Breast Surgery Corporate Medical Policy
|
|
|
- Alan Bradley
- 5 years ago
- Views:
Transcription
1 File name: Breast Surgery File code: UM.SURG.17 Origination: 2016 Last Review: 11/2018 (PA List Review) Next Review: 11/2019 Effective Date: 04/01/2018 Breast Surgery Corporate Medical Policy Description/Summary This policy focuses on breast-related procedures that include mastectomy for cancer, prophylactic mastectomy, reconstruction, the management of breast implants, breast reductions, corrections for certain asymmetries. BCBVT covers medically necessary procedures related to physiological dysfunction, such as breast cancer, congenital and developmental disorders, infection, trauma, surgical complications and macromastia causing physiological dysfunction in men and women. BCBSVT considers procedures that are only performed to reshape normal structures of the body in order to improve one s appearance or self-esteem only, to be cosmetic and therefore non-covered as benefit exclusions. Policy Coding Information Click the links below for attachments, coding tables & instructions. Attachment I CPT / Coding Table Requests for breast surgery should be accompanied by the following documentation: The name and date of the proposed surgery. Date of accident or injury, if applicable History of present illness and/or conditions including diagnoses Documentation of diagnosis, functional impairment, pain or significant anatomic variance How the treatment can be reasonably expected to improve the functional impairment If applicable, the description of and CPT coding for planned staged procedure following acute repair, within two years of previous stage or initial primary repair Any additional information listed for a specific procedure as indicated for the specific procedures listed below. BCBSVT will review procedures intended to correct complications from a cosmetic procedure, whether the original procedure was medically necessary or a non-covered service. In order Page 1 of 12
2 for these corrections to be considered medically necessary the subsequent surgery needs to be reconstructive in nature (i.e. procedures performed on abnormal structures of the body, caused by congenital defects, developmental abnormalities, trauma, infection, tumors or disease. It is generally performed to improve function, but may also be done to approximate normal appearance). The procedures in this medical policy are considered medically necessary in accordance with the Women s Health and Cancer Rights Act of 1998, when performed as a breast reconstruction procedure following or in connection with mastectomy, breast conservation therapy (BCT) or other diagnostic procedures causing deformity of the breast, in connection with breast cancer, evaluation of breast cancer or suspected breast cancer, to prevent development of breast cancer in high risk patients, reconstruction following breast tissue destruction due to accidental injury, trauma, infection or disease (including other cancers). If the intended service relates to gender reassignment services, please refer to the BCBSVT Transgender Services medical policy. Policy and Guidelines Breast Prosthetics ( codes, *L8020, *L8030, *L8031, L8039, L8499, L8699) We consider the procedure medically necessary for the following indications: - A or a history of breast cancer - Following a mastectomy for cancer - Following a prophylactic mastectomy - For absence of the breast due to trauma, disease or infection - A diagnosis of Poland s syndrome We consider the procedure cosmetic and therefore not covered as a benefit exclusion when: - None of the above indications are met. - Obtained only to improve appearance or to improve one s self-esteem. *When billed with a prior approval is not required. Mastectomy for Gynecomastia (CPT code 19300) - surgery due to development of abnormally large mammary gland in biologically male individuals. We consider the procedure medical necessary for the following: - With a ; OR - If the criteria for a Prophylactic Mastectomy are met. - When all of the following are met: o Documented symptoms, including pain or tenderness directly related to the breast tissue, and which has a clinically significant impact upon normal activities of daily living despite non-narcotic analgesics and anti-inflammatory agents; AND o Appropriate diagnostic evaluation has been done for possible underlying etiology; AND o The tissue removed must be glandular breast tissue; and Page 2 of 12
3 o The extra tissue must not be the result of obesity, adolescence, or reversible effects of drug treatment that can be discontinued. (This includes drug-induced Gynecomastia remaining unresolved six months after cessation of the causative drug therapy; o Additionally, for those under 18 years of age, one of the following must be submitted as evidence of puberty completion.* * Evidence of puberty completion: Documented tanner stage IV or V for members aged 15-18, AND Stable height measurements for 6 months, OR Puberty completion as shown on wrist radiograph. We consider the procedure not medically necessary when any of the following is present: - Conservative attempts to control the pain or tenderness, such as non- narcotic analgesics and anti-inflammatory agents, have not been attempted. - Use of a medication known to cause gynecomastia has not been discontinued. - The appropriate diagnostic evaluation for etiology has not been completed. We consider the procedure cosmetic and therefore not covered as a benefit exclusion for the following circumstances (not an all-inclusive list): - The tissue being removed is not glandular in nature; OR - The medically necessary criteria above is not met and the procedure is intended only to improve appearance or to improve one s emotional well-being. Prophylactic Mastectomy (CPT codes 19303, 19304) Surgical removal of breasts due to a high cancer risk. It is strongly recommended that all candidates for prophylactic mastectomy undergo counseling regarding cancer risks from a health professional skilled in assessing cancer risk other than the operating surgeon and discussion of the various treatment options, including increased surveillance or chemoprevention with the appropriate medication e.g. tamoxifen or raloxifene. Patients with a high risk of breast cancer may be defined as one or more of the following: a known BRCA1 or BRCA2 mutation or at high risk of BRCA1 or BRCA2 mutation due to a known presence of the mutation in relatives or another gene mutation associated with increased risk (eg, PTEN, TP53, CDH1, and STK11) or Li-Fraumeni syndrome or Cowden syndrome or Bannayan-Riley-Ruvalcaba syndrome or a first-degree relative with one of these syndromes or high risk (lifetime risk about 20% to 25% or greater) of developing breast cancer as identified by models that are largely defined by family history or received radiotherapy to the chest between 10 and 30 years of age We consider the procedure medically necessary for any of the following: - In patients at high risk of breast cancer as defined above. - In patients with inflammatory breast cancer. - In patients with lobular carcinoma in situ. Page 3 of 12
4 - In patients with such extensive mammographic abnormalities (i.e. calcifications) that adequate biopsy or excision is impossible. We consider the procedure investigational for all other indications, including, but not limited to contralateral prophylactic mastectomy in women with breast cancer who do not meet the high risk criteria as defined above. Breast Reconstruction (CPT codes 15777, 19340*, 19342*, 19350*, 19357*, 19361*, 19364*, 19366*, 19367*, 19368*, 19369*, 19380*). Utilization of natural or artificial tissue to reconstruct breasts following mastectomy, breast conservation therapy, burns, trauma and diagnostic deformity We consider reconstruction medically necessary for any of the following: - For the affected breast o When breast tissue is affected by disease, trauma, burns or infection o When performed in connection with cancer, the evaluation of cancer, the evaluation of suspected cancer (i.e. following biopsy or lumpectomy), or the prevention of breast cancer development in high risk patients; OR o For prostheses and physical complications* of all stages of mastectomy, breast conservation therapy (BCT) or other diagnostic procedures causing deformity (i.e. following biopsy or lumpectomy) including lymphedema treatment. *Physical complications of a staged mastectomy may include, but is not limited to, abdominal scar revision/release related to prior tissue needed for breast reconstruction. o Following the removal of a ruptured silicone gel-filled implant o For the unaffected breast in order to create a symmetrical appearance *When billed with a, following an approved mastectomy, prior approval is not required. o We consider the following cosmetic and therefore not covered as a benefit exclusion: o Breast reconstruction following mastectomy for gynecomastia. Notes: o Breast reconstruction utilizing autologous fat grafting to the breast with adipose-derived stem cells is considered investigational. CPT code should represent autologous fat grafting from direct harvest and is appropriate. o Allograft material for use in breast reconstructive therapy ( Q4100, Q4107, Q4116, Q4122,& Q4128) Implant repositioning Inverted nipple correction- (CPT 19355) Mastopexy (CPT 19316) o If the preceding criteria for Breast Reconstruction are not met, the following procedures are considered cosmetic and therefore not covered as a benefit exclusion: Mastopexy (CPT 19316) Inverted nipple correction (CPT 19355) Page 4 of 12
5 Implant repositioning Tattooing of the nipple and/or areola (CPT Codes 11920, 11921, 11922) Reduction Mammoplasty (CPT code 19318) Surgical reduction of breasts in women due to size and persistent symptoms. We consider the procedure medically necessary for the treatment of macromastia for the following: - Breast size is stable for six to twelve months prior to surgery. AND - A minimum of 6-weeks of two persistent well-documented symptoms which impair function such as shoulder, neck, or back pain or pain interfering with sleep related to macromastia that is not responsive to conservative therapy, such as an appropriate support bra, exercises, heat/cold treatment, and appropriate nonsteroidal anti-inflammatory agents/muscle relaxants OR - One of the above symptoms AND recurrent or chronic intertrigo between the pendulous breast and the chest wall that is resistant to topical treatment. - Additionally, for those under 18 years of age, one of the following must be submitted as evidence of puberty completion.* * Evidence of puberty completion: Documented tanner stage IV or V for members aged 15-18, AND Stable height measurements for 6 months, OR Puberty completion as shown on wrist radiograph. Breast Reduction (Reduction Mammaplasty CPT 19318) is considered medically necessary when in connection with breast reconstruction following a mastectomy. - We consider reduction mammoplasty cosmetic and therefore not covered as a benefit exclusion for any of the following: o Performed in order to improve athletic performance OR o Obtained only to improve appearance or to improve one s self-esteem - We consider the procedure investigational for all other indications not outlined above. Removal of implant(s) (CPT codes 19328, 19330); insertion of implant(s) (CPT codes 19340, 19342, C1789); Periprosthetic capsulotomy or capsulectomy (CPT codes 19370, 19371): the removal and replacement of breast implants originally placed following mastectomy. - Additional Documentation Required: o Date of implantation and type of implant o Objective evidence of leakage o Baker Contracture Class* - We consider the procedure medically necessary for the any of the following: o Explantation (removal of implant) of a silicone gel-filled or saline filled Page 5 of 12
6 implant with a documented implant rupture, extrusion, Baker Class IV* contracture, orsurgical treatment of cancer or other disease, infection, trauma or burn. - We consider the procedures not medically necessary for the any of the following: o Explantation of a when the original reconstruction was for cosmetic reasons and the medically necessary criteria above is not met. o Systemic symptoms, attributed to connective tissue diseases, autoimmune diseases, etc.; o Baker class III contractures in patients with implants for cosmetic purposes; o Rupture of a saline implant in patients with implants for cosmetic purposes; o Pain not related to contractures or rupture. - We consider the procedure cosmetic and therefore not covered as a benefit exclusion for the following: o Obtained only to improve appearance or to improve one s self-esteem. *Baker Classification of breast contractures: Grade I: Augmented breast feels as soft as a normal breast Grade II: Breast is less soft and the implant can be palpated but is not visible. Grade III: Breast is firm, palpable and the implant (or its distortion) is visible Grade IV: Breast is hard, painful, cold, tender and distorted Unilateral Breast Surgery for Asymmetry reduction mammoplasty (CPT code 19318) and/or augmentation mammoplasty (CPT codes & 19325) - surgical reconstruction in females of one breast by either reducing or enlarging. - Additional documentation Required: o History and physical findings o Height and weight o Size of each breast o Date of previous surgery, if applicable o Pathologic diagnosis, if applicable o Estimate of amount of tissue to be removed in a reduction or size of implant for augmentation. o Additionally, for those under 18 years of age, one of the following must be submitted as evidence of puberty completion.* * Evidence of puberty completion: Documented tanner stage IV or V for members aged 15-18, AND Stable height measurements for 6 months, OR Puberty completion as shown on wrist radiograph. - We consider the procedures medically necessary for any of the following Page 6 of 12
7 indications: o Biological females must be at least 15 years of age and have reached puberty*, and have a diagnosis of Poland s syndrome (congenital absence of breasts) OR o A disfiguring traumatic accident (e.g. burn) or complication of medical treatment (e.g. necrosis) OR o A breast infection resulting in disfigurement - We consider procedures for the following cosmetic and therefore not covered as a benefit exclusion: o Unilateral augmentation or reduction mammoplasty intended to create symmetry between otherwise normal breasts and the medically necessary criteria above is not met OR o Unilateral augmentation or reduction mammoplasty intended only to improve appearance or to improve a one s self-esteem Reference Resources 1. Blue Cross and Blue Shield Association. Medical Policy Reference Manual # Surgical Treatment of Bilateral Gynecomastia, last reviewed: February Blue Cross and Blue Shield Association. Medical Policy Reference Manual # Adipose-Derived Stem Cells in Autologous Fat Grafting to the Breast, last reviewed: October Blue Cross and Blue Shield Association. Medical Policy Reference Manual # Prophylactic Mastectomy, last reviewed: February Cuhaci, N., Polat, S.B., Evranos, B., Esroy, R. and Cakir, B. Gynecomastia: clinical evaluation and management. Indian Journal of Endocrinology and Metabolism Mar- Apr; 18(2): Kerrigan, C.L., Collins, E.D., Kim, H.M., Schnurr, P.L., Cunningham, B. and Lowery, J. Reduction mammoplasty: defining medical necessity. Medical Decision Making May- Jun; 22(3): Wolfswinkel, B.S., Lemaine, V., Weathers, W. M., Chike-Obi, C.J, Xue, A.S. and Heller, L. Hyperplastic Breast Anomalies in the Female Adolescent Breast. Seminars in Plastic Surgery Feb; Related Policies BCBSVT Medical Policy on Transgender Services BCBSVT Medical Policy on Bioengineered Skin and Soft Tissue Substitutes Document Precedence Blue Cross and Blue Shield of Vermont (BCBSVT) Medical Policies are developed to provide clinical guidance and are based on research of current medical literature and review of common medical practices in the treatment and diagnosis of disease. The applicable group/individual contract and member certificate language, or employer s benefit plan if an ASO group, determines benefits that are in effect at the time of service. Since medical practices and knowledge are constantly evolving, BCBSVT reserves the right to review and revise its medical policies periodically. To the extent that there may be any conflict between Page 7 of 12
8 medical policy and contract/employer benefit plan language, the member s contract/employer benefit plan language takes precedence Audit Information BCBSVT reserves the right to conduct audits on any provider and/or facility to ensure compliance with the guidelines stated in the medical policy. If an audit identifies instances of non-compliance with this medical policy, BCBSVT reserves the right to recoup all noncompliant payments. Benefit Determination Guidance Administrative and Contractual Guidance Prior approval is required and benefits are subject to all terms, limitations and conditions of the subscriber contract. Incomplete authorization requests may result in a delay of decision pending submission of missing information. To be considered compete, see policy guidelines above. NEHP/ABNE members may have different benefits for services listed in this policy. To confirm benefits, please contact the customer service department at the member s health plan. Coverage varies according to the member s group or individual contract. Not all groups are required to follow the Vermont legislative mandates. Member Contract language takes precedence over medical policy when there is a conflict. If the member receives benefits through an Administrative Services Only (ASO) group, benefits may vary or not apply. To verify benefit information, please refer to the member s employer benefit plan documents or contact the customer service department. Language in the employer benefit plan documents takes precedence over medical policy when there is a conflict. Policy Implementation/Update information 08/2016 New policy. 12/2016 Added CPT Code for clarification in medical policy. 08/2017 Reviewed and voted at HPC 08/07/2017 with the following: Updated related policies section, removed language under prophylactic mastectomy section, added CPT code as medically necessary, removed language under breast reconstruction added additional medical criteria under breast reconstruction, added coding table to align with codes contained within the medical policy, removed language under removal of implants, removed language under unilateral breast surgery for asymmetry. 07/2018 Added CPT code to require PA Page 8 of 12
9 11/2018 Added code C1789 & Q4122, to require prior authorization. codes L2999, L3999, L5999 & L7499 removed from body of policy. Added related policy section. Eligible providers Qualified healthcare professionals practicing within the scope of their license(s). Approved by BCBSVT Medical Directors Date Approved Joshua Plavin, MD, MPH, MBA Chief Medical Officer Kate McIntosh, MD, FAAP Senior Medical Director Attachment I CPT / Coding Table Code Type Number Brief Description Policy Instructions The following codes will be considered as medically necessary when applicable criteria have been met. Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; 6.0 sq cm or CPT less CPT Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; 6.1 to 20.0 sq cm CPT Tattooing, intradermal introduction of insoluble opaque pigments to correct color defects of skin, including micropigmentation; each additional 20.0 sq cm, or part thereof (List separately in addition to code for primary procedure) Page 9 of 12
10 Code Type Number Brief Description Policy Instructions CPT Implantation of biologic implant (eg, acellular dermal matrix) for soft tissue reinforcement (ie, breast, trunk)(list separately in addition to code for primary procedure) CPT Mastectomy for gynecomastia CPT Mastectomy, partial (eg, lumpectomy, tylectomy, quadrantectomy, segmentectomy) CPT Mastectomy, partial (eg, lumpectomy, tylectomy, quadrantectomy, segmentectomy); with axillary lymphadenectomy CPT Mastectomy, simple, complete CPT Mastectomy, subcutaneous CPT Mastopexy CPT Reduction mammaplasty CPT Mammaplasty, augmentation; without prosthetic implant CPT Mammaplasty, augmentation; with prosthetic implant CPT Removal of intact mammary implant CPT Removal of mammary implant material CPT Immediate insertion of breast prosthesis following mastopexy, mastectomy or in reconstruction CPT Delayed insertion of breast prosthesis following mastopexy, mastectomy or in reconstruction CPT Nipple/areola reconstruction CPT Correction of inverted nipples Page 10 of 12
11 Code Type Number Brief Description Policy Instructions CPT Breast reconstruction, immediate or delayed, with tissue expander, including subsequent expansion CPT CPT CPT CPT CPT CPT CPT CPT Breast reconstruction with latissimus dorsi flap, without prosthetic implant Breast reconstruction with free flap Breast reconstruction with other technique Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), single pedicle, including closure of donor site; with microvascular anastomosis (supercharging) Breast reconstruction with transverse rectus abdominis myocutaneous flap (TRAM), double pedicle, including closure of donor site Open periprosthetic capsulotomy, breast Preprosthetic capsulectomy, breast CPT Revision of reconstructed breast CPT CPT Preparation of moulage for custom breast implant Tissue grafts, other (eg, paratenon, fat, dermis) C1789 Prosthesis, breast (implantable) L8020 Breast prosthesis, mastectomy form Page 11 of 12
12 Code Type Number Brief Description Policy Instructions L8030 Breast prosthesis, silicone or equal, without integral adhesive L8031 Breast prosthesis, silicone or equal with integral adhesive L8039 Breast prosthesis, not otherwise specified L8499 Unlisted procedure for miscellaneous prosthetic services L8699 Q4100 Q4107 Prosthetic implant, not otherwise specified Skin substitute, not otherwise specified Graftjacket, per square centimeter Q4116 Alloderm, per square centimeter Q4122 DermACELL, per sq cm Q4128 S2066 S2067 S2068 FlexHD, Allopatch HD, or Matrix HD, per square centimeter Breast reconstruction with gluteal artery perforator (GAP) flap, including harvesting of the flap, microvascular transfer, closure of donor site and shaping the flap into a breast, unilateral Breast reconstruction of a single breast with stacked deep inferior epigastric perforator (DIEP) flap(s) and/or gluteal artery perforator (GAP) flap(s), including harvesting of the flap(s), microvascular transfer, closure of donor site(s) and shaping the flap into a breast, unilateral Breast reconstruction with deep inferior epigastric perforator (DIEP) flap, or superficial inferior epigastric artery (SIEA) flap, including harvesting of the flap, microvascular transfer, closure of donor site and shaping the flap into a breast, unilateral Page 12 of 12
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
Breast Reconstructive Surgery After Mastectomy Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Breast Reconstructive Surgery After Mastectomy PRE-DETERMINATION
Breast Reconstruction Surgery
 Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
Breast Reconstruction Surgery I. Policy University Health Alliance (UHA) will reimburse for Breast Reconstruction Surgery when it is determined to be medically necessary and when it meets the medical criteria
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: January 1, 2019 Table of Contents Page INSTRUCTIONS FOR USE...
Medical Policy Original Effective Date: Revised Date: Page 1 of 8
 Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
Page 1 of 8 Disclaimer Description Coverage Determination Refer to the member s specific benefit plan and Schedule of Benefits to determine coverage. This may not be a benefit on all plans, or the plan
BREAST RECONSTRUCTION POST MASTECTOMY
 UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
UnitedHealthcare Commercial Coverage Determination Guideline BREAST RECONSTRUCTION POST MASTECTOMY Guideline Number: SUR057 Effective Date: February 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
Medical Review Criteria Breast Surgeries Subject: Breast Surgeries Authorization: Prior authorization is required for the following procedures requested for members enrolled in HPHC commercial (HMO, POS,
Medical Review Criteria Breast Surgeries
 Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
Medical Review Criteria Breast Surgeries Effective Date: November 8, 2016 Subject: Breast Surgeries Policy: HPHC covers medically necessary breast surgeries including mastectomy, breast reconstruction,
BREAST RECONSTRUCTION/REMOVAL AND REPLACEMENT OF IMPLANTS
 Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent upon
Corporate Medical Policy
 Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 8/2017 8/2018 8/2017 Description of Procedure or Service Mastectomy
Corporate Medical Policy Breast Surgeries File Name: Origination: Last CAP Review: Next CAP Review: Last Review: breast_surgeries 1/2000 8/2017 8/2018 8/2017 Description of Procedure or Service Mastectomy
Breast Reconstruction Following Mastectomy or Lumpectomy
 Breast Reconstruction Following Mastectomy or Lumpectomy [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas
Breast Reconstruction Following Mastectomy or Lumpectomy [For the list of services and procedures that need preauthorization, please refer to www.mcs.com.pr go to Comunicados a Proveedores, and click Cartas
Reconstructive Breast Surgery and Management of Breast Implants
 Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Reconstructive Breast Surgery and Management of Breast Implants Policy Number: 7.01.22 Last Review: 1/2018 Origination: 3/1993 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Breast Reconstruction Surgery after Mastectomy or Lumpectomy
 Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Breast Reconstruction Surgery after Mastectomy or Lumpectomy Date of Origin: 11/1998 Last Review Date: 11/25/2017 Effective Date: 11/25/2017 Dates Reviewed: 08/2000, 09/2001, 11/2003, 11/2004, 12/2005,
Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus and Colon Corporate Medical Policy
 Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus and Colon Corporate Medical Policy File Name: Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders
Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders of the Small Bowel, Esophagus and Colon Corporate Medical Policy File Name: Wireless Capsule Endoscopy as a Diagnostic Technique in Disorders
Medical Policy Original Effective Date: Revised Date: 09/26/2018 Page 1 of 26
 Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
Contents: This includes the following items: 1. Breast Reconstruction Following Mastectomy: Page 1 of 26 2. Breast Implant Removal and/or Replacement and Capsulectomy: 3. Breast Reduction Mammaplasty for
Ambulatory Cardiac Monitors and Outpatient Telemetry Corporate Medical Policy
 Ambulatory Cardiac Monitors and Outpatient Telemetry Corporate Medical Policy File Name: Ambulatory Event Monitors and Mobile Cardiac Outpatient Telemetry File Code: UM.SPSVC.13 Origination: 10/2015 Last
Ambulatory Cardiac Monitors and Outpatient Telemetry Corporate Medical Policy File Name: Ambulatory Event Monitors and Mobile Cardiac Outpatient Telemetry File Code: UM.SPSVC.13 Origination: 10/2015 Last
Cochlear Implant Corporate Medical Policy
 Cochlear Implant Corporate Medical Policy File Name: Cochlear Implant & Aural Rehabilitation File Code: UM.REHAB.06 Origination: 03/2015 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019
Cochlear Implant Corporate Medical Policy File Name: Cochlear Implant & Aural Rehabilitation File Code: UM.REHAB.06 Origination: 03/2015 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019
Pediatric Neurodevelopmental and Autism Spectrum Disorder (ASD) Screening Corporate Medical Policy
 Pediatric Neurodevelopmental and Autism Spectrum Disorder (ASD) Screening Corporate Medical Policy File name: Pediatric Neurodevelopmental and Autism Spectrum Disorder (ASD) Screening File Number: UM.DIAG.03
Pediatric Neurodevelopmental and Autism Spectrum Disorder (ASD) Screening Corporate Medical Policy File name: Pediatric Neurodevelopmental and Autism Spectrum Disorder (ASD) Screening File Number: UM.DIAG.03
Nonpharmacologic Treatment of Rosacea Corporate Medical Policy
 Nonpharmacologic Treatment of Rosacea Corporate Medical Policy File Name: Nonpharmacologic Treatment of Rosacea. File Code: UM.SURG.11 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019
Nonpharmacologic Treatment of Rosacea Corporate Medical Policy File Name: Nonpharmacologic Treatment of Rosacea. File Code: UM.SURG.11 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019
Neuromuscular Electrical Stimulator (NMES) Corporate Medical Policy
 Neuromuscular Electrical Stimulator (NMES) Corporate Medical Policy File name: Neuromuscular Electrical Stimulator (NMES) File Code: UM.NS.04 Origination: 05/01/2007 Last Review: 06/2018 Next Review: 06/2019
Neuromuscular Electrical Stimulator (NMES) Corporate Medical Policy File name: Neuromuscular Electrical Stimulator (NMES) File Code: UM.NS.04 Origination: 05/01/2007 Last Review: 06/2018 Next Review: 06/2019
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Reconstructive Breast Surgery Policy Number: MP-052-MD-DE Approved By: Medical Management Medical Policy Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017
CLINICAL MEDICAL POLICY Policy Name: Reconstructive Breast Surgery Policy Number: MP-052-MD-DE Approved By: Medical Management Medical Policy Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017
Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC
 Downloaded from Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC What is Breast Reconstruction? Reconstruction of the breast involves recreating
Downloaded from Reconstruction of the Breast after Cancer An Overview of Procedures and Options by Karen M. Horton, MD, MSc, FRCSC What is Breast Reconstruction? Reconstruction of the breast involves recreating
External Insulin Pumps Corporate Medical Policy
 File Name: External Insulin Pumps File Code: UM.DME.02 Origination: 04/2006 Last Review: 11/2018 Next Review: 11/2019 Effective Date: 04/01/2019 External Insulin Pumps Corporate Medical Policy Description/Summary
File Name: External Insulin Pumps File Code: UM.DME.02 Origination: 04/2006 Last Review: 11/2018 Next Review: 11/2019 Effective Date: 04/01/2019 External Insulin Pumps Corporate Medical Policy Description/Summary
Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate surgical options
 A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
A Quality Initiative of the Program in Evidence-Based Care (PEBC), Cancer Care Ontario (CCO) Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate
Gastric Electrical Stimulation Corporate Medical Policy
 File name: Gastric Electrical Stimulation File code: UM.NS.06 Origination: 2007 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018 Description/Summary Gastric Electrical Stimulation Corporate
File name: Gastric Electrical Stimulation File code: UM.NS.06 Origination: 2007 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018 Description/Summary Gastric Electrical Stimulation Corporate
Occipital Nerve Stimulation Corporate Medical Policy
 Occipital Nerve Stimulation Corporate Medical Policy File Name: Occipital Nerve Stimulation File Code: UM.SPSVC.14 Origination: 2011 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018
Occipital Nerve Stimulation Corporate Medical Policy File Name: Occipital Nerve Stimulation File Code: UM.SPSVC.14 Origination: 2011 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018
Bariatric Surgery Corporate Medical Policy
 Bariatric Surgery Corporate Medical Policy File name: Bariatric Surgery File code: UM.SURG.01 Origination: 07/2008 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018 Description/Summary
Bariatric Surgery Corporate Medical Policy File name: Bariatric Surgery File code: UM.SURG.01 Origination: 07/2008 Last Review: 06/2018 Next Review: 06/2019 Effective Date: 10/01/2018 Description/Summary
Breast Reconstruction: Current Strategies and Future Opportunities
 Breast Reconstruction: Current Strategies and Future Opportunities Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery
Breast Reconstruction: Current Strategies and Future Opportunities Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery
Breast Reconstruction Options
 Breast Reconstruction Options Natural reconstruction using your ABDOMINAL tissue: TRAM Flap (Transverse Rectus Abdominis Myocutaneous) There are various forms of TRAM flap reconstruction that are commonly
Breast Reconstruction Options Natural reconstruction using your ABDOMINAL tissue: TRAM Flap (Transverse Rectus Abdominis Myocutaneous) There are various forms of TRAM flap reconstruction that are commonly
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Corporate Medical Policy
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Corporate Medical Policy File name: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures File code:
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures Corporate Medical Policy File name: Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures File code:
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. Oncoplastic and Reconstructive Surgery
 Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Oncoplastic and Reconstructive Surgery Plastic-reconstructive aspects after mastectomy Versions 2002 2017: Audretsch / Bauerfeind
Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer Oncoplastic and Reconstructive Surgery Plastic-reconstructive aspects after mastectomy Versions 2002 2017: Audretsch / Bauerfeind
Prophylactic Mastectomy & Reconstructive Implications
 Prophylactic Mastectomy & Reconstructive Implications Minas T Chrysopoulo, MD PRMA Center For Advanced Breast Reconstruction Prophylactic Mastectomy Surgical removal of one or both breasts to reduce the
Prophylactic Mastectomy & Reconstructive Implications Minas T Chrysopoulo, MD PRMA Center For Advanced Breast Reconstruction Prophylactic Mastectomy Surgical removal of one or both breasts to reduce the
Current Strategies in Breast Reconstruction
 Current Strategies in Breast Reconstruction Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery 12 th Annual School of
Current Strategies in Breast Reconstruction Hani Sbitany, MD Assistant Professor of Surgery University of California, San Francisco Division of Plastic and Reconstructive Surgery 12 th Annual School of
Breast Reconstruction
 Steven E. Copit, M.D. Chief- Division of Plastic Surgery Thomas Jefferson University Hospital Philadelphia, PA analysis of The Defect Skin Breast Volume Nipple Areola Complex analysis of The Defect the
Steven E. Copit, M.D. Chief- Division of Plastic Surgery Thomas Jefferson University Hospital Philadelphia, PA analysis of The Defect Skin Breast Volume Nipple Areola Complex analysis of The Defect the
NUTRITIONAL COUNSELING Corporate Medical Policy
 NUTRITIONAL COUNSELING Corporate Medical Policy File Name: Nutritional Counseling File Code: RB.NC.01 Origination: 04/2002 Last Review: 07/2018 Next Review: 07/2019 Effective Date: 11/01/2018 Description/Summary
NUTRITIONAL COUNSELING Corporate Medical Policy File Name: Nutritional Counseling File Code: RB.NC.01 Origination: 04/2002 Last Review: 07/2018 Next Review: 07/2019 Effective Date: 11/01/2018 Description/Summary
Medical Policy Breast Surgeries
 Medical Policy Breast Surgeries Document Number: 006 Commercial and MassHealth Connector/Qualified Health Plans Authorization required Breast Reconstruction Surgeries; Reduction Mammoplasty, Female Members;
Medical Policy Breast Surgeries Document Number: 006 Commercial and MassHealth Connector/Qualified Health Plans Authorization required Breast Reconstruction Surgeries; Reduction Mammoplasty, Female Members;
Breast Reconstruction. Westmead Breast Cancer Institute
 Breast Reconstruction Westmead Breast Cancer Institute What is breast reconstruction? Breast reconstruction is a surgical procedure that creates a shape on the chest wall following a mastectomy. Occasionally,
Breast Reconstruction Westmead Breast Cancer Institute What is breast reconstruction? Breast reconstruction is a surgical procedure that creates a shape on the chest wall following a mastectomy. Occasionally,
Chapter 11 Worksheet Code It
 Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Class: Date: Chapter 11 Worksheet 3 2 1 Code It True/False Indicate whether the statement is true or false. 1. Surgical destruction is considered part of the surgical procedure description. 2. Prepping
Name of Policy: Reduction Mammaplasty
 Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: November 2013 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross
Name of Policy: Reduction Mammaplasty Policy #: 056 Latest Review Date: November 2013 Category: Surgery Policy Grade: D Background/Definitions: As a general rule, benefits are payable under Blue Cross
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers
 ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
NUTRITIONAL COUNSELING Corporate Medical Policy
 NUTRITIONAL COUNSELING Corporate Medical Policy File name: Nutritional Counseling File code: RB.NC.01 Origination: 4/2002 Last Review: 10/2017 Next Review: 10/2018 Effective Date: 05/01/2018 Description/Summary
NUTRITIONAL COUNSELING Corporate Medical Policy File name: Nutritional Counseling File code: RB.NC.01 Origination: 4/2002 Last Review: 10/2017 Next Review: 10/2018 Effective Date: 05/01/2018 Description/Summary
Reconstructive and Cosmetic Services
 Reconstructive and Cosmetic Services Policy Number: 10.01.09 Last Review: 4/2018 Origination: 2/2006 Next Review: 4/2019 Policy Determination of whether a proposed therapy would be considered reconstructive
Reconstructive and Cosmetic Services Policy Number: 10.01.09 Last Review: 4/2018 Origination: 2/2006 Next Review: 4/2019 Policy Determination of whether a proposed therapy would be considered reconstructive
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons
 Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Advances and Innovations in Breast Reconstruction and Brest Surgery Presented by PCMC plastic surgeons Options for reconstruction after mastectomy Implants Autologous tissue = from your own body: skin
Breast Restoration Surgery After a mastectomy
 UW MEDICINE PATIENT EDUCATION Breast Restoration Surgery After a mastectomy This handout explains the most common procedures that are used at University of Washington Medical Center (UWMC) to restore a
UW MEDICINE PATIENT EDUCATION Breast Restoration Surgery After a mastectomy This handout explains the most common procedures that are used at University of Washington Medical Center (UWMC) to restore a
Prophylactic Mastectomy
 Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 09/01/2015 Section: Surgery Place(s) of Service:
Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 09/01/2015 Section: Surgery Place(s) of Service:
2017 PHYSICIAN CODING GUIDE
 2017 PHYSICIAN CODING GUIDE Breast Repair and/or Reconstruction Surgeon s Procedure Codes 11970 Replacement of tissue expander with permanent prosthesis 8.01 $628.05 +15777 Implantation of biologic implant
2017 PHYSICIAN CODING GUIDE Breast Repair and/or Reconstruction Surgeon s Procedure Codes 11970 Replacement of tissue expander with permanent prosthesis 8.01 $628.05 +15777 Implantation of biologic implant
Breast Reconstruction: Patient Information Document
 breastreconstructioncanada.ca Breast Reconstruction: Patient Information Document By Dr. Nicolas Guay Dr. Haemi Lee STANDARDIZED BREAST RECONSTRUCTION PATIENT INFORMATION TABLE OF CONTENTS Glossary...
breastreconstructioncanada.ca Breast Reconstruction: Patient Information Document By Dr. Nicolas Guay Dr. Haemi Lee STANDARDIZED BREAST RECONSTRUCTION PATIENT INFORMATION TABLE OF CONTENTS Glossary...
Classification System
 Classification System A graduate of the Breast Oncology training program should be able to care for all aspects of disease and/or provide comprehensive management. When referring to a discipline of training
Classification System A graduate of the Breast Oncology training program should be able to care for all aspects of disease and/or provide comprehensive management. When referring to a discipline of training
Prophylactic Mastectomy
 Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Surgery Place(s) of Service: Inpatient I.
Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Surgery Place(s) of Service: Inpatient I.
Prophylactic Mastectomy
 Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO 08/24/2012 Section: Surgery Place(s) of Service: Inpatient I.
Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO 08/24/2012 Section: Surgery Place(s) of Service: Inpatient I.
Advances in Breast Surgery. Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015
 Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
Advances in Breast Surgery Catherine Campo, D.O. Breast Surgeon Meridian Health System April 17, 2015 Objectives Understand the surgical treatment of breast cancer Be able to determine when a lumpectomy
Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C. Your Guide To BREAST RECONSTRUCTION
 Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C Your Guide To BREAST RECONSTRUCTION Introduction The diagnosis of breast cancer begins a journey of making many informed decisions
Neil J. Zemmel, MD, FACS Steven J. Montante, MD Megan J. Russell, PA-C Your Guide To BREAST RECONSTRUCTION Introduction The diagnosis of breast cancer begins a journey of making many informed decisions
Breast Cancer Imaging
 Breast Cancer Imaging I. Policy University Health Alliance (UHA) will cover breast imaging when such services meet the medical criteria guidelines (subject to limitations and exclusions) indicated below.
Breast Cancer Imaging I. Policy University Health Alliance (UHA) will cover breast imaging when such services meet the medical criteria guidelines (subject to limitations and exclusions) indicated below.
In a second stage or a second operation that tissue expander is removed through the same incision and the implant is placed within the chest pocket.
 Hello, I m Summer Hanson. I m an assistant professor in the Department of Plastics & Reconstructive Surgery at The University of Texas MD Anderson Cancer Center and today I m going to talk about the role
Hello, I m Summer Hanson. I m an assistant professor in the Department of Plastics & Reconstructive Surgery at The University of Texas MD Anderson Cancer Center and today I m going to talk about the role
Dry Needling of Myofascial Trigger Points Corporate Medical Policy
 Dry Needling of Myofascial Trigger Points Corporate Medical Policy File Name: Dry Needling of Myofascial Trigger Points File Code: UM.REHAB.09 Origination: 04/2015 Last Review: 09/2018 Next Review: 09/2019
Dry Needling of Myofascial Trigger Points Corporate Medical Policy File Name: Dry Needling of Myofascial Trigger Points File Code: UM.REHAB.09 Origination: 04/2015 Last Review: 09/2018 Next Review: 09/2019
The Case FOR Oncoplastic Surgery in Small Breasts. Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA
 The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
The Case FOR Oncoplastic Surgery in Small Breasts Barbara L. Smith, MD, PhD Massachusetts General Hospital Harvard Medical School Boston, MA USA Changing issues in breast cancer management Early detection
Selected Operative Procedure Categories for KNHSS SSI Surveillance
 Selected Operative Procedure Categories for KNHSS SSI Surveillance Breast Surgery Excision of lesion or tissue of breast including radical, modified, or quadrant resection, lumpectomy, incisional biopsy,
Selected Operative Procedure Categories for KNHSS SSI Surveillance Breast Surgery Excision of lesion or tissue of breast including radical, modified, or quadrant resection, lumpectomy, incisional biopsy,
Protocol. Reconstructive Breast Surgery/Management of Breast Implants
 Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
Protocol Reconstructive Breast Surgery/Management of Breast Implants Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization Yes Review Dates: 02/07, 02/08, 01/09, 01/10, 01/11,
National Medical Policy
 National Medical Policy Subject: Policy Number: Breast Reconstructive Surgery NMP492 Effective Date*: February 2013 Updated: April 2016 This National Medical Policy is subject to the terms in the IMPORTANT
National Medical Policy Subject: Policy Number: Breast Reconstructive Surgery NMP492 Effective Date*: February 2013 Updated: April 2016 This National Medical Policy is subject to the terms in the IMPORTANT
Supplementary Online Content
 Supplementary Online Content Abt NB, Flores JM, Baltodano PA, et al. Neoadjuvant chemotherapy and short-term in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. Published
Supplementary Online Content Abt NB, Flores JM, Baltodano PA, et al. Neoadjuvant chemotherapy and short-term in patients undergoing mastectomy with and without breast reconstruction. JAMA Surg. Published
Breast Cancer Reconstruction
 Breast Cancer Jerome H. Liu, MD Tom S. Liu, MD Jerome H. Liu, MD Undergraduate: Brown University Medical School: University of California, Los Angeles Residency: UCLA Medical Center Fellowship:UCLA Medical
Breast Cancer Jerome H. Liu, MD Tom S. Liu, MD Jerome H. Liu, MD Undergraduate: Brown University Medical School: University of California, Los Angeles Residency: UCLA Medical Center Fellowship:UCLA Medical
Breast debridement and closure cpt
 Breast debridement and closure cpt Close Breast debridement cpt code Medicare Billing Guidelines, Medicare payment and reimbursment, Medicare codes. Here is a list of CPT codes and Diagnoses that are.
Breast debridement and closure cpt Close Breast debridement cpt code Medicare Billing Guidelines, Medicare payment and reimbursment, Medicare codes. Here is a list of CPT codes and Diagnoses that are.
Breast Surgery Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives
 NHS Dorset Clinical Commissioning Group Breast Surgery Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives NHS DORSET CLINICAL COMMISSIONING GROUP BREAST SURGERY CRITERIA
NHS Dorset Clinical Commissioning Group Breast Surgery Criteria Based Access Protocol Supporting people in Dorset to lead healthier lives NHS DORSET CLINICAL COMMISSIONING GROUP BREAST SURGERY CRITERIA
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP
 INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
INFORMED CONSENT-BREAST RECONSTRUCTION WITH TRAM ABDOMINAL MUSCLE FLAP 2000 American Society of Plastic Surgeons. Purchasers of the Patient Consultation Resource Book are given a limited license to modify
Original Policy Date
 MP 7.01.06 Prophylactic Mastectomy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical Policy Index
MP 7.01.06 Prophylactic Mastectomy Medical Policy Section Surgery Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature search/12:2013 Return to Medical Policy Index
National Mastectomy & Breast Reconstruction Audit Datasheet - Mastectomy +/- Immediate Reconstruction
 Patient Registration data Surname Forename NHS/Private Hospital Number Date of birth Postcode Ethnicity Patient-reported outcomes consent Has this patient consented to being sent outcome questionnaires?
Patient Registration data Surname Forename NHS/Private Hospital Number Date of birth Postcode Ethnicity Patient-reported outcomes consent Has this patient consented to being sent outcome questionnaires?
AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION
 CHAPTER 18 AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION Ali A. Qureshi, MD and Smita R. Ramanadham, MD Aesthetic surgery of the breast aims to either correct ptosis with a mastopexy,
CHAPTER 18 AESTHETIC SURGERY OF THE BREAST: MASTOPEXY, AUGMENTATION & REDUCTION Ali A. Qureshi, MD and Smita R. Ramanadham, MD Aesthetic surgery of the breast aims to either correct ptosis with a mastopexy,
Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document.
 MRI OF THE BREAST Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
MRI OF THE BREAST Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Related Policies None
 Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Medical Policy MP 7.01.13 BCBSA Ref. Policy: 7.01.13 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies None DISCLAIMER Our medical policies are designed for informational
Post-mastectomy breast reconstruction
 Follow the link from the online version of this article to obtain certified continuing medical education credits Post-mastectomy breast reconstruction Paul T R Thiruchelvam, 1 Fiona McNeill, 2 Navid Jallali,
Follow the link from the online version of this article to obtain certified continuing medical education credits Post-mastectomy breast reconstruction Paul T R Thiruchelvam, 1 Fiona McNeill, 2 Navid Jallali,
Prostatic Urethral Lift Corporate Medical Policy
 Prostatic Urethral Lift Corporate Medical Policy File Name: Prostatic Urethral Lift File Code: UM.SURG.19 Origination: 05/2018 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019 Description/Summary
Prostatic Urethral Lift Corporate Medical Policy File Name: Prostatic Urethral Lift File Code: UM.SURG.19 Origination: 05/2018 Last Review: 01/2019 Next Review: 01/2020 Effective Date: 04/01/2019 Description/Summary
How To Make a Good Mastectomy for Reconstruction Based on the Anatomy. Zhang Jin, Ph.D MD
 How To Make a Good Mastectomy for Reconstruction Based on the Anatomy Zhang Jin, Ph.D MD Deputy Director and Professor Tianjin Medical University Cancer Institute and Hospital People s Republic of China
How To Make a Good Mastectomy for Reconstruction Based on the Anatomy Zhang Jin, Ph.D MD Deputy Director and Professor Tianjin Medical University Cancer Institute and Hospital People s Republic of China
Surgical Treatment of Bilateral Gynecomastia
 Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Surgical Treatment of Bilateral Gynecomastia Policy Number: 7.01.13 Last Review: 4/2018 Origination: 4/2006 Next Review: 4/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
MEDICAL POLICY No R10 BREAST RELATED PROCEDURES*
 BREAST RELATED PROCEDURES* Effective Date: June 19, 2017 Review Dates: 8/07, 8/08, 8/09, 4/10, 6/10, 8/10, 8/11, 8/12, 6/13, 8/14, 8/15, 2/16, 2/17 Date of Origin: August 8, 2007 Status: Current *This
BREAST RELATED PROCEDURES* Effective Date: June 19, 2017 Review Dates: 8/07, 8/08, 8/09, 4/10, 6/10, 8/10, 8/11, 8/12, 6/13, 8/14, 8/15, 2/16, 2/17 Date of Origin: August 8, 2007 Status: Current *This
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): July 26, 2011 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION
 Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION BREAST RECONSTRUCTION: A WOMAN S DECISION Options and Information Our approach to breast reconstruction entails a very
Frederick J. Duffy, Jr., MD, FACS and Brice W. McKane, MD, FACS BREAST RECONSTRUCTION BREAST RECONSTRUCTION: A WOMAN S DECISION Options and Information Our approach to breast reconstruction entails a very
PROS AND CONS OF IMMEDIATE PROSTHETIC IMPLANTS VS USE OF EXPANDER FOR POST MASTECTOMY BREAST RECONSTRUCTIONS
 PROS AND CONS OF IMMEDIATE PROSTHETIC IMPLANTS VS USE OF EXPANDER FOR POST MASTECTOMY BREAST Dr Tienie van Rooyen Mediclinic Kloof Hospital Pretoria IMMEDIATE Since 1990 s Skin sparing mastectomies proven
PROS AND CONS OF IMMEDIATE PROSTHETIC IMPLANTS VS USE OF EXPANDER FOR POST MASTECTOMY BREAST Dr Tienie van Rooyen Mediclinic Kloof Hospital Pretoria IMMEDIATE Since 1990 s Skin sparing mastectomies proven
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Surgical Treatment of Gynecomastia Page 1 of 6 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Surgical Treatment of Gynecomastia Professional Institutional Original
Prophylactic Mastectomy
 Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/01/2013 Section: Surgery Place(s) of Service:
Prophylactic Mastectomy Policy Number: Original Effective Date: MM.06.010 01/01/2009 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/01/2013 Section: Surgery Place(s) of Service:
Advances in Localized Breast Cancer
 Advances in Localized Breast Cancer Melissa Camp, MD, MPH and Fariba Asrari, MD June 18, 2018 Moderated by Elissa Bantug 1 Advances in Surgery for Breast Cancer Melissa Camp, MD June 18, 2018 2 Historical
Advances in Localized Breast Cancer Melissa Camp, MD, MPH and Fariba Asrari, MD June 18, 2018 Moderated by Elissa Bantug 1 Advances in Surgery for Breast Cancer Melissa Camp, MD June 18, 2018 2 Historical
Goals of Care. Restore shape and function after cancer
 Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
Goals of Care Restore shape and function after cancer Aid in physiological and psychological benefit Relationship with significant other Self esteem and positive body image Feeling of a whole body Avoid
Tanta University. Faculty of Medicine. Plastic and Reconstructive Surgery Department. Doctorate Degree in Plastic Surgery
 Componenets : Tanta University Faculty of Medicine Plastic and Reconstructive Surgery Department Doctorate Degree in Plastic Surgery Students should fulfill the designated number of credit hours, including
Componenets : Tanta University Faculty of Medicine Plastic and Reconstructive Surgery Department Doctorate Degree in Plastic Surgery Students should fulfill the designated number of credit hours, including
Prior Authorization Review Panel MCO Policy Submission
 Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
Prior Authorization Review Panel MCO Policy Submission A separate copy of this form must accompany each policy submitted for review. Policies submitted without this form will not be considered for review.
can see several late effects. Asymmetry is probably the most common and the thing that patients notice the most. We can also see implant wrinkling or
 Hello, I am Summer Hanson. I m an assistant professor with the Department of Plastic and Reconstructive Surgery at the University of Texas MD Anderson Cancer Center. And today I m going to talk to you
Hello, I am Summer Hanson. I m an assistant professor with the Department of Plastic and Reconstructive Surgery at the University of Texas MD Anderson Cancer Center. And today I m going to talk to you
Exercise & Breast Cancer Recovery
 Exercise & Breast Cancer Recovery LEARNING OBJECTIVES Demonstrate an understanding of the diagnosis and treatment of breast cancer Demonstrate an understanding of how breast cancer surgery and treatment
Exercise & Breast Cancer Recovery LEARNING OBJECTIVES Demonstrate an understanding of the diagnosis and treatment of breast cancer Demonstrate an understanding of how breast cancer surgery and treatment
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Prophylactic Mastectomy Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prophylactic Mastectomy Professional Institutional Original Effective Date:
Prophylactic Mastectomy Page 1 of 14 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Prophylactic Mastectomy Professional Institutional Original Effective Date:
Plastic surgery of the breast includes; augmentation, reduction, Plastic Surgery of the Breast. Abstract. Continuing Education Column
 Plastic Surgery of the Breast Keuk Shun Shin, M.D. Keuk SHUN SHIN s Asthetic Plastic Surgery E mail: drsks@drsks.co.kr Abstract Plastic surgery of the breast includes; augmentation, reduction, reconstruction
Plastic Surgery of the Breast Keuk Shun Shin, M.D. Keuk SHUN SHIN s Asthetic Plastic Surgery E mail: drsks@drsks.co.kr Abstract Plastic surgery of the breast includes; augmentation, reduction, reconstruction
rupture, you may notice silicone in their lymph nodes on radiographs. This may be seen and help us detect that there is a rupture.
 Hello. I m Melissa Crosby. I m an Associate Professor at The University of Texas MD Anderson Cancer Center in the Department of Plastic Surgery. I d like to discuss with you the Late Effects of Breast
Hello. I m Melissa Crosby. I m an Associate Professor at The University of Texas MD Anderson Cancer Center in the Department of Plastic Surgery. I d like to discuss with you the Late Effects of Breast
The Adult Exceptional Aesthetic Referral Protocol (AEARP) September 2011
 Aesthetic surgery is not routinely offered by the NHS and can only be provided on an exceptional case basis in line with the Please Note Patients should only be referred following a clinical assessment
Aesthetic surgery is not routinely offered by the NHS and can only be provided on an exceptional case basis in line with the Please Note Patients should only be referred following a clinical assessment
Author's response to reviews
 Author's response to reviews Title: Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of
Author's response to reviews Title: Classifying breast cancer surgery: a novel, complexity-based system for oncological, oncoplastic and reconstructive procedures, and proof of principle by analysis of
A Combined Practice. Why Its Worked. Barriers to Breast Reconstruction. As a breast oncologist the patient gets seemless care
 A Combined Practice A Combined Breast Oncology and Plastic Surgery Practice Why It Works Anne M. Wallace, MD, FACS Director, Comprehensive Breast Health Center Professor of Clinical Surgery, Surgical Oncology
A Combined Practice A Combined Breast Oncology and Plastic Surgery Practice Why It Works Anne M. Wallace, MD, FACS Director, Comprehensive Breast Health Center Professor of Clinical Surgery, Surgical Oncology
Commissioning Policy: Treatments Designed to Improve Aesthetic Appearance
 Commissioning Policy: Treatments Designed to Improve Aesthetic Appearance Policy Statement: Coventry and Rugby CCG consider funding of treatments designed to improve aesthetic appearance to be of low priority
Commissioning Policy: Treatments Designed to Improve Aesthetic Appearance Policy Statement: Coventry and Rugby CCG consider funding of treatments designed to improve aesthetic appearance to be of low priority
Timby/Smith: Introductory Medical-Surgical Nursing, 9/e
 Timby/Smith: Introductory Medical-Surgical Nursing, 9/e Chapter 60: Caring for Clients With Breast Disorders Slide 1 Infectious and Inflammatory Breast Disorders: Mastitis Pathophysiology and Etiology
Timby/Smith: Introductory Medical-Surgical Nursing, 9/e Chapter 60: Caring for Clients With Breast Disorders Slide 1 Infectious and Inflammatory Breast Disorders: Mastitis Pathophysiology and Etiology
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander.
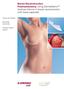 Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Breast Reconstruction Postmastectomy. Using DermaMatrix Acellular Dermis in breast reconstruction with tissue expander. Strong and flexible Bacterially inactivated Provides implant support Breast Reconstruction
Is Unilateral Implant or Autologous Breast Reconstruction Better in Obtaining Breast Symmetry?
 ORIGINAL ARTICLE Is Unilateral Implant or Autologous Breast Reconstruction Better in Obtaining Breast Symmetry? Oriana Cohen, MD, Kevin Small, MD, Christina Lee, BA, Oriana Petruolo, MD, Nolan Karp, MD,
ORIGINAL ARTICLE Is Unilateral Implant or Autologous Breast Reconstruction Better in Obtaining Breast Symmetry? Oriana Cohen, MD, Kevin Small, MD, Christina Lee, BA, Oriana Petruolo, MD, Nolan Karp, MD,
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference
 2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
2017 FlexHD Abdominal Wall Reconstruction Reimbursement Coding Reference Most Commonly Reported ICD-10-CM Procedure Codes and Descriptors ICD-10-CM Description 0WUF0KZ Supplement Abdominal Wall with Nonautologous
Updates in Breast Care. Truth or Hype. History of Breast Cancer Surgery. Dr Karen Barbosa 5/3/2017 4/20/2017
 Updates in Breast Care Dr Karen Barbosa 4/20/2017 Truth or Hype Princess Bust Developer Sears, Roebuck and Co. 1897 Promised to make the breast round, firm and beautiful History of Breast Cancer Surgery
Updates in Breast Care Dr Karen Barbosa 4/20/2017 Truth or Hype Princess Bust Developer Sears, Roebuck and Co. 1897 Promised to make the breast round, firm and beautiful History of Breast Cancer Surgery
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN
 MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
MENTORPROMISE AND MENTORPROMISE ENHANCED PROTECTION PLAN FOR MEMORYGEL BREAST IMPLANTS AND MEMORYSHAPE BREAST IMPLANTS This document describes the Mentor Worldwide LLC ( Mentor ) Product Replacement Policy
ONCOPLASTIC SURGERY. Dr. Sadir Alrawi Director of Surgical Oncology Services. Dr. Humaa Darr Surgical Oncology Fellow
 Hessa St ONCOPLASTIC SURGERY Dr. Sadir Alrawi Director of Surgical Oncology Services Dr. Humaa Darr Surgical Oncology Fellow Al Sufouh Rd AL SUFOUH AL SUFOUH Sharaf DG Mall of the Emirates Mall Of the
Hessa St ONCOPLASTIC SURGERY Dr. Sadir Alrawi Director of Surgical Oncology Services Dr. Humaa Darr Surgical Oncology Fellow Al Sufouh Rd AL SUFOUH AL SUFOUH Sharaf DG Mall of the Emirates Mall Of the
