Medical Policy Breast Surgeries
|
|
|
- Opal Underwood
- 6 years ago
- Views:
Transcription
1 Medical Policy Breast Surgeries Document Number: 006 Commercial and MassHealth Connector/Qualified Health Plans Authorization required Breast Reconstruction Surgeries; Reduction Mammoplasty, Female Members; Breast Implant Removal; Mastectomy; Nipple Repair; and Reduction Mammoplasty, Male Members Notification within 24 hours of service or next business day No notification or authorization Lumpectomy* Not covered *Elective inpatient admissions require prior authorization. Outpatient surgery resulting in inpatient admissions requires notification. Overview The purpose of this document is to describe the guidelines Neighborhood Health Plan (NHP) utilizes to determine medical appropriateness for breast surgery. The treating specialist must request prior authorization for breast surgery procedures. Prior authorization is required for all breast reduction and reconstruction surgeries, implant removal, nipple repair, and gynecomastia surgery and for mastectomy/lumpectomy procedures requiring an inpatient admission. Coverage Guidelines NHP covers mastectomy/lumpectomy for cancer and for cancer related prophylaxis in accordance with the benefits described in the individual benefit handbook or coverage of benefits when the attending physician determines that mastectomy is medically necessary. This includes prophylactic mastectomy for BRCA carriage or other well defined genetic predisposition to breast cancer. NHP covers breast reconstruction in accordance with the Women s Health and Cancer Rights Act of 1998 (WHCRA). NHP provides coverage for: Reconstruction of the breast on which a mastectomy/lumpectomy has been performed; Surgery and reconstruction of the other breast to produce symmetrical appearance; Prosthesis and treatment of physical complications at all stages of a mastectomy/lumpectomy, including lymphedema; and Tattooing of an areola as part of a nipple reconstruction following mastectomy/lumpectomy. NHP covers medically necessary mastectomy for gender dysphoria when a member is transitioning from female to male and meets relevant medical necessity criteria for coverage under the Gender Reassignment Surgery. NHP covers breast reconstruction, augmentation, reduction, implant removal, and gynecomastia surgery when it is recommended by the member s primary care physician or referring surgeon, the requested procedure can reasonably Breast Surgeries 006 Page 1 of 6
2 be expected to resolve the medical condition or complication and functional impairment, and the request meets medical necessity criteria indicated below. NHP reserves the right to deny coverage for any breast surgery procedures that; 1. Do not meet coverage criteria; 2. Are not in accordance with the WHCRA; 3. Are considered cosmetic, performed primarily to improve a person s appearance, and not medically necessary. Breast Reconstruction Surgery As of February 20, 2017 medical necessity for breast reconstruction surgery is determined through McKesson s InterQual criteria. To access the criteria, log in to NHP s provider website at NHP.Net and click the InterQual Criteria Lookup link under the Resources Menu. NHP also covers medically necessary breast reconstruction surgery in the following instances: 1. For treatment other than cancer related mastectomy/lumpectomy (photo documentation is required) for a member with: a. Severe disfigurement from Poland Syndrome or other disease; OR b. Gender dysphoria when a member is transitioning from male to female and meets relevant medical necessity criteria for coverage under the Gender Reassignment Surgery and the request is for augmentation mammoplasty. c. Severe breast asymmetry of at least 2 cup difference in breast size in a female patient who has reached full physical maturity, i.e., tanner stage V, typically age 15 and older. Reduction Mammoplasty, Female Members (photo documentation is required) As of February 20, 2017 medical necessity for reduction mammoplasty in female members is determined through McKesson s InterQual criteria. To access the criteria, log in to NHP s provider website at NHP.Net and click the InterQual Criteria Lookup link under the Resources Menu. NHP considers members <18 years of age eligible for reduction mammoplasty when they have reached full physical maturity i.e. tanner stage V, typically age 15 and older and when all other InterQual criteria are met. Mammogram Requirements Only women fifty years or older must have a negative mammogram for cancer performed within two years prior to the date of the planned surgery. This must be evidenced by one of the following: i. Copy of the mammogram report; ii. Verbal or written confirmation from a MD/RN/PA in the surgeon's or PCP's office; or iii. Verbal report from office support staff following instructions from MD/RN/PA (one of whom has reviewed the report). Note: Coverage for reduction mammoplasty is limited to one procedure per member per lifetime. Breast Implant Removal As of February 20, 2017 medical necessity for breast implant removal is determined through McKesson s InterQual criteria. To access the criteria, log in to NHP s provider website at NHP.Net and click the InterQual Criteria Lookup link under the Resources Menu. Nipple Repair NHP covers medically necessary nipple repair when there is medical record documentation supporting the following: 1. An inverted nipple is causing a demonstrated inability to breast feed and the requested procedure can reasonably be expected to restore this lost functionality; and 2. Other nipple procedures are authorized when they are medically necessary part of a NHP authorized breast reconstruction procedure. Reduction Mammoplasty, Male (Gynecomastia Surgery) (photo documentation is required) As of February 20, 2017 medical necessity for reduction mammoplasty in male members is determined through McKesson s InterQual criteria. To access the criteria, log in to NHP s provider website at NHP.Net and click the InterQual Criteria Lookup link under the Resources Menu. Exclusions Breast Surgeries 006 Page 2 of 6
3 NHP does not provide coverage for breast surgery for conditions that do not meet the criteria noted, including but not limited to: 1. Breast surgeries or procedures performed solely to enhance a member s appearance or to counteract appearance that occurs through the natural aging process, in the absence of any signs or symptoms of functional abnormalities and/or associated medical complication is considered cosmetic and is not a covered benefit, unless specifically noted in the coverage criteria. 2. Breast surgeries or procedures performed primarily for psychological or emotional reasons. 3. Mastopexy for breast reconstruction unless it is for cancer related mastectomy/lumpectomy. 4. Removal of a breast implant that has been placed for cosmetic purposes is not a covered benefit when performed due to: a. Pain without clearly defined abnormalities on exam or radiographically that meet the criteria above; b. Leakage of a saline implant; or c. Anxiety concerning a potential complication. 5. Replacement of an implant that has been removed for medical necessity that had been originally placed for cosmetic purposes is not a covered benefit. 6. Surgical treatment for gynecomastia is not considered medically necessary for any of the following reasons: a. There is laboratory drug screen evidence of illicit substance abuse that can cause gynecomastia (e.g. marijuana, heroin, amphetamines); b. There is a history of chronic alcohol abuse; c. There is a history of the use of supplements/herbal products/hormones that can cause gynecomastia, and which have not been prescribed by a licensed clinician to treat a medical condition; or d. Treatment of pseudogynecomastia (breast enlargement secondary to fatty tissue). 7. Breast surgeries not specifically noted as covered procedures in this medical policy or in the Gender Reassignment Surgery Policy. 8. Subsequent breast surgeries that are not part of an approved staged reconstruction plan and are intended for the sole purpose of cosmetic enhancement. Definitions Capsular contracture Baker Scale: Grade I the breast is normally soft and appears natural in size and shape Grade II the breast is a little firm, but appears normal Grade III the breast is firm and appears abnormal Grade IV the breast is hard, painful to the touch, and appears abnormal Cup Size: A cup size is usually estimated at 200 g (A = 200 g, B = 400 g, C = 600 g.) To estimate a cup size take the difference between the chest measurement at the nipple level and at the inframammary crease (IMC), with a difference of 5 as equal to an A cup with each additional inch another cup size. Example, if IMC measures 29 and the nipple level circumference is 35 in, this would be = 6, or a B cup. Gynecomastia: Abnormal proliferation of breast tissue in males. Gynecomastia Scale adapted from the McKinney and Simon, Hoffman and Kohn scales Grade I Small breast enlargement with localized button of tissue that is concentrated around the areola. Grade II Moderate breast enlargement exceeding areola boundaries with edges that are indistinct from the chest. o Grade IIA Moderate breast enlargement exceeding areola boundaries with edges that are indistinct from the chest without skin redundancy o Grade IIB Moderate breast enlargement exceeding areola boundaries with edges that are indistinct from the chest with skin redundancy Grade III Moderate breast enlargement exceeding areola boundaries with edges that are distinct from the chest with skin redundancy present. Grade IV Marked breast enlargement Breast Reduction Table: Based on a table adapted from a study by Schnur (1991). Breast Surgeries 006 Page 3 of 6
4 Regulation Women's Health and Cancer Rights Act of 1998 Sec Required Coverage for Reconstructive Surgery Following Mastectomies. (a) In General A group health plan, and a health insurance issuer providing health insurance coverage in connection with a group health plan, that provides medical and surgical benefits with respect to a mastectomy shall provide, in a case of a participant or beneficiary who is receiving benefits in connection with a mastectomy and who elects breast reconstruction in connection with such mastectomy, coverage for: All stages of reconstruction of the breast on which the mastectomy has been performed; Surgery and reconstruction of the other breast to produce a symmetrical appearance; Prostheses and physical complications of all stages of mastectomy, including lymphedemas; in a manner determined in consultation with the attending physician and the patient. Such coverage may be subject to annual deductibles and coinsurance provisions as may be deemed appropriate and as are consistent with those established for other benefits under the plan or coverage. Written notice of the availability of such coverage shall be delivered to the participant upon enrollment and annually thereafter. (b) Notice A group health plan and a health insurance issuer providing health insurance coverage in connection with a group health plan shall provide notice to each participant and beneficiary under such plan regarding the coverage required by this section in accordance 1078 with regulations promulgated by the Secretary. Such notice shall be in writing and prominently positioned in any literature or correspondence made available or distributed by the plan or issuer and shall be transmitted: In the next mailing made by the plan or issuer to the participant or beneficiary; As part of any yearly informational packet sent to the participant or beneficiary; or Not later than January 1, 1999; whichever is earlier. (c) Prohibitions A group health plan, and a health insurance issuer offering group health insurance coverage in connection with a group health plan, may not: Deny to a patient eligibility, or continued eligibility, to enroll or to renew coverage under the terms of the plan, solely for the purpose of avoiding the requirements of this section; and Penalize or otherwise reduce or limit the reimbursement of an attending provider, or provide incentives (monetary or otherwise) to an attending provider, to induce such provider to provide care to an individual participant or beneficiary in a manner inconsistent with this section (d) Rule of Construction Nothing in this section shall be construed to prevent a group health plan or a health insurance issuer offering group health insurance coverage from negotiating the level and type of reimbursement with a provider for care provided in accordance with this section. Related Policies Reconstructive and Cosmetic Procedures Dermatology Provider Payment Guideline Gender Reassignment Surgery Effective December 2017: Annual review. May 2017: Added photo documentation is required to subheads Reduction Mammoplasty, Female Members, and to Reduction Mammoplasty, Male (Gynecomastia Surgery). February 2017: Changes reflect the addition of InterQual breast reconstruction surgeries, breast implant removal, reduction mammoplasty (male), reduction mammoplasty (female), and mastectomy criteria. September 2016: Annual review. September 2015: Coverage for ruptured saline implant removal and replacement when placed for certain medical conditions, clarity regarding overlap and consistency with Gender Reassignment Surgery Medical Policy added September 2014: New medically necessary indicators added. May 2013: Added physical maturity to breast reduction criteria; added breast implant removal & surgery for gynecomastia criteria. June 2012: No change. May 2011: Annual Review. April 2010: Annual Review. Breast Surgeries 006 Page 4 of 6
5 April 2009: Annual Review. April 2008: Annual Review. April 2007: Annual Review. May 2006: Annual Review. May 2005: Effective date. References American Society of Plastic Surgeons, Recommended Criteria for Third Party Payer Coverage, downloaded from ended_insurance_coverage_criteria.html, retrieved 12/06 12/07, 12/08, 1/09, 1/10, 1/11, 1/12, 1/13, 3/14 American Society of Plastic Surgeons. Reduction Mammaplasty Recommended Criteria for Third Party Payer Coverage from the American Society of Plastic Surgeons (ASPS). May Available at URL address: Accessed, 2012, 2013, 4/13 American Society of Plastic Surgeons. Reduction Mammaplasty Recommended Criteria for Third Party Payer Coverage from the American Society of Plastic Surgeons (ASPS). March 9, 2002a. Available at URL address: Accessed, 2008, 2009, 2010, 2011, American Society of Plastic Surgeons. Reduction Mammaplasty. Practice Parameters. March 9, 2002b. Available at URL address: Accessed 2008, 2011, 2012, American Society of Plastic Surgeons. Consumer's Guide to Breast Reduction Breast Reduction Assessment of Value and Outcomes (BRAVO) Study. Accessed April, Available at URL address: Guide to Breast Reduction.cfm Division of Medical Assistance Guidelines for Medical Necessity Determination for Breast Reconstruction, March 9, 2009, retrieved 4/14 Division of Medical Assistance Guidelines for Medical Necessity Determination for Breast Reduction, July 1, 2005, retrieved 2008, 2009, 2010, 2011, 2012, /14, and 2016 Division of Medical Assistance Guidelines for Medical Necessity Determination for Mastectomy for Gynecomastia, February 22, 2012, retrieved 1/12 Schnur PL, Schnur DP, Petty PM, Hanson TJ, Weaver Al. Reduction mammoplasty: an outcome study. Plast Reconstr Surg Sep;100(4): Schnur, Paul L, et al., "Reduction Mammaplasty: Cosmetic or Reconstructive Procedure?" Annals of Plastic Surgery. Sept 1991; 27 (3): Chadbourne, E. B., Ahang, S., Gordon, M. J., Ro, E. Y., Ross, S. D., Schnur, P. L. & Schneider Redden, P. E. Clinical outcomes in reduction mammaplasty: a systematic review and met analysis of published studies. Mayo Clinic Proceedings 76:m , Kerrigan CL. Collins ED. Kim HM. Schnur PL. Wilkins E. Cunningham B. Lowery J. Reduction mammaplasty: Defining medical necessity. Medical Decision Making. Vol. 22(3) (pp ), Krieger, L.M., Lesavoy, M. A. Managed Care s Methods for Determining Coverage of Plastic Surgery Procedures: The Example of Reduction Mammaplasty. Plastic Reconstructive Surgery 200; 107: Nguyen, J., Wheatley, M., Schnur, P., Nguyen, T., Winn, S. Reduction mammaplasty: A review of managed care medical policy coverage criteria. Plastic and Reconstructive Surgery Vol 121 (4) PP , Independent practitioner review, Plastic surgeon review 2006 and 2007, Daniel Sigman, M.D. Independent practitioner review, Endocrinology review 2014 Breast Surgeries 006 Page 5 of 6
6 Hayes, Reduction Mammoplasty for breast Hypertrophy. Literature search of Medline and Embase completed February 7, Accessed 2008, 2009, Hayes, Reduction Mammoplasty. Literature search of Medline and Embase completed December 8, Accessed 2011, 2012, Hayes, Reduction Mammoplasty for Macromastia in Adolescents. November 26, Accessed 2013 Practice Surgery Practice April 2005, Two for One, Myron M. Persoff, MD, FACS, accessed Qutob, O., Elahi, B., Garimella, V., Ihsan, N., & Drew, P.J. Minimally invasive excision of gynaecomastia a novel and effective surgical technique. Ann R Coll Surg Engl April 92 (3): Available from < Accessed 2012 Breast Surgeries 006 Page 6 of 6
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Surgical Treatment of Bilateral Gynecomastia
 Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Breast Reconstruction Surgery
 Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Related Policies None
 Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
Breast Surgery Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives
 NHS Dorset Clinical Commissioning Group Breast Surgery Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives NHS DORSET CLINICAL COMMISSIONING GROUP BREAST SURGERY CRITERIA
NHS Dorset Clinical Commissioning Group Breast Surgery Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives NHS DORSET CLINICAL COMMISSIONING GROUP BREAST SURGERY CRITERIA
Corporate Medical Policy
 Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 8/2017 8/2018 8/2017 Description of Procedure or Service Mastectomy
Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 8/2017 8/2018 8/2017 Description of Procedure or Service Mastectomy
Medical Policy Original Effective Date: Revised Date: Page 1 of 8
 Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Reduction Mammoplasty
 Reduction Mammoplasty Date of Origin: 02/1999 Last Review Date: 06/28/2017 Effective Date: 06/28/2017 Dates Reviewed: 05/1999, 10/2000, 09/2001, 03/2002, 05/2002, 08/2002, 10/2003, 10/2004, 09/2005, 11/2005,
Reduction Mammoplasty Date of Origin: 02/1999 Last Review Date: 06/28/2017 Effective Date: 06/28/2017 Dates Reviewed: 05/1999, 10/2000, 09/2001, 03/2002, 05/2002, 08/2002, 10/2003, 10/2004, 09/2005, 11/2005,
Clinical Policy: Reduction Mammoplasty and Gynecomastia Surgery
 Clinical Policy: Reference Number: CP.MP.51 Last Review Date: 07/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications Revision Log Description
Clinical Policy: Reference Number: CP.MP.51 Last Review Date: 07/18 See Important Reminder at the end of this policy for important regulatory and legal information. Coding Implications Revision Log Description
Policy #: 114 Latest Review Date: March 2017
 Name of Policy: Gynecomastia Surgery Policy #: 114 Latest Review Date: March 2017 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross and
Name of Policy: Gynecomastia Surgery Policy #: 114 Latest Review Date: March 2017 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross and
Name of Policy: Reduction Mammaplasty
 Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: November 2013 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: November 2013 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross
Reduction mammaplasty is a surgical procedure designed to remove a variable proportion of breast tissue.
 Last Review Status/Date: March 2014 Page: 1 of 7 Description Reduction mammaplasty is a surgical procedure designed to remove a variable proportion of breast tissue. Background Macromastia, or gigantomastia,
Last Review Status/Date: March 2014 Page: 1 of 7 Description Reduction mammaplasty is a surgical procedure designed to remove a variable proportion of breast tissue. Background Macromastia, or gigantomastia,
NEIGHBORHOOD HEALTH PARTNERSHIP HMO SUMMARY OF BENEFITS
 . (EV-4) 25/45/1000 w/access Rider NEIGHBORHOOD HEALTH PARTNERSHIP HMO SUMMARY OF BENEFITS A quick glance at this Summary of Benefits will introduce you to the important advantages of the Neighborhood
. (EV-4) 25/45/1000 w/access Rider NEIGHBORHOOD HEALTH PARTNERSHIP HMO SUMMARY OF BENEFITS A quick glance at this Summary of Benefits will introduce you to the important advantages of the Neighborhood
Reduction Mammoplasty (193180)
 Reduction Mammoplasty (193180) POLICY Reduction mammoplasty or breast reduction surgery reduces the volume and weight of the female breasts by removing excess fat, glandular tissue and skin. Macromastia
Reduction Mammoplasty (193180) POLICY Reduction mammoplasty or breast reduction surgery reduces the volume and weight of the female breasts by removing excess fat, glandular tissue and skin. Macromastia
Breast Augmentation - Silicone Implants
 Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Breast Augmentation - Silicone Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics,
Breast Augmentation - Saline Implants
 Breast Augmentation - Saline Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy,
Breast Augmentation - Saline Implants Breast augmentation, or augmentation mammoplasty, is one of the most common plastic surgery procedures performed today. Over time, factors such as age, genetics, pregnancy,
Reduction Mammaplasty for Breast-Related Symptoms. Original Policy Date
 MP 7.01.16 Reduction Mammaplasty for Breast-Related Symptoms Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return
MP 7.01.16 Reduction Mammaplasty for Breast-Related Symptoms Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return
Breast Surgery Corporate Medical Policy
 File name: Breast Surgery File code: UM.SURG.17 Origination: 2016 Last Review: 11/2018 (PA List Review) Next Review: 11/2019 Effective Date: 04/01/2018 Breast Surgery Corporate Medical Policy Description/Summary
File name: Breast Surgery File code: UM.SURG.17 Origination: 2016 Last Review: 11/2018 (PA List Review) Next Review: 11/2019 Effective Date: 04/01/2018 Breast Surgery Corporate Medical Policy Description/Summary
Reduction Mammaplasty for Breast-Related Symptoms
 Reduction Mammaplasty for Breast-Related Symptoms Policy Number: 7.01.21 Last Review: 7/2014 Origination: 7/2005 Next Review: 7/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Reduction Mammaplasty for Breast-Related Symptoms Policy Number: 7.01.21 Last Review: 7/2014 Origination: 7/2005 Next Review: 7/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
BREAST REDUCTION. Based on my discussions with Dr Gutowski, I understand and agree to the following:
 INFORMED CONSENT FOR BREAST REDUCTION PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME Based on my discussions with Dr Gutowski, I understand and agree to the following: Dr. Gutowski
INFORMED CONSENT FOR BREAST REDUCTION PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME Based on my discussions with Dr Gutowski, I understand and agree to the following: Dr. Gutowski
The Adult Exceptional Aesthetic Referral Protocol (AEARP) September 2011
 Aesthetic surgery is not routinely offered by the NHS and can only be provided on an exceptional case basis in line with the Please Note Patients should only be referred following a clinical assessment
Aesthetic surgery is not routinely offered by the NHS and can only be provided on an exceptional case basis in line with the Please Note Patients should only be referred following a clinical assessment
Commissioning Policy: Treatments Designed to Improve Aesthetic Appearance
 Commissioning Policy: Treatments Designed to Improve Aesthetic Appearance Policy Statement: Coventry and Rugby CCG consider funding of treatments designed to improve aesthetic appearance to be of low priority
Commissioning Policy: Treatments Designed to Improve Aesthetic Appearance Policy Statement: Coventry and Rugby CCG consider funding of treatments designed to improve aesthetic appearance to be of low priority
No An act relating to health insurance coverage for early childhood developmental disorders, including autism spectrum disorders. (S.
 No. 158. An act relating to health insurance coverage for early childhood developmental disorders, including autism spectrum disorders. (S.223) It is hereby enacted by the General Assembly of the State
No. 158. An act relating to health insurance coverage for early childhood developmental disorders, including autism spectrum disorders. (S.223) It is hereby enacted by the General Assembly of the State
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Kadcyla) Reference Number: CP.PHAR.229 Effective Date: 06.01.16 Last Review Date: 05.18 Line of Business: Commercial, HIM-Medical Benefit, Medicaid Coding Implications Revision Log See
Clinical Policy: (Kadcyla) Reference Number: CP.PHAR.229 Effective Date: 06.01.16 Last Review Date: 05.18 Line of Business: Commercial, HIM-Medical Benefit, Medicaid Coding Implications Revision Log See
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN
 MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN
 MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS, MEMORYGEL XTRA BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC
MENTOR PROMISE AND MENTOR PROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS, MEMORYGEL XTRA BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC
INFORMED-CONSENT AUGMENTATION MAMMAPLASTY
 INFORMED-CONSENT AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks and alternative treatments.
INFORMED-CONSENT AUGMENTATION MAMMAPLASTY INSTRUCTIONS This is an informed-consent document that has been prepared to help inform you about augmentation mammaplasty, its risks and alternative treatments.
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
The Case FOR Oncoplastic Surgery in Small Breasts. Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA
 The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
Policy #: 056 Latest Review Date: March 2017
 Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: March 2017 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross and
Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: March 2017 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross and
Medical Policy Original Effective Date: Revised Date: 09/26/2018 Page 1 of 26
 Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
BREAST AUGMENTATION. Your complete guide to breast augmentation and enhancing your silhouette.
 BREAST AUGMENTATION Your complete guide to breast augmentation and enhancing your silhouette. (07) 3257 7950 drsamuelyang.com.au CONTENTS What is a Breast Augmentation? 3 Breast Augmentation Considerations
BREAST AUGMENTATION Your complete guide to breast augmentation and enhancing your silhouette. (07) 3257 7950 drsamuelyang.com.au CONTENTS What is a Breast Augmentation? 3 Breast Augmentation Considerations
INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY
 INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein
INFORMED-CONSENT-AUGMENTATION MAMMAPLASTY 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify documents contained herein
Based on my discussions with Dr Gutowski, I agree with, and choose to have the following options as part of my breast augmentation:
 INFORMED CONSENT FOR BREAST AUGMENTATION PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME Based on my discussions with Dr Gutowski, I agree with, and choose to have the following
INFORMED CONSENT FOR BREAST AUGMENTATION PLEASE REVIEW AND BRING WITH YOU ON THE DAY OF YOUR PROCEDURE PATIENT NAME Based on my discussions with Dr Gutowski, I agree with, and choose to have the following
Information about Breast Augmentation for patients who contact Dr. Medalie
 Information about Breast Augmentation for patients who contact Dr. Medalie Thank-you for contacting me through the internet. Below is some information regarding breast augmentation. Please also note the
Information about Breast Augmentation for patients who contact Dr. Medalie Thank-you for contacting me through the internet. Below is some information regarding breast augmentation. Please also note the
Reduction Mammaplasty for Breast-Related Symptoms
 Reduction Mammaplasty for Breast-Related Symptoms Policy Number: Original Effective Date: MM.06.012 11/12/2002 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 09/01/2017 Section:
Reduction Mammaplasty for Breast-Related Symptoms Policy Number: Original Effective Date: MM.06.012 11/12/2002 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 09/01/2017 Section:
Timby/Smith: Introductory Medical-Surgical Nursing, 9/e
 Timby/Smith: Introductory Medical-Surgical Nursing, 9/e Chapter 60: Caring for Clients With Breast Disorders Slide 1 Infectious and Inflammatory Breast Disorders: Mastitis Pathophysiology and Etiology
Timby/Smith: Introductory Medical-Surgical Nursing, 9/e Chapter 60: Caring for Clients With Breast Disorders Slide 1 Infectious and Inflammatory Breast Disorders: Mastitis Pathophysiology and Etiology
Plastic surgery of the breast includes; augmentation, reduction, Plastic Surgery of the Breast. Abstract. Continuing Education Column
 Plastic Surgery of the Breast Keuk Shun Shin, M.D. Keuk SHUN SHIN s Asthetic Plastic Surgery E mail: drsks@drsks.co.kr Abstract Plastic surgery of the breast includes; augmentation, reduction, reconstruction
Plastic Surgery of the Breast Keuk Shun Shin, M.D. Keuk SHUN SHIN s Asthetic Plastic Surgery E mail: drsks@drsks.co.kr Abstract Plastic surgery of the breast includes; augmentation, reduction, reconstruction
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Reduction Mammaplasty for Breast-Related Symptoms Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Reduction Mammaplasty for Breast-Related Symptoms
Reduction Mammaplasty for Breast-Related Symptoms Page 1 of 10 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Reduction Mammaplasty for Breast-Related Symptoms
Information about breast augmentation (enlargement) surgery Part 1 of 3
 Information about breast augmentation (enlargement) surgery Part 1 of 3 This leaflet explains breast augmentation surgery. It is important that you read this information carefully and completely. Please
Information about breast augmentation (enlargement) surgery Part 1 of 3 This leaflet explains breast augmentation surgery. It is important that you read this information carefully and completely. Please
Reconstructive Breast Surgery and Management of Breast Implants
 Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
INFORMED-CONSENT- AUGMENTATION MAMMOPLASTY
 INFORMED-CONSENT- AUGMENTATION MAMMOPLASTY Instructions This is an informed-consent document that has been prepared to help inform you about augmentation mammoplasty, its risks, and alternative treatments.
INFORMED-CONSENT- AUGMENTATION MAMMOPLASTY Instructions This is an informed-consent document that has been prepared to help inform you about augmentation mammoplasty, its risks, and alternative treatments.
Clinical Policy: Pertuzumab (Perjeta) Reference Number: CP.PHAR.227 Effective Date: Last Review Date: Line of Business: HIM, Medicaid
 Clinical Policy: (Perjeta) Reference Number: CP.PHAR.227 Effective Date: 06.01.16 Last Review Date: 05.18 Line of Business: HIM, Medicaid Coding Implications Revision Log See Important Reminder at the
Clinical Policy: (Perjeta) Reference Number: CP.PHAR.227 Effective Date: 06.01.16 Last Review Date: 05.18 Line of Business: HIM, Medicaid Coding Implications Revision Log See Important Reminder at the
Clinical Policy: Levetiracetam (Spritam) Reference Number: CP.CPA.156 Effective Date: Last Review Date: 11.18
 Clinical Policy: (Spritam) Reference Number: CP.CPA.156 Effective Date: 11.16.16 Last Review Date: 11.18 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Spritam) Reference Number: CP.CPA.156 Effective Date: 11.16.16 Last Review Date: 11.18 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Brigatinib (Alunbrig) Reference Number: CP.PHAR.342 Effective Date: 07.17.17 Last Review Date: 05.18 Line of Business: Commercial; Medicaid Revision Log See Important Reminder at the end
Clinical Policy: Brigatinib (Alunbrig) Reference Number: CP.PHAR.342 Effective Date: 07.17.17 Last Review Date: 05.18 Line of Business: Commercial; Medicaid Revision Log See Important Reminder at the end
Reduction Mammoplasty AHM
 Reduction Mammoplasty AHM Clinical Indications Breast reduction surgery is considered medically necessary for non-cosmetic indications [A] The condition not only must be unresponsive to dermatological
Reduction Mammoplasty AHM Clinical Indications Breast reduction surgery is considered medically necessary for non-cosmetic indications [A] The condition not only must be unresponsive to dermatological
INFORMED- CONSENT- BREAST IMPLANT REMOVAL SURGERY
 INFORMED- CONSENT- BREAST IMPLANT REMOVAL SURGERY INSTRUCTIONS This is an informed- consent document that has been prepared to help your plastic surgeon inform you concerning breast implant removal, its
INFORMED- CONSENT- BREAST IMPLANT REMOVAL SURGERY INSTRUCTIONS This is an informed- consent document that has been prepared to help your plastic surgeon inform you concerning breast implant removal, its
Medical Policy Bariatric Surgery. Document Number: 042 Commercial and Qualified Health Plans MassHealth Authorization required X X
 Medical Policy Bariatric Surgery Document Number: 042 Commercial and Qualified Health Plans MassHealth Authorization required X X No Prior Authorization Overview The purpose of this document is to describe
Medical Policy Bariatric Surgery Document Number: 042 Commercial and Qualified Health Plans MassHealth Authorization required X X No Prior Authorization Overview The purpose of this document is to describe
A separate consent form for the use of breast implants in conjunction with mastopexy is necessary.
 INFORMED-CONSENT-BREAST LIFT (MASTOPEXY) INSTRUCTIONS This is an informed-consent document that has been prepared to help your plastic surgeon inform you about mastopexy surgery, its risks, and alternative
INFORMED-CONSENT-BREAST LIFT (MASTOPEXY) INSTRUCTIONS This is an informed-consent document that has been prepared to help your plastic surgeon inform you about mastopexy surgery, its risks, and alternative
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Nerlynx) Reference Number: CP.PHAR.365 Effective Date: 09.05.17 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Nerlynx) Reference Number: CP.PHAR.365 Effective Date: 09.05.17 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Protocol. Reconstructive Breast Surgery/Management of Breast Implants
 Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Policy Reference Number: CP.PMN.53 Effective Date: 09.12.17 Last Review Date: 11.18 Line of Business: Medicaid, HIM-Medical Benefit Revision Log See Important Reminder at the end of this
Clinical Policy: Policy Reference Number: CP.PMN.53 Effective Date: 09.12.17 Last Review Date: 11.18 Line of Business: Medicaid, HIM-Medical Benefit Revision Log See Important Reminder at the end of this
Vertical mammaplasty has been developed
 BREAST Y-Scar Vertical Mammaplasty David A. Hidalgo, M.D. New York, N.Y. Background: Vertical mammaplasty is an effective alternative to inverted-t methods. Among other benefits, it results in a significantly
BREAST Y-Scar Vertical Mammaplasty David A. Hidalgo, M.D. New York, N.Y. Background: Vertical mammaplasty is an effective alternative to inverted-t methods. Among other benefits, it results in a significantly
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Pellet (Testopel) Reference Number: CP.PHAR.354 Effective Date: 08.01.17 Last Review Date: 11.18 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the
Clinical Policy: Pellet (Testopel) Reference Number: CP.PHAR.354 Effective Date: 08.01.17 Last Review Date: 11.18 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the
Clinical Policy: Secukinumab (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08/16 Last Review Date: 08/17
 Clinical Policy: (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Cosentyx) Reference Number: CP.PHAR.261 Effective Date: 08/16 Last Review Date: 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Reduction Mammaplasty for Breast- Related Symptoms
 Reduction Mammaplasty for Breast- Related Symptoms Policy Number: 7.01.21 Last Review: 7/2018 Origination: 7/2005 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Reduction Mammaplasty for Breast- Related Symptoms Policy Number: 7.01.21 Last Review: 7/2018 Origination: 7/2005 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide
Clinical Policy: Apremilast (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08/16 Last Review Date 08/17
 Clinical Policy: (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08/16 Last Review Date 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Otezla) Reference Number: CP.PHAR.245 Effective Date: 08/16 Last Review Date 08/17 Line of Business: Medicaid Revision Log See Important Reminder at the end of this policy for important
Non-voluntary dental (2-9) Texas
 Non-voluntary dental (2-9) Option 1 DMO Access Option 2 DMO Option 3 Freedom-of-Choice Monthly selection between the DMO and the PDN Plan Option 4 PDN Max Option 5 PDN 1500 DMO Copay 42 DMO 100/90/60 DMO
Non-voluntary dental (2-9) Option 1 DMO Access Option 2 DMO Option 3 Freedom-of-Choice Monthly selection between the DMO and the PDN Plan Option 4 PDN Max Option 5 PDN 1500 DMO Copay 42 DMO 100/90/60 DMO
Guide to Breast Augmentation: Everything You Need to Know
 Northwestern Specialists in Plastic Surgery Dr. Neil Fine, MD, FACS Dr. Clark Schierle, MD, PhD, FACS Contents 3 Introduction 4 Implant Shell 5 Implant Fill 6 Ideal Implant 7 Implant Shape 8 Implant Placement
Northwestern Specialists in Plastic Surgery Dr. Neil Fine, MD, FACS Dr. Clark Schierle, MD, PhD, FACS Contents 3 Introduction 4 Implant Shell 5 Implant Fill 6 Ideal Implant 7 Implant Shape 8 Implant Placement
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP
 INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
Therapeutic Mammoplasty. Breast Care
 Therapeutic Mammoplasty Breast Care We put our patients first by working as one team; leading and listening, and striving for the best. Together, we make the difference. Patient information Musgrove Park
Therapeutic Mammoplasty Breast Care We put our patients first by working as one team; leading and listening, and striving for the best. Together, we make the difference. Patient information Musgrove Park
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: No Coverage Criteria/Off-Label Use Policy Reference Number: CP.PMN.53 Effective Date: 07.01.18 Last Review Date: 05.01.18 Line of Business: Oregon Health Plan Revision Log See Important
Clinical Policy: No Coverage Criteria/Off-Label Use Policy Reference Number: CP.PMN.53 Effective Date: 07.01.18 Last Review Date: 05.01.18 Line of Business: Oregon Health Plan Revision Log See Important
UNDERSTANDiNG THE BREAST AUGMENTATION PROCEDURE
 UNDERSTANDiNG THE BREAST AUGMENTATION PROCEDURE Having fuller more voluptuous breasts is a very important part of feeling feminine and more confident for women. The breasts give the female body more proportion,
UNDERSTANDiNG THE BREAST AUGMENTATION PROCEDURE Having fuller more voluptuous breasts is a very important part of feeling feminine and more confident for women. The breasts give the female body more proportion,
A Combined Practice. Why Its Worked. Barriers to Breast Reconstruction. As a breast oncologist the patient gets seemless care
 A Combined Practice A Combined Breast Oncology and Plastic Surgery Practice Why It Works Anne M. Wallace, MD, FACS Director, Comprehensive Breast Health Center Professor of Clinical Surgery, Surgical Oncology
A Combined Practice A Combined Breast Oncology and Plastic Surgery Practice Why It Works Anne M. Wallace, MD, FACS Director, Comprehensive Breast Health Center Professor of Clinical Surgery, Surgical Oncology
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Xofluza) Reference Number: CP.PMN.185 Effective Date: 10.30.18 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Xofluza) Reference Number: CP.PMN.185 Effective Date: 10.30.18 Last Review Date: 11.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Dental Benefit Summary
 DMO Passive PPO Annual Deductible* Individual None $75 Family None $225 Preventive Service Covered Percent 100% 80% Basic Service Covered Percent 100% 50% Major Service Covered Percent 60% 50% Annual Benefit
DMO Passive PPO Annual Deductible* Individual None $75 Family None $225 Preventive Service Covered Percent 100% 80% Basic Service Covered Percent 100% 50% Major Service Covered Percent 60% 50% Annual Benefit
Medical Policy Hearing Devices. MassHealth* Connector/Qualified Health Plans* Authorization required X X No notification or authorization Not covered
 Medical Policy Hearing Devices Document Number: 019 Commercial and MassHealth* Connector/Qualified Health Plans* Authorization required X X No notification or authorization Not covered * Not all plans
Medical Policy Hearing Devices Document Number: 019 Commercial and MassHealth* Connector/Qualified Health Plans* Authorization required X X No notification or authorization Not covered * Not all plans
Coding Implications Revision Log. See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at the
Clinical Policy: (Eylea) Reference Number: CP.PHAR.184 Effective Date: 03.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at the
Clinical Policy: Digital Breast Tomosynthesis Reference Number: CP.MP.90
 Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.90 Effective Date: 01/18 Last Review Date: 12/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers
 ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
Non-voluntary dental (2-9) Nevada
 Non-voluntary dental (2-9) Option 1 DMO Access Option 2 Preventive Care PPO Option 3 PPO 1000 Option 4 PPO Active Option 5 PPO 2000 Plan 42 PPO 100/0/0 PPO 100/50/50 Preferred 100/80/50 Non-Preferred 80/60/50
Non-voluntary dental (2-9) Option 1 DMO Access Option 2 Preventive Care PPO Option 3 PPO 1000 Option 4 PPO Active Option 5 PPO 2000 Plan 42 PPO 100/0/0 PPO 100/50/50 Preferred 100/80/50 Non-Preferred 80/60/50
Dental Benefits Summary
 DMO Annual Deductible Individual Family Preventive Services 100% Basic Services 90% Major Services 60% Annual Benefit Maximum Office Visit Copay $5 Orthodontic Services Orthodontic Deductible Orthodontic
DMO Annual Deductible Individual Family Preventive Services 100% Basic Services 90% Major Services 60% Annual Benefit Maximum Office Visit Copay $5 Orthodontic Services Orthodontic Deductible Orthodontic
Clinical Policy: Pralatrexate (Folotyn) Reference Number: CP.PHAR.313 Effective Date: Last Review Date: Line of Business: Medicaid
 Clinical Policy: (Folotyn) Reference Number: CP.PHAR.313 Effective Date: 02.01.17 Last Review Date: 11.17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
Clinical Policy: (Folotyn) Reference Number: CP.PHAR.313 Effective Date: 02.01.17 Last Review Date: 11.17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
Dental Benefits Summary $1,000 Maximum
 Annual Deductible* Individual Family Preventive Services Basic Services Major Services Dental Benefits Summary $1,000 Maximum Participating (Negotiated Charge) $50 $100 100% 80% 50% Active PPO With PPO
Annual Deductible* Individual Family Preventive Services Basic Services Major Services Dental Benefits Summary $1,000 Maximum Participating (Negotiated Charge) $50 $100 100% 80% 50% Active PPO With PPO
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Imfinzi) Reference Number: CP.PHAR.339 Effective Date: 07.01.17 Last Review Date: 05.18 Line of Business: Medicaid, HIM-Medical Benefit Coding Implications Revision Log See Important
Clinical Policy: (Imfinzi) Reference Number: CP.PHAR.339 Effective Date: 07.01.17 Last Review Date: 05.18 Line of Business: Medicaid, HIM-Medical Benefit Coding Implications Revision Log See Important
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Sprycel) Reference Number: CP.PHAR.72 Effective Date: 07.01.18 Last Review Date: 05.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Sprycel) Reference Number: CP.PHAR.72 Effective Date: 07.01.18 Last Review Date: 05.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end of this policy
The number of breast reduction procedures COSMETIC. Liposuction Breast Reduction: A Prospective Trial in African American Women.
 COSMETIC Liposuction Breast Reduction: A Prospective Trial in African American Women Martin J. Moskovitz, M.D. Sherwood A. Baxt, M.D. Aridaman K. Jain, Ph.D. Robert E. Hausman, Ph.D. Paramus, N.J. Background:
COSMETIC Liposuction Breast Reduction: A Prospective Trial in African American Women Martin J. Moskovitz, M.D. Sherwood A. Baxt, M.D. Aridaman K. Jain, Ph.D. Robert E. Hausman, Ph.D. Paramus, N.J. Background:
Aetna Dental presents A Dental Benefit Summary for Michigan Voluntary Option 2; Freedom-of-Choice; No Ortho DMO
 Aetna Dental presents A Dental Benefit Summary for Michigan Voluntary Option 2; Freedom-of-Choice; No Ortho DMO PPO Max Annual Deductible* Individual None $75 Family None $225 Preventive Service Covered
Aetna Dental presents A Dental Benefit Summary for Michigan Voluntary Option 2; Freedom-of-Choice; No Ortho DMO PPO Max Annual Deductible* Individual None $75 Family None $225 Preventive Service Covered
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons
 Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Mastopexy. (Breast Uplift) Breast Care
 Mastopexy (Breast Uplift) Breast Care 1 Contents Introduction 2 What is a Mastopexy and what are the benefits? 2 Are there any alternatives to a Mastopexy? 3 Before the operation 3 The operation 3 After
Mastopexy (Breast Uplift) Breast Care 1 Contents Introduction 2 What is a Mastopexy and what are the benefits? 2 Are there any alternatives to a Mastopexy? 3 Before the operation 3 The operation 3 After
Authorized By: Elizabeth Connolly, Acting Commissioner, Department of Human
 HUMAN SERVICES 49 NJR 6(1) June 5, 2017 Filed May 10, 2017 DIVISION OF MENTAL HEALTH AND ADDICTION SERVICES Medical Necessity Review Tool for Substance Use Disorders Proposed New Rules: N.J.A.C. 10:163
HUMAN SERVICES 49 NJR 6(1) June 5, 2017 Filed May 10, 2017 DIVISION OF MENTAL HEALTH AND ADDICTION SERVICES Medical Necessity Review Tool for Substance Use Disorders Proposed New Rules: N.J.A.C. 10:163
Appendix C NEWBORN HEARING SCREENING PROJECT
 Appendix C NEWBORN HEARING SCREENING PROJECT I. WEST VIRGINIA STATE LAW All newborns born in the State of West Virginia must be screened for hearing impairment as required in WV Code 16-22A and 16-1-7,
Appendix C NEWBORN HEARING SCREENING PROJECT I. WEST VIRGINIA STATE LAW All newborns born in the State of West Virginia must be screened for hearing impairment as required in WV Code 16-22A and 16-1-7,
Page: 1. TRINET GROUP Effective Date: Dental Benefits Summary 80th OON R&C
 TRINET GROUP Effective Date: 10-01-2018 Dental Benefits Summary 80th OON R&C Active PPO With PPOII Network Participating Non-participating Annual Deductible* Individual $50 $100 Family $150 $300 Preventive
TRINET GROUP Effective Date: 10-01-2018 Dental Benefits Summary 80th OON R&C Active PPO With PPOII Network Participating Non-participating Annual Deductible* Individual $50 $100 Family $150 $300 Preventive
Clinical Policy: Netupitant and Palonosetron (Akynzeo) Reference Number: HIM.PA.113 Effective Date: Last Review Date: 05.
 Clinical Policy: (Akynzeo) Reference Number: HIM.PA.113 Effective Date: 05.01.17 Last Review Date: 05.18 Line of Business: HIM Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Akynzeo) Reference Number: HIM.PA.113 Effective Date: 05.01.17 Last Review Date: 05.18 Line of Business: HIM Revision Log See Important Reminder at the end of this policy for important
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Reference Number: HIM.PA.51 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this policy for
Clinical Policy: Reference Number: HIM.PA.51 Effective Date: 12/14 Last Review Date: 08/17 Line of Business: Health Insurance Marketplace Revision Log See Important Reminder at the end of this policy for
Clinical Policy: Naltrexone (Vivitrol) Reference Number: CP.PHAR.96 Effective Date: Last Review Date: Line of Business: Medicaid
 Clinical Policy: (Vivitrol) Reference Number: CP.PHAR.96 Effective Date: 03.01.12 Last Review Date: 02.19 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
Clinical Policy: (Vivitrol) Reference Number: CP.PHAR.96 Effective Date: 03.01.12 Last Review Date: 02.19 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
Clinical Policy: Naltrexone (Vivitrol) Reference Number: CP.PHAR.96 Effective Date: Last Review Date: Line of Business: Medicaid
 Clinical Policy: (Vivitrol) Reference Number: CP.PHAR.96 Effective Date: 03.01.12 Last Review Date: 02.18 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
Clinical Policy: (Vivitrol) Reference Number: CP.PHAR.96 Effective Date: 03.01.12 Last Review Date: 02.18 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
BREAST IMPLANT SURGERY INDIVIDUAL FUNDING REQUEST POLICY
 BREAST IMPLANT SURGERY INDIVIDUAL FUNDING REQUEST POLICY Version: Recommendation by: 1617.V2b Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 13 July 2016 Name of Originator/Author:
BREAST IMPLANT SURGERY INDIVIDUAL FUNDING REQUEST POLICY Version: Recommendation by: 1617.V2b Somerset CCG Clinical Commissioning Policy Forum (CCPF) Date Ratified: 13 July 2016 Name of Originator/Author:
Breast Lift (Mastopexy) Breast Lift (Mastopexy) Houston
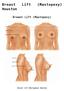 Breast Lift (Mastopexy) Houston Breast Lift (Mastopexy) Breast Lift (Mastopexy) Houston Breast Lift (Mastopexy) is a procedure that tightens firms and lifts the breasts. Patients who are satisfied with
Breast Lift (Mastopexy) Houston Breast Lift (Mastopexy) Breast Lift (Mastopexy) Houston Breast Lift (Mastopexy) is a procedure that tightens firms and lifts the breasts. Patients who are satisfied with
Breast Reconstruction Surgery after Mastectomy or Lumpectomy
 Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Medical Necessity Guidelines: Reconstructive and Cosmetic Surgery
 Medical Necessity Guidelines: Reconstructive and Cosmetic Surgery Effective: April 12, 2017 Clinical Documentation and Prior Authorization Coverage Guideline, No Prior Required Authorization Applies to:
Medical Necessity Guidelines: Reconstructive and Cosmetic Surgery Effective: April 12, 2017 Clinical Documentation and Prior Authorization Coverage Guideline, No Prior Required Authorization Applies to:
DENTAL CARE AND ORAL SURGERY
 UnitedHealthcare Benefits of Texas, Inc. 1. UnitedHealthcare of Oklahoma, Inc. 2. UnitedHealthcare of Oregon, Inc. 3. UnitedHealthcare of Washington, Inc. SIGNATUREVALUE BENEFIT INTERPRETATION POLICY DENTAL
UnitedHealthcare Benefits of Texas, Inc. 1. UnitedHealthcare of Oklahoma, Inc. 2. UnitedHealthcare of Oregon, Inc. 3. UnitedHealthcare of Washington, Inc. SIGNATUREVALUE BENEFIT INTERPRETATION POLICY DENTAL
