Based Series 2-4 Version 2 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Preoperative or Postoperative Therapy for the Management of Patients with
Evidence-Based Series 2-4 Version 2 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Preoperative or Postoperative Therapy for the Management of Patients with
Surgical Management of Advanced Stage Colon Cancer. Nathan Huber, MD 6/11/14
 Surgical Management of Advanced Stage Colon Cancer Nathan Huber, MD 6/11/14 Colon Cancer Overview Approximately 50,000 attributable deaths per year Colorectal cancer is the 3 rd most common cause of cancer-related
Surgical Management of Advanced Stage Colon Cancer Nathan Huber, MD 6/11/14 Colon Cancer Overview Approximately 50,000 attributable deaths per year Colorectal cancer is the 3 rd most common cause of cancer-related
Evaluation of the Efficacy of Modified De Gramont and Modified FOLFOX4 Regimens for Adjuvant Therapy of Locally Advanced Rectal Cancer
 Efficacy of Modified De Gramont and FOLFOX4 Regimens for Locally Advanced Rectal Cancer RESEARCH COMMUNICATION Evaluation of the Efficacy of Modified De Gramont and Modified FOLFOX4 Regimens for Adjuvant
Efficacy of Modified De Gramont and FOLFOX4 Regimens for Locally Advanced Rectal Cancer RESEARCH COMMUNICATION Evaluation of the Efficacy of Modified De Gramont and Modified FOLFOX4 Regimens for Adjuvant
Mini J.Elnaggar M.D. Radiation Oncology Ochsner Medical Center 9/23/2016. Background
 Mini J.Elnaggar M.D. Radiation Oncology Ochsner Medical Center 9/23/2016 Background Mostly adenocarcinoma (scc possible, but treated like anal cancer) 39, 220 cases annually Primary treatment: surgery
Mini J.Elnaggar M.D. Radiation Oncology Ochsner Medical Center 9/23/2016 Background Mostly adenocarcinoma (scc possible, but treated like anal cancer) 39, 220 cases annually Primary treatment: surgery
Carcinoma del retto: Highlights
 Carcinoma del retto: Highlights Stefano Cordio Struttura Complessa di Oncologia Medica ARNAS Garibaldi Catania Roma 17 Febbraio 2018 Disclosures Advisory Committee, research funding and speakers bureau
Carcinoma del retto: Highlights Stefano Cordio Struttura Complessa di Oncologia Medica ARNAS Garibaldi Catania Roma 17 Febbraio 2018 Disclosures Advisory Committee, research funding and speakers bureau
Current Status of Adjuvant Therapy for Colorectal Cancer
 Review Article [1] May 01, 2004 By Michael J. O connell, MD [2] Adjuvant therapy with chemotherapy and/or radiation therapy in addition to surgery improves outcome for patients with high-risk carcinomas
Review Article [1] May 01, 2004 By Michael J. O connell, MD [2] Adjuvant therapy with chemotherapy and/or radiation therapy in addition to surgery improves outcome for patients with high-risk carcinomas
Chemotherapy of colon cancers
 Chemotherapy of colon cancers Stage distribution Stage I : 15% T 1,2 NO Stage IV: 20 25% M+ Stage II : 20 30% T3,4 NO Stage III N+: 30 40% clinical stages I, II, or III colon cancer are at risk for having
Chemotherapy of colon cancers Stage distribution Stage I : 15% T 1,2 NO Stage IV: 20 25% M+ Stage II : 20 30% T3,4 NO Stage III N+: 30 40% clinical stages I, II, or III colon cancer are at risk for having
RECTAL CANCER CLINICAL CASE PRESENTATION
 RECTAL CANCER CLINICAL CASE PRESENTATION Francesco Sclafani Medical Oncologist, Clinical Research Fellow The Royal Marsden NHS Foundation Trust, London, UK esmo.org Disclosure I have nothing to declare
RECTAL CANCER CLINICAL CASE PRESENTATION Francesco Sclafani Medical Oncologist, Clinical Research Fellow The Royal Marsden NHS Foundation Trust, London, UK esmo.org Disclosure I have nothing to declare
Chemoradiation (CRT) Safety Analysis of ACOSOG Z6041: A Phase II Trial of Neoadjuvant CRT followed by Local Excision in ut2 Rectal Cancer
 Chemoradiation (CRT) Safety Analysis of ACOSOG Z6041: A Phase II Trial of Neoadjuvant CRT followed by Local Excision in ut2 Rectal Cancer Emily Chan, Qian Shi, Julio Garcia-Aguilar, Peter Cataldo, Jorge
Chemoradiation (CRT) Safety Analysis of ACOSOG Z6041: A Phase II Trial of Neoadjuvant CRT followed by Local Excision in ut2 Rectal Cancer Emily Chan, Qian Shi, Julio Garcia-Aguilar, Peter Cataldo, Jorge
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
Long Term Outcomes of Preoperative versus
 RESEARCH ARTICLE Long Term Outcomes of Preoperative versus Postoperative Concurrent Chemoradiation for Locally Advanced Rectal Cancer: Experience from Ramathibodi Medical School in Thailand Pichayada Darunikorn
RESEARCH ARTICLE Long Term Outcomes of Preoperative versus Postoperative Concurrent Chemoradiation for Locally Advanced Rectal Cancer: Experience from Ramathibodi Medical School in Thailand Pichayada Darunikorn
Preoperative adjuvant radiotherapy
 Preoperative adjuvant radiotherapy Dr John Hay Radiation Oncology Program BC Cancer Agency Vancouver Cancer Centre The key question for the surgeon Do you think that this tumour can be resected with clear
Preoperative adjuvant radiotherapy Dr John Hay Radiation Oncology Program BC Cancer Agency Vancouver Cancer Centre The key question for the surgeon Do you think that this tumour can be resected with clear
Case 1 Metastatic Pancreatic Adenocarcinoma: What Therapy Should I Select First?
 Case 1 Metastatic Pancreatic Adenocarcinoma: What Therapy Should I Select First? Marc Peeters, MD, PhD Head of the Oncology Department Antwerp University Hospital Antwerp, Belgium marc.peeters@uza.be 71-year-old
Case 1 Metastatic Pancreatic Adenocarcinoma: What Therapy Should I Select First? Marc Peeters, MD, PhD Head of the Oncology Department Antwerp University Hospital Antwerp, Belgium marc.peeters@uza.be 71-year-old
EASTERN COOPERATIVE ONCOLOGY GROUP
 EASTERN COOPERATIVE ONCOLOGY GROUP E5204 INTERGROUP RANDOMIZED PHASE III STUDY OF POSTOPERATIVE OXALIPLATIN, 5-FLUOROURACIL AND LEUCOVORIN VS OXALIPLATIN, 5-FLUOROURACIL, LEUCOV- ORIN AND BEVACIZUMAB FOR
EASTERN COOPERATIVE ONCOLOGY GROUP E5204 INTERGROUP RANDOMIZED PHASE III STUDY OF POSTOPERATIVE OXALIPLATIN, 5-FLUOROURACIL AND LEUCOVORIN VS OXALIPLATIN, 5-FLUOROURACIL, LEUCOV- ORIN AND BEVACIZUMAB FOR
Disclosures. Colorectal Cancer Update GAFP November Risk Assessment. Colon and Rectal Cancer The Challenge. Issues in Colon and Rectal Cancer
 Disclosures Colorectal Cancer Update GAFP November 2006 Robert C. Hermann, MD Georgia Center for Oncology Research and Education Northwest Georgia Oncology Centers, PC WellStar Health System Marietta,
Disclosures Colorectal Cancer Update GAFP November 2006 Robert C. Hermann, MD Georgia Center for Oncology Research and Education Northwest Georgia Oncology Centers, PC WellStar Health System Marietta,
Differential effect of concurrent chemotherapy regimen on clinical outcomes of preoperative chemoradiotherapy for locally advanced rectal cancer
 JBUON 2019; 24(2): 470-478 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Differential effect of concurrent chemotherapy regimen on clinical outcomes
JBUON 2019; 24(2): 470-478 ISSN: 1107-0625, online ISSN: 2241-6293 www.jbuon.com E-mail: editorial_office@jbuon.com ORIGINAL ARTICLE Differential effect of concurrent chemotherapy regimen on clinical outcomes
Radiation Therapy for Resectable Colon Cancer
 Review Article [1] February 01, 2006 By Brian G. Czito, MD [2], Johanna C. Bendell, MD [3], and Christopher G. Willett, MD [4] Colon cancer is a major public health problem. The primary treatment is resection.
Review Article [1] February 01, 2006 By Brian G. Czito, MD [2], Johanna C. Bendell, MD [3], and Christopher G. Willett, MD [4] Colon cancer is a major public health problem. The primary treatment is resection.
Re-irradiation in recurrent rectal cancer: single institution experience
 Original Article Re-irradiation in recurrent rectal cancer: single institution experience Rasha Mohammad Abdel Latif, Ghada E. El-Adawei, Wael El-Sada Clinical Oncology & Nuclear Medicine Department, Mansoura
Original Article Re-irradiation in recurrent rectal cancer: single institution experience Rasha Mohammad Abdel Latif, Ghada E. El-Adawei, Wael El-Sada Clinical Oncology & Nuclear Medicine Department, Mansoura
Oncologist. The. Gastrointestinal Cancer
 The Oncologist Gastrointestinal Cancer Addition of Bevacizumab to XELOX Induction Therapy Plus Concomitant Capecitabine-Based Chemoradiotherapy in Magnetic Resonance Imaging Defined Poor-Prognosis Locally
The Oncologist Gastrointestinal Cancer Addition of Bevacizumab to XELOX Induction Therapy Plus Concomitant Capecitabine-Based Chemoradiotherapy in Magnetic Resonance Imaging Defined Poor-Prognosis Locally
Neoadjuvant Therapy for Rectal Cancer is Overrated. Joon H. Lee, Research Resident University of Colorado 8/31/2009
 Neoadjuvant Therapy for Rectal Cancer is Overrated Joon H. Lee, Research Resident University of Colorado 8/31/2009 Objectives Brief overview of staging rectal cancer Current guidelines for evaluation and
Neoadjuvant Therapy for Rectal Cancer is Overrated Joon H. Lee, Research Resident University of Colorado 8/31/2009 Objectives Brief overview of staging rectal cancer Current guidelines for evaluation and
Retrospective analysis of the effect of CAPOX and mfolfox6 dose intensity on survival in colorectal patients in the adjuvant setting
 ORIGINAL ARTICLE CAPOX AND mfolfox6 DOSE INTENSITY AND CLINICAL OUTCOMES IN STAGE III CRC, Mamo et al. Retrospective analysis of the effect of CAPOX and mfolfox6 dose intensity on survival in colorectal
ORIGINAL ARTICLE CAPOX AND mfolfox6 DOSE INTENSITY AND CLINICAL OUTCOMES IN STAGE III CRC, Mamo et al. Retrospective analysis of the effect of CAPOX and mfolfox6 dose intensity on survival in colorectal
CREATE Trial Proposal: Survey of current practice and potential trial participation
 CREATE Trial Proposal: Survey of current practice and potential trial participation Approximately a quarter of newly diagnosed rectal cancer patients have features on pre-treatment pelvic MRI indicating
CREATE Trial Proposal: Survey of current practice and potential trial participation Approximately a quarter of newly diagnosed rectal cancer patients have features on pre-treatment pelvic MRI indicating
Rectal Cancer: Classic Hits
 Rectal Cancer: Classic Hits Charles M. Friel, MD Associate Professor of Surgery Section of Colon and Rectal Surgery University of Virginia September 28, 2016 None Disclosures 1 Objectives Review the Classic
Rectal Cancer: Classic Hits Charles M. Friel, MD Associate Professor of Surgery Section of Colon and Rectal Surgery University of Virginia September 28, 2016 None Disclosures 1 Objectives Review the Classic
Radiotherapy for rectal cancer. Karin Haustermans Department of Radiation Oncology
 Radiotherapy for rectal cancer Karin Haustermans Department of Radiation Oncology O U T L I N E RT with TME surgery? Neoadjuvant or adjuvant RT? 5 x 5 Gy or long-course CRT? RT with new drugs? Selection
Radiotherapy for rectal cancer Karin Haustermans Department of Radiation Oncology O U T L I N E RT with TME surgery? Neoadjuvant or adjuvant RT? 5 x 5 Gy or long-course CRT? RT with new drugs? Selection
The 2010 Gastrointestinal Cancers Symposium Oral Abstract Session: Cancers of the Pancreas, Small Bowel and Hepatobilliary Tract
 The 2010 Gastrointestinal Cancers Symposium : Cancers of the Pancreas, Small Bowel and Hepatobilliary Tract Abstract #131: Phase I study of MK 0646 (dalotuzumab), a humanized monoclonal antibody against
The 2010 Gastrointestinal Cancers Symposium : Cancers of the Pancreas, Small Bowel and Hepatobilliary Tract Abstract #131: Phase I study of MK 0646 (dalotuzumab), a humanized monoclonal antibody against
SMJ Singapore Medical Journal
 SMJ Singapore Medical Journal ONLINE FIRST PUBLICATION Online first papers have undergone full scientific review and copyediting, but have not been typeset or proofread. To cite this article, use the DOIs
SMJ Singapore Medical Journal ONLINE FIRST PUBLICATION Online first papers have undergone full scientific review and copyediting, but have not been typeset or proofread. To cite this article, use the DOIs
Targeted Therapies in Metastatic Colorectal Cancer: An Update
 Targeted Therapies in Metastatic Colorectal Cancer: An Update ASCO 2007: Targeted Therapies in Metastatic Colorectal Cancer: An Update Bevacizumab is effective in combination with XELOX or FOLFOX-4 Bevacizumab
Targeted Therapies in Metastatic Colorectal Cancer: An Update ASCO 2007: Targeted Therapies in Metastatic Colorectal Cancer: An Update Bevacizumab is effective in combination with XELOX or FOLFOX-4 Bevacizumab
11/21/13 CEA: 1.7 WNL
 Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Medicinae Doctoris. One university. Many futures.
 Medicinae Doctoris The Before and The After: Can chemotherapy revise the trajectory of gastric and esophageal cancers? Dr. David Dawe MD, FRCPC Medical Oncologist Assistant Professor Disclosures None All
Medicinae Doctoris The Before and The After: Can chemotherapy revise the trajectory of gastric and esophageal cancers? Dr. David Dawe MD, FRCPC Medical Oncologist Assistant Professor Disclosures None All
Pre-operative Chemoradiotherapy with Oral Capecitabine in Locally Advanced, Resectable Rectal Cancer
 Pre-operative Chemoradiotherapy with Oral Capecitabine in Locally Advanced, Resectable Rectal Cancer DIMITRIS P. KORKOLIS 1, CHRISTOS S. BOSKOS 2, GEORGE D. PLATANIOTIS 1, EMMANUEL GONTIKAKIS 1, IOANNIS
Pre-operative Chemoradiotherapy with Oral Capecitabine in Locally Advanced, Resectable Rectal Cancer DIMITRIS P. KORKOLIS 1, CHRISTOS S. BOSKOS 2, GEORGE D. PLATANIOTIS 1, EMMANUEL GONTIKAKIS 1, IOANNIS
Bevacizumab 5mg/kg Therapy 14 days
 INDICATIONS FOR USE: Bevacizumab 5mg/kg Therapy 14 days Regimen Code 00211a *Reimbursement status Hospital INDICATION ICD10 In combination with fluoropyrimidine-based chemotherapy C18 for treatment of
INDICATIONS FOR USE: Bevacizumab 5mg/kg Therapy 14 days Regimen Code 00211a *Reimbursement status Hospital INDICATION ICD10 In combination with fluoropyrimidine-based chemotherapy C18 for treatment of
Treatment strategy of metastatic rectal cancer
 35.Schweizerische Koloproktologie-Tagung Treatment strategy of metastatic rectal cancer Gilles Mentha University hospital of Geneva Bern, January 18th, 2014 Colorectal cancer is the third most frequent
35.Schweizerische Koloproktologie-Tagung Treatment strategy of metastatic rectal cancer Gilles Mentha University hospital of Geneva Bern, January 18th, 2014 Colorectal cancer is the third most frequent
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abdominal drainage, after hepatic resection, 159 160 Ablation, radiofrequency, for hepatocellular carcinoma, 160 161 Adenocarcinoma, pancreatic.
Index Note: Page numbers of article titles are in boldface type. A Abdominal drainage, after hepatic resection, 159 160 Ablation, radiofrequency, for hepatocellular carcinoma, 160 161 Adenocarcinoma, pancreatic.
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
Adjuvant therapies for large bowel cancer Wasantha Rathnayake, MD
 LEADING ARTICLE Adjuvant therapies for large bowel cancer Wasantha Rathnayake, MD Consultant Clinical Oncologist, National Cancer Institute, Maharagama, Sri Lanka. Key words: Large bowel; Cancer; Adjuvant
LEADING ARTICLE Adjuvant therapies for large bowel cancer Wasantha Rathnayake, MD Consultant Clinical Oncologist, National Cancer Institute, Maharagama, Sri Lanka. Key words: Large bowel; Cancer; Adjuvant
Department of Radiotherapy, Pt. BDS PGIMS, Rohtak, Haryana, India
 Bharti et al., IJPSR, 2010; Vol. 1 (11): 169-173 ISSN: 0975-8232 IJPSR (2010), Vol. 1, Issue 11 (Research Article) Received on 29 September, 2010; received in revised form 21 October, 2010; accepted 26
Bharti et al., IJPSR, 2010; Vol. 1 (11): 169-173 ISSN: 0975-8232 IJPSR (2010), Vol. 1, Issue 11 (Research Article) Received on 29 September, 2010; received in revised form 21 October, 2010; accepted 26
Rectal Cancer. GI Practice Guideline
 Rectal Cancer GI Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Dr. Francisco Perera MD, FRCPC (Radiation Oncologist) Dr. Jay Engel MD, FRCPC (Surgical Oncologist) Approval Date: 2006 This guideline
Rectal Cancer GI Practice Guideline Dr. Brian Dingle MSc, MD, FRCPC Dr. Francisco Perera MD, FRCPC (Radiation Oncologist) Dr. Jay Engel MD, FRCPC (Surgical Oncologist) Approval Date: 2006 This guideline
A Case of Marked Response to Preoperative Chemoradiotherapy for Rectal Cancer With Para-Aortic Lymph Node Metastasis
 Int Surg 2011;96:139 143 Case Report A Case of Marked Response to Preoperative Chemoradiotherapy for Rectal Cancer With Para-Aortic Lymph Node Metastasis Atsushi Horiuchi, Keiji Matsuda, Takuya Akahane,
Int Surg 2011;96:139 143 Case Report A Case of Marked Response to Preoperative Chemoradiotherapy for Rectal Cancer With Para-Aortic Lymph Node Metastasis Atsushi Horiuchi, Keiji Matsuda, Takuya Akahane,
RADIATION THERAPY WITH ONCE-WEEKLY GEMCITABINE IN PANCREATIC CANCER: CURRENT STATUS OF CLINICAL TRIALS
 doi:10.1016/s0360-3016(03)00449-8 Int. J. Radiation Oncology Biol. Phys., Vol. 56, No. 4, Supplement, pp. 10 15, 2003 Copyright 2003 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/03/$
doi:10.1016/s0360-3016(03)00449-8 Int. J. Radiation Oncology Biol. Phys., Vol. 56, No. 4, Supplement, pp. 10 15, 2003 Copyright 2003 Elsevier Inc. Printed in the USA. All rights reserved 0360-3016/03/$
Docetaxel. Class: Antineoplastic agent, Antimicrotubular, Taxane derivative.
 Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Index. Surg Oncol Clin N Am 14 (2005) Note: Page numbers of article titles are in boldface type.
 Surg Oncol Clin N Am 14 (2005) 433 439 Index Note: Page numbers of article titles are in boldface type. A Abdominosacral resection, of recurrent rectal cancer, 202 215 Ablative techniques, image-guided,
Surg Oncol Clin N Am 14 (2005) 433 439 Index Note: Page numbers of article titles are in boldface type. A Abdominosacral resection, of recurrent rectal cancer, 202 215 Ablative techniques, image-guided,
State of the art: Standard(s) of radio/chemotherapy for rectal cancer
 State of the art: Standard(s) of radio/chemotherapy for rectal cancer Dr Ian Chau Consultant Medical Oncologist The Royal Marsden Hospital London & Surrey Disclosure Advisory Board: Sanofi Oncology, Eli-
State of the art: Standard(s) of radio/chemotherapy for rectal cancer Dr Ian Chau Consultant Medical Oncologist The Royal Marsden Hospital London & Surrey Disclosure Advisory Board: Sanofi Oncology, Eli-
ADJUVANT CHEMOTHERAPY...
 Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
Colorectal Pathway Board: Non-Surgical Oncology Guidelines October 2015 Organization» Table of Contents ADJUVANT CHEMOTHERAPY... 2 DUKES C/ TNM STAGE 3... 2 DUKES B/ TNM STAGE 2... 3 LOCALLY ADVANCED
Radiotherapy for Rectal Cancer. Kevin Palumbo Adelaide Radiotherapy Centre
 Radiotherapy for Rectal Cancer Kevin Palumbo Adelaide Radiotherapy Centre Overview CRC are common (3 rd commonest cancer) rectal Ca approx 25-30% of all CRC. Presentation PR bleeding: beware attributing
Radiotherapy for Rectal Cancer Kevin Palumbo Adelaide Radiotherapy Centre Overview CRC are common (3 rd commonest cancer) rectal Ca approx 25-30% of all CRC. Presentation PR bleeding: beware attributing
Adjuvant Treatment of Pancreatic Cancer in 2009: Where Are We? Highlights from the 45 th ASCO Annual Meeting. Orlando, FL, USA. May 29 - June 2, 2009
 HIGHLIGHT ARTICLE - Slide Show Adjuvant Treatment of Pancreatic Cancer in 2009: Where Are We? Highlights from the 45 th ASCO Annual Meeting. Orlando, FL, USA. May 29 - June 2, 2009 Muhammad Wasif Saif
HIGHLIGHT ARTICLE - Slide Show Adjuvant Treatment of Pancreatic Cancer in 2009: Where Are We? Highlights from the 45 th ASCO Annual Meeting. Orlando, FL, USA. May 29 - June 2, 2009 Muhammad Wasif Saif
COLON AND RECTAL CANCER
 COLON AND RECTAL CANCER Mark Sun, MD Clinical Associate Professor of Surgery University of Minnesota No disclosures Objectives 1) Understand the epidemiology, management, and prognosis of colon and rectal
COLON AND RECTAL CANCER Mark Sun, MD Clinical Associate Professor of Surgery University of Minnesota No disclosures Objectives 1) Understand the epidemiology, management, and prognosis of colon and rectal
Bevacizumab 10mg/kg 14 days
 INDICATIONS FOR USE: Bevacizumab 10mg/kg 14 days Regimen Code 00212a *Reimbursement status Hospital INDICATION ICD10 In combination with fluoropyrimidine-based chemotherapy C18 for treatment of adult patients
INDICATIONS FOR USE: Bevacizumab 10mg/kg 14 days Regimen Code 00212a *Reimbursement status Hospital INDICATION ICD10 In combination with fluoropyrimidine-based chemotherapy C18 for treatment of adult patients
Terapia neoadyuvante en cáncer de recto Estado del arte Mauricio Lema Medina MD Clínica de Oncología Astorga / Clínica SOMA - Medellín, Colombia
 Terapia neoadyuvante en cáncer de recto Estado del arte Mauricio Lema Medina MD Clínica de Oncología Astorga / Clínica SOMA - Medellín, Colombia Temario Generalidades Adyuvancia en colon y recto FU / Capecitabina
Terapia neoadyuvante en cáncer de recto Estado del arte Mauricio Lema Medina MD Clínica de Oncología Astorga / Clínica SOMA - Medellín, Colombia Temario Generalidades Adyuvancia en colon y recto FU / Capecitabina
Treatment outcomes and prognostic factors of gallbladder cancer patients after postoperative radiation therapy
 Korean J Hepatobiliary Pancreat Surg 2011;15:152-156 Original Article Treatment outcomes and prognostic factors of gallbladder cancer patients after postoperative radiation therapy Suzy Kim 1,#, Kyubo
Korean J Hepatobiliary Pancreat Surg 2011;15:152-156 Original Article Treatment outcomes and prognostic factors of gallbladder cancer patients after postoperative radiation therapy Suzy Kim 1,#, Kyubo
RECTAL CANCER: Adjuvant Therapy. Maury Rosenstein, MD Montefiore Medical Center December 2012
 RECTAL CANCER: Adjuvant Therapy Maury Rosenstein, MD Montefiore Medical Center December 2012 Overview Indications for adjuvant therapy Preoperative Postoperative New Advances Epidemiology Approximately
RECTAL CANCER: Adjuvant Therapy Maury Rosenstein, MD Montefiore Medical Center December 2012 Overview Indications for adjuvant therapy Preoperative Postoperative New Advances Epidemiology Approximately
Patient Presentation. 32 y.o. female complains of lower abdominal mass CEA = 433, CA125 = 201
 Patient Presentation 32 y.o. female complains of lower abdominal mass CEA = 433, CA125 = 201 CT shows: Thickening of the right hemidiaphragm CT shows: Fluid in the right paracolic sulcus CT shows: Large
Patient Presentation 32 y.o. female complains of lower abdominal mass CEA = 433, CA125 = 201 CT shows: Thickening of the right hemidiaphragm CT shows: Fluid in the right paracolic sulcus CT shows: Large
Is adjuvant radiotherapy warranted in resected pt1-2 node-positive rectal cancer?
 Peng et al. Radiation Oncology 2013, 8:290 RESEARCH Open Access Is adjuvant radiotherapy warranted in resected pt1-2 node-positive rectal cancer? Junjie Peng 1,2, Xinxiang Li 1,2, Ying Ding 3, Debing Shi
Peng et al. Radiation Oncology 2013, 8:290 RESEARCH Open Access Is adjuvant radiotherapy warranted in resected pt1-2 node-positive rectal cancer? Junjie Peng 1,2, Xinxiang Li 1,2, Ying Ding 3, Debing Shi
Arm A: Induction Gemcitabine 1000 mg/m 2 IV once a week for 6 weeks.
 ECOG-4201 (RTOG Endorsed) ECOG 4201 Pancreas (RTOG Endorsed)-1 Protocol Status: Opened: April 10, 2003 Closed: December 15, 2005 Title: A Randomized Phase III Study of Gemcitabine in Combination with Radiation
ECOG-4201 (RTOG Endorsed) ECOG 4201 Pancreas (RTOG Endorsed)-1 Protocol Status: Opened: April 10, 2003 Closed: December 15, 2005 Title: A Randomized Phase III Study of Gemcitabine in Combination with Radiation
Short course radiation therapy for rectal cancer in the elderly: can radical surgery be avoided?
 Short communication Short course radiation therapy for rectal cancer in the elderly: can radical surgery be avoided? Michael A. Cummings 1, Kenneth Y. Usuki 1, Fergal J. Fleming 2, Mohamedtaki A. Tejani
Short communication Short course radiation therapy for rectal cancer in the elderly: can radical surgery be avoided? Michael A. Cummings 1, Kenneth Y. Usuki 1, Fergal J. Fleming 2, Mohamedtaki A. Tejani
Case Report Management of a Patient with Metastatic Colorectal Cancer and Liver Metastases
 Case Reports in Oncological Medicine, Article ID 790192, 4 pages http://dx.doi.org/10.1155/2014/790192 Case Report Management of a Patient with Metastatic Colorectal Cancer and Liver Metastases Muhammad
Case Reports in Oncological Medicine, Article ID 790192, 4 pages http://dx.doi.org/10.1155/2014/790192 Case Report Management of a Patient with Metastatic Colorectal Cancer and Liver Metastases Muhammad
Short-Course Radiation Versus Long-Course Chemoradiation for Rectal Cancer
 Original Article 1223 Short-Course Radiation Versus Long-Course Chemoradiation for Rectal Cancer Bruce D. Minsky, MD a ; Claus Rödel, MD b ; and Vincenzo Valentini, MD c Abstract The 2 broad approaches
Original Article 1223 Short-Course Radiation Versus Long-Course Chemoradiation for Rectal Cancer Bruce D. Minsky, MD a ; Claus Rödel, MD b ; and Vincenzo Valentini, MD c Abstract The 2 broad approaches
Management of Advanced Colorectal Cancer in Older Patients
 Review Article [1] April 15, 2005 By Stuart M. Lichtman, MD, FACP [2] Many elderly individuals have substantial life expectancy, even in the setting of significant illness. There is evidence to indicate
Review Article [1] April 15, 2005 By Stuart M. Lichtman, MD, FACP [2] Many elderly individuals have substantial life expectancy, even in the setting of significant illness. There is evidence to indicate
Index. Surg Oncol Clin N Am 16 (2007) Note: Page numbers of article titles are in boldface type.
 Surg Oncol Clin N Am 16 (2007) 465 469 Index Note: Page numbers of article titles are in boldface type. A Adjuvant therapy, preoperative for gastric cancer, staging and, 339 B Breast cancer, metabolic
Surg Oncol Clin N Am 16 (2007) 465 469 Index Note: Page numbers of article titles are in boldface type. A Adjuvant therapy, preoperative for gastric cancer, staging and, 339 B Breast cancer, metabolic
Randomized Phase II Study of Irinotecan and Cetuximab with or without Vemurafenib in BRAF Mutant Metastatic Colorectal Cancer
 Randomized Phase II Study of Irinotecan and Cetuximab with or without Vemurafenib in BRAF Mutant Metastatic Colorectal Cancer This is a two-arm, randomized phase II trial for patients with BRAF mutant
Randomized Phase II Study of Irinotecan and Cetuximab with or without Vemurafenib in BRAF Mutant Metastatic Colorectal Cancer This is a two-arm, randomized phase II trial for patients with BRAF mutant
Neoadjuvant treatment Evolution and Current Status
 Neoadjuvant treatment Evolution and Current Status Dr Andrew See Radiation Oncologist 2017 Rectal Cancer Symposium Friday 10 th November 2017 2 1 Major Randomised Trials Supporting Neoadjuvant CRT Trial
Neoadjuvant treatment Evolution and Current Status Dr Andrew See Radiation Oncologist 2017 Rectal Cancer Symposium Friday 10 th November 2017 2 1 Major Randomised Trials Supporting Neoadjuvant CRT Trial
Distal Margin Requirements After Preoperative Chemoradiotherapy for Distal Rectal Carcinomas: Are 1cm Distal Margins Sufficient?
 Annals of Surgical Oncology, 8(2):163 169 Published by Lippincott Williams & Wilkins 2001 The Society of Surgical Oncology, Inc. Distal Margin Requirements After Preoperative Chemoradiotherapy for Distal
Annals of Surgical Oncology, 8(2):163 169 Published by Lippincott Williams & Wilkins 2001 The Society of Surgical Oncology, Inc. Distal Margin Requirements After Preoperative Chemoradiotherapy for Distal
Il paziente anziano con malattia oncologica avanzata: il tumore del colon-retto
 Milano 05.10.2018 Il paziente anziano con malattia oncologica avanzata: il tumore del colon-retto Salvatore Corallo U.O.C. Oncologia Medica IRCCS Istituto Nazionale dei Tumori Milano CRC in elderly patients
Milano 05.10.2018 Il paziente anziano con malattia oncologica avanzata: il tumore del colon-retto Salvatore Corallo U.O.C. Oncologia Medica IRCCS Istituto Nazionale dei Tumori Milano CRC in elderly patients
Opportunity for palliative care Research
 Opportunity for palliative care Research Role of Radiotherapy in Multidisciplinary Management of Rectal Cancers Dr Sushmita Pathy Associate Professor Department of Radiation Oncology Dr BRA Institute Rotary
Opportunity for palliative care Research Role of Radiotherapy in Multidisciplinary Management of Rectal Cancers Dr Sushmita Pathy Associate Professor Department of Radiation Oncology Dr BRA Institute Rotary
Irinotecan. Class:Camptothecin. Indications : _Cervical cancer. _CNS tumor. _Esophageal cancer. _Ewing s sarcoma. _Gastric cancer
 Irinotecan Class:Camptothecin Indications : _Cervical cancer _CNS tumor _Esophageal cancer _Ewing s sarcoma _Gastric cancer _Nonsmall cell lung cancer _Pancreatic cancer _Small cell lung cancer _Colorectal
Irinotecan Class:Camptothecin Indications : _Cervical cancer _CNS tumor _Esophageal cancer _Ewing s sarcoma _Gastric cancer _Nonsmall cell lung cancer _Pancreatic cancer _Small cell lung cancer _Colorectal
Anus,Rectum and Colon
 JOURNAL OF THE Anus,Rectum and Colon http://journal-arc.jp REVIEW ARTICLE Recent advances in neoadjuvant chemoradiotherapy in locally advanced rectal cancer Kazushige Kawai, Soichiro Ishihara, Hiroaki
JOURNAL OF THE Anus,Rectum and Colon http://journal-arc.jp REVIEW ARTICLE Recent advances in neoadjuvant chemoradiotherapy in locally advanced rectal cancer Kazushige Kawai, Soichiro Ishihara, Hiroaki
Overview. What s New in the Treatment of Pancreatic Cancer? Lots! Steven J. Cohen, M.D. Fox Chase Cancer Center September 17, 2013
 What s New in the Treatment of Pancreatic Cancer? Lots! Steven J. Cohen, M.D. Fox Chase Cancer Center September 17, 2013 Overview Staging and Workup Resectable Disease Surgery Adjuvant therapy Locally
What s New in the Treatment of Pancreatic Cancer? Lots! Steven J. Cohen, M.D. Fox Chase Cancer Center September 17, 2013 Overview Staging and Workup Resectable Disease Surgery Adjuvant therapy Locally
Gallbladder Cancer. GI Practice Guideline. Michael Sanatani, MD, FRCPC (Medical Oncologist) Barbara Fisher, MD, FRCPC (Radiation Oncologist)
 Gallbladder Cancer GI Practice Guideline Michael Sanatani, MD, FRCPC (Medical Oncologist) Barbara Fisher, MD, FRCPC (Radiation Oncologist) Approval Date: September 2006 This guideline is a statement of
Gallbladder Cancer GI Practice Guideline Michael Sanatani, MD, FRCPC (Medical Oncologist) Barbara Fisher, MD, FRCPC (Radiation Oncologist) Approval Date: September 2006 This guideline is a statement of
Avastin Sample Coding
 First- and Second-line Metastatic Colorectal Cancer C18.0 Malignant neoplasm of the cecum C18.1 Malignant neoplasm of appendix C18.2-C18.9 C19 C20 C21.8 Malignant neoplasm of the colon, various sites Malignant
First- and Second-line Metastatic Colorectal Cancer C18.0 Malignant neoplasm of the cecum C18.1 Malignant neoplasm of appendix C18.2-C18.9 C19 C20 C21.8 Malignant neoplasm of the colon, various sites Malignant
Factors associated with delayed time to adjuvant chemotherapy in stage iii colon cancer
 Curr Oncol, Vol. 21, pp. 181-186 doi: http://dx.doi.org/10.3747/co.21.1963 DELAYED TIME TO ADJUVANT CHEMOTHERAPY ORIGINAL ARTICLE Factors associated with delayed time to adjuvant chemotherapy in stage
Curr Oncol, Vol. 21, pp. 181-186 doi: http://dx.doi.org/10.3747/co.21.1963 DELAYED TIME TO ADJUVANT CHEMOTHERAPY ORIGINAL ARTICLE Factors associated with delayed time to adjuvant chemotherapy in stage
Panitumumab After Resection of Liver Metastases From Colorectal Cancer in KRAS Wild-type Patients
 1 von 5 23.11.2011 10:52 Home Search Study Topics Glossary Full Text View Tabular View No Study Results Posted Related Studies Panitumumab After Resection of Liver Metastases From Colorectal Cancer in
1 von 5 23.11.2011 10:52 Home Search Study Topics Glossary Full Text View Tabular View No Study Results Posted Related Studies Panitumumab After Resection of Liver Metastases From Colorectal Cancer in
UCL. Rectum Adenocarcinoma. Quality of conformal radiotherapy Impact for the surgeon P. Scalliet & K. Haustermans
 Rectum Adenocarcinoma Quality of conformal radiotherapy Impact for the surgeon P. Scalliet & K. Haustermans Fifth Belgian Surgical Week May 6th, 2004, Oostende SOR rectum adenocarcinoma Indication of radiotherapy
Rectum Adenocarcinoma Quality of conformal radiotherapy Impact for the surgeon P. Scalliet & K. Haustermans Fifth Belgian Surgical Week May 6th, 2004, Oostende SOR rectum adenocarcinoma Indication of radiotherapy
Rectal Cancer. Madhulika G. Varma MD Associate Professor and Chief Section of Colorectal Surgery University of California, San Francisco
 Rectal Cancer Madhulika G. Varma MD Associate Professor and Chief Section of Colorectal Surgery University of California, San Francisco Modern Treatment for Rectal Cancer Improve Local Control Improved
Rectal Cancer Madhulika G. Varma MD Associate Professor and Chief Section of Colorectal Surgery University of California, San Francisco Modern Treatment for Rectal Cancer Improve Local Control Improved
Original Research Article. Manjunath I. Nandennavar 1, Shashidhar V. Karpurmath 1 *, Geeta S. Narayanan 2, Rajashree Aswath 2, Ganesh M. S.
 International Journal of Advances in Medicine http://www.ijmedicine.com pissn 2349-3925 eissn 2349-3933 Original Research Article DOI: http://dx.doi.org/10.18203/2349-3933.ijam20174640 Comparison of continuous
International Journal of Advances in Medicine http://www.ijmedicine.com pissn 2349-3925 eissn 2349-3933 Original Research Article DOI: http://dx.doi.org/10.18203/2349-3933.ijam20174640 Comparison of continuous
COLON AND RECTAL CANCER
 No disclosures COLON AND RECTAL CANCER Mark Sun, MD Clinical Assistant Professor of Surgery University of Minnesota Colon and Rectal Cancer Statistics Overall Incidence 2016 134,490 new cases 8.0% of all
No disclosures COLON AND RECTAL CANCER Mark Sun, MD Clinical Assistant Professor of Surgery University of Minnesota Colon and Rectal Cancer Statistics Overall Incidence 2016 134,490 new cases 8.0% of all
Radiation Oncology MOC Study Guide
 Radiation Oncology MOC Study Guide The following study guide is intended to give a general overview of the type of material that will be covered on the Radiation Oncology Maintenance of Certification (MOC)
Radiation Oncology MOC Study Guide The following study guide is intended to give a general overview of the type of material that will be covered on the Radiation Oncology Maintenance of Certification (MOC)
Concurrent Chemoradiation of Patients with Inoperable
 Med. J. Cairo Univ., VoL 81, No. 2, March: 29-34, 2013 www.medicaljournalofcairouniversity.com Concurrent Chemoradiation of Patients with Inoperable Non-Metastatic Pancreatic Cancer MOHAMED S. ELZAHI,
Med. J. Cairo Univ., VoL 81, No. 2, March: 29-34, 2013 www.medicaljournalofcairouniversity.com Concurrent Chemoradiation of Patients with Inoperable Non-Metastatic Pancreatic Cancer MOHAMED S. ELZAHI,
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Guidelines for Laparoscopic Resection of Curable Colon and Rectal Cancer
 SAGES Society of American Gastrointestinal and Endoscopic Surgeons http://www.sages.org Guidelines for Laparoscopic Resection of Curable Colon and Rectal Cancer Author : SAGES Webmaster PREAMBLE The following
SAGES Society of American Gastrointestinal and Endoscopic Surgeons http://www.sages.org Guidelines for Laparoscopic Resection of Curable Colon and Rectal Cancer Author : SAGES Webmaster PREAMBLE The following
Index. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Abdominoperineal excision, of rectal cancer, 93 111 current controversies in, 106 109 extent of perineal dissection and removal of pelvic floor,
Note: Page numbers of article titles are in boldface type. A Abdominoperineal excision, of rectal cancer, 93 111 current controversies in, 106 109 extent of perineal dissection and removal of pelvic floor,
Adjuvant and neoadjuvant chemotherapy for rectal cancer: Expensive but little gain
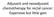 Adjuvant and neoadjuvant chemotherapy for rectal cancer: Expensive but little gain Outline The problem Adjuvant therapy Neoadjuvant therapy Options Conclusion The problem 30 years ago: Local recurrence
Adjuvant and neoadjuvant chemotherapy for rectal cancer: Expensive but little gain Outline The problem Adjuvant therapy Neoadjuvant therapy Options Conclusion The problem 30 years ago: Local recurrence
Advances in gastric cancer: How to approach localised disease?
 Advances in gastric cancer: How to approach localised disease? Andrés Cervantes Professor of Medicine Classical approach to localised gastric cancer Surgical resection Pathology assessment and estimation
Advances in gastric cancer: How to approach localised disease? Andrés Cervantes Professor of Medicine Classical approach to localised gastric cancer Surgical resection Pathology assessment and estimation
A multidisciplinary clinical treatment of locally advanced rectal cancer complicated with rectovesical fistula: a case report
 Zhan et al. Journal of Medical Case Reports 2012, 6:369 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access A multidisciplinary clinical treatment of locally advanced rectal cancer complicated with
Zhan et al. Journal of Medical Case Reports 2012, 6:369 JOURNAL OF MEDICAL CASE REPORTS CASE REPORT Open Access A multidisciplinary clinical treatment of locally advanced rectal cancer complicated with
Hagit Tulchinsky, MD, 1,2 Einat Shmueli, MD, 3 Arie Figer, MD, 3 Joseph M. Klausner, MD, 2 and Micha Rabau, MD 1,2
 Annals of Surgical Oncology DOI: 10.1245/s10434-008-9892-3 An Interval [7 Weeks between Neoadjuvant Therapy and Surgery Improves Pathologic Complete Response and Disease Free Survival in Patients with
Annals of Surgical Oncology DOI: 10.1245/s10434-008-9892-3 An Interval [7 Weeks between Neoadjuvant Therapy and Surgery Improves Pathologic Complete Response and Disease Free Survival in Patients with
ACR Appropriateness Criteria Resectable Rectal Cancer EVIDENCE TABLE
 . National Cancer Institute. Comprehensive Cancer Information. http://www.cancer.gov/cancertopics/types/ colon-and-rectal. Accessed 5 January 202. 2. Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM,
. National Cancer Institute. Comprehensive Cancer Information. http://www.cancer.gov/cancertopics/types/ colon-and-rectal. Accessed 5 January 202. 2. Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM,
Treatment of Locally Advanced Rectal Cancer: Current Concepts
 Treatment of Locally Advanced Rectal Cancer: Current Concepts James J. Stark, MD, FACP Medical Director, Cancer Program and Palliative Care Maryview Medical Center Professor of Medicine, EVMS Case Presentation
Treatment of Locally Advanced Rectal Cancer: Current Concepts James J. Stark, MD, FACP Medical Director, Cancer Program and Palliative Care Maryview Medical Center Professor of Medicine, EVMS Case Presentation
MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER
 10 MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER Recommendations from the EAU Working Party on Muscle Invasive and Metastatic Bladder Cancer G. Jakse (chairman), F. Algaba, S. Fossa, A. Stenzl, C. Sternberg
10 MUSCLE - INVASIVE AND METASTATIC BLADDER CANCER Recommendations from the EAU Working Party on Muscle Invasive and Metastatic Bladder Cancer G. Jakse (chairman), F. Algaba, S. Fossa, A. Stenzl, C. Sternberg
Protocol Abstract and Schema
 Protocol Abstract and Schema Phase II study of Bevacizumab plus Irinotecan (Camptosar ) in Children with Recurrent, Progressive, or Refractory Malignant Gliomas, Diffuse/Intrinsic Brain Stem Gliomas, Medulloblastomas,
Protocol Abstract and Schema Phase II study of Bevacizumab plus Irinotecan (Camptosar ) in Children with Recurrent, Progressive, or Refractory Malignant Gliomas, Diffuse/Intrinsic Brain Stem Gliomas, Medulloblastomas,
Staging Colorectal Cancer
 Staging Colorectal Cancer CT is recommended as the initial staging scan for colorectal cancer to assess local extent of the disease and to look for metastases to the liver and/or lung Further imaging for
Staging Colorectal Cancer CT is recommended as the initial staging scan for colorectal cancer to assess local extent of the disease and to look for metastases to the liver and/or lung Further imaging for
EXCLUSIONS: Suspected dihydropyrimidine dehydrogenase (DPD) deficiency (see Precautions)
 BC Cancer Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact
BC Cancer Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact
COLORECTAL CARCINOMA
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
Colorectal Cancer Treatment
 Scan for mobile link. Colorectal Cancer Treatment Colorectal cancer overview Colorectal cancer, also called large bowel cancer, is the term used to describe malignant tumors found in the colon and rectum.
Scan for mobile link. Colorectal Cancer Treatment Colorectal cancer overview Colorectal cancer, also called large bowel cancer, is the term used to describe malignant tumors found in the colon and rectum.
Follow this and additional works at: Part of the Neoplasms Commons
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2015 Will the Addition of Oxaliplatin to 5-Fluorouracil
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2015 Will the Addition of Oxaliplatin to 5-Fluorouracil
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel
 BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
