Identifying ALK+ NSCLC patients for targeted treatment
|
|
|
- Abel Moore
- 5 years ago
- Views:
Transcription
1 VENTANA (D5F3) CDx Assay Identifying + NSCLC patients for targeted treatment
2 VENTANA (D5F3) CDx Assay Identify + NSCLC patients eligible for treatment with XORI, ZYKADIA or ALECENSA NSCLC tissue samples stained with the VENTANA (D5F3) CDx Assay and OptiView DAB Detection and Amp Intended use VENTANA (D5F3) CDx Assay* is intended for the qualitative detection of the anaplastic lymphoma kinase () protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung carcinoma (NSCLC) tissue stained with a BenchMark XT or BenchMark ULTRA automated staining instrument. It is indicated as an aid in identifying patients eligible for treatment with XORI (crizotinib), ZYKADIA (ceritinib) or ALECENSA (alectinib). This product should be interpreted by a qualified pathologist in conjunction with histological examination, relevant clinical information, and proper controls. This product is intended for in vitro diagnostic (IVD) use. * (D5F3) Assay value points (D5F3) Assay stained with OptiView DAB IHC Detection and Amplification kits** detects the protein that is the target of therapy Clinical guidelines recommend rapid turnaround for earlier targeted therapies has comparable sensitivity and specificity relative to FISH More immediate treatment decisions can be made for advanced NSCLC patients by using the (D5F3) Assay XORI (crizotinib), ZYKADIA (ceritinib) and ALECENSA (alectinib) are clinically effective and recommended for the 10, 17, 19 treatment of -positive patients **OptiView DAB Detection and Amp Full automation Reproducible staining Standardization In-house testing Efficient workflow Rapid results Training Precision scoring Confidence
3 3 Lung cancer is the most common cancer worldwide with more than 1.8 million new cases 2 each year Lung cancer is the leading cause of cancer death for men and women globally with more than 4,000 people dying every day Main types of lung cancer 85 % non-small cell lung cancer (NSCLC) Poor 5-year survival rate when compared to other cancers 91 % 14 % small cell lung cancer (SCLC) 1% other breast cancer 18 % lung cancer About non-small cell lung cancer Lung cancer has been the most common cancer in the world for several decades and remains the leading cause of cancer deaths worldwide. It is estimated to account for 12.9% of all new cancer cases and is responsible for nearly 1.59 million deaths annually worldwide, or approximately one-in-five cancer-related deaths. 5 Although improvements have been made in diagnosis and therapy options, prognosis remains poor with low long-term survival rates for all stages. Over the past three decades, lung cancer has shown among the least improvement in survival rates when compared with other cancers. 4 Non-small cell lung cancer (NSCLC), one of the two major types of lung cancer, accounts for approximately 85% of all lung cancer cases. 4 In more than half of patients newly diagnosed with NSCLC, the disease has already metastasized, greatly decreasing the likelihood of survival. The five-year relative survival rate for NSCLC diagnosed as distant disease is 4.7%. 6 The majority of patients with NSCLC present with inoperable, locally advanced disease (Stage IIIB) or metastatic disease (Stage IV), neither of which currently has any curative treatment options. On average, these patients die within a year of diagnosis. Improvement in the clinical outcome of lung cancer is likely to be achieved through better understanding of the molecular events that underlie its pathogenesis, identifying new biomarker targets and developing new treatment options. Testing for lung cancer Clinical guidelines recommend routine testing for genetic mutations in all adenocarcinomas, including EML4 gene rearrangement. Testing is recommended immediately after establishing histology and is required prior to initiating targeted therapy for a patient. The current practice for testing include IHC and FISH. 4 EGFR mutation Stage IIIB IV NSCLC Adenocarcinoma /ROS1 rearrangement PD-L1 expression Figure 1. Common testing algorithm to determine indications for appropriate treatment in lung cancer
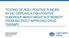
4 4 Lung cancer is the leading cause of death Lung cancer is the most prevalent form of cancer in the world. Each year, more than 1.8 million new cases are diagnosed. Lung cancer also has the highest mortality rate. Five-year survival rates are as low as 18%. Adenocarcinoma, a subset of NSCLC, is the most common, comprising approximately 40% of all lung disease. 7, 8 mutation in lung cancer Genetic mutations are known to play critical roles in the progression to metastatic lung disease. The majority of these mutations are found in adenocarcinoma of young non-smokers. is considered a key oncogenic driver in NSCLC. The gene codes for a transmembrane glycoprotein with tyrosine kinase activity. In-frame rearrangements with the known fusion partners place the kinase domain under the control of a different gene promoter. This fusion results in a chimeric protein (like EML4-) with constitutive tyrosine kinase activity that has been demonstrated to play a key role in controlling cell proliferation. This unique protein is also a potential target for 3, 5, 9 -specific tyrosine kinase inhibitor (TKI) therapy. no known genotype 49% KRAS 24% EGFR 13% 5% Figure 2. Five-year survival rate of patients after diagnosis with NSCLC is 18% 100% Figure 3. Breakdown of known gene mutations in NSCLC BRAF 4% PROFILE 1014 ASCEND 4 TRIAL ALEX STUDY Progression-Free Survival XORI (n=172) Chemotherapy (n=171) ZYKADIA (n=189) Chemotherapy (n=187) ALECENSA (n=152) XORI (n=151) Median, months (95% CI) [a] 10.9 (8.3, 13.9) 7.0 (6.8, 8.2) 16.6 (12.6, 27.2) 8.1 (5.8, 11.1) (17.7-not reached)* 11.1 (9.1,13.1) HR (95% CI) [b] 0.45 (0.35, 0.60) 0.55 (0.42, 0.73) 0.47 (0.34, 0.65) p-valued [c] <0.001 < < Figure 4. Clinical benefit of XORI, ZYKADIA and ALECENSA (progression free survival). HR=hazard ratio; CI=confidence interval; BIRC=Blinded Independent Review Committee; NR=not reached; NE=not estimable [a] Estimated using the Kaplan-Meier method. [b] A Cox regression model stratified by randomization stratification factors (WHO performance status: 0 vs. 1-2; presence or absence of BM, presence or absence of previous neo-/adjuvant chemotherapy)) was used to estimate the hazard ratio of PFS, along with 95% CI based on the Wald test. [c] Based on the stratified log-rank test (same stratification as [b]). *Median PFS not estimable due to insufficient patients demonstrating disease progression while on alectinib therapy. Treatment options for non-small cell lung cancer XORI, ZYKADIA and ALECENSA are indicated for the treatment of patients with metastatic NSCLC whose tumors are -positive as 10, 17, 19 detected by an approved testing method for. XORI (crizotinib) is indicated for the treatment of patients with positive metastatic NSCLC and other kinases. 10 ZYKADIA (ceritinib) is indicated for the treatment of patients with -positive metastatic NSCLC who have had no previous treatment, or who have progressed on, or who are intolerant to, crizotinib. 17 ALECENSA (alectinib) is indicated for the treatment of patients with -positive metastatic NSCLC who have had no previous treatment, or who have progressed on, or who are intolerant to, crizotinib. 19
5 5 Standardization of IHC testing: (D5F3) Assay and OptiView DAB Detection and Amp Patients with late-stage lung cancer need a fast, reliable and standardized way to assess treatment options. Roche developed the (D5F3) Assay to be used with OptiView DAB IHC Detection and Amp to identify these patients who are eligible for targeted therapy. A full range of human NSCLC tissue specimen types can be tested including resections, needle biopsies, bronchial biopsies and formalinfixed, paraffin-embedded cell blocks. Chemotherapy Targeted therapy Immunotherapy Cancer care options Surgery Radiation therapy Best in quality The (D5F3) Assay stained with OptiView DAB Detection and Amp scored high in External Quality Assurance testing vs. all other ready-to-use antibodies for demonstration of rearrangement. 11 Fast turnaround time The approved (D5F3) Assay stained with OptiView DAB Detection and Amp is a 41/2-hour, fully automated test to be stained with all other routine IHC testing for same-day results and to meet current CAP/ IASLC/AMP guidelines for testing patients with lung cancer. 4 Reagents required The (D5F3) Assay is fully optimized for use on the BenchMark IHC/ISH staining instrument. (D5F3) Assay Ref: Rabbit Monoclonal Negative Ref: Control Ig OptiView DAB IHC Detection Kit Ref: OptiView Amplification Kit Ref: Easy to score The sensitivity of the approved (D5F3) Assay stained with OptiView DAB Detection and Amp enables a reproducible, binary scoring system for evaluating staining results without the need for quantification of cells or staining. 1
6 6 (D5F3) Assay and Detection with Amp vs. FISH Technical benefits of IHC testing FISH can present technical challenges in evaluating patient results and offers the potential for false negatives. Recent studies indicate that the (D5F3) Assay stained with OptiView DAB Detection and Amp is sensitive and specific for determination of status, and a 12, 13, 14 better alternative to FISH. There are reports of IHC-positive, FISH-negative patients benefitting from treatment with XORI. Figure 4. Comparison of (D5F3) Assay stained with OptiView DAB Detection and Amp vs. FISH testing for mutation (D5F3) Assay with OptiView DAB Detection and Amp Easy to score Binary (+/-) scoring Any strong positive staining in any number of cells is positive for Faster turnaround times 41/2 hours, fully automated Routine IHC testing FISH Requires a dual-color scoring algorithm Requires 50 enumerable cells and specific cutoff ratios to be calculated 12+ hours, semi-automated Typically batch or send-out testing Brightfield vs. fluorescent staining Standard brightfield microscope Fully archivable results Full visibility of tumor morphology Requires a fluorescent microscope Staining and signal fade over time Loss of tissue morphology In one study, van der Wekken et al. found that Dichotomous -IHC is superior to -FISH on small biopsies and FNA to predict tumor response and survival to crizotinib for advanced NSCLC patients. 18 Visit IHC.com and contact your local Roche representative to learn more.
7 7 (D5F3) Assay with OptiView DAB Detection and Amp vs. other testing methods antibody clones There are a many available antibody clones and detection kits. Only (D5F3) Assay stained with OptiView DAB Detection and Amp is approved as an aid to identify patients eligible for treatment with XORI, ZYKADIA, or ALECENSA. 1 Figure 7. NSCLC tissue samples stained with different IHC methods VENTANA (D5F3) Assay with OptiView DAB Detection and Amp antibody, clone 5A4 with Polymer Detection Molecular testing Over ten genetic variants of EML4- mutations have been identified. (D5F3) Assay stained with OptiView DAB Detection and Amp identifies a conserved protein sequence common to known variants of the mutation. Current molecular testing does not identify all known genetic variants and is not recommended as an alternative testing method to select patients for inhibitor therapy. 4 Figure 8. Different variants of EML4- and non-eml4 fusion partners 19 The EML4 genetic sequence is diverse and creates multiple targets for PCR EML4- E13;A20 E20;A20 EML4 E18;A20 EML4 E17;A20 EML4 EML4 E6;A20 EML4 E14;A20 EML4 E15;A20 EML4 E2;A20 EML4 TFG- TFG A conserved protein sequence common to all known mutations is detected by (D5F3) Assay There are multiple genetic variants of the mutation that lead to NSCLC Molecular testing does not identify all gene variants and can miss positive cases D5F3 clone is specific to the common kinase domain of all mutations and should identify all genetic variants Molecular testing techniques rely upon good sample integrity and require sophisticated computational analysis to interpret results. Formalin-fixed, paraffin-embedded tissues provide a significant challenge as genetic material is known to degrade in sample preparation. Even when properly performed, interpreting the results of these techniques is not standardized. 4 testing with (D5F3) Assay with OptiView DAB Detection and Amp offers many benefits: A fully automated test to select patients for treatment with XORI, ZYKADIA or ALECENSA Fastest turnaround time to meet the current CAP/IASLC/AMP guidelines for testing lung patients Can be integrated into a routine IHC panel of antibodies to stratify NSCLC patients KIF5B- KIF5B Coiled-coil domain Tyrosine kinase domain
8 References 1. VENTANA (D5F3) Rabbit Monoclonal Primary Antibody [package insert] Tucson, AZ: Ventana Medical Systems, Inc.; Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. (2012). GLOBOCAN v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer; Available at: (last accessed September 2017). 3. Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol Feb1; 12 (2): doi: / S (10) Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol Jul; 8(7): doi: / JTO.0b013e f. 5. Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small cell lung cancer who harbor EML4-. J Clin Oncol. 2009; 27: NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. v Available at: physician_gls/pdf/nscl.pdf. Accessed August 1, World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in Lyon, France Default.aspx. Accessed September Lung Cancer Survival Rates and Prognosis. National Cancer Institute at the National Institutes of Health. Bethesda, MD. cancertopics/types/lung/cancer-survival-prognosis. Accessed August 1, Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4- fusion gene in non-small-cell lung cancer. Nature Aug 2; 448(7153): XORI (crizotinib) [package insert]. New York, NY: Pfizer; Zhou J, Zhao J, Sun K, Wang B, Wang L, et al. Accurate and Economical Detection of Positive Lung Adenocarcinoma with Semiquantitative Immunohistochemical Screening. PLoS ONE. 2014; 9(3): e doi: /journal.pone Shan L, Lian F, Guo L, Yang X, Ying J and Lin D. Combination of conventional immunohistochemistry and qrt-pcr to detect rearrangement. Diagn Pathol. 2014; 9:3. doi: / Ying J, Guo L, Qiu T, Shan L, LingY, Liu X, Lu N. Diagnostic value of a novel fully automated immunochemistry assay for detection of rearrangement in primary lung adenocarcinoma. Ann Oncol Oct 2; 24(10): Selinger CI, Rogers TM, Russell PA, et al. Testing for rearrangement in lung adenocarcinoma: a multicenter comparison of immunohistochemistry and fluorescent in situ hybridization. Mod Pathol. 2013; 26(12): doi: /modpathol ; published online 7 June Alì G, Proietti A, Pelliccioni S, et al. Rearrangement in a Large Series of Consecutive Non Small Cell Lung Cancers:Comparison Between a New Immunohistochemical Approach and Fluorescent In Situ Hybridization for the Screening of Patients Eligible for Crizotinib Treatment. Arch Pathol Lab Med June ZYKADIA (ceritinib) [package insert]. Whippany, NJ: Novartis Pharmaceuticals Corporation; van der Wekken, et al. Dichotomous -IHC is a better predictor for inhibition outcome than traditional -FISH in advanced non-small cell lung cancer, Clin Cancer Res Available at clincancerres.aacrjournals.org/content/early/2017/02/09/ CCR ALECENSA (alectinib) [package insert]. San Francisco, CA: Genentech; VENTANA, BENCHMARK and OPTIVIEW are trademarks of Roche. All other product names and trademarks are the property of their respective owners Roche PP-US Roche Diagnostics Corporation 9115 Hague Road Indianapolis, Indiana usdiagnostics.roche.com
VENTANA ALK (D5F3) Rabbit Monoclonal Primary Antibody. ALK IHC Biomarker Testing Aiding in patient diagnosis
 VENTANA (D5F3) Rabbit Monoclonal Primary Antibody IHC Biomarker Testing Aiding in patient diagnosis 2 IHC Biomarker Testing Lung cancer is the leading cause of death Lung cancer is the most prevalent form
VENTANA (D5F3) Rabbit Monoclonal Primary Antibody IHC Biomarker Testing Aiding in patient diagnosis 2 IHC Biomarker Testing Lung cancer is the leading cause of death Lung cancer is the most prevalent form
VENTANA PD-L1 (SP142) Assay Guiding immunotherapy in NSCLC
 VENTANA (SP142) Assay Guiding immunotherapy in NSCLC Hiker s path: VENTANA (SP142) Assay on non-small cell lung cancer tissue Location: Point Conception, CA VENTANA (SP142) Assay Assess NSCLC patient benefit
VENTANA (SP142) Assay Guiding immunotherapy in NSCLC Hiker s path: VENTANA (SP142) Assay on non-small cell lung cancer tissue Location: Point Conception, CA VENTANA (SP142) Assay Assess NSCLC patient benefit
Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment with Targeted Tyrosine Kinase Inhibitors
 Q: How is the strength of recommendation determined in the new molecular testing guideline? A: The strength of recommendation is determined by the strength of the available data (evidence). Strong Recommendation:
Q: How is the strength of recommendation determined in the new molecular testing guideline? A: The strength of recommendation is determined by the strength of the available data (evidence). Strong Recommendation:
An Interactive Guide to ALK+ Lymphoma. Our understanding of lung cancer has changed From one disease to many subtypes LCD
 An Interactive Guide to ALK+ Anaplastic Lymphoma Kinase Non-Small Cell Lung (NSCLC) Our understanding of lung cancer has changed From one disease to many subtypes Click to get started Learning about Lung
An Interactive Guide to ALK+ Anaplastic Lymphoma Kinase Non-Small Cell Lung (NSCLC) Our understanding of lung cancer has changed From one disease to many subtypes Click to get started Learning about Lung
Corporate Medical Policy
 Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Lin Yang 1, Yun Ling 1, Lei Guo 1, Di Ma 2, Xuemin Xue 1, Bingning Wang 1, Junling Li 2, Jianming Ying 1. Original Article.
 Original Article Detection of ALK translocation in non-small cell lung carcinoma (NSCLC) and its clinicopathological significance using the Ventana immunohistochemical staining method: a single-center
Original Article Detection of ALK translocation in non-small cell lung carcinoma (NSCLC) and its clinicopathological significance using the Ventana immunohistochemical staining method: a single-center
VENTANA MMR IHC Panel Interpretation Guide for Staining of Colorectal Tissue
 VENTANA MMR IHC Panel Interpretation Guide for Staining of Colorectal Tissue VENTANA anti-mlh1 (M1) Mouse Monoclonal Primary Antibody VENTANA anti-pms2 (A16-4) Mouse Monoclonal Primary Antibody VENTANA
VENTANA MMR IHC Panel Interpretation Guide for Staining of Colorectal Tissue VENTANA anti-mlh1 (M1) Mouse Monoclonal Primary Antibody VENTANA anti-pms2 (A16-4) Mouse Monoclonal Primary Antibody VENTANA
Molecular Testing in Lung Cancer
 Molecular Testing in Lung Cancer Pimpin Incharoen, M.D. Assistant Professor, Thoracic Pathology Department of Pathology, Ramathibodi Hospital Genetic alterations in lung cancer Source: Khono et al, Trans
Molecular Testing in Lung Cancer Pimpin Incharoen, M.D. Assistant Professor, Thoracic Pathology Department of Pathology, Ramathibodi Hospital Genetic alterations in lung cancer Source: Khono et al, Trans
Virtual Journal Club: Front-Line Therapy and Beyond Recent Perspectives on ALK-Positive Non-Small Cell Lung Cancer.
 Virtual Journal Club: Front-Line Therapy and Beyond Recent Perspectives on ALK-Positive Non-Small Cell Lung Cancer Reference Slides ALK Rearrangement in NSCLC ALK (anaplastic lymphoma kinase) is a receptor
Virtual Journal Club: Front-Line Therapy and Beyond Recent Perspectives on ALK-Positive Non-Small Cell Lung Cancer Reference Slides ALK Rearrangement in NSCLC ALK (anaplastic lymphoma kinase) is a receptor
VENTANA PD-L1 (SP142) Assay Guiding immunotherapy
 VENTANA PD-L1 (SP142) Assay Guiding immunotherapy Hiker s path: VENTANA PD-L1 (SP142) Assay on urothelial carcinoma tissue Location: Point Conception, CA VENTANA PD-L1 (SP142) Assay Identify patients most
VENTANA PD-L1 (SP142) Assay Guiding immunotherapy Hiker s path: VENTANA PD-L1 (SP142) Assay on urothelial carcinoma tissue Location: Point Conception, CA VENTANA PD-L1 (SP142) Assay Identify patients most
Read, Interpret, and Communicate Test Results
 Read, Interpret, and Communicate Test Results Effective interpretation of epidermal growth factor receptor (EGFR) T790M mutation test results at progression will help physicians to set patient expectations
Read, Interpret, and Communicate Test Results Effective interpretation of epidermal growth factor receptor (EGFR) T790M mutation test results at progression will help physicians to set patient expectations
Do You Think Like the Experts? Refining the Management of Advanced NSCLC With ALK Rearrangement. Reference Slides Introduction
 Do You Think Like the Experts? Refining the Management of Advanced NSCLC With ALK Rearrangement Reference Slides Introduction EML4-ALK Fusion Oncogene Key Driver in 3% to 7% NSCLC Inversion or Translocation
Do You Think Like the Experts? Refining the Management of Advanced NSCLC With ALK Rearrangement Reference Slides Introduction EML4-ALK Fusion Oncogene Key Driver in 3% to 7% NSCLC Inversion or Translocation
Non-Small Cell Lung Cancer:
 Non-Small Cell Lung Cancer: Where We Are Today Sila Shalhoub, PharmD PGY2 Oncology Pharmacy Resident Shalhoub.Sila@mayo.edu Pharmacy Grand Rounds September 26, 2017 2017 MFMER slide-1 Objectives Identify
Non-Small Cell Lung Cancer: Where We Are Today Sila Shalhoub, PharmD PGY2 Oncology Pharmacy Resident Shalhoub.Sila@mayo.edu Pharmacy Grand Rounds September 26, 2017 2017 MFMER slide-1 Objectives Identify
Refining Prognosis of Early Stage Lung Cancer by Molecular Features (Part 2): Early Steps in Molecularly Defined Prognosis
 5/17/13 Refining Prognosis of Early Stage Lung Cancer by Molecular Features (Part 2): Early Steps in Molecularly Defined Prognosis Johannes Kratz, MD Post-doctoral Fellow, Thoracic Oncology Laboratory
5/17/13 Refining Prognosis of Early Stage Lung Cancer by Molecular Features (Part 2): Early Steps in Molecularly Defined Prognosis Johannes Kratz, MD Post-doctoral Fellow, Thoracic Oncology Laboratory
GENETIC TESTING FOR TARGETED THERAPY FOR NON-SMALL CELL LUNG CANCER (NSCLC)
 CANCER (NSCLC) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
CANCER (NSCLC) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
Personalized Medicine: Lung Biopsy and Tumor
 Personalized Medicine: Lung Biopsy and Tumor Mutation Testing Elizabeth H. Moore, MD Personalized Medicine: Lung Biopsy and Tumor Mutation Testing Genomic testing has resulted in a paradigm shift in the
Personalized Medicine: Lung Biopsy and Tumor Mutation Testing Elizabeth H. Moore, MD Personalized Medicine: Lung Biopsy and Tumor Mutation Testing Genomic testing has resulted in a paradigm shift in the
LUNG CANCER. pathology & molecular biology. Izidor Kern University Clinic Golnik, Slovenia
 LUNG CANCER pathology & molecular biology Izidor Kern University Clinic Golnik, Slovenia 1 Pathology and epidemiology Small biopsy & cytology SCLC 14% NSCC NOS 4% 70% 60% 50% 63% 62% 61% 62% 59% 54% 51%
LUNG CANCER pathology & molecular biology Izidor Kern University Clinic Golnik, Slovenia 1 Pathology and epidemiology Small biopsy & cytology SCLC 14% NSCC NOS 4% 70% 60% 50% 63% 62% 61% 62% 59% 54% 51%
Lung Cancer Case. Since the patient was symptomatic, a targeted panel was sent. ALK FISH returned in 2 days and was positive.
 Lung Cancer Case Jonathan Riess, M.D. M.S. Assistant Professor of Medicine University of California Davis School of Medicine UC Davis Comprehensive Cancer Center 63 year-old woman, never smoker, presents
Lung Cancer Case Jonathan Riess, M.D. M.S. Assistant Professor of Medicine University of California Davis School of Medicine UC Davis Comprehensive Cancer Center 63 year-old woman, never smoker, presents
ALK positive Lung Cancer. Shirish M. Gadgeel, MD. Director of the Thoracic Oncology program University of Michigan
 ALK positive Lung Cancer Shirish M. Gadgeel, MD. Director of the Thoracic Oncology program University of Michigan Objectives What is ALK translocation? What drugs are used in what sequence? How many times
ALK positive Lung Cancer Shirish M. Gadgeel, MD. Director of the Thoracic Oncology program University of Michigan Objectives What is ALK translocation? What drugs are used in what sequence? How many times
Molecular Targets in Lung Cancer
 Molecular Targets in Lung Cancer Robert Ramirez, DO, FACP Thoracic and Neuroendocrine Oncology November 18 th, 2016 Disclosures Consulting and speaker fees for Ipsen Pharmaceuticals, AstraZeneca and Merck
Molecular Targets in Lung Cancer Robert Ramirez, DO, FACP Thoracic and Neuroendocrine Oncology November 18 th, 2016 Disclosures Consulting and speaker fees for Ipsen Pharmaceuticals, AstraZeneca and Merck
Advances in Pathology and molecular biology of lung cancer. Lukas Bubendorf Pathologie
 Advances in Pathology and molecular biology of lung cancer Lukas Bubendorf Pathologie Agenda The revolution of predictive markers Liquid biopsies PD-L1 Molecular subtypes (non-squamous NSCLC) Tsao AS et
Advances in Pathology and molecular biology of lung cancer Lukas Bubendorf Pathologie Agenda The revolution of predictive markers Liquid biopsies PD-L1 Molecular subtypes (non-squamous NSCLC) Tsao AS et
Transform genomic data into real-life results
 CLINICAL SUMMARY Transform genomic data into real-life results Biomarker testing and targeted therapies can drive improved outcomes in clinical practice New FDA-Approved Broad Companion Diagnostic for
CLINICAL SUMMARY Transform genomic data into real-life results Biomarker testing and targeted therapies can drive improved outcomes in clinical practice New FDA-Approved Broad Companion Diagnostic for
Quan Zhang, Na Qin, Jinghui Wang, Jialin Lv, Xinjie Yang, Xi Li, Jingying Nong, Hui Zhang, Xinyong Zhang, Yuhua Wu & Shucai Zhang
 Thoracic Cancer ISSN 1759-7706 ORIGINAL ARTICLE Crizotinib versus platinum-based double-agent chemotherapy as the first line treatment in advanced anaplastic lymphoma kinase-positive lung adenocarcinoma
Thoracic Cancer ISSN 1759-7706 ORIGINAL ARTICLE Crizotinib versus platinum-based double-agent chemotherapy as the first line treatment in advanced anaplastic lymphoma kinase-positive lung adenocarcinoma
Introduction ORIGINAL ARTICLE. Wang Zhiwei *, Jiang Yuan *, Yao Yihui *, Hou Xin, Chen Jingtao, Shi Lei & Duan Yongjian
 Thoracic Cancer ISSN 1759-7706 ORIGINAL ARTICLE Ventana immunohistochemistry assay for anaplastic lymphoma kinase gene rearrangement detection in patients with non-small cell lung cancer: A meta-analysis
Thoracic Cancer ISSN 1759-7706 ORIGINAL ARTICLE Ventana immunohistochemistry assay for anaplastic lymphoma kinase gene rearrangement detection in patients with non-small cell lung cancer: A meta-analysis
Delivering Value Through Personalized Medicine: An Industry Perspective
 Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
1. Q: What has changed from the draft recommendations posted for public comment in November/December 2011?
 Frequently Asked Questions (FAQs) in regard to Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors 1. Q: What has changed from the draft recommendations
Frequently Asked Questions (FAQs) in regard to Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors 1. Q: What has changed from the draft recommendations
7/6/2015. Cancer Related Deaths: United States. Management of NSCLC TODAY. Emerging mutations as predictive biomarkers in lung cancer: Overview
 Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
Evolution of Pathology
 1 Traditional pathology Molecular pathology 2 Evolution of Pathology Gross Pathology Cellular Pathology Morphologic Pathology Molecular/Predictive Pathology Antonio Benivieni (1443-1502): First autopsy
1 Traditional pathology Molecular pathology 2 Evolution of Pathology Gross Pathology Cellular Pathology Morphologic Pathology Molecular/Predictive Pathology Antonio Benivieni (1443-1502): First autopsy
Molecular Diagnosis of Lung Cancer
 Molecular Diagnosis of Lung Cancer Lucian R. Chirieac, M.D. Assistant Professor of Pathology Harvard Medical School Staff Pathologist, Department of Pathology Brigham and Women's Hospital 75 Francis Street
Molecular Diagnosis of Lung Cancer Lucian R. Chirieac, M.D. Assistant Professor of Pathology Harvard Medical School Staff Pathologist, Department of Pathology Brigham and Women's Hospital 75 Francis Street
Rearrangement of the anaplastic lymphoma kinase (ALK)
 Brief Report Clinical Implications of Variant ALK FISH Rearrangement Patterns Xin Gao, MD,* Lynette M. Sholl, MD,* Mizuki Nishino, MD,* Jennifer C. Heng, BS, Pasi A. Jänne, MD, PhD,* and Geoffrey R. Oxnard,
Brief Report Clinical Implications of Variant ALK FISH Rearrangement Patterns Xin Gao, MD,* Lynette M. Sholl, MD,* Mizuki Nishino, MD,* Jennifer C. Heng, BS, Pasi A. Jänne, MD, PhD,* and Geoffrey R. Oxnard,
Histology: Its Influence on Therapeutic Decision Making
 Histology: Its Influence on Therapeutic Decision Making Mark A. Socinski, MD Professor of Medicine and Thoracic Surgery Director, Lung Cancer Section, Division of Hematology/Oncology Co-Director, UPMC
Histology: Its Influence on Therapeutic Decision Making Mark A. Socinski, MD Professor of Medicine and Thoracic Surgery Director, Lung Cancer Section, Division of Hematology/Oncology Co-Director, UPMC
Anaplastic lymphoma kinase (ALK) gene rearrangement
 Original Article Heterogeneity of Anaplastic Lymphoma Kinase Gene Rearrangement in Non Small-Cell Lung Carcinomas A Comparative Study Between Small Biopsy and Excision Samples Hideyuki Abe, CT,* Akihiko
Original Article Heterogeneity of Anaplastic Lymphoma Kinase Gene Rearrangement in Non Small-Cell Lung Carcinomas A Comparative Study Between Small Biopsy and Excision Samples Hideyuki Abe, CT,* Akihiko
MICROSCOPY PREDICTIVE PROFILING
 Immunomodulatory therapy in NSCLC: a year into clinical practice Professor J R Gosney Consultant Thoracic Pathologist Royal Liverpool University Hospital Disclosure JRG is a paid advisor to and speaker
Immunomodulatory therapy in NSCLC: a year into clinical practice Professor J R Gosney Consultant Thoracic Pathologist Royal Liverpool University Hospital Disclosure JRG is a paid advisor to and speaker
Lung Cancer Genetics: Common Mutations and How to Treat Them David J. Kwiatkowski, MD, PhD. Mount Carrigain 2/4/17
 Lung Cancer Genetics: Common Mutations and How to Treat Them David J. Kwiatkowski, MD, PhD Mount Carrigain 2/4/17 Histology Adenocarcinoma: Mixed subtype, acinar, papillary, solid, micropapillary, lepidic
Lung Cancer Genetics: Common Mutations and How to Treat Them David J. Kwiatkowski, MD, PhD Mount Carrigain 2/4/17 Histology Adenocarcinoma: Mixed subtype, acinar, papillary, solid, micropapillary, lepidic
Clinical efficacy of crizotinib in Chinese patients with ALK-positive non-small-cell lung cancer with brain metastases
 Original Article Clinical efficacy of crizotinib in Chinese patients with ALK-positive non-small-cell lung cancer with brain metastases Yuan-Yuan Lei 1,2, Jin-Ji Yang 2, Wen-Zhao Zhong 2, Hua-Jun Chen
Original Article Clinical efficacy of crizotinib in Chinese patients with ALK-positive non-small-cell lung cancer with brain metastases Yuan-Yuan Lei 1,2, Jin-Ji Yang 2, Wen-Zhao Zhong 2, Hua-Jun Chen
Role of the pathologist in the diagnosis and mutational analysis of lung cancer Professor J R Gosney
 Role of the pathologist in the diagnosis and mutational analysis of lung cancer Professor J R Gosney Consultant Thoracic Pathologist Royal Liverpool University Hospital Disclosure JRG is a paid advisor
Role of the pathologist in the diagnosis and mutational analysis of lung cancer Professor J R Gosney Consultant Thoracic Pathologist Royal Liverpool University Hospital Disclosure JRG is a paid advisor
VENTANA ALK (D5F3) CDx Assay
 VENTANA ALK (D5F3) CDx Assay 790-4796 06687199001 50 Figure 1. VENTANA ALK (D5F3) CDx Assay staining in non-small cell lung carcinoma. INTENDED USE VENTANA ALK (D5F3) CDx Assay is intended for the qualitative
VENTANA ALK (D5F3) CDx Assay 790-4796 06687199001 50 Figure 1. VENTANA ALK (D5F3) CDx Assay staining in non-small cell lung carcinoma. INTENDED USE VENTANA ALK (D5F3) CDx Assay is intended for the qualitative
Joachim Aerts Erasmus MC Rotterdam, Netherlands. Drawing the map: molecular characterization of NSCLC
 Joachim Aerts Erasmus MC Rotterdam, Netherlands Drawing the map: molecular characterization of NSCLC Disclosures Honoraria for advisory board/consultancy/speakers fee Eli Lilly Roche Boehringer Ingelheim
Joachim Aerts Erasmus MC Rotterdam, Netherlands Drawing the map: molecular characterization of NSCLC Disclosures Honoraria for advisory board/consultancy/speakers fee Eli Lilly Roche Boehringer Ingelheim
HER2 status assessment in breast cancer. Marc van de Vijver Academic Medical Centre (AMC), Amsterdam
 HER2 status assessment in breast cancer Marc van de Vijver Academic Medical Centre (AMC), Amsterdam 13e Bossche Mamma Congres 17 th June 2015 Modern cancer therapies are based on sophisticated molecular
HER2 status assessment in breast cancer Marc van de Vijver Academic Medical Centre (AMC), Amsterdam 13e Bossche Mamma Congres 17 th June 2015 Modern cancer therapies are based on sophisticated molecular
Detection of Anaplastic Lymphoma Kinase (ALK) gene in Non-Small Cell lung Cancer (NSCLC) By CISH Technique
 Cancer and Clinical Oncology; Vol. 7, No. 1; 2018 ISSN 1927-4858 E-ISSN 1927-4866 Published by Canadian Center of Science and Education Detection of Anaplastic Lymphoma Kinase (ALK) gene in Non-Small Cell
Cancer and Clinical Oncology; Vol. 7, No. 1; 2018 ISSN 1927-4858 E-ISSN 1927-4866 Published by Canadian Center of Science and Education Detection of Anaplastic Lymphoma Kinase (ALK) gene in Non-Small Cell
Biomarcatori per la immunoterapia: cosa e come cercare Paolo Graziano
 Biomarcatori per la immunoterapia: cosa e come cercare Paolo Graziano Unit of Pathology Fondazione IRCCS Casa Sollievo della Sofferenza San Giovanni Rotondo, Foggia,Italy p.graziano@operapadrepio.it Disclosure
Biomarcatori per la immunoterapia: cosa e come cercare Paolo Graziano Unit of Pathology Fondazione IRCCS Casa Sollievo della Sofferenza San Giovanni Rotondo, Foggia,Italy p.graziano@operapadrepio.it Disclosure
Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader
 Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader Agenda PHC Approach Provides Better Patient Outcome FMI offers Comprehensive Genomic Profiling,
Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader Agenda PHC Approach Provides Better Patient Outcome FMI offers Comprehensive Genomic Profiling,
Enterprise Interest No
 Enterprise Interest No SY-05 Pulmonary Pathology: Options for targeted therapy in lung cancer Liquid biopsy in thoracic oncology Where are we now? Paul Hofman Laboratory of Clinical and Experimental Pathology
Enterprise Interest No SY-05 Pulmonary Pathology: Options for targeted therapy in lung cancer Liquid biopsy in thoracic oncology Where are we now? Paul Hofman Laboratory of Clinical and Experimental Pathology
VENTANA PD-L1 (SP142) Assay
 VENTANA (SP142) Assay Guiding immunotherapy Hiker s path: VENTANA (SP142) Assay on urothelial carcinoma tissue Location: Point Conception, CA VENTANA (SP142) Assay Assess UC patient benefit from TECENTRIQ
VENTANA (SP142) Assay Guiding immunotherapy Hiker s path: VENTANA (SP142) Assay on urothelial carcinoma tissue Location: Point Conception, CA VENTANA (SP142) Assay Assess UC patient benefit from TECENTRIQ
8/22/2016. Major risk factors for the development of lung cancer are: Outline
 Carcinomas of the Lung: Changes in Staging, Adenocarcinoma Classification and Genetics Grace Y. Lin, M.D., Ph.D. Outline Background Staging of Lung Cancer: Review of the 2010 7 th Edition of the AJCC Cancer
Carcinomas of the Lung: Changes in Staging, Adenocarcinoma Classification and Genetics Grace Y. Lin, M.D., Ph.D. Outline Background Staging of Lung Cancer: Review of the 2010 7 th Edition of the AJCC Cancer
Identification of Novel Variant of EML4-ALK Fusion Gene in NSCLC: Potential Benefits of the RT-PCR Method
 International journal of Biomedical science ORIGINAL ARTICLE Identification of Novel Variant of EML4-ALK Fusion Gene in NSCLC: Potential Benefits of the RT-PCR Method Martin K. H. Maus 1, 2, Craig Stephens
International journal of Biomedical science ORIGINAL ARTICLE Identification of Novel Variant of EML4-ALK Fusion Gene in NSCLC: Potential Benefits of the RT-PCR Method Martin K. H. Maus 1, 2, Craig Stephens
Quality in Control. ROS1 Analyte Control. Product Codes: HCL022, HCL023 and HCL024
 Quality in Control ROS1 Analyte Control Product Codes: HCL022, HCL023 and HCL024 Contents What is ROS1? 2 The Role of ROS1 in Cancer 3 ROS1 Assessment 3 ROS1 Analyte Control Product Details 4 ROS1 Analyte
Quality in Control ROS1 Analyte Control Product Codes: HCL022, HCL023 and HCL024 Contents What is ROS1? 2 The Role of ROS1 in Cancer 3 ROS1 Assessment 3 ROS1 Analyte Control Product Details 4 ROS1 Analyte
MEDICAL POLICY. Proprietary Information of YourCare Health Plan
 MEDICAL POLICY SUBJECT: HER-2 TESTING IN INVASIVE BREAST OR PAGE: 1 OF: 7 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
MEDICAL POLICY SUBJECT: HER-2 TESTING IN INVASIVE BREAST OR PAGE: 1 OF: 7 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases,
ALK Fusion Oncogenes in Lung Adenocarcinoma
 ALK Fusion Oncogenes in Lung Adenocarcinoma Vincent A Miller, MD Associate Attending Physician, Thoracic Oncology Service Memorial Sloan-Kettering Cancer Center New York, New York The identification of
ALK Fusion Oncogenes in Lung Adenocarcinoma Vincent A Miller, MD Associate Attending Physician, Thoracic Oncology Service Memorial Sloan-Kettering Cancer Center New York, New York The identification of
European Commission approves Roche s Alecensa (alectinib) as first-line treatment in ALK-positive lung cancer
 Media Release Basel, 21 December 2017 European Commission approves Roche s Alecensa (alectinib) as first-line treatment in ALK-positive lung cancer Alecensa provides a new treatment option for people with
Media Release Basel, 21 December 2017 European Commission approves Roche s Alecensa (alectinib) as first-line treatment in ALK-positive lung cancer Alecensa provides a new treatment option for people with
Molecular Testing Updates. Karen Rasmussen, PhD, FACMG Clinical Molecular Genetics Spectrum Medical Group, Pathology Division Portland, Maine
 Molecular Testing Updates Karen Rasmussen, PhD, FACMG Clinical Molecular Genetics Spectrum Medical Group, Pathology Division Portland, Maine Keeping Up with Predictive Molecular Testing in Oncology: Technical
Molecular Testing Updates Karen Rasmussen, PhD, FACMG Clinical Molecular Genetics Spectrum Medical Group, Pathology Division Portland, Maine Keeping Up with Predictive Molecular Testing in Oncology: Technical
Androgen Receptor Expression in Renal Cell Carcinoma: A New Actionable Target?
 Androgen Receptor Expression in Renal Cell Carcinoma: A New Actionable Target? New Frontiers in Urologic Oncology Juan Chipollini, MD Clinical Fellow Department of Genitourinary Oncology Moffitt Cancer
Androgen Receptor Expression in Renal Cell Carcinoma: A New Actionable Target? New Frontiers in Urologic Oncology Juan Chipollini, MD Clinical Fellow Department of Genitourinary Oncology Moffitt Cancer
MEDICAL POLICY. Proprietary Information of Excellus Health Plan, Inc. A nonprofit independent licensee of the BlueCross BlueShield Association
 MEDICAL POLICY SUBJECT: HER-2 TESTING IN INVASIVE BREAST OR PAGE: 1 OF: 7 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
MEDICAL POLICY SUBJECT: HER-2 TESTING IN INVASIVE BREAST OR PAGE: 1 OF: 7 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product,
Corporate Medical Policy
 Corporate Medical Policy Proteomic Testing for Targeted Therapy in Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomic_testing_for_targeted_therapy_in_non_small_cell_lung_cancer
Corporate Medical Policy Proteomic Testing for Targeted Therapy in Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: proteomic_testing_for_targeted_therapy_in_non_small_cell_lung_cancer
A study of EGFR wild-type non-small cell lung cancer ALK genetic mutation
 Original Article A study of EGFR wild-type non-small cell lung cancer ALK genetic mutation Shoufeng Wang, Naiquan Mao, Chuantian Zuo, Hong Pan, Tong Xie, Yaoyuan Huang, Qi Pan, Junwei Wu Department of
Original Article A study of EGFR wild-type non-small cell lung cancer ALK genetic mutation Shoufeng Wang, Naiquan Mao, Chuantian Zuo, Hong Pan, Tong Xie, Yaoyuan Huang, Qi Pan, Junwei Wu Department of
Non-Small Cell Lung Carcinoma - Myers
 Role of Routine Histology and Special Testing in Managing Patients with Non- Small Cell Lung Carcinoma Jeffrey L. Myers, M.D. A. James French Professor Director, Anatomic Pathology & MLabs University of
Role of Routine Histology and Special Testing in Managing Patients with Non- Small Cell Lung Carcinoma Jeffrey L. Myers, M.D. A. James French Professor Director, Anatomic Pathology & MLabs University of
Quality in Control ALK-Lung Analyte Control (EML4-ALK) ALK-Lymphoma Analyte Control (NPM-ALK)
 Quality in Control ALK-Lung Analyte Control (EML4-ALK) ALK-Lymphoma Analyte Control (NPM-ALK) 10 Product Codes: HCL007, HCL008 and HCL009 HCL010, HCL011 and HCL012 Page 1 of Contents 1. What is ALK?...
Quality in Control ALK-Lung Analyte Control (EML4-ALK) ALK-Lymphoma Analyte Control (NPM-ALK) 10 Product Codes: HCL007, HCL008 and HCL009 HCL010, HCL011 and HCL012 Page 1 of Contents 1. What is ALK?...
SUBJECT: GENOTYPING - EPIDERMAL GROWTH
 MEDICAL POLICY SUBJECT: GENOTYPING - EPIDERMAL GROWTH Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature
MEDICAL POLICY SUBJECT: GENOTYPING - EPIDERMAL GROWTH Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature
Trends in the incidence of thyroid cancer, Israel,
 Trends in the incidence of thyroid cancer, Israel, 1980-2012 Lital Keinan-Boker, MD, MPH, PhD, Barbara G. Silverman, MD, MPH Israel National Cancer Registry Israel Center for Disease Control Israel Ministry
Trends in the incidence of thyroid cancer, Israel, 1980-2012 Lital Keinan-Boker, MD, MPH, PhD, Barbara G. Silverman, MD, MPH Israel National Cancer Registry Israel Center for Disease Control Israel Ministry
Frequency of EGFR Mutation and EML4-ALK fusion gene in Arab Patients with Adenocarcinoma of the Lung
 HeSMO 6(2) 2015 19 23 DOI: 10.1515/fco-2015-0009 Forum of Clinical Oncology Frequency of EGFR Mutation and EML4-ALK fusion gene in Arab Patients with Adenocarcinoma of the Lung Hanan Ezzat Shafik 1 *,
HeSMO 6(2) 2015 19 23 DOI: 10.1515/fco-2015-0009 Forum of Clinical Oncology Frequency of EGFR Mutation and EML4-ALK fusion gene in Arab Patients with Adenocarcinoma of the Lung Hanan Ezzat Shafik 1 *,
Immunotherapy in NSCLC Pathologist role
 Immunotherapy in NSCLC Pathologist role Pimpin Incharoen, M.D. Assistant Professor, Thoracic Pathology Department of Pathology, Ramathibodi Hospital Genetic alterations in NSCLC Khono et al, Trans Lung
Immunotherapy in NSCLC Pathologist role Pimpin Incharoen, M.D. Assistant Professor, Thoracic Pathology Department of Pathology, Ramathibodi Hospital Genetic alterations in NSCLC Khono et al, Trans Lung
Interpretation Guide. Product Name: ALK Cell Line Analyte Control Product Codes: ALK2/CS and ALK2/CB. Page 1 of 9
 Interpretation Guide Product Name: ALK Cell Line Analyte Control Product Codes: ALK2/CS and ALK2/CB ALK2/CS/CB_IG_V_001 www.histocyte.com Page 1 of 9 Contents 1. What is ALK?... 2 2. Role of ALK in Cancer...
Interpretation Guide Product Name: ALK Cell Line Analyte Control Product Codes: ALK2/CS and ALK2/CB ALK2/CS/CB_IG_V_001 www.histocyte.com Page 1 of 9 Contents 1. What is ALK?... 2 2. Role of ALK in Cancer...
Disclosures Genomic testing in lung cancer
 Disclosures Genomic testing in lung cancer No disclosures Objectives Understand how FISH and NGS provide complementary data for the evaluation of lung cancer Recognize the challenges of performing testing
Disclosures Genomic testing in lung cancer No disclosures Objectives Understand how FISH and NGS provide complementary data for the evaluation of lung cancer Recognize the challenges of performing testing
Lung cancer is the leading cause of cancer-related
 Special Article Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors Guideline from the College of American Pathologists, International Association
Special Article Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors Guideline from the College of American Pathologists, International Association
The Non-$mall Cost of Non-Small Cell Lung Cancer
 The Non-$mall Cost of Non-Small Cell Lung Cancer CPT Zachary Leftwich, PharmD PHY-1 Managed Care Pharmacy Resident Defense Health Agency Pharmacy Operations Division, San Antonio, TX May 4 th 2018 At the
The Non-$mall Cost of Non-Small Cell Lung Cancer CPT Zachary Leftwich, PharmD PHY-1 Managed Care Pharmacy Resident Defense Health Agency Pharmacy Operations Division, San Antonio, TX May 4 th 2018 At the
MP Circulating Tumor DNA Management of Non-Small-Cell Lung Cancer (Liquid Biopsy)
 Medical Policy BCBSA Ref. Policy: 2.04.143 Last Review: 10/18/2018 Effective Date: 10/18/2018 Section: Medicine Related Policies 2.04.121 Miscellaneous Genetic and Molecular Diagnostic Tests 2.04.45 Molecular
Medical Policy BCBSA Ref. Policy: 2.04.143 Last Review: 10/18/2018 Effective Date: 10/18/2018 Section: Medicine Related Policies 2.04.121 Miscellaneous Genetic and Molecular Diagnostic Tests 2.04.45 Molecular
Introduction: Overview of Current Status of Lung Cancer Predictive Biomarkers
 Introduction: Overview of Current Status of Lung Cancer Predictive Biomarkers Program 7:15 7:40 Translocations as predictive biomarkers in lung cancer: Overview Mari Mino Kenudson, MD 7:40 8:05 Translocation
Introduction: Overview of Current Status of Lung Cancer Predictive Biomarkers Program 7:15 7:40 Translocations as predictive biomarkers in lung cancer: Overview Mari Mino Kenudson, MD 7:40 8:05 Translocation
FDA approves Roche s Alecensa (alectinib) as first-line treatment for people with specific type of lung cancer
 Media Release Basel, 07 November 2017 FDA approves Roche s Alecensa (alectinib) as first-line treatment for people with specific type of lung cancer Approval based on phase III results that showed Alecensa
Media Release Basel, 07 November 2017 FDA approves Roche s Alecensa (alectinib) as first-line treatment for people with specific type of lung cancer Approval based on phase III results that showed Alecensa
Integration of Genomics Into Clinical Pathways. Precision Medicine and Decision Support
 Integration of Genomics Into Clinical Pathways Precision Medicine and Decision Support Faculty Andrew Hertler, MD, FACP Chief Medical Officer New Century Health Andrew Hertler, MD, FACP is employed by
Integration of Genomics Into Clinical Pathways Precision Medicine and Decision Support Faculty Andrew Hertler, MD, FACP Chief Medical Officer New Century Health Andrew Hertler, MD, FACP is employed by
Media Release. FDA grants Roche s Perjeta accelerated approval for use before surgery in people with HER2-positive early stage breast cancer
 Media Release Basel, 1 October 2013 FDA grants Roche s Perjeta accelerated approval for use before surgery in people with HER2-positive early stage breast cancer The Perjeta regimen is the first treatment
Media Release Basel, 1 October 2013 FDA grants Roche s Perjeta accelerated approval for use before surgery in people with HER2-positive early stage breast cancer The Perjeta regimen is the first treatment
When Policy Topic is covered Crizotinib may be considered medically necessary in patients 18 years or older when the following criteria are met:
 Xalkori (crizotinib) Policy Number: 5.01.539 Last Review: 6/2018 Origination: 7/2012 Next Review: 6/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for crizotinib
Xalkori (crizotinib) Policy Number: 5.01.539 Last Review: 6/2018 Origination: 7/2012 Next Review: 6/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for crizotinib
D Ross Camidge, MD, PhD
 i n t e r v i e w D Ross Camidge, MD, PhD Dr Camidge is Director of the Thoracic Oncology Clinical Program and Associate Director for Clinical Research at the University of Colorado Cancer Center in Aurora,
i n t e r v i e w D Ross Camidge, MD, PhD Dr Camidge is Director of the Thoracic Oncology Clinical Program and Associate Director for Clinical Research at the University of Colorado Cancer Center in Aurora,
2 nd line Therapy and Beyond NSCLC. Alan Sandler, M.D. Oregon Health & Science University
 2 nd line Therapy and Beyond NSCLC Alan Sandler, M.D. Oregon Health & Science University Treatment options for advanced or metastatic (stage IIIb/IV) NSCLC Suitable for chemotherapy Diagnosis Unsuitable/unwilling
2 nd line Therapy and Beyond NSCLC Alan Sandler, M.D. Oregon Health & Science University Treatment options for advanced or metastatic (stage IIIb/IV) NSCLC Suitable for chemotherapy Diagnosis Unsuitable/unwilling
Lihong Ma 1 *, Zhengbo Song 2 *, Yong Song 1, Yiping Zhang 2. Original Article
 Original Article MET overexpression coexisting with epidermal growth factor receptor mutation influence clinical efficacy of EGFR-tyrosine kinase inhibitors in lung adenocarcinoma patients Lihong Ma 1
Original Article MET overexpression coexisting with epidermal growth factor receptor mutation influence clinical efficacy of EGFR-tyrosine kinase inhibitors in lung adenocarcinoma patients Lihong Ma 1
Targeted Therapy for NSCLC: EGFR and ALK Fadlo R. Khuri, MD
 EGFR and ALK Fadlo R. Khuri, MD President, American University of Beirut Professor of Medicine July 26, 2018 A great year end! Targeted Therapy for NSCLC: Evolving Landscape of Lung Adenocarcinoma NSCLC
EGFR and ALK Fadlo R. Khuri, MD President, American University of Beirut Professor of Medicine July 26, 2018 A great year end! Targeted Therapy for NSCLC: Evolving Landscape of Lung Adenocarcinoma NSCLC
Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY
 Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY Methodology The test is based on the reported 50-gene classifier algorithm originally named PAM50 and is performed on the ncounter Dx Analysis System
Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY Methodology The test is based on the reported 50-gene classifier algorithm originally named PAM50 and is performed on the ncounter Dx Analysis System
Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY
 Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY GENE EXPRESSION PROFILING WITH PROSIGNA What is Prosigna? Prosigna Breast Cancer Prognostic Gene Signature Assay is an FDA-approved assay which provides
Prosigna BREAST CANCER PROGNOSTIC GENE SIGNATURE ASSAY GENE EXPRESSION PROFILING WITH PROSIGNA What is Prosigna? Prosigna Breast Cancer Prognostic Gene Signature Assay is an FDA-approved assay which provides
Product Introduction
 Product Introduction Product Codes: HCL026, HCL027 and HCL028 Contents Introduction to HER2 2 HER2 immunohistochemistry 3 Cell lines as controls 5 HER2 Analyte Control DR IHC 7 HER2 Analyte Control DR
Product Introduction Product Codes: HCL026, HCL027 and HCL028 Contents Introduction to HER2 2 HER2 immunohistochemistry 3 Cell lines as controls 5 HER2 Analyte Control DR IHC 7 HER2 Analyte Control DR
Beyond ALK and EGFR: Novel molecularly driven targeted therapies in NSCLC Federico Cappuzzo AUSL della Romagna, Ravenna, Italy
 Beyond ALK and EGFR: Novel molecularly driven targeted therapies in NSCLC Federico Cappuzzo AUSL della Romagna, Ravenna, Italy Oncogenic drivers in NSCLC Certain tumours arise as a result of aberrant activation
Beyond ALK and EGFR: Novel molecularly driven targeted therapies in NSCLC Federico Cappuzzo AUSL della Romagna, Ravenna, Italy Oncogenic drivers in NSCLC Certain tumours arise as a result of aberrant activation
Predictive markers for treatment with Immune checkpoint inhibitors - PD-L1 et al -
 Predictive markers for treatment with Immune checkpoint inhibitors - PD-L1 et al - Lukas Bubendorf Pathology Improved overall survival as a result of combination therapy Predictive biomarkers for the treatment
Predictive markers for treatment with Immune checkpoint inhibitors - PD-L1 et al - Lukas Bubendorf Pathology Improved overall survival as a result of combination therapy Predictive biomarkers for the treatment
Disclosure of Relevant Financial Relationships NON-SMALL CELL LUNG CANCER: 70% PRESENT IN ADVANCED STAGE
 MORPHOLOGY AND MOLECULAR TESTING IN NON-SMALL CELL OF LUNG NEW FRONTIEIRS IN CYTOPATHOLOGY PRACTICE American Society for Cytopathology San Antonio, Texas Sunday March 5, 2017 Disclosure of Relevant Financial
MORPHOLOGY AND MOLECULAR TESTING IN NON-SMALL CELL OF LUNG NEW FRONTIEIRS IN CYTOPATHOLOGY PRACTICE American Society for Cytopathology San Antonio, Texas Sunday March 5, 2017 Disclosure of Relevant Financial
Media Release. Basel, 07 December 2017
 Media Release Basel, 07 December 2017 Phase III IMpower150 study showed Tecentriq (atezolizumab) and Avastin (bevacizumab) plus chemotherapy reduced the risk of disease worsening or death by 38 percent
Media Release Basel, 07 December 2017 Phase III IMpower150 study showed Tecentriq (atezolizumab) and Avastin (bevacizumab) plus chemotherapy reduced the risk of disease worsening or death by 38 percent
Plotting the course: optimizing treatment strategies in patients with advanced adenocarcinoma
 Pieter E. Postmus University of Liverpool Liverpool, UK Plotting the course: optimizing treatment strategies in patients with advanced adenocarcinoma Disclosures Advisor Bristol-Myers Squibb AstraZeneca
Pieter E. Postmus University of Liverpool Liverpool, UK Plotting the course: optimizing treatment strategies in patients with advanced adenocarcinoma Disclosures Advisor Bristol-Myers Squibb AstraZeneca
Original Articles. Implications for Optimal Clinical Testing
 Original Articles Comparison of Reverse Transcription-Polymerase Chain Reaction, Immunohistochemistry, and Fluorescence In Situ Hybridization Methodologies for Detection of Echinoderm Microtubule-Associated
Original Articles Comparison of Reverse Transcription-Polymerase Chain Reaction, Immunohistochemistry, and Fluorescence In Situ Hybridization Methodologies for Detection of Echinoderm Microtubule-Associated
Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors
 SPECIAL Article Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors Guideline from the College of American Pathologists, International Association
SPECIAL Article Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors Guideline from the College of American Pathologists, International Association
3/23/2017. Disclosure of Relevant Financial Relationships. Pathologic Staging Updates in Lung Cancer T STAGE OUTLINE SURVIVAL ACCORDING TO SIZE ONLY
 Pathologic Staging Updates in Lung Cancer Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence or control the content of CME
Pathologic Staging Updates in Lung Cancer Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence or control the content of CME
NCCN Non-Small Cell Lung Cancer V Meeting June 15, 2018
 Guideline Page and Request Illumina Inc. requesting to replace Testing should be conducted as part of broad molecular profiling with Consider NGS-based assays that include EGFR, ALK, ROS1, and BRAF as
Guideline Page and Request Illumina Inc. requesting to replace Testing should be conducted as part of broad molecular profiling with Consider NGS-based assays that include EGFR, ALK, ROS1, and BRAF as
Alectinib Versus Crizotinib for Previously Untreated Alk-positive Advanced Non-small Cell Lung Cancer : A Meta-Analysis
 Showa Univ J Med Sci 30 2, 309 315, June 2018 Original Alectinib Versus Crizotinib for Previously Untreated Alk-positive Advanced Non-small Cell Lung Cancer : A Meta-Analysis Ryo MANABE 1, Koichi ANDO
Showa Univ J Med Sci 30 2, 309 315, June 2018 Original Alectinib Versus Crizotinib for Previously Untreated Alk-positive Advanced Non-small Cell Lung Cancer : A Meta-Analysis Ryo MANABE 1, Koichi ANDO
ALK gene expression status in pleural effusion predicts tumor responsiveness to crizotinib in Chinese patients with lung adenocarcinoma
 Original Article ALK gene expression status in pleural effusion predicts tumor responsiveness to crizotinib in Chinese patients with lung adenocarcinoma Zheng Wang 1, Xiaonan Wu 2, Xiaohong Han 3, Gang
Original Article ALK gene expression status in pleural effusion predicts tumor responsiveness to crizotinib in Chinese patients with lung adenocarcinoma Zheng Wang 1, Xiaonan Wu 2, Xiaohong Han 3, Gang
Analysis of clinical characteristics and prognosis of patients with anaplastic lymphoma kinase-positive and surgically resected lung adenocarcinoma
 Thoracic Cancer ISSN 1759-7706 ORIGINAL ARTICLE Analysis of clinical characteristics and prognosis of patients with anaplastic lymphoma kinase-positive and surgically resected lung adenocarcinoma Hong
Thoracic Cancer ISSN 1759-7706 ORIGINAL ARTICLE Analysis of clinical characteristics and prognosis of patients with anaplastic lymphoma kinase-positive and surgically resected lung adenocarcinoma Hong
Xalkori. Xalkori (crizotinib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.12 Subject: Xalkori Page: 1 of 6 Last Review Date: June 22, 2017 Xalkori Description Xalkori (crizotinib)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.12 Subject: Xalkori Page: 1 of 6 Last Review Date: June 22, 2017 Xalkori Description Xalkori (crizotinib)
Roche receives FDA clearance for the VENTANA MMR IHC Panel for patients diagnosed with colorectal cancer
 Media Release Roche receives FDA clearance for the VENTANA MMR IHC Panel for patients diagnosed with colorectal cancer The VENTANA MMR IHC Panel 1 helps differentiate between sporadic colorectal cancer
Media Release Roche receives FDA clearance for the VENTANA MMR IHC Panel for patients diagnosed with colorectal cancer The VENTANA MMR IHC Panel 1 helps differentiate between sporadic colorectal cancer
Comprehensive Genomic Profiling, in record time. Accurate. Clinically Proven. Fast.
 Comprehensive Genomic Profiling, in record time Accurate. ly Proven. Fast. PCDx advantages Comprehensive genomic profiling, in record time PCDx Comprehensive Genomic Profiling (CGP) provides precise information
Comprehensive Genomic Profiling, in record time Accurate. ly Proven. Fast. PCDx advantages Comprehensive genomic profiling, in record time PCDx Comprehensive Genomic Profiling (CGP) provides precise information
TESTING OF ROS1-POSITIVE TUMORS BY IHC DISPLAYS A FISH-POSITIVE SUBGROUP WHICH MIGHT NOT BENEFIT FROM RECENTLY APPROVED DRUG THERAPY
 TESTING OF ROS1-POSITIVE TUMORS BY IHC DISPLAYS A FISH-POSITIVE SUBGROUP WHICH MIGHT NOT BENEFIT FROM RECENTLY APPROVED DRUG THERAPY Vanessa Rüsseler,MD Institut of Pathologie Uniklinik Köln Disclosure
TESTING OF ROS1-POSITIVE TUMORS BY IHC DISPLAYS A FISH-POSITIVE SUBGROUP WHICH MIGHT NOT BENEFIT FROM RECENTLY APPROVED DRUG THERAPY Vanessa Rüsseler,MD Institut of Pathologie Uniklinik Köln Disclosure
DM Seminar. ALK gene rearrangements & ALK targeted therapy in NSCLC Dr Sarat
 DM Seminar ALK gene rearrangements & ALK targeted therapy in NSCLC Dr Sarat Introduction Discovery of activating mutations in kinase domain of epidermal growth factor receptor (EGFR) opened a new era of
DM Seminar ALK gene rearrangements & ALK targeted therapy in NSCLC Dr Sarat Introduction Discovery of activating mutations in kinase domain of epidermal growth factor receptor (EGFR) opened a new era of
Targeted therapies for advanced non-small cell lung cancer. Tom Stinchcombe Duke Cancer Insitute
 Targeted therapies for advanced non-small cell lung cancer Tom Stinchcombe Duke Cancer Insitute Topics ALK rearranged NSCLC ROS1 rearranged NSCLC EGFR mutation: exon 19/exon 21 L858R and uncommon mutations
Targeted therapies for advanced non-small cell lung cancer Tom Stinchcombe Duke Cancer Insitute Topics ALK rearranged NSCLC ROS1 rearranged NSCLC EGFR mutation: exon 19/exon 21 L858R and uncommon mutations
Assessment Run C1 2017
 Assessment Run C1 2017 PD-L1 The first assessment in this new NordiQC Companion module C1 focused on the accuracy of the PD-L1 IHC assays performed by the participating laboratories to identify patients
Assessment Run C1 2017 PD-L1 The first assessment in this new NordiQC Companion module C1 focused on the accuracy of the PD-L1 IHC assays performed by the participating laboratories to identify patients
Medical Policy. MP Molecular Analysis for Targeted Therapy of Non-Small-Cell Lung Cancer
 Medical Policy MP 2.04.45 BCBSA Ref. Policy: 2.04.45 Last Review: 10/30/2017 Effective Date: 10/30/2017 Section: Medicine Related Policies 2.04.115 Molecular Panel Testing of Cancers to Identify Targeted
Medical Policy MP 2.04.45 BCBSA Ref. Policy: 2.04.45 Last Review: 10/30/2017 Effective Date: 10/30/2017 Section: Medicine Related Policies 2.04.115 Molecular Panel Testing of Cancers to Identify Targeted
State of the Art Treatment of Lung Cancer Ravi Salgia, MD, PhD
 State of the Art Treatment of Lung Cancer Ravi Salgia, MD, PhD Professor and Chair Arthur & Rosalie Kaplan Chair Medical Oncology and Therapeutics Research Nothing to disclose DISCLOSURE Objectives Lung
State of the Art Treatment of Lung Cancer Ravi Salgia, MD, PhD Professor and Chair Arthur & Rosalie Kaplan Chair Medical Oncology and Therapeutics Research Nothing to disclose DISCLOSURE Objectives Lung
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Circulating Tumor DNA Management of Non-Small-Cell Lung Cancer (Liquid Biopsy) Page 1 of 36 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Circulating Tumor DNA
Circulating Tumor DNA Management of Non-Small-Cell Lung Cancer (Liquid Biopsy) Page 1 of 36 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Circulating Tumor DNA
